Animal Models of Pain
Introduction
Animal modeling of the complex sensory, emotional, and ultimately subjective experience of pain poses a number of challenges. First, there is limited understanding of the pathophysiological mechanisms underlying the heterogeneous conditions that are the aim of the modeling. Other fundamental challenges are related to understanding the physical characteristics and physiological consequences of the stimulus used in the modeling paradigm, interpreting behavior as an expression of pain (or some other sensory experience), and understanding the relationship between stimuli and responses. Under physiological conditions a painful stimulus is likely to be transient, such as a pinprick or hot object, but under pathological conditions, particularly if chronic, pain may be generated or amplified from processes within the affected organs, be it an inflamed joint or an injured and dysfunctional nervous system. The processing of noxious signals and the experience of pain are certainly modulated by activity in the central nervous system. External stimuli are useful in probing the internal state, but we cannot a priori assume a direct relationship between evoked responses and the pain experience. Our methodology to quantify ongoing pain in animals is much less advanced than the armamentarium available for studying evoked responses, but there is growing interest in bridging the gap, and some approaches will be discussed in following sections.
The experience of pain can be inferred from an animal’s behavior. This is, of course, also the case with human beings not able to verbalize or communicate in the observer’s language. Although we may intuitively find it easier to interpret behavior displayed by a member of our own species, in reality, both verbal and non-verbal communication between humans is inherently imprecise and subject to interpretation (Staton et al 2007). People as well as animals may have reasons to exaggerate or suppress their expression of pain. Consequently, strictly objective measures of pain may not be achievable whether in animals or in human patients, and interpretation of behavior and verbal report will continue to play an important role in understanding and measuring pain. Some components of the pain experience may be unique to humans and therefore not possible to model in animals. This is particularly the case with chronic pain, where, for example, cognitive and emotional adaptation to the pain condition is less likely to be similar between species than, for instance, plastic changes in descending inhibition driven by noxious input. Recognizing these limitations is a prerequisite for effective use of animal models.
It is nevertheless unlikely that the ability to feel and experience pain, which has obvious survival value, would differ fundamentally between mammals. This is a basic assumption for animal modeling and has ethical implications. There is usually no conflict between good scientific practice and ethical treatment of laboratory animals, but it is important to formulate a clear, testable hypothesis, use adequate experimental designs, and minimize suffering both in terms of the individual animal and with regard to the number of animals. Another aspect is that the results should be published or lead to scientific progress in some other way. Reluctance to publish negative findings may contribute to an overall bias in the literature (Rice et al 2008) and possibly lead to unnecessary experiments.
Modeling may serve several purposes. Understanding the pathophysiology of pain with the aim of developing new prevention and treatment strategies, including novel analgesics, is probably the most cited motivation. Although the utility of animal modeling in analgesic drug development has been questioned (Quessy 2010), current models used carefully and with due attention to supporting data from the use of other methods have provided invaluable input to this process and will continue to do so in the foreseeable future (Mogil et al 2010a, Berge 2011). Animal models also expand our understanding of the normal physiology of nociceptive signaling—from molecular mechanisms to integrated systems. Basic science is valuable in its own right and an important foundation for applied research and generation of hypotheses with practical implications.
The number of animal models available for research on pain and analgesia is large and growing. Any model represents a simplification of the modeled condition, and it is important to recognize that the utility and validity of a model are fundamentally related to the specific hypothesis under investigation. Although a single model should not be expected to represent all aspects of a complex condition such as chronic neuropathic pain in humans, a set of models and supporting methods may provide decisive information about critical pathophysiological mechanisms.
Pain modeling is usually thought of in terms of behavior, but electrophysiology, functional imaging, biochemical biomarker analysis, genetics, and analysis of exposure and pharmacokinetics provide information that is indispensable for the elucidation of pain processes. A combination of several approaches is the most powerful way to address this complex area. This chapter focuses on commonly used behavioral methods, but other methodological tools will be mentioned or referred to as needed for discussion. Modeling will be described in terms of the stimuli used to evoke quantifiable responses and behavior, with critical discussion of some of the more popular methods of stimulation and quantification, and finally the manipulation that creates the relevant pathophysiology (the model per se). Rodent models are the main focus of this review, but it is appreciated that species differences in physiology, anatomy, and pathophysiology mean that other species are needed, such as for analysis of primary afferent functions translatable to human microneurography studies (Obreja and Schmelz 2010) or to overcome differences in receptor pharmacology (Leffler et al 2009).
Common Stimuli and Quantification of Evoked Responses
Nociceptive tests based on withdrawal responses evoked by stimuli at or near threshold intensity (e.g., thermal assays such as the tail-flick and hot plate tests) are frequently referred to as models of acute pain. This can be confusing since acute pain in clinical terminology typically refers to pain caused by accidental injury or surgery and involves inflammatory and sensitizing mechanisms not present in the basic implementations of these tests. Similarly, animal models of chronic pain are usually carried out in a time span of minutes to a few weeks, whereas in the clinic, chronic pain refers to conditions lasting at least 3 months. This distinction is not just semantic since some pathophysiological processes require more than a few weeks to develop. The formalin test may mimic some features of a particular chronic pain condition (e.g., paroxysmal peripheral input) but will not emulate, for example, long-term central nervous system plasticity. Conversely, inflammatory processes inherent in the model or its initiation may be more typical of a postoperative stage than of chronic neuropathy, for instance.
Quantification of pain and analgesic effect in animal models usually relies on responses evoked by physical or chemical stimuli or modification of motor behavior observed after tissue injury or inflammation. The use of controlled stimuli such as heat, cold, electric shock, or injection of chemical mediators may facilitate the specific activation of discrete groups of primary afferents, which are classified as mechanonociceptors, polymodal nociceptors, and mechanically insensitive nociceptors and further subdivided according to transduction and conduction properties (Schaible and Schmidt 1983, Perl 1996, Treede 1999, Koltzenburg 2000, Belmonte and Viana 2008, Beissner et al 2010; see also Chapter 1). These primary afferents are complemented by others responding to non-noxious levels of intensity. For mechanistic studies, specific activation of certain fiber types is a great asset, but using highly standardized and discrete methods may result in data and conclusions valid only for a limited set of circumstances. The difference between painful and non-painful is often a matter of stimulus intensity, and the criteria for whether a response reflects pain or some other modality may be arbitrary, particularly when graded stimuli are used.
Electric Stimuli
Electric stimuli are currently rarely used in pain research except for electrophysiological experiments and conditioning paradigms but have in the past been applied extensively in paradigms such as the flinch-jump test, vocalization tests, and jaw-opening models (Le Bars et al 2001). Electric stimuli are quantifiable and time-locked and can be repeated without damaging the stimulated tissue. Although delivery of an electric stimulus is easy to control, the impact on the target tissue is affected by a number of factors ranging from differences in tissue impedance (which may be affected by behavioral strategies of the subject) to contamination of the grid through which the stimulus is delivered. These factors may be difficult to recognize and control for, particularly when using conscious, unrestrained, or lightly restrained animals. Electric stimuli bypass the peripheral receptors and recruit all types of cutaneous nerve fibers, depending on stimulus intensity, with large-diameter fibers being excited at lower intensities than fine-diameter nociceptors. This means that a mixture of sensory modalities will be activated with a given stimulus, possibly engaging central inhibitory functions in addition to nociception. Human subjects tend to find electric stimuli aversive regardless of whether they are painful. In rodents, stimuli just above the detection threshold elicit an orienting response, and higher intensities elicit progressively more forceful responses. A pain threshold can be defined by using arbitrary criteria such as vocalization or jumping synchronized with the shock, but because of the aversive nature of electrical stimulation, even at low intensities, avoidance or escape responses are not sufficient to assume pain experience.
Heat
Heat is an adequate pain stimulus and a versatile modality that is used extensively in both animals and humans. The prototypical animal method based on radiant heat is the tail-flick test originally described by D’Amour and Smith (1941), which was preceded by the extensive use of radiant heat stimuli in human psychophysical studies (Beecher 1957). Various methods of heating are listed in Table 11-1.
The rate of increase in temperature in the exposed tissue depends on the type of heat source and the energy supplied. Most methods used in animal research are significantly influenced by the initial temperature of the skin. Depending on the tissue temperature achieved and the rate of heating, different classes of nociceptors are recruited, which can be used purposefully for mechanistic studies or contribute to unwanted variability and false interpretations if not adequately considered (Le Bars et al 2001). C fibers are activated at lower temperatures than Aδ fibers are, and slow heating rates will primarily recruit C fibers whereas more rapid heating will favor Aδ nociceptors (Yeomans et al 1996, Yeomans and Proudfit 1996, Schepers and Ringkamp 2009).
Heat stimuli are usually delivered by means of radiation. With this stimulus modality, transfer of heat to the layers of the nociceptive nerve endings is affected by a number of factors that need to be controlled. Skin color is important for heat absorption, and the heating rate can be accelerated by blackening of the skin (Winder et al 1946). Conversely, differences in skin color may be a source of variation and even a confounding factor if there are systematic differences between subjects (Wen et al 2009). As illustrated in Figure 11-1, skin temperature at the outset of stimulation is another important factor when radiant heat is used (Berge et al 1988, Tjølsen et al 1988, Luukko et al 1994).
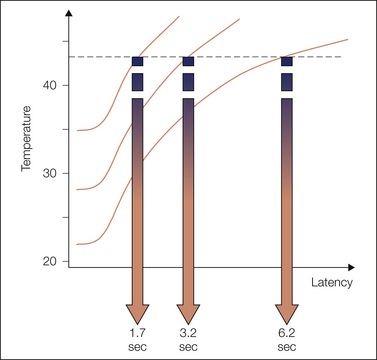
Figure 11-1 Relationship between initial skin temperature and latency to withdrawal when a radiant heat stimulus is applied.
These simulated data are based on actual recordings of subcutaneous skin temperature from the tail of an anesthetized rat stimulated by a standard tail-flick apparatus (Arne Tjølsen, thesis, University of Bergen, 1990). The threshold for withdrawal is indicated by the broken line. How fast the threshold is reached depends on the initial skin temperature and determines the response latency if other factors are equal. The rate of increase in temperature and the shape of the temperature curves depend on the heat source. The initial delay in heating can be minimized by preheating the projection bulb and using a shutter to control the stimulation.
