Tumors of the Nervous System
Brain Tumors
Brain tumors are frequently encountered in dogs and cats, with reported incidence rates of 14.5 dogs and 3.5 cats per 100,000 pets at risk, respectively.1,2 In addition to being more common in dogs than in cats, there is a wider variety of canine brain tumors than reported in cats; in addition, there is a wider range of histologic subtypes of canine meningioma reported compared with feline meningiomas.3,4
Most brain tumors encountered in clinical practice are primary tumors originating from brain parenchyma (e.g., glial cells and neurons, cells that line the interior and exterior brain surfaces [such as meningeal and ependymal cells]) or cells from vascular structures (e.g., choroid plexus). Meningioma is the most frequently reported primary brain tumor in dogs and cats, although gliomas are also frequently encountered in dogs.2,5-7 More than 50% of reported feline brain tumors are meningiomas.2,5,6 Gliomas are occasionally reported in cats.2,5 Other primary brain tumors less commonly reported in dogs include choroid plexus tumors; primary central nervous system (CNS) lymphosarcomas; primitive neuroectodermal tumors (PNETs), which typically includes neuroblastoma; primary CNS histiocytic sarcomas (also termed malignant histiocytosis); ependymomas; and vascular hamartomas. Other primary brain tumors occasionally encountered in cats include (in addition to gliomas): ependymomas, olfactory neuroblastomas, and choroid plexus tumors.2,5,7 In both dogs and cats, there are case reports of medulloblastomas, usually involving the cerebellum. Microglial tumors are considered rare in dogs and cats.1-3 Primary brain tumors in dogs and cats are typically solitary. Approximately 17% of cats with intracranial meningioma have more than one tumor.8,9 Multiple masses of the same primary brain tumor type in dogs are typically associated with choroid plexus tumors; in this scenario, tumor cells are disseminated or “dropped” via cerebrospinal fluid (CSF) flow in the ventricular system (“drop” metastases).5 The authors have encountered a number of Boxers with multiple intracranial gliomas. In one study, 10% of cats were found to have two different types of intracranial neoplasia concurrently.2 The occurrence of more than one histologic tumor type in dogs is considered rare.
Secondary brain tumors include metastatic neoplasia, as well as tumors that affect the brain by local extension. Examples of metastatic neoplasia include mammary, pulmonary, and prostatic carcinoma; hemangiosarcoma; malignant melanoma; and lymphosarcoma. Tumors that may extend into the brain from the periphery include nasal and frontal sinus carcinoma (adenocarcinoma, squamous cell carcinoma), calvarial tumors (e.g., osteosarcoma, chondrosarcoma, multilobular osteochondrosarcoma), pituitary tumors (e.g., pituitary macroadenomas in hyperadrenocorticism), and nerve sheath tumors (e.g., cranial nerve [CN] V tumors). In one large retrospective study of secondary brain tumors in dogs,10 the most common secondary brain tumor was hemangiosarcoma (29%), followed by pituitary tumors (25%), lymphosarcoma (12%), metastatic carcinoma (11%), and invasive nasal tumors (6%). In one report,2 secondary (multicentric) lymphoma and pituitary tumors were the second and third most common intracranial neoplasms found in cats. Lymphoma/lymphosarcoma is considered the most common secondary brain tumor type encountered in cats.
Brain tumors in dogs and cats can occur at any age, in either sex, and in any breed. However, brain tumors occur most commonly in older patients, with a median age of 9 years for dogs and older than 10 years for cats.2,5,6 Compared with other tumor types, dogs and cats with meningioma tend to be diagnosed at older ages.2,5-7 Male cats are more likely to develop intracranial meningioma than female cats.5,6 Golden retrievers and Boxers are considered to be predisposed to developing primary brain tumors.5-7 In general, dolichocephalic dog breeds are prone to develop meningiomas, whereas brachycephalic dog breeds are more likely to be afflicted with gliomas.5-7 There is no known breed predilection for cats to develop primary brain tumors.2,5,6 Cerebral tumors are more commonly encountered than tumors in the brainstem or cerebellum. Gliomas have a tendency to occur in the diencephalon and cerebellum, however.5,6
Pathology
As mentioned previously, brain tumors are broadly classified as primary (neoplastic cells originating from tissues intrinsic to the brain, its vasculature, and meninges/ependyma) or secondary (neoplastic cells originating from tissues extrinsic to those comprising primary brain tumors).5 Brain tumors exert their pathologic effects both by directly encroaching on and/or invading brain tissue and by secondary effects, such as peritumoral edema, inflammation, obstructive hydrocephalus, and hemorrhage.
The terms benign and malignant have different connotations when used in the context of intracranial neoplasia compared with tumors in other locations. Even slow-growing, readily removable brain tumors are not benign when considering the potential effect on the patient if therapy is not aggressively pursued in a timely fashion. Cytologic features of tumors are used to assess potential benignity or malignancy and include invasiveness, number of mitotic figures, and nuclear pleomorphism. Meningiomas are often regarded as benign. Considering the aggressive cytologic nature of many canine meningiomas (in comparison with human and feline meningiomas), this is probably an overstatement for this tumor type—especially in dogs.6,11
There are multiple histologic subtypes of meningiomas frequently encountered in dogs, including meningothelial, transitional, fibroblastic, psammomatous, angiomatous, microcystic, papillary, granular cell, myxoid, and anaplastic.3,4,6,11 In addition to these histologic subtypes, a grading system used in humans to predict the biologic behavior of intracranial meningiomas has recently been adapted to dogs; this grading system includes grade I (benign), grade II (atypical), and grade III (malignant).11 The repertoire of feline meningioma histologic subtypes is comparatively limited; the majority of feline meningiomas are meningothelial or psammomatous.2-4,6
Gliomas in dogs and cats include astrocytomas, oligodendrogliomas, and glioblastoma multiforme, in addition to undifferentiated gliomas. Gliomas as a group are considered to be malignant because these tumors tend to invade tissue and are typically resistant to all forms of definitive therapy.2,4,5,7,12
Clinicopathologic features of choroid plexus tumors in dogs have recently been reviewed.13 Choroid plexus tumors comprise choroid plexus papillomas (CPP) and choroid plexus carcinomas (CPC). Choroid plexus tumors in general tended to occur in the fourth ventricle in one report, and only CPCs occurred in the lateral ventricles.14 The two tumor subtypes are differentiated based on the presence or absence of local or distant metastases, as well as on histopathologic features.
In one large study,7 it was found that half of canine primary brain tumors occupy more than one anatomic region of the brain; this could lead to the false conclusion based on neurologic examination that a patient with a solitary brain mass has multifocal disease. Also in that study, it was found that 23% of dogs with primary brain tumors had concurrent, unrelated neoplasia (e.g., pulmonary carcinoma, hemangiosarcoma), most of which involved the thoracic or abdominal cavity; this finding underscores the importance of screening for concurrent unrelated neoplasia (via thoracic radiography and abdominal ultrasonography) prior to pursuing advanced diagnostics and definitive therapy for the brain tumor.
History and Clinical Signs
The diagnosis of brain tumor should be highly suspected in an elderly dog or cat with slowly progressive signs of brain dysfunction. A brain tumor should also be suspected in animals that experience a recent onset of seizure activity after 5 years of age, especially in certain breeds (e.g., golden retrievers, Boxers). Depending on the location and size of the tumor, such patients may appear neurologically normal interictally. Historic and presenting clinical signs for dogs and cats with brain tumors are variable and reflect both the location and the secondary effects (e.g., edema, hemorrhage) of the tumor. Seizures represent the most common presenting clinical sign of neurologic dysfunction in dogs with brain tumors, occurring in approximately half of the patients.5,6,15 In one study of feline brain tumors, the overall incidence of seizure activity was 23%, occurring more commonly with glioma (26.7%) and lymphoma (26.3%), in comparison with meningioma (15%).16 Cats with brain tumors are commonly presented to the veterinarian with a complaint of behavior change. In cats with primary brain tumors, nonspecific presenting clinical signs (i.e., signs not obviously referable to neurologic dysfunction) are fairly common, occurring in over 20% of cats in one large study.2 These clinical signs included lethargy, inappetence, and anorexia. Also in that study, it was found that approximately 19% of feline brain tumors were considered an incidental finding. Cerebral tumors are more common than tumors of the brainstem or cerebellum. Cerebral and diencephalic tumors tend to cause clinical signs of dysfunction such as seizure activity, behavior changes, circling, head-pressing, visual deficits, and hemiinattention (hemineglect) syndrome (ignoring environmental cues from the side opposite the tumor). Proprioceptive placing deficits and neck pain are often appreciable on neurologic examination. Tumors of the brainstem from midbrain through medulla often cause alterations of consciousness, dysfunction of cranial nerves (other than CN I and CN II), and obvious gait/proprioceptive abnormalities. Cerebellar tumors may result in clinical signs of dysfunction such as ataxia, dysmetria, intention tremors, vestibular abnormalities, and menace reaction deficits with normal vision.5,6
In most cases, clinical signs of neurologic dysfunction occur slowly and insidiously over time, especially with meningiomas. Owners of pets with meningiomas will often retrospectively realize that their pet had a behavior change for months to over a year prior to diagnosis. The subtle behavior changes are often attributed to “old age.” However, brain tumor patients can have subacute to acute development of neurologic dysfunction. These patients may experience sudden exhaustion of brain compensatory mechanisms or may suffer hemorrhage or acute obstructive hydrocephalus due to the tumor.5,6
Diagnostic Techniques and Work-Up
Signalment, history, and neurologic examination findings often provide a strong index of suspicion of a brain tumor as the cause of neurologic dysfunction in a dog or cat. However, there are a number of disorders (e.g., inflammatory/infectious, congenital, degenerative) that can produce similar signs of neurologic dysfunction in patients at risk for developing brain tumors. It is imperative therefore to follow an ordered diagnostic work-up in patients suspected of having brain tumors.
Minimum Database
Before pursuing advanced imaging, basic blood work (complete blood count [CBC] and chemistry profile) and a urinalysis should be performed. Thoracic radiographs should be taken to help rule out the possibility of metastatic cancer or a second unrelated neoplastic process. Considering the likelihood of concurrent neoplasia in dogs with brain tumors, performing abdominal ultrasound examination is also often advisable.
