chapter 97 Ultrasonography and Biopsy of the Prostate
Deaths due to prostate cancer in the United States were estimated to be almost 29,000 in 2008, representing a 25% decrease in the mortality rate compared with a decade ago (Jemal et al, 2008). Although the reasons for this improvement are often debated, early prostate cancer detection programs have likely played a role. Early detection has benefited greatly from prostate-specific antigen (PSA) screening efforts, the introduction and refinement of systematic transrectal ultrasonography (TRUS)–guided prostate biopsy techniques, and increased public awareness about prostate cancer.
TRUS of the prostate, first described by Watanabe and colleagues (1968), expanded to routine clinical use with improvements in ultrasound technology and the introduction of the TRUS-guided systematic sextant biopsy protocol by Hodge and associates (Hodge et al, 1989a, 1989b). Concurrent with improved biopsy techniques, the use of PSA screening increased the number of men undergoing early prostate cancer screening and prostate biopsy, with estimates as high as 800,000 biopsies annually in the United States alone (Halpern and Strup, 2000). Given the prevalence of prostate cancer and the frequency with which TRUS-guided prostate biopsies are performed, significant efforts have been focused on determining the appropriate indications for biopsy and the ideal technique by which to image and biopsy the prostate.
TRUS technology has become a mainstay of many image-guided prostate interventions, including prostate biopsy, brachytherapy, cryotherapy, and high-intensity focused ultrasonography (HIFU), as well as being used in the evaluation of appropriate patients for treatment of benign prostatic hyperplasia (BPH) (Beerlage, 2003). Fiducial gold seeds are being placed under ultrasound guidance to verify and correct the position of the prostate during megavoltage irradiation (Linden et al, 2009). In this chapter our focus is primarily on the use of TRUS and biopsy techniques for the diagnosis of prostate cancer.
Ultrasonographic Anatomy of the Prostate
The prostate lies between the bladder neck and the urogenital diaphragm, just anterior to the rectum, an ideal position to be imaged by TRUS. The prostate gland is traditionally described based on a pathologic zonal architecture. These divisions consist of the anterior fibromuscular stroma (AFS) that is devoid of glandular tissue, transition zone (TZ), central zone (CZ), periurethral zone, and peripheral zone (PZ). Unfortunately, these regions are not visible sonographically as distinct entities (Fig. 97–1).
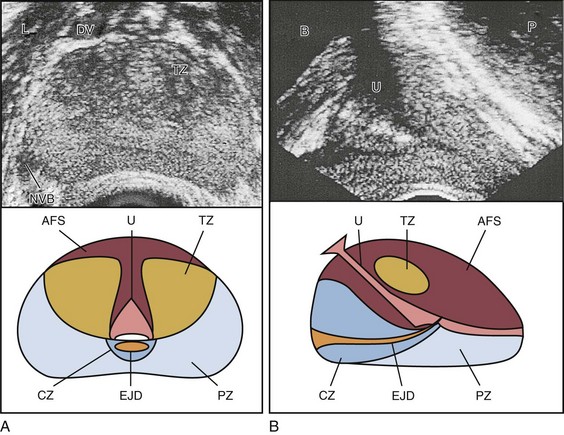
Figure 97–1 Normal prostate ultrasound images (top) with diagrams (bottom) at approximately the level of the verumontanum demonstrating zonal anatomy. A, Transverse view. B, Sagittal view. AFS, anterior fibromuscular stroma; CZ, central zone; DV, dorsal vein complex; EJD, ejaculatory ducts; NVB, neurovascular bundle; L, levator muscles; PZ, peripheral zone; TZ, transition zone; U, urethra.
However, the TZ may often be discernible from the PZ and CZ, particularly in glands with significant BPH. Located posteriorly, the normal CZ and PZ, from which a majority of adenocarcinomas arise, have a homogeneous echogenic appearance, whereas the anteriorly situated TZ is more heterogeneous. Frequently, calcifications along the surgical capsule known as “corpora amylacea” highlight the plane between the PZ and TZ (Halpern, 2002). Small, multiple diffuse calcifications are a normal, often incidental ultrasonographic finding in the prostate and represent a result of age rather than a pathologic entity. Larger prostatic calculi associated with symptoms may be related to underlying inflammation and require further evaluation and, possibly, treatment (Geramoutsos et al, 2004).
The prostatic urethra traverses the length of the gland in the midline and thus must be imaged in the sagittal plane to be simultaneously viewed along its entire course (Fig. 97–2A and B). The distended urethral lumen has a hypoechoic appearance, whereas periurethral calcifications may produce a thin echogenic outline. The smooth muscle of the internal sphincter extends from the bladder neck, encircling the urethra to the level of the verumontanum. These muscle fibers may be visualized sonographically as a hypoechoic ring around the upper prostatic urethra, giving it a funneled appearance proximally as it arises from the bladder neck. On reaching the verumontanum the urethra angles anteriorly and runs through the remainder of the gland to exit at the apex of the prostate. This angle gives the prostatic urethra an anteriorly concave appearance when viewed along its entire course in the sagittal plane.
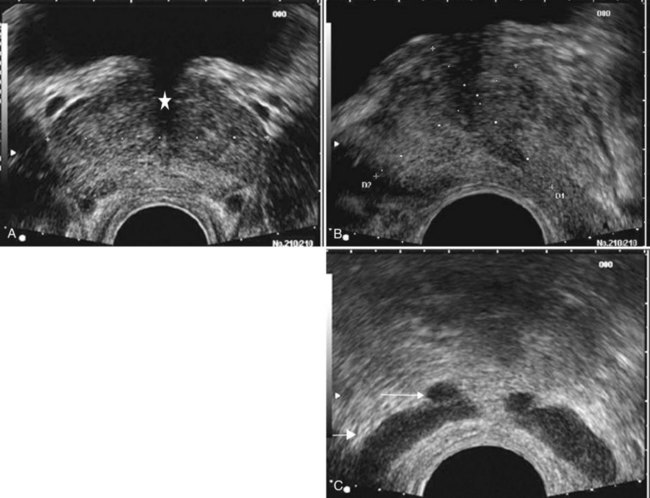
Figure 97–2 Classic gray-scale transrectal ultrasonography imaging of the prostate. A, In the transverse plane with the hypoechoic urethra centrally located (star) and dotted line representing transverse measurement. B, Midline sagittal view with the hypoechoic urethra running the length of the gland, D1 represents longitudinal and D2 anteroposterior measurement. C, Seminal vesicles (large arrow) and vasa deferentia (small arrow) in the transverse plane.
The paired seminal vesicles (SVs) are positioned posteriorly at the base of the prostate (Fig. 97–2C). They have a smooth, saccular appearance and should be symmetrical. The normal SV measures 4.5 to 5.5 cm in length and 2 cm in width. A cystic SV mass is presumptively benign, whereas a solid lesion has a small probability of being malignant, especially if the patient has a primary neoplasm elsewhere. Schistosomiasis should be considered when making a differential diagnosis in patients who live in areas where infestation is endemic with a solid SV mass (Al-Saeed et al, 2003). In the transverse plane, the vasa deferentia course just above their ipsilateral SV before diving caudally toward the prostate near the midline. Here they lie just medial to the tapering ipsilateral SV before the two structures fuse to form an ejaculatory duct. The ejaculatory ducts (occasionally seen as a hypoechoic structure) enter the gland posteriorly and empty into the urethra at the verumontanum (see Fig. 97–2C). Their course parallels that of the prostatic urethra distal to the verumontanum.
Gray-Scale Transrectal Ultrasonography (TRUS)
Gray-scale TRUS has become the most common imaging modality for the prostate. Most commonly used for prostate cancer detection, TRUS may also be used in the evaluation of other conditions such as infertility (see Chapter 21). Although the role of TRUS is expanding in directing the biopsy of prostate cancer, the role of staging localized prostate cancer using TRUS is very limited (Onur et al, 2004).
Commercially available endorectal probes are available in both side- and end-fire models and transmit frequencies of 6 to 10 MHz. Most modern ultrasound machines have optimized self-programming for TRUS and biopsy. Some newer biplane probes provide simultaneous sagittal and transverse imaging modes. Probes provide a scanning angle approaching 180 degrees to allow simultaneous visualization of the entire gland in both the transverse and sagittal planes. Increasing frequency yields increased resolution. As the frequency of the probe is increased, the portion of the image that is in focus (focal range) is closer to the transducer (Kossoff, 2000). The commonly used 7-MHz transducer produces a high-resolution image with a focal range from 1 to 4 cm from the transducer (best for PZ where most cancers arise). Lower-frequency transducers (e.g., older 4-MHz transducers) have a focal range from 2 to 8 cm but at lower resolution. Lower-frequency transducers improve anterior delineation of large glands, increasing the accuracy of volume measurements, but provide poor internal architecture visualization. Acoustic properties of soft tissue are similar to those of water, but clinically useful ultrasound energy does not propagate through air. For this reason, a water-density substance, termed a coupling medium, is used. The coupling medium, usually sonographic jelly or lubricant, is placed between the probe and the rectal surface. If the probe is covered with a protective condom, the coupling medium is placed between the probe and the condom, as well as between the condom and the rectal surface.
Techniques
The complete TRUS evaluation of the prostate includes scanning in both the sagittal and transverse planes to obtain a volume calculation. The CZ and PZ are inspected for hypoechoic lesions and contour abnormalities, and the SVs and vasa deferentia are fully visualized.
Machine Settings
The image magnification is adjusted so that most of the prostate is visible without the image being too small to allow detection of abnormalities. In general, the magnification is low during prostate measurements so that the entire gland is seen. During biopsies, magnification is maximal for visualization of needle passage. The ultrasonographer can manually alter the brightness (or gain) slightly with each new patient, and occasionally during imaging of different areas within the same prostate. The optimal brightness setting results in a medium-gray image of the normal PZ. This gray tone serves as the reference point for judging lesions as hypoechoic (darker than the normal PZ), isoechoic (similar to the normal PZ), hyperechoic (lighter than the normal PZ), or anechoic (completely black).
