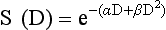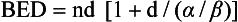Radiation Therapy
Radiation therapy has been used in veterinary medicine since shortly after the discovery of x-rays by Roentgen in 1895. Alois Pommer, an Austrian veterinarian, published extensively on the irradiation of benign and malignant diseases and established a radiation therapy protocol widely used for many years.1 Technologic advances improving our understanding of the radiation biology of normal and tumor tissues have enabled development of contemporary radiation therapy techniques and protocols.
Over half of human patients with serious cancers undergo radiation therapy at some point during treatment.2 Radiation therapy is an effective treatment modality for animal cancer patients with solid tumors; however, early use of the modality was limited due to the sparse availability of veterinary treatment centers. The last decade has been marked by the opening of numerous veterinary radiation therapy centers and the commissioning of more advanced radiation therapy technologies. More than 60 facilities in North America are actively treating animals with radiation therapy and the American College of Veterinary Radiology (Specialty in Radiation Oncology) has residency training programs at 17 treatment centers.3
The management of cancer patients is complex, and determining the best treatment modality or combination of modalities can be challenging. In most instances, when local control of a solid tumor cannot be obtained surgically without excessively compromising an animal’s function, appearance, or quality of life, a consultation with a radiation oncologist should be considered. In many such instances, combining surgery with radiation therapy will allow a more conservative surgery and yield comparable or better tumor control and/or functional outcome than either surgery or radiation alone. In other cases, radiation alone may be a preferred alternative to surgery (i.e., intranasal tumors).
In addition to treating serious cancers with what is referred to as “curative intent,” radiation therapy also plays an important role in the palliative treatment of advanced cancers, the treatment of endocrinopathies associated with endocrine adenomas, and as an adjuvant treatment for lymphoma patients.
New modalities such as stereotactic radiation therapy (SRT), image-guided radiation therapy (IGRT), and intensity-modulated radiation therapy (IMRT) are also changing the treatment paradigm by providing improved radiation options for tumors in a variety of locations. Keeping abreast of ongoing clinical evaluation of these modalities is important for optimal patient management.
Biologic Principles of Radiation Oncology
Radiation dose is described by the amount of energy absorbed by the tissue. The unit of absorbed dose is the Gray (Gy); one Gy equals one joule absorbed per kilogram of tissue. Ionizing radiation kills cells by damaging critical molecules in the cell, primarily DNA, which eventually leads to cell death. Megavoltage photons, the predominant form of radiation used in veterinary medicine, interact with tissue primarily by the Compton effect, producing high energy electrons that cause ionization events either to critical molecules (direct effect) or from water molecules located within nanometers of critical molecules (indirect effect). These events produce highly reactive free radicals that result in biologic damage that may kill the cell or render it incapable of reproducing. In most cells, death from exposure to ionizing radiation results from chromosomal aberrations.
Repair of Radiation Damage
A critical determinant of a cell population’s sensitivity to radiation is the ability of cells to repair DNA damage caused by radiation. One Gy of radiation from photons causes approximately 2500 base damages, 1000 single-strand breaks, and 40 double-strand breaks in DNA in each cell.4 Most of this damage is repaired by cells within 6 to 24 hours; the double-strand breaks are the most lethal because they may lead to severe chromosomal aberrations. A given dose of radiation is preferentially cytotoxic to proliferating cells, including tumor cells and renewing cell populations (e.g., epithelial stem cells), although slowly dividing and nondividing cells (e.g., bone and cells of the nervous system) are also affected by radiation.
Cell Cycle Effects
The period of the cell cycle in which DNA undergoes synthesis is known as S-phase. Before and after S-phase are periods without overt activity by the DNA; these periods are called G1 phase and G2 phase. G2 phase is followed by mitosis. Cells are distributed throughout the cell cycle in a tumor or tissue at a given time. The individual cell sensitivity to irradiation varies, depending on the phase of the cell cycle at the time of irradiation. Cells in late S-phase are most resistant to irradiation, and cells in late G2 or mitosis are most sensitive.
Oxygen Effects
Because of their rapid growth and abnormal vasculature, tumors often become partially hypoxic. This results in upregulation of hypoxia-inducible proteins that may prepare the tumor cells to handle stresses. Oxygen is also a critical factor in the response to irradiation because reactive oxygen species (ROS) generate much of the damage from radiation. As a result, normoxic cells are up to threefold more sensitive to radiation than hypoxic cells.
Relative Biologic Effectiveness
Although this chapter primarily discusses the effects of photons (x-rays and gamma rays) and electrons, there are many other forms of radiation that can be used in oncology, including protons and neutrons. The biologic effects of 1 Gy of electrons are the same as 1 Gy of photons, but 1 Gy of protons or neutrons may cause substantially more damage than 1 Gy of photons or electrons. This difference is known as the relative biologic effectiveness (RBE) of the type of radiation.
Time, Dose, and Fractionation
Early radiation oncologists found that higher total doses could be given if the doses were divided into smaller fractions. They observed that tumor response was improved, and less injury of normal tissue occurred. In veterinary medicine, standard fractionation denotes a regimen delivering 2.7 to 4 Gy per fraction, 3 to 5 times per week to a total dose of 42 to 57 Gy, although several other regimens are currently being used or investigated. Hyperfractionation refers to schedules in which the dose per fraction is reduced and the total dose is increased. Accelerated fractionation describes a treatment regimen in which the overall time of treatment is reduced, but the dose per fraction and total dose are unchanged. Hypofractionation describes the administration of high doses per fraction given in a small number of fractions to a lower total dose. The response of tumor and normal tissues between fractions throughout the course of radiation therapy has been described by Withers5 as the “four Rs” of radiation therapy: repair of DNA damage, redistribution of cells in the cell cycle, reoxygenation of tumor cells, and repopulation of tumor and normal tissues.
Division of the total radiation dose into fractions is important for a number of reasons. The first reason is to exploit potential differences in repair capabilities between tumors and normal tissues. Slowly dividing cells are somewhat less sensitive to small doses of radiation than more rapidly dividing cells; however, they appear to become relatively more sensitive if radiation is delivered in larger doses per fraction. If smaller doses per fraction are used, normal tissues with slowly dividing cells can be spared relative to tumor tissues with rapidly dividing cells.
Other events that occur between radiation fractions are cell cycle redistribution and reoxygenation. When a fraction of radiation therapy is administered, many of the cells in the sensitive portions of the cell cycle are killed. During the interval between fractions, cells from the late S-phase, which are more likely to be alive than other cells, progress to more sensitive parts of the cell cycle. This is known as redistribution. Reoxygenation also occurs during the interval between radiation fractions when many of the hypoxic tumor cells become aerobic and thus more sensitive to irradiation.
The length of time over which radiation therapy is administered is important, primarily because of tumor repopulation but also because of rapidly proliferating normal tissues, such as mucosa and skin. Tumor cells that have not been destroyed by irradiation continue to replicate during the course of therapy. This process is exacerbated by a phenomenon known as accelerated repopulation. Some have suggested that after approximately 4 weeks of therapy, tumors repopulate more rapidly than initially.6 The reason for this is not clear, but the phenomenon could be related to (1) a reduction in the cell cycle time, (2) an increase in the number of tumor cells that are actively dividing, or (3) a reduction in the number of tumor cells that normally die (cell loss factor). Regardless of the cause, when treatment lasts longer than 4 weeks, repopulation may affect the outcome unless total dose is increased to account for this phenomenon. Repopulation may have a greater impact on rapidly dividing tumors than on slowly dividing tumors.
Repopulation of proliferating (also known as acutely responding) normal tissues is also affected by time. The same total dose of radiation administered over a short period results in somewhat more severe acute effects than if administered over a longer course. Nonproliferating (late-responding) normal tissues are not significantly affected by the length of time over which therapy is administered. Fraction size and the interval between treatment fractions are more important in late-responding tissues. Fractions should be separated by at least 6 hours to allow repair of DNA damage to normal tissues. Cells of the brain and spinal cord may require additional time for complete repair, and the impact of multiple fractions per day on these late-responding tissues is not clearly understood.
The total dose administered to a patient should have a low probability for causing significant late normal tissue reactions in the region of therapy. However, the response of tissues also depends on the fraction size. For example, 48 Gy administered in 4 Gy fractions has a higher probability of causing late effects than 48 Gy administered in 3 Gy fractions (Figure 12-1). The probability of tumor control is not as affected because rapidly proliferating tissues, including tumors, are not as sensitive to the change in dose per fraction. The benefits of protocols that use small doses per fraction are clear: they allow a higher total dose to be administered without increasing the probability of damage to late-responding normal tissues.
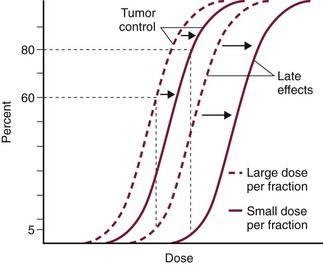
Figure 12-1 Radiotherapy delivered in small fractions (solid lines) can produce a higher probability of tumor control with the same level of late effects as radiotherapy delivered in large fractions (broken lines).
The total dose tolerated depends on the specific normal tissues present in the irradiated field. For example, brain and spinal cord are less tolerant to the effects of irradiation than muscle or bone. Another factor that must be considered when selecting the appropriate dose is the volume of tissue in the field. Large volumes of normal tissues are more susceptible to damage from irradiation than smaller volumes.
Although time, dose and fractionation are still the underpinnings of SRT, the paradigm is different than for fractionated radiation therapy. SRT involves the use of high doses per fraction but overcomes the radiobiologic limitations with stereotactically verified positioning and treatment delivery techniques that leave a minimal volume of normal tissue in the high dose area. Stereotactic radiation therapy, by definition, requires (1) a tumor for targeting (not microscopic disease), (2) treatment planning and administration that will provide a dramatic dose drop-off between the tumor and the surrounding normal tissue structures, and (3) a method of stereotactically verifying patient positioning. The result is that normal late-responding tissue structures are spared through dose avoidance rather than by administering small doses per fraction. The normal tissue structures still receive dose, and the dose per fraction is higher than for traditional radiation therapy. However, the total dose to the normal structures is lower than what is typical for fractionated radiation therapy. Normal tissue tolerance data are just evolving for SRT, as is long-term follow-up. Estimates of tolerance have been based on limited clinical data, toxicity observation, and educated guessing.7 It is inappropriate to extrapolate dose constraints from fractionated protocols. An additional difference between SRT and fractionated radiation therapy is that with SRT, acutely responding normal tissues in the surrounding region such as skin, esophagus, and colon may be susceptible to consequential late effects. A consequential late effect is a late effect that develops from severe acute effects that may be associated with stem cell depletion. Dose constraints therefore must also be applied to these tissues. SRT treatment is generally delivered in 1 to 5 fractions over a period of 1 week or less for most tumors; therefore accelerated repopulation is unlikely to impact tumor control. Although historically stereotactic treatment was delivered in a single fraction (referred to as stereotactic radiosurgery), current technology allowing precise repositioning makes limited fractionation feasible. Even this minimal fractionation should allow higher total doses to be administered safely to late-responding tissues in the region and presumably take advantage of tumor reoxygenation and redistribution. However, biologic response following SRT has not been comprehensively evaluated. From a practical standpoint, SRT minimizes the number of anesthesia episodes to these older and sometimes debilitated patients and is also generally more convenient for the owner. Acute effects are minimal and tumor-associated signs such as discomfort or dysfunction often improve rapidly. Long-term tumor control and late effects need to be quantitated.
No perfect radiation therapy protocol exists, and all protocols commonly used in veterinary and human medicine have advantages and disadvantages. It is not within the scope of this chapter to prescribe specific radiation doses or fractionation schedules because many factors must be considered. Rather, referring veterinarians must know what to expect when sending patients to a radiation oncology center, and they should be able to explain some of the fundamental principles to clients. The radiation oncologist should inform the referring veterinarian and owner of the probabilities of late effects and tumor control and estimate the degree of acute effects expected with a specific protocol. The goal of radiation therapy is to destroy the reproductive capacity of the tumor without excessive damage to surrounding normal tissues. This goal is best achieved by dividing the total dose into a number of smaller fractions (fractionation) that are administered over a period of time or by applying stereotactic technology. Regardless of the approach, the relationship of these three parameters (time-dose-fractionation) must be carefully considered in the development of radiation treatment plans for successful therapy.