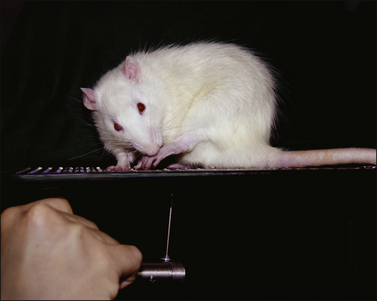
Since its introduction, the radiant heat paw withdrawal method (Hargreaves et al 1988) has rapidly become the standard method for measuring heat sensitivity in rodents, largely because it can easily be applied to the paw inflammation and sciatic neuropathy models of growing popularity. In this assay, a beam is projected through a transparent floor and (as in the tail-flick assay) the behavioral end point is the response latency, in this case withdrawal of the stimulated paw. Animals can be tested with a minimum of potentially stressful restraint, and the stimulus is directed to a specific and restricted part of the body while avoiding heterosegmental stimulation of nociceptors that might be a confounding factor, as in the hot plate test. The assay is, however, sensitive to certain factors, including posture, exact focus of the beam, initial temperature of the skin (which may be altered by inflammation or neuropathy, as well as by handling), conditions in the testing environment, and confounding pharmacological effects (Bennett and Xie 1988, Luukko et al 1994, Dirig et al 1997, Le Bars et al 2001). Preheating the floor to a holding temperature above the normal range of skin temperature will reduce or eliminate the influence of initial skin temperature and allow better reproducibility of the stimulus function. Importantly, starting from a higher temperature makes it possible to apply a slower heating rate without causing impractically long response latencies, and these factors may tune the assay toward C fiber–mediated responses. Description of the method used in publications is frequently unclear regarding whether adequate controls have been established for these potentially important variables.
Heat stimuli can also be delivered by direct contact with a heat source. Thermodes are common in human experimental work and on anesthetized animals but for practical reasons are used less frequently in behavioral studies. Thermodes can be used at a predetermined fixed temperature or to deliver a linear increase in temperature from a defined holding temperature. In principle, this is also the case with the rising-temperature hot plate assay, in which the temperature is increased at a slow rate (typically 2–3°C/min from a temperature of 32°C), thereby allowing estimation of an approximate response threshold as the temperature at which a predetermined response (e.g., licking of a hindpaw) occurs (Tjølsen et al 1991). However, because of the temperature gradient between the surface and the core, the observed threshold will be higher than the actual temperature at the nociceptors. The slow increase in temperature and the limited maximum temperature mean that the assay is likely to involve C fibers to a greater extent than conventional methods using a constant temperature source, as in the traditional hot plate assay or tail-dip and paw-dip assays. When compared with the conventional constant-temperature hot plate, it is also less sensitive to motor activity and stress (Hunskaar et al 1986), but exposure of several body parts to the hot plate means that heterosegmental interaction can take place.
These acute thermal assays can be used to study physiological nociception, and their utility and limitations have been discussed more extensively elsewhere (Le Bars et al 2001). Since the recruitment of different classes of heat nociceptors and the relative degree of non-nociceptive thermosensor activation depend on the heating profile, minor differences in protocol may be physiologically and pharmacologically relevant. Traditional implementations of acute heat pain assays in rodents tend to favor responses mediated through Aδ-fiber stimulation.
It has long been recognized that methods using acute thermal stimuli are sensitive to agonists at the μ-opioid receptor but not to other types of opiates (Taber 1974). The hot plate assay is, for instance, capable of predicting the potency ranking of spinal opioid analgesics corresponding to clinical dosing for postoperative pain (Yaksh 1997). The actual potency estimates of morphine, for instance, may differ 20–50-fold in a single paradigm, depending on the heating profile (Yeomans et al 1996). The assays are not sensitive to non-opiate analgesics unless very high, perhaps toxic doses are administered (Taber 1974, Liles and Flecknell 1992).
Cold
Cold stimuli are used primarily in the context of neuropathic pain models and in work aimed at characterizing physiological and molecular mechanisms of cold perception and cold pain. Research on cold transduction has made great progress in the last 10–15 years, but the way that signaling in different afferent fiber types relates to tissue temperature and sensory quality is less clear than that for heat perception. Although understanding of how these factors contribute to pathological pain in chronic conditions is incomplete, it is clear that as for heat, both the rate of change in temperature and the actual tissue temperature determine the recruitment of different fiber types and thus the mechanisms activated by a cold stimulus (Foulkes and Wood 2007, Belmonte et al 2009).
The most common way of inducing cooling and pain in rodent behavioral models is probably the application of acetone. The test is also used clinically; the stimulus is normally perceived as innocuous but may evoke allodynia in a minority of chronic pain patients (Rasmussen et al 2004). A typical testing protocol in the rat involves the application of a fixed volume of acetone to the plantar skin by means of plastic tubing and counting the frequency of responses, defined as brief withdrawal of the paw, obtained after a predefined number of evenly spaced applications (Choi et al 1994). Acetone spray and ethyl chloride spray are also used, thus adding a mechanical component to the stimulus, and the outcome variable is then usually the accumulated duration of paw withdrawal recorded for a period of up to 60 seconds after application (Dowdall et al 2005, Gustafsson and Sandin 2009). The degree of cooling and the contribution of the mechanical component may vary significantly between implementations of these methods, and therefore achieving reproducibility between different operators may be a challenge. Less frequently, cold sensitivity has been measured by means of a cold plate (Bennett and Xie 1988) or cold water bath (Pizziketti et al 1985), analogous to the heat stimulus paradigms, with latency to withdrawal or the frequency or duration of paw lifts being outcome measures.
Mechanical Stimulation
A diverse set of devices for mechanical stimulation exist, and we will discuss the most commonly used of these, von Frey filaments, which are used to deliver punctuate pressure. We also briefly discuss the pinprick method for punctuate hyperalgesia and approaches to measure deep pressure. In general, mechanical stimuli are difficult to apply in a standardized fashion even when standardized equipment is used, and the great number of devices and protocols make it difficult to compare results reported in different publications. In interpreting the data it is therefore important to understand the physiological consequences of the stimulation procedure in each case.
Von Frey Filaments and Similar Methods
Von Frey filaments were introduced in the 19th century to determine sensory thresholds in humans. The method is based on the principle that a monofilament will bend at a distinct force when applied perpendicular to a surface. A range of calibrated filaments can be used to calculate a response threshold or, alternatively, the response frequency (Chaplan et al 1994). When used in rodents, the animals are usually placed on a covered grid and probed from below, but in some cases the animals are restrained. Response is defined as brisk withdrawal of the paw, but a “hyperalgesia-like” response in which the animal elevates the paw for a second or more or shakes, grooms, licks, or chews the paw has been suggested to differentiate between aversive and non-aversive sensations when applied to neuropathic rats (Hogan et al 2004). This exaggerated behavior is rarely seen in control animals unless a very thin probe is used.
Von Frey filaments are used extensively but are associated with a number of drawbacks. Bove (2006) has provided a detailed analysis that can be summarized as follows:
These factors and the use of discrete filaments of fixed nominal bending force limit accurate estimation of thresholds and comparison of data between laboratories. Bove (2006) further refers to anecdotal data suggesting a discrepancy between the effect of filament stimulation and a set of other, perhaps more relevant mechanical stimuli, such as light or hard stroking and vigorous rubbing of the affected paw.
Some of these problems can be minimized by proper technique, such as stopping stimulation immediately when the filament bends, but there are additional complications related to the mechanical instability of conventional filaments. They are usually made of hygroscopic material and their bending properties will change rapidly in response to normally occurring fluctuations in relative humidity; a No. 3 hair at 26% relative humidity may bend at higher force than a No. 6 filament at 56%. They are also affected by temperature and deformation. As mentioned earlier, contact area geometry plays a role in stimulus function, and other factors being equal, probes of smaller diameter will be more effective (see Ängeby-Möller et al 1998 for references).
Using filaments made of non-hygroscopic material and with a fixed tip size can circumvent several of these problems (Song et al 1999, Fruhstorfer et al 2001). Force transducers based on von Frey–like devices (“electronic von Frey”; Fig. 11-2) are also available and facilitate standardization of stimulus application and avoid the problem of variable geometry and mechanical instability of the probe (Ängeby-Möller et al 1998). Standardized protocols that would reduce inter- and intraobserver variability have been suggested (e.g., Song et al 1999, Hogan et al 2004), with some salient factors to consider being the duration of application, stimulus interval, degree of bending, and site of stimulation.
Pinprick
Mechanical hyperalgesia is frequently tested by the pinprick method, which in its simplest form is performed by applying a sharp object such as a safety pin or an injection needle to the skin to cause an indentation but no penetration. The animals may be restrained for the procedure or, more commonly, placed on a grid. The response, either brisk withdrawal or a “hyperalgesia-like” response with sustained elevation, such as licking and grooming of the paw, can be quantified as the duration of such behavior in a predefined time frame (Erichsen and Blackburn-Munro 2002, Hogan et al 2004). In human experimental work, a more standardized procedure has been devised in which weighted pinprick stimulators with a fixed flat contact area of 0.2 mm in diameter produce force between 8 and 512 mN, which can be used to calculate threshold and stimulus response functions (Rolke et al 2006).
Deep Pressure
Sensitivity to deep pressure can be tested by applying constant or gradually increasing stimuli, the later being more common in animal research. The procedure is often referenced to a paper by Randall and Selitto (1957), who described a pneumatic device to deliver a controlled, progressive mechanical stimulus via a bullet-shaped wooden peg to a rat’s inflamed paw. The pressure reached when the rat started to struggle was taken as the behavioral readout. Since animals with an inflamed paw were used, the method could be shown to be sensitive to non-narcotic analgesics such as salicylates, in contrast to previously used deep pressure methods. Today, modified versions of the method are applied in models of neuropathic as well inflammatory hypersensitivity (Whiteside et al 2004, Pradhan et al 2010).
Chemical Stimuli
Irritants may cause pain by local administration to skin and other organs. In principle, they may act by direct activation of nociceptors and/or by causing inflammatory or toxic effects in the tissue. In either case, this type of stimuli is fundamentally different from acute physical stimuli in that the effect is protracted and sometimes lasts for several minutes and longer. The immediate effect is usually quantified by the behavior elicited (e.g., flinching, favoring, licking, or biting of an injected paw), and the altered threshold to mechanical or thermal stimuli as described earlier is used to study sensitization. The most popular of these methods is undoubtedly the formalin test, which is discussed in some detail.
