Cancer Pain
Treatment Overview
Introduction
Cancer pain, as discussed in Chapter 72, is a complex chronic pain that often has multiple physical components invoking different pain mechanisms, along with a significant affective component. It is therefore to be expected that treatment of patients with cancer pain should be multimodal and incorporate optimal combinations of analgesics and adjuvant analgesic drugs, psychological and social support, and specific cancer treatments. This is based on careful assessment of the individual components of a patient’s pain and individualization of the treatment program. A further important principle in the management of cancer pain is that the disease is a dynamic process that will evolve from day to day and week to week. This demands that all treatment programs be kept under careful review and different treatment modalities be introduced as the situation changes from that of a relatively fit ambulant patient to one at the end of life.
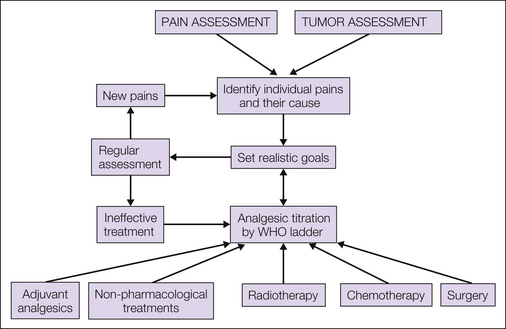
Management decisions should be based on a careful evaluation of the underlying pain, as described in Chapter 73, to define its components and the specific underlying cause of each of them. A careful psychological and social assessment of the patient should be included. In addition, the underlying disease requires assessment to determine whether the malignancy is potentially curable, whether it is advanced but amenable to palliative treatment when medium-term survival (i.e., several months) is expected, or whether the patient is approaching the end of life. An overview of management is presented in Figure 75-1.
Aims and Goals in Managing Cancer Pain
When embarking on the treatment of cancer pain it is important to establish a dialogue with patients and their caregivers and to clearly define expectations from treatment. This avoids unrealistic hopes compounding the already complex emotional response to advanced cancer and its underlying symptoms. As a simple rule of thumb it is usually possible to define four levels of pain relief that can be aimed for:
It is also important to have clear expectations with regard to the concept of “control” of pain and to understand what the patient understands by relief of pain and what the patient’s expectations are. Very few patients will require no analgesics and record pain scores of zero. The expectation in this population is that regular analgesics with or without adjuvant analgesics will be required; other specific interventions may also be necessary, but nonetheless, there may still be episodes of discomfort or a need for changes in lifestyle to ensure that pain is not a significant component of their experience.
Treatment Options
The treatments available for the management of patients with cancer pain are outlined in Figure 75-1. Most patients will require pharmacological intervention with regular analgesics with or without adjuvant analgesics. Anticancer therapy may be indicated in specific scenarios, for example, bone metastasis requiring radiotherapy or a pathological fracture of a long bone requiring surgery. In some selected cases, non-pharmacological interventional pain management will be necessary. All patients, however, are likely to benefit from psychological support during their illness, and optimal management of cancer pain requires a large multidisciplinary team that includes oncologists, palliative care physicians, specialist nurses, pain specialists, anesthetists, surgeons, psychologists, physiotherapists, occupational therapists, and spiritual counselors.
Pharmacological Pain Treatments
Analgesics have pain-relieving activity and work on the physiological mechanisms of pain. Adjuvant analgesics are drugs that have a primary indication for conditions other than pain but will be analgesic in certain painful conditions, for which they can modify the underlying pain process.
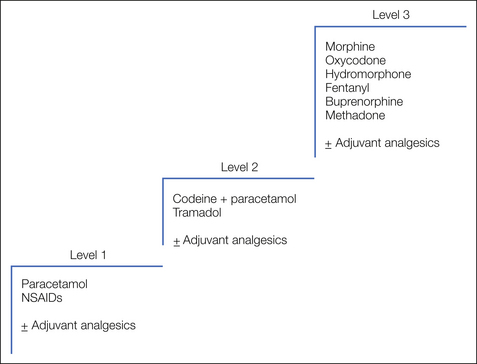
The use of analgesics for cancer pain is well established and follows clear guidelines based on the serial introduction of drugs with increasing analgesic potency titrated to pain relief as described by the World Health Organization (WHO) analgesic ladder (WHO 1996) illustrated in Figure 75-2. The ladder defines a number of fundamental principles:
The WHO Analgesic Ladder
The WHO analgesic ladder defines stepwise changes in medication through a hierarchy of increasingly potent analgesic drugs. This approach enables a simple stepwise escalation in analgesic potency until relief of pain is achieved. Although this approach has not been subject to validation in large randomized controlled trials, several large descriptive studies provide level III evidence of its efficacy. The largest of these included 2118 patients managed with strict adherence to the analgesic ladder. Step 1 analgesics were used on 11%, step 2 on 31%, and step 3 on 49% of treatment days. Seventy-six percent had good and 12% had satisfactory pain relief, whereas the remainder required additional measures such as neurolytic procedures to achieve pain control (Zech et al 1995). Further evidence in support of a formal escalation program comes from a randomized study involving 81 cancer patients in which allocation to a formal “multilevel treatment algorithm” was compared with “standard-practice” pain control. A significant reduction in “usual pain intensity” was seen in the structured algorithm group (DuPen et al 1999).
The original analgesic ladder used a straightforward three-step escalation from simple non-opioid analgesics to weak opioids to strong opioids, and this general principle has remained unchallenged for many years. The drugs within each step have evolved with the introduction of new agents, but the three-step approach allows these new agents to be appropriately incorporated into clinical practice. There is some evidence that the second step of the ladder may delay patients receiving strong opioids and thus achieving pain control, and some palliative care physicians use a two-step ladder in selected patients (Maltoni et al 2005). Most adhere to the three-step ladder, but a large randomized controlled trial to compare the two approaches is currently under way.
Step 1 Analgesics
Paracetamol
Paracetamol is the simplest and safest analgesic available at step 1 (Sykes and Hanks 1998). Alternatives include aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs), but they may be associated with more side effects. Despite being a widely available painkiller, paracetamol is an effective analgesic, and patients who have not undergone a trial of regular analgesia should have an initial period of regular full-dose paracetamol, 1 g every 6 hours. Paracetamol is predominantly a peripherally acting analgesic with antipyretic activity that works through central and peripheral non-opioid action that has yet to be fully defined. Significant toxicity is rare with therapeutic doses, and the only concern with paracetamol relates to its hepatotoxicity with an acute overdose or longer-term dosing above recommended levels.
Non-steroidal Anti-inflammatory Drugs
NSAIDs, including aspirin, are also appropriate analgesics at step 1 (Sykes and Hanks 1998). In addition, they have adjuvant analgesic activity in situations in which their anti-inflammatory action may be of value, such as musculoskeletal pain. The major drawback of NSAIDs relates to their wider toxicity profile. They will be discussed further in the section Adjuvant Analgesics.
Step 2 Analgesics
At step 2 of the analgesic ladder patients should have a weak opioid added to paracetamol. Many proprietary formulations of paracetamol and a weak opioid are available.
Codeine
Codeine, usually in the formulation codeine phosphate, is a weak opioid; chemically, it is methyl morphine. When given alone it is a relatively weak analgesic with a number needed to treat (NNT) of 10–20 to achieve 50% pain relief. Its NNT when used as a combination of codeine, 60 mg, and paracetamol, 1 g (as co-codamol 30/500), however, is 2.2 (Bandolier 2007). It is an opioid agonist and is associated with the usual spectrum of opioid-related side effects (i.e., constipation and nausea). It is recognized to have cough-suppressant activity, but clinically relevant respiratory depression is not seen in patients with normal renal function. Its oral bioavailability is around 35% and it is metabolized in the same way as morphine; indeed, approximately 10% of codeine is demethylated to the parent morphine molecule. This may account in part for its analgesic activity (Findlay et al 1978).
If co-codamol 30/500, two tablets every 6 hours, is ineffective, there is little value in trying alternative step 2 analgesics. This is an indication that a strong opioid is required.
Dihydrocodeine
A semisynthetic derivative of codeine, dihydrocodeine is equipotent to codeine given alone orally. It is the weak opioid component of co-dydramol in combination with paracetamol. It has no advantages over codeine and is inferior to paracetamol and codeine in combination. In single-dose studies, 30 mg of dihydrocodeine was found to have an NNT of at least 8.1 (Edwards et al 2000).
Tramadol
Tramadol has both weak opioid agonist activity and effects on noradrenaline and serotonin uptake in the spinal cord. A single 100-mg oral dose of tramadol is equivalent to 1000 mg of paracetamol. A dose of 100 mg has an NNT of 4.6 for at least 50% pain relief. In some countries it has the advantage of being outside the regulatory restrictions of strong opioid prescription, which can facilitate its use in the community. Otherwise, it is no different from the other drugs in this group, has lower efficacy than co-codamol 30/500, and has the same associated potential side effects when a standard dose of 50–100 mg every 6 hours is used (Brown et al 1989).
Step 3 Analgesics
When full-dose regular step 2 analgesia is ineffective, there is no value in switching to an alternative step 2 analgesic. More than 50% of patients ultimately require regular strong opioids (Hoskin and Hanks 1988), and even though there are a large number of drugs available in this class, morphine remains the drug of choice for most patients since it is cheap and readily available. There are still restrictions on its use in many parts of the world.
In the management of cancer-related pain, morphine should whenever possible be taken regularly by mouth, although in patients unable to take the drug orally, parenteral formulations are readily available and may be used. Rectal preparations are also available and may be useful occasionally.
Principles of Morphine Use for Cancer Pain
Morphine should be introduced when regular, full-dose step 2 analgesics are ineffective. The usual starting dose is 10 mg of normal-release morphine every 4 hours. Both liquid and tablet formulations are available, and they should be given according to the patient’s preference. Regular administration every 4 hours necessitates a dose in the middle of the night. The previous practice of giving a double dose as the patient’s last dose of the day is no longer recommended because it is not effective. Other important considerations include the following:
Dose Titration
The standard starting dose of morphine is 10 mg every 4 hours; however, the majority of patients will require larger doses, and the correct dose for an individual patient is achieved by careful “dose titration.” This requires close monitoring of the response of the patient’s pain to the medication and serial dose increments until adequate pain control is achieved within the limits of intrusive side effects. The median dose requirements in series of patients that have been published are in the range of 40–60 mg every 4 hours. Patients requiring escalation beyond this point should have their pain carefully reassessed and the role of other pain measures reviewed as detailed elsewhere in this chapter, alongside continued careful dose titration (Expert Working Group 1996).
Side Effects of Morphine
The side effects of morphine (and other strong opioids) may be either idiosyncratic or dose related. The majority of patients becomes constipated when taking morphine, and 30–50% will experience nausea and vomiting and, some, a dry mouth. Dose-related side effects increase if the patient’s dose has to be increased to control pain. Such effects include sedation, confusion, vivid dreams, hallucinations, myoclonic jerks, and finally, respiratory depression.
