Dentoalveolar Surgery
Dentoalveolar surgery is the surgical procedure that oral and maxillofacial surgeons perform most often. These procedures are associated with the dentate segment of the maxilla or mandible, termed the alveolar ridge. They include a variety of procedures, including simple tooth extractions, alveoplasty (recontouring of the alveolar bone), removal of tori, exposure of impacted teeth for orthodontic treatment, and extraction of impacted third molars. The origins and the current practice of oral and maxillofacial surgery are heavily based on dentoalveolar surgery. Such procedures account for more than 50% of the practice of oral and maxillofacial surgeons worldwide. Recently, placement of dental implants was added to the rehabilitative and reconstructive options for the maxillofacial region, replacing the more traditional preprosthetic procedures.
Since its establishment in 1918, the American Association of Oral and Maxillofacial Surgeons (AAOMS) has gone through extensive change, reflecting progressive changes in the specialty. Initially called the American Society of Exodontists, it was originally formed by a group of oral surgeons in Chicago after the National Dental Association meeting. As it has grown, the association has gone through several name changes, each reflecting the expansion of the specialty. Despite the wide scope of training of graduating oral and maxillofacial surgeons, as evidenced by the sections in this book, dentoalveolar surgery remains the foundation of our specialty.
In this chapter we present teaching cases representing some of the important aspects of this branch of oral and maxillofacial surgery. Three cases focus on complications of dentoalveolar surgery (dry socket, lingual nerve injury, and displacement of a tooth fragment during surgery), and two discuss the current issues in the treatment of impacted canines and third molars.
Third Molar Odontectomy
HPI
The patient recently completed his orthodontic therapy. For the past few weeks, he had experienced increasing discomfort in the posterior mandible. He was subsequently referred by his orthodontist for evaluation. He denies any fever, swelling, or drainage from the area.
PMHX/PDHX/Medications/Allergies/SH/FH
Noncontributory. Thorough past medical and dental histories are important to determine any potential concerns with general health, fitness for anesthesia, and possible anesthetic or surgical morbidities. The patient does not report any symptoms suggestive of temporomandibular joint dysfunction (TMD) and does not take any medications. Certain medications may promote bleeding risk (e.g., aspirin, warfarin, and clopidogrel) or delay or impede healing (e.g., steroids or bisphosphonates). The patient smokes approximately one pack of cigarettes per day (risk factor for the development of dry sockets). Al-Belasy has reported that the incidence of dry sockets to be reduced with smoking cessation.
Examination
General. The patient is a well-developed and well-nourished man in no apparent distress (higher levels of anxiety may require a deeper level of sedation/anesthesia).
Maxillofacial. There is no soft tissue abnormality or lymphadenopathy (LAD). The patient has a good range of mandibular motion with an maximal interincisal opening (MIO) of 45 mm. Examination of the TMJ reveals no abnormalities (clicks or pain upon palpation). The muscles of mastication are nontender to palpation (important to detect preexisting symptoms of TMD).
Intraoral. Oral soft tissue is free of lesions, and there is no evidence of acute infection. The mandibular third molars are partially erupted, with approximately 20% of the crown visible in the oral cavity with insufficient room for functional eruption. The overlying operculum appears slightly inflamed, with evidence of food debris and periodontal pockets of greater than 6 mm on the distal of the left and right mandibular second molars. The right and left maxillary third molars are partially erupted. Oral hygiene is fair. An examination of the oropharynx is without tonsillar hypertrophy, and the patient has a Mallampati score of 1.
Indications for the removal of third molars include tooth malposition, periodontal conditions (e.g., probing lengths greater than 4 mm at distal of second molars or third molars), pericoronitis, symptoms of pain, evidence of infection or caries, orthodontic considerations, lack of space, associated pathology, and inability to maintain oral hygiene, especially when the third molars are incompletely erupted. Although some controversy exists as to the timing of or necessity for removal of asymptomatic third molars, there is evidence that younger patients (under 25 years old) have a decreased risk of complications and improved recovery after surgery. The decision to remove asymptomatic third molars should be driven by evidence-based decision making; the predominant guiding principle should be the patient's preference. The clinical findings should be the main driving factors in treatment decisions.
Imaging
A panoramic radiograph is the minimum imaging modality necessary for the evaluation and treatment of impacted third molars. Computed tomography (CT) scans are not necessary for the routine evaluation, but they may be used in select cases of suspected maxillofacial pathology or for accurate determination of the inferior alveolar nerve anatomy. Partial odontectomy (coronectomy) may at times be an alternative treatment in patients requiring removal of a third molar that is in close proximity to the inferior alveolar nerve; however, this procedure does not eliminate the risk of inferior alveolar nerve injury and possible future infectious complications due to retention of root fragments.
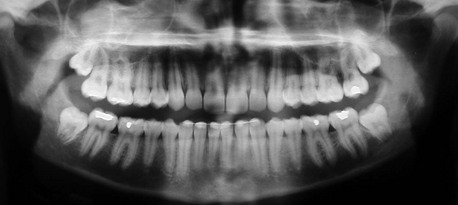
In the current patient, the panoramic radiograph reveals a partial lack of space to accommodate the eruption of the mildly mesioangularly impacted mandibular molars with 75% root development (Figure 5-1). The roots are not fused and do not extend below the level of the neurovascular bundle. The outlines of mandibular canals are easily discerned on the radiograph. There is no diversion of the inferior alveolar canal, darkening of the third molar root, or interruption of the cortical white line (risk factors associated with inferior alveolar nerve injury) (Box 5-1). The maxillary third molars are vertically positioned with partial bony impaction. The maxillary sinuses and the remainder of the radiograph are within normal limits.
Labs
No routine laboratory tests are indicated for the routine evaluation of impacted third molars unless dictated by underlying medical conditions.
Assessment
Partial bony impaction of the right and left maxillary and mandibular third molars with insufficient room for eruption; localized gingivitis and early periodontal pocketing noted around the left and right mandibular third molars.
Treatment
Two major professional organizations have made contradictory recommendations on the prophylactic removal of impacted third molars. The researchers for AAOMS Third Molar Clinical Trials published several scientific articles that linked third molars to future health problems in adults. In light of these findings, in 2005 the AAOMS suggested that removal of the third molars during young adulthood may be the most prudent option. In contrast, the National Health Service (NHS) of Great Britain and an associated agency, the National Institute of Clinical Excellence (NICE), published a series of guidelines recommending that “the practice of prophylactic removal of pathology-free impacted third molars should be discontinued in the NHS.” These guidelines, made public in 2000, did acknowledge the ongoing AAOMS Third Molar Clinical Trials. In 2012 Renton and colleagues published an article chronicling the United Kingdom's experience with retention of third molars. They concluded that “admissions for M3 [third molar] surgery activity under the NHS have decreased from the mid-1990s and into the 2000s, in association with professional and policy guidelines.” They found that the average age for third molar surgery had risen, and the indications for the surgery were “increasingly associated with other pathologic features such as dental caries or pericoronitis, in line with NICE guidelines.”
Although in some regions of the world, socioeconomic and available resources play a major role in the determination of guidelines for third molar extractions, current scientific evidence remains unchanged. The cumulative financial costs of treating the health complications of retained third molars in the older population should be considered. Although there is cost associated with the procedure to remove third molars, there is also the cost of monitoring retained third molars. Subsequent removal at an older age may also be associated with the cost of lost income in recuperation time, in addition to the greater risks of removal at an older age.
It is clear that the extraction of third molars poses some risks to the patient. However, the determination of extraction versus nonextraction of asymptomatic third molars must compare the cost and risks of surgical extraction with the lifetime health and cost benefits of preventing and eliminating any pathologic processes associated with retention of the third molars.
The effectiveness, safety, and relatively minimal cost of extraction of third molars using outpatient, office-based anesthesia, along with the currently available scientific evidence linking asymptomatic third molars to multiple health hazards, generally support the extraction of asymptomatic third molars in young adults; however, as mentioned, the patient's preference and an informed decision arrived at by the surgeon and patient are the most important deciding factors.
The current patient was seen in the clinic for extraction of his teeth under intravenous sedation. Monitors (pulse oximetry, capnography, blood pressure, and three-lead electrocardiography) were placed, and oxygen was delivered via a nasal mask at 4 L/min, followed by nitrous oxide. Midazolam and fentanyl were slowly titrated until a comfortable state of conscious sedation was achieved. A local anesthetic with epinephrine was injected, and adequate time was allowed for the local anesthetic block. A bite block was place for TMJ stabilization. An oral screen with loosely packed, moist gauze was placed to protect the airway from accidental aspiration. A full mucoperiosteal flap was elevated using a buccal envelope incision with a distal hockey-stick extension for the mandibular third molars. Special consideration was given to preventing trauma to the lingual tissue.
A buccal trough was made using a high-speed instrument (impaction drill and burr with irrigation), and the teeth were elevated and extracted. Careful attention must be given to avoiding violation of the lingual cortex (although at times, disrupting the lingual cortex is unavoidable). The neurovascular bundle was not visualized, and there was no excessive hemorrhage from the socket (visualization of the neurovascular bundle and excessive hemorrhage from the socket are associated with an increased risk of inferior alveolar nerve injury). The wound was irrigated with normal saline, and the flaps were closed with chromic suture, with careful attention paid to suturing only the superficial lingual mucosa and thus preventing lingual nerve injury.
The upper third molars were removed through an envelope mucoperiosteal flap. Care was taken to avoid the roots of the maxillary second molars (a possible complication). There was no evidence of an oral antral communication. The tooth follicles were removed, and the sites were irrigated. Gauze was placed between the teeth to promote hemostasis, and the patient was monitored in the recovery room until he was fully awake and alert.