The Formalin Test
Injection of formalin induces behavior suggestive of pain or irritation in several species (Tjølsen et al 1992, Raboisson and Dallel 2004). In humans, injection of formalin into the index finger or base of the hand was anecdotally reported to produce an intense, sharp, stinging, and burning pain (rated 3 out of 5 in intensity), which after about 5 minutes was replaced by a steady, throbbing ache that gradually disappeared over a period of 30–60 minutes and was followed by mild residual tenderness at the injection site (Dubuisson and Dennis 1977). The method, as largely implemented today, was first described in rats and cats and was developed to allow continuous painful stimulation (as opposed to commonly used transient stimuli such as electric shock and radiant heat) and to avoid restraint in the testing session, which might produce undue stress and interference with spontaneous behavior (Dubuisson and Dennis 1977). Dilute formalin was injected subcutaneously into a forepaw, and a pain intensity index was calculated based on the duration and weighting of protective behavior. Interestingly, cats did not display the clear biphasic response seen in rodents.
The formalin test is now used routinely in rats and mice. Formalin is usually injected into the dorsum or plantar tissue of a hindpaw, which makes it easier to differentiate the response from grooming than when a forepaw is used. In either way, the injection causes licking, flinching, shaking, and favoring of the affected paw that typically occurs in two phases, the early or first phase lasting up to 10 minutes after injection and the late or second phase for about 20–60 minutes. In rats it is common to calculate an index as originally described by Dubuisson and Dennis (1977), but more complex data-handling strategies, as well as reliance on a single parameter, usually flinching in rats or licking/biting of the paw in mice, are used (for review and discussion see Tjølsen et al 1992). Orofacial versions with injection into the upper lip of rats or mice have been developed to address mechanisms of the trigeminal system (Clavelou et al 1989, Luccarini et al 2006).
Both the concentration of formalin and the ambient temperature affect the time course of behavior. The second phase is not seen after lower doses and is greatly reduced at ambient temperatures that cause vasoconstriction. Conversely, higher ambient temperatures may increase the behavioral activity in the quiet period between the phases and make the biphasic pattern less clear. These factors also affect the tissue response and may influence the effect of pharmacological treatment (Rosland et al 1990, Tjølsen et al 1992, Damas and Liegeois 1999, Munro 2009).
Both phases of the behavioral response are associated with a primary afferent C-fiber drive that would be expected to initiate and maintain activity-dependent sensitization at a spinal level (McCall et al 1996). The electrophysiological response to formalin in the rat has been further characterized (Puig and Sorkin 1996). Single-fiber recordings of the sural nerve demonstrated activity in Aβ, Aδ, and high-threshold C nociceptive afferent fibers during the first phase. During the second phase, activity was observed in Aδ fibers with receptive fields in hairy skin and in mechanically sensitive C fibers, but also in mechanically insensitive fibers and Aδ and C fibers with receptive field centers outside the injection site. Activity during the second phase was suppressed by the administration of lidocaine in doses that produced non-anesthetic, clinically relevant exposure.
Behavioral studies show that both phases depend on the primary afferent drive (Taylor et al 1995), but central modulation may play a significant role in the behavioral response in the second phase of the test (Dickenson and Sullivan 1987, Abbadie et al 1997). This phase is generally more sensitive to pharmacological intervention. The human experience of changing pain quality and intensity as a function of time after injection, as well as the electrophysiological data cited earlier, may suggest that the phases differ with regard to pathology as well as intensity. Usually, the test is completed within 1 hour of formalin injection, but hypersensitivity to mechanical and thermal stimuli culminating 1–3 days after injection and lasting up to 4 weeks has been reported (Fu et al 2001). It appears that full-blown inflammation comes later than the behavior usually quantified. The contribution of inflammation to the behavior seen during the first and second phases is uncertain, and the test should not be referred to as inflammatory unless supported by other evidence, such as biomarker determination or relevant pharmacology in the same experiment or from experiments using an identical protocol. The formalin test is sensitive to non-steroidal anti-inflammatory drugs (NSAIDs) and mild analgesics only in relatively high doses and is probably best seen as a mechanistic model of pain driven by paroxysmal peripheral discharge, whether nociceptive or neuropathic in origin. There are many different implementations of the model in addition to the ones mentioned earlier, which hampers comparison of results across studies (Capone and Aloisi 2004).
The Writhing Test
The writhing test involves the intraperitoneal injection of an irritant that produces a behavioral response consisting of stretching and writhing, typically quantified as the number of episodes or the accumulated time that the behavior is displayed within a certain time frame. It is frequently referred to as a visceral pain model but is not specific for viscera because the peritoneum is affected. Both rats and mice have been used. Agents used to induce the response include acetic acid (probably the most frequently used agent), hydrochloric acid, phenylquinone, and potassium chloride, in addition to substances that may be more mechanistically selective, such as acetylcholine, adenosine triphosphate, bradykinin, noradrenaline, and oxytocin. The duration and intensity of the response depend on the agent and concentration. Strain differences in sensitivity may be substantial. Historically, various implementations of this paradigm have shown good sensitivity to analgesics of different classes, but the specificity is poor (Taber 1974). The method is therefore rarely used but may have some utility in mechanistic studies when agents that activate specific receptors are injected. For a detailed discussion of the pros and cons of this method see Le Bars et al (2001).
Injection of Specific Mediators
Injection or topical application of capsaicin to the skin causes transient pain followed by hypersensitivity to mechanical and thermal stimuli, as well as a local flare reaction. These effects can be quantified in several species, including the monkey, rat, and mouse (Butelman et al 2004, Joshi et al 2006), and constitute the basis for a popular human experimental pain model (Chizh et al 2009). Since capsaicin specifically activates transient receptor potential vanilloid 1 (TRPV1)-expressing nociceptors, the procedure is interesting from both a mechanistic and a translational perspective. Several other agents with selectivity for specific transduction mechanisms are appearing (Hwang and Oh 2007), and it has recently been claimed that formalin activates nociceptors at least in part through transient receptor potential A1 (TRPA1) (McNamara et al 2007).
Models of Arthritis
In rats, several models of polyarthritis are available to study disease and disease modification (Holmdahl et al 2001). Traditionally, polyarthritis induced by injection of Freund’s complete adjuvant (FCA, also referred to as CFA) into the base of the tail has also been used as a pain model. The procedure causes widespread tissue lesions and has detrimental effects on the general health of the subjects (for references and discussion, see Coderre and Wall 1987, Butler et al 1992). For pain research, the method has largely been abandoned to the benefit of various monarthritis and localized inflammation models.
The most popular of these models are based on injection of inflammatory agents into a knee or ankle joint or subcutaneously into the footpad of a rodent. Spontaneous and surgical models of osteoarthritis are available but not commonly used in pain studies (Ameye and Young 2006).
Intra-articular injection of sodium urate crystals has been used to produce joint inflammation and behavior indicative of pain in a range of species, and interestingly, the same procedure causes pain resembling an attack of gout in human volunteers and patients (Coderre and Wall 1987). In rats, behavioral signs and inflammation reach a maximum level within about 6 hours after injection into the ankle joint and start wearing off after 2 days. Injection of FCA into the ankle joint was introduced to produce a model of longer duration than the urate crystal model but without the generalized disease produced by systemic injection (Butler et al 1992). In the original study, behavioral and clinical signs of arthritis were observed between the second and sixth week after injection. Similar long-lasting hypersensitivity has been described after injection into the knee joint (Wilson et al 2006).
Typical implementation of the model in which lower doses of FCA are injected into the ankle joint (e.g., 0.05 mL containing 0.05 mg Mycobacterium tuberculosis) causes an inflammatory response lasting 1–2 weeks, which in most cases is sufficient for repeated administration of drugs. If a shorter duration is acceptable, carrageenan may be used as an induction agent since it produces an arthritis that peaks within 4–8 hours and resolves within 2–3 days (Schött et al 1994). Monarthritis models induced by FCA and carrageenan are also used in mice, with a similar time course as in rats (Heilborn et al 2007).
A more disease-like model of osteoarthritis is based on injection of the glycolysis inhibitor monosodium iodoacetate into the knee joint of the rat. The model was originally applied to the study of disease modification (Kalbhen 1987) but has occasionally been used as a pain model in rats. The procedure leads to signs of inflammation, particularly during the first week after induction, and to hypersensitivity to mechanical stimuli that lasts more than 4 weeks. An increased response to von Frey stimulation and deep pressure has been found to be partially sensitive to a high single dose of the NSAID diclofenac only during the first few days (Fernihough et al 2004). These data appear to be at variance with other findings in which several NSAIDs were effective in normalizing weight bearing 14 days after induction (Bove et al 2003), thus indicating that differences in protocol may affect pharmacological outcome significantly.
In arthritis models, hypersensitivity and drug effects are often quantified by observational rating of paw pressure against the floor during standing or walking, and even by evoked responses such as paw withdrawal, vocalization, and struggling to avoid thermal and mechanical stimuli (Coderre and Wall 1987, Yu et al 2002, Fernihough et al 2004). Several systems are available for automatic or semi-automatic quantification of weight bearing (Schött et al 1994, Bove et al 2003) or changes in gait (Ängeby-Möller et al 2008). These methods may provide more graded and differentiated readouts than purely observational and evoked response methods do and can thereby facilitate objective scoring. It cannot be assumed that the different quantification methods are equivalent in terms of engagement of sensory and pathophysiological mechanisms.
Carrageenan and FCA can also be injected subcutaneously, usually into the plantar surface of the rat hindpaw, which causes inflammation and hypersensitivity that can be quantified in a similar manner as in the monarthritis models and follow a similar time course. The implication of different injection sites is uncertain.
Models of Neuropathic Pain
Mechanisms revealed from models of neuropathic pain are discussed in Chapter 62. Here, the focus is on the models themselves.
Polyneuropathy Models
As with arthritis models, neuropathy models come in systemic and localized versions. Rodent models of diabetic-induced polyneuropathy are well established (Fox et al 1999, Obrosova 2009). These models may be used to study mechanisms related to neuropathic pain per se or to understand disease mechanisms. This distinction has implications for validation and validity of the models. General health changes may interfere with pain modeling and should be seen as a possible confounding factor. There are even models emerging for neuropathy induced by human immunodeficiency virus infection or antiretroviral therapy (Wallace et al 2007a, 2007b).
