Common Nerve Injuries About the Shoulder
▪ Treatment of these nerve injuries requires a thorough examination of the shoulder complex and electrophysiologic testing to identify the location and extent of nerve injury.
▪ Therapy plays a primary role in both surgical and nonoperative management of common shoulder injuries.
▪ Treatment plans for therapy should address scapular dysfunction and prevention of a frozen shoulder.
The shoulder is a complex joint with complicated kinematics that rely on muscle function and balance for mobility and stability. Nerve injury that affects the function of these muscles will significantly alter this balance. Shoulder problems overall are common. Nerve injuries about the shoulder are relatively uncommon but are a commonly undiagnosed cause of shoulder dysfunction. The presenting signs or symptoms may be attributed to a structural problem (e.g., rotator cuff tear, shoulder instability) but will not improve with standard treatment for these conditions. The symptoms of local abnormalities of the shoulder may also present distally in the arm and forearm, complicating diagnosis.
The spectrum of nerve injuries involving the shoulder includes both acute and chronic conditions. These problems coexist with other more common injuries. Nerve injuries may be primary, from pathology arising within the nerve, or secondary, from other ongoing processes within the shoulder resulting in compression or traction neuropathy. Injury to the neurologic system can arise at many anatomic locations, including cervical roots, brachial plexus, and peripheral nerves innervating the shoulder. Cervical spine pathology can lead to nerve injury at the root or spinal cord level. Existing systemic conditions (e.g., multiple sclerosis, malignancy, enthesopathies) may also make diagnosis difficult. For all these reasons, nerve injuries about the shoulder are often difficult to diagnose and manage.
The injury level and/or primary nerve pathology can be localized from the results of a detailed history and physical examination. Other diagnostic modalities, including electromyography (EMG), nerve conduction studies, spinal cord or cortical evoked potentials, magnetic resonance imaging (MRI), myelography, and computed tomography, are useful in confirming the clinical diagnosis and help guide treatment.
The goal of management is early correct diagnosis. Only after the correct diagnosis is made can appropriate treatment be instituted to avoid atrophy and/or contracture and to restore stability and mobility to the shoulder.
The neurologic elements of the upper extremity originate from the cervical portion of the spinal cord. The upper extremity receives contributions from cervical roots C5, C6, C7, and C8 and thoracic root T1. The brachial plexus is formed by the junctions of the ventral rami of these five roots. Occasionally, there may be contributions from C4 and less commonly from T2. The five cervical roots unite just above the clavicle, forming three trunks: the upper, consisting of roots C5 and C6; the middle trunk, consisting of root C7; and the lower trunk, consisting of roots C8 and T1 (Fig. 57-1). Just below the clavicle, each trunk divides into an anterior and posterior division. The anterior division of the upper and middle trunks forms the lateral cord. The anterior division of the lower trunk forms the medial cord, and the posterior divisions from the upper, middle, and lower trunks form the posterior cord. Note that the cords surround the subclavian artery and are named by their position relative to the artery. The lateral cord branches include the lateral pectoral nerve, musculocutaneous nerve, and median nerve. The medial cord branches include the medial pectoral nerve, medial brachial and antebrachial cutaneous nerves, and median and ulnar nerves. The posterior cord branches include the upper and lower subscapular nerves, the thoracodorsal, axillary, and radial nerves.
Other branches arise more proximally either from the cords, trunks, or roots to supply the shoulder girdle musculature. The dorsal scapular nerve, supplying the rhomboid muscles, arises directly from the C5 nerve root. The suprascapular nerve, supplying the supraspinatus and infraspinatus muscles, is a branch arising from the upper trunk. The long thoracic nerve, supplying the serratus anterior muscle, arises from contributions of the C5, C6, and C7 nerve roots (Table 57-1).