Complications
As mentioned, third molar extraction is the surgical procedure that oral and maxillofacial surgeons perform most often. A well-planned surgical approach, with the goal of prevention, is the best way to minimize complications. Yet, despite our best efforts, complications are expected, and it is best to counsel patients preoperatively for potential risks. Clinicians need to be aware of the risk factors associated with an increased risk of complications for this commonly performed procedure.
Pogrel concluded, “The age of 25 years appears in many studies to be a critical time after which complications increase more rapidly.” No studies indicate that complications decrease as age increases. In fact, the older a patient is, the more likely it is that the recovery from complications will be prolonged, less predictable, and less complete.
Sensory nerve injury is well documented. Injury to the inferior alveolar nerve can lead to a range of symptoms along its distribution (anesthesia, hypoesthesia, dysesthesia, or paresthesia). A review of the literature demonstrates an incidence of nerve injury between 0.4% and 5%. In one large study with 367,170 patients, the incidence of nerve injury was 0.4% (22% of whom had symptoms lasting longer than 12 months). The risk of nerve injury is greater with increasing patient age, degree of root development, degree of impaction, and the radiographic relationship of the roots to the inferior alveolar canal. The incidence of injury to the inferior alveolar nerve is slightly higher than that for the lingual nerve, but the inferior alveolar nerve has a higher incidence of spontaneous recovery (due to its position in the bony canal, which allows a greater possibility that the nerve endings will reapproximate); however, older patients are more likely to have incomplete recovery. Injury to the long buccal nerve is also possible, but it is less of a concern, causing minimal to no subjective disability. Patients with severe inferior alveolar nerve or lingual nerve injury should be referred to a microneurosurgeon for prompt evaluation and potential surgical intervention (decompression, neurolysis, or neurorrhaphy). Complications from local anesthesia also have been reported, probably due to direct needle trauma to the inferior alveolar nerve. The reported incidence ranges from 1 in 400,000 to 1 in 750,000 patients.
Not unlike with any other procedure, infections are commonly associated with third molar removal, both preoperatively and postoperatively. This appears to be more common after removal of partial and full bony impactions. Infections can occur as early as several days after the procedure, or they may present late (within several weeks). They can be localized to the area of the third molar or occasionally can spread to adjacent fascial spaces to cause life-threatening conditions. Most infections are easily managed with local measures and the use of antibiotics. The incidence of postoperative infection is approximately 3%. Increasing evidence supports the use of antibiotic prophylaxis, which has been shown to decrease the risk of postoperative infection. However, the decision on whether to prescribe antibiotics is multifactorial.
Localized osteitis (dry socket) is a well-known complication of tooth extractions and is discussed in detail elsewhere (see Alveolar Osteitis [Dry Socket] later in this chapter). Other complications associated with third molar surgery include periodontal complications, maxillary sinus involvement (oral antral communications, displacement of a fragment into the sinus), displacement of a tooth into adjacent fascial spaces, breaking of instruments, aspiration or swallowing of foreign objects, TMJ pain, maxillary tuberosity fractures, root fracture, injury to adjacent teeth, hemorrhage/hematoma, wound dehiscence, mandible fracture, and soft tissue emphysema.
Discussion
Indications for the removal of third molars are variable and influenced by many factors. Insufficient room for adequate eruption of the teeth can create difficulty with maintenance of oral hygiene in these areas, affecting the adjacent soft tissues and teeth. The increased difficulty and risks of third molar removal with increasing age, inadequate oral hygiene, and tooth position, in addition to periodontal health and orthodontic considerations, should be taken into account. Erupted or partially erupted third molars have been shown to have a negative impact on periodontal health. In a study by Dodson, attachment levels and probing depths improved after third molar removal. Pogrel reported that a periodontal condition may persist or may be created on the distal aspect of the second molar after third molar removal, especially in some older patients. Dodson has suggested that in this subgroup of patients, immediate reconstruction may be beneficial in the long term. However, the relationship between third molars and periodontal disease pathogenesis requires further study. There is no clear consensus on the ability of mandibular third molars to cause crowding of the anterior teeth. Although some investigators have shown a statistical association of third molars and late anterior crowding, this association is not strong. The majority of the literature does not support this hypothesis.
Offenbacher and colleagues published a study on periodontal disease and the risk of preterm delivery. The study involved 1,020 pregnant women who received antepartum and postpartum periodontal examinations. The findings clearly demonstrated that maternal periodontal disease increases the relative risk of preterm or spontaneous preterm birth. The mothers with third molar periodontal pathology had elevated serum markers of systemic inflammation (C-reactive protein, isoprostanes). Periodontal disease was also a predictor of more severe adverse pregnancy outcomes.
For extraction of third molars, there is a wide range of choices of anesthetic and surgical techniques related to the surgeon's training and experience. As the common dictum proclaims, “There is more than one way to do it.” Many different surgical flaps and instruments have been developed over the years. A variation of the buccal hockey-stick incision appears to be the most commonly used and has the lowest incidence of permanent neurosensory injury. Similarly, the choice of anesthesia can vary from local anesthesia, to intravenous sedation using a variety of medications, to general anesthesia with endotracheal intubation. This choice is influenced by many factors, including the patient's preference, available resources, surgeon's training, and practice patterns in the region. Various regimens of perioperative care are also followed. Common practices include the use of a long-acting local anesthetic (e.g., 0.5% Marcaine), corticosteroids, and nonsteroidal antiinflammatory drugs (NSAIDs) to improve postoperative pain management.
The anatomy of the inferior alveolar nerve is variable, but the canal is usually located inferior and buccal to the impacted mandibular third molars. In the largest cadaveric study of lingual nerve anatomy, by Behnia and associates, 669 nerves from 430 fresh cadavers were examined. In 94 cases (14%), the nerve was above the lingual crest, and in one case the nerve was in the retromolar pad region. In the remaining 574 cases (86%), the mean horizontal and vertical distances of the nerve to the lingual plate and the lingual crest were 2.1 mm and 3 mm, respectively. In 149 cases (22%), the nerve was in direct contact with the lingual plate of the alveolar process. The unpredictable anatomy of the lingual nerve in relation to the mandibular third molar increases this nerve's susceptibility to injury.
Al-Belasy, FA. The relationship of “Shisha” (water pipe) smoking to postextraction dry socket. J Oral Maxillofac Surg. 2004; 62:10.
Alling, CC. Dysesthesia of the lingual and inferior alveolar nerves following third molar surgery. J Oral Maxillofac Surg. 1986; 44:454.
Alling, C, Helfrick, J, Alling, R. Impacted teeth. Philadelphia: Saunders; 1993.
American Association of Oral and Maxillofacial Surgeons: Research study links wisdom teeth to health problems in young adults, September 20, 2005 (news release).
Bagheri, SC, Khan, HA. Extraction versus non-extraction management of third molars. Oral Maxillofac Surg Clin North Am. 2007; 19(1):15–21.
Behnia, H, Kheradvar, A, Shahrokhi, M. An anatomic study of the lingual nerve in the third molar region. J Oral Maxillofac Surg. 2000; 58(6):649–651.
Blaeser, BF, August, MA, Donoff, RB, et al. Panoramic radiographic risk factors for inferior alveolar nerve injury after third molar extraction. J Oral Maxillofac Surg. 2003; 61(4):417–421.
Blakey, GH, Jacks, MT, Offenbacher, S, et al. Progression of periodontal disease in the second/third molar region in subjects with asymptomatic third molars. J Oral Maxillofac Surg. 2006; 64(2):189–193.
Blakey, GH, Marciani, RD, Haug, RH, et al. Periodontal pathology associated with asymptomatic third molars. J Oral Maxillofac Surg. 2002; 60(11):1227–1233.
Cilasun, U, et al. Coronectomy in patients with high risk of inferior alveolar nerve injury diagnosed by computed tomography. J Oral Maxillofac Surg. 2011; 69:1557–1561.
Dodson, TB. Management of mandibular third molar extraction sites to prevent periodontal defects. J Oral Maxillofac Surg. 2004; 62(10):1213–1224.
Dodson, TB. Management of asymptomatic wisdom teeth: an evidence-based approach. In: Bagheri SB, Bell RB, Khan HA, eds. Current therapy in oral and maxillofacial surgery. Philadelphia: Elsevier/Saunders; 2011:122–126.
Dodson, TB. Surveillance as a management strategy for retained third molars: Is it desirable? J Oral Maxillofac Surg. 2012; 70(9):S20–S24.
Elter, JR, Cuomo, CJ, Offenbacher, S, et al. Third molars associated with periodontal pathology in the Third National Health and Nutrition Examination Survey. J Oral Maxillofac Surg. 2004; 62(4):440–445.
Hall, HD, Bildman, BS, Hand, CD. Prevention of dry socket with local application of tetracycline. J Oral Surg. 1971; 29:35.
Herpy, AK, Goupil, MT. A monitoring and evaluation study of third molar surgery complications at a major medical center. Military Medicine. 1991; 156:1.
Kiesselbach, JE, Chamberlain, JG. Clinical and anatomic observations on the relationship of the lingual nerve to the mandibular third molar. J Oral Surg. 1984; 42:565.
Koumaras, GM. What costs are associated with the management of third molars? J Oral Maxillofac Surg. 2012; 70(9):S8–S10.
Long, H, Zhou, Y, Liao, L, et al. Coronectomy vs total removal for third molar extraction: a systematic review. J Dent Res. 2012; 91(7):659–665.
