Sleep Dysfunction and Sleep-Disordered Breathing in Miscellaneous Neurological Disorders
The central nervous system (CNS) plays an important role in the regulation of sleep-wake cycles, and both the CNS and the peripheral nervous system play significant roles in the control of breathing in sleep. It is not therefore surprising that neurological disorders can have a profound impact on sleep and breathing.
Regulation of Sleep-Wake Cycles
Neuroanatomical substrates for wakefulness include the ascending reticular activating system terminating in the thalamus and extrathalamic regions of the basal forebrain. These ascending pathways project widely to the cerebral cortex to maintain wakefulness and a state of arousal. Major neurotransmitter pathways responsible for wakefulness include cholinergic neurons in the laterodorsal tegmentum (LDT) and pedunculopontine tegmentum (PPT), as well as the nucleus basalis of Meynert, noradrenergic neurons in the locus ceruleus (LC), serotonergic neurons in the dorsal raphe nucleus (DR), histaminergic posterior hypothalamic neurons, and the recently described neurons of the lateral hypothalamus and perifornical region that secrete orexin (also known as hypocretin). These hypocretinergic neurons have widespread ascending and descending projections throughout the CNS.
Generators of non–rapid eye movement (NREM) sleep are primarily located in the ventrolateral preoptic nucleus (VLPO) of the anterior hypothalamus, with a minor contribution from the nucleus tractus solitarius in the medulla. These neurons in the VLPO are γ-aminobutyric acid (GABA)ergic and galaninergic (both inhibitory neurotransmitters).
Rapid eye movement (REM) sleep is generated by neurons in the pons. The original McCarley-Hobson model for REM sleep generation mechanism by reciprocal interaction between REM-on (cholinergic LDT-PPT) neurons and REM-off (aminergic LC-DR) neurons was later superseded by the Lu-Saper and Luppi models. In the Lu-Saper model, REM-on GABAergic pontine neurons in the sublaterodorsal (SLD) nucleus (equivalent to peri-locus ceruleus alpha in cats and subceruleus in humans) and the REM-off GABAergic neurons in the ventrolateral periaqueductal gray (VLPAG) and lateral pontine region act as a flip-flop switch to generate REM-NREM cycles. In the Luppi model, REM-on SLD neurons are glutamatergic, whereas REM-off cells in VLPAG and the deep mesencephalic region are GABAergic. Dorsal SLD glutamatergic neurons project to the thalamus and cerebral cortex, causing electroencephalographic activation, and ventral SLD glutamatergic neurons are responsible for REM muscle atonia through both direct and indirect pathways via the ventromedial medulla, using glycinergic and GABAergic inhibitory projections to anterior horn cells of the spinal cord causing hyperpolarization.
Control of Breathing in Sleep and Wakefulness
The central respiratory neurons controlling respiration during sleep and wakefulness (metabolic or automatic system) are located in the medulla in the region of the nucleus tractus solitarius, nucleus ambiguus, and nucleus retroambigualis. The voluntary respiratory system is located in the cerebral cortex and projects partly to the metabolic system in the medulla but mostly descends to the upper cervical spinal cord where the metabolic and the voluntary systems are integrated. Therefore these anatomical locations make these structures controlling sleep-wakefulness and breathing highly vulnerable to the neurological lesions affecting the cortical and subcortical structures. Sleep-disordered breathing (SDB) therefore occurs in many central neurological disorders, as well as in peripheral neurological disorders such as polyneuropathies, neuromuscular junction disorders, and muscle diseases by causing weakness of the respiratory muscles. SDB has been described in many neurodegenerative disorders, “strokes,” other structural affections of the brainstem and upper cervical spinal cord, as well as in many neuromuscular diseases.
Sleep-Disordered Breathing and Other Sleep Dysfunction in Neurological Diseases
In this section of the atlas we are illustrating a few selected neurological disorders causing a variety of sleep dysfunction and SDB. SDB has been estimated to be present in 33% to 53% of Alzheimer’s disease (AD) patients (Fig. 12.1). Whether the prevalence of sleep apnea in AD patients is related to the advancing age of the patient and whether sleep apnea increases the severity of illness or more rapid progression of the disease remains to be determined. SDB causes cognitive impairment, and therefore it is most probable that the presence of SDB will adversely affect these demented patients, although there is some controversy about this. SDB (obstructive, central, and mixed apneas), as well as laryngeal stridor, may be more common in Parkinson’s disease (PD) patients than in age-matched controls. Those PD patients with autonomic dysfunction show increased incidence of SDB (Fig.12.2). Figure 12.3 schematically shows the spectrum of sleep dysfunction in PD. Multiple system atrophy (MSA), another degenerative disease of the autonomic and somatic neurons, initially presents with progressive autonomic failure followed by progressive somatic neurological manifestations affecting multiple systems. The term Shy-Drager syndrome is used to describe the condition in which autonomic failure is the predominant feature. The term striatonigral degeneration is used to describe the condition in which the predominant feature is parkinsonism, whereas sporadic olivopontocerebellar atrophy (OPCA) is used when cerebellar features are the predominant manifestations. A variety of sleep-related respiratory disturbances may occur, which include obstructive, central, and mixed apneas and hypopneas; dysrhythmic breathing; Cheyne-Stokes respiration; and nocturnal inspiratory stridor (Fig. 12.4). In addition to the sporadic OPCA, patients with dominant OPCA may also have central, upper airway obstructive, or mixed apneas, but these are less frequent and less intense in this condition than in MSA.
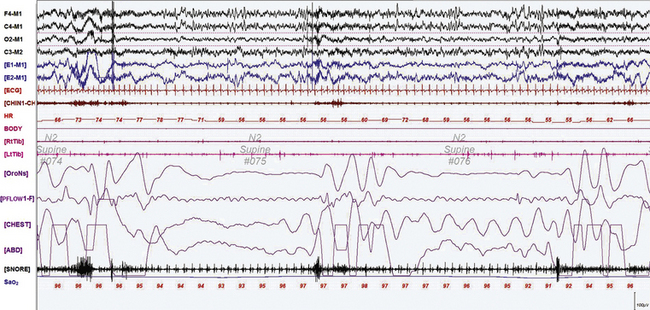
FIGURE 12.1 A case of Alzheimer’s dementia and sleep-disordered breathing.
Polysomnographic recording in a 75-year-old man in an advanced stage of Alzheimer’s disease with profound dementia, excessive daytime sleepiness, and severe nocturnal sleep disturbance. He had severe obstructive sleep apnea (apnea-hypopnea index 68/hr in general, O2 saturation nadir of 77%) with several obstructive, central, and mixed apneas. This 90-second epoch shows first a mixed and then an obstructive apnea. Top four channels, Electroencephalographic recording with electrodes placed according to the 10-20 international electrode placement system; E1-M1 and E2-M1, electro-oculogram channels; ECG, electrocardiogram; CHIN1-CHIN2, mentalis electromyogram; HR, heart rate; RtTib, LtTib, right and left tibialis anterior electromyogram; OroNs, oronasal airflow; PFLOW; nasal pressure transducer recording; CHEST and ABD, effort belts; Sao2, arterial oxygen saturation by finger oximetry. Also included is a snore channel.
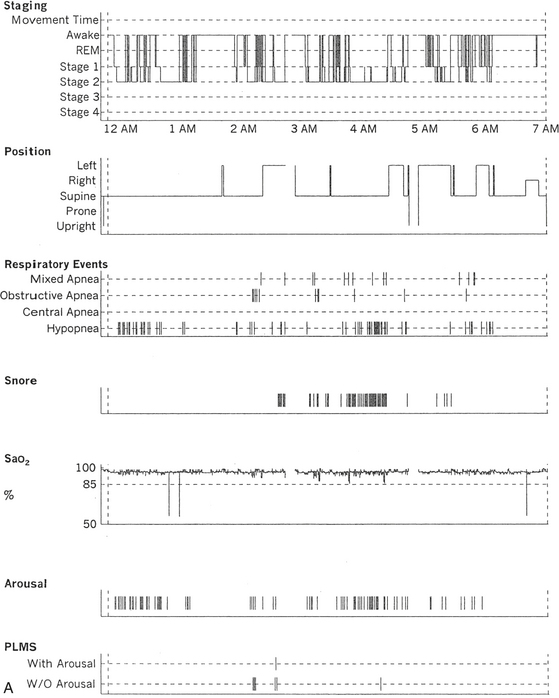
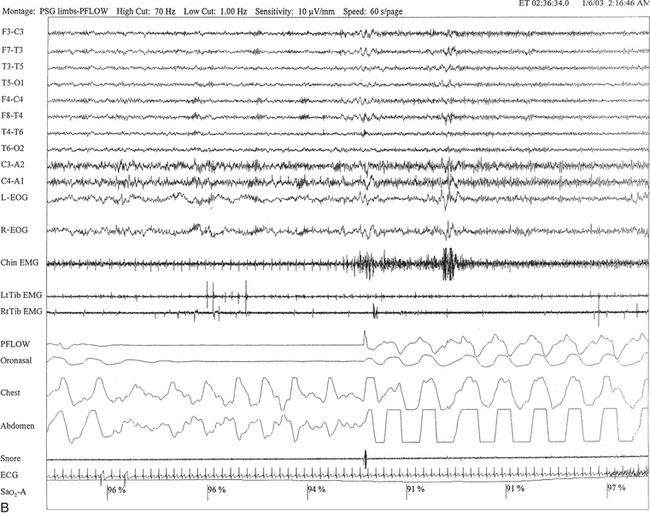
FIGURE 12.2 A case of Parkinson’s disease and sleep-disordered breathing.
