Primary Care of Flexor Tendon Injuries
▪ For an injury less than 3 weeks old, nonemergent repair is indicated unless there is active purulent infection.
▪ An injury greater than 6 weeks old is a relative contraindication to attempt primary repair.
▪ Multi-strand core suture technique with a running circumferential epitendinous suture is used
▪ A red rubber catheter should be ready if retrieval is required.
▪ The goal of repair and rehabilitation is a strong repair site that will not elongate beyond 3 mm with gentle active range-of-motion therapy designed to prevent adhesion formation.
▪ Lacerations of the slips of the FDS require different suture techniques than do FDP lacerations.
▪ Enter sheath between distal A2 and proximal A4.
▪ Retrieve proximal stump: skin hooks, reverse Esmarch, Sourmelis, direct exposure.
▪ Deliver distal stump by passive DIP flexion
▪ Transfix tendons, once delivered through pulleys with 25-gauge needle.
▪ Use a minimum of a four-strand core suture (4-0 FiberWire) technique with a running 6-0 Prolene epitendinous suture (nonlocked, deep bites into tendon).
▪ Use graded active rehabilitation protocol under supervision of a qualified therapist.
Restoring digital function after flexor tendon injury continues to be one of the great challenges in hand surgery.1-7 In recent years, important advancements in our understanding of tendon anatomy,8,9 biomechanics,10-12 nutrition,13-18 adhesion formation,19-23 and tendon repair techniques have led to enhanced results after flexor tendon repair. Despite the many gains, problems of stiffness, scarring, and functional impairment continue to frustrate the most experienced hand surgeons.
Appreciation of flexor tendon anatomy10 as well as the flexor retinacular sheath8,9,24-27 is critical to the surgeon’s ability to deal with flexor tendon injuries. The flexor digitorum profundus (FDP) arises from the proximal volar and medial surfaces of the ulna, the interosseous membrane, and occasionally the proximal radius. Along with the flexor pollicis longus (FPL), the FDP forms the deep muscle layer in the flexor compartment of the proximal forearm. In the midforearm, the muscle belly separates into a radial bundle and an ulnar bundle. In the distal third of the forearm, the radial bundle forms the index finger profundus tendon, and the ulnar bundle forms the profundus tendon to the ulnar three digits. The profundus tendons pass through the carpal canal, occupying the floor of the tunnel.
After traversing the carpal canal, the profundus tendons diverge to the digits. The lumbrical muscles originate at this level. The profundus tendons enter the flexor sheath deep to the superficialis tendons at the level of the metacarpophalangeal (MCP) joint. At the midproximal phalanx level, the profundus tendon becomes more palmar as it passes through the bifurcating superficialis tendon. It continues distally to insert into the palmar base of the distal phalanx.
The anterior interosseous branch of the median nerve innervates the FDP to the index and occasionally the long finger. The ulnar nerve innervates the FDP to the ring and small fingers.
The flexor digitorum superficialis (FDS) originates from two separate heads. The humeroulnar head arises from the medial humeral epicondyle and the coronoid process of the ulna. The radial head arises from the proximal shaft at the radius. In the proximal forearm, it occupies the intermediate layer of the flexor compartment superficial to the flexor profundus. In the middle third of the forearm, four separate muscles are identified. Four distinct tendons are seen in the distal third of the forearm. Presence of the small finger superficialis varies and may be absent in 21% of patients.28 At the carpal tunnel level, the long and ring finger superficialis tendons lie superficial and central to those of the index and small, which lie deeper and more peripheral.
At the level of the MCP joint, the superficialis tendon enters the flexor sheath palmar to the profundus tendon. At the proximal third of the proximal phalanx, the superficialis bifurcates around the profundus tendon. The two slips reunite deep to the profundus tendon at Camper’s chiasm, with 50% of fibers decussating and 50% remaining ipsilateral. The superficialis tendon then inserts through the radial and ulnar slips onto the proximal metaphysis of the middle phalanx. The median nerve provides sole innervation of the superficialis muscle.
The FPL originates from the proximal radius and interosseous membrane. In the proximal third of the forearm, it lies radial to the digital flexors in the flexor compartment’s deep layer. At the carpal tunnel level, the FPL lies on the radial floor. After traversing the carpal tunnel, it enters the palm by emerging between the adductor pollicis and the flexor pollicis brevis (FPB). The FPL is the only tendon that enters the thumb flexor sheath, and it inserts at the proximal palmar base of the distal phalanx. The anterior interosseous branch of the median nerve innervates the FPL.
The digital flexor sheath is a synovium-lined fibro-osseous tunnel. This system holds the flexor tendons in close opposition to the phalanges, ensuring efficient mechanical function in producing digital flexion. The flexor sheath is composed of synovial and retinacular tissue components, each with separate and distinct functions.24 The synovial component of the sheath consists of a visceral, or epitenon, layer that envelops the flexor tendon and a parietal, or outer, layer that lines the walls of the flexor sheath. These two layers are contiguous at the ends of the sheath, creating a double-walled, hollow tube that surrounds the flexor tendons. In the index, long, and ring fingers, the membranous sheath begins at the MCP joint and ends at the distal phalanx. At the thumb and small fingers, the synovial sheath continues proximally into the carpal tunnel as the radial and ulnar bursae, respectively.8,25 The synovial sheath provides a low-friction gliding system and nutrition to the tendon.
The retinacular portion of the sheath is characterized by fibrous bands that overlie the synovial sheath in segmental fashion (Fig. 35-1).8,9,26,27 Thickened transverse bands are termed annular pulleys, and thin flexible areas of criss-crossing fibers are termed cruciate pulleys. Stronger, broader annular pulleys provide mechanical stability to the system, ensuring optimal joint flexion for a given amount of tendon excursion. The more flexible cruciate pulleys permit flexibility to the system. The following pulleys have been identified: the palmar aponeurosis pulley, five annular pulleys, and three cruciate pulleys. The palmar aponeurosis pulley is formed from the transverse fibers9,27 of the palmar aponeurosis. It is located at the beginning of the membranous sheath and is anchored on each side of the sheath by vertical septa that attach to the deep transverse metacarpal ligament. The first annular pulley (A1) arises from the volar plate of the MCP joint. The second annular pulley (A2) arises from the volar aspect of the proximal half of the proximal phalanx. The first cruciate pulley (C1) extends from the A2 pulley to the third annular pulley (A3), which arises from the volar plate of the proximal interphalangeal (PIP) joint. The fourth annular pulley (A4) arises from the middle phalanx and is connected proximally to the A3 pulley by the second cruciate pulley (C2). The fifth annular pulley (A5) arises from the volar plate of the distal interphalangeal (DIP) joint. It is connected proximally to the A4 pulley by the third cruciate pulley (C3). Not all of the elements of the flexor sheath can be identified as described, particularly A3 and A5, which can be indistinct or absent.
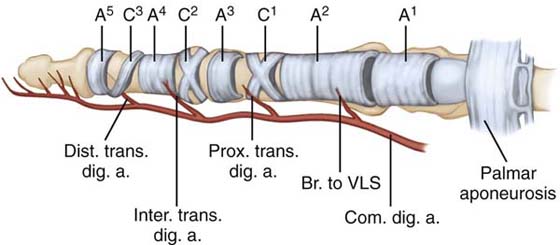
Figure 35-1 Flexor digital retinacular sheath and flexor tendon vascular supply. Br. to VLS; branch to the vinculum longus superficialis; Com. dig. a, common digital artery; Dist. trans. dig. a, distal transverse digital artery; Inter. trans. dig. a., interphalangeal transverse digital artery; Prox. trans. dig. a, proximal transverse digital artery.
The pulley system of the thumb is distinct from that of the digits25 (Fig. 35-2). One oblique and two annular pulleys have been identified. The first annular pulley of the thumb (A1) arises from the palmar plate of the MCP joint, and the second annular pulley (A2) arises from the palmar plate of the interphalangeal (IP) joint. The oblique pulley originates and inserts on the proximal phalanx in close association with the insertion of the adductor pollicis tendon.
Anatomic and clinical studies have demonstrated that the A2 and A4 pulleys are the most important components of the flexor sheath, their presence ensuring biomechanical efficiency of the system.10,11,24 The A3 and the palmar aponeurotic pulleys become important only when A2 and A4 have been damaged.9,11,24,27 The loss of all or portions of the pulley system may lead to flexor bowstringing. This leads to an increased mechanical moment arm, which can create late flexion contractures. In addition, increased flexor tendon excursions are required to produce full digital flexion. In the thumb, the oblique pulley is the most important. Loss of the thumb oblique pulley results in decreased IP joint motion. Incompetence of both the A1 and oblique pulleys of the thumb leads to a 30% loss of IP joint motion.25
Flexor tendon excursion in the retinacular sheath zone in cadaveric specimens has been calculated.29 DIP joint motion produces excursion of the FDP on the FDS of 1 mm for every 10 degrees of DIP flexion. PIP joint motion produces excursion of the FDS and the FDP together of 1.3 mm for every 10 degrees of PIP flexion relative to the retinacular sheath. Recently, postrepair clinical motion studies have demonstrated that this calculated excursion decreases.30 DIP joint motion of 10 degrees produces excursion of the FDP of only 0.3 mm, and PIP motion of 10 degrees produces excursion of the FDP and superficialis of 1.2 mm. This may explain why DIP motion is often suboptimal after flexor tendon repair.
