Ocular Tumors
Tumors of the eye, orbit, or adnexa can have devastating consequences for an animal’s vision, appearance, and comfort and may be harbingers of potentially life-threatening disease elsewhere in the body. By virtue of their location, even benign ocular tumors may cause blindness and loss of the eye. Although these tumors reportedly affected only 0.87% of all dogs and 0.34% of all cats recorded in the Veterinary Medical Data Base (VMDB) over a 10-year period, their actual frequency is undoubtedly greater because many presumably benign ocular tumors are not histologically examined. Additional insights into the relative frequency of ocular tumors can also be gained by reviewing submissions over several decades to the large ophthalmic pathology database compiled by the Comparative Ocular Pathology Laboratory of Wisconsin (COPLOW) (Figure 31-1). This chapter describes the more common ocular tumors in small animals and also uses the database of the COPLOW laboratory to estimate the relative frequency of various ocular tumors.
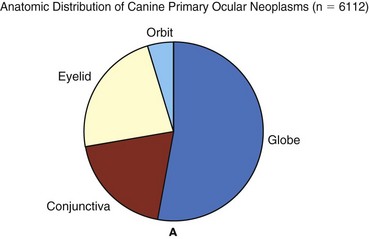
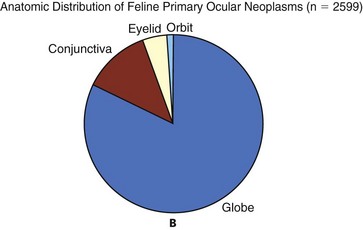
Figure 31-1 The distribution of canine (A) and feline (B) ocular tumors submitted to the Comparative Ocular Pathology Laboratory of Wisconsin.
Tumors of the Eyelids, Third Eyelid, Conjunctiva, and Ocular Surface
Benign adenomas and melanomas of the haired skin or eyelid margin make up 81% of eyelid tumors in the COPLOW database and tend to affect old dogs. One study suggests Boxers, collies, Weimaraners, cocker spaniels, and springer spaniels are at greater risk for eyelid neoplasia than the general hospital population,1 and another study suggests that beagles, Siberian huskies, and English setters are at greater risk than mixed-breed dogs.2 Canine juvenile histiocytomas affect the eyelid skin of young to middle-aged dogs1,2 but are relatively uncommon, comprising only 2% of eyelid biopsy samples submitted to COPLOW. Squamous cell carcinoma (SCC) comprises up to two-thirds of feline eyelid and third eyelid tumors and has a predilection for the lower eyelid and medial canthus of white cats.3 Ocular SCC is less frequent in dogs, but in both cats and dogs increased exposure to solar radiation, lack of adnexal pigmentation, and possibly chronic ocular surface irritation are believed to be predisposing factors (Figure 31-2).1,4,5
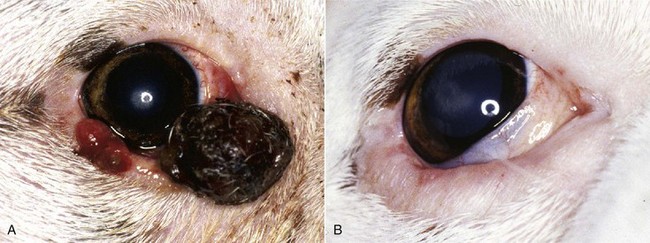
Figure 31-2 A, Squamous cell carcinoma affecting the lower eyelids of a Boxer. B, Same dog after cryosurgical ablation of the tumor. Lid function is spared.
Vascular endothelial cell tumors of the lateral limbus or the leading edge of the third eyelid constitute 27% of conjunctival tumors in dogs and tend to occur in the nonpigmented conjunctiva in Bassett hounds, springer spaniels and beagles. Melanomas often occur in dogs with a pigmented conjunctiva and are the most common malignant tumor of the canine conjunctiva, making up 17% of conjunctival tumor biopsies. Older (mean = 11 years), female, Weimaraner, and possibly German shepherd/large-breed dogs may be predisposed to conjunctival melanomas.6 Melanomas also have a propensity for the nictitating membrane and superior palpebral conjunctiva.6,7 Ocular viral papillomas compose about 3% of conjunctival tumors and tend to occur in young dogs and are believed to have a papillomavirus etiology (perhaps canine oral papillomavirus).8 Canine squamous papilloma is a benign papillary tumor of unknown etiology and makes up 9% of conjunctival tumor biopsies. Reactive papilloma is seen secondary to other conditions such as meibomian tumors, but they make up 18% of conjunctival tumor biopsies. Corneal tumors have a predilection for the limbus.
Pathology and Natural Behavior
Sebaceous or meibomian gland adenomas and epitheliomas, papillomas, and melanomas comprise more than 85% of canine eyelid and conjunctival neoplasms, and a substantial majority of these tumors are histologically benign.1,2 Even histologically malignant eyelid tumors in dogs rarely metastasize, although they are more likely to be locally invasive and recur following surgery.1,2 In contrast, most feline eyelid and ocular surface tumors such as SCC are malignant.3
Viral papillomas tend to be well demarcated and superficial, minimally altering deeper tissues. Surgical manipulation has occasionally been associated with dispersal of papillomas throughout the ocular surface.9,10 Papillomas, like histiocytomas, often spontaneously resolve in young dogs, although they may persist in the older dog. SCC may also develop superficially, but following malignant transformation the preinvasive actinic plaque can invade deeper tissues. SCC may spread to regional lymph nodes late in the course of the disease and uncommonly distantly metastasize. SCC of the third eyelid may more readily invade the orbit than corneal or eyelid SCC.
Adenocarcinomas of the gland of the third eyelid constitute 13% of conjunctival tumors in dogs. They are variable in morphology and often show moderate infiltrative growth.11 They may mimic prolapse of the gland of the nictitans (“cherry eye”) by appearing as localized, firm, smooth, pink swellings on the posterior surface of the nictitans, but a key differentiating feature is their occurrence in much older dogs (10 to 16 years). Although excision of the grossly visible tumor may initially appear adequate, recurrence is common if the entire gland is not removed, and metastasis, especially to the regional lymph nodes and orbit, is possible.11,12
The natural behavior of conjunctival vascular, melanocytic, and mast cell tumors is poorly understood, in part because they are uncommon. Conjunctival hemangiomas and hemangiosarcomas tend to remain relatively superficial but may recur following simple excision.13-17 Hemangiosarcomas may exhibit a more aggressive course and a primary ocular hemangiosarcoma must be differentiated from a metastatic lesion. However, metastasis of primary conjunctival vascular tumors, even when classified as hemangiosarcomas, appears to be rare.13-17
Feline conjunctival melanomas originate on the bulbar conjunctiva and invade the eyelid.18,19 Melanoma of the conjunctiva in dogs is most often morphologically malignant, but metastatic disease is not common. As in cats, canine conjunctival melanomas have been reported to recur locally following surgical excision in 55% of cases, and at least 17% of the dogs experienced orbital invasion or spread to the regional lymph nodes or lungs.6 Melanomas originating from the palpebral conjunctiva may have greater metastatic potential.6 Mitotic index, cell type, and degree of pigmentation are not useful predictors of malignancy for canine conjunctival melanomas.6 Subconjunctival mast cell tumors make up 4% of conjunctival tumors, but their natural history is poorly understood in part because they are uncommon. Nevertheless, they have been suggested to have a relatively benign course in dogs.20
History and Clinical Signs
Vascular tumors are often focal, raised, soft, red masses with visible feeder vessels arising from the surface of the conjunctiva or third eyelid.13-17 SCC of the eyelid, third eyelid, or ocular surface may appear as a focally thickened, roughened, and usually pink-to-red lesion in older animals or, more commonly, as an ulcerated lesion with a protracted course.3-5 In contrast, papillomas in young dogs appear verrucous and usually progress rapidly over weeks to a few months. Nonneoplastic conditions such as nodular granulomatous episcleritis can be mistaken for neoplasia.
