Surgical Oncology
Complete surgical removal of localized cancer cures more cancer patients than any other form of treatment,1 in part because this modality is generally applied as sole treatment for local disease, early stage disease, or tumors with limited potential to metastasize. In humans, 60% of patients who are cured of cancer are cured by surgery alone.2 Before this hope for cure can be realized in veterinary medicine, surgeons must have a thorough understanding of anatomy, physiology, resection, and reconstruction options for all organs; expected tumor behavior; and the various alternatives or adjuvants to surgery. Surgical oncologists should not only be good technical surgeons, but also dedicated tumor biologists. Surgery will likely play a role at one point or another in the management of most cancer patients. Surgical procedures may include any of the following: diagnosis (biopsy), resection for cure, palliation of symptoms, debulking (tumor cell cytoreduction), and a wide variety of ancillary procedures to enhance and complement other forms of treatment.
Surgical resection of cancer was introduced in the sixteenth century bce and remained relatively underutilized until general anesthesia (1840s), antisepsis (1860s), and pain management made aggressive resection safe and tolerable for the patient. Dr. William Halstead developed the basic principles of surgical oncology in the 1890s. The radical resection of the twentieth century has increasingly been customized to meet the needs of the patient and has frequently been reduced in magnitude. Further refinements in surgery have been made possible with newer equipment (e.g., staples, endoscopy), advanced imaging, pain management, use of blood products, and critical care services.
Most patients with cancer are “old.” However, “old” is a relative term, and a geriatric dog or cat with normal organ function should not be denied treatment simply on the basis of age. Age has not been shown to impact tumor-related prognosis. In fact, dogs with osteosarcoma that are less than 2 years of age do worse than dogs that are more than 2 years of age after amputation alone.3 “Old” animals, in most instances, will tolerate aggressive surgical intervention as well or as poorly as “young” patients.
Surgery for Diagnosis
Although biopsy principles are covered in Chapter 9, it bears emphasizing that properly timed, performed, and interpreted biopsies are one of the most crucial steps in the management of the cancer patient. Not only does the surgeon need to procure adequate and representative tissue to establish a diagnosis, but also the biopsy must not compromise subsequent curative surgical resection or radiation field planning.
Surgery for Cure
Before a surgeon can be in a position to provide the optimal operation for the patient with cancer, the following questions need to be considered:
1. What is the histologic type, stage, and grade of cancer to be treated?
2. What are the expected local and systemic effects of this tumor type, grade, and stage?
3. Is a cure possible and at what price in terms of cosmetics and function?
4. Is an operation indicated at all?
5. What are the options for alternative or planned combination treatment?
A recurring theme in surgical management of cancer is that the first surgery has the best chance of cure. Several mechanisms for this improvement in survival have been advanced. Untreated tumors have had less chronologic time to metastasize than recurrent primary cancer. Untreated tumors and proximate normal tissues have near normal anatomy, which will facilitate operative orientation and maneuvers. Recurrent tumors may have had seeding of previously noninvolved tissue planes, requiring wider resection than would have been required on the initial tumor. If one thinks about a given cancer as resembling a crab, incomplete surgery removes the body of the crab and leaves the legs behind. The “body” of most tumors is often quiescent and hypoxic, whereas the leading edge of the tumor (legs) is the most invasive and well vascularized. Subtotal removal may actually selectively leave behind the most aggressive components of the tumor. Patients with recurrent cancer will often have less normal tissue for closure. An ill-defined negative aspect of recurrent cancer is reported to be related to changes in vascularity and local immune responses. Regardless of the mechanism, curative-intent surgery is best performed at the first operation and the surgeon should have all the necessary diagnostic information in hand when constructing a treatment plan. Radiography and ultrasonography have been routine for many years, and the increased availability of computed tomography (CT) has added greatly to our ability to determine the extent of a solid tumor and optimize the surgical approach. CT allows good visualization of muscle bellies/fascial planes, intraabdominal/intrathoracic organs, lymph nodes, and bone detail. Three-dimensional (3D) CT reconstructions are particularly useful for planning procedures for skull-based tumors.
Advanced imaging has greatly enhanced the surgeon’s ability to assess the anatomic location and extent of various cancers; however, we must remember that imaging of any kind needs to be paired with clinical palpation, assessment of mobility, and expected biologic behavior. Some cancers deemed inoperable by imaging are in fact mobile and operable. Leading edges of some cancers are compressed against adjacent tissue and appear more invasive. Surgeons should always take the opportunity to palpate the local tumor before or after imaging, explore the history of the tumor’s growth pattern, and in many cases obtain a tissue sample (i.e., histopathology or cytology when appropriate) before declaring a mass inoperable. Positive prognostic factors typically include slow growth rate, mobility within proximate tissues, first attempt at surgery, discrete tumor borders, small tumor size, and low grade nature. Conversely, surgery may be less effective for the same tumor type and grade if the mass is ill-defined, is recurrent, or has a recent history of rapid growth. Palpation under heavy sedation or anesthesia may suggest that resection is possible in spite of imaging findings (e.g., unilateral thyroid masses, some soft tissue sarcomas). Deeper masses from locations such as ribs and liver may appear inoperable due to inflammation or compression of adjacent organs and structures; however, there actually may be no invasion present.
The surgical oncologist must be able to assimilate all of the information and make an informed decision. We must also remind ourselves and our clients that there is much we do not know (e.g., incomplete margins do not necessarily ensure later local recurrence4) and that surgical judgment regarding expected local behavior and likely resection is often qualitative and is an imperfect “science.”