Flexor tendon nutrition appears to occur through both direct vascular supply31-43 (see Fig. 35-1) and synovial diffusion.16,17,27,44-50 Proximal to the digital sheath, a longitudinal blood supply originates from within the proximal muscle tissue and is carried distally through the peritenon. Within the sheath, transverse branches of the digital arteries passing through the vincular system add segmental blood supply.39 These branches include a proximal vessel to the vinculum longus superficialis, a proximal digital transverse artery, an IP transverse digital artery, and a distal transverse digital artery. As the transverse branches pass to the midline, they merge to carry palmarly into the tendons via the vincula. The vinculum profundus brevis is a short triangular pedicle supplying the profundus tendon near its insertion. A similar short vinculum supplies the superficialis tendon at the neck of the proximal phalanx, but here the vessels continue to form the vinculum longus to the profundus tendon. The superficialis receives additional blood supply from the vinculum longus superficialis at the base of the proximal phalanx. Both tendons receive additional blood supply from their distal osseous attachments. Throughout the sheath, vessels enter the tendon from the dorsal surface, with the palmar third remaining relatively avascular.35 This anatomic fact has led to the surgical technique of palmar placement of sutures within the tendon to preserve blood supply. Finally, an avascular watershed zone of the FDP has been identified between the longitudinal and vincular vessels at the midproximal phalanx level.34,35
In addition to nourishment of the flexor tendons by vascular perfusion, experiments have demonstrated the importance of diffusional nutrition by the synovial fluid.16,17,27,44-49 Radioisotope tracer studies suggest a greater role of diffusion than perfusion.16,47 In addition, strong evidence has demonstrated that the superficial layers of isolated segments of tendon can heal in an isolated synovial environment without direct vascularity.8,17,51 This finding has led some authors to recommend sheath repair to restore synovial fluid.52-54 The relative significance of these dual nutritional pathways in the normal and repaired flexor tendon has yet to be completely clarified. Recent studies suggest that synovial diffusion associated with neovascularization of the healing site in the absence of ingrowth of peripheral vessels may play a role in the nourishment of the healing tendon.13
The subject of flexor tendon healing has traditionally been associated with controversy. Two theories have been proposed to help explain observed experimental phenomena. The first, the extrinsic healing theory, suggests that tendon healing occurs through cells extrinsic to the tendon through a fibroblastic response from surrounding tissue.55-60 This theory presupposes the necessity of surrounding peritendinous adhesions to allow complete healing of the tendon; thus, immobilization after flexor tendon repair was encouraged. Experimental clinical evidence of adhesions at the repair site has supported this concept. The sequence of healing by extrinsic mechanism begins with an inflammatory phase from 48 to 72 hours, formation of collagen fibers from 4 to 21 days, and scar remodeling after 21 days.
The second theory, intrinsic healing, suggests that healing is possible in the absence of cells and tissue extrinsic to the tendon.14,31,45,48,61-70 More recent experimental and clinical evidence to support this concept includes rounded ends of unrepaired tendons, tendon healing in the absence of adhesions, and in vitro healing of tendons in isolated, cell-free environments. Controlled mobilization of repaired tendons to allow healing but preventing peritendinous adhesions was the stated advantage of this healing theory. The sequence of intrinsic healing begins with the inflammatory phase, from 0 to 3 days after injury or repair with proliferation and thickening of epitenon cell layers. At 5 to 7 days, collagen formation and early vascular ingrowth ensue. A fibrous callus becomes visible by 10 days, and proliferation ingrowth of endotenon tenocytes occurs at 2 to 3 weeks.
Although the function of each type of tendon healing continues to require clarification, in the clinical situation, tendons probably heal by a combination of extrinsic and intrinsic cellular activity.20,62,71 Theoretically, the more intrinsic healing occurs, the fewer peritendinous adhesions form. This concept forms the basis of controlled mobilization programs after tendon repair.
One must consider the level of injury when performing flexor tendon repair. Five anatomic zones of injury have been identified based on Verdan’s original description of the flexor tendon system7,72-75 (Fig. 35-3). The level of injury should be recorded in relation to the position of tendon laceration in the sheath, with the finger in the extended position.
Zone I extends from the insertion of the FDS at the middle phalanx to that of the FDP at the distal phalanx. Injuries in this level may involve lacerations or avulsions of the FDP. Zone II involves that region in which both the FDS and FDP travel within the flexor sheath from the A1 pulley to the insertion of the FDS. This zone was termed “no man’s land” by Bunnell because of the poor prognosis associated with treatment of flexor tendon injuries at this level.1 A more descriptive term may be “some man’s land” because the more experienced hand surgeon can obtain satisfactory results with appropriate care. Zone III comprises the area between the distal border of the carpal tunnel and the A1 pulley of the flexor sheath. In addition to the common digital nerves, vessels, and both flexor tendons, the lumbrical muscles reside in this zone. Zone IV consists of that segment of flexor tendons covered by the transverse carpal ligament within the carpal tunnel. Injuries concomitant to the median and ulnar nerves may be associated with flexor tendon injuries in this zone. Zone V extends from the flexor musculotendinous junction in the forearm to the proximal border of the transverse carpal ligament. Associated neurovascular injuries may compromise results in this region as well.
The flexor tendon system in the thumb is predicated on only one flexor tendon. Zone I is at the insertion area of the FPL. Zone II coincides with the flexor retinaculum of the thumb, from the neck of the metacarpal to the neck of the proximal phalanx. Zone III is the area of the thenar muscles. Zone IV represents the area of the carpal canal. Finally, zone V is the anatomic area from the musculotendinous junction of the FPL to the transverse carpal ligament.
Knowledge of flexor tendon anatomy is necessary for diagnosing acute injury accurately. In the cooperative patient, diagnosis is usually not difficult. Because of the common muscle origin of the flexor profundi, FDS function can be assessed only by restraining the profundi by completely extending the other digits. An independently functioning superficialis is demonstrated by full flexion of the PIP joint of the affected finger. This test often is not applicable to the index finger because of the independent muscle belly of the FDP. The FDS to the index finger can be demonstrated through pulp-to-pulp pinch with the thumb and index finger.
Demonstration of index finger PIP joint flexion with the DIP joint fully extended or hyperextended confirms superficialis function to the index finger. As noted earlier, the presence of small-finger FDS varies and can be absent in 21% of patients.28 FDP function is demonstrated by means of active flexion of the DIP. Active flexion of the thumb IP joint indicates an intact FPL. If, when performing these tests, the patient demonstrates motion but experiences pain, the surgeon must entertain the possibility of partial flexor tendon injury.
In the uncooperative or unconscious patient or a child, additional diagnostic signs may be helpful. In the normal hand, a cascade of flexion of the digits is noted, increasing as one proceeds from the index to the small fingers. Abnormal posture or change in the normal cascade can indicate flexor tendon injury (Fig. 35-4). In addition, squeezing the forearm musculature to demonstrate flexion of the digits may be helpful. Finally, assessing tenodesis of flexor tendons with the wrist in extension demonstrates loss of finger flexion if flexor tendons are severed.
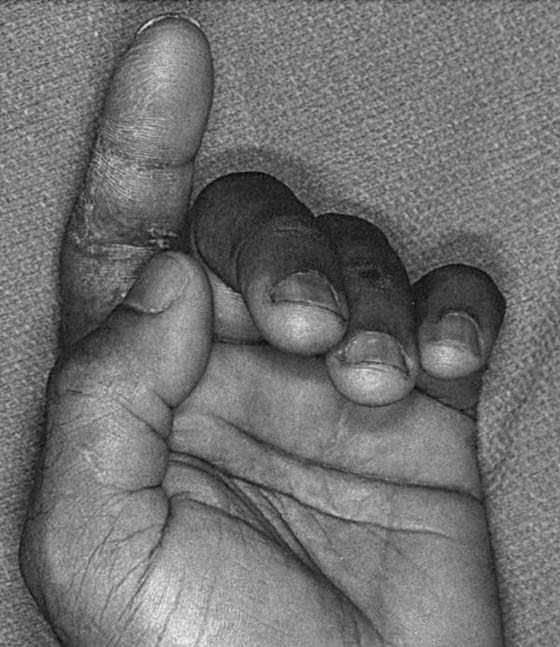
Figure 35-4 Laceration of the index finger. Posture of the index finger in extension suggests complete laceration of the flexor digitorum profundus and superficialis.
The examiner is responsible for determining that the flexor tendons are intact before discharging the patient. If the examiner is uncertain, exploration of the wound under operating room conditions may be required. The actual level of tendon laceration depends on the position of the fingers when the injury occurred. If the injury occurred with the finger in extension, the skin wound and both tendons will be lacerated at the same level. In fingers injured in flexion, the tendon injury will be distal to the skin wound. In addition, the FDP will be lacerated at a level different from that of the superficialis tendon because of their different excursions.
