chapter 99 Early Detection, Diagnosis, and Staging of Prostate Cancer
Prostate cancer rarely causes symptoms at an early stage. The presence of symptoms suggests locally advanced or metastatic disease. Manifestations of locally advanced prostate cancer include urinary symptoms, ureteral obstruction causing renal failure, hematospermia or decreased ejaculate volume, and, rarely, impotence. Manifestations of metastatic disease include bone pain, pathologic fractures, anemia, and lower extremity edema, and, less commonly, malignant retroperitoneal fibrosis, paraneoplastic syndromes, and disseminated intravascular coagulation (DIC). Locally advanced and metastatic disease are uncommon presentations due to widespread screening with prostate-specific antigen (PSA) and digital rectal examination (DRE). A histologic diagnosis of prostate cancer is typically made by prostate needle biopsy using transrectal ultrasound guidance.
Following a diagnosis of prostate cancer, the goal of staging is the accurate determination of disease extent for management decisions and prognostication. In addition to PSA and DRE, the pathologic features of a prostate biopsy (grade and extent) help inform management decisions. Imaging studies are useful in specific situations.
This chapter will review diagnostic and staging modalities for prostate cancer.
Early Detection
Screening
General Concepts of Screening
Screening refers to testing for disease in healthy, asymptomatic populations, whereas diagnosis is the identification of disease among individuals with signs or symptoms. The principal goal of screening is to improve overall health outcomes by identifying and treating disease at an earlier stage. Despite the common misperception that early diagnosis through screening is invariably beneficial, it also has the potential for harm (Welch, 2004). There is strong evidence that prostate cancer screening reduces the rates of advanced disease (van der Cruijsen-Koeter et al, 2006; Aus et al, 2007), but controversy exists regarding the balance of benefit and harm (Barry, 2009).
From 1993 to 2003 after the onset of widespread PSA testing, the mortality rate from prostate cancer declined by 32.5% (Surveillance, Epidemiology, and End Results [SEER] Program), along with a 75% reduction in the proportion of advanced-stage disease at diagnosis. It was estimated that PSA screening could have accounted for 45% to 70% of this reduction in prostate cancer mortality in the United States (Etzioni et al, 2008). However, randomized trials comparing the disease-specific outcomes of men who are screened and those who are not represent the highest level of evidence for screening.
Randomized Trials
The Prostate, Lung, Colon, and Ovary (PLCO) trial of the National Cancer Institute (NCI) and the European Randomized Trial of Prostate Cancer Screening (ERSPC) were initiated in 1993 to compare prostate cancer–specific mortality (primary end point) between screened and unscreened arms (Auvinen et al, 1996; de Koning et al, 2002; Schroder, 2003; Andriole et al, 2004). Reductions in high-grade cancer and locally advanced/metastatic disease with serial screening were reported in the ERSPC (van der Cruijsen-Koeter et al, 2006; Aus et al, 2007), as well as a 20% reduction in prostate cancer–specific mortality among those screened compared with controls at a median follow-up of 9 years (Schroder et al, 2009). However, in the ERSPC, it was estimated that to prevent one prostate cancer death would require screening 1410 men and treating an additional 48 men. With longer follow-up, the Goteborg randomized population-based screening trial reported a greater mortality benefit with screening. By comparison, there was no difference in prostate cancer mortality between the screened and control arms of the PLCO at a median follow-up of 11 years (Andriole et al, 2009). Both trials reported results early when considering the long natural history of prostate cancer; and, with further follow-up, the findings could change. Further, the disparate findings between the studies may result from high rates of screening in the control arm of the PLCO (contamination) and lesser ability to detect a mortality difference given that the PLCO had approximately threefold fewer events than the ERSPC (Barry, 2009).
These randomized trials emphasize the potential for overdiagnosis (detection of cancers that would have otherwise remained undetected) and overtreatment of prostate cancer with screening. Overtreatment is especially concerning among older men (age greater than 65 years), for whom treatment was associated with minimal benefit in a randomized trial of surgery versus watchful waiting (Bill-Axelson et al, 2008). Given that the average age at diagnosis today is approximately 67 years, the risk of overtreatment is high.
Specialty Group Recommendations
Professional groups have published statements and guidelines on prostate cancer screening (National Comprehensive Cancer Network, 2007; Lim and Sherin, 2008; Lin et al, 2008; U.S. Preventive Services Task Force, 2008). The U.S. Preventive Services Task Force (2008) concluded that the evidence is insufficient to assess the balance of benefit and harm of screening among men age 75 years or less, whereas screening is not recommended for men age 75 years or older. The American College of Preventive Medicine recommends against routine population screening with PSA and DRE (Lim and Sherin, 2008) and suggests shared decision making for men age 50 years or older with a life expectancy greater than 10 years. The American Cancer Society and the American Urological Association recommend annual prostate cancer screening beginning at age 50 years for average-risk men and earlier for higher-risk men (positive family history or black race). The National Comprehensive Cancer Network (2007) recommends offering baseline PSA screening at age 40 years with the frequency of follow-up testing based on PSA test results. The appropriate age to start and discontinue screening (Ross et al, 2005; Catalona et al, 2006; Schaeffer, 2009) and the appropriate interval between screens will continue to be a matter of debate (Carter et al, 1997; Ross et al, 2000; Hugosson et al, 2003b).
Despite the controversy associated with prostate cancer screening and disparate recommendations from professional organizations, opportunistic prostate cancer screening is highly prevalent in the United States (Lu-Yao et al, 2003; Sirovich et al, 2003; Ross et al, 2004; Schwartz et al, 2004; Chan et al, 2006; Walter et al, 2006). Although prostate cancer screening remains controversial, men who present for periodic health examinations should be made aware of the availability of the PSA test so that they can make an informed decision whether or not to be screened.
Diagnostic Modalities
Digital Rectal Examination
Before the availability of PSA, physicians relied solely on DRE for early detection of prostate cancer (Cooner et al, 1990; Catalona et al, 1994; Ellis et al, 1994; Schroder et al, 1998; Vis et al, 2001; Okotie et al, 2007). DRE has only fair reproducibility in the hands of experienced examiners (Smith and Catalona, 1995) and misses a substantial proportion of early cancers (Cooner et al, 1990; Catalona et al, 1994; Ellis et al, 1994). It has been suggested that the value of DRE for screening at PSA levels less than 3.0 ng/mL is limited (Schroder et al, 1998; Vis et al, 2001). Due to the risk of prostate cancer among men with abnormalities on DRE and the simplicity of the examination, most urologists use PSA and DRE together for prostate cancer detection.
Further, PSA improves the positive predictive value of DRE for cancer (Schroder et al, 1998). The positive predictive value of DRE ranged from 4% to 11% in men with PSA levels of 0 to 2.9 ng/mL, and from 33% to 83% in men with PSA levels of 3 to 9.9 ng/mL or more (Schroder et al, 1998).
Overall, when DRE and PSA are used in prostate cancer screening, detection rates are higher with PSA than with DRE and highest with both tests together (Catalona et al, 1991). Because DRE and PSA do not always detect the same cancers (Okotie et al, 2007), the tests are complementary and are recommended in combination as methods of assessing prostate cancer risk.
Prostate-Specific Antigen (PSA)
PSA is a member of the human kallikrein gene family, is secreted in high concentrations (mg/mL) into seminal fluid, and circulates in bound (complexed) and unbound (free) forms that can be measured using assays approved by the U.S. Food and Drug Administration (FDA).
Factors Influencing PSA
Serum PSA levels vary with age, race, and prostate volume. Blacks without prostate cancer have higher PSA values than whites (Morgan et al, 1996; Fowler et al, 1999). PSA increases 4% per milliliter of prostate volume; and 30% and 5% of the variance in PSA can be accounted for by prostate volume and age, respectively (Oesterling et al, 1993).