The actual surgical technique will vary with the site, size, and stage of the tumor, as well as the skill and experience of the surgeon. The same tumor type in dogs and cats may vary in surgical approach, technique, and prognosis. Some general statements that need to be emphasized with surgical oncology are as follows:
1. All incisional biopsy tracts should be excised in continuity with the primary tumor because tumor cells are capable of growth in these wounds. Fine-needle aspiration (FNA) cytology tracts are of minor, but not zero, concern, whereas punch biopsy tracts are of intermediate concern.5 With this in mind, all biopsies should be positioned in such a manner that they can be removed at surgery.
2. Early vascular ligation (especially venous) should be attempted to diminish release of large tumor emboli into the systemic circulation. This is probably only clinically meaningful for those tumors with a well-defined venous supply, such as splenic tumors, retained testicles, and lung tumors. Small numbers of cancer cells are constantly being released into the venous (and lymphatic) circulation by most tumors. Larger, macroscopic cell aggregates may be more dangerous, however, and these may be prevented from vascular escape with early venous ligation.
3. Local control of malignant cancer requires that a margin of normal tissue be removed around the tumor. Resection of the “bad from the good” can and should be classified in more detail than radical versus conservative (Table 10-1).6 Tumors with high probability of local recurrence (e.g., high-grade soft tissue sarcoma, high-grade mast cell tumors, feline mammary adenocarcinoma) should have 2- to 3-cm margins removed in three dimensions. Tumors are not flat, and wide removal in one plane does not ensure complete excision. Fixation of cancer to adjacent structures mandates removal of the adherent area in continuity with the tumor. This is commonly seen with oral cancer that is firmly adherent to the underlying mandible or maxilla. Invasive cancer should not be peeled out, shelled out, enucleated, or curetted if a cure is expected. Many cancers are surrounded by a pseudocapsule. This capsule is almost invariably composed of compressed and viable tumor cells, not healthy reactive host cells. If a malignant tumor is entered at the time of resection, or if the margins are incomplete, that procedure is often no better therapeutically than a large incisional biopsy. When possible, resection of the previous scar and the entire wound bed with “new” margins (never entering the old wound cavity) is indicated. One should strive for a level of dissection that is one tissue plane away from the mass (Figure 10-1). For example, invasion of cancer into the medullary cavity of a bone requires subtotal or total bone resection and not curettage.
Table 10-1
Classification and Resection of Wound Margins
| Type | Plane of Dissection | Result |
| Intracapsular | Tumor removed in pieces or curetted, “debulking” | Macroscopic disease left behind |
| Marginal | Removal just outside or on pseudocapsule or reactive capsule, “shelled out” | Usually leaves microscopic disease |
| Wide | Tumor and capsule never entered, normal tissue surrounds specimen | Possible skip lesions |
| Radical | Entire compartment or structure removed (e.g., amputation) | No local residual cancer |
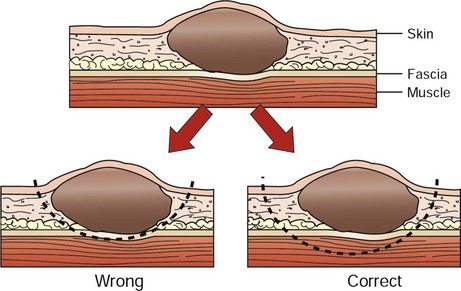
• Figure 10-1 Typical soft tissue cancer is in proximity to skin and underlying fascia. Inappropriate removal is to “peel it off” the deeper fascia, where microscopic extension is probable. Correct removal entails wide three-dimensional (3D) margins, including underlying skin and underlying fascia.
The width of surgical margins necessary for a complete excision for a given tumor type is an ongoing debate, and our current practices are based on little objective data. As a community, we have answered most of the questions about how much tissue we can safely remove, but it will serve our patients well to determine how little extra tissue is necessary to excise and consistently achieve the same success. We must challenge recommendations that are reported in the literature if based solely on a surgeon’s personal experience or opinion in the absence of objective findings.
4. Tumors should be handled gently to avoid risk of breaking off tumor cells into the operative wound, where they may thrive.7 Copious lavage of all cancer wound beds will help mechanically remove small numbers of exfoliated tumor cells but should not replace gentle tissue handling and avoidance of entering the tumor bed.
5. If more than one malignant mass is being removed, separate surgical packs should be used for each site to avoid iatrogenic tumor cell implantation from site to site.
