Although the incidence of nausea is low, treatment should be available. No studies, however, have specifically addressed the treatment of neuraxial analgesia-associated nausea and vomiting during labor. Metoclopramide, ondansetron, and droperidol have been used prophylactically in women who received neuraxial morphine for analgesia after cesarean delivery or nonobstetric surgery (see Chapter 28). Used in low doses, these agents have few significant side effects. When administered intravenously, metoclopramide should be administered slowly over 1 to 2 minutes to minimize feelings of restlessness and anxiety that may accompany rapid intravenous administration.308 A partial explanation for metoclopramide's efficacy may be its action in promoting gastric emptying. The package insert for droperidol contains a “black box” warning because of concern that the administration of this agent may increase the risk for severe cardiac dysrhythmias (secondary to prolongation of the QT interval and torsades de pointes). The warning suggests that patients be monitored for dysrhythmias for several hours after droperidol administration. Because maternal electrocardiographic monitoring is rarely undertaken in healthy parturients, the drug is now rarely used in the United States by obstetric anesthesia providers.
Fever
Both observational and randomized controlled trials have consistently noted a gradual rise in core temperature over several hours in laboring women receiving epidural analgesia that was not observed in women receiving no analgesia, inhaled nitrous oxide, or parenteral opioids.309 The mean increase in core temperature is typically small (< 1.0° C); however, women with epidural analgesia are more likely to have clinical fever (usually defined as core temperature ≥ 38° C) than those without epidural analgesia (risk ratio, 3.34; 95% CI, 2.63 to 4.23).3 In a retrospective study, Herbst et al.310 identified the use of epidural analgesia as a risk factor for intrapartum fever, along with prolonged labor and a prolonged interval between rupture of membranes and delivery. The incidence of clinical fever ranges from 20% to 30% in women randomized to receive epidural analgesia compared with 5% to 7% in women in the control groups.309
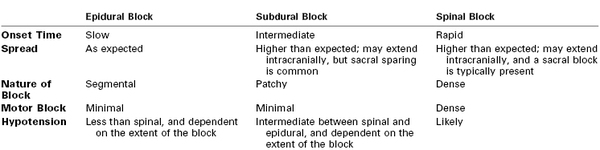
Newer evidence suggests that the slow increase in mean temperature observed in women with epidural analgesia may be an averaging artifact.311 In a prospective observational study of women with epidural analgesia, Goetzl et al.311 observed the incidence of fever was 22.2%. The mean temperature increase over 8 hours was 0.72° C, similar to that observed in earlier studies. However, the investigators noted that temperature increased in only a subset of women; the remaining cohort had no temperature increase (Figure 23-7). In the small subset of women who eventually developed clinical fever, core temperature began to rise within an hour of initiation of epidural analgesia. The researchers concluded that most women do not become febrile after epidural analgesia, and therefore it is unlikely that a perturbation in thermoregulation induced by epidural analgesia is the cause of epidural analgesia–associated fever.
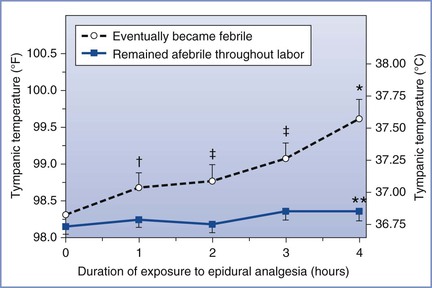
FIGURE 23-7 Maternal tympanic temperature in the 4 hours immediately after initiation of epidural analgesia, stratified by ultimate intrapartum fever status (febrile ≥ 38.0° C or afebrile < 38° C). *P < .001; †P < .05; ‡P < .01 (repeated measures analysis, febrile versus afebrile); **P = .26 (repeated measures analysis, afebrile group temperature change over time). (Modified from Goetzl L, Rivers J, Zighelboim I, et al. Intrapartum epidural analgesia and maternal temperature regulation. Obstet Gynecol 2007; 109:687-90.)
The mechanism of temperature elevation in some women who receive epidural labor analgesia is incompletely understood but likely reflects an inflammatory process. Several lines of evidence support this mechanism.309 Risk factors for intrapartum fever are similar to factors that are associated with the request for epidural analgesia, including nulliparity, prolonged rupture of membranes, and prolonged labor. In an observational study in women who self-selected the type of analgesia, the histologic diagnosis of placental inflammation was more common in women with epidural analgesia.312 However, the incidence of maternal fever was not different between women with and without epidural analgesia in the absence of placental inflammation. Additionally, Goetz et al.313 noted higher baseline maternal serum levels of interleukin-6 (IL-6), a marker of inflammation, in laboring women who eventually developed fever; final IL-6 levels were directly related to the duration of epidural analgesia.313 In a subsequent study, women with epidural analgesia randomized to receive maternal methylprednisolone (100 mg) had a lower rate of fever than those who received placebo, again suggesting that an inflammatory mechanism is involved.314
The significance of the temperature changes during labor is unclear. Maternal fever is associated with mode of delivery; the rate of instrumental and cesarean delivery is higher in women with intrapartum fever.315 Epidural analgesia during labor is associated with more neonatal sepsis evaluations but not with a higher incidence of neonatal sepsis.316,317 This link likely exists because the diagnosis of intrapartum chorioamnionitis is based on the presence of fever and usually one or two additional criteria (i.e., maternal leukocytosis, maternal tachycardia, uterine tenderness, foul-smelling amniotic fluid).318 Because maternal fever from any cause leads to maternal and fetal tachycardia, it may be difficult to differentiate women with actual infection (based on postpartum histologic placental examination) from women with fever associated with epidural analgesia. In the interests of maternal and fetal safety, intrapartum maternal fever typically prompts an intrapartum diagnosis of clinical chorioamnionitis. Revised 2010 guidelines from the U.S. Centers for Disease Control and Prevention stipulate that even well-appearing newborns whose mothers carry the diagnosis of suspected chorioamnionitis should undergo a limited evaluation (complete blood count [CBC] with differential cell count and blood culture) and antibiotic therapy pending the culture results.319
Of greater concern is the association between maternal or neonatal fever and serious adverse outcomes (i.e., neonatal seizures and encephalopathy, development of cerebral palsy).309 Evidence suggests that the mechanism of neonatal brain injury is inflammatory rather than fever per se.309,320 Whether epidural analgesia plays any role in these outcomes requires further research. However, because of the growing evidence that maternal inflammation and infection, which manifest as fever, can be detrimental to the fetal brain, anesthesia providers should not dismiss this apparent physiologic effect as a mere curiosity. When maternal fever occurs, good clinical practice dictates that efforts be made to lower maternal temperature and identify and treat a presumed maternal infection. (See Chapter 37 for a more complete discussion of this subject.)
Shivering
A number of factors, including hormonal factors, likely influence thermoregulatory response during labor and delivery. Shivering is frequently observed during labor and may occur more commonly after epidural analgesia.321 Panzer et al.322 performed an observational study of shivering during labor. Before delivery, 18% of women shivered, and 15% of these episodes were associated with normothermia and vasodilation, suggesting a nonthermoregulatory cause of the shivering. After delivery, shivering was observed in 16% of women, and in 28% of them, it was nonthermoregulatory. There was no difference in the incidence of shivering between women who chose epidural (bupivacaine/fentanyl) analgesia and those who chose systemic meperidine analgesia. The addition of an opioid to the local anesthetic solution may affect the shivering response.133,323 At least one study has suggested that the epidural administration of epinephrine increases shivering323; the etiology of this response is unknown.
Urinary Retention
Urinary retention is a troublesome side effect of neuraxial anesthesia/analgesia. The bladder and urethral sphincters receive sympathetic innervation from the low thoracic/high lumbar sympathetic fibers and parasympathetic innervation from sacral fibers. Neuraxial local anesthetics cause urinary retention through blockade of sacral nerve roots. Efferent and afferent nerve traffic via the S2, S3, and S4 nerve roots controls the detrusor muscle (responsible for urine storage and micturition) and internal and external sphincter function. Intrathecal opioids cause dose-dependent suppression of detrusor muscle contractility and decreased urge sensation via inhibition of sacral parasympathetic nervous system outflow.307,324 The onset of urinary retention appears to parallel the onset of analgesia.
It is difficult to determine the magnitude of this problem during labor, because parturients often require catheterization for other reasons. Postpartum bladder dysfunction was observed in 14% of women who had a normal spontaneous vaginal delivery and in 38% of women who underwent instrumental vaginal delivery, all without epidural analgesia.325
Several observational studies suggest that there is a higher risk for intrapartum and postpartum urinary retention in women who receive epidural labor analgesia than in those who receive nonepidural or no analgesia.326,327 Similarly, a meta-analysis of three small randomized controlled trials comparing neuraxial with systemic opioid analgesia, in which urinary retention was reported as a secondary outcome, also identified this association.3 Whether this higher risk reflects a cause-and-effect relationship or patient selection bias is not clear. Wilson et al.328 found that women randomized to receive neuraxial labor analgesia with low-concentration bupivacaine with opioid more often retained the ability to void spontaneously than women who received epidural analgesia with 0.25% bupivacaine (approximately 31% versus 11%), which suggests a dose-response relationship.
