Section D
Section D
Mesothelioma
Incidence and Risk Factors
Mesothelioma is a rare neoplasm of dogs and cats affecting the cells lining the coelomic cavities of the body. In 1962, Gerb et al cited reports of one case of mesothelioma in 1000 dogs and three cases in 5315 dogs.1 In dogs, primary mesothelial tumors affecting the thoracic cavity, abdominal cavity, pericardial sac, and vaginal tunics of the scrotum have been reported.2-6 In the cat, primary mesotheliomas have been reported in the pericardium, pleura, and peritoneum, as well as throughout the abdomen with lung and mediastinal lymph node metastases.7-12 Exposure to asbestos may be an important contributory factor to mesothelioma development in pet dog populations. Affected dogs often live with owners who have occupations or hobbies for which exposure to asbestos is a known risk.13 The level of asbestos fibers in lung tissues of affected dogs has been documented to be greater than controls.13,14 Asbestos refers to a family of silicate minerals that crystallize into long, flexible fibers. The fibers are categorized into two groups: thin rodlike amphibole and long curly serpentine, the main type being chrysotile. In humans, much greater risk has been related to amphibole asbestos compared to chrysotile exposure.15 Chrysotile now accounts for 90% of asbestos used worldwide.15
The underlying mechanisms of the neoplastic transformation of mesothelial cells, despite its association with asbestos, are not completely understood. Asbestos interacts with mesothelial cells via direct and indirect mechanisms and is associated with both phenotypic and genotypic changes in the affected cells. Chromosomal missegregation, aneuploidy, and deletions are reported.15,16 Loss of tumor suppressor gene products is thought to contribute to the transformation of mesothelial cells.16 Also, reactive oxygen and nitrogen species generated by macrophages as the cellular response to asbestos fiber phagocytosis and by the fibers themselves add to the genetic damage in the tumor precursor cells.15 Numerous growth factors (e.g., insulin-like growth factor-1 [IGF-1], platelet-derived growth factor [PDGF], and VEGF) produced by stimulated macrophages or mesothelial cells, as well as tumor suppressor genes, are likely important in the pathogenesis of mesothelioma.16-18 A recent report of five golden retrievers that developed pericardial mesothelioma after a long-term (30 to 54 months) history of idiopathic hemorrhagic pericardial effusion (IHPE) supports the concept that chronic inflammation may lead to neoplastic transformation in canine mesothelial cells.19
Mesothelial tumors occur most often in older animals; however, in cattle and sheep, newborn or young animals may be affected.20 Juvenile mesothelioma has been reported in two mixed-breed dogs under 1 year of age; no underlying etiology was identified.21,22 A report of a 7-week-old puppy with mesothelioma suggests a congenital form may exist.23
Pathology and Natural Behavior
The normal mesothelium is a monolayer of flattened mesothelial cells. These cells are distinguished by the presence of microvilli, desmosomes, and evidence of phagocytic potential. Disease conditions associated with inflammation or irritation of the lining of body cavities commonly result in a marked physiologic proliferation of mesothelial cells. Fluid accumulation in a body cavity promotes exfoliation and implantation of mesothelial cells. Mesotheliomas are considered malignant due to their ability to seed the body cavity, resulting in multiple tumor growths. Distant metastasis is rare.