Table 57-1 Branches of the Brachial Plexus
Peripheral Nerve |
Origin |
Long thoracic |
C5, C6, C7—from roots |
Dorsal scapular |
C5—from root |
Suprascapular |
C5, C6—from upper trunk |
Upper subscapular |
C5—posterior cord |
Lower subscapular |
C5, C6—posterior cord |
Thoracodorsal |
C7, C8—posterior cord |
Lateral pectoral |
C5, C6, C7—lateral cord |
Medial pectoral |
C8, T1—medial cord |
Medial brachial cutaneous |
C8, T1—medial cord |
Medial antebrachial cutaneous |
C8, T1—medial cord |
Axillary |
C5, C6—posterior cord |
Radial |
C6, C7, C8—posterior cord |
Median |
C5 through T1—medial and lateral cords |
Musculocutaneous |
C5, C6—lateral cord |
Ulnar |
C8, T1—medial cord |
Nerve injury can occur by laceration, traction, or compression. Laceration occurs by direct trauma through penetrating injury or as a result of a fracture or its treatment. Traction is a common mechanism that occurs with blunt trauma. A shoulder dislocation can result in a traction injury to the brachial plexus. A fall onto the shoulder with lateral deviation of the neck will result in a traction injury to the upper trunk of the brachial plexus. An injury with traction on the arm pulled in abduction will place traction on the lower plexus. Compression occurs either from a perineural scar or fracture callus in a post-trauma case or from an extrinsic lesion such as a ganglion or tumor.
The mechanism of injury is determined from the patient history. The degree of injury for each mechanism is determined by physical examination and electrophysiologic testing. The degree of nerve injury is characterized as one of three types based on the classification of Seddon37 and Sunderland.39,40
Neurapraxia: A minimal nerve injury characterized by a temporary, fully reversible nerve conduction block, with good prognosis
Axonotmesis: A moderate nerve injury characterized by a disruption of the axons and myelin sheath with the epineurium left intact to guide regeneration; has a fair prognosis
Neurotmesis: A severe injury characterized by complete destruction of the nerve with poor prognosis for regeneration
Another nerve injury classification system with more categories of nerve injury is described by Sunderland.39 The rate of nerve regeneration is about 1 inch per month, which must be considered when assessing nerves recovering various distances to muscles or skin.26
Examination for nerve injuries should be a part of every routine physical examination. The patient should be carefully observed with the trunk and upper extremities disrobed so that the normal side can be compared with the symptomatic side. Subtle areas of atrophy, deformity, discoloration, or swelling should be noted. The active and passive range of motion of the neck, shoulders, elbows, wrists, and hands should be measured and these joints tested for stability. The motor evaluation is one of the most important components of the neurologic examination. Each of the muscles of the shoulder should be tested, including those that move and stabilize the scapula. A comprehensive motor assessment can often define the site of a neurologic lesion. The nerve innervation of the muscles of the shoulder is outlined in Table 57-2.
Table 57-2 Nerves Commonly Injured about the Shoulder
Peripheral Nerve |
Muscle Innervated |
Long thoracic Suprascapular |
Serratus anterior Supraspinatus Infraspinatus |
Musculocutaneous |
Coracobrachialis Biceps Brachialis |
Axillary |
Deltoid Teres minor |
Spinal accessory |
Trapezius |
The strength of each muscle should be recorded using the muscle grading system of Sunderland40 (Table 57-3). Sensory deficits can occur in either a dermatomal (nerve root) distribution (Fig. 57-2) or along the distribution of peripheral nerves. If spinal cord lesions are suspected, a detailed evaluation of all sensory functions (pain, hot/cold, vibration, and position sense) is mandatory.
Table 57-3 Muscle Grading System
Grade |
Findings |
5 Normal |
Able to withstand full resistance |
4 Good |
Able to withstand some resistance |
3 Fair |
Move against gravity—no resistance |
2 Poor |
Move with gravity eliminated |
1 Trace |
Muscle contraction without movement |
0 Zero |
No muscle contraction |
Electrophysiologic examination remains the most commonly performed test for evaluation of neurologic injury. This test has two components: an electromyogram and a nerve conduction velocity (NCV) study. The combination of NCV and EMG allow localization of the nerve injury, assess the age and degree of injury, and provide evidence of nerve recovery. Chapter 15 provides detailed information on this diagnostic tool, but it is briefly reviewed in this chapter.
EMG is performed by placing a recording needle into the muscle being studied and observing the nature of the electrical activity in the muscle. A normal muscle is electrically silent at rest and produces a well-defined single-peaked recording when the muscle is contracted voluntarily (motor unit potential [MUP]). A nerve with an acute injury with denervation will produce spontaneous electrical signals at rest recorded as fibrillation potentials or positive sharp waves. The MUP will be altered or nonexistent, depending on the degree of axonal injury. These electrical changes in an acute injury do not become detectable by EMG until 3 weeks after injury. As regeneration occurs, the fibrillation potentials disappear. Intact motor nerves sprout to innervate denervated motor units, producing a polyphasic MUP with multiple peaks. The presence of polyphasic potentials indicate that the nerve injury is more than 3 months old.