Imaging
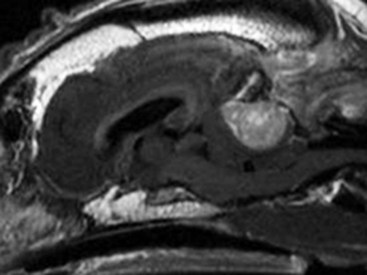
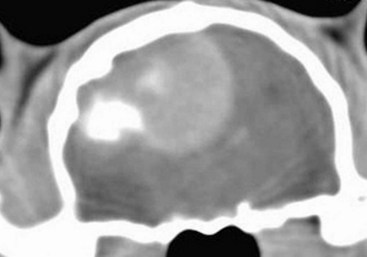
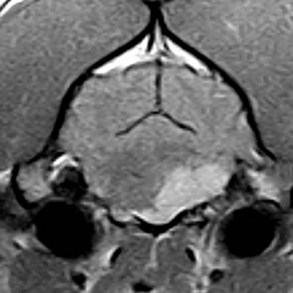
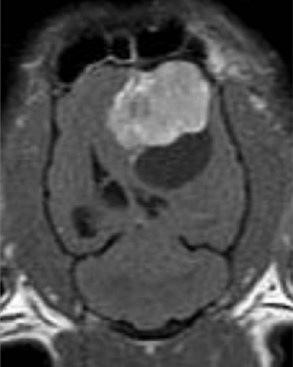
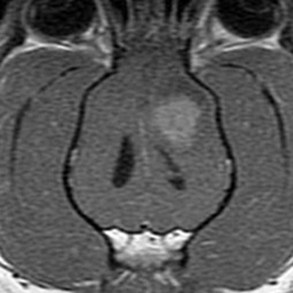
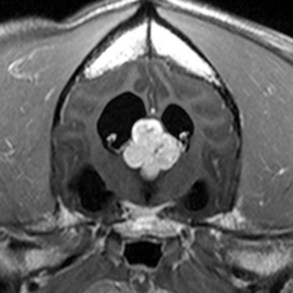
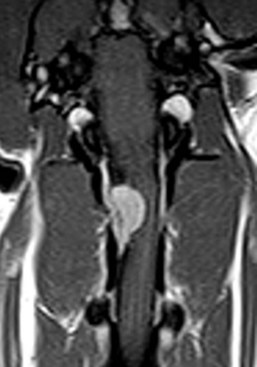
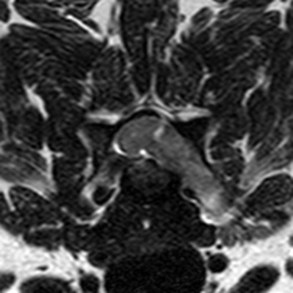
Radiographs of the skull typically do not provide useful clinical information in cases of brain tumor. Computed tomography (CT) and magnetic resonance imaging (MRI) are commonly used in the diagnosis of brain tumors, with MRI being the preferred imaging modality. Although specific types of brain tumors can vary in their appearance with these imaging modalities, there are some characteristic features that help distinguish meningiomas from gliomas. Meningiomas tend to have a broad-based, extraaxial attachment (they arise from the periphery of the brain and move inward, or axially), exhibit distinct tumor margins, and uniformly contrast enhance (Figure 30-1). Meningiomas tend to displace, rather than invade, parenchymal tissue; as discussed previously, however, many canine meningiomas do display some degree of invasiveness. Some meningiomas will calcify, which can be appreciated on a noncontrast CT image (Figure 30-2). The “dural tail” sign is an MRI feature typically associated with meningiomas, in which a contrast-enhancing meningeal-associated “tail” is seen extending from the main tumor mass (Figure 30-3). Meningiomas also may occasionally have a cystic component (cystic meningioma) extending from the main tumor mass (Figure 30-4). Gliomas tend to arise from an intraaxial location (from within the substance of the brain, moving outward), often lack distinct tumor margins (they tend to infiltrate, rather than displace normal tissue), and typically contrast enhance poorly and nonuniformly (Figure 30-5). Choroid plexus tumors and ependymomas tend to be intraventricular in location and often uniformly contrast enhance (Figure 30-6). The phenomenon of “ring enhancement,” in which a circular ring of contrast enhancement surrounds nonenhancing tissue, is nonspecific and has been associated with several neoplastic and nonneoplastic brain diseases. However, ring enhancement is often associated with gliomas (see Chapter 6). Meningeal contrast enhancement evident on MR images of the brain has been described but is not specific for brain tumors. These typical imaging features are guidelines only. Meningiomas can arise from the falx cerebri or the choroid plexus and appear intraaxial. Gliomas can be peripherally located and contrast enhancing. In a recent study, the accuracy of predicting primary brain tumor type based on MR images of 40 dogs was 70%.17 Stereotactic biopsy of brain tumors using MRI or CT images is now available at several veterinary referral centers.5 With this new technology, a definitive diagnosis can be obtained at the time of imaging without the need for major intracranial surgery.
Cerebrospinal Fluid Analysis
The utility of CSF evaluation for the suspected brain tumor patient is controversial. CSF is often abnormal in patients with brain tumors, but the white blood cell (WBC) counts and protein levels are variable and nonspecific for neoplasia. In fact, dogs and cats with meningiomas tend to have CSF with predominantly polymorphonuclear (neutrophilic) WBC counts. The authors often do not pursue CSF analysis if the CT or MR image strongly suggests a brain neoplasm. Although the risk of CSF procurement in the face of elevated intracranial pressure (ICP) in a brain tumor patient is often not great, the potential benefit of a nonspecific CSF result may not outweigh even a small danger of harming the patient with the procedure. Although CSF analysis tends to yield fairly nonspecific information in brain tumor cases, it may be helpful in distinguishing whether a choroid plexus tumor is a CPP or CPC. In one study,13 the more malignant CPC was significantly more likely to be associated with a CSF protein concentration greater than 100 mg/dL than the less malignant CPP tumor subtype. Regardless of whether CSF analysis is performed, imaging should always precede CSF analysis when a focal neoplasm is highly suspected. Anesthetizing a patient who is most likely to have a brain tumor solely for the purpose of obtaining CSF is generally contraindicated because the resultant information is unlikely to assist in either planning treatment or estimating prognosis.
Therapy
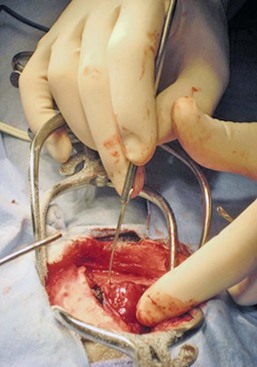
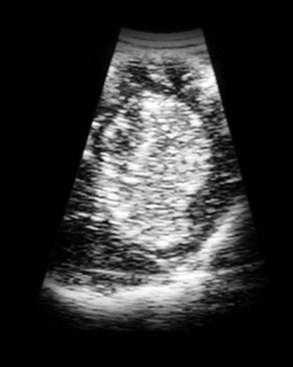
In addition to removing neoplastic tissue, surgical debulking/removal allows for a histologic diagnosis, as well as potentially providing an immediate decompressive effect (decreasing ICP). Feline meningiomas are typically located over the cerebral convexities and tend to “peel away” from normal brain tissue at surgery. In most cases, feline meningiomas can be relatively easily removed en masse. The authors have had considerable experience dealing with recurrent or repeat feline intracranial meningiomas; overall, these tumors are also typically readily removable. Surgical removal is the primary mode of definitive therapy for feline intracranial meningiomas.2,5,6,9 Canine meningiomas are also often located over the cerebral cortical surface and are thus surgically accessible (Figure 30-7). However, meningiomas in the cerebellar and brainstem regions are frequently encountered in dogs. Cerebellar meningiomas are often surgically accessible; meningiomas in the brainstem may not be accessible. Meningiomas in dogs are much less predictable than those in cats in terms of ease of surgical removal. Unlike cats, there are multiple histologic subtypes of canine meningiomas, and nearly one-third of these tumors are invasive.3-6,11 The authors have successfully used intraoperative ultrasound to assist in locating brain tumors and in judging completeness of removal (Figure 30-8). The authors do not recommend pursuing surgical removal of canine meningiomas if adjunctive therapy (e.g., radiation) is not planned to be used following removal. Some gliomas are surgically accessible, but surgical removal/debulking of gliomas is considerably more difficult than meningiomas. Gliomas tend to infiltrate normal brain parenchyma, and it is often difficult to discern tumor margin from brain tissue at surgery. Surgical removal of intracranial glioma is seldom attempted in dogs and cats because of the invasive nature of these neoplasms. Removal of other brain tumor types (e.g., choroid plexus tumor, ependymoma) is also uncommonly performed because of the intraaxial location of these masses. Surgical removal of secondary brain tumors is also not commonly performed because many of these tumors are invasive as are their extraneural source neoplasms. However, some calvarial tumors (e.g., multilobular osteochondrosarcoma [MLO]) are surgically resectable with good outcomes (see Chapter 24).5,18 On rare occasions, metastatic brain tumors are removed surgically.
Radiation Therapy
Radiation therapy (RT) is used in the treatment of primary brain tumors, as well as secondary brain tumors that arise by local extension or from metastasis from a distant site. Treatment options have advanced from early reports of the use of orthovoltage RT to current options, including intensity-modulated RT (IMRT) and stereotactic RT (SRT).19,20 Fractionated radiation protocols have largely transitioned from alternate day treatments to daily therapy (Monday through Friday), although there are reports on the application of hypofractionated protocols.21-23 Radiation dose per fraction, total dose of radiation, and the volume of the brain irradiated are important considerations when irradiating brain tumors.24,25 In one report of hypofractionated brain irradiation, 14.5% (12/83) of dogs died or were euthanized due to suspected delayed radiation side effects caused by delivery of a high dose per fraction.22 There are reports on the results of RT alone or in combination with surgery for canine primary brain tumors.19,24,26-29 RT improves survival over surgery alone or medical management alone.26,28 Median overall survival for 29 dogs (presumptive diagnosis of meningioma in 22 dogs, glioma in 4 dogs, and choroid plexus tumor in 3 dogs) that were treated with RT alone was 250 days, with 76% dying of presumptive recurrence or tumor progression.27 In another report of 46 dogs irradiated only for a range of different tumor types the median survival time (MST) was 699 days.24 It is a well-accepted principle that postoperative RT in the microscopic disease setting is generally more efficacious than treating macroscopic disease, including brain tumors. However, it may be difficult to determine if there are postoperative changes versus residual macroscopic disease when CT imaging is done for radiation treatment planning postoperatively.30 With no therapy or only supportive therapy, survival is reported to be 0.2 months in comparison to dogs irradiated with Cobalt-60 external-beam RT alone or in combination with other modalities with a median survival of 4.9 months.26 In another report comparing surgery alone to surgery plus RT, the median survival was 7 months (surgery alone and survival >1 week) compared to 16.5 months (combination therapy).28 In a report of 20 dogs with meningioma that had RT after incomplete resection, the median progression-free survival time was 35 months.29 In a retrospective study of endoscopic-assisted tumor removal in dogs (n = 33) and cats (n = 6), 44% were alive after more than 2 years, only 4 were irradiated postoperatively, and the MST for dogs with meningiomas was 2104 days.31
An alternative to conventional fractionated RT is stereotactic radiosurgery for the management of CNS tumors, which entails the precise delivery of a larger dose per fraction to the tumor in 1 to a few fractions for a total dose of 10 to 15 Gy.20 Limited information is available in the literature, but there are a number of veterinary facilities that now have equipment with this capability.
