Duration of Labor
First Stage of Labor
The effect of neuraxial labor analgesia on the duration of the first stage of labor was addressed as a secondary outcome variable in many of the randomized controlled trials. A 2011 meta-analysis3 of 11 studies found no difference in the duration of the first stage of labor between women who were randomly assigned to receive epidural analgesia and those assigned to receive systemic opioid analgesia, although the confidence interval was wide, indicating significant heterogeneity among studies (Table 23-12). There was significant heterogeneity in the outcome because of the mixed parity of the patient populations and differences among studies in the definition of the duration of the first stage of labor. In contrast, the individual meta-analysis of the Parkland Hospital data showed a significant prolongation of the first stage of labor (approximately 30 minutes) in nulliparous women who were randomly assigned to receive epidural analgesia.429
TABLE 23-12
Meta-Analyses of Duration of First and Second Stages of Labor
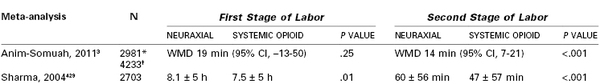
* First stage of labor.
† Second stage of labor.
CI, confidence interval; WMD, weighted mean difference
Data are from Anim-Somuah M, Smyth R, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev 2011; [12]:CD000331 and Sharma SK, McIntire DD, Wiley J, Leveno KJ. Labor analgesia and cesarean delivery: an individual meta-analysis of nulliparous women. Anesthesiology 2004; 100:142-8.
Wong et al.21 and Ohel et al.22 assessed duration of labor as a secondary outcome in their randomized controlled trials of the initiation of neuraxial analgesia during early labor. Both groups of investigators determined that the duration of the first stage of labor, and thus consequently the overall duration of labor, were significantly shorter in women randomly assigned to receive early labor neuraxial analgesia than in those assigned to receive systemic opioid analgesia. In the Wong et al. study,21 the median difference in the overall duration of labor between the early and late neuraxial analgesia groups was 81 minutes (95% CI, 28 to 123).
Determining the duration of labor requires that investigators document start and end times. The definition of the start time varies among studies but is usually consistent between groups within a study. The end of the first stage of labor is defined as the time of full (10 cm) cervical dilation. This point can be determined only with manual cervical examination. Most studies do not mandate regular cervical examinations by study protocol, or if they do, the intervals are fairly long (e.g., 1 to 2 hours). Clinically, full cervical dilation is diagnosed when a cervical examination is performed because the patient complains of rectal pressure. It is likely that women with effective epidural analgesia will complain of rectal pressure at a later time (and lower fetal station) than women with systemic opioid analgesia. In other words, the patient may be fully dilated for a significant time before cervical examination verifies full cervical dilation. This difference serves to artificially prolong the duration of the first stage of labor in the epidural group, although it shortens the apparent duration of the second stage of labor.
Other factors may also influence the duration of the first stage of labor. Some clinicians have noted enhanced uterine activity in some patients for approximately 30 minutes after the initiation of neuraxial analgesia, whereas uterine activity appears to be reduced in other patients. Schellenberg468 suggested that aortocaval compression is responsible for the transient decrease in uterine activity that occurs after the administration of epidural analgesia in some patients. He concluded that this effect does not occur if aortocaval compression is avoided. Cheek et al.469 noted that uterine activity decreased after the intravenous infusion of 1 L of crystalloid solution, but not after infusion of 0.5 L or maintenance fluid alone. There was no decrease in uterine activity after the administration of epidural analgesia. Zamora et al.36 made similar observations. Miller et al.470 hypothesized that a fluid bolus might inhibit antidiuretic hormone (vasopressin) release from the posterior pituitary gland. Because this organ also releases oxytocin, the production of that hormone might also be transiently suppressed; this possible decrease in oxytocin release may partially explain the transient changes in uterine contractility observed in association with epidural analgesia.
In a prospective but nonrandomized study, Rahm et al.471 observed that epidural analgesia (bupivacaine with sufentanil) was associated with lower plasma oxytocin levels at 60 minutes after initiation of analgesia than in healthy controls who did not receive epidural analgesia. Behrens et al.472 noted that epidural analgesia during the first stage of labor significantly reduced the release of prostaglandin F2α and “impede[d] the normal progressive increase in uterine activity.” In contrast, Nielsen et al.473 measured upper and lower uterine segment intrauterine pressures for 50 minutes before and after the administration of epidural bupivacaine analgesia in 11 nulliparous women during spontaneous labor. No significant difference in the number of contractions before and after epidural analgesia was observed. There was greater intrauterine pressure in the upper uterine segment than in the lower segment (consistent with fundal dominance) both before and after initiation of epidural analgesia. Further, fundal dominance was higher after epidural analgesia than in the preanalgesia period.
Increased uterine activity after the initiation of neuraxial analgesia has been hypothesized to be an indirect effect of neuraxial analgesia (see later discussion).474 Initiation of neuraxial analgesia is associated with an acute decrease in the maternal plasma concentration of circulating epinephrine.4 Epinephrine is a tocolytic, and the acute decrease in maternal concentration may result in greater uterine activity. This may be an explanation for the salutary effect on the progress of labor that is observed in some women with dysfunctional labor after the initiation of neuraxial analgesia475 or in women who are extremely anxious.476
The epidural administration of a local anesthetic with epinephrine is followed by systemic absorption of both drugs. Some physicians have expressed concern that the epinephrine may exert a systemic beta-adrenergic tocolytic effect and slow labor. Early studies, which used large doses of epinephrine, suggested that the caudal epidural administration of local anesthetic with epinephrine prolonged the first stage of labor and increased the number of patients who required oxytocin augmentation of labor.477 Subsequently, most studies have suggested that the addition of epinephrine 1.25 to 5 µg/mL (1 : 800,000 to 1 : 200,000) to the local anesthetic solution does not affect the progress of labor or method of delivery.*
There is no evidence that the specific local anesthetic or opioid used for neuraxial analgesia directly or indirectly affects the duration of labor.86,479 In a randomized controlled trial, Tsen et al.480 observed a higher rate of cervical dilation in women who received CSE analgesia than in those who received epidural analgesia. However, randomized controlled trials that compared CSE and epidural analgesia have not found a difference in the duration of labor between the two techniques.430-432,434,481
New evidence suggests that genetic polymorphism in the oxytocin receptor, catechol-O-methyltransferase (COMT), and β2-adrenergic receptor (ADRB2) genes affect the progress of labor.482,483 Whether these genotypes interact with neuraxial analgesia to affect the progress of labor requires further study with large numbers of parturients.
In summary, neuraxial analgesia appears to have a variable effect on the duration of the first stage of labor. It may shorten labor in some women and lengthen it in others. However, analgesia-related prolongation of the first stage of labor, if it occurs, is short, has not been shown to have adverse maternal or neonatal effects, and is probably of minimal clinical significance.
Second Stage of Labor
There is little doubt that effective neuraxial analgesia prolongs the second stage of labor. Meta-analyses of randomized controlled trials that compared neuraxial with systemic opioid analgesia support this clinical observation (see Table 23-12).3,429 The mean duration of the second stage was 15 to 20 minutes longer in women randomly assigned to receive neuraxial analgesia than in women assigned to receive systemic opioid analgesia.3,429
The ACOG has defined a prolonged second stage in nulliparous women as lasting more than 3 hours with neuraxial analgesia and more than 2 hours without neuraxial analgesia; for parous women, it is more than 2 hours in those with neuraxial analgesia and more than 1 hour in those without neuraxial analgesia.484 Zhang et al.485 performed a secondary analysis of data from the Consortium on Safe Labor, a large, multicenter study from 19 hospitals across the United States, to characterize the duration of labor in a contemporary cohort of American women. Data were abstracted for term parturients in spontaneous labor with a singleton gestation in the vertex presentation and with normal perinatal outcome. The 95th percentiles for duration of the second stage of were 3.6 and 2.8 hours for nulliparous women with and without epidural analgesia, respectively ( Table 23-13). Thus, these contemporary data suggest that a significant proportion of women will have a “prolonged” second stage, as defined by the ACOG criteria.
TABLE 23-13
Duration of Second Stage of Labor by Parity

Data are median (95th percentile) duration of the second stage of labor in spontaneous laboring women.
Data from Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol 2010; 116:1281-7.
Rouse et al.486 studied the relationship between second-stage duration and maternal and neonatal outcomes in nulliparous women by performing a secondary analysis of data collected as part of a multicenter study between 2002 and 2005. The rate of spontaneous vaginal delivery declined as the duration of the second stage of labor increased; however, over 55% of women whose second-stage duration was 3 hours or longer still went on to deliver vaginally.486 The risk for chorioamnionitis, third- or fourth-degree perineal laceration, and uterine atony was greater in women with a prolonged second stage duration; however, after adjusting for mode of delivery, adverse neonatal outcomes did not differ in women whose second stage duration was 3 hours or longer, compared with those women with a shorter second-stage labor duration. The authors concluded that the second stage of labor does not need to be terminated based on duration alone. Extending the duration of the second stage will allow a significant number of women to deliver vaginally.
Other studies have confirmed that a delay in delivery is not harmful to the infant or mother provided that (1) electronic FHR monitoring confirms the absence of nonreassuring fetal status, (2) the mother is well hydrated and has adequate analgesia, and (3) there is ongoing progress in the descent of the fetal head.484 The ACOG has stated that if progress is being made, the duration of the second stage alone does not mandate intervention.484 A 2012 workshop was convened by the National Institute of Child Health and Human Development, the Society for Maternal-Fetal Medicine, and the ACOG with the goal of recommending practices that prevent primary cesarean delivery. The group concluded that a cesarean delivery for second-stage arrest in nulliparous women with epidural analgesia should not be considered unless there is no progress (descent or rotation) for more than 4 hours (Box 23-10).487 Thus, the decision as to whether to perform an operative delivery in the second stage or allow continued observation should be made on the basis of clinical assessment of the woman and the fetus and the skill and training of the obstetrician.
Second-Stage Management: Immediate versus “Delayed” Pushing.
Many women are asked to begin “pushing” as soon as full cervical dilation has been confirmed, regardless of the fetal station. Some practitioners have suggested that “delayed” pushing might result in less maternal exhaustion and better maternal and fetal outcomes. Several studies have sought to determine whether immediate or delayed pushing for women with epidural analgesia during the second stage of labor affects labor duration and outcome.488-498 Data are conflicting. A 2012 meta-analysis of studies that compared early and delayed pushing included nine high-quality and three low-quality randomized controlled trials involving approximately 3000 women.499 Analysis of only the high-quality studies showed that delayed pushing did not influence the rate of spontaneous vaginal delivery (59.0% versus 54.9%; pooled RR, 1.07; 95% CI, 0.98 to 1.26) (Figure 23-14) or the rate of second-stage cesarean delivery. The total duration of the second stage was longer with delayed pushing (weighted mean difference, 57 min; 95% CI, 42 to 72), although duration of pushing was shorter. Heterogeneity in the reporting of neonatal outcomes among the trials precluded meta-analysis. One large study reported a higher incidence of low (< 7.10) umbilical arterial blood pH in the delayed pushing group491; however, other studies found no difference between groups in this outcome or in Apgar scores. The authors concluded that there are few clinical differences in outcomes between early and delayed pushing but that effects on maternal and neonatal outcomes remain unclear.
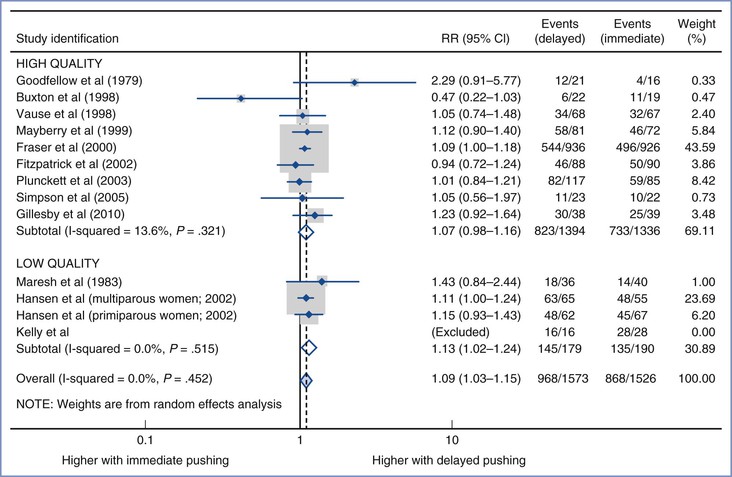
FIGURE 23-14 Meta-analysis of delayed versus immediate pushing on the rate of spontaneous vaginal delivery, stratified by quality of study. The circle represents the point estimate and the diamond is the point estimate for the pooled risk ratio. The number of events is the number of spontaneous vaginal deliveries. RR, risk ratio; CI, confidence interval. (Modified from Tuuli M, Frey HA, Odibo AO, et al. Immediate compared with delayed pushing in the second stage of labor: a systematic review and meta-analysis. Obstet Gynecol 2012; 120:660-8.)
Although there do not appear to be any major advantages to delayed pushing, it does not seem reasonable to ask the mother to push from a high fetal station. It is common for anesthesia providers to be asked to decrease or discontinue neuraxial analgesia because the mother does not feel the urge to push when she is fully dilated. However, women with effective neuraxial analgesia do not feel the urge to push at a high fetal station. The density of neuraxial analgesia should not be decreased until the fetus has descended. If evaluation at this point determines that the mother still does not feel the urge to push, the maintenance dose may be reduced. Discontinuing the maintenance of analgesia is rarely indicated because analgesia/anesthesia may be difficult to reestablish if the need for operative delivery arises.
Third Stage
Rosaeg et al.500 retrospectively reviewed the outcomes of 7468 women who underwent vaginal delivery at their hospital between 1996 and 1999. Epidural analgesia was not associated with a prolonged third stage of labor. The duration of the third stage of labor was shorter in women who received epidural analgesia and subsequently required manual removal of the placenta. The researchers suggested that epidural analgesia “provided a ‘permissive’ role”—in other words, epidural analgesia likely facilitated and/or encouraged earlier intervention by the obstetrician.
Other Factors and Progress of Labor
Oxytocin
Active management of labor is a concept that consists of a disciplined, standardized labor management protocol that includes early amniotomy and oxytocin augmentation if the cervix fails to dilate at a minimum rate (usually 1 cm/h in nulliparous women). Early studies suggested that this type of labor management decreased the rate of cesarean delivery.501 More recently, meta-analysis of a number of randomized controlled trials suggests that active management of labor may have little effect on the cesarean delivery rate.502 Although randomized trials of neuraxial compared with systemic opioid analgesia have consistently found that neuraxial analgesia does not cause an increase in the rate of cesarean delivery (see earlier discussion), Kotaska et al.503 questioned the external validity of these trials because of oxytocin management. In a search of the medical literature, they identified 16 randomized controlled trials; 8 of the 16 trials included descriptions of labor management and these trials were included in the analysis. Seven of the eight trials described active management of labor and found no difference in the mode of delivery between groups. Only one of eight trials described the use of low-dose oxytocin and reported a markedly higher rate of cesarean delivery in the neuraxial analgesia group. Kotaska et al.503 concluded that epidural analgesia in the setting of low-dose oxytocin probably increases the rate of cesarean delivery. The researchers were correct in stating that the role of oxytocin in neuraxial analgesia outcome studies has not been well controlled. However, their conclusion that epidural analgesia in the setting of low-dose oxytocin probably causes an increase in the rate of cesarean delivery is highly flawed because, in their analysis, the researchers did not include the eight studies that did not describe the management of labor. In all probability the management of labor in these studies was not active (e.g., did not include high-dose oxytocin administration), or this would have been described.
In randomized controlled trials that compared the effects of neuraxial and systemic opioid analgesia on the outcome of labor, women who received neuraxial opioids had a higher rate of oxytocin augmentation.3,429 In a meta-analysis that included 13 randomized trials, the risk ratio was 1.19 (95% CI, 1.03 to 1.39).3 The reason(s) for this observation are not clear.
Randomized controlled trials that compared early and late initiation of neuraxial analgesia have used markedly different oxytocin protocols, yet all have concluded that early initiation of neuraxial analgesia does not have an adverse effect on the outcome of labor. In the study of early CSE analgesia by Wong et al.,21 the rate of oxytocin use was high in both groups (approximately 93%). However, the maximum oxytocin infusion rate in the control (early systemic opioid) group was significantly higher than that in the early CSE group even though the median duration of labor was 81 minutes shorter in the CSE group. In the study of early epidural analgesia by Ohel et al.,22 the rate of oxytocin use in both groups was much lower (approximately 29%); however, as in the study by Wong et al.,21 the duration of labor was significantly shorter in the early neuraxial analgesia group. Taken together, the results of these studies do not support the hypothesis that oxytocin played a major role in the outcomes.