Neuropathy is a dose-limiting side effect of chemotherapy for cancer, and pain is an important symptom in this condition (Windebank and Grisold 2008). A number of models have been established in both rats and mice that use the more common chemotherapeutic agents such as vincristine, paclitaxel, cisplatin, and oxaliplatin. The compounds are administered both intravenously and intraperitoneally, and several administration paradigms are used, ranging from a single high-dose injection to daily or weekly injections for several weeks. For overviews see Authier and colleagues (2009) and Colleoni and Sacerdote (2010).
The majority of clinical drug trials involving neuropathic pain are performed in patients with polyneuropathy or post-herpetic neuralgia, and there is a need to develop and characterize models that reflect the pain-generating mechanisms of these conditions (Rice et al 2008). How well the diabetic and chemotherapy-induced models generalize to clinically painful diabetic neuropathy and other forms of polyneuropathy is currently unclear. A rat model of varicella-zoster virus–associated neuropathic pain exists but is not widely used, and it is not clear how closely it models post-herpetic neuralgia (Fleetwood-Walker et al 1999, Hasnie et al 2007).
Mononeuropathy Models
Notwithstanding a growing interest in polyneuropathy models for pain research, mononeuropathy models involving traumatic injury to a single nerve, usually the sciatic, dominate the field. Here we focus on the partial sciatic nerve lesion models as originally developed in rats. Mouse versions of these models and variants using branches of the trigeminal nerve have also been introduced (Vos et al 1994, Gustafsson et al 2003, Bourquin et al 2006, Kiso et al 2008, Colleoni and Sacerdote 2010) but will not be discussed specifically.
The chronic constriction injury (CCI) model was the first of a series of partial nerve lesion models. The injury is induced by applying loosely constrictive ligatures around the sciatic nerve trunk at the mid-thigh level (Bennett and Xie 1988). In the original publication, long-lasting changes in gait, posture, guarding, and spontaneous lifting of the affected paw were described, as well as a reduced rate of body weight gain, indicative of the presence of spontaneous pain. Sensitivity to heat, cold, and mustard oil but not to deep pressure was increased. The partial sciatic nerve ligation (PSL) model was developed to incorporate features of causalgia (Seltzer et al 1990, Shir and Seltzer 1990). In this model, approximately 50% of the sciatic nerve trunk is tied off by tight ligation. The procedure caused increased sensitivity to heat and touch. Neonatal capsaicin treatment prevented the development of thermal but not mechanical hypersensitivity, thus indicating differential involvement of myelinated and unmyelinated fibers. Involvement of the sympathetic system further indicated causalgia-like pathophysiology (Shir and Seltzer 1991).
There have been many attempts to simplify and standardize the procedures of the partial sciatic nerve lesion models. Using a polyethylene cuff instead of sutures in the CCI procedure leads to a similar model but possibly of a more reversible nature (Mosconi and Kruger 1996). Injury to the sciatic trunk by photochemically induced microinfarction was reported to produce a greater proportion of animals with pronounced tactile hypersensitivity than with the CCI and PSL methods (Gazelius et al 1996, Cui et al 2000). Comparing these models, it was found that the inflammatory cell count and pro-inflammatory cytokine levels were increased at the lesion site 14 days after surgery and that the inflammatory response correlated with tactile hypersensitivity in the CCI and PSL animals (Cui et al 2000). This finding is important since it implies that inflammation may play a role in the genesis of neuropathy in these models. Inflammation may also be a confounding factor producing symptoms and pharmacological responses not exclusively related to neuropathy when animals are tested within a few weeks of the original injury.
Another approach designed to standardize the procedure for partial nerve lesions is the spinal nerve ligation (SNL) model, in which spinal nerves L5 and L6 are tightly ligated distal to the dorsal root ganglia (Kim and Chung 1992). The authors found comparable increases in sensitivity to noxious heat and mechanical stimuli as in the PSL model and noticed behavior interpreted as signs of spontaneous pain (licking of the affected paw and overgrowth of nails). A study comparing the CCI, PSL, and SNL models found that mechanical hypersensitivity was more pronounced in the SNL and least in the CCI model whereas behavioral signs indicating ongoing pain were more prominent in the latter (Kim et al 1997). The behavioral signs of pain and hypersensitivity to sensory stimuli are reported to decrease after sympathectomy in all models, but more pronouncedly so in the SNL model. A theoretical advantage of the SNL model is a discrete pattern of denervation: the lateral part of the paw should be most affected, the central part is partially denervated, and the medial aspect is more intact. This pattern may be useful to elucidate the pathophysiological mechanisms involved, particularly the role of primary afferents not directly affected by the injury, but data are variable, perhaps reflecting differences in surgery and testing (Li et al 2000, Hogan et al 2004).
Even transection of different combinations of the three distal branches of the sciatic nerve (tibial, sural, and common peroneal) would potentially allow investigation of sensory changes in the innervation territory of both injured and neighboring intact sensory neurons. In one study, sectioning of the tibial and sural nerves while leaving the common peroneal nerve intact (TST) was found to be the more effective of different combinations in that it induces long-lasting increases in sensitivity to mechanical and thermal stimuli, as well as indications of spontaneous pain, in this case independent of sympathetic input (Lee et al 2000). Ligation plus transection of the tibial and common peroneal branches but sparing of the sural branch is known as the spared nerve injury (SNI) model (Decosterd and Woolf 2000).
The main features of the sciatic nerve lesion models have been reproduced across a great number of laboratories, but there are differences between models with regard to the technical difficulty of surgery and the duration and intensity of outcomes. Several studies have provided data comparing the methods as performed in a single laboratory. One of the more comprehensive of these studies compared the commonly used CCI, SNL, and PSL models with the TST model and complete transection of the sciatic nerve over an observation period of 56 days (Dowdall et al 2005). Animals were tested repeatedly during this period with a battery of tests consisting of an electronic von Frey method, pinprick, acetone spray, and scoring of paw lifting during 5 minutes on a cold- (−0.5°C), hot- (45°C), or neutral-temperature (22°C) plate.
The TST model produced robust responses to stimuli by the electronic von Frey, pinprick, and acetone procedures throughout the experimental period, whereas the response on the cold plate developed slowly and peaked around 4–5 weeks. There was little response on the neutral and hot plates—perhaps suggesting a low incidence of ongoing pain or dysesthesia and little thermal hyperalgesia in this model. The authors found that the surgery was relatively easy and consistent when compared with some of the other models.
The PSL and SNL models showed the same overall pattern across tests as the TST, but the effect tended to be of shorter duration, particularly for the PSL, and reproducibility of injury may be an issue with this model. SNL produced somewhat more response on the hot plate but was considered invasive and technically difficult to perform.
Of all the procedures, CCI produced the most pronounced response across tests. The variability between animals was high, however, with only a few animals responding on the hot and neutral plates. The animals that did respond to the neutral plate showed a clear-cut increase in paw lifting, possibly indicating ongoing pain or dysesthesia, although motor effects cannot be ruled out. The surgery was perceived as being technically difficult, and some animals fail to respond altogether.
Regarding readouts, the authors concluded that the mechanical thresholds obtained with the electronic von Frey procedure produced the most robust data in all models. Pinprick responses were also significant in all models, but with a more variable time course and greater variation between subjects. Some of the variability might have been due to difficulty standardizing the stimulus. The acetone spray test yielded robust and stable results, but a decline in responsiveness was observed after 3–4 weeks in the PSL and SNL models. Even the cold plate produced significant lifting in all models, but the response was variable and reached a stable plateau only in the CCI and SNL models. It was suggested that the neutral and hot plate tests might have some utility for screening of spontaneous pain but that they were not very useful in the context of these models.
It is clear from this study that the partial sciatic nerve lesion models may differ somewhat in the duration and magnitude of sensory changes and possibly in signs of spontaneous pain. The difference in technical difficulty and reproducibility may perhaps be of greater practical importance, however. The impact of surgical technique was investigated by Zeltser and co-workers (2000), who compared different methods of nerve sectioning in rats by using autotomy as a readout indicating pain, dysesthesia, or sensory disorder. They found higher rates of autotomy after cryoneurolysis and electrocauterization than after neurectomy with the CO2 laser, whereas tight ligation of the nerve or simple transection with scissors had an intermediate effect. The outcome may reflect the size of the injury discharge associated with the different procedures. These data, as well as other data discussed in the paper, indicate that surgical skill and procedure may have a significant impact on the outcome of neuropathy models and contribute to variability between surgeons and laboratories. Another factor shown to play a role is genetic differences between strains of animals, which represents a significant source of variability in model outcome (Mogil et al 1999b, Shir et al 2001, Xu et al 2001). Taken together, these factors may be more important than differences between the specific models.
In line with this supposition, a recent study comparing the CCI and SNL models found that the efficacy of some clinically used analgesics depended more on readout than on the model (Pradhan et al 2010). In this study, oxycodone, gabapentin, and amitriptyline were highly efficacious in reducing hypersensitivity to heat and deep pressure but only partially reversed the increased response to cold stimuli. Even the increased sensitivity to mechanical stimulation with von Frey filaments was only partially reversible, and amitriptyline was ineffective on this parameter, in line with some, but not all studies reviewed by Kontinen and Meert (2003). In contrast, Whiteside and colleagues (2008) found no effect of amitriptyline on paw pressure sensitivity in the SNL model.
How well do the Models Reflect Clinical Conditions?
As stated in the introduction, animal models may be used to elucidate fundamental mechanisms in nociception and pain, and the results from animal experimental studies may or may not have direct bearing on therapeutic advances in the area of clinical pain. The relatively short duration of the pathology in the models is a fundamental limitation that is difficult to fully overcome in a conventional laboratory setting, both for practical and ethical reasons. Even the models that are designated as chronic are often completed within 1 or 2 weeks and may have more features in common with clinical postoperative pain and pain caused by acute trauma and acute inflammatory disease. This means that processes that develop over a period of months and years may not be represented in most implementations of these models.