PSA expression is strongly influenced by androgens (Young et al, 1991; Henttu et al, 1992). Serum PSA becomes detectable at puberty with increases in luteinizing hormone and testosterone (Vieira et al, 1994). In hypogonadal men with low testosterone levels, serum PSA may be low because of decreased expression and may not reflect the presence of prostate disease such as cancer (Morgentaler et al, 1996).
Metabolic factors can influence the serum PSA concentration. Obese men have slightly lower PSA levels than nonobese men (Baillargeon et al, 2005), possibly due to hemodilution (Banez et al, 2007). Statin use may reduce PSA levels by lowering lipids (Hamilton et al, 2008).
Overall, the presence of prostate disease (prostate cancer, benign prostatic hyperplasia [BPH], and prostatitis) is the most important factor affecting serum PSA levels (Wang et al, 1981; Ercole et al, 1987; Dalton, 1989; Nadler et al, 1995). Although PSA elevations may indicate the presence of prostate disease, not all men with prostate disease have elevated PSA levels, and PSA elevations are not specific for cancer.
It is postulated that serum PSA elevations occur from disruption of the normal prostatic architecture, allowing PSA to gain access to the circulation. This can occur in the setting of prostate disease (BPH, prostatitis, prostate cancer) and with prostate manipulation (e.g., prostate massage, prostate biopsy, transurethral resection) (Klein and Lowe, 1997). Although DRE can lead to slight increases in serum PSA, the resultant change in PSA falls within the error of the assay and rarely causes false-positive tests (Crawford et al, 1992).
Studies examining the effect of ejaculation on serum PSA have reported conflicting results (Simak et al, 1993; Kirkali et al, 1995; Tchetgen et al, 1996; Heidenreich et al, 1997; Herschman et al, 1997; Stenner et al, 1998; Yavascaoglu et al, 1998). A repeat PSA after 48 hours of sexual abstinence may be helpful for interpreting serum PSA levels that are minimally elevated.
Prostate-directed treatments (for BPH or prostate cancer) can lower serum PSA by decreasing the volume of prostatic epithelium available for PSA production and by decreasing the amount of PSA produced per cell (Shingleton et al, 2000).
5α-Reductase inhibitors that are used for BPH treatment have been shown to lower PSA levels, including both type 2 isoenzyme inhibitors (finasteride) and dual type 1 and 2 isoenzyme inhibitors (dutasteride) (Guess et al, 1993; Roehrborn et al, 2002). Finasteride 1 mg (trade name Propecia) used for male pattern hair loss (androgenic alopecia) results in the same decline in serum PSA levels as the 5-mg dosage (D’Amico and Roehrborn, 2007).
Men initiating treatment with 5α-reductase inhibitors should first have a baseline PSA measurement and should be followed with serial measurements. Although the PSA level is often multiplied by two to estimate the “true” PSA level of a patient who has been taking a 5α-reductase inhibitor for 12 months or more (Andriole et al, 1998), the PSA response to finasteride treatment can be highly variable (Marks et al, 2006). It has been suggested that the PSA should instead be multiplied by a factor of 2.3 after 2 years and 2.5 after 7 years of treatment (Etzioni et al, 2005; Thompson et al, 2007; Walsh, 2008). Because this “moving target” can complicate the use of PSA in daily clinical practice, some have recommended using the PSA nadir on finasteride as the new baseline and performing a biopsy for subsequent PSA increases (Morgentaler, 2007).
Surgical therapy for BPH can lead to reductions in the serum PSA level (Shingleton et al, 2000) and “reset” the PSA baseline to a variable extent by removing the main contributor to PSA (transition zone).
Finally, prostate cancer treatments (medical or surgical), such as manipulation of the hormonal axis (e.g., luteinizing hormone–releasing hormone (LHRH) agonists, orchiectomy), radiation therapy, and radical prostatectomy lead to reductions in PSA.
The interpretation of PSA values should always take into account age, the presence of urinary tract infection or prostate disease, recent diagnostic procedures, and prostate-directed treatments.
Clinical Use for Diagnosis
The initial assays for PSA that were approved by the FDA in 1994 for early detection of prostate cancer detected both free PSA and PSA complexed to alpha 1 antichymotrypsin (ACT). Thus measurement of free and complexed PSA by these assays is generally referred to as the serum PSA level (Smith et al, 1996). Specific assays that detect free PSA alone and PSA complexed to ACT alone have been evaluated and approved for prostate cancer detection (see later).
It is now well-established that the use of PSA increases the detection of prostate cancers that are more likely to be organ-confined when compared with detection without PSA (Thompson et al, 1987; Mueller et al, 1988; Chodak et al, 1989; Rietbergen et al, 1999; Hoedemaeker et al, 2000). Observational studies and randomized trials have shown that both the future risk of prostate cancer and the chance of finding cancer on a prostate biopsy increase incrementally with the serum PSA level (Catalona et al, 1991, 1994; Brawer et al, 1992; Labrie et al, 1992; Gann et al, 1995; Fang et al, 2001; Thompson et al, 2004; Andriole et al, 2005; Whittemore et al, 2005; Loeb et al, 2006; Lilja et al, 2007).
Gann and associates (1995) were the first to demonstrate the association between the baseline PSA level and subsequent prostate cancer detection, which has been verified by others (Fang et al, 2001; Antenor et al, 2004; Whittemore et al, 2005; Loeb et al, 2006).
In addition to predicting future risk, PSA is directly associated with the present risk of prostate cancer. The probability of detecting prostate cancer on biopsy increases directly with PSA across the full spectrum of PSA levels (Table 99–1) (Thompson et al, 2004).
Table 99–1 Prostate Cancer Detection as a Function of Serum Prostate-Specific Antigen (PSA) Level and Digital Rectal Examination (DRE) Findings in Contemporary Series
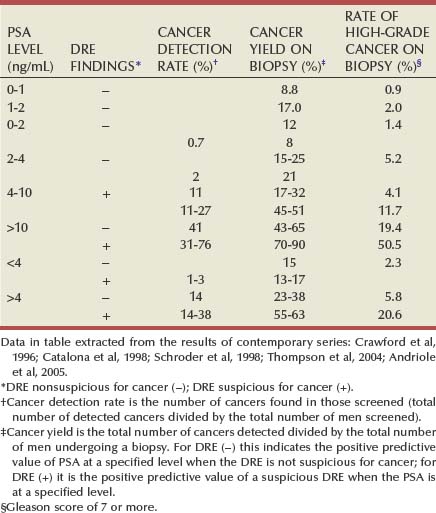
In summary, both PSA and DRE are used to assess prostate cancer risk. The addition of PSA to DRE increases both the detection rate of prostate cancer and detection of cancers with a more favorable prognosis.
Triggers for Biopsy
The choice of a PSA threshold for recommending a prostate biopsy is controversial (Catalona et al, 1994; Gann et al, 1995; Carter, 2004; Nadler et al, 2005; Thompson et al, 2005) and has recently been reviewed (Schroder et al, 2008). Gann (1995) pointed out that “dichotomization of PSA results into normal and abnormal obscures important information contained in levels below the usual cutoff.” Data from the Prostate Cancer Prevention Trial clearly show that the risk of prostate cancer is continuous as PSA increases (Thompson et al, 2005). Some investigators have recommended against referring to PSA as “elevated” or “abnormal,” and instead they advise using PSA together with other methods of risk assessment, such as family history, race, and DRE findings (Thompson et al, 2006). Nevertheless, most urologists continue to use total PSA thresholds for prostate biopsy decisions.
The use of higher PSA thresholds risks missing important cancers during the window for cure, whereas the use of lower thresholds increases the proportion of unnecessary biopsies and overdiagnosis. Although PSA was initially approved using 4 ng/mL as the upper limit of normal, many clinicians now use lower thresholds (2.5 to 3 ng/mL) to trigger a biopsy.
