Side Effects of Neuraxial Opioids
Respiratory Depression
Pharmacokinetics and Pharmacodynamics
Neuraxial opioids can depress the respiratory centers in the brainstem via direct and/or indirect mechanisms (Table 28-6).80,82,97,98,246-248 Respiratory depression after neuraxial morphine administration is biphasic.249 Early respiratory depression can occur 30 to 90 minutes after epidural morphine administration (owing to systemic vascular absorption),98 whereas delayed respiratory depression can occur 6 to 18 hours after epidural or intrathecal morphine administration (owing to rostral spread in CSF and slow penetration into the brainstem).118 In contrast, lipophilic opioids (e.g., fentanyl, sufentanil) do not cause delayed respiratory depression but may cause early-onset respiratory depression, typically within 30 minutes, because of significant vascular uptake and rostral spread in CSF and, potentially, direct transit in epidural veins.246,247
TABLE 28-6
Neuraxial Opioids and the Principal Mechanisms of Action Leading to Respiratory Depression
| Mechanism | Lipophilic Opioids (e.g., fentanyl) | Hydrophilic Opioids (e.g., morphine) |
| Vascular uptake (by the epidural or subarachnoid venous plexuses and circulation) to the respiratory center in the brainstem | +++ | + |
| Rostral spread via direct perimedullary vascular channels | ++ | + |
| Dural penetration of opioids | + | ++ |
| Rostral spread via the aqueous cerebrospinal fluid to the brainstem | + | +++ |
The + symbols denote the relative importance of the mechanism for the type of opioid.
Data from references 80, 81, and 214-216.
Incidence
Although rare, perioperative opioid-induced respiratory depression represents a significant concern that can lead to death or permanent brain damage.250,251 The reported incidence of respiratory depression after neuraxial opioid administration ranges from 0% to 3.4%.252 Differences in the observed incidence of respiratory depression may reflect differences in patient population, opioid dose, monitoring protocol, and definition of respiratory depression.253 The incidence is likely lower in healthy obstetric patients receiving low-dose neuraxial opioid analgesia. The incidence of respiratory depression after neuraxial morphine administration in obstetric patients ranges from 0% to 0.9%.254 The analgesic benefits derived from neuraxial opioids outweigh the risks of respiratory depression in most patients. No studies in the obstetric setting have reported serious morbidity, although some patients have required naloxone administration for treatment of respiratory depression.103 Early reports suggested that intrathecal morphine was more likely to cause delayed respiratory depression than epidural morphine.98 However, this likely reflected the higher intrathecal morphine doses (1 to 10 mg) used in early clinical studies.255 Subsequently, lower doses of intrathecal morphine were found to provide effective analgesia with a very low risk for clinically significant respiratory depression. The incidence of respiratory depression associated with systemic (intravenous or intramuscular) opioids is likely to be higher than that observed with neuraxial opioids.195,256,257
Extended-Release Epidural Morphine and Lipophilic Opioids
The incidence of respiratory depression with EREM may be higher than with standard epidural morphine, with a reported range of 2% to 16%.182,186,187 EREM is also associated with a significantly higher risk for respiratory depression compared with intravenous PCA (see earlier discussion).187 However, patients in these studies received large EREM doses (15 to 30 mg), were older, had more comorbidities, and often received general anesthesia for surgery. Studies of EREM in patients undergoing cesarean delivery have reported no clinically significant respiratory depression57,58,191; however, the small sample sizes in these studies and low incidence of respiratory depression did not allow for an accurate assessment of the incidence of respiratory depression. EREM use should be carefully considered prior to its administration to high-risk obstetric patients (e.g., obesity, obstructive sleep apnea, co-administration of magnesium sulfate). Its use requires more prolonged monitoring and nursing care.190,254
There are a few case reports of respiratory depression after neuraxial administration of a lipophilic agent in the obstetric setting. In one report, respiratory depression occurred 25 minutes after spinal anesthesia with intrathecal fentanyl 15 µg and required reversal with naloxone.258 Respiratory depression has been described after administration of epidural fentanyl 90 to 100 µg for cesarean delivery.259,260 Cohen et al.247 reported that epidural sufentanil 30 to 50 µg depressed the ventilatory response to CO2 after cesarean delivery. Although overt respiratory depression did not occur, the highest sedation scores and depression of CO2 response occurred 45 minutes after administration. Another group reported that epidural fentanyl 100 µg or sufentanil 10 to 50 µg added to lidocaine for cesarean delivery caused significant changes in respiratory rate and end-tidal CO2.261
Prevention
Identify Patients at Risk.
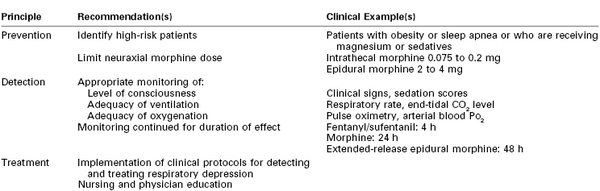
Risk factors for respiratory depression in the surgical setting include advanced age, obesity, cardiopulmonary disease, obstructive sleep apnea, and preoperative opioid tolerance.196,262 To identify patients at increased risk for respiratory depression, a history and physical examination directed at identifying sleep apnea and other relevant medical comorbidities should be performed prior to neuraxial opioid administration.262,263 Caution should be exercised when women are receiving magnesium sulfate, parenteral or oral opioids, or sedative drugs (e.g., diphenhydramine), because these agents may increase the risk for respiratory depression.251 Fortunately, most obstetric patients are relatively young and healthy and rarely have significant pulmonary disease or other risk factors for respiratory depression. However, opioid-induced respiratory depression occasionally occurs in healthy patients who have received standard doses of a neuraxial opioid,251 and vigilance is needed to prevent this rare but hazardous adverse outcome (Table 28-7).264
Limit Opioid Dose.
Historically, respiratory depression was more common because patients received doses of a neuraxial opioid greater than those currently used in modern practice. For cesarean delivery, neuraxial morphine appears to have a limit or “ceiling” in terms of analgesic efficacy. More specifically, effective doses of intrathecal and epidural morphine are 0.075 to 0.2 mg and 2 to 4 mg, respectively.51,104,199,252 Higher doses of neuraxial morphine may increase side effects without significant improving or prolonging postoperative analgesia.252
Monitoring, Detection, and Treatment
Monitor Respiration and Understand the Limitations of Monitoring Techniques.
A closed-claims analysis showed that 56% of respiratory events after neuraxial opioids could have been prevented.251 American Society of Anesthesiologists (ASA) guidelines address the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration.262 However, these guidelines do not specifically address obstetric patients.254 Opioid effects on respiration include reduced minute ventilation (decrease in respiratory rate, tidal volume, or both) and decreased response to hypoxemia and changes in PCO2.255 All patients receiving neuraxial opioids should be monitored for adequacy of ventilation, oxygenation, and level of consciousness.
Current monitoring technology and clinical observation practices have limitations.251,265 Intermittent evaluation of clinical signs (e.g., respiratory rate, level of sedation, pupil size) is often an unreliable predictor of respiratory depression.251,266 Intermittent respiratory monitoring may miss transient episodes of desaturation and bradypnea, because respiratory depression typically progresses slowly and is often preceded by increasing maternal sedation. The Anesthesia Patient Safety Foundation recommends continuous electronic monitoring in all hospitalized patients receiving opioids.251 Although pulse oximetry is considered the most useful electronic monitor currently available, it has poor sensitivity in detecting hypoventilation and hypercarbia, especially when supplemental oxygen is administered.251,265 Brief episodes of desaturation are common up to 24 hours after cesarean delivery; in one study, 71% of post–cesarean delivery patients had one or more episodes of desaturation (SpO2 less than 85%) after epidural morphine 5 mg.267 Continuous pulse oximetry is often inconvenient, owing to motion-artifact alarms that may affect patients' sleep and nursing care. Apnea monitors are also frequently associated with false alarms and do not detect hypoventilation. End-tidal CO2 monitoring in patients whose trachea is not intubated has significant limitations and is not universally available.
Despite these limitations, vigilant nursing care and hourly assessments of respiratory effort, respiratory rate, and somnolence are probably adequate for low-risk patients.103,254,268,269 Continuous pulse oximetry, although appropriate for the obstetric patient with risk factors for respiratory depression such as obesity, may be unnecessary in healthy postcesarean patients receiving small doses of neuraxial opioid (e.g., intrathecal morphine ≤ 0.2 mg, epidural morphine ≤ 4 mg).254 Greater surveillance and ventilation monitoring (e.g., capnography) may be warranted in patients at high risk for respiratory depression who are receiving supplemental oxygen.265
Monitor Respiration for an Appropriate Duration.
The duration of respiratory monitoring corresponds to the expected duration of action of the administered opioid. The onset of respiratory depression after neuraxial opioids is variable and has been reported to range from 2 to 12 hours.256 CO2 responsiveness is depressed for up to 24 hours after cesarean delivery after administration of epidural morphine 5 mg.149 The ASA recommends that respiratory monitoring after neuraxial administration of standard morphine should occur at least every hour for the first 12 hours and then every 2 hours for the next 12 hours (and then every 4 hours for another 24 hours in patients who have received EREM).186,262
Early-onset respiratory depression associated with lipophilic opioids usually occurs within 30 minutes of administration and is likely to occur in a high-visibility, controlled setting (e.g., operating or labor room). The ASA recommends that respiratory monitoring after administration of neuraxial fentanyl should continue for a minimum of 2 hours.262 However, it is prudent to continue monitoring for at least 3 to 4 hours with larger doses (e.g., intrathecal fentanyl > 20 µg, epidural fentanyl >100 µg), because delayed onset of respiratory depression (up to 180 minutes) has occurred after administration of lipophilic opioids.246,259 Patients receiving a continuous infusion of neuraxial opioid should be monitored during the infusion and for the expected residual duration of action after cessation of the infusion.262
Treating Respiratory Depression.
Physicians and nursing staff must be educated to prevent, recognize, and treat respiratory depression. Treatment protocols and mechanisms to ensure a rapid response to respiratory events are recommended. The patient who displays an altered level of consciousness, bradypnea, or hypoxemia should receive continuous supplemental oxygen until she is alert with no evidence of respiratory depression or hypoxemia. The routine use of supplemental oxygen is not advised because of the associated risk for prolonged apnea as well as limitations in the sensitivity of pulse oximetry to detect hypoventilation.251 An intravenous bolus dose of naloxone is indicated in patients with profound somnolence and respiratory depression who do not respond to arousal. Continued observation is advised after naloxone administration, because its half-life (43 to 90 minutes) may be shorter than the duration of effect for long-acting opioids. If naloxone fails to reverse severe respiratory depression or arrest, prompt mask ventilation and/or tracheal intubation should be performed. An intravenous infusion of naloxone should be maintained for as long as the patient remains symptomatic. It may be feasible to titrate an intravenous infusion of naloxone to treat respiratory depression without significantly reducing the quality of neuraxial analgesia.270-272 Although naloxone is a viable therapeutic option for reversing the opioid-induced respiratory depression, the routine administration of prophylactic naloxone is not recommended.269 Patients who require continuous positive airway pressure devices should be advised to continue using these devices during the postpartum period.
Nausea and Vomiting
Nausea and vomiting are common complaints after cesarean delivery, and the etiology of these symptoms is presumed to be multifactorial. A 2005 review highlighted the anesthetic and nonanesthetic causes of intraoperative nausea and vomiting (IONV) (Table 28-8).273 It is unclear whether patients are at increased risk for postoperative nausea and vomiting (PONV) if these symptoms occur intraoperatively. Neuraxial opioids may increase the risk for PONV after cesarean delivery. Nausea results either from the rostral spread of opioid in the CSF to the brainstem or from vascular uptake and delivery to the vomiting center and chemoreceptor trigger zone.118, 274 Palmer et al.199 found no difference in PONV between intrathecal morphine (0.025 to 0.5 mg) and placebo, nor a relationship between PONV and morphine dose. A similar study by the same group found no difference in the severity of PONV in patients receiving increasing doses of epidural morphine (1.25 to 5 mg).104 Importantly, neither study was adequately powered to investigate PONV as a primary outcome measure.
TABLE 28-8
Causes, Preventive Measures, and Treatment Measures for Intraoperative Nausea and Vomiting during Cesarean Delivery
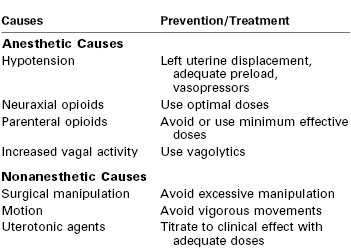
Modified from Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth 2005; 14:230-41.
Many studies have investigated different regimens to reduce PONV in patients receiving neuraxial opioids for cesarean delivery; however, these studies did not standardize PONV outcome measures and did not stratify patients according to risk for PONV. Information about the use of prophylactic antiemetic agents in actual clinical practice is lacking. A survey in Germany indicated that 82% of anesthesia departments did not provide any antiemetic prophylaxis for patients undergoing cesarean delivery.275
Individual Antiemetic Agents
Older-generation antiemetics, such as metoclopramide and droperidol, have been commonly used to prevent or treat neuraxial opioid-induced emesis in the obstetric setting. Metoclopramide 10 mg has been shown to decrease early PONV after intraoperative intravenous fentanyl and epidural morphine administration.276 In a meta-analysis of studies that assessed efficacy of antiemetic prophylaxis, metoclopramide was associated with a reduced incidence of IONV and early PONV compared with placebo (Figure 28-11).277 Metoclopramide antagonizes dopamine receptors in the chemoreceptor trigger zone. It is often administered preoperatively owing to its favorable prokinetic properties, and it is associated with a reduction in rates of IONV and PONV in patients receiving spinal anesthesia.278
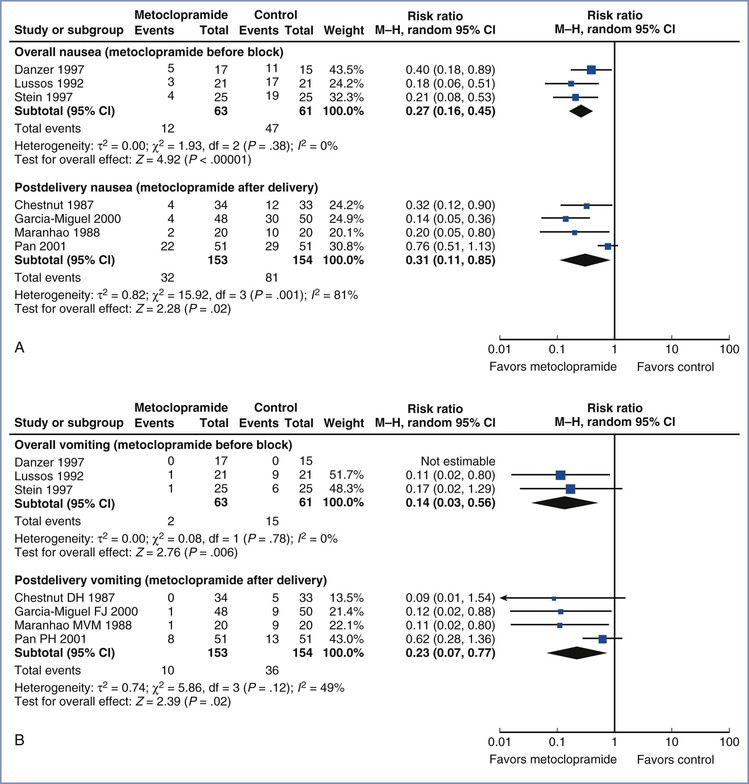
FIGURE 28-11 Meta-analysis of metoclopramide for nausea and vomiting prophylaxis during and after cesarean delivery. A, Forest plots for intraoperative nausea in patients undergoing cesarean delivery with neuraxial anesthesia. B, Forest plots for intraoperative vomiting in patients undergoing cesarean delivery with neuraxial anesthesia. In each series, separate analyses were performed according to whether metoclopramide was administered before block placement (overall incidence) or after umbilical cord clamping (postdelivery incidence). CI, confidence interval. (Forest plots from Ashraf Habib, Duke University, Durham, NC. See Mishriky BM, Habib AS. Impact of data by Fujii and colleagues on the meta-analysis of metoclopramide for antiemetic prophylaxis in women undergoing Caesarean delivery under neuraxial anaesthesia. Br J Anaesth 2012; 109:826.)
Droperidol is a butyrophenone that antagonizes dopaminergic (D2) receptors in the chemoreceptor trigger zone. Prophylactic administration of droperidol (0.625 to 2.5 mg) has been shown to decrease PONV after epidural anesthesia with epidural fentanyl279 and epidural morphine.280 Sedation and drowsiness may occur with droperidol, although their appearance does not appear to be a dose-related phenomenon. Droperidol has been less widely used by anesthesia providers since the FDA issued a “black box” warning in 2001, which highlighted concern related to conduction abnormalities (QT interval prolongation and an increased risk for development of torsades de pointes).
The use of a transdermal scopolamine patch may also lower the incidence of PONV after cesarean delivery. Early work by Kotelko et al.281 indicated that scopolamine is effective at reducing PONV during the first 10 hours after cesarean delivery. A transdermal scopolamine patch (1.5 mg) provided efficacy similar to that provided by ondansetron 4 mg in reducing postcesarean emesis among patients receiving spinal anesthesia (incidence of 40% and 42% respectively, versus 59% in a control group).282 However, transdermal scopolamine has a latency period of 3 to 4 hours, which limits its ability to treat early PONV. It also has a number of commonly reported side effects, including dry mouth, visual disturbances, dizziness, and agitation.