There has been controversy in the past over the efficacy of primary repair of flexor tendons, particularly in zone II,1,2,68,76 yet immediate or delayed primary repair is currently advocated for flexor tendon injuries with few exceptions.3-7,77-90 The advantages of primary repair over secondary grafting include less extensive surgery, decreased periods of disability, and restoration of normal tendon length.74
Specific contraindications to immediate or delayed primary repair include severe contamination where infection is a possibility.3,5,85,87-89 In addition, loss of palmar skin overlying the flexor system generally precludes tendon repair,3,5 although there have been some recent reports of concomitant tendon repair and soft tissue coverage procedures.91 Another contraindication to primary repair is extensive damage to the flexor retinaculum, where pulley reconstruction and one- or two-stage tendon reconstruction probably is required.92 Concurrent fracture or neurovascular injury, however, does not necessarily contraindicate tendon repair. If fracture stabilization can be obtained, then flexor tendon repair generally should ensue.
Researchers have effectively demonstrated that repair of both the FDP and the FDS rather than the FDP alone, even in zone II injury, provides the best result.7,75,77,78,93 The advantages of repairing and maintaining the FDS include maintenance of vincular blood supply to the FDP, retaining of a smooth gliding surface for the FDP, independent motion of the digit with stronger flexion power, and decreased possibility of hyperextension deformities of the PIP joint.
Schneider and colleagues94 demonstrated that tendon repairs delayed as long as 3 weeks after injury exhibited outcomes similar to those of tendons repaired more immediately. Although not statistically significant, repairs performed within the first 10 days after injury tended to be superior. Recent animal studies also have supported improved tendon excursion with early repairs.95 These studies demonstrate that, although tendon repair is not emergent, repair within the first few days after injury appears warranted.
Flexor tendon repair is ideaally performed by trained surgeons who know the anatomy of the flexor tendon system and the potential pitfalls of surgical repair. Repair is generally accomplished under regional or general anesthesia in a bloodless field. Traditionally, a tourniquet has been used. Recently, Lalonde and coworkers reported using elective injection of lidocaine with epinephrine into the operative field in the office setting. This “wide awake” technique creates a relatively bloodless field, without the need for a tourniquet, allowing use of local anesthetic only. They report the risk of digital infarction is remote, with the benefit of observing the repair with the patient’s active movement.96 Despite the appeal of performing surgery in the office, I have no experience with this technique.
The use of 2- to 4-power loupe magnification decreases inadvertent nerve or vessel injury. Lateral or palmar zigzag (Bruner’s) incisions are performed, depending on the surgeon’s preference. The Bruner incision, our preferred approach, offers excellent exposure but can cause scarring over the palmar surface of the digit.97,98 The midlateral incision is technically more demanding and may interfere with transverse digital branches supplying the vincula but has the advantage of decreased scarring over the flexor surface, which can lead to improved rehabilitation.78 Delicate use of instrumentation is required; pinching or crushing of the flexor tendon or sheath inevitably leads to suboptimal results.1,2 We generally prefer to handle the tendon at its cut end only, avoiding grasping of the epitenon, which can create later epitendinous adhesions. Minimal debridement of tendon ends is not usually required but may be performed if necessary using a knife or nerve-cutting instrument. Many suture techniques have been described.99-105 Traditionally, many surgeons have preferred a modified Kessler grasping-type two-strand core stitch based on the studies by Urbaniak and others.106-108 Although 3-0 or 4-0 braided synthetic material placed in the volar third of the tendon has been the most popular core suture material, I prefer the more recently developed 4-0 FiberWire (Arthrex, Naples, FL) because it is stronger than braided synthetic suture of the same caliber and not too bulky when knotted. Most authors currently recommend a four-strand core repair, crossing the repair site with epitendinous suture augmentation to afford sufficient strength to support a program of early active motion rehabilitation. A variety of multistrand core suture designs have been described, ranging from four to eight strands crossing the repair site. My preferred technique uses a single suture to create a four-strand repair as first described by Seiler (Fig. 35-5). A four-strand cruciate flexor repair has been described by McLarney and colleagues109 and is similar to my preferred suture technique (Fig. 35-6). Taras found that increasing suture caliber significantly increased the load to failure of core suture techniques. With 5-0 or 4-0 suture, the method of failure was suture rupture. With 3-0 and 2-0 suture, the failure was caused by suture pullout, clearly showing that repair technique with the modified Bunnell and double-grasping suture techniques securing the ends better than the modified Kessler technique.110 Recent studies have demonstrated that epitendinous suture significantly increases the repair strength.104,111-114 The epitendinous technique described by Silfverskiöld (Fig 35-7), employing a cross stitch has shown excellent results in reducing gapping by 10% to 50% at the repair site.115 The superficialis tendon is preferably repaired with a four-strand core suture depending on the size of the tendon and the location of the laceration. A recent study by Tran and associates found that in vitro a four-strand Tajima technique with a Silfverskiöld epitendinous cross stitch was superior to a four-strand Tajima core suture with running locking epitendinous technique and capable of handling the rigors of an early active rehabilitation protocol.116
Several techniques are available to atraumatically retrieve retracted tendon ends. With the wrist and MCP joints placed in maximal flexion, flexor muscle bellies can be milked manually to deliver the tendon ends. If this fails, alternatives are available. If a tendon end is visible in the flexor sheath, one may use a skin hook. The hooked end is slid along the surface of the sheath until it has passed the tendons, and the hook is then turned toward the tendons, engaging the most superficial one. As the instrument is pulled distally, the tendons follow. The tendons then can be held in position by a Keith or 25-gauge hypodermic needle placed through the skin and A2 pulley area for later repair.
Another common retrieval technique uses a red rubber urologic catheter, pediatric feeding tube, Hunter rod, or IV tubing, which is passed distal to proximal alongside the flexor tendons, which are left in situ. The catheter is sutured to both tendons 2 cm proximal to the A1 pulley through a second palmar incision. The catheter is advanced distally to deliver tendon ends into the repair site. A 25-gauge hypodermic needle is passed transversely through the skin and A2 pulley to maintain tendon position. Core sutures are then placed. The catheter tendon suture is then cut in the palm and withdrawn.117
Another often-used technique uses a catheter that is passed from distal to proximal through the flexor sheath. A stitch is placed into the tendon end, and the other end of the suture is placed into the end of the catheter. The catheter and suture, followed by the tendons, are pulled distally through the flexor sheath. Further retraction of tendons is prevented again by transfixing them to the skin and A2 pulley with a 25-gauge hypodermic needle.
For all of these techniques, the anatomic relationship of the profundus to the superficialis tendons must be maintained.53 One anatomic point that can aid the surgeon in maintaining this orientation and preventing tendon twisting is the fact that the vincula insert on the dorsal surface of the tendons.
Every attempt is made to preserve all pulleys of the flexor retinacular sheath. Small surgical windows into the sheath often are required to identify tendon ends. Whether the sheath should be subsequently repaired is controversial.19,23,31,41,54,118-120 Theoretical support for repair includes tendon gliding and nutrition. To date, no clinical studies have documented superiority of repair versus resection of the sheath.31,54 In addition, one prospective study comparing the two techniques demonstrated no superiority of sheath repair.119 Currently, we favor sheath repair when possible using 6-0 or 7-0 monofilament suture if there has been minimal loss of sheath substance and the repair can be performed easily without constriction of the sheath or the tendon repair.
Recently, a stainless steel device, TenoFix (Ortheon Medical, Columbus, OH) has been introduced for zone II flexor tendon repair. The authors reported similar results to a four-stranded core repair; however, smaller profundus tendons were unable to accommodate the device.121
In zone I, distal to the FDS insertion, only the FDP tendon is injured. The patient maintains PIP joint flexion but loses DIP flexion. Although adequate finger function can be maintained without repair in some circumstances, early direct repair is desirable. This is particularly true as one proceeds from the radial (precision grip) to the ulnar (power grip) side of the hand. In addition, the small finger superficialis is absent in a significant percentage of individuals, necessitating repair of the lacerated FDP tendon in that digit. With early repair, digital function can be near normal.
If more than 4 weeks have elapsed since the injury, direct repair usually cannot be performed due to FDP muscle contracture and degeneration of the lacerated ends of the tendon. Another pitfall in zone I is injury to the normally functioning FDS caused by excessive surgical manipulation. Finally, the surgeon must not advance the profundus tendon more than 1 cm at this level of injury to accomplish repair.122 This leads, particularly in the ulnar three digits, to unacceptable flexion contractures of the affected digit as well as incomplete flexion of the neighboring digits—a condition termed quadrigia syndrome.73
Three patterns of laceration occur in zone I. The first occurs when the short vincula of the FDP remain intact. Although this pattern is rare, the FDP remains just proximal to its insertion. Direct repairs can be performed late with this pattern of injury. The second pattern presents with the long vincula of the FDP intact. The severed end of the tendon lies at the FDS decussation. The prognosis for early repair is good. The third laceration pattern occurs when the FDP retracts into the palm, rupturing both vincula and resulting in loss of vincular blood supply. This type of repair requires more extensive surgical dissection and early repair. When this pattern of injury is diagnosed late, alternative treatments such as observation, tendon graft, DIP tenodesis, and arthrodesis must be entertained.