A PSA level that is considered suspicious for prostate cancer should be remeasured before performing a prostate biopsy, because of fluctuations in PSA that could create false-positive elevations (Eastham et al, 2003).
PSA Derivatives and Molecular Forms
Numerous variations on PSA-based screening have been proposed to improve test performance, including the adjustment of the PSA level for total prostate volume (PSA density) (Babaian et al, 1990; Veneziano et al, 1990; Littrup et al, 1991; Benson et al, 1992a, 1992b; Bazinet et al, 1994; Rommel et al, 1994; Catalona et al, 2000; Djavan et al, 2002; Egawa et al, 2002; Naya et al, 2002; Gjengsto et al, 2005) or transition zone volume (Djavan et al, 1999a, 1999b; Taneja et al, 2001; Singh et al, 2004; Gjengsto et al, 2005), and the evaluation of rate of change in PSA (PSA velocity) (Carter et al, 1992; Smith and Catalona, 1994; Fowler et al, 2000; Fang et al, 2002; D’Amico et al, 2004; Roobol et al, 2004; Berger et al, 2005; D’Amico et al, 2005; Schroder et al, 2006; Berger et al, 2007; Loeb et al, 2007a, 2007b, 2008c; Eggener et al, 2008; Vickers et al, 2009). The discovery that PSA circulates in both bound (complexed) and unbound (free) forms and development of assays to measure these forms separately, have resulted in the investigation of their use for prostate cancer detection (McCormack et al, 1995; Lilja, 1997, 2003; Polascik et al, 1999; Gretzer and Partin, 2003) and this topic has recently been reviewed (Jansen et al, 2009).
Volume-Based PSA Parameters
Distinguishing between men with PSA elevations driven by BPH or cancer is difficult, because PSA is not specific for cancer and the prevalence of BPH is high. Volume-based PSA parameters (with prostate volume typically determined by ultrasonography) have been evaluated to reduce confounding from BPH. These include PSA density (PSAD, PSA divided by prostate volume), complexed PSA density (complexed PSA divided by prostate volume), and PSA transition zone density (PSA divided by transition zone volume) (Babaian et al, 1990; Veneziano et al, 1990; Littrup et al, 1991; Benson et al, 1992a, 1992b; Bazinet et al, 1994; Rommel et al, 1994; Djavan et al, 1999a, 1999b, 2002; Catalona et al, 2000; Naya et al, 2002; Gjengsto et al, 2005).
Benson and colleagues (1992a, 1992b) suggested that adjusting PSA for prostate size by dividing PSA by prostate volume (PSAD) could help distinguish between PSA elevations caused by BPH and those caused by prostate cancer. A direct relationship between PSAD and the chance of cancer has been documented (Seaman et al, 1993; Bazinet et al, 1994; Rommel et al, 1994), and a PSAD of 0.15 or greater was proposed for recommending prostate biopsy in men with PSA levels between 4 and 10 ng/mL and a normal DRE (Seaman et al, 1993; Bazinet et al, 1994). The usefulness of PSAD in prostate cancer detection has not been confirmed in all studies (Cooner, 1994; Taneja et al, 2001). An advantage of PSAD is that it has been directly associated with prostate cancer aggressiveness (Carter et al, 2002, 2007; Kundu et al, 2007).
PSA has been adjusted for the transition zone volume (Kalish et al, 1994), the prostatic region that is the major determinant of serum PSA in men without prostate cancer (Lepor et al, 1994). Djavan and associates (1999b) found that PSA transition zone volume was the parameter with the highest overall sensitivity and specificity for prostate cancer detection when PSA was between 4 to 10 ng/mL.
In general, although PSAD and its associated measures are imperfect predictors of cancer, they represent an additional method of risk assessment with potential utility for counseling men with intermediate PSA levels (4 to 10 ng/mL) regarding the need for prostate biopsy (Benson and Olsson, 1994) or repeat biopsy if PSA is persistently elevated (Keetch et al, 1996).
Prostate-Specific Antigen Velocity
Short-term fluctuations in PSA can occur between measurements in the presence or absence of prostate cancer, primarily due to physiologic variation (Carter et al, 1992, 1995; Riehmann et al, 1993; Prestigiacomo and Stamey, 1996; Eastham et al, 2003). However, the rate of change in PSA (PSA velocity, or PSAV)—PSA corrected for the elapsed time between measurements (Carter et al, 1992)—is associated with the risk of prostate cancer (Carter et al, 1992; Smith and Catalona, 1994; D’Amico et al, 2004, 2005; Roobol et al, 2004; Berger et al, 2005, 2007; Sengupta et al, 2005; Schroder et al, 2006; Loeb et al, 2007a, 2007b, 2008a; Eggener et al, 2008; Vickers et al, 2009). Using frozen sera to measure PSA years before diagnosis, Carter and colleagues (1992) showed that a PSAV more than 0.75 ng/mL per year was a specific marker for the presence of prostate cancer in men with PSA levels between 4 and 10 ng/mL. Other studies have demonstrated that men with prostate cancer have more rapid rises in PSA than men without prostate cancer (Smith and Catalona, 1994; Carter et al, 1995; Raaijmakers et al, 2004; Thompson et al, 2004; Loeb et al, 2008a). More recently, it has been shown that PSAV might be useful for prostate cancer detection among men with PSA levels less than 4.0 ng/mL (Carter et al, 2006; Loeb et al, 2007b). Some investigators have suggested the use of lower PSAV thresholds for men with lower total PSA levels (Loeb et al, 2007b; Moul et al, 2007).
Some studies have failed to demonstrate the value of PSAV for prostate cancer prediction beyond that of a single PSA measurement (Roobol et al, 2004; Vickers et al, 2009). Differences between studies could be due to the method of calculating PSAV (Yu et al, 2006; Connolly et al, 2007) and the point in the PSA history at which PSAV is calculated (Carter et al, 1992; D’Amico et al, 2004).
PSAV may play a role in the prediction of life-threatening prostate cancer (D’Amico et al, 2004, 2005; Carter et al, 2006; Loeb et al, 2008a, 2008b). A PSAV greater than 0.35 ng/mL/year 10 to 15 years prior to diagnosis was associated with a fivefold increased risk of life-threatening prostate cancer more than a decade later (Carter et al, 2006), while a PSAV greater than 2 ng/mL/year during the year prior to a prostate cancer diagnosis was associated with prostate cancer–specific mortality following radical prostatectomy or radiation therapy (D’Amico et al, 2004, 2005; Sengupta et al, 2005). However, a recent meta-analysis suggested that PSAV prior to treatment provides no additional information regarding prostate cancer outcome when compared with PSA alone (Vickers et al, 2009).
Free Prostate-Specific Antigen
Men with prostate cancer generally have a greater fraction of serum PSA that is complexed—and therefore a lower percentage of total PSA circulating in the free (unbound) form—than men without prostate cancer (Christensson et al, 1993; Leinonen et al, 1993; Lilja, 1993; Stenman et al, 1994; Catalona et al, 1995, 1998, 2000; Keetch et al, 1997; Pannek et al, 1998; Woodrum et al, 1998; Gann et al, 2002; Roehl et al, 2002; Hugosson et al, 2003a; Raaijmakers et al, 2004). This difference is thought to be due to differential expression of PSA isoforms by transition zone (zone of origin of BPH) tissue compared with peripheral zone tissue (where most prostate cancers arise) (Chen et al, 1997; Mikolajczyk et al, 1997, 2000a, 2000b).
Free PSA levels vary directly by age and prostate volume, and vary indirectly with the total PSA level (Woodrum et al, 1998). In addition, because assays differ in their ability to determine both free and total PSA, results may differ depending on the assay or combination of assays used (Woodrum et al, 1998). The percentage of free PSA (%fPSA) does not appear to be significantly altered by race (Catalona et al, 2000) or 5α-reductase inhibitors (Keetch et al, 1997; Pannek et al, 1998).
