Chapter 705 Animal and Human Bites
Besides dogs and cats, many animals inflict bites, including large cats (tigers, lions, leopards) wild dogs, hyenas, wolves, crocodiles, and other reptiles. The profile of bites varies by country and region. Among an estimated 3-6 million animal bites per year in the USA, approximately 80-90% are from dogs, 5-15% from cats, 2-5% from rodents, and the remainder from rabbits, ferrets, farm animals, monkeys, and reptiles. Approximately 1% of dog bite wounds and 6% of cat bite wounds require hospitalization, with an annual cost of $100 million in health care expenses and lost income. Bites from dogs are also most common in Bangladesh, India, Pakistan, and Myanmar, whereas in Nepal, cattle and buffalo account for more than half of bites, followed by dogs, pigs, and horses.
Epidemiology
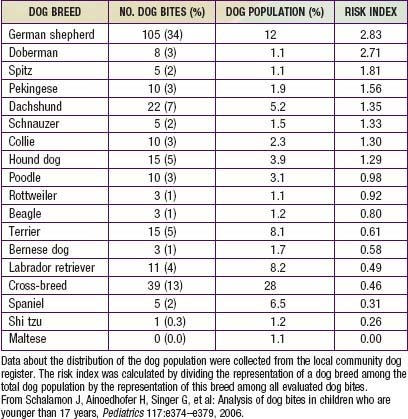
During the past 3 decades, there have been approximately 20 deaths per year in the USA from dog-inflicted injuries; 65% of these occurred in children younger than 11 yr. The breed of dog involved in attacks on children varies; Table 705-1 depicts the risk index by breed from one study of 341 dog bites. Rottweilers, pit bulls, and German shepherds accounted for more than 50% of all fatal bite-related injuries. Unaltered male dogs account for approximately 75% of attacks; nursing dams often inflict injury to humans when children attempt to handle one of their puppies.
The majority of dog-related attacks occur in children between the ages of 6 and 11 yr. Boys are attacked more often than girls (1.5 : 1). Approximately 65% of the attacks occur around the home, 75% of the biting animals are known by the children, and almost 50% of the attacks are said to be unprovoked. Similar statistics apply in Canada, where 70% of all bites reported in one study were sustained by children between 2 and 14 yr; 65% of the dogs involved in the biting were part of the family or extended family and occurred in someone’s home.
Of the approximately 450,000 reported cat bites per year occurring in the USA, nearly all are inflicted by known household animals. Because rat bites and gerbil bites are not reportable conditions, little is known about the epidemiology of these injuries or the incidence of infection after rodent-inflicted bites or scratches.
Few data exist on the incidence and demographics of human bite injuries in pediatric patients; however, preschool and early school-aged children appear to be at greatest risk of sustaining an injury from a bite by a human. Human bites are a common cause of injury in daycare centers in the USA, although in some series the proportion of human bites is highest among adolescents. In adolescents, fist-to-mouth (tooth) injuries are associated with fights.
Clinical Manifestations
Dog bite–related injuries can be divided into three, almost equal categories: abrasions, puncture wounds, and lacerations with or without an associated avulsion of tissue. Dog bites may be crush injuries. The most common type of injury from cat and rat bites is a puncture wound. Cat bites often penetrate to deep tissue. Human bite injuries are of two types: an occlusion injury that is incurred when the upper and lower teeth come together on a body part and, in older children and young adults, a clenched-fist injury that occurs when the injured fist, usually on the dominant hand, comes in contact with the tooth of another individual.
Diagnosis
Management of the bite victim should begin with a thorough history and physical examination. Careful attention should be paid to the circumstances surrounding the bite (e.g., type of animal [domestic or sylvatic], whether the attack was provoked or unprovoked, location of the attack); a history of drug allergies; and the immunization status of the child (tetanus) and animal (rabies). During physical examination, meticulous attention should be paid to the type, size, and depth of the injury; the presence of foreign material in the wound; the status of underlying structures; and, when the bite is on an extremity, the range of motion of the affected area. A diagram of the injury should be recorded in the patient’s medical record. A radiograph of the affected part should be obtained if there is likelihood that a bone or joint could have been penetrated or fractured or if foreign material is present. The possibility of a fracture or penetrating injury of the skull should be considered in individuals, particularly infants, who have sustained dog bite injuries to the face or head.