To study joint pain, the use of dogs or guinea pigs with naturally occurring osteoarthritis is an interesting possibility (Quessy 2010) but will at best solve only part of the problem. Quantification of hypersensitivity in more disease-like rodent models (e.g., K/BxN serum transfer arthritis) is another approach that may recruit mechanisms relevant to rheumatoid arthritis (Christianson et al 2010). Rodent modeling has access to both weight-bearing and gait analysis paradigms, which may reflect the activity-related pain common with chronic osteoarthritis. However, there is no generally accepted method for modeling spontaneous pain, which is typically seen in more advanced cases of osteoarthritis (Hunter et al 2008). In the clinical literature there is limited information on the relationship between ongoing pain and quantitative sensory testing parameters similar to the ones used in animal modeling, but increased sensitivity to stimuli, particularly pressure on a painful joint, has been reported and found to disappear together with spontaneous pain after joint replacement (Ordeberg 2004, Gwilym et al 2009). In terms of pharmacology, the monarthritis models are generally more responsive to analgesics than patients with joint pain are.
With regard to chronic neuropathic pain, ongoing pain is the major complaint, whereas modeling is focused mostly on evoked responses. Although signs of ongoing pain have been observed and reported with most of the common models, it is difficult to assess the impact since methods for quantification of ongoing pain remain rudimentary. Patients may or may not experience threshold changes in response to quantitative sensory testing stimuli resembling the ones used in animal models, but sensory loss is frequently observed. Increased sensitivity is not the rule on a population basis, and in a recent study of more than 1200 patients with neuropathy of various origin, increased sensitivity to cold was found in less than 20% of the patients, and the corresponding figures were 24% for heat, 20% for dynamic mechanical allodynia, 36% for blunt pressure, and 29% for pinprick (Maier et al 2010). However, in a study of 60 patients with peripheral traumatic neuropathy, 30 with pain and 30 without, 70% of the pain patients exhibited allodynia with light brushing, whereas none of the patients in the non-pain group were hypersensitive to this stimulus. Responses to heat and cold did not discriminate between the groups, with considerable hypersensitivity to cold being found even in the group with painless neuropathy (Kleggetveit and Jørum 2010).
Another study, this time on chronic pain of mixed cause, found that the intensity of ongoing pain correlated with cold allodynia (defined as >3 out of 10 on a visual analog scale) in a small subset of patients, regardless of whether their pain was of neuropathic origin (Rasmussen et al 2004). There is, in other words, no direct correlation between ongoing pain and hypersensitivity as studied in these examples, but the results may indicate that for some patients, the phenomena may be linked (Fig. 11-3).
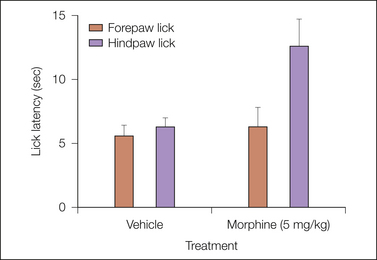
Figure 11-3 Forepaw lick versus hindpaw lick in a conventional hot plate paradigm.
Interpretation of behavior is sometimes a challenge: forepaw lick has been used as readout in this paradigm, as well as hindpaw lick. This experiment using rats, mentioned by Berge and colleagues (Berge et al 1983), was recorded on video and analyzed by an observer blinded to drug treatment. It turned out that many animals would lick their forepaws even when rearing, and the response was not dependent on previous contact with the hot plate. Forepaw lick is a heat-dissipating behavior in rodents and unsuitable as a readout in this type of test.
Behavioral Readouts
Regardless of the model and readout, interpretation of behavior is a challenge (see Fig. 11-3). The quality of the sensation eliciting an evoked response can be inferred only from the stimulus modality and characteristics of the response. It seems reasonable to assume that the reduced thresholds to von Frey stimulation seen in neuropathy and inflammation models reflect increased sensitivity to mechanical stimulation or a change in perceived quality of the stimulus, but the relevance of this measure in relation to pain or allodynia has been questioned (Le Bars et al 2001, Bove 2006). Using spontaneous motor activity, weight bearing, and gait analysis as a measure of pain seems to have utility in arthritis models but not in neuropathy, thus indicating that in the latter condition, these parameters reflect motor rather than sensory abnormalities (Ängeby-Möller et al 2008, Piesla et al 2009, Matson et al 2010, Mogil et al 2010b).
A number of alternatives to the conventional readouts described here have been investigated, and there is great interest in developing new approaches. Vierck has strongly advocated the use of operant methodology to obtain a readout more directly related to pain perception (e.g., Vierck et al 2008). A conditioned place avoidance paradigm has been used to demonstrate dissociation between sensory-discriminatory components, as reflected by conventional behavioral parameters, and affective components in a formalin model (Johansen et al 2001). A conditioned place preference model applied to the SNL and SNI models of neuropathic pain has provided evidence of tonic pain in these models (King et al 2009). Even drug discrimination has been used to demonstrate analgesic efficacy reflecting ongoing pain (Colpaert 1999). Recently, it was suggested that analysis of mice facial expression could provide a readout reflecting ongoing pain (Langford et al 2010). Unfortunately, the data supporting some of these exciting approaches are still relatively sparse, and further validation is needed. See also Chapter 62.
Use of Pharmacological Tools
Pharmacology is an important aspect of animal modeling not just with regard to prediction of the clinical efficacy of potential new drugs. Pharmacological tools are used to characterize models, to investigate physiological and pathophysiological mechanisms, and to discover potential new treatment strategies. For the results to be useful, several factors have to be controlled, among these unwanted or unexpected drug effects that can interfere with the outcome measures of the tests. Prediction is significantly strengthened by evidence linking effect to engagement of the molecular target; the compound should act at the right molecular target at the right place and at the right time. The more important of these issues are addressed in this section.
A general weakness in the literature is a scarcity of pharmacokinetic data or biomarkers that can provide evidence that the effect obtained is the effect intended, for instance, that the plasma and tissue concentration of a compound is in a relevant range or that a biomarker is affected in a manner compatible with the hypothesis. It is important to recognize that any model may be sensitive to pharmacodynamic actions unrelated to a compound’s intended therapeutic effect. This may be acceptable if the effect is recognized for what it is and can be related in some way to the therapeutic mechanism of action. An example is vasoconstriction mediated through the μ-opioid receptor, which may increase response latencies in thermal nociceptive tests after the intrathecal administration of opiates and thereby serves as a surrogate marker for antinociception (Le Bars et al 2001). Beecher (1957) discussed a range of what we may call biomarkers or surrogate markers for opioid action as objective readouts in both humans and animals. He suggested that respiratory depression in the rabbit might be a good preclinical predictor of opiate efficacy in the clinic. This type of approach is, of course, specific for a particular class of compounds and carries the risk that interpretation of data regarding exposure levels for clinical efficacy and therapeutic window may be misleading.
Side Effects and Confounders
Pharmacological compounds may have both off-target and on-target effects with potential confounding influences on the readouts of the models, in some cases rendering a specific model unsuitable for a certain test compound or pathway. The presence of confounding effects does not rule out that a compound may be useful as an analgesic or as a tool, but it does require supporting evidence (e.g., electrophysiological or biochemical data) compatible with an analgesic mode of action in an appropriate drug exposure range. A strategy to get around this problem is to use models or readouts that are differentially sensitive to unwanted effects, such as using pain-evoked and pain-suppressed behavior as outcome measures (Negus et al 2006). This approach is not always possible, and a number of devices such as rotarods, vertical grids, and motor activity monitoring equipment can be used for objective quantification of potentially detrimental motor effects (Jones and Roberts 1968, Näsström et al 1993, Crawley 1999). The primary disadvantages of dedicated systems are that they may be costly and will register a limited set of effects.
In the pharmaceutical industry, a much-used alternative for detection of side effects, particularly in the context of safety pharmacology and toxicology, is the Irwin screen (Irwin 1968). This systematic observation-based method requires few and simple tools and combines observation of spontaneous behavior and visible signs of autonomic disturbances with simple manipulations to test neurological and sensory functions. Originally described for mice, the method is well suited for rats as well. The procedure is comprehensive with about 50 different items, yet an animal can be screened in about 3 minutes according to the author. The procedure does, however, require extensive training, practice, and as with all observational methods, proper blinding and randomization.
For many purposes the number of items can be reduced and the procedure simplified for ease of use. The method can even be tailored and modified for phenotyping or quantification of motor deficits in neuropathy models. For the latter purpose, a subset of items with a neurological focus may suffice (Hao and Xu 1996).
Model Validation Using Pharmacological Tools and the Importance of Pharmacokinetics
Animal models have traditionally been used more or less in a black box manner; that is, a model has been developed by using face validity criteria, pharmacologically validated with clinically active analgesics, and then assumed to produce data that could be generalized to other drugs (at least within the same class) and pathophysiological mechanisms. This strategy has been applied successfully to the development of new opiates and NSAIDs, including selective cyclooxygenase-2 inhibitors, and even in predicting the efficacy of some novel pharmacological principles such as anti–nerve growth factor treatment of osteoarthritis, but in a number of other instances drugs supported by animal data have failed in the clinic (Berge 2011). Traditionally, validation and optimization rely on morphine and either anticonvulsants for neuropathy models or NSAIDs for arthritis models. On a population basis, these treatments are only moderately effective in the clinic, and there is therefore a risk that the modeling is optimized toward specific analgesic mechanisms already well served, as well as toward readouts that may or may not be related to analgesia. This can be illustrated by taking a closer look at traumatic neuropathy, which as discussed previously, dominates the animal modeling literature on neuropathic pain. In these conditions only opiates reliably show analgesic efficacy in the clinic, although the effect is variable and unimpressive in clinical trials (Finnerup et al 2010). Antidepressants and anticonvulsants have limited efficacy in these patient populations. In contrast, the literature shows relatively consistent efficacy of all these classes of compounds in the commonly used sciatic nerve lesion models (Kontinen and Meert 2003). Similarly, NSAIDs, which have moderate analgesic efficacy in clinical trials of osteoarthritic pain (Laine et al 2008), show excellent efficacy in many inflammatory pain models.
