Oral and Maxillofacial Radiology
• Multilocular Radiolucent Lesion in the Pericoronal Region (Keratocystic Odontogenic Tumor [Odontogenic Keratocyst])
• Unilocular Radiolucent Lesion of the Mandible
• Multilocular Radiolucent Lesion in the Periapical Region (Ameloblastoma)
• Unilocular Radiolucent Lesion in a Periapical Region (Periapical Cyst)
Interpretation of radiographs is a routine part of the daily practice of oral and maxillofacial surgery. Commonly obtained radiographs at the office include the periapical, occlusal, panoramic, and lateral cephalometric radiographs. Cone beam computed tomography (CBCT) scans are becoming more readily available in many offices. Although this technology is extremely useful, its indications, liabilities, and advantages have to be clearly recognized. As the future unfolds, the advancing technology will improve upon office imaging modalities that will facilitate diagnosis and treatment. Therefore, a knowledge of normal radiographic anatomy and clinical skill in recognizing pathologic conditions become even more essential.
Despite clinicians' ability to read and interpret many different imaging studies, the oral and maxillofacial radiologist will play an increasingly greater role in the practice of oral and maxillofacial surgeons.
This section includes the radiographic presentation of five important and representative pathologic processes, in addition to a new case demonstrating the use of CBCT. Included in each case is the differential diagnosis of associated conditions, to guide further study.
Figure 1-1 shows the most common location of several radiographically detectable maxillofacial pathologic processes.
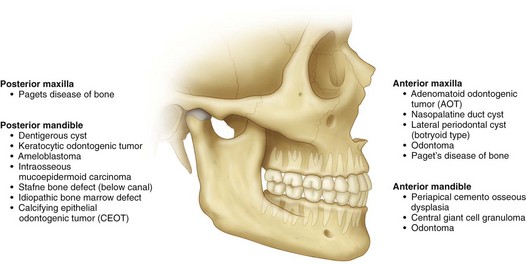
Figure 1-1 The most common location of several radiographically detectable maxillofacial pathologic processes.
Multilocular Radiolucent Lesion in the Pericoronal Region (Keratocystic Odontogenic Tumor [Odontogenic Keratocyst])
CC
A 20-year-old man is referred for evaluation of a swelling on his right mandible.
Keratocystic Odontogenic Tumor (KCOT)
Keratocystic odontogenic tumors (KCOTs) show a slight predilection for males and are predominantly found in individuals of Northern European descent. The peak incidence is seen between 11 and 40 years of age. Patients with larger lesions may present with pain secondary to infection of the cystic cavity. Smaller lesions are usually asymptomatic and are frequently diagnosed during routine radiographic examination.
The World Health Organization (WHO) has recommended the use of the term keratocystic odontogenic tumor (KCOT), rather than odontogenic keratocyst (OKC), because the former name better reflects the neoplastic behavior of the lesion. Genetically, the lesion shows a repeatable chromosomal abnormality (PTCH gene on chromosome 9q22.3-q31).
HPI
The patient complains of a 2-month history of progressive, nonpainful swelling of his right posterior mandible. (About 65% to 83% of KCOTs occur in the mandible, most often in the posterior body and ramus region. KCOTs account for approximately 3% to 14% of all oral cystic lesions.) The patient denies any history of pain in his right lower jaw, fever, purulence, or trismus. He does not report any neurosensory changes (which are generally not seen with KCOTs).
MHX/PDHX/Medications/Allergies/SH/FH
Noncontributory. There is no family history of similar presentations.
Nevoid basal cell carcinoma syndrome (NBCCS) is an autosomal dominant inherited condition with features that can include multiple basal cell carcinomas of the skin, multiple KCOTs, intracranial calcifications, and rib and vertebral anomalies. Many other anomalies have been reported with this syndrome (Box 1-1). The prevalence of NBCCS is estimated to be 1 in 57,000 to 1 in 164,000 persons.
Examination
Maxillofacial. The patient has slight lower right facial swelling isolated to the lateral border of the mandible and not involving the area below the inferior border. The mass is hard, nonfluctuant, and nontender to palpation (large cysts may rupture and leak keratin into the surrounding tissue, provoking an intense inflammatory reaction that causes pain and swelling). There are no facial or trigeminal nerve deficits (paresthesia of the inferior alveolar nerve would be more indicative of a malignant process). The intercanthal distance is 33 mm (normal), and there is no evidence of frontal bossing. His occipitofrontal circumference is normal (an intercanthal distance [the distance between the two medial canthi of the palpebral fissures] of greater than 36 mm is indicative of hypertelorism, and an occipitofrontal circumference greater than 55 cm is indicative of frontal bossing; both can be seen with NBCCS).
Neck. There are no palpable masses and no cervical or submandibular lymphadenopathy. Positive lymph nodes would be indicative of an infectious or a neoplastic process. A careful neck examination is paramount in the evaluation of any head and neck pathology.
Intraoral. Occlusion is stable and reproducible. The right mandibular third molar appears to be distoangularly impacted (KCOTs do not typically alter the occlusion). The interincisal opening is within normal limits. There is buccal expansion of the right mandible, extending from the right mandibular first molar area posteriorly toward the ascending ramus. Resorption of bone may include the cortex at the inferior border of the mandible, but this is observed at a slower rate than in intermedullary bone, which is less dense. For this reason, KCOTs characteristically extend anteroposteriorly than buccolingually. This pattern of expansion into less-dense bone explains why maxillary KCOTs show more buccal than palatal expansion and often expand into the maxillary sinus. There is no palpable thrill or audible bruit, both of which are seen with arteriovenous malformations. The oral mucosa is normal in appearance with no signs of acute inflammatory processes.
Thorax-abdomen-extremity. The patient has no findings suggestive of NBCCS (e.g., pectus excavatum, rib abnormalities, palmar or plantar pitting, and skin lesions; see Box 1-1).
Imaging
A panoramic radiograph is the initial screening examination of choice for patients presenting for evaluation of intraosseous mandibular pathology (10% to 20% of KCOTs are incidental radiographic findings). This provides an excellent overview of the bony architecture of the maxilla, mandible, and associated structures. CT scans can be obtained when large lesions are found. CT scans are valuable in that they provide additional information, such as the proximity of adjacent structures (e.g., the mandibular canal), the integrity of cortical plates, and the presence of perforations into adjacent soft tissues. CT scans provide accurate assessment of the size of the lesion and can demonstrate additional anatomic details (or lesions) that do not appear on panoramic radiographs.
A CBCT scan is appropriate for the evaluation of this lesion. Given its higher resolution, lower radiation dose (approximately 20% of the radiation of a conventional [helical] CT ), and lower cost, a CBCT can replace helical CT for evaluation and follow up of such a lesion. The CBCT scan can also be used to create a stereolithic model of the area of interest.
It has been demonstrated that T2-weighted magnetic resonance imaging (MRI) can detect KCOTs in 85% of new cases with a readily recognizable pattern. However, the use of MRI for management of suspected OKCs is not routine.
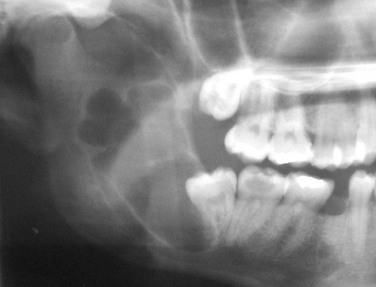
In this patient, the panoramic radiograph reveals a large, multilocular radiolucent lesion with possible displacement of the right mandibular third molar (Figure 1-2). There are also several carious teeth and a retained root tip of the right mandibular second bicuspid (tooth #29). (In a patient with a radiolucent lesion of the mandible presumed to be an odontogenic cystic lesion, a multilocular appearance is associated with a 12-fold increased risk for the diagnosis of KCOT; however, the presence of a unilocular lesion does not exclude the possibility of a KCOT diagnosis.)
Labs
No laboratory tests are indicated unless dictated by the medical history.
Fine-needle aspiration (FNA) biopsy and cytokeratin-10 immunocytochemical staining have been shown to differentiate KCOTs from dentigerous and other nonkeratinizing cysts. Despite their availability, these techniques are not routinely ordered.
Differential Diagnosis
The differential diagnosis of multilocular radiolucent lesions can be divided into lesions of cystic pathogenesis, neoplastic (benign or malignant) lesions, and vascular anomalies (least common). The differential diagnosis of multilocular radiolucent lesions is presented in Box 1-2 and can be further narrowed by the clinical presentation. Special consideration should be given to radiolucent lesions with poorly defined or ragged borders, which have a separate differential.
Biopsy
An incisional or excisional biopsy can be performed, depending on the size of the lesion. A smaller cystic lesion can be completely excised, whereas larger lesions require an incisional biopsy to guide final therapy. It is important to aspirate the lesion before incising into it (entering carefully through the cortical bone) to rule out a vascular lesion. The aspiration of bright red blood alerts the surgeon to the presence of a high-flow vascular lesion, such as an arteriovenous malformation, which could result in uncontrollable hemorrhage. In such a case, the procedure should be aborted to allow for further radiographic and angiographic studies to characterize the vasculature of the area. The aspiration of straw-colored (or clear) fluid is characteristic of a cystic lesion, and the absence of any aspirate may be seen with a solid mass (tumors).
Assessment
Expansile multilocular radiolucent mass of the posterior right mandible associated with an impacted right mandibular third molar (25% to 40% of cases are associated with an unerupted tooth).
With this patient under intravenous anesthesia, an incisional biopsy was performed after aspiration of straw-colored fluid that showed the classic histopathology of the KCOT. Histologic features include a thin squamous cell epithelial lining (eight cells or fewer). Because of the lack of rete ridges, the epithelial–connective tissue interface is flat. The epithelial surface is parakeratinized and often corrugated (wavy). The basal cell layer is hyperchromatic and composed of cuboidal cells, which show prominent palisading, giving a “tombstone” effect. The fibrous wall is usually thin and at times shows a mixed inflammatory response. Keratinization of the lumen is not a pathognomonic finding. The fibrous wall may contain epithelial islands that show central keratinization and cyst formation; these are known as daughter-satellite cells.
Treatment
Options that have been used to treat KCOTs include the following:
• Decompression by marsupialization
• Marsupialization followed by enucleation (surgical decompression of the cyst, followed by several months of daily irrigation with chlorhexidine via stents secured in the cystic cavity, followed by cystectomy)
• Enucleation with curettage alone
• Enucleation followed by chemoablation or cryotherapy
• Enucleation with peripheral ostectomy
• Enucleation with peripheral ostectomy and chemoablation or cryotherapy
Resection is advocated only if there have been multiple recurrences after enucleation with an adjunctive procedure (e.g., cryotherapy, Carnoy's solution, or peripheral ostectomy) or for a large KCOT exhibiting aggressive behavior, such as destruction of adjacent tissues. Several studies demonstrate that enucleation alone (when the diagnosis of KCOT has been established) has a high recurrence rate; therefore, many surgeons advocate enucleation with a local adjunctive procedure, such as cryotherapy, Carnoy's solution, and/or peripheral ostectomy.
