Bladder Infections
Uncomplicated Cystitis
Most cases of uncomplicated cystitis occur in women. Each year, approximately 10% of women report having had a UTI and more than 50% of all women have at least one such infection in their lifetime (Foxman et al, 2000). Uncomplicated cystitis occasionally occurs in prepubertal girls, but it increases greatly in incidence in late adolescence and during the second and fourth decades of life. Twenty-five to 30 percent of women 20 to 40 years of age have a history of UTIs (Kunin, 1987). Although it is much less common, young men may also experience acute cystitis without underlying structural or functional abnormalities of the urinary tract (Krieger et al, 1993). Risk factors (Table 10–15) include sexual intercourse and use of spermicides (Hooton et al, 1996; Foxman, 2002; Handley et al, 2002). Sexual transmission of uropathogens has been suggested by demonstrating identical E. coli in the bowel and urinary flora of sex partners (Johnson and Stamm, 1989).
Table 10–15 Risk Factors for UTIs
| Reduced Urine Flow |
| Promote Colonization |
| Facilitate Ascent |
Clinical Presentation
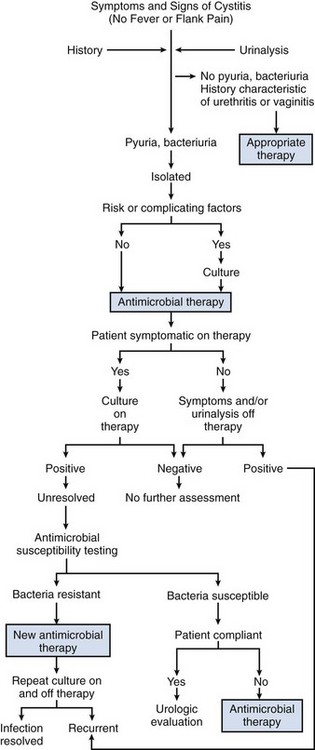
The presenting symptoms of cystitis are variable but usually include dysuria, frequency, and/or urgency (Fig. 10–13). Suprapubic pain, hematuria, or foul-smelling urine may develop. The probability of cystitis in a woman with these symptoms alone or in combination is 50% to 90%, respectively (Bent et al, 2002). When a woman who previously has had cystitis has symptoms suggesting a recurrence, the probability that an infection is present is about 90% (Gupta et al, 2001). Because acute cystitis, by definition, is a superficial infection of bladder mucosa, fever, chills, and other signs of dissemination are not present. Some patients may experience suprapubic tenderness, but most have no diagnostic physical findings. In women, physical examination should include the possibility of vaginitis, herpes, and urethral pathology, such as a diverticulum.
A remarkably narrow spectrum of etiologic agents with highly predictable profiles of antimicrobial susceptibility cause infections in young women with acute uncomplicated cystitis. E. coli is the causative organism in 75% to 90% of cases of acute cystitis in young women (Latham et al, 1983; Ronald, 2002). S. saprophyticus, a commensal organism of the skin, is the second most common cause of acute cystitis in young women, accounting for 10% to 20% of these infections (Jordan et al, 1980). Other organisms less commonly involved include Klebsiella and Proteus species and Enterococcus. In men, E. coli and other Enterobacteriaceae are the most commonly identified organisms.
Laboratory Diagnosis
The presumptive laboratory diagnosis of acute cystitis is based on microscopic urinalysis, which indicates microscopic pyuria, bacteriuria, and hematuria. Indirect dipstick tests for bacteria (nitrite) or pyuria (leukocyte esterase) may also be informative and more convenient but are less sensitive than microscopic examination of the urine. Dipsticks are most accurate when the presence of either nitrite or leukocyte esterase is considered a positive result. Urine culture remains the definitive test; and in symptomatic patients the presence of 102 cfu/mL or more of urine usually indicates infection (Stamm et al, 1982b).
However, routine urine cultures are often not necessary. It is generally more cost-effective to manage many patients who have symptoms and urinalysis findings characteristic of uncomplicated cystitis without an initial urine culture because treatment decisions are usually made and therapy is often completed before culture results are known (Komaroff, 1986). This position was supported by a cost-effectiveness study (Carlson and Mulley, 1985) in which it was estimated that the routine use of pretherapeutic urine cultures for lower UTI increases costs by 40% but decreases the overall duration of symptoms by only 10%.
Thus in women with recent onset of symptoms and signs suggesting acute cystitis and in whom factors associated with upper tract or complicated infection are absent, a urinalysis that is positive for pyuria, bacteriuria, or hematuria, or a combination, should provide sufficient documentation of UTI and a urine culture may be omitted (McIsaac et al, 2002). A urine culture should be obtained for patients in whom symptoms and urine examination findings leave the diagnosis of cystitis in doubt. Pretherapeutic cultures and susceptibility tests are also essential in the management of patients with recent antimicrobial therapy or UTI. In these situations, various pathogens may be present and antimicrobial therapy is less predictable and must be tailored to the individual organism (Stamm, 1986).
Differential Diagnosis
Cystitis must be differentiated from other inflammatory infectious conditions in which dysuria may be the most prominent symptom, including vaginitis, urethral infections caused by sexually transmitted pathogens, and miscellaneous noninflammatory causes of urethral discomfort (Komaroff, 1984). Characteristic features of the history, physical examination, and voided urine or other specimens allow patients with dysuria to be assigned to one of these diagnostic categories. Vaginitis is characterized by irritative voiding associated with vaginal irritation and is subacute in onset. A history of vaginal discharge or odor and multiple or new sexual partners is common. Frequency, urgency, hematuria, and suprapubic pain are not present. Physical examination reveals a vaginal discharge, and examination of vaginal fluid demonstrates inflammatory cells. Differential diagnosis includes herpes simplex virus infection, gonorrhea, infection with Chlamydia, trichomoniasis, yeast infection, and bacterial vaginosis. Urethritis causes dysuria that is usually subacute in onset and is associated with a history of discharge and new or multiple sexual partners. Frequency and urgency of urination may be present but are less pronounced than in patients with cystitis, and fever and chills are absent. Urethral discharge with inflammatory cells or initial pyuria in the male is characteristic. The common causes of urethritis include Neisseria gonorrhoeae, Chlamydia, herpes simplex virus, and trichomoniasis. Appropriate cultures and immunologic tests are indicated. Urethral injury associated with sexual intercourse, chemical irritants, or allergy may also cause dysuria. A history of trauma or exposure to irritants and a lack of discharge or pyuria are characteristic.
Management
Antimicrobial Selection
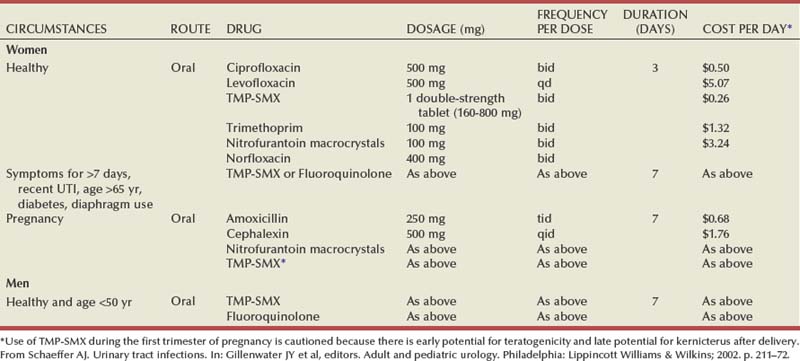
Oral antimicrobial agents for treatment of acute uncomplicated cystitis are listed in Table 10–16. TMP and TMP-SMX are effective and inexpensive agents for empirical therapy, resulting in bacteriologic cure (i.e., eradication of the pathogen from the urine) within 7 days after the start of treatment in approximately 94% of women (Warren et al, 1999). They are recommended in areas where the prevalence of resistance to these drugs among E. coli strains causing cystitis is less than 20% (Warren et al, 1999). The probability of resistant strains can be predicted in part from the history of recent antimicrobial usage. Women who have taken TMP-SMX recently are approximately 16 times as likely to be infected with an isolate resistant to this agent compared with women who have not taken the antimicrobial agent recently. In addition, those who have taken any other antimicrobial agent are more than twice as likely to be infected with a resistant isolate (Brown et al, 2002). With a 30% rate of resistance to TMP-SMX, the bacteriologic eradication rate is predicted to be 80% and the clinical cure rate is predicted to be 85% (Gupta et al, 2001). When used alone, TMP is as efficacious as TMP-SMX and is associated with fewer side effects, presumably because of the absence of the sulfa component (Harbord and Gruneberg, 1981). It can be prescribed to patients who are allergic to sulfa. However, TMP can cause hypersensitivity and rashes that may be erroneously attributed to sulfa (Alonso et al, 1992).
Nitrofurantoin has maintained an excellent level of activity over 4 decades and is well tolerated, but it is more expensive than TMP-SMX and it is considerably less active against aerobic gram-negative rods other than E. coli. Furthermore, it is usually prescribed for 7 days and may cause gastrointestinal upset. It is not associated with plasmid-mediated resistance, however, so it is an excellent choice for patients with recent exposure to most other antimicrobial agents. The high in-vitro resistance to ampicillin and sulfonamide and the high cost of amoxicillin/clavulanate and the cephalosporins limit their usefulness.
The fluoroquinolones offer excellent activity and are well tolerated. Resistance to the fluoroquinolones remains below 5% in most places (Fihn et al, 1988); however, it is increasing in certain areas. Twice-daily and once-daily extended-release fluoroquinolones are equally effective (Henry et al, 2002). Their use for uncomplicated cystitis should be limited to patients with allergy to TMP-SMX, to patients with previous exposure to antimicrobial agents causing bacterial resistance, and to areas where the prevalence of resistance to TMP or TMP-SMX is 20% or greater (Warren et al, 1999; Hooton et al, 2004).
The effects of an antimicrobial agent on the vaginal flora are also important in recurrence of bacteriuria (Fihn et al, 1988). The concentrations of TMP and the fluoroquinolones that have been studied in vaginal secretions are high, eradicating E. coli but minimally altering normal anaerobic and microaerophilic vaginal flora (Hooton and Stamm, 1991). Single-dose regimens using these drugs are less effective than multiple-day regimens in this regard (Fihn et al, 1988), which probably explains why there are more early recurrent infections after single-dose therapy with these drugs. Nitrofurantoin and β-lactam drugs are generally not effective in eliminating E. coli from the vagina.
Duration of Therapy
Three-day therapy is the preferred regimen for uncomplicated cystitis in women (Norrby, 1990; Warren et al, 1999). In an excellent review of more than 300 separate clinical trials of single-dose, 3-day, or 7-day treatment with TMP, TMP-SMX, fluoroquinolones, and β-lactam antimicrobial therapies, it was concluded that, irrespective of the antimicrobial used, 3-day therapy is more effective than single-dose therapy. Three-day therapy with TMP-SMX, TMP, amoxicillin, or cloxacillin has been associated with cure rates similar to longer courses of therapy and an incidence of adverse effects about as low as that seen with single-dose therapy and lower than seen with longer courses of therapy (Charlton et al, 1976; Kunin, 1985; McCue, 1986; Warren et al, 1999). Because 7-day therapy often causes more adverse effects, it is recommended only for women with symptoms of 1 week or more, men, and individuals with possible complicating factors. Other options include nitrofurantoin, perhaps as 7-day therapy, and fosfomycin single-dose therapy; each of these requires further study. β-Lactams as a group are less effective in treatment of cystitis than TMP, TMP-SMX, and the fluoroquinolones.
Seven-day therapy is the preferred regimen in uncomplicated cystitis in men.
Cost of Therapy
The cost of treating a UTI involves not only the initial evaluation and cost of the drug but also what occurs subsequently. The most important prediction of high cost-effectiveness is high efficacy against the most common urinary pathogen, E. coli. The lower the effectiveness against this bacterium, the greater the number of revisits, cases of progression to pyelonephritis, and follow-up costs. Antimicrobial cost is a poor prediction of cost-effectiveness, as illustrated by the finding that the most expensive and least expensive drugs, the fluoroquinolones and TMP-SMX, are approximately equally cost-effective (Rosenberg, 1999). Both of these drugs are more cost-effective than nitrofurantoin and amoxicillin.
Follow-Up
Approximately 90% of women are asymptomatic within 72 hours after initiating antimicrobial therapy (Fihn et al, 1988). Follow-up visit or culture is not required in young women who are asymptomatic after therapy. A follow-up visit, urinalysis, and urine culture are recommended in older women or those with potential risk factors and in men. Urologic evaluation is unnecessary in women and is usually unnecessary in young men who respond to therapy (Lipsky, 1989; Abarbanel et al, 2003). However, UTIs in most men should be considered complicated until proven otherwise. Andrews and associates (2002) showed that approximately 50% of men with UTIs have a significant abnormality. Furthermore, if a patient does not respond to therapy, appropriate microbiologic urologic evaluations should be undertaken for the causes of unresolved and complicated UTIs.
Asymptomatic Bacteriuria
Asymptomatic bacteriuria is a microbiologic diagnosis based on the isolation of a specified quantitative count of bacteria in a properly collected specimen of urine from a patient who is without symptoms or signs referable to UTI. In healthy individuals, the absence of symptoms is clear cut, but in, for example, catheterized or neurologically comprised patients it may be difficult to discern whether the UTI is truly asymptomatic. Kass (1962) originally proposed that for asymptomatic women two consecutive voided urine specimens with isolation of the same bacterial strain in quantitative counts of 105 cfu/mL is consistent with asymptomatic bacteriuria. In men, a single clean-catch voided specimen with similar counts is adequate. A single catheterized urine specimen with a solitary isolate with a quantitative count of 102 cfu/mL identifies bacteriuria in women or men (Nicolle et al, 2005). The prevalence of pyuria with asymptomatic bacteriuria ranges from approximately 30% in young women (Hooton et al, 2000) to 100% in catheterized patients. In addition, many coexisting factors, such as stones, can incite inflammation in these patients and therefore the presence or absence of pyuria is not sufficient to diagnose bacteriuria nor does it differentiate symptomatic from asymptomatic patients or provide indication for antimicrobial treatment (Nicolle et al, 2005).
The prevalence of asymptomatic bacteriuria varies widely and depends on the age, sex, and the presence of other genitourinary abnormalities (Table 10–17). E. coli is the most common isolate among patients with bacteriuria, and it contains fewer virulence characteristics than isolates from patients with symptomatic infections (Svanborg and Godaly, 1997). Other Enterobacteriaceae (e.g., P. mirabilis) and gram-positive uropathogens, including group B streptococci and coagulase-negative staphylococci, become more prevalent in concert with increased underlying abnormalities. For patients who are institutionalized and/or with indwelling urologic devices, P. aeruginosa, Proteus, and other highly resistant organisms are more prevalent.
Table 10–17 Prevalence of Asymptomatic Bacteriuria in Selected Populations
| POPULATION | PREVALENCE (%) | REFERENCE |
|---|---|---|
| Healthy, premenopausal women | 1.0-5.0 | Nicolle, 2003 |
| Pregnant women | 1.9-9.5 | Nicolle, 2003 |
| Postmenopausal women aged 50-70 years | 2.8-8.6 | Nicolle, 2003 |
| Diabetic patients | ||
| Women | 9.0-27 | Zhanel, 1991 |
| Men | 0.7-11 | Zhanel, 1991 |
| Elderly persons in the community | ||
| Women | 10.8-16 | Nicolle, 2003 |
| Men | 3.6-19 | Nicolle, 2003 |
| Elderly persons in a long-term care facility | ||
| Women | 25-50 | Nicolle, 1997 |
| Men | 14-50 | Nicolle, 1997 |
| Patients with spinal cord injuries | ||
| Intermittent catheter use | 23-89 | Bakke and Digranes, 1991 |
| Sphincterotomy and condom catheter in place | 57 | Waites, 1993 |
| Patients undergoing hemodialysis | 28 | Chaudhry, 1993 |
| Patients with indwelling catheter use | ||
| Short-term | 9-23 | Stamm, 1991 |
| Long-term | 100 | Warren, 1982 |
From Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643–54.
Management of asymptomatic bacteriuria is determined by the population and their risk for adverse outcome, which can be prevented with antimicrobial treatment of asymptomatic bacteriuria (Nicolle et al, 2005) (Table 10–18). These recommendations are based on the observation that in adult populations asymptomatic bacteriuria has not been shown to be harmful. Furthermore, although persons with bacteriuria are at increased risk of symptomatic urinary tractions, treatment of asymptomatic bacteriuria does not decrease the frequency of symptomatic infections or improve other outcomes. Thus, in populations other than those for whom treatment has been documented to be beneficial (e.g., pregnant women and patients undergoing urologic interventions), screening for or treatment of asymptomatic bacteriuria is not appropriate and should be discouraged (Nicolle et al, 2005).
Table 10–18 Screening for and Treatment of Asymptomatic Bacteriuria
| Premenopausal nonpregnant women | Not recommended |
| Pregnant women | Recommended |
| Diabetic women | Not recommended |
| Older persons residing in the community | Not recommended |
| Elderly institutionalized subjects | Not recommended |
| Subjects with spinal cord injuries | Not recommended |
| Patients with indwelling urethral catheters Note: Antimicrobial treatment of asymptomatic women with catheter-associated bacteriuria that persists 48 hours after catheter removal may be considered. |
Not recommended |
| Urologic interventions | Recommended |
| Immunocompromised patients and transplant patients | Not recommended |
From Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643–54.
Complicated Cystitis
Complicated UTIs are those that occur in a patient with a compromised urinary tract or that are caused by a very resistant pathogen (Table 10–19). These complicating factors may be readily apparent from the severity of the presenting illness or the past medical history. However, they may not be obvious at first and may only become evident from subsequent failure of the patient to respond to appropriate therapy (see discussion on unresolved or recurrent UTIs, later).
Table 10–19 Complicating Host Factors
The clinical spectrum ranges from mild cystitis to life-threatening kidney infections and urosepsis (kidney infections and urosepsis are discussed subsequently). These infections can be caused by a broad range of bacteria with resistance to multiple antimicrobial agents. Therefore urine cultures are mandatory to identify the bacteria and its antimicrobial susceptibility.
Because of the wide range of host conditions and pathogens and a lack of adequate controlled trials, guidelines for empirical therapy are limited. For patients with mild to moderate illness who can be treated as an outpatient with oral therapy, the fluoroquinolones provide a broad spectrum of activity with excellent urine and tissue levels and safety. If the susceptibility pattern of the pathogen is known, TMP-SMX may be effective (Table 10–20).
Table 10–20 Treatment of Complicated UTIs
| COMMON PATHOGENS | MITIGATING CIRCUMSTANCES | RECOMMENDED EMPIRICAL TREATMENT |
|---|---|---|
| E. coli, Proteus species, Klebsiella species, Pseudomonas species, | Mild-to-moderate illness, no nausea or vomiting—outpatient therapy | Oral* norfloxacin, ciprofloxacin, or ofloxacin for 10-14 days |
| Serratia species, enterococci, staphylococci | Severe illness or possible urosepsis— hospitalization required | Parenteral† ampicillin and gentamicin, ciprofloxacin, levofloxacin, ceftriaxone, aztreonam, ticarcillin-clavulanate or imipenem-cilastin until fever gone; then oral* trimethoprim-sulfamethoxazole, norfloxacin, ciprofloxacin, or levofloxacin for 14-21 days |
* Oral regimens for pyelonephritis and complicated UTI: trimethoprim-sulfamethoxazole, 160 to 800 mg q12h; norfloxacin, 400 mg q12h; ciprofloxacin, 500 mg q12h; levofloxacin, 500 mg/day.
† Parenteral regimens: ciprofloxacin, 400 mg q12h; levofloxacin, 500 mg/day; gentamicin, 1 mg/kg q8h; ceftriaxone, 1 to 2 g/day; ampicillin, 1 g q6h; imipenem-cilastin, 250 to 500 mg q6-8h; ticarcillin-clavulanate, 3.1 g q6h; and aztreonam, 1 g q8-12h.
Modified from Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med 1993;329:1328–34.
For patients requiring hospitalization, intravenous antimicrobial agents should be administered based on the susceptibility patterns of the known uropathogens at that institution.
Because therapy will be compromised without addressing the complicating factor(s), every effort should be made to correct any underlying urinary tract abnormalities and treat host factors that exacerbate the infection.