Acute and Late Effects
Reactions from radiation therapy are classified as acute (also called early) or late. Acute effects occur during or shortly after radiation therapy. Acute effects involve rapidly proliferating tissues, such as the oral mucosa, intestinal epithelium, and epithelial structures of the eyes and skin. Concurrent chemotherapy can exacerbate acute effects from radiation. These effects generally are self-limiting, and recovery is rapid. However, acute effects can be unpleasant for the patient and distressing to the owner, and in rare instances they can be life-threatening if the proper care is not given. The referring veterinarian often is called on to treat recently irradiated patients. Acute effects will heal without medical intervention in the vast majority of cases over the course of weeks or occasionally months. In veterinary patients, the most important provision to allow healing during this time is prevention of self-trauma of the radiation site by the patient. Therefore pain management plays an important role and should be addressed. Pain management for cancer patients is discussed in Chapter 15, Section A, of this text, and specific protocols have been published.8 Additional treatment is based on common sense, supportive care, and the knowledge that the signs will resolve with time.
Late effects involve more slowly proliferating tissues, such as bone, lung, heart, kidneys, and nervous system. The dose of radiation administered is limited by the tolerance of these normal tissue structures in the field. Late reactions can be difficult to treat; it is the radiation oncologist’s obligation to minimize the incidence of late effects with appropriate dose prescriptions and careful radiation planning and treatment. When late effects occur, they may be quite severe, resulting in fibrosis, necrosis, loss of function, or even death.9 Late effects occur from the loss of normal tissue stem cells with concurrent radiation-induced vascular changes and inflammation. These changes are multifactorial, but the cytokine transforming growth factor-β (TGF-β) is believed to play a critical role in radiation fibrosis. Strategies attempted in human radiation oncology to mitigate late radiation effects include the use of antioxidants and free radical scavengers (superoxide dismutase, vitamin E, thiol radioprotectors), vascular-directed therapies (clopidogrel, statins, pentoxifylline), antiinflammatory agents (corticosteroids), inhibitors of the renin-angiotensin system (angiotensin-converting enzyme [ACE] inhibitors), and stem cell therapies.9 In veterinary patients, severe late reactions such as fibrosis and tissue necrosis should be managed under the guidance of or referred to a surgeon and/or radiation oncologist experienced in dealing with radiation injury.
Radiation-Induced Neoplasia
Ionizing radiation is a complete carcinogen, capable of initiating, promoting, and progressing cellular changes that lead to cancer. Therefore it is possible to see radiation-induced neoplasia develop in a radiation treatment field. It appears that orthovoltage radiation and radiation with high linear energy transfer (high-LET) such as neutrons result in carcinogenesis at a higher frequency than the megavoltage photons typically used in veterinary radiation therapy.10 Other factors that influence the risk of radiation carcinogenesis include the age of the patient (young patients are more likely to develop subsequent tumors) and the tissues irradiated. Certain tissues are also more prone to development of radiation-induced tumors, such as the thyroid gland. For a tumor to be considered radiation induced, the following criteria must be met10,11:
1. The malignancy must arise within the irradiated field.
2. Sufficient latency must have elapsed between the time of irradiation and development of the tumor (typically at least 1 year).
3. The original tumor and the new tumor must have different histologic diagnoses.
4. The tissue in which the new tumor forms must have been normal prior to radiation exposure.
The overall incidence of radiation-induced tumors in patients treated with radiotherapy is thought to be extremely low (<1% to 2% of patients treated).11
Cell Survival After Irradiation
After a tissue or population of cells is exposed to any dose of radiation, a fraction of the cells will be killed. The proportion of remaining cells is known as the surviving fraction (S). The sensitivity of a tumor or tissue to radiation can be shown as a graph of the radiation dose (D) versus the surviving fraction (Figure 12-2).12 The relationship between a dose of radiation and the surviving fraction of cells is commonly described by the linear quadratic equation:
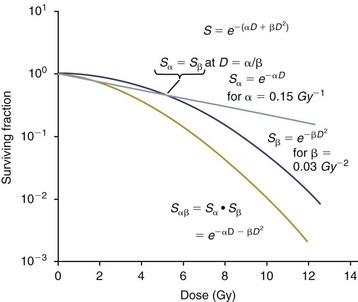
Figure 12-2 Illustration of the alpha/beta (α/β) model in which cell killing occurs by either a single-even process or a double-even process so that the overall killing by either process is the product of the two, and the (α/β) ratio is the dose at which both processes contribute equally to the total killing. Note that the upper curve is survival for the α component only, the middle curve is for the β component only, and the lower curve is for both components. (Redrawn from Wilson PF, Bedford JS. In Hoppe RT, Phillips TL, Roach M III, editors: Leibel and Phillips textbook of radiation oncology, ed 3, St. Louis, 2010, Elsevier.)
where S is the surviving fraction at a dose (D).13 Alpha (α) and beta (β) are constants that vary according to the tissue with α corresponding to the cell death that increases linearly with dose and β corresponding to cell death that increases in proportion to the square of the dose (also known as the quadratic component). The α/β ratio is a useful number that is the dose in Gy when cell kill from the linear and quadratic components of the cell survival curve is equal. Cells with a higher α/β ratio have a more linear appearance when plotted on a log scale, and cells with a low α/β ratio have a parabolic shape. The α/β ratio is also an important description of the radiosensitivity of a cell. At low-dose fractions, tissues or cells with low α/β ratio are relatively radiation resistant compared to tissues or cells with high α/β ratio. It has been suggested that tissues and cells with low α/β ratios have a greater capacity for repair of sublethal radiation damage. Sublethal radiation damage is defined as damage that can become lethal if it interacts with additional damage. Sublethal damage repair is the reason that cell survival increases when a radiation dose is split into two fractions separated by a time interval.
Most early responding tissues and tumors have a high α/β ratio, whereas late-responding tissues have a low α/β ratio.13 There are some tumors that may have a low α/β ratio, which can influence the optimal radiation prescription in terms of total dose, time, and fractionation. Tumors that may have lower α/β ratios include melanoma, prostatic tumors, soft tissue sarcomas, transitional cell carcinoma, and osteosarcomas.14-16
The concept of biologic effective dose (BED) is used to predict how changes in dose prescription may preferentially affect different cells or tissues based on their α/β ratio in the linear quadratic model of survival. The formula for BED is as follows:
where n is the number of fractions and d is the dose per fraction. If the α/β ratio of a tissue is known or can be estimated, one can calculate the BED for any dose prescription. It is possible to use this formula to assess how dosimetry changes or errors alter the effective dose of a protocol. It is important to note that there are several limitations to the use of this equation, including that it does not account for differences in the overall length of time of the radiation protocol or accelerated repopulation. There are other formulas that can be used to account for time. Also, the true α/β ratio of any cell or tissue is rarely known; therefore calculations made with this model involve making assumptions or predictions that may be incorrect. Nevertheless, this formula is a useful tool when considering hyperfractionating or hypofractionating a standard radiation protocol in order to create a new protocol that will have expected outcomes related to either tumor control or tissue complications. However, the validity of BED for SRT is unclear.
Alternative Mechanisms of Radiation Injury
In addition to direct radiation cytotoxicity, the extracellular matrix and local microenvironment also appear to play an important role in radiation response. Some evidence suggests that apoptosis of endothelial cells may precede damage to proliferating cells and cause some of the effects observed after radiation. Apoptosis of endothelium primarily occurs when high-dose fractions are administered and may play a more important role when hypofractionated and radiosurgical doses of radiation are delivered.
Chemical Modifiers of Radiation
Although rarely used clinically in veterinary medicine, many drugs can modify the cellular and tissue response to radiation. Radioprotectors (e.g., amifostine/WR-2721) are compounds that decrease the amount of radiation damage to targeted normal cells without providing similar protection to tumor cells. Radiosensitizers are chemicals that achieve greater tumor inactivation than would be expected from the additive effect of treatment with either radiation or the chemical alone. Mechanisms of action of radiation sensitizers include hypoxic cell sensitizers or cytotoxins and agents that damage or incorporate into DNA.
Palliative Radiation Therapy
Palliative radiation therapy is commonly used in human medicine, and its use in veterinary medicine has increased in recent years. Palliative radiation is generally hypofractionated compared to curative intent protocols, often administered in larger doses per fraction (6 to 10 Gy per fraction) in 1 to 4 total fractions once or twice weekly. Conversely, palliative therapy can be delivered in conventional doses or modestly hypofractionated daily or twice daily for a short but intense treatment regimen. The goal of palliative radiation therapy is not to provide long-term or definitive tumor control; rather, it is intended to relieve pain or improve function or quality of life in patients in which other factors (e.g., advanced metastatic disease) are likely to lead to early demise. Palliative radiation therapy has been used most often for metastatic or primary bone tumors, principally canine osteosarcoma (see Chapter 24). Palliative therapy is more convenient for the owner, and the cost is modest compared with curative radiation therapy because fewer fractions are administered. It is, however, important to remember that palliative radiation is not a substitute for curative-intent protocols despite the convenience. Some palliative therapy protocols may have an increased probability of causing late radiation effects but because they are prescribed to patients that have a poor long-term prognosis, these late effects may not have time to manifest. Curative-intent radiation protocols require strict adherence to radiation biologic principles; palliative radiation protocols, on the other hand, are far more flexible. As in human hospital settings, the protocols may vary dramatically from radiation center to radiation center.
Radiation Therapy Equipment
Ionizing radiation can be administered by an external source (teletherapy), through placement of radioactive isotopes interstitially (brachytherapy), or by systemic or cavitary injection of radioisotopes, such as iodine-131 (131I). Teletherapy, also referred to as external-beam radiation therapy, is the most commonly used method of radiation therapy in veterinary medicine. External-beam radiation therapy usually is classified as orthovoltage or megavoltage radiotherapy, based on the energy of the photon. Orthovoltage machines produce x-rays with an energy of 150 to 500 kVp; megavoltage radiation emits photons with an average energy greater than 1 million electron volts (1 MeV). Although some veterinary radiation oncology centers continue to treat with orthovoltage machines, megavoltage radiation is primarily used. Megavoltage radiation for therapy can be obtained from cobalt machines or linear accelerators. Because megavoltage radiation has excellent tissue-penetrating capabilities, radiation therapy can be performed on deeply seated tumors for which orthovoltage therapy would not be an option.
Orthovoltage x-rays, which have low energy, distribute maximum doses to the skin surface. Acute effects to the skin can be quite severe, causing discomfort to the patient, and late effects to the skin and subcutaneous tissues can be dose limiting. Megavoltage radiation has a higher energy than orthovoltage, and the photons must interact with tissues, allowing the dose to build up, before the maximum dose can be achieved. The skin therefore can receive a significantly weaker dose than the underlying tumor. This skin-sparing effect of megavoltage radiation allows the optimal dose to be administered to a more deeply seated tumor without causing severe reactions to the skin. When the tumor involves the skin or is in proximity of skin, megavoltage radiation can be used successfully by placing a sheet of tissue-equivalent material, called a bolus, over the tumor. This allows the dose build up to occur before reaching the skin, so that the skin and associated tumor can receive the maximum dose of radiation.
The absorption of megavoltage radiation, unlike that of orthovoltage radiation, is minimally dependent on the composition of the tissue. This characteristic permits even distribution of the dose throughout the tissues in the field. Orthovoltage radiation is preferentially absorbed by bone. If a tumor adjacent to or overlying bone is administered a meaningful dose of orthovoltage radiation, the probability that late effects to the bone (bone necrosis) will develop is quite high. Treatment with orthovoltage should be limited to small, superficial tumors such as nasal planum tumors or to superficial tumor beds after surgical excision. The interaction of megavoltage radiation with tissues is quite predictable, which has allowed the development of computerized treatment planning systems. These planning systems allow treatment of the tumor with multiple beams administered from different angles. Beam modifiers, such as wedges and blocks, can be incorporated into the treatment plan. Wedges are triangular-shaped pieces of lead that can be placed between the beam and the patient. Less radiation penetrates the thick side of the wedge, which modifies the dose distribution. The goal of computerized treatment planning is to ensure a desired minimum tumor dose to a region specified by the radiation oncologist and to spare normal tissue structures when possible. Conventional radiation therapy uses a limited number of computed tomography (CT) or magnetic resonance imaging (MRI) images, which are imported or contoured using a tablet. The summation of multiple beams coming from different directions provides a higher dose to the tumor than surrounding tissues.
