CHAPTER 36
Postoperative Management of Flexor Tendon Injuries
KAREN PETTENGILL, MS, OTR/L, CHT AND GWENDOLYN VAN STRIEN, LPT, MSC
CRITICAL POINTS
▪ Know the specifics of both injury and surgery.
▪ Calculate the “safe zone” and recalculate as needed.
▪ Base your rehabilitation program on both your evaluation and the known data on tendon anatomy, healing, excursion, and repair strength.
▪ Each patient responds differently, so do not use the same approach with every patient, and do not adhere rigidly to a protocol.
▪ Change your approach if it is not working.
▪ Aggressive treatment progression is essential, but that does not require aggressively vigorous treatment.
▪ Before advancing the program, identify all the tissues involved, and target those tissues appropriately.
▪ If you recognize today that the program should be changed, don’t wait until tomorrow because you’re too busy!
▪ Measure what you’re trying to change, for example, measure proximal interphalangeal (PIP) joint flexion contractures passively, because limited passive extension is the cause of limited active extension.
Postoperative management of the repaired flexor tendon requires a thorough understanding of the anatomy, physiology, biomechanics, and normal and pathologic healing not only of the flexor tendons but also of all adjacent injured structures. The therapist assesses tendon function through palpation, observation, and measurement. When the patient is referred for therapy, the surgeon communicates to the therapist the particulars of injury and of surgical and medical management; the therapist then questions both surgeon and patient to obtain any additional information. On the basis of this information, the therapist, in consultation with the referring surgeon, selects the appropriate therapeutic approach and modifies treatment when needed.
This chapter presents fundamental tendon management concepts and then reviews the aspects of flexor tendon anatomy, biomechanics, and mechanisms of nutrition and healing pertinent to postoperative management. Following that is a discussion of the three approaches to tendon management, with protocols or programs that exemplify each. Each protocol gives guidelines for management; all protocols should be evaluated and modified according to the patient’s individual needs.
The next section covers techniques for treating or preventing commonly encountered problems. The most important part of the chapter is the section on clinical decision making and application of the scientific evidence to the healing tendon. The chapter concludes with consideration of two special cases: flexor pollicis longus lacerations and laceration of multiple tendons and nerves at the wrist.
Fundamental Concepts
Goal: A Strong Repair That Glides Freely
Normal tendon function requires free gliding of the tendon without hindrance from surrounding tissues. Each tendon glides through a given amplitude of excursion to flex the digit completely and with adequate power. Because so many structures lie in such a constricted space within the hand, scar adhesions between adjacent structures can occur very easily after injury or surgery. Tendon adhesions can limit excursion to such an extent that tendon function is seriously compromised; intertendinous adhesions can further decrease function.
As it glides, a tendon encounters a certain normal amount of resistance or drag from surrounding tissues. For the first weeks after a repair, that drag is increased considerably by normal post-traumatic or postoperative edema, lacerated tissues, and the extra bulk of sutures and newly forming scar. Given the low strength of a newly repaired tendon, extra care must be taken to allow for increased drag during all immediate postoperative exercise. The effect of such factors as edema and adjacent injuries is to increase the work of flexion (the work necessary for the tendon to overcome the gliding resistance encountered). Calculation of work of flexion should take into account both gliding resistance between tendon and sheath and external forces (e.g., joint stiffness, edema) impeding gliding.1
In addition to unobstructed gliding, the tendon repair requires enough strength to withstand the normal forces acting on the tendon during daily activity. If subjected to excessive stress during early phases of healing, the repair may rupture or the tendon ends may pull apart without complete rupture. The gap may be filled with scar, and not only will it be weaker than a healthy repair, the tendon will also elongate, in effect putting it on slack. An elongated tendon requires even greater excursion to function as it should. Gap formation also has been shown to provoke increased adhesion formation in immobilized tendon repairs, although the functional significance of such gaps appears to be less in the repair that was mobilized early.2,3
Therefore the goal is for the tendon to heal without rupture or gap formation, with sufficient strength and excursion for daily activities. The therapist must understand and plan treatment to address all of the impediments to glide and the dangers to the repair for each patient. In addition to this chapter, the reader is encouraged to read Chapter 34 for a more comprehensive discussion of the variables of healing, nutrition, and gliding resistance that dictate individualization of flexor tendon treatment. Near the end of this chapter is a discussion of how to plan treatment in light of all these variables.
Evaluating Tendon Function
To plan effective therapy, the therapist evaluates tendon function in several ways. The most obvious is measurement of active and passive range of motion (AROM and PROM, respectively). If passive flexion greatly exceeds active flexion, the tendon is not functioning adequately. The repair may have ruptured or elongated, or it may be adherent. Groth4 has devised an adhesion grading scale to aid the therapist in determining the severity of adherence and the need to increase stress to the tendon to increase excursion (Table 36-1). She classifies adhesions as absent (≤5-degree discrepancy between active and passive flexion), responsive (≥10% resolution of active lag between therapy sessions), and unresponsive (≤10% resolution of active lag between therapy sessions). Using this scale, the therapist can evaluate the lag (number of degrees greater passive than active flexion) and assess response to intervention. If the lag is responsive, then the current program may be continued. If not, then the program should be stepped up to more vigorously address adherence. The presence and extent of adhesions alone is not enough information, however. The therapist now palpates along the course of the tendon to detect impediments to smooth gliding. Such impediments may be very subtle; patience and experience are needed to accurately assess glide, but this is one of the therapist’s most valuable skills.
Table 36-1 Adhesion Grading System
Absent |
Less than or equal to 5-degree discrepancy between digital active and passive flexion |
Responsive |
Greater than or equal to 10% resolution of active lag between therapy sessions |
Unresponsive |
Less than 10% resolution of active lag between therapy sessions |
An adherent tendon usually exhibits some excursion, however limited, but the entire excursion may be taken up by flexion of a single joint. For example, an adherent flexor digitorum profundus (FDP) tendon may produce active flexion of the distal interphalangeal (DIP) joint when the PIP and metacarpophalangeal (MCP) joints are held passively in extension, but when the proximal joints are left free, the limited excursion may not be sufficient to produce flexion at all joints.
An adherent tendon also limits passive composite extension, because it is in effect tethered to tissues at one level or more and cannot passively glide and lengthen to allow full extension. Figure 36-1 illustrates this phenomenon. The therapist evaluating limitations in motion also differentiates between those caused by impaired tendon function and those caused by articular or periarticular involvement.
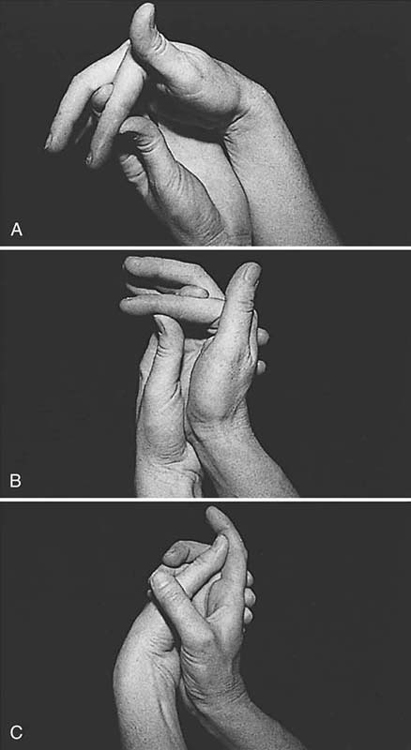
Figure 36-1 A, The proximal interphalangeal (PIP) joint can be extended completely when the wrist and metacarpophalangeal (MCP) joints are flexed. This means that there are no periarticular or articular restrictions to PIP joint extension. B, The PIP joint still can be extended completely when the wrist is extended, but we see the distal interphalangeal joint begin to flex, reflecting some tightening of the flexor digitorum profundus tendon. C, When the MCP joint and wrist are extended simultaneously, the PIP joint cannot be extended, indicating probable flexor tendon adhesions in the palm or at the level of the MCP joint or the proximal phalanx. (From Stewart KM. Tendon injuries. In: Stanley BG, Tribuzi SM, eds. Concepts in Hand Rehabilitation. Philadelphia: FA Davis, 1992;353–394.)
Three Approaches to Tendon Management
Each postoperative tendon management protocol falls within one of the following three categories:
Immobilization. This approach involves complete immobilization of the tendon repair, generally for 3 to 4 weeks, before beginning active and passive mobilization.
Early passive mobilization. This approach involves mobilizing the repair early (usually within the first week, often within 24 hours), either manually (by therapist or patient) or by dynamic flexion traction. Passive flexion pushes the tendon proximally, and limited active or passive extension pulls the tendon distally.
Early active mobilization. This involves mobilizing the repair (within a few days of repair) through active contraction of the involved flexor, with caution and within carefully prescribed limits.
Anatomy
Flexor tendon anatomy and zones were described in Chapter 1 and in Chapters 34 and 35. Following is a brief discussion of anatomic features according to the zone of injury pertinent to postoperative management.
Tendon injuries in zone 5 commonly become markedly adherent to overlying skin and fascia, but these adhesions are not generally problematic because adhesions form between the tendon and paratenon. Because the paratenon is a loose connective tissue, adhesions are not as restrictive as those that form between tendon and the firm, well-anchored flexor retinaculum or fibro-osseous tunnel.
Zone 4 injuries at the carpal canal are at risk for adhesion to synovial sheaths, to each other, and to the other structures lying within the constricted carpal tunnel space. As with zone 2 injuries, intertendinous adhesions limit differential glide and thus can severely limit hand function.
Zone 3 lacerations distal to the carpal tunnel are susceptible to adhesions to adjacent tendons, lumbricals, and interossei, and to overlying fascia and skin.
The anatomy of the flexor tendons in zone 2 is complex, and the reader is referred to Chapters 1 and 35 for a complete review.
With so many structures packed into the confines of the fibro-osseous tunnel, adhesions are highly likely between FDP and flexor digitorum superficialis (FDS); between tendon and sheath; and between tendon and bony, vascular, and other soft tissue structures. This risk is further compounded if both slips of FDS are repaired at the same level as FDP, as the bulk of the three sutures combine to increase gliding resistance. If only one slip of FDS was repaired, there is a risk of adherence to the unrepaired stumps of FDS, which may also catch on the edge of pulleys, thus impeding glide. The surgeon may minimize the risk by trimming stumps down and shaping the proximal end to remove the protruding unrepaired portion.
If tendons have retracted (in a repair delayed over 14 days, or if the finger was in flexion during injury so that muscle contraction pulled the proximal tendon stump into the palm), the tendon must be retrieved, inevitably contributing intraoperative trauma. In cases of delayed repair, the tendon may have shortened since injury and thus may be repaired under some tension.
In addition, damage to the pulleys may compromise tendon function, and injury to the vincula may compromise nutrition. Without the restraint of the pulleys, the tendon pulls away from bone with each muscle contraction, resulting in “bowstringing” (Fig. 36-2). Finally, loss of even a few millimeters of tendon excursion can mean a considerable functional deficit. It’s no wonder that so much time and effort have been expended in designing postoperative techniques to control adhesion formation, facilitate adequate nutrition and healing, and attain maximum excursion in the zone 2 injury.

Figure 36-2 Bowstringing of the flexor tendons is illustrated in this patient with absent pulleys because of a childhood injury.
In zone 1, the tendon does not have great excursion (only 5–7 mm), so loss of even a small amount of excursion can be functionally limiting. These injuries also are prone to adhesion of the repair to the A4 or A5 pulley and attenuation of the repair.
Nutrition
Flexor tendon nutrition is described in detail in Chapters 1 and 35.
It is important to note that in zone 2, where the tendons are surrounded by the pulley system and there are areas of relative avascularity, tendon nutrition comes from two sources: the blood supply and synovial diffusion.
Synovial fluid is apparently “forced” into the tendon under influence of high pressure against the pulleys during active flexion of the finger.5,6 The pumping mechanism, under influence of pressure of the tendon against the firm resistance of the pulleys, has been compared to the mechanism of synovial diffusion in articular cartilage.
A delicate balance between the two nutritional pathways exists. Nutrition to relatively avascular areas is supplied mostly by diffusion from the synovium. When injury occurs in these areas, the balance is disturbed and excessive adhesion formation may be seen. The adhesions bring the additional blood supply to the tendon necessary for the healing process, yet they limit free tendon glide. Injury to the vincula themselves also affects the nutritional balance, compromises tendon healing, and causes adhesion formation.
Basic Concepts of Tendon Healing
Three phases are described for wound healing: the exudative or inflammatory phase, the proliferative or fibroplasia phase, and the remodeling or maturation phase. In reality these phases do not occur as separate events, but rather as an intricate process during which the phases overlap considerably and are modified by intrinsic factors and external forces. The process of tendon healing is described in detail in Chapter 34.
An essential characteristic of the healing of repaired tendon is the fact that the entire wound actually involves more than just the tendon. All surrounding tissues, such as skin, subcutaneous tissues, and underlying tissues, also are involved in the wound-healing process. In the immobilized tendon, during the first few days after repair, the wound is filled with a cicatrix, consisting of ground substance and many types of cells. Scar formed in the first 3 weeks “glues” all involved tissue layers together, and independent function is lost. This was described by Peacock7 as the one-wound concept. The task of the therapist and surgeon is to restore gliding between soft tissues and thus recover their independent function.
Following are key events related to healing.
The exudative, or inflammatory, phase starts immediately after injury. Tensile strength of the immobilized tendon repair diminishes in the first 3 to 5 days because of softening of the tendon ends. As we shall see, this softening does not occur in tendons that have been mobilized in a controlled fashion during the first postoperative week.2,8
During the proliferative phase, fibroblasts migrate to the wound area and start production of tropocollagen approximately 5 days after injury. Tropocollagen is a triple-helix molecule with little tensile strength. After the weak hydrogen bonds of the tropocollagen molecule are replaced by stronger crosslinks between the three strands of the helix, collagen fibers are formed, and tensile strength starts to develop. The collagen molecules form a randomly oriented network, creating a bond between all tissues in the wound. From day 5 to day 21, tensile strength of the immobilized repair increases as the collagen matures and the intramolecular cross-linking continues. As we shall see, repairs that are subject to early controlled stress gain strength more rapidly.
In the third phase of wound healing (the remodeling phase), the tissues are differentiated, and dense, unyielding scar can be changed into more favorable scar. Scar remodeling is characterized by a balance between collagen production and collagen lysis. The randomly oriented collagen between tendon ends, under the influence of stress, is slowly replaced by newly formed collagen oriented along the long axis of the tendon, thus providing increased tensile strength. The randomly oriented fibers of the scar between tendon and surrounding tissues, however, must become loose and filmy to regain gliding function.
Extrinsic Versus Intrinsic Healing
It’s well accepted that tendons heal through a combination of intrinsic and extrinsic mechanisms.9-11
Extrinsic healing depends on formation of adhesions between tendon and surrounding tissue. These adhesions provide the blood supply and the cells (in particular, fibroblasts) needed for tendon healing. Unfortunately, they also prevent the tendon from gliding.12,13
Intrinsic healing occurs between the tendon ends only, without formation of limiting adhesions. This type of healing relies on the synovial fluid for nutrition and does not result in restricted motion of the tendon. The cells needed for tendon healing are supplied by the epitenon and endotenon itself.14-17
Although experimental research demonstrates that tendon healing is possible by either intrinsic or extrinsic means, in actual practice adhesions are seen to varying degrees, and the healing response is probably a balance between intrinsic and extrinsic healing mechanisms.
