Chapter 594 Pediatric Stroke Syndromes
Stroke has emerged as an important cause of acquired brain injury in newborns and children. The ischemic varieties of arterial ischemic stroke (AIS) and cerebral sinovenous thrombosis (CSVT) are more common than brain malignancy (incidence ∼5/100,000/yr) and affect 1 in 2,000 newborns. A similar number suffer from hemorrhagic stroke (HS) and other forms of cerebrovascular disease. Diagnosis is challenging and pathophysiology and risk factors are poorly understood. The frequent adverse neurologic outcomes suffered by most children who have strokes can be reduced by increasing pediatric physician awareness, facilitating early recognition, diagnosis, and specific treatment.
594.1 Arterial Ischemic Stroke (AIS)
Arterial blood reaches the brain via the anterior (internal carotid) and posterior (vertebrobasilar) circulations, converging at the circle of Willis. Strokes involve the middle cerebral artery (MCA) territory more frequently than either the anterior or posterior cerebral arteries. AIS is the focal brain infarction that results from occlusion of these arteries or their branches. AIS is a leading cause of acquired brain injury in children, with the perinatal period carrying the highest risk (see later).
In children the diagnosis of stroke is frequently delayed or missed. This is due to subtle and nonspecific clinical presentations, a complicated differential diagnosis (Chapter 594.4) and a lack of awareness by primary care pediatric physicians. The acute onset of a focal neurologic deficit in a child is stroke until proven otherwise. The most common focal presentation is hemiparesis but acute visual, speech, sensory, or balance deficits also occur. Children with these presentations require urgent neuroimaging and consultation with a child neurologist as emergency interventions may be indicated. AIS is a clinical and radiographic diagnosis. CT imaging can demonstrate larger mature AIS and exclude hemorrhage, however MRI identifies early and small infarcts and is therefore required to exclude ischemic stroke. Diffusion weighted MRI (DWI) can demonstrate AIS within minutes of onset, and MR angiography can confirm vascular occlusion and suggest arteriopathy as the underlying cause (Fig. 594-1).
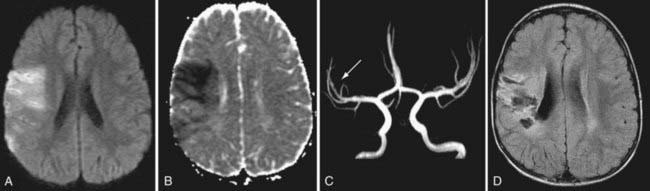
Figure 594-1 Arterial ischemic stroke. A healthy 3 yr old boy experienced the sudden onset of left-sided weakness. Examination also demonstrated left-sided hemisensory loss and neglect. Diffusion weighted MRI shows focal increased signal in the right temporal-parietal region in the territory of the middle cerebral artery (MCA, A). Apparent diffusion coefficient map confirms restricted diffusion consistent with infarction (ischemic stroke) (B). MR angiogram shows no occlusion or stenosis and decreased flow in the MCA (C). Follow-up MRI at 3 mo shows atrophy and gliosis in the same region (D).
Most etiologies for AIS are well-established; some represent only potential associations (Tables 594-1 and 594-2). AIS often remains idiopathic although most children have identifiable, frequently multiple risk factors. Three main categories of etiology should be considered: arteriopathic, cardiac, and hematological; full investigation often reveals multiple risk factors in a given individual.
Table 594-1 COMMON RISK FACTORS FOR ARTERIAL ISCHEMIC STROKE IN CHILDREN
| MAJOR CATEGORY | EXAMPLES |
|---|---|
| Arteriopathic | |
| Cardiac | |
| Hematologic | |
| Other |
Table 594-2 MISCELLANEOUS AND GENETIC RISK FACTORS FOR STROKE
From Roach ES, Golomb MR, Adams R, et al: Management of stroke in infants and children, Stroke 39:2644–2691, 2008, Table 2, p 6.
HERNS, hereditary endotheliopathy with retinopathy, nephropathy, and stroke; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes; MERRF, myoclonic epilepsy with ragged red fibers.