Probe Manipulation
Patients are typically scanned in the left lateral decubitus position (see Patient Positioning later). TRUS should be performed in both the transverse and the sagittal planes. There are two approaches to probe manipulation for transverse imaging (see Fig. 97–2A). With radial and some biplane probes, advancing the probe cephalad into the rectum images the prostate base, the SVs, and the bladder neck. Pulling the probe caudally toward the anal sphincter images the prostatic apex and proximal urethra. Transverse imaging with end-fire, side-fire, and some biplane probes is accomplished by angling the handle of the probe right or left using the anal sphincter as a fulcrum (see Fig. 97–2B). Angling the probe toward the scrotum produces more cephalad images, and angling the probe toward the sacrum produces more caudal images. There are also two approaches to probe manipulation for sagittal imaging. One method is rotation of the probe. Clockwise rotation yields images of the left side of the prostate, and counterclockwise rotation yields images of the right side. Alternatively, sagittal imaging can be accomplished by angling the probe up or down using the anal sphincter as a fulcrum. In the left lateral decubitus position, angling the handle of the probe down (toward the floor) images the right side of the prostate, and angling the handle of the probe up (toward the ceiling) images the left side. Urologists often prefer angling the probe because this method is similar to manipulation of a cystoscope and is less uncomfortable for the patient.
Volume Calculations
Prostate volume can be calculated through a variety of formulas. Volume calculation requires measurement of up to three prostate dimensions. In the axial plane, the transverse and anteroposterior (AP) dimensions are measured at the point of widest transverse diameter (see Fig. 97–2A and B). The longitudinal dimension is measured in the sagittal plane just off the midline, because the bladder neck may obscure the cephalad extent of the gland (see Fig. 97–2B). Most formulas assume that the gland conforms to an ideal geometric shape: either an ellipse (π/6 × transverse diameter × AP diameter × longitudinal diameter), sphere (π/6 × transverse diameter3), or a prolate (egg-shaped) spheroid (π/6 × transverse diameter2 × AP diameter). Despite the inherent inaccuracies that arise from these geometric assumptions, all formulas reliably estimate gland volume and weight, with correlation coefficients greater than 0.90 with radical prostatectomy specimen weights, because 1 cm3 equals approximately 1 g of prostate tissue (Terris and Stamey, 1991). The mature average prostate is between 20 and 25 g and remains relatively constant until about age 50, when the gland enlarges in many men (Griffiths, 1996).
When a more accurate determination of gland volume is required, such as during brachytherapy, planimetry may be employed. With the patient in the lithotomy position, the probe is mounted to a stepping device, and serial transverse images are obtained at set intervals (e.g., 3 to 5 mm) through the entire length of the gland. The surface area of each serial image is determined, and the sum of these measurements is then multiplied by total gland length to yield the prostate volume.
Once gland volume has been obtained, one can calculate derivatives such as the PSA density (PSAD = serum PSA/gland volume). An elevated PSAD of the entire gland has been shown to have a sensitivity and specificity of 75% and 44%, respectively, for predicting a positive cancer diagnosis on repeat biopsy (Djavan et al, 2000). Unfortunately, there is high interoperator and intraoperator variability in PSAD determinations, and similar predictive information can now be obtained using serum free:total PSA (Djavan et al, 2003).
Cystic Lesions of the Prostate
Cystic prostatic structures are common on TRUS. Simple cysts have the same sonographic appearance as in any other part of the body: They are thin walled, anechoic, and show acoustic enhancement posterior to the cyst. Prostatic cysts may be congenital or acquired but are rarely clinically significant regardless of etiology.
Congenital prostatic cystic lesions may arise from either müllerian (müllerian duct cysts and prostatic utricles) or wolffian (ejaculatory duct and seminal vesicle cysts) structures. An enlarged prostatic utricle represents a diverticular projection from the posterior urethra at the level of the verumontanum (Cochlin, 2002) and appears as a midline anechoic structure. These are associated with genital anomalies, including hypospadias (most common), ambiguous genitalia, undescended testes, and congenital urethral polyps (Gregg and Sty, 1989). Müllerian duct cysts also appear as midline anechoic lesions that result from failure of the müllerian ducts to fuse with the urethra. They are generally ovoid to pear shaped with the cyst neck oriented toward the verumontanum. When müllerian duct cysts are present, men should be evaluated for unilateral renal agenesis (McDermott et al, 1993).
Lateral paraprostatic cystic structures include seminal vesicle and vas deferens cysts (wolffian in origin). Ejaculatory duct cysts are typically small, lie off of the midline, and may accompany ejaculatory duct obstruction/obliteration with azoospermia (Fig. 97–3A and B). Seminal vesicle cysts can be caused by congenital or acquired obstruction of the ejaculatory duct and are associated with cystic renal disease; up to two thirds of men with seminal vesicle cysts may also have renal agenesis (King et al, 1991). Acquired cysts of the TZ result from hemorrhagic degeneration of BPH nodules (Hamper et al, 1990), whereas those of the outer gland have no proven etiology.
Prostate Cancer Imaging on TRUS
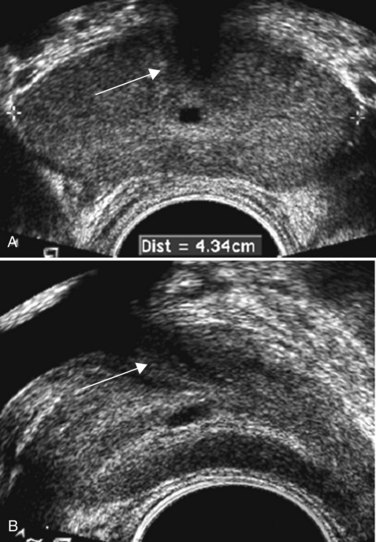
All hypoechoic lesions within the PZ should be noted and included in the biopsy material (Fig. 97–4). The lack of a distinct hypoechoic focus does not preclude proceeding with biopsy, because 39% of all cancers are isoechoic and up to 1% of tumors may be hyperechoic on conventional gray-scale TRUS (Shinohara et al, 1989). Despite the higher prevalence of cancers discovered in prostates with hypoechoic areas, the hypoechoic lesion itself was not associated with increased cancer prevalence compared with biopsy cores from isoechoic areas in a contemporary series of almost 4000 patients (Onur et al, 2004). Furthermore, other disease processes, such as granulomatous prostatitis (Terris et al, 1997a), prostatic infarct (Purohit et al, 2003), and lymphoma (Varghese and Grossfeld, 2000), may all produce hypoechoic lesions. TZ BPH nodules are typically hypoechoic but may contain isoechoic or even hyperechoic foci. A hypoechoic lesion is malignant in 17% to 57% of cases (Frauscher et al, 2002), highlighting the need to biopsy these lesions but recognizing they are not pathognomonic for cancer as once thought.
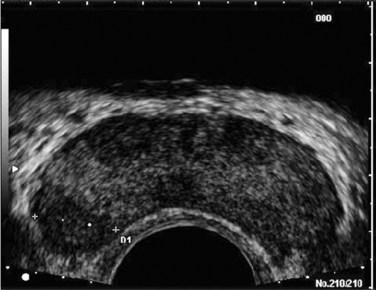
Figure 97–4 Classic hypoechoic peripheral zone (PZ) lesion (dotted line) in the right midgland that transrectal ultrasonography–guided biopsy proved to be a Gleason 3 + 3 = 6 adenocarcinoma.
Any focal contour abnormalities along the outer edge of the gland and any asymmetries in echotexture from the PZ of one lobe to that of the other are noted. Extracapsular extension of prostate cancer, although not well visualized if present as a microfocus, is suggested by a focal loss of the typically bright white periprostatic fat.
TRUS Appearance after Treatment
External-beam radiation therapy typically decreases volume by 6 months after treatment. Irradiated prostates are diffusely hypoechoic, with poorly defined anatomy. Large hypoechoic tumors, particularly those not responding to therapy, show little change in echogenicity once irradiated, but smaller foci responding well to therapy tend to become isoechoic (Egawa et al, 1991). In general, TRUS findings correlate poorly with pathologic findings and outcomes in irradiated prostates.
With interstitial brachytherapy, there is initial postimplantation edema followed by long-term changes as with external-beam radiation therapy (Whittington et al, 1999). With an ideal permanent implant, seeds should be distributed evenly throughout the gland. These seeds are hyperechoic and demonstrate posterior shadowing. The prostate volume declines significantly after treatment, with a 37% size reduction at 1 year after treatment and over 50% reduction 8 years after implantation (Stone and Stock, 2007). This decline is unaffected by neoadjuvant hormonal therapy.
Androgen ablation with luteinizing hormone–releasing hormone (LHRH) analogs will cause an average 30% volume decrease with androgen deprivation in prostates with and without cancer (Whittington et al, 1999). The decrease ranges up to 60% in large glands and as little as 10% in small glands. Volume decreases by approximately 21% at 6 months using agents such as finasteride (Marks et al, 1997).
Postradical prostatectomy TRUS is considered normal if there is smooth tapering of the bladder neck to the urethra (Kapoor et al, 1993). Many patients demonstrate a nodule of tissue anterior to the anastomosis, representing the ligated dorsal vein complex (Goldenberg et al, 1992). Any other hyperechoic or hypoechoic lesions or interruptions of the retroanastomotic fat plane are considered suspicious (Kapoor et al, 1993). Hypoechoic lesions have been reported in 75% to 95% of patients with locally recurrent cancer, and color Doppler has been used to improve cancer detection in the prostatic fossa (Sudakoff et al, 1996; Tamsel et al, 2006). Patients with detectable PSA who are candidates for salvage radiation therapy were once considered for routine biopsy of the anastomotic area. Biopsy of the anastomotic region with PSA recurrence in the absence of a palpable nodule is not usually informative (Scattoni et al, 2004). However, biopsy of an abnormality seen on TRUS, even with a normal digital rectal exam (DRE), can be diagnostic of locally recurrent disease (Naya et al, 2005).
