Spinal, Epidural, and Caudal Anesthesia
ANATOMY, PHYSIOLOGY, AND TECHNIQUE
Naveen Nathan MD, Cynthia A. Wong MD
Chapter Outline
Equipment and Placement of Needle/Catheter
Techniques to Minimize Local Anesthetic Toxicity
COMPLICATIONS OF NEURAXIAL TECHNIQUES
Unintentional Intravascular or Subarachnoid Injection
The art and science of neuraxial anesthesia requires a thorough appreciation of neuroanatomy and the physiologic effects imposed by medications commonly administered via the spinal and/or epidural route. The focus of this chapter is to characterize the anatomic and technical considerations for neuraxial anesthesia. The reader is referred to Chapter 23 for a corresponding discussion of the physiologic and untoward effects of neuraxial analgesia in laboring women and to Chapter 26 for a discussion of neuraxial anesthesia for cesarean delivery. Successful administration and management of neuraxial anesthesia requires well-developed technique moderated by sound clinical judgment.
Anatomy
Neuraxial Anatomy
The Spinal Cord, Spinal Canal, and Meninges
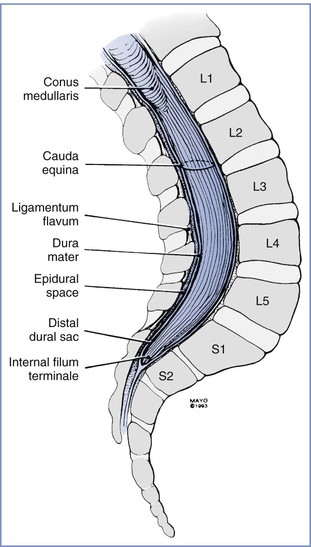
The cephalad aspect of the spinal cord is continuous with the brainstem through the foramen magnum. The spinal cord most often terminates as the conus medullaris at the level of the lower border of the first lumbar vertebral body. The conus medullaris is attached to the coccyx by means of a neural-fibrous band called the filum terminale, which is surrounded by the nerves of the lower lumbar and sacral roots, known as the cauda equina. Within the bony vertebral column are three membranes: the pia mater, the arachnoid mater, and the dura mater. The pia mater is a highly vascular membrane that closely invests the spinal cord. The arachnoid mater is a delicate, nonvascular membrane closely adherent to the third and outermost layer, the dura. The subarachnoid space, located between the pia mater and arachnoid mater, contains (1) cerebrospinal fluid (CSF), (2) spinal nerves, (3) a trabecular network between the two membranes, (4) blood vessels that supply the spinal cord, and (5) lateral extensions of the pia mater—the dentate ligaments. The dura mater is a membrane composed of collagen that encapsulates the spinal cord, the deeper meningeal layers, and the subarachnoid space. This layer forms a connective tissue sheath along the vertical axis of the central nervous system (CNS) that is contiguous with connective tissue covering the lateral extension of spinal nerve roots as they exit the intervertebral foramina. The interface between the dural and arachnoid layers has been described as a potential space capable of expansion subsequent to mechanical trauma. Unintentional injection of local anesthetic into this subdural space may explain some cases of failed spinal anesthesia. It may also explain the rare, slow-to-develop cases of high spinal anesthesia after the inadvertent subdural injection of larger volumes of local anesthetic intended for epidural administration.1 Although the spinal cord ends at the level of the bodies of L1 and L2 in most patients, the subarachnoid space and cauda equina continue to the S2 level (Figure 12-1).
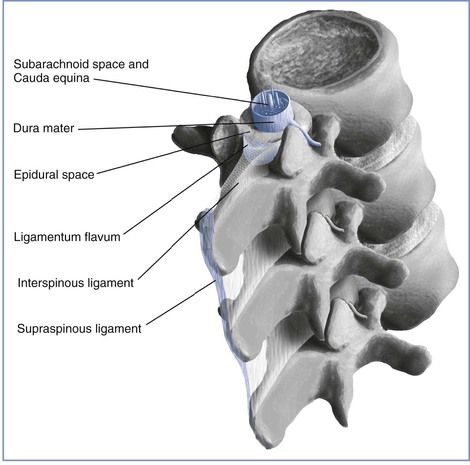
The Epidural Space
The epidural space is located external to the sac of the dura mater and contains loose connective tissue, adipose tissue, lymphatics, spinal nerve roots, and the internal vertebral venous plexus (Batson's plexus) (Figure 12-2). This space is bound by the posterior longitudinal ligament anteriorly, the ligamentum flavum and the periosteum of the lamina posteriorly, the pedicles of the vertebrae, and the intervertebral foramina with their contents laterally. The epidural space is closed at the foramen magnum where the spinal dura attaches to the dura of the cranium and at the sacral hiatus by the sacrococcygeal ligament. Frequently, anatomic references will illustrate neuraxial anatomy by way of sagittal and/or transverse cross section. This may result in the erroneous impression that the epidural space is a continuous columnar entity that envelops the dural sac at all points about its perimeter. Investigations using cryomicrotome sections and three-dimensional reconstruction of radiologic data verify that the epidural space is in fact discontinuous along the vertical and lateral axes of the spinal canal. It varies in anteroposterior thickness according to dermatomal distribution, being widest at the level of lumbar vertebrae and thinnest in the cervical region.2,3 Epiduroscopy and epidurography suggest the presence of a dorsal median connective tissue band in some individuals.4 Anatomic dissection and computed tomographic epidurography have also suggested the presence of epidural space septa. This band (or these septa) may provide an explanation for unilateral or incomplete epidural anesthesia.5 However, some investigators have suggested that the dorsal median band is an artifact of epidural space distention or an anatomic manifestation of the previously unappreciated epidural space segmentation.3
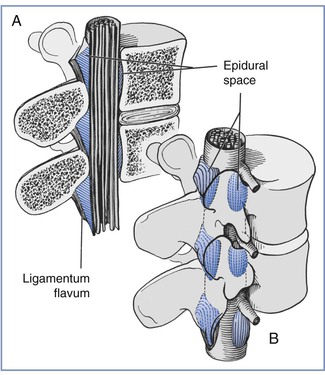
FIGURE 12-2 A, Sagittal section of the epidural space demonstrates that the contents of the epidural space depend on the level of the section. B, Three-dimensional drawing of the epidural space shows the discontinuity of the epidural contents. However, this potential space can be dilated by the injection of fluid into the epidural space. (Redrawn from Stevens RA. Neuraxial blocks. In Brown DL, editor. Regional Anesthesia and Analgesia. Philadelphia, WB Saunders, 1976:323).
The Vertebral Column and Ligaments
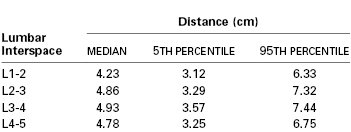
The ligamentum flavum lies posterior to the epidural space (Figure 12-3). The lamina, the spinous processes of the vertebral bodies, and the interspinous ligaments lie posterior to the ligamentum flavum. Posterior to these structures are the supraspinous ligament (which extends from the external occipital protuberance to the coccyx), subcutaneous tissue, and skin. Historically, some have described the ligamentum flavum as a single ligament. In actuality, however, it is composed of two curvilinear ligaments that join in the middle and form an acute angle with a ventral opening.3,6 Much like the epidural space, the ligamentum flavum is not uniform from skull to sacrum; indeed, it is not uniform even within a single intervertebral space. The thickness of the ligamentum flavum varies with vertebral level, body mass index, and age, as does the distance between the skin and the epidural space (Table 12-1).7,8
Anatomic Changes of Pregnancy
The normal anatomic changes of pregnancy affect the use of neuraxial anesthesia techniques. Uterine enlargement and vena caval compression result in engorgement of the epidural veins. Unintentional intravascular epidural catheter cannulation and injection of local anesthetic are more common in pregnant patients than in nonpregnant patients. In addition, the vertebral foraminal veins, which are contiguous with the epidural veins, are enlarged and obstruct one of the pathways for anesthetic egress from the epidural space during administration of epidural anesthesia. The enlarged epidural veins also may displace CSF from the thoracolumbar region of the subarachnoid space, as does the greater intra-abdominal pressure of pregnancy; this displacement partly explains the lowered dose requirement for spinal anesthesia in pregnant women.9 Subarachnoid dose requirements are also affected by the lower specific gravity of CSF in pregnant patients than in nonpregnant patients.10
The hormonal changes of pregnancy affect the perivertebral ligamentous structures, including the ligamentum flavum. The ligamentum flavum may feel less dense and “softer” in pregnant women than in nonpregnant patients; thus, sensing the passage of the epidural needle through the ligamentum flavum may be more difficult. It may also be more difficult for a pregnant woman to achieve flexion of the lumbar spine. Progressive accentuation of lumbar lordosis alters the relationship of surface anatomy to the vertebral column. At least three changes may occur. First, a pregnant woman's pelvis rotates on the long axis of the spinal column; thus, the line joining the iliac crests (Tuffier's line) assumes a more cephalad relationship to the vertebral column (e.g., this imaginary line might cross the vertebral column at the L3 to L4 interspace rather than the L4 to L5 interspace). Second, there is less space between adjacent lumbar spinous processes during pregnancy. It may be more difficult to use the midline approach to identify the epidural or subarachnoid space in pregnant women. (Thus the often-heard comment, “She has a narrow interspace.”) Third, magnetic resonance imaging has shown that the apex of the lumbar lordosis is shifted caudad during pregnancy, and the typical thoracic kyphosis in women is reduced during pregnancy.11 These changes may influence the spread of subarachnoid anesthetic solutions in supine patients, leading to a higher sensory level in the pregnant patient (Figure 12-4).12 Finally, labor pain makes it more difficult for some women to assume and maintain an ideal position while the anesthesia provider performs neuraxial anesthesia.
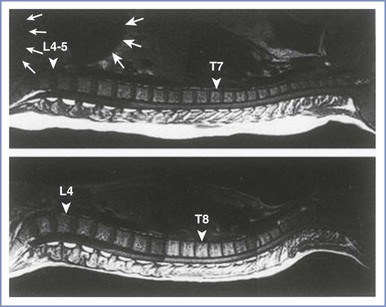
FIGURE 12-4 The curvature of the spinal column in the pregnant female (top) and nonpregnant female (bottom). The large and small white arrows indicate the uterus and fetal head, respectively. The apex of the lumbar lordosis moves caudad (triangular arrow), and the thoracic kyphosis is reduced and moves cephalad (triangular arrow) in the pregnant woman. (Reprinted with permission from Hirabayashi Y, Shimizu R, Fukuha H. Anatomical configuration of the spinal column in the supine position. II. Comparison of pregnant and non-pregnant women. Br J Anaesth 1995; 75:6-8.)
Physiology
Obstetric Pain Pathways
Pain during the first stage of labor results primarily from changes in the lower uterine segment and cervix. Pain is transmitted by visceral afferent nerve fibers that accompany the sympathetic nerves and enter the spinal cord at the T10 to L1 segments. During the late first stage and second stage of labor, pain results from distention of the pelvic floor, vagina, and perineum. Pelvic pain is transmitted by somatic nerve fibers, which enter the spinal cord at the S2 to S4 segments (Figure 12-5).
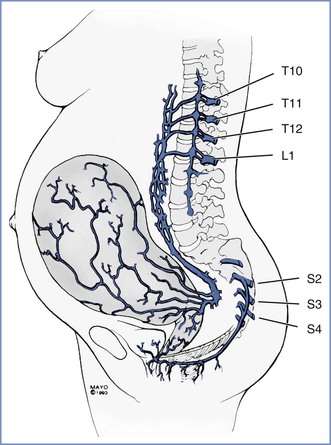
FIGURE 12-5 Pain pathways during labor and delivery. The afferent pain pathways from the cervix and uterus involve nerves that accompany sympathetic fibers and enter the neuraxis at T10 to L1. The pain pathways for the pelvic floor and perineum include the pudendal nerve fibers, which enter the neuraxis at S2 to S4.
During cesarean delivery, additional nociceptive pathways are involved in the transmission of pain, and a T4 level of anesthesia is required to provide adequate anesthesia. Most cesarean deliveries are performed with a horizontal (e.g., Pfannenstiel) skin incision, which involves the infraumbilical T11 to T12 dermatomes. During surgery, stretching of the skin may involve dermatomes two to four levels higher. Intraperitoneal manipulation and dissection involve poorly localized visceral pain pathways. Visceral pain may be transmitted by pathways as high as the celiac plexus. Additional somatic pain impulses may occur as a result of diaphragmatic stimulation because the intercostal nerves innervate a portion of the peripheral diaphragm.
Physiology of Neural Blockade
Hormonal changes, anatomic changes, and decreases in CSF specific gravity likely are responsible for the lower local anesthetic dose requirements for spinal anesthesia in pregnant women.10,13 Local anesthetics produce conduction blockade primarily by blocking sodium channels in nerve membranes, thereby preventing the propagation of neural impulses. Differential blockade is manifested as differences in the extent of cephalad blockade of temperature discrimination and vasomotor tone, sensory loss to pinprick, sensory loss to touch, and motor function. Temperature discrimination and vasomotor tone are blocked to the greatest extent (i.e., most cephalad level) and motor function to the least extent. During spinal anesthesia, local anesthetics act directly on neural tissue in the subarachnoid space. Regression of anesthesia can be explained by the simple vascular uptake of local anesthetic from the subarachnoid space and spinal cord.14 Epidural anesthesia has a much smaller zone of differential motor–sensory–sympathetic blockade; this difference suggests that the mechanism of epidural anesthesia must involve more than simple diffusion across the dura. For many years, nerve fiber size was presumed to be the primary determinant of susceptibility to local anesthetic blockade (i.e., smaller fibers are blocked more readily than larger fibers). However, later studies have shown that the length of nerve fiber exposed to local anesthetic is as important as the size of the nerve fiber. Fink15 hypothesized that the length of nerve fiber exposed to local anesthetic affects the extent of the differential zone of motor and sensory blockade. With spinal anesthesia, the local anesthetic concentration required to block sufficient sodium channels to affect motor, sensory, and sympathetic function is less than that needed for the better-protected nerves found in the epidural space; thus, a wider band of differential blockade occurs during spinal anesthesia than during epidural anesthesia.
The understanding of the mechanisms of spinal and epidural anesthesia likely remains oversimplified. Nonetheless, it seems clear that spinal anesthesia results primarily from the effects of local anesthetic on the spinal cord, whereas epidural anesthesia results from the effects of local anesthetic on nerve tissue within both the epidural and subarachnoid spaces.
Technique
Pre-procedural Considerations
Monitoring
The American Society of Anesthesiologists (ASA) has published guidelines for administration of neuraxial anesthesia in obstetric patients (see Appendix A). Among other things these recommendations address (1) the required presence of qualified anesthesia and obstetric care providers, (2) immediate availability of resuscitation medication and equipment (Box 12-1), (3) mandatory pre-procedural intravenous access, and (4) employment and documentation of maternal vital signs and fetal heart rate (FHR) monitoring.
During the initiation of neuraxial analgesia for labor, all patients are monitored with an automatic blood pressure cuff and a pulse oximeter to facilitate continuous assessment of the maternal heart rate and oxygenation. Maternal blood pressure is measured every 1 to 2 minutes after the administration of the test and therapeutic doses of local anesthetic for approximately 15 to 20 minutes, or until the mother is hemodynamically stable. Subsequently (during maintenance of neuraxial analgesia), maternal blood pressure is measured every 15 to 30 minutes or more frequently if hypotension ensues. Continuous pulse oximetry during maintenance analgesia is used in selected patients (e.g., patients with obstructive sleep apnea or cardiovascular disease). Rarely, invasive hemodynamic monitoring is necessary. The sensory level of analgesia and the intensity of motor block are assessed after the administration of the test and therapeutic doses of local anesthetic. Subsequently, sensory level and motor block are assessed at regular intervals.
Neither the ASA nor the American College of Obstetricians and Gynecologists (ACOG) provides a specific recommendation as to whether continuous FHR monitoring is necessary during performance of neuraxial anesthesia procedures. The ASA Task Force on Obstetric Anesthesia16 has stated:
The fetal heart rate should be monitored by a qualified individual before and after administration of neuraxial analgesia for labor. The Task Force recognizes that continuous electronic recording of the fetal heart rate may not be necessary in every clinical setting and may not be possible during initiation of neuraxial anesthesia.
The anesthesia provider cannot predict when hypotension will occur during the administration of neuraxial anesthesia. In addition, there is concern that intrathecal administration of an opioid is associated with a higher incidence of nonreassuring FHR patterns than other neuraxial techniques (see Chapter 23).17 Thus, we believe that continuous electronic FHR monitoring should be performed both during (if possible) and after the administration of neuraxial analgesia in all laboring women. In some cases, the mother's position or maternal obesity precludes the use of an external Doppler device to monitor the FHR. In such cases (especially when there is concern regarding fetal well-being), it is helpful for the obstetric provider to place a fetal scalp electrode to monitor the FHR.
Informed Consent, Patient-Procedure Verification, and Partner's Presence
Prior to initiation of neuraxial anesthesia, a pre-procedural verification process (i.e., “time-out”) is instituted as part of compliance with national patient safety recommendations from hospital accreditation organizations.18 The participation of the patient, the anesthesia care provider, and a third party such as a member of the nursing staff may lead to the discovery of concerns that should be addressed before the initiation of neuraxial anesthesia. The risk of neuraxial procedures relates to (1) the physical instrumentation of the spinal axis and (2) physiologic changes associated with medication administration via this anatomic route. Contraindications to needle or catheter placement include patient refusal or inability to cooperate, ongoing bleeding diathesis, infection either at the site of intended intervention or untreated systemic blood-borne illness, and increased intracranial pressure predisposing to cerebral herniation. Contraindications to injecting local anesthetics via the epidural or spinal route include severe hypovolemia and allergy to local anesthetics. It would be axiomatic that patient refusal presents an absolute contraindication to an elective procedure. A thorough preoperative assessment of current fetal well-being, maternal volume status, intrapartum systemic opioid use, antibiotic administration for ongoing chorioamnionitis or other infectious process, and a brief reiteration of known maternal disease states, including allergies, will readily identify most of the major concerns that would render neuraxial anesthesia potentially hazardous. The anesthesia provider should weigh the risks and benefits of neuraxial anesthesia for each patient.
