TABLE 36-5
Serious Maternal Complications in a Series of 442 Patients with Hemolysis, Elevated Liver Enzymes, and Low Platelets (HELLP) Syndrome
| Complication* | No. Patients | Percent |
| Disseminated intravascular coagulopathy | 92 | 21 |
| Placental abruption | 69 | 16 |
| Acute renal failure | 33 | 8 |
| Severe ascites | 32 | 8 |
| Pulmonary edema | 26 | 6 |
| Pleural effusions | 26 | 6 |
| Cerebral edema | 4 | 1 |
| Retinal detachment | 4 | 1 |
| Laryngeal edema | 4 | 1 |
| Subcapsular liver hematoma | 4 | 1 |
| Acute respiratory distress syndrome | 3 | 1 |
| Maternal death | 4 | 1 |
* Some women had multiple complications.
Modified from Sibai BM, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol 1993; 169:1000-6.
Because of a lack of universally accepted diagnostic criteria for HELLP syndrome, its incidence cannot be determined accurately. The existence of a subset of preeclampsia complicated by abnormal peripheral blood smear, abnormal liver function tests, and thrombocytopenia has been recognized for decades; in 1982, Weinstein231 described a series of 29 cases and coined the acronym HELLP. Women who do not demonstrate one or more of these clinical features are said to have “partial” HELLP syndrome.
Hemolysis, defined as the presence of microangiopathic hemolytic anemia, is the classic hallmark of HELLP syndrome; peripheral blood smear demonstrates schistocytes, burr cells, and echinocytes.232 Common histopathologic findings are periportal hepatic necrosis and hemorrhage.233 Sibai232 has proposed standardized laboratory diagnostic criteria, as outlined in Table 36-6. Maternal signs and symptoms include right upper quadrant or epigastric pain, nausea and vomiting, headache, hypertension, and proteinuria. Notably, clinical presentation varies; 12% to 18% of women may be normotensive, and proteinuria is absent in approximately 13% of affected women. Diagnosis can be especially challenging because numerous medical and surgical disorders, including acute fatty liver of pregnancy, hemolytic-uremic syndrome, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura, and lupus, can mimic HELLP syndrome (Box 36-4). Pregnant women likely to have preeclampsia, but who demonstrate atypical symptoms, should be screened with a complete blood cell count, platelet count, and liver enzyme assessment.
TABLE 36-6
Diagnostic Criteria for Hemolysis, Elevated Liver Enzymes, and Low Platelets (HELLP) Syndrome
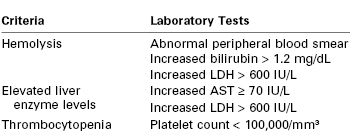
LDH, lactic dehydrogenase; AST, aspartate aminotransferase.
Modified from Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol 1990; 162:311-6.
In general, patients with HELLP syndrome are not considered candidates for expectant management155; however, deferred delivery for 24 to 48 hours to allow for corticosteroid administration to accelerate fetal lung maturity may be appropriate for women less than 34 weeks' gestation if the maternal and fetal condition remain stable.4 Women with HELLP syndrome who have not yet reached 34 weeks' gestation should be managed in a tertiary care facility with a neonatal intensive care unit capable of caring for a compromised preterm neonate.4 Clinical management is similar to that for severe preeclampsia and includes intravenous magnesium sulfate for seizure prophylaxis and antihypertensive medications to maintain a systolic blood pressure below 160 mm Hg and a diastolic blood pressure below 110 mm Hg.232 The first priority is to assess and stabilize the maternal condition, with particular attention given to hypertension and coagulation abnormalities. Next, the fetal condition should be assessed with FHR monitoring, Doppler ultrasonography of fetal vessels, a biophysical profile, or several of these options.
The platelet count can fall precipitously in the presence of HELLP syndrome, and it should be evaluated before the administration of neuraxial anesthesia. Women with a platelet count less than 50,000/mm3 are at significantly increased risk for bleeding,234 and general anesthesia is the method of choice for cesarean delivery. Data suggest treatment with dexamethasone may improve the platelet count in women with HELLP.207 If treatment with dexamethasone improves the patient's platelet count, the decision as to whether to use neuraxial anesthesia must weigh the risk of recurrent thrombocytopenia against the risk for a difficult airway and hypertension during induction of general anesthesia.
Platelet transfusions are indicated in the presence of significant bleeding and in all parturients with a platelet count less than 20,000/mm3. For women with a platelet count less than 40,000/mm3 who are scheduled for cesarean delivery, the preincision administration of 6 to 10 units of pooled random-donor platelets (or 1 to 2 units of apheresis platelets) has been recommended.235 The risk for postpartum hemorrhage is significantly increased in patients with HELLP syndrome; at least 2 red blood cell units should be type and crossmatched and large-bore intravenous access obtained.
Rupture of a subcapsular hematoma of the liver is a life-threatening complication of HELLP syndrome and severe preeclampsia236 that can manifest as abdominal pain, nausea and vomiting, and headaches; the pain worsens over time and becomes localized to the epigastric area or right upper quadrant. Hypotension and shock typically develop, and the liver is enlarged and tender.237 Diagnosis is confirmed with ultrasonography, computed tomography (CT), or magnetic resonance imaging of the liver (Figure 36-7). Subcapsular hematoma rupture with shock is a surgical emergency that requires immediate multidisciplinary treatment that includes intravascular volume resuscitation and blood and plasma transfusions.237 In some circumstances, selective arterial embolization by an interventional radiologist might be useful.236,238,239 Patients with fulminant hepatic failure may require liver transplantation.236 Prompt surgical intervention and refinements in surgical technique have substantially reduced the maternal mortality rate associated with spontaneous hepatic rupture to less than 20% in the past decade.236 The most common causes of death are coagulopathy and exsanguination.230,240
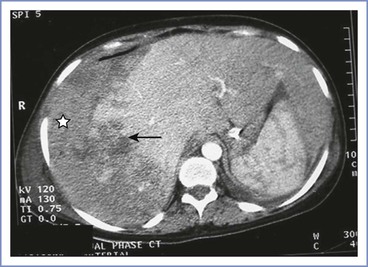
FIGURE 36-7 Contrast-enhanced computed tomography scan showing a large area of parenchymal hemorrhage with hepatic rupture (arrow) and subcapsular hematoma (star) in the right lobe of the liver. (From Das CJ, Srivastava DN, Debnath J, et al. Endovascular management of hepatic hemorrhage and subcapsular hematoma in HELLP syndrome. Indian J Gastroenterol 2007; 26:244-5.)
Conservative management is recommended for subcapsular hematoma or intraparenchymal hemorrhage without capsular rupture in stable women.235 Careful monitoring with ultrasonography or CT scan or both is required. An important component of conservative management is to avoid all potential trauma to the liver, including seizures, vomiting, and manual palpation of the abdomen. Patient transport and transfers should be conducted with care to avoid maneuvers that might result in rupture of a hematoma.
Anesthetic Management
The anesthetic management of the woman with preeclampsia without severe features differs little from the management of a healthy pregnant woman. However, the potential for rapid progression to the severe form of the disease mandates careful observation of the patient. The anesthesia provider must recognize the unpredictability of the development and progression of severe preeclampsia and should be prepared at all times for immediate cesarean delivery.
Preanesthetic Evaluation
The preanesthetic assessment of women with confirmed or suspected preeclampsia should focus on the airway examination, maternal hemodynamic and coagulation status, and fluid balance.
Airway.
Generalized edema can involve the airway and obscure visualization of anatomic landmarks at laryngoscopy.123 The anesthesia provider should anticipate the possibility of difficult airway management (see Chapter 30).
Hemodynamic Monitoring.
Systemic arterial blood pressure can change rapidly in women with severe preeclampsia, both as a result of disease progression and in response to the administration of intravenous fluids and antihypertensive drugs. In addition, preeclampsia is associated with variable degrees of intravascular volume depletion and the clinical assessment of intravascular volume status can be difficult. Therefore, the use of invasive vascular monitoring may be useful in the management of some women with severe preeclampsia.
The most frequent indications for radial artery catheter insertion are (1) poorly controlled maternal blood pressure; (2) need for frequent arterial blood gas measurements, especially in the context of pulmonary edema; (3) planned use of a rapid-acting vasodilator (e.g., sodium nitroprusside, nitroglycerin, nicardipine infusion); (4) use of calculated systolic pressure variation (SPV)241 to estimate intravascular volume status; and (5) need for continuous blood pressure monitoring during the induction of and emergence from general anesthesia in hypertensive women with severe preeclampsia.
Invasive central monitoring has been advocated in the assessment of oliguria and to monitor patient responses to fluid administration, but in practice it is rarely used. When invasive central monitoring is desired, a central venous pressure (CVP) catheter is adequate in the majority of cases; a pulmonary artery catheter is generally only used in patients with severe cardiac disease. The placement of an invasive CVP or pulmonary artery catheter is not a benign procedure. Well-recognized risks include arterial trauma, pneumothorax, venous air embolism, neuropathy, and cardiac arrhythmias. Additional risks for an indwelling pulmonary artery catheter include potentially fatal pulmonary artery hemorrhage, thromboembolism, sepsis, and endocardial damage.242
The 1991 to 1993 Report on Confidential Enquiries into Maternal Deaths in the United Kingdom described the postpartum death of a woman after several unsuccessful attempts at internal jugular line placement and a likely carotid artery puncture.243 Despite digital pressure and application of a pressure dressing after vessel puncture, she developed neck tightness and dyspnea 4 hours later. Attempts at reintubation and resuscitation were unsuccessful, and she died. In the 2003 to 2005 CEMACH report, an anesthesia-related maternal death occurred in a woman with fulminant preeclampsia and HELLP syndrome who developed a large right hemothorax after a subclavian line insertion.221 Because both immediate and delayed244 maternal deaths have been attributed to the use of invasive central catheters, insertion of these catheters should be done only after careful consideration of risks and benefits.
Further, the timing of the use of invasive central monitoring is important and requires clinical judgment. Many patients are better served by immediate transfer to the operating room for delivery rather than delaying delivery for central line placement. In one study of a critical care unit in an academic hospital, the time from the decision to proceed with placement of a pulmonary artery catheter until the first pressure measurement was obtained was always more than 45 minutes.245 Insertion time would likely exceed 45 minutes in most labor and delivery settings where the procedure is rarely performed and qualified assistance may not be available. In the majority of cases, the preoperative placement of an invasive central line will not change the intraoperative anesthetic management. Many obstetric care providers prefer to proceed immediately to delivery in women with persistent oliguria and to optimize fluid status after delivery.
Transthoracic echocardiography (TTE) can be used in place of invasive monitoring to quantify cardiac function and volume status.138 In the hands of experienced practitioners, TTE may be useful in guiding fluid management for women with severe preeclampsia at risk for pulmonary edema and with oliguria.246 Lung ultrasonography may also be useful in severe preeclampsia to diagnosis the presence of pulmonary edema.247 Pulse waveform analysis has been suggested as a minimally invasive method to measure cardiac output in patients with severe preeclampsia; cardiac output measurements using this approach closely match those obtained from thermodilution in preeclamptic patients.248
In summary, the presence of severe preeclampsia per se is not an indication for CVP or pulmonary artery pressure monitoring. There is no indication for central hemodynamic monitoring that is unique to preeclampsia. Preeclampsia is a disorder of the peripheral circulation, not the central circulation. Indications for invasive central monitoring are similar to those in other multisystem disorders such as severe sepsis, multisystem organ dysfunction, pulmonary edema, congenital heart disease, and cardiomyopathy. TTE and arterial waveform monitoring may provide less invasive means of assessing hemodynamic parameters in these patients. Some women will require transfer to an intensive care unit for specialized nursing care and management directed by a critical care medicine specialist.
Neuraxial Analgesia for Labor and Delivery
During labor, early administration of neuraxial analgesia is recommended for several reasons: (1) to avoid general anesthesia and the possibility of airway catastrophe and marked hypertension with laryngoscopy in the event of emergency cesarean delivery, (2) to optimize the timing of epidural catheter placement in the setting of a declining platelet count, and (3) to obtain the beneficial effects of neuraxial analgesia on uteroplacental perfusion.
