Human Immunodeficiency Virus
David J. Wlody MD
Chapter Outline
Gastrointestinal Abnormalities
Choice of Anesthetic Technique
STRATEGIES TO MINIMIZE TRANSMISSION OF HUMAN IMMUNODEFICIENCY VIRUS
In 1981, a cluster of cases of an unusual disorder, Pneumocystis carinii pneumonia (PCP), in five otherwise healthy men, initiated a search that culminated in the characterization of a new disease, acquired immunodeficiency syndrome (AIDS), and the identification of its causative agent, the human immunodeficiency virus (HIV). Subsequently, there has been an explosion of this disease in the United States. Geographically, a disease that once was limited to two or three urban areas is now found throughout the country. Further, the number of cases of HIV infection has reached epidemic levels. At the end of 2008, an estimated 1,178,000 persons were living with HIV in the United States, including more than 236,000 whose infection was undiagnosed.1 As of December 2011, more than 1,155,000 cases of AIDS had been reported to the U.S. Centers for Disease Control and Prevention (CDC). Some 636,000 people were reported to have died of AIDS and its complications by December 2010.2 HIV infection has exploded demographically from its initial isolation among homosexual men to its current endemic status among intravenous drug users, their sexual partners, and children born to women infected with HIV.
The impact of HIV infection in the developing world has been nothing less than catastrophic. As of 2012, the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimated that approximately 34 million people worldwide were infected with HIV (Figures 45-1 and 45-2). More than two thirds of these infections have occurred in sub-Saharan Africa, where nearly 5% of adults are infected with HIV and where 70% of all new infections that occurred worldwide during 2011 originated. It is encouraging that the number of new cases of HIV infection in sub-Saharan Africa has decreased by 25% between 2001 and 2011. Unfortunately, in at least nine countries, the number of new infections has increased by more than 25% in the same period.3
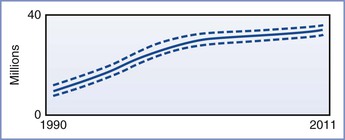
FIGURE 45-1 Estimated number of people living with HIV globally, 1990-2011. The bold line represents the estimate, and the dotted lines represent the high and low estimates. (From Global Report. UNAIDS report on the global AIDS epidemic 2012. Joint United Nations Programme on HIV/AIDS (UNAIDS), 2012. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf.)
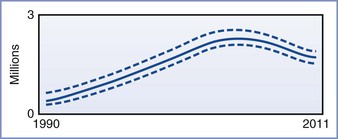
FIGURE 45-2 Adult and child deaths due to AIDS globally, 1990-2011. The bold line represents the estimate, and the dotted lines represent the high and low estimates. (From Global Report. UNAIDS Report on the global AIDS epidemic 2012. Joint United Nations Programme on HIV/AIDS (UNAIDS), 2012. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf.)
In the United States, although the rate of new infection in women appears to be decreasing, 21% of all new diagnoses of HIV infection identified in 2008 through 2011 occurred in women.2 Minority populations are disproportionately affected; 47% and 20% of all newly diagnosed infections in 2011 were seen in African-American and Hispanic populations, respectively.2 In 2009, HIV was the third leading cause of death in African-American women aged 35 to 44 years.4 Clearly, anyone providing anesthesia to pregnant women in the United States in the 21st century will care for patients who are infected with HIV. Neither medicolegal concerns nor fear of infection with HIV should prevent anesthesia providers from providing effective intrapartum analgesia and anesthesia to HIV-infected women.
Pathophysiology
HIV, previously known as lymphadenopathy-associated virus (LAV) and human T-cell lymphotropic virus type III (HTLV-III), is a member of the lentivirus subfamily of human retroviruses. The lentiviruses typically cause indolent infections in their hosts. These infections are notable for central nervous system (CNS) involvement, long periods of clinical latency, and persistent viremia caused by an impaired humoral immune response.5 HIV is a retrovirus (i.e., it carries the enzyme reverse transcriptase). This enzyme converts the single-stranded viral RNA into double-stranded DNA, which subsequently can be integrated into the DNA of the infected cell. This process is error prone, leading to rapid mutation of the virus, which significantly complicates drug therapy. HIV displays similarity to human immunodeficiency virus type 2 (HIV-II), a virus that is endemic in western Africa and that produces a similar syndrome. HIV-II is even more closely related to the simian immunodeficiency virus (SIV). It has been suggested that HIV arose in human populations through transmission of SIV via infected “bush meat,” the meat of chimpanzees and other primates consumed as food.6
For infection of the host cell to occur, HIV must bind to a cell-surface receptor, the CD4+ antigen complex.7 This protein molecule was first detected on helper T cells, and it subsequently was identified on B cells, macrophages, and monocytes.8 It also is found on placental cells9 and may provide a route of vertical transmission to the fetus during early pregnancy. The interaction between HIV and host cells requires an interaction with an additional cell-surface protein; binding with either the CCR5 or CXCR4 co-receptor is required for infection to occur. A number of therapeutic agents target this interaction.10
Infection of helper T cells is the key to immune suppression in HIV disease. These cells play a major role in the initial recognition of foreign antigen as well as in the activation of other immune system components.11 CD4+ monocytes and macrophages are also targeted by HIV. In addition to these T cell–mediated effects, both neutropenia and disturbances of neutrophil function are common in the later stages of HIV infection.12 Abnormalities of these elements of the immune system render the HIV patient vulnerable to bacterial, viral, fungal, parasitic, and mycobacterial infection. In addition, for reasons that are not entirely clear, patients infected with HIV are susceptible to several malignancies (e.g., Kaposi's sarcoma, B-cell lymphoma, invasive cervical carcinoma). AIDS-associated Kaposi's sarcoma is almost exclusively limited to homosexual men with HIV or to women whose male sexual partners are bisexual; this fact suggests that the malignancy is related to another sexually transmitted disease. In fact, DNA sequences from human herpesvirus-8 have been identified in AIDS-associated Kaposi's sarcoma.13,14
Diagnosis
The techniques for diagnosing HIV infection include viral culture, p24 antigen detection tests, nucleic acid amplification tests such as viral polymerase chain reaction (PCR), and immune function tests. Most often, the diagnosis is made on the basis of results of one of two antibody detection tests: enzyme immunoassay (EIA) and the Western blot technique. EIA measures the binding of anti-HIV antibody from the patient's serum to a mixture of antigens that typically have been obtained through recombinant DNA techniques (third-generation tests). The use of third-generation tests has improved the reliability of EIA, but false-positive results (caused by autoimmune disorders, influenza or hepatitis B immunization, and/or high parity) and false-negative results (caused by immunosuppressive therapy and various malignancies) can occur.11 For these reasons, a Western blot test usually is performed after a positive EIA result is obtained. False-positive Western blot tests can also occur, but they are less common than false-positive EIA tests. The Western blot technique allows the identification of antibodies to nine specific HIV antigens. Different organizations have different criteria for a positive Western blot test, but a positive Western blot test generally requires the presence of antibody to at least three different antigens. If there is no detectable antibody to any of these antigens, the result is negative.11 Identification of any combination of antibodies that does not meet the criteria for a positive result is considered an indeterminate result and an indication for retesting in 4 to 8 weeks.
Nucleic acid amplification tests can detect extremely low levels of infection. This technique can detect viremia as early as 1 to 2 weeks after exposure, during the period of primary symptomatic infection.11 Although this technique can be used to diagnose acute HIV infection, it is typically used to monitor the response to ongoing antiretroviral therapy.
Both the EIA and the Western blot tests rely on the detection of antibody to HIV antigens. Unfortunately, there may be an interval of several weeks to months after the initial infection before detectable levels of antibody are present. A patient infected with HIV who is tested during this “window period” has a negative test result but is fully capable of infecting others. In fact, exceptionally high levels of viremia are present during the initial period of infection, and transmission after needle-stick injury or other occupational exposure is more likely.15 This is a strong argument for instituting universal precautions. If barrier precautions are instituted only for patients with positive test results, health care workers will be exposed unnecessarily to seronegative but infectious patients.
