Preterm Labor and Delivery
Janelle R. Walton MD, William A. Grobman MD, MBA
Chapter Outline
INTERACTIONS BETWEEN TOCOLYTIC THERAPY AND ANESTHESIA
Indications for Anesthesia during and after Tocolytic Therapy
Beta-Adrenergic Receptor Agonists
Preterm delivery is defined as delivery before 37 weeks' gestation. It occurs in 12% to 13% of all pregnancies in the United States and in 5% to 9% of pregnancies in other developed countries.1 Preterm delivery is responsible for 75% to 80% of all neonatal deaths and significant neonatal morbidity.1,2 Birth statistics in the United States reveal a 20% increase in the preterm delivery rate between 1990 and 2006 (from 10.6% to 12.8%) and a 30% increase since 1981.3 Subsequently, the preterm delivery rate declined to 11.72% of all births in 2011 (Figures 34-1 and 34-2).4 Preterm births not only account for a significant degree of neonatal morbidity and mortality but also are responsible for a large economic burden to society. For example, in 2005, the costs associated with preterm birth were at least $26.2 billion.3
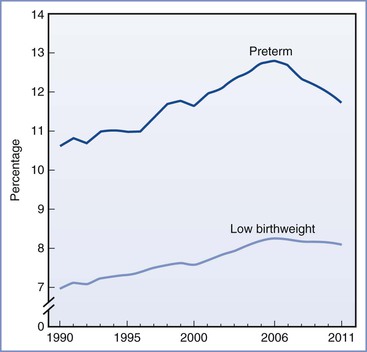
FIGURE 34-1 Preterm and low birth weight rates: United States, final data from 1990 to 2010, preliminary data from 2011. (From Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2011. Natl Vital Stat Rep 2012; 61[5].)
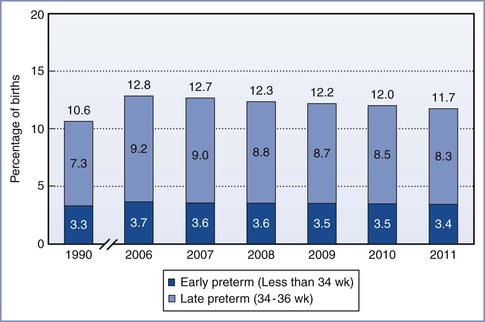
FIGURE 34-2 Total, early, and late preterm birth rates: United States 1990 and 2006 to 2010 (final) and 2011 (preliminary). Preterm birth is defined as less than 37 weeks' completed gestation. Early preterm is defined as less than 34 weeks' completed gestation. Late preterm is defined as 34 to 36 completed weeks' gestation. (Source: Centers for Disease Control and Prevention/NCHS, National Vital Statistics System; natality.)
In 2010, late preterm deliveries comprised 71% of all preterm births (see Figure 34-2). There is a notable racial/ethnic disparity in the frequency of preterm birth. In 2010, 10.8% of non-Hispanic whites, 17.1% of non-Hispanic blacks, and 11.8% of Hispanics delivered preterm. Women age 25 to 34 years were least likely to deliver preterm (11.4%), whereas women 40 years or older were most likely to deliver preterm (25.9%).5
The concern about preterm birth is not confined to the United States; the World Health Organization (WHO) and other nongovernmental organizations have identified the frequency of preterm birth as a critical health issue. The WHO uses low birth weight as an indicator of early delivery because true gestational age at delivery is often not available (see Figure 34-1). Worldwide, the incidence of low birth weight, defined as a birth weight less than 2500 g, is 15.5 per 1000 births.6 Africa and Asia have the highest incidence of low birth weight, at 14.3 and 18.3 per 1000, respectively. In contrast, in Europe and North America the incidence of low birth weight is 6.4 and 7.7 per 1000, respectively.6 Likewise, infant mortality rates are significantly higher in developing nations, particularly the sub-Saharan African countries. The United States has a higher infant mortality rate than Europe (6 versus 3 per 1000 births, respectively), which reflects the higher preterm birth rate in the United States.7
In 2006, the Institute of Medicine recommended that investigators focus on (1) better defining the problem; (2) developing treatments to prevent both preterm delivery and morbidity for children born preterm; (3) identifying the causes of preterm birth, including modifiable risk factors and the reasons for disparity among different ethnic, racial, and socioeconomic groups; and (4) developing policies and public programs that can be used to reduce the rate of preterm birth.3
Definitions
A preterm infant is defined as one who is born between 20 0/7 weeks and 36 6/7 weeks, inclusive, after the first day of the last menstrual period. If a good basis does not exist for establishing the gestational age from maternal history, the exact gestational age is difficult to determine. A low birth weight does not necessarily signify that a neonate has been born preterm, because some newborns have a low birth weight because they are small for gestational age (SGA) rather than preterm. A neonate who weighs less than 2500 g at birth is considered to have a low birth weight (LBW), regardless of gestational age. Likewise, an infant who weighs less than 1500 g at birth is considered to have a very low birth weight (VLBW), and an infant who weighs less than 1000 g at birth is considered to have an extremely low birth weight (ELBW).
Neonatal Mortality
The survival rate among neonates increases as the birth weight and/or gestational age increases (Figure 34-3; Table 34-1).8 After data are controlled for gestational age and weight, male infants have a higher mortality than female infants.9 During the past three decades, there has been a significant improvement in the survival rate for preterm infants, with the greatest improvement occurring in the subgroup with a birth weight between 501 and 1250 g.10 The rate of neonatal survival now exceeds 90% for infants born after 30 weeks' gestation, and a neonatal survival rate close to that of a term infant can be expected for those born after 32 weeks' gestation.
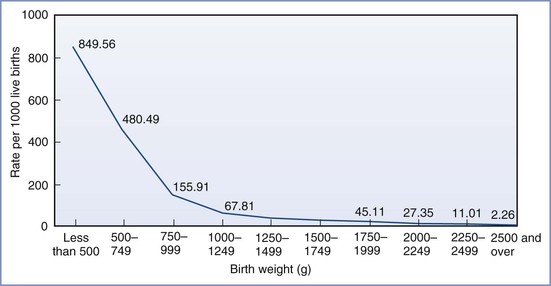
FIGURE 34-3 Infant mortality rates by birth weight: United States, 2004. (From Mathews TJ, MacDorman MF. Infant mortality statistics from the 2004 period linked birth/infant death data set. Natl Vital Stat Rep 2007; 55:1-32.)
TABLE 34-1
Neonatal Deaths by Gestational Age
| Completed Weeks' Gestation | Percentage of Deaths* |
| 22 | 94 |
| 23 | 74 |
| 24 | 45 |
| 25 | 28 |
| 26 | 16 |
| 27 | 12 |
| 28 | 8 |
* Death rate before discharge by gestational age among infants born in the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network Centers between 2003 and 2007.
Data from Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126:443-56.
However, despite this improvement, infants with a birth weight between 501 and 750 g continue to have high mortality rates (Table 34-2). For example, in a cohort of neonates delivered between 2000 and 2009, the mortality rate for infants weighing between 501 and 1500 g decreased from 14.3% to 12.4%.10 When stratified by birth weight, mortality ranged from 36.6% for infants who weighed between 501 and 750 g to 3.5% for infants who weighed between 1251 and 1500 g.10 A retrospective cohort study assessed survival rates for infants delivered between 24 and 26 weeks' gestation.11 Neonatal survival was 43%, 74%, and 83% at 24, 25, and 26 weeks' gestation, respectively. The majority of women received antenatal corticosteroids, and the majority of neonates received exogenous surfactant. A delay in delivery of even 1 week at this time in gestation leads to significantly better outcome and reduced cost. A similar study that examined a cohort of infants born between 1998 and 2002 showed a survival rate of 0% for infants born at 21 completed weeks' gestation; the survival rate rose steadily to 75% at 25 completed weeks' gestation (see Table 34-1).12
TABLE 34-2
Selected Outcomes for Extremely Preterm Infants*
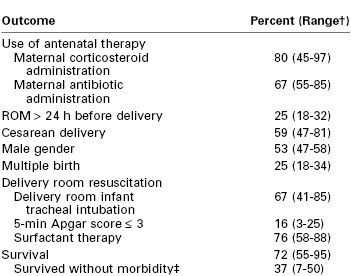
* Birth weight < 1000 g. Data are from the Eunice Kennedy Shriver National Institute of Child Health Development Neonatal Research Network for 9575 infants, gestational ages 22 to 28 weeks, birth weight 401 to 1500 g, between 2003 and 2007.
† Ranges are across 19 Neonatal Research Network centers.
‡ Morbidities included severe intraventricular hemorrhage, periventricular leukomalacia, bronchopulmonary dysplasia, necrotizing enterocolitis, infections, and retinopathy of prematurity (ROP) stage ≥ 3.
ROM, rupture of membranes.
Data from Stoll BJ, Nansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126:443-56.
Infants born at the threshold of viability (22 to 24 weeks' gestation) continue to have the greatest risk for poor outcome. A recent study examined outcome for infants born between 22 and 27 weeks' gestation between the years 2002 and 2008. The mortality rate for those born between 22 and 24 weeks' gestation was 61% compared with 19% for those born between 25 and 27 weeks' gestation.13 Compared with infants born at 22 to 24 weeks' gestation, infants born at 25 to 27 weeks' gestation were more likely to have been exposed to antenatal maternal corticosteroid administration (87% versus 62%), to be delivered by cesarean (70% versus 39%), and to be resuscitated at birth (99% versus 75%). It is estimated that 2% to 5% of VLBW infants who survive to hospital discharge die within 2 years because of medical complications of prematurity.13
Neonatal Morbidity
Approximately 90% of preterm births occur between 32 and 36 6/7 weeks' gestation. Compared with earlier gestational ages, mortality is less common, but morbidity is a relatively greater concern in this gestational age range. As with mortality, most morbidity decreases in frequency as the gestational age increases. For example, the incidence of high-grade (III or IV) intraventricular hemorrhage (IVH) diminishes rapidly after 27 weeks' gestation and grade III or IV intraventricular hemorrhages are very rare after 32 weeks' gestation. Likewise, neonatal morbidity from patent ductus arteriosus and necrotizing enterocolitis diminishes significantly after 32 weeks' gestation.12 Data from the National Institute of Child Health and Development (NICHD) Neonatal Research Network sites from 1997 through 2002 indicate that survival without complications (e.g., bronchopulmonary dysplasia, severe intraventricular hemorrhage, necrotizing enterocolitis, or a combination of these disorders) ranged from 20% for infants with a birth weight between 501 and 750 g to 89% for those with a birth weight between 1251 and 1500 g.2
Piecuch et al.14 reported data for a cohort of 138 nonanomalous infants delivered between 24 and 26 weeks' gestation between 1990 and 1994. The incidence of cerebral palsy did not differ significantly among the three groups born at 24, 25, and 26 weeks' gestation (11%, 20%, and 11%, respectively). However, the incidence of normal cognitive outcome rose with increasing gestational age at birth (28%, 47%, and 71% at 24, 25, and 26 weeks' gestation, respectively).
The EPICure study group assessed the association between extreme preterm delivery and long-term physical and mental disability in a cohort of infants delivered between 22 and 25 weeks' gestation during a 10-month period in 1995.15 These investigators noted rates of severe disability of 54%, 52%, and 45% among infants delivered at 23, 24, and 25 weeks' gestation, respectively. In a later cohort of infants, born between 1997 and 2002, the rates of severe disability were 33%, 21%, and 12% for infants delivered at 23, 24, and 25 weeks' gestation, respectively.16 A 6-year follow-up to the EPICure study cohort showed persistent severe disability in 25%, 29%, and 18% of infants born at 23, 24, and 25 weeks' gestation, respectively.17
Hack et al.18 monitored a cohort of ELBW infants born between 1992 and 1995 until they were 8 years old. The mean birth weight was 810 g, and the mean gestational age at delivery was 26 weeks. Compared with a cohort of age-matched children of normal birth weight, the ELBW group had a higher incidence of significant neurosensory impairment (16% versus 0%, respectively) and asthma (21% versus 9%). The ELBW children differed significantly from the cohort with normal birth weight in rates of suboptimal intelligence, academic achievement, motor skills, and adaptive functioning. These data illustrate the long-term medical, educational, and social services required by these children.
Correspondingly, the economic costs for the care of surviving preterm infants (especially VLBW infants) can be enormous. The Institute of Medicine estimated that the societal economic burden associated with preterm birth in the United States was at least $26.2 billion in 2005, or $51,600 per infant born preterm.3 These figures likely will continue to rise with the escalating cost of health care.
Preterm Labor
Risk Factors
Box 34-1 lists factors associated with preterm labor.1,19,20 These associations do not necessarily indicate cause-and-effect relationships. Significant risk factors include a history of preterm delivery, non-Hispanic black race (irrespective of socioeconomic status), and multiple gestation.
The process of normal parturition involves anatomic, physiologic, and biochemical changes that lead to (1) greater uterine contractility, (2) cervical ripening, and (3) membrane/decidual activation.19,20 The fetus also appears to play a role in parturition. It is hypothesized that the mature fetal hypothalamus secretes more corticotropin-releasing hormone (CRH), which in turn stimulates fetal adrenal production of adrenocorticotropic hormone (ACTH) and cortisol.19 Preterm labor results from the pathologic activation of one or more of these components (Figure 34-4).21 Preterm delivery results from (1) preterm premature rupture of membranes (preterm PROM) in approximately 30% of cases, (2) spontaneous preterm labor in approximately 45% of cases, and (3) maternal or fetal indications for early delivery in approximately 25% of cases.22 However, the “spontaneous” causes do not have a uniform underlying pathophysiology, and it appears that preterm labor is a syndrome with multiple causes influenced by a number of genetic, biologic, biophysical, psychosocial, and environmental factors.
