Problems of Early Pregnancy
Katherine W. Arendt MD
Chapter Outline
PHYSIOLOGIC CHANGES OF EARLY PREGNANCY
ABORTION AND INTRAUTERINE FETAL DEMISE
Clinical Presentation and Obstetric Management
CERVICAL INSUFFICIENCY OR INCOMPETENCE
GESTATIONAL TROPHOBLASTIC DISEASE
Obstetric disease of early pregnancy may result in significant maternal morbidity and even mortality. Safe care of patients with obstetric disease involves a thorough understanding of the physiologic changes of early pregnancy as well as the specific issues associated with each pathologic condition.
Physiologic Changes of Early Pregnancy
Respiratory System
The respiratory system undergoes profound physiologic changes during early pregnancy. Increased progesterone concentration stimulates respiratory efforts by increasing the sensitivity of the respiratory center to carbon dioxide. Minute ventilation increases by at least 15% by 12 weeks' gestation and by 25% by 20 weeks' gestation. This results from an increase in tidal volume (respiratory rate is unchanged) and exceeds the increase in oxygen consumption. The result is a respiratory alkalosis with maternal arterial partial pressure of carbon dioxide decreasing to 30 to 33 mm Hg by 10 to 12 weeks' gestation. Moreover, maternal arterial partial pressure of oxygen increases to 106 to 108 mm Hg in the first trimester. Decreased bicarbonate concentration partially compensates for the modest respiratory alkalosis that results from the physiologic hyperventilation, leading to a maternal pH that is slightly above normal (i.e., approximately 7.44). There is little or no change in lung capacities during the first half of pregnancy. Women in early pregnancy who undergo mechanical ventilation require increased minute ventilation.
Cardiovascular System
The cardiovascular system also undergoes profound changes early in pregnancy. Cardiac output increases 20% to 25% by 8 weeks' gestation and 35% to 40% by 20 weeks' gestation. Systemic vascular resistance decreases 30% by 8 weeks' gestation. Maternal mean arterial pressure decreases approximately 6 mm Hg at 16 to 24 weeks' gestation and returns to normal near term.
Aortocaval compression typically occurs after 18 to 20 weeks' gestation, when the uterine fundus reaches the umbilicus and is large enough to compress the aorta and vena cava when the patient is supine. Left uterine displacement is rarely needed in early pregnancy, but when the uterine size is equivalent to an 18- to 20-week gestation, left uterine displacement should be attained by elevating the right hip 15 degrees off midline with a wedge or blankets. The need for left uterine displacement occurs earlier in gestation in the presence of multiple gestation, polyhydramnios, or gestational trophoblastic disease.
Blood volume increases throughout pregnancy; the average prepregnancy blood volume of 4350 mL (76 mL/kg) increases to 4700 mL (81 mL/kg) at 12 weeks' gestation, to 5500 mL (89 mL/kg) at 20 weeks' gestation, and to approximately 6600 mL (97 mL/kg) at term. The increase in blood volume is primarily the result of greater plasma volume because red blood cell volume increases to a smaller degree (27 mL/kg). Because pregnant women have an expanded blood volume, they typically tolerate a blood loss of 500 to 1500 mL during the first half of pregnancy. A blood loss of 500 to 1500 mL rarely requires blood transfusion, provided that the blood loss is replaced with an adequate volume of crystalloid or colloid.
Gastrointestinal System
An increased progesterone level causes relaxation of lower esophageal sphincter tone as early as the first trimester. Fasting gastric volume is approximately 30 mL in both nonpregnant women and women in early pregnancy. Metoclopramide 10 mg, administered intravenously 15 to 30 minutes before anesthesia, can reduce this volume by 50%.1 In a study of 100 pregnant women undergoing general anesthesia by mask at 6 to 22 weeks' gestation, a pH electrode showed reflux of gastric contents into the esophagus in 17% of patients.2 Most episodes of reflux occurred in patients who experienced hiccups. Only 2% had regurgitation of gastric contents into the pharynx, and no patient demonstrated clinical evidence of pulmonary aspiration.
General anesthesia may be safely administered by means of a mask or a laryngeal mask airway by experienced anesthesia providers in selected obstetric patients during early pregnancy. Many anesthesia providers are comfortable managing an airway without tracheal intubation until 18 to 20 weeks' gestation, when the uterus moves out of the pelvis. The latter movement leads to anatomic and intragastric pressure changes that predispose to gastroesophageal reflux. Some anesthesia providers prefer to intubate the trachea of pregnant women who require general anesthesia as early as 12 to 14 weeks' gestation, given that hormonal changes leading to sphincter relaxation are present early in pregnancy. Patients who receive general anesthesia during the first half of pregnancy should be intubated if they are at increased risk for gastric content aspiration (e.g., history of gastroesophageal reflux, morbid obesity, food ingestion within 6 to 8 hours). Pharmacologic prophylaxis (e.g., sodium citrate, a histamine-2 (H2) receptor antagonist, and/or metoclopramide) is likely to further reduce the risk for aspiration pneumonia (see Chapter 29). Neuraxial anesthesia is associated with a lower risk for aspiration than general anesthesia.
Nervous System
During early pregnancy, the nervous system is more sensitive to general and local anesthetic agents. The minimum alveolar concentration (MAC) for volatile anesthetic agents is decreased by approximately 30%, although the underlying mechanism for this change is unclear. A recent study that compared patients undergoing cesarean delivery with nonpregnant patients undergoing elective gynecologic surgery found no difference between groups in electroencephalographic measures during general anesthesia with similar end-tidal concentrations of sevoflurane.3 Because it is well-proven that MAC decreases in pregnancy, this study implies that MAC in pregnant women may not correlate well with depth of anesthesia. Further research is needed in this area.
Ectopic Pregnancy
Ectopic pregnancy occurs when the fertilized ovum implants outside the endometrial lining of the uterus. Death, infertility, and recurrent ectopic pregnancy are possible sequelae. The frequency of ectopic pregnancy in the United States increased fourfold to fivefold between 1970 and 1992 but appears to have stabilized at a rate of approximately 16 per 1000 pregnancies.4,5 A higher prevalence of associated risk factors, especially pelvic inflammatory disease, as well as earlier diagnosis of previously unrecognized ectopic pregnancies may account for the reported increase.
Ruptured ectopic pregnancy is a leading cause of pregnancy-related maternal death during the first trimester and accounts for 6% of all pregnancy-related maternal deaths in the United States.6,7 Most deaths result from hemorrhage (93%); infection (2.5%), embolism (2.1%), and anesthetic complications (1.3%) are less common causes.8 More than 30% of women who have had an ectopic pregnancy subsequently suffer from infertility, and 5% to 23% have a second ectopic pregnancy.9
The number of deaths from ectopic pregnancy decreased in the United States from 1970 through 2007. The case-fatality rate decreased from 35.5 deaths per 10,000 ectopic pregnancies in 1970 to 3.8 per 10,000 in 1989,10 and the ectopic pregnancy mortality ratio decreased from 1.15 deaths per 100,000 live births in 1980 through 1984 to 0.5 death per 100,000 live births in 2003 through 2007.11 The U.S. Centers for Disease Control and Prevention attributes this decline to “improvements in the sensitivity, accuracy, and use of pregnancy testing, ultrasound for diagnosis, and improvements in therapeutic modalities, including laparoscopic surgery and medical management of ectopic pregnancy.”12 However, a recent cluster of 11 maternal deaths from ectopic pregnancy in Florida between 2009 and 2010 increased Florida's ectopic pregnancy mortality ratio from 0.6 death per 100,000 live births in 1999 through 2008 to 2.5 deaths per 100,000 live births in 2009 through 2010.12 Because these women collapsed (likely from acute rupture and hemorrhage) without ever seeking health care, it was believed that limited access to early care may have contributed to the adverse outcomes. Further, of the 11 women who died, 6 tested positive for illicit drugs at autopsy. Ectopic pregnancy deaths historically have been more common in teenagers10 and are 3 to 18 times higher in African-American women than in white women.6,8,10
Factors that alter the normal fallopian tube transport system for the fertilized ovum increase the risk for ectopic pregnancy. These factors include (1) previous ectopic pregnancy; (2) previous tubal surgery; (3) pelvic inflammation, especially infection with Chlamydia trachomatis; (4) congenital anatomic distortion such as that caused by exposure to diethylstilbestrol in utero; (5) previous pelvic or abdominal surgery; (6) use of a contraceptive intrauterine device, which may be associated with interstitial ectopic pregnancy; (7) delayed ovulation; (8) hormonal changes associated with ovulation induction or progestin-only oral contraceptives; (9) lifestyle factors (e.g., smoking, vaginal douching); (10) history of infertility; and (11) assisted reproductive technology (ART) procedures (e.g., zygote transfer into the fallopian tube or uterine cavity).13 However, one third of patients with ectopic pregnancies have no identifiable risk factors.
The fertilized ovum can implant anywhere along the path of migration or in the abdominal cavity (Figure 16-1). Most ectopic pregnancies (98%) are tubal (infundibular or fimbrial, 6%; ampullary, 78%; isthmic, 12%; interstitial or cornual, 2%). The remaining 2% implant on the cervix, vagina, or ovary or elsewhere in the abdomen.14 An increasing number of cesarean scar ectopic pregnancies, which may be on a continuum with early placenta accreta, are being reported.
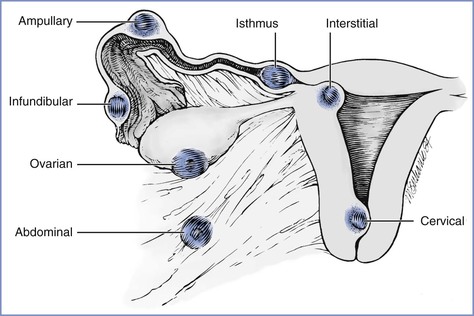
FIGURE 16-1 Potential locations of ectopic pregnancies. The majority occur in the ampullary portion of the fallopian tube. (Reprinted from Chantigian RC, Chantigian PDM. Problems in early pregnancy. In Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. Chestnut's Obstetric Anesthesia. 4th edition. Philadelphia, Mosby, 2009. Modified from DeCherney AH, Seifer DB. Ectopic pregnancy. In Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 2nd edition. New York, Churchill Livingstone, 1991:811.)