In addition to a mass lesion, other clinical signs of eyelid or ocular surface tumors may include epiphora, conjunctival vascular injection, mucopurulent ocular discharge, protrusion of the third eyelid, conjunctival/corneal roughening or ulceration, and corneal neovascularization or pigmentation. Occasionally, palpebral conjunctival masses protrude only when their bulk no longer can be accommodated by the space between the eyelid and globe, and very advanced tumors may create exophthalmia or enophthalmia if the orbit is invaded. Large tumors and sebaceous adenomas often have a substantial inflammatory component and may be secondarily infected. Mesenchymal hamartoma appears to have a predisposition for the skin of lateral canthus.21
Diagnostic Techniques and Work-Up
In addition to fluorescein staining and examination of the ocular surface with a cobalt filter or black light, the extent of involvement of the bulbar and palpebral conjunctiva should be determined by everting the eyelid (and third eyelid if affected). Careful palpation of the lesion by inserting a lubricated finger in the conjunctival cul-de-sac can be invaluable for determining the full extent of the tumor and whether bony involvement has occurred. Nasolacrimal lavage and possibly positive contrast dacryocystorhinography may help characterize medial canthal masses. In general, small eyelid and ocular surface tumors are best diagnosed and treated by excisional biopsy. Fine-needle aspiration (FNA) or incisional biopsies of larger tumors aid in determining prognosis and planning definitive therapy. Occasionally, orbital ultrasound, skull radiographs, computed tomography (CT), magnetic resonance imaging (MRI), regional lymph node cytology, and thoracic radiographs are required to localize or clinically stage potentially malignant tumors such as SCC, mast cell tumors, adenocarcinomas of the third eyelid, and conjunctival melanomas.
Therapy
Specific therapy varies with the type of tumor; its location, size, and extent; whether the eye still has useful vision; the animal’s expected lifespan; the degree of discomfort the mass is creating; and the owner’s financial limitations. All eyelid tumors, whether benign or malignant, have the potential to affect vision or ocular comfort. Indications for tumor removal include any eyelid tumor in a cat, rapid growth, ocular surface irritation, impaired eyelid function, owner concern, or an unappealing appearance. In young dogs, observation of nonirritating papillomas or histiocytomas, even if quite large, may be appropriate as spontaneous regression is common.
Tumors involving less than one-fourth to one-third of the length of the eyelid are best treated by a V-plasty (wedge) or four-sided excision.22 The latter technique affords superior apposition of the eyelid margins and wound stability, especially in tumors approaching the one-fourth to one-third limit, because the initial incision is made perpendicular to the eyelid margin rather than obliquely. In general, only one-third to one-fourth of the eyelid in dogs and one-fourth of the eyelid in cats can be removed with these techniques. Antibiotic or antiinflammatory therapy may reduce the size of large tumors that are infected or inflamed so that a wedge or four-sided excision becomes possible. Electrosurgical excision should be avoided because it may result in substantial scarring of the eyelids. Carbon dioxide (CO2) laser ablation may be appropriate for some tumors.
Tumors greater than one-fourth to one-third of the eyelid typically require more advanced reconstructive blepharoplasty or utilization of other therapeutic modalities. Some tumors may be responsive to systemic chemotherapy (e.g., lymphoma, mast cell tumors), local infiltration with chemotherapeutic agents such as cisplatin (e.g., SCC),23 and/or local radiation therapy (RT; e.g., SCC). In some cases, these modalities will completely eliminate the tumor or shrink it to the point in which a less extensive surgical procedure can be performed. Reconstructive blepharoplasty, however, is the procedure of choice if surgical cure is a possibility and these other modalities have failed or are unlikely to substantially impact the tumor or if the nature of the tumor indicates extensive margins are required.
Cryosurgery is an attractive alternative to extensive blepharoplasty and has been reported to be effective in several canine eyelid tumor types (see Figure 31-2, see also Chapter 10).2,24 It is quick, less technically demanding than reconstructive blepharoplasty, and usually permits preservation of the nasolacrimal puncta and canaliculus. In many old or debilitated patients, cryosurgery can be accomplished with only sedation or local/topical anesthesia. Following pretreatment with dexamethasone (0.1 mg/kg intravenous [IV]), the mass is isolated with a chalazion forceps (if possible) and debulked flush with the lid margin. Using liquid nitrogen and a closed probe that approximates the diameter of the mass as much as possible, a double freeze-thaw is performed so that the iceball extends 3 to 5 mm beyond the visible margins of the mass. Iceballs should overlap in large tumors. Freezing may be repeated a second or third time if complete regression is not achieved following the first session. Substantial postoperative swelling and usually transient depigmentation of the frozen tissue are to be expected.
Tumors involving the conjunctiva and third eyelid (especially conjunctival hemangiosarcomas, melanomas, and nictitans adenocarcinomas) are most effectively treated by wide surgical excision—perhaps to the point of exenterating the orbit. If the globe is to be spared, however, excision of the entire nictitans should not be taken lightly because undesirable sequelae such as ocular drying and chronic keratitis frequently result. Bulbar conjunctival tumors move freely and, if small, are generally amenable to excision under only topical anesthesia and perhaps sedation. Cryosurgery may permit the nictitans to be spared in the cases of papillomas and early SCC, or it can be used as an adjunct to excision in advanced canine conjunctival melanomas and SCC.6,24
Superficial keratectomy/sclerectomy is preferred for many corneal and scleral tumors, although some tumors require a full-thickness resection of the cornea or sclera. In the latter case, corneal or scleral allografts or autologous tissue grafts should be used to maintain ocular structural integrity. Limbal SCC and epibulbar melanoma may also be amenable to cryosurgery, although the iceball should be carefully monitored to avoid unnecessary freezing of intraocular structures.
Prognosis
The prognosis for most canine primary eyelid tumors is excellent, whether treated by excision or cryosurgery. Metastasis is rare, even in histologically malignant primary lid tumors, and recurrence rates are low (approximately 10% to 15%).2 New primary eyelid tumors are not uncommon and must be distinguished from recurrence. Because most eyelid tumors in cats are malignant, the prognosis is not as good as that for dogs, but it is unclear how prognosis correlates with the histologic features. Conjunctival melanomas and nictitans adenocarcinomas frequently recur following partial excision of the nictitans, even if all of the clinically visible tumor was removed.6 Conjunctival hemangiosarcomas appear to have a good prognosis because total excision may be curative, although recurrence and loss of the eye is still possible.13-17
Limbal (Epibulbar) Melanomas
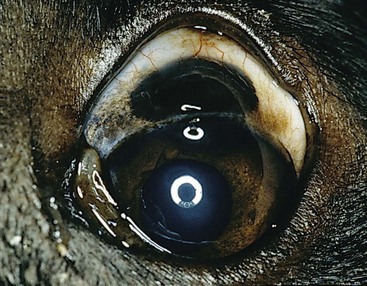
Limbal melanomas are typically benign, slightly raised, heavily pigmented masses originating from melanocytes in the sclera or subconjunctival connective tissue (Figure 31-3).25-30 They comprise 3.5% of all canine ocular tumors and 1% of feline ocular tumor submissions to COPLOW. The majority of these slow-growing tumors originate in the superior limbal region, suggesting exposure to solar radiation may be a risk factor.13 Affected dogs average 5 to 6 years old (cats 8+ years), and a female sex and German shepherd, golden retriever and Labrador retriever breed predilection has been inconsistently reported.25-29 Confirmed metastasis has not been reported in dogs or cats and mitotic figures are rarely encountered; although in one study, two of four cats also had feline leukemia virus (FeLV)-associated lymphoma or leukemia, and a third cat had a second intraocular pigmented mass unassociated with the limbal tumor.29 Lightly pigmented spindle cells capable of division are seen histologically, but the dominant cell is presumably a hypermature spindle cell that is large, round, pigment laden, and benign.26 These masses are often only incidentally noted and the clinical signs are typically minimal, although local corneal invasion, epiphora, and mild conjunctival irritation may be seen.27,28 Differential diagnoses include conjunctival melanoma, invasive uveal melanoma, metastatic melanoma, and staphyloma or coloboma. Gonioscopy aids in differentiating invasive intraocular tumors from limbal melanomas.