TRUS and Other Malignancies
Prostatic involvement with transitional cell carcinoma (TCC) from the bladder is generally not detectable by TRUS, but 71% of prostatic stromal TCC lesions are hypoechoic. Prostatic TCC detected by TRUS must be confirmed by biopsy, because granulomas resulting from instillation of bacille Calmette-Guérin are common in TCC patients and are also hypoechoic (Terris et al, 1997a).
Extension to the prostate from the bladder, or urethral squamous cell carcinoma (SCC), is much more common than is primary prostatic SCC. Prostatic SCC appears as an irregular, anterior mass demonstrating relative hyperechogenicity (Terris, 1999).
Adenoid cystic/basal cell carcinoma of the prostate is rare but potentially fatal. Histologically, cribriform or adenoid cystic patterns predominate. Numerous cystic glands give this tumor an unusual appearance on TRUS, characterized by multiple, evenly distributed, small, anechoic cysts (Iczkowski et al, 2003).
Prostatic sarcoma is a rare complication of prostatic irradiation, and the TRUS appearance is typified by an irregular, hypoechoic mass with an anechoic area consistent with necrosis (Terris, 1998). Unlike radiation-induced sarcoma, the echogenicity of rhabdomyosarcoma is similar to that of normal prostate tissue.
Hematologic and lymphoid malignancies involving the prostate are generally not visualized with TRUS (Terris and Freiha, 1998). Biopsies may demonstrate a lymphocytic infiltrate, but this is often attributed to chronic inflammation if no suspicion of nonprostate malignancy exists.
Prostate Biopsy Techniques and Outcomes
Indications for Prostate Biopsy
Before TRUS improvements and serum PSA testing became widespread, clinicians relied mainly on digital rectal examination to establish a suspicion of prostate cancer and performed digitally directed lesional biopsies. Today, PSA-based screening of asymptomatic men has resulted in the adaptation of TRUS biopsy as the standard of care for routine prostate biopsy. The presence of focal nodules on digital rectal examination still will prompt a biopsy using the TRUS technique regardless of PSA levels. TRUS-directed prostate needle biopsy remains the gold standard for diagnosis of prostate cancer.
Key Points: Gray-Scale Transrectal Ultrasonography (TRUS)
Early prostate cancer detection has been markedly improved by PSA-based screening programs. These initiatives have been shown to significantly increase the rate of organ-confined and potentially curable disease (Catalona et al, 1993). Currently, most clinicians recommend biopsy once a patient’s serum PSA rises above 4.0 ng/mL, although significant research efforts are ongoing to identify the optimal PSA threshold to recommend prostate biopsy in the asymptomatic patient. Evidence for lowering the PSA threshold from work by Catalona’s group showed higher rates of organ-confined disease at the time of radical retropubic prostatectomy in samples from PSAs in the 2.6- to 4.0-ng/mL range (Krumholtz et al, 2002). These findings have led many urologists to now recommend prostate biopsy to men younger than 60 years of age once their PSA level rises above 2.5 ng/mL. Despite this downward shift in the PSA cutoff for younger men, there remains a general trend toward allowing older men (70 years or older) to have slightly higher “normal” PSAs, in the range of 5.5 to 6.5 ng/mL, although this is not universally accepted (American Urological Association, 2000; Scardino, 2005). Subsequent data from the Prostate Cancer Prevention Trial have shown that there is no safe PSA threshold that can rule out prostate cancer in any age range (Thompson et al, 2005). When examining men whose serum PSA was less than or equal to 4.0, significant numbers of men were diagnosed with prostate cancer at all PSA levels, with an overall prostate cancer detection rate of 15% for all men with a value less than 4.0, and nearly 15% having a Gleason score of 7 or greater (Thompson et al, 2004).
Adjuncts to serum PSA testing have been advocated to improve the performance characteristics of PSA for prostate cancer detection, including measuring the free:total PSA, PSA velocity, PSA density (PSAD), and PSAD of the transition zone (PSAD-TZ) (Djavan et al, 2002). For patients with a serum PSA value between 4.0 and 10.0 ng/mL, using a percentage of free PSA threshold of less than 25% allowed detection of 95% of cancers while eliminating 20% unnecessary biopsies, and within this group the risk of prostate cancer increased dramatically as the percentage of free PSA level declined (Catalona et al, 1998).
Regardless of initial PSA value, a PSA velocity greater than 0.75 ng/mL/year is frequently associated with prostate cancer and warrants biopsy (Carter et al, 1992). Lower PSA velocity ranges, as low as 0.35 ng/mL/year, may be indicative of occult prostate cancer (Carter et al, 2006). The clinical utility of PSA velocity to detect clinically significant prostate cancer remains controversial, however, with a recent European Organization for Research and Treatment of Cancer (EORTC) study indicating the PSA velocity, on multivariable analysis, was not an independent predictor of detecting prostate cancer or clinically significant prostate cancer on transrectal biopsy (Wolters et al, 2009). An elevated PSAD and PSAD-TZ have both been shown to increase the likelihood of diagnosing prostate cancer on repeat biopsy (Djavan et al, 2000). The indications for TRUS and prostate biopsy are rapidly evolving and being continually refined (Table 97–1). Details concerning screening for prostate cancer and the role of prostate biopsy are discussed in Chapter 99.
Table 97–1 Commonly Cited Recommendations for Transrectal Ultrasonography (TRUS) and/or Biopsy
ASAP, atypical small acinar proliferation; PIN, prostatic intraepithelial neoplasia; PSA, prostate-specific antigen.
Data from American Urological Association (AUA, 2000) and National Comprehensive Cancer Network (Scardino, 2005).
Prostate biopsy may also be indicated on the basis of the pathologic analysis of previous biopsy specimens. In men who have undergone prostate biopsy and are found to have only high-grade prostatic intraepithelial neoplasia (HGPIN) or atypical small acinar proliferation (ASAP), a follow-up biopsy should be performed. HGPIN represents a premalignant lesion and carries a 23% to 35% risk of diagnosing prostate cancer on subsequent biopsy (Davidson et al, 1995; Kronz et al, 2001). The natural history of ASAP is less well defined than that of HGPIN, but, if ASAP is present in the initial biopsy specimen, the risk of diagnosing prostate cancer on subsequent biopsy is significantly increased (Iczkowski et al, 1998; Ouyang et al, 2001). Thus irrespective of follow-up PSA values, current recommendations are to rebiopsy all patients with either HGPIN or ASAP in their initial biopsy specimen within 3 to 6 months.
Contraindications to Prostate Biopsy
Significant coagulopathy, painful anorectal conditions, severe immunosuppression, and acute prostatitis are all contraindications to prostate biopsy.
Preparing Patients for Biopsy
Patients should be informed of the risks and benefits of the procedure and provide informed consent. All anticoagulant therapy (warfarin, clopidogrel, aspirin/nonsteroidal anti-inflammatory drugs [NSAIDs], herbal supplements) should be stopped 7 to 10 days before prostate biopsy. For those patients with underlying coagulopathy or who are on warfarin, prostatic biopsy should not be performed until the international normalized ratio has been corrected below 1.5. A small amount of urine in the bladder can facilitate the examination.
Antibiotic Prophylaxis
A wide variety of prophylactic regimens have been studied using both oral and intravenous antibiotics, with widely varying opinions of the use of antibiotics and the choice of agents. The American Urological Association Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis advocates antibiotic prophylaxis prior to transrectal prostate biopsy (Wolf et al, 2008). Our current practice is to give patients a dose of an oral fluoroquinolone 30 to 60 minutes before biopsy and continue therapy for 2 to 3 days. By using similar protocols, recent large studies have reported minimal infectious complications, although bacteremia/sepsis still occurs in 0.1% to 0.5% of patients (Djavan et al, 2001b; Raaijmakers et al, 2002). The postbiopsy duration of antibiotics also remains controversial even in those who agree the antibiotics are indicated; single-dose oral fluoroquinolone prophylactic regimens demonstrated equivalent efficacy to 3-day regimens in preventing infections (Kapoor et al, 1998; Sabbagh et al, 2004; Wolf et al, 2008). For those patients at risk of developing endocarditis or infection of prosthetic joints, pacemakers, and automated implanted cardiac defibrillators, prophylaxis should consist of intravenous ampicillin (vancomycin, if penicillin allergic) and gentamicin preoperatively, followed by 2 to 3 days of an oral fluoroquinolone.
Cleansing Enema
We routinely have patients self-administer a cleansing enema at home before biopsy. This practice decreases the amount of feces in the rectum, thereby producing a superior acoustic window for prostate imaging. However, many clinicians may elect not to use an enema because this option may allow more spontaneous performance of a prostate biopsy. The enema’s effect on reducing infections is debatable, but it seems logical that a cleansing enema and empty rectal vault may reduce bacterial seeding of the prostate.
Analgesia
Comments on periprocedural pain control using topical lidocaine jelly have been published (Issa et al, 2000; Obek et al, 2004); in our experience and in the literature, this provides only suboptimal analgesia. Data now suggest that infiltration anesthesia around the nerve bundles with local anesthetic may provide excellent pain control, which is increasingly important when using extended biopsy techniques (Berger et al, 2003; Trucchi et al, 2005). A local prostatic block is achieved using 2% lidocaine, a long spinal needle (7-inch, 22-gauge), and TRUS guidance along the biopsy channel of the transducer. Multiple variations exist for the infiltration of local anesthetic for transrectal biopsy (Nash et al, 1996; Soloway and Obek, 2000). We have found that injecting 5 mL of lidocaine at the level of the seminal vesicles near the bladder base, at the hyperechoic fat pad that demarcates the junction of the seminal vesicles and the prostate bilaterally, produces an excellent block. Other approaches include infiltration of 10 mL starting at the junction of the seminal vesicles and infiltration along the lateral aspect of the prostate from base to apex. Direct infiltration into the prostate (intraprostatic injection) can augment the anesthetic benefit seen with periprostatic injection (Lee et al, 2007; Cam et al, 2008). One must be cautious, however, to avoid direct intravascular injection, because of the risk of systemic lidocaine absorption. Local anesthesia for transperineal biopsies should include infiltration of the skin and subcutaneous tissues of the perineum initially. Ultrasound guidance may then be employed to aid infiltration of deeper tissues along the anticipated tracts of the biopsy needle. Postbiopsy analgesic regimens, if used, should avoid aspirin and NSAIDs because of increased risk of bleeding.