The aggressiveness of resection should only rarely be tempered by fears of wound closure. It is better to leave a wound partially or even in some cases completely open with no cancer than closed with residual cancer. Numerous innovative reconstructive techniques are available for closure of cancer wounds, and the surgeon is only limited by his or her ingenuity.8 Reliable microvascular-free composite transfers of muscle and skin are somewhat hampered due to unique canine skin/muscle anatomy but are being developed.9
Lymph Node Removal
Controversy surrounds the surgical management of regional lymph nodes draining the primary tumor site.10,11 As a general rule, epithelial cancers are more likely to metastasize to lymph nodes than are mesenchymal cancers. However, any enlarged regional lymph node requires investigation for complete staging. Lymphadenopathy may be from metastasis of cancer (firm, irregular, and sometimes fixed to surrounding tissue) or from hyperplasia and reactivity to various tumor factors, infection, or inflammation.12 The former cause is a poor prognostic sign, and the latter may be a beneficial host response. Enlarged lymph nodes as a result of cancer metastasis and invasion are generally effaced by tumor cells and can often be diagnosed by FNA. Histologically, positive lymph nodes at diagnosis usually are a sign of impending emergence of systemic metastasis. Removal of lymph nodes should be considered under the following circumstances:
1. If the lymph node is positive for cancer and not fixed to surrounding normal tissues, it may be possible to remove the node with some therapeutic intent. Frequently, however, many lymph nodes drain a primary tumor site (e.g., oral cavity) and lymphadenectomy is incomplete. Lymph node metastasis at the time of initial diagnosis is a poor prognostic sign. However, patients that develop metastasis in a delayed fashion (1 to 2 years) after local tumor control may benefit from lymphadenectomy. Although it is usually not practical, removal of the primary tumor, intervening lymphatic ducts, and draining lymph node has been recommended (en bloc resection). En bloc resection may be possible for a malignant toe tumor with metastasis to the popliteal lymph node but is usually only accomplished with amputation. A mastectomy that includes the regional lymph node (e.g., glands four and five with the inguinal lymph node) is another example of en bloc resection. Few other anatomic sites are routinely amenable to this therapy. A specific instance where local lymphadenectomy may be beneficial is in removal of iliac/sublumbar lymph nodes in patients with metastatic apocrine or sebaceous gland adenocarcinomas of the perineum. Although removal of these lymph nodes is rarely curative, it may help increase the benefit of adjuvant radiation or chemotherapy and should help alleviate, at least in the short term, the paraneoplastic syndrome of hypercalcemia by reducing levels of parathormone-like substances. Regional lymphadenectomy may also prevent or improve obstruction of the large bowel and urinary tract and can be repeated as necessary in the absence of overt systemic metastasis (e.g., metastasis to lungs, liver).
2. It is well estabished that normal-sized lymph nodes may contain micrometastasis, and this point should always be explained clearly to a client. Normal-appearing lymph nodes that are known to drain a primary tumor site may be randomly sampled (biopsy or cytology) to gain further staging information. This is particularly important if adjuvant therapy decisions (irradiation or chemotherapy) would be predicated on confirmation of residual or metastatic cancer. Intrathoracic or intraabdominal lymph nodes are perhaps most crucial because they are not readily accessible to histologic or cytologic follow-up examination. However, in some instances, lymph nodes that are not enlarged cannot be sampled safely due to proximity to vital structures (e.g., sublumbar lymph nodes at the aortic bifurcation), even under ultrasound guidance. In such cases, the surgeon must educate the client about the situation and either remove the primary tumor without further knowledge of regional lymph node involvement or recommend removal of the normal-sized nodes concurrently for staging (and possibly therapeutic) purposes. For dogs with malignant anal sac tumors, this approach involves an exploratory surgery of the sublumbar nodal bed and dissection/removal of all nodal tissue encountered.
In human medicine, the concept of the sentinel lymph node (first node to receive lymphatic flow from a primary tumor) has become important in the management of select malignancies, most notably breast cancer.12 Basically, the area of the primary tumor is injected with blue dye or a low dose of a radionuclide or both. The first draining node is detected visually or with a handheld gamma camera probe and removed for frozen section analysis. If the first node is negative for metastasis, subsequent nodal dissection often is avoided. If the sentinel node is positive, further nodal dissection is performed. Targeting the sentinel node is most valuable in an anatomic location in which there is an extensive nodal bed12 and hence numerous potential paths for regional metastasis. The benefits of such an approach in humans are readily apparent because many patients have been spared extensive nodal resections if the sentinel lymph node has been determined to be free of cancer cells. The topic of the sentinel lymph node has only recently emerged in veterinary medicine but is gathering some momentum.13 Several techniques have been described, including microbubble detection via ultrasound,14 fluorescein, and blue dye.15 Although sentinel lymph node staging may not yet have the relevance and importance that have been demonstrated in humans due to less complex nodal networks, there are several potential indications (e.g., tumors of the head and neck) that should be investigated in clinical studies. Histologically positive lymph nodes will alter prognosis and stage and will also be informative for decisions related to postoperative chemotherapy and radiation.
Lymph node removal is generally not performed under the following circumstances:
1. Lymph nodes in critical areas (retropharyngeal, hilar, mesenteric) that have eroded through the capsule and become adherent (fixed) to surrounding tissues. In this scenario, lymph nodes may not be removeable without leaving residual disease in the wound bed (necessitating adjuvant therapy to achieve local control) or an attempt at removal may cause serious harm to the patient by injuring important adjacent structures. In such instances, it is usually prudent to aspirate or biopsy the node to confirm involvement in the disease process and leave the node in situ or treat with other modalities. One example of an exception to this scenario is metastasis of limb and paw tumors to prescapular and popliteal lymph nodes that can be removed with amputation (radical en bloc resection).
2. Prophylactic removal of “normal” draining lymph nodes or chains of lymph nodes (as opposed to sampling for stage) is not beneficial and may be harmful.9 Regional lymph nodes may in fact be the initiator of favorable local and systemic immune responses, and elective removal has been associated with poor survival in certain human cancers.10,16,17
Surgery for Distant Disease
Metastasectomy for pulmonary metastasis of sarcoma in some instances has been accepted therapy in humans and dogs. Resection of liver metastasis for carcinomas (especially gastrointestinal cancers) is increasing in human oncology. As more effective adjuvant therapies evolve and minimally invasive techniques are further developed, the need for cytoreductive metastasectomy will increase.