Mesothelial cells appear morphologically as epithelial cells; however, their derivation is from mesoderm. Mesothelioma can appear histologically as epithelial, mesenchymal, or biphasic, which is a combination of the two.24 The epithelial form, which resembles carcinoma or adenocarcinoma, is by far the most common form in small animals. There are also several reports of a variation of the mesenchymal form, which resembles sarcoma and is referred to as sclerosing mesothelioma.4,25,26 The biphasic form of mesothelioma has been reported in two dogs.27,28 A cystic peritoneal mesothelioma has also been reported in the dog. This is a rare, benign, slowly progressive form of mesothelioma in humans, which is treated with surgical excision when the disease is localized.29
History and Clinical Signs
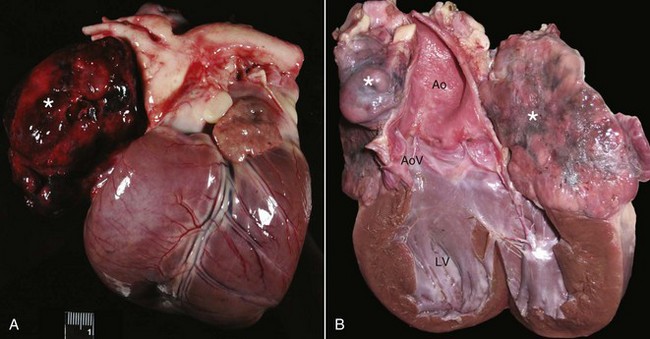
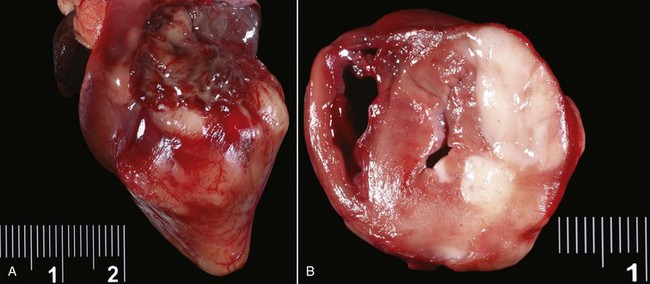
Classic mesotheliomas occur as a diffuse nodular mass or multifocal masses covering the surfaces of the body cavity (Figure 33-8). Extensive effusions occur due to exudation from the tumor surface or from tumor-obstructed lymphatics; therefore the most common presenting sign is dyspnea from pleural effusion or a distended abdomen from peritoneal effusion. Dogs with pericardial or heart-base mesotheliomas can present with acute tamponade and right-sided heart failure.30-32
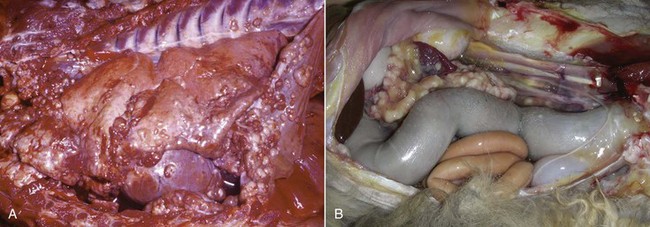
Figure 33-8 A, Pleural, parietal, and pericardial surfaces of a dog at necropsy illustrating nodular lesions histologically confirmed as mesothelioma. B, Mesothelioma involving the peritoneal surfaces of a cat at necropsy.
Sclerosing mesothelioma is a variation of mesothelial tumor seen primarily in male dogs, with German shepherd dogs being overrepresented.4,25,26,33 These tumors present as thick fibrous linings in the abdominal and/or pleural cavities. Restriction occurs around organs in the affected area, and in the abdomen such changes can impinge on organs and lead to vomiting and urinary tract signs.