RT has also been used successfully to irradiate a calvarial allograft used in the repair of a calvarial defect in a cat with an intracranial meningioma.32 It is important to consider the potential impact on radiation dose distribution in patients that have undergone surgery and require reconstruction that may include the use of metal implants.33-36 This is potentially of more concern when larger metal implants (e.g., plates, rods, screws) are used to stabilize the spine.37
Chemotherapy
There are a limited number of reports on the use and efficacy of chemotherapy in the management of brain tumors in companion animals, including individual case reports and small case series.21,38-44 Lomustine, carmustine, and hydroxyurea have been utilized in the treatment of canine brain tumors, but there are limited data to provide validation of the utility of chemotherapy. Based on a retrospective study of oral hydroxyurea in combination with glucocorticoids, 33 dogs with MRI-diagnosed meningioma had a significantly longer survival time than observed in 10 dogs treated with glucocorticoids alone.45 The MST for the combination therapy group was 28 weeks versus 14 weeks for dogs treated with glucocorticoids alone.45 A report on the use of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) in 206 dogs with a number of different tumor types, including 11 dogs with brain tumors, documented toxicity of this chemotherapeutic agent but did not provide response data.38 Chemotherapy (carmustine, lomustine) alone or in combination with RT or cytoreductive surgery in dogs with astrocytomas has resulted in survival times of 3 to 8 months.21,40
Other Therapies
There is limited information on other therapeutic approaches to the management of canine brain tumors, including the use of whole body hyperthermia in conjunction with RT and/or chemotherapy, radioactive iodine-125 implants in combination with other therapeutic approaches, gene therapy, and convection-enhanced delivery of liposomal nanoparticles containing topoisomerase inhibitor CPT-11, which is still being developed.26,46-52 The use of whole body hyperthermia in conjunction with external-beam irradiation did not alter survival.46 Dogs with meningiomas that were irradiated had a mean survival of 314 days versus 288 days for dogs treated with RT plus whole body hyperthermia.46 Gene therapy strategies in dogs have included the delivery of an adeno-associated viral vector containing prodrug activating genes that confer sensitivity to toxic metabolites and cytokine-based gene therapy using an intravenously delivered cationic DNA lipid complex containing murine endostatin gene in a DNA plasmid to perturb tumor angiogenesis.47,48 There is limited information on the application of brachytherapy in conjunction with excisional biopsy, but it may represent another potential avenue of treatment.26,53
Based on the association between vascular endothelial growth factor (VEGF) expression and survival in dogs with intracranial meningiomas, administration of antiangiogenic agents may have future therapeutic potential.54,55
There is information available that indicates that dynamic contrast-enhanced CT may have utility in evaluating and classifying intracranial lesions and for determining response to therapy.56
Prognosis
Prognosis is poor with no therapy or only supportive therapy or palliative therapy in dogs with a MST of 0.2, or less than 2 months.23,26 Results of RT based on a subset of the reports in the literature are difficult to interpret because a definitive histopathologic diagnosis is not always obtained and tumor types are grouped together (including pituitary tumors).19,24,26,27 A presumptive diagnosis may be made based on either CT or MRI findings.27,57 However, this may not be a reliable means of arriving at a presumptive diagnosis.58 The interpretation of results is improving with the advent of minimally invasive techniques for brain tumor biopsies or cytology, as well as advancements in surgical techniques and tumor extirpation for histopathologic analysis, including endoscopic-assisted intracranial tumor removal.31,59-64 The ability to definitively diagnose the specific histopathologic type of brain tumor prior to surgery may aid both clinicians and clients in deciding on a course of treatment and in understanding the potential prognosis.48 Additional potential positive prognostic factors that have been identified include solitary versus multiple lesions, limited neurologic dysfunction, and treatment with RT or a combination of surgery and RT.26,28 Tumor location may also impact survival; MST for forebrain versus caudal brain meningiomas was 2104 days and 702 days, respectively.31 Pneumonia can develop due to aspiration postoperatively and may have a negative impact on survival.65
Meningiomas are the most common intracranial tumor in dogs, but attempts to correlate tumor grade or histologic subtype and MRI features largely have not identified any significant associations that would allow preoperative prognostication.11 In a study of 112 dogs with intracranial meningiomas using criteria of the human WHO international histologic classification system 56% were classified as benign, 43% were atypical, and 1% were malignant meningiomas; the atypical and malignant meningiomas were noted to behave more aggressively with a higher potential for recurrence.11 In one report of 17 dogs treated surgically for meningioma, survival was correlated with histologic tumor type: anaplastic 0 days, fibroblastic 10 days, psammomatous more than 313 days, meningothelial more than 523 days, and transitional 1254 days.44 Of interest is that use of a surgical aspirator to resect intracranial meningiomas resulted in a MST of 1254 days.44 None of the dogs were irradiated, and only 2 of 17 received postoperative chemotherapy (hydroxyurea) when there were MRI findings suggestive of recurrence.44 In a study of 17 dogs with benign meningiomas treated with surgery and postoperative hypofractionated RT, significantly shorter survival times were associated with greater VEGF expression.54 The MST was 748 days for dogs with tumors with 75% or fewer cells staining for VEGF compared with 442.5 days for dogs with tumors with more than 75% of cell staining for VEGF.54 Furthermore, dogs with more intense staining had a significantly shorter survival time.54 In a more recent study, tumor proliferation defined by reactivity of the monoclonal antibody MIB-1 to the Ki67 antigen was not associated with survival, and there was no association between VEGF and tumor proliferation.66 In this study, 70 dogs underwent surgery and postoperative hypofractionated RT, and the overall MST from the date of diagnosis was 514 days.66 Progesterone and estrogen receptor expression has been evaluated in meningiomas in dogs and cats; further investigation will be necessary to determine if there is prognostic significance or a role for targeted intervention.29,67 In one study, progesterone receptor expression was inversely related to tumor proliferative fraction measured by immunohistochemical detection of proliferating cell nuclear antigen (PFPCNA index), which was predictive of survival in dogs with meningiomas after surgery and postoperative RT.29 The 2-year progression-free survival was 43% for tumors with a high PFPCNA index and 91% for tumors with a low PFPCNA index. Furthermore, tumors with a high PFPCNA index were 9.1 times more likely to recur.29 Cyclooxygenase-2 (COX-2) expression has been demonstrated in the majority of intracranial meningiomas (21/24 or 87%), but the role in tumorigenesis and the potential for therapeutic intervention with COX-2 inhibitors have not been elucidated.68 Conversely, in a study of 20 canine gliomas, none of the tumors expressed COX-2.69 In this same study that also evaluated c-kit overexpression, the authors speculated that c-kit inhibitors may have an antiangiogenic effect in high-grade tumors due to presence of intramural vascular expression.69
Stereotactic radiosurgery may provide comparable or improved tumor control with a fewer number of anesthetic episodes but is limited in its application due to a limited number of facilities with this capability.20 Two dogs with meningiomas survived 227 and 56 weeks after radiosurgery; a dog with an oligodendroglioma survived 66 weeks.20 Additional information should be forthcoming with increased availability of RT units with advanced capability to deliver radiation.