The ACOG supports the use of oxytocin for the treatment of dystocia or arrest of labor in the first or second stage, whether or not the patient is receiving neuraxial analgesia.484
Ambulation
Observational studies suggest that ambulation may be associated with less pain and a shorter duration of labor.504 However, randomized controlled trials that compared ambulation and bed rest during the first stage of labor in women with neuraxial analgesia have not demonstrated any advantages of ambulation with regard to the progress or outcome of labor. Nageotte et al.432 randomly assigned 505 nulliparous women to receive CSE analgesia either with or without ambulation. There was no difference between groups in the mode of delivery or duration of labor. These results agree with those of a meta-analysis of five randomized controlled trials involving 1161 women.505 In addition, there were no differences between groups in the use of oxytocin augmentation, satisfaction with analgesia, or Apgar scores. No adverse effects were reported. These results are similar to those of trials that compared ambulation and bed rest in women without neuraxial analgesia.506
Effects of Neuraxial Analgesia on the Fetus and Neonate
Neuraxial analgesia may affect the fetus directly, indirectly, or both. First, systemic absorption of the anesthetic agents may be followed by transplacental transfer of the drug, which has a direct effect on the fetus. Second, the effects of neuraxial blockade on the mother may affect the fetus indirectly. Effects of local anesthetics and opioids on the fetus and neonate are discussed in detail in Chapter 13.
Direct Effects
Direct fetal effects include intrapartum drug effects on the FHR as well as possible respiratory depression after delivery. The determinants of maternal plasma drug concentration, transfer across the placenta, and effects on the neonate are discussed in Chapters 4 and 13. Determinants of maternal plasma drug concentration include dose, site of administration, metabolism and excretion of the drug, and the presence of adjuvants (e.g., epinephrine). Factors that influence placental transfer include maternal and fetal placental perfusion, the physicochemical characteristics of the drug, concentration of the free drug in maternal plasma, and permeability of the placenta. Most anesthetic and analgesic drugs, including local anesthetics and opioids, readily cross the placenta.
Fetal Heart Rate
Effects of local anesthetics and opioids on FHR may be direct and indirect (see earlier discussion)474,479; however, there is little evidence for a direct effect when these drugs are administered as components of neuraxial analgesia. Transient changes in FHR variability and periodic decelerations have been observed during epidural labor analgesia with bupivacaine and other local anesthetics.479,507,508 These FHR decelerations were not associated with maternal hypotension. However, Loftus et al.509 did not observe FHR decelerations in women who received epidural bupivacaine for elective cesarean delivery, despite the use of larger doses of bupivacaine and the occurrence of more extensive sympathetic blockade in comparison with epidural labor analgesia. Of interest, one study noted that the administration of either epidural bupivacaine or intrathecal sufentanil was followed by a similar incidence of FHR decelerations (23% and 22%, respectively) in laboring women.510 Other studies have not observed a higher incidence of FHR decelerations associated with epidural administration of bupivacaine during labor.511 Further, the reports of FHR decelerations after bupivacaine did not demonstrate adverse neonatal outcome; thus, the significance of these decelerations is unclear. There are no published data on the relationship between the concentration of bupivacaine used for intrapartum epidural analgesia and the incidence of FHR decelerations. Altogether, these data suggest that epidural local anesthetics have minimal, if any, direct effect on FHR.
Similarly, neuraxial opioid administration has little direct effect on the FHR.109,512,513 In contrast, systemic meperidine analgesia was associated with a greater reduction of FHR variability and fewer FHR accelerations than epidural bupivacaine analgesia.514 Spinal administration of local anesthetics and opioids results in lower maternal plasma concentrations of drug(s) than epidural administration and is therefore even less likely to cause a direct fetal effect.
Neonatal Depression
Systemic absorption of local anesthetic or opioid may have neonatal effects. This occurs more often after the systemic administration of opioid for labor analgesia.21,515 The neonatal depressant effects of drugs administered to the mother in the intrapartum period are usually assessed with neurobehavioral testing. Unfortunately, these tests are quite subjective and lack specificity. Additionally, scientifically rigorous studies are lacking, and most of the local anesthetic studies were performed in the era when high-dose epidural analgesia was common; these observational studies found that local anesthetics administered as components of epidural analgesia were sometimes associated with minor, transient effects on neonatal behavior.86,479,516
When given by continuous epidural infusion, epidural opioid administration rarely results in accumulation of the drug and subsequent neonatal respiratory depression.* Bader et al.220 noted that a continuous epidural infusion of 0.125% bupivacaine with fentanyl 2 µg/mL over a period of 1 to 15 hours did not result in significant fetal drug accumulation or adverse neonatal effects (in this study, the maximal cumulative dose of fentanyl was 300 µg). Porter et al.221 reported no adverse effect of fentanyl on neurobehavioral scores or other indices of fetal welfare when patients received an epidural infusion of 0.0625% bupivacaine with or without fentanyl 2.5 µg/mL. The mean ± SD maternal dose of fentanyl was 183 ± 75 µg (range, 53 to 400 µg). Loftus et al.109 observed only a modest reduction in NACS at 24 hours in neonates whose mothers had received epidural fentanyl during labor; neonates exposed to sufentanil during labor had a somewhat higher NACS at 24 hours, and sufentanil was detected in the umbilical arterial blood in only one of nine samples. Vertommen et al.107 observed no difference in Apgar scores or NACS in neonates whose mothers were randomly assigned to receive epidural sufentanil (up to 30 µg) during the course of labor and a control group that did not receive sufentanil.107 Maternal sufentanil levels were below the sensitivity of the assay (0.1 ng/mL) after an epidural bolus of 10 µg.114
Intrathecal administration of an opioid during labor would be expected to have even fewer direct effects on the fetus than epidural administration. Smaller doses of opioid are administered, and less drug is absorbed systemically.
Indirect Effects
The indirect fetal effects of epidural and intrathecal opioids may be more significant than the direct effects. Obviously, if the mother has severe respiratory depression and hypoxemia, fetal hypoxemia and hypoxia will follow.357 More common is the occurrence of fetal bradycardia after initiation of neuraxial analgesia. The presumed cause is that the rapid onset of analgesia results in decreased plasma concentrations of catecholamines.474 Epinephrine causes uterine relaxation by stimulating β2-adrenergic uterine receptors. A reduced circulating concentration of epinephrine may result in increased uterine tone. Because uteroplacental perfusion occurs during periods of uterine diastole (i.e., uterine relaxation), uterine tachysystole may result in decreased uteroplacental perfusion and fetal hypoxia.
Published observations suggest that uterine tachysystole and fetal bradycardia may follow the administration of either intrathecal or epidural analgesia during labor. Abrão et al.518 randomized 72 laboring women to receive either CSE or epidural analgesia, and they observed the incidence of FHR abnormalities (prolonged deceleration or bradycardia) and an elevation in uterine tone (defined as an increase of 10 mm Hg or more in basal uterine pressure). The incidences of FHR abnormalities (32% versus 6%), and FHR abnormalities combined with an increase in uterine pressure (27% versus 3%), were significantly higher in the CSE group than in the epidural group. However, a significant limitation of this study is that the outcomes were assessed for only 15 minutes after the initiation of analgesia and the analgesic techniques were not equipotent.519 The overall high incidence of FHR abnormalities noted in the study may have been due to the initiation of analgesia in women in advanced labor.
Fortunately, fetal bradycardia after labor analgesia does not appear to increase the overall risk for adverse outcome. Albright and Forster520 retrospectively reviewed outcomes for 2560 women who delivered at their hospital between March 1995 and April 1996. Approximately half of the patients received CSE analgesia (10 to 15 µg of intrathecal sufentanil), and the other half received either systemic opioids or no medication. There was no difference between the two groups in the incidence of emergency cesarean delivery (1.3% versus 1.4%, respectively). Mardirosoff et al.521 performed a systematic review of reports of randomized comparisons of intrathecal opioid analgesia with any nonintrathecal opioid regimen in laboring women. The investigators noted that intrathecal opioid analgesia was associated with a significant increase in the risk for fetal bradycardia (OR, 1.8; 95% CI, 1.0 to 3.1). However, the risk for cesarean delivery for FHR abnormalities was similar in the two groups (6.0% versus 7.8%, respectively). Van de Velde et al.522 randomly assigned laboring women to one of the following three treatment regimens: intrathecal sufentanil 7.5 µg, intrathecal sufentanil 1.5 µg/bupivacaine 2.5 mg/epinephrine 2.5 µg, and epidural bupivacaine 12.5 mg/sufentanil 7.5 µg/epinephrine 12.5 µg. Although the incidence of FHR abnormalities was higher in the high-dose intrathecal sufentanil group, there was no difference among groups in the need for emergency cesarean delivery.
Given the risk for fetal bradycardia with neuraxial analgesia in laboring women, the FHR should be monitored during and after the administration of either epidural or intrathecal analgesia. Treatment of fetal bradycardia includes (1) relief of aortocaval compression; (2) discontinuation of intravenous oxytocin; (3) administration of supplemental oxygen; (4) treatment of maternal hypotension, if present; and (5) fetal scalp stimulation. Persistent uterine tachysystole should also prompt the administration of a tocolytic drug (e.g., terbutaline or nitroglycerin).
Conclusions and Recommendations
Philosophy of Labor Analgesia
An unacceptably high number of women involuntarily experience severe pain during labor. As noted by the ASA and the ACOG, “There is no other circumstance where it is considered acceptable for a person to experience severe pain, amenable to safe intervention, while under a physician's care.”18,19 Unfortunately, labor represents one of the few circumstances in which the provision of effective analgesia is alleged to interfere with the parturient's and obstetrician's goal (e.g., spontaneous vaginal delivery). Dense neuraxial anesthesia may adversely affect the progress of labor in some patients. Indeed, given the complicated neurohumoral and mechanical processes involved in childbirth, it would be unreasonable to expect that neuroblockade of the lower half of the body would not have an effect on this process, whether positive or negative. However, maternal-fetal factors and obstetric management—not the use of neuraxial analgesia—are the most important determinants of the outcome of labor. Anesthesia providers should identify those methods of analgesia that provide the most effective pain relief without unduly increasing the risk for obstetric intervention. Operative delivery increases the risk for maternal morbidity and mortality and is more expensive than spontaneous vaginal delivery. Randomized trials suggest that the use of neuraxial analgesia does not increase the cesarean delivery rate but may adversely influence the instrumental vaginal delivery rate.3 Further, neuraxial analgesia may occasionally, either directly or indirectly, have adverse—usually temporary—effects on the fetus.
Despite these risks, many women opt for neuraxial analgesia because no other method of labor analgesia provides its benefits (almost complete analgesia), and the risks are acceptably low. Even no analgesia may be more hazardous to some women than neuraxial analgesia (e.g., patients with an anticipated difficult airway or those at high risk for emergency cesarean delivery). Therefore, it is the duty of the anesthesia provider to provide appropriate (albeit not always total) pain relief during the first and second stages of labor. Analgesia should be tailored to the individual patient's labor, medical condition, preferences, and goals. Most women strongly dislike dense motor blockade, and many prefer to maintain some sensation of uterine contractions and perineal pressure, especially during the second stage of labor. However, a few women may accept the probable increase in risk for instrumental vaginal delivery in exchange for dense analgesia.
A Practical Guide to Neuraxial Labor Analgesia
Initiation of Analgesia
Neuraxial labor analgesia may be initiated with either the intrathecal (CSE) or the epidural injection of analgesic/anesthetic agents. The decision regarding the specific technique and choice of drugs and doses is individualized for each parturient. Parity, stage and phase of labor, use of intravenous oxytocin, and the presence of any coexisting disease(s), as well as the status of the fetus, are all considered in the decision.
In healthy nulliparous women in early labor (< 4 to 5 cm cervical dilation), my colleagues and I often initiate CSE analgesia with an intrathecal opioid alone (e.g., fentanyl 25 µg or sufentanil 5 µg), followed by placement of an epidural catheter and administration of a standard lidocaine 45 mg/epinephrine 15 µg epidural test dose. Some anesthesia providers initiate intrathecal analgesia with both an opioid and a local anesthetic. The addition of a local anesthetic is unnecessary for achieving complete spinal analgesia during early labor; it may increase the risk for hypotension and result in motor blockade in some patients, particularly if it is followed by injection of an epidural test dose that contains a local anesthetic. However, the intrathecal administration of both an opioid and a local anesthetic achieves a longer duration of analgesia and lower incidence and severity of pruritus than intrathecal injection of an opioid alone.
Alternatively, epidural analgesia can be initiated with injection of a low-concentration local anesthetic solution (bupivacaine 0.0625% to 0.125%) combined with an opioid (fentanyl 50 to 100 µg). The epidural catheter is sited and a standard epidural test dose is injected, followed by administration of 5 to 15 mL of the local anesthetic/opioid solution, injected in 5-mL increments. Ten to 15 mL provides satisfactory analgesia for most nulliparous women in early labor; injection of 20 mL may be necessary if a dilute solution (e.g., 0.0625% bupivacaine) is used. A smaller dose is necessary if administered after a standard test dose.
We typically give an epinephrine-containing test dose before initiation of epidural analgesia in laboring women. Some anesthesia providers elect to omit the epidural test dose when initiating epidural labor analgesia, particularly if a woman wishes to ambulate in early labor. The omission of the epidural test dose requires that the therapeutic dose of local anesthetic be injected slowly, incrementally, and cautiously, because the therapeutic dose functions as the test dose. These precautions should be followed with all bolus injections of local anesthetic through an epidural catheter.
For nulliparous women in the active phase of the first stage of labor, CSE analgesia is usually initiated with the intrathecal injection of an opioid combined with a local anesthetic (fentanyl 15 µg and bupivacaine 2.5 mg). Alternatively, epidural analgesia can be initiated with a local anesthetic (bupivacaine 0.125%) combined with an opioid (fentanyl 100 µg). Women in active labor may require a higher total volume of epidural local anesthetic solution (15 to 20 mL) than women in early labor (10 to 15 mL) as well as a higher local anesthetic concentration (e.g., 0.125% rather than 0.0625% bupivacaine).
Labor typically progresses at a faster rate in parous women, who often require a more rapid onset of analgesia and more extensive neuroblockade than nulliparous women when neuraxial analgesia is initiated at the same cervical dilation. Therefore, in healthy parous women CSE analgesia is usually initiated with an intrathecal opioid combined with a local anesthetic, regardless of the stage and phase of labor. Alternatively, epidural analgesia is initiated with bupivacaine 0.125% combined with fentanyl 100 µg.
CSE analgesia with both a local anesthetic and an opioid is particularly advantageous for parous women in the late active phase of the first stage of labor and in all women in whom neuraxial analgesia is initiated in the second stage of labor. Sacral neuroblockade is required for complete analgesia during the second stage of labor; this neuroblockade is difficult to accomplish in a timely fashion with an initial (de novo) lumbar epidural injection of analgesic/anesthetic agents. (For initiation of lumbar epidural anesthesia in late labor, the injection of a large volume [≥ 20 mL] of local anesthetic solution may be required to achieve sacral analgesia, and this injection often results in a mid- or high-thoracic neuroblockade that is more extensive than desired. Therefore, when initiating neuraxial analgesia in late labor, a CSE technique is preferred).
Maintenance epidural analgesia is typically initiated soon after the initiation of analgesia (within 15 to 30 minutes) rather than waiting for the neuroblockade to regress. There are several advantages to this technique. Most women experience seamless analgesia (i.e., there is no window of pain as the initial block regresses). The workload for the anesthesia provider is lessened, because he or she can set up and initiate the epidural infusion while monitoring the patient for hypotension after initiation of neuroblockade. Finally, an epidural bolus of local anesthetic is not required to reestablish or extend neuroblockade, possibly enhancing safety.
Analgesia is typically maintained with a dilute solution of an amide local anesthetic and an opioid, administered by continuous infusion or PCEA. My colleagues and I prefer PCEA because it allows patient titration of neuroblockade and entails less risk for breakthrough pain. Patient satisfaction is better and the workload for the anesthesia provider is decreased. At our institution, the PCEA infusion pump parameters are the same for all laboring women, so there are fewer errors in pump setup. However, when a continuous infusion is used without PCEA to maintain analgesia, it may be necessary to titrate the continuous infusion rate to individual patient needs. For example, women in early labor require less drug to maintain analgesia (6 to 10 mL/h), whereas women in more advanced labor may require a higher infusion rate (8 to 15 mL/h). Similarly, a parous patient may require a higher infusion rate than a nulliparous patient, even though analgesia is initiated at the same stage of labor.
