Specialized Techniques
14A
Multiple Sleep Latency Testing
The most common indication for referring a patient for laboratory assessment is excessive daytime sleepiness (EDS), although sleep onset and sleep maintenance insomnia is the most common complaint in the general population. The initial step in assessment of a patient with EDS is detailed sleep and other histories, and physical examination. For assessment of persistent sleepiness, the Epworth Sleepiness Scale (ESS) is often used to assess a general level of sleepiness. This is a subjective propensity to sleepiness assessed by the patient under eight situations on a scale of 0 to 3, with 3 indicating a situation when chances of dozing off are highest. The maximum score is 24, and a score of 10 suggests the presence of EDS. This test has been weakly correlated with multiple sleep latency test (MSLT) scores. The ESS and MSLT, however, test different types of sleepiness. MSLT tests the propensity to sleepiness objectively, and ESS tests the general feeling of sleepiness or subjective propensity to sleepiness. The Stanford Sleepiness Scale is a 7-point analog scale to measure subjective sleepiness, but it does not measure persistent sleepiness. Visual Analog Scale is the other scale used to assess alertness and well-being, in which subjects indicate their feelings of alertness at an arbitrary point on a line of 0- to 100-mm scale, with 100 being the maximum sleepiness and 0 being the most alert.
Technique of Multiple Sleep Latency Test
The MSLT has been standardized and includes several general and specific procedures. The general procedures before the actual recording include keeping a sleep diary for 1 to 2 weeks before the test, which records the information about bedtime, time of rising, napping, and any drug use. The test is preceded by an overnight polysomnographic (PSG) study, and MSLT is scheduled about 2 to 3 hours after the conclusion of the overnight PSG study. The actual test consists of four to five opportunities for napping at 2-hour intervals, and each recording session lasts for 20 minutes. Between tests subjects must remain awake. The subjects must not smoke for 30 minutes before lights are turned off. Physiological calibrations (i.e., grit teeth, blink your eyes, look up, look down, look to the right, look to the left, open your eyes, and close your eyes) are then performed, and the patient is instructed to relax and fall asleep, and the lights are turned off. The test must be conducted in a quiet, dark room. The specific recording includes three or more channels of electroencephalography (EEG), submental electromyography (EMG), and electro-oculography (EOG) recordings. Three to four channels of EEG (F3-A2, C3-A2, 01-A2, and C4-A1) are recommended to document alpha activity in relaxed wakefulness in adults and its disappearance at sleep onset.
The measurements include average sleep-onset latency and the presence of sleep-onset rapid eye movements (SOREMs). If no sleep occurs, then the test is concluded 20 minutes after lights are turned off. The time from lights out to the first onset of any stage of sleep is defined as sleep latency. The test is terminated 15 minutes after the first 30-second epoch of any stage of sleep. If the finding is indefinite, then it is better to continue the test than to end it prematurely. Mean sleep latency is calculated from the sum of the latency to sleep onset for each of the four to five naps. Mean sleep latency of less than 8 minutes is consistent with pathological sleepiness. A mean sleep latency of 8 to 10 minutes is consistent with mild sleepiness. The occurrence of rapid eye movement (REM) sleep within 15 minutes of sleep onset is defined as SOREMs.
Repeat MSLT is required if the patient is strongly suspected of having narcolepsy but did not show the characteristic findings, as may be seen in a certain percentage of narcolepsy patients. MSLT may not be diagnostic in the initial test, and the diagnostic yield increases after the second test. The other situation for repeating MSLT is when the findings are ambiguous and the sleep onset or REM sleep cannot be adequately interpreted. Finally, if the MSLT guidelines have not been followed, the test results may be invalid.
Indications for Multiple Sleep Latency Test
The American Academy of Sleep Medicine (AASM) Standards of Practice Committee recommended indications for MSLT. Narcolepsy is the single most important indication for performing the MSLT (Fig. 14A.1). A mean sleep latency of less than 8 minutes combined with SOREMs in two or more of the four to five recordings during MSLT is strongly suggestive of narcolepsy, although REM sleep dysregulation and circadian rhythm sleep disorders may also lead to such findings.
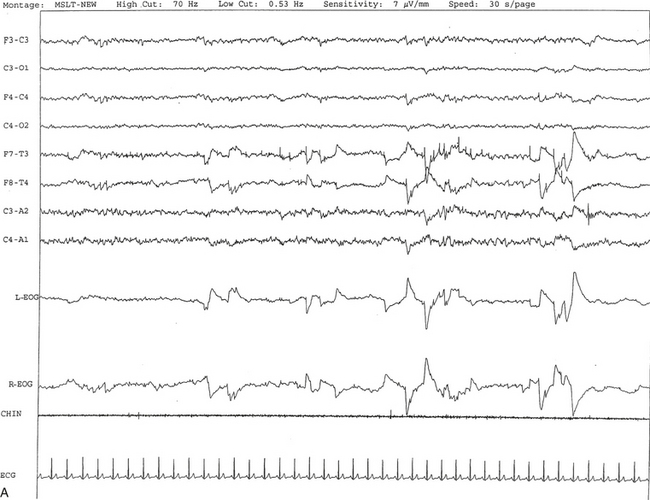
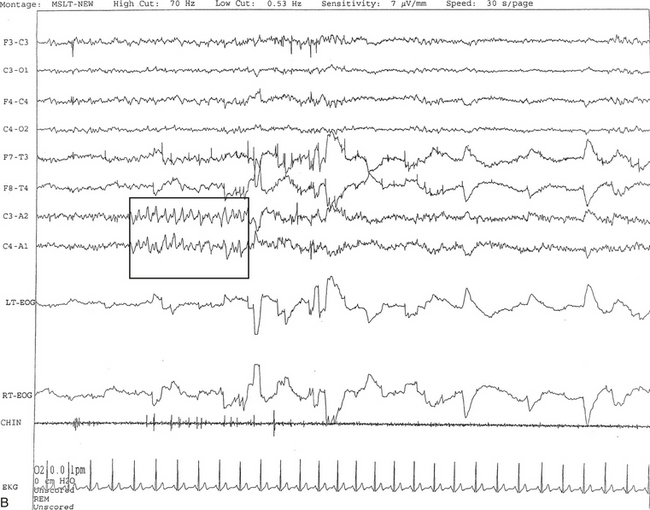
FIGURE 14A.1 A case of narcolepsy.
A 60-year-old woman with new onset of intermittent episodes of sudden transient bilateral leg weakness, excessive daytime sleepiness, and intermittent periods of transient confusion. A daytime electroencephalogram (EEG) was normal. Overnight polysomnography was significant for sleep architecture changes with an immediate sleep-onset latency, presence of only one rapid eye movement (REM) cycle, with a decreased REM sleep percentage (7%), an increased arousal index of 23 without associated apnea or periodic limb movements, and with excessive fragmentary myoclonus in non-REM and REM sleep. The multiple sleep latency test (MSLT) showed a mean sleep latency of 1.6 minutes consistent with pathological sleepiness and the presence of two (out of four) sleep-onset REM naps, suggestive of REM sleep dysregulation as seen in narcolepsy. A, A 30-second epoch from the MSLT showing the presence of sleep-onset REM occurring 7 minutes after sleep onset. Prominent REMs are seen in the electro-oculogram (EOG) channels and anterior temporal EEG electrodes. B, A 30-second epoch taken from the same sleep nap as in A, showing the presence of prominent sawtooth waves (boxed) in C3 and C4 electrode channels referenced to contralateral ears. Eye movements characteristic of REM sleep are noted as described earlier. Top eight channels, EEG; L-EOG and R-EOG, left and right EOG; CHIN, electromyography of chin; ECG, electrocardiography.
In patients with upper airway obstructive sleep apnea syndrome (OSAS), the MSLT is not routinely indicated in the initial evaluation and diagnosis or in assessment of change following treatment with nasal continuous positive airway pressure (CPAP). However, those patients previously diagnosed with OSAS or other sleep-related breathing disorder, periodic limb movement disorder, or mood disorders who continue to have excessive sleepiness despite optimal treatment may require evaluation by the MSLT to exclude associated narcolepsy. The coexistence of OSAS and narcolepsy is well known.
An MSLT is indicated in patients suspected of having idiopathic hypersomnia; in this condition the MSLT findings will be consistent with pathological sleepiness but without SOREMs.
In patients with medical and neurological disorders (other than narcolepsy), insomnia, or circadian rhythm disorders, the MSLT is not routinely indicated for evaluation of sleepiness.
The following are the recommended indications for repeat MSLT: (1) extraneous circumstances or inappropriate conditions affecting the initial MSLT, (2) presence of ambiguous or uninterpretable findings, (3) initial MSLT without polygraphic confirmation in a patient suspected of having narcolepsy.
Reliability, Validity, and Limitation of the Multiple Sleep Latency Test
The sensitivity and specificity of the MSLT in detecting sleepiness have not been clearly determined. The test-retest reliability of the MSLT, however, has been documented in both normal subjects and patients with narcolepsy. In subjects with sleepiness caused by circadian rhythm sleep disorders, sleep deprivation, and ingestion of hypnotics and alcohol, pathological sleepiness has been validated by the MSLT. However, there is poor correlation between the MSLT and ESS. The patient’s psychological and behavioral state also interferes with the MSLT results. If the patient suffers from severe anxiety or psychological disturbances causing behavioral stimulation, MSLT may not show sleepiness even in a patient complaining of EDS. Day-to-day variability in the degree of sleepiness and an inability to cooperate or understand instructions are other factors for unreliability. Furthermore, pathological sleepiness with two or more SOREMs may occasionally be seen in other conditions (e.g., OSAS, REM sleep dysregulation, circadian rhythm sleep disorders). In addition, the MSLT measures propensity to fall asleep in an environment (e.g., sleep laboratory) conducive to sleep but not in other conditions (e.g., work or driving). One should also beware of false-positive and false-negative test results. In one study only 80% of cases of narcolepsy were diagnostically positive on the initial MSLT. Repeat studies showed positive results in 85% to 90%, but a small percentage of narcolepsy-cataplexy cases may not show two or more s even on repeated studies.
Maintenance of Wakefulness Test
The maintenance of wakefulness test (MWT) is a variant of the MSLT that measures an individual’s ability to stay awake. It should be clearly understood that the MSLT and MWT assess separate functions: The MSLT unmasks physiological sleepiness, which depends on both circadian and homeostatic factors; in contrast, the MWT is a reflection of the individual’s capability to resist sleep and is influenced by physiological sleepiness.
Technique of the MWT
The MWT protocols require four trials at 2-hour intervals to test an individual’s ability to stay awake. Based on studies published in the peer-reviewed literature, the Standards of Practice Committee of the AASM recommended the MWT 40-minute protocol. Unlike the MSLT, the MWT does not require prior overnight polysomnography. The test is performed about 1½ to 3 hours after the individual’s usual wake-up time. The recording montage is similar to that used for the MSLT. The patient calibrations before each test are similar to those used for the MSLT. Before the beginning of the recording the patients are asked to sit still in bed with a back and headrest and remain awake as long as possible. They are not allowed to read, use headphones to listen to music, use a mobile phone, watch television, or use any digital device to read during each trial of 40 minutes. Sleep onset is defined as the time between lights off in the beginning of the recording and the onset of three consecutive epochs of stage 1 non-REM (NREM) or one epoch of any other stage of sleep. The test should be terminated after sleep onset or after 40 minutes if no sleep occurs. A mean sleep latency (the arithmetic mean of the four trials) of less than 8 minutes is considered abnormal as recommended by the Standards of Practice Committee of the AASM; values greater than this but less than 40 minutes are of uncertain significance.
Indications for the MWT
The AASM Standards of Practice Committee recommended the following indications for the MWT:
• Assessment of individuals employed in occupations involving public transportation or safety for their ability to remain awake
• Assessment of response to treatment (e.g., response to stimulants in narcolepsy and CPAP titration in OSAS patients)
There is a clear need for further research to obtain well-defined normative data, sensitivity, and specificity of the MWT to assess ability to stay awake.
Aldrich, M. S. Sleep Medicine. New York: Oxford University Press; 1999.
Arand, D., Bonnet, M., Hurwitz, T., et al. The clinical use of the MSLT and MWT. Sleep. 2005; 28:123–144.
Carskadon, M. A., Dement, W. C., Mitler, M., et al. Guidelines for the multiple sleep latency test MSLT: A standard measure of sleepiness. Sleep. 1986; 9:519–524.
Chervin, R. Assessment of sleepiness. In Chokroverty S., Montagna P., Allen R. P., Walters A. S., eds. : Sleep and Movement Disorders, 2nd ed, New York: Oxford University Press, 2013.
Doghramji, K. The maintenance of wakefulness test. In: Chokroverty S., ed. Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects. 3rd ed. Philadelphia: Saunders/Elsevier; 2009:224–228.
Littner, M. R., Kushida, C., Wise, M., et al. Practice parameters for clinical use of the multiple sleep latency tests and the maintenance of wakefulness test. Sleep. 2005; 28:113–121.
14B
Actigraphy
An actigraph, also known as an actometer or actimeter, monitors body movements and other activities continuously for days, weeks, or even months. This can be worn on the wrist or alternatively on the ankle for recording arm, leg, and body movements. An actigraph uses piezoelectric sensors that function as accelerometers to record acceleration or deceleration of movements rather than the actual movement. The mechanical movements are converted into electrical signals, which are then sampled every tenth second over a predetermined time or epoch and then retrieved and analyzed in a computer. The principle of analysis is based on the fact that increased movements (as indicated by black bars in the actigraph) are seen during wakefulness in contrast to markedly decreased movements or no movements (as indicated by the white area interrupting the black bars) during sleep, although normal physiological body and limb movements and postural shifts during sleep will cause interruptions (black bars) of the white background (Fig. 14B.1). Several actigraph models are in the development stage to carefully regulate the sampling frequencies and duration, filters, sensitivities, and dynamic range to detect and quantify periodic limb movements in sleep (PLMS), but no generally accepted standardized technique of quantifying and identifying PLMS that differentiates them from other movements (e.g., those resulting from parasomnias, nocturnal seizures, and other dyskinesias) is currently available. Two of these devices (i.e., Actiwatch and PAM-RL) showed a reasonable correlation with PLMS observed with PSG study. Further validation studies in a large number of patients are needed before these can be used reliably to detect and quantify PLMS. Currently several devices are available (e.g., ambulatory monitoring, Actiwatch, PAM-RL) along with appropriate software, with or without light or position sensors, to determine if the subject is sitting, lying, or upright. These devices can provide a direct assessment of the amount of activity or body movement at the site of attachment of the device. Studies have been conducted using actigraph and PSG recordings simultaneously to validate the ability of the actigraph to distinguish sleep from wakefulness. Computer algorithms are available for automatic sleep-wake scoring; however, visual inspection of the raw data is necessary. The reliability and validity of the data are available only for a specific actigraph model; no universally validated data are available. There is poor agreement between sleep characteristics obtained by actigraphy and subjective data from a sleep diary. Actigraphs can differentiate indirectly sleep from wakefulness (see Fig. 14B.1) but cannot differentiate REM from NREM sleep and cannot identify different NREM sleep stages. Actigraphs and sleep logs are complementary.