Therapy should be considered if the tumor has invaded the eye or if growth is rapid. Given its benign nature and usually slow growth rate (imperceptible growth over 18 months has been described), observation alone may be appropriate in older dogs. If intervention is required, lamellar keratectomy/sclerectomy with graft placement is often curative.31 Beta-irradiation and cryosurgery have been used as adjuncts to surgery.31,32 Cryosurgery alone or laser photocoagulation30 has also been described as effective means of treatment. Regrowth following local surgical excision occurs in approximately 30% of patients, but 2 to 3 years may pass before the anterior chamber is invaded and enucleation is required.26-28 The addition of adjunctive therapy such as cryotherapy or beta-irradiation substantially reduces the risk of recurrence following local excision.31,32 Enucleation is curative and indicated if painful intraocular disease is present.26
Primary Ocular Tumors
Canine Anterior Uveal Melanomas
Incidence and Risk Factors
In the Armed Forces Institute of Pathology collection of tumors from the canine eye, orbit, and adnexa, intraocular melanomas constitute 12%, other primary intraocular tumors 14%, and metastatic intraocular neoplasms 9%.33 In the COPLOW archive, uveal melanocytic tumors make up 27% of all ocular tumor submissions. Any age is at risk, but most affected dogs are older than 7 years of age, and breed or sex predilections are inconsistent.26
Pathology and Natural Behavior
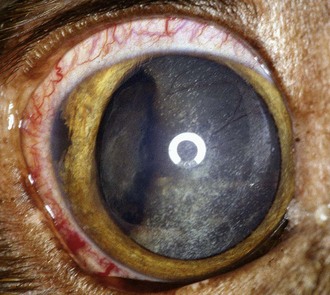
Approximately 78% of canine intraocular melanomas are benign, and 93% arise from the iris or ciliary body (Figure 31-4).26,27,34 The most clinically useful classification scheme classifies these tumors simply as melanocytoma (benign) and melanoma (potentially malignant) based on nuclear features of the tumor cells—with mitotic rate being the most important.26 Benign tumors have fewer than 2 mitotic figures/10 HPF (mitotic index), and malignant tumors demonstrate nuclear pleomorphism and a mitotic index of at least 4 and often more than 30. Destruction of the eye is not by itself sufficient for a diagnosis of malignancy.26 The overall rate of metastasis of intraocular melanomas is approximately 4%34 and usually occurs via the hematogenous route. Local spread along ocular vessels and nerves or via direct penetration of the sclera or cornea also occurs. Benign tumors tend to be more darkly pigmented than malignant tumors.26
Circumscribed, nevus-like pigmented iridal growths have been described in young dogs (7 months to 2 years old).27 The natural history of these lesions is variable because enlargement may not occur over several years.27,33 Some, however, are capable of rapid growth, but to date all are clinically and histologically benign.
Ocular melanosis of Cairn terriers resembles feline diffuse iris melanoma in some respects.35 This disorder is probably an autosomal-dominant condition with a variable age of onset and rate of progression. It results in a thickening and pigmentation of the iris, release of pigment into the aqueous, pigment deposition in the sclera/episclera, and to a lesser extent posterior segment pigment deposition. Secondary glaucoma is common, and overt uveal melanocytic neoplasia occurs in a small percentage of dogs.35
History and Clinical Signs
Common presentations of intraocular tumors include a visible intraocular or scleral mass, glaucoma, hyphema, anterior uveitis, or extrabulbar spread, or they can be an incidental finding during an ophthalmic examination.26,34 Because glaucoma or hyphema are often the only overtly visible clinical signs,26,34 intraocular neoplasia should always be considered in animals with hyphema, glaucoma, or both when there is no history of trauma or coagulopathy. Small masses frequently create few symptoms other than pupillary distortion. Pigmentation is variable and not a reliable indicator of tumor type.
Diagnostic Techniques and Work-Up
Usually, the clinical or ultrasonographic appearance (if the media are opaque) is strongly suggestive of intraocular neoplasia, although it may be difficult to arrive at a definitive diagnosis without invading the eye or removing it because organizing blood clots are not always distinguishable from neoplastic mass lesions. Because most anterior uveal brown or black masses are cystic and not neoplastic, transillumination should be attempted before more invasive procedures. Uveal cysts typically permit bright light to pass through them, are roughly spherical, and may be attached to the ciliary body or free-floating in the anterior chamber. Once suspected, most primary canine intraocular tumors are observed for progression, although occasionally FNA (with its risks of inflammation, infection, and hemorrhage) or attempts at intraocular resection or enucleation are used for diagnostic purposes. The possibility of metastasis from another primary site (i.e., oral cavity or nail bed) to the eye or from the eye to other organs should be eliminated.
Therapy
Canine primary intraocular tumors are generally carefully observed. Digital photographs are a valuable aid in assessing progression. Enucleation is advised if there is concern about malignancy or if complications such as intractable uveitis or secondary glaucoma occur.36 In the COPLOW collection, 14% of canine globes with glaucoma that are removed also had melanoma. The low risk of metastasis and unproved efficacy of enucleation at preventing metastasis in the few malignant tumors that have been reported, however, make it difficult to automatically advise enucleation of normotensive, noninflamed, visual eyes.26 Isolated primary masses involving only the iris or a portion of the ciliary body may be amenable to local resection by sector iridectomy/cyclectomy in order to preserve the eye and vision.27,33 These intraocular procedures, however, require an accomplished ophthalmic surgeon and often have unsatisfactory long-term results. Transscleral and transcorneal Nd:YAG or diode laser therapy has induced remission in some small- to moderate-sized primary intraocular tumors.36,37 Specialized goniolens may also allow laser treatment of masses that have invaded into the iridocorneal angle. Although the results were variable, perhaps because these tumors varied histologically in nature, laser therapy holds promise for the palliation or potential cure of a number of intraocular tumor types while also preserving vision. Metastasis was not observed following this procedure, although this obviously remains a risk when the tumor is malignant.36
Prognosis
Although the data in most studies are heavily “censored,” the prognosis for histologically benign melanomas appears to be excellent. Enucleation is curative, but attempts at local excision or laser photoablation may be only palliative, especially if the ciliary body or trabecular meshwork is involved. The presence of black, nonsolid material within the orbit following the enucleation of benign melanomas with scleral invasion apparently does not affect prognosis because these cells appear incapable of continued growth.27 In one study, approximately 25% of histologically malignant melanomas demonstrated metastasis, typically within 3 months of enucleation, and most dogs with metastasis are euthanatized within 6 months of enucleation.26 This surprisingly poor prognosis has not been the experience of cases followed recently in the COPLOW data set, and in a larger study, dogs with tumors classified as malignant were reported to have only a somewhat decreased survival time compared to dogs with melanocytoma and dogs from a control population.38
Choroidal Melanomas
Choroidal melanomas are rare intraocular melanocytic tumors, comprising only 4% to 7% of canine uveal melanomas, with no clear breed or sex predisposition.39 Middle-aged (6 to 7 years), medium- to large-breed dogs predominate.39 Generally, these tumors are well-delineated, raised subretinal pigmented masses with tapering margins, bulging centers, and a propensity for the peripapillary region and optic nerve.39,40 In some cases, the tumor may remain virtually static for many years, whereas others exhibit infiltration into the overlying retina, through the sclera, up the optic nerve, and into the orbit.39 Nuclear anaplasia is minimal and generally mitotic figures are absent.40 Despite these benign cytologic features, metastasis has been described in one dog 21 months after exenteration, and follow-up in most studies is incomplete.41 In general, however, these tumors appear to be benign in the vast majority of dogs. Most dogs with tumors involving a limited portion of the choroid are asymptomatic, and the mass is noted incidentally on ophthalmoscopy. Larger tumors frequently present with chronic uveitis, secondary glaucoma, retinal detachment, intraocular hemorrhage, or blindness.39,40 Extension into the orbit can occur, and documentation of this is important in planning for enucleation surgery. Ocular ultrasonography may demonstrate mass lesions if anterior segment changes or retinal detachment obscures an underlying mass. Therapy usually consists of enucleation once progression has been documented or if the eye is painful. Diode laser ablation may offer an alternative to enucleation if the lesion is small and does not involve the optic nerve. Optic nerve or scleral invasion may warrant a more cautious prognosis.