In patients who undergo ART procedures, ectopic pregnancies have been reported in approximately 2% of pregnancies.13 Most of these pregnancies are tubal; however, approximately 6% are ovarian, abdominal, or cervical, and 12% to 15% are heterotopic (see later discussion).14
Clinical Presentation
The clinical presentation of the patient with an ectopic pregnancy depends on the gestational age, site of implantation, and extent of hemorrhage. Prior to rupture, the signs and symptoms are often subtle. Classic clinical signs of impending rupture or a ruptured tubal pregnancy include abdominal or pelvic pain (95%), delayed menses (75% to 95%), and vaginal bleeding (60% to 80%). Vaginal bleeding results from the breakdown and shedding of the decidual lining of the uterine wall, which is probably associated with decreased hormone production by the corpus luteum and inadequate human chorionic gonadotropin (hCG) production by the ectopic trophoblast. Pain often precedes vaginal bleeding. Patients with hemorrhage (with or without tubal rupture) may experience dizziness or syncope, may have the urge to defecate because of the effect of blood in the cul-de-sac, and may have shoulder pain from diaphragmatic irritation by intra-abdominal blood.
Physical findings include abdominal tenderness with or without rebound (80% to 95%), a uterus that is smaller than expected for dates (30%), and a tender adnexal mass (30% to 50%). A bulging cul-de-sac suggests hemoperitoneum. With significant hemorrhage there may be signs of shock, but some patients may appear hemodynamically stable despite a hemoperitoneum volume of 1000 to 1500 mL; presumably, these patients have an ectopic pregnancy with slow bleeding and are able to compensate for the gradual blood loss.
Diagnosis
Ectopic pregnancy should be excluded in any patient who has pelvic pain and a positive pregnancy test. In a woman of reproductive age, the symptoms of ectopic pregnancy must be differentiated from (1) a threatened, inevitable, or incomplete abortion; (2) infection after attempted abortion; (3) pelvic inflammatory disease; (4) a degenerating fibroid; (5) appendicitis and other gastrointestinal diseases; (6) ovarian torsion; (7) a ruptured or bleeding ovarian cyst; (8) a trapped retroverted uterus in pregnancy; and (9) nephrolithiasis.
Current tests allow early diagnosis of ectopic pregnancy and prompt treatment that decreases morbidity and mortality.9 Diagnostic algorithms include the following guidelines:
1. Ultrasonography can reliably confirm only the presence of an intrauterine pregnancy. The ectopic pregnancy itself may be difficult to visualize.15 Transvaginal ultrasonography is the current modality of choice because it can detect an intrauterine gestational sac as soon as 21 days after conception (when the beta-hCG concentration is greater than 1400 mIU/mL with use of the International Reference Preparation [IRP] standard). Transabdominal ultrasonography can visualize an intrauterine pregnancy when the serum beta-hCG concentration is higher than 6000 to 6500 mIU/mL IRP.16
2. A serial beta-hCG concentration that decreases, plateaus, or shows a subnormal increase (< 53% over 48 hours) usually indicates a nonviable pregnancy—either an ectopic pregnancy or an impending abortion.17 With a spontaneous abortion, a decline in beta-hCG concentration of at least 21% to 35% should be seen over 2 days. A slower decline is suggestive of an ectopic pregnancy. A beta-hCG concentration greater than 100,000 mIU/mL is usually associated with a viable intrauterine pregnancy.18
3. A serum progesterone concentration greater than 25 ng/mL is usually associated with a viable pregnancy. A concentration less than or equal to 5 ng/mL usually indicates a nonviable pregnancy but cannot distinguish a spontaneous abortion from an ectopic pregnancy.19 Most ectopic pregnancies are associated with progesterone levels between 5 and 25 ng/mL, a fact that limits the usefulness of this test.
4. Uterine curettage can be performed when nonviability is established. Identification of trophoblastic villi confirms miscarriage of an intrauterine pregnancy. Absence of villi signals either a complete spontaneous abortion (confirmed by rapidly decreasing beta-hCG concentration) or an ectopic pregnancy.
In the past, culdocentesis was used to aid in the diagnosis of hemoperitoneum and ectopic pregnancy. Although a positive result is highly predictive of hemoperitoneum, the advent of pelvic ultrasonography and rapid quantitative beta-hCG tests limits its value in the diagnosis of ectopic pregnancy.
Obstetric Management
Management options for ectopic pregnancy are expectant, medical, and surgical. Management choice depends on the symptoms and diagnostic findings.
Expectant management may be used for selected asymptomatic patients with early tubal ectopic pregnancies and stable or decreasing beta-hCG levels. Successful resolution has been reported in approximately 50% of these selected patients.4 If expectant management is unsuccessful, a medical or surgical approach is required.
The American College of Obstetricians and Gynecologists (ACOG) as well as the American Society of Reproductive Medicine have published guidelines for the medical management of ectopic pregnancy.20,21 Systemic, intramuscular, oral, and intragestational forms of chemotherapy have been used successfully in the medical management of ectopic pregnancy. Methotrexate, a folate antagonist that interrupts DNA synthesis and thus cell replication, inhibits growth of trophoblastic cells of the placenta and is commonly used to treat ectopic pregnancy. Because methotrexate is toxic to all rapidly-dividing tissues of the body, there are many contraindications to medical treatment of ectopic pregnancy, including immunodeficiency and pulmonary, liver, renal, or hematologic disease. Further, the ACOG has recommended that only early tubal pregnancies (i.e., no cardiac activity, a gestational sac with a diameter < 3.5 to 4.0 cm, and no evidence of tubal rupture or hemoperitoneum) be treated with methotrexate.
Methotrexate treatment protocols include a single-dose regimen, a two-dose regimen, and a fixed multidose regimen; the multidose regimen is reserved for patients with high beta-hCG levels (i.e., > 5000 mIU/mL). From day 4 to day 7 after methotrexate treatment, a decrease in beta-hCG level of 15% must be present to consider the treatment successful. Otherwise, repeat methotrexate treatment or surgical intervention is required. Follow up and close monitoring until beta-hCG level reaches nonpregnant values is imperative because of the risk for rupture and hemorrhage. Side effects of methotrexate can be severe and include abdominal pain, vomiting, stomatitis, severe neutropenia, and pneumonitis. Compared with surgical management, medical management of ectopic pregnancy provides no difference in overall tubal preservation, tubal patency, risk for repeat ectopic pregnancy, or success of future pregnancies.
Surgical management depends on the location of the pregnancy, the hemodynamic stability of the patient, the available equipment, and the surgeon's expertise. Most often, diagnostic laparoscopy is performed to confirm the diagnosis and locate the ectopic pregnancy. For tubal ectopic pregnancies, a salpingostomy, salpingotomy, or salpingectomy (usually partial) is performed by means of laparoscopy or laparotomy. To aid hemostasis during laparoscopic removal of the ectopic pregnancy, some obstetricians inject dilute vasopressin into the surface of the fallopian tube. This agent causes marked blanching of the tube and results in a relatively bloodless surgical field. If the vasopressin is accidentally injected intravenously, a marked increase in maternal blood pressure may occur.
A laparotomy is indicated if the surgeon is not trained in operative laparoscopy, laparoscopic removal is anticipated to be difficult (e.g., tube diameter > 6 cm or an interstitial location of the ectopic pregnancy), or there is uncontrollable bleeding. Laparotomy should be performed immediately if there is hemodynamic instability; these cases often require a partial or total salpingectomy. If a partial salpingectomy is performed, tubal repair may be performed primarily or during a second operation. Although some experts have noted that outcomes from randomized trials comparing salpingostomy and salpingectomy are lacking,22 the risk for persistent ectopic pregnancy may be higher after salpingostomy than after salpingectomy.23
Interstitial, cervical, cesarean scar, and abdominal ectopic pregnancies as well as early placenta accreta may present significant diagnostic and therapeutic challenges, resulting in delay of diagnosis and treatment. There is potential for massive hemorrhage because of disruption of organs and adjacent tissues. The desire to preserve fertility may result in greater blood loss as tissue and organ preservation are attempted.
Interstitial pregnancy often goes unrecognized and may manifest as uterine wall rupture, massive hemorrhage, and shock. Conservative surgery (e.g., cornual resection) may be attempted, but hysterectomy may be required if uterine damage is severe.
Cervical pregnancy often results in massive hemorrhage because of the inability of the cervix to contract. In the past, most cervical pregnancies necessitated hysterectomy to control hemorrhage. More current management options that have greater likelihood of maintaining fertility include (1) methotrexate therapy, (2) local excision, (3) cerclage and tamponade, (4) ligation of the hypogastric arteries or the cervical branches of the uterine arteries, and (5) angiographic embolization of the uterine arteries followed by a dilation and evacuation (D and E) procedure (see later discussion).24
Cesarean scar pregnancy occurs when a gestational sac implants in the uterine scar defect (niche) at the site of a previous cesarean delivery. Cesarean scar pregnancy has a high complication rate. Although relatively rare, its incidence is rising with increasing cesarean delivery rates and currently may be as high as 1 in 1800 pregnancies.25,26 Jurkovic et al.26 described two types of cesarean scar pregnancies: (1) implantation on the scar with enlargement into the uterine cavity, and (2) implantation into a scar defect with growth into the myometrium. Depending on its progression, the former type may grow normally or may be treated medically. Scar implantation results in an increased risk for hemorrhage at delivery. Growth into the myometrium may lead to eventual rupture and bleeding in the first trimester; prompt surgical intervention is preferred over medical management in this situation.
In a review of 112 cases of cesarean scar pregnancies, Rotas et al.25 found that approximately half occurred in women with only one previous cesarean delivery. Many patients have vaginal bleeding, abdominal cramps, and/or lower abdominal pain. Up to one third of cases may be asymptomatic and are diagnosed during routine ultrasonography. A review of 751 published cases of cesarean scar pregnancy found that the diagnosis was missed in 107 of 751 cases (13.6%).27 Transvaginal ultrasonography was the best diagnostic tool. There were a total of 31 different primary treatment approaches, which included hysterectomy, dilation and curettage (D and C), hysteroscopic excision, uterine artery embolization, and intra-gestational aspiration or injection of methotrexate or potassium. Complications occurred in 331 of the 751 cases (44.1%), of which the most common was hemorrhage. The authors noted that local methotrexate and hysteroscopic-directed procedures had the lowest complication rates and that curettage, systemic methotrexate therapy, or embolization as single treatments should be avoided.
