Ascending Projection Systems
Introduction
This chapter summarizes our current knowledge on ascending pathways from the spinal cord to the brain relevant to nociception and pain. The cells of origin in the spinal cord and their projection targets in the brain stem and thalamus are described. Information on spinal and cortical substrates is also available in Chapters 4-7. Specialized craniofacial activity in the trigeminal system is likewise described. We focus on data in humans and non-human primates but briefly mention comparative evidence in other animals.
Ascending Nociceptive Pathways
Stimuli and tissue conditions that cause pain generally activate nociceptive spinal neurons that project in ascending pathways. The association of pain with these pathways is based on anatomic and functional properties, as well as on correlations with the effects of stimulation or blockade in behaving animals and human patients. Particular ascending pathways from the spinal cord to sites in the brain stem and thalamus are important. Spinal projections to other sites (e.g., cerebellum, lateral reticular nucleus, inferior olive, or tectum) are primarily involved in sensorimotor integration. The ascending pathways important for pain include
There are also indirect pathways to the forebrain through the brain stem, particularly the post-synaptic dorsal column (PSDC) system and the spinocervicothalamic (SCT) pathway. Pathways similar to these also originate from the trigeminal sensory nuclei in the medulla that represent facial structures.
The functional and anatomical characteristics of these ascending pathways are described below, specifically, the connectivity and physiological characteristics of the cells of origin, the locations of their ascending axons, and the distribution of their terminations. Other reviews can be consulted for more comprehensive literature references (Willis 1985, Fields 1987, Lenz and Dougherty 1997, Craig and Dostrovsky 1999, Sessle 2000, Craig 2003a).
Spinothalamic Projection
The direct spinothalamic (and trigeminothalamic) projection is the pathway most closely associated with pain, temperature, and itch sensation. It has been known for more than 100 years that lesions involving this pathway (at the spinal, medullary, mesencephalic, or thalamic levels) result clinically in contralateral loss of these sensations (i.e., analgesia [or hypalgesia] and thermanesthesia) (White and Sweet 1969, Craig et al 2002). Considerable information is now available regarding this pathway that is consistent with this classic association.
Cells of Origin
STT cells have been identified in various mammals by using anatomical tracers that label neuronal cell bodies by retrograde transport from the thalamus (Craig and Dostrovsky 1999, Craig 2003a). Comparable evidence was obtained in humans by examining chromatolytic spinal cells in autopsy material subsequent to cordotomy in terminal patients (Kuru 1949). The STT is not a monolithic pathway; it originates in three distinct regions of the spinal gray matter (Fig. 12-1):
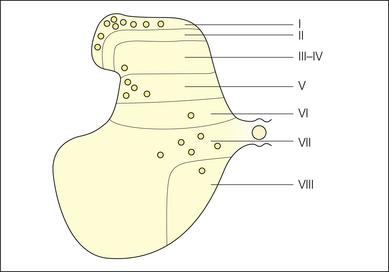
Figure 12-1 Schematic diagram summarizing the locations of the three main concentrations of spinothalamic tract cells in mammals: the marginal zone (lamina I), the neck of the dorsal horn (laminae IV–V), and the intermediate zone and ventral horn (laminae VII–VIII).
Although minor differences exist between species, in primates almost half of STT cells are located in lamina I, about one-quarter are found in laminae IV–V, and the remaining quarter are in laminae VII–VIII. Approximately 85–90% of STT cells are found on the contralateral side, with 10–15% on the ipsilateral side. There are approximately 10,000 STT cells that project to the thalamus from one side. STT cells are most numerous in the cervical and lumbosacral enlargements. Another large population of STT cells is widely distributed bilaterally throughout the C1–2 segments.
Each of the three main populations of STT neurons is dominated by afferent input from a different constellation of primary afferent fibers. Accordingly, these populations of STT cells display different patterns of functional activity. This evidence is based on microelectrode recordings of single STT cells identified by antidromic activation from their axonal terminations in the thalamus.
Lamina I STT Cells
Lamina I cells, which are medium-sized neurons in the most superficial layer of the dorsal horn that arborize longitudinally in the horizontal plane, receive input from small-diameter (Aδ and C) primary afferent fibers that innervate all tissues of the body, including skin, muscle, joint, and viscera (and also specialized trigeminal structures). The fundamental role of lamina I seems to be distribution of modality-selective homeostatic afferent activity related to the physiological status of the tissues of the body, which includes specific activity related to pain, temperature, and itch sensations. Based on cutaneous stimulation, three major classes of modality-selective lamina I STT cells are recognized (Craig 2003a):
In addition, there are histamine-selective (ITCH) and mustard oil–selective cells (Andrew and Craig 2001) and cells selectively sensitive to low-threshold C-fiber tactile stimulation (Light 1992). Lamina I cells of the three major classes have distinctive somatodendritic morphologies: NS cells are fusiform neurons (with unmyelinated axons), HPC cells are multipolar neurons, and COOL cells are pyramidal neurons. Each of these three classes forms approximately one-third of the population of lamina I STT cells.
Laminae IV–V Cells
Laminae IV–V cells, which are large neurons in the neck of the dorsal horn that have dorsally and mediolaterally radiating dendrites, receive input primarily from large-diameter (Aβ) fibers from the skin. Many receive monosynaptic input from nociceptive Aδ fibers and polysynaptic input from C fibers from skin, muscle, or viscera. Although some laminae IV–V STT cells respond predominantly to low-threshold (LT) or non-noxious mechanical cutaneous stimuli, such as brushing hair or graded pressure, others respond predominantly to high-threshold (HT) noxious stimuli such as pinch, heat, and deep squeeze—yet most are so-called wide–dynamic range (WDR) nociceptive cells because they respond to both LT and HT stimuli (Willis 1985, Price 1988).
Individual neurons can have a graded discharge from the innocuous into the noxious range, or they may poorly reflect stimulus intensity; however, as a population, these STT cells reflect the “intensive trajectory” of somatic stimulation and apparently serve as cumulative integrators of the entire spectrum of somatic afferent inflow to the dorsal horn (Wall 1973). Cells with (polysynaptic) C-fiber input can respond to repetitive C-fiber activation with a “wind up” discharge (i.e., they rapidly increase to a sustained plateau level if the stimulation is delivered at a rate faster than 0.3 Hz). Lamina V STT cells often receive convergent deep and visceral input, and most have large excitatory and inhibitory receptive fields. They are organized musculotopically rather than somatotopically during development by movement-induced patterns of primary afferent activity, and they are intimately involved in motor reflex activity (Schouenborg et al 1995). Evidence indicates that they respond tonically to multijoint limb position (Craig 2004b), consistent with afferent input from group II slowly adapting muscle afferents (Bannatyne et al 2006). Lamina I cells may also influence the activity of lamina V cells (McMahon and Wall 1988, Khasabov et al 2002).
Laminae VII–VIII Cells
Laminae VII–VIII cells, which are very large neurons in the intermediate zone that have widely radiating dendrites (Meyers and Snow 1982), generally receive convergent input from large-diameter skin and deep (muscle, joint) input, as well as other (polysynaptic) input. These complex cells respond to innocuous or noxious stimulation within large, bilateral, or widely separated somatic regions. They can have very large inhibitory fields and can be excited or inhibited by different modes of somatic stimulation, such as proprioceptive or visceral input (Giesler et al 1981). These cells are thought to inform higher levels regarding the integrative state of spinal interneuron pools important for locomotion. Cells in the most medial intermediate zone, near the central canal (lamina X), also receive small-diameter visceral input.
Organization of Ascending STT Axons
The axons of STT cells generally cross in the dorsal and ventral spinal commissures to reach the white matter of the contralateral spinal cord within one or two segments rostral to the cells of origin. Ascending STT axons are concentrated in two locations: the middle of the lateral funiculus (the classic “lateral” spinothalamic tract) and the middle of the anterior (ventral) funiculus (the classic “anterior” spinothalamic tract) (Craig et al 2002). These bundles were first observed histologically by using silver stains for degenerating fibers in human autopsy and monkey material following spinal hemisection. Kuru’s (1949) report that the lateral STT originates predominantly from lamina I cells and the anterior STT originates from deeper laminae V and VII cells has been confirmed by recent observations. The lateral STT can be visualized with immunohistochemical staining for calbindin (a particular calcium-binding protein), which labels lamina I cells, as well as their terminations in the thalamus. There is considerable individual variability in the precise location and extent of the lateral STT.
The lateral STT is crudely organized somatotopically. The fibers that join the lateral STT at each segment laterally displace the axons ascending from more caudal levels, with the result being that axons from caudal body regions tend to be located more laterally (i.e., superficially) in the white matter whereas those from rostral body regions are located more medially (closer to midline). At the spinomedullary junction, the lateral and anterior STT bundles coalesce in the ventrolateral aspect of the medulla. Trigeminothalamic axons join the medial aspect of the STT at this level. At the caudal end of the pons the STT shifts dorsally and ascends at the lateral aspect of the parabrachial region (the superior cerebellar peduncle) and then occupies a position ventrolateral to the brachium of the inferior colliculus at the lateral aspect of the mesencephalon. The ascending STT axons show weak topographic organization as they ascend in this position to the thalamus.
STT Projection Sites
Based on anterograde tracing experiments in primates and silver-stained degeneration subsequent to cordotomy in humans, it is known that the STT terminates in six distinct regions of the thalamus, which are represented in Figure 12-2 (Craig and Dostrovsky 1999, Craig 2003a). Using nomenclature common to the primate brain, these regions are
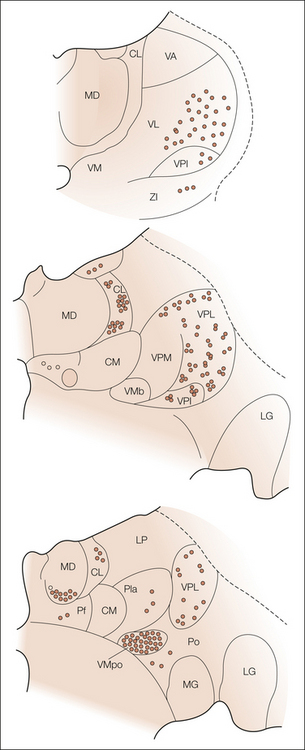
Figure 12-2 Schematic diagram summarizing the distribution and relative density of spinothalamic tract terminations in the macaque monkey at three frontal levels, from caudal to rostral.
Abbreviations of thalamic nuclei: CL, central lateral; CM, center median; LG, lateral geniculate; LP, lateral posterior; MD, medial dorsal; MG, medial geniculate; Pf, parafascicular; Pla, anterior pulvinar; Po, posterior; VA, ventral anterior; VL, ventral lateral; VM, ventral medial; VMb, basal part of the ventral medial; VMpo, posterior part of the ventral medial; VPI, ventral lateral inferior; VPL, ventral posterior lateral; VPM, ventral posterior medial; ZI, zona incerta.
These regions are similarly named in the human, but atlases vary significantly (Jones 1985, 1990). In the human, the VPM and VPL nuclei have been called the internal and external portions of the ventral caudal (Vc) nucleus, and the VPI has been called the parvicellular part of the ventral caudal nuclei (Vcpc). The recently recognized VMpo nucleus (Blomqvist et al 2000), which is located at the posterior–inferior aspect of VP (or Vc) thalamus, was previously included in the caudal VP, the Vc portae, or the posterior complex.
Posterior Part of the Ventral Medial Nucleus (VMpo)
The densest STT termination field occurs in the VMpo, which lies immediately posterior and inferior to the VP nucleus and is contiguous rostrally with the basal part of the VM nucleus (VMb). The VMpo serves as a thalamocortical relay nucleus for lamina I STT cells (Craig 2003a, 2004a; Craig and Zhang 2006). It is the primary projection target of lamina I STT cells in the primate, and lamina I STT cells are essentially the exclusive source of its ascending input. This projection is organized topographically from posterior to anterior, with lumbar input being most posterior and cervical and trigeminal input successively more anterior.