Patients may be chronically infected with HIV for many years yet appear clinically well or have only minor evidence of immune suppression, such as oral candidiasis or recurrent herpes zoster. The diagnosis of AIDS is made when any one of a number of AIDS-indicator conditions develops (Box 45-1).
Clinical Manifestations
In the early years of the AIDS epidemic, the predominant symptoms were those of immune suppression (e.g., opportunistic infections, unusual malignancies). Disturbances of gastrointestinal function were also prominent. As improvements in prophylaxis and treatment of opportunistic infections have increased longevity, it has become apparent that HIV eventually affects multiple organ systems. The aggressive use of highly active antiretroviral therapy (HAART) can significantly prolong the symptom-free interval, and it is highly unusual for a pregnant patient to have significant organ system involvement due to HIV.
Neurologic Abnormalities
Neurologic involvement can occur at any time during HIV infection (Box 45-2). Viral particles can be isolated from the cerebrospinal fluid (CSF) at the time of primary infection.16 The manifestations of nervous system involvement vary with the stage of the disease.
During initial systemic HIV infection, a variety of CNS disorders may occur. Headache, photophobia, and retro-orbital pain are common. Cranial and peripheral neuropathies, demyelinating polyneuropathy, and septic meningoencephalitis have been reported.17 Cognitive and affective changes (e.g., depression, irritability) may be noted. Most of these disorders are self-limited, but persistent neurologic dysfunction may occur.17
A subset of patients remains neurologically asymptomatic during the latent phase of HIV infection. Nevertheless, these patients typically have CSF abnormalities, including the local synthesis of HIV antibody and the presence of HIV particles or viral nucleic acid.18 This is an important consideration when one is determining the risk for introducing virus into the CNS during the performance of neuraxial anesthesia in an asymptomatic patient. It is almost certain that CNS infection has already occurred.
Finally, the late stages of HIV infection are marked by significant neurologic deterioration in almost all patients. Meningitis is common, and its causes include tuberculosis, infection with Cryptococcus, metastatic lymphoma, and direct infection of the meninges by HIV. Diffuse encephalopathy can occur; cytomegalovirus (CMV), herpes simplex virus (HSV), and toxoplasmosis typically produce a simultaneous impairment of both cognition and alertness. Diffuse encephalopathy may also be seen as a consequence of systemic disease, such as sepsis or hypoxemia secondary to respiratory disease. Patients with the AIDS dementia complex also have a diffuse encephalitic picture; however, unlike other forms of encephalitis in which cognitive function is diminished, the level of alertness remains unimpaired. In addition, the complex is associated with impairment of motor function and behavioral changes (apathy, agitation). Focal brain disorders can occur secondary to toxoplasmosis, primary CNS lymphoma, and progressive multifocal leukoencephalopathy, an opportunistic viral infection that causes selective destruction of white matter tracts. Myelopathy is common; it can manifest in an acute, segmental form, as in the transverse myelitis produced by varicella infection, or as a more progressive and diffuse disorder—vacuolar myelopathy—which is marked by a progressive, painless gait disturbance and spasticity. A distal, predominantly sensory peripheral neuropathy is quite common in late HIV infection. The etiology is unknown; it has been suggested to occur as a result of cytokine-mediated neurotoxicity.19 Sensory and motor dysfunction typically are minimal, but pain can be severe enough to prevent walking. CMV infection can also lead to a polyradiculopathy that usually responds to anti-CMV therapy. Autonomic neuropathy can manifest as mild postural hypotension or severe cardiovascular instability during invasive procedures. Autonomic dysfunction also can contribute to the chronic diarrhea that occurs in some patients with AIDS. An inflammatory myopathy resembling dermatomyositis has been reported, although this disorder is less common than the neuropathies.19 Finally, neurologic side effects of antiretroviral and other therapies also may occur (see later discussion).
Pulmonary Abnormalities
The pulmonary manifestations of HIV disease are caused not by a direct effect of the virus but, rather, by the opportunistic infections associated with the disease. The most prominent of these is Pneumocystis jiroveci (formerly known as Pneumocystis carinii), a fungal organism that is seen in a wide variety of mammals and appears to be carried asymptomatically by many humans.20 Despite this evidence of widespread exposure to the organism, symptomatic Pneumocystis pneumonia (PCP) is typically seen only in patients with severe immune suppression. The clinical picture is similar to the adult respiratory distress syndrome, consisting of severe hypoxemia and a pattern of diffuse interstitial infiltrates on chest radiography. The mortality rate of patients with PCP who require tracheal intubation may be as high as 75%.21 Early initiation of corticosteroid therapy decreases the likelihood of progression to respiratory failure.22 Patients who survive the disease are at risk for the development of pneumatoceles; subsequent rupture leading to pneumothorax is common. Survivors of PCP also are at risk for developing chronic airway disease, including chronic bronchitis and bronchiectasis.23
Reactivation of latent tuberculosis is common in patients with HIV infection because of the impairment of cellular immunity that ordinarily keeps the disease in a quiescent state; HIV-infected individuals also may be more susceptible to acquiring tuberculosis when they are exposed to an infectious individual.24 The impairment of humoral immunity is responsible for a higher incidence of bacterial pneumonia caused by encapsulated organisms (e.g., Streptococcus pneumoniae, Haemophilus influenzae).25 Finally, although less common than PCP, pneumonia secondary to other fungal organisms (e.g., Aspergillus, Cryptococcus, Coccidioides) is much more common in patients infected with HIV than in the general population.25
Gastrointestinal Abnormalities
Gastrointestinal disturbances occur at some time in almost all patients with HIV infection (Box 45-3). Painful or difficult swallowing is common and is typically caused by herpetic, CMV, or candidal esophagitis; the contribution of these disorders to gastroesophageal reflux is unclear.26 Severe diarrhea resulting from infection with CMV, HSV, Shigella, Salmonella, Candida, Cryptosporidia, Giardia, Mycobacterium avium complex (MAC), or HIV itself can lead to significant cachexia and electrolyte abnormalities. Finally, hepatobiliary disease is common. Causes of parenchymal liver disease include hepatitis B and C, CMV, mycobacterial infection (both Mycobacterium tuberculosis and MAC), and Cryptococcus. Kaposi's sarcoma and non-Hodgkin's lymphoma may involve the liver. Biliary tract disease can develop in patients with advanced HIV infection; although several pathogens have been associated with this disorder (cryptosporidium, CMV), treatment of those pathogens is seldom effective.27 Endoscopic retrograde cholangiopancreatography (ERCP)–guided stenting has been successfully used to treat this disorder.28
Hematologic Abnormalities
HIV infection is associated with hematologic abnormalities that affect each of the peripheral cell lines.12 Leukopenia is a hallmark of the disease, especially the depletion of CD4+ lymphocytes; qualitative alterations in the functions of neutrophils and macrophages also occur. Anemia is quite common. Causes include direct HIV infection of erythroid precursors, suppression of erythropoiesis due to inappropriate release of tumor necrosis factor, infiltration of bone marrow with MAC or malignancy, and occult gastrointestinal blood loss.
Coagulation disturbances are common in patients with HIV. Immune thrombocytopenia is common and typically is only mildly symptomatic. Platelet production may be impaired because of direct infection of megakaryocytes with HIV. Thrombocytopenia frequently responds to the initiation of antiretroviral therapy. The response to corticosteroid therapy is variable. Intravenous immune globulin produces a rapid but transient effect, and it may be indicated in patients with life-threatening hemorrhage. The activated partial thromboplastin time may be prolonged because of the presence of the lupus anticoagulant; this finding is linked to a higher incidence of major thromboembolic events in HIV-infected patients. Finally, many of the antiretroviral agents and other drugs used in these patients have hematologic toxicity.