Arteriopathy refers to disorders of the cerebral arteries and has emerged as the leading cause of childhood AIS, present in >50% of children. Idiopathic arterial stenosis has been termed focal cerebral arteriopathy (FCA), and may be more specfically identified as transient cerebral arteriopathy (TCA) or postvaricella angiopathy (PVA). These diseases likely represent focal, localized, unilateral vasculitis. More diffuse or bilateral vasculitis may be primary or associated with systemic inflammatory conditions (Table 594-3). Arterial dissection can be spontaneous or post-traumatic and can affect extracranial internal carotid or vertebral arteries, or intracranial arteries. Moyamoya disease may be idiopathic or associated with other conditions (NF-1, trisomy 21, sickle cell anemia, radiation therapy) and demonstrates progressive occlusion of the distal internal carotid arteries (Table 594-4, Fig. 594-2). Congenital malformations of the craniocervical arteries, including PHACES syndrome, may predispose to AIS.
From Roach ES, Golomb MR, Adams R, et al: Management of stroke in infants and children, Stroke 39:2644–2691, 2008, Table 5, p 8.
Table 594-4 RISK FACTORS FOR MOYAMOYA DISEASE
| PATIENTS (n) | |
|---|---|
| No associated conditions (idiopathic) | 66 |
| Neurofibromatosis type 1 | 16 |
| Asian heritage | 16 |
| Cranial therapeutic radiation | 15 |
| Hypothalamic-optic system glioma | 8 |
| Craniopharyngioma | 4 |
| Medulloblastoma, with Gorlin syndrome | 1 |
| Acute lymphocytic leukemia, intrathecal chemotherapy | 2 |
| Down syndrome | 10 |
| Congenital cardiac anomaly, previously operated | 7 |
| Renal artery stenosis | 4 |
| Hemoglobinopathy (2 sickle cell anemia, 1 “Bryn Mawr”) | 3 |
| Other (hematologic: 1 spherocytosis, 1 idiopathic thrombocytopenic purpura) | 2 |
| Giant cervicofacial hemangiomas | 3 |
| Shunted hydrocephalus | 3 |
| Idiopathic hypertension requiring medication | 3 |
| Hyperthyroidism (1 with Graves syndrome) | 2 |
Other syndromes, 1 patient each: Reye (remote), Williams, Alagille, cloacal exstrophy, renal artery fibromuscular dysplasia, and congenital cytomegalic inclusion virus infection (remote). Two patients had unclassified syndromic presentations. There were 4 blacks, 2 of whom had sickle cell disease.
From Roach ES, Golomb MR, Adams R, et al: Management of stroke in infants and children, Stroke 39:2644–2691, 2008, Table 6, p 11.
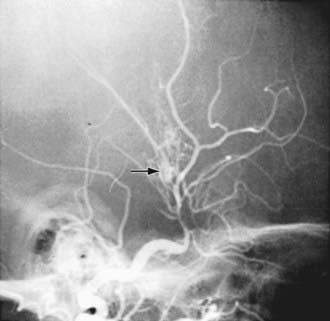
Figure 594-2 Cerebral angiogram showing idiopathic supraclinoid–internal carotid arteriopathy with classic moyamoya collaterals (arrow).
Cardioembolic stroke comprises about 25% of childhood AIS with embolism occurring either spontaneously, or during catheterization or surgical repair. AIS complicates 1 in 185 pediatric cardiac surgeries, and re-operation increases the risk. Complex congenital heart diseases are the most frequent cause for AIS, however acquired conditions including arrhythmia, cardiomyopathy, and infective endocarditis should also be considered. The presence of a patent foramen ovale provides the possibility of paradoxical venous thromboembolism. All children with suspected AIS require thorough cardiovascular examination, electrocardiogram, and echocardiogram.
Hematologic disorders include iron deficiency anemia and sickle cell anemia (SCA). In SCA the risk of AIS is increased 400-fold. Coagulation disorders are also frequently identified in AIS. They include hereditary (e.g., factor V Leiden) and acquired (e.g., antiphospholipid antibodies) prothrombotic states and prothrombotic medications, including oral contraceptives and asparaginase chemotherapy. Additional AIS risk factors include migraine, acute childhood illnesses, chronic systemic illnesses, illicit drugs and toxins, and rare inborn errors of metabolism.