The lamina I STT projection co-localizes with a dense field of terminal fibers immunoreactive for calbindin, a reflection of the strong calbindin immunoreactivity of lamina I cells and lateral STT axons (although not with all antibodies). This feature was used to verify the cytoarchitectonic identification of VMpo in the human thalamus (Blomqvist et al 2000) and its correspondence with the zone of dense STT terminations demonstrated in human cordotomy patients.
The VMpo nucleus is rudimentary or non-existent in non-primates and well developed only in humans. At the ultrastructural level, glutamatergic lamina I STT terminations in the VMpo form multiple contacts—triadic arrangements with relay cell dendrites and GABAergic presynaptic dendrites; this provides the basis for high synaptic security and temporal fidelity. The VMpo projects topographically to the dorsal margin of the posterior insular cortex buried within the lateral sulcus. Together with the parallel pathway for parasympathetic visceral afferent activity (i.e., vagal and gustatory input) via the VMb, the VMpo projection to the insular cortex constitutes an interoceptive sensory representation of the physiological condition of the body. This view is consistent with the general view of the insula as limbic sensory cortex associated with autonomic activity. Embedded within this projection are distinct, highly resolved representations of several “feelings” from the body, including pain, temperature, itch, muscle and visceral sensations, and sensual (C-fiber) touch.
Ventral Posterior Nuclei
The pronounced clusters of STT terminations (“archipelago”) that occur within the VP nuclei were historically the first STT terminations clearly described in human material. These clusters are particularly dense along the rostral border of VP with VL and along its caudal border with the pulvinar and posterior group (Stepniewska et al 2002). They are concentrated near the major fiber laminae that subdivide VP and are roughly topographically organized in parallel with the precise somatotopic organization of the mechanoreceptive lemniscal representation in VP such that trigeminothalamic cells project to the VPM, cervical STT cells project to the medial VPL, and lumbar STT cells project to the lateral VPL.
The STT terminations in VP originate primarily from cells in laminae IV–V. These terminations occur around VP neurons whose somata are immunopositive for calbindin, whereas lemniscal input (from the dorsal column nuclei and the principal trigeminal nucleus) is associated with VP cells that are immunoreactive for parvalbumin (Rausell and Jones 1991). The biological significance of this distinction is not understood, but it emphasizes the likelihood that such input is processed differently. It has also been reported that the STT terminations in VP differ from the lemniscal terminations in that they do not form ultrastructural triads with GABAergic presynaptic dendrites (Ralston and Ralston 1994) and that the neurons that they contact project to the superficial rather than to the middle layers of the sensorimotor cortex (Rausell and Jones 1991). STT axons that terminate in VP can have a collateral terminal in the CL (Giesler et al 1981), and most have a collateral terminal in the VL (Craig 2008). In addition, the VPI nucleus, a cell-sparse region ventral to VPL and VPM, receives STT input that originates from both lamina I and laminae IV–V STT cells and is topographically organized posterior to anterior (Craig 2006). This nucleus also receives vestibular input, and it projects to the region of the retroinsular (vestibular) cortex posterior to the second somatosensory area in the lateral sulcus. A long-held view is that STT input to VP has a role in pain, yet the recent recognition of VMpo and other findings suggest the alternative interpretation that it has a role in sensorimotor integration (see below).
Ventral Lateral Nucleus (VL)
There is moderately dense STT input to the VL, rostral to VP and overlapping with cerebellothalamic projections (Stepniewska et al 2002), that originates from laminae V and VII STT cells (Craig 2008). It provides the basis for some somatosensory responsiveness in this region (Mackel et al 1992). The VL projects to the motor cortex, and this STT component is certainly associated with sensorimotor activity.
Central Lateral Nucleus (CL)
There is dense STT input to portions of the CL, particularly in its caudal aspect. This projection, which also arises from laminae V and VII STT cells (Giesler et al 1981, Craig and Dostrovsky 1999), does not appear to have a simple topography; rather, individual cell clusters within the CL receive STT input from different portions of the spinal cord. The CL also receives dense input from the cerebellum, substantia nigra, tectum, globus pallidus, mesencephalic tegmentum, and motor cortex. The majority of cells in this portion of the intralaminar thalamus project to the basal ganglia, but others project to superficial and deep layers of the motor and posterior parietal cortices (Jones 1985). This STT component may be involved in control of orientation and attention, as well as motor set.
Parafascicular Nucleus (Pf)
There is a weak STT projection to the Pf that originates from laminae I and V cells. The neighboring center median (CM) was once thought to receive STT input, but modern evidence indicates that it does not. The connections of the Pf and CM are generally motor related (basal ganglia, substantia nigra, and motor cortex; Sadikot et al 1992).
Medial Dorsal Nucleus (MD)
There is a moderately dense STT projection to the ventral caudal part of the medial dorsal nucleus (MDvc). It has an anteroposterior topography, with trigeminal input located most posterior (Ganchrow 1978). This STT projection originates from lamina I cells (Albe-Fessard et al 1975). Cells in the MDvc project to area 24c in the cortex at the fundus of the anterior cingulate sulcus (limbic motor cortex) rather than to the orbitofrontal and prefrontal cortex, where the remainder of the MD projects (Ray and Price 1993, Craig 2003a). This STT component is important for the affective/motivational aspect of pain (see below).
Species Differences
Several important phylogenetic differences in STT terminations have been noted. These differences must be related to behavioral and evolutionary conditions (see Craig 2003a), but they also provide clues to the functional role of different projections.
In the cat the major STT terminations in the somatosensory thalamus occur along the ventral aspect of the VMb, VPI, and VPL. In contrast to primates, there is only a weak lamina V STT projection, and almost no STT terminations occur within VP. In the rat, on the other hand, there are many lamina V STT cells and STT terminations occur throughout VP, probably reflecting the broad overlap of somatosensory and motor cortices in rodents. Lamina I input to the ventral VMb in the cat seems to constitute a primordial homologue of the primate VMpo because it is important for discriminative thermal sensation (Norrsell and Craig 1999) and projects to the insular cortex (Clascá et al 1997). There is almost no lamina I input to the homologous region in the rat (Gauriau and Bernard 2004). In the cat and rat, lamina I projects to the medial thalamic nucleus submedius (Sm), which originates developmentally from the pronucleus of the MD; this constitutes a stark phylogenetic difference from the lamina I projection to the MDvc in the primate because the Sm projects to the ventral lateral orbital cortex rather than to the cingulate cortex. Spinal input to the anterior cingulate passes through the ventral VP (cat; Musil and Olson 1988) or indirectly by way of parabrachial (PB) input to the medial thalamus (cat and rat) instead of through the MDvc (Devinsky et al 1995). In the rat, input to the Sm originates from trigeminal cells at the junction of the caudalis and interpolaris subnuclei, as well as from trigeminal and cervical lamina I cells and other cells in the spinal cord (Yoshida et al 1992).
Spinobulbar Projections
Spinal projections to the brain stem are important for the integration of nociceptive activity with processes that subserve homeostasis and behavioral state. There are also pathways that indirectly convey nociceptive activity to the forebrain following integration in the brain stem. In addition, spinal input to the brain stem influences the modulation of both spinal and forebrain activity, which can affect the experience of pain.
Cells of Origin
Retrograde labeling studies indicate that spinobulbar cells have a similar distribution to STT cells, specifically, laminae I, V, and VII in the monkey, cat, and rat (Wiberg et al 1987). Similar response categories have been described for spinobulbar and STT cells (Yezierski and Schwartz 1986, Ammons 1987, Wilson et al 2002). These similarities suggest that STT and spinobulbar projections could originate from the same cells. However, identification of spinal cells that project to the brain stem by retrograde labeling or by antidromic activation has been confounded technically by the passing STT fibers that ascend through the brain stem, which can cause spurious labeling or activation. Recent evidence based on double retrograde labeling indicates that spinomedullary and STT neurons are closely overlapping but almost completely separate populations (Andrew et al 2003). This means that there may be distinct physiological types of cell that differentiate these two populations (cf. Wilson et al 2002), thus implying that the nervous system could potentially exercise differential control over the activity of ascending spinal projections that convey similar afferent information to supraspinal sites having different functions. Whereas spinal input to the thalamus is contralateral, spinal projections to the medulla are bilateral, and those to the pons and mesencephalon have a contralateral dominance.
Spinobulbar Terminations
Anatomical evidence indicates that ascending spinobulbar projections terminate mainly in four major areas of the brain stem, as indicated in Figure 12-3 (Wiberg et al 1987, Craig 2003a):
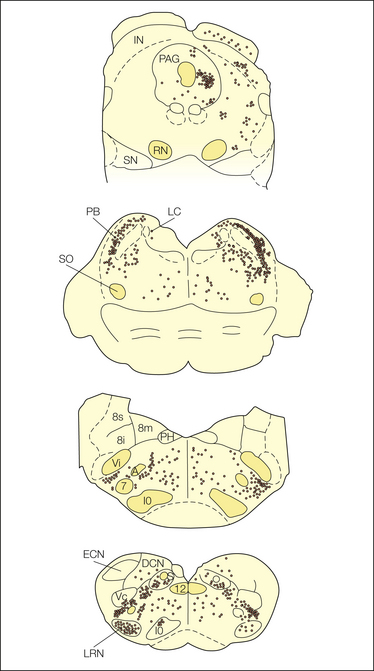
Figure 12-3 Schematic diagram summarizing the distribution and relative density of spinobulbar terminations in the macaque monkey at four transverse levels, from rostral to caudal.
Abbreviations of brain stem nuclei: A, ambiguus; DCN, dorsal column nuclei; ECN, external cuneate; IN, intercollicular; IO, inferior olive; LC, locus coeruleus; LRN, lateral reticular; PAG, periaqueductal gray; PB, parabrachial; PH, praepositus hypoglossi; RN, red nucleus; S, solitary complex; SN, substantia nigra; SO, superior olive; Vc, trigeminal nucleus caudalis; Vi, trigeminal nucleus interpolaris; 7, facial; 8i, inferior vestibular; 8m, medial vestibular; 8s, superior vestibular; 12, hypoglossal.
Lamina I cells project to the first three sites, but not to the reticular formation. Spinal laminae V and VII cells project mainly to the reticular formation, the lateral reticular nucleus, and the tectum, with relatively minor projections to the PB, the ventrolateral medulla, and the region of the PAG (Yezierski 1988, Andrew et al 2003).
Catecholamine Cell Groups
There is moderately dense spinal input to brain stem regions that contain the catecholamine cell groups: the ventrolateral medulla (A1, C1, A5), the nucleus of the solitary tract (A2), the locus coeruleus (A6), and the subcoeruleus and Kölliker–Füse regions in the dorsolateral pons (A7). These are integration sites for cardiorespiratory and homeostatic function that contain pre-autonomic bulbospinal neurons that drive sympathetic outflow (Loewy and Spyer 1990). Spinal projections to the ventrolateral medulla activate spino–bulbo–spinal somato-autonomic reflex arcs (Sato and Schmidt 1973), as well as descending modulatory (including pro- and antinociceptive) mechanisms. Lamina I terminations in the caudal ventrolateral medulla are especially dense around cells of the A1 group; the A1 projection to the hypothalamus is responsible for the release of adrenocorticotropic hormone and vasopressin in response to trauma and noxious stimulation. Spinal input to the solitary complex provides an ascending pathway for visceral nociceptive activity. Lamina I projections to the A6 and A7 groups in the dorsolateral pons influence noradrenergic and enkephalinergic bulbospinal cells that modulate nociceptive and autonomic spinal activity (Basbaum and Fields 1978, Westlund et al 1984, Hermanson and Blomqvist 1997).
