Hematopoietic Tumors
 Section A
Section A
Canine Lymphoma and Lymphoid Leukemias
David M. Vail, Marie E. Pinkerton, and Karen M. Young
Lymphoma
The lymphomas (malignant lymphoma or lymphosarcoma) are a diverse group of neoplasms that have in common their origin from lymphoreticular cells. They usually arise in lymphoid tissues such as lymph nodes, spleen, and bone marrow; however, they may arise in almost any tissue in the body. Although the annual incidence of lymphoma is difficult to predict in the absence of a national canine tumor registry, it is clear that it represents one of the most common neoplasms seen in the dog. The annual incidence has been estimated to range between 13 to 24 per 100,000 dogs at risk.1-3 The annual incidence rates at specific ages are estimated to be 1.5 per 100,000 for dogs less than 1.0 year of age and 84 per 100,000 in the 10- to 11-year-old group. Lymphoma comprises approximately 7% to 24% of all canine neoplasia and 83% of all canine hematopoietic malignancies.4,5 In a review of the Veterinary Medical Data Base Program (VMDP) at Purdue University from 1987 to 1997, the frequency of canine lymphoma patients presented to 20 veterinary institutions increased from 0.75% of total case load to 2.0%, and it appears the frequency is continuing to increase. A similar trend is present in physician-based oncology; non-Hodgkin’s lymphoma (NHL) represents 5% of all new cancer cases, the fifth leading cause of cancer death, and the second fastest growing cancer in terms of mortality in humans.6 Middle-aged to older (median age of 6 to 9 years) dogs are primarily affected. A decreased risk for lymphoma is reported for intact females.7 Breeds reported to have a higher incidence include Boxers, bull mastiffs, basset hounds, St. Bernards, Scottish terriers, Airedales, and bulldogs; breeds at lower risk include dachshunds and Pomeranians.8,9
Etiology
The etiology of canine lymphoma is likely multifactorial and largely unknown; however, investigations are currently shedding significant light on the subject.
Genetic and Molecular Factors
Recent advances in molecular cytogenetics (see Chapter 1, Section A), including gene microarray techniques, have been and are currently being applied to investigations of chromosomal aberrations in dogs with lymphoma.10-16 The publication of the canine genome and the commercial availability of canine gene microarrays (GeneChip Canine Genome 2.0 Array, Affymetrix, Inc.) have led to advances in our understanding of genetic events occurring in lymphoma.17 Breen’s group has documented gain of dog chromosomes 13 and 31 and loss of chromosome 14 as the most common aberrations in a group of 25 cases analyzed.11 Chromosomal aberrations have also been associated with prognosis in dogs with lymphoma. A study of 61 dogs with lymphoma demonstrated a prognostic advantage in dogs with trisomy of chromosome 13 (25% of the dogs studied) as evidenced by increase in duration of first remission and overall survival time.18 Germline and somatic genetic mutations and altered oncogene/tumor suppressor gene expression, epigenetic changes (e.g., DNA hypomethylation), signal transduction, and death-pathway alterations (e.g., Bcl-2 family) are common in human lymphomas and have been reported in the dog as well (see Chapter 1, Section A, and Chapter 14, Section B). These include N-ras, p53, Rb, and p16 cyclin-dependent kinase aberrations.19-24 Additionally, differences in the prevalence of immunophenotypic subtypes of lymphoma among different breeds indicate heritable risks.25 Additionally, telomerase activity (see Chapter 2) has been documented in canine lymphoma tissues.26-28
Infectious Factors
The hypothesis that a retrovirus may be involved in the pathogenesis of canine lymphoma has not been confirmed. However, serologic detection of Epstein-Barr virus infection, linked to some forms of lymphoma in humans, has been documented in dogs with lymphoma and is currently being investigated.29
In humans, a direct association between Helicobacter sp. infections and development of gastric lymphoma has been made.30 Although this has not been definitively shown in dogs, there is evidence of Helicobacter sp. infection in laboratory beagle dogs resulting in gastric lymphoid follicle formation that is considered a precursor of mucosa-associated lymphoid tissue (MALT) lymphoma in humans.31
Environmental Factors
In humans, evidence has accumulated implicating phenoxyacetic acid herbicides, in particular 2, 4-dichlorophenoxyacetic acid (2, 4-D), in the development of NHL. A published hospital-based case-control study of dogs indicated that owners in households with dogs that develop malignant lymphoma applied 2, 4-D herbicides to their lawn and/or employed commercial lawn care companies to treat their yard more frequently than owners of dogs without lymphoma.32 The risk of canine lymphoma was reported to rise twofold (odds ratio [OR] = 1.3) with four or more yearly owner applications of 2, 4-D. The results of this study have come under criticism, and three additional follow-up investigations have not validated assertions of increased risk.33-35 In another study, dogs exposed to lawn treatment within 7 days of application were greater than 50 times more likely to have urine levels of 2, 4-D at 50 µg/L or higher.36 The highest concentration was noted 2 days after application. In an environmental case-control study performed in Europe, two variables, residency in industrial areas and use of chemicals (defined as paints or solvents) by owners, modestly increased the risk of developing lymphoma; however, no link was found with pesticide use.37
A weak association between lymphoma in dogs and exposure to strong magnetic fields was observed in a preliminary epidemiologic study.38 In this hospital-based case-control study, the risk of developing lymphoma categorized into high or very high exposure was increased (odds ratio = 1.8). More thorough studies are necessary to evaluate this association further. Proximity to environmental waste was implicated in two European studies; however, it was felt to be a risk indicator rather than a risk factor and would require further case-control investigations.39,40
Immunologic Factors
Impaired immune function has also been implicated in dogs with lymphoma. Immune system alterations in the dog such as immune-mediated thrombocytopenia, independent of age and sex, have been associated with a higher risk of subsequently developing lymphoma when compared to the normal population.41,42 Additional evidence comes from observations in human and feline transplantation patients. In a case-control study of cats undergoing renal transplant, 24% of cases developed cancer (36% of those were lymphoma) while on cyclosporine immunosuppressive therapy compared to 5.1% of control cats, none of which developed lymphoma (OR, 6.1; p = 0.001).43 A case of lymphoma developing in a dog following treatment with cyclosporine also exists.44 One report suggests an association between the immunodysregulation observed in dogs with atopic dermatitis and the risk of developing epitheliotropic T-cell lymphoma; whether this is associated with the disease or the immunomodulatory treatments commonly applied is unknown.45
Classification and Pathology
Classification of malignant lymphoma in dogs is based on anatomic location, histologic criteria, and immunophenotypic characteristics. The most common anatomic forms of lymphoma, in order of decreasing prevalence, are multicentric, gastrointestinal (GI), mediastinal, and cutaneous forms.46 Primary extranodal forms, which can occur in any location outside the lymphatic system, include the eyes, central nervous system (CNS), bone marrow, bladder, heart, and nasal cavity. The pathologic characteristics of the various anatomic classifications will be discussed in this section and clinical characteristics will be described in subsequent sections.
Eighty-four percent of dogs with lymphoma develop the multicentric form, which is usually characterized by the presence of superficial lymphadenopathy (Figure 32-1).46 The alimentary form of lymphoma is much less common, accounting for 5% to 7% of all canine lymphomas. This form is reported to be more common in male dogs than female dogs.6 Primary GI lymphoma in dogs may occur focally but more often affects multiple segments, with thickening of the wall, narrowing of the lumen, and frequently mucosal ulceration.47,48 Histologically, there is infiltration of neoplastic lymphocytes throughout the mucosa and submucosa, with occasional transmural infiltration. Liver and local lymph nodes are often secondarily involved. Lymphocytic-plasmacytic enteritis (LPE) can be seen adjacent to or distant from the primary tumor. Pathologically, some of these neoplasms may resemble plasma cell tumors, and aberrant production of immunoglobulins may occur. Histopathologically, distinguishing between GI lymphoma and LPE can be difficult. Some have suggested that LPE may be a prelymphomatous change in the GI tract. A syndrome of immunoproliferative intestinal disease characterized by LPE has been described in Basenjis, which subsequently develop GI lymphoma.49 In addition, plasma cell–rich areas with heterogeneous lymphomatous infiltration may resemble lesions of LPE. Only a few reports specifically identify the immunophenotype of the lymphocyte subpopulations in alimentary lymphoma in dogs. Historically, it was presumed that they most likely originate from B cells; however, recent evidence suggests that most GI lymphomas in dogs arise from T cells and often exhibit epitheliotropism.48,50 The Boxer and Shar-pei breeds may be overrepresented in cases of alimentary lymphoma.50,51
The mediastinal form of the disease occurs in approximately 5% of cases.46 This form is characterized by enlargement of the cranial mediastinal lymph nodes, thymus, or both (Figure 32-2). Hypercalcemia is reported to occur in 10% to 40% of dogs with lymphoma and is most common with the mediastinal form. In a study of 37 dogs with lymphoma and hypercalcemia, 16 (43%) had mediastinal lymphoma.52 The mediastinal form in dogs is most commonly associated with a T-cell phenotype.53,54
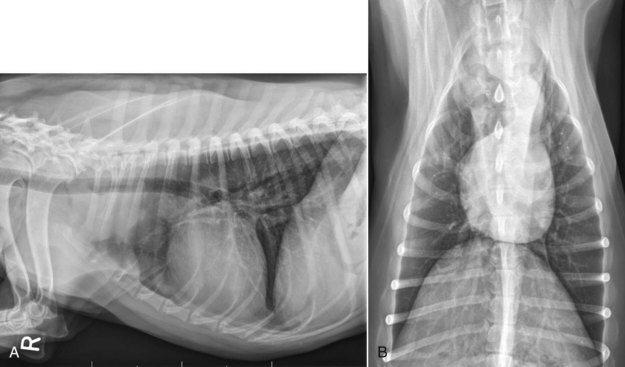
Figure 32-2 A, Lateral radiographic projection of a dog with mediastinal lymphoma. B, Ventrodorsal projection of the same dog.
Cutaneous lymphoma can be solitary or more generalized and usually is classified as epitheliotropic (mycosis fungoides) or nonepitheliotropic.55 Canine epitheliotropic cutaneous lymphoma originates from T-cells,55-60 similar to its development in humans. In dogs, these more commonly represent CD8+ cells, whereas in humans they are typically CD4+ cells. A rare form of cutaneous T-cell lymphoma, characterized by skin involvement with evidence of peripherally circulating large (15 to 20 µm in diameter) malignant T-cells with folded, grooved nuclei, has been described. In humans, this is referred to as Sézary syndrome and has been reported in both dogs and cats.61-63 Nonepitheliotropic cutaneous lymphomas form single or multiple dermal or subcutaneous nodules or plaques; histologically, they spare the epidermis and papillary dermis and affect the middle and deep portions of the dermis and subcutis.55
Atypical Anatomic Forms of Lymphoma
Hepatosplenic lymphoma is a relatively uncommon, distinct presentation in the dog marked by a lack of significant peripheral lymphadenopathy in the face of hepatic, splenic, and bone marrow infiltration with malignant lymphocytes, usually of T-cell origin.64,65 Biologically, this form of lymphoma is extremely aggressive and poorly responsive to therapy. In humans the tumor usually is composed of γδT-cells (i.e., T-cells that express the γδT-cell receptor), and this immunophenotype has been confirmed in at least one dog in the veterinary literature.65
Intravascular (angiotropic, angioendotheliomatosis) lymphoma is a distinct form of lymphoma defined as proliferations of neoplastic lymphocytes within the lumen and wall of blood vessels in the absence of a primary extravascular mass or leukemia. It has been reported several times in the veterinary literature, and in most cases it involves the CNS and peripheral nervous system (PNS), including the eye.66-71 The B-cell immunophenotype is most common in humans; however, in most reported cases in dogs, the origin is either T-cell or null cell (neither B- nor T-cell), although one case of a B-cell phenotype has been reported.
Pulmonary lymphomatoid granulomatosis (PLG) is a rare pulmonary infiltrative and/or nodular disorder characterized by a heterogenous accumulation of lymphocytes (both B and T, although some evidence suggests primarily a T-cell origin), neutrophils, plasma cells, and macrophages, often arranged angiocentrically.72-75 Whether this syndrome is a true lymphoma or a prelymphoma state is debatable. Clinical signs are related to respiratory compromise, and various chemotherapeutic protocols have been used with reported results varying from rapid progression to long-term clinical remissions.