Any difference in bladder function appears to be short-lived; differences between groups in one study had resolved by postpartum day 1.326 In two studies, patients were randomly assigned to receive epidural analgesia with or without an opioid; there was no difference between groups in the incidence of intrapartum329 or postpartum327 urinary retention.
Parturients should be regularly observed during labor for evidence of bladder distention, especially if they complain of suprapubic pain during contractions. The differential diagnosis of breakthrough pain during neuraxial labor analgesia should include bladder distention. Personal observation suggests that many women can void in the presence of low-dose neuroblockade if placed on a bedpan or escorted to the toilet, even if they do not perceive a full bladder. Inability to void and bladder distention should prompt catheterization to empty the bladder.
Recrudescence of Herpes Simplex Virus
The seroprevalence of herpes simplex virus (HSV) among pregnant women was 72% in the period 1999 to 2002.330 HSV type 1 (HSV-1) is typically found in the trigeminal ganglia and causes orofacial lesions, whereas HSV-2 is more commonly found in the lumbosacral ganglia. However, either of these viruses can infect any region of the body.
The common cold sore or fever blister is a manifestation of the reactivation of latent infection. Reactivation can occur after exposure to ultraviolet light, fever, immunosuppression, or trauma. Prospective randomized studies have demonstrated a higher incidence of postpartum oral HSV reactivation in women randomly assigned to receive neuraxial (epidural,331,332 intrathecal333) morphine than among women assigned to receive systemic morphine for post–cesarean delivery analgesia. Case reports have associated intraspinal administration of meperidine and fentanyl with the subsequent recurrence of HSV infection.334,335
To our knowledge, postcesarean reactivation of HSV infection after neuraxial opioid administration has not resulted in clinically significant maternal or neonatal complications.336 In addition, we are unaware of any study that has investigated whether epidural or intrathecal opioid administration during labor increases the incidence of recurrent oral HSV infection after vaginal delivery. Therefore, we do not withhold neuraxial opioids during labor in women with a history of oral herpes.
Delayed Gastric Emptying
Labor may result in delayed gastric emptying, which may be exacerbated by opioid administration (see Chapter 29).337,338 Intravenous or intramuscular opioid administration results in delayed gastric emptying in laboring women. Studies suggest that epidural fentanyl combined with bupivacaine and administered as part of a continuous epidural infusion does not result in delayed gastric emptying compared with infusion of bupivacaine alone339,340; however, delayed gastric emptying may occur with epidural fentanyl administered as a bolus (50 to 100 µg)341,342 or with a prolonged infusion.339 In another study, intrathecal fentanyl 25 µg resulted in delayed gastric emptying compared with epidural fentanyl 50 µg plus bupivacaine or bupivacaine alone.343 Delayed gastric emptying may predispose a patient to nausea and vomiting. In addition, it may result in a greater volume of gastric contents, which—in theory—might be problematic in patients who require induction of general anesthesia for emergency cesarean delivery.
Complications of Neuraxial Analgesia
Inadequate Analgesia
The reported failure rate for neuraxial analgesia varies according to the definition of “failure.”30,344,345 In survey studies the rate of epidural catheter replacement has ranged from 5% to 13%.30,344,345 Successful location of the epidural space is not always possible, and satisfactory analgesia does not always occur, even when the epidural space has been identified correctly. Factors such as patient age and weight, the specific technique, the type of epidural catheter, and the skill of the anesthesia provider are associated with the rate of failure of neuraxial analgesia.30,345 Failure to provide adequate analgesia not only results in a dissatisfying experience for the patient but also may lead to litigation.346 The risk for failed anesthesia and the potential need to place a second epidural catheter should be discussed with the patient during the preanesthetic evaluation, before placement of the first epidural catheter.
Pan et al.30 used quality assurance data to retrospectively assess the failure rate among more than 12,000 neuraxial procedures performed for labor analgesia over a 3-year period (Table 23-8). The overall failure rate of 12% included procedures that resulted in no or inadequate analgesia, unintentional dural puncture with an epidural needle or catheter, intravenous cannulation with the epidural catheter, or replacement of the catheter for any reason. After initial adequate analgesia, 6.8% of the catheters were replaced, although eventually 98.8% of women received adequate pain relief. The rate of failed analgesia was significantly lower after CSE than after epidural analgesia (10% versus 14%, respectively; P < .001).
TABLE 23-8
Characteristics of Neuraxial Analgesia Failures*
* Retrospective audit of all neuraxial analgesic procedures for labor analgesia at a single teaching institution over a 3-year period. Most of the procedures were performed by residents.
† Dural puncture with epidural needle or catheter.
‡ Epidural catheter initially functional but was replaced during the course of labor.
NA, not applicable.
Modified from Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. Int J Obstet Anesth 2004; 13:227-33.
Typically, failed analgesia after injection of intrathecal or epidural anesthetics results in no neuroblockade, unilateral blockade or missed segments, or inadequate density of neuroblockade. Patient complaints of pain should prompt timely evaluation and treatment (Box 23-7). The progress of labor should be assessed, and the patient should be queried as to the nature of the pain. Typically, pain becomes more intense as labor progresses. An epidural block that was adequate at 4-cm cervical dilation may not be adequate at 8-cm cervical dilation. Expectations and treatment may be different for women in latent versus active or second-stage labor. The bladder should be checked and emptied if distended. The position of the epidural catheter at the skin should be assessed to exclude the possibility of catheter migration out of the epidural space. Inadequate analgesia may also result from migration of the epidural catheter into a vein or movement of the catheter outside of the epidural space. Before giving a bolus dose of local anesthetic, the anesthesia provider should give a test dose to exclude intravenous migration of the catheter.
The extent of neuroblockade should be assessed with a cold or sharp stimulus that starts over the lateral thighs (the dermatomal level at which the tip of the epidural catheter is sited) and moves both cephalad and caudad on both sides. Inexperienced anesthesia providers often fail to check for the presence of sacral blockade. In the case of no sensory blockade, the epidural catheter should be replaced. If the extent of neuroblockade is inadequate (in either the cephalad or caudad direction), or if there is unilateral blockade or missed segments, the injection of a large volume (10 to 15 mL) of a dilute local anesthetic solution (e.g., 0.0625% to 0.125% bupivacaine) may result in satisfactory analgesia. An advantage of using a more dilute solution of local anesthetic is the ability to increase the administered volume to ensure adequate spread of analgesia.
Some women appear to have adequate extent of sensory blockade but still complain of pain. These women may require more dense analgesia; a larger dose of local anesthetic (10- to 15-mL bolus of 0.125% bupivacaine or a 5- to 10-mL bolus of 0.25% bupivacaine) often successfully reestablishes analgesia. Alternatively, a lipid-soluble opioid (e.g., fentanyl 50 µg) may be added to the solution. The opioid is especially helpful if the parturient is experiencing back pain because the fetus is in the occiput posterior position. In some European practices, epidural clonidine (75 µg) is used to treat breakthrough pain that occurs during/after epidural administration of the standard local anesthetic-opioid solution.
Some anesthesia providers advocate pulling the epidural catheter 1 to 2 cm out of the epidural space before administering the bolus injection. Beilin et al.347 investigated this practice by randomly assigning women with incomplete analgesia to one of two treatments: (1) immediate injection of 0.25% bupivacaine 5 mL or (2) withdrawal of the (multi-orifice) epidural catheter 1 cm followed by injection of the same dose of bupivacaine. There was no difference in the ability to rescue analgesia between the two treatments (74% versus 77%, respectively).
Although available data are inconsistent, maternal position has little effect on the development of an asymmetric block after a bolus dose of anesthetic solution into the epidural space.348,349 Husemeyer and White348 administered 10 mL of epidural 1.5% lidocaine to pregnant women who were in the lateral position; they observed greater spread (two to three spinal segments) of anesthesia on the dependent side. In contrast, others have observed that posture has little influence on the spread of local anesthetic within the epidural space.281,350 It is likely that the position of the epidural catheter in relation to other epidural space structures (e.g., connective tissue, fatty tissue, blood vessels) affects the spread and quality of analgesia to a greater extent than maternal position. Anatomic barriers (e.g., a longitudinal connective tissue band between the dura and ligamentum flavum) or placement of the catheter tip in the anterior epidural space or paravertebral space may explain some cases of single nerve root, unilateral, or asymmetric blockade.351-354
The response to the bolus dose should be assessed in a timely fashion, and the epidural catheter should be replaced (with the patient's consent) if satisfactory analgesia is not obtained.
Unintentional Dural Puncture
In a meta-analysis of 13 studies that involved more than 300,000 obstetric patients, Choi et al.355 determined that the rate of unintentional dural puncture with an epidural needle or catheter was 1.5% (95% CI, 1.5% to 1.5%) (see Chapter 31). Dural puncture may be detected at the time of insertion of the epidural needle or after placement of the catheter. If dural puncture is detected with the epidural needle, the anesthesia provider has two primary options. He or she may elect to remove the needle and place an epidural catheter at another interspace; if CSE analgesia was planned, the intrathecal dose may be injected through the epidural needle before it is removed and re-sited at a different interspace. Alternatively, the anesthesia provider may place a catheter in the subarachnoid space and administer continuous spinal analgesia for labor and delivery. This latter technique is particularly advantageous for patients at high risk for repeat dural puncture on a second attempt or in cases in which it may be difficult to enter either the epidural or subarachnoid space successfully at an alternative interspace (e.g., in obese women or in patients with abnormal anatomy of the lumbar spine). It is very important to append a label that clearly identifies the catheter as a spinal catheter to decrease the risk for injecting an epidural dose of local anesthetic into the subarachnoid space. The parturient and all providers on the labor and delivery unit, including nurses, midwives, and other anesthesia providers, must be made aware of the intrathecal catheter, and this information must be communicated during any hand-off of care to another provider.
