Chapter 96 Digestive System Disorders
Vomiting
Vomiting or, more often, regurgitation is a relatively frequent symptom during the neonatal period. In the 1st few hours after birth, infants may vomit mucus, occasionally blood streaked. This vomiting rarely persists after the 1st few feedings; it may be due to irritation of the gastric mucosa by material swallowed during delivery. If vomiting is protracted, gastric lavage with physiologic saline solution may relieve it.
When vomiting occurs shortly after birth and is persistent, the possibilities of intestinal obstruction, metabolic disorders, and increased intracranial pressure must be considered. A history of maternal polyhydramnios suggests upper gastrointestinal (esophageal, duodenal, ileal) atresia. Bile-stained emesis suggests intestinal obstruction beyond the duodenum but may also be idiopathic. Abdominal radiographs (kidney-ureter-bladder [KUB] and cross-table lateral views) should be performed in neonates with persistent emesis and in all infants with bile-stained emesis to detect air-fluid levels, distended bowel loops, characteristic patterns of obstruction (double bubble: duodenal atresia), and pneumoperitoneum (intestinal perforation). A contrast swallow roentgenogram with small bowel follow-through is indicated in the presence of bilious emesis.
Obstructive lesions of the digestive tract are the most frequent gastrointestinal anomalies (Chapters 311, 321, 322, and 324). Vomiting (and drooling) from esophageal obstruction occurs with the 1st feeding. The diagnosis of esophageal atresia can be suspected if unusual drooling from the mouth is observed and if resistance is encountered during an attempt to pass a catheter into the stomach. The diagnosis should be made before the infant has trouble with oral feedings and aspiration pneumonia develops. Infantile achalasia (cardiospasm), a rare cause of vomiting in newborn infants, is demonstrable radiographically as obstruction at the cardiac end of the esophagus without organic stenosis. Regurgitation of feedings because of continuous relaxation of the esophageal-gastric sphincter, or chalasia, is a cause of vomiting. Keeping the infant in a semi-upright position, thickening the feeding, or administering prokinetic drugs can control it.
Vomiting due to obstruction of the small intestine usually begins on the 1st day of life and is frequent, persistent, usually nonprojectile, copious, and, unless the obstruction is above the ampulla of Vater, bile-stained; it is associated with abdominal distention, visible deep peristaltic waves, and reduction or absence of bowel movements. Malrotation with obstruction from midgut volvulus is an acute emergency that must be not only considered but also urgently evaluated by an upper gastrointestinal contrast radiographic series. Radiographs of the abdomen show the distribution of air in the intestine, which may point to the anatomic location of an obstruction; malrotation can be identified only by contrast studies. Normally, air can be demonstrated by radiographs in the jejunum by 15-60 min, in the ileum by 2-3 hr, and in the colon by 3 hr after birth. Absence of rectal gas at 24 hr is abnormal. Persistent vomiting may occur with congenital diaphragmatic hernia. The vomiting associated with pyloric stenosis may begin any time after birth but does not assume its characteristic pattern before the 2nd-3rd wk. Vomiting with obstipation is a common early sign of Hirschsprung disease. Vomiting may occur with many other disturbances that do not obstruct the digestive tract, such as milk allergy, adrenal hyperplasia of the salt-losing variety, galactosemia, hyperammonemias, organic acidemias, increased intracranial pressure, septicemia, meningitis, and urinary tract infection. In many infants, it is simply regurgitation from overfeeding or from failure to permit the infant to eructate swallowed air. (See Chapter 315 for a discussion of gastric emptying and gastroesophageal reflux.)
Constipation
More than 90% of full-term newborn infants pass meconium within the 1st 24 hr. The possibility of intestinal obstruction should be considered in any infant who does not pass meconium by 24-36 hr. Intestinal atresia, stricture, or stenosis; Hirschsprung disease; milk bolus obstruction; meconium ileus; or meconium plugs may manifest as constipation or, more often, obstipation. About 20% of very low birthweight (VLBW) infants do not pass meconium within the 1st 24 hr. Constipation not present from birth but appearing during the 1st mo of life may be a sign of short-segment congenital aganglionic megacolon, hypothyroidism, strictures after necrotizing enterocolitis (NEC), or anal stenosis. It must be kept in mind that infrequent bowel movements do not necessarily mean constipation. A breast-fed infant usually has frequent bowel movements, whereas a formula-fed infant may have 1-2 movements a day or every other day.
Meconium Plugs
Lower colonic or anorectal plugs (Fig. 96-1) with a lower than normal water content may cause intestinal obstruction. Rarely, a firm mass of meconium may form elsewhere in the intestine and cause intrauterine intestinal obstruction and meconium peritonitis unrelated to cystic fibrosis (CF). Anorectal plugs may also cause mucosal ulceration and intestinal perforation. Meconium plugs are associated with small left colon syndrome in infants of diabetic mothers and with CF, rectal aganglionosis, maternal opiate use, and magnesium sulfate therapy for preeclampsia. The plug may be evacuated by glycerin suppository or rectal irrigation with isotonic saline. Enemas with the iodinated contrast medium Gastrografin usually induce passage of the plug, presumably because the high osmolarity (1,900 mOsm/L) of the solution draws fluid rapidly into the intestinal lumen and loosens inspissated material. Such rapid loss of fluid into the bowel may result in acute dehydration and shock, so it is advisable to dilute the contrast material with an equal amount of water, correct any existing dehydration, and provide intravenous fluids during and for several hours after the procedure. After removal of a meconium plug, the infant should be observed closely for the possible presence of congenital aganglionic megacolon.
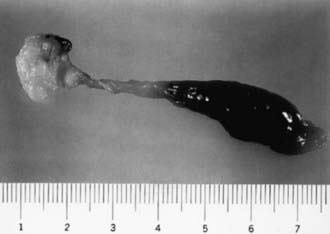
Figure 96-1 This plug of meconium and mucus (scale in cm) caused bowel obstruction in a premature infant. A radiograph showed marked gaseous distention and multiple fluid levels at 30 hr of age. Dramatic improvement occurred when the plug was passed after an enema.
(From: The abnormal fetus. In Beischer NA, Mackay EV, Colditz PB, editors: Obstetrics and the newborn, ed 3, Philadelphia, 1997, WB Saunders.)
96.1 Meconium Ileus in Cystic Fibrosis
Akhil Maheshwari and Waldemar A. Carlo
Impaction of meconium causes intestinal obstructions and may be associated with CF. The absence of fetal pancreatic enzymes in CF limits normal digestive activities in the intestine, and meconium becomes viscid and mucilaginous. It clings to the intestinal wall and moves with difficulty. The inspissated and impacted meconium fills the intestinal canal but is most concentrated in the lower part of the ileum. Clinically, the pattern is that of congenital intestinal obstruction with or without intestinal perforation. Abdominal distention is prominent, and vomiting becomes persistent. Infrequently, one or more inspissated meconium stools may be passed shortly after birth.
The differential diagnosis involves other causes of intestinal obstruction, including intestinal pseudo-obstruction and other causes of pancreatic insufficiency (Chapter 341). A presumptive diagnosis can be made on the basis of a history of CF in a sibling, via palpation of doughy or cordlike masses of intestines through the abdominal wall, and from the radiographic appearance. In contrast to the generally evenly distended intestinal loops above an atresia, the loops may vary in width and are not as evenly filled with gas. At points of heaviest meconium concentration, the infiltrated gas may create a bubbly granular appearance (Figs. 96-2 and 96-3). It is technically difficult to perform a sweat test in a neonate. Genetic testing confirms the diagnosis of CF.
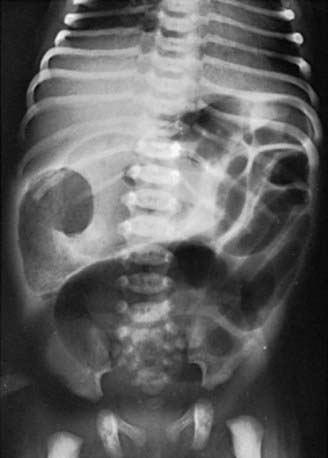
Figure 96-2 Meconium ileus. Impacted meconium with small amounts of air interspersed can be seen in loops of intestine on the right side of the abdomen. The intestinal loops above this impaction are greatly distended.

Figure 96-3 Meconium ileus. The colon, outlined by contrast material, is small because meconium has not reached it.
Treatment for meconium ileus is high Gastrografin enema as described previously for meconium plugs. If the procedure unsuccessful or perforation of the bowel wall is suspected, laparotomy is performed, and the ileum opened at the point of greatest diameter of the impaction. Approximately 50% of these infants have associated intestinal atresia, stenosis, or volvulus that requires surgery. The inspissated meconium is removed by gentle and patient irrigation with warm isotonic sodium chloride or acetylcysteine (Mucomyst) solution through a catheter passed between the impaction and the bowel wall. Most infants with meconium ileus survive the neonatal period. If meconium ileus is associated with CF, the long-term prognosis depends on the severity of the underlying disease (Chapter 395).