Patient Positioning
Patients are usually placed in the left lateral decubitus position with knees and hips flexed 90 degrees. An armboard attached parallel to the table and a pillow between the knees helps maintain this position. The buttocks should be flush with the end of the table to allow manipulation of the probe and biopsy gun without obstruction. If necessary, the right lateral decubitus or lithotomy position can be used. The lithotomy position is used by some clinicians and is preferred for transperineal biopsies, brachytherapy treatment planning, or placement of fiducial gold markers for external-beam therapy (Dehnad et al, 2003). Because the distribution of color Doppler flow within the prostate is dependent on patient position, the lithotomy position is preferred when color Doppler imaging is used to identify areas of hyperemia for targeted biopsy of the prostate (Halpern et al, 2002b).
Transrectal Prostate Biopsy Techniques
The prostate volume is assessed, and imaging of the prostate in both the transverse and sagittal planes is begun. The examination usually starts at the base of the gland and extends to the apex. Most modern ultrasound units are automatically set for optimal prostate visualization, and the TRUS gray-scale exam of the prostate is conducted as previously described, noting the location and characteristics of any lesions (i.e., hypoechoic, hyperechoic, calcifications, contour abnormalities, cystic structures).
A spring-driven, 18-gauge, needle core biopsy device or biopsy gun, which can be passed through the needle guide attached to the ultrasound probe, is most often used. Most ultrasound units provide best visualization of the biopsy needle path in the sagittal plane. Images are typically superimposed with a ruled puncture path that corresponds to the needle guide of the TRUS unit. The biopsy gun advances the needle 0.5 cm and samples the subsequent 1.5 cm of tissue with the tip extending 0.5 cm beyond the area sampled (Kaye, 1989). Therefore when sampling the PZ, the needle tip may be placed 0.5 cm posterior to the prostate capsule before firing; advancing the needle to or through the capsule can result in sampling of more anterior tissue, missing the most common location of cancers. Avoiding adjustment of the probe position while the biopsy needle is in contact with the rectal surface and applying pressure with the probe to compress the rectal mucosa before biopsy can help avoid rectal bleeding. Pressing the probe against the rectum also minimizes the discomfort of the biopsy needle traversing the rectal mucosa, similar to pulling the skin tight to minimize the discomfort of phlebotomy.
The biopsy sample is typically placed in 10% formalin or per local protocol. There is currently no uniformly accepted method for submission of the biopsy samples (e.g., all samples submitted individually or multiple samples placed in one container). Some pathologists believe strongly that each site should be specifically identified, because certain locations may be predisposed to cancer “look-alikes” (e.g., Cowper gland at the apex, seminal vesicles at the base) (Levin, 2004). At the very least, samples should be segregated into left- and right-sided containers or processed by local guidelines.
Sextant Biopsy
The original sextant biopsy scheme (one core, bilaterally, from the base, mid, and apex) significantly improved cancer detection for digital-directed biopsy of palpable nodules and ultrasound-guided biopsy of specific hypoechoic lesions (Hodge et al, 1989a, 1989b). Taken in the parasagittal plane, these cores sampled a portion of the PZ but also included a significant amount of tissue from the TZ, with subsequent studies of radical prostatectomy specimens demonstrating that the vast majority of adenocarcinomas arise in the posterolateral PZ (McNeal et al, 1988), thus explaining some of the false-negative results of standard sextant biopsy (Eskew et al, 1997).
Extended Core Biopsy Techniques
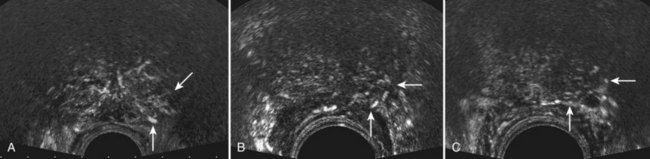
Modifications to the standard sextant biopsy scheme have focused on the importance of laterally directed cores (Terris et al, 1992). Numerous studies have shown improved cancer detection rates by incorporating additional laterally directed cores into the standard systematic sextant technique, ultimately taking anywhere from 8 to 13 cores (Eskew et al, 1997; Chang et al, 1998a; Levine et al, 1998; Babaian et al, 2000; Brossner et al, 2000; Presti et al, 2000; Durkan et al, 2002; Fink et al, 2003). Selected extended biopsy series are compared in Table 97–2. In a prospective study of 483 patients, Presti and colleagues (2000) found that adding laterally directed cores from the base and midgland bilaterally improved cancer detection from 80% with standard sextant to 96% with this 10-core scheme (only 4% of cancers were detected on the lesionally directed or TZ biopsy). At present, 6 cores are considered inadequate for routine prostate biopsy for cancer detection. Figure 97–5 depicts the originally proposed sextant technique and several common extended core biopsy strategies. TZ and SVs are not routinely sampled, because these regions have been shown to have consistently low yields for cancer detection at initial biopsy (Epstein et al, 1997; Terris et al, 1997b), but TZ and anteriorly directed biopsies may occasionally prove necessary to diagnose prostate cancer in those patients with persistently elevated PSA levels and prior negative biopsies (Mazal et al, 2001). However, there may be a role for TZ biopsies in men with gland size of more than 50 mL, with an additional yield of 15% cancer detection in these larger prostates (Chang et al, 1998b). Seminal vesicle biopsy is not routinely performed unless there is a palpable abnormality, with some authors recommending SV biopsy when the PSA value is greater than 30 or if brachytherapy is being considered (Gohji et al, 1995).
Table 97–2 Increasing Prostate Cancer Detection Rates with Extended Core Biopsy Protocols
| STUDY | NO. OF CORES | CANCER DETECTION RATE |
|---|---|---|
| Eskew et al, 1997 | 6 | 26.1% |
| 13 | 40.3% | |
| Naughton et al, 2000 | 6 | 26% |
| 12 | 27% | |
| Presti et al, 2000 | 6 | 33.5% |
| 8 | 39.7% | |
| 10 | 40.2% | |
| Babaian et al, 2000 | 6 | 20% |
| 11 | 30% |
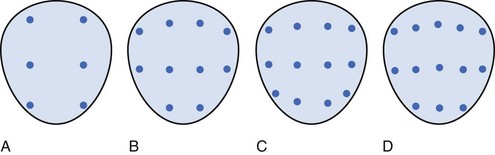
Figure 97–5 Various reported systematic biopsy schemes. A, Sextant biopsy scheme originally proposed by Hodge and associates (Hodge et al, 1989b). B, The 10-core biopsy of Presti and coworkers (2000). C, The 12-core, or double sextant, biopsy. D, The 13-core “5-region biopsy” of Eskew and colleagues (1997). Base is at the top of figure, apex is at bottom.
Repeat and Saturation Prostate Biopsy
Physicians are frequently presented with the dilemma of a patient who has had one or more negative prostate biopsies yet continues to have an elevated PSA value or abnormal digital rectal examination of concern for prostate cancer. Often these patients have undergone multiple biopsies despite the well-documented decline in cancer detection with each successive biopsy (Djavan et al, 2003). Keetch and coworkers (1994) reported an initial positive biopsy rate of 34% in 1136 men from their PSA-based prostate cancer screening program. Cancer detection rates then fell to 19%, 8%, and 7% on biopsy 2, 3, and 4, respectively. These findings were confirmed by results from the European Prostate Cancer Detection Study. In this cohort of 1051 men with PSA values between 4.0 and 10.0 ng/mL, the initial cancer detection rate with sextant biopsy was 22%. Positive cores were then found in only 10%, 5%, and 4% of patients on subsequent biopsies 2, 3, and 4, respectively (Djavan et al, 2001a).
These diminishing returns coupled with improved cancer detection rates on initial biopsy with extended core protocols have led some researchers to examine “saturation biopsy” techniques in this difficult subset of patients. In a study of 57 men with an average of two prior negative sextant biopsies, a cancer detection rate of 30% was obtained with an average of 22.5 cores per patient (Borboroglu et al, 2000). Similar protocols from the Mayo Clinic (Stewart et al, 2001) and Toronto (Fleshner and Klotz, 2002) demonstrated improved cancer detection rates. A drawback to these techniques is that additional anesthetic requirements often require these “saturation” biopsies to be performed in a hospital setting. More recent reports question the benefit of a saturation biopsy scheme, considering the attendant increased cost and potential morbidity. In a reassessment of their previous work on saturation biopsy, investigators at the Mayo Clinic performed a large prospective study of standard systematic and saturation biopsy techniques and did not find a significant increase in prostate cancer detection (Ashley et al, 2008).
Our study suggests that equally improved cancer detection rates can be achieved in this setting using contrast medium–enhanced TRUS (CE-TRUS) and a targeted biopsy protocol of only 10 cores in an outpatient setting (Halpern et al, 2005), thereby minimizing the morbidity and costs associated with saturation biopsies. The use of the free and total PSA may also allow patient classifications of low probability or high probability of having prostate cancer with a PSA level less than 10 ng/mL and determine the need for additional prostate biopsy after an initial negative result (Catalona et al, 1998).
When should we stop ordering biopsies for the patient who has a high degree of suspicion for undiagnosed prostate cancer? Unfortunately there is no clear answer. By using available data from large series such as the European Prostate Cancer Detection Study, we can consider some reasonable recommendations to our patients. In this series of over 1000 men, despite differences in location and multifocality, pathologic and biochemical features of cancers detected on first and second biopsy were similar, suggesting similar biologic behavior. Cancers found on third and fourth biopsies had a lower grade, stage, and cancer volume compared with cancers on first and repeat biopsies. Morbidity of first and repeat biopsies was similar, whereas third and fourth biopsies had a slightly higher complication rate. The use of a second prostate biopsy in all cases of a negative finding on initial biopsy appears justified. Third and fourth repeat biopsies, however, should only be obtained in selected patients with high suspicion of cancer and/or poor prognostic factors on the first or second biopsy (Djavan et al, 2005).