A volar zigzag incision from the PIP joint crease to distal to the DIP joint is utilized. Every effort is made to preserve the A4 pulley. If only a short distal stump of FDP remains, opening the tendon sheath at the C3-A5 pulley level may be required. If the proximal FDP stump has retracted to the level of the PIP joint, a window at the C2 pulley may be fashioned to retrieve it. If the proximal profundus has retracted to the level of the palm, the zigzag incision may be extended proximally, or a separate transverse incision in the palm proximal to the A1 pulley may be required. Retracted proximal FDP tendon ends are retrieved atraumatically as described previously. If the injury is recent, the FDP may be passed through the FDS decussation. In older lacerations, one slip of the superficialis may be sacrificed to facilitate passage of the FDP through a tight flexor sheath. Under no circumstances should a normal FDS be completely excised to repair the FDP.
Every effort is made to repair to the distal tendon stump to avoid overadvancement. As previously discussed, the FDP is not advanced more than 1 cm to permit repair. If the repair catches at the end of the A4 pulley, a small portion of the sheath may be vented.
If the distal stump is extremely short or nonexistent, the tendon must be repaired to the distal phalanx (Fig. 35-8). One develops a periosteal flap at the base of the distal phalanx, carefully avoiding the palmar plate of the DIP joint. The cortex is prepared with a curette to provide a bleeding bone surface that will encourage tendon-to-bone healing. A synthetic monofilament suture, such as 2-0 Prolene, is inserted into the tendon in “unlocked” fashion to facilitate later removal. The ends of the suture are threaded onto a Keith needle. Regardless of whether a short FDP stump is present, I fasten the sutures using an “around-the-bone” technique. With the use of a needle holder, the Keith needles are passed around both sides of the distal phalanx to emerge through the middle third of the nail plate. Effort should be made to avoid the germinal nail matrix; the ideal point of exit through the nail plate should be 3 to 4 mm distal to the lunula and approximately 2 mm from the midline. The Keith needles are withdrawn, and the sutures are tied directly over the nail. The pullout suture is removed 6 weeks after initial repair. Alternatively, a bone anchor is reported to have been used at the distal phalanx level for this injury. The length of the bone anchor must avoid the nail bed. The use of a bone anchor technique has been reported to make no significant difference in outcome versus pullout suture technique without the morbidity of this method.123
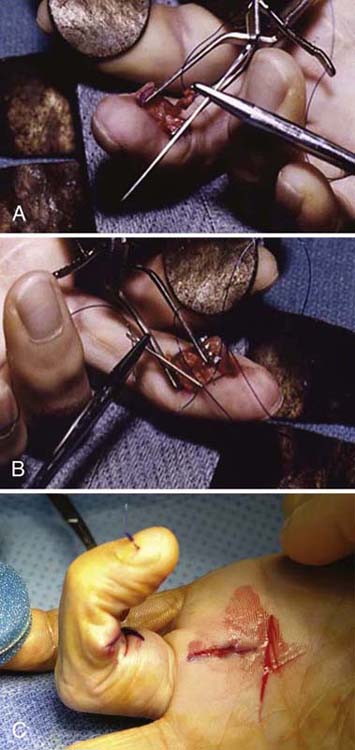
Figure 35-8 Reinsertion technique for flexor digitorum profundus avulsion at distal phalanx in zone I injury.
Classically termed “no man’s land,” injuries at zone II are associated with the greatest technical difficulties in obtaining maximal function. Atraumatic technique is nowhere more important than in zone II, where both flexor tendons are confined within the flexor retinacular sheath. Early repair of both the FDP and FDS is indicated when wound conditions permit.
Preferred Technique. The area is exposed with a volar zigzag or midlateral incision incorporating the laceration. One identifies the flexor sheath, carefully protecting the neurovascular bundles. If nerve injury is identified, the nerve is prepared for repair after repair of the flexor tendons. Every effort must be made to maintain the annular pulleys, especially some portion of the A2 and A4 pulleys. If possible, repair should be made through windows in the cruciate–synovial areas through small lateral or transverse incisions. The distal profundus and superficialis tendons usually can be identified and delivered into the wound by acutely flexing the digit. The proximal tendons are atraumatically identified and delivered into the wound by one of the methods previously described. When retrieving the proximal tendons, one should maintain the superficialis and profundus tendons together so as not to interrupt their vascular connections. In zone II, the surgeon must understand the spiral nature of the FDS as it bifurcates around the profundus to reinsert on the middle phalanx at Camper’s chiasm. If the superficialis is lacerated after its bifurcation, proximal and distal ends can rotate 180 degrees in different directions. If this anatomic orientation is not corrected, then profundus tendon excursion can be less than optimal.
After the tendons are delivered into the wound, a Keith or 25-gauge hypodermic needle is placed across the skin and annular pulley to maintain tendon position. A 3-0 or 4-0 core suture is placed in a four-strand single-cross grasp as described by Seiler and Noguchi et al.124 Although traditionally the suture has been placed volarly in the tendon to avoid the blood supply, recent studies show some biomechanical benefit to dorsal placement.125,126 The core suture is then tied with the tension being removed from the repair by flexing the distal joint of the digit. Then a 6-0 Prolene suture is placed in an epitendinous cross-stitch fashion as described by Silfverskiöld. “If the superficialis is lacerated distal to its birfurcation, two-strand locking sutures in each limb usually suffice. If the superficialis is lacerated proximal to the bifurcation, I prefer a four-strand core suture. Every attempt should be made to repair the superficialis tendons. However, if the superficialis tendon is too bulky to glide under the A2 pulley, we recommend resection of one slip of the superficialis tendon rather than resecting the A2 pulley.” The flexor retinaculum is repaired with 6-0 or 7-0 Prolene if possible. If this cannot be achieved and a bulky repair is snagging on a pulley, a portion of the pulley should be vented with a lateral release to allow unimpaired motion after surgery. Finally, at the proximal level of the flexor sheath, one may consider converting a zone II to a zone III injury by excising the A1 pulley. This is particularly appropriate if a bulky repair is performed at the A1 pulley level. If pulleys require reconstruction, we currently favor staged tendon reconstruction at this level.
Injuries in zone III carry a good prognosis because zone III is located out of the fibro-osseous sheath and is therefore less prone to adhesion formation. Injuries to the common digital nerves may accompany the tendon injury. Both superficialis and profundus tendons should be repaired. Delayed primary repair can be performed up to 3 weeks or more after injury because the proximal end of the profundus tendon is held by the lumbrical origin.
The zigzag approach to the palm is used for surgical repairs in zone III. Excision of local palmar fascia may be necessary for exposure. Careful protection of associated neurovascular structures is essential. Repair of both tendons with 3-0 or 4-0 core suture and a 6-0 epitendinous Prolene suture is standard.
Flexor tendon injuries in zone IV (within the carpal tunnel) are less common because of the anatomic protection of the medial and lateral bony pillars as well as the stout transverse carpal ligament. Injuries to the median and ulnar nerves, ulnar artery, and superficial palmar arch are often associated because of their proximity to the flexor tendons. If possible, repair of all tendons should be undertaken. If swelling precludes repair of all tendons, the superficialis to the small finger can be excised. If anatomic confines preclude even further repair, all the superficialis tendons can be excised as necessary. Delayed primary repair should be performed within 3 weeks of injury because myostatic contraction prevents repair after this.
Zone IV injury is approached through a volar zigzag incision, incorporating a carpal tunnel–type incision. One may consider a Z-plasty lengthening of the transverse carpal ligament for later repair, although some authors have not found bowstringing to be a problem if an appropriate postoperative orthosis is used with the wrist in neutral position.78 If the motor branch of the ulnar nerve is lacerated, one should consider repairing it first because it is the deepest structure requiring repair in the wound. All zone IV flexor tendons are repaired with four strands of 4-0 FiberWire core suture. Frequently, swollen or hemorrhagic synovium must be resected to allow repair. Closing the transverse carpal ligament without Z-plasty lengthening is not wise because it may constrict the median nerve. After surgery, a controlled mobilization program with the wrist near neutral position and the MCP joints flexed to 70 degrees is maintained to prevent bowstringing and avoid placing stress on nerve repairs.
Deep forearm lacerations proximal to the transverse carpal ligament typically involve multiple structures, including tendons, median and ulnar nerves, and the radial and ulnar arteries. Primary repair of all structures is recommended. Return of satisfactory motion is the rule in this zone, but it may take several months.