%fPSA has been shown to significantly improve the ability to distinguish between individuals with and without prostate cancer, compared with total PSA alone (Christensson et al, 1993). The %fPSA cutoff that optimizes sensitivity and specificity for cancer detection depends on prostate size, because overlap is greatest among men with enlarged prostates, with or without concomitant prostate cancer (Catalona et al, 1995).
%fPSA appears to be most useful in distinguishing between those with and without prostate cancer at intermediate total PSA levels. In men with PSA levels of 4 to 10 ng/mL and palpably benign prostate glands, a %fPSA cutoff of 25% detected 95% of cancers while avoiding 20% of unnecessary biopsies (Catalona et al, 1998). The value of %fPSA to predict prostate cancer at levels less than 4.0 ng/mL is unclear (Gann et al, 2002; Roehl et al, 2002; Hugosson et al, 2003a; Raaijmakers et al, 2004).
%fPSA (at a cutoff of 25%) and PSAD (using a threshold of 0.078) have been shown to have comparable specificity (at a sensitivity of 95%) although %fPSA does not require transrectal ultrasonography (TRUS) (Catalona et al, 2000). Thus %fPSA can be used to counsel men with PSA elevations in the range 4 to 10 ng/mL regarding their risk of cancer.
Complexed Prostate-Specific Antigen
Because men with prostate cancer have a greater fraction of total PSA that is complexed to protease inhibitors than men without prostate cancer, measurement of complexed PSA (cPSA) has been studied as a marker for detection (Brawer et al, 2000, 2002; Okegawa et al, 2000; Parsons et al, 2004). When total PSA levels were between 4 and 10 ng/mL, cPSA provided improved specificity compared with total PSA, and similar specificity compared with the percentage of free PSA at a sensitivity of 95% (Brawer et al, 2000), findings that were subsequently confirmed (Okegawa et al, 2000). Similar results were reported in the 2.6 to 4.0 ng/mL PSA range (Parsons et al, 2004). Overall, at a high sensitivity, cPSA provides higher specificity compared with total PSA and comparable specificity to %fPSA in prostate cancer detection. A potential advantage of cPSA is the requirement for one assay.
PSA Isoforms
PSA is secreted from the prostatic luminal epithelium in a precursor form (pPSA or proPSA) (see Chapter 98) (Mikolajczyk et al, 2001, 2004; Peter et al, 2001; Catalona et al, 2003, 2004; Gretzer and Partin, 2003; Khan et al, 2003; Lilja, 2003; Canto et al, 2004; Lein et al, 2005; Makarov et al, 2009). Active free PSA can be further cleaved to BPSA or intact PSA (iPSA) that is inactive and not complexed. Research assays have been developed for measuring BPSA and pPSA (both native and truncated forms).
The relative concentration of these isoforms differs in the presence of prostatic disease. BPSA is found preferentially in nodular BPH tissue from the transition zone and can be considered a marker for BPH (Mikolajczyk et al, 2000b; Canto et al, 2004), whereas a larger relative proportion of proPSA has been associated with prostate cancer (Mikolajczyk et al, 1997, 2000a, 2001; Peter et al, 2001). Some studies have suggested that proPSA may improve the identification of prostate cancer among men with PSA levels of 2 to 4 ng/mL (Catalona et al, 2003, 2004), 4 to 10 ng/mL (Khan et al, 2003; Mikolajczyk et al, 2004), and 2 to 10 ng/mL (Catalona et al, 2003), while other studies have not shown incremental predictive value of specific subtypes beyond %fPSA (Lein et al, 2005).
hK2
hK2 is a closely related serine protease in the PSA/kallikrein gene family that has also been evaluated for prostate cancer detection (Kwiatkowski et al, 1998; Partin et al, 1999; Becker et al, 2000, 2003; Haese et al, 2003; Bangma et al, 2004; Steuber et al, 2005; Vickers et al, 2008). Expression of hK2 is higher in more poorly differentiated cancer tissues than in normal and benign tissues (Tremblay et al, 1997). Although some studies have suggested that the ratio of hK2 and free PSA might improve the ability of PSA to identify men with prostate cancer (Kwiatkowski et al, 1998; Partin et al, 1999; Becker et al, 2000; Vickers et al, 2008), other analyses have not (Becker et al, 2003; Bangma et al, 2004). hK2 does appear to correlate directly with grade and cancer volume and could be useful in patient assessment after diagnosis (Haese et al, 2003; Steuber et al, 2005).
Other Markers (see Chapter 98)
Prostate cancer gene 3 (PCA-3) is a noncoding prostate-specific mRNA overexpressed in prostate cancer tissue compared with benign tissue (Bussemakers et al, 1999; Marks et al, 2007; Deras et al, 2008; Haese et al, 2008; Nakanishi et al, 2008; Sokoll et al, 2008; van Gils et al, 2008; Whitman et al, 2008). Urine assays have been developed to measure PCA-3 mRNA (Sokoll et al, 2008), which is associated with the likelihood of a positive initial or repeat prostate biopsy (Marks et al, 2007; Deras et al, 2008; Haese et al, 2008). There are conflicting results on the association between PCA-3 with prostate cancer aggressiveness (Nakanishi et al, 2008; van Gils et al, 2008; Whitman et al, 2008). In the future, it is likely that panels of biomarkers will be used in combination with standard measures of risk (age, family history, race) to selectively identify men who should undergo further evaluation for the presence of prostate cancer (Etzioni et al, 2003).
Staging
General Concepts of Staging
The clinical staging of prostate cancer uses pretreatment parameters to predict the extent of disease, both for assessment of prognosis and to inform decisions regarding appropriate treatment. Available pretreatment modalities used to predict disease extent in men with prostate cancer include DRE (T stage), PSA and its derivatives, prostate cancer features on needle biopsy, and radiologic imaging. Because imaging modalities cannot reliably visualize microscopic disease, they are not helpful in most newly diagnosed cases. Pelvic lymph node biopsy is rarely performed prior to definitive therapy.
Clinical versus Pathologic Staging
Clinical staging is the assessment of disease extent using pretreatment parameters (DRE, PSA, needle biopsy findings, and radiologic imaging); whereas pathologic stage is determined after prostate removal and involves histologic analysis of the prostate, seminal vesicles, and pelvic lymph nodes if lymphadenectomy is performed. Pathologic staging more accurately estimates disease burden and is more useful than clinical staging for outcome prediction (Pound et al, 1997). Biochemical recurrence-free survival and cancer-specific survival are both inversely related to the pathologic stage of disease (Roehl et al, 2004). The most important pathologic criteria that predict prognosis after radical prostatectomy are tumor grade, surgical margin status, extracapsular disease, seminal vesicle invasion, and pelvic lymph node involvement (Jewett, 1975; Walsh and Jewett, 1980; Epstein et al, 1990, 1993a, 1993b; Partin et al, 1993; Pound et al, 1997).
Classifications
The Whitmore and Jewett staging system is now of historical interest (Jewett 1956; Whitmore 1956). Today, clinical staging is based upon the tumor-node-metastases (TNM) classification system (Table 99–2). This system was first adopted in 1975 by the American Joint Committee on Cancer (AJCC) and has since undergone numerous modifications (Schroder et al, 1992). The most recent 1997 modification reduced the subdivision of T2 disease from three categories (T2a, T2b, and T2c) to two categories by combining single-lobe disease (T2a and T2b) into a single stage (Schroder et al, 1992). However, some believe that a distinction between stages T2a and T2b is clinically important (Iyer et al, 1999; Han et al, 2000). Also, a nonpalpable lesion identified by TRUS is considered T2 by the current TNM clinical staging system. However, TRUS findings do not predict tumor extent in PSA-detected nonpalpable lesions (Epstein et al, 1994; Ferguson et al, 1995) so that many urologists classify men with nonpalpable disease as T1c regardless of TRUS findings.