A 67-year-old man with history of loud snoring for several years, mild daytime sleepiness, and light-headedness on standing up in the last 2 to 3 months. His past medical history is significant for Parkinson’s disease diagnosed about 2 years ago (currently stage II Hoehn-Yahr scale) and hypertension. A prior sleep study performed approximately 3 years ago had diagnosed severe obstructive sleep apnea syndrome. He tried continuous positive airway pressure treatment unsuccessfully and has been using a dental appliance every night for the last 2 years, which has somewhat decreased his daytime sleepiness. A, Hypnogram shows sleep architectural changes with frequent stage shifts and awakenings resulting in a decreased sleep efficiency of 54%. Slow-wave sleep and rapid eye movement (REM) sleep are conspicuously lacking. Frequent respiratory events, particularly hypopneas, are recorded throughout the night associated with mild O2 desaturation and arousals. The apnea-hypopnea index is moderately increased at 25/hr. The multiple sleep latency test shows a mean sleep latency of 1.75 minutes consistent with pathological sleepiness. Several sleep complaints, commonly sleep maintenance insomnia, hypersomnia, and parasomnias, particularly REM behavior disorder, are reported in Parkinson’s disease patients. Some patients may also have obstructive and central sleep apnea, but adequate studies have not been undertaken to see if apneas are part of the intrinsic disease process or related to aging. B, A 60-second excerpt from an overnight polysomnogram showing an obstructive sleep apnea in stage II non-REM sleep associated with mild O2 desaturation and followed by an arousal. Top 10 channels, Electroencephalogram; L-EOG and R-EOG, left and right electro-oculograms; Chin EMG, electromyography of chin; LtTib, RtTib, left and right tibialis anterior electromyogram; PFLOW, nasal pressure transducer; oronasal thermistor; chest and abdomen effort channels; snore monitor; ECG, electrocardiography; Sao2, oxygen saturation by finger oximetry; PLMS, periodic limb movements in sleep.
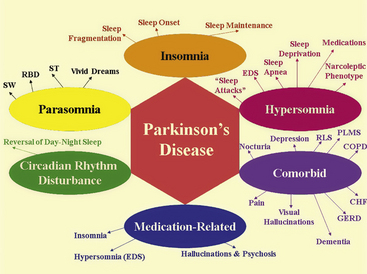
FIGURE 12.3 The spectrum of sleep dysfunction in Parkinson’s disease.
CHF, Congestive heart failure; COPD, chronic obstructive pulmonary disease; EDS, excessive daytime sleepiness; GERD, gastroesophageal reflux disease; PLMS, periodic limb movements in sleep; RBD, rapid eye movement sleep behavior disorder; RLS, restless legs syndrome; ST, sleep terror; SW, sleep walking.
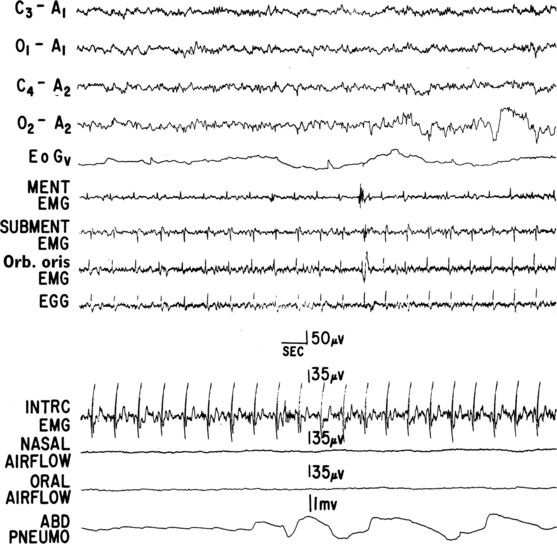
FIGURE 12.4 A case of multiple systems atrophy (Shy-Drager syndrome) and sleep-disordered breathing.
Polysomnogram (PSG) recording in a 58-year-old patient with olivopontocerebellar atrophy presenting with ataxic gait, scanning dysarthria, nystagmus, marked finger-nose and heal-knee incoordination, and bilateral extensor plantar responses. Laboratory test results showed evidence of dysautonomia. PSG shows a portion of an episode of mixed apnea during stage II non-REM sleep. Top four channels, Electroencephalograms; EOGv, vertical electro-oculogram; MENT, mentalis; EMG, electromyography; SUBMENT, submentalis; Orb. oris, orbicularis oris; EGG, electroglossogram; INTRC, intercostal muscles; ABD PNEUMO, abdominal pneumogram. (From Chokroverty S, Sachdeo R, Masdeu J. Autonomic dysfunction and sleep apnea in olivopontocerebellar degeneration. Arch Neurol. 1984;41:509, with permission.)
In several multiple sclerosis patients, SDB abnormalities have been described, including predominant central sleep apnea and ataxic or Biot’s breathing (Fig. 12.5). SDB in such a condition may result from a demyelinating plaque involving the hypnogenic and respiratory neurons in the brainstem. In Arnold-Chiari malformation, particularly in type I malformation, several patients have been described with central and upper airway obstructive sleep apneas, as well as profound sleep hypoventilation (Fig. 12.6).
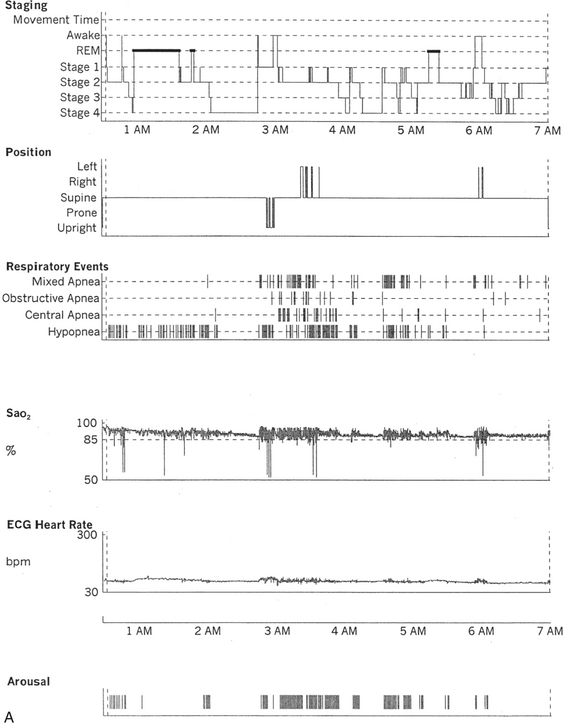
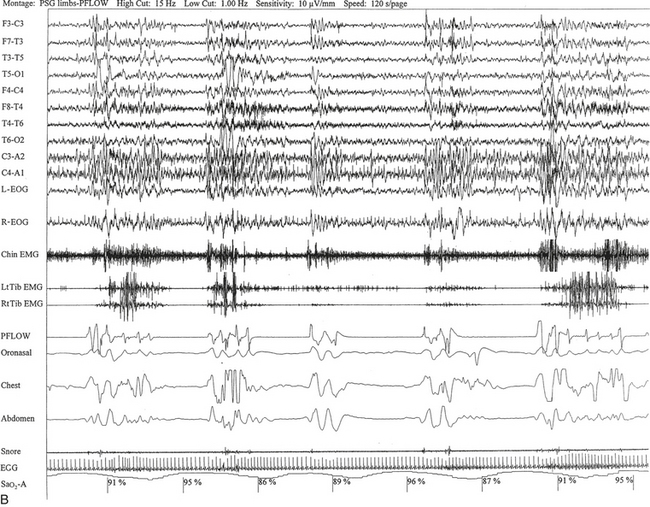
FIGURE 12.5 A case of multiple sclerosis and sleep-disordered breathing (SDB).