Complications
Infection is the most common complication of bite injuries, regardless of the species of biting animal. The decision to obtain material for culture from a wound depends on the species of the biting animal, the length of time that has elapsed since the injury, the depth of the wound, the presence of foreign material contaminating the wound, and whether there is evidence of infection. Although potentially pathogenic bacteria have been isolated from up to 80% of dog bite wounds that are brought to medical attention within 8 hr after the bite, the infection rate for wounds receiving medical attention in <8 hr is small (2.5-20%). Thus, unless they are deep and extensive, dog bite wounds that are less than 8 hr old do not need to be cultured unless there is evidence of contamination or early signs of infection or the patient is immunocompromised. Species of Capnocytophaga canimorsus, uncommon pathogens in bite-inflicted injuries, have been isolated from nearly 5% of infected wounds in immunocompromised patients. The infection rate in cat bite wounds that receive early medical attention is at least 50%; therefore, it is prudent to obtain material for culture from all but the most trivial cat-inflicted wounds and from all other animal bite wounds that are not brought to medical attention within 8 h, regardless of species of the biting animal.
The rate of infection after rodent bite injuries is not known. Most of the oral flora of rats is similar to that of other mammals; however, approximately 50% and 25% of rats harbor strains of Streptobacillus moniliformis and Spirillum minus, respectively, in their oral flora. Each of these agents has the potential to cause infection (Chapter 705.1).
All human bite wounds, regardless of the mechanism of injury, should be regarded as carrying high risk for infection and should be cultured. Because of the high incidence of anaerobic infection after bite wounds, it is important to obtain material for anaerobic as well as aerobic cultures.
Common causes of soft tissue bacterial infections after dog, cat, or human bites are noted in Table 705-2. High risk for infection after a bite is associated with wounds in the hand, foot, or genitals, penetration of bone or tendons, human or cat bites, delay in treatment longer than 24 hr, presence of foreign material, immunosuppression (asplenia), and crush or deep puncture wounds.
Table 705-2 MICROORGANISMS ASSOCIATED WITH BITES
DOG BITES
CAT BITES
HERBIVORE BITES
SWINE BITES
RODENT BITES—RAT BITE FEVER
PRIMATE BITES
LARGE REPTILE (CROCODILE, ALLIGATOR) BITES
Adapted from Perkins Garth A, Harris NS: Animal bites (website). http://emedicine.medscape.com/article/768875-overview. Accessed December 3, 2010. Reprinted with permission from eMedicine.com, 2009.
Treatment (Table 705-3)
After the appropriate material has been obtained for culture, the wound should be anesthetized, cleaned, and vigorously irrigated with copious amounts of normal saline. Irrigation with antibiotic-containing solutions provides no advantage over irrigation with saline alone and may cause local irritation of the tissues. Puncture wounds should be thoroughly cleansed and gently irrigated with a catheter or blunt-tipped needle; high-pressure irrigation should not be employed. Avulsed or devitalized tissue should be debrided and any fluctuant areas incised and drained.
Table 705-3 PROPHYLACTIC MANAGEMENT OF HUMAN OR ANIMAL BITE WOUNDS TO PREVENT INFECTION
| CATEGORY OF MANAGEMENT | MANAGEMENT |
|---|---|
| Cleansing | Sponge away visible dirt. Irrigate with a copious volume of sterile saline solution by high-pressure syringe irrigation.* |
| Do not irrigate puncture wounds. Standard precautions should be used. | |
| Wound culture | No for fresh wounds, unless signs of infection exist. |
| Yes for wounds more than 8-12 hr old and wounds that appear infected.† | |
| Radiographs | Indicated for penetrating injuries overlying bones or joints, for suspected fracture, or to assess foreign body inoculation. |
| Debridement | Remove devitalized tissue. |
| Operative debridement and exploration | Yes for one of the following conditions: |
| Wound closure | Yes for selected fresh, nonpuncture bite wounds (see text) |
| Assess tetanus immunization status | Yes |
| Assess risk of rabies from animal bites | Yes |
| Assess risk of hepatitis B virus infection from human bites | Yes |
| Assess risk of human immunodeficiency virus from human bites | Yes |
| Initiate antimicrobial therapy | Yes for any of the following: |
| Follow-up | Inspect wound for signs of infection within 48 hr |
* Use of 18-gauge needle with a large-volume syringe is effective. Antimicrobial or anti-infective solutions offer no advantage and may increase tissue irritation.
