Assessment of Pain Beliefs, Coping, and Function
Introduction
People display and describe pain in many different ways, from stoical minimization to neurotic exaggeration. Although exceptions do occur, pain, particularly chronic pain, alters daily life, often changing how the person spends his or her time. As pain continues, the impact of the patient’s pain extends to family, social, and work interactions, often challenging the sufferer’s sense of self and personal worth. Even with acute pain—the pain of childbirth or pain following surgery—the meaning of the pain and the emotional dimension of pain influence the description and expression of the pain, as well as what treatments the patient is willing to pursue. Given the prevalence of pain, the chronicity of many painful conditions, and the aging population, pain clinicians and researchers alike need to pay close attention to these other responses to pain—emotional, cognitive, and social responses—in addition to the pathophysiology of the condition underlying the persistent pain. Simplistic interpretations of behavior in the clinical setting can lead to errors in assessment and treatment planning (Main and Waddell 1998). Although an extensive literature demonstrates the role of these factors in predicting persistent disabling low back pain (Chou and Shekelle 2010), more and more evidence indicates that these psychosocial factors predict persistent postoperative pain (Hinrichs-Rocker et al 2009, Katz and Seltzer 2009), as well as response to pain treatments (Celestin et al 2009). Unfortunately, assessment of the psychosocial factors discussed in this chapter is often relegated to the last resort after organic approaches and treatments have been exhausted. Ideally, assessment of at least some of the domains addressed in this chapter should be standard in all pain settings, including basic research studies involving human subjects, because the impact of these factors on pain and function is consistent and compelling and continues to elucidate individual differences in the experience of pain and its effect on daily living (see Chapters 21, 22, and 23 for assessment of pain in different age groups).
Pain Beliefs and Attitudes
Pain beliefs—how we and our patients understand pain and its causes and treatments; the meaning of pain to the patient, family, employer, and treatment team; and our attitudes toward disability and the social and interpersonal effects of pain—can be powerful determinants of a person’s response to pain and the development of pain-related disability. The breadth of concepts and measures currently available to assess pain beliefs and attitudes attests to the value of these constructs in extending our understanding of pain. The range includes patients’ concerns about reporting pain and using pain medications (Ward et al 1993) to acceptance of pain (Thompson and McCraken 2011). These beliefs and attitudes affect the experience of both acute and chronic pain and may increase the risk that acute pain becomes chronic. Though activated by the experience of pain, these beliefs are seen across cultures, are influential during childhood, and are not simply artifacts of chronicity or disability. DeGood and Cook (2011) provide an excellent, detailed discussion of the range of pain beliefs examined in chronic pain, particularly measures of beliefs that are pain specific rather than general personality dimensions. The select group of measures presented here has been chosen because of their common use in both clinical and research settings.
General Measures of Pain Beliefs and Attitudes
The Survey of Pain Attitudes (SOPA; Jensen et al 1994b) measures attitudes and beliefs that pain can be controlled, that emotions influence pain, that pain signals harm, and that a medical cure will be found, as well as attitudes and beliefs about using pain medications, about disability, and about needing care and attention. The original 57-item scale has been shortened to 35 items and shows excellent psychometric properties that are comparable to scores generated by the original scale, with the exception of the harm subscale (Jensen et al 1999). Validation of many of the SOPA subscales has accumulated over a number of studies. Cancer patients reporting strong beliefs about using pain medications and low perceptions of controlling pain report greater adherence in using pain medications (Lai et al 2002). Following multidisciplinary treatment, beliefs that pain is disabling and harmful decrease and perceptions of pain as controllable increase (Jensen et al 1994a), although these gains are not sustained in about 30% of patients (Jensen et al 2007). To facilitate broad use of the SOPA measure in the context of a comprehensive assessment, reliable and valid one- and two-item subscales have been identified (Jensen et al 2003).
Specific Measures of Pain Beliefs and Attitudes
Self-efficacy, or confidence that one can cope with pain and its impact on daily life, is one of the most consistent predictors of pain-related outcomes (Benyon et al 2010), although it is not consistently related to pain intensity (Nicholas 2007, Benyon et al 2010). Individuals with high self-efficacy report lower levels of disability and depression, and a recent review found that high self-efficacy predicted which patients would benefit most from self-management interventions (Miles et al 2011). Measurement of self-efficacy generally includes multiple dimensions, with most scales including specific types of behavior, as well as broader concepts such as “coping with pain.” Since most measures do not instruct the patient to consider the level of pain when rating confidence in performing a task, Nicholas (2007) developed the Pain Self-Efficacy Questionnaire, which includes confidence ratings of general activities (such as “socialize with my friends or family members as often as I used to do despite the pain”). Other commonly used scales include the Chronic Pain Self Efficacy Scale (Anderson et al 1995) and the Arthritis Self Efficacy Scale (Lorig et al 1989). The Arthritis Self Efficacy Scale has been the most widely studied and shows consistent relationships with pain, fatigue, and physical disability in patients with arthritis (Prindahl et al 2011).
Acceptance
In the past decade, acceptance of pain (McCracken 1998, McCracken et al 2004) has received increasing attention and empirical support as a psychological process associated with better outcomes in patients with chronic pain (Thompson and McCracken 2011), including less avoidance and disability (McCracken et al 2007). The strength of these associations has contributed to the reshaping of cognitive–behavioral therapies for pain to incorporate mindfulness, acceptance, values, and committed action with the goal of enhancing psychological flexibility (Vowels et al 2009, Veehof et al 2011), although the specific role of acceptance as opposed to increased perception of control over pain remains unclear in the psychological treatment of chronic pain (Wetherell et al 2011). The original Chronic Pain Acceptance Questionnaire (McCracken 1998) was later revised to include 20 items that include Measurement of Pain Willingness and Activity Engagement (Vowels et al 2008).
Fear Avoidance
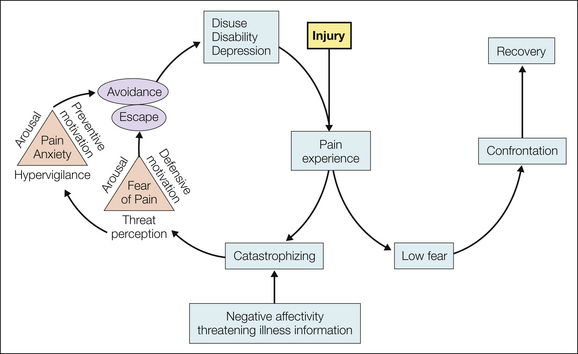
Because of the aversive nature of pain, conditioning of fear and then avoidance of certain events or activities, particularly those that evoke pain, can occur when pain becomes chronic. Distinct from the dysphoria or negative affectivity often seen with chronic pain, pain-related anxiety and fear contribute uniquely to adaptation. Vlaeyen and colleagues developed (1995) and later revised (Leeuw et al 2007) a cognitive–behavioral model that depicts a chain of events that can transpire in response to painful experiences brought on by a back injury (Fig. 24-1). Particularly in people who experience high levels of negative affectivity and perceive pain as threatening, pain can activate catastrophizing (a complex cognitive–emotional response to pain—see later), which then activates pain-related anxiety and fear of continued pain and leads to avoidance and attempts to escape from the pain and anxiety. People in this cycle may become less physically active and engaged in their daily life, show diminished muscle strength and restricted movements, and ultimately report functional limitations and depression (Leeuw et al 2007). When pain does not activate catastrophizing and the individual confronts the pain, recovery is more likely to occur. Fear-avoidance beliefs capture the dimension that pain is to be avoided rather than confronted. A rich literature demonstrates the disabling nature of these beliefs, largely in individuals with low back pain, and the relationship with disability is typically stronger than that with pain intensity. These beliefs may have broad applicability to many types of chronic musculoskeletal pain conditions, including fibromyalgia and osteoarthritis (Roelofs et al 2011), as well as long-term postoperative outcomes (Archer et al 2011). Fear-avoidance beliefs are most commonly measured with the Tampa Scale of Kinesiophobia (unpublished report by Miller et al 1991) or the Fear-Avoidance Beliefs Questionnaire (Waddell et al 1993), which is specific to back pain. Items for the 17-item Tampa Scale of Kinesiophobia are provided in an appendix to Vlaeyan and colleagues’ paper validating the Dutch translation (1995), and recent work has validated a shorter (11 item) scale with two primary factors: somatic focus and activity avoidance (Roelofs et al 2007).
Pain Coping
How individuals cope with chronic pain is a consistent predictor of various dimensions of the experience of pain, including pain severity, disability and physical function, and psychological adaptation (Jensen et al 1991, Boothby et al 1999), although evidence supporting the adaptive value of any individual coping strategy is weak (Benyon et al 2010). The conceptual issues in measuring coping and determining the maladaptive or adaptive nature of any individual or composite measure of coping are complex (Boothby et al 1999). Consider the possibility that a superficially maladaptive coping strategy may serve a subtle but valuable role in the individual’s life. For example, rest may be unproductive and even harmful and can result in deconditioning and physical incapacity when used to cope with months or years of low back pain. However, rest may play an important, adaptive role for that individual in that it can enhance a sense of control over the pain by providing pain relief and predictability or providing quiet time away from the stress of a disrupted family life. Any pain-coping strategy may be adaptive in one domain and maladaptive in another (Tan et al 2011).
General Measures of Pain Coping
Current technologies for measuring pain-coping strategies have strengths and weaknesses. The most frequently used measures of pain-coping strategies in the chronic pain literature are the Coping Strategies Questionnaire (CSQ; Rosenstiel and Keefe 1983), which focuses primarily on cognitive coping strategies; the Vanderbilt Pain Management Inventory (Brown and Nicassio 1987), which measures active and passive pain-coping strategies; and the Chronic Pain Coping Inventory (CPCI; Jensen et al 1995), which provides a measure of specific behavioral pain-coping strategies—rest, relaxation, exercise and stretching, and seeking social support—to complement the primarily cognitive focus of the CSQ. A 42-item scale has been developed to facilitate repeated assessments (Romano et al 2003). One- and two-item subscales for both the CSQ and the CPCI have been published (Jensen et al 2003).
