Hypertensive Disorders
Brian T. Bateman MD, MSc, Linda S. Polley MD
Chapter Outline
CLASSIFICATION OF HYPERTENSIVE DISORDERS
Clinical Presentation and Diagnosis
Resuscitation and Seizure Control
Hypertension is the most common medical disorder of pregnancy, affecting 6% to 10% of pregnancies.1-4 It is a leading cause of maternal mortality, accounting for approximately 26% of maternal deaths in Latin America and the Caribbean, 9% of deaths in Africa and Asia, and 16% of deaths in the developed world.5 Hypertensive disorders are an important risk factor for fetal complications, including preterm birth, fetal growth restriction (also known as intrauterine growth restriction), and fetal/neonatal death.4,6-7
Classification of Hypertensive Disorders
Hypertensive disorders of pregnancy encompass a range of conditions—chronic hypertension, gestational hypertension, preeclampsia, preeclampsia superimposed on chronic hypertension, and eclampsia—that can be difficult to diagnose because the clinical presentation is often similar despite complex differences in their underlying pathophysiologies and prognoses. Adding to the challenges for clinicians and researchers alike was a long-standing absence of consensus guidelines for categorizing hypertensive disorders. This lack resulted in the use of conflicting definitions that confounded attempts to compare and interpret data from many older clinical studies. This problem was resolved in 2000, when the National High Blood Pressure Education Program (NHBPEP) Working Group on High Blood Pressure in Pregnancy3 published a classification scheme establishing definitions that subsequently gained wide international acceptance (Box 36-1). This classification was updated in 2013 when the American College of Obstetricians and Gynecologists (ACOG) Taskforce on Hypertension in Pregnancy reviewed available literature and published a summary of current knowledge and recommendations.4
Gestational hypertension is the most frequent cause of hypertension during pregnancy, affecting approximately 5% of parturients.2,8,9 When mild, it results in outcomes that are generally similar to those of normotensive pregnancies,10,11 but when severe, it can result in rates of adverse pregnancy outcome that approximate those observed in preeclamptic women.12 Gestational hypertension presents as elevated blood pressure after 20 weeks' gestation without proteinuria (in the absence of chronic hypertension) that resolves by 12 weeks postpartum.13,14 Most cases of gestational hypertension develop after 37 weeks' gestation. Approximately one fourth of patients diagnosed with gestational hypertension will develop preeclampsia. A definitive diagnosis of gestational hypertension can be made only in retrospect after delivery when the diagnosis of chronic hypertension can be excluded based on a return to a normotensive state.
Preeclampsia is defined as the new onset of hypertension and proteinuria after 20 weeks' gestation (Box 36-2). The NHBPEP recommends that clinicians also consider the diagnosis of preeclampsia in the absence of proteinuria when any of the following signs or symptoms of end-organ involvement are present: (1) persistent epigastric or right upper quadrant pain, (2) persistent cerebral symptoms, (3) fetal growth restriction, (4) thrombocytopenia, or (5) elevated serum liver enzymes.3 The term eclampsia is used when central nervous system (CNS) involvement results in the new onset of seizures in a woman with preeclampsia. HELLP syndrome refers to the development of hemolysis, elevated liver enzymes, and a low platelet count in a woman with preeclampsia. This condition may be a variant of severe preeclampsia, but this classification is controversial because the disease may represent a pathophysiologically distinct entity.
Chronic hypertension involves either (1) prepregnancy systolic blood pressure of 140 mm Hg or higher and/or diastolic blood pressure of 90 mm Hg or higher or (2) elevated blood pressure that fails to resolve after delivery. Chronic hypertension develops into preeclampsia in approximately one fifth to one fourth of affected patients. However, even in the absence of preeclampsia, chronic hypertension is an important risk factor for adverse maternal and fetal pregnancy outcomes.6,15
Chronic hypertension with superimposed preeclampsia occurs when preeclampsia develops in a woman with chronic hypertension before pregnancy. The diagnosis is made in the presence of a new onset of proteinuria or a sudden increase in proteinuria or hypertension or both, or when other manifestations of severe preeclampsia appear. Morbidity is increased for both mother and fetus compared with preeclampsia alone.16
The clinical findings in chronic hypertension, gestational hypertension, and preeclampsia are compared in Table 36-1.
TABLE 36-1
Hypertensive Disorders of Pregnancy
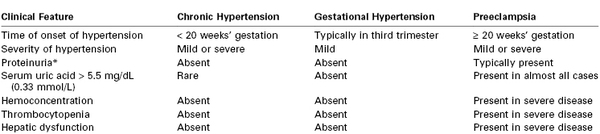
* Defined as ≥ 300 mg in a 24-h urine collection, urine protein-creatinine ratio ≥ 0.3, or ≥ 1+ result on urine dipstick testing.
From Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med 1996; 335:257-65; and American College of Obstetricians and Gynecologists Taskforce on Hypertension in Pregnancy. Hypertension in pregnancy. ACOG. Washington, DC, 2013.
Preeclampsia
Preeclampsia is a multisystem disease unique to human pregnancy. It is characterized by diffuse endothelial dysfunction with maternal complications including placental abruption, pulmonary edema, acute renal failure, liver failure, stroke, and neonatal complications, including indicated preterm delivery, fetal growth restriction, hypoxic-neurologic injury, and perinatal death.17 Although significant advances have been made in the understanding of the pathophysiology of the disease, the specific proximal cause remains unknown. Management is supportive; delivery of the infant and placenta remains the only definitive cure.
The clinical syndrome of preeclampsia is defined as the new onset of hypertension and proteinuria after 20 weeks' gestation. Previous definitions included edema, but edema is no longer part of the diagnostic criteria because it lacks specificity and occurs in many healthy pregnant women. Preeclampsia is classified as preeclampsia without severe features or severe (see Box 36-2). The ACOG now discourages use of the term “mild” for preeclampsia without severe features because preeclampsia is progressive, and appropriate management involves frequent reevaluation for severe features.4
Some authors suggest classifying preeclampsia into the early form (type I), with symptom onset before 34 weeks' gestation, or the late form (type II), with symptom onset after 34 weeks' gestation (Table 36-2).18 Early-onset preeclampsia begins with abnormal placentation, has a high rate of recurrence, and has a strong genetic component.19-21 In contrast, late-onset preeclampsia generally occurs in women metabolically predisposed to the disease, and abnormal placentation may feature less prominently in the pathogenesis. These women, who often have long-standing hypertension, obesity, diabetes, or other forms of microvascular disease, are challenged to meet the demands of the growing fetoplacental unit and decompensate near term. Decompensation manifests as late-onset or, less frequently, postpartum preeclampsia.18
TABLE 36-2
Differences between Early- and Late-Onset Preeclampsia
| Early Onset | Late Onset | |
| Onset of clinical symptoms | < 34 weeks' gestation | > 34 weeks' gestation |
| Relative frequency | 20% of cases | 80% of cases |
| Risk for adverse outcome | High | Negligible |
| Association with fetal growth restriction | Yes | No |
| Clear familial component* | Yes | No |
| Placental morphology | Abnormal† | Normal† |
| Etiology | Primarily placental‡ | Primarily maternal§ |
| Risk factor (relative risk) | Family history (2.9) | Diabetes (3.56) Multiple pregnancy (2.93) Increased blood pressure at registration (1.38) Increased body mass index (2.47) Maternal age ≥ 40 years (1.96) Cardiovascular disorders (3.84) |
* Defined as recurrence across generations and occurrence within families.
† From Egbor M, Ansari T, Morris N, et al. Morphometric placental villous and vascular abnormalities in early- and late-onset preeclampsia with and without fetal growth restriction. BJOG 2006; 113:580-9.
‡ Reduced extravillous trophoblast invasion.
§ Predisposed maternal constitution reflecting microvascular disease or predisposed genetic constitution with cis- or trans-acting genomic variations subject to interaction.
From Oudejans CB, van Dijk M, Oosterkamp M, et al. Genetics of preeclampsia: paradigm shifts. Hum Genet 2007; 120:607-12.
Epidemiology
Preeclampsia occurs in 3% to 4% of pregnancies in the United States.2,9 Delivery of the infant and placenta is the only definitive treatment; thus, preeclampsia is a leading cause of indicated preterm delivery in developed countries.22 Low-birth-weight and preterm infants born to preeclamptic mothers present major medical, social, and economic burdens to families and societies.23 Preterm delivery is the most common indication for admission to the neonatal intensive care unit.24 Preeclampsia is also a leading indication for maternal peripartum admission to an intensive care unit.25,26
The clinical findings of preeclampsia can manifest as a maternal syndrome (e.g., hypertension and proteinuria with or without other systemic abnormalities) with or without an accompanying fetal syndrome (e.g., fetal growth restriction, oligohydramnios, abnormal oxygen exchange).3,17 In approximately 75% of cases, preeclampsia occurs without severe features near term or during the intrapartum period.17 In contrast, disease onset prior to 34 weeks' gestation correlates with increased disease severity and worse outcomes for both mother and fetus.