Meconium Peritonitis
Perforation of the intestine may occur in utero or shortly after birth. Frequently, the intestinal perforation seals naturally with relatively little meconium leakage into the peritoneal cavity. In some cases, with long-standing perforation, meconium peritonitis is more pronounced. Perforations occur most often as a complication of meconium ileus in infants with CF but are occasionally due to a meconium plug or in utero intestinal obstruction of another cause. Cases at the most severe end of the spectrum may be diagnosed on prenatal ultrasonography with fetal ascites, polyhydramnios, bowel dilatation, intra-abdominal calcifications, and hydrops fetalis. At the other end are cases in which an intestinal perforation may seal spontaneously with only a minor meconium leak, so the event may never be detected except when meconium becomes calcified and is later discovered on radiographs of the abdomen. Alternatively, the clinical picture may be dominated by the signs of intestinal obstruction (as in meconium ileus) or chemical peritonitis. Characteristic clinical findings include abdominal distention, vomiting, and absence of stools. Treatment consists primarily of elimination of the intestinal obstruction and drainage of the peritoneal cavity.
96.2 Neonatal Necrotizing Enterocolitis
NEC is the most common life-threatening emergency of the gastrointestinal tract in the newborn period. The disease is characterized by various degrees of mucosal or transmural necrosis of the intestine. The cause of NEC remains unclear but is most likely multifactorial. The incidence of NEC is 1-5% of infants in neonatal intensive care units (NICUs). Both incidence and case fatality rates increase with decreasing birthweight and gestational age. Because very small, ill preterm infants are particularly susceptible to NEC, a rising incidence may reflect improved survival of this high-risk group of patients.
Pathology and Pathogenesis
Many factors may contribute to the development of a necrotic segment of intestine, gas accumulation in the submucosa of the bowel wall (pneumatosis intestinalis), and progression of the necrosis to perforation, peritonitis, sepsis, and death. The distal part of the ileum and the proximal segment of colon are involved most frequently; in fatal cases, gangrene may extend from the stomach to the rectum. Although NEC is a multifactorial disease primarily associated with intestinal immaturity, the concept of “risk factors” for NEC is controversial. The triad of intestinal ischemia (injury), enteral nutrition (metabolic substrate), and bacterial translocation has classically been linked to NEC. The greatest risk factor for NEC is prematurity. The disorder probably results from an interaction between loss of mucosal integrity due to a variety of factors (ischemia, infection, inflammation) and the host’s response to that injury (circulatory, immunologic, inflammatory), leading to necrosis of the affected area. Coagulation necrosis is the characteristic histologic finding in intestinal specimens. Clustering of cases suggests a primary role for an infectious agent. Various bacterial and viral agents, including Escherichia coli, Klebsiella, Clostridium perfringens, Staphylococcus epidermidis, astrovirus, norovirus, and rotavirus, have been recovered from cultures. Nonetheless, in most situations, no pathogen is identified. NEC rarely occurs before the initiation of enteral feeding and is much less common in infants fed human milk. Aggressive enteral feeding may predispose to the development of NEC.
Although nearly 90% of all cases of NEC occur in premature infants, the disease can occur in full-term neonates. NEC in term infants is often a “secondary” disease, seen more frequently in infants with history of birth asphyxia, Down syndrome, congenital heart disease, rotavirus infections, and Hirschsprung disease.
Clinical Manifestations
Infants with NEC have a variety of signs and symptoms and may have an insidious or sudden catastrophic onset (Table 96-1). The onset of NEC is usually in the 2nd or 3rd week of life but can be as late as 3 mo in VLBW infants. Age of onset is inversely related to gestational age. The 1st signs of impending disease may be nonspecific, including lethargy and temperature instability, or related to gastrointestinal pathology, such as abdominal distention and gastric retention. Obvious bloody stools are seen in 25% of patients. Because of nonspecific signs, sepsis may be suspected before NEC. The spectrum of illness is broad, ranging from mild disease with only guaiac-positive stools to severe illness with bowel perforation, peritonitis, systemic inflammatory response syndrome, shock, and death. Progression may be rapid, but it is unusual for the disease to progress from mild to severe after 72 hr.
Diagnosis
A very high index of suspicion in treating preterm at-risk infants is crucial. Plain abdominal radiographs are essential to make a diagnosis of NEC. The finding of pneumatosis intestinalis (air in the bowel wall) confirms the clinical suspicion of NEC and is diagnostic; 50-75% of patients have pneumatosis when treatment is started (Fig. 96-4). Portal venous gas is a sign of severe disease, and pneumoperitoneum indicates a perforation (Figs. 96-4 and 96-5). Hepatic ultrasonography may detect portal venous gas despite normal abdominal roentgenograms.
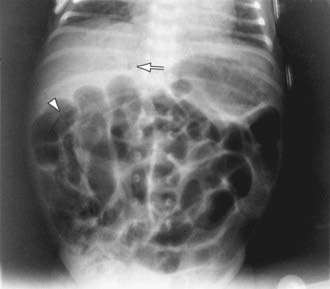
Figure 96-4 Necrotizing enterocolitis. A kidney-ureter-bladder film demonstrates abdominal distention, hepatic portal venous gas (arrow), and a bubbly appearance of pneumatosis intestinalis (arrowhead; right lower quadrant). The latter two signs are thought to be pathognomonic for neonatal necrotizing enterocolitis.

Figure 96-5 Intestinal perforation. A cross-table abdominal roentgenogram in a patient with a neonatal necrotizing enterocolitis demonstrates marked distention and massive pneumoperitoneum as evidenced by the free air below the anterior abdominal wall.
The differential diagnosis of NEC includes specific infections (systemic or intestinal), gastrointestinal obstruction, volvulus, and isolated intestinal perforation. Idiopathic focal intestinal perforation can occur spontaneously or after the early use of postnatal steroids and indomethacin. Pneumoperitoneum develops in such patients, but they are usually less ill than those with NEC.
Treatment
Rapid initiation of therapy is required for suspected as well as proven cases of NEC. There is no definitive treatment for established NEC, so, therapy is directed at giving supportive care and preventing further injury with cessation of feeding, nasogastric decompression, and administration of intravenous fluids. Careful attention to respiratory status, coagulation profile, and acid-base and electrolyte balances are important. Once blood has been drawn for culture, systemic antibiotics (with broad coverage based on the antibiotic sensitivity patterns of the gram-positive, gram-negative, and anaerobic organisms in the particular NICU) should be started immediately. If present, umbilical catheters should be removed, but good intravenous access is maintained. Ventilation should be assisted in the presence of apnea or if abdominal distention is contributing to hypoxia and hypercapnia. Intravascular volume replacement with crystalloid or blood products, cardiovascular support with fluid boluses and/or inotropes, and correction of hematologic, metabolic, and electrolyte abnormalities are essential to stabilize the infant with NEC.
The patient’s course should be monitored closely by means of frequent physical assessments; sequential anteroposterior and cross-table lateral or lateral decubitus abdominal radiographs to detect intestinal perforation; and serial determinations of hematologic, electrolyte, and acid-base status. Gown and glove isolation and grouping of infants at similar increased risks into cohorts separate from other infants should be instituted to contain an epidemic.
A surgeon should be consulted early in the course of treatment. Indications for surgery include evidence of perforation on abdominal roentgenograms (pneumoperitoneum) or positive result of abdominal paracentesis (stool or organism on Gram stain preparation from peritoneal fluid). Failure of medical management, a single fixed bowel loop on radiographs, abdominal wall erythema, and a palpable mass are relative indications for exploratory laparotomy. Ideally, surgery should be performed after intestinal necrosis develops but before perforation and peritonitis occur. In unstable premature infants with perforated NEC, peritoneal drainage can be cautiously considered as an alternative to exploratory laparotomy, although the best surgical approach in these infants remains unresolved. The type of surgical operation did not influence survival or other clinically important early outcomes in one multicenter study, but another large randomized trial showed that a majority of infants who were initially treated with peritoneal drains required a delayed secondary laparotomy. There are also some concerns about the long-term outcome (death or neurodevelopmental outcome) for infants treated with peritoneal drainage.
Patients with isolated intestinal perforation tend to have a lower birthweight, are less likely to be receiving oral feeding, and are prone to perforation at an earlier postnatal age than are patients with perforation related to NEC. In many patients with isolated intestinal perforation treated by drainage, no further surgical procedure is needed; a small subgroup may require later surgery to repair an intestinal stricture or fistula.