In recent discussions, theoreticians have maligned the use of summary checklists to assess this complex construct, which is thought to change rapidly over time as the individual’s life transpires. Daily diaries (Lefebvre and Keefe 2002) illuminate the complexities of coping with pain and allow detailed examination of the impact of a coping attempt over a period of hours or days. Revelations that paper diaries are frequently not completed in a timely fashion (Stone et al 2002) support the value of extending modern electronic monitoring devices to the assessment of coping (Peters et al 2000, Sorbi et al 2006). These technologies are extremely valuable to the researcher and may be positively perceived by busy clinicians, although they are not consistently integrated into treatment decision making (Marceau et al 2010). Even though diaries are useful in many ways, most of our knowledge of how individuals cope with pain, particularly chronic pain, derives from checklist methods that despite their limitations, have provided a wealth of information about coping with pain.
Specific Dimensions of Pain Coping
Catastrophizing, a negative emotional–cognitive–attitudinal response to pain, is a pain-coping strategy that has received a great deal of attention in the psychosocial pain literature (e.g., Haythornthwaite 2009, Edwards et al 2011), primarily because of its consistently strong relationship with poorer functioning. Catastrophizing correlates with pain, pain-related interference and disability, and depressive symptoms across studies, laboratories, and patient groups (Sullivan et al 2001, Marceau et al 2007). Although most data on catastrophizing are cross-sectional, longitudinal analyses support its role in determining the level of pain, pain-related function, and depressive symptoms months later (Haythornthwaite et al 2003, Sullivan et al 2011), and an isolated study suggests a beneficial effect of catastrophizing on pain-related function and depressive symptoms in the months immediately following an amputation (Jensen et al 2002). Fairly consistently, catastrophizing is reduced following multidisciplinary or behavioral treatment, and longitudinal analyses suggest that reductions in catastrophizing occur before improvements in other outcomes (Miller et al 1991, Burns et al 2003).
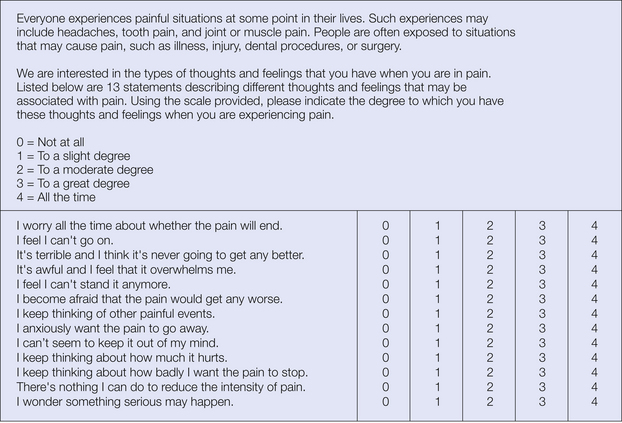
Two measures of catastrophizing are most widely used: the catastrophizing subscale of the CSQ (Rosenstiel and Keefe 1983) and the Pain Catastrophizing Scale (Sullivan et al 1995). The CSQ consists of 50 items that assess how the individual copes with pain, including a number of primary scales (coping self-statements, diverting attention, ignoring sensations, reinterpreting sensations, praying and hoping, increasing activity, and catastrophizing). Although composite coping scores are often used, individual scales provide more information and are generally recommended (Jensen and Karoly 1992). The catastrophizing subscale of the CSQ includes six items (e.g., “I worry all the time about whether the pain will end”) measuring the general construct of helplessness. Multiple factor analyses of the CSQ support the validity of the catastrophizing subscale in particular (e.g., Robinson et al 1997). The 13-item Pain Catastrophizing Scale (Fig. 24-2) includes a rumination subscale (e.g., “I can’t seem to keep it out of my mind”) and a magnification subscale (e.g., “I become afraid that the pain will get worse”), in addition to the original CSQ subscale measuring helplessness. This factor structure has been confirmed in later analyses (Van Damme et al 2002), although psychometric analysis of a racially diverse group of workers’ compensation claimants suggested a two-factor structure in the African American group (Chibnall and Tait 2005).
Distracting Attention, Ignoring Pain, or Distancing
Distraction and ignoring pain are frequently measured by the CSQ (distraction, “I try to think of something pleasant”; ignoring pain, “I don’t think about the pain”). The findings to date on distraction as a pain-coping strategy are mixed; distraction may be more useful for managing acute pain than chronic pain (Boothby et al 1999). Although music distraction may not relieve severe acute pain (Haythornthwaite et al 2001), a growing literature supports virtual reality distraction as an effective analgesic for burn pain (Sharar et al 2007) and other painful medical procedures (Mahrer and Gold 2009). Ignoring pain, though conceptually related to distraction and diverting attention, has typically been measured as a separate dimension of pain coping. Similar to distraction, ignoring pain occasionally displays significant associations with pain and pain-related functioning (Boothby et al 1999).
The reinterpreting pain sensations subscale of the CSQ—which includes items such as “I try to feel distant from the pain, almost as if the pain was in someone else’s body”—has been reconceptualized as a slightly broader factor measuring distancing from pain and includes some items from the original reinterpreting pain sensations subscale and one item from the diverting attention subscale (Robinson et al 1997). As is the case with distraction and ignoring pain sensations, reinterpreting pain sensations shows no or at best small correlations with various outcomes, with relatively few studies showing any consistent benefit with this strategy. Overall, these strategies may have some benefit in managing acute pain but appear to provide little benefit in managing chronic pain (Boothby et al 1999).
Task Persistence
The extent to which people continue their usual daily activities as a strategy to manage pain has been labeled task persistence (Jensen et al 1995). Task persistence is one of the few pain-coping strategies that has fairly consistently been associated with positive outcomes, including disability and depression (Jensen et al 1995, Tan et al 2005, Garcia-Campayo et al 2007, Jensen et al 2011), and it is one of the more frequently endorsed pain-coping strategies (Ersek et al 2006). These findings derive from use of the task persistence subscale of the CPCI (Jensen et al 1995, 2003; Romano et al 2003).
Coping Self-Statements
Some studies have found positive self-statements to be associated with adaptive functioning (e.g., Haythornthwaite et al 1998). As with other pain-coping strategies, the positive impact of coping self-statements is not consistent across studies and outcome domains. Reviews conclude that positive coping self-statements do not generally show a consistent relationship with reduced pain and improved functioning (Boothby et al 1999, DeGood and Cook 2011). However, these coping self-statements are an integral component of most psychological interventions for pain management and show change with treatment. These positive coping statements can be measured by using the coping self-statements subscales of either the CSQ (e.g., “I tell myself that I can overcome the pain”) or the CPCI (e.g., “Told myself things will get better”). Both scales have one- and two-item versions (Jensen et al 2003).
Spirituality and Religiosity
Prayer is one the most frequently endorsed pain-coping strategies, and although spiritual and religious beliefs contribute to many positive types of coping behavior, these beliefs can also direct negative coping behavior (Pargament et al 2000, Wachholtz and Pearce 2009). The most commonly used measure of religiosity as a pain-coping strategy is the praying and hoping scale from the CSQ. This domain of coping is measured with items that generally have a passive focus (e.g., “I pray to God it won’t last long”). Higher scores on this scale are generally correlated with greater pain severity, disability, and distress (Andersson 2008), although the results are inconsistent (Boothby et al 1999). The correlational nature of this research does not illuminate whether people pray and hope more in response to difficult times or whether this coping strategy contributes actively to poorer adaptation to chronic pain (Boothby et al 1999). Patients with rheumatoid arthritis have reported large day-to-day variation in the frequency and nature of daily religious/spiritual coping, and some dimensions of religious/spiritual pain coping were associated with higher positive and lower negative mood (Keefe et al 2001). Research on acceptance of chronic pain suggests that spirituality may be a key dimension of accepting pain when the spiritual dimension is presented as positive and guiding (Risdon et al 2003). Interest in the health benefits of spirituality/religiosity (Hill and Pargament 2003) and the frequent use of spiritual/religious coping strategies for managing pain suggest that continued, expanded investigation of this domain might be informative in future studies. For this to be fruitful, new scales will have to be developed or adapted from other areas of investigation (Pargament et al 2000).
Function
The concept of function as it relates to pain is complex and multidimensional, including at a minimum the impact of pain on daily activities and the individual’s level of function in emotional, occupational, and social roles. In its broadest conceptualization, function also encompasses types of pain behavior, health-related quality of life (HRQoL), disability, and health care utilization. Some of the earliest assessments of physical activity and function relied on diaries of “uptime,” or the amount of time patients spend sitting, standing, or walking (Fordyce et al 1973). Though integral to the early behavioral analysis of pain and evaluation of rehabilitative treatments, unfortunately, such diaries are not frequently used in the modern-day multidisciplinary clinic. Recent developments in using electronic devices to quantify activity level may prove useful in both research and clinical settings, either to monitor (Bussman et al 1998) or increase physical activity (Talbot et al 2003) or to track increases associated with treatment of pain (Agarwal et al 2007). In some instances, measurement of actual physical function has been performed in the clinical office (Harding et al 1994), and the use of similar measures in epidemiological studies (Fried et al 2001) suggests that such measures may be feasible for monitoring physical capabilities in clinical settings. However, widespread use of these direct measurements continues to be hampered largely by issues of feasibility.
For these reasons, all the widely used measures of function involve self-report by the patient. Many of these scales measure the patient’s perception of how much pain interferes with a category of activity (e.g., recreational activity or work) rather than measuring how often the individual engages in a specific behavior (e.g., walking, sitting, or climbing stairs) or how much pain interferes with specific types of behavior. Some disease-specific measures, such as the Western Ontario and McMaster (WOMAC) Universities Osteoarthritis Index for knee osteoarthritis (Bellamy 2005), do measure the impact of pain on specific types of behavior that elicit pain, such as walking up stairs. The specificity of this latter approach is a clear advantage of these scales but not appropriate for other painful conditions that do not affect that particular activity in most patients.
This section includes a discussion of some of the more commonly used scales that measure pain-related function, psychosocial function including mood, and HRQoL. The measures included here were chosen because of their use in many different painful conditions but may not be appropriate when a disease-specific measure is available, commonly used, or recommended by consensus panels (e.g., Bellamy et al 1997).
Pain-Related Function
Pain-related function is one of the most important outcomes in the pain literature, second only to pain severity, with which it is highly correlated. Pain-related function typically includes ratings by patients of the extent to which pain causes disability or interferes with activities. Most measures of pain-related function assess multiple domains of function, including daily activities, work, socializing, and recreation, and many include ratings of the impact of pain on mood and in most instances include ratings of the impact of pain on sleep. Thus, many of these measures confound the assessment of physical and psychosocial function.