Histologic Classification Systems
Lymphomas arise from clonal expansion of lymphoid cells with distinctive morphologic and immunophenotypic features. Many histologic systems have been used to classify NHL in humans, and some of these have been applied to lymphoma in the dog and other species. The National Cancer Institute (NCI) Working Formulation76 and the updated Kiel system77 have been adapted to canine tumors with some success. The World Health Organization (WHO) also publishes a histologic classification scheme, which uses the revised European American lymphoma (REAL) system as a basis for defining histologic categories of hematopoietic and lymphoid tumors in domestic animals.78 This system incorporates anatomic, histologic, and immunophenotypic criteria (B- and T-cell immunophenotype), with the goal of enabling accurate and reproducible diagnosis of specific neoplastic disease entities. This theoretically should assist in better tailoring of treatment protocols, better correlation of prognosis, and better comparative capabilities. Table 32-1 shows some of the WHO categories in three different surveys, including a 2-year survey (2008-2009) of canine necropsy and biopsy cases at the University of Wisconsin-Madison Veterinary Medical Teaching Hospital pathology service79,80; some of the less common categories in the WHO system were not represented and are not listed. The WHO system provides accurate and consistent reproducible diagnostic results similar to the system used in human pathology; accuracy among a group of pathologists examining 300 cases was at 83% agreement, and accuracy in evaluating the six most common diagnoses (80% of the cases) was 87%.81 Clinical studies are needed to correlate the various categories of disease with biologic behavior, response to treatment, and prognosis. Preliminary results indicate dogs with indolent lymphoma (e.g., marginal zone lymphoma, follicular lymphoma, B- or T-cell small cell lymphoma, T-cell–rich B-cell lymphoma, and T zone lymphoma) maintain normal activity and appetite levels even during advanced stages of disease and experience long-term survival even with limited or no therapy.81-84
Table 32-1
World Health Organization Classification System for Canine Lymphoma
*Non-B, non-T lymphomas.
†Three B-cell and four T-cell cutaneous lymphomas, not specified as epitheliotropic/nonepitheliotropic.
‡Includes one T-zone lymphoma.
Modified from Sueiro FAR, Alessi AC, Vassallo J: Canine lymphomas: A morphological and immunohistochemical study of 55 cases, with observations on p53 immunoexpression, J Comp Pathol 131:207–213, 2004; and Vezzali E, Parodi AL, Marcato PS, et al: Histopathological classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO, Vet Comp Oncol 8:36–49, 2009.
The Working Formulation (WF) was developed to allow investigators to “translate” among the numerous classification systems so that clinical trials could be compared in humans. Most of the larger compilations agree that most canine lymphomas are intermediate or high grade; however, diffuse immunoblastic forms appear to predominate in the United States, whereas the follicular large cell variations predominate in Europe. A comparison of European and American classifications is warranted based on this discrepancy. The WF categorizes tumors according to pattern (diffuse or follicular) and cell type (e.g., small cleaved cell, large cell, immunoblastic), but it does not include information about the immunophenotype of the tumor.76 The WF subtypes are related to the biology of the tumor and patient survival. The updated Kiel classification includes the architectural pattern, morphology (centroblastic, centrocytic, or immunoblastic), and immunophenotype (B-cell or T-cell) of the tumor cells.77 In both systems, the tumors can then be categorized as low-grade, intermediate grade, or high-grade malignancies. Low-grade lymphomas composed of small cells with a low mitotic rate typically progress slowly and are associated with long survival times but are ultimately incurable. High-grade lymphomas with a high mitotic rate progress rapidly but are more likely to respond initially to chemotherapy and, in humans, are potentially curable.
Several features of canine lymphomas become apparent when these classification systems are applied. The most striking difference between canine and human lymphomas is the scarcity of follicular lymphomas in the dog.79,80 Some diffuse lymphomas in the dog may initially be follicular, but these may progress to the more aggressive, diffuse form by the time of diagnostic biopsy. The most common form of canine lymphoma is diffuse large-cell lymphoma, a high-grade tumor most commonly of B-cell origin.80,81,85 Only a small percentage of canine lymphomas (5.3% to 29%) are considered low-grade tumors.
High-grade lymphomas occur frequently if diffuse large-cell lymphomas, classified as intermediate grade in the WF, are considered high-grade, as in the updated Kiel classification (in which they are labeled as diffuse centroblastic lymphomas). A documented difference exists in the prevalence of the various immunophenotypes based on breed.25 For example, cocker spaniels and Doberman pinschers are more likely to develop B-cell lymphoma, Boxers are more likely to have T-cell lymphoma, and golden retrievers appear to have an equal likelihood of B- and T-cell tumors.
To be clinically useful, these classification systems in the end must yield information about response to therapy, maintenance of remission, and survival. Some studies suggest that the subtypes in the WF can be correlated with survival, and the Kiel system may be useful for predicting relapse.86,87 In most studies, high-grade lymphomas achieve a complete response (CR) to chemotherapy significantly more often than low-grade tumors. However, dogs with low-grade tumors may live a long time without aggressive chemotherapy.83,84 Dogs with T-cell lymphomas have shown a lower rate of CR to chemotherapy and shorter remission and survival times than dogs with B-cell tumors (with the exception of low-grade T-cell subtypes).53,54,86,88 Furthermore, T-cell lymphomas tend to be associated with hypercalcemia.89,90
In the veterinary literature, 60% to 80% of canine lymphomas are of B-cell origin; T-cell lymphomas account for 10% to 38%; mixed B- and T-cell lymphomas account for as many as 22%; and null cell tumors (i.e., neither B-cell nor T-cell immunoreactive) represent fewer than 5%.53,54,91-93 The development of monoclonal antibodies to detect specific markers on canine lymphocytes has made immunophenotyping of tumors in dogs routinely available in many commercial laboratories. Such techniques can be performed on paraffin-embedded samples, from tissue microarrays, on cytologic specimens obtained by fine-needle aspiration (FNA) of lesions, or by flow cytometric analysis of cellular fluid samples (e.g., peripheral blood, effusions) and lesion aspirates.
The Rappaport classification system, proposed in 1956 for human NHL, describes the architectural pattern (follicular or diffuse) and the cytologic features (well differentiated, poorly differentiated, or histiocytic) of lymphoma.94,95 This system has not proved useful in providing prognostic information or in guiding therapy in dogs with lymphoma because of the low number of follicular lymphomas in dogs, the problematic “histiocytic” subgroup, and the failure to account for different morphologic and immunologic cell types.
One criticism of the Rappaport, Kiel, and WF classification systems is that they fail to include extranodal lymphomas as a separate category. The WHO system does include anatomic location as a factor in determining certain categories. Although differences between nodal and extranodal tumors in biologic behavior and prognosis are well recognized, comparative information about the histogenesis of these tumors is lacking. For example, in humans small-cell lymphomas arising from MALT are composed of cells with a different immunophenotype than that of other small-cell lymphomas (i.e., MALT lymphomas typically are negative for both CD5 and CD10). Except for cutaneous lymphoid neoplasms, detailed characterization of extranodal lymphomas in dogs has not been done. Although cutaneous lymphoma is a heterogeneous group of neoplasms that includes an epitheliotropic form resembling mycosis fungoides and a nonepitheliotropic form, most cutaneous lymphomas have a T-cell phenotype.64,96
To summarize, it is important to determine the histologic grade of canine lymphomas as low (small lymphocytic or centrocytic lymphomas) or intermediate to high (diffuse large cell, centroblastic, and immunoblastic lymphomas) and the architecture as diffuse or follicular. Furthermore, determining the immunophenotype of the tumor provides useful information. Response rates to chemotherapy are, in general, better in animals with B-cell tumors and intermediate- to high-grade lymphomas. Dogs with low-grade lymphomas can have long survival times without aggressive therapy.
History and Clinical Signs
The clinical signs associated with canine lymphoma are variable and depend on the extent and location of the tumor. Multicentric lymphoma, the most common form, is usually distinguished by the presence of generalized painless lymphadenopathy (see Figure 32-1). Enlarged lymph nodes are usually painless, rubbery, and discrete and may initially include the mandibular and prescapular nodes. In addition, hepatosplenomegaly and bone marrow involvement occur commonly. Most dogs with multicentric lymphoma present without dramatic signs of systemic illness (WHO substage a) (Box 32-1); however, a large array of nonspecific signs such as anorexia, weight loss, vomiting, diarrhea, emaciation, ascites, dyspnea, polydipsia, polyuria, and fever can occur (WHO substage b). Dogs presented with T-cell lymphoma are more likely to have constitutional (i.e., substage b) signs. Polydipsia and polyuria are particularly evident in dogs with hypercalcemia of malignancy. Dogs may also be presented with clinical signs related to blood dyscrasias secondary to marked tumor infiltration of bone marrow (myelophthisis) or paraneoplastic anemia, thrombocytopenia, or neutropenia. These could include fever, sepsis, anemia, and hemorrhage. Diffuse pulmonary infiltration is seen in 27% to 34% of dogs with the multicentric form, as detected by radiographic changes (Figure 32-3).97,98 Based on bronchoalveolar lavage, the actual incidence of lung involvement may be higher.99,100
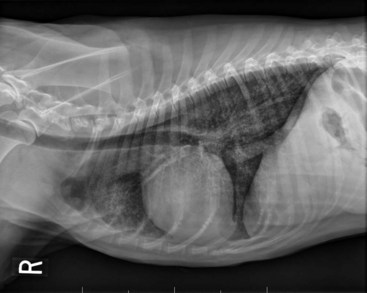
Figure 32-3 Lateral projection of a thoracic radiograph of a dog with diffuse interstitial infiltration with lymphoma secondary to multicentric lymphoma.
Dogs with GI or alimentary lymphoma are usually presented with nonspecific GI signs, such as vomiting, diarrhea, weight loss, and malabsorption.47,101,102 Mesenteric lymph nodes, spleen, and liver may be involved.
The mediastinal form of lymphoma is characterized by enlargement of the cranial mediastinal structures and/or thymus (see Figure 32-2), and clinical signs are associated with the extent of disease with resulting respiratory compromise or polydipsia/polyuria from hypercalcemia. Commonly, dogs are presented with respiratory distress caused by a space-occupying mass and pleural effusion, exercise intolerance, and possibly regurgitation. Additionally, dogs with mediastinal lymphoma may present with precaval syndrome, characterized by pitting edema of the head, neck, and forelimbs secondary to tumor compression or invasion of the cranial vena cava (Figure 32-4).
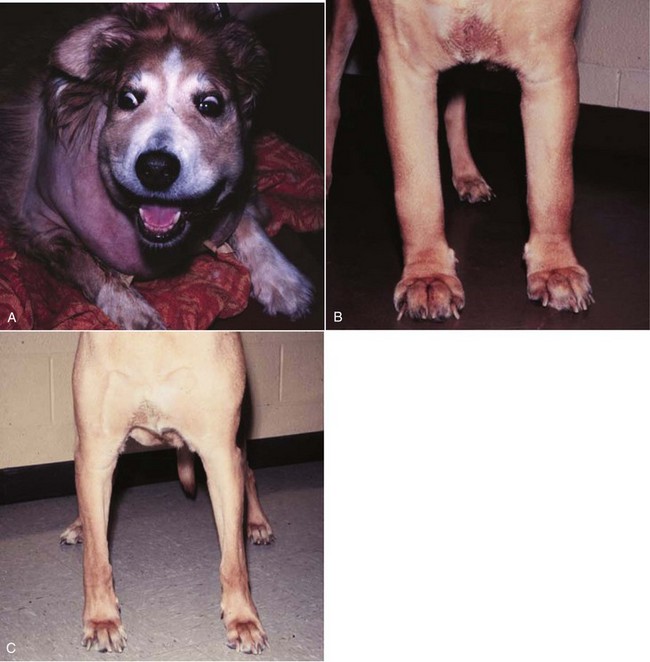
Figure 32-4 A, Facial edema in a dog with precaval syndrome secondary to mediastinal lymphoma. B, Forelimb edema in a dog with precaval syndrome secondary to mediastinal lymphoma. C, The dog in B 24 hours after radiation therapy to the cranial mediastinal mass, showing resolution of pitting edema.
Signs in dogs with extranodal lymphoma depend on the specific organ involved. Cutaneous lymphoma is usually generalized or multifocal.55-57 Tumors occur as nodules, plaques, ulcers, and erythemic or exfoliative dermatitis with focal hypopigmentation and alopecia. Epitheliotropic T-cell lymphoma (e.g., mycosis fungoides) typically has a clinical course with three apparent clinical stages. Initially, there will be scaling, alopecia, and pruritus (Figure 32-5, A), which can mimic a variety of other skin conditions. As the disease progresses, the skin becomes more erythematous, thickened, ulcerated, and exudative. The final stage is characterized by proliferative plaques and nodules with progressive ulceration (Figure 32-5, B). Oral involvement may also occur and this can appear as multicentric erythematous plaque-like lesions or nodules associated with the gum and lips (Figure 32-5, C). Extracutaneous involvement can also occur, most often in the lymph nodes, spleen, liver, and bone marrow. Nonepitheliotropic cutaneous lymphomas form single or multiple dermal or subcutaneous nodules or plaques; histologically, they spare the epidermis and papillary dermis and affect the middle and deep portions of the dermis and subcutis.55
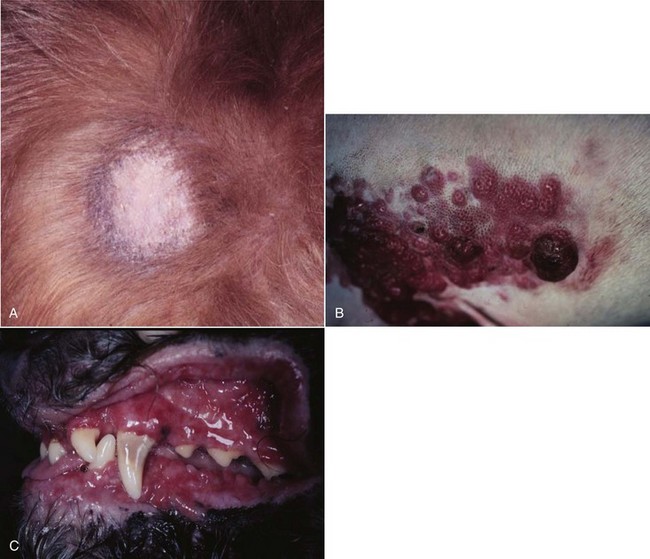
Figure 32-5 A, Early epitheliotropic cutaneous lymphoma in the scaly, plaque stage in a dog. B, Advanced epitheliotropic cutaneous lymphoma in the nodular stage in a dog. C, Oral mucosal epitheliotropic cutaneous lymphoma in a dog.