Constipation is universal with opioid medication and should be prevented with the regular use of laxatives. Its main cause is related to smooth muscle relaxation, and therefore a bowel stimulant together with a fecal softener is usually necessary. Proprietary preparations are available that contain both these components in a single formulation, which may be more convenient for the patient. Combinations of senna or bisacodyl with docusate sodium or lactulose are equally effective and do allow differential titration of the softener and stimulant. Opioid-induced constipation should be manageable with oral agents, but sometimes patients will also require rectal measures such as suppositories and enemas.
Methylnaltrexone is a methylated form of the μ-opioid antagonist naltrexone that blocks the peripheral effects of opioids without reversing centrally mediated analgesia. It is administered subcutaneously and leads to 57% of patients having a bowel movement within 4 hours (Lipman et al 2011). It is associated with some nausea and abdominal pain. Methylnaltrexone may be useful for some patients with difficult opioid-induced constipation, but most patients should be managed with adequate laxatives given by mouth.
Nausea and vomiting occur in 30–50% of patients starting regular morphine. The majority will respond to regular antiemetics. The mechanism of opioid-induced nausea is predominantly through a central mechanism via the chemoreceptor trigger zone, although peripheral smooth muscle relaxation may also be a component. In the first instance, antiemetics such as haloperidol or cyclizine, which act predominantly centrally, are recommended while recognizing that they may in themselves have additional side effects, in particular, some drowsiness and dry mouth with cyclizine. When these drugs are ineffective, metoclopramide with additional peripheral activity may be of value. If these agents are unhelpful and vomiting continues, subcutaneous levomepromazine may be considered.
Dry mouth has been reported as a morphine-related side effect but will also be compounded by the use of other drugs with anticholinergic activity, for example, cyclizine and antidepressants. This may require attention to oral hygiene and the use of sips of water, ice chips, chewing gum, or artificial saliva.
Drowsiness or a degree of sedation is common when starting morphine or when undergoing dose escalation. It is usually self-limited and best managed by careful explanation and reassurance. As physical tolerance develops, these effects usually regress. Because of this, however, patients should be advised not to drive or undertake other tasks that require similar skills for a few days after initiating or changing the morphine dose (Vainio et al 1995). There is no contraindication to such activity once on a stable dose. In patients in whom dose escalation leads to unacceptable side effects, particularly sedation, despite best efforts to control them, a switch to an alternative strong opioid may produce lesser effects. The psychostimulants dextroamphetamine and methylphenidate have been used to manage the sedative side effects and cognitive impairment associated with opioids. A recent systematic review concluded that methylphenidate may have a role in managing opioid-induced sedation or cognitive impairment, or both, in patients for whom opioid dose reduction or switching to an alternative opioid is not possible, but the recommendation is weak because of a poor-quality evidence base (Stone and Minton 2010).
Cognitive impairment may lead to confusion, and some patients experience particularly vivid and frightening dreams. They may also have visual or auditory hallucinations. These adverse effects often respond to haloperidol given as a single dose of up to 5 mg at night or switching to an alternative opioid.
Myoclonic jerks are manifestations of flexor myoclonus that occur in patients with opioid toxicity, usually in the arms, but they can also occur in the legs. In patients who become drowsy and cognitively impaired while taking opioids, opioid toxicity is often thought to be the cause; however, patients with cancer pain will frequently have many drug- and non–drug-related reasons for their deterioration, such as infection. The presence of myoclonic jerks is helpful in making the diagnosis of opioid toxicity. Patients may need to be cautioned to handle hot drinks with care, and relatives should be reassured that the myoclonus is not due to pain, although patients with severe movement-related pain may find that severe myoclonic jerking precipitates pain. Myoclonus usually settles with time or as the opioid dose is reduced, but if it remains troublesome, benzodiazepines may reduce the myoclonus.
Respiratory depression is often quoted by health care professionals as a concern in patients taking high regular doses of morphine. In practice, “pain is the physiological antagonist” to the respiratory-depressant effects of morphine, and giving patients inadequate doses of analgesics for their pain is an infinitely greater problem than respiratory depression. Systematic evaluation of changes in blood gas findings in patients taking regular morphine for cancer pain reveals no evidence of carbon dioxide retention or other parameters of respiratory failure (Estfan et al 2007).
Addiction is also still quoted as a concern with the use of morphine for cancer pain. There is a wealth of clinical experience on the use of regular high-dose morphine by cancer patients that confirms that addiction is extremely rare in this setting (Porter and Jick 1980). Physical dependence does occur as a physiological response to opioid receptor stimulation with the regular administration of opioid agonists, and hence a slow increase in morphine dose is observed in some longitudinal studies. This is not invariable in clinical practice. Abrupt cessation of morphine in patients who may, for example, undergo a surgical procedure to relieve their pain will result in physical withdrawal symptoms in about 10% of patients. This can also occur with the inadvertent or inexpert administration of an opioid antagonist such as naloxone. If the pain can be managed by a surgical, anesthetic, or neurolytic procedure, gradual withdrawal of morphine over a short period is entirely possible without the development of withdrawal symptoms.
Addiction is a more complex phenomenon related to psychological dependence and habituation alongside physical dependence that results in drug craving and sociological changes to enable continuing and ever-increasing supplies of the drug. This is not a recognized phenomenon in the use of morphine for cancer pain.
Opioid pseudo-addiction has been described and refers to a phenomenon that reflects inadequate use of morphine in a patient suffering severe pain. In this setting, patients will seek to persuade their carers of the severity of their pain and, if denied appropriate analgesics, will go to extreme lengths to seek attention and obtain analgesics, thereby creating a situation of mistrust on both sides; the patient requires what appears to be ever-increasing doses of analgesics for the pain, whereas carers observe abnormal behavior and what they interpret as exaggeration of symptoms to acquire analgesics. This situation should not evolve in modern pain care settings in which appropriate regular use of analgesics according to the analgesic ladder is in operation; sadly, however, it is still seen.
Morphine Toxicity
Long-term side effects with morphine are unusual when it is used for cancer pain. Nausea and constipation should be controlled with appropriate adjuvant medication, drowsiness will resolve in most patients, and formal assessment of psychomotor function in cancer patients with established long-term oral morphine treatment has revealed no major differences from controls who took no regular analgesics (Vainio et al 1995).
Used by the oral route, given regularly, and taken with appropriate medication to avoid predictable side effects, morphine is an extremely safe analgesic drug when taken in appropriate doses to control chronic cancer pain. Toxicity arises in one of three situations:
Modified-Release Preparations
Modified-release preparations have obvious advantages for patients requiring regular chronic use of a drug, and they are available for morphine with timed release over a period of 12 and 24 hours. Twice-daily preparations are most commonly used. Randomized controlled trials have confirmed that these preparations achieve analgesia equivalent to the same dose of normal-release morphine every 4 hours and can be substituted on an equivalent total 24-hour dose basis; for example, a patient requiring 20 mg of normal-release morphine every 4 hours will require 60 mg of modified-release morphine every 12 hours. Initial treatment in the hospital is usually with normal-release morphine, and once the patient’s 24-hour dose requirement has been established, treatment can be switched to a modified-release preparation. Randomized controlled trial evidence has shown that no loading dose is required and a simple dose-for-dose switch can be made (Hoskin et al 1989b).
An important principle of using modified-release preparations is that breakthrough medication should be available between the prolonged dosing intervals and be in the form of normal-release morphine at a dose every 4 hours equivalent to that required to achieve the total 24-hour dose. For instance, a patient requiring 120 mg of morphine over a 24-hour period will require a 20-mg breakthrough dose of normal-release morphine. Regular use of normal-release breakthrough medication should lead to adjustment of the total modified-release dose to the new 24-hour dose requirement, provided that breakthrough medication has not been taken to pre-empt movement-related pain, for example.
Modified-release preparations may have a role in initial therapy, with normal-release breakthrough medication used to titrate the dose against a background level. There are limited trial data to suggest that this may be equally effective as titrating a normal-release formulation throughout (Klepstad et al 2003), but this is common practice when initiating opioids in the community.
Pain Poorly Responsive to Opioids
Some patients have pain that is poorly responsive to opioids, although the number of patients who have no pain response to opioids is low. Some patients experience side effects that limit dose escalation of morphine, in particular, sedation and cognitive impairment (Hanks et al 2001) and in some cases nausea, which may be less prominent if treatment is switched to an alternative opioid. This is distinct from pain that is truly poorly responsive to opioids, which should be considered in patients who are requiring dose escalation into the upper 10th percentile of the dose level, typically in this population above the equivalent of 110 mg of morphine every 4 hours (Hoskin and Hanks 1988) or those who have opioid-induced dose-related side effects without relief of pain. Neuropathic pain and incident (movement-related) musculoskeletal pain can be particularly difficult to control, and additional adjuvant intervention is required. Opioid medication is, however, usually necessary in combination with these adjuvant interventions.
A small group of patients do have true opioid-resistant pain. The pathophysiology may be characterized by hyperalgesia or allodynia, possibly as a consequence of central sensitization in which the N-methyl-D-aspartate (NMDA) receptor plays an important role (Woolf and Thompson 1991). Therefore, NMDA antagonist drugs may be useful in the management of this condition. Ketamine is the most commonly used drug in this setting (Mercadante 1996). It is a competitive NMDA antagonist, allows resetting of the NMDA receptors, and restores the normal sensitivity to pain and opioid analgesia. It should, however, be used only after careful assessment of the patient and confirmation that the pain does not respond to morphine or alternative opioids. It must be administered in a controlled inpatient setting with careful monitoring. In palliative care, a continuous subcutaneous infusion commencing at 0.1–2.5 mg/kg/hr and increasing in 50- to 100-mg/24-hr increments is used. The most disturbing toxicity is a florid psychotomimetic disturbance, which usually requires the co-administration of drugs such as midazolam or haloperidol. There may also be associated hypotension. Since it works by restoring opioid sensitivity, it is important to maintain opioid medication during the use of ketamine.
Dextromethorphan also has NMDA receptor antagonist activity (Elliot et al 1995) but does not appear to be useful in this context. Similarly, the d-isomer of methadone (Davis and Inturissi 1999) and some of the newer NSAIDs (Ebert et al 1998) are recognized to influence NMDA activity, but this remains under evaluation.
Finally, it is important to exclude from the labels “opioid poorly responsive” or “opioid-resistant” pain those patients with “opioid-irrelevant” pain, which is pain in which the affective component is the predominant cause of the patient’s distress. In this setting, careful evaluation and assessment of the underlying psychological distress, appropriate use of psychological support, and administration of anxiolytic and antidepressant drugs will be more fruitful approaches than ever-increasing doses of analgesics.
Other Step 3 Strong Opioid Analgesics
Oxycodone is an alternative to morphine in patients in whom dose escalation is limited by side effects. It may also have a role in patients with moderate renal impairment. It may be given orally or parenterally (or rectally) with equivalent formulations to those available for morphine, including every-4-hour normal-release and every-12-hour modified-release tablets. Oxycodone is twice as potent as morphine given orally, and therefore when switching to oxycodone, the morphine dose should be divided by two. It is twice as potent parenterally as orally. Its overall side effect profile is very similar to that of morphine; however, for individual patients it may be better tolerated than morphine.