Multidimensional Pain Inventory
The theoretically driven West Haven–Yale Multidimensional Pain Inventory (Kerns et al 1985) was slightly expanded and became the Multidimensional Pain Inventory (MPI; Rudy 1989). Widespread use of this scale, which has been translated into numerous languages, has yielded an extensive literature on the psychosocial aspects of pain. The scale includes 12 subscales, many of which measure psychosocial function, including social support, negative mood, life control, specific responses from the significant other (solicitous, distracting, or punishing responses to pain), and activity level. Though used widely, there is some debate regarding the extent to which the original subscales are independent (Deisinger et al 2001). Consistent with its theoretical underpinnings, a prominent dimension of the MPI is measurement of the patient’s perception of the social environment—both support from significant others and specific responses that a significant other might have to pain (e.g., attention and caretaking, distraction, or punishment).
The perceived interference scale is embedded in the first section of the instrument and includes 11 items that assess interference or change in satisfaction in day-to-day activities, sleep, work, social and recreational activities, marriage and family activities, household chores, and friendships (Rudy 1989). The instructions do not include any specific time frame, and most items do not specify a time frame. Though recommended by the IMMPACT group as a measure of physical function (Dworkin et al 2005), the MPI interference scale is really a measure of combined physical and psychosocial function. A second scale from the MPI that deserves consideration as a potential measure of pain-related function is the general activity subscale. This scale is typically used as a summary of four activity scales in which social activities, activities away from home, household chores, and outdoor work are assessed with 18 items. Validation of these two subscales is provided by an extensive literature from multiple countries and many different types of pain conditions documenting expected relationships with other measures of interference, activity level, disability, and function. The MPI interference subscale has been validated against daily diary ratings of interference caused by pain, and the same study showed low correlations between daily activities and the MPI general activity subscale. Bicycle ergometer performance correlates with the MPI general activity subscale (Lousberg et al 1999). Improvements in treadmill capacity and reductions in downtime correlated with increases in general activity in patients with musculoskeletal pain enrolled in a multidisciplinary rehabilitation program (Burns et al 1998). Further construct validation work using the MPI has provided important information about the impact of negative mood on pain-related function, including both interference and general activity, and both scales show validity in predicting future outcomes of acute pain.
These scales have been used extensively to measure outcomes of multidisciplinary rehabilitation, psychological treatments of chronic pain, and even a brief (1.5 day) intensive treatment of fibromyalgia (Worrel et al 2001). Importantly, the MPI interference scale may have greater ability to detect changes in pain-related physical function than other measures such as the Oswestry scale have (Turk et al 1998). However, not all studies show the expected changes when using these scales (Nielson et al 1992). Randomized, controlled clinical trials have used these scales extensively to document improvements following psychological and rehabilitative treatments in a number of chronic pain populations. The results of these trials are generally mixed (e.g., Raja et al 2002), and few studies demonstrate a treatment effect on the MPI general activity subscale.
Brief Pain Inventory—Interference Scale
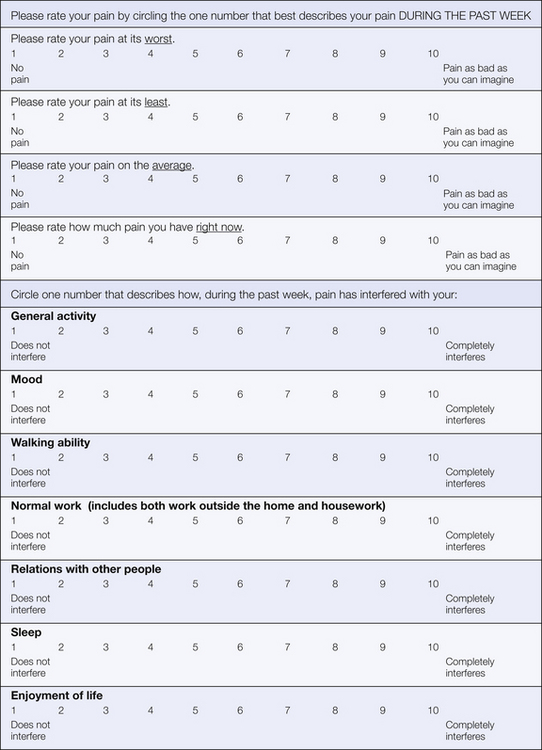
The Brief Pain Inventory (BPI; Anderson et al 2001) was originally developed and has been used extensively to measure pain severity and pain-related interference in patients with cancer (Jensen 2003). It has been translated into many different languages and its use extended to a wide range of chronically painful conditions (Dworkin et al 2005). The BPI interference subscale includes seven areas: general activity, mood, walking ability, normal work including work outside the home and housework, relationships with other people, enjoyment of life, and sleep (Fig. 24-3; Cleeland and Ryan 1994). Some investigators have added additional domains: self-care, recreational activities, and social activities (Jensen et al 2002). Analyses have demonstrated two dimensions of the BPI interference scale after controlling for worst pain intensity: affect (relationships with others, mood, enjoyment of life) and activity (walking, work, general activity, sleep; Cleeland et al 1996). The BPI interference scale demonstrates excellent psychometric properties, and a large literature supports its validity. Scores correlate with other measures of disability and negative mood in the expected directions, and both pain severity and mood contribute to ratings of total BPI interference scores (Portenoy et al 1992). The BPI may be particularly suited to the assessment of episodic or fluctuating pain states, such as can occur with pain from cancer (Owen et al 2000). The BPI interference scale has been used to track responses to a variety of pain management interventions, including implementation of pain guidelines, open-label studies of pharmacological treatments, and randomized clinical trials.
Pain Disability Index
The Pain Disability Index (PDI) was developed as a brief measure of the degree to which chronic pain interferes with normal role functioning (Tait et al 1987, Chibnall and Tait 1994) and has been used to measure function and disability in a wide range of chronically painful conditions. The PDI includes seven items assessing perceived disability: family and home responsibilities, recreation, social activity, occupation, sexual behavior, self-care (e.g., taking a shower, driving, getting dressed), and life support activity (e.g., eating, sleeping, breathing). Most work suggests that these items form a single factor (Chibnall and Tait 1994), and scores on the PDI correlate with other indices of physical function. The PDI has been used to track responses to treatment in a broad range of painful conditions across a number of different treatment modalities.
Pain Behavior
Pain behavior—the actions that communicate pain—has long been assessed clinically, and now a systematic, empirical literature has amassed from a variety of systematic methodologies developed for observing and coding overt pain behavior (Labus et al 2003). Much of this work has grown out of the behavioral treatment literature. Coding systems provide methods for sampling behavior, definitions of behavior codes, methods for training observers, and methods for determining reliability and validity (Keefe 2000). Although some systems incorporate assessment into the medical examination (Main and Waddell 1998), most systems have been developed for research purposes and include scoring of videotaped records (Keefe 2000) rather than real-time assessments (Prkachin et al 2002). A meta-analysis of this literature revealed a moderately positive relationship between self-reports of pain intensity and overt pain behavior (Labus et al 2003). The relationship is quite variable and depends on whether the pain is acute or chronic (correlation is higher when pain is acute), the timing of the pain assessment (pain assessed following observation of behavior shows a stronger relationship than do pain reports obtained before the observation), and the range of behaviors—guarding, bracing, rubbing, grimacing—observed (composite ratings show higher correlations than any single behavior does; Labus et al 2003). Although self-report measures of pain behavior have been developed (Kerns et al 1991, Revicki et al 2009), the value of this dimension of physical function lies in the inclusion of behavior that is more automatic and less under the control of voluntary processes (Hadjistavropoulos and Craig 2002).
Psychosocial Function
Extensive laboratory and clinical research has documented the complex role that emotions and mood play in the experience of pain (Lumley et al 2011). Mood states, as well as clinical mood disorders, influence laboratory pain sensitivity, clinical pain and its treatment, and new-onset pain conditions and incur risk for persistent pain following surgery or trauma (Dickens et al 2002, Mallen et al 2007, Asmundson and Katz 2009, Chou and Shekelle 2010). Although the incidence of mood disorders is elevated (Gureje et al 1998, Bair et al 2003, Asmundson and Katz 2009), many patients with chronic pain will not have a psychiatric disorder, and the emotional functioning of a patient with pain should be considered carefully even when a mood disorder is not present. Measurement of mood generally falls into two categories—measures that screen for clinically significant emotional problems, which typically create a dichotomy using cutoffs, and measures that conceptualize mood as a continuous dimension. The discussion here focuses on measures that are commonly used in medical settings for painful conditions rather than on the large number of measures that are primarily used in psychiatric settings. Even though the specific emotion of anger is highly relevant to pain, particularly chronic pain, anger has not received broad attention in the clinical literature, although an excellent review (Bruehl et al 2009) nicely summarizes recent findings and discusses the potential neural basis for an association between anger expression and increased pain sensitivity. The discussion here also includes sleep as a dimension of psychosocial function since sleep is typically disrupted in patients with chronic pain (Smith and Haythornthwaite 2004), is a symptom of depression and anxiety, and may be an important outcome of pain treatment (Stacey and Swift 2006).
Multidimensional Measures of Mood
The Profile of Mood States (POMS; McNair et al 1992) assesses six dimensions of mood (tension–anxiety, depression–dejection, anger–hostility, vigor–activity, fatigue–inertia, and confusion–bewilderment) and has been used most widely as an outcome measure in pharmacological clinical trials (e.g., Rowbotham et al 1998, Raja et al 2002, Dworkin et al 2005). The Symptom Checklist–90 Revised (SCL-90R; Derogatis 1983) measures 10 dimensions of emotional and physical symptoms (depression, anxiety, hostility, phobic anxiety, interpersonal sensitivity, obsessions and compulsions, somatization, psychoticism, paranoid ideation, and other), which can also be summarized as a Global Severity Index or scores within the individual domains. Though convenient for many clinical settings, the validity of the original scoring for patients with chronic pain has been debated and alternative scoring methods developed (Williams et al 1995). Of note, the somatization scale from the SCL-90 has been used extensively in some specific pain conditions, such as temporomandibular joint disorder (Ohrbach et al 2010), and high scores on somatization predict poor outcomes after lumbar surgery and spinal cord stimulation (Cleestin et al 2009). The General Hospital Questionnaire (GHQ) is available in a number of versions varying from 12 items, which is useful for epidemiological screening (Benjamin et al 2000), to the original 60-item version (Goldberg et al 1976). Unlike other instruments that provide a multidimensional assessment of mood, the GHQ is primarily used as a screening tool and is helpful in identifying individuals who probably have significant emotional distress, if not a frank psychiatric disorder.