Informed consent should include a frank discussion about anesthetic procedures and risks. Surveys of postpartum women have demonstrated that most parturients want to know the possible complications of epidural analgesia, even those that are rare.19,20 It is best to relay this information before the onset of labor (e.g., during antenatal classes),21 or early in the intrapartum period, although doing so is not always feasible. Some anesthesia providers fear that distressed, desperate, or sedated parturients may not understand the discussion of anesthetic procedures. However, adequacy of consent can be demonstrated not only by documentation of information provided to the patient but also by the lack of patient objection to a procedure and the cooperation provided by the patient during the procedure.22 In a survey of North American anesthesiologists, Brull et al.23 found that a majority of clinicians did not disclose the most severe risks of central neuraxial anesthesia to their patients. Additionally, most anesthesiologists cited inaccurate incidences of these complications. We do not find it difficult to explain the procedure and the risks of neuraxial analgesia to a laboring woman. The preanesthetic evaluation allows the physician to communicate a sense of concern and to demonstrate a commitment to the patient's care. Most laboring women understand the need for informed consent, and they appreciate the opportunity to participate in decisions about their care.
Management of the pregnant patient occurs in a unique clinical care environment in which the presence of the patient's spouse or family member must be addressed. Most often, the dictates of local institutions will establish whether a partner's presence during neuraxial labor analgesia is acceptable. However, hospital policy may be vague enough that the discretion lies in the hands of the anesthesia provider. Intuition may suggest that a partner who remains present during the conduct of neuraxial analgesia may help alleviate the patient's ongoing anxiety regarding the procedure. Conversely, the partner may be so apprehensive or disruptive that the partner's presence becomes counterproductive to the care of the patient. Orbach-Zinger et al.24 randomized 84 nulliparous women to either presence or absence of their partner during labor epidural catheter placement. Interestingly, patient and partner anxiety, as measured by a validated anxiety questionnaire, were reduced when partners were absent during the procedure.
Patient Positioning
Pregnant women have an exaggerated lumbar lordosis, and it is more difficult for them to flex the lumbar spine. However, most pregnant women are young, and youth usually allows sufficient flexibility to facilitate the insertion of a needle into the epidural or subarachnoid space. Whether the block is initiated in the lateral or sitting position is a matter of provider and patient preference. Notable advantages of the lateral position include (1) orthostatic hypotension is less likely and (2) the position often facilitates continuous FHR monitoring during placement of the epidural catheter. Vincent and Chestnut25 performed a study in which they observed that neither the sitting nor the lateral position was consistently superior with regard to patient comfort. However, pregnant women who preferred the left lateral decubitus position weighed less and had lower body mass indices than women who preferred the sitting position. The sitting position is likely associated with a higher incidence of orthostatic hypotension and syncope. However, the sitting position is preferred—and may be required—in obese parturients, in whom identification of the midline is usually significantly easier in the sitting position. Further, morbidly obese women may experience hypoxemia when placed in the lateral decubitus position.
One study demonstrated a greater reduction in maternal cardiac output with maximal lumbar flexion in the lateral decubitus position than in the sitting position during identification of the epidural space in laboring women.26 The researchers speculated that maximal lumbar flexion in the lateral decubitus position results in concealed aortocaval compression. In contrast, they suggested that the uterus falls forward (and thus does not cause aortocaval compression) when the patient assumes the sitting flexed position. They recommended that “the tight fetal curl position be avoided,” especially when the patient assumes the lateral decubitus position for identification of the epidural or intrathecal space.
Aortocaval compression must be avoided at all times. The gravid uterus can occlude the inferior vena cava and aorta when the parturient assumes the supine position.27-29 This position may cause maternal hypotension30,31 and reduce uteroplacental perfusion,32 even in the absence of anesthesia. Increased venous tone in the lower extremities helps overcome partial occlusion of the inferior vena cava in unanesthetized pregnant women. If maternal hydration is inadequate and if aortocaval compression is not avoided, the onset of anesthesia-induced sympathetic blockade may result in decreased venous return, cardiac output, and uteroplacental perfusion.29
Maternal position during placement of the epidural catheter does not seem to affect the incidence of unintentional dural puncture. However, adoption of the lateral recumbent head-down position for epidural catheter placement may reduce the incidence of epidural venous puncture.33
When spinal or epidural anesthesia is performed with the patient in a lateral position, the patient's back should lie at, and parallel to, the edge of the bed, for at least two reasons. First, the edge is the most firm section of the mattress. If the patient lies away from the edge of the bed, the patient's weight will depress the mattress, and the anesthesia provider must work in a “downhill” direction. Second, this position allows anesthesia providers to keep their elbows flexed, facilitating control of fine hand and wrist muscle movements. The plane of the entire back should be perpendicular to the mattress. When asked to flex the lower back, patients typically roll the top shoulder forward, an action that rotates the spine (which is undesirable) but does not flex the lower back.
Similarly, patients positioned sitting should have their feet supported by a stool with the backs of their knees against the edge of the bed, a maneuver that helps position the patient's back closer to the anesthesia provider. The shoulders should be relaxed symmetrically over the hips and buttocks. Beds in obstetric units often break at the foot, and the split in the mattress encourages the patient's seat to slope downhill if she is straddling the mattress split; this position will cause spine rotation and may make the procedure more difficult.
When spinal anesthesia is performed, the patient's posture relative to the baricity of the anesthetic solution should be considered, because it influences the extent of blockade, the latency of blockade, and the incidence of hypotension. The incidence, timing, and extent of hypotension in the period immediately after initiation of the block depend on the type of block (e.g., spinal, epidural, or combined spinal-epidural [CSE]), drug characteristics (e.g., baricity, concentration), patient position during the procedure, and patient position in the period following the procedure. For example, when spinal anesthesia is initiated with a hyperbaric solution for instrumental vaginal delivery, it often makes sense for the patient to be sitting to ensure the rapid onset of sacral anesthesia. Conversely, spinal anesthesia for cervical cerclage can be initiated with the patient in the steep lateral Trendelenburg position with a hypobaric anesthetic solution.
Posture has less influence on the spread of epidural anesthesia.34-36 During epidural anesthesia, a unilateral block more likely results from the malposition of the catheter (or perhaps an anatomic barrier within the epidural space) than from patient position, particularly after a bolus injection. Norris and Dewan34 observed that gravity did not augment the spread of anesthesia in patients receiving epidural anesthesia for cesarean delivery, and they concluded that posture does not need to be manipulated to ensure adequate bilateral epidural anesthesia. In at least two studies it was noted that the use of the sitting position is not necessary for the development of good sacral anesthesia when large volumes of epidural local anesthetic are given for cesarean delivery.34,36 However, Reid and Thorburn36 observed that use of the sitting position appeared to delay the spread of anesthesia to the midthoracic dermatomes. In comparison with the bolus administration of epidural local anesthetic, the extent of blockade may be more gravity dependent when the anesthetic is administered as a continuous infusion over a prolonged period.
Some anesthesiologists contend that maternal position after epidural catheter placement affects the efficacy of epidural analgesia, although this is a matter of some dispute. Beilin et al.37 observed that the placement of the laboring woman in the supine position with a 30-degree leftward tilt was associated with better epidural analgesia than maintenance of the left lateral decubitus position. In contrast, Preston et al.38 observed no difference in analgesia and a significantly higher incidence of fetal bradycardia with the supine wedged position than with the full lateral position.
Caudal anesthesia is used infrequently in modern obstetric anesthesia practice. However, there remain some circumstances in which a caudal technique is useful and/or advantageous. It is a good choice for the second stage of labor in selected patients in whom the lumbar epidural approach is hazardous or contraindicated (e.g., fusion or instrumentation of the lumbar spine). In most cases, caudal anesthesia can be successfully performed with the patient in a lateral decubitus position.
Aseptic Technique
There appears to be a great degree of practice variation among anesthesia providers with respect to aseptic technique during administration of neuraxial anesthesia.39-41 This lack of consensus may result from an underappreciation of the gravity of infectious complications related to neuraxial anesthesia.42-44 Indeed the incidence of epidural abscess and spinal meningitis is generally so low that many of the available recommendations are based on evidence from other domains of infection control (e.g., surgical wound site and central venous catheter–related infection).45-47 Nonetheless, neurologic compromise resulting from neuraxial infection can be a devastating complication.
Infection of the epidural space tends to result in the formation of an abscess, most commonly formed by Staphylococcus aureus found in the epidermis of either the patient or the anesthesia provider. In contrast, meningitis associated with neuraxial procedures is most commonly caused by Streptococcus viridans. Viridans species of streptococcus may reside in the oronasopharyngeal tract of providers or patients or in the vagina. Potential routes of infection include the (1) epidural catheter track, (2) bloodstream, (3) equipment, and (4) injectate. A more in-depth discussion of neuraxial infection is found in Chapter 32.
Guidelines describing aseptic technique for regional and neuraxial anesthetic procedures have been published by professional anesthesiology organizations, including the ASA, the Association of Anaesthetists of Great Britain and Ireland, and the American Society of Regional Anesthesia and Pain Medicine.47-49 The following recommendations deserve emphasis:
1. Given that the oropharyngeal and skin flora of the anesthesia provider are implicated in many cases of neuraxial infection, the provider should don a surgical facemask and hat before initiation of spinal/epidural anesthesia. Microbial sampling in laminar-flow operating theaters has shown a 22-fold increase in bacterial counts when a facemask and hat are not worn.50
2. Washing hands with an alcohol-based antiseptic solution is recommended because this has been shown to be superior to antimicrobial soap.51 Jewelry (e.g., rings, watches) should be removed before and sterile gloves worn after hand cleansing.
3. The patient's skin should be decontaminated, preferably with a chlorhexidine-in-alcohol solution.47 An abundance of evidence affirms the superior bacteriocidal and bacteriostatic efficacy of chlorhexidine compared with povidone-iodine.46,52 If chlorhexidine is not available, then povidone-iodine with alcohol, rather than povidone-iodine alone, is preferred.47,53 Of importance, the anesthesia provider is encouraged to exercise patience in allowing the antiseptic to dry, because a major mechanism of antisepsis is the desiccating action of alcohol.
Equipment and Placement of Needle/Catheter
Spinal Anesthesia
The first equipment decision involves determining whether to perform a single-shot or continuous technique. Continuous spinal anesthesia is not a new technique; indeed, some physicians performed continuous spinal anesthesia more than 50 years ago. Currently, a large-bore epidural needle and catheter must be used for continuous spinal anesthesia, because the U.S. Food and Drug Administration rescinded approval for the use of small-bore microcatheters in 1992.54 Therefore, the risk for post–dural puncture headache is significant. This technique is useful after unintentional dural puncture with an epidural needle. In the morbidly obese patient, it may be easier to manipulate and advance a rigid epidural needle than a more flexible spinal needle; thus the technique is useful for establishing continuous analgesia or anesthesia in this patient population, particularly when the need for anesthesia is urgent. However, for most obstetric patients, a single-shot technique is preferred for spinal anesthesia.
The primary equipment choice for spinal anesthesia concerns the type and size of the spinal needle. Cutting-bevel needles (e.g., Quincke) are rarely used in contemporary obstetric anesthesia practice because of the unacceptably high incidence of post–dural puncture headache associated with their use.55 Instead, non-cutting needles (e.g., Whitacre, Sprotte, Gertie Marx) are used almost exclusively (Figure 12-6). Some anesthesia providers refer to the non-cutting needles as “pencil-point needles.” It is now believed that the pencil-point needles cause more trauma to the dura, which then results in a more intense inflammatory response than occurs with cutting-bevel needles. Presumably, the inflammation results in more rapid closure of the dural defect.56
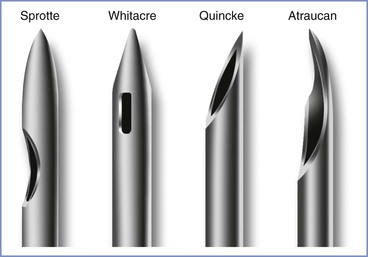
FIGURE 12-6 Spinal needle assortment often used in parturients. Each needle is shown in an open-bevel view and an oblique orientation. The Whitacre and Sprotte needles have cone-shaped bevels, whereas the Quincke has a cutting bevel. (Other sizes are available in some of these needle designs.)
Needle size must also be determined. Larger needles offer a greater fidelity of tactile feedback as the anesthesia provider traverses tissue planes of variable impedance when performing spinal anesthesia. Furthermore, larger needles are more likely to withstand the high resistance encountered when contacting bone without bending or shearing. In general, the “ease-of-use” advantages associated with larger needles must be balanced against a lower incidence of post–dural puncture headache with smaller needles. Most anesthesia providers use 25- or 27-gauge non-cutting needles for routine spinal anesthesia in obstetric patients. However, anesthesia providers should make individual decisions based on their own skills, practice setting, and the patient. The urgency of the procedure may also influence the choice of needle size. For example, a 27-gauge needle might be chosen for spinal anesthesia for an elective procedure, and a larger (e.g., 22-gauge) needle might be chosen when the subarachnoid space must be entered quickly because of severe fetal compromise.
With a small-gauge needle (i.e., 24-gauge or smaller), use of an introducer needle is preferable. The introducer needle engages the interspinous ligament and more accurately guides the trajectory of the smaller spinal needle than is possible with use of a small-gauge spinal needle alone. The introducer needle also aids with skin puncture; it is often difficult to puncture the skin with non-cutting needles.
Either the midline or the paramedian approach can be used to enter the subarachnoid space. The midline approach requires the patient to reduce her lumbar lordosis to allow access to the subarachnoid space between adjacent spinous processes (usually L3 to L4, sometimes L4 to L5 or L2 to L3). The interspinous space may be identified with one (usually the thumb or index finger) or two fingers (usually the index and middle fingers) of the anesthesia provider's nondominant hand. The single finger “slides” along the skin in the midline from cephalad to caudad until it “settles” into an interspinous space. The two fingers identify the interspinous space by palpating the caudad border of the more cephalad spine. The fingers identify the midline by rolling in a medial-to-lateral direction (Figure 12-7).
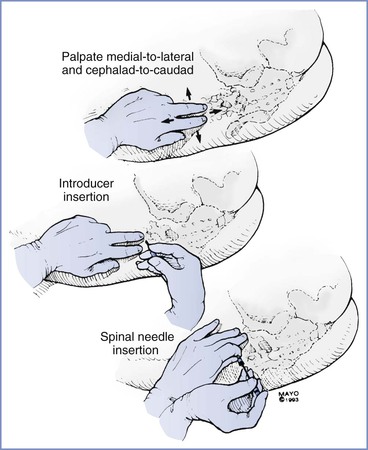
FIGURE 12-7 The midline approach for spinal needle insertion requires accurate identification of a lumbar interspinous space. The palpating fingers are rolled in a medial-to-lateral and cephalad-to-caudad direction; an introducer is then inserted through the interspinous space almost perpendicular to the lumbar spinous process. Once the introducer is seated in the interspinous ligament, the spinal needle is inserted; the needle is stabilized in a tripod fashion during insertion (much like a dart being thrown).
Next, the anesthesia provider injects local anesthetic intradermally and subcutaneously. The introducer needle is inserted into the substance of the interspinous ligament. It is helpful if the introducer needle is embedded in the interspinous ligament; therefore, obese patients may require a longer introducer needle. The introducer needle should lie in the sagittal midline plane. It is then grasped and steadied with the fingers of the nondominant hand while the dominant hand holds the spinal needle like a dart. The fifth finger may be used as a tripod against the patient's back to prevent patient movement from causing unintentional needle insertion to a level deeper than intended, and to “brake” the needle. As the needle passes through the ligamentum flavum and the dura, characteristic changes in resistance are noted. A “pop” is often perceived as the needle tip traverses the ligamentum flavum. A subsequent and more pronounced pop is perceived as the needle tip exits the dura-arachnoid. The stylet is removed, and CSF should appear in the needle hub. If CSF does not appear, the stylet is replaced, and the needle is advanced a few millimeters and again checked for CSF flow. This process continues until either bone is encountered or CSF returns through the needle. If neither occurs, the needle and introducer are withdrawn and the process is repeated.