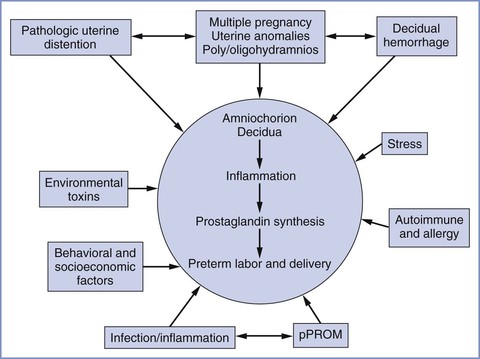
FIGURE 34-4 Major etiologic factors in preterm birth, including activation of the maternal or fetal hypothalamic-pituitary axis (stress), inflammation, decidual hemorrhage, and pathologic distention of the myometrium. The pathways are not mutually exclusive and may overlap, and they share a common biochemical pathway. pPROM, preterm premature rupture of membranes. (From Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand 2008; 87:590-600.)
Two emerging factors of interest are the influences of infection and uterine distention on initiation of myometrial contractility. Infection is thought to be present in up to 40% of preterm deliveries.1 Commonly identified organisms include Ureaplasma urealyticum, Bacteroides species, Neisseria gonorrhoeae, Chlamydia trachomatis, group B streptococci, Staphylococcus aureus, Treponema pallidum, and enteropharyngeal bacteria.23-25 Although approximately 50% of preterm deliveries occur in women with no apparent risk factors, subclinical infection may precipitate preterm labor in some of these cases.24
In the past three decades there has been a significant rise in the incidence of multiple gestation (see Chapter 35). The twin birth rate increased by 76% from 1980 to 2009.5 The triplet and higher-order multiple birth rate rose by over 400% during the 1980s and 1990s but has declined 29% since the 1998 peak.4 The increased incidence of multiple gestation is due, in part, to the significant increase in the use of assisted reproductive technologies (ARTs).26 Over 50% of all twins and 90% of all triplets are born preterm.5 Consequently, multiple gestations account for 17% of all preterm births.27 ART pregnancies are also associated with an increase in risk for preterm delivery, even for singleton pregnancies.28 A 2004 meta-analysis of 15 studies, which compared outcomes for 12,283 ART singleton pregnancies with outcomes for 1.9 million spontaneously conceived singleton pregnancies, demonstrated a higher risk for preterm and SGA deliveries in the ART group.28 Placenta previa, gestational diabetes, preeclampsia, and neonatal intensive care unit admission were also more prevalent in the ART group.28
Approximately 25% of all preterm deliveries do not result from spontaneous preterm labor or preterm PROM. The obstetrician may choose to perform indicated delivery for maternal or fetal indications, such as severe preeclampsia or a nonreassuring fetal status.
Prediction of Preterm Labor
The ability to prevent spontaneous preterm birth would be facilitated if it were possible to intervene prophylactically to prevent preterm labor or to effectively treat preterm labor once it occurs. Both prophylaxis and treatment would be facilitated if it were possible to accurately predict which asymptomatic or symptomatic patients would have spontaneous preterm delivery. Several methods of predicting preterm delivery have been proposed, including home uterine activity monitoring, salivary estriol measurement, fetal fibronectin screening, and transvaginal cervical ultrasonography.29
The use of home uterine activity monitoring to identify women at risk for preterm labor has been investigated in a number of randomized controlled trials.29 Likewise, salivary estriol levels have been assessed as a marker of risk for preterm delivery. Neither has been shown to be clinically useful in the prevention of preterm birth.
Fetal fibronectin (fFN) is a basement membrane glycoprotein produced by the fetal membranes. It functions as an adhesive protein of the placental membranes to the decidua.30 fFN is normally absent from vaginal secretions from 20 weeks' gestation until near term. Detection of elevated levels of fFN is associated with an increased risk for preterm delivery. It is hypothesized that the presence of fFN is a marker of choriodecidual disruption. A study from the Maternal-Fetal Medicine Units Network documented that a positive fFN test result at 22 to 24 weeks' gestation had a sensitivity of 63% in predicting preterm labor before 28 weeks' gestation.31 If fFN is absent (i.e., a negative result), the risk for preterm delivery within 1 or 2 weeks is less than 1%.32 The high negative predictive value of this test may make it a useful tool to help triage symptomatic patients more efficiently, although this remains to be demonstrated in randomized trials33; currently, there is no role for this test in screening low-risk asymptomatic women.29
Short cervical length, as assessed by transvaginal ultrasonography, also is associated with a greater risk for preterm delivery.34 In a 2006 systematic analysis, Kagan et al.35 concluded that cervical length is associated with preterm delivery (i.e., the shorter the cervix the greater the risk for preterm delivery) in symptomatic women. Further, multiple studies have shown an increased risk for preterm delivery in asymptomatic women with a shortened cervix.29 A Maternal-Fetal Medicine Units Network study of nearly 3000 women found that the risk for spontaneous preterm delivery is increased in women with evidence of a short cervix detected by transvaginal ultrasonography between 24 and 28 weeks' gestation. A cervical length below the 10th percentile had a sensitivity of 37% and a specificity of 92% in predicting preterm birth before 35 weeks' gestation, with a corresponding positive predictive value of 18% and a negative predictive value of 97%.34
A history of cervical surgery, including conization and loop electrosurgical excision procedure, traditionally has been thought to be a risk factor for preterm birth because of associated cervical injury. However, this relationship may be related to environmental factors and/or behavioral factors that underlie the progression of cervical dysplasia. Uterine instrumentation, such as dilation and curettage, also has been associated with an increased risk for preterm birth in some, but not all, studies; the mechanism is unclear, but it may be a result of intrauterine microbial colonization, injury to the endometrium, or both, together with host and environmental factors.29
Prevention of Preterm Labor
Antenatal screening for risk factors for preterm labor and delivery is of value only if interventions are available that can decrease the frequency of preterm delivery and improve neonatal outcome.29 Unfortunately, few if any interventions have been shown to definitively reduce these outcomes. Interventions that have been studied include detection and suppression of uterine contractions, antimicrobial therapy, prophylactic cervical cerclage, maternal nutritional supplements, and reduction of maternal stress.20 It is not surprising that most of these simple interventions have not been shown to alter outcome, given that preterm labor is increasingly understood to be a complex syndrome with multiple, overlapping causes.20
Prophylactic cervical cerclage in the early second trimester has been performed to prevent preterm birth, typically in women with a history of mid-trimester pregnancy loss. Evidence that supports the efficacy of this practice is weak.36 There remains controversy with regard to whether cerclage should be placed in response to transvaginal ultrasonographic evidence of a short cervix in the second half of the mid trimester. Data do not support such a practice in a general population,37 but there is some evidence that such a practice may be beneficial among high-risk women, such as those with a prior preterm birth.38
Evidence does not support the administration of prophylactic antibiotics in asymptomatic women at risk for preterm labor.39 Likewise, evidence does not support the prophylactic use of beta-adrenergic receptor agonists to prevent preterm labor in high-risk women.40
In contrast, evidence suggests that progesterone therapy may be effective in reducing the rate of preterm birth in some patient populations. The Maternal-Fetal Medicine Units Network performed a randomized controlled trial that compared prophylactic intramuscular 17α-hydroxyprogesterone caproate (17P) (250 mg weekly beginning at 16 to 20 weeks' gestation, and continued until delivery or 36 weeks' gestation) with placebo in women with a history of spontaneous preterm delivery.41 The risk for delivery before 37 weeks' gestation was reduced in the 17P group (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54 to 0.81). Similarly, da Fonseca et al.42 demonstrated a decreased risk for preterm delivery (< 34 weeks' gestation) in high-risk women randomly assigned to receive either vaginal progesterone (100 mg daily) or placebo. A systematic review of 11 randomized controlled trials (n = 2425) also concluded that progesterone administration was associated with a significant reduction in recurrent preterm birth in women with a history of spontaneous preterm delivery.43
Progesterone therapy also has been shown to be efficacious in preventing preterm delivery among women with a short cervix identified by transvaginal ultrasonography. In two double-blind, placebo-controlled trials, women with a mid-trimester diagnosis of a short cervix (< 15 mm in one trial44 and 10 to 20 mm in the other45) were randomized to receive either vaginal progesterone or placebo. Women who received vaginal progesterone experienced a significant reduction in the frequency of preterm delivery before 33 weeks' gestation.44,45 In contrast, in a trial that enrolled nulliparous women with a cervical length less than 30 mm, women randomized to receive 17P did not experience a reduction in preterm delivery compared with women who received placebo.46
A number of studies have examined whether progesterone is efficacious in reducing preterm birth among women with multiple gestation. Uniformly, progesterone therapy has not been shown to be beneficial in this population.47-49
Even among women for whom progesterone is thought to be indicated, the optimal type, timing, and dosing of progesterone is unclear. Based on existing evidence, the American College of Obstetricians and Gynecologists (ACOG)29 has concluded that vaginal progesterone should be offered to asymptomatic women with a singleton gestation without a previous preterm delivery, who have a very short cervical length (i.e., ≤ 20 mm at or before 24 weeks' gestation).
Diagnosis
Determining whether a woman is in early preterm labor or in false labor is often difficult. Criteria for the diagnosis of preterm labor include gestational age between 20 0/7 and 36 6/7 weeks' gestation and regular uterine contractions accompanied by a change in cervical dilation, effacement, or both (or initial presentation with regular contractions and cervical dilation of 2 cm or more).19 Less than 10% of women with the clinical diagnosis of preterm labor actually give birth within 7 days of presentation.19
Assessment and Therapy
Initial assessment of the patient with possible preterm labor includes physical examination and external monitoring of contractions with a tocodynometer (and fetal heart rate if indicated by the gestational age). Acute conditions associated with preterm labor should be considered, including infection and placental abruption. Maternal physical examination may include a sterile speculum examination to exclude preterm PROM if symptoms or signs indicate this possibility. In many women who have preterm uterine contractions, these contractions will cease spontaneously. In the past, clinicians assumed that intravenous hydration was a useful component of therapy. However, there is no evidence that intravenous hydration reduces the chance of preterm delivery.50
Once the diagnosis of preterm labor is established, the obstetric care provider must decide whether intervention is warranted. The therapeutic agents currently thought to be associated with improved neonatal outcomes include antenatal maternal corticosteroid administration to accelerate maturation of fetal lungs and other developing organ systems, and the targeted use of magnesium sulfate for fetal neuroprotection (see later discussion).51,52 Although widely used before 34 weeks' gestation, acute tocolytic therapy remains a source of controversy. There is no consistent evidence that the use of acute tocolysis reduces the chance of preterm birth or improves neonatal outcome. However, because acute tocolysis has been associated with a short (approximately 48 hour) prolongation of pregnancy, it may be used to facilitate transfer of the patient from a community hospital to a tertiary care facility that can provide optimal care for the preterm neonate. Moreover, a short course of tocolytic therapy may delay delivery for 24 to 48 hours, allowing maternal administration of (1) a corticosteroid to accelerate fetal lung maturity and (2) antibiotic therapy to prevent neonatal group B streptococcal infection. Thus, the ACOG has supported the use of acute tocolysis to allow administration of a complete course of antenatal corticosteroids, but the ACOG discourages the continued use of tocolysis after corticosteroid administration is complete.19
Criteria for the use of tocolytic therapy include (1) gestational age after viability and before 34 weeks' gestation, (2) reassuring fetal status, and (3) no overt clinical signs of infection. The potential benefits of delaying delivery of the preterm infant (i.e., decreased neonatal morbidity and mortality) must be weighed against the maternal and fetal risks (e.g., maternal side effects of tocolytic drugs, deterioration of a compromised fetus). Box 34-2 lists contraindications to the inhibition of labor.
In the setting of preterm PROM, obstetricians have worried that tocolytic therapy might increase the risk for maternal and/or fetal infection. It also seems logical that tocolytic therapy is less effective in patients with preterm PROM. Prospective, randomized studies have shown that tocolytic therapy does not improve neonatal outcome compared with conservative expectant management in patients with preterm PROM.53
Antenatal Administration of Corticosteroids
The neonatal benefits of corticosteroid administration (Table 34-3) before preterm delivery have been clearly demonstrated in large clinical trials. The NICHD Neonatal Research Network evaluated outcomes for 11,718 preterm infants delivered after antenatal maternal corticosteroid administration between 1988 and 1992. Antenatal corticosteroid treatment significantly reduced the incidence of neonatal respiratory distress syndrome, intraventricular hemorrhage, and neonatal death in all subgroups of the population studied (including male and female infants, African and Caucasian race infants, and infants delivered before 30 weeks' gestation).54 The reduction in neonatal morbidity and mortality from antenatal corticosteroid administration is additive to the reduction observed with the use of neonatal surfactant alone.55
TABLE 34-3
Antenatal Corticosteroid Therapy
| Drug | Dose and Route | Frequency/Duration |
| Betamethasone | 12 mg IM | Every 24 h × 2 |
| Dexamethasone | 6 mg IM | Every 12 h × 4 |
IM, intramuscular.