A problem with analysis of literature data is the limited reporting of drug exposure levels in the animal literature, and hypothetically, the good sensitivity may simply be due to overdosing of drugs leading to plasma and target tissue levels not possible to achieve in the clinic. Kontinen and Meert (2003) did not have access to data on drug exposure for the studies in their review, but another study comparing data from published clinical trials with data from rat experiments found that antidepressants were either ineffective in the rat SNL model of neuropathic pain or required 10–40 times higher plasma levels than in the clinic whereas the anticonvulsants gabapentin, lamotrigine, and carbamazepine were active at concentrations 1–3 times the human levels (Whiteside et al 2008). With regard to inflammatory pain, effective plasma levels of celecoxib and indomethacin were only slightly higher in the rat FCA paw inflammation model than in humans. Thus, as used in this study, the animal models were sensitive to some but not all drugs. Tricyclic antidepressants, which constitute the overall most efficacious drug class for neuropathic pain in the clinic (Finnerup et al 2010), came out poorly in the rat model, but as discussed, neither anticonvulsants nor tricyclic antidepressants are particularly effective in neuropathic pain of traumatic origin.
In the cited study, as well as in most of the preclinical literature, all drugs were given as a single dose, which may have worked against drug effects that develop over time. Drug distribution may also differ significantly, depending on whether a compound is given as a single dose or repeatedly to achieve a steady state. Gabapentin, for instance, is taken up from the gastrointestinal tract to blood, as well as from blood to cerebrospinal fluid, by a saturable transport mechanism (Stewart et al 1993, Luer et al 1999), and a relatively small fraction of the drug enters the central nervous system after a single dose (Welty et al 1993). A minimum effective single dose in the rat yields plasma concentrations about three times higher than human maintenance dosing (Whiteside et al 2008). The higher concentration may recruit peripheral mechanisms not or only minimally accessible under normal clinical dosing conditions and may, at least hypothetically, be a reason why the compound is much more efficacious in models than in real life. These examples should serve to illustrate that the relationship between effects in animal models and in the clinic is far from straightforward.
To increase the predictive capability of modeling it is important to establish a quantitative relationship between drug exposure in relevant tissues, target engagement, and behavioral readout (i.e., by evidence that the effect observed is obtained by the intended receptor interaction). Tools such as biochemical biomarkers, electrophysiology, and functional histochemistry and imaging can be used to this end. There is growing awareness in the scientific community that predictive pharmacokinetic–pharmacodynamic modeling requires a set of biomarkers to establish a consistent chain of events from target drug interaction to clinical effect (Danhof et al 2005). Given the diversity of clinical pain conditions, the predictive validity of any model will remain uncertain unless it can be mechanistically linked to human pathophysiology. There is substantial interest in developing tools and algorithms for mechanism-based patient segmentation in neuropathic pain by means of sensory profiling and symptoms (Attal et al 2008, Maier et al 2010), but the underlying pathophysiology is still incompletely understood. This is clearly a limiting factor in determining the construct validity of animal models and ultimately their predictive validity.
Thus, although predictive validity would ideally imply modeling of pharmacodynamic–pharmacokinetic relationships with exact predictions of doses and efficacy from experimental species to crudely defined human patient populations, this is not achievable in a single step from animal to human by current modeling and probably never will be. Factors such as species differences in drug target molecular composition and structural differences in pain signaling pathways and higher-order processing will pose hurdles. Even more important, however, terms such as “post-herpetic neuropathic pain” or “pain due to osteoarthritis” cover heterogeneous patient populations in which some individuals respond well to some available treatments whereas the majority do not. The efficacy of a drug in such heterogeneous populations can therefore not be predicted by a single model. By choice of reference compounds for a certain model, we may optimize for a certain subsegment of a clinical indication but not for a wider population.
Conclusion
As evident from this discussion, minor changes in experimental protocols and testing parameters may have profound effects on the mechanisms activated and outcome variables, both in terms of basic responses and with regard to the effect of pharmacological and other manipulations. Unfortunately, the quality of experimental design and reporting of results is variable across the animal experimental literature, and it is frequently impossible to compare data from different publications, let alone be sure that the conclusions are valid based on the data presented. Basic features such as formulation of the hypothesis, as well as information regarding the number and characteristics of subjects, and statistical analysis are frequently inadequate, and description of randomization and blinding procedures is the exception rather than the rule (Kilkenny et al 2009). Uncontrolled experimental bias has been suggested to have an impact on the interpretation of pain-modeling outcomes such as hypersensitivity estimates and drug effects (Lindner et al 2003, Eisenach and Lindner 2004, Hogan et al 2004, Bove 2006, Lindner 2007).
Higher and more uniform standards for experimental design and reporting would not only improve the quality of the science in individual papers but also facilitate meta-analyses and systematic reviews. Recommendations for reduction of bias and reporting standards in preclinical pain research have been published (Rice et al 2008), and work is continuing within the European Union Innovative Medicines Initiative (http://imi.europa.eu/calls-01_en.html). A set of general guidelines (ARRIVE—Animals in Research: Reporting In Vivo Experiments) with a checklist of important information that should be addressed in scientific publications on animal experimental studies was recently published (Kilkenny et al 2010). Hopefully, these recommendations and standards will lead to a situation closer to the one achieved in the clinical area, where standardization of reporting has facilitated best practice and made true meta-analysis of data possible (Dworkin et al 2005).
On the positive side, preclinical pain research has access to a great armamentarium of methods and models, which makes it possible to address specific mechanistic hypotheses and to generate new ones. This can be illustrated by the use of animal modeling and genetics to identify genes and mechanisms of relevance for clinical pain in humans. Characterization of genetic susceptibility to neuropathic pain in mice indicated that genetic risk factors may be important (Mogil et al 1999a). Taking this a step further by expression profiling studies using dorsal root ganglia neurons of rats with different types of neuropathy, it has been possible to identify genes that are regulated in a similar way across models and show that at least two of these genes play a role in susceptibility to the development of chronic pain in humans (Tegeder et al 2006, Costigan et al 2010). Although the track record for predictive validity concerning novel analgesics is mixed, there are a number of examples where modeling has been successful and resulted in therapeutic advances (Berge 2011). Some areas where animal modeling tends to fall short today, such as with regard to establishing pharmacokinetic–pharmacodynamic relationships and controlling for confounding effects, may to a great extent be addressed with available methodology. Given the continuing interest in animal modeling of pain, we can with all likelihood look forward to novel approaches addressing some other identified challenges, such as the measurement of ongoing pain, the limited number of clinically relevant biomarkers, and perhaps models incorporating more disease-like components.
The references for this chapter can be found at www.expertconsult.com.
References
Abbadie C., Taylor B.K., Peterson M.A., et al. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–110.
Ameye L.G., Young M.F. Animal models of osteoarthritis: lessons learned while seeking the “holy grail”. Current Opinion in Rheumatology. 2006;18:537–547.
Ängeby-Möller K., Berge O.G., Hamers F.P. Using the CatWalk method to assess weight-bearing and pain behaviour in walking rats with ankle joint monoarthritis induced by carrageenan: effects of morphine and rofecoxib. Journal of Neuroscience Methods. 2008;174:1–9.
Ängeby-Möller K., Johansson B., Berge O.G. Assessing mechanical allodynia in the rat paw with a new electronic algometer. Journal of Neuroscience Methods. 1998;84:41–47.
Attal N., Fermanian C., Fermanian J., et al. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138:343–353.
Authier N., Balayssac D., Marchand F., et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6:620–629.
Beecher H.K. The measurement of pain. Pharmacological Reviews. 1957;9:59–209.
Beissner F., Brandau A., Henke C., et al, Quick discrimination of Adelta and C fiber mediated pain based on three verbal descriptors. PLoS One 2010;5:e12944. Available at, http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0012944
Belmonte C., Brock J.A., Viana F. Converting cold into pain. Experimental Brain Research. 2009;196:13–30.
Belmonte C., Viana F. Molecular and cellular limits to somatosensory specificity. Molecular Pain. 2008;4:14.
Bennett G.J., Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107.
Berge O.G. Predictive validity of behavioral animal models for chronic pain. British Journal of Pharmacology. 2011;164:1195–1206.
Berge O.G., Fasmer O.B., Hole K. Serotonin receptor antagonists induce hyperalgesia without preventing morphine antinociception. Pharmacology, Biochemistry, and Behavior. 1983;19:873–878.
Berge O.G., Garcia-Cabrera I., Hole K. Response latencies in the tail-flick test depend on tail skin temperature. Neuroscience Letters. 1988;86:284–288.
Bourquin A.F., Suveges M., Pertin M., et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 122, 2006. 14–14, 2006
Bove G. Mechanical sensory threshold testing using nylon monofilaments: the pain field’s “tin standard”. Pain. 2006;124:13–17.
Bove S.E., Calcaterra S.L., Brooker R.M., et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate–induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830.
Butelman E.R., Harris T.J., Kreek M.J. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. Journal of Pharmacology and Experimental Therapeutics. 2004;311:155–163.
Butler S.H., Godefroy F., Besson J.M., et al. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81.
Capone F., Aloisi A.M. Refinement of pain evaluation techniques. The formalin test. Annali dell’Istituto Superiore di Sanita. 2004;40:223–229.
Chaplan S.R., Bach F.W., Pogrel J.W., et al. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63.
Chizh B.A., Priestley T., Rowbotham M., et al. Predicting therapeutic efficacy—experimental pain in human subjects. Brain Research Reviews. 2009;60:243–254.
Choi Y., Yoon Y.W., Na H.S., et al. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376.
Christianson C.A., Corr M., Firestein G.S., et al. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain. 2010;151:394–403.
Clavelou P., Pajot J., Dallel R., et al. Application of the formalin test to the study of orofacial pain in the rat. Neuroscience Letters. 1989;103:349–353.
Coderre T.J., Wall P.D. Ankle joint urate arthritis (AJUA) in rats: an alternative animal model of arthritis to that produced by Freund’s adjuvant. Pain. 1987;28:379–393.
Colleoni M., Sacerdote P., Murine models of human neuropathic pain. Biochimica et Biophysica Acta 2010;1802:924–933. Available at http://dx.doi.org/10.1016/j.bbadis.2009.10.012.
Colpaert F.C. Drug discrimination in neurobiology. Pharmacology, Biochemistry, and Behavior. 1999;64:337–345.
Costigan M., Belfer I., Griffin R.S., et al. Multiple chronic pain states are associated with a common amino acid–changing allele in KCNS1. Brain. 2010;133:2519–2527.
Crawley J.N. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Research. 1999;835:18–26.
Cui J., Holmin S., Mathiesen T., et al. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248.
Damas J., Liegeois J.F. The inflammatory reaction induced by formalin in the rat paw. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1999;359:220–227.