Therapy is usually continued for 10 to 14 days and switched from parenteral to oral therapy when the patient is afebrile and clinically stable. Repeat urine cultures should be performed if the patient fails to respond to therapy.
Unresolved UTIs
Clinical Presentation
Unresolved infection indicates that initial therapy has been inadequate in eliminating symptoms and/or bacterial growth in the urinary tract. If the symptoms of UTI do not resolve by the end of treatment or if symptoms recur shortly after therapy, urinalysis and urine culture with susceptibility testing should be obtained. If the patient’s symptoms are significant, empirical therapy with a fluoroquinolone is appropriate pending results of the culture and susceptibility testing.
The causes of unresolved bacteriuria during antimicrobial therapy are shown in Table 10–21. Most commonly, the bacteria are resistant to the antimicrobial agent selected to treat the infection. Typically, the patient has received the antimicrobial therapy in the recent past and developed bowel colonization with resistant bacteria. β-Lactams, tetracycline, and sulfonamides are notorious for causing plasmid-mediated R factors that simultaneously carry resistance to multiple antimicrobial agents. The second most common cause is development of resistance in a previously susceptible population of bacteria during the course of treatment of UTIs. This problem occurs in approximately 5% of the patients receiving antimicrobial therapy. It is easy to recognize clinically because the culture on therapy shows that the previous susceptible population has been replaced by resistant bacteria of the same species. It can be shown that resistant organisms were actually present before contact with the initial antimicrobial agent, but they were present in such low numbers that it was impossible to detect by in-vitro susceptibility studies before therapy. When the antimicrobial concentration in the urine is insufficient to kill all the bacteria present, the more resistant forms will emerge. This characteristically is seen in patients who are underdosed or who are poorly compliant and hence have inadequate dose regimens. The third cause is the presence of an unsuspected, second pathogen that was present initially and is resistant to the antimicrobial therapy chosen. Treatment of the dominant organism unmasks the presence of the second strain. The fourth cause is rapid reintroduction of a new resistant species while the patient is undergoing initial therapy. Rapid reinfection that mimics unresolved bacteriuria should alert the clinician to the possibility of an enterovesical fistula.
Table 10–21 Causes of Unresolved Bacteriuria, in Descending Order of Importance
|
Rapid reinfection with a new, resistant species during initial therapy for the original susceptible organism
|
If the culture obtained on therapy shows that the initial species is still present and susceptible to the antimicrobial chosen to treat the infection, the unresolved infection must be caused by either inability to deliver an adequate concentration of antimicrobial agents into the urinary tract or an excessive number of bacteria that “override” the antimicrobial activity. In patients with azotemia, a determination of urinary antimicrobial concentrations usually shows that the level of the drug is below the minimal inhibitory concentration of the infecting organism.
In patients with papillary necrosis, severe defects in the medullary concentrating ability dilute the antimicrobial agent. A large mass of bacteria within the urinary tract is most commonly associated with a giant staghorn calculus. Even though adequate urinary levels of bactericidal drugs are present, the concentration is inadequate to sterilize the urine. This occurs because even susceptible bacteria cannot be inhibited once they reach a certain critical density, particularly if attached to a foreign body.
The last cause of unresolved bacteriuria occurs in those patients who have variants of Munchausen syndrome. These patients secretly inoculate their bladders with uropathogens or omit their oral antimicrobial agents while steadfastly asserting that they never miss a dose. The patient with Munchausen syndrome presents with an inconsistent clinical history and invariably a normal urinary tract on urologic imaging. Careful bacteriologic observations usually indicate the implausibility of the clinical picture.
Laboratory Diagnosis
Urinalysis and urine culture are mandatory to determine the cause of unresolved bacteriuria. The first four causes that are associated with resistant bacteria require no further evaluation. However, if reculture shows that the bacteria is sensitive to the antimicrobial agent the patient is taking, renal function and radiologic evaluation should be performed to identify renal or urinary tract abnormalities.
Management
Initial empirical antimicrobial selection should be based on the assumption that the bacteria are resistant. Therefore an antimicrobial agent different from the original agent should be selected. Fluoroquinolones offer excellent coverage in most cases and should be given for 7 days. When the bacterial susceptibilities are available, adjustments can be made if necessary. Urine cultures should be performed during and 7 days after therapy to ensure microbiologic efficacy.
Recurrent UTIs
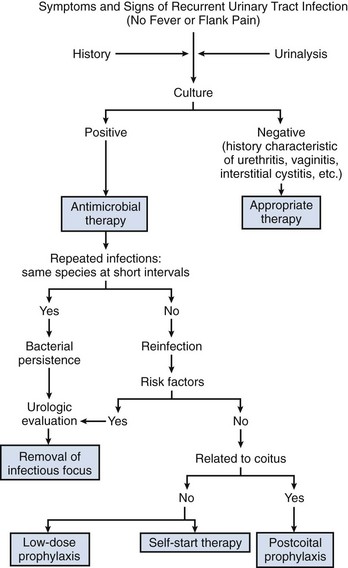
Recurrent UTIs are caused by either reemergence of bacteria from a site within the urinary tract (bacterial persistence) or new infections from bacteria outside the urinary tract (reinfection). Clinical identification of these two types of recurrence is based on the pattern of recurrent infections (Fig. 10–14). Bacterial persistence must be caused by the same organism in each instance, and infections that occur at close intervals are characteristic. Conversely, reinfections usually occur at varying and sometimes long intervals and often are caused by different species. The distinction between bacterial persistence and reinfection is important in management because patients with bacterial persistence can usually be cured of the recurrent infections by identification and surgical removal or correction of the focus of infection. Conversely, women with reinfection usually do not have an alterable urologic abnormality and require long-term medical management. Reinfections in men are uncommon and may be associated with an underlying abnormality, such as urethral stricture; therefore, at a minimum, endoscopic evaluation is indicated.
Bacterial Persistence
Once the bacteriuria has resolved (i.e., the urine shows no growth for several days after the antimicrobial agent has been stopped), recurrence with the same organism can arise from a site within the urinary tract that was excluded from the high urine concentrations of the antimicrobial agent. The 12 correctable urologic abnormalities that cause bacteria to persist within the urinary tract between episodes of recurrent bacteriuria are listed in Table 10–6. The relationship of these abnormalities to bacterial persistence, as well as the documentation that surgical excision removes the infection as a source of recurrent bacteriuria, is presented elsewhere in detail (Stamey, 1980). Once the urologist recognizes that the cause of the patient’s recurrent bacteriuria is bacterial persistence, Table 10–6 should serve as a checklist for known, correctable causes. Some of the causes are subtle, and many require cystoscopic localization of the infection with ureteral catheters to accurately define the focus of bacterial persistence.
Although patients with bacterial persistence are relatively uncommon, their identification is important because they represent the only surgically curable cause of recurrent UTIs. A systematic radiologic and endoscopic evaluation of the urinary tract is mandatory. CT and cystoscopy provide the initial screening. Retrograde urography may be required in selected patients to delineate abnormalities, such as diverticulum or nonrefluxing ureteral stump.
Urea-Splitting Bacteria That Cause Struvite Renal Stones
The infection that ultimately leads to an infection stone commonly begins inconspicuously as inadequately treated cystitis. Most patients with P. mirabilis cystitis do not form struvite stones. But struvite stones form in those patients who have a protracted infection with P. mirabilis, an infection that is often asymptomatic or minimally symptomatic. P. mirabilis causes intense alkalinization of the urine with precipitation of calcium, magnesium, ammonium, and phosphate salts and the subsequent formation of branched struvite renal stones. Bacteriuria in most of these patients with struvite stones recurs almost immediately on stopping antimicrobial therapy, usually within 5 to 7 days. The bacteriologic consequences are substantial because the bacteria persist inside these struvite stones even when the urine shows no growth. Indeed, struvite infection stones, together with the occasional oxalate or apatite stone that becomes secondarily colonized, constitute the major cause of bacterial persistence in women in the absence of azotemia.
Underlying urinary tract abnormalities are not a prerequisite for this type of infection. However, patients with indwelling catheters, urinary diversions, or other urinary tract abnormalities are particularly susceptible to these infections. Urea-splitting organisms, such as P. mirabilis, cause infection stones that are relatively radiolucent. If such a stone is suspected, plain film tomograms or CT scans without contrast medium enhancement should be obtained (Greenberg et al, 1982). Medical management with continued suppressive antimicrobial therapy and acidification temporarily relieves symptoms and retards deterioration of renal function in some patients. Complete removal of the calculus is generally required for bacteriologic cure and to prevent renal damage due to obstruction (Silverman and Stamey, 1983). Percutaneous nephrolithotomy and extracorporeal shockwave lithotripsy are now the preferred treatment for most renal and upper ureteral calculi.
When extracorporeal shockwave lithotripsy is used to fragment infection stones, the patient should be maintained on appropriate antimicrobial therapy until the fragments pass. Occasionally, long-term antimicrobial therapy can result in eradication of bacteriuria even if some fragments persist after lithotripsy, presumably because the shockwaves have rendered the entrapped bacteria more susceptible to antimicrobial therapy (Michaels et al, 1988). If percutaneous or open surgery is used, all the residual particles of struvite stones must be removed at surgery to prevent recurrent bacteriuria from bacterial persistence in the calculus. Rocha and Santos (1969) have shown that soaking these stones in iodine and alcohol for 6 hours will not kill the bacteria within the interior of the stone. The importance of recognizing this fact is twofold: (1) The bacteria cannot be killed by antimicrobial therapy, even though the urine may show no growth for months or even years (Shortliffe et al, 1984), and (2) any fragments left behind at the time of surgical removal leave residual bacteria within the interstices of the stone; these bacteria ensure recurrence of the staghorn calculus with its attendant morbidity.
If fragments remain after surgery, a small, multiholed polyethylene catheter should be left for postoperative irrigation with Renacidin or Suby G solution (Silverman and Stamey, 1983). Follow-up radiographs are essential to ensure that all the stone fragments are removed, and cultures must demonstrate that the urease-splitting bacteria are eradicated.
Most of the other congenital or acquired abnormalities listed in Table 10–6 require surgical removal for eradication of the source of bacterial persistence. Chronic bacterial prostatitis is treated initially with long-term antimicrobial therapy and, in select cases, by radical transurethral resection (Mears, 1978).
In patients in whom the focus of infection cannot be eradicated, long-term, low-dose antimicrobial suppression is necessary to prevent symptoms of infection. The antimicrobial drugs used for low-dose prophylaxis will also be effective for bacterial suppression if the persistent strain is susceptible. These include nitrofurantoin, TMP-SMX, cephalexin, and the fluoroquinolones.
Reinfections
Patients with recurrent infections caused by different species or occurring at long intervals almost invariably have reinfections. These reinfections most often occur in women and girls and are associated with ascending colonization from the bowel flora. Reinfections in men are often associated with a urinary tract abnormality. The possibility of a vesicoenteric or vesicovaginal fistula should be considered when the patient has any history of pneumaturia, fecaluria, diverticulitis, obstipation, previous pelvic surgery, or radiation therapy. Evaluation of the patient with presumed reinfections must be individualized.
Failure to recognize and correct abnormalities that reduce formation, transmission, and elimination of urine by the urinary tract increases the incidence of reinfection in susceptible patients and reduces the effectiveness of antimicrobial therapy. Abnormalities should be corrected and urinary tract function restored by medical, pharmacologic, or surgical management. A thorough urologic evaluation is essential in all men and in women with evidence of upper tract infections (fevers, chills, flank pain, hemorrhagic cystitis, or other risk factors, such as history of unexplained hematuria, obstructive symptoms, neurogenic bladder dysfunction, renal calculi, fistula, analgesic abuse, or severe disease such as diabetes mellitus). In women, diaphragm-spermicide use has been associated with an increased risk of UTI and vaginal colonization with E. coli (Hooton et al, 1991b). Spermicides containing the active ingredient nonoxynol-9 may provide a selective advantage in colonizing the vagina, perhaps by a reduction in vaginal lactobacilli and through enhancement of adherence of E. coli to epithelial cells (Gupta et al, 2000; Hooton et al, 1991a). Thus spermicides should be discontinued in women with recurrent UTI and other forms of contraception should be used.
Postmenopausal women have frequent reinfections (Hooton and Stamm, 1991; Raz and Stamm, 1993). These infections are sometimes attributable to residual urine after voiding, which is often associated with bladder or uterine prolapse. In addition, the lack of estrogen causes marked changes in the vaginal microflora, including a loss of lactobacilli and increased colonization by E. coli (Raz and Stamm, 1993). Estrogen replacement frequently restores the normal vaginal environment, allows recolonization with lactobacilli, and thus eliminates bacterial uropathogenic colonization. A reduced incidence of UTIs has been documented with this approach (Raz and Stamm, 1993).
Urinary tract imaging will demonstrate the anatomy of the urinary tract and provide reasonable assessment of its functional status. In healthy women, upper tract abnormalities associated with reinfections are very rare; therefore routine urologic imaging is not indicated. Cystoscopy should be performed in men or women who have frequent reinfections and symptoms suggestive of obstruction, bladder dysfunction, and fistula. If the patient has residual urine that is judged to be significant (e.g., 100 mL) and due to a narrowing of the urethra, a single dilation of the urethra to improve bladder emptying would appear appropriate. There is little evidence, however, that repeated urethral dilation is indicated in the routine management of most women.
Antimicrobial management in women who have had two or more symptomatic UTIs over a 6-month period or three or more episodes within a 12-month period involves one of three regimens: low-dose continuous prophylaxis, self-start intermittent therapy, or postintercourse prophylaxis.
Low-Dose Continuous Prophylaxis
Biologic Basis of Successful Prophylaxis: Antimicrobial Effect on Bowel and Vaginal Bacterial Flora
The success of prophylaxis depends, in large part, on the effect an antimicrobial agent has on the introital and bowel reservoirs of pathogenic bacteria. Antimicrobial agents that eliminate pathogenic bacteria from these sites and/or do not cause bacterial resistance at the sites can be effective for antimicrobial prophylaxis of UTIs (Table 10–22).
Table 10–22 Low-Dose Prophylaxis for Recurrent UTIs in Women
| INVESTIGATORS | REGIMEN | INFECTIONS PER PATIENT-YEAR |
|---|---|---|
| Bailey et al (1971) | Nitrofurantoin, 50 or 100 mg daily | 0.09 |
| Nitrofurantoin, 50 mg daily | 0.19 | |
| Placebo | 2.1 | |
| Harding and Ronald (1974) | Sulfamethoxazole, 500 mg daily | 2.5 |
| TMP-SMX, 40 and 200 mg daily | 0.1 | |
| Methenamine mandelate, 2 g daily, plus ascorbic acid, 2 g | 1.6 | |
| Kasanen et al (1974) | Nitrofurantoin, 50 mg daily | 0.32 |
| Methenamine hippurate, 1 g daily | 0.39 | |
| Trimethoprim, 100 mg daily | 0.13 | |
| TMP-SMX, 80 and 400mg daily | 0.19 | |
| Gower (1975) | Cephalexin, 125 mg daily | 0.10 |
| Stamey et al (1977) | TMP-SMX, 40 and 200 mg daily | 0.00 |
| Nitrofurantoin macrocrystals, 100 mg daily | 0.74 | |
| Harding et al (1979) | TMP-SMX, 40 and 200 mg daily three times weekly | 0.1 |
| Stamm et al (1980) | TMP-SMX, 40 and 200 mg daily | 0.15 |
| Trimethoprim, 100 mg daily | 0.00 | |
| Nitrofurantoin macrocrystals, 100 mg daily | 0.14 | |
| Placebo | 2.8 | |
| Brumfitt et al (1981) | Nitrofurantoin, 50 mg twice daily | 0.19 |
| Methenamine hippurate, 1 g twice daily | 0.57 | |
| Harding et al (1982) | TMP-SMX, 40 and 200 mg three times weekly | 0.14 |
| Brumfitt et al (1983) | Trimethoprim, 100 mg daily | 1.53 |
| Methenamine hippurate, 1 g daily | 1.38 | |
| Povidone-iodine wash, twice daily | 1.79 | |
| Wong et al (1985) | TMP-SMX, 40 and 200 mg daily | 0.2 |
| Self-administered cotrimoxazole, 4 × 80 and 400 mg | 2.2 | |
| Martinez et al (1985) | Cephalexin, 250 mg daily | 0.18 |
| Brumfitt et al (1985) | Trimethoprim, 100 mg daily | 1.00 |
| Nitrofurantoin macrocrystals, 100 mg daily | 0.16 | |
| Nicolle et al (1989) | Nitrofurantoin, 200 mg daily | 0.00 |
| Norfloxacin, 200 mg daily | 0.00 | |
| Raz and Boger (1991) | Norfloxacin, 200 mg daily | 0.04 |
TMP-SMX, trimethoprim-sulfamethoxazole.
From Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am 1987;1:793–806.
Winberg and his colleagues were among the first to emphasize that oral antimicrobial therapy causes resistant strains in the bowel flora and subsequent resistant UTIs (Lincoln et al, 1970; Winberg et al, 1973). The increase in resistant strains of E. coli, as well as the proliferation of other Enterobacteriaceae species, Candida albicans, enterococci, and other pathogenic bacteria in the bowel and vaginal flora that accompanies even short-term, full-dose oral administration of tetracyclines, ampicillin, sulfonamides, amoxicillin, and cephalexin is well documented (Sharp, 1954; Daikos et al, 1968; Hinton, 1970; Lincoln et al, 1970; Datta et al, 1971; Gruneberg et al, 1973; Winberg et al, 1973; Toivanen et al, 1976; Ronald et al, 1977; Preiksaitis et al, 1981). These ecologic changes may interfere with antimicrobial prophylaxis in the urinary tract and must be considered in the choice of prophylactic agents.
Effective Drugs
The oral antimicrobial agents with minimal adverse effects on the bowel and vaginal flora are TMP-SMX or TMP alone, nitrofurantoin, cephalexin (in minimal dosage), and the fluoroquinolones.
TMP-SMX eradicates gram-negative aerobic flora from the bowel and vaginal fluid. Vaginal fluid measurements of TMP and SMX in patients showed that TMP infused across the noninflamed vaginal wall and produced concentrations that exceeded serum levels (Stamey and Condy, 1975); SMX was undetectable in vaginal fluid. These observations on diffusion and concentration of TMP and vaginal fluid and on the effects of TMP-SMX in clearing Enterobacteriaceae from the rectal and vaginal flora clearly indicate why TMP-SMX is such a powerful prophylactic agent for the prevention of reinfections in the female. These important biologic effects occur in addition to the bactericidal levels of TMP-SMX that are present in the urine during nightly prophylaxis.
Kasanen and his colleagues (1978) in Finland studied the bowel flora in volunteers and patients who took 100 mg of TMP per day for periods of 3 weeks to 36 months; 4 of 20 patients treated for long periods developed coliforms resistant to TMP (>8 µg/mL). Svensson and his associates (1982) gave 100 mg of TMP once daily for 6 months to 26 patients with recurrent UTIs. The infection recurrence rate before prophylaxis was 26 per 100 months compared with 3.3 recurrences per 100 months during prophylaxis (P = .001). The postprophylactic infection rate returned to 23 recurrences per 100 months. It is important to note that all E. coli UTIs after prophylaxis were sensitive to TMP, the number of rectal Enterobacteriaceae was markedly reduced during prophylaxis, and, although a 10% incidence of TMP-resistant organisms from rectal swabs was observed less than 1 month into prophylaxis there was no significant further accumulation of resistant bacteria.
These studies on TMP alone suggest that it should be as effective as TMP-SMX for prophylactic prevention of recurrent UTIs. Stamm and coworkers (1980a) noted only one resistant strain of E. coli in 316 rectal, urethral, and vaginal isolates from 15 patients receiving 100 mg of TMP and 15 others receiving 40 mg of TMP with 200 mg of SMX nightly for 6 months; their unbelievably low recovery of TMP-resistant E. coli was due to their method of sampling, which did not include streaking cultures from these colonization sites directly onto media containing TMP.
These studies on TMP-SMX and TMP prophylactic therapy usually have been limited to 6 months to test continuing susceptibility in patients with reinfections. Two studies (Pearson et al, 1979; Harding et al, 1982), however, continued TMP-SMX prophylaxis from 2 to 5 years without showing any increase in “breakthrough” infections or any increase in TMP-resistant recurrent infections. Indeed, in the 15 patients treated for 2 years with one-half tablet of TMP-SMX thrice weekly (Harding et al, 1982), 100 of 116 cultures from the periurethral area (91%) and 60 of 97 cultures from the anal canal (68%) showed no aerobic gram-negative bacilli at these colonization sites.