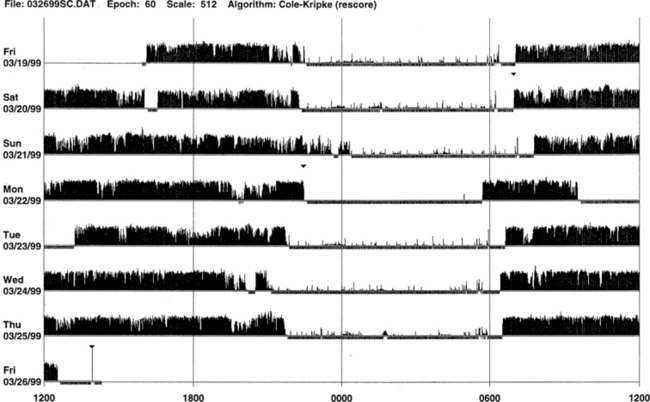
FIGURE 14B.1 Normal sleep-wake schedule.
Wrist actigraphic recording from a 55-year-old healthy woman without sleep complaints. This shows a fairly regular sleep-wake schedule except one weekend night (third from the top). She goes to bed between 10:30 PM and 11:00 PM and wakes up around 7:00 AM except on the third day. Physiological body shifts and movements during sleep are indicated by a few black bars in the white areas. The waking period is indicated by black bars.
Indications for Actigraph
The AASM Standards of Practice Committee made the following recommendations for actigraph:
• Actigraph may be a useful adjunct to history, physical examination, and sleep logs in patients with insomnia, including paradoxical sleep (sleep state misperception) and inadequate sleep hygiene (Figs. 14B.2 to 14B.7), and circadian rhythm sleep disorders (Figs. 14B.8 to 14B.13).
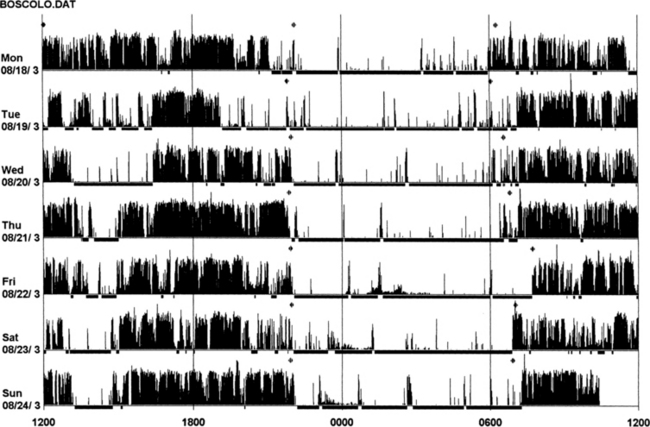
FIGURE 14B.2 Actigraphy in insomnia (sleep state misperception).
A 59-year-old man complaining of insomnia since the age of 12 years. His DSM IV axis 2 personality disorder (dependent personality) and panic attacks were diagnosed in the past and treated with benzodiazepines (chlordesmethyldiazepam 3 mg; flurazepam 30 mg) and zolpidem 10 mg. He denies any symptoms of restless legs syndrome, excessive daytime sleepiness, or daytime sleep attacks. Subjective sleep duration is 3 to 4 hours per night. In the past he had numerous drugs for sleep amelioration, but no clear and stable subjective improvement was noted. An actigraphic monitoring (during drug-reduction, chlordesmethyldiazepam 2 mg, flurazepam 15 mg, and no zolpidem) shows a clear misperception of sleep duration and quality. The recording shows normal nocturnal motor activity and sleep efficiency and duration. Note sleep period during the afternoon. He complained of sleeping not more than 3 hours each night. Polysomnography on the third night: total sleep time, 387 minutes; sleep efficiency, 73.5; wake after sleep onset, 122 minutes; number of awakenings, 17; slow wake sleep percentage, 1.3; periodic limb movements in sleep index, 1.9. DSM IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
FIGURE 14B.3 A case of chronic insomnia.
Wrist actigraph from a 71-year-old woman with complaints of sleep onset and maintenance insomnia for 30 years. She has had many hypnotics over the years with temporary benefits. Despite increased doses, the effects wore off over the years. She has had many “street drugs,” and now she takes cocaine every night at bedtime. She also complains of excessive daytime somnolence. The actigraph shows many periods of wakefulness (intrusion of black bars) during sleep period (white area) and intermittent brief periods of somnolence (intrusion of white areas into the black bars) during daytime wakefulness. This is an example of hypnotic-dependent and stimulant-dependent sleep disorder.
FIGURE 14B.4 A patient with sleep-onset insomnia and sleep apnea.
Wrist actigraph from a 31-year-old man who has been complaining of sleep-onset insomnia and excessive daytime sleepiness. The actigraph shows irregular sleep onset (delayed) and irregular wake-up time. There are periods of somnolence (intrusion of white areas into black bars) during waking period (black bars). Overnight polysomnographic study showed moderately severe upper airway obstructive sleep apnea (apnea-hypopnea index of 39.8) with oxygen desaturation.
FIGURE 14B.5 Parkinson’s disease and sleep disorder (see also Fig. 12.2).
Actigraphic recording from the right ankle of a 67-year-old patient with idiopathic Parkinson’s disease (Hoehn-Yahr stage 2: mild-moderate). The patient complains of insomnia and daytime hypersomnia. The actigraph shows disorganization of sleep-wake schedule with irregular sleep-wake onset and offset, increased activity (black bars in the white area) during nighttime sleep, and many periods of daytime somnolence (intrusions of white areas within the black bars). Overnight polysomnography shows evidence of upper airway obstructive sleep apnea syndrome (apnea-hypopnea index of 25) and oxygen desaturation.
FIGURE 14B.6 A patient with advanced Parkinson’s disease and chronic sleep deprivation.
This ankle actigraph, from a 65-year-old man with advanced Parkinson’s disease (Hoehn-Yahr stage 4), shows a complete disorganization of sleep-wake cycling. Between midnight and 7:00 AM (sleep period) there are frequent short episodes of sleepiness (white areas) and wakefulness (black bars). During waking period (7:00 AM to midnight) there are episodes of sleepiness (white areas intruding into black bars).
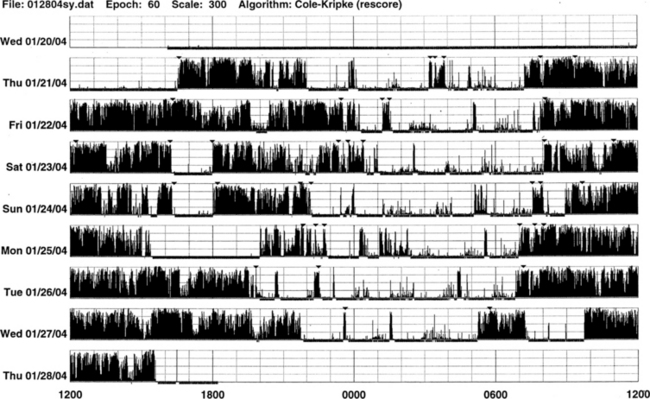
FIGURE 14B.7 Wrist actigraph from a 52-year-old psychologist with type I Arnold-Chiari malformation, who presented with sleep difficulties because of repeated awakenings and excessive daytime somnolence for 15 years (see Fig. 12.6). Overnight polysomnography showed rapid eye movement hypoventilation. The actigraph shows increased activity and periods of wakefulness during nighttime sleep (intrusions of black bars into white areas), irregular sleep onset at approximately 10:00 PM (day 1), midnight (day 2), 1:00 AM (day 3), 10:00 PM (day 4), 11:00 PM (day 5), 8:00 PM (day 6), and 10:00 PM (day 7), and variable wake-up times as indicated by sustained black bars. There are also many brief episodes of daytime somnolence (intrusions of white areas into black bars). On day 5 the actigraph was taken off during part of the waking period as indicated by abrupt onset of white area (no activity).
FIGURE 14B.8 Primary delayed sleep phase syndrome.
Wrist actigraphic recording from a 29-year-old man with a lifelong history of delayed sleep onset and delayed wake-up time. The actigraph shows his typical sleep period from 3:00 AM to 4:00 AM to 9:00 AM to noon (white areas). If he has to wake up early in the morning, he feels exhausted and sleepy all day. He feels fine if he is allowed to follow his own schedule. Melatonin at night did not help him. Morning bright light therapy was suggested, but the patient declined.
FIGURE 14B.9 Delayed sleep phase.
A 15-year-old girl complaining of difficulties falling asleep since the age of 2 to 3 years with tendency to sleep, at that time, from 11 PM to 10 AM. She complained of difficulties in falling asleep at the social time schedule for a teenager, with sleep latency of up to 2 hours with a time in bed at 11 PM and more difficulty waking up in the morning for school. Moreover, she had some problems with progress at school and sometimes experienced a “completely awake night.” Actigraphy for 6 free-running days showed a tendency to delay sleep onset (from midnight to 2 AM, more during the weekend) and awakening after 9 AM to 10 AM, excluding the last night. Estimated sleep efficiency results were very good in all of the nights (ranging from 92% to 98%).
FIGURE 14B.10 Delayed sleep phase.
The same patient as in Fig. 14B.9, treated with melatonin 1 mg per night (at 8 PM). Note in a free-running 6-day period an advance of 1 to 1½ hours for sleep onset and 2 hours for awakening except for occasional delays (third and fourth nights) during the weekend that were behaviorally induced.
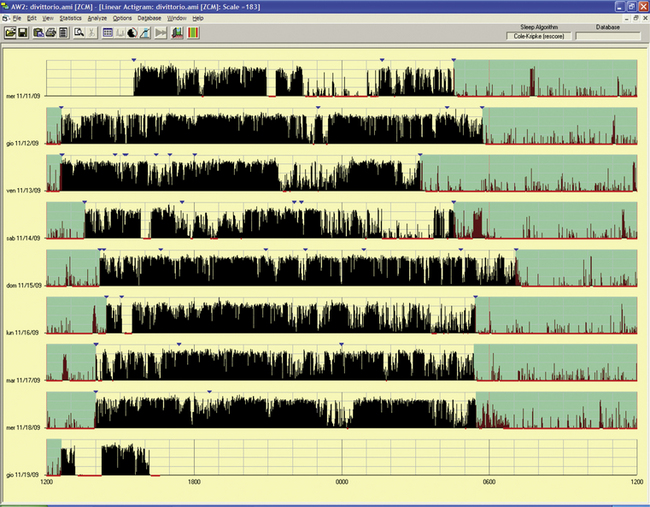
FIGURE 14B.11 Delayed sleep phase disorder.
A 55-year-old woman with a tendency to have difficulty falling asleep at night and a tendency to fall asleep during the day since childhood. She experienced progression of the disorder with age and chose a work schedule from 11 AM to 7 PM. There is a family history for evening type (brother). The sleep diary showed sleep from 4:30 AM to 5 AM until noon to 1 PM if free running. Actigraphy for eight day-night periods confirmed a delayed sleep (range 4:30 AM to 6 AM to 12.30 PM to 2 PM) with an estimated sleep efficiency above 90% in all of the nights except the last night (79.8%). Note an attempt to sleep in the evening/night on two occasions (first and fourth nights), with short duration and increased activity, indicating poor sleep quality. Treatment with melatonin in the evening and bright light in the morning was started.
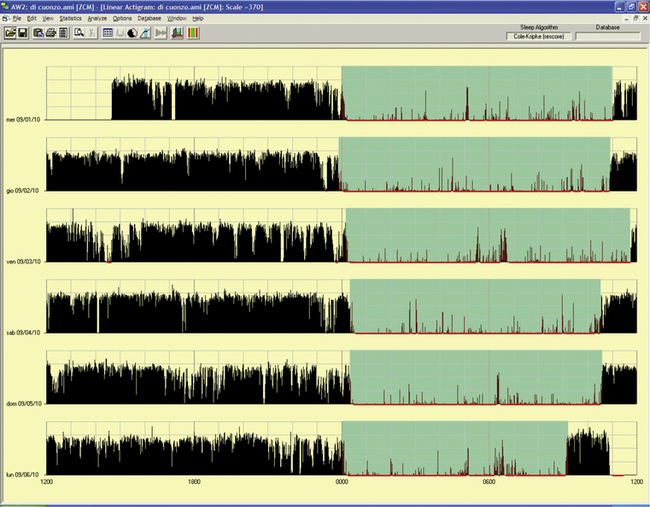
FIGURE 14B.12 Delayed sleep phase rhythm.
A 10-year-old-girl whose mother described the girl's difficulty falling asleep before midnight and trouble awakening in the morning for school. During weekends or vacations the girl sleeps from 1 AM to noon. She reported nightmares or fearful dreaming. The father has an evening type of sleep-wake rhythm, and three siblings have normal day-night rhythms. Actigraphy showed a very stable sleep-wake schedule in a free-running 6-day period: sleep onset around midnight and awakening ranging from 10:30 AM to 11:30 AM. The last night’s recording showed a provoked awakening at 9 AM to deliver the actigraphy results.