Cardiovascular Abnormalities
When echocardiography and autopsy evidence of lymphocytic infiltration of the myocardium are used as evidence of cardiovascular involvement in patients with HIV, the prevalence of such involvement is as high as 50%.29 Nevertheless, clinically significant cardiovascular disease is rare in patients with HIV. Pericarditis has been reported to be the most prevalent cardiovascular disorder seen in HIV-infected patients. The most common etiology appears to be mycobacterial infection; CMV, HSV, Kaposi's sarcoma, malignant lymphoma, and HIV itself have also been implicated.30 Pulmonary hypertension can develop secondary to repeated episodes of PCP and can also be a consequence of cytokine-mediated endothelial injury.29 Direct myocardial involvement—typically, focal myocarditis—is identified in 15% to 50% of autopsy studies, but clinical myocarditis or cardiomyopathy is rare.29 Infective endocarditis among patients with HIV occurs almost exclusively in intravenous drug users. Finally, the elevations in serum cholesterol and triglyceride concentrations produced by antiretroviral agents appear to increase the risk for coronary artery disease in patients receiving these drugs.31
Endocrine Abnormalities
Endocrine dysfunction can result from HIV infection, opportunistic infections, or drug therapy.32 There is a relatively high incidence of pathologic findings in the adrenal gland at autopsy, yet clinical evidence of glucocorticoid insufficiency is rare. Patients with AIDS frequently have abnormal thyroid function test results, similar to the findings in patients with other chronic illnesses, yet clinical hypothyroidism is unusual. Insulin resistance and diabetes are increasingly recognized as consequences of HIV infection and antiretroviral treatment.33
Renal Abnormalities
Patients with HIV are at risk for acute renal failure secondary to sepsis, dehydration, and drug toxicity.34 A common cause of chronic renal insufficiency is proliferative glomerulonephritis secondary to deposition of immune complexes containing HIV antigen within the glomeruli. Renal failure may also occur because of a specific disorder, HIV-associated nephropathy.35 This entity, seen almost exclusively in African-American patients, is characterized by a focal segmental glomerulosclerosis. Hypertension is uncommon, deterioration of renal function is extremely rapid, and the long-term prognosis is worse than that seen in renal failure from other causes. The underlying cause appears to be direct infection of renal cells by HIV. Antiretroviral therapy appears to modify the course of the disease.35
Interaction with Pregnancy
In 1991 and in 1995, the nationwide seroprevalence rate of HIV during pregnancy was reported to be 1.5 and 1.7 per 1000 pregnant women, respectively.36,37 There was considerable geographic variation in these figures; the highest rates of seroprevalence were found in New York (5.8 per 1000), Washington, DC (5.5 per 1000), and New Jersey (4.9 per 1000). In 1991, seropositive women were identified in all but 2 of the 39 reporting areas.36 In New York City, the prevalence of HIV infection among pregnant women was 6.2 per 1000 in 1999-2000, having declined by 49% over the previous decade. This decrease was markedly greater in white women than in African-American women.38 According to the CDC, the number of HIV-infected women giving birth in the United States increased from 6000 to 7000 in 2000 to 8700 in 2006, an increase of approximately 30%.39
The diagnosis of HIV infection in the offspring of HIV-infected mothers has been hampered by the persistence of passively acquired maternal antibody in the infant for as long as 18 months. Until 18 months of age, an infant's HIV status must be confirmed by viral culture or DNA polymerase chain reaction (PCR). Measurement of circulating p24 antigen has been used for rapid diagnosis of neonatal HIV infection,40 but this test is no longer recommended because it is less sensitive than PCR and is associated with false-positive results.41
There is intense interest in the identification of factors that promote perinatal transmission of HIV from mother to the newborn (i.e., vertical transmission) (Box 45-4).42 Clinical severity of maternal disease is associated with an increased risk for transmission, as reflected by a higher rate of infection in newborns of women with symptomatic AIDS.43 Maternal viral burden correlates with transmission. In one study, 13 of 13 women with more than 80,000 viral RNA copies per mL of plasma transmitted the disease, but none of the 63 women with less than 20,000 copies per mL transmitted HIV.44 Other factors that have been implicated in vertical transmission are the presence of ruptured membranes for more than 4 hours,45 coexisting sexually transmitted diseases,46 chorioamnionitis,47 and invasive procedures such as amniocentesis and cerclage.46 At least one study has demonstrated that fetal scalp blood sampling and the use of fetal scalp electrodes do not increase vertical transmission48; however, the documented presence of HIV in maternal cervical secretions has made some clinicians reluctant to use this monitoring technique.49 Finally, there is considerable evidence that breast-feeding may double the rate of perinatal transmission in women with established HIV infection.50 Thus, breast-feeding should be discouraged unless bottle-feeding is not a safe alternative, as is true in many developing countries.
In addition to identifying risk factors for vertical transmission, there is a significant effort to determine which active interventions might decrease the transmission of HIV. The first such intervention that was identified is the administration of zidovudine (ZDV, formerly azidothymidine [AZT]). In a study known as the AIDS Clinical Trial Group (ACTG) Protocol 076, administration of ZDV orally during pregnancy, intravenously during labor, and orally to the infant for the first 6 weeks of life decreased the transmission rate from 25.5% to 8.3%. No significant adverse effects were noted in these infants.51 Because of the success of HAART in reducing perinatal transmission to 1% to 2%—compared with 10% for ZDV monotherapy52—it has been suggested that all HIV-infected pregnant women should receive an aggressive treatment regimen, regardless of viral load.42
Because neonatal infection rates are low when the time interval between rupture of membranes and delivery is shortened, it has been suggested that cesarean delivery might decrease vertical transmission. At least four studies have suggested that elective cesarean delivery may decrease the rate of transmission by as much as 80%.53 The American College of Obstetricians and Gynecologists (ACOG) has recommended that HIV-infected women be offered the option of elective cesarean delivery to decrease the rate of transmission below the rate that would be expected with ZDV therapy alone.54 Although the ACOG acknowledged that the data were insufficient to demonstrate a benefit for women with viral loads less than 1000 viral copies per milliliter of plasma, there is some evidence that abdominal delivery may be beneficial even in the setting of viral loads below that threshold55; thus, it has been suggested that elective cesarean delivery be offered to patients in this group as well.53
A number of studies have assessed the effect of HIV infection on pregnancy outcome. Alger et al.56 followed 97 seronegative and 101 seropositive but asymptomatic women throughout pregnancy. There was no difference between groups in the incidence of low-birth-weight (LBW) (< 2500 g), small-for-gestational-age (SGA) infants, or low 5-minute Apgar scores.56 However, in a study of 315 seropositive and 311 seronegative women in Kenya, HIV seropositivity was associated with an increased risk for preterm delivery and LBW infants but not with an increased incidence of SGA infants.57 These different results may reflect the higher incidence of symptomatic HIV disease in the Kenyan patients. Another study noted that the incidence of serious infectious complications (e.g., PCP, CNS toxoplasmosis) is greater than 30% in pregnant women with advanced HIV infection (CD4+ lymphocyte counts < 300 cells/mm3).58 The fetal implications of such infections are obvious. Drug therapy per se does not seem to affect pregnancy outcome; specifically, evidence suggests that there is no higher incidence of preterm delivery, LBW infants, low Apgar scores, or stillbirth in women receiving therapy than in controls.59
There also is concern that pregnancy itself may have an adverse effect on the progression of HIV infection. However, no evidence suggests that pregnancy accelerates clinical deterioration in the HIV-infected patient or that viral RNA load changes significantly during pregnancy.56,60,61
Drug Therapy
There is an ever-increasing number of medications used to treat HIV infection, administered in innumerable multidrug regimens; in addition to HIV medications, patients may receive a number of other drugs to treat or prevent opportunistic infections. The side effects of the most commonly used antiretroviral agents and medications used for prophylaxis and treatment of opportunistic infections are listed in Tables 45-1 and 45-2, respectively.