From National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: Repeat courses—National Institutes of Health Consensus Development Conference Statement, August 17-18, 2000. Obstet Gynecol 2001; 98:144-50.
Although there is little controversy about the efficacy of a single course of antenatal corticosteroids, there remains debate over the use of multiple courses of corticosteroids for women who remain undelivered 7 days after the initial dose of corticosteroids. A 2001 review56 and a National Institutes of Health (NIH) consensus panel statement57 did not recommend multiple courses of corticosteroids; however, both documents cited some evidence of their possible benefit. These documents also identified possible risks, including a higher incidence of neonatal infection and potentially deleterious effects on neuronal and organ growth.56,57 A large study performed by the Maternal-Fetal Medicine Units Network randomly assigned women at risk for preterm delivery between 23 and 32 weeks' gestation to receive either a single course or repeated (weekly) courses of antenatal corticosteroids.58 Weekly corticosteroid administration did not significantly reduce the composite primary morbidity outcome, but it significantly reduced the need for neonatal surfactant, mechanical ventilation, and continuous positive airway pressure (CPAP), as well as the incidence of pneumothorax. In contrast, weekly corticosteroid administration was associated with an increase in the delivery of SGA infants, and there was a significant reduction in the birth weight of the infants whose mothers received four or more courses of corticosteroids.
To balance the potential beneficial effects and risks of additional courses of corticosteroids, some have advocated for a single “rescue” course (i.e., a second course of corticosteroids), which is administered at the time of a second episode of preterm labor with a high probability of preterm delivery. One randomized trial demonstrated that additional neonatal benefit could be derived from a single “rescue” course of corticosteroids.59 The investigators reserved this intervention for patients with intact membranes, whose antecedent corticosteroid treatment had been given at least 2 weeks before the “rescue” course, whose gestational age was less than 33 weeks, and who were judged by the clinician as likely to give birth within the next week. A meta-analysis60 concluded that a single “rescue” course of antenatal corticosteroids should be considered in women whose prior course of antenatal corticosteroids was administered at least 7 days previously and who were at acute risk for preterm delivery before 34 weeks' gestation. The ACOG51 has stated that one “rescue” course of corticosteroids may be considered in these specific populations. However, regularly scheduled repeat courses or multiple courses (more than two) of corticosteroids are not currently recommended.51
Antibiotic Therapy
The results of a large multicenter randomized controlled trial61 and a meta-analysis62 do not support the use of prophylactic antibiotic therapy in the management of preterm labor in patients with intact membranes as a method to reduce the likelihood of preterm birth. In fact, in one study that assessed long-term outcome for offspring born to women who participated in a randomized placebo-controlled trial of antibiotic administration in the setting of preterm labor, children born to women who received antibiotic treatment had more functional health impairment.63 Accordingly, the ACOG19 does not recommend empirical antibiotic therapy in this population. It should be noted, however, that prophylactic antibiotic administration remains appropriate in women who are positive for group B streptococcus (GBS) and who are thought to be in preterm labor.64
In contrast, in patients with preterm PROM, randomized controlled trials and a meta-analysis have concluded that antimicrobial therapy prolongs pregnancy and reduces both maternal and neonatal morbidity.65,66 Thus, when preterm PROM is diagnosed, the ACOG67 recommends a 7-day course of antimicrobial therapy; the best antibiotic regimen is not known with certainty, although intravenous ampicillin and erythromycin (48 hours), followed by oral amoxicillin and erythromycin (5 days), is a commonly used regimen for women with preterm PROM who are receiving expectant management.67
Neuroprotection
Several clinical trials have provided evidence that maternal administration of magnesium sulfate provides fetal neuroprotection when given to women at risk for preterm delivery. In 2003, Crowther et al.68 reported the results of a multicenter randomized, placebo-controlled study of 1062 women (1255 infants) at less than 30 weeks' gestation, in whom delivery was planned or expected within 24 hours. The investigators observed no significant difference between groups in the primary outcomes, which included total pediatric mortality, cerebral palsy, or both, at a corrected age of 2 years. However, they observed a significantly reduced rate of substantial gross motor dysfunction, as well as a reduced combined rate of death or substantial gross motor dysfunction, in the children exposed to magnesium sulfate in utero.68 Similarly, a randomized controlled trial of magnesium sulfate administration in 573 pregnant women at less than 33 weeks' gestation, and in whom delivery was planned or expected within 24 hours, found that infants exposed to magnesium sulfate had a reduced rate of total neonatal mortality, severe cerebral white matter injury (which is associated with cerebral palsy), and the combination of severe white matter injury and/or death, but the differences were not statistically significant.69 In the largest randomized trial,70 2241 women at imminent risk for delivery before 32 weeks' gestation were randomized to receive magnesium sulfate or placebo. The offspring who had been exposed to magnesium sulfate in utero were significantly less likely to develop moderate/severe cerebral palsy (1.9% versus 3.5%; RR, 0.55; 95% CI, 0.32 to 0.95).70
A recent meta-analysis has synthesized the data from the clinical trials and suggests that prenatal administration of magnesium sulfate reduces the occurrence of cerebral palsy when given with neuroprotective intent (RR, 0.71; 95% CI, 0.55 to 0.91).71 The ACOG52 recently stated that, based on available evidence, magnesium sulfate—given before anticipated early preterm birth—reduces the risk for cerebral palsy in surviving infants. Because the best regimen of magnesium sulfate administration remains unclear, physicians electing to use magnesium sulfate for fetal neuroprotection should develop specific guidelines regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring based on the protocols of the larger published trials.52
Selection of Tocolytic Agents
Once the obstetrician has decided to begin tocolytic therapy, an appropriate agent must be selected (Table 34-4). (Each specific class of tocolytic agent is discussed in detail later in this chapter.) A 2003 analysis of studies that compared the four classes of tocolytic agents currently in use (i.e., beta-adrenergic receptor agonists, calcium entry–blocking agents, magnesium sulfate, and nonsteroidal anti-inflammatory drugs [NSAIDs]) concluded that all are more effective than placebo in prolonging pregnancy, but the investigators found no evidence of a beneficial effect on neonatal morbidity or mortality.72 A more recent analysis suggested that magnesium sulfate specifically is not efficacious and should not be used for tocolysis.73
TABLE 34-4
Tocolytic Drugs for Preterm Labor
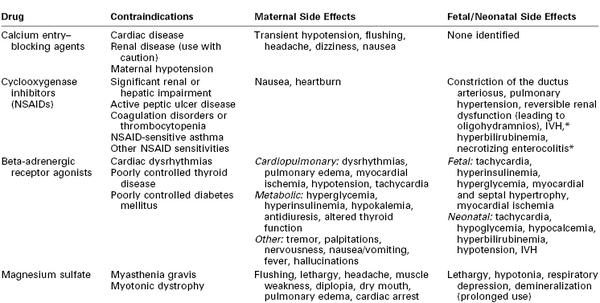
* Data are conflicting as to whether cyclooxygenase inhibitors increase risk.
IVH, intraventricular hemorrhage; NSAIDs, nonsteroidal anti-inflammatory drugs.
From Muir HA, Wong CA. Preterm labor and delivery. In Chestnut DH, Tsen LC, Polley LS, Wong CA, editors. Chestnut's Obstetric Anesthesia. 4th edition. Philadelphia, Mosby, 2009. Modified from Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol 2000; 43:787-801.
Physiology of Uterine Contractions
The contractile elements in myometrial smooth muscle consist of thick (myosin) and thin (actin) filaments that interact and slide past one another, generating the contractile force for uterine contractions. The myometrium has pacemaker cells with spontaneous contractile ability, which spread activity throughout the rest of the uterus by means of gap junctions between myometrial cells. Myometrial contractions are preceded by a rise in intracellular calcium concentration through the influx of calcium across the sarcolemma and/or release from internal stores such as the sarcoplasmic reticulum. Hormones and neurotransmitters may play a role in the regulation of uterine activity by causing agonist-induced entry of calcium or other ions by means of receptor-controlled channels and the release of calcium from internal stores.74
The rise in intracellular calcium results in the formation of a complex between calcium and calmodulin (a regulatory enzyme), which activates myosin light-chain kinase (MLCK). Activated MLCK then phosphorylates the light-chain subunit of myosin, allowing actin to bind to myosin and activate myosin adenosine triphosphatase. Adenosine triphosphate (ATP) is then hydrolyzed, and muscle shortening or contraction results. Relaxation of smooth muscle results from a reduction in the intracellular calcium concentration and/or dephosphorylation of the myosin light chain by myosin light-chain phosphatase. Increases in intracellular cyclic adenosine monophosphate (cAMP) also can result in muscle relaxation by two mechanisms: (1) activation of a cAMP-dependent protein kinase, which decreases the activity of MLCK; and (2) a reduction of the intracellular calcium concentration.
The control of labor and the processes for signaling its onset are complex and incompletely understood. During pregnancy, the uterus remains in a state of functional quiescence as a result of the activity of various inhibitors, including progesterone, prostacyclin, relaxin, nitric oxide, parathyroid hormone–related peptide, corticotropin-releasing hormone, human placental lactogen, calcitonin gene–related peptide, adrenomedullin, and vasoactive intestinal peptide (Figure 34-5).25 Before term the uterus goes through an activation phase in response to uterotropins, including estrogen. This activation phase is characterized by (1) greater expression of a series of contraction-associated proteins (including myometrial receptors for prostaglandins and oxytocin), (2) activation of certain ion channels, and (3) an increase in connexin-43 concentration. Once activated, the uterus can be stimulated to contract by the action of uterotonics such as oxytocin and prostaglandins E2 and F2α. A parturition cascade likely removes the mechanisms that have maintained uterine quiescence and recruits factors that promote uterine activity.
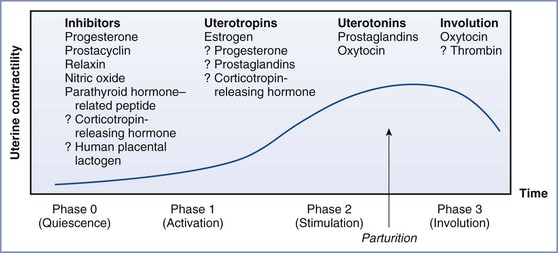
FIGURE 34-5 The regulation of uterine activity during pregnancy and labor can be divided into four distinct physiologic phases—quiescence, activation, stimulation, and involution—that are, or may be, influenced by a number of stimulatory and inhibitory factors. (From Liao JB, et al. Normal labor: mechanism and duration. Obstet Gynecol Clin North AM 2005; 32:146; adapted from Challis JRG, Gibb W. Control of parturition. Prenat Neonat Med 1996; 1:283.)
Once the uterus has been “activated,” endocrine, paracrine, and autocrine factors from the fetoplacental unit initiate a change in the pattern of uterine activity from irregular to regular contractions. Evidence from animal models suggests that the fetus may coordinate this change in activity through (1) its influence on the production of placental steroid hormones, (2) mechanical distention of the uterus, and (3) secretion of neurohypophyseal hormones and other stimulators of prostaglandin synthesis. The final common pathway for labor in all species is thought to be the activation of the fetal hypothalamic-pituitary-adrenal axis. Of interest, however, is the observation that spontaneous labor occurs in women with an anencephalic fetus (with no residual pituitary/adrenal function), suggesting that intact neurohypophyseal function is not a prerequisite for the onset of human labor.75
Preterm labor may result from a loss of inhibitory factors on uterine quiescence or may represent a short-circuiting of the normal parturition cascade through the overproduction of a critical factor. As the understanding of the physiology of uterine activity increases, the approach to both predicting and treating preterm labor will become more focused. Multimodal therapy may become a standard, given that evidence now suggests that labor is initiated by the interaction of multiple factors.
Significant attention has focused on the role of progesterone in parturition. In nonhuman mammals, the onset of labor is associated with progesterone withdrawal.75 Although the understanding of its role in human labor remains incomplete, a number of isoforms of the progesterone receptor (PGR) have been identified. One isoform, PGR-B, may play a role in quiescence, whereas another, PGR-A, may act to initiate functional progesterone withdrawal. PGR-A receptors are increasingly expressed with the onset of labor. In concert with the increase in PGR-A relative to PGR-B, an increase in estrogen receptor transcripts is observed. This change may lead to an increase in estrogen responsiveness. A third isoform, PGR-C, may also contribute to antagonism of uterine quiescence. Through a complex interplay of co-regulators such as cAMP, progesterone receptors seem to be responsible for both uterine quiescence and the stimulation of the onset of labor.75
Gap junctions facilitate the propagation of electrical impulses and movement of small molecules between cells. Gap junction protein alpha-1 (GJA1, or connexin-43) is one of the main protein components of myometrial gap junctions. The appearance of gap junctions in myometrium is thought to herald the onset of labor.75 GJA1 expression is stimulated by estradiol and is inhibited by progesterone. Microarray studies of human myometrium (i.e., preterm myometrium, term myometrium not in labor, term myometrium in labor) have added to the knowledge of the genetic modulation that occurs during pregnancy and labor.76 Differential expression of 118 genes has been identified. A process of remodeling and maturation of the uterus, and the differential expression of the genes that regulate this process, are evident throughout gestation. Further work is needed to fully elucidate the signaling pathways that contribute to human labor.76
Efficacy of Tocolytic Therapy
There is general consensus that acute tocolytic therapy for the treatment of preterm labor offers only limited benefit and does not reduce the rate of preterm birth.77 A meta-analysis suggested that calcium entry–blocking agents such as nifedipine are as efficacious as beta-adrenergic receptor agonists, with fewer maternal and fetal side effects.78 The mechanism of action is thought to be inhibition of voltage-dependent calcium channels, which results in decreased calcium influx into smooth muscle cells, as well as decreased release of intracellular calcium stores into the myoplasm.