In dogs with choroid plexus tumors, it should be noted that local spread of the tumor within the ventricular system can occur and distant metastasis via the subarachnoid space occurs in approximately 50% of dogs.13 Additionally, at necropsy in one study of 56 dogs with choroid plexus tumors, 19% had evidence of gross and/or microscopic spinal cord metastases that should impact considerations of diagnostics, approaches to treatment, and prognosis.13
There are descriptive reports but limited prognostic information available for other specific tumor types, such as for astrocytomas.12,70,71 In a report of 86 dogs treated for brain tumors that included 27 meningiomas, 7 astrocytomas, and 6 choroid plexus tumors, survival based on tumor type was evaluated as meningioma versus other; dogs with meningiomas lived significantly longer.26 In a study of dogs irradiated with a hypofractionated protocol, the MST for 34 dogs with intraaxial tumors (presumably gliomas) was 40.4 weeks versus 49.7 weeks for 41 dogs with extraaxial tumors (presumably meningioma, including schwannoma and choroid plexus tumor).22
Histiocytic sarcoma can affect the CNS in dogs with systemic disease, but there are a limited number of reports of localized CNS histiocytic sarcoma, with one report with necropsy information that confirmed the localized nature.72-76 Rapid disease progression and euthanasia was the outcome for two dogs: one with primary spinal cord involvement and the other with brain involvement.76 Surgical intervention has been reported in 12 dogs with subdural histiocytic sarcomas, although no follow-up information was provided.75
There are a number of studies that have been conducted to further elucidate the mechanisms of tumorigenesis and determination of the immunohistochemical profiles of brain tumors.77-83 Such efforts may ultimately lead to a better understanding and ability to prognosticate for individual patients based on identification of specific markers and for determination of appropriate targeted therapy. However, to date, targeted therapies in humans with meningiomas have only realized modest success and are associated with toxicity.84
It should be noted that multiple meningiomas, multifocal oligodendroglioma, and synchronous brain tumors (oligodendroglioma and meningioma) have been reported, as well as synchronous primary and metastatic tumors in dogs.43,85-89 Based on one small case series, survival may be comparable for dogs with multiple meningiomas as seen for dogs treated for a solitary meningioma.43 Additionally, dogs with a primary brain tumor may have another primary tumor that may affect prognosis.7 In a report of 170 dogs with primary intracranial neoplasia, 38/170 (23%) had another tumor unrelated to the brain tumor.7 In a study of 28 dogs with meningiomas 7 (25%) had another tumor in addition to the meningioma.87 Although rare, pulmonary metastasis in three dogs with intracranial meningioma has been reported.90 Cats are also reported to have multiple or two different types of intracranial tumors and, in fact, more commonly have multiple meningiomas (17%) than dogs.2,8,91,92 It has been questioned whether multiple meningiomas represent multicentric disease or metastasis. Dogs with metastatic intracranial neoplasms most commonly have metastatic hemangiosarcoma or carcinomas, representing 50% and 20% of metastatic tumors, respectively, with an anticipated poor prognosis.10,93 Meningeal carcinomatosis results from spread of a solid tumor that can be an intracranial primary tumor or of distant origin (e.g., mammary gland carcinoma) and is associated with a very poor prognosis.94-96 Metastatic disease is usually recognized as multiple mass lesions, but a solitary lesion may represent metastasis. Full staging is necessary to rule out other disease because secondary intracranial neoplasia may be more common than primary intracranial neoplasia.10
Cats respond well to surgery alone for intracranial meningiomas, which is the most common primary intracranial tumor in cats, and have a MST of approximately 2 years.2,9,97,98 In one report, the MST for cats treated surgically was 685 days compared to 18 days for cats that did not have surgery.2 Recurrence is documented to occur; 20.6% in one report had a median postoperative time to recurrence of 285 days (range 123 to 683 days; n = 6 cats); second surgeries are feasible in cats.2,9 Cats with multiple meningiomas based on one report have long-term survival when treated surgically with follow-up chemotherapy (hydroxyurea) for 2 (n = 3 cats) or 4 (n = 1 cat) meningiomas.91
Cats with intracranial lymphoma more commonly have multicentric disease; fewer cats have primary intracranial lymphoma.2 In cats treated palliatively with systemic corticosteroids, the MST was 21 days (range 9 to 270 days in nine cats).2
Two cats with oligodendrogliomas were euthanized at the time of diagnosis or 6 weeks after diagnosis due to their poor neurologic status.99 Of six cats with oligodendrogliomas, three had surgery (n = 2) and/or received corticosteroids (n = 1), with survival times of 1 day, 5 days, and one cat that had surgery was lost to follow-up at 1 month.2 Four cats with cerebral astrocytomas (three of which were managed medically) lived for 1 to 3 years with anticonvulsant therapy.100 Two of six cats with astrocytomas had surgery, followed by RT in one cat with survival times of 1 day and 179 days; a third cat treated with only corticosteroids survived 35 days.2 One cat with an ependymoma that had two surgeries ultimately survived for 667 days; another cat treated with corticosteroids was euthanized at 685 days.2
Comparative Aspects
Humans are afflicted with the same overall cadre of brain tumor types that are reported in dogs and cats. Overall, the incidence of brain tumors in people is approximately 19 per 100,000 person-years, with about 60% of these being benign tumors; the most common benign brain tumor in people is meningioma and the most common malignant brain tumor in people is glioblastoma multiforme (also called grade IV astrocytoma).101,102 Intracranial meningiomas in humans are often classified based on three histologic grades (benign, atypical, malignant) that predict biologic behavior. Approximately 80% of human meningiomas are considered benign according to this scheme, with atypical and malignant grades accounting for 8% and less than 3%, respectively. Although the specific histologic subtypes (e.g., transitional, psammomatous) of canine and human meningiomas are very similar, the percentage of atypical tumors is much higher in dogs at 43%.11,103 Treatment modalities used for human brain tumors are similar to those used for canine and feline brain tumors. Surgical removal is the primary treatment method used for human meningiomas, and survival is linked to the completeness of surgical removal. Adjunctive RT improves survival rates in people for incompletely resected meningiomas.11,101,102,104 Oral hydroxyurea therapy may be an effective treatment for human meningioma as well, although this is not as well established as RT.104 The prognosis for long-term control or cure of human intracranial meningioma is good.11,104 The morbidity and mortality for human gliomas is poor overall, with survival times often ranging from 7 to 24 months, even with combination (surgery, radiation, chemotherapy) therapy.102
Spinal Tumors
The actual incidence of spinal tumors in dogs and cats is unknown but appears to be considerably less than that of brain tumors. As with brain tumors, spinal tumors are broadly classified as primary or secondary. Spinal tumors are usually also classified in terms of their anatomic location: extradural, intradural/extramedullary, intramedullary, or mixed compartment. The most common primary spinal cord tumor in dogs is meningioma, and in cats the most common primary spinal cord tumor is lymphoma.6,105-109 Hemangiosarcoma is the most commonly reported secondary spinal cord tumor in dogs, and osteosarcoma is the most commonly reported secondary spinal cord tumor in cats.105,106 In one large retrospective study of 399 histopathologically confirmed spinal cord tumors in dogs, 48% were extradural, 13% were intradural/extramedullary, 6% were intramedullary, and 33% were mixed compartment.110
Canine spinal tumors tend to occur in large breeds, typically at an older age (e.g., 9 to 10 years).105,106 Meningiomas and malignant nerve sheath tumors (MNSTs) arise most frequently in the cervical spinal cord, and MNSTs are especially prominent in the cervical intumescence area.105,106 Boxers may be predisposed to spinal meningiomas.109 More aggressive meningiomas (grade II versus grade I) are likely to occur in the thoracolumbar region and in younger dogs.109 Spinal nephroblastoma typically occurs in young dogs (between 6 months and 3 years of age); this tumor also has a predilection for golden retrievers and German shepherd dogs and is usually located between the T10 and L2 spinal cord segments.105,106,111,112 Spinal lymphoma often affects young cats, is often associated with the feline leukemia virus (FeLV), tends to occur in the thoracic and lumbar spine, and is usually present in multiple extraneural locations.105-108 Vertebral osteosarcoma in cats is usually reported in older patients (e.g., >8 years).107,108
Pathology
The category of extradural tumors includes primary and secondary (metastatic or local invasion) vertebral and soft tissue tumors. Primary vertebral tumors such as osteosarcoma, chondrosarcoma, myeloma (plasma cell tumor), fibrosarcoma, and hemangiosarcoma are common extradural tumors encountered in dogs.105,106 In one study, vertebral tumors were slightly less prevalent than lymphosarcoma in cats, with osteosarcoma being the most common.107 Other vertebral tumors reported in cats include fibrosarcoma, undifferentiated sarcoma, and plasma cell tumors. The most common primary vertebral body tumor in dogs is also osteosarcoma.105,110 It may be difficult in some cases to ascertain whether a vertebral tumor is primary or metastatic. Other tumors may occur in the epidural space, without directly involving the vertebrae. Common among these are sarcomas—most frequently osteosarcoma and hemangiosarcoma. Lymphosarcoma can be primary or metastatic and is often located in the extradural space, particularly in cats. Meningioma and MNSTs usually are typically located intradurally but occasionally will exhibit an extradural pattern on imaging. Metastatic carcinomas (e.g., mammary carcinoma, prostatic carcinoma) may localize to the extradural space. A number of fatty tumors have been reported to affect the spinal cord in dogs, including lipoma, myelolipoma, infiltrative lipoma, and liposarcoma. These all generally occur in an extradural location.105,106,110,113-115
Meningiomas and MNSTs are the two most common neoplasms in the intradural/extramedullary location category, with meningiomas predominating. An uncommon blast cell tumor of young dogs called nephroblastoma also typically displays an intradural/extramedullary pattern on spinal imaging.*
Intramedullary tumors are infrequently encountered and include primary spinal parenchymal tumors (e.g., astrocytoma, oligodendroglioma, ependymoma) and intramedullary metastases. The most common intramedullary metastases in dogs are thought to be hemangiosarcoma and lymphosarcoma.105,106 In one study, hemangiosarcoma was the most common tumor type in the intramedullary category.110
History and Clinical Signs
Spinal tumors typically cause progressive signs of a myelopathy, but acute or subacute development of spinal cord dysfunction often occurs, especially with feline lymphosarcoma and intramedullary neoplasms. Rapid onset of clinical signs may be due to factors such as pathologic fracture of a cancerous vertebra, acute hemorrhage or necrosis of a tumor, or rapid growth of a neoplasm with subsequent damage to spinal cord parenchyma (more likely with intramedullary tumors). Spinal cord tumors are typically solitary and can occur anywhere along the length of the spine. As with brain tumors, the specific neurologic deficits associated with a spinal tumor will depend on the specific neuroanatomic location of that tumor.
A prominent feature of extradural and intradural/extramedullary spinal neoplasia is spinal hyperesthesia, which often precedes the onset of proprioceptive and voluntary motor deficits. Spinal hyperesthesia is often not a prominent early clinical feature in patients with intramedullary spinal tumors, probably due to the lack of meningeal involvement. In MNSTs of the cervical intumescence, a history of unilateral thoracic limb lameness (on the side of the tumor) preceding the development of clinical signs of myelopathy is common.6,105,106
Diagnostic Techniques and Work-Up
The minimum database for a patient with a suspected spinal neoplasm is similar to that for a patient with a suspected brain tumor. Blood work (CBC and chemistry profile) and urinalysis should be performed, and survey radiographs should be procured to help rule out metastatic disease or a secondary neoplastic process.
Imaging
Survey spinal radiographs are generally recommended to identify the presence or absence of any obvious bony lesions (e.g., bone lysis and/or proliferation). In the majority of soft tissue spinal neoplasms, plain radiographs of the spine are normal. In some cases, however, the expanding neoplasm leads to expansion of either the vertebral canal or the intervertebral foramen, with or without obvious thinning of the surrounding bone. As with brain tumors, the preferred imaging modality for investigation of suspected spinal neoplasia is MRI (Figure 30-9). CT, alone or in combination with myelography, can also be used to diagnose spinal cord tumors but is considered to be inferior to MRI in most cases. Lesions that are difficult to distinguish as being either intradural/extramedullary or intramedullary on myelographic images may be better delineated on MRI or CT/myelography; however, some spinal tumors have enough associated spinal cord swelling that this distinction may be difficult to make, even on MR images. Also, intradural/extramedullary spinal tumors will occasionally infiltrate the spinal cord parenchyma (mixed compartment mass), which may contribute to the development of an intramedullary imaging pattern (Figure 30-10).105,106,110
Cerebrospinal Fluid Analysis
CSF analysis is often pursued in combination with spinal imaging. As with brain tumors, CSF analysis is likely to be abnormal but usually does not provide specific information about the disease process. With the possible exception of spinal lymphosarcoma, CSF evaluation rarely reveals neoplastic cells and may reveal increased protein levels, with or without elevated cell counts (more likely with tumors with meningeal involvement).6,105,106
Therapy
Therapeutic options for spinal cord tumors are similar to those available for brain tumor management. Surgical removal may be advisable for those neoplasms that have not infiltrated spinal cord parenchyma.