The incidence of early placenta accreta is also rising as a result of increasing cesarean delivery rates. It is defined as penetration of the placenta into the myometrium, which is discovered in the first or early second trimester. Because of similarities in pathogenesis, it is thought—although not confirmed—that early placenta accreta may develop from cesarean scar pregnancy. A recent review found that 15 of 47 (32%) patients with early placenta accreta had spontaneous uterine rupture, in most cases followed by bleeding and shock, which resulted in laparotomy, hysterectomy, or uterine artery embolization.27 Although the gestational age is early, it is imperative that the anesthesia team is aware of the risk for hemorrhage during surgical intervention for cesarean scar pregnancy and early placenta accreta.
Abdominal pregnancy is defined as implantation in the peritoneal cavity, not including the fallopian tubes, ovaries, or ligaments, and is associated with a high incidence of maternal morbidity and fetal demise.28 In a recently published series of advanced extrauterine pregnancies, Worley et al.29 identified ten women who presented with extrauterine pregnancies beyond 18 weeks' gestation, of whom three met the diagnostic criteria for abdominal pregnancy. All patients had difficult surgery, nine patients required blood transfusion, and only five fetuses survived after complicated courses.
Diagnosis of abdominal pregnancy can be difficult, historically being missed in as many as one of nine cases.28 The diagnosis was missed prior to delivery in four of the ten cases in the series of Worley et al.29 Abdominal pain, vaginal bleeding, symptoms consistent with partial bowel obstruction, shock, or death may be the first indication of this unusual type of pregnancy. Ultrasonography is useful but may miss the diagnosis in more than 50% of cases. Magnetic resonance imaging may prove to be a more sensitive diagnostic tool.
If an extrauterine pregnancy is suspected in early gestation, laparoscopy can be used to diagnose and remove gestational products. If the extrauterine pregnancy is not identified until late gestation, it is associated with decreased placental perfusion (which typically results in fetal growth restriction) and oligohydramnios (which often results in pulmonary hypoplasia and anatomic deformities). In 1993, Stevens30 reviewed published cases of abdominal pregnancy since 1809 and found that 63% of infants survived when born after 30 weeks' gestation.
Management of an advanced extrauterine pregnancy consists of laparotomy and delivery of the fetus. Once the fetus is delivered, management of the placenta is controversial and fraught with hazard. Removal of the placenta is associated with massive hemorrhage, prolonged and complicated surgery (e.g., bowel resection), and an increased risk for maternal mortality. A decision to leave the placenta in situ results in a higher risk for infectious morbidity as well as a potential greater need for additional surgery.30,31 In the series of Worley et al.,29 the placenta was left in situ in two patients, both of whom developed serious complications. The site of placental implantation and the ability to adequately ligate the blood supply often dictates the obstetrician's decision about management of the placenta.
Heterotopic pregnancy describes the simultaneous occurrence of an ectopic and an intrauterine pregnancy. Historically, it was thought to occur in 1 in 30,000 spontaneous pregnancies.32 However, in patients undergoing ART, 0.2% to 3% of pregnancies may be heterotopic.14,33 Difficulty visualizing the entire fallopian tube on ultrasonography, combined with normal or slightly elevated beta-hCG measurements (i.e., low serum levels from the ectopic pregnancy combined with normal levels from the intrauterine pregnancy), make the early diagnosis of heterotopic pregnancy difficult.34 This diagnosis should be suspected in cases in which clinical signs of an ectopic pregnancy and a confirmed intrauterine pregnancy coexist. In most cases, the ectopic pregnancy is removed surgically, which can be difficult when trying to maintain the intrauterine pregnancy. Alternatively, transvaginal ultrasonography–guided injection of potassium chloride into the ectopic pregnancy has been performed successfully; however, as many as 55% of patients may require subsequent surgery.35 The patient often sustains the normal intrauterine pregnancy to term.
Patients with ectopic pregnancies who are Rh-negative should receive Rh0(D) immune globulin.36
Anesthetic Management
Patients with an unruptured tubal pregnancy usually have normal intravascular volume, minimal bleeding before and during surgery, and low anesthetic and surgical risk. Anesthetic considerations for laparoscopy or laparotomy are summarized in Box 16-1. Although most patients may prefer general anesthesia, neuraxial anesthesia with an upper sensory level to at least T4 may be an alternative in selected patients. Shoulder pain from diaphragmatic irritation may occur and can be treated with intravenous analgesics (e.g., fentanyl 1 to 2 µg/kg).
A ruptured ectopic pregnancy may be associated with significant preoperative blood loss, but estimation of the extent of this is difficult because young women may have normal blood pressure despite a markedly reduced circulating blood volume. General anesthesia, with preparation for hemorrhage, is preferred if significant bleeding has occurred (e.g., ruptured tubal pregnancy) or is likely to occur (e.g., cervical, interstitial, cornual, cesarean scar, or abdominal ectopic pregnancy, or early placenta accreta). Intraoperative autologous blood transfusion can be used, and it may be useful especially in developing countries, where blood bank supplies are limited and women typically present late with significant hemoperitoneum and/or hypovolemic shock.37 The desire to preserve fertility often results in greater blood loss as tissue and organ preservation are attempted.
Abortion and Intrauterine Fetal Demise
Abortion refers to a pregnancy loss or termination, either before 20 weeks' gestation or when the fetus weighs less than 500 g. It can occur spontaneously or may be performed electively for personal or medical reasons. A total of 825,564 elective abortions were reported in the United States in 2008, a rate of 16 abortions per 1000 women and a ratio of approximately 234 abortions per 1000 live births.38 The total number of abortions and rate of abortions (number of abortions per 1000 women) in the United States steadily declined from 1999 through 2007 and then remained static from 2007 to 2008.38 Between 1999 and 2008, the number, rate, and ratio (number per 1000 live births) of elective abortions in the United States declined by 3%, 4%, and 10%, respectively.38
In 2008, 62.8% of elective abortions were performed before 8 weeks' gestation, 91.4% were performed before 13 weeks' gestation, and 1.3% were performed after 21 weeks' gestation.38 Most (75.9%) were performed by D and C at less than 13 weeks' gestation, although some (14.6%) were induced medically, most commonly using methotrexate and misoprostol, or mifepristone and misoprostol before 8 weeks' gestation.38
Of the 4693 reported pregnancy-related maternal deaths from 1998 to 2005 in the United States, 3% were the result of induced or spontaneous abortion.39 Deaths are usually the result of sepsis, hemorrhage, or embolism.40 Of the 20 abortion-related maternal deaths in the United States in 2003, 10 were related to spontaneous abortion and 10 women died after legal elective abortion (6 after surgical procedures and 4 after medical or nonsurgical procedures).
From a global perspective, abortion is a significant cause of maternal death. A review of causes of maternal death from 1997 to 2002 by the World Health Organization (WHO) reported that in some areas of Latin America and the Caribbean as many as 30% of maternal deaths are caused by abortion.41 In 2006, the WHO estimated that 12, 23, and 37 maternal deaths per 100,000 live births occur as a result of induced abortion in South Asia, Latin America/Caribbean, and sub-Saharan Africa, respectively.41 It is likely that many of these abortions are performed by unskilled individuals in a nonsterile environment that does not meet minimal medical standards. The exact number of maternal deaths that result from induced abortion is unknown and likely underreported.41,42
Spontaneous abortion occurs in 10% to 15% of clinically recognized pregnancies; when subclinical pregnancies are also considered, the incidence of spontaneous pregnancy loss may be as high as 60%.43 Although most spontaneous abortions manifest clinically at 8 to 14 weeks' gestation, ultrasonography suggests that fetal demise usually occurs before 8 weeks' gestation. If the fetus is viable at 8 weeks' gestation, the incidence of subsequent fetal loss is only 3%.
The etiology of spontaneous abortion varies among patients. Chromosomal abnormalities are responsible for at least 50% to 80% of all spontaneous abortions.44 Other causes include (1) immunologic mechanisms, (2) maternal infections, (3) endocrine abnormalities (e.g., poorly-controlled diabetes mellitus), (4) uterine anomalies, (5) incompetent cervix, (6) debilitating maternal disease, (7) maternal clotting disorders, (8) trauma, and possibly (9) environmental exposures (e.g., irradiation, smoking, certain drugs).
Although some studies (conducted before scavenging of anesthetic gases was routine) suggested a higher incidence of spontaneous abortion among women who were exposed to trace concentrations of anesthetic agents in operating rooms,45 reevaluation of these data demonstrated significant flaws in study design, casting doubt on the original conclusions.46 Later studies have shown no increased incidence of spontaneous abortion in women working in operating rooms.47
Clinical Presentation and Obstetric Management
The clinical presentation and management of spontaneous abortion vary. A threatened abortion is defined as uterine bleeding without cervical dilation before 20 weeks' gestation. Bleeding may be accompanied by cramping or backache. Once the diagnosis is confirmed, the patient's activities are restricted until symptoms resolve. Approximately 25% of pregnancies are complicated by a threatened abortion; approximately half of affected women progress to a spontaneous abortion.48
An inevitable abortion is defined as cervical dilation or rupture of membranes without expulsion of the fetus or placenta. Spontaneous expulsion of the uterine contents usually occurs, but infection can be a complication.
A complete abortion is defined as a total, spontaneous expulsion of the fetus and placenta. Partial expulsion of the uterine contents (i.e., an incomplete abortion) is more common after 8 weeks' gestation. Persistent bleeding and cramping after expulsion of tissue are signs of an incomplete abortion. An incomplete abortion usually requires a D and E or a D and C procedure to remove any remaining fetal or placental tissue. A D and C procedure refers to dilation of the cervix followed by curettage, which is typically by suction. A D and E procedure involves greater cervical dilation followed by evacuation of the uterus with surgical instruments. Typically, the latter is required after ossification of the fetal bones, which usually occurs around 13 to 15 weeks' gestation. A D and E procedure is associated with more complications. Oxytocin and/or an ergot alkaloid (e.g., methylergonovine) increases uterine tone and may be administered intraoperatively and/or postoperatively to decrease the amount of uterine bleeding.