Palliative Surgery
Palliative surgery is an attempt to improve the quality of the patient’s life (pain relief or improved function) but not necessarily the length of the patient’s life.18 This type of surgery requires careful consideration of the expected morbidity of the procedure versus the expected gain to the patient and the client. In essence, it comes down to a decision of when to discontinue therapy. One of the most difficult decisions in surgical oncology is the decision not to operate. Treatment of any kind should never be worse than no treatment.
Certain situations do exist, however, in which palliative surgery may be beneficial. If an infected and draining mammary tumor in a patient with asymptomatic lung metastasis is the limiting factor in the patient’s life, mastectomy may still be a logical procedure. Splenectomy for ruptured hemangiosarcoma is commonly performed but probably has little impact on long-term survival and can be considered palliative because it will stop the immediate threat of hemorrhage.
Cytoreductive Surgery
Incomplete removal of a tumor (planned or unplanned) is referred to as debulking or cytoreductive surgery. It is commonly performed but rarely indicated.19 Its theoretical indication is to enhance the efficacy of other treatment modalities. Debulking is a practical consideration prior to cryosurgery to decrease the amount of tissue to freeze and the time it will take. It may also help the treatment planning and dosimetry with certain forms of irradiation, but the improved cancer control achieved is more a result of geometric and dosimetry considerations than intentional and incomplete removal of tumor cells. Removing 99.9% of a 1-cm tumor (1 × 109 cells, one billion) still leaves a million cancer cells behind. Immunotherapy and chemotherapy could theoretically be helped by tumor volume reduction (such as lymph node removal for oral melanoma with the use of a melanoma vaccine),20,21 but few well-controlled clinical trials have shown a benefit to date in veterinary medicine. A variety of soft tissue sarcomas in dogs and cats will have better local control when radiation is given adjuvantly rather than for bulky measurable disease. Amputation or limb sparing of dogs with osteosarcoma is essentially an extreme cytoreductive procedure and clearly requires postoperative chemotherapy for prolongation of life. If tumors are debulked with the anticipation of postoperative radiation therapy, the margins of known tumor or the operative field should be marked with radiopaque metal clips to allow proper treatment planning from radiographs or CT. The orientation of the incision should be considered carefully if radiation therapy is possible postoperatively.
Nonsurgical Locally Ablative Procedures
Ablative techniques to eradicate local (or metastatic) disease have a place in oncology but often suffer from “sales before science” and are only rarely based on evidence of outcomes.22 Several of these techniques will be mentioned later, and the reader is referred to accompanying references for details. Indications for the use of local ablative therapy vary but are generally limited to “small” (less than 2 cm diameter) discrete lesions. A limitation of all of the techniques is that completeness of cell kill and margin analysis cannot be determined after treatment. If the operator picked the correct tumor, site, and “dose” delivery, then local control may be achieved; however, monitoring for regrowth is the only way to ensure this. As a general rule, recurrent disease tends to be more invasive and difficult to control than the primary intervention, so the first ablative maneuver is hopefully well planned and executed. All of the local ablative treatments require special equipment and training to properly perform. Selective tumor cell kill while sparing all normal tissue is often claimed for these techniques but is unlikely to be true.
Radiofrequency Ablation
The most common nonsurgical locally ablative procedures in human medicine include radiofrequency (RF), microwave, and cryoablation. RF ablation involves delivery of a high frequency (300 to 500 KHz) alternating current via a needle-like probe. The procedure is typically performed via image guidance (e.g., ultrasound or CT) and is commonly used to treat both primary and secondary hepatic tumors. The minimally invasive nature of the technique makes it ideal for the latter application. With hepatic metastatic colorectal cancer, RF rivals surgery for increasing 5-year survival. Microwave ablation is similar to RF, but higher frequency current (900 to 2459 MHz) is delivered through the probe. It is a newer technology with less evidence supporting its use but is often used for the same indications as RF ablation. Neither the RF nor microwave procedures have been evaluated for clinical applications in veterinary patients23,24; however, some of the work done to evaluate the safety of these procedures in humans was performed experimentally in dogs.
Cryoablation
Cryosurgery, or cryoablation, is a much older form of local therapy that impacts tumors via direct cell kill and vascular collapse. It has been used extensively in veterinary medicine, most commonly for skin, nasal planum, eyelid, perianal, and oral cavity neoplasms.25-27 It continues to be an attractive treatment option for clients not interested in invasive procedures and for palliation of advanced (i.e., nonresectable or metastatic) oral disease and can be used as an adjunct to debulking surgery. There is a recent report of image-guided cryoablation of a nasal mass in a dog that recurred following intensity-modulated radiation therapy (IMRT).28 In humans, laparoscopic and percutaneous cryoablation of select neoplasms (e.g., breast and renal tumors) has become an attractive, minimally invasive treatment option.29,30
Hyperthermia
Hyperthermia is the elevation of tissue temperature above normal physiologic levels. Thus the term hyperthermia encompasses a wide range of temperatures and modalities. A variety of methods and devices are used clinically to induce hyperthermia in tissues. Noninvasive methods using RF currents, microwaves, or ultrasound are the most common. Heating of solid tumors is typically nonuniform, due in part to the heating devices available and in part to nonuniform distribution of blood flow in the tumor and heat-dissipating activity of surrounding normal tissue. A number of studies demonstrate improved outcome when hyperthermia is added to radiation therapy of solid tumors in dogs.31-34 The ideal strategy for clinical hyperthermia treatment, including thermal dose, fractionation, and time and temperature goals, has yet to be identified.