Dogs with primary CNS lymphoma may be presented with either multifocal or solitary involvement.103-105 Seizures, paralysis, and paresis may be noted. Ocular lymphoma is characterized by infiltration and thickening of the iris, uveitis, hypopyon, hyphema, posterior synechia, and glaucoma.106,107 In one study of 94 cases of canine multicentric lymphoma, 37% had ocular changes consistent with lymphoma, and in a series of 102 cases of uveitis in dogs, 17% were secondary to lymphoma.107 Anterior uveitis was most commonly seen in advanced stage of disease (stage V). Dogs with intravascular lymphoma usually present with signs relative to CNS, PNS, or ocular involvement.66-71 These include paraparesis, ataxia, hyperesthesia, seizures, blindness, lethargy, anorexia, weight loss, diarrhea, polyuria, polydipsia, and intermittent fever. Finally, dogs with pure hepatosplenic lymphoma usually are presented with nonspecific signs of lethargy, inappetence, and weakness and often are icteric.64,65
The differential diagnosis of lymphadenopathy depends on the dog’s travel history (i.e., relative to infectious disease) and the size, consistency, and location of affected lymph nodes. Other causes of lymphadenopathy include infections caused by bacteria, viruses, parasites (Toxoplasma sp., Leishmania sp.), rickettsial organisms (Salmon-poisoning, Ehrlichia sp.), and fungal agents (Blastomyces and Histoplasma sp.). The potential for hypercalcemia to accompany systemic fungal diseases may further complicate differentiation from lymphoma. Discrete, hard, asymmetric lymph nodes, particularly if they are fixed to underlying tissues, may indicate metastatic tumors such as mast cell tumor or carcinoma. Immune-mediated diseases (e.g., pemphigus, systemic lupus erythematosus) also may result in mild-to-moderately enlarged lymph nodes. The various differential diseases or conditions that can resemble canine lymphoma are listed in Table 32-2.
Table 32-2
Differential Diseases or Conditions That Can Resemble Canine Lymphoma
*The existence of this disease is controversial; in most cases, the disease has been reclassified as a lymphoid neoplasm.
Canine lymphoma also may be associated with paraneoplastic syndromes (see Chapter 5). Anemia is the most common lymphoma-related paraneoplastic syndrome.108 Paraneoplastic hypercalcemia is also common and is characterized clinically by anorexia, weight loss, muscle weakness, lethargy, polyuria, polydipsia, and rarely CNS depression and coma. Lymphoma-induced hypercalcemia in most cases results from parathyroid hormone–related peptide (PTHrP), elaborated by neoplastic cells; however, it can also be related to the production of several other humoral factors, including interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and vitamin D analogs (e.g., 1,25-dihydroxyvitamin D).89,109,110 As previously discussed, hypercalcemia is most commonly associated with the T-cell immunophenotype. Other paraneoplastic syndromes that may be encountered include monoclonal gammopathies, neuropathies, and cancer cachexia.
Diagnostics and Clinical Staging
For dogs suspected of having lymphoma, the diagnostic evaluation should include a thorough physical examination; complete blood count (CBC), with a differential cell count, including a platelet count; a serum biochemical profile; and urinalysis. Optimally, ionized calcium should be measured. Ultimately, obtaining tissue or cytologic specimens for a definitive diagnosis is essential.
Physical Examination
A thorough physical examination should include palpation of all assessable lymph nodes, including a rectal examination; in the authors’ experience, a significant proportion of dogs will have rectal polyps consisting of aggregates of neoplastic lymphocytes. Inspection of mucous membranes for pallor, icterus, petechiae, and ulceration should be undertaken as these signs may indicate anemia or thrombocytopenia secondary to myelophthisis or immune-mediated disease or may be evidence of major organ failure or uremia. Abdominal palpation may reveal organomegaly, intestinal wall thickening, or mesenteric lymphadenopathy. The presence of a mediastinal mass and/or pleural effusion can be suspected following thoracic auscultation. An ocular examination, including funduscopic assessment, may reveal abnormalities (e.g., uveitis, retinal hemorrhage, ocular infiltration) in approximately one-third to one-half of dogs with lymphoma.107,111
Complete Blood Count, Biochemistry Profile, and Urinalysis
Anemia, the most common lymphoma-related hematologic abnormality, is usually normochromic and normocytic (nonregenerative), consistent with anemia of chronic disease.108 However, hemorrhagic and hemolytic anemias may also occur, and regenerative anemias may reflect concomitant blood loss or hemolysis. Additionally, if significant myelophthisis is present, anemia may be accompanied by thrombocytopenia and leukopenia.112,113 In animals with anemia or evidence of bleeding, in addition to a platelet count, a reticulocyte count and coagulation testing may be indicated. Thrombocytopenia may be seen in 30% to 50% of cases, but bleeding is seldom a clinical problem. Neutrophilia can be seen in 25% to 40% of dogs and lymphocytosis occurs in approximately 20% of affected dogs.108 Circulating atypical lymphocytes may be indicative of bone marrow involvement and leukemia. It is important to differentiate multicentric lymphoma with bone marrow involvement (i.e., stage V disease) from primary lymphoblastic leukemia (see later), as the prognosis for each is entirely different. Hypoproteinemia is observed more frequently in animals with alimentary lymphoma. In dogs with a high total protein or evidence of an increased globulin fraction on a chemistry profile, serum proteins may be evaluated by serum electrophoresis. Monoclonal gammopathies have been reported to occur in approximately 6% of dogs with lymphoma.114
Serum biochemical abnormalities often reflect the anatomic site involved, as well as paraneoplastic syndromes such as hypercalcemia. In cases of hypercalcemia of unknown origin, lymphoma should always be considered high on the differential disease list, and diagnostic testing directed at this possibility should be undertaken (see Chapter 5). In addition, the presence of hypercalcemia can serve as a biomarker for response to therapy and early recurrence. Increased urea nitrogen and creatinine concentrations can occur secondary to renal infiltration with tumor, hypercalcemic nephrosis, or prerenal azotemia from dehydration. Increases in liver-specific enzyme activities or bilirubin concentrations may result from hepatic parenchymal infiltration. Increased serum globulin concentrations, usually monoclonal, occur infrequently with B-cell lymphoma.
Urinalysis is part of the minimum database used to assess renal function and the urinary tract. For example, isosthenuria and proteinuria in the absence of an active sediment may indicate renal disease, and hematuria may result from a hemostatic abnormality. It is important to remember that isosthenuria in azotemic dogs with hypercalcemia is not necessarily indicative of renal disease as the high calcium levels interfere with tubular concentration capabilities through disruption of antidiuretic hormone (ADH) control.
Several abnormalities in serum have been explored as biomarkers of lymphoma in the dog. Examples include alpha-fetoprotein, alpha-1 glycoprotein levels, zinc, chromium, iron, endostatin, vascular endothelial growth factor (VEGF), lactate dehydrogenase, C-reactive protein haptoglobin, and antioxidants/oxidative stress markers.115-122 The clinical, biologic, and prognostic significance of these alterations is yet to be definitively characterized.
Histologic and Cytologic Evaluation of Lymph Nodes
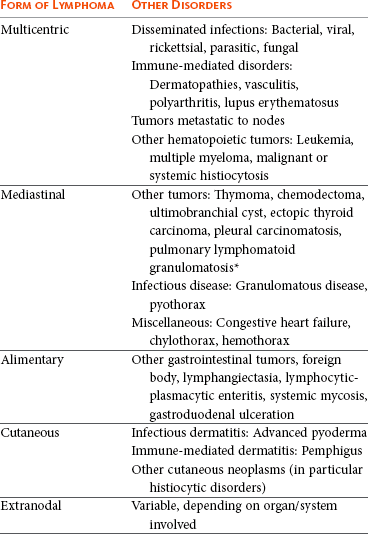
Morphologic examination of the tissue and cells that constitute the tumor is essential to the diagnosis of lymphoma. Care should be taken to avoid lymph nodes from reactive areas (e.g., mandibular lymph nodes), unless those nodes are the only ones enlarged; the prescapular or popliteal lymph nodes are preferable if also involved. Also, lymphoid cells are fragile, and in preparing smears of aspirated material only gentle pressure should be applied in spreading material on the slides. In most cases, a diagnosis of lymphoma can be made on evaluation of fine-needle aspirates of affected lymph nodes or other tissues. Typically, most of the cells are large lymphoid cells (>2 times the diameter of a red blood cell [RBC] or larger than neutrophils), and they may have visible nucleoli and basophilic cytoplasm (Figure 32-6, A) or fine chromatin with indistinct nucleoli. Because tissue architecture is not maintained in cytologic specimens, effacement of the node or capsular disruption cannot be detected. Therefore marked reactive hyperplasia characterized by increased numbers of large lymphoid cells may be difficult to distinguish from lymphoma, and small cell lymphomas may have few cytologic clues that point to malignancy. Also, classification of lymphoma, which has been attempted using cytologic appearance and immunophenotypic analysis,123 into subcategories that make up the low-, intermediate-, and high-grade forms is performed most accurately on histologic sections (discussed previously).
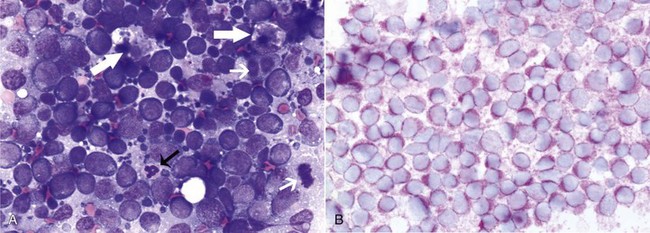
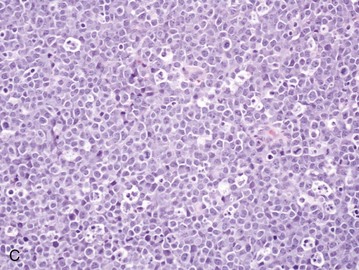
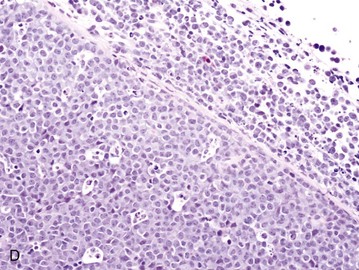
Figure 32-6 Lymph nodes from dogs with lymphoma. A, Fine-needle aspirate. Note the homogenous population of large lymphoid cells with prominent nucleoli and basophilic cytoplasm. These cells are larger than the neutrophil (black arrow) in the field. Mitotic figures (thin white arrows) and tingible body macrophages (thick white arrows) also are present. (Wright’s stain, ×60 objective.) B, Fine-needle aspirate stained for immunoreactivity for CD79a. Note that nearly all of the lymphocytes express CD79A. The diagnosis was B-cell lymphoma. (Alkaline phosphatase/fast red, ×60 objective.) C, Histologic section. Note effacement of normal architecture. The white spaces are macrophages, giving a “starry sky” appearance to the lymph node. (H&E, ×20 objective.) D, Histologic section. Note the presence of tumor cells outside the capsule of the lymph node. (H&E, ×20 objective.)
For accurate histopathologic evaluation, an entire lymph node, including the capsule, should be removed, placed in buffered formalin, and submitted to a pathologist. Although needle core biopsies may be satisfactory, it is important to avoid crush artifact or inadequate sample size. Most pathologists prefer whole node biopsies because they provide the maximal amount of information. Effacement of normal nodal architecture by neoplastic lymphocytes and capsular disruption are characteristic findings (Figure 32-6, C and D). Diagnostic ultrasonography and ultrasound-guided FNA or needle biopsy have been useful for evaluation of involvement of the liver, spleen, or abdominal lymph nodes.124-126 Aspiration of ultrasonographically normal splenic tissue is rarely contributory to a diagnosis.124 If possible, the diagnosis should be made by sampling peripheral nodes, avoiding percutaneous biopsies of the liver and spleen. However, if there is no peripheral node involvement, it is appropriate to biopsy affected tissues in the abdominal cavity.
Histologic and Cytologic Evaluation of Extranodal Sites
When GI lymphoma is suspected, an open surgical wedge biopsy of the intestine is preferred in most cases to differentiate lymphoma from lymphocytic enteritis. If associated abdominal lymph nodes also appear involved, image-guided biopsies may be associated with less morbidity than intestinal biopsies. Multiple samples may be necessary to accurately diagnose segmental disease. Endoscopic biopsies may be inadequate as only a superficial specimen is obtained; however, more aggressive endoscopic biopsy techniques combined with more accurate histopathologic, immunophenotypic, and molecular assessments are improving the diagnostic yield of these less invasive techniques.127-130 In many dogs with primary GI lymphoma, an inflammatory nonneoplastic infiltrate (i.e., LPE) may be misdiagnosed on biopsy specimens that are too superficial. The application of assays for clonal expansion (e.g., PARR—see next section on molecular diagnostic techniques) does not appear as yet to be as accurate for endoscopically derived intestinal biopsies as with other solid lymphoid tumors in dogs.