Nitrofurantoin, which does not alter the bowel flora, is present for brief periods at high concentrations in the urine and leads to repeated elimination of bacteria from the urine, presumably interfering with bacterial initiation of infection. Because of either its complete absorption in the upper intestinal tract or its degradation and inactivation in the intestinal tract, it produces minimal effects on bowel flora (Stamey et al, 1977). Unlike the situation in prophylaxis with TMP-SMX that eliminates colonization, in prophylaxis with nitrofurantoin colonization of the vaginal introitus with Enterobacteriaceae continues throughout therapy. The bacteria colonizing the vagina nearly always remain susceptible because of the lack of bacterial resistance in the bowel flora. Patients on long-term therapy should be monitored for adverse reactions (e.g., pulmonary fibrosis). The risk of an adverse reaction increases with age, with the greatest number occurring in patients older than 50 years. If a patient develops a chronic cough, the drug should be discontinued and a chest radiograph obtained.
Fairley and his associates (1974) first reported on the prophylactic efficacy of 500 mg of cephalexin per day in preventing recurrent infections during a 6-month period of observation. Of the 22 patients, 17 remained free of infection, an impressive record because several patients had papillary necrosis, chronic pyelonephritis, and even renal calculi. Gower (1975) treated 25 women with 125 mg of cephalexin nightly for 6 to 12 months and found only 1 infection, whereas 13 of 25 women receiving a placebo had infection.
Martinez and coworkers (1985) studied the effect on the vaginal and rectal flora of 250 mg of cephalexin nightly for 6 months in 23 patients with reinfections of the urinary tract. Throughout prophylaxis, 22 of the 23 patients maintained a sterile urine; a single patient developed two enterococcal UTIs, both of which responded to nitrofurantoin. No change was detected in the rectal or vaginal carriage of Enterobacteriaceae. More importantly, not a single resistant strain of E. coli was detected in 154 cultures obtained at monthly intervals during cephalexin therapy. These results are in contrast to those of Preiksaitis and colleagues (1981), who found rectal Enterobacteriaceae resistance in 38% of patients when cephalexin was administered at a dose of 500 mg four times daily for 14 days. Cephalexin at 250 mg or less nightly is an excellent prophylactic agent because bowel flora resistance does not develop at this low dosage.
With short-course fluoroquinolone therapy (Hooton et al, 1989), eradication of Enterobacteriaceae from the bowel and vaginal (Nord, 1988; Tartaglione et al, 1988) flora has been documented, observations that have been exploited in the use of these agents for prophylaxis. More recently, Nicolle and coworkers (1989) documented the prophylactic efficacy of norfloxacin for the prevention of recurrent UTIs in women. Of 11 women who completed 1 year of prophylaxis (with 200 mg orally), all remained free of infection. By comparison, the majority of individuals receiving placebos developed UTIs. The drug was well tolerated. In addition to preventing symptomatic UTIs, norfloxacin virtually eradicated periurethral and bowel colonization with aerobic gram-negative organisms. A larger study by Raz and Boger (1991) confirmed these results.
Because the fluoroquinolones are expensive and can be used only in nonpregnant women, we favor their use only when antimicrobial resistance or patient intolerance to TMP-SMX, TMP, nitrofurantoin, or cephalexin occurs. Further studies are required to determine the minimal effective regimen and efficacy of the fluoroquinolones for prophylaxis of recurrent UTIs in women.
Efficacy of Prophylaxis
Low-dose continuous prophylaxis is indicated when the urine culture shows no growth (usually when a patient has completed antimicrobial therapy). Nightly therapy is then begun with one of the following drugs: (1) nitrofurantoin, 50 to 100 mg half-strength (HS) (Stamey et al, 1977); (2) TMP-SMX, 40 to 200 mg (Stamm et al, 1982a); (3) TMP, 50 mg (Stamm et al, 1982a); or (4) cephalexin (Keflex), 250 mg (Martinez et al, 1985). Prophylactic therapy has been repeatedly documented as being effective in the management of women with recurrent UTIs, with recurrences decreased by 95% when compared with placebo or with the patients’ prior experiences as controls. These reported results of prophylaxis, together with agents and doses, have been summarized by Nicolle and Ronald (1987) (see Table 10–22). These studies consistently show a remarkable reduction in the reinfection rate from 2.0 to 3.0 per patient-year to 0.1 to 0.4 per patient-year with the use of prophylaxis. Urinary antiseptics, such as methenamine mandelate or hippurate, have resulted in some decrease in recurrences, but they are not as effective as antimicrobial agents.
Every-other-night therapy is also effective and is probably practiced by most patients. When breakthrough infections occur, they are not necessarily accompanied by symptoms; therefore we advocate monitoring for infections every 1 to 3 months, even in asymptomatic patients. Breakthrough infections usually respond to full-dose therapy with the drug used for prophylaxis. However, cultures and susceptibility tests may indicate that another drug is indicated. After the infection is cured, prophylaxis may be reinstituted. Low-dose prophylaxis is usually discontinued after about 6 months, and the patient is monitored for reinfection. Approximately 30% of women will have spontaneous remissions that last up to 6 months (Kraft and Stamey, 1977). Unfortunately, many of the remissions are followed by reinfections, and low-dose prophylaxis must be reinstituted. At this point, many patients prefer an alternative form of management.
Self-Start Intermittent Therapy
With self-start intermittent therapy, the patient is given a dip slide device to culture the urine and is instructed to perform a urine culture when symptoms of UTI occur (Schaeffer and Stuppy, 1999; Blom et al, 2002). The patient is also provided a 3-day course of empirical, full-dose antimicrobial therapy to be started immediately after performing the culture. It is important that the antimicrobial agent selected for self-start therapy have a broad spectrum of activity and achieve high urinary levels to minimize development of resistant mutants. In addition, there should be minimal or no side effects on the bowel flora. Fluoroquinolones are ideal for self-start therapy because they have a spectrum of activity broader than any of the other oral agents and are superior to many parenteral antimicrobials, including aminoglycosides. Nitrofurantoin and TMP-SMX are acceptable alternatives, although they are somewhat less effective. Antimicrobial agents such as tetracycline, ampicillin, SMX, and cephalexin in full doses should be avoided because they can give rise to resistant bacteria (Wong et al, 1985).
The culture is brought to the office as soon as possible. If the culture is positive and the patient is asymptomatic, a culture is performed 7 to 10 days after therapy to determine efficacy. In most cases, the therapy is limited to two inexpensive dip slide cultures and a short course of antimicrobial therapy. If the patient has symptoms that do not respond to initial antimicrobial therapy, a repeat culture and susceptibility testing of the initial culture specimen are performed and therapy adjusted accordingly. If symptoms of infection are not associated with positive cultures, urologic evaluation should be performed to rule out other causes of irritative bladder symptoms, including carcinoma in situ, interstitial cystitis, and neurogenic bladder dysfunction. The authors’ experience with this technique has been very favorable, and is particularly attractive to patients who have less frequent infections and are willing to play an active role in their diagnosis and management.
Postintercourse Prophylaxis
Antimicrobial management through postintercourse prophylaxis is based on research establishing that sexual intercourse can be an important risk factor for acute cystitis in women (Nicolle et al, 1982). Diaphragm users have a significantly greater risk of UTI than do women who use other contraceptive methods (Fihn et al, 1985). Postintercourse therapy with antimicrobial agents, such as nitrofurantoin, cephalexin, TMP-SMX, or a fluoroquinolone taken as a single dose, will effectively reduce the incidence of reinfection (Pfau et al, 1983; Melekos et al, 1997).
Other Strategies
Cranberry juice contains proanthocyanidins that block adherence of pathogens to uroepithelial cells in vitro (Foo et al, 2000). Randomized trials in low-risk patients show that 200 to 750 mL daily of cranberry or lingonberry juice or cranberry-concentrate tablets reduce the risk of symptomatic, recurrent infection by 12% to 20% (Avorn et al, 1994; Kontiokari et al, 2001; Stothers, 2002; McMurdo et al, 2009). However, the actual cranberry content of juices and tablets varies substantially; therefore their efficacy is not predictable (Consumer Reports, 2001; Klein, 2002). Furthermore, other trials of cranberry products show no benefit and there is no evidence that they are effective for treatment of UTIs (Jepson et al, 2001; Raz et al, 2004).
Other factors, such as hygiene, frequency and timing of voiding, wiping patterns, use of hot tubs, and type of undergarments, have not been shown to predispose women to recurrent infection and there is no rationale for giving women specific instructions regarding them.
Kidney Infections
Renal Infection (Bacterial Nephritis)
Although renal infection is less prevalent than bladder infection it often is a more difficult problem for the patient and his or her physician because of its often varied and morbid presentation and course, the difficulty in establishing a firm microbiologic and pathologic diagnosis, and its potential for significantly impairing renal function. Although the classic symptoms of acute onset of fever, chills, and flank pain are usually indicative of renal infection, some patients with these symptoms do not have renal infection. Conversely, significant renal infection may be associated with an insidious onset of nonspecific local or systemic symptoms or it may be entirely asymptomatic. Therefore a high clinical index of suspicion and appropriate radiologic and laboratory studies are required to establish the diagnosis of renal infection.
Unfortunately, the relationship between laboratory findings and the presence of renal infection often is poor. Bacteriuria and pyuria, the hallmarks of UTI, are not predictive of renal infection. Conversely, patients with significant renal infection may have sterile urine if the ureter draining the kidney is obstructed or the infection is outside the collecting system.
The pathologic and radiologic criteria for diagnosing renal infection may also be misleading. Interstitial renal inflammation, once thought to be caused predominantly by bacterial infection, is now recognized as a nonspecific histopathologic change associated with a variety of immunologic, congenital, or chemical lesions that usually develop in the absence of bacterial infection. Infectious granulomatous diseases of the kidney often have either radiologic or pathologic characteristics that mimic renal cystic disease, neoplasia, or other renal inflammatory disease.
The effect of renal infection on renal function is varied. Acute or chronic pyelonephritis may transiently or permanently alter renal function, but nonobstructive pyelonephritis is no longer recognized as a major cause of renal failure (Baldassarre and Kaye, 1991; Fraser et al, 1995). However, pyelonephritis, when associated with urinary tract obstruction or granulomatous renal infection, may lead rapidly to significant inflammatory complications, renal failure, or even death.
Pathology
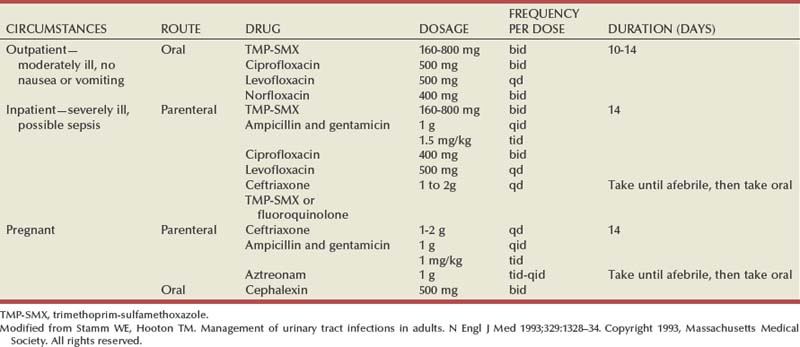
The opportunity for pathologic confirmation of acute bacterial nephritis is rare. The kidney may be edematous. Focal acute suppurative bacterial nephritis caused by hematogenous dissemination of bacteria to the renal cortex is characterized by multiple focal areas of suppuration on the surface of the kidney (Fig. 10–15). Histologic examination of the renal cortex shows focal suppurative destruction of glomeruli and tubules. Adjacent cortical structures and the medulla are not involved in the inflammatory reaction. Acute ascending pyelonephritis is characterized by linear bands of inflammation extending from the medulla to the renal capsule (Fig. 10–16). Histologic examination usually reveals a focal wedge-shaped area of acute interstitial inflammation with the apex of the wedge in the renal medulla. Polymorphonuclear leukocytes or a predominantly lymphocytic and plasma cell response are seen. Bacteria also may be present.
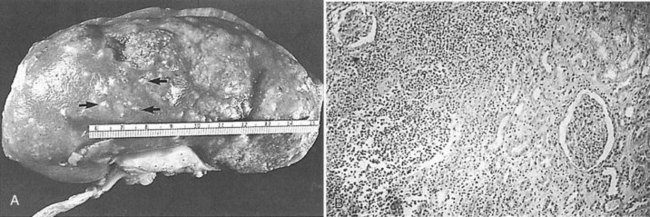
Figure 10–15 Acute focal suppurative bacterial nephritis. A, Surface of kidney. Arrows indicate focal areas of suppuration. B, Renal cortex showing focal suppuration destruction of glomeruli and tubules.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY, et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
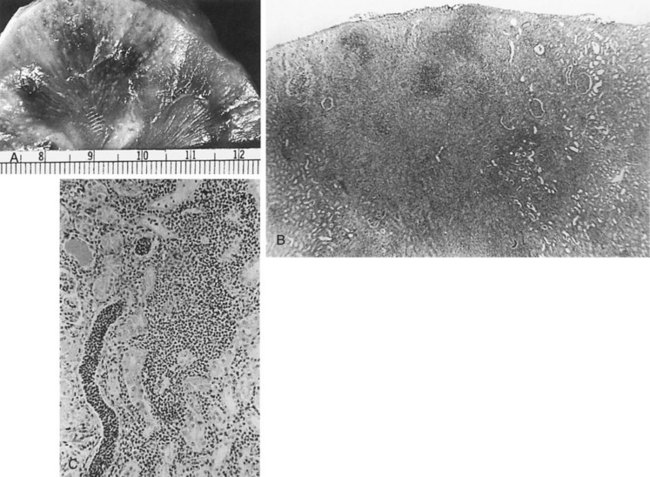
Figure 10–16 Acute ascending pyelonephritis. A, Cortical structures, tubules, and collecting ducts diffusely infiltrated with inflammatory cells. B, Section of the renal cortex showing wedge-shaped destruction of renocortical structures as a result of ascending infiltration with inflammatory cells. C, Thickened and inflamed tissue surrounding the collecting ducts in the medulla. A polymorphonuclear cast of segmented neutrophils is clearly visible.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
The changes that appear to be most specific for chronic pyelonephritis are evident on careful gross examination of the kidney and consist of a cortical scar associated with retraction of the corresponding renal papilla (Hodson, 1965a, 1965b; Heptinstall, 1974; Freedman, 1979). The kidney shows evidence of patchy involvement with numerous chronic inflammatory foci mainly confined to the cortex but also involving the medulla (Fig. 10–17).
Figure 10–17 Chronic pyelonephritis. The renal cortex shows thickened fibrous capsule and focal retracted scar on surface of kidney. Focal destruction of tubules in center of picture is accompanied by periglomerular fibrosis and scarring.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
The scars may be separated by intervening zones of normal parenchyma, causing a grossly irregular renal outline. The microscopic appearance, as with most chronic interstitial disease, includes the presence of lymphocytes and plasma cells. Although glomeruli within scars may be surrounded by a cuff of fibrosis or be partially or completely hyalinized, glomeruli outside these severely scarred zones are relatively normal. Vascular involvement is variable, but in patients with hypertension, nephrosclerosis may be found. Papillary abnormalities include deformity, sclerosis, and sometimes necrosis. Studies in animals have clearly indicated the critical role of the papilla in the initiation of pyelonephritis (Freedman and Beeson, 1958). However, these changes are not necessarily specific for bacterial infection and may occur in the absence of infection as a result of other disorders such as analgesic abuse, diabetes, and sickle cell disease.
Acute Pyelonephritis
Although pyelonephritis is defined as inflammation of the kidney and renal pelvis, the diagnosis is clinical. True infection of the “upper urinary tract” can be proved by catheterization tests (ureteral catheterization or bladder washout) as described in this chapter, but these are impractical and unnecessary in most patients with acute pyelonephritis. None of the noninvasive tests that have been developed to determine infection in the kidney or bladder is totally reliable.
Clinical Presentation
The clinical spectrum ranges from gram-negative sepsis to cystitis with mild flank pain (Stamm and Hooton, 1993). The classic presentation is an abrupt onset of chills, fever (100° F or greater), and unilateral or bilateral flank or costovertebral angle pain and/or tenderness. These so-called upper tract signs are often accompanied by dysuria, increased urinary frequency, and urgency.
Although some authors regard loin pain and fever in combination with significant bacteriuria as diagnostic of acute pyelonephritis, it is clear from localization studies using ureteral catheterization (Stamey and Pfau, 1963) or the bladder washout technique (Fairley et al, 1967) that clinical symptoms correlate poorly with the site of infection (Stamey et al, 1965; Eykyn et al, 1972; Fairley, 1972; Smeets and Gower, 1973).
In a large study of 201 women and 12 men with recurrent UTIs, Busch and Huland (1984) showed that fever and flank pain are no more diagnostic of pyelonephritis than they are of cystitis. Of patients with flank pain and/or fever, over 50% had lower tract bacteriuria. Conversely, patients with bladder symptoms or no symptoms frequently had upper tract bacteriuria. Approximately 75% of patients give a history of previous lower UTIs.
On physical examination there often is tenderness to deep palpation in the costovertebral angle. Variations of this clinical presentation have been recognized. Acute pyelonephritis may also simulate gastrointestinal tract abnormalities with abdominal pain, nausea, vomiting, and diarrhea. Asymptomatic progression of acute pyelonephritis to chronic pyelonephritis, particularly in compromised hosts, may occur in the absence of overt symptoms. Acute renal failure may be present in the rare case (Richet and Mayaud, 1978; Olsson et al, 1980).
Laboratory Diagnosis
The patient may have leukocytosis with predominance of neutrophils. Urinalysis usually reveals numerous WBCs, often in clumps, and bacterial rods or chains of cocci. Leukocytes exhibiting brownian motion in the cytoplasm (glitter cells) may be present if the urine is hypotonic, but they are not in themselves diagnostic of pyelonephritis. The presence of large amounts of granular or leukocyte casts in the urinary sediment is suggestive of acute pyelonephritis. A specific type of urinary cast characterized by the presence of bacteria in its matrix has been demonstrated in the urine of patients who have had acute pyelonephritis (Fig. 10–18) (Lindner et al, 1980). Bacteria in the casts were not easily distinguished by simple brightfield microscopy without special staining of the sediment. Staining of the sediment with a basic dye such as dilute toluidine blue or KOVA stain (I.C.L. Scientific, Fountain Valley, CA) demonstrated the bacteria in casts without difficulty. Blood tests may show leukocytosis with a predominance of neutrophils, increased erythrocyte sedimentation rate, elevated C-reactive protein levels, and elevated creatinine levels if renal failure is present. In addition, creatinine clearance may be decreased. Blood cultures may be positive.
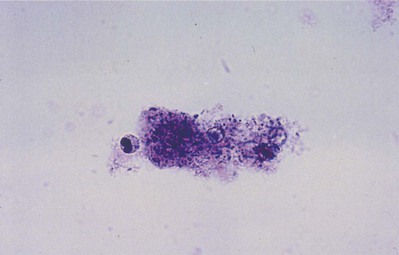
Figure 10–18 Brightfield micrograph of a mixed bacterial leukocyte cast from patient with acute pyelonephritis. Only the bacteria and the nucleus of a leukocyte stain strongly. Many bacteria are clearly demonstrated by through-focusing (toluidine blue O, ×640).
(From Lindner LE, Jones RN, Haber MH. A specific urinary cast in acute pyelonephritis. Am J Clin Pathol 1980;73:809–11.)
Bacteriology
Urine cultures are positive, but about 20% of patients have urine cultures with fewer than 105 cfu/mL and, therefore, negative results on Gram staining of the urine (Rubin et al, 1992).
E. coli, which constitutes a unique subgroup that possesses special virulence factors, accounts for 80% of cases. If vesicoureteral reflux is absent, a patient bearing the P blood group phenotype may have special susceptibility to recurrent pyelonephritis caused by E. coli that have P pili and bind to the P blood group antigen receptors (Lomberg et al, 1983). Bacterial K antigens and endotoxins also may contribute to pathogenicity (Kaijser et al, 1977). Many cases of community-acquired pyelonephritis are caused by a limited number of multiple-antimicrobial–resistant clonal groups (Manges et al, 2004).