Nakamori, K, Fujiwara, K, et al. Clinical assessment of the relationship between the third molar and the inferior alveolar canal using panoramic images and computed tomography. J Oral Maxillofac Surg. 2008; 66:2308–2313.
Nakayama, K, Nonoyama, M, Takaki, Y, et al. Assessment of the relationship between impacted mandibular third molars and inferior alveolar nerve with dental 3-dimensional computed tomography. J Oral Maxillofac Surg. 2009; 67:2587–2591.
National Institute for Clinical Excellence. Guidance on the extraction of wisdom teeth. www. nice. org. uk/pdf/wisdomteethguidance. pdf, 2013.
Offenbacher, S, Boggess, KA, Murtha, AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006; 107(1):29–36.
Offenbacher, S, Beck, JD, Moss, KL, et al. What are the local and systemic implications of third molar retention? J Oral Maxillofac Surg. 2012; 70(9):S58–S65.
Osborn, TP, Frederickson, G, Small, IA, et al. A prospective study of complications related to mandibular third molar surgery. J Oral Maxillofac Surg. 1985; 43:767–771.
Patel, V, Gleeson, CF, Kwok, J, et al. Coronectomy practice. Paper 2. Complications and long term management. Br J Oral Maxillofac Surg. 2012; 50(8):739–744.
Piecuch, JF. What strategies are helpful in the operative management of third molars? J Oral Maxillofac Surg. 2012; 70(9):S25–S32.
Pogrel, MA. Complications of third molar surgery. In: Kaban LB, Pogrel MA, Perrott DH, eds. Complications of oral and maxillofacial surgery. Philadelphia: Saunders, 1997.
Pogrel, MA. What are the risks of operative intervention? J Oral Maxillofac Surg. 2012; 70(9):S33–S36.
Pogrel, MA. What is the effect of timing of removal on the incidence and severity of complications? J Oral Maxillofac Surg. 2012; 70(9):S37–S40.
Renton, T, et al. What has been the United Kingdom's experience with retention of third molars? J Oral Maxillofac Surg. 2012; 70(9):S48–S57.
Rood, JP, Shehab, BA. The radiological predictors of inferior alveolar nerve injury during third molar surgery. Br J Oral Maxillofac Surg. 1990; 28:20.
Sweet, JB, Butler, DP. Predisposing and operative factors: effect on the incidence of localized osteitis in mandibular third molar surgery. J Oral Surg. 1978; 46:206.
White, RP, Jr., Madianos, PN, Offenbacher, S, et al. Microbial complexes detected in the second/third molar region in patients with asymptomatic third molars. J Oral Maxillofac Surg. 2002; 60(11):1234–1240.
Alveolar Osteitis (Dry Socket)
CC
A 19-year-old woman presents to your clinic 5 days after removal of four impacted third molars. She complains of increasing pain that is difficult to control with prescription pain medications.
HPI
The patient underwent removal of four difficult full bony impactions. Postoperatively, she was given a prescription for an opioid/acetaminophen combination medication for pain control. On the fifth postoperative day, she describes a worsening, dull, aching pain that radiates to her left ear. She complains of a bad odor (halitosis) and taste in her mouth emanating from her lower jaw. She reports that she adhered closely to the postoperative instructions and that this pain is different from her immediate postoperative pain.
PMHX/PDHX/Medications/Allergies/SH/FH
The patient uses birth control pills and smokes one pack of cigarettes per day (both risk factors for the development of alveolar osteitis) (Box 5-2).
Examination
General. The patient appears to be in mild distress secondary to pain. The accompanying parent is very concerned.
Vital signs. Her vital signs are within normal limits (dry sockets do not cause fever).
Maxillofacial. There is bilateral edema of the lower face consistent with the patient's surgery. There are no cranial nerve deficits.
Intraoral. The extraction sockets of the maxillary third molars and the right mandibular third molar appear to be healing adequately with no evidence of exposed alveolar bone. The extraction socket of the left mandibular third molar shows evidence of exposed bone, food debris, and sensitivity upon irrigation. There is no purulence (alveolar osteitis is not an infection).
Imaging
A panoramic radiograph is not routinely indicated for the evaluation of alveolar osteitis; it is used if there is suspicion of retained bony or tooth fragments in the sockets. Nonresorbable dry socket packs must contain a radiopaque material to ensure removal of any packing material upon subsequent visits, which can be confirmed with radiographs.
The preoperative panoramic radiograph should be evaluated for the proximity of the inferior alveolar nerve before application of eugenol-based medicaments because of the possible neurotoxic effects of this medication.
Assessment
Alveolar osteitis (dry socket) of the extraction socket of the left mandibular third molar.
Treatment
The primary goal of treatment is relief of pain through this phase of delayed healing. Medicaments such as eugenol and lidocaine jelly have been advocated for packing the socket, although dry sockets resolve with symptomatic therapy alone. If the clinician chooses to use packs, they can be changed every day or every other day for approximately 3 to 6 days until the pain has resolved. It is imperative to avoid using eugenol-based packs if the alveolar nerve is suspected to be in close proximity, as mentioned, because of eugenol's neurotoxic properties. It is important to evaluate the preoperative panoramic radiograph and the operative note for evidence of close proximity to or exposure of the inferior alveolar nerve to the roots and/or sockets of the third molars. In such cases, alternatives such as in 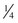 -inch gauze strips saturated with lidocaine jelly may be used. Once the pain has subsided, the packing should be removed to avoid the development of a foreign body reaction.
-inch gauze strips saturated with lidocaine jelly may be used. Once the pain has subsided, the packing should be removed to avoid the development of a foreign body reaction.
Previously it was thought that curettage of the socket could induce bleeding and therefore healing. This has not been shown to promote healing. Aggressive curettage of the socket is unnecessary and contraindicated. There is no indication for the use of antibiotics for treatment of dry sockets.
In the current patient, treatment involved gentle irrigation with warm saline (without curettage), placement of iodoform/eugenol-based packing into the socket, and prescription antiinflammatory medication. The patient had improvement of her pain within minutes, and she returned 48 hours later with a marked reduction of pain. The packing was removed, and the socket was gently irrigated. She was instructed to maintain good oral hygiene and rinse with warm saline until the socket no longer collected debris.
Complications
The vast majority of cases of alveolar osteitis heal without any intervention. Most medicaments placed in the socket (which usually contain eugenol) are palliative and may not necessarily expedite recovery. However, the clinician must identify chronic nonhealing extraction sockets, especially in patients at risk for the development of osteomyelitis (diabetes or chronic steroid use), osteoradionecrosis (history of radiation therapy to the surgical site), or bisphosphonate-induced osteonecrosis. Patients who do not respond to routine care of diagnosed alveolar osteitis must undergo further evaluation for other pathologic processes.
Discussion
The onset of alveolar osteitis is variable but commonly occurs 3 to 7 days after extraction. The etiology, prevention, and treatment of alveolar osteitis are up for debate. The prevailing theory of the pathogenesis of alveolar osteitis is that the initial clot is destroyed by fibrinolytic activity, impairing the production of granulation tissue that in turn promotes the formation of the initial fibrillar bone and ultimately mature bone. Bacteria may play an important but not clearly defined role. The incidence of alveolar osteitis is between 1% and 3% of all extraction sockets. The condition is most common with mandibular molars; the next most commonly involved teeth are the mandibular premolars, followed by the maxillary premolars, molars, canines, and incisors.
It is well known that smoking, oral contraceptive pills, a history of existing pericoronitis, traumatic extractions (correlating with the surgeon's experience), older age, and inadequate irrigation at the time of surgery are strongly associated with the development of alveolar osteitis. Based on several studies, preoperative rinsing with chlorhexidine and thorough intraoperative lavage with physiologic saline reduce the incidence of alveolar osteitis by up to 50%. The findings of studies on the efficacy of topical/intra-alveolar antibiotics and their benefits are the subject of debate. Recent studies have not demonstrated any benefit. Similarly, the use of systemic perioperative antibiotics has not been shown to be of any benefit. At present, the literature does not support the use of perioperative antibiotics in reducing the incidence of alveolar osteitis.
Contrary to current thinking, factors such as the use of a vasoconstrictive drug, seasonal allergies, bacterial load in the socket, flap design, and negative pressure dislodgement of the clot (use of a straw or spitting) have not been shown to increase the incidence of dry socket. As mentioned, there is evidence that the use of oral contraceptives is a risk factor, but female gender alone is not.
Alexander, RE. Dental extraction wound management: a case against medicating postextraction sockets. J Oral Maxillofac Surg. 2000; 58(5):538–551.
Betts, NJ, Makowski, G, Shen, YH, et al. Evaluation of topical viscous 2% lidocaine jelly as an adjunct during the management of alveolar osteitis. J Oral Maxillofac Surg. 1995; 53(10):1140–1144.
Caso, A, Hung, LK, Beirne, OR. Prevention of alveolar osteitis with chlorhexidine: a meta-analytic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 99(2):155–159.
Garcia, AG, Grana, PM, Sampedro, FG, et al. Does oral contraceptive use affect the incidence of complications after extraction of a mandibular third molar? Br Dent J. 2003; 194(8):453–455.
Halabi, D, Escobar, J, Munoz, C, et al. Logistic regression analysis of risk factors for the devel0pment of alveolar osteitis. J Oral Maxillofac Surg. 2012; 70:1040–1044.
Hermesch, CB, Hilton, TJ, Biesbrock, AR, et al. Perioperative use of 0. 12% chlorhexidine gluconate for the prevention of alveolar osteitis: efficacy and risk factor analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85(4):381–387.