Continuous lumbar epidural analgesia or combined-spinal epidural (CSE) analgesia are the preferred methods of pain management during labor in women with preeclampsia. Advantages include (1) provision of high quality analgesia, which attenuates the hypertensive response to pain249; (2) a reduction in levels of circulating catecholamines and stress-related hormones250; (3) possible improvement in intervillous blood flow251; and (4) provision of a means for administration of local anesthetic for emergency cesarean delivery, thus obviating the need for general anesthesia with its attendant risks.
One disadvantage of the CSE technique is that epidural catheter function cannot be fully evaluated until after resolution of the intrathecal analgesia. For this reason, some anesthesia providers avoid the CSE technique in favor of a standard epidural technique in women with severe preeclampsia who have an increased risk for emergency cesarean delivery. Use of a standard epidural technique allows for verification of catheter function within 15 to 20 minutes.
Continuous epidural infusion of local anesthetic has been used in the antepartum period to optimize uteroplacental blood flow in the hope of prolonging pregnancy and avoiding preterm delivery in preeclamptic women remote from term. Kanayama et al.252 studied 20 severely preeclamptic women at 28 to 32 weeks' gestation who were assigned by physician choice to receive either long-term epidural bupivacaine combined with routine supportive management or routine supportive management alone. Gestational age at delivery and birth weight were greater in the treatment group, and maternal blood pressure and platelet count were also improved, compared with the routine management group. Although promising, this technique remains unproven, and randomized controlled trials are needed to verify that antepartum epidural local anesthetic infusion may facilitate expectant management of women with preeclampsia remote from term.
For the most part, the clinical administration of epidural analgesia to women with preeclampsia does not differ from that in healthy pregnant women without preeclampsia (see Chapter 23). The choice of local anesthetic, method of epidural space identification, and maintenance of analgesia are not affected by the presence of preeclampsia. However, four special considerations exist in preeclamptic women: (1) assessment of coagulation status, (2) intravenous hydration before the epidural administration of a local anesthetic, (3) treatment of hypotension, and (4) use of epinephrine-containing local anesthetic solutions.
Coagulation Status.
Platelets contribute to coagulation and hemostasis in two important ways. First, their adhesive and cohesive functions lead to the formation of the hemostatic plug. Second, they activate the coagulation process by exposing a phospholipid surface and acting as a catalytic site for subsequent coagulation and consolidation of the initial platelet plug. Activated platelets release adenosine diphosphate, serotonin, thromboxane A2, and other adhesive proteins, coagulation factors, and growth factors.253
Women with preeclampsia without severe features are usually hypercoagulable relative to women with an uncomplicated pregnancy and should not be denied neuraxial labor analgesia.140 Women with severe preeclampsia (particularly those with HELLP syndrome) may develop thrombocytopenia, which increases the risk for bleeding into the epidural or spinal space with a neuraxial procedure (see Chapter 32). Neuraxial hematoma formation can result in permanent neurologic sequelae. Therefore, documentation of the platelet count is necessary before provision of epidural analgesia in women with severe preeclampsia. The incidence of neuraxial hematoma cannot be precisely determined because not all cases are reported and because there is no accurate method to determine the denominator of all preeclamptic women who have received neuraxial anesthesia. That said, the incidence of epidural hematoma in contemporary obstetric anesthesia practice is exceptionally low.254
In the past, a platelet count greater than or equal to 100,000/mm3 was considered necessary for the safe administration of neuraxial anesthesia. This threshold probably originated from the results of a 1972 study that correlated platelet counts with bleeding times.255 Critical appraisal of 1,083 human studies concluded that the bleeding time is not a reliable method of assessing the risk for bleeding for a single individual.256 Coagulopathy is rare in preeclamptic women with a platelet count exceeding 100,000/mm3, and in the absence of other risk factors for coagulopathy, neuraxial block placement is considered safe without further coagulation testing.156
Currently, many anesthesiologists agree that neuraxial procedures may be initiated in pregnant women without other risk factors if the platelet count is higher than 80,000/mm3.234 There is general consensus among anesthesia providers that a platelet count less than 50,000/mm3 precludes the administration of neuraxial anesthesia. For women with a platelet count between 50,000 and 80,000/mm3, the risks and benefits of neuraxial anesthesia must be weighed against the risks of general anesthesia for the individual patient if emergency cesarean delivery is required, including whether anatomic features of the patient's airway are favorable. Two additional considerations include the trend in the platelet count and any coexisting coagulopathy.
In certain cases, the platelet count decreases rapidly and the nadir in the platelet count cannot be identified prospectively. If serial platelet counts are stable and remain in the normal range, platelet count measurement every 24 to 48 hours is adequate to monitor women undergoing expectant management of severe preeclampsia remote from term.4,161 Once a decision is made to induce labor, platelet count determination at least every 6 hours will ensure a timely measurement has been obtained at the time of request for neuraxial analgesia. If the platelet count is low (80,000 to 100,000/mm3), early epidural catheter insertion is recommended in anticipation of worsening thrombocytopenia. If an epidural catheter is inserted before labor pain becomes significant, dilute solutions of local anesthetic may be infused at a low rate.
If the platelet count is less than 100,000/mm3, other hemostatic abnormalities, including prolonged PT and aPTT and hypofibrinogenemia, may be present.156 Further coagulation studies may be useful, particularly if risk factors for DIC are present (e.g., placental abruption, HELLP syndrome).156 Thus, in the presence of thrombocytopenia or abnormal results of liver function tests, the PT and aPTT should be assessed before the initiation of neuraxial anesthesia.257
Viscoelastic monitors of coagulation may expedite decision-making about neuraxial block administration for women with preeclampsia-related hemostatic dysfunction. Although thromboelastography (TEG) has shown some promise in the assessment of coagulation status in pregnant patients with thrombocytopenia,140 it has been criticized for its inability to diagnose specific coagulation defects, particularly for impairments in primary hemostasis.258 Platelet function analysis (PFA-100) appears to be more sensitive to coagulation dysfunction in severe preeclampsia.142 However, with both TEG and PFA, the ability to predict the risk for epidural hematoma after the administration of neuraxial anesthesia is unproven and requires further study.259
The risk for epidural hematoma formation exists not only at the time of epidural catheter placement but also at the time of its removal. In patients with thrombocytopenia, the catheter should not be withdrawn from the epidural space until there is evidence of an acceptable (and increasing) platelet count. A platelet count of 75,000 to 80,000/mm3 seems reasonable for epidural catheter removal. The platelet count in women with HELLP syndrome usually reaches a nadir on the second or third postpartum day and then gradually returns to the patient's normal baseline.
If the decision is made to proceed with a neuraxial technique when the platelet count is less than 100,000/mm3, the following suggestions may help reduce the risk for epidural hematoma and its sequelae:
1. The most skilled anesthesia provider available should perform the neuraxial procedure to minimize the number of needle passes and subsequent bleeding.
2. A spinal technique may be preferable to an epidural technique (when appropriate) because of the smaller needle size, although supporting data are lacking.
3. Use of a flexible wire-embedded epidural catheter may reduce epidural vein trauma.
4. The patient should be carefully monitored after delivery for neurologic signs that may signal bleeding into the epidural space.
5. The platelet count should be checked for evidence of a return toward normal measurements (at least 75,000 to 80,000/mm3) before removal of the epidural catheter. Epidural vein trauma at the time of catheter discontinuation can result in epidural bleeding and perhaps epidural hematoma.
6. Imaging studies and neurologic or neurosurgical consultation should be obtained immediately if there is any question of an epidural hematoma. Prompt surgical intervention may be required to avoid permanent neurologic injury.
Intravenous Hydration.
In the past, when high concentrations of local anesthetic solution (e.g., 0.25% to 0.5% bupivacaine) were administered during labor, intravenous crystalloid hydration preceded epidural local anesthetic administration to prevent or ameliorate hypotension. In contemporary practice, lower concentrations of local anesthetic are used (e.g., 0.0625% to 0.125% bupivacaine in combination with an opioid), hypotension is less common, and fluid administration at the time of analgesia initiation is of less clinical importance. Furthermore, the rapid administration of a large bolus of intravenous fluid (preload) results in only a transient increase in CVP and has little impact on the risk for hypotension.260 Although the use of a fluid bolus has decreased with current techniques for labor analgesia, careful attention to intravenous fluid infusion rates is necessary in women with severe preeclampsia because of the increased risk for pulmonary edema in these patients.
Treatment of Hypotension.
A retrospective study found that parturients with severe preeclampsia were more susceptible to hypotension after initiation of epidural labor analgesia than healthy parturients.261 The hypotension was associated with an increase in FHR abnormalities,261 suggesting the need for close attention to maternal blood pressure at the time of initiation of analgesia. Women with preeclampsia may be treated with phenylephrine or ephedrine (although the latter is no longer the agent of choice in healthy women—see Chapter 26). There is an often-expressed concern that severely preeclamptic women may have an exaggerated response to vasopressors that might result in a sharp increase in blood pressure.262,263 However, supportive data are lacking. The anesthesia provider should initiate treatment with low doses of phenylephrine (25 to 50 µg) or ephedrine (5 to 10 mg) to assess maternal blood pressure response before administration of a larger dose. With careful dosing, increased sensitivity to vasopressors is rarely a clinical problem.
Epinephrine.
It has been suggested that local anesthetic solutions containing epinephrine (including the standard epinephrine-containing test dose) should be avoided during the administration of epidural analgesia in preeclamptic women. This concern arises from observations that preeclamptic women exhibit an increased sensitivity to vasopressors, including angiotensin II,264,265 norepinephrine and epinephrine,133,266 and a thromboxane A2-mimetic agent.267 In addition, clinical studies have demonstrated that smaller doses of ephedrine and phenylephrine are required to restore maternal blood pressure during spinal anesthesia in preeclamptic women compared with healthy women.262,263,268,269 One case report described a hypertensive crisis in a preeclamptic woman after the incremental administration of 30 mL of freshly mixed 2% lidocaine with epinephrine 5 µg/mL for planned cesarean delivery.270 However, the onset and duration of hypertension were atypical and a drug error could not be excluded.270 In contrast, several other case series have used the same solution without adverse effects in women with preeclampsia.271,272
No randomized controlled trials have assessed the effects of epidural epinephrine in women with severe preeclampsia. In the absence of malignant hypertension, epinephrine is unlikely to pose a significant risk for hypertensive crisis, given the absence of confirmed reports after decades of its use in obstetric anesthesia practice. Although not necessarily harmful for women with preeclampsia, the use of epinephrine in epidural test doses or analgesic solutions may not be particularly helpful. Patients who have received beta-adrenergic receptor antagonists (e.g., labetalol) do not demonstrate the typical tachycardic response to intravascular administration of epinephrine.273 This lack of response will decrease the sensitivity of the epinephrine test dose to detect intravascular administration of local anesthetic solution, and alternative testing strategies to detect intravascular catheter location may be preferred.274 The addition of epinephrine to local anesthetic solutions results in a modest local anesthetic-sparing effect, at best,275 and increases the density of motor blockade.276 Finally, consideration should be given to the consequences of an unintentional intravascular injection of an epinephrine-containing test dose in a patient with baseline hypertension.
Anesthesia for Cesarean Delivery
The administration of neuraxial anesthesia for cesarean delivery in women with preeclampsia does not differ greatly from administration in healthy pregnant women (see Chapter 26). Hepatic dysfunction can result in reduced drug clearance but has little clinical impact on choice of anesthetic or analgesic agents.
The choice of local anesthetic, method of epidural space identification, and maintenance of anesthesia are not affected by the presence of preeclampsia. However, there are three special considerations in preeclamptic women undergoing cesarean delivery: (1) choice of anesthetic technique, (2) technique for induction of general anesthesia, and (3) the interaction between magnesium sulfate and nondepolarizing muscle relaxants.
Neuraxial Anesthesia.
In the Seventh and Eighth Reports of the Confidential Enquiries into Maternal Deaths from the United Kingdom,212,221 the leading cause of death in women with preeclampsia was intracranial hemorrhage. Disadvantages of general anesthesia in the presence of preeclampsia include the risk for intracranial hemorrhage from the hypertensive response to both tracheal intubation and extubation. In a study involving more than 300,000 women in Taiwan, general anesthesia for cesarean delivery was associated with a greater than twofold increase in the risk for stroke after adjusting for confounders compared with neuraxial anesthesia.277 Neuraxial anesthesia also avoids the possibility of difficult tracheal intubation secondary to airway edema. Therefore, neuraxial anesthesia is preferred whenever clinical circumstances permit its use.