† Both aerobic and anaerobic bacterial culture should be performed.
Modified from American Academy of Pediatrics. Bite wounds. In Pickering LK, Baker CJ, Long SS, et al, editors: Red book: 2006 report of the committee on infectious disease, ed 27, EIk Grove Village, IL, 2006, AAP, pp 191–195.
There is much controversy about, and few data exist to determine, whether bite wounds should undergo primary closure or delayed primary closure (3-5 days) or should be allowed to heal by secondary intention. Factors to be considered are the type, size, and depth of the wound; the anatomic location; the presence of infection; the time since the injury; and the potential for cosmetic disfigurement. Surgical consultation should be obtained for all patients with deep or extensive wounds; wounds involving the face or bones and joints; and infected wounds that require open drainage. Although there is general agreement that infected wounds and those that are older than 24 hr should not be sutured, there are disagreement about and varying clinical experience with the efficacy and safety of closing wounds that are less than 8 hr old with no evidence of infection. Because all hand wounds are at high risk for infection, particularly if there has been disruption of the tendons or penetration of the bones, delayed primary closure is recommended for all but the most trivial bite wounds of the hands. Facial lacerations are at smaller risk for secondary infection because of the more luxuriant blood supply to this region. Many plastic surgeons advocate primary closure of facial bite wounds that have been brought to medical attention within 6 hr and have been thoroughly irrigated and debrided.
Few studies unequivocally demonstrate the efficacy of antimicrobial agents for prophylaxis of bite injuries. There is general consensus that antibiotics should be administered to all victims of human bites and all but the most trivial of dog, cat, and rat bite injuries, regardless of whether there is evidence of infection. The bacteriology of bite wound infections is primarily a reflection of the oral flora of the biting animal and, to a lesser extent, of the skin flora of the victim (see Table 705-2). Because each of the multitudes of aerobic and anaerobic bacterial species that colonize the oral cavity of the biting animal has the potential to invade local tissue, multiply, and cause tissue destruction, most bite wound infections are polymicrobial. Evidence suggests that as many as five different species may be isolated from infected dog bite wounds.
Despite the large degree of homology in the bacterial flora of the oral cavity among humans, dogs, and cats, important differences exist between the biting species, and they are reflected in the type of wound infections that occur. The predominant bacterial species isolated from infected dog bite wounds are Staphylococcus aureus (20-30%), Pasteurella multocida (20-30%), Staphylococcus intermedius (25%), and C. canimorsus; approximately one half of dog bite wound infections contain mixed anaerobes. Similar species are isolated from infected cat bite wounds; however, P. multocida is the predominant species in at least 50% of cat bite wound infections. At least 50% of rats harbor strains of Streptobacillus moniliformis in the oropharynx, and approximately 25% harbor Spirillum minor, an aerobic gram-negative organism. In human bite wounds, nontypable strains of Haemophilus influenzae, Eikenella corrodens, S. aureus, α-hemolytic streptococci, and β-lactamase–producing aerobes (about 50%) are the predominant species. Clenched-fist injuries are particularly prone to infection by Eikenella spp. (25%) and anaerobic bacteria (50%).
The choice between oral and parenteral antimicrobial agents should be based on the severity of the wound, the presence and degree of overt infection, signs of systemic toxicity, and the patient’s immune status. Amoxicillin-clavulanate is an excellent choice for empirical oral therapy for human and animal bite wounds because of its activity against most of the strains of bacteria that have been isolated from infected bite injuries. Similarly, ticarcillin-clavulanate or ampicillin and sulbactam are preferred for patients who require empirical parenteral therapy. Procaine penicillin remains the drug of choice for prophylaxis and treatment of rat-inflicted injuries. First-generation cephalosporins have limited activity against P. multocida and E. corrodens and, therefore, should not be used for prophylaxis or empirical initial therapy of bite wound infections. The therapeutic alternatives for penicillin-allergic patients are limited, because the traditional alternative agents are generally inactive against one or more of the multiple pathogens that cause bite wound infections. Although erythromycin is commonly recommended as an alternative agent for penicillin-allergic patients who have suffered dog and cat bites, it has incomplete activity against strains of P. multocida and S. moniliformis and is not effective against E. corrodens. Similarly, clindamycin and the combination trimethoprim-sulfamethoxazole have limited activity against strains of P. multocida and anaerobic bacteria, respectively. Azithromycin and the ketolide antibiotics may be considered because they have activity against aerobic and anaerobic bacteria that are present in infected bite wounds. Tetracycline is the drug of choice for penicillin-allergic patients who have sustained rat bite injuries.