Larsen, PE. Alveolar osteitis after surgical removal of impacted mandibular third molars: identification of the patient at risk. Oral Surg Oral Med Oral Pathol. 1992; 73(4):393–397.
Poeschl, PW, Eckel, D, Poeschl, E. Postoperative prophylactic antibiotic treatment in third molar surgery—a necessity? J Oral Maxillofac Surg. 2004; 61(1):3–8.
Rood, JP, Shehab, BA. The radiological prediction of inferior alveolar nerve injury during third molar surgery. Br J Oral Maxillofac Surg. 1990; 28(1):20–25.
Sanchis, JM, Sáez, U, Peñarrocha, M, et al. Tetracycline compound placement to prevent dry socket: a postoperative study of 200 impacted mandibular third molars. J Oral Maxillofac Surg. 2004; 62(5):587–591.
Surgical Exposure of an Impacted Maxillary Canine
CC
A 14-year-old boy is referred to your office by his orthodontist for exposure and bracketing of an impacted left maxillary canine (the canines normally erupt between 11 and 12 years of age). The maxillary canines are the second most commonly impacted teeth (the most common are the third molars).
HPI
The patient has a history of premature loss of the primary left maxillary canine secondary to trauma (premature loss of teeth with subsequent arch length reduction is one of the many causes of impaction). Orthodontic treatment has begun, and sufficient arch space has been accommodated for the guided eruption of the impacted canine. The patient has no history of any other impacted or congenitally missing teeth and presents with an otherwise full dentition.
Examination
General. The patient is a well-developed and well-nourished boy in no apparent distress.
Maxillofacial. He has a symmetrical facial appearance with no obvious skeletal abnormalities.
Intraoral. Orthodontic bands, brackets, and arch wires are in place. A well-healed edentulous space is present in the area of the left maxillary cuspid with an adequate alveolar ridge. A small, painless, palpable bony buccal protuberance can be noted in the area of the left maxillary cuspid, consistent with the crown of the impacted canine (clinical evaluation to determine palatal or buccal impaction is important and often sufficient to determine the approach for access to the tooth). The gingival and palatal tissues both appear healthy, with no notable periodontal defects.
Imaging
A panoramic radiograph is the initial screening study of choice for evaluating impacted teeth. It provides an excellent overview of the dentition, associated dentoalveolar structures, and location of impacted teeth. Periapical “shift shots” can help determine whether the tooth is buccal/labial or palatal/lingual (the SLOB rule [same lingual, opposite buccal] is frequently used to determine the position of the tooth on the subsequent x-ray film as the cone of the x-ray machine is moved anteriorly or posteriorly). Occlusal films, lateral cephalometric films, or CT scans can be used for precisely locating the position and orientation of impacted teeth.
In-office, small field cone-beam computed tomography (CBCT) provides perhaps the most convenient and valuable imaging method; it demonstrates not only the canine position, but also the details of angulation, orientation, and relationship to adjacent structures (see the figures in the section on cone-beam computed tomography [CBCT] in Chapter 1). This information can be beneficial for the surgeon's treatment plan and the choice of surgical approach, in addition to aiding the orthodontist in determining the path of eruption. CBCT may also detect root resorption of adjacent teeth that is not evident on panoramic radiographs. Haney reported significant changes in position diagnosis, root resorption detection, orthodontic vector determination, and surgical access planning by a group of orthodontists and oral surgeons who reviewed CBCT images compared with review of traditional radiographs of the same patients.
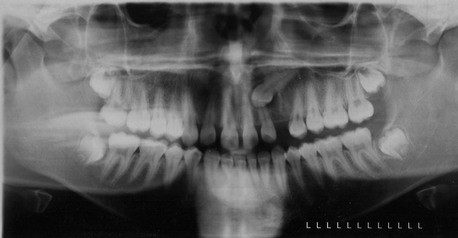
In the current patient, the panoramic radiograph shows a fully formed impacted left maxillary cuspid with a mesioangular orientation. Figure 5-2 demonstrates the position of the impacted canine before the initiation of orthodontic therapy. The crown of the canine appears to have a pericoronal radiolucent lesion consistent with a hyperplastic dental follicle (although a dentigerous cyst or other pathologic processes are also possible). No crestal bone loss is noted in the surrounding region. The full bony impacted third molars are also noted.
Labs
No laboratory studies are indicated for routine exposure and orthodontic bracket placement of impacted teeth unless dictated by the medical history.
Treatment
Current popular treatments of impacted canines can be divided into open and closed surgical techniques, differing slightly in regard to palatal versus labial impactions. Autotransplantation and extraction with implant replacement are less commonly used techniques and are described later with other historical techniques. Extraction of the primary canine may be considered if the patient is between 10 and 13 years old and sufficient arch space has been created, allowing observation for normal eruption of the permanent canine. Serial radiographs can be used to monitor eruption, and if no movement is observed over 12 months, alternative techniques should be performed.
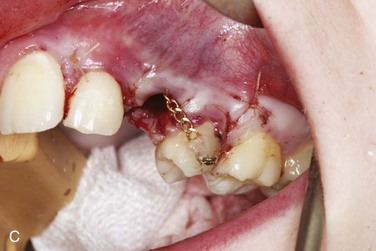
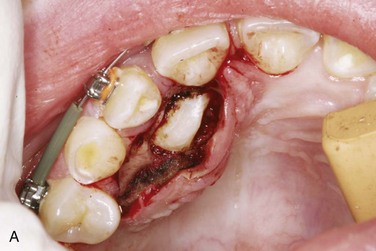
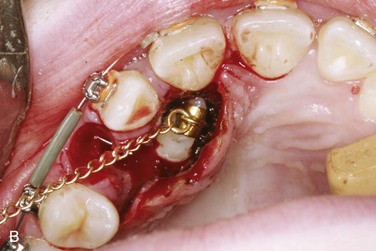
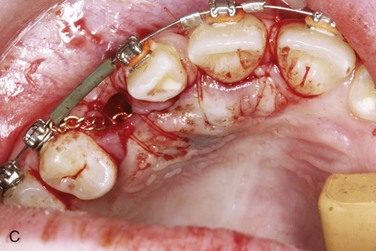
Open techniques. These surgical techniques are indicated when the crown of the impacted canine is in an appropriate location near the alveolar process, allowing exposure and access for orthodontic bracket placement. For palatal impactions, the excision of overlying soft tissue may be performed with a surgical blade or electrocautery as a “window.” Care should be taken to preserve sufficient soft tissue between the “window” and the cervical margin of surrounding erupted teeth to avoid potential tissue necrosis and periodontal complications. Bone removal may be performed with a rotary instrument, rongeurs, or hand instruments to expose the crown to the level of the cervical margin. Complete exposure of the crown may not be feasible in cases in which the crown is in close proximity to incisor roots. Any dental follicle remnants should be excised at this time, and gentle luxation of the tooth may be performed to rule out ankylosis. An orthodontic bracket with gold chain may be etched and bonded to the crown with the chain attached passively to existing orthodontic arch wires. The wound may be left open or packed with a periodontal dressing for a period of 4 to 5 days. It is generally accepted that a period of 6 to 8 weeks is observed for both palatal and labial impactions to allow for spontaneous eruption prior to the application of orthodontic forces. The apically repositioned flap is the open technique of choice for labially impacted canines. Electrocautery, or a “window” excision of overlying soft tissue, should be avoided with labial impactions, because it usually results in a lack of attached gingiva after eruption, with a possible need for a secondary graft procedure. A full thickness mucoperiosteal flap with vertical releasing incisions is raised to the level of the vestibular sulcus, followed by bone removal, follicle removal, and crown exposure and luxation as previously described. The distal aspect of the flap is positioned apically and sutured with chromic gut at the level of the cervical margin, thus placing attached gingiva at the level of the cementoenamel junction. Again, the bracket with chain may be bonded at this time.
Closed techniques. These surgical techniques are indicated when the crown is not near the alveolar process or is in a position that inhibits the apical repositioning of a flap (Figures 5-3 and 5-4). In both palatal and labial impactions, a full thickness mucoperiosteal flap is raised, allowing subsequent crown exposure, gentle luxation, and bonding of the orthodontic bracket with a chain. At this point, the chain may be brought through the distal aspect of the flap (or through a stab incision in the body of the flap), and the full flap is repositioned and sutured. In closed techniques, orthodontic forces may be applied after 1 week to allow for soft tissue healing.
Complications
The most prevalent complication associated with surgical exposure of impacted canines is failure of the orthodontic bracket bond or fracture of the chain. This is of greater consequence in closed techniques, because it requires surgical reexposure of the crown before replacement of the bracket. Moisture in the surgical field during bracket bonding may be the likely cause of this complication. Reexposure also is required with the occurrence of gingival overgrowth in open techniques.
Periodontal defects may occur as a result of inappropriate flap design and/or bone loss adjacent to the surgical site. Damage to the erupting tooth and adjacent tooth roots, including root resorption, may occur secondary to difficulty controlling the path of eruption. Devitalization of the pulp of the impacted tooth or neighboring incisors requires cessation of orthodontic movement and evaluation for possible endodontic treatment.
Ankylosis of the impacted tooth should be considered if no movement is observed after sufficient application of orthodontic forces and adequate time. Intrusion of the anchoring dentition may be observed in this situation. Some suggest that the act of gently luxating the tooth at the time of exposure may cause ankylosis as a result of subsequent bleeding and inflammation.
Other complications include infection, flap necrosis secondary to poor design or contact with acid etch, lack of keratinized tissue secondary to poor flap design, and paresthesia of the palate (if the nasopalatine nerve is injured) or of the lower lip, chin, mandibular incisors, and gingiva in the case of impacted mandibular canines.