Advances in treatment planning and imaging over the past decade have led to the development of image-based, three-dimensional (3D) conformal radiation therapy (3DCRT), which permits better conformity between the irradiated high-dose volume and the geometric shape of the tumor (Figure 12-3). 3DCRT requires importation of CT, MRI, or positron emission tomography (PET) imaging into the treatment planning system. The animal must be positioned for the imaging in a fashion that can be replicated precisely on a day-to-day basis for treatment. Alpha cradles, acrylic face masks, bite plates, and/or Vac-Lok Cushions (Figure 12-4) often are used as positional aids. The radiation oncologist identifies important normal tissue structures, as well as the gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) on these images. By definition, the GTV only includes gross tumor, and the CTV includes the GTV plus an expansion based on the known clinical behavior of the specific tumor to account for regional microscopic disease. For example, the CTV expansion for a sarcoma is generally larger than for a carcinoma. If the patient has had cytoreductive surgery and only microscopic tumor remains, there is no GTV, and the CTV is based on the scar, regions of surgical disruption, and an expansion for microscopic disease beyond the surgical site. In addition to the GTV and CTV, the PTV includes expansion for an internal margin (IM) that accounts for variations in size and shape relative to anatomic landmarks (filling of bladder, respiratory movements) and set-up margin (SM). The SM accounts for uncertainties in patient positioning and alignment during planning imaging and subsequent treatments. The better the immobilization device, the smaller the SM expansion can be. SM expansions will vary based on the location of the tumor because some sites such as the head are more amenable to rigid immobilization devices such as bite blocks, which provide better replicability. The IM expansion is impacted more by the radiation therapy device. Machines with on-board-imaging devices such as kV x-ray or cone-beam CT (CBCT) can have more confidence in smaller IM expansions. Decreasing the PTV expansion by using good immobilization and available imaging impacts the volume of normal tissue treated and is a key component to successful 3DCRT and is critical to other advanced treatment modalities. More sophisticated beam shaping is performed by taking advantage of fixed multileaf collimators or custom-made blocks. A major advantage of 3DCRT is that dose-volume histograms can be obtained for the tumor and normal tissue structures. This provides a quantitative method of evaluating treatment plans and enhances quality assurance. Defined dosimetric parameters may be useful predictors of outcome.
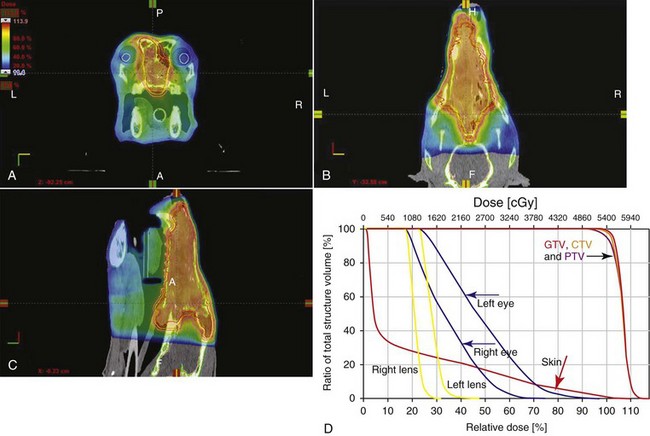
Figure 12-3 A to C, The axial, dorsal, and sagittal sections through a canine nasal tumor. Dose is in color wash, with the prescribed dose in orange. D, The dose-volume histogram. Note the high dose to the tumor compared to regional normal tissue structures.
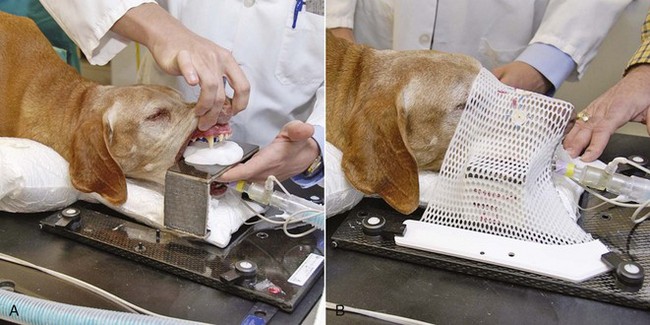
Figure 12-4 A, Teeth being placed in preformed acrylic bite block that is inserted into a carbon fiber–indexed frame. Neck is resting on a vacuum lock bag. B, Acrylic face mask being placed.
IMRT and related modalities such as tomotherapy allow even greater sculpting of the radiation dose. These modalities require strategies for patient positioning and immobilization. IMRT requires a specific treatment planning system that uses inverse planning. Inverse planning requires that the various tumor structures (GTV, CTV, and PTV), as well as critical normal tissue structures, be identified and contoured into the planning system. Optimization objectives for each structure are entered, and a sophisticated algorithm attempts to meet all objectives. This is the standard of care for treatment of prostate tumors, head and neck cancers, vertebral cancers, some brain cancers, and pelvic cancers in humans. A major benefit associated with IMRT is that the dose to adjacent normal tissue structures can be minimized, dramatically reducing acute effects. Patients are more comfortable and require less pain medication.17 In addition, the tumor dose can be increased without exceeding normal tissue tolerance, presumably leading to improved tumor control. Fractionation schedules similar to those for conventional radiation therapy are used. Tomotherapy, a form of IMRT that uses a helical delivery system to sculpt the beam, is also being used in veterinary medicine.18,19 IMRT is proving useful for the treatment of tumors or tumor beds with complex geometry located near important normal tissues, such as nasal tumors, oral tumors, urogenital tumors, and in cats, vaccine-associated sarcomas.17
SRT describes an emerging field in radiation oncology that uses advanced technology to achieve a different biologic approach. It was originally coined stereotactic radiosurgery (SRS) when the gamma knife was first developed for treatment of inoperable brain tumors in the 1950s by Lars Leksell.20 The gamma knife uses hundreds of small cobalt sources that can converge in 3 dimensions to focus precisely on a small volume of tumor. The gamma knife initially required a rigid frame-based positioning device that was bolted into the patient’s skull prior to imaging and treatment planning. Therefore it was primarily used as a single fraction treatment. CyberKnife is a robotic radiosurgery system incorporating a small linear accelerator on a movable robotic arm, integrating advances in robotic technology and real-time computer-tracking technology.21 The small linear accelerator is moved around the patient and tumor by the robotic device, while the tracking system verifies position by tracking fiducial markers placed in the tumor prior to treatment planning. This frameless tracking system makes repeat fractions feasible, allowing the benefits of modest fractionation. It also makes treating tumors in other parts of the body possible. SRT can also be delivered by specially designed linear accelerators that have designated beams with attributes conducive to small fields and on-board imaging (OBI) capability. Patients are placed in positioning devices such as bite blocks, face masks, and Vac-Lok cushions prior to imaging. For tumors associated with bony structures such as nasal or brain tumors, orthogonal kV images are obtained and the operating software allows the patient’s treatment position to be “matched” to the original imaging positioning. The couch will then make an automatic adjustment. For tumors that may move relative to adjacent bone, CBCT is used and a 3D match is used to verify tumor position.22 The benefits of accelerator-based SRT are that treatment times are generally short, almost any tumor location is accessible, and tumors with larger volumes can be treated.
A number of acronyms are used to describe hypofractionated stereotactic radiation treatment. SRS refers primarily to treatment of brain tumors and generally, but not always, refers to a single fraction treatment. Stereotactic body radiation therapy (SBRT), SRT, and stereotactic ablative radiation therapy (SAbR) are interchangeable terms used to describe 1 to 5 fraction treatment regimens, regardless of method of administration. Of key importance is the recognition that these stereotactic procedures require gross tumor as a target, must have dramatic dose drop-off between tumor and normal tissues, and must have a method of stereotactic verification.
Radiosurgery has been used on a limited basis in veterinary medicine for brain and bone tumors23-25 and more recently for nasal tumors, multilobular osteochondromas, pituitary tumors, thyroid tumors, heart-based tumors, sarcomas, and tumors in the pelvic canal (personal communications, James Custis, Susan LaRue).26 Technologic advances such as those described previously are likely to become available on a limited basis over the next decade. An important consideration is identifying which tumor types will benefit most from such approaches.
Tumors Commonly Treated with Radiation Therapy
Many oral tumors (see Chapter 22, Section A) are responsive to radiation therapy. The region is anatomically complex, and aggressive surgery often can leave functional and cosmetic abnormalities. For many oral tumors, combining surgery and radiation will provide the best outcomes; however, the optimal time to begin irradiation after surgery (or surgery after irradiation) has not been determined. If difficulty in obtaining primary wound healing appears likely, radiation can be delayed. However, the therapeutic gain from combining modalities will be lost if the tumor recurs in the interim. Initiating radiation therapy immediately after surgery does not appear to be a problem if the suture line is tension free and well vascularized. Mucosal flaps can be used successfully to close surgical defects in the oral cavity of patients that will receive postoperative radiotherapy, but complications are common.27
Efficacy of Treatment
Acanthomatous ameloblastomas, previously called adamantinomas or acanthomatous epulides, are very radiation responsive. Tumor control with radiation therapy can be close to 90%.28 A relationship has been demonstrated between tumor (T) stage and local control.29 The reported 3-year, progression-free survival (PFS) for T1 tumors (less than 2 cm) and T2 tumors (2 to 4 cm) is 86%; it is only about 30% for T3 tumors (over 4 cm).29 In 2004 a retrospective study of 57 dogs with epulides that were treated with irradiation reported that the overall median time to first event and overall survival were 1210 and 1441 days, respectively.30 Dogs younger than 8.3 years old (the median age in the study) had a significantly longer median survival time (2322 days) than dogs older than 8.3 years (1106 days). Dogs that received doses higher than 40 Gy had significantly longer survival times than dogs that received 40 Gy or less (2994 days versus 143 days).
Canine oral squamous cell carcinomas (SCCs) are responsive to radiation, although the prognosis is site dependent with tumors located more rostrally having better probability of control.31 Tumors of the base of the tongue and tonsil are highly metastatic and are likely to recur locally or regionally. In these locations, radiation therapy has an advantage over surgery because it includes associated lymphatic structures in the treatment field. A 1987 study of oral SCC treated with a coarse fractionation protocol of 10 4.5-Gy fractions resulted in tumor control at 1 year of about 75%.32 Another study of oral tumors reported a PFS at 1 and 3 years of 72% and 40% for SCC of all T stages.33 A 1996 study of fractionated radiotherapy (48 to 57 Gy in 3 to 4 Gy fractions) in 14 dogs with SCC reported a median disease-free interval and survival of 365 and 450 days, respectively.34
In cats, oral SCC has a very poor prognosis.35 Although many cats show an initial response and may even show dramatic reductions in tumor size, rapid tumor recurrence is common. Combining curative-intent radiation with etanidazole or mitoxantrone therapy has resulted in median survival times of 116 to 170 days.36,37 In one study, seven cats with mandibular SCCs that were treated with hemimandibulectomy and mandibular node excision followed by radiation therapy had a median survival time of 420 days.38 Although the numbers are limited, mandibular SCCs may have a better prognosis than sublingual SCC. Recent studies have indicated that palliative (coarsely fractioned) radiation therapy, with or without chemotherapy, for cats with oral SCC is of dubious value.39,40 Another approach investigated in nine cats with oral SCC was accelerated radiotherapy (14 fractions of 3.5 Gy in 9 days), which resulted in a median survival of 86 days although three cats had a complete response with a median survival of 298 days.41 Although radiation is likely to play a role in the treatment of this disease, new approaches are indicated.
Oral fibrosarcomas are unlikely to metastasize but can be difficult to control locally. The histologic appearance can be deceptive; tumors diagnosed as fibromas or low-grade fibrosarcomas can be extremely aggressive locally.42 If clinical evidence of rapid growth, invasion into bone, or tumor recurrence exists, the tumor should be treated aggressively in spite of more benign pathologic features. En bloc surgical resection often is curative but difficult to accomplish because of the invasive nature of the tumor. Oral fibrosarcomas are less radiosensitive than epulides and SCC, although tumor control probabilities ranging from 33% to 67% at 1 year have been reported.43,44 One study of oral fibrosarcomas reported a PFS at 1 year and 3 years of 76% and 55%, respectively.33 Surgical cytoreduction improves the probability of tumor control by radiation therapy, and this should be taken into consideration during surgical planning with a focus on removing clinical disease (avoiding extensive local dissection, which increases the radiation field size) and obtaining a tension-free closure provided that all macroscopic tumor is removed. Forrest and colleagues45 reported a median survival time of 540 days in eight dogs with oral sarcomas treated with surgery followed by irradiation. In tumors too large to be surgically resected to a subclinical level, radiation therapy alone is indicated; the probability of long-term tumor control is low with conventional radiation therapy, but new strategies using IMRT or SRT are currently being evaluated.