A zigzag or curvilinear skin incision is used to expose the injured structures. Depending on the level of laceration, a carpal tunnel release may be performed. A four-strand core repair with 4-0 FiberWire or 3-0 Ethibond is favored based on the caliber of the tendons at this level. A surgical caveat in this zone applies; if multiple tendons and nerves are lacerated, it is prudent to identify and tag structures as one progresses from superficial to deep. This organization averts the mistake of mismatching proximal and distal tendon ends, because the proximal tendon ends can lose their normal anatomic alignment as the dissection is performed. Circumferential epitendinous suture can be performed if time permits, although it is not necessary if multiple tendons require repair during a long procedure.
The anatomic differences between the FPL and the digital tendons and sheath have been described. The FPL spans only two digital joints and travels alone in its flexor sheath. These factors may explain improved results in FPL repair.127-129 The FPL has only one vinculum and no associated lumbrical muscles; therefore, FPL lacerations in zones I and II are more likely to retract proximally to the palm or wrist level. Direct repair of the FPL is recommended at all levels as wound conditions permit if no more than 3 or 4 weeks have elapsed since the injury.
Lacerations in zones I and II of the FPL are approached in a volar zigzag incision. The flexor sheath is identified, and windows are made for identification of the tendon ends as required. The A1 or oblique pulley must be maintained to prevent late bowstringing.
If the tendon is lacerated at its insertion, a pullout suture to the distal phalanx, as previously described, is used. More proximally in the thumb, if the proximal tendon end is easily identified, it is usually still being held by an intact vinculum. If the proximal tendon is not identified in the thumb, it is retracted into the palm or wrist. We prefer not to explore the carpal tunnel or thenar eminence to retrieve the proximal end for risk of damaging sensory or motor branches of the thumb; rather, a separate transverse or curvilinear incision in the distal forearm is used to identify the proximal tendon. Passing a tube or catheter from the distal incision to the proximal one, using one of the techniques described earlier, can atraumatically retrieve the tendon. Standard end-to-end repair is accomplished with an additional epitendon suture.
The treatment of partial flexor tendon lacerations is controversial.130-139 Early experimental studies demonstrated that if more than 60% of a tendon is transected, rupture is likely under applied stress.133 Other reported complications of untreated partial flexor tendon lacerations include triggering, rupture, and entrapment.131,134 On the other hand, several researchers have noted that placement of core sutures into a largely intact tendon weakens the tendon, increasing the likelihood of tendon rupture.136,138 More recently, researchers have demonstrated that early protected mobilization after partial tendon laceration demonstrated improved tensile strengths. In addition, they recommended repair of partial flexor tendon lacerations when more than 60% of the width of the tendon is lacerated.140-142
A volar zigzag approach is used to expose tendon injury, and the partial laceration is assessed. It has been our practice, based on the available data, to repair partial lacerations greater than 60% of the tendon’s substance with a modified Kessler core suture followed by an epitendinous 6-0 suture. Lacerations of 25% to 60% of the tendon are repaired with a 6-0 running epitendinous suture. Partial lacerations of less than 25% of the tendon’s diameter are treated by minimal handling of the tendon and beveling of sharp edges to prevent catching. A dynamic controlled mobilization program is used postoperatively.
Rupture of the FDP is caused by forced extension at the DIP joint while the profundus muscle is maximally contracting. The injury commonly occurs in football or rugby players as they grab an opponent’s jersey. The patient describes pain and swelling over the area of distal avulsion. Because the superficialis still provides function to the PIP joint, the injury is often missed, losing the opportunity for early repair. Radiographs should always be obtained because, occasionally, a fragment of distal bone is also avulsed, which can localize the profundus tendon end.
Although any digit, including the thumb, has been reported to be involved, this injury most often involves the ring finger. Several theories have been advanced to explain the propensity for ring finger involvement, including a lack of independent extension of the ring finger because of its junctura tendinae and another demonstrating that the ring finger has the weakest profundus insertion.143
Avulsion of the profundus tendon has been classified by Leddy78 and Leddy and Packer144 into three main types. In type I, the tendon retracts into the palm, where a substantial blood supply is lost because of vincular rupture. Tendon repair must be performed early, within 7 to 10 days, before it becomes contracted. In type II avulsions, the tendon retracts to the PIP joint. The vincula are intact, and some synovial nutrition remains. Although early repair is recommended, repair up to 3 months after injury can result in satisfactory results. Type III injuries involve a large bone fragment. The A4 pulley prevents retraction of the tendon. Early reinsertion or, with large fragments, open reduction and internal fixation (ORIF) provide satisfactory results. Finally, type IIIA injuries describe a simultaneous avulsion of the profundus tendon and a fracture fragment distally.78,145,146 This injury requires ORIF of the bony fragment and reinsertion of the avulsed tendon.
If a case of flexor profundus avulsion is missed and left untreated beyond the period of repair, options for treatment include observation, tendon grafting, a DIP joint tenodesis, or arthrodesis.78
A palmar zigzag or midlateral incision is used, exposing the flexor sheath just proximal to the PIP joint to the insertion of the profundus tendon. For a type I injury, a transverse window is made just distal to the A2 pulley. If the tendon is not seen, it has retracted into the palm, and an additional incision is required in the palm proximal to the A1 pulley. The proximal tendon is atraumatically passed through the sheath and the superficialis decussation by one of the methods described previously. It is then reinserted to the distal phalanx with 3-0 nonlocking Prolene suture tied over a button on the nail plate distal to the germinal matrix. For type II injuries, the tendon end is noted at the incision just distal to the A2 pulley, held by its vincula. No palmar incision is required. The tendon is reinserted distally as described. For type III or type IIIA incisions, the bony fragment is noted just distal to the A4 pulley. A transverse sheath incision distal to the A2 pulley is not required. ORIF of the bony fragment usually provides satisfactory results. In the case of a IIIA injury, after ORIF of the bone fragment, the distal tendon is reinserted as described previously.
Adhesion is the most common complication of flexor tendon repair. The techniques employed in postoperative orthosis use and tendon rehabilitation contribute significantly to the functional outcome of flexor tendon repair. Historically, flexor tendon rehabilitation programs have evolved from postoperative protective orthotic immobilization to protect the repair and promote healing to controlled tendon activity to restore tendon tensile strength and promote tendon gliding. The reader is referred to Chapter 36 for a comprehensive discussion of postoperative tendon management.
It is currently generally accepted that a flexor tendon rehabilitation regimen depends on the strength of the surgical repair. Therapists are encouraged to contact the treating surgeon and obtain surgical reports, whenever possible, so that a tendon rehabilitation program formulated on the specific strength of the tendon repair can be instituted. In all cases, orthosis use and therapy should begin as early in the postoperative period as possible. The therapist should bear in mind that edema greatly increases the stress placed on the repaired tendon through movement and that premorbid conditions such as diabetes can alter individual tolerances for force placed on the postoperative tendon. If the repair technique is unknown to the therapist, it is advisable to institute a traditional protected range of motion (ROM) tendon program.
Recently, we moved to the use of the pyramid model of tendon rehabilitation described by Groth147 and Steelman and coworkers148 for our patient population. No timelines are used to introduce activity according to this program. Instead, this program requires assessment of tissue response during each therapy session so that the patient can progress through the program as tissue response warrants. The program begins at postoperative day two or three. The patient is fitted with an orthosis like that described for the two previous flexor tendon rehabilitation programs discussed earlier. Therapy interventions begin at the base of the pyramid and progress upward to the apex. The major tissue response utilized to determine when the patient can progress through the steps of the pyramid is an active joint lag, which is defined as passive joint ROM that exceeds active joint ROM. When the ROM lag does not respond to treatment, the patient progresses up the pyramid (Fig. 35-9), one step per treatment session, until the desired treatment effect is achieved. When adverse reactions such as swelling or inflammation occur, the patient is backed down the pyramid accordingly. Patients demonstrating no active lag and acceptable ROM may be discharged, never progressing past active composite fisting. Conversely, a patient with significant scar adhesion may progress up the pyramid to isolated joint motion within a few treatment sessions. We have had excellent outcomes and few adverse reactions in our flexor tendon repair population using the pyramid model. It is an excellent tool to guide clinical decision making in flexor tendon rehabilitation for the experienced hand therapist.