More resistant species, such as Proteus, Klebsiella, Pseudomonas, Serratia, Enterobacter, or Citrobacter should be suspected in patients who have recurrent UTIs, are hospitalized, or have indwelling catheters, as well as in those who required recent urinary tract instrumentation. Except for E. faecalis, S. epidermidis, and S. aureus, gram-positive bacteria rarely cause pyelonephritis.
Blood cultures are positive in about 25% of cases of uncomplicated pyelonephritis in women, and the majority replicate the urine culture and do not influence decisions regarding therapy. Therefore blood cultures should not be routinely obtained for the evaluation of uncomplicated pyelonephritis in women. However, they should be performed in men and women with systemic toxicity or in those requiring hospitalization or with risk factors such as pregnancy (Velasco et al, 2003).
Renal Ultrasonography and Computed Tomography
These studies are commonly used to evaluate patients initially for complicated UTIs or factors or to reevaluate patients who do not respond after 72 hours of therapy (see later). Ultrasonography (Fig. 10–19) and CT show renal enlargement, hypoechoic or attenuated parenchyma, and a compressed collecting system. They also may delineate focal bacterial nephritis and obstruction. When parenchymal destruction becomes pronounced, a more disorganized parenchyma and abscess formation associated with complicated renal and perirenal infections may be identified (Soulen et al, 1989).
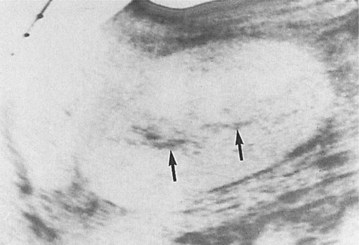
Figure 10–19 Acute pyelonephritis. Ultrasound image of the right kidney demonstrates renal enlargement, hypoechoic parenchyma, and compressed central collecting complex (arrows).
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott William & Wilkins; 2002. p. 211–72.)
Differential Diagnosis
Acute appendicitis, diverticulitis, and pancreatitis can cause a similar degree of pain, but the location of the pain often is different. Results of the urine examination are usually normal. Herpes zoster can cause superficial pain in the region of the kidney but is not associated with symptoms of UTI; the diagnosis will be apparent when shingles appear.
Management
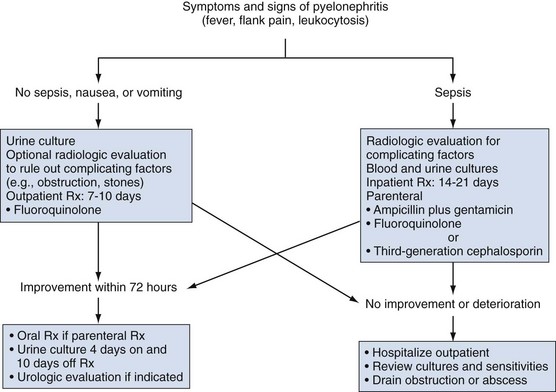
Initial Management
Infection in patients with acute pyelonephritis can be subdivided into (1) uncomplicated infection that does not warrant hospitalization, (2) uncomplicated infection in patients with normal urinary tracts who are ill enough to warrant hospitalization for parenteral therapy, and (3) complicated infection associated with hospitalization, catheterization, urologic surgery, or urinary tract abnormalities (Fig. 10–20).
It is critical to determine whether the patient has an uncomplicated or complicated UTI because significant abnormalities have been found in 16% of patients with acute pyelonephritis (Shen and Brown, 2004). In patients with presumed uncomplicated pyelonephritis that will be managed as outpatients, initial radiologic evaluation can usually be deferred. However, if there is any reason to suspect a problem or if the patient will not have reasonable access to imaging if there should be no change in condition, we prefer renal ultrasound evaluation to rule out stones or obstruction. In patients with known or suspected complicated pyelonephritis, CT provides excellent assessment of the status of the urinary tract and the severity and extent of the infection.
For patients who will be managed as outpatients, single-drug oral therapy with a fluoroquinolone is more effective than TMP-SMX for patients with domiciliary infections (Talan et al, 2000). Many physicians administer a single parenteral dose of an antimicrobial agent (ceftriaxone, gentamicin, or a fluoroquinolone) before initiating oral therapy (Israel et al, 1991; Pinson et al, 1994). If a gram-positive organism is suspected, amoxicillin or amoxicillin/clavulanic acid is recommended (Warren et al, 1999).
If a patient has an uncomplicated infection but is sufficiently ill to require hospitalization (high fever, high WBC count, vomiting, dehydration, evidence of sepsis), has complicated pyelonephritis, or fails to improve during the initial outpatient treatment period, a parenteral fluoroquinolone, an aminoglycoside with or without ampicillin, or an extended-spectrum cephalosporin with or without an aminoglycoside is recommended (Warren et al, 1999) (Table 10–23). If gram-positive cocci are causative, ampicillin/sulbactam with or without an aminoglycoside is recommended.
Hospitalization, initially with complete bed rest, intravenous fluids, and antipyretics, is required.
An obstructed kidney has difficulty concentrating and excreting antimicrobial agents. Any substantial obstruction must be relieved expediently by the safest and simplest means.
A Gram stain of the urine sediment is helpful to guide the selection of the initial empirical antimicrobial therapy. In all cases, antimicrobial therapy should be active against potential uropathogens and achieve antimicrobial levels in renal tissue as well as urine.
Subsequent Management
Even though the urine usually becomes sterile within a few hours of starting antimicrobial therapy, patients with acute uncomplicated pyelonephritis may continue to have fever, chills, and flank pain for several more days after initiation of successful antimicrobial therapy (Behr et al, 1996). They should be observed.
Ambulatory patients should be treated with a fluoroquinolone for 7 days (Talan et al, 2000). Fluoroquinolone therapy is associated with greater bacteriologic and clinical cure rates than 14-day TMP-SMX therapy (Talan et al, 2000). Alterations in antimicrobial therapy may be made depending on the patient’s clinical response and the results of the culture and susceptibility tests. Susceptibility tests should also be used to replace potentially toxic drugs, such as aminoglycosides, with less toxic drugs, such as the fluoroquinolones, aztreonam, and cephalosporins.
Patients with complicated pyelonephritis and positive blood cultures should be treated with parenteral therapy until clinically stable. If blood cultures are negative, 2- to 3-day parenteral therapy is sufficient. Following parenteral therapy, an appropriate oral antimicrobial drug (fluoroquinolone, TMP, TMP-SMX, or amoxicillin or amoxicillin/clavulanic acid for gram-positive organisms) should be continued in full dosage for an additional 10 to 14 days.
Unfavorable Response to Therapy
When the response to therapy is slow or the urine continues to show infection, an immediate reevaluation is mandatory. Urine and blood cultures must be repeated and appropriate alterations in antimicrobial therapy made on the basis of susceptibility testing. CT is indicated to attempt to identify unsuspected obstructive uropathy, urolithiasis, or underlying anatomic abnormalities that may have predisposed the patient to infection, prevented a rapid therapeutic response, or caused complications of the infectious process, such as renal or perinephric abscess. In patients with fever lasting longer than 72 hours, CT is most helpful for ruling out obstruction and identifying renal and perirenal infections (Soulen et al, 1989). Radionuclide imaging may be useful to demonstrate functional changes associated with acute pyelonephritis (decrease in renal blood flow, delay in peak function, and delay in excretion of the radionuclide) (Fischman and Roberts, 1982) and cortical defects associated with vesicoureteral reflux.
Follow-Up
Repeat urine cultures should be performed on the fifth to the seventh day of therapy and 10 to 14 days after discontinuing antimicrobial therapy to ensure that the urinary tract remains free of infections. Between 10% and 30% of individuals with acute pyelonephritis relapse after a 14-day course of therapy. Patients who experience relapse usually are cured by a second 14-day course of therapy, but occasionally a 6-week course is necessary (Tolkoff-Rubin et al, 1984; Johnson and Stamm, 1987).
Depending on the clinical presentation and response and initial urologic evaluation, some patients may require additional evaluation (e.g., voiding cystourethrogram, cystoscopy, bacterial localization studies) and correction of an underlying abnormality of the urinary tract. Raz and colleagues (2003) evaluated the long-term impact of acute pyelonephritis in women. Scanning with 99mTc-dimercaptosuccinic acid (99mTc-DMSA) 10 to 20 years after acute pyelonephritis revealed scars in approximately 50% of the patients but changes in renal function were minimal and not associated with renal scarring.
Acute Focal or Multifocal Bacterial Nephritis
Acute focal or multifocal bacterial nephritis is an uncommon, severe form of acute renal infection in which a heavy leukocyte infiltrate is confined to a single renal lobe (focal) or multiple lobes (multifocal).
Clinical Presentation
The clinical presentation of patients with acute bacterial nephritis is similar to that of patients with acute pyelonephritis but usually is more severe. About half of the patients are diabetic, and sepsis is common. Generally, leukocytosis and UTI resulting from gram-negative organisms are found; more than 50% of the patients are bacteremic (Wicks and Thornbury, 1979).
Radiologic Findings
The diagnosis must be made by radiologic examination. The mass has slightly less nephrographic density than the surrounding normal renal parenchyma.
Ultrasonography and CT establish the diagnosis. On ultrasonography the lesion is typically poorly marginated and relatively sonolucent with occasional low-amplitude echoes that disrupt the cortical medullary junction (Corriere and Sandler, 1982) (Fig. 10–21A). Enhancement with a contrast agent is necessary with CT studies because the lesion is difficult to visualize on the unenhanced study (see Fig. 10–21B). Wedge-shaped areas of decreased enhancement are seen. No definite wall is evident, and frank liquefaction is absent. Conversely, abscesses tend to have liquid centers, are usually round, and are present both before and after contrast medium enhancement. More chronic abscesses may also show a ring-shaped area of increased enhancement surrounding the lesion (Corriere and Sandler, 1982). Gallium scanning reveals uptake that is in the region of and larger than the previously demonstrated mass (Rosenfield et al, 1979). In patients with multifocal disease the findings are similar but multiple lobes are involved.
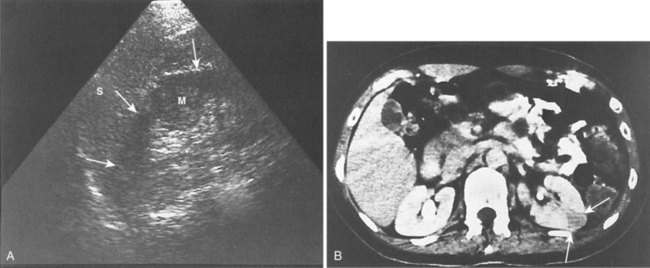
Figure 10–21 Acute focal bacterial nephritis. A, Ultrasound image: longitudinal view of the left kidney demonstrates spleen (S) and left kidney (arrows). Note irregular midpole mass (M) of slightly higher echo texture than surrounding normal renal parenchyma. B, Contrast medium–enhanced CT scan demonstrates a wedge-shaped area of low density (arrows) in the middle portion of the left kidney. The findings resolved after antimicrobial therapy.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
Management
Acute bacterial nephritis probably represents a relatively early phase of frank abscess formation. In a series of cases reported by Lee and coworkers (1980) a patient with acute focal bacterial nephritis progressed to abscess formation. Treatment includes hydration and intravenous antimicrobial agents for at least 7 days, followed by 7 days of oral antimicrobial therapy. Patients with bacterial nephritis typically respond to medical therapy, and follow-up studies will show resolution of the wedge-shaped zones of diminished attenuation. Failure to respond to antimicrobial therapy is an indication for appropriate studies to rule out obstructive uropathy, renal or perirenal abscess, renal carcinoma, or acute renal vein thrombosis. Long-term follow-up studies performed in a few patients with multifocal disease have demonstrated a decrease in renal size and focal calyceal deformities suggestive of papillary necrosis (Davidson and Talner, 1978).
Emphysematous Pyelonephritis
Emphysematous pyelonephritis is a urologic emergency characterized by an acute necrotizing parenchymal and perirenal infection caused by gas-forming uropathogens. The pathogenesis is poorly understood. Because the condition usually occurs in diabetic patients, it has been postulated that the high tissue glucose levels provide the substrate for microorganisms such as E. coli, which are able to produce carbon dioxide by the fermentation of sugar (Schainuck et al, 1968). Although glucose fermentation may be a factor, the explanation does not account for the rarity of emphysematous pyelonephritis despite the high frequency of gram-negative UTI in diabetic patients, nor does it explain the rare occurrence of the condition in nondiabetic patients.
In addition to diabetes, many patients have urinary tract obstruction associated with urinary calculi or papillary necrosis and significant renal functional impairment. The overall mortality rate has been reported to be between 43% (Freiha et al, 1979) and 19% (Huang and Tseng, 2000).
Clinical Presentation
All of the documented cases of emphysematous pyelonephritis have occurred in adults (Hawes, 1983). Juvenile diabetic patients do not appear to be at risk. Women are affected more often than men.
The usual clinical presentation is severe, acute pyelonephritis, although in some instances a chronic infection precedes the acute attack. Almost all patients display the classic triad of fever, vomiting, and flank pain (Schainuck et al, 1968). Pneumaturia is absent unless the infection involves the collecting system. Results of urine cultures are invariably positive. E. coli is most commonly identified. Klebsiella and Proteus are less common.
Radiologic Findings
The diagnosis is established radiographically. Tissue gas that is distributed in the parenchyma may appear on abdominal radiographs as mottled gas shadows over the involved kidney (Fig. 10–22). This finding is often mistaken for bowel gas. A crescentic collection of gas over the upper pole of the kidney is more distinctive. As the infection progresses, gas extends to the perinephric space and retroperitoneum. This distribution of gas should not be confused with cases of emphysematous pyelitis in which air is in the collecting system of the kidney. Emphysematous pyelitis is secondary to a gas-forming bacterial UTI, often occurs in nondiabetic patients, is less serious, and usually responds to antimicrobial therapy.
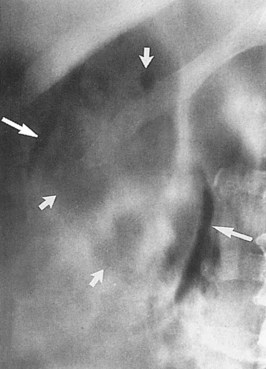
Figure 10–22 Emphysematous pyelonephritis; plain film. Extensive perinephric (long arrows) and intraparenchymal (short arrows) gas secondary to acute bacterial pyelonephritis.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott William & Wilkins; 2002. p. 211–72.)
Ultrasonography usually demonstrates strong focal echoes suggesting the presence of intraparenchymal gas (Brenbridge et al, 1979; Conrad et al, 1979). CT is the imaging procedure of choice in defining the extent of the emphysematous process and guiding management (Figs. 10-23 and 10-24). An absence of fluid in CT images or the presence of streaky or mottled gas with or without bubbly and loculated gas appears to be associated with rapid destruction of renal parenchyma and a 50% to 60% mortality rate (Wan et al, 1996; Best et al, 1999). The presence of renal or perirenal fluid, the presence of bubbly or loculated gas or gas in the collecting system, and the absence of streaky or mottled gas patterns is associated with a less than 20% mortality rate. Obstruction is demonstrated in approximately 25% of the cases. A nuclear renal scan should be performed to assess the degree of renal function impairment in the involved kidney and the status of the contralateral kidney.
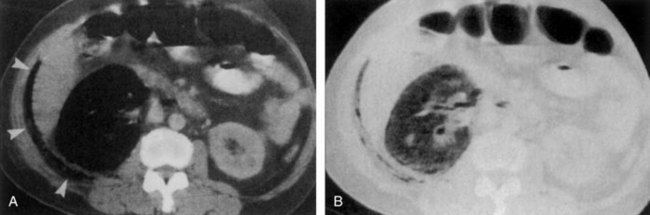
Figure 10–23 Type I emphysematous pyelonephritis with complete renal destruction in a 49-year-old woman. A, CT scan of the right kidney shows complete destruction with gas (arrowheads) extending beyond the renal fascia. B, CT scan with a modified lung window display shows the characteristic streaky gas in the completely destroyed kidney. The patient died on arrival in the emergency department.
(A and B, From Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas-producing bacterial renal infection: correlation between imaging findings and clinical outcome. Radiology 1996;198:433–8.)
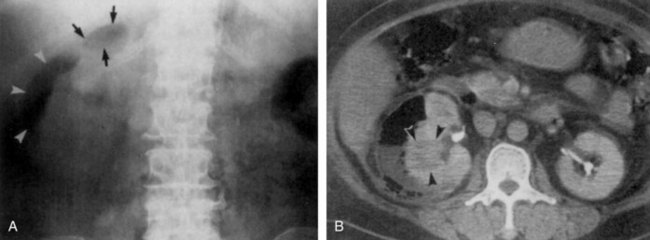
Figure 10–24 Type II emphysematous pyelonephritis in a 57-year-old woman. A, Radiograph shows crescent-shaped (arrowheads) and loculated (arrows) gas in the right renal area. B, CT scan obtained after administration of contrast material shows a low-attenuation area (arrowheads) in the right kidney due to acute pyelonephritis as well as a subcapsular abscess with fluid and bubbly and loculated gas. The patient survived after percutaneous drainage was performed.
(A and B, From Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas-producing bacterial renal infection: correlation between imaging findings and clinical outcome. Radiology 1996;198:433–8.)
Management
Emphysematous pyelonephritis is a surgical emergency. Most patients are septic, and fluid resuscitation and broad-spectrum antimicrobial therapy are essential. If the kidney is functioning, medical therapy can be considered (Wan et al, 1996; Best et al, 1999). Nephrectomy is recommended for patients who do not improve after a few days of therapy (Malek and Elder, 1978). If the affected kidney is nonfunctioning and not obstructed, nephrectomy should be performed because medical treatment alone is usually lethal. If a kidney is obstructed, catheter drainage must be instituted. If the patient’s condition improves, nephrectomy may be deferred pending a complete urologic evaluation. Although there are isolated case reports of retention of renal function after medical therapy combined with relief of obstruction, most patients require nephrectomy (Hudson et al, 1986).
Renal Abscess
Renal abscess or carbuncle is a collection of purulent material confined to the renal parenchyma. Before the antimicrobial era, 80% of renal abscesses were attributed to hematogenous seeding by staphylococci (Campbell, 1930). Although experimental and clinical data document the facility for abscess formation in normal kidneys after hematogenous inoculation with staphylococci, widespread use of antimicrobial agents since about 1950 appears to have diminished the propensity for gram-positive abscess formation (DeNavasquez, 1950; Cotran, 1969).
Since about 1970, gram-negative organisms have been implicated in the majority of adults with renal abscesses. Hematogenous renal seeding by gram-negative organisms may occur, but this is not likely to be the primary pathway for gram-negative abscess formation. Clinically there is no evidence that gram-negative septicemia antedates most lesions. Furthermore, gram-negative hematogenous pyelonephritis is virtually impossible to produce in animals unless the kidney is traumatized or completely obstructed (Cotran, 1969; Timmons and Perlmutter, 1976). The partially obstructed kidney rejects blood borne gram-negative inocula as well as a normal kidney. Thus ascending infection associated with tubular obstruction from prior infections or calculi appears to be the primary pathway for the establishment of gram-negative abscesses. Two-thirds of gram-negative abscesses in adults are associated with renal calculi or damaged kidneys (Salvatierra et al, 1967). Although the association of pyelonephritis with vesicoureteral reflux is well established, the association of renal abscess with vesicoureteral reflux has been infrequently noted (Segura and Kelalis, 1973). More recent observations, however, indicate that reflux is frequently associated with renal abscesses and persists long after sterilization of the urinary tract (Timmons and Perlmutter, 1976).
Clinical Presentation
The patient may present with fever, chills, abdominal or flank pain, and occasionally with weight loss and malaise. Symptoms of cystitis may occur. Occasionally these symptoms may be vague and delay diagnosis until surgical exploration or, in more severe cases, autopsy (Anderson and McAninch, 1980). A thorough history may reveal a gram-positive source of infection 1 to 8 weeks before the onset of urinary tract symptoms. The infection may have occurred in any area of the body. Multiple skin carbuncles and intravenous drug abuse introduce gram-positive organisms into the bloodstream. Other common sites are the mouth, lungs, and bladder (Lyons et al, 1972). Complicated UTIs associated with stasis, calculi, pregnancy, neurogenic bladder, and diabetes mellitus also appear to predispose the patient to abscess formation (Anderson and McAninch, 1980).