Re-siting the epidural catheter in a different interspace eliminates the problem of mistaking an intrathecal catheter for an epidural catheter. However, local anesthetic or opioid injected through the epidural catheter may pass through the dural puncture site and into the subarachnoid space, resulting in unexpectedly high neuroblockade.356 This complication is more likely to occur with the bolus injection of local anesthetic than with an epidural infusion of local anesthetic.
If dural puncture is not recognized until CSF is aspirated from the catheter, or if administration of the test dose results in spinal anesthesia, the anesthesia provider has the following two options: (1) replace the epidural catheter at an alternative interspace or (2) provide continuous spinal analgesia through the existing catheter.
Respiratory Depression
The administration of opioids by any route entails risk for respiratory depression. Factors that affect the risk for respiratory depression after neuraxial opioid administration include the choice and dose of drug and its interaction with systemically administered opioids and other central nervous system depressants (see Chapter 13). The most important factor affecting the onset of respiratory depression is the lipid solubility of the drug.307 In general, if respiratory depression is going to occur, it will do so within 2 hours of the injection of a lipid-soluble opioid such as fentanyl or sufentanil. When a lipid-soluble opioid gains access to the CSF, it is quickly absorbed by lipophilic body tissues. Subsequent clearance and elimination are similar to those associated with intravenous injection of the same drug. Thus, with spinal or epidural injection of a lipid-soluble opioid, the “time window” for respiratory depression is short. Conversely, with a hydrophilic drug such as morphine, the onset of respiratory depression is delayed. Once a hydrophilic drug such as morphine enters the CSF, it tends to stay in the CSF. Rostral migration and absorption into the respiratory centers occur over several hours, so respiratory depression may not occur until 6 to 12 hours after injection of the drug (see Figure 13-13).
The dose of opioid is a major determinant of the risk for respiratory depression.177 Herman et al.177 observed an increase in end-tidal CO2 concentration with intrathecal fentanyl doses of 15 µg or higher. The time of maximum end-tidal CO2 was approximately 30 minutes after the intrathecal injection. A risk factor for respiratory depression is previous parenteral opioid administration. Several reports have implicated prior intravenous opioid administration as a contributing factor to the respiratory arrest that occurred after intrathecal sufentanil 10 µg administration in laboring women.357,358 (This dose is higher than the currently recommended intrathecal dose range.) For this reason, we refrain from administering a bolus dose of epidural or spinal opioid to women who have recently received systemic opioid analgesia.
Intravascular Injection of Local Anesthetic
The incidence of fatal local anesthetic systemic toxicity (LAST) appears to have declined in the past quarter century.359 In a prospective audit from the United Kingdom of more than 145,000 obstetric epidural procedures, the incidence of intravascular injection was 1 in 5000 (Table 23-9). Bupivacaine 0.75% is no longer used for epidural anesthesia in obstetric patients. In the United States, lidocaine or 2-chloroprocaine is most often used when a high-concentration local anesthetic is required for operative epidural anesthesia, and low concentrations of local anesthetic are now routinely used for labor analgesia. Nonetheless, local anesthetic systemic toxicity remains a serious potential complication during the administration of epidural anesthesia in obstetric patients.
TABLE 23-9
Incidence of Unintentional Intravascular, Intrathecal, and Subdural Injections during Attempted Epidural Labor Analgesia*
| Event | Incidence | Rate (%)† |
| Intravascular injection | 1 : 5000 | 0.020 (0.014-0.029) |
| Intrathecal injection | 1 : 2900 | 0.035 (0.027-0.046) |
| Subdural injection | 1 : 4200 | 0.025 (0.017-0.033) |
| High/total spinal anesthesia | 1 : 16,200 | 0.006 (0.003-0.012) |
* Prospective data collection of 145,550 epidural procedures for obstetric patients in 14 maternity units in the South West Thames Region (United Kingdom) over a 17-year period.
† 95% confidence intervals shown in parentheses.
Modified from Jenkins JG. Some immediate serious complications of obstetric analgesia and anaesthesia: a prospective study of 145,550 epidurals. Int J Obstet Anesth 2005; 14:37-42.
Intravenous injection of a large dose of local anesthetic causes central nervous system symptoms (e.g., restlessness, dizziness, tinnitus, perioral paresthesia, difficulty speaking, seizures, loss of consciousness) (see Chapter 13). Cardiovascular effects may progress from increased blood pressure (as a result of sympathetic stimulation) to bradycardia, depressed ventricular function, and ventricular tachycardia and fibrillation. Bupivacaine cardiotoxicity may be fatal in pregnant women.360
Steps for the management of the unintentional intravascular injection of local anesthetic are listed in Box 23-8. They include treatment of convulsions, supporting oxygenation and ventilation, and initiating advanced cardiac life support, if indicated. Lidocaine should not be administered for the treatment of life-threatening ventricular arrhythmias. Early delivery of the infant should be considered, because it may improve the likelihood of successful resuscitation.
In its 2010 Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care,361 the American Heart Association recommended that providers consider the administration of lipid emulsion in cases of suspected LAST. Both the American Society of Regional Anesthesia and Pain Medicine362 and the Association of Anaesthetists of Great Britain and Ireland363 have incorporated the administration of lipid emulsion into their guidelines for managing LAST. At least one case report describes the successful resuscitation of a parturient who developed LAST after the epidural injection of bupivacaine using lipid emulsion.364
High Neuroblockade and Total Spinal Anesthesia
An unexpectedly high level of anesthesia may result in one of several situations. High (or total) spinal blockade may occur after the unintentional and unrecognized injection of local anesthetic (via a needle or catheter) into either the subarachnoid or subdural space during the planned initiation of epidural analgesia/anesthesia. Alternatively, the epidural catheter may migrate into the subarachnoid or subdural space during the course of labor and delivery. Finally, high spinal blockade may result from an overdose of local anesthetic in the epidural space. Crawford356 reported 6 cases of high or total spinal anesthesia in a series of nearly 27,000 cases of lumbar epidural anesthesia administered during labor (an incidence of approximately 1 in 4500). Paech et al.344 reported 8 cases of unexpectedly high neuroblockade in a series of 10,995 epidural blocks in obstetric patients (an incidence of approximately 1 in 1400). Two patients required tracheal intubation and mechanical ventilation. Jenkins365 reported an incidence of 1 in 16,200 procedures (see Table 23-9).
Aspiration alone, particularly through a single-orifice catheter, is a not completely reliable method of excluding subarachnoid placement of the catheter. Administration of an appropriate test dose and careful assessment of the patient's response to the test dose should minimize the chance of unintentional injection of a large dose of local anesthetic into the subarachnoid space.
High or total spinal anesthesia results in agitation, profound hypotension, dyspnea, the inability to speak, and loss of consciousness. Loss of consciousness usually results from hypoperfusion of the brain and brainstem, not from brain anesthesia. Evidence of spinal anesthesia may be apparent shortly after intrathecal injection of a local anesthetic, but the maximal spread may not be evident for several minutes. This delay underscores the need for the anesthesia provider to carefully assess the effects of both the test and therapeutic doses of local anesthetic. If total spinal anesthesia should occur, the anesthesia provider must be prepared to maintain oxygenation, ventilation, and circulation (Box 23-9). Immediate management consists of avoidance of aortocaval compression, ventilation with 100% oxygen, tracheal intubation, and administration of intravenous fluids and vasopressors to support the blood pressure as needed. The FHR should be monitored continuously.
Extensive neuroblockade may also result from subdural injection of a local anesthetic.366-368 A subdural injection may be difficult to diagnose because onset is later than that with an intrathecal injection and more closely resembles that associated with epidural neuroblockade.
The subdural space is a potential space between the dura mater and the arachnoid mater. A retrospective review of 2182 lumbar epidural injections for pain management found that clinical signs of subdural catheter placement occurred in approximately 0.82% of patients366; the true incidence is not known, but may be as high as 10%.369 Subdural injection of local anesthetic typically results in unexpectedly high (but patchy) blockade with an onset time that is intermediate between that of spinal anesthesia and epidural anesthesia (i.e., 10 to 20 minutes) (Table 23-10).369 Cranial spread is more extensive than caudal spread of the local anesthetic, so sacral analgesia typically is absent. The block may involve the cranial nerves. (The subdural space, unlike the epidural space, extends intracranially.) Thus, apnea and unconsciousness can occur during a subdural block. Horner's syndrome has been reported.367 A subdural block usually results in less intense motor blockade than the blockade that occurs with high or total spinal anesthesia. This difference may reflect the limited spread of the local anesthetic within the subdural space, which helps spare the anterior motor fibers.368 Subdural block results in less severe hypotension than that with high or total spinal anesthesia, most likely because subdural injection leads to less sympathetic blockade than spinal anesthesia. The unpredictable spread of local anesthetic, the slower onset of maximal spread (in comparison with spinal anesthesia), the patchy nature of the block, and the sacral sparing make it difficult to use a subdural catheter safely during labor and delivery. If it is suspected that a catheter is positioned within the subdural space, it should be replaced with an epidural catheter.