Hydromorphone is a further alternative to morphine. It should also be considered in patients who have troublesome side effects with morphine that limit dose escalation or in those with moderate renal impairment. It is available in equivalent normal- and modified-release preparations via the oral and parenteral routes. Hydromorphone is 5–10 times as potent given orally as oral morphine, and the suggested conversion ratio is 7.5. It may be given parenterally and can also therefore be seen as an alternative to diamorphine for parenteral use. It is twice as potent parenterally as it is when given by mouth (Houde 1986). As with oxycodone, its side effect profile is similar to that of morphine, but individual patients may tolerate it better.
Fentanyl is a semisynthetic opioid that is available in transdermal and transmucosal formulations. It is highly potent as an opioid agonist with an analgesic dose ratio equivalent to that of morphine of 1:80–100 when given in single parenteral doses. Its greater lipid solubility enables absorption across the skin, and when given parenterally it has a more rapid and extensive distribution with an elimination half-life of up to 12 hours.
Transdermal fentanyl is particularly useful in patients unable to take oral drugs because of difficulty swallowing or nausea and vomiting. It may be relatively less constipating (Ahmedzai and Brooks 1997). The transdermal patches need to be attached to hairless skin, usually on the upper part of the trunk or arm. Fentanyl is released in controlled fashion from the patch and forms a subcutaneous depot of drug. Therapeutic drug levels are reached in 12–24 hours and the patch is changed only every 72 hours. The lowest-dose patch releases 25 μg/hr, which is approximately equivalent to 10–20 mg of morphine every 4 hours. A 12-μg/hr patch is available for pediatric use. Transdermal fentanyl is therefore inflexible for dose titration, and most specialists suggest that patients be titrated to pain control with a normal-release opioid preparation before conversion to transdermal administration. They should also have ready access to breakthrough medication, the drug of choice usually being morphine in an appropriate dose. Transdermal fentanyl is not suitable for patients with unstable pain because of its 72-hour dosing schedule. Local factors may also affect absorption from the skin, and a clear temperature dependence has been demonstrated, with increased absorption in febrile patients (Southam 1995). This may result in unexpected toxicity if not taken into account.
Since plasma levels take 12–24 hours to reach their plateau, patients switching from high doses of morphine to fentanyl will require ready access to normal-release morphine for relief of pain for the first 12–24 hours of wearing the patch before reducing administration to breakthrough doses only (Portenoy et al 1993). Up to 10% of patients have been reported to experience a morphine withdrawal reaction, usually diarrhea, on switching from alternative opioids to transdermal fentanyl (Zenz et al 1994).
Transmucosal fentanyl preparations have arisen out of a desire to have a formulation that offers rapid-onset and rapid-offset analgesia for patients with movement-related or incident and therefore often short-lived pain. Fentanyl is ideal for these preparations because it is lipid soluble and potent and thus easily absorbed across the mucosal membranes of the nose or mouth. Various preparations are available: transmucosal lozenges, buccal and sublingual tablets, oral effervescent preparations, and nasal sprays. There is little to choose between them. They all provide more rapid onset of analgesia than oral morphine does with improvement in pain scores within 10 minutes; however, like morphine, the duration of analgesia is about 4 hours (Christie et al 1998).
Buprenorphine is a partial agonist at the μ-opioid receptor and is available as sublingual tablets and transdermal patches that last 4 or 7 days. Like transdermal fentanyl, it takes 11–21 hours to reach therapeutic levels. Four-day patches are available that deliver 35, 52.5, or 70 μg/hr, with 70 μg/hr of buprenorphine being equivalent to approximately 180 mg of morphine over a 24-hour period. The 7-day patches deliver 5, 10, and 20 μg/hr, with 20 μg/hr of buprenorphine being equivalent to approximately 45 mg of morphine over a 24-hour period. At these doses buprenorphine is probably functioning as a pure μ-opioid receptor agonist. Theoretically, sublingual buprenorphine is the opioid of choice for breakthrough pain since as a partial agonist, buprenorphine would displace the more potent morphine at the μ-opioid receptor. In clinical practice, the use of morphine for breakthrough pain does not seem to be problematic. The morphine equivalence of the transdermal buprenorphine patches is low, so their use for cancer pain is limited, and there is certainly no evidence that transdermal fentanyl or buprenorphine should be used as first-line treatment of cancer pain (Tassinari et al 2011).
Methadone is an strong opioid alternative to morphine and may be advantageous in patients with neuropathic or other pain demonstrating relative resistance to morphine since it has weak NMDA antagonist activity together with inhibitory effects on serotonin and noradrenaline uptake mechanisms. It can be given orally but has very variable pharmacokinetics even within patients, with an elimination half-life of up to 5 days, particularly in the elderly. Switching from alternative opioids is complex, and a number of regimens are available; however, regular dosing is given at 6- to 12-hour intervals with careful monitoring and adjustment. It is essential that one be particularly alert for opioid toxicity with sedation and impaired cognitive function suggestive of drug accumulation, which is managed by increasing the dosing interval. There is also variation in the relative potency of methadone to morphine (Ripamonti et al 1998). Single-dose data suggest that methadone is equipotent, but with chronic use it may be relatively more potent, and equianalgesic dose ratios of up to 15:1 morphine to methadone have been quoted. The dose equivalence seems to be dose related, with the greatest methadone sensitivity seen in patients taking the highest doses of morphine before switching. It is a useful and effective drug but requires considerable skill, experience, and careful monitoring in its initiation and titration.
Tapentadol is a newer centrally acting analgesic that acts as an agonist at the μ-opioid receptor and a norepinephrine reuptake inhibitor. It is approximately 2.5 times less potent than morphine. Tapentadol has been used mainly for chronic non-cancer pain and provides at least similar analgesia to equivalent doses of other strong opioids. There is some evidence that it produces fewer gastrointestinal (GI) side effects (Riemsma et al 2011). Its role in cancer pain is unproven thus far.
Diamorphine, or diacetylmorphine, is chemically closely related to morphine, and when taken orally it functions simply as a prodrug for morphine. Diacetylation occurs during the first pass through the liver. It is used rarely orally because it has a very short shelf life. The main advantage of diamorphine is the ability to administer it parenterally because of its much greater solubility, which enables a smaller volume of dilution than can be achieved with morphine; in addition, as a result of its greater lipid solubility it is the opioid of choice (where available) for spinal administration. When given parenterally it has more rapid entry into the central nervous system because of its greater lipid solubility and therefore has a more rapid onset of action, which is the basis of its effect among drug misusers. In the management of chronic cancer pain it is not an essential drug but a useful alternative to morphine to keep injection volumes low; the recommended maximum concentration of diamorphine for subcutaneous use is 250 mg/mL, as opposed to just 30 mg/mL for morphine. The use of subcutaneous opioids often allows a patient to be discharged home to die, where the oral route is not available and intravenous use would not be practical in the community. Diamorphine is, however, available in only a few European countries. Parenteral morphine is equally effective and can be used where diamorphine is not available.
Other Strong Opioids
A number of other strong opioid drugs are available, none of which has significant advantages over those just described and some of which have distinct disadvantages—for example, pethidine with its short half-life and potential accumulation of the toxic metabolite nor-pethidine with chronic use. It is therefore recommended that for the management of cancer pain, the number of strong opioid drugs used by any individual practitioner or unit be limited to those detailed earlier, with morphine being the strong opioid of choice for oral or parenteral administration and oxycodone or hydromorphone being alternative formulations when morphine is not tolerated and transdermal fentanyl when this is preferred. Diamorphine can be used to substitute for morphine when parenteral medication is indicated but has no intrinsic advantages other than its high solubility; morphine in a larger infusion volume or oxycodone or hydromorphone, which can also be given in smaller infusion volumes, are equally effective in equianalgesic doses.
Opioid Rotation or Switching
The availability of a range of strong opioid drugs has led to the proposal that when one member of the class is ineffective, rotation or switching to a different drug may provide better pain control. This approach is supported by animal data, which demonstrate that although all members of this class have μ-opioid receptor agonist activity, there is considerable heterogeneity in receptor binding profile and receptor affinity (Pasternak and Standifer 1995). Genetic studies suggest various populations of opioid receptors with different drug specificity. Mice deficient in opioid receptors have now been developed that will still respond to other opioid drugs such as diamorphine or fentanyl (Rossi et al 1995). Firm evidence of the efficacy of this approach in patients responding poorly to morphine is still lacking, with no phase III trials comparing this approach with other maneuvers such as continued dose titration, adjuvant analgesics, and non-pharmacological intervention. The superiority of opioid rotation for difficult pain control over optimal use of a single opioid drug with appropriate adjuvant pain treatment remains untested by randomized controlled trials. A recent systematic review concluded that even though the evidence is poor, opioid switching may be a useful maneuver in some patients (Dale et al 2011). It is, however, practiced widely and generally accepted. Many patients referred to palliative care teams will have already undergone one opioid switch, usually from morphine to oxycodone in the United Kingdom. Further switching preferably requires specialist advice, and a randomized assessment of its value is awaited.
Parenteral Opioids
The mainstay in the management of chronic cancer pain is regular oral administration of analgesics. This is convenient and effective and does not require intensive supervision at the point of administration, although as described earlier, careful monitoring with reappraisal of drug use is an important principle in the overall management of these patients. Provided that drug absorption is normal in the GI tract, there is no reason that parenteral analgesics will be any more effective than oral analgesics in equianalgesic doses. It is of course true that when given parenterally the same dose of morphine that has an oral bioavailability of only 35% (Hoskin et al 1989a) will be substantially more effective, but this is a simple dose–response observation, not a feature of the parenteral route itself.
In general, therefore, the parenteral route should be considered only when there are specific indications and oral administration is not possible—for example, in patients with severe nausea and vomiting, patients with difficulty swallowing or intestinal obstruction, or those with reduced levels of consciousness approaching the end of life. Rectal administration can be a useful alternative to oral administration in the community and, in a randomized crossover trial, was found to produce more rapid and prolonged pain relief than the same dose by mouth (DeConno et al 1995); however, its use is increasingly rare. The availability of transdermal fentanyl has provided a further alternative route to parenteral administration that is often simpler and more acceptable (Hanks et al 2001). Only in the acute situation of severe uncontrolled pain when it is desirable to achieve rapid delivery of opioid is there any other indication for parenteral administration.
When parenteral medication is used, the subcutaneous route is recommended since use of opioids intravenously on a regular basis has been shown to produce more rapid physical tolerance and requires ever-increasing doses to achieve the same level of analgesia. When it is anticipated that subcutaneous doses may be required for only a short time—for example, in the last few hours of life or in an acute situation because of vomiting—intermittent subcutaneous injections at the same dose interval as oral medications are appropriate. If longer-term medication is anticipated, a subcutaneous syringe driver is more convenient and allows the addition of antiemetics and other drugs such as midazolam or haloperidol, which may be of value for agitation and confusion in patients with advanced cancer at the end of life.
Spinal Opioids
Spinal opioids are used only occasionally in patients with pain from advanced cancer and should be considered only by skilled individuals working within a unit that is experienced in their use. The epidural, intrathecal, and subarachnoid routes have all been used. Drugs are usually delivered via epidural or intrathecal catheters; intrathecal drug delivery is generally considered preferable to epidural administration because of practical difficulties with epidural catheter fibrosis and a higher dose requirement. Therefore, the intrathecal route is preferable to the epidural route for longer-term use. Intrathecal catheters can lead to granuloma formation, although this is more problematic in patients with non-cancer pain who may require prolonged spinal administration.