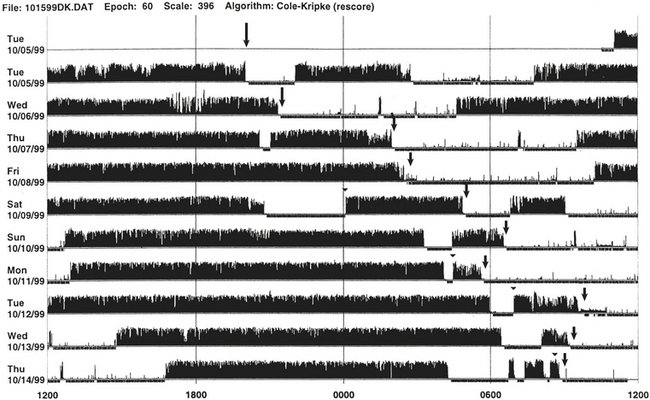
FIGURE 14B.13 Sleep-wake schedule disorder in a patient with acquired immunodeficiency syndrome (AIDS).
Wrist actigraph from a 46-year-old man with AIDS. The patient presented with sleep difficulty caused by inability to fall asleep and wake at desired bedtime and wake-up time. This 10-day recording shows disorganization of the sleep-wake schedule. There is a suggestion of non–24-hour (hypernychthemeral) syndrome with progressive delay of sleep onset (arrows) from day 1 to day 6, and again delayed sleep onset (arrows) from day 7 to day 10.
• Actigraph may be a useful adjunct to detect the rest-activity patterns during modified portable sleep apnea testing.
• Actigraph is useful to document rest-activity patterns over days and weeks when a sleep log is not able to provide such data.
Although not adequately standardized, actigraph may have a role in patients with restless legs syndrome and PLMS (Figs. 14B.14 to 14B.16). It may also be useful to document objectively periodic hypersomnias as seen in patients with Kleine-Levin syndrome (Fig. 14B.17).
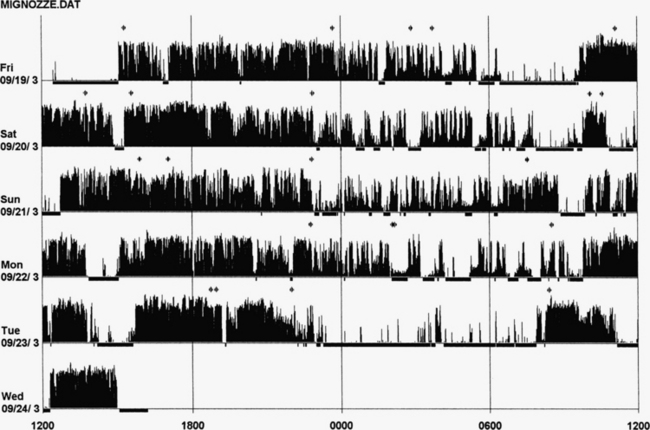
FIGURE 14B.14 Actigraphy in restless legs syndrome (RLS).
A 50-year-old woman with a 2-year history of RLS and a recent increase in severity (International Restless Legs Syndrome Study Group [IRLSSG] score of 32), of symptoms, and of difficulty in falling asleep (mean 2 hours). Her father and brother are both affected, and she is unresponsive to benzodiazepines (lorazepam, clonazepam) and zolpidem. Five nights of monitoring with actigraphy at the calf, 4 days without treatment, and the fifth with dopamine agonist (pramipexole 0.125 mg 2 hours before bedtime). Actigraph shows an increase in nocturnal leg activity during the night (probably periodic limb movements) and an increase in number of awakenings in 4 nights. The last night shows very clear increase in sleep duration and quality with a decrease in nocturnal motor activity. Polysomnography on the fourth night: total sleep time, 356 minutes; sleep efficiency, 56.1; wake after sleep onset, 224; 19 awakenings; periodic limb movements in sleep index, 82.2.
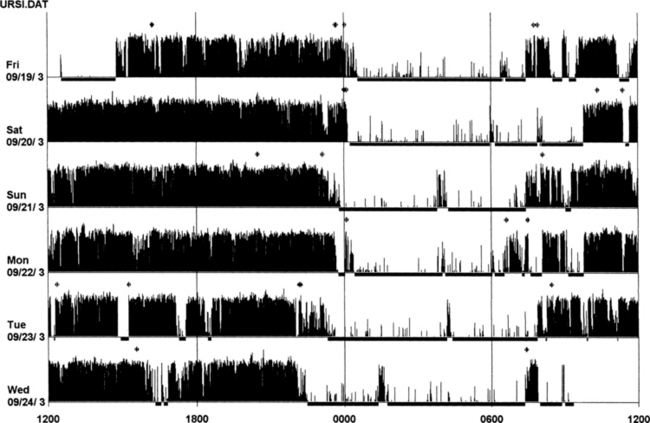
FIGURE 14B.15 Actigraphy at the calf in restless legs syndrome (RLS).
A 59-year-old woman with a 9-year history of mild to moderate RLS (International Restless Legs Syndrome Study Group [IRLSSG] score of 22), positive family history for RLS, treated with lorazepam 1 mg for several years. One week of actigraphic monitoring at the calf with an increase in nocturnal motor activity in four out of six nights and reduced sleep efficiency in the same nights. Polysomnography on the fourth night: total sleep time, 345 minutes; sleep efficiency, 92.7; wake after sleep onset, 24 minutes; number of awakenings, 7; periodic limb movements in sleep index, 4.9. Compare these findings with those in Figure 14B.14 (severe form of RLS).
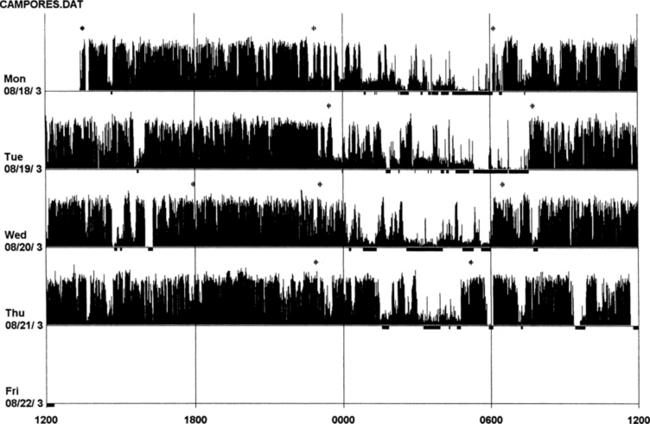
FIGURE 14B.16 Actigraphy of severe restless legs syndrome (RLS).
A 60-year-old woman with familial idiopathic RLS since the age of 30 years but increased in severity for the past 6 years. Symptoms are prominent between 8 PM and 11 PM. Sometimes RLS also occurs during the day. She has received treatment with ropinirole (slight effect), L-dopa (augmentation after 2 months), and pramipexole as an add-on to L-dopa for 2 months. She now experiences RLS during the day and night with 3 to 4 hours (maximum) of sleep per night (International Restless Legs Syndrome Study Group [IRLSSG] score of 35). Polysomnography (the second night of actigraphic monitoring, during treatment with L-dopa 250 mg and pramipexole 0.50 mg): total sleep time, 421 minutes; sleep efficiency, 81.3; wake after sleep onset, 96 minutes; number of awakenings, 19; periodic limb movements in sleep index, 108.
FIGURE 14B.17 Periodic hypersomnia (Kleine-Levin syndrome type).
A 29-year-old man with a history of alternate periods of insomnia and hypersomnia, with irregular sleep-wake rhythms associated with hyperphagia; this was treated as a psychiatric problem. Polysomnogram during an out-of-phase period showed no abnormalities in multiple sleep latency test (sleep latency, 13 minutes) and nocturnal sleep (only a reduced rapid eye movement sleep percentage). Actigraphy for an eight-night/day period had a chance to record a period of hypersomnia (last three lines): the patient complained of being unable to wake and stand up in the morning and of hyperphagia and some manifestations of hypersexuality during the day. Note the difference between the first three nights with a long sleep period, but with increased motor activity, and the next two nights of insomnia before going into the hypersomniac period.
An important application of actigraphy is in field studies to assess sleep-wakefulness where PSG is not feasible.
Advantages of Actigraph Over Polysomnography
The advantages include the following: easy accessibility; inexpensive recording over extended periods for days, weeks, or even months; recording of 24-hour activities at all sites (home, work, or laboratories); usefulness in uncooperative and demented patients when laboratory PSG study is not possible; ability to conduct longitudinal studies during therapeutic intervention (behavioral or pharmacological treatment) in patients with insomnia; usefulness in sleep state misperception (see Fig. 14B.2); ability to document delayed or advanced sleep phase syndrome or non–24-hour circadian rhythm disorders, although sleep logs may suggest such a diagnosis; and documentation of excessive daytime sleepiness and repeated episodes of sleepiness (lasting more than a few minutes).
Disadvantages and Limitations of Actigraph Recordings
The disadvantages and limitations include inability to diagnose sleep apnea and to clarify the cause of insomnia; overestimation of sleep when some insomniacs may lie down in bed for prolonged periods without moving; and inability to identify subjects who are feigning a sleep problem, to discriminate types of movements such as PLMS from other body movements, and to provide any information about other physiological characteristics (e.g., EEG, EOG, respiration).
The lack of standardization of placement of the actigraph may be viewed as a pitfall. Most commonly the actigraph is placed on the nondominant side. Activity is somewhat different between the two sides with more activities being recorded from the dominant than the nondominant limbs. However, overall agreement for the sleep period between the two sides is not significantly different when compared with the PSG data.
In conclusion, the actigraph is an inexpensive useful method for longitudinal assessment of sleep-wake pattern, it can differentiate individuals with normal sleep pattern from those with disturbed sleep caused by insomnia or sleep-disordered breathing, and it can differentiate normal sleep from sleep-wake schedule disturbances by recording over a prolonged period with longitudinal monitoring.
Gschliesser, V., Frauscher, B., Brandauer, E., et al. PLM detection by actigraphy compared to polysomnography: a validation and comparison of two actigraphs. Sleep Med. 2009; 10:306–311.
Jean-Louis, G., Kripke, D. F., Cole, R. J., et al. Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav. 2001; 72:21.
Sadeh, A., Hauri, P. J., Kripke, D. F., et al. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995; 18:288–302.
Standards of Practice Committee. An American Sleep Disorders Association report: Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. Sleep. 1995; 18:285–287.
Tyron, W. W. Activity Measurement in Psychology and Medicine. New York, NY: Plenum Press; 1991.
14C
Recommendations for Practical Use of Pulse Transit Time as a Tool for Respiratory Effort Measurements During Sleep and Microarousal Recognition
The identification and quantification of events characteristic of obstructive sleep apnea and related conditions is a task both laborious and time consuming. The standard polysomnography (PSG) provides the easy detection of events, such as apneas, using basic respiratory effort measurements (thoracic and abdominal movements), although the correct identification of hypopneas and upper airway resistance episodes requires sensitive and quantitative measures of inspiratory effort and airflow. For respiratory effort, methods such as esophageal pressure (Pes) manometry are expensive, cumbersome, and invasive and require the investigations to be performed in a sleep laboratory. Thus the inadequacy of current equipment for the characterization of sleep-disordered breathing has led to the use of the promising new technique of pulse transit time (PTT) for measuring respiratory effort and detecting microarousals (MAs).
There is great uncertainty about the level of arousal from sleep sufficient enough to cause sleep fragmentation. Sforza et al have demonstrated the existence of a hierarchical arousal response from autonomic arousals to bursts of delta activity and finally to alpha activity. Thus a predefined arousal stimulus produces, or not, a visible cortical arousal. It is possible that in certain circumstances only the first step of the hierarchical arousal response is identifiable, that is, autonomic activation. Thus one can choose to detect MAs only by using autonomic markers such as blood pressure (BP) rises, heart rate variations, or transient dips in PTT. It has been demonstrated that autonomic MAs are as sensitive as EEG for detecting sleep disruption.
In summary, the recording of PTT has provided us with a noninvasive method both specific and sensitive in the analysis of variations in sleep fragmentation and respiratory effort during the three stages of sleep. The signal used is not only commercially available, relatively economical to buy, and portable but also produces a trace, which can be swiftly and efficiently analyzed. The purpose of this review is to describe in a very practical way the interpretation of the signal in clinical practice.
Definition
The “true” PTT value is the time taken for the pulse wave to travel from the aortic valve to the periphery. The speed of this wave is directly proportional to the inverse of the systolic blood pressure. Thus a slight increase in blood pressure causes an increase in vascular tone, which in turn stiffens the arterial wall, causing the PTT to shorten (a transient dip in the baseline of the PTT trace). Therefore PTT is a sensitive tool for measuring transient changes in autonomic tone. For practical reasons the opening of the aortic valve is assimilated to the R wave on the electrocardiogram (ECG) (Fig. 14C.1). There is a slight delay between the opening of the aortic valve and the R wave, corresponding to the isometric contraction time of the left ventricle. Inspiratory effort causes a prolongation of the isometric contraction time, which in turn amplifies the PTT signal.
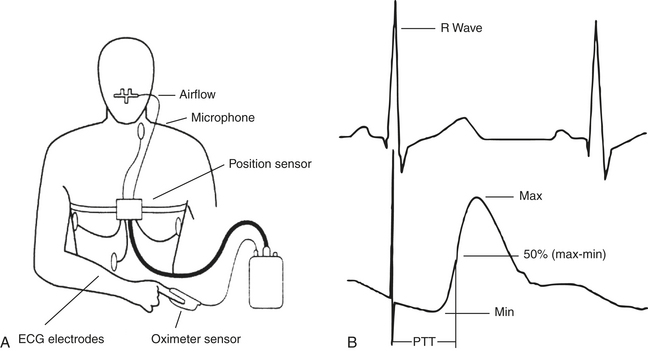
FIGURE 14C.1 Schematic diagram demonstrating the position of the sensors (A) and the calculation of pulse transit time using the electrocardiogram R wave as the starting point reference and the arterial pulse wave as the end point (B). (Modified from Smith R, Argod J, Pépin J-L, Lévy P. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;54:452-457.)
Method of Measurement
Measuring PTT needs to incorporate ECG leads for the recognition of the R wave and oximetric photoplethysmography (usually a finger probe) to assess the arrival of the pulse wave in the periphery (see Fig. 14C.1, C). PTT is measured as the interval between the ECG R wave and the subsequent arrival of the pulse wave at the finger or ear lobe (usually the point on the pulse waveform that is 50% the height of the maximum value). PTT is approximately 200 to 300 msec when using the finger probe and is measured to an accuracy of 2 msec. PTT values are available with every heartbeat and are typically oversampled at 5 Hz to ensure that no values are neglected.