Effects of Motion and Force on Tendon Healing
Beneficial effects of early mobilization and stress applied to tendon anastomoses have been demonstrated in a number of laboratory experiments. Although concluding that the risks outweighed the potential benefits, Mason and Allen,8 in 1941, noted that motion created a stronger repair in the wrist flexor tendons of some of their canine subjects. Tensile strength increased after the seventh day, especially when mobilization was protected.
Gelberman and others18-22 performed a series of experimental studies of early passive mobilization of tendons in dogs. The authors reported that compared with tendons subjected to immobilization or delayed mobilization, the tensile strength and excursion of mobilized tendons were superior, probably as a result of improved intrinsic healing and consequently restored gliding surfaces. These studies support the hypothesis that motion has a beneficial effect on tendon nutrition, tenocyte metabolism, or both.
In 1987, Hitchcock and colleagues2 studied healing of chicken flexor profundus tendons, comparing immobilized tendons with those allowed immediate controlled mobilization. They found that the immobilized tendons healed as described by previous investigators,8 with a decrease in strength as tendon ends softened during the exudative phase as early as 5 days after repair. In contrast, the mobilized tendons did not go through a definable exudative phase, and therefore the tendon ends did not soften as occurs with an immobilized repair. Instead, repairs gained strength, appearing to heal through intrinsic mechanisms, with a notable lack of adhesion formation. Numerous other studies have confirmed the benefits of early mobilization.23-30
The Future of Tendon Healing
Many researchers have attempted to improve the quality of tendon healing by influencing quantity and type of adhesion formation. To limit fixed adhesions, weak healing is needed between tendon and surrounding tissues. In contrast, strong healing is needed between the tendon ends to transmit muscle power. This type of differential wound healing seems necessary to recover a free-gliding and functioning tendon after flexor tendon repair.
Over the past 15 years research has increased dramatically on the influence of growth factors on tendon healing. Growth factors are molecules that influence various cellular functions, regulating various steps along the cascade of tendon healing. The actual clinical applications of this research in tendon healing is still unclear.31-36 In an effort to decrease gliding resistance of the repaired tendon, recent studies have investigated the application of lubricants such as lubricin and hyaluronic acid.37-39 This research is explored in Chapter 34.
Postoperative Management
Factors Affecting Healing and Rehabilitation
Many variables may affect the outcome of a tendon repair. Among these are individual patient characteristics, factors related to injury or surgery, and aspects of the therapy.
Patient-Related Factors
Age. The only documented age-related factor is the number of vincula, which decreases as the patient grows older.6 As a result, larger areas within the tendon lack blood supply, with a consequent decrease in healing potential. In addition, in theory, cell aging could lead to decreased healing capacity of tenocytes.5
General Health and Healing Potential. In general, patients in good health heal well. Certain lifestyles or dietary habits, however, adversely affect healing. For example, a cigarette smoker often experiences delayed healing.5,40-42
Rate and Quality of Scar Formation. In practice, clinicians often observe that of two patients with virtually identical injury, surgery, and therapy, one may form scar rapidly and heavily and have great difficulty mobilizing the tendon as a result, but the other may form scar slowly and then form very light scar. The latter patient runs a greater risk of tendon rupture.
Patient Motivation. The patient’s motivation and ability to follow the postoperative program are critical determinants of the end result of a primary flexor tendon repair. One cannot expect careful and conscientious performance of the therapy program by a patient who does not understand his central role in his own rehabilitation. Each patient’s goal is different and often is dictated by his occupation. The therapist must identify the patient’s goals and make them a part of the overall therapy goals.
Patient education can decrease the danger of rupture, prevent overzealous patients from exercising too much or too forcefully, and perhaps help less-motivated patients to understand the importance of adhering to the home program.
Socioeconomic Factors. A patient’s family life, her economic status, and other socioeconomic factors can help or hinder in rehabilitation. The patient may have no health insurance and no income, or may be supporting a family but unable to work. Her family may be unsupportive of her rehabilitation efforts or may simply be unable to help her. She may live alone and be unable to handle all of the daily responsibilities while performing a complex home program. If these factors are not taken into account in planning treatment, therapy may fail.
Injury- and Surgery-Related Factors
Level of Injury. The effect of injury varies depending on the zone of injury. These variations were described earlier.
Type of Injury. The nature of the injury is another important determinant of final outcome. In an untidy laceration or crush injury, subsequent infection may prolong the inflammatory process and delay healing. Crushing or blunt injuries usually cause more associated injuries to surrounding tissues and lead to more scar formation. Crush injuries also frequently involve vascular injury, and this can impair healing, especially with injury to the vincula. When adjacent injured tissues must be protected (as with fractures or injuries to neurovascular bundles), treatment is modified, subsequently compromising the ultimate result.
An isolated FDP injury heals with fewer adhesions, partly because there is only a single repair rather than two contiguous repairs that are prone to intertendinous adhesion. If both slips of FDS were injured and repaired along with FDP, the added bulk may make excursion and intertendinous gliding harder to attain. If FDS is injured, there is likelihood of greater vincular damage, and vascularity is impaired, again increasing the risk of adhesion formation (Fig. 36-3, online)
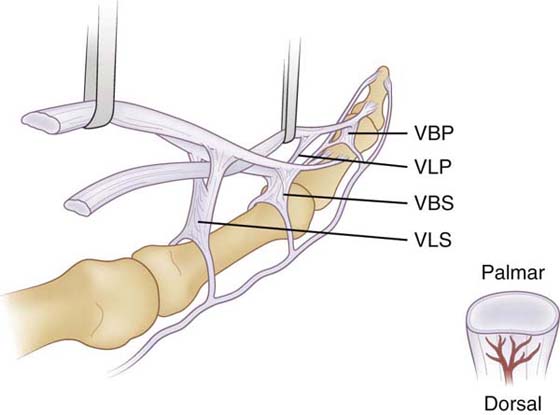
Figure 36-3 The flexor digitorum superficialis lies volar to the flexor digitorum profundus as the tendons enter the sheath. At the level of the proximal phalanx, the superficialis tendon splits and the two slips pass around dorsal to the profundus tendon, merging and splitting again before inserting on the middle phalanx (Camper’s chiasm). Both tendons have a short and long vinculum. The vinculum longus profundus (VLP) is a continuation of the vinculum brevis superficialis (VBS). The cross-section view shows the greater dorsal vascularity of the flexors in zones I and II. VBP, vinculum brevis profundus; VLS, vinculum longus superficialis. (From Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg. 2000;25A:214.)
The prognosis also may be better for a partial laceration than for a complete laceration, because vascularity is generally better preserved. There is controversy in the literature about whether to repair the partial laceration.43-45 Triggering or entrapment may occur when the irregular surface of an untreated partially lacerated tendon catches on the sheath, and theoretically the unrepaired partially lacerated tendon may rupture. Some evidence indicates that partial lacerations may be better left untouched except for beveling to prevent gliding problems.46
If the finger was flexing powerfully when injured, the contracting muscle will pull the proximal portion proximally like a rubber band cut under tension. The vincula may be ruptured or stretched, impairing vascularity. The surgeon must retrieve the proximal tendon stump before repair. The very retrieval may be traumatic to the tendon and surrounding sheath.
Position of the finger when injured also affects outcome in that a given point on the tendon glides proximally during flexion and distally during extension. For example, suppose a test tube breaks in a patient’s hand, lacerating the FDP and FDS of one finger. The finger is flexed when injured. If the digit is extended, the cut distal portion of each tendon could be pulled distally by as much as 3 or 4 cm, depending on the excursion of the tendon in that patient and the actual level of injury. Thought should be given to postoperative positioning to avoid resting the repair(s) adjacent to the original wound or other involved tendon.
Sheath Integrity. The sheath and pulleys often are involved in a zone 1 or 2 injury.
Injury to the pulley system decreases the mechanical advantage of the tendon.47-50 Pulley injury also affects tendon nutrition because of the role the pulleys play in synovial diffusion.
Several studies have shown no advantage to sheath repair.51-55 Apparently a single cell layer much like the sheath regenerates in the first postoperative days.11,55 Many surgeons attempt to repair the sheath, however, to prevent the possibility of triggering of the tendon repair site on the open sheath.
Surgical Technique. Meticulous surgical technique can minimize the amount of additional tissue trauma and hematoma, thereby reducing the number of adhesions. Excessive postoperative hematoma causes increased inflammatory and cellular responses. An increase in the amount of hematoma therefore may increase the number of adhesions surrounding the repaired tendon.
Tissues Must Be Handled Delicately. Potenza56 has demonstrated that even the marks of the forceps on the epitenon can trigger adhesion formation. The effect of different surgical variables on adhesion formation in repaired tendons also has been investigated. Matthews and Richards57 demonstrated an increase in adhesion formation when suture material was added to a gliding tendon. Injury to the sheath and use of an orthosis were other variables investigated and found to increase adhesion formation. Sutures may “strangulate” the intratendinous vessels and provoke adhesion formation. In fact, sutures often are placed in the relatively avascular volar aspect of the tendon to avoid damage to the dorsally placed intratendinous vessels.
Suture Strength Is a Crucial Variable. It was only when both strong and atraumatic sutures were developed that early mobilization could be contemplated. The suture must be strong enough to prevent gapping while allowing gentle stress to the repair. The development of early active mobilization techniques depends even more heavily on adequate suture strength. Although a discussion of specific sutures is beyond the scope of this chapter, several recently developed sutures clearly are strong enough to withstand early active mobilization if performed with carefully controlled force. The literature indicates that given a grasping suture that is technically well placed, strength is directly proportional to the number of strands crossing the repair.58-61 This is illustrated in Figure 36-4. Several studies also have indicated that a well-designed circumferential suture will add strength to any repair.62-68 Suture knot placement may also affect strength.69,70 Another consideration is the work of flexion, or resistance to gliding due to placement, bulk, or other design aspects of the core or circumferential suture.62,71,72
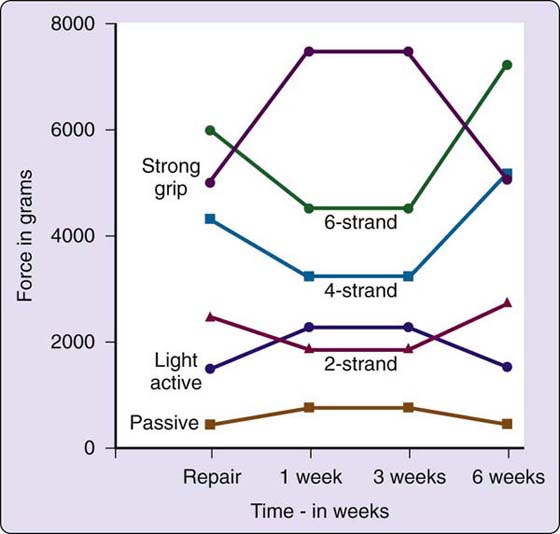
Figure 36-4 This graph plots the strength of two-, four- and six-strand repairs against the forces exerted by passive flexion, light active flexion, and strong grip, with a margin allowed for friction, edema, and stress. (From Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg. 2000 25A:214.)
Timing of Repair. The longer a tendon repair is delayed, the more difficult the rehabilitation may be.73 By 2 weeks after injury, the cut tendon ends have scarred down to surrounding tissues and must be dissected free before repair. In addition, the entire musculotendinous unit shortens and pulls the tendon proximally, which may place tension on the repair and increase the risk of gapping or rupture; shortening also increases the risk of later flexion contractures. Injury- and surgery-related factors are summarized in Box 36-1.
Box 36-1 Injury- and Surgery-Related Factors Affecting Healing and Rehabilitation
Level of injury determines which anatomic, healing, and biomechanical features to consider in planning treatment.
Type of injury
Untidy laceration/crush injuries. Infection may prolong the inflammatory process and delay healing. Associated injuries may increase the risk of adhesions
Isolated FDP injury. Easier to regain gliding because only one tendon involved
Combined FDP/FDS injury/repair
Risk of poor gliding because of adhesions between repairs
Repair of separate FDS slips. Increased bulk, and increased risk of adhesions
FDS laceration. Chance of greater vincular damage leads to increased risk of adhesions
Partial laceration. Prognosis may be better, depending on surgical management
Position of the finger when injured
If finger injured when flexed tendon retracts proximally, retrieval is difficult and may cause more scarring.
Avoid positioning repair at original injury site to prevent adherence
Sheath integrity. Repair of sheath may not improve healing of repaired tendon but may improve gliding
Injury to the pulley system
Decreases mechanical advantage of tendon
Affects synovial tendon nutrition
Surgical technique
Tissue handling. Atraumatic surgical technique decreases risk of adhesions
Suture technique. Suture may strangulate intratendinous vessels and cause more adhesion formation
Suture strength. Is it appropriate for your chosen treatment approach?
Timing of repair. Delayed repair causes scarring of tendon ends or shortening of musculotendinous unit (or both), both increase risk of adhesions
Therapy-Related Factors
Timing. As noted before, an immobilized tendon repair loses strength initially, whereas early mobilization strengthens the repair. Therefore, if early mobilization is to be used, therapy should begin as soon as possible. If mobilization begins at 1 week after repair, the repair will already have weakened enough to be at increased risk for rupture or deformation. Adhesions also will have begun to form, adding to the stress placed on the weakened repair. A 1995 study by Tottenham and coworkers74 found better results in patients whose repaired zone II tendons were mobilized passively within 1 week than in those mobilized between 1 and 3 weeks after repair. However, in a severely edematous digit, starting early mobilization on the day of surgery would be dangerous. Inflammation and edema subside after a day or so of rest and elevation in the compressive postoperative dressing, which reduces the work of flexion.75
Another aspect of timing is progression according to tendon healing status. Although not always described as such, every protocol can be divided into three phases or stages. The early stage is a protective period that includes the inflammatory and proliferative or fibroplasia phases and sometimes the beginning of the remodeling phase of wound healing, when the repair is at its weakest. This lasts for 3 to 4 weeks. Next is the intermediate stage, when stress to the tendon is increased, either by mobilizing for the first time, or by decreasing protection during mobilization. The late stage generally begins at 6 to 8 weeks and continues to the end of therapy. Stress to the tendon is increased, and muscle strengthening and job simulation are added gradually.
Technique. As will be clear from the following discussion, the postoperative rehabilitation technique must be selected with care to match the needs of the patient. Not every tendon injury can be treated with the identical protocol, and often the best approach is a combination of techniques from various protocols.
Expertise. The therapist’s expertise must be taken into account in selecting the postoperative rehabilitation approach. No therapist should undertake a treatment program without sufficient preparation, experience, and any supervision needed. This may seem evident, but despite the multitude of available options for tendon management, many therapists attempt to use approaches that they simply do not understand, following rigid protocols without consideration of individual variables and the rationale for treatment. Nowhere is it more vital to fully understand the rationale for treatment than in tendon management.
The uninformed reader may be misled easily by results of various studies presented in the literature. In addition to evaluating research design, instrumentation, and other components of any study, the therapist must bear in mind the differences between the population in the study and the patient now being treated. For example, military patients are a “captive audience” and can be assumed to be more compliant than civilians. The health care delivery system may also make a difference in the outcome. Some studies involve patients who are hospitalized and therefore more closely supervised. If your patient is restricted by insurance coverage to one visit per week, your choice of approach may need to change or the way you educate the patient and monitor progress.
In addition, studies use varying means of assessing the results of flexor tendon repair. The interested reader is referred to Elliot and Harris’ comprehensive review of the different methods of assessing results of tendon repair.76
Following are the rationale, indications, and representative protocols for each of the three basic approaches to flexor tendon management: immobilization, early passive mobilization, and early active mobilization. The discussion of immobilization is the most concrete and detailed, including concepts and techniques that may be used in other approaches.