Diagnostic Techniques and Work-Up
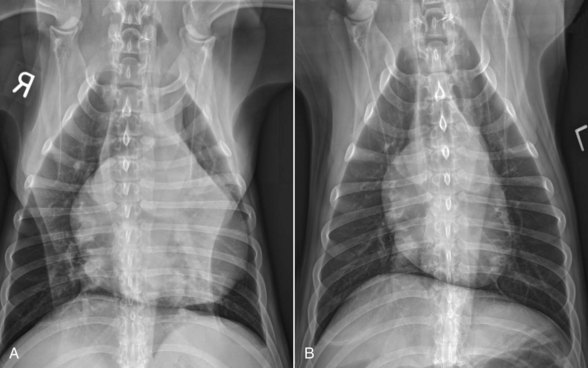
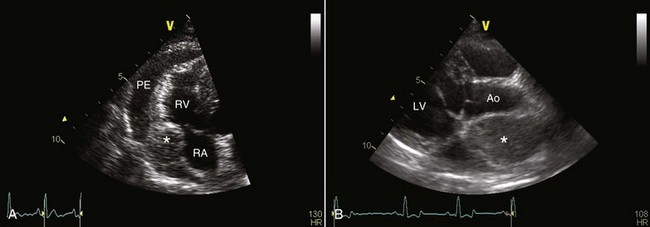
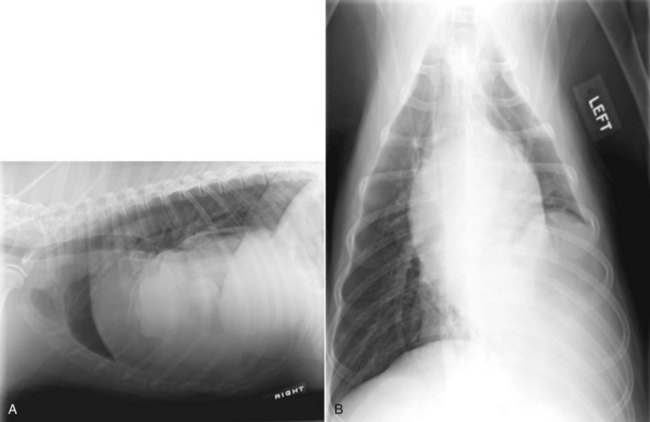
Mesothelioma should be suspected in adult dogs presenting with a history of chronic, nonspecific disease and fluid accumulation in any of the body cavities. Routine echocardiography and abdominal ultrasound are not typically helpful because the tumor cells cling to epithelial surfaces and a mass lesion is rarely noted.34 In a recent study of echocardiography of dogs with pericardial effusion, only 5 of 15 dogs with pericardial effusion due to mesothelioma had a discrete cardiac mass identified.32 Thoracic CT may be of benefit in identification of nodular lesions and in assessment of lung parenchyma in the face of pleural effusion35,36 (Figure 33-9).
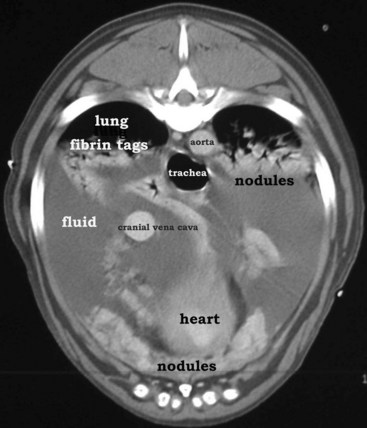
Figure 33-9 Thoracic CT (with contrast) from a dog with histologically confirmed mesothelioma. The effusion resolved following the first of five doxorubicin chemotherapy (30 mg/m2 every 3 weeks, IV) treatments.