The traditional view was that spinal anesthesia is relatively contraindicated in severe preeclampsia because of the possibility of marked hypotension as a result of the rapid onset of spinal anesthesia–induced sympathetic blockade. However, data that have emerged over the past decade suggest that this concern is not supported by evidence, and that both spinal and epidural anesthesia are reasonable anesthetic modalities for women with severe preeclampsia. Wallace et al.278 randomized 80 women with severe preeclampsia who required cesarean delivery to receive general, epidural, or CSE anesthesia. Notably, the initial spinal dose in the CSE group (hyperbaric bupivacaine 11.25 mg) is a dose comparable to that often used for a single-shot spinal technique. There was no significant difference between the CSE and epidural anesthesia groups in maternal mean arterial pressure over time. Another small prospective study randomized women with severe preeclampsia to receive either spinal or epidural anesthesia, with similar results.279 Hood and Curry280 retrospectively reviewed cesarean delivery records for 138 women with severe preeclampsia who received either spinal or epidural anesthesia and found that the lowest mean blood pressure measurements did not differ between groups. Because of the retrospective study design, the possibility that the groups were dissimilar cannot be excluded (i.e., the anesthesia providers may have chosen to administer epidural anesthesia to the more severely ill women). Nonetheless, the expected marked hypotension after spinal anesthesia did not occur. These studies lend support to the safety of spinal anesthesia in women with severe preeclampsia.
In two prospective cohort studies of women undergoing cesarean delivery, Aya et al.262,268 compared women with severe preeclampsia to healthy pregnant women (both preterm and at term) and found that the risk for significant spinal anesthesia–induced hypotension (defined as requiring the administration of ephedrine) was significantly lower in the preeclampsia group than in the control groups. The authors speculated that the known increased vascular sensitivity to vasoconstrictors may explain the infrequent incidence of hypotension after spinal anesthesia and the ease with which mean arterial blood pressure can be restored to baseline with small doses of vasopressor.
A randomized multicenter study281 comparing the hemodynamic effects of spinal anesthesia with epidural anesthesia for cesarean delivery in women with severe preeclampsia found that significantly more women in the spinal anesthesia group experienced hypotension. However, the duration of hypotension was less than 1 minute in both groups and, although there was more ephedrine use in the spinal group than the epidural group, hypotension was easily treated in both groups. In addition, there was no significant difference in neonatal outcome between infants whose mothers received spinal anesthesia compared with those whose mothers received epidural anesthesia.282 Another study suggested that spinal anesthesia has little effect on cardiac output in severely preeclamptic women.263 These data, taken together, suggest that the use of spinal anesthesia for cesarean delivery in women with severe preeclampsia is appropriate.
General Anesthesia.
General anesthesia is less desirable than neuraxial anesthesia because of (1) the possibility of difficult tracheal intubation secondary to airway edema and (2) the transient but severe hypertension that accompanies tracheal intubation and extubation. Nonetheless, there are situations in which general anesthesia is the best anesthetic option. Clinical indications include severe ongoing maternal hemorrhage, sustained fetal bradycardia with a reassuring maternal airway examination, and severe thrombocytopenia or other coagulopathy, or a combination of these indications. The platelet count can fall dramatically with rapidly progressing severe preeclampsia or HELLP syndrome and may mandate administration of general anesthesia. Major placental abruption, intrauterine fetal demise, and preeclampsia all increase the risk for DIC. The safe administration of general anesthesia in women with preeclampsia requires an advanced state of readiness and careful preparation.
Once the decision has been made to proceed with general anesthesia, the anesthesia provider faces three specific challenges: (1) the potential difficulty of securing the airway, (2) the hypertensive response to direct laryngoscopy and tracheal intubation, and (3) the effects of magnesium sulfate on neuromuscular transmission and uterine tone. A suggested technique for the administration of general anesthesia is outlined in Box 36-5.
Airway Considerations.
Before proceeding with general anesthesia, careful airway examination is mandatory. Airway edema may be present even with a relatively reassuring airway examination; thus, many anesthesia providers try to avoid emergency administration of general anesthesia if there is any suspicion of a difficult airway. Endotracheal tubes in various sizes and difficult airway equipment should be immediately available (see Chapter 30). In unusually difficult situations, it may be prudent to have a surgeon immediately available to establish a surgical airway, if needed. One of the dangers of repeated tracheal intubation attempts is the risk for traumatic bleeding in the airway, which may make ventilation difficult or even impossible. It is wise to avoid repeated attempts and proceed with insertion of a supraglottic airway device (e.g., laryngeal mask airway) before the airway is irretrievably lost. Because the supraglottic airway devices do not protect the patient from pulmonary aspiration of gastric contents, the obstetrician should be encouraged to complete the procedure as quickly as possible.
If indicated, an awake fiberoptic tracheal intubation should be used to secure the airway. Ideally, invasive blood pressure monitoring should be initiated before tracheal intubation and the induction of general anesthesia in patients with severe preeclampsia so that hypertension can be promptly recognized and treated. Effective topical anesthesia of the airway with nebulized or atomized lidocaine can enhance patients' comfort and decrease the hypertensive response to airway manipulation. Airway injections (including glossopharyngeal nerve blocks and transtracheal injections) are usually best avoided because of coagulopathy in this population.
Hypertensive Response to Laryngoscopy.
The hemodynamic instability associated with rapid-sequence induction and tracheal intubation presents a serious problem. The transient but severe hypertension that may accompany tracheal intubation can result in cerebral hemorrhage or pulmonary edema, both potentially fatal complications. Continuous arterial blood pressure monitoring is required for severely hypertensive women to monitor the effects of antihypertensive drugs administered before and after tracheal intubation and to allow rapid detection of adverse hemodynamic responses to laryngoscopy.
Medications that have been used to blunt the hemodynamic response to laryngoscopy include labetalol, esmolol, nitroglycerin, sodium nitroprusside, and remifentanil.283-287 The goal of treatment is to reduce the arterial blood pressure to approximately 140/90 mm Hg before the induction of general anesthesia and to maintain the systolic blood pressure between 140 to 160 mm Hg and the diastolic blood pressure between 90 to 100 mm Hg throughout laryngoscopy and tracheal intubation.166 If possible, the FHR should be monitored during intravenous antihypertensive therapy.
Most anesthesia providers consider labetalol to be the drug of choice for attenuating the hypertensive response to laryngoscopy in women with severe preeclampsia. Ramanathan et al.285 compared intravenously administered labetalol with no treatment in a randomized study of preeclamptic women who received general anesthesia for cesarean delivery. Mean arterial blood pressure increased after tracheal intubation in both study groups, but the hypertensive response was significantly less pronounced in the labetalol group. Women in the control group also developed tachycardia (in response to laryngoscopy and tracheal intubation), which did not occur in the labetalol group. Labetalol can be administered using either a bolus technique or a continuous intravenous infusion, or both.
There is also evidence of safe short-term administration of esmolol in this setting. A randomized double-blind study of 80 hypertensive women presenting for cesarean delivery demonstrated that intravenous esmolol—in doses as high as 2 mg/kg—can be safely used to dampen the hemodynamic response to laryngoscopy and tracheal intubation.288
Nitroglycerin has many desirable properties for blunting the hypertensive response to tracheal intubation. It is a direct vasodilator with a rapid onset, is rapidly metabolized, and has no apparent maternal or fetal toxicity. In a randomized controlled trial, Hood et al.284 administered intravenous nitroglycerin (200 µg/mL), which decreased mean arterial blood pressure by approximately 20%, before the induction of general anesthesia. Compared with women who did not receive nitroglycerin, the maximal blood pressure with tracheal intubation was significantly lower in the nitroglycerin group. Both Apgar scores and umbilical cord blood gas and acid-base measurements were similar in the two groups.
Sodium nitroprusside infusions have also been used to attenuate hemodynamic responses to tracheal intubation in women with severe preeclampsia. An intravenous infusion can be initiated at 0.5 µg/kg/min and titrated to blood pressure response. Short-term infusions are considered safe for the fetus.
The short-acting opioid remifentanil is rapidly metabolized by both mother and neonate by nonspecific blood and tissue esterases and has been administered to preeclamptic women. A clear advantage of remifentanil compared with other opioids is the rapid onset and short duration of the drug; the limited duration of action should not interfere with the resumption of spontaneous ventilation if tracheal intubation is unsuccessful. Ngan Kee et al.287 randomly allocated 40 pregnant women without preeclampsia who required general anesthesia for cesarean delivery to receive either a one-time intravenous dose of remifentanil 1 µg/kg or saline immediately before induction of anesthesia. The primary outcome was the maximum increase in systolic blood pressure (compared with a baseline measurement). Administration of remifentanil significantly decreased the maximum increase in systolic blood pressure. However, remifentanil crosses the placenta, and two neonates in the remifentanil group required naloxone administration for poor respiratory effort. Park et al.289 randomized 48 patients with severe preeclampsia to receive remifentanil 0.5 µg/kg or 1 µg/kg before tracheal intubation. Both doses prevented a hypertensive response, but three patients treated with the higher dose required ephedrine to treat hypotension. Apgar scores and umbilical cord blood gas measurements were comparable in both groups. The lower remifentanil dose, 0.5 µg/kg, is therefore likely preferable.
Effects of Magnesium Sulfate.
Most severely preeclamptic women will present to the operating room after receiving magnesium sulfate for seizure prophylaxis. The magnesium infusion should continue throughout surgery to minimize the risk of eclampsia.4 The primary anesthetic considerations for women receiving magnesium sulfate are (1) interaction with nondepolarizing muscle relaxants, (2) effects on uterine tone, and (3) interaction with calcium entry–blocking agents.
Magnesium inhibits the release of acetylcholine at the neuromuscular junction, decreases the sensitivity of the neuromuscular junction to acetylcholine, and depresses the excitability of the muscle fiber membrane. Magnesium sulfate increases the potency and duration of vecuronium, rocuronium, and mivacurium.290-292 Several case reports have described a requirement for overnight mechanical ventilation after administration of routine doses of vecuronium in women receiving magnesium sulfate.290,293 Thus, if nondepolarizing muscle relaxants are used, they should be administered in very small doses and the response should be monitored carefully with a peripheral nerve stimulator. Many practitioners avoid the use of depolarizing neuromuscular blocking agents in this setting because of the concern for residual postoperative neuromuscular blockade, which can lead to respiratory complications. Because of abdominal wall distention in the term parturient, neuromuscular blockade is rarely required to facilitate surgical closure after cesarean delivery.
Even though succinylcholine mimics acetylcholine at the nerve terminal, the onset and duration of a single intubating dose is not prolonged when administered concurrently with a magnesium sulfate infusion294; a routine intubating dose of 1 to 1.5 mg/kg should be used during rapid-sequence induction of anesthesia.
Used for many years as a tocolytic agent, magnesium depresses smooth muscle contractions and inhibits CNS catecholamine release.295-297 Therefore, it seems intuitive that the risk for uterine atony and excessive blood loss might be increased in women receiving magnesium sulfate.193 However, studies193,203 have not found an increased risk for blood loss in women receiving magnesium sulfate. A blood sample for a type and screen should be sent to the blood bank before cesarean delivery, and uterotonic agents should be immediately available. Although some reports have suggested that coadministration of a calcium entry–blocking agent and magnesium may cause hypotension and/or neuromuscular blockade,176-179 more recent information suggests that these medications can be used safely together.180
Postoperative Analgesia.
Options for postoperative analgesia are the same as for healthy pregnancies and include patient-controlled intravenous opioids, neuraxial opioids (single injection), and continuous epidural infusion of analgesic agents. Many anesthesia providers prefer neuraxial opioid administration for postcesarean analgesia (see Chapter 28). For women whose postpartum hypertension persists longer than one day, the ACOG has suggested that nonsteroidal anti-inflammatory medications be replaced by alternative analgesics, as nonsteroidal anti-inflammatory drugs may contribute to hypertension.4 In the rare case of a woman with continuing severe refractory hypertension, continuous epidural analgesia is attractive for its blood pressure–modulating properties.
Regardless of the postoperative analgesic technique, all women should be carefully monitored with pulse oximetry for signs of respiratory depression or airway obstruction.