Measures of Depression
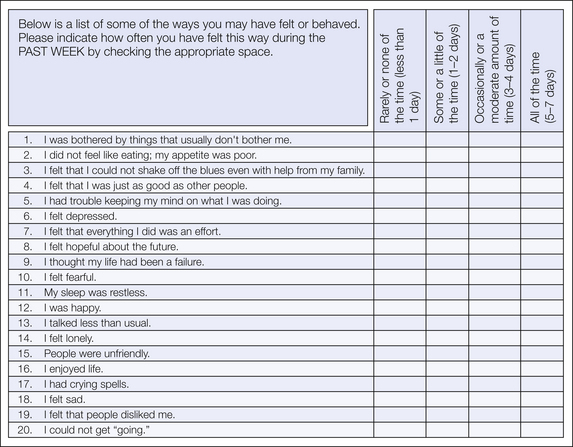
Measurement of depressive symptoms in the pain literature has largely included three instruments: the Center for Epidemiologic Studies–Depression Scale (CES-D; Radloff 1977), the Beck Depression Inventory (BDI; Beck et al 1961), and the Hospital Anxiety and Depression Scale (HADS; Zigmond and Snaith 1983). All are brief, have excellent psychometric properties, discriminate patients with major mood disorders, and are responsive to treatment effects. The CES-D is a 20-item scale (Fig. 24-4; Radloff 1977) designed for use in community samples and focuses on the affective and cognitive symptoms of depression, including 4 items that are worded positively. The BDI is a 21-item instrument in which patients indicate which of 4 clustered statements describe their current experience. Although cutoffs have been recommended for determining the severity of depressive symptoms (none, mild, moderate, severe) and used to determine the sensitivity and specificity of scores in identifying clinical depression, concern about the content of many symptom clusters has fueled debate on the interpretation of specific scores in patients with chronic pain (Morley et al 2002). Following a confirmatory factor analysis of items on the BDI, item scores for a heterogeneous group of patients with chronic pain reflecting a negative view of self were consistently lower than item scores for a group of patients referred for mental health care (Morley et al 2002). These findings, in the context of the larger literature on the BDI, suggest that the quality of the depressive symptoms experienced by patients with chronic pain may be quite distinct from that of patients without chronic pain who are seen in mental health settings. Particularly in clinical settings, the BDI-II (Beck et al 1996), which adapted the BDI to align more closely with revisions in diagnostic criteria made in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association 1994), may be used more frequently. Recent work has confirmed a similar factor structure in people with chronic pain that includes three first-order constructs (negative attitude, performance difficulty, and somatic elements) and a single second-order construct of depression (Harris and D’Eon 2008). The HADS, a 14-item scale, screens for the two most common disturbances seen in medical settings—depression and anxiety—and avoids the use of somatic symptoms to reduce false-positive scores. The HADS shows good psychometric properties in a wide variety of settings (Herrmann 1997), and although the score provides a probability of psychiatric disturbance (i.e., “caseness”), two factor scores capture the dimensionality of anxiety and depression. Although the anxiety and depression scales show high intercorrelations, each scale demonstrates clinical utility; Herrmann (1997) provides an excellent summary of the broad use of this scale in many countries and across a wide array of medical conditions, including chronic pain.
Measures of Anxiety
In addition to the measures of fear-avoidance discussed earlier, general measures of anxiety should be considered for use in assessment of mood. In addition to the HADS, the Spielberger State–Trait Anxiety Inventory (STAI; Spielberger et al 1983) provides a dimensional assessment of anxiety. The STAI consists of two (one presently—state—and one generally—trait) 20-item lists of brief statements describing symptoms of anxiety (e.g., “I feel nervous” or “I feel calm”). Although the STAI has good psychometric properties and is responsive to change as a result of pain treatment, as is the case with the HADS, the STAI often shows a worrisomely high correlation with measures of depression.
Measurement of specific anxiety symptoms experienced in response to the individual’s pain condition may also be useful. The Pain Anxiety Symptoms Scale (PASS; McCracken et al 1992) consists of 40 items measuring avoidance (“I try to avoid activities causing pain”), physiological arousal (“When I sense pain, I feel dizzy or faint”), cognitive responses (“When I feel pain, I can’t think straight”), and fearful thoughts (“I think that if my pain gets too severe, it will never decrease”). The PASS has been applied broadly to many painful conditions, has been shown to have effects independent of other dimensions of negative affect (e.g., depression or trait anxiety), and is responsive to the effects of treatment.
Measures of Sleep
Patients with chronically painful conditions report high rates of sleep disturbance (Smith et al 2000, Haythornthwaite et al 2003). The standardized measures of sleep used in the sleep literature, such as the Pittsburgh Sleep Quality Index (PSQI; Menefee et al 2000, Smith et al 2000) and sleep diaries (Haythornthwaite et al 1991, Stacey and Swift 2006), are important tools available to the pain clinician and may provide more valuable information about the impact of pain on sleep. The sleep ratings used typically focus on the extent to which pain interfered with sleep, either in diary form (Rowbotham et al 1998) or as part of the overall assessment of pain-related function (e.g., a sleep item is included in the MPI, BPI, and PDI). Successful treatment of pain can be reflected in improvements in sleep, often assessed as pain-related interference with sleep, but this improvement may not be reflected in more general measures of sleep function (Harati et al 1998). Specific treatment of insomnia secondary to chronic pain (Currie et al 2000) and fibromyalgia (Edinger et al 2005, Jungquist et al 2010) that includes cognitive–behavioral therapy for sleep improves an array of sleep measures, and treatment of sleep dysfunction before surgery reduces pain medication use (Tompkins et al 2011).
Health-Related Quality of Life
In many situations, HRQoL measures complement and may even be preferable to pain-specific measures. As measures of disease burden, scores on these instruments will be affected by co-morbid conditions, which may limit their responsivity to change in the context of pain treatment. Alternatively, the broad nature of these measures may capture iatrogenic effects of treatment such as side effects, and they provide information that allows comparison of samples to populations (healthy individuals or groups with another painful or non-painful condition).
Sickness Impact Profile
The Sickness Impact Profile (SIP; Bergner et al 1981) was developed as a broad measure of the behavioral impact of sickness. After extensive refinement, the final version includes 136 items in 12 categories of function and yields 3 summary scores—psychosocial, physical, and other impairment (Bergner et al 1981). The SIP has been used extensively in the pain literature for a very broad range of painful conditions. Early work applying the SIP to chronic pain validated the physical impairment scale against daily activity logs by demonstrating a significant inverse correlation between uptime and SIP physical function score (Follick et al 1985). SIP physical impairment scores correlate with clinical ratings of knee function and physical disability (de Bock et al 1996). They show expected increases across groups with osteoarthritis reporting sporadic versus episodic versus chronic joint pain (Hopman-Rock et al 1996), and total SIP scores predict the development of chronic pain (Epping-Jordan et al 1998). Most studies using the SIP as an outcome measure of pain treatment have used the total SIP score. The physical impairment scale does track improvements following multidisciplinary rehabilitation (Jensen et al 1994a) and spinal cord stimulation (Burchiel et al 1996), but in randomized, controlled trials this scale has not consistently demonstrated responsivity to treatment (e.g., Moulin et al 1996).
Early in its application, 24 of the original SIP items were developed as a measure of function in patients with back pain by adding the following stem to each statement: “because of my back pain”—the Roland Morris Disability Scale (Roland and Morris 1983, Roland and Fairbank 2000). Selection of items was based on the probable impact that back pain would have on physical function; however, not all items are from the SIP physical impairment scale. Items include assessment of irritability, appetite, and housework. This measure has become one of a select group of standard outcome measures in the back pain literature (Deyo 1986, Deyo et al 1998).
Medical Outcome Study Short Form
The 36-item short form of the Medical Outcome Study instrument (SF-36; McHorney et al 1993) is one of the most widely used measures of HRQoL and includes subscales measuring physical health, mental health, social functioning, role functioning, general health, pain, and vitality. From these individual scales, two summary scores—the Physical Component Summary Score and the Mental Component Summary Score—can be derived. This instrument shows excellent psychometric properties and is widely used for assessment of HRQoL. Shorter versions have been designed, but the 36-item version has been used most frequently in the pain literature (Dworkin et al 2005) and has been shown to be sensitive to the effects of treatment in randomized controlled trials (e.g., Robotham et al 1998).
Nottingham Health Profile
The Nottingham Health Profile (NHP; Hunt et al 1980) includes 38 true/false items measuring six domains (mobility, pain, energy, sleep, emotional reactions, and social isolation) and an optional second part that includes additional items about sex, work, hobbies, and social relationships. The NHP is simple, comprehensive, and widely used, particularly in Europe. Under some circumstances the NHP may be more sensitive than the SF-36 to treatment-related changes (Klevsgard et al 2002) and includes a specific sleep scale and more pain items than the SF-36 does (Meyer-Rosberg et al 2001); however, in some circumstances the SF-36 may show greater responsivity to treatment (Beaton et al 1997).
European Quality of Life Instrument
The European Quality of Life Instrument (EQ; EuroQol 1990) is a brief, single-index measure that includes five questions (mobility, self-care, usual activity, pain or distress, and depression or anxiety) and a summary visual analog rating of overall health. One of 243 unique health states can be derived from the patient’s response, and responses to the five dimensions can be scored with a utility-weighting algorithm for economic evaluations (Williams 1995) that yields scores ranging from 0–100 (EQ-5D Index), in addition to the summary rating scale, which is also scored from 0–100 (EuroQol 1990). Though quite useful for population-based studies of HRQoL, the EQ may be too global a measure to capture subtle changes in response to treatment, thus making it more useful for economic evaluations than for HRQoL-related evaluations (Brazier et al 1999, Melloh et al 2008).
Cost Outcomes
The cost of pain, particularly chronic pain, is high. Although many studies do not measure use of health services as an outcome of pain treatment (Morley et al 1999), this is one domain of measurement that needs to be expanded in clinical pain research. In addition to self-report summaries of health care use (Turner et al 2005a), diary methods, even over a 4-week period (Smeets et al 2009), may provide complementary information, particularly about certain types of health care visits (Goossens et al 2000).