Immobilization
Rationale and Indications
No matter how sophisticated our therapeutic and surgical care becomes, there will probably always be a need for immobilization of flexor tendon repairs in some circumstances. Early mobilization protocols are appropriate for alert, motivated patients who understand the exercise program and precautions. For that reason, immobilization is still the treatment of choice for patients who are too young, those with cognitive deficits, and those who for any other reason are clearly unable or unwilling to participate in a complex rehabilitation program. These patients benefit far more from protection of the repair until adequate healing and adhesion formation have taken place. Some tendons also must be immobilized to protect other injured structures. In some cases the patient is not referred to therapy for a postoperative orthosis but simply remains in the postoperative cast until sent to therapy at 3 to 4 weeks after surgery. Therefore all therapists must be prepared to treat the immobilized tendon with skill and care. It may be very difficult to mobilize these repairs because of heavy adhesion formation.
Treating the Immobilized Tendon Repair
The following guidelines are based on those developed by Cifaldi Collins and associates,77 to ensure sufficiently aggressive therapy after immobilization. We describe here several techniques and concepts applicable to all flexor tendon management, regardless of the approach used.
Early Stage (from 0 to 3 or 4 weeks)
Orthosis. The dorsal forearm-based postoperative orthosis or cast holds the wrist in 10 to 30 degrees of flexion, the MCP joints in 40 to 60 degrees of flexion, and the interphalangeal (IP) joints in full extension.
Exercise. At home patients perform range-of-motion (ROM) exercises of uninvolved joints (elbow, shoulder) to prevent stiffness. The orthosis is worn 24 hours a day, except for therapy visits once or twice a week when the orthosis may be removed for gentle protected PROM by the therapist. For protected PROM, the therapist holds adjacent joints in flexion while extending and flexing each joint. Thus, for example, for PIP joint extension, the wrist and MCP and DIP joints are kept flexed to give some slack to the flexor tendons. If the adjacent MCP and DIP joints were allowed to remain extended during PIP joint extension, the slack would be taken up and PIP joint extension might stretch or rupture a repair at the proximal phalanx level. The concept of protected passive motion is applied in all flexor tendon management protocols.
During the therapy session the therapist may remove the orthosis to clean the patient’s skin and, after the sutures are removed and the incision is well healed, perform massage to the scar. As the scar heals, massage may help modify both skin and tendon adhesions. Elastomer or other pressure dressings are helpful for flattening unusually bulky scars but generally should be used only at night, to avoid restricting mobility during the day. In some patients such pressure can be applied during the early stage without removing the orthosis.
Intermediate Stage (starting at 3 to 4 weeks)
Orthosis. At 3 to 4 weeks, the orthosis is modified to bring the wrist to neutral (0 degrees). The patient is taught to remove the orthosis hourly for exercise.
Exercise. With the wrist at 10 degrees of extension, the patient performs passive digit flexion and extension, followed by active differential tendon gliding exercises (Fig. 36-5). These exercises elicit maximum total and differential flexor tendon glide at the wrist–palm level.78 The straight fist, with the MCP joints and PIP joints flexed but the DIP joints extended, elicits maximum FDS glide in relation to surrounding structures. The full fist, with the MCP, PIP, and DIP joints flexed, does the same for the FDP tendon. In the hook fist, with the MCP joints extended while the IP joints flex, maximum differential gliding between the two tendons is achieved. For the Cifaldi Collins and associates’ protocol, the exercises incorporate synergistic wrist motion (SWM): the wrist extends when the digits flex, and flexes when the digits extend, to increase the excursion attained and prevent simultaneous full wrist and digit flexion.
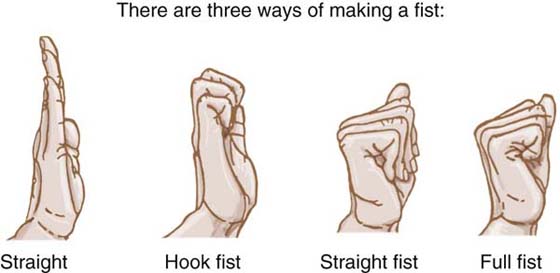
Figure 36-5 The three different positions of tendon gliding exercises: hook fist, straight fist, and full fist.
After 3 or 4 days of these exercises, tendon function is evaluated. The therapist measures active and passive flexion, totaling the degrees of flexion achieved at the MCP and IP joints for total active and passive flexion. If there is a discrepancy of more than 50 degrees between total active and total passive flexion, poor gliding and heavy adhesion formation are assumed, and the patient is moved on to the next phase of therapy. According to the original published guidelines, if the discrepancy is less than 50 degrees, the patient continues with the current phase of therapy until 6 weeks after repair. The authors of this chapter do not wait until 6 weeks unless the discrepancy is far less (see also Groth’s Pyramid of Force Progression,4 discussed later on, and Fig 36-19).
Late Stage (starting at 4 weeks or later, depending on tendon glide)
Orthosis. The dorsal blocking orthosis is discontinued. If flexor muscle–tendon unit shortening is a problem, a forearm-based palmar nighttime orthosis may be worn, holding wrist and fingers in maximum comfortable extension (Fig. 36-6). The orthosis, made of plaster or thermoplastic material, is serially remade or adjusted to accommodate any improvements in extension.
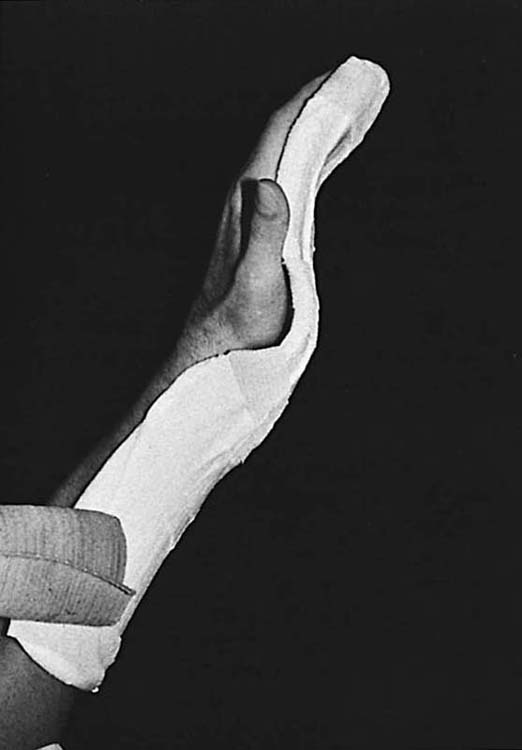
Figure 36-6 A forearm-based orthosis or plaster “stretcher” maintains maximum comfortable extension at night. It is serially remolded or remade to accommodate gains in extension.
Exercise. The patient begins gentle blocking exercises for isolated FDP and FDS glide. For isolated FDP gliding, the MCP and PIP joints are held in extension, thus preventing FDS glide, while the FDP functions alone to flex the DIP joint (Fig. 36-7). Some patients are unable to perform this exercise without exerting excessive force and risking rupture or repair deformation. Alternative interventions can be found discussed under the sections “Treating Adhesion Problems” and “Clinical Decision Making.” For isolated FDS glide, the adjacent fingers are held in full extension, thus holding FDP tendons (which have a common muscle belly) at their full length and making it virtually impossible for them to assist as the FDS flexes the PIP joint (Fig. 36-8). The index finger often has a separate FDP muscle belly, allowing FDP glide even when the adjacent middle finger is held in extension, but often the patient can be taught to use only the FDS tendon, with extension of the middle finger as a cue.

Figure 36-7 Blocking exercise for flexor digitorum profundus gliding. The proximal interphalangeal (PIP) joint is gently maintained in passive PIP joint extension to prevent flexor digitorum superficialis glide. Blocking exercises are performed carefully, avoiding forceful distal interphalangeal joint flexion that may apply excessive stress to newly healed tendons. (From Stewart KM. Review and comparison of current trends in the postoperative management of tendon repair. Hand Clin. 1991;7:447–460.)
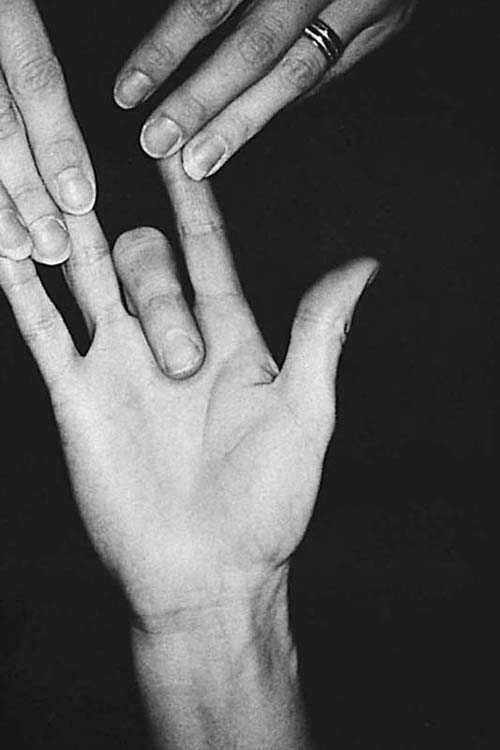
Figure 36-8 Active isolated superficialis exercise—flexing one finger at a time at the proximal interphalangeal joint while holding the other fingers in extension with the uninvolved hand.
If active flexion does not improve rapidly enough (in 1 week according to the original publication), the program is upgraded to include activities involving increased stress to the repair to elicit greater excursion. From there the program is gradually progressed to add greater stress as needed. If the patient begins to make faster progress, the progression of the program is delayed, to avoid excessive stress in the presence of improving tendon excursion.
Greater resistance to flexion elicits a stronger muscle contraction and therefore assists in elongating tendon adhesions and improving glide, but excessive resistance may rupture a tendon even as late as 3 months after injury. In general, the more adherent the tendon, the safer it is to apply resistance to glide. The tendon that is gliding well does not need that additional resistance. Most tendons are not ready for heavy resistance (e.g., lifting over 10 pounds, using grip exercisers) and manual labor job simulation until 10 to 12 weeks.
Early Passive Mobilization
Rationale and Indications
If applied with care, early passive mobilization (starting within a few days of repair) has been shown to produce superior results, apparently because early mobilization inhibits restrictive adhesion formation, promotes intrinsic healing and synovial diffusion, and produces a stronger repair, preventing the decrease in tensile strength of repairs noted in immobilized tendons by Mason and Allen and by Urbaniak and colleagues.2,8,15,20,26,79,80 In a study using metal markers in repaired flexor tendons, Silfverskiöld and coworkers81 demonstrated that measurable passive excursion occurs with passive IP joint flexion. Related studies by May and associates82 and Silfverskiöld and colleagues83 found a significant correlation between early passive IP joint flexion and later active flexion measured in long-term follow-up. Drawing on the results of these studies and others,21,84-88 Strickland89 summarizes the passive excursion of the long finger flexors as follows: In uninjured flexors, passive MCP joint motion does not provide any excursion, while DIP joint motion provides 1 to 2 mm of FDP excursion per 10 degrees of joint flexion, and PIP joint flexion provides 1.5 mm of glide per 10 degrees of joint flexion. In contrast, in the case of repaired flexors, 10 degrees of passive DIP joint flexion produces only 0.3 mm of excursion (a substantial decrease), and 10 degrees of passive PIP joint flexion gives 1.3 mm of excursion (a much smaller decrease compared with the unrepaired tendon). Clearly the repaired tendon’s excursion is affected by the increased bulk and other factors contributing to gliding resistance.
Published Protocols
There are two basic types of early passive mobilization programs. One approach is based on the work of Kleinert and colleagues,90,91 and the other on that of Duran and Houser.92 Hand specialists have worked many variations on these two approaches, and in fact, few experienced therapists adhere closely to one protocol or the other.93-96
In both approaches, a forearm-based dorsal orthosis blocks the MCP joints and wrist in flexion to place the flexor tendons on slack, and the IP joints are left free or allowed to extend to neutral within the orthosis. The orthosis allows passive flexion of the fingers but does not allow extension beyond the limits of the orthosis. Dynamic traction maintains the fingers in flexion, to further relax the tendon and prevent inadvertent active flexion. The dynamic traction may be provided by rubber bands, elastic threads, springs, or other devices; the traction is applied to the fingernail either by placing a suture through the nail in surgery, or by gluing to the fingernail a dress hook, Velcro, a piece of soft leather or moleskin, or the rubber band itself.
Duran and Houser
Early Stage (from 0 to 4.5 weeks)
Orthosis. The wrist is held in 20 degrees of flexion and the MCP joints in a relaxed position of flexion.
Exercise. Duran and Houser92 determined through clinical and experimental observation that 3 to 5 mm of glide was sufficient to prevent formation of firm tendon adhesions; the exercises (six to eight repetitions twice a day) are designed to achieve this. With the MCP and PIP joints flexed, the DIP joint is passively extended, thus moving the FDP repair distally, away from an FDS repair. Then with the DIP and MCP joints flexed, the PIP joint is extended; both repairs glide distally away from the site of repair and any surrounding tissues to which they might otherwise form adhesions (Fig. 36-9).
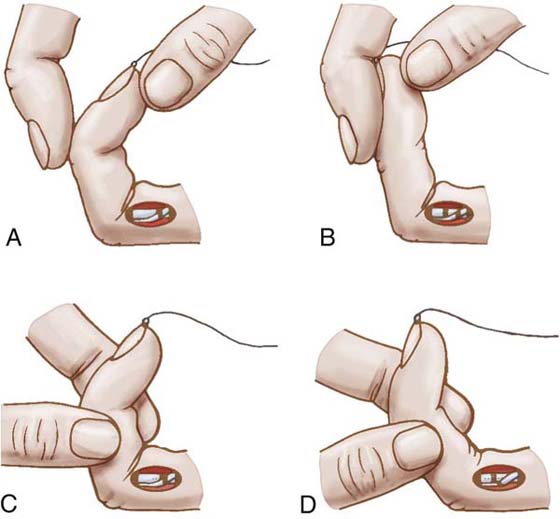
Figure 36-9 Duran and Houser’s exercises for passive flexor tendon gliding. With the metacarpophalangeal (MCP) joint and proximal interphalangeal (PIP) joint flexed (A), the distal interphalangeal (DIP) joint is passively extended (B), thus moving the flexor digitorum profundus repair distally, away from a flexor digitorum superficialis repair. Then with DIP and MCP joints flexed (C), the PIP joint is extended (D); both repairs glide distally away from the site of repair and any surrounding tissues to which they might otherwise form adhesions. (From Duran RJ, et al: Management of flexor tendon lacerations in zone 2 using controlled passive motion postoperatively. In Hunter JM, et al, editors: Rehabilitation of the Hand, ed 3. St Louis: Mosby, 1990.)
Intermediate Stage (from 4.5 weeks to 7.5 or 8 weeks)
Orthosis. After 4.5 weeks, the orthosis is replaced with a wristband to which rubber-band traction is attached (Fig. 36-10)
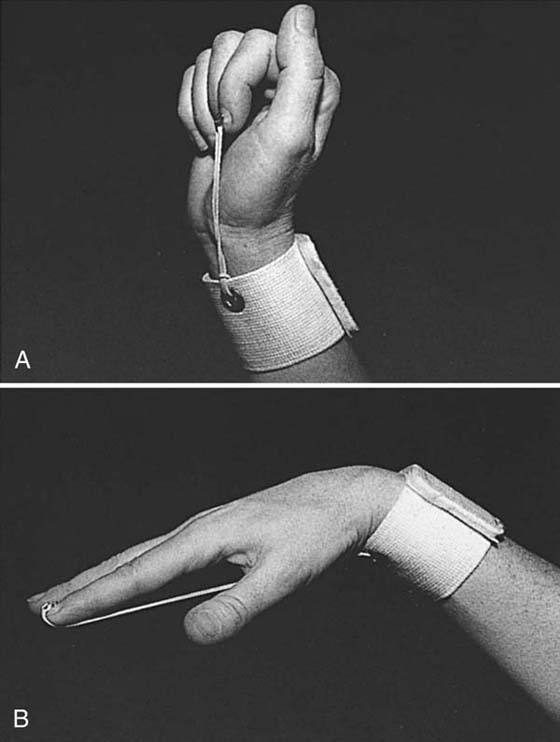
Figure 36-10 Elastic traction from the wrist band prevents simultaneous wrist and finger extension. A, When the wrist is extended, the fingers are passively flexed by the elastic traction. B, When the fingers extend, the wrist is passively flexed. (From Stewart KM. Tendon injuries. In: Stanley BG, Tribuzi SM, eds. Concepts in Hand Rehabilitation. Philadelphia: FA Davis, 1992;353–394.)