Feline Primary Intraocular Melanomas (Feline Diffuse Iris Melanoma)
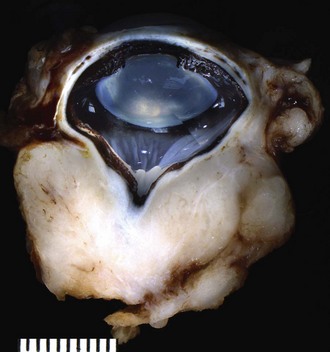
Anterior uveal melanomas are the most common primary intraocular tumor in cats (Figure 31-5).42 They account for 49% of all neoplastic submissions to the COPLOW in cats. There appears to be no breed or sex predisposition, and most cats are more than 9 years of age at the time of diagnosis,42,43 although the prodromal period for many of these tumors may be quite long.
Pathology and Natural Behavior
In the malignant form of uveal melanoma, the rate of metastasis (frequently to the liver and lungs) has been reported to vary from 55% to 66% or higher.42-45 Iridal hyperpigmentation, however, frequently takes months to years to progress to the extent to which the eye must be enucleated, and an additional 1 to 3 years after enucleation are required before metastatic disease may become evident.42,43,45,46 No single morphologic feature is predictive of outcome, but metastasis has been linked to a greater mitotic index, larger tumors, and extension through the iris into the ciliary body stroma and involvement of the scleral venous plexus.42,43
Most ophthalmologists have noted unilateral or occasionally bilateral slowly progressive iridal pigmentary changes (especially in older orange cats) over many years to a decade or more that apparently do not lead to disease beyond the pigmentation, although eventual removal of these eyes can show melanoma. It is possible in some cats that these initially benign-appearing accumulations of small, angular pigmented cells on the anterior iridal surface undergo transformation to the larger, rounded cells typical of the potentially malignant diffuse iris melanoma. Of concern to the clinician waiting to document progression before advising treatment, however, is that malignant transformation is not readily observable clinically and that these cells, once transformed, appear to be capable of quickly dropping off into the anterior chamber and entering the drainage apparatus and vasculature.43
History, Clinical Signs, Diagnostic Techniques, and Work-Up
Slowly progressive, diffuse iridal hyperpigmentation is the most common clinical sign, although occasionally a pigmented iridal nodule or amelanotic mass is seen. Secondary glaucoma will eventually occur, and the diffuse form may be mistaken for chronic anterior uveitis with iridal hyperpigmentation.
The diagnosis of melanoma, generally made clinically, requires demonstration of progression and iridal thickening or irregularity of the iris surface or pupil. The prognostic and diagnostic value of fine-needle aspirates of the iridal surface or iridal biopsies is unclear and worthy of further study.
Therapy
The treatment of feline uveal melanomas is controversial. Ideally, enucleation would be delayed until just prior to malignant transformation, invasion into other ocular structures, secondary glaucoma, or metastasis. Such precise timing, however, is seldom attainable in a clinical setting, and enucleation is commonly performed if iridal pigment changes have been demonstrated to progressively increase to the point that virtually the entire iridal surface is involved, pigmented cells are present in the trabecular meshwork, the pupil is distorted (indicating iridal invasion), ciliary body or scleral invasion is threatened, uveitis is present, or glaucoma is impending. Although it would seem logical that early enucleation would optimize survival, this is unproved. In one study, enucleation has been shown to markedly enhance the rate of metastasis in cats with feline sarcoma virus–induced uveal melanomas.47 The applicability of this experimental model to spontaneous disease, however, is unclear and neither feline sarcoma virus nor FeLV were found in a study of 10 eyes with spontaneous diffuse iris melanoma.48 Recently, some ophthalmologists have attempted to ablate small, focal, hyperpigmented foci on the iris of cats with a diode laser, thereby preserving vision and the eye. The long-term success rate and side effects of this procedure, however, are not known. Finally, most slowly progressing lesions are simply monitored, ideally by comparison to baseline photographs. This option is particularly suitable for older cats with other diseases that limit their expected lifespan. In many cats, progression may be so slow as to permit the patient to be followed for many years to a decade or more without apparent metastasis.
Prognosis
In one study, the metastatic potential of feline uveal melanomas has been correlated with the extent of ocular involvement seen histologically.43 Because the tumor is relatively slow-growing, however, the period until metastatic disease becomes apparent may be measured in years, and even then substantial additional time may be required before the metastasis is life threatening. Cats with tumors confined to iris stroma and trabecular meshwork at the time of enucleation have survival times comparable to those of age-matched controls. Enucleation after the tumor has invaded into the ciliary body but not the sclera warrants a poorer prognosis, but median survival time (MST) is still approximately 5 years.43 Enucleation after the tumor has invaded into the ciliary body and the sclera merits an even poorer prognosis, with a MST of approximately 1.5 years.43 MST is also reduced if secondary glaucoma has occurred.43
Feline Ocular Posttraumatic Sarcoma
Sarcomas following ocular trauma, although uncommon, are second only to melanomas in frequency as a primary ocular tumor of cats.49-52 In the COPLOW collection, 8% of feline ocular tumors are posttraumatic sarcoma. These tumors are subdivided into three morphologic subtypes: spindle cell sarcoma (the most common), round cell sarcoma, and osteosarcoma/chondrosarcoma. All three have similar histories leading up to tumor presentation. Cats that are 7 to 15 years of age are most commonly affected, the latency period following trauma averages 5 years,50 and 67% of affected cats are males or neutered males. Damage to the lens and chronic uveitis may be risk factors.49-51 Because of the risk of posttraumatic sarcoma, many clinicians are cautious about cataract surgery or the use of an intrascleral prosthesis in cats. The cell of origin in the three subtypes is not definitively known, but it is likely that the released lens epithelial cell is the cell of origin in the spindle cell variant and the round cell variant likely represents a form of lymphoma.53 Chronic inflammation may support neoplastic transformation of a pluripotent cell.49-51 These tumors, often within the same eye, exhibit a spectrum of changes ranging from granulation tissue to fibrosarcoma, osteosarcoma, and anaplastic spindle cell sarcoma.50,51 All of these tumors tend to circumferentially line the choroid and quickly infiltrate the retina and optic nerve.50,51 The round cell variant tends to infiltrate the retina early.53,54 White or pinkish discoloration of the affected eye or change in the shape or consistency of the globe are the most common presenting signs. Skull radiographs may demonstrate bone involvement or metallic foreign bodies.52
Because this tumor is uncommon, many ophthalmologists will not remove a comfortable phthisical feline eye unless it changes appearance. The advanced stage at which many of these tumors are first identified, however, and the propensity for early optic nerve involvement indicate that enucleation at this point may be only palliative and not prolong life. This has led some authors to advocate prophylactic enucleation of phthisical feline eyes or of feline eyes that are blind and have been severely traumatized or are chronically inflamed.49,52 Further support for this approach comes from the observation that approximately 8% of globes removed prophylactically in the COPLOW collection already have tumors. Extension beyond the sclera or into the optic nerve may occur and are poor prognostic indicators, further supporting the concept of early enucleation. As much of the optic nerve as possible should be removed during enucleation for confirmed or suspected ocular sarcoma so that the extent of infiltrative disease and prognosis may be accurately determined. There is reason to believe that the prognosis is considerably better if enucleation is performed before the tumor invades the optic nerve or extends beyond the sclera.55 To date, there have been no reports of treatment by radiation or chemotherapy.