The broad impact of pain on function in many chronically painful conditions and the societal costs of disability support an emphasis in the treatment of pain on returning the patient to work, particularly individuals involved in compensation systems. In the United States, most injured autoworkers do not file claims following a musculoskeletal work injury, and income, work dissatisfaction, and factors associated with the severity of the condition predict who files for compensation (Rosenman et al 2000). Receiving disability compensation is a strong predictor of not returning to work (MacKenzie et al 1998), and pain and physical function at 3 months predict long-term work disability following lower limb trauma (MacKenzie et al 2006). Return to work has been a historically important outcome in the chronic pain literature, particularly in evaluating multidisciplinary pain treatment (e.g., Flor et al 1992).
Another interesting work-related outcome that is receiving increased attention is on-the-job productivity. Although absenteeism and return to work are clearly important for some patients, reduced productivity while at work (i.e., presenteeism) may be an important index for individuals with chronically painful conditions who continue working. Lost productive time (Loeppke et al 2003) can be assessed through structured interviews (Stewart et al 2003) or questionnaires (Prasad et al 2004), and in 2003 the cost of lost productive time at work in the United States because of common pain conditions (back pain, arthritis and other musculoskeletal pain, and headache) was estimated to be $61.2 billion (Stewart et al 2003). Various instruments are available for the measurement of work productivity, but no single instrument appears to be psychometrically better than another or responsive to treatment effects (Prasad et al 2004); a panel of experts identified five different scales that are recommended for the assessment of general health-related work productivity (Loeppke et al 2003).
Conclusion
As an overview, this chapter has attempted to orient the reader to the broad array of measures available to assess key aspects of pain attitudes, coping, and function. Multidimensional assessment of someone with a chronically painful condition should include at least one measure in each category discussed (pain attitudes, coping, and function) and ideally will include a number of scales within each category. The advent of briefer versions of some well-investigated scales (e.g., one- and two-item scales of pain attitudes and coping; Jensen et al 2003) and development of the Patient-Reported Outcomes Measurement Information System (PROMIS) item bank for the assessment of pain impact (Amtmann et al 2010), as well as the assessment of mood (Pilkonis et al 2011), have provided a highly efficient assessment methodology with the advantage of having access to normative data on large samples for comparison purposes.
Comprehensive reviews of each category are available in other chapters and published papers identified throughout this discussion. Clinicians and researchers alike are encouraged to select psychometrically sound measures—either from the foregoing discussion or from the empirical literature—that have a history of use for the painful condition of interest. This said, however, it is important to also acknowledge the shortcomings of existing measures, which often require refinement as our understanding of these constructs improves. These largely self-report measures that summarize the individual’s experience over time provide valuable information but, as summary measures, may be subject to biases inherent in the recall of personal experience. Although the measures reviewed in this chapter generally have extensive validity data supporting their continued use for the assessment of pain, the emergence of technological advances such as electronic diaries may shift attention away from summary scales to measures that capture individuals’ experience during daily life in their natural environment (Stone et al 2003). Use of these technologies in clinical practice does not appear to be on the near horizon, but pain and sleep diaries (Rowbotham et al 1998, Raja et al 2002) may become the new standard for clinical trials of pain treatments. Extension of the other measures reviewed earlier—those of coping and function—to the use of daily diaries for measurement of treatment outcome (Turner et al 2005b) is an exciting direction for future research.
The references for this chapter can be found at www.expertconsult.com.
References
Agarwal S., Polydefkis M., Block B., et al. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Medicine. 2007;8:554–562.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1994.
Amtmann D., Cook K.F., Jensen M.P., et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182.
Anderson K.O., Dowds B.N., Pelletz R.E., et al. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63:77–83.
Anderson K.O., Syrjala K.L., Cleeland C.S. How to assess cancer pain. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. New York: Guilford Press; 2001:579–600.
Andersson G. Chronic pain and praying to a higher power: useful or useless? Journal of Religion and Health. 2008;47:176–187.
Archer K.R., Wegener S.T., Seebach C., et al. The effect of fear-avoidance beliefs on pain and disability after surgery for lumbar and cervical degenerative conditions. Spine. 2011;36:1554–1562.
Asmundson G.J.G., Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depression and Anxiety. 2009;26:888–901.
Bair M.J., Robinson R.L., Katon W., et al. Depression and pain comorbidity: a literature review. Archives of Internal Medicine. 2003;163:2433–2445.
Beaton D.E., Hogg-Johnson S., Bombardier C. Evaluating changes in health status: reliability and responsiveness of five generic health status measures in workers with musculoskeletal disorders. Journal of Clinical Epidemiology. 1997;50:79–93.
Beck A.T., Steer R.A., Brown G.K. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
Beck A.T., Ward E., Mendelson M., et al. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571.
Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clinical and Experimental Rheumatology. 2005;23:S148–S153.
Bellamy N., Kirwan J., Boers M., et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. Journal of Rheumatology. 1997;24:799–802.
Benjamin S., Morris S., McBeth J., et al. The association between chronic widespread pain and mental disorder: a population-based study. Arthritis and Rheumatism. 2000;43:561–567.
Benyon K., Hill S., Zadurian N., et al. Coping strategies and self-efficacy as predictors of outcome in osteoarthritis: a systematic review. Musculoskeletal Care. 2010;8:224–236.
Bergner M., Bobbitt R.A., Carter W.B., et al. The sickness impact profile: development and final revision of a health status measure. Medical Care. 1981;19:787–805.
Boothby J.L., Thorn B.E., Stroud M.W., et al. Coping with pain. In: Gatchel R.J., Turk D.C., eds. Psychosocial factors in pain. New York: Guilford Press; 1999:343–359.
Brazier J.E., Harper R., Munro J., et al. Generic and condition-specific outcome measures for people with osteoarthritis of the knee. Rheumatology (Oxford). 1999;38:870–877.
Brown G.K., Nicassio P.M. Development of a questionnaire for the assessment of active and passive coping strategies in chronic pain patients. Pain. 1987;31:53–64.
Bruehl S., Burns J.W., Chung O.Y., et al. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neuroscience and Biobehavioral Reviews. 2009;33:475–491.
Burchiel K.J., Anderson V.C., Brown F.D., et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21:2786–2794.
Burns J.W., Johnson B.J., Mahoney N., et al. Cognitive and physical capacity process variables predict long-term outcome after treatment of chronic pain. Journal of Consulting and Clinical Psychology. 1998;66:434–439.
Burns J.W., Kubilus A., Bruehl S., et al. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? A cross-lagged panel analysis. Journal of Consulting and Clinical Psychology. 2003;71:81–91.
Bussmann J.B., van de Laar Y.M., Neeleman M.P., et al. Ambulatory accelerometry to quantify motor behaviour in patients after failed back surgery: a validation study. Pain. 1998;74:153–161.
Celestin J., Edwards R.R., Jamison R.N. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Medicine. 2009;10:639–653.
Chibnall J.T., Tait R.C. The Pain Disability Index: factor structure and normative data. Archives of Physical Medicine and Rehabilitation. 1994;75:1082–1086.
Chibnall J.T., Tait R.C. Confirmatory factor analysis of the Pain Catastrophizing Scale in African American and Caucasian workers’ compensation claimants with low back injuries. Pain. 2005;113:369–375.
Chou R., Shekelle P. Will this patient develop persistent disabling low back pain? JAMA: The Journal of the American Medical Association. 2010;303:1295–1302.
Cleeland C.S., Nakamura Y., Mendoza T.R., et al. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67:267–273.
Cleeland C.S., Ryan K.M. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine Singapore. 1994;23(2):129–138.
Currie S.R., Wilson K.G., Pontefract A.J., et al. Cognitive-behavioral treatment of insomnia secondary to chronic pain. Journal of Consulting and Clinical Psychology. 2000;68:407–416.
de Bock G.H., Hermans J., van Marwijk H.W., et al. Health-related quality of life assessments in osteoarthritis during NSAID treatment. Pharmacy World & Science. 1996;18:130–136.
DeGood D.E., Cook A.J. Psychosocial assessment: comprehensive measures and measures specific to pain beliefs and coping. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. New York: Guilford Press; 2011:67–97.
Deisinger J.A., Cassisi J.E., Lofland K.R., et al. An examination of the psychometric structure of the Multidimensional Pain Inventory. Journal of Clinical Psychology. 2001;57:765–783.
Derogatis L. The SCL-90R: administration, scoring, and procedures manual. Towson, MD: Clinical Psychometric Research; 1983.
Deyo R.A. Comparative validity of the sickness impact profile and shorter scales for functional assessment in low-back pain. Spine. 1986;11:951–954.
Deyo R.A., Battie M., Beurskens A.J., et al. Outcome measures for low back pain research. A proposal for standardized use. Spine. 1998;23:2003–2013.
Dickens C., McGowan L., Clark-Carter D., et al. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosomatic Medicine. 2002;64:52–60.
Dworkin R.H., Turk D.C., Farrar J.T., et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19.
Edinger J.D., Wohlgemuth W.K., Krystal A.D., et al. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Archives of Internal Medicine. 2005;165:2527–2535.
Edwards R.R., Cahalan C., Mensing G., et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nature Reviews. Rheumatology. 2011;7:216–224.
Epping-Jordan J.E., Wahlgren D.R., Williams R.A., et al. Transition to chronic pain in men with low back pain: predictive relationships among pain intensity, disability, and depressive symptoms. Health Psychology. 1998;17:421–427.
Ersek M., Turner J.A., Kemp C.A. Use of the Chronic Pain Coping Inventory to assess older adults’ pain coping strategies. Journal of Pain. 2006;7:833–842.
European Quality of Life instrument. EuroQol—a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208.
Flor H., Fydrich T., Turk D.C. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain. 1992;49:221–230.
Follick M.J., Smith T.J., Ahern D.K. The sickness impact profile: a global measure of disability in chronic low back pain. Pain. 1985;21:67–76.
Fordyce W.E., Fowler R.S., Lehmann J.F., et al. Operant conditioning in the treatment of chronic pain. Archives of Physical Medicine and Rehabilitation. 1972;54:399–408.
Fried L.P., Young Y., Rubin G., et al. Self-reported preclinical disability identifies older women with early declines in performance and early disease. Journal of Clinical Epidemiology. 2001;54:889–901.