Cytologic examination of cerebrospinal fluid (CSF), thoracic fluid, or mass aspirates is indicated in animals with CNS disease, pleural effusion, or an intrathoracic mass, respectively. In one study of dogs with CNS involvement, CSF analysis was diagnostic in seven of eight dogs.103 Characteristics of the CSF included an increased nucleated cell count in the seven dogs, and 95% to 100% of the cells were atypical lymphocytes. The CSF protein concentration was increased in five of the dogs, ranging from 34 to 310 mg/dL (reference interval: <25 mg/dL).
For cutaneous lymphoma, punch biopsies (4 to 8 mm) should be taken from the most representative and infiltrative, but not secondarily infected, skin lesions. Application of immunophenotypic and clonality assessments of cutaneous biopsies can aid in differentiating lymphoma from benign lymphocytic lesions.58,131,132
Molecular Diagnostic Techniques
Molecular techniques can be used to establish a diagnosis of lymphoma or to further characterize the tumor after the initial diagnosis is made. Tissues and cells from peripheral blood, lymph nodes, nonlymphoid sites, and effusions can be analyzed by various molecular means to aid in cases that represent a more difficult diagnostic challenge, particularly in cases where reactive lymphocytosis and lymphoma are both possible based on standard histologic or cytologic assessment. These include histochemical and cytochemical, immunohistochemical and immunocytochemical, flow cytometric, and polymerase chain reaction (PCR) techniques. For example, the immunophenotype (B-cell versus T-cell),93,133-140 proliferation rate (e.g., expression of Ki67, proliferating cell nuclear antigen [PCNA] expression, argyrophilic nucleolar organizer regions [AgNOR]),53,133,141-145 and clonality (PCR for antigen receptor gene rearrangement [PARR])146-154 of the tumor can be determined. The availability of such analyses is increasing; however, at present, only immunophenotype and PARR clonality assays are routinely used in dogs to inform clinical decision making.
Immunophenotyping
Immunophenotyping is used to determine the type of cells that comprise the tumor, but this technique also can be helpful for making the initial diagnosis.133-140,155 When a heterogenous population of lymphocytes is expected in a tissue, documentation of a homogeneous population of the same immunophenotype is supportive of a neoplastic process. The immunophenotype of a lymphocyte is identified by determining the expression of molecules specific for B-cells (e.g., CD79a, CD20) and T-cells (e.g., CD3). Although tumor cells sometimes have morphologic characteristics that typify a particular immunophenotype, exceptions occur, and morphologic appearance cannot be used as the sole determinant of immunophenotype. For example, in a series of nine high-grade T-cell lymphomas and leukemias in dogs, the cells had a plasmacytoid appearance, typically associated with B-cell lymphoma.156 Similarly, anatomic location does not always predict the immunophenotype.
For accurate determination of immunophenotype, antibodies against lymphocyte markers are applied to tissue sections (immunohistochemistry), cytologic specimens (immunocytochemistry), or individual cells in a fluid medium (flow cytometry). Flow cytometric evaluation of cells obtained by needle aspiration is also feasible. For T-cells, markers include CD3 (pan T), CD4 (helper T), and CD8 (cytotoxic T); for B-cells, the markers are CD79a (Figure 32-6, B), CD20, and CD21. Increasingly, aberrant expression of CD molecules has been reported in canine lymphoma. In a study of 59 dogs with lymphoma, tumor cells from six dogs were positive for both T- and B-cell markers; however, a clonality assay (see later) revealed clonality either of the T-cell or the immunoglobulin receptor but not both. This indicates that in some cases, the malignant cells may co-express B- and T-cell markers.93 Antibodies against these molecules are used to determine the immunophenotype; however, they also have potential utility as a therapeutic modality if tumor cells could be targeted using these antibodies.
Other Immunohistochemical and Immunocytochemical Assessments
Assessments of markers of multidrug resistance and apoptotic pathways (e.g., P-glycoprotein, p53, Bcl-2 proteins) have been evaluated in dogs with lymphoma.19,79,142,156a,156b However, their clinical significance and utility await further evaluation.
Clonality Assays
Occasionally, diagnosis of lymphoma and differentiation of malignant versus benign proliferation of lymphocytes are not possible based on standard histologic and cytologic criteria. In these cases, advanced molecular analyses may be helpful to confirm a diagnosis. Clonality is the hallmark of malignancy; that is, the malignant cell population theoretically should be derived from expansion of a single malignant clone characterized by a particular DNA region unique to that tumor. For example, in a dog with T-cell lymphoma, all the malignant cells theoretically should have the same DNA sequence for the variable region of the T-cell receptor gene. Likewise, in a dog with B-cell lymphoma, the tumor cells should have identical DNA sequences in the variable region of the immunoglobulin (Ig) receptor gene. Conversely, in reactive lymphocytosis, the cells are polyclonal for their antigen receptors. Using this knowledge, investigators have used PCR technology to amplify the variable regions of the T-cell and immunoglobulin receptor genes to detect the presence of clonal lymphocyte populations in dogs (see Figure 8-4 of Chapter 8). These techniques are reviewed in Chapter 8 and elsewhere.150 In physician-based medicine, such assays of clonality are approximately 70% to 90% sensitive and have a false-positive rate of approximately 5%, and recent studies report similar rates in dogs. False-negative and false-positive results can occur with clonality assays. For example, cells from a dog with lymphoma may be negative for clonality if the clonal segment of DNA is not detected with the primers used, if the malignant cells are natural killer (NK) cells (rare), or if the malignant cells are present in too low a frequency to be detected. False positives occur rarely in some infectious diseases (e.g., ehrlichiosis and Lyme disease). In these cases, a diagnosis should be made only after considering the results of all the diagnostic tests, including histologic/cytologic evaluation, immunophenotyping, and clonality studies in conjunction with signalment and physical examination findings. These molecular techniques, although helpful for diagnosis, could also have utility in detecting early recurrence and in determining more accurate clinical stage and so-called “molecular remission rates” because they are more sensitive than standard cytologic assessment of peripheral blood, bone marrow, or lymph nodes (covered subsequently in section on treatment response).
Proteomics
Proteomics comprises, simplistically, methodologies that analyze the entire protein component or protein signature of cells (the proteome). Protein components of a cell (normal or malignant) change over time with upregulation and downregulation of gene expression in response to varied stimuli (e.g., growth factors, environmental cues). It may therefore be possible to use the field of proteomics to identify serum biomarkers of malignancy (i.e., cancer-specific protein markers) and to further analyze response to therapy or even to predict which therapies are appropriate for an individual patient’s tumor. Although in its infancy in veterinary oncology, preliminary investigations of the proteome of dogs with lymphoma have been reported157-160; however, they have yet to reach the level of sophistication in which useful output would have an impact on clinical decision making.
Staging
After a diagnosis has been established, the extent of disease should be determined and categorized by the clinical stage of disease. The WHO staging system routinely used to stage dogs with lymphoma is presented in Box 32-1. Most dogs (>80%) are presented in advanced stages (III to IV). Diagnostic imaging and assessment of bone marrow involvement may be indicated for staging. The degree to which thorough staging is implemented depends on whether the result will alter the treatment plan, whether relevant prognostic information is gleamed, and whether the clients need to know the stage prior to initiating (or declining) a treatment plan. Additionally, when comparing different treatment protocols with respect to efficacy, consistent and similar staging diagnostics should be used to avoid so-called “stage migration,” which results when one staging methodology is more accurate than another.161 The impact of stage migration on prognosis should be considered when comparing different published outcomes.
Bone Marrow Evaluation
A bone marrow aspirate or biopsy (from proximal humerus or iliac crest) is recommended for complete staging and prognostication and is indicated in dogs with anemia, lymphocytosis, peripheral lymphocyte atypia, or other peripheral cytopenias. In one study of 53 dogs with lymphoma, 28% had circulating malignant cells and were considered leukemic, whereas bone marrow examination indicated involvement in 57% of the dogs.162 The presence of a few prolymphocytes and large lymphocytes with nucleoli in the circulation of dogs with lymphoma may indicate bone marrow involvement. It is important to remember these cells also can be seen with GI parasitism, immune-mediated hemolytic anemia, and other immune-mediated and infectious diseases. As discussed previously, tumor cells within the peripheral and bone marrow compartments can also be identified using clonality assays (PARR) that are more sensitive than routine microscopic examination in detecting malignant cells; however, the prognostic significance of the knowledge gained with more sensitive staging methodologies is yet to be determined. Although bone marrow evaluation may offer prognostically valuable information, it is not necessary to perform the procedure if the client is committed to treat regardless of stage.
Imaging
Evaluation of thoracic and abdominal radiographs may be important in determining the extent of internal involvement (Figure 32-7). Approximately 60% to 75% of dogs with multicentric lymphoma have abnormalities on thoracic radiographs, with one-third having evidence of pulmonary infiltrates (see Figure 32-3) and two-thirds having thoracic lymphadenopathy (sternal and tracheobronchial lymph nodes [see Figure 32-7]) and widening of the cranial mediastinum (see Figure 32-2).97,98 Pulmonary infiltrates usually are represented by an interstitial and/or alveolar pattern; however, nodules (rarely) and bronchial infiltrates can also occur.163 Pleural effusion may also be present. Cranial mediastinal lymphadenopathy is detected in 20% of dogs with lymphoma.97,163 Abdominal radiographs reveal evidence of involvement of medial iliac (sublumbar) and/or mesenteric lymph node, spleen, or liver in approximately 50% of cases. In the authors’ practice, for the typical cases of canine multicentric lymphoma, imaging is limited to thoracic radiographs as there is no prognostic difference between dogs with stage III and IV disease (i.e., liver/spleen involvement), whereas the presence of cranial mediastinal lymphadenopathy is of prognostic significance (see prognosis section). However, if there are clinical signs attributable to abdominal disease or if complete staging is necessary (e.g., for clinical trial inclusion), further imaging of the abdomen is warranted. Abdominal ultrasonography can be important for obtaining ultrasound-guided intraabdominal samples for diagnosis. It may also be useful for the diagnosis of GI, abdominal nodal, and hepatosplenic lymphoma.125 Ultrasonographic (including Doppler ultrasound) assessment of peripheral lymph nodes has also been explored126; however, its clinical applicability is questionable because cytologic assessment of peripheral nodes is easy, inexpensive, and of higher diagnostic utility.
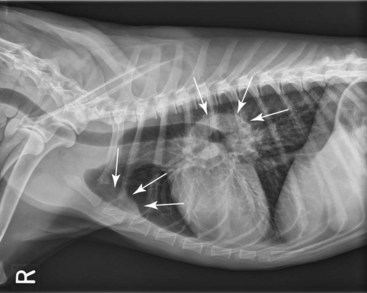
Figure 32-7 Lateral radiographic projection of a dog with sternal and hilar lymphadenopathy due to lymphoma.
Advanced imaging modalities, including computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), or PET/CT imaging, are becoming more commonplace in veterinary practice and their utility is only now being determined.164-169 PET/CT imaging is the current standard of care for following and indeed predicting durability of treatment response in human patients with lymphoma, and both [18F]fluorothymidine (18FLT) PET/CT and [18F]fluoro-D-glucose (18FDG) PET imaging have been reported in dogs with lymphoma.164-166 18FLT-PET/CT functional and anatomic imaging shows promise for the evaluation of response to cytotoxic chemotherapy in dogs with lymphoma and for predicting relapse before standard clinical and clinicopathologic confirmation (Figure 32-8).
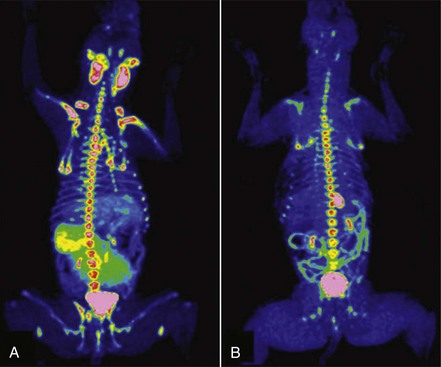
Figure 32-8 A, FLT-PET/CT image of a 3-year-old MN Hound cross illustrating FLT uptake in the peripheral nodes, bone marrow, kidneys, bladder, and spleen. B, FLT-PET/CT image of the same dog 3 weeks after his final dose of chemotherapy. The lymph nodes were small on CT with minimal FLT uptake on PET images. Note the persistent uptake in the bone marrow, kidneys, and bladder. (Reprinted with permission from Lawrence J, Vanderhoek M, Barbee D, et al: Use of 3′-deoxy-3′-[18F]fluorothymidine PET/CT for evaluating response to cytotoxic chemotherapy in dogs with non-Hodgkin’s lymphoma, Vet Radiol Ultrasound 50:660–668, 2009.)
Treatment of Multicentric Lymphoma
The therapeutic approach to a particular patient with lymphoma is determined by the stage and substage of disease, the presence or absence of paraneoplastic disease, the overall physiologic status of the patient, financial and time commitment of the clients, and their level of comfort with respect to likelihood of treatment-related success and/or side effects.