Although tetanus occurs only rarely after human or animal bite injuries, it is important to obtain a careful immunization history and to provide tetanus toxoid to all patients who are incompletely immunized or those in whom it has been longer than 10 yr since the last immunization. The need for postexposure rabies vaccine in victims of dog and cat bites depends on whether the biting animal is known to have been vaccinated and, most importantly, on local experience with rabid animals in the community (Chapter 266). In developing countries, bites from dogs, cats, foxes, skunks, and raccoons carry a high risk of rabies. Annually worldwide, animal bites result in more than10 million postexposure treatments. An estimated 55,000 deaths due to rabies occur each year, although this number probably represents an underestimation. Most deaths occur in low-income families in developing countries. The local health department should be consulted for advice in all instances in which the vaccination status of the biting animal is unknown and whether there is known endemic rabies in the community. Postexposure prophylaxis for hepatitis B should be considered in the rare instance in which an individual has sustained a human bite from an individual who is at high risk for hepatitis B (Chapter 350).
All but the most trivial bite wounds of the hand should be immobilized in position of function for 3-5 days, and patients with bite wounds of an extremity should be instructed to keep the affected extremity elevated for 24-36 hr or until the edema has resolved. All bite wound victims should be reevaluated within 24-36 hr after the injury.
Prevention
It is possible to reduce the risk of injury with anticipatory guidance. Parents should be routinely counseled during prenatal visits and routine health maintenance examinations about the risks of having potentially biting pets in the household. All patients should be cautioned against harboring exotic animals for pets. Additionally, parents should be made aware of the proclivity of certain breeds of dogs to inflict serious injuries and the protective instincts of nursing dams. All young children should be closely supervised, particularly when in the presence of animals, and from a very early age should be taught to respect animals and to be aware of their potential to inflict injury (Tables 705-4 and 705-5).
Table 705-4 CODE OF BEHAVIOR WHEN HANDLING A DOG
| DOG CHARACTERISTICSS | RECOMMENDED HUMAN BEHAVIOR |
|---|---|
| Dogs sniff as a means of communication. | Before petting a dog, let it sniff you. |
| Dogs like to chase moving objects. | Do not run past dogs. |
| Dogs run faster than humans. | Do not try to outrun a dog. |
| Screaming may incite predatory behavior. | Remain calm if a dog approaches. |
| The order of precedence needs to be in evidence. | Do not hug or kiss a dog. |
| Direct eye contact may be interpreted as aggression. | Avoid direct eye contact. |
| Dogs tend to attack extremities, face, and neck. | If attacked, stand still (feet together) and protect neck and face with arms and hands. |
| Lying on the ground provokes attacks. | Stand up. If attacked while lying, keep face down and cover the ears with the hands. Do not move. |
| Fighting dogs bite at anything that is near. | Do not try to stop 2 fighting dogs. |
From Schalamon J, Ainoedhofer H, Singer G, et al: Analysis of dog bites in children who are younger than 17 years, Pediatrics 117:e374–e379, 2006.
Table 705-5 MEASURES FOR PREVENTING DOG BITES
From Centers for Disease Control and Prevention: Dog bite-related fatalities—United States, 1995-1996, MMWR Morb Mortal Wkly Rep 46:463–467, 1997.
Reduction of the rate of human bite injuries, particularly in daycare centers and schools, can be achieved by good surveillance of the children and adequate supervisory personnel-to-child ratios.
Bibliography
Benson L, Edwards S, Schiff A, et al: Dog and cat bites to the hand: treatment and cost assessment, J Hand Surg 31:468–473, 2006.