TABLE 45-1
Side Effects of Antiretroviral Agents
| Drug | Side Effects |
| Zidovudine (ZDV, AZT, Retrovir) | Headache, N&V, bone marrow suppression |
| Didanosine (ddI, Videx) | N&V, peripheral neuropathy, pancreatitis, lactic acidosis |
| Zalcitabine (ddC, HIVID) | Peripheral neuropathy, pancreatitis |
| Stavudine (d4T, Sterit) | Peripheral neuropathy, pancreatitis, lactic acidosis |
| Lamivudine (3TC, Epivir) | N&V, headache, pancreatitis (children) |
| Emtricitabine (FTC, Emtriva) | Headache, N&V |
| Abacavir (ABC, Ziagen) | Systemic hypersensitivity reaction, headache, N&V |
| Delavirdine (Rescriptor) | Rash, elevated liver function test results |
| Nevirapine (NVP, Viramune) | Rash, hepatotoxicity |
| Efavirenz (EFV, Sustiva) | Dizziness, lightheadedness, vivid dreams, rash |
| Saquinavir (SQV, Invirase) | N&V, diarrhea, abdominal pain, hyperglycemia, increased triglycerides, fat redistribution |
| Ritonavir (RTV, Norvir) | N&V, diarrhea, abdominal pain, hyperglycemia, increased triglycerides, fat redistribution |
| Indinavir (IDV, Crixivan) | Nephrolithiasis, gastrointestinal intolerance, hyperglycemia, increased triglycerides, fat redistribution |
| Nelfinavir (NFV, Viracept) | N&V, diarrhea, hyperglycemia, increased triglycerides, fat redistribution |
| Amprenavir (APV, Agenerase) | Headache, N&V, hyperglycemia, increased triglycerides, fat redistribution |
| Lopinavir/ritonavir (LPV/RTV, Kaletra) | N&V, diarrhea, hyperglycemia, increased triglycerides, fat redistribution |
| Atazanavir (ATV, Reyataz) | N&V, headache, smaller increase in triglycerides than with other protease inhibitors |
| Tenofovir (TDF, Viread) | N&V, headache |
| Enfuvirtide (T20, Fuzeon) | Injection site reaction |
N&V, Nausea and vomiting.
Modified from Pau AK, Robertson S. AIDS-related medications. In Dolin R, Masur H, Saag MS, editors. AIDS Therapy. 3rd edition. St. Louis, Churchill Livingstone, 2008:1407-40.
TABLE 45-2
Side Effects of Agents Used for Prophylaxis/Treatment of Opportunistic Infections
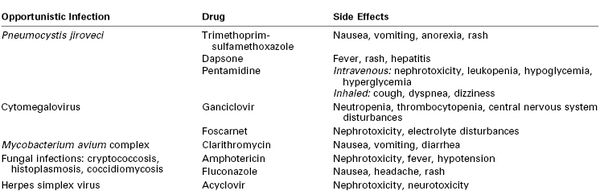
From Pau AK, Robertson S. AIDS-related medications. In Dolin R, Masur H, Saag MS, editors. AIDS Therapy. 3rd edition. St. Louis, Churchill Livingstone, 2008:1407-40.
Fetal Side Effects
There are few published data regarding the use of HIV medications in pregnant women. Fortunately, clinical experience suggests that fetal risk is minimal. This is best demonstrated by ACTG Protocol 076, which showed a significant reduction of vertical transmission of HIV with maternal ZDV therapy.51 There was no difference between the ZDV and placebo groups in the number and type of birth defects. The only apparent difference in neonatal outcome was a mild transient anemia (which required no treatment) in the ZDV group. Ongoing observation of these infants is planned, but no difference in growth or neurodevelopmental status has been identified in the ZDV group. As of July 2012, the Antiretroviral Pregnancy Registry62 had collected records of more than 14,000 women who had received these agents during pregnancy in the United States; the data indicate no increase in overall risk for birth defects or risk for specific defects for the overall population exposed to antiretroviral drugs. However, exposure to didanosine or nelfinavir was associated with a modest but statistically significant increase in overall rates of birth defects. The clinical relevance of this finding is unclear, and no pattern of birth defects has been observed with either drug.62
Historically, physicians have been concerned that the use of trimethoprim-sulfamethoxazole during the third trimester might increase the risk for neonatal kernicterus. This complication has not been reported, and trimethoprim-sulfamethoxazole should be continued until delivery in women who tolerate the drug.63
None of the antiretroviral agents or drugs used to treat opportunistic infections during pregnancy are listed in U.S. Food and Drug Administration pregnancy category A, which signifies a lack of fetal effect in controlled human trials.42 Despite the reassuring findings of the Antiretroviral Pregnancy Registry, the use of these drugs requires a full discussion of the risks and benefits with the mother.64 Minkoff and DeHovitz 63 concluded, “The guiding principle in the use of medications by HIV-infected women who become pregnant is to adhere strictly to standards promulgated for nonpregnant women, unless there are documented and compelling fetal concerns that would justify a modification of those standards.”
Anesthetic Management
Coexisting Diseases
Many pregnant women infected with HIV have health problems that are related to the behaviors that led to their infection with HIV. The most significant of these is substance abuse. A significant proportion of women with HIV contract the disease through intravenous drug use. It can be expected that many of these patients also abuse alcohol and crack cocaine.
The HIV-positive parturient is at high risk for harboring other sexually transmitted diseases. From the anesthesia provider's perspective, the most significant of these diseases is syphilis, because of its neurologic effects in later stages. If neuraxial anesthesia is performed, a careful neurologic examination should be completed and documented. Hepatitis B also is a sexually transmitted disease, and it should be investigated in HIV-positive parturients. Severe hepatic impairment affects anesthetic management. Of equal importance are the infectivity of hepatitis B and the high likelihood of its transmission after needle-stick injury. For this reason, it is unacceptable for health care workers to remain unvaccinated against hepatitis B.
Choice of Anesthetic Technique
There are concerns with the use of either general or neuraxial anesthesia. Overall, both techniques are safe, keeping in mind the precautions outlined in this chapter.65
Neuraxial Anesthesia
Whether HIV-infected pregnant women are more prone to the occurrence of infection after administration of neuraxial anesthesia is an important concern. Hughes et al.66 performed a study of 30 HIV-positive parturients, of whom 18 received neuraxial anesthesia and 12 did not; there was no evidence of accelerated disease progression in either group, and there were no neurologic or infectious complications in either group immediately after delivery and at 4 to 6 months postpartum.66 A later study demonstrated no postoperative changes in viral load or CD4+/CD8+ lymphocyte ratio and no increased hemodynamic instability or blood loss in HIV-infected patients undergoing elective cesarean delivery with spinal anesthesia.67 The prevention of infectious complications of neuraxial anesthesia depends on the maintenance of strict aseptic technique, which should include careful hand washing and wearing a face mask (see Chapter 12). Although the American Society of Anesthesiologists (ASA) practice advisory68 does not specifically recommend that anesthesia providers wear a sterile gown during initiation of neuraxial anesthesia, the U.S. CDC has recommended that a gown should be worn to prevent the transmission of HIV to the health care worker “during invasive procedures likely to result in the splashing of blood or other bodily fluids.”69
Some physicians may question whether it is prudent to administer neuraxial anesthesia to a patient who almost certainly will develop neurologic deficits at some time in the future and whether these deficits might be ascribed to the neuraxial anesthetic technique. Because such deficits are unlikely to be temporally related to the anesthesia, this does not seem to be a significant concern. Further, it seems cruel to deny the most effective intrapartum analgesic techniques to HIV-positive women simply because of fear of future litigation.