The beta-adrenergic receptor agonists (e.g., ritodrine, terbutaline) have been widely used as tocolytic agents for many years but have fallen out of favor because other tocolytic agents (e.g., nifedipine, oxytocin antagonists) are equally efficacious with fewer side effects.78,79 Intravenous ritodrine, for example, is no longer marketed in the United States. Beta-adrenergic receptor agonists relax smooth muscle via β2-adrenergic receptor stimulation. A 2004 meta-analysis included 17 studies, 11 of which compared beta-adrenergic agonists with placebo.80 Use of beta-adrenergic agonists reduced the number of women who delivered within 48 hours, but there was no decrease in the number of births within 7 days or in perinatal death or neonatal morbidity. Tocolysis was significantly associated with adverse maternal side effects (see Table 34-4). In 2011, the United States Food and Drug Administration (FDA) issued a warning regarding terbutaline use. Specifically, it stated that injectable terbutaline should not be used in pregnant women for prolonged treatment (beyond 48 to 72 hours) of preterm labor in either the hospital or outpatient setting because of the potential for serious maternal heart problems and death. It also warned that oral terbutaline should not be used for prevention or treatment of preterm labor because it had not been shown to be effective and was associated with similar safety concerns.81
Prostaglandins are mediators in the final pathways of uterine contraction. They increase intracellular calcium concentrations, increase activation of MLCK, and promote gap junction formation.19 The nonselective cyclooxygenase inhibitor indomethacin is the agent in this class most often studied as a tocolytic agent. Serious maternal side effects are uncommon. Fetal concerns, in particular in the setting of prolonged use (> 48 hours), include a risk for constriction of the ductus arteriosus and oligohydramnios (due to fetal renal dysfunction).
The oxytocin receptor antagonist atosiban has received attention as a tocolytic agent. Although available in Europe, the drug was not approved by the FDA because of a higher rate of fetal deaths in the atosiban arm of a randomized controlled trial.82 However, this finding may have been due to an imbalance in the number of women less than 26 weeks' gestation who were randomly assigned to receive atosiban. A 2005 meta-analysis concluded that atosiban did not achieve a lower rate of preterm birth than placebo or beta-adrenergic agonists. Several small, randomized controlled trials comparing atosiban with nifedipine have suggested that the drugs are equally efficacious for acute tocolysis.83,84
Magnesium sulfate also has been used as a tocolytic agent. A 2002 meta-analysis of nine high-quality randomized controlled trials that compared magnesium with placebo concluded that magnesium is ineffective in delaying or preventing preterm birth.73
The nitric oxide donor nitroglycerin has also been studied. A meta-analysis suggested that nitroglycerin does not delay delivery or improve neonatal outcome in comparison with placebo or other tocolytic agents.85
The ACOG77 has stated that evidence supports the use of tocolytic treatment with beta-adrenergic agonist therapy, calcium entry–blocking agents, or NSAIDs for short-term prolongation of pregnancy (up to 48 hours) to allow for antenatal maternal corticosteroid administration. Multiple clinical trials have demonstrated that prolonged use of tocolytic agents (as prophylactic therapy or after completion of acute treatment) does not alter outcome. No matter which tocolytic agent is chosen, the risk for side effects increases when more than one tocolytic agent is administered simultaneously. Thus, combining tocolytic agents is not routinely recommended.
The Preterm Infant
Physiology
Several, but not all, studies have suggested that the incidence of intrapartum acidosis is greater in the preterm fetus than in the term fetus.86,87 The preterm fetus has lower hemoglobin concentration and oxygen-carrying capacity than a term fetus.86 Nonetheless, these characteristics do not translate into a higher risk for intrapartum fetal neurologic injury.
Preterm infants are at risk for a number of complications, including respiratory distress syndrome, hyperbilirubinemia, necrotizing enterocolitis, intraventricular hemorrhage, perinatal infection, retinopathy of prematurity, patent ductus arteriosus, pulmonary hypertension, water and electrolyte imbalances, acid-base disturbances, anemia, and hypoglycemia.2 In the long term, preterm infants also are more likely to experience adverse outcomes such as bronchopulmonary dysplasia, reactive airway disease, failure to thrive, cerebral palsy, neurodevelopmental delay, hearing loss, blindness, pulmonary hypertension, adult hypertension, and impaired glucose regulation.2
Method of Delivery
Evidence does not support a conclusion that routine cesarean delivery of preterm infants improves outcome. Malloy et al.88 analyzed birth and death certificate information from Missouri for the years 1980 to 1984. The cesarean delivery rate for VLBW infants (500 to 1499 g) rose from 24% to 44%. During the same period, the cesarean delivery rate increased from 21% to 26% for infants weighing 1500 to 2499 g and from 14% to 18% for infants weighing 2500 g or more. The first-day death rates were significantly higher among the smallest infants (weighing 500 to 749 g) that were delivered vaginally than among those delivered by cesarean (59% and 33%, respectively), although this finding may very well be related to confounding by indication (i.e., cesarean delivery was more likely to be deferred for those judged to have the least chance of survival). In contrast, the mortality rates for the two methods of delivery for these infants did not differ after the first 6 days of life. There was no association between the method of delivery and first-day death rates for infants weighing between 750 and 1500 g. The investigators concluded that the use of cesarean delivery did not improve overall survival for VLBW infants.
Malloy et al.89 later reviewed the incidence of intraventricular hemorrhage and neonatal mortality in 1765 VLBW infants admitted to seven neonatal intensive care units between 1987 and 1988. After adjusting the data for gestational age at birth and other maternal and fetal factors, these investigators concluded that cesarean delivery did not lower the risk for either mortality or intraventricular hemorrhage for infants who weighed less than 1500 g at birth.
A systematic review of six randomized controlled trials comparing elective with selective cesarean delivery for preterm infants (n = 122) found no difference in outcomes between groups, although the confidence intervals were wide because of the small number of patients included in the analysis.90 A retrospective analysis of 2466 VLBW preterm infants in the state of Washington between 1994 and 2003 did not demonstrate any benefit of cesarean delivery for improving survival.91 In a study published in 2012, Reddy et al.92 examined neonatal outcomes by attempted route of delivery for singleton births that occurred between 24 0/7 and 31 6/7 weeks' gestation. Among women who delivered between 24 0/7 and 27 6/7 weeks' gestation with a vertex presentation, 77.2% attempted vaginal delivery (85% success rate) with the remainder undergoing scheduled cesarean delivery. No difference was observed in neonatal mortality or other adverse neonatal outcomes between the two groups. Similarly, when examining outcomes in women with a vertex presentation between 28 0/7 and 31 6/7 weeks' gestation, no difference in neonatal mortality was found in women who attempted vaginal delivery compared with those who underwent planned cesarean delivery.92
Aside from operative risks in the index pregnancy, preterm cesarean delivery may increase maternal risk in subsequent pregnancies. In an observational study that involved 26,454 women with previous cesarean delivery, the Maternal-Fetal Medicine Units Network noted that women with a prior preterm cesarean delivery were at higher risk for uterine rupture than women with a prior term cesarean delivery (odds ratio [OR], 1.6; 95% CI, 1.01 to 2.50; P = .043).93
Most obstetricians perform cesarean delivery for the delivery of VLBW singleton fetus in a breech presentation.88,89 Head entrapment behind an incompletely dilated cervix is more common in preterm singleton fetuses with a breech presentation because the head is larger than the wedge formed by the buttocks and thighs. Similarly, cesarean delivery has been recommended for preterm twins in whom the presenting fetus has a nonvertex presentation, although there are no prospective, controlled studies to support this practice.94 The management of preterm twins when twin A is vertex and twin B is nonvertex is more controversial; there are no good data from clinical trials, and the results from observational studies suffer from potential selection bias.95 Thus, a definitive recommendation regarding whether a breech second twin should be delivered by cesarean remains elusive; it seems reasonable that practitioners with experience can individualize care, taking into account the clinical circumstances, and offer certain patients the option of delivering preterm twins vaginally if the first twin has a vertex presentation.
The survival rate remains low for infants with a birth weight between 500 and 750 g. In these cases, obstetricians must decide whether to recommend cesarean delivery for fetal indications, such as in cases of nonreassuring fetal status or breech presentation. The neonatologist is frequently asked to speak with the patient about the risk for neonatal morbidity and mortality so that the patient can make an informed decision about the method of delivery. Regardless of the mode of delivery, if resuscitation is planned, additional support personnel (ideally a neonatologist and a neonatal resuscitation team) should be prepared and present for the delivery.
Ethical Issues
The antenatal maternal administration of corticosteroids, the application of advanced neonatal ventilation techniques, the use of neonatal surfactant therapy, and the use of extracorporeal membrane oxygenation (ECMO) have reduced mortality and morbidity for preterm neonates. However, below a certain gestational age (i.e., < 23 0/7 weeks), survival is not typically possible and the relationship between new treatments and reduced mortality is not relevant. Around the time when survival becomes at least possible, the chance of survival, and particularly survival without long-term major adverse outcomes, remains low and difficult to predict for any individual neonate. These uncertainties often lead to controversy about the decision to resuscitate (or not resuscitate) a preterm infant. Parents, obstetricians, and neonatologists should all be involved in the decision-making process.
Anesthesia providers may find themselves in the middle of these ethical dilemmas if they are practicing in a location at which the anesthesia provider is responsible for neonatal resuscitation. Although no firm rules exist, some basic principles can be applied. First, the parents should have a critical role in the decision-making process. Second, as much data as possible should be obtained to provide a prognostic assessment. Third, discussion of these issues ideally should be held before delivery, not in the moment of crisis. The ACOG95 has published general recommendations about the care of infants on the threshold of viability but has not made specific recommendations for neonatal resuscitation on the basis of gestational age. In contrast, the Canadian Paediatric Society and the Society of Obstetricians and Gynaecologists of Canada have issued relatively specific recommendations.96 For an infant born at 22 to 23 weeks' gestation, they suggest that resuscitation efforts be initiated only if uncertainty about gestational age exists or fully informed parents request that resuscitation be performed. For an infant born at 23 to 24 weeks' gestation, resuscitation can be offered as long as parents are informed of the need to reassess this decision at critical intervals and possibly withdraw therapy. For an infant born at 25 weeks' gestation, full resuscitation in the absence of lethal anomalies is recommended.96 The American Heart Association and the American Academy of Pediatrics have stated that, with few exceptions, resuscitation is not indicated if the infant is delivered at less than 23 completed weeks' gestation or with a birth weight less than 400 g.97 Revised neonatal resuscitation guidelines have addressed the ethical issues of non-initiation or discontinuation of resuscitation in the delivery room.16,97 In some cases, a trial of therapy may be appropriate, but such a trial does not always mandate continued support.
Fetal Heart Rate Monitoring
Most obstetricians use continuous electronic fetal heart rate (FHR) monitoring once preterm labor becomes established and the gestational age and circumstances are consistent with the possibility of neonatal survival. Preterm gestation may complicate the interpretation of FHR patterns, given that the FHR pattern of preterm fetuses may have relatively decreased variability and magnitude of accelerations compared with the FHR pattern of term fetuses.98
The value of continuous electronic FHR monitoring over intermittent auscultation of the FHR remains controversial. Luthy et al.99 performed a randomized trial comparing continuous electronic FHR monitoring (with selective fetal blood gas assessment) with periodic auscultation of the FHR during preterm labor in women with fetuses weighing between 700 and 1750 g. There was no significant difference between groups in the incidence of cesarean delivery, low 5-minute Apgar scores, intrapartum acidosis, intracranial hemorrhage, or perinatal death. At 18 months of age, the incidence of cerebral palsy was significantly higher in the electronic FHR group than in the intermittent auscultation group (20% versus 8%, respectively).100
Anesthetic Management
Anesthesia providers often participate in the care of preterm parturients. Many women who deliver preterm request neuraxial analgesia for labor and vaginal delivery. These patients may also require cesarean delivery, for example, in situations of nonreassuring fetal status, and may require urgent administration of anesthesia.