Postpartum Management
The risks of severe preeclampsia do not end with delivery. Postpartum women are at significant risk for pulmonary edema, sustained hypertension, stroke, venous thromboembolism, airway obstruction, and seizures and should receive close monitoring of blood pressure, fluid intake, and urinary output. In addition, severe preeclampsia, the HELLP syndrome, and eclampsia can present for the first time in the postpartum period, with delayed presentation as late as 4 weeks after delivery.4 A study of almost 4000 women diagnosed with preeclampsia found that the incidence of postpartum onset of disease was 5.7%,116 and hypertension is the leading indication for postpartum hospital readmission.298
The risk for pulmonary edema is also highest in the postpartum period. The resolution of preeclampsia usually occurs within 5 days of delivery and is heralded by a marked diuresis that follows mobilization of extracellular fluid and an increase in the intravascular volume. As a consequence, women with severe preeclampsia, particularly those with early-onset disease, renal insufficiency, or pulmonary capillary leak, are at increased risk for the development of postpartum pulmonary edema.163
In contrast to women with gestational hypertension, who typically become normotensive within a week of delivery, women with severe preeclampsia may have a longer duration of hypertension; the risk for cerebrovascular accident is highest during this time.168,208,209,299,300 Given the increased risk of postpartum cerebrovascular accident, the ACOG recommends antihypertensive therapy in the postpartum period when systolic blood pressure persistently exceeds 150 mm Hg or diastolic blood pressure exceeds 100 mm Hg.4 Antihypertensive therapy should be continued, started, or resumed for these women, and blood pressure should be closely monitored. For women in the postpartum period who develop new-onset hypertension associated with headaches or other neurologic symptoms, or new-onset severe hypertension, a 24-hour course of magnesium sulfate administration may help prevent eclampsia or a cerebrovascular accident.4
Postpartum venous thromboembolism (VTE) has an estimated incidence of 1 to 18 per 1000 cesarean deliveries301,302 and is a leading cause of maternal mortality in pregnancy.303 The risk factors for antepartum and postpartum events differ, suggesting a different pathophysiology for each.304 Both cesarean delivery304-307 and preeclampsia304,305 are independent risk factors for postpartum VTE. An emergency cesarean delivery doubles the risk for VTE compared with a nonemergent cesarean delivery.308
The 2012 American College of Chest Physicians Evidence-Based Clinical Practice Guidelines recommend that women with a risk for postcesarean VTE greater than 3% receive either pharmacologic thromboprophylaxis or mechanical prophylaxis (elastic stockings or intermittent pneumatic compression) during hospitalization after cesarean delivery.301 Preeclampsia in the absence of fetal growth restriction is considered a minor risk factor for VTE. VTE prophylaxis is indicated in patients with two or more minor risk factors (e.g., BMI > 30 kg/m2, use of > 10 cigarettes per day, multiple pregnancy, emergency cesarean delivery, postpartum hemorrhage > 1 L) (see Box 39-4).301 Preeclampsia with fetal growth restriction is considered a major risk factor (with risk for VTE > 3%), such that all patients with this condition merit VTE prophylaxis after cesarean delivery.301 The ACOG recommends pneumatic compression devices for all patients undergoing cesarean delivery.309
Preeclampsia has been associated with increased upper airway resistance and an increased risk of obstructive sleep apnea.310,311 A review of anesthesia-related maternal deaths in Michigan312 described a series of postoperative and postpartum deaths attributed to airway obstruction or hypoventilation, including the death of one women with severe preeclampsia and sleep-disordered breathing who likely experienced opioid-related respiratory depression while receiving patient-controlled intravenous analgesia after cesarean delivery. Such findings highlight the need for close monitoring and consistent vigilance in the postoperative care of women with severe preeclampsia—particularly those with generalized edema, known airway swelling, snoring, and obesity.
Long-Term Outcomes
Women with a history of preeclampsia are at increased risk for chronic hypertension and cardiovascular disease, including ischemic heart disease and stroke, later in life,59,61,313-316 and an earlier onset of cardiovascular disease than women with healthy pregnancies.60,317,318 Risks for ischemic heart disease and stroke are elevated approximately twofold.61
In addition, there is evidence of a dose-response relationship between preeclampsia and cardiovascular disease. Women with severe and/or early-onset preeclampsia, and whose pregnancies are complicated by both preeclampsia (the maternal syndrome) and fetal growth restriction (the fetal syndrome) are at higher risk than women with preeclampsia without severe features or gestational hypertension.59 Women with preeclampsia in both their first and second pregnancies are at even greater risk for future ischemic heart disease.318
The mechanism of increased risk for cardiovascular disease in preeclampsia is unclear. It is possible that preeclampsia causes permanent damage to the endothelium and hastens the onset of cardiovascular disease. Women with a history of preeclampsia have persistent impairment of brachial artery endothelium-dependent vascular relaxation at 1 to 3 years after delivery compared with healthy control women.319,320 A more likely explanation is that preeclampsia and cardiovascular disease have a common pathogenesis because of shared risk factors. Common risk factors for preeclampsia and atherosclerosis include hypertension, obesity, insulin resistance, advanced age, hypercholesterolemia, and dyslipidemia.317,321,322 Cigarette smoking is the notable exception in that it is an established risk factor for cardiovascular disease but is protective against preeclampsia.67,68 Preeclampsia may be a cardiovascular risk marker in women with an underlying predisposition to vascular disease; the hemodynamic and metabolic stress of pregnancy causes the predisposition to manifest as preeclampsia. After pregnancy, women return to a normal state until the threshold for disease development is exceeded in later life.
Regardless of the mechanism of increased risk, these observations represent a potential opportunity for primary disease prevention and risk factor modification. In a 2004 multinational study, 90% of the risk for a first myocardial infarction was attributed to potentially modifiable risk factors.323 Possible interventions include earlier cardiovascular disease screening and individual counseling regarding the importance of smoking cessation, regular exercise, and a diet low in saturated fat and high in antioxidants.
In contrast to the increased risk for cardiovascular disease, a history of preeclampsia has been associated with a decreased risk for cancer. Several earlier studies have suggested that women who have been diagnosed with preeclampsia have a slightly lower risk for breast cancer in later life compared with other parous women.324,325 However, a 2007 systematic review and meta-analysis of almost 200,000 women with a history of preeclampsia found no association between preeclampsia and future cancer risk.61
Evidence indicates that preeclampsia may also result in psychological sequelae. A woman with a history of severe preeclampsia (particularly with early-onset disease and preterm delivery) has experienced a serious complication that threatened her life and the life of her child. In one study, approximately one fourth of women developed post-traumatic stress disorder (PTSD) after early-onset preeclampsia.326 This association may be mediated by the condition of the offspring after preterm delivery.327-329 Further research is required to characterize women at risk for PTSD and to investigate strategies for PTSD prevention and intervention.
Eclampsia
Eclampsia is defined as the new onset of seizures or unexplained coma during pregnancy or the postpartum period in a woman with signs and symptoms of preeclampsia and without a preexisting neurologic disorder.330-333
Epidemiology
Findings from population-based studies in the past 10 years suggest that the incidence of eclampsia varies from 0.1 to 5.9 per 10,000 pregnancies in developed countries.9,38,334-338 The variation in rates of eclampsia among studies likely reflects reporting differences among countries or differences in treatment for severe preeclampsia.335,339 On average, studies have shown a decrease in the incidence of eclampsia in developed countries over time; this decrease is likely attributable to an increase in the use of magnesium for seizure prophylaxis.335,337,338
Eclampsia can occur suddenly at any point in the puerperium; however, most seizures occur intrapartum or within the first 48 hours after delivery. Late eclampsia is defined as seizure onset from 48 hours after delivery to 4 weeks postpartum.331,340 The majority of eclamptic women have evidence of severe preeclampsia, but in 10% to 15% of cases, hypertension is absent or modest and/or proteinuria is not detected.340 Reported risks include young maternal age, nulliparity, multiple gestation, molar pregnancy, triploidy, preexisting hypertension, renal or cardiac disease, previous severe preeclampsia or eclampsia, nonimmune hydrops fetalis, and systemic lupus erythematosus.338,341 Major maternal complications of eclampsia include pulmonary aspiration, pulmonary edema, cerebrovascular accident, cardiopulmonary arrest, venous thromboembolism, acute renal failure, and death.333-335,338 Eclampsia is associated with a high perinatal death rate and has also been associated with placental abruption, severe fetal growth restriction, and extreme prematurity.330,334,335,338
Clinical Presentation and Diagnosis
Any of the pathophysiologic changes of preeclampsia can be present in eclampsia. About 80% of patients will have premonitory neurologic symptoms, the most common of which are headache and visual disturbances.342 Other premonitory signs and symptoms can include photophobia, epigastric or right upper quadrant pain, hyperreflexia, and altered mental status330,340; these symptoms can occur before or after the onset of seizures.330
Seizures have an abrupt onset, typically beginning as facial twitching that is followed by a tonic phase that persists for 15 to 20 seconds. This phase progresses to a generalized clonic phase characterized by apnea, which lasts approximately 1 minute. Breathing generally resumes with a long stertorous inspiration, and the patient enters a postictal state with a variable period of coma. Cardiorespiratory arrest and pulmonary aspiration of gastric contents can complicate a seizure. Although the definitive diagnosis for eclampsia is a sudden seizure in a pregnant woman who has signs and symptoms of preeclampsia, a woman who lapses into coma without witnessed convulsions can also be classified as eclamptic.330
The mechanism of eclamptic seizures remains poorly understood.332 It may involve a loss of the normal cerebral autoregulatory mechanism, resulting in hyperperfusion and leading to interstitial or vasogenic cerebral edema and decreased cerebral blood flow.213,214,332 Neuroradiologic studies suggest that eclampsia might be a form of PRES.121,122
Until proven otherwise, the occurrence of seizures during pregnancy should be considered eclampsia. Conditions simulating eclampsia include seizure disorder, stroke, hypertensive encephalopathy, ischemia or hypoxia, cerebral space-occupying lesion, systemic disease (e.g., systemic lupus erythematosus, sickle cell anemia), infection (e.g., meningitis, encephalitis), electrolyte and endocrine disturbances, PRES, vasculitis or angiopathy, amniotic fluid embolism, medications (withdrawal, illicit drug use), and organ failure.330,331
Obstetric Management
Immediate goals are to stop convulsions, establish a patent airway, and prevent major complications (e.g., hypoxemia, aspiration). Further obstetric management includes antihypertensive therapy, induction or augmentation of labor, and expeditious (preferably vaginal) delivery. Fetal bradycardia typically begins during or immediately after a seizure but does not mandate immediate delivery unless it is persistent.
Resuscitation and Seizure Control
During the seizure, oxygenation may prove impossible but supplemental oxygen should be delivered by means of a facemask (Box 36-6). Attempts to insert an oral airway should be withheld until the seizure abates. As soon as breathing resumes, ventilation may be gently augmented with a bag-mask device. Pulse oximetry should be used to assess maternal oxygenation. Blood pressure and the electrocardiogram should be monitored to identify hypertension, arrhythmia, or cardiac arrest. While initial resuscitation is underway, an assistant should establish intravenous access, which may be difficult in a combative postictal woman. Judicious sedation might be required to allow further treatment in some patients.
Magnesium sulfate is the preferred drug for the prevention of further seizures in eclampsia.189,190 The administration of magnesium sulfate in eclamptic women is associated with significantly lower maternal death rates. An initial intravenous bolus of 4 to 6 g is administered, followed by an infusion at 1 to 2 g/h, assuming the patient has adequate renal function.343 Recurrent convulsions should prompt administration of an additional bolus of 2 to 4 g, infused over 5 to 10 minutes.343 The patient should be carefully monitored for signs of magnesium toxicity.
Anesthetic Management
The preanesthetic management of an eclamptic woman parallels that of a patient with severe preeclampsia. Considerations330,344 specific to the woman with eclampsia are as follows:
1. Assessment of seizure control and neurologic function. The possibility of increased intracranial pressure is not a cause for concern if the patient remains conscious, alert, and free of seizures. Persistent coma and localizing signs may indicate a major intracranial pathologic process that could affect anesthetic management.
2. Maintenance of fluid balance. Intake should be restricted to 75 to 100 mL/h to minimize the risk for exacerbating cerebral edema.