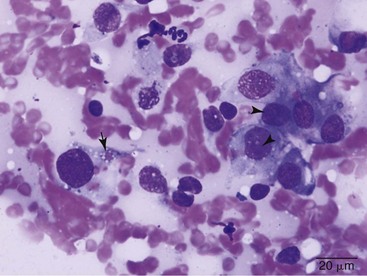
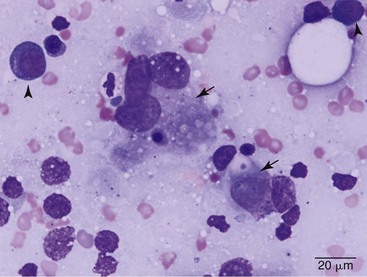
Cytologic evaluation of fluid can be diagnostic for other disease processes such as infection or lymphoma but will not conclusively diagnose mesothelioma. Mesothelial cells proliferate under any circumstance associated with fluid accumulation in the body cavity, making the distinction between physiologic mesothelial proliferation and neoplasia difficult. Although malignant mesothelial cells easily exfoliate into effusion fluid, they are hard to distinguish from reactive hypertrophic mesothelial cells cytologically. Reactive mesothelial cells display many cytologic features of malignancy, making a definitive diagnosis of neoplasia via cytology impossible in most cases. Although one study found pericardial fluid pH analysis to be a discriminatory test to differentiate benign from malignant effusions, subsequent studies found too much overlap in the pH values for the test to be of benefit.37-39 Fibronectin concentrations have also been evaluated in pleural effusions in dogs and cats and were found to differentiate malignant or inflammatory causes from cardiogenic effusions. Elevation in fibronectin levels is a sensitive but nonspecific test for malignant effusions, and mesothelioma can be ruled out if fibronectin levels are not increased.40
Establishing a definitive diagnosis of malignant mesothelioma may be difficult, particularly early in the disease. The diagnosis of mesothelioma requires adequate tissue sampling, preferably from an open, visually directed biopsy. Increasing availability of thoracoscopy and laparoscopy for small animals provides a less invasive means to evaluate these cases.41 In either procedure, the clinician is encouraged to biopsy any body cavity lining when an obvious cause for fluid accumulation is not found. Sclerosing mesothelioma must be distinguished from chronic inflammatory diseases of the body cavity, such as chronic peritonitis, and histologic examination of biopsy material is essential to establish the diagnosis. Additionally, embolized, nonneoplastic mesothelial cells within lymph nodes is a rare finding in humans with cavity effusions and has been reported in dogs affected with idiopathic hemorrhagic pericardial effusion; therefore care must be taken so as not to overinterpret these cells as indicative of a metastatic process.42
The most useful criteria in establishing a diagnosis of mesothelioma is to demonstrate that the tumor is primarily a neoplasm of the coelomic cavity lining and that the method of tumor spread is by transcoelomic implantation. Therefore mesothelioma should be considered when the bulk of the neoplastic tissue exists on the coelomic surface. Histologically, mesotheliomas need to be differentiated from carcinomas, adenocarcinomas, or sarcomas, depending on the morphologic type of the mesothelioma. Unfortunately, there are no cellular markers that conclusively define the mesothelial cell. Recent advances in IHC staining have provided additional ways to examine neoplastic cells to help differentiate mesothelioma from other epithelial or mesenchymal tumors in humans. Podoplanin and D2-40 were found to be highly sensitive IHC markers for sarcomatoid mesotheliomas.43 Differentiation of malignant epithelial mesotheliomas from adenocarcinomas can be aided by application of a panel of different immunohistochemical stains, including calretinin, Wilms’ tumor-1, and cytokeratin 5/6 for which mesotheliomas, but not adenocarcinomas, are strongly positive.44
Treatment and Prognosis
No satisfactory treatment exists for mesothelioma. Radical excision may benefit some animals, but usually the tumors are too advanced locally and have spread by implantation early in the course of disease. In one case report, a 2-year-old Siberian Husky with a solitary sclerosing mesothelioma affecting the left thoracic diaphragmatic surface with pericardial and mediastinal adhesions was treated with aggressive surgical resection and diaphragmatic reconstruction using the transversus abdominis muscle.45 The dog recovered well, but subcutaneous masses at the surgery site, as well as hepatic and renal masses, were noted 54 days postoperatively, leading to euthanasia. Pericardiectomy may palliate mesothelioma patients that present with cardiac tamponade; two dogs treated with surgery alone survived 4 and 9 months in one study.46 In another report, the median survival in five dogs treated with pericardiectomy was 13.6 months; three of these dogs received adjuvant IV chemotherapy (two DOX, one mitoxantrone).47 A dog treated with pericardiectomy, intrathoracic and IV cisplatin, and IV DOX remained free of disease at 27 months.48 In a report of eight dogs with pericardial mesothelioma, the MST was 60 days(range 15 to 300 days) following partial pericardiectomy. The one dog that survived 300 days was treated with DOX and intracavitary cisplatin for the 4 months preceding death.34 Thoracoscopic partial pericardiectomy is a less invasive procedure than open thoracic surgical pericardiectomy and has been successfully performed in dogs with malignant pericardial effusions, including four dogs with mesotheliomas.49 Portal site seeding with the mesothelioma is a potential complication of this procedure.50 Median survival for animals with untreated mesotheliomas in any location is difficult to assess from reports because the tumors are rare and animals frequently are euthanized at the time of diagnosis.