Conventional wisdom holds that the preterm fetus is more vulnerable than the term fetus to the depressant effects of analgesic and anesthetic drugs, for the following reasons: (1) less protein available for drug binding, leading to a reduction in protein-drug affinity; (2) higher levels of bilirubin, which may compete with the drug for protein binding; (3) greater drug access to the central nervous system (CNS) because of the presence of an incomplete blood-brain barrier; (4) decreased ability to metabolize and excrete drugs; and (5) a higher incidence of acidosis during labor and delivery.87,101 However, few controlled studies have documented the maternal and fetal pharmacokinetics and pharmacodynamics of anesthetic agents throughout gestation. The preterm fetus may be less vulnerable to the depressant effects of local anesthetics than originally thought. The human fetal liver cytochrome P450 system is present as early as 14 weeks' gestation and has the capability to oxidize several drugs.102,103
Teramo et al.104 noted that the amount of lidocaine necessary to produce seizure activity in preterm fetal lambs was greater than that required in older fetal lambs. These investigators also observed that the cardiovascular response to lidocaine (i.e., increases in blood pressure and heart rate) was less severe in fetuses with a younger gestational age. Pedersen et al.105 evaluated the effects of gestational age on the pharmacokinetics and pharmacodynamics of lidocaine in gravid ewes and fetal lambs. They studied two groups of animals, preterm (119 ± 1 days' gestation or 0.8 of term pregnancy) and near-term (138 ± 1 days' gestation or 0.95 of term pregnancy). They administered an intravenous infusion of lidocaine to obtain a maternal steady-state plasma concentration of 2 µg/mL. Transplacental transfer of lidocaine did not adversely affect fetal cardiac output, organ blood flow, or blood gas and acid-base measurements in either group. Tissue uptake of lidocaine was similar in the two groups of fetal lambs, except that it was greater in the lungs and liver of the term fetuses. The investigators concluded that there was no significant difference in the pharmacokinetics and pharmacodynamics of lidocaine between the two gestational ages studied.105
Smedstad et al.106 also concluded that there was no difference in fetal blood pressure, heart rate, or blood gas measurements in response to maternal intravenous infusion of lidocaine or bupivacaine between early preterm (119 days' gestation) and late preterm (132 days' gestation) fetal lambs. In addition, the plasma concentrations of bupivacaine and lidocaine and the fetal-to-maternal ratios of both drugs were similar in the two groups of fetuses.
None of these studies evaluated the effects of anesthetic agents on the acidotic preterm fetus. Asphyxia may increase the risk for adverse effects by causing the following changes in the fetal environment: (1) reduced plasma protein-binding capacity (which increases the proportion of free drug available)107; (2) greater maternal-fetal hydrogen ion difference, which causes “ion trapping” of weak bases (e.g., amide local anesthetics, opioids) on the fetal side of the circulation108; (3) greater blood-brain barrier permeability109; and (4) enhanced susceptibility to the myocardial depressant effects of local anesthetics.110,111
Morishima et al.110 subjected a group of preterm fetal lambs (0.8 of term gestation) to asphyxia by causing partial occlusion of the umbilical cord. They subsequently administered either lidocaine or saline-control intravenously to the gravid ewes for 180 minutes. The mean (± SD) maternal and fetal steady-state plasma lidocaine concentrations were 2.32 ± 0.12 and 1.23 ± 0.17 µg/mL, respectively. (These concentrations are similar to those that occur during administration of epidural anesthesia in humans.) Umbilical cord occlusion resulted in the typical fetal compensatory response to hypoxia (i.e., decreased FHR and increased blood flow to the fetal brain, heart, and adrenal glands). Maternal administration of saline-control did not result in additional deterioration of the fetus. However, maternal administration of lidocaine resulted in a significant increase in PaCO2, and decreases in pH, mean arterial pressure (MAP), and blood flow to the brain, myocardium, and adrenal glands. Thus, lidocaine attenuated the normal fetal compensatory response to asphyxia.
In an earlier study, the same investigators observed that lidocaine did not affect the fetal compensatory response to asphyxia in term fetuses.111 They concluded that “the immature fetus loses its cardiovascular adaptation to asphyxia when exposed to clinically acceptable plasma concentrations of lidocaine obtained transplacentally from the mother.”110 Limitations of this study include (1) a failure to compare the fetal response to lidocaine with the response to other anesthetic, analgesic, or sedative drugs and (2) consideration of only the effects of a steady-state concentration of lidocaine in the presence of asphyxia. That is, the investigators did not evaluate the potential benefits derived from epidural anesthesia, such as reduced maternal concentrations of catecholamines and the ability of epidural anesthesia to facilitate a controlled, atraumatic delivery of the preterm infant.
Bupivacaine has a low fetal-to-maternal plasma concentration ratio because of its relatively high (96%) maternal protein binding; therefore, the potential for fetal toxicity seems minimal.101 Studies of the effects of bupivacaine on the compensatory response to asphyxia in preterm fetal lambs have demonstrated results similar to those seen with lidocaine. Santos et al.112 observed that bupivacaine abolished the compensatory increase in blood flow to vital organs in asphyxiated preterm fetal lambs. However, bupivacaine did not affect fetal heart rate, blood pressure, or acid-base measurements. The investigators suggested that these changes were less severe than those seen with lidocaine in their earlier study.110,112
Ropivacaine and bupivacaine have almost identical dissociation constants (pKB of 8.0 and 8.2, respectively), but ropivacaine's protein binding is slightly less than that for bupivacaine (92% versus 96%, respectively), and it is substantially less lipid soluble than bupivacaine.113 These differences may affect maternal and fetal free plasma concentrations of drug. Investigators have documented higher maternal and fetal plasma concentrations with ropivacaine than with bupivacaine.114,115 Studies suggest that ropivacaine is less cardiotoxic than bupivacaine. However, no study has evaluated the effect of ropivacaine on the fetal compensatory response to hypoxia.
2-Chloroprocaine also is a good choice of local anesthetic in preterm patients because it is rapidly metabolized in both the maternal plasma and fetal plasma.116 Further, placental transfer of 2-chloroprocaine is not increased by fetal acidosis.117
Vaginal Delivery
Neither pudendal nerve block nor local infiltration of the perineum provides profound relaxation of the levator ani and bulbocavernosus muscles. Thus, for women in whom more profound analgesia is desired, either for elective or medical indications, neuraxial analgesia is the technique of choice during labor and vaginal delivery.
Neuraxial analgesia decreases maternal concentrations of catecholamines, and in some patients, it may improve uteroplacental perfusion as long as hypotension is avoided.118 No prospective, controlled studies have evaluated the effect of neuraxial analgesia on preterm infant outcome. The timing of the intrapartum administration of neuraxial analgesia in preterm parturients may be problematic for several reasons. First, there may be uncertainty as to whether women who have contractions are in labor. Second, even women with a clear diagnosis of preterm labor often have a prolonged latent phase of labor, with or without the use of tocolytic agents. Third, once active labor begins, patients often progress though labor very quickly. Thus, in some cases, it may be appropriate to establish neuraxial analgesia even before it is clear that a preterm delivery will soon occur. An advantage of early initiation of neuraxial analgesia is the ability to rapidly convert labor analgesia to surgical anesthesia if emergency cesarean delivery should be necessary.
Cesarean Delivery
Administration of general anesthesia for preterm cesarean delivery is similar to that for parturients at term (see Chapter 26). Most anesthetic agents that are used for induction and maintenance of general anesthesia cross the placenta. If cesarean delivery is necessary, conventional wisdom holds that it is preferable to give either epidural or spinal anesthesia to avoid the depressant effects of agents given for general anesthesia. Rolbin et al.119 observed that preterm infants exposed to epidural anesthesia for cesarean delivery had higher 1- and 5-minute Apgar scores than similar infants exposed to general anesthesia. Laudenbach et al.120 performed a secondary analysis of prospectively gathered data from a population-based cohort study of all deliveries before 33 weeks' gestation in nine regions in France in 1997 (n = 1338). Of concern, after controlling for known confounders, infants born by cesarean delivery to mothers who received spinal anesthesia had a higher mortality rate than those born to mothers who received general or epidural anesthesia (adjusted OR, 1.7; 95% CI, 1.1 to 2.6).120 However, the authors noted that the secondary analysis of a preexisting database did not allow them to adjust for confounders known to affect anesthetic outcome (e.g., intraoperative hypotension, choice of vasopressor, fluid management). Nonetheless, for these high-risk births, it seems reasonable that anesthesia providers should pay meticulous attention to maternal hemodynamic variables regardless of the type of anesthesia that is administered.
Many anesthesiologists have considered the maternal administration of supplemental oxygen during cesarean delivery essential, regardless of the choice of anesthetic technique. Recent studies, however, have questioned this practice. Fetal or neonatal hyperoxia may lead to production of oxygen free radicals that ultimately may result in neuronal damage.121 Investigators have not demonstrated any clear improvement in neonatal outcome with maternal supplemental oxygen administration, even after a prolonged uterine incision-to-delivery interval.122,123 Currently, it is not clear whether the maternal administration of supplemental oxygen affects neonatal outcome.
Some data from animal studies have suggested that exposure of the immature brain to anesthetic agents such as propofol, thiopental, ketamine, and inhalation agents can trigger significant brain cell apoptosis in the developing fetal/neonatal brain and cause functional learning deficits later in life.124,125 However, in these animal studies, the duration of exposure to anesthetic agents was much longer than is typical for cesarean delivery in humans. Whether clinical exposure to anesthetic agents during general anesthesia for cesarean delivery results in clinically significant brain cell apoptosis in humans remains to be determined (see Chapters 10 and 17).
In summary, at the current time there is no evidence to support altering the anesthetic technique for cesarean delivery merely because the infant is preterm. Further study is necessary to determine whether one technique (e.g., spinal or epidural) or specific drug(s) (e.g., ephedrine or phenylephrine) have specific risks or benefits relative to the preterm infant.
Interactions between Tocolytic Therapy and Anesthesia
Indications for Anesthesia during and after Tocolytic Therapy
There are several situations in which obstetric patients require analgesia or anesthesia during or after tocolytic therapy. First, preterm labor may progress and delivery may occur despite tocolysis. In this case, the patient may desire pain relief during labor and vaginal delivery or may require anesthesia for cesarean delivery. Second, some obstetricians give a tocolytic agent before and during the performance of cervical cerclage. Third, some obstetricians advocate the bolus injection of a tocolytic agent when there is a tetanic uterine contraction or tachysystole in the setting of an FHR abnormality. Fourth, many obstetricians administer tocolysis when attempting external cephalic version, a procedure that may also involve the request for neuraxial analgesia.
Calcium Entry–Blocking Agents
Among this class of drugs, nifedipine has undergone the most extensive evaluation as a tocolytic agent. Many obstetricians have suggested that calcium entry–blocking drugs should become first-line therapy in the treatment of preterm labor because of their low incidence of significant maternal and fetal side effects. Typical nifedipine dose regimens for preterm labor are listed in Table 34-5.
TABLE 34-5
Tocolytic Dose Regimens for Preterm Labor
| Drug | Initiation Dose | Maintenance Dose |
| Nifedipine | 20-30 mg PO | 10-20 mg every 4-6 h |
| Cyclooxygenase inhibitors (NSAIDs)*: | ||
| Indomethacin | 50-100 mg PO or per rectum | 25-50 mg every 4 h |
| Ketorolac | 60 mg IM | 30 mg every 6 h |
| Sulindac | 200 mg PO | 200 mg every 12 h |
| Terbutaline† | 0.25 mg SQ | 0.25 mg every 20 min to 3 h |
| Magnesium sulfate | 4-6 g IV bolus over 20 min | 2-4 g/h continuous IV infusion |
* NSAID administration should be limited to 48 to 72 hours and restricted to gestations < 32 weeks.
† Terbutaline is given until uterine quiescence occurs or maternal heart rate reaches 120 bpm.
IM, intramuscularly; IV, intravenously; NSAIDs, nonsteroidal anti-inflammatory drugs; PO, per os (orally); SQ, subcutaneously.
Data from references 19 and 143. Modified from Muir HA, Wong CA. Preterm labor and delivery. In Chestnut DH, Tsen LC, Polley LS, Wong CA, editors. Chestnut's Obstetric Anesthesia. 4th edition. Philadelphia, Mosby, 2009.
Mechanism of Action
Calcium entry–blocking drugs act by blocking the aqueous voltage-dependent cell membrane channels that are selective for calcium. They also act by preventing calcium release from the sarcoplasmic reticulum. The net result is a decrease in available intracellular calcium, which inhibits MLCK activity. This inhibition leads to decreased actin-myosin interaction, which results in relaxation of smooth muscle (including myometrial smooth muscle).126,127
Side Effects
Nifedipine has fewer side effects than beta-adrenergic receptor agonists. Hypotension is the most common side effect. Other side effects include headache, flushing, dizziness, and nausea.128 Most effects are mild, but pulmonary edema129 and myocardial infarction130 have been reported after calcium entry–blocking agent therapy for preterm labor.
Although early animal studies noted that nifedipine and nicardipine decreased uterine blood flow and resulted in fetal hypoxemia and acidosis,131,132 clinical studies have suggested that short-term administration of nifedipine does not adversely affect the uteroplacental or fetal circulation.133,134
Anesthetic Management
Although nifedipine has fewer effects on cardiac conduction than some of the other calcium entry–blocking agents, it has the potential to cause vasodilation, hypotension, myocardial depression, and conduction defects when used in combination with one of the volatile halogenated anesthetic agents.135 One report noted that administration of both nifedipine and magnesium sulfate was associated with neuromuscular blockade in a preeclamptic patient at 28 weeks' gestation.136
Cyclooxygenase Inhibitors
Indomethacin is the prototype cyclooxygenase inhibitor used for tocolysis. Both sulindac and ketorolac also have been evaluated and found to be effective. Typical doses of this class of medication used for tocolysis are listed in Table 34-5.