Photodynamic Therapy
The practice of using sunlight to treat disease is ancient, but modern refinements have allowed the interactions between light and drugs to evolve into a highly effective cancer treatment called photodynamic therapy (PDT). PDT relies on light of an appropriate activating wavelength, oxygen, and a photosensitizer (PS) that accumulates within a tumor. The excited PS interacts with molecular oxygen, creating reactive oxygen species that are responsible for causing vascular stasis and necrosis, membrane damage, and apoptosis and for initiating a signaling cascade resulting in an influx of inflammatory cells. Although initially studied as a single modality, PDT may also be useful when combined with other cancer treatments. Early studies show that a combination of PDT and low-dose cisplatin increases efficacy in both in vitro and murine models, and similar synergy has been observed when doxorubicin is combined with PDT.35,36 In veterinary medicine, PDT has been most commonly used in the treatment of squamous cell carcinoma (SCC). Most SCCs are superficial, localized, and do not metastasize until late in the course of the disease, making them well suited for treatment with PDT. An early description of chloro-aluminum sulfonated phthalocyanine–based PDT in 10 cats with superficial SCC or carcinoma in situ reported a 70% complete response rate, demonstrating the potential for PDT as a skin cancer treatment.37 With a number of recent technologic improvements, PDT has the potential to become integrated into the mainstream of cancer treatment.38
Surgery and Chemotherapy
The combined use of chemotherapy and surgery is becoming more commonplace in veterinary oncology, and the knowledge an oncologic surgeon must possess to master the use of combination therapy is ever expanding.39,40 Many chemotherapy agents will impede wound healing to some extent. In spite of this risk, few clinically relevant problems occur when surgery is performed on a patient receiving chemotherapy.41,42 General recommendations are to wait 7 to 10 days after surgery to begin chemotherapy, especially for high-risk procedures such as intestinal anastomosis.43 The use of intraoperative44or perioperative chemotherapy is receiving increased attention45,46 and could have greater implications for wound healing. Neoadjuvant chemotherapy is also becoming more popular and in some instances may greatly facilitate excision of a solid tumor. Such an approach is commonly used with some canine mast cell tumors, but there remain unanswered questions, such as what characteristics identify the indications for such an approach? Also, if a tumor reduction occurs, should the location of the original tumor border be used to make measurements for margins or the new outer edge?
Surgery and Radiation
Theoretical advantages can be advanced for both preoperative and postoperative radiation.40,47 Either way, some impairment of wound-healing potential will exist and need to be considered.48 Radiation damage to normal tissues (stem cells, blood vessels, lymphatics) may be progressive and potentially permanent as total radiation dose, dose per fraction, and field size increase. Therefore close collaboration between the radiation oncologist and surgeon is critical when designing the most effective regimen. If radiation therapy is given preoperatively, surgery can be performed after acute radiation reactions have resolved (generally 3 to 4 weeks). Postoperative radiation is recommended after a 7- to 14-day delay to allow for adequate wound healing. In spite of the theoretical problems, surgery can often be safely performed on irradiated tissues and complications are not prohibitive.
The benefit of surgery and radiation is clear for some tumor types; however, in some instances the improvement in outcome over single modality therapy is controversial. One such debate is that of canine nasal tumors.49,50 Early reports did not show benefit of postoperative radiation over radiation alone. A recent report demonstrated preoperative radiation as beneficial when followed by exenteration of the nasal cavity 6 to 10 weeks later.50 With the advent of stereotactic radiation therapy (SRT) and IMRT (see Chapter 12), such approaches are even more attractive because the overlying skin or underlying mucosa in the case of nasal tumors can be spared from the full effects of the radiation, thereby diminishing concerns about incisional healing. In the past, surgeons would be reluctant to operate in a radiation field; thus these more focused forms of radiation therapy will hopefully allow the surgeon to operate with fewer wound healing complications and create novel treatment plans that combine radiation and surgery for select tumors. Radiation side effects are greatly diminished with a more conformal approach, and this in turn makes clients more willing to have their pets undergo radiotherapy.
Access to more sophisticated radiation techniques such as SRT and IMRT is rapidly increasing throughout the world, and with this development, a new paradigm is emerging in veterinary radiation oncology. For example, bone sarcomas in locations not amenable to limb-sparing surgery can now be treated with curative intent therapy51 and treatment protocols for large solid tumors previously deemed nonresectable are currently being investigated. Combinations of SRT and surgery are also being explored. However, while these new treatment options represent great advances, familiar challenges remain. In the case of dogs with appendicular osteosarcoma, fracture may occur after SRT and while some of these cases are amenable to surgical stabilization, healing of the fracture does not occur normally due to the effects of radiation on bone healing. Thus the role of the surgeon continues to evolve in the management of cases treated with radiation.
Prevention of Cancer
Certain common cancers in dogs and cats can be prevented. The recent elucidation of the canine genome as it relates to genetic susceptibility will likely increase the surgical indications for prevention. It is well known that early (<1 year) oophorectomy will reduce the risk of mammary cancer in the dog by 200-fold compared to intact bitches (and to a lesser degree the cat). Castration of the male dog will help prevent perianal adenomas and obviously testicular cancer. Removal of in situ SCC (precancerous) from the skin of white cats or removal of in situ adenomatous polyps from the rectum of dogs may also prevent subsequent development of cancer. Elective removal of cryptorchid testes, which are at high risk for tumor development, is another example of preventive surgery.