Although with time and practice the tactile feedback produced by advancing a needle through tissues of variable resistance will become utterly familiar to the anesthesia provider, the novice may be unsure of the anatomic position of the needle tip, especially if unexpected resistance (i.e., contact with bone) or an unexpected and premature “pop sensation” is encountered during needle advancement. A stepwise problem-solving approach is reasonable. First, the anesthesia provider should reconfirm that (1) the patient has normal anatomy (i.e., not scoliotic), (2) she is acceptably positioned at an appropriate height for the anesthesia provider, and (3) the chosen point of needle insertion is the true midline plane. If these assertions are true, and the needle tip encounters bone, it is highly likely that the osseous structure is either the inferior or superior spinous process. One of two maneuvers may overcome this barrier. After slight withdrawal of the needle, simple angulation in a cephalad or caudad direction may redirect the needle trajectory sufficiently to achieve access to the central neuraxial canal. One must appreciate the “toughness” of the interspinous ligament. Even a 17-gauge epidural needle can be bent if the angle is changed without some prior retraction of the needle. Furthermore, if a spinal needle/introducer complex is used, care must be exercised that angulation of the spinal needle does not occur without first withdrawing it into the lumen of the introducer. Thereafter, the entire spinal needle/introducer complex is angulated before the spinal needle is re-advanced. Angulation of the spinal needle without first withdrawing it into the introducer creates a fulcrum at the junction of the introducer tip where the spinal needle emerges and can potentially damage or even shear the delicate spinal needle (Figure 12-8).57
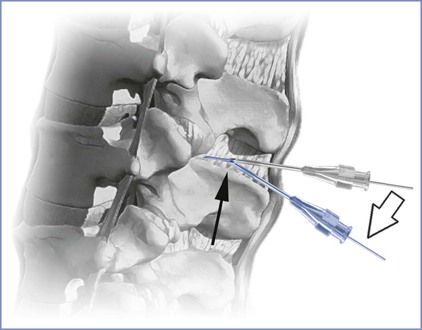
FIGURE 12-8 Change of needle trajectory during spinal anesthesia. Note that if both the spinal needle and its introducer needle are manipulated without prior retraction of the spinal needle into the lumen of the introducer (open arrow), a fulcrum is created (dark arrow) where the risk for bending or shearing the delicate spinal needle may occur. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Alternatively, the needle may be withdrawn fully into subcutaneous tissue and either raised or lowered (cephalad or caudad) while still maintaining an angulation that is parallel to the original trajectory. Which approach is more effective may depend on the reason for the initial bone contact. If the patient has very narrow interspaces, then careful raising or lowering of the needle while maintaining a trajectory parallel to the floor may be appropriate. However, if the patient is overly flexed forward, it is possible that her lumbar spinous processes are projecting in a slightly upward angulation (relative to the perpendicular transverse plane). This may require that the needle be re-angulated accordingly. If bone is still encountered despite all these considerations, it is likely that the needle tip is in fact not in the midline plane and is contacting the vertebral lamina. This may occur if the initial skin puncture is not in the midline, the needle tip deviates from the midline as it is advanced, or the patient's spine is rotated (either from poor positioning or scoliosis). Clues that the needle tip is not midline include (1) the patient complaining of lateralizing pain, (2) lack of CSF flow despite appropriate needle depth, and (3) the perception of “soft” or “mushy” tissue during needle advancement (paraspinous tissue) rather than the more “rigid” ligamentous tissue, or even a false “pop” as the needle tip exits the interspinous ligament laterally into paraspinous tissue. Much like the progressive modifications described earlier for correct alignment in the superoinferior plane, so too can these approaches be employed for redirecting in the lateral plane (Figure 12-9). The novice is advised to make systematic changes in a stepwise fashion, rather than indiscriminately changing needle direction without first considering the anatomic problem.
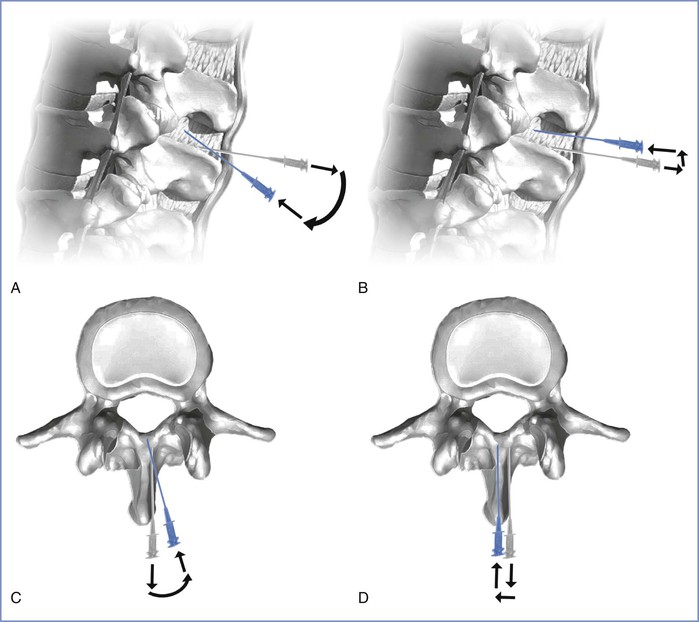
FIGURE 12-9 Troubleshooting contact with bony structures during needle placement. The gray needle represents the initial needle trajectory; the blue needle represents the adjusted needle trajectory. A, Assuming correct midline needle placement, the needle can be retracted slightly and angulated to overcome a spinous process. B, Alternatively, the needle may be “lifted” after slight retraction while keeping the original trajectory constant. C, Assuming the needle is deviating from the midline plane and contacting lamina, an action similar to that in A may be executed. D, Alternatively a stepwise lateral shift similar in concept to that shown in B may correctly achieve midline alignment. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Once CSF is freely dripping from the needle hub, the dorsum of the provider's nondominant hand steadies the spinal needle against the patient's back while the syringe with local anesthetic is attached to the needle. After aspirating to ensure the free flow of CSF, the anesthesia provider injects the local anesthetic at a rate of approximately 0.2 mL per second. After completion of the injection, some anesthesia providers again aspirate approximately 0.2 mL of CSF and reinject it into the subarachnoid space. This last step reconfirms the needle location and clears the needle of the remaining local anesthetic. The patient is then repositioned as appropriate.
For most patients, the midline approach is faster and less painful than the paramedian approach. The midline approach is also easier to teach than the paramedian approach, because it requires mental projection of the anatomy in only two planes, whereas the paramedian approach requires appreciation of a third plane and estimation of the depth of the subarachnoid space from the skin (Figure 12-10).
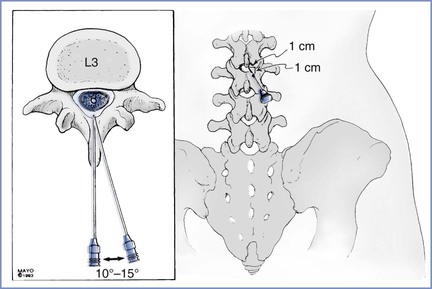
FIGURE 12-10 Vertebral anatomy of midline and paramedian approaches for spinal and epidural anesthesia. The midline approach requires anatomic projection in only two planes: sagittal and horizontal. The paramedian approach also requires consideration of the oblique plane. However, the paramedian approach requires less patient cooperation in reducing lumbar lordosis to allow for successful needle insertion. The paramedian needle insertion site is made 1 cm lateral and 1 cm caudad to the caudad edge of the more cephalad spinous process. The paramedian needle is inserted 10 to 15 degrees off the sagittal plane (inset).
Nevertheless, the paramedian approach is a useful technique that allows for the successful identification of the subarachnoid or epidural space in difficult cases. The paramedian approach does not require that the patient fully reduce her lumbar lordosis. This approach exploits the larger target that is available when the needle is inserted slightly off the midline.
A common error that is made with the paramedian approach is the insertion of the needle too far off the midline; the vertebral lamina then becomes a barrier to needle insertion. With the paramedian approach, the palpating fingers should again identify the caudad edge of the more cephalad spinous process. A skin wheal is raised 1 cm lateral and 1 cm caudad to this point; a longer needle is then used to infiltrate the deeper tissues in a cephalomedial plane. This step contrasts to the midline approach, in which the local anesthetic is not injected beyond the subcutaneous tissue. The spinal introducer is then inserted 10 to 15 degrees off the sagittal plane in a cephalomedial direction, and the spinal needle is advanced through the introducer needle toward the subarachnoid space. Another common error is to use an excessive cephalad angle with initial needle insertion. When the needle is inserted correctly and contacts bone, it is redirected slightly cephalad. If bone is again encountered, but at a deeper level, the slight stepwise increase in cephalad angulation is continued, and the needle is “walked” up and off the lamina. As with the midline approach, the characteristic feel of the ligamentum flavum and dura can be appreciated. The aim of the paramedian approach is to puncture the dura in the midline, even though the needle is inserted off the midline. Use of the paramedian approach requires insertion of a greater length of needle. Once CSF is obtained, the block is performed as it is with the midline approach.
During the performance of any nerve block technique, needle advancement should stop if the patient complains of pain. If pain is the result of inadequate soft tissue anesthesia, additional local anesthetic should be injected. Pain or paresthesias may also result from needle contact with central nerves or the spinal cord. Patient perception of paresthesias during the initiation of spinal anesthesia may indicate that the needle tip is in the subarachnoid space. The anesthesia provider should remove the stylet and check for CSF. If the paresthesia has resolved, the local anesthetic may be injected. If the paresthesia persists, however, the needle should be withdrawn and repositioned. In any case, the anesthesia provider should never inject the local anesthetic if the patient is complaining of paresthesias or lancinating pain, either of which may signal injection into a nerve or the spinal cord.
Epidural Anesthesia
Special equipment for epidural analgesia or anesthesia includes an epidural needle, an epidural catheter (for a continuous technique), and a loss-of-resistance syringe (for the loss-of-resistance technique to identify the epidural space). Single-shot epidural anesthesia is rarely used in obstetric practice, because the major advantage of epidural over spinal anesthesia is the ability to provide continuous anesthesia or analgesia without puncturing the dura with a large needle. An epidural needle with a lateral opening (e.g., Hustead, Tuohy) is most commonly used because it allows a catheter to be threaded through its orifice (Figure 12-11).
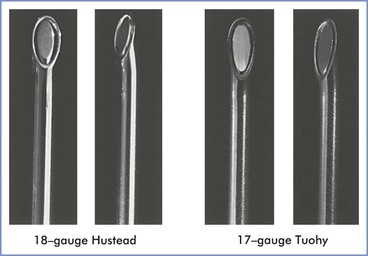
FIGURE 12-11 Epidural needles often used in parturients. Each needle is shown in an open-bevel view and an oblique orientation. The 18-gauge Hustead and 17-gauge Tuohy needles have lateral-facing openings, which direct epidural catheters to enter the epidural space more easily than if a single-shot Crawford needle design is used. (Other sizes and needle designs are available for obstetric epidural anesthesia.)
Two methods are used to identify the epidural space during needle advancement: (1) hanging drop method and (2) loss-of-resistance method. The majority of anesthesia providers use the loss-of-resistance method (Figure 12-12).
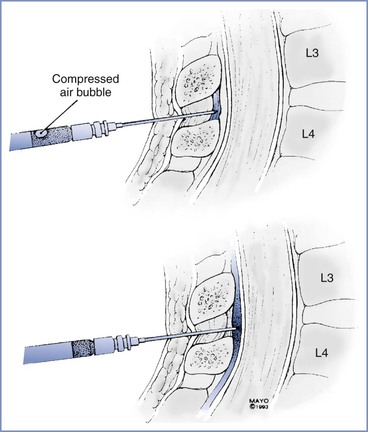
FIGURE 12-12 Loss-of-resistance technique for identifying the epidural space. The needle is first inserted into the interspinous ligament or ligamentum flavum, and a syringe containing an air bubble in saline is attached to the hub. After compression of the air bubble by pressure on the syringe-plunger, the needle is carefully advanced until a loss of resistance to syringe-plunger pressure is noted as the needle enters the epidural space.
The traditional loss-of-resistance syringe is a finely ground glass syringe with a Luer-Lok connector. Plastic syringes are now available, and the choice is generally a matter of the anesthesia provider's preference. The syringe is filled with 2 to 4 mL of saline, air, or saline with a small (0.25 to 0.5 mL) air bubble. There is some controversy regarding the use of air versus saline for detecting the point of loss of resistance.58 Saline causes some syringe plungers to stick and may be confused with CSF during initiation of CSE anesthesia. Conversely, injection of air into the epidural space may contribute to patchy anesthesia,59 and unintentional pneumocephalus may increase the risk for post–dural puncture headache.60
Prior investigations have suggested superiority of loss-of-resistance with saline over air with respect to block success. This conclusion is further supported by the results of a meta-analysis that pooled six controlled trials (n = 1037) in which laboring women were randomized to receive loss-of-resistance to air versus liquid (saline or local anesthetic solution).61 This analysis suggested an increased risk for unblocked segments when loss-of-resistance to air was used, presumably owing to air bubbles leaking through the intervertebral foramina and residing adjacent to nerve roots.62 No differences were found with respect to the occurrence of paresthesias or unintentional dural puncture or the need for additional analgesic medication or epidural catheter replacement. The authors recommended a cautious interpretation of their analysis because many of the endpoints studied (e.g., “block success”) do not have widely accepted definitions. In contrast, Schier et al.63 report a meta-analysis that included both obstetric and nonobstetric patients (n = 4422), which suggested no significant difference between loss-of-resistance to air versus saline in the occurrence of difficult catheter insertion, paresthesias, intravascular catheter insertion, unintentional dural puncture, post–dural puncture headache, and partial block. Lastly, in a retrospective study of loss-of-resistance to air versus saline by Segal and Arendt,64 no significant differences in block success were found in 929 patients. The authors intentionally chose a retrospective approach to the question; they stated that because “it is impossible to mask the anesthesiologist to the medium used for loss-of-resistance, [they] hypothesized that randomized controlled trials might overestimate the difference between air and saline by forcing the operator to use a less-preferred technique in half of the subjects.”
Regardless of the technique used, success depends on correct placement of the needle tip within the ligamentum flavum. The needle should be advanced sufficiently into the interspinous ligament before the syringe is attached or before the hanging drop of solution is placed into the needle hub. This approach has at least three advantages. First, it encourages the anesthesia provider to use proprioception while directing and advancing the needle. Second, it shortens the time required for successful identification of the epidural space. Third, it lowers the likelihood of a false-positive loss of resistance. Undoubtedly, this false-positive identification of the epidural space is responsible for many cases of unsuccessful epidural anesthesia; it is even possible to insert a catheter between the interspinous ligament and the ligamentum flavum.
During advancement of the needle-syringe assembly, the needle should be moved toward the epidural space by the provider's nondominant hand while the thumb of the dominant hand applies constant pressure on the syringe plunger, thereby compressing the small air bubble. Alternatively, the intermittent, oscillating technique is typically employed when using the loss-of-resistance to air technique. When the needle enters the epidural space, the pressure applied to the syringe plunger causes the solution or air to flow easily into the epidural space (see Figure 12-12).
In most obstetric cases, the anesthesia provider inserts a catheter and uses an intermittent bolus or continuous infusion technique to maintain analgesia. Most practitioners insert the catheter before injecting local anesthetic to allow for the slow, incremental injection of local anesthetic/opioid solution and the more controlled development of epidural anesthesia. If the principal reason for using an epidural technique is the provision of continuous analgesia, it seems most practical to insert the catheter before injecting the therapeutic dose of local anesthetic so that correct catheter placement can be verified promptly.
Several types of single-use, disposable epidural catheters are available. Catheters are made from plastic materials and differ as to the degree of “stiffness.” Wire-embedded catheters are more flexible and are associated with a lower incidence of paresthesias and intravascular placement during catheter insertion.33,65,66 The single-orifice catheter has one opening at its tip, whereas the multi-orifice catheter has a closed “bullet” tip with three lateral orifices between 0.5 and 1.5 cm from the tip (Figure 12-13).
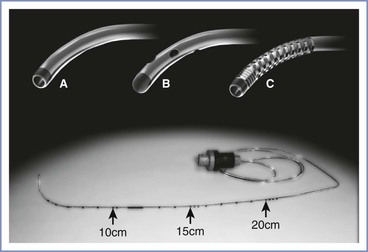
FIGURE 12-13 Epidural catheters. A, Single-orifice catheter; B, multi-orifice catheter with bullet tip; C, coiled wire reinforced catheter. Bottom, Epidural catheter with centimeter markings along distal end and Luer-Lok connector at proximal end. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
The proposed advantage of single-orifice, open-end catheters is that the injection of drugs is restricted to a single anatomic site. In theory, this arrangement should facilitate the detection of intravenous or subarachnoid placement of the catheter. Likewise, a theoretical disadvantage of multi-orifice, closed-end catheters is that local anesthetic may be injected into more than one anatomic site (e.g., both the epidural and subarachnoid spaces). A catheter initially placed in the epidural space can migrate into a vein or the subdural or subarachnoid space. Fortunately, this does not seem to be a common clinical problem. Regardless of the choice of catheter, aspiration should be performed before each dose of local anesthetic is injected.
An advantage of the multi-orifice catheter over the single-orifice catheter is the consistent ability to aspirate fluid (either blood or CSF) when the catheter is in a vessel or the subarachnoid space.67 Multi-orifice catheters may lead to more even distribution of local anesthetic and a lower incidence of “patchy” or unilateral anesthesia when the anesthetic is injected as a bolus.68 However, during an infusion into the epidural space, the solution exits only the most proximal hole,69 and multi-orifice catheters thus behave like single-orifice catheters.
If the catheter is placed before the test and therapeutic doses of local anesthetic, it may be helpful to inject 5 to 10 mL of saline before threading the catheter, because this may reduce the incidence of epidural vein cannulation,33 particularly when using stiffer epidural catheters. Rolbin et al.70 noted that there was no advantage to the injection of 3 mL of fluid into the epidural space before insertion of the epidural catheter.
Six to eight centimeters of catheter are threaded into the epidural space before the epidural needle is removed. The catheter may then be pulled back until it is at the desired distance at the skin. Occasionally, the anesthesia provider will have difficulty advancing the catheter past the tip of the epidural needle. This difficulty may indicate that the epidural needle tip is not in the epidural space. However, if the provider is convinced that the needle is correctly placed, several maneuvers may facilitate catheter advancement. Often, having the patient take a deep breath allows catheter advancement. Saline may be injected through the epidural needle if this has not been done. Although some providers rotate the epidural needle in an attempt to successfully advance the catheter, we do not recommend this maneuver, because it may increase the risk for dural puncture. Instead, the epidural needle should be withdrawn 0.5 to 1 cm and again advanced into the epidural space.