Discussion
Excluding third molars, canines are the most commonly treated impactions by the oral and maxillofacial surgeon. The frequency of impacted canines has been reported as 2% to 5%, with palatal impactions occurring more frequently than labial impactions. The male to female ratio is commonly reported as 1 : 2, and mandibular impactions have a 0.4% reported frequency. Most impacted canines have an unknown etiology; however, numerous etiologies have been suggested, including mechanical obstruction by adjacent teeth, pathology, arch length discrepancy (more prevalent in labial impactions), premature loss of deciduous teeth, associated syndromes, questionable genetic predispositions, and endocrine abnormalities, such as hypothyroidism and hypopituitarism.
Historical treatments of impacted canines include the placement of crown forms or cervical wires. Adapted aluminum or plastic crown forms were commonly placed after exposure using a closed technique, with resulting erosion of overlying soft tissue secondary to a foreign body reaction. Once visible in the oral cavity, the crown form was removed and orthodontic brackets were placed. Wires secured around the cervical neck of the canine were also popularly used, but this was a more technically demanding procedure, sometimes requiring excessive manipulation of the tooth, and erosion of the canine at the cervical neck has been reported with this technique.
Additional surgical options include autotransplantation, segmental osteotomy, and extraction with subsequent implant placement. Autotransplantation may be indicated in circumstances of deep impactions and involves the creation of a bony socket for the extracted and transplanted canine. Survival rates have been reported at 70%, and as high as 94% when the periodontal membrane is intact at the time of transplantation. This technique, however, is not commonly used, because it is less predictable, and several cases of external root resorption have been reported. Segmental osteotomy is seldom performed and carries the risks of a more technical procedure. The incidence of extraction and replacement with osseointegrated implants has increased in recent years, but the use of implants in growing children is still controversial. Implants have been shown to migrate and may become submerged as growth continues vertically. The latter complication may be avoided by placement of the implant after vertical growth of the alveolus is complete. Despite these concerns, successful restoration using implants has been demonstrated in numerous studies and warrants further investigation.
The decision between an open or a closed technique is often left up to the practitioner and may be one of personal choice. As mentioned previously, certain physical location factors of the involved impacted tooth may dictate one technique over another. Some studies have reported a twofold higher rate of complications for closed techniques compared with open techniques. The primary complication with closed techniques is bond or wire failure, and the primary complication with open techniques is soft tissue overgrowth. More recent studies show no significant differences in surgical outcomes. Bond failure in open techniques is a minor complication, and some suggest delaying bracket placement until after the observational period, allowing greater control of moisture in the surgical field. No significant differences in subsequent periodontal complications have been reported between the two groups. The consensus appears to be that both techniques are acceptable and provide predictable results for the treatment of impacted canines.
Alberto, PL. Management of the impacted canine and second molar. Oral Maxillofac Surg Clin North Am. 2007; 19:59–68.
Bass, T. Observation on the misplaced upper canine tooth. Dent Pract Dent Rec. 1967; 18:25–33.
Caminiti, MF, Sandor, GK, Giambattistini, C, et al. Outcomes of the surgical exposure, bonding and eruption of 82 impacted maxillary canines. J Can Dent Assoc. 1998; 64(8):572–579.
Celikoglu, M, Kamak, H, Oktay, H, et al. Investigation of transmigrated and impacted maxillary and mandibular canine teeth in an orthodontic patient population. J Oral Maxillofac Surg. 2010; 68:1001–1006.
Chaushu, S, et al. Patient's perception of recovery after exposure of impacted teeth: a comparison of closed- versus open-eruption techniques. J Oral Maxillofac Surg. 2005; 63:323–329.
Felsenfeld, AL, Aghaloo, T. Surgical exposure of impacted teeth. Oral Maxillofac Surg Clin North Am. 2002; 14:187–199.
Ferguson, JW, Parvizi, F. Eruption of palatal canines following surgical exposure: a review of outcomes in a series of consecutively treated cases. Br J Orthod. 1997; 24(3):203–207.
Haney, E, Gansky, SA, Lee, JS, et al. Comparative analysis of traditional radiographs and cone beam computed-tomography volumetric images in the diagnosis and treatment planning of maxillary impacted canines. Am J Orthod Dentofacial Orthop. 2010; 137:590–597.
Jacobs, SG. The impacted maxillary canine: further observations on aetiology, radiographic localization, prevention/interception of impaction, and when to suspect impaction. Aust Dent J. 1996; 41(15):310–316.
Johnson, W. Treatment of palatally impacted canine teeth. Am J Orthod. 1969; 56(6):589–596.
Moss, JP. Autogenous transplantation of maxillary canines. Br J Oral Surg. 1968; 26:775.
Parkin, NA, Deery, C, Smith, AM, et al. No difference in surgical outcomes between open and closed exposures of palatally displaced maxillary canines. J Oral Maxillofac Surg. 2012; 70:2026–2034.
Pearson, MH, Robinson, SN, Reed, R, et al. Management of palatally impacted canines: the findings of a collaborative study. Eur J Orthod. 1997; 19(5):511–515.
Pogrel, MA. Evaluation of over 400 autogenous tooth transplants. J Oral Maxillofac Surg. 1987; 45:205–211.
Tiwanna, PS, Kushner, GM. Management of impacted teeth in children. Oral Maxillofac Surg Clin North Am. 2005; 17:365–373.
Lingual Nerve Injury
CC
An 18-year-old female is referred to a microneurosurgeon for evaluation of numbness of her left tongue.
HPI
The patient had all four third molars surgically removed by an oral and maxillofacial surgeon 13 weeks before presentation. Upon follow-up at 7 days with the referring surgeon, the patient complained of persistent loss of feeling in her left tongue and altered taste sensation. No neurosensory testing was done at that time. Six weeks after surgery, the patient continued to report profound numbness of the left tongue and no improvement in taste perception. All surgical wounds were healed. Neurosensory testing (NST) of the tongue (pinprick and light touch) demonstrated total anesthesia (absence of perception of any stimulation of the mucosa) of the anterior two thirds of the left tongue, floor of the mouth, and lingual gingiva. Photographic documentation of the affected area of the tongue was obtained. An appointment was made for reevaluation of the patient in 4 weeks. At follow-up (10 weeks postsurgery), repeat NST revealed no change (persistent total anesthesia) from the previous examination. The patient was subsequently referred to a microneurosurgeon for evaluation of left lingual nerve (LN) injury.
The patient also complained of pain radiating into her left tongue when chewing food or brushing her left lower teeth (allodynia) and frequent accidental biting of her tongue. (Allodynia is defined as pain due to a stimulus that does not normally produce pain. Dysesthesia is an unpleasant abnormal sensation, either spontaneous or evoked, and anesthesia dolorosa is pain in an area or a region that is anesthetic.)
Examination
General. The patient is a well-developed and well-nourished adolescent female in no apparent distress.
Maxillofacial. There is no cervical lymphadenopathy. Maximum interincisal opening is 51 mm without mandibular deviation, and all extraction/surgical sites are healed. There are no oral masses or ulcerations; no fasciculations, deviation, or atrophic changes of the tongue; and no evidence of recent tongue trauma (scars or lacerations). Inspection of the lingual and buccal aspects of the mandible reveals no abnormalities (texture, color, and consistency of mucosa are within normal limits). Palpation and percussion of the lingual surface of the posterior mandible adjacent to the third molar area produced a localized painful sensation that radiated to the left tongue (positive Tinel's sign: a provocative test of regenerating nerve sprouts in which light percussion over the nerve elicits a distal tingling sensation; it is often interpreted as a sign of small fiber recovery, but following LN injury with complete severance, this response likely represents proximal stump neuroma formation and phantom pain).
Clinical neurosensory. This examination is performed at three levels: A, B, and C (Box 5-3). Cranial nerves II through XII were intact except for the left LN distribution, mandibular division (V3 of the left trigeminal nerve [CN V]). The patient showed no response to any of the three levels of NST, which supports a diagnosis of anesthesia.
In patients with abnormal pain sensations (allodynia, anesthesia dolorosa, dysesthesia), a local anesthetic block of the involved nerve may be helpful in making treatment decisions. If the pain is abolished during the duration of the local anesthetic block, there is a reasonable possibility of pain relief from microneurosurgical repair of the injured nerve.
Imaging
Panoramic radiograph (11 weeks postsurgery) reveals no evidence of retained root fragment or foreign bodies. The outline of the socket of the right mandibular third molar is well demarcated and is appropriate for the stage of healing. Assessment of the LN is possible with an MRI study, but this is not generally necessary in making treatment decisions.
Labs
No routine or special laboratory tests are indicated for microneurosurgical evaluation unless dictated by the medical history.
Assessment
Left LN injury, exhibiting complete anesthesia to NST at 13 weeks after injury, is a neurotmesis, or Sunderland fifth-degree injury (i.e., nerve injury with anatomic disruption of all axonal and sheath elements and/or physiologic block of all impulse transmission, producing wallerian degeneration and probable neuroma formation) (Table 5-1).
Surgical intervention is indicated for microrepair of the left LN (i.e., excision of the proximal stump neuroma and, most likely, neurorrhaphy [repair of a severed nerve by suturing the two nerve ends together] or, less likely, reconstruction of a nerve gap with a graft).