A 51-year-old woman with history of multiple sclerosis diagnosed 7 years ago. Her sleep difficulties started approximately 3 years ago and are described as frequent night awakenings, sleepwalking, and brief episodes consisting of sudden sleepiness or impairment of consciousness resulting in falls and multiple fractures but never accompanied by jerky movements of the limbs, tongue biting, or incontinence, with spontaneous recovery in 5 to 10 minutes without residual confusion. These episodes occur during early morning hours, as well as during the day. Her neurological examination is significant for the presence of decreased visual acuity and impaired saccades bilaterally, horizontal nystagmus on looking to the left, intention tremor (left more than right) on finger-to-nose testing, mild ataxia in the lower extremities on heel-to-shin testing, tandem ataxia, and impaired joint and position sense in the toes bilaterally. She was clinically evaluated with a differential diagnosis of SDB related to multiple sclerosis, narcolepsy-cataplexy secondary to multiple sclerosis, and sleepwalking. Unusual nocturnal seizures remained unlikely given the clinical features, the several negative electroencephalograms (EEGs), and the negative long-term epilepsy monitoring. A, Hypnogram significant for rapid eye movement (REM) sleep distribution abnormality (longest REM in the early part of the night); frequent obstructive, mixed, and central apneas and hypopneas both during non-REM and REM sleep; mild-moderate O2 desaturation; and frequent arousals. Sleep-related respiratory dysrhythmias caused by brainstem involvement are a common finding in multiple sclerosis patients. B, A 120-second excerpt from overnight polysomnographic recording showing repeated central apneas with O2 desaturation. Note that some of these events have the characteristics of Biot’s breathing (a variant of ataxic breathing). An increase in muscle tone is noted on chin and tibialis anterior electromyogram (EMG) channels following some central events. Top 10 channels, EEG; L-EOG and R-EOG, left and right electro-oculograms; Chin EMG, electromyography of chin; LtTib, RtTib, left and right tibialis anterior electromyography; PFLOW, peak flow; oronasal thermistor; chest and abdomen effort channels; snore monitor; ECG, electrocardiography; Sao2, oxygen saturation by finger oximetry.
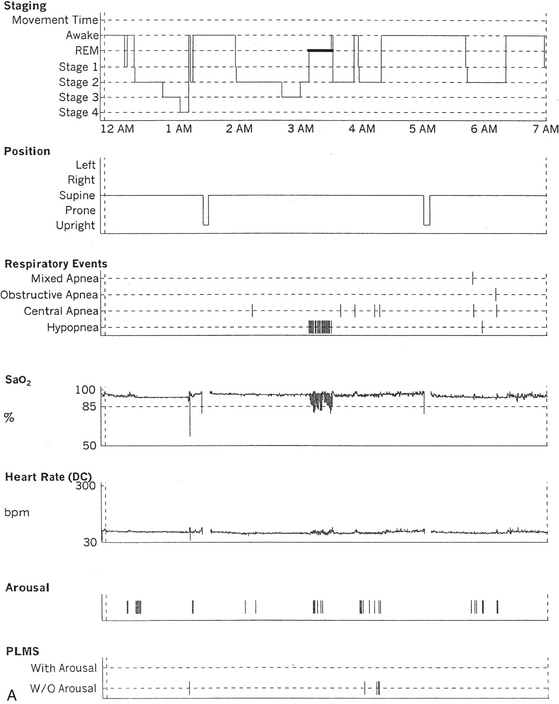
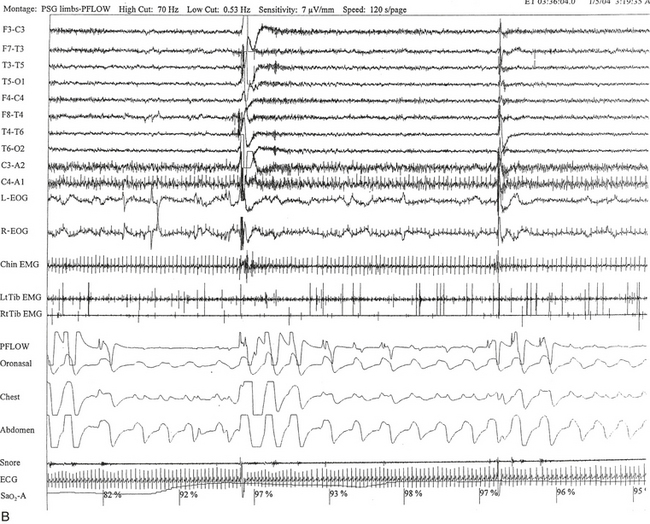
FIGURE 12.6 A case of Arnold-Chiari malformation and sleep-disordered breathing.
A 52-year-old man with history of tiredness and excessive daytime sleepiness for many years but no cataplexy, sleep paralysis, or hypnagogic hallucinations. At the age of 27 years, he complained of gait problems, and magnetic resonance imaging examination revealed Arnold-Chiari malformation type I. His neurological examination is significant for the presence of a coarse horizontal nystagmus, minimal right lower facial weakness, minimal right wrist extensor muscle weakness, minimal to mild ataxia in upper and lower extremities on coordination testing, and presence of ataxia on tandem gait. A, Hypnogram shows a few periods of apneas and hypopneas accompanied by mild-moderate oxygen desaturation and arousals limited exclusively to a single rapid eye movement (REM) sleep period recorded during the night. A supine posture is maintained throughout the polysomnographic recording. These findings are suggestive of REM sleep–related hypoventilation. Central sleep apnea, obstructive sleep apnea, and hypoventilation have all been described in patients with Arnold-Chiari malformation, likely from brainstem involvement. B, A 120-second excerpt from REM sleep showing one obstructive apnea followed by two sequential hypopneas; O2 desaturation of 82%, likely from a prior respiratory event, is recorded at the onset of the epoch. The first two events are followed by an arousal response, and the epoch does not include the complete recovery phase of the third event. Phasic eye movements of REM sleep are noted on the electro-oculogram channels in the early part of the epoch. Phasic muscle twitches of REM sleep are noted on both tibialis anterior electromyogram (EMG) channels. Chin EMG channel shows electrocardiography artifact. Top 10 channels, Electroencephalogram; L-EOG and R-EOG, left and right electro-oculograms; Chin EMG, electromyography of chin; LtTib, RtTib, left and right tibialis anterior electromyography; PFLOW, peak flow; oronasal thermistor; chest and abdomen effort channels; snore monitor; ECG, electrocardiography; Sao2, oxygen saturation by finger oximetry; PLMS, periodic limb movements in sleep.
Rapid eye movement behavior disorder (RBD) is characterized by dream-enacting behavior (DEB) arising out of REM sleep and polysomnographic (PSG) documentation of REM without atonia or excessive phasic muscle bursts during REM sleep. The yield is improved with a multiple muscle montage (Fig. 12.7; see Table 1.4). Electromyographic (EMG) abnormalities may not be seen in all muscles in RBD patients, explaining EMG heterogeneity (Fig. 12.8) and the importance of multiple muscle recordings in such patients. Patients often complain that their dreams are violent and unpleasant (although some RBD patients may have nonviolent dreams), and the acting out is often forceful and may cause injury to the patient or bed partner. There is a male predominance. RBD has been described in synucleinopathies (e.g., PD, MSA, diffuse Lewy body dementia) and occasionally in tauopathies (e.g., AD, progressive supranuclear palsy, corticobasal degeneration), brainstem strokes, and myotonic dystrophy type 2 (see Fig. 12.7). RBD may precede the appearance of clinically identifiable neurodegenerative disease by several years or even decades. According to some investigators, the commonest cause of acute RBD is antidepressant use (particularly selective serotonin reuptake inhibitors and tricyclic antidepressants). It has also been seen in patients withdrawing from alcohol, and in narcolepsy. Rare cases of DEB have been described arising out of NREM sleep (see Fig. 9.17).
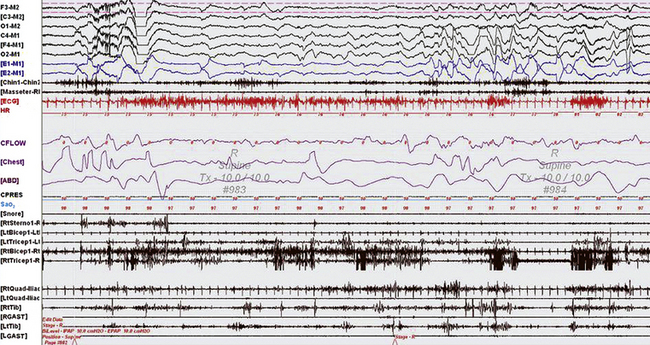
FIGURE 12.7 A case of rapid eye movement (REM) behavior disorder (RBD) in myotonic dystrophy type 2.
