Chapter 386 Other Distal Airway Diseases
386.1 Bronchiolitis Obliterans
Epidemiology
Bronchiolitis obliterans (BO) is a rare chronic obstructive lung disease of the bronchioles and smaller airways. An insult to the lower respiratory tract occurs, resulting in fibrosis of the small airways. In the nontransplant patient, BO most commonly occurs in the pediatric population after respiratory infections, particularly adenovirus, but also Mycoplasma, measles, legionella, influenza, and pertussis; other causes include inflammatory diseases (juvenile rheumatoid arthritis, systemic lupus erythematosus [Chapter 152], scleroderma [Chapter 154], Stevens-Johnson syndrome [Chapter 146]), and inhalation of toxin fumes (NO2, NH3) (see Table 386-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). Bronchiolitis obliterans syndrome (BOS), a clinical entity that relates to graft deterioration after transplantation due to progressive airway, is increasingly recognized as a long-term complication of lung and bone marrow transplantation; more than one third of survivors of lung transplant can develop this disorder. BO occurs in all age groups, and the prevalence in 1 pediatric autopsy series was 2/1,000. BOS appears to be more common among older children and adolescents than infants and toddlers. There is some evidence that postinfectious obliterans may be more common in the southern hemisphere and among persons of Asian descent.
Table 386-1 ETIOLOGY OF BRONCHIOLITIS OBLITERANS
POSTINFECTIOUS
POSTTRANSPLANT
CONNECTIVE TISSUE DISEASE
TOXIC FUME INHALATION
CHRONIC HYPERSENSITIVITY PNEUMONITIS
ASPIRATION
DRUGS
STEVENS-JOHNSON SYNDROME
From Moonnumakal SP, Fan LL: Bronchiolitis obliterans in children, Curr Opin Pediatr 20:272–278, 2008.
Pathogenesis
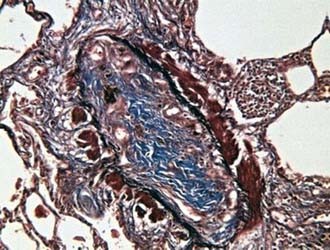
After the initial insult, inflammation affecting terminal bronchioles, respiratory bronchioles, and alveolar ducts can result in the obliteration of the airway lumen (Fig. 386-1). Epithelial damage resulting in abnormal repair is characteristic of BO. Complete or partial obstruction of the airway lumen can result in air trapping or atelectasis. Bronchiolitis obliterans organizing pneumonia (BOOP) is a fibrosing lung disease that includes the histologic features of BO with extension of the inflammatory process from distal alveolar ducts into alveoli and proliferation of fibroblasts. BOS appears histologically similar to BO. The etiology of BOS is unclear, however, and may be unrelated to the mechanisms responsible for BO in nontransplant patients.
Clinical Manifestations and Diagnosis
Cough, fever, cyanosis, dyspnea, chest pain, and respiratory distress followed by initial improvement may be the initial signs of BO. In this phase, BO is easily confused with pneumonia, bronchitis, or bronchiolitis. Progression of the disease can ensue, with increasing dyspnea, chronic cough, sputum production, and wheezing. Physical examination findings are usually nonspecific and can include wheezing, hypoxemia, and crackles. Chest radiographs may be relatively normal compared with the extent of physical findings but can demonstrate hyperlucency and patchy infiltrates. Occasionally, a Swyer-James syndrome (unilateral hyperlucent lung; Chapter 384) develops. Pulmonary function tests demonstrate variable findings but typically show signs of airway obstruction. Ventilation-perfusion scans reveal a typical moth-eaten appearance of multiple matched defects in ventilation and perfusion. Chest CT often demonstrates patchy areas of hyperlucency and bronchiectasis. Physical and radiologic signs can wax and wane over weeks or months. Open lung biopsy or transbronchial biopsy remains the best means of establishing the diagnosis of BO or BOOP.