Serotonin (5-HT3) receptor antagonists have been used for both prophylaxis and treatment of PONV. These drugs specifically bind to 5-HT3 receptors in the chemoreceptor trigger zone and at vagal afferents in the gastrointestinal tract. Prophylactic administration of ondansetron 4 to 8 mg has been shown to have a better antiemetic profile in the first 24 hours after intrathecal and epidural opioid administration compared with placebo (Figure 28-12).283,284 Research evaluating the antiemetic profile of granisetron in patients receiving spinal anesthesia for cesarean delivery is limited. Balki et al.285 found no difference in the rate of IONV in patients receiving granisetron 1 mg compared with placebo (20% versus 17%). A recent meta-analysis of six trials found that the use of 5-HT3 receptor antagonists reduced the incidence of PONV and the need for rescue antiemetic treatment in women who received intrathecal opioids for cesarean delivery.286
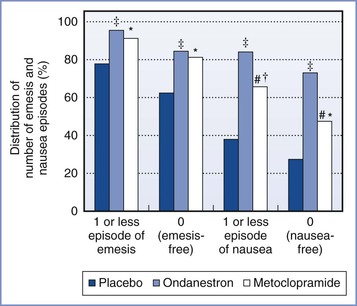
FIGURE 28-12 Randomized trial of postoperative nausea and emesis in patients undergoing cesarean delivery with epidural anesthesia (2% lidocaine with epinephrine and fentanyl) and who received prophylactic ondansetron, metoclopramide, or placebo. Distribution of number of nausea and emesis episodes. Values are given as percentages. #Group metoclopramide versus ondansetron; P < .05. *Group metoclopramide versus placebo; P < .05. †Group metoclopramide versus placebo; P < .005. ‡Group ondansetron versus placebo; P < .005. (Data from Pan PH, Moore CM. Comparing the efficacy of prophylactic metoclopramide, ondansetron, and placebo in cesarean section patients given epidural anesthesia. J Clin Anesth 2001; 13:430-5.)
Administration of the corticosteroid dexamethasone has been successful in preventing and treating emesis after chemotherapy, and subsequently the drug has become more popular as an antiemetic agent in anesthesia practice. Corticosteroids have various effects in the CNS, including the regulation of transmitter levels, receptor densities, and neuronal configurations.287 Corticosteroid receptors have been identified in areas important to the signal processing of nausea and vomiting, including the nucleus of the solitary tract, the nucleus of raphe, and the area postrema. Tzeng et al.287 reported that intravenous dexamethasone 8 mg and droperidol 1.25 mg provided similar efficacy in the prevention of PONV. Wang et al.288 suggested that dexamethasone 5 mg is the minimum effective dose for preventing PONV. In both of these studies, patients received epidural morphine 3 mg. In a recent meta-analysis of studies of obstetric and gynecologic patients who received neuraxial morphine, prophylactic dexamethasone (2.5 to 10 mg) was associated with a reduced risk for PONV and need for antiemetic rescue therapy compared with placebo.289 Administration of cyclizine 50 mg has been reported to be associated with significantly fewer episodes of PONV (0 to 12 hours after cesarean delivery) than administration of dexamethasone 8 mg after intrathecal opioid (fentanyl and morphine) administration.290
Combination Antiemetic Regimens
Few studies have compared the effects of individual drugs with those of combination antiemetic regimens. Administration of drugs acting at two different receptor sites may improve antiemetic efficacy through synergism.291 Drug combinations may also facilitate a concomitant reduction in drug doses. Wu et al.292 reported lower rates of PONV after intrathecal morphine administration with use of a combination of dexamethasone 8 mg and droperidol 0.625 mg than with use of dexamethasone 8 mg or droperidol 1.25 mg alone.
Nonpharmacologic Techniques
A number of studies have investigated the prophylactic use of acupressure (using wrist bands with a plastic bead placed bilaterally on the P6 [HG-6] acupoint) in reducing PONV after neuraxial anesthesia for cesarean delivery. Ho et al.293 reported significantly less PONV with acupressure in cesarean delivery patients who received epidural morphine (3 mg increments, 8 mg average total dose) in the postanesthesia care unit. A similar effect was seen in a study of patients who received intrathecal morphine 0.2 mg.294 However, other studies investigating prophylactic acupressure before spinal anesthesia reported no reduction in PONV in patients who received intrathecal morphine 0.25 mg and fentanyl 10 µg295 or in patients who did not receive neuraxial opioids.296 A meta-analysis of six studies (649 patients) that assessed the effect of P6 stimulation versus placebo to reduce IONV and PONV revealed inconsistent results, thereby limiting any definitive conclusions regarding the efficacy of this intervention.297
Pruritus
Pruritus is a common side effect of neuraxial opioid administration in obstetric patients. A retrospective review of 4880 cesarean delivery patients who received epidural morphine 2 to 5 mg observed that 58% of patients reported pruritus.103 However, a sample of patients who received spinal anesthesia for cesarean delivery ranked pain, nausea, and vomiting as more undesirable than pruritus (see Table 28-1).10 The incidence and severity of pruritus are likely influenced by the opioid dose, route of administration (more common after intrathecal administration), and method of assessment.298 Approximately 40% of patients reporting pruritus after receiving epidural morphine request treatment.103,299,300
Pruritus may manifest in the dermatomal distribution of neuraxial opioid spread as well as nonspecific areas of the head and neck; specific symptoms and severity vary among patients.301 These effects typically occur within a few hours of neuraxial opioid administration. Although opioid-induced histamine release from mast cells is well described, this does not appear to be the causative mechanism for pruritus after neuraxial opioid administration. Plasma opioid and histamine levels are clinically insignificant at the time of symptom presentation (3 to 6 hours after intraspinal morphine administration).98,118,302 In addition, sufentanil and fentanyl can produce pruritus but do not stimulate histamine release. At present, the mechanisms of spinal and epidural opioid–induced pruritus remain unclear. Postulated theories of causation include (1) direct or indirect excitatory effects on central µ-opioid receptors; (2) cephalad migration of the opioid within the CSF to the trigeminal nucleus (which contains the subnucleus caudalis, integrates facial sensory input, and exhibits high opioid receptor density); (3) excitatory effects on dorsal or ventral horn neurons; and (4) other mechanisms (e.g., effects on dopamine-2 [D2] receptors, prostaglandin system, serotonin 5-HT3 receptors, and central nervous system gamma-aminobutyric acid [GABA] and glycine receptors).302 Pregnant patients may be more susceptible as a result of possible estrogenic interactions with opioid receptors.303 Pharmacogenetics may also play a role. Polymorphisms at the human µ-opioid receptor gene have been implicated as a potential explanatory factor because central-type pruritus induced by neuraxial opioids may be influenced by tonic inhibitory control of pain signaling.304 The incidence of moderate-to-severe pruritus with epidural morphine given for postcesarean analgesia has been reported to be lower in patients homozygous for the G118G polymorphism in the µ-opioid receptor gene (OPRM1) than in patients with the A118G or A118A genotypes (incidence 5%, 42%, and 53%, respectively).305
Drug Therapy
There is little consensus regarding treatment of neuraxial opioid-induced pruritus after cesarean delivery.306 Further, there are currently no validated or consistent methods for assessing pruritus, which limits the analysis of data from studies investigating the efficacy of antipruritic regimens.
Opioid antagonists are commonly employed to treat opioid-related pruritus. The efficacy of opioid antagonists depends, in part, on the drug-opioid receptor interaction (antagonist versus mixed agonist-antagonist). Studies comparing opioid antagonists for the treatment of pruritus have demonstrated mixed results. Cohen et al.300 compared naloxone (0.2 mg, with a maximum of three doses) with nalbuphine (5 mg, with a maximum of three doses) after administration of epidural morphine 5 mg for postcesarean analgesia. Nalbuphine significantly reduced the severity of pruritus after 30 minutes, and fewer patients in the nalbuphine group required additional doses for treatment of persistent pruritus. Somrat et al.307 suggested that smaller doses of nalbuphine (2 to 3 mg) could adequately treat moderate-to-severe pruritus after intrathecal morphine administration. Butorphanol has attracted interest as an antipruritic agent. Wu et al.308 found that butorphanol 1 mg followed by an infusion of 0.2 mg/h was associated with reduced postcesarean pruritus in patients who received intrathecal morphine compared with a saline-control group.
Pretreatment with opioid antagonists has also been investigated as a method of reducing the incidence of opioid-induced pruritus. Morgan et al.309 reported that pretreatment with intravenous nalbuphine (20 mg, at skin closure) with subsequent postoperative administration (40 mg, in divided doses) was ineffective in reducing pruritus in patients receiving epidural morphine. Similarly, pretreatment with subcutaneous naloxone (0.4 mg) did not significantly reduce the incidence of pruritus in patients receiving intrathecal fentanyl and morphine for elective cesarean delivery.310 Naloxone may be more efficacious as an infusion, and Luthman et al.311 reported reductions in the severity and incidence of pruritus using a naloxone infusion (0.1 mg/h) after cesarean delivery. Naloxone and nalbuphine in patient-controlled bolus doses, combined with a background infusion, have also been found to reduce the incidence of pruritus after cesarean delivery in patients who received epidural morphine 5 mg.299 Abboud et al.312 observed that the use of the long-acting opioid antagonist naltrexone (9 mg, administered orally) was associated with a lower incidence of pruritus compared with placebo after cesarean delivery in patients who received epidural morphine 4 mg. However, these investigators also noted a significant increase in the incidence of unsatisfactory analgesia. A similar trend was reported with a 6-mg dose of oral naltrexone after administration of intrathecal morphine 0.25 mg.313 A cautious approach is needed when considering high-dose naloxone therapy for treating pruritus to avoid reversing the analgesic effect of neuraxial opioids.300
The effects of neuraxially administered opioid antagonists have also been investigated. Jeon et al.314 reported less pruritus in patients receiving epidural naloxone (1.2 mg over 48 hours) with epidural 0.1% bupivacaine and morphine (6 mg over 48 hours) than in a control group not receiving naloxone. Similarly, Culebras et al.230 investigated the effects of three different doses of intrathecal nalbuphine (0.2, 0.8, and 1.6 mg) and found a significantly lower incidence of pruritus in all of the nalbuphine groups compared with a control group that received intrathecal morphine without nalbuphine. However, the duration of analgesia was significantly shorter among patients in the nalbuphine groups.230 Studies in animals and nonobstetric patients have suggested no adverse neurologic effects after neuraxial administration of opioid antagonists. Of note, the use of experimental drugs and drugs not approved for neuraxial administration continues to raise concerns regarding the potential for adverse neurotoxic effects. These concerns have limited the clinical assessment of neuraxially administered drugs for the prevention and treatment of opioid-induced pruritus and other side effects. To avoid reversal of opioid-induced analgesia, further work is necessary to identify the optimal dose of each opioid antagonist for the prevention and treatment of pruritus.
NSAIDs are often incorporated into multimodal analgesic regimens for patients undergoing cesarean delivery. Some investigators have postulated that prostaglandins are involved in the etiology of pruritus after neuraxial opioid administration, owing to their ability to enhance C-fiber transmission to the CNS and release histamine. However, there is limited evidence that NSAIDs reduce the occurrence of opioid-induced pruritus. A study evaluating the effect of oral celecoxib 200 mg after intrathecal morphine 0.3 mg reported no significant difference in the severity of pruritus or the need for rescue medications between the treatment and placebo groups.315
Propofol has been reported to relieve pruritus caused by neuraxial opioids in nonobstetric patients after a 10-mg bolus dose without an infusion316 and after a 10-mg bolus dose followed by a 30 mg/24 h infusion.317 The antipruritic effect of propofol has been proposed to occur as a result of inhibitory effects on posterior horn transmission rather than specific antagonism of the opioid receptors.316 However, this effect has not been observed in obstetric patients who received subhypnotic doses of propofol (10 to 20 mg) for treatment of intrathecal morphine–induced pruritus.318,319 A comparative study demonstrated that intravenous nalbuphine 3 mg is superior to propofol 20 mg for treating pruritus after administration of intrathecal morphine.320
The use of 5-HT3 receptor antagonists for prophylaxis of neuraxial opioid–induced pruritus after cesarean delivery has attracted interest. A meta-analysis of studies of surgical patients receiving neuraxial opioids concluded that prophylaxis with a 5-HT3 antagonist results in a reduced risk for postoperative pruritus, compared with placebo (OR, 0.44; 95% CI, 0.29 to 0.68).321 Direct stimulation of 5-HT3 receptors found in the dorsal horn of the spinal cord and in the nucleus of the spinal tract of the trigeminal nerve in the medulla may occur after subarachnoid opioid administration. Intravenous ondansetron 4 to 8 mg has been shown to be more effective than placebo in reducing the incidence of postcesarean pruritus after intrathecal administration of morphine 0.15 to 0.2 mg.322,323 However, other studies that compared ondansetron 8 mg with placebo found no significant reduction in pruritus after intrathecal administration of morphine (0.1 to 0.2 mg) alone324 or in combination with a lipophilic opioid (sufentanil or fentanyl).283,325 The lack of antipruritic effect may be due to the peak effect of ondansetron occurring much sooner (at 15 minutes) than that of intrathecal morphine. The antipruritic effects associated with ondansetron may depend on the dose, lipophilicity, and duration of action of the intrathecal opioid.326 A study of epidurally administered ondansetron (8 mg over 2 days) in patients who received epidural ropivacaine and morphine after cesarean delivery demonstrated a reduction in the incidence of pruritus.327 The investigators found no histologic evidence of neurotoxic sequelae after intrathecal ondansetron administration in rats.327 There are conflicting data on the antipruritic effect of granisetron in patients receiving intrathecal morphine for postcesarean analgesia. Siddik-Sayyid et al.324 found no significant differences in the incidence or severity of pruritus among patients who received granisetron 3 mg or ondansetron 8 mg and those in a control group. In contrast, Tan et al.328 observed that the severity of pruritus was reduced at 8 and 24 hours after cesarean delivery in patients who received granisetron 3 mg compared with those who received ondansetron 8 mg.
A meta-analysis of studies (published up to 2008) in women receiving spinal anesthesia for cesarean delivery indicated that prophylactic administration of a 5-HT3 antagonist did not reduce the risk for pruritus compared with a control group; however, administration of a 5-HT3 antagonist reduced the severity of pruritus and the need for rescue treatment compared with placebo.286 Heterogeneity and small sample sizes in the studies included in this systematic review limited detailed analysis of the efficacy of prophylactic administration of a 5-HT3 antagonist. Large-scale prospective studies are needed to better investigate the prophylactic antipruritic efficacy of neuraxial 5-HT3 antagonists. Few studies have assessed the therapeutic effect of 5-HT3 antagonists for managing postcesarean pruritus induced by neuraxial opioids. In one study, ondansetron 4 mg had a high rate of success for the treatment of moderate-to-severe pruritus compared with placebo (80% and 36%, respectively).329
Pentazocine, a κ-opioid receptor agonist and partial µ-opioid receptor agonist, may be a potentially useful drug for treating opioid-induced pruritus.330 Tamdee et al.331 found that pentazocine 15 mg was more effective than ondansetron 4 mg for the treatment of moderate-to-severe pruritus in patients who received intrathecal morphine for postcesarean analgesia.
Historically, antihistamines have been a popular first choice for treatment of pruritus. However, the efficacy of these agents has been questioned in patients receiving neuraxial opioids that do not cause histamine release (e.g., fentanyl, sufentanil). Using a tailored treatment algorithm, Alhashemi et al.332 demonstrated that diphenhydramine was less effective than nalbuphine (higher itching scores and more treatment failures) after administration of intrathecal morphine 0.2 mg. Yeh et al.323 found that the incidence of pruritus was comparable among patients receiving diphenhydramine 30 mg and a placebo (80% and 85%, respectively); however, both groups had a higher incidence of pruritus than did a group that received ondansetron 0.1 mg/kg (25%). In contrast, Siddik-Sayyid et al.333 found that the therapeutic success rates for ondansetron 4 mg and diphenhydramine 25 mg were identical (70% for each drug), with similar recurrence rates in successfully treated patients (28% versus 35%, respectively). Differences in study methodology and drug-dosing regimens may explain the inconsistent antipruritic effect of diphenhydramine observed in these studies.