Microarousal Recognition Using Pulse Transit Time (Figures 14C.2 and 14C.3)
An MA is systematically associated with a surge in BP, and because PTT is inversely correlated with BP, a PTT MA is defined as a transient dip from the baseline.
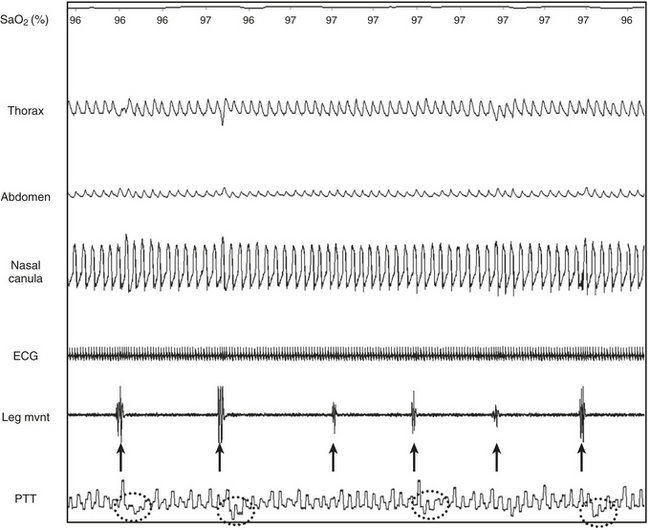
FIGURE 14C.2 Periodic limb movements (PLMs) and associated nonrespiratory microarousals (MAs).
Arrows indicate the typical pattern associated with MAs occurring immediately after the leg movement. The leg movement induces an MA, which is associated with a rise in blood pressure, which in turn induces a transient dip in pulse transit time. The leg movement is probably underestimated by electromyography (EMG) but suspected by the concurrent MAs. Note that no significant respiratory events occur. This figure demonstrates arterial oxygen saturation (SaO2), thorax, and abdominal inductance plethysmography, flow (nasal canula), pulse transit time (PTT), and leg electromyography (leg mvnt).
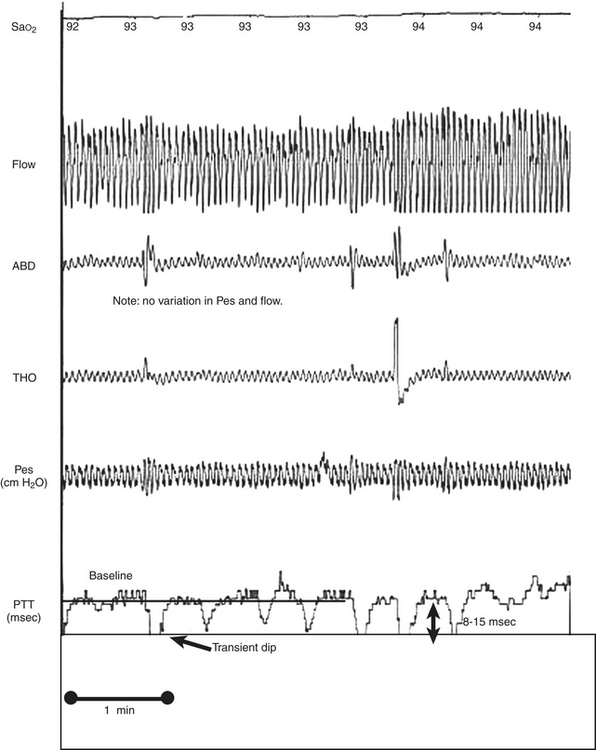
FIGURE 14C.3 Nonrespiratory arousals detected by pulse transit time (PTT).
Microarousals (MAs) were demonstrated by transient dips from the baseline of 8 to 15 msec during stage N2 despite no significant variations in esophageal pressure (Pes) or flow. This patient exhibited pain-related MAs owing to rheumatoid arthritis. This figure demonstrates arterial oxygen saturation (SaO2), thorax (THO) and abdominal (ABD) inductance plethysmography, Pes, and PTT.
PTT is able to assess sleep fragmentation caused by nonrespiratory causes, whether the cause is immediately obvious, as in the case of periodic limb movements (PLM; see Fig. 14C.2) or not so obvious (see Fig. 14C.3). The PTT arousal dip from baseline (ΔPTT) usually ranges from 8 to 15 msec (see Fig. 14C.3) in stage N1 to N2 and from 6 to 8 msec during stage N3, although there is no clear threshold from one patient to another. Therefore the exact measurement of ΔPTT (number of milliseconds) is less important than the visual aspect, that is, an obvious transient dip from the baseline (see Fig. 14C.3). A PTT arousal can be confirmed using corroborative signals such as ECG (increase in heart rate) or BP measurements when available (Fig. 14C.4, A; surges in BP), such as by Finapres. To accurately define a PTT arousal, it should be noted that the shape of the area above the curve of the PTT is precisely related to the time course evolution of BP. For example, a pointed increase in BP (as seen using a Finapres) is associated with an abrupt transient dip in PTT and a rapid return to baseline presented as a relatively linear PTT trace under the curve (Fig. 14C.4, B). In contrast, a more progressive increase in BP is related to a different pattern on the PTT (Fig. 14C.4, C). However, at present when analyzing the trace, the amplitude of the dip should be the only tool used to define a PTT microarousal (i.e., between 8 msec and 15 msec during stage N2 sleep). The area of the trace (this corresponds to the mirror picture of BP evolution during and after MAs) and the length of the dips are probably of importance, but their significance remains unknown for the time being.
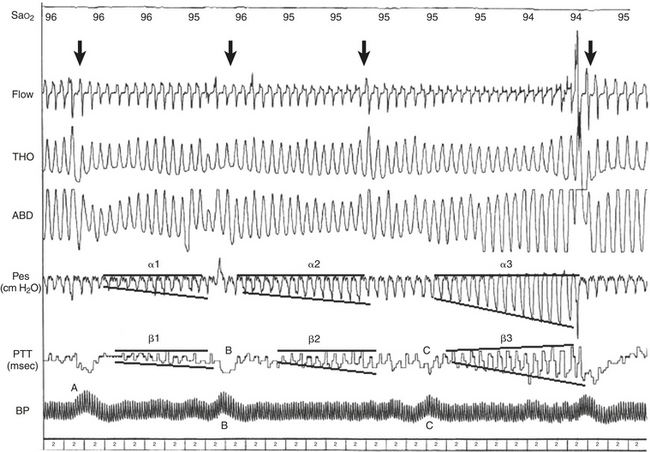
FIGURE 14C.4 Classic obstructive hypopneas terminated by a microarousal (MA).
The increases in ΔPTT are proportional to the increases in esophageal pressure (Pes); α1 and β1 demonstrate a smaller increase than α2 and β2, which demonstrate a smaller increase than α3 and β3. The corroborative signal of blood pressure (BP) measurements by Finapres (A) help confirm an arousal because the time course evolution of BP is precisely related to the shape of the area under the pulse transit time (PTT) curve. Note a pointed increase in BP (B) is associated with an abrupt transient dip in PTT and a rapid return to baseline presented as a relatively linear PTT trace under the curve. In contrast, a more progressive increase in BP (C) is related to a different pattern on the PTT. Arrows indicate the disappearance of inspiratory flow limitation occurring concurrently with the MA. This figure demonstrates arterial oxygen saturation (SaO2), thorax (THO) and abdominal (ABD) inductance plethysmography, Pes, PTT, and blood pressure using a Finapres (BP). (From Pépin J-L, Delavie N, Pin I, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005;127[3]:722-730.)
During REM sleep, variations in sympathetic activity are spontaneously very high and therefore the PTT baseline is highly variable. Thus the recognition of true MAs during REM sleep is a lot less specific than in other sleep stages (Fig. 14C.5).
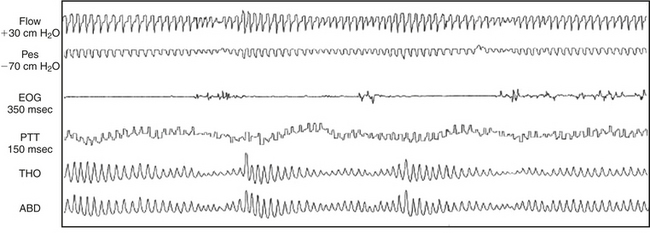
FIGURE 14C.5 Difficulties of interpretation arising during rapid eye movement (REM) sleep.
Note the huge variation in pulse transit time (PTT) related to physiological fluctuations of autonomic tone during REM sleep. In these conditions, transient dips from the baseline related to microarousals (MAs) are more difficult to identify. REM sleep is associated with marked physiological variations in respiratory effort and airflow, so identification of the classic pattern of PTT or esophageal pressure (Pes) changes associated with obstructive respiratory events is more difficult. REM sleep is also associated with marked changes in blood pressure and, as can be clearly seen, this causes the baseline PTT value to fluctuate, compounding further the interpretation difficulties. This figure demonstrates Pes, electro-oculography (EOG), PTT, and thorax (THO) and abdominal (ABD) inductance plethysmography. (From Smith R, Argod J, Pépin J-L, Lévy P. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;56:452-457.)
Respiratory Effort (Figures 14C.4, 14C.6, and 14C.7)
Pes is the gold standard measurement used to analyze changes in intrathoracic pressure and, because the esophagus is situated in the thorax, Pes is highly effective at reflecting and quantifying variations in intrathoracic pressure. However, this method of analysis is both invasive and frequently unacceptable to the patient. PTT can be used to analyze respiratory effort and moreover is specific when defining certain respiratory events (hypopneas, upper airway resistance episodes, and central events). The heart and large vessels are also located in the thoracic cavity and are thus affected by variations in thoracic volume and pressure. During inspiration the volume of the thoracic cavity increases, reducing thoracic pressure, which in turn reduces the compression of the heart and large vessels (vena cava and aorta), decreasing BP and slowing PTT (PTT dips from the baseline). The opposite is true for expiration. As the thoracic pressure increases, the heart is compressed and BP increases and PTT quickens.
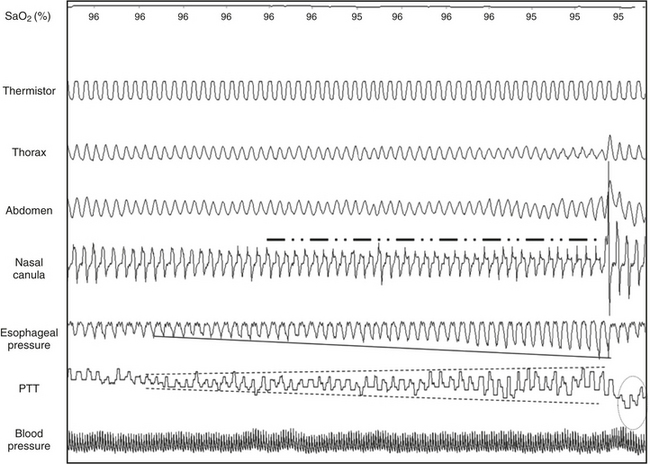
FIGURE 14C.6 Illustrative respiratory effort–related arousal.
The progressive decrease in intrathoracic pressure on esophageal pressure is well disclosed by the progressive increase in the pulse transit time (PTT) arousal dip from baseline (ΔPTT), with a marked arousal terminating the event.
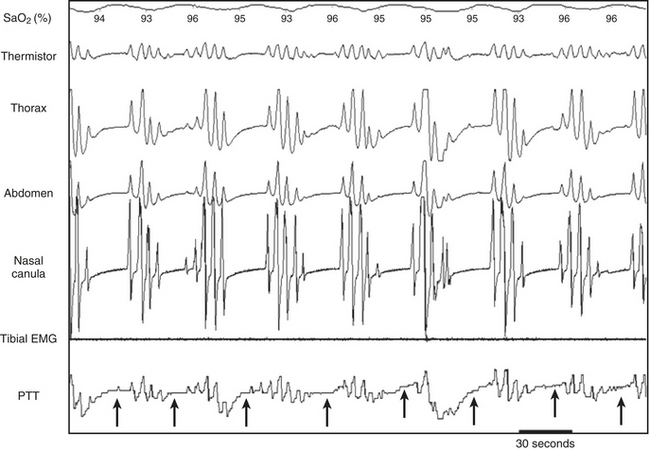
FIGURE 14C.7 Cheyne-Stokes respiration.
During Cheyne-Stokes respiration, a clear increase in the pulse transit time (PTT) arousal dip from baseline (ΔPTT) may be observed during the crescendo pattern. In contrast, during central apneas (arrows), ΔPTT values are minimal. This figure demonstrates arterial oxygen saturation (SaO2), thorax (THO) and abdominal (ABD) inductance plethysmography, and PTT.
Obstructive Events or Upper Airway Resistance Episodes (see Figures 14C.4 and 14C.6)
Obstructive events (see Fig. 14C.4) or upper airway resistance episodes (see Fig. 14C.6) may be recorded on the demonstration of a steady increase in ΔPTT terminated by an MA and then the normalization of the trace (see Figure 14C.4, α1, α2, α3). This is observed simultaneously with a steady increase in Pes to a more negative value (or abdominal thoracic movements with or without a modification of the phase angle or paradoxical movements) terminated by an MA and the normalization of the trace (β1, β2, β3). The increases in ΔPTT are proportional to the increases in Pes; α1 and β1 demonstrate a smaller increase than α2 and β2, which demonstrate a smaller increase than α3 and β3. It is for this reason that PTT has a good specificity, sensitivity, and negative predictive value at differentiating obstructive from central apneas and hypopneas.
Central Events (see Figure 14C.7)
ΔPTT reduces during a central event in a similar fashion to the reduction in esophageal pressure. It should be noted that central hypopneas can be judged on the reduction in amplitude of PTT proportional to the reduction in flow. Except for Pes, PTT is the only tool validated for the recognition of central hypopneas. This pattern of decreasing ΔPTT is clearly demonstrated during Cheyne-Stokes respiration (see Fig. 14C.7).
Limitations of the Technique (Figure 14C.8)
A drawback with this method of analyzing respiratory effort is that a PTT value is only available every QRS complex. Therefore measurements may fall on either side of the peak or trough of the BP oscillations associated with respiratory effort, which results in a tendency to undersample. In certain conditions the minimal value of Pes does not occur concurrently with a QRS complex. This is demonstrated in Figure 11C.8, wherein a given maximum PTT value may (see Fig. 14C.8, A) or may not (see Fig. 14C.8, B) correspond to the Pes nadir.