Table 99–2 1997 and 1992 TNM Clinical Staging Systems for Prostate Cancer
| 1997 | 1992 | DESCRIPTION |
|---|---|---|
| TX | TX | Primary tumor cannot be assessed |
| T0 | T0 | No evidence of primary tumor |
| T1 | T1 | Nonpalpable tumor—not evident by imaging |
| T1a | T1a | Tumor found in tissue removed at TUR; 5% or less is cancerous and histologic grade <7 |
| T1b | T1b | Tumor found in tissue removed at TUR; >5% is cancerous or histologic grade >7 |
| T1c | T1c | Tumor identified by prostate needle biopsy due to elevation in PSA |
| T2 | T2 | Palpable tumor confined to the prostate |
| T2a | Tumor involves one lobe or less | |
| T2a | Tumor involves less than half of one lobe by normal tissue on all sides | |
| T2b | Tumor involves more than one lobe | |
| T2b | Tumor involves more than half of a lobe but not both lobes | |
| None | T2c | Tumor involves more than one lobe |
| T3 | T3 | Palpable tumor beyond prostate |
| T3a | T3a | Unilateral extracapsular extension |
| T3b | T3b | Bilateral extracapsular extension |
| T3c | T3c | Tumor invades seminal vesicle(s) |
| T4 | T4 | Tumor is fixed or invades adjacent structures (not seminal vesicles) |
| T4a | T4a | Tumor invades bladder neck, external sphincter, and/or rectum |
| T4b | T4b | Tumor invades levator muscle and/or fixed to pelvic wall |
| N(+) | N(+) | Involvement of regional lymph nodes |
| NX | NX | Regional lymph nodes cannot be assessed |
| N0 | N0 | No lymph node metastases |
| N1 | N1 | Metastases in single regional lymph node, ≤2 cm in dimension |
| N2 | N2 | Metastases in single (>2 but ≤5 cm) or multiple with none >5 cm |
| N3 | N3 | Metastases in regional lymph node >5 cm in dimension |
| M(+) | M(+) | Distant metastatic spread |
| MX | MX | Distant metastases cannot be assessed |
| M0 | M0 | No evidence of distant metastases |
| M1 | M1 | Distant metastases |
| M1a | M1a | Involvement of nonregional lymph nodes |
| M1b | M1b | Involvement of bones |
| M1c | M1c | Involvement of other distant sites |
TNM, tumor-node-metastasis; TUR, transurethral resection.
Prediction of Tumor Extent
Prostate-Specific Antigen
Despite controversy over its correlation with prostate cancer volume (Stamey et al, 2004), PSA is associated directly with pathologic stage and tumor extent (Stamey et al, 1987, 1989). PSA cannot be used alone to accurately predict disease extent for an individual patient due to significant overlap in PSA levels between stages, the variable contribution from BPH to PSA, and the fact that, on average, poorly differentiated tumors produce less PSA per gram of tumor (Partin et al, 1990). Despite these confounding factors, pathologic organ-confined disease is found in 80% of men with a PSA less than 4.0 ng/mL, 66% of those with PSA levels between 4.0 and 10.0 ng/mL, and fewer than 50% of men with PSA greater than 10.0 ng/mL (Catalona et al, 1997; Rietbergen et al, 1999). Also, 20% of men with PSA greater than 20 ng/mL and 75% of those with PSA greater than 50 ng/mL are found to have pelvic lymph node involvement.
In addition to the total PSA level, free PSA, hK2, proPSA, PSAD, and PSAV (see above) have been evaluated as predictors of prostate cancer grade and extent (Carter et al, 1997, 2006, 2007; Southwick et al, 1999; D’Amico et al, 2004, 2005; Kundu et al, 2007; Loeb et al, 2008b). Although prostatic acid phosphatase (PAP) has been associated with pathologic stage and progression following radical prostatectomy, (Moul et al, 1998; Han et al, 2001) the closer relationship between PSA and disease extent has virtually eliminated the clinical use of this parameter (Heller, 1987).
Digital Rectal Examination
DRE is used to determine whether a lesion is palpable and is associated with local disease extent (clinical T stage). Also, an abnormal DRE was associated with an increased risk of detecting high-grade (Gleason 8 to 10) prostate cancer in a screened population (Gosselaar et al, 2008).
However, because of its poor sensitivity and lack of reproducibility, DRE can both overestimate and underestimate the extent of disease (Turner and Belt, 1957; Byar and Mostofi, 1972; Walsh and Jewett, 1980). In one series of 565 men with presumed organ-confined disease based upon DRE, the sensitivity and specificity were 52% and 81%, respectively, for prediction of organ-confined disease (Partin et al, 1993). Nevertheless, DRE can be used in combination with other parameters to help predict tumor extent.
Prostate Needle Biopsy
Histologic grade is the most important information obtained from prostate needle biopsy and the Gleason grading system is the most commonly used (Gleason, 1966). At low-power magnification, the sum of a grade (1 to 5) assigned to the predominant pattern (occupying the largest area of the specimen) and the second most common pattern yields a score ranging from 2 to 10.
Recent studies have shown that tertiary Gleason patterns may affect prognosis (Patel et al, 2007), leading a 2005 consensus conference to recommend modification of the Gleason grading system (see Chapter 96). Accordingly, a biopsy Gleason score of 3 + 4 or 4 + 3 with a tertiary pattern 5 would be considered Gleason 3 + 5 and 4 + 5, respectively (Epstein et al, 2005).
Although a higher Gleason grade is associated with worse prognosis, it is not used alone for risk prediction (Stein et al, 1991; Epstein et al, 1993a, 1993b; Partin et al, 1993; Zincke et al, 1994). Other biopsy findings provide information regarding the extent of disease, including the number of positive cores, percentage of positive cores, and presence of perineural invasion (PNI). These features are associated with radical prostatectomy findings, and have been used to guide the selection of candidates for active surveillance programs (Egan and Bostwick, 1997; de la Taille et al, 1999a; Carter et al, 2002; O’Malley et al, 2002; Bismar et al, 2003).
Findings of seminal vesicle invasion or involvement of the periprostatic fat on prostate needle biopsy are associated with worse prognosis (Stone et al, 1998). Although some authors have recommended biopsy of the seminal vesicles and/or prostatic capsule to improve staging (Terris et al, 1993; Ravery et al, 1994; Vallancien et al, 1994; Stone et al, 1995, 1998), others suggest biopsy of these structures only when there is a large palpable tumor located at the base of the prostate (Guillonneau et al, 1997; Terris et al, 1997).
Combined Use of Pretreatment Parameters
Nomograms and algorithms have been developed to integrate multiple clinical parameters for improved staging. Considering the primary T stage based on DRE findings, serum PSA level, and Gleason grade, these algorithms/nomograms have been shown to more accurately predict both cancer extent and long-term outcomes after treatment compared with use of a single parameter (Humphrey et al, 1991; Kleer et al, 1993; Kleer and Oesterling, 1993; Partin et al, 1993, 1997, 2001; Bluestein et al, 1994; Kattan et al, 1998; Han et al, 2003; Stephenson et al, 2006; Makarov et al, 2007).
Several classification schemes have also been proposed that correlate with clinical outcomes. D’Amico demonstrated that stratification into low-risk (clinical stage T1 to 2a, PSA 10 ng/mL or less and Gleason score 6 or less), intermediate-risk (stage T2b, PSA greater than 10 but less than 20 ng/mL or Gleason score 7), and high-risk disease (stage T2c, PSA greater than 20 ng/mL or Gleason score 8 to 10) (D’Amico et al, 1998) was significantly associated with freedom from disease at 10 years after radical prostatectomy; 83% for low-risk, 46% for intermediate-risk, and 29% for high-risk disease (D’Amico et al, 2001). Other validated classification schemes have since been developed, including the Cancer of the Prostate Risk Assessment (CAPRA) score (Cooperberg et al, 2005, 2006; May et al, 2007). Pretreatment risk stratification using multiple parameters is useful for patient counseling.