Malignant melanoma is the most common oral tumor in dogs and is associated with a high rate of regional and distant metastasis.46 Higher doses of radiation per fraction (4 Gy and above) are believed to improve response rates for melanoma. In one study, 38 dogs with oral melanoma without evidence of metastasis were treated with 48 Gy delivered in 4 Gy fractions on a Monday-Wednesday-Friday schedule.33 The median PFS was 17.8 months for all dogs and was stage dependent; for T1 tumors, it was 38 months; for T2 tumors, 11.7 months, and for T3 tumors, 12 months. A retrospective study of 140 dogs evaluated a population of dogs, most of which had regional or distant metastasis at presentation.47 Coarsely fractionated (9 to 10 Gy weekly fractions to a total dose of 30 to 36 Gy) and conventionally fractionated (2 to 4 Gy fractions to as high as 45 Gy or more) protocols were used with or without surgery or chemotherapy. The median times to first event and survival were 5 and 7 months, respectively. Tumor recurrence was the first event in only 27% of the dogs, with new metastases or death accounting for the other 63%. In a retrospective study of 39 dogs with incompletely resected oral melanoma treated with coarsely fractionated radiation therapy plus platinum-based chemotherapy, the median survival time was 363 days.48 The dogs received 6 weekly fractions of 6 Gy, with cisplatin (10 to 30 mg/m2) or carboplatin (90 mg/m2) administered 1 hour before irradiation. Fifteen percent of the dogs failed locally, and the median time to metastasis was 311 days.
Melanoma is frequently treated with immunotherapy to potentially prevent or delay the onset of metastasis. Immunotherapy is unlikely to be successful if the primary tumor is not controlled locally with radiation therapy and/or surgery. It is not currently known whether concurrent administration of a melanoma vaccine with radiation therapy may enhance or diminish the effectiveness or toxicities associated with each treatment.
Five cats with oral malignant melanoma were treated with 8 Gy delivered on days 0, 7, and 21. All died from progressive disease, and median survival was 146 days.49
Radiation Considerations
Reproducibility of treatment fields for oral tumors can be aided by positioning and immobilization devices. Depending on the size and location of the target, IMRT allows better sparing of surrounding normal structures, such as the eyes and salivary glands. Melanoma may have a low α/β ratio, making them more responsive to coarsely fractionation protocols.50
Treatment-Related Toxicities
Treatment toxicity depends on the time-dose-fractionation of the protocol and the specific normal structures in the treatment field. For oral tumors, this can include the skin, nasal cavity, and eyes, which are discussed in subsequent sections. The major acute complication associated with radiation treatment of the oral cavity is mucositis. Mucositis always occurs to some degree in patients that have received irradiation to the oral cavity, pharynx, and/or esophagus. Mucositis typically begins during the second week of therapy and reaches a maximum severity during or shortly after the last week of therapy. Clinical signs include thickened saliva and tenderness of the mouth. These patients occasionally become reluctant to eat or drink and require supportive care. Low-salt foods are more palatable and less irritating to the oral mucosa than regular commercial diets. Rarely, placement of a gastrostomy or esophagostomy tube may be necessary to facilitate feeding. Oral mucositis should subside 1 to 2 weeks after therapy. Rarely, animals treated with chemotherapy subsequent to a course of radiotherapy can develop a return of radiation side effects such as mucositis, which is a phenomenon known as radiation recall.
Late complications of radiation specific to the oral cavity include osteoradionecrosis, xerostomia, and oronasal fistula development. Xerostomia (dryness of the mouth due to salivary dysfunction) is a common complication in human patients undergoing radiation therapy of the head and neck region; however, clinically significant xerostomia is not commonly recognized in animals. IMRT technology should provide improved sparing of salivary glands and may decrease the volume of tissue with mucositis. Osteoradionecrosis or development of nonvital bone susceptible to pathologic fracture after radiotherapy is an uncommon complication that can occur in any bone in a radiation field, typically years after treatment. The mandible is the most susceptible bone to osteoradionecrosis but as with most late effects, the risk can be minimized by administering radiation in lower dose per fraction. Oronasal fistula development after radiation is rare unless the hard or soft palate has been disrupted by an aggressive tumor or oral surgery. At one time, it was suggested that radiation therapy could result in transformation of epulides into malignant epithelial tumors, but this possibility has since been refuted.28,30
Nasal Tumors
Nasal tumors (see Chapter 23, Section B) in dogs are difficult to control. Surgery, chemotherapy, cryosurgery, or immunotherapy alone does not appear to improve survival over no treatment.51-56 Radiation therapy provides the best reported tumor control for canine nasal tumors and likely needs to be part of any curative-intent treatment regimen.
Efficacy of Treatment
Radiation therapy has long been the standard of care for nasal and sinonasal tumors. The location is not generally amenable to complete surgical resection, and most reports combining radiation therapy and surgery have not demonstrated therapeutic gain compared to radiation alone. Interestingly, over the last 20 years, advancement in treatment planning technology from point calculations to 2-dimensional (2D), 2.5-dimensional, and 3DCRT technologies has not been associated with improved tumor control or survival. Megavoltage radiation therapy alone has been reported to provide a median survival of about 1 year.57-59 Histologic subpopulations may be prognostically significant; however, most publications are hampered by inadequate patient numbers. Nasal SCCs, undifferentiated carcinomas, and anaplastic carcinomas have been reported to have survival times of 4 to 6 months, and chondrosarcomas have been reported to have survival times up to 15 months. Cribriform erosion has been associated with a poor prognosis.60 Adams and colleagues61 reported a median survival time of 47.7 months in a small group of dogs that underwent surgery in cases in which a nasal tumor did not regress at least 80% 6 weeks after radiation. Late effects were significant; 9 of 13 dogs developed rhinitis, and 4 progressed to osteonecrosis. Surgery and coarse fractionation reduce normal tissue tolerance to radiation, which likely contributed to the high percentage of patients with late effects in this study. The role of chemotherapy combined with radiation therapy for nasal tumors is still unclear. Studies using conventional or low-dose chemotherapy have not improved the outcome over radiation alone.52,58 Radiation therapy combined with slow-release cisplatin implanted intramuscularly in a small group of dogs significantly improved survival compared with a group of historic controls with sarcomas and carcinomas treated with radiation therapy alone.62 The median survival time for 14 dogs in the combined radiation-cisplatin protocol was 580 days, compared to 325 days for the 13 dogs in the radiation-only group. In a follow-up study of 51 dogs treated with the same radiation-cisplatin regimen, the median survival time was 474 days.63
Two recent publications evaluated outcome in dogs with nasal tumors treated with IMRT.17,64 Thirty-one dogs treated with IMRT were compared to 36 historic controls treated with the same prescription using 2D treatment planning. Duration of survival was 420 days and 410 days, respectively.64 In another report, twelve dogs treated with IMRT to a dose commonly used for 2D or 3DCRT planning had a median survival of 446 days.17 Patient numbers in both studies were low, and there was no stratification for prognostic factors. Although conclusions cannot be made regarding tumor control, the decrease in acute effects to the region in both studies was profound. Although it seems intuitively obvious that improved tumor control would require dose escalation, reducing acute effects and keeping patients more comfortable during treatment may allow more patients to complete treatment as prescribed.
SRT, delivered in 3 daily fractions of 9 to 10 Gy, has been used to treat over 40 dogs with nasal tumors. Treatment was well tolerated with few acute effects, and early improvement in clinical signs was noted. Median survival time was 411 days.65 Lymphoproliferative nasal tumors in cats respond well and durably to radiation therapy (these are addressed later in the chapter in the section on lymphoma; also see Chapter 32).66 Carcinomas and sarcomas in cats respond comparably to nasal tumors in dogs: 48 Gy administered in 4 Gy fractions over 4 weeks had a 1-year survival rate of 44.3% and a 2-year survival rate of 16.6%.67 The histologic type and clinical stage of the tumor did not affect the prognosis.
Nine dogs with recurrent nasal tumors previously treated with 3DCRT (median 50 Gy), were reirradiated.68 Overall median survival from initial treatment was 31 months with an increased incidence of late effects. Retreatment becomes a more viable option with new modalities that spare local normal tissues.
The response to planum nasale SCC is affected by the tumor type and stage.69 Cats with T1 tumors had a 1-year survival rate of 85% and a 5-year survival rate of 56%, and the mean was 53 months (the median was not calculable). However, larger, more invasive tumors showed a less favorable response when treated with 40 Gy in 4 Gy fractions over 3.5 weeks. Tumor control should be improved by reducing the dose per fraction and increasing the total dose. For small, superficial SCC lesions of the planum nasale in cats, strontium (Sr)-90 plesiotherapy is a viable option. Because Sr-90 emits a low-energy β particle, very high doses can be administered to the surface of a lesion without unacceptable complications or damage to underlying tissue. In one study, 49 cats with SCC of the planum nasale were administered a median of 128 Gy in a single fraction, with a complete response rate of 88% and median progression-free interval of 1710 days. Larger, invasive SCC lesions are usually not amenable to treatment with Sr-90.70
Radiation Considerations
Canine and feline sinonasal tumors are challenging to treat with radiation because they are anatomically complex; they frequently involve the nasal sinuses, the cribriform plate, and the nasal pharynx. The geometry of the nasal cavity is problematic because the target is larger caudally than rostrally, making it difficult to achieve even dose distribution. The dose-sculpting benefits of IMRT and SRT decrease acute effects. Optimizing protocols, including modest dose escalation, may lead to improved tumor control. Evidence-based studies need to thoroughly evaluate the impact of IMRT and SRT on acute responding tissues, tumor control, and late effects.
Treatment-Related Toxicities
Because nasal tumors are immediately adjacent to the oral cavity, radiation toxicities of nasal treatment are similar to those described previously for the oral cavity. Radiation side effects in the nasal cavity may mimic signs of the tumor, such as nasal discharge, sneezing, and epistaxis. In most dogs, these clinical signs actually improve rather than worsen during treatment despite acute radiation effects because of the response of the tumor. Recurrence of clinical signs of an intranasal tumor (nasal discharge, sneezing, epistaxis) may indicate chronic radiation effects or tumor recurrence. Ocular radiation complications are extremely common because of the proximity of the nasal cavity and frontal sinus to the orbit. Effects to the eyes are dose related and vary in severity.71 Acute effects include blepharitis, blepharospasm, conjunctivitis, and the development of keratoconjunctivitis sicca (KCS). KCS is treated with artificial tears and steroids to prevent corneal ulceration. If corneal ulceration is present, healing may be delayed as a result of radiation damage to the corneal stem cells. KCS may be temporary or permanent, depending on the dose administered and the sensitivity of the patient. Late effects include vascular changes, which may have subtle effects on vision but in most cases do not result in blindness. Radiation-induced cataracts may occur, and the latent period is related to dose. These cataracts can be removed with phacoemulsification. Eyes that are in the field of irradiation may receive the full treatment dose. At doses above 40 Gy, degenerative angiopathy of retinal vessels can progress over 2 years and result in retinal degeneration. Optic nerve axonal degeneration has occurred secondary to the retinal changes.72 IMRT decreases dose to the ocular region, resulting in limited acute and late toxicities.64
Brain Tumors and Pituitary Tumors
Brain tumors can be treated successfully with radiation therapy (see Chapter 30). In the treatment of brain tumors, surgery may be indicated to relieve life-threatening clinical signs. Although appropriate studies are still needed, for animals with less severe clinical signs, reported survival times after radiation for brain tumors in dogs are frequently comparable to surgery alone. Adjuvant radiation is indicated in patients with incomplete surgical resection; combined surgery and radiation may provide the best long-term control. Radiation therapy alone should be performed in dogs with cancer at surgically inaccessible sites or in locations in which surgical morbidity is high. SRT is being evaluated for treatment of a wide variety of brain tumors in dogs and cats.
Efficacy of Treatment
Published survival times of canine brain tumors treated with radiotherapy compare favorably to surgery, although directly comparable data is lacking, and combined surgical and radiation treatment may be superior. In one study, 46 dogs with brain tumors that initially had shown neurologic signs were treated with radiation alone.69 The median overall survival time was 23.3 months; 69% of the dogs survived 1 year, and 47% survived 2 years. The outcome in this study was superior to those from previous reports, in which the median survival time was about 1 year. No prognostic clinical factors (e.g., tumor size or location or clinical signs) were identified.73-75 Differences may be due to improved treatment planning capabilities. In another study by Axlund et al of 31 dogs with meningioma, postoperative radiation improved the median survival time from 7 months with surgery alone to 16.5 months with surgery followed by radiation therapy. Stereotactic radiation therapy has been used to treat brain tumors.23 In a recent preliminary summary of 20 dogs with presumed meningiomas treated with 3 fractions of SRT, median survival was 594 days, and treatment was well tolerated (personal communication, Lynn Griffin, CSU).