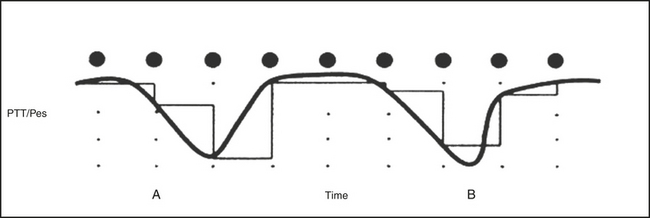
FIGURE 14C.8 Undersampling error of pulse transit time (PTT) as reflected by esophageal pressure (Pes).
The points represent each pulse. Breaths corresponding to a selected obstructive respiratory event are represented by a thick sinuous line (Pes) and a thin squared line (PTT). The trace shows two respiratory cycles of obstructive sleep apnea. The nadir of the first cycle happens to coincide with the arterial pulse wave, and therefore the PTT amplitude is an accurate representation of the Pes swing (A). This second respiratory cycle does not coincide with the pulse wave, and consequently the PTT swing underestimates the Pes swing (B). (From Smith R, Argod J, Pépin J-L, Lévy P. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;54:452-457.)
False Pulse Transit Time Values (Figure 14C.9)
Unfortunately, because the PTT measurements begin with the R wave on the ECG, false values of PTT occur when the patient suffers from cardiac arrhythmia. Extrasystole produces acute variations in PTT despite the absence of a respiratory event or MA.
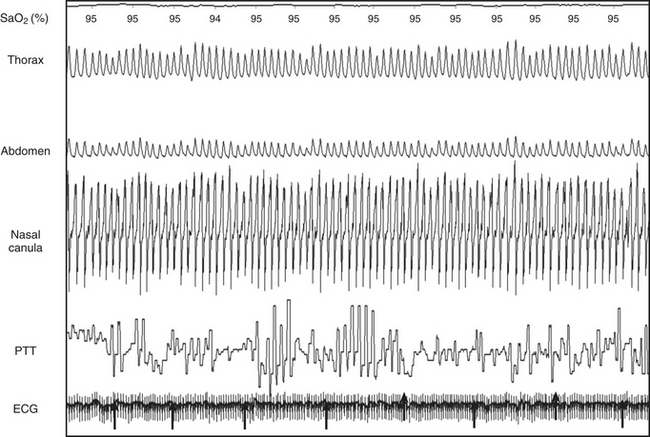
FIGURE 14C.9 Pulse transit time (PTT) artifacts as a consequence of ventricular extrasystoles.
This trace was recorded during a period of normal breathing. Note the false result produced by the PTT simultaneously to extrasystoles. This figure demonstrates thorax (THO) and abdominal (ABD) inductance plethysmography, PTT, electrocardiography (ECG), flow (nasal canula), and oxygen saturation (SAT).
Medication
Personal experience has demonstrated that the PTT trace is not generally affected by any cardiac medication such as β-blockers; however, there is no account of this in the literature.
Pulse Transit Time as a Measure of Respiratory Effort Under Noninvasive Ventilation (Figures 14C.10 and 14C.11)
Noninvasive ventilation (NIV) is widely accepted as a long-term treatment for chronic hypercapnic respiratory failure. Recently, an increasing awareness of nocturnal respiratory events occurring under NIV has led to a wider use of polysomnography (PSG) to improve adjustment of long-term NIV settings. The most frequent problems detected are unintentional leaks (i.e., not related to exhalation valve of interface), patient-ventilatory asynchrony, and obstructive or central events (either residual or induced by NIV). Differentiating between central and obstructive events on PG or PSG tracings under NIV is more challenging than in spontaneous breathing and requires appropriate measurements of respiratory effort. PTT has been demonstrated as an interesting tool in this situation.
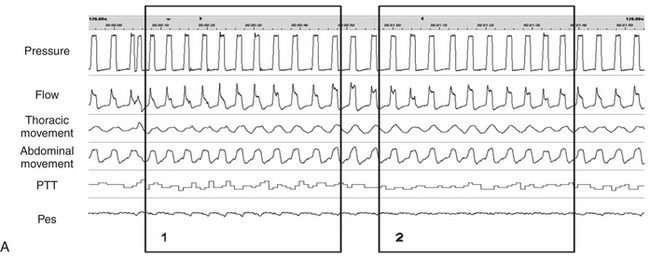
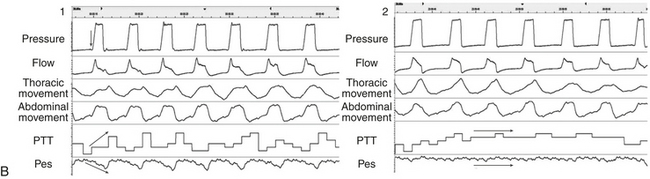
Figure 14C.10 The ability of pulse transit time (PTT) to characterize respiratory effort during respiratory cycles triggered or not triggered by the patients
A: Box 1, Respiratory cycles are triggered by the patient. Box 2, There is no inspiratory effort from the patient; all respiratory cycles are controlled by the ventilator. B: 1, Respiratory cycles are triggered by the patient as demonstrated by the changes in pressure signal before pressurization (arrow). There is a clear increase in respiratory effort as demonstrated by esophageal pressure and an increase in inspiratory-expiratory differences on PTT signal. 2, All the respiratory cycles are controlled by the ventilator, with an obvious reduction in effort both on PTT and Pes as compared with B-1 (arrows).
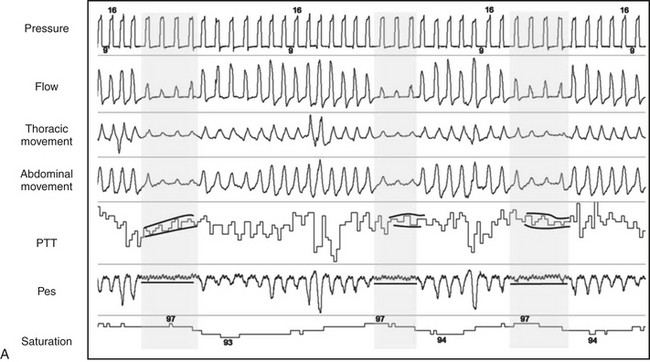
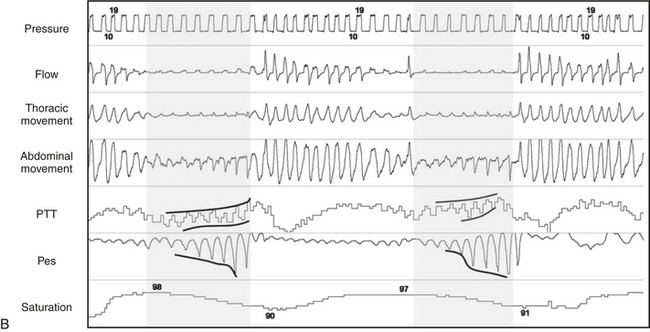
Figure 14C.11 During 3-minute epoch, illustrative examples of how to identify central versus obstructive events using pulse transit time (PTT) or esophageal pressure (Pes) under noninvasive ventilation.
A, Central respiratory event: for both Pes and PTT signals, respiratory oscillations are markedly reduced or disappear. B, Obstructive respiratory event: swings of Pes become increasingly negative during an obstructive event, with a simultaneous increase in oscillations of PTT between inspiration and expiration (dark lines).
Conclusion
In conclusion, the measurements of inspiratory swings by PTT can provide quantitative and qualitative information during NREM sleep in patients suffering from sleep respiratory disorders, thus omitting the necessity for invasive or cumbersome methods of analysis, such as Pes. Both respiratory and nonrespiratory MAs can be identified with reasonable sensitivity and specificity using this technique. Care should be taken not to use this portable device on patients with cardiac arrhythmias because of resultant artifacts.
Ali, N., Davies, R. J., Fleetham, J. A., Stradling, J. R. Periodic movements of the leg during sleep associated with rises in systemic blood pressure. Sleep. 1991; 14:163–165.
Argod, J., Pépin, J. -L., Lévy, P. Differentiating obstructive and central sleep respiratory events through pulse transit time. Am J Respir Crit Care Med. 1998; 158:1778–1783.
Argod, J., Pépin, J. -L., Smith, R. P., Lévy, P. Comparison of esophageal pressure with pulse transit time as a measure of respiratory effort for scoring obstructive nonapneic respiratory events. Am J Respir Crit Care Med. 2000; 162:87–93.
Contal, O., Carnevale, C., Borel, J. C., et al. Pulse transit time as a measure of respiratory effort under noninvasive ventilation. Eur Respir J. 2013; 41:346–353.
Pépin, J. -L., Delavie, N., Pin, I., et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005; 127(3):722–730.
Pitson, D., Sandell, A., van den Hout, R., Stradling, J. R. Use of pulse transit time as a measure of inspiratory effort in patients with obstructive sleep apnoea. Eur Respir J. 1995; 8:1669–1674.
Pitson, D., Stradling, J. R. Autonomic markers of arousal during sleep in patients undergoing investigation for obstructive sleep apnoea, their relationship to EEG arousals, respiratory events and subjective sleepiness. J Sleep Res. 1998; 7:53–59.
Sforza, E., Jouny, C., Ibanez, V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000; 111:1611–1619.
Smith, R., Argod, J., Pépin, J. -L., Lévy, P. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999; 54:452–457.
14D
The Cyclic Alternating Pattern
Phasic Events of Non–Rapid Eye Movement Sleep
Sleep staging is based on the application of rules according to which epochs of 20 or 30 seconds are assigned a score that most appropriately characterizes the prevalent pattern occurring during that interval. In the last 35 years, this scoring system has been a methodological landmark allowing a universal approach to and a standardized training for the analysis of human sleep. There is, however, a huge body of evidence that indicates sleep stages are nonhomogeneous states, which contain a mass of neurophysiological phenomena that adjust continuously the restless flow of cerebral activities. Indeed, sleep hosts several phasic events, which play a dynamic role in the structural organization of sleep-modulating autonomic and motor correlates (Figs. 14D.1 and 14D.2). When these phasic events appear in a periodic manner, the resulting EEG feature is called cyclic alternating pattern, or CAP.
Definition of Cyclic Alternating Pattern
CAP is a periodic EEG activity of NREM sleep characterized by the repetitive occurrence of phasic events described in Figures 14D.1 and 14D.2. CAP is composed of sequences of CAP cycles. A CAP cycle is composed of a phase A and the following phase B (Fig. 14D.3). A CAP sequence begins with a phase A and ends with a phase B. Each phase of CAP is 2 to 60 seconds in duration.
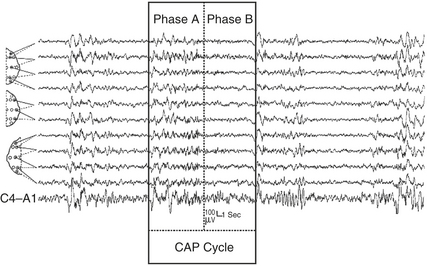
FIGURE 14D.3 A cyclic alternating pattern (CAP) sequence in stage II sleep with a highlighted CAP cycle (black box). The CAP cycle is defined by a phase A (aggregate of phasic events) and by a phase B (interval between two successive A phases). Bipolar electroencephalographic (EEG) derivations using international electrode placement; channels 1 to 6 from top: FP2-F4, F4-C4, C4-P4, P4-O2, F8-T4, T4-T6; channels 7 to 11 from top: FP1-F3, F3-C3, C3-P3, P3-O1, C4-A1.
CAP is a global EEG phenomenon involving extensive cortical areas. Under normal conditions, CAP does not occur in REM sleep, where the phase A features, consisting mainly of rapid low-amplitude rhythms, are separated by a mean interval of 3 to 4 minutes.
Definition of Non–Cyclic Alternating Pattern
The absence of CAP for more than 60 seconds is scored as non–CAP. An isolated phase A, that is, preceded or followed by another phase A by more than 60 seconds, is classified as non-CAP (Fig. 14D.4; ![]() Video Vignette 30).
Video Vignette 30).
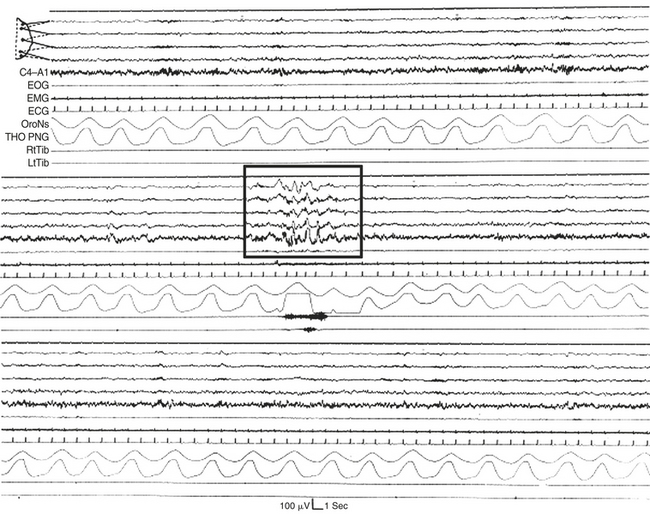
FIGURE 14D.4 Long stretch of stage II sleep characterized by a stable electroencephalographic (EEG) background (non-CAP) with an isolated phase A (box). EOG, Electro-oculogram; EMG, electromyogram; ECG, electrocardiogram; OroNs, oronasal airflow; THO-PNG, thoracic pneumogram; RtTib, right anterior tibialis muscle; LtTib, left anterior tibialis muscle. EEG channel derivations as in Figure 14D.1 (top segment).