Intracavitary cisplatin has shown palliative potential in the dog; it was well tolerated and greatly decreased mesothelioma-associated thoracic fluid accumulation in three dogs in one study.51 The treatments also appeared to arrest tumor growth for a limited time. Two doses of intracavitary carboplatin were safely administered to a cat with suspected pleural mesothelioma and resulted in transient resolution of clinical signs for a total of 54 days, at which point the owners discontinued therapy.52 Unfortunately, local penetration of intracavitary chemotherapy only occurs to a limited depth (2 to 3 mm); thus large masses will not be affected significantly, other than from ultimate exposure to the systemically absorbed intracavitary drug. In such cases, combining debulking surgery or systemic chemotherapy such as DOX or mitoxantrone with intracavitary cisplatin may be beneficial. For peritoneal mesothelioma, four doses of intracavitary cisplatin in two dogs and carboplatin in one cat, combined with piroxicam administration, resolved the effusion in all cases.53 One of the dogs had debulking surgery first; this dog was still in remission at 2 years, whereas the other dog and the cat lived 8 and 6 months, respectively. IV chemotherapy may provide benefit in some patients; single-agent IV cisplatin administered every 3 weeks was reported to improve clinical signs in a dog with bicavitary epithelial mesothelioma, until sudden death occurred 5 months after treatment initiation.54
Comparative Aspects
In humans, mesothelioma is closely linked to exposure to aerosolized asbestos fibers. Approximately 70% to 80% of cases have a history of occupational exposure, with the type of employment significantly affecting relative risk.17,55,56 Occupations such as construction work, ship building, heating trades, asbestos mining, and insulation work are strong risk factors in the development of mesothelioma in humans.17 Family members of exposed industrial workers are at risk due to asbestos fiber exposure from the workers’ clothing. Affected individuals routinely have greatly increased counts of asbestos fibers in parenchymal lung tissue.55 The latency period from time of exposure to tumor development is long, with reports ranging from 12 to 50 years.17 Other risk factors discussed in the development of mesotheliomas include past radiation, exposure to certain chemicals or nonasbestos fibers, and genetic tendency.15,17 In addition, the DNA tumor virus Simian 40 (SV40) is suspected to act as a cocarcinogen with asbestos in causing mesotheliomas.16,57,58 SV40 was introduced into a large percentage of humans through contaminated polio vaccines between 1955 and 1963. SV40 can transform cells and experimentally leads to mesothelioma development in laboratory animals. However, there is strong evidence against the proposed connection between SV40 and mesothelioma and data support that the high prevalence of SV40 found in human mesotheliomas in previous studies was likely due to false-positive molecular assays.59,60
In humans, the median survival is approximately 1 year from symptom onset. A prognostic index, based on a combination of prognostic factors and gene expression testing, allows for the determination of high- and low-risk patients with respective median survivals of 6.9 versus 31.9 months.56 There is no consensus in the literature regarding surgery and RT for treatment, although recently several chemotherapy options have been shown to improve survival time and quality of life.56 The current gold standard drugs are antifolates, specifically pemetrexed and raltitrexed, often combined with cisplatin or carboplatin.56 Gemcitabine and vinorelbine have also shown some efficacy in humans with mesothelioma. Several novel therapies are also under investigation. Unfortunately, some therapies (e.g., epidermal growth factor receptor [EGFR] inhibitors) performing well in preclinical models did not show activity in clinical mesothelioma patients.61 Trials of other novel therapies, including anti-VEGF antibody, cyclin-dependent kinase (CDK) inhibitor, antimesothelin antibody, and proteasome inhibitors, are ongoing. Multimodality therapy, including novel agents, will hopefully improve survival time in the future.57,61