Unexpectedly high neuroblockade may result from the migration of an epidural catheter into the subdural or subarachnoid space.368 The mechanism by which a soft epidural catheter penetrates the dura or dura-arachnoid is unclear. Disposable epidural needles are sharp, and insertion of the needle into the epidural space may result in an unrecognized nick in the dura, which may create a site for delayed migration of the catheter into the subdural or subarachnoid space. Subdural or subarachnoid injection of local anesthetic also may occur if a multi-orifice catheter is used, and one orifice is located within the epidural space while another is located within the subdural or subarachnoid space. In this situation, the force of injection determines the ultimate destination of the local anesthetic. Thus, each bolus injection of local anesthetic should serve as a test dose. During the continuous infusion of a local anesthetic, a gradual increase in the level of anesthesia and intensity of motor blockade may indicate the intrathecal infusion of the local anesthetic solution.
Extensive Motor Blockade
Clinically significant motor block may occur after repeated bolus doses146 or after many hours of a continuous infusion of local anesthetic into the epidural space.370 The administration of bupivacaine with epinephrine may result in a greater likelihood of motor blockade than the administration of bupivacaine alone.146,371 Extensive motor blockade is often bothersome for the patient, and it may impair maternal expulsive efforts during the second stage of labor and increase the likelihood of instrumental vaginal delivery (see later discussion). Some obstetricians argue that pelvic floor relaxation prevents rotation of the fetal head and increases the likelihood of an abnormal position of the vertex at delivery.
If intense motor blockade develops during the continuous epidural infusion of local anesthetic, the infusion can be discontinued for a short period (e.g., 30 minutes). Subsequently, the infusion can be restarted at a reduced rate or with a more dilute solution of local anesthetic. Extensive motor blockade does not occur with administration of a very dilute solution of local anesthetic combined with an opioid.
Prolonged Neuroblockade
Rarely, the duration of neuraxial analgesia/anesthesia exceeds the time expected. Most cases of unexpectedly prolonged neuroblockade follow the epidural administration of a high concentration of local anesthetic with epinephrine.372 Abnormal neurologic findings after the administration of neuraxial anesthesia should prompt the anesthesia provider to look for evidence of peripheral nerve injury or an epidural hematoma (see Chapter 32). Factors that argue against the presence of an epidural hematoma include (1) the absence of back pain, (2) a unilateral block, and (3) regression (rather than progression) of the symptoms. Peripheral nerve injuries typically result in a neurologic deficit in the distribution of a specific peripheral nerve. Neurologic or neurosurgical consultation and immediate imaging studies should be obtained if there is any question about the etiology of prolonged anesthesia. Avoiding the use of a high concentration of local anesthetic should help minimize the incidence of prolonged neuroblockade during and after labor and vaginal delivery.
Sensory Changes
In one of the early studies of intrathecal opioid administration during labor, Cohen et al.294 observed sensory changes in women who received intrathecal sufentanil. Subsequent studies have demonstrated that these sensory changes do not result from a local anesthetic effect of sufentanil. Sensory changes do not predict the quality or duration of analgesia or the extent of hemodynamic change.373 Further, intrathecal sufentanil does not cause a sympathectomy.374 Wang et al.375 have provided the best explanation for these sensory changes. They showed that intrathecal opioids block the afferent information from A-delta and C fibers to the spinal cord but that efferent nerve impulses are unaffected. These sensory changes can be clinically significant, especially when they extend to the cervical dermatomes. In such cases, patients may feel that they cannot breathe or swallow, a sensation that can be quite distressing. Fortunately, neither intrathecal sufentanil nor fentanyl affects the efferent limb of the nervous system and so does not impair motor function. Affected patients should be reassured that respiratory efforts are not compromised and that these symptoms will subside in 30 to 60 minutes.376,377
In addition to sensory changes, case reports have described mental status changes, aphasia, and automatisms after the intrathecal injection of fentanyl378 and sufentanil.379 These symptoms seem to be related to an opioid effect. In one case, the symptoms were partially reversed by naloxone.378
Back Pain
Approximately 50% of women complain of back pain during pregnancy and the puerperium.380,381 The most significant risk factors for postpartum back pain are antepartum back pain and inability to reduce weight to prepregnancy levels.380,382,383 Early retrospective studies identified an association between epidural anesthesia and an increased risk for postpartum back pain.384,385 However, retrospective studies suffer not only from patient recall bias (i.e., patients with a problem are much more likely to complete and return the questionnaire) but also from selection bias in the epidural and nonepidural groups. Patients who select epidural analgesia for labor may have obstetric, orthopedic, social, or other unidentified factors that predispose them to postpartum back pain.
In an attempt to assess anesthetic factors that might contribute to postpartum backache (e.g., motor blockade), Russell et al.383 randomly assigned laboring women requesting epidural analgesia to receive either bupivacaine alone or bupivacaine plus an opioid. Despite the expected differences in motor blockade, the incidence of backache did not differ between the two anesthetic groups (bupivacaine alone, 39%; bupivacaine plus an opioid, 30%). In addition, the incidence of backache in both epidural groups was similar to that found in a nonrandomized control group of women who labored without epidural analgesia (31%).
Prospective reports have not shown a significant relationship between the use of epidural analgesia and long-term backache. Breen et al.382 observed no difference in the incidence of postpartum backache among women who delivered vaginally with or without epidural analgesia. A prospective Canadian study assessed the relationship between postpartum backache and patient-selected intrapartum analgesia.386 The rate of low back pain was greater in the epidural group (53%) than in the nonepidural group (43%) on the first postpartum day, but the rates were similar on postpartum day 7 and at 6 weeks and 1 year.386 These investigators suggested that the higher incidence of backache immediately after delivery may have resulted from tissue trauma during epidural needle placement. Finally, Loughnan et al.387 enrolled 310 women in a randomized controlled trial that compared epidural bupivacaine with systemic meperidine analgesia. The primary outcome was back pain 6 months after delivery. There was no difference between the two groups in the incidence of backache (epidural 48%, meperidine 50%). Similarly, another randomized controlled trial of epidural versus nonepidural analgesia found no difference in the incidence of backache at 3 and 12 months388 and several years389 after delivery.
In summary, prospective studies have consistently shown that no causal relationship exists between epidural analgesia and the development of long-term postpartum backache. Short-term backache (several days) may be related to local tissue trauma at the site of skin puncture.
Pelvic Floor Injury
Few studies have evaluated the possible effects of epidural analgesia on postpartum pelvic floor function. In a case-control study, Christianson et al.390 did not identify epidural analgesia as a risk factor for third- or fourth-degree perineal lacerations. Similarly, Sartore et al.391 observed no significant difference in the incidence of stress urinary incontinence, anal incontinence, or vaginal prolapse 3 months after vaginal delivery between those women who did and those who did not receive epidural analgesia. In a small randomized trial that compared epidural with systemic administration of meperidine, there was no difference in the rate of perineal trauma between groups.388
Any factor that increases the likelihood of instrumental vaginal delivery might be expected to increase the risk for pelvic floor injury and subsequent pelvic floor dysfunction (see later discussion). However, to our knowledge, there is no evidence that epidural analgesia per se predisposes to pelvic floor injury.
Effects of Neuraxial Analgesia on the Progress of Labor
Neuraxial analgesia during labor is associated with a prolonged labor and operative delivery. (The term operative delivery refers to both cesarean delivery and instrumental vaginal delivery [e.g., forceps delivery or vacuum extraction]). Controversy exists as to whether there is a cause-and-effect relationship between the use of these analgesic techniques and prolonged labor or operative delivery. The understanding of this subject has been limited by the difficulty of performing controlled trials in which parturients are randomly assigned to neuraxial analgesia or a control group. Ideally, if one wants to study the effect of neuraxial analgesia on the progress and outcome of labor, the control group would receive no analgesia. However, such a study is not ethical, and even if it were and women volunteered to participate in it, the crossover rate would probably be high and the data consequently would not be interpretable. Therefore, controlled trials have randomly assigned parturients to receive neuraxial analgesia or an alternative form of pain relief, usually systemic opioid analgesia. However, even when the control group receives some type of analgesia, the crossover rate may be high because the quality of neuraxial analgesia is markedly superior to that of all other modes of labor analgesia.392
The difficulty in performing and interpreting the results of labor analgesia trials was aptly described by Noble et al.,393 who assessed obstetric outcome in 245 patients randomly assigned to receive either epidural analgesia or “conventional” analgesia (i.e., meperidine, nitrous oxide, or no analgesia). The investigators made the following comments393:
Of 245 selected patients, 43 had to be removed from the trial after labour ensued.…Most of the patients removed from the non-epidural group were apparently experiencing severe pain; they were usually primigravidae whose baby presented in the occipito-posterior position.…The majority of patients removed from the epidural group were apparently normal and usually multigravidas; their labours were so rapid it was not possible to arrange for an epidural block.
In other words, patients at low risk for operative delivery were excluded from the epidural group, and patients at high risk were excluded from the nonepidural group. The investigators' candid comments illustrate that, even when a prospective, randomized study is performed, it is difficult to maintain conditions that allow for the comparison of women at equal risk for abnormal labor and operative delivery.