Parabrachial Nucleus (PB)
There is dense spinal input concentrated in the lateral part of the PB and sparse input in the medial and dorsal parts overlapping with visceral afferent input from the solitary nucleus (Burton et al 1979, Craig 2003a). The projection is weakly organized topographically (Feil and Herbert 1995). Spinal input to the PB originates primarily from lamina I neurons, with a weak contribution from laminae IV–VI cells. Findings in rats indicate that the PB has numerous interconnections with brain stem reticular formation cells (including the catecholamine cell groups), appropriate for its role in homeostasis and autonomic integration (Chamberlin and Saper 1992).
The PB projects to the hypothalamus, amygdala, midline and intralaminar thalamus, and a portion of the ventrobasal thalamus (VMb, or VPMpc) that serves as a relay to the insular cortex for general and special visceral (gustatory) sensory activity (Bernard et al 1993). Nociceptive PB neurons that project to the amygdala or the hypothalamus have response characteristics similar to lamina I neurons (Bernard and Besson 1990). Lamina I input to the PB thus provides a substrate for integration of nociceptive activity with general visceral (homeostatic) afferent activity, as well as an indirect relay to forebrain autonomic, neuroendocrine, and emotional control regions.
Periaqueductal Gray (PAG)
Moderately dense spinal input occurs in the lateral and ventrolateral (caudal) portions of the PAG and adjacent tegmentum. It originates primarily, but not exclusively, from lamina I. It is topographically organized in the lateral, but not in the ventrolateral, PAG, with a rostrocaudal (trigeminal, cervical, lumbar) sequence (Wiberg et al 1987). The ventrolateral PAG also receives bilateral input from laminae VII–VIII cells in C1–2. The PAG is a major mesencephalic site for homeostatic control and limbic motor output that has both ascending and descending projections. Stimulation of different portions of the PAG can elicit aversive behavior, cardiovascular changes, and (opiatergic or non-opiatergic) antinociceptive modulation, in parallel with the topography of spinal and trigeminal input and in a context-dependent manner appropriate for different behavioral states (Depaulis et al 1992). Spinal input to the PAG may be integrated with descending antinociceptive modulation of the spinal cord by way of PAG projections to the rostral ventromedial nucleus (raphe magnus), dorsolateral pons, and ventrolateral medulla (Basbaum and Fields 1978, Fields 1987). Notably, the portions of the PAG that receive spinal input also have ascending projections to the hypothalamus and medial thalamus (Mantyh 1983).
Reticular Formation
Scattered spinal input to the reticular formation was observed with early silver degeneration techniques, but this projection has not been examined with more modern methods. Nociceptive neurons have been recorded in the reticular formation. Many neurons in the rostral brain stem project to the thalamus, so it has been suggested that some indirectly convey nociceptive spinal activity to the forebrain. A so-called spinoreticulothalamic pathway was hypothesized as a multisynaptic, alternative pathway for pain-related activity that could serve the motivational and arousal aspects of pain (Melzack and Casey 1968). However, brain stem reticular neurons that project to the forebrain do not seem to receive spinal input (Blomqvist and Berkley 1992); rather, spinal projections to the PB and PAG provide the major indirect routes for spinal input to reach the forebrain by way of the brain stem.
A portion of the dorsomedial reticular formation of the medulla (subnucleus reticularis dorsalis) receives spinal input from laminae I and V cells and contains neurons with nociceptive responses from large receptive fields; however, these cells project back to the dorsal horn or rostrally to the ventromedial thalamus and thence to layer 1 over the entire frontal cortex in the rat (Bernard et al 1990, Villanueva et al 1998). A modulatory role for such cells has been proposed in pain behavior.
Spinohypothalamic Pathway
Retrograde labeling evidence in the rat indicates the presence of a spinohypothalamic tract (SHT) that originates bilaterally from cells in laminae I, V, VII, and X over the entire length of the cord (Dado et al 1994). There is also sparse spinal input to other forebrain regions, such as the nucleus accumbens, the septal nuclei, and the pallidum. Extensive antidromic mapping studies indicate that SHT axons often pursue a tortuous course through the contralateral diencephalon, decussate in the optic chiasm, and then descend ipsilaterally through the hypothalamus and as far as the brain stem. Many of these are STT axons that have a collateral branch extending into the anterior and lateral portions of the hypothalamus (Burstein et al 1996). The SHT has been proposed to be a general mammalian pathway; however, actual terminations have not been identified with anterograde labeling (suggesting that they are only fibers of passage), it is only sparsely represented in the cat, and little evidence has been obtained of such a projection in the monkey. Nevertheless, if a significant SHT termination exists in primates, it could have potentially important implications for the autonomic, neuroendocrine, and emotional aspects of pain.
Other Indirect Pathways
Two additional pathways to the forebrain have been identified that are potentially pain related: the post-synaptic dorsal column (PSDC) system and the spinocervicothalamic (SCT) pathway. Both originate from second-order cells in the spinal dorsal horn, primarily in laminae IV–VI and lamina X. PSDC axons ascend in the deep dorsal columns or the superficial dorsolateral funiculus and terminate in the ventral and rostral portions of the dorsal column nuclei (DCN: gracile and cuneate nuclei). These portions of the DCN contain GABAergic interneurons and also neurons that project to motoric regions of the brain stem rather than to the somatosensory thalamus (Berkley et al 1986). Accordingly, this pathway may engage inhibitory interneurons that reduce activity in mechanoreceptive relay cells and that relate such background levels to other sensorimotor sites.
SCT cells project via the dorsolateral funiculus to the lateral cervical nucleus in C1–2, which projects to the VP thalamus via the medial lemniscus (Boivie 1983). This pathway is large in carnivores (cat, raccoon), but diminutive in primates. Activity in the PSDC and the SCT is dominated by low-threshold mechanoreceptors, but nociceptive neurons have been recorded in both pathways. Recent evidence indicates that the PSDC can convey visceral nociceptive activity to DCN neurons that project to the thalamus in the rat, and correlative studies have examined this possibility in the monkey and humans (Al-Chaer et al 1998, but see Villanueva and Nathan 2000). However, the pattern of cortical sites activated during visceral pain in humans is inconsistent with this hypothesis (e.g., Strigo et al 2003).
The Trigeminal Brain Stem Complex and Nociception
The craniofacial region contains several specialized structures (tooth pulp, cerebral vasculature, nasal mucosa, and cornea) from which pain is the primary sensation and a frequent clinical complaint. Craniofacial afferent fibers terminate in the trigeminal brain stem complex, which consists of the principal sensory nucleus and the subnuclei of the spinal tract (oralis, interpolaris, and caudalis). The caudal-most region, the subnucleus caudalis, is frequently referred to as the medullary dorsal horn because it is essentially a rostral continuation of the cervical spinal dorsal horn. It receives small-diameter trigeminal primary afferent input, and nociceptive neurons in its superficial and deep layers have properties and projections similar to the spinal neurons described above in lamina I and lamina V (Sessle 1987, Hutchison et al 1997).
Clinical lesions of the trigeminal afferent fibers and the nucleus caudalis at the level of the obex (trigeminal tractotomy; Wallenberg’s syndrome) reduce orofacial pain and temperature sensation. Nociceptive neurons in the nucleus caudalis that receive input from specialized non-cutaneous tissues (temporomandibular joint, nasal mucosa, cornea, tooth pulp, and cerebral vasculature) usually have cutaneous receptive fields too, which provides a likely basis for the common referral and radiation of pain arising from these deep structures (Strassman et al 1986, Broton et al 1988, Meng et al 1997).
The more rostral trigeminal subnuclei can also be activated by orofacial nociceptive input. Following trigeminal tractotomy at the obex level, there can be residual pain sensation from the intraoral and perioral regions (Young 1982). Lesions of the subnucleus oralis can interfere with nociceptive behavior in animals, and nociceptive neurons have been recorded in these subnuclei that respond to tooth pulp, muscle, or dura stimulation (Davis and Dostrovsky 1988).
Functional Role of Anterolateral Tract Axons
Early clinical investigators concluded that the two ascending bundles of STT fibers—the lateral STT and the anterior STT—are associated with different functions (“pain and temperature” versus “crude touch and movement”) based on the effects of small lesions of the spinal white matter made, in some cases, in awake pain patients under local anesthesia (Kuru 1949, Craig et al 2002). Modern clinical findings have verified that cordotomy lesions in the middle of the lateral funiculus (at the level of the central canal) produce contralateral loss of pain, temperature, itch, and sensual touch sensations that begin within two segments caudal to the lesion (Fig. 12-4). Similar findings have been obtained in monkeys. This corresponds with the location of some ascending lamina I STT fibers (Craig et al 2002), and because the same symptoms can be produced by interrupting these fibers at the bulbar and thalamic levels, this supports the conclusion that they have a critical role in these sensations. Furthermore, pain or thermal (cool, warm) sensations can be elicited directly by electrical stimulation of these ascending fibers during percutaneous cordotomy or spinothalamic tractotomy, and only pain, temperature, and crude touch sensations remain intact if the entire spinal cord is transected except for the anterolateral quadrant.
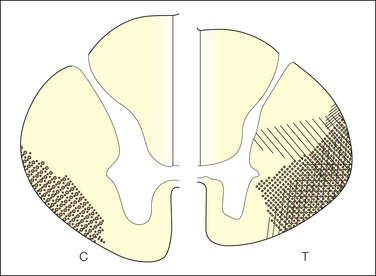
Figure 12-4 Diagram summarizing the locations of cordotomy incisions that caused dense analgesia in a limited region of the body in a sample of pain patients.
Left, cervical cord (C); right, thoracic cord (T). Each case of cordotomy has the lesion illustrated by a different form of cross-hatching (From Nathan PW, Smith MC 1979 Clinico-anatomical correlation in anterolateral cordotomy. In: Bonica JJ (ed) Advances in pain research and therapy, vol 3. Raven Press, New York, p 921–926.)
Nevertheless, accumulated experience led neurosurgeons to conclude that cordotomies should be made through the entire anterolateral quadrant—and only for terminal cancer patients—because even if initially successful, the pain could return, or worse, a central pain syndrome could develop, which is a distinct condition that is described further below (White and Sweet 1969, Pagni 1998). Variable success in lesion placement was certainly a major cause of variability in outcome, but individual anatomical variability in the location of the lateral STT fibers was also implied by the clinical observations, and such variability has recently been documented in humans (Craig et al 2002). It has also been postulated that the neural representation of pain must be redundant and plastic and that other pathways might have substantial involvement in pain in addition to the lateral STT (Melzack and Casey 1968).
Investigators endorsed the view that WDR lamina V cells that project primarily in the anterior STT must have a major role in pain, as proposed by the “Gate Control Theory” (Wall 1973). Some of these neurons show graded responses to innocuous and noxious mechanical, noxious heat, noxious cold, and noxious muscle or visceral stimuli. Their responses usually increase (“wind-up”) with strong noxious stimulation (using intradermal capsaicin or repetitive C-fiber stimuli) and then show sensitization to innocuous stimuli in a manner that closely resembles the increased pain, hyperalgesia, and allodynia experienced by humans following such stimuli. It was emphasized that their discharge properties correlate well with the behavioral response speed of awake, well-trained monkeys to incremental noxious heat stimuli applied to the face (Maixner et al 1989).
A unique study that used two-pulse electrical stimulation of anterolateral fibers during percutaneous cordotomy reported that human pain correlated with the axonal conduction properties of WDR lamina V STT axons in monkeys (Mayer et al 1975). Another study claimed that only WDR lamina V cells show maintained responses to tonic noxious stimuli, similar to human sensation, whereas superficial NS cells show adaptation, similar to the responses of C-fiber nociceptors. Nevertheless, several issues confound these interpretations (Craig 2003a).