A significant increase in the incidence of hypertensive disorders of pregnancy has occurred over the past decade (Figure 36-1), including an alarming 30% increase in severe preeclampsia/eclampsia.2 These increases are at least partially explained by major shifts in the demographics and clinical conditions of pregnant women in the United States and other developed countries. Average maternal age is increasing; advanced maternal age is a recognized risk factor for preeclampsia. Both the growing epidemic of obesity and the increased prevalence of diabetes and chronic hypertension in the developed world may also contribute to this trend. An increase in the use of assisted reproductive techniques and the use of donated gametes is contributory; these techniques increase risk for the disease by altering the maternal-fetal immune reaction27 and by increasing the incidence of multiple gestation. Last, improvements in record-keeping and the use of consistent disease definitions since 2000 may have contributed to the increased number of reported cases.3
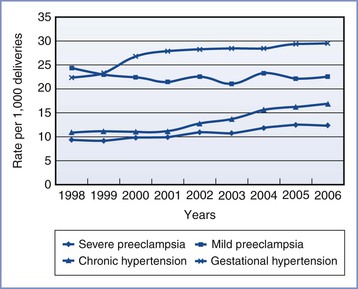
FIGURE 36-1 Age-adjusted prevalence of hypertensive disorders during delivery hospitalization in the United States. (From Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009; 113:1299-306.)
Numerous preconception and pregnancy-related risk factors associated with the development of preeclampsia have been identified (Box 36-3). Risk factors for preeclampsia can be divided into maternal demographic factors, genetic factors, medical conditions, obstetric conditions, behavioral factors, and partner-related factors.
Risk Factors
Demographic Factors.
Advanced maternal age has consistently been shown to be a risk factor for preeclampsia, with women who are 40 years or older having an approximately twofold increase in risk compared with women between 20 and 29 years of age.28,29 This risk may be independent of the increased prevalence of medical conditions and obesity that accompany advancing age.28 Teenage pregnancy may also be a risk factor for preeclampsia,30-32 but data are mixed.33
Black women constitute a high-risk group, with increased rates of chronic hypertension,34,35 obesity,35,36 and preeclampsia.37-40 Black women with severe preeclampsia demonstrate more extreme hypertension, require more antihypertensive therapy,41 and are more likely to die of the condition, compared with women of other racial backgrounds.42 Hispanic ethnicity may also confer increased risk for developing preeclampsia.43,44
Genetic Factors.
Maternal genetic factors are known to be important risk factors for the development of preeclampsia. Pregnant women with a family history of preeclampsia are approximately twice as likely to develop the disorder.45,46 It is estimated that about one third of the variance in liability to preeclampsia is caused by maternal genetic factors.47
In a study of 1.7 million births in the Medical Birth Registry of Norway, men who fathered one preeclamptic pregnancy were found to be nearly twice as likely to father a preeclamptic pregnancy in a different woman, irrespective of her previous obstetric history.48 Therefore, paternal genes (in the fetus) contribute significantly to a pregnant woman's risk for preeclampsia. It is estimated that approximately one fifth of the variance in liability for preeclampsia is conferred through the fetal genes.47
Women with a history of preeclampsia in a previous pregnancy are at increased risk for preeclampsia in a subsequent pregnancy,29,49 particularly if the preeclampsia was of early-onset in the previous pregnancy.50 Risk for recurrence increases with multiple affected pregnancies.50 In addition, women with a history of previous placental abruption and fetal growth restriction are at increased risk for preeclampsia in a subsequent pregnancy,51 and women with a history of preeclampsia are at risk for these outcomes even in the absence of recurrent preeclampsia.52 These associations suggest that some women harbor a susceptibility (potentially genetically mediated) to obstetric conditions caused by placental dysfunction, which manifests differently in different pregnancies.
Medical and Obstetric Conditions.
Obesity is an important risk factor for preeclampsia, and risk escalates with increasing body mass index (BMI).53,54 A systematic review found that an increase in BMI of 5 to 7 kg/m2 was associated with a twofold increased risk for preeclampsia.53 Obesity is strongly associated with insulin resistance, another risk factor for preeclampsia. As the prevalence of obesity continues to increase worldwide, it is likely that the incidence of preeclamptic pregnancies will increase as well.
Women with chronic hypertension are also at increased risk for preeclampsia. A 2012 population-based study found that primary hypertension increased the odds of developing preeclampsia 10-fold and that secondary hypertension increased the odds nearly 12-fold.6 Chronic hypertension in association with other risk factors, including diabetes, renal disease, and collagen vascular disease, confers particularly elevated risk.6 As women in developed countries delay childbirth, the impact of chronic hypertension will increase because of the increased prevalence of hypertension with advancing age.55 Indeed, recent data from the United States show a substantial increase in the prevalence of chronic hypertension during pregnancy.2,6
Diabetes mellitus is associated with the development of preeclampsia.6,29 In a study of 334 diabetic pregnancies, the incidence of preeclampsia was 9.9%, compared with 4.3% in nondiabetic control pregnancies. The incidence of preeclampsia also increased with the severity of diabetes as determined by the White classification.56
The metabolic syndrome, which occurs in 7% of women of childbearing age in the United States, is characterized by the presence of obesity, hyperglycemia, insulin resistance, and hypertension.57 The metabolic syndrome increases risk for preeclampsia.58 The insulin resistance and microvascular dysfunction observed in this condition have been implicated as a common factor in both preeclampsia and cardiovascular disease; these conditions may partially mediate the association of preeclampsia and increased risk for cardiovascular disease later in life.59-61
Additional maternal medical conditions that are well recognized risk factors for preeclampsia include chronic renal disease,62,63 antiphospholipid antibody syndrome,29 and systemic lupus erythematosus.6,64 Pregnancy-related conditions that increase placental mass, including multifetal gestation29,65 and hydatidiform mole,66 are associated with higher rates of preeclampsia as well.
Behavioral Factors.
Paradoxically, cigarette smoking during pregnancy has been associated with a decreased risk for preeclampsia,67,68 an effect consistently observed across studies in various countries. Women who smoke during pregnancy have a 30% to 40% lower risk for developing preeclampsia compared with women who do not smoke. The protective effect is dose related67; heavier smokers have a lower incidence of preeclampsia than those who smoke fewer cigarettes.
The duration of this protective effect after smoking cessation has been studied with conflicting results. Its biologic mechanism remains unknown, but it is believed that the mechanism may include nicotine inhibition of thromboxane A2 synthesis,69 simulation of nitric oxide release,70 or a combination of these factors. Further research of this protective mechanism may help elucidate the pathogenesis of preeclampsia.
Recreational Physical Activity.
Recreational physical activity during pregnancy has been associated with a decrease in the risk for preeclampsia,71,72 particularly in nonobese women.73 Mechanistically, this may occur through exercise promoting placenta growth, decreasing oxidative stress, enhancing endothelial function, and modulating the immune and inflammatory response.72
Partner-Related Risk Factors.
The unifying theme among partner-related risk factors is limited maternal exposure to paternal sperm antigens before conception, which suggests an immunologic role in the pathophysiology of preeclampsia. A leading risk factor for preeclampsia is nulliparity; the incidence is approximately threefold higher compared with parous women.29 Long considered a disease of primigravid women, preeclampsia is also more common in (1) parous women who have conceived with a new partner, (2) women who have used barrier methods of contraception prior to conception, and (3) women who have conceived with donated sperm.74,75 Long-term sperm exposure with the same partner appears to be protective; this protective effect is lost in a pregnancy conceived with a new partner.
Pathogenesis
The exact pathogenic mechanisms responsible for the initiation and progression of preeclampsia are not known. The placenta is the focus of hypotheses regarding disease pathogenesis; delivery of the placenta results in the resolution of disease, and the disease can occur in the absence of a fetus (e.g., a molar pregnancy).76
Preeclampsia as a Two-Stage Disorder
Contemporary hypotheses generally conceptualize preeclampsia as a two-stage disorder.77 The asymptomatic first stage occurs early in pregnancy with impaired remodeling of the spiral arteries (the end branches of the uterine artery that supply the placenta).76 In normal pregnancy, embryo-derived cytotrophoblasts invade the decidual and myometrial segments of the spiral arteries, replacing endothelium and causing remodeling of vascular smooth muscle and the inner elastic lamina (Figure 36-2).78,79 The luminal diameter of the spiral arteries increases fourfold, resulting in the creation of flaccid tubes that provide a low-resistance vascular pathway to the intervillous space. Furthermore, the remodeled arteries are unresponsive to vasoactive stimuli. These alterations in maternal vasculature ensure adequate blood flow to nourish the growing fetus and placenta.