Treatment
No definitive therapy exists for BO. Administration of corticosteroids may be beneficial. Immunomodulatory agents such as sirolimus, tacrolimus, aerosolized cyclosporine, and macrolide antibiotics have been used in post–lung transplant recipients with BO with variable success. Infliximab, a monoclonal antibody that binds tumor necrosis factor-α (TNF-α), has been successful in 1 patient with BO after a bone marrow transplant. Azithromycin may be effective in patients with BOS. For BOOP, use of oral corticosteroids for up to 1 yr has been advocated as first-line therapy for symptomatic and progressive disease. Patients with asymptomatic or nonprogressive BOOP can be observed.
Prognosis
Some patients with BO experience rapid deterioration in their condition and die within weeks of the initial symptoms; most nontransplant patients survive with chronic disability. BOS has a higher mortality rate. Unlike in BO, total recovery is seen in 60-80% of patients with BOOP, although this depends on the underlying systemic disease. BOOP can relapse, especially if treatment is <1 yr and is amenable to repeat courses of oral corticosteroids. Unlike the more common idiopathic BOOP, progressive BOOP characterized by acute respiratory distress syndrome (ARDS) is rare but is aggressive in its clinical course, leading to death.
Epler GR. Bronchiolitis obliterans organizing pneumonia. Arch Intern Med. 2001;161:158-164.
Fullmer JJ, Fan LL, Dishop MK, et al. Successful treatment of bronchiolitis obliterans in a bone marrow transplant patient with tumor necrosis factor-α blockade. Pediatrics. 2005;116:767-770.
Hardy KA, Schidlow DV, Zaeri N. Obliterative bronchiolitis in children. Chest. 1988;93:460-466.
Kurland G, Michelson P. Bronchiolitis obliterans in children. Pediatr Pulmonol. 2005;39:193-208.
Moonnumakal SP, Fan LL. Bronchiolitis obliterans in children. Curr Opin Pediatr. 2008;20:272-278.
Zhang L, Irion K, Kozakewich H, et al. Clinical course of postinfectious bronchiolitis obliterans. Pediatr Pulmonol. 2000;29:341-350.
386.2 Follicular Bronchitis
Follicular bronchitis (FB) is a lymphoproliferative lung disorder characterized by the presence of lymphoid follicles coursing alongside the airways (bronchi or bronchioles) and infiltration of the walls of bronchi and bronchioles. Although the cause is unknown, an infectious etiology (viral) has been proposed. It can occur in adults and children; in children, onset of symptoms generally occurs by 6 wk of age and peaks between 6 and 18 mo. Cough, moderate respiratory distress, fever, and fine crackles are common clinical findings. Fine crackles generally persist over time, and recurrence of symptoms is common. Chest radiographs may be relatively benign initially (air trapping, peribronchial thickening) but evolve into the typical interstitial pattern. Chest CT can show a fine reticular pattern. Definitive diagnosis is made by open lung biopsy. Some patients with FB respond to therapy with corticosteroids. Prognosis is variable, with some patients having significant progression of pulmonary disease and others developing only mild obstructive airway disease. In children it is generally associated with immunodeficiency; the differential diagnosis includes the pulmonary complications of HIV infection.
Bramson RT, Cleveland R, Blickman JG, et al. Radiographic assessment of follicular bronchitis in children. AJR Am J Roentgenol. 1996;166:1447-1450.
Kinane BT, Mansell AL, Zwerdling RG, et al. Follicular bronchitis in the pediatric population. Chest. 1993;104:1183-1186.
Yousem SA, Colby TV, Carrington CB. Follicular bronchitis/bronchiolitis. Hum Pathol. 1985;16:700-706.
386.3 Pulmonary Alveolar Microlithiasis
Approximately 400 cases of pulmonary alveolar microlithiasis (PAM), an unusual disorder, have been reported. Although the underlying cause of PAM is unknown, the disease is characterized by the formation of lamellar concretions of calcium phosphate or “microliths” within the alveoli, creating a classic pattern on the radiograph (see Fig. 386-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).