EMG can detect the effects of denervation caused by a lower motor lesion. EMG cannot define the location of the lesion between the spinal cord and muscle, unless multiple muscles are individually tested to map the location of the nerve injury. EMG can determine whether the pathologic process involves more than one peripheral nerve. It can also identify whether the observed pathology is part of a generalized peripheral neuropathy or chronic myopathy (e.g., muscular dystrophy).
The NCV is a direct measurement of nerve conduction through the extremity. Myelin is the insulating material for the nerve axons. As myelin is lost as a result of compression or injury, the conduction across the injured segment will decrease. This test helps to define areas of compression or injury along a peripheral nerve. These measurements are obtained by direct stimulation of a nerve proximally in the extremity and measurement of the time required for impulse conduction to a point in the nerve more distal in the extremity. The measurement is recorded in meters per second. Abnormal values (decreased conduction velocity) are determined by comparison with the uninvolved extremity or published normal standards.21 Nerve conduction can also be measured by stimulating the nerve at a fixed distance from an anatomic landmark and tracking (from a distal muscle) the time from stimulation to recording of a MUP. This measurement is a motor latency and is recorded in milliseconds. A sensory latency is measured by stimulating the fingertip and recording from the nerve at a fixed distance proximally. These measurements, if prolonged, imply abnormal nerve conduction in the anatomic region being studied.
The spinal accessory nerve is the 11th cranial nerve and the primary motor nerve to the trapezius and sternocleidomastoid muscles. It is susceptible to injury because of its superficial course through the subcutaneous tissues on the floor of the posterior cervical triangle. This anatomic structure is bound by the trapezius, sternocleidomastoid, and clavicle (Fig. 57-3). Injury occurs from blunt trauma directly to the nerve, traction injury to the nerve from a force depressing the shoulder while the head is forced in the opposite direction,45 or iatrogenic injury from surgical procedures in the posterior cervical triangle, most commonly cervical node biopsy. Injury to the spinal accessory nerve results in paralysis of the trapezius muscle.
Spinal accessory palsy presents as a dull ache or pain, with drooping of the shoulder and noticeable weakness in arm elevation and abduction (Fig. 57-4). Atrophy of the trapezius muscle is present. The sternocleidomastoid muscle may also be atrophied and nonfunctional if the injury is proximal. The patient is unable to shrug the affected shoulder. Winging of the scapula can be seen when viewed from behind because of the lack of the suspensory action of the trapezius.
When the injury is caused by blunt trauma or traction, initial treatment is conservative, with sling immobilization and a therapy regimen to prevent a frozen shoulder and to strengthen the trapezius and surrounding muscles.
These patients may present to therapy with a diagnosis of shoulder impingement. The impingement is secondary to drooping of the shoulder from the spinal accessory palsy and unrelated to acromioclavicular joint pathology. It is difficult to compensate for the lack of trapezius function simply by strengthening adjacent muscles, and the impingement symptoms fail to improve with traditional therapy prescribed for classic impingement syndrome.
The deficit in closed nerve injuries may persist for 3 to 12 months. If no electrophysiologic evidence of regeneration is present by 6 months, the trapezius palsy can be considered permanent.36
When the injury is iatrogenic or caused by open trauma, neurolysis and nerve repair are most successful when performed within 6 months of injury.16 If surgical exploration reveals a nerve transection, nerve repair is performed by either direct repair or nerve grafting if a tension-free repair cannot be performed. If a neuroma-in-continuity is found at surgical exploration, intraoperative nerve conduction studies are performed to determine whether neurolysis alone is effective or whether resection with primary repair is needed. A recovery period of about 1 year is required to assess the results of surgical intervention.
Kim et al.22 reported on the surgical outcomes of 111 spinal accessory nerve injuries. The most frequent injury mechanism was iatrogenic (103) with 82 of these patients having a lymph node biopsy as the index procedure. Eight were caused by stretch injury and three by traumatic laceration. Nineteen patients at operative exploration were found to have the nerve in continuity. They were treated with neurolysis when intraoperative electrical stimulation demonstrated conduction along the nerve. When the nerve was found to have been lacerated, 26 patients were treated by end-to-end repair and 58 patients were treated by nerve graft. The average graft length was 1.5 inches. Of the patients treated by neurolysis, 95% regained grade 4 or higher trapezius muscle strength. Of the patients treated by suture repair or nerve graft, 77% regained grade 3 or higher strength.