Radiation therapy should be used in dogs and cats with pituitary macroadenomas because pituitary tumors generally are responsive to radiation, and surgical access is limited. Dogs with pituitary tumors have been reported to have median survival times varying between 1 and 2 years.76,77 A study comparing 19 dogs with pituitary tumors receiving radiation therapy (48 Gy in 16 daily fractions of 3 Gy) to untreated dogs found 1-, 2-, and 3-year survival rates of 93%, 87%, and 55% in the irradiated group and 45%, 32%, and 25% in the unirradiated group. Tumor size as assessed by relative tumor area to brain area was prognostic in the irradiated patients. Fractionated pituitary irradiation in dogs is more effective at delaying tumor growth than in controlling adrenocorticotropic hormone (ACTH) secretion. Eucortisolism is seen in some patients after irradiation; however, pre-ACTH and post-ACTH cortisol levels should be monitored at regular intervals so that medications can be modified, if indicated.
Cats seem to have marked clinical improvement of associated endocrinopathies. In one study, eight cats with pituitary tumors were treated with radiation therapy, and neurologic signs improved within 2 months in all cats.78 Endocrinopathies, including hyperadrenocorticism, acromegaly, and insulin-resistant diabetes, were dramatically improved. The median survival time, regardless of cause of death, was 17.4 months. A study evaluating conformal radiation–focused treatment of 11 cats (single dose of 15 to 20 Gy) found improved regulation of diabetes mellitus in 5/9 cats and improved neurologic function in 2/2 cats, with a median survival time of 25 months. SRT was administered in 3 to 4 fractions to 19 acromegalic cats with macroscopic or microscopic pituitary tumors. Median survival has not been reached, with 90% of cats alive at 1 and 2 years. Insulin-resistant diabetes and other associated endocrinopathies improved. The treatment was well tolerated acutely26 (see Chapter 25).
Radiation Considerations
The integration of 3D imaging, patient-positioning devices, and advanced treatment planning techniques have the potential to improve tumor targeting and sparing of normal brain tissue. For many brain tumors, IMRT will not provide an improved dose distribution compared to 3DCRT; however, dose sculpting may be beneficial for cranial nerve tumors. An additional consideration in patients undergoing radiation therapy for a brain tumor is anesthetic risk related to increased intracranial pressure or brainstem disease; an appropriate anesthetic regimen should be selected to minimize the risk of complications. Specifically, patients should be ventilated while under anesthesia to decrease partial pressure of carbon dioxide (pco2) in the blood, and anesthetic agents that decrease (or at least do not increase) intracranial pressure should be selected. SRT limits the number of anesthetic episodes, which may be beneficial in unstable patients. SRT can be used with curative intent or in a single fraction for palliative purposes.
Treatment-Related Toxicities
For most brain tumors, acute effects to the skin can be avoided with megavoltage treatments. Occasionally, ocular and auricular side effects or mucositis to the caudal oral cavity may be seen if in or adjacent to the treatment field. The radiation tolerance is lower when the entire brain is treated; this limits prescription of a dose that is adequate for tumor control but still has an acceptable probability of late effects. The radiation tolerance of brain and spinal tissues is generally considered to be less than that of other commonly treated tissues, and volume may be an important factor for brain and spinal lesions. Early delayed effects can occur 1 to 3 months after treatment and may be due to transient demyelination. Animals with early delayed effects may have signs similar to those of the initial presentation, or they may be generally stuporous. Early delayed effects occur in up to 40% of humans undergoing brain irradiation; symptoms include headache, lethargy, and exacerbation of focal neurologic signs.79 In animals, clinical signs often are transient, but response time can be slow. Sometimes administration of systemic cortisone and aggressive supportive care are required. CT and MRI may show an apparent increase in tumor size and tumor enhancement during this time. Focal enhancement in normal brain associated with edema and demyelination may also be present.80
Late effects probably occur in veterinary patients more often than identified and should be considered as a differential diagnosis for signs that occur during that interval. Late delayed effects (more commonly referred to simply as late effects) generally occur at least 6 months after treatment but can also occur years later. Late effects are associated with brain necrosis. The probability of late brain effects depends on the total dose, the fraction size, and the volume of brain irradiated. In a study of 83 dogs with brain masses treated with a hypofractionated protocol (38 Gy administered in 5 weekly fractions), brain necrosis was confirmed or suspected in 14% of dogs (32.7 months).81 The signs are similar to those associated with early delayed effects, although the response to steroids is limited. Clinically, distinguishing between late effects and tumor recurrence can often be difficult. CT or MRI evaluation can be misleading. Not all brain tumors completely recede after treatment; therefore the presence of a mass does not always indicate a recurring tumor. A prudent course is to obtain a CT or MRI evaluation 6 months after treatment to serve as a reference if clinical signs develop in the future.
Superficial Tumors of the Trunk and Extremities
Many tumors involving the trunk or extremities are amenable to treatment by radiation therapy. Combining radiation therapy with surgery enhances tumor control and improves the functional outcome better than surgery or radiation alone. For tumors in nonresectable locations, radiation alone may provide a good outcome depending on tumor type and volume. The surgeon and radiation oncologist should consult before therapeutic intervention is started to develop an overall treatment approach.
Hemangiopericytomas, fibrosarcomas, neurofibrosarcomas, myxosarcomas, and nerve sheath tumors are classified together as soft tissue sarcomas because of their similar biologic behavior (see Chapter 21). Metastases are uncommon with grade 1 and grade 2 sarcomas; therefore local tumor control is the primary concern. Soft tissue sarcomas are locally invasive, and tumor cells may extend far beyond the bulk of the tumor. Surgery alone is curative if the tumor can be removed completely.82 If surgical resection is attempted, the margins should be closely examined by a pathologist for evidence of tumor infiltration. If tumor cells extend out to the margin, radiation therapy should be recommended if further resection is not possible. If it is apparent that a tumor cannot be excised completely, treatment combining radiation and surgery can be beneficial. Radiation therapy can be administered first with the hope of converting an inoperable tumor into an operable one. This approach has the benefit of reducing the volume of normal tissues irradiated. As an alternative, surgical excision can be performed first as a cytoreductive procedure and then followed by radiation therapy to kill the residual subclinical disease. In one study, 48 dogs with soft tissue sarcomas were treated with surgical cytoreduction followed by radiation therapy; only 8 dogs (16%) developed tumor recurrence, and the 5-year survival rate was 78%.83 In a different study, which involved 38 dogs with soft tissue sarcomas of the body and extremities, treatment with surgery followed by irradiation provided a median survival time of 2270 days.45 In comparison, soft tissue sarcomas treated by narrow surgical excision alone have recurrence rates of 17% to 40%.84-89
Soft tissue sarcomas can be treated with radiation therapy alone; however, tumor control is not as durable as with a combination of radiation therapy and surgery.43 Radiation therapy alone is useful for tumors near the pads, where surgical options are limited. Pads in the irradiation field initially may slough; however, if appropriate fractionation schemes are used, the pads regrow and can function normally. Local hyperthermia has been used effectively with radiation therapy to improve local tumor control in canine soft tissue sarcomas.90 Currently, no facilities are able to administer hyperthermia. Adjuvant chemotherapy has been considered for grade 3 soft tissue sarcomas because of the higher metastatic potential of these tumors; however, definitive evidence of improvement in local tumor control or survival is lacking.
Cutaneous mast cell tumors (grades 1 and 2) can be treated successfully with radiation therapy (see Chapter 20). The obvious advantage is that greater margins can be obtained with radiation than with surgery. The probability of control may be improved if surgical cytoreduction is performed first. In a study involving 37 dogs with grade 2 tumors treated with cytoreduction and radiation therapy, tumor control at 1 and 2 years exceeded 90%.91 In 56 dogs with incompletely resected mast cell tumors, the medium disease-free interval was 32.7 months.92 Radiotherapy also is indicated for cutaneous mast cell tumors with regional lymph node metastasis. In one study, 19 dogs with mast cell tumors and regional node involvement were treated with surgical cytoreduction of the primary site, radiation to the primary tumor and regional node, and prednisone.93 The median disease-free survival time was 1240 days. Palliative radiation therapy is commonly used to treat locoregional mast cell tumors in dogs when systemic spread has occurred.
Vaccine-associated soft tissue sarcomas are a significant problem in cats (see Chapter 21). These tumors are challenging to control locally and seem unresponsive to aggressive radiation therapy or conservative surgery alone. In one study, 33 cats with histologically confirmed fibrosarcomas were treated with radiation therapy followed by surgery.94 The median disease-free interval and the overall survival time were 398 and 600 days, respectively. In another study, 25 cats with subclinical disease after surgery were treated with radiation therapy alone (57 Gy delivered in 3 Gy fractions) and in some cases with adjuvant chemotherapy.95 The overall median survival time was 701 days. Recurrence was seen in 7 cases (5 of 18 [27.8%] in the group treated with doxorubicin and 2 of 7 [28.6%] in the group not treated with doxorubicin). In the recurrence cases, one tumor developed outside the treatment field and the others arose in the area treated with radiation. Metastases to the lung or other sites were not seen in this group of cats. Similar findings were evident in 78 cats treated with surgical cytoreduction followed by radiation.96 In this study, cats that underwent only one surgery before radiation had a lower recurrence rate than cats that had more than one surgery. The survival time and the disease-free interval shortened as the time between surgery and the start of radiation therapy lengthened. In a study of 79 cats treated with either preoperative or postoperative radiation therapy, packed cell volume (PCV) higher than 25 was associated with better outcome (median survival 760 days) than cats with PCV lower than 25 (306 days).97
Cats with surgically nonresectable disease present a greater challenge. Escalation of the radiation dose by delivering the dose in smaller fractions probably is necessary for these patients. IMRT or SRT may be beneficial for obtaining adequate dose to the tumor because appropriate sparing of the lungs, viscera, and spinal cord is critical in these patients.
Radiation Considerations
Many tumors of the limbs and trunk extend very close to the skin surface. Application of an appropriate thickness of a bolus material over the skin is frequently needed when treating with megavoltage photons in order to avoid underdosing the superficial region. When treating a sloped surface such as around a limb, side-scatter equilibrium is lost, resulting in heterogeneous dose distribution that can lead to significant underdosing of tumor and/or overdosing of the skin. The use of tapered bolus can reduce such dose inhomogeneity. Whenever possible, when an extremity is in a radiation field, a 1 to 2 cm strip of tissue should be shielded to avoid the risk of lymphedema, which can present as painful swelling of the distal limb.
Treatment-Related Toxicities
When treating superficial tumors, early effects to the skin are expected and are restricted to the radiation field. The severity of effect is dose related, and the patient may have a variety of lesions. Epilation is common and in some cases may be permanent. The hair may not return for several months, and the amount of regrowth varies in relation to the dose administered to the skin and the individual patient’s sensitivity. Damage to the melanocytes may result in hypopigmentation or hyperpigmentation of the skin and/or alteration of the coat color when regrowth occurs, often resulting in whitish-gray fur (leukotrichia). Dry desquamation may accompany epilation; this generally does not cause any problem or discomfort for the patient and usually is not treated. Moist desquamation, which usually appears 3 to 5 weeks after the start of therapy, is associated with pruritus, which can vary in severity. Self-inflicted mutilation exacerbates the problem and may lead to ulceration or necrosis. Severe late effects to the skin are rare in fractionated therapy but include fibrosis and necrosis.
Bone Tumors
Although osteosarcomas are not considered highly radiation-responsive tumors, radiation may be considered as part of a multimodality therapy when surgical excision is not an option. (Radiation therapy for osteosarcoma is discussed at length in Chapter 24.) Radiation therapy can be combined with chemotherapy and surgery for limb-sparing protocols.98,99 In a retrospective study of multimodality therapy for axial skeletal osteosarcoma, dogs that underwent curative-intent radiation protocols had a longer duration of tumor control (265 days) than those treated with a palliative regimen (79 days).100 SRT is currently being evaluated as a limb-sparing alternative. In one study, stereotactic radiosurgery was performed on 11 dogs with appendicular osteosarcomas,24 and although radiation dose varied and not all dogs received adjuvant chemotherapy, good limb function and tumor control were observed in some of the patients. Fifty dogs with appendicular osteosarcoma underwent treatment with SRT followed by chemotherapy. Sites included distal radius and proximal and distal humerus, femur, and tibia. All dogs had improved limb function with no clinical recurrences.25 Fracture was observed in dogs with severely lytic lesions at or before 90 days.101 Criteria were established for patient selection, including evaluation of radiographs and CT scans, reducing fracture rate to less than 20% (personal communication, James Custis). Prophylactic stabilization is indicated in dogs with severely lytic lesions. The mechanism of the amelioration of pain caused by bony neoplasia is not completely understood. Relief of pain may occur almost immediately or may be delayed, sometimes as long as 2 weeks. Human studies have indicated that single, coarsely fractionated radiation may be comparable or superior to multifraction protocols using more conventional doses per fraction.102 In a recent study of 58 dogs, 8 Gy was administered on 2 consecutive days, providing onset of pain relief within 2 days in 91% of patients.103 Commonly in veterinary medicine, 7 to 10 Gy fractions have been administered on days 0, 7, and 21.104,105 In one study, 12 of 15 dogs with appendicular bone tumors treated with palliative radiation therapy had improved limb function, and the median survival time was 130 days.105 In another study, dogs with appendicular osteosarcoma were given either 3 fractions of 10 Gy or 2 fractions of 8 Gy.106 Seventy of the 95 dogs experienced pain relief, with a median duration of 73 days. No difference in response was found between the two treatment groups. Sometimes, localized pain recurs before metastatic disease becomes life limiting. Palliative radiation can be readministered as long as the owners understand that continued administration of large doses per fraction eventually leads to late effects.