D’Amour F.E., Smith D.L. A method for determining loss of pain sensation. Journal of Pharmacology and Experimental Therapeutics. 1941;72:74–79.
Danhof M., Alvan G., Dahl S.G., et al. Mechanism-based pharmacokinetic-pharmacodynamic modeling—a new classification of biomarkers. Pharmaceutical Research. 2005;22:1432–1437.
Decosterd I., Woolf C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158.
Dickenson A.H., Sullivan A.F. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neuroscience Letters. 1987;83:207–211.
Dirig D.M., Salami A., Rathbun M.L., et al. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. Journal of Neuroscience Methods. 1997;76:183–191.
Dowdall T., Robinson I., Meert T.F. Comparison of five different rat models of peripheral nerve injury. Pharmacology, Biochemistry, and Behavior. 2005;80:93–108.
Dubuisson D., Dennis S.G. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174.
Dworkin R.H., Turk D.C., Farrar J.T., et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19.
Eisenach J.C., Lindner M.D. Did experimenter bias conceal the efficacy of spinal opioids in previous studies with the spinal nerve ligation model of neuropathic pain? Anesthesiology. 2004;100:765–767.
Erichsen H.K., Blackburn-Munro G. Pharmacological characterisation of the spared nerve injury model of neuropathic pain. Pain. 2002;98:151–161.
Fernihough J., Gentry C., Malcangio M., et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93.
Finnerup N.B., Sindrup S.H., Jensen T.S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581.
Fleetwood-Walker S.M., Quinn J.P., Wallace C., et al. Behavioural changes in the rat following infection with varicella-zoster virus. Journal of General Virology. 1999;80:2433–2436.
Foulkes T., Wood J.N. Mechanisms of cold pain. Channels (Austin). 2007;1:154–160.
Fox A., Eastwood C., Gentry C., et al. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain. 1999;81:307–316.
Fruhstorfer H., Gross W., Selbmann O. Von Frey hairs: new materials for a new design. European Journal of Pain. 2001;5:341–342.
Fu K.Y., Light A.R., Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. Journal of Pain. 2001;2:2–11.
Gazelius B., Cui J.G., Svensson M., et al. Photochemically induced ischaemic lesion of the rat sciatic nerve. A novel method providing high incidence of mononeuropathy. Neuroreport. 1996;7:2619–2623.
Gustafsson H., Flood K., Berge O.G., et al. Gabapentin reverses mechanical allodynia induced by sciatic nerve ischemia and formalin-induced nociception in mice. Experimental Neurology. 2003;182:427–434.
Gustafsson H., Sandin J. Oral pregabalin reverses cold allodynia in two distinct models of peripheral neuropathic pain. European Journal of Pharmacology. 2009;605:103–108.
Gwilym S.E., Keltner J.R., Warnaby C.E., et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis and Rheumatism. 2009;61:1226–1234.
Hao J.X., Xu X.J. Treatment of a chronic allodynia-like response in spinally injured rats: effects of systemically administered excitatory amino acid receptor antagonists. Pain. 1996;66:279–285.
Hargreaves K., Dubner R., Brown F., et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88.
Hasnie F.S., Breuer J., Parker S., et al. Further characterization of a rat model of varicella zoster virus–associated pain: relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508.
Heilborn U., Berge O.G., Arborelius L., et al. Spontaneous nociceptive behaviour in female mice with Freund’s complete adjuvant– and carrageenan-induced monoarthritis. Brain Research. 2007;1143:143–149.
Hogan Q., Sapunar D., Modric-Jednacak K., et al. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–487.
Holmdahl R., Lorentzen J.C., Lu S., et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184–202.
Hunskaar S., Berge O.G., Hole K. A modified hot-plate test sensitive to mild analgesics. Behavioral Brain Research. 1986;21:101–108.
Hunter D.J., McDougall J.J., Keefe F.J. The symptoms of osteoarthritis and the genesis of pain. Rheumatic Diseases Clinics of North America. 2008;34:623–643.
Hwang S.W., Oh U. Current concepts of nociception: nociceptive molecular sensors in sensory neurons. Current Opinion in Anaesthesiology. 2007;20:427–434.
Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257.
Johansen J.P., Fields H.L., Manning B.H. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8077–8082.
Jones B.J., Roberts D.J. A rotarod suitable for quantitative measurements of motor incoordination in naive mice. Naunyn-Schmiedebergs Archiv fur Pharmakologie. 1968;259:211.
Joshi S.K., Hernandez G., Mikusa J.P., et al. Comparison of antinociceptive actions of standard analgesics in attenuating capsaicin and nerve-injury-induced mechanical hypersensitivity. Neuroscience. 2006;143:587–596.
Kalbhen D.A. Chemical model of osteoarthritis—a pharmacological evaluation. Journal of Rheumatology. 1987;14:130–131.
Kilkenny C., Browne W.J., Cuthill I.C., et al, Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010. Jun 29, 2010. Available at, http://www.plosbiology.org/article/info%3Adoi%2F10.1371%2Fjournal.pbio.1000412
Kilkenny C., Parsons N., Kadyszewski E., et al, Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 2009;4:e7824. Available at, http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0007824
Kim K.J., Yoon Y.W., Chung J.M. Comparison of three rodent neuropathic pain models. Experimental Brain Research. 1997;113:200–206.
Kim S.H., Chung J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363.
King T., Vera-Portocarrero L., Gutierrez T., et al. Unmasking the tonic-aversive state in neuropathic pain. Nature Neuroscience. 2009;12:1364–1366.
Kiso T., Watabiki T., Tsukamoto M., et al. Pharmacological characterization and gene expression profiling of an L5/L6 spinal nerve ligation model for neuropathic pain in mice. Neuroscience. 2008;153:492–500.
Kleggetveit I.P., Jørum E. Large and small fiber dysfunction in peripheral nerve injuries with or without spontaneous pain. Journal of Pain. 2010;11:1305–1310.
Koltzenburg M. Neural mechanisms of cutaneous nociceptive pain. Clinical Journal of Pain. 2000;16:S131–S138.
Kontinen V.K., Meert T.F., Predictive validity of neuropathic pain models in pharmacological studies with a behavioral outcome in the rat: a systematic review. Dostrovsky J.O., Carr D.B., Kontinen V.K., eds. Proceedings of the 10th Congress on Pain, 24. Seattle: IASP Press. Progress in Pain Research and Management; 2003:489–498.
Laine L., White W.B., Rostom A., et al. COX-2 selective inhibitors in the treatment of osteoarthritis. Seminars in Arthritis and Rheumatism. 2008;38:165–187.
Langford D.J., Bailey A.L., Chanda M.L., et al. Coding of facial expressions of pain in the laboratory mouse. Nature Methods. 2010;7:447–449.
Le Bars D., Gozariu M., Cadden S.W. Animal models of nociception. Pharmacological Reviews. 2001;53:597–652.
Lee B.H., Won R., Baik E.J., et al. An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport. 2000;11:657–661.
Leffler A., Ahlstedt I., Engberg S., et al. Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. Biochemical Pharmacology. 2009;77:1522–1530.
Li Y., Dorsi M.J., Meyer R.A., et al. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000;85:493–502.
Liles J.H., Flecknell P.A. The use of non-steroidal anti-inflammatory drugs for the relief of pain in laboratory rodents and rabbits. Laboratory Animals. 1992;26:241–255.
Lindner M.D. Clinical attrition due to biased preclinical assessments of potential efficacy. Pharmacology & Therapeutics. 2007;115:148–175.
Lindner M.D., Frydel B.R., Francis J.M., et al. Analgesic effects of adrenal chromaffin allografts: contingent on special procedures or due to experimenter bias? Journal of Pain. 2003;4:64–73.
Luccarini P., Childeric A., Gaydier A.M., et al. The orofacial formalin test in the mouse: a behavioral model for studying physiology and modulation of trigeminal nociception. Journal of Pain. 2006;7:908–914.
Luer M.S., Hamani C., Dujovny M., et al. Saturable transport of gabapentin at the blood-brain barrier. Neurological Research. 1999;21:559–562.
Luukko M., Konttinen Y., Kemppinen P., et al. Influence of various experimental parameters on the incidence of thermal and mechanical hyperalgesia induced by a constriction mononeuropathy of the sciatic nerve in lightly anesthetized rats. Experimental Neurology. 1994;128:143–154.
Maier C., Baron R., Tölle T.R., et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450.
Matson D.J., Broom D.C., Cortright D.N. Locomotor activity in a novel environment as a test of inflammatory pain in rats. Methods in Molecular Biology (Clifton, N. J.). 2010;617:67–78.
McCall W.D., Tanner K.D., Levine J.D. Formalin induces biphasic activity in C-fibers in the rat. Neuroscience Letters. 1996;208:45–48.
McNamara C.R., Mandel-Brehm J., Bautista D.M., et al. TRPA1 mediates formalin-induced pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13525–13530.
Mogil J.S., Davis K.D., Derbyshire S.W. The necessity of animal models in pain research. Pain. 2010;151:12–17.
Mogil J.S., Graham A.C., Ritchie J., et al, Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Molecular Pain, 6;34. 2010. Available at, http://www.molecularpain.com/content/6/1/34
Mogil J.S., Wilson S.G., Bon K., et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82.
Mogil J.S., Wilson S.G., Bon K., et al. Heritability of nociception II. “Types” of nociception revealed by genetic correlation analysis. Pain. 1999;80:83–93.
Mosconi T., Kruger L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterations. Pain. 1996;64:37–57.
Munro G. Pharmacological assessment of the rat formalin test utilizing the clinically used analgesic drugs gabapentin, lamotrigine, morphine, duloxetine, tramadol and ibuprofen: influence of low and high formalin concentrations. European Journal of Pharmacology. 2009;605:95–102.
Näsström J., Karlsson U., Berge O.G. Systemic or intracerebroventricular injection of NMDA receptor antagonists attenuates the antinociceptive activity of intrathecally administered NMDA receptor antagonists. Brain Research. 1993;623:47–55.
Negus S.S., Vanderah T.W., Brandt M.R., et al. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. Journal of Pharmacology and Experimental Therapeutics. 2006;319:507–514.
Obreja O., Schmelz M. Single-fiber recordings of unmyelinated afferents in pig. Neuroscience Letters. 2010;470:175–179.
Obrosova I.G. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647.