KCOTs do not invade the epineurium; therefore, the inferior alveolar nerve can be separated and preserved. Furthermore, any perforations of the keratinized mucosa should be excised, because they may contain additional epithelial rests, which can lead to recurrences. Aggressive soft tissue excision is not required, because KCOTs do not usually infiltrate adjacent structures. If the cyst is removed in one unit, there is no need for curettage, unless the lining has been shredded or torn.
Some controversy exists regarding the optimal management (extraction versus retention) of teeth involved with an KCOT. It is generally accepted that a KCOT with a scalloped radiographic appearance should have the associated teeth removed, because it is considered impossible to completely remove the thin-walled cystic lining. However, if the KCOT is successfully removed in one unit, the teeth may be spared without compromising recurrence. In most instances there is no need for endodontic therapy, despite surgical denervation. The teeth may not become devitalized due to perfusion of the pulp via accessory canals through the periodontal ligaments.
Some surgeons advocate the application of Carnoy's solution after enucleation and peripheral ostectomy with application of methylene blue. Carnoy's solution is composed of 6 ml of absolute alcohol, 3 ml of ferric chloride, and 1 ml of 100% acetic acid. Chloroform is no longer recommended due to its carcinogenic potential. Carnoy's solution penetrates the bone to a depth of 1.54 mm after a 5-minute application. It is difficult to obtain and needs to be mixed fresh. It does not fixate the inferior alveolar nerve, but some clinicians cover the nerve with sterile petrolatum as a caution.
Synchronous bone grafting is not carried out with this technique. Cryotherapy with liquid nitrogen is an acceptable alternative to the use of Carnoy's solution. Liquid nitrogen is sprayed within the cavity and penetrates to a depth of about 1.5 mm. Suggested protocols include spraying the cavity for 1 minute and then allowing the bone to thaw. This can be repeated two or three times.
Synchronous grafting with cancellous bone can be accomplished after cryotherapy. Patients should be cautioned since liquid nitrogen does weaken the mandible, and this may result in a pathologic fracture. Sensory nerves within the field may show paresthesia; however, the majority recover within 3 to 6 months.
With both techniques, adjacent soft tissue needs to be protected. An alternate technique is used in cases of buccal or lingual plate perforation and with sinus involvement.
This patient was treated under general anesthesia with enucleation of the lesion followed by the application of methylene blue to guide peripheral ostectomy. The patient was placed on a soft diet to reduce the risk of jaw fracture. The postoperative panoramic radiograph confirmed that the inferior border of the mandible remained intact.
The final pathology report confirmed the diagnosis of a KCOT consistent with the initial incisional biopsy specimen. The patient was placed on a strict recall schedule—every 6 months for the first 5 years and then yearly. The recurrence rate for KCOTs has been reported to range from 5% to 60%. It has been reported that most recurrences are seen within 5 years, although they can develop at any time. Recurrences that arise secondary to residual cyst left in the bone may be apparent within 18 months of surgery.
Complications
The KCOT has been described as having clinical features that include potentially aggressive behavior and a high recurrence rate. Because recurrence is a major concern, clinicians vary in their surgical approach. Resection results in the lowest recurrence rate; however, there is considerable morbidity associated with this radical treatment. The primary mechanisms for recurrence have been postulated to be incomplete removal of all the cystic lining; new primary cyst formation from additional activated rests; or the development of a new KCOT in an adjacent area that is interpreted as a recurrence.
KCOTs have been reported to undergo transformation into ameloblastoma and squamous cell carcinoma, although this occurrence is rare. Other common postprocedural complications include inferior alveolar nerve paresthesia, postoperative infection and, with larger lesions, pathologic mandibular fracture (the highest risk is during the first few weeks after enucleation).
Discussion
Ever since the histologic features of the KCOT were established, many investigators have recognized that two major variants exist, based on microscopic findings: a cyst with a parakeratinized epithelial lining and a cyst with an orthokeratinized epithelial lining.
Crowley and colleagues undertook a comparison of the orthokeratin and parakeratinized variants. In their review, they found that the parakeratinized variant occurred more commonly than the orthokeratinized variant (frequency of 86.2% for the parakeratinized variant compared with 12.2% for the orthokeratinized variant); 1.6% of cysts had both orthokeratin and parakeratin features.
These researchers also found that the parakeratinized variant demonstrated a 42% recurrence rate, compared to only 2.2% for the orthokeratinized variant. In addition, the orthokeratinized variant was more frequently associated with impacted teeth. Given the different clinical behaviors of these two entities, many authors designate them as separate pathologic lesions, with the orthokeratinized variant known as an orthokeratinized odontogenic cyst (OOC). A lesion with both orthokeratin and parakeratin features should be treated as a parakeratinized KCOT (OKC).
As was mentioned earlier, in 2005 WHO designated the OKC a keratocystic odontogenic tumor (KCOT). The lesion was defined as a benign, unicystic or multicystic intraosseous tumor of odontogenic origin with a characteristic lining of parakeratinized, stratified squamous epithelium and the potential for aggressive infiltrative behavior.
Stimulation of residual epithelial cells is a common feature in the development of any cyst. In the case of the KCOT, the epithelial cells implicated are from the rest of Serres or Malassez or from the reduced enamel epithelium. Aberration of a PTCH gene is thought to be a genetic cause for the etiology of the KCOT. Collagenase activity in the cyst's epithelium, with its resorptive properties, appears to regulate the ability of the lesion to grow expansively in bone.
Identification of individuals who may have NBCCS allows the clinician to arrange for appropriate referrals. NBCCS should be suspected when multiple lesions exist. The diagnosis is confirmed upon finding any two of the major criteria, or one major criterion plus two minor criteria (see Box 1-1). Some abnormalities are pertinent only to the diagnosis and do not require any specific therapy. Other abnormalities may pose further risk to the patient and require the input of other specialists. Spina bifida and central nervous system tumors require referral to a neurosurgeon. In addition, genetic counseling for all patients afflicted with NBCCS is recommended. KCOTs associated with this syndrome are treated in the same manner as an isolated KCOT; however, these lesions have a higher rate of recurrence when associated with NBCCS (which may represent new lesions). KCOTs are often associated with the follicle of a potentially functional tooth and, when possible, marsupialization with orthodontic guidance should be considered.
August, M, Caruso, PA, Faquin, WC. Report of a case: an 18-year-old man with a one-month history of nontender left mandibular swelling. N Engl J Med. 2005; 353:2798–2805.
August, M, Faquin, WC, Troulis, M, et al. Differentiation of odontogenic keratocysts from nonkeratinizing cysts by use of fine-needle aspiration biopsy and cytokeratin-10 staining. J Oral Maxillofac Surg. 2000; 58:935–940.
Barnes L, Eveson JW, Reichart P, et al, eds. Pathology and genetics: head and neck tumours (IARC World Health Organization classification of tumors). Lyon, France: IARC Press, 2005.
Buckley, P, Seldin, E, Dodson, T, et al. Multilocularity as a radiographic marker of the keratocystic odontogenic tumor. J Oral Maxillofac Surg. 2012; 70:320.
Crowley, T, Kaugras, GE, Gunsolley, JC. Odontogenic keratocysts: a clinical and histologic comparison of the parakeratin and orthokeratin variants. J Oral Maxillofac Surg. 1992; 50:22–26.
Evans, DG, Ladusans, EJ, Rimmer, S, et al. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993; 30:460–464.
Kolokythas, A. OKC: to decompress or not to decompress—a comparative study between decompression and enucleation vs resection/peripheral ostectomy. J Oral Maxillofac Surg. 2007; 65(4):640–644.
Madras, J. Keratocystic odontogenic tumor: reclassification of the odontogenic keratocyst from cyst to tumor. J Can Dent Assoc. 2008; 74:2.
Marx, RE, Stern, D. Oral and maxillofacial pathology: a rationale for diagnosis and treatment. Chicago: Quintessence; 2003.
Pindborg, JJ, Hansen, J. Studies on odontogenic cyst epithelium: clinical and roentgenographic aspects of odontogenic keratocysts. Acta Pathol Microbial Scand. 1963; 58:283.
Pogrel, MA. Keratocystic odontogenic tumor. In: Bagheri S, ed. Current therapy in oral and maxillofacial surgery. St Louis: Mosby, 2012.
Pogrel, MA, Jordan, RC. Marsupialization as a definitive treatment for the odontogenic keratocyst. J Oral Maxillofac Surg. 2004; 62:651–655.
Pogrel, MA, Schmidt, BL. The odontogenic keratocyst. Oral Maxillofacial Surg Clin N Am. 15(3): xi, 2003.
Shear, M. Primordial cysts. J Dent Assoc S Afr. 1960; 152:1.
Tolstunov, L, Treasure, T. Surgical treatment algorithm for odontogenic keratocyst: combined treatment of odontogenic keratocyst and mandibular defect with marsupialization, enucleation, iliac crest bone graft and dental implants. J Oral Maxillofac Surg. 2008; 66:1025.
Quereshy, F, Barnum, G, Demko, C, et al. Applications of cone beam computed tomography in the practice of oral and maxillofacial surgery. J Oral Maxillofac Surg. 2008; 66:791.
Unilocular Radiolucent Lesion of the Mandible
HPI
Approximately 2 months earlier, the patient noticed a nonpainful swelling of the right posterior mandible (dentigerous cysts can cause expansion but are typically nonpainful unless secondarily infected). The most common location of dentigerous cysts is the mandibular third molar region; other frequently involved teeth include the maxillary canines, maxillary third molars, and mandibular second premolars. The patient was seen by the referring general dentist, who discovered a radiolucent lesion on a periapical radiograph. The patient denies any history of pain in his right lower jaw, fever, purulence, or trismus (inability to open the mouth due to contraction of the muscles of mastication, commonly a sign of inflammatory infiltration of the muscles secondary to infection).
PMHX/PDHX/Medications/Allergies/SH/FH
Noncontributory. There is no history of similar presentations in his family (there is no familial predisposition).
Examination
General. The patient is a well-developed and well-nourished anxious man (patients are often anxious because they fear a malignant process).
Maxillofacial. There is noticeable right lower facial swelling isolated to the lateral border of the mandible not involving the area below the inferior border. The mass is hard, nonfluctuant, and nontender to palpation, consistent with a noninflammatory process. There are no facial or trigeminal nerve deficits (paresthesia of the right inferior alveolar nerve would be indicative of a malignant process).