Treatment of childhood AIS is multifaceted and 3 consensus-based guidelines are now available. Emergency thrombolysis is not yet established for children as there are no safety data available. Antithrombotic strategies depend on the suspected cause but include anticoagulation with heparins or antiplatelet strategies such as aspirin. Neuroprotective strategies are essential to prevent progressive ischemic brain injury. These include careful control of blood glucose, temperature, and seizures and aggressive maintenance of cerebral perfusion pressure, with systolic blood pressures maintained in the high normal range. Malignant cerebral edema in the initial 72 hr is life-threatening and more common in children; emergency surgical decompression can be lifesaving. Disease-specific treatments include transfusion therapy in SCA, immunosuppression in vasculitis, and revascularization surgery in moyamoya disease. Long-term treatment goals include secondary stroke prevention, including antiplatelet therapy or, for cardiogenic causes, anticoagulation. Multimodal, family-centered rehabilitation programs are required for most survivors targeting motor deficits, language and intellectual impairments, behavioral and social disabilities, and epilepsy. Long term attention to arterial health lifestyle factors (avoiding obesity and smoking) is also important. Outcomes after childhood stroke include death in 6-10%, neurological deficits in 60-70%, and seizure disorders in 15%.
Perinatal stroke, the leading cause of term-born cerebral palsy (congenital hemiplegia), requires separate consideration. Acute neonatal stroke typically presents with seizures alone. Presumed perinatal ischemic stroke presents with gradual evolution of hemiparesis in later infancy and remote AIS on neuroimaging. Etiologies for both include cardiac and prothrombotic conditions discussed earlier, but additional maternal, prenatal, perinatal, placental, and neonatal factors must also be considered. Acute neonatal AIS requires neuroprotective treatment, but antithrombotic agents are only provided for cardiogenic embolism. Long-term morbidity is present in most children and requires comprehensive rehabilitation, as deficits continue to emerge with maturation.
Amlie-Lefond C, Sebire G, Fullerton HJ. Recent developments in childhood arterial ischaemic stroke. Lancet Neurol. 2008;7:425-435.
Benseler SM, Silverman E, Aviv RI, et al. Primary central nervous system vasculitis in children. Arthritis Rheum. 2006;54:1291-1297.
Bernard TJ, Goldenberg NA, Armstrong-Wells J, et al. Treatment of childhood arterial ischemic stroke. Ann Neurol. 2008;63:679-696.
Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. 2009;66:704-709.
Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomized placebo-controlled trial. Lancet Neurol. 2011;10:123-130.
Danchaivijitr Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620-626.
De Tiege X, Van Bogaert P, Aeby A, et al. Primary angitis of the central nervous system: neurologic deterioration despite treatment. Pediatrics. 2011;127:el086-el090.
deVeber G, Kirkham F. Guidelines for the treatment and prevention of stroke in children. Lancet Neurol. 2008 Nov;7:983-985.
Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet. 2008;371:1612-1622.
Elbers J, Benseler SM. Central nervous system vasculitis in children. Curr Opin Rheumatol. 2008;20:47-54.
Farrar MA, Lin CSY, Krishnan AV, et al. Acute, reversible axonal energy failure during stroke-like episodes in MELAS. Pediatrics. 2010;126(3):e734-e739.
Goldenberg NA, Bernard TJ, Fullerton HJ, et al. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8:1120-1127.
Herak DC, Antolic MR, Krleza JL, et al. Inherited prothrombotic risk factors in children with stroke, transient ischemic attack, or migraine. Pediatrics. 2009;123:e653-e660.
Ikram MA, Seshadri S, Bis JC, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718-1728.
Jea A, Smith ER, Robertson R, et al. Moyamoya syndrome associated with down syndrome: outcome after surgical revascularization. Pediatrics. 2005;116:e694-e701.
Kirton A, deVeber G. Advances in perinatal ischemic stroke. Pediatr Neurol. 2009;40:205-214.
Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke. Neurology. 2010;74:792-797.
Mallick AA, Ganesan V, O’Callaghan FJK. Mortality from childhood stroke in England and Wales, 1921-2000. Arch Dis Child. 2010;95:12-19.
Mitre N, Mack K, Babovic-Vuksanovic D, et al. Ischemic stroke as the presenting symptom of primary hyperparathyroidism due to multiple endocrine neoplasia type 1. J Pediatr. 2008;153:582-585.
Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:887S-968S.
Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76:444-450.