Some parturients experience breakthrough pain. After evaluating the nature of the pain, the extent of neuroblockade, and the progress of labor, we usually treat breakthrough pain with a bolus epidural injection of bupivacaine 0.125%, 10 to 15 mL, administered in 5-mL increments. The patient may benefit from additional instruction about the optimal use of PCEA. Occasionally, we may elect to use a more concentrated local anesthetic solution (e.g., bupivacaine 0.25%), particularly in the presence of an abnormal fetal position or dysfunctional labor. In this case, the concentration of the maintenance solution may also need to be increased.
This maintenance technique usually results in satisfactory perineal analgesia for delivery. Occasionally, women with epidural analgesia require additional (more dense) analgesia for delivery, particularly if an instrumental vaginal delivery is planned. In this case, we often administer 5 to 12 mL of 1% to 2% lidocaine or 2% to 3% 2-chloroprocaine. This usually results in satisfactory sacral anesthesia in a patient with preexisting epidural labor analgesia.
There is no single correct way to provide neuraxial labor analgesia, although for particular patients and specific clinical conditions some methods may have advantages over others. Frequent communication among members of the anesthesia, obstetric, and nursing teams is essential to the safe and satisfactory provision of neuraxial labor analgesia. In addition, within each labor and delivery unit, consistency among anesthesia providers in their choice of techniques, specific drugs, and drug doses/concentrations is likely to result in fewer errors and higher satisfaction among other caregivers and patients.
References
1. Jones L, Othman M, Dowswell T, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database Syst Rev. 2012;(3).
2. Paech MJ. The King Edward Memorial Hospital 1,000 mother survey of methods of pain relief in labour. Anaesth Intensive Care. 1991;19:393–399.
3. Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;(12).
4. Shnider SM, Abboud TK, Artal R, et al. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. Am J Obstet Gynecol. 1983;147:13–15.
5. Lederman RP, Lederman E, Work B, McCann DS. Anxiety and epinephrine in multiparous labor: relationship to duration of labor and fetal heart rate pattern. Am J Obstet Gynecol. 1985;153:870–877.
6. Jouppila R, Hollmén A. The effect of segmental epidural analgesia on maternal and foetal acid-base balance, lactate, serum potassium and creatine phosphokinase during labour. Acta Anaesth Scand. 1976;20:259–268.
7. Levinson G, Shnider SM, deLorimier AA, Steffenson JL. Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology. 1974;40:340–347.
8. Peabody JH. Transcutaneous oxygen measurement to evaluate drug effects. Clin Perinatol. 1979;6:109–121.
9. Capogna G, Camorcia M, Stirparo S. Expectant fathers’ experience during labor with or without epidural analgesia. Int J Obstet Anesth. 2007;16:110–115.
10. Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol. 2011;117:69–74.
11. Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
12. Osterman MJ, Martin JA. Epidural and spinal anesthesia use during labor: 27-state reporting area, 2008. Natl Vital Stat Rep. 2011;59(no. 5):1–13.
13. National Health Service Maternity Statistics 2010-11. [Available at] http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=1815 [Accessed December 2012] .
14. Huang C, Macario A. Economic considerations related to providing adequate pain relief for women in labour: comparison of epidural and intravenous analgesia. Pharmacoeconomics. 2002;20:305–318.
15. Macario A, Scibetta WC, Navarro J, Riley E. Analgesia for labor pain: a cost model. Anesthesiology. 2000;92:841–850.
16. American Society of Anesthesiologists. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–863.
17. American College of Obstetricians and Gynecologists. Pain relief during labor. ACOG Committee Opinion No. 295. Washington, DC, July 2004 (Reaffirmed 2008). Obstet Gynecol. 2004;104:213.
18. American Society of Anesthesiologists. Statement on pain relief during labor. [Available at] http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx; 2010 [Accessed December 2012] .
19. American College of Obstetricians and Gynecologists. Analgesia and cesarean delivery rates. ACOG Committee Opinion No. 339. Washington, DC, July 2006 (Reaffirmed 2010). Obstet Gynecol. 2006;107:1487–1488.
20. Chestnut DH, McGrath JM, Vincent RD, et al. Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80:1201–1208.
21. Wong CA, Scavone BM, Peaceman AM, et al. The risk of cesarean delivery with neuraxial analgesia given early versus late in labor. N Engl J Med. 2005;352:655–665.
22. Ohel G, Gonen R, Vaida S, et al. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006;194:600–605.
23. Chestnut DH, Vincent RD, McGrath JM, et al. Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are receiving intravenous oxytocin? Anesthesiology. 1994;90:1193–1200.
24. Wang F, Shen X, Guo X, et al. Epidural analgesia in the latent phase of labor and the risk of cesarean delivery: a five-year randomized controlled trial. Anesthesiology. 2009;111:871–880.
25. Wassen MM, Zuijlen J, Roumen FJ, et al. Early versus late epidural analgesia and risk of instrumental delivery in nulliparous women: a systematic review. BJOG. 2011;118:655–661.
26. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (third edition). Reg Anesth Pain Med. 2010;35:64–101.
27. Simmons SW, Taghizadeh N, Dennis AT, et al. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev. 2012;(10).
28. Sia AT, Chong JL, Tay DH, et al. Intrathecal sufentanil as the sole agent in combined spinal-epidural analgesia for the ambulatory parturient. Can J Anaesth. 1998;45:620–625.
29. Norris MC. Are combined spinal-epidural catheters reliable? Int J Obstet Anesth. 2000;9:3–6.
30. Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. Int J Obstet Anesth. 2004;13:227–233.
31. Lee S, Lew E, Lim Y, Sia AT. Failure of augmentation of labor epidural analgesia for intrapartum cesarean delivery: a retrospective review. Anesth Analg. 2009;108:252–254.
32. Cappiello E, O’Rourke N, Segal S, Tsen LC. A randomized trial of dural puncture epidural technique compared with the standard epidural technique for labor analgesia. Anesth Analg. 2008;107:1646–1651.
33. Minty RG, Kelly L, Minty A, Hammett DC. Single-dose intrathecal analgesia to control labour pain: is it a useful alternative to epidural analgesia? Can Fam Physician. 2007;53:437–442.
34. Kinsella SM, Pirlet M, Mills MS, et al. Randomized study of intravenous fluid preload before epidural analgesia during labour. Br J Anaesth. 2000;85:311–313.
35. Collins KM, Bevan DR, Beard RW. Fluid loading to reduce abnormalities of fetal heart rate and maternal hypotension during epidural analgesia in labour. Br Med J. 1978;2:1460–1461.
36. Zamora JE, Rosaeg OP, Lindsay MP, Crossan ML. Haemodynamic consequences and uterine contractions following 0.5 or 1.0 litre crystalloid infusion before obstetric epidural analgesia. Can J Anaesth. 1996;43:347–352.
37. Banerjee A, Stocche RM, Angle P, Halpern SH. Preload or coload for spinal anesthesia for elective Cesarean delivery: a meta-analysis. Can J Anaesth. 2010;57:24–31.
38. Fisher AJ, Huddleston JF. Intrapartum maternal glucose infusion reduces umbilical cord acidemia. Am J Obstet Gynecol. 1997;177:765–769.
39. Cerri V, Tarantini M, Zuliani G, et al. Intravenous glucose infusion in labor does not affect maternal and fetal acid-base balance. J Matern Fetal Med. 2000;9:204–208.
40. Shrivastava VK, Garite TJ, Jenkins SM, et al. A randomized, double-blinded, controlled trial comparing parenteral normal saline with and without dextrose on the course of labor in nulliparas. Am J Obstet Gynecol. 2009;200:379 e1–6.
41. Norris MC, Ferrenbach D, Dalman H, et al. Does epinephrine improve the diagnostic accuracy of aspiration during labor epidural analgesia? Anesth Analg. 1999;88:1073–1076.
42. Calimaran AL, Strauss-Hoder TP, Wang WY, et al. The effect of epidural test dose on motor function after a combined spinal-epidural technique for labor analgesia. Anesth Analg. 2003;96:1167–1172.
43. Cohen SE, Yeh JY, Riley ET, Vogel TM. Walking with labor epidural analgesia: the impact of bupivacaine concentration and a lidocaine-epinephrine test dose. Anesthesiology. 2000;92:387–392.
44. Birnbach DJ, Chestnut DH. The epidural test dose in obstetric patients: has it outlived its usefulness? Anesth Analg. 1999;88:971–972.
45. Chestnut DH, Owen CL, Brown CK, et al. Does labor affect the variability of maternal heart rate during induction of epidural anesthesia? Anesthesiology. 1988;68:622–625.
46. Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci USA. 1976;73:3729–3733.
47. Rush B. Medical Inquiries and Observations. 4th edition. T&G Palmer: Philadelphia; 1805.
48. Justins DM, Francis D, Houlton PG, Reynolds F. A controlled trial of extradural fentanyl in labour. Br J Anaesth. 1982;54:409–414.
49. Belfrage P, Berlin A, Raabe N, Thalme B. Lumbar epidural analgesia with bupivacaine in labor: drug concentration in maternal and neonatal blood at birth and during the first day of life. Am J Obstet Gynecol. 1975;123:839–844.
50. Eisenach JC, Grice SC, Dewan DM. Epinephrine enhances analgesia produced by epidural bupivacaine during labor. Anesth Analg. 1987;66:447–451.
51. Capogna G, Celleno D, Lyons G, et al. Minimum local analgesia concentration of extradural bupivacaine increases with progression of labor. Br J Anaesth. 1998;80:11–13.
52. Polley LS, Columb MO, Wagner DS, Naughton NN. Dose-dependent reduction of the minimum local analgesic concentration of bupivacaine by sufentanil for epidural analgesia in labor. Anesthesiology. 1998;89:626–632.
53. Christiaens F, Verborgh C, Dierick A, Camu F. Effects of diluent volume of a single dose of epidural bupivacaine in parturients during the first stage of labor. Reg Anesth Pain Med. 1998;23:134–141.
54. Lyons GR, Kocarev MG, Wilson RC, Columb MO. A comparison of minimum local anesthetic volumes and doses of epidural bupivacaine (0.125% w/v and 0.25% w/v) for analgesia in labor. Anesth Analg. 2007;104:412–415.
55. Ginosar Y, Davidson EM, Firman N, et al. A randomized controlled trial using patient-controlled epidural analgesia with 0.25% versus 0.0625% bupivacaine in nulliparous labor: effect on analgesia requirement and maternal satisfaction. Int J Obstet Anesth. 2010;19:171–178.
56. Katz JA, Bridenbaugh PO, Knarr DC, et al. Pharmacodynamics and pharmacokinetics of epidural ropivacaine in humans. Anesth Analg. 1990;70:16–21.
57. Santos AC, Arthur GR, Roberts DJ, et al. Effect of ropivacaine and bupivacaine on uterine blood flow in pregnant ewes. Anesth Analg. 1992;74:62–67.
58. Santos AC, Arthur GR, Pedersen H, et al. Systemic toxicity of ropivacaine during ovine pregnancy. Anesthesiology. 1991;75:137–141.
59. Moller R, Covino BG. Cardiac electrophysiologic properties of bupivacaine and lidocaine compared with those of ropivacaine, a new amide local anesthetic. Anesthesiology. 1990;72:322–329.
60. Santos AC, Arthur GR, Wlody D, et al. Comparative systemic toxicity of ropivacaine and bupivacaine in nonpregnant and pregnant ewes. Anesthesiology. 1995;82:734–740.
61. Polley LS, Columb MO, Naughton NN, et al. Relative analgesic potencies of ropivacaine and bupivacaine for epidural analgesia in labor: implications for therapeutic indexes. Anesthesiology. 1999;90:944–950.
62. Capogna G, Celleno D, Fusco P, et al. Relative potencies of bupivacaine and ropivacaine for analgesia in labour. Br J Anaesth. 1999;82:371–373.
63. Ngan Kee WD, Ng FF, Khaw KS, et al. Determination and comparison of graded dose-response curves for epidural bupivacaine and ropivacaine for analgesia in laboring nulliparous women. Anesthesiology. 2010;113:445–453.
64. Owen MD, D’Angelo R, Gerancher JC, et al. 0.125% ropivacaine is similar to 0.125% bupivacaine for labor analgesia using patient-controlled epidural infusion. Anesth Analg. 1998;86:527–531.
65. Owen MD, Thomas JA, Smith T, et al. Ropivacaine 0.075% and bupivacaine 0.075% with fentanyl 2 µg/mL are equivalent for labor epidural analgesia. Anesth Analg. 2002;94:179–183.
66. Beilin Y, Guinn NR, Bernstein HH, et al. Local anesthetics and mode of delivery: bupivacaine versus ropivacaine versus levobupivacaine. Anesth Analg. 2007;105:756–763.
67. Meister GC, D’Angelo R, Owen M, et al. A comparison of epidural analgesia with 0.125% ropivacaine with fentanyl versus 0.125% bupivacaine with fentanyl during labor. Anesth Analg. 2000;90:632–637.
68. Chua NP, Sia AT, Ocampo CE. Parturient-controlled epidural analgesia during labour: bupivacaine vs. ropivacaine. Anaesthesia. 2001;56:1169–1173.
69. Parpaglioni R, Capogna G, Celleno D. A comparison between low-dose ropivacaine and bupivacaine at equianalgesic concentrations for epidural analgesia during the first stage of labor. Int J Obstet Anesth. 2000;9:83–86.
70. Fernandez-Guisasola J, Serrano ML, Cobo B, et al. A comparison of 0.0625% bupivacaine with fentanyl and 0.1% ropivacaine with fentanyl for continuous epidural labor analgesia. Anesth Analg. 2001;92:1261–1265.
71. Brockway MS, Bannister J, McClure JH, et al. Comparison of extradural ropivacaine and bupivacaine. Br J Anaesth. 1991;66:31–37.
72. Griffin RP, Reynolds F. Extradural anaesthesia for caesarean section: a double-blind comparison of 0.5% ropivacaine with 0.5% bupivacaine. Br J Anaesth. 1995;74:512–516.
73. Lacassie HJ, Habib AS, Lacassie HP, Columb MO. Motor blocking minimum local anesthetic concentrations of bupivacaine, levobupivacaine, and ropivacaine in labor. Reg Anesth Pain Med. 2007;32:323–329.
74. Halpern SH, Breen TW, Campbell DC, et al. A multicenter, randomized, controlled trial comparing bupivacaine with ropivacaine for labor analgesia. Anesthesiology. 2003;98:1431–1435.
75. Halpern SH, Walsh V. Epidural ropivacaine versus bupivacaine for labor: a meta-analysis. Anesth Analg. 2003;96:1473–1479.
76. Beilin Y, Halpern S. Focused review: ropivacaine versus bupivacaine for epidural labor analgesia. Anesth Analg. 2010;111:482–487.
77. Yoshida M, Matsuda H, Fukuda I, Furuya K. Sudden cardiac arrest during cesarean section due to epidural anaesthesia using ropivacaine: a case report. Arch Gynecol Obstet. 2008;277:91–94.
78. Bardsley H, Gristwood R, Baker H, et al. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1998;46:245–249.
79. Vanhoutte F, Vereecke J, Verbeke N, Carmeliet E. Stereoselective effects of the enantiomers of bupivacaine on the electrophysiological properties of the guinea-pig papillary muscle. Br J Pharmacol. 1991;103:1275–1281.
80. Lyons G, Columb M, Wilson RC, Johnson RV. Epidural pain relief in labour: potencies of levobupivacaine and racemic bupivacaine. Br J Anaesth. 1998;81:899–901.
81. Polley LS, Columb MO, Naughton NN, et al. Relative analgesic potencies of levobupivacaine and ropivacaine for epidural analgesia in labor. Anesthesiology. 2003;99:1354–1358.
82. Benhamou D, Ghosh C, Mercier FJ. A randomized sequential allocation study to determine the minimum effective analgesic concentration of levobupivacaine and ropivacaine in patients receiving epidural analgesia for labor. Anesthesiology. 2003;99:1383–1386.
83. Lacassie HJ, Columb MO. The relative motor blocking potencies of bupivacaine and levobupivacaine in labor. Anesth Analg. 2003;97:1509–1513.
84. Kennedy RL, Bell JU, Miller RP, et al. Uptake and distribution of lidocaine in fetal lambs. Anesthesiology. 1990;72:483–489.