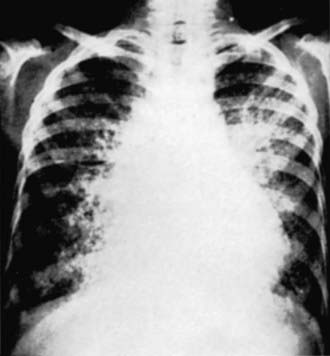
Figure 386-2 Radiograph of the chest of a 7 yr old boy with pulmonary alveolar microlithiasis.
(From Clark RB III, Johnson FC: Idiopathic pulmonary alveolar microlithiasis: a case report and brief review of the literature, Pediatrics 28:650, 1961.)
Epidemiology and Etiology
Although the mean age at time of diagnosis is in the mid 30s, the onset of the disease can occur in childhood. A strong familial association is seen in 50% of the reported cases, with a probable autosomal recessive pattern of inheritance. In some families, progression of disease is rapid. An equal male and female incidence is noted. A relatively large proportion of patients with PAM have Turkish ancestry. The nonfamilial or sporadic form appears to be associated with other medical conditions including mitral stenosis, nephrolithiasis, pulmonary fibrosis, milk alkali syndrome, or environmental factors (sand).
Clinical Manifestations
When symptomatic, patients with PAM usually complain of dyspnea on exertion and nonproductive cough. Physical examination of the lungs can reveal fine inspiratory crackles and diminished breath sounds. Clubbing occurs, although this is usually a more advanced sign. Discordance between the clinical and radiographic manifestations is common. Children are often asymptomatic on initial presentation. Complications of pneumothorax, pleural adhesions and calcifications, pleural fibrosis, apical bullae, and extrapulmonary sites of microliths have been reported (kidneys, prostate, sympathetic chain, and testes).
Diagnosis
Chest radiography typically reveals bilateral infiltrates with a fine sandlike micronodular appearance or “sandstorm” appearance with greater density in the lower and middle lung fields (see Fig. 386-2). CT of the chest shows diffuse micronodular calcified densities, with thickening of the microliths along the septa and around distal bronchioles, especially in the inferior and posterior regions. Diffuse uptake of technetium-99 methylene diphosphonate (99mTc) by nuclear scan has been reported. Open lung and transbronchial lung biopsy reveal 0.1-0.3 mm laminated calcific concretions within the alveoli. Although the alveoli are often normal initially, progression to pulmonary fibrosis with advancing disease usually ensues. Sputum expectoration might reveal small microliths, although this finding is not diagnostic for PAM and is not typically seen in children. Pulmonary function testing reveals restrictive lung disease with impaired diffusing capacity as the disease progresses, whereas exercise testing demonstrates arterial oxygen desaturation. The differential diagnosis includes sarcoidosis, miliary tuberculosis, hemosiderosis, healed disseminated histoplasmosis, pulmonary calcinosis, and metastatic pulmonary calcifications.
Treatment
No specific treatment is effective, although some clinicians have used glucocorticosteroids, etidronate disodium, and bronchopulmonary lavage with limited success. Lung transplantation has been performed for this condition, although it is unknown whether the disease recurs after transplantation.
Prognosis
Progressive cardiopulmonary disease can ensue, leading to cor pulmonale, superimposed infections, and subsequent death in mid-adulthood. Because of the familial nature of this disease, counseling and chest radiographs of family members are indicated.
Edelman JD, Bavaria J, Kaiser LR, et al. Bilateral sequential lung transplantation for pulmonary alveolar microlithiasis. Chest. 1997;112:1140-1144.
Erelel M, Kiyan E, Cuhadaroglu C, et al. Pulmonary alveolar lithiasis in two siblings. Respiration. 2001;68:327-330.
Mariotta S, Ricci A, Papale M, et al. Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vase Diffuse Lung Dis. 2004;21(3):173-181.
Schmidt H, Lorcher U, Kitz R, et al. Pulmonary alveolar microlithiasis in children. Pediatr Radiol. 1996;26:33-36.
Wallis C, Whitehead B, Malone M, et al. Pulmonary alveolar microlithiasis in childhood: Diagnosis by transbronchial biopsy. Pediatr Pulmonol. 1996;21:62-64.