Neck. The patient does not have palpable masses or cervical or submandibular lymphadenopathy. Positive lymph nodes would be indicative of an infectious or neoplastic etiology, so a careful neck examination is paramount in the evaluation of any head and neck pathology.
Intraoral. The occlusion is stable and reproducible. There does not appear to be displacement of the dentition in the involved area (dentigerous cysts do not typically alter the occlusion). Interincisal opening is within normal limits. There is significant buccal expansion of the right mandible, extending from the mental foramen posteriorly and ascending into the ramus (large cysts may be associated with a painless expansion of the bone, but most are asymptomatic and do not cause expansion). The patient does not have a palpable thrill or an audible bruit (both are seen with arteriovenous malformations). The oral mucosa is normal in appearance with no signs of any acute inflammatory processes.
Imaging
When evaluating large lesions of the mandible, the panoramic radiograph is an excellent initial study for assessment of the bony and dental anatomy. A CT scan is not essential, but it can better delineate the three-dimensional bony and regional architecture, including involvement of the cortices of the mandible (i.e., cortical perforation is seen with some tumors and locally aggressive cysts) and the lesion's position in relation to the inferior alveolar canal.
In this patient, a panoramic radiograph (Figure 1-3, A) demonstrates a well-corticated unilocular radiolucent lesion of the right posterior mandible extending from the area of tooth #31 up to the sigmoid notch and coronoid process. The right mandibular third molar (tooth #32) is displaced inferiorly, and the lesion involves the roots of tooth #31, with some resorption and superior displacement of the tooth. After aspiration and incisional biopsy, teeth #31 and teeth #32 were extracted and the cyst was enucleated and curettaged (Figure 1-3, B to E). Six- and 16-week postoperative orthopantograms demonstrate good progressive bony fill of the defect (Figure 1-3, F and G).
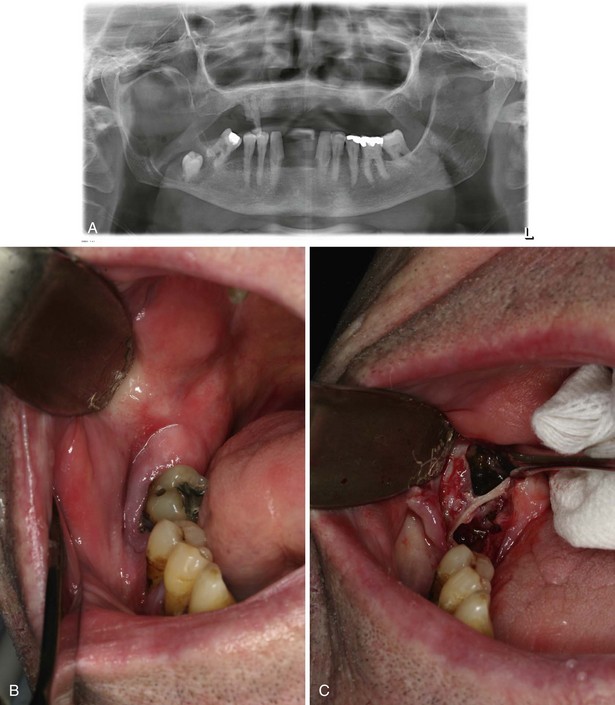
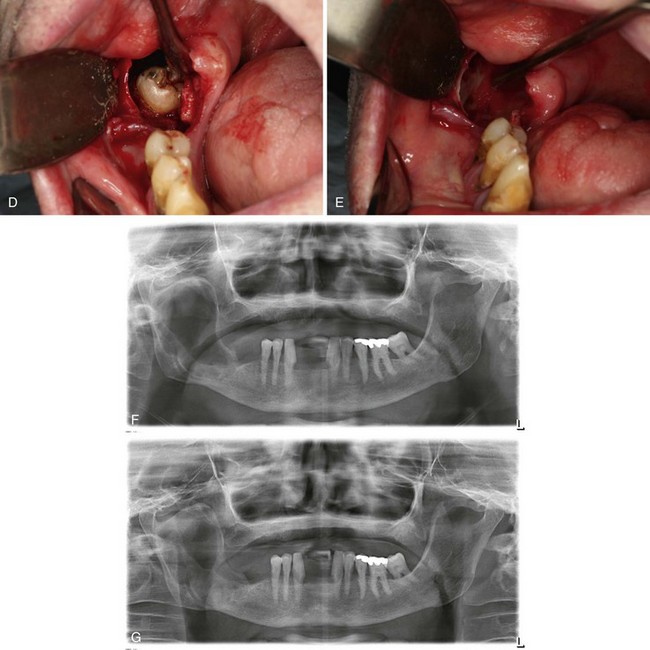
Figure 1-3 A, Unilocular radiolucency from posterior mandibular body to sigmoid notch. B, Preoperative photograph demonstrating absence of tooth #32. C, Initial exposure of the lesion. D, Unroofing of the lesion and exposure of tooth #32. E, Surgical defect after enucleation and curettage of the lesion. F, Orthopantogram 6 weeks after enucleation and curettage of the lesion. G, Orthopantogram 16 weeks after enucleation and curettage of the lesion.
Labs
No laboratory tests are indicated unless dictated by the medical history. If brown tumor of hyperparathyroidism is in the differential diagnosis, serum calcium levels should be obtained. This tumor is derived from primary hyperparathyroidism, which leads to osseous lesions with abundant hemorrhage and hemosiderin deposition within the tumor (giving it a brown color). Removal of the hyperplastic parathyroid tissue is necessary in this condition.
Differential Diagnosis
The differential diagnosis can be divided into lesions of cystic pathogenesis, neoplastic (benign or malignant) lesions, and vascular anomalies (least common). Well-defined borders and the lack of a multilocular appearance are more suggestive of a cystic process, but this distinction is not predictable. The differentials presented in Box 1-3 should be considered; the first three are the most likely.
Biopsy
An incisional biopsy would be indicated to guide final therapy for this lesion. This can be done under local, intravenous sedation or general anesthesia, depending on the surgeon's preference and the medical indications. It is important to obtain an aspirate of the mass before perforating the bony cortex. Bright red blood would alert the surgeon to the presence of a high-flow vascular lesion that could result in uncontrollable hemorrhage. In such a case, the procedure should be aborted to allow for further radiographic and angiographic studies to characterize the vasculature of the area. Straw-colored fluid is characteristic of a cystic lesion, and the absence of any aspirate may be seen with tumors of the jaw.
Assessment
Expansile radiolucent mass of the posterior mandible associated with impacted right mandibular second and third molars
In this case, under intravenous anesthesia, an incisional biopsy was performed after aspiration (straw-colored fluid), demonstrating classic dentigerous cyst histopathology (epithelial lining of nonkeratinized, stratified squamous epithelium and a loosely arranged fibrous connective tissue wall; see Figure 1-3, A to D).
Treatment
Complete removal of the cyst by enucleation, along with removal of the unerupted tooth, is the preferred treatment for dentigerous cysts. If eruption of the involved tooth into a functional position is feasible (with or without orthodontic guidance), enucleation of the cyst can be performed without removal of the tooth. The inferior alveolar neurovascular bundle is commonly displaced by the cyst and should be preserved if possible. Large cysts may be treated with marsupialization when enucleation and curettage would likely result in neurosensory dysfunction or a pathologic fracture of the mandible. Postoperative maxillomandibular fixation may be prudent to allow remodeling of the bone before function, especially in larger lesions.
Marsupialization permits decompression of the lesion and shrinkage of the cyst and bony defect. Definitive removal can be performed at a later date. A disadvantage of marsupialization is the need for greater patient compliance with an open cystic cavity between treatments. In addition, it does not reliably produce a reduction in the size of the cyst.
This patient was taken to the operating room and underwent enucleation and curettage via an intraoral incision. The right mandibular second and third molars were maintained due to the high risk of fracture and inferior alveolar nerve paresthesia; although this may increase the possibility of recurrence, that fact should be weighed against the possible complications of tooth removal.
The patient was placed on a soft diet to reduce the risk of jaw fracture. The postoperative panoramic radiograph confirmed that the inferior border of the mandible remained intact.
Marsupialization, mentioned earlier, is an alternative treatment; however, the patient must be compliant in serial appointments thereafter and must often undergo final definitive treatment of the shrunken cyst.
The final pathology report confirmed the diagnosis of a dentigerous cyst consistent with the initial incisional biopsy specimen.
Complications
With adequate treatment, the prognosis for a dentigerous cyst is excellent, and recurrence is rare. Left untreated, these cysts may lead to tooth displacement, resorption of adjacent tooth roots, and extensive bone destruction as they increase in size. The most common complications include postoperative infection, inferior alveolar nerve paresthesia and, with larger cysts, mandibular fracture. Neoplastic transformation into an ameloblastoma, a squamous cell carcinoma, and a mucoepidermoid carcinoma has been reported in fewer than 2% of cases. For this reason, a complete histopathologic examination with enucleation may be preferable to marsupialization, which could delay the diagnosis of a neoplastic transformation.
Discussion
A dentigerous cyst is developmental in origin; it forms as a consequence of the buildup of fluid between the dental follicle and the crown of an unerupted tooth. It is the second most common odontogenic cyst after the periapical cyst. Arising from stellate reticulum and forming from the cementoenamel junction, they are the most common type of developmental odontogenic cyst, making up about 20% of all epithelium-lined cysts in the jaws. The pathogenesis is unknown but is possibly related to the accumulation of fluid between the reduced enamel epithelium and the tooth crown. Dentigerous cysts most commonly involve impacted mandibular third molars but may also involve maxillary canine, maxillary third, and mandibular second molars. This cyst is most commonly discovered between the ages of 10 and 30 years. It has a slight male predilection and a higher prevalence for Caucasians than for African Americans.
Dentigerous cysts are commonly asymptomatic on presentation but can grow to a significant size, resulting in painless expansion of the bone in the involved area. Large dentigerous cysts can become secondarily infected, with associated pain and swelling.
Radiographically dentigerous cysts usually show a unilocular radiolucent area that is associated with an unerupted tooth. A large dentigerous cyst may give the impression of a multilocular process because of the bony trabeculations. However, these cysts are grossly and histopathologically a unilocular process.
Angadi, PV, Rekha, K. Calcifying epithelial odontogenic tumor (Pindborg tumor). Head Neck Pathol. 2011; 5(2):137–139.
Benn, A, Altini, M. Dentigerous cysts of inflammatory origin: a clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 81(2):203–209.
Boffano, P, Gallesio, C, Barreca, A, et al. Surgical treatment of odontogenic myxoma. J Craniofac Surg. 2011; 22(3):982–987.