Broder J, Jerrard D, Olshaker J, et al: Low risk of infection in selected human bites treated without antibiotics, Am J Emerg Med 22:10–12, 2004.
Centers for Disease Control and Prevention: Nonfatal dog bite-related injuries treated in hospital emergency departments—United States, 2001, MMWR Morb Mortal Wkly Rep 52:605, 2003.
Chapman S, Righetti J, Sung L: Preventing dog bites in children: randomized controlled trial of an educational intervention, BMJ 320:1512, 2000.
Chen E, Hornig S, Shepherd SM, et al: Primary closure of mammalian bites, Acad Emerg Med 7:157–161, 2000.
Ertl HCJ: Novel vaccines to human rabies, PLoS Negl Trop Dis 39:e515, 2009.
Goel S, Gupta H, Mazta SR: Epidemiological profile of bite cases admitted at a 50 bedded Community Health Centre of Himachal Pradesh, India, Internet J Health 7:1, 2008.
Merchant RC, Fuerch J, Becker BM, et al: Comparison of the epidemiology of human bites evaluated at three US pediatric emergency departments, Pediatr Emerg Care 21:833–838, 2005.
Monroy A, Behar P, Nagy M, et al: Head and neck dog bites in children, Otolaryngol Head Neck Surg 140:354–357, 2009.
Morgan M, Palmer J: Dog bites, BMJ 334:413–417, 2007.
Schalamon J, Ainoedhofer H, Singer G, et al: Analysis of dog bites in children who are younger than 17 years, Pediatrics 117:e374–e379, 2006.
Stefanopoulos PK, Tarantzopoulou AD: Facial bite wounds: management update, Int J Oral Maxillofoc Surg 34:464–472, 2005.
Talan DA, Abrahamian M, Moran GJ, et al: Clinical presentation and bacteriologic analysis of infected human bites in patients presenting to emergency departments, Clin Infect Dis 37:1481–1489, 2003.
Warrell MJ: Emerging aspects of rabies infection: with a special emphasis on children, Curr Opin Infect Dis 21:251–257, 2008.
705.1 Rat Bite Fever
Etiology
Rat bite fever is a generic term that has been applied to at least two distinct clinical syndromes, each caused by a different microbial agent.
Rat bite fever due to Streptobacillus moniliformis is most commonly reported in the USA as well as Brazil, Canada, Mexico, Paraguay, Great Britain, and France; it has been identified elsewhere in Europe and in Australia. S. moniliformis is a gram-negative bacillus that is present in the nasopharyngeal flora of approximately 10-100% of healthy laboratory rats and 50-100% of healthy wild rats. Infection with S. moniliformis most commonly occurs following the bite of a rat; however, infection has also been reported in individuals who have been scratched by rats, in those who have handled dead rats, and in those who have ingested milk contaminated with the bacterium (Haverhill fever). Rat bite fever may also be transmitted by bites from wild mice. Although there have been occasional reports of the disease due to bites from other animals, these reports have not been confirmed.
Rat bite fever caused by Spirillum minus, called sodoku, is most commonly reported in Asia. S. minus is a small spiral, aerobic gram-negative organism. The incubation period of sodoku is longer (14-21 days) than that of the streptobacillary form of disease, and myalgia and arthritis are less common manifestations.
Reports of rat bite fever from Africa are rare, probably reflecting underrecognition rather than absence of the disease.
Clinical Course
The incubation period for the streptobacillary form of rat bite fever is variable, ranging from 3-10 days. The illness is characterized by an abrupt onset of fever up to 41°C (fever occurring in >90% of reported cases), severe throbbing headache, intense myalgia, chills, and vomiting. In virtually all instances, the lesion at the cutaneous inoculation site has healed by the time the systemic systems first appear. Shortly after the onset of the fever, a polymorphic rash occurs in up to 75% of patients. In most patients, the rash consists of blotchy, red maculopapular lesions that often have a petechial component; the distribution of the rash is variable, but it usually is most dense on the extremities (Fig. 705-1). Hemorrhagic vesicles may develop on the hands and feet, and are very tender to palpation (Fig. 705-2).