Fetal death may go unrecognized for several weeks in a patient with a missed abortion. Occasionally, coagulation defects such as disseminated intravascular coagulation (DIC) may complicate intrauterine fetal death; this possibility is more likely when fetal demise occurs at an advanced gestational age. If spontaneous expulsion of the uterine contents does not occur after a brief period of observation, evacuation of the uterus is indicated. Management options include placement of intracervical laminaria or intravaginal or intracervical placement of a prostaglandin E2 (PGE2) preparation. This can be followed by induction of labor or a D and C or D and E procedure. Side effects of prostaglandins include nausea, vomiting, diarrhea, and fever. Intra-amniotic instillation of hypertonic saline is not recommended in cases of intrauterine fetal death because coagulation defects may be induced or enhanced.
Recurrent or habitual abortion refers to the occurrence of three or more consecutive spontaneous abortions in the same patient.
Obstetric Complications
Complications of D and C and D and E procedures include cervical laceration, uterine perforation, hemorrhage, retained products of conception, and infection. The risk for these complications is increased in pregnancies that have progressed beyond the first trimester and is greater for D and E procedures than for D and C procedures. Vasovagal events, postabortal syndrome (i.e., intrauterine blood clots with uterine atony, associated with lower abdominal pain, tachycardia, and diaphoresis), DIC, and unrecognized ectopic pregnancy can also occur. Management of uterine perforation may involve simple observation or immediate laparotomy with repair. Management depends on the suspected severity of injury to the uterus and adjacent structures.
Serious infection (e.g., septic abortion) complicates approximately 1 in 200,000 spontaneous abortions. It is more common after induced abortion, particularly illegal abortion.49 Septic abortion causes significant morbidity and is life threatening. Blood cultures should be taken, and broad-spectrum intravenous antibiotics should be administered promptly. Patients with hemodynamic instability may require invasive hemodynamic monitoring to guide fluid, blood, and vasoactive drug therapy. Lower genital tract or bowel injury should be excluded, and the uterus should promptly be re-evacuated. Occasionally, hysterectomy is necessary and may be lifesaving.
Rh0(D) immune globulin should be administered to prevent Rh sensitization in Rh-negative woman who abort. It should also be given to Rh-negative women with a threatened abortion because a positive Kleihauer-Betke test result (indicating transplacental hemorrhage of fetal blood into the maternal circulation) has been demonstrated in 11% of these patients.50
Women who suffer spontaneous abortion are at increased risk for depressive disorders during the 6 months after miscarriage51 (see Chapter 51).
Anesthetic Management
Several anesthetic techniques are appropriate for D and C and D and E procedures (Box 16-2). The choice depends on several factors (e.g., whether the cervix is dilated; the gestational age and ossification of the fetus; the presence of significant blood loss, sepsis, or a full stomach; and the emotional state and preference of the patient). Dilation of the cervix is relatively painful, whereas suction and curettage are less painful. If the cervix is dilated and the products of conception can be curettaged and suctioned, sedation with or without a paracervical block may suffice. If the cervix is not dilated, paracervical block and sedation, spinal or epidural anesthesia (with sensory blockade from T10 to S4), or general anesthesia should be used. General anesthesia may be most appropriate if the patient is emotionally upset or if the gestational age is 13 to 15 weeks or greater (which requires a greater degree of cervical dilation for a D and E procedure). Selected patients may benefit from premedication with a short-acting benzodiazepine (e.g., midazolam).
Typically, the cervix is already dilated in patients who have had significant preoperative bleeding; rarely does a patient with a closed cervix have significant bleeding. In the presence of significant bleeding, intravascular volume should be restored first. A paracervical block and sedation may then be adequate. Substantial hemorrhage represents a relative contraindication to the use of spinal or epidural anesthesia, which probably should also be avoided in patients with evidence of sepsis.
General anesthesia may be induced with propofol or thiopental, although ketamine or etomidate may be preferred in patients with significant bleeding. Large doses (1.5 to 2.0 mg/kg) of ketamine increase uterine tone,52 which may be advantageous in patients who require evacuation of the uterus.
Drugs administered for general anesthesia may influence blood loss during the procedure. Volatile anesthetic agents cause dose-dependent relaxation of uterine smooth muscle53 and have been associated with increased uterine bleeding.54,55 In two studies that compared blood loss during general anesthesia for elective first-trimester abortion, blood loss was greater when anesthesia was maintained with isoflurane compared with propofol.54,55 However, the differences were small considering the blood volume expansion that occurs during pregnancy. Some obstetricians contend that relaxation of the uterus (caused by administration of a volatile anesthetic agent) increases the risk for uterine perforation, and they prefer that administration of a volatile anesthetic agent during a D and C or D and E procedure be avoided.
General anesthesia is commonly maintained with oxygen, nitrous oxide, and an opioid. A propofol infusion or a low concentration (< 0.5 MAC) of a volatile agent may be added. The volatile agent should be avoided or discontinued if there is any evidence of uterine atony. In most cases, oxytocin diluted in crystalloid is administered intravenously to increase uterine tone and decrease blood loss.
The D and C or D and E procedure is performed with the patient in the lithotomy position. After the procedure is completed and the patient's legs are lowered, hypotension may develop in patients who have lost a substantial amount of blood, especially if neuraxial anesthesia has been used.
Cervical Insufficiency or Incompetence
An inherent or traumatic deficiency in the structure or function of the uterine cervix results in cervical insufficiency or incompetence, which is defined as the inability to sustain a pregnancy to full term. Cervical insufficiency is characterized by recurrent second-trimester pregnancy losses with (1) painless cervical dilation; (2) herniation followed by rupture of the fetal membranes; and (3) a short labor with delivery of a live, immature infant.
The true incidence of cervical insufficiency is difficult to determine owing to a lack of objective clinical findings and definitive diagnostic tests. The reported incidence varies from 1 in 100 to 1 in 2000. The U.S. National Vital Statistics Report for 2004 cited a rate of 4.4 cervical cerclages performed per 1000 live births.56 Lidegaard57 reported an incidence of cervical incompetence of 4.6 women per 1000 births in Denmark between 1980 and 1990.
Potential causes of cervical insufficiency include cervical trauma, congenital abnormalities, intrauterine infection, endocervical inflammation, deficiencies in cervical collagen and elastin, and hormonal abnormalities.58 A common cause of cervical insufficiency is trauma, occurring at the time of a previous vaginal delivery or a surgical procedure (e.g., D and C, conization of the cervix, partial amputation or resection of the cervix, cervical cauterization). Congenital abnormalities of the reproductive tract (e.g., unicornuate or bicornuate uterus) may be present in as many as 2% of patients with cervical insufficiency. Some anomalies may result from maternal exposure to diethylstilbestrol in utero.
Warren et al.59 found that more than 25% of women with a diagnosis of cervical insufficiency have a positive family history of cervical insufficiency as well as a greater frequency of two genes associated with abnormalities of connective tissue, collagen, and extracellular matrix. Other recent studies have found high rates of intra-amniotic inflammation (measured by elevated amniotic fluid matrix metalloproteinase-8 concentration) mostly without signs of infection in patients with acute cervical insufficiency or an asymptomatic shortened cervix.60,61 The presence of this inflammation was a risk factor for impending preterm delivery.60,61 Further research in this area could determine if there is potential for this observation to translate into therapy to prevent preterm labor.
Diagnosis
Cervical insufficiency remains a clinical diagnosis. A definitive diagnosis is made when herniating fetal membranes are visualized or palpated through a partially dilated cervix during the second trimester. A characteristic history from a previous pregnancy allows the presumptive diagnosis of cervical insufficiency, once other causes of recurrent pregnancy loss have been excluded. History suggestive of the diagnosis of cervical insufficiency consists of two or more second-trimester pregnancy losses, loss of each successive pregnancy at an earlier gestational age, painless cervical dilation to 4 to 6 cm, and cervical trauma or anomaly.62 Uterine contractions, vaginal bleeding, or chorioamnionitis during a previous pregnancy suggests that other mechanisms are responsible for pregnancy loss.
Symptoms of cervical insufficiency include increased vaginal discharge, lower abdominal or back pressure or discomfort, vaginal fullness, and urinary frequency.
A higher risk for preterm birth has been associated with shortened cervical length at early gestational age.63,64 Recent trials have indicated that vaginal progesterone treatment in women with a shortened cervix is effective in prevention of preterm birth and neonatal morbidity.65,66 Physical examination may reveal cervical shortening and/or cervical abnormalities. Historically, it has been debated whether serial ultrasonographic evaluations of cervical length and dilation should be considered in the early second trimester for pregnant women at high risk for cervical insufficiency.62,67,68 However, a recent decision and economic analysis concluded that, of four alternatives examined, universal transvaginal ultrasonographic assessment of cervical length at the time of routine anatomic ultrasonography and subsequent treatment with daily vaginal progesterone for women with a short cervix was the most cost-effective strategy and was associated with the greatest reduction in preterm birth less than 34 weeks' gestation.69
Obstetric Management
Management of cervical insufficiency remains controversial.58,70,71 A meta-analysis by Berghella et al.72 concluded that cerclage does not prevent preterm birth in all women and can actually be detrimental in multifetal pregnancies. Current evidence, however, shows significant benefit in reducing preterm birth in women with singleton gestation who have had a previous preterm birth, have a shortened cervical length, and are less than 24 weeks' gestation.73 It was estimated that in these patients, 20 cerclages are needed to prevent one perinatal death and more than 6500 infants per year could be saved in the United States by this management.74
The most common cerclage procedures are the modified Shirodkar cerclage and the McDonald cerclage, both of which are performed transvaginally. A ligature (e.g., polyester fiber [Mersilene] tape) is placed around the cervix at or near the level of the internal cervical os. In the more invasive modified Shirodkar procedure, the cervical mucosa is incised anteriorly and posteriorly, the bladder may be advanced, the ligature is placed submucosally and then tied, and the mucosal incisions are closed. The cervical mucosa is left intact with the McDonald cerclage; a purse-string ligature is placed around the cervix and then tied (Figure 16-2). These two procedures result in comparable rates of fetal survival in patients with no history of a previous cerclage.75 In one study, better outcome (i.e., more advanced gestational age) was obtained when a Shirodkar cerclage was performed in patients who had a previous cerclage.76
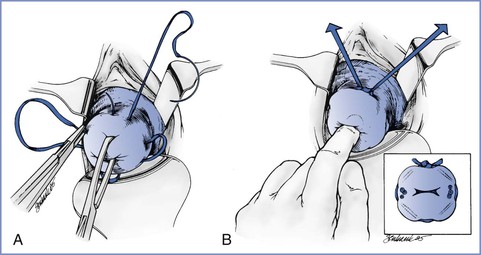
FIGURE 16-2 Placement of sutures for McDonald cervical cerclage. A, A double-headed polyester fiber (Mersilene) band with four “bites” is placed in the cervix, avoiding the vessels. B, The suture is placed high up on the cervix, close to the cervical-vaginal junction, approximately at the level of the internal os. (Reprinted from Chantigian RC, Chantigian PDM. Problems in early pregnancy. In Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. Chestnut's Obstetric Anesthesia. 4th edition. Philadelphia, Mosby, 2009. Modified from Iams JD. Preterm birth. In Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 4th edition. New York, Churchill Livingstone, 2002:803.)