Despite the use of stronger suture repair techniques and the application of early controlled mobilization, adhesion formation remains the most common complication after flexor tendon surgery. Surgical tenolysis is the preferred treatment if an appropriate period of therapy (3–6 months) has failed to restore tendon gliding.71,149,150
Tendon rupture after primary repair is uncommon. Although rupture can occur as late as 8 weeks after surgery, in my experience it is usually noted around the seventh to tenth postoperative day. Early reexploration and repair is the usual treatment of choice for tendon rupture.78,151,152 If the FDP is advanced and repaired greater than 1 cm, quadrigia syndrome can develop.73,121 This is based on the limited proximal excursion of the remaining FDP tendons, which have a common muscle belly. A flexion contracture of the affected digit is noted, and the patient often complains of a weak grasp because of lack of full motion of surrounding digits. Other less common complications include pulley failure and bowstringing, triggering, and swan-neck deformity.153
Early active mobilization or controlled active motion is now established as a reliable method for the postoperative management of flexor tendon repairs.75,114,154 Several factors must be considered when these protocols are used in clinical practice. The tensile strength of the suture material must be adequate, and the design of the suture must enable it to withstand active mobilization without rupture or gapping. Several recent studies have addressed these issues.102,155-165 It is clear from these studies that tendon repair strength is roughly proportional to the number of strands that cross the repair site.166 Multiple-core suture designs have been described. It is believed that a core repair of at least four strands in conjunction with an epitendinous suture is required to withstand the rigors of an early active motion protocol. This knowledge must be tempered by the fact that the work of flexion is also proportional to the amount of suture material present at the repair site.167 The epitendinous stitch has assumed an increasingly important role both in ultimate strength and prevention of gap formation after flexor tendon repair.104,110-113
Current themes in flexor tendon research include understanding the healing process from the cellular, molecular, and genetic levels. Studies using mesenchymal stem cells on a scaffold to bridge tendon gaps have shown some promise.168,169 Before the era of molecular medicine, researchers had attempted to modify adhesion formation with mechanical barriers, such as silicone, hydroxyapatite, and polyethylene, between the healing tendon and sheath. Others have tried chemical means, including corticosteroids, ibuprofen, 5-flourouracil, and hyaluronic acid; however, these media have not been adopted in clinical practice. Molecular techniques targeting cytokines such as transforming growth factor beta (TGF-β), which has been implicated in scar formation after repair, have shown promise in reducing adhesions and increasing ROM.170 Other prospects include introducing a bone morphogenic protein (BMP-13) to improve tendon healing.167
1. Bunnell S. Repair of tendons in the fingers and description of two new instruments. Surg Gynecol Obstet. 1918;26:103–110.
2. Bunnell S. Repair of tendons in the fingers. Surg Gynecol Obstet. 1922;35:88–98.
3. Kleinert HE, Cash SL. Management of acute flexor tendon injuries in the hand. Instr Course Lect. 1985;34:361–372.
4. Kleinert HE, Kutz JE, Cohen MJ. Primary repair of zone 2 flexor tendon lacerations. In: American Academy of Orthopaedic Surgeons: Symposium on Tendon Surgery in the Hand. St Louis: Mosby; 1975.
5. Kleinert HE, Schepels S, Gill T. Flexor tendon injuries. Surg Clin North Am. 1981;61:267–268.
6. Kleinert HE, Kutz JE, Ashbell TS, Martinez E. Primary repair of flexor tendons in “no man’s land.”. J Bone Joint Surg. 1967;49A:577–578.
7. Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am. 1973;4:865–876.
8. Doyle JR. Anatomy of the flexor tendon sheath and pulley system. J Hand Surg. 1988;13A:473–484.
9. Doyle JR. Anatomy and function of the palmar aponeurosis pulley. J Hand Surg. 1990;15A:78–82.
10. Idler RS. Anatomy and biomechanics of the digital flexor tendons. Hand Clin. 1985;1:3–11.
11. Idler RS, Strickland JW. The effects of pulley resection on the biomechanics of the proximal interphalangeal joint. Univ Pa Orthop J. 1986;2:20–23.
12. Schuind F, Garcis-Elias M, Cooney WP 3rd, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg. 1992;17A:291–298.
13. Gelberman RH, Khabie V, Cahill CJ. The revascularization of healing flexor tendons in the digital sheath. J Bone Joint Surg. 1991;73A:868–881.
14. Gelberman RH, Vandeberg JS, Lundborg GN, et al. Flexor tendon healing and restoration of the gliding surface: an ultrastructural study in dogs. J Bone Joint Surg. 1983;65A:70–80.
15. Gelberman RH, Steinberg D, Amiel D, Akeson W. Fibroblast chemotaxis after tendon repair. J Hand Surg. 1991;16A:686–693.
16. Manske PR, Lesker PA. Biochemical evidence of flexor tendon participation in the repair process: an in vitro study. J Hand Surg. 1984;9B:117–120.
17. Manske PR, Lesker PA. Histologic evidence of intrinsic flexor tendon repair in various experimental animals: an in vitro study. Clin Orthop Relat Res. 1984;182:297–304.
18. Seradge H. Elongation of the repair configuration following tendon repair. J Hand Surg. 1983;8:182–185.
19. Gelberman RH, Woo SL, Amiel D, et al. Influences of flexor sheath continuity and early motion on tendon healing in dogs. J Hand Surg. 1990;15A:69–77.
20. Ketchum LD. Effects of triamcinolone on tendon healing and function. Plast Reconstr Surg. 1971;47:471–482.
21. Kulick MI, Smith S, Hadler K. Oral ibuprofen: evaluation of its effect on peritendinous adhesions and the breaking strength of a tenorrhaphy. J Hand Surg. 1986;11:110–120.
22. Kulick MI, Brazlow R, Smith S, Hentz VR. Injectable ibuprofen: preliminary evaluation of its ability to decrease peritendinous adhesions. Ann Plast Surg. 1984;13:459–467.
23. Peterson WW, Manske PR, Dunlap J, et al. Effect of various methods of restoring flexor sheath integrity on the formation of adhesions after tendon injury. J Hand Surg. 1990;15A:48–56.
24. Doyle RF, Blythe W. The finger flexor tendon sheath and pulleys: anatomy and reconstruction. In: American Academy of Orthopaedic Surgeons: Symposium on Tendon Surgery in the Hand. St Louis: Mosby; 1975.
25. Doyle RF, Blythe W. Anatomy of the flexor tendon sheath and pulleys of the thumb. J Hand Surg. 1977;2:149–151.
26. Hunter JM, Cook JF, Ochiai N, et al. The pulley system. J Hand Surg. 1980;5:283–289.
27. Manske PR, Lesker PA. Palmar aponeurosis pulley. J Hand Surg. 1983;8:259–263.
28. Austin GJ, Leslie BM, Ruby LK. Variations of the flexor digitorum superficialis of the small finger. J Hand Surg. 1989;14A:262–267.
29. McGrouther DA, Ahmed MR. Flexor tendon excursions in “no-man’s land.”. Hand. 1981;13:129–141.
30. Silfverskiöld KL, May EJ, Tornvall AH. Flexor digitorum profundus tendon excursions during controlled motion after flexor tendon repair in zone II: a prospective clinical study. J Hand Surg. 1992;17A:122–131.
31. Amadio PC, Hunter JM, Jaeger SH, et al. The effect of vincular injury on the results of flexor tendon surgery in zone 2. J Hand Surg. 1985;10A:626–672.
32. Brockis JG. The blood supply of the flexor and extensor tendons of the fingers in man. J Bone Joint Surg. 1953;35B:131–138.
33. Edwards DA. The blood supply and lymphatic drainage of tendons. J Anat. 1946;80:147–152.
34. Hergenroeder PT, Gelberman RH, Akeson WA. The vascularity of the flexor pollicis longus tendon. Clin Orthop Relat Res. 1982;162:298–303.
35. Lundborg G, Myrhage R, Rydevik B. The vascularization of human flexor tendons within the digital synovial sheath region: structural and functional aspects. J Hand Surg. 1977;2:417–427.
36. Mayer L. The physiological method of tendon transplantation. I. Historical: anatomy and physiology of tendons. Surg Gynecol Obstet. 1916;22:182–197.
37. Mayer L. The physiological method of tendon transplantation. II. Operative technique. Surg Gynecol Obstet. 1916;22:298–306.
38. Mayer L. The physiological method of tendon transplantation. III. Experimental and clinical experiences. Surg Gynecol Obstet. 1916;22:472–481.
39. Ochiai N, Matsui T, Miyaji N, et al. Vascular anatomy of flexor tendons: I. Vincular system and blood supply of the profundus tendon in the digital sheath. J Hand Surg. 1979;4:321–330.
40. Peacock EE. A study of the circulation in normal tendons and healing grafts. Ann Surg. 1959;149:415–428.
41. Penington DG. The influence of attendance sheath integrity vincula blood supply on adhesion formation following tendon repairing in hands. Br J Plast Surg. 1939;32:302.
42. Smith JW. Blood supply of tendons. Am J Surg. 1965;109:272–276.
43. Young L, Weeks PM. Profundus tendon blood supply within the digital sheath. Surg Forum Plast Surg. 1971;21:504–506.
44. Eiken O, Lundborg G, Rank F. The role of the digital synovial sheath in tendon grafting. Scand J Plast Reconstr Surg. 1975;9:182–189.
45. Lundborg G, Rank F. Experimental intrinsic healing of flexor tendons based upon synovial fluid nutrition. J Hand Surg. 1978;3:21–31.
46. Manske PR, Lesker PA. Nutrient pathways of flexor tendon in primates. J Hand Surg. 1982;7:436–444.
47. Manske PR, Lesker PA. Diffusion as a nutrient pathway to the flexor tendon. In: Hunter JM, Schneider LH, Mackin EJ, eds. Tendon Surgery in the Hand. St Louis: Mosby; 1987:86–90.
48. Manske PR, Gelberman RH, Vandeberg JS, Lesker PA. Intrinsic flexor-tendon repair. J Bone Joint Surg. 1984;66A:385–396.