3. Blood pressure control. Antihypertensive therapy should be instituted if the systolic pressure is 160 mm Hg or higher, or if the diastolic pressure is 110 mm Hg or higher.
4. Continuous pulse oximetry monitoring of maternal oxygenation.
6. Laboratory investigations mimic those for preeclampsia. Additionally, coagulation studies should be obtained regardless of the platelet count.
The anesthetic plan is tailored to each individual case. In conscious, eclamptic women with no evidence of increased intracranial pressure and whose seizures are well controlled, neuraxial analgesia/anesthesia can be considered. In a retrospective review of 66 stable eclamptic South African women, Moodley et al.345 found no difference in maternal and neonatal outcomes in women who received epidural anesthesia compared with general anesthesia for cesarean delivery.
Eclamptic seizures are likely associated with an increase in intracranial pressure. In the rare instance of requirement for immediate delivery in a woman with ongoing seizures, a technique similar to that used for neuroanesthesia in patients with increased intracranial pressure should be considered. Intravenous induction agents such as propofol346 or thiopental347 will reduce the cerebral metabolic rate and cerebral blood flow, with a consequent decrease in cerebral blood volume and intracranial pressure. These agents are also effective in terminating seizures.348,349 Because hyperventilation reduces cerebral blood flow without a reduction in cerebral metabolic rate, it should be employed with caution. On the other hand, hypoventilation is associated with hypercarbia, which can lower the seizure threshold. To prevent further neurologic injury, it is important not to be overly aggressive in the reduction of systemic pressure because cerebral perfusion pressure is the difference between mean arterial pressure and intracranial pressure.350 Avoidance of hypoxia, hyperthermia, and hyperglycemia is also important in avoiding an exacerbation of neurologic injury.351 The tracheas of patients who have not recovered neurologically should remain intubated, and these patients should be monitored in an intensive care unit. If unconsciousness persists, further neurologic evaluation with electroencephalography and brain imaging to rule out persistent seizures and/or other underlying neurologic problems should be performed.
Long-Term Outcomes
Neurologic abnormalities occurring in patients with eclampsia (e.g., cortical blindness, focal motor deficits, coma) do not usually result in permanent neurologic deficits.330 However, recent studies suggest that formerly eclamptic women had significantly poorer neurocognitive function as well as an increase in visual impairment years after the index pregnancy, both of which may be attributable to permanent white matter changes caused by eclampsia.352-354
References
1. Brown MA, Lindheimer MD, de Swiet M, et al. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX–XIV.
2. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–1306.
3. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22.
4. American College of Obstetricians and Gynecologists Taskforce on Hypertension in Pregnancy. Hypertension in pregnancy. ACOG: Washington, DC; 2013.
5. Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074.
6. Bateman BT, Bansil P, Hernandez-Diaz S, et al. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134 e1–8.
7. American College of Obstetricians and Gynecologists. Chronic hypertension in pregnancy. [ACOG Practice Bulletin No. 125. Washington, DC] Obstet Gynecol. 2012;119:396–407.
8. Walker RL, Hemmelgarn B, Quan H. Incidence of gestational hypertension in the Calgary Health Region from 1995 to 2004. Can J Cardiol. 2009;25:e284–e287.
9. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21:521–526.
10. Roberts CL, Algert CS, Morris JM, et al. Hypertensive disorders in pregnancy: a population-based study. Med J Aust. 2005;182:332–335.
11. Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Intensification of fetal and maternal surveillance in pregnant women with hypertensive disorders. Int J Gynaecol Obstet. 1998;61:127–133.
12. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66–71.
13. Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension: calcium for preeclampsia prevention study group. Obstet Gynecol. 2000;95:24–28.
14. Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184:979–983.
15. Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;339:667–671.
16. Giannubilo SR, Dell’Uomo B, Tranquilli AL. Perinatal outcomes, blood pressure patterns and risk assessment of superimposed preeclampsia in mild chronic hypertensive pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;126:63–67.
17. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799.
18. Oudejans CB, van Dijk M, Oosterkamp M, et al. Genetics of preeclampsia: paradigm shifts. Hum Genet. 2007;120:607–612.
19. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594.
20. Skjaerven R, Vatten LJ, Wilcox AJ, et al. Recurrence of pre-eclampsia across generations: exploring fetal and maternal genetic components in a population based cohort. BMJ. 2005;331:877.
21. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958.
22. Basso O, Rasmussen S, Weinberg CR, et al. Trends in fetal and infant survival following preeclampsia. JAMA. 2006;296:1357–1362.
23. Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: quantification by gestational age and birth weight. Obstet Gynecol. 2003;102:488–492.
24. Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network: 1996-1997. Pediatrics. 2000;106:1070–1079.
25. Umo-Etuk J, Lumley J, Holdcroft A. Critically ill parturient women and admission to intensive care: a 5-year review. Int J Obstet Anesth. 1996;5:79–84.
26. Small MJ, James AH, Kershaw T, et al. Near-miss maternal mortality: cardiac dysfunction as the principal cause of obstetric intensive care unit admissions. Obstet Gynecol. 2012;119:250–255.
27. Keegan DA, Krey LC, Chang HC, Noyes N. Increased risk of pregnancy-induced hypertension in young recipients of donated oocytes. Fertil Steril. 2007;87:776–781.
28. Bianco A, Stone J, Lynch L, et al. Pregnancy outcome at age 40 and older. Obstet Gynecol. 1996;87:917–922.
29. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
30. Usta IM, Zoorob D, Abu-Musa A, et al. Obstetric outcome of teenage pregnancies compared with adult pregnancies. Acta Obstet Gynecol Scand. 2008;87:178–183.
31. Saftlas AF, Olson DR, Franks AL, et al. Epidemiology of preeclampsia and eclampsia in the United States, 1979-1986. Am J Obstet Gynecol. 1990;163:460–465.
32. Baker AM, Haeri S. Estimating risk factors for development of preeclampsia in teen mothers. Arch Gynecol Obstet. 2012;286:1093–1096.
33. de Vienne CM, Creveuil C, Dreyfus M. Does young maternal age increase the risk of adverse obstetric, fetal and neonatal outcomes: a cohort study. Eur J Obstet Gynecol Reprod Biol. 2009;147:151–156.
34. Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165:2098–2104.
35. Bateman BT, Shaw KM, Kuklina EV, et al. Hypertension in women of reproductive age in the United States: NHANES 1999-2008. PLoS One. 2012;7:e36171.
36. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–2850.
37. Samadi AR, Mayberry RM, Reed JW. Preeclampsia associated with chronic hypertension among African-American and White women. Ethn Dis. 2001;11:192–200.
38. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–212.
39. Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet Gynecol. 2005;106:156–161.
40. Shen JJ, Tymkow C, MacMullen N. Disparities in maternal outcomes among four ethnic populations. Ethn Dis. 2005;15:492–497.
41. Goodwin AA, Mercer BM. Does maternal race or ethnicity affect the expression of severe preeclampsia? Am J Obstet Gynecol. 2005;193:973–978.
42. Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The black-white disparity in pregnancy-related mortality from 5 conditions: differences in prevalence and case-fatality rates. Am J Public Health. 2007;97:247–251.
43. Wolf M, Shah A, Jimenez-Kimble R, et al. Differential risk of hypertensive disorders of pregnancy among Hispanic women. J Am Soc Nephrol. 2004;15:1330–1338.
44. Gong J, Savitz DA, Stein CR, Engel SM. Maternal ethnicity and pre-eclampsia in New York City, 1995-2003. Paediatr Perinat Epidemiol. 2012;26:45–52.
45. Mogren I, Hogberg U, Winkvist A, Stenlund H. Familial occurrence of preeclampsia. Epidemiology. 1999;10:518–522.
46. Esplin MS, Fausett MB, Fraser A, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867–872.
47. Cnattingius S, Reilly M, Pawitan Y, Lichtenstein P. Maternal and fetal genetic factors account for most of familial aggregation of preeclampsia: a population-based Swedish cohort study. Am J Med Genet A. 2004;130A:365–371.
48. Lie RT, Rasmussen S, Brunborg H, et al. Fetal and maternal contributions to risk of pre-eclampsia: population based study. BMJ. 1998;316:1343–1347.
49. Hnat MD, Sibai BM, Caritis S, et al. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparas. Am J Obstet Gynecol. 2002;186:422–426.
50. Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255.
51. Ananth CV, Peltier MR, Chavez MR, et al. Recurrence of ischemic placental disease. Obstet Gynecol. 2007;110:128–133.
52. Wikstrom AK, Stephansson O, Cnattingius S. Previous preeclampsia and risks of adverse outcomes in subsequent nonpreeclamptic pregnancies. Am J Obstet Gynecol. 2011;204:148 e1–6.
53. O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374.
54. Samuels-Kalow ME, Funai EF, Buhimschi C, et al. Prepregnancy body mass index, hypertensive disorders of pregnancy, and long-term maternal mortality. Am J Obstet Gynecol. 2007;197:490e1–6.
55. Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445.
56. Garner PR, D’Alton ME, Dudley DK, et al. Preeclampsia in diabetic pregnancies. Am J Obstet Gynecol. 1990;163:505–508.
57. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359.
58. Mazar RM, Srinivas SK, Sammel MD, et al. Metabolic score as a novel approach to assessing preeclampsia risk. Am J Obstet Gynecol. 2007;197:411 e1.
59. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803.
60. Funai EF, Friedlander Y, Paltiel O, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215.
61. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974.
62. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598.
63. Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR. Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis. 2004;43:415–423.
64. Smyth A, Oliveira GH, Lahr BD, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5:2060–2068.
65. Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:428 e1–6.
66. Koga K, Osuga Y, Tajima T, et al. Elevated serum soluble fms-like tyrosine kinase 1 (sFlt1) level in women with hydatidiform mole. Fertil Steril. 2010;94:305–308.
67. Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181:1026–1035.
68. England LJ, Levine RJ, Qian C, et al. Smoking before pregnancy and risk of gestational hypertension and preeclampsia. Am J Obstet Gynecol. 2002;186:1035–1040.
69. Ylikorkala O, Viinikka L, Lehtovirta P. Effect of nicotine on fetal prostacyclin and thromboxane in humans. Obstet Gynecol. 1985;66:102–105.
70. Rama Sastry BV, Hemontolor ME, Olenick M. Prostaglandin E2 in human placenta: its vascular effects and activation of prostaglandin E2 formation by nicotine and cotinine. Pharmacology. 1999;58:70–86.
71. Sorensen TK, Williams MA, Lee IM, et al. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41:1273–1280.
72. Genest DS, Falcao S, Gutkowska J, Lavoie JL. Impact of exercise training on preeclampsia: potential preventive mechanisms. Hypertension. 2012;60:1104–1109.
73. Magnus P, Trogstad L, Owe KM, et al. Recreational physical activity and the risk of preeclampsia: a prospective cohort of Norwegian women. Am J Epidemiol. 2008;168:952–957.
74. Einarsson JI, Sangi-Haghpeykar H, Gardner MO. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol. 2003;188:1241–1243.
75. Smith GN, Walker M, Tessier JL, Millar KG. Increased incidence of preeclampsia in women conceiving by intrauterine insemination with donor versus partner sperm for treatment of primary infertility. Am J Obstet Gynecol. 1997;177:455–458.
76. Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869.
77. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644.
78. Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579.
79. Keogh RJ, Harris LK, Freeman A, et al. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–841.
80. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. BJOG. 1986;93:1049–1059.
81. Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591.
82. Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192.
83. Mattar R, Amed AM, Lindsey PC, et al. Preeclampsia and HIV infection. Eur J Obstet Gynecol Reprod Biol. 2004;117:240–241.
84. Lockwood CJ, Matta P, Krikun G, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452.
85. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249.
86. Saftlas AF, Beydoun H, Triche E. Immunogenetic determinants of preeclampsia and related pregnancy disorders: a systematic review. Obstet Gynecol. 2005;106:162–172.
87. Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952.
88. Walther T, Wallukat G, Jank A, et al. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279.
89. Dechend R, Homuth V, Wallukat G, et al. AT1 receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387.
90. Xia Y, Wen H, Bobst S, et al. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10:82–93.
91. Dechend R, Viedt C, Muller DN, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639.
92. Zhou CC, Zhang Y, Irani RA, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862.
93. Moretti M, Phillips M, Abouzeid A, et al. Increased breath markers of oxidative stress in normal pregnancy and in preeclampsia. Am J Obstet Gynecol. 2004;190:1184–1190.