Meningioma is reported to be the most common intraspinal tumor in dogs and can be treated effectively with surgery with or without postoperative external-beam RT.109,116-118 RT results in an improved outcome and can contribute to prevention of or delay in local recurrence.109,116 With surgery alone for intraspinal meningioma, the mean survival time has been reported to be 19 months for 8 of 10 dogs that had evidence of postoperative recurrence.109 In a report of the results of surgery in nine dogs with spinal meningioma, five were alive longer than 6 months postoperatively.119 In two dogs treated surgically for spinal meningiomas, the survival time was 1410 and 1440 days.118 In seven dogs that underwent a combination of surgery and postoperative RT, survival times ranged from 18 to 78 months.109 Six dogs that had postoperative RT for spinal meningioma had a MST of 13.5 months (range 8 to 25 months).116 One dog that had recurrence of an intraspinal meningioma 15 months postoperatively was irradiated with almost complete resolution of neurologic deficits and was ultimately euthanized for recurrence 19 months after RT.117 As with RT for intracranial tumors, the trend has been to reduce the dose per fraction and increase the total dose. The goal is to maximize the dose of radiation that can be delivered while minimizing the risk of radiation myelopathy. Also, there is a dose-volume relationship, and the length of the spinal cord irradiated has to be considered when prescribing the dose.120
Dogs with spinal nephroblastomas can be treated effectively with a combination of surgery and RT.111,112,121 The results reported for surgery alone are widely variable with MSTs of 70.5 days and 380.5 days.111,112,122 It should be noted that since this is a tumor that develops in young dogs, RT carries the risk for the development of radiation-induced tumors.121 There is one report of possible intraspinal metastasis of a canine spinal cord nephroblastoma, which alternatively may have represented multifocal disease.123
A report of two dogs with intramedullary spinal cord hemangioma included information on one dog treated with lomustine (no specific details); this dog was euthanized 15 months after the initial diagnosis.124
Three dogs treated for spinal lymphoma had a MST of 560 days (range = 560 to 1030 days).118 Two of the dogs were treated with RT and chemotherapy in addition to surgery.
There is limited information on results of treatment of spinal meningiomas in cats.107,125 In one report, a cat that underwent decompressive surgery for a spinal meningioma was doing well but was lost to follow-up 7 months postoperatively.107 The MST for 16 cats that underwent surgery for spinal meningiomas was 518 days.125
There are reports on spinal lymphoma in cats, although there is limited information on the results of treatment.107,126,127 Lymphoma is the most common tumor affecting the spinal cord in cats and often affects multiple regions of the spinal cord and brain.107,128 Treatment options include surgery, RT, and/or chemotherapy.
Spinal nerve sheath tumors treated surgically in seven dogs (with one dog also treated with radiation and chemotherapy) had a MST of 180 days (range of 21 to 300 days).118 One dog irradiated postoperatively for a spinal nerve sheath tumor was alive but lost to follow-up at 25 months.116 Spinal nerve sheath tumors in three cats treated surgically resulted in survival times of 67 days (failed to improve), 112 days, and 275 days (both had local recurrence).125
Outcomes for other types of spinal cord tumors in cats are relatively unknown because of the limited number of cases, the tendency to euthanize due to a presumed poor prognosis, or a poor neurologic condition. A tetraparetic cat with a spinal cord glioma at cervical spinal cord segments C3-C4 was euthanized at the time of diagnosis.129
Prognosis
The majority of dogs (17/21, 81%) with intraspinal meningiomas improve neurologically postoperatively.109 Some reports provide histopathologic grade for meningiomas, although this is not done consistently.109,116,130 Based on relatively small numbers of dogs, there does not appear to be a correlation between grade and long-term outcome.109 Perioperative complications are more commonly encountered for lesions in the cranial cervical region. It should be noted that outcome in dogs that decline neurologically after RT may represent either local tumor recurrence or late radiation damage to the spinal cord, and without necropsy examination it is difficult to discern the cause of neurologic impairment.116 Nine dogs (meningioma in six dogs, and one each nerve sheath tumor, ependymoma, and neuroepithelioma) treated postoperatively with RT had MSTs of 17 months (range 6.5 to 70 months).116 There is overall limited information on the results of treatment of spinal cord tumors in dogs or cats.
In cats with lymphoma of the spinal cord, it is important to note that in one study of 26 cats that had necropsy examination, lymphoma was identified in extraneural locations in 22 (84.6%), with the most common locations bone marrow, kidneys, liver, skeletal muscle, and spleen.107 This is important in considering treatment options, as well as prognosis, in cats with spinal lymphoma. Six cats treated with a combination of vincristine, cyclophosphamide, and prednisone had a complete remission rate of 50%; median duration of remission was 14 weeks.127 Another cat treated with decompressive surgery in combination with chemotherapy had a remission of 62 weeks.127 In another report of four cats with spinal lymphoma, three were treated with l-asparaginase, vincristine, and prednisone after RT and one cat had surgery; three cats were euthanized or died within 20 weeks and one cat was alive at 13 months.126
There are limited reports on the outcome of surgery in cats with spinal meningioma.125,131 Of five cats with spinal cord meningioma, one cat was alive 1400 days postoperatively, and the MST for the other four cats was 180 days (range = 30 to 600 days).131 A MST of 426 days (range 211 to 842 days) was reported for 16 cats treated surgically for spinal cord meningioma.125 One cat with a spinal cord nerve sheath tumor treated surgically was alive 2190 days postoperatively.131
Comparative Aspects
In humans, primary spinal cord tumors are approximately 10 to 15 times less common than primary brain tumors, comprising 2% to 4% of all primary tumors of the CNS.132 Primary spinal tumors are typically intradural (intradural extramedullary and intramedullary); extradural spinal tumors in humans are most often metastatic.132-134 The most common intramedullary spinal tumors in people are ependymoma and low-grade astrocytoma. Ependymomas are typically readily resectable as are some astrocytomas. These tumors carry a fair-to-good prognosis, with surgical resection often combined with radiation and chemotherapy.132 The most common intradural extramedullary spinal tumors in people are meningiomas and nerve sheath tumors (schwannoma, neurofibroma, and malignant peripheral nerve sheath tumor [MPNST]); with the exception of MPNST, the prognosis for patients with these tumors is favorable with surgical removal.132
Extradural spinal metastases generally portend a poor prognosis in humans. In addition to histologic features of the specific tumor, neurologic status at the time of diagnosis influences the likelihood of treatment response. Treatment of metastatic extradural spinal metastases in people typically involves RT and chemotherapy, with or without surgical intervention.133,134
Peripheral Nerve Tumors
In general, peripheral nerve tumors (also called peripheral nerve sheath tumors [PNSTs]) are infrequently reported in dogs and rarely in cats. Peripheral nerve tumors arise in the cranial nerves, spinal nerve roots, and peripheral nerves. These tumors occur most commonly in middle-aged to older dogs of medium and large breeds. The most common cranial nerve affected is the trigeminal (CN V), and the most common spinal nerve roots affected are in the region of the brachial plexus (C6-T2).106,135-137 Tumors of the thoracic and lumbar spinal nerve roots also occur with some frequency and tend to cause signs of spinal cord compression as an early clinical sign of disease. Secondary peripheral nerve tumors (lymphoma, malignant sarcomas, hamartomas) can occasionally involve peripheral nerves as well.106,135
Pathology
Peripheral nerve tumors occur mainly in the cranial, spinal, and associated peripheral nerves and less commonly in the autonomic nervous system (e.g., sympathetic nerves and ganglia). These tumors arise from Schwann cells, perineurial fibroblasts, or a combination of these two cell types.135-138 The traditional nomenclature for these neoplasms is confusing and of limited clinical use. These neoplasms have been classified as schwannomas, neurofibromas and neurofibrosarcomas. They have also been more broadly classified as benign peripheral nerve sheath tumors (BPNSTs) and malignant peripheral nerve sheath tumors (MPNSTs).135-138 This latter terminology is more useful from a clinical standpoint, especially considering that the majority of reported PNSTs in dogs are histologically and biologically aggressive masses.138,139 PNSTs in cats are uncommon, but the proportion of benign tumor types may be higher than that reported for dogs.140
History and Clinical Signs
As with brain and spinal tumors, the clinical signs of peripheral nerve tumors reflect the anatomic location of the tumor. The majority of reported canine peripheral nerve tumors involve the nerve roots and/or nerves of the brachial plexus region; in such cases, the typical scenario is a chronic progressive unilateral thoracic limb lameness that often eludes diagnosis for months. These dogs are often evaluated for possible orthopedic conditions prior to evaluation for a potential peripheral nerve tumor. A palpable mass may be found in the axillary region in some of these dogs (37% in one report),137 and pain is usually easily elicited when palpating this region on the lame side. If the mass invades the vertebral canal and causes spinal cord compression, clinical signs of an asymmetric C6-T2 myelopathy may be apparent.106,135-138 Less commonly, a peripheral nerve tumor will involve the thoracolumbar region of the spine; in these cases, a more rapidly progressive development of a T3-L3 myelopathy is common.106,135 Peripheral nerve tumors also will occasionally affect nerve roots of the cauda equina, leading to clinical signs of progressive unilateral pelvic limb lameness (similar to the situation described for brachial plexus tumors).106,135
As mentioned previously, the cranial nerve typically affected by peripheral nerve tumor is the trigeminal nerve (CN V). Dogs afflicted by CN V nerve sheath tumors typically develop unilateral atrophy of the muscles of mastication (e.g., temporalis and masseter muscles) over weeks to months. Other clinical features associated with CN V involvement may include decreased-to-absent facial sensation and Horner’s syndrome. If the intracranial portion of CN V becomes compressive, clinical signs of brainstem compression (e.g., hemiparesis, dysphagia) may develop.135,141,142
Diagnostic Techniques and Work-Up
Peripheral nerve tumors, especially those involving the brachial plexus region, can be challenging to diagnose. There should be a high index of suspicion for dogs with chronic thoracic limb lameness for which an obvious musculoskeletal cause cannot be found. The most useful imaging tool for diagnosing peripheral nerve tumors is MRI, whether the tumor involves a plexus, the spinal cord, or a cranial nerve. It is important in cases of brachial plexus nerve sheath tumors to image the axillary region, as well as the cervical spinal cord. Small brachial plexus tumors may be difficult to impossible to see on MR images; in some cases, subtle asymmetry in the suspected abnormal region is appreciated without an obvious mass being apparent.106,135,141-143 Some dogs may have axillary masses that are visible via ultrasonography, in which case a needle aspirate may be obtained.144,145 In the authors’ opinion, CT is a poor second choice for imaging patients with suspected nerve sheath tumors. Myelography is unlikely to provide adequate information in such cases and should be avoided if possible. Electrodiagnostics may be useful in cases of suspected nerve sheath tumor. Abnormal electromyographic (EMG) findings may distinguish atrophied muscles from a primary neurogenic or myopathic disorder from atrophy due to musculoskeletal disease. Nerve conduction studies performed on peripheral nerves of limbs may also indicate abnormalities. In cases for which a peripheral nerve tumor is highly suspected but diagnostic tests are all negative or equivocal, exploratory surgery (e.g., brachial plexus exploratory) may be an option.106,135,146
Therapy
Surgery is the reported approach for the treatment of PNSTs in dogs and cats, although complete resection is difficult to achieve.137,140,141,147 Amputation may be necessary alone or in conjunction with laminectomy to accomplish tumor resection as high up as possible for nerve root tumors.137,147 Adjunctive RT is a postoperative option for residual microscopic disease, but there are only anecdotal reports regarding the success of treatment. Historically, dogs have been reportedly euthanized intraoperatively due to the recognition of the extent of disease during surgical exploration.147 Preoperative cross-sectional imaging is recommended to define the extent of disease and for preplanning, particularly when considering combination therapy. Chemotherapy and/or RT is an option for lymphoma involving peripheral nerves.