The main indication for spinal opioids for relief of cancer pain is when systemic opioids are providing effective pain relief but severe side effects and movement-related incident pain are prominent and not adequately controlled by oral or parenteral administration. Other drugs may be co-administered by this route, and the use of clonidine has been advocated for neuropathic pain (Eisenach et al 1995) and local anesthetic for incident pain (Mercadante 1999). Morphine is considered the opioid of choice for spinal use, but hydromorphone, fentanyl, and sufentanil are equally effective. It should be noted, however, that subcutaneous, intravenous, and intrathecal delivery of fentanyl is equipotent because of its poor solubility in cerebrospinal fluid. Systemic side effects related to opioid receptor stimulation are still encountered, including nausea, vomiting, drowsiness, cognitive impairment, and psychotomimetic effects. Relatively common when using the spinal route are urinary retention and pruritus as side effects, and cranial migration may rarely cause delayed respiratory depression. Physical withdrawal symptoms have also been described in patients receiving high doses of oral or parenteral morphine who switch to spinal opioids. In occasional patients, however, this route may have a significant impact on an otherwise difficult pain control situation, and in these patients chronic administration with tunneled catheters and infusion pumps may be appropriate and is technically feasible with skilled supervision.
Adjuvant Analgesics
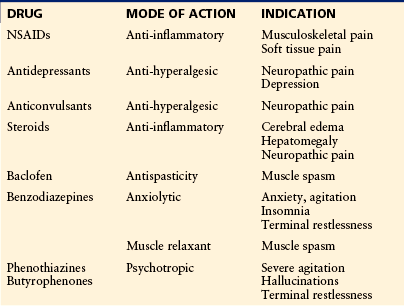
Although regular analgesics are the mainstay of pharmacological pain relief in patients with advanced cancer, many other medications may contribute to the overall success of management, particularly drugs that will modify the underlying mechanisms contributing to the overall pain that the patient experiences. These drugs are listed in Table 75-1.
Non-steroidal Anti-inflammatory Drugs
NSAIDs have intrinsic analgesic activity and are included in step 1 of the WHO analgesic ladder but may be used for their anti-inflammatory activity alongside stronger analgesics or, indeed, simple paracetamol. They are particularly recommended in circumstances associated with musculoskeletal and soft tissue visceral pain. There are a large number of individual members of this class of drugs that act through blocking cyclooxygenase (COX) activity and thereby inhibiting the synthesis of prostaglandin from arachidonic acid. Aspirin was the original member of this class of drugs and remains an effective analgesic that is used in doses of 600-650 mg four times a day, but it is associated with more frequent and serious side effects than newer members of this class of drug.
The major toxicity of concern in this patient population is GI hemorrhage, with an incidence varying from 2% with ibuprofen to 23.7% with ketoprofen and the highest incidence being reported with the use of subcutaneous ketorolac (Langman et al 1994). The drugs with the lowest risk for GI toxicity, with an odds ratio of 2 (95% confidence interval [CI], 1.4–2.8), are ibuprofen or diclofenac, and these are considered the drugs of choice in this setting for initial management. Naproxen has intermediate GI toxicity with an odds ratio estimate of 9.1 (95% CI, 5.5–15.1) for GI bleeding, and azapropazone, ketorolac, and ketoprofen are very high-risk drugs with odds ratios estimated at between 23 and 31 (Graham et al 1988). The role of GI prophylaxis with NSAIDs remains uncertain. There is randomized controlled trial evidence that misoprostol will reduce the incidence of GI bleeding with ibuprofen, piroxicam, or naproxen to just 1.4% from 21.7% with placebo (Langman et al 1994). It is common to use proton pump inhibitor drugs in this setting, either omeprazole or lansoprazole. Other factors that may influence the choice of NSAIDs relate to their half-life. Aspirin, diclofenac, and ketorolac have short half-lives, which necessitates more frequent administration. This may be less convenient in this group of patients who will be taking multiple medications.
COX has two major isoenzymes, of which COX-2 is thought to be specific for the anti-inflammatory effects and COX-1 for the GI effects. It was hoped that specific COX-2 inhibitors would provide a good analgesic profile with fewer GI side effects and thus an improved safety profile. Their efficacy was demonstrated; however, they were subsequently withdrawn because of increased risk for myocardial infarction and stroke, which was thought to be due to a relative increase in thromboxane.
A meta-analysis of the use of conventional NSAIDs for cancer pain has been undertaken; 25 randomized controlled trials that included 16 different drug types and 15,445 patients were evaluated (Eisenberg et al 1994). Of these, 13 trials were evaluating single-dose efficacy; 9 trials, multiple-dose efficacy; and 3 trials, both single and multiple doses. Overall, their efficacy in single-dose studies for metastatic pain was equivalent to 5–10 mg of morphine when given in the recommended maximum dose, with no dose response noted beyond this. The total pain relief score for NSAIDs was 52% as opposed to 45% with morphine. Undoubtedly, their main use is in combination with analgesics of appropriate strength. A small randomized trial has reported a significant reduction in the morphine dose, when adjuvant ketorolac was used in the titration phase, from a mean final dose of 249 mg in the control group to 173 mg in the group receiving adjuvant ketorolac (Mercadante et al 2002).
In addition to GI toxicity, fluid retention, renal impairment, and bronchospasm are recognized problems with this group of drugs, and care is required in patients with borderline renal function and underlying asthma.
There is level III evidence from case reports that continuous subcutaneous infusion of ketorolac may be effective for otherwise refractory pain, particularly of a musculoskeletal origin (Blackwell et al 1993), but this formulation must be used with careful monitoring and for only a limited period along with GI tract prophylaxis in view of the high reported incidence of GI bleeding with this drug.
Various topical formulations of NSAIDs are available. There has been no randomized evaluation of their efficacy for cancer pain, but for superficial joint-related pain they may have value and carry the advantage of avoiding potential systemic side effects.
Psychotropic Drugs
Cancer pain has both physical and affective components, and careful assessment of the emotional response of patients to their situation is a vital part of pain management. It follows, therefore, that psychotropic drugs may have a vital role in the pharmacological treatment of cancer pain in the few patients in whom anxiety and depression are exerting a major impact on their ability to deal with pain. In addition, there may be specific indications, such as the use of antidepressants as adjuvant analgesics for neuropathic pain.
Anxiolytics
Benzodiazepines are the most common group of drugs used when there is clear evidence that anxiety and fear are hampering a patient’s ability to cope with pain or when restlessness, agitation, and insomnia occur as a result of the pain or exacerbate the perception of pain. The choice of individual benzodiazepine can be based on the indications and the relative half-life of the drugs. When insomnia is a problem and sedation alone is required, a benzodiazepine with a short half-life such as temazepam is appropriate. Zopiclone, which belongs to the cyclopyrrolone group of drugs, is an alternative that may be better tolerated, particularly in the elderly. In a patient who has insomnia with associated mild anxiety, a drug with a longer half-life such as lorazepam, which has an effective half-life of 8–12 hours, may be more appropriate. In patients with severe anxiety and agitation requiring long-term anxiolytic therapy, diazepam is more appropriate. At the end of life, terminal agitation and restlessness may be an additional problem and midazolam is an alternative benzodiazepine that can be given by subcutaneous infusion together with strong opioids as necessary.
For patients with severe agitation or confusion and hallucinations, neuroleptic drugs, including phenothiazines such as levomepromazine and butyrophenones such as haloperidol, may be more effective. There is some evidence that levomepromazine has intrinsic analgesic activity, and acute single-dose data also suggest that for postoperative pain or myocardial infarction it is equivalent to morphine (Lasagna and DeKornfeld 1961). It may be of value for terminal agitation when it can be given by subcutaneous infusion with opioids in a syringe driver. Haloperidol may be used in patients requiring oral medication, although they should be observed for extrapyramidal effects and parkinsonian-type symptoms.
Antidepressants
In the setting of cancer-related neuropathic pain the tricyclic antidepressants (TCAs) are effective adjuvant analgesics and have been shown in randomized controlled trials to be superior to placebo. There are, however, few data on neuropathic pain related to cancer, with typical models being diabetic neuropathy, post-herpetic neuralgia, and atypical facial pain. Across the different conditions that are relieved by TCAs, meta-analysis has defined the NNT as being between 2 and 3. The serotonin–norepinephrine reuptake inhibitor venlafaxine has an NNT of 4 for non–cancer-related painful polyneuropathies and the selective serotonin reuptake inhibitors an NNT of 7 for cancer-related neuropathic pain. Duloxetine is now the drug of choice for non–cancer-related neuropathic pain with an NNT of 4.1; however, its role in cancer-related neuropathic pain remains unclear (Saarto and Wiffen 2008). The TCAs therefore remain the antidepressants of choice for neuropathic pain. Antidepressants have a clear role in patients with associated depression, and in this setting the newer group of serotonin uptake inhibitors such as fluoxetine, paroxetine, and citalopram are valuable.
Anticonvulsants
Anticonvulsants have a place as adjuvant analgesics in the management of neuropathic pain that may arise in patients with advanced cancer as a result of peripheral nerve infiltration or compression. Patients with advanced malignancy are also prone to the development of herpes zoster, and post-herpetic neuralgia may be a significant problem. Axillary or pelvic surgery may also cause post-surgical neuralgia. The most commonly used anticonvulsants for neuropathic pain in current practice are gabapentin and pregabalin, which have largely replaced drugs such as carbamazepine, phenytoin, sodium valproate, and clonazepam. However, the latter drugs can be tried in patients who have pain that is lancinating, shooting, sharp, or stabbing in nature and in whom gabapentin and pregabalin have been ineffective or poorly tolerated. The NNT for gabapentin in chronic neuropathic pain is 5.8 and that for pregabalin is 4.2 (Finnerup et al 2005). Other new drugs in this group such as lamotrigine and vigabatrin have not been fully evaluated, although a recent systematic review suggested that lamotrigine has no role in neuropathic pain (Wiffen et al 2011). It should be remembered that the NNT for morphine in relieving neuropathic pain is 2.5, and although there is a trend to using anticonvulsants, particularly gabapentin and pregabalin, as first-line adjuvant analgesics for neuropathic pain, the evidence suggests that TCAs remain the drugs of first choice.
Corticosteroids
Corticosteroids are widely used in patients with advanced cancer both for their non-specific beneficial effects in improving well-being and appetite and for specific indications in pain relief. In patients with soft tissue swelling and edema, corticosteroids are effective in reducing the swelling and edema and as a consequence may improve pain control. Specific examples are the pain associated with raised intracranial pressure from cerebral metastasis and upper abdominal pain from widespread liver metastasis. Corticosteroids may also have a role in neuropathic pain and other scenarios in which tumors are causing compressive symptoms, such as in the abdomen or pelvis and in the head and neck region.