Exercise. Active extension exercises begin within the limitations imposed by the wristband. Active flexion (blocking, FDS gliding, and fisting) is initiated on removal of the wristband at 5.5 weeks.
Late Stage (starting at 7.5 to 8 weeks). Resisted flexion waits until 7.5 to 8 weeks. The program is upgraded following the principles outlined under the section on “Immobilization.”
Modified Duran
The Duran protocol is not frequently used in its standard form by hand therapists. Some therapists (including the authors of this chapter) now use what is often called a “modified Duran” approach. This applies a dorsal protective orthosis (we prefer 40–50 degrees of flexion at the MCP joints and from 20 degrees of extension to 20 degrees of flexion at the wrist, depending on the quality of the repair and other factors), but the rubber-band traction is omitted and the IP joints are strapped in extension between exercises or at night (Fig. 36-11). Patients perform passive individual and composite flexion and extension, and active composite extension exercises (manually blocking the MCP joint in greater flexion for more complete active IP joint extension) as well as the passive flexion and extension exercises advocated by Duran and Houser. Only during therapy is the orthosis removed for careful protected SWM exercises (passive or assisted simultaneous wrist flexion and finger extension, alternating with simultaneous wrist extension and finger flexion). Obviously performance of SWM exercises depends on the zone of injury and relative safety of this maneuver for the patient.
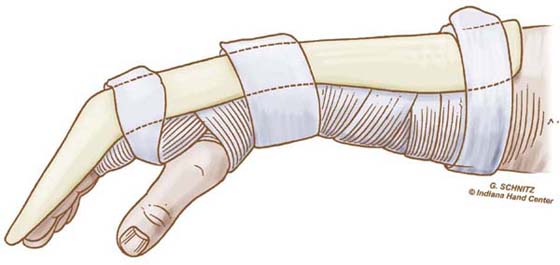
Figure 36-11 Dorsal blocking orthosis used for modified Duran protocol. Wrist and metacarpophalangeal joints are flexed, and fingers are strapped in interphalangeal joint extension when not exercising. (From Cannon N. Indiana Hand Cen News. 1993;1:13.)
Kleinert
Duran and Houser use dynamic traction to rest the digit in flexion, but Kleinert and colleagues uses the rubber band to resist full active extension, based on findings of electromyographic silence in the flexors during resisted digit extension.91,97 Others have questioned this finding in more recent research, as noted later.
The original protocol is not described in detail, as it is no longer used much in the original form.
Orthosis. In the original Kleinert protocol, the dorsal blocking orthosis blocked the wrist in 45 degrees of flexion and the MCP joints in 10 to 20 degrees (since modified to less wrist flexion [20 degrees] and more MCP joint flexion [40 degrees]). Rubber-band traction was directed to the fingernail from the wrist or just proximal to the wrist (more recently modified to direct traction through a palmar pulley, as we shall see in May’s program discussed in the following section and later in this chapter).
Exercise. Every hour, the patient actively extends the fingers to the limits of the orthosis 10 times, allowing the rubber bands to flex the fingers.
At 3 to 6 weeks (depending on the quality of tendon glide) gentle active flexion may begin (intermediate stage), although resisted exercise waits until 6 to 8 weeks (late stage).
May and colleagues95 published an early passive mobilization protocol that they called the “four-finger” method. The wrist is flexed 30 to 45 degrees and the MCP joints 50 to 70 degrees. The dorsal orthosis extends only to the PIP joints, to allow full active PIP joint extension. Rubber-band traction passes through a palmar pulley, and patients perform hourly active IP joint extension exercises. All four fingers are included in traction, even if not injured. Manual pressure to all four fingers is used to attain the final degrees of passive flexion during exercise. Patients use the uninvolved hand to decrease resistance from the rubber bands by pulling them distally during the active extension part of the exercises. At night the rubber bands are detached, and a volar component is added holding the IP joints in extension.
The orthosis is discontinued at 4 weeks, and active flexion and extension is initiated (intermediate stage).
As previously noted, all early passive mobilization programs use a dorsal blocking orthosis for at least 3 weeks, and all involve some form of passive flexion and active extension. Except for the Duran, all use frequent exercise: every 1 to 2 hours. Over the years, there has been a tendency to use less and less wrist flexion; severe wrist flexion angles are uncomfortable and may lead to difficulty regaining wrist extension. In the zone 4 or 5 injury, excessive flexion can lead to serious flexion contractures.
Early Active Mobilization
Rationale
Early mobilization is applied to recently injured, edematous tendons with added bulk at the suture sites. The tendons are mobilized within surrounding sheath or other structures that are also edematous and that often do not provide a smooth gliding surface. In early passive mobilization programs, the tendon is pushed proximally; as has been pointed out, this is akin to pushing a piece of cooked linguine down a tube. The tendon is likely to fold or bunch up rather than glide. Early active mobilization involves active contraction of the injured flexor muscle, pulling the tendon proximally, and logically this should produce better glide. Certainly the results of such programs so far have been very encouraging, supporting the observation made earlier that some of the best early passive mobilization results come when patients “cheat” and add a little active motion. Studies81,87,98,99 have found that passive IP joint flexion does provide passive FDP glide. However, one study found that although passive PIP joint flexion “mobilized the tendon with an efficiency of 90%” compared with active motion, the efficiency of DIP joint flexion was only 36%.100 This could mean a poorer prognosis for zone II FDP injuries over the middle phalanx when managed with passive mobilization.
Kubota and coworkers,26 investigating the breaking strength and increase in cellular activity produced by early mobilization and tension to tendon repairs, found that early mobilization without tension on the repair was not as effective as a combination of the two (such as would occur in active mobilization). We conclude that active mobilization produces a stronger repair with better excursion, especially at the level of the middle phalanx. Furthermore, if the tendon attains better excursion, the “milking” effect increases, thus enhancing the nutrition through synovial diffusion.
Repair techniques have improved vastly in recent years: we now have stronger, less bulky sutures that glide much more easily. Clearly, whenever feasible, early active mobilization is preferable to early passive mobilization. The literature is growing rapidly and contains a diversity of postoperative approaches.24,58,94,101-109 It can be difficult to sort out the relative value of one approach over another, or to select the appropriate patient for early active mobilization. This should not stop the experienced therapist or surgeon from exploring this promising avenue, as long as we remember one caveat: Just as a piece of cooked linguine bunches up if pushed through a tube, it also tears if pulled too hard! Early active mobilization is only appropriate if both therapist and surgeon possess skill and experience in tendon management, if they communicate closely with each other, if the suture used was of adequate strength, and if the patient is highly reliable and understands the program thoroughly.
Published Protocols
Most early active mobilization programs were developed for zone 2 injuries. Almost all use a dorsal blocking orthosis like those employed for early passive mobilization. Exercises and exercise frequency vary, but all programs protect the tendon by limiting active flexion for the first 3 to 6 weeks. Following is a discussion of the distinctions between a few selected protocols. Some of these undoubtedly have been updated since publication. Wherever possible, changes have been incorporated in the following material.
Active Mobilization
Belfast and Sheffield. Authors from the United Kingdom have published a group of related early active mobilization programs. Two similar original protocols102,107 were modified subsequently by other authors.103,104,110,111 Following is one of the more detailed of the recently published versions by Gratton.104
Early Stage (from 0 to 4 or 6 weeks)
ORTHOSIS. The postoperative cast maintains the wrist at 20 degrees of flexion and the MCP joints at 80 to 90 degrees of flexion, allowing full IP joint extension. The cast extends 2 cm beyond the fingertips to inhibit use of the hand. A radial plaster “wing” wraps around the wrist just proximal to the thumb to prevent the cast from migrating distally. On initiation of therapy, the postoperative dressing is debulked to allow exercise.
EXERCISE. For zone 3 injuries therapy is initiated 24 hours after repair, but zone 2 repairs are allowed to rest until 48 hours after surgery to allow postoperative inflammation to subside. Exercises, performed every 4 hours within the orthosis, include all digits and consist of two repetitions each of full passive flexion, active flexion, and active extension. The first week’s goal is full passive flexion, full active extension, and active flexion to 30 degrees at the PIP joint and 5 to 10 degrees at the DIP joint. Active flexion is expected to gradually increase over following weeks, reaching 80 to 90 degrees at the PIP joint and 50 to 60 degrees at the DIP joint by the fourth week. In actual practice these precise flexion measurements are not used; instead the patient is taught to place the four fingers of the opposite hand across the palm of the involved hand, with the little finger touching the palm. The patient is asked to flex the involved finger(s) to touch the contralateral index finger, which is closest to the tips of the involved fingers. To advance the program the patient progressively places three fingers in the palm instead of four (to set a new goal for increased flexion), then two fingers, and then one. This gives a gradually moving goal that progresses to flexion to the palm.112
Intermediate Stage (starting at 4 to 6 weeks)
ORTHOSIS. The orthosis is discontinued at 4 weeks if tendon glide is poor (not achieving expected goals given earlier), at 5 weeks for most patients, or at 6 weeks for patients with unusually good tendon gliding (full fist developing within the first 2 weeks). Three weeks after orthotic positioning is discontinued, any residual flexion contractures are treated with finger-based dynamic extension orthoses.
EXERCISE. The only exercise specified for this period is protected passive IP joint extension (with the MCP joint held in flexion) in the presence of flexion contractures. Presumably patients continue active flexion and extension exercises, and the program is progressed from this point as it would be for any tendon protocol. Small and associates107 do speak of using blocking exercises to increase tendon glide at 6 weeks, and Cullen and colleagues102 initiate progressive resistive exercise and heavier hand use at 8 weeks, with full function expected by 12 weeks.
Note that in published versions of this approach, the patients were hospitalized initially to ensure compliance. This is no longer the case for most patients in Britain. There are no published results for this program without hospitalization.
Active Hold/Place Hold Mobilization
Strickland/Cannon. Various authors24,80,113,114 have attempted to quantify the force or muscle tension of the flexors during such motions as passive and active flexion and flexion against varying amounts of resistance. Each author has used a different method and arrived at different numbers. For their early mobilization protocol, Strickland115 and Cannon101 assumed forces similar to those measured by Urbaniak and coworkers80: for FDP, 500 g for passive motion (Urbaniak and coworkers: 200–300 g) and 1500 g for “light grip” (Urbaniak and coworkers: 1500 g for flexion against moderate resistance). For FDS, the values are 15% to 30% of the values for FDP. Their protocol assumes that some margin must be allowed for postoperative edema and other factors, and they rely on tensile strength of 2150 to 4300 g during the first 3 weeks with the Tajima repair plus a horizontal mattress (or an equivalent four-strand suture59) and a running lock circumferential suture. They further decrease the load on the tendons by holding the wrist in extension and keeping the MCP joints flexed for active flexion exercise. This is based on work by Savage,113 who found that the force required for active digit flexion (work of flexion) decreased when the wrist was held at 45 degrees of extension and the MCPs at 90 degrees of flexion.
This is properly speaking an “active hold” or “place hold active mobilization” program. The digits are passively placed in flexion, and the patient then maintains the flexion with a gentle muscle contraction. Patients learn to use only minimal force by practicing with the uninjured hand and also use biofeedback to monitor the strength of contraction (<10 mV on a Cyborg model biofeedback unit).
Early Stage (from 0 to 4 weeks)
ORTHOSIS. Two different orthoses are used. A dorsal blocking orthosis is worn most of the time, with the wrist at 20 degrees of flexion and the MCP joints at 50 degrees (Figs. 36-12 and 36-13). The exercise orthosis has a hinged wrist, allowing full wrist flexion, but wrist extension is limited to 30 degrees. Full digit flexion and full IP joint extension are allowed, but MCP joint extension is limited to 60 degrees. The orthosis used for distal FPL repairs (zone T1) is similar but allows IP joint extension to only 25 degrees (as in the Evans zone 1 protocol) to prevent repair deformation and problems with glide deep to the A2 pulley. In current practice, the authors sometimes use only the exercise orthosis, adding a block between the two hinged components to maintain wrist flexion between exercise sessions.116
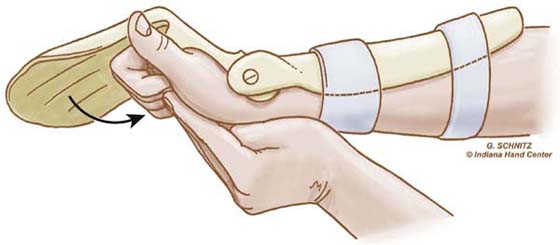
Figure 36-12 The patient extends the wrist actively with simultaneous passive digit flexion. (From Cannon N. Indiana Hand Cen News. 1993;1:13–17.)
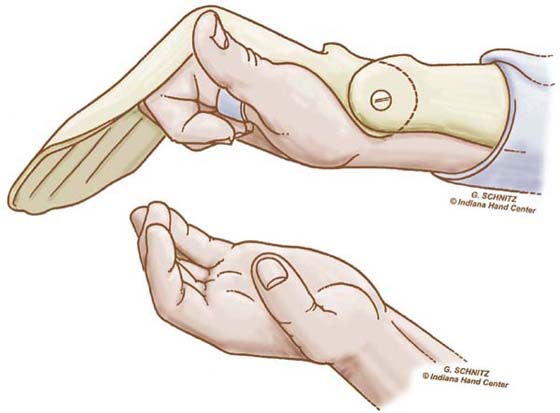
Figure 36-13 The patient maintains digit flexion with a gentle active muscle contraction. (From Cannon N. Indiana Hand Cen News. 1993;1:13–17.)
EXERCISE. Every hour patients perform the modified Duran exercises in the dorsal blocking orthosis, followed by place-and-hold digit flexion in the exercise orthosis. The patient extends the wrist actively with simultaneous passive digit flexion (see Fig. 36-12) and actively maintains digit flexion for 5 seconds (see Fig. 36-13). The patient then relaxes and allows the wrist to flex and digits to extend within the limits of the orthosis (Fig. 36-14). Note again the use of SWM to both protect the tendon and increase excursion.
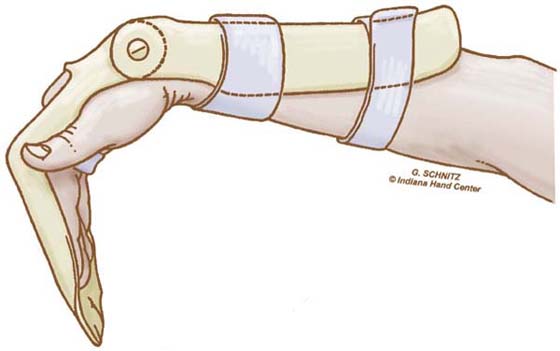
Figure 36-14 The wrist is allowed to relax into flexion with simultaneous digit extension (limited to 60 degrees at the metacarpophalangeal joints). (From Cannon N. Indiana Hand Cen News. 1993;1:13–17.)