Another concern is the external validity of these studies. Women who agree to participate in research trials may be inherently different from women who refuse to participate. Many women make a decision regarding labor analgesia well before the onset of labor and are unwilling to let chance randomization determine the type of labor analgesia. Thus, the study results may not be generalizable to the general obstetric population.
Ironically, the effect of systemic opioids on the progress and outcome of labor has not been well studied. Furthermore, there may be differences among the opioids.394 Finally, neuraxial analgesia is not a generic procedure. Conclusions about the effect of one technique on the progress of labor may not be applicable to other techniques (see later discussion).
Additional factors prevent rigorous scientific study of this issue. Ideally, a randomized controlled trial should be double blinded. This is not possible for studies that compare neuraxial analgesia with another mode of analgesia, because of the marked difference in the quality of analgesia. Therefore, the potential for bias on the part of the parturient, nurses, and anesthesia and obstetric providers is substantial. Additionally, a number of factors are known to affect or to be associated with the progress and outcome of labor, including parity, artificial rupture of membranes, use of oxytocin, and payer status; these factors should be controlled in well-conducted studies.
One factor known to markedly influence the outcome of labor is the obstetric provider. Neuhoff et al.395 retrospectively reviewed the records of 607 nulliparous women at term gestation and compared the mode of delivery in “clinic” patients (whose care was given primarily by residents) and private patients (whose care was provided primarily by private obstetricians). Approximately 42% of patients received epidural analgesia during labor. Five percent of patients in the clinic group and 17% of patients in the private group underwent cesarean delivery (P < .001). More striking was the difference between groups in the incidence of cesarean delivery for dystocia (0.5% versus 13.7%, respectively; P < .001). Similarly, Guillemette and Fraser396 observed marked obstetrician variation in cesarean delivery rates, despite similarities in the use of oxytocin and epidural analgesia.
Several groups of investigators have noted that the timing of cesarean delivery conforms to a “circadian” rhythm.397,398 For example, investigators in Japan noted a delivery time rhythm in hospitals, but not birthing centers, suggesting that obstetric intervention, not biologic rhythm, partly determines the timing of delivery.399
Retrospective studies are difficult to interpret because they suffer from selection bias. In some cases, distinguishing between anesthesia administered for pain relief during labor and anesthesia administered in preparation for operative delivery is difficult. Moreover, women at higher risk for prolonged labor and operative delivery are more likely to request and receive epidural analgesia during labor than women who have a rapid, uncomplicated labor.400 Wuitchik et al.400 observed a relationship between pain and cognitive activity during early labor and the subsequent progress of labor in 115 healthy nulliparous women. During the latent phase, higher levels of pain were predictive of longer latent and active phases of labor. Those women who reported “horrible” or “excruciating” pain during the latent phase were more than twice as likely to require instrumental delivery as women who only had “discomfort.” In addition, women who reported “distress” rather than “coping” had a fivefold higher incidence of abnormal FHR patterns and a fourfold higher requirement for assistance from pediatricians during neonatal resuscitation.
Greater pain intensity during labor appears to be a risk factor for operative delivery. This fact will significantly bias observational studies of labor analgesia because women with greater pain intensity request analgesia, specifically neuraxial analgesia, at a higher rate than women with less intense pain. Alexander et al.401 performed a secondary analysis of data from a randomized controlled trial in which one group of laboring women received patient-controlled intravenous meperidine analgesia. The rate of cesarean delivery for dystocia was 14% in women who self-administered 50 mg/h or more of meperidine, compared with 1.4% in women who self-administered less than 50 mg/h. In a retrospective study of factors that predict operative delivery in laboring women, Hess et al.402 found that the cesarean delivery rate in women who had significant breakthrough pain during low-dose bupivacaine/fentanyl epidural analgesia was more than twice as high as the rate in women with less breakthrough pain (odds ratio, 2.62; 95% CI, 2.01 to 3.43).
Taken together, these studies suggest that the early onset of severe pain and the requirement of high doses of analgesic agents predict higher risks for abnormal labor, FHR abnormalities, and operative delivery. These findings may explain the observed association between neuraxial analgesia and operative delivery.
Cesarean Delivery Rate
Randomized Controlled Trials
A number of randomized controlled trials have studied the effect of neuraxial (primarily epidural) and systemic opioid (primarily meperidine) analgesia on the cesarean delivery rate.388,392,403-427 These trials differ in a number of ways, including (1) the population studied (e.g., nulliparous women or women of mixed parity); (2) onset of labor (spontaneous labor alone or a mix of spontaneous and induced labors); (3) type of neuraxial analgesia; (4) density of neuraxial analgesia; (5) route of administration of systemic analgesia (although all the studies included meperidine with or without an adjuvant); (6) the crossover rate; and (7) management of labor (e.g., active management of labor, including electronic FHR monitoring, artificial rupture of membranes, and oxytocin infusion). All but one of these studies found no difference in the rate of cesarean delivery between women randomly assigned to receive either neuraxial or systemic opioid analgesia.
Four prospective, randomized trials were performed at the University of Texas Southwestern Medical Center, Parkland Hospital, in Dallas.392,408,422,423 This institution is unique among many others that have performed randomized trials, in that the population is composed largely of indigent women whose labor is managed by the same group of resident physicians and midwives, supervised by the same core group of attending obstetricians. In the first study, 1330 women of mixed parity were randomly assigned to receive either epidural bupivacaine/fentanyl or intravenous meperidine for labor analgesia.392 Approximately one third of the women did not receive the assigned treatment. The cesarean delivery rates were 9.0% in women who received epidural analgesia and 3.9% in women who received intravenous meperidine. However, the investigators did not report an intent-to-treat analysis of these data; thus, it was unclear whether there was a higher incidence of cesarean delivery in the women randomly assigned to the epidural analgesia group. Subsequently, the investigators published a reanalysis of the data that included an intent-to-treat analysis (Table 23-11).428 The cesarean delivery rate in both groups was 6%. These analyses support the conclusion that women who choose epidural analgesia have an inherent risk factor(s) for cesarean delivery and that the administration of neuraxial analgesia per se does not alter this risk.
TABLE 23-11
Parkland Hospital Randomized Controlled Trial of Epidural Versus Systemic Opioid Analgesia and Rate of Cesarean Delivery: Actual Treatment Versus Intent-to-Treat Analysis*
| Type of Analysis | Cesarean Delivery Rate (%) | |
| EPIDURAL ANALGESIA (n = 664) | SYSTEMIC OPIOID ANALGESIA (n = 666) | |
| Actual treatment† | 9.0 | 3.9‡ |
| Intent-to-treat | 6 | 6 |
* In the systemic opioid group, 103 women requested and received epidural analgesia because opioid analgesia was inadequate. The initial analysis was published in 1995. The intent-to-treat analysis of the same data was published in 2000.
† The protocol violation rate was 35%.
‡ P < .05 compared with epidural analgesia group.
Data from Ramin SM, Gambling DR, Lucas MJ, et al. Randomized trial of epidural versus intravenous analgesia during labor. Obstet Gynecol 1995; 86:783-9; and Sharma SK, Leveno KJ. Update: Epidural analgesia does not increase cesarean births. Curr Anesthesiol Rep 2000; 2:18-24.
In an attempt to lower the rate of crossover by providing better analgesia to the control (meperidine) group, the Parkland Hospital investigators performed another study in which meperidine was administered by PCIA.422 A significant number of women in both groups did not receive their assigned treatment, although the reason in all cases was rapid labor. Only 5 of 357 women randomly assigned to the meperidine group crossed over to receive epidural analgesia. Using an intent-to-treat analysis, the investigators observed no difference between the groups in the incidence of cesarean delivery (4% in the epidural group and 5% in the PCIA group). There was no difference between the two groups in neonatal outcome, except that more neonates of women in the PCIA group received naloxone to reverse respiratory depression at birth.
Only one randomized trial has compared CSE and systemic opioid analgesia.408 In this large study (n = 1223), patients of mixed parity were randomly assigned to receive CSE analgesia (intrathecal sufentanil 10 µg, followed by epidural bupivacaine with fentanyl at the second request for analgesia) or intravenous meperidine (50 mg every hour on request). Approximately 60% of patients complied with the protocol. An intent-to-treat analysis showed that there was no difference between groups in the rate of cesarean delivery (CSE 6%, systemic opioid 5.5%).
The studies comparing neuraxial with systemic opioid analgesia have been systematically reviewed in several meta-analyses.3,429 The latest meta-analysis covered outcomes for 8417 women randomized to receive neuraxial or no neuraxial/no analgesia (control) from 27 trials (Figure 23-8).3 The risk ratio for cesarean delivery in women randomly assigned to receive neuraxial analgesia compared with those assigned to the control group was 1.10 (95% CI, 0.97 to 1.25).3 In an individual patient meta-analysis of the studies performed at Parkland Hospital (n = 4465),429 the odds ratio was 1.04 (95% CI, 0.81 to 1.34).