If trapezial function does not return, reconstructive procedures are performed. For isolated, complete, permanent trapezial injuries, muscle transfers are recommended.2,25,42 The Eden-Lange procedure36 involves transfer of the levator scapulae muscle to the acromion and transfer of the rhomboid muscle to the infraspinatus fossa of the scapula. Romero and Gerber36 reported a series of 12 patients treated with the Eden-Lange procedure with a mean follow-up of 32 years. Nine were classified as excellent, two were fair, and one was poor. Pain was adequately controlled in 11 patients. Overhead activity was restored in nine patients. The patient with a poor outcome had a concomitant long thoracic nerve palsy.
Scapular suspension or scapulothoracic fusion are preferred8,26 in patients with neuromuscular disorders and multiple nerve injuries involving the parascapular muscles.
The long thoracic nerve originates from the ventral rami of C5, C6, and C7 and travels beneath the brachial plexus and clavicle over the first rib.4 The nerve then courses along the lateral aspect of the chest wall to innervate the serratus anterior muscle (Fig. 57-5). Because of its superficial course and length, this nerve is susceptible to damage from blows to the shoulder or chest wall or from prolonged traction on the shoulder.
The serratus anterior stabilizes the scapula on the posterior thoracic wall, providing a firm point for muscles originating from the scapula to move the arm. In addition, the serratus anterior, together with the trapezius and levator scapulae, acts to upwardly rotate the scapula, allowing greater glenohumeral elevation.
The clinical presentation includes a dull ache or pain around the shoulder, winging of the scapula, and decreased active shoulder motion.10,20 Often, the patient may present with painless winging of the scapula. This winging deformity of the scapula is most accentuated by forward pushing on a wall (Fig. 57-6) or when the arm is loaded and then elevated. More severe pain usually indicates more diffuse involvement of regional muscles and an associated brachial plexus neuropathy.18 An idiopathic form of serratus anterior paralysis has been described, termed neuralgic amyotrophy, which consists of a syndrome of paralytic brachial neuritis that occasionally may affect only the long thoracic nerve.32 The differential diagnosis for long thoracic nerve paralysis must include polymyositis, muscular dystrophy, and cervical spondylosis, all of which must be ruled out.
Electrophysiologic studies are invaluable in the diagnosis and prognosis of this injury. EMG determines the location of nerve involvement. It will determine whether the injury involves the cervical roots or brachial plexus or is isolated to the long thoracic nerve.
Although there is no consensus as to the appropriate treatment of this condition, conservative treatment with rest and therapy is a common approach. Therapy should strive to strengthen the serratus anterior and surrounding muscles and maintain the range of motion of the shoulder. These patients may also present to therapy with an incorrect diagnosis of shoulder impingement, secondary to the winging deformity of the shoulder from long thoracic palsy. The winged scapula rotates forward, decreasing the space between the acromium and greater tuberosity and resulting in rotator cuff impingement.
The overall prognosis for serratus anterior paralysis is good, with progressive recovery occurring up to 2 years after the injury.14 A serratus palsy can be judged permanent26 if no clinical or electrical evidence of recovery is seen by 12 months after injury.
A number of surgical options are available for persistent deficits:
1. Neurolysis.9 Nath28 reported 50 patients treated by neurolysis of the supraclavicular portion of the long thoracic nerve. The average follow-up was 25.7 months. Neurolysis improved scapular winging in 49 (98%) of the 50 cases. Pain reduction was good or excellent in 43 (86%) of the cases.
2. Muscle transfer.6,27 Perlmutter and Leffert33 reported the results of 16 patients treated with pectoralis major tendon transfer to the scapula. Eight patients were asymptomatic with normal function, five had intermittent mild discomfort, and one had frequent mild pain with no scapular winging. Two patients failed after a traumatic event.
3. Scapulothoracic arthrodesis. Stabilization of the scapula to the chest wall is an option for pain relief but with significant loss of shoulder motion.17
The suprascapular nerve originates from the upper trunk of the brachial plexus, with contributions from the fifth and sixth cervical roots. The nerve courses through the posterior cervical triangle, passing under the body of the omohyoid muscle and anterior border of the trapezius muscle. The nerve passes through the suprascapular notch, covered by the transverse scapular ligament, to innervate the supraspinatus muscle. At this point, it also gives off sensory fibers to the capsular ligamentous structures of the shoulder and acromioclavicular joint. The suprascapular nerve continues around the lateral border of the spine of the scapula, through the spinoglenoid notch, to innervate the infraspinatus muscle (Fig. 57-7).