Transvaginal cerclage can be performed in most patients with an incompetent cervix. However, if no substantial cervical tissue is present (e.g., severe cervical laceration, congenital or traumatic cervical shortening) or if a previous transvaginal cerclage has failed, a transabdominal cerclage may be performed, either before or during pregnancy.77 Although a posterior colpotomy and division of the transabdominal cerclage occasionally are performed in an attempt to allow vaginal delivery, most patients with transabdominal cerclage undergo cesarean delivery. The transabdominal cerclage can remain in situ if further pregnancies are desired, or it can be removed at the time of cesarean delivery.
Although the efficacy of perioperative antibiotics and/or tocolytic drugs has not been confirmed, some obstetricians may choose to use them.62
Contraindications to cerclage procedures include preterm labor, vaginal bleeding, fetal anomalies, fetal death, rupture of membranes, placental abruption, and chorioamnionitis. Some obstetricians obtain specimens for culture of the amniotic fluid and/or cervix before placement of a cerclage.
Cerclage can be performed (1) prophylactically, before or during pregnancy (interval or primary cerclage); (2) therapeutically, when cervical changes are noted in the current pregnancy (secondary cerclage); or (3) emergently, in patients with marked cervical changes, including membrane exposure to the vaginal milieu (tertiary cerclage).
Interval cerclage may increase the risk for infertility and may prevent easy evacuation of the uterus in the case of a first-trimester spontaneous abortion. Because prophylactic cerclage is more effective than emergency cerclage (historical data show fetal survival is 78% to 87% versus 42% to 68%, respectively78,79), most obstetricians perform prophylactic cerclage in the at-risk patient at 12 to 18 weeks' gestation, once fetal viability is established. A cervical dilation of 2 cm or more is associated with a greater risk of premature rupture of membranes and/or preterm delivery.76
The greatest risk during the performance of emergency cerclage is rupture of the membranes. Several techniques have been described to facilitate replacement of the bulging fetal membranes into the uterus. Uterine relaxation is essential, which can be facilitated by administration of a volatile anesthetic agent. Alternatively, a tocolytic drug (e.g., terbutaline) may be administered. The steep Trendelenburg position allows for gravity assistance.
To assist in reduction of herniated membranes, some obstetricians fill the urinary bladder with sterile saline. Insertion of a 16-mm Foley catheter (with the tip removed) into the cervical canal with subsequent inflation of the balloon with 30 to 60 mL of saline has also been described.80 The balloon is deflated and the catheter is removed at the end of the procedure.
Cervical cerclage is associated with a 0.6% risk for perioperative complications.81 Immediate complications include rupture of the fetal membranes, hemorrhage, and preterm labor. Delayed complications include infection, suture displacement, cervical stenosis secondary to scarring, and cervical lacerations and uterine rupture if labor proceeds with the cerclage in place. Rarely, sepsis may result in death. Overall, patients who have had a cerclage have a higher rate of cesarean delivery. The Shirodkar procedure is associated with a rate of cesarean delivery almost double that associated with a McDonald cerclage (31% versus 17%, respectively).76
Anesthetic Management
Transvaginal cervical cerclage is usually performed under spinal, epidural, or general anesthesia (Box 16-3). The degree of cervical dilation may influence the choice of anesthesia. If the cervix is not dilated, spinal, epidural, or general anesthesia may be administered. Although McCulloch et al.82 described the use of pudendal nerve block for McDonald cerclage, this may not provide adequate anesthesia for many patients. Spinal anesthesia provides a rapid, predictable onset of sacral anesthesia, which is desirable for these procedures. Sensory blockade from sacral dermatomes to T10 is necessary, because both the cervix (L1 to T10) and vagina and perineum (S2 to S4) require anesthesia.
If the cervix is dilated—and especially if the fetal membranes are bulging—the choice of anesthesia is less straightforward. The advantages and disadvantages of each anesthetic technique must be weighed carefully. It is important to produce adequate analgesia for the mother and to prevent an increase in intra-abdominal and intrauterine pressure that may lead to further bulging and possible rupture of the fetal membranes.
General anesthesia may be preferred in the patient with a dilated cervix and bulging fetal membranes. Administration of a volatile anesthetic agent relaxes uterine smooth muscle and results in a decrease in intrauterine pressure. A decrease in intrauterine pressure facilitates replacement of the bulging membranes and placement of the cerclage. On occasion, an amniocentesis may be performed before or during a cerclage procedure in an attempt to decrease intrauterine pressure and facilitate reduction of the fetal membranes. During induction and maintenance of general anesthesia, it is important to avoid endotracheal tube–induced coughing, which might raise intrauterine pressure. In addition, vomiting significantly raises intrauterine pressure.
Administration of neuraxial anesthesia obviates the need for tracheal intubation and the possibility of coughing on the endotracheal tube. Although some physicians worry that the acute dorsiflexion needed during initiation of the neuraxial blockade may raise intrauterine pressure, many prefer the avoidance of general anesthesia during pregnancy whenever possible.
Few clinical studies have compared obstetric outcomes after administration of neuraxial anesthesia and general anesthesia for cerclage. One retrospective study observed no difference in fetal outcome after administration of either general anesthesia (375 cases) or epidural anesthesia (114 cases).83 Another study found no significant difference in plasma oxytocin levels or postoperative uterine activity between women who received either spinal or general anesthesia for a Shirodkar cerclage.84
Fetal heart rate monitoring should be considered during the procedure if the pregnancy is sufficiently advanced to allow monitoring to be performed easily. In theory, it is possible that replacement of bulging membranes and closure of the cervix may raise intrauterine pressure with a subsequent reduction in placental blood flow. In this case, it would be reasonable to give a tocolytic agent to help reduce intrauterine pressure.
The transvaginal cerclage is removed at 37 to 38 weeks' gestation, or earlier if rupture of membranes or onset of labor occurs. Removal of a McDonald cerclage often requires no anesthesia. Anesthesia (e.g., paracervical block, spinal anesthesia, epidural anesthesia) is usually necessary for removal of a Shirodkar cerclage. If the Shirodkar cerclage is epithelialized, some obstetricians elect to leave it intact and perform an elective cesarean delivery.
Labor often begins within a few hours or days after suture removal. If an epidural catheter was placed for cerclage removal, the epidural anesthetic can be allowed to regress while the patient is observed for evidence of cervical dilation and the onset of labor. When labor begins, epidural labor analgesia can be initiated by injection of drugs through the in situ catheter.
Gestational Trophoblastic Disease
In normal pregnancy, trophoblastic tissue forms the placenta. Abnormal trophoblastic proliferation results in gestational trophoblastic disease (GTD). When GTD persists after the pregnancy is concluded (diagnosed by persistent elevation of beta-hCG), it is called persistent gestational trophoblastic disease (PGTD).
Prior to 1970, most cases of PGTD were fatal. Early diagnosis and effective chemotherapy have reduced the mortality rate to 0.1% in Great Britain and the Netherlands and to 1% in the United States.85,86 This improvement may be related to the fact that trophoblastic cells produce beta-hCG, which provides an easily assayed biochemical marker to aid in detection and to monitor treatment. Further, it is thought that cytotoxic chemotherapeutic drugs are particularly effective against PGTD because the latter arises from a genotype that is not entirely that of the patient, thus facilitating rejection of the trophoblastic cells and leaving them vulnerable to this therapy.87
However, some women still die of GTD, often as a result of late presentation and drug resistance or concomitant human immunodeficiency virus infection.88 GTD caused 0.3% of all pregnancy-related maternal deaths in the United States between 1991 and 1999.8 Risk factors for GTD include advanced or very young maternal age, previous molar pregnancy, and, possibly, nutritional factors.
Categorization and Etiology
The classification and terminology applied to GTD are varied and can be confusing because GTD encompasses a heterogeneous group of diseases. GTD is also called gestational trophoblastic tumor or gestational trophoblastic neoplasia, although in this context neoplasia simply means “new grown” and does not necessarily differentiate benign from malignant forms of GTD.
In 2003, the WHO classified GTD into eight categories (Box 16-4). The pathologic and clinical features of GTD are summarized in Table 16-1.89 Discussion of placental site trophoblastic tumor, exaggerated placental site, placental site nodules and plaques, as well as miscellaneous and unclassified trophoblastic lesions, is beyond the scope of this chapter. Here the focus is mainly on molar pregnancies. Overall, it is helpful to simply remember that GTD always involves trophoblastic cells that have abnormally proliferated and/or invaded and/or metastasized to distant sites in the body.
TABLE 16-1
Pathologic and Clinical Features of Gestational Trophoblastic Disease
| Gestational Trophoblastic Disease | Pathologic Features | Clinical Features |
| Hydatidiform mole, complete | 46,XX (mainly); 46,XY Absent fetus/embryo Diffuse swelling of villi Diffuse trophoblastic hyperplasia | 15%-20% trophoblastic sequelae Beta-hCG often > 100,000 mIU/mL Medical complications |
| Hydatidiform mole, partial | Triploid (69,XXY; 69,XYY; 69,XXX) Abnormal fetus/embryo Focal swelling of villi Focal trophoblastic hyperplasia | < 5% trophoblastic sequelae Beta-hCG usually < 100,000 mIU/mL Rare medical complications |
| Invasive mole | Myometrial invasion Swollen villi Hyperplastic trophoblast | 15% metastatic, to lung/vagina Most often diagnosed clinically, rather than pathologically |
| Choriocarcinoma | Abnormal trophoblastic hyperplasia and anaplasia Absent villi Hemorrhage, necrosis | Vascular spread to distant sites: lung/brain/liver Malignant disease |
| Placental site trophoblastic tumor | Tumor cells infiltrate myometrium with vascular/lymphatic invasion Intermediate cells/absent villi Less hemorrhage and necrosis Tumor cells stain positive for hPL | Extremely rare Beta-hCG levels less reliable indicator Relatively chemoresistant Mainly surgical treatment |
hCG, human chorionic gonadotropin; hPL, human placental lactogen.