Another question is whether an anesthesia provider can ethically or legally refuse to provide care to an HIV-positive patient. Specifically, can an anesthesia provider refuse to provide epidural analgesia during labor? The American Medical Association has taken the position that physicians have an ethical duty to treat HIV-positive patients. Refusing to treat a patient with HIV also places the physician at legal risk, because numerous federal, state, and local statutes prohibit discrimination against patients with HIV disease. Any physician who refuses to provide care for patients with HIV must participate in a referral system ensuring that such patients receive prompt medical care.70
Despite the use of small-gauge, pencil-point spinal needles, and despite careful technique during administration of epidural anesthesia, post–dural puncture headache (PDPH) remains a problem in pregnant patients (see Chapter 31). Clearly, the onset of headache and photophobia in an immunosuppressed patient who has recently received a major neuraxial anesthetic can be worrisome, but the typical postural nature of a PDPH should allay fears of bacterial meningitis. Once the diagnosis of PDPH is made, an initial course of conservative therapy is indicated. It typically consists of bed rest, analgesics, and oral hydration. Although dehydration can worsen PDPH, there is no evidence that forced oral or intravenous overhydration has any beneficial effect.
Should conservative therapy for PDPH fail, a number of additional pharmacologic interventions have been proposed, including intravenous or oral caffeine, adrenocorticotropic hormone, and the 5-HT3 receptor agonist sumatriptan; all have varying degrees of success. However, the “gold standard” for treatment of PDPH is an autologous epidural blood patch. Such treatment can be expected to produce permanent and complete pain relief in the great majority of patients; a second epidural blood patch typically produces relief in most patients for whom the initial procedure fails.
Some physicians have expressed concern that the introduction of HIV-infected blood into the neuraxis might lead to the introduction of HIV into a previously uninfected CNS.71 Demonstration of subarachnoid extension of an epidural blood patch on magnetic resonance imaging heightens these concerns.72 However, it is likely that CNS infection occurs quite early in the course of HIV disease, even in asymptomatic patients. Nevertheless, it seems prudent to acknowledge the possibility that an epidural blood patch could accelerate the CNS manifestations of the disease. This question was addressed in a study of six seropositive patients who experienced PDPH after diagnostic lumbar puncture and who subsequently received an epidural blood patch.73 These patients subsequently underwent serial neuropsychological testing for as long as 2 years. The investigators stated that “none of these six subjects had a decline in neurocognitive performance or other adverse neurologic or infectious sequelae” during the period of the study. Although these numbers are small and this study has never been repeated, it provides the best evidence to date of the safety of epidural blood patch in an HIV-infected patient.
An alternative to autologous epidural blood patch is the epidural infusion of normal saline or colloidal solutions such as hetastarch. Unfortunately, the relief obtained from this technique is often transient, lasting only as long as the infusion continues. Further, the safety of epidural colloid infusion has not been established. Another proposed alternative is an epidural blood patch with fresh allogeneic whole blood; however, there are no published data on this technique.
General Anesthesia
As with neuraxial anesthesia, it is appropriate to ask whether patients with HIV might be more susceptible to the infectious (e.g., pulmonary) complications of general anesthesia. No published study has addressed this question. However, it seems appropriate to handle the endotracheal tube in as sterile a manner as possible and to minimize the duration of postoperative ventilation.
Another question involves the effect of general anesthesia on immune function. Several published studies suggest that general anesthesia can transiently depress immune function, but this depression appears to be clinically insignificant in healthy patients.74 It is appropriate to ask whether this effect might be exaggerated to the point of clinical significance in patients with HIV disease. Studies on this issue are lacking. At present, it would be inappropriate to recommend one anesthetic technique over another on the basis of their effects on immune function.75
Strategies to Minimize Transmission of Human Immunodeficiency Virus
To the Uninfected Patient
Any survey of HIV and anesthesia must include a discussion of those measures that can decrease the risk for HIV transmission to the uninfected patient. The most common iatrogenic cause of such transmission is the transfusion of infected blood. The use of nucleic acid amplification techniques to test donor blood should reduce the risk for transfusing HIV-infected blood. Nonetheless, the most significant impact that anesthesia providers can have on disease transmission is to minimize the transfusion of allogeneic blood.
In healthy patients, oxygen delivery is satisfactorily maintained at hemoglobin levels much lower than the historical transfusion threshold of 10 g/dL. The Consensus Development Conference on Perioperative Red Blood Cell Transfusion concluded that healthy patients tolerate a hemoglobin concentration as low as 7 g/dL.76 Patients with chronic anemia have a higher concentration of 2,3-diphosphoglycerate, which allows effective oxygen delivery at low hemoglobin concentrations. ASA guidelines77 state the following:
The information needed to precisely define when a blood transfusion should be given is not available….Red blood cells should usually be administered when the hemoglobin concentration is low (e.g., less than 6 g/dL in a young, healthy patient), especially when the anemia is acute. Red blood cells are usually unnecessary when the hemoglobin concentration is more than 10 g/dL….The determination of whether intermediate hemoglobin concentrations (i.e., 6 to 10 g/dL) justify or require RBC transfusion should be based on any ongoing indication of organ ischemia, potential or actual ongoing bleeding (rate and magnitude), the patient's intravascular volume status, and the patient's risk factors for complications of inadequate oxygenation. These risk factors include a low cardiopulmonary reserve and high oxygen consumption.
It is not clear how these guidelines should be applied to pregnant women. An association between preterm delivery and hemoglobin concentrations of less than 10 g/dL has been reported,78 as has a similar association between LBW infants and hemoglobin concentrations lower than 9 g/dL.79 However, whether this is a causal relationship or whether anemia serves as a marker of poor nutrition or lower socioeconomic status that may independently lead to perinatal morbidity is unknown. Although it is impossible to designate a minimum acceptable hemoglobin level during pregnancy, anemia is clearly undesirable. Once a cause is determined, appropriate therapy should be initiated, including transfusion if the anemia is life threatening for the mother or fetus.
Patients often want to use blood specifically donated by friends or relatives for their use (directed donation). There are disadvantages of directed donation. First, a directed unit is unavailable to another patient who may need it more urgently. Second, directed donation may discourage the routine voluntary donation of blood. Third, the directed donor sacrifices anonymity and legal protection. More pertinent to the issue of HIV transmission, there is no evidence that blood from designated donors is safer than anonymously donated bank blood80; this finding may be related to the slightly higher rate of HIV seropositivity among first-time donors.81 Further, fatal graft-versus-host disease has been reported in patients receiving blood from first-degree relatives.82
Another approach is the use of autologous blood donation during pregnancy in patients at high risk for peripartum hemorrhage, such as those with placenta previa or suspected placenta accreta. Several studies have demonstrated the safety of autologous donation in pregnant women with a hematocrit of at least 34%.83,84 However, it may be impossible to identify the patients who are more likely to require transfusion. In one study, only 4 (1.6%) of 251 high-risk patients eventually required transfusion. Further, only 2 of 13 patients who did receive blood during the peripartum period had identifiable risk factors. These results cast doubt on the benefits and cost-effectiveness of autologous blood donation during pregnancy.85
In patients at risk for hemorrhage during cesarean delivery, the use of acute normovolemic hemodilution may reduce the need for transfusion. This approach involves the collection of blood immediately before surgery with the simultaneous infusion of an appropriate volume of crystalloid or colloid to maintain normovolemia. In one study of 38 patients at risk for hemorrhage, 750 to 1000 mL of blood was removed with the simultaneous infusion of an equal volume of pentastarch. The hemoglobin concentration dropped from 10.9 to 8.3 g/dL; fetal monitoring revealed no change in the fetal heart rate pattern. The blood was reinfused during surgery. Neonatal outcome was satisfactory, and only one patient required allogeneic blood.86 In contrast, a meta-analysis in nonpregnant patients did not support the use of normovolemic hemodilution as an effective strategy for avoiding allogeneic transfusion.87
A final option for minimizing allogeneic transfusion is intraoperative blood salvage through the use of a cell salvage device (see Chapter 38). In the past, the use of intraoperative blood salvage in obstetric patients has been limited, in part by concern that transfusion of salvaged blood might precipitate amniotic fluid embolism. Waters88 and others have argued that this rare but devastating complication is not an embolic disease but rather an anaphylactoid reaction that would occur with or without transfusion of cell-salvaged blood, given that amniotic fluid is routinely entrained into the maternal circulation at the time of delivery.