Most injuries involve entrapment of the suprascapular nerve at the suprascapular notch by the transverse scapular ligament before it innervates the supraspinatus muscle.7,15 An injury here would produce symptoms of both supraspinatus and infraspinatus weakness. Another common area of entrapment is at the spinoglenoid notch. Because the supraspinatus has already been innervated, an injury here produces only infraspinatus weakness.
Injury to the suprascapular nerve is most commonly caused by direct shoulder trauma, including fracture or dislocation,46 but can also occur from a traction injury.12 The clinical presentation includes a dull ache or pain along the posterior aspect of the scapula. Weakness of abduction and external rotation of the arm38 is found on physical examination. Atrophy of the supraspinatus or infraspinatus muscles is found in more advanced cases (Fig. 57-8).
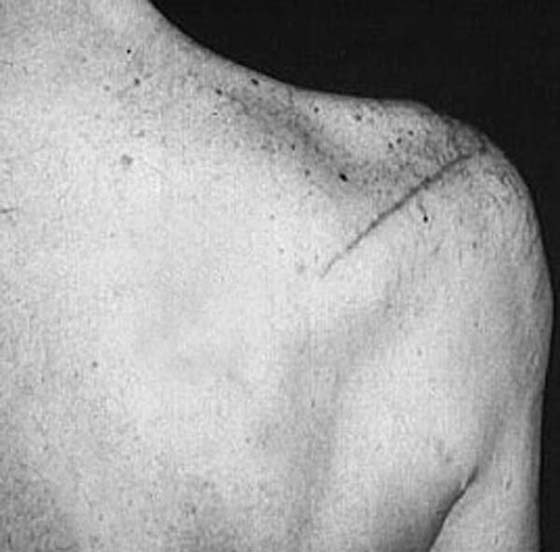
Figure 57-8 Atrophy of the supraspinatus and infraspinatus muscles resulting from suprascapular nerve injury.
The differential diagnosis should include rotator cuff tears, cervical radiculopathy, myopathy, and brachial plexus neuropathy. Patients often initially receive a diagnosis of a rotator cuff injury and undergo a workup for this diagnosis. However, the diagnosis of suprascapular nerve entrapment should be suspected if the MRI study shows a structurally intact rotator cuff with selective atrophy of the supraspinatus and infraspinatus muscles. EMG and NCV studies can confirm the clinical impression of suprascapular nerve entrapment and determine the location at either the suprascapular or spinoglenoid notch. MRI is performed to look for a ganglion arising from the glenohumeral joint compressing the nerve.
Initial treatment is conservative, consisting of rest, anti-inflammatory agents, and therapy to strengthen the involved and surrounding muscles while maintaining shoulder motion. Surgical decompression may be required if an anatomic lesion is identified.41 Explorations of the suprascapular nerve have revealed hypertrophy of the transverse scapular ligament and compression of the nerve at the suprascapular notch and spinoglenoid notch.13,35 Ganglion cysts are a common cause of compression of the suprascapular nerve at the spinoglenoid notch of the scapula.19,29,43 The clinical response to surgical decompression varies from no improvement to complete pain relief with full restoration of muscle bulk and power, depending on the form and duration of the causal lesion.15,44 Kim et al.23 reported a series of 42 patients treated by surgical decompression of the suprascapular nerve. Three patients were treated by ganglion excision with good return of function, and 39 patients were treated with neurolysis. Thirty-one presented with mild to moderate shoulder pain and muscle weakness. Preoperative supraspinatus and infraspinatus muscle function was grade 0 to 2. Postoperative supraspinatus muscle function improved to grade 4 or better in 28 (90%) of the patients. Infraspinatus muscle function improved to better than grade 3 in 10 (23%) of patients. Eight patients presented with severe pain and grade 3 muscle strength. Postoperatively, 7 (88%) of the patients in this group reported improvement in pain. All patients had muscle strength that remained the same or improved to grade 4.