References
1. Geib, LW, DeNarvaez, F, Eby, CH. Pleural mesothelioma in a dog. J Am Vet Med Assoc. 1962;140:1317–1319.
2. Cihak, RW, Roen, DR, Klaassen, J. Malignant mesothelioma of the tunica vaginalis in a dog. J Comp Pathol. 1986;96:459–462.
3. Vascellari, M, Carminato, A, Camali, G, et al. Malignant mesothelioma of the tunica vaginalis testis in a dog: histological and immunohistochemical characterization. J Vet Diagn Invest. 2011;23:135–139.
4. Dubielzig, RR. Sclerosing mesothelioma in five dogs. J Am Anim Hosp Assoc. 1979;15:745–748.
5. Thrall, DE, Goldschmidt, MH. Mesothelioma in the dog: six case reports. J Am Vet Radial Soc. 1978;19:107–115.
6. Morini, M, Bettini, G, Morandi, F, et al. Deciduoid peritoneal mesothelioma in a dog. Vet Pathol. 2006;43:198–201.
7. Schaer, M, Meyer, D. Benign peritoneal mesothelioma, hyperthyroidism, nonsuppurative hepatitis, and chronic disseminated intravascular coagulation in a cat: a case report. J Am Anim Hosp Assoc. 1988;24:195–202.
8. Kobayashi, Y, Usuda, H, Ochiai, K, et al. Malignant mesothelioma with metastases and mast cell leukaemia in a cat. J Comp Path. 1994;111:453–458.
9. Carpenter, JL, Andrews, LK, Holzworth, J. Tumors and tumor-like lesions. Philadelphia: WB Saunders; 1987.
10. Tilley, LP, Owens, JM, Wilkins, RJ, et al. Pericardial mesothelioma with effusion in a cat. J Am Anim Hosp Assoc. 1975:60–65.
11. Umphlet, RC, Bertoy, RW. Abdominal mesothelioma in a cat. Mod Vet Pract. 1988;69:71–73.
12. Bacci, B, Morandi, F, De Meo, M, et al. Ten cases of feline mesothelioma: an immunohistochemical and ultrastructural study. J Comp Pathol. 2006;134:347–354.
13. Glickman, LT, Domanski, LM, Maguire, TG, et al. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ Res. 1983;32:305–313.
14. Harbison, ML, Godleski, JJ. Malignant mesothelioma in urban dogs. Vet Pathol. 1983;20:531–540.
15. Hughes, RS. Malignant pleural mesothelioma. Am J Med Sci. 2005;329:29–44.
16. Jaurand, MC, Fleury-Feith, J. Pathogenesis of malignant pleural mesothelioma. Respirology. 2005;10:2–8.
17. Pistolesi, M, Rusthoven, J. Malignant pleural mesothelioma: update, current management, and newer therapeutic strategies. Chest. 2004;126:1318–1329.
18. Belli, C, Anand, S, Tassi, G, et al. Translational therapies for malignant pleural mesothelioma. Expert Rev Respir Med. 2010;4:249–260.
19. Machida, N, Tanaka, R, Takemura, N, et al. Development of pericardial mesothelioma in golden retrievers with a long-term history of idiopathic haemorrhagic pericardial effusion. J Comp Pathol. 2004;131:166–175.