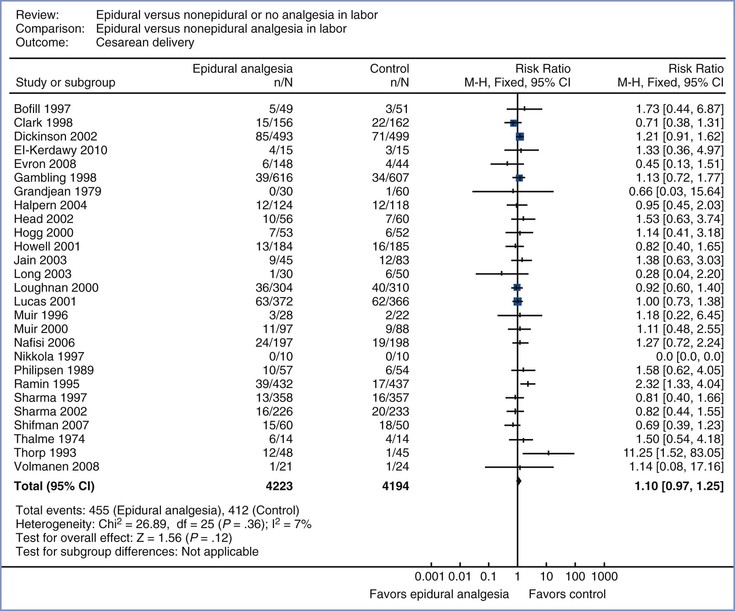
FIGURE 23-8 Meta-analysis of cesarean delivery rate in women randomized to neuraxial or non-neuraxial labor analgesia. The number of women who had a cesarean delivery, the risk ratio, and 95% confidence interval (CI) of the risk ratio (fixed effect model) are shown for each study. For studies with no cesarean deliveries the risk ratio could not be calculated. Control, nonepidural analgesia. n, number of events (cesarean delivery) in the neuraxial or non-neuraxial group; N, total number of subjects in the neuraxial or non-neuraxial group. The scale is logarithmic. (Modified from Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev 2011; [12]:CD000331.)
Mode and Density of Neuraxial Analgesia and Effect on Cesarean Delivery Rate.
If neuraxial analgesia adversely affects the outcome of labor, one would expect to observe a dose-response effect. The COMET study randomly assigned more than 1000 parturients to one of three groups: (1) “high-dose” epidural analgesia (traditional epidural analgesia with bupivacaine 0.25%); (2) “low-dose” epidural analgesia (bupivacaine 0.1%/fentanyl 2 µg/mL bolus, followed by a continuous epidural infusion); and (3) “low-dose” CSE analgesia (intrathecal bupivacaine/fentanyl followed by intermittent boluses of epidural bupivacaine 0.1%/fentanyl 2 µg/mL).430 There was no difference in cesarean delivery rates among groups (Figure 23-9). Similarly, several other studies that compared traditional epidural analgesia (using bupivacaine 0.25%) and low-dose CSE techniques found no difference between groups in the cesarean delivery rate.431-433 The results of these studies suggest that “high-dose” neuraxial analgesia does not entail a higher risk for cesarean delivery than “low-dose” techniques; in other words, no dose-response effect has been observed.
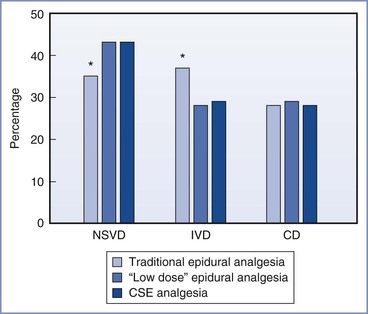
FIGURE 23-9 Outcome of labor in the COMET study. Parturients were randomly assigned to traditional epidural analgesia or to one of two “low-dose” neuraxial techniques (see text). There was no difference among groups in the cesarean delivery (CD) rate. *Women who received traditional epidural analgesia had a higher rate of instrumental vaginal delivery (IVD) than those who received either “low-dose” technique (P = .04). CSE, combined spinal-epidural; NSVD, normal spontaneous vaginal delivery. (Data from Comparative Obstetric Mobile Epidural Trial Study Group UK. Effect of low-dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet 2001; 358:19-23.)
There is no evidence that CSE analgesia influences the mode of delivery, when compared with epidural analgesia alone. Large randomized controlled trials comparing CSE analgesia with epidural analgesia alone have found no difference between groups in the rate of cesarean delivery.430-432,434
Impact Studies
Some physicians have questioned whether prospective, randomized studies provide an accurate representation of the effect of neuraxial analgesia on the mode of delivery in actual clinical practice. They have suggested the possibility that prospective studies may introduce a Hawthorne effect (which may be defined as the appearance or disappearance of a phenomenon on initiation of a study to confirm or exclude its existence). An alternative study design is to assess obstetric outcome immediately before and after a sentinel event, such as the introduction of an epidural analgesia service in a given hospital. The results of these studies may be generalizable to the general population because patients have not chosen to participate in a study. It also eliminates the problem of treatment group crossover because epidural analgesia was not available in the control period. One limitation of this study design is that it assumes that there were no other changes in obstetric management in the “after” period.
In 1999, Yancey et al.435 published an impact study using data from the Tripler Army Medical Center in Hawaii. Because of relative homogeneity in socioeconomic status, universal access to health care, and the availability of dedicated health care providers in the population served by this hospital, its rate of cesarean delivery may not be subject to influences common to other hospitals. Prior to 1993 the rate of epidural analgesia was less than 1% at Tripler Army Medical Center. In 1993 a policy change within the U.S. Department of Defense mandated on-demand availability of neuraxial labor analgesia in military hospitals. In nulliparous women in spontaneous labor with a singleton infant with a vertex presentation, the rate of epidural labor analgesia rose from less than 1% to approximately 80% in a 1-year period.436 The rate of cesarean delivery was unchanged during the same period (14.4% versus 12.1%, respectively; adjusted RR, 0.8; 95% CI, 0.6 to 1.2).
In another impact study, Impey et al.437 compared obstetric outcome for the first 1000 nulliparous women (term gestation, singleton fetus, cephalic presentation, spontaneous labor) who delivered at the National Maternity Hospital in 1987 with the outcome for a similar group of women who delivered in 1992 and 1994. The epidural analgesia rate rose from 10% in 1987 to 45% in 1992 and 57% in 1994. In each of these 3 years, 82% of women underwent spontaneous vaginal delivery. The cesarean delivery rate was 4% in 1987, 5% in 1992, and 4% in 1994 (P = NS) (Figure 23-10). The investigators concluded that the consistency of the operative delivery rates in each of 3 years with very different epidural rates suggests that epidural analgesia does not increase the cesarean delivery rate.
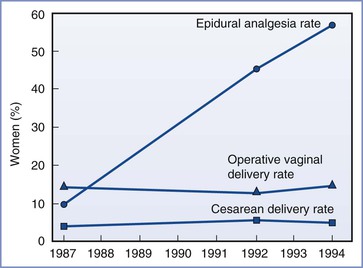
FIGURE 23-10 Epidural analgesia and cesarean and instrumental vaginal delivery rates for 1000 consecutive nulliparous women in spontaneous labor at term during 3 different years at the National Maternity Hospital in Dublin, Ireland. (Modified from Impey L, MacQuillan K, Robson M. Epidural analgesia need not increase operative delivery rates. Am J Obstet Gynecol 2000; 182:358-63.)
Socol et al.438 evaluated the impact of three initiatives to reduce the cesarean delivery rate in their hospital. First, they strongly encouraged a trial of labor and vaginal birth after cesarean delivery. Second, after the 1988 calendar year, they circulated data showing the cesarean delivery rate of every obstetrician to all obstetricians. Third, they recommended the active management of labor as the preferred method of labor management for term nulliparous women. The rates of total, primary, and repeat cesarean deliveries dropped from 27%, 18%, and 9% in 1986 to 17%, 11%, and 6%, respectively, in 1991 (P < .001 for all three comparisons). Meanwhile, the use of epidural analgesia rose from 28% in 1986 to 48% in 1991 (P < .001). There was no change in the incidence of instrumental vaginal delivery (13% in 1986 versus 13% in 1991).
In a meta-analysis, Segal et al.439 identified nine impact studies involving a total of 37,753 patients. These researchers found no increase in the rate of cesarean delivery with the increase in availability of epidural analgesia (Figure 23-11). Thus, the before-after impact studies support the results of randomized controlled trials—namely, that neuraxial analgesia does not cause an increase in the cesarean delivery rate.
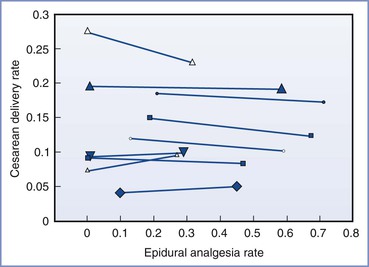
FIGURE 23-11 Rates of cesarean delivery during periods of higher and lower availability of epidural analgesia in nine studies (n = 37,753) subjected to meta-analysis. Each pair of symbols shows data from one investigation (the left symbol is the epidural analgesia rate and cesarean delivery rate during the period of low epidural analgesia availability, and the right symbol is the epidural analgesia rate and cesarean delivery rate during the period of high epidural availability). The size of the plot symbol is proportional to the number of patients in the analysis. (Modified from Segal S, Su M, Gilbert P. The effect of a rapid change in availability of epidural analgesia on the cesarean delivery rate: a meta-analysis. Am J Obstet Gynecol 2000; 183:974-8.)