Patients with severe, longstanding atrophy and denervation potentials on EMG have a poor prognosis for recovery with surgical decompression. Because the supraspinatus stabilizes the humeral head in the glenoid during arm elevation and the infraspinatus provides 90% of the external rotation power of the shoulder, residual weakness will limit activities of daily living and often preclude return to competitive sports.30
The axillary nerve is a branch from the posterior cord of the brachial plexus receiving its nerve fibers from the C5 and C6 nerve roots. The nerve courses laterally and inferiorly, anterior to the subscapularis muscle, passing along the medioinferior aspect of the shoulder joint, around the neck of the humerus into the quadrilateral space. The quadrilateral space is on the posterior aspect of the shoulder, bordered by the teres minor superiorly, teres major inferiorly, humeral shaft laterally, and the long head of the triceps muscle medially. The axillary nerve then passes around the posterior and lateral portion of the proximal humerus, divides into anterior and posterior branches, and innervates the deltoid and teres minor muscles. A sensory cutaneous branch of the nerve supplies the lateral aspect of the upper arm.
Injury to the axillary nerve is usually a result of a proximal humerus fracture, shoulder dislocation, or direct trauma.1,3 Iatrogenic injury to the nerve can result from any operative procedure involving the inferior aspect of the shoulder capsule, by either compression from a retractor or direct injury during the capsular release. The axillary nerve runs horizontally 5 cm inferior to the acromion; a deltoid-splitting procedure that exceeds this 5 cm will injure the nerve and denervate the anterior deltoid muscle. The high frequency of involvement of this nerve with shoulder injuries is explained by its anatomic location, making it more susceptible to stretch or tension.
The clinical presentation can vary considerably, including poorly localized anterior shoulder pain, deltoid atrophy with weakness in arm elevation and abduction, and numbness along the lateral aspect of the upper arm (Fig. 57-9). Abduction weakness may not be clinically apparent because of effective substitution by the supraspinatus muscle. When an axillary nerve injury is diagnosed, the remaining branches of the posterior cord must be evaluated to determine the location of the nerve lesion. This requires examination of the muscles innervated by the thoracodorsal nerve (latissimus dorsi muscle) and radial nerve (wrist and finger extensor muscles). Electrophysiologic testing is helpful in determining the location and extent of the axillary nerve injury.
If a partial injury exists, treatment consists of rest and therapy. Therapy should focus on strengthening of the shoulder muscles, specifically the deltoid and teres minor muscles, and maintaining shoulder range of motion. Because of the short course of this nerve, some degree of recovery should be observed within 3 to 4 months. If no recovery is evident after this period, neurolysis or nerve grafting produces a good outcome.11,34 Kline and Kim24 reported a series of 56 isolated axillary nerve palsies of which 90% had nerve lesions in continuity and were treated with operative exploration. If nerve action potentials were recorded crossing the zone of injury, neurolysis was performed. If no conduction was evident, the lesion was resected and nerve grafted. The neurolysis group attained a mean grade of 4 muscle recovery. The nerve graft group attained a mean grade of 3.7 muscle recovery.
Quadrilateral space syndrome involves compression of the posterior humeral circumflex artery and axillary nerve5 as they pass through the quadrilateral space. It manifests with axillary nerve deficits, as discussed earlier, and a diminished radial pulse with the arm positioned in abduction, elevation, and external rotation (provocative maneuver). This diagnosis can be confirmed by arteriography while performing the provocative maneuver. Once this problem is diagnosed, surgical decompression is usually necessary to relieve symptoms. Cahill and Palmer5 reported a series of 18 patients treated with surgical decompression of the quadrilateral space. Eight patients reported complete relief of symptoms, eight reported improvement of symptoms, and two patients showed no improvement.
The musculocutaneous nerve is a terminal branch of the lateral cord, receiving contributions from the C5 and C6 roots. It supplies the coracobrachialis, brachialis, and biceps muscles; innervates the elbow joint; and becomes the lateral antebrachial cutaneous nerve in the forearm. Isolated musculocutaneous nerve injury is uncommon. It usually occurs in association with shoulder trauma. Injury may occur iatrogenically during shoulder operative procedures (e.g., creating an anterior portal for shoulder arthroscopy). A “safe zone” has been described intraoperatively because the nerve is usually noted to cross obliquely to enter the brachioradialis muscle 5 cm below the coracoid process. However, this interval of safety may be diminished by abduction of the arm and with anatomic variation. Iatrogenic injury can also occur from arm traction during an operative procedure.