Published experience with intraoperative blood salvage suggests that it is safe in obstetric patients.89,90 Allam et al.89 reviewed the use of intraoperative blood salvage in obstetric patients; at the time of their review in 2008, approximately 400 published cases had been reported. (In most published cases, the total volume of transfused salvaged blood was small.) These investigators concluded that “no single serious complication leading to poor maternal outcome has been directly attributed to its use.” They suggested that large prospective studies are needed to evaluate the efficacy and safety of cell salvage in obstetric patients.89 Arguments against use of intraoperative blood salvage in obstetric patients include (1) because of the low use of blood salvage procedures in obstetrics, ongoing operator (user) competency may be problematic and the risk for error may be increased; and (2) the number of patients studied to date is not sufficient to detect rare adverse events.91 Uncertainty remains about the cause of the syndrome of amniotic fluid embolism (including uncertainty as to which agent[s] trigger[s] the syndrome and which patients are at risk). Also, the blood salvage procedure does not eliminate fetal red blood cells, so the Rh-negative mother is at risk for isoimmunization from transfusion of salvaged blood.
The revised ASA Practice Guidelines for Obstetric Anesthesia92 recommend that “in cases of intractable hemorrhage when banked blood is not available or the patient refuses banked blood, intraoperative cell salvage should be considered if available.” And a 2012 ACOG committee opinion93 concluded that “autologous blood salvage devices have proved safe and the use of these devices may be a valuable adjunct during…surgery [for placenta accreta].” At best, however, intraoperative blood salvage will reduce or eliminate exposure to allogeneic blood in a minority of parturients who require transfusion94 (see Chapter 38 for a more complete discussion of intraoperative blood salvage).
Standards for Equipment Disinfection
The ASA Subcommittee on Infection Control Policy95 has made specific recommendations about the disinfection of reusable anesthesia equipment that comes in contact with mucous membranes. In practice, this equipment consists of laryngoscope blades, endoscopes, and face masks. The ASA Subcommittee recommends that such items be washed as soon as possible to remove gross contamination, followed by either high-level disinfection or sterilization. Functionally, each of these procedures kills fungi, viruses, and vegetative bacteria (including mycobacteria). In addition, sterilization kills larger numbers of endospores.95
In many institutions, disposable carbon dioxide absorbers and unidirectional valves are used for the anesthetizing of HIV-infected patients. However, no evidence suggests that HIV is transmitted in respiratory aerosols.96 This practice is unnecessary and is not recommended by the ASA subcommittee. An exception involves the HIV patient with active pulmonary tuberculosis; if a disposable absorber is not used for such a patient, the entire assembly distal to the fresh gas source must be disassembled and sterilized.
The rate of nosocomial transmission of HIV is negligible, and the only documented cases of such transmission apparently occurred in a setting in which disinfection procedures were notably lax.96 Commonsense measures should effectively reduce the rate of nosocomial transmission to zero.
To the Health Care Worker
The primary means of preventing the transmission of HIV to health care workers is the mandatory use of universal blood and body fluid barrier precautions. This policy has three crucial components. First, it must be universal. Establishing a higher level of concern for dealing with known HIV-positive patients implies a lower level of concern in the care of patients not known to be HIV positive. Unfortunately, a patient who is infected with HIV may be living within the window between the acquisition of infection and seroconversion. Further, all care providers should be equally concerned with the transmission of other bloodborne infections of higher infectivity and sometimes equal deadliness, such as hepatitis B and C.
Second, this policy must be followed whenever contact with infectious material is anticipated. Blood obviously is the primary source of exposure, but other potentially infectious body fluids include amniotic fluid, CSF, synovial fluid, pleural fluid, and pericardial fluid. Saliva is not believed to be infectious, but manipulations of the oral mucosa (e.g., laryngoscopy, tracheal intubation) probably lead to the contamination of saliva with blood.
Third, barrier precautions must be effective. Barriers include gloves, mask, and eye shields. The use of gloves prevents 98% of an anesthesia provider's contact with patient blood.97 When gross contamination is likely (e.g., during neonatal resuscitation), full-length gowns are indicated.
An additional component of universal precautions is the avoidance of needle-stick injuries. The recapping of needles is the most common cause of needle-stick injuries. Contaminated needles, including needles that have been injected into intravenous tubing, should not be recapped by hand. If recapping is necessary, a mechanical protective device should be used. The use of needleless systems can be expected to significantly decrease the risk for injury, and the use of such systems should be encouraged.98
Postexposure Prophylaxis for Health Care Workers
Occupational exposure to HIV is perhaps the most frightening work-related injury that an anesthesia provider can sustain. The risk for seroconversion after percutaneous exposure to HIV-infected blood is approximately 0.3%,99 but this statistic provides little reassurance to the exposed health care worker in view of the presumed 100% fatality rate of HIV infection. As of June 2000, the CDC had received voluntary reports of 56 U.S. health care providers with documented HIV seroconversion temporally related to occupational exposure, and an additional 138 reports of seroconversion that were considered possibly a result of occupational exposure.100 There have been no confirmed cases of occupational transmission since 1999.101
Certain measures should be taken after any parenteral exposure to potentially infectious body fluids, even those of the HIV-negative patient (Box 45-5). Although it is of unclear efficacy in preventing HIV seroconversion, local wound care with an antiseptic solution is indicated.102 In view of the exceedingly high transmission rate of hepatitis B infection, it is mandatory to determine the health care worker's antibody status after parenteral exposure to body fluids from a patient known to have or to be at high risk for hepatitis B. A previously vaccinated health care worker with absence or insufficiency of antibodies to hepatitis B should receive a booster dose of vaccine and hepatitis B immune globulin to provide protection until an adequate antibody response develops. A health care worker with no history of vaccination and an absence of antibodies should undergo primary immunization and should receive hepatitis B immune globulin.99
Several primate studies have demonstrated that the administration of antiretroviral drugs shortly after inoculation with SIV or HIV-2 can prevent seroconversion.103,104 Further, although prospective data are lacking, a retrospective case-control study demonstrated an 81% reduction in transmission of HIV to exposed health care workers who received ZDV prophylaxis.105 Finally, the reduction of vertical transmission by ZDV therapy demonstrated by ACTG Protocol 076 was only partly a result of the reduction of maternal viral load; inhibition of viral replication clearly played some role.99 Altogether, these results suggest that postexposure prophylaxis may play a significant role in preventing seroconversion.
The U.S. Public Health Service has issued postexposure prophylaxis guidelines for health care workers who have experienced either percutaneous or permucosal exposure to HIV.99 These recommendations attempt to determine the relative risk of transmission on the basis of (1) the nature of the material to which the worker was exposed, (2) the size of the inoculum, (3) the route of exposure, and (4) the presumed viral titer in the inoculum. Although encouraging results have been obtained, the primary strategy for the prevention of occupational transmission should focus on the prevention of exposure, especially the prevention of needle-stick injuries.
References
1. Centers for Disease Control and Prevention. HIV surveillance—United States, 1981-2008. MMWR Morb Mortal Wkly Rep. 2011;60:689–693.
2. Centers for Disease Control and Prevention. HIV surveillance report. [vol. 23. Available at] http://www.cdc.gov/hiv/topics/surveillance/resources/reports/; 2011 [Accessed March 1, 2013] .