Carneiro, JT, Jr., Carreira, AS, Felix, VB, et al. Pathologic fracture of jaw in unicystic ameloblastoma treated with marsupialization. J Craniofac Surg. 2012; 23(6):e537–e539.
Curran, AE, Damm, DD, Drummond, JF. Pathologically significant pericoronal lesions in adults: histopathologic evaluation. J Oral Maxillofac Surg. 2002; 60(6):613–617.
de Matos, FR, Nonaka, CF, Pinto, LP, et al. Adenomatoid odontogenic tumor: retrospective study of 15 cases with emphasis on histopathologic features. Head Neck Pathol. 2012; 6(4):430–437.
Elo, JA, Slater, LJ, Herford, AS, et al. Squamous cell carcinoma radiographically resembling a dentigerous cyst: report of a case. J Oral Maxillofac Surg. 2007; 65(12):2559–2562.
Gbolahan, O, Fatusi, O, Owotade, F, et al. Clinicopathology of soft tissue lesions associated with extracted teeth. J Oral Maxillofac Surg. 2008; 66(11):2284–2289.
Gomes, CC, Diniz, MG, Duarte, AP, et al. Molecular review of odontogenic myxoma. Oral Oncol. 2011; 47(5):325–328.
Koca, H, Esin, A, Aycan, K. Outcome of dentigerous cysts treated with marsupialization. J Clin Pediatr Dent. 2009; 34(2):165–168.
Marciani, RD. Is there pathology associated with asymptomatic third molars? J Oral Maxillofac Surg. 2012; 70(9 Suppl 1):S15–S19.
Peacock, ZS, Jordan, RC, Schmidt, BL. Giant cell lesions of the jaws: does the level of vascularity and angiogenesis correlate with behavior? J Oral Maxillofac Surg. 2012; 70(8):1860–1866.
Sandhu, SV, Narang, RS, Jawanda, M, et al. Adenomatoid odontogenic tumor associated with dentigerous cyst of the maxillary antrum: a rare entity. J Oral Maxillofac Pathol. 2010; 14(1):24–28.
Simiyu, BN, Butt, F, Dimba, EA, et al. Keratocystic odontogenic tumours of the jaws and associated pathologies: a 10-year clinicopathologic audit in a referral teaching hospital in Kenya. J Craniomaxillofac Surg. 2013; 41(3):230–234.
Smith, JL, II., Kellman, RM. Dentigerous cysts presenting as head and neck infections. Otolaryngol Head Neck Surg. 2005; 133(5):715–717.
Tabrizi, R, Ozkan, BT, Dehgani, A, et al. Marsupialization as a treatment option for the odontogenic keratocyst. J Craniofac Surg. 2012; 23(5):e459–e461.
Williams, MD, Hanna, EY, El-Naggar, AK. Anaplastic ameloblastic fibrosarcoma arising from recurrent ameloblastic fibroma: restricted molecular abnormalities of certain genes to the malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104(1):72–75.
Multilocular Radiolucent Lesion in the Periapical Region (Ameloblastoma)
CC
A 34-year-old woman complaining of a painless swelling in the lower jaw (ameloblastomas are often asymptomatic) is referred by her general dentist.
HPI
For the past 2 months, the patient has noticed a progressively enlarging “hard mass” in her mandible. There have been no neurosensory changes associated with the swelling (sensory changes are particularly common in malignancies and are not usually seen in benign lesions such as ameloblastomas). On consultation, her general dentist noticed a significant buccal and lingual bony expansion adjacent to normal-appearing first and second molars on the right posterior mandible, in addition to a multilocular radiolucent lesion on the panoramic radiograph (ameloblastomas occur most frequently in the mandible [80% of the time], often in the posterior mandible).
Examination
General. The patient is well developed and well nourished and appears distressed about her possible diagnosis.
Vital Signs. Vital signs are stable, and the patient is afebrile.
Maxillofacial. There is mild right facial enlargement that is most pronounced at the angle of the mandible, with no evidence of trismus. No cervical lymphadenopathy is present. (Ameloblastomas are benign tumors and in general do not cause lymphadenopathy, which may be seen with malignant tumors.) Neurosensory testing reveals normal mandibular nerve (V3) function bilaterally and no other focal neurologic deficits (ameloblastomas generally do not invade the neurovascular bundle).
Intraoral. There is buccal and lingual expansion of the posterior right mandible with mild tenderness but no evidence of fluctuance or purulent secretions. The mesiobuccal tip of the right mandibular third molar is partially visible. The right mandibular second molar is not mobile; however, there are periodontal pocket depths greater than 10 mm on the distal aspect.
Imaging
The panoramic radiograph is the initial imaging study of choice for evaluation of a mandibular mass. CT scans are particularly useful for outlining the three-dimensional anatomy to demonstrate the amount of expansion and areas of bony perforation implying subsequent soft tissue involvement. Accurate stereolithographic models can be fabricated from the CT scan and can assist in both resection and reconstruction.
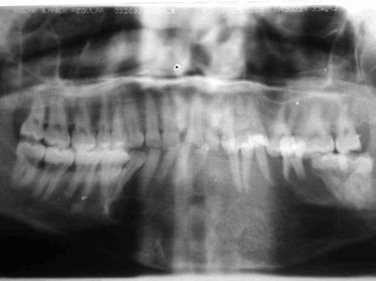
In this patient, the panoramic radiograph demonstrates a 5 × 3.5-cm multilocular, cystic-appearing lesion extending from the distal aspect of the impacted right mandibular third molar, involving the entire ramus up to the level of the sigmoid notch. The bone at the inferior border of the mandible has a normal appearance, without loss of continuity (Figure 1-4).
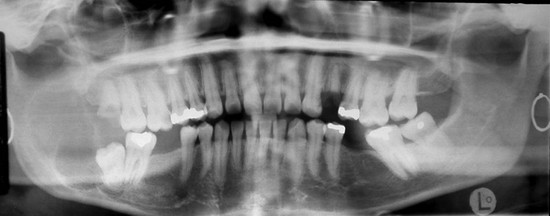
Figure 1-4 Panoramic radiograph demonstrating a multilocular radiolucent lesion of the right posterior mandible.
The CT scan shows an expansile lesion of the right posterior mandible, with cortical perforation seen on axial and coronal sections at the anterior border of the ramus. There is no evidence of lymphadenopathy, and no areas of abnormal enhancement are seen (contrast-enhanced imaging provides improved delineation of soft tissue and can aid in determining any associated vascular malformations).
Labs
Baseline hemoglobin and hematocrit levels should be obtained before major maxillofacial surgical interventions. Other laboratory tests are obtained as dictated by the medical history.
Differential Diagnosis
The differential diagnosis of a multilocular radiolucent lesion of the posterior mandible is best categorized as lesions of cystic, neoplastic, or vascular origin, with the latter being less common. Although the presentation just described is classic for an ameloblastoma (i.e., bony expansion of the posterior mandible with a multilocular or “soap bubble” appearance), this lesion cannot be distinguished on clinical and radiographic parameters. A complete differential diagnosis should be considered (see Differential Diagnosis in the section Multilocular Radiolucent Lesion in the Pericoronal Region [Odontogenic Keratocyst], earlier in the chapter).
Biopsy
For diagnosis of this multilocular and expansile lesion, aspiration followed by an incisional biopsy was performed with local anesthesia and intravenous sedation in the clinic. Needle aspiration was negative for blood or any clear fluids and therefore suggestive of a mass lesion. A typical buccal mucoperiosteal third molar incision was reflected (it is important to make the incision where the definitive surgical incision would ultimately be made in order to minimize dehiscence at the time of definitive surgery). No purulence was noted. A cystic lesion with a keratin-like substance was encountered in the large bony concavity. A large sample of the cyst lining was taken from two different locations. The wound was closed with 3-0 chromic interrupted sutures, and a specimen was sent for histopathological examination.
Assessment
Microscopic evaluation reveals islands of epithelium that resemble enamel organ in a fibrous connective tissue stroma; attached to the basement membrane surrounding the islands are tall columnar cells exhibiting reversed polarity.
This is consistent with the diagnosis of a multicystic, follicular ameloblastoma.
Treatment
The treatment of ameloblastomas has raised some controversy. In general, treatment must focus on the ability of the tumor to invade surrounding bone tissue. The average extension into surrounding bone beyond the normal tumor margin is 4.5 mm, with a range of 2 to 8 mm. With this in mind, resection must be at least 10 mm beyond the bony (and radiographic) margin of the tumor for large, multicystic-type ameloblastomas. Resected tumors seldom recur; the cure rate for primary tumors is 95% to 98%. In contrast, there is a high incidence of recurrence (approximately 70%) for treatment with enucleation and curettage alone. Regardless of the reconstructive measure, close patient follow-up is necessary to monitor for recurrence, especially in patients who do not undergo a resection. Because ameloblastomas can recur within a variable time frame, a cure rate for an ameloblastoma does not necessarily correlate with a 5-year disease-free period, compared with other neoplastic processes. Long-term follow-up is necessary.
For this patient, given the size and extensive, aggressive nature of the lesion, a segmental reconstruction was undertaken via an intraoral approach. Tumor resection was elected because of the close anatomic proximity of the tumor margin at the inferior boarder, in addition to the cortical perforation and involvement of mucosa at the anterior border and near the sigmoid notch. Special attention was paid to the site of perforation, in which a supraperiosteal resection was performed, and the overlying mucosa and periosteum were resected with the specimen. The condyle was retained, and the ramus and angle were reconstructed using a standard reconstruction plate. The inferior alveolar nerve was dissected free and preserved (ameloblastoma cells do not necessarily penetrate the nerve unless there is gross involvement of the inferior alveolar canal, which can theoretically allow the tumor cells to penetrate the perineural tissue).
Other reported treatment alternatives include enucleation, curettage and cryotherapy with or without bone grafting, and excision of the tumor with peripheral ostectomy. Marsupialization of unicystic variants have also been reported. These treatments have not been proved to be curative, are not widely advocated, and have a higher recurrence rate compared with resection.
The mandibular defect in this patient was subsequently reconstructed using an iliac crest cancellous bone graft, performed at 4 months (to allow time for soft tissue healing), via an extraoral approach. A segmental resection with an osteotomized, vascularized fibula free flap and reconstruction plate is a good alternative, especially with recurrences or large, aggressive tumors.