Without treatment, most dogs with lymphoma will die of their disease in 4 to 6 weeks after diagnosis, although significant variability exists.114 With few exceptions, canine lymphoma is considered a systemic disease and therefore requires systemic therapy in order to achieve remission and prolong survival. The majority of canine multicentric lymphomas are intermediate to high grade, and, currently, histopathologic and immunophenotypic characterization has not played a significant role in determining the initial treatment protocol. It is hoped that in the near future, sufficient data will emerge to better tailor treatment protocols chosen for dogs with lymphoma based on these and other yet to be characterized parameters. That being said, systemic multiagent chemotherapy continues to be the therapy of choice for canine lymphoma. In general, combination chemotherapy protocols are superior in efficacy to single-agent protocols. Single-agent protocols result in lower response rates that are not as durable as combination chemotherapy, which is summarized in Table 11-2 in Chapter 11. In rare cases in which lymphoma is limited to one site (especially an extranodal site), the animal can be treated with a local modality such as surgery or radiation therapy (RT) as long as the client and clinician are committed to diligent reevaluation to document subsequent progression to systemic involvement, should it occur.
Multidrug Combination Protocols
Many chemotherapeutic protocols for dogs with lymphoma have been developed over the past 15 to 20 years (Table 32-3).91,170-190 Significant limitations arise when comparing efficacy studies in the veterinary literature for the various published protocols. Few of these studies include sufficient numbers for adequate statistical power and even fewer compare treatment protocols in a randomized prospective fashion. In addition, staging, inclusion, and response criteria vary considerably between reports. Therefore evaluations of efficacy among various protocols are subject to substantial bias, making direct comparisons difficult and indeed precarious. A recurring theme in the concluding statement in most of these published protocols is some variation of “prospective randomized trials will be required to confirm these suggestive findings.” In an attempt to better standardize response criteria and outcome reporting of future trials, the Veterinary Cooperative Oncology Group (VCOG) has recently published response evaluation criteria (v1.0)169 (see subsequent response evaluation section). The greatest obstacle to the performance of prospective randomized comparative lymphoma trials in veterinary oncology is financial; that is, clinical trials are inherently costly, and because most of the known effective drugs are unregistered off-label human generic (i.e., off patent) drugs, the incentive for pharmaceutical-funded, sufficiently powered, randomized field trials is low, resulting in a general lack of comparative data.
Table 32-3
Summary of First Remission Outcomes of Combination or Single-Agent Doxorubicin Lymphoma Chemotherapy Protocols (Minimum of 30 Cases Required for Inclusion)*
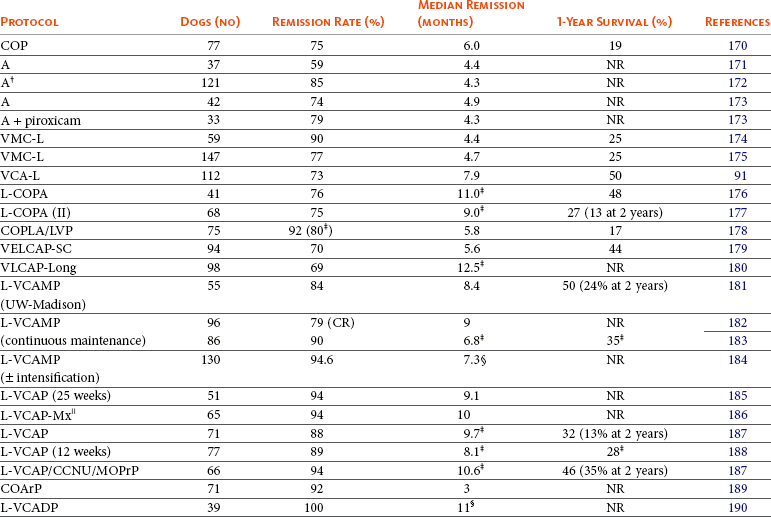
L, L-Asparaginase; V, vincristine; C, cyclophosphamide; M, methotrexate; Mx, mitoxantrone; O, Oncovin (vincristine); P, prednisone; A, Adriamycin (doxorubicin); D, dactinomycin; Pr, procarbazine; Ar, cytosine arabinoside; NR, not reported; CR, complete response.
*Few of these protocols include sufficient numbers for adequate statistical power and fewer compare treatment protocols in a randomized prospective fashion. In addition, staging, inclusion, and response criteria vary considerably between protocols presented. Therefore evaluations of efficacy between the various protocols are subject to bias, making direct comparisons difficult and indeed precarious.
†With COP rescue.
‡Only durations of cases achieving CR reported.
§Time to progression.
Despite the plethora of available combination protocols, most are modifications of CHOP protocols initially designed for human oncologic use, and currently randomized prospective evidence does not exist to clearly recommend one over the other as long as the basic “CHOP” components are present. CHOP represents combinations of cyclophosphamide (C), doxorubicin (H, hydroxydaunorubicin), vincristine (O, Oncovin), and prednisone (P). Conventional CHOP-based chemotherapy induces remission in approximately 80% to 95% of dogs, with overall median survival times (MSTs) of 10 to 12 months. Approximately 20% to 25% of treated dogs will be alive 2 years after initiation of these protocols (Figure 32-9). Response rates and duration of response vary according to the presence or absence of prognostic factors discussed subsequently in the section on prognosis in this chapter. The relative cost of the various protocols to the client depends on the drug(s) selected, the size of the animal, the frequency of administration, and the laboratory tests required to monitor adverse events and response.
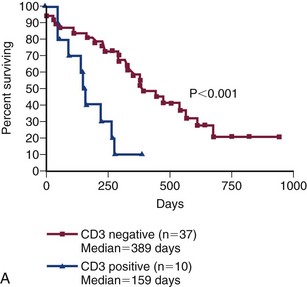
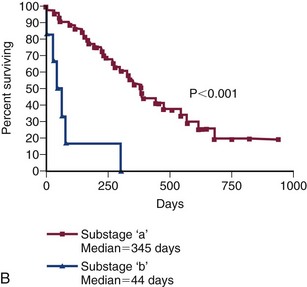
Figure 32-9 A, Kaplan-Meier survival duration estimates for a group of 55 dogs with lymphoma treated with an identical CHOP-based combination chemotherapy protocol. Dogs with CD3 immunoreactive (T-cell) lymphoma had significantly shorter survival durations. B, Kaplan-Meier survival duration estimates for a group of 55 dogs with lymphoma treated with an identical CHOP-based combination chemotherapy protocol at the University of Wisconsin. Dogs with substage b disease (i.e., clinically ill) had significantly shorter survival durations. (From Vail DM: Hematopoietic tumors. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, St. Louis, 2005, Elsevier.)
Dogs responding to chemotherapy and undergoing complete remission are usually free of clinical signs associated with lymphoma and subsequently return to a very good quality of life. Treating dogs with lymphoma is initially gratifying because a high percentage enjoy a complete response. Most dogs tolerate chemotherapy well, and although dose reductions and treatment breaks (“treatment holidays”) are not uncommonly required in individual cases, only a minority of dogs develop significant adverse events requiring hospitalization.191,192 Studies assessing client perceptions of medical treatment for cancer in general and lymphoma in particular report a positive experience; most owners feel treatment was worthwhile, that it resulted in improvement in the well-being of their pet, and that quality of life during treatment was good.193,194 Very few clients express regret about treating lymphoma using a multidrug protocol.
With lymphoma, the fundamental goals of chemotherapy are to induce a complete durable (>6 months) first remission (termed induction), to reinduce a remission when the tumor recrudesces (or the patient relapses) following achievement of a remission (termed reinduction), and, finally, to induce remissions when the cancer fails to respond to induction or reinduction using drugs not present in the initial protocols (termed rescue).
An unanswered question in the treatment of lymphoma has been whether long-term maintenance chemotherapy is useful following an initial course of aggressive induction chemotherapy lasting 6 months or less. Long-term maintenance chemotherapy has not been shown to be of significant value in humans with most forms of NHL; however, in humans, the initial induction course of chemotherapy is much more aggressive than that used in veterinary patients. Although no randomized prospective studies have been performed to address the therapeutic benefit of long-term maintenance chemotherapy in dogs, most comparisons of dogs treated with CHOP-based protocols do not show any clear advantage for a maintenance or consolidation phase after induction therapy.* Indeed, in most reports, dogs receiving shorter, less costly protocols that do not include a prolonged maintenance phase have comparable remission and progression-free survival (PFS) durations and appear to more readily achieve second remissions when they relapse following completion of chemotherapy than their counterparts receiving long-term maintenance. These data, taken together, suggest that maintenance therapy is not beneficial for most dogs with lymphoma. Until well-designed randomized prospective trials indicate otherwise, the author (DMV) prefers protocols that utilize an aggressive induction without maintenance.
Single-Agent Chemotherapy with Known Activity for Dogs with Lymphoma
The most effective, currently available chemotherapeutic agents for canine lymphoma include doxorubicin, L-asparaginase, vincristine, cyclophosphamide, and prednisone—most of which are represented to one degree or another in most first-line multiagent chemotherapy protocols. Other drugs that have documented activity are often considered second-line agents and include lomustine, vinblastine, actinomycin-D, mitoxantrone, mustargen, chlorambucil, methotrexate, dacarbazine (DTIC), 9-aminocamptothecin, ifosfamide, cytosine arabinoside, and gemcitabine. Of these, cytosine arabinoside,199 ifosfamide,200 and gemcitabine201 appear to have only minimal activity. With the exception of doxorubicin, induction therapy with single-agent chemotherapy does not typically result in durable remission durations when compared with standard combination protocols (see Table 11-2, Chapter 11). Incorporation of other standard cytotoxic drugs with single-agent activity into standard CHOP-based protocols has not resulted in significant gains, and most are reserved for subsequent rescue settings.
Overall Chemotherapy Recommendations for Multicentric Lymphoma (Author Preference)
Several factors should be considered and discussed with caregivers on a case-by-case basis when choosing the protocol to be used. These factors include cost, time commitment involved, efficacy, adverse event profiles, and experience of the clinician with the protocols under consideration.
Induction in Treatment-Naïve Patients
It is now clearly established that “standard of care” combination protocols used in dogs with lymphoma are essentially variations of “CHOP” protocols (see Table 32-3). Specific details regarding dose and timing of the CHOP protocol currently preferred by the author (DMV) are outlined in Box 32-2. This protocol does not have a maintenance therapy arm, and all treatments cease at 19 weeks, provided the animal is in complete remission. Although several other CHOP-based protocols include L-asparaginase either at initiation or at varying times throughout the protocol, several studies suggest this does not result in clinically relevant increases in remission rate, speed of attaining remission, or first-remission duration, and therefore the author reserves its use for rescue situations.172,195,202,203
If client or other considerations preclude a CHOP-based protocol, single-agent doxorubicin (30 mg/m2, intravenous [IV], every 3 weeks for 5 total treatments) is offered along with a 4-week tapering oral prednisone regimen (same prednisone regimen in Box 32-2) as a less aggressive and less costly approach. The expected CR rate will range from 50% to 75%, with an anticipated median survival of 6 to 8 months.171,172,204,205 The addition of oral cyclophosphamide (50 mg/m2 daily for 3 days starting on the same day as doxorubicin) to single-agent doxorubicin resulted in a numerically but not statistically superior outcome in a recent randomized trial205 comparing doxorubicin/prednisone with doxorubicin/cyclophosphamide/prednisone (PFS of 5.6 months versus 8.2 months, respectively). This trial was only powered to detect a threefold difference in PFS; therefore larger trials should be undertaken to confirm any benefit.
If clients are reticent to include IV medications, the author often recommends a protocol of oral lomustine (CCNU; 70 mg/m2 by mouth [PO] every 3 weeks for 5 treatments) and prednisone. This protocol has been associated with short median remissions (40 days) in only one small case series206; however, in the author’s experience, a subset of dogs have remained in remission for several months on this protocol when clients decline IV medication.
If financial or other client concerns preclude the use of systemic chemotherapy, prednisone alone (2 mg/kg PO, daily) will often result in short-lived remissions of approximately 1 to 2 months. In these cases, it is important to educate clients that, should they decide to pursue more aggressive therapy at a later date, dogs receiving single-agent prednisone therapy are more likely to develop multidrug resistance (MDR) and experience shorter remission and survival durations with subsequent combination protocols.207-209 This is especially true following long-term prednisone use or in dogs that have experienced a recurrence while receiving prednisone. Therefore the earlier that clients opt for more aggressive therapy, the more likely a durable response will result.
A CBC should be performed prior to each chemotherapy treatment. A minimum of 1500 neutrophils/µL (some oncologists use a cut-off of 2000 neutrophils/µL) and 50,000 platelets/µL should be present prior to the administration of myelosuppressive chemotherapy. If the neutrophil count is lower than 1500/µL, it is best to wait 5 to 7 days and repeat the CBC; if the neutrophil count has increased to more than 1500 cells/µL, the drug can be safely administered. A caveat to these restrictions is that for dogs presented prior to initiation of chemotherapy with low neutrophil and platelet counts due to bone marrow effacement, myelosuppressive chemotherapy is instituted in the face of cytopenias in order to clear the bone marrow of neoplastic cells and allow hematopoiesis to normalize.