Imaging
Numerous imaging modalities have been evaluated for staging prostate cancer. No technique is reliably sensitive to detect extraprostatic disease. The inability to image microscopic disease limits the accuracy of current modalities.
Radionuclide bone scan (bone scintigraphy) is the most sensitive modality for the detection of skeletal metastases (Terris et al, 1991). Bone survey films (skeletal radiography) have lower sensitivity for the identification of distant spread and are typically used only to confirm a positive bone scan in men at low risk for bone metastases. Because bone metastases at diagnosis are rare in asymptomatic men in the PSA era, the routine use of bone scans in this population may lead to false-positive results, unnecessary anxiety, and cost (Chybowski et al, 1991). Accordingly, recent guidelines recommend the use of bone scans for patients with a PSA greater than 20 ng/mL, a Gleason score of 8 to 10, clinical stage T3 or T4, or clinical symptoms (National Comprehensive Cancer Network, 2009).
The use of computed tomography (CT) and magnetic resonance imaging (MRI) to evaluate the local extent of disease and the possibility of nodal involvement is not routinely recommended due to low sensitivity (Rifkin, 1990; Tempany et al, 1994; Wolf et al, 1995). Cross-sectional imaging may be reserved for high-risk patients, such as those with clinical stage greater than or equal to T3 disease or greater than 20% nomogram probability of lymph node metastases. Given the rarity of lymph node involvement in contemporary screened populations, it appears that imaging is being overused (Kindrick et al, 1998; Cooperberg et al, 2002; Abraham et al, 2007).
Combined MRI and MRI spectroscopy have been evaluated for staging prostate cancer, but there is no evidence that these methods will overcome the current limitations of imaging microscopic disease (Yu et al, 1999; Kurhanewicz et al, 2000). Specialized techniques such as high-resolution MRI used in tandem with the intravenous administration of lymphotropic superparamagnetic nanoparticles may allow the detection of small and otherwise undetectable lymph node metastases in patients with prostate cancer (Harisinghani et al, 2003). These techniques, however, require further clinical evaluation before widespread use.
Advances in ultrasound imaging are also being studied for improving prostate cancer detection (Purohit et al, 2003). Color ultrasonography with power Doppler to evaluate the blood flow within prostate vessels and three-dimensional Doppler using contrast agents could improve the visualization of more subtle tissue alterations caused by cancer.
Finally, monoclonal antibody radioimmunoscintigraphy (radiolabeled monoclonal antibody scan) have been used for identification of microscopic cancer deposits in regional and distant sites. The ProstaScint scan (Cytogen, Princeton, NJ) uses this technology but has had limited accuracy in the detection of lymph node metastases because the antibody targets an intracellular epitope that is only exposed in dying or dead cells (Troyer et al, 1997; Chang et al, 1999). Future generations of this technology circumventing this limitation are under development.
Molecular Staging
Molecular staging has focused on the detection of circulating prostate cancer cells either directly through centrifugation/immunostaining methods or indirectly by identifying the genetic message (messenger RNA [mRNA]) for prostate-specific biomarkers (e.g., PSA) from circulating prostate cells (Moreno et al, 1992; Ts’o et al, 1997). Although these polymerase chain reaction (PCR)–based assays have been associated with pathologic stage, sensitivity for detecting circulating cancer cells is variable between studies (Cama et al, 1995; Israeli et al, 1995; de la Taille et al, 1999b). With the recent FDA approval of the semiautomated CellSearch system (Veridex, Raritan, NJ) for monitoring metastatic breast and prostate cancer, considerable investigation is underway to determine whether circulating tumor cells have a role in the staging of early disease (Davis et al, 2008; Helo et al, 2009).
Pelvic Lymphadenectomy
The presence of lymph node metastasis in men diagnosed with clinically localized prostate cancer portends a poor prognosis. Identification of patients harboring nodal metastases could have important implications for the initiation of adjuvant therapy. Although the prevalence of pelvic lymph node metastases correlates directly with T stage, serum PSA level, and biopsy grade, pelvic lymphadenectomy (PLND) remains the most accurate way to detect occult nodal involvement (Parker et al, 1999).
PSA screening has resulted in a steady decline in the rates of lymph node metastases from 20% to 40% in the 1970s and 1980s to less than 4% today (Partin et al, 1997; Parker et al, 1999). Currently, lymphadenectomy is often omitted before curative treatment (e.g., radical prostatectomy, radiation therapy) (Bishoff et al, 1995; Kawakami et al, 2006). Laparoscopic pelvic lymphadenectomy prior to treatment is typically reserved for patients with a Gleason score greater than 8, extraprostatic extension on DRE, PSA greater than 20 ng/mL, or suspicion of enlarged lymph nodes on radiologic evaluation.
Given the individual variation in prostatic lymphatic drainage patterns (Mattei et al, 2008), some investigators favor an extended pelvic lymphadenectomy in lieu of a limited dissection (Bader et al, 2002; Burkhard et al, 2006). Given the greater complication rates with a more extended PLND, risks may outweigh benefits for most men diagnosed with low-risk cancer today (Klein et al, 2008). Information regarding the therapeutic value of these strategies is confounded by stage migration and is difficult to evaluate without prospective trials.
Key Points
Andriole GL, Grubb RL3rd, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310-1319.
Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267(16):2215-2220.
Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156-1161.
Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273(4):289-294.
Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270(7):860-864.
Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320-1328.
Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246.
Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368-374.
Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90(10):766-771.
Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150(1):110-114.
Abraham N, Wan F, Montagnet C, et al. Decrease in racial disparities in the staging evaluation for prostate cancer after publication of staging guidelines. J Urol. 2007;178(1):82-87. discussion 87
Andriole GL, Grubb RL3rd, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310-1319.
Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar long-term efficacy and safety study. Urology. 1998;52(2):195-201. discussion 201–2
Andriole GL, Levin DL, Crawford ED, et al. Prostate Cancer Screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst. 2005;97(6):433-438.
Andriole GL, Reding D, Hayes RB, et al. The prostate, lung, colon, and ovarian (PLCO) cancer screening trial: status and promise. Urol Oncol. 2004;22(4):358-361.
Antenor JA, Han M, Roehl KA, et al. Relationship between initial prostate specific antigen level and subsequent prostate cancer detection in a longitudinal screening study. J Urol. 2004;172(1):90-93.
Aus G, Bergdahl S, Lodding P, et al. Prostate cancer screening decreases the absolute risk of being diagnosed with advanced prostate cancer—results from a prospective, population-based randomized controlled trial. Eur Urol. 2007;51(3):659-664.
Auvinen A, Rietbergen JB, Denis LJ, et al. Prospective evaluation plan for randomised trials of prostate cancer screening. The International Prostate Cancer Screening Trial Evaluation Group. J Med Screen. 1996;3(2):97-104.
Babaian RJ, Fritsche HA, Evans RB. Prostate-specific antigen and prostate gland volume: correlation and clinical application. J Clin Lab Anal. 1990;4(2):135-137.
Bader P, Burkhard FC, Markwalder R, et al. Is a limited lymph node dissection an adequate staging pro FC cedure for prostate cancer? J Urol. 2002;168(2):514-518. discussion 518
Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103(5):1092-1095.
Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298(19):2275-2280.
Bangma CH, Wildhagen MF, Yurdakul G, et al. The value of (-7, -5)pro-prostate-specific antigen and human kallikrein-2 as serum markers for grading prostate cancer. BJU Int. 2004;93(6):720-724.
Barry MJ. Screening for prostate cancer—the controversy that refuses to die. N Engl J Med. 2009;360(13):1351-1354.