Recent findings support the view that lamina I STT cells have a critical role in the representation of several distinct sensations from the body, including sharp pain, burning pain, cool, warm, itch, muscle and visceral sensations, sensual touch, and so on (Craig 2003a). Lamina I STT axons ascend in the lateral STT, where clinical cordotomy reduces such sensations, and project to thalamocortical regions that are specifically associated with these sensations (see below). Thermoreceptive-specific and histamine-selective lamina I STT cells are the only ascending cells that correspond with cool, warm, and itch sensations. In stark contrast to the modality-ambiguous WDR lamina V cells, lamina I cells provide distinct sensory channels for the qualitatively different pain sensations from skin (i.e., first, sharp pain, and second, burning pain), muscle, and other tissues. In contrast to WDR cells, modality-selective lamina I cells provide a clear explanation for the fact that innocuous touch normally does not cause pain. Lamina I HPC cells uniquely explain the dull burning pain elicited by pinch, heat, or cooling (but not touch) during a peripheral nerve block of A-fiber conduction. Their activity also uniquely explains the burning, ice-like pain unmasked by the thermal grill illusion (Craig and Bushnell 1994), which is elicited by innocuous cool and warm stimuli that do not activate WDR lamina V cells.
Finally, recent evidence indicates that lamina I STT cell activity explains the augmentation and “re-set” of the burning pain elicited psychophysically by the repeated brief-contact heat paradigm whereas lamina V WDR STT cells cannot (Craig and Andrew 2002, Craig 2003b). This dissociation may mean that humans report pain when HPC cells are active but WDR cells are not; conversely, they do not report pain when WDR cells are active but HPC cells are not. Lamina V WDR cells are most notable for their sensitization, which seems to correspond with clinical allodynia and hyperalgesia, yet lamina I NS and HPC cells can readily be sensitized to innocuous mechanical and cool stimuli by repeated noxious stimulation (Craig and Kniffki 1985).
In light of these findings, an alternative suggestion for the role of WDR lamina V cells is that they serve as cumulative integrators of all somatic afferent activity for the purpose of sensorimotor integration (Perl 1984b). This idea is supported by their musculotopic organization in the dorsal horn (Schouenborg et al 1995), their intercalation in the flexor reflex pathway (Bannatyne et al 2006), their projection to the cerebellum and to motoric regions of the lateral and medial thalamus, and their role in inhibition of mechanoreceptive cells in the DCN. This view is also consistent with the inhibition by noxious heat of mechanosensory activity in cortical area 3b, where WDR lamina V cells project to superficial layers by way of the VP (Tommerdahl et al 1996), which directly contrasts with the simultaneous activation of area 3a, where a lamina I pathway terminates (see below). Their potential role in neuropathic pain (Wall 1973, Willis 1985, Price 1988) has not been supported by recent studies that directly associate lamina I activity with allodynia and hyperalgesia in rat models of pain-like behavior (Nichols et al 1999, Bester et al 2000, Gorman et al 2001). Finally, lamina I STT neurons, but not lamina V STT neurons, show responses in the monkey that correlate with the human second pain sensation (Craig 2004b), whereas lamina V STT cells show tonic sensitivity to limb position, consistent with a demonstrated input from group II muscle afferent fibers (Bannatyne et al 2006). Nevertheless, the potential central effects of WDR lamina V STT cells in arousal and motivation clearly require further study because despite their modality-ambiguous nature, their robust activation during noxious stimulation must have an effect on supraspinal processing that can influence the pain experience.
The most parsimonious view is that the activity of all ascending pathways is integrated in the forebrain in the context of current conditions and past experience in order for all aspects of the sensation of pain to be generated. Elimination of a portion of this system or a particular pathway, such as a partial lesion in the periphery, spinal cord, or forebrain, can cause an imbalance with variable effects on integrated sensation. For example, it may result in pain in the absence of any objective stimulus, such as phantom pain or central pain (see below). Therefore, we emphasize the inherent complexity of the spinal and supraspinal interconnections that must be involved in the human experience of pain, which remain to be fully elucidated.
The Thalamus and Pain
Comparative Evidence in Primates
Here, we briefly summarize the anatomical and physiological evidence in monkeys on thalamic regions that are implicated in pain (Fig. 12-5). These thalamic regions receive STT projections, and some receive input from other pathways as well. Evidence from other animals is briefly mentioned. Evidence from studies involving the human thalamus is summarized subsequently.
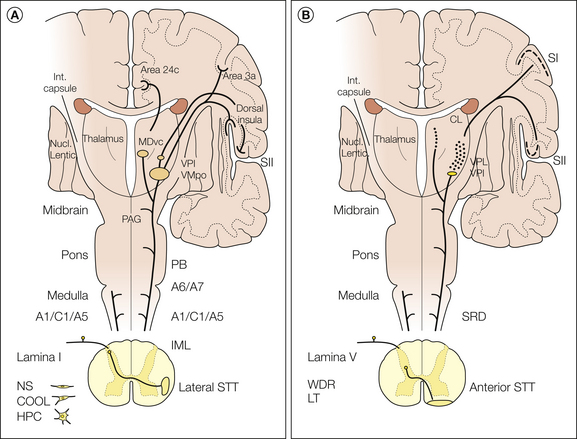
Figure 12-5 A, Schematic diagram summarizing the ascending projections of lamina I cells in the macaque monkey. The three major classes of lamina I cells (COOL, polymodal nociceptive [HPC], and nociceptive specific [NS]) are indicated. Their axons decussate and ascend in the lateral spinothalamic tract (STT). Terminations occur in the brain stem in the ventrolateral medulla (A1/C1/A5), the dorsolateral pons (A6/A7), the parabrachial nucleus (PB), and the periaqueductal gray (PAG). In the thalamus, lamina I STT terminations occur in the posterior part of the ventral medial nucleus (VMpo), the ventral lateral inferior nucleus (VPI), and the ventral caudal part of the medial dorsal nucleus (MDvc), whose cortical projections are shown. B, Schematic diagram summarizing the ascending projections of laminae IV–V cells in the macaque monkey. The axons of both low-threshold (LT) and wide–dynamic range (WDR) cell types decussate and ascend in the anterior STT. Terminations occur in the brain stem in the subnucleus reticularis dorsalis (SRD) and other sites, probably including the reticular core. In the thalamus, laminae IV–V STT terminations occur in the ventral posterior inferior nucleus (VPI), in the ventral posterior lateral nucleus (VPL), in the ventral lateral nucleus, and in the central lateral nucleus (CL).
VMpo
The posterior thalamus was formerly regarded as undifferentiated, but modern observations in monkeys and humans have documented a distinct nucleus, the VMpo (the posterior part of the ventral medial nucleus), that serves as a specific lamina I STT thalamocortical relay for feelings from the body, including pain, temperature, itch, and so on (Craig et al 1994, Craig 2003a). It is rudimentary or non-existent in non-primates and is small in monkeys (some investigators have difficulty finding it), but it is enormously enlarged in humans. VMpo neurons have small receptive fields that are topographically organized rostrocaudally, in correspondence with the anatomical organization of dense lamina I STT terminations in the VMpo. They show selective response properties similar to lamina I STT cells (i.e., NS, HPC, COOL, etc.; Fig. 12-6) and are segregated into separate maps according to these classes. Single-unit recordings obtained in awake monkeys from the VMpo showed strong correlations with behavioral detection of cooling and noxious heat stimuli applied to the face, and lidocaine (lignocaine) deactivation of this region selectively reduced such behavioral responses (Bushnell et al 1993); these recording sites were unfortunately originally misinterpreted as the VPM (Craig, unpublished observations).
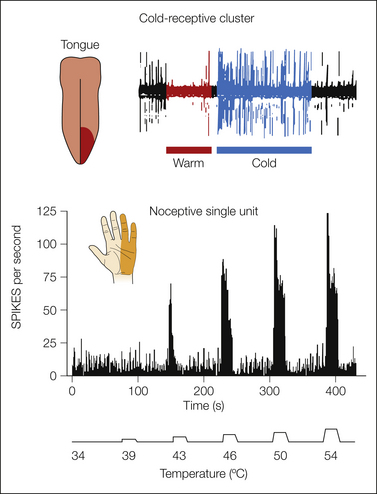
Figure 12-6 Examples of the responses of neurons in the posterior part of the ventral medial nucleus (VMpo) of the monkey.
The clustered thermoreceptive-specific neurons (top) had ongoing activity that was inhibited by radiant warming, and they were excited by cooling (application of a wet ice cube) and by no other stimuli from a receptive field on the contralateral tongue. The histogram (bottom) shows the graded responses of a single nociceptive-specific neuron to noxious heat pulses applied with a thermode to the receptive field on the ulnar side of the contralateral hand. (From Craig AD, Bushnell MC, Zhang ET, et al 1994 A thalamic nucleus specific for pain and temperature sensation. Nature 372:770–773.)
The VMpo projects topographically to the dorsal margin of the posterior insula, where preliminary functional magnetic resonance imaging (fMRI) observations in monkeys showed strong activation by noxious stimuli that is uniquely graded with stimulus intensity (Keltner et al 2006) and with repeated-heat augmentation (Staud et al 2008). This interoceptive cortex (so called because it is functionally related to homeostasis rather than to skeletal movement) is organized rostrocaudally, distinct from the mediolateral topography of the exteroceptive (mechanoreceptive) SII/PV (second somatosensory and parietal ventral) cortical fields in the adjacent parietal operculum (Craig 2010, Henderson et al 2010). The VMpo also provides a corollary projection to area 3a in the fundus of the central sulcus.
In the cat, comparative evidence indicates the existence of nociceptive cells in a less well differentiated posterior complex (Po) that have very large receptive fields and convergent visceral input (Poggio and Mountcastle 1960). The portion of the Po dorsal to the VP in the cat differs in that it projects to area 5a (Jones 1985), but it also contains a few nociceptive cells. In the rat, the Po also contains neurons responsive to noxious stimuli, possibly mediated by descending input from the somatosensory cortices (but see Gauriau and Bernard 2004). In the cat (and perhaps the rat), a narrow region along the ventral aspect of the VMb contains nociceptive and visceroceptive neurons and projects to the insular cortex; this may be a rudimentary homologue of the primate’s VMpo (Clascá et al 1997, Norrsell and Craig 1999). Lamina I STT input has been described in this region in the cat but only sparsely in the rat.
VP
The ventral posterior nuclei are the main thalamic somatosensory nuclei. They relay a somatotopic representation of cutaneous mechanoreceptors from the DCN and the principal trigeminal nucleus to the main somatosensory (SI) cortex, areas 3b and 1. A dorsally and anteriorly adjacent region, the ventroposterior superior nucleus (VPS), relays proprioceptive (muscle and joint afferent) activity to areas 3a and 2 (Kaas et al 1984, Jones 1990). A ventrally adjacent region (VPI) relays input to an area posterior to the second somatosensory cortical region (SII/PV) in the retroinsular (vestibular) cortex.
The view has long been held that the VP must be involved in pain sensation because it receives STT input (in primates, though not in carnivores) and because the sensory aspects (localization, intensity discrimination) of pain were thought to require involvement of the discriminative somatosensory system (Melzack and Casey 1968). In support of this view, nociceptive neurons exist among the many mechanoreceptive neurons in the VP in monkeys, amounting to about 10% of the VP neurons (Willis 1985, Treede et al 1999). Nearly all are WDR neurons. Their moderate to large receptive fields (often half or more of the face or arm) are roughly in register with the VP somatotopy. They are concentrated in the posterior aspect of VP near the major fiber laminae, consistent with the concentrations of STT terminations there. A few WDR cells in the VP have been antidromically activated from areas 3b and 1; however, anatomical data suggest that they project to the most superficial layers (consistent with a modulatory role), in contrast to the middle layer projection of most VP relay cells (Shi et al 1993). Visceral noxious stimuli activate some VP WDR cells but also activate low-threshold mechanoreceptive VP cells without regard to somatotopy (Al-Chaer et al 1998). Both WDR and NS cells are found in the VPI, where there may be a separate topographic representation of the body, but the VPI receives vestibular input as well (Guldin and Grusser 1998).