Treatment
The two most important factors in successful decision making regarding treatment of peripheral trigeminal nerve injuries are prompt evaluation of suspected nerve injuries and correct patient selection (diagnosis). There is a time constraint on the interval after injury in which a peripheral nerve can be repaired with reasonable expectation of success. After injury, severed axons in the nerve undergo wallerian degeneration over a period of 1 to 2 months. If the distal endoneurial sheaths of the necrotic axons are not recannulated with new axonal sprouts from the proximal nerve stump within a critical interval (probably 9 to 12 months after injury), the endoneurial sheaths collapse and are replaced with scar tissue, making reinnervation unlikely or impossible. The selection of patients who might benefit from surgical intervention is based on a standardized neurosensory examination. Patients suffering from unacceptable partial or complete loss of sensation, with or without pain symptoms, are most likely to benefit from microsurgical repair of nerve injuries.
Surgical treatment of peripheral nerve injuries follows a stereotypical series of steps, performed in order. These include external decompression, internal neurolysis, preparation of nerve stumps (including excision of scar tissue and neuromas), neurorrhaphy, reconstruction of a nerve gap, and other steps, if the nerve is found not to be repairable) (Table 5-2). Specific intraoperative findings dictate the surgical treatment modality.
Table 5-2
Steps in Microsurgical Peripheral Nerve Repair
| Procedural Steps* | Description |
| 1. External decompression | Removal of bone, scar tissue, and foreign material (e.g., root canal filling material, missile fragments, internal fixation wires, screws or plates); exposure of nerve |
| 2. Internal neurolysis | Incision of epineurium, inspection of internal nerve structure, removal of scar tissue, repair of individual fascicles |
| 3. Preparation of nerve stumps | Excision of neuroma or scar tissue, exposure of viable nerve tissue in nerve stumps, mobilization of proximal and distal nerve limbs to allow approximation |
| 4. Neurorrhaphy | Approximation and suturing of nerve stumps without tension |
| 5. Reconstruction of nerve gap | Autogenous nerve graft; processed allogeneic nerve graft; alloplastic nerve guide |
| 6. Nerve-sharing procedure | When proximal nerve is not available, anastomosis of proximal stump of nearby nerve (e.g., great auricular nerve) to viable distal stump of injured nerve, using a bridging autogenous nerve graft (e.g., sural nerve) |
| 7. Irreparable nerve injury | Nerve capping; nerve redirection; neurectomy (only for pain of terminal malignancy) |
*All steps are performed in consecutive order, as shown. The operation can be concluded at any step at which the surgeon decides the procedure has been completed.
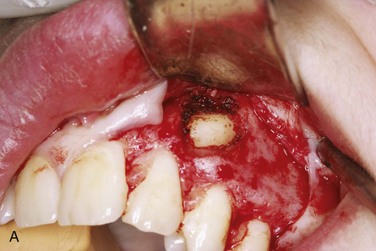
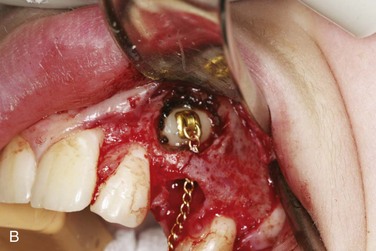
In the current patient, under general nasal endotracheal anesthesia, bupivacaine with epinephrine was injected into the soft tissue of the operative field (in addition to an inferior alveolar nerve block for vasoconstriction of the associated proximal vessels). Using ×3.5 loop magnification (or an operating microscope can be used) and fiberoptic lighting, incisions were made along the gingival margins of the premolar and molar teeth on both the buccal and lingual aspects of the left mandible and extended posterolaterally up the ascending ramus. The mucoperiosteum was elevated from the region of the bicuspids and posteriorly. There was a defect in the lingual cortex of the left mandible. The lingual periosteum was sharply incised with microscissors, and the left LN was identified and dissected free to reveal a total transection adjacent to the previously removed third molar with a stump (or amputation) neuroma on the proximal segment. The distal and proximal nerve stumps were freed of surrounding scar tissue, the proximal neuroma was excised, and the distal nerve stump was freshened to visualize viable fascicles (Figure 5-5, A). The proximal and distal nerve limbs were mobilized by dissecting them free of surrounding scar and connective tissue. This dissection enabled the nerve endings to be brought together without tension and sutured (neurorrhaphy) using 8-0 ophthalmic nylon (Figure 5-5, B). (Tension across the suture line of greater than 25g significantly compromises regeneration.) The anastomosis was encircled with a resorbable flexible collagen nerve cuff to prevent fibrous tissue ingrowth (Figure 5-5, C). The mucosal incision was closed with chromic sutures, and the patient was extubated.
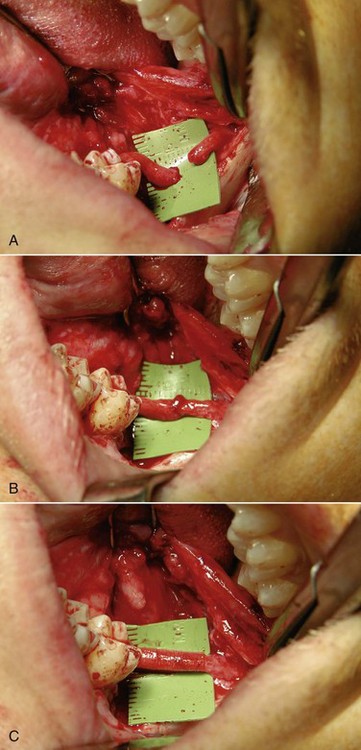
Figure 5-5 A, Proximal and distal nerve stumps before reanastomosis. B, Neurorrhaphy with 8-0 nylon sutures. C, Repair protected by a resorbable flexible collagen nerve cuff.
Postoperatively, the patient was closely monitored for adequate wound healing, and physical therapy was prescribed to restore normal mandibular opening and range of motion. Four months after the operation, the patient began to experience spontaneous tingling sensations in her left tongue, and she could perceive the hot or cold temperature of ingested liquids. One month later, the anterior two thirds of the left tongue and lingual mandibular gingiva responded to painful stimuli (level C) and static light touch (level B). At that time, daily sensory reeducation exercises (SREs) were prescribed for the tongue and lingual gingiva, which the patient performed three times daily. At 1-year follow-up, the patient demonstrated both subjective and objective signs of left lingual nerve sensory function. She continued the SREs for several more months, after which two-point discrimination (level A) in the left tongue was equal to that in the normal right tongue. Subjectively, the left tongue seemed nearly normal to the patient, and she was dismissed from care.
Complications
Like other surgical procedures, microneurosurgical intervention is not without risks. Careful patient selection is of paramount importance. The indications for microneurosurgical intervention are not always consistent in the literature. However, common indications for surgical exploration and repair of the lingual nerve include the following:
• Spontaneous or stimulus-evoked hyperesthesia (a group of painful responses to stimuli that includes allodynia [a painful response to a stimulus that is ordinarily not painful, such as stroking with a cotton wisp], hyperpathia [delayed onset of pain in response to repetitive stimuli, such as tapping with a blunt object, with continuation of the pain for seconds or minutes after withdrawal of the stimulus], and hyperalgesia [an increased response to a stimulus that is normally painful]) that is abolished temporarily by a local anesthetic block of the suspected nerve
• Constant, deep pain in an anesthetic (anesthesia dolorosa) or a hypoesthetic area (e.g., the tongue) that is abolished by a local anesthetic block of the suspected nerve
• Intolerable or unacceptable (to the patient) anesthesia or hypoesthesia, with or without pain, that shows no signs of recovery (as determined by interval NST) and persists beyond 3 months after injury
Patients with acceptable anesthesia/hypoesthesia or with satisfactory neurosensory recovery without intolerable pain or dysfunction are generally not candidates for surgical nerve exploration. It is possible for such patients to experience a worse outcome, such as the development of anesthesia dolorosa in a previously anesthetic but nonpainful region. Fortunately, this appears to be a rare event. Likewise, most patients with nerve injury whose presurgical symptom is numbness rather than pain do not develop painful sensations after microsurgical nerve repair. More commonly, failure of peripheral trigeminal microneurosurgery is related to inability to restore the preinjury sensory function. In cases of total nerve severance (neurotmesis, or Sunderland fifth-degree injury), the time lapse from injury to repair, proper surgical technique (e.g., tension-free closure), and the patient's age and health status are among the most important factors influencing success. Best results are seen when repair is performed within 6 months of the date of injury. In cases of a witnessed nerve severance, immediate primary nerve repair is indicated unless the surgical site is contaminated (e.g., gunshot wound), the patient's current medical status is compromised, or the surgeon does not have the training or instrumentation to complete the repair at that time. In such instances, either a delayed repair is done, after the injury site shows early signs of healing without infection, or the patient is referred to a surgeon with microsurgical training for completion of the nerve repair. This delay of a few days or weeks seems not to result in a statistically significant reduction in the success rate of repair of peripheral nerve injuries.
Discussion
The inferior alveolar nerve and the lingual nerve are the sensory nerves most commonly injured during surgical treatment by oral and maxillofacial surgeons. Injury to these nerves is not always avoidable, despite a good knowledge of the anatomy and meticulous surgical technique. The lingual nerve has a more variable and less predictable course. Studies based on anatomic cadaveric dissections show that the lingual nerve is positioned above the lingual alveolar crest at the retromolar area in 14% of cases (see the section on third molar odontectomy earlier in this chapter). In other instances, the lingual nerve travels through or inferior to the submandibular salivary gland and courses anteriorly adjacent to the submandibular salivary duct. Removal of a mandibular third molar tooth (M3) is the surgical procedure most commonly associated with injuries to the LN and the inferior alveolar nerve (IAN), with those to the LN occurring less frequently than those to the IAN. However, the lingual nerve, which is located entirely within soft tissue, is less likely to spontaneously recover from injury compared with the inferior alveolar nerve. This is hypothesized to be due to the position of the inferior alveolar nerve in the bony canal, which might serve as a conduit for nerve regeneration, although successful spontaneous regeneration of the IAN does not occur predictably.