Urinary Retention
The mechanisms by which neuraxial opioids affect specific components of micturition (urge sensation, detrusor and sphincter function) are not fully understood, although spinal and supraspinal sites of action are likely to be involved. Kuipers et al.334 performed urodynamic studies of healthy male volunteers receiving intrathecal sufentanil and morphine. Both opioids caused dose-dependent decreases in detrusor contractility and the “urgency to void.” Patients receiving intrathecal sufentanil had earlier recovery of lower urinary tract function than those receiving intrathecal morphine.334 Intrathecal local anesthetics (bupivacaine and lidocaine) have been shown to cause complete absence of detrusor contractility and urge sensation until the dermatomal block regresses to S2 to S3, with no partial recovery until this regression has occurred.335
Although neuraxial opioids may increase the risk for postpartum urinary retention after cesarean delivery, there is a lack of consensus on the definition of postpartum urinary retention in this setting. Postpartum urinary retention has been previously described as “no spontaneous voiding within 6 hours of removal of an indwelling catheter (more than 24 hours after cesarean delivery).”336 Some authorities advocate a diagnosis based on clinical diagnostic features (e.g., “the sudden inability to void”) or postvoid residual bladder volume (PVRV). However, there is marked variability in defining “significant” PVRV values (40 to 500 mL) associated with postpartum urinary retention.337
Few studies have investigated the incidence of urinary retention in patients who have received a neuraxial opioid for postcesarean analgesia. Evron et al.338 performed an observational study investigating the effects of epidural morphine and methadone in 120 women undergoing cesarean delivery. Difficulty in micturition and need for bladder catheterization were greater in the morphine group (58%) compared with the methadone group (3%).338 A similar study by Liang et al.339 reported a higher incidence of postcesarean urinary retention and urinary catheterization (22%) among patients receiving epidural morphine compared with other analgesia modalities (PCEA with ropivacaine-fentanyl [7%]; intramuscular meperidine [3%]). In a study of male volunteers, naloxone reversed the impact of neuraxial morphine on urodynamic function.340 To avoid impairment of bladder/detrusor function, urinary catheterization should be considered if voiding has not occurred within 6 hours.341 Risk factors for postcesarean urinary retention include low body mass index and multiparity.342
Hypothermia and Shivering
Perioperative and postoperative hypothermia and shivering are commonly observed in patients who receive neuraxial anesthesia for cesarean delivery and are caused by a number of interrelated processes. The true incidence of core hypothermia and shivering in this setting is unclear; however, results from earlier studies suggest that these complications may occur in up to 66% and 85% of patients, respectively.343,344 Core-to-periphery heat redistribution is the major cause of core hypothermia after spinal or epidural anesthesia and is due to the effects of sympathetic and motor nerve blockade.345 A study of healthy volunteers reported that core temperature can decrease by a mean (± SD) of 0.8° ± 0.3° C in the first hour of anesthesia.346 Neuraxial anesthesia also impairs centrally mediated thermoregulatory control, lowers the vasoconstriction and shivering thresholds, and promotes greater environmental heat loss than metabolic heat production.347-349
The onset and the severity of hypothermia and shivering vary according to the anesthetic technique, the anesthetic agents administered, and baseline thermal status of the patient. Saito et al.350 found that spinal anesthesia reduces initial core temperature more rapidly than epidural anesthesia during cesarean delivery. Interestingly, there was no difference in the incidence of shivering between groups, but the severity of shivering was significantly less in the group receiving spinal anesthesia. The more intense sensory block observed with spinal anesthesia inhibits central thermoregulatory control more than epidural anesthesia, which can affect shivering thresholds and intensity.351
The effect of neuraxial opioids on thermoregulation and shivering in patients undergoing cesarean delivery is not fully understood. One study demonstrated that patients who received intrathecal morphine 0.15 mg had a greater degree of hypothermia than patients who received no intrathecal opioid.352 However, intrathecal administration of both fentanyl and morphine is associated with a lower incidence of shivering than single-dose intrathecal morphine alone.353 Hong and Lee354 reported that intrathecal meperidine 10 mg produced fewer and less intense shivering episodes than intrathecal morphine (0.1 and 0.2 mg). Core hypothermia occurred to a similar extent in all study groups. In a different study, intrathecal meperidine (12.5 or 25 mg) was independently associated with a lower incidence of shivering compared with a no-meperidine control group; however, rates of PONV were significantly higher in the meperidine groups.355 The effect of epidural opioids on thermoregulation may be more consistent, with a number of studies reporting a reduction in the incidence and severity of shivering after epidural meperidine, butorphanol, fentanyl, and sufentanil.356-359
Preoperative patient warming with forced air has been shown to reduce the incidence of perioperative and postoperative core hypothermia and shivering in patients undergoing cesarean delivery with epidural anesthesia.360 However, a subsequent study found that perioperative forced-air warming does not prevent maternal hypothermia after cesarean delivery with spinal anesthesia that includes fentanyl and morphine.343 It is likely that forced-air warming cannot compensate for the initial rapid drop in core temperature (from heat redistribution) after administration of spinal anesthesia.
Neuraxial Nonopioid Analgesic Adjuvants
The addition of neuraxial nonopioid adjuvants to local anesthetic agents may improve the quality of both intraoperative and postcesarean analgesia. Nonopioid neuraxial adjuvants have different sites and mechanisms of actions, and interactions between neuraxial opioids and nonopioid adjuvants may be additive or synergistic. Potential advantages of neuraxial drug combinations include a reduction in dose of individual drugs (with subsequent reductions in dose-dependent side effects), in particular a reduction in postoperative opioid requirements and opioid-related side effects.361
Alpha-Adrenergic Agonists
α2-Adrenergic agonists bind to presynaptic and postsynaptic α2-adrenergic receptors at peripheral, spinal (dorsal horn), and brainstem sites. Epidural and intrathecal α2-adrenergic agonists provide analgesia by mimicking the activity of the descending noradrenergic system.362,363 This process subsequently leads to norepinephrine release, which in turn modulates pain processing in the dorsal horn by inhibiting the release of substance P and increasing acetylcholine levels to produce analgesia.364,365 Clonidine, an α2-adrenergic agonist, provides a more potent analgesic response when administered neuraxially than when administered systemically. Clonidine is also associated with more profound sensory and motor block when administered with epidural local anesthetics and acts additively or synergistically with intraspinal opioids.361,365 In combination with an intrathecal local anesthetic, intrathecal clonidine may also prolong the regression of sensory block, improve postoperative analgesia, and decrease the risk for intraoperative pain.366 However, a combination of intrathecal clonidine and local anesthetic may increase the risk for hypotension in a non–dose-responsive manner.366 Pregnancy may further enhance the analgesic effects of α2-adrenergic agonists.367
Initial clinical studies of epidural clonidine 700 to 900 µg demonstrated a rapid onset of analgesia (within 20 minutes) lasting approximately 5 hours.368 Mendez et al.369 compared the analgesic efficacy of low-dose clonidine (400 µg bolus) with that of high-dose clonidine (800 µg bolus), followed by an epidural clonidine infusion at 10 or 20 µg/h after cesarean delivery. The investigators found a dose-dependent analgesic effect in the first 6 hours and dose-dependent sedation and motor block in the first 3 hours postoperatively. These time-dependent side effects might lead to delays in the discharge of the patient from the postanesthesia care unit. Huntoon et al.370 reported similar postcesarean analgesia in patients receiving epidural clonidine 400 or 800 µg after epidural bupivacaine anesthesia. Absent or reduced postcesarean analgesia was observed in separate groups that received epidural 2-chloroprocaine anesthesia with epidural clonidine 400 or 800 µg. This finding may have been due to the calcium chelator (disodium EDTA) present in the 2-chloroprocaine solution (Figure 28-13). These investigators also evaluated the effect of a postoperative epidural clonidine infusion (40 µg/h). Postcesarean analgesia was sustained in patients who had received epidural bupivacaine; in the 2-chloroprocaine group, analgesia was prolonged only in patients who received epidural clonidine 800 µg.370
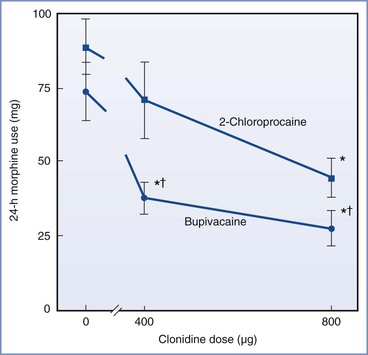
FIGURE 28-13 Randomized trial of epidural 0.5% bupivacaine compared with 3% 2-chloroprocaine with epidural clonidine 400 µg or 800 µg: 24-hour morphine use. 2-chloroprocaine (▪) or bupivacaine (•). *P < .05 versus saline control (0 µg clonidine). †P < .05 versus 2-chloroprocaine group. (From Huntoon M, Eisenach JC, Boese P. Epidural clonidine after cesarean section: appropriate dose and effect of prior local anesthetic. Anesthesiology 1992; 76:187-93.)
Few studies have compared epidural clonidine with systemic or neuraxial opioid analgesia. Narchi et al.371 found that postcesarean pain scores were lower after individual bolus doses of epidural clonidine 150 to 300 µg than with intramuscular morphine 10 mg in the first 3 hours after cesarean delivery. The investigators reported that epidural clonidine 300 µg was paradoxically associated with higher pain scores and greater episodes of obstructive apnea with desaturation (SpO2 ≤ 90%) than occurred with clonidine 150 µg. Several studies have investigated epidural clonidine in combination with epidural opioids for optimizing postcesarean analgesia. An isobolographic evaluation of epidural clonidine (in doses ranging from 50 to 400 µg) with fentanyl (15 to 135 µg) did not demonstrate synergy between clonidine and fentanyl in patients recovering from cesarean delivery.372 Results of this study suggest that these two drugs interact in an additive rather than a synergistic manner in humans. However, marked variability in drug response and failure of high doses to produce complete analgesia limited the validity of dose-response and ED50 analyses. Capogna et al.373 observed that the addition of clonidine 75 to 150 µg to epidural morphine 2 mg significantly lengthened the duration of postcesarean analgesia without increasing the incidence of side effects. Vercauteren et al.374 compared three different PCEA regimens with an epidural background infusion (sufentanil 2 µg/mL; sufentanil 2 µg/mL with epinephrine 2.5 µg/mL; sufentanil 2 µg/mL with clonidine 3 µg/mL) in patients who had undergone cesarean delivery. Although 24-hour sufentanil consumption was lowest in the clonidine admixture group, there were no significant differences among groups with regard to pain scores (at 10 or 24 hours), sedation, or hypotension.
A number of studies have evaluated the potential role of intrathecal clonidine for postcesarean analgesia. Filos et al.375 observed that patients undergoing general anesthesia for cesarean delivery who were randomly assigned to receive intrathecal clonidine 150 µg at 45 minutes after extubation experienced earlier onset of analgesia (within 20 minutes), lower maximal pain scores at 90 minutes, and more prolonged analgesia (> 6 hours) than patients who received intrathecal saline (the control group). However, patients in the clonidine group had higher sedation scores, a greater maximal decrease in mean arterial pressure, and more complaints of dry mouth than the control group. Utilizing a similar study design to compare different doses of intrathecal clonidine (150, 300, or 450 µg), the same investigators observed that both the onset and duration of analgesia and sedation were dose dependent.376 Intrathecal clonidine was also associated with a significant reduction in mean arterial pressure. Van Tuijl et al.377 assessed postcesarean analgesia in patients who received intrathecal clonidine 75 µg combined with bupivacaine before surgery versus intrathecal bupivacaine alone. Early postoperative analgesia (1 to 2 hours) was improved with clonidine; however, no difference was found in 24-hour morphine consumption between the groups.
Studies of intrathecal opioids in combination with clonidine have investigated the contribution of each drug to the subsequent analgesia and side-effect profile. Benhamou et al.378 evaluated postcesarean analgesic outcomes in patients who received hyperbaric bupivacaine alone or bupivacaine and clonidine 75 µg with and without fentanyl 12.5 µg. Patients receiving the clonidine-fentanyl combination reported less intraoperative pain and more prolonged postcesarean analgesia (time to first analgesia request 215 minutes) than those receiving bupivacaine-clonidine and bupivacaine alone (183 and 137 minutes, respectively). However, significantly higher rates of pruritus and sedation were reported for the clonidine-fentanyl group. Paech et al.379 performed a six-arm study assessing postcesarean analgesia after intrathecal bupivacaine 12.5 mg with fentanyl 15 µg and one of the following regimens: clonidine 150 µg; morphine 0.1 mg; and morphine 0.1 mg with clonidine 30, 60, 90, or 150 µg. The investigators concluded that the morphine-clonidine regimens provided optimal postcesarean analgesia with significantly lower pain scores at rest and with coughing in the first 4 hours. The minimum effective intrathecal dose of clonidine was 30 to 60 µg when combined with bupivacaine, fentanyl 15 µg, and morphine 0.1 mg. However, a significant increase in intraoperative sedation was observed in all groups receiving clonidine. Lavand'homme et al.380 compared postcesarean antihyperalgesic effects in patients receiving intrathecal clonidine 150 µg with bupivacaine, clonidine 75 µg with bupivacaine-sufentanil, or bupivacaine-sufentanil. The bupivacaine-clonidine group had a significantly reduced area of peri-incisional hyperalgesia and a lower incidence of hyperalgesia compared with the other study groups. However, no between-group differences were observed in postoperative morphine consumption or in pain scores before and after discharge. The relationship between postoperative wound hyperalgesia and chronic wound pain after cesarean delivery remains unclear (see Chapter 27).
In summary, neuraxial clonidine does not appear to offer substantial improvement in analgesia over that provided by neuraxial opioids. Epidural clonidine (150 to 800 µg) may improve postcesarean analgesia when given in combination with epidural opioids. Intrathecal clonidine (75 to 450 µg) has modest efficacy and a relatively short duration of action. Ongoing concern about the adverse side-effect profile of epidural or intrathecal clonidine—notably sedation and hypotension—limit the neuraxial administration of this agent in patients undergoing cesarean delivery. Additionally, in the United States, epidural clonidine has a “black box” warning stating that it is not recommended for obstetric, postpartum, or perioperative pain management due to the risk for hemodynamic instability, especially hypotension and bradycardia. In selected cases the anesthesiologist may conclude that the potential benefits may outweigh the possible risks.
No published studies have assessed the administration of neuraxial dexmedetomidine in pregnant patients. A study in patients undergoing bladder surgery who received spinal anesthesia with bupivacaine and clonidine 30 µg or dexmedetomidine 3 µg found similar sensory and motor block duration, with no hemodynamic compromise or sedation.381 However, when dexmedetomidine was applied to strips of pregnant human myometrium in vitro, significant increases in uterine contractility were observed.382
Neostigmine
By interfering with the breakdown of acetylcholine, neostigmine indirectly stimulates spinal nicotinic and muscarinic receptors and the release of nitric oxide. The resulting analgesia is most likely due to central and peripheral alterations in pain modulation and transmission. Initial studies of intrathecal neostigmine in animals and human volunteers have demonstrated analgesic effects without neurotoxic effects.235,383-385 However, despite producing dose-dependent analgesia, intrathecal neostigmine at doses greater than 25 µg also results in nausea that is resistant to traditional antiemetic treatment (droperidol, ondansetron) and cholinergic antagonists.384
Krukowski et al.386 reported that escalating doses of intrathecal neostigmine (10 to 100 µg) improved analgesia in a dose-independent manner when given after epidural anesthesia for cesarean delivery. The reduction in morphine requirements lasted up to 10 hours. The incidence of nausea varied from 50% to 100%. Chung et al.387 showed that the postoperative analgesia provided by intrathecal neostigmine 25 µg was similar to that observed with intrathecal morphine 0.1 mg. The investigators observed that the combination of neostigmine 12.5 µg with morphine 0.05 mg prolonged the analgesia in an additive (rather than a synergistic) manner, was associated with less need for supplemental analgesia, and had fewer side effects than observed with either drug administered alone in higher doses (Figure 28-14). Pan et al.388 compared analgesic outcomes after intrathecal bupivacaine given either alone or in combination with three different admixtures: intrathecal neostigmine 50 µg, intrathecal clonidine 150 µg, or a neostigmine-clonidine combination (same doses of each). Although patients in the clonidine-neostigmine group had lower pain scores in the first 10 postoperative hours, they experienced more significant side effects, including a prolongation of motor block, a higher incidence of hypotension, and a higher incidence (with greater severity) of nausea and vomiting.
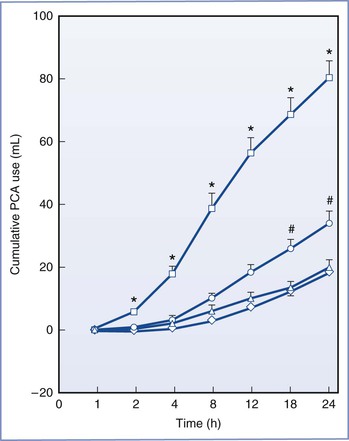
FIGURE 28-14 Randomized trial of postcesarean analgesia with intrathecal saline (□), neostigmine 25 µg (○), morphine 0.1 mg (△), or the combination of neostigmine 12.5 µg and morphine 0.05 mg (⋄) with hyperbaric bupivacaine 12 mg. Cumulative patient-controlled analgesia (PCA) consumption. Intravenous PCA was started with fentanyl 500 µg and ketorolac 150 mg in a total volume of 100 mL. The PCA device was set to deliver a bolus of 5 mL (i.e., fentanyl 25 µg and ketorolac 7.5 mg), with a lockout interval of 10 minutes and no basal infusion. Each value represents the mean ± SE. *P < .05 versus the other three groups; #P < .05 versus the combination group. (From Chung CJ, Kim JS, Park HS, Chin YJ. The efficacy of intrathecal neostigmine, intrathecal morphine, and their combination for post-cesarean section analgesia. Anesth Analg 1998; 87:341-6.)
Kaya et al.389 assessed the analgesic efficacy of epidural neostigmine administration after cesarean delivery. In this study, a CSE technique was employed with intrathecal bupivacaine 8 mg and fentanyl 10 µg, and patients subsequently received epidural neostigmine doses of 75, 150, or 300 µg after delivery. The investigators reported modest, short-lived, and dose-independent reductions in postoperative pain in the neostigmine groups.389 No differences among groups in 24-hour morphine consumption after surgery were observed.
In summary, dose-dependent side effects of intrathecal neostigmine limit its use as a single neuraxial adjunct for postcesarean analgesia. Intrathecal neostigmine 12.5 to 25 µg may be used in combination regimens to improve analgesia and reduce side effects. The use of epidural neostigmine is not currently recommended until additional studies substantiate greater postcesarean analgesic benefits with fewer side effects. Although data regarding the maternal and fetal safety profile of epidural neostigmine are reassuring,390 additional studies are needed to substantiate whether epidural neostigmine can be considered as a viable alternative or adjunct to opioids for postcesarean analgesia.