Radiation Considerations
Radiation is unlikely to have a substantial palliative effect if a pathologic fracture from a bone tumor is already present. Additional padding and support during anesthetic recovery is important to prevent a recovering patient from trying to stand prematurely and injuring the affected limb.
Treatment-Related Toxicities
The most common and concerning bone tumor treatment–related toxicity is pathologic fracture. Most likely, the bone tumor itself rather than any effects of radiation makes the bone susceptible to fracture; however, the pain-relieving effects of radiation and/or other treatments may make animals more likely to put significant weight on the affected limb, resulting in fracture.
Other Tumors
Radiation therapy is used for a variety of tumors in the thoracic and abdominal cavities (Table 12-1). The principles of patient selection for radiation therapy with tumors in these regions are the same as for any other region. Radiation therapy should be considered for any tumor that cannot be excised completely. In one study, dogs with thyroid carcinomas treated with 48 Gy delivered in 4 Gy fractions had PFS rates of 80% at 1 year and 72% at 3 years.119 Thymomas are radiation responsive in human patients.120 In a study of seven cats with thymoma that were treated with radiation therapy, the median survival time was close to 2 years (see Chapter 33, Section B).
Table 12-1
Tumors Commonly Treated with Radiation Therapy
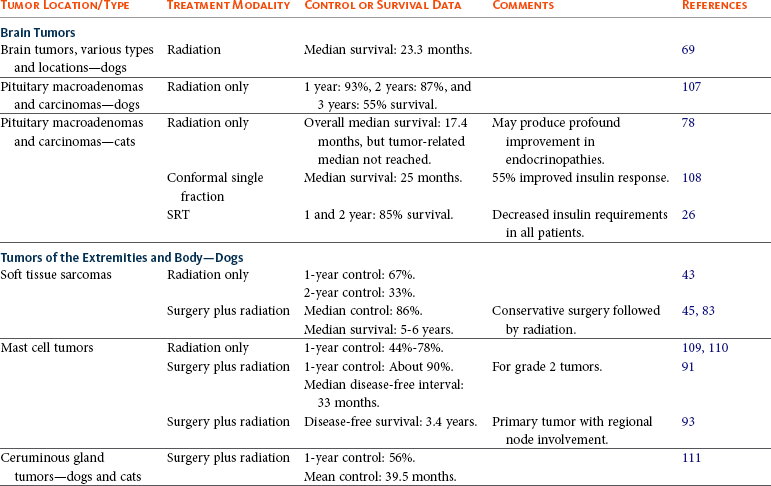
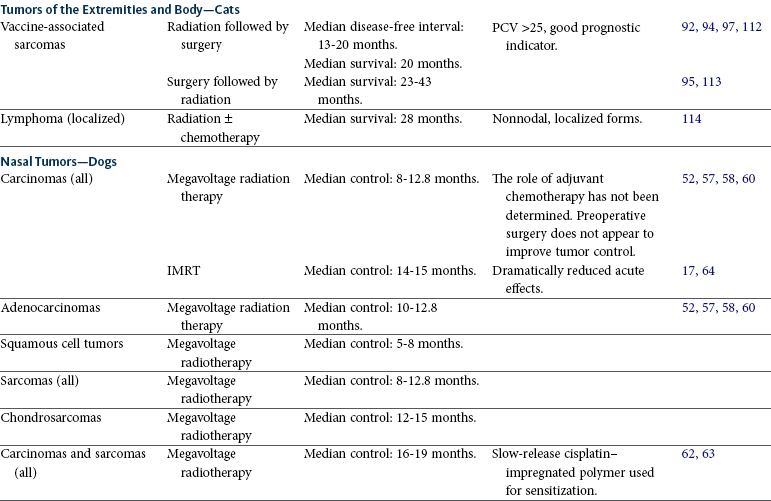
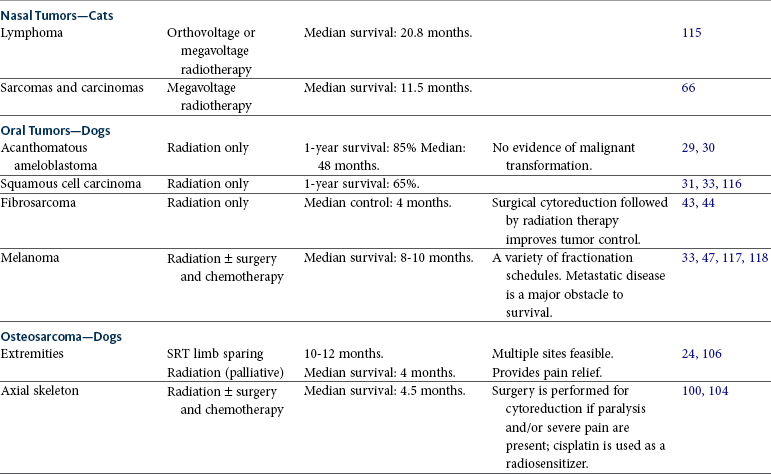
SRT, Stereotactic radiation therapy; IMRT, intensity-modulated radiation therapy; PCV, packed cell volume.
Palliative radiation therapy can be useful for tumors that may be causing airway, bowel, or urinary tract obstruction or neurologic dysfunction. Mediastinal lymphoma often responds rapidly to irradiation. Relief from respiratory distress can be achieved within hours of a single dose of radiation.
Eighteen dogs with primary disease of the urinary bladder (7), urethra (1), or prostate (10) were treated with IMRT assisted by image guidance to verify tumor position (personal communication, Michael Nolan). The majority of patients were treated with adjuvant chemotherapy and nonsteroidal antiinflammatory drugs (NSAIDs). In all dogs, the radiation dose ranged from 54 to 58 Gy, delivered in 20 daily fractions. Acute and late tissue toxicity was limited, and treatment was well tolerated. Overall median survival time was 654 days. Location of primary tumor had no demonstrable effect on either local tumor control or survival.
Perianal adenocarcinomas and anal sac adenocarcinomas (see Chapter 22, Section H) can be difficult to control locally with surgery alone and are likely to spread to regional lymph nodes. One hundred and thirteen dogs with carcinomas of the apocrine gland were treated with surgery, radiation therapy, chemotherapy, or multimodal treatment. Overall median survival was 544 days. Dogs treated with chemotherapy alone had significantly shorter survival than those receiving other treatments, whereas the addition of surgery was beneficial.121 Late effects from radiation therapy to the pelvic region can occur and be clinically significant.122 This can be addressed by administering the radiation in smaller doses per fraction. Perianal gland tumors are generally slow to disseminate systemically, so full-course treatment of involved regional nodes may be warranted. Intraoperative therapy as a boost to external-beam radiation therapy may also be useful in the treatment of the regional nodes or associated tumor bed following excision.
Mucositis also can occur whenever any portion of the alimentary system receives radiation therapy. Colitis is a common acute effect during radiation therapy for bladder or colorectal tumors. Severe large bowel diarrhea may be seen. Anusitis from irradiation is worsened by the diarrhea, making the patient quite uncomfortable. High-bulk diets are recommended, along with good hygiene in the region. Steroid enemas seem beneficial in some patients with colitis.123
Lymphoma
Radiation therapy can be important in the treatment of localized lymphoma, and it has an emerging role as an adjuvant therapy in the treatment of systemic lymphoma. Lymphocytes are exquisitely radiation sensitive124 and may undergo apoptosis in addition to classic mitotic death after exposure to radiation.125 Human patients with non-Hodgkin’s lymphoma commonly receive combined modality treatment with radiation therapy and chemotherapy, primarily for stage I or stage II disease,126 although this treatment may have a role in more advanced stages as well.127 Combined modality treatment or radiotherapy alone also is beneficial in humans for primary lymphomas of bone, cutaneous B-cell lymphoma, and mycosis fungoides.128-131
In one study, a series of feline extranodal lymphomas (nasal cavity, retrobulbar area, mediastinum, subcutaneous tissue, maxilla, and mandible) were treated with megavoltage radiotherapy with or without concurrent chemotherapy.114 Eight of 10 cats attained a complete local remission; one cat with retrobulbar involvement and one with mandibular involvement attained a partial remission. The median remission time for the cats that attained complete remission was 114 weeks, and three cats were alive and disease free at 131 weeks. Three cats developed disease outside the radiation field, which suggests that adjuvant chemotherapy or extending the field for part of the treatment to include additional nodes may improve locoregional control.
Radiation has also been used to treat cutaneous lymphoma in dogs. Radiotherapy of localized lymphoma has been reported to result in prolonged remission times.132 Humans with cutaneous B-cell lymphoma commonly undergo radiation therapy, which generally results in long-term control of disease. The extent of skin involvement and the presence of extracutaneous disease are prognostic.131
Mycosis fungoides is sometimes treated with total skin electron irradiation in humans. As with cutaneous B-cell lymphoma, the stage of disease is prognostic, but human patients with disease confined to the skin may have prolonged remission times.
Interest has developed in the use of half-body radiotherapy to treat canine lymphoma. A dosage of 8 Gy to lymphoma cells reduces the surviving fraction to 0.005, which is much greater than the estimated cell kill from one cycle of chemotherapy.124 The rationale for adding radiation to a chemotherapy protocol is that the projected improvement in cell kill would improve the duration of remission and perhaps result in a cure. However, because of the impact of radiation on normal bone marrow cells, the radiation can be administered only to one-half of the body at a time. (8 Gy delivered to the entire body is lethal.) In one study, induction chemotherapy (11 weeks) was administered to 94 dogs, 52 of which received subsequent half-body irradiation.133 Half-body irradiation was administered in 2 daily fractions of 4 Gy to the cranial half of the body; 1 month later, the same protocol was used for the caudal half of the body. No additional chemotherapy was administered because the main purpose of the study was to see whether the half-body irradiation could serve as a substitute for long-term chemotherapy. The median survival of 311 days was comparable to but no better than many chemotherapy-only protocols.
In another study, half-body irradiation was interposed within a 25-week protocol based on cyclophosphamide, doxorubicin, Oncovin, and prednisone (CHOP). The radiation was well tolerated, and the median remission and survival times were 455 and 560 days, respectively. Although these results are very encouraging, the study involved only eight dogs, and the role of radiation in the treatment of systemic lymphoma requires further evaluation. Nevertheless, the addition of irradiation represents a new approach to a disease for which the duration of remission with chemotherapy alone has not improved over the past 10 years. Interestingly, the two half-body studies described previously differed from earlier reports in which each half-body treatment was delivered in a single 7 to 8 Gy fraction (personal communication, A. Abrams-Ogg). Dividing the dose into 4 Gy fractions delivered on consecutive days reduced the number and severity of acute effects. Diarrhea, the most common effect, was seen in about two-thirds of the dogs after the caudal half of the body was treated.134
An emerging approach in the management of dogs with lymphoma is total body irradiation (TBI) with autologous transplantation of hematopoietic peripheral blood progenitor cells. In one protocol, following treatment with a standard chemotherapy regimen, dogs are given 500 to 650 mg/m2 of cyclophosphamide intravenously. They are then treated with recombinant human granulocyte colony-stimulating factor (rhG-CSF) and undergo apheresis to separate CD34+ progenitor cells followed by two 5 Gy fractions of TBI, 3 hours apart. Immediately after TBI, the cell harvest from apheresis is infused intravenously. In a toxicity study of 10 dogs, all dogs experienced grade 4 neutropenia, lymphopenia, and thrombocytopenia. Neutrophils recovered to at least 500 neutrophils/µL by day 12, but thrombocytopenia often persisted for weeks. Collection of long-term outcome data is still ongoing, as are attempts to escalate TBI dose.135
References
1. Pommer, A. X-ray therapy in veterinary medicine. In: Brandly CA, Jungher EL, eds. Advances in veterinary science. New York: Academic Press, 1958.