85. Scanlon JW, Brown WU Jr, Weiss JB, Alper MH. Neurobehavioral responses of newborn infants after maternal epidural anesthesia. Anesthesiology. 1974;40:121–128.
86. Abboud TK, Afrasiabi A, Sarkis F, et al. Continuous infusion epidural analgesia in parturients receiving bupivacaine, chloroprocaine, or lidocaine—maternal, fetal, and neonatal effects. Anesth Analg. 1984;63:421–428.
87. Kuhnert BR, Harrison MJ, Linn PL, Kuhnert PM. Effects of maternal epidural anesthesia on neonatal behavior. Anesth Analg. 1984;63:301–308.
88. Grice SC, Eisenach JC, Dewan DM. Labor analgesia with epidural bupivacaine plus fentanyl: enhancement with epinephrine and inhibition with 2-chloroprocaine. Anesthesiology. 1990;72:623–628.
89. Corke BC, Carlson CG, Dettbarn WD. The influence of 2-chloroprocaine on the subsequent analgesic potency of bupivacaine. Anesthesiology. 1984;60:25–27.
90. Hess PE, Snowman CE, Hahn CJ, et al. Chloroprocaine may not affect epidural morphine for postcesarean delivery analgesia. J Clin Anesth. 2006;18:29–33.
91. Marcus RJ, Wong CA, Lehor A, et al. Postoperative epidural morphine for postpartum tubal ligation analgesia. Anesth Analg. 2005;101:876–881.
92. Vella LM, Willatts DG, Knott C, et al. Epidural fentanyl in labour: an evaluation of the systemic contribution to analgesia. Anaesthesia. 1985;40:741–747.
93. D’Angelo R, Gerancher JC, Eisenach JC, Raphael BL. Epidural fentanyl produces labor analgesia by a spinal mechanism. Anesthesiology. 1998;88:1519–1523.
94. Polley LS, Columb MO, Naughton NN, et al. Effect of intravenous versus epidural fentanyl on the minimum local analgesic concentration of epidural bupivacaine in labor. Anesthesiology. 2000;93:122–128.
95. Ginosar Y, Columb MO, Cohen SE, et al. The site of action of epidural fentanyl infusions in the presence of local anesthetics: a minimum local analgesic concentration infusion study in nulliparous labor. Anesth Analg. 2003;97:1439–1445.
96. Ginosar Y, Riley ET, Angst MS. The site of action of epidural fentanyl in humans: the difference between infusion and bolus administration. Anesth Analg. 2003;97:1428–1438.
97. Steinberg RB, Dunn SM, Dixon DE, et al. Comparison of sufentanil, bupivacaine, and their combination for epidural analgesia in obstetrics. Reg Anesth. 1992;17:131–138.
98. Chestnut DH, Owen CL, Bates JN, et al. Continuous infusion epidural analgesia during labor: a randomized, double-blind comparison of 0.0625% bupivacaine/0.0002% fentanyl versus 0.125% bupivacaine. Anesthesiology. 1988;68:754–759.
99. Lyons G, Columb M, Hawthorne L, Dresner M. Extradural pain relief in labour: bupivacaine sparing by extradural fentanyl is dose dependent. Br J Anaesth. 1997;78:493–497.
100. Robinson AP, Lyons GR, Wilson RC, et al. Levobupivacaine for epidural analgesia in labor: the sparing effect of epidural fentanyl. Anesth Analg. 2001;92:410–414.
101. Buyse I, Stockman W, Columb M, et al. Effect of sufentanil on minimum local analgesic concentrations of epidural bupivacaine, ropivacaine and levobupivacaine in nullipara in early labour. Int J Obstet Anesth. 2007;16:22–28.
102. Palm S, Gertzen W, Ledowski T, et al. Minimum local analgesic dose of plain ropivacaine vs. ropivacaine combined with sufentanil during epidural analgesia for labour. Anaesthesia. 2001;56:526–529.
103. Polley LS, Columb MO, Lyons G, Nair SA. The effect of epidural fentanyl on the minimum local analgesic concentration of epidural chloroprocaine in labor. Anesth Analg. 1996;83:987–990.
104. Reynolds F, O'Sullivan G. Epidural fentanyl and perineal pain in labour. Anaesthesia. 1989;44:341–344.
105. van Steenberge A, Debroux HC, Noorduin H. Extradural bupivacaine with sufentanil for vaginal delivery: a double-blind trial. Br J Anaesth. 1987;59:1518–1522.
106. Celleno D, Capogna G. Epidural fentanyl plus bupivacaine 0.125 per cent for labour: analgesic effects. Can J Anaesth. 1988;35:375–378.
107. Vertommen JD, Vandermeulen E, Van Aken H, et al. The effects of the addition of sufentanil to 0.125% bupivacaine on the quality of analgesia during labor and on the incidence of instrumental deliveries. Anesthesiology. 1991;74:809–814.
108. Connelly NR, Parker RK, Vallurupalli V, et al. Comparison of epidural fentanyl versus epidural sufentanil for analgesia in ambulatory patients in early labor. Anesth Analg. 2000;91:374–378.
109. Loftus JR, Hill H, Cohen SE. Placental transfer and neonatal effects of epidural sufentanil and fentanyl administered with bupivacaine during labor. Anesthesiology. 1995;83:300–308.
110. Herman NL, Sheu KL, Van Decar TK, et al. Determination of the analgesic dose-response relationship for epidural fentanyl and sufentanil with bupivacaine 0.125% in laboring patients. J Clin Anesth. 1998;10:670–677.
111. Capogna G, Camorcia M, Columb MO. Minimum analgesic doses of fentanyl and sufentanil for epidural analgesia in the first stage of labor. Anesth Analg. 2003;96:1178–1182.
112. Coda BA, Brown MC, Schaffer R, et al. Pharmacology of epidural fentanyl, alfentanil, and sufentanil in volunteers. Anesthesiology. 1994;81:1149–1161.
113. Yau G, Gregory MA, Gin T, et al. The addition of fentanyl to epidural bupivacaine in first stage labour. Anaesth Intensive Care. 1990;18:532–535.
114. Steinberg RB, Powell G, Hu XH, Dunn SM. Epidural sufentanil for analgesia for labor and delivery. Reg Anesth. 1989;14:225–228.
115. Capogna G, Parpaglioni R, Lyons G, et al. Minimum analgesic dose of epidural sufentanil for first-stage labor analgesia: a comparison between spontaneous and prostaglandin-induced labors in nulliparous women. Anesthesiology. 2001;94:740–744.
116. Conell-Price J, Evans JB, Hong D, et al. The development and validation of a dynamic model to account for the progress of labor in the assessment of pain. Anesth Analg. 2008;106:1509–1515.
117. Camorcia M, Capogna G, Stirparo S, et al. Effect of mu-opioid receptor A118G polymorphism on the ED50 of epidural sufentanil for labor analgesia. Int J Obstet Anesth. 2012;21:40–44.
118. Chassard D, Duflo F, de Queiroz Siqueira M, et al. Chronobiology and anaesthesia. Curr Opin Anaesthesiol. 2007;20:186–190.
119. Scavone BM, McCarthy RJ, Wong CA, Sullivan JT. The influence of time of day of administration on duration of opioid labor analgesia. Anesth Analg. 2010;111:986–991.
120. Shafer SL, Lemmer B, Boselli E, et al. Pitfalls in chronobiology: a suggested analysis using intrathecal bupivacaine analgesia as an example. Anesth Analg. 2010;111:980–985.
121. Connelly NR, Parker RK, Pedersen T, et al. Diluent volume for epidural fentanyl and its effect on analgesia in early labor. Anesth Analg. 2003;96:1799–1804.
122. Lirzin JD, Jacquinot P, Jorrot JC, et al. Effect of diluting fentanyl on epidural bupivacaine during labor analgesia. Reg Anesth. 1989;14:279–281.
123. Hughes SC, Rosen MA, Shnider SM, et al. Maternal and neonatal effects of epidural morphine for labor and delivery. Anesth Analg. 1984;63:319–324.
124. Abboud TK, Afrasiabi A, Zhu J, et al. Epidural morphine or butorphanol augments bupivacaine analgesia during labor. Reg Anesth. 1989;14:115–120.
125. Hill DA, McCarthy G, Bali IM. Epidural infusion of alfentanil or diamorphine with bupivacaine in labour—a dose finding study. Anaesthesia. 1995;50:415–419.
126. Cooper RA, Devlin E, Boyd TH, Bali IM. Epidural analgesia for labour using a continuous infusion of bupivacaine and alfentanil. Eur J Anaesthesiol. 1993;10:183–187.
127. Sinatra RS, Eige S, Chung JH, et al. Continuous epidural infusion of 0.05% bupivacaine plus hydromorphone for labor analgesia: an observational assessment in 1830 parturients. Anesth Analg. 2002;94:1310–1311.
128. Parker RK, Connelly NR, Lucas T, et al. The addition of hydromorphone to epidural fentanyl does not affect analgesia in early labour. Can J Anaesth. 2002;49:600–604.
129. Mhyre JM. Strategies to induce labor analgesia with epidural hydromorphone. Int J Obstet Anesth. 2008;17:81–82.
130. Liu S, Carpenter R. Lipid solubility and epidural opioid efficacy. Anesthesiology. 1995;83:427–428.
131. Jaffe RA, Rowe MA. A comparison of the local anesthetic effects of meperidine, fentanyl, and sufentanil on dorsal root axons. Anesth Analg. 1996;83:776–781.
132. Handley G, Perkins G. The addition of pethidine to epidural bupivacaine in labour—effect of changing bupivacaine strength. Anaesth Intensive Care. 1992;20:151–155.
133. Brownridge P. Shivering related to epidural blockade with bupivacaine in labour, and the influence of epidural pethidine. Anaesth Intensive Care. 1986;14:412–417.
134. Massad IM, Khadra MM, Alkazaleh FA, et al. Bupivacaine with meperidine versus bupivacaine with fentanyl for continuous epidural labor analgesia. Saudi Med J. 2007;28:904–908.
135. Hunt CO, Naulty JS, Malinow AM, et al. Epidural butorphanol-bupivacaine for analgesia during labor and delivery. Anesth Analg. 1989;68:323–327.
136. Hatjis CG, Meis PJ. Sinusoidal fetal heart rate pattern associated with butorphanol administration. Obstet Gynecol. 1986;67:377–380.
137. McLeod GA, Munishankar B, Columb MO. An isobolographic analysis of diamorphine and levobupivacaine for epidural analgesia in early labour. Br J Anaesth. 2007;98:497–502.
138. Lowson SM, Eggers KA, Warwick JP, et al. Epidural infusions of bupivacaine and diamorphine in labour. Anaesthesia. 1995;50:420–422.
139. Abboud TK, Sheik-ol-Eslam A, Yanagi T, et al. Safety of efficacy of epinephrine added to bupivacaine for lumbar epidural analgesia in obstetrics. Anesth Analg. 1985;64:585–591.
140. Polley LS, Columb MO, Naughton NN, et al. Effect of epidural epinephrine on the minimum local analgesic concentration of epidural bupivacaine in labor. Anesthesiology. 2002;96:1123–1128.
141. Abboud TK, David S, Nagappala S, et al. Maternal, fetal, and neonatal effects of lidocaine with and without epinephrine for epidural anesthesia in obstetrics. Anesth Analg. 1984;63:973–979.
142. Reynolds F, Taylor G. Plasma concentrations of bupivacaine during continuous epidural analgesia in labour: the effect of adrenaline. Br J Anaesth. 1971;43:436–440.
143. Reynolds F, Laishley R, Morgan B, Lee A. Effect of time and adrenaline on the feto-maternal distribution of bupivacaine. Br J Anaesth. 1989;62:509–514.
144. Craft JB Jr, Epstein BS, Coakley CS. Effect of lidocaine with epinephrine versus lidocaine (plain) on induced labor. Anesth Analg. 1972;51:243–246.
145. Matadial L, Cibils LA. The effect of epidural anesthesia on uterine activity and blood pressure. Am J Obstet Gynecol. 1976;125:846–854.
146. Yarnell RW, Ewing DA, Tierney E, Smith MH. Sacralization of epidural block with repeated doses of 0.25% bupivacaine during labor. Reg Anesth. 1990;15:275–279.
147. Soetens FM, Soetens MA, Vercauteren MP. Levobupivacaine-sufentanil with or without epinephrine during epidural labor analgesia. Anesth Analg. 2006;103:182–186.
148. Albright GA, Jouppila R, Hollmen AI, et al. Epinephrine does not alter human intervillous blood flow during epidural anesthesia. Anesthesiology. 1981;54:131–135.
149. Skjöldebrand A, Garle M, Gustafsson LL, et al. Extradural pethidine with and without adrenaline during labour: wide variation in effect. Br J Anaesth. 1982;54:415–420.
150. Landau R, Schiffer E, Morales M, et al. The dose-sparing effect of clonidine added to ropivacaine for labor epidural analgesia. Anesth Analg. 2002;95:728–734.
151. Tremlett MR, Kelly PJ, Parkins J, et al. Low-dose clonidine infusion during labour. Br J Anaesth. 1999;83:257–261.
152. Dewandre PY, Kirsch M, Bonhomme V, et al. Impact of the addition of sufentanil 5 µg or clonidine 75 µg on the minimum local analgesic concentration of ropivacaine for epidural analgesia in labour: a randomized comparison. Int J Obstet Anesth. 2008;17:315–321.
153. Dewandre PY, Decurninge V, Bonhomme V, et al. Side effects of the addition of clonidine 75 µg or sufentanil 5 µg to 0.2% ropivacaine for labour epidural analgesia. Int J Obstet Anesth. 2010;19:149–154.
154. Chassard D, Mathon L, Dailler F, et al. Extradural clonidine combined with sufentanil and 0.0625% bupivacaine for analgesia in labour. Br J Anaesth. 1996;77:458–462.
155. Paech MJ, Pavy TJ, Orlikowski CE, Evans SF. Patient-controlled epidural analgesia in labor: the addition of clonidine to bupivacaine-fentanyl. Reg Anesth Pain Med. 2000;25:34–40.
156. Claes B, Soetens M, Van Zundert A, Datta S. Clonidine added to bupivacaine-epinephrine-sufentanil improves epidural analgesia during childbirth. Reg Anesth Pain Med. 1998;23:540–547.
157. Wallet F, Clement HJ, Bouret C, et al. Effects of a continuous low-dose clonidine epidural regimen on pain, satisfaction and adverse events during labour: a randomized, double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27:441–447.
158. Bazin M, Bonnin M, Storme B, et al. Addition of clonidine to a continuous patient-controlled epidural infusion of low-concentration levobupivacaine plus sufentanil in primiparous women during labour. Anaesthesia. 2011;66:769–779.
159. Buggy DJ, MacDowell C. Extradural analgesia with clonidine and fentanyl compared with 0.25% bupivacaine in the first stage of labour. Br J Anaesth. 1996;76:319–321.
160. Roelants F, Lavand’homme PM, Mercier-Fuzier V. Epidural administration of neostigmine and clonidine to induce labor analgesia: evaluation of efficacy and local anesthetic-sparing effect. Anesthesiology. 2005;102:1205–1210.
161. Van de Velde M, Berends N, Kumar A, et al. Effects of epidural clonidine and neostigmine following intrathecal labour analgesia: a randomised, double-blind, placebo-controlled trial. Int J Obstet Anesth. 2009;18:207–214.
162. Aveline C, El Metaoua S, Masmoudi A, et al. The effect of clonidine on the minimum local analgesic concentration of epidural ropivacaine during labor. Anesth Analg. 2002;95:735–740.
163. Roelants F, Rizzo M, Lavand’homme P. The effect of epidural neostigmine combined with ropivacaine and sufentanil on neuraxial analgesia during labor. Anesth Analg. 2003;96:1161–1166.
164. Roelants F, Lavand’homme PM. Epidural neostigmine combined with sufentanil provides balanced and selective analgesia in early labor. Anesthesiology. 2004;101:439–444.
165. Naguib M, Yaksh TL. Antinociceptive effects of spinal cholinesterase inhibition and isobolographic analysis of the interaction with mu and alpha 2 receptor systems. Anesthesiology. 1994;80:1338–1348.
166. Ross VH, Pan PH, Owen MD, et al. Neostigmine decreases bupivacaine use by patient controlled epidural analgesia during labor: a randomized controlled study. Anesth Analg. 2009;109:524–531.