In those breeds likely to have MDR1 gene mutations (e.g., collies; see Chapter 11) and therefore to be at risk for serious chemotherapeutic toxicity,210 the author will initiate a CHOP protocol out of sequence, beginning with non-MDR1–associated drugs, such as cyclophosphamide. This ensures treatment of the lymphoma while allowing sufficient time for analysis of MDR1 gene mutations prior to initiating MDR1 substrate drugs. No specific protocols have been scrutinized for treating dogs that are double-mutant for MDR1; however, if using MDR1 substrate drugs, the author initiates at a 40% dose reduction. Subsequent dose modifications (increased or decreased dosage) can be implemented, depending on adverse event levels observed, particularly neutrophil counts at nadir.
The Case for Treating T-Cell Lymphoma Differently
With some exceptions, multicentric T-cell lymphoma, when compared with multicentric B-cell lymphoma, is associated with similar initial response rates, but significantly lower response durability (e.g., PFS) following chemotherapy (including CHOP-based protocols).* Additionally, the effectiveness of a single treatment of doxorubicin in the treatment of naïve dogs with lymphoma in one retrospective case series suggested a lower initial response rate for T-cell, compared with B-cell, immunophenotypes.213 This has led many to question whether dogs diagnosed with T-cell lymphoma should be treated with standard CHOP-based protocols or with alternative protocols. This is a valid question; however, the answer remains elusive because adequately powered randomized controlled trials do not currently exist in the literature to show superiority for an alternate protocol in this scenario. A retrospective study of an L-asparaginase and MOPP (M, mechlorethamine; O, Oncovin; P, procarbazine; P, prednisone) protocol suggested improvement in PFS in dogs with either confirmed T-cell lymphoma or lymphoma with hypercalcemia and no immunophenotypic classification.214 However, differences in determining PFS, response evaluation, and study population in this retrospective study did not definitively confirm superiority.211 Further, some have advocated early inclusion of lomustine (CCNU) into protocols for treating multicentric T-cell lymphoma based on moderate success of lomustine-based rescue protocols in dogs failing CHOP. As yet, no randomized trials have documented superiority with this approach. Ultimately, superior protocol development for T-cell lymphoma awaits careful, randomized, prospective trial assessment. Until such time, the author prefers to initiate CHOP-based induction and switch to lomustine-based rescue at the first sign of progression.
Treatment Response Evaluation for Lymphoma
VCOG has recently published response evaluation criteria (v1.0)169 to standardize reporting of outcome results and comparisons among protocols for peripheral nodal disease. The most important of these outcome measures and the preferred temporal outcome criterion for assessing protocol activity is now considered to be PFS, which is defined as being from the time of treatment initiation until tumor progression or death from any cause. This brings veterinary outcome reporting more in line with human standards. Because the majority of dogs with lymphoma eventually experience recurrence following chemotherapy-induced remissions and because methodology for differentiating complete and partial responses is analysis dependent, PFS removes many sources of bias. Further, overall survival in published reports invariably includes patients who go on to receive varied rescue protocols that bias the overall result, making it a less comparable outcome. Widespread application of these standardized criteria should allow more suitable comparisons in the future.
Superior methods of detection of minimal residual disease (MRD) or early recurrence have been investigated in dogs with lymphoma and include advanced imaging and detection of molecular and biologic markers of minimal disease. Advanced functional and anatomic imaging (i.e., PET/CT) are the current standard for assessing treatment response and early relapse of lymphoma in humans and have also been investigated in dogs (see Figure 32-8).164-166,168 As this technology becomes available to a broader veterinary population, its clinical application will surely increase. Molecular detection of MRD applies clonality and PCR techniques previously discussed in this chapter. Beyond diagnostic applications, these techniques have been applied to determine cytoreductive efficacy of various chemotherapeutic drugs and to document and predict early relapse in patients prior to more conventional methods.215-219 Regarding biomarkers of MRD, preliminary investigations have suggested serum lactate dehydrogenase activity,120 thymidine kinase 1 activity,220 and serum C-reactive protein221 may be candidates in the dog.
As we become more proficient at defining MRD, the pressing clinical question becomes how we use this information. Theoretically, such information could suggest when more aggressive therapy or alternative therapy should be instituted in patients who have not achieved a “molecular remission” or who are undergoing early relapse; however, until we determine what these interventions should be, their clinical utility remains theoretical.
Reinduction and Rescue Chemotherapy
Eventually, the majority of dogs that achieve a remission will relapse or experience recrudescence of lymphoma. This usually represents the emergence of tumor clones or tumor stem cells222 (see Chapter 2) that are inherently more resistant to chemotherapy than the original tumor, the so-called MDR clones that either were initially drug resistant or became so following exposure to selected chemotherapy agents. Evidence suggests that in dogs with recurrent lymphoma, tumor cells are more likely to express the MDR1 gene that encodes the protein transmembrane drug pump often associated with MDR.156a,156b,225 MDR1 represents only one of the plethora of mechanisms that lead to drug-resistant disease (see Chapter 11). Other causes for relapse following chemotherapy include inadequate dosing and frequency of administration of chemotherapy, failure to achieve high concentrations of chemotherapeutic drugs in certain sites such as the CNS, and initial treatment with prednisone alone.
At the first recurrence of lymphoma, it is recommended that reinduction be attempted first by reintroducing the induction protocol that was initially successful, provided the recurrence occurred temporally far enough from the conclusion of the initial protocol (e.g., ≥2 months) to make reinduction likely. Attention must be given to the cumulative dose of doxorubicin that will result from reinduction, and baseline cardiac assessment, the use of cardioprotectants, alternative drug choices, and client education should all be considered. In general, the length of the reinduction will be half that encountered in the initial therapy; however, a subset of animals will enjoy long-term reinductions, especially if the dog completed the initial induction treatment protocol and was currently not receiving chemotherapy for several months when relapse occurred. Nearly 80% to 90% reinduction rates can be expected in dogs that have completed CHOP-based protocols and then relapse while not receiving therapy.185,226 The duration of a second CHOP-based remission in one report was predicted by the duration of the interval between protocols and the duration of the first remission.226
If reinduction fails or the dog does not respond to the initial induction, the use of so-called “rescue” agents or “rescue” protocols may be attempted. These are single drugs or drug combinations that are typically not found in standard CHOP protocols and are withheld for use in the drug-resistant setting. The most common rescue protocols used in dogs include single-agent use or a combination of actinomycin D, mitoxantrone, doxorubicin (if doxorubicin was not part of the original induction protocol), dacarbazine (DTIC), temozolomide, lomustine (CCNU), L-asparaginase, mechlorethamine, vincristine, vinblastine, procarbazine, prednisone, and etoposide. Some rescue protocols are easy and convenient single-agent treatments, whereas others are more complicated (and expensive) multiagent protocols, such as MOPP. Overall rescue response rates of 40% to 90% are reported; however, responses are usually not durable, with median responses of 1.5 to 2.5 months being typical, regardless of the complexity of the protocol. A small (<20%) subset of animals will enjoy longer rescue durations. Table 32-4 provides a summary of canine rescue protocols and published results.227-238 Current published data from rescue protocols do not include sufficient numbers for adequate statistical power nor do they compare protocols in a randomized prospective fashion. Therefore evaluations of efficacy among various protocols are subject to substantial bias, making direct comparisons difficult and indeed precarious. Choice of a particular rescue protocol should depend on several factors, including cost, time commitment required, efficacy, toxicity, and experience of the clinician with the protocols in question. As the complexity of rescue protocols does not yet appear to be associated with significant gains in rescue durability, the author tends to choose simpler and less costly protocols (e.g., CCNU/L-asparaginase/prednisone) (Table 32-5). However, the use of multiple varied rescue protocols, switching as needed based on response, continues as long as clients are comfortable with their dog’s quality of life. This sequential application of several different rescue protocols can result in several months of extended survival with acceptable quality of life.
Table 32-4
Summary of Response for Rescue Protocols (Minimum 25 Cases)*
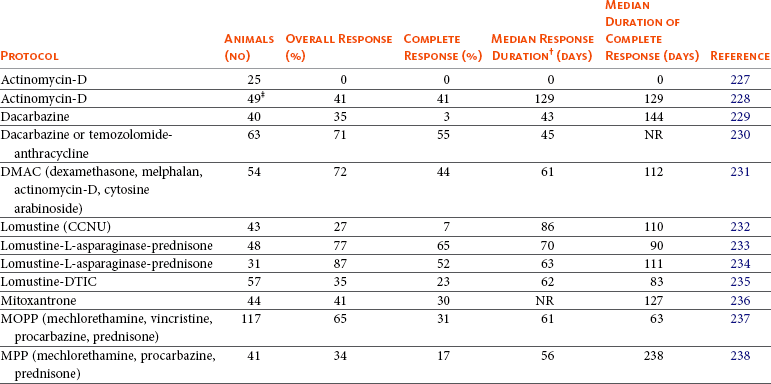
*Few of these protocols include sufficient numbers for adequate statistical power and fewer compare treatment protocols in a randomized prospective fashion. In addition, staging, inclusion, and response criteria vary considerably between protocols presented. Therefore evaluations of efficacy between the various protocols are subject to bias, making direct comparisons difficult and indeed precarious.
†Various temporal response endpoints were used, including disease-free interval, time to progression, and progression-free survival.
‡Prednisone often used concurrently.
Table 32-5
First-Line Rescue Protocol*
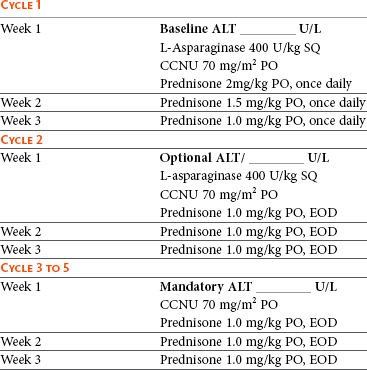
ALT, Alanine aminotransferase; SQ, subcutaneous; PO, by mouth; EOD, every other day.
*Treatment discontinuation criteria:
Strategies to Enhance Effectiveness of Therapy in Lymphoma
Despite the plethora of published chemotherapeutic protocols for dogs with lymphoma, it appears we have achieved as much as we can from currently available chemotherapeutics in standard settings. The 12-month median survival “wall” and the 20% to 25% 2-year survival rates have not improved dramatically. Further advances in remission and survival durations await the development of new methods of delivering or targeting traditional chemotherapeutic drugs, new generations of chemotherapeutic drugs, or novel nonchemotherapeutic treatment modalities. Mechanisms of avoiding or abrogating MDR, enhancing tumor apoptosis (programmed cell death), tumor ablation, and immune-system reconstitution, as well as novel immunomodulatory therapies for lymphoma, are all active areas of investigation in both human and veterinary medicine.
Mechanisms of Drug Resistance
Drug resistance can be inherent in cancer cells or develop following exposure to selected chemotherapeutic agents and often is associated with increased expression of members of the adenosine triphosphate (ATP)-binding cassette (ABC) transporter superfamily (e.g., P-glycoprotein pump), many of which efflux various chemotherapeutic compounds from cells (see Chapter 11).239 P-glycoprotein is under the control of the MDR1 gene. MDR has been reported in canine lymphoma following exposure to chemotherapy.156a,225,240 Expression levels of mRNA encoding the canine MDR1 gene have been characterized in canine cell lines and lymphomas. Although expression of MDR1 mRNA correlated with in vitro drug sensitivity, it did not correlate with in vivo doxorubicin sensitivity in dogs with lymphoma in this study. Additionally, quantitative analysis of mRNA for 10 different drug-resistance factors was performed in 23 dogs with lymphoma.241 These dogs were divided into drug “sensitive” and “resistant” categories based on response to a CHOP-based protocol; however, significant differences in expression were not observed in this small study.
Altering Drug Pharmacokinetics
Methods of increasing the time that tumor cells are exposed to chemotherapeutics should theoretically enhance tumor killing. These methods could include long-term continuous infusions (impractical in many veterinary situations), increasing the frequency of treatments, or enhancing the circulation time of drugs used. In one study, dogs with lymphoma received lower dose doxorubicin weekly rather than a higher dose every 3 weeks (thereby decreasing Cmax, which is associated with cardiotoxicity) in order to potentially increase the time of drug exposure.242 No benefit was noted, and, in fact, remission rates were inferior. Studies evaluating pegylated long-circulating doxorubicin-containing liposome drug delivery systems in dogs with lymphoma have also been performed.243,244 Although efficacy was established, enhancement of remission or survival durations over equivalent doses of native doxorubicin was not observed.
Treatment Approaches Using Immunologic or Biologic Agents
Monoclonal Antibody Approaches
In the past decade, enhanced durability of first remissions in humans with non-Hodgkin’s B-cell lymphoma has been achieved primarily through the institution of monoclonal antibody (MAb)-based therapies (so-called R-CHOP protocols); the “R” refers to rituximab, a recombinant chimeric murine/human antibody directed against the CD20 antigen, a hydrophobic transmembrane protein located on normal pre-B and mature B lymphocytes. Following binding, rituximab triggers a host cytotoxic immune response against CD20-positive cells. Unfortunately, rituximab does not have therapeutic activity in dogs due to a lack of external recognition of a similar antigen on canine lymphoma cells and the inherent antigenicity of human-derived antibodies in dogs.245,246 Another immunotherapy approach involved MAb-231, a murine-derived anticanine MAb (IgG2a). It mediates antibody-dependent cellular cytotoxicity (ADDC) and complement-mediated cellular cytotoxicity (CMCC).247-249 It also prevented outgrowth of canine lymphoma xenografts in nude mice. In a noncontrolled clinical study of 215 dogs treated with CHOP-based chemotherapy and MAb-231, enhanced overall survival was observed; however, the antibody was removed from the commercial market in the mid-1990s without definitive randomized trials being performed. Several laboratories throughout the world are currently working to characterize and develop effective MAb therapies for use in dogs.