Extraocular extension is common, as is recurrence following orbital exenteration.49,52 Continued growth up the remainder of the optic nerve into the chiasm and brain, with vision loss or other neurologic signs, involvement of regional lymph nodes, and distant metastasis, has been reported.40,50 The vast majority of animals die from local invasion and recurrence, typically within several months of enucleation.49,52
Spindle Cell Tumors of Blue-Eyed Dogs
Dogs with a blue, or partially blue, iris appear to be at risk of developing a spindle cell sarcoma in the uvea.56 These tumors usually involve the iris but can originate or extend into the ciliary body, choroid, and even the vitreous. Breeds that commonly have blue irides are more likely to develop an iridal spindle cell sarcoma, but any dog with any blue in its iris appears to be at risk. The origin of these tumors is thought to be Schwann cells of nonmyelinated peripheral nerves.57 The cells stain positive with glial fibrillary acidic protein (GFAP), as do the Schwann cells of nonmyelinating nerves. In a case series involving 11 dogs, more than half of the tumors were not clinically recognized and the diagnosis of neoplasia was not made until histopathology was performed. Metastatic disease has not been seen; however, local recurrence within the scleral shell was seen in a dog that had been treated by evisceration and placement of an intrascleral prosthesis.56
Iridociliary Epithelial Tumors
Primary iridociliary epithelial tumors (ciliary body adenomas, adenocarcinomas, pleomorphic adenocarcinoma, and less commonly, medulloepitheliomas) are infrequent in dogs and rare in cats.58 The two main histologic forms that have been described are papillary (57% of cases in one study) and solid tumors (43%).58 In the authors’ experience, these tumors often appear nonpigmented clinically, but histologically at least some pigmented cells are present in approximately one-half of cases. Pigmented tumors of the ciliary body may be grossly indistinguishable from anterior uveal melanomas. Middle-aged to older dogs are the most commonly affected and golden and Labrador retrievers may be predisposed, for they comprised 27% of dogs with iridociliary epithelial tumors in one survey.58 Most of these tumors appear to be benign, fairly well-delineated, sometimes pedunculated, slow-growing masses that originate in the pars plicata of the ciliary body or the iris epithelium.58,59 Although approximately 60% invade the uveal tract, only 21% invade the sclera.58 Tumors that invade the sclera are typically classified as adenocarcinomas and have anaplastic features, but metastasis is uncommon and occurs late in the course of the disease, if at all.58-61 A small series of truly malignant pleomorphic adenocarcinomas with potentially fatal metastasis has been described in dogs.62 Dogs affected with this rare form usually have long-standing disease thought to be inflammatory or traumatic, and 4 of 25 cases have a prior history of an intravitreous gentamicin injection for the treatment of glaucoma.62
Clinical signs include a retro-iridal mass that may displace the iris or lens by expansive growth, and if the tumor is large, secondary glaucoma, ocular pain, and intraocular hemorrhage may be noted.60 The diagnostic work-up and differential diagnosis are similar to that of anterior uveal melanomas. Given the high frequency of ciliary body cysts in some predisposed breeds (especially the golden retriever), it is essential to differentiate ciliary body tumors from a benign cystic lesion prior to enucleation. Cystic lesions, which rarely require any intervention, are usually seen as lightly pigmented, ovoid, retro-iridal masses that can be shown to be hollow by transillumination or hypoechoic by ultrasonography. Early enucleation of ciliary body tumors has been recommended, although benign adenomas may remain static for years, making enucleation controversial for small tumors unassociated with secondary ocular disease. Local intraocular resection or laser photoablation may permit vision and ocular comfort to be maintained.36 Systemic administration of 5-fluorouracil (5-FU) has been described as an adjunct to local resection of ciliary body tumors, but the efficacy of this therapy is unknown.61
Secondary Uveal Neoplasms
Numerous malignant tumors, especially adenocarcinomas, have been reported to metastasize to the highly vascular uveal tract. Lymphoma is the most common secondary intraocular tumor in the dog and cat, and ocular lesions are present in approximately one-third of dogs with the disease.60,63-65 Common presentations include severe uveitis, glaucoma, retinal hemorrhages, hyphema, conjunctivitis, and keratitis characterized by corneal infiltrates, edema, vascularization, and intrastromal hemorrhage.60,63-65 Exophthalmia resulting from orbital invasion by the tumor and vision loss due to optic nerve or central nervous system (CNS) disease may also be present. Posterior segment lesions may include retinal vascular tortuosity, papilledema, multiple intraretinal hemorrhages, and retinal detachment. In one study, the lifespan of dogs with intraocular lymphoma was only 60% to 70% as long as dogs without ocular involvement when treated with cyclophosphamide, vincristine, and prednisolone (COP), or with doxorubincin.64 Topical or systemic corticosteroid therapy or enucleation is palliative. (See Chapter 32 for the definitive therapy of lymphoma.) Ophthalmic disease, especially intraocular or retinal hemorrhage, may also be the presenting complaint in animals with multiple myeloma.66
Tumors of the Orbit and Optic Nerve
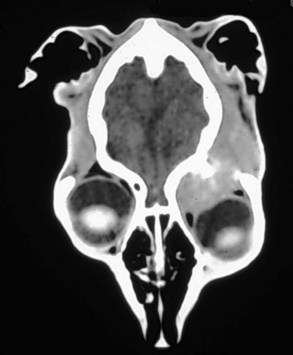
Risk factors other than middle to old age, possibly large-breed dogs,67 and possibly sex (female dogs, male cats) have not been described.67-75 Tumors involving the optic nerve are rare, although secondary invasion occurs in feline posttraumatic sarcomas, feline SCC, and canine choroidal melanomas. Canine orbital meningioma is the most common tumor of the optic nerve but comprises only 3% of all meningiomas in dogs (Figure 31-6).71,73 Lobular adenomas of unspecified glandular origin have been recently reported to involve the anterior orbit in dogs.72
Pathology and Natural Behavior
Orbital neoplasia may be primary (most common in dogs), secondary to extension of adjacent tumors into the orbit (most common in cats), or the result of distant metastasis. In cats and dogs, more than 90% of orbital tumors are malignant, and regional infiltration (including into the CNS) or distant metastasis is common.67-70 At least 26 types of orbital tumors, roughly equally divided among connective tissue, bone, epithelial, and hemolymphatic origins, have been reported in dogs.70 Osteosarcomas, mast cell tumors, reticulum cell sarcomas, fibrosarcomas, and neurofibrosarcomas are the most common canine primary orbital tumors.70 More than two-thirds of feline orbital tumors are epithelial in origin, with SCC being the most common,69 but at least 15 other tumor types have been described in cats.
Canine orbital meningiomas exhibit predictable biologic behavior. They are slowly progressive and rarely metastasize; they may be osteolytic and invade surrounding tissues, including the CNS via the optic foramen.70,72,73 Primary optic nerve tumors in dogs include glioma and meningioma.70,71,73,74 Retinal and optic nerve gliomas may be considered as differential diagnoses of intraocular and orbital masses. The metastatic potential of gliomas appears to be low, but ascending invasion into the ventral aspect of the brain is possible.75 Lobular orbital adenomas are made up of multiple friable lobules in the anterior orbit, making complete surgical excision difficult.72
History and Clinical Signs
Slowly progressive exophthalmia, absent to minimal pain on opening the mouth, difficulty in retropulsing the eye, and deviation of the globe typify orbital neoplasia. Sudden erosion of nasal or sinus tumors into the orbit occasionally results in acute exophthalmia and substantial orbital pain. Enophthalmia may occur if the mass is anterior to the equator of the globe. Lobular adenomas may present as soft, raised, subconjunctival masses and create either enophthalmia or exophthalmia. Chronic epiphora secondary to obstruction of the nasolacrimal duct, exposure keratoconjunctivitis, palpable orbital masses following enucleation, or unexplained orbital pain also suggests orbital neoplasia.67-70 Measurement of corneal diameters and intraocular pressure (IOP) aids in differentiating glaucomatous ocular enlargement (large corneal diameter, high IOP) from exophthalmia (normal corneal diameter and IOP).
Optic nerve lesions may result in unilateral or bilateral blindness (the latter if the optic chiasm is affected), optic nerve head pallor, papilledema, or marked protrusion and congestion of the optic disc on ophthalmoscopy. A relatively mild degree of exophthalmia with vision loss suggests optic nerve neoplasia because tumors of other orbital tissues typically cause profound exophthalmos before visual loss. Tumors affecting the retrobulbar, intracanalicular, or chiasmal portions of the optic nerve may not result in exophthalmia or a visible change in the optic nerve head.
Diagnostic Techniques and Work-Up
It is essential to differentiate nonneoplastic orbital inflammatory diseases (granulomas, cellulitis, abscesses, myositis of the extraocular and masticatory muscles) from neoplasia. Animals with inflammatory disease typically exhibit significant pain on opening the mouth. The location of an orbital mass can usually be determined by careful physical examination, including retropulsion of the globe, oral examination caudal to the last molar, and determination of the direction of malposition of the eye.