A 60-second epoch from the overnight continuous positive airway pressure titration study of a 63-year-old woman with myotonic dystrophy type 2. She had a history of dream-enacting behavior (DEB) and a prior diagnosis of obstructive sleep apnea. She was not on medications traditionally known to cause RBD, such as selective serotonin reuptake inhibitors, nor did she have a history of alcohol use. The study captured an episode of DEB with clear recall (she recalls dreaming that she was being strangled and was fighting her attacker ![]() [Video Vignette 20]). This epoch represents rapid eye movement (REM) sleep, as evidenced by rapid eye movements in the electro-oculogram (EOG) channels and a desynchronized, low-voltage electroencephalogram (EEG); however, there are excessive phasic bursts in the chin and limb electromyogram (EMG) channels and persistence of muscle tone in the Chin EMG. This activity was present in more than 50% of REM epochs, meeting the criteria for REM without atonia. RBD, commonly seen with neurodegenerative diseases, particularly synucleinopathies, is not known to occur in myotonic disorders; RBD in this case may have resulted from brainstem involvement of her multisystem generalized membrane disorder. Top six channels, EEG recording with electrodes placed according to the international 10-20 electrode placement system; E1-M1 and E2-M1, left and right EOG channels with reference to the left mastoid (M1); Chin1-Chin2, EMG activity from the mentalis muscle. Multiple muscle recording also includes EMG activity recorded from the following muscles: Right Masseter muscule (Masseter RM) right sternocleidomastoid (RtSterno1), bilateral biceps and triceps, and bilateral quadriceps femoris referenced to the respective iliac crests (RtQuad-Iliac, LtQuad-Iliac), bilateral gastrocnemius (GAST) and tibialis anterior (Tib) muscles. Chest and abdominal effort were measured using respiratory inductive plethysmography belts. ECG, Electrocardiography; HR, heart rate; Sao2, oxygen saturation by finger oximetry. Snore channel is also displayed. The CFLOW channel demonstrates obstructive apneas with arousals. (Reproduced with permission from Chokroverty S, Bhat S, Rosen D, Farheen A. REM behavior disorder in myotonic dystrophy type 2. Neurology. 2012;78[24]:2004.)
[Video Vignette 20]). This epoch represents rapid eye movement (REM) sleep, as evidenced by rapid eye movements in the electro-oculogram (EOG) channels and a desynchronized, low-voltage electroencephalogram (EEG); however, there are excessive phasic bursts in the chin and limb electromyogram (EMG) channels and persistence of muscle tone in the Chin EMG. This activity was present in more than 50% of REM epochs, meeting the criteria for REM without atonia. RBD, commonly seen with neurodegenerative diseases, particularly synucleinopathies, is not known to occur in myotonic disorders; RBD in this case may have resulted from brainstem involvement of her multisystem generalized membrane disorder. Top six channels, EEG recording with electrodes placed according to the international 10-20 electrode placement system; E1-M1 and E2-M1, left and right EOG channels with reference to the left mastoid (M1); Chin1-Chin2, EMG activity from the mentalis muscle. Multiple muscle recording also includes EMG activity recorded from the following muscles: Right Masseter muscule (Masseter RM) right sternocleidomastoid (RtSterno1), bilateral biceps and triceps, and bilateral quadriceps femoris referenced to the respective iliac crests (RtQuad-Iliac, LtQuad-Iliac), bilateral gastrocnemius (GAST) and tibialis anterior (Tib) muscles. Chest and abdominal effort were measured using respiratory inductive plethysmography belts. ECG, Electrocardiography; HR, heart rate; Sao2, oxygen saturation by finger oximetry. Snore channel is also displayed. The CFLOW channel demonstrates obstructive apneas with arousals. (Reproduced with permission from Chokroverty S, Bhat S, Rosen D, Farheen A. REM behavior disorder in myotonic dystrophy type 2. Neurology. 2012;78[24]:2004.)
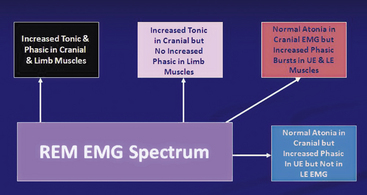
FIGURE 12.8 The heterogeneity of electromyographic (EMG) findings in rapid eye movement (REM) behavior disorder.
LE, Lower extremites; UF, upper extremities.
Sleep, epilepsy, breathing, and sleep apnea are all interrelated, but seizures may adversely affect breathing in sleep, and disordered breathing during sleep may in turn adversely affect seizures. Sleep and sleep deprivation may trigger seizures in susceptible individuals (see Chapter 11). Generalized seizures and seizure discharges in the limbic-hypothalamic system may cause SDB. Reciprocal connections between the central respiratory neurons in the medulla and the limbic, hypothalamic, and other forebrain structures explain why epileptic seizures are triggered during sleep and the discharges in the limbic-hypothalamic region may interfere with respiratory regulation, causing SDB. The association of epilepsy and sleep apnea is being increasingly recognized (Fig. 12.9). Sleep fragmentation caused by Sleep fragmentation caused by SDB is an important cause of triggering seizures, and several studies have shown that treatment of SDB in these patients improves seizure control.
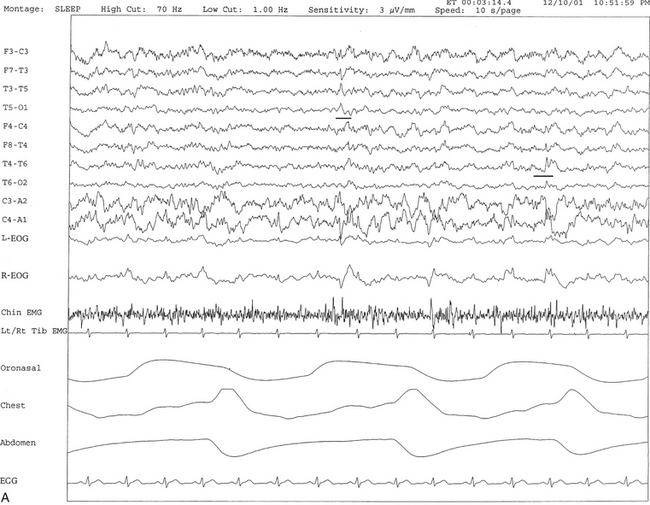
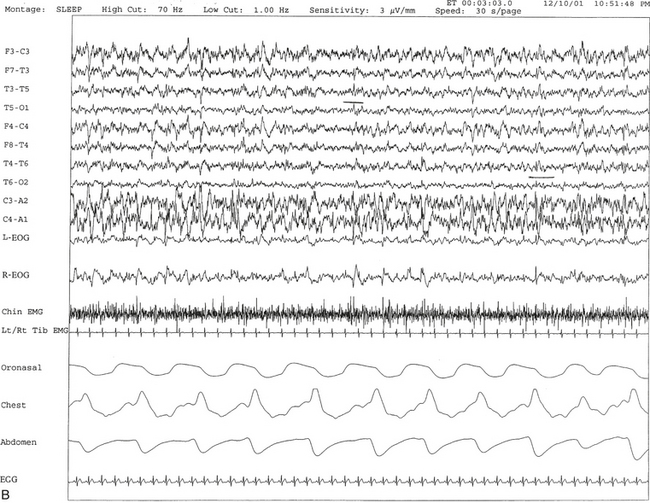
FIGURE 12.9 A case of seizure disorder and sleep-disordered breathing.
A 53-year-old man with history of frequent night awakenings and excessive daytime sleepiness. Over the last 2 months he reports awakening with choking and “fighting for breath” along with episodes of transient dizziness and unsteadiness during the day. In addition, he reports several episodes of nocturnal tongue biting and two episodes of nocturnal enuresis over the last 20 years. The only significant finding on neurological examination is the presence of postural tremor of outstretched hands bilaterally. Differential diagnostic possibilities of sleep apnea and nocturnal seizures are raised. Magnetic resonance imaging of the brain revealed no intracranial pathological condition. Overnight polysomnogram (PSG) showed the presence of mild sleep apnea with an apnea-hypopnea index of 10.4/hr. The presence of spike and wave activity (underlined) independently over both temporal regions, left more often than right, was also noted and further confirmed on a prolonged daytime EEG. A and B, Ten- and 30-second excerpts, respectively, from an overnight PSG recording showing the presence of independent left and right temporal spike and wave discharges (underlined). Top 10 channels, Electroencephalogram; L-EOG and R-EOG, left and right electro-oculograms; electromyography of chin, LtTib, RtTib, left and right tibialis anterior electromyography; oronasal thermistor; chest and abdomen effort channels; ECG, electrocardiography.