Phase A Subtypes (Figure14D.5)
Phase A activities can be classified into three subtypes. Subtype classification is based on the reciprocal proportion of high-voltage slow waves (EEG synchrony) and low-amplitude fast rhythms (EEG desynchrony) throughout the entire phase A duration. The three phase A subtypes are as follows:
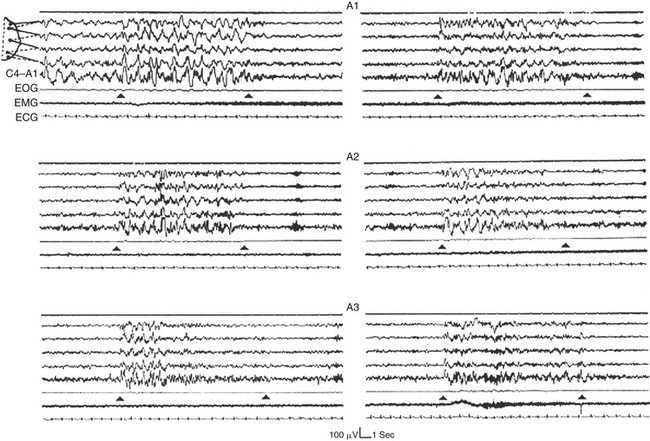
FIGURE 14D.5 Six specimens of phase A subtypes confined within black triangles.
The A1 subtypes (top) are dominated by high-voltage low-frequency electroencephalographic (EEG) frequencies. The A3 subtypes (bottom) contain a prevalent amount of low-voltage high-frequency EEG activities. The A2 subtypes (middle) are characterized by a balanced mixture of EEG synchrony and EEG desynchrony. Notice that in most cases the high-voltage low-frequency EEG activities precede the onset of the fast low-amplitude rhythms. EOG, Electro-oculogram; EMG, electromyogram; ECG, electrocardiogram. EEG derivations as in Figure 14D.1 (top segment).
Subtype A1: EEG synchrony is the predominant activity. If present, EEG desynchrony occupies less than 20% of the entire phase A duration. Subtype A1 includes delta bursts, K complex sequences, vertex sharp transients, and polyphasic bursts (clusters of high-voltage delta waves, intermixed with theta, alpha, or beta rhythms) with less than 20% EEG desynchrony.
Subtype A2: The EEG activity is a mixture of slow and fast rhythms with 20% to 50% of phase A occupied by EEG desynchrony. Subtype A2 specimens include polyphasic bursts with more than 20% but less than 50% EEG desynchrony.
Subtype A3: The EEG activity is predominantly rapid low-voltage rhythms with more than 50% percent of phase A occupied by EEG desynchrony. Subtype A3 specimens include K-alpha, EEG arousals, and polyphasic bursts with more than 50% EEG desynchrony. A movement artifact within a CAP sequence is also classified as subtype A3.
The majority of EEG arousals occurring in NREM sleep (87%) are inserted within the unstable background offered by CAP, where arousals basically coincide with a phase A2 or A3.
The regular oscillations that accompany the transition from light sleep to deep stable sleep are basically expressed by the A1 subtypes (Fig. 14D.6), whereas the breakdown of deep sleep and the introduction of REM sleep are mostly associated with subtype A2 (Fig. 14D.7) or A3 of CAP.
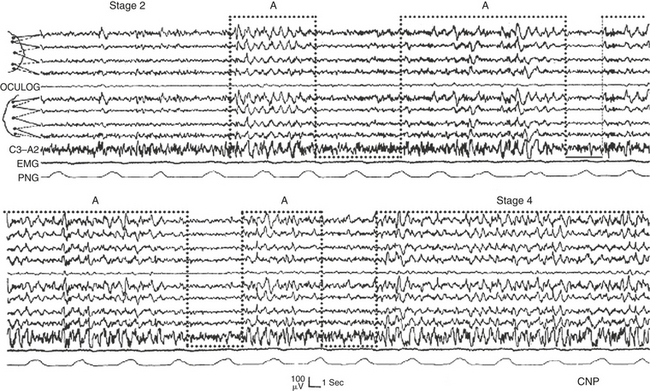
FIGURE 14D.6 Transition from stage II to stage IV accompanied and smoothed by a cyclic alternating pattern sequence composed of phase A1 subtypes (bottom-open dotted boxes). OCULOG, Oculogram; EMG, electromyogram; PNG, pneumogram. EEG derivations as in Figure 14D.1 (top and middle segments).
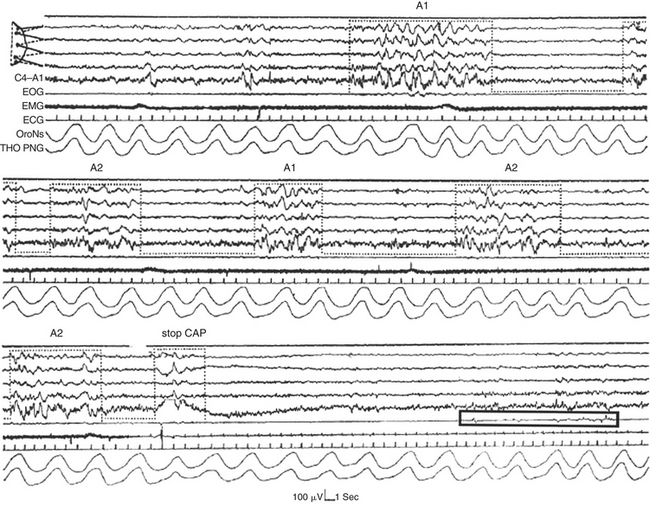
FIGURE 14D.7 Onset of rapid eye movement (REM) sleep heralded by a cyclic alternating pattern (CAP) sequence in stage II.
The transition to REM sleep is defined by the abrupt drop of muscle tone, the interruption of the CAP sequence (stop CAP), and the occurrence of a burst of rapid eye movements (REMs; black box). EOG, Electro-oculogram; EMG, electromyogram; ECG, electrocardiogram; OroNs, oronasal airflow; THO-PNG, thoracic pneumogram. EEG channel derivations as in Figure 14D.1 (top segment).
Cyclic Alternating Pattern and Nonrestorative Sleep
CAP is a marker of sleep instability swinging between poles of greater arousal (phase A) and lesser arousal (phase B). CAP is increased by perturbing conditions such as acoustic stimulation (Fig. 14D.8) and correlates with the subjective sensation of nonrestorative sleep. The amount of CAP and the representation of phase A subtypes can be deeply influenced in the different sleep disorders.
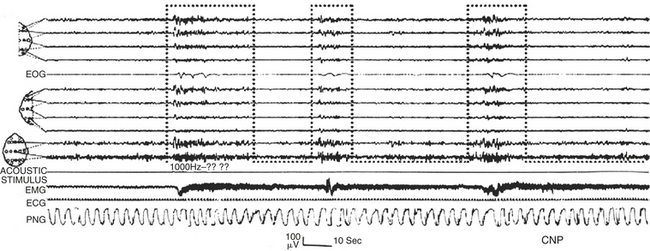
FIGURE 14D.8 A rapid shift from stage II non–cyclic alternating pattern (non-CAP; left side of the trace) to stage II CAP, induced by a single acoustic stimulus. The A phases of the CAP (bottom-open dotted boxes) are associated with a reinforcement of muscle tone. Electroencephalographic channel derivations: channels 1 to 4 as in Figure 14D.1 (top segment); channels 6 to 9 as in Figure 14D.1 (middle segment); channels 10, 11: Fz-Cz, Cz-Pz. EOG, Electro-oculogram EMG, electromyogram; ECG, electrocardiogram; PNG, pneumogram.
Primary insomnias (both psychophysiological and paradoxical insomnia) are characterized by an exaggeration of the physiological amounts of CAP. Sleep-promoting medication reduces the excessive amount of CAP in both primary and secondary insomnia. CAP parameters are also useful tools to monitor the effects of intermittent hypnotic treatment.
The amount of CAP is also increased in psychiatric-related insomnia (major depression, dysthymia), in primary generalized epilepsy, in nocturnal frontal lobe epilepsy, in PLM disorders, in sleep apnea syndrome, and when sleep is recovered in the morning hours
The Cyclic Alternating Pattern Phases and Sleep Disorders
Phase A of CAP is associated with an activation of cardiac and autonomic functions (![]() Fig. 14D.9; VideoVignettes 32 to 35). It reflects a gate through which pathological events are more likely to occur. A gating effect has been established for interictal EEG bursts (
Fig. 14D.9; VideoVignettes 32 to 35). It reflects a gate through which pathological events are more likely to occur. A gating effect has been established for interictal EEG bursts (![]() Figure 14D.10; Video Vignette 31), epileptic motor events (
Figure 14D.10; Video Vignette 31), epileptic motor events (![]() Fig. 14D.11; VideoVignettes 32 and 33), periodic limb movements
Fig. 14D.11; VideoVignettes 32 and 33), periodic limb movements ![]() (Fig. 11D.12; Video Vignette 34), sleep bruxism (
(Fig. 11D.12; Video Vignette 34), sleep bruxism (![]() Fig. 14D.13), and postapnea respiratory recovery (Fig. 14D.14; Video Vignettes 35 and 36) and Cheyne Stokes respiration (
Fig. 14D.13), and postapnea respiratory recovery (Fig. 14D.14; Video Vignettes 35 and 36) and Cheyne Stokes respiration (![]() Video Vignette 37). The coupling of CAP with modulations in respiratory and autonomic functions can allow quantification of sleep stability using simple tools (e.g., ECG signal).
Video Vignette 37). The coupling of CAP with modulations in respiratory and autonomic functions can allow quantification of sleep stability using simple tools (e.g., ECG signal).
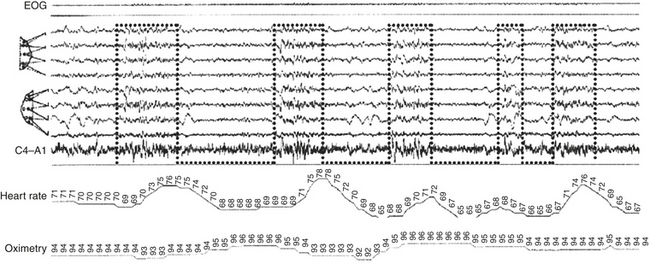
FIGURE 14D.9 Cyclic alternating pattern sequence coupled with online monitoring of heart rate and oximetry (patient with periodic limb movements and mild sleep-disordered breathing). Notice the close time relation between the onset of the A phases (bottom-open dotted boxes) and the acceleration of cardiac activity (parallel with the delayed saturation fall). EOG, Electro-oculogram. Top four electroencephalographic (EEG) channels as in Figure 14D.1 (top segment); EEG channels 5 to 8 as in Figure 14D.1 (middle segment).
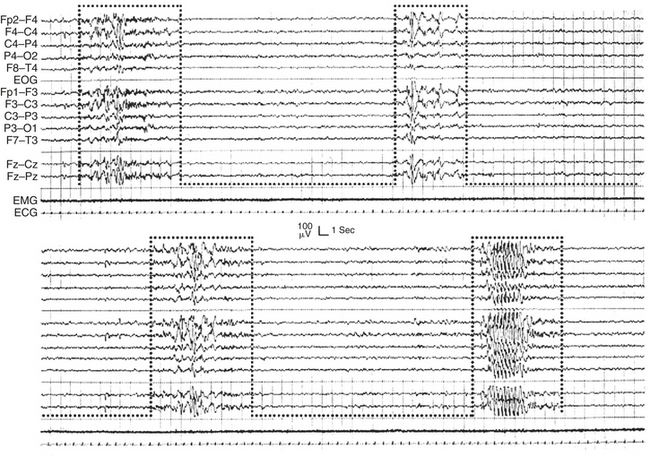
FIGURE 14D.10 Primary generalized electroencephalographic (EEG) discharges recurring in clusters boxed within the A phases of a cyclic alternating pattern sequence. Notice the absence of EEG bursts during phase B. EMG, Electromyogram; ECG, electrocardiogram; EOG, electro-oculogram.
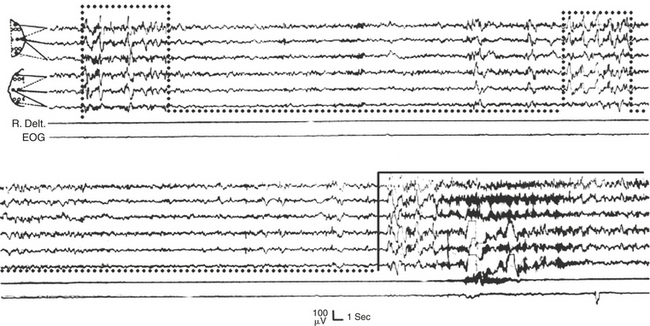
FIGURE 14D.11 Nocturnal motor seizure (black continuous outline) in stage II sleep preceded by a cyclic alternating pattern sequence with A phases (dotted bottom-open boxes) containing interictal electroencephalographic (EEG) abnormalities. R. Delt., Right deltoid muscle; EOG, electro-oculogram. EEG channels 1 to 6 from the top: FP2-C4, FP2-T4, T4-O2; FP1-C3, FP1-T3, T3-O1.
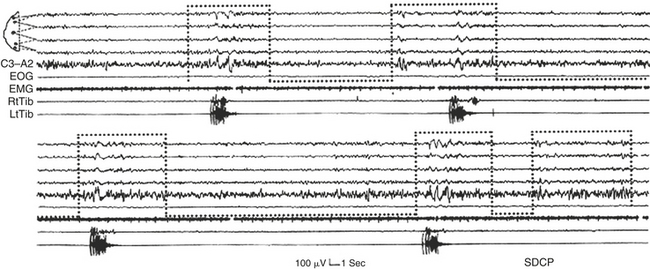
FIGURE 14D.12 A cyclic alternating pattern (CAP) sequence associated with periodic limb jerks that occur in close relation with the A phases of CAP (and never during the B phases). The motor phenomena coincide with or follow the onset of the A phases (dotted bottom-open boxes). Notice that the last phase A is not associated with any muscle event. EOG, Electro-oculogram; EMG, electromyogram; RtTib, right anterior tibialis muscle; LtTib, left anterior tibialis muscle; SDCP, Sleep Disorders Center of Parma. Electroencephalographic channel derivations as in Figure 14D.1 (middle segment).