Prognosis
The prognosis for PNSTs in dogs depends in part on tumor location and whether or not the tumor is amenable to surgery (amputation, laminectomy, or combination), with very limited information available on the results of adjunctive therapy.137,141,147,148 The prognosis for dogs treated surgically has been reported to be guarded to poor, although earlier detection after the onset of clinical signs may improve outcome.137 Long-term survival has been reported (>18 months, >27 months, >42 months).141,147,148 Of seven dogs with trigeminal nerve sheath tumors that were not treated, the survival time was up to 21 months after onset of clinical signs, although several died or were euthanized at the time of presentation.141 Postoperative RT is feasible, but there is limited information on results of treatment. There is no information available on the use of chemotherapy for the treatment of PNSTs. Failure is typically due to neurologic signs associated with local recurrence. Although usually solitary, multicentric disease involving multiple cranial nerve roots has been reported.149 Additionally, metastasis of PNSTs can occur but is relatively rare.141The prognosis for cats is more favorable than what has been reported for dogs. In a report of 53 cats with 59 PNSTs, follow-up information was available for 45 cats that had surgical excision and/or amputation.140 Of the 45 cats, 9 (20%) had local recurrence with histologically malignant tumors more likely to recur than histologically benign tumors, and 3 recurred locally twice. The median follow-up period was 21 months; 13 cats were euthanized at 2 weeks to 52 months (median 21.5 months; mean 22.6 months) after surgery.140 Although PNSTs in cats can recur postoperatively, metastasis has not been documented to occur; euthanasia when disease related is due to local disease.140
Comparative Aspects
People are affected by peripheral nerve tumors of the same cellular origins as those that affect dogs. However, the vast majority of peripheral nerve tumors in humans are biologically benign (most are schwannomas and neurofibromas). These tumors are usually readily excised and rarely recur.150,151 In comparison, most peripheral nerve tumors in dogs are aggressive and tend to recur following surgical excision.135-138
References
1. Vandevelde, M, Brain tumors in domestic animals: an overview. Research Triangle Park, NC. Proceedings of the Conference on Brain Tumors in Man and Animals September 5-6, 1984.
2. Troxel, MT, Vite, CH, Van Winkle, TJ, et al. Feline intracranial meningioma: a retrospective review of 160 cases (1985-2001). J Vet Intern Med. 2003;17:850–859.
3. Koestner, A, Bilzer, T, Fatzer, R, et al. Histological classification of tumors of the nervous system of domestic animals. Washington, DC: Armed Forces Institute of Pathology; 1999.
4. Summers, BA, Cummings, JF, deLahunta, A. Tumors of the central nervous system. In: Summers BA, Cummings JF, deLahunta A, eds. Veterinary neuropathology. St Louis: Mosby, 1995.
5. Dewey, CW. Encephalopathies: disorders of the brain. In Dewey CW, ed.: A practical guide to canine and feline neurology, ed 2, Ames, Iowa: Wiley-Blackwell, 2008.
6. Adamo, PF, Forrest, L, Dubielzig, R. Canine and feline meningiomas: diagnosis, treatment and prognosis. Compend Contin Educ Pract Vet. 2004;27:951–966.
7. Snyder, JM, Shofer, FS, Van Winkle, TJ, et al. Canine intracranial primary neoplasia: 173 cases (1986-2003). J Vet Intern Med. 2006;20:669–675.
8. Nafe, LA. Meningiomas in cats: a retrospective clinical study of 36 cases. J Am Vet Med Assoc. 1979;174:1224–1227.
9. Gordon, LE, Thacher, C, Matthiesen, DT, et al. Results of craniotomy for the treatment of cerebral meningioma in 42 cats. Vet Surg. 1994;23:94–100.
10. Snyder, JM, Lipitz, L, Skorupski, KA, et al. Secondary intracranial neoplasia in the dog: 177 cases (1986-2003). J Vet Intern Med. 2008;22:172–177.
11. Sturges, BK, Dickinson, PJ, Bollen, AW, et al. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J Vet Intern Med. 2008;22:586–595.
12. Lipsitz, D, Higgins, RJ, Kortz, GD, et al. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol. 2003;40:659–669.
13. Westworth, DR, Dickinson, PJ, Vernau, W, et al. Choroid plexus tumors in 56 dogs (1985-2007). J Vet Intern Med. 2008;22:1157–1165.
14. Thankey, K, Faissler, A, Kavirayani, A, et al. Clinical presentation and outcome in dogs with histologically confirmed choroid plexus papillomas. J Vet Intern Med. 2006;20:782–783. [(abstract)].
15. Bagley, RS, Gavin, PR, Moore, MP, et al. Clinical signs associated with brain tumors in dogs: 97 cases (1992-1997). J Am Vet Med Assoc. 1999;215:818–819.
16. Tomek, A, Cizinauskas, S, Doherr, M, et al. Intracranial neoplasia in 61 cats: localization, tumour types and seizure patterns. J Feline Med Surg. 2006;8:243–253.
17. Rodenas, S, Pumarola, M, Gaitero, L, et al. Magnetic resonance imaging findings in 40 dogs with histologically confirmed intracranial tumours. Vet J. 2011;187:85–91.
18. Dernell, WS, Straw, RC, Cooper, MF, et al. Multilobular osteochondrosarcoma in 39 dogs: 1979-1993. J Am Anim Hosp Assoc. 1998;34:11–18.
19. Evans, SM, Dayrell-Hart, B, Powlis, W, et al. Radiation therapy of canine brain masses. J Vet Intern Med. 1993;7:216–219.
20. Lester, NV, Hopkins, AL, Bova, FJ, et al. Radiosurgery using a stereotactic headframe system for irradiation of brain tumors in dogs. J Am Vet Med Assoc. 2001;219:1562–1567.
21. Jeffrey, N, Brearley, MJ. Brain tumours in the dog: treatment of 10 cases and review of recent literature. J Small Anim Pract. 1993;34:367–372.
22. Brearley, MJ, Jeffery, ND, Phillips, SM, et al. Hypofractionated radiation therapy of brain masses in dogs: A retrospective analysis of survival of 83 cases (1991-1996). J Vet Intern Med. 1999;13:408–412.
23. Turrel, JM, Fike, JR, LeCouteur, RA, et al. Radiotherapy of brain tumors in dogs. J Am Vet Med Assoc. 1984;184:82–86.
24. Bley, CR, Sumova, A, Roos, M, et al. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med. 2005;19:849–854.
25. Schultheiss, TE, Kun, LE, Ang, KK, et al. Radiation response of the central nervous system. Int J Radiation Oncology Biol Phys. 1995;31:1093–1112.
26. Heidner, GL, Kornegay, JN, Page, RL, et al. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J Vet Intern Med. 1991;5:219–226.
27. Spugnini, EP, Thrall, DE, Price, GS, et al. Primary irradiation of canine intracranial masses. Vet Radiol Ultrasound. 2000;41:377–380.
28. Axlund, TW, McGlasson, ML, Smith, AN. Surgery alone or in combination with radiation therapy for treatment of intracranial meningiomas in dogs: 31 cases (1989-2002). J Am Vet Med Assoc. 2002;221:1597–1600.
29. Théon, AP, LeCouteur, RA, Carr, EA, et al. Influence of tumor cell proliferation and sex-hormone receptors on effectiveness of radiation therapy for dogs with incompletely resected meningiomas. J Am Vet Med Assoc. 2000;216:701–707.
30. Bergman, R, Jones, J, Lanz, O, et al. Post-operative computed tomography in two dogs with cerebral meningioma. Vet Radiol Ultrasound. 2000;41:425–432.
31. Klopp, LS, Rao, S. Endoscopic-assisted intracranial tumor removal in dogs and cats: long-term outcome of 39 cases. J Vet Intern Med. 2009;23:108–115.
32. O’Brien, CS, Bagley, RS, Hicks, DG, et al. Gamma-irradiated calvarium allograft cranioplasty in a cat following brain tumor removal. J Am Anim Hosp Assoc. 2010;46:268–273.
33. Bordelon, JT, Rochat, MC. Use of a titanium mesh for cranioplasty following radical rostrotentorial craniectomy to remove an ossifying fibroma in a dog. J Am Vet Med Assoc. 2007;231:1692–1695.
34. Gordon, PN, Kornegay, JN, Lattimer, JC, et al. Use of a rivet-like titanium clamp closure system to replace an external frontal bone flap after transfrontal craniotomy in a dog. J Am Vet Med Assoc. 2005;226:752–755.
35. Bryant, KJ, Steinberg, H, McAnulty, JF. Cranioplasty by means of molded polymethylmethacrylate prosthetic reconstruction after radical excision of neoplasms of the skull in two dogs. J Am Vet Med Assoc. 2003;223:67–72.
36. Son, SH, Kang, YN, Ryu, MR. The effect of metallic implants on radiation therapy in spinal tumor patients with metallic spinal implants. Med Dosim. 2011;37(1):98–107.
37. Pekmezci, M, Dirican, B, Yapici, B, et al. Spinal implants and radiation therapy: the effect of various configurations of titanium implant systems in a single-level vertebral metastasis model. J Bone Joint Surg. 2006;88:1093–1100.
38. Heading, KL, Brockley, LK, Bennett, PF. CCNU (lomustine) toxicity in dogs: a retrospective study (2002-07). Aust Vet J. 2011;89:109–116.
39. Fulton, LM, Steinberg, HS. Preliminary study of lomustine in the treatment of intracranial masses in dogs following localization by imaging techniques. Sem Vet Med Surg (Sm Anim). 1990;5:241–245.