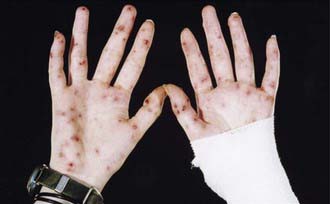
Figure 705-1 Maculopapular rash with small dark red eruptions on hand of person with rat-bite fever.
(From Van Nood E, Peters SH: Rat-bite fever, Neth J Med 63:319–321, 2005.)
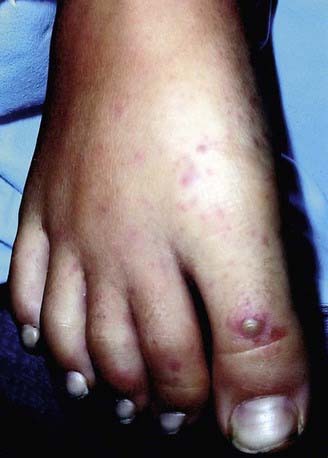
Figure 705-2 Hemorrhagic vesicles on the first and third toes of a patient with advanced rat bite fever.
(From Elliott SP: Rat bite fever and Streptobacillus moniliformis, Clin Microbiol Rev 20:13–22, 2007.)
Approximately 50% of patients have arthritis, which first manifests toward the end of the first week of disease; early on, the arthritis may be migratory. If untreated, the fever, rash and arthritis last from 14-21 days; often, there is a biphasic pattern to the fever and arthritis. A wide range of complications have been reported in patients with rat bite fever, the most common being pneumonia, persistent arthritis, brain and soft tissue abscesses, and, less commonly, myocarditis or endocarditis. The mortality rate of untreated rat bite fever is estimated to be about 13%.
The hallmark of Spirillum-induced disease is fever associated with an indurated, often suppurative, nonhealing lesion at the bite site. Lymphadenitis and lymphadenopathy invariably are present in the regional nodes that drain the inoculation site, and many patients have a generalized macular rash that is most prominent when fever is present. In untreated patients, sodoku has a relapsing course; symptoms abate after 5-7 days of chills and fever but recur 7-10 days later. There may be multiple cycles if the disease is not recognized and treated.
Diagnosis
Diagnosis of the streptobacillary form of rat bite fever is difficult, because the disease is uncommon and is often confused with Rocky Mountain spotted fever (Chapter 220) or, less commonly, meningococcemia (Chapter 184). Furthermore, S. moniliformis is difficult both to isolate and to identify with classic bacteriologic techniques. The organism is fastidious, requires enriched media for growth, and is inhibited by sodium polyanetholsulfonate, an additive present in most commercial blood culture bottles. A definitive diagnosis is made when the organism is recovered from blood or joint fluid or is identified in human samples with molecular technology such as polymerase chain reaction (PCR) analysis, which has been used successfully with humans and laboratory animals.
Diagnosis of sodoku is made on clinical grounds, because there are no diagnostic serologic tests and S. minus has not been cultured on artificial media. Rarely, the organism may be identified in gram-stained smears of pus from the infected inoculation site.
Treatment
Penicillin is the drug of choice for both forms of rat bite fever. Intravenous penicillin G is recommended for 7-10 days, followed by oral penicillin V for an additional 7 days. If the patient has had a prompt response and has improved in 5-7 days without evidence of endocarditis, the transition to oral penicillin can be made at that time. Tetracycline or streptomycin is an effective alternative for penicillin-allergic patients. Patients with endocarditis due to S. moniliformis require high-dose penicillin G and streptomycin or gentamicin.
Dong JP, Olano JW, McBride JW, et al. Emerging pathogens: challenges and successes of molecular diagnostics. J Mol Diagn. 2008;20:185-197.
Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13-22.
Gaastra W, Boot R, Ho HT, et al. Rat bite fever [review]. Vet Microbiol. 2009;133:211-228.
Ojukwu IC, Christy C. Rat-bite fever in children: case report and review. Scand J Infect Dis. 2002;34:474-477.
van Nood E, Peters SH. Rat-bite fever. Neth J Med. 2005;63:319-321.