Many techniques are available for securing the epidural catheter at the skin entry site. If a catheter will be used for prolonged intrapartum or postoperative analgesia, care providers should be able to assess the skin surrounding the catheter. A transparent, sterile adhesive dressing applied over the catheter after application of skin adhesive generally works well, and the periphery of the dressing can be reinforced with tape. The position of the epidural catheter may change significantly with patient movement from the sitting-flexed to the sitting-upright or lateral decubitus position.71 D'Angelo et al.72 found that the risk for catheter dislodgement was higher when catheters were inserted 2 cm into the epidural space, but the risk for unilateral blockade was greater when catheters were inserted 6 to 8 cm. Therefore, if the catheter is to be used for a short period (e.g., during cesarean delivery), it should be left 2 to 4 cm into the epidural space. In contrast, if the catheter will be used for many hours (e.g., during labor), it should be left 4 to 6 cm into the space. To minimize catheter movement at the skin, the patient should be positioned sitting upright or in the lateral position before the catheter is secured, especially if the patient is obese.71
The potential for the contamination of local anesthetic solutions has prompted the use of micropore filters during the administration of continuous epidural analgesia for labor. There is no evidence that filters decrease the rate of infection or of injection of undesirable foreign substances.73 Additionally, filters may reduce the reliability of aspiration74 and absorb local anesthetic solution, unless they are primed.75 We believe that micropore filters have little use in clinical obstetric anesthesia practice.
Combined Spinal-Epidural Anesthesia
Combined spinal-epidural anesthesia combines the advantages and mitigates the disadvantages of single-shot spinal anesthesia and continuous epidural anesthesia (Box 12-2). Anesthesia is initiated with a subarachnoid injection of local anesthetic and maintained via an epidural catheter. It is useful for both cesarean delivery anesthesia and labor analgesia. For cesarean delivery, the injection of the smaller dose of local anesthetic required for spinal (compared with epidural) anesthesia is inherently safer with regard to the possibility of unintentional intravascular injection. Additionally, the anesthesia provider can inject a local anesthetic dose that is lower than the ED95 (effective dose in 95% of cases) without fear of inadequate anesthesia. These lower doses result in less maternal hypotension.76 If surgical anesthesia is inadequate, the block can be “rescued” with epidural administration of local anesthetic. For example, a randomized trial comparing 7, 8, and 9 mg of intrathecal bupivacaine administered as part of a CSE technique for cesarean delivery produced equivalent latencies to T4 sensory block with lower rates of maternal hypotension in the lowest dose group.77 The shorter duration of action seen in the low-dose (7 mg) group was easily addressed through the administration of local anesthetic via the indwelling epidural catheter. Compared with conventional epidural anesthesia for cesarean delivery, CSE anesthesia is associated with a more rapid onset of surgical anesthesia, less intraoperative pain and discomfort (e.g., a more dense block), better muscle relaxation, and less shivering and vomiting.78
During labor, CSE analgesia is associated with a faster onset of analgesia and is generally achieved with an opioid alone or an opioid combined with a small dose of local anesthetic. Studies differ as to whether CSE analgesia is associated with higher maternal satisfaction and fewer requests for supplemental analgesia. Goodman et al.79 randomized 100 parous women in early labor to receive either CSE or conventional epidural analgesia. There were no differences in requests for supplemental analgesia, although pain scores were lower in the CSE group within the first 30 minutes. This study harbors limitations echoed by many of its predecessors, including (1) question of equipotency between the techniques, (2) inadequate sample size for assessment of secondary outcomes, and (3) inability to truly blind the study (the difference in analgesia latency readily identifies group allocation). A 2007 systematic review comparing CSE and epidural labor analgesia concluded that onset was faster with the CSE technique, but that there was no evidence for differences in maternal satisfaction, mode of delivery, ability to ambulate, or incidence of hypotension between the two techniques.80 Several studies have found a lower incidence of failed epidural analgesia after the initiation of analgesia with a CSE technique.81,82 Presumably, verification of the correct placement of the spinal needle by visualization of CSF increases the likelihood that the tip of the epidural needle is correctly placed in the epidural space.
A disadvantage of the CSE technique is that the correct placement of the epidural catheter in the epidural space cannot be verified until spinal anesthesia wanes. Therefore, if a functioning epidural catheter is important to the safe care of the mother and fetus (e.g., in the setting of a suspected difficult airway or nonreassuring fetal status), a CSE technique may not be the technique of choice.
There are several techniques for initiation of CSE anesthesia/analgesia.83 The most popular is the needle-through-needle technique, in which the epidural needle is sited in the epidural space and serves as an introducer for the spinal needle. The spinal needle passes through the epidural needle to puncture the dura. After injection of the subarachnoid dose, the spinal needle is removed, and the epidural catheter is threaded through the epidural needle. An alternative technique uses two skin punctures and two different interspaces: the spinal needle and epidural needle and catheter are introduced sequentially in two different interspaces.
The needle-through-needle technique requires a long spinal needle. Typically, a small (25-gauge or smaller) non-cutting needle is used to minimize the risk for post–dural puncture headache. The tip of the spinal needle must protrude 12 to 17 mm beyond the tip of the epidural needle when the two needles are fully engaged (Figure 12-14). Failure to puncture the dura and visualize CSF occurred in 25% of patients when the spinal needle protruded 9 mm, compared with no patients when the needle protruded 17 mm.84 A 127-mm spinal needle is commonly used with a standard 9-cm epidural needle. However, because of differences in hub configurations among needles, the two hubs may not “mesh,” and spinal needle protrusion may vary with specific needle combinations. Alternatively, manufacturers now sell CSE needle “kits,” in which the spinal needle is designed for a specific epidural needle. An additional small non–Luer-Lok syringe (1 to 3 mL) is required for the spinal dose.
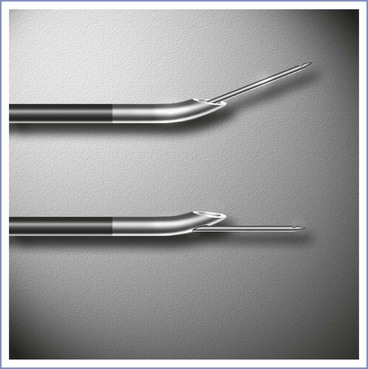
FIGURE 12-14 Combined spinal-epidural needle configuration. Top, Spinal needle exits the epidural needle through the normal epidural needle bevel. Because the epidural needle bevel opening faces sideways, the spinal needle exits the epidural needle at a slight angle to the long axis of the epidural needle. Bottom, Spinal needle exits the epidural needle through a special orifice. The axes of the spinal and epidural needles are aligned. The spinal needle must protrude from the tip of the epidural 12 to 17 mm when the hubs are engaged, or the ability to puncture the dura with the spinal needle is compromised. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
CSE anesthesia is initiated much like epidural anesthesia. The epidural needle is sited in the epidural space (Figure 12-15). Prior to inserting the epidural catheter, the spinal needle is introduced through the epidural needle with the anesthesia provider's dominant hand, while the nondominant hand is anchored against the patient's back to serve as a brake for further advancement of the spinal needle. The provider usually perceives the tip of the spinal needle passing the tip of the epidural needle as a slight increase in resistance. Spinal needle advancement should stop immediately after the anesthesia provider perceives the dural puncture “pop.” Dural puncture is verified by visualization of CSF after removal of the spinal needle stylet. The provider's nondominant hand is anchored on the patient's back, and the spinal and epidural needle hubs are grasped together between the thumb and index finger of this hand. The dominant hand attaches the spinal syringe and injects the drug. We do not attempt to aspirate CSF, because it may not be possible to do so through long, small-bore needles and because attempted aspiration may result in movement of the spinal needle. After removal of the spinal syringe and needle as a unit, the epidural catheter is threaded in the usual fashion.
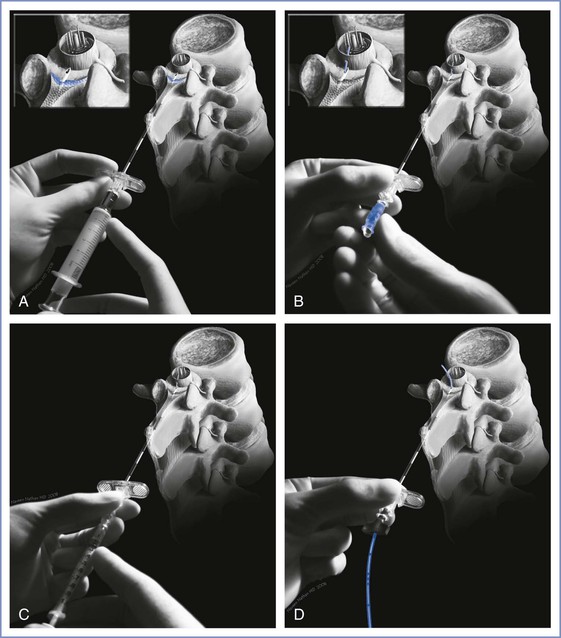
FIGURE 12-15 Needle-through-needle combined spinal-epidural technique. A, The epidural needle is sited in the epidural space. B, The long spinal needle is passed through the epidural needle and punctures the dura mater. The operator's nondominant hand stabilizes the spinal and epidural needles, and the spinal needle stylet is withdrawn. Cerebrospinal fluid is seen spontaneously dripping from the spinal needle. C, The syringe is attached to the spinal needle, and the intrathecal dose is injected. D, The spinal needle is withdrawn, and the epidural catheter is threaded through the epidural needle into the epidural space. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Failure to puncture the dura with the spinal needle may occur in several circumstances (Figure 12-16). The epidural needle tip may not be located in the epidural space, or the needle tip may be correctly placed, but the spinal needle may fail to puncture the dura or may not reach the dura because of the depth of the posterior epidural space. Alternatively, the epidural needle may be angled away from the midline or in a sagittal plane off the midline and the spinal needle may traverse the lateral epidural space without puncturing the dura. In this latter circumstance, the anesthesia provider may elect to abandon the CSE technique and continue with epidural anesthesia (if convinced that the epidural needle tip is in the epidural space) or to reposition the epidural needle and reattempt the CSE technique.
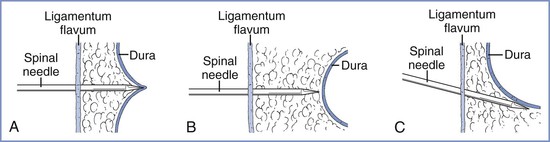
FIGURE 12-16 Reasons for failure of the combined spinal-epidural technique. A, The spinal needle tents the dura but does not puncture it. B, The spinal needle does not reach the dura. C, The spinal needle passes to the side of the dural sac. (Redrawn with permission from Riley ET, Hamilton CL, Ratner EF, Cohen SE. A comparison of the 24-gauge Sprotte and Gertie Marx spinal needles for combined spinal-epidural analgesia during labor. Anesthesiology 2002; 97:574.)
Caudal Anesthesia
Equipment for caudal anesthesia is similar to that used for lumbar epidural techniques, except that a needle with a lateral-faced opening is not needed. A blunt-tipped needle is satisfactory even when a catheter is used, because the angle of needle insertion allows insertion of the catheter. Successful administration of caudal anesthesia requires the accurate identification of the sacral hiatus. The sacrococcygeal ligament (an extension of the ligamentum flavum) overlies the sacral hiatus between the sacral cornua. Identification of the posterior superior iliac spines facilitates the identification of the sacral cornua; the location of the sacral hiatus is approximated by using the line between them as one side of an equilateral triangle (Figure 12-17). Once the sacral hiatus is identified, the palpating fingers are placed on the cornua, the skin is anesthetized, and the caudal needle is inserted with the hub at an angle approximately 45 degrees from the skin. A decrease in resistance is noted when the needle enters the caudal canal. The needle is advanced until it contacts bone (i.e., the dorsal aspect of the ventral plate of the sacrum). Next, the needle is withdrawn slightly and redirected so that the angle of insertion relative to the skin surface is decreased. In pregnant women, the final angle is approximately 15 degrees from a plane parallel to the sacrum.
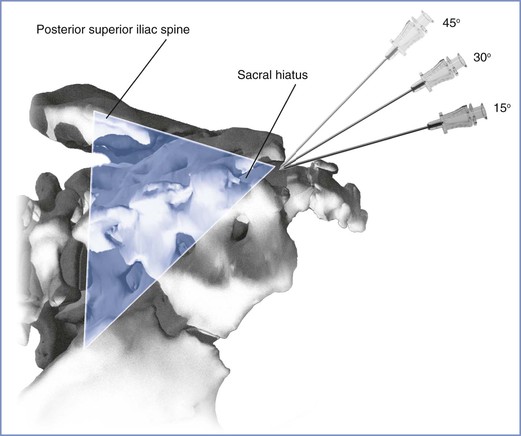
FIGURE 12-17 The location of the sacral hiatus for caudal anesthesia is facilitated by the identification of the posterior superior iliac spines. The posterior superior spines are marked, and a line drawn between them forms one edge of an equilateral triangle. If the triangle is completed as illustrated, the sacral hiatus should underlie the caudad tip of the equilateral triangle. Once the sacral hiatus is identified, the needle is inserted by insertion and withdrawal in a stepwise fashion from an initial 45-degree angle off the coronal plane. In pregnant women, the needle eventually enters the caudal canal at an angle approximately 15 degrees off the coronal plane. If the needle is placed properly, no subcutaneous “lump” develops after the injection of the local anesthetic solution. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Accurate placement of the caudal needle is verified primarily from the “feel” of the needle passing through the sacrococcygeal ligament. An additional maneuver may help providers with less experience to verify correct needle placement: Once the needle is believed to be within the caudal canal, 5 mL of saline is rapidly injected through the needle while the anesthesia provider's other hand is placed over the dorsum of the sacrum. If the needle is placed correctly, no mass or pressure wave is detected over the midline of the sacrum. Conversely, if the needle is malpositioned (often posterior to the caudal canal), a fluid mass or pressure wave is felt by the palpating hand.
The needle should be advanced only 1 to 2 cm into the caudal canal. Dural puncture or unintentional intravascular cannulation is more likely to occur with deeper insertion. A test dose similar to that used during administration of lumbar epidural anesthesia should be administered.
Ultrasonographic Guidance
Traditionally, surface anatomy visualization and palpation have been used to assess landmarks before initiation of the neuraxial procedure. This landmark-guided technique is effective for the vast majority of patients and, as a consequence, the use of ultrasonography has not played as prominent a role in neuraxial anesthesia as it has in peripheral nerve blockade. There exist, however, unique clinical circumstances in which immediate pre-procedural imaging of epidural anatomy can be highly beneficial. Such may be the case in patients with morbid obesity, derangements of spinal anatomy such as scoliosis or spinal stenosis, or a history of spinal instrumentation, and in patients in whom identification of specific vertebral levels might be warranted (e.g., known preexisting disc herniation or nerve root compression at a specific interspace). Unlike the use of ultrasonography for vascular access and peripheral nerve block techniques, ultrasonography for neuraxial techniques is not used in real time. Indeed, the real-time use of ultrasonography during administration of neuraxial anesthesia can be prohibitively cumbersome, is fraught with a tangible risk for violating aseptic technique, and raises unanswered questions about what untoward effects may result should ultrasound coupling medium (gel) breach neuraxial structures. Most often ultrasonography is a pre-procedural tool to aid the operator in the assessment of needle insertion site, needle angle, and estimated depth of the epidural space.
It remains to be seen whether neuraxial ultrasonography can significantly impact the clinical success of neuraxial procedures, whether that is measured in terms of time to completion of the spinal or epidural anesthesia procedure, or block success and patient satisfaction. Additionally, reductions in the incidence of complications of neuraxial anesthesia, such as unintentional dural puncture, have yet to be realized.
In the obstetric population, four randomized controlled trials have explored outcomes related to block success using the traditional landmark-based technique versus the ultrasound-aided technique.85-88 Only one, however, divorced the unblinded ultrasonographer from those supervising the block and rendering assessments of success.88 In this investigation,88 370 parturients receiving epidural labor analgesia were randomized to receive pre-procedural ultrasonographic imaging for determination of depth to epidural space or no imaging (control group). The estimated depth to the epidural space was conveyed to 15 first-year residents with little or no previous neuraxial anesthesia experience, who performed the blocks under the supervision of a staff anesthesiologist who was blinded to the group assignment. Obese patients were not excluded from this study, and there were no significant differences between groups in demographic factors. The authors found a significant reduction in the number of attempts needed to place the epidural catheter and in the need for catheter replacement in the ultrasonography group. There was no difference in the rate of unintentional dural puncture, although the study was underpowered for assessment of this outcome. The calculated number of epidural catheter placements with ultrasonography needed to avoid replacing one failed catheter (number needed to treat) was 26. Of note, the residents were not themselves randomized, nor were they tracked according to the number of cumulative procedures with and without ultrasonography. From the perspective of introducing trainees to the technique of neuraxial anesthesia procedures, ultrasonographic imaging may yet play a more substantial educational role.
How does pre-procedural ultrasonographic imaging affect neuraxial anesthesia success for patients anticipated to endure difficult needle placement? Chin et al.89 identified 120 patients presenting for elective lower extremity orthopedic surgery who were predicted to endure difficult administration of spinal anesthesia. Their basis for qualifying these patients as “difficult” included a body mass index greater than 35 kg/m2 with nonpalpable spinous processes, the presence of moderate to severe scoliosis, or a history of spinal surgery involving excision of two or more lumbar spinous processes. These subjects were randomized to receive pre-procedural ultrasonographic imaging of epidural anatomy or a conventional landmark-based technique. The total number of attempts required before successful dural puncture was twofold higher in the landmark control group than in the ultrasonography group. Although the average time spent completing the spinal anesthesia procedure was longer in the control group than in the ultrasonography group (mean = 7.3 minutes versus 5.0 minutes, respectively), if one accounted for the pre-procedural ultrasonographic examination, the total procedure time was shorter in the control group (7.9 minutes versus 12.2 minutes).