Miscellaneous Oncologic Surgery
Veterinary surgeons are being called on increasingly to facilitate the medical management of cancer patients. The placement of long-term vascular access catheters for delivery of fluids, chemotherapy, or anesthesia and pain relief agents has become commonplace, and ports are routinely placed to aid in the evacuation of malignant thoracic effusions (e.g., mesothelioma). Operative placement of various enteral and parenteral feeding tubes is also commonly performed.
Surgeons and radiotherapists may work together for the operative exposure of nonresectable cancer so that large doses of irradiation may be delivered to the tumor or tumor bed after exclusion of radiosensitive tissues. Surgical intervention for oncologic emergencies such as intractable pain, bleeding, pathologic fracture, infection, and bowel perforation or obstruction may also arise.
The comprehensive veterinary oncology team also now includes the discipline of interventional radiology to help manage/palliate certain malignancies. Examples include the use of self-expanding nitinol stents to treat dogs with malignant urethral obstruction52 and double pigtail stents for the treatment of malignant ureteral obstructions resulting from trigonal transitional cell carcinoma (TCC) of the urinary bladder.53
Equipment advances are facilitating tumor excisions (e.g., harmonic scalpel54 and LigaSure for splenectomy and liver masses), and laparoscopic and thoracoscopic evaluation of body cavities for staging is increasingly being performed on animals. Cancer resections with this technique are also on the rise and given the potential for decreased morbidity, veterinary surgeons may feel more comfortable performing surgery in the face of advanced disease for certain solid cancers (e.g., thoracoscopic removal of a solitary metastatic lung nodule at the time of amputation for an appendicular osteosarcoma). A few other examples of minimally invasive surgery performed in companion animals for cancerous diseases include prostatic biopsy,55 thoracoscopic pericardiectomy for heart-based tumors, laparoscopy-assisted intestinal biopsy, liver biopsy, pancreatic biopsy, splenectomy,56 and adrenalectomy.57 Further definition of appropriate case selection and increased access to equipment and training will ultimately expand these techniques because the rising popularity of minimally invasive surgery in the pet-owning public is driving this technology.
Discussion
It is clear that surgery will be the mainstay of local or regionally confined cancer treatment in veterinary medicine for many years to come. It is also clear that just because a surgical procedure is possible, this is not the best reason to do it. It was not long ago that surgical resection of the external genitalia was routine treatment for dogs with transmissible venereal tumors. It is now recognized that chemotherapy alone is curative in over 90% of dogs, and surgery is needed for biopsy only. Simple versus radical mastectomy in the dog and humans does not influence survival for most mammary gland tumors, but more aggressive surgery may indeed be beneficial in the cat.58-60 More surgery is not always better surgery. Long-term follow-up of well-staged and graded tumors with defined surgical technique and margins is necessary to demonstrate the true value of any operation. A great deal of progress in surgical technique and surgical thinking needs to take place before the use of surgery can be optimized.
It is hoped that a better understanding of expected tumor biology and more precise staging methods (e.g., molecular diagnostics, angiograms, ultrasound, CT scans, magnetic resonance imaging [MRI], positron emission tomography [PET]/CT) will facilitate more precise surgical operations to be performed. Surgical techniques will continue to improve and undergo refinements,54,61-63 but until surgeons become biologists, the big breakthroughs will be slow in coming. Surgeons should be investigating the influence of anesthesia, infection, immune function, blood transfusions, growth factors, oncogenes, and cytokines, to name a few, on the outcome of our patients.64-70 In spite of these anticipated advances in technology and biology, the most difficult aspect to learn is surgical judgment. “Biology is king; selection of cases is queen, and the technical details of surgical procedures are the princes and princesses of the realm who frequently try to overthrow the powerful forces of the king or queen, usually to no long-term avail, although with some temporary apparent victories.”71
References
1. Chabner, BA, Curt, GA, Hubbard, SM. Surgical oncology research development: the perspective of the National Cancer Institute. Cancer Treat Rep. 1984;68:825–829.
2. Poston, GJ. Is there a surgical oncology? In: Textbook of surgical oncology. London: Informa Healthcare; 2007.
3. Spodnick, GJ, Berg, J, Rand, WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J Am Vet Med Assoc. 1992;200:995–999.
4. Bacon, NJ, Dernell, WS, Ehrhart, N, et al. Evaluation of primary re-excision after recent inadequate resection of soft tissue sarcomas in dogs: 41 cases (1999–2004). J Am Vet Med Assoc. 2007;230(4):548–554.
5. Withrow, SJ. Risk associated with biopsies for cancer. Kirk’s current veterinary therapy, XII. In: Bonagura J, ed. Small animal practice. Philadelphia: Saunders, 1995.
6. Enneking, WF. Musculoskeletal tumor surgery. New York: Churchill-Livingstone; 1983.
7. Gilson, SK, Stone, EA. Surgically induced tumor seeding in eight dogs and two cats. J Am Vet Med Assoc. 1990;196:1811–1815.
8. Pavletic, M. Atlas of small animal reconstructive surgery. Philadelphia: JB Lippincott; 1993.
9. Dundas, JM, Fowler, JD, Schmon, CL. Modification of the superficial cervical axial pattern skin flap for oral reconstruction. Vet Surg. 2005;34:206–213.