Clinical presentation of a musculocutaneous nerve injury includes weakness of elbow flexion, especially with the forearm fully supinated, and sensory loss over the lateral volar forearm. Unless it is known with certainty that the nerve is divided, patients with immediate postoperative musculocutaneous nerve deficits are treated nonoperatively with therapy for the first 3 weeks. Therapy should involve strengthening the shoulder muscles, focusing on the involved coracobrachialis, brachialis, and biceps muscles, and maintaining shoulder motion. Three weeks after surgery, electrophysiologic studies can be performed to further assess the state of the nerve. Signs of nerve recovery should be evident within 3 months of injury. If no recovery is noted by 3 to 6 months, surgical exploration and repair are recommended. Osborne et al.31 reported a series of 85 patients with repair of traumatic lesions of the musculocutaneous nerve. There were 57 patients with good results, 17 patients with fair results, and 11 patients with poor results. The type of injury was the most important factor in determining the result. Twelve of 13 patients with clean, sharp lacerations had good results compared with 30 of 48 patients with closed traction lesions who had good results. The results were better when repaired within 14 days of the injury and when the nerve grafts were less than 10 cm long. The results were worse in the presence of a bony or arterial injury.
Nerve injuries about the shoulder are uncommon but are a commonly undiagnosed cause of shoulder dysfunction. Injuries to the nerve coexist with other more common shoulder problems. The goal of management is early diagnosis by careful physical examination and electrophysiologic studies.
The correct diagnosis must be made to guide treatment to restore stability and mobility to the shoulder. Therapy relies heavily on the appropriate management of scapular dysfunction, which is thoroughly discussed in Chapter 93.
1. Berry H, Bril V. Axillary nerve palsy following blunt trauma to the shoulder region: a clinical and electrophysiological review. J Neurol Neurosurg Psychiatry. 1982;45:1027.
2. Bigliani LU, Perez-Sanz JR, Wolf IN. Treatment of trapezius paralysis. J Bone Joint Surg. 1985;67A:871.
3. Blom S, Dahlback LO. Nerve injuries in dislocations of the shoulder joint and fractures of the neck and humerus. Acta Chir Scand. 1970;136:461.
4. Bertelli JA, Ghizoni MF. Long thoracic nerve: anatomy and functional assessment. J Bone Joint Surg Am. 2005;87(5):993–998.
5. Cahill BR, Palmar PE. Quadrilateral space syndrome. J Hand Surg. 1983;8:65–69.
6. Chavez JP. Pectoralis minor transplanted for paralysis of the serratus anterior. J Bone Joint Surg. 1951;33B:2128.
7. Clein LJ. Suprascapular entrapment neuropathy. J Neurosurg. 1975;43:337.
8. Dewar FP, Harris RI. Restoration of function of the shoulder following paralysis of the trapezius by fascial sling fixation and transplantation of the levator scapulae. Ann Surg. 1950;132:1111.
9. Disa JJ, Wang B, Dellon AL. Correction of scapular winging by suprascapular neurolysis of the long thoracic nerve. J Reconstr Microsurg. 2001;17(2):79–84.
10. Foo CL, Swann M. Isolated paralysis of the serratus anterior. J Bone Joint Surg. 1983;65B:552.
11. Friedman AH., et al. Repair of isolated axillary nerve lesions after infraclavicular brachial plexus injuries: case reports. Neurosurgery. 1990;27:403.
12. Ganzhorn RW., et al. Suprascapular nerve entrapment. J Bone Joint Surg. 1981;63:492.
13. Garcia G, McQueen D. Bilateral suprascapular nerve entrapment syndrome. J Bone Joint Surg. 1981;63A:491.
14. Goodman CE, Kenrick MM, Blum MV. Long thoracic nerve palsy: a follow-up study. Arch Phys Med Rehabil. 1975;56:352.
15. Hadley MN. Suprascapular nerve entrapment. J Neurosurg. 1986;64:843.
16. Harris HH, Dickey JR. Nerve grafting to restore function of the trapezius muscle after radical neck dissection. Ann Otolaryngol. 1965;74:880.
17. Hawkins RJ, Willis RB, Litchfield RB. Scapulothoracic arthrodesis for scapular winging. In: Post M, Morrey BF, Hawkins RJ, eds. Surgery of the Shoulder. St Louis: Mosby; 1990.