Mechanism of Action
Cyclooxygenase inhibitors inhibit cyclooxygenase and thus prevent the synthesis of prostaglandins from the precursor, arachidonic acid. Prostaglandins E2 and F2α play an important role in the stimulation of uterine contractions by increasing intracellular calcium and activation of MLCK and promoting gap junction formation.19
Side Effects
Maternal side effects from indomethacin are minimal when it is used for tocolytic therapy. Indomethacin does not alter maternal heart rate or blood pressure. The most common complaints are nausea and heartburn.137 Inhibition of cyclooxygenase results in decreased production of thromboxane A2 and abnormal platelet aggregation. In contrast to aspirin, which permanently inhibits cyclooxygenase and therefore inhibits platelet aggregation for the lifetime of the platelet (7 to 10 days), indomethacin and other cyclooxygenase inhibitors reversibly inhibit cyclooxygenase; thus, their effect on platelet function is only transient.138
Maternal administration of a cyclooxygenase inhibitor may cause premature closure of the ductus arteriosus in utero. Indomethacin is often used to promote closure of the ductus arteriosus in the preterm neonate.137 Moise et al.139 used fetal echocardiography to evaluate the fetal response to short-term (< 72 hours) indomethacin therapy. They observed evidence of transient ductal constriction in 7 of 14 fetuses between 26 and 31 weeks' gestation. Tricuspid regurgitation was also noted in three fetuses. These changes were reversed within 24 hours of discontinuation of indomethacin. Clinical studies suggest that indomethacin is less likely to cause intrauterine closure of the ductus arteriosus at earlier gestational ages.137,140-142 Also, studies suggest that adverse neonatal effects (including closure of the ductus arteriosus) are unlikely if indomethacin is used in short courses (e.g., 24 to 48 hours).137,141,142 For example, investigators142 retrospectively analyzed fetal echocardiograms performed in 44 patients with preterm labor or polyhydramnios who were treated with indomethacin. The frequency of ductal constriction was relatively low (5% to 10%) until 32 weeks' gestation, when it rose to approximately 50%. Vermillion et al.142 concluded that ductal constriction can occur at any gestational age but that it is reversible with early identification followed by timely discontinuation of indomethacin therapy.
Indomethacin administration may result in fetal oligohydramnios secondary to decreased fetal urine output.143 Kirshon et al.144 noted reduced fetal urine output after short-term145 and long-term (15 to 28 days) maternal administration of indomethacin for tocolytic therapy. Amniotic fluid volume reaccumulated within 1 week after the discontinuation of indomethacin. Indomethacin may be used to treat polyhydramnios in selected cases. One proposed mechanism for the decrease in fetal urine output is an enhanced antidiuretic hormone effect after inhibition of cyclooxygenase.146 Wurtzel147 evaluated renal function during the first 10 postnatal days in 14 preterm infants exposed to indomethacin in utero and in 10 control infants. This investigator found that maternal administration of indomethacin did not significantly alter neonatal renal function.
Data are conflicting as to whether maternal cyclooxygenase inhibitor administration increases the risk for adverse neonatal outcomes, including intraventricular hemorrhage and necrotizing enterocolitis. A 2005 meta-analysis concluded that maternal administration of indomethacin at less than 34 weeks' gestation did not lead to an increased risk for adverse events.148 However, the investigators cautioned that the significant heterogeneity in study design makes it difficult to draw definitive conclusions.
The current recommendation for use of cyclooxygenase inhibitors is to limit the course of therapy to less than 72 hours. This approach may delay delivery and allow maternal administration of corticosteroids to accelerate fetal lung maturity.
Anesthetic Management
The effects of indomethacin on platelet function are transient. Several large studies have demonstrated the safety of epidural and spinal anesthesia in patients receiving low-dose aspirin or one of a variety of cyclooxygenase inhibitors.149-151 The Consensus Conference on Neuraxial Anesthesia and Anticoagulation (sponsored by the American Society of Regional Anesthesia and Pain Medicine) concluded that such therapy is not a contraindication to administration of neuraxial anesthesia.152
Beta-Adrenergic Receptors Agonists
In the past, ritodrine and terbutaline, among other agents, were commonly used beta-adrenergic tocolytic agents; however, their use has declined substantially. Maternal side effects continue to be a limiting factor in their use.80
Mechanism of Action
All beta-adrenergic tocolytic drugs have both β1-receptor and β2-receptor effects, but in different proportions. β2-Receptors are found in smooth muscle (uterus, blood vessels, bronchi, intestine, detrusor, and spleen capsule), adipose tissue, liver, skeletal muscle, pancreas, and salivary glands. Ritodrine and terbutaline are relatively selective for β2-receptors; stimulation of these receptors in the myometrium results in relaxation of uterine smooth muscle. Unfortunately, other undesired β2-adrenergic agonist effects (e.g., vasodilation) and β1-effects still occur. β1-Receptors are located predominantly in the heart and adipose tissue. β1-Receptor stimulation has clinically significant cardiovascular side effects, such as increased maternal heart rate and cardiac output.153
Beta-adrenergic agonists interact with β2-receptor sites on the outer membrane of uterine myometrial cells, activating the enzyme adenyl cyclase. This enzyme catalyzes the conversion of ATP to cAMP, causing a rise in the intracellular concentration of cAMP. The higher cAMP concentration decreases the available intracellular concentration of calcium and inhibits MLCK activity. This inhibition, in turn, decreases the interaction between actin and myosin, resulting in myometrial relaxation.143
Treatment Regimen
Before beginning tocolytic therapy with a beta-adrenergic agonist, the obstetrician should determine baseline maternal vital signs and weight and exclude significant cardiovascular or pulmonary disease. Prolonged administration results in down-regulation (or desensitization) of the myometrial β2-receptors.154 There is no evidence that a continuous long-term infusion of terbutaline alters outcome.155
Side Effects
The administration of beta-adrenergic tocolytic therapy may result in the following maternal side effects: (1) hypotension; (2) tachycardia, with or without cardiac arrhythmias and myocardial ischemia; (3) pulmonary edema; (4) hyperglycemia; and (5) hypokalemia.143 The reported frequency of these side effects has varied. Earlier studies reported an incidence of 2% to 9%.156,157 However, Perry et al.158 performed a retrospective review of outcomes for 8709 patients who had received a low-dose, continuous infusion of terbutaline. These investigators noted adverse cardiopulmonary effects in only 47 of 8709 patients, an incidence of 0.54%.
Other uncommon maternal side effects reported with the use of beta-adrenergic agents are elevations in serum transaminase levels,159 paralytic ileus,160 cerebral vasospasm in patients with a previous history of migraine,161 and respiratory arrest due to increased muscle weakness in a patient with myasthenia gravis.162
Anesthetic Management
It would seem ideal to delay administration of anesthesia until maternal tachycardia subsides. A delay of 15 minutes often results in slowing of the maternal heart rate. However, advanced labor, an abnormal presentation, and/or nonreassuring fetal status often require emergency administration of anesthesia. Published reports of anesthetic management after administration of a beta-adrenergic agent are scarce.163-167 Ravindran et al.165 reported one case each of intraoperative pulmonary edema, sinus tachycardia, and ventricular arrhythmia during general anesthesia in patients who had received terbutaline therapy immediately before or 15 minutes after the induction of anesthesia. These investigators recommended that induction of general anesthesia be delayed at least 10 minutes after discontinuation of the beta-adrenergic agent.
Chestnut et al.168 performed a controlled study to determine whether administration of ritodrine worsens maternal hypotension during epidural anesthesia in gravid ewes. Prior administration of ritodrine did not worsen maternal hypotension during epidural lidocaine anesthesia in gravid ewes.
Theoretically, induction of epidural analgesia or anesthesia after beta-adrenergic agonist therapy may cause less hemodynamic compromise than spinal anesthesia because of the slower onset of sympathetic blockade. However, this theory remains unproven. Patients receiving beta-adrenergic agonist therapy are at risk for development of pulmonary edema (as discussed earlier). Therefore, aggressive hydration should be avoided before and during the induction of anesthesia in these patients.
If general anesthesia is required in a patient who has recently received beta-adrenergic tocolysis, agents that might exacerbate maternal tachycardia (e.g., atropine, glycopyrrolate, pancuronium) should be avoided. Residual maternal tachycardia may make it more difficult to assess volume status and depth of anesthesia. Halothane, which sensitizes the myocardium to catecholamine-induced arrhythmias, should not be used. Hyperventilation should be avoided, because it may exacerbate hypokalemia and potentiate the hyperpolarization of the cell membrane. In nonpregnant patients, Slater et al.169 found that terbutaline pretreatment shortened the onset time and recovery of succinylcholine-induced neuromuscular blockade. It seems prudent to monitor neuromuscular function with a peripheral nerve stimulator during general anesthesia.
Oxytocin Antagonists
Atosiban (1-deamino-2-D-Tyr-[OEt]-4-Thr-8-Orn-vasotocin/oxytocin) is an oxytocin antagonist. It is a competitive inhibitor of oxytocin that binds to both myometrial and decidual receptors. It does not alter the subsequent sensitivity of the myometrium to oxytocin.170 Clinically, this feature represents a major advantage; it should reduce the risk for postpartum uterine atony and hemorrhage.
Phase II and III studies have shown that atosiban is an effective tocolytic agent.82,171-175 It has few maternal side effects, undergoes minimal placental transfer, and does not increase maternal blood loss at delivery.82,171 Studies have suggested that atosiban has efficacy similar to that of beta-adrenergic agonists174,175 and nifedipine176 in obtaining and maintaining uterine quiescence. However, a meta-analysis of studies comparing atosiban with either placebo or beta-adrenergic agonists did not demonstrate that atosiban resulted in a reduction in preterm birth or improved neonatal outcome,177 although side effects were fewer with atosiban. The FDA has not approved atosiban for use in the United States because of a higher rate of perinatal deaths in the atosiban arm of the study that it reviewed.82
There are no data on the interaction between atosiban and anesthetic agents. However, given the hemodynamic profile of this agent, one would not expect significant interactions. Atosiban is widely used in Europe.
Magnesium Sulfate
Mechanism of Action
Extracellular magnesium functions as a competitive antagonist of calcium either at the motor end plate or cell membrane, thus reducing calcium influx into the myocyte.19,143 It also competes with calcium for low-affinity calcium-binding sites on the outside of the sarcoplasmic reticulum membrane and prevents the rise in free intracellular calcium concentration. Hypermagnesemia results in abnormal neuromuscular function. Magnesium also decreases the release of acetylcholine at the neuromuscular junction and the sensitivity of the end plate to acetylcholine.
Side Effects
Most studies have reported that magnesium sulfate results in less frequent and less severe cardiovascular side effects than the beta-adrenergic tocolytic agents.178-180 Nonetheless, magnesium sulfate may have side effects similar to those that occur during beta-adrenergic tocolytic therapy. Chest pain and tightness, palpitations, nausea, transient hypotension, blurred vision, sedation, and pulmonary edema have been reported.179,180 Hypermagnesemia may attenuate the normal compensatory responses to hemorrhage in the mother and fetus.181,182
Magnesium is eliminated almost entirely by renal excretion. Therefore, patients with abnormal renal function should be monitored carefully if they receive magnesium sulfate.
Anesthetic Management
It has been suggested that magnesium sulfate should be discontinued before the administration of epidural anesthesia because magnesium may increase the likelihood of hypotension through its generalized vasodilating properties. Vincent et al.183 observed that magnesium sulfate reduced maternal mean arterial pressure but not uterine blood flow or fetal oxygenation during epidural lidocaine anesthesia in gravid ewes. This study suggests that hypermagnesemia may increase the likelihood of modest hypotension during neuraxial anesthesia in normotensive parturients.
Magnesium attenuates the release of acetylcholine at the neuromuscular junction, reduces the sensitivity of the end plate to acetylcholine, and decreases the excitability of the muscle membrane. The drug potentiates the action of both depolarizing and nondepolarizing muscle relaxants.184 A defasciculating dose of a nondepolarizing muscle relaxant should not be given before administration of succinylcholine in hypermagnesemic women. A standard intubating dose of muscle relaxant (e.g., succinylcholine 1 mg/kg) should be used because the extent of potentiation by magnesium sulfate is variable.185 However, a lower dose of a nondepolarizing muscle relaxant should be administered during the maintenance of anesthesia.
Parturients receiving magnesium sulfate often appear sedated. Thompson et al.186 evaluated the anesthetic effects of magnesium sulfate and ritodrine on the minimum alveolar concentration of halothane in pregnant and nonpregnant rats and reported a 20% decrease with serum magnesium levels of 7 to 11 mg/dL. A more detailed discussion of magnesium sulfate and its interactions with anesthetic agents is found in Chapter 36.
References
1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84.
2. Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358:1700–1711.
3. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. [Preterm Birth: Causes, Consequences, and Prevention. Available at] http://www.ncbi.nlm.nih.gov/books/NBK11362/ [Accessed March 2013] .
4. Hamilton BE, Hoyert DL, Martin JA, et al. Annual summary of vital statistics: 2010-2011. Pediatrics. 2013;131:548–558.
5. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2007;61.
6. Wardlaw T, Blanc A, Zupan J, Ahman E. Low Birthweight Country, Regional and Global Estimates. [UNICEF/WHO. Available at] http://www.unicef.org/nutrition/index_24840.html [Accessed April 2013] .
7. UNICEF. The State of the World's Children 2007: Women and Children: The Double Dividend of Gender Equality. [Available at] http://www.unicef.org/sowc07/docs/sowc07.pdf [Accessed April 2013] .
8. Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456.
9. Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1.
10. Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129:1019–1026.
11. Kilpatrick SJ, Schlueter MA, Piecuch R, et al. Outcome of infants born at 24-26 weeks’ gestation: I. Survival and cost. Obstet Gynecol. 1997;90:803–808.
12. Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000-2002. Pediatrics. 2007;119:37–45.
13. Smith PB, Ambalavanan N, Li L, et al. Approach to infants born at 22 to 24 weeks’ gestation: relationship to outcomes of more-mature infants. Pediatrics. 2012;129:e1508–e1516.