Garcia-Campayo J., Pascual A., Alda M., et al. Coping with fibromialgia: usefulness of the Chronic Pain Coping Inventory-42. Pain. 2007;132:S68–S76.
Goldberg D.P., Rickels K., Downing R., et al. A comparison of two psychiatric screening tests. British Journal of Psychiatry. 1976;129:61–67.
Goossens M.E., Rutten-van Molken M.P., Vlaeyen J.W., et al. The cost diary: a method to measure direct and indirect costs in cost-effectiveness research. Journal of Clinical Epidemiology. 2000;53:688–695.
Gureje O., Von Korff M., Simon G.E., et al. Persistent pain and well-being: a World Health Organization study in primary care. [Published erratum appears in JAMA 280(13):1142, 1998.]. JAMA: The Journal of the American Medical Association. 1998;280:147–151.
Hadjistavropoulos T., Craig K.D. A theoretical framework for understanding self-report and observational measures of pain: a communications model. Behaviour Research and Therapy. 2002;40:551–570.
Harati Y., Gooch C., Swenson M., et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50:1842–1846.
Harding V.R., Williams A.C., Richardson P.H., et al. The development of a battery of measures for assessing physical functioning of chronic pain patients. Pain. 1994;58:367–375.
Harris C.A., D’Eon J.L. Psychometric properties of the Beck Depression Inventory-Second Edition (BDI-II) in individuals with chronic pain. Pain. 2008;137:609–622.
Haythornthwaite J.A. It’s a belief. It’s an appraisal. It’s coping. No, it’s catastrophizing. In: Castro-Lopes J, ed. Current topics in pain: 12th World Congress on Pain. Seattle: IASP Press; 2009:271–288.
Haythornthwaite J.A., Clark M.R., Pappagallo M., et al. Pain coping strategies play a role in the persistence of pain in post-herpetic neuralgia. Pain. 2003;106:453–460.
Haythornthwaite J.A., Hegel M.T., Kerns R.D. Development of a sleep diary for chronic pain patients. Journal of Pain and Symptom Management. 1991;6:65–72.
Haythornthwaite J.A., Lawrence J.W., Fauerbach J.A. Brief cognitive interventions for burn pain. Annals of Behavioral Medicine. 2001;23:42–49.
Haythornthwaite J.A., Menefee L.A., Heinberg L.J., et al. Pain coping strategies predict perceived control over pain. Pain. 1998;77:33–39.
Herrmann C. International experiences with the Hospital Anxiety and Depression Scale—a review of validation data and clinical results. Journal of Psychosomatic Research. 1997;42:17–41.
Hill P.C., Pargament K.I. Advances in the conceptualization and measurement of religion and spirituality. Implications for physical and mental health research. American Psychologist. 2003;58:64–74.
Hinrichs-Rocker A., Schulz K., Järvinen I., et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP)—a systematic review. European Journal of Pain. 2009;13:719–730.
Hopman-Rock M., Odding E., Hofman A., et al. Physical and psychosocial disability in elderly subjects in relation to pain in the hip and/or knee. Journal of Rheumatology. 1996;23:1037–1044.
Hunt S.M., McKenna S.P., McEwen J., et al. A quantitative approach to perceived health status: a validation study. Journal of Epidemiology and Community Health. 1980;34:281–286.
Jensen M.P. The validity and reliability of pain measures in adults with cancer. Journal of Pain. 2003;4:2–21.
Jensen M.P., Ehde D.M., Hoffman A.J., et al. Cognitions, coping and social environment predict adjustment to phantom limb pain. Pain. 2002;95:133–142.
Jensen M.P., Karoly P. 1992 Pain-specific beliefs, perceived symptom severity, and adjustment to chronic pain. Clinical Journal of Pain. 1992;8:123–130.
Jensen M.P., Keefe F.J., Lefebvre J.C., et al. One- and two-item measures of pain beliefs and coping strategies. Pain. 2003;104:453–469.
Jensen M.P., Moore M.R., Bockow T.B., et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Archives of Physical Medicine and Rehabilitation. 2011;92:146–160.
Jensen M.P., Turner M.A., Romano J.M. Correlates of improvement in multidisciplinary treatment of chronic pain. Journal of Consulting and Clinical Psychology. 1994;62:172–179.
Jensen M.P., Turner J., Romano J. Pain belief assessment: a comparison of the short and long versions of the Survey of Pain Attitudes. Journal of Pain. 1999;1:138–150.
Jensen M.P., Turner J.A., Romano J.M. Changes after multidisciplinary pain treatment in patient pain beliefs and coping are associated with concurrent changes in patient functioning. Pain. 2007;131:38–47.
Jensen M.P., Turner J.A., Romano J.M., et al. Coping with chronic pain: a critical review of the literature. Pain. 1991;47:249–283.
Jensen M.P., Turner J.A., Romano J.M., et al. Relationship of pain specific beliefs to chronic pain adjustment. Pain. 1994;57:301–309.
Jensen M.P., Turner J.A., Romano J.M., et al. The Chronic Pain Coping Inventory: development and preliminary validation. Pain. 1995;60:203–216.
Jungquist C.R., O’Brien C., Matteson-Rusby S., et al. 2010 The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Medicine. 2010;11:302–309.
Katz J., Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Review of Neurotherapeutics. 2009;9:723–744.
Keefe F.J. Pain behavior observation: current status and future directions. Current Review of Pain. 2000;4:12–17.
Keefe F.J., Affleck G., Lefebvre J., et al. Living with rheumatoid arthritis: the role of daily spirituality and daily religious and spiritual coping. Journal of Pain. 2001;2:101–110.
Kerns R., Turk D., Rudy T. The West Haven–Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23:345–356.
Kerns R.D., Haythornthwaite J., Rosenberg R., et al. The Pain Behavior Check List (PBCL): factor structure and psychometric properties. Journal of Behavioral Medicine. 1991;14:155–167.
Klevsgard R., Froberg B.L., Risberg B., et al. Nottingham Health Profile and Short-Form 36 Health Survey questionnaires in patients with chronic lower limb ischemia: before and after revascularization. Journal of Vascular Surgery. 2002;36:310–317.
Labus J.S., Keefe F.J., Jensen M.P. Self-reports of pain intensity and direct observations of pain behavior: when are they correlated? Pain. 2003;102:109–124.
Lai Y.H., Keefe F.J., Sun W.Z., et al. Relationship between pain-specific beliefs and adherence to analgesic regimens in Taiwanese cancer patients: a preliminary study. Journal of Pain and Symptom Management. 2002;24:415–423.
Leeuw M., Goossens M.E., Linton S.J., et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of Behavioral Medicine. 2007;30:77–94.
Lefebvre J.C., Keefe F.J. Memory for pain: the relationship of pain catastrophizing to the recall of daily rheumatoid arthritis pain. Clinical Journal of Pain. 2002;18:56–63.
Loeppke R., Hymel P.A., Lofland J.H., et al. Health-related workplace productivity measurement: general and migraine-specific recommendations from the ACOEM Expert Panel. Journal of Occupational and Environmental Medicine. 2003;45:349–359.
Lorig K., Chastain R.L., Ung E., et al. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis and Rheumatism. 1989;32:37–44.
Lousberg R., Van Breukelen G.J., Groenman N.H., et al. Psychometric properties of the Multidimensional Pain Inventory, Dutch language version (MPI-DLV). Behaviour Research and Therapy. 1999;37:167–182.
Lumley M.A., Cohen J.L., Borszcz G.S., et al. Pain and emotion: a biopsychosocial review of recent research. Journal of Clinical Psychology. 2011;67:942–968.
MacKenzie E.J., Bosse M.J., Kellam J.F., et al. Early predictors of long-term work disability after major limb trauma. Journal of Trauma. 2006;61:688–694.
MacKenzie E.J., Morris J.A., Jr., Jurkovich G.J., et al. Return to work following injury: the role of economic, social, and job-related factors. American Journal of Public Health. 1998;88:1630–1637.
Mahrer N.E., Gold J.I. The use of virtual reality for pain control: a review. Current Pain and Headache Reports. 2009;13:100–109.
Main C.J., Waddell G. Behavioral responses to examination. A reappraisal of the interpretation of “nonorganic signs,”. Spine. 1998;23:2367–2371.
Mallen C.D., Peat G., Thomas E., et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. British Journal of General Practice. 2007;57:655–661.
Marceau L.D., Link C., Jamison R.N., et al. Electronic diaries as a tool to improve pain management: is there any evidence? Pain Medicine. 2007;8(Suppl 3):S101–S109.
Marceau L.D., Link C.L., Smith L.D., et al. In-clinic use of electronic pain diaries: barriers of implementation among pain physicians. Journal of Pain and Symptom Management. 2010;40:391–404.
McCracken L.M. Learning to live with the pain: acceptance of pain predicts adjustment in persons with chronic pain. Pain. 1998;74:21–27.
McCracken L.M., Carson J.W., Eccleston C., et al. Acceptance and change in the context of chronic pain. Pain. 2004;109:4–7.
McCracken L.M., Samuel V.M. The role of avoidance, pacing, and other activity patterns in chronic pain. Pain. 2007;130:119–125.
McCracken L.M., Zayfert C., Gross R.T. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain. 1992;50:67–73.
McHorney C.A., Ware J.E., Jr., Raczek A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31:247–263.
McNair D.M., Lorr M., Droppleman L.F. POMS manual profile of mood states. EdiTS/Educational and Industrial Testing Service. San Diego: Calif; 1992.
Melloh M., Röder C., Elfering A., et al. Differences across health care systems in outcome and cost-utility of surgical and conservative treatment of chronic low back pain: a study protocol. BMC Musculoskeletal Disorders. 2008;9:81.
Menefee L.A., Frank E.D., Doghramji K., et al. Self-reported sleep quality and quality of life for individuals with chronic pain conditions. Clinical Journal of Pain. 2000;16:290–297.
Meyer-Rosberg K., Burckhardt C.S., Huizar K., et al. A comparison of the SF-36 and Nottingham Health Profile in patients with chronic neuropathic pain. European Journal of Pain. 2001;5:391–403.
Miles C.L., Pincus T., Carnes D., et al. Can we identify how programmes aimed at promoting self-management in musculoskeletal pain work and who benefits? A systematic review of sub-group analysis within RCTs. European Journal of Pain. 2011;15:775. e1–e11
Miller R.P., Kori S.H., Todd D.D. The Tampa Scale. Tampa, FL: Unpublished report; 1991.