Complications
Complications associated with resection and reconstruction of this tumor include mandibular nerve anesthesia, graft failure, unacceptable facial symmetry, poor bony height and width for implant reconstruction, and donor site morbidity. Plate exposure, plate fracture, and intraoral dehiscence are also possibilities. Recurrence is the most worrisome long-term complication. Recurrence can be due to persistence of the original tumor that was not resected or actual recurrence of new neoplastic cells. Aggressive surgical therapy does not necessarily eliminate the chance of tumor recurrence. In theory, the more aggressive the initial treatment, the lesser the likelihood of tumor recurrence. However, this comes at the expense of a larger residual defect and more complicated reconstructive measures. Surgeons need to determine the extent of the resection and preservation of structures based on the available evidence, tumor biology, patient's preference and availability for follow-up, surgeon experience, and other individual factors.
Discussion
There are seven histologic types of ameloblastomas: follicular, plexiform, acanthomatous, granular cell, desmoplastic, basal cell, and unicystic variant; the first two types are the most common. The desmoplastic variant often presents in the anterior maxilla or mandible and can appear more radiopaque because of the high amount of dense connective tissue. Ameloblastomas can be either solid or multicystic, but they frequently demonstrate both characteristics. The tumor can arise from embryonic remnants of odontogenic cysts, dental lamina, enamel organ, or stratified squamous epithelium of the oral cavity.
Although the majority of the tumors originate from within the maxilla or mandible, they can also be peripheral. The different histological variants do not significantly alter treatment considerations except for the unicystic and the peripheral types, which can typically be treated with enucleation and curettage. Unicystic ameloblastomas represent about 10% to 15% of intraosseous ameloblastomas and can be misdiagnosed as dentigerous cysts. These lesions commonly occur in younger patients and have three distinct variants (luminal, intraluminal, and mural). Luminal and intraluminal types can typically be treated with enucleation and close observation, whereas mural types should be treated more aggressively. Peripheral ameloblastomas are very uncommon, representing approximately 1% of all ameloblastomas, and are usually successfully treated with local surgical excision because of their nonaggressive behavior. Malignant ameloblastomas are extremely rare and usually metastasize to the lungs (probably from aspiration of oral tumor). The malignant variant has a poor prognosis.
Bianchi, B, Ferri, A, Ferrari, S, et al. Mandibular resection and reconstruction in the management of extensive ameloblastoma. J Oral Maxillofac Surg. 2013; 71:528–537.
Carlson, ER, Marx, RE. The ameloblastoma: primary, curative surgical management. J Oral Maxillofac Surg. 2006; 64:484–494.
Carlson, ER, Monteleone, K. Analysis of inadvertent perforations of mucosa and skin concurrent with mandibular reconstruction. J Oral Maxillofac Surg. 2004; 62:1103–1107.
Chapelle, K, Stoelinga, P, de Wilde, P, et al. Rational approach to diagnosis and treatment of ameloblastoma and odontogenic keratocysts. Br J Oral Maxillofac Surg. 2004; 42:381–390.
Gerzenshtein, J, Zhang, F, Caplan, J, et al. Immediate mandibular reconstruction with microsurgical fibula flap transfer following wide resection for ameloblastoma. Plast Reconstr Surg. 2006; 17:178–182.
Gold, L, Williams, TP. Odontogenic tumors: surgical pathology and management. In: Fonseca RJ, ed. Oral and maxillofacial surgery. ed 2. Philadelphia: Saunders/Elsevier; 2009:466–538.
Laskin, DM, Giglio, JA, Ferrer-Nuin, LF. Multilocular lesion in the body of the mandible: clinicopathologic conference. J Oral Maxillofac Surg. 2002; 60:1045–1048.
Nakamura, N, Mitsuyasu, T, Higuchi, Y, et al. Growth characteristics of ameloblastoma involving the inferior alveolar nerve: a clinical and histopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91:557–562.
Neville, BW, Damm, DD, Allen, CM, et al. Oral and maxillofacial pathology, ed 2. New York: Saunders; 2002.
Sachs, SA. Surgical excision with peripheral ostectomy: a definitive, yet conservative, approach to the surgical management of ameloblastoma. J Oral Maxillofac Surg. 2006; 64:476–483.
Sampson, DE, Pogrel, MA. Management of mandibular ameloblastoma: the clinical basis for a treatment algorithm. J Oral Maxillofac Surg. 1999; 57:1074–1077.
Tung-Yiu, W, Jehn-Shyun, H, Ching-Hung, C. Epineural dissection to preserve the inferior alveolar nerve in excision of an ameloblastoma of mandible: case report. J Oral Maxillofac Surg. 2000; 58:1159–1161.
Unilocular Radiolucent Lesion in a Periapical Region (Periapical Cyst)
CC
A 37-year-old woman is referred by her general dentist for evaluation of a periapical radiolucency associated with the right mandibular second bicuspid, which was discovered during a new patient examination.
HPI
The patient reports having “a cavity for a long time,” with subsequent fracture, approximately 6 months earlier, of a portion of the crown of the right mandibular second bicuspid, which was part of a three-unit bridge. The right mandibular second molar and the right mandibular second bicuspid were treated endodontically 9 years earlier without complications. The patient denies any history of swelling or purulence in the region and is asymptomatic (periapical cysts seldom present with any clinical symptoms, but infected cysts can present with a draining fistula). The patient also denies a history of trauma in the area (periapical cysts are associated with pulpal necrosis secondary to either caries or trauma).
Examination
Maxillofacial. There is no discernible facial asymmetry or swelling (periapical cysts are rarely associated with any cortical expansion).
Neck. No cervical or submandibular lymphadenopathy can be detected (positive node findings could be indicative of an infectious or a neoplastic process).
Intraoral. The right mandibular second bicuspid is grossly carious and fractured at the gingival margin. There is no gingival swelling or palpation tenderness along the buccal or lingual cortices.
Imaging
A panoramic radiograph is the initial study of choice for any intraosseous lesion, because it provides an excellent overview of the bony anatomy and architecture of the maxilla and mandible and demonstrates the relationship to adjacent anatomic structures. A periapical radiograph can be obtained for small lesions, providing a more detailed outline of the borders and trabecular pattern. More extensive imaging, such as CT scanning, is seldom required for management of a periapical cyst unless the diagnosis is in question.
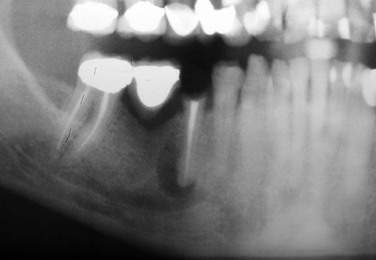
In this patient, a panoramic radiograph demonstrated a well-circumscribed, radiolucent lesion associated with the right mandibular second bicuspid (Figure 1-5). The lesion is approximately 1 cm in diameter (periapical cysts are generally between 0.5 and 1.5 cm in diameter but may enlarge to fill an entire quadrant). There is no associated root resorption (although root resorption is uncommon in association with a periapical cyst, it can be seen, especially with larger cysts).
Labs
No routine laboratory tests are indicated for the work-up of a periapical cyst unless dictated by the medical history.
Differential Diagnosis
The differential diagnosis of a periapical radiolucent lesion is greatly influenced by the clinical history and vitality of the associated tooth. If the associated tooth is nonvital and there is radiographic evidence of pulpal pathology, a periapical cyst is the most likely diagnosis. However, a diagnosis based on histopathological examination is warranted, because tumors, developmental cysts, metastatic disease, and early fibro-osseous lesions may also occur in a periapical location. The differential diagnosis is outlined Box 1-4.
Biopsy
Biopsy and treatment of small periapical cysts are usually synonymous and excisional in nature and vary, depending on the restorative plan for the involved tooth. If extraction is planned, then the cyst can be removed through the extraction socket. If the tooth is to be restored or the lesion is particularly large, a periapical approach through the buccal cortex can be used.
Assessment
Distinct periapical radiolucent lesion associated with the carious and nonvital right mandibular second bicuspid. The lesion is closely associated with the mental foramen.
In this case, the tooth was extracted and the underlying lesion was carefully removed to avoid injury to the inferior alveolar neurovascular bundle. The specimen was sent for histopathological examination and demonstrated a cystic lining of nonkeratinized, stratified squamous epithelium of varying thickness with numerous curved and round hyaline bodies (Rushton bodies). The wall of the cyst showed dense fibrous connective tissue and significant mixed inflammatory infiltrate of lymphocytes, plasma cells, neutrophils, and histocytes. The central lumen was found to contain proteinaceous fluid and necrotic cellular debris. These clinical and histologic findings are consistent with a periapical cyst.
Treatment
Periapical cysts are treated by enucleation and curettage, either through an extraction socket or via a periapical surgical approach when the tooth is restorable or the lesion is greater than 2 cm in diameter. If the tooth is to be preserved, endodontic treatment is necessary, if it has not been done. Some literature supports nonsurgical, conservative endodontic treatment of smaller lesions, ensuring the complete removal of causative organisms, and regular radiographic follow-up to evaluate healing and monitor potential lesion enlargement. Excised cysts should always be sent for histologic evaluation to rule out other possible pathologies. Recurrence is uncommon.
Complications
There are few complications associated with the treatment of a periapical cyst. Extraction of a tooth without removal of the cyst, or incomplete removal of the cystic lining, can result in a residual cyst. As mentioned previously, residual cysts are histologically identical to periapical cysts and treated with enucleation. Failed conservative endodontic treatment shows a persistent periapical cyst. Endodontic re-treatment may be attempted before a curative surgical apicoectomy and enucleation are performed. When both the buccal and lingual cortices are involved, it is possible for the area to heal with fibrous tissue (periapical scar). No treatment is necessary for periapical scars.
Complications associated with surgical removal of the cyst can be related to the regional anatomy. Neurosensory disturbances secondary to injury to the inferior alveolar nerve or branches of the mental nerve can be seen, especially with larger lesions, but these are usually temporary. Postoperative infection can occur with any surgical intervention.
Discussion
The periapical cyst, or radicular cyst, results from inflammatory stimulation of the rests of Malassez within the periodontal ligament. It is relatively common, accounting for a reported 50% of all oral cysts. Its development is preceded by a periapical granuloma that forms at the tooth apex in response to pulpal bacterial infection and necrosis. A periapical granuloma consists of an outer dense fibrous tissue capsule surrounding a central area of granulation tissue. Expansion of the granuloma leads to central ischemic necrosis and development of a central lumen surrounded by an epithelial membrane. The necrotic cellular debris within the lumen creates an osmotic gradient, drawing in fluid and causing enlargement of the cyst and resorption of surrounding bone secondary to hydrostatic pressure.