Laboratory Diagnosis
The patient typically has marked leukocytosis. The blood cultures are usually positive. Pyuria and bacteriuria may not be evident unless the abscess communicates with the collecting system. Because gram-positive organisms are most commonly blood borne, urine cultures in these cases typically show no growth or a microorganism different from that isolated from the abscess. When the abscess contains gram-negative organisms, the urine culture usually demonstrates the same organism isolated from the abscess.
Ultrasonography and CT distinguish abscess from other inflammatory renal diseases. Ultrasonography is the quickest and least expensive method to demonstrate a renal abscess. An echo-free or low-echodensity space-occupying lesion with increased transmission is found on the ultrasound image (Fig. 10–25). The margins of an abscess are indistinguishable in the acute phase, but the structure contains a few echoes and the surrounding renal parenchyma is edematous (Fiegler, 1983). Subsequently, the appearance tends to be that of a well-defined mass. The internal appearance, however, may vary from a virtually solid lucent mass to one with large numbers of low-level internal echoes (Schneider et al, 1976). The number of echoes depends on the amount of cellular debris within the abscess. The presence of air results in a strong echo with a shadow. Differentiation between an abscess and a tumor is impossible in many cases. Arteriography is used infrequently to demonstrate abscesses. The center of the mass tends to be hypervascular or avascular, with increased vascularity at the cortical margins and lack of vascular displacement and neovascularity.
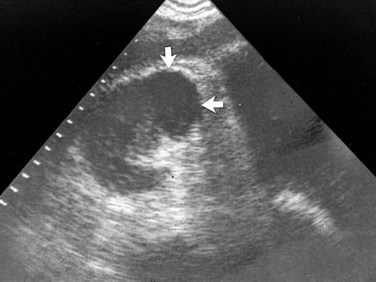
Figure 10–25 Acute renal abscess. Transverse ultrasound image of the right kidney demonstrates a poorly marginated rounded focal hypoechoic mass (arrows) in the anterior portion of the kidney.
CT appears to be the diagnostic procedure of choice for renal abscesses, because it provides excellent delineation of the tissue. On CT, abscesses are characteristically well defined both before and after contrast agent enhancement. The findings depend in part on the age and severity of the abscess (Baumgarten and Baumgartner, 1997). Initially, CT shows renal enlargement and focal, rounded areas of decreased attenuation (Fig. 10–26). After several days of the onset of the infection, a thick fibrotic wall begins to form around the abscess. An echo-free or slightly echogenic mass due to the presence of necrotic debris is seen. CT of a chronic abscess shows obliteration of adjacent tissue planes, thickening of the Gerota fascia, a round or oval parenchymal mass of low attenuation, and a surrounding inflammatory wall of slightly higher attenuation that forms a ring when the scan is enhanced with contrast material (Fig. 10–27). The ring sign is caused by the increased vascularity of the abscess wall (Callen, 1979; Gerzof and Gale, 1982).
Figure 10–26 Acute renal abscess. Nonenhanced CT scan through the mid pole of the right kidney demonstrates right renal enlargement and an area of decreased attenuation (arrows). After antimicrobial therapy, a follow-up scan showed complete regression of these findings.
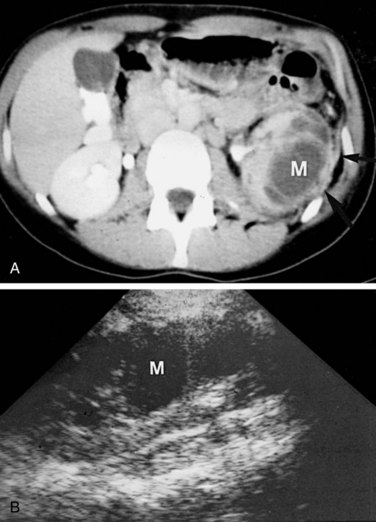
Figure 10–27 Chronic renal abscess. A, Enhanced CT scan shows an irregular septated low-density mass (M) extensively involving the left kidney. Note thickening of perinephric fascia (arrowheads) and extensive compression of the renal collecting system. Findings are typical of renal abscess. B, Ultrasound longitudinal image demonstrates a septated hypoechoic mass (M) occupying much of the renal parenchymal volume.
Radionuclide imaging with gallium or indium is sometimes useful in evaluating patients with renal abscesses (see earlier sections in this chapter and Chapter 4).
Management
Although the classic treatment for an abscess has been percutaneous or open incision and drainage there is good evidence that the intravenous use of antimicrobial agents and careful observation of a small abscess less than 3 cm in diameter, if begun early enough in the course of the process, may obviate surgical procedures (Hoverman et al, 1980; Levin et al, 1984; Shu et al, 2004). CT- or ultrasound-guided needle aspiration may be necessary to differentiate an abscess from a hypervascular tumor. Aspirated material should be cultured and appropriate antimicrobial therapy instituted on the basis of the findings.
The selection of empirical antimicrobial therapy is dependent on the presumed source of the infection and the resistance patterns within the hospital. When hematogenous dissemination is suspected, the pathogenic organism most frequently is penicillin-resistant Staphylococcus, and the antimicrobial of choice therefore is a penicillinase-resistant penicillin (Schiff et al, 1977). If a history of penicillin hypersensitivity is present, the recommended drug is vancomycin. Cortical abscesses that occur in the abnormal urinary tract are associated with more typical gram-negative pathogens and should be treated empirically with intravenous third-generation cephalosporins, antipseudomonal penicillins, or aminoglycosides until specific therapy can be instituted. Patients should have serial examinations with ultrasonography or CT until the abscess resolves. A clinical course contrary to this should lead to the suspicion of misdiagnosis or an uncontrolled infection with the development of perinephric abscess or infection with an organism resistant to the antimicrobial agents used in therapy.
Abscesses 3 to 5 cm in diameter and smaller abscesses in immunocompromised hosts or those that do not respond to antimicrobial therapy should be drained percutaneously (Fernandez et al, 1985; Fowler and Perkins, 1994; Siegel et al, 1996). Surgical drainage, however, currently remains the procedure of choice for most renal abscesses greater than 5 cm in diameter.
Infected Hydronephrosis and Pyonephrosis
Infected hydronephrosis is bacterial infection in a hydronephrotic kidney. The term pyonephrosis refers to infected hydronephrosis associated with suppurative destruction of the parenchyma of the kidney, in which there is total or nearly total loss of renal function (Fig. 10–28). Where infected hydronephrosis ends and pyonephrosis begins is difficult to determine clinically. Rapid diagnosis and treatment of pyonephrosis are essential to avoid permanent loss of renal function and to prevent sepsis.
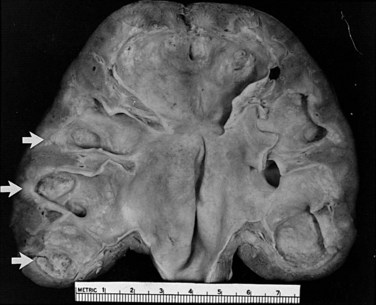
Figure 10–28 Pyonephrosis: gross specimen. The kidney shows marked thinning of the renal cortex and medulla, suppurative destruction of the parenchyma (arrows), and distention of the pelvis and calyces. Previous incision released a large quantity of purulent material. The ureter showed obstruction distal to the point of section.
Clinical Presentation
The patient is usually very ill, with high fever, chills, flank pain, and tenderness. Occasionally, however, a patient may have only an elevated temperature and a complaint of vague gastrointestinal discomfort. A previous history of urinary tract calculi, infection, or surgery is common. Bacteriuria may not be present if the ureter is completely obstructed.
Radiologic Findings
The ultrasonographic diagnosis of infected hydronephrosis depends on demonstration of internal echoes within the dependent portion of a dilated pyelocalyceal system. CT is nonspecific but may show thickening of the renal pelvis, stranding of the perirenal fat, and a striated nephrogram. Ultrasonography demonstrates hydronephrosis and fluid debris levels within the dilated collecting system (Corriere and Sandler, 1982) (Fig. 10–29A). The diagnosis of pyonephrosis is suggested if focal areas of decreased echogenicity are seen within the hydronephrotic parenchyma.
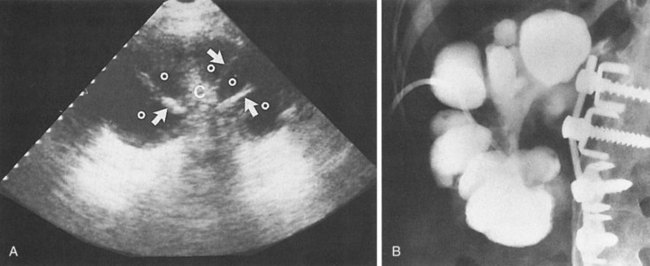
Figure 10–29 Pyonephrosis. A, Longitudinal ultrasound image of the right kidney demonstrates echogenic central collecting complex (C) with radiating echogenic septa (arrows) and thinned hypoechoic parenchyma. Multiple dilated calyces (o) with diffuse low-level echoes are seen. B, Antegrade pyelogram performed through a percutaneous nephrostomy catheter correlates well with the ultrasound image. Dilated pus-filled calyces are demonstrated. The renal pelvis is obliterated by chronic scarring and stone disease. The kidney did not regain function.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
Management
Once the diagnosis of pyonephrosis is made, treatment is initiated with appropriate antimicrobial drugs and drainage of the infected pelvis. A ureteral catheter can be passed to drain the kidney, but if the obstruction prevents this, a percutaneous nephrostomy tube should be placed (Camunez et al, 1989) (see Fig. 10–29B). When the patient becomes hemodynamically stable, other procedures are usually needed to identify and treat the source of the obstruction.
Perinephric Abscess
Perinephric abscess usually results from rupture of an acute cortical abscess into the perinephric space or from hematogenous seeding from sites of infection. Patients with pyonephrosis, particularly when a calculus is present in the kidney, are susceptible to perinephric abscess formation. Diabetes mellitus is present in approximately one third of patients with perinephric abscess (Thorley et al, 1974; Edelstein and McCabe, 1988). In about one third of the cases, perinephric abscess is caused by hematogenous spread, usually from sites of skin infection. A perirenal hematoma can become secondarily infected by the hematogenous route or by direct extension of a primary renal infection. When a perinephric infection ruptures through the Gerota fascia into the pararenal space, the abscess becomes paranephric. Paranephric abscesses may also result from infectious disorders of the bowel, pancreas, or pleural cavity. Conversely, perinephric or psoas abscess may be the result of bowel perforation, Crohn disease, or spread of osteomyelitis from the thoracolumbar spine. E. coli, Proteus, and S. aureus account for most infections.
Clinical Presentation
The onset of symptoms is typically insidious. Symptoms are present for more than 5 days in most patients with perinephric abscess compared with only about 10% of patients with pyelonephritis. The clinical presentation may be similar to that of pyelonephritis; however, more than one third of patients may be afebrile. An abdominal or flank mass can be felt in about half of the cases. Psoas abscess should be suspected if the patient has a limp and flexion and external rotation of the ipsilateral hip. Laboratory features include leukocytosis, elevated levels of serum creatinine, and pyuria in more than 75% of cases. Edelstein and McCabe (1988) showed that results of urine cultures predicted perinephric abscess isolates in only 37% of cases; a blood culture, particularly with multiple organisms, was often indicative of perinephric abscess but identified all organisms in only 42% of cases. Therefore therapy based on the results of urine and blood cultures often may be inadequate. Pyelonephritis usually responds within 4 to 5 days of appropriate antimicrobial therapy; perinephric abscess does not. Thus perinephric abscess should be suspected in a patient with UTI and abdominal or flank mass or persistent fever after 4 days of antimicrobial therapy.
CT is particularly valuable for demonstrating the primary abscess. In some cases, the abscess is confined to the perinephric space; however, extension to the flank or psoas muscle may occur (Fig. 10–30). CT is able to show with exquisite anatomic detail the route of spread of infection into the surrounding tissues (Fig. 10–31). This information may be helpful in planning the approach for surgical drainage. Ultrasonography demonstrates a diverse appearance ranging from a nearly anechoic mass displacing the kidney to an echogenic collection that tends to blend with normally echogenic fat within the Gerota fascia (Corriere and Sandler, 1982). Occasionally a retroperitoneal or subdiaphragmatic infection may spread to the paranephric fat that is outside this fascia. The clinical symptoms of insidious onset of fever, flank mass, and tenderness are indistinguishable from those associated with perinephric abscess. UTI, however, is absent. Ultrasonography and CT can usually delineate the abscess outside the Gerota fascia.
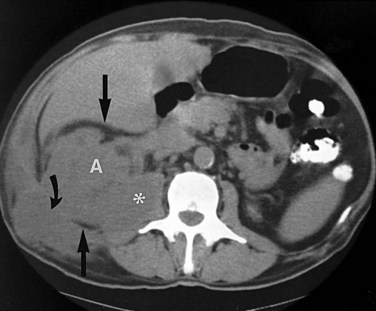
Figure 10–30 Nonenhanced CT scan through the lower pole of the right kidney (previous left nephrectomy) shows extensive perinephric abscess. Extensive abscess (A) distorts and enlarges the renal contour, infiltrates perinephric fat (straight arrows), and also extends into the psoas muscle (asterisk) and the soft tissues of the flank (curved arrow). Also note that normal renal collecting system fat has been obliterated by the process.
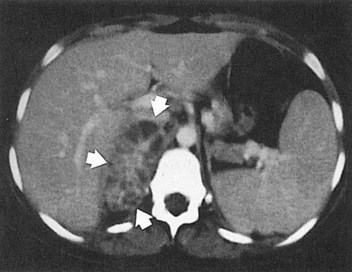
Figure 10–31 Perinephric abscess involving the right adrenal gland. CT scan shows large right pararenal mass (arrows) with multiple low-density areas within. At surgery, a large pararenal abscess with extensive involvement of the right adrenal was found.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
Management
Although antimicrobial agents are useful to control sepsis and to prevent spread of infection, the primary treatment for perinephric abscess is drainage; reports of successful treatment by antimicrobial agents alone are unusual (Herlitz et al, 1981). A detailed analysis of 52 perinephric abscess patients by Thorley and colleagues (1974) supports this tenet. In this study, half the patients were admitted to medical services and the other half to surgical services; 65% of those admitted to medical services died, whereas 23% of those admitted to surgical services died. These mortality rates reflect differences in the population of patients. Those admitted to the medical services were usually sicker and had higher temperatures, more underlying diseases, and vaguer symptoms. More importantly, none of those admitted to medical wards had an admission diagnosis of perinephric abscess, whereas 73% of those admitted to surgical wards did. Although 71% of all the patients had eventual surgical treatment of their perinephric abscesses, the diagnostic delay of those patients admitted to medical services postponed definitive treatment and consequently caused higher mortality.
Although surgical drainage, or nephrectomy if the kidney is nonfunctioning or severely infected, is the classic treatment for perinephric abscesses, renal ultrasonography and CT make percutaneous aspiration and drainage of small perirenal collections possible (Haaga and Weinstein, 1980; Elyaderani et al, 1981; Edelstein and McCabe, 1988). Haaga and Weinstein (1980), however, consider percutaneous drainage to be contraindicated in large abscess cavities filled with thick, purulent fluid.
Gram stain identifies the pathogenesis and guides antimicrobial therapy. An aminoglycoside together with an antistaphylococcal agent, such as methicillin or oxacillin, should be started immediately. If the patient has a penicillin hypersensitivity, cephalothin or vancomycin may be used.
Once the perinephric abscess has been drained, the underlying problem must be dealt with. Some conditions such as renal cortical abscess or enteric communication require prompt attention. Nephrectomy for pyonephrosis may be performed concurrent with drainage of the perinephric abscess if the patient’s condition is good. In other instances it is best to drain the perinephric abscess first and correct the underlying problem or perform a nephrectomy when the patient’s condition has improved.
Perinephric Abscess versus Acute Pyelonephritis
It has already been emphasized that the greatest obstacle to the treatment of perinephric abscess is the delay in diagnosis. In the series of Thorley and colleagues (1974) a common misdiagnosis was acute pyelonephritis. In their review they found that two factors differentiated perinephric abscess and acute pyelonephritis: (1) most patients with uncomplicated pyelonephritis were symptomatic for less than 5 days before hospitalization, whereas most with perinephric abscesses were symptomatic for longer than 5 days; and (2) no patient with acute pyelonephritis remained febrile for longer than 4 days once appropriate antimicrobial agents were started. All patients with perinephric abscesses had a fever for at least 5 days, with a median of 7 days. Similar results were noted by Fowler and Perkins (1994).
Patients with polycystic renal disease who undergo hemodialysis may be particularly susceptible to the progression from acute UTIs to perinephric abscess. Of 445 patients undergoing chronic hemodialysis at the Regional Kidney Disease Program in Minneapolis, 5.4% had polycystic kidney disease and 33.3% of these patients developed symptomatic UTIs (Sweet and Keane, 1979). Eight (62.5%) developed perinephric abscesses, and 3 of these patients died. According to the investigators, all UTIs, even those that progressed to perinephric abscesses, were promptly treated with appropriate antimicrobial agents, and all patients in this group became afebrile and asymptomatic when the agents were stopped. Yet later, after various times, symptoms attributable to perinephric abscess developed in 8 of the patients. The mechanism of this process is not clear, but the limited bioavailability of some antimicrobial agents in cysts is variable and could contribute to the progression of renal infection.
Chronic Pyelonephritis
In patients without underlying renal or urinary tract disease, chronic pyelonephritis secondary to UTI is a rare disease and an even more rare cause of chronic renal failure. In patients with underlying functional or structural urinary tract abnormalities, however, chronic renal infection can cause significant renal impairment. Thus it is essential that appropriate studies be used to diagnose, localize, and treat chronic renal infection.
The prevalence of chronic pyelonephritis has also been assessed in patients undergoing dialysis for end-stage renal disease. Despite a 2% to 5% prevalence of bacteriuria in women, pyelonephritis uncomplicated by obstruction or urinary tract malformation does not cause end-stage renal disease. Schechter and colleagues (1971) analyzed the cause for renal failure in 170 patients referred to them for dialysis. Chronic pyelonephritis was the primary cause of end-stage renal disease in 22 (13%) but was usually associated with an underlying structural defect. Unequivocal nonobstructive chronic pyelonephritis was not found. The authors also observed that symptomatic infections tended to occur before the onset of azotemia in most patients with chronic pyelonephritis. Similarly, Huland and Busch (1982) evaluated 161 patients with end-stage renal disease and found that 42 had chronic pyelonephritis. However, in addition to a history of UTIs, these 42 patients had complicating defects, such as vesicoureteral reflux, analgesic abuse, nephrolithiasis, or obstruction. Nonobstructive uncomplicated UTI alone was never found to be the cause of renal insufficiency. Thus, using end-stage renal disease seen at autopsy or at the dialysis clinic as an indicator, the prevalence of uncomplicated chronic bacterial pyelonephritis is rare.
In addition, the role of bacterial infection in development of chronic renal disease can be assessed in patients with renal interstitial and tubular damage similar to that which has classically been called chronic pyelonephritis. The frequency with which various potential causes of interstitial damage are operative in patients with interstitial nephritis was assessed by Murray and Goldberg (1975). These investigators not only concluded that UTI is rarely the sole cause of chronic renal disease in the adult but also observed that 89% of their azotemic patients had a readily identifiable primary cause of their interstitial nephritis. Thus, when patients with a clinical diagnosis of chronic interstitial nephritis are selected as the starting point, it is easy to associate many factors with this disease but UTI does not seem to be one of them.
Clinical Presentation
There are no symptoms of chronic pyelonephritis until it produces renal insufficiency, and then the symptoms are similar to those of any other form of chronic renal failure. If a patient’s chronic pyelonephritis is thought to be an end result of many episodes of acute pyelonephritis, a history of intermittent symptoms of fever, flank pain, and dysuria may be elicited. Similarly, urinary findings and the presence of renal infection correlate poorly. Bacteriuria and pyuria, the hallmarks of UTI, are not predictive of renal infection. Conversely, patients with significant renal infection may have sterile urine if the ureter draining the kidney is obstructed or the infection is outside the collecting system.