Morley S., Eccleston C., Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1–13.
Morley S., Williams A.C., Black S. A confirmatory factor analysis of the Beck Depression Inventory in chronic pain. Pain. 2002;99:289–298.
Moulin D.E., Iezzi A., Amireh R., et al. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347:143–147.
Nicholas M.K. The pain self-efficacy questionnaire: taking pain into account. European Journal of Pain. 2007;11:153–163.
Nielson W.R., Walker C., McCain G.A. Cognitive behavioral treatment of fibromyalgia syndrome: preliminary findings. Journal of Rheumatology. 1992;19:98–103.
Ohrbach R., Turner J.A., Sherman J.J., et al. The research diagnostic criteria for temporomandibular disorders. IV: evaluation of psychometric properties of the axis II measures. Journal of Orofacial Pain. 2010;24:48–62.
Owen J.E., Klapow J.C., Casebeer L. Evaluating the relationship between pain presentation and health-related quality of life in outpatients with metastatic or recurrent neoplastic disease. Quality of Life Research. 2000;9:855–863.
Pargament K.I., Koenig H.G., Perez L.M. The many methods of religious coping: development and initial validation of the RCOPE. Journal of Clinical Psychology. 2000;56:519–543.
Peters M.L., Sorbi M.J., Kruise D.A., et al. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain. 2000;84:181–192.
Pilkonis P.A., Choi S.W., Reise S.P., et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18:263–283.
Portenoy R.K., Miransky J., Thaler H.T., et al. Pain in ambulatory patients with lung or colon cancer. Prevalence, characteristics, and effect. Cancer. 1992;70:1616–1624.
Prasad M., Wahlqvist P., Shikiar R., et al. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics. 2004;22:225–244.
Primdahl J., Wagner L., Horslev-Petersen K. Self-efficacy as an outcome measure and its association with physical disease-related variables in persons with rheumatoid arthritis: a literature review. Musculoskeletal Care. 2011;9:125–140.
Prkachin K.M., Hughes E., Schultz I., et al. Real-time assessment of pain behavior during clinical assessment of low back pain patients. Pain. 2002;95:23–30.
Radloff L.S. The CES-D scale: a self report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401.
Raja S.N., Haythornthwaite J.A., Pappagallo M., et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59:1015–1021.
Revicki D.A., Chen W.H., Harnam N., et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158–169.
Risdon A., Eccleston C., Crombez G., et al. How can we learn to live with pain? A Q-methodological analysis of the diverse understandings of acceptance of chronic pain. Social Science & Medicine. 2003;56:375–386.
Robinson M.E., Riley J.L., III., Myers C.D., et al. The Coping Strategies Questionnaire: a large sample, item level factor analysis. Clinical Journal of Pain. 1997;13:43–49.
Roelofs J., Sluiter J.K., Frings-Dresen M.H.W., et al. Fear of movement and (re)injury in chronic musculoskeletal pain: evidence for an invariant two-factor model of the Tampa Scale for Kinesiophobia across pain diagnoses and Dutch, Swedish, and Canadian samples. Pain. 2007;131:181–190.
Roelofs J., van Breukelen G., Sluiter J., et al. Norming of the Tampa Scale for kinesiophobia across pain diagnoses and various countries. Pain. 2011;152:1090–1095.
Roland M., Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115–3124.
Roland M., Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144.
Romano J.M., Jensen M.P., Turner J.A. The Chronic Pain Coping Inventory-42: reliability and validity. Pain. 2003;104:65–73.
Rosenman K.D., Gardiner J.C., Wang J., et al. Why most workers with occupational repetitive trauma do not file for workers’ compensation. Journal of Occupational and Environmental Medicine. 2000;42:25–34.
Rosenstiel A.K., Keefe F.J. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44.
Rowbotham M., Harden N., Stacey B., et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA: The Journal of the American Medical Association. 1998;280:1837–1842.
Rudy TE. Multiaxial assessment of pain. Multidimensional pain inventory. Computer program user’s manual version 2.1. University of Pittsburgh School of Medicine, Pittsburgh, pp 1–72
Sharar S.R., Carrougher G.J., Nakamura D., et al. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: preliminary results from 3 ongoing studies. Archives of Physical Medicine and Rehabilitation. 2007;88:S43–S49.
Smeets R.J., Severens J.L., Beelen S., et al. More is not always better: cost-effectiveness analysis of combined, single behavioral and single physical rehabilitation programs for chronic low back pain. European Journal of Pain. 2009;13:71–81.
Smith M.T., Haythornthwaite J.A. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8:119–132.
Smith M.T., Perlis M.L., Smith M.S., et al. Sleep quality and presleep arousal in chronic pain. Journal of Behavioral Medicine. 2000;23:1–13.
Sorbi M.J., Peters M.L., Kruise D.A., et al. Electronic momentary assessment in chronic pain II: pain and psychological pain responses as predictors of pain disability. Clinical Journal of Pain. 2006;22:67–81.
Spielberger C.D., Gorsuch R.L., Lushene R., et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1993.
Stacey B.R., Swift J.N. Pregabalin for neuropathic pain based on recent clinical trials. Current Pain and Headache Reports. 2006;10:179–184.
Stewart W.F., Ricci J.A., Chee E., et al. Lost productive time and cost due to common pain conditions in the US workforce. JAMA: The Journal of the American Medical Association. 2003;290:2443–2454.
Stone A.A., Broderick J.E., Schwartz J.E., et al. Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain. 2003;104:343–351.
Stone A.A., Shiffman S., Schwartz J.E., et al. Patient non-compliance with paper diaries. BMJ. 2002;324:1193–1194.
Sullivan M.J., Bishop S.R., Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532.
Sullivan M., Tanzer M., Reardon G., et al. The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain. 2011;152:2287–2293.
Sullivan M.J., Thorn B., Haythornthwaite J.A., et al. Theoretical perspectives on the relation between catastrophizing and pain. Clinical Journal of Pain. 2001;17:52–64.
Tait R.C., Pollard C.A., Margolis R.B., et al. The Pain Disability Index: psychometric and validity data. Archives of Physical Medicine and Rehabilitation. 1987;68:438–441.
Talbot L.A., Gaines J.M., Huynh T.N., et al. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. Journal of the American Geriatrics Society. 2003;51:387–392.
Tan G., Nguyen Q., Anderson K.O., et al. Further validation of the chronic pain coping inventory. Journal of Pain. 2005;6:29–40.
Tan G., Teo I., Anderson K.O., et al. Adaptive versus maladaptive coping and beliefs and their relation to chronic pain adjustment. Clinical Journal of Pain. 2011;27:769–774.
Thompson M., McCracken L.M. Acceptance and related processes in adjustment to chronic pain. Current Pain and Headache Reports. 2011;15:144–151.
Tompkins M., Plante M., Monchik K., et al. The use of a non-benzodiazepine hypnotic sleep-aid (zolpidem) in patients undergoing ACL reconstruction: a randomized controlled clinical trial. Knee Surgery, Sports Traumatology. Arthroscopy. 2011;19:787–791.
Turk D.C., Okifuji A., Sinclair J.D., et al. Interdisciplinary treatment for fibromyalgia syndrome: clinical and statistical significance. Arthritis Care Research. 1998;11:186–195.
Turner J.A., Brister H., Huggins K., et al. Catastrophizing is associated with clinical examination findings, activity interference, and health care use among patients with temporomandibular disorders. Journal of Orofacial Pain. 2005;19:291–300.
Turner J.A., Mancl L., Aaron L.A. Brief cognitive-behavioral therapy for temporomandibular disorder pain: effects on daily electronic outcome and process measures. Pain. 2005;117:377–387.
Van Damme S., Crombez G., Bijttebier P., et al. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319–332.
Veehof M.M., Oskam M.J., Schreurs K.M., et al. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152:533–542.
Vlaeyen J.W., Kole-Snijders A.M., Boeren R.G., et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372.
Vowles K.E., McCracken L.M., McLeod C., et al. The Chronic Pain Acceptance Questionnaire: confirmatory factor analysis and identification of patient subgroups. Pain. 2008;140:284–291.
Vowles K.E., Wetherell J.L., Sorrell J.T. Targeting acceptance, mindfulness, and values-based action in chronic pain: findings of two preliminary trials of an outpatient group-based intervention. Cognitive Behavioural Practice. 2009;16:49–58.
Wachholtz A.B., Pearce M.J. Does spirituality as a coping mechanism help or hinder coping with chronic pain? Current Pain and Headache Reports. 2009;13:127–132.
Waddell G., Newton M., Henderson I., et al. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–168.
Ward S.E., Goldberg N., Miller-McCauley V., et al. Patient-related barriers to management of cancer pain. Pain. 1993;52:319–324.
Wetherell J.L., Afari N., Rutledge T., et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain. 2011;152:2098–2107.
Williams A. The measurement and validation of health: a chronicle. York, UK: University of York; 1995. Discussion Paper 136
Williams D.A., Urban B., Keefe F.J., et al. Cluster analyses of pain patients’ responses to the SCL-90R. Pain. 1995;61:81–91.
Worrel L.M., Krahn L.E., Sletten C.D., et al. Treating fibromyalgia with a brief interdisciplinary program: initial outcomes and predictors of response. Mayo Clinic Proceedings. 2001;76:384–390.
Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370.
Amtmann D., Cook K.F., Jensen M.P., et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182.
Anderson K.O., Dowds B.N., Pelletz R.E., et al. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63:77–83.
Asmundson G.J.G., Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depression and Anxiety. 2009;26:888–901.
Bair M.J., Robinson R.L., Katon W., et al. Depression and pain comorbidity: a literature review. Archives of Internal Medicine. 2003;163:2433–2445.
Beaton D.E., Hogg-Johnson S., Bombardier C. Evaluating changes in health status: reliability and responsiveness of five generic health status measures in workers with musculoskeletal disorders. Journal of Clinical Epidemiology. 1997;50:79–93.
Benyon K., Hill S., Zadurian N., et al. Coping strategies and self-efficacy as predictors of outcome in osteoarthritis: a systematic review. Musculoskeletal Care. 2010;8:224–236.