Prognosis
Medical management fails in about 20-40% of patients with pneumatosis intestinalis at diagnosis; of these, 10-30% die. Early postoperative complications include wound infection, dehiscence, and stomal problems (prolapse, necrosis). Later complications include intestinal strictures, which develop at the site of the necrotizing lesion in about 10% of surgically or medically managed patients. Resection of the obstructing stricture is curative. After massive intestinal resection, complications from postoperative NEC include short-bowel syndrome (malabsorption, growth failure, malnutrition), complications related to central venous catheters (sepsis, thrombosis), and cholestatic jaundice. Premature infants with NEC who require surgical intervention or who have concomitant bacteremia are at increased risk for adverse growth and neurodevelopmental outcome.
Prevention
Newborns exclusively breast-fed have a reduced risk of NEC. There have been concerns about early and aggressive increase in feeding volumes in raising the risk of NEC in VLBW infants, although a safe feeding regimen remains unknown. Gut stimulation protocols consisting of minimal enteral feeds followed by judicious volume advancement decreased the incidence of NEC in smaller study cohorts, but significant benefits were not detected in a meta-analysis of all randomized studies. Prophylactic enteral antibiotics can reduce the risk of NEC, although concerns about adverse outcomes persist, particularly related to the development of resistant bacteria. Probiotic preparations may also decrease the incidence of NEC; enteral supplementation of probiotics reduces the risk of severe NEC (stage II or higher) and mortality in preterm infants. The safety and efficacy of these supplements needs further evaluation in infants <1,000 g birthweight.
Afrazi A, Sodhi CP, Richardson W, et al. New insights into the pathogenesis and treatment of necrotizing enterocolitis toll-like receptors and beyond. Pediatr Res. 2011;69(3):183-188.
Alfaleh K, Bassler D: Probiotics for prevention of necrotizing enterocolitis in preterm infants, Cochrane Database Syst Rev (1):CD005496, 2008.
Bagci S, Eis-Hubinger AM, Franz AR, et al. Detection of astrovirus in premature infants with necrotizing enterocolitis. Pediatr Infect Dis J. 2008;27:347-350.
Blakely ML, Tyson JE, Lally KP, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth rate infants: outcomes through 18 mo adjusted age. Pediatrics. 2006;117:e680-e687.
Bombell S, McGuire W: Early trophic feeding for very low birth weight infants, Cochrane Database Syst Rev (3):CD000504, 2009.
Deshpande G, Rao S, Patole S, Balsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125(5):921-930.
Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1-147.e8.
Figueras-Aloy J, Rodriguez-Miguelez JM, Iriondo-Sanz M, et al. Intravenous immunoglobulin and necrotizing enterocolitis in newborns with hemolytic disease. Pediatrics. 2010;125:139-144.
Henderson G, Craig S, Brocklehurst P, et al. Enteral feeding regimens and necrotizing enterocolitis in preterm infants: a multicentre case control study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F120-F123.
Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopment and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:1-8.
Meinzen-Derr J, Morrow AL, Hornung RW, et al. Epidemiology of necrotizing enterocolitis temporal clustering in two neonatal practices. J Pediatr. 2009;154:656-661.
Meinzen-Derr J, Poindexter B, Wrage L, et al. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol. 2009;29:57-62.
Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354:2225-2234.
Murdoch EM, Sinha AK, Shanmugalingam ST, et al. Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics. 2006;118:1999-2003.
Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255-264.
Pickard SS, Feinstein JA, Popat RA, et al. Short- and long-term outcomes of necrotizing enterocolitis in infants with congenital heart disease. Pediatrics. 2009;123:e901-e906.
Pietz J, Achanti B, Lilien L, et al. Prevention of necrotizing enterocolitis in preterm infants: a 20-year experience. Pediatrics. 2007;119:e164-e170.
Rees CM, Eaton S, Kiely EM, et al. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008;248:44-51.
Sharma R, Tepas JJIII, Hudak ML, et al. Portal venous gas and surgical outcome of neonatal necrotizing enterocolitis. J Pediatr Surg. 2005;40:371-376.
Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357-2365.
Turcios-Ruiz RM, Axelrod PSt, John K, et al. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr. 2008;153:339-344.
Young C, Sharma R, Handfield M, et al. Biomarkers for infants at risk for necrotizing enterocolitis: clues to prevention? Pediatr Res. 2009;65:91R-97R.
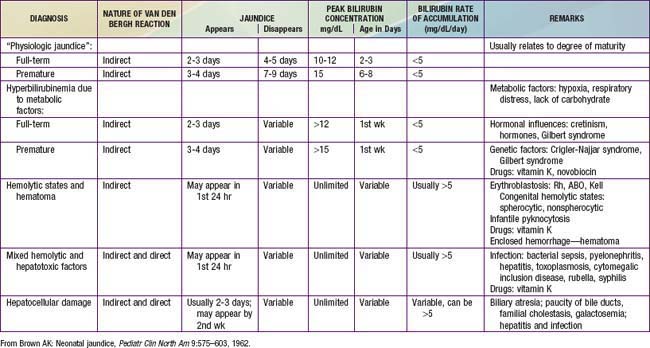
96.3 Jaundice and Hyperbilirubinemia in the Newborn
Hyperbilirubinemia is a common and, in most cases, benign problem in neonates. Jaundice is observed during the 1st wk of life in approximately 60% of term infants and 80% of preterm infants. The yellow color usually results from the accumulation of unconjugated, nonpolar, lipid-soluble bilirubin pigment in the skin. This unconjugated bilirubin (designated indirect-acting by nature of the Van den Bergh reaction) is an end product of heme-protein catabolism from a series of enzymatic reactions by heme-oxygenase and biliverdin reductase and nonenzymatic reducing agents in the reticuloendothelial cells. It may also be due in part to deposition of pigment from conjugated bilirubin, the end product from indirect, unconjugated bilirubin that has undergone conjugation in the liver cell microsome by the enzyme uridine diphosphoglucuronic acid (UDP)–glucuronyl transferase to form the polar, water-soluble glucuronide of bilirubin (direct-reacting). Although bilirubin may have a physiologic role as an antioxidant, elevations of indirect, unconjugated bilirubin are potentially neurotoxic. Even though the conjugated form is not neurotoxic, direct hyperbilirubinemia indicates a potentially serious hepatic disorders or a systemic illness.
Etiology
During the neonatal period, metabolism of bilirubin is in transition from the fetal stage, during which the placenta is the principal route of elimination of the lipid-soluble, unconjugated bilirubin, to the adult stage, during which the water-soluble conjugated form is excreted from hepatic cells into the biliary system and gastrointestinal tract. Unconjugated hyperbilirubinemia may be caused or increased by any factor that (1) increases the load of bilirubin to be metabolized by the liver (hemolytic anemias, polycythemia, bruising or internal hemorrhage, shortened red blood cell life as a result of immaturity or transfusion of cells, increased enterohepatic circulation, infection); (2) damages or reduces the activity of the transferase enzyme or other related enzymes (genetic deficiency, hypoxia, infection, thyroid deficiency); (3) competes for or blocks the transferase enzyme (drugs and other substances requiring glucuronic acid conjugation); or (4) leads to an absence or decreased amounts of the enzyme or to reduction of bilirubin uptake by liver cells (genetic defect, and prematurity). The toxic effects of elevated serum concentrations of unconjugated bilirubin are increased by factors that reduce the retention of bilirubin in the circulation (hypoproteinemia, displacement of bilirubin from its binding sites on albumin by competitive binding of drugs such as sulfisoxazole and moxalactam, acidosis, and increased free fatty acid concentration secondary to hypoglycemia, starvation, or hypothermia). Neurotoxic effects are directly related not only to the permeability of the blood-brain barrier and nerve cell membranes but also to neuronal susceptibility to injury, all of which are adversely influenced by asphyxia, prematurity, hyperosmolality, and infection. Early and frequent feeding decreases, whereas breast-feeding and dehydration increase, serum levels of bilirubin. Delay in passage of meconium, which contains 1 mg bilirubin/dL, may contribute to jaundice by enterohepatic recirculation after deconjugation by intestinal glucuronidase (Fig. 96-6). Drugs such as oxytocin (in the mother) and chemicals used in the nursery such as phenolic detergents may also produce unconjugated hyperbilirubinemia. Risk factors for unconjugated hyperbilirubinemia are listed in Table 96-2. Additional risk factors include polycythemia, infection, prematurity, and having a diabetic mother.