The pathologic and radiologic criteria for diagnosing renal infection may also be misleading. Asscher (1980) has tabulated eight long-term follow-up studies from the literature on kidneys of adults with UTIs. The data from these reports on 901 patients show that bacteriuria present in otherwise healthy adults for long periods may be associated with nonexistent or extremely minimal evidence of kidney damage. Conversely, patients who have chronic pyelonephritis may have negative urine cultures.
Radiologic Findings
The diagnosis of chronic pyelonephritis can be made with the greatest confidence on the basis of pyelographic findings. The essential features are asymmetry and irregularity of the kidney outlines, blunting and dilation of one or more calyces, and cortical scars at the corresponding site (Fig. 10–32). In the absence of stones, obstruction, and tuberculosis, and with the single exception of analgesic nephritis with papillary necrosis (which can be readily excluded by history), chronic pyelonephritis is virtually the only disease that produces a localized scar over a deformed calyx (Stamey, 1980). In advanced pyelonephritis, calyceal distortion and irregularity together with cortical scars complete the picture. Hodson (1965a) pointed out that renal infarction, an extremely rare condition, may closely resemble pyelonephritic scars but that the renal pyramid remains with renal infarction in contradistinction to pyelonephritis.
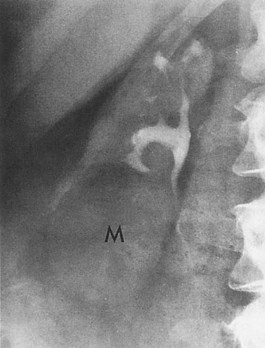
Figure 10–32 Chronic pyelonephritis. Ten-minute excretory urogram demonstrates irregular renal outline with upper pole parenchymal atrophy. Note significant loss of renal cortical thickness over blunted and dilated calyces. Lower pole mass (M) is a simple cyst.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
Pathology
In chronic pyelonephritis, the gross kidney is often diffusely contracted, scarred, and pitted. The scars are Y-shaped, flat, broad-based depressions with red-brown granular bases. The scarring is often polar with underlying calyceal blunting. The parenchyma is thin, and the corticomedullary demarcation is lost. Histologic changes are patchy. There is usually an interstitial infiltrate of lymphocytes, plasma cells, and occasional polymorphonuclear cells. Portions of the parenchyma may be replaced by fibrosis, and, although glomeruli may be preserved, periglomerular fibrosis is often seen. In some affected areas, glomeruli may be completely fibrosed and tubules atrophied. Leukocyte and hyaline casts are sometimes present in the tubules; the latter may cause resemblance to the thyroid colloid, hence the description renal thyroidization (Braude, 1973). In general, the changes are nonspecific; they also may be seen in toxic exposures, postobstructive atrophy, hematologic disorders, postirradiation nephritis, ischemic renal disease, and nephrosclerosis.
Management
Management of radiographic evidence of pyelonephritis should be directed at treating infection, if present; preventing future infections; and monitoring and preserving renal function. The treatment of existing infection must be based on careful antimicrobial susceptibility tests and selection of drugs that can achieve bactericidal concentrations in the urine and yet are not nephrotoxic. Achievement of acceptable bactericidal levels of a drug in the urine of a patient with chronic pyelonephritis may be difficult because the diminished concentrating ability of pyelonephritis may impair excretion and concentration of the antimicrobial agent. The duration of antimicrobial therapy is often prolonged to maximize the chance of cure. With patients in whom renal damage develops or progresses in the presence of UTI, the working hypothesis should be that there is an underlying renal, usually papillary, lesion or underlying urologic condition, such as obstruction or calculus, that has increased susceptibility to renal damage. Appropriate nephrologic and urologic evaluation should be undertaken to identify and, if possible, correct these abnormalities.
Bacterial “Relapse” from a Normal Kidney
The concept that bacteria persist in the renal parenchyma between bacteriuric episodes and cause “relapsing” UTIs was based on a study by Turck and colleagues (1968) that suggested that bacterial persistence could be recognized by simply identifying two consecutive recurrent infections with the same organism. Unfortunately this study did not indicate whether the urine was cultured during therapy to ensure that the original infection had actually been eradicated. It is possible that some of these so-called relapses were in fact unresolved initial infections and that ureteral edema associated with catheterization may have impeded clearance of the initial infecting strain.
Subsequent studies summarized by Stamey (1980) and Forland and associates (1977) have shown that in a normal urinary tract recurrent infections are not caused by relapse from bacterial persistence in the kidney. With ureteral catheterization techniques, Cattell (1973) localized the site of bacteriuria in 42 patients who had follow-up for 6 months after therapy. He analyzed the response to antimicrobial therapy of 2 weeks’ duration. Of the 26 patients who were cured of their initial infection, 16 had recurrence with the same organism, 8 had upper tract infections, and 8 had bladder bacteriuria.
Most of the changes of chronic pyelonephritis seem to occur in infancy, probably because the growing kidney is most susceptible to scarring. In a review that examined the long-term effect of UTIs in adults it was concluded that renal damage is rare in nonobstructive UTIs (Stamey, 1980) but that it does occur (Bailey et al, 1969; Davies et al, 1972; Davidson and Talner, 1973; Feldberg, 1982).
The association between hypertension and the pyelonephritic kidney has been addressed by Pfau and Rosenmann (1978), who concluded that the association of chronic pyelonephritis and hypertension is usually coincidental. Their conclusion agrees with that of a study by Parker and Kunin (1973) that examined 74 women who had been admitted to the hospital 10 to 20 years previously for pyelonephritis. Only 14.5% of these women had hypertension, a rate similar to that found in a random female population of the same age.
Infectious Granulomatous Nephritis
Xanthogranulomatous Pyelonephritis
Xanthogranulomatous pyelonephritis is a rare, severe, chronic renal infection typically resulting in diffuse renal destruction. Most cases are unilateral and result in a nonfunctioning, enlarged kidney associated with obstructive uropathy secondary to nephrolithiasis. Xanthogranulomatous pyelonephritis is characterized by accumulation of lipid-laden foamy macrophages. It begins within the pelvis and calyces and subsequently extends into and destroys renal parenchymal and adjacent tissues. It has been known to imitate virtually every other inflammatory disease of the kidney, as well as renal cell carcinoma, on radiographic examination (Malek and Elder, 1978; Tolia et al, 1980). In addition, the microscopic appearance of xanthogranulomatous pyelonephritis has been confused with clear cell adenocarcinoma of the kidney on frozen section and has led to radical nephrectomy (Anhalt et al, 1971; Malek and Elder, 1978; Flynn et al, 1979; Lorentzen and Nielsen, 1980; Tolia et al, 1980). The entity is uncommon and is found in only about 0.6% (Malek et al, 1972) to 1.4% (Ghosh, 1955) of patients with renal inflammation who are evaluated pathologically.
Pathogenesis
The primary factors involved in the pathogenesis of xanthogranulomatous pyelonephritis are nephrolithiasis, obstruction, and infection (Gregg et al, 1999). Nephrolithiasis has been noted in as many as 83% of the patients in various series; approximately half of the renal stones have been of the staghorn type (Parsons et al, 1983; Chuang et al, 1992; Nataluk et al, 1995). It has been proposed clinically and demonstrated experimentally that primary obstruction followed by infection with E. coli can lead to tissue destruction and collections of lipid material by macrophages (Povysil and Konickova, 1972). These macrophages (xanthoma cells) are distributed in sheets around parenchymal abscesses and calyces and are intermixed with lymphocytes, giant cells, and plasma cells. The bacteria appear to be of low virulence because spontaneous bacteremia has rarely been described. Other possible interrelated factors include venous occlusion and hemorrhage, abnormal lipid metabolism, lymphatic blockage, failure of antimicrobial therapy in UTI, altered immunologic competence, and renal ischemia (Friedenberg and Spjut, 1963; Mering et al, 1973; Goodman et al, 1979; McDonald, 1981; Tolia et al, 1981). The concept that xanthogranulomatous pyelonephritis is related to incomplete bacterial degradation and altered host response has received mixed support (Nielsen and Lorentzen, 1981; Khalyl-Mawad et al, 1982). Thus it appears that there is probably no single factor that is instrumental in the pathogenesis of this disease. Rather, there is an inadequate host acute inflammatory response within an obstructed, ischemic, or necrotic kidney.
Pathology
The kidney is usually massively enlarged and has a normal contour. Xanthogranulomatous pyelonephritis may be diffuse, as in approximately 80% of the patients, or segmental. In the diffuse form of the disease the entire kidney is involved, whereas in segmental xanthogranulomatous pyelonephritis only the parenchyma surrounding one or more calyces or one pole of a duplicated collecting system is involved. On sectioning, the kidney usually demonstrates nephrolithiasis and peripelvic fibrosis. The calyces are dilated and filled with purulent material, but fibrosis surrounding the pelvis usually prevents dilation. The papillae are often destroyed by papillary necrosis (Goodman et al, 1979). In advanced stages of the disease, multiple parenchymal abscesses are filled with viscous pus and lined by yellowish tissue (Fig. 10–33A). The cortex is often thin and replaced by xanthogranulomatous tissue. The capsule is often thickened, and extension of the inflammatory process into the perinephric or paranephric space is common (Goodman et al, 1979; McDonald, 1981; Gregg et al, 1999).
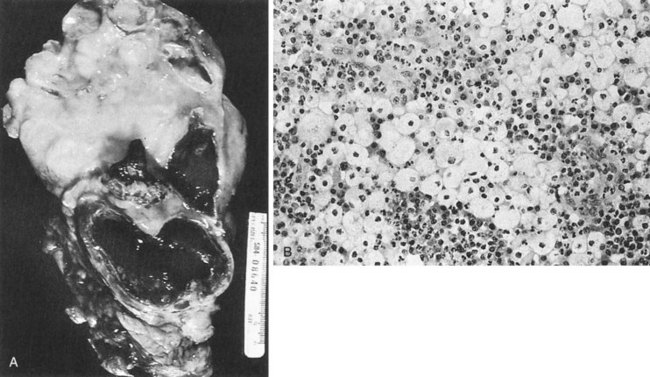
Figure 10–33 Xanthogranulomatous pyelonephritis. A, Gross specimen. Kidney is massively enlarged, measuring 23 × 12 cm; the normal architecture is replaced by a shaggy yellow upper pole mass corresponding to xanthogranulomatous inflammation and numerous distorted and dilated calyces. B, Microscopically, the shaggy yellow tissue is composed primarily of lipid-laden histiocytes mixed with other inflammatory cells.
(From Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.)
On microscopic examination the yellowish nodules that line the calyces and surround the parenchymal abscesses contain dark sheets of lipid-laden macrophages (foamy histiocytes with small, dark nuclei and clear cytoplasm) intermixed with lymphocytes, giant cells, and plasma cells (see Fig. 10–33B). Xanthogranulomatous cells are not specific to xanthogranulomatous pyelonephritis but may be present anywhere inflammation or obstruction coexists. The origin of the fatty substance is disputed. Cholesterol esters that make up a part of the lipid might be derived from lysis of erythrocytes after hemorrhage (Saeed and Fine, 1963).
Clinical Presentation
Xanthogranulomatous pyelonephritis should be suspected in patients with UTIs and a unilateral enlarged nonfunctioning or poorly functioning kidney with a stone or a mass lesion indistinguishable from malignant tumor. Most patients present with flank pain (69%), fever and chills (69%), and persistent bacteriuria (46%) (Malek and Elder, 1978). Additional vague symptoms, such as malaise, may be present. On physical examination, 62% of the patients had a flank mass and 35% had previous calculi (Malek and Elder, 1978). Less commonly, hypertension, hematuria, or hepatomegaly is the presenting complaint. The medical history is often positive for UTIs and urologic instrumentation (Malek and Elder, 1978; Flynn et al, 1979; Goodman et al, 1979; Grainger et al, 1982; Yazaki et al, 1982; Petronic et al, 1989; Eastham et al, 1994; Nataluk et al, 1995). Diabetics also appear to be at greater risk of developing the disease (Eastham et al, 1994). Although it may occur at any age, the peak incidence of xanthogranulomatous pyelonephritis is in the fifth to the seventh decade. Women are more commonly affected than men. There is no predilection for either kidney.
Bacteriology and Laboratory Diagnosis
Although review of the literature shows Proteus to be the most common organism involved with xanthogranulomatous pyelonephritis (Anhalt et al, 1971; Tolia et al, 1981), E. coli is also common. The prevalence of Proteus organisms may reflect their association with stone formation and subsequent chronic obstruction and irritation. Malek and Elder (1978), in their analysis of 26 cases, found that renal tissue cultures grew bacteria in 22 of 23 cases. Anaerobes also have been cultured (Malek and Elder, 1978).
Approximately 10% of patients have mixed cultures. About one third of patients have no growth in their urine, probably because many patients have recently taken or are taking antimicrobial agents when cultures are obtained. The infecting organism may be revealed only by tissue cultures obtained during surgery. Urinalysis usually shows pus and protein. In addition, blood tests often reveal anemia and may show hepatic dysfunction in up to 50% of the patients (Malek and Elder, 1978).
Xanthogranulomatous pyelonephritis is almost always unilateral; therefore azotemia or frank renal failure is uncommon (Goodman et al, 1979; Gregg et al, 1999).
CT is probably the most useful radiologic technique in evaluating patients with xanthogranulomatous pyelonephritis (Fig. 10–34). Fifty to 80 percent of patients show the classic triad of unilateral renal enlargement with little or no function and a large calculus in the renal pelvis (Elder, 1984). CT usually demonstrates a large, reniform mass with the renal pelvis tightly surrounding a central calcification but without pelvic dilatation (Solomon et al, 1983; Goldman et al, 1984; Hartman, 1985). Renal parenchyma is replaced by multiple water-density masses representing dilated calyces and abscess cavities filled with varied amounts of pus and debris. On enhanced scans the walls of these cavities demonstrate a prominent blush owing to the abundant vascularity within the granulation tissue. The cavities themselves, however, fail to enhance, whereas tumors and other inflammatory lesions usually do. The CT scan is particularly helpful in demonstrating the extent of renal involvement and may indicate whether adjacent organs or the abdominal wall are involved by xanthogranulomatous pyelonephritis (Eastham et al, 1994; Kaplan et al, 1997).
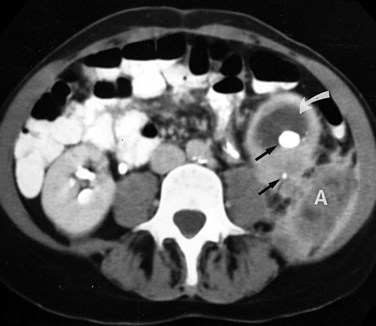
Figure 10–34 Xanthogranulomatous pyelonephritis. Enhanced CT scan shows collecting system and parenchymal calculi (straight arrows) with lower pole pyonephrosis (curved arrow) and an irregular, predominantly low-density perinephric abscess (A) extending into the soft tissues of the flank.
Ultrasonography usually demonstrates global enlargement of the kidney (Merenich and Popky, 1991). The normal renal architecture is replaced by multiple hypoechoic fluid-filled masses that correspond to debris-filled, dilated calyces or foci of parenchymal destruction (Fagerholm, 1983; Hartman et al, 1984). With focal involvement, a solid mass involving a segment of the kidney is demonstrated with an associated calculus in the collecting system or ureter. Renal cell carcinoma and other solid renal lesions must be considered in the differential diagnosis (Elder, 1984).
Radionuclide renal scanning using 99mTc-DMSA is used to confirm and quantify the differential lack of function in the involved kidney (Gregg et al, 1999). MRI has not yet superseded CT in the evaluation of renal inflammation but it provides some advantages in delineating extrarenal extension of inflammation (Soler et al, 1997). Lesions of xanthogranulomatous pyelonephritis may appear as cystic foci of intermediate signal intensity on T1-weighted images and of hyperintensity on T2-weighted images. Arteriography shows hypervascular areas, but there may be some hypovascular areas (Malek and Elder, 1978; Van Kirk et al, 1980; Tolia et al, 1981). Therefore radiologic studies, although distinctive, often cannot be used to differentiate between xanthogranulomatous pyelonephritis and renal cell carcinoma.
Differential Diagnosis
Diagnosis of segmental xanthogranulomatous pyelonephritis without calculi may be difficult. Xanthogranulomatous pyelonephritis in association with massive pelvic dilation cannot be distinguished from pyonephrosis. When xanthogranulomatous pyelonephritis occurs within a small contracted kidney, the radiographic findings are nonspecific and nondiagnostic. Renal parenchymal malacoplakia may show renal enlargement and multiple inflammatory masses replacing the normal renal parenchyma, but calculi are usually not present. Renal lymphoma may be associated with multiple hypoechoic masses surrounding the contracted, nondilated pelvis, but lymphoma is usually clinically obvious, and renal involvement is usually bilateral and not associated with calculi (Hartman, 1985).
Management
The primary obstacle to the correct treatment of xanthogranulomatous pyelonephritis is incorrect diagnosis. Today, with CT technology, the diagnosis of xanthogranulomatous pyelonephritis is made preoperatively nearly 90% of the time (Eastham et al, 1994; Nataluk et al, 1995). Antimicrobial therapy may be necessary to stabilize the patient preoperatively, and, occasionally, long-term antimicrobial therapy will eradicate the infection and restore renal function (Mollier et al, 1995). Because the renal abnormality may be diagnosed preoperatively as a renal tumor and/or is diffuse, nephrectomy is usually performed. If localized xanthogranulomatous pyelonephritis is diagnosed preoperatively or at exploration, it is amenable to partial nephrectomy (Malek and Elder, 1978; Tolia et al, 1980; Osca et al, 1997).
The lipid-laden macrophages associated with xanthogranulomatous pyelonephritis, however, closely resemble clear cell adenocarcinoma and may be difficult to distinguish solely on the basis of frozen section. Furthermore, xanthogranulomatous pyelonephritis has been associated with renal cell carcinoma, papillary transitional cell carcinoma of the pelvis or bladder, and infiltrating squamous cell carcinoma of the pelvis (Schoborg et al, 1980; Pitts et al, 1981; Tolia et al, 1981); thus if malignant renal tumor cannot be excluded, nephrectomy should be performed. When diffuse and extensive disease into the retroperitoneum exists, removal of the kidney and perinephric fat may be needed. Under these circumstances the surgery may be difficult and may involve dissection of granulomatous tissue from the diaphragm, great vessels, and bowel (Malek and Elder, 1978; Flynn et al, 1979). It is important to remove the entire inflammatory mass because in nearly three fourths of patients xanthogranulomatous tissue is infected. If incision and drainage alone are performed rather than nephrectomy the patient may continue to suffer from protracted debilitating illness and may develop a renal cutaneous fistula; an even more difficult nephrectomy will then be necessary. In one early case-matched series of laparoscopic nephrectomies performed for xanthogranulomatous pyelonephritis it was concluded that the benefits of laparoscopic surgery do not extend to the treatment of this disease (Bercowsky et al, 1999); however, a larger review of a modern xanthogranulomatous pyelonephritis experience suggests that laparoscopic nephrectomy is a reasonable treatment approach.
Malacoplakia
Malacoplakia, from the Greek word meaning “soft plaque,” is an unusual inflammatory disease that was originally described to affect the bladder but has been found to affect the genitourinary and gastrointestinal tracts, skin, lungs, bones, and mesenteric lymph nodes. It is an inflammatory lesion described originally by Michaelis and Gutmann (1902). It was characterized by von Hansemann (1903) as soft, yellow-brown plaques with granulomatous lesions in which the histiocytes contain distinct basophilic lysosomal inclusion bodies or Michaelis-Gutmann bodies. Although its exact pathogenesis is unknown, malacoplakia probably results from abnormal macrophage function in response to a bacterial infection, which is most often due to E. coli.
Pathogenesis
The pathogenesis is unknown, but several theories are popular. In 93 patients who had cultures of urine, diseased tissue, or blood, 89.4% had coliform infections (Stanton and Maxted, 1981). Moreover, 40% of the patients in this review had an immunodeficiency syndrome, autoimmune disease, carcinoma, or another systemic disorder. This association of coliform infections and compromised health status in patients with malacoplakia is well recognized.