(From Lurain JR. Gestational trophoblastic disease. I. Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol 2010; 203:531-9.)
GTD can be categorized according to pathologic features of the trophoblastic cells, malignant potential of the trophoblastic cells, or the genetic makeup of the trophoblastic cells.
Pathologically, although all GTD arises from trophoblast, hydatidiform moles and gestational choriocarcinoma arise from villous trophoblast, whereas exaggerated placental site, placental site nodule, placental site trophoblastic tumor, and epithelioid trophoblastic tumor arise from intermediate trophoblast. Further differentiation of the pathologic features of GTD is outlined in Table 16-1. Gestational choriocarcinoma typically results from a single trophoblastic cell line that has become malignant and metastasized to distant sites in the body. It can occur after a molar pregnancy, a normal pregnancy, or even a pregnancy loss.
The spectrum from the most benign to the most malignant form of GTD is as follows: (1) partial hydatidiform mole, (2) complete hydatidiform mole, (3) invasive mole, and (4) gestational choriocarcinoma. Benign forms of GTD include complete or partial molar pregnancies that have not demonstrated myometrial invasion. Malignant forms of GTD include invasive mole (complete or partial molar pregnancies that have demonstrated myometrial invasion), gestational choriocarcinoma, and placental site trophoblastic tumor. The latter diseases metastasize, most often to the lungs and brain, and are fatal if not treated. Complete molar pregnancies have a higher rate of associated complications and a higher rate of subsequent PGTD than partial molar pregnancies. Approximately 20% of patients with complete molar pregnancy have postmolar nonmetastatic PGTD (70% to 90%) or malignant PGTD (10% to 30%) and require chemotherapy; in contrast, only 5% of patients with partial molar pregnancy require chemotherapy.90
GTD can also be classified according to the genetic makeup of the trophoblastic cells. The various means by which the unusual genetic events occur in GTD are outlined here; further details are available elsewhere.87 Typically, the genetic makeup of trophoblastic cells in complete hydatidiform molar pregnancy is androgenic, meaning nearly all of the genome of the trophoblastic cell arises from the sperm. It can be either diploid (two sets of 23 chromosomes [e.g., 46,XY]) or triploid (three sets of 23 chromosomes [e.g., 69,XXY]). An ovum lacking chromosomes is fertilized most commonly by one sperm cell with reduplication (46,XX androgenic) or by two sperm cells (dispermy, 46,XX or 46,XY androgenic). No fetus develops. Approximately 90% of hydatidiform moles are complete.91
Typically, the genetic makeup of a partial hydatidiform molar pregnancy is diandric, meaning the chromosomes arise from both the egg and the sperm but is complicated by triploid conception (69,XXX or 69,XXY). One set of haploid chromosomes is maternal, and there is either reduplication of the paternal donation after fertilization by a single sperm or the egg is fertilized by two separate sperm. Because both maternal and paternal chromosomes are present, it is possible for a fetus to form with a partial mole. As a result, patients with partial mole may have a preoperative diagnosis of incomplete or missed abortion.
Invasive mole is also called chorioadenoma destruens. Because this describes any mole (complete or partial) that has invaded into the myometrium, it can have the genetic makeup of either a complete or partial mole. However, most invasive moles result from complete moles.
Complete and Partial Hydatidiform Mole
The reported incidence of hydatidiform mole varies. In the United States, it is detected in 1 in 600 elective abortions and 1 in 1500 pregnancies. Rates of 1 in 400 pregnancies are reported in Korea and Indonesia and among Native Americans.85,92,93 Coexistence of an intact fetus with molar components is extremely rare, occurring in 1 in 22,000 to 1 in 100,000 pregnancies.92
Patients may have a complete molar pregnancy diagnosed during first trimester ultrasonography, or they may have vaginal bleeding after delayed menses, suggestive of a threatened, missed, or incomplete abortion. They may spontaneously pass hydropic vesicles. The absence of fetal cardiac activity, a uterus large for gestational age, and a marked elevation of beta-hCG strongly suggest the diagnosis of hydatidiform mole. Diagnosis may be made after a D and C for an incomplete abortion92; baseline chest radiography and quantitative beta-hCG levels should be obtained promptly after surgery in such cases.
Molar pregnancies produce hCG in amounts proportional to the neoplastic volume. Excessive uterine size is associated with a marked elevation of serum beta-hCG concentration (> 100,000 mIU/mL) secondary to a large tumor volume. Large ovarian cysts, hyperemesis gravidarum, and early onset of gestational hypertension are also strongly suggestive of GTD. Ultrasonography may show characteristic multi-echogenic regions that represent hydropic villi or hemorrhagic foci.
Persistent and Malignant Gestational Trophoblastic Disease
Persistent or malignant GTD can develop after any gestational event, including normal pregnancy, spontaneous or elective abortion, ectopic pregnancy, and molar pregnancy. Histologic forms of postmolar PGTD include noninvasive trophoblastic proliferation, invasive mole, gestational choriocarcinoma, and placental site trophoblastic tumor. Diagnosis of postmolar PGTD is made when beta-hCG levels plateau or rise.92,93
Signs and symptoms of malignant GTD after a nonmolar gestational event are very subtle and diagnosis may be delayed. A quantitative beta-hCG measurement should be performed in any patient with continued or abnormal vaginal bleeding 6 weeks after the end of gestation. In any woman of reproductive age with metastatic disease from an unknown primary tumor, the diagnosis of gestational choriocarcinoma should be considered, given the fact that metastases of gestational choriocarcinoma can occur anywhere. The vagina, liver, lung, and brain are the most frequently involved sites. Signs and symptoms are related to the affected site. Biopsy of metastases is rarely needed and can result in profuse bleeding. The diagnosis of metastatic GTD is suggested with a positive beta-hCG result and no pregnancy.85,92,93
Details on staging and risk-factor scoring of GTD and their implications for therapy and prognosis are available elsewhere.90,94,95
Medical Complications
Routine use of ultrasonography has led to earlier diagnosis of molar pregnancy, which has reduced the incidence of medical complications. However, excessive uterine size occurs in up to half of patients with complete molar pregnancy and is associated with a higher incidence of medical complications. Medical complications occur in about 25% of patients with uterine size of more than 14 to 16 weeks' gestation; they include ovarian theca-lutein cysts, hyperemesis gravidarum, gestational hypertension, anemia, hyperthyroidism, DIC, and sepsis (Table 16-2).90,92-94,96-99
TABLE 16-2
Complications of Complete Molar Pregnancies
| Complication | Incidence (%) |
| Excessive uterine size | 30-53 |
| Ovarian theca-lutein cysts (> 6 cm) | 4-50 |
| Hyperemesis gravidarum | 14-29 |
| Gestational hypertension | 11-27 |
| Anemia (hemoglobin < 10 g/dL) | 10-54 |
| Hyperthyroidism | 1-7 |
| Trophoblastic emboli | 2-7 |
| Acute cardiopulmonary distress | 6-27 |
| Malignant sequelae (metastasis) | 4-36 |
| Other (renal, disseminated intravascular coagulation, infection) | Rare |
Data from references 88, 90, 94, 96-98, and 100-102. Reprinted from Chantigian RC, Chantigian PDM. Problems in early pregnancy. In Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. Chestnut's Obstetric Anesthesia. 4th edition. Philadelphia, Mosby, 2009.
Ovarian theca-lutein cysts occur primarily in patients with extremely high serum beta-hCG concentration (>100,000 mIU/mL).97 They typically regress over 2 to 3 months; rarely, torsion, rupture, or infarction may necessitate oophorectomy. Patients with theca-lutein cysts and a uterus more than 4 weeks larger than expected (for dates) have a 50% likelihood of development of postmolar GTN.85
Hyperemesis gravidarum can lead to significant electrolyte disturbances and volume depletion, all of which should be corrected before surgery.
Gestational hypertension occurs in up to 27% of women with molar pregnancy, typically in patients with an excessively large uterus.98 Preeclampsia may be diagnosed if proteinuria accompanies hypertension, although early presentation does not fit usual definitions (see Chapter 36). Although it is thought that convulsions rarely occur in these patients,97 prophylactic use of magnesium sulfate should be considered. An antihypertensive agent (e.g., hydralazine, labetalol) should be given as required to reduce blood pressure. GTD should be strongly suspected in any patient who develops hypertension during early pregnancy.
Anemia frequently complicates a complete molar pregnancy. The visible vaginal bleeding may underrepresent the total amount of hemorrhage. Occult bleeding into and around the tumor results in multiple hemorrhagic foci. Because blood loss may occur gradually, the patient may have a normal intravascular volume despite the presence of severe anemia. In the 1970s and 1980s, blood transfusion was required in as many as 32% to 45% of patients.96,99 With earlier diagnosis using ultrasonography, the incidence of transfusion may be less than in the past. Nonetheless, blood typing and antibody screening should be performed preoperatively.
Although it occurs infrequently, hyperthyroidism may result from a marked elevation of hCG,100 which can have a thyrotropin-like effect. Alternatively, hyperthyroidism may result from some other thyrotropic substance produced by the neoplasm.100 Anesthesia or surgery can precipitate thyroid storm (i.e., sinus tachycardia, atrial fibrillation, hyperthermia, cardiovascular collapse), which is treated using a beta-adrenergic antagonist.