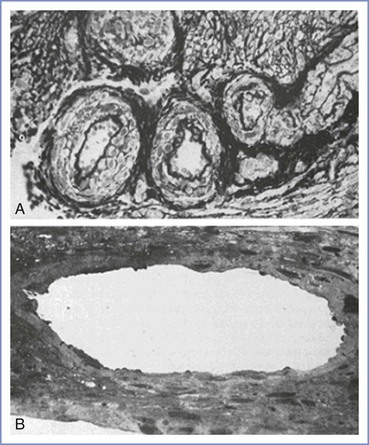
FIGURE 36-2 Sections through spiral arteries (A) at the myometrial-endometrial junction of the nonpregnant uterus and (B) at the myometrial-decidual junction in late normal pregnancy (×150). (From Sheppard BL, Bonnar J. Uteroplacental arteries and hypertensive pregnancy. In Bonnar J, MacGillivray I, Symonds G, eds. Pregnancy Hypertension. Baltimore, University Park Press, 1980:205.)
In contrast, in preeclamptic pregnancies, cytotrophoblast invasion is incomplete and only the decidual segments undergo change; the myometrial spiral arteries are not invaded and remodeled and thus remain small, constricted, and hyperresponsive to vasomotor stimuli.76 This failure of normal angiogenesis results in superficial placentation. Abnormal placentation results in decreased placental perfusion and placental infarcts, predisposing the fetus to growth restriction (Figure 36-3). Placental ischemia worsens throughout pregnancy as narrowed vessels are increasingly unable to meet the needs of the growing fetoplacental unit.
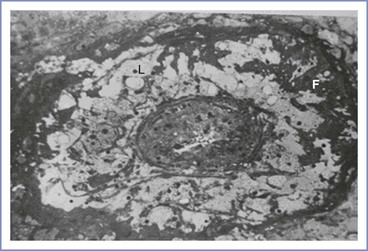
FIGURE 36-3 This figure shows lipid-laden cells (L) and fibrin deposition (F) in this occluded decidual vessel characteristic of both severe preeclampsia and severe fetal growth restriction (×150). (From Sheppard BL, Bonnar J. Uteroplacental arteries and hypertensive pregnancy. In Bonnar J, MacGillivray I, Symonds G, eds. Pregnancy Hypertension. Baltimore, University Park Press, 1980:205.)
In some women, the reduced perfusion of the intervillous space in the first stage leads to the symptomatic second stage, which is characterized by the release of antiangiogenic factors from the intervillous space into the maternal circulation; these factors cause widespread maternal endothelial dysfunction and an accentuated systemic inflammatory response. In the absence of preeclampsia, healthy endothelium prevents platelet activation, activates circulating anticoagulants, buffers the response to pressors, and maintains fluid in the intravascular compartment. These normal functions are disrupted in preeclampsia. As a result, the pregnant woman develops hypertension and proteinuria, and is at risk for other manifestations of severe systemic disease (e.g., HELLP syndrome, eclampsia, end-organ damage). These clinical manifestations usually occur after 20 weeks' gestation.
Not all women with impaired placental perfusion develop preeclampsia. The same failure of uterine vascular remodeling occurs in women with isolated fetal growth restriction80 and in approximately one third of cases of spontaneous preterm birth without maternal clinical manifestations of preeclampsia.81
Abnormal Placentation
The basis for abnormal uteroplacental development has not been fully elucidated and is likely due to a complex interaction of immunologic, vascular, environmental, and genetic factors. The hypothesis that immune maladaptation may play a central role in predisposing to abnormal placentation and subsequent preeclampsia is supported by evidence showing that long-term exposure to paternal antigens in sperm is protective. Furthermore, the importance of an intact immune system in the development of preeclampsia is demonstrated by the lower incidence of preeclampsia in women with untreated human immunodeficiency virus; the incidence returns to baseline after treatment with antiretroviral therapy.82,83
The immune cells present in the decidua—the endometrium in the nonpregnant state becomes the decidua in pregnancy—include macrophages, dendritic cells, and natural killer (NK) cells. Macrophages and dendritic cells are found in greater density in preeclamptic placentas than in control placentas.82,84 Similarly, levels of chemokines that attract these immune cells are also elevated.82,84 Excess macrophages in the decidua are associated with impaired trophoblast invasion, suggesting that excess inflammation may be one of the causal components of impaired placentation.84 NK cells may also be important in regulating vascular development during placentation. NK cells interact with fetal trophoblast cell markers via killer immunoglobulin receptors (KIR) to influence trophoblastic invasion. Specific genotypic combinations of maternal KIR and trophoblastic human leukocyte antigen C (HLA-C) may increase the risk for preeclampsia.85 A systematic review of 22 studies examining the association between HLA type and the risk for preeclampsia suggested that HLA-DR correlates with preeclampsia, but it is unclear if this, or any other HLA genotype, is causally related to preeclampsia risk; the authors called for additional studies with larger sample sizes to examine maternal-fetal HLA combinations and the risk for preeclampsia.86
Agonistic autoantibodies to the angiotensin receptor-1 (AT1) are present in many preeclamptic women in association with defective remodeling of the uteroplacental vasculature.87,88 These autoantibodies activate AT1 receptors on trophoblast cells, endothelial cells, and vascular smooth muscle cells.89-91 They appear to block trophoblastic invasion90 and may induce the production of reactive oxygen species91 and thus play a significant role in the pathophysiology of preeclampsia (Figure 36-4). Furthermore, introduction of these autoantibodies into pregnant mice increases production of soluble fms-like tyrosine kinase-1 (sFlt-1) and results in hypertension and proteinuria.92 Thus, these autoantibodies may play an important role in the pathogenesis of preeclampsia at several different stages.
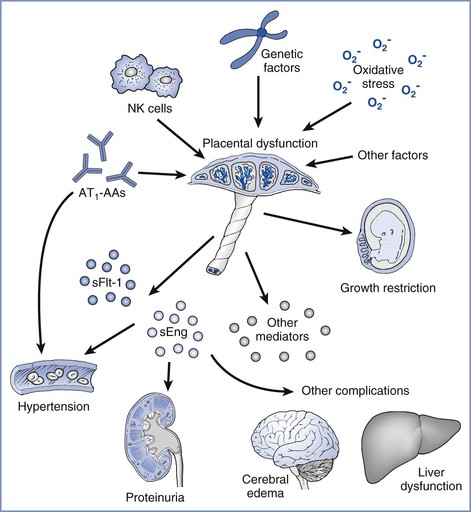
FIGURE 36-4 Angiotensin receptor autoantibodies (AT1-AAs) in preeclampsia. AT1-AAs and other factors (e.g., oxidative stress and genetic factors) may cause placental dysfunction, which, in turn, leads to the release of antiangiogenic factors (e.g., sFlt-1 and sEng) and other inflammatory mediators to induce preeclampsia. AT1-AAs may also act directly on the maternal vasculature to enhance angiotensin II sensitivity and hypertension. (From Parikh SM, Karumanchi SA. Putting pressure on preeclampsia. Nat Med 2008; 14:810-2.)