94. Carty DM, Delles C, Dominiczak AF. Preeclampsia and future maternal health. J Hypertens. 2010;28:1349–1355.
95. Maynard SE, Min J-Y, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658.
96. Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642.
97. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683.
98. Buhimschi CS, Norwitz ER, Funai E, et al. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005;192:734–741.
99. Jeyabalan A, Powers RW, Durica AR, et al. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am J Hypertens. 2008;21:943–947.
100. Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005.
101. Solomon CG, Seely EW. Preeclampsia—searching for the cause. N Engl J Med. 2004;350:641–642.
102. McElrath TF, Lim KH, Pare E, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207:407 e1–7.
103. Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1123.
104. Palmer K, Saglam B, Whitehead C, et al. Severe early-onset preeclampsia is not associated with a change in placental catechol O-methyltransferase (COMT) expression. Am J Pathol. 2011;178:2484–2488.
105. Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–1798.
106. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2004;(1).
107. Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–414.
108. Subtil D, Goeusse P, Puech F, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 1). BJOG. 2003;110:475–484.
109. Belizan JM, Villar J, Repke J. The relationship between calcium intake and pregnancy-induced hypertension: up-to-date evidence. Am J Obstet Gynecol. 1988;158:898–902.
110. Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2010;(8).
111. Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337:69–76.
112. Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev. 2008;(1).
113. Rumbold AR, Crowther CA, Haslam RR, et al. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806.
114. Beazley D, Ahokas R, Livingston J, et al. Vitamin C and E supplementation in women at high risk for preeclampsia: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2005;192:520–521.
115. Poston L, Briley AL, Seed PT, et al. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154.
116. Matthys LA, Coppage KH, Lambers DS, et al. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190:1464–1466.
117. Okanloma KA, Moodley J. Neurological complications associated with the pre-eclampsia/eclampsia syndrome. Int J Gynaecol Obstet. 2000;71:223–225.
118. Zeeman GG. Neurologic complications of pre-eclampsia. Semin Perinatol. 2009;33:166–172.
119. Belfort MA, Varner MW, Dizon-Townson DS, et al. Cerebral perfusion pressure, and not cerebral blood flow, may be the critical determinant of intracranial injury in preeclampsia: a new hypothesis. Am J Obstet Gynecol. 2002;187:626–634.
120. Liman TG, Bohner G, Heuschmann PU, et al. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol. 2012;19:935–943.
121. Wagner SJ, Acquah LA, Lindell EP, et al. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clin Proc. 2011;86:851–856.
122. Brewer J, Owens MY, Wallace K, et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208:468 e1–6.
123. Munnur U, de Boisblanc B, Suresh MS. Airway problems in pregnancy. Crit Care Med. 2005;33(10 Suppl):S259–S268.
124. Rout CC. Anaesthesia and analgesia for the critically ill parturient. Best Pract Res Clin Obstet Gynaecol. 2001;15:507–522.
125. Perlow JH, Kirz DS. Severe preeclampsia presenting as dysphonia secondary to uvular edema: a case report. J Reprod Med. 1990;35:1059–1062.
126. Sibai BM, Mabie BC, Harvey CJ, Gonzalez AR. Pulmonary edema in severe preeclampsia-eclampsia: analysis of thirty-seven consecutive cases. Am J Obstet Gynecol. 1987;156:1174–1179.
127. Zinaman M, Rubin J, Lindheimer M. Serial plasma oncotic pressure levels and echoencephalography during and after delivery in severe pre-eclampsia. Lancet. 1985;325:1245.
128. Benedetti TJ, Carlson RW. Studies of colloid osmotic pressure in pregnancy-induced hypertension. Am J Obstet Gynecol. 1979;135:308–311.
129. Benedetti TJ, Kates R, Williams V. Hemodynamic observations in severe preeclampsia complicated by pulmonary edema. Am J Obstet Gynecol. 1985;152:330–334.
130. Thornton CE, von Dadelszen P, Makris A, et al. Acute pulmonary oedema as a complication of hypertension during pregnancy. Hypertens Pregnancy. 2011;30:169–179.
131. Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56.
132. Kobayashi T, Tokunaga N, Isoda H, et al. Vasospasms are characteristic in cases with eclampsia/preeclampsia and HELLP syndrome: proposal of an angiospastic syndrome of pregnancy. Semin Thromb Hemost. 2001;27:131–135.
133. VanWijk MJ, Boer K, van der Meulen ET, et al. Resistance artery smooth muscle function in pregnancy and preeclampsia. Am J Obstet Gynecol. 2002;186:148–154.
134. Mushambi MC, Halligan AW, Williamson K. Recent developments in the pathophysiology and management of pre-eclampsia. Br J Anaesth. 1996;76:133–148.
135. Cotton DB, Lee W, Huhta JC, Dorman KF. Hemodynamic profile of severe pregnancy-induced hypertension. Am J Obstet Gynecol. 1988;158:523–529.
136. Mabie WC, Ratts TE, Sibai BM. The central hemodynamics of severe preeclampsia. Am J Obstet Gynecol. 1989;161:1443–1448.
137. Visser W, Wallenburg HC. Central hemodynamic observations in untreated preeclamptic patients. Hypertension. 1991;17:1072–1077.
138. Dennis AT, Castro J, Carr C, et al. Haemodynamics in women with untreated pre-eclampsia. Anaesthesia. 2012;67:1105–1118.
139. Clark SL, Cotton DB. Clinical indications for pulmonary artery catheterization in the patient with severe preeclampsia. Am J Obstet Gynecol. 1988;158:453–458.
140. Sharma SK, Philip J, Whitten CW, et al. Assessment of changes in coagulation in parturients with preeclampsia using thromboelastography. Anesthesiology. 1999;90:385–390.
141. Harlow FH, Brown MA, Brighton TA, et al. Platelet activation in the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2002;187:688–695.
142. Davies JR, Fernando R, Hallworth SP. Hemostatic function in healthy pregnant and preeclamptic women: an assessment using the platelet function analyzer (PFA-100) and thromboelastograph. Anesth Analg. 2007;104:416–420.
143. Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev. 2009;23:167–176.
144. Rinehart BK, Terrone DA, Magann EF, et al. Preeclampsia-associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999;54:196–202.
145. Moran P, Baylis PH, Lindheimer MD, Davison JM. Glomerular ultrafiltration in normal and preeclamptic pregnancy. J Am Soc Nephrol. 2003;14:648–652.
146. Sims EA, Krantz KE. Serial studies of renal function during pregnancy and the puerperium in normal women. J Clin Invest. 1958;37:1764–1774.
147. Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18:152–161.
148. Siemens J, Bogert L. The uric acid content of maternal and fetal blood. J Biol Chem. 1917;32:63–67.
149. Schaffer NK, Dill LV, Cadden JF. Uric acid clearance in normal pregnancy and pre-eclampsia. J Clin Invest. 1943;22:201–206.
150. Powers RW, Bodnar LM, Ness RB, et al. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194:160.
151. Trudinger BJ, Giles WB, Cook CM, et al. Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. BJOG. 1985;92:23–30.
152. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374:979–988.
153. Magee LA, Yong PJ, Espinosa V, et al. Expectant management of severe preeclampsia remote from term: a structured systematic review. Hypertens Pregnancy. 2009;28:312–347.
154. Churchill D, Duley L. Interventionist versus expectant care for severe pre-eclampsia before term. Cochrane Database Syst Rev. 2002;(3).
155. Society for Maternal-Fetal Medicine Publications Committee, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205:191–198.
156. Leduc L, Wheeler JM, Kirshon B, et al. Coagulation profile in severe preeclampsia. Obstet Gynecol. 1992;79:14–18.
157. Romero R, Vizoso J, Emamian M, et al. Clinical significance of liver dysfunction in pregnancy-induced hypertension. Am J Perinatol. 1988;5:146–151.
158. Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119:484–492.
159. Thangaratinam S, Ismail KM, Sharp S, et al. Accuracy of serum uric acid in predicting complications of pre-eclampsia: a systematic review. BJOG. 2006;113:369–378.
160. Koopmans CM, van Pampus MG, Groen H, et al. Accuracy of serum uric acid as a predictive test for maternal complications in pre-eclampsia: bivariate meta-analysis and decision analysis. Eur J Obstet Gynecol Reprod Biol. 2009;146:8–14.
161. Haddad B, Sibai BM. Expectant management of severe preeclampsia: proper candidates and pregnancy outcome. Clin Obstet Gynecol. 2005;48:430–440.
162. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–1373.
163. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–192.
164. Yoon BH, Lee CM, Kim SW. An abnormal umbilical artery waveform: a strong and independent predictor of adverse perinatal outcome in patients with preeclampsia. Am J Obstet Gynecol. 1994;171:713–721.
165. Pattinson RC, Norman K, Odendaal HJ. The role of Doppler velocimetry in the management of high risk pregnancies. BJOG. 1994;101:114–120.
166. American College of Obstetricians and Gynecologists. Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. [ACOG Committee Opinion No. 514. Washington, DC] Obstet Gynecol. 2011;118:1465–1468.
167. Barton JR, Sibai BM. Acute life-threatening emergencies in preeclampsia–eclampsia. Clin Obstet Gynecol. 1992;35:402–413.
168. Martin JN Jr, Thigpen BD, Moore RC, et al. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–254.
169. Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2006;(3).
170. MacCarthy EP, Bloomfield SS. Labetalol: a review of its pharmacology, pharmacokinetics, clinical uses and adverse effects. Pharmacotherapy. 1983;3:193–219.
171. Magee LA, Cham C, Waterman EJ, et al. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:955–960.
172. Coppage KH, Sibai BM. Treatment of hypertensive complications in pregnancy. Curr Pharm Des. 2005;11:749–757.
173. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335:257–265.
174. Vigil-De Gracia P, Lasso M, Ruiz E, et al. Severe hypertension in pregnancy: hydralazine or labetalol: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2006;128:157–162.
175. Waisman GD, Mayorga LM, Camera MI, et al. Magnesium plus nifedipine: potentiation of hypotensive effect in preeclampsia? Am J Obstet Gynecol. 1988;159:308–309.
176. Impey L. Severe hypotension and fetal distress following sublingual administration of nifedipine to a patient with severe pregnancy induced hypertension at 33 weeks. BJOG. 1993;100:959–961.
177. Snyder SW, Cardwell MS. Neuromuscular blockade with magnesium sulfate and nifedipine. Am J Obstet Gynecol. 1989;161:35–36.
178. Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. BJOG. 1994;101:262–263.
179. Magee LA, Miremadi S, Li J, et al. Therapy with both magnesium sulfate and nifedipine does not increase the risk of serious magnesium-related maternal side effects in women with preeclampsia. Am J Obstet Gynecol. 2005;193:153–163.
180. Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–969.
181. Nij Bijvank SW, Duvekot JJ. Nicardipine for the treatment of severe hypertension in pregnancy: a review of the literature. Obstet Gynecol Surv. 2010;65:341–347.
182. Ignarro LJ, Ross G, Tillisch J. Pharmacology of endothelium-derived nitric oxide and nitrovasodilators. West J Med. 1991;154:51–62.
183. Grossi L, D’Angelo S. Sodium nitroprusside: mechanism of NO release mediated by sulfhydryl-containing molecules. J Med Chem. 2005;48:2622–2626.
184. Eisenach JC, Castro MI. Maternally administered esmolol produces fetal beta-adrenergic blockade and hypoxemia in sheep. Anesthesiology. 1989;71:718–722.
185. Larson CP Jr, Shuer LM, Cohen SE. Maternally administered esmolol decreases fetal as well as maternal heart rate. J Clin Anesth. 1990;2:427–429.
186. Losasso TJ, Muzzi DA, Cucchiara RF. Response of fetal heart rate to maternal administration of esmolol. Anesthesiology. 1991;74:782–784.
187. Ducey JP, Knape KG. Maternal esmolol administration resulting in fetal distress and cesarean section in a term pregnancy. Anesthesiology. 1992;77:829–832.
188. Eisenach JC, Mandell G, Dewan DM. Maternal and fetal effects of labetalol in pregnant ewes. Anesthesiology. 1991;74:292–297.