Steroids are associated with a significant range of side effects and their use should therefore be limited to the lowest effective dose for the shortest period. Additional care is required in patients with underlying diabetes and peptic ulceration, for whom gastric protection is recommended. Weight gain, peripheral edema, and cushingoid features can appear rapidly, and the change in habitus can be disturbing to patients in the last weeks of life. Fluid retention can be a problem in patients with borderline heart failure, and in a proportion of patients acute psychotomimetic effects, both steroid psychosis and depression, can cause severe disturbance. In many patients restlessness and difficulty sleeping limit their use.
The two main formulations in use are prednisolone and dexamethasone. Dexamethasone has the advantage of being more potent and therefore fewer tablets need to be given, with doses of 4–8 mg often being sufficient. It may, however, be more prone to precipitate psychotomimetic effects, in contrast to prednisolone, which is more likely to be associated with fluid retention.
Muscle Relaxants
Muscle spasm can cause considerable pain and distress. It may be associated with local tumor infiltration into soft tissue regions or adjacent bone metastasis. A wide range of drugs may have muscle relaxant properties, including the benzodiazepines, of which diazepam is the most commonly used in this setting; others include baclofen, dantrolene, and quinine. Smooth muscle spasm causing abdominal or pelvic pain may be helped by anticholinergic drugs such as hyoscine or oxybutynin.
Bisphosphonates
Bisphosphonates are increasingly being used for the management of metastatic bone pain. They are drugs with poor oral bioavailability and are usually given as intravenous infusions, with pamidronate and clodronate being the most commonly used, although they are often replaced by the more potent drugs zoledronate and ibandronate. Currently, only clodronate is available as an oral formulation, but it is frequently poorly tolerated, causes GI symptoms, and has variable absorption. There is good evidence that in the adjuvant setting bisphosphonates reduce morbidity from bone metastasis, and three randomized trials of patients with prostate and lung cancer (Ernst et al 1992, Coleman et al 1995, Robertson et al 1995) and Cochrane reviews on breast cancer (Pavlakis and Stockler 2002) and bone metastases from various tumors (Wong and Wiffen 2002) support their role in established metastatic bone pain, the latter suggesting an NNT of 11 at 4 weeks and 7 at 12 weeks. When administered by intravenous infusion they are well tolerated with little toxicity other than mild fever, and they have an emerging role in the management of metastatic bone pain.
Non-Pharmacological Measures for Relief of Pain
Most patients will be treated with a combination of analgesics along with adjuvant analgesics, supplemented by non-pharmacological therapy. Many of these therapies are used on an empirical basis and have often not been subjected to rigorous clinical trials.
Peripheral Nerve Block
Peripheral nerve blocks can be extremely valuable in carefully selected patients who have pain associated with irritation of a specific local nerve root. Common examples include celiac plexus blockade in patients with pancreatic carcinoma, which has been validated in randomized controlled trials and a meta-analysis as being more effective than analgesics alone (Eisenberg et al 1995). Intercostal nerve block, brachial plexus and lumbosacral plexus nerve block, and trigeminal nerve blocks are also used for pain in the respective nerve distributions.
Ablative Neurosurgical Procedures
These procedures are discussed in detail in Chapter 39. They are often regarded as a last resort in patients with intractable pain but, again, in carefully selected cases can be of considerable value—for example, cordotomy in patients with unilateral pain secondary to mesothelioma—although their use for pain from advanced cancer is relatively rare.
Transcutaneous Electrical Nerve Stimulation
Transcutaneous electrical nerve stimulation (TENS) is widely available for both cancer-related and non–cancer-related pain, particularly pain with a neuropathic element. It relies on active stimulation of the large-diameter nerve fibers in skin and subcutaneous tissue that inhibit pain stimuli. TENS machines can be purchased readily and TENS may prove to be a valuable non-invasive technique because it allows patients to participate in active management of their pain. Objective evaluation, however, is problematic, partly because of the difficulty in designing an appropriate control and the heterogeneity of the studies available. At present, there is no robust objective evidence to support the efficacy of TENS in relieving cancer-related pain (Robb et al 2008).
Other Non-pharmacologic Measures
Music therapy as part of relaxation strategies may improve anxiety and has moderate effects on pain, mood, and quality of life in people with cancer (Bradt et al 2011).
Massage is often available in hospice and palliative care settings. Although there is limited evidence that it confers short-term psychological benefit to patients with cancer, any effect on physical symptoms is less clear (Wilkinson et al 2008).
Acupuncture may be used as well, and despite some evidence for its role in the management of chemotherapy-induced nausea and vomiting (Ezzo et al 2006), there is no evidence that it benefits patients with cancer pain (Paley et al 2011).
Specific Cancer Treatments
Cancer treatment includes locoregional treatment, either surgery or radiotherapy, and systemic treatment with chemotherapy or hormone therapy.
Surgery
Major surgery is rarely appropriate in patients with advanced cancer and metastatic pain. However, certain scenarios are clear indications for surgical intervention, and in such cases it results in dramatic pain relief; these indications are shown in Table 75-2.
Obstructive hydrocephalus secondary to tumor in the posterior fossa or midbrain region can cause a progressive headache that is difficult to control with analgesics or steroids. Shunting, either internally or with a ventriculoperitoneal shunt if the patient is well enough, will resolve the situation rapidly and not only relieve the pain but also improve other neurological sequelae of hydrocephalus.
Pathological fracture of a long bone is a clear indication for internal surgical fixation, following which rapid pain relief and restoration of function can be achieved.
Progressive ascites can cause persistent abdominal pain and discomfort. Repeated paracentesis may not be possible or appropriate, and a LeVeen shunt to drain the ascitic fluid into the superior vena cava can be a valuable means of resolving this situation.
Radiotherapy
Radiotherapy has considerable application in the palliative management of metastatic cancer pain. It is usually delivered as external beam treatment; common indications are shown in Table 75-3. Radiation may also be delivered by systemic radioisotopes, and this is particularly indicated for the management of scattered metastatic bone pain, for which bone-seeking isotopes such as strontium 89 (89Sr) or samarium (153Sm) ethylene diamine tetramethylene phosphonate (EDTMP) can be given intravenously to target sites of bone damage from metastatic disease and deliver radiotherapy to these sites. Such treatments are predominantly used for primary tumors associated with osteoblastic metastases, such as prostate and breast cancer.
Table 75-3
Indications for Radiotherapy in the Management of Cancer Pain
PAIN |
CAUSE |
| Bone pain | Metastases Pathological fracture (non-surgical, e.g., rib, pelvis) |
| Headache | Primary cerebral tumor Brain metastases |
| Abdominal pain | Hepatomegaly |
| Pelvic pain | Local tumor infiltration |
| Chest pain | Primary lung cancer Mesothelioma |
| Soft tissue pain | Local tumor infiltration |
Chemotherapy
Chemotherapy may also provide valuable pain relief in patients with widespread metastatic disease; common indications are shown in Table 75-4. Its principal limitation is related to the limited tumor chemosensitivity encountered in advanced and recurrent cancer. Common tumors such as breast cancer, non–small cell lung cancer (NSCLC), and colorectal cancer are responsive to chemotherapy, but resistance evolves after one or two exposures to treatment, and thereafter the efficacy of chemotherapy is often outweighed by its potential toxicity. However, in some tumors, particularly those that are more sensitive, chemotherapy has a major palliative role. Examples include multiple myeloma, which is frequently associated with widespread severe metastatic bone pain; small cell lung cancer (SCLC), which is typically widespread and affects bones, brain, and the liver; and breast cancer, for which second-, third-, or even fourth-line chemotherapy may have efficacy and an important role in palliation of pain.
Table 75-4
Indications for Chemotherapy in the Management of Cancer Pain
PAIN |
CAUSE |
PRIMARY TUMOR TYPES |
| Bone pain | Bone metastases | Myeloma Breast cancer Lung cancer (small cell and non–small cell) |
| Headache | Brain metastases | Germ cell tumors Lymphoma and leukemias [Breast cancer] [Small cell lung cancer] |
| Abdominal pain | Ascites Subacute obstruction Pancreatic pain |
Ovarian Colorectal Gastric Pancreatic |
| Pelvic pain | Local tumor infiltration | Colorectal Ovarian Cervical |
| Chest pain | Local tumor infiltration | Lung cancer (small cell and non–small cell) Metastases from chemosensitive sites, e.g., breast, colorectal [Mesothelioma] |
Note: Brackets indicate tumors with only modest (<50%) response rates, for which other modalities (e.g., radiotherapy) may be preferred.
Hormone Therapy
A small number of cancers are hormone sensitive, but these include the common cancers of the breast and prostate, which account for a large number of patients with metastatic disease and cancer pain. Antiandrogen therapy for prostate cancer results in dramatic relief of pain for many patients, with response rates greater than 90% on initial exposure. Unfortunately, this is only for a finite duration, with a median response period of 18 months to 2 years, following which further hormone maneuvers are rarely of great benefit. Breast cancer, however, may respond to second- and third-line hormone treatment with antiestrogen drugs such as tamoxifen or toremifene, aromatase inhibitors such as anastrozole and letrozole, progestogens such as megestrol or medroxyprogesterone acetate, and occasionally androgens. These hormone maneuvers may be used sequentially and achieve useful responses in patients with widespread disease and metastatic pain. The other cancer that is sensitive to hormone therapy is endometrial cancer, and metastatic disease from this site will respond in 30–40% of patients to progestogen treatment or luteinizing hormone–releasing hormone analogue treatment.
Specific Pain Problems in Cancer Patients
Musculoskeletal pain in cancer patients is most commonly associated with bone metastases. The metastases arise from blood-borne dissemination and therefore serve as a multifocal somatic source of pain that varies in its intensity between individual sites in random fashion. Some patients will have a single solitary site of severe pain with other documented bone metastases being asymptomatic, whereas in others, scattered multifocal pain, often of a flitting nature from one area to another, is the clinical scenario. Treatments based on the above discussion of available modalities will encompass a combination of pharmacological and non-pharmacological management, with radiotherapy generally being recognized as the most effective treatment in this setting; an overview is shown in Figure 75-3.
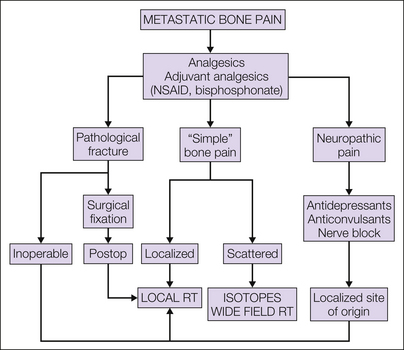
Figure 75-3 Management of metastatic bone pain.
NSAID, non-steroidal anti-inflammatory drug; RT, radiotherapy.
A review of large randomized trials of radiotherapy for bone pain revealed some information on the pattern of analgesic use in this group of patients. Between 9 (Nielsen et al 1998) and 30% (Bone Pain Trial Working Party 1999) required no analgesia and 13–38% required mild analgesia or NSAIDs alone. The range reported reflects the different patient population groups in these studies.
Moving through the analgesic ladder, the use of simple analgesics such as paracetamol, including NSAIDs as step 1 analgesia or adjuvant analgesics, will be the mainstay of pharmacological management. Other drugs that may have a role as adjuvant analgesics in this setting include skeletal muscle relaxants such as diazepam and baclofen in patients with associated muscle spasm, corticosteroids for intractable scattered pain, and bisphosphonates. Neuropathic pain may be a feature particularly related to vertebral metastasis and requires other specific treatment.