Fine-Needle Aspiration Biopsy
The use of transrectal fine-needle aspiration (FNA) of palpable abnormalities of the prostate still is advocated in many countries outside the United States, because it is less expensive, faster, and easier to perform and results in lower morbidity than any other technique. High sensitivity, specificity, and efficacy depend on appropriate training in performing transrectal FNA of the prostate and in interpreting the smears (Perez-Guillermo et al, 2005). Controversy exists as to whether FNA is as reliable as core biopsy for grading purposes (Algaba et al, 1996).
Transperineal Prostate Biopsy
Transperineal biopsy offers an approach to the prostate in those patients lacking a rectum (e.g., surgical extirpation, congenital anomaly). The patient is positioned in dorsal lithotomy with the perineum shaved and prepared as for a sterile surgical procedure. An end-fire ultrasound transducer is used. Despite significant limitations in visualization using this technique compared with TRUS, the prostate can and should be imaged in both the coronal and sagittal planes with calculation of the gland volume. Focal PZ hypoechoic lesions are difficult to visualize through the transperineal window, as are the SVs. The urethra will appear as a hypoechoic midline structure and may be readily identified by following the corpus spongiosum proximally from the base of the penis. Once the boundaries of the gland have been clearly delineated in the coronal plane, six cores should be taken, three from either side of midline.
The diagnostic yield of transperineal ultrasound–guided prostate biopsy was compared with that of TRUS-guided biopsies in a routine biopsy setting (Vis et al, 2000). By using radical prostatectomy specimens with TRUS biopsy–detected prostate cancer, simulated transperineal biopsies were performed and transrectal biopsies were repeated. Significantly, 82.5% of the known tumors were detected with the longitudinal transperineal approach versus 72.5% of cancer detection with repeat transrectal biopsy. The authors postulate that the longitudinal orientation of their cores allows more efficient sampling of the PZ, thereby improving cancer detection. By using this approach in the repeat biopsy setting, researchers at Mayo Clinic in Jacksonville, Florida, obtained a mean of 21.2 cores (range, 12 to 41) in 210 men using a template perineal biopsy (Pinkstaff et al, 2005). The transperineal approach enhanced identification of transition zone cancers not detected by previous transrectal prostate biopsy in high-risk patients. Conversely, in a recent Japanese randomized trial comparing transrectal and transperineal techniques for initial prostate biopsy, the cancer detection rate was similar for both, with higher complications noted in the transperineal approach (Hara et al, 2008). Therefore the authors concluded that transrectal prostate biopsy should be the preferred technique for initial prostate biopsy.
Transurethral Prostate Biopsy
Transuretheral resection biopsy was once advocated for the diagnosis of TZ cancers or after negative TRUS sampling. In contemporary series, solitary TZ cancers, without concomitant peripheral zone tumors, are estimated to occur in less than 5% of prostate cancer patients (Pelzer et al, 2005). With improved TRUS techniques including local anesthesia, the TZ can be adequately sampled, and the value of transurethral biopsy has been questioned for the vast majority of patients (Bratt, 2006).
Risks and Complications of Prostate Biopsy
Postbiopsy Infections
Most infectious complications after TRUS biopsy are limited to symptomatic urinary tract infection and low-grade febrile illness, which can be readily treated with oral or intravenous antibiotics; however, rare case reports of fatal septicemia after prostate biopsy have been published (Breslin et al, 1978; Brewster et al, 1993; Bates et al, 1998; da Silva et al, 1999). Historical series prior to the routine use of antibiotic prophylaxis found bacteriuria in 32% to 36% of patients and bacteremia/febrile illness in 48% to 69% of patients undergoing TRUS biopsy (Brown et al, 1981; Crawford et al, 1982). Recent studies show that 2% of patients will go on to develop a febrile urinary tract infection, bacteremia, or acute prostatitis and require hospitalization for intravenous antibiotics (Kapoor et al, 1998; Lindert et al, 2000). Additional infections such as epididymitis have been reported infrequently (Donzella et al, 2004). There is considerable concern, however, regarding the rising incidence of community-acquired antibiotic-resistant organisms resistant to standard fluoroquinolone prophylaxis (Patel and Kirby, 2008).
Bleeding
Even with normal coagulation parameters, bleeding is the most common complication seen after prostate biopsy. As noted, any potential medications that can alter coagulation parameters, including herbal remedies, should be held for 5 to 7 days before biopsy and those on warfarin managed as noted. Two large European screening programs noted hematuria in 23% to 63% of men after sextant biopsy, with clot retention developing in 0.7% (Djavan et al, 2001b; Raaijmakers et al, 2002). Rectal bleeding is common and seen in 2.1% to 21.7% of patients (Enlund and Varenhorst, 1997; Djavan et al, 2001b). Rectal bleeding is typically minor and readily controlled with direct pressure by the ultrasound probe or digitally; persistent brisk hematochezia may require anoscopic intervention for control. Hematospermia, commonly seen postbiopsy, is of minimal clinical importance but can be cause for significant concern on the part of the patient if not discussed at the time of biopsy; 9.8% to 50.4% of men experience some blood in their ejaculate (Djavan et al, 2001b; Raaijmakers et al, 2002), which may persist for 4 to 6 weeks after prostate biopsy.
Other Complications
Excessive anxiety and discomfort from the endorectal probe may produce a moderate or severe vasovagal response in 1.4% to 5.3% of patients (Rodriguez and Terris, 1998; Djavan et al, 2001b) and may require termination of the procedure. Placing the patient in the Trendelenburg position and use of intravenous hydration usually resolve these symptoms, with further intervention as clinically indicated.
Acute urinary retention requiring temporary catheterization develops in 0.2% to 0.4% of patients after TRUS biopsy (Enlund and Varenhorst, 1997; Raaijmakers et al, 2002). Men with significantly enlarged glands and those with significant lower urinary tract symptoms (e.g., high International Prostate Symptoms Score), are more prone to develop retention (Rodriguez and Terris, 1998; Raaijmakers et al, 2002).
Advanced Ultrasonographic Techniques for Prostate Imaging
Color and Power Doppler TRUS
Color Doppler imaging is based on the frequency shift in the reflected sound waves from the frequency of insonation and thus depicts the velocity of blood flow in a directionally dependent manner (Fig. 97–6A). Color assignment is based on the direction of blood flow related to the orientation of the transducer receiving the signal; flow toward the transducer is depicted in shades of red and flow away in shades of blue; the color is not specific for arterial or venous flow. Power Doppler imaging (also known as enhanced color Doppler, color amplitude imaging [CAI], or color angiography) uses amplitude shift to detect flow in a velocity and directionally independent manner (Bude and Rubin, 1996) (see Fig. 97–6B). The advantages of power Doppler imaging are its ability to detect slower flow and to have less reliance on the Doppler angle, making it more suitable for detection of prostate cancer neovascularity. Although power Doppler imaging offers improved sensitivity to small amounts of flow, neither modality has yet proved itself superior to the other for cancer detection. In 251 patients, Halpern and Strup (2000) found color Doppler sensitivity and specificity of 14.6% and 93.9%, respectively, to identify cancer. Whereas Doppler modes showed an improved diagnosis versus gray-scale TRUS, 45% of cancers still went unidentified by any sonographic modality. Others have shown increased cancer detection rates using Doppler-targeted biopsy strategies (Kelly et al, 1993; Rifkin et al, 1993; Newman et al, 1995; Sakarya et al, 1998; Cornud et al, 2000; Okihara et al, 2000; Shigeno et al, 2000), but none is sufficiently accurate to replace systematic biopsy (Halpern et al, 2002a). Enhancements in the technical aspects of color Doppler TRUS, including the use of contrast agents (see later), may provide the necessary improvements to specifically identify cancer sites in the future.
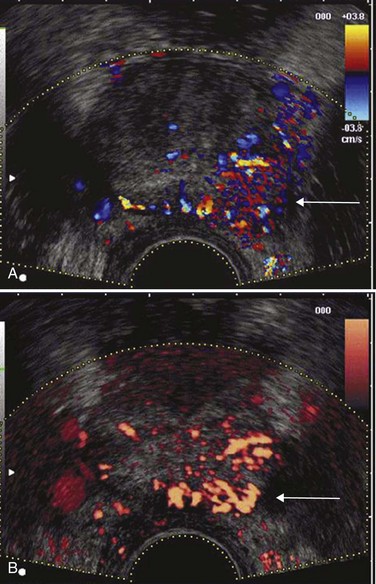
Figure 97–6 Color Doppler (A) transrectal ultrasonography (TRUS) and power Doppler (B) TRUS identify a Gleason 4 + 4 = 8 adenocarcinoma in the left midgland.
Multiple studies have shown that angioneogenesis and the resultant increase in microvessel density that occurs within foci of prostatic adenocarcinoma correlates with the presence of metastasis (Weidner et al, 1993), stage of disease (Fregene et al, 1993; Brawer et al, 1994; Bostwick et al, 1996), and disease-specific survival (Lissbrant et al, 1997; Borre et al, 1998). Interest in using color and power Doppler TRUS to aid in prostate cancer detection stems from studies of radical prostatectomy specimens demonstrating that foci of adenocarcinoma possess an increased density of microvessels compared with surrounding normal parenchyma (Bigler et al, 1993).
Patients with detectable color Doppler flow within their dominant tumor at the time of TRUS-guided biopsy are at a 10-fold increased risk for PSA recurrence after radical retropubic prostatectomy (Ismail et al, 1997). The presence of increased flow was also associated with a higher Gleason grade, increased incidence of SV invasion, and a lower biochemical disease-free (bNED) survival rate versus subjects without increased flow on preoperative TRUS (50% vs. 96% bNED at 31 months) (Ismail et al, 1997). Other investigators have also shown the association of power Doppler flow signals as an indicator of microvessel density with a higher Gleason score and have suggested a correlation with outcome (Wilson et al, 2004).