How accurately do pre-procedural ultrasonoanatomic measurements correlate to the actual distance from the skin to the epidural space? In general, although the correlation is high, ultrasonographic measurements of neuraxial anatomy may underestimate the true distance from skin to epidural space in the context of neuraxial procedures.88,90-92 The factors that influence the small disparity between the two include (1) differences between the angulation of the imaging beam versus the angulation of the needle; (2) differences between the degree of exerted pressure and skin compression of the ultrasound probe versus the needle; (3) current lack of fidelity in the ability of ultrasonography to discriminate between ligamentum flavum, epidural space, and dura mater; and (4) operator dependency of typical onscreen measurement tools such as digital calipers. In a general obstetric population, the mean difference between the needle depth and ultrasonographic depth to the epidural space was 0.01 cm (95% confidence interval, −0.67 to 0.69 cm),92 whereas in a second study by the same investigators in which subjects were limited to obese parturients, the mean difference was 0.3 cm (95% confidence interval, −0.7 cm to 1.3 cm).90
A low-frequency (2- to 5-Hz) curvilinear probe allows visualization of neuraxial structures beneath the skin. Low-frequency waves are preferable owing to the requisite depth of penetration. The curvilinear array allows enough ultrasonic scope to capture lateral structures such as the transverse processes. The ultrasound beam can be used to identify first the spinous processes if these are not palpable, then the interspinous spaces, and, finally, ligamentous structures. The ligamentum flavum and dura mater are dense tissues and will appear hyperechoic (white), like bone, whereas the less dense epidural and subarachnoid spaces will appear hypoechoic (black). A variety of imaging planes are now well described. Transverse and median sagittal as well as paramedian sagittal approaches have been documented. The transverse-interlaminar view is likely to be of most use when attempting neuraxial procedures using the midline approach. On the other hand, the paramedian sagittal view may be of use if a paramedian approach is anticipated (Figure 12-18). With the median sagittal approach, the spinous process will produce a shadow when the beam is placed directly over it, thus reducing the ability to appreciate any ligaments beyond it.
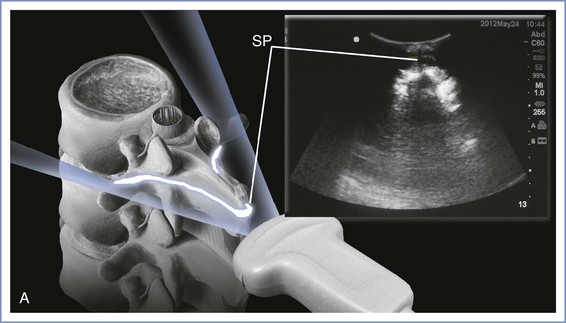
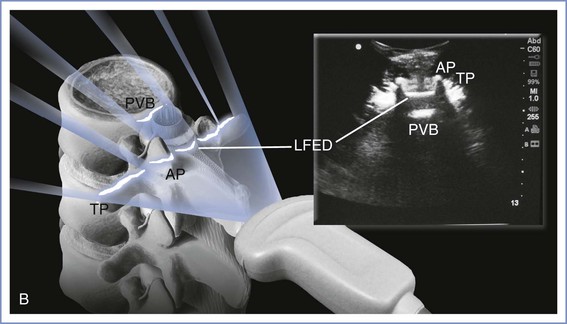
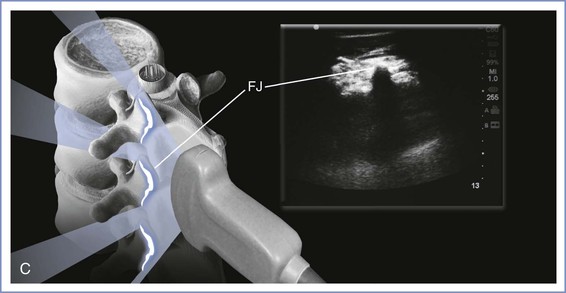
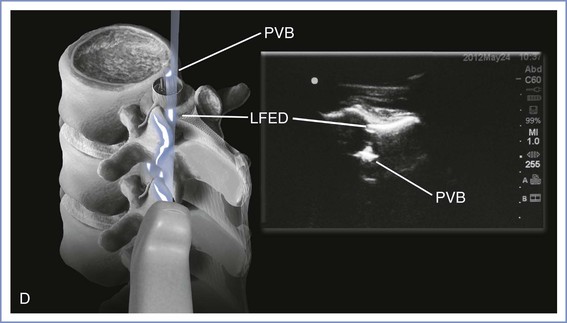
FIGURE 12-18 Use of ultrasonography. A, Transverse spinous process view: A long, dark shadow is cast with little to no discernible anatomy beyond the osseous tip of the spinous process (SP). B, Transverse interlaminar view: ultrasound waves propagate through the interspinous ligament leading to signals partially reflected by the ligamentum flavum–epidural space–dura mater complex (LFED). The rays that continue to transmit through this complex encounter the posterior wall of the vertebral body (PVB). Articular (AP) and transverse processes (TP) can be seen as well. C, Paramedian view: a so-called sawtooth pattern may be seen as the lamina and facet joints (FJ) are captured by this view, typically achieved 1 cm lateral to the midline sagittal plane. D, Paramedian view: by angulating the ultrasound probe, the ultrasound waves may escape through the interlaminar and transforaminal “windows” and capture the ligamentum flavum-epidural space-dura mater complex (LFED) as well as the posterior surface of the vertebral body (PVB). (Drawings by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Epidural Test Dose
Epidural catheter placement may be complicated by blood vessel or dural puncture with the needle or catheter. To prevent possible local anesthetic toxicity and high or total spinal anesthesia, the anesthesia provider must recognize the unintentional intravenous or subarachnoid placement of the needle or catheter. The purpose of the test dose is to allow early recognition of a malpositioned catheter. The ideal test dose must be readily available, safe, and effective. Its use should have a high sensitivity (i.e., low false-negative rate) and a high specificity (i.e., low false-positive rate). The intravascular and intrathecal test doses may be combined (a single injection to test for both intravascular and subarachnoid placement) or administered separately. A negative response to an epidural test dose does not guarantee the correct placement of the epidural catheter in the epidural space, nor does it guarantee that the catheter is not malpositioned in a blood vessel or the subarachnoid space. Rather, it decreases the likelihood that the catheter tip is in a blood vessel or the subarachnoid space.
Intravascular Test Dose
The ideal method for excluding intravenous placement of the catheter is controversial. Intravascular placement of the epidural catheter may occur in as many as 7% to 8.5% of obstetric patients.93 Failure to recognize intravenous placement of the epidural catheter and subsequent intravenous injection of a large dose of local anesthetic may lead to systemic local anesthetic toxicity, with CNS symptoms, seizures, cardiovascular collapse, and death.94
The most common intravascular test dose contains epinephrine 15 µg. In normal volunteers, intravenous injection of epinephrine 15 µg (3 mL of a 1 : 200,000 solution) reliably causes tachycardia.95,96 An increase in heart rate of 20 beats per minute (bpm) within 45 seconds was 100% sensitive and specific for intravascular injection in unpremedicated patients.95 An increase in systolic blood pressure of between 15 and 25 mm Hg was also observed. Some anesthesiologists have expressed concerns about the use of an epinephrine-containing test dose in laboring women. Intravenous epinephrine may cause a transient decline in uterine blood flow as a result of alpha-adrenergic receptor–mediated constriction of the uterine arteries (Figure 12-19).97-99
FIGURE 12-19 The effect of intravenous epinephrine (EPI), lidocaine (LIDO), and lidocaine with epinephrine on uterine artery blood flow velocity (UBFV) in the pregnant guinea pig. The dose of lidocaine was 0.4 mg/kg. Values are presented as mean ± standard error of mean (SEM) percentage of baseline. (From Chestnut DH, Weiner CP, Martin JG, et al. Effect of intravenous epinephrine on uterine artery blood flow velocity in the pregnant guinea pig. Anesthesiology 1986; 65:633-6.)
However, this decrease in uterine blood flow is transient and comparable to the decrease that occurs during a uterine contraction. Youngstrom et al.100 noted that this intravenous dose of epinephrine did not worsen fetal condition in acidotic fetal lambs. In healthy parturients, any transient effect of epinephrine on uterine blood flow likely represents a less severe insult than systemic local anesthetic toxicity. An epinephrine-containing test dose, however, may not be appropriate in parturients with severe hypertension or uteroplacental insufficiency.
Some anesthesiologists argue that the epinephrine-containing test dose lacks specificity in laboring women. The maternal tachycardic response to intravenous injection of epinephrine cannot always be distinguished from other causes of tachycardia (e.g., pain during a uterine contraction) (Figure 12-20).101,102
FIGURE 12-20 Heart rate of a laboring patient (maternal heart rate [MHR]), fetal heart rate, and uterine contractions (UC) are shown. This tracing was obtained with the use of an FHR monitor with dual heart rate capacity. Note the variability of the MHR with uterine contractions. An intravenous injection of bupivacaine 12.5 mg and epinephrine 12.5 µg was given (arrow). Note the marked increase in MHR in response to intravenous injection of the test dose. The maternal tachycardia had a duration of approximately 40 seconds. (From Van Zundert AA, Vaes LE, De Wolf AM. ECG monitoring of mother and fetus during epidural anesthesia. Anesthesiology 1987; 66:584-5.)
Cartwright et al.101 noted that 12% of laboring women had an increase in heart rate of at least 30 bpm after epidural injection of 3 mL of 0.5% bupivacaine without epinephrine. A study in laboring women compared the intravenous injection of epinephrine (10 to 15 µg) with that of saline; the sensitivity was 100%, the area under the receiver operator curve was 0.91 to 0.93, and the negative predictive value was 100%.103 However, the positive predictive value was 55% to 73%. The results suggested that if a positive heart rate response to an epinephrine-containing test dose occurs in 20% of patients, 5% to 9% of epidural catheters would be identified incorrectly as intravascular and removed unnecessarily. Colonna-Romano and Nagaraj104 concluded that the intravenous injection of an epinephrine-containing test dose results in “a sudden and fast acceleration in maternal heart rate within one minute.” Thus, careful assessment of the rate of increase in maternal heart rate may help distinguish a contraction-induced increase in heart rate from the effect of intravenously injected epinephrine, thereby improving the specificity of the epinephrine test. It is unclear whether such an assessment is clinically practical or will actually reduce the incidence of false-positive results.105
Additionally, some anesthesiologists argue that the epinephrine-containing test dose lacks sensitivity (the ability to elicit a predictable increase in heart rate when the catheter is intravascular). An increase in maternal heart rate of 25 bpm occurring within 2 minutes of drug injection and lasting at least 15 seconds was observed in only 5 of 10 laboring women who received intravenous epinephrine 15 µg.106 Detection of intravenous epinephrine injection was improved when the authors retrospectively defined a positive maternal tachycardic response as a 10-bpm increase above the maximum maternal heart rate observed in the 2-minute period preceding the epinephrine injection. Others have confirmed that these revised criteria improve the sensitivity of the epinephrine-containing test dose in laboring women.103 These findings stress the importance of tracking the chronotropic variability that occurs in laboring patients during neuraxial anesthesia procedures to reduce the risk for misinterpretation of the test dose.
The usefulness of an epinephrine-containing test dose also improves if additional information is obtained. For example, investigators administered intravenous bupivacaine 12.5 mg with epinephrine 12.5 µg or saline to laboring women.107 They correctly identified the test solution in 39 of 40 women when they assessed maternal heart rate, blood pressure, uterine contractions, the timing of the injection, the presence of analgesia, and subjective signs and symptoms of intravascular injection (e.g., palpitations, lightheadedness, dizziness). The tachycardic response to intravenous epinephrine is not a reliable indicator of intravascular injection in patients who have received a beta-adrenergic receptor antagonist.95
Other means of identifying intravascular placement of an epidural catheter have been proposed and may be clinically useful in specific patients (Table 12-2). Intravenous administration of isoproterenol 5 µg consistently results in tachycardia in pregnant women.108,109 Data from animals99,110 and noninvasive measurements in parturients109 suggest that isoproterenol is devoid of the adverse effects of epinephrine on uterine blood flow, and limited neurotoxicity evaluations have not revealed adverse effects.110,111 However, isoproterenol has not been approved for epidural or intrathecal administration. Given the lack of adequate information regarding potential neurotoxicity, we do not recommend the use of isoproterenol as an epidural test dose.
TABLE 12-2
Epidural Test Dose Regimens*
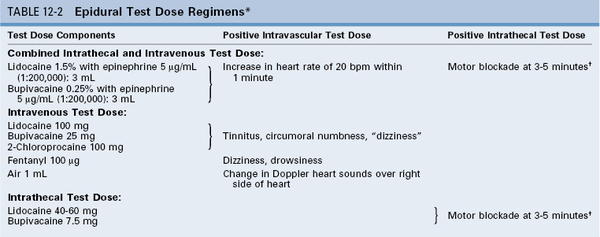
* Test doses may be less sensitive in premedicated patients, patients treated with a beta-adrenergic receptor antagonist, pregnant patients, and anesthetized patients.
†Weakness in hip flexion.
Modified from Yilmaz M, Wong CA. Technique of neuraxial anesthesia. In Wong CA, editor. Spinal and Epidural Anesthesia. New York, McGraw-Hill, 2007:27-73.
Leighton et al.112 have advocated the use of air as an objective marker of intravascular injection. Intravenous injection of 1 or 2 mL of air through a single-orifice catheter consistently produces changes in heart sounds as detected by the use of precordial Doppler ultrasonography.112 (The external FHR monitor can be used for this purpose.) False-negative results may occur when small volumes of air are injected through a multi-orifice epidural catheter; thus, the air test is not a reliable test for intravascular injection when multi-orifice epidural catheters are used.113
Local anesthetic–induced symptoms of subclinical CNS toxicity have also been evaluated as a means of recognizing the unintentional intravenous injection of epidural medications. Colonna-Romano et al.114 administered intravenous saline, lidocaine 100 mg, or 2-chloroprocaine 100 mg to laboring women. Observers blinded as to which substance was administered recorded the presence of CNS symptoms (i.e., dizziness, tinnitus, funny taste) after intravenous injection of local anesthetic. Lidocaine 100 mg was a reliable marker of intravenous injection when the symptoms of tinnitus and funny taste were considered (sensitivity 100%; specificity 81%). 2-Chloroprocaine was less reliable (sensitivity 81% to 94%; specificity 69% to 81%). In a volunteer study, a dose of 1.5 mg/kg of 2-chloroprocaine was necessary to produce a probability of 90% that the subject would report symptoms of intravenous injection.115
Administration of fentanyl 100 µg has been described as a test for intravenous injection.116 Morris et al.117 evaluated the accuracy and reliability of the fentanyl test dose in a double-blind study in which either intravenous or epidural fentanyl 100 µg was administered to parturients, and the investigators sought evidence of the occurrence of sedation, dizziness, euphoria, and/or analgesia. Dizziness was the most reliable symptom of intravenous fentanyl injection, with a sensitivity of 92% and a specificity of 92%.
Some situations reduce the reliability of subjective symptoms as a signal of intravenous injection of a drug. Tests that rely on the self-reporting of subjective symptoms require clear communication with the patient and thus are less useful when the anesthesia provider and patient speak different languages. Patient exhaustion and/or prior opioid administration also may affect the reliability of the test.
Lastly, the epidural catheter design and speed of injection may affect the reliability of the epidural test dose. An epinephrine-containing test dose should be injected rapidly; otherwise rapid redistribution and metabolism of the drug decrease the actual dose administered to chronoreceptors. Multi-orifice epidural catheters have three potential sites of exit for injected fluid or air, and the orifices may lie within two different body compartments. If injected too slowly, air or fluid preferentially exits the proximal orifice. The speed of injection used in clinical practice typically exceeds that required to ensure that fluid will exit all three orifices. In contrast, air must be injected at a much greater speed to ensure that it exits all three orifices; this speed is not practical for clinical use. The distal orifice is both the most difficult to test and the one most likely to be positioned outside the epidural space.118
Intrathecal Test Dose
The intrathecal test dose should allow easy identification of subarachnoid (intrathecal) placement of the catheter without causing high or total spinal anesthesia and hemodynamic compromise. Bupivacaine (7.5 mg) and lidocaine (45 to 60 mg) are the local anesthetics most often used for an intrathecal test dose (see Table 12-2; Figure 12-21).119,120
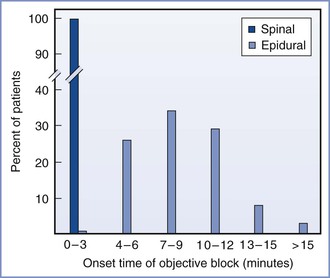
FIGURE 12-21 Percentage of pregnant patients who demonstrated objective evidence of anesthesia (defined as the loss of sensation to pinprick) after epidural injection of 2 to 3 mL of hyperbaric 1.5% lidocaine with 1 : 200,000 epinephrine (light teal bars) (n = 250) or after intrathecal injection of 2 mL of hyperbaric 1.5% lidocaine with 1 : 200,000 epinephrine (dark teal bar) (n = 15). (From Abraham RA, Harris AP, Maxwell LG, et al. The efficacy of 1.5% lidocaine with 7.5% dextrose and epinephrine as an epidural test dose for obstetrics. Anesthesiology 1986; 64:116-9.)