Vagal nerve stimulation (VNS), a Food and Drug Administration–approved treatment modality for refractory epilepsy, is an effective adjuvant therapy. The device consists of a generator subcutaneously implanted in the anterior chest wall and a lead that wraps around the left vagus nerve. Intermittent vagal nerve stimulation is thought to cause desynchronization of thalamocortical impulses, thus exerting an antiepileptic effect. However, VNS has also been known to affect breathing in sleep (Fig. 12.10). Cyclical hypopneas corresponding to VNS activation and an increase in central apneas have been reported in patients with VNS implantation. Investigators have found that decreasing VNS frequency improved VNS-induced SDB. In another report, increasing VNS off-time improved arterial oxygen desaturations occurring with VNS-induced SDB. It is not clear why VNS devices induce respiratory events in sleep; both central and peripheral factors seem to play a role.
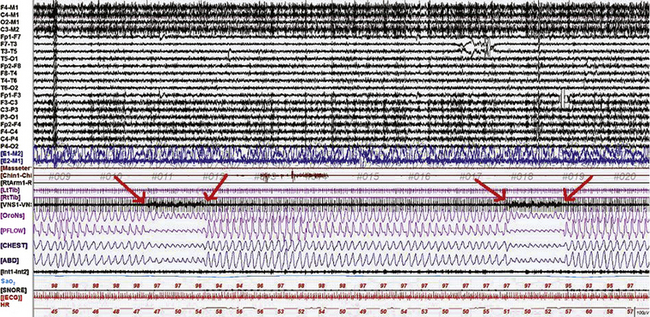
FIGURE 12.10 The effects of vagal nerve stimulation (VNS) on breathing in sleep.
A 360-second epoch of N2 sleep from the overnight polysomnogram of a 22-year-old man referred to the sleep laboratory for snoring and weight gain, with a suspicion of obstructive sleep apnea. He had also undergone VNS implantation for intractable epilepsy. Note the occurrence of respiratory events corresponding to VNS stimulation (as noted by artifact in the VNS channel, between the arrows). Events occurred every 3 minutes, corresponding to off-time (VNS settings were a current of 2 mA, frequency 30 Hz, pulse width 500 microseconds, on-time 30 seconds, off-time 3 minutes). These events are accompanied by SaO2 desaturations of 3%. Also noted is a mild tachypnea during the event. The etiology of VNS-induced sleep-disordered breathing is unclear, and both central and peripheral mechanisms have been implicated. Top 20 channels, Extended electroencephalogram channels in referential and bipolar montage (international 10-20 electrode nomenclature); E1-M2, E2-M1, left and right electro-oculograms; Masseter, masseter electromyogram (EMG); Chin 1-Chin 2, chin EMG; RtArm, right biceps brachii EMG; LtTib, RtTib, left and right tibialis anterior EMG; VNS1-VNS2,VNS channel (an electrode was placed over the lead in the left neck and a reference electrode a short distance away to capture VNS stimulations); OroNs and PFLOW, respiratory air flow; CHEST and ABD, respiratory effort; Sao2, arterial oxygen saturation by pulse oximetry; ECG, electrocardiogram; HR, heart rate. Also included is a snore channel.
Patients with narcolepsy-cataplexy syndrome may also have sleep apnea, which is present in up to 30% of narcoleptic patients (Fig. 12.11). SDB most commonly occurs as central apneas, but patients may also have obstructive and mixed apneas. Sleep attacks with narcolepsy may be aggravated by such associated sleep apnea, and therefore recognition of this is important in these patients because they may require additional treatment with continuous positive airway pressure for relief of apnea and excessive daytime somnolence.
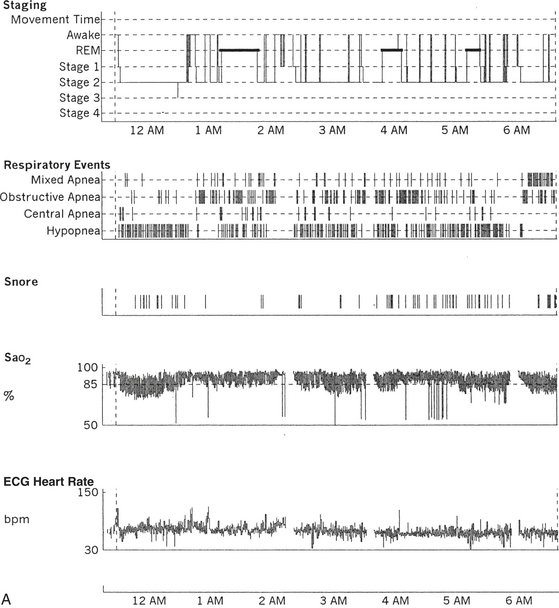
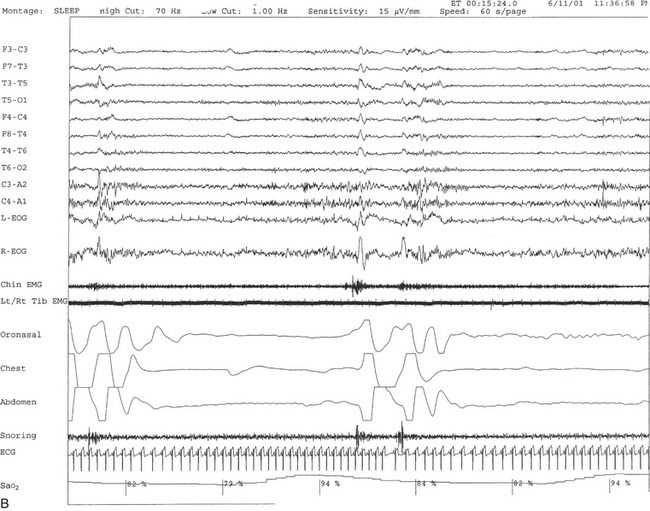
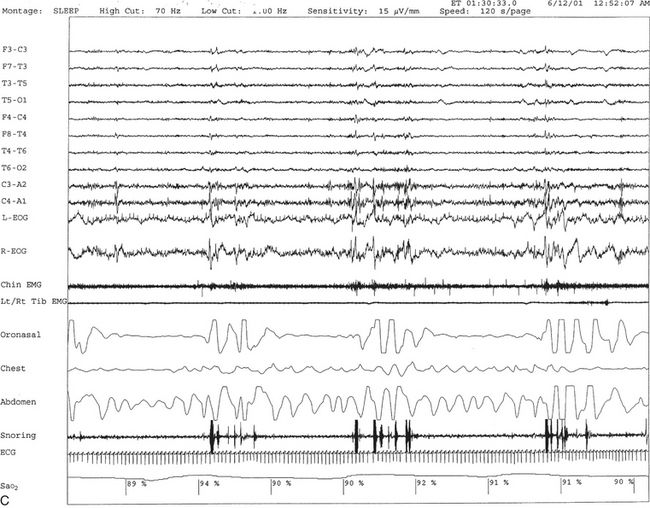
FIGURE 12.11 A case of narcolepsy and sleep-disordered breathing.
A 27-year-old man complaining of fatigue and excessive daytime sleepiness with snoring and disturbed sleep at night for several years. Daytime sleepiness was characterized by feeling sleepy during classes in high school and while driving, but there was no history of cataplexy, sleep paralysis, or hypnagogic hallucinations. Results of evaluation of the throat and neurological examinations were normal. Overnight polysomnogram (PSG) in 1998 was significant for slightly increased respiratory disturbance index at 8.7 with some nonspecific sleep architectural changes and absence of oxygen desaturation consistent with mild sleep apnea. The mean sleep latency on multiple sleep latency test (MSLT), however, was 1.12 minutes, consistent with pathological sleepiness, and he had two sleep-onset rapid eye movement (REM) periods. Given the clinical history and disparity between PSG and MSLT findings, he was diagnosed with narcolepsy and started on stimulant treatment. He responded well, and his daytime sleepiness decreased. In 2001 his daytime sleepiness and fatigue returned despite taking the stimulant. He had disturbed sleep with cessation of breathing and frequent awakenings at night. He was also reported to snore loudly in sleep. He reported having put on approximately 20 pounds of weight in the last 6 months. A repeat PSG study in 2001 showed recurrent episodes of obstructive, mixed, and central apneas and hypopneas during non-REM and REM sleep accompanied by moderate-severe O2 desaturation, repeated arousal, snoring, and a severely increased apnea-hypopnea index of 85/hr. The hypnogram is shown in A. Thus sleep apnea in conjunction with narcolepsy was diagnosed. Sleep apnea, predominantly central, has been described in patients with narcolepsy. B, A 60-second excerpt from overnight PSG showing two central apneas accompanied by mild-moderate O2 desaturation.