Boothby J.L., Thorn B.E., Stroud M.W., et al. Coping with pain. In: Gatchel R.J., Turk D.C., eds. Psychosocial factors in pain. New York: Guilford Press; 1999:343–359.
Bruehl S., Burns J.W., Chung O.Y., et al. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neuroscience and Biobehavioral Reviews. 2009;33:475–491.
Burchiel K.J., Anderson V.C., Brown F.D., et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21:2786–2794.
Celestin J., Edwards R.R., Jamison R.N. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Medicine. 2009;10:639–653.
Chibnall J.T., Tait R.C. The Pain Disability Index: factor structure and normative data. Archives of Physical Medicine and Rehabilitation. 1994;75:1082–1086.
Chou R., Shekelle P. Will this patient develop persistent disabling low back pain? JAMA: The Journal of the American Medical Association. 2010;303:1295–1302.
Cleeland C.S., Nakamura Y., Mendoza T.R., et al. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67:267–273.
Cleeland C.S., Ryan K.M.: Pain assessment: global use of the Brief Pain Inventory, Annals of the Academy of Medicine, Singapore 23:129–138, 1994
DeGood D.E., Cook A.J. Psychosocial assessment: comprehensive measures and measures specific to pain beliefs and coping. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. New York: Guilford Press; 2011:67–97.
Dickens C., McGowan L., Clark-Carter D., et al. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosomatic Medicine. 2002;64:52–60.
Dworkin R.H., Turk D.C., Farrar J.T., et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19.
Edwards R.R., Cahalan C., Mensing G., et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nature Reviews. Rheumatology. 2011;7:216–224.
Epping-Jordan J.E., Wahlgren D.R., Williams R.A., et al. Transition to chronic pain in men with low back pain: predictive relationships among pain intensity, disability, and depressive symptoms. Health Psychology. 1998;17:421–427.
Flor H., Fydrich T., Turk D.C. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain. 1992;49:221–230.
Goossens M.E., Rutten-van Molken M.P., Vlaeyen J.W., et al. The cost diary: a method to measure direct and indirect costs in cost-effectiveness research. Journal of Clinical Epidemiology. 2000;53:688–695.
Gureje O., Von Korff M., Simon G.E., et al. Persistent pain and well-being: a World Health Organization study in primary care. [published erratum appears in JAMA 1998 Oct 7;280(13):1142]. JAMA: The Journal of the American Medical Association. 1998;280:147–151.
Hadjistavropoulos T., Craig K.D. A theoretical framework for understanding self-report and observational measures of pain: a communications model. Behaviour Research and Therapy. 2002;40:551–570.
Harris C.A., D’Eon J.L. Psychometric properties of the Beck Depression Inventory-Second Edition (BDI-II) in individuals with chronic pain. Pain. 2008;137:609–622.
Haythornthwaite J.A. It’s a belief. It’s an appraisal. It’s coping.… No, it’s catastrophizing. In: Castro-Lopes J, ed. Current topics in pain: 12th World Congress on Pain. Seattle: IASP Press; 2009:271–288.
Haythornthwaite J.A., Hegel M.T., Kerns R.D. Development of a sleep diary for chronic pain patients. Journal of Pain and Symptom Management. 1991;6:65–72.
Herrmann C. International experiences with the Hospital Anxiety and Depression Scale—a review of validation data and clinical results. Journal of Psychosomatic Research. 1997;42:17–41.
Hill P.C., Pargament K.I. Advances in the conceptualization and measurement of religion and spirituality. Implications for physical and mental health research. American Psychologist. 2003;58:64–74.
Hinrichs-Rocker A., Schulz K., Järvinen I., et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP)—a systematic review. European Journal of Pain. 2009;13:719–730.
Jensen M.P., Ehde D.M., Hoffman A.J., et al. Cognitions, coping and social environment predict adjustment to phantom limb pain. Pain. 2002;95:133–142.
Jensen M.P., Keefe F.J., Lefebvre J.C., et al. One- and two-item measures of pain beliefs and coping strategies. Pain. 2003;104:453–469.
Jensen M.P., Moore M.R., Bockow T.B., et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Archives of Physical Medicine and Rehabilitation. 2011;92:146–160.
Jensen M.P., Turner J.A., Romano J.M., et al. Coping with chronic pain: a critical review of the literature. Pain. 1991;47:249–283.
Jensen M.P., Turner J., Romano J. Pain belief assessment: a comparison of the short and long versions of the Survey of Pain Attitudes. Journal of Pain. 1999;1:138–150.
Katz J., Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Review of Neurotherapeutics. 2009;9:723–744.
Keefe F.J. Pain behavior observation: current status and future directions. Current Review of Pain. 2000;4:12–17.
Kerns R., Turk D., Rudy T. The West Haven–Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23:345–356.
Labus J.S., Keefe F.J., Jensen M.P. Self-reports of pain intensity and direct observations of pain behavior: when are they correlated? Pain. 2003;102:109–124.
Leeuw M., Goossens M.E., Linton S.J., et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of Behavioral Medicine. 2007;30:77–94.
Lefebvre J.C., Keefe F.J. Memory for pain: the relationship of pain catastrophizing to the recall of daily rheumatoid arthritis pain. Clinical Journal of Pain. 2002;18:56–63.
Loeppke R., Hymel P.A., Lofland J.H., et al. Health-related workplace productivity measurement: general and migraine-specific recommendations from the ACOEM Expert Panel. Journal of Occupational and Environmental Medicine. 2003;45:349–359.
Lumley M.A., Cohen J.L., Borszcz G.S., et al. Pain and emotion: a biopsychosocial review of recent research. Journal of Clinical Psychology. 2011;67:942–968.
MacKenzie E.J., Morris J.A., Jr., Jurkovich G.J., et al. Return to work following injury: the role of economic, social, and job-related factors. American Journal of Public Health. 1998;88:1630–1637.
Mahrer N.E., Gold J.I. The use of virtual reality for pain control: a review. Current Pain and Headache Reports. 2009;13:100–109.
Main C.J., Waddell G. Behavioral responses to examination. A reappraisal of the interpretation of “nonorganic signs,”. Spine. 1998;23:2367–2371.
Mallen C.D., Peat G., Thomas E., et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. British Journal of General Practice. 2007;57:655–661.
Marceau L.D., Link C., Jamison R.N., et al. Electronic diaries as a tool to improve pain management: is there any evidence? Pain Medicine. 2007;8(Suppl 3):S101–S109.
McCracken L.M. Learning to live with the pain: acceptance of pain predicts adjustment in persons with chronic pain. Pain. 1998;74:21–27.
Miles C.L., Pincus T., Carnes D., et al. Can we identify how programmes aimed at promoting self-management in musculoskeletal pain work and who benefits? A systematic review of sub-group analysis within RCTs. European Journal of Pain. 2011;15:775. e1–e11
Morley S., Eccleston C., Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1–13.
Morley S., Williams A.C., Black S. A confirmatory factor analysis of the Beck Depression Inventory in chronic pain. Pain. 2002;99:289–298.
Nicholas M.K. The pain self-efficacy questionnaire: taking pain into account. European Journal of Pain. 2007;11:153–163.
Ohrbach R., Turner J.A., Sherman J.J., et al. The research diagnostic criteria for temporomandibular disorders. IV: evaluation of psychometric properties of the axis II measures. Journal of Orofacial Pain. 2010;24:48–62.
Pilkonis P.A., Choi S.W., Reise S.P., et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18:263–283.
Prasad M., Wahlqvist P., Shikiar R., et al. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics. 2004;22:225–244.
Primdahl J., Wagner L., Horslev-Petersen K. Self-efficacy as an outcome measure and its association with physical disease-related variables in persons with rheumatoid arthritis: a literature review. Musculoskeletal Care. 2011;9:125–140.
Revicki D.A., Chen W.H., Harnam N., et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158–169.
Roelofs J., van Breukelen G., Sluiter J., et al. Norming of the Tampa Scale for kinesiophobia across pain diagnoses and various countries. Pain. 2011;152:1090–1095.
Romano J.M., Jensen M.P., Turner J.A. The Chronic Pain Coping Inventory-42: reliability and validity. Pain. 2003;104:65–73.
Rosenstiel A.K., Keefe F.J. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44.
Smeets R.J., Severens J.L., Beelen S., et al. More is not always better: cost-effectiveness analysis of combined, single behavioral and single physical rehabilitation programs for chronic low back pain. European Journal of Pain. 2009;13:71–81.
Smith M.T., Haythornthwaite J.A. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8:119–132.
Stacey B.R., Swift J.N. Pregabalin for neuropathic pain based on recent clinical trials. Current Pain and Headache Reports. 2006;10:179–184.
Stewart W.F., Ricci J.A., Chee E., et al. Lost productive time and cost due to common pain conditions in the US workforce. JAMA: The Journal of the American Medical Association. 2003;290:2443–2454.
Sullivan M.J., Bishop S.R., Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532.
Sullivan M.J., Thorn B., Haythornthwaite J.A., et al. Theoretical perspectives on the relation between catastrophizing and pain. Clinical Journal of Pain. 2001;17:52–64.
Tait R.C., Pollard C.A., Margolis R.B., et al. The Pain Disability Index: psychometric and validity data. Archives of Physical Medicine and Rehabilitation. 1987;68:438–441.
Thompson M., McCracken L.M. Acceptance and related processes in adjustment to chronic pain. Current Pain and Headache Reports. 2011;15:144–151.
Turner J.A., Brister H., Huggins K., et al. Catastrophizing is associated with clinical examination findings, activity interference, and health care use among patients with temporomandibular disorders. Journal of Orofacial Pain. 2005;19:291–300.
Turner J.A., Mancl L., Aaron L.A. Brief cognitive-behavioral therapy for temporomandibular disorder pain: effects on daily electronic outcome and process measures. Pain. 2005;117:377–387.
Van Damme S., Crombez G., Bijttebier P., et al. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319–332.
Veehof M.M., Oskam M.J., Schreurs K.M., et al. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152:533–542.
Vowles K.E., McCracken L.M., McLeod C., et al. The Chronic Pain Acceptance Questionnaire: confirmatory factor analysis and identification of patient subgroups. Pain. 2008;140:284–291.
Wachholtz A.B., Pearce M.J. Does spirituality as a coping mechanism help or hinder coping with chronic pain? Current Pain and Headache Reports. 2009;13:127–132.
Wetherell J.L., Afari N., Rutledge T., et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain. 2011;152:2098–2107.