167. Paech M, Pan P. New recipes for neuraxial labor analgesia: simple fare or gourmet combos? Int J Obstet Anesth. 2009;18:201–203.
168. Wong CA, Scavone BM, Loffredi M, et al. The dose-response of intrathecal sufentanil added to bupivacaine for labor analgesia. Anesthesiology. 2000;92:1553–1558.
169. Wong CA, Scavone BM, Slavenas JP, et al. Efficacy and side effect profile of varying doses of intrathecal fentanyl added to bupivacaine for labor analgesia. Int J Obstet Anesth. 2004;13:19–24.
170. Saito Y, Kaneko M, Kirihara Y, et al. Interaction of intrathecally infused morphine and lidocaine in rats (part I): synergistic antinociceptive effects. Anesthesiology. 1998;89:1455–1463.
171. Penning JP, Yaksh TL. Interaction of intrathecal morphine with bupivacaine and lidocaine in the rat. Anesthesiology. 1992;77:1186–2000.
172. Campbell DC, Camann WR, Datta S. The addition of bupivacaine to intrathecal sufentanil for labor analgesia. Anesth Analg. 1995;81:305–309.
173. Stocks GM, Hallworth SP, Fernando R, et al. Minimum local analgesic dose of intrathecal bupivacaine in labor and the effect of intrathecal fentanyl. Anesthesiology. 2001;94:593–598.
174. Sia AT, Chong JL, Chiu JW. Combination of intrathecal sufentanil 10 µg plus bupivacaine 2.5 mg for labor analgesia: is half the dose enough? Anesth Analg. 1999;88:362–366.
175. Palmer CM, Cork RC, Hays R, et al. The dose-response relation of intrathecal fentanyl for labor analgesia. Anesthesiology. 1998;88:355–361.
176. Nelson KE, Rauch T, Terebuh V, D’Angelo R. A comparison of intrathecal fentanyl and sufentanil for labor analgesia. Anesthesiology. 2002;96:1070–1073.
177. Herman NL, Choi KC, Affleck PJ, et al. Analgesia, pruritus, and ventilation exhibit a dose-response relationship in parturients receiving intrathecal fentanyl during labor. Anesth Analg. 1999;89:378–383.
178. Nelson KE, Houle TT, Eisenach JC. Blood pressure, but not cerebrospinal fluid fentanyl concentration, predicts duration of labor analgesia from spinal fentanyl. Anesthesiology. 2010;112:174–180.
179. Herman NL, Calicott R, Van Decar TK, et al. Determination of the dose-response relationship for intrathecal sufentanil in laboring patients. Anesth Analg. 1997;84:1256–1261.
180. Nelson KE, D’Angelo R, Foss ML, et al. Intrathecal neostigmine and sufentanil for early labor analgesia. Anesthesiology. 1999;91:1293–1298.
181. Arkoosh VA, Cooper M, Norris MC, et al. Intrathecal sufentanil dose response in nulliparous patients. Anesthesiology. 1998;89:364–370.
182. Camann W, Abouleish A, Eisenach J, et al. Intrathecal sufentanil and epidural bupivacaine for labor analgesia: dose-response of individual agents and in combination. Reg Anesth Pain Med. 1998;23:457–462.
183. Landau R, Kern C, Columb MO, et al. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14.
184. Wong CA, McCarthy RJ, Blouin J, Landau R. Observational study of the effect of mu-opioid receptor genetic polymorphism on intrathecal opioid labor analgesia and post-cesarean delivery analgesia. Int J Obstet Anesth. 2010;19:246–253.
185. Wong CA. The promise of pharmacogenetics in labor analgesia…tantalizing, but not there yet. Int J Obstet Anesth. 2012;21:105–108.
186. Van de Velde M, Dreelinck R, Dubois J, et al. Determination of the full dose-response relation of intrathecal bupivacaine, levobupivacaine, and ropivacaine, combined with sufentanil, for labor analgesia. Anesthesiology. 2007;106:149–156.
187. Baraka A, Noueihid R, Hajj S. Intrathecal injection of morphine for obstetric analgesia. Anesthesiology. 1981;54:136–140.
188. Abboud TK, Shnider SM, Dailey PA, et al. Intrathecal administration of hyperbaric morphine for the relief of pain in labour. Br J Anaesth. 1984;56:1351–1360.
189. Abouleish E. Apnoea associated with the intrathecal administration of morphine in obstetrics: a case report. Br J Anaesth. 1988;60:592–594.
190. Vasudevan A, Snowman CE, Sundar S, et al. Intrathecal morphine reduces breakthrough pain during labour epidural analgesia. Br J Anaesth. 2007;98:241–245.
191. Hess PE, Vasudevan A, Snowman C, Pratt SD. Small dose bupivacaine-fentanyl spinal analgesia combined with morphine for labor. Anesth Analg. 2003;97:247–252.
192. Yeh HM, Chen LK, Shyu MK, et al. The addition of morphine prolongs fentanyl-bupivacaine spinal analgesia for the relief of labor pain. Anesth Analg. 2001;92:665–668.
193. Hein A, Rosblad P, Norman M, et al. Addition of low-dose morphine to intrathecal bupivacaine/sufentanil labour analgesia: a randomised controlled study. Int J Obstet Anesth. 2010;19:384–389.
194. Kafle SK. Intrathecal meperidine for elective caesarean section: a comparison with lidocaine. Can J Anaesth. 1993;40:718–721.
195. Honet JE, Arkoosh VA, Norris MC, et al. Comparison among intrathecal fentanyl, meperidine, and sufentanil for labor analgesia. Anesth Analg. 1992;75:734–739.
196. Booth JV, Lindsay DR, Olufolabi AJ, et al. Subarachnoid meperidine (Pethidine) causes significant nausea and vomiting during labor. Anesthesiology. 2000;93:418–421.
197. Kestin IG, Madden AP, Mulvein JT, Goodman NW. Analgesia for labour and delivery using incremental diamorphine and bupivacaine via a 32-gauge intrathecal catheter. Br J Anaesth. 1992;68:244–247.
198. Vaughan DJ, Ahmad N, Lillywhite NK, et al. Choice of opioid for initiation of combined spinal epidural analgesia in labour—fentanyl or diamorphine. Br J Anaesth. 2001;86:567–569.
199. Asokumar B, Newman LM, McCarthy RJ, et al. Intrathecal bupivacaine reduces pruritus and prolongs duration of fentanyl analgesia during labor: a prospective, randomized controlled trial. Anesth Analg. 1998;87:1309–1315.
200. Whitty R, Goldszmidt E, Parkes RK, Carvalho JC. Determination of the ED95 for intrathecal plain bupivacaine combined with fentanyl in active labor. Int J Obstet Anesth. 2007;16:341–345.
201. Camorcia M, Capogna G, Columb MO. Minimum local analgesic doses of ropivacaine, levobupivacaine, and bupivacaine for intrathecal labor analgesia. Anesthesiology. 2005;102:646–650.
202. Richardson MG, Wissler RN. Densities of dextrose-free intrathecal local anesthetics, opioids, and combinations measured at 37 degrees C. Anesth Analg. 1997;84:95–99.
203. Richardson MG, Thakur R, Abramowicz JS, Wissler RN. Maternal posture influences the extent of sensory block produced by intrathecal dextrose-free bupivacaine with fentanyl for labor analgesia. Anesth Analg. 1996;83:1229–1233.
204. Ferouz F, Norris MC, Arkoosh VA, et al. Baricity, needle direction, and intrathecal sufentanil labor analgesia. Anesthesiology. 1997;86:592–598.
205. Gage JC, D’Angelo R, Miller R, Eisenach JC. Does dextrose affect analgesia or the side effects of intrathecal sufentanil? Anesth Analg. 1997;85:826–830.
206. Rofaeel A, Lilker S, Fallah S, et al. Intrathecal plain vs hyperbaric bupivacaine for labour analgesia: efficacy and side effects. Can J Anaesth. 2007;54:15–20.
207. Gautier PE, De Kock M, Fanard L, et al. Intrathecal clonidine combined with sufentanil for labor analgesia. Anesthesiology. 1998;88:651–656.
208. Mercier FJ, Dounas M, Bouaziz H, et al. The effect of adding a minidose of clonidine to intrathecal sufentanil for labor analgesia. Anesthesiology. 1998;89:594–601.
209. Missant C, Teunkens A, Vandermeersch E, Van de Velde M. Intrathecal clonidine prolongs labour analgesia but worsens fetal outcome: a pilot study. Can J Anaesth. 2004;51:696–701.
210. Sia AT. Optimal dose of intrathecal clonidine added to sufentanil plus bupivacaine for labour analgesia. Can J Anaesth. 2000;47:875–880.
211. D’Angelo R, Dean LS, Meister GC, Nelson KE. Neostigmine combined with bupivacaine, clonidine, and sufentanil for spinal labor analgesia. Anesth Analg. 2001;93:1560–1564.
212. Chiari A, Lorber C, Eisenach JC, et al. Analgesic and hemodynamic effects of intrathecal clonidine as the sole analgesic agent during first stage of labor: a dose-response study. Anesthesiology. 1999;91:388–396.
213. Owen MD, Ozsarac O, Sahin S, et al. Low-dose clonidine and neostigmine prolong the duration of intrathecal bupivacaine-fentanyl for labor analgesia. Anesthesiology. 2000;92:361–366.
214. Campbell DC, Banner R, Crone LA, et al. Addition of epinephrine to intrathecal bupivacaine and sufentanil for ambulatory labor analgesia.
215. Vercauteren MP, Jacobs S, Jacquemyn Y, Adriaensen HA. Intrathecal labor analgesia with bupivacaine and sufentanil: the effect of adding epinephrine 2.25 µg to epinephrine. Reg Anesth Pain Med. 2001;26:473–477.
216. Gurbet A, Turker G, Kose DO, Uckunkaya N. Intrathecal epinephrine in combined spinal-epidural analgesia for labor: dose-response relationship for epinephrine added to a local anesthetic-opioid combination. Int J Obstet Anesth. 2005;14:121–125.
217. Palmer SK, Bosnjak ZJ, Hopp FA, et al. Lidocaine and bupivacaine differential blockade of isolated canine nerves. Anesth Analg. 1983;62:754–757.
218. Hess PE, Pratt SD, Oriol NE. An analysis of the need for anesthetic interventions with differing concentrations of labor epidural bupivacaine: an observational study. Int J Obstet Anesth. 2006;15:195–200.
219. Beilin Y, Nair A, Arnold I, et al. A comparison of epidural infusions in the combined spinal/epidural technique for labor analgesia. Anesth Analg. 2002;94:927–932.
220. Bader AM, Fragneto R, Terui K, et al. Maternal and neonatal fentanyl and bupivacaine concentrations after epidural infusion during labor. Anesth Analg. 1995;81:829–832.
221. Porter J, Bonello E, Reynolds F. Effect of epidural fentanyl on neonatal respiration. Anesthesiology. 1998;89:79–85.
222. Bernard JM, Le Roux D, Barthe A, et al. The dose-range effects of sufentanil added to 0.125% bupivacaine on the quality of patient-controlled epidural analgesia during labor. Anesth Analg. 2001;92:184–188.
223. Association of Women's Health Obstetric and Neonatal Nurses. Role of the registered nurse in the care of the pregnant woman receiving analgesia and anesthesia by catheter techniques. J Obstet Gynecol Neonatal Nurs. 2012;41:455–457.
224. Lamont RF, Pinney D, Rodgers P, Bryant TN. Continuous versus intermittent epidural analgesia. Anaesthesia. 1989;44:893–896.
225. Bogod DG, Rosen M, Rees GA. Extradural infusion of 0.125% bupivacaine at 10 mL/hr to women during labor. Br J Anaesth. 1987;59:325–330.
226. Li DF, Rees GAD, Rosen M. Continuous extradural infusion of 0.0625% or 0.125% bupivacaine for pain relief in primigravid labour. Br J Anaesth. 1985;57:264–270.
227. Hicks JA, Jenkins JG, Newton MC, Findley IL. Continuous epidural infusion of 0.075% bupivacaine for pain relief in labour. Anaesthesia. 1988;43:289–292.
228. Smedstad KG, Morison DH. A comparative study of continuous and intermittent epidural analgesia for labour and delivery. Can J Anaesth. 1988;35:234–241.
229. Boutros A, Blary S, Bronchard R, Bonnet F. Comparison of intermittent epidural bolus, continuous epidural infusion and patient controlled-epidural analgesia during labor. Int J Obstet Anesth. 1999;8:236–241.
230. Gambling DR, Yu P, Cole C, et al. A comparative study of patient controlled epidural analgesia (PCEA) and continuous infusion epidural analgesia (CIEA) during labour. Can J Anaesth. 1988;35:249–254.
231. Curry PD, Pacsoo C, Heap DG. Patient-controlled epidural analgesia in obstetric anaesthetic practice. Pain. 1994;57:125–128.
232. Collis RE, Plaat FS, Morgan BM. Comparison of midwife top-ups, continuous infusion and patient-controlled epidural analgesia for maintaining mobility after a low-dose combined spinal-epidural. Br J Anaesth. 1999;82:233–236.
233. Purdie J, Reid J, Thorburn J, Asbury AJ. Continuous extradural analgesia: comparisons of midwife top-ups, continuous infusions and patient controlled administration. Br J Anaesth. 1992;68:580–584.
234. Tan S, Reid J, Thorburn J. Extradural analgesia in labour: complications of three techniques of administration. Br J Anaesth. 1994;73:619–623.
235. Gambling DR, Huber CJ, Berkowitz J, et al. Patient-controlled epidural analgesia in labour: varying bolus dose and lockout interval. Can J Anaesth. 1993;40:211–217.
236. Ferrante FM, Lu L, Jamison SB, Datta S. Patient-controlled epidural analgesia: demand dosing. Anesth Analg. 1991;73:547–552.
237. Ferrante FM, Rosinia FA, Gordon C, Datta S. The role of continuous background infusions in patient-controlled epidural analgesia for labor and delivery. Anesth Analg. 1994;79:80–84.
238. van der Vyver M, Halpern S, Joseph G. Patient-controlled epidural analgesia versus continuous infusion for labour analgesia: a meta-analysis. Br J Anaesth. 2002;89:459–465.
239. Paech MJ. Patient-controlled epidural analgesia in labour—is a continuous infusion of benefit? Anaesth Intensive Care. 1992;20:15–20.
240. Petry J, Vercauteren M, Van Mol I, et al. Epidural PCA with bupivacaine 0.125%, sufentanil 0.75 µg and epinephrine 1/800.000 for labor analgesia: is a background infusion beneficial? Acta Anaesthesiol Belg. 2000;51:163–166.
241. Boselli E, Debon R, Cimino Y, et al. Background infusion is not beneficial during labor patient-controlled analgesia with 0.1% ropivacaine plus 0.5 µg/mL sufentanil. Anesthesiology. 2004;100:968–972.
242. Vallejo MC, Ramesh V, Phelps AL, Sah N. Epidural labor analgesia: continuous infusion versus patient-controlled epidural analgesia with background infusion versus without a background infusion. J Pain. 2007;8:970–975.
243. Missant C, Teunkenst A, Vandermeersch E, Van de Velde M. Patient-controlled epidural analgesia following combined spinal-epidural analgesia in labour: the effects of adding a continuous epidural infusion. Anaesth Intensive Care. 2005;33:452–456.
244. Bremerich DH, Waibel HJ, Mierdl S, et al. Comparison of continuous background infusion plus demand dose and demand-only parturient-controlled epidural analgesia (PCEA) using ropivacaine combined with sufentanil for labor and delivery. Int J Obstet Anesth. 2005;14:114–120.
245. Lim Y, Ocampo CE, Supandji M, et al. A randomized controlled trial of three patient-controlled epidural analgesia regimens for labor. Anesth Analg. 2008;107:1968–1972.
246. Brogly N, Schiraldi R, Vazquez B, et al. A randomized control trial of patient-controlled epidural analgesia (PCEA) with and without a background infusion using levobupivacaine and fentanyl. Minerva Anestesiol. 2011;77:1149–1154.
247. Okutomi T, Saito M, Mochizuki J, et al. A double-blind randomized controlled trial of patient-controlled epidural analgesia with or without a background infusion following initial spinal analgesia for labor pain. Int J Obstet Anesth. 2009;18:28–32.
248. Halpern SH, Carvalho B. Patient-controlled epidural analgesia for labor. Anesth Analg. 2009;108:921–928.
249. Sng BL, Sia AT, Lim Y, et al. Comparison of computer-integrated patient-controlled epidural analgesia and patient-controlled epidural analgesia with a basal infusion for labour and delivery. Anaesth Intensive Care. 2009;37:46–53.