There is some debate in the literature as to radiographic distinctions between periapical granulomas and cysts, but the consensus is that they are indistinguishable. It has been shown that there is no significant relationship between preoperative symptoms and lesion type; however, lesions that present with an associated swelling demonstrate worse postoperative bone regeneration. There is a wide range of reported incidences of periapical granulomas versus cysts. The percentage of periapical radiolucent lesions that are attributed to periapical cysts is reported as close to 50%. However, studies with strict histological criteria put the incidence of periapical cysts at about 5% to 22% and that of periapical granulomas at 50% to 85%. Admittedly, this debate might be considered irrelevant, because the eventual treatment of the two lesions is the same. Nonetheless, it has been found that postoperative bone regeneration is diminished in cases involving cysts, compared to granulomas, and that a poorer prognosis is related to larger lesions, which may be a factor in the long-term outcome of endodontic therapy.
Periapical cysts or granulomas are clearly common lesions seen by oral and maxillofacial surgeons. A careful history, the clinical and radiographic presentation and, most important, assessment of the vitality of the associated tooth aid in determining the appropriate diagnosis and management of this common pathologic lesion.
Carrillo, C, Penarrocha, M, Bagan, JV, et al. Relationship between histological diagnosis and evolution of 70 periapical lesions at 12 months, treated by periapical surgery. J Oral Maxillofac Surg. 2008; 66:1606–1609.
Gallego Romero, D, Torres Lagares, D, Garcia Calderon, M, et al. Differential diagnosis and therapeutic approach to periapical cysts in daily dental practice. Med Oral. 2002; 7:54–58.
Mass, DR, Bhambhani, SM. A clinical and histopathological study of radicular cysts associated with primary molars. J Oral Pathol Med. 1995; 24:458–461.
Nair, PN. New perspectives on radicular cysts: do they heal? Int Endod J. 1998; 31:155–160.
Omoregie, OF, Saheeb, BDO, Odykoya, O, et al. A clinicopathologic correlation in the diagnosis of periradicular lesions of extracted teeth. J Oral Maxillofac Surg. 2009; 67:1387–1391.
Philipsen, HP, Reichart, PA, Ogawa, I, et al. The inflammatory paradental cyst: a critical review of 342 cases from the literature survey, including 17 new cases from the author's file. J Oral Pathol Med. 2004; 33:147–155.
Penarrocha, M, Carrillo, C, Penarrocha, M, et al. Symptoms before periapical surgery related to histologic diagnosis and postoperative healing at 12 months for 178 periapical lesions. J Oral Maxillofac Surg. 2011; 69:e31–e37.
Ramachandran Nair, PN, Pajorola, G, Schroeder, HE. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 81:93–102.
Regezi, JA, Sciubba, JJ, Jordan, RCK. Oral pathology: clinical pathologic correlations, ed 6. St Louis: Saunders; 2012.
Sanchis, JM, Penarrocha, M, Bagan, JV, et al. Incidence of radicular cysts in a series of 125 chronic periapical lesions: histopathologic study. Rev Stomatol Chir Maxillofac. 1998; 98:354–358.
Spatafore, CM, Griffin, JA, Jr., Keyes, GG, et al. Periapical biopsy: an analysis of 10-year period. J Endod. 1990; 16:239–241.
Stockdale, CR, Chandler, NP. The nature of the periapical lesion: a review of 1,108 cases. J Dent. 1988; 16:123–129.
Trope, M, Pettigrew, J, Petras, J, et al. Differentiation of radicular cyst and granulomas using computerized tomography. Endod Dent Traumatol. 1989; 5:69–72.
Zaiv, RB, Roswati, N, Ismail, K. Radiographic features of periapical cysts and granulomas. Singapore Dent J. 1989; 14:29–32.
Mixed Radiolucent-Radiopaque Lesion (Ossifying Fibroma)
CC
A 32-year-old woman is referred for evaluation of a mandibular lesion. She states, “I have a tumor in my lower jaw.”
HPI
Approximately 3 years earlier, the patient noticed a painless bony expansion of her anterior mandible (ossifying fibroma is much more common in the mandible than in the maxilla and rarely presents with pain or paresthesia). The patient states that the swelling has been slowly enlarging over the past 3 years and that she was previously scared to seek treatment. Now that the mass is so large and disfiguring, she wants treatment (ossifying fibromas that are left untreated can become very large). She complains of difficulty talking and chewing because of the size of the mass.
Examination
General. The patient is well developed and well nourished and in no apparent distress.
Maxillofacial. There is an obvious enlargement of the anterior mandible. A firm, bony mass is palpable that extends from first premolar to first premolar. The mass has expanded the buccal and inferior cortices (“downward bowing” is common in large ossifying fibromas of the mandible). Sensory examination shows that the mental nerve distributions are intact bilaterally (perineural invasion is not seen with ossifying fibroma).
Neck. No lymphadenopathy is noted (cervical lymphadenopathy is not seen in benign neoplastic processes).
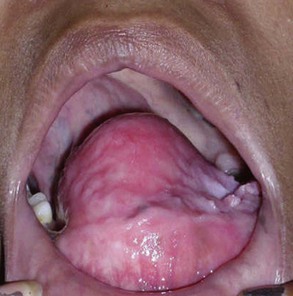
Intraoral. There is a significant amount of bony expansion of the buccal and lingual cortices in the anterior mandible, displacing the tongue posteriorly (Figure 1-6). The left and right mandibular lateral and central incisors are displaced and mobile (larger lesions may cause tooth displacement and root divergence, resorption, or both). The overlying attached gingiva and mucosa are normal in appearance (mucosal ulcerations can be a sign of a malignant process; however, traumatic ulcerations can occur within large, expansible, benign lesions).
Imaging
A panoramic radiograph is the initial screening study of choice. It provides an excellent overview of the bony anatomy and architecture of the maxilla and mandible. Osseous lesions are well characterized on a panoramic film, allowing the clinician to make a working differential diagnosis based on the lesion's location, radiodensity, locular or trabecular pattern, border demarcation, size, and effect on adjacent structures (i.e., root resorption, root divergence, scalloping, cortical expansion, cortical erosion, or destruction).
CT is not always essential during the work-up or treatment of a mixed radiopaque-radiolucent lesion that is suspected to be benign. However, when there are radiographic signs of a malignant process (e.g., poorly defined radiolucency, mottled or “moth-eaten” appearance, unilateral widening of the periodontal ligament space, floating teeth, cortical perforation, or “spiked roots”), CT of the mandible and neck is required. CT provides additional information (e.g., lingual or buccal cortex thinning or perforation, location of the inferior alveolar canal) and is especially useful when the lesion is difficult to assess on plain films. A three-dimensional stereolithographic model is useful to prebend a reconstruction plate in anticipation of resection.
The radiographic appearance of ossifying fibroma varies, depending on the degree of maturity of the lesion. Early ossifying fibromas are radiolucent, and as they mature, they become mixed radiolucent-radiopaque and may eventually become predominantly radiopaque. Untreated, these tumors are likely to reach large proportions as they continue to grow. Expansion of the lesion is symmetric and has a spherical or egg-shaped appearance on the CT scan.
In this patient, the panoramic radiograph reveals a well-defined, expansile, mixed radiolucent-radiopaque mass in the anterior mandible extending from the right premolar to the left molar regions (Figure 1-7). The borders are smooth and well defined. The anterior mandibular teeth show significant displacement and root resorption. A CT scan reveals a large, circular, expansile mass, in the anterior mandible, of a heterogeneous nature with radiopaque areas (of the same density as bone) within the lesion. There are no air- or fluid-filled spaces noted. The mass is well demarcated from surrounding structures. The stereolithographic model reconstructed from the CT scan illustrates the size of the lesion (Figure 1-8).
Labs
Baseline hemoglobin and hematocrit levels should be obtained before tumor resection. No other laboratory tests are indicated unless dictated by the medical history.
Differential Diagnosis
The differential diagnosis of a mixed radiolucent-radiopaque mass includes odontogenic cyst, odontogenic tumor, osteogenic tumor, or a process secondary to infection. Many osseous lesions can change in radiographic characteristics and radiodensity as they mature and progress from a radiolucent to a mixed or radiopaque lesion. The differential diagnosis of a mixed radiolucent-radiopaque lesion can be narrowed when radiographic findings are correlated with clinical findings. Several mixed radiolucent-radiopaque lesions other than an ossifying fibroma are listed in Box 1-5.
Biopsy
Benign tumors of bone cannot be distinguished on clinical and radiographic information alone and require histological assessment for a definitive diagnosis. In this case, an incisional biopsy would be indicated to guide final therapy. This can be done under local, intravenous sedation or general anesthesia, depending on the surgeon's preference and medical indications. In general, an ossifying fibroma is well demarcated from the surrounding bone but is not encapsulated. During biopsy, it is imperative to preserve the cortical-lesional relationship.
Assessment
A large, expansile, mixed radiopaque-radiolucent lesion of the mandible.
In this case, the histopathological examination showed a predominance of proliferative fibrous connective tissue with no clear capsule (ossifying fibroma can be seen with a capsule). The stroma appears avascular with some regularly shaped blood vessels (ossifying fibroma can have a mixed vascularity). A mild degree of osteoblastic activity and trabeculae of lamellar bone are also noted. Cementum-like deposits are present in combination with the lamellar bone. Radiographic, clinical, history, and histopathologic findings are consistent with an ossifying fibroma.
Treatment
Treatment varies, depending on the size and clinical appearance of the lesion. Small tumors may be treated with enucleation and curettage with 1- to 2-mm margins, as long as they have not lost their encapsulation and the margins remain well demarcated from the surrounding bone. However, resection with 5-mm margins is recommended if:
• Tumors have reached a larger size.
• There is loss of the encapsulation (radiographically or clinically).
• Tumor is within 1 cm of the inferior border of the mandible.
• There is involvement of the maxillary sinus or nasal cavities.
If enucleation and curettage is used, the defect can be left open to heal by secondary intent or closed primarily, using resorbable packs to eliminate the dead space. Packing the defect with materials such as iodoform gauze or various bone regeneration preparations to expedite bone regeneration has not been shown to be effective.
When bony reconstruction is required, various techniques (immediate or secondary cancellous marrow bone graft or immediate vascularized free flap) can be used, depending on the clinical situation. When cancellous marrow graft is used, secondary bony reconstruction is recommended at least 3 months after the resection to allow for sufficient mucosal healing and tensile strength to prevent mucosal perforation and graft contamination.
In this patient, a partial mandibulectomy with 5-mm margins was performed, with immediate stabilization using a prebent reconstruction plate. Immediate bony reconstruction (cancellous–marrow bone graft) would not be recommended in this case, because the resection involved a dentate segment that would allow oral contaminates to penetrate the graft site. Second-stage bony reconstruction was performed after 3 months of adequate soft tissue healing. Although recurrence is unlikely, the patient should be followed for at least 10 years with serial panoramic radiographs because of the slow-growing nature of this lesion.