49. Matthews P. The pathology of flexor tendon repair. Hand. 1979;11:233–242.
50. McDowell CL, Snyder DM. Tendon healing: an experimental model in the dog. J Hand Surg. 1977;2:122–126.
51. Gelberman RH, Nunley JA 2nd, Osterman AL, et al. Influences of the protected passive mobilization interval on flexor tendon healing. Clin Orthop Relat Res. 1991;264:189–196.
52. Lister GD. Incision and closure of the flexor tendon sheath during primary tendon repair. Hand. 1979;15:123–135.
53. Lister GD. Pitfalls and complications of flexor tendon surgery. Hand Clin. 1985;1:133–146.
54. Lister GD, Tonkin M. The results of primary flexor tendon repair with closure of the tendon sheath. J Hand Surg. 1986;11A:767.
55. Mason ML, Allen HS. The rate of healing of tendons: experimental study of tensile strength. Ann Surg. 1941;113:424–459.
56. Peacock EE. Fundamental aspects of the wound healing relating to the restoration of gliding function after tendon repair. Surg Gynecol Obstet. 1964;119:241–250.
57. Peacock EE. Biological principles in the healing of long tendons. Surg Clin North Am. 1965;45:461–476.
58. Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg. 1962;44A:49–64.
59. Potenza AD. The healing of autogenous tendon grafts within the flexor digital sheath in dogs. J Bone Joint Surg. 1964;46A:1462–1484.
60. Potenza AD. Flexor tendon injuries. Orthop Clin North Am. 1970;1:355–373.
61. Eiken O, Hagberg L, Lundborg G. Evolving biologic concepts as applied to tendon surgery. Clin Plast Surg. 1981;8:1–12.
62. Flynn JE, Graham JH. Healing following tendon suture and tendon transplants. Surg Gynecol Obstet. 1962;360:467–472.
63. Furlow LT. The role of tendon tissues in tendon healing. Plast Reconstr Surg. 1976;57:39–49.
64. Gelberman RH, Woo SL, Lothringer K, et al. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg. 1982;7:170–175.
65. Gelberman RH, Vandeberg JS, Manske PR, Akeson WH. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg. 1985;10A:776–784.
66. Lindsay WK, Thomson HG, Walker FG. Digital flexor tendons: an experimental study. Br J Plast Surg. 1960;13:1–9.
67. Lundborg G. Experimental flexor tendon healing without adhesion formation. Hand. 1976;8:235–238.
68. Mass DP, Tuel RJ. Intrinsic healing of the laceration site in human superficialis flexor tendons in vitro. J Hand Surg. 1991;16A:24–30.
69. Matthews P, Richards H. Factors in the adherence of flexor tendon after repair: an experimental study in the rabbit. J Bone Joint Surg. 1976;58B:230–236.
70. Slattery PG. The modified Kleinert splint in zone II flexor tendon injuries. J Hand Surg. 1988;13B:273–276.
71. Strickland JW. Flexor tendon injuries. Part 2: flexor tendon repair. Orthop Rev. 1986;15:49–68.
72. Verdan C. Primary repair of flexor tendons. J Bone Joint Surg. 1960;42A:647–657.
73. Verdan C. Practical considerations for primary and secondary repair in flexor tendon injuries. Surg Clin North Am. 1964;44:951–970.
74. Verdan C. Primary and secondary repair of flexor and extensor tendon injuries. In: Flynn JE, ed. Hand Surgery. Baltimore: Williams & Wilkins; 1966:251–258.
75. Verdan C. Half a century of flexor-tendon surgery: current status and changing philosophies. J Bone Joint Surg. 1972;54A:472–491.
76. Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Joint Surg. 1960;42A:637–646.
77. Gault DT. A review of repaired flexor tendons. J Hand Surg. 1987;12B:321–325.
78. Leddy JP. Flexor tendons: acute injuries. In: Green DP, ed. Green’s Operative Hand Surgery. New York: Churchill Livingstone; 1993:1823–1852.
79. Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg. 1977;2:441–451.
80. Madsen E. Delayed primary suture of flexor tendons cut in the digital sheath. J Bone Joint Surg. 1970;52B:264–267.
81. McFarlane RM, Lamon R, Jarvis G. Flexor tendon injuries within the finger. J Trauma. 1968;8:987–1003.
82. Nielsen AB, Jensen PO. Primary flexor tendon repair in “no man’s land.”. J Hand Surg. 1984;9B:279–281.
83. Peacock EE, Madden JW, Trier WC. Postoperative recovery of flexor tendon function. Am J Surg. 1971;122:686–692.
84. Schneider LH, Bush DC. Primary care of flexor tendon injuries. Hand Clin. 1989;5:383–394.
85. Schneider LH, McEntee PE. Flexor tendon injuries: treatment of the acute problem. Hand Clin. 1986;2:119–131.
86. Singer M, Maloon S. Flexor tendon injuries: the results of primary repair. J Hand Surg. 1988;13B:269–272.
87. Strickland JW. Management of acute flexor tendon injuries. Orthop Clin North Am. 1983;14:827–849.
88. Strickland JW. Flexor tendon injuries. Part 2: flexor tendon repair. Orthop Rev. 1986;15:49–68.
89. Strickland JW. Biologic rationale, clinical application, and results of early motion following flexor tendon repair. J Hand Ther. 1989;2:71–83.
90. Vahvanen V, Gripenberg L, Nuutinen P. Flexor tendon injury of the hand in children. Scand J Plast Reconstr Surg. 1981;15:43–48.
91. Gault DT, Quaba AA. The role of cross-finger flaps in the primary management of untidy flexor tendon injuries. J Hand Surg. 1988;13B:62–65.
92. Schneider LH, Hunter JM. Flexor tendons: late reconstruction. In: Green DP, ed. Green’s Operative Hand Surgery. New York: Churchill Livingstone; 1983.
93. Ejeskar A. Finger flexion force and hand grip strength after tendon repair. J Hand Surg. 1982;7:61–65.
94. Schneider LH, Hunter JM, Norris TR, Nadeau PO. Delayed flexor tendon repair in no-man’s land. J Hand Surg. 1977;2:452–455.
95. Gelberman RH, Siegel BD, Woo SL, et al. Healing of digital flexor tendons: importance of the interval from injury to repair. J Bone Joint Surg. 1991;73A:66–75.
96. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg. 2005;30A:1061–1067.
97. Bruner JM. The zig-zag palmar-digital incision for flexor-tendon surgery. Plast Reconstr Surg. 1967;40:571–574.
98. Bruner JM. Surgical exposure of the flexor pollicis longus tendon. Hand. 1975;7:241–245.
99. Ikuta Y, Tsuge K. Postoperative results of looped nylon suture used in injuries of the digital flexor tendons. J Hand Surg. 1985;10B:67–72.
100. Kessler I. The “grasping” technique for tendon repair. Hand. 1973;5:253–255.
101. Kessler I, Nissim F. Primary repair without immobilization of flexor tendon division within the digital sheath. Acta Orthop Scand. 1969;40:587–601.
102. Mashadi ZB, Amis AA. The effect of locking loops on the strength of tendon repair. J Hand Surg. 1991;16B:35–39.
103. Savage R, Risitano G. Flexor tendon repair using a “six strand” method of repair and early active mobilization. J Hand Surg. 1989;14B:396–399.
104. Tsuge K, Ikuta Y, Matsuishi Y. Intra-tendinous tendon suture in the hand: a new technique. Hand. 1975;7:250–254.
105. Wade PJF, Wetherell RG, Amis AA. Flexor tendon repair: significant gain in strength from the Halsted peripheral suture technique. J Hand Surg. 1989;14B:232–235.
106. Ketchum LD. Suture materials and suture techniques used in tendon repair. Hand Clin. 1985;1:43–53.
107. Urbaniak JR, Cahill JD, Mortenson RA. Tendon suturing methods: analysis of tensile strengths. In: American Academy of Orthopaedic Surgeons: Symposium on Tendon Surgery in the Hand. St Louis: Mosby; 1975:70–80.
108. Wray RC, Weeks PM. Experimental comparison of techniques of tendon repair. J Hand Surg. 1980;5:144–148.
109. McLarney E, Hoffman H, Wolfe SW. Biomechanical analyses of the cruciate four-strand flexor tendon repair. J Hand Surg. 1999;24A:295–301.
110. Taras JS, Raphael JS, Marczyk S, Bauerle WB. Evaluation of suture caliber in flexor tendon repair. J Hand Surg. 2001;26A:1100–1104.
111. Lin GT, An KN, Amadio PC, et al. Biomechanical studies of running suture for flexor tendon repair in dogs. J Hand Surg. 1988;13A:553–558.
112. Mashadi ZB, Amis AA. Strength of the suture in the epitenon and within the tendon fibres: development of stronger peripheral suture technique. J Hand Surg. 1992;17B:172–175.
113. Sanders WE. Advantages of “epitenon first” suture placement technique in flexor tendon repair. Clin Orthop. 1992;280:198–199.