In other species, the distribution of nociceptive neurons in the region of the VP differs markedly. In the cat, there are essentially no nociceptive cells within the VP proper. Instead, they are found in the dorsal and ventral aspects of the VP, including in particular the VPI and the ventral aspect of the VMb, where STT terminations occur (see above). These include both NS and WDR cells. They are crudely topographically organized mediolaterally, in parallel (but not in register) with the somatotopy of the adjacent VP. Some receive convergent input from the skin, muscle, tooth pulp, viscera, or cranial vasculature (Davis and Dostrovsky 1988), and some project to the somatosensory cortex (area 3a or the SII region) or to the anterior cingulate cortex (see Craig and Dostrovsky 2001). In the rat, nociceptive cells (NS and WDR) are found throughout the VP intermixed (and in topographic register) with low-threshold mechanoreceptive neurons. They generally have large, often bilateral receptive fields, and some receive convergent visceral input. In rats with experimentally induced arthritis or neuropathies, there is an increase in the number of WDR thalamocortical cells.
MD
Topographically organized lamina I STT input terminates in the ventral caudal part of the medial dorsal nucleus (MDvc), which projects to the anterior cingulate cortex. Recordings in anesthetized monkeys indicate that the MDvc contains a discrete concentration of NS neurons with large, sometimes bilateral receptive fields (Craig 2003a). Their ongoing activity can be inhibited by innocuous thermal (cool, warm) stimuli, consistent with a role in the cold-induced inhibition of pain and in the thermal grill illusion of pain (see below). Nociceptive responses were recorded in the medial thalamus of awake monkeys that were enhanced by attention and reduced by cooling, and although the neurons were ascribed to the CM, Pf, and CL, they probably included MDvc cells (Fig. 12-7) (Bushnell and Duncan 1989).
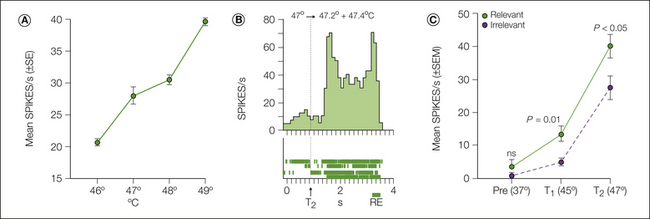
Figure 12-7 Responses of a neuron in the posteromedial thalamus of an awake monkey to noxious thermal stimuli applied to the face.
A, Mean firing rate with increasing temperatures. B, Peristimulus histogram with an associated dot raster display showing the increased firing when the temperature increased from 47 to 47.2 or 47.4°C. In this particular example the monkey did not respond until the end of the trial (RE, button release). C, The neuron responded more intensely during a task in which the monkey was rewarded for detecting a 47°C stimulus (T2; solid line, n = 10) versus a task in which the monkey was rewarded for ignoring the thermal stimulus and waiting for a later change in a visual stimulus (dashed line, n = 5). (From Bushnell MC, Duncan GH 1989 Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Experimental Brain Research 78:415–418.)
In the cat and rat, there is indirect spinal input to the medial thalamus by way of the PB, but there is also a prominent STT projection to the nucleus submedius (Sm). The Sm is developmentally related to the MD, but it projects to the ventral lateral orbital cortex rather than to the anterior cingulate. Nociceptive neurons have been recorded in the Sm in the cat and rat (Kawakita et al 1993), some with responses to deep tissues. In the arthritic rat, many Sm cells are responsive to innocuous joint movements. In the cat, both nociceptive and thermoreceptive lamina I STT cells project to the Sm (Craig and Dostrovsky 2001); this convergence may provide the basis for cold-induced inhibition of nociceptive processing, similar to the MDvc of the primate. Lesion and stimulation studies indicate that the Sm and ventral lateral orbital cortex (VLO) are involved in activation of descending antinociceptive controls by way of the VLO (sometimes termed the rostral agranular insula) and the PAG (Zhang et al 1998). In addition, several studies have suggested that the habenula (adjacent to the MD) may be involved in pain modulation (Cohen and Melzack 1993), which could be related to its limbic connections and its documented role in homeostatic behavior.
Intralaminar Nuclei
In monkeys, cats, and rats, responses to noxious electric, mechanical, or heat stimuli have been recorded throughout the intralaminar thalamus, particularly the CL and Pf. Most such cells have large receptive fields; cells with graded responses to noxious heat have been observed. These cells could be related to attention; many intralaminar neurons discharge with eye movements, consistent with strong ascending input from the cerebellum and the superior colliculus and a postulated role in gaze orientation (see Jones 1985).
Other Structures
Amygdala
Nociceptive neurons have been identified in the central nucleus of the amygdala in the rat; these studies support the efficacy of nociceptive PB neurons that project there (Bernard et al 1993), although the amygdala also receives input from the rat’s posterior thalamus. Increased glucose metabolism in the amygdala was observed in a neuropathic pain model in the rat. The amygdala may be significant for the analgesic effects of systemic morphine and for fear-conditioned descending antinociception (Helmstetter et al 1993). However, lesions of the amygdala in primates cause memory deficits, and effects on pain sensibility have not been reported clinically.
Basal Ganglia
Nociceptive neurons have been recorded in these sensorimotor structures in the rat (Chudler et al 1993), but not in the cat or the primate. Nociceptive responses have also been obtained in the substantia nigra that are sensitive to systemic morphine. Clinical lesions of the basal ganglia and diseases that affect these structures (e.g., Parkinson’s or Huntington’s disease) may have some effect on pain perception.
Hypothalamus
Nociceptive neurons have not been well studied in the hypothalamus, but cells that respond to visceral or tooth pulp stimulation were recorded in the rat. In humans, both stimulation and lesions of the PAG involving the posterior hypothalamus have been used to alleviate pain (Gybels and Sweet 1989).
Direct Evidence in Humans
The unique opportunity afforded by functional stereotactic surgery to record and stimulate in the thalamus of awake patients has provided some interesting findings and validation of subhuman primate studies related to thalamic function in pain. Unfortunately, the inherent limitations of these studies (time constraints, ethical considerations, and lack of histological confirmation) limit interpretation of the findings. The studies attempted to address the following questions:
This section briefly summarizes the findings pertaining to these questions.
Nociceptive Neurons in the Lateral Thalamus
The existence of nociceptive neurons in the VP (often termed the Vc in humans) and adjacent regions has been reported by Lenz and colleagues (for review, see Lenz and Dougherty 1997). In addition to the mechanoreceptive lemniscal cells within the VP, neurons responding to both low- and high-intensity mechanical stimuli were found (WDR neurons); some of these also responded to noxious thermal stimuli (Lee et al 1999). These neurons were primarily located in the posterior–inferior portion of the VP. Interestingly, in the adjoining posterior–inferior area, which includes the VMpo (Blomqvist et al 2000), they identified NS neurons that responded to noxious heat, and none of the neurons in this area responded to innocuous tactile stimuli. Thus NS neurons were found only in the posterior–inferior region, whereas only WDR and mechanoreceptive neurons were found in the VP.
Stimulation-Induced Pain
One of the unique aspects of electrophysiological studies in human patients is the ability to question the patient about sensations evoked by electrical stimulation within the brain. Electrical stimulation within the VP and adjacent regions of the thalamus usually evokes innocuous paresthesia. However, several early studies documented that stimulation in the area posterior–inferior to the VP elicits reports of painful sensations in some patients (Hassler and Riechert 1959, Tasker 1984). Recent studies have examined the effects of stimulation in much greater detail (Davis et al 1996, Lenz and Dougherty 1997, Dostrovsky et al 2000). These studies show that pain and innocuous thermal sensations can be evoked from a region at the posterior–inferior border of the VP and extending several millimeters posterior, inferior, and medial (Figs. 12-8 and 12-9).
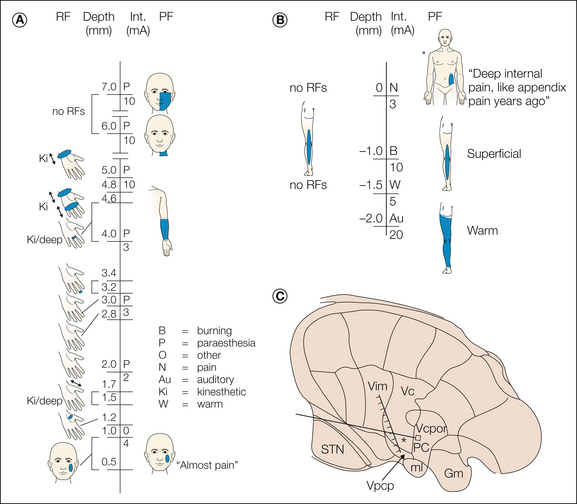
Figure 12-8 Reconstruction of the data obtained during an electrode trajectory through the lateral thalamus in a patient with essential tremor.
The receptive fields (RFs) of neurons encountered during the penetration are shown to the left of the vertical line. The stimulus intensity (Int.) and evoked sensation and projected fields (PFs) are shown to the right of the vertical line. The patient’s description of certain evoked sensations is indicated in quotation marks. The shaded bar indicates the presumed tactile region of the ventral caudal nucleus (Vc) based on neuronal responses to tactile stimuli. The inset shows the thalamic map determined by the patient’s anterior and posterior commissures and presumed location of the trajectory based on stereotactic coordinates. The asterisk indicates the site where stimulation evoked a visceral pain response. Gm, medial geniculate nucleus; ml, medial lemniscus; PC, posterior commissure; STN, subthalamic nucleus; Vcpc, parvocellular ventrocaudal nucleus; Vcpor, Vc portae; Vim, ventral intermediate nucleus. (From Davis KD, Tasker RR, Kiss ZHT, et al 1995 Visceral pain evoked by thalamic microstimulation in humans. Neuroreport 6:369–374.)
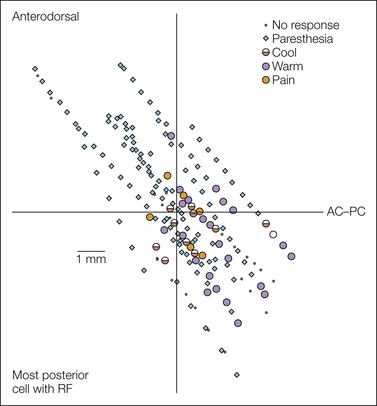
Figure 12-9 Locations of sites in the thalamus of awake human patients where paresthetic or thermal/pain sensations were evoked.
The quality of the evoked sensation is as listed in the key. The sites are shown in a sagittal reconstruction relative to the anterior commissure–posterior commissure line (AC–PC, horizontal line) and a vertical line defined as the anteroposterior location of the most posterior cell responding to innocuous somatosensory stimulation. Therefore, the region located posterior to the vertical line is the posterior–inferior region, which presumably corresponds to the posterior part of the ventral medial nucleus (VMpo). RF, receptive field. (Used with permission from Davis KD, Lozano AM, Manduch M, et al 1999 Thalamic relay site for cold perception in humans. Journal of Neurophysiology 81:1970–1973.)
The incidence of evoked pain/thermal sensations is much higher in the posterior–inferior area than within the VP (except in poststroke pain patients—see below). Unlike the paresthetic (tingling, buzzing, and “electric shock”) sensations evoked in the VP, the pain/thermal sensations are usually reported as quite natural. They are always perceived on the contralateral side of the body, and the projected fields can be quite small. The painful sensations are frequently described as burning pain. In a few cases, sensations of pain referred to deep and visceral sites have been elicited. Lenz and colleagues have reported that microstimulation within the VP (at sites where WDR neurons responding to noxious mechanical stimuli were found) rarely results in pain whereas at the sites in the region posterior–inferior to VP where microstimulation evoked pain, there was a high likelihood of finding nociceptive neurons (see Lenz and Dougherty 1997). Histological confirmation of these stimulation and recording sites has not been obtained in such patients, but it seems likely that the physiologically localized region posterior–inferior to VP corresponds anatomically to the VMpo.