Oxidative stress is another mechanism that has been postulated as an important component of impaired placentation.82 Oxidative stress and the resultant free radicals are known to contribute to atherosclerosis and thus may contribute to placental atherosis.82 Volatile organic compounds measured in a breath test, a marker for oxidative stress, are found in greater quantity in preeclamptic women compared with healthy pregnant controls.93 Enthusiasm for this theory is tempered by the failure of antioxidant supplementation to decrease the risk for preeclampsia in clinical trials.82
Maternal Systemic Disease
The symptomatic second stage of preeclampsia is marked by widespread endothelial activation/dysfunction, and the signs and symptoms of preeclampsia are attributable to the manifestations of endothelial dysfunction specific to each organ system.76 This notion is supported by studies showing increased levels of biomarkers indicating endothelial activation or injury, or both, in preeclamptic women, including endothelin-1, fibronectin, von Willebrand factor, and thrombomodulin.76 The central role of endothelial dysfunction is further evidenced by the fact that chronic conditions that cause prepregnancy endothelial injury, including chronic hypertension, preexisting diabetes, and renal disease, are risk factors for preeclampsia. A predilection for endothelial dysfunction may similarly explain the association of preeclampsia and future cardiovascular disease.76,94
The mechanistic link between abnormal placentation and subsequent widespread endothelial dysfunction is an area of great interest and ongoing investigation. The prevailing hypothesis is that as the pregnancy progresses, the placenta becomes relatively hypoxic and this change results in an overexpression and release into the maternal circulation of placentally derived antiangiogenic factors, including sFlt-1 and soluble endoglin (sEng).76,77
The vascular endothelium requires proangiogenic factors for normal function. sFlt-1 antagonizes the angiogenic growth factors vascular endothelial growth factor (VEGF) and placental growth factor (PlGF).95,96 Evidence for a central role for sFlt-1 in the pathogenesis of preeclampsia comes from both animal and human studies. Maynard et al.95 demonstrated that sFlt-1 levels increase during gestation and fall after delivery and that increased circulating sFlt-1 levels reduce circulating levels of free VEGF and PlGF, causing endothelial dysfunction that can be rescued by exogenous VEGF and PlGF. Furthermore, these investigators found that the administration of sFlt-1 to pregnant rats induced hypertension, proteinuria, and glomerular endotheliosis, which is the classic renal lesion of preeclampsia. When administered in vitro, VEGF and PlGF cause rat renal arteriolar relaxation, which is blocked by sFlt-1. In response to increased circulating levels of sFlt-1, VEGF and PlGF levels are reduced, resulting in endothelial dysfunction in maternal vessels (Figure 36-5). In a study in humans, elevated sFlt-1 levels and reduced levels of PlGF predicted the subsequent development of preeclampsia before the development of any maternal symptoms.97 Subsequent studies have confirmed the importance of the sFlt-1-to-PlGF ratio as a marker of preeclampsia.98 Of interest, cigarette smoking, which is known to be protective against preeclampsia, is associated with lower maternal sFlt-1 concentrations during pregnancy compared with nonsmokers.99
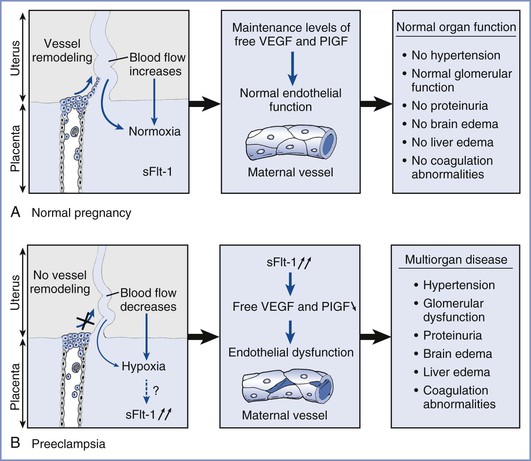
FIGURE 36-5 Hypothesis on the role of soluble fms-like tyrosine kinase (sFlt-1) in preeclampsia. A, During normal pregnancy, the uterine spiral arteries are infiltrated and remodeled by endovascular invasive trophoblasts, thereby increasing blood flow significantly to meet the oxygen and nutrient demands of the fetus. B, In the placenta of preeclamptic women, trophoblast invasion does not occur and blood flow is reduced, resulting in placental hypoxia. In addition, increased amounts of soluble sFlt-1 are produced by the placenta and scavenge vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), thereby lowering circulating levels of unbound VEGF and PlGF. This altered balance causes generalized endothelial dysfunction, resulting in multiorgan disease. (From Luttun A, Carmeliet P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest 2003; 111:600-2.)
sEng is another placentally derived antiangiogenic protein that appears to be important in the pathogenesis of preeclampsia.96 Circulating levels of sEng are markedly increased in women who subsequently develop preeclampsia. Furthermore, if women have both elevated sEng and increased sFlt-1/PlGF ratios, their risk for preeclampsia is elevated approximately 30-fold compared with women with normal levels of both factors/ratios.100
The study of antiangiogenic proteins is an active area of current research, and rapid progress is being made in understanding the role of these proteins in the pathogenesis of preeclampsia. However, the importance of recent findings is tempered by the knowledge that preeclampsia does not develop in all women with high sFlt-1 and low PlGF levels, and the syndrome occurs in some women with low sFlt-1 and high PlGF levels.97,101 These observations are consistent with those from a large, longitudinal study involving 2246 singleton pregnancies that found that PlGF and sFlt-1 levels had limited sensitivity, specificity, and positive predictive value for predicting the development of preeclampsia.102
Kanasaki et al.103 hypothesized that a molecular defect upstream from the soluble factors contributes to preeclampsia. The investigators demonstrated that pregnant mice deficient in catechol-O-methyltransferase (COMT) demonstrate a preeclampsia-like phenotype in response to the absence of 2-methoxyestradiol (2-ME), a natural metabolite of estradiol that is elevated during the third trimester of normal pregnancy. Administration of 2-ME to COMT-deficient mice suppresses placental hypoxia and sFlt-1 elevation. In addition, women with severe preeclampsia have significantly lower levels of COMT and 2-ME than women with healthy pregnancies. However, a subsequent study in humans failed to find a significant difference in placental COMT expression in women with early-onset, severe preeclampsia compared with normotensive women104; therefore, further research is needed to determine what, if any, role COMT plays in the pathogenesis of preeclampsia.
Genetic Factors
There is a strong genetic basis underlying the risk for preeclampsia that is attributable to both maternal and fetal genetic factors. It is estimated that approximately one fifth of the variance in disease risk is attributable to fetal genetic effects, and one third is attributable to maternal genetic factors.47 Despite this, with the possible exception of thrombophilia genes, no genetic variants have been robustly associated with preeclampsia. Because preeclampsia is extremely likely to be polygenic, genome-wide association studies may provide a useful approach to identify preeclampsia genes. A large, international collaborative is currently undertaking a genome-wide association study of this disease and results are expected soon. In addition to increasing the understanding of the genetic architecture of preeclampsia, the identification of genetic variants associated with the disease may provide new avenues to understanding the basic pathophysiology of this disorder.
Prophylaxis
Administration of low-dose aspirin has been proposed for the prevention of preeclampsia based on the observation that thromboxane is increased relative to prostacyclin in preeclamptic pregnancies. Aspirin inhibits the synthesis of prostaglandins by the irreversible acetylation and inactivation of cyclooxygenase. Thromboxane and prostacyclin are arachidonic acid metabolites and physiologic antagonists important in vasoregulation. Thromboxane is a potent vasoconstrictor, and prostacyclin is a strong vasodilator. Aspirin inhibits the biosynthesis of platelet thromboxane A2, and it has been hypothesized that preeclampsia could be prevented by preventing the imbalance in the thromboxane-to-prostacyclin ratio. Meta-analysis of available data points to a 10% to 20% reduction in the risk for developing preeclampsia in women treated with aspirin or other antiplatelet agents.105,106 The reduction in risk is most evident in women at moderate or high risk for preeclampsia who initiate aspirin prophylaxis at 16 weeks' gestation or earlier.107,108 The ACOG suggests daily low-dose aspirin, beginning in the late first trimester, specifically for women with a history of preeclampsia leading to prior preterm delivery before 34 weeks' gestation, or preeclampsia in more than one prior pregnancy.4
Calcium supplementation has been studied for preeclampsia prophylaxis based on observations that dietary calcium intake is inversely related to the incidence of preeclampsia.109 A 2010 meta-analysis of available randomized controlled clinical trial data showed that calcium supplementation decreased the risk for preeclampsia by approximately 50%.110 The reduction in risk was most pronounced in high-risk women and those with low baseline calcium intake.110 However, in a large multicenter, randomized, placebo-controlled trial involving 2589 healthy, nulliparous women conducted in the United States, ingestion of 2 g of elemental calcium daily did not reduce the occurrence of preeclampsia or gestational hypertension overall or in a subset of women with low baseline calcium intake.111 The ACOG does not recommend calcium supplementation to prevent preeclampsia for women with normal dietary calcium intake.4
Antioxidant supplementation has also been investigated as prophylaxis because of the important role oxidative stress is thought to play in the pathogenesis of preeclampsia. Numerous studies have been conducted to investigate a possible prophylactic or therapeutic role for antioxidant supplementation in the hypertensive disorders of pregnancy. Although some early, small studies suggested that this may be a promising approach, subsequent larger, high-quality studies have not shown a benefit.112 In randomized, controlled clinical trials, supplementation with 1000 mg of vitamin C and 400 IU of vitamin E did not reduce the incidence of preeclampsia in healthy nulliparous women113 or in women at increased risk for preeclampsia.114,115 One of these trials even showed evidence of harm, with a greater incidence of (1) low birth weight, (2) unexplained fetal death after 24 weeks' gestation, and (3) umbilical cord blood acidemia in patients randomized to the antioxidant group.115 The ACOG does not recommend the administration of vitamin C or vitamin E to prevent preeclampsia.4
Clinical Presentation
Preeclampsia occurs more frequently in nulliparous women and most commonly presents during the third trimester, often near term. Women with early-onset disease (before 34 weeks' gestation) have worse outcomes than women with late-onset disease. The disease typically regresses rapidly after delivery, with resolution of symptoms within 48 hours. However, preeclampsia can also manifest postpartum with hypertension, proteinuria, or the occurrence of seizures (eclampsia). Postpartum preeclampsia usually presents within 7 days of delivery.116
Disease manifestations of severe preeclampsia occur in all body systems as the result of widespread endothelial dysfunction.