Ordeberg G. Characterization of joint pain in human OA. Novartis Foundation Symposium. 2004;260:105–115.
Perl E.R. Cutaneous polymodal receptors: characteristics and plasticity. Progress in Brain Research. 1996;113:21–37.
Piesla M.J., Leventhal L., Strassle B.W., et al. Abnormal gait, due to inflammation but not nerve injury, reflects enhanced nociception in preclinical pain models. Brain Research. 2009;1295:89–98.
Pizziketti R.J., Pressman N.S., Geller E.B., et al. Rat cold water tail-flick: a novel analgesic test that distinguishes opioid agonists from mixed agonist-antagonists. European Journal of Pharmacology. 1985;119:23–29.
Pradhan A.A., Yu X.H., Laird J.M. Modality of hyperalgesia tested, not type of nerve damage, predicts pharmacological sensitivity in rat models of neuropathic pain. European Journal of Pain. 2010;14:503–509.
Puig S., Sorkin L.S. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355.
Quessy S.N. The challenges of translational research for analgesics: the state of knowledge needs upgrading and some uncomfortable deficiencies remain to be urgently addressed. Journal of Pain. 2010;11:698–700.
Raboisson P., Dallel R. The orofacial formalin test. Neuroscience and Biobehavioral Reviews. 2004;28:219–226.
Randall L.O., Selitto J.J. A method for measurement of analgesic activity on inflamed tissue. Archives of International Pharmacodynamic Therapy. 1957;111:409–419.
Rasmussen P.V., Sindrup S.H., Jensen T.S., et al. Symptoms and signs in patients with suspected neuropathic pain. Pain. 2004;110:461–469.
Rice A.S., Cimino-Brown D., Eisenach J.C., et al. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247.
Rolke R., Magerl W., Campbell K.A., et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. European Journal of Pain. 2006;10:77–88.
Rosland J.H., Tjølsen A., Mæhle B., et al. The formalin test in mice: effect of formalin concentration. Pain. 1990;42:235–242.
Schaible H.G., Schmidt R.F. Responses of fine medial articular nerve afferents to passive movements of knee joints. Journal of Neurophysiology. 1983;49:1118–1126.
Schepers R.J., Ringkamp M. Thermoreceptors and thermosensitive afferents. Neuroscience and Biobehavioral Reviews. 2009;33:205–212.
Schött E., Berge O.G., Ängeby-Möller K., et al. Weight bearing as an objective measure of arthritic pain in the rat. Journal of Pharmacological and Toxicological Methods. 1994;31:79–83.
Seltzer Z., Dubner R., Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218.
Shir Y., Seltzer Z. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neuroscience Letters. 1990;115:62–67.
Shir Y., Seltzer Z. Effects of sympathectomy in a model of causalgiform pain produced by partial sciatic nerve injury in rats. Pain. 1991;45:309–320.
Shir Y., Zeltser R., Vatine J.J., et al. Correlation of intact sensibility and neuropathic pain–related behaviors in eight inbred and outbred rat strains and selection lines. Pain. 2001;90:75–82.
Song X.J., Hu S.J., Greenquist K.W., et al. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. Journal of Neurophysiology. 1999;82:3347–3358.
Staton L.J., Panda M., Chen I., et al. When race matters: disagreement in pain perception between patients and their physicians in primary care. Journal of the National Medical Association. 2007;99:532–538.
Stewart B.H., Kugler A.R., Thompson P.R., et al. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharmaceutical Research. 1993;10:276–281.
Taber R.I. Predictive value of analgesic assays in mice and rats. Advances in Biochemical Psychopharmacology. 1974;8:191–211.
Taylor B.K., Peterson M.A., Basbaum A.I. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. Journal of Neuroscience. 1995;15:7575–7584.
Tegeder I., Costigan M., Griffin R.S., et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nature Medicine. 2006;12:1269–1277.
Tjølsen A., Berge O.G., Eide P.K., et al. Apparent hyperalgesia after lesions of the descending serotonergic pathways is due to increased tail skin temperature. Pain. 1988;33:225–231.
Tjølsen A., Berge O.G., Hunskaar S., et al. The formalin test: an evaluation of the method. Pain. 1992;51:5–17.
Tjølsen A., Rosland J.H., Berge O.G., et al. The increasing-temperature hot-plate test: an improved test of nociception in mice and rats. Journal of Pharmacological Methods. 1991;25:241–250.
Treede R.D. Transduction and transmission properties of primary nociceptive afferents. Rossiiskii Fiziologicheskii Zhurnal Imeni I. M. Sechenova. 1999;85:205–211.
Vierck C.J., Hansson P.T., Yezierski R.P. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10.
Vos B.P., Strassman A.M., Maciewicz R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. Journal of Neuroscience. 1994;14:2708–2723.
Wallace V.C., Blackbeard J., Pheby T., et al. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133:47–63.
Wallace V.C., Blackbeard J., Segerdahl A.R., et al. Characterization of rodent models of HIV-gp120 and anti-retroviral–associated neuropathic pain. Brain. 2007;130:2688–2702.
Welty D.F., Schielke G.P., Vartanian M.G., et al. Gabapentin anticonvulsant action in rats: disequilibrium with peak drug concentrations in plasma and brain microdialysate. Epilepsy Research. 1993;16:175–181.
Wen T., Ansonoff M.A., Pintar J.E. The tail pigmentation pattern of C57BL/6J mice affects nociception/pain quantification in the tail flick test. European Journal of Pain. 2009;13:564–567.
Whiteside G.T., Adedoyin A., Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology. 2008;54:767–775.
Whiteside G.T., Harrison J., Boulet J., et al. Pharmacological characterisation of a rat model of incisional pain. British Journal of Pharmacology. 2004;141:85–91.
Wilson A.W., Medhurst S.J., Dixon C.I., et al. An animal model of chronic inflammatory pain: pharmacological and temporal differentiation from acute models. European Journal of Pain. 2006;10:537–549.
Windebank A.J., Grisold W. Chemotherapy-induced neuropathy. Journal of the Peripheral Nervous System. 2008;13:27–46.
Winder C.V., Pfeiffer C.C., Maison G.L. The nociceptive contraction of the cutaneous muscle of the guinea pig as elicited by radiant heat, with observations on the mode of action of morphine. Archives of International Pharmacodynamic Therapy. 1946;72:329–359.
Xu X., Plesan A., Yu W., et al. Possible impact of genetic differences on the development of neuropathic pain–like behaviors after unilateral sciatic nerve ischemic injury in rats. Pain. 2001;89:135–145.
Yaksh T.L. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiolica Scandinavica. 1997;41:94–111.
Yeomans D.C., Pirec V., Proudfit H.K. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–140.
Yeomans D.C., Proudfit H.K. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–150.
Yu Y.C., Koo S.T., Kim C.H., et al. Two variables that can be used as pain indices in experimental animal models of arthritis. Journal of Neuroscience Methods. 2002;115:107–113.
Zeltser R., Beilin B., Zaslansky R., et al. Comparison of autotomy behavior induced in rats by various clinically-used neurectomy methods. Pain. 2000;89:19–24.
Authier N., Balayssac D., Marchand F., et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6:620–629.
Berge O.G. Predictive validity of behavioral animal models for chronic pain. British Journal of Pharmacology. 2011;164:1195–1206.
Bove G. Mechanical sensory threshold testing using nylon monofilaments: the pain field’s “tin standard”. Pain. 2006;124:13–17.
Capone F., Aloisi A.M. Refinement of pain evaluation techniques. The formalin test. Annali dell’Istituto Superiore di Sanita. 2004;40:223–229.
Coderre T.J., Wall P.D. Ankle joint urate arthritis (AJUA) in rats: an alternative animal model of arthritis to that produced by Freund’s adjuvant. Pain. 1987;28:379–393.
Colleoni M., Sacerdote P., Murine models of human neuropathic pain. Biochimica et Biophysica Acta 2010;1802:924–933. Available at http://dx.doi.org/10.1016/j.bbadis.2009.10.012.
Danhof M., Alvan G., Dahl S.G., et al. Mechanism-based pharmacokinetic-pharmacodynamic modeling—a new classification of biomarkers. Pharmaceutical Research. 2005;22:1432–1437.
Decosterd I., Woolf C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158.
Dowdall T., Robinson I., Meert T.F. Comparison of five different rat models of peripheral nerve injury. Pharmacology, Biochemistry, and Behavior. 2005;80:93–108.
Dubuisson D., Dennis S.G. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174.
Finnerup N.B., Sindrup S.H., Jensen T.S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581.
Fox A., Eastwood C., Gentry C., et al. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain. 1999;81:307–316.
Hargreaves K., Dubner R., Brown F., et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88.
Hogan Q., Sapunar D., Modric-Jednacak K., et al. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–487.
Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257.
Kilkenny C., Parsons N., Kadyszewski E., et al, Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 2009;4:e7824. Available at, http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0007824
Kontinen V.K., Meert T.F., Predictive validity of neuropathic pain models in pharmacological studies with a behavioral outcome in the rat: a systematic review. Dostrovsky J.O., Carr D.B., Kontinen V.K., eds. Proceedings of the 10th Congress on Pain, 24. Seattle: IASP Press; 2003:489–498. Progress in Pain Research and Management
Le Bars D., Gozariu M., Cadden S.W. Animal models of nociception. Pharmacological Reviews. 2001;53:597–652.
Lindner M.D. Clinical attrition due to biased preclinical assessments of potential efficacy. Pharmacology & Therapeutics. 2007;115:148–175.
Rice A.S., Cimino-Brown D., Eisenach J.C., et al. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247.
Schepers R.J., Ringkamp M. Thermoreceptors and thermosensitive afferents. Neuroscience and Biobehavioral Reviews. 2009;33:205–212.
Taber R.I. Predictive value of analgesic assays in mice and rats. Advances in Biochemical Psychopharmacology. 1974;8:191–211.
Tjølsen A., Berge O.G., Hunskaar S., et al. The formalin test: an evaluation of the method. Pain. 1992;51:5–17.
Whiteside G.T., Adedoyin A., Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology. 2008;54:767–775.
Yeomans D.C., Pirec V., Proudfit H.K. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–140.
Zeltser R., Beilin B., Zaslansky R., et al. Comparison of autotomy behavior induced in rats by various clinically-used neurectomy methods. Pain. 2000;89:19–24.