Bazinet M, Meshref AW, Trudel C, et al. Prospective evaluation of prostate-specific antigen density and systematic biopsies for early detection of prostatic carcinoma. Urology. 1994;43(1):44-51. discussion 51–2
Becker C, Piironen T, Pettersson K, et al. Clinical value of human glandular kallikrein 2 and free and total prostate-specific antigen in serum from a population of men with prostate-specific antigen levels 3.0 ng/mL or greater. Urology. 2000;55(5):694-699.
Becker C, Piironen T, Pettersson K, et al. Testing in serum for human glandular kallikrein 2, and free and total prostate specific antigen in biannual screening for prostate cancer. J Urol. 2003;170(4 Pt. 1):1169-1174.
Benson MC, Olsson CA. Prostate specific antigen and prostate specific antigen density. Roles in patient evaluation and management. Cancer. 1994;74(6):1667-1673.
Benson MC, Whang IS, Olsson CA, et al. The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol. 1992;147(3 Pt. 2):817-821.
Benson MC, Whang IS, Pantuck A, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147(3 Pt. 2):815-816.
Berger AP, Deibl M, Steiner H, et al. Longitudinal PSA changes in men with and without prostate cancer: assessment of prostate cancer risk. Prostate. 2005;64(3):240-245.
Berger AP, Deibl M, Strasak A, et al. Large-scale study of clinical impact of PSA velocity: long-term PSA kinetics as method of differentiating men with from those without prostate cancer. Urology. 2007;69(1):134-138.
Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
Bishoff JT, Reyes A, Thompson IM, et al. Pelvic lymphadenectomy can be omitted in selected patients with carcinoma of the prostate: development of a system of patient selection. Urology. 1995;45(2):270-274.
Bismar TA, Lewis JSJr, Vollmer RT, et al. Multiple measures of carcinoma extent versus perineural invasion in prostate needle biopsy tissue in prediction of pathologic stage in a screening population. Am J Surg Pathol. 2003;27(4):432-440.
Bluestein DL, Bostwick DG, Bergstralh EJ, et al. Eliminating the need for bilateral pelvic lymphadenectomy in select patients with prostate cancer. J Urol. 1994;151(5):1315-1320.
Brawer MK. Clinical usefulness of assays for complexed prostate-specific antigen. Urol Clin North Am. 2002;29(1):193-203. xi
Brawer MK, Cheli CD, Neaman IE, et al. Complexed prostate specific antigen provides significant enhancement of specificity compared with total prostate specific antigen for detecting prostate cancer. J Urol. 2000;163(5):1476-1480.
Brawer MK, Chetner MP, Beatie J, et al. Screening for prostatic carcinoma with prostate specific antigen. J Urol. 1992;147(3 Pt. 2):841-845.
Burkhard FC, Schumacher MC, Studer UE. An extended pelvic lymph-node dissection should be performed in most patients if radical prostatectomy is truly indicated. Nat Clin Pract Urol. 2006;3(9):454-455.
Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59(23):5975-5979.
Byar DP, Mostofi FK. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer. 1972;30(1):5-13.
Cama C, Olsson CA, Raffo AJ, et al. Molecular staging of prostate cancer. II. A comparison of the application of an enhanced reverse transcriptase polymerase chain reaction assay for prostate specific antigen versus prostate specific membrane antigen. J Urol. 1995;153(5):1373-1378.
Canto EI, Singh H, Shariat SF, et al. Serum BPSA outperforms both total PSA and free PSA as a predictor of prostatic enlargement in men without prostate cancer. Urology. 2004;63(5):905-910. discussion 910–11
Carter HB. Prostate cancers in men with low PSA levels—must we find them? N Engl J Med. 2004;350(22):2292-2294.
Carter HB, Epstein JI, Chan DW, et al. Recommended prostate-specific antigen testing intervals for the detection of curable prostate cancer. JAMA. 1997;277(18):1456-1460.
Carter HB, Ferrucci L, Kettermann A, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98(21):1521-1527.
Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178(6):2359-2364. discussion 2364–5
Carter HB, Morrell CH, Pearson JD, et al. Estimation of prostatic growth using serial prostate-specific antigen measurements in men with and without prostate disease. Cancer Res. 1992;52(12):3323-3328.
Carter HB, Partin AW, Luderer AA, et al. Percentage of free prostate-specific antigen in sera predicts aggressiveness of prostate cancer a decade before diagnosis. Urology. 1997;49(3):379-384.
Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267(16):2215-2220.
Carter HB, Pearson JD, Waclawiw Z, et al. Prostate-specific antigen variability in men without prostate cancer: effect of sampling interval on prostate-specific antigen velocity. Urology. 1995;45(4):591-596.
Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167(3):1231-1234.
Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro prostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/mL. J Urol. 2003;170(6 Pt. 1):2181-2185.
Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro-prostate specific antigen preferentially detects aggressive prostate cancers in men with 2 to 4 ng/mL prostate specific antigen. J Urol. 2004;171(6 Pt. 1):2239-2244.
Catalona WJ, Hudson MA, Scardino PT, et al. Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152(6 Pt. 1):2037-2042.
Catalona WJ, Loeb S, Han M. Viewpoint: expanding prostate cancer screening. Ann Intern Med. 2006;144(6):441-443.
Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279(19):1542-1547.
Catalona WJ, Partin AW, Slawin KM, et al. Percentage of free PSA in black versus white men for detection and staging of prostate cancer: a prospective multicenter clinical trial. Urology. 2000;55(3):372-376.
Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151(5):1283-1290.
Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277(18):1452-1455.
Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156-1161.
Catalona WJ, Smith DS, Wolfert RL, et al. Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995;274(15):1214-1220.
Catalona WJ, Southwick PC, Slawin KM, et al. Comparison of percent free PSA, PSA density, and age-specific PSA cutoffs for prostate cancer detection and staging. Urology. 2000;56(2):255-260.
Chan EC, Barry MJ, Vernon SW, et al. Brief report: physicians and their personal prostate cancer-screening practices with prostate-specific antigen. A national survey. J Gen Intern Med. 2006;21(3):257-259.
Chang SS, Reuter VE, Heston WD, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192-3198.
Chen Z, Chen H, Stamey TA. Prostate specific antigen in benign prostatic hyperplasia: purification and characterization. J Urol. 1997;157(6):2166-2170.
Chodak GW, Keller P, Schoenberg HW. Assessment of screening for prostate cancer using the digital rectal examination. J Urol. 1989;141(5):1136-1138.
Christensson A, Bjork T, Nilsson O, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150(1):100-105.
Chybowski FM, Keller JJ, Bergstralh EJ, et al. Predicting radionuclide bone scan findings in patients with newly diagnosed, untreated prostate cancer: prostate specific antigen is superior to all other clinical parameters. J Urol. 1991;145(2):313-318.
Connolly D, Black A, Murray LJ, et al. Methods of calculating prostate-specific antigen velocity. Eur Urol. 2007;52(4):1044-1050.
Cooner WH. Prostate cancer. J Urol. 1994;151(1):103-104.
Cooner WH, Mosley BR, Rutherford CLJr, et al. Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination and prostate specific antigen. J Urol. 1990;143(6):1146-1152. discussion 1152–4
Cooperberg MR, Freedland SJ, Pasta DJ, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006;107(10):2384-2391.
Cooperberg MR, Lubeck DP, Grossfeld GD, et al. Contemporary trends in imaging test utilization for prostate cancer staging: data from the cancer of the prostate strategic urologic research endeavor. J Urol. 2002;168(2):491-495.
Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938-1942.
Crawford ED, DeAntoni EP, Etzioni R, et al. Serum prostate-specific antigen and digital rectal examination for early detection of prostate cancer in a national community-based program. The Prostate Cancer Education Council. Urology. 1996;47(6):863-869.
Crawford ED, Schutz MJ, Clejan S, et al. The effect of digital rectal examination on prostate-specific antigen levels. JAMA. 1992;267(16):2227-2228.