705.2 Monkeypox
Etiology
Monkeypox virus, causing the disease monkeypox, is the most important member for humans of the genus Orthopoxvirus since the eradication of smallpox, caused by variola virus. The disease was first described in monkeys in a zoo in Denmark more than a half century ago. Monkeys are the predominant host for the virus; however, it appears to be endemic in African squirrels in the rain forest and is present in African rats, mice, domestic pigs, hedgehogs, and opossums. It also has been identified in and transmitted by prairie dogs in the USA and has affected elephants in zoos. The severity varies by strain and by host; it is relatively mild in Cynomolgus monkeys but severe in orangutans.
Monkeypox virus was first observed in humans from West and Central Africa in the 1970s at the time that smallpox had been eradicated from the area. In the 1970s, the secondary attack rate was around 3%, much lower than the 80% seen in unvaccinated smallpox contacts. Few cases were observed over the next two decades; however, during a subsequent outbreak in the 1990s when smallpox titers were no longer present in the population, the secondary attack rate exceeded 75%. Monkeypox outbreaks have also been reported in the Sudan. Monkeypox was accidently introduced into the USA in 2003, presumably through rodents from Ghana that infected prairie dogs who were distributed as pets. More than 70 humans were infected, typically members of the households of the infected prairie dogs and workers in the pet stores or animal hospitals caring for the pets. Primary transmission of the disease from infected animal to human is by bite or by human contact with an infected animal’s blood, wound discharge, or body fluid. Human-to-human transmission of infection is uncommon but is believed to have been an important source for transmission of new cases during the U.S. outbreak.
Clinical Course
The clinical signs, symptoms, and course of monkeypox are similar to those of smallpox, although usually milder. After a 10- to 14-day incubation period during which the virus replicates in lymphoid tissues, humans experience an abrupt onset of malaise, fever, myalgia, headache, and severe backache. A nonproductive cough, nausea, vomiting, and abdominal pain may be present. Generalized lymphadenopathy, a rare finding in smallpox, is invariably present during the acute stages of the illness. After a 2- to 4-day prodrome, an exanthem appears in cephalad-to-caudal progression. As the rash progresses, the high spiking fever begins to abate. The initial rash generally first appears on the face and consists of erythematous macules. Within hours of first appearance, the macules transform into firm papules that rapidly vesiculate and become pustular over 2-3 days. Unlike smallpox lesions, but similar to chickenpox lesions, the lesions of monkeypox tend to occur in crops. Late into the second week of illness, the lesions begin to desiccate, crust, scab, and fall off.
Monkeypox should be suspected in any child who has the characteristic prodrome associated with an atypical form of chickenpox and a history of contact with prairie dogs or exotic mammals such as Gambian rats and rope squirrels. Any one of the following criteria establishes a definitive diagnosis:
Treatment
There is no proven effective therapy for monkeypox. In spite of evidence that preexposure administration of smallpox vaccine is 85% effective in preventing or attenuating the disease, the rarity of the disease does not warrant universal vaccination. In instances of known exposure or in epidemic situations, there may be an indication for administering the vaccine. Consideration should be given to vaccinating close family contacts and health care workers who provide care to infected individuals. Vaccine is said to be preventative if given within 2 wk of exposure. Individuals with a compromised immune system and those who have had life-threatening allergies to latex or to smallpox vaccine or any of its components (polymyxin B, streptomycin, chlortetracycline, neomycin) also should not receive the smallpox vaccine.
Although there are data that indicate that cidofovir has in vitro virocidal activity against monkeypox virus and has also been effective in preventing monkeypox infection in animals, there are no data to support its effectiveness in humans.
Careful attention should be paid to skin hygiene, maintenance of adequate nutrition and hydration, and prompt implementation of local or systemic therapy of secondary bacterial infection that may occur. To prevent human-to-human spread of disease, a combination of the Centers for Disease Control and Prevention guidelines for droplet and airborne infection control should be implemented.
Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182.
Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Med. 2010;140:229-236.
Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280.
Maskalyk J. Monkeypox outbreak among pet owners. CMAJ. 2003;169:44.
Parker S, Handley L, Buller RM. Therapeutic and prophylactic drugs to treat orthopoxvirus infections. Future Virol. 2008;3:595-612.
Sejvar JJ, Chowdary Y, Schomogyi M, et al. Human monkeypox infection: a family cluster in the midwestern United States. J Infect Dis. 2004;190:1833.