Intermediate Stage (from 4 weeks to 7 or 8 weeks)
ORTHOSIS. The exercise orthosis is discontinued. The patient still wears the dorsal blocking orthosis except for active flexion exercises.
EXERCISE. The SWM exercises (Fig. 36-15) continue every 2 hours, but now flexion is active. The patient is instructed to avoid simultaneous wrist and digit extension. FDS gliding also may be added. At 5 to 6 weeks, blocking and hook fists may be added if needed to improve tendon gliding.
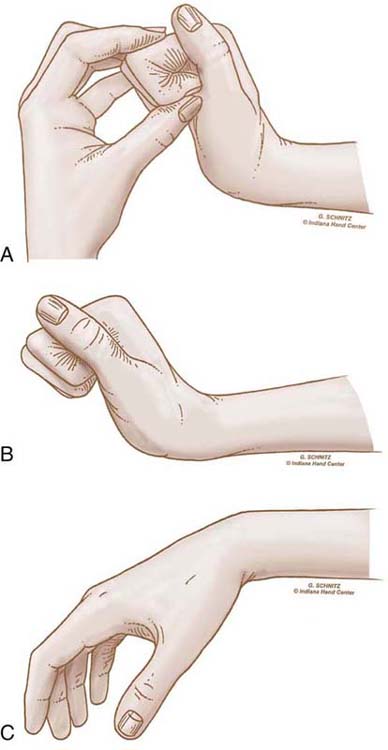
Figure 36-15 The exercise orthosis is discontinued at 4 weeks, but the patient continues tenodesis exercises. A, The wrist is extended, with passive digit flexion, maintained actively (B). C, The patient then allows the wrist to relax into flexion with simultaneous digit extension. (From Cannon N. Indiana Hand Cen News. 1993;1:13–17.)
Late Stage (starting at 7 to 8 weeks)
ORTHOSIS. The orthosis is discontinued.
EXERCISE. Progressive resistive exercise is initiated. The patient gradually resumes activities of daily living, with no restrictions by 14 weeks. FPL is moved more aggressively than digit flexors, and flexors to the small finger are moved the least aggressively, in the light of the authors’ clinical observation that repairs of these tendons are the most prone to deformation and rupture.117
Silfverskiöld and May.118 These authors have added an active hold component to their previously published early passive mobilization protocol (see the May and colleagues’ protocol,95 discussed earlier) in patients whose zone II FDP tendons were repaired with a modified Kessler core suture and a new epitenon suture (the “cross-stitch”). The wrist is positioned in neutral instead of 30 to 45 degrees of flexion, but the orthosis is otherwise identical to the early passive mobilization protocol (MCP joints in 50–70 degrees flexion and orthosis extending only to the PIP joints). Exercises are similar to those outlined for the four-finger program, but after using the uninvolved hand to push the fingers of the involved hand into full flexion, the patient uses an active muscle contraction to maintain flexion of the involved fingers for 2 to 3 seconds. The program is progressed in the same manner as the four-finger protocol. In a prospective clinical study of 47 patients with 56 injured fingers, the authors demonstrated promising results. Note that in the original Swedish study, patients were hospitalized initially to ensure close compliance with the program. Nowadays Swedish patients performing this program are no longer hospitalized; there are no recently published results.
Evans and Thompson. Evans and Thompson24 have examined the biomechanical aspects of early active hold mobilization using the concept of “minimal active muscle–tendon tension” (MAMTT), the minimal tension required to overcome the viscoelastic resistance of the antagonistic muscle–tendon unit. They calculated the drag encountered by each flexor tendon, the force necessary to overcome that drag, and the force normally exerted by the finger in flexion in various positions. Two central findings were that flexion forces increase dramatically at the end of the flexion range (in a full fist) and when digit flexion is combined with wrist flexion. The authors then surveyed currently used tendon repair sutures and designated guidelines for early place hold mobilization of the tendon with any suture used in combination with an epitenon running suture. They presented a retrospective review of 165 tendons (both flexor and extensor tendons) treated with their guidelines.
The MAMTT exercises are performed only under a therapist’s supervision, while the patient follows an early passive mobilization program at home (which protocol is not specified by the authors; a dorsal blocking orthosis is used, without rubber band traction). For MAMTT exercise, the orthosis is removed. The wrist is passively placed in 20 degrees of extension and the finger passively flexed to 83 degrees at the MCP joint, 75 degrees at the PIP joint, and 40 degrees at the DIP joint. The patient is then asked to maintain the position with as gentle a muscle contraction as possible. The force of the muscle contraction is measured with a small (<150 g) Haldex pinch gauge. A loop of string passes perpendicularly around the gauge arm of the pinch meter and around the finger tip. The patient flexes the finger with a force of 50 g or less.
Unlike the Strickland/Cannon protocol, the Evans and Thompson guidelines suggest that few patients can perform this program at home. However, Evans119 does state that she has patients perform active hold exercises with the uninvolved fingers at home, thus probably eliciting some active muscle–tendon tension in the involved fingers.
Assisted Active
Sandow and McMahon. These authors120 take a rather unusual approach. They position the wrist in 20 degrees of extension to decrease passive extensor resistance to active flexion, and the MCP joints in 90 degrees of flexion, allowing full IP joint extension. They initiate assisted active flexion in the operating room. Exercises are performed hourly. The patient is seen weekly in therapy for adjustment to the program as needed. The orthosis is discontinued at 6 weeks, and buddy straps are applied for interim protection. The program is progressed in the same manner as other tendon management programs.
Timing of Initiation of Early Active Mobilization
Based on studies indicating that early motion increases repair strength,2,22,26 most published protocols start motion at 24 to 48 hours after surgery. Several studies have now been published supporting a longer delay in initiating active motion. These studies all examine the work of flexion encountered during active motion at various points in the first week. Halikis and colleagues75 compared immobilized repairs to those mobilized immediately, those mobilized at 3 days, and those mobilized at 5 days. They found that the work of flexion increased markedly in tendons mobilized immediately, whereas the work of flexion increased the least for tendons initiating active mobilization at 3 days. Other authors have recommended delaying initiation to 5 days121,122 or 7 days.123
Treating Adhesion Problems
Restrictive adhesions are the most common complication after immobilization of the repaired flexor tendon. There are many techniques for mobilizing the adherent tendon, all aimed at gradually lengthening adhesions to allow greater glide. The object is not to break adhesions, because this internal trauma may lead to greater fibrosis and new adhesions.
The best way to treat adhesions is to prevent them or to catch them at a very early stage. To select the best method of treatment, the therapist first identifies the location and extent of adhesions, as described earlier. Precise identification of adhesions assists in planning precise intervention: if a single FDS tendon is adherent, exercise could focus on gliding of that single tendon, with or without resistance (see Fig. 36-8). If all FDS tendons are adherent, DIP joint extension orthoses may be worn during active and resistive exercises to aid in eliciting FDS glide.
A number of different exercises are designed to elicit greater excursion. A commonly used example is blocking exercises (as described earlier under “Immobilization”) to isolate motion of a particular tendon. Blocking exercises can be dangerous for a newly healed tendon if not performed correctly. This is true particularly in the case of isolated DIP joint flexion. If the patient does not concentrate on flexing only the DIP joint but instead “fights” the fingers holding the PIP joint in extension, this active exercise becomes a strongly resisted exercise. When the finger is edematous and the FDP tendon is gliding poorly, the patient may have difficulty resisting the temptation to exercise too vigorously. Patient education includes the danger of rupture with overzealous blocking exercise; some patients may not be appropriate candidates for blocking until 2 or 3 weeks later, when the tendon repair is stronger.
Tendon gliding exercises (see Fig. 36-5) facilitate gliding of FDP and FDS as well as differential gliding to control intertendinous adhesions. If the patient has difficulty understanding how much force is safe to exert, light prehension with synergistic wrist motion may be helpful. If the patient does not understand the concept of synergistic wrist motion or appears to be flexing with excessive force, the following technique may be helpful. The patient sits with elbow on the table, wrist relaxed forward into flexion. He is instructed to extend the wrist while keeping the fingers relaxed. If he is able to do this, then the fingers automatically assume a midflexion position. If instead he extends his fingers along with his wrist, he is asked to extend the wrist while touching his fingers to his thumb or grasping a light object such as a pencil, held by the therapist. Often a functional grasp is much easier for an apprehensive patient to comprehend than a structured exercise.
Sustained grip activities such as dowel sanding, use of a rake or other garden implement, or bicycling elicit isometric flexor contraction. Some patients find it helpful to carry with them at all times a lipstick, a pill bottle, or other small cylinder that they can barely grip. Frequently throughout the day they grip the cylinder 10 times for 10 to 30 seconds.
Therapy putty squeezing or scraping resists tendon excursion and elicits stronger flexor contractions. Whenever possible, functional motions are used in place of exercise: “piano playing” on the tabletop, towel walking, paper crumpling, handling dice, grasping handfuls of packing “popcorn” or large beans, handling light objects of varying size and shape. The patient should be encouraged to resume daily activities as soon as it is safe, not only to facilitate reintegration of the involved fingers, but also to incorporate functional grasp activities to facilitate tendon excursion at home.
One of the most common mistakes made by patients is overdoing resistive exercise or a favorite activity, however light the resistance to flexion involved. This can provoke inflammation and lead to increased fibrosis and stiffness. Patients may develop trigger fingers (stenosing tenosynovitis) through excessive repetitive gripping or squeezing (as with putty exercise). The therapist must not only warn patients of this danger but also routinely palpate for triggering at the A1 pulley.
Neuromuscular electrical stimulation may be used to provoke a stronger muscle contraction; this is appropriate within 1 week of initiating resisted exercise. Ultrasound applied over the area of tendon adherence may be used to provide deep heat and when combined with passive extension active flexor tendon gliding can help to modify and lengthen adhesions. Interested readers are referred to Chapter 118 for further discussion. Superficial and deep scar respond well to soft tissue mobilization techniques such as cross-frictional massage. The patient also may actively contract the affected muscle while massaging over the adherent tendon; the tendon pulls proximally while the patient gently pushes the skin distally, stressing local adhesions.
If flexor adhesions limit extension, orthotic positioning and gentle passive extension may be necessary to regain functional extension. Orthotic options include dynamic and static-progressive extension orthoses for intermittent daytime use, and serial-static orthoses for night use.
Beyond Protocols: Clinical Decision Making
With all of the many tools and techniques at our disposal, the hardest decision is when to use which technique. All of the protocols described are meant to be guidelines rather than recipes. Following are examples of modifications to popular protocols, new directions in research, and new tools for assisting in application of rehabilitation techniques using clinical reasoning and integration of more recent research.
Prevention of Proximal Interphalangeal Joint Flexion Contractures
When the fingers are maintained in PIP joint flexion with dynamic traction, PIP joint flexion contractures often result. One solution is to remove the traction at night and strap the fingers in IP joint extension.82,96,124 Therapists employ variations on night and intermittent day orthotic positioning to correct incipient PIP joint flexion contractures in both Kleinert and “modified Duran” protocols, particularly if a delicate digital nerve repair or other injury has mandated positioning the PIP joint in slight flexion initially.124,125 A static PIP joint extension orthosis may be inserted between the dorsal blocking orthosis and the dorsum of the finger to address this problem when it first develops. Prevention, however, is the best treatment.
Another reason for PIP joint flexion contractures may be the difficulty of extending the injured finger fully against excessive resistance. Recent studies126,127 indicate that increasing the strength of dynamic flexion traction has no advantage; in fact, these electromyographic studies disagree with that of Lister and coworkers,97 finding that flexor contraction is inconsistently inhibited no matter how great the resistance to extension (thus throwing some doubt on any use of resisted extension in early passive mobilization programs). Studies have shown that rubber bands offer increasing resistance as finger extension stretches the elastic further.128-130 Burge and Brown128 found that this increase could be moderated by use of a palmar pulley or by positioning the MCP joint in no more than 20 degrees of flexion. Patients may be instructed to manually release some rubber-band tension during exercise to ease extension as in the May and associates’95 protocol.
Another proposed solution is to change the means of dynamic traction within the orthosis design. In the Washington regimen93,129,131,132 (also known as modified Kleinert) two rubber bands are used, one of which is cut in half so that it forms a single strand. Before performing active extension exercises, the patient detaches proximally the intact rubber band so that only the single-strand elastic resists extension, making full extension easier to achieve. The finger rests in complete flexion to the distal palmar crease when not exercising. An orthosis design proposed by Werntz and colleagues130 incorporates a coiled lever to offer a more constant resistance and make full extension easier to achieve. However, this orthosis is not commonly used, given the less expensive alternatives available.
Even in the absence of dynamic flexion traction, the patient may develop PIP joint flexion contractures if the volar wound and surgical incisions are allowed to limit PIP joint extension as they form scar and contract; if there is excessive edema; or if the patient has difficulty extending fully due to pain, apprehension, or an orthosis that blocks full extension. Unfortunately it is common for permanent PIP joint flexion contractures to develop. Any patient showing this tendency should be positioned in complete PIP joint extension as needed, using some combination of dynamic, static, or serial-static PIP joint extension positioning. If a patient is discharged from therapy with a residual PIP joint flexion contracture, night positioning in extension is crucial for the first few months to a year.
Force and Excursion Issues
Excursion
McGrouther and Ahmed88 found that complete excursion of the FDP and differential excursion between FDP and FDS could be accomplished only through flexion of the DIP joint; later this principle was found to be true also for the flexor pollicis longus (FPL). In other words, to achieve glide of a repair, it is necessary to flex the joints distal to the repair. Horibe’s cadaver study of FDP excursion in zone II confirmed the importance of distal joint flexion but also found that PIP joint flexion appears to produce the greatest excursion relative to the tendon sheath.87 Silfverskiöld and colleagues, in their series of in vivo studies, initially found results agreeing with Horibe’s.100 Later, using a larger number of cases, they found a significant correlation between controlled passive DIP joint ROM and FDP excursion.81 All of these studies taken together suggest that early passive mobilization programs should incorporate the greatest possible degree of flexion in the IP joints. Remember also the difference noted by Strickland and others21,86-89,133 regarding the reduced passive excursion in the repaired flexor tendon.
In the standard dynamic flexion orthosis as first designed by Kleinert, the rubber-band traction is directed from the wrist or distal forearm to the fingernail. This flexes the MCP joint and to a lesser extent the PIP joint but leaves the DIP joint in virtual extension (Fig. 36-16). Slattery and McGrouther134 proposed adding a palmar pulley to redirect dynamic traction and thus fully flex the DIP joint (Fig. 36-17). This orthosis innovation (also known as modified Kleinert) has become standard practice with zone II FDP injuries. Brown and McGrouther84 later suggested that similarly, in FPL lacerations the thumb MCP joint be immobilized and efforts directed toward mobilizing the IP joint.
Figure 36-16 In the Kleinert orthosis as originally designed, the metacarpophalangeal joint and proximal interphalangeal joint are held in flexion by rubber-band traction, but the distal interphalangeal joint rests in almost complete extension.
Figure 36-17 A simple palmar pulley can be provided by a safety pin attached to a palmar strap at distal palmar crease level. The line passes through the “eye” of the safety pin to direct the pull precisely.