Central Nervous System
Although the term preeclampsia suggests that eclampsia is the end stage of preeclampsia, it is more accurate to consider eclampsia as the outward manifestation of disease progression in the brain, similar to other organ involvement. Central nervous system manifestations include severe headache, hyperexcitability, hyperreflexia, and coma.3,117 Visual disturbances can include scotoma, amaurosis, and blurred vision.118
Noninvasive measurements of cerebral blood flow and resistance, along with other neuroimaging approaches, suggest that the loss of cerebral vascular autoregulation and vascular barotrauma occur with preeclampsia and eclampsia.118,119 Hyperperfusion of the brain, particularly in the setting of the endothelial dysfunction present in preeclampsia, causes vasogenic edema. Failure of autoregulation occurs most commonly in the posterior circulation, such that the changes in the brain with severe preeclampsia/eclampsia result in the posterior reversible leukoencephalopathy syndrome (PRES).118,120-122
Airway
In pregnant women, the internal diameter of the trachea is reduced because of mucosal capillary engorgement. In women with preeclampsia, these changes can be exaggerated with upper airway narrowing as a result of pharyngolaryngeal edema; these changes may compromise visualization of airway landmarks during direct laryngoscopy.123 Subglottic edema can cause airway obstruction. Signs of airway obstruction include dysphonia, hoarseness, snoring, stridor, and hypoxemia.124,125
Pulmonary
Pulmonary edema is a severe complication that occurs in approximately 3% of women with preeclampsia.126 It is relatively infrequent in healthy, younger women; the risk increases in older multigravid women, in women with preeclampsia superimposed on chronic hypertension or renal disease, and among those whose preeclampsia leads to oliguria.
Plasma colloid osmotic pressure is reduced in normal pregnancy because of decreased plasma albumin concentration, and it is decreased even further in preeclamptic women.127 Women with normal pregnancies have a mean osmotic pressure of approximately 22 mm Hg in the third trimester and approximately 17 mm Hg during the early postpartum period. In contrast, a study of women with preeclampsia demonstrated a mean colloid osmotic pressure of approximately 18 mm Hg before delivery and 14 mm Hg after delivery.128 Decreased colloid osmotic pressure, in combination with increased vascular permeability and the loss of intravascular fluid and protein into the interstitium, increases the risk for pulmonary edema.129 Excess intravenous fluid is an important risk factor for pulmonary edema in preeclamptic patients.130
Cardiovascular
Women with preeclampsia have increased vascular tone and increased sensitivity to vasoconstrictor influences, which result in the clinical manifestations of hypertension, vasospasm, and end-organ ischemia.131 Preeclampsia is characterized by severe vasospasm as well as exaggerated hemodynamic responses to circulating catecholamines.132,133 Characteristically, blood pressure and systemic vascular resistance are elevated. In preeclampsia without severe features, plasma volume may be normal; however, it may be reduced as much as 40% in women with severe disease.134
Severe preeclampsia is usually a hyperdynamic state. Many studies have attempted to characterize the hemodynamic characteristics of preeclampsia using invasive monitoring techniques or echocardiography.135-138 Interpretation and comparison of the results of these studies have been difficult because of variation in patient populations, definitions of preeclampsia, disease severity, prior treatment, and the presence or absence of concomitant comorbid disease. Hemodynamic characteristics in preeclamptic women are more complex than originally thought, in part, because hemodynamic measurements change with treatment and disease progression. Overall, studies have found that the majority of affected women exhibit increased cardiac output,138 hyperdynamic left ventricular function,135 and mild to moderately increased systemic vascular resistance.135,138 A smaller group of women comprise a high-risk group who present with decreased left ventricular function, markedly decreased systemic vascular resistance, and severely decreased intravascular volume.135,139
Hematologic
Thrombocytopenia is the most common hematologic abnormality in women with preeclampsia. Platelet counts less than 100,000/mm3 occur most commonly in women with severe disease or HELLP syndrome17 and correlate with the severity of the disease process.
Studies using thromboelastography have found that women with preeclampsia without severe features are hypercoagulable relative to women without preeclampsia and that those with severe disease are relatively hypocoagulable.140 In contrast to normal pregnancies and other hypertensive disorders, platelets are activated in preeclampsia141; subsequent platelet degranulation is believed to account for the decreases in platelet function, and aggregation appears to account for the decrease in platelet count.142
The syndrome of disseminated intravascular coagulation (DIC) occurs in some women with preeclampsia, generally in the setting of severe liver involvement, intrauterine fetal demise, placental abruption, or postpartum hemorrhage.143 Activation of the coagulation system is marked by consumption of procoagulants, increased levels of fibrin degradation products, and end-organ damage secondary to microthrombi formation. In advanced DIC, procoagulants (e.g., fibrinogen, platelets) decrease to a level that may lead to spontaneous hemorrhage.
Hepatic
Hepatic manifestations include periportal hemorrhage and fibrin deposition in hepatic sinusoids. Hepatic involvement frequently presents as right upper quadrant or epigastric pain. Damage ranges from mild hepatocellular necrosis to the more ominous HELLP syndrome and can be associated with subcapsular bleeding and risk for hepatic rupture. Spontaneous hepatic rupture is rare but is associated with a 32% maternal mortality rate.144
Renal
Renal manifestations of preeclampsia include persistent proteinuria, changes in the glomerular filtration rate, and hyperuricemia. The presence of proteinuria is a defining element of preeclampsia. The characteristic renal histologic lesion of preeclampsia is glomerular capillary endotheliosis and manifests as glomerular enlargement and endothelial and mesangial cell swelling. Increasing urinary excretion of protein likely results from changes in the pore size or charge selectivity of the glomerular filter and impaired proximal tubular reabsorption.145
During normal pregnancy, the glomerular filtration rate (GFR) increases by 40% to 60% during the first trimester,146,147 with a resulting decrease in the serum markers of renal clearance, including blood urea nitrogen (BUN), creatinine, and uric acid. In preeclampsia, this increase in GFR is blunted compared with normal pregnancy.145 Notably, women with preeclampsia may have BUN and creatinine measurements in the normal range for nonpregnant women despite significantly decreased GFR relative to healthy pregnant women.
The association between preeclampsia and hyperuricemia was recognized as early as 1917.148 Most evidence suggests that decreased renal clearance is the primary mechanism for elevated uric acid levels.149 Because levels of serum uric acid begin to increase as early as 25 weeks' gestation,150 it has been investigated as a possible early predictor of preeclampsia.
Oliguria is a possible late manifestation of severe preeclampsia and parallels the severity of disease. Persistent oliguria (< 500 mL urine output in 24 hours) requires immediate assessment of intravascular volume status. Progression to renal failure is rare and is typically preceded by hypovolemia, placental abruption, or DIC.
Uteroplacental Perfusion
Uteroplacental perfusion can be impaired in pregnancies complicated by preeclampsia. In contrast to normal pregnancy, downstream resistance in the uteroplacental bed increases, diastolic flow velocity decreases, and the systolic-to-diastolic flow velocity ratio increases.151 The systolic-to-diastolic ratio, calculated from Doppler ultrasonographic determination of blood flow velocities, reflects intrinsic arterial resistance. Pathophysiologic changes can result in fetal growth restriction (the fetal syndrome) in some pregnancies complicated by severe preeclampsia.
Obstetric Management
Optimal management of the woman with preeclampsia requires a team approach. There is considerable overlap in areas of concern to the obstetrician and the anesthesia provider.