114. Wade PJF, Muir IFK, Hutcheon LL. Primary flexor tendon repair: the mechanical limitations of the modified Kessler technique. J Hand Surg. 1986;11B:71–76.
115. Silfverskiöld KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg. 1994;19A:53–60.
116. Tran NT, Cannon DL, Lieber RL, Abrams RA. In vivo cyclic tensile testing of combined peripheral and core flexor tenorrhaphy suture techniques. J Hand Surg. 2002;27A:518–524.
117. Sourmelis SG, McGrouther DA. Retrieval of the retracted flexor tendon. J Hand Surg. 1987;12B:109–111.
118. Peterson WW, Manske PR, Lesker PA. The effect of flexor sheath integrity on nutrient uptake by primate flexor tendons. J Hand Surg. 1986;11A:413–416.
119. Peterson WW, Manske PR, Kain CC, Lesker PA. Effect of flexor sheath integrity on tendon gliding: a biomechanical and histologic study. J Orthop Res. 1986;4:458–465.
120. Saldana MJ, Ho PK, Lichtman DM, et al. Flexor tendon repair and rehabilitation in zone II open sheath technique versus closed sheath technique. J Hand Surg. 1987;12A:1110–1114.
121. Su BW, Solomons M, Barrow A, et al. A device for zone II flexor tendon repair. Surgical technique. J Bone Joint Surg. 2006;88A(Suppl Part I):37–39.
122. Malerich MM, Baird RA, McMaster W, Erickson JM. Permissible limits of flexor digitorum profundus tendon advancement: an anatomic study. J Hand Surg. 1987;12A:30–33.
123. McAllister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg. 2006;31:246–251.
124. Noguchi M, Seiler JG III, Gelberman RH, et al. In vitro biomechanical analysis of suture. methods for flexor tendon repair. J Orthop Res. 1993;11:603–611.
125. Soejima O, Diao E, Lotz JC, Hariharan JS. Comparative mechanical analysis of dorsal versus palmar placement of core suture for flexor tendon repair. J Hand Surg. 1995;20A:801–807.
126. Stein T, Ali A, Hamman J, Mass DP. A randomized biomechanical study of zone II human flexor tendon repairs analyzed in an in vitro model. J Hand Surg. 1998;23A:1046–1051.
127. Noonan KJ, Blair WF. Long-term follow-up of primary flexor pollicis longus tenorrhaphies. J Hand Surg. 1991;16A:653–662.
128. Nunley JA, Levin LS, Devito D, et al. Direct end-to-end repair of flexor pollicis longus tendon lacerations. J Hand Surg. 1992;17A:118–121.
129. Urbaniak JR, Goldner JL. Laceration of the flexor pollicis longus tendon: delayed repair by advancement, free graft or direct suture. J Bone Joint Surg. 1973;55A:1123–1148.
130. Bunker TD, Potter B, Barton NJ. Continuous passive motion following flexor tendon repair. J Hand Surg. 1989;14B:406–411.
131. Hitchcock TF, Candel AG, Light TR, Blevens AD. New technique for producing uniform partial lacerations of tendons. J Orthop Res. 1989;7:451–455.
132. Kleinert HE. Should an incomplete severed tendon be sutured? Plast Reconstr Surg. 1976;57:36–38.
133. McGeorge DD, Stilwell JH. Partial flexor tendon injuries: to repair or not. J Hand Surg. 1992;17B:176–177.
134. McMaster PE. Tendon and muscle ruptures: clinical and experimental studies on the causes and locations of subcutaneous rupture. J Bone Joint Surg. 1933;15:705–722.
135. Schlenker JD, Lister GD, Kleinert HE. Three complications of untreated partial laceration of flexor tendon: entrapment, rupture, and triggering. J Hand Surg. 1981;6:392–398.
136. Weeks PM. Invited comment on “three complications of untreated partial lacerations of flexor tendon: entrapment; rupture, and triggering.”. J Hand Surg. 1981;64:396–398.
137. Wray RC, Holtmann B, Weeks PM. Clinical treatment of partial tendon lacerations without suturing and with early motion. Plast Reconstr Surg. 1977;59:231–234.
138. Wray RC, Ollinger H, Weeks PM. Effects of mobilization on tensile strength of partial tendon lacerations. Surg Forum. 1975;26:557–558.
139. Wray RC, Weeks PM. Treatment of partial tendon lacerations. Hand. 1980;12:163–166.
140. Bishop AT, Cooney WP, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26:301–312.
141. Cooney WP, Weidman K, Malo D, Wood MB. Management of acute flexor tendon injury in the hand. Instruct Course Lect. 1985;34:373–381.
142. Cooney WP, Weidman KA, Malo DS, et al. Partial flexor tendon lacerations. In: Hunter JM, Schneider LH, Mackin EJ, eds. Tendon Surgery in the Hand. St. Louis: Mosby; 1987:148–155.
143. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: an experimental study. Hand. 1978;10:52–55.
144. Leddy JP, Packer JW. Avulsion of the profundus insertion in athletes. J Hand Surg. 1977;2:66–69.
145. Buscemi MJ Jr, Page BJ 2nd. Flexor digitorum profundus avulsions with associated distal phalanx fractures: a report of four cases and review of the literature. Am J Sports Med. 1987;15:366–370.
146. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx. J Hand Surg. 1981;6:600–601.
147. Groth GN. Pyramid of progressive force exercises to the injured flexor tendon. J Hand Ther. 2004;17:31–42.
148. Steelman PJ, Groth GN, Taras JT. Individualized rehabilitation program for flexor tendon repair: from pyramid to algorithm. Oper Tech Orthop. 2007;17:148–154.
149. Whitaker JH, Strickland JW, Ellis RK. The role of flexor tenolysis in the palm and digits. J Hand Surg. 1977;2:462–470.
150. Wray RC, Moucharafieh B, Weeks PM. Experimental study of the optimal time for tenolysis. Plast Reconstr Surg. 1978;61:184–189.
151. Allen BN, Frykman GK, Unsell RS, Wood VE. Ruptured flexor tenorrhaphies in zone II: repair and rehabilitation. J Hand Surg. 1987;12:18–21.
152. Parkes A. The “lumbrical plus” finger. J Bone Joint Surg. 1971;53B:236–239.
153. Lilly SI, Messer TM. Complications after treatment of flexor tendon injuries. J AAOS. 2006;14:387–396.
154. Baktir A, Türk CY, Kabak S, et al. Flexor tendon repair in zone II followed by early active mobilization. J Hand Surg. 1996;21B:624–628.
155. Cullen KW, Tolhurst P, Lang D, Page RE. Flexor tendon repair in zone 2 followed by controlled active mobilization. J Hand Surg. 1989;14B:392–395.
156. Gelberman RH, Botte MJ, Spiegelman JJ, Akeson WH. The excursion and deformation of repaired flexor tendons treated with protected early motion. J Hand Surg. 1986;11A:106–110.
157. Gelberman RH, Manske PR, Akeson WH, et al. Flexor tendon repair. J Orthop Res. 1986;4:119–128.
158. Lee H. Double loop locking suture: a technique of tendon repair for early active mobilization. J Hand Surg. 1990;15A:953–958.
159. Mathews JP. Early mobilisation after flexor tendon repair. J Hand Surg. 1989;14B:363–367.
160. Pruitt DL, Manske PR, Fink B. Cyclic stress analysis of flexor tendon repair. J Hand Surg. 1991;16A:701–707.
161. Riaz M, Hill C, Kahn K, Small JO. Long term outcome of early active mobilization following flexor tendon repair in zone II. J Hand Surg. 1999;24B:157–160.
162. Robertson GA, Al-Qattan MM. A biomechanical analysis of a new interlock suture technique for flexor tendon repair. J Hand Surg. 1992;17B:92–93.
163. Savage R. The influence of wrist position on the minimum force required for active movement of the interphalangeal joints. J Hand Surg. 1988;13B:262–268.
164. Small JO, Brennen MD, Colville J. Early active mobilization following flexor tendon repair in zone 2. J Hand Surg. 1989;14B:383–391.
165. Trail IA, Powell ES, Noble J. The mechanical strength of various suture techniques. J Hand Surg. 1992;17B:89–91.
166. Thurman RT, Trumble TE, Hanel DP, et al. Two-, four-, and six-strand zone II flexor tendon repairs: an in situ biomechanical comparison using a cadaver model. J Hand Surg. 1998;23A:261–265.
167. Aoki M, Manske PR, Pruitt DL, Lasdon BJ. Work of flexion after tendon repair. J Hand Surg. 1995;20B:310–313.
168. Luo J, Mass DP, Phillips CS, He TC. The future of flexor tendon surgery. Hand Clin. 2005;25:267–273.
169. Cao Y, Liu W, Liu Y, et al. Bridging tendon defects using autologous tenocytes engineered tendon in a hen model. Plast Reconstr Surg. 2002;110:1280–1289.
170. Chang J, Thunder R, Most D, et al. Studies in flexor tendon wound healing: neutralizing antibody to TGF-β increases postoperative range of motion. J Hand Surg. 1998;23A:1052–1058.