Sleep disturbances in neuromuscular disorders are usually the result of sleep-related respiratory dysrhythmias, and in some cases direct dysfunction of the respiratory premotor and motor neurons in the brainstem and spinal cord are responsible for sleep complaints. Involvement of the respiratory muscles, the phrenic and intercostal nerves, or the neuromuscular junctions of the respiratory and oropharyngeal muscles may cause SDB in neuromuscular disorders, causing excessive daytime somnolence, repeated arousals, and sleep fragmentation (Figs. 12.12 and 12.13). SDB has been described in many patients with neuromuscular disorders, including myotonic dystrophy (both types 1 and 2) and other primary muscle diseases, motor neuron disease or amyotrophic lateral sclerosis, and neuromuscular junctional disorders and polyneuropathies. Many reports of central, mixed, and upper airway obstructive sleep apneas and alveolar hypoventilation associated with excessive daytime somnolence have been described in such patients. Respiratory disturbances are generally noted in the advanced stage of such disorders, but sometimes SDB may be the presenting complaint in patients with motor neuron disease or with acid maltase deficiency. In amyotrophic lateral sclerosis, SDB may result from weakness of the upper airway and the diaphragmatic and intercostal muscles because of involvement of the bulbar, phrenic, and intercostal motor nuclei (see Fig. 12.12). In addition, degeneration of central respiratory neurons may occur, causing central and obstructive sleep apneas in this condition. Patients with myotonic dystrophy type 1 often have excessive daytime sleepiness with hypersomnolence and sleep-onset REM periods (SOREMPs) on their multiple sleep latency test (MSLT). Investigators recently reported increased arousals, decreased sleep efficiency, alpha-delta sleep, obstructive apneas, and paradoxical breathing in REM sleep in six patients with myotonic dystrophy type 2. MSLT performed in four of these patients showed reduced mean sleep latency without SOREMPs.
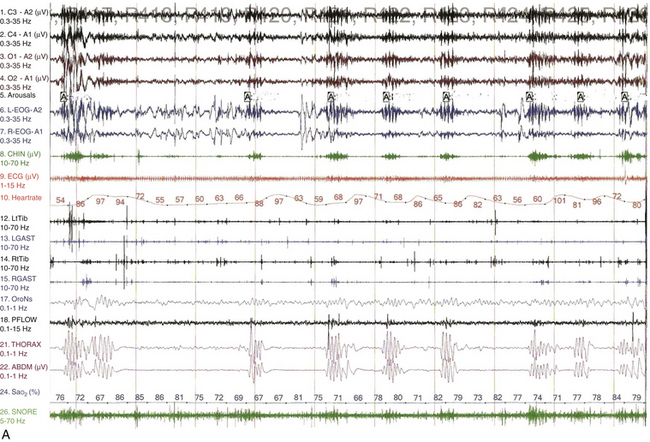
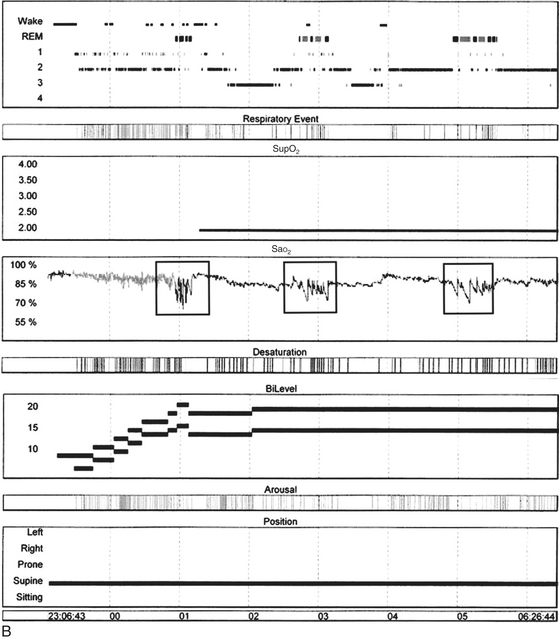
FIGURE 12.12 Sleep-disordered breathing in amyotrophic lateral sclerosis (ALS).
A, Overnight polysomnographic recording from a patient with ALS presenting with upper and lower motor neuron signs, including bulbar palsy, showing recurrent periods of central apneas, many of which are prolonged, followed by irregular ventilatory cycles resembling ataxic breathing accompanied by severe oxygen desaturation and sleep hypoxemia during rapid eye movement (REM) sleep. Top four channels, Electroencephalogram, international electrode placement system; L-EOG, left electro-oculogram; R-EOG, right electro-oculogram; CHIN, submental electromyogram (EMG); ECG, electrocardiogram; LtTib, left tibialis anterior EMG; LGAST, left gastrocnemius EMG; RtTib, right tibialis anterior EMG; RGAST, right gastrocnemius EMG; OroNs, oronasal air flow; PFLOW, nasal pressure recording for airflow; THORAX, thoracic breathing effort; ABD, abdominal breathing effort; Sao2%, arterial oxygen saturation (percentage) by finger oximetry; SNORE, snoring recording. B, Overnight hypnogram from this patient’s subsequent bilevel titration study. Even at the highest pressure tried (20/15 cm), residual obstructive sleep apnea (OSA) (with respiratory events marked in the hypnogram) accompanied by desaturations occurred in REM sleep, although they were better controlled in non-REM (NREM) sleep. However, arterial oxygen saturation (SaO2) remained below 90% even in the absence of OSA and despite the addition of supplemental O2 (Sup O2) at 2 L/min. There is superimposed cyclical worsening of SaO2 in REM sleep (boxes). This pattern was caused by sleep hypoxemia, with superimposed event-related hypoxemia in REM sleep caused by OSA. In patients with neuromuscular weakness, this pattern is the result of alveolar hypoventilation caused by diaphragmatic weakness, with superimposed OSA caused by upper airway muscle atonia, both of which are worse in REM sleep. (Reproduced with permission from Bhat S, Gupta D, Chokroverty S. Sleep disorders in neuromuscular diseases. Neurol Clin. 2012;30[4]:1359-1387.)
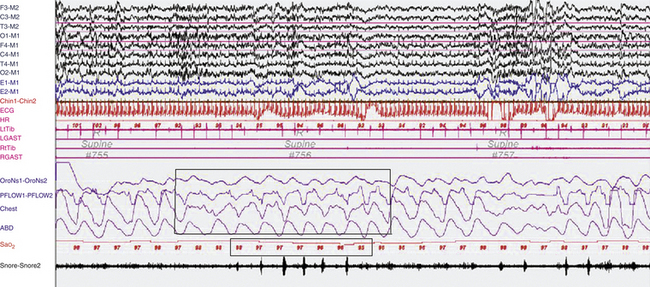
FIGURE 12.13 Paradoxical breathing in rapid eye movement (REM) sleep in a patient with limb girdle muscular dystrophy.
A representative 90-second epoch of REM sleep from the overnight polysomnographic (PSG) recording of an 18-year-old woman with limb girdle muscular dystrophy. She complained of orthopnea, insomnia caused by poor sleep continuity with frequent awakenings, excessive daytime sleepiness, and cognitive concerns. On examination she had generalized, symmetrical weakness, rated (right/left) 1/1 deltoids, 1/1 arm extensors, 2/2 elbow flexors, 3/3 elbow extensors, 3/3 wrist and finger extensors, 4/4 finger flexors, 1/1 hip flexors, 4/4 knee extensors, 4/4 knee flexors, 0/0 dorsiflexion at the ankles, 4/4 plantar flexion bilaterally. Reflexes were intact and symmetrical throughout. She was nonambulatory and wheelchair bound. Her PSG showed mild obstructive sleep apnea, with an apnea-hypopnea index of 10.3/hr and an arterial oxygen saturation (SaO2) nadir of 93%. Note the occurrence of paradoxical breathing (upper box) in REM sleep, with alternating movements of the chest and abdominal effort belts, accompanied by an SaO2 desaturation of 3% (lower box). A second, similar event without accompanying SaO2 desaturation is noted toward the end of the epoch. In non-REM (NREM) sleep, she had mostly short cycle central apneas. Top eight channels, Electroencephalographic recording with electrodes placed according to the 10-20 international electrode placement system; E1-M1 and E2-M1, electro-oculogram channels; Chin1-Chin2, submental electromyogram (EMG); ECG, electrocardiogram; HR, heart rate; LtTib, RtTib, left and right tibialis anterior EMG; LGAST, RGAST; left and right gastrocnemius EMG; OroNs1-OroNS2, oronasal airflow; PFLOW, nasal pressure transducer recording; Chest and ABD, effort belts; Sao2, arterial oxygen saturation by finger oximetry. Also included is a snore channel. (Reproduced with permission from Bhat S, Gupta D, Chokroverty S. Sleep disorders in neuromuscular diseases. Neurol Clin. 2012;30[4]:1359-1387.)