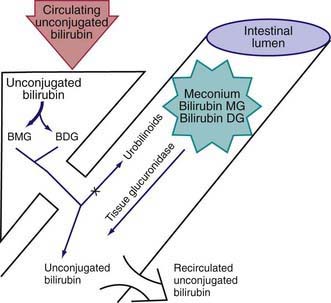
Figure 96-6 The neonatal production rate of bilirubin is 6-8 mg/kg/24 hr (in contrast to 3-4 mg/kg/24 hr in adults). Water-insoluble bilirubin is bound to albumin. At the plasma-hepatocyte interface, a liver membrane carrier (bilitranslocase) transports bilirubin to a cytosolic binding protein (ligandin or Y protein, now known to be glutathione S-transferase), which prevents back-absorption to plasma. Bilirubin is converted to bilirubin monoglucuronide (BMG). Neonates excrete more BMG than adults do. In the fetus, conjugated lipid-insoluble BMG and bilirubin diglucoronide (BDG) must be deconjugated by tissue β-glucuronidases to facilitate placental transfer of lipid-soluble unconjugated bilirubin across the placental lipid membranes. After birth, intestinal or milk-containing glucuronidases contribute to the enterohepatic recirculation of bilirubin and possibly to the development of hyperbilirubinemia.
Table 96-2 RISK FACTORS FOR DEVELOPMENT OF SEVERE HYPERBILIRUBINEMIA IN INFANTS ≥35 WEEKS OF GESTATION (IN APPROXIMATE ORDER OF IMPORTANCE)
MAJOR RISK FACTORS
MINOR RISK FACTORS
DECREASED RISK (these factors are associated with decreased risk of significant jaundice, listed in order of decreasing importance)
TcB, transcutaneous bilirubin; TSB, total serum bilirubin.
* Race as defined by mother’s description.
From AAP Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation, Pediatrics 114:297–316, 2004.
Clinical Manifestations
Jaundice may be present at birth or may appear at any time during the neonatal period, depending on etiology. Jaundice usually becomes apparent in a cephalocaudal progression, starting on the face and progressing to the abdomen and then the feet, as serum levels increase. Dermal pressure may reveal the anatomic progression of jaundice (face, ≈5 mg/dL; mid-abdomen, ≈15 mg/dL; soles, ≈20 mg/dL), but clinical examination cannot be depended on to estimate serum levels. Jaundice to the mid-abdomen, signs or symptoms, high-risk factors that suggest nonphysiologic jaundice, or hemolysis must be evaluated further (Tables 96-2 and 96-3). Noninvasive techniques for transcutaneous measurement of bilirubin (TcB) that correlate with serum levels may be used to screen infants, but determination of serum bilirubin level is indicated in patients with elevated age-specific transcutaneous bilirubin measurement, progressing jaundice, or risk for either hemolysis or sepsis. Whereas jaundice from deposition of indirect bilirubin in the skin tends to appear bright yellow or orange, jaundice of the obstructive type (direct bilirubin) has a greenish or muddy yellow cast. Infants with severe hyperbilirubinemia may present with lethargy and poor feeding and, without treatment, can progress to acute bilirubin encephalopathy (kernicterus) (Chapter 96.4).
Table 96-3 LABORATORY EVALUATION OF THE JAUNDICED INFANT ≥35 WEEKS OF GESTATION
| INDICATIONS | ASSESSMENTS |
|---|---|
| Jaundice in first 24 hr | Measure TcB and/or TSB |
| Jaundice appears excessive for infant’s age | Measure TcB and/or TSB |
| Infant receiving phototherapy or TSB rising rapidly (i.e., crossing percentiles [see Fig. 96-8]) and unexplained by history and physical examination | |
| TSB concentration approaching exchange levels or not responding to phototherapy | Perform reticulocyte count, G6PD, albumin, ETCOc, if available |
| Elevated direct (or conjugated) bilirubin level | |
| Jaundice present at or beyond age 3 wk, or sick infant |
ETCOc, end tidal carbon monoxide concentration; G6PD, glucose-6-phosphate dehydrogenase; TcB, transcutaneous bilirubin; TSB, total serum bilirubin.
From AAP Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation, Pediatrics 114:297–316, 2004.
Differential Diagnosis
Jaundice, consisting of either indirect or direct bilirubin, that is present at birth or appears within the 1st 24 hr of life requires immediate attention and may be due to erythroblastosis fetalis, concealed hemorrhage, sepsis, or congenital infections, including syphilis, cytomegalovirus, rubella, and toxoplasmosis. Hemolysis is suggested by a rapid rise in serum bilirubin concentration (>0.5 mg/dL/hr), anemia, pallor, reticulocytosis, hepatosplenomegaly, and a positive family history. An unusually high proportion of direct-reacting bilirubin may characterize jaundice in infants who have received intrauterine transfusions for erythroblastosis fetalis. Jaundice that first appears on the 2nd or 3rd day is usually physiologic but may represent a more severe form. Familial nonhemolytic icterus (Crigler-Najjar syndrome) and early-onset breast-feeding jaundice are seen initially on the 2nd or 3rd day. Jaundice appearing after the 3rd day and within the first week suggests bacterial sepsis or urinary tract infection; it may also be due to other infections, notably syphilis, toxoplasmosis, cytomegalovirus, and enterovirus. Jaundice secondary to extensive ecchymosis or blood extravasation may occur during the 1st day or later, especially in premature infants. Polycythemia may also lead to early jaundice.
There is a long differential diagnosis for jaundice first recognized after the 1st wk of life, including breast milk jaundice, septicemia, congenital atresia or paucity of the bile ducts, hepatitis, galactosemia, hypothyroidism, cystic fibrosis, and congenital hemolytic anemia crises related to red blood cell morphology and enzyme deficiencies (Fig. 96-7). The differential diagnosis for persistent jaundice during the first month of life includes hyperalimentation-associated cholestasis, hepatitis, cytomegalic inclusion disease, syphilis, toxoplasmosis, familial nonhemolytic icterus, congenital atresia of the bile ducts, galactosemia, and inspissated bile syndrome following hemolytic disease of the newborn. Rarely, physiologic jaundice may be prolonged for several weeks, as in infants with hypothyroidism or pyloric stenosis.
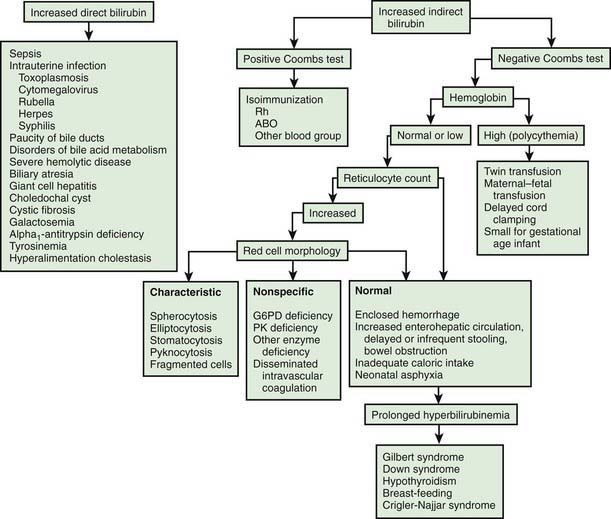
Figure 96-7 Schematic approach to the diagnosis of neonatal jaundice. G6PD, glucose-6-phosphate dehydrogenase; PK, pyruvate kinase.
(From Oski FA: Differential diagnosis of jaundice. In Taeusch HW, Ballard RA, Avery MA, editors: Schaffer and Avery’s diseases of the newborn, ed 6, Philadelphia, 1991, WB Saunders.)
Full-term, low-risk, asymptomatic infants with jaundice may be evaluated by monitoring of total serum bilirubin (TSB) levels. Regardless of gestation or time of appearance of jaundice, patients with significant hyperbilirubinemia and those with symptoms or signs require a complete diagnostic evaluation, which includes determination of direct and indirect bilirubin fractions, hemoglobin, reticulocyte count, blood type, Coombs test, and examination of a peripheral blood smear. Indirect hyperbilirubinemia, reticulocytosis, and a smear with evidence of red blood cell destruction suggest hemolysis (see Table 96-3). In the absence of blood group incompatibility, nonimmunologically induced hemolysis should be considered. If the reticulocyte count, Coombs test result, and direct bilirubin value are normal, physiologic or pathologic indirect hyperbilirubinemia may be present (see Fig. 96-7). If direct hyperbilirubinemia is present, hepatitis, congenital bile duct disorders (atresia, paucity, Byler disease), cholestasis, inborn errors of metabolism, cystic fibrosis, and sepsis are diagnostic possibilities.
Physiologic Jaundice (Icterus Neonatorum)
Under normal circumstances, the level of indirect bilirubin in umbilical cord serum is 1-3 mg/dL and rises at a rate of <5 mg/dL/24 hr; thus, jaundice becomes visible on the 2nd or 3rd day, usually peaking between the 2nd and 4th days at 5-6 mg/dL and decreasing to <2 mg/dL between the 5th and 7th days of life. Jaundice associated with these changes is designated physiologic and is believed to be the result of increased bilirubin production from the breakdown of fetal red blood cells combined with transient limitation in the conjugation of bilirubin by the immature neonatal liver.