2. DeVita, VTJr. Progress in cancer management. Keynote address. Cancer. 1983;51:2401–2409.
3. Farrelly, J, McEntee, MC, A survey of veterinary radiation facilities in the United States during 2010. In 2011 Annual Meeting of the American College of Veterinary Radiology 2011. [Albuquerque, New Mexico].
4. Ward, JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125.
5. Withers, HR. The four R’s of radiotherapy. In: Lett J, Adler H, eds. Advances in radiation biology. New York: Academic Press, 1975.
6. Schmidt-Ullrich, RK, Contessa, JN, Dent, P, et al. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat Oncol Investig. 1999;7:321–330.
7. Benedict, SH, Yenice, KM, Followill, D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101.
8. Carsten, RE, Hellyer, PW, Bachand, AM, et al. Correlations between acute radiation scores and pain scores in canine radiation patients with cancer of the forelimb. Vet Anaesth Analg. 2008;35:355–362.
9. Stewart, FA, Dorr, W. Milestones in normal tissue radiation biology over the past 50 years: from clonogenic cell survival to cytokine networks and back to stem cell recovery. Int J Radiat Biol. 2009;85:574–586.
10. Halperin, EC, Perez, CA, Brady, LW. The discipline of radiation oncology. In Halperin EC, Perez CA, Brady LW, eds.: Perez and Brady’s principles and practice of radiation oncology, ed 5, Philadelphia, PA: Lippincott Willams & Wilkins, 2008.
11. Hall, EJ, Wuu, CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88.
12. Wilson, PF, Bedford, JS. Radiobiological principles. In Hoppe RT, Phillips TL, Roach M, III., eds.: Leibel and Phillips textbook of radiation oncology, ed 3, St. Louis: Elsevier, 2010.
13. Fowler, JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694.
14. van den Aardweg, GJMJ, Kilic, E, de Klein, N, et al. Dose fractionation effects in primary and metastatic human uveal melanoma cell lines. Invest Ophthalmol Vis Sci. 2003;44:4660–4664.
15. Fitzpatrick, CL, Farese, JP, Milner, RJ, et al. Intrinsic radiosensitivity and repair of sublethal radiation-induced damage in canine osteosarcoma cell lines. Am J Vet Res. 2008;69:1197–1202.
16. Parfitt, SL, Milner, RJ, Salute, ME, et al. Radiosensitivity and capacity for radiation-induced sublethal damage repair of canine transitional cell carcinoma (TCC) cell lines. Vet Comp Oncol. 2011;9:232–240.
17. Hunley, DW, Mauldin, GN, Shiomitsu, K, et al. Clinical outcome in dogs with nasal tumors treated with intensity-modulated radiation therapy. Can Vet J. 2010;51:293–300.
18. Yang, JN, Mackie, TR, Reckwerdt, P, et al. An investigation of tomotherapy beam delivery. Med Phys. 1997;24:425–436.
19. Forrest, LJ, Mackie, TR, Ruchala, K, et al. The utility of megavoltage computed tomography images from a helical tomotherapy system for setup verification purposes. Int J Radiat Oncol Biol Phys. 2004;60:1639–1644.
20. Leksell, L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316–319.
21. Adler, JR, Jr., Chang, SD, Murphy, MJ, et al. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124–128.
22. Nieset, JR, Harmon, JF, LaRue, SM. Characterization of canine bladder variations to optimize fractionated radiation therapy protocols using cone-beam computed tomography. Vet Radiol Ultrasound. 2009;51(2):235.
23. Lester, NV, Hopkins, AL, Bova, FJ, et al. Radiosurgery using a stereotactic headframe system for irradiation of brain tumors in dogs. J Am Vet Med Assoc. 2001;219:1562–1567.
24. Farese, JP, Milner, R, Thompson, MS, et al. Stereotactic radiosurgery for treatment of osteosarcomas involving the distal portions of the limbs in dogs. J Am Vet Med Assoc. 2004;225:1567–1572.
25. Ryan, SD, Ehrhart, NE, Worley, D, et al. Stereotactic radiation therapy for appendicular bone tumors. Vet Radiol Ultrasound. 2010;51(2):234.
26. Lunn, KF, LaRue, SM. Endocrine function in cats after stereotactic radiosurgery treatment for acromegally. J Vet Intern Med. 2009;23:698.
27. Seguin, B, McDonald, DE, Kent, MS, et al. Tolerance of cutaneous or mucosal flaps placed into a radiation therapy field in dogs. Vet Surg. 2005;34:214–222.
28. Thrall, DE. Orthovoltage radiotherapy of acanthomatous epulides in 39 dogs. J Am Vet Med Assoc. 1984;184:826–829. [7].
29. Theon, AP, Rodriguez, C, Griffey, S, et al. Analysis of prognostic factors and patterns of failure in dogs with periodontal tumors treated with megavoltage irradiation. J Am Vet Med Assoc. 1997;210:785–788.
30. McEntee, MC, Page, RL, Theon, A, et al. Malignant tumor formation in dogs previously irradiated for acanthomatous epulis. Vet Radiol Ultrasound. 2004;45:357–361.
31. Evans, SM, Shofer, F. Canine oral nontonsillar squamous cell carcinomas: Prognostic factors for recurrence and survival following orthovoltage radiation therapy. Vet Radiol. 1988;29:133–137.
32. Gillette, EL, McChesney, SL, Dewhirst, MW, et al. Response of canine oral carcinomas to heat and radiation. Int J Radiat Oncol Biol Phys. 1987;13:1861–1867.
33. Theon, AP, Rodriquez, C, Madewell, BR. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. J Am Vet Med Assoc. 1997;210:778–784.
34. LaDueMiller, T, Price, GS, Page, RL, et al. Radiotherapy of canine non-tonsillar squamous cell carcinoma. Vet Radiol Ultrasound. 1996;37:74–77.
35. Postorino-Reeves, NC, Turrel, JM, Withrow, SJ. Oral squamous cell carcinoma in the cat. J Am Anim Hosp Assoc. 1993;29:1–4.
36. Evans, SM, LaCreta, F, Helfand, S, et al. Technique, pharmacokinetics, toxicity, and efficacy of intratumoral etanidazole and radiotherapy for treatment of spontaneous feline oral squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 1991;20:703–708.
37. Ogilvie, GK, Moore, AS, Obradovich, JE. Toxicoses and efficacy associated with administration of mitoxantrone to cats with malignant tumors. J Am Vet Med Assoc. 1993;202:1839–1844.
38. Hutson, CA, Willauer, CC, Walder, EJ, et al. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987–1989). J Am Vet Med Assoc. 1992;201:777–781.
39. Bregazzi, VS, LaRue, SM, Powers, BE, et al. Response of feline oral squamous cell carcinoma to palliative radiation therapy. Vet Radiol Ultrasound. 2001;42:77–79.
40. Jones, PD, de Lorimier, LP, Kitchell, BE, et al. Gemcitabine as a radiosensitizer for nonresectable feline oral squamous cell carcinoma. J Am Anim Hosp Assoc. 2003;39:463–467.
41. Fidel, JL, Sellon, RK, Houston, RK, et al. A nine-day accelerated radiation protocol for feline squamous cell carcinoma. Vet Radiol Ultrasound. 2007;48:482–485.
42. Ciekot, PA, Powers, BE, Withrow, SJ, et al. Histologically low-grade, yet biologically high-grade, fibrosarcomas of the mandible and maxilla in dogs: 25 cases (1982–1991). J Am Vet Med Assoc. 1994;204:610–615. [4].
43. McChesney, SL, Withrow, SJ, Gillette, EL, et al. Radiotherapy of soft tissue sarcomas in dogs. J Am Vet Med Assoc. 1989;194:60–63. [1].
44. Gillette, SM, Dewhirst, MW, Gillette, EL, et al. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia: a randomized phase II study. Int J Hyperthermia. 1992;8:309–320.
45. Forrest, LJ, Chun, R, Adams, WM, et al. Postoperative radiotherapy for canine soft tissue sarcoma. J Vet Intern Med. 2000;14:578–582.
46. Todoroff, RJ, Brodey, RS. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J Am Vet Med Assoc. 1979;175:567–571.
47. Proulx, DR, Ruslander, DM, Dodge, RK, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound. 2003;44:352–359.
48. Freeman, KP, Hahn, KA, Harris, FD, et al. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987–1997). J Vet Intern Med. 2003;17:96–101.
49. Farrelly, J, Denman, DL, Hohenhaus, AE, et al. Hypofractionated radiation therapy of oral melanoma in five cats. Vet Radiol Ultrasound. 2004;45:91–93.
50. Bentzen, SM, Overgaard, J, Thames, HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989;16:169–182.
51. Hahn, KA, Knapp, DW, Richardson, RC, et al. Clinical response of nasal adenocarcinoma to cisplatin chemotherapy in 11 dogs. J Am Vet Med Assoc. 1992;200:355–357.
52. Henry, CJ, Brewer, WG, Jr., Tyler, JW, et al. Survival in dogs with nasal adenocarcinoma: 64 cases (1981–1995). J Vet Intern Med. 1998;12:436–439.
53. Holmberg, DL, Fries, C, Cockshutt, J, et al. Ventral rhinotomy in the dog and cat. Vet Surg. 1989;18:446–449.
54. Langova, V, Mutsaers, AJ, Phillips, B, et al. Treatment of eight dogs with nasal tumours with alternating doses of doxorubicin and carboplatin in conjunction with oral piroxicam. Aust Vet J. 2004;82:676–680.
55. MacEwen, G, Withrow, SJ, Patnaik, AK. Nasal tumors in the dog: retrospective evaluation of diagnosis, prognosis and treatment. J Am Vet Med Assoc. 1977;170:45–48.
56. MacMillan, R, Withrow, SJ, Gillette, EL. Surgery and regional irradiation for treatment of canine tonsillar squamous cell carcinoma: retrospective review of eight cases. J Am Anim Hosp Assoc. 1982;18:311–314.
57. McEntee, MC, Page, RL, Heidner, GL, et al. A retrospective study of 27 dogs with intranasal neoplasms treated with cobalt radiation. Vet Radiol. 1991;32:135–139. [3].
58. Nadeau, M, Kitchell, BE, Rooks, RL, et al. Cobalt radiation with or without low-dose cisplatin for treatment of canine naso-sinus carcinomas. Vet Radiol Ultrasound. 2004;45:362–367.
59. Theon, AP, Madewell, BR, Harb, MF, et al. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J Am Vet Med Assoc. 1993;202:1469–1475.
60. Adams, WM, Withrow, SJ, Walshaw, R, et al. Radiotherapy of malignant nasal tumors in 67 dogs. J Am Vet Med Assoc. 1987;191:311–315. [3].
61. Adams, WM, Bjorling, DE, McAnulty, JE, et al. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1990–2002). J Am Vet Med Assoc. 2005;227:936–941.
62. Lana, SE, Dernell, WS, LaRue, SM, et al. Slow release cisplatin combined with radiation for the treatment of canine nasal tumors. Vet Radiol Ultrasound. 1997;38:474–478.
63. Lana, SE, Dernell, WS, Lafferty, MH, et al. Use of radiation and a slow-release cisplatin formulation for treatment of canine nasal tumors. Vet Radiol Ultrasound. 2004;45:577–581.
64. Lawrence, JA, Forrest, LJ, Turek, MM, et al. Proof of principle of ocular sparing in dogs with sinonasal tumors treated with intensity-modulated radiation therapy. Vet Radiol Ultrasound. 2010;51:561–570.
65. Custis, JT, Harmon, JF, Ryan, SD, et al, Canine nasal tumors: A stereotactic radiation therapy approach. Presented at the ACVIM, Denver, June 16 2011.
66. Straw, RC, Withrow, SJ, Gillette, EL, et al. Use of radiotherapy for the treatment of intranasal tumors in cats: six cases (1980–1985). J Am Vet Med Assoc. 1986;189:927–929. [8].
67. Theon, AP, Peaston, AE, Madewell, BR, et al. Irradiation of nonlymphoproliferative neoplasms of the nasal cavity and paranasal sinuses in 16 cats. J Am Vet Med Assoc. 1994;204:78–83.
68. Bommarito, DA, Kent, MS, Selting, KA, et al. Reirradiation of recurrent canine nasal tumors. Vet Radiol Ultrasound. 2011;52:207–212.
69. Bley, CR, Sumova, A, Roos, M, et al. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med. 2005;6:849–854.
70. Hammond, GM, Gordon, IK, Theon, AP, et al. Evaluation of strontium Sr 90 for the treatment of superficial squamous cell carcinoma of the nasal planum in cats: 49 cases (1990–2006). J Am Vet Med Assoc. 2007;231:736–741.