Current unenhanced Doppler modalities are not able to identify the microvessels of prostate cancer, which are typically 10 to 15 µm in diameter. The flow signals associated with malignant foci detected by unenhanced color and power Doppler imaging are due to detection of larger feeding vessels (Ismail and Gomella, 2001). Intravenous microbubble ultrasound contrast agents, similar to those currently approved and used in echocardiography, have been infused systemically during gray-scale and TRUS Doppler imaging to amplify flow signals within the microvasculature of prostate tumors, allowing selective visualization of malignant foci in clinical trials (Halpern et al, 2000; Ismail and Gomella, 2001). These intravenous “bubble” contrast agents are constructed with air or higher-molecular-weight gas agents encapsulated (albumin or polymer hard shell, lipid- or surfactant-coated) for longevity and are generally 1 to 10 µm.
Using contrast-enhanced TRUS (CE-TRUS) for prospective prostate cancer detection, Halpern and associates (Halpern et al, 2001) demonstrated an increase in sensitivity from 38% to 65% versus baseline unenhanced imaging, without significantly altering specificity. Subsequent studies by our group and others have improved sonographic detection of malignant foci using CE-TRUS and targeted biopsy of enhancing lesions (Frauscher et al, 2001; Halpern et al, 2002a; Roy et al, 2003; Halpern et al, 2005; Heijmink and Barentsz, 2007). In a multi-institutional trial involving several European centers, CE-TRUS has been recommended for routine care in prostate biopsy (Wink et al, 2008). Imaging using microbubble contrast agents combined with three-dimensional image reconstruction of enhanced power Doppler images also demonstrated increased diagnostic accuracy (Unal et al, 2000) (Fig. 97–7).Gray-scale harmonic imaging is a relatively newer method for imaging ultrasound contrast agents that provides better spatial and temporal resolution compared with color Doppler imaging. A variation on gray-scale harmonic imaging, flash-replenishment imaging, provides improved visualization of neovessels that are below the standard resolution of even gray-scale ultrasonography. Flash-replenishment imaging uses a combination of high-power flash pulses to destroy contrast microbubbles, followed by low- power pulses to demonstrate contrast replenishment. A composite image is constructed depicting the vascular architecture through maximum intensity capture of temporal data in consecutive low-power images, and it can be used for real-time targeted transrectal biopsy of areas of increased or abnormal vascularity. Using this technique, we have demonstrated much finer vascular detail for targeting biopsy, and targeted biopsy cores were significantly more likely to be cancerous than random systematic biopsy cores (Linden et al, 2007) (Fig. 97–8). Future developments in these and other imaging modalities that can selectively visualize prostate cancers based on the presence of angioneogenesis may ultimately allow more accurate localization of the sites of cancer.
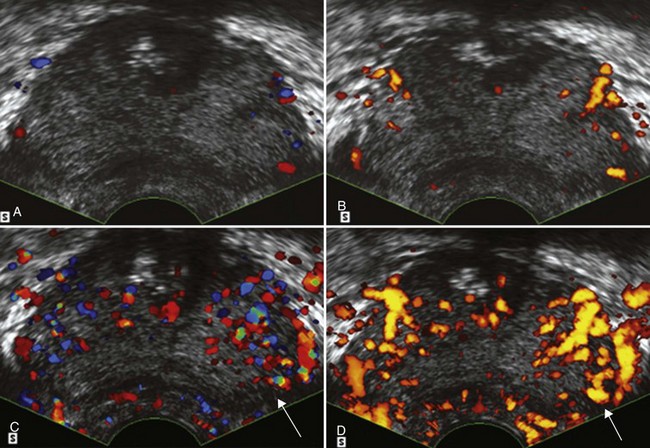
Figure 97–7 Unenhanced color (A) transrectal ultrasonography (TRUS) and power Doppler (B) TRUS fail to detect evidence of an underlying malignancy. After infusion of a microbubble contrast agent, color (C) TRUS and power Doppler (D) TRUS demonstrate an area of increased flow in the left midgland that proved to be a Gleason 3 + 4 = 7 adenocarcinoma on targeted biopsy (arrows).
Other Investigational Techniques
Artificial neural networks are another potential way to enhance TRUS images and identify malignant foci. Investigational automated image analysis, including pattern recognition and artificial neural networks applied to TRUS images, may successfully identify lesions that cannot be seen by the human eye (Loch et al, 1999).
A new sonographic technique known as elastography may prove to be superior to color Doppler imaging in the identification of malignant areas in the prostate (Nelson et al, 2007; Sumura et al, 2007). This technique employs real-time sonographic imaging of the prostate at baseline and under varying degrees of compression (Fig. 97–9). Through computerized calculations, differences in displacement between ultrasonic images from baseline and during compression may be visualized, and regions with decreased tissue elasticity may be tagged as suggestive of malignancy. In a preliminary study of 404 cases, with 151 cases positive for prostate cancer, the malignancy was found in 127 patients (84.1%) with real-time elastography directing the biopsy (Konig et al, 2005).
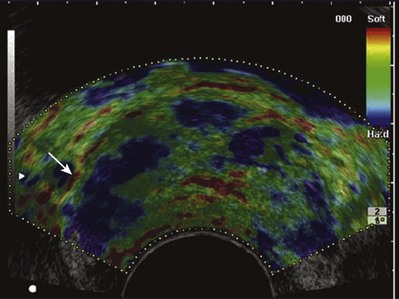
Figure 97–9 Elastography demonstrates an area of decreased compliance in the right base consistent with an underlying malignancy (blue near arrow). Note color scale in upper right corner indicating relative tissue “firmness.” Targeted biopsy of this region revealed a Gleason 4 + 4 = 8 adenocarcinoma.
Endorectal magnetic resonance imaging (MRI) and MR spectroscopy as combined modalities might be able to guide and therefore limit the number of iterative biopsies and cores for patients (Amsellem-Ouazana et al, 2005). Use of MRI will require modifications in instrumentation and the technique of biopsy (Beyersdorff and Hamm, 2005). These MRI-directed biopsy techniques require expensive equipment that is not widely available for biopsy procedures.
New Doppler, contrast medium–enhanced, and other developing techniques have the potential to allow accurate localization and diagnosis of prostate cancer and minimize or eliminate the need for multiple biopsy sites to diagnose prostate cancer in the future. However, until these techniques are proved superior in the localization of prostate cancer, systemic TRUS gray-scale core needle biopsy will continue to be regarded as the gold standard for the diagnosis of prostate cancer.
Key Points: Advanced Ultrasonographic Techniques for Prostate Imaging
Djavan B, Waldert M, et al. Safety and morbidity of first and repeat transrectal ultrasound-guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol. 2001;166:856-860.
Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71-74. discussion 74–5
Ismail M, Gomella LG. Ultrasound and enhanced ultrasound in the management of prostate cancer. Curr Opin Urol. 2001;11:471-477.
Linden RA, Halpern EJ. Advances in transrectal ultrasound imaging of the prostate. Semin Ultrasound CT MR. 2007;28(4):249-257.
Presti JC, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163(1):163-166. discussion 166–7
Trucchi A, De Nunzio C, et al. Local anesthesia reduces pain associated with transrectal prostatic biopsy: a prospective randomized study. Urol Int. 2005;74:209-213.
Al-Saeed O, Sheikh M, et al. Seminal vesicle masses detected incidentally during transrectal sonographic examination of the prostate. J Clin Ultrasound. 2003;31(4):201-206.
Algaba F, Epstein JI, et al. Assessment of prostate carcinoma in core needle biopsy—definition of minimal criteria for the diagnosis of cancer in biopsy material. Cancer. 1996;78(2):376-381.
American Urological Association. Prostate-specific antigen (PSA) best practice policy. Oncology (Williston Park). 2000;14(2):267-272. 277–8, 280 passim
Amsellem-Ouazana D, Younes P, et al. Negative prostatic biopsies in patients with a high risk of prostate cancer. Is the combination of endorectal MRI and magnetic resonance spectroscopy imaging (MRSI) a useful tool? A preliminary study. Eur Urol. 2005;47(5):582-586.
Ashley RA, Inman BA, et al. Reassessing the diagnostic yield of saturation biopsy of the prostate. Eur Urol. 2008;53(5):976-981.
Babaian RJ, Toi A, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163(1):152-157.
Bates TS, Porter T, et al. Prophylaxis for transrectal prostatic biopsies: a randomized controlled study of intravenous co-amoxiclav given as a single dose compared with an intravenous dose followed by oral co-amoxiclav for 24 h. Br J Urol. 1998;81(4):529-531.
Beerlage HP. Alternative therapies for localized prostate cancer. Curr Urol Rep. 2003;4(3):216-220.
Berger AP, Frauscher F, et al. Periprostatic administration of local anesthesia during transrectal ultrasound-guided biopsy of the prostate: a randomized, double-blind, placebo-controlled study. Urology. 2003;61(3):585-588.
Beyersdorff D, Hamm B. [MRI for troubleshooting detection of prostate cancer]. Rofo. 2005;177(6):788-795.
Bigler SA, Deering RE, et al. Comparison of microscopic vascularity in benign and malignant prostate tissue. Hum Pathol. 1993;24(2):220-226.
Borboroglu PG, Comer SW, et al. Extensive repeat transrectal ultrasound guided prostate biopsy in patients with previous benign sextant biopsies. J Urol. 2000;163(1):158-162.
Borre M, Offersen BV, et al. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998;78(7):940-944.
Bostwick DG, Wheeler TM, et al. Optimized microvessel density analysis improves prediction of cancer stage from prostate needle biopsies. Urology. 1996;48(1):47-57.
Bratt O. The difficult case in prostate cancer diagnosis—when is a diagnostic TURP indicated? Eur Urol. 2006;49(5):769-771.
Brawer MK, Deering RE, et al. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer. 1994;73(3):678-687.
Breslin JA, Turner BI, et al. Anaerobic infection as a consequence of transrectal prostatic biopsy. J Urol. 1978;120(4):502-503.