Innocuous Cool Neurons and Sensations
Cells responding to innocuous thermal stimuli are also of great interest and highly relevant because of the well-known association of the pain and temperature pathways. Cooling-specific neurons are found only in lamina I of the spinal and trigeminal dorsal horns and have been shown to project to the VMpo in the monkey (Dostrovsky and Craig 1996). In animal studies, cooling neurons in the thalamus have been reported only in the VMpo (monkey) and medial VPM (cat). Cooling-specific neurons in the human thalamus (Fig. 12-10) were located in the region medial and posterior–inferior to the VP, which probably corresponds to the human VMpo (Davis et al 1999). Of particular interest was the finding that stimulation at such sites evoked cooling sensations that were graded by stimulus intensity and that were referred to the same cutaneous region as the receptive fields of the cooling-specific neurons recorded at the site. Stimulation in this posterior–inferior region can also elicit pain (see above), and as shown by Lenz and colleagues (see Lenz and Dougherty 1997), this region also contains NS neurons.
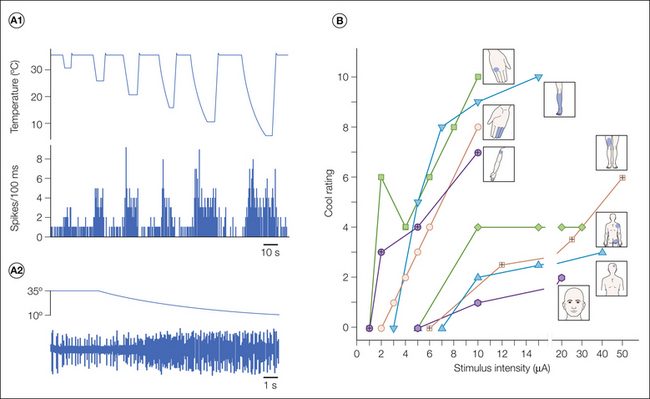
Figure 12-10 A1, Responses of a single neuron in the presumed posterior part of the ventral medial (VMpo) region of a human patient responding to cooling stimuli applied to a receptive field on the fifth digit. The top trace shows the temperature of the thermode with increasing cooling steps. The bottom trace is a histogram of the neuronal firing showing graded responses to increasing cooling steps. A2, Segment of a raw trace of neuronal recording from A1 showing response to the first part of a cooling step. B, Thalamic stimulation-evoked cool sensations. Verbal ratings (0–10 scale) of the innocuous cool sensations evoked by threshold and suprathreshold intensities of thalamic microstimulation were obtained in eight patients. Figurines adjacent to each line depict the location of the thalamic stimulation-evoked sensation at threshold. (From Davis KD, Lozano AM, Manduch M, et al 1999 Thalamic relay site for cold perception in humans. Journal of Neurophysiology 81:1970–1973.)
Medial Thalamus
Much less is known regarding the role of the medial thalamus in human pain, largely because of the fact that there are few opportunities to record and stimulate in this region during functional stereotactic surgery. There are several reports of the existence of nociceptive responses in the medial thalamus. However, it is difficult to evaluate these findings because few details were provided by the authors, and more recent studies have failed to replicate the findings (see Lenz and Dougherty 1997 for references). Certain studies reported that stimulation in the posterior aspect of the medial thalamus can evoke pain (Jeanmonod et al 1994), but in most cases large tipped electrodes and high intensities were used for stimulation, so widespread activation by current spread is a confounding issue. More recent studies also failed to replicate these findings.
Thalamic Bursting Activity
Several groups reported the existence of thalamic neurons in chronic pain patients that fired in a bursting pattern similar to the low-threshold calcium spike-mediated bursting activity reported during sleep in animal studies. Although such activity is common in the medial thalamus, it is also observed in the lateral thalamus, including the VP. It has been proposed that such firing may be the result of and/or the cause of chronic pain (Lenz and Dougherty 1997). However, stimulation in areas with such bursting activity does not reliably evoke pain in awake human patients, and comparable firing in similar regions can be observed in patients without pain. Thus, the role of such activity in mediating central neuropathic pain is unclear.
Physiological Observations in Clinical Pain Patients
In pain patients with deafferentation as a result of amputation or spinal cord injury, there is evidence of plasticity in the somatosensory system. In particular, the thalamic mechanoreceptive representation of the intact regions can be enlarged into thalamic regions of the VP that have been deafferentated. Stimulation in such regions frequently elicits sensations arising from the phantom limb or the deafferentated body region. Although such stimulation does not usually result in pain, this type of reorganization (plasticity) provides a possible basis for mechanisms related to chronic pain in these patients (Davis et al 1998).
Recordings and stimulation in patients with poststroke central pain indicate that the probability of evoking pain by electrical stimulation is greatly increased, in particular for stimulation within the VP at sites where neurons respond to tactile stimuli. In the posterior–inferior region there is also an increase in sites where pain is evoked; interestingly, this occurs in conjunction with a corresponding decrease of sites where stimulation evokes innocuous thermal stimulation. These findings suggest that alterations occurred in the processing of somatosensory information in these patients that led to increased pain. In contrast, in some patients electrical stimulation of the VP through implanted electrodes can be highly effective in alleviating deafferentation and other pain conditions (Siegfried 1987).
Effects of Lesions
Infarcts or surgical lesions of the posterior–inferior region that contains the VMpo in humans can produce analgesia and thermanesthesia (Head and Holmes 1911, Hassler and Riechert 1959, Tasker 1984). In about half of such cases, however, a central pain syndrome develops in which burning, dysesthetic pain is referred to deep tissue in the analgesic region, as addressed below (Pagni 1998).
Neurosurgical lesions have been made in the medial thalamus with the intent of alleviating the emotional aspect of chronic pain. Some of these lesions were large and could have involved both the VMpo and MDvc (Jeanmonod et al 1994, Lenz and Dougherty 1997), but whether this might explain the variable success of such lesions is unknown. Lesions in the medial thalamus reportedly do not cause thalamic pain syndrome.
An Overview of Spinothalamocortical Systems and Pain
The critical role of the thalamus in pain sensation was first recognized by Head and Holmes (1911) in their analysis of patients with thalamic pain syndrome. Known today as central pain (Pagni 1998, Craig 2003a), this syndrome is characterized by ongoing pain, often described as burning, that is referred to a portion of the body in which there may be a paradoxical loss of cutaneous pain sensitivity (analgesia) and, virtually always, thermanesthesia or thermosensory dysfunction. Head and Holmes believed that a lesion of the posterolateral thalamus destroyed a specific sensory substrate for pain and temperature and that the consequent loss of discriminative pain released (disinhibited) the emotional aspect of pain (ascribed to the medial thalamus). They suggested that the somatosensory cortex had only a modulatory role in pain, knowing that lesions of the somatosensory cortex rarely produce analgesia (White and Sweet 1969), and they speculated about alternative pathways that might explain exacerbation of the pain that their patients reported in response to cold or weak mechanical stimuli (allodynia).
Their observations originated the enduring notion that the lateral thalamus is involved in discriminative pain and the medial thalamus in the motivational aspects of pain (Melzack and Casey 1968). Their report also led to the misinterpretation that pain sensation occurs in the thalamus, whereas we now know that the thalamus is intimately interconnected with the cerebral cortex and cannot be considered in isolation.
The functional anatomy of ascending pain pathways, as summarized above, indicates that pain is associated with multiple pathways; activity in multiple regions of the forebrain must therefore be integrated with past experience and the present context to result in the complete, multidimensional pain experience. Although particular neurons and pathways may have a predominant contribution to one or another aspect of the pain experience, it is the constellation of activity across the entire brain that must constitute the basis for the conscious experience of pain. The areas involved would predictably include the pathways and regions described above and potentially many other areas of the human brain.
Modern functional imaging studies using positron emission tomography (PET) and fMRI in human subjects have identified several forebrain areas, which are discussed in detail in Chapter 7. Noxious hot and cold stimuli activate four main cortical sites (see Craig 2003a; Apkarian et al 2005):
In addition, activation has often been reported in subcortical sites, such as the PAG, hypothalamus, amygdala, hippocampus, and cerebellum. All these regions (including the cerebellum) receive ascending nociceptive activity, as described above.
In particular, the cortical projection target of the specific thalamic pain and temperature relay nucleus, the VMpo, in the dorsal posterior insular cortex (usually misidentified as “SII”) is strongly activated in all imaging studies of pain and temperature sensation (Bingel et al 2003, Craig 2003a, Iannetti et al 2003, Henderson et al 2010). It is the earliest site activated in studies of laser-evoked pain. This is consistent with the concept that this lamina I spinothalamocortical pathway serves as an interoceptive representation of the physiological condition of the body that includes specific, distinct sensations of pain, temperature, itch, sensual touch, and so on (Craig 2003a). Clinically, lesions of the parieto-insular cortex or the underlying internal capsule produce hypalgesia and thermanesthesia (Schmahmann and Liefer 1992). Electrical stimulation of this region causes discrete pain sensations in awake human patients, similar to stimulation in the region of the VMpo (Frot and Mauguiere 2003, Mazzola et al 2009). The buried location of the insular cortex means that it would not be damaged by superficial head wounds, and thus clinical documentation of the effects of such lesions was not possible until imaging technology became available. Notably, this region is re-represented in the right (non-dominant) anterior insula, which provides a substrate for subjective appreciation of feelings from the body (Craig 2003a, 2009), and lesions of the anterior insula are associated with pain asymbolia.
Activation of the region of “SI” has been observed in about half of all imaging studies of pain (Bushnell et al 1999, Craig 2003a, Apkarian et al 2005). Nociceptive WDR VP cells apparently project to the supragranular layers of areas 3b and 1 in the SI cortex, and similar cells have been recorded in monkeys in these areas (Treede et al 1999). Accordingly, such cells are thought by many to be responsible for the functional imaging activation of “SI.” However, optical imaging evidence in the monkey indicates that noxious heat activates area 3a and actually inhibits mechanically evoked activity in areas 3b and 1 (Tommerdahl et al 1996). Physiological recordings documenting clusters of NS neurons support the view that activation of area 3a by noxious stimuli is responsible for the activation ascribed to “SI” in human studies (Craig 2003a). Lesions of area 3a may account for the few clinical observations of reduced pain sensation following superficial cortical lesions (Perl 1984a). It is likely that this evidence reflects the projection to area 3a from the VMpo, but identification of this activation site as area 3a in functional imaging studies has been below the resolution of PET and fMRI studies thus far.
The MDvc projection to area 24c in the fundus of the anterior cingulate sulcus is probably one source of activation observed in nearly all imaging and laser-evoked potential studies of pain in humans. Activation of the anterior cingulate cortex (ACC) is uniquely associated with the sensation of burning, ice-like pain elicited by the thermal grill illusion of pain (based on spatially interlaced warm and cool stimuli), which depends on activation of HPC lamina I STT cells (Craig and Bushnell 1994, Craig 2003a). Activation of the ACC is also strongly correlated with hypnotic modulation of pain affect (“unpleasantness”) (Rainville et al 1997). A few nociceptive neurons have been recorded in the ACC in humans (Hutchison et al 1999). Clinically, lesions of the ACC can have significant but variable effects on pain affect; however, localization of such lesions has not been well controlled, which is a significant factor because the morphology of the cingulate cortex in humans is highly variable.