250. Siddik-Sayyid SM, Aouad MT, Jalbout MI, et al. Comparison of three modes of patient-controlled epidural analgesia during labour. Eur J Anaesthesiol. 2005;22:30–34.
251. Stratmann G, Gambling DR, Moeller-Bertram T, et al. A randomized comparison of a five-minute versus fifteen-minute lockout interval for PCEA during labor. Int J Obstet Anesth. 2005;14:200–207.
252. Carvalho B, Cohen SE, Giarrusso K, et al. “Ultra-light” patient-controlled epidural analgesia during labor: effects of varying regimens on analgesia and physician workload. Int J Obstet Anesth. 2005;14:223–229.
253. Bernard JM, Le Roux D, Frouin J. Ropivacaine and fentanyl concentrations in patient-controlled epidural analgesia during labor: a volume-range study. Anesth Analg. 2003;97:1800–1807.
254. Gogarten W, Van de Velde M, Soetens F, et al. A multicentre trial comparing different concentrations of ropivacaine plus sufentanil with bupivacaine plus sufentanil for patient-controlled epidural analgesia in labour. Eur J Anaesthesiol. 2004;21:38–45.
255. Boselli E, Debon R, Duflo F, et al. Ropivacaine 0.15% plus sufentanil 0.5 µg/mL and ropivacaine 0.10% plus sufentanil 0.5 µg/mL are equivalent for patient-controlled epidural analgesia for labor. Anesth Analg. 2003;96:1173–1177.
256. Nikkola E, Laara A, Hinkka S, et al. Patient-controlled epidural analgesia in labor does not always improve maternal satisfaction. Acta Obstet Gynecol Scand. 2006;85:188–194.
257. Sia AT, Ruban P, Chong JL, Wong K. Motor blockade is reduced with ropivacaine 0.125% for parturient-controlled epidural analgesia during labour. Can J Anaesth. 1999;46:1019–1023.
258. Hogan Q. Distribution of solution in the epidural space: examination by cryomicrotome section. Reg Anesth Pain Med. 2002;27:150–156.
259. Wong CA, Ratliff JT, Sullivan JT, et al. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg. 2006;102:904–909.
260. Lim Y, Sia AT, Ocampo C. Automated regular boluses for epidural analgesia: a comparison with continuous infusion. Int J Obstet Anesth. 2005;14:305–309.
261. Chua SM, Sia AT. Automated intermittent epidural boluses improve analgesia induced by intrathecal fentanyl during labour. Can J Anaesth. 2004;51:581–585.
262. Fettes PD, Moore CS, Whiteside JB, et al. Intermittent vs continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. Br J Anaesth. 2006;97:359–364.
263. Sia AT, Lim Y, Ocampo C. A comparison of a basal infusion with automated mandatory boluses in parturient-controlled epidural analgesia during labor. Anesth Analg. 2007;104:673–678.
264. Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg. 2011;113:826–831.
265. George RB, Allen TK, Habib AS. Intermittent epidural bolus compared to continuous infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg. 2013;116:133–144.
266. Birnbach DJ, Vincent CA. A matter of conscience: a call to action for system improvements involving epidural and spinal catheters. Anesth Analg. 2012;114:494–496.
267. National Patient Safety Agency. Safer spinal (intrathecal), epidural and regional devices, Ref. Number 1310. [Available at] http://www.nrls.npsa.nhs.uk/resources/?EntryId45=94529 [Accessed December 2012] .
268. Palmer CM. Continuous spinal anesthesia and analgesia in obstetrics. Anesth Analg. 2010;111:1476–1479.
269. Rigler MI, Drasner K, Krejcie TC, et al. Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg. 1991;72:275–281.
270. Rigler ML, Drasner K. Distribution of catheter-injected local anesthetic in a model of the subarachnoid space. Anesthesiology. 1991;75:684–692.
271. Arkoosh VA, Palmer CM, Yun EM, et al. A randomized, double-masked, multicenter comparison of the safety of continuous intrathecal labor analgesia using a 28-gauge catheter versus continuous epidural labor analgesia. Anesthesiology. 2008;108:286–298.
272. Pavy TJ. Patient-controlled spinal analgesia for labour and cesarean delivery. Anaesth Intensive Care. 2001;29:58–61.
273. Van de Velde M, Budts W, Vandermeersch E, Spitz B. Continuous spinal analgesia for labor pain in a parturient with aortic stenosis. Int J Obstet Anesth. 2003;12:51–54.
274. Okutomi T, Kikuchi S, Amano K, et al. Continuous spinal analgesia for labor and delivery in a parturient with hypertrophic obstructive cardiomyopathy. Acta Anaesthesiol Scand. 2002;46:329–331.
275. Collis RE, Baxandall ML, Srikantharajah ID, et al. Combined spinal epidural analgesia with ability to walk throughout labour. Lancet. 1993;341:767–768.
276. Buggy D, Hughes N, Gardiner J. Posterior column sensory impairment during ambulatory extradural analgesia in labour. Br J Anaesth. 1994;73:540–542.
277. Parry MG, Fernando R, Bawa GPS, Poulton BB. Dorsal column function after epidural and spinal blockade: implications for the safety of walking following low-dose regional analgesia for labour. Anaesthesia. 1998;53:382–387.
278. Davies J, Fernando R, McLeod A, et al. Postural stability following ambulatory regional analgesia for labor. Anesthesiology. 2002;97:1576–1581.
279. Weiniger CF, Yaghmour H, Nadjari M, et al. Walking reduces the post-void residual volume in parturients with epidural analgesia for labor: a randomized-controlled study. Acta Anaesthesiol Scand. 2009;53:665–672.
280. Merry AF, Cross JA, Mayadeo SV, Wild CJ. Posture and the spread of extradural analgesia in labour. Br J Anaesth. 1983;55:303–307.
281. Park WY, Hagins FM, Massengale MD, Macnamara TE. The sitting position and anesthetic spread in the epidural space. Anesth Analg. 1984;63:863–864.
282. Erdemir HA, Soper LE, Sweet RB. Studies of factors affecting peridural anesthesia. Anesth Analg. 1965;44:400–404.
283. Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–765.
284. Kinsella SM, Black AM. Reporting of ‘hypotension’ after epidural analgesia during labour. Effect of choice of arm and timing of baseline readings. Anaesthesia. 1998;53:131–135.
285. Preston R, Crosby ET, Kotarba D, et al. Maternal positioning affects fetal heart rate changes after epidural analgesia for labour. Can J Anaesth. 1993;40:1136–1141.
286. Beilin Y, Abramovitz SE, Zahn J, et al. Improved epidural analgesia in the parturient in the 30 degree tilt position. Can J Anaesth. 2000;47:1176–1181.
287. Kubli M, Shennan AH, Seed PT, O'Sullivan G. A randomised controlled trial of fluid pre-loading before low dose epidural analgesia for labour. Int J Obstet Anesth. 2003;12:256–260.
288. Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–926.
289. Hughes SC, Ward MG, Levinson G, et al. Placental transfer of ephedrine does not affect neonatal outcome. Anesthesiology. 1985;63:217–219.
290. Wright RG, Shnider SM, Levinson G, et al. The effect of maternal administration of ephedrine on fetal heart rate and variability. Obstet Gynecol. 1981;57:734–738.
291. Palmer CM, Van Maren G, Nogami WM, Alves D. Bupivacaine augments intrathecal fentanyl for labor analgesia. Anesthesiology. 1999;91:84–89.
292. Lee BB, Ngan Kee WD, Hung VYS, Wong ELY. Combined spinal-epidural analgesia in labour: comparison of two doses of intrathecal bupivacaine with fentanyl. Br J Anaesth. 1999;83:868–871.
293. Lim EH, Sia AT, Wong K, Tan HM. Addition of bupivacaine 1.25 mg to fentanyl confers no advantage over fentanyl alone for intrathecal analgesia in early labor. Can J Anaesth. 2002;49:57–61.
294. Cohen SE, Cherry CM, Holbrook RH Jr, et al. Intrathecal sufentanil for labor analgesia—sensory changes, side effects, and fetal heart rate changes. Anesth Analg. 1993;77:1155–1160.
295. Norris MC, Grieco WM, Borkowski M, et al. Complications of labor analgesia: epidural versus combined spinal epidural techniques. Anesth Analg. 1994;79:529–537.
296. Douglas MJ, Kim JH, Ross PL, McMorland GH. The effect of epinephrine in local anaesthetic on epidural morphine-induced pruritus. Can Anaesth Soc J. 1986;33:737–740.
297. Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF. Primer of postoperative pruritus for anesthesiologists. Anesthesiology. 2005;103:168–178.
298. Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333.
299. Scott PV, Fischer HB. Spinal opiate analgesia and facial pruritus: a neural theory. Postgrad Med J. 1982;58:531–535.
300. Beilin Y, Bernstein HH, Zucker-Pinchoff B, et al. Subhypnotic doses of propofol do not relieve pruritus induced by intrathecal morphine after cesarean section. Anesth Analg. 1998;86:310–313.
301. Charuluxananan S, Somboonviboon W, Kyokong O, Nimcharoendee K. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Reg Anesth Pain Med. 2000;25:535–539.
302. Tamdee D, Charuluxananan S, Punjasawadwong Y, et al. A randomized controlled trial of pentazocine versus ondansetron for the treatment of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2009;109:1606–1611.
303. Siddik-Sayyid SM, Yazbeck-Karam VG, Zahreddine BW, et al. Ondansetron is as effective as diphenhydramine for treatment of morphine-induced pruritus after cesarean delivery. Acta Anaesthesiol Scand. 2010;54:764–769.
304. George RB, Allen TK, Habib AS. Serotonin receptor antagonists for the prevention and treatment of pruritus, nausea, and vomiting in women undergoing cesarean delivery with intrathecal morphine: a systematic review and meta-analysis. Anesth Analg. 2009;109:174–182.
305. Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012;114:813–822.
306. Cohen SE, Ratner EF, Kreitzman TR, et al. Nalbuphine is better than naloxone for treatment of side effects after epidural morphine. Anesth Analg. 1992;75:747–752.
307. Chaney MA. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891–903.
308. Metoclopramide injection. [Available at] http://www.drugs.com/pro/metoclopramide-injection.html [Accessed November 2012] .
309. Segal S. Labor epidural analgesia and maternal fever. Anesth Analg. 2010;111:1467–1475.
310. Herbst A, Wolner-Hanssen P, Ingemarsson I. Risk factors for fever in labor. Obstet Gynecol. 1995;86:790–794.
311. Goetzl L, Rivers J, Zighelboim I, et al. Intrapartum epidural analgesia and maternal temperature regulation. Obstet Gynecol. 2007;109:687–690.
312. Dashe JS, Rogers BB, McIntire DD, Leveno KJ. Epidural analgesia and intrapartum fever: placental findings. Obstet Gynecol. 1999;93:341–344.
313. Goetzl L, Evans T, Rivers J, et al. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. Am J Obstet Gynecol. 2002;187:834–838.
314. Goetzl L, Zighelboim I, Badell M, et al. Maternal corticosteroids to prevent intrauterine exposure to hyperthermia and inflammation: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2006;195:1031–1037.
315. Lieberman E, Cohen A, Lang J, et al. Maternal intrapartum temperature elevation as a risk factor for cesarean delivery and assisted vaginal delivery. Am J Public Health. 1999;89:506–510.
316. Goetzl L, Cohen A, Frigoletto F Jr, et al. Maternal epidural use and neonatal sepsis evaluation in afebrile mothers. Pediatrics. 2001;108:1099–1102.
317. Yancey MK, Zhang J, Schwarz J, et al. Labor epidural analgesia and intrapartum maternal hyperthermia. Obstet Gynecol. 2001;98:763–770.
318. Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–354.
319. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36.
320. Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–1126.
321. Kapusta L, Confino E, Ismajovich B, et al. The effect of epidural analgesia on maternal thermoregulation in labor. Int J Gynaecol Obstet. 1985;23:185–189.
322. Panzer O, Ghazanfari N, Sessler DI, et al. Shivering and shivering-like tremor during labor with and without epidural analgesia. Anesthesiology. 1999;90:1609–1616.
323. Shehabi Y, Gatt S, Buckman T, Isert P. Effect of adrenaline, fentanyl and warming of injectate on shivering following extradural analgesia in labour. Anaesth Intensive Care. 1990;18:31–37.
324. Kuipers PW, Kamphuis ET, van Venrooij GE, et al. Intrathecal opioids and lower urinary tract function: a urodynamic evaluation. Anesthesiology. 2004;100:1497–1503.
325. Grove LH. Backache, headache and bladder dysfunction after delivery. Br J Anaesth. 1973;45:1147–1149.
326. Weiniger CF, Wand S, Nadjari M, et al. Post-void residual volume in labor: a prospective study comparing parturients with and without epidural analgesia. Acta Anaesthesiol Scand. 2006;50:1297–1303.
327. Olofsson CI, Ekblom AO, Ekman-Ordeberg GE, Irestedt LE. Post-partum urinary retention: a comparison between two methods of epidural analgesia. Eur J Obstet Gynecol Reprod Biol. 1997;71:31–34.
328. Wilson MJ, Macarthur C, Shennan A. Urinary catheterization in labour with high-dose vs mobile epidural analgesia: a randomized controlled trial. Br J Anaesth. 2009;102:97–103.
329. Evron S, Muzikant G, Rigini N, et al. Patient-controlled epidural analgesia: the role of epidural fentanyl in peripartum urinary retention. Int J Obstet Anesth. 2006;15:206–211.
330. Xu F, Markowitz LE, Gottlieb SL, Berman SM. Seroprevalence of herpes simplex virus types 1 and 2 in pregnant women in the United States. Am J Obstet Gynecol. 2007;196:43 e1–6.
331. Crone LA, Conly JM, Storgard C, et al. Herpes labialis in parturients receiving epidural morphine following cesarean section. Anesthesiology. 1990;73:208–213.
332. Boyle RK. Herpes simplex labialis after epidural or parenteral morphine: a randomized prospective trial in an Australian obstetric population. Anaesth Intensive Care. 1995;23:433–437.
333. Davies PW, Vallejo MC, Shannon KT, et al. Oral herpes simplex reactivation after intrathecal morphine: a prospective randomized trial in an obstetric population. Anesth Analg. 2005;100:1472–1476.
334. Valley MA, Bourke DL, McKenzie AM. Recurrence of thoracic and labial herpes simplex virus infection in a patient receiving epidural fentanyl. Anesthesiology. 1992;76:1056–1057.
335. Acalovschi I. Herpes simplex after spinal pethidine. Anaesthesia. 1986;41:1271–1272.
336. Bauchat JR. Focused review: neuraxial morphine and oral herpes reactivation in the obstetric population. Anesth Analg. 2010;111:1238–1241.
337. Murphy DF, Nally B, Gardiner J, Unwin A. Effect of metoclopramide on gastric emptying before elective and emergency caesarean section. Br J Anaesth. 1984;56:1113–1116.
338. Carp H, Jayaram A, Stoll M. Ultrasound examination of the stomach contents of parturients. Anesth Analg. 1992;74:683–687.
339. Porter JS, Bonello E, Reynolds F. The influence of epidural administration of fentanyl infusion on gastric emptying in labour. Anaesthesia. 1997;52:1151–1156.
340. Zimmermann DL. Adding fentanyl 0.0002% to epidural bupivacaine 0.125% does not delay gastric emptying in laboring patients. Anesth Analg. 1996;82:612–616.
341. Ewah B, Yau K, King M, et al. Effect of epidural opioids on gastric emptying in labour. Int J Obstet Anesth. 1993;2:125–128.
342. Wright PMC, Allen RW, Moore J, Donnelly JP. Gastric emptying during lumbar extradural analgesia in labor: effect of fentanyl supplementation. Br J Anaesth. 1992;68:248–251.
343. Kelly MC, Carabine UA, Hill DA, Mirakhur RK. A comparison of the effect of intrathecal and extradural fentanyl on gastric emptying in laboring women. Anesth Analg. 1997;85:834–838.
344. Paech MJ, Godkin R, Webster S. Complications of obstetric epidural analgesia and anaesthesia: a prospective analysis of 10,995 cases. Int J Obstet Anesth. 1998;7:5–11.
345. Eappen S, Blinn A, Segal S. Incidence of epidural catheter replacement in parturients: a retrospective chart review. Int J Obstet Anesth. 1998;7:220–225.