Obstetric management of preeclampsia centers on the following: (1) fetal and maternal surveillance, (2) treatment of hypertension, (3) seizure prophylaxis, and (4) decisions regarding the timing and route of delivery. Delivery remains the only cure for preeclampsia. Obstetric care of women with preeclampsia without severe features differs little from routine management of healthy pregnant women, with the exception of careful monitoring to detect the progression of disease to severe preeclampsia. Data suggest induction of labor for pregnancies beyond 37 weeks' gestation in women with gestational hypertension or preeclampsia without severe features is associated with improved maternal outcomes compared with expectant management.4,152 Outcomes in these pregnancies are similar to those in uncomplicated pregnancies.4,13,77
In general, induction of labor is recommended for women presenting with severe preeclampsia at 34 weeks' gestation or later.4,17 For women at less than 34 weeks' gestation, expectant management may improve fetal outcomes without substantially endangering the mother,77,153 but data are few.154 Delay of delivery for 24 to 48 hours allows for the administration of corticosteroids to facilitate fetal lung maturity and transfer to a facility with maternal and neonatal intensive care resources. Expedited delivery, regardless of corticosteroid administration, is indicated for patients with eclampsia, pulmonary edema, DIC, placental abruption, abnormal fetal surveillance, a previable or nonviable fetus, or intrauterine fetal demise (Figure 36-6).4,155 If a woman develops refractory severe hypertension despite maximum doses of antihypertensive agents or persistent cerebral symptoms while receiving magnesium sulfate, delivery should occur within 24 to 48 hours, regardless of gestational age or corticosteroid administration.4,155 Expectant management before 34 weeks' gestation should be undertaken at facilities with neonatal and maternal intensive care resources.4
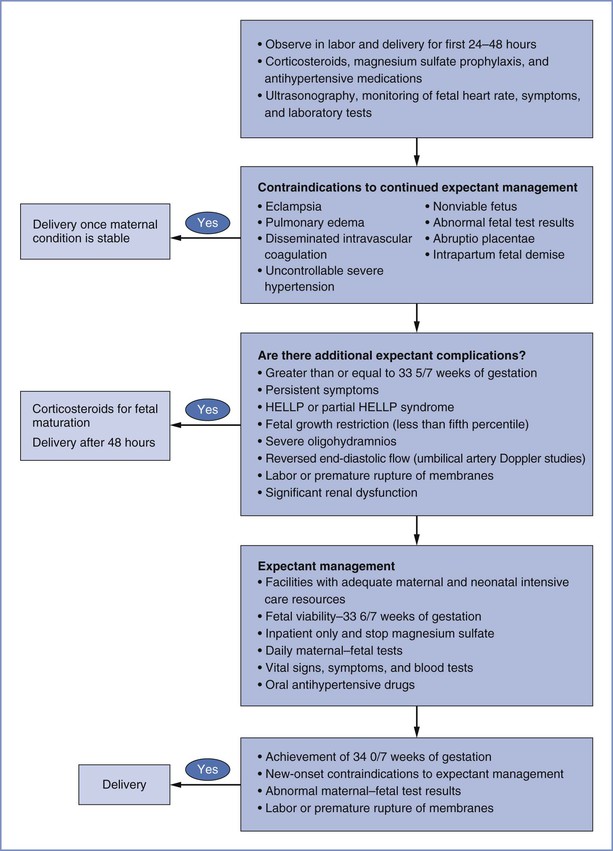
FIGURE 36-6 Suggested algorithm for the management of severe preeclampsia at less than 34 weeks' gestation. HELLP, hemolysis, elevated liver enzymes, and low platelet count. (From American College of Obstetricians and Gynecologists Taskforce on Hypertension in Pregnancy. Hypertension in pregnancy. ACOG. Washington, DC, 2013.)
Maternal and Fetal Surveillance
Maternal surveillance is indicated for all preeclamptic women. In women with preeclampsia without severe features, the goal is early detection of severe disease. In women with severe disease, the goal is detection of worsening organ dysfunction. All women should be evaluated for signs or symptoms indicating end-organ involvement, including (1) severe headache, (2) visual disturbances, (3) altered mentation, (4) dyspnea, (5) right upper quadrant or epigastric pain, (6) nausea and vomiting, (7) decreased urine output, and (8) CNS hyperexcitability.3
Initial laboratory investigations for the pregnant woman who develops hypertension after 20 weeks' gestation are listed in Table 36-3. The admission platelet count is an excellent predictor of subsequent thrombocytopenia.156 For preeclamptic women with a platelet count exceeding 100,000/mm3, further coagulation testing is not required because coagulopathy is rarely present in severely preeclamptic women who have a normal platelet count.156 If the platelet count is less than 100,000/mm3, other hemostatic abnormalities (e.g., prolonged prothrombin time [PT] and activated partial thromboplastin time [aPTT], reduced fibrinogen concentration) may be present.156 Further coagulation studies may be useful, particularly if risk factors for DIC are present (e.g., placental abruption, liver dysfunction, HELLP syndrome). Liver function tests are obtained in all women with preeclampsia because abnormal levels indicate more severe disease and may prompt delivery. Approximately 20% of preeclamptic women have elevated serum aminotransferase levels.157 The value of uric acid testing is controversial, with conflicting evidence regarding its association with increased fetal or maternal risk for complications.158-160
TABLE 36-3
Initial Laboratory Investigations for Women in Whom Hypertension Develops after 20 Weeks' Gestation
| Test | Rationale |
| Hemoglobin and hematocrit | Hemoconcentration supports diagnosis of preeclampsia and is an indicator of severity. Values are decreased if hemolysis is present. |
| Platelet count | Thrombocytopenia suggests severe preeclampsia. |
| Urine protein-creatinine ratio or 24-h urine protein excretion | Presence of proteinuria distinguishes preeclampsia from gestational hypertension. |
| Serum creatinine level | Abnormal or rising creatinine level suggests severe preeclampsia, especially in presence of oliguria. |
| Serum aminotransferase levels | Elevated serum aminotransferase levels suggest severe preeclampsia with hepatic involvement. |
Modified from Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000; 183:S1-S22; and American College of Obstetricians and Gynecologists Taskforce on Hypertension in Pregnancy. Hypertension in pregnancy. ACOG. Washington, DC, 2013.
In general, the frequency of subsequent laboratory evaluation will be guided by the initial findings and the severity of illness and disease progression.155 A diagnosis of preeclampsia without severe features should prompt at least weekly laboratory investigations, with the frequency modified based on subsequent clinical findings.4 In the expectant management of severe preeclampsia, hemoglobin, platelet count, liver function tests, creatinine, and coagulation parameters should be assessed daily or every other day.4,161 For women undergoing induction of labor for whom initial laboratory measurement or daily platelet counts demonstrate thrombocytopenia, serial platelet counts at least every 6 hours may be useful to detect declining platelet counts and to guide decision-making about the timing of delivery and analgesic or anesthetic technique. Finally, for women with indicated delivery, an active type and screen is indicated, with consideration of type and crossmatch of 2 units of packed red blood cells or more, because these women are at increased risk for postpartum hemorrhage.162
Preeclampsia is a known risk factor for perinatal death. The ACOG Taskforce on Hypertension in Pregnancy recommends daily fetal movement counts with either nonstress testing or biophysical profile testing at the time of diagnosis and at regular intervals thereafter.4,163 Ultrasonography is used to estimate fetal weight and amniotic fluid volume. Doppler ultrasonography is used to measure fetal blood flow velocimetry when fetal growth restriction is suspected.4,164,165
Treatment of Acute Hypertension
Antihypertensive medications are used to treat severe hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 mm Hg) with the goal of preventing adverse maternal sequelae such as hypertensive encephalopathy, cerebrovascular hemorrhage, myocardial ischemia, and congestive heart failure.163,166
Although acute control of maternal blood pressure is critical, rapid changes in maternal perfusion pressure may adversely affect uteroplacental perfusion and oxygen delivery to the fetus. Antihypertensive medications should be carefully titrated to avoid abrupt changes in maternal blood pressure. The aim of therapy is to lower the mean arterial blood pressure by no more than 15% to 25%, with a target systolic blood pressure between 120 and 160 mm Hg and a diastolic blood pressure between 80 and 105 mm Hg.4,167,168 Commonly used drugs include labetalol, hydralazine, and nifedipine. Nicardipine, sodium nitroprusside, and esmolol may be considered second-line agents (Table 36-4). In usual clinical doses, all are considered safe for the fetus.
TABLE 36-4
Treatment of Acute Severe Hypertension* In Preeclampsia/Eclampsia
| Medication | Onset of Action† | Dose |
| Labetalol | 5-10 min | 20 mg IV, then 40-80 mg every 10 min up to maximum dose of 220 mg IV |
| Hydralazine | 10-20 min | 5 mg IV every 20 min up to maximum dose of 20 mg IV |
| Nifedipine | 10-20 min | 10 mg PO every 20 min up to a maximum dose of 50 mg |
| Nicardipine | 10-15 min | Initial infusion 5 mg/h, increase by 2.5 mg/h every 5 min to a maximum of 15 mg/h |
| Sodium nitroprusside‡ | 0.5-1 min | 0.25-5.0 µg/kg/min IV infusion |
* Systolic blood pressure ≥ 160 mm Hg, diastolic blood pressure ≥ 110 mm Hg, or both, if sustained.
† From Stoelting R, Hillier S. Pharmacology & Physiology in Anesthetic Practice. Philadelphia, Lippincott Williams & Wilkins, 2006.
‡ Risk for fetal cyanide poisoning with treatment > 4 hours.
IV, intravenously; PO, per os.