Thus, the major cortical sites that show activation with pain sensation in functional imaging, laser stimulation, and lesion studies largely correspond with the lamina I spinothalamocortical projections described above. The multiple sites activated by this pathway are probably related to the different functional roles of these cortical regions in pain; specifically, activation of the interoceptive insular cortex may engender the distinct sensory qualities of pain and its homeostatic control functions, activation of area 3a in the sensorimotor cortex may be related to the somatic motor aspects of pain sensation (and perhaps localization), and activation of the ACC may engender the affective/motivational aspect of pain. However, these areas are interrelated with subcortical sites (e.g., amygdala, hypothalamus, PAG, and ventral striatum) that receive indirect ascending input and are also involved in the experience of pain. Elucidating the interrelationships of these areas is a crucial challenge for understanding the forebrain processing of pain.
The importance of these interrelationships is emphasized by the phenomenon of the central (thalamic) pain syndrome. Central pain is correlated with lesions that interrupt the ascending lamina I–lateral STT–spinothalamocortical pathway by way of the VMpo to the dorsal posterior insula (Pagni 1998, Schmahmann and Liefer 1992, Craig 2003a). Lesions of this pathway can produce analgesia, but in about half of such cases this disruption results (either immediately or after a variable delay) in the appearance of ongoing pain in the deafferentated region. This must stem from an imbalance among the various forebrain regions involved in pain sensation because of disruption of their interactions at the cortical and subcortical levels. One of these interactions is the inhibitory effect of thermosensory (and thermoregulatory) integration on pain (i.e., inhibition of pain induced by cooling). It has been proposed that interruption of this particular interaction could be a possible cause of central pain based on similarities with the thermal grill illusion of pain, in which a reduction in cooling-specific activity unmasks the cold-activated burning pain elicited by the polymodal nociceptive (HPC) lamina I pathway. This could explain the clinical observation that in central pain, the ongoing burning pain is often focused in a region in which there is a demonstrable loss of thermal sensibility. The recent demonstration of the absence of the thermal grill illusion in a central pain patient supports this hypothesis (Morin et al 2002), as do the characteristics of patients with syringomyelia (Ducreux et al 2006). Future research on the interactions between the multiple sites of forebrain pain activity and the multiple ascending pathways activated by noxious stimuli will illuminate the complex representation of pain in the forebrain.
The references for this chapter can be found at www.expertconsult.com.
References
Albe-Fessard D., Boivie J., Grant G., et al. Labelling of cells in the medulla oblongata of the monkey after injections of horseradish peroxidase in the thalamus. Neuroscience Letters. 1975;1:75–80.
Al-Chaer E.D., Feng Y., Willis W.D. A role for the dorsal column in nociceptive visceral input into the thalamus of primates. Journal of Neurophysiology. 1998;79:3143–3150.
Ammons W.S. Characteristics of spinoreticular and spinothalamic neurons with renal input. Journal of Neurophysiology. 1987;58:480–495.
Andrew D., Craig A.D. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neuroscience. 2001;4:72–77.
Andrew D., Krout K., Craig A.D. Differentiation of lamina I spinomedullary and spinothalamic neurons in the cat. Journal of Comparative Neurology. 2003;458:257–271.
Apkarian A.V., Bushnell M.C., Treede R.D., et al. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–484.
Bannatyne B.A., Edgley S.A., Hammar I., et al. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. Journal of Neuroscience. 2006;26:2871–2880.
Basbaum A.I., Fields H.L. Endogenous pain control mechanisms: review and hypothesis. Annals of Neurology. 1978;4:451–462.
Berkley K.J., Budell R.J., Blomqvist A., et al. Output systems of the dorsal column nuclei in the cat. Brain Research Bulletin. 1986;11:199–225.
Bernard J.F., Alden M., Besson J- M. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. Journal of Comparative Neurology. 1993;329:201–229.
Bernard J.F., Besson J.M. The spino(trigemino) pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. Journal of Neurophysiology. 1990;63:473–490.
Bernard J.F., Villanueva L., Carroué J., et al. Efferent projections from the subnucleus reticularis dorsalis (SRD): a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience Letters. 1990:116–262.
Bester H., Beggs S., Woolf C.J. Changes in tactile stimuli–induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. Journal of Comparative Neurology. 2000;428:45–61.
Bingel U., Quante M., Knab R., et al. Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. NeuroImage. 2003;18:740–748.
Blomqvist A., Berkley K.J. A re-examination of the spino-reticulo-diencephalic pathway in the cat. Brain Research. 1992;579:17–31.
Blomqvist A., Zhang E.-T., Craig A.D. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the VMpo nucleus, in the human thalamus. Brain. 2000;123:601–619.
Boivie J. Anatomic and physiologic features of the spino-cervico-thalamic pathway. In: Macchi G., Rustioni A., Spreafico R., eds. Somatosensory integration in the thalamus. Amsterdam: Elsevier; 1983:63–106.
Broton J.G., Hu J.W., Sessle B.J. Effects of temporomandibular joint stimulation on nociceptive and nonnociceptive neurons of the cat`s trigeminal subnucleus caudalis (medullary dorsal horn). Journal of Neurophysiology. 1988;59:1575–1589.
Burstein R., Falkowsky O., Borsook D., et al. Distinct lateral and medial projections of the spinohypothalamic tract of the rat. Journal of Comparative Neurology. 1996;373:549–574.
Burton H., Craig A.D., Jr., Poulos D.A., et al. Efferent projections from temperature sensitive recording loci within the marginal zone of the nucleus caudalis of the spinal trigeminal complex in the cat. Journal of Comparative Neurology. 1979;183:753–788.
Bushnell M.C., Duncan G.H. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Experimental Brain Research. 1989;78:415–418.
Bushnell M.C., Duncan G.H., Hofbauer R.K., et al. Pain perception: is there a role for primary somatosensory cortex? Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7705–7709.
Bushnell M.C., Duncan G.H., Tremblay N. Thalamic VPM nucleus in the behaving monkey. I. Multimodal and discriminative properties of thermosensitive neurons. Journal of Neurophysiology. 1993;69:739–752.
Chamberlin N.L., Saper C.B. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. Journal of Comparative Neurology. 1992;326:245–262.
Chudler E.H., Sugiyama K., Dong W.K. Nociceptive responses in the neostriatum and globus pallidus of the anesthetized rat. Journal of Neurophysiology. 1993;69:1890–1903.
Clascá F., Llamas A., Reinoso-Suárez F. Insular cortex and neighboring fields in the cat: a redefinition based on cortical microarchitecture and connections with the thalamus. Journal of Comparative Neurology. 1997;384:456–482.
Cohen S.R., Melzack R. The habenula and pain: repeated electrical stimulation produces prolonged analgesia but lesions have no effect on formalin pain or morphine analgesia. Behavioural Brain Research. 1993;54:171–178.
Craig A.D. Pain mechanisms: labeled lines versus convergence in central processing. Annual Review of Neuroscience. 2003;26:1–30.
Craig A.D. Comparison of laminae I and V STT neurons in the monkey with human second pain sensation. Society for Neuroscience Abstract online. 2003;260:13.
Craig A.D. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. Journal of Comparative Neurology. 2004;477:119–148.
Craig A.D. Lamina I, but not lamina V, spinothalamic neurons exhibit responses that correspond with burning pain. Journal of Neurophysiology. 2004;92:2604–2609.
Craig A.D. Retrograde analyses of spinothalamic projections in the macaque monkey: input to ventral posterior nuclei. Journal of Comparative Neurology. 2006;499:965–978.
Craig A.D. Retrograde analyses of spinothalamic projections in the macaque monkey: input to the ventral lateral nucleus. Journal of Comparative Neurology. 2008;508:315–328.
Craig A.D. How do you feel—now? The anterior insula and human awareness. Nature Reviews. Neuroscience. 2009;10:59–70.
Craig A.D. The sentient self. Brain Structure & Function. 2010;214:563–577.
Craig A.D., Andrew D. Responses of spinothalamic lamina I neurons to repeated brief contact heat stimulation in the cat. Journal of Neurophysiology. 2002;87:1902–1914.
Craig A.D., Bushnell M.C. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265:252–255.
Craig A.D., Bushnell M.C., Zhang E.-T., et al. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770–773.
Craig A.D., Dostrovsky J.O. Medulla and thalamus. In: Wall P.D., Melzack R., eds. Textbook of pain. ed 4. Edinburgh: Churchill Livingstone; 1999:183–214.
Craig A.D., Dostrovsky J.O. Differences in the projection targets of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. Journal of Neurophysiology. 2001;86:856–870.
Craig A.D., Jr., Kniffki K.-D. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. Journal of Physiology (London). 1985;365:197–221.
Craig A.D., Zhang E.T. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. Journal of Comparative Neurology. 2006;499:953–964.
Craig A.D., Zhang E.T., Blomqvist A. Association of spinothalamic lamina I neurons and their ascending axons with calbindin-immunoreactivity in monkey and human. Pain. 2002;97:105–115.
Dado R.J., Katter J.T., Giesler G.J., Jr. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. I. Locations of antidromically identified axons in the thalamus and hypothalamus. Journal of Neurophysiology. 1994;71:959–980.
Davis K.D., Dostrovsky J.O. Responses of feline trigeminal spinal tract nucleus neurons to stimulation of the middle meningeal artery and sagittal sinus. Journal of Neurophysiology. 1988;59:648–666.
Davis K.D., Kiss Z.H.T., Luo L., et al. Phantom sensations generated by thalamic microstimulation. Nature. 1998;391:385–387.
Davis K.D., Kiss Z.H.T., Tasker R.R., et al. Thalamic stimulation-evoked sensations in chronic pain patients and in nonpain (movement disorder) patients. Journal of Neurophysiology. 1996;75:1026–1037.
Davis K.D., Lozano A.M., Manduch M., et al. Thalamic relay site for cold perception in humans. Journal of Neurophysiology. 1999;81:1970–1973.
Davis K.D., Tasker R.R., Kiss Z.H.T., et al. Visceral pain evoked by thalamic microstimulation in humans. Neuroreport. 1995;6:369–374.
Depaulis A., Keay K.A., Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Experimental Brain Research. 1992;90:307–318.
Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306.
Dostrovsky J.O., Craig A.D. Cooling-specific spinothalamic neurons in the monkey. Journal of Neurophysiology. 1996;76:3656–3665.
Dostrovsky J.O., Manduch M., Davis K.D., et al. Thalamic stimulation-evoked pain and temperature sites in pain and non-pain patients. In: Devor M., Rowbotham M.C., Wiesenfeld-Hallin Z., eds. Proceedings of the 9th World Congress on Pain. Seattle: IASP Press; 2000:419–425.
Ducreux D., Attal N., Parker F., et al. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129:963–976.
Feil K., Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kölliker–Fuse nuclei. Journal of Comparative Neurology. 1995;353:506–528.
Fields H.L. Pain. New York: McGraw-Hill; 1987.
Frot M., Mauguiere F. Dual representation of pain in the operculo-insular cortex in humans. Brain. 2003;126:438–450.
Ganchrow D. Intratrigeminal and thalamic projections of nucleus caudalis in the squirrel monkey (Saimiri sciureus): a degeneration and autoradiographic study. Journal of Comparative Neurology. 1978;178:281–312.
Gauriau C., Bernard J.F. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. Journal of Comparative Neurology. 2004;468:24–56.
Giesler G.J., Jr., Yezierski R.P., Gerhart K.D., et al. Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. Journal of Neurophysiology. 1981;46:1285–1308.
Gorman A.L., Yu C.G., Ruenes G.R., et al. Conditions affecting the onset, severity and progression of a spontaneous pain-like behavior following excitotoxic spinal cord injury. Journal of Pain. 2001;2:229–240.
Guldin W.O., Grusser O.J. Is there a vestibular cortex? Trends in Neuroscience. 1998;21:254–259.
Gybels J.M., Sweet W.H., Neurosurgical treatment of persistent pain. Physiological and pathological mechanisms of human pain. Pain and headache, vol. 11. Basel: Karger; 1989.