Pappachan J, Kirkham FJ. Cerebrovascular disease and stroke. Arch Dis Child. 2008;93:890-898.
Perry JJ, Stiell IG, Sivilotti MLA, et al. High risk clinical characteristics for subarachnoid haemorrhage in patients with acute headache: prospective cohort study. BMJ. 2010;341:c5204.
Rea D, Brandsema JF, Arnstrong D, et al. Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics. 2009;124:e476-e483.
Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children. Stroke. 2008;39:2644-2691.
Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-1237.
Sebire G. Transient cerebral arteriopathy in childhood. Lancet. 2006;368:8-10.
Veeravagu A, Guzman R, Patil CG, et al. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurg Focus. 2008;24:1-9.
594.2 Cerebral Sinovenous Thrombosis (CSVT)
Cerebral venous drainage occurs via superficial (cortical veins, superior sagittal sinus) and deep (internal cerebral veins, straight sinus) systems that converge at the torcula to exit via the paired transverse and sigmoid sinuses and jugular veins. In CSVT, thrombotic occlusion of these venous structures can create increased intracranial pressure, cerebral edema, and, in 50% of cases, venous infarction (stroke). CSVT may be more common in children than in adults, and risk is greatest risk in the neonatal period.
Clinical presentations are often more gradual, variable, and nonspecific compared to AIS. Neonates typically present with diffuse neurologic signs and seizures. Children may present with progressive headache, papilledema, diplopia secondary to 6th nerve palsies (frequently misdiagnosed as idiopathic intracranial hypertension), or acute focal deficits. Seizures, lethargy, and confusion are common. Diagnosis requires a high clinical suspicion and purposeful imaging of the cerebral venous system. Nonenhanced CT is very insensitive for CSVT, and contrast CT venography (CTV) is usually necessary to demonstrate filling defects in the cerebral venous system (Fig. 594-3). However MRI includes diffusion imaging of the brain parenchyma and modern MR venography (MRV) can be comparable to CTV in accuracy. Intraventricular hemorrhage in term infants suggests CSVT.
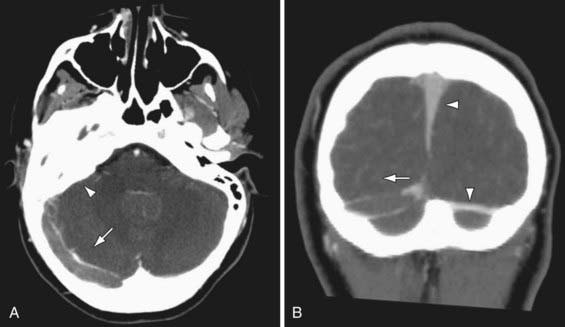
Figure 594-3 Cerebral sinovenous thrombosis. A 9 yr old girl presented with fever and progressive right-sided headache. She complained of double vision and had papilledema on examination. Axial (A) and coronal (B) CT venography demonstrates a large thrombus in the right transverse sinus that fails to opacify with contrast (arrows). Note normal filling superior sagittal and left transverse sinuses (arrowheads in B) and opacification of the mastoid air cells (arrowhead in A). Cause was otitis media/mastoiditis with septic thrombophlebitis of transverse sinus.
The Virchow triad is helpful in understanding the risk factors for CSVT (Table 594-5). Hypercoagulable states are frequently associated with childhood venous thrombosis including CSVT. Prothrombotic states frequently detected in childhood CSVT include inherited (e.g., prothrombin gene 20210A mutation) and acquired (e.g., antiphospholipid antibodies) conditions, prothrombotic medications (asparaginase, oral contraceptives), and common childhood illnesses, including iron deficiency anemia and severe dehydration. Systemic diseases associated with increased CSVT risk include leukemia, inflammatory bowel disease, and nephrotic syndrome.
Table 594-5 COMMON RISK FACTORS FOR CEREBRAL SINOVENOUS THROMBOSIS IN CHILDREN
| MAJOR CATEGORIES (VIRCHOW TRIAD) | EXAMPLES |
|---|---|
| Blood coagulation | |
| Blood vessel |
Head and neck disorders can directly involve cerebral veins and sinuses causing CSVT. Common infections including meningitis, otitis media, and mastoiditis can cause septic thrombophlebitis of venous channels. CSVT can complicate head trauma especially adjacent to skull fractures. Neurosurgical procedures in proximity to cerebral venous structures may lead to injury and CSVT. Finally, obstruction of the cervical jugular veins and proximal stasis may result in CSVT. In neonates the unfused status of cranial sutures enables compression of underlying venous sinuses during delivery, or postnatally during supine lying when the occipital bone can compress the posterior sagittal sinus, possibly predisposing to CSVT.