Modified from Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000; 183:S1-S22; Marik PE, Rivera R. Hypertensive emergencies: an update. Curr Opin Crit Care 2011; 17:569-80; and Shekhar S, Sharma C, Thakur S, Verma S. Oral nifedipine or intravenous labetalol for hypertensive emergency in pregnancy: a randomized controlled trial. Obstet Gynecol 2013; 122:1057-63.
Systematic review and meta-analysis of available studies show insufficient data regarding the relative efficacy of these commonly used agents and recommend selection based on clinician familiarity and what is known about adverse effects.169 The systematic review does, however, suggest that some agents are inferior and recommends avoiding diazoxide, katanserin, nimodipine, and magnesium sulfate for the treatment of severe hypertension in pregnancy (although magnesium is recommended for seizure prophylaxis).169 A 2011 ACOG Committee Opinion recommends labetalol or hydralazine as first-line treatment for acute-onset, severe hypertension in pregnant or postpartum patients.166
Labetalol.
Labetalol is a combined alpha- and beta-adrenergic receptor antagonist with a 1-to-7 ratio of alpha- to beta-adrenergic receptor antagonism when administered intravenously. Labetalol should be avoided in women with severe asthma or congestive heart failure.170
A systematic review169 and a meta-analysis171 of small, randomized controlled trials concluded that intravenous labetalol has efficacy similar to intravenous hydralazine but with fewer maternal side effects.
Hydralazine.
Hydralazine has been used safely in pregnant women for decades and is also considered a first-line drug for treating severe hypertension in pregnancy.166 Hydralazine exerts a potent direct vasodilating effect. Plasma volume expansion before administration decreases the risk for maternal hypotension. Other side effects include tachycardia, palpitations, headache, and neonatal thrombocytopenia.172,173 In a randomized clinical trial, hydralazine was associated with more maternal tachycardia and palpitations and less neonatal bradycardia and hypotension than labetalol,174 but both antihypertensive drugs are considered safe and effective for the treatment of severe hypertension in pregnant women.
Nifedipine.
Nifedipine is a calcium entry–blocking agent that lowers blood pressure by relaxing arterial and arteriolar smooth muscle. It can be administered as a long-acting oral medication once the severe hypertension has stabilized. Nifedipine immediate-release capsules should never be administered to women with known coronary artery disease, long-standing diabetes mellitus or aortic stenosis, or to women older than 45 years of age because of an increased risk for sudden cardiac death.77 Although earlier reports suggested that the coadministration of nifedipine with magnesium sulfate causes adverse effects in both mother and fetus, including severe maternal hypotension,175,176 neuromuscular blockade,177,178 and nonreassuring FHR patterns,175,176 subsequent data suggest that these drugs can be used together safely.179,180
Other Agents.
If labetalol, hydralazine, or nifedipine are not effective in controlling blood pressure, consideration may be given to using a nicardipine or labetalol infusion or other antihypertensive agents.166
Nicardipine is a calcium entry–blocking agent that can be administered by intravenous infusion and has been shown to achieve rapid decreases in systolic and diastolic blood pressures in pregnant women.181 It is an excellent option for treating severe hypertension that is not responsive to labetalol or hydralazine.166
Sodium nitroprusside is a potent smooth muscle vasodilator that interacts with sulfhydryl groups on endothelial cells and results in the release of nitric oxide.182,183 It relaxes arterial vessels and reduces both afterload and venous return, with an almost instantaneous onset. The drug is used in pregnant women with severe hypertension who do not respond to hydralazine or labetalol therapy. It should be used only in emergency situations and then for the shortest time period possible because sodium nitroprusside metabolism produces cyanide, which undergoes placental transfer, thus exposing the fetus to potential cyanide toxicity. However, fetal harm is unlikely to result from short-term use of sodium nitroprusside in doses of 2 µg/kg/min or less. Use of the drug requires careful titration; continuous intra-arterial blood pressure monitoring is mandatory.
Esmolol is a short-acting beta-adrenergic receptor antagonist that can be used to treat acute hypertension. Concerns regarding the use of esmolol during pregnancy arose in 1989 after a report of dose-dependent prolonged fetal bradycardia in a study of gravid ewes receiving esmolol by stepped infusion.184 Subsequent human case reports have reported variable responses,185-187 but in most cases fetal bradycardia was transient and FHR returned to baseline after discontinuation of the drug. Placental transfer is rapid and the anesthesia provider should expect to observe the clinical effects of beta-adrenergic receptor blockade in the fetus. Maternal administration of esmolol produces a greater degree of beta-adrenergic receptor blockade in the fetal lamb than that observed after maternal administration of an equipotent dose of labetalol.184,188
Seizure Prophylaxis
The routine use of magnesium sulfate for seizure prophylaxis in women with severe preeclampsia is an established obstetric practice in the United States and has gained popularity throughout the world. There is clear evidence that magnesium sulfate is the best available agent for prevention of recurrent seizures in women with eclampsia189,190; thus, its use has been extended to seizure prophylaxis in women with severe preeclampsia.4
A 2010 meta-analysis of the available data identified six trials involving 11,444 women that compared magnesium sulfate for the treatment of preeclampsia with either placebo or no anticonvulsant.191 Magnesium decreased the risk for developing eclampsia (relative risk [RR], 0.41; 95% confidence interval [CI], 0.29 to 0.58); there was also a trend toward lower risk for maternal death (RR, 0.54; 95% CI, 0.26 to 1.10) but no effect on serious maternal morbidity.191 Additionally, magnesium therapy reduced the risk for placental abruption but did not adversely affect fetal and/or neonatal outcomes, including stillbirth, perinatal death, or neurosensory disability.191 Treatment with magnesium increased the risk for maternal respiratory depression (RR, 1.98; 95% CI, 1.24 to 3.15) and cesarean delivery (RR, 1.05; 95% CI, 1.01 to 1.10).191 Other side effects that were significantly more common in those treated with magnesium included feeling warm or flushed, nausea/vomiting, muscle weakness, hypotension, dizziness, drowsiness/confusion, and headache.191
There are insufficient data to justify the use of magnesium sulfate for seizure prevention in preeclampsia without severe features.4 Studies have failed to show a difference in the number of women who progressed to severe preeclampsia.192-195
The mechanism of the anticonvulsant effect of magnesium is not well understood. It was previously believed that eclamptic seizures were the result of cerebral vasospasm, and it was also believed that the cerebral vasodilating properties of magnesium reduced the rate of eclamptic seizures by relieving vasospasm.196 However, more recent evidence suggests that abrupt, sustained blood pressure elevation overwhelms myogenic vasoconstriction and causes forced dilation of the cerebral vessels, hyperperfusion, and cerebral edema.119,196-198 This evidence raises the question of how magnesium sulfate—a vasodilator—could be effective in seizure prophylaxis; magnesium would be expected to worsen cerebral hyperperfusion and edema. Using a rat model, Euser and Cipolla196 demonstrated that the mesenteric vessels are more sensitive to magnesium-induced vasodilation than are cerebral vessels, suggesting that part of the effect may be mediated through decreasing peripheral vascular resistance.199 Magnesium may also protect the blood-brain barrier,199 decrease cerebral edema,199 or act centrally at N-methyl-D-aspartate (NMDA) receptors to raise the seizure threshold.200
No consensus exists regarding the following: (1) the ideal time to initiate treatment with magnesium sulfate, (2) the best loading and maintenance doses, and (3) the optimal duration of therapy. Many obstetricians administer a loading dose of 4 to 6 g over 20 to 30 minutes, followed by a maintenance infusion of 1 to 2 g/h. The infusion is commonly initiated once the decision is made to deliver and is continued for 24 hours postpartum. Expert opinion recommends that severely preeclamptic women undergoing cesarean delivery should receive magnesium sulfate at least 2 hours before the procedure, during surgery, and for 12 hours postpartum.4,194
Magnesium sulfate is eliminated almost entirely by renal excretion,201 and serum levels may become dangerously high in the presence of renal insufficiency. Side effects include chest pain and tightness, palpitations, nausea, blurred vision, sedation, transient hypotension, and, rarely, pulmonary edema.202,203 In untreated patients, the normal range for serum magnesium concentrations is 1.7 to 2.4 mg/dL. The therapeutic range lies between 5 and 9 mg/dL.193 Reflex testing is used as a clinical screen for hypermagnesemia; when deep tendon reflexes are preserved, the more serious side effects are usually avoided. Patellar reflexes are lost at serum magnesium levels of approximately 12 mg/dL. Respiratory arrest occurs at levels of 15 to 20 mg/dL, and asystole occurs when the level exceeds 25 mg/dL.204 Preeclamptic women with renal impairment should be monitored closely because magnesium toxicity can occur with usual dosing regimens. Serial measurement of serum magnesium levels may be helpful in the management of women with renal dysfunction.