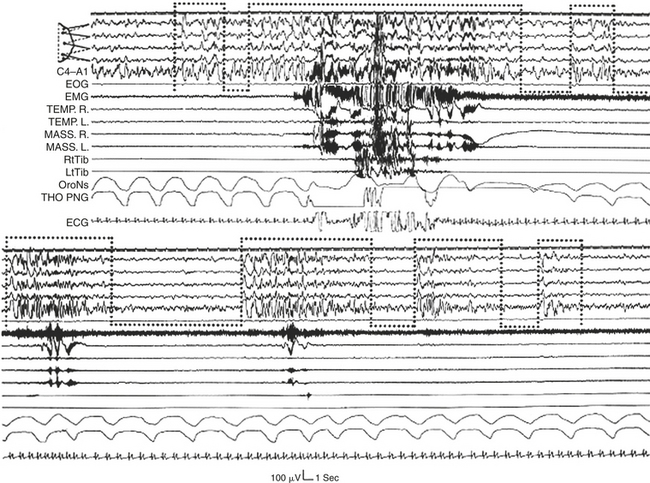
FIGURE 14D.13 Bruxism episodes of different intensities associated with the recurring A phases of cyclic alternating pattern (dotted bottom-open boxes) in the transition from deep to light non–rapid eye movement sleep. Motor events and cardiorespiratory activation occur after the onset of the A phases dominated by desynchronized electroencephalographic (EEG) patterns (especially A3 subtypes). EOG, Electro-oculogram; EMG, electromyogram; TEMP. R, right temporalis muscle; TEMP. L, left temporalis muscle; MASS. R, right masseter muscle; MASS. L, left masseter muscle; RtTib, right anterior tibialis muscle; LtTib, left anterior tibialis muscle; OroNs, oronasal airflow; THO-PNG, thoracic pneumogram; ECG, electrocardiogram. EEG channel derivations as in Figure 14D.1 (top segment).
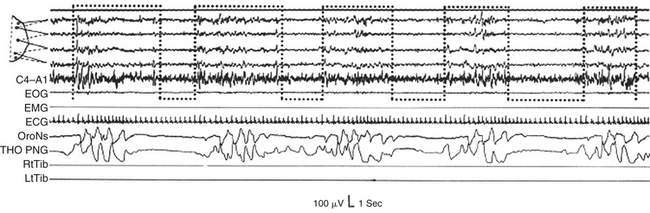
FIGURE 14D.14 Repetitive sequence of different respiratory events (obstructive, mixed, and central), which always occur during a phase B and are interrupted by a phase A (dotted bottom-open boxes). EOG, Electrooculogram; EMG, electromyogram; ECG, electrocardiogram; OroNs, oronasal airflow; THO-PNG, thoracic pneumogram; RtTib, right anterior tibialis muscle; LtTib, left anterior tibialis muscle. EEG channel derivations as in Figure 14D.1 (top segment).
Phase B exerts a powerful inhibition on generalized (see Fig. 14D.10; Video Vignette 31) and focal lesional (![]() Fig. 14D.15) EEG discharges, on myoclonic jerks (see Fig. 14D.12; Video Vignette 34), and on sleep bruxism (see
Fig. 14D.15) EEG discharges, on myoclonic jerks (see Fig. 14D.12; Video Vignette 34), and on sleep bruxism (see ![]() Fig. 14D.13). Phase B triggers breathing interruption in sleep apnea syndrome (see
Fig. 14D.13). Phase B triggers breathing interruption in sleep apnea syndrome (see ![]() Fig. 14D.14; Video Vignette 35).
Fig. 14D.14; Video Vignette 35).
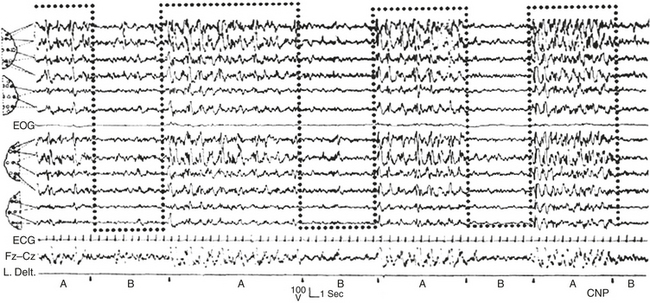
FIGURE 14D.15 Secondary generalization of interictal electroencephalographic (EEG) discharges (right anterior cerebral focus) modulated by the A phases of cyclic alternating pattern (dotted bottom-open boxes). EOG, Electro-oculogram; ECG, electrocardiogram; L. Delt., left deltoid muscle. EEG channel derivations using international electrode placement as in Figure 14D.3 (channels 1 to 6 and 8 to 11 from top); channels 12, 13 from top: F7-T3, T3-T5.
Halasz, P., Terzano, M. G., Parrino, L., Bodizs, R. The nature of arousal in sleep. J Sleep Res. 2004; 13:1–23.
Hirshkowitz, M. Standing on the shoulders of giants: The Standardized Sleep Manual after 30 years. Sleep Med Rev. 2000; 4:169–179.
Parrino, L., Smerieri, A., Spaggiari, M. C., Terzano, M. G. Cyclic alternating pattern (CAP) and epilepsy during sleep: how a physiological rhythm modulates a pathological event. Clin Neurophysiol. 2000; 111(suppl 2):S39–S46.
Parrino, L., Halasz, P., Tassinari, C. A., Terzano, M. G. CAP, epilepsy and motor events during sleep: the unifying role of arousal. Sleep Med Rev. 2006; 10:267–285.
Parrino, L., Ferri, R., Bruni, O., Terzano, M. G. Cyclic alternating pattern (CAP): The marker of sleep instability. Sleep Med Rev. 2012; 16:27–45.
Physionet. CAP Sleep Database. www. physionet. org. [Accessed July 18, 2012].
Rechtschaffen A., Kales A., eds. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: U. S. Government Printing Office, NIH Publication No. 204, 1968.
Terzano, M. G., Mancia, D., Salati, M. R., Costani, G., Decembrino, A., Parrino, L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep. 1985; 8:137–145.
Terzano, M. G., Parrino, L. Clinical applications of cyclic alternating pattern. Physiol Behav. 1993; 54:807–813.
Terzano, M. G., Parrino, L. Origin and significance of the cyclic alternating pattern (CAP). Sleep Med Rev. 2000; 4:101–123.
Terzano, M. G., Parrino, L., Smerieri, A., et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep: Consensus report. Sleep Med. 2002; 3:187–199.
Terzano, M. G., Parrino, L., Spaggiari, M. C., Palomba, V., Rossi, M., Smerieri, A. CAP variables and arousals as sleep electroencephalogram markers for primary insomnia. Clin Neuro-physiol. 2003; 114:1715–1723.
14E
Peripheral Arterial Tonometry
Transient arousal from sleep is associated with increased bursts of sympathetic nerve activity (SNA) in normal adults. This leads to increased heart rate and blood pressure, as well as peripheral vasoconstriction. Sleep fragmentation resulting from frequent arousals has been implicated in daytime impairment of cognitive and psychomotor performance based on investigation of experimental sleep fragmentation in healthy adults. Thus, quantifying brief arousals may be important for the assessment of sleep quality, independent of alternative sleep quality measures such as sleep latency, sleep efficiency, and wake after sleep onset. This response is nonspecific, not dependent on the cause of arousal, but in the appropriate clinical context (such as obesity, heart failure) or with the appropriate phase-linked associations (such as oxygen desaturations), disease states may be predicted.
Theoretical Principles
Exposure to cold results in reflex digital vasoconstriction, whereas heat exposure produces vasodilatation. The digit vascular bed has a dense network of arteriovenous anastomoses that allow considerable variation in skin blood flow. The finger pulse wave amplitude decreases with brachial artery infusion of norepinephrine during wakefulness in a dose-dependent manner. The sympathetic discharges associated with recurrent transient arousals from sleep induce recurrent cycles of digital vasoconstriction, providing an alternative method to EEG for arousal detection in those with a functioning sympathetic nervous system. The peripheral arterial tonometry (PAT) signal is attenuated in response to obstructive breathing during sleep. The signal’s attenuation at the termination of apnea has been shown to be reduced in a dose-dependent manner using the α-receptor antagonist phentolamine (Fig. 14E.1). Thus the finger pulse wave amplitude reflecting digital vasoconstriction is α-adrenoreceptor mediated. O’Donnell et al evaluated the effects of experimental airflow limitation and arousal on digital vascular tone in human subjects with OSA using PAT signal recordings. Subjects were maintained at a therapeutic positive airway, and then nasal positive pressure was reduced over a range of pressures for three to five breaths during NREM sleep as shown in Figure 14E.2. EEG arousal in response to airflow obstruction attenuated the PAT signal more (23.3% reduction) than in the absence of arousal (7.7% reduction). This suggests acute digital vasoconstriction is accentuated in the presence of EEG arousal.
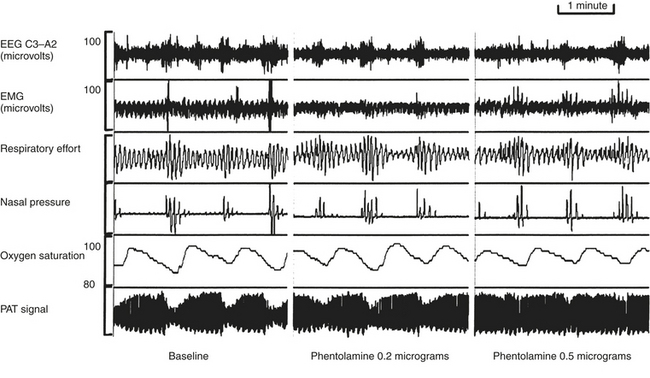
FIGURE 14E.1 Peripheral arterial tonometry (PAT) signal in sleep-disordered breathing.
The cyclic attenuation of the PAT signal is seen to occur phase-locked with respiratory recovery (left panel). Low-dose phentolamine infusion attenuates (middle panel) and higher dose eliminates digital vasoconstriction. Thus normal sympathetic function and peripheral innervation is necessary to detect the signal, which is a limitation in patients with severe autonomic neuropathy. (From Zou D, Grote L, Eder DN, Peker Y, Hedner J. Obstructive apneic events induce alpha-receptor mediated digital vasoconstriction. Sleep. 2004;27:485-489.)
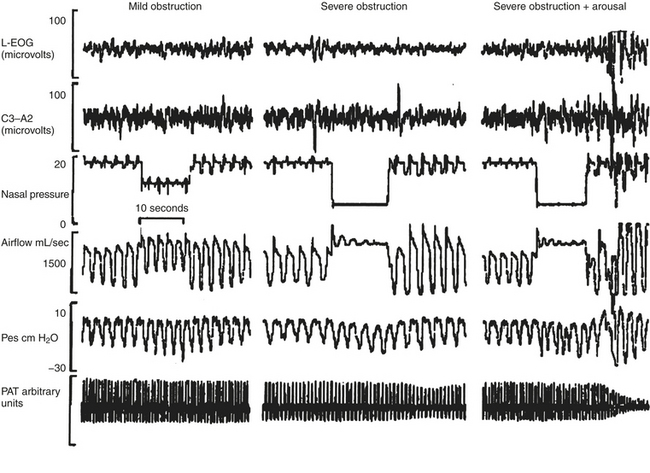
FIGURE 14E.2 Interaction of obstruction and arousal.
Sleep, airflow, nasal positive airway pressure, esophageal pressure (Pes), and the peripheral arterial tonometry (PAT) signal are recorded simultaneously. Mild obstruction induced by partial lowering of positive airway pressure does not result in an electroencephalographic arousal attenuation of the PAT signal (left panel). Dropping the pressure to induce an obstructive apnea results in severe obstruction and mild attenuation of the PAT signal (middle panel), whereas the occurrence of an arousal results in severe digital vasoconstriction (right panel). (From O’Donnell CP, Allan L, Atkinson P, Schwartz AR. The effect of upper airway obstruction and arousal on peripheral arterial tonometry in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:965-971.)
The signal detected has no overlap with pulse transit time, but the basic target is identical—the identification of bursts of sympathetic activity. Similar information may be obtained from the recording of muscle or skin SNA, skin blood flow, and heart rate variability. However, sympathetic innervation and discharge patterns have regional differences, and not all activators result in an increased autonomic flow to all targets.
Summary of Technology
PAT is a plethysmographic technique that uses a finger-mounted pneumo-optical sensor for the continuous measurement of the digital arterial pulse wave volume. The finger sensor applies a uniform, subdiastolic pressure field to the distal two thirds of the finger and fingertip that (1) clamps the probe to the finger, (2) facilitates the unloading of arterial wall tension, which increases the dynamic range of the PAT signal, and (3) prevents distal venous pooling and distention to avoid the induction of venoarterial-mediated vasoconstriction. Thus attenuation of the PAT signal reflects digital vasoconstriction and increased SNA and can serve as a marker for arousal from sleep. It has also been reported that the PAT signal can detect the peripheral vasoconstriction associated with REM sleep.
Clinical Applications
A number of reports have investigated automated analysis of the PAT signal (attenuation and pulse rate), oxyhemoglobin saturation, and wrist actigraphy for detecting episodes of obstructive sleep-disordered breathing. One investigation included standard PSG laboratory studies to define the severity of OSA and concurrent PAT technology studies in the laboratory and home studies on a separate night with only the PAT technology. Based on the likelihood ratios (LRs) for the home study results, the system yielded a very large increase (LR+, 8) in the probability of having moderate-severe OSA (respiratory disturbance index [RDI], 15 events/hr) and a large reduction (LR−, 0.05; 95% CI, 0.01 to 0.31) in the probability of having moderate-severe OSA. Investigations by Pillar et al assessed automated PAT signal detection of arousals and then validated their technique against American Academy of Sleep Medicine (AASM)-defined arousals using concurrent laboratory PSG recordings. Using the Watch-PAT device for the detection of OSA, there was a significant correlation (r, 0.87, P < .001) between ASDA-defined arousals derived from the PSG and autonomic arousals detected with the PAT signal. The sensitivity and specificity of PAT in detecting patients with at least 20 arousals per hour of sleep were 0.80 and 0.79, respectively. The autonomic profile detected by PAT technology also allows a measure of sleep and sleep quality. One example each of a severely and minimally abnormal screen for sleep apnea is shown (Figs 14E.3 and 14E.4).