It is hypothesized that bacteria or bacterial fragments form the nidus for the calcium phosphate crystals that laminate the Michaelis-Gutmann bodies. Most investigations into the pathogenesis of this disease support theories that a defect in intraphagosomal bacterial digestion accounts for the unusual immunologic response that causes malacoplakia.
Pathology
The diagnosis is made by biopsy. The lesion is characterized by large histiocytes, known as von Hansemann cells, and small basophilic, extracytoplasmic, or intracytoplasmic calculospherules called Michaelis-Gutmann bodies, which are pathognomonic (Fig. 10–35). Electron microscopy has revealed intact coliform bacteria and bacterial fragments within phagolysosomes of the foamy-appearing malacoplakic histiocytes (Lewin et al, 1976; Stanton and Maxted, 1981). In their review of the subject, Stanton and Maxted (1981) and Esparza and associates (1989) emphasized that, although pathognomonic for the disease, Michaelis-Gutmann bodies may be absent in early malacoplakia and are not necessary for the diagnosis.
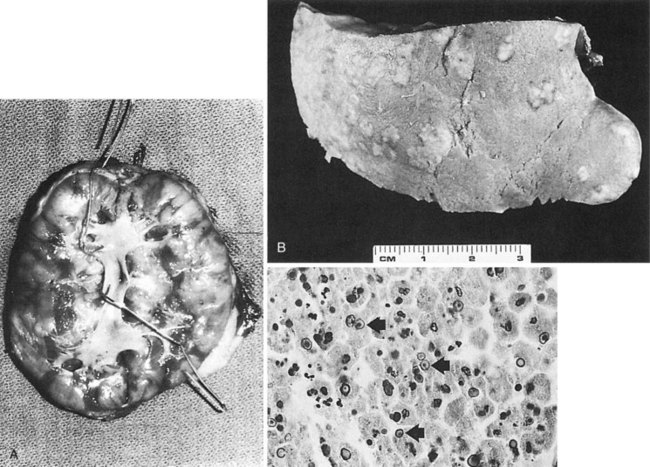
Figure 10–35 Renal parenchymal malacoplakia. A, Cut surface demonstrates extensive cortical and upper medullary replacement by multifocal, confluent, tumor-like masses. B, Cortical surface exhibits multiple, firm, plaquelike lesions. C, Hallmark of malacoplakia is demonstration of the Michaelis-Gutmann body (arrows), which represents incompletely destroyed bacteria surrounded by lipoprotein membrane (hematoxylin-eosin stain).
(From Hartman DS. Radiologic pathologic correlation of the infectious granulomatous diseases of the kidney: I and II. Monogr Urol 1985;6:3.)
It has been shown that macrophages in malacoplakia involving the kidney and bladder contain large amounts of immunoreactive α1-antitrypsin (Callea et al, 1982). The amount of α1-antitrypsin remains unchanged during the morphogenetic stages of the pathologic process. Macrophages from other pathologic processes, closely resembling malacoplakia but without Michaelis-Gutmann bodies, do not contain α1-antitrypsin except for a few macrophages in tuberculosis and xanthogranulomatous pyelonephritis. Therefore immunohistochemical staining for α1-antitrypsin may be a useful test for an early and accurate differential diagnosis of malacoplakia.
Clinical Presentation
Most patients are older than 50 years. The ratio of females to males with malacoplakia within the urinary tract is 4:1, but this disparity does not occur in other body tissues (Stanton and Maxted, 1981). The patients often are debilitated, are immunosuppressed, and have other chronic diseases. The symptoms of bladder malacoplakia are bladder irritability and hematuria. Cystoscopy reveals mucosal plaques or nodules. As these lesions progress they may become fungating, firm, sessile masses that cause filling defects of the bladder, ureter, or pelvis on excretory urograms. The distal ureter may become strictured or stenotic and cause subsequent renal obstruction or nonfunction (Sexton et al, 1982). A typical patient with renal parenchymal disease may have one or more radiographic masses and chronic E. coli infections. Renal parenchymal malacoplakia may be complicated by renal vein thrombosis and inferior vena cava thrombosis (McClure, 1983). When malacoplakia involves the testis, epididymo-orchitis is present. Malacoplakia of the prostate is rare, but, when it occurs, it may be confused with carcinoma clinically (Shimizu et al, 1981). Mortality can exceed 50%, and the morbidity can be substantial (Stanton and Maxted, 1981).
Radiologic Findings
Multifocal malacoplakia on excretory urography typically presents as enlarged kidneys with multiple filling defects. Renal calcification, lithiasis, and hydronephrosis are absent. The multifocal nature is best appreciated by using ultrasonography, CT, or arteriography. Ultrasound examination may demonstrate renal enlargement and distortion of the central echo complex. The masses are often confluent, resulting in an overall increase in the echogenicity of the renal parenchyma (Hartman et al, 1980). On CT the foci of malacoplakia are less dense than the surrounding enhanced parenchyma (Hartman, 1985). Arteriography typically reveals a hypovascular mass without peripheral neovascularity (Cavins and Goldstein, 1977; Trillo et al, 1977).
Unifocal malacoplakia on excretory urography appears as a noncalcified mass that is indistinguishable from other inflammatory or neoplastic lesions. Ultrasonography and CT may demonstrate a solid or cystic structure, depending on the degree of internal necrosis. Angiography may demonstrate neovascularity (Trillo et al, 1977). Extension beyond the kidney, which can occur with either multifocal or uniform malacoplakia, is best demonstrated by CT.
Differential Diagnosis
The differential diagnosis includes renal cystic disease, neoplasia, and renal inflammatory disease (Hartman, 1985). Malacoplakia should be considered when one or more renal masses are observed, particularly in female patients with recurrent UTIs with E. coli, altered immune response syndromes, or cystoscopic evidence of malacoplakia or filling defects in the collecting system (Charboneau, 1980). Malacoplakia should also be suspected when these radiographic findings occur in a renal transplant patient who has persistent UTI despite appropriate antimicrobial therapy. Cystic disease generally can be excluded by careful ultrasound and CT evaluations. Renal involvement with metastatic disease or lymphomas usually occurs late in the course of the disease, which is well established. Multifocal renal cell carcinoma is most often seen in the context of von Hippel-Lindau disease with its other clinical manifestations. Patients with xanthogranulomatous pyelonephritis usually have signs and symptoms of UTI. As with malacoplakia, the involved kidney is enlarged but renal calculi and obstruction are common. Multiple renal abscesses are often associated with hematogenous dissemination resulting from cardiac disease.
Management
Management of malacoplakia should be directed at control of the UTIs, which should stabilize the disease process. This subject is well reviewed by Stanton and Maxted (1981). Although multiple long-term antimicrobial agents, including many antituberculosis agents, have been used, the sulfonamides, rifampin, doxycycline, and TMP are thought to be especially useful because of their intracellular bactericidal activity (Maderazo et al, 1979). Fluoroquinolones are taken up by macrophages directly and have also proven effective in the management of malacoplakia (Vallorosi et al, 1999). Other investigators have used ascorbic acid and cholinergic agents such as bethanechol in conjunction with antimicrobial therapy and have reported good results (Abdou et al, 1977; Zornow et al, 1979; Stanton et al, 1983). Both agents are thought to increase intracellular cyclic guanosine monophosphate levels, which have been postulated as the biologic defect causing macrophage dysfunction. Surgical intervention, however, may be necessary if the disease progresses in spite of antimicrobial treatment. Nephrectomy is usually performed for the treatment of symptomatic unilateral renal lesions.
The long-term prognosis appears to be related to the extent of the disease. When parenchymal renal malacoplakia is bilateral or occurs in the transplanted kidney, death usually occurs within 6 months (Bowers and Cathey, 1971; Deridder et al, 1977). Patients with unilateral disease usually have a long-term survival after nephrectomy.
Renal Echinococcosis
Echinococcosis is a parasitic infection caused by the larval stage of the tapeworm Echinococcus granulosus. The disease is prevalent in dogs, sheep, cattle, and humans in South Africa, Australia, New Zealand, Mediterranean countries (especially Greece), and some parts of the former Soviet Union. In the United States the disease is rare, but it is found in immigrants from Eastern Europe or other foreign endemic areas or as an indigenous infection among American Indians in the Southwest and in Eskimos (Plorde, 1977).
Pathogenesis and Pathology
Echinococcosis is produced by the larval form of the tapeworm, which in its adult form resides in the intestine of the dog, the definitive host. The adult worm is 3 to 9 mm long. The ova in the feces of the dog contaminate grass and farmlands and are ingested by sheep, pigs, or humans, the intermediate hosts. Larvae hatch, penetrate venules in the wall of the duodenum, and are carried by the bloodstream to the liver. Those larvae that escape the liver are next filtered by the lungs. Approximately 3% of the organisms that escape entrapment in the liver and lungs may then enter the systemic circulation and infect the kidneys. The larvae undergo vesiculation, and the resultant hydatid cyst gradually develops at a rate of about 1 cm/yr. Thus the cyst may take 5 to 10 years to reach pathologic size.
Echinococcosis cysts of the kidney are usually single and located in the cortex (Nabizadeh et al, 1983). The wall of the hydatid cyst has three zones: a peripheral zone of fibroblasts derived from tissues of the host becomes the adventitia and may calcify; an intermediate laminated layer becomes hyalinized; and a single inner layer is composed of nucleated epithelium and is called the germinal layer. The germinal layer gives rise to brood capsules that increase in number, become vacuolated, and remain attached to the germinal membrane by a pedicle. New larvae (scoleces) develop in large numbers from the germinal layer within the brood capsule (Fig. 10–36). The hydatid cyst is also filled with fluid. When brood capsules detach, they enlarge and move freely in the fluid and are then called daughter cysts. Hydatid sand is composed of free larvae and daughter cysts.
Figure 10–36 Echinococcosis. A, Gross specimen. A cystic mass measuring 7 × 11 cm in the lower pole. Smaller daughter cysts are identified within the larger cystic mass. B, Gross specimen. Daughter cysts represent brood capsules that have detached and move freely. C, Photomicrograph. Brood capsules (B) arising from the germinal layer (G) contain viable and degenerating scoleces (S).
(From Hartman DS. Radiologic pathologic correlation of the infectious granulomatous diseases of the kidney: III and IV. Monogr Urol 1985;6:26.)
Clinical Presentation
The symptoms of echinococcosis are those of a slowly growing tumor. Most patients are asymptomatic or have a flank mass, dull pain, or hematuria (Gilsanz et al, 1980; Nabizadeh et al, 1983). Because the cyst is focal, it rarely affects renal function. Rarely, the cyst ruptures into the collecting system, and the patient may experience severe colic and passage of debris resembling grape skins in the urine (hydatiduria). The cyst may also rupture into an adjacent viscus or the peritoneal cavity. The fluid is extremely antigenic (Hartman, 1985).
Laboratory Diagnosis
If cyst rupture occurs, the definitive diagnosis can be established by identifying daughter cysts in the urine or by identifying the laminated wall of the cyst (Sparks et al, 1976). Fewer than half of the patients have eosinophilia. The most reliable diagnostic test uses partially purified hydatid arc 5 antigens in a double-diffusion test (Coltorti and Varela-Diaz, 1978). Complement fixation, HA, and the Casoni intradermal skin tests are less reliable but, when combined, are positive in about 90% of patients (Sparks et al, 1976).
Radiologic Findings
Excretory urography typically shows a thick-walled cystic mass, occasionally calcified (Buckley et al, 1985). If the cyst ruptures into the collecting system, daughter cysts may be outlined in the pelvis as an irregular mass or as multiple solitary lesions (Gilsanz et al, 1980). Occasionally, direct filling of the cyst with contrast medium occurs.
Ultrasonography and CT are useful in characterizing the mass. Ultrasonography usually demonstrates a multicystic or multiloculated mass. A sudden change in position may demonstrate bright falling echoes corresponding to hydatid sand, which can be observed during real-time evaluation of hydatid cysts (Saint Martin and Chiesa, 1984).
On CT several patterns of renal echinococcosis may be recognized. The most specific is a cystic mass with discrete, round daughter cysts and a well-defined enhancing membrane (Martorana et al, 1981). The less-specific pattern is that of a thick-walled multiloculated cystic mass (Gilsanz et al, 1980). The presence of daughter cysts within the mother cyst differentiates the lesion from a simple renal cyst and from renal abscesses, infected cysts, and necrotic neoplasm.
Both CT and ultrasonography are useful in evaluating the liver. Angiography is seldom required. Diagnostic aspiration should not be performed because of the danger of rupture and spillage of the highly antigenic cyst contents and risk of fatal anaphylaxis. Nevertheless, Baijal and coworkers (1995) described a percutaneous management of renal hydatidosis as a minimally invasive diagnostic and therapeutic option.
Management
The prognosis of echinococcosis is good but depends on the site and size of the cysts. Medical treatment with benzimidazole compounds such as mebendazole or albendazole has shown limited success with significant side effects (Nabizadeh et al, 1983).
Surgery remains the mainstay of treatment of renal echinococcosis (Poulios, 1991). The cyst should be removed without rupture to reduce the chance of seeding and recurrence. If the cyst wall is calcified, the larvae are probably dead and the risk of seeding is low, although a daughter cyst may be viable. If the cyst ruptures or cannot be removed and marsupialization is required, then the contents of the cyst initially should be aspirated and filled with a scolicidal agent such as 30% sodium chloride, 0.5% silver nitrate, 2% formalin, or 1% iodine for approximately 5 minutes to kill the germinal portion (Sparks et al, 1976; Nabizadeh et al, 1983; Shetty et al, 1992).
Key Points
Kidney Infections
Bacteremia, Sepsis, and Septic Shock
Sepsis is a clinical syndrome characterized by extremes of body temperature, heart rate, respiratory rate, and WBC count that occur in response to an infection. A typical host response to infection involves localized containment and elimination of bacteria and repair of damaged tissue. This process is facilitated by macrophages and dendritic cells and orchestrated by CD4+ T helper cells via the release of both proinflammatory and anti-inflammatory molecules (cytokines, chemokines, interferons). Sepsis occurs when a local infectious process becomes an uncontrolled systemic blood-borne inflammatory response resulting in damage to tissues or organs remote from the initial site of infection or injury.
Definitions
Table 10–24 Characteristics of Sepsis
| General |
| Inflammatory |
| Organ Dysfunction |
| Tissue Perfusion |
INR, international normalized ratio; aPTT, activated partial thromboplastin time.
From Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–6.
Pathophysiology
Initial studies of pathophysiologic features of septic shock concentrated on the interactions of lipopolysaccharides (LPS) from the gram-negative bacterial cell wall with various innate immune system pathways. More recent investigations now focus on understanding the activation and regulation of both the innate and acquired immune systems and the array of cytokines that are released during localized and systemic inflammatory responses.
Bacterial Cell Wall Components in Septic Shock
The exotoxins produced by some bacteria (e.g., exotoxin A produced by P. aeruginosa) can initiate septic shock. However, the bacteria themselves, and in particular their cell wall components, are primarily responsible for the development of septic shock. These components activate numerous innate immunologic pathways, including macrophages, neutrophils, and dendritic cells and the complement system. The prime initiator of gram-negative bacterial septic shock is endotoxin, an LPS component of the bacterial outer membrane. Endotoxin can directly activate the coagulation, complement, and fibrinolytic systems, leading to the release of small molecules that cause vasodilation and increased endothelial permeability (Tapper and Herwald, 2000).
Cytokine Network
Monocytic cells appear to have a pivotal role in mediation of the biologic effects of SIRS and septic shock. Monocytes can remove and detoxify LPS and be beneficial to the host. However, LPS-stimulated monocytes produce cytokines such as tumor necrosis factor (TNF) and interleukin (IL)-1. The intravascular activation of inflammatory systems involved in septic shock is mainly the consequence of an overproduction of these and other cytokines. Production of these cytokines is modulated by CD4+ T helper cells. Type 1 CD4+ T helper cells release proinflammatory cytokines including TNF-α, interferon-γ, and IL-2. These cytokines are also produced by macrophages, endothelial cells, and other cells stimulated by microbial products. The systemic release of large amounts of the cytokine TNF is associated with death from septic shock in humans (Waage et al, 1987; Calandra et al, 1988; Girardin et al, 1988). However, despite the fact that TNF is classically regarded as a central mediator of pathophysiologic changes associated with sepsis, the role of attenuation of this and other proinflammatory cytokines remains unclear. For example, in one animal model of peritonitis, survival was worsened by the administration of antibodies blocking TNF (Eskandari et al, 1992). Also, patients suffering from rheumatoid arthritis treated with TNF-α agents remain susceptible to the development of septic shock. Lastly, a meta-analysis of clinical trials utilizing anti-inflammatory agents in sepsis suggested these agents were generally harmful in all but a small subset of patients (Hotchkiss and Karl, 2003). More recently, anti-inflammatory cytokines, including IL-4 and IL-10, released by type II CD4+ T helper cells, have also been noted to be elevated in sepsis, further illustrating the complex regulation of the both proinflammatory and anti-inflammatory cytokines in a septic patient. In summary, both proinflammatory and anti-inflammatory cytokines are elements of early sepsis; however, the role of cytokine modulation in the treatment of sepsis remains unclear.
Clinical Presentation and Diagnosis
Early signs of the systemic inflammatory response syndrome include temperature extremes (>38° C [100.4° F] or <36° C [96.8° F]), tachycardia (heart rate >90 beats per minute), tachypnea, and altered mental status. The classic bedside findings differentiating septic shock from other types of shock include a warm patient, brisk capillary refill, and a bounding pulse reflecting pyrexia, peripheral vasodilation, and decreased systemic vascular resistance. Other diagnostic criteria include evidence of organ dysfunction such as hypotension, oliguria, or ileus and laboratory abnormalities including leukocytosis or leukopenia, hyperbilirubinemia, hyperlactatemia, hyperglycemia, coagulation abnormalities, and elevated C-reactive protein and procalcitonin (see Table 10–24 for complete list of Consensus Conference criteria). The classic clinical presentation of fever and chills followed by hypotension is manifest only in about 30% of patients with gram-negative bacteremia (McClure, 1983). Even before temperature extremes and the onset of chills, bacteremic patients often begin to hyperventilate. Thus the earliest metabolic change in septicemia is a resultant respiratory alkalosis. In critically ill patients, the sudden onset of hyperventilation should lead to blood drawing for culture and careful evaluation of the patient. Changes in mental status can also be important clinical clues. Although the most common pattern is lethargy or obtundation, an occasional patient may become excited, agitated, or combative. Cutaneous manifestations such as the bull’s-eye lesion associated with P. aeruginosa may be identified.
Metastatic infections secondary to genitourinary tract bacteremia have been described (Siroky et al, 1976). In this review of 137 patients who developed metastatic infections from bacteremia with a genitourinary source, 79% had undergone prior urologic instrumentation, 59% developed skeletal infections, mainly of the spine; and 29% developed endocarditis, most commonly caused by E. faecalis.
Bacteriology
In classic studies of sepsis syndrome and septic shock, gram-negative bacteria were predominant organisms isolated in 30% to 80% of cases and gram-positive bacteria in 5% to 24% (Ispahani et al, 1987; Calandra et al, 1988; Bone, 1991). Although E. coli is the most common organism causing gram-negative bacteremia, many nosocomial catheter-associated infections are caused by highly resistant gram-negative organisms: P. aeruginosa, Proteus, Providencia, and Serratia. Acinetobacter and Enterobacter are also emerging as important nosocomial pathogens. In a large series, E. coli caused about one third of the cases; the Klebsiella-Enterobacter-Serratia family, approximately 20%; and Pseudomonas, Proteus, Providencia, and anaerobic species, approximately 10% each (Kreger et al, 1980). Anaerobic organisms may cause bacteremia when the source is a postsurgical intra-abdominal abscess or transrectal prostatic biopsy. More recent studies suggest the incidence of sepsis caused by both gram-positive bacterial and fungal organisms is increasing (Martin et al, 2003) and reinforce the need for initial broad-spectrum antimicrobial coverage.
Management
The principles of management of sepsis include resuscitation, supportive care, monitoring, administration of broad-spectrum antimicrobial agents, and drainage or elimination of infection (Dellinger et al, 2004; Sessler et al, 2004). Although the identification and early intervention of sepsis by the urologist is important, the use of expert consultants is also recommended because management of sepsis and the critically ill patient is complex and always evolving.