20. Head, KW. Tumors of the alimentary tract, ed 3. Berkeley: University of Calif Press; 1990.
21. Kim, JH, Choi, YK, Yoon, HY, et al. Juvenile malignant mesothelioma in a dog. J Vet Med Sci. 2002;64:269–271.
22. Vural, SA, Ozyildiz, Z, Ozsoy, SY. Pleural mesothelioma in a nine-month-old dog. Ir Vet J. 2007;60:30–33.
23. Leisewitz, AL, Nesbit, JW. Malignant mesothelioma in a seven-week-old puppy. J S Afr Vet Assoc. 1992;63:70–73.
24. Corson, JM. Pathology of mesothelioma. Thorac Surg Clin. 2004;14:447–460.
25. Geninet, C, Bernex, F, Rakotovao, F, et al. Sclerosing peritoneal mesothelioma in a dog - a case report. J Vet Med A Physiol Pathol Clin Med. 2003;50:402–405.
26. Schoning, P, Layton, CE, Fortney, WD, et al. Sclerosing peritoneal mesothelioma in a dog evaluated by electron microscopy and immunoperoxidase techniques. J Vet Diagn Invest. 1992;4:217–220.
27. Dias Pereira, P, Azevedo, M, Gartner, F. Case of malignant biphasic mesothelioma in a dog. Vet Rec. 2001;149:680–681.
28. Sato, T, Miyoshi, T, Shibuya, H, et al. Peritoneal biphasic mesothelioma in a dog. J Vet Med A Physiol Pathol Clin Med. 2005;52:22–25.
29. DiPinto, MN, Dunstan, RW, Lee, C. Cystic, peritoneal mesothelioma in a dog. J Am Anim Hosp Assoc. 1995;31:385–389.
30. Cobb, MA, Brownlie, SE. Intrapericardial neoplasia in 14 dogs. J Small Anim Pract. 1992;33:309–316.
31. McDonough, SP, MacLachlan, NJ, Tobias, AH. Canine pericardial mesothelioma. Vet Pathol. 1992;29:256–260.
32. MacDonald, KA, Cagney, O, Magne, ML. Echocardiographic and clinicopathologic characterization of pericardial effusion in dogs: 107 cases (1985-2006). J Am Vet Med Assoc. 2009;235:1456–1461.
33. Gumber, S, Fowlkes, N, Cho, DY. Disseminated sclerosing peritoneal mesothelioma in a dog. J Vet Diagn Invest. 2011;23:1046–1050.
34. Stepien, RL, Whitley, NT, Dubielzig, RR. Idiopathic or mesothelioma-related pericardial effusion: clinical findings and survival in 17 dogs studied retrospectively. J Small Anim Pract. 2000;41:342–347.
35. Echandi, RL, Morandi, F, Newman, SJ, et al. Imaging diagnosis—canine thoracic mesothelioma. Vet Radiol Ultrasound. 2007;48:243–245.
36. Reetz, JA, Buza, EL, Krick, EL. CT features of pleural masses and nodules. Vet Radiol Ultrasound. 2011. [November 18. Epub ahead of print].
37. Edwards, NJ. The diagnostic value of pericardial fluid pH determination. J Am Anim Hosp Assoc. 1996;32:63–67.
38. Fine, DM, Tobias, AH, Jacob, KA. Use of pericardial fluid pH to distinguish between idiopathic and neoplastic effusions. J Vet Intern Med. 2003;17:525–529.
39. de Laforcade, AM, Freeman, LM, Rozanski, EA, et al. Biochemical analysis of pericardial fluid and whole blood in dogs with pericardial effusion. J Vet Intern Med. 2005;19:833–836.
40. Hirschberger, J, Pusch, S. Fibronectin concentrations in pleural and abdominal effusions in dogs and cats. J Vet Intern Med. 1996;10:321–325.