The total encasement of the LN within soft tissue offers one important advantage in the surgical repair of this nerve. The LN has a rather tortuous course, especially distally from the adjacent third molar area into the floor of the mouth. By identifying and carefully dissecting the distal limb of a severed LN, the surgeon can straighten this tortuosity, thereby gaining up to 2 cm of length. This additional length often allows the proximal and distal nerve limbs to be brought together without tension. Therefore, the vast majority of LN injuries, except in cases of substantial avulsive or ablative loss of nerve tissue, can be repaired by neurorrhaphy, rather than requiring the additional surgery needed for reconstruction of a nerve gap with a nerve graft.
The reported incidence of temporary paresthesia of the lingual nerve from third molar surgery is between 2% and 6%; fewer than about 1% of these injuries result in a permanent deficit. Several factors may be associated with an increased risk of lingual nerve injury, including lingual bone–splitting technique, aggressive curettage of the follicular sac or granulation tissue, excessive lingual bone removal, lingual plate perforation by a drill or an instrument, and deeply placed lingual sutures. Placement of a lingual retractor increases the incidence of temporary lingual nerve paresthesia but most likely decreases the incidence of permanent nerve injury.
Upon injury to a nerve, the distal nerve segment undergoes wallerian degeneration. The severed distal axons rapidly become necrotic and are phagocytosed within 1 to 2 months, leaving the endoneurial superstructure initially intact. New axonal sprouts extend from the proximal nerve stump and attempt to recannulate the distal endoneurial tubules. If this does not occur within a variable period of time (estimated in humans to be between 9 and 15 months), the endoneurial tubules progressively degenerate and are replaced by scar tissue. Once scar tissue has fully replaced the connective tissue framework, the regenerating proximal axons can no longer recannulate the endoneurial tubules and reinnervate their target tissue. Therefore, the best results for microneurosurgical repair of nerve severance are achieved when surgery is performed as soon as the diagnosis is confirmed and the patient is willing to proceed with the procedure, given the risks and benefits. Within 6 months of the injury, repair has a reasonable chance of success (80% to 90%) (defined as response to pressure and light touch at normal thresholds, two-point discrimination at a threshold of less than 15 mm, and no hyperesthesia), whereas beyond 12 months, the likelihood of success is very low.
Bagheri, SC, Meyer, RA, Ali Khan, H, et al. Microsurgical repair of the peripheral trigeminal nerve after mandibular sagittal split ramus osteotomy. J Oral Maxillofac Surg. 2010; 68(11):2770.
Bagheri, SC, Meyer, RA, Ali Khan, H, et al. Retrospective review of microsurgical repair of 222 lingual nerve injuries. J Oral Maxillofac Surg. 2010; 68(4):715.
Bagheri, SC, Meyer, RA. Management of trigeminal nerve injuries. In: Bagheri SC, Bell RB, Khan HA, eds. Current therapy in oral and maxillofacial surgery. St Louis: Saunders; 2011:224–237.
Essick, GK. Comprehensive clinical evaluation of perioral sensory function. Oral Maxillofac Surg Clin North Am. 1992; 4(2):503.
Gregg, JM. Surgical management of lingual nerve injuries. Oral Maxillofac Surg Clin North Am. 1992; 4(2):417.
LaBanc, JP. Classification of nerve injuries. Oral Maxillofac Surg Clin North Am. 1992; 4(2):285.
Meyer, RA. Applications of microneurosurgery to the repair of trigeminal nerve injuries. Oral Maxillofac Surg Clin North Am. 1992; 4(2):405.
Meyer, RA, Bagheri, SC. Clinical evaluation of nerve injuries. In: Miloro M, ed. Trigeminal nerve injuries. Heidelberg: Springer, 2013.
Meyer, RA, Bagheri, SC. Clinical evaluation of peripheral trigeminal nerve injuries. Oral Maxillofac Surg Clin North Am. 2011; 19(1):15.
Meyer, RA, Bagheri, SC. Etiology and prevention of nerve injuries. In: Miloro M, ed. Trigeminal nerve injuries. Heidelberg: Springer, 2013.
Meyer, RA, Bagheri, SC. Nerve injuries from mandibular third molar removal. Oral Maxillofac Surg Clin North Am. 2011; 19(1):63.
Meyer, RA, Rath, EM. Sensory rehabilitation after trigeminal injury or nerve repair. Oral Maxillofac Surg Clin North Am. 2001; 13(2):365.
Miloro, M, Kolokythas, A. Inferior alveolar and lingual nerve imaging. Oral Maxillofac Surg Clin North Am. 2011; 19(1):35.
Phillips, C, Blakey, G, Essick, GK. Sensory retraining: a cognitive behavioral therapy for altered sensation. Oral Maxillofac Surg Clin North Am. 2011; 19(1):109.
Robert, RC, Bacchetti, P, Pogrel, MA. Frequency of trigeminal nerve injuries following third molar removal. J Oral Maxillofac Surg. 2005; 63(6):732–735.
Ziccardi, VB. Microsurgical techniques for repair of the inferior alveolar and lingual nerves. Oral Maxillofac Surg Clin North Am. 2011; 19(1):79.
Zuniga, JR, Meyer, RA, Gregg, JM, et al. The accuracy of clinical neurosensory testing for nerve injury diagnosis. J Oral Maxillofac Surg. 1998; 56:2.
Displaced Root Fragments During Dentoalveolar Surgery
CC
A 41-year-old man is referred to your office for extraction of a nonrestorable left maxillary first molar.
HPI
Four years earlier the patient had undergone a root canal procedure because of extensive caries on the left maxillary first molar, without any complications (extractions of endodontically treated teeth have a greater probability of root fracture and displacement). He did not pursue restoration of the tooth due to financial reasons and has now been referred for extraction of the failed root canal. He presented to his general dentist with a complaint of pain and mild gingival swelling adjacent to the left maxillary first molar.
PMHX/PDHX/Medications/Allergies/SH/FH
Noncontributory. The patient does not use tobacco.
Medical comorbidities that compromise wound healing (e.g., chronic steroid therapy, smoking cigarettes, diabetes, radiation therapy, and malnutrition) may increase the likelihood of persistent oral antral communications, requiring repeat surgical closure. However, the regional anatomy of the area, such as the length of the roots, extent of sinus pneumatization, and amount and quality of surrounding bone, is also important.
Examination
Intraoral. The patient has localized gingival edema and erythema of the left maxillary first molar, with no vestibular fluctuance. There is a 2-mm draining fistula on the buccal gingiva. A large carious lesion is present on the mesial-occlusal surface of the tooth. The left maxillary second and third molars (teeth #15 and #16) are missing, with significant resorption of the posterior maxillary ridge.
Imaging
The periapical or panoramic radiograph is the minimal imaging modality necessary before the extraction of a tooth. The panoramic radiograph allows better evaluation of the surrounding structures (e.g., the maxillary sinus). Evaluation of the size and shape of the tooth, degree of sinus pneumatization, and amount of bone is important for assessment of possible risks for oral antral exposure or root fracture.
For the current patient, the panoramic radiograph reveals a long palatal root of the left maxillary first molar that appears to partially project into the sinus. There is a loss of continuity of the maxillary sinus in the area of the palatal root (suggestive of a periapical scar secondary to the previous root canal or a pathologic process involving the maxillary sinus).
Labs
No laboratory testing is indicated before routine dentoalveolar surgery unless dictated by the medical history.
Assessment
Nonrestorable carious left maxillary first molar requiring extraction.
Preoperative assessment of this patient should alert the surgeon to the increased likelihood of root fracture and/or oral antral communication upon surgical removal of the left maxillary first molar. Well-informed patients are more accepting of necessary secondary procedures (e.g., oral antral closure, root retrieval from the sinus, or nerve repair).
Treatment
After injection of a local anesthetic with epinephrine, extraction of the left maxillary first molar was attempted using an elevator and forceps. Removal of the tooth revealed fracture of the palatal root with the root fragment retained within the palatal socket. A root tip pick was used to retrieve the fragment. During elevation, the root tip suddenly disappeared from the surgical field. Evaluation of the socket revealed a dark hole, suggesting that the fragment has dislodged into the maxillary sinus.
Upon diagnosis of a displaced root into the maxillary sinus, several maneuvers may be attempted to retrieve the fragment. It is possible for a fragment to be displaced below the schneiderian membrane without actual dislodgment into the maxillary antrum. If the membrane appears intact, this diagnosis should be considered. In cases of dislodgment into the sinus, a perforation into the antrum may be visible. Asking the patient to exhale while pinching the nose may demonstrate air or bubbles exiting the socket, confirming the diagnosis of sinus perforation. Immediately upon diagnosis, a small suction tip can be placed at the apex of the extraction socket in an attempt to remove the fragment. The procedure can be repeated with the patient placed in an upright position. If this maneuver fails, the maxillary sinus can be irrigated with normal saline, followed by suctioning to allow root retrieval. If the root fragment cannot be visualized, the procedure should be aborted. The following two treatment approaches should be considered:
• Closure of the sinus communication, leaving the root fragment in place. The patient is subsequently monitored with panoramic radiographs to document the position of the root. In patients who are asymptomatic, with small fragments that are fixed in the antrum, it is possible to simply observe the root with serial radiographs.