Overall, 6-7% of full-term infants have indirect bilirubin levels >13 mg/dL and less than 3% have levels >15 mg/dL. Risk factors for elevated indirect bilirubin include maternal age, race (Chinese, Japanese, Korean, and Native American), maternal diabetes, prematurity, drugs (vitamin K3, novobiocin), altitude, polycythemia, male sex, trisomy 21, cutaneous bruising, blood extravasation (cephalohematoma), oxytocin induction, breast-feeding, weight loss (dehydration or caloric deprivation), delayed bowel movement, and a family history of or a sibling who had physiologic jaundice (see Table 96-2). In infants without these variables, indirect bilirubin levels rarely rise above 12 mg/dL, whereas infants with several risk factors are more likely to have higher bilirubin levels. A combination of breast-feeding, variant-glucuronosyl transferase activity (1A1), and alterations of the organic anion transporter 2 gene increases the risk in Asian children. Predicting which neonates are at risk for exaggerated physiologic jaundice can be based on hour-specific bilirubin levels in the first 24-72 hr of life (Fig. 96-8). Transcutaneous measurements of bilirubin are linearly correlated with serum levels and can be used for screening. Indirect bilirubin levels in full-term infants decline to adult levels (1 mg/dL) by 10-14 days of life. Persistent indirect hyperbilirubinemia beyond 2 wk suggests hemolysis, hereditary glucuronyl transferase deficiency, breast milk jaundice, hypothyroidism, or intestinal obstruction. Jaundice associated with pyloric stenosis may be due to caloric deprivation, deficiency of hepatic UDP-glucuronyl transferase, or an increase in the enterohepatic circulation of bilirubin from the ileus. In premature infants, the rise in serum bilirubin tends to be the same or somewhat slower but of longer duration than in term infants. Peak levels of 8-12 mg/dL are not usually reached until the 4th-7th day, and jaundice is infrequently observed after the 10th day, corresponding to the maturation of mechanisms for bilirubin metabolism and excretion.
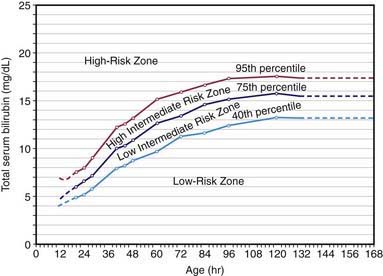
Figure 96-8 Risk designation of term and near-term well newborns based on their hour-specific serum bilirubin values. The high-risk zone is subdivided by the 95th percentile track. The intermediate -risk zone is subdivided into upper and lower risk zones by the 75th percentile track. The low-risk zone has been electively and statistically defined by the 40th percentile track.
(From Bhutani VK, Johnson L, Sivieri EM: Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns, Pediatrics 103:6–14, 1999.)
The diagnosis of physiologic jaundice in term or preterm infants can be established only by excluding known causes of jaundice on the basis of the history, clinical findings, and laboratory data (Table 96-4). In general, a search to determine the cause of jaundice should be made if (1) it appears in the first 24-36 hr of life, (2) serum bilirubin is rising at a rate faster than 5 mg/dL/24 hr, (3) serum bilirubin is >12 mg/dL in a full-term infant (especially in the absence of risk factors) or 10-14 mg/dL in a preterm infant, (4) jaundice persists after 10-14 days of life, or (5) direct bilirubin fraction is >2 mg/dL at any time. Other factors suggesting a nonphysiologic cause of jaundice are family history of hemolytic disease, pallor, hepatomegaly, splenomegaly, failure of phototherapy to lower the bilirubin level, vomiting, lethargy, poor feeding, excessive weight loss, apnea, bradycardia, abnormal vital signs (including hypothermia), light-colored stools, dark urine positive for bilirubin, and signs of kernicterus (Chapter 96.4).
Pathologic Hyperbilirubinemia
Jaundice and its underlying hyperbilirubinemia are considered pathologic if the time of appearance, duration, or pattern varies significantly from that of physiologic jaundice or if the course is compatible with physiologic jaundice but other reasons exist to suspect that the infant is at special risk for neurotoxicity. It may not be possible to determine the precise cause of an abnormal elevation of unconjugated bilirubin, but many infants with this finding have associated risk factors such as Asian race, prematurity, breast-feeding, and weight loss. Frequently, the terms exaggerated physiologic jaundice and hyperbilirubinemia of the newborn are used in infants whose primary problem is probably a deficiency or inactivity of bilirubin glucuronyl transferase (Gilbert syndrome) rather than an excessive load of bilirubin for excretion (see Table 96-2). The combination of glucose-6-phosphate dehydrogenase (G6PD) deficiency and a mutation of the promoter region of UDP-glucuronyl transferase-1 produces indirect hyperbilirubinemia in the absence of signs of hemolysis. Nonphysiologic hyperbilirubinemia may also be caused by mutations in the gene for bilirubin UDP-glucuronyl transferase.
The greatest risk associated with indirect hyperbilirubinemia is the development of bilirubin-induced neurologic dysfunction, which typically occurs with high indirect bilirubin levels (Chapter 96.4). The development of kernicterus (bilirubin encephalopathy) depends on the level of indirect bilirubin, duration of exposure to bilirubin elevation, the cause of jaundice, and the infant’s well-being. Neurologic injury including kernicterus may occur at lower bilirubin levels in preterm infants and in the presence of asphyxia, intraventricular hemorrhage, hemolysis, or drugs that displace bilirubin from albumin. The exact serum indirect bilirubin level that is harmful for VLBW infants is unclear.
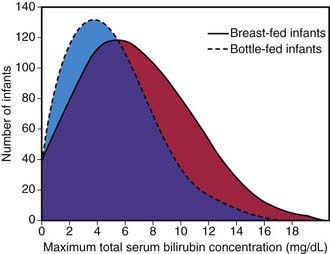
Jaundice Associated with Breast-Feeding
Significant elevation in unconjugated bilirubin (breast milk jaundice) develops in an estimated 2% of breast-fed term infants after the 7th day of life, with maximal concentrations as high as 10-30 mg/dL reached during the 2nd-3rd week. If breast-feeding is continued, the bilirubin gradually decreases but may persist for 3-10 wk at lower levels. If nursing is discontinued, the serum bilirubin level falls rapidly, reaching normal range within a few days. With resumption of breast-feeding, bilirubin seldom returns to previously high levels. Phototherapy may be of benefit (Chapter 96.4). Although uncommon, kernicterus can occur in patients with breast milk jaundice. The etiology of breast milk jaundice is not entirely clear but may be attributed to the presence of glucuronidase in some breast milk.
This syndrome should be distinguished from an early-onset, accentuated unconjugated hyperbilirubinemia known as breast-feeding jaundice, which occurs in the 1st week of life in breast-fed infants, who normally have higher bilirubin levels than formula-fed infants (Fig. 96-9). Hyperbilirubinemia (>12 mg/dL) develops in 13% of breast-fed infants in the 1st wk of life and may be due to decreased milk intake with dehydration and/or reduced caloric intake. Prophylactic supplements of glucose water to breast-fed infants are associated with higher bilirubin levels, in part because of reduced intake of the higher–caloric density breast milk. Frequent breast-feeding (>10/24 hr), rooming-in with night feeding, and ongoing lactation support may reduce the incidence of early breast-feeding jaundice. Even when breast-feeding jaundice develops, breast-feeding should be continued if possible. It is an option to temporarily interrupt breast-feedings and substitute formula for a day or two. In addition, frequent feeding and supplementation with formula or expressed breast milk is appropriate if the intake seems inadequate, weight loss is excessive, or the infant appears dehydrated.
Congenital Atresia of the Bile Ducts
See Chapter 348.1. Jaundice persisting for more than 2 wk or associated with acholic stools and dark urine suggests biliary atresia. All infants with such findings must undergo an immediate diagnostic evaluation, including determination of direct bilirubin.
Agrawal SK, Kumar P, Rathi R, et al. UGT1A1 gene polymorphisms in north Indian neonates presenting with unconjugated hyperbilirubinemia. Pediatr Res. 2009;65:675-680.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
Fay DL, Schellhase KG, Suresh GK. Bilirubin screening for normal newborns: a critique of the hour-specific bilirubin nomogram. Pediatrics. 2009;124:1203-1205.
Ip S, Chung M, Kulig J, et al. An evidenced-base review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
Johnson L, Bhutani VK, Karp K, et al. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J Perinatol. 2009;29:S25-S45.