189. Duley L, Henderson-Smart DJ, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev. 2010;(1).
190. Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;(12).
191. Duley L, Gulmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;(11).
192. Witlin AG, Friedman SA, Sibai BM. The effect of magnesium sulfate therapy on the duration of labor in women with mild preeclampsia at term: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1997;176:623–627.
193. Livingston JC, Livingston LW, Ramsey R, et al. Magnesium sulfate in women with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2003;101:217–220.
194. Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520–1526.
195. Cahill AG, Macones GA, Odibo AO, Stamilio DM. Magnesium for seizure prophylaxis in patients with mild preeclampsia. Obstet Gynecol. 2007;110:601–607.
196. Euser AG, Cipolla MJ. Resistance artery vasodilation to magnesium sulfate during pregnancy and the postpartum state. Am J Physiol Heart Circ Physiol. 2005;288:H1521–H1525.
197. Manfredi M, Beltramello A, Bongiovanni LG, et al. Eclamptic encephalopathy: imaging and pathogenetic considerations. Acta Neurol Scand. 1997;96:277–282.
198. Engelter ST, Provenzale JM, Petrella JR. Assessment of vasogenic edema in eclampsia using diffusion imaging. Neuroradiology. 2000;42:818–820.
199. Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40:1169–1175.
200. Cotton DB, Hallak M, Janusz C, et al. Central anticonvulsant effects of magnesium sulfate on N-methyl-D-aspartate-induced seizures. Am J Obstet Gynecol. 1993;168:974–978.
201. Cruikshank DP, Pitkin RM, Donnelly E, Reynolds WA. Urinary magnesium, calcium, and phosphate excretion during magnesium sulfate infusion. Obstet Gynecol. 1981;58:430–434.
202. Elliott JP, O’Keeffe DF, Greenberg P, Freeman RK. Pulmonary edema associated with magnesium sulfate and betamethasone administration. Am J Obstet Gynecol. 1979;134:717–719.
203. Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–1890.
204. James MF. Magnesium in obstetric anesthesia. Int J Obstet Anesth. 1998;7:115–123.
205. World Health Organization. Managing complications in pregnancy and childbirth: a guide for midwives and doctors. [Available at] http://whqlibdoc.who.int/publications/2007/9241545879_eng.pdf; 2013 [Accessed July] .
206. Amorim MM, Santos LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283–1288.
207. Woudstra DM, Chandra S, Hofmeyr GJ, Dowswell T. Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy. Cochrane Database Syst Rev. 2010;(9).
208. Bateman BT, Schumacher HC, Bushnell CD, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. 2006;67:424–429.
209. Bateman BT, Olbrecht VA, Berman MF, et al. Peripartum subarachnoid hemorrhage: nationwide data and institutional experience. Anesthesiology. 2012;116:324–333.
210. Del Zotto E, Giossi A, Volonghi I, et al. Ischemic stroke during pregnancy and puerperium. Stroke Res Treat. 2011;2011:606780.
211. Tang CH, Wu CS, Lee TH, et al. Preeclampsia-eclampsia and the risk of stroke among peripartum in Taiwan. Stroke. 2009;40:1162–1168.
212. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
213. Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190:714–720.
214. Schaefer PW, Buonanno FS, Gonzalez RG, Schwamm LH. Diffusion-weighted imaging discriminates between cytotoxic and vasogenic edema in a patient with eclampsia. Stroke. 1997;28:1082–1085.
215. Dennis AT, Solnordal CB. Acute pulmonary oedema in pregnant women. Anaesthesia. 2012;67:646–659.
216. Dildy GA 3rd, Cotton DB. Management of severe preeclampsia and eclampsia. Crit Care Clin. 1991;7:829–850.
217. Mabie WC, Hackman BB, Sibai BM. Pulmonary edema associated with pregnancy: echocardiographic insights and implications for treatment. Obstet Gynecol. 1993;81:227–234.
218. Desai DK, Moodley J, Naidoo DP, Bhorat I. Cardiac abnormalities in pulmonary oedema associated with hypertensive crises in pregnancy. BJOG. 1996;103:523–528.
219. DiFederico EM, Burlingame JM, Kilpatrick SJ, et al. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or of nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925–933.
220. Young P, Johanson R. Haemodynamic, invasive and echocardiographic monitoring in the hypertensive parturient. Best Pract Res Clin Obstet Gynaecol. 2001;15:605–622.
221. Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mother's Lives: reviewing maternal deaths to make motherhood safer—2003-2005. CEMACH: London; 2007.
222. Abraham KA, Kennelly M, Dorman AM, Walshe JJ. Pathogenesis of acute renal failure associated with the HELLP syndrome: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2003;108:99–102.
223. Nwoko R, Plecas D, Garovic VD. Acute kidney injury in the pregnant patient. Clin Nephrol. 2012;78:478–486.
224. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy: pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;162:777–783.
225. Drakeley AJ, Le Roux PA, Anthony J, Penny J. Acute renal failure complicating severe preeclampsia requiring admission to an obstetric intensive care unit. Am J Obstet Gynecol. 2002;186:253–256.
226. Stratta P, Canavese C, Colla L, et al. Acute renal failure in preeclampsia-eclampsia. Gynecol Obstet Invest. 1987;24:225–231.
227. Ganesan C, Maynard SE. Acute kidney injury in pregnancy: the thrombotic microangiopathies. J Nephrol. 2011;24:554–563.
228. Lindqvist PG, Happach C. Risk and risk estimation of placental abruption. Eur J Obstet Gynecol Reprod Biol. 2006;126:160–164.
229. von Dadelszen P, Payne B, Li J, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377:219–227.
230. Sibai BM, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000–1006.
231. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159–167.
232. Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981–991.
233. Barton JR, Riely CA, Adamec TA, et al. Hepatic histopathologic condition does not correlate with laboratory abnormalities in HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count). Am J Obstet Gynecol. 1992;167:1538–1543.
234. Douglas M. The use of neuraxial anesthesia in parturients with thrombocytopenia: what is an adequate platelet count? Halpern S, Douglas M. Evidence-Based Obstetric Anaesthesia. Blackwell Publishing: Oxford; 2005:165–177.
235. Barton J, Sibai B. Diagnosis and management of hemolysis, elevated liver enzymes, and low platelets syndrome. Clin Perinatol. 2004;31:807–833.
236. Vigil-De Gracia P, Ortega-Paz L. Pre-eclampsia/eclampsia and hepatic rupture. Int J Gynaecol Obstet. 2012;118:186–189.
237. Sheikh RA, Yasmeen S, Pauly MP, Riegler JL. Spontaneous intrahepatic hemorrhage and hepatic rupture in the HELLP syndrome: four cases and a review. J Clin Gastroenterol. 1999;28:323–328.
238. Boulleret C, Chahid T, Gallot D, et al. Hypogastric arterial selective and superselective embolization for severe postpartum hemorrhage: a retrospective review of 36 cases. Cardiovasc Intervent Radiol. 2004;27:344–348.
239. Chauleur C, Fanget C, Tourne G, et al. Serious primary post-partum hemorrhage, arterial embolization and future fertility: a retrospective study of 46 cases. Hum Reprod. 2008;23:1553–1559.
240. Onrust S, Santema JG, Aarnoudse JG. Pre-eclampsia and the HELLP syndrome still cause maternal mortality in The Netherlands and other developed countries; can we reduce it? Eur J Obstet Gynecol Reprod Biol. 1999;82:41–46.
241. Preisman S, Pfeiffer U, Lieberman N, Perel A. New monitors of intravascular volume: a comparison of arterial pressure waveform analysis and the intrathoracic blood volume. Intensive Care Med. 1997;23:651–657.
242. American Society of Anesthesiologists Task Force on Pulmonary Artery Catheterization. Practice guidelines for pulmonary artery catheterization. Anesthesiology. 2003;99:988–1014.
243. Hibbard B, Anderson M, Drife J, et al. Report on Confidential Enquiries into Maternal Deaths in the United Kingdom, 1991-1993. HMSO: London; 1996.
244. Chestnut DH, Lumb PD, Jelovsek F, Killam AP. Nonbacterial thrombotic endocarditis associated with severe preeclampsia and pulmonary artery catheterization: a case report. J Reprod Med. 1985;30:497–500.
245. Lefrant JY, Muller L, Bruelle P, et al. Insertion time of the pulmonary artery catheter in critically ill patients. Crit Care Med. 2000;28:355–359.
246. Dennis AT. Transthoracic echocardiography in obstetric anaesthesia and obstetric critical illness. Int J Obstet Anesth. 2011;20:160–168.
247. Zieleskiewicz L, Lagier D, Contargyris C, et al. Lung ultrasound–guided management of acute breathlessness during pregnancy. Anaesthesia. 2012;68:97–101.
248. Dyer RA, Piercy JL, Reed AR, et al. Comparison between pulse waveform analysis and thermodilution cardiac output determination in patients with severe pre-eclampsia. Br J Anaesth. 2011;106:77–81.
249. Simmons SW, Cyna AM, Dennis AT, Hughes D. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev. 2007;(3).
250. Ramanathan J, Coleman P, Sibai B. Anesthetic modification of hemodynamic and neuroendocrine stress responses to cesarean delivery in women with severe preeclampsia. Anesth Analg. 1991;73:772–779.
251. Jouppila P, Jouppila R, Hollmen A, Koivula A. Lumbar epidural analgesia to improve intervillous blood flow during labor in severe preeclampsia. Obstet Gynecol. 1982;59:158–161.
252. Kanayama N, Belayet HM, Khatun S, et al. A new treatment of severe pre-eclampsia by long-term epidural anaesthesia. J Hum Hypertens. 1999;13:167–171.
253. Ryan D. Examination of the blood. Lichtman M, Beutler E, Kipps T, et al. Williams Hematology. 7th edition. McGraw-Hill: New York; 2005.
254. Bateman BT, Mhyre JM, Ehrenfeld J, et al. The risk and outcomes of epidural hematomas after perioperative and obstetric epidural catheterization: a report from the multicenter perioperative outcomes group research consortium. Anesth Analg. 2013;116:1380–1385.
255. Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972;287:155–159.
256. Rodgers RP, Levin J. A critical reappraisal of the bleeding time. Semin Thromb Hemost. 1990;16:1–20.
257. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35:64–101.
258. Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth. 2012;109:851–863.
259. Watson HG. Thromboelastography should be available in every labour ward. Int J Obstet Anesth. 2005;14:325–327.
260. Karinen J, Rasanen J, Alahuhta S, et al. Maternal and uteroplacental haemodynamic state in pre-eclamptic patients during spinal anaesthesia for Caesarean section. Br J Anaesth. 1996;76:616–620.
261. Vricella LK, Louis JM, Mercer BM, Bolden N. Epidural-associated hypotension is more common among severely preeclamptic patients in labor. Am J Obstet Gynecol. 2012;207:335 e1–7.
262. Aya AG, Vialles N, Tanoubi I, et al. Spinal anesthesia-induced hypotension: a risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005;101:869–875.
263. Dyer RA, Piercy JL, Reed AR, et al. Hemodynamic changes associated with spinal anesthesia for cesarean delivery in severe preeclampsia. Anesthesiology. 2008;108:802–811.
264. Gant NF, Daley GL, Chand S, et al. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689.
265. Baker PN, Kilby MD, Broughton Pipkin F. The effect of angiotensin II on platelet intracellular free calcium concentration in human pregnancy. J Hypertens. 1992;10:55–60.
266. Nisell H, Hjemdahl P, Linde B. Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol. 1985;5:479–493.
267. Vedernikov YP, Belfort MA, Saade GR, Garfield RE. Inhibition of cyclooxygenase but not nitric oxide synthase influences effects on the human omental artery of the thromboxane A2-mimetic U46619 and 17-beta-estradiol. Am J Obstet Gynecol. 2001;185:182–189.
268. Aya AG, Mangin R, Vialles N, et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: a prospective cohort comparison. Anesth Analg. 2003;97:867–872.
269. Clark VA, Sharwood-Smith GH, Stewart AV. Ephedrine requirements are reduced during spinal anaesthesia for caesarean section in preeclampsia. Int J Obstet Anesth. 2005;14:9–13.
270. Hadzic A, Vloka J, Patel N, Birnbach D. Hypertensive crisis after a successful placement of an epidural anesthetic in a hypertensive parturient: case report. Reg Anesth. 1995;20:156–158.