Anticoagulation therapy plays an important role in childhood CSVT treatment. Despite no randomized trials, substantial indirect evidence has led to consensus across published guidelines which uniformly recommend anticoagulation with unfractionated or low molecular weight heparins in most children. Hemorrhagic transformation of venous infarcts is not an absolute contraindication to anticoagulation. Treatment is often continued for 3 mo at which time re-imaging either confirms recanalization (treatment usually discontinued) or persistent thrombus (treatment usually extended to 6 mo). However anticoagulation of neonates is more controversial and guidelines differ. New evidence suggests that protocol-based anticoagulants are safe in neonatal CSVT. About 30% of untreated neonates will extend their thrombosis in the 1st week postdiagnosis and additional venous infarction can result. Therefore if anticoagulation is withheld, early repeat venous imaging is paramount. Protocols supporting initial anticoagulation recommend shorter treatment durations in neonates (i.e., 6 weeks, 3 mo). Children with persistent risk factors carry risk for recurrence and may require long-term anticoagulation. Neuroprotective and supportive interventions include aggressive management of infection, maintenance of hydration, and neuroprotective measures (normothermia, normotension, seizure control). Optic neuropathy secondary to increased intracranial pressure is an important and often overlooked complication of CSVT. Regular fundoscopic examination by an ophthalmologist and measures to reduce intracranial pressure (e.g., acetazolamide, serial lumbar puncture) may be required. Most neurologic morbidity is suffered by those incurring venous infarction, and children with bilateral injuries can be devastated. Consistent with other forms of childhood stroke, a comprehensive neurorehabilitation program is required for children with venous infarction.
deVeber G, Andrew M, Canadian Pediatric Ischemic Stroke Study Group. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417-423.
Kenet G, Kirkham F, Niederstadt T, et al. Risk factors for recurrent venous thromboembolism in the European collaborative paediatric database on cerebral venous thrombosis: a multicentre cohort study. Lancet Neurol. 2007;6:595-603.
Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:887S-968S.
Moharir MD, Shroff M, Stephens D, et al. Anticoagulants in pediatric cerebral sinovenous thrombosis: a safety and outcome study. Ann Neurol. 2010;67:590-599.
Sebire G, Tabarki B, Saunders DE, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477-489.
Smith R, Hourihan MD. Investigating suspected cerebral venous thrombosis. BMJ. 2007;334:794-795.
594.3 Hemorrhagic Stroke (HS)
Hemorrhagic stroke (HS) includes nontraumatic intracranial hemorrhage and is classified by the intracranial compartment containing the hemorrhage. Intraparenchymal bleeds may occur in any location within the brain. Intraventricular hemorrhage may be primary or an extension of intraparenchymal hemorrhage. Bleeding outside the brain may occur in the subarachnoid, subdural, or epidural spaces.
Clinical presentations vary according to location, cause, and rate of bleeding. Acute hemorrhages may feature instantaneous or thunderclap headache, loss of consciousness, and nuchal rigidity in addition to focal neurologic deficits and seizures. HS can be rapidly fatal. In bleeds associated with vascular malformations, pulsatile tinnitus, cranial bruit, macrocephaly, and high-output heart failure may be present. Diagnosis relies on imaging, and CT is highly sensitive to acute HS. However lumbar puncture may be required to exclude subarachnoid hemorrhage. Modern MRI is highly sensitive to even small amounts of acute hemorrhage and it images residual blood products long-term following most parenchymal bleeds (Fig. 594-4). Angiography by CT, MR, or conventional means is often required to exclude underlying vascular abnormalities (e.g., vascular malformations, aneurysms).