In addition to physical examination, cytology of regional lymph nodes, orbital ultrasound, and skull and thoracic radiographs should be performed. In one study of cats with orbital neoplasia, 59% had radiographic signs of orbital bone lesions and 15% had evidence of metastasis on thoracic radiographs.69 CT or MRI offer superior depictions of the orbit and facilitate planning of either radiation or surgical therapy (Figure 31-7). Histologic characterization by FNA or needle core biopsies (performed via the mouth or through the orbital skin), with ultrasound guidance if necessary, are helpful in arriving at a definitive diagnosis. The globe, major orbital blood vessels, and optic nerve should be avoided. Because 50% of orbital tumors may have a nondiagnostic FNA, especially in cases of SCC,69 exploratory orbitotomy via a number of approaches76-80 or exenteration may be required to characterize the mass and resect it if possible. Cerebrospinal fluid taps may aid in distinguishing optic nerve neoplasia from optic neuritis.
Therapy
Primary orbital and optic nerve tumors that lack metastasis or regional lymph node involvement may be amenable to surgical excision. If bony involvement is not present, orbital exenteration by widely dissecting around the mass (stripping periorbita if necessary) is usually the preferred procedure, as the advanced stage of the tumor at the time of diagnosis typically makes it impossible to excise the mass completely and preserve a functional or comfortable eye. If periorbital bones are involved, radical “orbitectomy,” which resects the affected orbital tissues and surrounding bones, should be considered.78 When treating optic nerve tumors, as much of the ipsilateral optic nerve as possible should be removed in an attempt to obtain complete excision.70
If preservation of a comfortable eye and vision appears possible, a variety of orbitotomy techniques, ranging from small incisions through the eyelid or mouth to reflection of the zygomatic arch, temporalis muscle elevation, and zygomatic process osteotomy, have been described.76-80 Postoperative complications are common and may include secondary enophthalmia with entropion and possibly diplopia (double vision). Surgical debulking can be palliative, and some dogs may survive a year or more with minimal therapy.
The role of chemotherapy and RT, either alone or as an adjunct to surgery, is yet to be defined in the treatment of orbital tumors, although chemotherapy for orbital lymphoma may be effective. Systemic corticosteroids may permit some patients with optic nerve meningioma to maintain vision for several weeks to months. RT may be helpful in the case of nasal tumors with orbital extension, in subtotally excised or recurrent meningiomas, and in other select cases.
Prognosis
With conservative treatment the prognosis for most tumors involving the orbit and optic nerve is poor,69,70 especially if there is bony involvement on skull radiographs. Recurrence at the primary site and involvement of adjacent or distant sites are common, often occurring within weeks to a few months. Even benign-appearing tumors such as lobular orbital adenomas and orbital meningiomas may be locally invasive and have a propensity for recurrence following wide excison.67-73,78 In one study, however, radical orbitectomy (with or without chemotherapy or RT) provided a local disease-free interval of more than 1 year in more than 50% of patients and a 70% survival rate for the first year.78 In another study, the mean survival time for cats with orbital tumors treated by RT, chemotherapy, or surgery that included resection of affected orbital bones was only 4.3 months.69 In a study of 23 dogs with orbital tumors, most of whom were treated by exenteration with or without adjunct therapy, only 3 survived 3 years or longer.70 The majority of these animals died as a direct result of the tumor or were euthanatized at the time of diagnosis.67-70
Ocular Effects of Cancer Therapeutic Modalities
The ocular effect of external-beam RT for nasal and periocular tumors can have a substantial impact on an animal’s quality of life. Common complications include chronic keratoconjunctivitis, corneal ulceration, “dry eye,” enophthalmia, entropion, cataracts, retinal hemorrhages, retinal detachments, and blindness.81,82 Many of these conditions respond poorly to treatment, and vigorous attempts at prevention should be made in order to avoid chronic ocular pain and blindness. Recently, intensity-modulated RT (IMRT), which uses conformal avoidance, has been shown to significantly decrease the ocular toxicity seen in dogs treated by RT for spontaneous sinonasal tumors (Figure 31-8).83,84
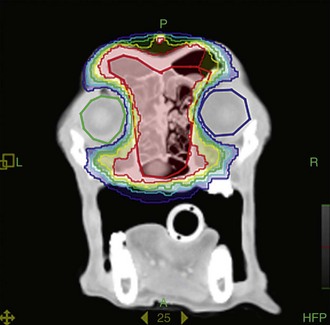
Figure 31-8 Intensity-modulated radiation therapy (IMRT), which uses conformal avoidance, can spare the eyes (green and blue circles) and has been shown to significantly decrease the ocular toxicity of radiation therapy for sinonasal tumors.
In humans, blurred vision, partial visual field defects, loss of color vision, and diplopia have been associated with several antineoplastic drugs.85 Similar effects probably occur in animals but would be difficult to detect. Additionally, in humans, the bacillus Calmette-Guérin (BCG) has been associated with uveitis; cyclophosphamide has been associated with dry eye; cisplatin has been associated with neuroretinal toxicity; doxorubicin has been associated with excessive lacrimation and conjunctivitis; 5-FU has been associated with blurred vision, excessive lacrimation, blepharitis, conjunctivitis, and keratitis; and vincristine has been associated with cranial nerve palsies, optic neuropathy, and cortical and night blindness.85 Monoclonal antibodies directed at the vasculature supporting the tumor also have been associated with uveitis.86
Comparative Aspects
Malignant melanoma of the choroid is the most common primary ocular malignancy in adult humans. Initially, it was believed that enucleation of these patients may enhance the risk of metastasis; thus a large, randomized clinical trial (the Collaborative Ocular Melanoma Study) was conducted comparing enucleation to iodine-125 brachytherapy, which left the globe intact.87,88 Both treatment modalities were found to yield similar results, although many patients still died from metastatic melanoma, and it appears that significant improvement in survival rates will depend on developing effective systemic therapeutic modalities for melanoma.87,88 Retinoblastoma is the most common malignant intraocular tumor of children and has a genetic basis. No cases of retinoblastoma have been described in nonhuman primates, and only one case of retinoblastoma has been described in a dog.89 With therapy, long-term survival in children is over 85%, but many patients develop second tumors, especially osteosarcoma.90 Cancer-associated retinopathy is an uncommon, immune-mediated paraneoplastic phenomenon in humans in which antibodies are directed against specific retinal autoantigens, such as recoverin.91-93 In this condition, patients with small-cell lung carcinoma and other tumors may develop blurred vision, impaired color vision, substantial visual field defects, or complete blindness as tumor antigens cross-react with specific retinal components.91-93 Treatment with IV immunoglobulin has been reported to return vision to some patients.91 Although cancer-associated retinopathy has been suggested to occur in dogs, especially those with sudden acquired retinal degeneration syndrome (SARDS), definitive proof is lacking and one study did not identify antibody activity against retinal proteins or evidence of neoplasia in dogs with SARDS.94
References
1. Krehbiel, JD, Langham, RF. Eyelid neoplasms in dogs. Am J Vet Res. 1975;36:115–119.
2. Roberts, SM, Severin, GA, Lavach, JD. Prevalence and treatment of palpebral neoplasms in the dog: 200 cases (1975-1983). J Am Vet Med Assoc. 1986;189:1355–1359.
3. McLaughlin, SA, Whitley, RD, Gilger, BC, et al. Eyelid neoplasms in cats: A review of demographic data (1979 to 1989). J Am Anim Hosp Assoc. 1983;29:63–67.
4. Barrie, KP, Gelatt, KN, Parshall, CP. Eyelid squamous cell carcinoma in four dogs. J Am Anim Hosp Assoc. 1982;18:123–127.
5. Bernays, ME, Flemming, D, Peiffer, RL. Primary corneal papilloma and squamous cell carcinoma associated with pigmentary keratitis in four dogs. J Am Vet Med Assoc. 1999;214:215–217.
6. Collins, BK, Collier, LL, Miller, MA, et al. Biologic behavior and histologic characteristics of canine conjunctival melanoma. Prog Vet Comp Ophthalmol. 1993;3:135–140.
7. Roels, S, Ducatelle, R. Malignant melanoma of the nictitating membrane in a cat (Felix vulgaris). J Comp Pathol. 1998;119:189–193.
8. Brandes, K, Fritsche, J, Mueller, N, et al. Detection of canine oral papillomavirus DNA in conjunctival epithelial hyperplastic lesions of three dogs. Vet Pathol. 2009;46:34–38.