• Closure of the sinus perforation, followed by immediate or delayed removal of the root fragment via a Caldwell-Luc, transalveolar, or endoscopic sinus surgery.
These treatment options are addressed in more detail in the Discussion section.
Complications
Displacement of a tooth or root fragment into the maxillary sinus in a known complication of maxillary dentoalveolar surgery. Although several preoperative findings (described earlier) can identify patients at risk, this complication can occur in any patient. Other possible complications of dentoalveolar surgery are listed in Box 5-4.
Pain and swelling are inevitable consequences of any surgical intervention. However, measures to minimize pain and swelling (preoperative steroids, short operative time, and careful surgical technique) may increase the patient's comfort and satisfaction.
Discussion
The palatal root of the maxillary first molar is the most likely root to be pushed into the maxillary sinus. There is some controversy regarding the optimal management of root fragments displaced into the maxillary sinus. Many surgeons advocate removal of all root fragments from the sinus, regardless of any preexisting sinus or periapical pathology. It is hypothesized that a root tip may act as a foreign body in the sinus, leading to polyps or sinusitis. No randomized trials have evaluated this issue, and most authors argue that the decision needs to be made on a case-by-case basis. It is recommended that if the root tip is small (less than 3 mm) and the sinus and the tooth demonstrate no preexisting pathology, only minimal attempts should be made to retrieve the root. The majority of root fragments are fibrosed into the sinus membrane, without any long-term sequelae. Case reports of retrieved maxillary implants that had migrated or perforated the sinus mucosa have demonstrated no inflammatory changes in the mucosa (both clinically and radiographically). However, other case reports have found that migration of a cover screw has caused acute sinusitis.
A standard procedure used to retrieve foreign bodies from the maxillary sinus is the Caldwell-Luc procedure. A vestibular incision is used to access the canine fossa. A perforation is made in the anterior maxillary wall, allowing visualization of the sinus. This can be enlarged to gain access to the sinus as needed. Careful attention to the infraorbital nerve prevents postoperative hypoesthesia.
Access to the maxillary sinus can also be gained via a transalveolar approach, by extending the opening of the extraction socket. Removal of buccal bone beyond the apex of the socket allows exposure of the antral mucosa. If the membrane has not been violated, this tissue plane may be explored; otherwise, an opening can be made through the membrane to allow sinus exploration. The opening is closed primarily using a buccal flap. This technique provides superior exposure to the antral floor (exposing the most likely position of the dislodged tooth). However, if the patient is interested in replacing the edentulous area with an implant, this approach would compromise the alveolar ridge bone, which is important for implant restorations.
Prevention of root displacement is the best treatment. If a root tip is fractured and the clinician suspects the possibility of displacement into the sinus, blind attempts at elevation of the fragment should be avoided. The use of adequate lighting (headlight) and full exposure of the area usually allow successful retrieval of the root from the socket. A variety of methods, including the use of endodontic files to remove root tips, have been described.
It is generally recommended that exposure of the sinus via the oral cavity warrants antibiotic therapy and “sinus precautions,” regardless of the decision to retain or retrieve a tooth fragment. The sinus flora includes the bacteria Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Nasal decongestants, such as oxymetazoline (Afrin) or pseudoephedrine, are used to improve sinus drainage. Topical application of oxymetazoline (an α-agonist) causes arteriolar vasoconstriction, resulting in nasal mucosal shrinkage, which allows for improved drainage. Oxymetazoline should not be used for longer than 3 to 5 days secondary to the development of rhinitis medicamentosa, causing rebound nasal congestion. Decongestants containing pseudoephedrine (a sympathomimetic, α-adrenergic agonist) cause vasoconstriction by selectively acting on the peripheral α-receptors, without the central nervous system side effects. These medications are frequently available in combination with an antihistamine or antitussive agents.
In addition to root tip displacement into the maxillary sinus, case reports have found displacement of implants, either during placement or at a later time (Figure 5-6). The same techniques have been reported for retrieval as those used for root tips; however, waiting is not recommended. Generally either immediate or early retrieval is warranted, because the implant can act as a nidus of infection. There is a limitation on the size of the objects that can be removed using an endoscopic technique. A transnasal approach is limited both by the endoscope's inability to pick up an object greater than 20 mm and by the inability to remove such an object through the narrow and complicated pathway. An endoscope can be used in combination with a Caldwell-Luc access to minimize the amount of surgical trauma. It is possible to retrieve the implant via the initial osteotomy site; however, this could compromise future implant placement.
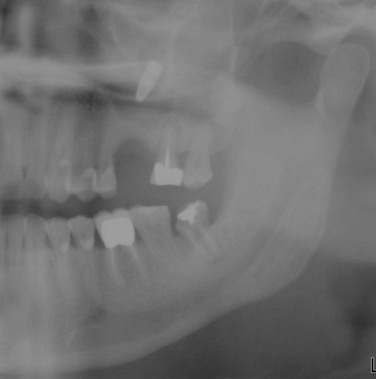
Figure 5-6 Panoramic radiograph showing displaced maxillary implant into the sinus 4 months after placement of the implant.
Although commonly discussed but rarely reported, displacement of the maxillary third molar into the infratemporal fossa is a potential complication. A number of case reports have discussed various treatment techniques. The infratemporal fossa is the space inferior and medial to the zygomatic arch, with possible superior extension superficial or deep to the temporalis muscle. A CBCT scan can demonstrate the position of the tooth (it also shows whether the tooth was displaced into the maxillary antrum or the infratemporal fossa); however, MRI or contrast-enhanced CT can better determine the location of the tooth in relation to the muscular (and soft tissue) anatomy. Upon displacement of the tooth, the surgical incision can be enlarged to attempt extraction of the tooth via the displacement tract. If the tooth can be palpated, a spinal needle can also be used to push the tooth inferiorly toward the oral cavity for retrieval. If this is not successful, the patient can be placed on antibiotics, and retrieval can be attempted in the operating room using endotracheal intubation. If possible, delaying the procedure for 6 weeks allows fibrosis and encapsulation of the tooth, which minimizes movement during removal. Other techniques include a coronal approach (including a Gilles approach) and intraoral access by removal of the coronoid process. Intraoperative navigation guided by CT can be used via an intraoral or a small temporal incision, or a combination of the two. This approach is technique sensitive and may be more costly.
Extraction of mandibular molars can be complicated by displacement of root tips through a perforated lingual cortex into the submandibular or sublingual space (depending on the attachment of the mylohyoid muscle). The lingual plate at the area of the mandibular third molars can be very thin and, in some instances, fenestrated. Upon identification of a tooth fragment that is likely to be dislodged from the socket, placement of a finger along the medial aspect of the lingual cortex can frequently prevent this complication.
If a tooth becomes dislodged into the submandibular/sublingual space, attempts at removal should be made through the extraction socket or the perforation. If this maneuver is unsuccessful, a lingual mucoperiosteal flap can be elevated to allow exploration of the immediate region. Care should be taken not to injure the lingual nerve. In the event of failure to identify the tooth, the procedure should be aborted and the patient placed on antibiotics. A waiting period of 4 to 6 weeks has been recommended to allow the development of fibrosis around the tooth to facilitate removal. A CT scan may be obtained to visualize the exact position of the tooth, allowing careful preoperative planning. For small root fragments (less than 5 mm) that are not associated with any pathology, the surgeon may elect to observe the tooth and remove the fragment only if it becomes symptomatic.
Barclay, JK. Root in the maxillary sinus. Oral Surg Oral Med Oral Pathol. 1987; 64:162–164.
Bodner, L, Zion, B, Puterman, M. Removal of a maxillary third molar from the infratemporal fossa. a case report. J Med Cases. 2012; 3(2):97–99.
Campbell, A, Costello, B. Retrieval of a displaced third molar using navigation and active image guidance. J Oral Maxillofac Surg. 2010; 68:480–485.
Chrcanovic, B, Custódio, A. Surgical removal of dental implants into the maxillary sinus: a case report. Serbian Dental Journal. 2009; 56:139–144.
Friedlich, J, Rittenberg, BN. Endoscopically assisted Caldwell-Luc procedure for removal of a foreign body from the maxillary sinus. J Can Dent Assoc. 2005; 71:2000–2001.
Gulbrandsen, SR, Jackson, IT, Turlington, EG. Recovery of a maxillary molar from the infratemporal space via a hemicoronal approach. J Oral Maxillofac Surg. 1987; 45(3):279–282.
Iida, S, Tanaka, N, Kogo, M, et al. Migration of dental implant into the maxillary sinus: a case report. Int J Oral Maxillofac Surg. 2000; 29(5):358–359.
Lee, FM. Management of the displaced root in the maxillary sinus. Int J Oral Maxillofac Surg. 1978; 7:374–379.
Nakamura, N, Mitsuyasu, T, Ohishi, M. Endoscopic removal of a dental implant displaced into the maxillary sinus: technical note. Int J Oral Maxillofac Surg. 2004; 33:195–197.
Peterson, LJ, Ellis, E, Hupp, JR, et al. Contemporary oral and maxillofacial surgery. St Louis: Mosby; 1993.
Selvi, F, Cakarer, S, Keskin, C, et al. Delayed removal of a maxillary molar accidentally displaced into the infratemporal fossa. J Craniofac Surg. 2011; 22(4):1391–1393.
Speilman, AI, Laufer, D. Use of a Hedstrom file for removal of fractured root tips. J Am Dent Assoc. 1985; 111(6):970.
Ueda, M, Kaneda, T. Maxillary sinusitis caused by a dental implant: report of two cases. J Oral Maxillofac Surg. 1992; 50:285–287.