N-Methyl-D-Aspartate Antagonists
Ketamine
Anesthetic and subanesthetic doses of ketamine have analgesic properties as a result of noncompetitive antagonism of N-methyl-D-aspartate (NMDA) receptors. Animal studies suggest that NMDA receptor blockade can prevent opioid tolerance and reduce the progressive increase in action potential discharge known as the “wind-up phenomenon.”391-393 Research in dogs has indicated that no clinical or histologic disturbance in spinal tissue or the meninges occurs after exposure to a single intrathecal injection of preservative-free S(+)-ketamine at a dose of 1 mg/kg.394
Limited data exist for the role of neuraxial ketamine in the provision of postcesarean analgesia. In patients undergoing cesarean delivery randomly assigned to receive intrathecal bupivacaine alone or in combination with S(+) ketamine 0.05 mg/kg or fentanyl 25 µg,395 significantly prolonged and better quality analgesia was observed in the fentanyl group. No differences in side effects were observed between the ketamine and fentanyl groups.395 It is unclear whether the S(+) or R(–) isomers of ketamine have analgesic advantages over the racemate. Currently, the use of intrathecal ketamine does not appear to offer an analgesic benefit for postcesarean analgesia; moreover, the potential for neurotoxicity for both preservative-containing and preservative-free ketamine has not been adequately studied.396
Two systematic reviews have evaluated postoperative analgesia with perioperative epidural ketamine administration in patients undergoing nonobstetric surgery.397,398 Subramaniam et al.397 analyzed results from eight studies that compared a combination of epidural ketamine with opioids to epidural opioids alone. Although marked heterogeneity was observed among studies, the pain scores at rest were moderately lower in the patients who received epidural ketamine. No overall difference between the groups was observed in pain scores with movement. A Cochrane review also reported marked heterogeneity and mixed analgesic outcomes in studies that assessed preoperative and postoperative epidural administration of ketamine.398 To date, no published studies have evaluated perioperative epidural ketamine administration in patients undergoing cesarean delivery. In a study of patients undergoing gynecologic surgery, Kawana et al.399 noted that low-dose epidural ketamine (4, 6, or 8 mg) provided inferior analgesia compared with epidural morphine 3 mg.
In summary, epidural ketamine provides limited postcesarean analgesia and cannot be recommended for patients undergoing cesarean delivery. Further research is needed to evaluate the role of epidural and intrathecal ketamine as part of a multimodal regimen for postcesarean analgesia.
Magnesium
Magnesium is an NMDA receptor antagonist that may alter pain signaling by preventing central sensitization after nociceptive stimulation.400 The antinociceptive properties of magnesium are due to its noncompetitive NMDA receptor antagonism resulting in ion channel blockage in a voltage-dependent manner. Studies investigating intrathecal or epidural magnesium have shown variable analgesic effects after cesarean delivery. Sun et al.401 compared the postcesarean analgesic profile of four different epidural solutions administered in the perioperative period. All patients received 0.1% bupivacaine 10 mL with one of the following: morphine 1.5 mg, magnesium 500 mg, morphine 1.5 mg and magnesium 500 mg, or placebo. Patients who received all three drugs (bupivacaine, magnesium, and morphine) had significantly lower postoperative pain scores at rest and with movement, an increased time to first analgesic request, and increased satisfaction at 24 hours after surgery compared with women who received only two drugs. In women who received a CSE technique with intrathecal bupivacaine 10 mg and epidural bupivacaine 25 mg with fentanyl 100 µg, postoperative analgesic requirements were lower for women who received magnesium sulfate 500 mg compared with a control (no magnesium) group.402
Intrathecal magnesium sulfate 50 mg prolongs the duration of spinal anesthesia and improves postoperative analgesia in patients undergoing nonobstetric surgery with bupivacaine and fentanyl spinal anesthesia.403,404 There are limited data regarding the analgesic effects of intrathecal magnesium sulfate in women undergoing cesarean delivery. No difference in the first request for postcesarean analgesia was found among patients who were randomized to receive intrathecal magnesium sulfate 50 mg compared with placebo (median time, 100 minutes versus 105 minutes, respectively), and patients who received intrathecal fentanyl 25 µg had a longer time to first request for analgesia (132 minutes) compared with the magnesium group.405
Intravenous magnesium sulfate, given in either a “low-dose” regimen (25 mg/kg bolus and a 24-hour infusion at 1 g/h) or a “high-dose” regimen (50 mg/kg bolus and a 24-hour infusion at 2 g/h), was evaluated in patients undergoing spinal anesthesia for cesarean delivery.406 No differences in sequential pain scores or cumulative opioid consumption were found up to 48 hours postoperatively. Some investigators have postulated that the blood-brain barrier may affect the rate of transfer of magnesium into the CSF, a possibility that may explain why CSF magnesium concentrations do not directly reflect plasma concentrations.404
In summary, epidural administration of magnesium, co-administered with neuraxial opioids, may have a favorable analgesic effect in patients after cesarean delivery. However, more research, including dose-response studies, are needed to more formally assess the analgesic efficacy of epidural and intrathecal magnesium in the postcesarean period.
Epinephrine
Epinephrine has a direct analgesic effect by binding to alpha-adrenergic receptors and may potentiate local anesthetics by inducing local vasoconstriction and decreased drug clearance. A number of clinical studies have investigated epinephrine as a spinal or epidural adjunct. Robertson et al.83 reported that epidural epinephrine 25 µg prolonged the duration of analgesia with epidural fentanyl 100 µg but increased the incidence of pruritus. Similar improvements in duration of analgesia have been observed when epinephrine (5 to 30 µg/mL) was combined with either epidural diamorphine or sufentanil; however, the incidence of side effects (including vomiting that required treatment) was increased.145,407 In contrast, McMorland et al.408 found that epidural epinephrine did not enhance the efficacy of postcesarean analgesia provided by epidural sufentanil.
In a study assessing postoperative outcome measures for PCEA, patients who received 0.01% bupivacaine with epinephrine (0.5 µg/mL) and fentanyl reported better analgesia than those who received either fentanyl or fentanyl-epinephrine.409 No significant differences in side effects were reported between PCEA regimens with and without epinephrine. In another study, no improvement in analgesia and no reduction in opioid consumption were found with the addition of epinephrine 5 µg/mL to PCEA meperidine 5 mg/mL.410 Patients in the epinephrine group reported more nausea at 2 and 24 hours as well as higher pruritus scores at 2 hours than patients in the no-epinephrine group. The investigators attributed the epinephrine-associated increase in side effects to enhanced transfer of meperidine into the CSF. Studies of epidural 2% lidocaine or 0.5% bupivacaine with epinephrine (5 µg/mL) have not demonstrated any detrimental effects of epinephrine on umbilical artery blood flow-velocity waveforms, uteroplacental or fetal vascular resistance, fetal myocardial function, or fetal heart rate.411,412
The use of intrathecal epinephrine as an adjuvant to local anesthetics, with or without opioids, has been evaluated in a number of studies. The addition of epinephrine 200 µg to hyperbaric spinal bupivacaine improved perioperative analgesia but was associated with a longer duration of residual sensory and motor block.413 In a separate study, a combined intrathecal regimen of epinephrine 200 µg with morphine 0.2 mg did not significantly improve postoperative analgesia compared with intrathecal morphine 0.2 mg alone.414 Zakowski et al.415 found earlier and higher peak plasma bupivacaine concentrations with the addition of spinal epinephrine 200 µg to spinal bupivacaine in patients undergoing cesarean delivery. The investigators postulated that epinephrine might have a vasodilatory or biphasic action on certain vascular beds. In contrast, plasma levels of morphine were approximately 66% lower in the epinephrine group than in the control group.415
In summary, the use of epidural epinephrine (2.5 to 30 µg/mL) seems to prolong the duration of analgesia with epidural opioids but may increase opioid-related side effects. The use of intrathecal epinephrine 200 µg does not seem to enhance neuraxial opioid analgesia and is associated with prolonged sensory and motor block.
Newer Agents
In the future, newer agents and adjuvants may enhance postoperative pain management strategies in patients receiving neuraxial anesthesia for cesarean delivery. Adenosine (and adenosine analogues) may have antinociceptive effects that involve spinal adenosine A1 receptors.416 Intrathecal adenosine may enhance the effect of intrathecal clonidine, ketamine, and morphine. However, studies have not demonstrated improved analgesia with intrathecal adenosine administration in patients undergoing hysterectomy.417,418 Among pregnant women, no beneficial effects in the quality or duration of analgesia have been observed in patients who received both intrathecal adenosine 500 µg and sufentanil compared with intrathecal sufentanil alone.419
A direct relationship may exist between central potassium channels and antinociception. Several animal studies have investigated potassium channel openers (nicorandil, sildenafil) administered by the intrathecal420 or epidural421 route. These drugs may also enhance the analgesic effects of neuraxial opioids and α2-adrenergic agonists.
Intrathecal midazolam produces analgesia by acting on GABAA receptors and reducing dorsal (sensory) and motor horn excitability. Midazolam's solubility in water is pH dependent, and the likelihood of drug precipitation is high at physiologic pH and concentrations greater than 1 mg/mL.422,423 There has been growing interest in the use of midazolam as an intrathecal agent for treating acute postoperative pain. A meta-analysis of studies that assessed the clinical benefit and side effects of intrathecal midazolam in obstetric and nonobstetric patients indicated that it has a favorable pharmacologic profile.424 Intrathecal midazolam was associated with significantly improved analgesia and a reduced risk for PONV. Important side effects (including respiratory depression, prolonged motor block, and neurotoxicity) were reported as rare and were not significantly increased in patients who received intrathecal midazolam, compared with those who received placebo. Tucker et al.425 performed a cohort study to investigate potential neurotoxic effects of intrathecal midazolam 2 mg in 1100 patients receiving spinal anesthesia; they reported no increased risk for neurologic or urologic symptoms up to 1 month after neuraxial block. In the obstetric setting, one study demonstrated that the combination of intrathecal midazolam 2 mg and intrathecal fentanyl 10 µg reduced labor pain to a greater degree than was observed with either drug given alone.426 No adverse maternal or fetal events and no clinical evidence of neurologic impairment were reported among subjects in this study. After cesarean delivery, patients who received intrathecal midazolam 2 mg (without neuraxial opioids) had more prolonged postoperative analgesia and lower pain scores for 6 hours after surgery compared with patients who received either intrathecal midazolam 1 mg or no midazolam.427 The impact of multimodal regimens that include intrathecal midazolam on postcesarean analgesia remains unclear.
Several neuraxially administered drugs have been shown to produce antinociceptive effects by altering calcium channel conductance at the spinal level. Intrathecal gabapentin reduced incision-induced allodynia in rats,428,429 and epidural verapamil lowered postoperative opioid consumption after lower abdominal surgery.430 Ziconotide, a neuronal N-type-selective voltage-sensitive calcium entry–blocking agent, has been shown to have analgesic effects after intrathecal administration.431
Before recommendations can be made about the potential use of new adjuncts, neurotoxicity studies are necessary to ensure these agents' safety for neuraxial administration. In addition, studies assessing analgesic efficacy, side effects, and toxicity must demonstrate that these agents result in significant improvement over the neuraxial local anesthetic and opioid regimens currently used in clinical practice.
References
1. MacDorman MF, Menacker F, Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol. 2008;35:293–307.
2. Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2010. National Vital Statistics Report. November 17, 2011;60.
3. Niino Y. The increasing cesarean rate globally and what we can do about it. Biosci Trends. 2011;5:139–150.
4. Fassoulaki A, Gatzou V, Petropoulos G, Siafaka I. Spread of subarachnoid block, intraoperative local anaesthetic requirements and postoperative analgesic requirements in Caesarean section and total abdominal hysterectomy. Br J Anaesth. 2004;93:678–682.
5. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540.
6. Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002;89:409–423.
7. Dahl J, Gordon DB. The JCAHO pain standards: a progress report. American Pain Society Bulletin. 2002;12:11–12.
8. Kinsella M. Obstetric services. Section 8.9: Pain relief after caesarean section. [Available at] Colvin JR, Peden CJ. Raising the standard: a compendium of audit recipes. 2013 http://www.rcoa.ac.uk/ARB2012.
9. Wrench IJ, Sanghera S, Pinder A, et al. Dose response to intrathecal diamorphine for elective caesarean section and compliance with a national audit standard. Int J Obstet Anesth. 2007;16:17–21.
10. Carvalho B, Cohen SE, Lipman SS, et al. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg. 2005;101:1182–1187.
11. Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
12. Jenkins JG, Khan MM. Anaesthesia for Caesarean section: a survey in a UK region from 1992 to 2002. Anaesthesia. 2003;58:1114–1118.
13. Aiono-Le Tagaloa L, Butwick AJ, Carvalho B. A survey of perioperative and postoperative anesthetic practices for cesarean delivery. Anesthesiol Res Pract. 2009;2009:510642.
14. Ng K, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev. 2004;(2).
15. Riley ET, Cohen SE, Macario A, et al. Spinal versus epidural anesthesia for cesarean section: a comparison of time efficiency, costs, charges, and complications. Anesth Analg. 1995;80:709–712.
16. Butwick AJ, Carvalho B, Danials C, Riley ET. Retrospective analysis of anesthetic interventions for obese patients undergoing elective cesarean delivery. J Clin Anesth. 2010;22:519–526.
17. Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079–1088.
18. Cohen SE, Subak LL, Brose WG, Halpern J. Analgesia after cesarean delivery: patient evaluations and costs of five opioid techniques. Reg Anesth. 1991;16:141–149.
19. Lim Y, Jha S, Sia AT, Rawal N. Morphine for post-caesarean section analgesia: intrathecal, epidural or intravenous? Singapore Med J. 2005;46:392–396.
20. Harrison DM, Sinatra R, Morgese L, Chung JH. Epidural narcotic and patient-controlled analgesia for post-cesarean section pain relief. Anesthesiology. 1988;68:454–457.
21. Eisenach JC, Grice SC, Dewan DM. Patient-controlled analgesia following cesarean section: a comparison with epidural and intramuscular narcotics. Anesthesiology. 1988;68:444–448.
22. Bonnet MP, Mignon A, Mazoit JX, et al. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain. 2010;14:894 e1–9.
23. Mishriky BM, George RB, Habib AS. Transversus abdominis plane block for analgesia after Cesarean delivery: a systematic review and meta-analysis. Can J Anaesth. 2012;59:766–778.
24. Abdallah FW, Halpern SH, Margarido CB. Transversus abdominis plane block for postoperative analgesia after Caesarean delivery performed under spinal anaesthesia? A systematic review and meta-analysis. Br J Anaesth. 2012;109:679–687.
25. Lavand’homme P. Postcesarean analgesia: effective strategies and association with chronic pain. Curr Opin Anaesthesiol. 2006;19:244–248.
26. Pan PH. Post cesarean delivery pain management: multimodal approach. Int J Obstet Anesth. 2006;15:185–188.
27. Ranta PO, Ala-Kokko TI, Kukkonen JE, et al. Incisional and epidural analgesia after caesarean delivery: a prospective, placebo-controlled, randomised clinical study. Int J Obstet Anesth. 2006;15:189–194.
28. O’Neill P, Duarte F, Ribeiro I, et al. Ropivacaine continuous wound infusion versus epidural morphine for postoperative analgesia after cesarean delivery: a randomized controlled trial. Anesth Analg. 2012;114:179–185.
29. Bamigboye AA, Hofmeyr GJ. Local anaesthetic wound infiltration and abdominal nerves block during caesarean section for postoperative pain relief. Cochrane Database Syst Rev. 2009;(3).
30. Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203:914–932.
31. Carvalho B, Clark DJ, Yeomans DC, Angst MS. Continuous subcutaneous instillation of bupivacaine compared to saline reduces interleukin 10 and increases substance P in surgical wounds after cesarean delivery. Anesth Analg. 2010;111:1452–1459.
32. Meylan N, Elia N, Lysakowski C, Tramer MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth. 2009;102:156–167.
33. Lavand’homme PM, Roelants F, Vanderbeck B, Alluin L. Risk to develop chronic pain after elective cesarean delivery in young healthy parturients. Anesthesiology. 2005;102:A18.
34. Nikolajsen L, Sorensen HC, Jensen TS, Kehlet H. Chronic pain following Caesarean section. Acta Anaesthesiol Scand. 2004;48:111–116.
35. Almeida EC, Nogueira AA, Candido dos Reis FJ, Rosa e Silva JC. Cesarean section as a cause of chronic pelvic pain. Int J Gynaecol Obstet. 2002;79:101–104.
36. Lavand’homme PM, Roelants F, Waterloos H, De Kock MF. Postoperative analgesic effects of continuous wound infiltration with diclofenac after elective cesarean delivery. Anesthesiology. 2007;106:1220–1225.
37. Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94.
38. Kainu JP, Sarvela J, Tiippana E, et al. Persistent pain after caesarean section and vaginal birth: a cohort study. Int J Obstet Anesth. 2010;19:4–9.
39. Loos MJ, Scheltinga MR, Mulders LG, Roumen RM. The Pfannenstiel incision as a source of chronic pain. Obstet Gynecol. 2008;111:839–846.
40. Sng BL, Sia AT, Quek K, et al. Incidence and risk factors for chronic pain after caesarean section under spinal anaesthesia. Anaesth Intensive Care. 2009;37:748–752.
41. Eisenach JC, Pan P, Smiley RM, et al. Resolution of pain after childbirth. Anesthesiology. 2013;118:143–151.
42. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery: a review of predictive factors. Anesthesiology. 2000;93:1123–1133.
43. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625.
44. Lavand’homme P. Chronic pain after vaginal and cesarean delivery: a reality questioning our daily practice of obstetric anesthesia. Int J Obstet Anesth. 2010;19:1–2.
45. De Kock M, Lavand’homme P, Waterloos H. The short-lasting analgesia and long-term antihyperalgesic effect of intrathecal clonidine in patients undergoing colonic surgery. Anesth Analg. 2005;101:566–572.
46. Lavand’homme P, De Kock M, Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology. 2005;103:813–820.
47. Brandsborg B, Nikolajsen L, Hansen CT, et al. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology. 2007;106:1003–1012.
48. Cade L, Ashley J, Ross AW. Comparison of epidural and intravenous opioid analgesia after elective caesarean section. Anaesth Intensive Care. 1992;20:41–45.
49. Terajima K, Onodera H, Kobayashi M, et al. Efficacy of intrathecal morphine for analgesia following elective cesarean section: comparison with previous delivery. J Nippon Med Sch. 2003;70:327–333.