Evans94 published her analysis of the excursion data and results of an early passive mobilization program specifically designed for zone I injuries, which are prone to excursion problems, given the limited excursion available in the uninjured zone I FDP. She keeps the DIP joint positioned in flexion with a small orthosis within the larger dorsal protective orthosis. She has also used a similar approach with early active mobilization.135
Cooney and others87,98 conducted a cadaver study comparing the total FDP, total FDS, and differential FDP/FDS excursion obtained at three levels (zones II, III, and V) using three different methods. The first was original Kleinert traction directed from the distal forearm. The second was an orthosis with a palmar pulley (Brooke Army orthosis or modified Kleinert orthosis). Third was a new orthosis using SWM to produce a dynamic tenodesis effect: wrist flexion producing finger extension and wrist extension producing finger flexion. The study found that although all three orthoses produced adequate total excursion (judged according to Duran and Houser’s recommended 3–5 mm), the Brooke Army orthosis produced more than did the Kleinert, and the SWM orthosis produced the greatest excursion of all. Surprisingly, in view of previously cited tendon excursion studies,87,88,100 the traditional Kleinert orthosis produced more differential gliding than did the Brooke Army; the SWM orthosis again outperformed both of the other orthoses.
More recent studies have examined the relative excursion and force in varying combinations of wrist and finger motion. Several authors136-140 have concluded the following: passive flexion and extension of the digits produces low excursion with low force to the repair if the wrist is held in flexion, high excursion with high force to the repair if the wrist is held in extension, and high excursion with low force to the repair if the wrist moves synergistically (wrist extends with finger flexion and flexes with finger extension).
In 2005 Amadio and Tanaka141,142 each published studies that propose a modified SWM exercise for increased excursion with early passive mobilization (Fig. 36-18). The hand starts in full finger and wrist flexion. The first step is passive extension and flexion of the involved digit. The next is extension of the wrist with the fingers held in flexion. Finally, with the wrist still held in extension and the IP joints held in flexion, the MCP joint is extended as far as possible. Then the steps are reversed: MCP joint flexed, wrist flexed, and fingers extended. All of these steps are performed gradually and gently, with respect to soft tissue resistance and always following gentle and persistent protected joint passive range of motion and edema control measures.
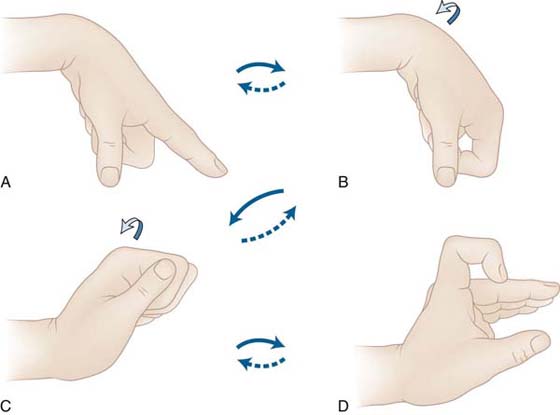
Figure 36-18 Modified passive synergistic wrist motion protocol. A, Passive full extension of metacarpophalangeal (MCP), PIP and DIP joints with wrist at 60 degrees flexion, to pull the tendon distally. B, Passive composite flexion of the finger, keeping the wrist flexed 60 degrees. C, Slow, careful wrist extension to 60 degrees (or maximum comfortable extension), with finger remaining flexed. D, Slow, careful MCP joint extension to 45 degrees hyperextension (or maximum comfortable extension). Steps are repeated in reverse order. (From Amadio PC. Friction of the gliding surface. Implications for tendon surgery and rehabilitation. J Hand Ther. 2005;18:112.)
Motion Versus Tension
There is an ongoing debate regarding the relative importance of the two different kinds of stress applied to the healing tendon: the motion imparted to the tendon and the tension or force placed on the repair. As noted before, Kubota and coworkers26 found that the rate of cellular activity at the repair site was greater with either motion or tension than with neither, but greatest with a combination of the two.
Closer examination of excursion and force variables suggests that in a canine model, increased excursion (>1.7 mm) does not improve ultimate excursion or tensile properties of the repair.138 This contradicts the earlier finding of Silfverskiöld and colleagues81 that the greater the passive excursion (≤6–9 mm, after which the benefit is less) the greater will be the ultimate active excursion. Furthermore, high force (simultaneous extension and flexion of wrist and digits, or force increased from 5 to 17 N) does not appear to improve healing or increase the rate at which the repair gains strength.143,144 As a result of these studies, the therapist must conclude the following: (1) synergistic wrist motion is the safest (high excursion and low force) means of attaining the highest excursion during exercise, whether active or passive; (2) although both motion and stress to the repair are responsible for the known benefits of early mobilization, there is no evidence that high force is more effective than lower force in improving strength or excursion of the tendon; there is no definitive answer about how much early passive or active excursion is need for optimal results.
Choosing the Right Approach and Progressing the Program Appropriately
The most important question in therapy following flexor tendon repair is how to treat the patient in the first few weeks. Simplistically speaking, this boils down to immobilization, early passive mobilization, and early active mobilization. In a perfect world, with a perfect patient, surgeon, and therapist, early active mobilization is the treatment of choice. However, this is not possible for all patients. With a patient who is unable to follow an early mobilization program (because of youth, illness, or other factors), immobilization is the only choice. With a patient who can follow a complex program, early active or passive mobilization should be used. If early mobilization is the choice, then further decisions are dictated by a number of factors, including strength of repair; edema and other sources of resistance to tendon glide; the frequency with which patient can attend therapy; surgical preference; and the therapist’s preference, clinical reasoning skills, and degree of autonomy in making decisions. A recent article by Groth145 examines clinical decision making and therapists’ autonomy in making clinical decisions regarding flexor tendon management.
Surgeon–therapist team A may decide to use early passive mobilization with a patient who appears able to participate but a little overenthusiastic: a possible risk of rupture in the event of inadvertent “cheating” on an early active mobilization program.
Surgeon–therapist team B may decide on early active mobilization for their patient because the suture is strong, the edema well-controlled, and the patient apparently very cautious.
In both of these cases, either tissue response or patient response may not be as anticipated, and the plan may need changing on the subsequent visits. It’s incumbent on surgeons and therapists to understand that just as not every patient can follow a certain protocol, many patients need a more nuanced approach to treatment, with the plan changing as needed in accordance with findings at each visit. In addition, using one or more of several available clinical decision-making models, the therapist and surgeon can carefully examine the available information and design a program that allows for increasing or decreasing force and excursion, switching from passive to active mobilization and otherwise upgrading the program in a logical and safe fashion.
Groth4 devised the Pyramid of Progressive Force Exercises, a model for systematic evaluation and progression of stress applied to the healing tendon, based on existing studies of tendon excursion and loading. She used the concept of a pyramid with nine levels (Fig. 36-19) to illustrate the model. The broad base of the pyramid represents exercises applying the lowest force to the repair, and therefore prescribed most frequently; the apex of the pyramid represents exercises applying the highest force, and therefore prescribed least frequently. The nine levels are further divided into exercises performed with the wrist protected (the lowest five levels) and the wrist unprotected. The adhesion grading system discussed earlier (see Table 36-1) aids the therapist in determining when to progress the program. Essentially, the program is progressed when a flexion lag is present and does not respond adequately to current treatment.
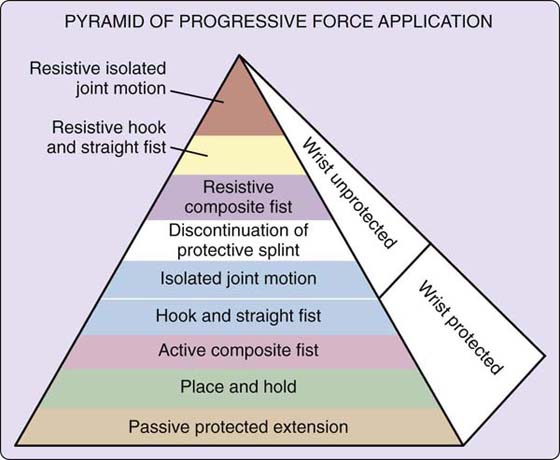
Figure 36-19 Progressive application of force to the repaired intrasynovial flexor tendon. (From Groth G. Pyramid of progressive force exercises to the injured flexor tendon. J Hand Ther. 2004;17:31.)
A brief description of the nine levels and their rationale follows. Passive protected digital extension consists of passive flexion and extension of the IP joints, both independently and in composite motion, together with SWM to increase excursion and decrease force applied to the repair. The next level is place-and-hold finger flexion in 20 degrees of wrist extension and partial finger flexion, similar to the Evans and Thompson exercises, designed to gain composite active flexion with low stress. The third level exercise is full active composite flexion (increased force to the repair) with slight wrist extension. At the fourth level, hook and straight-fist exercises are added to increase total and differential tendon excursion. Isolated joint motion is the fifth level, adding blocking for FDP gliding, PIP joint flexion and FDS gliding to increase the force to the tendon repair. The remaining four levels, discontinuance of protective positioning, resistive composite fist, resistive hook and straight fist, and resistive isolated joint motion, all eliminate wrist protection (whether by incorporation of synergistic wrist motion or by holding a given wrist position during finger motion) and place successively increasing stress to the repair. Although excursion and force data are still limited, Groth’s program uses what data are available to logically relate tendon response to treatment options. Thus she provides a useful tool for planning treatment according to tendon status, rather than according to a timed protocol. More recently von der Heyde presented her version of the pyramid, using essentially the same data to support synergistic wrist motion as proposed by Cannon.146
Another potentially useful tool is the concept of a safe zone, which the therapist and surgeon can use to balance surgical, patient, and therapeutic considerations in moving the tendon appropriately. This model, proposed by Amadio,141 incorporates data on force, gliding resistance, and tendon repair strength, to illustrate the range of tendon loads that produce excursion but do not cause a rupture or tendon gap. In Figure 36-20 we see a narrow safe zone between forces (in newtons) capable of producing excursion and excessive forces with risk of gapping or rupture. Figure 36-21 illustrates how the safe zone is expanded with increased repair strength or reduction of resistance to gliding.
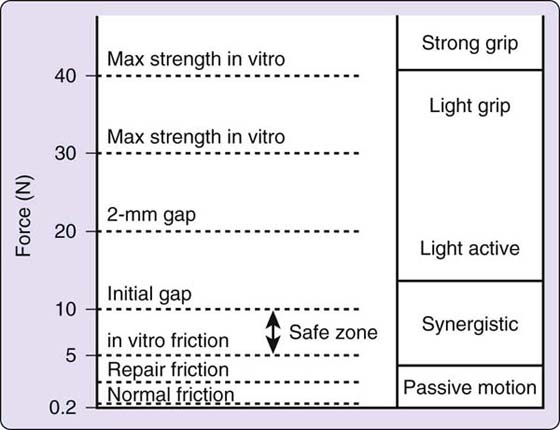
Figure 36-20 Small safe zone for a four-strand repair with 40-N (9-pound) breaking strength. (From Amadio PC. Friction of the gliding surface. Implications for tendon surgery and rehabilitation. J Hand Ther. 2005;18:112.)
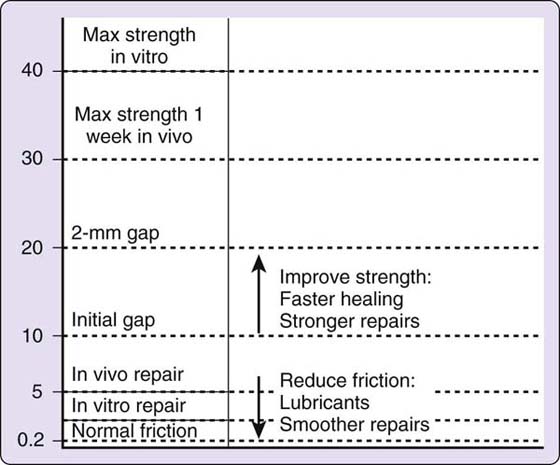
Figure 36-21 Larger safe zone with increased suture repair strength and faster healing, and reduced friction through the use of lubricants and repairs with smooth gliding surfaces. (From Amadio PC. Friction of the gliding surface. Implications for tendon surgery and rehabilitation. J Hand Ther. 2005;18:112.)
Groth’s and Amadio’s models can be used together. The clinician can determine the safe zone at initiation of therapy and then decide how to initiate motion while staying within that safe zone. If complications arise (e.g., unexpected increase in edema), the safe zone contracts, and therefore the plan must be modified to place less stress on the repair or to stay at the same level of stress until the safe zone can be expanded (e.g., if edema decreases). The adhesion-grading system helps the clinician decide when levels of stress must be modified, and the force pyramid gives a structured way to choose how to increase or control stress to the repair.
Although the steps delineated by Groth are appropriate, they need not be adhered to unquestioningly. For example, some patients have difficulty learning how firmly to stabilize the PIP joint when isolating DIP joint motion and may endanger the FDS repair by actually resisting FDS. The same patients may more easily apply an appropriate amount of stress using functional grasp activities such as picking up and transferring large pieces of polyester batting.
The force pyramid and the concept of the safe zone can be applied to existing programs such as the Indianapolis hinged orthosis program. There is no requirement that the patient delay active mobilization and start with passive mobilization. The therapist may choose active (place-and-hold) mobilization in a hinged orthosis, as long as the safe zone allows.
We prefer to start therapy with postoperative orthosis application as soon as possible after surgery. If we are using active mobilization, we start with gentle passive motion and edema control and delay initiation of active mobilization until 3 to 5 days after surgery to allow inflammation to subside somewhat and reduce the work of flexion. We always precede active flexion exercises with edema control measures and passive flexion to reduce tissue resistance and always integrate a modified Duran approach with active mobilization.
Flexor Pollicis Longus
Management of flexor pollicis longus (FPL) repairs presents a different set of problems to the therapist, for several reasons. Lacerated FPL tendons tend to retract further and more quickly than do the finger flexors. This may be due to the lack of a lumbrical origin to restrain retraction, fewer interconnections with the adjacent tendons, or, as Elliot and Southgate have speculated,147 to an inherent tendency of the FPL muscle itself to shorten rapidly. Even a short delay to surgery may require not only a separate proximal incision at or near the wrist to retrieve the proximal FPL, but also a repair performed under tension. Tendon-lengthening procedures can decrease tension on the repair, but additional adhesions may result from lengthening as well as from the proximal incision and the process of pulling the proximal stump into place for repair. To complicate matters further, there are often concurrent injuries to digital neurovascular structures, thenar muscles, and the branches of median and ulnar nerves in the thenar eminence. One study found that 74% of FPL injuries studied were in zone II, and 82% involved injury to one or both neurovascular bundles.148 The challenges presented, therefore, are (1) to repair the tendon as early as possible, under as little tension as possible, and with the strongest suture feasible; (2) to restore motion with a program that is aggressive enough to produce functional excursion by preventing or modifying dense adhesions and yet cautious enough to prevent rupture; and (3), in the presence of irreparable digital nerve injury, to restore thumb function without return of full sensation.
As we plan our treatment, it’s vital to understand the balance of muscle activity controlling the thumb and the functional results of residual strength and ROM deficits. An electromyographic study by Cooney and colleagues149 found that the FPL was the primary muscle involved in isometric flexion and one of the primary muscles (along with adductor pollicis, flexor pollicis brevis and opponens pollicis) involved in key pinch, but it played a secondary role in other kinds of pinch. In addition, there remains disagreement as to the functional impairment posed by limited IP joint flexion, with some stating that limited IP joint extension (−25 degrees) and flexion (30 degrees) does not significantly impede function.148
Given the relative paucity of published studies on rehabilitation of FPL repairs, most therapists position the wrist in some degree of flexion, the thumb in mild carpometacarpal (CMC) joint flexion and palmar abduction, the MCP joint in flexion, and the IP joint allowed full extension. For passive mobilization, some use an adapted Kleinert technique, but others use a form of modified Duran. Whichever passive technique is used for zone I and II repairs, the therapist should incorporate immobilization of the MCP joint during passive IP joint flexion, based on the work of Brown and McGrouther,84 who found no FPL gliding over the proximal phalanx with MCP joint flexion, compared with considerable glide with the MCP joint immobilized and the IP joint passively flexed and extended.