Agrypnia excitata is a syndrome characterized by severely reduced or absent sleep (agrypnia) associated with generalized motor and autonomic overactivation (excitata). It is caused by a thalamolimbic system dysfunction and comprises three different conditions: fatal familial insomnia (FFI), delirium tremens, and Morvan's syndrome. These patients may lapse into peculiar oneiric stupor episodes during which they display simple quiet repetitive motor gestures mimicking daily-life activities, such as gesticulating or searching for nonexistent objects. When questioned, they are able to recall the content of their oneiric scene just enacted spontaneously or on probing. These oneiric stupor episodes may occur with open or closed eyes and initially last only a few seconds. PSG studies during these episodes show a mixture of stage 1 and REM sleep (Figs.12.14 to 12.16) and should not be confused with RBD, which correlates with REM sleep without atonia (see earlier).
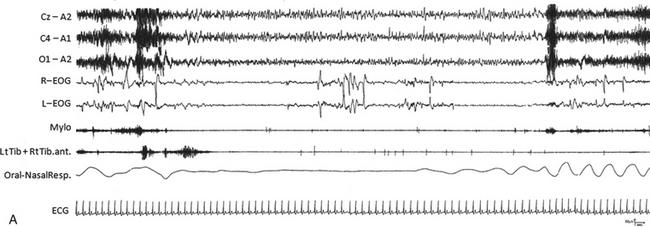
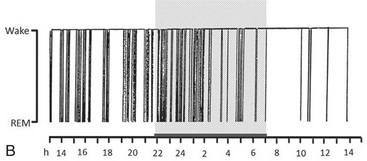
FIGURE 12.14 Fatal familial insomnia (FFI).
An epoch from the polysomnographic (PSG) recording (A) as well as a hypnogram (B) from a patient with FFI. Sleep in FFI patients is characterized by an early and progressive reduction in sleep spindles and K complexes, a reduction in total sleep time, and disruption of the cyclical organization of sleep. Slow wave sleep is lost first, then rapid eye movement (REM) disengages from its circadian cycle and intrudes into the waking state. Twenty-four hour PSG recordings demonstrate a progressive inability to generate physiological electroencephalographic (EEG) sleep patterns. Sleep spindles, EEG activities of thalamic origin characterizing physiological sleep onset, and the shift from non-REM to REM sleep and vice versa disappear very early in the course of the disease, and only brief episodes of REM sleep emerge directly and abnormally from wakefulness. The hypnogram is characterized by continuous fluctuations from wakefulness to brief episodes of REM sleep with or without atonia. Cz-A2, C4-A1, 01-A2, Electroencephalogram; R-EOG, L-EOG, right and left electro-oculogram; RtTib, LtTib, right and left tibialis anterior EMG; Oral-Nasal Resp, oronasal airflow; ECG, electrocardiogram. ![]() (See Video Vignette 9.)
(See Video Vignette 9.)
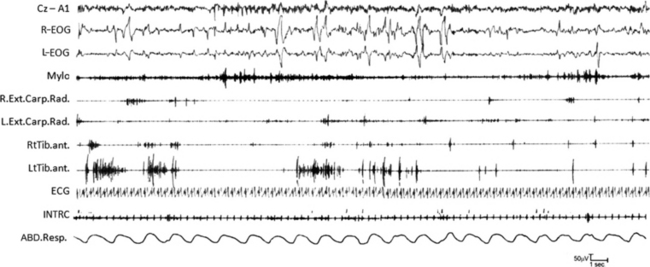
FIGURE 12.15 Delirium tremens.
A 56-year-old man, an alcohol abuser since the age of 30 years, spontaneously stopped drinking. Within a few days, he developed abnormal motor and verbal activities throughout the day and night: jerks, fragmented movements, violent fighting behavior, talking, and mimicking daytime actions such as shaving or hair combing. If awakened from these episodes, he reported a vivid oneiric scene. Video-polysomnography documented that the abnormal behavior appeared during abnormal long-lasting recurrent episodes of rapid eye movement sleep without atonia characterized by increased axial muscle tone and marked limb myoclonic activity. Cz-A1, Electroencephalogram; L-EOG and R-EOG, left and right electro-oculograms; Mylo, mylohyoideus EMG; Ext. Carp. Rad., extensor carpi radialis EMG; RtTib, LtTib, right and left tibialis anterior EMG; ECG, electrocardiogram; INTRC, intercostalis; ABD Resp., abdominal respirogram. ![]() (See Video Vignette 10.)
(See Video Vignette 10.)
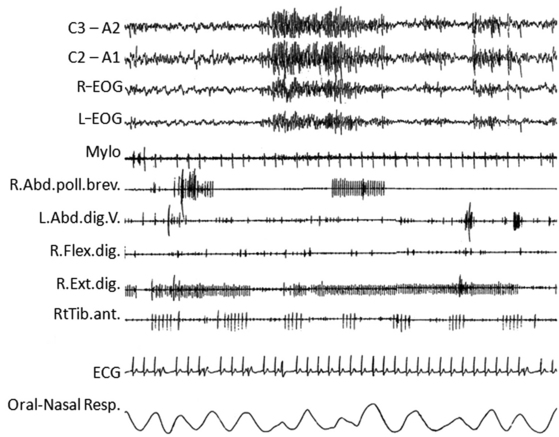
FIGURE 12.16 Morvan’s syndrome.
A 76-year-old man presented with insomnia, muscle twitching, profuse sweating, and weight loss. Throughout the day and night he manifested episodes with complex and quasi-purposeful gestures mimicking daily-life activities (oneiric stupor). When questioned, he often reported a mental content consistent with the mimicked gestures. The polysomnogram (during which the patient is agitated and presents oneiric stupor) is characterized by wake-activity rhythms, muscle artifacts, and the characteristic continuous muscle-fiber activity on electromyogram. Electrocardiogram shows multiple arrhythmic abnormalities. C3-A2, C2-A1, Electroencephalogram; R-EOG and L-EOG, right and left electro-oculograms; Mylo, mylohyoideus; Abd.Poll.Brev., abductor pollicis brevis; Abd.digit.V, abductor digiti quinti; Flex Digit, flexor digiti; Ext.Digit, extensor digiti; RtTib, right tibialis anterior; ECG, electrocardiogram; Resp., respirogram. ![]() (See Video Vignette 11.)
(See Video Vignette 11.)
FFI is an autosomal dominant prion disease with a mean age of onset of around 50 years and presenting with loss of sleep, sympathetic and motor overactivity, and progressive attenuation of autonomic and endocrine circadian oscillations. The typical duration of FFI is 7 to 36 months with a mean of 18 months. The characteristic pathological condition is thalamic degeneration with selective involvement of dorsomedial and anteroventral thalamic nuclei; in addition, there may be involvement of cerebellum and inferior olivary nuclei and there may be spongiform changes in the cerebral cortex. Figure 12.14 shows the PSG and hypnogram from a patient with FFI.
Postural orthostatic tachycardia syndrome (POTS) is an entity of selective autonomic failure characterized by a sustained heart rate increase of 30 beats/min or more or an absolute heart rate of 120 beats/min or more within the first 5 minutes of assuming an upright position, associated with symptoms of orthostatic intolerance but without significant orthostatic hypotension. Sleep dysfunction, an oft-neglected but important non–orthostatic intolerance component, reveals heterogeneity (insomnia, hypersomnia, sleep apnea, and circadian rhythm disturbances), similar to the protean manifestations of POTS.