50. Swart M, Sewell J, Thomas D. Intrathecal morphine for caesarean section: an assessment of pain relief, satisfaction and side-effects. Anaesthesia. 1997;52:373–377.
51. Dahl JB, Jeppesen IS, Jorgensen H, et al. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91:1919–1927.
52. Tziavrangos E, Schug SA. Regional anaesthesia and perioperative outcome. Curr Opin Anaesthesiol. 2006;19:521–525.
53. Guay J. The benefits of adding epidural analgesia to general anesthesia: a metaanalysis. J Anesth. 2006;20:335–340.
54. Richman JM, Wu CL. Epidural analgesia for postoperative pain. Anesthesiol Clin North America. 2005;23:125–140.
55. Kettner SC, Willschke H, Marhofer P. Does regional anaesthesia really improve outcome? Br J Anaesth. 2011;107(Suppl 1):i90–i95.
56. Cohen SE, Woods WA. The role of epidural morphine in the postcesarean patient: efficacy and effects on bonding. Anesthesiology. 1983;58:500–504.
57. Carvalho B, Riley E, Cohen SE, et al. Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg. 2005;100:1150–1158.
58. Carvalho B, Roland LM, Chu LF, et al. Single-dose, extended-release epidural morphine (DepoDur®) compared to conventional epidural morphine for post-cesarean pain. Anesth Analg. 2007;105:176–183.
59. Rosaeg OP, Lui AC, Cicutti NJ, et al. Peri-operative multimodal pain therapy for caesarean section: analgesia and fitness for discharge. Can J Anaesth. 1997;44:803–809.
60. Stenkamp SJ, Easterling TR, Chadwick HS. Effect of epidural and intrathecal morphine on the length of hospital stay after cesarean section. Anesth Analg. 1989;68:66–69.
61. Kujovich JL. Hormones and pregnancy: thromboembolic risks for women. Br J Haematol. 2004;126:443–454.
62. Ramanathan J, Coleman P, Sibai B. Anesthetic modification of hemodynamic and neuroendocrine stress responses to cesarean delivery in women with severe preeclampsia. Anesth Analg. 1991;73:772–779.
63. Rawal N, Sjostrand U, Christoffersson E, et al. Comparison of intramuscular and epidural morphine for postoperative analgesia in the grossly obese: influence on postoperative ambulation and pulmonary function. Anesth Analg. 1984;63:583–592.
64. Tuman KJ, McCarthy RJ, March RJ, et al. Effects of epidural anesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesth Analg. 1991;73:696–704.
65. Yeager MP, Glass DD, Neff RK, Brinck-Johnsen T. Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology. 1987;66:729–736.
66. Armitage EN. Postoperative pain—prevention or relief? Br J Anaesth. 1989;63:136–138.
67. Yarnell RW, Polis T, Reid GN, et al. Patient-controlled analgesia with epidural meperidine after elective cesarean section. Reg Anesth. 1992;17:329–333.
68. Cooper DW, Ryall DM, McHardy FE, et al. Patient-controlled extradural analgesia with bupivacaine, fentanyl, or a mixture of both, after Caesarean section. Br J Anaesth. 1996;76:611–615.
69. Parker RK, Sawaki Y, White PF. Epidural patient-controlled analgesia: influence of bupivacaine and hydromorphone basal infusion on pain control after cesarean delivery. Anesth Analg. 1992;75:740–746.
70. Atweh SF, Kuhar MJ. Autoradiographic localization of opiate receptors in rat brain. I. Spinal cord and lower medulla. Brain Res. 1977;124:53–67.
71. Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984;228:1–12.
72. Kitahata LM, Kosaka Y, Taub A, et al. Lamina-specific suppression of dorsal-horn unit activity by morphine sulfate. Anesthesiology. 1974;41:39–48.
73. Yaksh TL, Reddy SV. Studies in the primate on the analgetic effects associated with intrathecal actions of opiates, alpha-adrenergic agonists and baclofen. Anesthesiology. 1981;54:451–467.
74. Cousins MJ, Mather LE, Glynn CJ, et al. Selective spinal analgesia. Lancet. 1979;1:1141–1142.
75. Yaksh TL. Spinal opiate analgesia: characteristics and principles of action. Pain. 1981;11:293–346.
76. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–151.
77. Bernards CM, Hill HF. Morphine and alfentanil permeability through the spinal dura, arachnoid, and pia mater of dogs and monkeys. Anesthesiology. 1990;73:1214–1219.
78. Bernards CM, Hill HF. Physical and chemical properties of drug molecules governing their diffusion through the spinal meninges. Anesthesiology. 1992;77:750–756.
79. George MJ. The site of action of epidurally administered opioids and its relevance to postoperative pain management. Anaesthesia. 2006;61:659–664.
80. Gourlay GK, Cherry DA, Plummer JL, et al. The influence of drug polarity on the absorption of opioid drugs into CSF and subsequent cephalad migration following lumbar epidural administration: application to morphine and pethidine. Pain. 1987;31:297–305.
81. Bernards CM, Shen DD, Sterling ES, et al. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (part 2): effect of epinephrine. Anesthesiology. 2003;99:466–475.
82. Bernards CM, Shen DD, Sterling ES, et al. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (part 1): differences among opioids. Anesthesiology. 2003;99:455–465.
83. Robertson K, Douglas MJ, McMorland GH. Epidural fentanyl, with and without epinephrine for post-Caesarean section analgesia. Can Anaesth Soc J. 1985;32:502–505.
84. Glass PS, Estok P, Ginsberg B, et al. Use of patient-controlled analgesia to compare the efficacy of epidural to intravenous fentanyl administration. Anesth Analg. 1992;74:345–351.
85. Ellis DJ, Millar WL, Reisner LS. A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after cesarean section. Anesthesiology. 1990;72:981–986.
86. Ionescu TI, Taverne RH, Houweling PL, et al. Pharmacokinetic study of extradural and intrathecal sufentanil anaesthesia for major surgery. Br J Anaesth. 1991;66:458–464.
87. D’Angelo R, Gerancher JC, Eisenach JC, Raphael BL. Epidural fentanyl produces labor analgesia by a spinal mechanism. Anesthesiology. 1998;88:1519–1523.
88. Liu SS, Gerancher JC, Bainton BG, et al. The effects of electrical stimulation at different frequencies on perception and pain in human volunteers: epidural versus intravenous administration of fentanyl. Anesth Analg. 1996;82:98–102.
89. Ginosar Y, Riley ET, Angst MS. The site of action of epidural fentanyl in humans: the difference between infusion and bolus administration. Anesth Analg. 2003;97:1428–1438.
90. Cohen S, Pantuck CB, Amar D, et al. The primary action of epidural fentanyl after cesarean delivery is via a spinal mechanism. Anesth Analg. 2002;94:674–679.
91. Stevens RA, Petty RH, Hill HF, et al. Redistribution of sufentanil to cerebrospinal fluid and systemic circulation after epidural administration in dogs. Anesth Analg. 1993;76:323–327.
92. Nordberg G, Hedner T, Mellstrand T, Dahlstrom B. Pharmacokinetic aspects of epidural morphine analgesia. Anesthesiology. 1983;58:545–551.
93. Youngstrom PC, Cowan RI, Sutheimer C, et al. Pain relief and plasma concentrations from epidural and intramuscular morphine in post-cesarean patients. Anesthesiology. 1982;57:404–409.
94. Weddel SJ, Ritter RR. Serum levels following epidural administration of morphine and correlation with relief of postsurgical pain. Anesthesiology. 1981;54:210–214.
95. Nordberg G, Hedner T, Mellstrand T, Dahlstrom B. Pharmacokinetic aspects of intrathecal morphine analgesia. Anesthesiology. 1984;60:448–454.
96. Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–753.
97. Gourlay GK, Murphy TM, Plummer JL, et al. Pharmacokinetics of fentanyl in lumbar and cervical CSF following lumbar epidural and intravenous administration. Pain. 1989;38:253–259.
98. Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310.
99. van den Hoogen RH, Colpaert FC. Epidural and subcutaneous morphine, meperidine (pethidine), fentanyl and sufentanil in the rat: analgesia and other in vivo pharmacologic effects. Anesthesiology. 1987;66:186–194.
100. Brill S, Gurman GM, Fisher A. A history of neuraxial administration of local analgesics and opioids. Eur J Anaesthesiol. 2003;20:682–689.
101. Chumpathong S, Santawat U, Saunya P, et al. Comparison of different doses of epidural morphine for pain relief following cesarean section. J Med Assoc Thai. 2002;85(Suppl 3):S956–S962.
102. Rosen MA, Hughes SC, Shnider SM, et al. Epidural morphine for the relief of postoperative pain after cesarean delivery. Anesth Analg. 1983;62:666–672.
103. Fuller JG, McMorland GH, Douglas MJ, Palmer L. Epidural morphine for analgesia after caesarean section: a report of 4880 patients. Can J Anaesth. 1990;37:636–640.
104. Palmer CM, Nogami WM, Van Maren G, Alves DM. Postcesarean epidural morphine: a dose-response study. Anesth Analg. 2000;90:887–891.
105. Asantila R, Eklund P, Rosenberg PH. Epidural analgesia with 4 mg of morphine following caesarean section: effect of injected volume. Acta Anaesthesiol Scand. 1993;37:764–767.
106. Eisenach JC, Schlairet TJ, Dobson CE 2nd, Hood DH. Effect of prior anesthetic solution on epidural morphine analgesia. Anesth Analg. 1991;73:119–123.
107. Karambelkar DJ, Ramanathan S. 2-Chloroprocaine antagonism of epidural morphine analgesia. Acta Anaesthesiol Scand. 1997;41:774–778.
108. Hess PE, Snowman CE, Hahn CJ, et al. Chloroprocaine may not affect epidural morphine for postcesarean delivery analgesia. J Clin Anesth. 2006;18:29–33.
109. Camann WR, Hartigan PM, Gilbertson LI, et al. Chloroprocaine antagonism of epidural opioid analgesia: a receptor-specific phenomenon? Anesthesiology. 1990;73:860–863.
110. Zakowski MI, Ramanathan S, Turndorf H. A two-dose epidural morphine regimen for cesarean section patients: therapeutic efficacy. Acta Anaesthesiol Scand. 1992;36:698–701.
111. Duale C, Frey C, Bolandard F, et al. Epidural versus intrathecal morphine for postoperative analgesia after Caesarean section. Br J Anaesth. 2003;91:690–694.
112. Sarvela J, Halonen P, Soikkeli A, Korttila K. A double-blinded, randomized comparison of intrathecal and epidural morphine for elective cesarean delivery. Anesth Analg. 2002;95:436–440.
113. Carvalho B, Coleman L, Saxena A, et al. Analgesic requirements and postoperative recovery after scheduled compared to unplanned cesarean delivery: a retrospective chart review. Int J Obstet Anesth. 2010;19:10–15.
114. Chadwick HS, Bernards CM, Kovarik DW, Tomlin JJ. Subdural injection of morphine for analgesia following cesarean section: a report of three cases. Anesthesiology. 1992;77:590–594.
115. Grass JA, Sakima NT, Schmidt R, et al. A randomized, double-blind, dose-response comparison of epidural fentanyl versus sufentanil analgesia after cesarean section. Anesth Analg. 1997;85:365–371.
116. Preston PG, Rosen MA, Hughes SC, et al. Epidural anesthesia with fentanyl and lidocaine for cesarean section: maternal effects and neonatal outcome. Anesthesiology. 1988;68:938–943.
117. Yee I, Carstoniu J, Halpern S, Pittini R. A comparison of two doses of epidural fentanyl during caesarean section. Can J Anaesth. 1993;40:722–725.
118. Bromage PR, Camporesi EM, Durant PA, Nielsen CH. Rostral spread of epidural morphine. Anesthesiology. 1982;56:431–436.
119. Wolfe MJ, Nicholas AD. Selective epidural analgesia. Lancet. 1979;2:150–151.
120. King MJ, Bowden MI, Cooper GM. Epidural fentanyl and 0.5% bupivacaine for elective caesarean section. Anaesthesia. 1990;45:285–288.
121. Naulty JS, Datta S, Ostheimer GW, et al. Epidural fentanyl for postcesarean delivery pain management. Anesthesiology. 1985;63:694–698.
122. Sevarino FB, McFarlane C, Sinatra RS. Epidural fentanyl does not influence intravenous PCA requirements in the post-caesarean patient. Can J Anaesth. 1991;38:450–453.
123. Tejwani GA, Rattan AK, McDonald JS. Role of spinal opioid receptors in the antinociceptive interactions between intrathecal morphine and bupivacaine. Anesth Analg. 1992;74:726–734.
124. Penning JP, Yaksh TL. Interaction of intrathecal morphine with bupivacaine and lidocaine in the rat. Anesthesiology. 1992;77:1186–2000.
125. Birnbach DJ, Johnson MD, Arcario T, et al. Effect of diluent volume on analgesia produced by epidural fentanyl. Anesth Analg. 1989;68:808–810.
126. Cohen S, Lowenwirt I, Pantuck CB, et al. Bupivacaine 0.01% and/or epinephrine 0.5 µg/mL improve epidural fentanyl analgesia after cesarean section. Anesthesiology. 1998;89:1354–1361.
127. Rosen MA, Dailey PA, Hughes SC, et al. Epidural sufentanil for postoperative analgesia after cesarean section. Anesthesiology. 1988;68:448–454.
128. Perriss BW, Latham BV, Wilson IH. Analgesia following extradural and i.m. pethidine in post-caesarean section patients. Br J Anaesth. 1990;64:355–357.
129. Brownridge P, Frewin DB. A comparative study of techniques of postoperative analgesia following caesarean section and lower abdominal surgery. Anaesth Intensive Care. 1985;13:123–130.
130. Paech MJ. Epidural pethidine or fentanyl during caesarean section: a double-blind comparison. Anaesth Intensive Care. 1989;17:157–165.
131. Ngan Kee WD, Lam KK, Chen PP, Gin T. Epidural meperidine after cesarean section: the effect of diluent volume. Anesth Analg. 1997;85:380–384.
132. Ngan Kee WD, Lam KK, Chen PP, Gin T. Epidural meperidine after cesarean section: a dose-response study. Anesthesiology. 1996;85:289–294.
133. Khaw KS, Ngan Kee WD, Critchley LA. Epidural meperidine does not cause hemodynamic changes in the term parturient. Can J Anaesth. 2000;47:155–159.
134. Rosaeg OP, Lindsay MP. Epidural opioid analgesia after caesarean section: a comparison of patient-controlled analgesia with meperidine and single bolus injection of morphine. Can J Anaesth. 1994;41:1063–1068.
135. Paech MJ, Pavy TJ, Orlikowski CE, et al. Postoperative intraspinal opioid analgesia after caesarean section; a randomised comparison of subarachnoid morphine and epidural pethidine. Int J Obstet Anesth. 2000;9:238–245.
136. Ngan Kee WD. Epidural pethidine: pharmacology and clinical experience. Anaesth Intensive Care. 1998;26:247–255.
137. de Leon-Casasola OA, Lema MJ. Postoperative epidural opioid analgesia: what are the choices? Anesth Analg. 1996;83:867–875.
138. Dougherty TB, Baysinger CL, Henenberger JC, Gooding DJ. Epidural hydromorphone with and without epinephrine for post-operative analgesia after cesarean delivery. Anesth Analg. 1989;68:318–322.
139. Henderson SK, Matthew EB, Cohen H, Avram MJ. Epidural hydromorphone: a double-blind comparison with intramuscular hydromorphone for postcesarean section analgesia. Anesthesiology. 1987;66:825–830.
140. Chestnut DH, Choi WW, Isbell TJ. Epidural hydromorphone for postcesarean analgesia. Obstet Gynecol. 1986;68:65–69.
141. Halpern SH, Arellano R, Preston R, et al. Epidural morphine vs hydromorphone in post-caesarean section patients. Can J Anaesth. 1996;43:595–598.
142. Quigley C. Hydromorphone for acute and chronic pain. Cochrane Database Syst Rev. 2002;(1).
143. Rawal N, Allvin R. Acute pain services in Europe: a 17-nation survey of 105 hospitals. The EuroPain Acute Pain Working Party. Eur J Anaesthesiol. 1998;15:354–363.
144. Haynes SR, Davidson I, Allsop JR, Dutton DA. Comparison of epidural methadone with epidural diamorphine for analgesia following caesarean section. Acta Anaesthesiol Scand. 1993;37:375–380.
145. Semple AJ, Macrae DJ, Munishankarappa S, et al. Effect of the addition of adrenaline to extradural diamorphine analgesia after caesarean section. Br J Anaesth. 1988;60:632–638.
146. Roulson CJ, Bennett J, Shaw M, Carli F. Effect of extradural diamorphine on analgesia after caesarean section under subarachnoid block. Br J Anaesth. 1993;71:810–813.
147. Macrae DJ, Munishankrappa S, Burrow LM, et al. Double-blind comparison of the efficacy of extradural diamorphine, extradural phenoperidine and i.m. diamorphine following caesarean section. Br J Anaesth. 1987;59:354–359.
148. Wee MY, Brown H, Reynolds F. The National Institute of Clinical Excellence (NICE) guidelines for caesarean sections: implications for the anaesthetist. Int J Obstet Anesth. 2005;14:147–158.
149. Abboud TK, Moore M, Zhu J, et al. Epidural butorphanol or morphine for the relief of post-cesarean section pain: ventilatory responses to carbon dioxide. Anesth Analg. 1987;66:887–893.
150. Palacios QT, Jones MM, Hawkins JL, et al. Post-caesarean section analgesia: a comparison of epidural butorphanol and morphine. Can J Anaesth. 1991;38:24–30.