10. Cady, B. Lymph node metastases: Indicators, but not governors of survival. Arch Surg. 1984;119:1067–1072.
11. Gilson, SD. Clinical management of the regional lymph node. Vet Clin North Am Small Anim Pract. 1995;25:149–167.
12. Nyman, HT, Kristensen, AT, Skovgaard, IM, et al. Characterization of normal and abnormal canine superficial lymph nodes using gray-scale B-mode, color flow mapping, power, and spectral Doppler ultrasonography: a multivariate study. Vet Radiol Ultrasound. 2005;46:404–410.
13. Tuohy, JL, J Milgram, J, Worley, DR, et al. A review of sentinel lymph node evaluation and the need for its incorporation into veterinary oncology. Vet Comp Oncol. 2009;7(2):81–91.
14. Lurie, DM, Seguin, B, Schneider, PD, et al. Contrast-assisted ultrasound for sentinel lymph node detection in spontaneously arising canine head and neck tumors. Invest Radiol. 2006;41(4):415–421.
15. Wells, S, Bennett, A, Walsh, P, et al. Clinical usefulness of intradermal fluorescein and patent blue violet dyes for sentinel lymph node identification in dogs. Vet Comp Oncol. 2006;4(2):114–122.
16. Olson, RM, Woods, JE, Soule, EH. Regional lymph node management and outcome in 100 patients with head and neck melanoma. Am J Surg. 1981;142:470–473.
17. Veronesi, U, Adamus, J, Bandiera, DC, et al. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982;49:2420–2430.
18. Milch, RA. Surgical palliative care. Semin Oncol. 2005;32:165–168.
19. Moore, GE. Debunking debulking. Surg Gyn Obstet. 1980;150:395–396.
20. Morton, DL. Changing concepts of cancer surgery: surgery as immunotherapy. Am J Surg. 1978;135:367–371.
21. Broomfield, S, Currie, A, van der Most, RG, et al. Partial, but not complete, tumor-debulking surgery promotes protective antitumor memory when combined with chemotherapy and adjuvant immunotherapy. Cancer Res. 2005;65:7580–7584.
22. Withrow, SJ, Poulson, JM, Lucroy, MD. Miscellaneous treatments for solid tumors in Withrow and MacEwen’s small animal clinical oncology, ed 4. St Louis: Saunders; 2007.
23. Huang, J, Li, T, Liu, N, et al. Safety and reliability of hepatic radiofrequency ablation near the inferior vena cava: an experimental study. Int J Hyperthermia. 2011;27(2):116–123.
24. Qiu-Jie, S, Zhi-Yu, H, Xiao-Xia, N. Feasible temperature of percutaneous microwave ablation of dog liver abutting the bowel. Int J Hyperthermia. 2011;27(2):124–131.
25. Harvey, HJ. Cryosurgery of oral tumors in dogs and cats. Vet Clin North Am Small Anim Pract. 1980;10(4):821–830.
26. Holmberg, DL. Cryosurgical treatment of canine eyelid tumors. Vet Clin North Am Small Anim Pract. 1980;10(4):831–836.
27. Fernandez De Queiroz, G, Matera, JM, Dagli, M. Clinical study of cryosurgery efficacy in the treatment of skin and subcutaneous tumors in dogs and cats. Vet Surg. 2008;37:438–443.
28. Murphy, SM, Lawrence, JA, Schmiedt, CW, et al. Image-guided transnasal cryoablation of a recurrent nasal adenocarcinoma in a dog. J Small Anim Pract. 2011;52(6):329–333.
29. Manenti, G, Perretta, T, Gaspari, E, et al. Percutaneous local ablation of unifocal subclinical breast cancer: clinical experience and preliminary results of cryotherapy. Eur Radiol. 2011;21(11):2344–2353.
30. Klatte, T, Grubmüller, B, Waldert, M, et al. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: Systematic review and cumulative analysis of observational studies. Eur Urol. 2011;60(3):435–443.
31. Dewhirst, MW, Sim, DA, Sapareto, S, et al. Importance of mini- mum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res. 1984;44:43–50.
32. Thrall, DE, LaRue, SM, Yu, D, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2005;11(14):5206–5214.
33. Gillette, EL, McChesney, SL, Dewhirst, MW, et al. Response of canine oral carcinomas to heat and radiation. Int J Radiat Oncol Biol Phys. 1987;13:1861–1867.
34. Gillette, SM, Dewhirst, MW, Gillette, EL, et al. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia: a randomized phase II study. Int J Hyperthermia. 1992;8:309–320.
35. Casas, A, Fukuda, H, Riley, P, et al. Enhancement of aminolevulinic acid based photodynamic by Adriamycin. Cancer Lett. 1997;121:105.
36. Lanks, KW, Gao, JP, Sharma, T. Photodynamic enhancement of doxorubicin cytotoxicity. Cancer Chemother Pharmacol. 1994;35:17.
37. Roberts, WG, Klein, MK, Loomis, M, et al. Photodynamic therapy of spontaneous cancers in felines, canines, and snakes with chloro-aluminum sulfonated phthalocyanine. J Natl Cancer Inst. 1991;83:18.
38. Agostinis, P, Berg, K, Cengel, KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281.
39. Cornell, K, Waters, DJ. Impaired wound healing in the cancer patient: effects of cytotoxic therapy and pharmacologic modulation by growth factors. Vet Clin North Am Small Anim Pract. 1995;25:111–131.
40. McEntee, MC. Principles of adjunct radiotherapy and chemotherapy. Vet Clin North Am Small Anim Pract. 1995;25:133–148.