In a study of older, nonpregnant patients receiving continuous spinal anesthesia for surgery, Colonna-Romano et al.120 used plain lidocaine 45 mg plus epinephrine 15 µg and assessed patient perception of lower extremity warmth and heaviness, sensory loss to pinprick, and ability to perform a straight-leg raise. Patients usually perceived warmth in their legs within 1 minute of the intrathecal injection; however, impaired straight-leg raise 4 minutes after an intrathecal test dose injection was the only test that had a sensitivity of 100% for intrathecal injection. The application of these data to pregnant patients is unclear.
Richardson et al.121 described the rapid onset (1 to 3 minutes) of high levels of spinal anesthesia with motor block and hypotension in five parturients who had received a test dose of plain lidocaine 45 mg plus epinephrine 15 µg. This solution is slightly hypobaric relative to CSF at body temperature; thus, the upright posture of these parturients during the injection may have contributed to the high levels of spinal anesthesia. The anesthesia provider must recognize the possible range of responses to the dose of local anesthetic used to assess the position of an epidural catheter and should perform a careful assessment of sensory, motor, and sympathetic function 3 to 5 minutes after administration of the test dose before concluding that the intrathecal test dose result is negative. Ropivacaine 15 mg is not a useful intrathecal test dose because the slow onset of motor blockade precludes timely diagnosis of intrathecal injection.122 2-Chloroprocaine is the only local anesthetic that can be used as a single, combined intravascular and intrathecal test dose; in most patients, 100 mg results in dense, but not total, spinal anesthesia after intrathecal injection and produces systemic signs of subclinical toxicity (tinnitus, funny taste) after intravascular injection.
The epidural injection of local anesthetic for the purpose of ruling out intrathecal or intravascular catheter placement augments epidural analgesia and should be considered in the calculation of the initial therapeutic dose of local anesthetic. Several groups of investigators demonstrated that the test dose enhanced the density of epidural blockade and adversely affected the ability to ambulate. A test dose containing lidocaine 45 mg/epinephrine 15 µg adversely affected the ability to ambulate when injected immediately before initiation of epidural analgesia with 0.125% bupivacaine (18.75 mg) with sufentanil (10 µg).123 Similarly, the same test dose interfered with ambulation when administered immediately after the intrathecal injection of bupivacaine 2.5 mg and fentanyl 25 µg.124 Finally, a lidocaine 60 mg/epinephrine 15 µg test dose adversely affected the ability to ambulate in women who received neostigmine 500 µg with sufentanil 10 µg for initiation of analgesia.125
Techniques to Minimize Local Anesthetic Toxicity
No perfect test dose exists. Some anesthesia providers elect not to administer a test dose because it contributes to motor blockade.123,124 In addition, aspiration of a multi-orifice epidural catheter for blood has 98% sensitivity for detection of an intravascular location.67 Inadvertent intravascular injection of a solution containing a low concentration of local anesthetic is unlikely to result in serious morbidity. However, this conclusion depends heavily on the use of small doses of local anesthetic.126 Laboring women may need large doses of local anesthetic for operative delivery. In some cases, large doses are administered quickly (without incremental injection) for emergency cesarean delivery. The anesthesia provider wants to determine as soon as possible that the epidural catheter is correctly positioned within the epidural space. Even if no morbidity results, it is inconvenient for both the patient and the anesthesia provider to have to repeat the procedure and replace the catheter once the drape has been removed and the patient has been repositioned. The epinephrine-containing test dose provides an objective marker of intravascular injection that has stood the test of time. Thus, we typically give a test dose that contains either bupivacaine 7.5 mg or lidocaine 45 to 60 mg with 15 µg of epinephrine. No matter whether a test dose is injected, drugs should be injected incrementally into the epidural space, because no test is 100% sensitive and catheters may migrate during use. Each incremental dose should be treated as a “test dose” (i.e., the dose should be small enough that it will not cause systemic toxicity if unintentionally injected intravascularly or total spinal anesthesia if injected intrathecally). Pregnant women are very difficult to resuscitate from local anesthetic cardiovascular toxicity.94
Steps to minimize the possibility of local anesthetic toxicity are summarized in Box 12-3. They include observation for passive return of CSF or blood (by lowering the proximal end of the epidural catheter below the insertion site), administration of the test dose between contractions, aspiration before each dose, incremental dose administration, maintaining verbal contact with the patient, and assessment for an appropriate level and density of sensory and motor blockade (which indicates correct placement of the catheter in the epidural space).
Choice of Drug*
The dose requirement of both epidural and spinal local anesthetics is decreased approximately 25% in pregnancy owing to anatomic and physiologic changes (see Chapter 2). These alterations begin to revert to the prepregnancy state within hours of delivery.
Spinal Anesthesia
Anesthesia providers may give spinal anesthesia for cerclage, nonobstetric surgery during pregnancy, instrumental vaginal delivery, cesarean delivery, removal of a retained placenta, or postpartum tubal ligation. Spinal analgesia may be used for labor analgesia. Cesarean delivery represents the most common indication for spinal anesthesia in pregnant women. Most anesthesia providers administer a hyperbaric solution of local anesthetic for spinal anesthesia in obstetric patients. Use of a hyperbaric solution as compared with an isobaric solution results in a faster onset of block and a higher maximum sensory level with a shorter duration of blockade.127 The urgency and anticipated duration of surgery dictate the choice of local anesthetic agent. The most common choice in the United States is bupivacaine. Other agents include lidocaine and tetracaine. Ropivacaine and levobupivacaine may be used but are not approved for spinal administration in the United States, and levobupivacaine is not available in the United States. Lidocaine provides a short to intermediate duration of action. Bupivacaine, tetracaine, levobupivacaine, and ropivacaine provide intermediate to long durations of action.
Anesthesia providers often add an opioid to the local anesthetic to improve the quality of anesthesia, particularly with regard to visceral stimulation, and to provide postoperative analgesia.128,129 The addition of an opioid to the local anesthetic decreases the incidence of intraoperative nausea and vomiting. The short-acting, lipid-soluble opioids (i.e., fentanyl, sufentanil) contribute to intraoperative anesthesia, and morphine is often administered for postoperative analgesia. Epinephrine may be added to prolong block duration and perhaps improve block density. It was hoped that other adjuncts (e.g., clonidine, neostigmine) might allow for the administration of a lower dose of local anesthetic and thereby minimize sympatholytic side effects and hasten recovery. Side effects from these other adjuncts, however, have precluded their wide use in obstetric anesthesia practice (see Chapters 23, 26, and 28).
Epidural Anesthesia
Local anesthetic agents available for epidural administration in obstetric patients include 2-chloroprocaine, lidocaine, mepivacaine, bupivacaine, ropivacaine, and etidocaine. Mepivacaine and etidocaine are used infrequently in obstetric anesthesia practice.
Bupivacaine remains the most popular local anesthetic for analgesia during labor and vaginal delivery because of its differential sensory blockade, long duration of action, low frequency of tachyphylaxis, and low cost. Anesthesia providers infrequently administer bupivacaine for cesarean delivery because of the risk for cardiac toxicity and maternal mortality after unintentional intravascular injection of the drug.
Ropivacaine has gained popularity as an agent for epidural analgesia and anesthesia because it may result in less cardiac toxicity and greater differential sensory blockade than bupivacaine.130
Levobupivacaine also has a more favorable safety profile than bupivacaine. Clinical trials have shown that ropivacaine and levobupivacaine have potency131 and analgesic qualities similar to those of bupivacaine,132,133 with the probable exception of less motor nerve block.134
Bupivacaine, ropivacaine, and levobupivacaine all have longer durations of action than lidocaine, and they may be preferred over shorter-acting agents when a longer duration of anesthesia or analgesia is desirable. They are more commonly used for maintenance of epidural labor analgesia, whereas the shorter-acting agents are used for epidural surgical anesthesia. Despite some variation among reports, published clinical studies suggest no more than slight differences in onset and potency, and no differences in quality or duration of neural blockade, among the three drugs. However, bupivacaine is more cardiotoxic than the other agents in vitro and probably after unintentional intravascular administration.135 It would seem prudent to use ropivacaine or levobupivacaine rather than bupivacaine when a bolus dose of a concentrated solution is being given. When administered as a low concentration infusion, improved safety has not been demonstrated with ropivacaine and levobupivacaine compared with bupivacaine.
The most popular choice of local anesthetic for epidural anesthesia for cesarean delivery is 2% lidocaine with epinephrine. The addition of epinephrine (5 µg/mL) causes a modest prolongation of the block. The major advantage of epinephrine is that it improves the quality of epidural lidocaine anesthesia. Lam et al.136 have shown that epidural labor analgesia can be extended to surgical anesthesia for cesarean delivery in 5.2 ± 1.5 minutes (mean ± SD) with the addition of bicarbonate and fentanyl to 2% lidocaine with epinephrine.
Many anesthesia providers reserve 2-chloroprocaine for cases in which rapid extension of epidural anesthesia for vaginal delivery or urgent cesarean delivery is necessary. The onset of surgical anesthesia was several minutes faster with 2-chloroprocaine compared with lidocaine with freshly mixed epinephrine and sodium bicarbonate in the setting of urgent caesarean delivery after epidural labor analgesia.137 Therefore, when time is of the essence, 2-chloroprocaine is the drug of choice. Typically, in an emergency, a large volume of concentrated local anesthetic solution is injected quickly. An additional advantage of 2-chloroprocaine in this situation is that it is rapidly metabolized by plasma esterases. Therefore, the unintentional intravascular injection of a large volume of 2-chloroprocaine may be less likely to have serious adverse consequences. A potential disadvantage of 2-chloroprocaine is that it may interfere with the subsequent actions of opioids138 and bupivacaine,139 although this possibility is controversial.140
As in spinal anesthesia, epidural opioids work synergistically with local anesthetics. Fentanyl 50 to 100 µg or sufentanil 5 to 10 µg is frequently added to an amide local anesthetic for both labor analgesia (allowing a lower dose of local anesthetic and less motor block) and cesarean delivery (resulting in a denser block with better blockade of visceral stimulation). Sodium bicarbonate may be added to lidocaine141 and 2-chloroprocaine142 (1 mEq/10 mL local anesthetic) to decrease latency.
Caudal Anesthesia
The drugs used for caudal epidural anesthesia are identical to those used for lumbar epidural block. However, a much larger volume (e.g., 25 to 35 mL) of local anesthetic solution must be administered to extend a caudal block for cesarean delivery or labor analgesia. Such large volumes entail a greater risk for systemic local anesthetic toxicity. Additionally, the caudal epidural space is highly vascular, which further predisposes to intravasation of drugs administered via this route.
Complications of Neuraxial Techniques
Unintentional Dural Puncture
Unintentional dural puncture with an epidural needle occurs at a rate of approximately 1.5% in the obstetric population.143 Approximately 52% of women will experience a post–dural puncture headache after puncture with an epidural needle (see Chapter 31). Techniques to minimize the incidence of unintentional dural puncture include (1) identification of the ligamentum flavum during epidural needle advancement; (2) understanding the likely depth of the epidural space in an individual patient; (3) advancement of the needle between contractions, when unexpected patient movement is less likely; (4) adequate control of the needle-syringe assembly during advancement of the needle; and (5) clearing the needle of clotted blood or bone plugs. Norris et al.144 observed that post–dural puncture headache after unintentional dural puncture was less likely to result in headache if the epidural needle bevel faced lateral rather than cephalad. In contrast, Richardson and Wissler145 found no difference between the two orientations. An in vitro study using cadaver dura found that the fluid leakage rate through dural tears was not dependent on the orientation of the dura relative to the needle bevel.146 We prefer to insert the epidural needle with the bevel oriented in a cephalad direction so that there is no need to rotate the needle bevel within the epidural space. Cephalad bevel orientation also increases the likelihood of successful epidural anesthesia.147
The management of unintentional dural puncture depends on the clinical setting. If advancement of an epidural needle results in dural trespass and the free flow of CSF is perceived in the barrel of the epidural syringe, the anesthesia provider should halt advancement of the epidural needle and either remove the epidural needle if the plan is to resite the epidural catheter or replace the stylet back into the lumen of the epidural needle to prevent further egress of CSF if the plan is to administer intrathecal medication or perform a continuous spinal technique. Reinjection of CSF contained in the syringe should not be entertained because there is a high likelihood that air will concomitantly be introduced into the subarachnoid space and cause pneumocephalus. The risks and benefits of administering an intrathecal dose of anesthetic should be considered. Although intrathecal administration of local anesthetic through the epidural needle will result in rapid analgesia for an uncomfortable patient, the rapid efflux of CSF may render the injectate ineffective. Additionally, spinal analgesia may mask paresthesias during subsequent attempts at neuraxial anesthesia. Furthermore, spinal anesthesia may cause profound hypotension. If the patient is sitting, and likely to remain so for the next few minutes, the anesthesia provider may struggle to manage two problems instead of one (i.e., a challenging neuraxial procedure and maternal hypotension). However, rapid provision of analgesia may enable the patient to better assume an optimal position without moving. One option is to administer intrathecal opioid, thus providing analgesia without the risk for hypotension.
The anesthesia provider has two options after unintentional dural puncture: site the epidural catheter within the subarachnoid space and use a continuous spinal anesthetic technique or site the epidural catheter in a different interspace (i.e., starting afresh). Evidence is conflicting as to whether the insertion of an epidural catheter through the dural puncture site decreases the incidence of post–dural puncture headache.148 Continuous spinal anesthesia is an attractive option if identification of the epidural space has been difficult or if the anticipated duration of epidural anesthesia or analgesia is relatively short (e.g., cesarean delivery, or vaginal delivery in parous women). The major disadvantage of an intrathecal catheter is the risk that it may be mistaken for an epidural catheter. Given that the local anesthetic dose required for epidural anesthesia is many times greater than that required for spinal anesthesia, unintentional administration of an epidural dose into the subarachnoid space will lead to total spinal anesthesia. Therefore, on a busy labor and delivery unit where multiple providers are giving anesthesia care, it may be safer to use an epidural catheter rather than an intrathecal catheter in women in whom prolonged analgesia is anticipated.
Thus, the anesthesia provider may elect to initiate epidural anesthesia in another lumbar epidural interspace. However, even if the attempt results in a correctly placed catheter, the provider must be wary of an unexpectedly high level of anesthesia after the epidural administration of usual doses of local anesthetic.149,150 Leach and Smith150 reported a patient who had an extensive block after unintentional dural puncture and subsequent epidural injection of bupivacaine. They presented radiologic evidence of the spread of local anesthetic from the epidural space to the subarachnoid space. The extent to which a dural tear affects the movement of substances from the epidural space to the subarachnoid space depends on the size of the hole, the lipophilicity of the drug (highly lipophilic drugs cross quickly regardless of the presence of a hole, whereas water-soluble drugs cross more quickly in the presence of a hole),151 and whether the drug is administered into the epidural space as a bolus or an infusion. Rapid bolus administration of medications through an epidural catheter in a patient with a known dural puncture with a large-bore needle significantly increases the likelihood of high spinal anesthesia.
Unfortunately, there is no reliable method to decrease the risk for post–dural puncture headache once dural puncture occurs. Obese patients appear to be at lower risk than lean patients for the development of headache.152 A prophylactic blood patch (injection of autologous blood before removal of the epidural catheter and before onset of a headache) does not reduce the risk for post–dural puncture headache.148,153
Occasionally, unintentional dural puncture is not recognized until the epidural catheter is threaded and CSF spontaneously appears at the proximal end of the catheter, or the catheter aspiration or intrathecal test dose is positive. Finally, an epidural catheter that has been correctly sited in the epidural space may migrate into the subarachnoid space. The most significant clinical threat in this scenario is the continued use of a large volume infusion of local anesthetic drug intended for epidural administration. Thus, during prolonged epidural labor analgesia, the patient should be monitored for evidence of high or dense neuraxial anesthesia.
Unintentional Intravascular or Subarachnoid Injection
The unintentional injection of large doses of local anesthetics into the subarachnoid space can lead to catastrophe. The rapid onset of high or total spinal anesthesia results in profound hypotension, loss of consciousness, and apnea secondary to hypoperfusion of the brain stem. Prompt treatment necessitates assisted ventilation, volume resuscitation, and pharmacologic support of blood pressure. Administration of chronotropic agents such as epinephrine may also be necessary if blockade of cardiac sympathetic drive results in bradycardia. The patient is at high risk for awareness in this setting, and the judicious use of an amnestic agent such as midazolam should be considered once cardiorespiratory stability has been restored.
The incidence of intravascular catheter placement varies according to catheter type,33,65 patient population,154 and proper placement of the epidural needle tip in the midline. Pregnant women are at higher risk for unintentional intravenous cannulation due to the engorgement of epidural veins. Intravascular injection of a local anesthetic may initially result in altered sensorium, tinnitus, and perioral numbness. Higher blood concentrations may result in seizures, and even higher concentrations may cause dysrhythmias and cardiovascular collapse (see Chapter 13). Management of intravascular toxicity includes prompt institution of advanced cardiac life support (ACLS), gamma-aminobutyric acid (GABA)–potentiating agents such as benzodiazepines to mitigate seizure activity, and the use of intralipid emulsion to detoxify the patient. Several modifications have been made to the conventional ACLS protocol for the specific treatment of local anesthetic-induced cardiac arrest.155 These include using small, judicious doses of intravenous epinephrine for circulatory support (10 to 100 µg boluses in adults), avoidance of vasopressin, and the use of amiodarone in place of local anesthetics for treatment of ventricular arrhythmias.
Inadequate Anesthesia
Pain during anesthesia represents a higher proportion of obstetric malpractice claims than of nonobstetric claims.156 During labor, inadequate epidural analgesia may result from the inadequate extent of sensory blockade, nonuniform blockade, or inadequate density of blockade. When called to evaluate breakthrough pain, the anesthesia provider should first evaluate the extent of bilateral sensory blockade in both the cephalad and caudad directions. Particularly if labor is progressing quickly, the extent of sacral blockade may not be adequate. In this case, epidural injection of a large volume of local anesthetic may improve sacral blockade. In contrast, if the extent of sensory blockade is adequate but the patient is still experiencing pain, the density of blockade may be insufficient. In this case, the provider should reestablish and maintain analgesia using a more concentrated solution of local anesthetic.