The published studies on rehabilitation of FPL repairs use a variety of means of mobilization and methods of assessing results, making them difficult to compare. The majority of studies examine immobilization or early passive mobilization (or both). Interestingly no studies have large enough populations to recommend one over the other.
A recent series of articles by Elliott, Sirotakova, and colleagues103,147,150,151 have taken a systematic look at early active mobilization, raising some interesting questions. They compared four different groups of patients with variations on suture and postoperative management. Results improved with three specific elements: (1) immobilization of the fingers between exercise sessions (to prevent the inadvertent thumb IP joint flexion that accompanies grasp and may have increased the rupture rate), (2) wrist position in 10 degrees of ulnar deviation and 10 degrees of wrist extension (to reduce stress at the point where the FPL changes course at the distal border of the carpal canal), and (3) the stronger suture (four-strand core and cross-stitch circumferential suture). Although the authors note no evidence comparing early passive to early active mobilization, and therefore do not recommend one over the other, they do recommend a four-strand core and cross-stitch circumferential suture.
The active mobilization program as most recently published147 involves active opposition of the thumb tip first to the tip of the middle finger in the first week, to the tip of the ring finger in the second week, and then as far as possible thereafter. Flexion was assisted and more vigorous passive flexion was added as needed.112
A Special Case: Multiple Tendon and Nerve Lacerations in Zone V
Laceration of multiple flexor tendons in zone V presents a special problem in management. As noted earlier, in zone V (from proximal edge of carpal tunnel to the musculotendinous junction) the flexor tendons lie in close proximity both to each other and to major nerves (median and ulnar) and arteries (radial and ulnar). This type of injury has been labeled “spaghetti wrist,” “suicide wrist,” and “full-house syndrome” by various authors.111,152-156 As with all flexor tendon injuries besides those in zone II, there is very little in the literature to indicate the best postoperative management. However, to design the appropriate program, we can draw on experience, a good understanding of how the involved structures heal, and both specific and related literature.
The psychosocial ramifications of such injuries are significant, but luckily not insurmountable. A certain percentage of these injuries are due to suicide attempts, so that one might expect a poor prognosis due to psychological and emotional issues. However, some authors have found favorable results and improved psychological status after such injuries. Many sustain these injuries on broken glass, often when an angry young man punches his fist through a glass window. Again the immaturity and poor impulse control of the patient seem to predict compromised results, but youth is to the patient’s advantage, with better results having been noted in younger patients by some authors.
These patients, correctly treated, generally attain satisfactory or full digit flexion, since the extrasynovial tendons can produce full flexion even with excursion limited by relatively heavy adhesions to the overlying skin and surrounding tissues. Independent FDS glide is difficult to achieve, but has not been found to impair function. In fact, recent research indicates that in the normal hand, FDS may not glide as independently as one might assume.157 Wrist and digit extension are commonly limited to some extent by flexor adhesions. There may be limitations in isolated wrist extension as well as in composite wrist and digit extension. In most cases, either passive extension can be regained over time through diligent treatment, or the patient finds that the lack of extension is not a functional impairment.
A greater problem is reinnervation. Both sensory and intrinsic muscle reinnervation are needed for a full functional recovery. One small study suggests that ulnar nerve injuries may have a poorer prognosis than median nerve injuries.156 In any case, in addition to impaired flexor tendon function, these patients lack part or all of their intrinsic muscle function (depending on which nerve is injured) as well as median or ulnar nerve sensibility. Unless and until reinnervation occurs, patients may injure their insensate fingers when attempting to use their hands normally and may have difficulty with prehension and strong grip due to lack of normal sensory feedback. They lack full grip strength and pinch strength in the absence of intrinsic muscle power and miss the control and balance afforded by the intrinsics for fine dexterity. Over time, if not carefully treated, they may develop deformities (“claw hand” or “ape hand,” PIP joint flexion contractures, thumb adduction contracture) that are both unattractive and an impediment to function.
The literature includes both early passive mobilization152-156,158 and early active mobilization111 approaches. As always, the approach should be selected according to the individual patient’s characteristics, with preference given to early mobilization of some sort. All of the published protocols keep the wrist in some flexion, but as with injuries at other levels, the recent trend is toward less wrist flexion. Too much wrist flexion can make it very difficult to regain extension with an injury so close to the wrist and to the flexor retinaculum, a prime source for flexor adhesions.
Our preference is to protect the patient with wrist as close to neutral as possible and the MCP joints flexed about 40 degrees. Holding the MCP joints in flexion might encourage some intrinsic tightness, which is a bonus when a long wait for return of nerve function is expected. Nerves repaired under tension must be very carefully protected with more wrist flexion.
Early management is similar to that for injuries in other zones, with the following special considerations. As soon as possible after active mobilization is initiated, the program should include differential tendon gliding. Because passive extension is so often a problem, these patients may need nighttime serial static extension positioning (see Fig. 36-6) as early as 4 weeks after repair, and dynamic extension positioning during the day beginning within the following week. Often the flexor tightness limits the “clawing” produced by intrinsic palsies, but if not, an “anticlaw” orthosis of some sort (preventing MCP joint hyperextension but allowing full wrist and finger flexion) may be needed to prevent development of a full-fledged claw deformity. The therapist may opt as well to forego intrinsic stretch exercises in the protective stage, so that some intrinsic tightness is allowed to develop as noted earlier.
Summary
This chapter has reviewed the fundamentals of tendon management and explored a range of management approaches. No single source can prepare the clinician to treat the repaired flexor tendon. Readers are urged to read both the original references cited in discussion of each protocol and the research that validates each approach.
As we study suture strength and gliding properties, measure muscle–tendon force, gather data, and choose the protocol based on scientific principles, we must not lose sight of the most important variable of all: the patient. After all, our job is patient care. Without a motivated patient who is able to participate fully in her rehabilitation, all our efforts are in vain. So understanding is really the key to effective postoperative management of the flexor tendon, understanding the scientific basis for our intervention, and understanding the patient herself.
REFERENCES
1. Tanaka T, Amadio PC, Zhao C, et al. Gliding resistance versus work of flexion—two methods to assess flexor tendon repair. J Orthop Res. 2003;21:813–818.
2. Hitchcock TF, Light TR, Bunch WH, et al. The effect of immediate constrained digital motion on the strength of flexor tendon repairs in chickens. J Hand Surg [Am]. 1987;12:590–595.
3. Silfverskiöld KL, May EJ. Gap formation after flexor tendon repair in zone II. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27:263–268.
4. Groth GN. Pyramid of progressive force exercises to the injured flexor tendon. J Hand Ther. 2004;17:31–42.
5. Amadio P, Jaeger S, Hunter J. Nutritional aspects of tendon healing. In: Hunter J, Schneider L, Mackin E, Callahan A, eds. Rehabilitation of the Hand. 3rd edition St. Louis: C.V. Mosby Co.; 1990:373–378.
6. Amadio PC, Hunter JM. Prognostic factors in flexor tendon surgery in zone 2. In: Hunter JM, Schneider LH, Mackin EM, eds. Tendon Surgery in the Hand. St. Louis: The C.V. Mosby Co.; 1987:138–141.
7. Peacock EE. Biological principles in the healing of long tendons. Surg Clin North Am. 1965;45:2.
8. Mason J, Allen H. The rate of healing of tendons: an experimental study of tensile strength. Ann Surg. 1941;113:424.
9. Manske PR. Flexor tendon healing. J Hand Surg [Br]. 1988;13:237–245.
10. Schepel SJ. Intrinsic healing of flexor tendons in primates. In: Hunter JM, Schneider LH, Mackin EJ, eds. Tendon Surgery in the Hand. St. Louis: The C.V. Mosby Co.; 1987:61–66.
11. Seyfer AE, Bolger WE. Effects of unrestricted motion on healing: a study of posttraumatic adhesions in primate tendons. Plast Reconstr Surg. 1989;83:122–128.
12. Potenza A. Critical evaluation of flexor tendon healing and adhesion formation within artificial digital sheaths. An experimental study. J Bone Joint Surg [Am]. 1963;45A:1217.
13. Potenza AD. Tendon healing within the flexor digital sheath in the dog. An experimental study. J Bone Joint Surg [Am]. 1962;44:49.
14. Abrahamsson SO, Lundborg G, Lohmander LS. Tendon healing in vivo. An experimental model. Scand J Plast Reconstr Surg Hand Surg. 1989;23:199–205.
15. Gelberman RH, Manske PR, Akeson WH, et al. Flexor tendon repair. J Orthop Res. 1986;4:119–128.
16. Lundborg G, Rank F, Heinau B. Intrinsic tendon healing. A new experimental model. Scand J Plast Reconstr Surg. 1985;19:113–117.
17. Manske PR, Lesker PA, Gelberman RH, Rucinsky TE. Intrinsic restoration of the flexor tendon surface in the nonhuman primate. J Hand Surg [Am]. 1985;10:632–637.
18. Gelberman RH, Amifl D, Gonsalves M, Woo S, Akeson WH. The influence of protected passive mobilization on the healing of flexor tendons: a biochemical and microangiographic study. The Hand. 1981;13:120–128.
19. Gelberman RH, Nunley JA, Osterman AL, et al. Influences of the protected passive mobilization interval on flexor tendon healing. A prospective randomized clinical study. Clin Orthop. 1991.189–196.
20. Gelberman RH, Steinberg D, Amiel D, Akeson W. Fibroblast chemotaxis after tendon repair. J Hand Surg [Am]. 1991;16:686–693.
21. Gelberman RH, Woo SL, Lothringer K, et al. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg [Am]. 1982;7:170–175.
22. Gelberman RH, Woo SL-Y. The physiological basis for application of controlled stress in the rehabilitation of flexor tendon injuries. J Hand Ther. 1989;2:66–70.
23. Buckwalter JA. Effects of early motion on healing of musculoskeletal tissues. Hand Clin. 1996;12:13–24.
24. Evans RB, Thompson DE. The application of force to the healing tendon. J Hand Ther. 1993;6:266–284.
25. Feehan LM, Beauchene JG. Early tensile properties of healing chicken flexor tendons: early controlled passive motion versus postoperative immobilization. J Hand Surg [Am]. 1990;15:63–68.
26. Kubota H, Manske PR, Aoki M, et al. Effect of motion and tension on injured flexor tendons in chickens. J Hand Surg [Am]. 1996;21:456–463.
27. Tanaka H, Manske PR, Pruitt DL, Larson BJ. Effect of cyclic tension on lacerated flexor tendons in vitro. J Hand Surg [Am]. 1995;20A:467–473.
28. Woo SL, Gelberman RH, Cobb NG, et al. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–622.
29. Wray RC Jr., Ollinger H, Lowrey R, Weeks PM. Effect of continuous load on the mechanical properties of tendon adhesions. The Hand. 1981;13:92–96.
30. Aoki M, Kubota H, Pruitt DL, Manske PR. Biomechanical and histologic characteristics of canine flexor tendon repair using early postoperative mobilization. J Hand Surg [Am]. 1997;22:107–114.
31. Costa MA, Wu C, Pham BV, et al. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Engineering. 2006;12:1937–1943.
32. Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg [Am]. 2004;29:551–563.
33. Mehta V, Mass D. The use of growth factors on tendon injuries. J Hand Ther. 2005;18:87–92. quiz 93
34. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Medicine. 2003;33:381–394.
35. Thomopoulos S, Harwood FL, Silva MJ, et al. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg [Am]. 2005;30:441–447.
36. Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–1368.
37. Taguchi M, Sun YL, Zhao C, et al. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg [Am]. 2008;90:129–135.
38. Uchiyama S, Amadio PC, Berglund LJ, An KN. Analysis of the gliding pattern of the canine flexor digitorum profundus tendon through the A2 pulley. J Biomechanics. 2008;41:1281–1288.
39. Zhao C, Sun YL, Amadio PC, et al. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg [Am]. 2006;88:2181–2191.
40. Krueger JK, Rohrich RJ. Clearing the smoke: the scientific rationale for tobacco abstention with plastic surgery. Plast Reconstr Surg. 2001;108:1063–1073. discussion 1074-1067
41. Manassa EH, Hertl CH, Olbrisch RR. Wound healing problems in smokers and nonsmokers after 132 abdominoplasties. Plast Reconstr Surg. 2003;111:2082–2087. discussion 2088-2089
42. van Adrichem LN, Hovius SE, van Strik R, van der Meulen JC. The acute effect of cigarette smoking on the microcirculation of a replanted digit. J Hand Surg [Am]. 1992;17:230–234.
43. Bishop AT, Cooney WPd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26:301–312.
44. McCarthy DM, Tramaglini DM, Chan SS, et al. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. J Hand Surg [Am]. 1995;20:795–800.
45. McGeorge DD, Stilwell JH. Partial flexor tendon injuries: to repair or not. J Hand Surg [Br]. 1992;17:176–177.
46. al-Qattan MM. Conservative management of zone II partial flexor tendon lacerations greater than half the width of the tendon. J Hand Surg [Am]. 2000;25:1118–1121.
47. Mitsionis G, Bastidas JA, Grewal R, et al. Feasibility of partial A2 and A4 pulley excision: effect on finger flexor tendon biomechanics. J Hand Surg [Am]. 1999;24:310–314.
48. Peterson WW, Manske PR, Bollinger BA, et al. Effect of pulley excision on flexor tendon biomechanics. J Orthop Res. 1986;4:96–101.
49. Doyle JR, Blythe WF. The finger flexor tendon sheath and pulleys: anatomy and reconstruction. In AAOS Symposium on Tendon Surgery. St. Louis: The C.V. Mosby Co.; 1975.
50. Idler RS. Anatomy and biomechanics of the digital flexor tendons. Hand Clin. 1985;1:3–11.
51. Gelberman RH, Woo SL, Amiel D, Horibe S, Lee D. Influences of flexor sheath continuity and early motion on tendon healing in dogs. J Hand Surg [Am]. 1990;15:69–77.
52. Peterson WW, Manske PR, Dunlap J, et al. Effect of various methods of restoring flexor sheath integrity on the formation of adhesions after tendon injury. J Hand Surg [Am]. 1990;15:48–56.
53. Peterson WW, Manske PR, Kain CC, Lesker PA. Effect of flexor sheath integrity on tendon gliding: a biomechanical and histologic study. J Orthop Res. 1986;4:458–465.
54. Peterson WW, Manske PR, Lesker PA. The effect of flexor sheath integrity on nutrient uptake by chicken flexor tendons. Clin Orthop. 1985.259–263.
55. Peterson WW, Manske PR, Lesker PA. The effect of flexor sheath integrity on nutrient uptake by primate flexor tendons. J Hand Surg [Am]. 1986;11:413–416.
56. Potenza AD. Prevention of adhesions to healing digital flexor tendons. JAMA. 1964;187:99.
57. Matthews P, Richards H. Factors in the adherence of flexor tendon after repair: an experimental study in the rabbit. J Bone Joint Surg [Br]. 1976;58:230–236.
58. Strickland JW. Flexor tendon injuries: I. Foundations of treatment. J Am Acad Orthop Surg. 1995;3:44–54.
59. Strickland JW. Flexor tendon injuries: II. Operative technique. J Am Acad Orthop Surg. 1995;3:55–62.
60. Winters SC, Gelberman RH, Woo SL, et al. The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg [Am]. 1998;23:97–104.
61. Strickland JW. The scientific basis for advances in flexor tendon surgery. J Hand Ther. 2005;18:94–110. quiz 111
62. Kubota H, Aoki M, Pruitt DL, Manske PR. Mechanical properties of various circumferential tendon suture techniques. J Hand Surg [Br]. 1996;21:474–480.