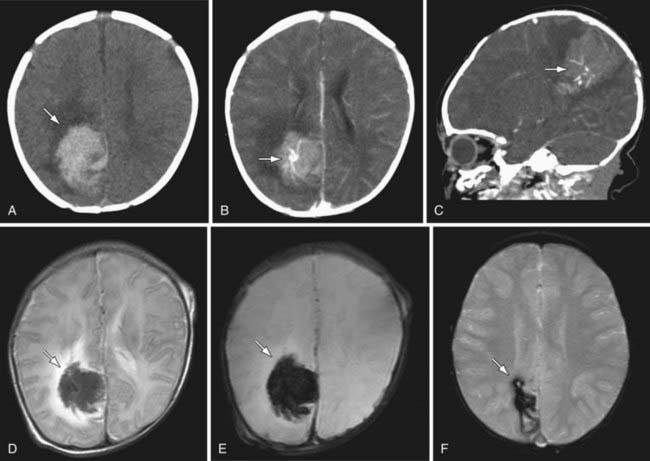
Figure 594-4 Hemorrhagic stroke due to vascular malformation. A healthy 1 mo old presented with sudden onset irritability followed by focal left body seizures. Plain CT head demonstrates a large hyperdense lesion in the right parietal region with surrounding edema consistent with acute hemorrhage (A). Axial (B) and sagittal (C) contrast CT scans suggest an abnormal cluster of vessels in the center of the hemorrhage. T2-weighted MRI differentiates the acute hemorrhage from surrounding edema (D). Gradient ECHO MRI, both acutely (E) and at 3 mo (F), demonstrates the presence of blood product.
Abusive (nonaccidental) head trauma with intracranial bleeding in children may present as primary subdural or parenchymal hemorrhage with no apparent history of trauma. Subtle scalp or ear bruising, retinal hemorrhages in multiple retinal layers, and chronic failure to thrive should always be sought, and in small infants with subdural bleeds, x-rays performed to rule out fractures. Epidural hematoma is nearly always due to trauma, including middle meningeal artery injury typically associated with skull fracture. Subdural hematoma can occur spontaneously in children with brain atrophy due to stretching of bridging veins.
Causes of HS include vascular malformations and systemic disorders (Table 594-6). Arteriovenous malformations (AVM) are the most common cause of childhood subarachnoid and intraparenchymal HS and may occur anywhere in the brain. Risk of AVM bleeding is approximately 2-4% per year throughout life. Other vascular malformations leading to HS include cavernous angiomas (cavernomas), dural arteriovenous fistulas, and vein of Galen malformations. Cerebral aneurysms are an uncommon cause of subarachnoid hemorrhage in children and may suggest an underlying disorder (e.g., polycystic kidney disease, infective endocarditis). A common cause for HS is bleeding from a pre-existing brain tumor. Arterial diseases that usually cause ischemic stroke can also predispose to HS including the central nervous system (CNS) vasculitides and moyamoya disease. Additional causes of parenchymal HS include hypertensive hemorrhage and hematologic disorders such as thrombocytopenic purpura, hemophilia, acquired coagulopathies (e.g., disseminated intravascular coagulation, liver failure), anticoagulant therapy (e.g., warfarin), or illicit drug use. Ischemic infarcts may undergo hemorrhagic transformation, particularly in CSVT, and can be difficult to differentiate from primary HS.
Table 594-6 POTENTIAL RISK FACTORS FOR HEMORRHAGIC STROKE IN CHILDREN
| MAJOR CATEGORIES | EXAMPLES |
|---|---|
| Vascular disorder | |
| Blood disorder | |
| Trauma |
Management of childhood HS may include emergent neurosurgical intervention for large or rapidly expanding lesions. The same principles of neuroprotection for vulnerable brain suggested in the ischemic stroke sections also apply to HS. Reversal of anticoagulant therapy may be required (e.g., vitamin K, fresh frozen plasma) but the role of other medical interventions such as factor VII are unstudied in children. Recurrence risk for those with structural lesions is significant and serial imaging may be required. Outcomes from childhood HS are not well studied but likely depend on lesion size, location, and etiology. Compared with ischemic stroke, death is more frequent in HS, however greater degrees of recovery from the initial deficit can be expected.
Neonatal hemorrhagic strokes are different. Cranial ultrasound can detect most significant neonatal bleeds. In the preterm infant, germinal matrix bleeding and intraventricular hemorrhage are common (Chapter 93.3). Subarachnoid and subdural blood may be imaged in up to 25% of normal term newborns. Term HS is poorly studied and includes the etiologies listed earlier, but may be idiopathic in >50% of cases. Term intraventricular bleeding is often secondary to either deep CSVT or choroid plexus angiomas.