Historically, acute cardiopulmonary distress was observed after evacuation of molar pregnancy in as many as 27% of patients.97,101-103 A higher risk for cardiopulmonary complications occurs in patients with a uterine size of 16 weeks or greater.101,104 Signs and symptoms include chest pain, cough, tachycardia, tachypnea, hypoxemia, diffuse rales, and chest radiographic evidence of bilateral pulmonary infiltrates. It is thought that trophoblastic embolization may be the etiology of cardiopulmonary distress in more than half of cases.103,104 Other causes include (1) high-output cardiac failure from thyrotoxicosis, (2) pulmonary congestion from severe anemia, (3) gestational hypertension or preeclampsia, (4) aspiration pneumonitis, and (5) iatrogenic fluid overload.103,104 Symptoms usually develop within 12 hours of uterine evacuation.103 Some patients require tracheal intubation, mechanical ventilation, and invasive hemodynamic monitoring. Symptoms usually subside within 72 hours; however, massive embolization or adult respiratory distress syndrome may result in death. If the patient survives trophoblastic embolization, malignant sequelae often develop.94,103,105
Obstetric Management
The following preoperative tests are recommended for patients with suspected hydatidiform mole: (1) complete blood count including platelet count, (2) coagulation studies, (3) renal and liver function studies, (3) blood typing and antibody screening, (4) quantitative beta-hCG level, and (5) chest radiography.92,93 Prompt molar evacuation should be instituted, because a delay in uterine evacuation may raise the risk for complications. Once the patient is stabilized, suction curettage is performed to evacuate the uterus in patients who want to preserve fertility. Real-time ultrasonography may help the obstetrician perform a complete evacuation of the uterus in patients with excessive uterine size.106 Hysterectomy is performed in patients who have completed childbearing. Hysterotomy and medical induction of labor are not recommended because they are associated with increased blood loss and a higher incidence of postmolar PGTD.93 Rh0(D) immune globulin should be administered to Rh-negative patients.
After uterine evacuation, the beta-hCG level should be measured weekly until it is undetectable for 3 weeks, then monthly for 6 months and every 2 months for another 6 months. Frequent pelvic examinations are performed while beta-hCG levels remain high. Thorough evaluation of the patient with GTD includes screening for evidence of metastasis (e.g., vagina, liver, lung, brain) and other potential complications. Prevention of pregnancy for 12 months is recommended.85
Malignant GTD should be managed by an experienced team in a trophoblastic center to minimize mortality.85,92-94 Chemotherapy is indicated in patients with (1) histologic evidence of invasive mole or choriocarcinoma, (2) an increase in beta-hCG levels of 10% or greater in three or more samples taken over at least 2 weeks, (3) a plateau of beta-hCG levels in four or more samples taken over 3 consecutive weeks, (4) persistence of measurable beta-hCG levels 6 months after molar evacuation, or (5) evidence of metastasis.95 Some patients may require delayed hysterectomy, thoracotomy for resection of pulmonary metastasis, and/or liver or brain irradiation.
Anesthetic Management
Preoperative assessment of the patient with a molar pregnancy consists of evaluation for specific complications of molar pregnancy, including hyperemesis gravidarum, gestational hypertension and preeclampsia, anemia, and thyrotoxicosis. The main anesthetic considerations are the potential for rapid and significant blood loss and the risk for cardiopulmonary distress with uterine evacuation. The anesthesia provider should establish adequate intravenous access, and blood products should be immediately available. Invasive arterial pressure and/or central venous pressure monitoring may be indicated in the patient with hypoxemia, severe anemia, hemorrhage, severe gestational hypertension or preeclampsia, hyperthyroidism, or a uterus of more than 16 weeks' size.107
Although neuraxial anesthesia has been described, general anesthesia is often preferred because of the potential for rapid, substantial blood loss and cardiopulmonary distress during evacuation of the uterus (Box 16-5). For patients with acute hemorrhage and hypovolemia, induction with thiopental or propofol may cause marked hypotension. In hyperthyroid patients, ketamine may result in marked tachycardia.108 Etomidate is an excellent choice for patients with preoperative bleeding and preoperative evidence of hyperthyroidism. Anesthesia can be maintained using either an inhalational or intravenous technique, although it may be necessary to avoid volatile anesthetic agents in some patients to optimize uterine contractility,109 and care should be exercised with the use of a propofol infusion in hemodynamically unstable patients.
An intravenous oxytocin infusion (20 IU/L of crystalloid) is begun either before97,101 or during94 uterine evacuation. Oxytocin helps the uterus contract, facilitating safe curettage and reducing blood loss. Some obstetricians have speculated that oxytocin may decrease trophoblastic embolization by constricting the uterine veins.101 Postoperatively, the patient should be monitored closely for any evidence of uterine hemorrhage or cardiopulmonary distress.
Hyperemesis Gravidarum
As many as 50% to 80% of women experience nausea and vomiting during pregnancy; this is the most common indication for admission to the hospital during the first trimester of pregnancy. Symptoms are often worse during the morning hours, thus the term morning sickness. Symptoms typically improve or resolve by the end of the first trimester.
On rare occasions, pregnant women experience a persistent and severe form of nausea and vomiting called hyperemesis gravidarum. This is believed to be an extreme form of normal nausea and vomiting of pregnancy, although there is no single accepted definition. These women may develop dehydration, ketonuria, nutritional compromise, weight loss, electrolyte abnormalities, and/or transient hepatic and renal dysfunction. Intravenous rehydration, correction of electrolyte abnormalities, antiemetics, and, rarely, hyperalimentation are indicated.
Hyperemesis gravidarum may be associated with multiple gestation, thyrotoxicosis, and/or GTD. Diagnosis is by exclusion, and many other underlying diseases should be ruled out, including gastrointestinal diseases (e.g., hepatitis, cholecystitis, pancreatitis, partial bowel obstruction), genitourinary diseases (e.g., pyelonephritis, uremia, ovarian torsion, kidney stones), metabolic disorders, neurologic or psychiatric disorders, acute fatty liver of pregnancy, drug toxicity, and preeclampsia.
Corpus Luteum Cysts
Symptomatic corpus luteum cysts occasionally occur during early pregnancy. Typically, they resolve over several weeks. In some cases, hemorrhage or ovarian torsion necessitates ovarian cystectomy or oophorectomy. After the cyst is removed, the fetus usually is not affected, provided that supplemental progesterone is administered until 10 to 12 weeks' gestation.
References
1. Wyner J, Cohen SE. Gastric volume in early pregnancy: effect of metoclopramide. Anesthesiology. 1982;57:209–212.
2. Vanner RG. Gastro-oesophageal reflux and regurgitation during general anaesthesia for termination of pregnancy. Int J Obstet Anesth. 1992;1:123–128.
3. Ueyama H, Hagihira S, Takashina M, et al. Pregnancy does not enhance volatile anesthetic sensitivity on the brain: an electroencephalographic analysis study. Anesthesiology. 2010;113:577–584.
4. Walker JJ. Ectopic pregnancy. Clin Obstet Gynecol. 2007;50:89–99.
5. Van Den Eeden SK, Shan J, Bruce C, Glasser M. Ectopic pregnancy rate and treatment utilization in a large managed care organization. Obstet Gynecol. 2005;105:1052–1057.
6. Anderson FW, Hogan JG, Ansbacher R. Sudden death: ectopic pregnancy mortality. Obstet Gynecol. 2004;103:1218–1223.
7. Minino AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119.
8. Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance—United States, 1991-1999. MMWR Surveill Summ. 2003;52:1–8.
9. Carson SA, Buster JE. Ectopic pregnancy. N Engl J Med. 1993;329:1174–1181.
10. Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42:73–85.
11. Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837–843.
12. Centers for Disease Control and Prevention. Ectopic pregnancy mortality—Florida, 2009-2010. MMWR Morb Mortal Wkly Rep. 2012;61:106–109.
13. Clayton HB, Schieve LA, Peterson HB, et al. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006;107:595–604.
14. Pisarska MD, Carson SA. Incidence and risk factors for ectopic pregnancy. Clin Obstet Gynecol. 1999;42:2–8.
15. Levine D. Ectopic pregnancy. Radiology. 2007;245:385–397.
16. Fossum GT, Davajan V, Kletzky OA. Early detection of pregnancy with transvaginal ultrasound. Fertil Steril. 1988;49:788–791.
17. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107:399–413.
18. Stovall TG, Ling FW, Carson SA, Buster JE. Serum progesterone and uterine curettage in differential diagnosis of ectopic pregnancy. Fertil Steril. 1992;57:456–457.
19. McCord ML, Muram D, Buster JE, et al. Single serum progesterone as a screen for ectopic pregnancy: exchanging specificity and sensitivity to obtain optimal test performance. Fertil Steril. 1996;66:513–516.
20. American College of Obstetricians and Gynecologists. Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479–1485.
21. Practice Committee of the American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy. Fertil Steril. 2006;86:S96–102.
22. Barnhart KT. Clinical practice. Ectopic pregnancy. N Engl J Med. 2009;361:379–387.
23. Bangsgaard N, Lund CO, Ottesen B, Nilas L. Improved fertility following conservative surgical treatment of ectopic pregnancy. BJOG. 2003;110:765–770.
24. Kirk E, Condous G, Haider Z, et al. The conservative management of cervical ectopic pregnancies. Ultrasound Obstet Gynecol. 2006;27:430–437.
25. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373–1381.
26. Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol. 2003;21:220–227.
27. Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207:14–29.
28. Atrash HK, Friede A, Hogue CJ. Abdominal pregnancy in the United States: frequency and maternal mortality. Obstet Gynecol. 1987;69:333–337.
29. Worley KC, Hnat MD, Cunningham FG. Advanced extrauterine pregnancy: diagnostic and therapeutic challenges. Am J Obstet Gynecol. 2008;198:297 e1–7.
30. Stevens CA. Malformations and deformations in abdominal pregnancy. Am J Med Genet. 1993;47:1189–1195.
31. Naim NM, Ahmad S, Siraj HH, et al. Advanced abdominal pregnancy resulting from late uterine rupture. Obstet Gynecol. 2008;111:502–504.
32. Reece EA, Petrie RH, Sirmans MF, et al. Combined intrauterine and extrauterine gestations: a review. Am J Obstet Gynecol. 1983;146:323–330.
33. Ko JK, Cheung VY. A 12-year experience of the management and outcome of heterotopic pregnancy at Queen Mary Hospital, Hong Kong, China. Int J Gynaecol Obstet. 2012;119:194–195.
34. Barrenetxea G, Barinaga-Rementeria L, Lopez de Larruzea A, et al. Heterotopic pregnancy: two cases and a comparative review. Fertil Steril. 2007;87:417 e9–15.
35. Goldstein JS, Ratts VS, Philpott T, Dahan MH. Risk of surgery after use of potassium chloride for treatment of tubal heterotopic pregnancy. Obstet Gynecol. 2006;107:506–508.
36. American College of Obstetricians and Gynecologists. Medical management of tubal pregnancy. Practice Bulletin No. 3, December 1998. Int J Gynaecol Obstet. 1999;65:97–103.