3. UNAIDS. Global report: UNAIDS report on the Global AIDS epidemic 2012. [Available at] http://www.unaids.org/en/resources/campaigns/20121120_globalreport2012/globalreport/ [Accessed February 22, 2013] .
4. Centers for Disease Control and Prevention. Leading causes of death in females. [Available at] http://www.cdc.gov/women/lcod/ [Accessed March 3, 2013] .
5. Geleziunas R, Greene WC. Molecular insights into HIV-1 infection and pathogenesis. Sande MA, Volberding PA. The Medical Management of AIDS. 6th edition. WB Saunders: Philadelphia; 1999:23–39.
6. Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614.
7. Kilby JM. Enfuvirtide. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:401–413.
8. Staprans SI, Feinberg MB. Natural history and immunopathogenesis of HIV-1 disease. Sande MA, Volberding PA. The Medical Management of AIDS. 5th edition. WB Saunders: Philadelphia; 1997:29–56.
9. Maury W, Potts BJ, Rabson AB. HIV-1 infection of first-trimester and term human placental tissue: a possible mode of maternal-fetal transmission. J Infect Dis. 1989;160:583–588.
10. Kuhmann SE, Gulick RM, Moore JP. Inhibiting the entry of R5 and X4 HIV-1 phenotypic variants. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:415–447.
11. Branson BM, McDougal JS. Establishing the diagnosis of HIV infection. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1–22.
12. Moore RD. Hematologic disease. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1187–1205.
13. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869.
14. Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and those without HIV infection. N Engl J Med. 1995;332:1181–1185.
15. Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409.
16. Denning DW, Anderson J, Rudge P, Smith H. Acute myelopathy associated with primary infection with human immunodeficiency virus. Br Med J (Clin Res Ed). 1987;294:143–144.
17. Mehandru S, Markowitz M. Acute HIV Infection. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:559–577.
18. Garcia F, Niebla G, Romeu J, et al. Cerebrospinal fluid HIV-1 RNA levels in asymptomatic patients with early stage chronic HIV-1 infection: support for the hypothesis of local viral replication. AIDS. 1999;13:1491–1496.
19. Spudich SS, Price RW. Neurological disease. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1075–1101.
20. Huang L, Masur H. Pneumocystis pneumonia. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:637–658.
21. Wachter RM, Luce JM, Safrin S, et al. Cost and outcome of intensive care for patients with AIDS, Pneumocystis carinii pneumonia, and severe respiratory failure. JAMA. 1995;273:230–235.
22. Bozette SA, Sattler FR, Chiu J, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med. 1990;323:1451–1457.
23. Huang L, Stansell JD. Pneumocystis carinii pneumonia. Sande MA, Volberding PA. The Medical Management of AIDS. 6th edition. WB Saunders: Philadelphia; 1999:305–330.
24. Dobbs TE, Kimerling ME. Mycobacterium tuberculosis. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:711–736.
25. Huang L. Respiratory disease. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1225–1252.
26. Wilcox CM. Diseases of the esophagus, stomach, and small bowel. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1337–1354.
27. Martin NM, Chung RT, Sherman KE. Hepatic and hepatobiliary diseases. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1355–1381.
28. Devarbhavi H, Sebastian T, Seetharamu SM, Karanth D. HIV/AIDS cholangiopathy: clinical spectrum, cholangiographic features and outcome in 30 patients. J Gastroenterol Hepatol. 2010;25:1656–1660.
29. Cheitlin MD. Cardiovascular complications of HIV infection. Sande MA, Volberding PA. The Medical Management of AIDS. 6th edition. WB Saunders: Philadelphia; 1999:275–284.
30. Barbaro G. Cardiovascular manifestations of HIV infection. Circulation. 2002;106:1420–1425.
31. Lundgren JD, Sjol A. Cardiovascular disease in HIV. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1207–1224.
32. Lo JC, Schambelan M. Adrenal, gonadal, and thyroid disorders. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1325–1336.
33. Hadigan C, Grinspoon S. Diabetes and insulin resistance. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1265–1272.
34. Winston JA, Klotman PE. HIV-related renal disease. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:1253–1264.
35. Herman ES, Klotman PE. HIV-associated nephropathy: epidemiology, pathogenesis, and treatment. Semin Nephrol. 2003;23:200–208.
36. Gwinn M, Pappaioanou M, George JR, et al. Prevalence of HIV infection in childbearing women in the United States: surveillance using newborn blood samples. JAMA. 1991;265:1704–1708.
37. U.S. Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. MMWR Recomm Rep. 1995;44(RR-7):1–15.
38. Pulver WP, Glebatis D Wade N, et al. Trends from an HIV seroprevalence study among childbearing women in New York State from 1988 through 2000: a valuable epidemiologic tool. Arch Pediatr Adolesc Med. 2004;158:443–448.
39. Centers for Disease Control and Prevention. HIV among pregnant women, infants, and children in the United States. [Available at] http://www.cdc.gov/hiv/topics/perinatal/ [Accessed April 9, 2013] .
40. Miles SA, Balden E, Magpantay L, et al. Rapid serologic testing with immune-complex-dissociated HIV p24 antigen for early detection of HIV infection in neonates. N Engl J Med. 1993;328:297–302.
41. Pavia AT, Christenson JC. Pediatric AIDS. Sande MA, Volberding PA. The Medical Management of AIDS. 6th edition. WB Saunders: Philadelphia; 1999:525–535.
42. McIntyre J. Managing pregnant patients. Dolin R, Masur H, Saag MS. AIDS Therapy. 3rd edition. Churchill Livingstone: St. Louis; 2008:595–635.
43. Ryder RW, Nsa W, Hassig SE, et al. Perinatal transmission of the human immunodeficiency virus type 1 to infants of seropositive women in Zaire. N Engl J Med. 1989;320:1637–1642.
44. Dickover RE, Garratty EM, Herman SA, et al. Identification of levels of maternal HIV-1 RNA associated with risk of perinatal transmission: effect of maternal zidovudine treatment on viral load. JAMA. 1996;275:599–605.
45. Landesman SH, Kalish LA, Burns DN, et al. Obstetrical factors and the transmission of human immunodeficiency virus type 1 from mother to child. N Engl J Med. 1996;334:1617–1623.
46. Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175:661–667.
47. St. Louis ME, Kamenga M, Brown C, et al. Risk for perinatal HIV-1 transmission according to maternal immunologic, virologic, and placental factors. JAMA. 1996;269:2853–2859.
48. Viscarello RR, Copperman AB, DeGennaro NJ. Is the risk of perinatal transmission of human immunodeficiency virus increased by the intrapartum use of spiral electrodes or fetal pH sampling? Am J Obstet Gynecol. 1994;170:740–743.
49. Beckerman KP. Conception, pregnancy, and parenthood: maternal health care and the HIV epidemic. Sande MA, Volberding PA. The Medical Management of AIDS. 6th edition. WB Saunders: Philadelphia; 1999:555–573.
50. Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340:585–588.
51. Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180.
52. Cooper ER, Charurat M, Mofensen L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494.
53. Minkoff H. Human immunodeficiency virus infection in pregnancy. Obstet Gynecol. 2003;101:797–810.
54. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Scheduled cesarean delivery and the prevention of vertical transmission of HIV infection. ACOG Committee Opinion No. 234. [Washington, DC] May 2000.
55. Ioannidis JP, Abrams EJ Ammann A, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA viral loads <1000 copies/ml. J Infect Dis. 2001;183:539–545.
56. Alger LS, Farley JJ, Robinson BA, et al. Interactions of human immunodeficiency virus infection and pregnancy. Obstet Gynecol. 1993;82:787–796.
57. Temmerman M, Chomba EN, Ndinya-Achola J, et al. Maternal human immunodeficiency virus-1 infection and pregnancy outcome. Obstet Gynecol. 1994;83:495–501.