Keren R, Luam Z, Friedman S, et al. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near-term infants. Pediatrics. 2008;121:e170-e179.
Kuzniewicz MW, Escobar GJ, Wi S, et al. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: a nested case-control study. J Pediatr. 2008;153:234-240.
Lin Z, Fontaine J, Watchko JF. Coexpression of gene polymorphisms involved in bilirubin production and metabolism. Pediatrics. 2008;122:e156-e162.
Morris BH, Oh W, Tyson JE, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359:1885-1896.
Watchko JF. Hyperbilirubin toxicity in the late preterm infant. Clin Perinatol. 2006;33:839-852.
96.4 Kernicterus
Kernicterus, or bilirubin encephalopathy, is a neurologic syndrome resulting from the deposition of unconjugated (indirect) bilirubin in the basal ganglia and brainstem nuclei. The pathogenesis of kernicterus is multifactorial and involves an interaction between unconjugated bilirubin levels, albumin binding and unbound bilirubin levels, passage across the blood-brain barrier, and neuronal susceptibility to injury. Disruption of the blood-brain barrier by disease, asphyxia, and other factors and maturational changes in blood-brain barrier permeability affect risk.
The precise blood level above which indirect-reacting bilirubin or free bilirubin will be toxic for an individual infant is unpredictable, but in a large series, kernicterus occurred only in infants with a bilirubin >20 mg/dL. Ninety percent of the infants in whom kernicterus developed were in previously healthy, predominantly breast-fed term and near-term infants. The duration of exposure to high bilirubin levels needed to produce toxic effects are unknown. The more immature the infant is, the greater the susceptibility to kernicterus. Factors that potentiate the movement of bilirubin across the blood-brain barrier and into brain cells are discussed in Chapter 96.3.
Clinical Manifestations
Signs and symptoms of kernicterus usually appear 2-5 days after birth in term infants and as late as the 7th day in premature infants, but hyperbilirubinemia may lead to encephalopathy at any time during the neonatal period. The early signs may be subtle and indistinguishable from those of sepsis, asphyxia, hypoglycemia, intracranial hemorrhage, and other acute systemic illnesses in a neonate. Lethargy, poor feeding, and loss of the Moro reflex are common initial signs. Subsequently, the infant may appear gravely ill and prostrate, with diminished tendon reflexes and respiratory distress. Opisthotonos with a bulging fontanel, twitching of the face or limbs, and a shrill high-pitched cry may follow. In advanced cases, convulsions and spasm occur, with affected infants stiffly extending their arms in an inward rotation with the fists clenched (Table 96-5). Rigidity is rare at this late stage.
Table 96-5 CLINICAL FEATURES OF KERNICTERUS
ACUTE FORM
CHRONIC FORM
From Dennery PA, Seidman DS, Stevenson DK: Neonatal hyperbilirubinemia, N Engl J Med 344:581–590, 2001.
Many infants who progress to these severe neurologic signs die; the survivors are usually seriously damaged but may appear to recover and for 2-3 mo show few abnormalities. Later in the 1st yr of life, opisthotonos, muscle rigidity, irregular movements, and convulsions tend to recur. In the 2nd yr, the opisthotonos and seizures abate, but irregular, involuntary movements, muscle rigidity, or, in some infants, hypotonia increase steadily. By 3 yr of age, the complete neurologic syndrome is often apparent; it consists of bilateral choreoathetosis with involuntary muscle spasms, extrapyramidal signs, seizures, mental deficiency, dysarthric speech, high-frequency hearing loss, squinting, and defective upward eye movements. Pyramidal signs, hypotonia, and ataxia occur in a few infants. In mildly affected infants, the syndrome may be characterized only by mild to moderate neuromuscular incoordination, partial deafness, or “minimal brain dysfunction,” occurring singly or in combination; these problems may be inapparent until the child enters school (see Table 96-5).
Incidence and Prognosis
By pathologic criteria, kernicterus develops in 30% of infants (all gestational ages) with untreated hemolytic disease and bilirubin levels >25-30 mg/dL. The incidence at autopsy in hyperbilirubinemic premature infants is 2-16% and is related to the risk factors discussed in Chapter 96.3. Reliable estimates of the frequency of the clinical syndrome are not available because of the wide spectrum of manifestations. Overt neurologic signs have a grave prognosis; more than 75% of infants die, and 80% of affected survivors have bilateral choreoathetosis with involuntary muscle spasms. Mental retardation, deafness, and spastic quadriplegia are common.
Prevention
Although kernicterus has been thought to be a disease of the past, there are reports of neurotoxic effects of bilirubin in term and near-term infants who were discharged as healthy newborns. Some but not all experts recommend universal screening for hyperbilirubinemia in the 1st 24-48 hr of life to detect infants at high risk for severe jaundice and bilirubin-induced neurologic dysfunction.
Effective prevention requires ongoing vigilance and a practical, system-based approach in order to distinguish infants with benign newborn jaundice from those whose course may be less predictable and potentially harmful. Protocols using the hour-specific bilirubin nomogram (see Fig. 96-8), physical examination, and clinical risk factors have been successful in identifying patients at risk for hyperbilirubinemia and candidates for targeted management. The American Academy of Pediatrics (AAP) has identified potentially preventable causes of kernicterus, as follows: (1) early discharge (<48 hr) with no early follow-up (within 48 hr of discharge); this problem is particularly important in near-term infants (35-37 wk of gestation); (2) failure to check the bilirubin level in an infant noted to be jaundiced in the first 24 hr; (3) failure to recognize the presence of risk factors for hyperbilirubinemia; (4) underestimation of the severity of jaundice by clinical (visual) assessment; (5) lack of concern regarding the presence of jaundice; (6) delay in measuring the serum bilirubin level despite marked jaundice or delay in initiating phototherapy in the presence of elevated bilirubin levels; and (7) failure to respond to parental concern regarding jaundice, poor feeding, or lethargy. An evidence-based management algorithm for infants is shown in Figure 96-10. In addition, it is recommended to determine before discharge each infant’s risk factors from established protocols (see Table 96-2).
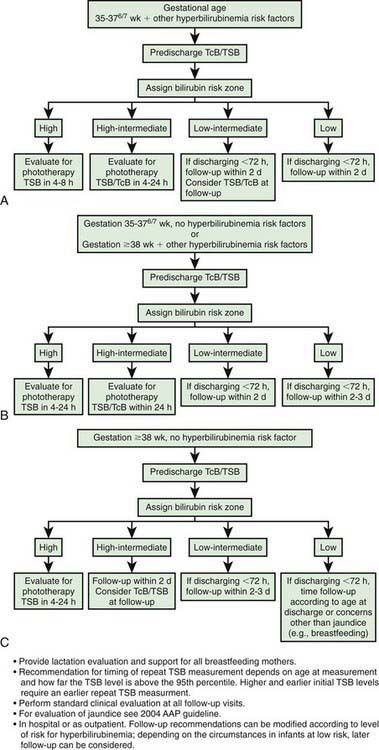
Figure 96-10 Algorithm providing recommendations for management and follow-up according to predischarge bilirubin measurements, gestation, and risk factors for subsequent hyperbilirubinemia. TcB, transcutaneous bilirubin; TSB, total serum bilirubin.
(From Maisels MJ, Bhutani VK, Bogen D, et al: Hyperbilirubinemia in the newborn infant ≥ 35 weeks’ gestation: an update with clarifications, Pediatrics 124:1193–1198, 2009.)
The following approach is further recommended: (1) any infant who is jaundiced before 24 hr requires measurement of serum bilirubin level and, if it is elevated, evaluation for possible hemolytic disease and (2) follow-up should be provided within 2-3 days of discharge to all neonates discharged earlier than 48 hr after birth. Early follow-up is particularly important for infants younger than 38 wk of gestation. The timing of follow-up depends on the age at discharge and the presence of risk factors. In some cases, follow-up within 24 hr is necessary. Post-discharge follow-up is essential for early recognition of problems related to hyperbilirubinemia and disease progression. Parental communication with regard to concerns about infant’s skin color and behavioral activities should be addressed early and frequently, including education about potential risks and neurotoxicity. Ongoing lactation promotion, education, support, and follow-up services are essential throughout the neonatal period. Mothers should be advised to nurse their infants every 2-3 hr and to avoid routine supplementation with water or glucose water in order to ensure adequate hydration and caloric intake.