346. Davies JM, Posner KL, Lee LA, et al. Liability associated with obstetric anesthesia: a closed claims analysis. Anesthesiology. 2009;110:131–139.
347. Beilin Y, Zahn J, Bernstein HH, et al. Treatment of incomplete analgesia after placement of an epidural catheter and administration of local anesthetic for women in labor. Anesthesiology. 1998;88:1502–1506.
348. Husemeyer RP, White DC. Lumbar extradural injection pressures in pregnant women: an investigation of relationships between rate of infection, injection pressures and extent of analgesia. Br J Anaesth. 1980;52:55–60.
349. Apostolou GA, Zarmakoupis PK, Mastrokostopoulos GT. Spread of epidural anesthesia and the lateral position. Anesth Analg. 1981;60:584–586.
350. Norris MC, Leighton BL, DeSimone CA, Larijani GE. Lateral position and epidural anesthesia for cesarean section. Anesth Analg. 1988;67:788–790.
351. Savolaine ER, Pandya JB, Greenblatt SH, Conover SR. Anatomy of the human lumbar epidural space: new insights using CT-epidurography. Anesthesiology. 1988;68:217–220.
352. Asato F, Goto F. Radiographic findings of unilateral epidural block. Anesth Analg. 1996;83:519–522.
353. Blomberg RG, Olsson SS. The lumbar epidural space in patients examined with epiduroscopy. Anesth Analg. 1989;68:157–160.
354. McCrae AF, Whitfield A, McClure JH. Repeated unilateral epidural blockade. Anaesthesia. 1992;47:859–861.
355. Choi PT, Galinski SE, Takeuchi L, et al. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Can J Anaesth. 2003;50:460–469.
356. Crawford JS. Some maternal complications of epidural analgesia for labour. Anaesthesia. 1985;40:1219–1225.
357. Ferouz F, Norris MC, Leighton BL. Risk of respiratory arrest after intrathecal sufentanil. Anesth Analg. 1997;85:1088–1090.
358. Lu JK, Manullang TR, Staples MH, et al. Maternal respiratory arrests, severe hypotension, and fetal distress after administration of intrathecal sufentanil and bupivacaine after intravenous fentanyl. Anesthesiology. 1997;87:170–172.
359. Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology. 1997;86:277–284.
360. Albright GA. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51:285–287.
361. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S829–S861.
362. Neal JM, Mulroy MF, Weinberg GL. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2012 version. Reg Anesth Pain Med. 2012;37:16–18.
363. Association of Anaesthetists of Great Britain and Ireland. AAGBI Safety Guideline: The Management of Local Anaesthetic Toxicity. [Available at] http://www.aagbi.org/sites/default/files/la_toxicity_2010_0.pdf [Accessed December 2012] .
364. Spence AG. Lipid reversal of central nervous system symptoms of bupivacaine toxicity. Anesthesiology. 2007;107:516–517.
365. Jenkins JG. Some immediate serious complications of obstetric epidural analgesia and anaesthesia: a prospective study of 145,550 epidurals. Int J Obstet Anesth. 2005;14:37–42.
366. Lubenow T, Keh-Wong E, Kristof K, et al. Inadvertent subdural injection: a complication of an epidural block. Anesth Analg. 1988;67:175–179.
367. Rodríguez J, Bárcena M, Taboada-Muñiz M, Alvarez J. Horner syndrome after unintended subdural block: a report of 2 cases. J Clin Anesth. 2005;17:473–477.
368. Abouleish E, Goldstein M. Migration of an extradural catheter into the subdural space: a case report. Br J Anaesth. 1986;58:1194–1197.
369. Hoftman N. Unintentional subdural injection: a complication of neuraxial anesthesia/analgesia. Anesthesiol Clin. 2011;29:279–290.
370. Chestnut DH, Bates JN, Choi WW. Continuous infusion epidural analgesia with lidocaine: efficacy and influence during the second stage of labor. Obstet Gynecol. 1987;69:323–327.
371. Leonard SA, Flynn R, Kelleher N, Shorten GD. Addition of epinephrine to epidural ropivacaine during labour—effects on onset and duration of action, efficacy, and systemic absorption of ropivacaine. Int J Obstet Anesth. 2002.
372. Cuerden C, Buley R, Downing JW. Delayed recovery after epidural block in labour: a report of four cases. Anaesthesia. 1977;32:773–776.
373. Riley ET, Ratner EF, Cohen SE. Intrathecal sufentanil for labor analgesia: do sensory changes predict better analgesia and greater hypotension? Anesth Analg. 1997;84:346–351.
374. Riley ET, Walker D, Hamilton CL, Cohen SE. Intrathecal sufentanil for labor analgesia does not cause a sympathectomy. Anesthesiology. 1997;87:874–878.
375. Wang C, Chakrabarti MK, Whitwam JG. Specific enhancement by fentanyl of the effects of intrathecal bupivacaine on nociceptive afferent but not on sympathetic efferent pathways in dogs. Anesthesiology. 1993;79:766–773.
376. Hamilton CL, Cohen SE. High sensory block after intrathecal sufentanil for labor analgesia. Anesthesiology. 1995;83:1118–1121.
377. Abu Abdou W, Aveline C, Bonnet F. Two additional cases of excessive extension of sensory blockade after intrathecal sufentanil for labor analgesia. Int J Obstet Anesth. 2000;9:48–50.
378. Scavone BM. Altered level of consciousness after combined spinal-epidural labor analgesia with intrathecal fentanyl and bupivacaine. Anesthesiology. 2002;96:1021–1022.
379. Fragneto RY, Fisher A. Mental status change and aphasia after labor analgesia with intrathecal sufentanil/bupivacaine. Anesth Analg. 2000;90:1175–1176.
380. To WW, Wong MW. Factors associated with back pain symptoms in pregnancy and the persistence of pain 2 years after pregnancy. Acta Obstet Gynecol Scand. 2003;82:1086–1091.
381. Malmqvist S, Kjaermann I, Andersen K, et al. Prevalence of low back and pelvic pain during pregnancy in a Norwegian population. J Manipulative Physiol Ther. 2012;35:272–278.
382. Breen TW, Ransil BJ, Groves PA, Oriol NE. Factors associated with back pain after childbirth. Anesthesiology. 1994;81:29–34.
383. Russell R, Dundas R, Reynolds F. Long term backache after childbirth: prospective search for causative factors. BMJ. 1996;312:1384–1388.
384. MacArthur C, Lewis M, Knox EG, Crawford JS. Epidural anaesthesia and long term backache after childbirth. BMJ. 1990;301:9–12.
385. MacArthur C, Lewis M, Knox EG. Investigation of long term problems after obstetric epidural anaesthesia. BMJ. 1992;304:1279–1282.
386. MacArthur A, MacArthur C, Weeks S. Epidural anesthesia and low back pain after delivery: a prospective cohort study. BMJ. 1995;311:1336–1339.
387. Loughnan BA, Carli F, Romney M, et al. Epidural analgesia and backache: a randomized controlled comparison with intramuscular meperidine for analgesia during labour. Br J Anaesth. 2002;89:466–472.
388. Howell CJ, Kidd C, Roberts W, et al. A randomised controlled trial of epidural compared with non-epidural analgesia in labour. BJOG. 2001;108:27–33.
389. Howell CJ, Dean T, Lucking L, et al. Randomised study of long term outcome after epidural versus non-epidural analgesia during labour. BMJ. 2002;325:357.
390. Christianson LM, Bovbjerg VE, McDavitt EC, Hullfish KL. Risk factors for perineal injury during delivery. Am J Obstet Gynecol. 2003;189:255–260.
391. Sartore A, Pregazzi R, Bortoli P, et al. Effects of epidural analgesia during labor on pelvic floor function after vaginal delivery. Acta Obstet Gynecol Scand. 2003;82:143–146.
392. Ramin SM, Gambling DR, Lucas MJ, et al. Randomized trial of epidural versus intravenous analgesia during labor. Obstet Gynecol. 1995;86:783–789.
393. Noble AD, Craft IL, Bootes JA, et al. Continuous lumbar epidural analgesia using bupivacaine: a study of the fetus and newborn child. J Obstet Gynaecol Br Commonw. 1971;78:559–563.
394. Yoo KY, Lee J, Kim HS, Jeong SW. The effects of opioids on isolated human pregnant uterine muscles. Anesth Analg. 2001;92:1006–1009.
395. Neuhoff D, Burke MS, Porreco RP. Cesarean birth for failed progress in labor. Obstet Gynecol. 1989;73:915–920.
396. Guillemette J, Fraser WD. Differences between obstetricians in caesarean section rates and the management of labour. Br J Obstet Gynaecol. 1992;99:105–108.
397. Goldstick O, Weissman A, Drugan A. The circadian rhythm of “urgent” operative deliveries. Isr Med Assoc J. 2003;5:564–566.
398. Fraser W, Usher RH, McLean FH, et al. Temporal variation in rates of cesarean section for dystocia: does “convenience” play a role? Am J Obstet Gynecol. 1987;156:300–304.
399. Morita N, Matsushima N, Ogata N, et al. Nationwide description of live Japanese births by day of the week, hour, and location. J Epidemiol. 2002;12:330–335.
400. Wuitchik M, Bakal D, Lipshitz J. The clinical significance of pain and cognitive activity in latent labor. Obstet Gynecol. 1989;73:35–42.
401. Alexander JM, Sharma SK, McIntire DD, et al. Intensity of labor pain and cesarean delivery. Anesth Analg. 2001;92:1524–1528.
402. Hess PE, Pratt SD, Soni AK, et al. An association between severe labor pain and cesarean delivery. Anesth Analg. 2000;90:881–886.
403. Bofill JA, Vincent RD, Ross EL, et al. Nulliparous active labor, epidural analgesia, and cesarean delivery for dystocia. Am J Obstet Gynecol. 1997;177:1465–1470.
404. Clark A, Carr D, Loyd G, et al. The influence of epidural analgesia on cesarean delivery rates: a randomized, prospective clinical trial. Am J Obstet Gynecol. 1998;179:1527–1533.
405. Dickinson JE, Paech MJ, McDonald SJ, Evans SF. The impact of intrapartum analgesia on labour and delivery outcomes in nulliparous women. Aust N Z J Obstet Gynaecol. 2002;42:59–66.
406. El-Kerdawy H, Farouk A. Labor analgesia in preeclampsia: remifentanil patient controlled intravenous analgesia versus epidural analgesia. Middle East J Anesthesiol. 2010;20:539–545.
407. Evron S, Ezri T, Protianov M, et al. The effects of remifentanil or acetaminophen with epidural ropivacaine on body temperature during labor. J Anesth. 2008;22:105–111.
408. Gambling DR, Sharma SK, Ramin SM, et al. A randomized study of combined spinal-epidural analgesia versus intravenous meperidine during labor: impact on cesarean delivery rate. Anesthesiology. 1998;89:1336–1344.
409. Grandjean H, de Mouzon J, Cabot JA, et al. Peridural analgesia and by phenoperidine in normal labor. Therapeutic trial with a control series. Arch Fr Pediatr. 1979;36(9 Suppl):LXXV–LXXXI.
410. Halpern SH, Muir H, Breen TW, et al. A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor. Anesth Analg. 2004;99:1532–1538.
411. Head BB, Owen J, Vincent RD Jr, et al. A randomized trial of intrapartum analgesia in women with severe preeclampsia. Obstet Gynecol. 2002;99:452–457.
412. Hogg B, Owen J, Shih G, et al. A randomised control trial of intrapartum analgesia in women with severe preeclampsia. Am J Obstet Gynecol. 2000;182:S148.
413. Jain S, Arya VK, Gopalan S, Jain V. Analgesic efficacy of intramuscular opioids versus epidural analgesia in labor. Int J Gynaecol Obstet. 2003;83:19–27.
414. Long J, Yue Y. Patient controlled intravenous analgesia with tramadol for labor pain relief. Chin Med J (Engl). 2003;116:1752–1755.
415. Loughnan BA, Carli F, Romney M, et al. Randomized controlled comparison of epidural bupivacaine versus pethidine for analgesia in labour. Br J Anaesth. 2000;84:715–719.
416. Lucas MJ, Sharma SK, McIntire DD, et al. A randomized trial of labor analgesia in women with pregnancy-induced hypertension. Am J Obstet Gynecol. 2001;185:970–975.
417. Muir HA, Shukla R, Liston R, Writer D. Randomized trial of labor analgesia: a pilot study to compare patient-controlled intravenous analgesia with patient-controlled epidural analgesia to determine if analgesic method affects delivery outcome. Can J Anaesth. 1996;43:A60.
418. Muir H, Breen T, Campbell D, et al. A multicentre study of the effects of analgesia on the progress of labour. Anesthesiology. 2000;92(Suppl).
419. Nafisi S. Effects of epidural lidocaine analgesia on labor and delivery: a randomized, prospective, controlled trial. BMC Anesthesiol. 2006;6:15.
420. Nikkola EM, Ekblad UU, Kerno PO, et al. Intravenous fentanyl PCA during labour. Can J Anaesth. 1997;44:1248–1255.
421. Philipsen T, Jensen N-H. Epidural block or parenteral pethidine as analgesic in labour: a randomized study concerning progress in labour and instrumental deliveries. Eur J Obstet Gynecol Reprod Biol. 1989;30:27–33.
422. Sharma SK, Sidawi JE, Ramin SM, et al. Cesarean delivery: a randomized trial of epidural versus patient-controlled meperidine analgesia during labor. Anesthesiology. 1997;87:487–494.
423. Sharma SK, Alexander JM, Messick G, et al. Cesarean delivery: a randomized trial of epidural analgesia versus intravenous meperidine analgesia during labor in nulliparous women. Anesthesiology. 2002;96:546–551.
424. Shifman EM, Butrov AV, Floka SE, Got IB. Transient neurological symptoms in puerperas after epidural analgesia during labor. Anesteziol Reanimatol. 2007;17–20.
425. Thalme B, Belfrage P, Raabe N. Lumbar epidural analgesia in labour. I. Acid-base balance and clinical condition of mother, fetus and newborn child. Acta Obstet Gynecol Scand. 1974;53:27–35.
426. Thorp JA, Hu DH, Albin RM, et al. The effect of intrapartum epidural analgesia on nulliparous labor: a randomized, controlled, prospective trial. Am J Obstet Gynecol. 1993;169:851–858.
427. Volmanen P, Sarvela J, Akural EI, et al. Intravenous remifentanil vs. epidural levobupivacaine with fentanyl for pain relief in early labour: a randomised, controlled, double-blinded study. Acta Anaesthesiol Scand. 2008;52:249–255.
428. Sharma SK, Leveno KJ. Update: Epidural analgesia does not increase cesarean births. Curr Anesthesiol Rep. 2000;2:18–24.
429. Sharma SK, McIntire DD, Wiley J, Leveno KJ. Labor analgesia and cesarean delivery: an individual patient meta-analysis of nulliparous women. Anesthesiology. 2004;100:142–148.
430. Comparative Obstetric Mobile Epidural Trial Study Group UK. Effect of low-dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet. 2001;358:19–23.
431. Collis RE, Davies DW, Aveling W. Randomised comparison of combined spinal-epidural and standard epidural analgesia in labour. Lancet. 1995;345:1413–1416.
432. Nageotte MP, Larson D, Rumney PJ, et al. Epidural analgesia compared with combined spinal-epidural analgesia during labor in nulliparous women. N Engl J Med. 1997;337:1715–1719.
433. Olofsson C, Ekblom A, Ekman-Ordeberg G, Irestedt L. Obstetric outcome following epidural analgesia with bupivacaine-adrenaline 0.25% or bupivacaine 0.125% with sufentanil—a prospective randomized controlled study in 1000 parturients. Acta Anaesthesiol Scand. 1998;42:284–292.
434. Norris MC, Fogel ST, Conway-Long C. Combined spinal-epidural versus epidural labor analgesia. Anesthesiology. 2001;95:913–920.
435. Yancey MK, Pierce B, Schweitzer D, Daniels D. Observations on labor epidural analgesia and operative delivery rates. Am J Obstet Gynecol. 1999;180:353–359.
436. Zhang J, Yancey MK, Klebanoff MA, et al. Does epidural analgesia prolong labor and increase risk of cesarean delivery? A natural experiment. Am J Obstet Gynecol. 2001;185:128–134.
437. Impey L, MacQuillan K, Robson M. Epidural analgesia need not increase operative delivery rates. Am J Obstet Gynecol. 2000;182:358–363.
438. Socol ML, Garcia PM, Peaceman AM, Dooley SL. Reducing cesarean births at a primarily private university hospital. Am J Obstet Gynecol. 1993;168:1748–1758.