Why do some obstetric epidural anesthetics fail over time? Collier157 administered epidural radiocontrast dye in 25 parturients reporting unsatisfactory analgesia. The two major causes of inadequate block in this small study were transforaminal migration of the catheter tip and an obstructive barrier in the epidural space. Total block failure usually results from failure to identify the epidural space correctly or from malposition of the catheter tip outside the epidural space (e.g., in a neuroforamen). A unilateral block may occur despite the use of good technique. Unilateral blocks can often be prevented by limiting the length of catheter within the epidural space to 3 cm or less. The problem with limited insertion of the catheter is that, in some patients, the catheter tends to migrate outward over time. (Patients undergoing surgery remain still; by contrast, laboring women change position frequently.) Obese women seem to be at higher risk for outward migration of the catheter tip. Prospective studies suggest that 4 to 6 cm is the optimal depth of epidural catheter insertion in laboring women.71,72,158
Whether catheter withdrawal in the setting of breakthrough pain is beneficial is not clear. Beilin et al.159 compared catheter withdrawal followed by injection of local anesthetic with injection of local anesthetic without catheter withdrawal for the treatment of breakthrough pain. The ability to rescue analgesia was not different between the groups. Gielen et al.160 performed a radiologic study in which they observed no consistent relationship between catheter position and the asymmetric onset of sympathetic blockade. Unilateral or patchy sensory blockade likely results from the nonuniform distribution of local anesthetic solution in the epidural space.161 Injection of a large volume of dilute local anesthetic solution into the epidural space usually corrects this problem, regardless of the location of the tip of the epidural catheter (provided it is actually in the epidural space). If analgesia cannot be rescued with a second injection, the catheter should be removed and replaced at another interspace.
The management of inadequate anesthesia is more problematic during cesarean delivery. Failure of spinal anesthesia may result from the maldistribution of local anesthetic within the subarachnoid space.162,163 If inadequate spinal anesthesia is noted before incision, the anesthesia provider may augment the block with additional local anesthetic by either performing a second spinal anesthetic procedure or placing an epidural catheter. However, care must be taken if performing a second spinal anesthetic procedure. In the ASA Closed-Claims Project database, Drasner and Rigler162 identified three cases of cauda equina syndrome complicating spinal anesthesia. In two cases, “failed spinal” anesthesia had occurred, followed by a repeat injection of local anesthetic. The researchers recommended that anesthesia providers determine the presence of anesthesia in the sacral dermatomes before giving additional local anesthetic into the subarachnoid space. Additionally, they stated that if CSF was aspirated during the original procedure, it should be assumed that local anesthetic was delivered into the subarachnoid space, and the total dose of local anesthetic be limited to the maximum dose a clinician would consider reasonable to administer in a single injection.162 If partial blockade is present (even if it is limited to the sacral dermatomes), the second dose should be reduced accordingly. It may also be advisable to perform the second procedure at a different interspace or make other changes to the original procedure (e.g., alter the patient's position, use a local anesthetic with different baricity, or straighten the lumbosacral curvature).
If the patient complains of pain after incision, the anesthesia provider must decide between the administration of inhalation or intravenous analgesia and the administration of general anesthesia. Supplemental analgesia may be provided by giving 60% nitrous oxide in oxygen, small incremental boluses of ketamine (0.1 to 0.25 mg/kg), or small boluses of intravenous opioid. Supplemental infiltration of the wound with local anesthetic is sometimes helpful, especially when spinal anesthesia regresses near the end of an unexpectedly long operation. The anesthesia provider must ensure that the patient remains sufficiently alert to protect her airway. In most cases, severe pain unrelieved by modest doses of analgesic drug requires rapid-sequence induction of general anesthesia followed by endotracheal intubation and general anesthesia.
In some cases, inadequate epidural anesthesia results from failure to give a sufficient dose of local anesthetic or failure to wait a sufficient time after its administration. For example, after the epidural administration of 0.5% bupivacaine, approximately 20 minutes must pass to achieve an adequate level of anesthesia, and additional local anesthetic may be needed to achieve an adequate density of blockade. In urgent cases or in cases with a “missed” segment, local infiltration with a local anesthetic often results in satisfactory anesthesia. Sometimes it is difficult to separate the beneficial effect of the local infiltration from the beneficial effect of waiting for the obstetrician to obtain, prepare, and inject the local anesthetic solution. Finally, the anesthesia provider should exercise caution when initiating spinal anesthesia after failure of epidural anesthesia because of a greater incidence of high spinal anesthesia in this setting.164 Presumably, the large volume of local anesthetic within, or near, the epidural space results in decreased lumbar CSF volume, which predisposes to high spinal anesthesia. It may be advisable to reduce the dose of intrathecal local anesthetic, particularly in the presence of partial epidural blockade.
Equipment Problems
The frequency of major equipment malfunction is very low during the administration of neuraxial anesthesia. Most anesthesia providers in the United States use disposable needles, and the plastic needle hubs are attached to the needles' shafts with epoxy. Rarely, a needle breaks at the hub-shaft junction.165 If a needle should break, the portion of the needle that remains in the patient should be removed, because it may migrate and cause injury.
An epidural or spinal catheter may shear and break off if the catheter is withdrawn through a needle; thus an epidural or spinal catheter should never be withdrawn in this manner. Rather, if the catheter must be withdrawn, the needle and catheter should be withdrawn as a unit. It is also possible to break a catheter during attempts at removing it, although this is rare. If resistance to catheter removal is encountered, the patient should assume a position that reduces lumbar lordosis, thereby lessening the kinking of the catheter between perivertebral structures. If position change is not successful, the catheter should be taped under tension to the patient's back and left undisturbed for several hours. The catheter usually works its way out and is then easy to remove. Once the catheter has been removed successfully, it should be examined to ensure that it has been removed completely. Complete removal of the catheter should be documented in the medical record.
Rarely, catheters do break on removal. We favor aggressive attempts to remove broken spinal catheters. However, it may be unnecessary to remove broken epidural catheters; rather, in these circumstances, the patient can be informed of the complication and observed over time. The incidence of catheter migration or other delayed sequelae appears to be low. Computed tomography may help identify the precise location of a broken catheter.166
During use, an epidural catheter occasionally becomes disconnected from the catheter connector. Options include replacing the epidural catheter or reconnecting the connector to the catheter. Langevin et al.167 used an in vitro model to investigate whether microbial contamination precludes reconnection. They found that an area of the interior of the catheter distal to the disconnection may remain sterile for up to 8 hours if the fluid column within the catheter remains static (i.e., if “fluid does not move within the catheter when it is raised above the level of the patient”).167 Therefore, they concluded that it may be safe to decontaminate the exterior of the catheter, cut the catheter with a sterile instrument, and reconnect it to a new sterile connector. However, given the potential catastrophic consequences of neuraxial infection, we recommend replacing the catheter. Also, wire-embedded catheters cannot be cut.
References
1. Reynolds F, Speedy HM. The subdural space: the third place to go astray. Anaesthesia. 1990;45:120–123.
2. Hogan QH. Epidural anatomy examined by cryomicrotome section: influence of age, vertebral level, and disease. Reg Anesth. 1996;21:395–406.
3. Hogan QH. Lumbar epidural anatomy: a new look by cryomicrotome section. Anesthesiology. 1991;75:767–775.
4. Blomberg R. The dorsomedian connective tissue band in the lumbar epidural space of humans: an anatomical study using epiduroscopy in autopsy cases. Anesth Analg. 1986;65:747–752.
5. Savolaine ER, Pandya JB, Greenblatt SH, Conover SR. Anatomy of the human lumbar epidural space: new insights using CT-epidurography. Anesthesiology. 1988;68:217–220.
6. Zarzur E. Anatomic studies of the human ligamentum flavum. Anesth Analg. 1984;63:499–502.
7. Harrison GR, Clowes NW. The depth of the lumbar epidural space from the skin. Anaesthesia. 1985;40:685–687.
8. Altinkaya N, Yildirim T, Demir S, et al. Factors associated with the thickness of the ligamentum flavum: is ligamentum flavum thickening due to hypertrophy or buckling? Spine. 2011;36:E1093–E1097.
9. Hogan QH, Prost R, Kulier A, et al. Magnetic resonance imaging of cerebrospinal fluid volume and the influence of body habitus and abdominal pressure. Anesthesiology. 1996;84:1341–1349.
10. Richardson MG, Wissler RN. Density of lumbar cerebrospinal fluid in pregnant and nonpregnant humans. Anesthesiology. 1996;85:326–330.
11. Hirabayashi Y, Shimizu R, Fukuda H, et al. Anatomical configuration of the spinal column in the supine position. II. Comparison of pregnant and non-pregnant women. Br J Anaesth. 1995;75:6–8.
12. Fassoulaki A, Gatzou V, Petropoulos G, Siafaka I. Spread of subarachnoid block, intraoperative local anaesthetic requirements and postoperative analgesic requirements in Caesarean section and total abdominal hysterectomy. Br J Anaesth. 2004;93:678–682.
13. Datta S, Hurley RJ, Naulty JS, et al. Plasma and cerebrospinal fluid progesterone concentrations in pregnant and nonpregnant women. Anesth Analg. 1986;65:950–954.
14. Burm AG, van Kleef JW, Gladines MP, et al. Spinal anesthesia with hyperbaric lidocaine and bupivacaine: effects of epinephrine on the plasma concentration profiles. Anesth Analg. 1987;66:1104–1108.
15. Fink BR. Mechanisms of differential axial blockade in epidural and subarachnoid anesthesia. Anesthesiology. 1989;70:851–858.
16. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–863.
17. Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. Br J Obstet Gynaecol. 2002;109:274–281.
18. The Joint Commission. 2012 Hospital Accreditation Standards. The Joint Commission: Oak Brook Terrace, IL; 2012.
19. Jackson A, Henry R, Avery N, et al. Informed consent for labour epidurals: what labouring women want to know. Can J Anaesth. 2000;47:1068–1073.
20. Bethune L, Harper N, Lucas DN, et al. Complications of obstetric regional analgesia: how much information is enough? Int J Obstet Anesth. 2004;13:30–34.
21. Swan HD, Borshoff DC. Informed consent—recall of risk information following epidural analgesia in labour. Anaesth Intensive Care. 1994;22:139–141.
22. Knapp RM. Legal view of informed consent for anesthesia during labor. Anesthesiology. 1990;72:211.
23. Brull R, McCartney CJ, Chan VW, et al. Disclosure of risks associated with regional anesthesia: a survey of academic regional anesthesiologists. Reg Anesth Pain Med. 2007;32:7–11.
24. Orbach-Zinger S, Ginosar Y, Sverdlik J, et al. Partner's presence during initiation of epidural labor analgesia does not decrease maternal stress: a prospective randomized controlled trial. Anesth Analg. 2012;114:654–660.
25. Vincent RD, Chestnut DH. Which position is more comfortable for the parturient during identification of the epidural space? Int J Obstet Anesth. 1991;1:9–11.
26. Andrews PJ, Ackerman WE 3rd, Juneja MM. Aortocaval compression in the sitting and lateral decubitus positions during extradural catheter placement in the parturient. Can J Anaesth. 1993;40:320–324.
27. Bieniarz J, Curuchet E, Crottogini JJ, et al. Aortocaval compression by the uterus in late human pregnancy. Am J Obstet Gynecol. 1978;100:203–217.
28. Kerr MG, Scott DB, Samuel E. Studies of the inferior vena cava in late pregnancy. Br Med J. 1964;1:532–533.
29. Scott DB. Inferior vena caval occlusion in late pregnancy and its importance in anaesthesia. Br J Anaesth. 1968;40:120–128.
30. Eckstein KL, Marx GF. Aortocaval compression and uterine displacement. Anesthesiology. 1974;40:92–96.
31. Marx GF, Husain FJ, Shiau HF. Brachial and femoral blood pressures during the prenatal period. Am J Obstet Gynecol. 1980;136:11–13.
32. Ellington C, Katz VL, Watson WJ, Spielman FJ. The effect of lateral tilt on maternal and fetal hemodynamic variables. Obstet Gynecol. 1991;77:201–203.
33. Mhyre JM, Greenfield ML, Tsen LC, Polley LS. A systematic review of randomized controlled trials that evaluate strategies to avoid epidural vein cannulation during obstetric epidural catheter placement. Anesth Analg. 2009;108:1232–1242.
34. Norris MC, Dewan DM. Effect of gravity on the spread of extradural anaesthesia for caesarean section. Br J Anaesth. 1987;59:338–341.
35. Norris MC, Leighton BL, DeSimone CA, Larijani GE. Lateral position and epidural anesthesia for cesarean section. Anesth Analg. 1988;67:788–790.
36. Reid JA, Thorburn J. Extradural bupivacaine or lignocaine anaesthesia for elective caesarean section: the role of maternal posture. Br J Anaesth. 1988;61:149–153.
37. Beilin Y, Abramovitz SE, Zahn J, et al. Improved epidural analgesia in the parturient in the 30 degree tilt position. Can J Anaesth. 2000;47:1176–1181.
38. Preston R, Crosby ET, Kotarba D, et al. Maternal positioning affects fetal heart rate changes after epidural analgesia for labour. Can J Anaesth. 1993;40:1136–1141.
39. Panikkar KK, Yentis SM. Wearing of masks for obstetric regional anaesthesia: a postal survey. Anaesthesia. 1996;51:398–400.
40. Sellors JE, Cyna AM, Simmons SW. Aseptic precautions for inserting an epidural catheter: a survey of obstetric anaesthetists. Anaesthesia. 2002;57:593–596.
41. Skinner MW, Sutton BA. Do anaesthetists need to wear surgical masks in the operating theatre? A literature review with evidence-based recommendations. Anaesth Intensive Care. 2001;29:331–338.
42. Baer ET. Post-dural puncture bacterial meningitis. Anesthesiology. 2006;105:381–393.
43. Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179–190.
44. Ruppen W, Derry S, McQuay H, Moore RA. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology. 2006;105:394–399.
45. O'Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23:759–769.
46. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26.
47. American Society of Anesthesiologists. Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques: a report by the American Society of Anesthesiologists Task Force on Infectious Complications Associated with Neuraxial Techniques. Anesthesiology. 2010;112:530–545.
48. Hebl JR. The importance and implications of aseptic techniques during regional anesthesia. Reg Anesth Pain Med. 2006;31:311–323.
49. Association of Anaesthetists of Great Britain and Ireland. Infection control in anaesthesia. Anaesthesia. 2008;63:1027–1036.
50. Hubble MJ, Weale AE, Perez JV, et al. Clothing in laminar-flow operating theatres. J Hosp Infect. 1996;32:1–7.
51. McNeil SA, Foster CL, Hedderwick SA, Kauffman CA. Effect of hand cleansing with antimicrobial soap or alcohol-based gel on microbial colonization of artificial fingernails worn by health care workers. Clin Infect Dis. 2001;32:367–372.
52. Kinirons B, Mimoz O, Lafendi L, et al. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children: a randomized, controlled trial. Anesthesiology. 2001;94:239–244.
53. Birnbach DJ, Meadows W, Stein DJ, et al. Comparison of povidone iodine and DuraPrep, an iodophor-in-isopropyl alcohol solution, for skin disinfection prior to epidural catheter insertion in parturients. Anesthesiology. 2003;98:164–169.
54. Benson JSUS. Food and Drug Administration safety alert: cauda equina syndrome associated with use of small-bore catheters in continuous spinal anesthesia. AANA J. 1992;60:223.
55. Shutt LE, Valentine SJ, Wee MY, et al. Spinal anaesthesia for caesarean section: comparison of 22-gauge and 25-gauge Whitacre needles with 26-gauge Quincke needles. Br J Anaesth. 1992;69:589–594.
56. Reina MA, de Leon-Casasola OA, Lopez A, et al. An in vitro study of dural lesions produced by 25-gauge Quincke and Whitacre needles evaluated by scanning electron microscopy. Reg Anesth Pain Med. 2000;25:393–402.
57. Thomsen AF, Nilsson CG. Broken small-gauge spinal needle. Anesth Analg. 1997;85:230–231.
58. Shenouda PE, Cunningham BJ. Assessing the superiority of saline versus air for use in the epidural loss of resistance technique: a literature review. Reg Anesth Pain Med. 2003;28:48–53.
59. Beilin Y, Arnold I, Telfeyan C, et al. Quality of analgesia when air versus saline is used for identification of the epidural space in the parturient. Reg Anesth Pain Med. 2000;25:596–599.
60. Aida S, Taga K, Yamakura T, et al. Headache after attempted epidural block: the role of intrathecal air. Anesthesiology. 1998;88:76–81.
61. Murphy JD, Ouanes JP, Togioka BM, et al. Comparison of air and liquid for use of loss-of-resistance technique during labor epidurals: a meta-analysis. J Anesth Clin Res. 2011;2:175.
62. Dalens B, Bazin JE, Haberer JP. Epidural bubbles as a cause of incomplete analgesia during epidural anesthesia. Anesth Analg. 1987;66:679–683.
63. Schier R, Guerra D, Aguilar J, et al. Epidural space identification: a meta-analysis of complications after air versus liquid as the medium for loss of resistance. Anesth Analg. 2009;109:2012–2021.
64. Segal S, Arendt KW. A retrospective effectiveness study of loss of resistance to air or saline for identification of the epidural space. Anesth Analg. 2010;110:558–563.