Several studies have assessed whether there is a relationship between an individual obstetrician's cesarean delivery rate and the rate of epidural analgesia for his or her patients.440,441 For example, Lagrew et al.440 divided obstetricians into two groups according to whether their individual cesarean delivery rates were more than 15% (the control group) or less than 15% (the target group). Obstetricians in the target group used epidural analgesia more often than obstetricians in the control group. In other words, the target group of obstetricians was able to achieve a lower cesarean delivery rate despite their greater use of epidural analgesia.
Timing of Initiation of Neuraxial Analgesia
Review of observational data suggests an association between cesarean delivery and the initiation of neuraxial analgesia during early labor (often defined as a cervical dilation < 4 to 5 cm).426,432,442 For example, in a retrospective study of 1917 nulliparous women, the rate of cesarean delivery was twice as high in women who received neuraxial analgesia at a cervical dilation less than 4 cm than in those in whom neuraxial analgesia was initiated at a cervical dilation of 4 cm or more (18.9% versus 8.9%, respectively).442 As a result of these data, for many years the ACOG suggested that women delay requesting epidural analgesia “when feasible, until the cervix is dilated to 4 to 5 cm.”443 However, as with the cause-and-effect question raised by the association of neuraxial labor analgesia and cesarean delivery, the question arises as to whether the early initiation of neuraxial labor analgesia causes a higher risk for cesarean delivery or whether the request for early labor analgesia is a marker for some other risk factor(s) for cesarean delivery.
A number of randomized controlled trials have addressed the question of whether initiation of neuraxial analgesia during early labor adversely affects the mode of delivery.20-24,444,445 All except one small study444 compared early labor neuraxial analgesia with systemic opioid analgesia, which was followed by neuraxial analgesia when cervical dilation reached 4 to 5 cm (the control group in the Luxman et al.444 study received no analgesia). In 1994, Chestnut et al.20,23 reported two trials in which nulliparous women were randomly assigned to receive early epidural analgesia or early intravenous nalbuphine analgesia followed by epidural analgesia when cervical dilation reached 5 cm. The median cervical dilation at the time of initiation of analgesia was 3.5 cm23 and 4 cm20 in the two studies. There was no difference between groups in the cesarean delivery rate.
Subsequently, Wong et al.21 and Ohel et al.22 reported randomized trials that compared early labor neuraxial analgesia with systemic opioid analgesia; the median cervical dilation at initiation of analgesia was 2 cm. As in the previous studies, there was no difference between the two groups in rate of cesarean delivery or in rate of instrumental vaginal delivery. The study protocols differed in that the treatment group in one study received CSE analgesia in early labor,21 whereas the treatment group in the second study received epidural analgesia alone.22 The use of oxytocin augmentation was markedly different in the two studies (94%21 and 29%22).
Subsequent to the publication of the later studies, the ACOG446 published an updated Committee Opinion entitled Analgesia and Cesarean Delivery Rates. This revised opinion includes the following statement:
Neuraxial analgesia techniques are the most effective and least depressant treatments for labor pain. The American College of Obstetricians and Gynecologists previously recommended that practitioners delay initiating epidural analgesia in nulliparous women until the cervical dilation reached 4-5 cm. However, more recent studies have shown that epidural analgesia does not increase the risks of cesarean delivery. The choice of analgesic technique, agent, and dosage is based on many factors, including patient preference, medical status, and contraindications. The fear of unnecessary cesarean delivery should not influence the method of pain relief that women can choose during labor.
Later randomized trials in nulliparous women in both spontaneous24 and induced445 labor, as well as a 2011 meta-analysis (five randomized controlled trials; n = 14,836),25 replicated these results. The researchers concluded that early initiation of neuraxial analgesia does not increase the rate of cesarean delivery (RR, 1.02 ; 95% CI, 0.96 to 1.08) (Figure 23-12).25
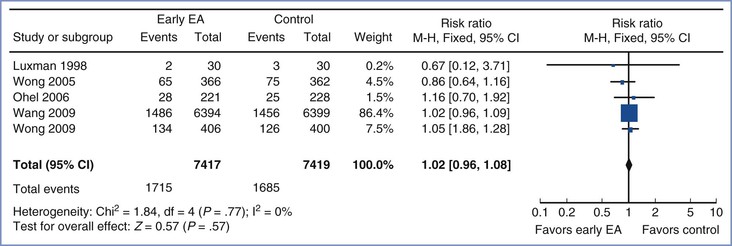
FIGURE 23-12 Meta-analysis of cesarean delivery in women randomized to receive early labor initiation of neuraxial analgesia (cervical dilation < 4 cm) or late initiation (cervical dilation ≥ 4 cm). The size of the box at the point estimate for each study is proportional to the number of patients in the study. The diamond represents the point estimate of the pooled risk ratio, and the length of the diamond is proportional to the confidence interval. Control, late epidural analgesia; EA, epidural analgesia; n, number of events (cesarean delivery) in the treatment or control group; N, total number of patients in the treatment or control group. (Modified from Wassen MM, Zuijlen J, Roumen FJ, et al. Early versus late epidural analgesia and risk of instrumental delivery in nulliparous women: a systematic review. BJOG 2011; 118:655-61.)
Instrumental Vaginal Delivery Rate
Observational data associate neuraxial labor analgesia with a higher rate of instrumental (forceps or vacuum extraction) vaginal delivery. The effect of neuraxial analgesia on mode of vaginal delivery has not been assessed as a primary outcome in randomized controlled trials, although it has been assessed as a secondary outcome in multiple trials. Interpretation of these results is clouded by the fact that most studies have not assessed the quality of analgesia during the second stage of labor. Further, most investigators did not define the criteria for the performance of instrumental vaginal delivery. In clinical practice, and in study interpretation, it is often difficult to distinguish “indicated” instrumental deliveries from elective instrumental deliveries. Indeed, we have observed that indications for instrumental vaginal delivery vary markedly among obstetricians. An obstetrician is more likely to perform an elective instrumental delivery in a patient with satisfactory anesthesia than in a patient without analgesia. In addition, most randomized controlled trials are conducted in teaching institutions that have an obligation to teach obstetric residents how to perform instrumental vaginal delivery. Instrumental vaginal deliveries performed for the purpose of teaching are more likely to be done in women with adequate analgesia.
Multiple randomized, controlled studies comparing epidural analgesia with systemic opioid analgesia have assessed the rate of instrumental vaginal delivery as a secondary outcome variable.* Most systematic reviews have concluded that epidural analgesia is associated with a higher risk for instrumental vaginal delivery than systemic analgesia.3,429 For example, in a 2011 meta-analysis of 23 studies (n = 7935),3 the risk ratio for instrumental vaginal delivery in women randomly assigned to receive epidural analgesia or nonepidural/no analgesia was 1.42 (95% CI, 1.28 to 1.57) (Figure 23-13). Similarly, in the individual patient meta-analysis reported by Sharma et al.,429 the adjusted odds ratio was 1.86 (95% CI, 1.43 to 2.40).
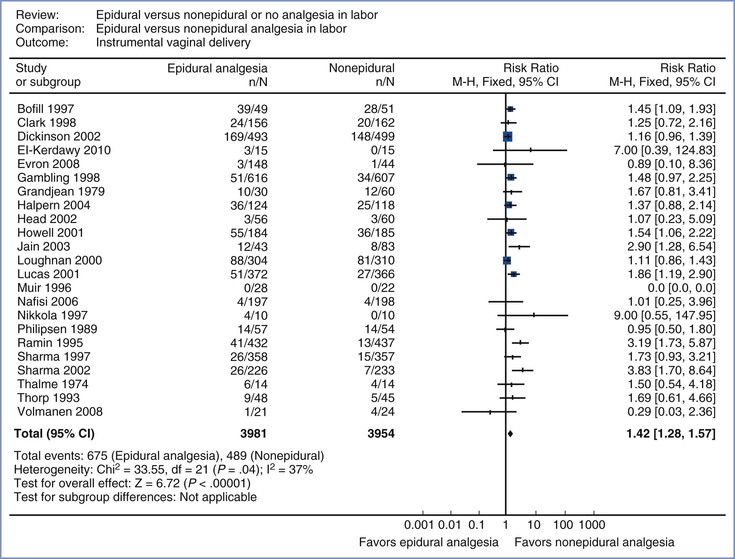
FIGURE 23-13 Meta-analysis of instrumental vaginal delivery rate in women randomized to neuraxial or non-neuraxial labor analgesia. The number of women who had an instrumental vaginal delivery, the risk ratio, and 95% confidence interval (CI) of the risk ratio (fixed effect model) are shown for each study. For studies with no instrumental vaginal deliveries the risk ratio could not be calculated. n, number of events (instrumental vaginal delivery) in the neuraxial or non-neuraxial group; N, total number of subjects in the neuraxial or non-neuraxial group. The scale is logarithmic. (Modified from Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev 2011; [12]:CD000331.)
In contrast to these studies, many of the impact studies observed no difference in the instrumental vaginal delivery rate between the control and study periods.435-437,447 For example, despite a rise in the epidural analgesia rate from 1% to almost 80% at Tripler Army Medical Center, the rate of instrumental vaginal delivery did not change (11.1% versus 11.9%).435 Similarly, despite a more than fivefold increase in the epidural analgesia rate at the National Maternity Hospital in Dublin (see earlier discussion), the instrumental vaginal delivery rate remained unchanged (see Figure 23-10). A systematic review of seven impact studies439 involving more than 28,000 patients did not identify a difference in instrumental vaginal delivery rates between periods of low and periods of high epidural analgesia rates (mean change, 0.76%; 95% CI, −1.2 to 2.8).