When pathological fracture of a long bone is encountered, internal surgical fixation remains the optimal management. In most other patients, radiotherapy to deliver definitive treatment will be considered. External beam radiotherapy is the usual modality for localized bone pain, whereas for more scattered sites of pain, either wide-field external beam radiotherapy or radioisotope therapy is given.
Localized External Beam Radiotherapy
Localized external beam radiotherapy for metastatic bone pain has been the subject of a large number of randomized controlled trials and two Cochrane reviews (McQuay et al 1997, Sze et al 2002). These studies confirm its efficacy with complete response rates of 32–34% and an NNT for a complete response of 3.9 (95% CI, 3.5–4.4). At least 50% pain relief was achieved by 60% with an NNT of 3.6 (95% CI, 3.2–3.9).
The optimal dose of radiation for effective pain relief remains an area of some debate, but most authorities, supported by a large body of phase III randomized trial data, conclude that single doses of 8–10 Gy are as effective as more prolonged high-dose schedules. In some studies, renal cancer and lung cancer have had lower response rates than other primary sites, but in general the underlying histological type of the primary tumor is not thought to be a major predictor of response. Toxicity is mild and related to the site of treatment. Areas that include significant amounts of bowel (e.g., the lumbosacral spine and pelvis) will result in nausea and increased bowel frequency in 20–30% of patients (Bone Pain Trial Working Party 1999). This will respond to medical management and is self-limited over a period of 10–14 days. Peripheral sites in the upper and lower limbs are not generally associated with any significant side effects.
The pattern of pain relief after external beam radiotherapy for localized bone pain has consistently been shown to evolve over a period of 4 to 6 weeks after treatment, with 50% of patients achieving a response within 2 weeks of treatment and reaching a plateau 2–4 weeks later, when on actuarial analysis around 80% of patients will have recorded a response.
Pathological fracture may be treated with external beam radiotherapy when the fracture is not surgically operable—for example, ribs, vertebral bodies, and pelvic bones. Following doses similar to those given for local bone pain, bone healing occurs over a period of 6–12 weeks after treatment but is preceded by early relief of bone pain.
Wide-Field External Beam Radiotherapy
Wide-field external beam radiotherapy is used for the treatment of multiple sites of bone pain, typically defined as upper hemibody radiotherapy covering the ribs and cervicodorsal spine or lower hemibody radiotherapy covering the lumbosacral spine, pelvis, and lower limbs, but customized variations are possible, such as moving the radiation field, which will typically be 40 cm2, to cover the majority of painful sites. This technique can be used sequentially to treat the entire skeleton, but a 4- to 6-week interval is required to allow recovery of bone marrow in the treated area before exposing the remainder of the bone marrow to radiation. Similar response rates to external beam radiotherapy are reported with a pattern of response that is much more rapid; 25% of patients have responded within the first 24 hours in some studies. One of the meta-analyses (McQuay et al 1997) included wide-field radiotherapy in the outcome data for overall response to external beam radiotherapy as detailed above. Evaluation of this therapy in a wide range of health care settings has proved its efficacy and universal application via a simple two-fraction schedule delivering 8 Gy in 2 days (Salazar et al 2001). Overall response rates are similar to those achieved with local treatments but are characteristically seen more rapidly, often within 24 hours of treatment.
Inevitably, treating larger volumes results in more toxicity when this technique is used, and around two-thirds of patients will report nausea and increased bowel frequency. Bone marrow depression is universal but rarely reaches levels of clinical significance, although in comparative series 8% of patients are reported to require blood transfusion after this technique (Quilty et al 1994). Whole-lung irradiation as delivered with upper hemibody fields can cause pneumonitis, but when the total dose is less than 6 Gy at standard dose rates, this problem is extremely rare with a reported incidence of approximately 1%. Radiation pneumonitis in this setting, however, is universally fatal.
Radioisotope Treatment
Radioisotope treatment involves the intravenous administration of a bone-seeking radioisotope to target localized radiotherapy to multiple sites of bone metastasis. This is achieved by using isotopes that are physiologically attracted to sites of bone mineralization. Strontium (89Sr) is the most commonly used currently. An alternative approach is to incorporate an appropriate radioisotope with a bisphosphonate compound, EDTMP being the most commonly used. Samarium and rhenium are available in this form. The principal radiation modality with these isotopes is β-particle radiation, which has a short range of a few millimeters and therefore concentrates the radiation dose within the site of bone damage caused by invasion of cancer cells. Samarium and rhenium also produce γ-irradiation, which enables imaging of their distribution with a gamma camera but also introduces additional radioprotection restrictions. Strontium, a pure β-particle emitter, can be given as a single outpatient intravenous injection and the patient allowed to travel home without restriction because of its short-range delivery.
Radioisotope treatment of metastatic bone pain has efficacy similar to that of wide-field external beam irradiation but is associated with less toxicity and lower transfusion requirements. Meta-analyses (McQuay et al 1997, Sze et al 2002) did not define an individual NNT for radioisotope therapy. Though of similar efficacy to external beam wide-field radiotherapy, its better toxicity profile and relative ease of delivery have meant that in a wealthy health care system radioisotope therapy has become the treatment of choice in this setting. However, when not available, wide-field external beam radiotherapy can achieve equivalent pain relief.
A further specific role for radioisotope therapy relates to bone metastases from thyroid carcinoma. Around 80% of differentiated thyroid cancers will concentrate radioiodine, and this therefore provides a potential therapeutic isotope for these metastases at any site in the body. Radioiodine is given orally in this setting in doses of 3000–5000 megabecquerels following ablation of the thyroid gland. Even though large series of patients have been reported in terms of disease-free and overall survival from thyroid cancer, only limited information on the specific response of bone metastases has been published. One paper addressing this question suggested that paradoxically, external beam radiotherapy in this setting may be more effective than radioisotope therapy (Brown et al 1984).
Chemotherapy
Chemotherapy can also be of considerable value in the management of metastatic bone pain when a tumor is chemosensitive. Examples would include myeloma, both SCLC and NSCLC, and breast cancer, with the natural history of these tumors being widespread dissemination, including bone as a common site of metastasis.
Hormone Therapy
Hormone therapy is also of value in hormone-sensitive tumors, and both breast cancer and prostate cancer commonly spread to bone, in the latter bone being the most common site and pattern of metastasis. Quite dramatic responses within a few days of starting antiandrogen therapy can be achieved for prostate cancer. Response in patients with metastatic breast cancer is generally slower, and thus additional measures for pain relief are usually required in the first few weeks of starting hormone therapy.
Intrathoracic Pain
Common causes of intrathoracic pain in patients with malignancy are NSCLC and mesothelioma. The pain is often poorly localized with respect to the primary tumor site, and with mesothelioma, neuropathic pain resulting from local infiltration of the intercostal nerves may become a prominent feature. Associated symptoms such as cough and dyspnea will also exacerbate the pain and provoke increasing anxiety, which will further compound the overall picture of intrathoracic pain from these tumors.
The general approach outlined above—the use of dose-escalating analgesics through the analgesic ladder—will therefore be required in most patients, along with supplementation with other more specific therapies as shown in Figure 75-4. When chest wall infiltration has occurred, NSAIDs may be of value, and with neuropathic pain, antidepressants and anticonvulsants will have an important role. Intercostal nerve blocks are also very effective in selected patients.
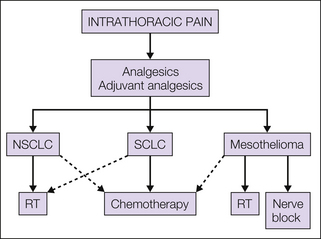
Figure 75-4 Management of intrathoracic pain.
Dashed lines, second-line options; NSCLC, non–small cell lung cancer; RT, radiotherapy; SCLC, small cell lung cancer.
For NSCLC an extensive literature of randomized controlled trials supports the role of radiotherapy for pain relief, which is achieved in about 70% of patients with single doses of 10 Gy or more prolonged fractionation schedules of 17 Gy in 2 fractions, 30 Gy in 10 fractions, or 39 Gy in 13 fractions (Timothy et al 2001). The role of radiotherapy for chest wall pain from mesothelioma is less well studied, and only small series and case reports provide evidence for this. Some efficacy with doses of 21 Gy in three fractions or more protracted fractionated courses has been reported, but the overall response appears to be less favorable than with NSCLC (Bissett et al 1991). Indeed, the pain picture presented with mesothelioma tends to be much more severe and complex.
Chemotherapy has a role in NCSLC and results in both improved quality of life and modest improvements in survival of a few months. Typical schedules will include cisplatin with gemcitabine, vinorelbine, or paclitaxel (Schiller et al 2002). Specific pain responses are difficult to distill from the published literature, but the overall improvement reported in quality of life suggests that it is an effective treatment. These tumors are not hormone sensitive, and unless localized, for which primary surgery will be the treatment of choice, surgical resection for pain from advanced disease is not indicated.
For SCLC, chemotherapy is the treatment of choice, with high response rates achieved even in patients with advanced disease; unfortunately, responses are rarely sustained, but later relapse may well respond to second- or third-line chemotherapy (Spiro et al 1989). Effective schedules for SCLC include cyclophosphamide, Adriamycin (doxorubicin), and vincristine (CAV) or etoposide and cisplatin (EP). For local pain problems, palliative radiotherapy using doses similar to those for NSCLC is also effective.
Abdominopelvic Pain
Abdominal pain in malignancy is typically visceral and is due to hepatic metastasis or bowel obstruction. Pelvic pain may have a visceral component but is also likely to have a neuropathic element from infiltration of cancer into the lumbosacral plexus. An overview of management is shown in Figure 75-5.
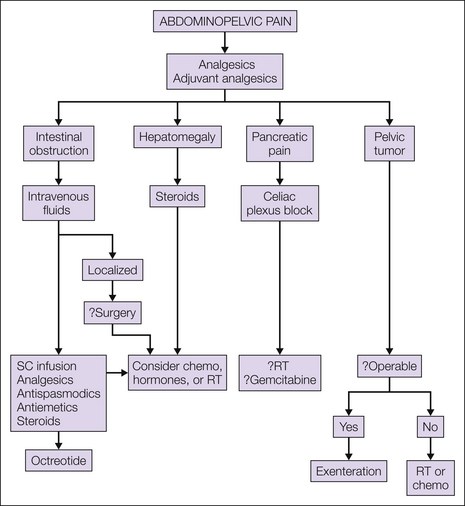
Figure 75-5 Management of abdominopelvic pain.
chemo, chemotherapy; RT, radiotherapy; SC, subcutaneous.