151. Pokharel K, Rahman TR, Singh SN, et al. The efficacy and safety of low dose epidural butorphanol on postoperative analgesia following cesarean delivery. JNMA J Nepal Med Assoc. 2008;47:57–61.
152. Camann WR, Loferski BL, Fanciullo GJ, et al. Does epidural administration of butorphanol offer any clinical advantage over the intravenous route? A double-blind, placebo-controlled trial. Anesthesiology. 1992;76:216–220.
153. Rawal N, Nuutinen L, Raj PP, et al. Behavioral and histopathologic effects following intrathecal administration of butorphanol, sufentanil, and nalbuphine in sheep. Anesthesiology. 1991;75:1025–1034.
154. Eisenach J. Opioid antagonist adjuncts to epidural morphine for postcesarean analgesia: maternal outcomes. Anesth Analg. 1994;79:611–612.
155. Yaksh T, Birnbach DJ. Intrathecal nalbuphine after cesarean delivery: are we ready? Anesth Analg. 2000;91:505–508.
156. Camann WR, Hurley RH, Gilbertson LI, et al. Epidural nalbuphine for analgesia following caesarean delivery: dose-response and effect of local anaesthetic choice. Can J Anaesth. 1991;38:728–732.
157. Parker RK, Holtmann B, White PF. Patient-controlled epidural analgesia: interactions between nalbuphine and hydromorphone. Anesth Analg. 1997;84:757–763.
158. Palmer CM, Voulgaropoulos D, Alves D. Subarachnoid fentanyl augments lidocaine spinal anesthesia for cesarean delivery. Reg Anesth. 1995;20:389–394.
159. Vincent RD Jr, Chestnut DH, Choi WW, et al. Does epidural fentanyl decrease the efficacy of epidural morphine after cesarean delivery? Anesth Analg. 1992;74:658–663.
160. Cooper DW, Garcia E, Mowbray P, Millar MA. Patient-controlled epidural fentanyl following spinal fentanyl at Caesarean section. Anaesthesia. 2002;57:266–270.
161. Dottrens M, Rifat K, Morel DR. Comparison of extradural administration of sufentanil, morphine and sufentanil-morphine combination after caesarean section. Br J Anaesth. 1992;69:9–12.
162. Sinatra RS, Sevarino FB, Chung JH, et al. Comparison of epidurally administered sufentanil, morphine, and sufentanil-morphine combination for postoperative analgesia. Anesth Analg. 1991;72:522–527.
163. Wittels B, Glosten B, Faure EA, et al. Opioid antagonist adjuncts to epidural morphine for postcesarean analgesia: maternal outcomes. Anesth Analg. 1993;77:925–932.
164. Lawhorn CD, McNitt JD, Fibuch EE, et al. Epidural morphine with butorphanol for postoperative analgesia after cesarean delivery. Anesth Analg. 1991;72:53–57.
165. Gambling DR, Howell P, Huber C, Kozak S. Epidural butorphanol does not reduce side effects from epidural morphine after cesarean birth. Anesth Analg. 1994;78:1099–1104.
166. Pongraweewan O, Santawata U, Weerasarn L, et al. Epidural nalbuphine for post cesarean epidural morphine induced pruritus. J Med Assoc Thai. 2009;92:782–786.
167. Lubenow TR, Tanck EN, Hopkins EM, et al. Comparison of patient-assisted epidural analgesia with continuous-infusion epidural analgesia for postoperative patients. Reg Anesth. 1994;19:206–211.
168. Nightingale JJ, Knight MV, Higgins B, Dean T. Randomized, double-blind comparison of patient-controlled epidural infusion vs nurse-administered epidural infusion for postoperative analgesia in patients undergoing colonic resection. Br J Anaesth. 2007;98:380–384.
169. Liu SS, Allen HW, Olsson GL. Patient-controlled epidural analgesia with bupivacaine and fentanyl on hospital wards: prospective experience with 1,030 surgical patients. Anesthesiology. 1998;88:688–695.
170. Paech MJ, Moore JS, Evans SF. Meperidine for patient-controlled analgesia after cesarean section. Intravenous versus epidural administration. Anesthesiology. 1994;80:1268–1276.
171. Fanshawe MP. A comparison of patient controlled epidural pethidine versus single dose epidural morphine for analgesia after caesarean section. Anaesth Intensive Care. 1999;27:610–614.
172. Ngan Kee WD, Lam KK, Chen PP, Gin T. Comparison of patient-controlled epidural analgesia with patient-controlled intravenous analgesia using pethidine or fentanyl. Anaesth Intensive Care. 1997;25:126–132.
173. Goh JL, Evans SF, Pavy TJ. Patient-controlled epidural analgesia following caesarean delivery: a comparison of pethidine and fentanyl. Anaesth Intensive Care. 1996;24:45–50.
174. Yu PY, Gambling DR. A comparative study of patient-controlled epidural fentanyl and single dose epidural morphine for post-caesarean analgesia. Can J Anaesth. 1993;40:416–420.
175. Matsota P, Batistaki C, Apostolaki S, Kostopanagiotou G. Patient-controlled epidural analgesia after Caesarean section: levobupivacaine 0.15% versus ropivacaine 0.15% alone or combined with fentanyl 2 µg/mL: a comparative study. Arch Med Sci. 2011;7:685–693.
176. Parker RK, White PF. Epidural patient-controlled analgesia: an alternative to intravenous patient-controlled analgesia for pain relief after cesarean delivery. Anesth Analg. 1992;75:245–251.
177. Cohen S, Amar D, Pantuck CB, et al. Postcesarean delivery epidural patient-controlled analgesia. Fentanyl or sufentanil? Anesthesiology. 1993;78:486–491.
178. Vercauteren MP, Coppejans HC, ten Broecke PW, et al. Epidural sufentanil for postoperative patient-controlled analgesia (PCA) with or without background infusion: a double-blind comparison. Anesth Analg. 1995;80:76–80.
179. Boudreault D, Brasseur L, Samii K, Lemoing JP. Comparison of continuous epidural bupivacaine infusion plus either continuous epidural infusion or patient-controlled epidural injection of fentanyl for postoperative analgesia. Anesth Analg. 1991;73:132–137.
180. Vercauteren M, Vereecken K, La Malfa M, et al. Cost-effectiveness of analgesia after Caesarean section: a comparison of intrathecal morphine and epidural PCA. Acta Anaesthesiol Scand. 2002;46:85–89.
181. Howell SB. Clinical applications of a novel sustained-release injectable drug delivery system: DepoFoam® technology. Cancer J. 2001;7:219–227.
182. Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam®: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45:1153–1176.
183. Mantripragada S. A lipid based depot (DepoFoam® technology) for sustained release drug delivery. Prog Lipid Res. 2002;41:392–406.
184. Viscusi ER, Gambling DR, Hughes TL, Manvelian GZ. Pharmacokinetics of extended-release epidural morphine sulfate: pooled analysis of six clinical studies. Am J Health Syst Pharm. 2009;66:1020–1030.
185. Vanterpool S, Coombs R, Fecho K. Continuous epidural infusion of morphine versus single epidural injection of extended-release morphine for postoperative pain control after arthroplasty: a retrospective analysis. Ther Clin Risk Manag. 2010;6:271–277.
186. Nagle PC, Gerancher JC. DepoDur®: Extended-release epidural morphine: a review of an old drug in a new vehicle. Tech Reg Anesth Pain Manag. 2007;11:9–18.
187. Sumida S, Lesley MR, Hanna MN, et al. Meta-analysis of the effect of extended-release epidural morphine versus intravenous patient-controlled analgesia on respiratory depression. J Opioid Manag. 2009;5:301–305.
188. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting. Anesthesiology. 2012;116:248–273.
189. Gerancher JC, Nagle PC. Management of accidental spinal administration of extended-release epidural morphine. Anesthesiology. 2008;108:1147–1149.
190. Keck S, Glennon C, Ginsberg B. DepoDur® extended-release epidural morphine: reshaping postoperative care. What perioperative nurses need to know. Orthop Nurs. 2007;26:86–93.
191. Atkinson Ralls L, Drover DR, Clavijo CF, Carvalho B. Prior epidural lidocaine alters the pharmacokinetics and drug effects of extended-release epidural morphine (DepoDur®) after cesarean delivery. Anesth Analg. 2011;113:251–258.
192. Gambling D, Hughes T, Gould E, Manvelian G. A pharmacokinetic and pharmacodynamic study of a single dose of epidural DepoDur® following epidural bupivacaine: a randomized controlled trial in patients undergoing lower abdominal surgery (abstract). Reg Anesth Pain Med. 2006;30:A29.
193. DepoDur® (Morphine Sulfate Extended-Release Liposome Injection). Bedminster, NJ, Full Prescribing Information, EKR Therapeutics, Inc, 2007.
194. Chadwick HS, Ready LB. Intrathecal and epidural morphine sulfate for post-cesarean analgesia—a clinical comparison. Anesthesiology. 1988;68:925–929.
195. Abboud TK, Dror A, Mosaad P, et al. Mini-dose intrathecal morphine for the relief of post-cesarean section pain: safety, efficacy, and ventilatory responses to carbon dioxide. Anesth Analg. 1988;67:137–143.
196. Abouleish E, Rawal N, Rashad MN. The addition of 0.2 mg subarachnoid morphine to hyperbaric bupivacaine for cesarean delivery: a prospective study of 856 cases. Reg Anesth. 1991;16:137–140.
197. Sibilla C, Albertazz P, Zatelli R, Martinello R. Perioperative analgesia for caesarean section: comparison of intrathecal morphine and fentanyl alone or in combination. Int J Obstet Anesth. 1997;6:43–48.
198. Popping DM, Elia N, Marret E, et al. Opioids added to local anesthetics for single-shot intrathecal anesthesia in patients undergoing minor surgery: a meta-analysis of randomized trials. Pain. 2012;153:784–793.
199. Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–444.
200. Milner AR, Bogod DG, Harwood RJ. Intrathecal administration of morphine for elective Caesarean section: a comparison between 0.1 mg and 0.2 mg. Anaesthesia. 1996;51:871–873.
201. Yang T, Breen TW, Archer D, Fick G. Comparison of 0.25 mg and 0.1 mg intrathecal morphine for analgesia after Cesarean section. Can J Anaesth. 1999;46:856–860.
202. Uchiyama A, Nakano S, Ueyama H, et al. Low dose intrathecal morphine and pain relief following caesarean section. Int J Obstet Anesth. 1994;3:87–91.
203. Wong JY, Carvalho B, Riley ET. Intrathecal morphine 100 and 200 µg for post-cesarean delivery analgesia: a trade-off between analgesic efficacy and side effects. Int J Obstet Anesth. 2013;22:36–41.
204. Girgin NK, Gurbet A, Turker G, et al. Intrathecal morphine in anesthesia for cesarean delivery: dose-response relationship for combinations of low-dose intrathecal morphine and spinal bupivacaine. J Clin Anesth. 2008;20:180–185.
205. Mikuni I, Hirai H, Toyama Y, et al. Efficacy of intrathecal morphine with epidural ropivacaine infusion for postcesarean analgesia. J Clin Anesth. 2010;22:268–273.
206. Belzarena SD. Clinical effects of intrathecally administered fentanyl in patients undergoing cesarean section. Anesth Analg. 1992;74:653–657.
207. Dahlgren G, Hultstrand C, Jakobsson J, et al. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997;85:1288–1293.
208. Siddik-Sayyid SM, Aouad MT, Jalbout MI, et al. Intrathecal versus intravenous fentanyl for supplementation of subarachnoid block during cesarean delivery. Anesth Analg. 2002;95:209–213.
209. Meyer RA, Macarthur AJ, Downey K. Study of equivalence: spinal bupivacaine 15 mg versus bupivacaine 12 mg with fentanyl 15 µg for cesarean delivery. Int J Obstet Anesth. 2012;21:17–23.
210. Siti Salmah G, Choy YC. Comparison of morphine with fentanyl added to intrathecal 0.5% hyperbaric bupivacaine for analgesia after caesarean section. Med J Malaysia. 2009;64:71–74.
211. Hunt CO, Naulty JS, Bader AM, et al. Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology. 1989;71:535–540.
212. Chu CC, Shu SS, Lin SM, et al. The effect of intrathecal bupivacaine with combined fentanyl in cesarean section. Acta Anaesthesiol Sin. 1995;33:149–154.
213. Chen X, Qian X, Fu F, et al. Intrathecal sufentanil decreases the median effective dose (ED50) of intrathecal hyperbaric ropivacaine for caesarean delivery. Acta Anaesthesiol Scand. 2010;54:284–290.
214. Courtney MA, Bader AM, Hartwell B, et al. Perioperative analgesia with subarachnoid sufentanil administration. Reg Anesth. 1992;17:274–278.
215. Braga Ade F, Braga FS, Poterio GM, et al. Sufentanil added to hyperbaric bupivacaine for subarachnoid block in Caesarean section. Eur J Anaesthesiol. 2003;20:631–635.
216. Karaman S, Kocabas S, Uyar M, et al. The effects of sufentanil or morphine added to hyperbaric bupivacaine in spinal anaesthesia for caesarean section. Eur J Anaesthesiol. 2006;23:285–291.
217. Qian XW, Chen XZ, Li DB. Low-dose ropivacaine-sufentanil spinal anaesthesia for caesarean delivery: a randomised trial. Int J Obstet Anesth. 2008;17:309–314.
218. Lee JH, Chung KH, Lee JY, et al. Comparison of fentanyl and sufentanil added to 0.5% hyperbaric bupivacaine for spinal anesthesia in patients undergoing cesarean section. Korean J Anesthesiol. 2011;60:103–108.
219. Kafle SK. Intrathecal meperidine for elective caesarean section: a comparison with lidocaine. Can J Anaesth. 1993;40:718–721.
220. Nguyen Thi TV, Orliaguet G, Ngu TH, Bonnet F. Spinal anesthesia with meperidine as the sole agent for cesarean delivery. Reg Anesth. 1994;19:386–389.
221. Yu SC, Ngan Kee WD, Kwan AS. Addition of meperidine to bupivacaine for spinal anaesthesia for Caesarean section. Br J Anaesth. 2002;88:379–383.
222. Imarengiaye CO, Asudo FD, Akpoguado DD, et al. Subarachnoid bupivacaine and pethidine for caesarean section: assessment of quality of perioperative analgesia and side effects. Niger Postgrad Med J. 2011;18:200–204.
223. Kelly MC, Carabine UA, Mirakhur RK. Intrathecal diamorphine for analgesia after caesarean section: a dose finding study and assessment of side-effects. Anaesthesia. 1998;53:231–237.
224. Stacey R, Jones R, Kar G, Poon A. High-dose intrathecal diamorphine for analgesia after Caesarean section. Anaesthesia. 2001;56:54–60.
225. Cowan CM, Kendall JB, Barclay PM, Wilkes RG. Comparison of intrathecal fentanyl and diamorphine in addition to bupivacaine for caesarean section under spinal anaesthesia. Br J Anaesth. 2002;89:452–458.
226. Lane S, Evans P, Arfeen Z, Misra U. A comparison of intrathecal fentanyl and diamorphine as adjuncts in spinal anaesthesia for Caesarean section. Anaesthesia. 2005;60:453–457.
227. Saravanan S, Robinson AP, Qayoum Dar A, et al. Minimum dose of intrathecal diamorphine required to prevent intraoperative supplementation of spinal anaesthesia for Caesarean section. Br J Anaesth. 2003;91:368–372.
228. Husaini SW, Russell IF. Intrathecal diamorphine compared with morphine for postoperative analgesia after caesarean section under spinal anaesthesia. Br J Anaesth. 1998;81:135–139.
229. Hallworth SP, Fernando R, Bell R, et al. Comparison of intrathecal and epidural diamorphine for elective caesarean section using a combined spinal-epidural technique. Br J Anaesth. 1999;82:228–232.
230. Culebras X, Gaggero G, Zatloukal J, et al. Advantages of intrathecal nalbuphine, compared with intrathecal morphine, after cesarean delivery: an evaluation of postoperative analgesia and adverse effects. Anesth Analg. 2000;91:601–605.
231. Chung JH, Sinatra RS, Sevarino FB, Fermo L. Subarachnoid meperidine-morphine combination: an effective perioperative analgesic adjunct for cesarean delivery. Reg Anesth. 1997;22:119–124.
232. Draisci G, Frassanito L, Pinto R, et al. Safety and effectiveness of coadministration of intrathecal sufentanil and morphine in hyperbaric bupivacaine-based spinal anesthesia for cesarean section. J Opioid Manag. 2009;5:197–202.
233. Cooper DW, Lindsay SL, Ryall DM, et al. Does intrathecal fentanyl produce acute cross-tolerance to i.v. morphine? Br J Anaesth. 1997;78:311–313.
234. Carvalho B, Drover DR, Ginosar Y, et al. Intrathecal fentanyl added to bupivacaine and morphine for cesarean delivery may induce a subtle acute opioid tolerance. Int J Obstet Anesth. 2012;21:29–34.
235. Eisenach JC, James FM 3rd, Gordh T Jr, Yaksh TL. New epidural drugs: primum non nocere. Anesth Analg. 1998;87:1211–1212.
236. Yaksh TL, Grafe MR, Malkmus S, et al. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–427.
237. Yaksh TL, Rathbun M, Jage J, et al. Pharmacology and toxicology of chronically infused epidural clonidine-HCl in dogs. Fundam Appl Toxicol. 1994;23:319–335.
238. Sabbe MB, Grafe MR, Mjanger E, et al. Spinal delivery of sufentanil, alfentanil, and morphine in dogs: physiologic and toxicologic investigations. Anesthesiology. 1994;81:899–920.
239. Abouleish E, Barmada MA, Nemoto EM, et al. Acute and chronic effects of intrathecal morphine in monkeys. Br J Anaesth. 1981;53:1027–1032.