Treatment of Hyperbilirubinemia
Regardless of the cause, the goal of therapy is to prevent neurotoxicity related to indirect-reacting bilirubin while not causing undue harm. Phototherapy and, if it is unsuccessful, exchange transfusion remain the primary treatment modalities used to keep the maximal total serum bilirubin below pathologic levels (Figs. 96-11 and 96-12; Table 96-6). The risk of injury to the central nervous system from bilirubin must be balanced against the potential risk of treatment. There is lack of consensus regarding the exact bilirubin level at which to initiate phototherapy. Because phototherapy may require 6-12 hr to have a measurable effect, it must be started at bilirubin levels below those indicated for exchange transfusion. When identified, underlying medical causes of elevated bilirubin and physiologic factors that contribute to neuronal susceptibility should be treated, with antibiotics for septicemia and correction of acidosis (Table 96-7).
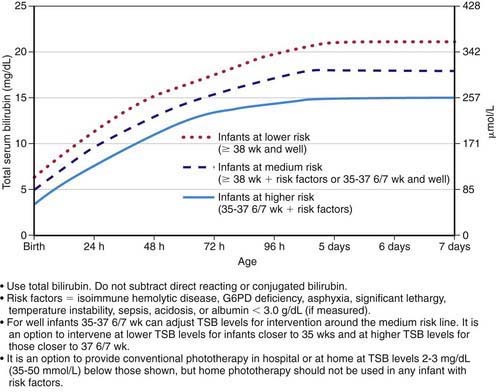
Figure 96-11 Guidelines for phototherapy in hospitalized infants of ≥35 weeks of gestation. Note: These guidelines are based on limited evidence, and the levels shown are approximations. The guidelines refer to the use of intensive phototherapy, which should be used when the total serum bilirubin (TSB) exceeds the line indicated for each category. Infants are designated as “higher risk” because of the potential negative effects of the conditions listed on albumin binding of bilirubin, the blood-brain barrier, and the susceptibility of the brain cells to damage by bilirubin. “Intensive phototherapy” implies irradiance in the blue-green spectrum (wavelengths approximately 430-490 nm) of at least 30 µW/cm2/nm (measured at the infant’s skin directly below the center of the phototherapy unit) and delivered to as much of the infant’s skin surface area as possible. Note that irradiance measured below the center of the light source is much greater than that measured at the periphery. Measurements should be made with a radiometer specified by the manufacturer of the phototherapy system. If TSB levels approach or exceed the exchange transfusion line (see Fig. 96-12), the sides of the bassinette, incubator, or warmer should be lined with aluminum foil or white material, to increase both the surface area of the infant exposed and the efficacy of phototherapy. The presence of hemolysis is strongly suggested if the TSB does not decrease or continues to rise in an infant who is receiving intensive phototherapy. Infants who receive phototherapy and have an elevated direct-reacting or conjugated bilirubin value (cholestatic jaundice) may inconsistently have the bronze-baby syndrome. G6PD, glucose-6-phosphate dehydrogenase.
(From American Academy of Pediatrics Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation, Pediatrics 114:297–316, 2004.)
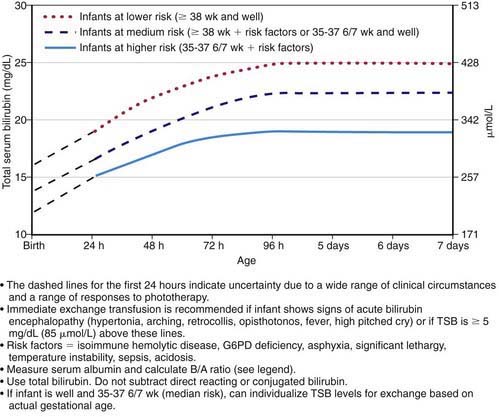
Figure 96-12 Guidelines for exchange transfusion in hospitalized infants of ≥35 weeks of gestation. Note: These suggested levels represent a consensus of most of the committee but are based on limited evidence, and the levels shown are approximations. During birth hospitalization, exchange transfusion is recommended if the total serum bilirubin (TSB) rises to these levels despite intensive phototherapy. In a readmitted infant, if the TSB level is above the exchange level, TSB measurement should be repeated every 2-3 hr; exchange transfusion should be considered if the TSB remains above the levels indicated after intensive phototherapy for 6 hr. The following B/A ratios can be used together with, but not in lieu of, the TSB level as an additional factor in determining the need for exchange transfusion. G6PD, glucose-6-phosphate dehydrogenase.
(From American Academy of Pediatrics Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation, Pediatrics 114:297–316, 2004.)
Table 96-6 SUGGESTED MAXIMAL INDIRECT SERUM BILIRUBIN CONCENTRATIONS (mg/dL) IN PRETERM INFANTS
| BIRTHWEIGHT (g) | UNCOMPLICATED* | COMPLICATED* |
|---|---|---|
| <1,000 | 12-13 | 10-12 |
| 1,000-1,250 | 12-14 | 10-12 |
| 1,251-1,499 | 14-16 | 12-14 |
| 1,500-1,999 | 16-20 | 15-17 |
| 2,000-2,500 | 20-22 | 18-20 |
* Complications include perinatal asphyxia, acidosis, hypoxia, hypothermia, hypoalbuminemia, meningitis, intraventricular hemorrhage, hemolysis, hypoglycemia, or signs of kernicterus. Phototherapy is usually started at 50-70% of the maximal indirect level. If values greatly exceed this level, if phototherapy is unsuccessful in reducing the maximal bilirubin level, or if signs of kernicterus are evident, exchange transfusion is indicated.
Table 96-7 EXAMPLE OF A CLINICAL PATHWAY FOR MANAGEMENT OF THE NEWBORN INFANT READMITTED FOR PHOTOTHERAPY OR EXCHANGE TRANSFUSION
TREATMENT
Use intensive phototherapy and/or exchange transfusion as indicated in Figs. 96-11 and 96-12
LABORATORY TESTS
INTERVENTIONS
FOR INFANTS RECEIVING INTENSIVE PHOTOTHERAPY:
TSB, total serum bilirubin.
From AAP Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation, Pediatrics 114:297–316, 2004.
Phototherapy
Clinical jaundice and indirect hyperbilirubinemia are reduced by exposure to a high intensity of light in the visible spectrum. Bilirubin absorbs light maximally in the blue range (420-470 nm). Broad-spectrum white, blue, and special narrow-spectrum (super) blue lights have been effective in reducing bilirubin levels. Bilirubin in the skin absorbs light energy, causing several photochemical reactions. One major product from phototherapy is a result of a reversible photo-isomerization reaction converting the toxic native unconjugated 4Z,15Z-bilirubin into an unconjugated configurational isomer, 4Z,15E-bilirubin, which can then be excreted in bile without conjugation. The other major product from phototherapy is lumirubin, which is an irreversible structural isomer converted from native bilirubin that can be excreted by the kidneys in the unconjugated state.
The therapeutic effect of phototherapy depends on the light energy emitted in the effective range of wavelengths, the distance between the lights and the infant, and the surface area of exposed skin, as well as the rate of hemolysis and in vivo metabolism and excretion of bilirubin. Available commercial phototherapy units vary considerably in spectral output and the intensity of radiance emitted; therefore, the wattage can be accurately measured only at the patient’s skin surface. Dark skin does not reduce the efficacy of phototherapy. Maximal intensive phototherapy should be used when indirect bilirubin levels approach those noted in Figure 96-11 and Table 96-7. Such therapy includes using “special blue” fluorescent tubes, placing the lamps within 15-20 cm of the infant, and putting a fiberoptic phototherapy blanket under the infant’s back to increase the exposed surface area. Aggressive phototherapy may improve neurodevelopmental outcome in infants <1,000 g.
The use of phototherapy has decreased the need for exchange transfusion in term and preterm infants with hemolytic and nonhemolytic jaundice. When indications for exchange transfusion are present, phototherapy should not be used as a substitute; however, phototherapy may reduce the need for repeated exchange transfusions in infants with hemolysis. Conventional phototherapy is applied continuously, and the infant is turned frequently for maximal skin surface area exposure. It should be discontinued as soon as the indirect bilirubin concentration has reduced to levels considered safe with respect to the infant’s age and condition. Serum bilirubin levels and hematocrit should be monitored every 4-8 hr in infants with hemolytic disease and those with bilirubin levels near toxic range for the individual infant. Others, particularly older infants, may be monitored less frequently. Serum bilirubin monitoring should continue for at least 24 hr after cessation of phototherapy in patients with hemolytic disease, because unexpected rises in bilirubin may occur, requiring further treatment. Skin color cannot be relied on for evaluating the effectiveness of phototherapy; the skin of babies exposed to light may appear to be almost without jaundice in the presence of marked hyperbilirubinemia. Although not necessary for all affected infants, intravenous fluid supplementation added to oral feedings may be beneficial in dehydrated patients or infants with bilirubin levels nearing those requiring exchange transfusion.