271. Heller PJ, Goodman C. Use of local anesthetics with epinephrine for epidural anesthesia in preeclampsia. Anesthesiology. 1986;65:224–226.
272. Dror A, Henriksen E. Accidental epidural magnesium sulfate injection. Anesth Analg. 1987;66:1020–1021.
273. Guinard JP, Mulroy MF, Carpenter RL, Knopes KD. Test doses: optimal epinephrine content with and without acute beta-adrenergic blockade. Anesthesiology. 1990;73:386–392.
274. Guay J. The epidural test dose: a review. Anesth Analg. 2006;102:921–929.
275. Polley LS, Columb MO, Naughton NN, et al. Effect of epidural epinephrine on the minimum local analgesic concentration of epidural bupivacaine in labor. Anesthesiology. 2002;96:1123–1128.
276. Soetens FM, Soetens MA, Vercauteren MP. Levobupivacaine-sufentanil with or without epinephrine during epidural labor analgesia. Anesth Analg. 2006;103:182–186.
277. Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Caesarean delivery: a population-based study. Br J Anaesth. 2010;105:818–826.
278. Wallace DH, Leveno KJ, Cunningham FG, et al. Randomized comparison of general and regional anesthesia for cesarean delivery in pregnancies complicated by severe preeclampsia. Obstet Gynecol. 1995;86:193–199.
279. Sharwood-Smith G, Clark V, Watson E. Regional anaesthesia for caesarean section in severe preeclampsia: spinal anaesthesia is the preferred choice. Int J Obstet Anesth. 1999;8:85–89.
280. Hood DD, Curry R. Spinal versus epidural anesthesia for cesarean section in severely preeclamptic patients: a retrospective survey. Anesthesiology. 1999;90:1276–1282.
281. Visalyaputra S, Rodanant O, Somboonviboon W, et al. Spinal versus epidural anesthesia for cesarean delivery in severe preeclampsia: a prospective randomized, multicenter study. Anesth Analg. 2005;101:862–868.
282. Santos AC, Birnbach DJ. Spinal anesthesia for cesarean delivery in severely preeclamptic women: don't throw out the baby with the bathwater!. Anesth Analg. 2005;101:859–861.
283. Stempel JE, O’Grady JP, Morton MJ, Johnson KA. Use of sodium nitroprusside in complications of gestational hypertension. Obstet Gynecol. 1982;60:533–538.
284. Hood DD, Dewan DM, James FM 3rd, et al. The use of nitroglycerin in preventing the hypertensive response to tracheal intubation in severe preeclampsia. Anesthesiology. 1985;63:329–332.
285. Ramanathan J, Sibai BM, Mabie WC, et al. The use of labetalol for attenuation of the hypertensive response to endotracheal intubation in preeclampsia. Am J Obstet Gynecol. 1988;159:650–654.
286. Baker AB. Management of severe pregnancy-induced hypertension, or gestosis, with sodium nitroprusside. Anaesth Intensive Care. 1990;18:361–365.
287. Ngan Kee WD, Khaw KS, Ma KC, et al. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: a randomized, double-blind, controlled trial. Anesthesiology. 2006;104:14–20.
288. Bansal S, Pawar M. Haemodynamic responses to laryngoscopy and intubation in patients with pregnancy-induced hypertension: effect of intravenous esmolol with or without lidocaine. Int J Obstet Anesth. 2002;11:4–8.
289. Park BY, Jeong CW, Jang EA, et al. Dose-related attenuation of cardiovascular responses to tracheal intubation by intravenous remifentanil bolus in severe pre-eclamptic patients undergoing Caesarean delivery. Br J Anaesth. 2011;106:82–87.
290. Sinatra RS, Philip BK, Naulty JS, Ostheimer GW. Prolonged neuromuscular blockade with vecuronium in a patient treated with magnesium sulfate. Anesth Analg. 1985;64:1220–1222.
291. Kussman B, Shorten G, Uppington J, Comunale ME. Administration of magnesium sulphate before rocuronium: effects on speed of onset and duration of neuromuscular block. Br J Anaesth. 1997;79:122–124.
292. Hodgson RE, Rout CC, Rocke DA, Louw NJ. Mivacurium for caesarean section in hypertensive parturients receiving magnesium sulphate therapy. Int J Obstet Anesth. 1998;7:12–17.
293. Yoshida A, Itoh Y, Nagaya K, et al. Prolonged relaxant effects of vecuronium in patients with deliberate hypermagnesemia: time for caution in cesarean section. J Anesth. 2006;20:33–35.
294. Baraka A, Yazigi A. Neuromuscular interaction of magnesium with succinylcholine-vecuronium sequence in the eclamptic parturient. Anesthesiology. 1987;67:806–808.
295. Lipman J, James MF, Erskine J, et al. Autonomic dysfunction in severe tetanus: magnesium sulfate as an adjunct to deep sedation. Crit Care Med. 1987;15:987–988.
296. James MF, Beer RE, Esser JD. Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth Analg. 1989;68:772–776.
297. Sipes SL, Weiner CP, Gellhaus TM, Goodspeed JD. The plasma renin-angiotensin system in preeclampsia: effects of magnesium sulfate. Obstet Gynecol. 1989;73:934–937.
298. Belfort MA, Clark SL, Saade GR, et al. Hospital readmission after delivery: evidence for an increased incidence of nonurogenital infection in the immediate postpartum period. Am J Obstet Gynecol. 2010;202:35 e1–7.
299. Walters BN, Walters T. Hypertension in the puerperium. Lancet. 1987;2:330.
300. Cantu-Brito C, Arauz A, Aburto Y, et al. Cerebrovascular complications during pregnancy and postpartum: clinical and prognosis observations in 240 Hispanic women. Eur J Neurol. 2011;18:819–825.
301. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S–736S.
302. Gherman RB, Goodwin TM, Leung B, et al. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94:730–734.
303. Berg CJ, Callaghan WM, Henderson Z, Syverson C. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2011;117:1230.
304. Jacobsen AF, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium—a register-based case-control study. Am J Obstet Gynecol. 2008;198:233e1–7.
305. Lindqvist P, Dahlback B, Marsal K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. 1999;94:595–599.
306. Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108:56–60.
307. Ros HS, Lichtenstein P, Bellocco R, et al. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? Am J Obstet Gynecol. 2002;186:198–203.
308. Macklon NS, Greer IA. Venous thromboembolic disease in obstetrics and gynaecology: the Scottish experience. Scott Med J. 1996;41:83–86.
309. American College of Obstetricians and Gynecologists. Thromboembolism in pregnancy. [ACOG Practice Bulletin No. 123. Washington, DC] Obstet Gynecol. 2011;118:718–729.
310. Chen YH, Kang JH, Lin CC, et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206:136 e1–5.
311. O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207:487 e1–9.
312. Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
313. Haukkamaa L, Salminen M, Laivuori H, et al. Risk for subsequent coronary artery disease after preeclampsia. Am J Cardiol. 2004;93:805–808.
314. McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930.
315. Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: a retrospective cohort study of 119,668 births. Am J Epidemiol. 2004;159:336–342.
316. Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112:1486–1491.
317. Ramsay JE, Stewart F, Greer IA, Sattar N. Microvascular dysfunction: a link between pre-eclampsia and maternal coronary heart disease. BJOG. 2003;110:1029–1031.
318. Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845.
319. Chambers JC, Fusi L, Malik IS, et al. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612.
320. Hamad RR, Eriksson MJ, Silveira A, et al. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens. 2007;25:2301–2307.
321. Newstead J, von Dadelszen P, Magee LA. Preeclampsia and future cardiovascular risk. Expert Rev Cardiovasc Ther. 2007;5:283–294.
322. Magee LA, von Dadelszen P. Pre-eclampsia and increased cardiovascular risk. BMJ. 2007;335:945–946.
323. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952.
324. Troisi R, Weiss HA, Hoover RN, et al. Pregnancy characteristics and maternal risk of breast cancer. Epidemiology. 1998;9:641–647.
325. Cohn BA, Cirillo PM, Christianson RE, et al. Placental characteristics and reduced risk of maternal breast cancer. J Natl Cancer Inst. 2001;93:1133–1140.
326. Engelhard IM, van Rij M, Boullart I, et al. Posttraumatic stress disorder after pre-eclampsia: an exploratory study. Gen Hosp Psychiatry. 2002;24:260–264.
327. Furuta M, Sandall J, Bick D. A systematic review of the relationship between severe maternal morbidity and post-traumatic stress disorder. BMC Pregnancy Childbirth. 2012;12:125.
328. Hoedjes M, Berks D, Vogel I, et al. Symptoms of post-traumatic stress after preeclampsia. J Psychosom Obstet Gynaecol. 2011;32:126–134.
329. Stramrood CA, Wessel I, Doornbos B, et al. Posttraumatic stress disorder following preeclampsia and PPROM: a prospective study with 15 months follow-up. Reprod Sci. 2011;18:645–653.
330. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402–410.
331. Hirshfeld-Cytron J, Lam C, Karumanchi SA, Lindheimer M. Late postpartum eclampsia: examples and review. Obstet Gynecol Surv. 2006;61:471–480.
332. Shah AK, Rajamani K, Whitty JE. Eclampsia: a neurological perspective. J Neurol Sci. 2008;271:158–167.
333. Mattar F, Sibai BM. Eclampsia. VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307–312.
334. Andersgaard AB, Herbst A, Johansen M, et al. Eclampsia in Scandinavia: incidence, substandard care, and potentially preventable cases. Acta Obstet Gynecol Scand. 2006;85:929–936.
335. Knight M. Eclampsia in the United Kingdom 2005. BJOG. 2007;114:1072–1078.
336. Zwart J, Richters J, Öry F, et al. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. BJOG. 2008;115:842–850.
337. O’Connor HD, Hehir MP, Kent EM, et al. Eclampsia: trends in incidence and outcomes over 30 years. Am J Perinatol. 2013;30:661–664.
338. Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118:987–994.
339. Ekholm E, Salmi MM, Erkkola R. Eclampsia in Finland in 1990-1994. Acta Obstet Gynecol Scand. 1999;78:877–882.
340. Karumanchi SA, Lindheimer MD. Advances in the understanding of eclampsia. Curr Hypertens Rep. 2008;10:305–312.
341. Sibai BM. Eclampsia. VI. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163:1049–1054.
342. Cooray SD, Edmonds SM, Tong S, et al. Characterization of symptoms immediately preceding eclampsia. Obstet Gynecol. 2011;118:995–999.
343. Aagaard-Tillery KM, Belfort MA. Eclampsia: morbidity, mortality, and management. Clin Obstet Gynecol. 2005;48:12–23.
344. Brodie H, Malinow AM. Anesthetic management of preeclampsia/eclampsia. Int J Obstet Anesth. 1999;8:110–124.
345. Moodley J, Jjuuko G, Rout C. Epidural compared with general anaesthesia for caesarean delivery in conscious women with eclampsia. BJOG. 2001;108:378–382.
346. Oshima T, Karasawa F, Satoh T. Effects of propofol on cerebral blood flow and the metabolic rate of oxygen in humans. Acta Anaesthesiol Scand. 2002;46:831–835.
347. Kassell NF, Hitchon PW, Gerk MK, et al. Alterations in cerebral blood flow, oxygen metabolism, and electrical activity produced by high dose sodium thiopental. Neurosurgery. 1980;7:598–603.
348. Wood PR, Browne GP, Pugh S. Propofol infusion for the treatment of status epilepticus. Lancet. 1988;1:480–481.
349. Ramsay RE. Treatment of status epilepticus. Epilepsia. 1993;34(Suppl 1):S71–S81.
350. Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–821.
351. Bissonnette B. Cerebral protection. Pediatr Anaesth. 2004;14:403–406.
352. Aukes AM, Wessel I, Dubois AM, et al. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol. 2007;197:365e1–6.
353. Postma IR, Wessel I, Aarnoudse JG, Zeeman GG. Neurocognitive functioning in women with a history of eclampsia: executive functioning and sustained attention. Am J Perinatol. 2010;27:685–690.
354. Wiegman MJ, de Groot JC, Jansonius NM, et al. Long-term visual functioning after eclampsia. Obstet Gynecol. 2012;119:959–966.