65. Banwell BR, Morley-Forster P, Krause R. Decreased incidence of complications in parturients with the Arrow (FlexTip Plus) epidural catheter. Can J Anaesth. 1998;45:370–372.
66. Jaime F, Mandell GL, Vallejo MC, Ramanathan S. Uniport soft-tip, open-ended catheters versus multiport firm-tipped close-ended catheters for epidural labor analgesia: a quality assurance study. J Clin Anesth. 2000;12:89–93.
67. Norris MC, Ferrenbach D, Dalman H, et al. Does epinephrine improve the diagnostic accuracy of aspiration during labor epidural analgesia? Anesth Analg. 1999;88:1073–1076.
68. D'Angelo R, Foss ML, Livesay CH. A comparison of multiport and uniport epidural catheters in laboring patients. Anesth Analg. 1997;84:1276–1279.
69. Fegley AJ, Lerman J, Wissler R. Epidural multiorifice catheters function as single-orifice catheters: an in vitro study. Anesth Analg. 2008;107:1079–1081.
70. Rolbin SH, Halpern SH, Braude BM, et al. Fluid through the epidural needle does not reduce complications of epidural catheter insertion. Can J Anaesth. 1990;37:337–340.
71. Hamilton CL, Riley ET, Cohen SE. Changes in the position of epidural catheters associated with patient movement. Anesthesiology. 1997;86:778–784.
72. D'Angelo R, Berkebile BL, Gerancher JC. Prospective examination of epidural catheter insertion. Anesthesiology. 1996;84:88–93.
73. Tyagi A, Kumar R, Bhattacharya A, Sethi AK. Filters in anaesthesia and intensive care. Anaesth Intensive Care. 2003;31:418–433.
74. Charlton GA, Lawes EG. The effect of micropore filters on the aspiration test in epidural analgesia. Anaesthesia. 1991;46:573–575.
75. Westphal M, Hohage H, Buerkle H, et al. Adsorption of sufentanil to epidural filters and catheters. Eur J Anaesthesiol. 2003;20:124–126.
76. Van de Velde M, Van Schoubroeck D, Jani J, et al. Combined spinal-epidural anesthesia for cesarean delivery: dose-dependent effects of hyperbaric bupivacaine on maternal hemodynamics. Anesth Analg. 2006;103:187–190.
77. Leo S, Sng BL, Lim Y, Sia AT. A randomized comparison of low doses of hyperbaric bupivacaine in combined spinal-epidural anesthesia for cesarean delivery. Anesth Analg. 2009;109:1600–1605.
78. Choi DH, Kim JA, Chung IS. Comparison of combined spinal epidural anesthesia and epidural anesthesia for cesarean section. Acta Anaesthesiol Scand. 2000;44:214–219.
79. Goodman SR, Smiley RM, Negron MA, et al. A randomized trial of breakthrough pain during combined spinal-epidural versus epidural labor analgesia in parous women. Anesth Analg. 2009;108:246–251.
80. Simmons SW, Cyna AM, Dennis AT, Hughes D. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev. 2007.
81. Norris MC. Are combined spinal-epidural catheters reliable? Int J Obstet Anesth. 2000;9:3–6.
82. Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. Int J Obstet Anesth. 2004;13:227–233.
83. Cook TM. Combined spinal-epidural techniques. Anaesthesia. 2000;55:42–64.
84. Riley ET, Hamilton CL, Ratner EF, Cohen SE. A comparison of the 24-gauge Sprotte and Gertie Marx spinal needles for combined spinal-epidural analgesia during labor. Anesthesiology. 2002;97:574–577.
85. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45:766–771.
86. Grau T, Leipold RW, Conradi R, et al. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14:169–175.
87. Grau T, Leipold RW, Fatehi S, et al. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21:25–31.
88. Vallejo MC, Phelps AL, Singh S, et al. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19:373–378.
89. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115:94–101.
90. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108:1876–1881.
91. Tran D, Kamani AA, Lessoway VA, et al. Preinsertion paramedian ultrasound guidance for epidural anesthesia. Anesth Analg. 2009;109:661–667.
92. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104:1188–1192.
93. Mulroy MF, Norris MC, Liu SS. Safety steps for epidural injection of local anesthetics: review of the literature and recommendations. Anesth Analg. 1997;85:1346–1356.
94. Albright GA. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51:285–287.
95. Guinard JP, Mulroy MF, Carpenter RL, Knopes KD. Test doses: optimal epinephrine content with and without acute beta-adrenergic blockade. Anesthesiology. 1990;73:386–392.
96. Moore DC, Batra MS. The components of an effective test dose prior to epidural block. Anesthesiology. 1981;55:693–696.
97. Chestnut DH, Weiner CP, Martin JG, et al. Effect of intravenous epinephrine on uterine artery blood flow velocity in the pregnant guinea pig. Anesthesiology. 1986;65:633–636.
98. Hood DD, Dewan DM, James FM 3rd. Maternal and fetal effects of epinephrine in gravid ewes. Anesthesiology. 1986;64:610–613.
99. Marcus MA, Vertommen JD, Van Aken H, Wouters PF. Hemodynamic effects of intravenous isoproterenol versus epinephrine in the chronic maternal-fetal sheep preparation. Anesth Analg. 1996;82:1023–1026.
100. Youngstrom P, Hoyt M, Veille JC, et al. Effects of intravenous test dose epinephrine on fetal sheep during acute fetal stress and acidosis. Reg Anesth. 1990;15:237–241.
101. Cartwright PD, McCarroll SM, Antzaka C. Maternal heart rate changes with a plain epidural test dose. Anesthesiology. 1986;65:226–228.
102. Chestnut DH, Owen CL, Brown CK, et al. Does labor affect the variability of maternal heart rate during induction of epidural anesthesia? Anesthesiology. 1988;68:622–625.
103. Colonna-Romano P, Lingaraju N, Godfrey SD, Braitman LE. Epidural test dose and intravascular injection in obstetrics: sensitivity, specificity, and lowest effective dose. Anesth Analg. 1992;75:372–376.
104. Colonna-Romano P, Nagaraj L. Tests to evaluate intravenous placement of epidural catheters in laboring women: a prospective clinical study. Anesth Analg. 1998;86:985–988.
105. Mulroy M, Glosten B. The epinephrine test dose in obstetrics: note the limitations. Anesth Analg. 1998;86:923–925.
106. Leighton BL, Norris MC, Sosis M, et al. Limitations of epinephrine as a marker of intravascular injection in laboring women. Anesthesiology. 1987;66:688–691.
107. Gieraerts R, Van Zundert A, De Wolf A, Vaes L. Ten ml bupivacaine 0.125% with 12.5 micrograms epinephrine is a reliable epidural test dose to detect inadvertent intravascular injection in obstetric patients: a double-blind study. Acta Anaesthesiol Scand. 1992;36:656–659.
108. Leighton BL, DeSimone CA, Norris MC, Chayen B. Isoproterenol is an effective marker of intravenous injection in laboring women. Anesthesiology. 1989;71:206–209.
109. Marcus MA, Vertommen JD, Van Aken H, et al. Hemodynamic effects of intravenous isoproterenol versus saline in the parturient. Anesth Analg. 1997;84:1113–1116.
110. Norris MC, Arkoosh VA, Knobler R. Maternal and fetal effects of isoproterenol in the gravid ewe. Anesth Analg. 1997;85:389–394.
111. Marcus MA, Vertommen JD, Van Aken H, et al. The effects of adding isoproterenol to 0.125% bupivacaine on the quality and duration of epidural analgesia in laboring parturients. Anesth Analg. 1998;86:749–752.
112. Leighton BL, Norris MC, DeSimone CA, et al. The air test as a clinically useful indicator of intravenously placed epidural catheters. Anesthesiology. 1990;73:610–613.
113. Leighton BL, Topkis WG, Gross JB, et al. Multiport epidural catheters: does the air test work? Anesthesiology. 2000;92:1617–1620.
114. Colonna-Romano P, Lingaraju N, Braitman LE. Epidural test dose: lidocaine 100 mg, not chloroprocaine, is a symptomatic marker of i.v. injection in labouring parturients. Can J Anaesth. 1993;40:714–717.
115. Rathmell JP, Viscomi CM, Ashikaga T. Detection of intravascular epidural catheters using 2-chloroprocaine: influence of local anesthetic dose and nalbuphine premedication. Reg Anesth. 1997;22:113–118.
116. Yoshii WY, Miller M, Rottman RL, et al. Fentanyl for epidural intravascular test dose in obstetrics. Reg Anesth. 1993;18:296–299.
117. Morris GF, Gore-Hickman W, Lang SA, Yip RW. Can parturients distinguish between intravenous and epidural fentanyl? Can J Anaesth. 1994;41:667–672.
118. Power I, Thorburn J. Differential flow from multihole epidural catheters. Anaesthesia. 1988;43:876–878.
119. Abraham RA, Harris AP, Maxwell LG, Kaplow S. The efficacy of 1.5% lidocaine with 7.5% dextrose and epinephrine as an epidural test dose for obstetrics. Anesthesiology. 1986;64:116–119.
120. Colonna-Romano P, Padolina R, Lingaraju N, Braitman LE. Diagnostic accuracy of an intrathecal test dose in epidural analgesia. Can J Anaesth. 1994;41:572–574.
121. Richardson MG, Lee AC, Wissler RN. High spinal anesthesia after epidural test dose administration in five obstetric patients. Reg Anesth. 1996;21:119–123.
122. Ngan Kee WD, Khaw KS, Lee BB, et al. The limitations of ropivacaine with epinephrine as an epidural test dose in parturients. Anesth Analg. 2001;92:1529–1531.
123. Cohen SE, Yeh JY, Riley ET, Vogel TM. Walking with labor epidural analgesia: the impact of bupivacaine concentration and a lidocaine-epinephrine test dose. Anesthesiology. 2000;92:387–392.
124. Calimaran AL, Strauss-Hoder TP, Wang WY, et al. The effect of epidural test dose on motor function after a combined spinal-epidural technique for labor analgesia. Anesth Analg. 2003;96:1167–1172.
125. Roelants F, Mercier-Fuzier V, Lavand'homme PM. The effect of a lidocaine test dose on analgesia and mobility after an epidural combination of neostigmine and sufentanil in early labor. Anesth Analg. 2006;103:1534–1539.
126. Birnbach DJ, Chestnut DH. The epidural test dose in obstetric patients: has it outlived its usefulness? (editorial). Anesth Analg. 1999;88:971–972.
127. Khaw KS, Ngan Kee WD, Wong M, et al. Spinal ropivacaine for cesarean delivery: a comparison of hyperbaric and plain solutions. Anesth Analg. 2002;94:680–685.
128. Choi DH, Ahn HJ, Kim MH. Bupivacaine-sparing effect of fentanyl in spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25:240–245.
129. Dahlgren G, Hulstrand C, Jakobsson J, et al. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for Cesarean section. Anesth Analg. 1997;85:1288–1293.
130. Lyons G, Reynolds F. Toxicity and safety of epidural local anaesthetics. Int J Obstet Anesth. 2001;10:259–262.
131. Lyons G, Columb M, Wilson RC, Johnson RV. Epidural pain relief in labour: potencies of levobupivacaine and racemic bupivacaine. Br J Anaesth. 1998;81:899–901.
132. Beilin Y, Guinn NR, Bernstein HH, et al. Local anesthetics and mode of delivery: bupivacaine versus ropivacaine versus levobupivacaine. Anesth Analg. 2007;105:756–763.
133. Camorcia M, Capogna G. Epidural levobupivacaine, ropivacaine and bupivacaine in combination with sufentanil in early labour: a randomized trial. Eur J Anaesthesiol. 2003;20:636–639.
134. Lacassie HJ, Habib AS, Lacassie HP, Columb MO. Motor blocking minimum local anesthetic concentrations of bupivacaine, levobupivacaine, and ropivacaine in labor. Reg Anesth Pain Med. 2007;32:323–329.
135. Finucane BT. Ropivacaine cardiac toxicity—not as troublesome as bupivacaine. Can J Anaesth. 2005;52:449–453.
136. Lam DT, Ngan Kee WD, Khaw KS. Extension of epidural blockade in labour for emergency Caesarean section using 2% lidocaine with epinephrine and fentanyl, with or without alkalinisation. Anaesthesia. 2001;56:790–794.
137. Gaiser RR, Cheek TG, Gutsche BB. Epidural lidocaine versus 2-chloroprocaine for fetal distress requiring urgent Cesarean section. Int J Obstet Anesth. 1994;3:208.
138. Eisenach JC, Schlairet TJ, Dobson CE, Hood DH. Effect of prior anesthetic solution on epidural morphine analgesia. Anesth Analg. 1991;73:119–123.
139. Corke BC, Carlson CG, Dettbarn WD. The influence of 2-chloroprocaine on the subsequent analgesic potency of bupivacaine. Anesthesiology. 1984;60:25–27.
140. Hess PE, Snowman CE, Hahn CJ, et al. Chloroprocaine may not affect epidural morphine for postcesarean delivery analgesia. J Clin Anesth. 2006;18:29–33.
141. DiFazio CA, Carron H, Grosslight KR, et al. Comparison of pH-adjusted lidocaine solutions for epidural anesthesia. Anesth Analg. 1986;65:760–764.
142. Ackerman WE, Juneja MM, Denson DD, et al. The effect of pH and Pco2 on epidural analgesia with 2% 2-chloroprocaine. Anesth Analg. 1989;68:593–598.
143. Choi PT, Galinski SE, Takeuchi L, et al. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Can J Anaesth. 2003;50:460–469.
144. Norris MC, Leighton BL, DeSimone CA. Needle bevel direction and headache after inadvertent dural puncture. Anesthesiology. 1989;70:729–731.
145. Richardson MG, Wissler RN. The effects of needle bevel orientation during epidural catheter insertion in laboring parturients. Anesth Analg. 1999;88:352–356.
146. Angle PJ, Kronberg JE, Thompson DE, et al. Dural tissue trauma and cerebrospinal fluid leak after epidural needle puncture. Anesthesiology. 2003;99:1376–1382.
147. Huffnagle SL, Norris MC, Arkoosh VA, et al. The influence of epidural needle bevel orientation on spread of sensory blockade in the laboring parturient. Anesth Analg. 1998;87:326–330.
148. Apfel CC, Saxena A, Cakmakkaya OS, et al. Prevention of postdural puncture headache after accidental dural puncture: a quantitative systematic review. Br J Anaesth. 2010;105:255–263.
149. Hodgkinson R. Total spinal block after epidural injection into an interspace adjacent to an inadvertent dural perforation. Anesthesiology. 1981;55:593–595.
150. Leach A, Smith GB. Subarachnoid spread of epidural local anaesthetic following dural puncture. Anaesthesia. 1988;43:671–674.
151. Bernards CM, Kopacz DJ, Michel MZ. Effect of needle puncture on morphine and lidocaine flux through the spinal meninges of the monkey in vitro: implications for combined spinal-epidural anesthesia. Anesthesiology. 1994;80:853–858.
152. Faure E, Moreno R, Thisted R. Incidence of postdural puncture headache in morbidly obese parturients (letter). Reg Anesth. 1994;19:361–363.
153. Scavone BM, Wong CA, Sullivan JT, et al. Efficacy of a prophylactic epidural blood patch in preventing post dural puncture headache in parturients after inadvertent dural puncture. Anesthesiology. 2004;101:1422–1427.
154. Bahar M, Chanimov M, Cohen ML, et al. The lateral recumbent head-down position decreases the incidence of epidural venous puncture during catheter insertion in obese parturients. Can J Anaesth. 2004;51:577–580.
155. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152–161.
156. Chadwick HS, Posner K, Caplan RA, et al. A comparison of obstetric and nonobstetric anesthesia malpractice claims. Anesthesiology. 1991;74:242–249.
157. Collier CB. Why obstetric epidurals fail: a study of epidurograms. Int J Obstet Anesth. 1996;5:19–31.
158. Beilin Y, Bernstein HH, Zucker-Pinchoff B. The optimal distance that a multiorifice epidural catheter should be threaded into the epidural space. Anesth Analg. 1995;81:301–304.
159. Beilin Y, Zahn J, Bernstein HH, et al. Treatment of incomplete analgesia after placement of an epidural catheter and administration of local anesthetic for women in labor. Anesthesiology. 1998;88:1502–1506.
160. Gielen MJ, Slappendel R, Merx JL. Asymmetric onset of sympathetic blockade in epidural anaesthesia shows no relation to epidural catheter position. Acta Anaesthesiol Scand. 1991;35:81–84.
161. Hogan Q. Distribution of solution in the epidural space: examination by cryomicrotome section. Reg Anesth Pain Med. 2002;27:150–156.
162. Drasner K, Rigler ML. Repeat injection after a “failed spinal”: at times, a potentially unsafe practice. Anesthesiology. 1991;75:713–714.
163. Rigler ML, Drasner K. Distribution of catheter-injected local anesthetic in a model of the subarachnoid space. Anesthesiology. 1991;75:684–692.
164. Furst SR, Reisner LS. Risk of high spinal anesthesia following failed epidural block for cesarean delivery. J Clin Anesth. 1995;7:71–74.
165. Schlake PT, Peleman RR, Winnie AP. Separation of the hub from the shaft of a disposable epidural needle. Anesthesiology. 1988;68:611–613.
166. Moore DC, Artru AA, Kelly WA, Jenkins D. Use of computed tomography to locate a sheared epidural catheter. Anesth Analg. 1987;66:795–796.
167. Langevin PB, Gravenstein N, Langevin SO, Gulig PA. Epidural catheter reconnection. Safe and unsafe practice. Anesthesiology. 1996;85:883–888.
* Chapter 13 contains a detailed discussion of anesthetic agents used for neuraxial anesthetic techniques.