58. Minkoff H, Willoughby A, Mendez H, et al. Serious infections during pregnancy among women with advanced human immunodeficiency virus infection. Am J Obstet Gynecol. 1990;162:30–34.
59. Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346:1863–1870.
60. Burns DN, Landesman S, Minkoff H, et al. The influence of pregnancy on human immunodeficiency virus type 1 infection: antepartum and postpartum changes in human immunodeficiency virus type 1 viral load. Am J Obstet Gynecol. 1998;178:355–359.
61. Bessinger R, Clark R, Kissinger P, et al. Pregnancy is not associated with the progression of HIV disease in women attending an HIV outpatient program. Am J Epidemiol. 1998;147:434–440.
62. The antiretroviral pregnancy registry. [Available at] http://www.apregistry.com/who.htm [Accessed March 3, 2013] .
63. Minkoff HL, DeHovitz JA. Care of women infected with the human immunodeficiency virus. JAMA. 1991;266:2253–2258.
64. Minkoff HL, Moreno JD. Drug prophylaxis for human immunodeficiency virus–infected pregnant women: ethical considerations. Am J Obstet Gynecol. 1990;163:1111–1114.
65. Hignett R, Fernando R. Anesthesia for the pregnant HIV patient. Anesthesiol Clin. 2008;26:127–143.
66. Hughes SC, Dailey PA, Landers D, et al. Parturients infected with human immunodeficiency virus and regional anesthesia: clinical and immunologic response. Anesthesiology. 1995;82:32–37.
67. Avidan MS, Groves P, Blott M, et al. Low complication rate associated with cesarean section under spinal anesthesia for HIV-1–infected women on antiretroviral therapy. Anesthesiology. 2002;97:320–324.
68. American Society of Anesthesiologists Task Force on Infectious Complications Associated with Neuraxial Techniques. Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques: a report by the American Society of Anesthesiologists Task Force on infectious complications associated with neuraxial techniques. Anesthesiology. 2010;112:530–545.
69. Centers for Disease Control. Recommendations for prevention of HIV transmission in health-care settings. MMWR Morb Mortal Wkly Rep. 1987;36(Suppl 2):1S–18S.
70. Kern JMD, Croy BB. AIDS litigation for the primary care physician. Sande MA, Volberding PA. The Medical Management of AIDS. 3rd edition. WB Saunders: Philadelphia; 1992:477–483.
71. Gibbons JJ. Post-dural puncture headache in the HIV-positive patient (letter). Anesthesiology. 1991;74:953.
72. Griffiths AG, Beards SC, Jackson A, Horsman EL. Visualization of extradural blood patch for post lumbar puncture headache by magnetic resonance imaging. Br J Anaesth. 1993;70:223–225.
73. Tom DJ, Gulevich SJ, Shapiro HM, et al. Epidural blood patch in the HIV-positive patient: review of clinical experience. San Diego HIV Neurobehavioral Research Center. Anesthesiology. 1992;76:943–947.
74. Schneemilch CE, Schilling T, Bank U. Effects of general anesthesia on inflammation. Best Pract Res Clin Anaesthesiol. 2004;18:493–507.
75. Gershon RY, Manning-Williams D. Anesthesia and the HIV-infected parturient: a retrospective study. Int J Obstet Anesth. 1997;6:76–81.
76. Consensus conference: perioperative red blood cell transfusion. JAMA. 1998;260:2700–2703.
77. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208 https://ecommerce.asahq.org/p-116-practice-guidelines-for-perioperative-blood-transfusion-and-adjuvant-therapies.aspx.
78. Klein L. Premature birth and maternal prenatal anemia. Am J Obstet Gynecol. 1962;83:588–590.
79. Kaltreider DF, Johnson JW. Patients at high risk for low-birth-weight delivery. Am J Obstet Gynecol. 1976;124:251–256.
80. Cordell RR, Yalon VA, Cigahn-Haskell C, et al. Experience with 11,916 designated donors. Transfusion. 1986;26:484–486.
81. Cumming PD, Wallace EL, Schorr JB, Dodd RY. Exposure of patients to human immunodeficiency virus through the transfusion of blood components that test antibody-negative. N Engl J Med. 1989;321:941–946.
82. Thaler M, Shamiss A, Orgad S, et al. The role of blood from HLA-homozygous donors in fatal transfusion-associated graft-versus-host disease after open-heart surgery. N Engl J Med. 1989;321:25–28.
83. Herbert WN, Owen HG, Collins ML. Autologous blood storage in obstetrics. Obstet Gynecol. 1988;72:166–170.
84. McVay PA, Hoag RW, Hoag MS, Toy PT. Safety and use of autologous blood donation during the third trimester of pregnancy. Am J Obstet Gynecol. 1989;160:1479–1488.
85. Andres RL, Piacquadio KM, Resnik R. A reappraisal of the need for autologous blood donation in the obstetric patient. Am J Obstet Gynecol. 1990;163:1551–1553.
86. Grange CS, Douglas MJ, Adams TJ, Wadsworth LD. The use of acute hemodilution in parturients undergoing cesarean section. Am J Obstet Gynecol. 1998;178:156–160.
87. Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. Anesth Analg. 1998;86:9–15.
88. Waters JH. Is cell salvage a safe technique for the obstetric patient? Pro. Soc Obstet Anesth Perinatol Newsl. 2005;Fall:7–8.
89. Allam J, Cox M, Yentis SM. Cell salvage in obstetrics. Int J Obstet Anesth. 2008;17:37–45.
90. Sullivan JV, Crouch ME, Stocken G, Lindow SW. Blood cell salvage during cesarean delivery. Int J Gynaecol Obstet. 2011;115:161–163.
91. Santrach PJ. Is cell salvage a safe technique for the obstetric patient? Con. Soc Obstet Anesth Perinatol Newsl. 2005;Fall:9.
92. American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia. Anesthesiology. 2007;106:843–863.
93. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Placental accreta. ACOG Committee Opinion No. 529. [Washington, DC] July 2012.
94. Fong J, Gurewitsch ED, Kang HJ, et al. An analysis of transfusion practice and the role of intraoperative red blood cell salvage during cesarean delivery. Anesth Analg. 2007;104:666–672.
95. American Society of Anesthesiologists Subcommittee on Infection Control Policy. Recommendations for Infection Control for the Practice of Anesthesiology. ASA: Park Ridge, IL; 1994.
96. Gerberding JL. Limiting the risks to health care workers. Sande MA, Volberding PA. The Medical Management of AIDS. 5th edition. WB Saunders: Philadelphia; 1997:75–85.
97. Kristensen MS, Sloth E, Jensen TK. Relationship between anesthetic procedure and contact of anesthesia personnel with patient body fluids. Anesthesiology. 1990;73:619–624.
98. Greene ES, Berry AJ, Arnold WP, Jagger J. Percutaneous injuries in anesthesia personnel. Anesth Analg. 1996;83:273–278.
99. Panlilio AL, Cardo DM, Grohskopf LA, et al. U.S. Public Health Service. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):1–17.
100. U.S. Public Health Service; Updated Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1–52.
101. Centers for Disease Control and Prevention. Occupational HIV transmission and prevention among health care workers. [Available at] http://www.cdc.gov/hiv/resources/factsheets/PDF/hcw.pdf [Accessed March 2013] .
102. Henderson DK, Fahey BJ, Willy M, et al. Risk for occupational transmission of human immunodeficiency virus type 1 (HIV-1) associated with clinical exposures: a prospective evaluation. Ann Intern Med. 1990;113:740–746.
103. Böttiger D, Johansson NG, Samuelsson B, et al. Prevention of simian immunodeficiency virus, SIVsm, or HIV-2 infection in cynomolgus monkeys by pre- and postexposure administration of BEA-005. AIDS. 1997;11:157–162.
104. Otten RA, Smith DK, Adams DR, et al. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J Virol. 2000;74:9771–9775.
105. Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–1490.