71. Roberts, SM, Lavach, JD, Severin, GA, et al. Ophthalmic complications following megavoltage irradiation of the nasal and paranasal cavities in dogs. J Am Vet Med Assoc. 1987;190:43–47. [1].
72. Ching, SV, Gillette, SM, Powers, BE, et al. Radiation-induced ocular injury in the dog: a histological study. Int J Radiat Oncol Biol Phys. 1990;19:321–328.
73. Evans, SM, Dayrell-Hart, B, Powlis, W, et al. Radiation therapy of canine brain masses. J Vet Intern Med. 1993;7:216–219.
74. Theon, AP, LeCouteur, RA, Carr, EA, et al. Influence of tumor cell proliferation and sex-hormone receptors on effectiveness of radiation therapy for dogs with incompletely resected meningiomas. J Am Vet Med Assoc. 2000:701–707. [216].
75. Turrel, JM, Fike, JR, LeCouteur, RA, et al. Radiotherapy of brain tumors in dogs. J Am Vet Med Assoc. 1984;184:82–86.
76. Dow, SW, LeCouteur, RA, Rosychuk, RAW, et al. Response of dogs with functional pituitary macroadenomas and macrocarcinomas to radiation. J Small Anim Pract. 1990;31:287–294.
77. Theon, AP, Feldman, EC. Megavoltage irradiation of pituitary macrotumors in dogs with neurologic signs. J Am Vet Med Assoc. 1998;213:225–231.
78. Mayer, MN, Greco, DS, LaRue, SM. Outcomes of pituitary tumor irradiation in eight cats. J Vet Intern Med. 2006;20:1151–1154.
79. Leibel, SA, Sheline, GE. Tolerance of the central and peripheral nervous system in therapeutic irradiation. In: Lett JT, Altman KI, eds. Advances in Radiation Biology. New York: Academic Press, 1987.
80. Graeb, DA, Steinbok, P, Robertson, WD. Transient early computed tomographic changes mimicking tumor progression after brain tumor irradiation. Radiology. 1982;144:813–817.
81. Brearley, MJ. Hypofractionated radiation therapy of brain masses in dogs: a retrospective analysis of survival in 83 cases (1991–1996). J Vet Intern Med. 1999;13(5):408–412.
82. Kuntz, CA, Dernell, WS, Powers, BE, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986–1996). J Am Vet Med Assoc. 1997;211:1147–1151.
83. McKnight, JA, Mauldin, GN, McEntee, MC, et al. Radiation treatment for incompletely resected soft-tissue sarcomas in dogs. J Am Vet Med Assoc. 2000;217:205–210.
84. Bostock, DE, Dye, MT. Prognosis after surgical excision of canine fibrous connective tissue sarcomas. Vet Pathol. 1980;17:581–588.
85. Graves, GM, Bjorling, DE, Mahaffey, E. Canine hemangiopericytoma: 23 cases (1967–1984). J Am Vet Med Assoc. 1988;192:99–102.
86. Ettinger, SN, Scase, TJ, Oberthaler, KT, et al. Association of argyrophilic nucleolar organizing regions, Ki-67, and proliferating cell nuclear antigen scores with histologic grade and survival in dogs with soft tissue sarcomas: 60 cases (1996–2002). J Am Vet Med Assoc. 2006;228:1053–1062.
87. Stefanello, D, Morello, E, Roccabianca, P, et al. Marginal excision of low-grade spindle cell sarcoma of canine extremities: 35 dogs (1996–2006). Vet Surg. 2008;37:461–465.
88. McSporran, KD. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Vet Pathol. 2009;46:928–933.
89. Chase, D, Bray, J, Ide, A, et al. Outcome following removal of canine spindle cell tumours in first opinion practice: 104 cases. J Small Anim Pract. 2009;50:568–574.
90. Thrall, DE, LaRue, SM, Yu, D, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2005;11:5206–5214.
91. Frimberger, AE, Moore, AS, LaRue, SM, et al. Radiotherapy of incompletely resected, moderately differentiated mast cell tumors in the dog: 37 cases (1989–1993). J Am Anim Hosp Assoc. 1997;33:320–324.
92. Ladue, T, Price, GS, Dodge, R, et al. Radiation therapy for incompletely resected canine mast cell tumors. Vet Radiol Ultrasound. 1998;39:57–62.
93. Chaffin, K, Thrall, DE. Results of radiation therapy in 19 dogs with cutaneous mast cell tumor and regional lymph node metastasis. Vet Radiol Ultrasound. 2002;43:392–395.
94. Cronin, K, Page, RL, Spodnick, G, et al. Radiation therapy and surgery for fibrosarcoma in 33 cats. Vet Radiol Ultrasound. 1998;39:51–56.
95. Bregazzi, VS, LaRue, SM, McNiel, E, et al. Treatment with a combination of doxorubicin, surgery, and radiation versus surgery and radiation alone for cats with vaccine-associated sarcomas: 25 cases (1995–2000). J Am Vet Med Assoc. 2001;218:547–550.
96. Cohen, M, Wright, JC, Brawner, WR, et al. Use of surgery and electron beam irradiation, with or without chemotherapy, for treatment of vaccine-associated sarcomas in cats: 78 cases (1996–2000). J Am Vet Med Assoc. 2001;219:1582–1589.
97. Mayer, MN, Treuil, PL, LaRue, SM. Radiotherapy and surgery for feline soft tissue sarcoma. Vet Radiol Ultrasound. 2009;50:669–672.
98. LaRue, SM, Withrow, SJ, Powers, BE, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc. 1989;195:1734–1744.
99. Thrall, DE, Dewhirst, MW, Page, RL, et al. A comparison of temperatures in canine solid tumours during local and whole-body hyperthermia administered alone and simultaneously. Int J Hyperthermia. 1990;6:305–317.
100. Dickerson, ME, Page, RL, LaDue, TA, et al. Retrospective analysis of axial skeleton osteosarcoma in 22 large-breed dogs. J Vet Intern Med. 2001;15:120–124.
101. Custis, JT, Ryan, SD, Valdes-Martinez, A, et al. Identifying factors predictive of osteosarcoma related pathologic fracture following stereotactic radiation therapy. Vet Radiol Ultrasound. 2009;51(2):234.
102. Steenland, E, Leer, JW, van Houwelingen, H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109.
103. Knapp-Hoch, HM, Fidel, JL, Sellon, RK, et al. An expedited palliative radiation protocol for lytic or proliferative lesions of appendicular bone in dogs. J Am Anim Hosp Assoc. 2009;45:24–32.
104. Dernell, WS, Van Vechten, BJ, Straw, RC, et al. Outcome following treatment of vertebral tumors in 20 dogs (1986–1995). J Am Anim Hosp Assoc. 2000;36:245–251.
105. McEntee, MC, Page, RL, Novotney, CA, et al. Palliative radiotherapy for canine appendicular osteosarcoma. Vet Radiol Ultrasound. 1993;34:367–370.
106. Ramirez, O, III., Dodge, RK, Page, RL, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound. 1999;40:517–522.
107. Kent, MS, Bommarito, D, Feldman, E, et al. Survival, neurologic response, and prognostic factors in dogs with pituitary masses treated with radiation therapy and untreated dogs. J Vet Intern Med. 2007;21:1027–1033.
108. Sellon, RK, Fidel, J, Houston, R, et al. Linear-accelerator-based modified radiosurgical treatment of pituitary tumors in cats: 11 cases (1997–2008). J Vet Intern Med. 2009;23:1038–1044.
109. Allan, GS, Gillette, EL. Response of canine mast cell tumors to radiaton. J Natl Cancer Inst. 1979;63(3):691–694.
110. Turrel, JM. Prognostic factors for radiation treatment of mast cell tumor in 85 dogs. J Am Vet Med Assoc. 1988;193:936–940.
111. Theon, AP, Barthez, PY, Madewell, BR, et al. Radiation therapy of ceruminous gland carcinomas in dogs and cats. J Am Vet Med Assoc. 1994;205:566–569.
112. Kobayashi, T, Hauck, ML, Dodge, R, et al. Preoperative radiotherapy for vaccine associated sarcoma in 92 cats. Vet Radiol Ultrasound. 2002;43:473–479.
113. Eckstein, C, Guscetti, F, Roos, M, et al. A retrospective analysis of radiation therapy for the treatment of feline vaccine-associated sarcoma. Vet Comp Oncol. 2009;7:54–68.
114. Elmslie, RE, Ogilvie, GK, Gillette, EL, et al. Radiotherapy with and without chemotherapy for localized lymphoma in 10 cats. Vet Radiol. 1991;32:277–280.
115. North, SM, Meleo, KA, Mooney, S, et al, Radiation therapy in the treatment of nasal lymphoma in cats. Proceedings of the Veterinary Cancer Society 14th Annual Conference Townsend, Tennessee, 21 1994.
116. Gillette, EL, McChesney, SL, Dewhirst, MW, et al. Response of canine oral carcinomas to heat and radiation. Int J Radiat Oncol Biol Phys. 1987;13:1861–1867.
117. Bateman, KE, Catton, PA, Pennock, PW, et al. 0-7-21 Radiation therapy for the treatment of canine oral melanoma. J Vet Intern Med. 1994;8:267–272.
118. Blackwood, L, Dobson, JM. Radiotherapy of oral malignant melanomas in dogs. J Am Vet Med Assoc. 1996;209:98–102.
119. Theon, AP, Marks, SL, Feldman, ES, et al. Prognostic factors and patterns of treatment failure in dogs with unresectable differentiated thyroid carcinomas treated with megavoltage irradiation.[see comment]. J Am Vet Med Assoc. 2000;216:1775–1779.
120. Ohara, K, Tatsuzaki, H, Fuji, H, et al. Radioresponse of thymomas verified with histologic response. Acta Oncologica. 1998;37:471–474.
121. Williams, LE, Gliatto, JM, Dodge, RK, et al. Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985–1995). J Am Vet Med Assoc. 2003;223:825–831.
122. Anderson, CR, McNiel, EA, Gillette, EL, et al. Late complications of pelvic irradiation in 16 dogs. Vet Radiol Ultrasound. 2002;43:187–192.
123. Fuccio, L, Guido, A, Laterza, L, et al. Randomised clinical trial: preventive treatment with topical rectal beclomethasone dipropionate reduces post-radiation risk of bleeding in patients irradiated for prostate cancer. Aliment Pharmacol Ther. 2011;34:628–637.
124. Fertil, B, Malaise, E. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: Analysis of 101 published survival curves. Int J Rad Onc Biol Phys. 1985;11(9):1699–1707.
125. Bump, EA, Braunhut, SJ, Palayoor, ST, et al. Novel concepts in modification of radiation sensitivity. Int J Radiat Oncol Biol Phys. 1994;29:249–253.
126. Vose, JM. Current approaches to the management of non-Hodgkin’s lymphoma. Semin Oncol. 1998;25:483–491.
127. Yahalom, J. Radiation therapy in the treatment of lymphoma. Curr Opin Oncol. 1999;11:370–374.
128. Fidias, P, Spiro, I, Sobczak, ML, et al. Long-term results of combined modality therapy in primary bone lymphomas. Int J Radiat Oncol Biol Phys. 1999;45:1213–1218.
129. Giger, U, Evans, SM, Hendrick, MJ, et al. Orthovoltage radiotherapy of primary lymphoma of bone in a dog. J Am Vet Med Assoc. 1989;195:627–630.
130. Kirova, YM, Piedbois, Y, Haddad, E, et al. Radiotherapy in the management of mycosis fungoides: indications, results, prognosis. Twenty years experience. Radiother Oncol. 1999;51:147–151.
131. Kirova, YM, Piedbois, Y, Le Bourgeois, JP. Radiotherapy in the management of cutaneous B-cell lymphoma. Our experience in 25 cases. Radiother Oncol. 1999;52:15–18.
132. Meleo, KA. The role of radiotherapy in the treatment of lymphoma and thymoma. Vet Clin North Am Small Anim Pract. 1997;27(1):115–129.
133. Williams, LE, Johnson, JL, Hauck, ML, et al. Chemotherapy followed by half-body radiation therapy for canine lymphoma. J Vet Intern Med. 2004;18:703–709.
134. Gustafson, NR, Lana, SE, Mayer, MN, et al. A preliminary assessment of whole-body radiotherapy interposed within a chemotherapy protocol for canine lymphoma. Vet Comp Oncol. 2005;2:125–131.
135. Escobar, C, Grindem, C, Neel, JA, et al. Hematologic changes after total body irradiation and autologous transplantation of hematopoietic peripheral blood progenitor cells in dogs with lymphoma. Vet Pathol. 2012;49(2):341–343.