Studies of early versus late initiation of neuraxial labor analgesia have not identified an increased risk for instrumental vaginal delivery in the early analgesia group.21,22,24,445
Obstetricians and anesthesiologists have suggested that multiple factors (e.g., station and position of the fetal vertex, maternal pain and the urge to bear down, and neuraxial analgesia–induced motor blockade) may contribute to the outcome of the second stage of labor. The contribution of these factors to the mode of vaginal delivery, and their interactions, are not well understood and these factors have not been well controlled in many studies.
Several studies have specifically assessed the effect of maintenance of neuraxial analgesia until delivery with regard to the duration and outcome of the second stage of labor.370,448-452 Chestnut et al.370 randomly assigned women already receiving epidural analgesia at 8 cm of cervical dilation to receive a continuous epidural infusion of 0.75% lidocaine or saline until delivery. There was no difference between groups in the rate of instrumental vaginal delivery, but women in the lidocaine group as well as the saline group had inadequate second-stage analgesia. In a similar study in which patients were randomly assigned to receive epidural bupivacaine 0.125% or saline (control),449 second-stage analgesia was clearly better in the bupivacaine group than in the control group, but the rate of instrumental vaginal delivery was nearly double (52% versus 27%, respectively; P < .05), and the duration of the second stage was longer. In a third study, second-stage analgesia was maintained with 0.0625% bupivacaine with fentanyl 2 µg/mL or saline-placebo.448 There was no difference between groups in the instrumental vaginal delivery rate, but analgesia was only marginally better in the treatment group.
The effect of neuraxial analgesia on the outcome of the second stage of labor may be influenced by the density of neuraxial analgesia. High concentrations of epidural local anesthetic may cause maternal motor blockade, leading to relaxation of pelvic floor musculature, which in turn may interfere with fetal rotation during descent. Abdominal muscle relaxation may decrease the effectiveness of maternal expulsive efforts. The effects of specific analgesic techniques, concentration of local anesthetic, total dose of local anesthetic, and degree of motor blockade on the risk for instrumental vaginal delivery are overlapping and difficult to study. For example, some studies suggest that administration of epidural analgesia using higher concentrations of bupivacaine is associated with a higher risk for instrumental vaginal delivery compared with use of lower concentrations.430,432,433,453 James et al.453 randomly assigned parturients to receive intermittent epidural bupivacaine 0.25% or bupivacaine 0.1% with fentanyl 2 µg/mL. The severity of motor blockade and the incidence of instrumental vaginal delivery were less in the low-dose group than in the high-dose group (6% versus 24%, respectively; P = .03). Similarly, in a much larger study that compared CSE (low-dose) with traditional high-dose epidural analgesia, the rate of instrumental vaginal delivery was lower in the CSE group.432 In another large study, Olofsson et al.433 noted a lower instrumental vaginal delivery rate in women randomly assigned to “low-dose” bupivacaine 0.125% with sufentanil than in those receiving “high-dose” bupivacaine 0.25% with epinephrine.
In contrast, Collis et al.431 observed no difference in mode of delivery between women randomly assigned to receive either a high-dose or a low-dose neuraxial technique. Even more confusing, the COMET investigators reported a lower rate of instrumental vaginal delivery in the two groups of women randomly assigned to receive either the low-dose epidural or CSE technique than in the group that received 0.25% bupivacaine (see earlier discussion and Figure 23-9).430 However, the total bupivacaine dose in the traditional “high-dose” epidural group did not actually differ from that in the “low-dose” epidural group because the former was administered by intermittent injection and the latter by continuous infusion. In contrast, the total bupivacaine dose was significantly lower in the CSE group. Finally, in a meta-analysis of studies that compared CSE and epidural analgesia,27 the instrumental vaginal delivery rate was lower in the CSE group than in the traditional “high-dose” epidural analgesia groups (risk ratio 0.80; 95% CI, 0.65 to 0.98), but there was no difference between “low-dose” epidural and CSE analgesia (risk ratio 1.06; 95% CI, 0.87 to 1.30). Together, these data suggest that the specific analgesia technique may influence the risk for instrumental vaginal delivery.
In general, the dose of bupivacaine is significantly lower if epidural analgesia is maintained with an intermittent bolus technique rather than a continuous infusion technique (see earlier discussion). Most investigators have noted a difference in motor blockade between the two techniques; higher total bupivacaine doses (i.e., continuous infusion techniques) are associated with a greater degree of motor blockade. However, the relationship between motor blockade and instrumental vaginal delivery is inconsistent. Smedstad and Morison228 reported a higher incidence of instrumental vaginal delivery when bupivacaine 0.25% was administered as a continuous epidural infusion than as intermittent bolus injections. In contrast, the COMET investigators observed no difference in the instrumental vaginal delivery rate in the two groups who received “low-dose” bupivacaine/fentanyl, one by infusion and the other by intermittent bolus.430 Similarly, in a meta-analysis of PCEA (without background infusion) compared with continuous epidural infusion analgesia,238 the dose of bupivacaine and degree of motor blockade were significantly lower in the PCEA group, but the rates of instrumental vaginal delivery did not differ.
It is possible that the inconsistent results can be explained by the actual absolute differences in bupivacaine dose and motor blockade. For example, the differences in dose and motor blockade may have clinically significant adverse effects on the outcome of the second stage of labor if bupivacaine 0.25% is compared with bupivacaine 0.125% but not if bupivacaine 0.125% is compared with bupivacaine 0.0625%. Many of the randomized controlled trials included in the meta-analysis that compared epidural with systemic opioid analgesia used concentrated solutions of bupivacaine for both the loading and infusion doses (e.g., bupivacaine 0.25% for the loading dose, bupivacaine 0.125% by continuous infusion for maintenance of analgesia.)3
Motor blockade may increase the risk for malrotation of the fetal vertex. Robinson et al.454 and Le Ray et al.455 observed a higher incidence of occiput malposition at delivery in patients who received epidural analgesia before engagement of the fetal head. In contrast, Yancey et al.456 and Sheiner et al.457 noted that the administration of on-demand epidural analgesia did not increase the frequency of malposition of the fetal head at delivery; the incidence of instrumental vaginal delivery was not related to fetal station at initiation of analgesia. In a prospective cohort study using ultrasonography, Lieberman et al.458 reported that fetal position changed frequently during labor but that epidural analgesia was associated with a higher incidence of occiput posterior position at delivery (13% versus 3%, P < .002). However, these results should be interpreted with caution as women were not randomly assigned to the treatment group. Factors that cause women to request analgesia when the fetal head is high may also be independent risk factors for instrumental vaginal delivery. A study from Italy459 in which ultrasonography was used to assess fetal head position found no association between use of early labor neuraxial analgesia and fetal head position at delivery.
In an editorial, Chestnut460 concluded that effective second-stage analgesia increases the risk for instrumental vaginal delivery. However, effective analgesia is a spectrum that ranges from complete absence of sensory input (dense analgesia) to perception of uterine contraction “pressure” without pain (less dense analgesia). Minimizing the risk for instrumental vaginal delivery while maximizing analgesia is both an art and a science and requires the attention of the anesthesia provider to the individual needs of the patient. A single analgesic technique or single dose/concentration of drug(s) is not likely to have optimal results for everyone. I believe the best technique incorporates the use of PCEA with a low-rate background infusion (4 to 8 mL/h) using a dilute solution of local anesthetic combined with an opioid. Use of a dilute local anesthetic solution without PCEA leads to inadequate analgesia for many women. Increasing the infusion dose improves analgesia and reduces the workload for the anesthesia provider but will result in overly dense analgesia for some patients, thus potentially increasing the risk for instrumental vaginal delivery. In the future, new pump technology may allow timed bolus and/or closed feedback-loop anesthetic administration that will better tailor anesthetic dose to patient need.
Why should anesthesia providers give attention to the effects of analgesia on the method of vaginal delivery? Lack of effective maternal effort associated with inadequate progress of labor (descent of the fetus) is an indication for operative vaginal delivery.461 Studies suggest that vacuum extraction is associated with a higher neonatal risk for cephalohematoma, subgaleal hemorrhage, and intraventricular hemorrhage than spontaneous vaginal or forceps delivery, whereas forceps delivery is associated with an increased risk for facial trauma.462 However, there is no evidence of adverse long-term outcome with operative vaginal compared with spontaneous vaginal delivery.463
The risk for maternal trauma is also greater with operative vaginal delivery (e.g., third- and fourth-degree vaginal lacerations, which are associated with a small but not negligible risk for rectovaginal fistula). Robinson et al.464 observed that epidural analgesia was associated with an increased rate of severe perineal trauma because of the more frequent use of instrumental vaginal delivery and episiotomy in nulliparous women who received epidural analgesia. In contrast, several large observational studies suggest that epidural analgesia is associated with a decreased risk for anal sphincter laceration in nulliparous women.465,466 Regardless of the presence or magnitude of the risks of maternal or neonatal injury, many women want to minimize the likelihood of operative delivery and they perceive that a higher risk for instrumental vaginal delivery is undesirable. Of concern is a decline in the number of obstetricians skilled at operative vaginal delivery.467 The concern is that loss of these skills will lead to an increase in second-stage cesarean delivery rates.