Complications associated with phototherapy include loose stools, erythematous macular rash, purpuric rash associated with transient porphyrinemia, overheating, dehydration (increased insensible water loss, diarrhea), hypothermia from exposure, and a benign condition called bronze baby syndrome (which occurs in the presence of direct hyperbilirubinemia; see later). Phototherapy is contraindicated in the presence of porphyria. Before phototherapy is initiated, the infant’s eyes should be closed and adequately covered to prevent light exposure and corneal damage. Body temperature should be monitored, and the infant should be shielded from bulb breakage. Irradiance should be measured directly. In infants with hemolytic disease, care must be taken to monitor for the development of anemia, which may require transfusion. Anemia may develop despite lowering of bilirubin levels. Clinical experience suggests that long-term adverse biologic effects of phototherapy are absent, minimal, or unrecognized.
The term bronze baby syndrome refers to a sometimes-noted dark, grayish brown skin discoloration in infants undergoing phototherapy. Almost all infants observed with this syndrome have had significant elevation of direct-reacting bilirubin and other evidence of obstructive liver disease. The discoloration may be due to photo-induced modification of porphyrins, which are often present during cholestatic jaundice and may last for many months. Despite the bronze baby syndrome, phototherapy can continue if needed.
Intravenous Immunoglobulin
The administration of intravenous immunoglobulin is an adjunctive treatment for hyperbilirubinemia due to isoimmune hemolytic disease. Its use is recommended when serum bilirubin is approaching exchange levels despite maximal interventions including phototherapy. Intravenous immunoglobulin (0.5-1.0 g/kg/dose; repeat in 12 hr) has been shown to reduce the need for exchange transfusion in both ABO and Rh hemolytic disease, presumably by reducing hemolysis.
Metalloporphyrins
A potentially important alternative therapy is the use of metalloporphyrins for hyperbilirubinemia. The metalloporphyrin Sn-mesoporphyrin (SnMP) offers promise as a drug candidate. The proposed mechanism of action is competitive enzymatic inhibition of the rate-limiting conversion of heme-protein to biliverdin (an intermediate metabolite in the production of unconjugated bilirubin) by heme-oxygenase. A single intramuscular dose on the 1st day of life may reduce the need for subsequent phototherapy. Such therapy may be beneficial when jaundice is anticipated, particularly in patients with ABO incompatibility or G6PD deficiency or when blood products are objected to, as with Jehovah’s Witness patients. Complications from metalloporphyrins include transient erythema if the infant is receiving phototherapy. Administration of SnMP may reduce bilirubin levels and decrease both the need for phototherapy and the duration of hospital stay; however, it remains unclear whether treatment with metalloporphyrins for unconjugated hyperbilirubinemia will alter the risk of kernicterus or long-term neurodevelopment impairment. Data on efficacy, toxicity, and long-term benefit are currently being evaluated.
Exchange Transfusion
Double-volume exchange transfusion is performed if intensive phototherapy has failed to reduce bilirubin levels to a safe range and if the risk of kernicterus exceeds the risk of the procedure. Potential complications from exchange transfusion are not trivial and include metabolic acidosis, electrolyte abnormalities, hypoglycemia, hypocalcemia, thrombocytopenia, volume overload, arrhythmias, NEC, infection, graft versus host disease, and death. This widely accepted treatment is repeated if necessary to keep indirect bilirubin levels in a safe range (see Fig. 96-12 and Table 96-7). See Exchange Transfusion in Chapter 97.
Various factors may influence the decision to perform a double-volume exchange transfusion in an individual patient. The appearance of clinical signs suggesting kernicterus is an indication for exchange transfusion at any level of serum bilirubin. A healthy full-term infant with physiologic or breast milk jaundice may tolerate a concentration slightly higher than 25 mg/dL with no apparent ill effect, whereas kernicterus may develop in a sick premature infant at a significantly lower level. A level approaching that considered critical for the individual infant may be an indication for exchange transfusion during the 1st or 2nd day of life when a further rise is anticipated, but not typically on the 4th day in a term infant or on the 7th day in a premature infant because an imminent fall may be anticipated as the hepatic conjugating mechanism becomes more effective.
American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Clinical practice guideline: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
Amin SB, Prinzing D, Myers G. Hyperbilirubinemia and language delay in premature infants. Pediatrics. 2009;123:327-331.
Bhutani V, Johnson L, Sivier E. Predictive ability of a predischarge hour specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
Bhutani VK, Johnson LH, Keren R, et al. Diagnosis and management of hyperbilirubinemia in the term neonate: for a safer first week. Pediatr Clin North Am. 2004;51:843-861.
Boo NY, Lee HT. Randomized controlled trial of oral versus intravenous fluid supplementation on serum bilirubin level during phototherapy of term infants with severe hyperbilirubinaemia. J Paediatr Child Health. 2002;38:151-155.
Burke BL, Robbins JM, Bird TM, et al. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988–2005. Pediatrics. 2009;123:524-532.
Chen SM, Chang MH, Du JC, et al. Screening for biliary atresia by infant stool color card in Taiwan. Pediatrics. 2006;117:1147-1154.
Jangaard KA, Fell DB, Dodds L, et al. Outcomes in a population of healthy term and near-term infants with serum bilirubin levels of ≥ 325 µmol/L (≥19 mg/dL) who were born in Nova Scotia, Canada between 1994 and 2000. Pediatrics. 2008;122:119-124.
Kaplan M, Hammerman C, Vreman HJ, et al. Hemolysis and hyperbilirubinemia in antiglobulin positive, direct ABO blood group heterospecific neonates. J Pediatr. 2010;157:772-777.
Kaplan M, Herschel M, Hammerman C, et al. Neonatal hyperbilirubinemia in African American males: the importance of glucose-6-phosphate dehydrogenase deficiency. J Pediatr. 2006;149:83-88.
Kaplan M, Muraca M, Vreman HJ, et al. Neonatal bilirubin production-conjugation imbalance: effect of glucose-6-phosphate dehydrogenase deficiency and borderline prematurity. Arch Dis Child Fetal Neonatal Ed. 2005;90:F123-F127.
Kappas A, Drummond GS, Valaes T. A single dose of Sn-mesoporphyrin prevents development of severe hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns. Pediatrics. 2001;108:25-30.
Kappas A, Munson DP, Marshall JR. Sn-mesoporphyrin interdiction of severe hyperbilirubinemia in Jehovah’s Witness newborns as an alternative to exchange transfusion. Pediatrics. 2001;108:1374-1377.
The Lancet. Detection and treatment of neonatal jaundice. Lancet. 2010;375:1845.
Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant ≥ 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med. 2002;156:669-672.
Maisels MJ, Kring E. The contribution of hemolysis to early jaundice in normal newborns. Pediatrics. 2006;118:276-279.
Maisels MJ, Kring E. Transcutaneous bilirubin levels in the first 96 hours in a normal newborn population of > or = 35 weeks’ gestation. Pediatrics. 2006;117:1169-1173.
Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358:920-928.
Manning D. Neonatal jaundice: in the eye of the beholder? Arch Dis Child Fetal Neonatal Ed. 2009;94:F314-F316.
McDonagh AF. Bilirubin, copper-porphyrins, and the bronze baby syndrome. J Pediatr. 2011;158:160-164.
Mehta S, Kumar P, Narang A. A randomized controlled trial of fluid supplementation in term neonates with severe hyperbilirubinemia. J Pediatr. 2005;147:781-785.
Morris BH, Oh W, Tyson JE, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359:1885-1896.
Newman TB, Liljestrand P, Jeremy RJ, et al. Outcomes among newborns with total serum bilirubin levels of 25 mg per deciliter or more. N Engl J Med. 2006;354:1889-1900.
Okumura A, Kidoforo H, Shoji H, et al. Kernicterus in preterm infants. Pediatrics. 2009;123:e1052-e1058.
Patra K, Storfer-Isser A, Siner B, et al. Adverse events associated with neonatal exchange transfusion in the 1990s. J Pediatr. 2004;144:626-631.
Rennie J, Burman-Roy S, Murphy MS. Neonatal jaundice: summary of NICE guidance. BMJ. 2009;338:b1805.
Suresh GK, Martin CL, Soll RF: Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates, Cochrane Database Syst Rev (2):CD004207, 2003.
Trikalinos TA, Cheung M, Lau J, et al. Systematic review of screening for bilirubin encephalopathy in neonates. Pediatrics. 2009;124:1162-1171.
US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US preventive services task force recommendation statement. Pediatrics. 2009;124:1172-1177.
Walsh S, Molloy EJ. Is intravenous immunoglobulin superior to exchange transfusion in the management of hyperbilirubinaemia in term neonates? Arch Dis Child. 2009;94:739-741.
Wennberg RP, Ahlfors CE, Bhutani VD, et al. Toward understanding kernicterus: a challenge to improve the management of jaundiced newborns. Pediatrics. 2006;117:474-485.