Complications
With proper treatment, the prognosis for ossifying fibroma is excellent and recurrence is rare. The potential complications generally reflect the presenting size of the lesion. Smaller lesions often can be treated by enucleation and curettage without complications. Larger lesions requiring resection and reconstruction have the potential for more complex complications (e.g., wound dehiscence, wound infection, hardware failure, graft failure, facial or trigeminal nerve injury, cosmetic deformity). In particular, reconstruction of a continuity defect of the anterior mandible is challenging.
Discussion
Ossifying fibroma (OF) is a slow-growing, benign lesion of bone most commonly associated with the jaws. There have been occurrences in other bones, most commonly the tibia. Many names have been used to describe this lesion (osteofibroma, fibro-osteoma, cementifying fibroma) secondary to the tumor's cell of origin. Because it is not possible to determine whether the cell of origin is cementum or bone (both are derived from mesenchymal stem cells), it has become an irrelevant point. Therefore, the name ossifying fibroma is used. A variety of chromosomal abnormalities have been described in the small number of OF tumors submitted for cytogenetic analysis.
Multiple studies have shown that OF has a predilection for females (70%) between the second and fourth decades of life. About 58% occur in Caucasians; 23% in African Americans; and 12% in Hispanic individuals. Ossifying fibromas occur predominantly in the mandible (77%), with a greater propensity for the molar regions, followed by the premolar regions.
Juvenile ossifying fibroma is a variant of the ossifying fibroma. It is an uncommon lesion of juveniles and adolescents. It is typically more aggressive than an ossifying fibroma and usually affects the paranasal sinuses and bones around the orbit. It is further subdivided into two histopathologic variants: a psammamatous type and a trabecular type. The psammamatous type mainly involves the bones of the orbit and paranasal sinuses, whereas the trabecular type commonly involves the jaws, although there is controversy as to which type has a greater predilection for the maxilla versus the mandible. The psammamatous type occurs in an older and wider age range, compared to the trabecular type, which occurs in children and adolescents.
Frequent clinical features of juvenile ossifying fibroma include proptosis or exophthalmos and nasal symptoms. The lesion may require more aggressive treatment, with larger margins than are needed for the adult counterpart. However, some studies have indicated that smaller lesions may be treated by enucleation and curettage.
Histopathologically, the lesion may contain irregularly mineralized, cellular osteoid strands lined by plump osteoblasts. Juvenile ossifying fibromas have a reported recurrence rate between 38% and 50%.
Abrams, AM, Melrose, RJ. Fibro-osseous lesion. J Oral Pathol. 1975; 4:158–165.
Barnes L, Eveson JW, Reichart P, et al, eds. Pathology and genetics: head and neck tumors (IARC World Health Organization classification of tumors). IARC Press: Lyon, France, 2005:319–322.
Brannon, RB, Fowler, CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol. 2001; 8:126–143.
Eversole, LR. Craniofacial fibrous dysplasia and ossifying fibroma. Oral Maxillofac Surg Clin North Am. 1997; 9:625–642.
Eversole, LR, Leider, AS, Nelson, K. Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral Surg Oral Med Oral Pathol. 1985; 60:505–511.
Eversole, LR, Merrell, PW, Strub, D. Radiographic characteristics of central ossifying fibroma. Oral Surg Oral Med Oral Pathol. 1985; 59:522–527.
Eversole, LR, Sabes, WR, Rovin, S. Fibrous dysplasia: a nosologic problem in the diagnosis of fibro-osseous lesions of the jaws. J Oral Pathol. 1972; 1:189–220.
Fechner, RE. Problematic lesions of the craniofacial bones. Am J Surg Pathol. 1989; 13(Suppl 1):17–30.
Kramer IRH, Pindborg JJ, Shear M, eds. WHO international histological classification of tumors. ed 2. WHO, Geneva, 1992:27–29.
Marx, RE, Stern, D. Oral and maxillofacial pathology: a rationale for diagnosis and treatment. Chicago: Quintessence; 2003.
Parham, DM, Bridge, JA, Lukacs, JL, et al. Cytogenetic distinction among benign fibro-osseous lesions of bone in children and adolescents: value of karyotypic findings in differential diagnosis. Pediatr Dev Pathol. 2004; 7:148.
Said, AL, Surwillo, E. Florid osseous dysplasia of the mandible: report of a case. Compendium. 1999; 20:1017–1030.
Sciubba, JJ, Younai, F. Ossifying fibroma of the mandible and maxilla: review of 18 cases. J Oral Pathol Med. 1989; 18:315–321.
Sissons, HA, Steiner, GC, Dorfman, HD. Calcified spherules in fibro-osseous lesions of bone. Arch Pathol Lab Med. 1993; 117:284–290.
Su, L, Weathers, D, Waldron, CA. Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifying fibromas. II. A clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 84:540–549.
Su, L, Weathers, SD, Waldron, CA. Distinguishing features of focal cemento-osseous dysplasias and cemento-ossifying fibromas. I. A pathologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 84:301–309.
Summerlin, D, Tomich, C. Focal cemento-osseous dysplasia: a clinicopathologic study of 221 cases. Oral Surg Oral Med Oral Pathol. 1994; 78:611–620.
Waldron, C. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1993; 51:828–835.
Waldron, C, Giansanti, J. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. II. Benign fibro-osseous lesions of periodontal ligament origin. Oral Surg Oral Med Oral Pathol. 1973; 35:340–350.
Wenig, B. Atlas of head and neck pathology. Philadelphia: Saunders; 1993.
Williams, HK, Mangham, C, Speight, PM. Juvenile ossifying fibroma: an analysis of eight cases and a comparison with other fibro-osseous lesions. J Oral Pathol Med. 2000; 29:13.
Cone-Beam Computed Tomography (CBCT)
HPI
The patient's parent states that she had her primary cuspids removed at a younger age, with subsequent failure of her canines to erupt. Her orthodontist has recommended exposure and bracketing of the impacted canines to facilitate correct eruption and to avoid possible root resorption of her adjacent lateral incisors. The patient is otherwise asymptomatic.
Examination
General. The patient is well developed, well nourished, and in no apparent distress.
Intraoral. Teeth #6 and #11 are not visible in the mouth. There is a questionable bulge of the maxillary anterior palate on the left and right sides; however, definitive localization of the impacted canines by palpation is not readily apparent.
Maxillary canines are the second most frequently impacted teeth, after the third molars. The prevalence is 1% to 3% of the population. Approximately 60% to 85% of these impactions are palatal. Impacted canines are seen more commonly in females.
Imaging
Panoramic imaging revealed that teeth #6 and #11 were impacted and in close association with the adjacent lateral incisors (Figure 1-9). The third molars, also present, were full bone impactions. Given these findings, the risks and benefits of additional imaging were discussed with the patient's parents, who agreed to a CBCT scan to evaluate the maxillary canines (Figure 1-10).
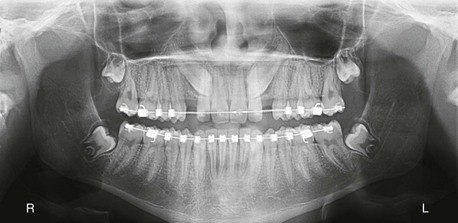
Figure 1-9 Preoperative panoramic view showing impacted teeth #6 and #11. Note the close association with the adjacent lateral incisors.
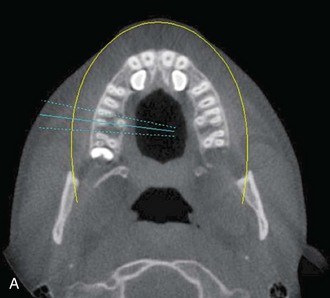
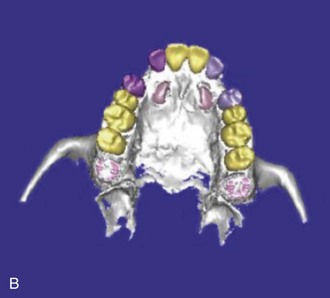
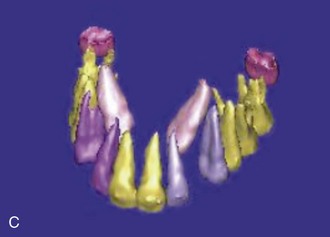
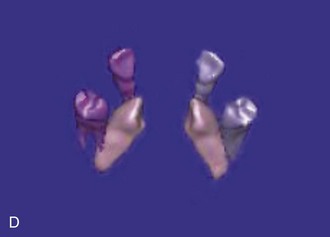
Figure 1-10 Axial view (A) and CBCT reconstruction (B to D) with different-colored masks assigned to the different anatomic structures in the FOV. Segmentation of the adjacent anatomy allows a better appreciation of the region of interest.
In approximately 38% to 67% of cases, impacted canines can cause varying degrees of resorption of adjacent teeth, especially the lateral incisor. Root resorption can be difficult to diagnose with traditional two-dimensional (2D) radiography, especially when the canine is in a direct palatal or facial position to the lateral incisor roots.
Two-dimensional imaging for surgical or orthodontic planning has several limitations, such as image magnification and distortion, superimposition of structures, and misinterpretation. Three-dimensional (3D) imaging allows the surgeon to determine the best clinical approach and reduces the invasiveness of surgery. Additionally, it allows the orthodontist to determine what orthodontic force vector should be applied to move the canine efficiently, thus reducing involvement of adjacent teeth.
CBCT offers a 3D view that can provide more accurate information about the size, shape, angulation, associated pathology (cysts, tumors, resorption of adjacent teeth), and relationship to adjacent structures (inferior alveolar nerve [IAN] canal, sinus). CBCT software allows anatomic entities in the 3D image to be differentiated by assigning each a color (known as a mask). The masks can be turned off, allowing the clinician a better appreciation of the anatomy. This type of reconstruction can be time-consuming, but it can be referred to third-party companies.
Assessment
Impacted maxillary canines needing surgically assisted exposure and bracketing for orthodontic correction.
Treatment
The precise location of the maxillary canines was determined. No readily apparent resorption of the lateral incisors was noted. (It is possible to underestimate root resorption, owing to inadequate visualization secondary to the limitations of CBCT, such as selecting a large field of view [FOV], which diminishes the resolution of the image.) The 3D reconstruction served two important purposes. It allowed the surgeon to easily appreciate the anatomy, and it also provided a visual aid that enabled the patient to easily understand the anatomic configuration of her problem; this, in turn, facilitated discussion of the procedure and its risks and benefits with the patient and her parents. Subsequently, the impacted canines were exposed and bracketed without incident in a standard fashion. (See Surgical Exposure of an Impacted Maxillary Canine in Chapter 5, Dentoalveolar Surgery.)