Brewster SF, Rooney N, et al. Fatal anaerobic infection following transrectal biopsy of a rare prostatic tumour. Br J Urol. 1993;72(6):977-978.
Brossner C, Bayer G, et al. Twelve prostate biopsies detect significant cancer volumes (> 0.5 mL). BJU Int. 2000;85(6):705-707.
Brown RW, Warner JJ, et al. Bacteremia and bacteriuria after transrectal prostatic biopsy. Urology. 1981;18(2):145-148.
Bude RO, Rubin JM. Power Doppler sonography. Radiology. 1996;200(1):21-23.
Cam K, Sener M, et al. Combined periprostatic and intraprostatic local anesthesia for prostate biopsy: a double-blind, placebo controlled, randomized trial. J Urol. 2008;180(1):141-144. discussion 144–5
Carter HB, Ferrucci L, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98(21):1521-1527.
Carter HB, Pearson JD, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267(16):2215-2220.
Catalona WJ, Partin AW, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279(19):1542-1547.
Catalona WJ, Smith DS, et al. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270(8):948-954.
Chang JJ, Shinohara K, et al. Prospective evaluation of lateral biopsies of the peripheral zone for prostate cancer detection. J Urol. 1998;160(6 Pt. 1):2111-2114.
Chang JJ, Shinohara K, et al. Prospective evaluation of systematic sextant transition zone biopsies in large prostates for cancer detection. Urology. 1998;52(1):89-93.
Cochlin DL. Cysts and congenital anomalies of the prostate and ejaculatory ducts. In: Halpern EJ, Cochlin DL, Golberg BB, editors. Imaging of the prostate. London: Martin Dunitz; 2002:115-128.
Cornud F, Hamida K, et al. Endorectal color Doppler sonography and endorectal MR imaging features of nonpalpable prostate cancer: correlation with radical prostatectomy findings. AJR Am J Roentgenol. 2000;175(4):1161-1168.
Crawford ED, Haynes ALJr, et al. Prevention of urinary tract infection and sepsis following transrectal prostatic biopsy. J Urol. 1982;127(3):449-451.
da Silva E, Pereiro Alvarez B, et al. [Peritonitis following transrectal biopsy of the prostate]. Arch Esp Urol. 1999;52(2):167-168.
Davidson D, Bostwick DG, et al. Prostatic intraepithelial neoplasia is a risk factor for adenocarcinoma: predictive accuracy in needle biopsies. J Urol. 1995;154(4):1295-1299.
Dehnad H, Nederveen AJ, et al. Clinical feasibility study for the use of implanted gold seeds in the prostate as reliable positioning markers during megavoltage irradiation. Radiother Oncol. 2003;67(3):295-302.
Djavan B, Milani S, et al. Prostate biopsy: who, how and when. An update. Can J Urol. 2005;12(Suppl. 1):44-48. discussion 99–100
Djavan B, Ravery V, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166(5):1679-1683.
Djavan B, Remzi M, et al. Complexed prostate-specific antigen, complexed prostate-specific antigen density of total and transition zone, complexed/total prostate-specific antigen ratio, free-to-total prostate-specific antigen ratio, density of total and transition zone prostate-specific antigen: results of the prospective multicenter European trial. Urology. 2002;60(4 Suppl. 1):4-9.
Djavan B, Remzi M, et al. When to biopsy and when to stop biopsying. Urol Clin North Am. 2003;30(2):253-262. viii
Djavan B, Waldert M, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol. 2001;166(3):856-860.
Djavan B, Zlotta A, et al. Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1,051 men. J Urol. 2000;163(4):1144-1148. discussion 1148–9
Donzella JG, Merrick GS, et al. Epididymitis after transrectal ultrasound-guided needle biopsy of prostate gland. Urology. 2004;63(2):306-308.
Durkan GC, Sheikh N, et al. Improving prostate cancer detection with an extended-core transrectal ultrasonography-guided prostate biopsy protocol. BJU Int. 2002;89(1):33-39.
Egawa S, Wheeler TM, et al. The sonographic appearance of irradiated prostate cancer. Br J Urol. 1991;68(2):172-177.
Enlund AL, Varenhorst E. Morbidity of ultrasound-guided transrectal core biopsy of the prostate without prophylactic antibiotic therapy. A prospective study in 415 cases. Br J Urol. 1997;79(5):777-780.
Epstein JI, Walsh PC, et al. Use of repeat sextant and transition zone biopsies for assessing extent of prostate cancer. J Urol. 1997;158(5):1886-1890.
Eskew LA, Bare RL, et al. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157(1):199-202. discussion 202–3
Fink KG, Hutarew G, et al. One 10-core prostate biopsy is superior to two sets of sextant prostate biopsies. BJU Int. 2003;92(4):385-388.
Fleshner N, Klotz L. Role of saturation biopsy in the detection of prostate cancer among difficult diagnostic cases. Urology. 2002;60(1):93-97.
Frauscher F, Klauser A, et al. Detection of prostate cancer with a microbubble ultrasound contrast agent. Lancet. 2001;357(9271):1849-1850.
Frauscher F, Klauser A, et al. Advances in ultrasound for the detection of prostate cancer. Ultrasound Q. 2002;18(2):135-142.
Fregene TA, Khanuja PS, et al. Tumor-associated angiogenesis in prostate cancer. Anticancer Res. 1993;13(6B):2377-2381.
Geramoutsos I, Gyftopoulos K, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol. 2004;45(3):333-337. discussion 337–8
Gohji K, Morisue K, et al. Correlation of transrectal ultrasound imaging and the results of systematic biopsy with pathological examination of radical prostatectomy specimens. Br J Urol. 1995;75(6):758-765.
Goldenberg SL, Carter M, et al. Sonographic characteristics of the urethrovesical anastomosis in the early post-radical prostatectomy patient. J Urol. 1992;147(5):1307-1309.
Gregg DC, Sty JR. Sonographic diagnosis of enlarged prostatic utricle. J Ultrasound Med. 1989;8(1):51-52.
Griffiths K. Molecular control of prostate growth. In: Kirby R, editor. Textbook of benign prostatic hyperplasia. Oxford (UK): Isis Medical Media, 1996.
Halpern EJ. Anatomy of the prostate gland. In: Halpern EJ, Cochlin DL, Goldberg BB, editors. Imaging of the prostate. London: Martin Dunitz; 2002:3-15.
Halpern EJ, Frauscher F, et al. Directed biopsy during contrast-enhanced sonography of the prostate. AJR Am J Roentgenol. 2002;178(4):915-919.
Halpern EJ, Frauscher F, et al. High-frequency Doppler US of the prostate: effect of patient position. Radiology. 2002;222(3):634-639.
Halpern EJ, Ramey JR, et al. Detection of prostate carcinoma with contrast-enhanced sonography using intermittent harmonic imaging. Cancer. 2005;104(11):2373-2383.
Halpern EJ, Rosenberg M, et al. Prostate cancer: contrast-enhanced us for detection. Radiology. 2001;219(1):219-225.
Halpern EJ, Strup SE. Using gray-scale and color and power Doppler sonography to detect prostatic cancer. AJR Am J Roentgenol. 2000;174(3):623-627.
Halpern EJ, Verkh L, et al. Initial experience with contrast-enhanced sonography of the prostate. AJR Am J Roentgenol. 2000;174(6):1575-1580.
Hamper UM, Epstein JI, et al. Cystic lesions of the prostate gland. A sonographic-pathologic correlation. J Ultrasound Med. 1990;9(7):395-402.
Hara R, Jo Y, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008;71(2):191-195.
Heijmink SW, Barentsz JO. Contrast-enhanced versus systematic transrectal ultrasound-guided prostate cancer detection: an overview of techniques and a systematic review. Eur J Radiol. 2007;63(3):310-316.
Hodge KK, McNeal JE, et al. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142(1):66-70.
Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142(1):71-74. discussion 74–5
Iczkowski KA, Bassler TJ, et al. Diagnosis of suspicious for malignancy in prostate biopsies: predictive value for cancer. Urology. 1998;51(5):749-757. discussion 757–8
Iczkowski KA, Ferguson KL, et al. Adenoid cystic/basal cell carcinoma of the prostate: clinicopathologic findings in 19 cases. Am J Surg Pathol. 2003;27(12):1523-1529.
Ismail M, Gomella LG. Ultrasound for prostate imaging and biopsy. Curr Opin Urol. 2001;11(5):471-477.
Ismail M, Petersen RO, et al. Color Doppler imaging in predicting the biologic behavior of prostate cancer: correlation with disease-free survival. Urology. 1997;50(6):906-912.
Issa MM, Bux S, et al. A randomized prospective trial of intrarectal lidocaine for pain control during transrectal prostate biopsy: the Emory University experience. J Urol. 2000;164(2):397-399.
Jemal A, Siegel R, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
Kapoor DA, Klimberg IW, et al. Single-dose oral ciprofloxacin versus placebo for prophylaxis during transrectal prostate biopsy. Urology. 1998;52(4):552-558.
Kapoor DA, Wasserman NF, et al. Value of transrectal ultrasound in identifying local disease after radical prostatectomy. Urology. 1993;41(6):594-597.
Kaye KW. Prostate biopsy using automatic gun. Technique for determination of precise biopsy site. Urology. 1989;34(2):111-112.
Keetch DW, Catalona WJ, et al. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151(6):1571-1574.
Kelly IM, Lees WR, et al. Prostate cancer and the role of color Doppler US. Radiology. 1993;189(1):153-156.
King BF, Hattery RR, et al. Congenital cystic disease of the seminal vesicle. Radiology. 1991;178(1):207-211.
Konig K, Scheipers U, et al. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174(1):115-117.
Kossoff G. Basic physics and imaging characteristics of ultrasound. World J Surg. 2000;24(2):134-142.
Kronz JD, Allan CH, et al. Predicting cancer following a diagnosis of high-grade prostatic intraepithelial neoplasia on needle biopsy: data on men with more than one follow-up biopsy. Am J Surg Pathol. 2001;25(8):1079-1085.