14. Piecuch RE, Leonard CH, Cooper BA, Sehring SA. Outcome of extremely low birth weight infants (500 to 999 grams) over a 12-year period. Pediatrics. 1997;100:633–639.
15. Wood NS, Marlow N, Costeloe K, et al. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343:378–384.
16. Keogh J, Sinn J, Hollebone K, et al. Delivery in the ‘grey zone’: collaborative approach to extremely preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:273–278.
17. Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19.
18. Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294:318–325.
19. Iams J, Romero R. Preterm birth. Gabbe S, Niebyl J, Simpson J. Obstetrics: Normal and Problem Pregnancies. 5th edition. Churchill Livingstone: Philadelphia; 2007:669–712.
20. Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600.
21. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42.
22. Krupa FG, Faltin D, Cecatti JG, et al. Predictors of preterm birth. Int J Gynaecol Obstet. 2006;94:5–11.
23. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507.
24. Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528.
25. Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med. 1999;341:660–666.
26. Dickey RP. The relative contribution of assisted reproductive technologies and ovulation induction to multiple births in the United States 5 years after the Society for Assisted Reproductive Technology/American Society for Reproductive Medicine recommendation to limit the number of embryos transferred. Fertil Steril. 2007;88:1554–1561.
27. American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Multiple gestation: complicated twin, triplet, and high-order multifetal pregnancy. [ACOG Practice Bulletin No. 56. Washington, DC, October 2004 (reaffirmed 2009)] Obstet Gynecol. 2004;104:869–883.
28. Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563.
29. American College of Obstetricians and Gynecologists. Prediction and prevention of preterm birth. [ACOG Practice Bulletin No. 130. Washington, DC, October 2012] Obstet Gynecol. 2012;120:964–973.
30. Feinberg RF, Kliman HJ, Lockwood CJ. Is oncofetal fibronectin a trophoblast glue for human implantation? Am J Pathol. 1991;138:537–543.
31. Goldenberg RL, Mercer BM, Meis PJ, et al. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996;87:643–648.
32. Smith V, Devane D, Begley CM, et al. A systematic review and quality assessment of systematic reviews of fetal fibronectin and transvaginal length for predicting preterm birth. Eur J Obstet Gynecol Reprod Biol. 2007;133:134–142.
33. Grobman WA, Welshman EE, Calhoun EA. Does fetal fibronectin use in the diagnosis of preterm labor affect physician behavior and health care costs? A randomized trial. Am J Obstet Gynecol. 2004;191:235–240.
34. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–572.
35. Kagan KO, To M, Tsoi E, Nicolaides KH. Preterm birth: the value of sonographic measurement of cervical length. BJOG. 2006;113(Suppl 3):52–56.
36. Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2012;(4).
37. To MS, Alfirevic Z, Heath VC, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–1853.
38. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375 e1–8.
39. Simcox R, Shennan A. Cervical cerclage in the prevention of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2007;21:831–842.
40. Whitworth M, Quenby S. Prophylactic oral betamimetics for preventing preterm labour in singleton pregnancies. Cochrane Database Syst Rev. 2008;(1).
41. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385.
42. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424.
43. Dodd JM, Flenady VJ, Cincotta R, Crowther CA. Progesterone for the prevention of preterm birth: a systematic review. Obstet Gynecol. 2008;112:127–134.
44. Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469.
45. Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31.
46. Grobman WA, Thom EA, Spong CY, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207:390 e1–8.
47. Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461.
48. Caritis SN, Rouse DJ, Peaceman AM, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113:285–292.
49. Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–2040.
50. Stan C, Boulvain M, Hirsbrunner-Amagbaly P, Pfister R. Hydration for treatment of preterm labour. Cochrane Database Syst Rev. 2002;(2).
51. American College of Obstetricians and Gynecologists. Antenatal corticosteroid therapy for fetal maturation. [ACOG Committee Opinion No. 475. Washington, DC, February 2011] Obstet Gynecol. 2011;117:422–424.
52. American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Magnesium sulfate before anticipated preterm birth for neuroprotection. [ACOG Committee Opinion No. 455. Washington, DC, March 2010] Obstet Gynecol. 2010;115:669–671.
53. Garite TJ, Keegan KA, Freeman RK, Nageotte MP. A randomized trial of ritodrine tocolysis versus expectant management in patients with premature rupture of membranes at 25 to 30 weeks of gestation. Am J Obstet Gynecol. 1987;157:388–393.
54. Wright LL, Verter J, Younes N, et al. Antenatal corticosteroid administration and neonatal outcome in very low birth weight infants: the NICHD Neonatal Research Network. Am J Obstet Gynecol. 1995;173:269–274.
55. Andrews EB, Marcucci G, White A, Long W. Associations between use of antenatal corticosteroids and neonatal outcomes within the Exosurf Neonatal Treatment Investigational New Drug Program. Am J Obstet Gynecol. 1995;173:290–295.
56. Murphy K, Aghajafari F, Hannah M. Antenatal corticosteroids for preterm birth. Semin Perinatol. 2001;25:341–347.
57. National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: repeat courses—National Institutes of Health Consensus Development Conference Statement, August 17-18, 2000. Obstet Gynecol. 2001;98:144–150.
58. Wapner RJ, Sorokin Y, Thom EA, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–642.
59. Garite TJ, Kurtzman J, Maurel K, et al. Impact of a ‘rescue course’ of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol. 2009;200:248 e1–9.
60. Crowther CA, McKinlay CJ, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev. 2011;(6).
61. Kenyon SL, Taylor DJ, Tarnow-Mordi W, Oracle Collaborative Group. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. Lancet. 2001;357:989–994.
62. King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev. 2002;(4).
63. Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008;372:1319–1327.
64. Verani JR, McGee L, Schrag SJ, et al. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36.
65. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes: a randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989–995.
66. Kenyon SL, Taylor DJ, Tarnow-Mordi W, Oracle Collaborative Group. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. Lancet. 2001;357:979–988.
67. American College of Obstetricians and Gynecologists. Premature rupture of membranes. [ACOG Practice Bulletin No. 80. Washington, DC, April 2007 (reaffirmed 2012)] Obstet Gynecol. 2007;109:1007–1019.
68. Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676.
69. Marret S, Marpeau L, Zupan-Simunek V, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG. 2007;114:310–318.
70. Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905.
71. Doyle LW, Crowther CA, Middleton P, Marret S. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2007;(1).
72. Berkman ND, Thorp JM Jr, Lohr KN, et al. Tocolytic treatment for the management of preterm labor: a review of the evidence. Am J Obstet Gynecol. 2003;188:1648–1659.
73. Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2002;(4).
74. Wray S. Uterine contraction and physiological mechanisms of modulation. Am J Physiol. 1993;264:C1–18.
75. Lopez Bernal A. Overview. Preterm labour: mechanisms and management. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S2.
76. Breuiller-Fouche M, Charpigny G, Germain G. Functional genomics of the pregnant uterus: from expectations to reality, a compilation of studies in the myometrium. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S4.
77. American College of Obstetricians and Gynecologists. Management of preterm labor. [ACOG Practice Bulletin No. 127. Washington, DC, June 2012] Obstet Gynecol. 2012;119:1308–1317.
78. King JF, Flenady VJ, Papatsonis DN, et al. Calcium channel blockers for inhibiting preterm labour. Cochrane Database Syst Rev. 2003;(1).
79. Husslein P, Cabero Roura L, Dudenhausen JW, et al. Atosiban versus usual care for the management of preterm labor. J Perinat Med. 2007;35:305–313.
80. Anotayanonth S, Subhedar NV, Garner P, et al. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev. 2004;(4).
81. U.S. Food and Drug Administration. FDA drug safety communication: new warnings against use of terbutaline to treat preterm labor. [Available at] http://www.fda.gov/drugs/drugsafety/ucm243539.htm [Accessed April 2013] .
82. Romero R, Sibai BM, Sanchez-Ramos L, et al. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. Am J Obstet Gynecol. 2000;182:1173–1183.
83. Kashanian M, Akbarian AR, Soltanzadeh M. Atosiban and nifedipine for the treatment of preterm labor. Int J Gynaecol Obstet. 2005;91:10–14.
84. Al-Omari WR, Al-Shammaa HB, Al-Tikriti EM, Ahmed KW. Atosiban and nifedipine in acute tocolysis: a comparative study. Eur J Obstet Gynecol Reprod Biol. 2006;128:129–134.
85. Duckitt K, Thornton S. Nitric oxide donors for the treatment of preterm labour. Cochrane Database Syst Rev. 2002;(3).
86. Bowes WA Jr. Delivery of the very low birth weight infant. Clin Perinatol. 1980;8:183–195.
87. Low JA, Wood SL, Killen HL, et al. Intrapartum asphyxia in the preterm fetus less than 2000 gm. Am J Obstet Gynecol. 1990;162:378–382.
88. Malloy MH, Rhoads GG, Schramm W, Land G. Increasing cesarean section rates in very low-birth weight infants: effect on outcome. JAMA. 1989;262:1475–1478.
89. Malloy MH, Onstad L, Wright E. The effect of cesarean delivery on birth outcome in very low birth weight infants. National Institute of Child Health and Human Development Neonatal Research Network. Obstet Gynecol. 1991;77:498–503.
90. Grant A, Penn ZJ, Steer PJ. Elective or selective caesarean delivery of the small baby? A systematic review of the controlled trials. Br J Obstet Gynaecol. 1996;103:1197–1200.
91. Wylie BJ, Davidson LL, Batra M, Reed SD. Method of delivery and neonatal outcome in very low-birthweight vertex-presenting fetuses. Am J Obstet Gynecol. 2008;198:640.e1–640.e7.
92. Reddy UM, Zhang J, Sun L, et al. Neonatal mortality by attempted route of delivery in early preterm birth. Am J Obstet Gynecol. 2012;207:117 e1–8.
93. Sciscione AC, Landon MB, Leveno KJ, et al. Previous preterm cesarean delivery and risk of subsequent uterine rupture. Obstet Gynecol. 2008;111:648–653.
94. Chervenak FA, Johnson RE, Youcha S, et al. Intrapartum management of twin gestation. Obstet Gynecol. 1985;65:119–124.
95. American College of Obstetricians and Gynecologists. Perinatal care at the threshold of viability. [ACOG Practice Bulletin No. 38. Washington, DC, September 2002 (reaffirmed 2012)] Obstet Gynecol. 2002;100:617–624.
96. Canadian Paediatric Society, Society of Obstetricians and Gynaecologists of Canada. Management of the woman with threatened birth of an infant of extremely low gestational age. CMAJ. 1994;151:547–553.
97. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: neonatal resuscitation guidelines. Pediatrics. 2006;117:e1029–e1038.
98. Westgren M, Holmquist P, Svenningsen NW, Ingemarsson I. Intrapartum fetal monitoring in preterm deliveries: prospective study. Obstet Gynecol. 1982;60:99–106.
99. Luthy DA, Shy KK, van Belle G, et al. A randomized trial of electronic fetal monitoring in preterm labor. Obstet Gynecol. 1987;69:687–695.
100. Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322:588–593.
101. Thomas J, Long G, Moore G, Morgan D. Plasma protein binding and placental transfer of bupivacaine. Clin Pharmacol Ther. 1976;19:426–434.
102. Rane A, Sjoqvist F, Orrenius S. Cytochrome P-450 in human fetal liver microsomes. Chem Biol Interact. 1971;3:305.
103. Rane A, Sjoqvist F, Orrenius S. Drugs and fetal metabolism. Clin Pharmacol Ther. 1973;14:666–672.
104. Teramo K, Benowitz N, Heymann MA, Rudolph AM. Gestational differences in lidocaine toxicity in the fetal lamb. Anesthesiology. 1976;44:133–138.
105. Pedersen H, Santos AC, Morishima HO, et al. Does gestational age affect the pharmacokinetics and pharmacodynamics of lidocaine in mother and fetus? Anesthesiology. 1988;68:367–372.
106. Smedstad KG, Morison DH, Harris WH, Pascoe P. Placental transfer of local anesthetic in the premature sheep fetus. Int J Obstet Anesth. 1993;2:34–38.
107. Tucker GT, Mather LE. Clinical pharmacokinetics of local anaesthetics. Clin Pharmacokinet. 1979;4:241–278.
108. Biehl D, Shnider SM, Levinson G, Callender K. Placental transfer of lidocaine: effects of fetal acidosis. Anesthesiology. 1978;48:409–412.
109. Ritter DA, Kenny JD, Norton HJ, Rudolph AJ. A prospective study of free bilirubin and other risk factors in the development of kernicterus in premature infants. Pediatrics. 1982;69:260–266.
110. Morishima HO, Pedersen H, Santos AC, et al. Adverse effects of maternally administered lidocaine on the asphyxiated preterm fetal lamb. Anesthesiology. 1989;71:110–115.
111. Morishima HO, Santos AC, Pedersen H, et al. Effect of lidocaine on the asphyxial responses in the mature fetal lamb. Anesthesiology. 1987;66:502–507.
112. Santos AC, Yun EM, Bobby PD, et al. The effects of bupivacaine, L-nitro-L-arginine-methyl ester, and phenylephrine on cardiovascular adaptations to asphyxia in the preterm fetal lamb. Anesth Analg. 1997;85:1299–1306.
113. Arthur GR, Feldman HS, Covino BG. Comparative pharmacokinetics of bupivacaine and ropivacaine, a new amide local anesthetic. Anesth Analg. 1988;67:1053–1058.