18. Hershman EB, Wilbourn AJ, Bergfeld JA. Acute brachial neuropathy in athletes. Am J Sports Med. 1989;17:655.
19. Hirayama T, Takemitsu Y. Compression of the suprascapular nerve by ganglion at the suprascapular notch. Clin Orthop. 1981;155:95.
20. Johnson JTH, Kendall HO. Isolated paralysis of the serratus anterior muscle. J Bone Joint Surg. 1955;37A:567.
21. Kamura J. Electrodiagnosis in Disease of Nerve and Muscle. Philadelphia: FA Davis; 1983.
22. Kim DH, Cho YJ, Tiel RL, Kline DG. Surgical outcomes of 111 spinal accessory nerve injuries. Neurosurgery. 2003;53(5):1106–1112.
23. Kim DH, Murovic JA, Tiel RL, Kline DG. Management and outcomes of 42 surgical suprascapular nerve injuries and entrapments. Neurosurgery. 2005;57(1):120–127.
24. Kline DG, Kim DH. Axillary nerve repair in 99 patients with 101 stretch injuries. J Neurosurg. 2003;99(4):630–636.
25. Langenskiold A, Ryoppy S. Treatment of paralysis of the trapezius muscles by the Eden-Lange operation. Acta Orthop Scand. 1973;44:383.
26. Leffert RD. Nerve lesions about the shoulder. Orthop Clin North Am. 2000;31:331.
27. Marmor L, Bechtal CO. Paralysis of the serratus anterior due to electric shock relieved by transplantation of the pectoralis major muscle: a case report. J Bone Joint Surg. 1983;45A:156.
28. Nath RK, Lyons AB, Bietz G. Microneurolysis and decompression of long thoracic nerve injury are effective in reversing scapular winging: long term results in 50 cases. BMC Musculoskelet Disord. 2007;8:25.
29. Neviaser TJ., et al. Suprascapular nerve denervation secondary to attenuation by a ganglion cyst. J Bone Joint Surg. 1986;68A:627.
30. Nicholas JA, Hershman EB. The Upper Extremity in Sports Medicine. St Louis: Mosby; 1995.
31. Osborne AW, Birch RM, Munshi P, Bonney G. The musculocutaneous nerve. J Bone Joint Surg. 2000;82(8):1140–1142.
32. Parsonage MJ, Turner JWA. Neuralgic amyotrophy: the shoulder girdle syndrome. Lancet. 1948;1:973.
33. Perlmutter GS, Leffert RD. Results of transfer of the pectoralis major tendon to treat paralysis of the serratus anterior muscle. J Bone Joint Surg Am. 1999;81(3):377–384.
34. Petrucci FS, Morelli A, Raimohdi PL. Axillary nerve injuries: 21 cases treated by nerve graft and neurolysis. J Hand Surg. 1982;7:271.
35. Rask MR. Suprascapular nerve entrapment: a report of two cases treated by suprascapular notch resection. Clin Orthop. 1978;134:266.
36. Romero J, Gerber C. Levator scapulae and rhomboid transfer for paralysis of trapezius. The Eden-Lange procedure. J Bone Joint Surg Br. 2003;85(8):1141–1145.
37. Seddon HJ. Three types of nerve injury. Brain. 1943;66:237.
38. Strohm BR, Colachis SC Jr. Shoulder joint dysfunction following injury to the suprascapular nerve. Phys Ther. 1965;45:106.
39. Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491.
40. Sunderland S. Nerves and Nerve Injuries. 2nd ed Edinburgh: Churchill Livingstone; 1978.
41. Swafford AR, Lichtman DH. Suprascapular nerve entrapment: case report. J Hand Surg. 1982;7:57.
42. Teboul F, Bizot P, Kakkar R, Sedel L. Surgical management of trapezius palsy. J Bone Joint Surg Am. 2004;86-A(9):1884–1890.
43. Thompson RC Jr, Schneider W, Kennedy T. Entrapment neuropathy of the inferior branch of the suprascapular nerve by ganglia. Clin Orthop. 1982;166:185.
44. Vastamaki M. Suprascapular nerve entrapment. Paper presented at AAOS 54th Annual Meeting, Scientific program, 1987.
45. Wright YA. Accessory spinal nerve injury. Clin Orthop. 1975;108:15.
46. Zoltan JD. Injury to the suprascapular nerve associated with anterior dislocation of the shoulder: case report and review of the literature. J Trauma. 1979;19:203.