Krumholtz JS, Carvalhal GF, et al. Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002;60(3):469-473. discussion 473–4
Lee HY, Lee HJ, et al. Effect of intraprostatic local anesthesia during transrectal ultrasound guided prostate biopsy: comparison of 3 methods in a randomized, double-blind, placebo controlled trial. J Urol. 2007;178(2):469-472. discussion 472
Levin HS. Current issues in pathologic evaluation. In: Klein EA, editor. Current clinical management of prostate cancer. Totowa (NJ): Humana, 2004.
Levine MA, Ittman M, et al. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998;159(2):471-475. discussion 475–6
Linden RA, Trabulsi EJ, et al. Contrast enhanced ultrasound flash replenishment method for directed prostate biopsies. J Urol. 2007;178(6):2354-2358.
Linden RA, Weiner PR, et al. Technique of outpatient placement of intraprostatic fiducial markers before external beam radiotherapy. Urology. 2009;73(4):881-886.
Lindert KA, Kabalin JN, et al. Bacteremia and bacteriuria after transrectal ultrasound guided prostate biopsy. J Urol. 2000;164(1):76-80.
Lissbrant IF, Stattin P, et al. Vascular density is a predictor of cancer-specific survival in prostatic carcinoma. Prostate. 1997;33(1):38-45.
Loch T, Leuschner I, et al. Artificial neural network analysis (ANNA) of prostatic transrectal ultrasound. Prostate. 1999;39(3):198-204.
Marks LS, Partin AW, et al. Prostate tissue composition and response to finasteride in men with symptomatic benign prostatic hyperplasia. J Urol. 1997;157(6):2171-2178.
Mazal PR, Haitel A, et al. Spatial distribution of prostate cancers undetected on initial needle biopsies. Eur Urol. 2001;39(6):662-668.
McDermott V, Orr JD, et al. Duplicated mullerian duct remnants associated with unilateral renal agenesis. Abdom Imaging. 1993;18(2):193-195.
McNeal JE, Redwine EA, et al. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12(12):897-906.
Nash PA, Bruce JE, et al. Transrectal ultrasound guided prostatic nerve blockade eases systematic needle biopsy of the prostate. J Urol. 1996;155(2):607-609.
Naughton CK, Miller DC, et al. A prospective randomized trial comparing 6 versus 12 prostate biopsy cores: impact on cancer detection. J Urol. 2000;164(2):388-392.
Naya Y, Okihara K, et al. Efficacy of prostatic fossa biopsy in detecting local recurrence after radical prostatectomy. Urology. 2005;66(2):350-355.
Nelson ED, Slotoroff CB, et al. Targeted biopsy of the prostate: the impact of color Doppler imaging and elastography on prostate cancer detection and Gleason score. Urology. 2007;70(6):1136-1140.
Newman JS, Bree RL, et al. Prostate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology. 1995;195(1):86-90.
Obek C, Ozkan B, et al. Comparison of 3 different methods of anesthesia before transrectal prostate biopsy: a prospective randomized trial. J Urol. 2004;172(2):502-505.
Okihara K, Kojima M, et al. Transrectal power Doppler imaging in the detection of prostate cancer. BJU Int. 2000;85(9):1053-1057.
Onur R, Littrup PJ, et al. Contemporary impact of transrectal ultrasound lesions for prostate cancer detection. J Urol. 2004;172(2):512-514.
Ouyang RC, Kenwright DN, et al. The presence of atypical small acinar proliferation in prostate needle biopsy is predictive of carcinoma on subsequent biopsy. BJU Int. 2001;87(1):70-74.
Patel U, Kirby R. Infections after prostate biopsy and antibiotic resistance. BJU Int. 2008;101(10):1201-1202.
Pelzer AE, Bektic J, et al. Are transition zone biopsies still necessary to improve prostate cancer detection? Results from the Tyrol Screening Project. Eur Urol. 2005;48(6):916-921. discussion 921
Perez-Guillermo M, Acosta-Ortega J, et al. The continuing role of fine-needle aspiration of the prostate gland into the 21st century: a tribute to Torsten Lowhagen. Diagn Cytopathol. 2005;32(5):315-320.
Pinkstaff DM, Igel TC, et al. Systematic transperineal ultrasound-guided template biopsy of the prostate: three-year experience. Urology. 2005;65(4):735-739.
Presti JCJr, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163(1):163-166. discussion 166–7
Purohit RS, Shinohara K, et al. Imaging clinically localized prostate cancer. Urol Clin North Am. 2003;30(2):279-293.
Raaijmakers R, Kirkels WJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60(5):826-830.
Rifkin MD, Sudakoff GS, et al. Prostate: techniques, results, and potential applications of color Doppler US scanning. Radiology. 1993;186(2):509-513.
Rodriguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: a prospective study and review of the literature. J Urol. 1998;160(6 Pt. 1):2115-2120.
Roy C, Buy X, et al. Contrast enhanced color Doppler endorectal sonography of prostate: efficiency for detecting peripheral zone tumors and role for biopsy procedure. J Urol. 2003;170(1):69-72.
Sabbagh R, McCormack M, et al. A prospective randomized trial of 1-day versus 3-day antibiotic prophylaxis for transrectal ultrasound guided prostate biopsy. Can J Urol. 2004;11(2):2216-2219.
Sakarya ME, Arslan H, et al. The role of power Doppler ultrasonography in the diagnosis of prostate cancer: a preliminary study. Br J Urol. 1998;82(3):386-388.
Scardino P. Update: NCCN prostate cancer Clinical Practice Guidelines. J Natl Compr Canc Netw. 2005;3(Suppl. 1):S29-S33.
Scattoni V, Montorsi F, et al. Diagnosis of local recurrence after radical prostatectomy. BJU Int. 2004;93(5):680-688.
Shigeno K, Igawa M, et al. The role of colour Doppler ultrasonography in detecting prostate cancer. BJU Int. 2000;86(3):229-233.
Shinohara K, Wheeler TM, et al. The appearance of prostate cancer on transrectal ultrasonography: correlation of imaging and pathological examinations. J Urol. 1989;142(1):76-82.
Soloway MS, Obek C. Periprostatic local anesthesia before ultrasound guided prostate biopsy. J Urol. 2000;163(1):172-173.
Stewart CS, Leibovich BC, et al. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2001;166(1):86-91. discussion 91–2
Stone NN, Stock RG. The effect of brachytherapy, external beam irradiation and hormonal therapy on prostate volume. J Urol. 2007;177(3):925-928.
Sudakoff GS, Smith R, et al. Color Doppler imaging and transrectal sonography of the prostatic fossa after radical prostatectomy: early experience. AJR Am J Roentgenol. 1996;167(4):883-888.
Sumura M, Shigeno K, et al. Initial evaluation of prostate cancer with real-time elastography based on step-section pathologic analysis after radical prostatectomy: a preliminary study. Int J Urol. 2007;14(9):811-816.
Tamsel S, Killi R, et al. The potential value of power Doppler ultrasound imaging compared with grey-scale ultrasound findings in the diagnosis of local recurrence after radical prostatectomy. Clin Radiol. 2006;61(4):325-330. discussion 323–4
Terris MK. Transrectal ultrasound appearance of radiation-induced prostatic sarcoma. Prostate. 1998;37(3):182-186.
Terris MK. Transrectal ultrasound appearance of squamous cell carcinoma involving the prostate. Urol Int. 1999;63(2):133-135.
Terris MK, Freiha FS. Transrectal ultrasound appearance of hematolymphoid malignancies involving the prostate. Urology. 1998;51(2):339-341.
Terris MK, Macy M, et al. Transrectal ultrasound appearance of prostatic granulomas secondary to bacillus Calmette-Guerin instillation. J Urol. 1997;158(1):126-127.
Terris MK, McNeal JE, et al. Detection of clinically significant prostate cancer by transrectal ultrasound-guided systematic biopsies. J Urol. 1992;148(3):829-832.
Terris MK, Pham TQ, et al. Routine transition zone and seminal vesicle biopsies in all patients undergoing transrectal ultrasound guided prostate biopsies are not indicated. J Urol. 1997;157(1):204-206.
Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol. 1991;145(5):984-987.
Thompson IM, Ankerst DP, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294(1):66-70.
Thompson IM, Pauler DK, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246.
Trucchi A, De Nunzio C, et al. Local anesthesia reduces pain associated with transrectal prostatic biopsy. A prospective randomized study. Urol Int. 2005;74(3):209-213.
Unal D, Sedelaar JP, et al. Three-dimensional contrast-enhanced power Doppler ultrasonography and conventional examination methods: the value of diagnostic predictors of prostate cancer. BJU Int. 2000;86(1):58-64.
Varghese SL, Grossfeld GD. The prostatic gland: malignancies other than adenocarcinomas. Radiol Clin North Am. 2000;38(1):179-202.
Vis AN, Boerma MO, et al. Detection of prostate cancer: a comparative study of the diagnostic efficacy of sextant transrectal versus sextant transperineal biopsy. Urology. 2000;56(4):617-621.
Watanabe H, Kato H, et al. [Diagnostic application of ultrasonotomography to the prostate]. Nippon Hinyokika Gakkai Zasshi. 1968;59(4):273-279.
Weidner N, Carroll PR, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143(2):401-409.
Whittington R, Broderick GA, et al. The effect of androgen deprivation on the early changes in prostate volume following transperineal ultrasound guided interstitial therapy for localized carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1999;44(5):1107-1110.
Wilson NM, Masoud AM, et al. Correlation of power Doppler with microvessel density in assessing prostate needle biopsy. Clin Radiol. 2004;59(10):946-950.
Wink M, Frauscher F, et al. Contrast-enhanced ultrasound and prostate cancer; a multicentre European research coordination project. Eur Urol. 2008;54(5):982-992.
Wolf JSJr, Bennett CJ, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179(4):1379-1390.
Wolters T, Roobol MJ, et al. Is prostate-specific antigen velocity selective for clinically significant prostate cancer in screening? European Randomized Study of Screening for Prostate Cancer (Rotterdam). Eur Urol. 2009;55(2):385-392.