240. Hodgson PS, Neal JM, Pollock JE, Liu SS. The neurotoxicity of drugs given intrathecally (spinal). Anesth Analg. 1999;88:797–809.
241. Eisenach JC, Yaksh TL. Safety in numbers: how do we study toxicity of spinal analgesics? Anesthesiology. 2002;97:1047–1049.
242. Yaksh TL, Collins JG. Studies in animals should precede human use of spinally administered drugs. Anesthesiology. 1989;70:4–6.
243. Coombs DW, Colburn RW, Allen CD, et al. Toxicity of chronic spinal analgesia in a canine model: neuropathologic observations with dezocine lactate. Reg Anesth. 1990;15:94–102.
244. Hughes SC, Rosen MA, Shnider SM, et al. Maternal and neonatal effects of epidural morphine for labor and delivery. Anesth Analg. 1984;63:319–324.
245. Baraka A, Noueihid R, Hajj S. Intrathecal injection of morphine for obstetric analgesia. Anesthesiology. 1981;54:136–140.
246. Negre I, Gueneron JP, Ecoffey C, et al. Ventilatory response to carbon dioxide after intramuscular and epidural fentanyl. Anesth Analg. 1987;66:707–710.
247. Cohen SE, Labaille T, Benhamou D, Levron JC. Respiratory effects of epidural sufentanil after cesarean section. Anesth Analg. 1992;74:677–682.
248. Bernards CM. Recent insights into the pharmacokinetics of spinal opioids and the relevance to opioid selection. Curr Opin Anaesthesiol. 2004;17:441–447.
249. Kafer ER, Brown JT, Scott D, et al. Biphasic depression of ventilatory responses to CO2 following epidural morphine. Anesthesiology. 1983;58:418–427.
250. Koo CY, Eikermann M. Respiratory effects of opioids in perioperative medicine. Open Anesthesiol J. 2011;5(Suppl 1):23–34.
251. Weinger MB. Dangers of postoperative opioids. Anesth Patient Safety Found Newsl. 2006;21:61.
252. Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs. 2011;71:1807–1819.
253. Ko S, Goldstein DH, VanDenKerkhof EG. Definitions of “respiratory depression” with intrathecal morphine postoperative analgesia: a review of the literature. Can J Anaesth. 2003;50:679–688.
254. Carvalho B. Respiratory depression after neuraxial opioids in the obstetric setting. Anesth Analg. 2008;107:956–961.
255. Etches RC, Sandler AN, Daley MD. Respiratory depression and spinal opioids. Can J Anaesth. 1989;36:165–185.
256. Shapiro A, Zohar E, Zaslansky R, et al. The frequency and timing of respiratory depression in 1524 postoperative patients treated with systemic or neuraxial morphine. J Clin Anesth. 2005;17:537–542.
257. Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–223.
258. Palmer CM. Early respiratory depression following intrathecal fentanyl-morphine combination. Anesthesiology. 1991;74:1153–1155.
259. Brockway MS, Noble DW, Sharwood-Smith GH, McClure JH. Profound respiratory depression after extradural fentanyl. Br J Anaesth. 1990;64:243–245.
260. Noble DW, Morrison LM, Brockway MS, McClure JH. Adrenaline, fentanyl or adrenaline and fentanyl as adjuncts to bupivacaine for extradural anaesthesia in elective caesarean section. Br J Anaesth. 1991;66:645–650.
261. Madej TH, Strunin L. Comparison of epidural fentanyl with sufentanil: analgesia and side effects after a single bolus dose during elective caesarean section. Anaesthesia. 1987;42:1156–1161.
262. American Society of Anesthesiologists Task Force on Neuraxial Opioids. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 2009;110:218–230.
263. The Joint Commission. Safe use of opioids in hospitals. [Available at] http://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf [Accessed February 2013] .
264. Hughes SC. Respiratory depression following intraspinal narcotics: expect it!. Int J Obstet Anesth. 1997;6:145–146.
265. Weinger MB. No patient shall be harmed by opioid-induced respiratory depression. Anesth Patient Safety Found Newsl. 2011;26:21.
266. Bailey PL, Rhondeau S, Schafer PG, et al. Dose-response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59.
267. Brose WG, Cohen SE. Oxyhemoglobin saturation following cesarean section in patients receiving epidural morphine, PCA, or im meperidine analgesia. Anesthesiology. 1989;70:948–953.
268. Celleno D, Capogna G, Sebastiani M, et al. Epidural analgesia during and after cesarean delivery: comparison of five opioids. Reg Anesth. 1991;16:79–83.
269. Ready LB, Loper KA, Nessly M, Wild L. Postoperative epidural morphine is safe on surgical wards. Anesthesiology. 1991;75:452–456.
270. Johnson A, Bengtsson M, Soderlind K, Lofstrom JB. Influence of intrathecal morphine and naloxone intervention on postoperative ventilatory regulation in elderly patients. Acta Anaesthesiol Scand. 1992;36:436–444.
271. Thind GS, Wells JC, Wilkes RG. The effects of continuous intravenous naloxone on epidural morphine analgesia. Anaesthesia. 1986;41:582–585.
272. Cannesson M, Nargues N, Bryssine B, et al. Intrathecal morphine overdose during combined spinal-epidural block for Caesarean delivery. Br J Anaesth. 2002;89:925–927.
273. Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth. 2005;14:230–241.
274. Chaney MA. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891–903.
275. Marcus HE, Fabian A, Dagtekin O, et al. Pain, postdural puncture headache, nausea, and pruritus after cesarean delivery: a survey of prophylaxis and treatment. Minerva Anestesiol. 2011;77:1043–1049.
276. Chestnut DH, Vandewalker GE, Owen CL, et al. Administration of metoclopramide for prevention of nausea and vomiting during epidural anesthesia for elective cesarean section. Anesthesiology. 1987;66:563–566.
277. Mishriky BM, Habib AS. Impact of data by Fujii and colleagues on the meta-analysis of metoclopramide for antiemetic prophylaxis in women undergoing Caesarean delivery under neuraxial anaesthesia. Br J Anaesth. 2012;109:826.
278. Lussos SA, Bader AM, Thornhill ML, Datta S. The antiemetic efficacy and safety of prophylactic metoclopramide for elective cesarean delivery during spinal anesthesia. Reg Anesth. 1992;17:126–130.
279. Pan PH, Moore CH. Intraoperative antiemetic efficacy of prophylactic ondansetron versus droperidol for cesarean section patients under epidural anesthesia. Anesth Analg. 1996;83:982–986.
280. Sanansilp V, Areewatana S, Tonsukchai N. Droperidol and the side effects of epidural morphine after cesarean section. Anesth Analg. 1998;86:532–537.
281. Kotelko DM, Rottman RL, Wright WC, et al. Transdermal scopolamine decreases nausea and vomiting following cesarean section in patients receiving epidural morphine. Anesthesiology. 1989;71:675–678.
282. Harnett MJ, O’Rourke N, Walsh M, et al. Transdermal scopolamine for prevention of intrathecal morphine-induced nausea and vomiting after cesarean delivery. Anesth Analg. 2007;105:764–769.
283. Yazigi A, Chalhoub V, Madi-Jebara S, et al. Prophylactic ondansetron is effective in the treatment of nausea and vomiting but not on pruritus after cesarean delivery with intrathecal sufentanil-morphine. J Clin Anesth. 2002;14:183–186.
284. Pan PH, Moore CH. Comparing the efficacy of prophylactic metoclopramide, ondansetron, and placebo in cesarean section patients given epidural anesthesia. J Clin Anesth. 2001;13:430–435.
285. Balki M, Kasodekar S, Dhumne S, Carvalho JC. Prophylactic [corrected] granisetron does not prevent postdelivery nausea and vomiting during elective cesarean delivery under spinal anesthesia. Anesth Analg. 2007;104:679–683.
286. George RB, Allen TK, Habib AS. Serotonin receptor antagonists for the prevention and treatment of pruritus, nausea, and vomiting in women undergoing cesarean delivery with intrathecal morphine: a systematic review and meta-analysis. Anesth Analg. 2009;109:174–182.
287. Tzeng JI, Wang JJ, Ho ST, et al. Dexamethasone for prophylaxis of nausea and vomiting after epidural morphine for post-Caesarean section analgesia: comparison of droperidol and saline. Br J Anaesth. 2000;85:865–868.
288. Wang JJ, Ho ST, Wong CS, et al. Dexamethasone prophylaxis of nausea and vomiting after epidural morphine for post-Cesarean analgesia. Can J Anaesth. 2001;48:185–190.
289. Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anest Analg. 2012;114:813–822.
290. Nortcliffe SA, Shah J, Buggy DJ. Prevention of postoperative nausea and vomiting after spinal morphine for Caesarean section: comparison of cyclizine, dexamethasone and placebo. Br J Anaesth. 2003;90:665–670.
291. Heffernan AM, Rowbotham DJ. Postoperative nausea and vomiting—time for balanced antiemesis? Br J Anaesth. 2000;85:675–677.
292. Wu JI, Lo Y, Chia YY, et al. Prevention of postoperative nausea and vomiting after intrathecal morphine for Cesarean section: a randomized comparison of dexamethasone, droperidol, and a combination. Int J Obstet Anesth. 2007;16:122–127.
293. Ho CM, Hseu SS, Tsai SK, Lee TY. Effect of P-6 acupressure on prevention of nausea and vomiting after epidural morphine for post-cesarean section pain relief. Acta Anaesthesiol Scand. 1996;40:372–375.
294. Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and prevention of nausea and vomiting during and after spinal anaesthesia for caesarean section. Br J Anaesth. 2000;84:463–467.
295. Duggal KN, Douglas MJ, Peteru EA, Merrick PM. Acupressure for intrathecal narcotic-induced nausea and vomiting after caesarean section. Int J Obstet Anesth. 1998;7:231–236.
296. Ho CM, Tsai HJ, Chan KH, Tsai SK. P6 acupressure does not prevent emesis during spinal anesthesia for cesarean delivery. Anesth Analg. 2006;102:900–903.
297. Allen TK, Habib AS. P6 stimulation for the prevention of nausea and vomiting associated with cesarean delivery under neuraxial anesthesia: a systematic review of randomized controlled trials. Anesth Analg. 2008;107:1308–1312.
298. Slappendel R, Weber EW, Benraad B, et al. Itching after intrathecal morphine: incidence and treatment. Eur J Anaesthesiol. 2000;17:616–621.
299. Kendrick WD, Woods AM, Daly MY, et al. Naloxone versus nalbuphine infusion for prophylaxis of epidural morphine-induced pruritus. Anesth Analg. 1996;82:641–647.
300. Cohen SE, Ratner EF, Kreitzman TR, et al. Nalbuphine is better than naloxone for treatment of side effects after epidural morphine. Anesth Analg. 1992;75:747–752.
301. Morgan M. The rational use of intrathecal and extradural opioids. Br J Anaesth. 1989;63:165–188.
302. Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333.
303. LaBella FS, Kim RS, Templeton J. Opiate receptor binding activity of 17-alpha estrogenic steroids. Life Sci. 1978;23:1797–1804.
304. Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186.
305. Tsai FF, Fan SZ, Yang YM, et al. Human opioid µ-receptor A118G polymorphism may protect against central pruritus by epidural morphine for post-cesarean analgesia. Acta Anaesthesiol Scand. 2010;54:1265–1269.
306. Kjellberg F, Tramer MR. Pharmacological control of opioid-induced pruritus: a quantitative systematic review of randomized trials. Eur J Anaesthesiol. 2001;18:346–357.
307. Somrat C, Oranuch K, Ketchada U, et al. Optimal dose of nalbuphine for treatment of intrathecal-morphine induced pruritus after caesarean section. J Obstet Gynaecol Res. 1999;25:209–213.
308. Wu Z, Kong M, Wang N, et al. Intravenous butorphanol administration reduces intrathecal morphine-induced pruritus after cesarean delivery: a randomized, double-blind, placebo-controlled study. J Anesth. 2012;26:752–757.
309. Morgan PJ, Mehta S, Kapala DM. Nalbuphine pretreatment in cesarean section patients receiving epidural morphine. Reg Anesth. 1991;16:84–88.
310. Lockington PF, Fa’aea P. Subcutaneous naloxone for the prevention of intrathecal morphine induced pruritus in elective Caesarean delivery. Anaesthesia. 2007;62:672–676.
311. Luthman JA, Kay NH, White JB. Intrathecal morphine for post caesarean section analgesia: does naloxone reduce the incidence of pruritus? Int J Obstet Anesth. 1992;1:191–194.
312. Abboud TK, Afrasiabi A, Davidson J, et al. Prophylactic oral naltrexone with epidural morphine: effect on adverse reactions and ventilatory responses to carbon dioxide. Anesthesiology. 1990;72:233–237.
313. Abboud TK, Lee K, Zhu J, et al. Prophylactic oral naltrexone with intrathecal morphine for cesarean section: effects on adverse reactions and analgesia. Anesth Analg. 1990;71:367–370.
314. Jeon Y, Hwang J, Kang J, et al. Effects of epidural naloxone on pruritus induced by epidural morphine: a randomized controlled trial. Int J Obstet Anesth. 2005;14:22–25.
315. Lee L, Irwin MG, Lim J, Wong CK. The effect of celecoxib on intrathecal morphine-induced pruritus in patients undergoing caesarean section. Anaesthesia. 2004;59:876–880.
316. Borgeat A, Wilder-Smith OH, Saiah M, Rifat K. Subhypnotic doses of propofol relieve pruritus induced by epidural and intrathecal morphine. Anesthesiology. 1992;76:510–512.
317. Torn K, Tuominen M, Tarkkila P, Lindgren L. Effects of sub-hypnotic doses of propofol on the side effects of intrathecal morphine. Br J Anaesth. 1994;73:411–412.
318. Beilin Y, Bernstein HH, Zucker-Pinchoff B, et al. Subhypnotic doses of propofol do not relieve pruritus induced by intrathecal morphine after cesarean section. Anesth Analg. 1998;86:310–313.
319. Warwick JP, Kearns CF, Scott WE. The effect of subhypnotic doses of propofol on the incidence of pruritus after intrathecal morphine for caesarean section. Anaesthesia. 1997;52:270–275.
320. Charuluxananan S, Kyokong O, Somboonviboon W, et al. Nalbuphine versus propofol for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2001;93:162–165.
321. Bonnet MP, Marret E, Josserand J, Mercier FJ. Effect of prophylactic 5-HT3 receptor antagonists on pruritus induced by neuraxial opioids: a quantitative systematic review. Br J Anaesth. 2008;101:311–319.
322. Charuluxananan S, Kyokong O, Somboonviboon W, et al. Nalbuphine versus ondansetron for prevention of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2003;96:1789–1793.
323. Yeh HM, Chen LK, Lin CJ, et al. Prophylactic intravenous ondansetron reduces the incidence of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2000;91:172–175.
324. Siddik-Sayyid SM, Aouad MT, Taha SK, et al. Does ondansetron or granisetron prevent subarachnoid morphine-induced pruritus after cesarean delivery? Anesth Analg. 2007;104:421–424.
325. Sarvela PJ, Halonen PM, Soikkeli AI, et al. Ondansetron and tropisetron do not prevent intraspinal morphine- and fentanyl-induced pruritus in elective cesarean delivery. Acta Anaesthesiol Scand. 2006;50:239–244.
326. Yazigi A, Chalhoub V, Madi-Jebara S, Haddad F. Ondansetron for prevention of intrathecal opioids-induced pruritus, nausea and vomiting after cesarean delivery. Anesth Analg. 2004;98:264.
327. Han DW, Hong SW, Kwon JY, et al. Epidural ondansetron is more effective to prevent postoperative pruritus and nausea than intravenous ondansetron in elective cesarean delivery. Acta Obstet Gynecol Scand. 2007;86:683–687.
328. Tan T, Ojo R, Immani S, et al. Reduction of severity of pruritus after elective caesarean section under spinal anaesthesia with subarachnoid morphine: a randomised comparison of prophylactic granisetron and ondansetron. Int J Obstet Anesth. 2010;19:56–60.
329. Charuluxananan S, Somboonviboon W, Kyokong O, Nimcharoendee K. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Reg Anesth Pain Med. 2000;25:535–539.
330. Ko MC, Lee H, Song MS, et al. Activation of kappa-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther. 2003;305:173–179.
331. Tamdee D, Charuluxananan S, Punjasawadwong Y, et al. A randomized controlled trial of pentazocine versus ondansetron for the treatment of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2009;109:1606–1611.
332. Alhashemi JA, Crosby ET, Grodecki W, et al. Treatment of intrathecal morphine-induced pruritus following caesarean section. Can J Anaesth. 1997;44:1060–1065.
333. Siddik-Sayyid SM, Yazbeck-Karam VG, Zahreddine BW, et al. Ondansetron is as effective as diphenhydramine for treatment of morphine-induced pruritus after cesarean delivery. Acta Anaesthesiol Scand. 2010;54:764–769.
334. Kuipers PW, Kamphuis ET, van Venrooij GE, et al. Intrathecal opioids and lower urinary tract function: a urodynamic evaluation. Anesthesiology. 2004;100:1497–1503.
335. Kamphuis ET, Ionescu TI, Kuipers PW, et al. Recovery of storage and emptying functions of the urinary bladder after spinal anesthesia with lidocaine and with bupivacaine in men. Anesthesiology. 1998;88:310–316.
336. Kermans G, Wyndaele JJ, Thiery M, De Sy W. Puerperal urinary retention. Acta Urol Belg. 1986;54:376–385.
337. Yip SK, Sahota D, Pang MW, Chang A. Postpartum urinary retention. Acta Obstet Gynecol Scand. 2004;83:881–891.
338. Evron S, Samueloff A, Simon A, et al. Urinary function during epidural analgesia with methadone and morphine in post-cesarean section patients. Pain. 1985;23:135–144.