439. Segal S, Su M, Gilbert P. The effect of a rapid change in availability of epidural analgesia on the cesarean delivery rate: a meta-analysis. Am J Obstet Gynecol. 2000;183:974–978.
440. Lagrew DC Jr, Adashek JA. Lowering the cesarean section rate in a private hospital: comparison of individual physicians’ rates, risk factors, and outcomes. Am J Obstet Gynecol. 1998;178:1207–1214.
441. Segal S, Blatman R, Doble M, Datta S. The influence of the obstetrician in the relationship between epidural analgesia and cesarean section for dystocia. Anesthesiology. 1999;91:90–96.
442. Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol. 1999;94:600–607.
443. American College of Obstetricians and Gynecologists. Obstetric analgesia and anesthesia. ACOG Practice Bulletin No. 36. Washington, DC, July 2002. Obstet Gynecol. 2002;100:177–191.
444. Luxman D, Wolman I, Groutz A, et al. The effect of early epidural block administration on the progression and outcome of labor. Int J Obstet Anesth. 1998;7:161–164.
445. Wong CA, McCarthy RJ, Sullivan JT, et al. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113:1066–1074.
446. American College of Obstetricians and Gynecologists. Analgesia and cesarean delivery rates. ACOG Committee Opinion No. 339. Washington, DC, June 2006. Obstet Gynecol. 2006;107:1487.
447. Lyon DS, Knuckles G, Whitaker E, Salgado S. The effect of instituting an elective labor epidural program on the operative delivery rate. Obstet Gynecol. 1997;90:135–141.
448. Chestnut DH, Laszewski LJ, Pollack KL, et al. Continuous epidural infusion of 0.0625% bupivacaine-0.0002% fentanyl during the second stage of labor. Anesthesiology. 1990;72:613–618.
449. Chestnut DH, Vandewalker GE, Owen CL, et al. The influence of continuous epidural bupivacaine analgesia on the second stage of labor and method of delivery in nulliparous women. Anesthesiology. 1987;66:774–780.
450. Phillips KC, Thomas TA. Second stage of labour with or without extradural analgesia. Anaesthesia. 1983;38:972–976.
451. Johnsrud ML, Dale PO, Lovland B. Benefits of continuous infusion epidural analgesia throughout vaginal delivery. Acta Obstet Gynecol Scand. 1988;67:355–358.
452. Luxman D, Wolman I, Niv D, et al. Effect of second-stage 0.25% epidural bupivacaine on the outcome of labor. Gynecol Obstet Invest. 1996;42:167–170.
453. James KS, McGrady E, Quasim I, Patrick A. Comparison of epidural bolus administration of 0.25% bupivacaine and 0.1% bupivacaine with 0.0002% fentanyl for analgesia during labour. Br J Anaesth. 1998;81:501–510.
454. Robinson CA, Macones GA, Roth NW, Morgan MA. Does station of the fetal head at epidural placement affect the position of the fetal vertex at delivery? Am J Obstet Gynecol. 1996;175:991–994.
455. Le Ray C, Carayol M, Jaquemin S, et al. Is epidural analgesia a risk factor for occiput posterior or transverse positions during labour? Eur J Obstet Gynecol Reprod Biol. 2005;123:22–26.
456. Yancey MK, Zhang J, Schweitzer DL, et al. Epidural analgesia and fetal head malposition at vaginal delivery. Obstet Gynecol. 2001;97:608–612.
457. Sheiner E, Sheiner EK, Segal D, et al. Does the station of the fetal head during epidural analgesia affect labor and delivery? Int J Gynaecol Obstet. 1999;64:43–47.
458. Lieberman E, Davidson K, Lee-Parritz A, Shearer E. Changes in fetal position during labor and their association with epidural analgesia. Obstet Gynecol. 2005;105:974–982.
459. Malvasi A, Tinelli A, Brizzi A, et al. Intrapartum sonography head transverse and asynclitic diagnosis with and without epidural analgesia initiated early during the first stage of labor. Eur Rev Med Pharmacol Sci. 2011;15:518–523.
460. Chestnut DH. Epidural anesthesia and instrumental vaginal delivery. Anesthesiology. 1991;74:805–808.
461. Society of Obstetricians and Gynaecologists of Canada. Guidelines for operative vaginal birth. No. 148, May 2004. Int J Gynaecol Obstet. 2005;88:229–236.
462. O’Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev. 2010;(11).
463. Seidman DS, Laor A, Gale R, et al. Long-term effects of vacuum and forceps deliveries. Lancet. 1991;337:1583–1585.
464. Robinson JN, Norwitz ER, Cohen AP, et al. Episiotomy, operative vaginal delivery, and significant perinatal trauma in nulliparous women. Am J Obstet Gynecol. 1999;181:1180–1184.
465. Baumann P, Hammoud AO, McNeeley SG, et al. Factors associated with anal sphincter laceration in 40,923 primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:985–990.
466. Dahl C, Kjolhede P. Obstetric anal sphincter rupture in older primiparous women: a case-control study. Acta Obstet Gynecol Scand. 2006;85:1252–1258.
467. Goetzinger KR, Macones GA. Operative vaginal delivery: current trends in obstetrics. Womens Health (Lond Engl). 2008;4:281–290.
468. Schellenberg JC. Uterine activity during lumbar epidural analgesia with bupivacaine. Am J Obstet Gynecol. 1977;127:26–31.
469. Cheek TG, Samuels P, Miller F, et al. Normal saline i.v. fluid load decreases uterine activity in active labour. Br J Anaesth. 1996;77:632–635.
470. Miller AC, DeVore JS, Eisler EA. Effects of anesthesia on uterine activity and labor. Shnider SM, Levinson G. Anesthesia for Obstetrics. 3rd edition. Williams & Wilkins: Baltimore, MD; 1993:53–69.
471. Rahm VA, Hallgren A, Hogberg H, et al. Plasma oxytocin levels in women during labor with or without epidural analgesia: a prospective study. Acta Obstet Gynecol Scand. 2002;81:1033–1039.
472. Behrens O, Goeschen K, Luck HJ, Fuchs AR. Effects of lumbar epidural analgesia on prostaglandin F2 alpha release and oxytocin secretion during labor. Prostaglandins. 1993;45:285–296.
473. Nielsen PE, Abouleish E, Meyer BA, Parisi VM. Effect of epidural analgesia on fundal dominance during spontaneous active-phase nulliparous labor. Anesthesiology. 1996;84:540–544.
474. Clarke VT, Smiley RM, Finster M. Uterine hyperactivity after intrathecal injection of fentanyl for analgesia during labor: a cause of fetal bradycardia? Anesthesiology. 1994;81:1083.
475. Moir DD, Willocks J. Management of incoordinate uterine action under continuous epidural analgesia. Br Med J. 1967;3:396–400.
476. Lederman RP, Lederman E, Work BA Jr, McCann DS. The relationship of maternal anxiety, plasma catecholamines, and plasma cortisol to progress in labor. Am J Obstet Gynecol. 1978;132:495–500.
477. Gunther RE, Bauman J. Obstetrical caudal anesthesia. I. A randomized study comparing 1 per cent mepivacaine with 1 per cent lidocaine plus epinephrine. Anesthesiology. 1969;31:5–19.
478. Yau G, Gregory MA, Gin T, Oh TE. Obstetric epidural analgesia with mixtures of bupivacaine, adrenaline and fentanyl. Anaesthesia. 1990;45:1020–1023.
479. Abboud TK, Khoo SS, Miller F, et al. Maternal, fetal, and neonatal responses after epidural anesthesia with bupivacaine, 2-chloroprocaine, or lidocaine. Anesth Analg. 1982;61:638–644.
480. Tsen LC, Thue B, Datta S, Segal S. Is combined spinal-epidural analgesia associated with more rapid cervical dilation in nulliparous patients when compared with conventional epidural analgesia? Anesthesiology. 1999;91:920–925.
481. Gambling D, Berkowitz J, Farrell T, Shay D. A randomized controlled comparison of epidural analgesia and combined spinal-epidural analgesia in a private practice setting: pain scores during first and second stages of labor and at delivery. Anesth Analg. 2013;116:636–643.
482. Reitman E, Conell-Price J, Evansmith J, et al. Beta2-adrenergic receptor genotype and other variables that contribute to labor pain and progress. Anesthesiology. 2011;114:927–939.
483. Terkawi AS, Jackson WM, Thiet MP, et al. Oxytocin and catechol-O-methyltransferase receptor genotype predict the length of the first stage of labor. Am J Obstet Gynecol. 2012;207:184 e1–8.
484. American College of Obstetricians and Gynecologists. Dystocia and augmentation of labor. ACOG Practice Bulletin No. 49. Washington, DC, December 2003. Obstet Gynecol. 2003;102:1445–1454.
485. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281–1287.
486. Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1–357.e7.
487. Buxton E, Redman C, Obhrai M. Delayed pushing with lumbar epidural in labour—does it increase the incidence of spontaneous delivery? J Obstet Gynaecol. 1988;8:258–261.
488. Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery. Summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120:1181–1193.
489. Vause S, Congdon HM, Thornton JG. Immediate and delayed pushing in the second stage of labor for nulliparous women with epidural analgesia: a randomized controlled trial. Br J Obstet Gynaecol. 1998;105:186–188.
490. Mayberry LJ, Hammer R, Kelly C, et al. Use of delayed pushing with epidural anesthesia: findings from a randomized, controlled trial. J Perinatol. 1999;19:26–30.
491. Fraser WD, Marcoux S, Krauss I, et al. Multicenter, randomized, controlled trial of delayed pushing for nulliparous women in the second stage of labor with continuous epidural analgesia. The PEOPLE (Pushing Early or Pushing Late with Epidural) Study Group. Am J Obstet Gynecol. 2000;182:1165–1172.
492. Fitzpatrick M, Harkin R, McQuillan K, et al. A randomised clinical trial comparing the effects of delayed versus immediate pushing with epidural analgesia on mode of delivery and faecal continence. BJOG. 2002;109:1359–1365.
493. Plunkett BA, Lin A, Wong CA, et al. Management of the second stage of labor in nulliparas with continuous epidural analgesia. Obstet Gynecol. 2003;102:109–114.
494. Simpson KR, James DC. Effects of immediate versus delayed pushing during second-stage labor on fetal well-being: a randomized clinical trial. Nurs Res. 2005;54:149–157.
495. Gillesby E, Burns S, Dempsey A, et al. Comparison of delayed versus immediate pushing during second stage of labor for nulliparous women with epidural anesthesia. J Obstet Gynecol Neonatal Nurs. 2010;39:635–644.
496. Maresh M, Choong KH, Beard RW. Delayed pushing with lumbar epidural analgesia in labour. Br J Obstet Gynaecol. 1983;90:623–627.
497. Hansen SL, Clark SL, Foster JC. Active pushing versus passive fetal descent in the second stage of labor: a randomized controlled trial. Obstet Gynecol. 2002;99:29–34.
498. Kelly M, Johnson E, Lee V, et al. Delayed versus immediate pushing in second stage of labor. MCN Am J Matern Child Nurs. 2010;35:81–88.
499. Tuuli MG, Frey HA, Odibo AO, et al. Immediate compared with delayed pushing in the second stage of labor: a systematic review and meta-analysis. Obstet Gynecol. 2012;120:660–668.
500. Rosaeg OP, Campbell N, Crossan ML. Epidural analgesia does not prolong the third stage of labour. Can J Anaesth. 2002;49:490–492.
501. O'Driscoll K, Meagher D, Boylan P. Active Management of Labor. 3rd edition. Mosby–Year Book: London; 1993.
502. Wei S, Wo BL, Qi HP, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2012;(9).
503. Kotaska AJ, Klein MC, Liston RM. Epidural analgesia associated with low-dose oxytocin augmentation increases cesarean births: a critical look at the external validity of randomized trials. Am J Obstet Gynecol. 2006;194:809–814.
504. Lupe PJ, Gross TL. Maternal upright posture and mobility in labor—a review. Obstet Gynecol. 1986;67:727–734.
505. Roberts CL, Algert CS, Olive E. Impact of first-stage ambulation on mode of delivery among women with epidural analgesia. Aust N Z J Obstet Gynaecol. 2004;44:489–494.
506. Bloom SL, McIntire DD, Kelly MA, et al. Lack of effect of walking on labor and delivery. N Engl J Med. 1998;339:76–79.
507. Lavin JP, Samuels SV, Miodovnik M, et al. The effects of bupivacaine and chloroprocaine as local anesthetics for epidural anesthesia of fetal heart rate monitoring parameters. Am J Obstet Gynecol. 1981;141:717–722.
508. Boehm FH, Woodruff LF Jr, Growdon JH Jr. The effect of lumbar epidural anesthesia on fetal heart rate baseline variability. Anesth Analg. 1975;54:779–782.
509. Loftus JR, Holbrook RH, Cohen SE. Fetal heart rate after epidural lidocaine and bupivacaine for elective cesarean section. Anesthesiology. 1991;75:406–412.
510. Nielsen PE, Erickson JR, Abouleish EI, et al. Fetal heart rate changes after intrathecal sufentanil or epidural bupivacaine for labor analgesia: incidence and clinical significance. Anesth Analg. 1996;83:742–746.
511. Pello LC, Rosevear SK, Dawes GS, et al. Computerized fetal heart rate analysis in labor. Obstet Gynecol. 1991;78:602–610.
512. Viscomi CM, Hood DD, Melone PJ, Eisenach JC. Fetal heart rate variability after epidural fentanyl during labor. Anesth Analg. 1990;71:679–683.
513. Wilhite AO, Moore CH, Blass NH, Christmas JT. Plasma concentration profile of epidural alfentanil. Bolus followed by continuous infusion technique in the parturient: effect of epidural alfentanil and fentanyl on fetal heart rate. Reg Anesth. 1994;19:164–168.
514. Hill JB, Alexander JM, Sharma SK, et al. A comparison of the effects of epidural and meperidine analgesia during labor on fetal heart rate. Obstet Gynecol. 2003;102:333–337.
515. Smith CV, Rayburn WF, Allen KV, et al. Influence of intravenous fentanyl on fetal biophysical parameters during labor. J Matern Fetal Med. 1996;5:89–92.
516. Scanlon JW, Ostheimer GW, Lurie AO, et al. Neurobehavioral responses and drug concentrations in newborns after maternal epidural anesthesia with bupivacaine. Anesthesiology. 1976;45:400–405.
517. Elliott RD. Continuous infusion epidural analgesia for obstetrics: bupivacaine versus bupivacaine-fentanyl mixture. Can J Anaesth. 1991;38:303–310.
518. Abrão KC, Francisco RP, Miyadahira S, et al. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol. 2009;113:41–47.
519. Landau R, Carvalho B, Wong C, et al. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol. 2009;113:1374.
520. Albright GA, Forster RM. Does combined spinal-epidural analgesia with subarachnoid sufentanil increase the incidence of emergency cesarean delivery? Reg Anesth. 1997;22:400–405.
521. Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. Br J Obstet Gynaecol. 2002;109:274–281.
522. Van de Velde M, Teunkens A, Hanssens M, et al. Intrathecal sufentanil and fetal heart rate abnormalities: a double-blind, double placebo-controlled trial comparing two forms of combined spinal epidural analgesia with epidural analgesia in labor. Anesth Analg. 2004;98:1153–1159.
523. Sia AT, Chong JL. Epidural 0.2% ropivacaine for labour analgesia: parturient-controlled or continuous infusion? Anaesth Intensive Care. 1999;27:154–158.
524. Smedvig JP, Soreide E, Gjessing L. Ropivacaine 1 mg/mL, plus fentanyl 2 µg/mL for epidural analgesia during labour. Is mode of administration important? Acta Anaesthesiol Scand. 2001;45:595–599.
* The Institute of Safe Medicine Practices (ISMP) has recommended that health care providers never use µg as an abbreviation for micrograms, but rather they should use mcg (http://www.ismp.org/tools/errorproneabbreviations.pdf, accessed February 2013). The use of the symbol µg is frequently misinterpreted and involved in harmful medication errors. The abbreviation may be mistaken for mg (milligrams), which would result in a 1000-fold overdose. The symbol µg should never be used when communicating medical information, including pharmacy and prescriber computer order entry screens, computer-generated labels, labels for drug storage bins, and medication administration records. However, most scholarly publications have continued to use the abbreviation µg. The editors have chosen to retain the use of the abbreviation µg throughout this text. However, the editors recommend the use of the abbreviation mcg in clinical practice.