Antitumor Vaccine Approaches
Several antitumor vaccine approaches have been applied in dogs with lymphoma. A tumor vaccine extract using killed lymphoma cells combined with Freund’s adjuvant was administered to a small number of dogs after remission induction with combination chemotherapy.250 Prolongation of median survival was noted in the treatment group; however, a subsequent study revealed that prolongation was likely due to the Freund’s adjuvant.251 An autologous killed lymphoma tumor cell vaccine has been intralymphatically administered to dogs placed in remission using a combination chemotherapy protocol, and, although modest gains were reported in remission times, no survival advantage was found.252 An exploratory vaccine study targeting telomerase253 (see Chapter 14, Section D) and one using RNA-loaded CD40-activated B cells254 in dogs with lymphoma have also been conducted. These studies involved small numbers of nonrandomized patients and lacked controlled populations for comparison. In a randomized study of 60 dogs with lymphoma comparing CHOP-based chemotherapy with CHOP-based chemotherapy and a human granulocyte-macrophage colony-stimulating factor (GM-CSF) DNA cationic-lipid complexed autologous whole tumor cell vaccine, a small measure of immunomodulation was documented by delayed-type hypersensitivity; however, significant improvement in clinical outcome was not noted.255 Although little well-supported activity is reported to date with these immunomodulatory approaches, our basic understanding of methodologies is expanding.
Surgery
Most dogs with lymphoma have multicentric disease and therefore require systemic chemotherapy to effectively treat their disease. However, surgery has been used to treat solitary lymphoma (early stage I) or solitary extranodal disease. Careful staging is necessary in such cases to rule out multicentric involvement prior to treating local disease.
The benefit of surgical removal of the spleen in dogs with massive splenomegaly remains unclear.82,83,256 In an older report, 16 dogs with lymphoma underwent splenectomy to remove a massively enlarged spleen and were subsequently treated with chemotherapy.256 Within 6 weeks of splenectomy, 5 of the 16 dogs died of disseminated intravascular coagulation (DIC) and sepsis. The remaining 11 dogs (66%) had a CR, and 7 dogs had a MST of 14 months. No staging or histologic information was provided, so the information appears of limited usefulness, although those with follow-up lived approximately 1 year. In two reports of indolent nodular lymphoma of the spleen (marginal zone lymphoma [MZL] and mantle cell lymphoma [MCL]), outcome was available on seven MZL cases, including three cases that did not receive adjuvant chemotherapy after surgery,82,83 and only one died of lymphoma following splenectomy. In a recent report of indolent lymphomas, four splenic lymphomas (three MZL and one MCL) underwent splenectomy alone and all survived greater than 1 year with none dying of their primary disease.84 Splenectomy should be considered if the lymphoma is not documented in other sites following thorough staging, if lymphoma is an indolent form histologically, or if splenic rupture has occurred. Of note, no control population consisting of dogs that did not undergo splenectomy exists, so the natural history of indolent splenic lymphoma remains uncertain.
Radiation Therapy
Radiation therapy, although its use is limited in the treatment of lymphoma, may be indicated in selected cases.257 Indications are as follows:
1. Curative intent therapy for stage I lymph node and solitary extranodal disease (i.e., nasal, cutaneous, spinal lymphoma).
2. Palliation for local disease (e.g., mandibular lymphadenopathy, rectal lymphoma, mediastinal lymphoma where precaval syndrome is present, localized bone involvement).
3. Total body radiation combined with bone marrow or stem cell transplantation.
4. Whole or staged half-body RT following chemotherapy-induced remissions.
In the latter case, staged half-body irradiation sandwiched between chemotherapy cycles or following the attainment of remission by induction chemotherapy has been preliminarily investigated as a form of consolidation or maintenance.198,258-262 Radiation therapy was delivered to either the cranial or the caudal half of the dog’s body in 4 to 8 Gy fractions, and following a 2- or 4-week rest the other half of the body was irradiated in a similar fashion. Although these preliminary investigations were not randomized, they suggest that RT applied when dogs are in either complete or partial remission is safe and warrants further investigation to determine if a significant therapeutic gain can be realized. A pilot study of low-dose (1 Gy) single-fraction total body irradiation in seven dogs with relapsed drug-resistant lymphoma, although safely applied, resulted in only partial nondurable (1 to 4 week) remissions.263
Total body irradiation (and/or ablative chemotherapy) for complete or partial bone marrow ablation followed by reconstitution with bone marrow or stem-cell transplant in dogs, although a recognized model in comparative research settings,264,265 is still in its early phases of development and application in clinical veterinary practice.266,267 Because of the high cost, limited accessibility to relatively sophisticated equipment, and management requirements, these types of procedures are limited to preliminary investigations at a few centers. Currently, long-term results in significant numbers of treated cases have yet to be presented.
Treatment of Extranodal Lymphoma
In general, the veterinary literature suffers from a paucity of information on treating various extranodal forms of lymphoma in dogs, and our ability to predict outcome is thus limited. In general, it is recommended that, following extensive staging, in those cases where disease is shown to be localized to a solitary site, local therapies (e.g., surgery, local RT) can be used. In contrast, if multiple extranodal sites are involved or they are part of a more generalized process, systemic chemotherapy should be chosen.
Alimentary Lymphoma
Most dogs with alimentary lymphoma are presented with diffuse involvement of the intestinal tract, and involvement of local lymph nodes and liver is common. Chemotherapy in dogs with diffuse disease has been reported to be unrewarding for the most part47,268,269; however, more aggressive CHOP-based protocols used extensively for multicentric lymphoma in dogs have resulted in durable remissions in a small subset of cases. Solitary alimentary lymphoma is rare in the dog; however, if the tumor is localized and can be surgically removed, results (with or without follow-up chemotherapy) can be encouraging.
Primary Central Nervous System Lymphoma
CNS lymphoma in dogs usually results from extension of multicentric lymphoma. However, primary CNS lymphoma (PCNSL) has been reported.103-105 If tumors are localized, local RT should be considered. Few studies have reported the use of chemotherapy. In one study, cytosine arabinoside (Ara-C) at a dosage of 20 mg/m2 was given intrathecally; this treatment was combined with systemic chemotherapy and CNS radiation.103 Overall, the response rates are low and of short duration (several weeks to months).
Cutaneous Lymphoma
Treatment of cutaneous lymphoma depends on the extent of disease. Solitary lesions may be treated with surgical excision or RT. Fractionated RT (to a total dose of 30 to 45 Gy) has been associated with long-term control.270 Diffuse cutaneous lymphoma is best managed with combination chemotherapy, although the rate and durability of response is generally less than in multicentric lymphoma. The most widely used protocols for epitheliotropic cutaneous T-cell lymphoma include CCNU (60 to 70 mg/m2 PO, every 3 weeks) along with prednisone.271,272 Although response rates approach 80%, median remission is approximately 3 months; occasionally, durable remissions are encountered. The author has added L-asparaginase to this protocol (see Table 32-5), and although anecdotally it appears to improve response, comparative data are not available. Sporadic reports of other therapies for cutaneous lymphoma in small numbers of cases include the use of COAP (cyclophosphamide, vincristine [Oncovin], Ara-C, and prednisone),273 retinoic acid analogs (e.g., Accutane, etretinate),274 L-asparaginase and pegylated L-asparaginase,275 topical mechlorethamine (Mustargen),276 and recombinant human α-interferon.277 All of these reports involved small numbers of cases and resulted in limited response rates with short durations.
A form of cutaneous lymphocytic infiltration has recently been characterized as an indolent T-cell lymphoma based on clonality.131 It is associated with slow progression and long-term survival following corticosteroid management; however, it does have the potential to progress to high-grade lymphoma.
Prognosis
The prognosis for dogs with lymphoma is highly variable and depends on a wide variety of factors documented or presumed to affect response to therapy. Although rarely curable (<10% of cases), CRs and a good quality of life during extended remissions and survival are typical. Factors that have been shown to influence treatment response and survival are summarized in Tables 32-6* and 32-7.† The two prognostic factors most consistently identified are immunophenotype and WHO substage (see Figure 32-9). Many reports have confirmed that dogs with CD3-immunoreactive tumors (i.e., T-cell derivation) are associated with significantly shorter remission and survival durations.‡ This holds true primarily for dogs with multicentric lymphoma because the immunophenotype of solitary or extranodal forms of lymphoma has not been thoroughly investigated with respect to prognosis. Additionally, it has been shown that dogs with B-cell lymphomas that express lower than normal levels of B5 antigen (expressed in 95% of nonneoplastic lymphocytes) also experience shorter remission and survival durations.54 Recently, low levels of class II MHC expression on B-cell lymphoma predicted poor outcomes.279 Dogs presented with WHO substage b disease (i.e., clinically ill) also do poorly when compared with dogs with substage a disease.53,86,91,181,278 Dogs with stage I and II disease have a better prognosis than those dogs in more advanced stages (stage III, IV, and V).
Histologic grade (subtype) has been found to influence prognosis in some studies; however, our ability to predict outcome based on subtype is still quite limited. Dogs with lymphoma classified as intermediate or high grade (large cell, centroblastic, and immunoblastic) tend to respond to chemotherapy but can relapse early. Dogs with low-grade lymphomas (small lymphocytic or centrocytic) have a poorer response rate to chemotherapy, yet have a survival advantage over dogs with intermediate- and high-grade lymphomas (Figure 32-10) in that the disease may be more indolent. Several case compilations have documented that dogs with indolent lymphoma (e.g., MZL, MCL, T-zone) experience prolonged survivals, often in the absence of any or aggressive chemotherapy.82-84 Proliferative assays such as analysis of bromodeoxyuridine (BrdU) uptake, Ki67 antibody reactivity, and argyrophilic nucleolar organizer region (AgNOR) indices to measure proliferative activity of tumor cells have been shown to provide prognostic information in dogs treated with combination chemotherapy. Results of different studies are contradictory, however. In two trials, dogs having tumors with short doubling times, high AgNOR frequencies, or high Ki67 immunoreactivity had a better prognosis than those with tumors with long doubling times or low AgNOR frequencies.53,142 In other trials, the low-proliferating tumor groups were associated with a better prognosis.283,284 Additionally, in one trial, the proportion of tumor cells undergoing apoptosis was modestly predictive of remission duration.142

Figure 32-10 Kaplan-Meier curves illustrating time to relapse adjusted for clinical stage and immunophenotype among dogs treated for low-grade (n = 17) (blue line) or high-grade (n = 51) (red line) Kiel classification lymphoma. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (From Teske E, van Heerde P, Rutteman GR, et al: Prognostic factors for treatment of malignant lymphoma in dogs, J Am Vet Med Assoc 205:1722–1728, 1994.) J Am Vet Med Assoc
The anatomic site of disease is also of considerable prognostic importance. Primary diffuse cutaneous, diffuse GI, hepatosplenic, and primary CNS lymphomas tend to be associated with a poor prognosis. Dogs with indolent cutaneous T-cell lymphocytic infiltration experience long-term survivals.131 Sex has been shown to influence prognosis in some studies.175, 181 Neutered females tend to have a better prognosis; male dogs may have a higher incidence of the T-cell phenotype, which may account for the poorer prognosis.
Reported biomarkers of prognosis, summarized in Table 32-7, include circulating levels of glutathione-S-transferase, thymidine kinase, lactate dehydrogenase, serum C-reactive proteins, and VEGF. Finally, one report suggests that a history of chronic inflammatory disease of several types predicts likelihood of early relapse.291 These putative prognostic indicators require further confirmation in larger trials.
Table 32-7
Circulating (Serum/Plasma) Biomarkers as Prognostic Indices in Dogs with Lymphoma
| Biomarker | Comments | References |
| Lactate dehydrogenase activity | Increased activity predicted early recurrence. | 120 |
| Thymidine kinase activity | Increases associated with diminished prognosis. | 220, 286 |
| Serum VEGF levels | Small study suggests pretreatment levels predictive of remission duration. | 119, 287 |
| Glutathione-S-transferase | Increases associated with diminished prognosis. | 288 |
| Hypercalcemia | Negative factor if associated with T-cell subtype and reduced renal function. | 91, 289, 290 |
| Serum cobalamine | Hypocobalaminemia associated with poor outcome. | 290 |
| Serum C-reactive protein | Although it may be used to characterize remission status, variable levels preclude utility. | 221 |
Lymphocytic Leukemia
Lymphocytic leukemia is typically defined as proliferation of neoplastic lymphocytes in bone marrow. Neoplastic cells usually originate in the bone marrow, but occasionally in the spleen, and may or may not be circulating in the peripheral blood. Although our ability to diagnose lymphocytic leukemias using flow cytometric and molecular diagnostic techniques has increased significantly in the past decade, little information on treatment and prognosis is available except for chronic lymphocytic leukemia (CLL). Differentiating between true leukemia and stage V lymphoma can be difficult and arbitrary and is often based on lack of significant lymphadenopathy, degree of blood and bone marrow involvement, and immunophenotypic characteristics.