37. Selo-Ojeme DO, Feyi-Waboso PA. Salvage autotransfusion versus homologous blood transfusion for ruptured ectopic pregnancy. Int J Gynaecol Obstet. 2007;96:108–111.
38. Pazol K, Zane SB, Parker WY, et al. Abortion surveillance—United States, 2008. MMWR Surveill Summ. 2011;60:1–41.
39. Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–1309.
40. Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991-1997. Obstet Gynecol. 2003;101:289–296.
41. Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074.
42. Ronsmans C, Graham WJ. Maternal mortality: who, when, where, and why. Lancet. 2006;368:1189–1200.
43. Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194.
44. Strom CM, Ginsberg N, Applebaum M, et al. Analyses of 95 first-trimester spontaneous abortions by chorionic villus sampling and karyotype. J Assist Reprod Genet. 1992;9:458–461.
45. Occupational disease among operating room personnel: a national study. Report of an Ad Hoc Committee on the Effect of Trace Anesthetics on the Health of Operating Room Personnel, American Society of Anesthesiologists. Anesthesiology. 1974;41:321–340.
46. Tannenbaum TN, Goldberg RJ. Exposure to anesthetic gases and reproductive outcome: a review of the epidemiologic literature. J Occup Med. 1985;27:659–668.
47. McGregor DG. Occupational exposure to trace concentrations of waste anesthetic gases. Mayo Clin Proc. 2000;75:273–277.
48. Stabile I. Spontaneous abortion: a clinical perspective. Female Patient. 1992;17:14–30.
49. Stubblefield PG, Grimes DA. Septic abortion. N Engl J Med. 1994;331:310–314.
50. Von Stein GA, Munsick RA, Stiver K, Ryder K. Fetomaternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383–386.
51. Neugebauer R, Kline J, Shrout P, et al. Major depressive disorder in the 6 months after miscarriage. JAMA. 1997;277:383–388.
52. Oats JN, Vasey DP, Waldron BA. Effects of ketamine on the pregnant uterus. Br J Anaesth. 1979;51:1163–1166.
53. Munson ES, Embro WJ. Enflurane, isoflurane, and halothane and isolated human uterine muscle. Anesthesiology. 1977;46:11–14.
54. Hall JE, Ng WS, Smith S. Blood loss during first trimester termination of pregnancy: comparison of two anaesthetic techniques. Br J Anaesth. 1997;78:172–174.
55. Kumarasinghe N, Harpin R, Stewart AW. Blood loss during suction termination of pregnancy with two different anaesthetic techniques. Anaesth Intensive Care. 1997;25:48–50.
56. Martin JA, Menacker F. Expanded health data from the new birth certificate, 2004. Natl Vital Stat Rep. 2007;55:1–22.
57. Lidegaard O. Cervical incompetence and cerclage in Denmark 1980-1990: a registry-based epidemiological survey. Acta Obstet Gynecol Scand. 1994;73:35–38.
58. Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9.
59. Warren JE, Silver RM, Dalton J, et al. Collagen 1Alpha1 and transforming growth factor-beta polymorphisms in women with cervical insufficiency. Obstet Gynecol. 2007;110:619–624.
60. Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633.e1–633.e8.
61. Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (≤15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433 e1–8.
62. American College of Obstetricians and Gynecologists. Cervical insufficiency. ACOG Practice Bulletin No. 48, November 2003. (Obstet Gynecol. 2003;102:1091–1099.
63. Althuisius SM, Dekker GA, Hummel P, et al. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–1112.
64. Berghella V, Roman A, Daskalakis C, et al. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110:311–317.
65. Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469.
66. Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124 e1–19.
67. Andrews WW, Copper R, Hauth JC, et al. Second-trimester cervical ultrasound: associations with increased risk for recurrent early spontaneous delivery. Obstet Gynecol. 2000;95:222–226.
68. Golan A, Barnan R, Wexler S, et al. Incompetence of the uterine cervix. Obstet Gynecol Surv. 1989;44:96–107.
69. Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol. 2010;202:548 e1–8.
70. Berghella V. Cerclage decreases preterm birth: finally the level I evidence is here. Am J Obstet Gynecol. 2011;205:89–90.
71. Repke JT. Women with prior preterm birth and short cervix: do NOT cerclage. Am J Obstet Gynecol. 2011;205:89–90.
72. Berghella V, Odibo AO, To MS, et al. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181–189.
73. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1–375.e8.
74. Berghella V, Rafael TJ, Szychowski JM, et al. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117:663–671.
75. Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24:55–60.
76. Treadwell MC, Bronsteen RA, Bottoms SF. Prognostic factors and complication rates for cervical cerclage: a review of 482 cases. Am J Obstet Gynecol. 1991;165:555–558.
77. Zaveri V, Aghajafari F, Amankwah K, Hannah M. Abdominal versus vaginal cerclage after a failed transvaginal cerclage: a systematic review. Am J Obstet Gynecol. 2002;187:868–872.
78. Harger JH. Comparison of success and morbidity in cervical cerclage procedures. Obstet Gynecol. 1980;56:543–548.
79. Magrina JF, Kempers RD, Williams TJ. Cervical cerclage: 20 years’ experience at the Mayo Clinic. Minn Med. 1983;66:599–602.
80. Rust OA, Roberts WE. Does cerclage prevent preterm birth? Obstet Gynecol Clin North Am. 2005;32:441–456.
81. Drassinower D, Poggi SH, Landy HJ, et al. Perioperative complications of history-indicated and ultrasound-indicated cervical cerclage. Am J Obstet Gynecol. 2011;205:53.e1–53.e5.
82. McCulloch B, Bergen S, Pielet B, et al. McDonald cerclage under pudendal nerve block. Am J Obstet Gynecol. 1993;168:499–502.
83. Crawford JS, Lewis M. Nitrous oxide in early human pregnancy. Anaesthesia. 1986;41:900–905.
84. Yoon HJ, Hong JY, Kim SM. The effect of anesthetic method for prophylactic cervical cerclage on plasma oxytocin: a randomized trial. Int J Obstet Anesth. 2008;17:26–30.
85. Benedet JL, Bender H, Jones H 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–262.
86. Practice KohornE. bulletin No. 53. Diagnosis and treatment of gestational trophoblastic disease. Obstet Gynecol. 2004;104:1422.
87. Hoffner L, Surti U. The genetics of gestational trophoblastic disease: a rare complication of pregnancy. Cancer Genet. 2012;205:63–77.
88. Moodley M, Budhram S, Connolly C. Profile of mortality among women with gestational trophoblastic disease infected with the human immunodeficiency virus (HIV): argument for a new poor prognostic factor. Int J Gynecol Cancer. 2009;19:289–293.
89. Lurain JR. Gestational trophoblastic disease. I. Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203:531–539.
90. Kohorn EI. Gestational trophoblastic neoplasia and evidence-based medicine. J Reprod Med. 2002;47:427–432.
91. Jones WB, Lauersen NH. Hydatidiform mole with coexistent fetus. Am J Obstet Gynecol. 1975;122:267–272.
92. American College of Obstetricians and Gynecologists. Diagnosis and treatment of gestational trophoblastic disease. ACOG Practice Bulletin No. 53, June 2004. (Obstet Gynecol. 2004;103:1365–1377.
93. Soper JT. Gestational trophoblastic disease. Obstet Gynecol. 2006;108:176–187.
94. Soper JT, Lewis JL Jr, Hammond CB. Gestational trophoblastic disease. Hoskins WJ, Perez CA, Young RC. Principles and Practice of Gynecologic Oncology. 2nd edition. Lippincott-Raven: Philadelphia; 1997:1039–1077.
95. Kohorn EI. Negotiating a staging and risk factor scoring system for gestational trophoblastic neoplasia: a progress report. J Reprod Med. 2002;47:445–450.
96. Beischer NA, Bettinger HF, Fortune DW, Pepperell R. Hydatidiform mole and its complications in the state of Victoria. J Obstet Gynaecol Br Commonw. 1970;77:263–276.
97. Berkowitz RS, Goldstein DP. Diagnosis and management of the primary hydatidiform mole. Obstet Gynecol Clin North Am. 1988;15:491–503.
98. Curry SL, Hammond CB, Tyrey L, et al. Hydatidiform mole: diagnosis, management, and long-term followup of 347 patients. Obstet Gynecol. 1975;45:1–8.
99. Schlaerth JB, Morrow CP, Montz FJ, d'Ablaing G. Initial management of hydatidiform mole. Am J Obstet Gynecol. 1988;158:1299–1306.
100. Amir SM, Osathanondh R, Berkowitz RS, Goldstein DP. Human chorionic gonadotropin and thyroid function in patients with hydatidiform mole. Am J Obstet Gynecol. 1984;150:723–728.
101. Cotton DB, Bernstein SG, Read JA, et al. Hemodynamic observations in evacuation of molar pregnancy. Am J Obstet Gynecol. 1980;138:6–10.
102. Hammond CB. Diagnosis and management of hydatidiform mole. Postgrad. Obstet Gynecol. 1982;2:1–6.
103. Twiggs LB, Morrow CP, Schlaerth JB. Acute pulmonary complications of molar pregnancy. Am J Obstet Gynecol. 1979;135:189–194.
104. Kohorn EI. Clinical management and the neoplastic sequelae of trophoblastic embolization associated with hydatidiform mole. Obstet Gynecol Surv. 1987;42:484–488.
105. Orr JW Jr, Austin JM, Hatch KD, et al. Acute pulmonary edema associated with molar pregnancies: a high-risk factor for development of persistent trophoblastic disease. Am J Obstet Gynecol. 1980;136:412–415.
106. Evers JL, Schijf CP, Kenemans P, Martin CB Jr. Real-time ultrasound as an adjunct in the operative management of hydatidiform mole. Am J Obstet Gynecol. 1981;140:469–471.
107. Kim JM, Arakawa K, McCann V. Severe hyperthyroidism associated with hydatidiform mole. Anesthesiology. 1976;44:445–448.
108. Kaplan JA, Cooperman LH. Alarming reactions to ketamine in patients taking thyroid medication—treatment with propranolol. Anesthesiology. 1971;35:229–230.
109. Ackerman WE III. Anesthetic considerations for complicated hydatidiform molar pregnancies. Anesth Rev. 1984;11:20–24.