Complications
Clinicians must abide by the “as low as reasonably achievable” (ALARA) principle when ordering an imaging modality for a patient. Exposing the patient to the radiation must provide an image with a diagnostic value that is greater than the detriment the radiation exposure may cause. Not every patient requires CBCT, because the technique does expose the patient to radiation and results in increased cost. The American Dental Association (ADA) Council on Scientific Affairs suggests that CBCT use should be based on professional judgment, and clinicians must optimize technical factors, such as using the smallest FOV possible for diagnostic purposes and using appropriate personal protective shielding.
The radiation dose per U.S. resident has been increasing over the past 30 years. Ionizing radiation is also found in the natural environment in the form of cosmic rays or radon. At doses used in diagnostic and interventional procedures, ionizing radiation may cause DNA damage and increase the risk for future cancer. The probability of effects arising from ionizing radiation (e.g., future cancer, cataracts) is a function of the total radiation dose, although the severity of such effects is also influenced by other factors, such as genetics. The linear no threshold model (LNT) is the most widely used theoretical dose-response model that assumes that any exposure to ionizing radiation can induce future cancer. (Some studies suggest that low doses may not have a detrimental effect.)
The concentration of ionizing radiation in a specific volume of air is a measure of radiation exposure and is expressed in roentgens (R). The amount of radiation absorbed by a specific tissue is measured in grays (Gy) or rads. The effective dose is measured in sieverts (Sv) (1 Sv = 1,000 mSv = 1,000,000 µSv), which provides a quantification of the potential radiobiologic detriment caused by radiation. Calculating the effective dose allows for comparison across different imaging modalities. The International Commission on Radiological Protection (IRCP) estimates a 4% to 5% increased relative risk of fatal cancer after an average person receives a whole-body radiation dose of 1 Sv. Some models predict that 1 in 1,000 persons exposed to 10 mSv (10,000 µSv) will develop cancer as a result of that single exposure.
For comparison purposes, consider the following estimated effective doses:
• Single chest x-ray: 0.02 mSv
• Transcontinental flight: 0.02 mSv
• Annual individual radiation dose from the natural background: 3 mSv
Table 1-1 shows the effective dose estimates for common dental radiographic examinations.
Table 1-1
Effective Dose Estimates for Common Dental Radiographic Examinations and Cone-Beam Computed Tomography (CBCT) Imaging*

*Estimates are for adult patients based on data from Paulwels and colleagues, the SEDENTEXCT Project, and Ludlow and colleagues.
†Measured in microsieverts.
‡Median values for effective dose provided parenthetically.
From the American Dental Association Council on Scientific Affairs: The use of cone-beam computed tomography in dentistry, JADA 143(8):899-902, 2012.
The radiation risk with many newer CBCT machines is lower than that for the most common intraoral full mouth series; therefore, it may be possible, when indicated, to use CBCT with select intraoral images as an option for dental treatment planning in the future.
Currently the results for the use of CBCT for caries detection are mixed. CBCT for this purpose is limited to nonrestored teeth. Because of beam hardening, CBCT for caries detection has a high sensitivity. For periodontics, CBCT promises to be superior to 2D imaging for the visualization of bone topography and lesion architecture (intrabony defects and furcations), but no more accurate than 2D imaging for bone height. Restorations within the dentition can obscure views of the alveolar crest.
CBCT for endodontic purposes is the most promising modality. It has been shown to be useful for detecting apical lesions and root fractures, canal identification, characterizing internal and external resorption, and facilitating apical surgery. In orthodontics, CBCT can be used to evaluate the shape and function of the maxillofacial complex, and it provides a powerful tool for the visualization of root angulations.
Adjacent anatomy outside the region of interest is usually captured with CBCT. Given the volume of tissue that is exposed and readily available for review, there is a moral, ethical, and legal responsibility attached to the interpretation of the volumetric data set. Because of the complexity of the anatomy of the maxillofacial area, review of the images by an appropriately trained radiologist is prudent.
The most common algorithm for reconstructing 3D objects (cone-beam reconstruction) from cone-beam projections is the Feldkemp, Davis, and Kress (FDK) method, which is used by many research groups and commercial vendors. This algorithm has some limitations, such as distortion in the noncentral transverse plane, resolution degradation in the longitudinal direction, and a high computational time required to perform reconstructions. To address these problems, other algorithm and cone-beam geometries are being developed that will likely be incorporated into future machines.
A Web site that provides comparative information on the currently available CBCT machines can be found at www.conebeam.com/cbctchart.
Discussion
Dedicated CBCT of the maxillofacial region has created a revolution in all fields of dentistry and has expanded the role of imaging from diagnosis to image guidance for many surgical procedures. CBCT has eliminated some of the inherent limitations of 2D images, such as magnification, distortion, superimposition, and misrepresentation.
CBCT uses a cone-beam–shaped source of ionizing radiation, and the beam is directed through the middle of the area of interest (field of view, or FOV). The beam covers the entire FOV; therefore, only one rotation of the gantry is required. Traditional medical CT uses a fan-shaped beam to acquire individual image slices (each slice requires a separate scan), which are then stacked to obtain a 3D representation. CBCT usually results in a lower dose of radiation than CT, but doses vary widely among different systems and among different imaging protocols (slice thickness, FOV, mAs, kVp, scan time). It is recommended that clinicians use appropriate selection criteria, along with imaging protocols that use the minimal doses that ensure acceptable diagnostic qualities (Table 1-2).
CBCT image production requires four components.
a. X-ray generation: A pulsed or constant beam of radiation can be used. This is one of the reasons for variation in cone-beam dosimetry between different units.
b. Field of view: This depends on the detector size and shape, beam projection geometry, and the ability to collimate the beam. CBCT can be categorized by the available FOV, which usually ranges from 4 to 30 cm. The larger the FOV, the poorer the resolution.
c. Scan factors: During the scan, single exposures (known as basis, frame, or raw images) are made. (These are similar to the lateral view of PA cephalometric images.) The complete series is known as projection data. The number of images comprising a projection data set is determined by the frame rate (i.e., the number of images acquired per second; a faster frame rate results in better image quality, but it also exposes the patient to more radiation); the speed of rotation; and the completeness of the trajectory arc. Most CBCT machines scan for a full 360 degrees to acquire projection data. However, some machines limit the scanning arc, thus reducing the time, radiation dose, and mechanical components required. The disadvantages of this approach are greater noise and a higher possibility of artifacts.
Current CBCT machines can be divided into two groups based on the detector type: image intensifier tube/charge-coupled device (IIT/CCD) or flat panel imager. The flat panel imager is thought to create less distortion and have fewer artifacts. Flat panel performance limitations are most noticeable at lower and higher exposures.
The projection data must be reconstructed to create a usable volumetric data set. This is computationally complex and can involve two computers (an acquisition computer and a processing [workstation] computer). This phase is divided into two stages: the acquisition stage (usually 160 to 600 basis images are collected) and the reconstruction stage (in which algorithms such as the FDK algorithm are used to recombine the data for visualization).
CBCT is most widely used for implant planning. Fusion of the CBCT scan data with three-dimensional clinical data (e.g., CBCT of a plaster cast, optical scan of the cast, or optical scan of the oral cavity) facilitates the fabrication of surgical guides (with fiducial markers or, more recently, with corresponding anatomic points). Fusion is necessary to obtain an accurate guide because of the presence of scatter artifact; fusion also allows more accurate visualization of the gingival level.
If CBCT is used for implant planning, the radiographic examination of a potential implant site can include cross-sectional imaging:
• CBCT should be considered as the imaging modality of choice for the preoperative cross-sectional imaging of potential implant sites.
• CBCT is also considered when the initial exam indicates the need for site development (e.g., block grafting, sinus lifting).
• CBCT should be considered for evaluating the results of site development (if bone augmentation procedures were performed before implant placement). In the absence of clinical signs or symptoms, periapical radiographs are appropriate for postoperative assessment.
• CBCT is indicated in the immediate postoperative period if there is altered sensation or implant mobility. CBCT is not indicated for periodic review of asymptomatic implants.
• CBCT can be considered if implant retrieval is anticipated.
In summary, the use of CBCT should be judged based on sound clinical and radiographic parameters. It can provide very valuable information for diagnosis and treatment planning. However, overzealous use of CBCT can result in unnecessary radiation exposure and added cost.
American Dental Association Council on Scientific Affairs. The use of cone-beam computed tomography in dentistry. JADA. 2012; 143(8):899–902.
Bouwens, D, Cevidanes, L, Ludlow, J, et al. Comparison of mesiodistal root angulation with posttreatment panoramic radiographs and cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2011; 139:126–132.
Friedland, B, Donoff, B, Chenin, D. Virtual technologies in dentoalveolar evaluation and surgery. Atlas Oral Maxillofacial Surg Clin North Am. 2012; 20:37–52.
Kim, JW, Cha, IH, Kim, SJ, et al. Which risk factors are associated with neurosensory deficits of inferior alveolar nerve after mandibular third molar extraction? J Oral Maxillofac Surg. 2012; 70(11):2508–2514.
Lee, C, Elmore, J. Radiation related risks of imaging studies. www. uptodate. com, 2013.
Ludlow, JB, Davies-Ludlow, LE, White, SC. Patient risk related to common dental radiographic examinations: the impact of 2007 International Commission on Radiological Protection recommendations regarding dose calculation. JADA. 2008; 139(9):1237.
Meara, DJ. Evaluation of third molars: clinical examination and imaging techniques. Atlas Oral Maxillofacial Surg Clin North Am. 2012; 20:163–168.
Oberoi, S, Knueppel, S. Three-dimensional assessment of impacted canines and root resorption using cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012; 113(2):260.
Okano, T, Sur, J. Radiation dose and protection in dentistry. Jpn Dent Sci Rev. 2010; 6:112–121.
Palomo, L, Palomo, JM. Cone beam CT for diagnosis and treatment planning in trauma cases. Dent Clin North Am. 2009; 53:717–727.
Pauwels, R, Beinsberger, J, Collaert, B, et al. Effective dose range for dental cone beam computed tomography scanners. Eur J Radiol. 2012; 81(2):267–272.
Scarfe, W, Farman, A. What is cone-beam CT and how does it work? Dent Clin North Am. 2008; 52:707–730.
The SEDENTEXCT Project. Radiation Protection: Cone Beam CT for Dental and Maxillofacial Radiology: Evidence Based Guidelines 2011 (v2. 0 Final). www. sedentexct. eu/files/guidelines_final. pdf.
Tyndall, D, Rathore, S. Cone-beam CT diagnostic applications: caries, periodontal bone assessment and endodontic applications. Dent Clin North Am. 2008; 52:825–841.
Tyndall, D, Price, JB, Tetradis, S, et al. Position statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:817–826.
White, S, Pharoah, MJ. The evolution and application of dental maxillofacial imaging modalities. Dent Clin North Am. 2008; 52:689–705.