41. Ferguson, MK. The effect of antineoplastic agents on wound healing. Surg Gyn Obstet. 1982;154:421–429.
42. Graves, G, Cunningham, P, Raaf, JH. Effect of chemotherapy on the healing of surgical wounds. Clin Bull. 1980;10:144–149.
43. Laing, EJ. The effects of antineoplastic agents on wound healing: guidelines for combined use of surgery and chemotherapy. Compend Contin Educ Pract Vet. 1989;11:136–143.
44. Dernell, WS, Withrow, SJ, Straw, RC, et al. Intracavitary treatment of soft tissue sarcomas in dogs using cisplatin in a biodegradable polymer. Anticancer Res. 1997;17:4499–4506.
45. Fisher, B, Gunduz, N, Saffer, EA. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 1983;43:1488–1492.
46. Fisher, B. Cancer surgery: A commentary. Cancer Treat Rep. 1984;68:31–41.
47. Tepper, J, Million, RR. Radiation therapy and surgery. Cancer Treat Symposia. 1984;1:111–117.
48. Sequin, B, McDonald, DE, Kent, MS, et al. Tolerance of cutaneous or mucosal flaps placed into a radiation therapy field of dogs. Vet Surg. 2005;34:214–222.
49. MacEwen, EG, Withrow, SJ, Patnaik, AK. Nasal tumors in the dog: retrospective evaluation of diagnosis, prognosis, and treatment. J Am Vet Med Assoc. 1977;170:45–48.
50. Adams, WM, Bjorling, DE, McAnulty, JF, et al. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1990–2002). J Am Vet Med Assoc. 2005;227:936–941.
51. Farese, JP, Milner, R, Thompson, MS, et al. Stereotactic radiosurgery for the treatment of lower extremity osteosarcoma in dogs. J Am Vet Med Assoc. 2004;225(10):1567–1572.
52. Weisse, C, Berent, A, Todd, K, et al. Evaluation of palliative stenting for management of malignant urethral obstructions in dogs. J Am Vet Med Assoc. 2006;229(2):226–234.
53. Berent, AC, Weisse, C, Beal, MW, et al. Use of indwelling, double-pigtail stents for treatment of malignant ureteral obstruction in dogs: 12 cases (2006–2009). J Am Vet Med Assoc. 2011;238(8):1017–1025.
54. Royals, SR, Ellison, GW, Adin, CA. Use of an ultrasonically activated scalpel for splenectomy in 10 dogs with naturally occurring splenic disease. Vet Surg. 2005;34:174–178.
55. Holak, P, Adamiak, Z, Jały ski, M, et al. Laparoscopy-guided prostate biopsy in dogs–a study of 13 cases. Pol J Vet Sci. 2010;13(4):765–766.
ski, M, et al. Laparoscopy-guided prostate biopsy in dogs–a study of 13 cases. Pol J Vet Sci. 2010;13(4):765–766.
56. Collard, F, Nadeau, ME, Carmel, EN. Laparoscopic splenectomy for treatment of splenic hemangiosarcoma in a dog. Vet Surg. 2010;39:870–872.
57. Mayhew, PD. Advanced laparoscopic procedures (hepatobiliary, endocrine) in dogs and cats. Vet Clin North Am Small Anim Pract. 2009;39(5):925–939.
58. MacEwen, EG, Hayes, AA, Harvey, HJ, et al. Prognostic factors for feline mammary tumors. J Am Vet Med Assoc. 1984;185:201–204.
59. Golinger, RC. Breast cancer controversies: surgical decisions. Semin Oncol. 1980;7:444–459.
60. Kurzman, ID, Gilbertson, SR. Prognostic factors in canine mammary tumors. Semin Vet Med Surg. 1988;1:25–32.
61. Bartels, KE. Lasers in veterinary medicine: where have we been, and where are we going? Vet Clin North Am Small Anim Pract. 2002;32:495–515.
62. Gillams, AR. Mini-review: The use of radiofrequency in cancer. Br J Cancer. 2005;92:1825–1829.
63. Kennedy, JE. High-intensity focused ultrasound in the treatment of solid tumours. Nature Rev. 2005;5:321–327.
64. Kodama, M, Kodama, T, Nishi, Y, et al. Does surgical stress cause tumor metastasis? Anticancer Res. 1992;12:1603–1616.
65. Blumberg, N, Heal, JM. Perioperative blood transfusion and solid tumor recurrence: a review. Cancer Invest. 1987;5:615–625.
66. Pollock, RE, Lotzová, E, Stanford, SD. Surgical stress impairs natural killer cell programming of tumor for lysis in patients with sarcomas and other solid tumors. Cancer. 1992;70:2192–2202.
67. Medleau, L, Crowe, DT, Dawe, DL. Effect of surgery on the in vitro response of canine peripheral blood lymphocytes to phytohemagglutinin. Am J Vet Res. 1983;44:859–860.
68. Murthy, SM, Goldschmidt, RA, Rao, LN, et al. The influence of surgical trauma on experimental metastasis. Cancer. 1989;64:2035–2044.
69. Navarro, M, Lozano, R, Román, A, et al. Anesthesia and immunosuppression in an experimental model. Eur Surg Res. 1990;22:317–322.
70. Meakins, JL. Surgeons, surgery, and immunomodulation. Arch Surg. 1991;126:494–498.
71. Cady, B. Basic principles in surgical oncology. Arch Surg. 1997;132:338–346.