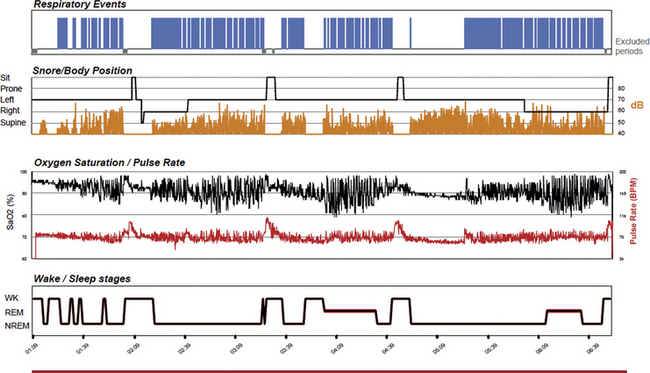
FIGURE 14E.3 Severely abnormal peripheral arterial tonometry (PAT)-based sleep apnea screening test.
A 52-year-old obese (body mass index, 42 kg/m2) man. The 4% oxygen desaturation index is 41/hr of sleep, the PAT-derived respiratory disturbance index is 48 events/hr, and the apnea-hypopnea index is 45.6/hr of sleep. The raw signals may also be viewed, though the system generates the indices in an automated mode.
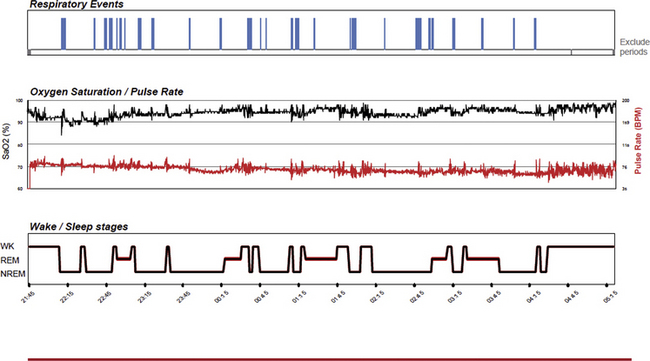
FIGURE 14E.4 Mildly abnormal peripheral arterial tonometry (PAT)-based sleep apnea screening test.
A 46-year-old woman with snoring and mild daytime fatigue. The screening study shows intermittent snoring and relatively mild sleep apnea. The PAT-derived respiratory disturbance index is 12/hr, the apnea-hypopnea index is 5/hr, and the 4% oxygen desaturation index is 4.3/hr. Rapid eye movement sleep was estimated to be 26%.
The PAT technology has been adopted for a measure of vascular function (Endo-PAT 2000). The principle is that the hyperemia after occlusion, resulting in an increase in PAT signal amplitude, reflects endothelial function, and a reactive hyperemia index can be generated. The contralateral upper extremity is used as each subject’s control. The index of endothelial function is calculated as the postocclusion to preocclusion ratio. PAT-derived endothelial function is widely used in research studies, including large epidemiological studies.
Conclusions
The PAT signal can detect digital vasoconstriction associated with bursts of sympathetic activity that are characteristic of transient arousals from sleep. The signal provides one measure of autonomic activation. Integration of such signals into the clinical practice of sleep medicine may provide a complementary role in assessing the detection and clinical impact of disturbed sleep.
Bar, A., Pillar, G., Dvir, I., Sheffy, J., Schnall, R. P., Lavie, P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003; 123:695–703.
Davies, R. J., Belt, P. J., Roberts, S. J., Ali, N. J., Stradling, J. R. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993; 74:1123–1130.
Grote, L., Zou, D., Kraiczi, H., Hedner, J. Finger plethysmography—a method for monitoring finger blood flow during sleep disordered breathing. Respir Physiol Neurobiol. 2003; 136:141–152.
Hamburg, N. M., Benjamin, E. J. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009; 19:6–11.
Hamburg, N. M., Palmisano, J., Larson, M. G., et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011; 57:390–396.
Lavie, P., Schnall, R. P., Sheffy, J., Shlitner, A. Peripheral vasoconstriction during REM sleep detected by a new plethysmographic method. Nat Med. 2000; 6:606.
Morgan, B. J., Crabtree, D. C., Puleo, D. S., Badr, M. S., Toiber, F., Skatrud, J. B. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol. 1996; 80:1627–1636.
O’Donnell, C. P., Allan, L., Atkinson, P., Schwartz, A. R. The effect of upper airway obstruction and arousal on peripheral arterial tonometry in obstructive sleep apnea. Am J Respir Crit Care Med. 2002; 166:965–971.
Pillar, G., Bar, A., Shlitner, A., Schnall, R., Shefy, J., Lavie, P. Autonomic arousal index: an automated detection based on peripheral arterial tonometry. Sleep. 2002; 25:543–549.
Pittman, S. D., Ayas, N. T., MacDonald, M. M., Malhotra, A., Fogel, R. B., White, D. P. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004; 27:923–933.
Schnall, R. P., Shlitner, A., Sheffy, J., Kedar, R., Lavie, P. Periodic, profound peripheral vasoconstriction—a new marker of obstructive sleep apnea. Sleep. 1999; 22:939–946.
Somers, V. K., Dyken, M. E., Mark, A. L., Abboud, F. M. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993; 328:303–307.
Zou, D., Grote, L., Eder, D. N., Peker, Y., Hedner, J. Obstructive apneic events induce alpha-receptor mediated digital vasoconstriction. Sleep. 2004; 27:485–489.
14F
The Electrocardiogram-Spectrogram
The cardiopulmonary coupling technique is based on a continuous ECG signal, and it employs Fourier-based techniques to analyze two signal features: (1) the variability of the cardiac interbeat (R-R) interval series and (2) the fluctuations in QRS amplitude induced by respiration, also known as the ECG-derived respiration (EDR) signal. These signals have two basic patterns: a high-frequency component caused by physiological sinus arrhythmia that reflects breath-to-breath fluctuations, and a low-frequency component that reflects cyclic variation across multiple breaths. Using the Fourier transform, the R-R interval time series and the associated EDR signals are first decomposed into a set of sinusoidal oscillations with specific amplitudes and phases at each frequency.
Two factors are considered in evaluating the strength of the coupling between these two signals. First, if both signals have relatively large oscillation amplitudes at a given frequency, then it is likely that they are coupled with each other. This can be measured by computing the cross-spectral power (i.e., the product of the powers of the two individual signals at a given frequency). Second, if two oscillations are synchronized with each other at a given frequency (i.e., they maintain a constant phase relationship), this can be measured by computing the coherence of these signals. A time series of normal-to-normal sinus (N-N) intervals and the time series of the EDR associated with these N-N intervals are then extracted from the original R-R interval time series. The cross-spectral power and coherence of these two signals are calculated over a 1024 sample (8.5-minute) window. To quantify the low- and high-frequency coupling power distributions, in each 1024 window the coherence and cross-power product is used in calculating the ratio of the sum of the two maximal coherent cross-power peaks in the low-frequency (0.01 to 0.1 Hz) band to the sum of the two maximal peaks in the high-frequency (0.1 to 0.4 Hz) band. Periods of continuous ECG can then be characterized as high, low, and very low frequency coupling. The night-to-night variability of spectrogram metrics is small (intraclass coefficients 0.8 or higher) over 2 weeks of recording.
A subset of low-frequency coupling is elevated–low-frequency coupling, the detection of which requires that the minimum low-frequency power be greater than 0.05 normalized units and that the low-to-high-frequency ratio be greater than 30 to define periods of probable apnea/hypopnea or fragmented sleep. When consecutive spectral windows have a near-identical frequency, it reflects self-similar oscillations of autonomic drive and respiration; this is typical of central sleep apnea and periodic breathing. Thus sleep-breathing phenotypes (obstructive versus central influences) can be identified. The individual respiratory events may be “obstructive,” but if the timing and morphologic characteristics are metronomic/self-similar and self-sustained (nearly 18 minutes), narrow-band coupling will be detected.
High-frequency coupling is reduced and low-frequency coupling increased in states of fragmented sleep, which may occur in depression, fibromyalgia, sleep apnea, and heart failure. Sleep spectrogram biomarkers are heritable and are associated with hypertension and stroke. The EEG correlate of high-frequency coupling, non-CAP, is increased in the sleep following sleep deprivation, during positive pressure titration, following sleep restriction, and with the use of benzodiazepines. High-frequency coupling could be considered a biomarker of sleep effectiveness, an index of the “goodness” of sleep.
The ECG-spectrogram has potential utility as a sleep quality assessment device in the appropriate context (e.g., insomnia, depression, pain) and to detect and track strong respiratory chemoreflex effects on sleep respiration (central sleep apnea, complex apnea, periodic breathing).
The clinical application of the ECG-spectrogram has been enhanced by the development of the M1, a small wearable monitor that can record five to seven nights of ECG, body position, actigraphy, and snoring vibration. A cloud computing approach enables analysis and graphing. The output includes some normative data, which is a work in progress.
Acknowledgment
This chapter is dedicated to the memory of Joseph E. Mietus, who developed the ECG-spectrogram software. As great ones do, he opened my (our) eyes to a new reality.
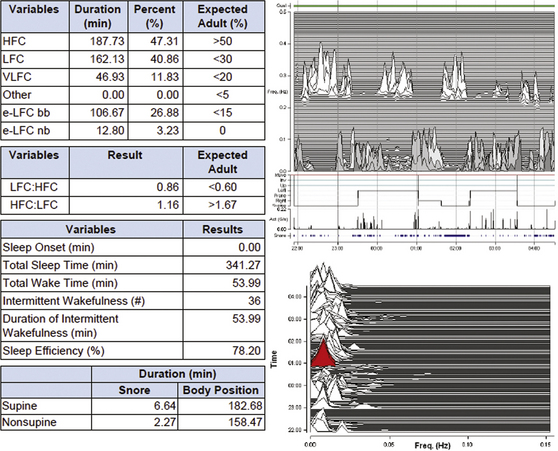
FIGURE 14F.2 Complex sleep apnea—mild residual.
A 46-year-old man with complex apnea, treated with continuous positive airway pressure, dead space, and acetazolamide (Diamox). Note that the amount of high-frequency coupling (HFC) is slightly reduced relative to healthy subjects (also compare with Fig. 14F.3). The clinical outcome is that of major improvement but residual fatigue. Note the small amount of “narrow-band coupling,” in red, consistent with persistent periods of consecutive central apneas or periodic breathing. There is also more “snoring” in the supine position, indicating that pressure increases may be necessary. The table on the left provides summary metrics. bb, Broad band coupling (obstructive apnea); e, elevated; LFC, low-frequency coupling; nb, narrow band coupling (chemoreflex-modulated sleep apnea); VLFC, very low frequency coupling.
FIGURE 14F.1 Intraindividual stability of the electrocardiogram (ECG)-spectrogram.
A 24-year-old man (left) and a 32-year-old woman (right), who are healthy subjects. Samples (every other night, first to last in top to bottom sequence) of a continuous 14-night assessment of the ECG-spectrogram using the M1. Note that the amount of high-frequency coupling, for example, is different between subjects but overall night-to-night intraindividual consistency is noted. Vertical axis is coupling frequency—high-frequency coupling occurs at respiratory frequency. The horizontal axis is time from start of the recording.
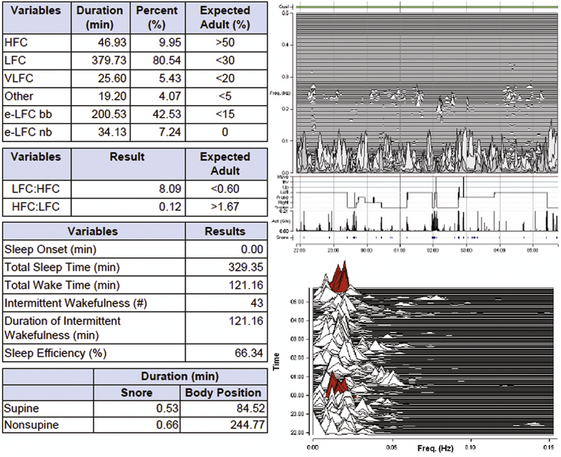
FIGURE 14F.3 Complex sleep apnea—severe residual disease.
A 65-year-old man with congestive heart failure, using continuous positive airway pressure, supplemental O2, zolpidem, and dead space. Note severe fragmented sleep (minimum high-frequency coupling) and multiple periods of narrow band coupling. The table on the left provides summary metrics as in Figure 14F.2.
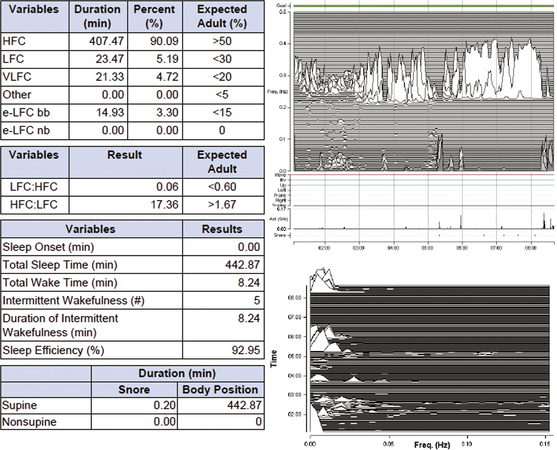
FIGURE 14F.4 Rebound sleep effects.
Changes in sleep homeostatic drive may be captured with the electrocardiogram-spectrogram, and stable periods of non–rapid eye movement sleep are enriched following sleep deprivation recovery or successful positive pressure titration. Note the late (second half) prominence of high-frequency coupling. This patient, a 52-year-old man, has delayed sleep phase syndrome (recording started at 1 AM), resulting in a shift of consolidated sleep later into the night. There is also likely chronic partial sleep deprivation. Though asked to continue sleeping until natural awakening, the patient used an alarm to wake up around 9 AM. The table on the left provides summary metrics as in Figure 14F.2.
Pogach, M. S., Punjabi, N. M., Thomas, N., Thomas, R. J. Electrocardiogram-based sleep spectrogram measures of sleep stability and glucose disposal in sleep disordered breathing. Sleep. 2012; 35:139–148.
Thomas, R. J., Weiss, M. D., Mietus, J. E., Peng, C. K., Goldberger, A. L., Gottlieb, D. J. Prevalent hypertension and stroke in the Sleep Heart Health Study: association with an ECG-derived spectrographic marker of cardiopulmonary coupling. Sleep. 2009; 32:897–904.
Thomas, R. J., Mietus, J. E., Peng, C. K., et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007; 30:1756–1769.
Thomas, R. J., Mietus, J. E., Peng, C. K., Goldberger, A. L. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005; 28:1151–1161.
Sleepimage. http://www. sleepimage. com. Accessed February 26, 2013.