Al-Shahi Salman R, Labovitz DL, Stapf C. Spontaneous intracerebral haemorrhage. BMJ. 2009;339:284-289.
Gomis P, Graftieau P, Sercombe R, et al. Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2010;112:681-688.
Gupta SN, Kechli AM, Kanamalla US. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr Neurol. 2009;40:1-12.
Lo WD, Lee J, Rusin J, et al. Intracranial hemorrhage in children: an evolving spectrum. Arch Neurol. 2008;65:1629-1633.
Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25:416-421.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632-1642.
Runchey S, McGee S. Does this patient have a hemorrhagic stroke? JAMA. 2010;313:2280-2286.
van Gijn J, Kerr RS, Rinkel GJE. Subarachnoid haemorrhage. Lancet. 2007;369:306-318.
594.4 Differential Diagnosis of Strokelike Events
Adam Kirton and Gabrielle deVeber
The diagnosis of stroke in childhood requires a high index of suspicion, balanced with awareness of the differential diagnosis. Acute onset of a focal neurologic deficit or in neonates, seizures, should be considered stroke until proven otherwise and assessed with neuroimaging. Pediatric stroke must be differentiated from other strokelike disorders (Table 594-7) that may require their own urgent specific treatment.
Table 594-7 DIFFERENTIAL DIAGNOSIS OF STROKELIKE EPISODES IN CHILDREN
| DISORDER | CLINICAL DISTINCTION FROM STROKE | IMAGING DISTINCTION FROM STROKE |
|---|---|---|
| Migraine | Evolving or “marching” symptoms, short duration, complete resolution, headache, personal or family history of migraine | Typically normal Migrainous infarction is rare |
| Seizure | Positive symptoms, Todd paralysis is postseizure and limited | Normal or may identify source of seizures (e.g., malformation, old injury) |
| Infection | Fever, encephalopathy, gradual onset, meningismus | Normal or signs of encephalitis/cerebritis, which are typically diffuse and bilateral AIS and CSVT can occur in bacterial meningitis |
| Demyelination | Gradual onset, multifocal symptoms, encephalopathy Accompanying optic neuritis or transverse myelitis | Multifocal lesions, typical appearance (e.g., patchy in ADEM, ovoid in MS), typical locations (e.g., pericallosal in MS), less likely to show restricted diffusion |
| Hypoglycemia | Risk factor (e.g., insulin therapy), related to meals, additional systemic symptoms | Bilateral, symmetric May see restricted diffusion Posterior dominant pattern |
| Watershed infarction due to global HIE | Risk factor (e.g., hypotension, sepsis, heart disease), bilateral deficits | Bilateral, symmetric restricted diffusion in border zones between major arteries (watersheds) |
| Hypertensive encephalopathy (PRES) | Documented hypertension, bilateral visual symptoms, encephalopathy | Posterior dominant, bilateral, patchy lesions involving gray and white matter; usually no restricted diffusion |
| Inborn errors of metabolism | Pre-existing delays/regression, multisystem disease, abnormal biochemical profiles | May have restricted diffusion lesions but bilateral, symmetrical, not within vascular territories MR spectroscopy changes (e.g., high lactate in MELAS) |
| Vestibulopathy | Symptoms limited to vertigo, imbalance (i.e., no weakness) Gradual onset | Normal |
| Acute cerebellar ataxia | Sudden onset bilaterally symmetric ataxia postviral | Normal |
| Channelopathy | Syndromic cluster of symptoms not localizing to single lesion Gradual onset, progressive evolution |
Normal |
| Alternating hemiplegia | History contralateral events Choreoathetosis/dystonia |
Normal |
ADEM, acute disseminated encephalomyelitis; AIS, arterial ischemic stroke; CSVT, cerebral sinovenous thrombosis; HIE, hypoxic-ischemic encephalopathy; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes; MR, magnetic resonance; MS, multiple sclerosis; PRES, posterior reversible leukoencephalopathy syndrome.