Treatment of suspected magnesium toxicity includes immediate discontinuation of the infusion and the intravenous administration of calcium gluconate (1 g) over 10 minutes.205 In the rare event of respiratory compromise, the patient may require tracheal intubation and mechanical ventilation until spontaneous ventilation returns.
Route of Delivery
Vaginal delivery should be attempted in all women with preeclampsia without severe features, assuming no other indications for cesarean delivery exist. Vaginal delivery should also be attempted in most women with severe disease, especially those beyond 34 weeks' gestation. The report of the NHBPEP Working Group on High Blood Pressure in Pregnancy states3:
Vaginal delivery is preferable to cesarean delivery for women with preeclampsia, because it avoids addition of the stress of surgery to the multiple physiologic aberrations [of the disease]. Acute palliation for several hours does not increase maternal risk if performed appropriately. Labor induction should be carried out aggressively once the decision for delivery has been made. In gestation remote from term in which delivery is indicated, and with fetal and maternal conditions stable enough to permit pregnancy to be prolonged 48 hours, glucocorticoids can be safely administered to accelerate fetal pulmonary maturity.
Cesarean delivery is appropriate when the maternal or fetal condition mandates immediate delivery or when other indications for cesarean delivery exist.
Corticosteroid Administration for Severe Preeclampsia or HELLP Syndrome
To accelerate fetal lung maturity, all women who develop severe preeclampsia or HELLP syndrome between 24 and 34 weeks' gestation should receive a course of corticosteroid therapy. A randomized double-blind trial of 218 women with severe preeclampsia at 26 to 34 weeks' gestation found that the infants of those receiving betamethasone, compared with the infants of those receiving placebo, exhibited a significant reduction in the rate of the neonatal respiratory distress syndrome and reduced rates of neonatal intraventricular hemorrhage, infection, and death.206 The available data also suggest that treatment with corticosteroids results in improvement in the maternal platelet count in women with HELLP, with dexamethasone being more efficacious than betamethasone.207 However, these studies do not show clear benefit of corticosteroid treatment on the endpoints of severe maternal morbidity or mortality.207
Complications
Severe preeclampsia is associated with an increased risk for maternal morbidity and mortality, including HELLP syndrome, cerebrovascular accident, pulmonary edema, renal failure, placental abruption, and eclampsia. In general, these complications are more common in women with early-onset preeclampsia and in women with prepregnancy medical conditions, including diabetes mellitus, chronic renal disease, and thrombophilia.38
Cerebrovascular Accident
Although the absolute risk for cerebrovascular accident is low, preeclampsia confers markedly increased risk for intracerebral and subarachnoid hemorrhage208,209 and ischemic stroke.210,211 Stroke remains the leading cause of death in women with preeclampsia.212 In the 2006-2008 Confidential Enquiry into Maternal and Child Health (CEMACH) report, 19 deaths were attributed to eclampsia and preeclampsia; 9 resulted from intracranial hemorrhage. Failure to adequately control hypertension was noted in most of these cases. The CEMACH report emphasized the importance of urgent treatment of preeclamptic women with a systolic blood pressure in excess of 150 to 160 mm Hg or at lower pressures if the clinical condition suggests rapid deterioration.212
The endothelial dysfunction of preeclampsia can promote edema, vascular tone instability, platelet activation, and local thrombosis. Reversible cerebral edema is the most common CNS feature of preeclampsia or eclampsia. The loss of cerebral autoregulation causes hyperperfusion that, compounded by endothelial disruption, leads to interstitial or vasogenic edema.76,213,214 The presence of HELLP syndrome or DIC increases the risk for a hemorrhagic event.
As noted in the CEMACH recommendations,212 there is growing recognition that mean arterial blood pressure and diastolic blood pressure may not reflect the true risk for stroke. A review of 28 case histories of severely preeclamptic women who suffered a stroke revealed that (1) systolic blood pressure in excess of 160 mm Hg was a far superior predictor of stroke than diastolic hypertension or mean arterial pressure, (2) the majority of strokes were hemorrhagic (93%) as opposed to thrombotic (7%), and (3) the majority of strokes (57%) occurred in the postpartum period.168 Close attention to blood pressure control throughout the peripartum period is the mainstay of stroke prevention.166 In keeping with this goal, ergot alkaloids should be avoided in hypertensive patients because their administration can result in severe hypertension.212
Pulmonary Edema
Pulmonary edema is a severe complication of preeclampsia that occurs in approximately 3% of affected women.126 It is relatively infrequent in younger (previously healthy) women; risk increases in older, multigravid women and in women with preeclampsia superimposed on chronic hypertension or renal disease. The clinical presentation is characterized by worsening dyspnea and orthopnea with concomitant signs of respiratory compromise, including tachypnea, rales, and hypoxemia. Causes of pulmonary edema include low colloid osmotic pressure, increased intravascular hydrostatic pressure, and increased pulmonary capillary permeability.215 All of these factors may coexist in a single patient.
A large proportion of cases of pulmonary edema occur postpartum, usually within 2 to 3 days after delivery, and management is directed toward the underlying cause (e.g., fluid overload, sepsis, cardiac failure).216 Echocardiography can be helpful in the diagnosis of cardiogenic causes of pulmonary edema.217,218 Initial treatment includes administration of supplemental oxygen, fluid restriction, and diuretic therapy (e.g., furosemide). A retrospective study of 86 women with peripartum pulmonary edema found that even in the presence of extensive radiographic infiltrates and severe hypoxemia, resolution was typically rapid, with a limited need for intensive care unit admission.219 Placement of a pulmonary artery catheter can facilitate management of patients with severe refractory pulmonary edema, but a high rate of complications of these catheters in hypertensive patients is reported, and their use should only follow careful assessment of the risks and benefits.220 Notably, in the last two CEMACH reports,212,221 there were no deaths attributed solely to pulmonary causes. Presumably, this trend reflects improvements in the fluid management of women with severe preeclampsia.
Renal Failure
Acute renal failure is a rare but serious complication of severe preeclampsia and HELLP syndrome.222 The true incidence remains unknown. Acute renal failure is divided into three categories: (1) prerenal, which refers to renal hypoperfusion; (2) intrarenal, which suggests intrinsic renal parenchymal damage; and (3) postrenal, which implies obstructive uropathy.223 The majority of cases (83% to 90%) of acute renal failure in preeclampsia result from prerenal and intrarenal pathologic processes (most commonly acute tubular necrosis) and resolve completely after delivery.224,225 In contrast, bilateral renal cortical necrosis is a rare and serious condition associated with considerable maternal and perinatal morbidity and mortality. It occurs most commonly in association with known renal parenchymal disease, chronic hypertension with superimposed preeclampsia, placental abruption, DIC, HELLP syndrome, sepsis, or fetal death.226,227
Placental Abruption
Placental abruption occurs in approximately 2% of women with preeclampsia and results in increased perinatal morbidity and mortality. A 2006 retrospective case-control study of 161 women with placental abruption and 2000 women without abruption found a threefold increased risk for placental abruption in women with preeclampsia.228 The incidence is also increased in women with underlying chronic hypertension.216 Management depends on the extent of abruption and associated hypotension, coagulopathy, or fetal compromise (see Chapter 38). Placental abruption is also associated with the development of DIC.
Prediction of Adverse Maternal Outcome
Identification of women at greatest risk for adverse maternal outcomes is potentially useful in guiding triage to high-risk centers and weighing the risks and benefits of expectant management. A 2011 multicenter, prospective study involving 2023 women with preeclampsia admitted to tertiary care centers described a model for predicting which women would develop fatal or life-threatening complications.229 Adverse outcomes occurred in 261 patients. Predictors of adverse maternal outcome included early gestational age, chest pain or dyspnea, oxygen saturation, platelet count, and creatinine and aspartate aminotransferase concentrations. The model showed excellent discrimination with an area under the receiver operating characteristics (ROC) curve of 0.88 for adverse events within 48 hours of admission. It continued to perform well in predicting adverse events up to 7 days after admission.229
HELLP Syndrome
The HELLP syndrome is characterized by hemolysis, elevated levels of liver enzymes, and a low platelet count. It may be a variant of severe preeclampsia, but this is controversial because a substantial fraction of HELLP syndrome patients do not have hypertension or proteinuria. It is associated with increased rates of maternal morbidities, including DIC, placental abruption, pulmonary edema, acute renal failure, liver hemorrhage or failure, acute respiratory distress syndrome, sepsis, stroke, and death (Table 36-5).230 Additionally, 70% of HELLP syndrome patients deliver preterm230; prematurity-related neonatal complications increase the risk for perinatal morbidity and mortality. The onset of HELLP syndrome occurs antepartum in 70% of cases and postpartum in 30%.