40. Dimski, DS, Cook, JR. Carmustine-induced partial remission of an astrocytoma in a dog. J Am Anim Hosp Assoc. 1990;26:179–182.
41. Tamura, S, Tamura, Y, Ohoka, A, et al. A canine case of skull base meningioma treated with hydroxyurea. J Vet Med Sci. 2007;69:1313–1315.
42. Jung, D, Kim, H, Park, C, et al. Long-term chemotherapy with lomustine of intracranial meningioma occurring in a miniature schnauzer. J Vet Med Sci. 2006;68:383–386.
43. McDonnell, JJ, Kalbko, K, Keating, JH, et al. Multiple meningiomas in three dogs. J Am Anim Hosp Assoc. 2007;43:201–208.
44. Greco, JJ, Aiken, SA, Berg, JM, et al. Evaluation of intracranial meningioma resection with a surgical aspirator in dogs: 17 cases (1996-2004). J Am Vet Med Assoc. 2006;229:394–400.
45. Cautela, MA, Dewey, CW, Cerda-Gonzalez, S, et al. Oral hydroxyurea therapy for dogs with suspected intracranial meningioma: a retrospective cohort study (2004-2009). J Vet Intern Med. 2009;23:737.
46. Thrall, DE, LaRue, SM, Powers, BE, et al. Use of whole body hyperthermia as a method to heat inaccessible tumours uniformly: a phase III trial in canine brain masses. Int J Hyperthermia. 1999;15:383–398.
47. Chauvet, AE, Kesava, PP, Goh, CS, et al. Selective intraarterial gene delivery into a canine meningioma. J Neurosurg. 1998;88:870–873.
48. LeCouteur, RA. Current concepts in the diagnosis and treatment of brain tumors in dogs and cats. J Small Anim Pract. 1999;40:411–416.
49. Candolfi, M, Pluhar, GE, Kroeger, K, et al. Optimization of adenoviral vector-mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro. Neuro Oncol. 2007;9:245–258.
50. Oh, S, Pluhar, GE, McNeil, EA, et al. Efficacy of nonviral gene transfer in the canine brain. J Neurosurg. 2007;107:136–144.
51. Dickinson, PJ, LeCouteur, RA, Higgins, RJ, et al. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol. 2010;12:928–940.
52. Dickinson, PJ, LeCouteur, RA, Higgins, RJ, et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging. J Neurosurg. 2008;108:989–998.
53. Packer, RA, Freeman, LJ, Miller, MA, et al. Evaluation of minimally invasive excisional brain biopsy and intracranial brachytherapy catheter placement in dogs. Am J Vet Res. 2011;72:109–121.
54. Platt, SR, Scase, TJ, Adams, V, et al. Vascular endothelial growth factor expression in canine intracranial meningiomas and association with patient survival. J Vet Intern Med. 2006;20:663–668.
55. Platt, SR. Angiogenesis and cerebral neoplasia. Vet Comp Oncol. 2005;3:123–138.
56. MacLeod, AG, Dickinson, PJ, LeCouteur, RA, et al. Quantitative assessment of blood volume and permeability in cerebral mass lesions using dynamic contrast-enhanced computed tomography in the dog. Acad Radiol. 2009;16:1187–1195.
57. Wisner, ER, Dickinson, PJ, Higgins, RJ. Magnetic resonance imaging features of canine intracranial neoplasia. Vet Radiol Ultrasound. 2011;52:S52–S61.
58. Singh, JB, Oevermann, A, Lang, J, et al. Contrast media enhancement of intracranial lesions in magnetic resonance imaging does not reflect histopathologic findings consistently. Vet Radiol Ultrasound. 2011;52:619–626.
59. Vernau, KM, Higgins, RJ, Bollen, AW, et al. Primary canine and feline nervous system tumors: intraoperative diagnosis using the smear technique. Vet Pathol. 2001;38:47–57.
60. Koblik, PD, LeCouteur, RA, Higgins, RJ, et al. CT-guided brain biopsy using a modified Pelorus mark III stereotactic system: experience with 50 dogs. Vet Radiol Ultrasound. 1999;40:434–440.
61. Koblik, PD, LeCouteur, RA, Higgins, RJ, et al. Modification and application of a Pelorus mark II stereotactic system for CT-guided brain biopsy in 50 dogs. Vet Radiol Ultrasound. 1999;40:424–433.
62. Moissonnier, P, Blot, S, Devauchelle, P, et al. Stereotactic CT-guided brain biopsy in the dog. J Small Anim Pract. 2002;43:115–123.
63. De Lorenzi, D, Mandara, MT, Tranquillo, M, et al. Squash-prep cytology in the diagnosis of canine and feline nervous system lesions: a study of 42 cases. Vet Clin Pathol. 2006;35:208–214.
64. Klopp, LS, Ridgway, M. Use of an endoscope in minimally invasive lesion biopsy and removal within the skull and cranial vault in two dogs and one cat. J Am Vet Med Assoc. 2009;234:1573–1577.
65. Fransson, BA, Bagley, RS, Gay, JM, et al. Pneumonia after intracranial surgery in dogs. Vet Surg. 2001;30:432–439.
66. Matiasek, LA, Platt, SR, Adams, V, et al. Ki-67 and vascular endothelial growth factor expression in intracranial meningiomas in dogs. J Vet Intern Med. 2009;23:146–151.
67. Adamo, PF, Cantile, C, Steinberg, H. Evaluation of progesterone and estrogen receptor expression in 15 meningiomas of dogs and cats. Am J Vet Res. 2003;64:1310–1318.
68. Rossmeisl, JH, Robertson, JL, Zimmerman, KL, et al. Cyclooxygenase-2 (COX-2) expression in canine intracranial meningiomas. Vet Comp Oncol. 2009;7:173–180.
69. Jankovsky, JM, Newkirk, KM, Ilha, MR, et al. COX-2 and c-kit expression in canine gliomas. Vet Comp Oncol. 23 Nov 2011. [Epub].
70. Stoica, G, Levine, J, Wolff, J, et al. Canine astrocytic tumors: a comparative review. Vet Pathol. 2011;48:266–275.
71. Frenier, SL, Kraft, SL, Moore, MP, et al. Canine intracranial astrocytomas and comparison with the human counterpart. Comp Cont Educ Small Anim. 1990;12:1422–1433.
72. Chandra, AM, Ginn, PE. Primary malignant histiocytosis of the brain in a dog. J Comp Pathol. 1999;121:77–82.
73. Uchida, K, Morozumi, M, Yamaguchi, R, et al. Diffuse leptomeningeal malignant histiocytosis in the brain and spinal cord of a Tibetan terrier. Vet Pathol. 2001;38:219–222.
74. Zimmerman, K, Almy, F, Carter, L, et al. Cerebrospinal fluid from a 10-year-old dog with a single seizure episode. Vet Clin Pathol. 2006;35:127–131.
75. Ide, T, Uchida, K, Kagawa, Y, et al. Pathological and immunohistochemical features of subdural histiocytic sarcomas in 15 dogs. J Vet Diagn Invest. 2011;23:127–132.
76. Tzipory, L, Vernau, KM, Sturges, BK, et al. Antemortem diagnosis of localized central nervous system histiocytic sarcoma in 2 dogs. J Vet Intern Med. 2009;23:369–374.
77. Courtay-Cahen, C, Platt, SR, De Risio, L, et al. Preliminary analysis of genomic abnormalities in canine meningiomas. Vet Comp Oncol. 2008;6:182–192.
78. Dickinson, PJ, Roberts, BN, Higgins, RJ, et al. Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRα and c-Met in canine primary brain tumours. Vet Comp Oncol. 2006;4:132–140.
79. Dickinson, PJ, Surace, EI, Campbell, M, et al. Expression of the tumor suppressor genes NF2, 4.1B, and TSLC1 in canine meningiomas. Vet Pathol. 2009;46:884–892.
80. Dickinson, PJ, Sturges, BK, Higgins, RJ, et al. Vascular endothelial growth factor mRNA expression and peritumoral edema in canine primary central nervous system tumors. Vet Pathol. 2008;45:131–139.
81. Higgins, RJ, Dickinson, PJ, LeCouteur, RA, et al. Spontaneous canine gliomas: overexpression of EGFR PDGFRα and IGFBP2 demonstrated b tissue microarray immunophenotyping. J Neurooncol. 2010;98:49–55.
82. Thomson, SAM, Kennerly, E, Olby, N, et al. Microarray analysis of differentially expressed genes of primary tumors in the canine central nervous system. Vet Pathol. 2005;42:550–558.
83. York, D, Higgins, RJ, LeCouteur, RA, et al. TP53 mutations in canine brain tumors. Vet Pathol. 2012 March 12. [Epub].
84. Chamberlain, MC, Barnholtz-Sloan, JS. Medical treatment of recurrent meningiomas. Expert Rev Neurother. 2011;11:1425–1432.
85. Stacy, BA, Stevenson, TL, Lipsitz, D, et al. Simultaneously occurring oligodendroglioma and meningioma in a dog. J Vet Intern Med. 2003;17:357–359.
86. Lobetti, RG, Nesbit, JW, Miller, DB. Multiple malignant meningiomas in a young cat. J S Afr Vet Assoc. 1997;68:62–65.
87. Patnaik, AK, Kay, WJ, Hurvitz, AI. Intracranial meningioma: a comparative pathologic study of 28 dogs. Vet Pathol. 1986;23:369–373.
88. Koch, MW, Sánchez, MD, Long, S. Multifocal oligodendroglioma in three dogs. J Am Anim Hosp Assoc. 2011;47:77–85.
89. Alves, A, Prada, J, Almeida, JM, et al. Primary and secondary tumours occurring simultaneously in the brain of a dog. J Small Anim Pract. 2006;47:607–610.
90. Schulman, FY, Ribas, JL, Carpenter, JL, et al. Intracranial meningioma with pulmonary metastasis in three dogs. Vet Pathol. 1992;29:196–202.
91. Forterre, F, Tomek, A, Konar, M, et al. Multiple meningiomas: clinical, radiological, surgical and pathological findings with outcome in four cats. J Feline Med Surg. 2007;9:36–43.
92. Zaki, FA, Hurvitz, AI. Spontaneous neoplasms of the central nervous system of the cat. J Small Anim Pract. 1976;17:773–782.
93. Waters, DJ, Hayden, DW, Walter, PA. Intracranial lesions in dogs with hemangiosarcoma. J Vet Intern Med. 1989;3:222–230.