Migraine
Careful history and examination can often suggest migraine as the cause of acute focal deficits. Migraine auras should last between 5 and 60 min and resolve completely. The neurologic deficit representing the aura of migraine typically evolves slowly compared with stroke, with sensory disturbance or weakness “marching” from distal to proximal limb over minutes. Evolution into a migrainous headache is expected, however headache may also accompany acute infarction. A group of uncommon migraine subtypes can more closely mimic stroke in children, including familial hemiplegic migraine, basilar migraine, and migraine aura without headache. Migraine can rarely cause a stroke, referred to as migrainous infarction.
Seizure
Prolonged focal seizure activity is frequently followed by a period of focal neurologic deficit (Todd paralysis) which typically resolves within an hour. Very rarely, focal seizures can manifest with only negative symptoms producing acute onset, focal neurologic deficits. A history of jerks or tonic posturing at onset, a known past history of seizures, and EEG findings may be helpful. Imaging is required in all new cases of seizure with persisting Todd paralysis because stroke in children frequently is associated with seizures at onset.
Infection
Life-threatening and treatable brain infections can be mistaken for stroke including bacterial meningitis and herpes encephalitis. However, symptom onset in primary CNS infection is typically more gradual and less focal with fever as a consistent feature. Children with bacterial meningitis are at risk for both venous and arterial stroke.
Demyelination
Acute disseminated encephalomyelitis (ADEM), clinically isolated syndrome (CIS), multiple sclerosis, and other demyelinating conditions can present with acute focal neurological deficits. Symptom onset and initial progression is more gradual (over hours or days) compared with stroke onset (over minutes). Multifocal deficits, or concurrent encephalopathy in the case of ADEM, would decrease the probability of stroke.
Hypoglycemia
Acute drops in blood glucose can produce focal deficits mimicking stroke. New onset hypoglycemia in otherwise healthy children is rare, but predisposing conditions include insulin-dependent diabetes, adrenal insufficiency and steroid withdrawal, hypopituitarism, and ketogenic diet.
Global Hypoxic-Ischemic Encephalopathy (HIE)
Generalized decreases in cerebral perfusion can produce focal areas of watershed brain infarction which can mimic stroke. Watershed ischemic injury should be accompanied by recognized hypotension or conditions predisposing to low cerebral perfusion such as sepsis, dehydration, or cardiac dysfunction. Clinical presentations would involve more generalized and bilateral cerebral dysfunction compared to stroke and the anatomic location of the infarct is in typical bilateral watershed zones rather than a single arterial territory.
Hypertensive Encephalopathy (PRES)
Posterior reversible leukoencephalopathy syndrome (PRES) is seen in children with hypertension, often in the context of an acute rise in blood pressure. Posterior regions are selectively involved, typically resulting in symptoms of bilateral cortical visual dysfunction in addition to encephalopathy and seizures.
Inborn Errors of Metabolism
Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS; Chapter 591.2) is the classic example, though other mitochondrial disease can mimic stroke. Features favoring MELAS would include a history of developmental regression, posterior (and often bilateral) lesions not respecting vascular territories on MRI, and elevated serum or cerebrospinal fluid lactate (MR spectroscopy). In contrast to these types of “metabolic infarction,” children with Fabry disease and homocystinuria are at risk of true ischemic stroke.
Vestibulopathy/Ataxia
Acute onset vertigo and/or ataxia can be confused with brainstem or cerebellar stroke. Simple bedside tests of vestibular function with otherwise intact brainstem functions are reassuring. This differential diagnosis includes acute vestibular neuronopathy, viral labyrinthitis, and the benign paroxysmal vertigos as well as acute cerebellar ataxia and episodic ataxias.
Channelopathies
An increasing number of nervous system ion channel mutations are described that feature sudden focal neurologic deficits, thereby mimicking stroke. These include the migraine syndromes mentioned earlier as well as a growing list of episodic ataxias. A strong family history raises suspicion but most require additional investigation.
Alternating Hemiplegia of Childhood (AHC)
AHC typically presents in infancy with acute intermittent episodes of hemiplegia lasting hours (range minute to weeks) that alternate from 1 side of the body to the other. The hemiplegia persists for minutes to weeks and then resolves spontaneously. Choreoathetosis and dystonic movements are commonly observed in the hemiparetic extremity. Signs spontaneously regress with sleep but recur with awakening. Neuroimaging including MR angiography should be completed to exclude moyamoya disease. AHC has been linked to mutations in certain ion channels.