9. Bonney, CH, Koch, SA, Dice, PF, et al. Papillomatosis of conjunctiva and adnexa in dogs. J Am Vet Med Assoc. 1980;176:48–51.
10. Collier, LL, Collins, BK. Excision and cryosurgical ablation of severe periocular papillomatosis in a dog. J Am Vet Med Assoc. 1994;204:881–885.
11. Wilcock, B, Peiffer, R. Adenocarcinoma of the gland of the third eyelid in seven dogs. J Am Vet Med Assoc. 1988;193:1549–1550.
12. Schäffer, EH, Pfleghaar, S, Gordon, S, et al. Malignant nictitating membrane tumors in dogs and cats. Tierarztliche Praxis. 1994;22:382–389.
13. Hargis, AM, Lee, AC, Thomassen, RW. Tumor and tumor-like lesions of perilimbal conjunctiva in laboratory dogs. J Am Vet Med Assoc. 1978;173:1185–1190.
14. Mughannam, AJ, Hacker, DV, Spangler, WL. Conjunctival vascular tumors in six dogs. Vet Comp Ophthalmol. 1997;7:56–59.
15. Multari, D, Vascellari, M, Mutinelli, F. Hemangiosarcoma of the third eyelid in a cat. Vet Ophthalmol. 2002;5:273–276.
16. Pirie, CG, Knollinger, AM, Thomas, CB, et al. Canine conjunctival hemangioma and hemangiosarcoma: a retrospective evaluation of 108 cases (1989-2004). Vet Ophthalmol. 2006;9:215–226.
17. Pirie, CG, Dubielzig, RR. Feline conjunctival hemangioma and hemangiosarcoma: a retrospective evaluation of eight cases (1993-2004). Vet Ophthalmol. 2006;9:227–231.
18. Patnaik, AK, Mooney, S. Feline melanoma: a comparative study of ocular, oral and dermal neoplasms. Vet Pathol. 1988;25:105–112.
19. Schobert, CS, Labelle, P, Dubielzig, RR. Feline conjunctival melanoma: histopathological characteristics and clinical outcomes. Vet Ophthalmol. 2010;13:43–46.
20. Johnson, BW, Brightman, WhiteleyHE. Conjunctival mast cell tumor in two dogs. J Am Anim Hosp Assoc. 1988;24:439–442.
21. Kafarnik, C, Calvarese, S, Dubielzig, RR. Canine mesenchymal hamartoma of the eyelid. Vet Ophthalmol. 2010;13:94–98.
22. Maggs, DJ. Eyelids. In Maggs DJ, Miller PE, Ofri R, eds.: Slatter’s fundamentals of veterinary ophthalmology, ed 4, St. Louis: Elsevier, 2008.
23. Guiliano, EA. Equine ocular adnexal and nasolacrimal disease. In Gilger BC, ed.: Equine ophthalmology, ed 2, St. Louis: Elsevier, 2011.
24. Holmberg, DL, Withrow, SJ. Cryosurgical treatment of palpebral neoplasms: clinical and experimental results. Vet Surg. 1979;8:68–73.
25. Donaldson, D, Sansom, J, Scase, T, et al. Canine limbal melanoma: 30 cases (1992-2004). Part 1. Signalment, clinical and histological features and pedigree analysis. Vet Ophthalmol. 2006;9:115–119.
26. Wilcock, BP, Peiffer, RL. Morphology and behavior of primary ocular melanomas in 91 dogs. Vet Pathol. 1986;23:418–424.
27. Diters, RW, Dubielzig, RR, Aquirre, GD, et al. Primary ocular melanoma in dogs. Vet Pathol. 1983;20:379–395.
28. Diters, RW, Ryan, AM. Canine limbal melanoma. Vet Med Small Anim Clin. 1983;78:1529–1534.
29. Harling, DE, Peiffer, RL, Cook, CS, et al. Feline limbal melanoma: four cases. J Am Anim Hosp Assoc. 1986;22:795–802.
30. Sullivan, TC, Nasisse, MP, Davidson, MG, et al. Photocoagulation of limbal melanoma in dogs and cats: 15 cases (1989-1993). J Am Vet Med Assoc. 1996;208:891–894.
31. Featherstone, HJ, Renwick, P, Heinrich, CL, et al. Efficacy of lamellar resection, cryotherapy, and adjunctive grafting for the treatment of canine limbal melanoma. Vet Ophthalmol. 2009;12(Suppl 1):65–72.
32. Donaldson, D, Sansom, J, Scase, T, et al. Canine limbal melanoma: 30 cases (1992-2004). Part 2. Treatment with lamellar resection and adjunctive strontium-90 beta plesiotherapy—efficacy and morbidity. Vet Ophthalmol. 2006;9:179–185.
33. Gelatt, KN, Johnson, KA, Peiffer, RL. Primary iridal pigmented masses in three dogs. J Am Anim Hosp Assoc. 1979;15:339–344.
34. Bussanich, NM, Dolman, PJ, Rootman, J, et al. Canine uveal melanomas: series and literature review. J Am Anim Hosp Assoc. 1987;23:415–422.
35. Petersen-Jones, SM, Forcier, J, Mentzer, AL. Ocular melanosis in the Cairn Terrier: clinical description and investigation of mode of inheritance. Vet Ophthalmol. 2007;10(Suppl 1):63–69.
36. Nasisse, MP, Davidson, MG, Olivero, DK, et al. Neodymium:YAG laser treatment of primary canine intraocular tumors. Prog Vet Comp Ophthalmol. 1993;3:152–157.
37. Cook, CS, Wilkie, DA. Treatment of presumed iris melanoma in dogs by diode laser photocoagulation: 23 cases. Vet Ophthalmol. 1999;2:217–225.
38. Giuliano, EA, Chappell, R, Fischer, B, et al. A matched observational study of canine survival with primary melanocytic neoplasia. Vet Ophthalmol. 1999;2:185–190.
39. Collinson, PN, Peiffer, RL. Clinical presentation, morphology, and behavior of primary choroidal melanomas in eight dogs. Prog Vet Comp Ophthalmol. 1993;3:158–164.
40. Dubielzig, RR, Aquirre, GD, Gross, SL, et al. Choroidal melanomas in dogs. Vet Pathol. 1985;22:582–585.
41. Hyman, JA, Koch, SA, Wilcock, BP. Canine choroidal melanoma with metastases. Vet Ophthalmol. 2002;5:113–117.
42. Duncan, DE, Peiffer, RL. Morphology and prognostic indicators of anterior uveal melanomas in cats. Prog Vet Comp Ophthalmol. 1991;1:25–32.
43. Kalishman, JB, Chappell, R, Flood, LA, et al. A matched observational study of survival in cats with enucleation due to diffuse iris melanoma. Vet Ophthalmol. 1998;1:21–24.
44. Bellhorn, RW, Henkind, P. Intraocular malignant melanomas in domestic cats. J Small Anim Pract. 1970;10:631–637.
45. Patnaik, AK, Mooney, S. Feline melanoma: A comparative study of ocular, oral and dermal neoplasms. Vet Pathol. 1988;25:105–112.
46. Acland, GM, McLean, IW, Aquirre, GD, et al. Diffuse iris melanoma in cats. J Am Vet Med Assoc. 1980;176:52–56.
47. Niederkorn, JY, Shadduck, JA, Albert, DM. Enucleation and the appearance of second primary tumors in cats bearing virally induced intraocular tumors. Invest Ophthalmol Vis Sci. 1982;23:719–725.
48. Cullen, CL, Haines, DM, Jackson, ML, et al. Lack of detection of feline leukemia and feline sarcoma viruses in diffuse iris melanomas of cats by immunohistochemistry and polymerase chain reaction. J Vet Diagn Invest. 2002;14:340–343.
49. Peiffer, RL, Monticello, T, Bouldin, TW. Primary ocular sarcomas in the cat. J Small Anim Pract. 1988;29:105–116.
50. Dubielzig, RR, Everitt, J, Shadduck, JA, et al. Clinical and morphologic features of post-traumatic ocular sarcomas in cats. Vet Pathol. 1990;27:62–65.