339. Liang CC, Chang SD, Wong SY, et al. Effects of postoperative analgesia on postpartum urinary retention in women undergoing cesarean delivery. J Obstet Gynaecol Res. 2010;36:991–995.
340. Rawal N, Mollefors K, Axelsson K, et al. An experimental study of urodynamic effects of epidural morphine and of naloxone reversal. Anesth Analg. 1983;62:641–647.
341. Rathmell JP, Lair TR, Nauman B. The role of intrathecal drugs in the treatment of acute pain. Anesth Analg. 2005;101(Suppl 5S):S30–S43.
342. Liang CC, Chang SD, Chang YL, et al. Postpartum urinary retention after cesarean delivery. Int J Gynaecol Obstet. 2007;99:229–232.
343. Butwick AJ, Lipman SS, Carvalho B. Intraoperative forced air-warming during cesarean delivery under spinal anesthesia does not prevent maternal hypothermia. Anesth Analg. 2007;105:1413–1419.
344. Roy JD, Girard M, Drolet P. Intrathecal meperidine decreases shivering during cesarean delivery under spinal anesthesia. Anesth Analg. 2004;98:230–234.
345. Sessler DI. Perioperative heat balance. Anesthesiology. 2000;92:578–596.
346. Matsukawa T, Sessler DI, Christensen R, et al. Heat flow and distribution during epidural anesthesia. Anesthesiology. 1995;83:961–967.
347. Emerick TH, Ozaki M, Sessler DI, et al. Epidural anesthesia increases apparent leg temperature and decreases the shivering threshold. Anesthesiology. 1994;81:289–298.
348. Ozaki M, Kurz A, Sessler DI, et al. Thermoregulatory thresholds during epidural and spinal anesthesia. Anesthesiology. 1994;81:282–288.
349. Kurz A, Sessler DI, Schroeder M, Kurz M. Thermoregulatory response thresholds during spinal anesthesia. Anesth Analg. 1993;77:721–726.
350. Saito T, Sessler DI, Fujita K, et al. Thermoregulatory effects of spinal and epidural anesthesia during cesarean delivery. Reg Anesth Pain Med. 1998;23:418–423.
351. Leslie K, Sessler DI. Reduction in the shivering threshold is proportional to spinal block height. Anesthesiology. 1996;84:1327–1331.
352. Hui CK, Huang CH, Lin CJ, et al. A randomised double-blind controlled study evaluating the hypothermic effect of 150 µg morphine during spinal anaesthesia for Caesarean section. Anaesthesia. 2006;61:29–31.
353. Techanivate A, Rodanant O, Tachawattanawisal W, Somsiri T. Intrathecal fentanyl for prevention of shivering in cesarean section. J Med Assoc Thai. 2005;88:1214–1221.
354. Hong JY, Lee IH. Comparison of the effects of intrathecal morphine and pethidine on shivering after Caesarean delivery under combined-spinal epidural anaesthesia. Anaesthesia. 2005;60:1168–1172.
355. Khan ZH, Zanjani AP, Makarem J, Samadi S. Antishivering effects of two different doses of intrathecal meperidine in caesarean section: a prospective randomised blinded study. Eur J Anaesthesiol. 2011;28:202–206.
356. Macintyre PE, Pavlin EG, Dwersteg JF. Effect of meperidine on oxygen consumption, carbon dioxide production, and respiratory gas exchange in postanesthesia shivering. Anesth Analg. 1987;66:751–755.
357. Juneja M, Ackerman WE 3rd, Heine MF, et al. Butorphanol for the relief of shivering associated with extradural anesthesia in parturients. J Clin Anesth. 1992;4:390–393.
358. Sevarino FB, Johnson MD, Lema MJ, et al. The effect of epidural sufentanil on shivering and body temperature in the parturient. Anesth Analg. 1989;68:530–533.
359. Sutherland J, Seaton H, Lowry C. The influence of epidural pethidine on shivering during lower segment caesarean section under epidural anaesthesia. Anaesth Intensive Care. 1991;19:228–232.
360. Horn EP, Schroeder F, Gottschalk A, et al. Active warming during cesarean delivery. Anesth Analg. 2002;94:409–414.
361. Walker SM, Goudas LC, Cousins MJ, Carr DB. Combination spinal analgesic chemotherapy: a systematic review. Anesth Analg. 2002;95:674–715.
362. Kitahata LM. Spinal analgesia with morphine and clonidine. Anesth Analg. 1989;68:191–193.
363. Taiwo YO, Fabian A, Pazoles CJ, Fields HL. Potentiation of morphine antinociception by monoamine reuptake inhibitors in the rat spinal cord. Pain. 1985;21:329–337.
364. Kuraishi Y, Hirota N, Sato Y, et al. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182.
365. Eisenach JC, De Kock M, Klimscha W. Alpha2-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995). Anesthesiology. 1996;85:655–674.
366. Elia N, Culebras X, Mazza C, et al. Clonidine as an adjuvant to intrathecal local anesthetics for surgery: systematic review of randomized trials. Reg Anesth Pain Med. 2008;33:159–167.
367. Iwasaki H, Collins JG, Saito Y, et al. Low-dose clonidine enhances pregnancy-induced analgesia to visceral but not somatic stimuli in rats. Anesth Analg. 1991;72:325–329.
368. Eisenach JC, Lysak SZ, Viscomi CM. Epidural clonidine analgesia following surgery: phase I. Anesthesiology. 1989;71:640–646.
369. Mendez R, Eisenach JC, Kashtan K. Epidural clonidine analgesia after cesarean section. Anesthesiology. 1990;73:848–852.
370. Huntoon M, Eisenach JC, Boese P. Epidural clonidine after cesarean section: appropriate dose and effect of prior local anesthetic. Anesthesiology. 1992;76:187–193.
371. Narchi P, Benhamou D, Hamza J, Bouaziz H. Ventilatory effects of epidural clonidine during the first 3 hours after caesarean section. Acta Anaesthesiol Scand. 1992;36:791–795.
372. Eisenach JC, D’Angelo R, Taylor C, Hood DD. An isobolographic study of epidural clonidine and fentanyl after cesarean section. Anesth Analg. 1994;79:285–290.
373. Capogna G, Celleno D, Zangrillo A, et al. Addition of clonidine to epidural morphine enhances postoperative analgesia after cesarean delivery. Reg Anesth. 1995;20:57–61.
374. Vercauteren MP, Vandeput DM, Meert TF, Adriaensen HA. Patient-controlled epidural analgesia with sufentanil following caesarean section: the effect of adrenaline and clonidine admixture. Anaesthesia. 1994;49:767–771.
375. Filos KS, Goudas LC, Patroni O, Polyzou V. Intrathecal clonidine as a sole analgesic for pain relief after cesarean section. Anesthesiology. 1992;77:267–274.
376. Filos KS, Goudas LC, Patroni O, Polyzou V. Hemodynamic and analgesic profile after intrathecal clonidine in humans: a dose-response study. Anesthesiology. 1994;81:591–601.
377. van Tuijl I, van Klei WA, van der Werff DB, Kalkman CJ. The effect of addition of intrathecal clonidine to hyperbaric bupivacaine on postoperative pain and morphine requirements after Caesarean section: a randomized controlled trial. Br J Anaesth. 2006;97:365–370.
378. Benhamou D, Thorin D, Brichant JF, et al. Intrathecal clonidine and fentanyl with hyperbaric bupivacaine improves analgesia during cesarean section. Anesth Analg. 1998;87:609–613.
379. Paech MJ, Pavy TJ, Orlikowski CE, et al. Postcesarean analgesia with spinal morphine, clonidine, or their combination. Anesth Analg. 2004;98:1460–1466.
380. Lavand’homme PM, Roelants F, Waterloos H, et al. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesth Analg. 2008;107:948–955.
381. Ala-Kokko TI, Pienimaki P, Lampela E, et al. Transfer of clonidine and dexmedetomidine across the isolated perfused human placenta. Acta Anaesthesiol Scand. 1997;41:313–319.
382. Sia AT, Kwek K, Yeo GS. The in vitro effects of clonidine and dexmedetomidine on human myometrium. Int J Obstet Anesth. 2005;14:104–107.
383. Bouaziz H, Tong C, Eisenach JC. Postoperative analgesia from intrathecal neostigmine in sheep. Anesth Analg. 1995;80:1140–1144.
384. Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–343.
385. Hood DD, Eisenach JC, Tong C, et al. Cardiorespiratory and spinal cord blood flow effects of intrathecal neostigmine methylsulfate, clonidine, and their combination in sheep. Anesthesiology. 1995;82:428–435.
386. Krukowski JA, Hood DD, Eisenach JC, et al. Intrathecal neostigmine for post-cesarean section analgesia: dose response. Anesth Analg. 1997;84:1269–1275.
387. Chung CJ, Kim JS, Park HS, Chin YJ. The efficacy of intrathecal neostigmine, intrathecal morphine, and their combination for post-cesarean section analgesia. Anesth Analg. 1998;87:341–346.
388. Pan PM, Huang CT, Wei TT, Mok MS. Enhancement of analgesic effect of intrathecal neostigmine and clonidine on bupivacaine spinal anesthesia. Reg Anesth Pain Med. 1998;23:49–56.
389. Kaya FN, Sahin S, Owen MD, Eisenach JC. Epidural neostigmine produces analgesia but also sedation in women after cesarean delivery. Anesthesiology. 2004;100:381–385.
390. Ross VH, Pan PH, Owen MD, et al. Neostigmine decreases bupivacaine use by patient-controlled epidural analgesia during labor: a randomized controlled study. Anesth Analg. 2009;109:524–531.
391. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769.
392. Price DD, Mayer DJ, Mao J, Caruso FS. NMDA-receptor antagonists and opioid receptor interactions as related to analgesia and tolerance. J Pain Symptom Manage. 2000;19:S7–11.
393. Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217.
394. Rojas AC, Alves JG, Moreira ELR, et al. The effects of subarachnoid administration of preservative-free S(+)-ketamine on spinal cord and meninges in dogs. Anesth Analg. 2012;114:450–455.
395. Unlugenc H, Ozalevli M, Gunes Y, et al. A double-blind comparison of intrathecal S(+) ketamine and fentanyl combined with bupivacaine 0.5% for Caesarean delivery. Eur J Anaesthesiol. 2006;23:1018–1024.
396. Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111–125.
397. Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg. 2004;99:482–495.
398. Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006;(1).
399. Kawana Y, Sato H, Shimada H, et al. Epidural ketamine for postoperative pain relief after gynecologic operations: a double-blind study and comparison with epidural morphine. Anesth Analg. 1987;66:735–738.
400. Tramer MR, Schneider J, Marti RA, Rifat K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology. 1996;84:340–347.
401. Sun J, Wu X, Xu X, et al. A comparison of epidural magnesium and/or morphine with bupivacaine for postoperative analgesia after cesarean section. Int J Obstet Anesth. 2012;21:310–316.
402. Yousef AA, Amr YM. The effect of adding magnesium sulphate to epidural bupivacaine and fentanyl in elective caesarean section using combined spinal-epidural anaesthesia: a prospective double blind randomised study. Int J Obstet Anesth. 2010;19:401–404.
403. Ozalevli M, Cetin TO, Unlugenc H, et al. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anaesthesia. Acta Anaesthesiol Scand. 2005;49:1514–1519.
404. Arcioni R, Palmisani S, Tigano S, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce post-operative analgesic requirements: a prospective, randomized, double-blind, controlled trial in patients undergoing major orthopedic surgery. Acta Anaesthesiol Scand. 2007;51:482–489.
405. Unlugenc H, Ozalevli M, Gunduz M, et al. Comparison of intrathecal magnesium, fentanyl, or placebo combined with bupivacaine 0.5% for parturients undergoing elective cesarean delivery. Acta Anaesthesiol Scand. 2009;53:346–353.
406. Paech MJ, Magann EF, Doherty DA, et al. Does magnesium sulfate reduce the short- and long-term requirements for pain relief after caesarean delivery? A double-blind placebo-controlled trial. Am J Obstet Gynecol. 2006;194:1596–1602.
407. Leicht CH, Kelleher AJ, Robinson DE, Dickerson SE. Prolongation of postoperative epidural sufentanil analgesia with epinephrine. Anesth Analg. 1990;70:323–325.
408. McMorland GH, Douglas MJ, Kim JH, et al. Epidural sufentanil for post-caesarean section analgesia: lack of benefit of epinephrine. Can J Anaesth. 1990;37:432–437.
409. Cohen S, Lowenwirt I, Pantuck CB, et al. Bupivacaine 0.01% and/or epinephrine 0.5 µg/mL improve epidural fentanyl analgesia after cesarean section. Anesthesiology. 1998;89:1354–1361.
410. Ngan Kee WD, Khaw KS, Ma ML. The effect of the addition of adrenaline to pethidine for patient-controlled epidural analgesia after caesarean section. Anaesthesia. 1998;53:1012–1016.
411. McLintic AJ, Danskin FH, Reid JA, Thorburn J. Effect of adrenaline on extradural anaesthesia, plasma lignocaine concentrations and the feto-placental unit during elective caesarean section. Br J Anaesth. 1991;67:683–689.
412. Alahuhta S, Rasanen J, Jouppila R, et al. Effects of extradural bupivacaine with adrenaline for caesarean section on uteroplacental and fetal circulation. Br J Anaesth. 1991;67:678–682.
413. Abouleish EI. Epinephrine improves the quality of spinal hyperbaric bupivacaine for cesarean section. Anesth Analg. 1987;66:395–400.
414. Abouleish E, Rawal N, Tobon-Randall B, et al. A clinical and laboratory study to compare the addition of 0.2 mg of morphine, 0.2 mg of epinephrine, or their combination to hyperbaric bupivacaine for spinal anesthesia in cesarean section. Anesth Analg. 1993;77:457–462.
415. Zakowski MI, Ramanathan S, Sharnick S, Turndorf H. Uptake and distribution of bupivacaine and morphine after intrathecal administration in parturients: effects of epinephrine. Anesth Analg. 1992;74:664–669.
416. Gan TJ, Habib AS. Adenosine as a non-opioid analgesic in the perioperative setting. Anesth Analg. 2007;105:487–494.
417. Rane K, Sollevi A, Segerdahl M. Intrathecal adenosine administration in abdominal hysterectomy lacks analgesic effect. Acta Anaesthesiol Scand. 2000;44:868–872.
418. Sharma M, Mohta M, Chawla R. Efficacy of intrathecal adenosine for postoperative pain relief. Eur J Anaesthesiol. 2006;23:449–453.
419. Rane K, Sollevi A, Segerdahl M. A randomised double-blind evaluation of adenosine as adjunct to sufentanil in spinal labour analgesia. Acta Anaesthesiol Scand. 2003;47:601–603.
420. Araiza-Saldana CI, Reyes-Garcia G, Bermudez-Ocana DY, et al. Effect of diabetes on the mechanisms of intrathecal antinociception of sildenafil in rats. Eur J Pharmacol. 2005;527:60–70.
421. Asano T, Dohi S, Iida H. Antinociceptive action of epidural K+(ATP) channel openers via interaction with morphine and an alpha2-adrenergic agonist in rats. Anesth Analg. 2000;90:1146–1151.
422. Yaksh TL, Allen JW. The use of intrathecal midazolam in humans: a case study of process. Anesth Analg. 2004;98:1536–1545.
423. Yaksh TL, Allen JW. Preclinical insights into the implementation of intrathecal midazolam: a cautionary tale. Anesth Analg. 2004;98:1509–1511.
424. Ho KM, Ismail H. Use of intrathecal midazolam to improve perioperative analgesia: a meta-analysis. Anaesth Intensive Care. 2008;36:365–373.
425. Tucker A, Lai C, Nadeson R, Goodchild C. Intrathecal midazolam. I. A cohort study investigating safety. Anesth Analg. 2004;98:1512–1520.
426. Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS. Intrathecal midazolam. II. Combination with intrathecal fentanyl for labor pain. Anesth Analg. 2004;98:1521–1527.
427. Prakash S, Joshi N, Gogia AR, Singh R. Analgesic efficacy of two doses of intrathecal midazolam with bupivacaine in patients undergoing cesarean delivery. Reg Anesth Pain Med. 2006;31:221–226.
428. Cheng JK, Chen CC, Yang JR, Chiou LC. The antiallodynic action target of intrathecal gabapentin: Ca2+ channels, KATP channels or N-methyl-D-aspartic acid receptors? Anesth Analg. 2006;102:182–187.
429. Cheng JK, Lai YJ, Chen CC, et al. Magnesium chloride and ruthenium red attenuate the antiallodynic effect of intrathecal gabapentin in a rat model of postoperative pain. Anesthesiology. 2003;98:1472–1479.
430. Choe H, Kim JS, Ko SH, et al. Epidural verapamil reduces analgesic consumption after lower abdominal surgery. Anesth Analg. 1998;86:786–790.
431. Schug SA, Saunders D, Kurowski I, Paech MJ. Neuraxial drug administration: a review of treatment options for anaesthesia and analgesia. CNS Drugs. 2006;20:917–933.
* The Institute of Safe Medicine Practices (ISMP) has recommended that health care providers never use µg as an abbreviation for micrograms, but rather they should use mcg (http://www.ismp.org/tools/errorproneabbreviations.pdf, Accessed February 2013). The use of the symbol µg is frequently misinterpreted and involved in harmful medication errors. The abbreviation may be mistaken for mg (milligrams), which would result in a 1000-fold overdose. The symbol µg should never be used when communicating medical information, including pharmacy and prescriber computer order entry screens, computer-generated labels, labels for drug storage bins, and medication administration records. However, most scholarly publications have continued to use the abbreviation µg. The editors have chosen to retain the use of the abbreviation µg throughout this text. However, the editors recommend the use of the abbreviation mcg in clinical practice.