Hepatic metastasis typically causes pain as a result of enlargement of the liver and subsequent stretching of the capsule, where the sensory innervation is found. Use of analgesics in accordance with the analgesic ladder is recommended. In general, unless there is gross hepatic dysfunction, the common drugs in the ladder, paracetamol, codeine-based compounds, and strong opioids, are not affected by the presence of liver metastasis. Steroids may be of value in reducing hepatic edema and liver pain. When a chemosensitive tumor is present, reduction of the size of the liver with chemotherapy may improve pain. However, although hormone therapy may reduce hepatomegaly from liver metastasis, the response is often slow, with several months needed to achieve it. Two randomized controlled trials addressed the role of hepatic irradiation for advanced malignancy and concluded that effective palliation of pain can be achieved in 80% and relief of systemic symptoms in 45% of selected cases (Borgelt at al 1981).
Pain and abdominal distention from obstruction of the small or large bowel may be a feature of primary bowel tumors or, more frequently, intra-abdominal metastases typically from ovarian or breast cancer. Appropriate analgesia using the analgesic ladder is indicated. In the case of a primary tumor or a single metastatic lesion, surgical resection or bypass of an obstructing lesion may be possible. Metastatic disease is typically multifocal with several sites of obstruction and is rarely amenable to surgical measures. Medical management of intestinal obstruction will include initial intravenous fluid replacement (although most patients can be managed without long-term fluid replacement), analgesics, and anticholinergic drugs such as hyoscine. High-dose steroids are often tried, but evidence of their efficacy is weak. Subcutaneous octreotide may decrease nausea and vomiting for a period (Mercadante et al 2007).
Splenomegaly may also be a cause of abdominal pain. Typically, splenomegaly will be due to a hematological malignancy such as chronic granulocytic leukemia or lymphoma. These are chemosensitive tumors and this will be the main line of attack. High-dose steroids will also be of value, and on occasion in patients with chemoresistant disease, either surgical splenectomy or splenic irradiation will have a role in relief of pain.
Pancreatic pain is a characteristic severe visceral pain radiating into the back and often poorly controlled with analgesics, even with titration of strong opioids. Randomized controlled trial evidence has confirmed the positive role of neurolytic celiac plexus block in this setting, with superior results in terms of pain relief over analgesics alone. A meta-analysis of both controlled trials and reported series found that 59% of patients reported complete relief of pain by 2 weeks after the procedure and between 73 and 92% had continued pain relief until death (Eisenberg et al 1995).
Pelvic pain, if not caused by bone metastases, will most commonly be due to presacral recurrence of rectal carcinoma or pelvic recurrence of cervical cancer. Lumbosacral plexus infiltration is common and results in severe pain with a major neuropathic component. In addition to analgesic escalation through the analgesic ladder, early implementation of amitriptyline or gabapentin may be of value. Alternative antidepressants or anticonvulsants should be considered if these drugs are ineffective. Steroids may also have a role in intractable cases, and in selected patients an epidural anaesthetic may be of value.
Headache
Headache from malignant disease may arise as a result of raised intracranial pressure secondary to brain metastasis or progressive incurable primary brain tumors. It may also be a result of hydrocephalus, typically from a tumor in the midbrain region or posterior fossa obstructing the aqueduct. Diffuse meningeal disease may cause a communicating hydrocephalus that is less commonly associated with headache. It is important to remember that headache may also be due to anxiety and depression and that other common non-malignant causes of headache may be found in patients with advanced cancer—for example, tension headache and migraine. A general approach is shown in Figure 75-6.
Figure 75-6 Management of headache.
chemo, chemotherapy; OA, osteoarthritis; RT, radiotherapy; SCLC, small cell lung cancer.
Analgesic treatment according to the analgesic ladder is again recommended. When intracranial pressure is raised, steroids are of value. Randomized controlled data suggest that relatively low doses of dexamethasone are as effective as higher doses, with 4 mg being equivalent to 8 or 16 mg and associated with fewer steroid-induced side effects (Vecht et al 1994).
Brain metastasis can be palliated successfully with brain irradiation (Hoskin and Brada 2000). A solitary metastasis may best be treated by surgical decompression and postoperative radiotherapy and multiple metastases with whole-brain radiotherapy. Despite several large randomized controlled trials there has been no meta-analysis of the pooled data. Single-study results suggest response rates of around 70–80% for headache as a symptom when using modest palliative radiotherapy doses of 12 Gy in 2 fractions, 20 Gy in 5 fractions, 30 Gy in 10 fractions, or 40 Gy in 15 fractions. No clear dose–response relationship for symptom control has been shown, and median survival is short, typically around 18–20 weeks.
Chemotherapy is also of value in brain metastasis when a chemosensitive tumor is present and should always be considered for hematological malignancies, including non-Hodgkin’s lymphoma, germ cell tumors, SCLC, and breast cancer. Specific response rates for headache are difficult to define from the published literature.
Primary brain tumors are best managed by surgical debulking followed by postoperative radiotherapy. Dexamethasone—and in acute situations mannitol—may be required to control intracranial pressure, which is the usual cause of headache. High-dose radiotherapy for primary gliomas is a recognized treatment, and a demonstrable advantage has been shown in patients with good performance status when treated with doses of 60 Gy as opposed to a lesser dose of 45 Gy in one randomized controlled trial (Bleehan et al 1991). Specific response rates for headache are not quoted, but improvement in quality of life is achieved. The median survival in this group of patients is short, typically less than 1 year.
Obstructive hydrocephalus is best treated by surgical decompression followed by appropriate local treatment of the tumor, which will often include radiotherapy. An internal shunt may be effective when decompression is not possible.
Other associated causes of headache should also be considered, including cervical spine metastasis, for which local radiotherapy will have an important role, and tumors of the head and neck region, particularly those involving the sinuses or orbit. Appropriate surgical resection or radiotherapy will be considered for these tumors alongside pharmacological management of their pain.
The references for this chapter can be found at www.expertconsult.com.
References
Ahmedzai S., Brooks D. Transdermal fentanyl versus sustained release oral morphine in cancer pain: preference, efficacy, and quality of life. The TTS-Fentanyl Comparative Trial Group. Journal of Pain and Symptom Management. 1997;13:254–261.
Bandolier, The Oxford League table of analgesic efficacy, 2007. Available at, http://www.medicine.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/Leagtab.html, 2007. Accessed December 1, 2011
Bissett D., Macbeth F.R., Cram I. The role of palliative radiotherapy in malignant mesothelioma. Clinical Oncology. 1991;3:315–317.
Blackwell N., Bangham L., Hughes M., et al. Subcutaneous ketorolac—a new development in pain control. Palliative Medicine. 1993;7:63–65.
Bleehan N., Stenning S.P. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. British Journal of Cancer. 1991;64:769–774.
Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiotherapy and Oncology. 1999;52:111–121.
Borgelt B., Gelber R., Brady L.W., et al. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. International Journal of Radiation Oncology, Biology, Physics. 1981;7:587–591.
Bradt J., Dileo C., Grocke D., et al. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database of Systematic Reviews. 2011;8:CD006911.
Brown A.P., Greening W.P., McCready V.R., et al. Radioiodine treatment of metastatic thyroid carcinoma: the Royal Marsden Hospital experience. British Journal of Radiology. 1984;57:323–327.
Brown P., Mehlisch D.R., Minn F. Tramadol hydrochloride: efficacy compared to codeine sulfate, acetaminophen with dextropropoxyphene and placebo in dental-extraction pain. British Journal of Pharmacology. 1989;98:441.
Christie J.M., Simmond M., Patt R., et al. Dose-titration multicentre study of oral transmucosal fentanyl citrate for the treatment of breakthrough pain in cancer patients using transdermal fentanyl for persistent pain. Journal of Clinical Oncology. 1998;16:3238–3245.
Coleman R.E., Vinholes J., Abbey M.E. Double blind randomised trial of pamidronate for the palliative treatment of metastatic bone pain. Journal of Clinical Oncology. 1995;15:528.
Dale O., Moksnes K., Kaasa S. European Palliative Care Research Collaborative pain guidelines: opioid switching to improve analgesia or reduce side effects. a systematic review, Palliative Medicine. 2011;25:494–503.
Davis A.M., Inturissi C.E. D-methadone blocks morphine tolerance and N-methyl-D-aspartate–induced hyperalgesia. Journal of Pharmacology and Experimental Therapeutics. 1999;289:1048–1053.
DeConno F., Ripamonti C., Saita L., et al. Role of rectal route in treating cancer pain: a randomised crossover clinical trial of oral versus rectal morphine administration in opioid naïve cancer patients with pain. Journal of Clinical Oncology. 1995;13:1004–1008.
DuPen S.L., Du Pen A.R., Polissar N., et al. Implementing guidelines for cancer pain management: results of a randomised controlled trial. Journal of Clinical Oncology. 1999;17:361–370.
Ebert B., Thorkildsen C., Andersen S., et al. Opioid analgesics as non-competitive N-methyl-D-aspartate (NMDA) antagonists. Biochemical Pharmacology. 1998;56:553–559.
Edwards J.E., McQuay H.J., Moore R.A. Single dose dihydrocodeine for acute postoperative pain. Cochrane Database of Systematic Reviews. 2000;4:CD002760.
Eisenach J.C., DuPen S., Dubois M., et al. Epidural clonidine analgesia for intractable cancer pain. Pain. 1995;61:391–399.
Eisenberg E., Berkey C.S., Carr D.B., et al. Efficacy and safety of nonsteroidal anti-inflammatory drugs for cancer pain: a meta-analysis. Journal of Clinical Oncology. 1994;12:2756–2765.
Eisenberg E., Carr D.B., Chalmers C.T. Neurolytic coeliac plexus block for treatment of cancer pain—a meta-analysis. Anaesthesia and Analgesia. 1995;80:290–295.
Elliot K., Kest B., Man A., et al. N-methyl-D-aspartate (NMDA) receptors, mu and kappa opioid tolerance and perspectives on new analgesic drug development. Neuropsychopharmacology. 1995;13:347–356.
Ernst S.D., MacDonald R.N., Paterson A.H.G., et al. A double-blind crossover trial of intravenous clodronate in metastatic bone pain. Journal of Pain and Symptom Management. 1992;7:4–11.
Estfan B., Mahmoud F., Shaheen P., et al. Respiratory function during parenteral opioid titration for cancer pain. Palliative Medicine. 2007;21:81–86.
Expert Working Group of the European Association for Palliative Care. Morphine in cancer pain: modes of administration. British Medical Journal. 1996;312:823–826.
Ezzo J., Richardson M.A., Vickers A., et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database of Systematic Reviews. 2006;2:CD002285.
Findlay J.W.A., Jones E.C., Butz R.F., et al. Plasma codeine and morphine concentrations after therapeutic oral doses of codeine-containing analgesics. Clinical Pharmacology and Therapeutics. 1978;24:60–68.
Finnerup N.B., Otto M., McQuay H.J., et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305.
Graham D.Y., Agrawal N.M., Roth S.H. Prevention of NSAID-induced gastric ulcer with misoprostol: a multicentre, double-blind placebo-controlled trial. Lancet. 1988;2:1277–1280.
Hanks G.W., deConno F., Cherny N., et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. British Journal of Cancer. 2001;84:587–593.
Hanks G.W., Hoskin P.J., Aherne G.W., et al. Explanation for potency of repeated oral doses of morphine. Lancet. 1987;2:723–725.
Hoskin P.J., Brada M. On behalf of the participants of the Second Workshop on Palliative Radiotherapy and Symptom Control, London. Clinical Oncology. 2000;13:91–94.