Patients on long-term opiates treatment may develop central sleep apnea, including Cheyne-Stokes respirations and ataxic or Biot’s breathing (see Fig. 4.28) associated with oxygen desaturations, as a result of depression or destabilization of central respiratory controllers in the brainstem. Clinicians treating patients with opiates for long periods of time must regularly monitor them for sleep-disordered breathing. Treatment includes reduction or elimination of opiates and positive pressure ventilation, generally using adaptive servo ventilation or a bilevel device with or without a backup rate.
SDB causing sleep disturbance is well known in patients during the acute and convalescent stage of poliomyelitis. Sleep disorders, however, in postpolio syndrome are less well known. Patients with postpolio syndrome present with increasing weakness, wasting of previously affected muscles, and additional involvement of the unaffected regions of the body. Patients may present with excessive daytime somnolence and fatigue as a result of sleep-related hypoventilation or apneas (Fig. 12.17).
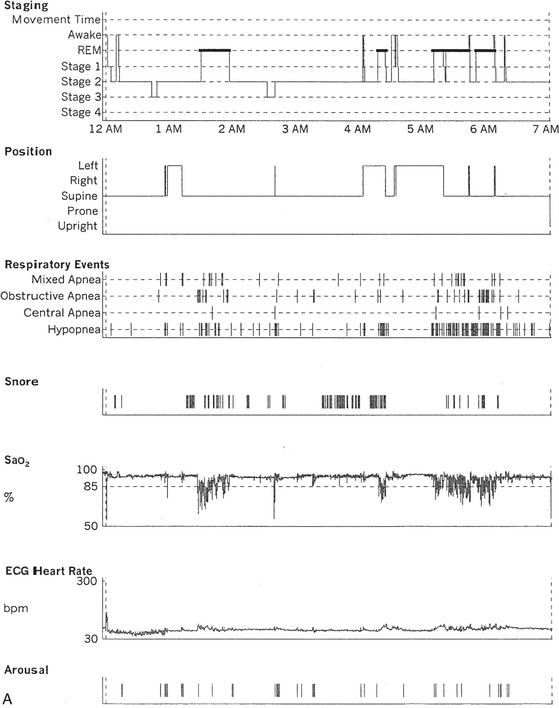
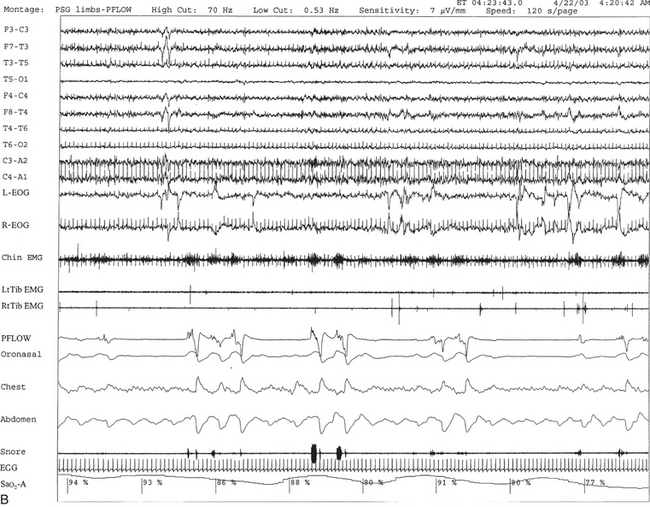
FIGURE 12.17 A case of postpolio syndrome and sleep-disordered breathing (SDB).
A 56-year-old woman with a 2- to 3-year history of shortness of breath and choking sensation when she lies in bed at night. Past medical history is significant for poliomyelitis at the age of 17 years, which paralyzed her from the neck down. She slowly recovered to a great extent and could eventually walk without aid but continued to have residual weakness in both legs. In the last 2 to 3 years she has noticed weakness in the upper extremities as well and pains all over her body. Her recent complaints are consistent with diagnosis of postpolio syndrome with SDB, which is commonly seen in patients with neuromuscular disorders. She also has adult-onset diabetes mellitus, high blood pressure, hypercholesterolemia, and recently diagnosed left breast carcinoma pending mastectomy. A, Hypnogram is significant for frequent respiratory events, particularly hypopneas most marked during rapid eye movement (REM) sleep, which is commonly noted in patients with SDB secondary to neuromuscular disorder. The total apnea-hypopnea index is 30.3/hr but 86.7/hr in REM sleep. This is accompanied by moderate-severe oxygen desaturation most marked in REM sleep. A minimum oxygen saturation of 61% and a mean of 87% during REM sleep are noted. A REM sleep distribution abnormality (with a long REM sleep period recorded in the early part of the night) is also recorded on sleep staging. This can be frequently encountered in patients with disturbed night sleep. B, A 120-second excerpt from overnight polysomnographic recording showing repeated obstructive apneas in REM sleep. Top 10 channels, Electroencephalogram; L-EOG and R-EOG, left and right electro-oculograms; Chin EMG, electromyography of chin; RtTib, LtTib, right and left tibialis anterior EMG; PFLOW, nasal pressure transducer; oronasal thermistor; chest and abdomen effort channels; snore monitor; ECG, electrocardiogram; Sao2, arterial oxygen saturation by finger oximetry.
In summary, it is important to recognize SDB in a variety of neurological conditions because appropriate treatment may improve the quality of life and improve the long-term prognosis of the primary neurological disorder. The clinical clues to SDB in neurological disorders include sleep disturbances at night and presence of excessive daytime sleepiness for which no obvious cause is found. It is not appropriate to simply attribute these complaints to the underlying neurological disorder, which unfortunately is often still done.
The figures in this chapter provide PSG samples and hypnograms with brief clinical information in patients with a variety of neurological disorders.
Alattar, M. A., Scharf, S. M. Opioid associated central sleep apnea: a case series. Sleep Breath. 2009; 13:201–206.
Bhat, S., Chokroverty, S., Kabak, B., et al. Dream-enacting behavior in non-rapid eye movement sleep. Sleep Med. 2012; 13(4):445–456.
Bhat, S., Gupta, D., Chokroverty, S. Sleep disorders in neuromuscular diseases. Neurol Clin. 2012; 30(4):1359–1387.
Bhat, S., Lysenko, L., Neiman, E. S., Rao, G. K., Chokroverty, S. Increasing off-time improves sleep disordered breathing induced by vagal nerve stimulation. Epileptic Disord. 2012; 14(4):432–437.
Bhat, S., Sander, H. W., Grewal, R. P., Chokroverty, S. Sleep disordered breathing and other sleep dysfunction in myotonic dystrophy type 2. Sleep Med. 2012; 13(9):1207–1208.
Cersósimo, R. O., Bartuluchi, M., Fortini, S., et al. Vagus nerve stimulation: effectiveness and tolerability in 64 paediatric patients with refractory epilepsies. Epileptic Disord. 3(4), 2011. [382-328].
Chokroverty, S., Bhat, S., Rosen, D., Farheen, A. REM behavior disorder in myotonic dystrophy type 2. Neurology. 78(24), 2012. [2004].
Chokroverty, S. Sleep and neurodegenerative diseases. Semin Neurol. 2009; 29(4):446–467.
Chokroverty, S., Montagna, P. Sleep, breathing and neurological disorders. In: Chokroverty S., ed. Sleep Disorders Medicine: Basic Science, Technical Considerations, and Clinical Aspects. 3rd ed. Philadelphia: Saunders/Elsevier; 2009:436–498.
Diederich, N. J., Comella, C. L. Sleep disturbances in Parkinson’s disease. In Chokroverty S., Montagna P., Allen R., Walters A., eds. : Sleep and Movement Disorders, 12th ed, New York: Oxford University Press, 2013. [In press].
Iranzo, A., Santamaria, J., Tolosa, E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009; 13:385–401.
Lu, J., Sherman, D., Devor, M., et al. A putative flip-flop switch for control of REM sleep. Nature. 2006; 441:589–594.
Luppi, P. H., Clement, O., Sapin, E., et al. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011; 15:153–163.
Malow, B. A., Edwards, J., Marzec, M., et al. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000; 55(10):1450–1454.
McCarley, R. W. Neurobiology of rapid eye movement and non-rapid eye movement sleep. In: Chokroverty S., ed. Sleep Disorders Medicine: Basic Science, Technical Considerations, and Clinical Aspects. 3rd ed. Philadelphia: Saunders/Elsevier; 2009:29–58.
Polos, P., Kabak, B., Bhat, S., et al. Central sleep apnea in patients on long-term narcotics. Chest. 2010; 138(suppl 4):635A.
Yu, H., Laberge, L., Jaussent, I., et al. Daytime sleepiness and REM sleep characteristics in myotonic dystrophy: a case-control study. Sleep. 2011; 34(2):165–170.