Incidence, Risk Factors, and Etiology
Lymphocytic leukemia is more common than acute myeloid leukemia and myeloproliferative disorders (MPD), but the true incidence is unknown. German shepherd dogs and golden retrievers may be overrepresented.137,292 Lymphocytic leukemia can occur in dogs of any age but typically occurs in middle-aged to older dogs (mean of 7 to 10 years); CLL usually occurs in older dogs (mean of 10 years).137,280,292,293 A significant sex predilection is not reported. As with lymphoma, the etiology of lymphocytic leukemia is for the most part unknown. Genetic factors likely play a role and have been compared between dogs and humans.16 Retroviruses have been implicated in diverse animal species such as cats, cattle, fish, snakes, birds, rodents, nonhuman primates, and humans; however, there is no proven evidence implicating a retroviral cause in dogs. In humans, acute lymphocytic leukemia (ALL) has been associated with genetic factors and exposure to radiation, benzene, phenylbutazone, and antineoplastic agents. Extrapolation of predisposing factors across species is not warranted; in fact, etiologic factors in dogs may be quite different from those for humans given the difference in the predominant immunophenotype of the neoplastic cells (see later).
Pathology and Classification
Lymphocytic leukemias can be subdivided based on cell size, maturity, genetic aberrations, microRNA expression, and immunophenotype.* The simplest classification divides leukemia into two groups: chronic (small cells with a mature cytologic phenotype) and acute (large cells with an immature cytologic phenotype). Immunophenotypic assessment using flow cytometric and molecular assays can further characterize these two major subtypes; however, some discordance exists in the veterinary literature.
Three primary subtypes of CLL are reported in dogs, based primarily on immunophenotyping137,280,293: (1) T-CLL, which is the most common form, with cells in the majority of cases being CD8+ granular lymphocytes; (2) B-CLL, which is the next most common subtype; and (3) atypical CLL, which represents a combination of immunophenotypes (CD3−, CD8+; CD3+, CD4−, CD8−; CD3+, CD4+, CD8+; and CD3+ + CD21+*). This is in contrast to CLL in humans, which is primarily a disease of B-cells. In CLL, lymphocytes often are indistinguishable morphologically from normal small lymphocytes (Figure 32-11) and have a low rate of proliferation; accumulation of lymphocytes likely results from their prolonged lifespan.
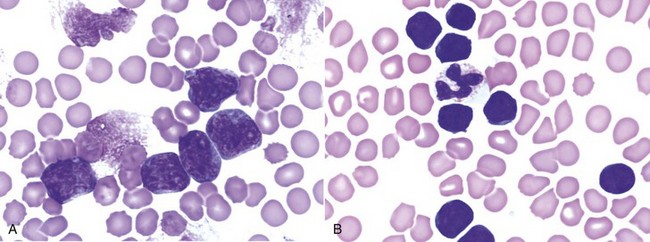
Figure 32-11 A, Peripheral blood from a dog with acute lymphoblastic leukemia (ALL). Note the large lymphoid cells with visible nucleoli. Chromatin from disintegrated cells also is visible. (Wright’s stain, ×60 objective.) B, Peripheral blood from a dog with chronic lymphocytic leukemia (CLL). Note the small lymphocytes of normal morphology (smaller than the neutrophil). (Wright’s stain, ×60 objective.)
The immunophenotype of ALL typically is B-cell (CD21+, CD3−, CD4−, CD8−), although a smaller percentage (<10%) are of T-cell origin (CD3+,CD4−, CD8−, CD21−).137 In general, these cells tend to be intermediate-sized or large cells with moderate amounts of basophilic cytoplasm. Perhaps the most distinguishing feature of lymphoblasts is the nuclear chromatin pattern, which typically is more condensed than the chromatin in myeloblasts. Lymphoblasts are larger than neutrophils, have a high nuclear : cytoplasmic ratio, and contain blue cytoplasm that in some cases is intensely basophilic (see Figure 32-11). Nucleoli, although present, are less prominent in lymphoblasts than in myeloblasts. Nevertheless, these cells cannot be distinguished easily from blast cells of other hematopoietic lineages, and identification of lineage-specific markers by immunocytochemical or flow cytometric analysis is required to ascertain the lineage. If the cells express CD34, a stem cell marker, an acute phenotype is implied137,280; however, both myeloid and lymphoid lineages express CD34, and our ability to differentiate ALL from acute myeloid leukemia (AML) relies on detection of other markers, including T- and B-cell markers and myeloperoxidase, a myeloid marker.
History and Clinical Signs
Dogs with CLL are often asymptomatic, but some owners report lethargy and decreased appetite. Mild lymphadenopathy and splenomegaly may be present, although late in the disease splenomegaly may be marked.295 The white blood cell (WBC) count is usually greater than 30,000 cells/µL but can vary from normal to greater than 100,000 cells/µL because of an increase in circulating mature lymphocytes. Lymphocytosis is persistent and granulocytes are usually present in normal numbers. Other than lymphocytosis, hemograms of dogs with CLL tend to have few abnormalities when lymphocytes are less than 30,000/µL.137,280,293 In some dogs, the disease is identified incidentally when the animal is undergoing evaluation for an unrelated problem. Mild anemia, neutropenia, and thrombocytopenia are common but may become marked as the disease progresses and lymphocyte counts increase above 30,000/µL. Despite the well-differentiated appearance of the lymphocytes in CLL, these cells may function abnormally. Paraneoplastic syndromes include monoclonal gammopathies, immune-mediated hemolytic anemia, pure red cell aplasia, and, rarely, hypercalcemia.296,297 In one report of 22 dogs with CLL, 68% had monoclonal gammopathies (usually IgM or IgA).296 The immunophenotypes were not reported, but a monoclonal gammopathy would be more likely to occur in B-CLL.
Dogs with ALL usually are presented with clinical signs of anorexia, weight loss, and lethargy. Splenomegaly is typical and other physical abnormalities may include hemorrhage, lymphadenopathy, and hepatomegaly.298 Infiltration of bone marrow by neoplastic lymphoblasts may be extensive, resulting in significant depression of normal hematopoietic elements or myelophthisis.137,280,292,298,299 Anemia, neutropenia, and thrombocytopenia are typically much more severe than with CLL and may become life threatening. Infiltration of extramedullary sites such as the CNS, bone, and GI tract may also occur and can result in neuropathies, bone pain, and GI signs, respectively.
Diagnostics and Clinical Staging
Consideration of signalment, history, physical findings, and morphologic appearance and immunophenotype of cells is essential in making an accurate diagnosis. It is helpful to know the profile of lymphocyte subsets in the peripheral blood of normal dogs to determine if a particular subset has expanded. Approximately 80% of circulating lymphocytes in normal dogs are T-cells, and about 15% are B-cells. NK cells and double-negative (CD4−, CD8−) T-cells constitute the remaining fraction. In the T-cell fraction, helper T-cells (CD4+) outnumber cytotoxic T-cells (CD8+).296 Lymphocytic leukemia should be a consideration if atypical lymphocytes are in circulation, the immunophenotype of the lymphocytes in circulation is homogenous as determined by flow cytometric analysis, a phenotype typically present in low frequency has increased, or if clonality is documented (e.g., by PARR analysis). Other differential diagnoses for lymphocytosis include infectious diseases, such as chronic ehrlichiosis, postvaccinal responses in young dogs, IL-2 administration, and transient physiologic or epinephrine-induced lymphocytosis. In some cases, reactive and neoplastic lymphocytosis are difficult to distinguish.
Expansion of neoplastic lymphocytes in bone marrow is the hallmark of ALL and, in most cases, CLL. Careful examination of peripheral blood and bone marrow by an experienced cytopathologist is important in establishing a diagnosis of lymphoid leukemia; in cases of marked lymphocytosis with atypia, peripheral blood can be used for analysis of immunophenotype and clonality, and examination of bone marrow is not essential. If diagnostic bone marrow cannot be adequately obtained by aspiration, bone marrow core biopsy should be performed. In ALL, lymphoblasts predominate in the bone marrow and are also present in peripheral blood, and other lineages are decreased. In B- and T-cell CLL, the lymphocytes are small mature cells that occur in excessive numbers in bone marrow (≥30% of all nucleated cells) early in the disease.295 In T-CLL, lymphocytes may contain pink granules. Infiltration becomes more extensive as the disease slowly progresses, and eventually the neoplastic cells replace normal marrow.
A separate clinical staging system has not been developed for lymphoid leukemia. Currently, all dogs with leukemia are classified as stage V based on the WHO Staging System for lymphoma as presented in Table 32-2.
Treatment of Chronic Lymphocytic Leukemia
Because of the indolent and often asymptomatic nature of CLL, the decision to treat is often based on the clinical and laboratory findings in the individual dog. Most oncologists recommend active surveillance (monthly or bimonthly physical examination and CBC) over active therapy for patients when CLL is identified incidentally, there are no accompanying clinical signs, and other significant hematologic abnormalities are not identified. If the animal is significantly anemic or thrombocytopenic, is showing evidence of significant lymphadenopathy or hepatosplenomegaly, or has an excessively high lymphocyte count (e.g., >60,000/µL), therapy should be instituted. The definition of “excessively high” varies among oncologists, and a standard has not been established in veterinary medicine. The author (DMV) prefers to base treatment decisions on the presence of significant constitutional signs and peripheral cytopenias. Currently, the most effective drug available for treatment of CLL is chlorambucil.295 Chlorambucil is given orally at a dose of 0.2 mg/kg or 6 mg/m2 PO once daily for 7 to 14 days; the dose can then be reduced to 0.1 mg/kg or 3 mg/m2 PO daily. For long-term maintenance, a dose of 2.0 mg/m2 every other day can be used. The dose is adjusted based on clinical response and bone marrow tolerance. Oral prednisone is used concurrently with chlorambucil at doses of 1 mg/kg daily for 1 to 2 weeks, then 0.5 mg/kg every other day thereafter. The addition of vincristine or the substitution of cyclophosphamide for chlorambucil has been advocated in animals that do not respond to chlorambucil.
Treatment of CLL is primarily palliative with rare complete remissions. Owing to the indolent nature of this disease, however, survival times have been in the range of 1 to 3 years with a good quality of life.295,300 The phenotypic expression of CLL is usually stable over months to years. However, the disease may evolve into an acute phase, and some dogs will develop a form of lymphoma that is rapidly progressive and characterized by the presence of pleomorphic immunoblasts; in humans, this is termed Richter’s syndrome.301 The prognosis for response to treatment is poor for this form of lymphoma.
Treatment of Acute Lymphoblastic Leukemia
Much of the morbidity in dogs with ALL results from effacement of bone marrow (myelophthisis) and subsequent life-threatening peripheral cytopenias. Neutropenia, thrombocytopenia, and anemia may be severe. Patients often require supportive therapy, such as fresh whole-blood transfusions, broad-spectrum antibiotics, fluid therapy, and nutritional support. Careful monitoring for sepsis, hemorrhage, and DIC is important. Specific treatment of ALL requires aggressive chemotherapy. Consistently efficacious protocols for ALL have not been developed in veterinary medicine, and there are few published reports. CHOP-based protocols, similar to those used for lymphoma (see Table 32-4), have been used by the author (DMV) for dogs with ALL; however, responses and durability of response are generally disappointing. The standard of care in humans with acute leukemia generally involves bone marrow ablative treatments with stem cell or marrow replacement, a protocol not generally available in veterinary oncology.
Prognosis
In general, CLL is a slowly progressive disease, and some animals will not require therapy for some time after diagnosis; one dog was reported to survive almost 2 years without treatment.302 For those dogs that are treated, normalization of lymphocyte counts can be expected in 70% of cases. In one report of 17 dogs treated with vincristine, chlorambucil, and prednisone, MST was approximately 12 months with an expected 30% survival at 2 years.295 In larger compilations of cases that include immunophenotypic analysis, treatment protocols were poorly documented, although most received chlorambucil and prednisone; in 43 dogs with follow-up, for dogs with T-CLL, B-CLL, and atypical CLL, median survival was 930, 480, and only 22 days, respectively.293 In this group of dogs, young age and anemia were also associated with a poor prognosis. In another series with limited treatment information, dogs with CLL of a CD8+ immunophenotype that presented with less than 30,000 lymphocytes/µL or greater than 30,000 lymphocytes/µL had median survivals of 1098 and 131 days, respectively.
Prognosis for dogs with ALL is generally very poor. In a study of 21 dogs treated with vincristine and prednisone, the dogs achieving complete or partial remission (29%) had a MST of 120 days, and few dogs survived longer than 8 months with that protocol.298 In one report of 46 cases of ALL with a CD34+ phenotype, dogs had a median survival of 16 days (ranged from 3 to 128 days), even though the majority received a CHOP-based treatment protocol.280 Additionally, dogs with B-cell ALL (CD21+) in which the lymphocytes were large cells (forward scatter lymphocyte/forward scatter neutrophil ratio of >0.58 by flow cytometric analysis) had a median survival of only 129 days, independent of treatment protocol.280