114. Datta S, Camann W, Bader A, VanderBurgh L. Clinical effects and maternal and fetal plasma concentrations of epidural ropivacaine versus bupivacaine for cesarean section. Anesthesiology. 1995;82:1346–1352.
115. Ala-kokko TI, Alahuta S, Jouppila P, et al. Feto-maternal distribution of ropivacaine and bupivacaine after epidural administration for cesarean section. Int J Obstet Anesth. 1997;6:147–152.
116. Kuhnert BR, Kuhnert PM, Reese AL, et al. Maternal and neonatal elimination of CABA after epidural anesthesia with 2-chloroprocaine during parturition. Anesth Analg. 1983;62:1089–1094.
117. Philipson EH, Kuhnert BR, Syracuse CD. Fetal acidosis, 2-chloroprocaine, and epidural anesthesia for cesarean section. Am J Obstet Gynecol. 1985;151:322–324.
118. Hollmen AI, Jouppila R, Jouppila P, et al. Effect of extradural analgesia using bupivacaine and 2-chloroprocaine on intervillous blood flow during normal labour. Br J Anaesth. 1982;54:837–842.
119. Rolbin SH, Cohen MM, Levinton CM, et al. The premature infant: anesthesia for cesarean delivery. Anesth Analg. 1994;78:912–917.
120. Laudenbach V, Mercier FJ, Roze JC, et al. Anaesthesia mode for caesarean section and mortality in very preterm infants: an epidemiologic study in the EPIPAGE cohort. Int J Obstet Anesth. 2009;18:142–149.
121. Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18–23.
122. Khaw KS, Ngan Kee WD, Lee A, et al. Supplementary oxygen for elective Caesarean section under spinal anaesthesia: useful in prolonged uterine incision-to-delivery interval? Br J Anaesth. 2004;92:518–522.
123. Ngan Kee WD, Khaw KS, Ma KC, et al. Randomized, double-blind comparison of different inspired oxygen fractions during general anaesthesia for Caesarean section. Br J Anaesth. 2002;89:556–561.
124. Perouansky M. General anesthetics and long-term neurotoxicity. Handb Exp Pharmacol. 2008;143–157.
125. Jevtovic-Todorovic V, Olney JW. PRO: Anesthesia-induced developmental neuroapoptosis: status of the evidence. Anesth Analg. 2008;106:1659–1663.
126. Forman A, Andersson KE, Ulmsten U. Inhibition of myometrial activity by calcium antagonists. Semin Perinatol. 1981;5:288–294.
127. Struyker-Boudier HA, Smits JF, De Mey JG. The pharmacology of calcium antagonists: a review. J Cardiovasc Pharmacol. 1990;15(Suppl 4):S1–10.
128. Glock JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol. 1993;169:960–964.
129. Bal L, Thierry S, Brocas E, et al. Pulmonary edema induced by calcium-channel blockade for tocolysis. Anesth Analg. 2004;99:910–911.
130. Oei SG, Oei SK, Brolmann HA. Myocardial infarction during nifedipine therapy for preterm labor. N Engl J Med. 1999;340:154.
131. Blea CW, Barnard JM, Magness RR, et al. Effect of nifedipine on fetal and maternal hemodynamics and blood gases in the pregnant ewe. Am J Obstet Gynecol. 1997;176:922–930.
132. Ducsay CA, Thompson JS, Wu AT, Novy MJ. Effects of calcium entry blocker (nicardipine) tocolysis in rhesus macaques: fetal plasma concentrations and cardiorespiratory changes. Am J Obstet Gynecol. 1987;157:1482–1486.
133. Mari G, Kirshon B, Moise KJ Jr, et al. Doppler assessment of the fetal and uteroplacental circulation during nifedipine therapy for preterm labor. Am J Obstet Gynecol. 1989;161:1514–1518.
134. Pirhonen JP, Erkkola RU, Ekblad UU, Nyman L. Single dose of nifedipine in normotensive pregnancy: nifedipine concentrations, hemodynamic responses, and uterine and fetal flow velocity waveforms. Obstet Gynecol. 1990;76:807–811.
135. Tosone SR, Reves JG, Kissin I, et al. Hemodynamic responses to nifedipine in dogs anesthetized with halothane. Anesth Analg. 1983;62:903–908.
136. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol. 1994;101:262–263.
137. Niebyl JR, Witter FR. Neonatal outcome after indomethacin treatment for preterm labor. Am J Obstet Gynecol. 1986;155:747–749.
138. Kocsis JJ, Hernandovich J, Silver MJ, et al. Duration of inhibition of platelet prostaglandin formation and aggregation by ingested aspirin or indomethacin. Prostaglandins. 1973;3:141–144.
139. Moise KJ Jr, Huhta JC, Sharif DS, et al. Indomethacin in the treatment of premature labor: effects on the fetal ductus arteriosus. N Engl J Med. 1988;319:327–331.
140. Dudley DK, Hardie MJ. Fetal and neonatal effects of indomethacin used as a tocolytic agent. Am J Obstet Gynecol. 1985;151:181–184.
141. Vermillion ST, Scardo JA, Lashus AG, Wiles HB. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol. 1997;177:256–259.
142. Moise KJ Jr. Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol. 1993;168:1350–1353.
143. Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol. 2000;43:787–801.
144. Kirshon B, Moise KJ Jr, Mari G, Willis R. Long-term indomethacin therapy decreases fetal urine output and results in oligohydramnios. Am J Perinatol. 1991;8:86–88.
145. Kirshon B, Moise KJ Jr, Wasserstrum N, et al. Influence of short-term indomethacin therapy on fetal urine output. Obstet Gynecol. 1988;72:51–53.
146. Anderson RJ, Berl T, McDonald KM, Schrier RW. Prostaglandins: effects on blood pressure, renal blood flow, sodium and water excretion. Kidney Int. 1976;10:205–215.
147. Wurtzel D. Prenatal administration of indomethacin as a tocolytic agent: effect on neonatal renal function. Obstet Gynecol. 1990;76:689–692.
148. Loe SM, Sanchez-Ramos L, Kaunitz AM. Assessing the neonatal safety of indomethacin tocolysis: a systematic review with meta-analysis. Obstet Gynecol. 2005;106:173–179.
149. de Swiet M, Redman CW. Aspirin, extradural anaesthesia and the MRC Collaborative Low-dose Aspirin Study in Pregnancy (CLASP). Br J Anaesth. 1992;69:109–110.
150. Horlocker TT, Wedel DJ, Schroeder DR, et al. Preoperative antiplatelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth Analg. 1995;80:303–309.
151. Horlocker TT, Wedel DJ, Offord KP. Does preoperative antiplatelet therapy increase the risk of hemorrhagic complications associated with regional anesthesia? Anesth Analg. 1990;70:631–634.
152. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35:64–101.
153. Kleinman G, Nuwayhid B, Rudelstorfer R, et al. Circulatory and renal effects of beta-adrenergic-receptor stimulation in pregnant sheep. Am J Obstet Gynecol. 1984;149:865–874.
154. Berg G, Andersson RG, Ryden G. Beta-adrenergic receptors in human myometrium during pregnancy: changes in the number of receptors after beta-mimetic treatment. Am J Obstet Gynecol. 1985;151:392–396.
155. Nanda K, Cook LA, Gallo MF, Grimes DA. Terbutaline pump maintenance therapy after threatened preterm labor for preventing preterm birth. Cochrane Database Syst Rev. 2002;(4).
156. Benedetti TJ, Hargrove JC, Rosene KA. Maternal pulmonary edema during premature labor inhibition. Obstet Gynecol. 1982;59(Suppl 6):S33–S37.
157. Hatjis CG, Swain M. Systemic tocolysis for premature labor is associated with an increased incidence of pulmonary edema in the presence of maternal infection. Am J Obstet Gynecol. 1988;159:723–728.
158. Perry KG Jr, Morrison JC, Rust OA, et al. Incidence of adverse cardiopulmonary effects with low-dose continuous terbutaline infusion. Am J Obstet Gynecol. 1995;173:1273–1277.
159. Lotgering FK, Lind J, Huikeshoven FJ, Wallenburg HC. Elevated serum transaminase levels during ritodrine administration. Am J Obstet Gynecol. 1986;155:390–392.
160. Nair GV, Ghosh AK, Lewis BV. Letter: Bowel distension during treatment of premature labour with beta-receptor agonists. Lancet. 1976;1:907.
161. Rosene KA, Featherstone HJ, Benedetti TJ. Cerebral ischemia associated with parenteral terbutaline use in pregnant migraine patients. Am J Obstet Gynecol. 1982;143:405–407.
162. Catanzarite VA, McHargue AM, Sandberg EC, Dyson DC. Respiratory arrest during therapy for premature labor in a patient with myasthenia gravis. Obstet Gynecol. 1984;64:819–822.
163. Schoenfeld A, Joel-Cohen SJ, Duparc H, Levy E. Emergency obstetric anaesthesia and the use of beta2-sympathomimetic drugs. Br J Anaesth. 1978;50:969–971.
164. Crowhurst JA. Salbutamol, obstetrics and anaesthesia: a review and case discussion. Anaesth Intensive Care. 1980;8:39–43.
165. Ravindran R, Viegas OJ, Padilla LM, LaBlonde P. Anesthetic considerations in pregnant patients receiving terbutaline therapy. Anesth Analg. 1980;59:391–392.
166. Shin YK, Kim YD. Ventricular tachyarrhythmias during cesarean section after ritodrine therapy: interaction with anesthetics. South Med J. 1988;81:528–530.
167. Suppan P. Tocolysis and anaesthesia for caesarean section. Br J Anaesth. 1982;54:1007.
168. Chestnut DH, Pollack KL, Thompson CS, et al. Does ritodrine worsen maternal hypotension during epidural anesthesia in gravid ewes? Anesthesiology. 1990;72:315–321.
169. Slater RM, From RP, Sum Ping JS, Pank JR. Changes in plasma potassium and neuromuscular blockade following suxamethonium in patients pre-treated with terbutaline. Eur J Anaesthesiol. 1991;8:281–286.
170. Phaneuf S, Asboth G, MacKenzie IZ, et al. Effect of oxytocin antagonists on the activation of human myometrium in vitro: atosiban prevents oxytocin-induced desensitization. Am J Obstet Gynecol. 1994;171:1627–1634.
171. Goodwin TM, Millar L, North L, et al. The pharmacokinetics of the oxytocin antagonist atosiban in pregnant women with preterm uterine contractions. Am J Obstet Gynecol. 1995;173:913–917.
172. Goodwin TM, Valenzuela GJ, Silver H, Creasy G. Dose ranging study of the oxytocin antagonist atosiban in the treatment of preterm labor. Atosiban Study Group. Obstet Gynecol. 1996;88:331–336.
173. Moutquin JM, Sherman D, Cohen H, et al. Double-blind, randomized, controlled trial of atosiban and ritodrine in the treatment of preterm labor: a multicenter effectiveness and safety study. Am J Obstet Gynecol. 2000;182:1191–1199.
174. Worldwide Atosiban versus Beta-agonists Study Group. Effectiveness and safety of the oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of preterm labour. BJOG. 2001;108:133–142.
175. European Atosiban Study Group. The oxytocin antagonist atosiban versus the beta-agonist terbutaline in the treatment of preterm labor. A randomized, double-blind, controlled study. Acta Obstet Gynecol Scand. 2001;80:413–422.
176. Salim R, Garmi G, Nachum Z, et al. Nifedipine compared with atosiban for treating preterm labor: a randomized controlled trial. Obstet Gynecol. 2012;120:1323–1331.
177. Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev. 2005;(3).
178. Beall MH, Edgar BW, Paul RH, Smith-Wallace T. A comparison of ritodrine, terbutaline, and magnesium sulfate for the suppression of preterm labor. Am J Obstet Gynecol. 1985;153:854–859.
179. Hollander DI, Nagey DA, Pupkin MJ. Magnesium sulfate and ritodrine hydrochloride: a randomized comparison. Am J Obstet Gynecol. 1987;156:631–637.
180. Chau AC, Gabert HA, Miller JM Jr. A prospective comparison of terbutaline and magnesium for tocolysis. Obstet Gynecol. 1992;80:847–851.
181. Chestnut DH, Thompson CS, McLaughlin GL, Weiner CP. Does the intravenous infusion of ritodrine or magnesium sulfate alter the hemodynamic response to hemorrhage in gravid ewes? Am J Obstet Gynecol. 1988;159:1467–1473.
182. Reynolds JD, Chestnut DH, Dexter F, et al. Magnesium sulfate adversely affects fetal lamb survival and blocks fetal cerebral blood flow response during maternal hemorrhage. Anesth Analg. 1996;83:493–499.
183. Vincent RD Jr, Chestnut DH, Sipes SL, et al. Magnesium sulfate decreases maternal blood pressure but not uterine blood flow during epidural anesthesia in gravid ewes. Anesthesiology. 1991;74:77–82.
184. De Vore JS, Asrani R. Magnesium sulfate prevents succinylcholine-induced fasciculations in toxemic parturients. Anesthesiology. 1980;52:76–77.
185. James MF, Cork RC, Dennett JE. Succinylcholine pretreatment with magnesium sulfate. Anesth Analg. 1986;65:373–376.
186. Thompson SW, Moscicki JC, DiFazio CA. The anesthetic contribution of magnesium sulfate and ritodrine hydrochloride in rats. Anesth Analg. 1988;67:31–34.