Hoskin P.J., Hanks G.W. The management of symptoms in advanced cancer: experience in a hospital-based continuing care unit. Journal of the Royal Society of Medicine. 1988;81:341–344.
Hoskin P.J., Hanks G.W., Aherne G.W., et al. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. British Journal of Clinical Pharmacology. 1989;27:499–505.
Hoskin P.J., Poulain P., Hanks G.W. Controlled-release morphine in cancer pain: is a loading dose required when the formulation is changed? Anaesthesia. 1989;44:897–901.
Houde R.W., Clinical analgesic studies of hydromorphone. Foley K.M., Inturrisi C.E., eds. Advances in pain research and therapy, 8. New York: Raven Press; 1986:129–136. 1986
King S., Forbes K., Hanks G.W., et al. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliative Medicine. 2011;25:525–552.
Klepstad P., Kaasa S., Jystad A., et al. Immediate or sustained release morphine for dose finding during start of morphine to cancer patients: a randomised, double blind trial. Pain. 2003;101:193–198.
Langman M.J.S., Weil J., Wainwright P., et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:1075–1078.
Lasagna L., DeKornfeld T.J. Methotrimeprazine, a new phenothiazine derivative with analgesic properties. JAMA: Journal of the American Medical Association. 1961;178:887–890.
Lipman A.G., Carver S., Cooney G.A., et al. Methylnaltrexone for opioid-induced constipation in patients with advanced illness: a 3-month open-label treatment extension study. Journal of Pain & Palliative Care Pharmacotherapy. 2011;25:136–145.
Maltoni M., Scarpi E., Modonesi C., et al. A validation study of the WHO analgesic ladder: a two-step vs three step strategy. Supportive Care in Cancer. 2005;13:888–894.
McQuay H., Carroll D., Moore R.A. Radiotherapy for painful bone metastases: a systematic review. Clinical Oncology. 1997;9:150–154.
Mercadante S. Ketamine in cancer pain: an update. Palliative Medicine. 1996;10:225–230.
Mercadante S. Problems of long term spinal opioid treatment in advanced cancer patients. Pain. 1999;79:1–13.
Mercadante S., Cassucio A., Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. Journal of Pain and Symptom Management. 2007;33:217–223.
Mercadante S., Fulfaro F., Casuccio A. A randomised controlled study on the use of anti-inflammatory drugs in patients with cancer pain on morphine therapy: effects on dose escalation and a pharmacoeconomic analysis. European Journal of Cancer. 2002;38:1358–1363.
Nielsen O.S., Bentzen S.M., Sandberg E., et al. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiotherapy and Oncology. 1998;47:233–240.
Osborne J.R., Joel S.P., Slevin M.L. Morphine intoxication in renal failure: the role of morphine-6-glucuronide. British Medical Journal. 1986;292:1548–1549.
Paley C.A., Johnson M.I., Tashani O.A., et al. Acupuncture for cancer pain in adults. Cochrane Database of Systematic Reviews. 2011;1:CD007753.
Pasero C., McCaffery M. Pain: clinical manual. St Louis: Mosby; 1999.
Pasternak G.W., Standifer K.M. Mapping of opioid receptor using antisense oligonucleotides: correlating their molecular biology and pharmacology. Trends in Pharmacological Sciences. 1995;6:334–350.
Pavlakis N., Stockler M. Bisphosphonates in breast cancer. Cochrane Database of Systematic Reviews. 2002;1:CD003474.
Portenoy R.K., Southam M.A., Gupta S.K., et al. Transdermal fentanyl for cancer pain: repeated dose pharmacokinetics. Anesthesiology. 1993;78:36–43.
Porter J., Jick H. Addiction rare in patients treated with narcotics. New England Journal of Medicine. 1980;302:123.
Quilty P.M., Kirk D., Bolger J.J., et al. A comparison of the palliative effects of strontium 89 and external beam radiotherapy in metastatic prostate cancer. Radiotherapy and Oncology. 1994;31:33–40.
Riemsma R., Forbes C., Harker J., et al. Systematic review of tapentadol in chronic severe pain. Current Medical Research and Opinion. 2011;27:1907–1930.
Ripamonti C., Groff L., Brunelli C., et al. Switching from morphine to oral methadone in treating cancer pain: what is the equianalgesic dose ratio? Journal of Clinical Oncology. 1998;16:3216–3221.
Robb K.A., Bennett M.I., Johnson M.I., et al. Transcutaneous electric nerve stimulation (TENS) for cancer pain in adults. Cochrane Database of Systematic Reviews. 2008;3:CD006276.
Robertson A.G., Reed N.S., Ralston S.H. Effect of oral clodronate on metastatic bone pain: a double blind placebo controlled trial. Journal of Clinical Oncology. 1995;13:2427–2430.
Rossi G.C., Pan Y.X., Brown G.P., et al. Antisense mapping the MOR-1 opioid receptor: evidence for alternative splicing and a novel morphine-6 beta glucuronide receptor. FEBS Letters. 1995;369:192–196.
Saarto T., Wiffen P.J. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews. 2008;1:CD005454.
Salazar O.M., Sandhu T., DaMotta N.W., et al. Fractionated half-body irradiation (HBI) for the rapid palliation of widespread, symptomatic metastatic bone disease: a randomised phase III trial of the International Atomic Energy Agency (IAEA), International Journal of Radiation Oncology. Biology, Physics. 2001;50:765–775.
Schiller J.H., Harrington D., Belani C.P., et al. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. New England Journal of Medicine. 2002;346:92–98.
Southam M.A. Transdermal fentanyl therapy: system design, pharmacokinetics and efficacy. Anti-Cancer Drugs. 1995;6(Suppl 3):26–34.
Spiro S.G., Souhami R.L., Geddes D.M., et al. Duration of chemotherapy in small cell lung cancer: a Cancer Research Campaign trial. British Journal of Cancer. 1989;59:578–583.
Stone P., Minton O. European Palliative Care Research collaborative pain guidelines: central side-effects management: what is the evidence to support best practice in the management of sedation, cognitive impairment and myoclonus? Palliative Medicine. 2010;25:431–441.
Sykes J.V., Hanks G.W. Non-opioid analgesics in the treatment of pain due to malignant disease. Pain Reviews. 1998;5:32–50.
Sze W.M., Shelley M., Held I., et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy. Cochrane Database of Systematic Reviews. 2002;1:CD004721.
Tassinari D., Drudi F., Rosati M., et al. Transdermal opioids as front line treatment for moderate to severe cancer pain: a systematic review. Palliative Medicine. 2011;25:478–487.
Timothy A.R., Girling D.J., Saunders M.I., et al. Radiotherapy for inoperable lung cancer. Clinical Oncology. 2001;13:86–87.
Vainio A., Ollila J., Rosenberg P., et al. Driving ability in cancer patients receiving long term morphine analgesia. Lancet. 1995;346:667–670.
Vecht C.J., Hovestadt A., Verbiest H.B.C., et al. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumours: a randomised study of 4.8 and 16 mg per day. Neurology. 1994;44:675–680.
Wiffen P.J., Derry S., Moore R.A. Lamotrigine for acute and chronic pain. Cochrane Database of Systematic Reviews. 2011;2:CD006044.
Wilkinson S., Barnes K., Storey L. Massage for symptom relief in patients with cancer: a systematic review. Journal of Advanced Nursing. 2008;63:430–439.
Wong R.K.S., Wiffen P.J. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database of Systematic Reviews. 2002;2:CD002068.
Woolf C.J., Thompson S.W.N. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299.
World Health Organization. Cancer pain relief, ed 2. Geneva: World Health Organization; 1996.
Zech D.F., Grond S., Lynch J., et al. Validation of World Health Organization guidelines for cancer pain relief: a 10 year prospective study. Pain. 1995;63:65–76.
Zenz M., Donner B., Strumpf M. Withdrawal symptoms during therapy with transdermal fentanyl (fentanyl TTS)? Journal of Pain and Symptom Management. 1994;9:54–55.
Ahmedzai S., Brooks D. Transdermal fentanyl versus sustained release oral morphine in cancer pain: preference, efficacy, and quality of life. The TTS-Fentanyl Comparative Trial Group. Journal of Pain and Symptom Management. 1997;13:254–261.
Bandolier, The Oxford League table of analgesic efficacy, 2007. Available at, http://www.medicine.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/Leagtab.html, 2007. Accessed December 1, 2011
Bleehan N., Stenning S.P. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. British Journal of Cancer. 1991;64:769–774.
Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiotherapy and Oncology. 1999;52:111–121.
Christie J.M., Simmond M., Patt R., et al. Dose-titration multicentre study of oral transmucosal fentanyl citrate for the treatment of breakthrough pain in cancer patients using transdermal fentanyl for persistent pain. Journal of Clinical Oncology. 1998;16:3238–3245.
Dale O., Moksnes K., Kaasa S. European Palliative Care Research Collaborative pain guidelines: opioid switching to improve analgesia or reduce side effects. a systematic review, Palliative Medicine. 2011;25:494–503.
DuPen S.L., Du Pen A.R., Polissar N., et al. Implementing guidelines for cancer pain management: results of a randomised controlled trial. Journal of Clinical Oncology. 1999;17:361–370.
Estfan B., Mahmoud F., Shaheen P., et al. Respiratory function during parenteral opioid titration for cancer pain. Palliative Medicine. 2007;21:81–86.
Expert Working Group of the European Association for Palliative Care. Morphine in cancer pain: modes of administration. British Medical Journal. 1996;312:823–826.
Finnerup N.B., Otto M., McQuay H.J., et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305.
Hanks G.W., deConno F., Cherny N., et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. British Journal of Cancer. 2001;84:587–593.
Hoskin P.J., Brada M. On behalf of the participants of the Second Workshop on Palliative Radiotherapy and Symptom Control, London. Clinical Oncology. 2000;13:91–94.
King S., Forbes K., Hanks G.W., et al. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliative Medicine. 2011;25:525–552.
Maltoni M., Scarpi E., Modonesi C., et al. A validation study of the WHO analgesic ladder: a two-step vs three-step strategy. Supportive Care in Cancer. 2005;13:888–894.
McQuay H., Carroll D., Moore R.A. Radiotherapy for painful bone metastases: a systematic review. Clinical Oncology. 1997;9:150–154.
Pavlakis N., Stockler M. Bisphosphonates in breast cancer. Cochrane Database of Systematic Reviews. 2002;1:CD003474.
Saarto T., Wiffen P.J. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews. 2008;1:CD005454.
Sze W.M., Shelley M., Held I., et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy. Cochrane Database of Systematic Reviews. 2002;1:CD004721.
Vainio A., Ollila J., Rosenberg P., et al. Driving ability in cancer patients receiving long term morphine analgesia. Lancet. 1995;346:667–670.
Wong R.K.S., Wiffen P.J. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database of Systematic Reviews 2:CD002068. 2002.
World Health Organization. Cancer pain relief, ed 2. Geneva: World Health Organization; 1996.
Zech D.F., Grond S., Lynch J., et al. Validation of World Health Organization guidelines for cancer pain relief: a 10 year prospective study. Pain. 1995;63:65–76.