Principles of resuscitation include support of the airway and breathing and optimization of perfusion. Intubation and mechanical ventilation may be required in patients who are obtunded and are unable to protect their airway. Supplemental oxygen may be instituted, but supranormal oxygen delivery is no longer considered a goal of therapy (Dellinger et al, 2004). Tissue perfusion should first be optimized with fluid resuscitation to restore mean circulating filling pressures. If additional blood pressure support is needed, vasoactive agents including phenylephrine, norepinephrine, and dopamine can be instituted; however, low-dose dopamine administration for renal protection is no longer recommended by critical care experts. Other principles of resuscitation and supportive care include optimization of oxygen delivery, the use of stress-dose corticosteroid therapy, correction of coagulopathies if clinically significant, maintenance of blood glucose levels below 150 mg/dL, and implementation of hemofiltration as needed.
Identification of the presumptive source of infection and cultures from corresponding fluids and blood should be obtained before the initiation of antimicrobial therapy. Multiple blood cultures for aerobic and anaerobic organisms should be obtained. In addition, all potential sources of bacteremia must be cultured (i.e., urine, sputum, and wounds). Careful attempts to identify the source of infection should be made, because the choice of appropriate antimicrobial coverage depends on the organisms that are thought most likely to cause the infection. The severity of the underlying disease and the possibility of synergistic interactions are also important considerations. If the urinary tract is the most likely portal of entry, a broad-spectrum antimicrobial agent either alone or in combination with an aminoglycoside should be administered. Three clinical factors have been predictive of the subsequent isolation of a resistant pathogen: (1) the use of an antimicrobial drug in the last month, (2) advanced age, and (3) male sex (Leibovici et al, 1992). If the infection is hospital acquired, or if the patient has had multiple infections or is immunocompromised or severely ill, an aminoglycoside and anti-Pseudomonas β-lactam or a third-generation cephalosporin should be used. When identification and drug susceptibilities of the offending organism are known, antimicrobial therapy should be changed to use the lowest cost, least toxic antimicrobial with the narrowest antimicrobial coverage. Antimicrobial treatment should be continued until the patient has been afebrile for 3 to 4 days and is clinically stable. Local infections that may have provided the focus for the bacteremia should be treated individually as appropriate.
Early studies suggested that recombinant human activated protein C (drotrecogin alfa), an inhibitor of multiple inflammatory and coagulation pathway components, could be used to lower mortality in sepsis (Bernard et al, 2001). However, meta-analysis of two large randomized trials raised doubt about the clinical usefulness of activated protein C in reducing mortality in sepsis (Wiedermann and Kaneider, 2005).
Bacteriuria in Pregnancy
Asymptomatic bacteriuria is one of the most common infectious complications of pregnancy. The prevalence of bacteriuria does not change initially with the occurrence of pregnancy and ranges from 2% to 7% (Hooton et al, 2000). The risk of acquiring bacteriuria during pregnancy increases with the progression of pregnancy (Norden and Kass, 1968; Campbell-Brown et al, 1987; Stenqvist et al, 1989), lower socioeconomic class, multiparity, and sickle cell traits (Patterson and Andriole, 1987; Stenqvist et al, 1989).
The site of bacteriuria in pregnant female patients probably also reflects the situation before conception. In two studies that localized the origin of the bacteriuria, one using the Stamey ureteral catheterization technique and the other the Fairley bladder washout, upper tract infections were found in 44% and 24.5% of pregnant female patients, respectively (Fairley et al, 1966; Heineman and Lee, 1973). In nonpregnant females with recurrent bacteriuria, Stamey (1980) has reported about a 50% probability that the origin is in the upper tract. With other techniques, which may reflect the severity of tissue infection rather than the location of infection, the results are similar; approximately 50% of women with screening bacteriuria of pregnancy are fluorescent antibody-positive (Fa+) and thus have evidence of upper tract infection (Harris et al, 1976). Fairley and his group (1973) found that the site of infection is unrelated to the likelihood that pyelonephritis will develop during pregnancy.
Spontaneous resolution of bacteriuria in pregnant women is unlikely unless treated. Nonpregnant patients often clear their asymptomatic bacteriuria (Hooton et al, 2000), but pregnant women become symptomatic more frequently and tend to remain bacteriuric (Elder et al, 1971).
Pyelonephritis develops in 1% to 4% of all pregnant women (Sweet, 1977) and in 20% to 40% of pregnant women with untreated bacteriuria (Pedler and Bint, 1987; Wright et al, 1993). Of the women who develop pyelonephritis during pregnancy, 60% to 75% acquire it during the third trimester (Cunningham et al, 1973), when hydronephrosis and stasis in the urinary tract are most pronounced. From 10% to 20% of pregnant women who get pyelonephritis develop it again before or just after the delivery (Cunningham et al, 1973; Gilstrap et al, 1981). Moreover, a third of pregnant women who develop pyelonephritis have a documented prior history of pyelonephritis (Gilstrap et al, 1981). Treatment of screening bacteriuria of pregnancy decreases the incidence of acute pyelonephritis during pregnancy from a range of 13.5% to 65% to a range of 0% to 5.3% (Sweet, 1977).
In Sweet’s excellent review (1977) of bacteriuria and pyelonephritis during pregnancy, he suggests that patients with a renal source of bacteriuria are more likely to have persistent postpartum bacteriuria than those with cystitis alone. In addition, those women with persistent bacteriuria may have an increased incidence of impaired creatinine clearance and urinary concentrating ability and an increased incidence of radiographic changes compatible with chronic pyelonephritis. His review of 12 studies revealed that follow-up excretory urograms in pregnant women with bacteriuria showed an 8% to 33% incidence of radiologic changes compatible with chronic pyelonephritis; the incidence of all urinary tract abnormalities in this group was 18% to 80%.
Zinner and Kass (1971) estimated that approximately 10% of bacteriuric pregnant females have pyelographic evidence of pyelonephritis, and, in their study, these abnormalities were most common in women who had bacteriuria 10 to 14 years after pregnancy. The highest incidence of radiographic changes of pyelonephritis was found in women who had their infections localized to the upper urinary tract (Fairley et al, 1966). In this study, abbreviated excretory urograms were performed within a few days of localization of the infection by ureteral catheterization. Six women (30%) with upper UTIs revealed radiographic renal abnormalities compatible with chronic pyelonephritis on the side to which the infection was localized, and two (10%) had nonexcretion of one kidney with infection in the contralateral one. No patient with an infection localized to the bladder showed radiographic evidence of pyelonephritis.
In their evaluation of renal function, Zinner and Kass (1971) found that women who had had bacteriuria during pregnancy and had it again 10 to 14 years later on follow-up showed significantly lower mean maximal urine osmolalities than those who were not bacteriuric on follow-up. Others have found evidence of decreased creatinine clearance and concentrating ability in bacteriuric women post partum (Sweet, 1977). It is unlikely, however, that uncomplicated bacteriuria in pregnant women produces changes in kidney appearance or function different from those found in nonpregnant bacteriuric women. After observing 40 bacteriuric women during pregnancy, Kincaid-Smith (1978) stated that there was no difference in renal size or function between 6 months and 4 years after delivery. Pregnancy, therefore, may provide the opportunity for bacteriuria to be discovered, but this bacteriuria probably reflects only a susceptibility to UTI that was present at conception. The increased likelihood that bacteriuria may progress to acute pyelonephritis during pregnancy alters the morbidity of bacteriuria for this group. Treatment of asymptomatic bacteriuria found early in pregnancy has been shown to decrease the prevalence of subsequent acute pyelonephritis from 28% to less than 3% (Sweet, 1977).
Pathogenesis
The anatomic and physiologic changes induced by the gravid state significantly alter the natural history of bacteriuria (Patterson and Andriole, 1987). These changes may cause pregnant women to be more susceptible to pyelonephritis and may require alteration of therapy. These changes have been well summarized in several reviews (Davidson and Talner, 1978; Waltzer, 1981).
Anatomic and Physiologic Changes during Pregnancy
Increase in Renal Size
Renal length increases approximately 1 cm during normal pregnancy. It is thought that this does not represent true hypertrophy but is the result of increased renal vascular and interstitial volume. No histologic changes have been identified in renal biopsy specimens (Waltzer, 1981).
Smooth Muscle Atony of the Collecting System and Bladder
The collecting system, especially the ureters, undergoes decreased peristalsis during pregnancy, and most women in their third trimester show significant ureteral dilatation (Davison and Lindheimer, 1978; Kincaid-Smith, 1978; Waltzer, 1981) (Fig. 10–37). This hydroureter has been attributed both to the muscle-relaxing effects of increased progesterone during pregnancy and to mechanical obstruction of the ureters by the enlarging uterus at the pelvic brim. Progesterone-induced smooth muscle relaxation also may cause an increased bladder capacity (Waltzer, 1981). Later in pregnancy, the dilation may be the result of the obstructive effect of the enlarging uterus (Poole and Thorsen, 1999).
Figure 10–37 Progressive hydroureter and hydronephrosis observed on IVGs during a normal pregnancy. A, 15 weeks; B, 18 weeks; C, 22 weeks; D, 26 weeks; E, 34 weeks; F, 39 weeks; G, 1 week post partum; H, 6 weeks post partum. Bilateral hydroureter and hydronephrosis are shown as early as 15 weeks (A). B to H, Successive urograms are from one patient during a normal pregnancy. Dilation occurs mainly on the right side, and both urinary tracts are normal by 6 weeks after delivery.
(A to H, From Hundley JM, Walton HJ, Hibbits JT, et al. Physiologic changes occurring in the urinary tract during pregnancy. Am J Obstet Gynecol 1935;30:625–49.)
Bladder Changes
The enlarging uterus displaces the bladder superiorly and anteriorly. The bladder becomes hyperemic and may appear congested endoscopically (Waltzer, 1981). Estrogen stimulation probably causes bladder hypertrophy as well as squamous changes of the urethra (Waltzer, 1981).
Augmented Renal Function
The transient increases in glomerular filtration rate and renal plasma flow during pregnancy have been well summarized by several authors and are probably secondary to the increase in cardiac output (Zacur and Mitch, 1977; Davison and Lindheimer, 1978; Kincaid-Smith, 1978; Waltzer, 1981). Glomerular filtration increases by 30% to 50%, and urinary protein excretion increases. The significance of these physiologic changes is apparent when the normal serum creatinine and urea nitrogen values for pregnant women are surveyed (Table 10–25). Values considered normal in nonpregnant women may represent renal insufficiency during pregnancy.
Table 10–25 Average Values for Serum Creatinine and Urea Nitrogen
| NONPREGNANT FEMALES (mg/dL) | PREGNANT FEMALES (mg/dL) | |
|---|---|---|
| Serum creatinine | 0.7 | 0.5 |
| Urea nitrogen | 13.0 | 9.0 |
Data from Davison JM, Lindheimer MD. Renal disease in pregnant women. Clin Obstet Gynecol 1978;21:411.
Davison and Lindheimer (1978) recommend that pregnant patients with serum creatinine levels greater than 0.8 mg/dL or urea nitrogen levels greater than 13 mg/dL undergo further evaluation of renal function. Similarly, urinary protein in pregnancy is not considered abnormal until greater than 300 mg of protein in 24 hours is excreted.
These significant physiologic changes in pregnancy, which may develop as early as the first trimester, lead to urinary stasis and mild hydroureteronephrosis, and contribute to development of pyelonephritis.
Recent studies of E. coli adhesins and their respective specific tissue receptors have established an adhesin-based mechanism of pyelonephritis-induced preterm births and low birth weights in mice (Kaul et al, 1999). There is a higher incidence of E. coli-bearing Dr adhesins during the third trimester of pregnancy in women with gestational pyelonephritis (Nowicki et al, 1994) and an upregulation of Dr adhesin in the kidney, endometrium, and placenta during the third trimester of pregnancy (Martens et al, 1993). When infected intravesically with E. coli-bearing Dr adhesin, nearly 90% of mice that were hyporesponsive to bacterial lipopolysaccharide and had a deficient immune response delivered preterm, compared with 10% of mice infected with E. coli without Dr adhesin. Also, there was a significant reduction in fetal birth weight in the Dr adhesin-infected group. Bacterial tissue culture showed systemic spread of the E. coli-bearing Dr adhesins to the placentae and fetuses.
Complications Associated with Bacteriuria during Pregnancy
Prematurity and Prenatal Mortality
In the preantimicrobial era, pregnant women with symptomatic UTIs and bacterial pyelonephritis were reported to have a high incidence of prematurity, low birth weight, and death (Gilstrap et al, 1981). The relationship between asymptomatic bacteriuria and prematurity is less clear. Gilstrap and colleagues (1981) found no difference in pregnancy among patients treated for asymptomatic bacteriuria as compared with nonbacteriuric controls. However, because women with asymptomatic bacteriuria are at higher risk for developing a symptomatic UTI that results in adverse fetal sequelae, complications associated with bacteriuria during pregnancy, and pyelonephritis and its possible sequelae, such as sepsis in the mother, all women with asymptomatic bacteriuria should be treated (Smaill, 2001).
Maternal Anemia
Although several studies suggest that bacteriuria untreated during pregnancy is associated with maternal anemia, not all studies support this. Some difficulties in interpreting the results of these surveys have been caused by inadequate documentation of bacteriuria. In one survey in which urine cultures were obtained by suprapubic aspiration, the data suggest that pregnant patients requiring three or more treatments for bacteriuria have lower levels of serum hemoglobin and folate than controls (McFadyen et al, 1973). In another study from England, investigators showed a statistically significant difference in the incidence of anemia between 410 bacteriuric pregnant women and 409 control pregnant women (Williams et al, 1973). In this survey, 14.6% of bacteriuric women and 10% of control women were anemic at the first prenatal visit. This separation increased during the third trimester (32 weeks), when 25% of women treated with placebo alone had anemia but only 16.8% of those women treated with antimicrobial agents had anemia. Furthermore, in the 31 untreated (placebo-treated) bacteriuric women who subsequently developed pyelonephritis, the incidence of anemia was 45.2%. These investigators concluded that “untreated bacteriuria increases the likelihood of developing anemia during pregnancy and that this risk is enhanced by the development of acute pyelonephritis, even if it is treated promptly.”
Laboratory Diagnosis
Significant false-negative rates occur if screening is conducted by urinalysis or reagent strip testing (Preston et al, 1999; McNair et al, 2000). Therefore an initial screening culture should be performed in all pregnant women during the first trimester (Stenqvist et al, 1989). If the culture shows no growth, repeat cultures are generally unnecessary because patients who have no growth in their urine early in their pregnancy are unlikely to develop bacteriuria later (Norden and Kass, 1968; McFadyen et al, 1973). Pregnant women with a history of recurrent UTI or vesicoureteral reflux may benefit from antimicrobial prophylaxis (Bukowski et al, 1998).
Management
Selection of an antimicrobial agent to treat the bacteriuria must be made, however, with special considerations given to maternal and fetal toxicity. The physiologic changes of pregnancy may decrease tissue and serum drug concentrations. Maternal expanded fluid volume, the distribution of the drug in the fetus, increased renal blood flow, and increased glomerular filtration decrease the serum drug concentration. If the culture is positive, special consideration must be given to the selection of antimicrobial agents chosen to treat infection to prevent fetal toxicity. The pathogens are similar to those seen in nonpregnant women (MacDonald et al, 1983). Table 10–26 lists the antimicrobial agents and dosing for use in pregnancy. The aminopenicillins and cephalosporins are considered safe and generally effective throughout pregnancy. In patients with penicillin allergy, nitrofurantoin is a reasonable alternative. It may be used safely during the first two trimesters in patients without glucose-6-phosphate dehydrogenase deficiency. Given the low efficacy of short-course β-lactam therapy in nonpregnant women, it is prudent to describe a full 3- to 7-day course of therapy in pregnant women. Follow-up cultures should be obtained to document absence of infection. If the culture is positive, the cause of bacteriuria must be determined to be lack of resolution, bacterial persistence, or reinfection. If the infection is unresolved, proper selection and administration of another drug probably will solve the problem. If the problem is bacterial persistence or rapid reinfection, antimicrobial suppression of infection or prophylaxis (Pfau and Sacks, 1992) throughout the remainder of the pregnancy should be considered.
Table 10–26 Oral Antimicrobial Agents Used in Pregnancy
| DRUG | DOSAGE | COMMENTS |
|---|---|---|
| Agents Considered Safe | ||
| Penicillins | ||
| Ampicillin | 500 mg qid | Extensively used |
| Amoxicillin | 250 mg tid | Safe and effective |
| Penicillin V | 500 mg qid | Used less frequently but achieves excellent urinary levels |
| Cephalosporins | ||
| Cephalexin | 500 mg qid | Extensively used |
| Cefaclor | 500 mg qid | Somewhat more effective against gram-negative organisms |
| Nitrofurantoin | 100 mg qid | May be used during the first two trimesters; may result in hemolytic anemia in patients with G6PD deficiency |
| Sulfasoxazole | 1 g, followed by 500 mg qid | May cause kernicterus in the newborn; also may cause hemolytic anemia when G6PD deficiency is present; especially avoid in last few weeks of gestation |
| Agents That Should Be Avoided | ||
| Fluoroquinolones | Possible damage to immature cartilage | |
| Chloramphenicol | Associated with “gray baby” syndrome | |
| Trimethoprim | May cause megaloblastic anemia because of anti–folic acid action | |
| Erythromycin | Associated with maternal cholestatic jaundice | |
| Tetracyclines | May cause acute liver decompensation in the mother and inhibition of new bone growth in the fetus | |
G6PD, glucose-6-phosphate dehydrogenase.
Adapted from Schaeffer AJ. Urinary tract infections. In: Gillenwater JY, Grayhack JT, Howards SS, Mitchell ME, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 211–72.
Pregnant women with acute pyelonephritis should be hospitalized and treated initially with parenteral antimicrobial agents. More than 95% of these patients respond within 24 hours using ampicillin and an aminoglycoside (Cunningham et al, 1973) or cephalosporins (Sanchez-Ramos et al, 1995). Appropriate oral agents should then be given for at least 14 days (Faro et al, 1984). After the treatment course is completed, low-dose prophylaxis with nitrofurantoin, amoxicillin, or cephalexin has been shown to be effective in preventing reinfection (Van Dorsten et al, 1987; Sandberg and Brorson, 1991). The efficacy of postcoital prophylaxis with either cephalexin (250 mg) or nitrofurantoin (50 mg) has been reported (Pfau and Sacks, 1992).
Drugs that are relatively contraindicated during pregnancy include the fluoroquinolones, TMP, chloramphenicol, erythromycin, tetracycline, sulfonamides, and sometimes nitrofurantoin (Nicolle, 1987). Fluoroquinolones are contraindicated because of their effects on immature cartilage. TMP may have teratogenic effects and should be avoided, especially in the first trimester. The “gray baby” syndrome is a toxic effect of chloramphenicol on neonates resulting from the inability of the infant to metabolize or excrete the drug. Erythromycin may cause cholestatic jaundice in the mother. Tetracycline may cause fetal malformations and maternal liver decompensation. Sulfonamides may cause kernicterus and neonatal hyperbilirubinemia and should be avoided in the third trimester. As mentioned earlier, nitrofurantoin can cause hemolytic anemia in both mother and child when glucose-6-phosphate dehydrogenase deficiency is present (Nicolle, 1987).
Pregnancy in Women with Renal Insufficiency
With current management of recurrent UTIs, infections alone are no contraindication to pregnancy. In patients who have renal insufficiency with or without UTIs, Davison and Lindheimer (1978) emphasize that renal function should be carefully evaluated by both serum creatinine levels and creatinine clearance before a woman is counseled about conceiving or continuing a pregnancy. Although little is known about the outcome of pregnancies with differing degrees of renal insufficiency, it is known that normal pregnancy is rare if the preconception serum creatinine level exceeds 3 mg/dL (about 30 mL/min clearance).
The degree of renal function impairment is the major determinant for pregnancy outcome. The fetal survivors of pregnant women with mild or moderate renal disease (serum creatinine <1.4 mg/dL and from 1.4 mg/dL to 2.4 to 2.8 mg/dL, respectively) is only slightly diminished, and irreversible deterioration of maternal renal function is uncommon. However, the perinatal mortality is approximately four times higher with severe disease. The rate of perinatal morbidity due to low birth weight or prematurity doubles from mild to moderate renal disease, and again from moderate to severe disease (Vidaeff et al, 2008).
Key Points
Bacteriuria in Pregnancy