Hassler R., Riechert T. Klinische und anatomische Befunde bei stereotaktischen Schmerzoperationen im thalamus. Archives of Psychiatry. 1959;200:93–122.
Head H., Holmes G. Sensory disturbances from cerebral lesions. Brain. 1911;34:102–254.
Helmstetter F.J., Bellgowan P.S., Tershner S.A. Inhibition of the tail flick reflex following microinjection of morphine into the amygdala. Neuroreport. 1993;4:471–474.
Henderson L.A., Rubin T.K., Macefield V.G. Within-limb somatotopic representation of acute muscle pain in the human contralateral dorsal posterior insula. Human Brain Mapping. 2011;32:1592–1601.
Hermanson O., Blomqvist A. Subnuclear localization of FOS-like immunoreactivity in the parabrachial nucleus after orofacial nociceptive stimulation of the awake rat. Journal of Comparative Neurology. 1997;387:114–123.
Hutchison W.D., Davis K.D., Lozano A.M., et al. Pain-related neurons in the human cingulate cortex. Nature Neuroscience. 1999;2:403–405.
Hutchison W.D., Tsoukatos J., Dostrovsky J.O. Quantitative analysis of orofacial thermoreceptive neurons in the superficial medullary dorsal horn of the rat. Journal of Neurophysiology. 1997;77:3252–3266.
Iannetti G.D., Truini A., Romaniello A., et al. Evidence of a specific spinal pathway for the sense of warmth in humans. Journal of Neurophysiology. 2003;89:562–570.
Jeanmonod D., Magnin M., Morel A. A thalamic concept of neurogenic pain. In: Gebhart G.F., Hammond D.L., Jensen T.S., eds. Proceedings of the 7th Congress on Pain. Seattle: IASP Press; 1994:767–787.
Jones E.G. The thalamus. New York: Plenum; 1985.
Jones E.G. Correlation and revised nomenclature of ventral nuclei in the thalamus of human and monkey. Stereotactic and Functional Neurosurgery. 1990;54–55:1–20.
Kaas J.H., Nelson R.J., Sur M., et al. The somatotopic organization of the ventroposterior thalamus of the squirrel monkey, Saimiri sciureus. Journal of Comparative Neurology. 1984;226:111–140.
Kawakita K., Dostrovsky J.O., Tang J.S., et al. Responses of neurons in the rat thalamic nucleus submedius to cutaneous, muscle and visceral nociceptive stimuli. Pain. 1993;55:327–338.
Keltner J.R., Furst A., Fan C., et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. Journal of Neuroscience. 2006;26:4437–4443.
Khasabov S.G., Rogers S.D., Ghilardi J.R., et al. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. Journal of Neuroscience. 2002;22:9086–9098.
Kuru M. The sensory paths in the spinal cord and brain stem of man. Tokyo: Sogensya; 1949.
Lee J.I., Dougherty P.M., Antezana D., et al. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. Journal of Comparative Neurology. 1999;410:541–555.
Lenz F.A., Dougherty P.M., Pain processing in the human thalamus. Steriade M., Jones E.G., McCormick D.A., eds. Thalamus, vol. II, Experimental and clinical aspects. Amsterdam: Elsevier; 1997:617–652.
Lenz F.A., Seike M., Richardson R.T., et al. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. Journal of Neurophysiology. 1993;70:200–212.
Light A.R. The initial processing of pain and its descending control: spinal and trigeminal systems. Basel: Karger; 1992.
Loewy A.D., Spyer K.M. Central regulation of autonomic functions. New York: Oxford University Press; 1990.
Mackel R., Iriki A., Brink E.E. Spinal input to thalamic VL neurons: evidence for direct spinothalamic effects. Journal of Neurophysiology. 1992;67:132–144.
Maixner W., Dubner R., Kenshalo D.R., Jr., et al. Responses of monkey medullary dorsal horn neurons during the detection of noxious heat stimuli. Journal of Neurophysiology. 1989;62:437–449.
Mantyh P.W. Connections of midbrain periaqueductal gray in the monkey. I: ascending efferent projections. Journal of Neurophysiology. 1983;49:567–581.
Mayer D.J., Price D.D., Becker D.P. Neurophysiological characterization of the anterolateral spinal cord neurons contributing to pain perception in man. Pain. 1975;1:51–58.
Mazzola L., Isnard J., Peyron R., et al. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146:99–104.
McMahon S.B., Wall P.D. Descending excitation and inhibition of spinal cord lamina I projection neurons. Journal of Neurophysiology. 1988;59:1204–1219.
Melzack R., Casey K.L. Sensory, motivational, and central control determinants of pain. In: Kenshalo D.R., ed. The skin senses. Springfield, IL: Thomas; 1968:423–443.
Meng I.D., Hu J.W., Benetti A.P., et al. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. Journal of Neurophysiology. 1997;77:43–56.
Meyers D.E.R., Snow P.J. The morphology of physiologically identified deep spinothalamic tract cells in the lumbar spinal cord of the cat. Journal of Physiology (London). 1982;329:372–388.
Morin C., Bushnell M.C., Luskin M.B., et al. Disruption of thermal perception in a multiple sclerosis patient with central pain. Clinical Journal of Pain. 2002;18:191–195.
Musil S.Y., Olson C.R. Organization of cortical and subcortical projections to anterior cingulate cortex in the cat. Journal of Comparative Neurology. 1988;272:203–218.
Nathan P.W., Smith M.C., Clinico-anatomical correlation in anterolateral cordotomy. Bonica J.J., ed. Advances in pain research and therapy, vol. 3. New York: Raven Press; 1979:921–926.
Nichols M.L., Allen B.J., Rogers S.D., et al. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561.
Norrsell U., Craig A.D. Behavioral thermosensitivity after lesions of thalamic target areas of a thermosensory spinothalamic pathway in the cat. Journal of Neurophysiology. 1999;82:611–625.
Pagni C.A. Central pain: a neurosurgical challenge. Turin: Ediziona Minerva Medica; 1998.
Perl E.R., Pain and nociception. Darian-Smith I., ed. Handbook of physiology, section 1, The nervous system, vol. III. Bethesda, MD: Sensory processes. American Physiological Society; 1984:915–975.
Perl E.R. Why are selectively responsive and multireceptive neurons both present in somatosensory pathways? In: Ottoson D., ed. Somatosensory mechanisms. New York: Plenum; 1984:141–161.
Poggio G.F., Mountcastle V.B. A study of the functional contributions of the lemniscal and spinothalamic systems to somatic sensibility. Bulletin of the Johns Hopkins Hospital. 1960;106:266–316.
Price D.D. Psychological and neural mechanisms of pain. New York: Raven Press; 1988.
Rainville P., Duncan G.H., Price D.D., et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971.
Ralston H.J., III., Ralston D.D. Medial lemniscal and spinal projections to the macaque thalamus: an electron microscopic study of differing GABAergic circuitry serving thalamic somatosensory mechanisms. Journal of Neuroscience. 1994;14:2485–2502.
Rausell E., Jones E.G. Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. Journal of Neuroscience. 1991;11:226–237.
Ray J.P., Price J.L. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1993;337:1–31.
Sadikot A.F., Parent A., François C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. Journal of Comparative Neurology. 1992;315:137–159.
Sato A., Schmidt R.F. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiological Reviews. 1973;53:916–947.
Schmahmann J.D., Leifer D. Parietal pseudothalamic pain syndrome: clinical features and anatomic correlates. Archives of Neurology. 1992;49:1032–1037.
Schouenborg J., Weng H.R., Kalliomäki J., et al. A survey of spinal dorsal horn neurones encoding the spatial organization of withdrawal reflexes in the rat. Experimental Brain Research. 1995;106:19–27.
Sessle B.J. The neurobiology of facial and dental pain: present knowledge, future directions. Journal of Dental Research. 1987;66:962–981.
Sessle B.J. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Critical Reviews in Oral Biology and Medicine. 2000;11:57–91.
Shi T., Stevens R.T., Tessier J., et al. Spinothalamocortical inputs nonpreferentially innervate the superficial and deep cortical layers of SI. Neuroscience Letters. 1993;160:209–213.
Siegfried J. Stimulation of thalamic nuclei in human: sensory and therapeutical aspects. In: Besson J.-M., Guilbaud G., Peschanski M., eds. Thalamus and pain. Amsterdam: Excerpta Medica; 1987:271–278.
Staud R., Craggs J.G., Perlstein W.M., et al. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. European Journal of Pain. 2008;12:1078–1089.
Stepniewska I., Sakai S.T., Qi H.X., et al. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: a potential substrate for parkinsonian tremor. Journal of Comparative Neurology. 2002;455:378–395.
Strassman A., Mason P., Moskowitz M., et al. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Research. 1986;379:242–250.
Strigo I.A., Duncan G.H., Boivin M., et al. Differentiation of visceral and cutaneous pain in the human brain. Journal of Neurophysiology. 2003;89:3294–3303.
Tasker R.R. Stereotaxic surgery. In: Wall P.D., Melzack R., eds. Textbook of pain. Edinburgh: Churchill Livingstone; 1984:639–655.
Tommerdahl M., Delemos K.A., Vierck C.J., Jr., et al. Anterior parietal cortical response to tactile and skin-heating stimuli applied to the same skin site. Journal of Neurophysiology. 1996;75:2662–2670.
Treede R.D., Kenshalo D.R., Gracely R.H., et al. The cortical representation of pain. Pain. 1999;79:105–111.
Villanueva L., Desbois C., Le Bars D., et al. Organization of diencephalic projections from the medullary subnucleus reticularis dorsalis and the adjacent cuneate nucleus: a retrograde and anterograde tracer study in the rat. Journal of Comparative Neurology. 1998;390:133–160.
Villanueva L., Nathan P.W. Multiple pain pathways. In: Devor M., Rowbotham M.C., Wiesenfeld-Hallin Z., eds. Proceedings of the 9th World Congress on Pain. Seattle: IASP Press; 2000:371–386.
Wall P.D. Dorsal horn electrophysiology. In: Iggo A., ed. Handbook of sensory physiology—somatosensory system. Berlin: Springer-Verlag, 1973.
Westlund K.N., Bowker R.M., Ziegler M.G., et al. Origins and terminations of descending noradrenergic projections to the spinal cord of monkey. Brain Research. 1984;292:1–16.
White J.C., Sweet W.H. Pain and the neurosurgeon: a forty-year experience. Springfield: Thomas; 1969.
Wiberg M., Westman J., Blomqvist A. Somatosensory projection to the mesencephalon: an anatomical study in the monkey. Journal of Comparative Neurology. 1987;264:92–117.
Willis W.D. The pain system. Basel: Karger; 1985.
Wilson L.B., Andrew D., Craig A.D. Activation of spinobulbar lamina I neurons by static muscle contraction. Journal of Neurophysiology. 2002;87:1641–1645.
Yezierski R.P. Spinomesencephalic tract: projections from the lumbosacral spinal cord of the rat, cat, and monkey. Journal of Comparative Neurology. 1988;267:131–146.
Yezierski R.P., Schwartz R.H. Response and receptive-field properties of spinomesencephalic tract cells in the cat. Journal of Neurophysiology. 1986;55:76–96.
Yoshida A., Dostrovsky J.O., Chiang C.Y. The afferent and efferent connections of the nucleus submedius in the rat. Journal of Comparative Neurology. 1992;324:115–133.
Young R.F. Effect of trigeminal tractotomy on dental sensation in humans. Journal of Neurosurgery. 1982;56:812–818.
Zhang S., Tang J.S., Yuan B., et al. Inhibitory effects of glutamate-induced activation of thalamic nucleus submedius are mediated by ventrolateral orbital cortex and periaqueductal gray in rats. European Journal of Pain. 1998;2:153–163.