63. Lin GT, An KN, Amadio PC, Cooney WPd. Biomechanical studies of running suture for flexor tendon repair in dogs. J Hand Surg [Am]. 1988;13:553–558.
64. Mashadi ZB, Amis AA. The effect of locking loops on the strength of tendon repair. J Hand Surg [Br]. 1991;16:35–39.
65. Mashadi ZB, Amis AA. Strength of the suture in the epitenon and within the tendon fibres: development of stronger peripheral suture technique. J Hand Surg [Br]. 1992;17:172–175.
66. Pruitt DL, Manske PR, Fink B. Cyclic stress analysis of flexor tendon repair. J Hand Surg [Am]. 1991;16:701–707.
67. Silfverskiöld KL, Andersson CH. Two new methods of tendon repair: an in vitro evaluation of tensile strength and gap formation. J Hand Surg [Am]. 1993;18:58–65.
68. Wade PJ, Wetherell RG, Amis AA. Flexor tendon repair: significant gain in strength from the Halsted peripheral suture technique. J Hand Surg [Br]. 1989;14:232–235.
69. Aoki M, Pruitt DL, Kubota H, Manske PR. Effect of suture knots on tensile strength of repaired canine flexor tendons. J Hand Surg [Br]. 1995;20:72–75.
70. Pruitt DL, Aoki M, Manske PR. Effect of suture knot location on tensile strength after flexor tendon repair. J Hand Surg [Am]. 1996;21:969–973.
71. Aoki M, Manske PR, Pruitt DL, et al. Canine cadaveric study of flexor tendon repair using tendon splint: tensile strength and the work of flexion. Nippon Seikeigeka Gakkai zasshi. 1995;69:332–341.
72. Aoki M, Manske PR, Pruitt DL, Larson BJ. Work of flexion after tendon repair with various suture methods. A human cadaveric study. J Hand Surg [Br]. 1995;20:310–313.
73. Gelberman RH, Siegel DB, Woo SL, et al. Healing of digital flexor tendons: importance of the interval from injury to repair. A biomechanical, biochemical, and morphological study in dogs. J Bone Joint Surg [Am]. 1991;73:66–75.
74. Tottenham VM, Wilton-Bennett K, Jeffrey J. Effects of delayed therapeutic intervention following zone II flexor tendon repair. J Hand Ther. 1995;8:23–26.
75. Halikis MN, Manske PR, Kubota H, Aoki M. Effect of immobilization, immediate mobilization, and delayed mobilization on the resistance to digital flexion using a tendon injury model. J Hand Surg [Am]. 1997;22:464–472.
76. Elliot D, Harris SB. The assessment of flexor tendon function after primary tendon repair. Hand Clin. 2003;19:495–503.
77. Cifaldi Collins D, Schwarze L. Early progressive resistance following immobilization of flexor tendon repairs. J Hand Ther. 1991;4:111–116.
78. Wehbe MA, Hunter JM. Flexor tendon gliding in the hand. Part II. Differential gliding. J Hand Surg [Am]. 1985;10:575–579.
79. Gelberman RH, Manske PR. Factors influencing flexor tendon adhesions. Hand Clin. 1985;1:35–42.
80. Urbaniak JR, Cahill JD, Mortenson RA. Tendon suturing methods: analysis of tensile strengths. In AAOS Symposium on Tendon Surgery in the Hand. St. Louis: C.V. Mosby Co.; 1975.
81. Silfverskiöld KL, May EJ, Tornvall AH. Tendon excursions after flexor tendon repair in zone. II: results with a new controlled-motion program. J Hand Surg [Am]. 1993;18:403–410.
82. May EJ, Silfverskiöld KL, Sollerman CJ. The correlation between controlled range of motion with dynamic traction and results after flexor tendon repair in zone II. J Hand Surg [Am]. 1992;17:1133–1139.
83. Silfverskiöld KL, May EJ, Oden A. Factors affecting results after flexor tendon repair in zone II: a multivariate prospective analysis. J Hand Surg [Am]. 1993;18:654–662.
84. Brown CP, McGrouther DA. The excursion of the tendon of flexor pollicis longus and its relation to dynamic splintage. J Hand Surg [Am]. 1984;9:787–791.
85. Greenwald D, Shumway S, Allen C, Mass D. Dynamic analysis of profundus tendon function. J Hand Surg [Am]. 1994;19:626–635.
86. Hagberg L, Selvik G. Tendon excursion and dehiscence during early controlled mobilization after flexor tendon repair in zone II: an x-ray stereophotogrammetric analysis. J Hand Surg [Am]. 1991;16:669–680.
87. Horibe S, Woo SL, Spiegelman JJ, et al. Excursion of the flexor digitorum profundus tendon: a kinematic study of the human and canine digits. J Orthop Res. 1990;8:167–174.
88. McGrouther DA, Ahmed M. Flexor tendon excursions in “no man’s land.”. The Hand. 1981;13:129.
89. Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg [Am]. 2000;25:214–235.
90. Kleinert HE, Kutz JE, Ashbell S, Martinez E. Primary repair of lacerated flexor tendons in no-man’s-land. J Bone Joint Surg [Am]. 1967;49A:577.
91. Kleinert HE, Kutz JE, Cohen MJ. Primary repair of zone 2 flexor tendon lacerations. In AAOS Symposium on Tendon Surgery in the Hand. St. Louis: C.V. Mosby Co.; 1975.
92. Duran R, Houser R. Controlled passive motion following flexor tendon repair in zones 2 and 3. In AAOS Symposium on Tendon Surgery in the Hand. St. Louis: The C.V. Mosby Co.; 1975.
93. Dovelle S, Heeter P. The Washington regimen: rehabilitation of the hand following flexor tendon injuries. Phys Ther. 1989;69:1034.
94. Evans R. A study of the zone I flexor tendon injury and implications for treatment. J Hand Ther. 1990;3:133–148.
95. May EJ, Silfverskiöld KL, Sollerman CJ. Controlled mobilization after flexor tendon repair in zone II: a prospective comparison of three methods. J Hand Surg [Am]. 1992;17:942–952.
96. Pettengill KM. The evolution of early mobilization of the repaired flexor tendon. J Hand Ther. 2005;18:157–168.
97. Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg [Am]. 1977;2:441–451.
98. Cooney WP, Lin GT, An K-N. Improved tendon excursion following flexor tendon repair. J Hand Ther. 1989;2:102–106.
99. Horii E, Lin GT, Cooney WP, et al. Comparative flexor tendon excursion after passive mobilization: an in vitro study. J Hand Surg [Am]. 1992;17:559–566.
100. Silfverskiöld KL, May EJ, Tornvall AH. Flexor digitorum profundus tendon excursions during controlled motion after flexor tendon repair in zone II: a prospective clinical study. J Hand Surg [Am]. 1992;17:122–131.
101. Cannon N. Post flexor tendon repair motion protocol. Indiana Hand Center Newsletter. 1993;1:13–17.
102. Cullen KW, Tolhurst P, Lang D, Page RE. Flexor tendon repair in zone 2 followed by controlled active mobilisation. J Hand Surg [Br]. 1989;14:392–395.
103. Elliot D, Moiemen NS, Flemming AF, et al. The rupture rate of acute flexor tendon repairs mobilized by the controlled active motion regimen. J Hand Surg [Br]. 1994;19:607–612.
104. Gratton P. Early active mobilization after flexor tendon repairs. J Hand Ther. 1993;6:285–289.
105. Marin Braun F, Foucher G, Buch Jaeger N, Sammut D. Reparation du flechisseur profond et du long flechisseur du pouce par la “fixation en rappel.” Resultats d’une serie de soixante-dix-sept cas. Annales de chirurgie de la Main et du Membre Superieur. 1991;10:13–21.
106. Riaz M, Hill C, Khan K, Small JO. Long term outcome of early active mobilization following flexor tendon repair in zone 2. J Hand Surg [Br]. 1999;24:157–160.
107. Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg [Br]. 1989;14:383–391.
108. Steelman P. Treatment of flexor tendon injuries: therapist’s commentary. J Hand Ther. 1999;12:149–151.
109. Strickland JW. Flexor tendon injuries. Part 5. Flexor tenolysis, rehabilitation and results. Orthop Rev. 1987;16:137–153.
110. Bainbridge LC, Robertson C, Gillies D, Elliot D. A comparison of post-operative mobilization of flexor tendon repairs with “passive flexion-active extension” and “controlled active motion” techniques. J Hand Surg [Br]. 1994;19:517–521.
111. Yii NW, Urban M, Elliot D. A prospective study of flexor tendon repair in zone 5. J Hand Surg [Br]. 1998;23:642–648.
112. Harris S. Personal communication. 2008.
113. Savage R. The influence of wrist position on the minimum force required for active movement of the interphalangeal joints. J Hand Surg [Br]. 1988;13:262–268.
114. Schuind F, Garcia-Elias M, Cooney WPd, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg [Am]. 1992;17:291–298.
115. Strickland J. Flexor tendon repair: Indiana method. Indiana Hand Center Newsletter. 1993;1:1.
116. Cannon N. PC re EAM flexors. Personal communication, 2008.
117. Cannon N. PC re EAM flexors. Personal communication, 1993.
118. Silfverskiöld KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg [Am]. 1994;19:53–60.
119. Evans. PC re MAMTT home program etc. Personal communication, 1993.
120. Sandow MJ, McMahon MM. Single-cross grasp six-strand repair for acute flexor tenorrhaphy: modified Savage technique. Atlas of the Hand Clinics. 1996;1:41–64.
121. Zhao C, Amadio PC, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg [Am]. 2004;86:320–327.
122. Zhao C, Amadio PC, Tanaka T, et al. Short-term assessment of optimal timing for postoperative rehabilitation after flexor digitorum profundus tendon repair in a canine model. J Hand Ther. 2005;18:322–329. quiz 329
123. Cao Y, Tang JB. Resistance to motion of flexor tendons and digital edema: an in vivo study in a chicken model. J Hand Surg [Am]. 2006;31:1645–1651.
124. Rosenblum N, Robinson S. Advances in flexor and extensor tendon management. Clin Phys Ther. 1986.17–24.
125. Stegink Janson C, Minerbo G. A comparison between early dynamically controlled mobilization and immobilization after flexor tendon repair in zone 2 of the hand. J Hand Ther. 1990;3:20–25.
126. Citron ND, Forster A. Dynamic splinting following flexor tendon repair. J Hand Surg [Br]. 1987;12:96–100.
127. van Alphen JC, Oepkes CT, Bos KE. Activity of the extrinsic finger flexors during mobilization in the Kleinert splint. J Hand Surg [Am]. 1996;21:77–84.
128. Burge PD, Brown M. Elastic band mobilisation after flexor tendon repair; splint design and risk of flexion contracture. J Hand Surg [Br]. 1990;15:443–448.
129. Chow J, Stephens M, Ngai W, et al. A splint for controlled active motion after flexor tendon repair: design, mechanical testing and preliminary clinical results. J Hand Surg [Am]. 1990;15A:645.
130. Werntz J, Chesher S, Breidenbach W, et al. A new dynamic splint for postoperative treatment of flexor tendon injury. J Hand Surg [Am]. 1989;14:559–566.
131. Chow JA, Thomes LJ, Dovelle S, et al. A combined regimen of controlled motion following flexor tendon repair in “no man’s land.”. Plast Reconstr Surg. 1987;79:447–455.
132. Chow JA, Thomes LJ, Dovelle S, et al. Controlled motion rehabilitation after flexor tendon repair and grafting. A multi-centre study. J Bone Joint Surg [Br]. 1988;70:591–595.
133. Green DP. Radial nerve palsy. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th edition Philadelphia: Elsevier Churchill Livingstone; 2005:1113–1130.
134. Slattery PG, McGrouther DA. A modified Kleinert Controlled Mobilization Splint following flexor tendon repair. J Hand Surg [Br]. 1984;9:217–218.
135. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18:128–140.
136. Lieber RL, Amiel D, Kaufman KR, et al. Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg [Am]. 1996;21:957–962.
137. Lieber RL, Silva MJ, Amiel D, Gelberman RH. Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomechanics. 1999;32:175–181.
138. Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783.
139. Zhao C, Amadio PC, Momose T, et al. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg [Am]. 2002;84-A:78–84.
140. Zhao C, Amadio PC, Zobitz ME, et al. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop. 2002.223–230.
141. Amadio PC. Friction of the gliding surface. Implications for tendon surgery and rehabilitation. J Hand Ther. 2005;18:112–119.
142. Tanaka T, Amadio PC, Zhao C, et al. Flexor digitorum profundus tendon tension during finger manipulation. J Hand Ther. 2005;18:330–338. quiz 338
143. Boyer MI, Gelberman RH, Burns ME, et al. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg [Am]. 2001;83-A:891–899.
144. Goldfarb CA, Harwood F, Silva MJ, et al. The effect of variations in applied rehabilitation force on collagen concentration and maturation at the intrasynovial flexor tendon repair site. J Hand Surg [Am]. 2001;26:841–846.
145. Groth GN. Clinical decision making and therapists’ autonomy in the context of flexor tendon rehabilitation. J Hand Ther 2008. 2008;21(3):254–259. quiz 260
146. von der Heyde R. Evidence supporting wrist motion following flexor tendon repair. In ASHT Annual Meeting. Boston, 2008.
147. Elliot D, Southgate CM. New concepts in managing the long tendons of the thumb after primary repair. J Hand Ther. 2005;18:141–156.
148. Nunley JA, Levin LS, Devito D, et al. Direct end-to-end repair of flexor pollicis longus tendon lacerations. J Hand Surg [Am]. 1992;17:118–121.
149. Cooney WP 3rd, An KN, Daube JR, Askew LJ. Electromyographic analysis of the thumb: a study of isometric forces in pinch and grasp. J Hand Surg [Am]. 1985;10:202–210.
150. Sirotakova M, Elliot D. Early active mobilization of primary repairs of the flexor pollicis longus tendon. J Hand Surg [Br]. 1999;24:647–653.
151. Sirotakova M, Elliot D. Early active mobilization of primary repairs of the flexor pollicis longus tendon with two Kessler two-strand core sutures and a strengthened circumferential suture. J Hand Surg [Br]. 2004;29:531–535.
152. Hudson DA, de Jager LT. The spaghetti wrist. Simultaneous laceration of the median and ulnar nerves with flexor tendons at the wrist. J Hand Surg [Br]. 1993;18:171–173.
153. Puckett CL, Meyer VH. Results of treatment of extensive volar wrist lacerations: the spaghetti wrist. Plast Reconstr Surg. 1985;75:714–721.
154. Stefanich RJ, Putnam MD, Peimer CA, Sherwin FS. Flexor tendon lacerations in zone V. J Hand Surg [Am]. 1992;17:284–291.
155. Weinzweig N, Chin G, Mead M, Gonzalez M. “Spaghetti wrist”: management and results [published erratum appears in Plast Reconstr Surg 1998 Aug;102:2 following table of contents]. Plast Reconstr Surg. 1998;102:96–102.
156. Widgerow AD. Full-house/spaghetti wrist injuries. Analysis of results. S Afr J Surg. 1990;28:6–10.
157. Leijnse JN. A generic morphological model of the anatomic variability in the m. flexor digitorum profundus, m. flexor pollicis longus and mm. lumbricales complex. Acta Anat (Basel). 1997;160:62–74.
158. Rogers GD, Henshall AL, Sach RP, Wallis KA. Simultaneous laceration of the median and ulnar nerves with flexor tendons at the wrist. J Hand Surg [Am]. 1990;15:990–995.




