41. Reggeti, F, Brisson, B, Ruotsalo, K, et al. Invasive epithelial mesothelioma in a dog. Vet Pathol. 2005;42:77–81.
42. Peters, M, Tenhundfeld, J, Stephan, I, et al. Embolized mesothelial cells within mediastinal lymph nodes of three dogs with idiopathic haemorrhagic pericardial effusion. J Comp Pathol. 2003;128:107–112.
43. Chirieac, LR, Pinkus, GS, Pinkus, JL, et al. The immunohistochemical characterization of sarcomatoid malignant mesothelioma of the pleura. Am J Cancer Res. 2011;1:14–24.
44. Chirieac, LR, Corson, JM. Pathologic evaluation of malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009;21:121–124.
45. Liptak, JM, Brebner, NS. Hemidiaphragmatic reconstruction with a transversus abdominis muscle flap after resection of a solitary diaphragmatic mesothelioma in a dog. J Am Vet Med Assoc. 2006;228:1204–1208.
46. Kerstetter, KK, Krahwinkel, DJ, Millis, DL, et al. Pericardiectomy in dogs: 22 cases (1978-1994). J Am Vet Med Assoc. 1997;211:736–740.
47. Dunning, D, Monnet, E, Orton, EC, et al. Analysis of prognostic indicators for dogs with pericardial effusion: 46 cases (1985-1996). J Am Vet Med Assoc. 1998;212:1276–1280.
48. Closa, JM, Font, A, Mascort, J. Pericardial mesothelioma in a dog: long-term survival after pericardiectomy in combination with chemotherapy. J Small Anim Pract. 1999;40:383–386.
49. Jackson, J, Richter, K, Launer, D. Thoracoscopic partial pericardiectomy in 13 dogs. J Vet Intern Med. 1999;13:529–533.
50. Brisson, BA, Reggeti, F, Bienzle, D. Portal site metastasis of invasive mesothelioma after diagnostic thoracoscopy in a dog. J Am Vet Med Assoc. 2006;229:980–983.
51. Moore, AS, Kirk, C, Cardona, A. Intracavitary cisplatin chemotherapy experience with six dogs. J Vet Intern Med. 1991;5:227–231.
52. Sparkes, A, Murphy, S, McConnell, F, et al. Palliative intracavitary carboplatin therapy in a cat with suspected pleural mesothelioma. J Feline Med Surg. 2005;7:313–316.
53. Spugnini, EP, Crispi, S, Scarabello, A, et al. Piroxicam and intracavitary platinum-based chemotherapy for the treatment of advanced mesothelioma in pets: preliminary observations. J Exp Clin Cancer Res. 2008;27:6.
54. Seo, KW, Choi, US, Jung, YC, et al. Palliative intravenous cisplatin treatment for concurrent peritoneal and pleural mesothelioma in a dog. J Vet Med Sci. 2007;69:201–204.
55. Zellos, L, Christiani, DC. Epidemiology, biologic behavior, and natural history of mesothelioma. Thorac Surg Clin. 2004;14:469–477. [viii].
56. Campbell, NP, Kindler, HL. Update on malignant pleural mesothelioma. Semin Respir Crit Care Med. 2011;32:102–110.
57. Carbone, M, Fisher, S, Powers, A, et al. New molecular and epidemiological issues in mesothelioma: role of SV40. J Cell Physiol. 1999;180:167–172.
58. Fisher, SG, Weber, L, Carbone, M. Cancer risk associated with simian virus 40 contaminated polio vaccine. Anticancer Res. 1999;19:2173–2180.
59. Lopez-Rios, F, Illei, PB, Rusch, V, et al. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet. 2004;364:1157–1166.
60. Gee, GV, Stanifer, ML, Christensen, BC, et al. SV40 associated miRNAs are not detectable in mesotheliomas. Br J Cancer. 2010;103:885–888.
61. Raja, S, Murthy, SC, Mason, DP. Malignant pleural mesothelioma. Curr Oncol Rep. 2011;13:259–264.