51. Dubielzig, RR, Hawkins, KL, Toy, KA, et al. Morphologic features of feline ocular sarcomas in 10 cats: Light microscopy, ultrastructure, and immunohistochemistry. Vet Comp Ophthalmol. 1994;4:7–12.
52. Håkansson, N, Shively, JN, Reed, RE, et al. Intraocular spindle cell sarcoma following ocular trauma in a cat: case report and literature review. J Am Anim Hosp Assoc. 1990;26:63–66.
53. Naranjo, C, Schobert, CS, Dubielzig, RR. Round cell variant of feline ocular posttraumatic sarcoma: a retrospective study (abstract). Vet Ophthalmol. 2007;10:399.
54. Dubielzig RR, Zeiss C: Feline post-traumatic ocular sarcoma: Three morphologic variants and evidence that some are derived from lens epithelial cells. Proceedings of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, 2004.
55. Dubielzig, RR. Feline post-traumatic ocular sarcoma: a review of 110 cases. Proceedings American College of Veterinary Pathologists, New Orleans. Vet Pathol. 2002;39:619.
56. Klauss, G, Dubielzig, RR. Characteristics of primary spindle cell neoplasm of the anterior uveal tract: 11 dogs. Proceedings American College of Veterinary Pathologists, Salt Lake City. Vet Pathol. 2001;38:574.
57. Zarfoss, MK, Klauss, G, Newkirk, K, et al. Uveal spindle cell tumor of blue-eyed dogs: an immunohistochemical study. Vet Pathol. 2007;44:276–284.
58. Dubielzig, RR, Steinberg, H, Garvin, H, et al. Iridociliary epithelial tumors in 100 dogs and 17 cats: a morphological study. Vet Ophthalmol. 1998;1:223–231.
59. Peiffer, RL. Ciliary body epithelial tumors in the dog and cat: a report of thirteen cases. J Small Anim Pract. 1983;24:347–370.
60. Gwin, RM, Gelatt, KN, Williams, LW. Ophthalmic neoplasms in the dog. J Am Anim Hosp Assoc. 1982;18:853–866.
61. Clerc, B. Surgery and chemotherapy for the treatment of adenocarcinoma of the iris and ciliary body in five dogs. Vet Comp Ophthalmol. 1996;6:265–270.
62. Bell, CM, Dubielzig, RR. Canine iridociliary epithelial tumors: a morphologic review of 702 cases (abstract). Vet Pathol. 2009;46:1064.
63. Williams, LW, Gelatt, KN, Gwin, RM. Ophthalmic neoplasms in the cat. J Am Anim Hosp Assoc. 1981;17:999–1008.
64. Krohne, SG, Henderson, NM, Richardson, RC, et al. Prevalence of ocular involvement in dogs with multicentric lymphoma: prospective evaluation of 94 cases. Vet Comp Ophthalmol. 1994;4:127–135.
65. Corcoran, KA, Peiffer, RL, Koch, SA. Histopathologic features of feline ocular lymphosarcoma: 49 cases (1978-1992). Vet Comp Ophthalmol. 1995;5:35–41.
66. Hendrix, DV, Gelatt, KN, Smith, PJ, et al. Ophthalmic disease as the presenting complaint in five dogs with multiple myeloma. J Am Anim Hosp Assoc. 1998;34:121–128.
67. Attali-Soussay, K, Jegou, JP, Clerc, B. Retrobulbar tumors in dogs and cats: 25 cases. Vet Ophthalmol. 2001;4:19–27.
68. Mauldin, EA, Deehr, AJ, Hertzke, D, et al. Canine orbital meningiomas: a review of 22 cases. Vet Ophthalmol. 2000;3:11–16.
69. Gilger, BC, McLaughlin, SA, Whitley, RD, et al. Orbital neoplasms in cats: 21 cases (1974-1990). J Am Vet Med Assoc. 1992;201:1083–1086.
70. Kern, TJ. Orbital neoplasia in 23 dogs. J Am Vet Med Assoc. 1985;186:489–491.
71. Braund, KG, Ribas, JL. Central nervous system meningiomas. Compend Contin Educ Pract Vet. 1986;8:241–248.
72. Headrick, JK, Bentley, E, Dubielzig, RR. Canine lobular orbital adenoma: a report of 15 cases with distinctive features. Vet Ophthalmol. 2004;7:47–51.
73. Dugan, SJ, Schwarz, PD, Roberts, SM, et al. Primary optic nerve meningioma and pulmonary metastasis in a dog. J Am Anim Hosp Assoc. 1993;29:11–16.
74. Spiess, BM, Wilcock, BP. Glioma of the optic nerve with intraocular and intracranial involvement in a dog. J Comp Pathol. 1987;97:79–84.
75. Naranjo, C, Schobert, C, Dubielzig, RR. Canine ocular gliomas: a retrospective study. Vet Ophthalmol. 2008;11:356–362.
76. Slatter, DH, Abdelbaki, Y. Lateral orbitotomy by zygomatic arch resection in the dog. J Am Vet Med Assoc. 1979;175:1179–1182.
77. Gilger, BC, Whitely, RD, McLaughlin, SA. Modified lateral orbitotomy for removal of orbital neoplasms in two dogs. Vet Surg. 1994;23:53–58.
78. O’Brien, MG, Withrow, SJ, Straw, RC, et al. Total and partial orbitectomy for the treatment of periorbital tumors in 24 dogs and 6 cats: a retrospective study. Vet Surg. 1996;25:471–479.
79. Håkansson, NW, Håkansson, BW. Transfrontal orbitotomy in the dog: an adaptable three-step approach to the orbit. Vet Ophthalmol. 2010;13:377–383.
80. Bartoe, JT, Brightman, AH, Davidson, HJ. Modified lateral orbitotomy for vision-sparing excision of a zygomatic mucocele in a dog. Vet Ophthalmol. 2007;10:127–131.
81. Adams, WM, Miller, PE, Vail, DM, et al. An accelerated technique for irradiation of malignant canine nasal and paranasal sinus tumors. Vet Radiol Ultrasound. 1998;5:475–481.
82. Roberts, SM, Lavach, JD, Severin, GA, et al. Ophthalmic complications following megavoltage irradiation of the nasal and paranasal cavities in dogs. J Am Vet Med Assoc. 1987;190:43–47.
83. Miller PE, Turek MM, Forrest LJ, et al: Ocular sparing using intensity modulated radiation therapy (IMRT) in a canine model of spontaneous sinonasal cancer: Proof of principle of conformal avoidance. Proceedings of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, 2005.
84. Lawrence, JA, Forrest, LJ, Turek, MM, et al. Proof of principle of ocular sparing in dogs with sinonasal tumors treated with intensity-modulated radiation therapy. Vet Radiol Ultrasound. 2010;51:561–570.
85. Imperia, PS, Lazarus, HM, Lass, JH. Ocular complications of systemic cancer chemotherapy. Surg Ophthalmol. 1989;34:209–230.
86. Martin, PL, Miller, PE, Mata, M, et al. Ocular inflammation in cynomolgus macaques following intravenous administration of a human monoclonal antibody. J Toxicol. 2009;28:5–16.
87. Robertson, DM. Changing concepts in the management of choroidal melanoma. Am J Ophthalmol. 2003;136:161–170.
88. Diener-West, M, Earle, JD, Fine, SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969–982.
89. Syed, NA, Nork, TM, Poulsen, GL, et al. Retinoblastoma in a dog. Arch Ophthalmol. 1997;115:758–763.
90. Shields, CL, Meadows, AT, Leahey, AM, et al. Continuing challenges in the management of retinoblastoma with chemotherapy. Retina. 2004;24:849–862.
91. Guy, J, Aptiauri, N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol. 1999;117:471–477.
92. Subramanian, L, Polan, AS. Cancer-related diseases of the eye: The role of calcium-binding proteins. Biochem Biophys Res Commun. 2004;322:1153–1165.
93. Ohgura, H, Yokoi, Y, Ohguro, I, et al. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol. 2004;137:1117–1119.
94. Gilmour, MA, Cardenas, MR, Blaik, MA, et al. Evaluation of a comparative pathogenesis between cancer-associated retinopathy in humans and sudden acquired retinal degeneration syndrome in dogs via diagnostic imaging and western blot analysis. Am J Vet Res. 2006;67:877–881.