94. Mandara, MT, Rossi, F, Lepri, E, et al. Cerebellar leptomeningeal carcinomatosis in a dog. J Small Anim Pract. 2007;48:504–507.
95. Mateo, I, Lorenzo, V, Munoz, A, et al. Meningeal carcinomatosis in a dog: magnetic resonance imaging features and pathological correlation. J Small Anim Pract. 2010;51:43–48.
96. Patnaik, AK, Erlandson, RA, Lieberman, PH, et al. Choroid plexus carcinoma with meningeal carcinomatosis in a dog. Vet Pathol. 1980;17:381–385.
97. Gallagher, JG, Berg, J, Knowles, KE, et al. Prognosis after surgical excision of cerebral meningiomas in cats: 17 cases (1986-1992). J Am Vet Med Assoc. 1993;203:1437–1440.
98. Lawson, DC, Burk, RL, Prata, RG. Cerebral meningioma in the cat: diagnosis and surgical treatment of ten cases. J Am Anim Hosp Assoc. 1984;20:333–342.
99. Dickinson, PJ, Keel, MK, Higgins, RJ, et al. Clinical and pathologic features of oligodendrogliomas in two cats. Vet Pathol. 2000;37:160–167.
100. Sarfaty, D, Carrillo, JM, Patnaik, AK. Cerebral astrocytoma in four cats: clinical and pathological findings. J Am Vet Med Assoc. 1987;191:976–978.
101. Ostrum, QT, Barnholtz-Sloan, JS. Current state of our knowledge on brain tumor epidemiology. Curr Neurol Neurosci Rep. 2011;11:329–335.
102. Preusser, M, deRibaupierre, S, Wohrer, A, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70:9–21.
103. Mawrin, C, Perry, A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99:379–391.
104. Wen, PY, Quant, E, Drappatz, J, et al. Medical therapies for meningiomas. J Neurooncol. 2010;99:365–378.
105. Dewey, CW. Myelopathies: disorders of the spinal cord. In Dewey CW, ed.: A practical guide to canine and feline neurology, ed 2, Ames, Iowa: Wiley-Blackwell, 2008.
106. Bagley, RS. Spinal neoplasms in small animals. Vet Clin Small Anim. 2010;40:915–927.
107. Marioni-Henry, K, Van Winkle, TJ, Smith, SH, et al. Tumors affecting the spinal cord of cats: 85 cases (1980-2005). J Am Vet Med Assoc. 2008;232:237–243.
108. Marioni-Henry, K. Feline spinal cord diseases. Vet Clin Small Anim. 2010;40:1011–1028.
109. Petersen, SA, Sturges, BK, Dickinson, PJ, et al. Canine intraspinal meningiomas: imaging features, histopathologic classification, and long-term outcome in 34 dogs. J Vet Intern Med. 2008;22:946–953.
110. Johnson, KB, Manhart, K, Vite, C, et al. 399 spinal tumors in dogs (abstract). J Vet Intern Med. 2007;21:639–640.
111. Brewer, DM, Cerda-Gonzalez, S, Dewey, CW, et al. Spinal cord nephroblastoma in dogs: 11 cases (1985-2007). J Am Vet Med Assoc. 2011;238:618–624.
112. Liebel, FX, Rossmeisl, JH, Lanz, OI, et al. Canine spinal nephroblastoma: long-term outcomes associated with treatment of 10 cases (1996-2009). Vet Surg. 2011;40:244–252.
113. Ueno, H, Miyake, T, Kobayashi, Y, et al. Epidural spinal myelolipoma in a dog. J Am Anim Hosp Assoc. 2007;43:132–135.
114. Rodenas, S, Valin, I, Devauchelle, P, et al. Combined use of surgery and radiation in the treatment of an intradural myxoid liposarcoma in a dog. J Am Anim Hosp Assoc. 2006;42:386–391.
115. Morgan, LW, Toal, R, Siemering, G, et al. Imaging diagnosis-infiltrative lipoma causing spinal cord compression in a dog. Vet Rad Ultrasound. 2007;48:35–37.
116. Siegel, S, Kornegay, JN, Thrall, DE. Postoperative irradiation of spinal cord tumors in 9 dogs. Vet Radiol Ultrasound. 1996;37:150–153.
117. Bell, FW, Feeney, DA, O’Brien, TJ, et al. External beam radiation therapy for recurrent intraspinal meningioma in a dog. J Am Anim Hosp Assoc. 1992;28:318–322.
118. Levy, MS, Kapatkin, AS, Patnaik, AK, et al. Spinal tumors in 37 dogs: clinical outcome and long-term survival (1987-1994). J Am Anim Hosp Assoc. 1997;33:307–312.
119. Fingeroth, JM, Prata, RG, Patnaik, AK. Spinal meningiomas in dogs: 13 cases (1972-1987). J Am Vet Med Assoc. 1987;191:720–726.
120. Powers, BE, Beck, ER, Gillette, EL, et al. Pathology of radiation injury to the canine spinal cord. Int J Radiation Oncology Biol Phys. 1992;23:539–549.
121. Dickinson, PJ, McEntee, MC, Lipsitz, D, et al. Radiation induced vertebral osteosarcoma following treatment of an intradural extramedullary spinal cord tumor in a dog. Vet Radiol Ultrasound. 2001;42:463–470.
122. Macri, NP, Van Alstine, W, Coolman, RA. Canine spinal nephroblastoma. J Am Anim Hosp Assoc. 1997;33:302–306.
123. Terrell, SP, Platt, SR, Chrisman, CL, et al. Possible intraspinal metastasis of a canine spinal cord nephroblastoma. Vet Pathol. 2000;37:94–97.
124. Jull, P, Walmsley, GL, Benigni, L, et al. Imaging diagnosis – spinal cord hemangioma in two dogs. Vet Radiol Ultrasound. 2011;52:653–657.
125. Rossmeisl, JH, Lanz, OI, Waldron, DR, et al. Surgical cytoreduction for the treatment of non-lymphoid vertebral and spinal cord neoplasms in cats: retrospective evaluation of 26 cases (1990-2005). Vet Com Oncol. 2006;4:411–450.
126. Lane, SB, Kornegay, JN, Duncan, JR, et al. Feline spinal lymphosarcoma: a retrospective evaluation of 23 cats. J Vet Intern Med. 1994;8:99–104.
127. Spodnick, GJ, Berg, J, Moore, FM, et al. Spinal lymphoma in cats: 21 cases (1976-1989). J Am Vet Med Assoc. 1992;200:373–376.
128. Marioni-Henry, K, Vite, CH, Newton, AL, et al. Prevalence of disease of the spinal cord of cats. J Vet Intern Med. 2004;18:851–858.
129. Haynes, JS, Leininger, JR. A glioma in the spinal cord of a cat. Vet Pathol. 1982;19:713–715.
130. Barnhart, KF, Wojcieszyn, J, Storts, RW. Immunohistochemical staining patterns of canine meningiomas and correlation with published immunophenotypes. Vet Pathol. 2002;39:311–321.
131. Levy, MS, Mauldin, G, Kapatkin, AS, et al. Nonlymphoid vertebral canal tumors in cats: 11 cases (1987-1995). J Am Vet Med Assoc. 1997;210:663–664.
132. Chamberlain, MC, Tredway, TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep. 2011;11:320–328.
133. Taylor, JW, Schiff, D. Metastatic epidural spinal cord compression. Semin Neurol. 2010;30:245–253.
134. Quraishi, NA, Gokaslan, ZL, Boriani, S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg. 2010;92B:1054–1060.
135. Dewey, CW. Disorders of the peripheral nervous system: mononeuropathies and polyneuropathies. In Dewey CW, ed.: A practical guide to canine and feline neurology, ed 2, Ames, Iowa: Wiley-Blackwell, 2008.
136. Targett, MP, Dyce, J, Houlton, JEF. Tumours involving the nerve sheaths of the forelimb in dogs. J Small Anim Pract. 1993;34:221–225.
137. Brehm, DM, Vite, CH, Steinberg, HS, et al. A retrospective evaluation of 51 cases of peripheral nerve sheath tumors in the dog. J Am Anim Hosp Assoc. 1995;31:349–359.
138. Summers, BA, Cummings, JF, deLahunta, A. Diseases of the peripheral nervous system. In: Summers BA, Cummings JF, deLahunta A, eds. Veterinary neuropathology. St Louis: Mosby, 1995.
139. Chijiwa, K, Uchida, K, Tateyama, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet Pathol. 2004;41:307–318.
140. Schulman, FY, Johnson, TO, Facemire, PR, et al. Feline peripheral nerve sheath tumors: histologic, immunohistochemical, and clinicopathologic correlation (59 tumors in 53 cats). Vet Pathol. 2009;46:1166–1180.
141. Bagley, RS, Wheeler, SJ, Klopp, L, et al. Clinical features of trigeminal nerve-sheath tumor in 10 dogs. J Am Anim Hosp Assoc. 1998;34:19–25.
142. Schultz, RM, Tucker, RL, Gavin, PR, et al. Magnetic resonance imaging of acquired trigeminal nerve disorders in six dogs. Vet Rad Ultrasound. 2007;48:101–104.
143. Kraft, S, Ehrhart, EJ, Gall, D, et al. Magnetic resonance imaging characteristics of peripheral nerve sheath tumors of the canine brachial plexus in 18 dogs. Vet Rad Ultrasound. 2007;48:1–7.
144. Rose, S, Long, C, Knipe, M, et al. Ultrasonographic evaluation of brachial plexus tumors in five dogs. Vet Rad Ultrasound. 2005;46:514–517.
145. daCosta, RC, Parent, JM, Dobson, H, et al. Ultrasound-guided fine needle aspiration in the diagnosis of peripheral nerve sheath tumors in 4 dogs. Can Vet J. 2008;49:77–81.
146. Sharp, NJH. Craniolateral approach to the canine brachial plexus. Vet Surg. 1988;17:18–21.
147. Bradley, RL, Withrow, SJ, Snyder, SP. Nerve sheath tumors in the dog. J Am Anim Hosp Assoc. 1982;18:915–921.
148. Bailey, CS. Long-term survival after surgical excision of a schwannoma of the sixth cervical spinal nerve in a dog. J Am Vet Med Assoc. 1990;196:754–756.
149. Zachary, JF, O’Brien, DP, Ingles, BW, et al. Multicentric nerve sheath fibrosarcomas of multiple cranial nerve roots in two dogs. J Am Vet Med Assoc. 1986;188:723–726.
150. Woertler, K. Tumors and tumor-like lesions of peripheral nerves. Semin Musculoskel Radiol. 2010;14:547–558.