D’Amico AV, Chen MH, Roehl KA, et al. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351(2):125-135.
D’Amico AV, Renshaw AA, Sussman B, et al. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA. 2005;294(4):440-447.
D’Amico AV, Roehrborn CG. Effect of 1 mg/day finasteride on concentrations of serum prostate-specific antigen in men with androgenic alopecia: a randomised controlled trial. Lancet Oncol. 2007;8(1):21-25.
D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-974.
D’Amico AV, Whittington R, Malkowicz SB, et al. Predicting prostate specific antigen outcome preoperatively in the prostate specific antigen era. J Urol. 2001;166(6):2185-2188.
Dalton DL. Elevated serum prostate-specific antigen due to acute bacterial prostatitis. Urology. 1989;33(6):465.
Davis JW, Nakanishi H, Kumar VS, et al. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: initial results in early prostate cancer. J Urol. 2008;179(6):2187-2191. discussion 2191
de Koning HJ, Auvinen A, Berenguer Sanchez A, et al. Large-scale randomized prostate cancer screening trials: program performances in the European Randomized Screening for Prostate Cancer trial and the Prostate, Lung, Colorectal and Ovary cancer trial. Int J Cancer. 2002;97(2):237-244.
de la Taille A, Katz A, Bagiella E, et al. Perineural invasion on prostate needle biopsy: an independent predictor of final pathologic stage. Urology. 1999;54(6):1039-1043.
de la Taille A, Olsson CA, Katz AE. Molecular staging of prostate cancer: dream or reality? Oncology (Williston Park). 1999;13(2):187-194. discussion 194–8, 204–5 pas
Deras IL, Aubin SM, Blase A, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol. 2008;179(4):1587-1592.
Djavan B, Remzi M, Zlotta AR, et al. Complexed prostate-specific antigen, complexed prostate-specific antigen density of total and transition zone, complexed/total prostate-specific antigen ratio, free-to-total prostate-specific antigen ratio, density of total and transition zone prostate-specific antigen: results of the prospective multicenter European trial. Urology. 2002;60(4 Suppl. 1):4-9.
Djavan B, Zlotta A, Kratzik C, et al. PSA, PSA density, PSA density of transition zone, free/total PSA ratio, and PSA velocity for early detection of prostate cancer in men with serum PSA 2.5 to 4.0 ng/mL. Urology. 1999;54(3):517-522.
Djavan B, Zlotta AR, Remzi M, et al. Total and transition zone prostate volume and age: how do they affect the utility of PSA-based diagnostic parameters for early prostate cancer detection? Urology. 1999;54(5):846-852.
Eastham JA, Riedel E, Scardino PT, et al. Variation of serum prostate-specific antigen levels: an evaluation of year-to-year fluctuations. JAMA. 2003;289(20):2695-2700.
Egan AJ, Bostwick DG. Prediction of extraprostatic extension of prostate cancer based on needle biopsy findings: perineural invasion lacks significance on multivariate analysis. Am J Surg Pathol. 1997;21(12):1496-1500.
Egawa S, Suyama K, Matsumoto K, et al. Prospective evaluation of prostate cancer detection by prostate specific antigen related parameters: comparison in serum and plasma samples. J Urol. 2002;167(1):97-102.
Eggener SE, Yossepowitch O, Roehl KA, et al. Relationship of prostate-specific antigen velocity to histologic findings in a prostate cancer screening program. Urology. 2008;71(6):1016-1019.
Ellis WJ, Chetner MP, Preston SD, et al. Diagnosis of prostatic carcinoma: the yield of serum prostate specific antigen, digital rectal examination and transrectal ultrasonography. J Urol. 1994;152(5 Pt. 1):1520-1525.
Epstein JI. Evaluation of radical prostatectomy capsular margins of resection. The significance of margins designated as negative, closely approaching, and positive. Am J Surg Pathol. 1990;14(7):626-632.
Epstein JI, Allsbrook WCJr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228-1242.
Epstein JI, Carmichael MJ, Pizov G, et al. Influence of capsular penetration on progression following radical prostatectomy: a study of 196 cases with long-term followup. J Urol. 1993;150(1):135-141.
Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71(11):3582-3593.
Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368-374.
Ercole CJ, Lange PH, Mathisen M, et al. Prostatic specific antigen and prostatic acid phosphatase in the monitoring and staging of patients with prostatic cancer. J Urol. 1987;138(5):1181-1184.
Etzioni R, Kooperberg C, Pepe M, et al. Combining biomarkers to detect disease with application to prostate cancer. Biostatistics. 2003;4(4):523-538.
Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19(2):175-181.
Etzioni RD, Howlader N, Shaw PA, et al. Long-term effects of finasteride on prostate specific antigen levels: results from the prostate cancer prevention trial. J Urol. 2005;174(3):877-881.
Fang J, Metter EJ, Landis P, et al. Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001;58(3):411-416.
Fang J, Metter EJ, Landis P, et al. PSA velocity for assessing prostate cancer risk in men with PSA levels between 2.0 and 4.0 ng/mL. Urology. 2002;59(6):889-893. discussion 893–4
Ferguson JK, Bostwick DG, Suman V, et al. Prostate-specific antigen detected prostate cancer: pathological characteristics of ultrasound visible versus ultrasound invisible tumors. Eur Urol. 1995;27(1):8-12.
Fowler JEJr, Bigler SA, Kilambi NK, et al. Relationships between prostate-specific antigen and prostate volume in black and white men with benign prostate biopsies. Urology. 1999;53(6):1175-1178.
Fowler JEJr, Bigler SA, Miles D, et al. Predictors of first repeat biopsy cancer detection with suspected local stage prostate cancer. J Urol. 2000;163(3):813-818.
Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273(4):289-294.
Gann PH, Ma J, Catalona WJ, et al. Strategies combining total and percent free prostate specific antigen for detecting prostate cancer: a prospective evaluation. J Urol. 2002;167(6):2427-2434.
Gjengsto P, Paus E, Halvorsen OJ, et al. Predictors of prostate cancer evaluated by receiver operating characteristics partial area index: a prospective institutional study. J Urol. 2005;173(2):425-428.
Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50(3):125-128.
Gosselaar C, Roobol MJ, Roemeling S, et al. The role of the digital rectal examination in subsequent screening visits in the European randomized study of screening for prostate cancer (ERSPC), Rotterdam. Eur Urol. 2008;54(3):581-588.
Gretzer MB, Partin AW. PSA markers in prostate cancer detection. Urol Clin North Am. 2003;30(4):677-686.
Guess HA, Heyse JF, Gormley GJ, et al. Effect of finasteride on serum PSA concentration in men with benign prostatic hyperplasia. Results from the North American phase III clinical trial. Urol Clin North Am. 1993;20(4):627-636.
Guillonneau B, Debras B, Veillon B, et al. Indications for preoperative seminal vesicle biopsies in staging of clinically localized prostatic cancer. Eur Urol. 1997;32(2):160-165.
Haese A, de la Taille A, van Poppel H, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol. 2008;54(5):1081-1088.
Haese A, Graefen M, Becker C, et al. The role of human glandular kallikrein 2 for prediction of pathologically organ confined prostate cancer. Prostate. 2003;54(3):181-186.
Hamilton RJ, Goldberg KC, Platz EA, et al. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100(21):1511-1518.
Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517-523.
Han M, Piantadosi S, Zahurak ML, et al. Serum acid phosphatase level and biochemical recurrence following radical prostatectomy for men with clinically localized prostate cancer. Urology. 2001;57(4):707-711.
Han M, Walsh PC, Partin AW, et al. Ability of the 1992 and 1997 American Joint Committee on Cancer staging systems for prostate cancer to predict progression-free survival after radical prostatectomy for stage T2 disease. J Urol. 2000;164(1):89-92.
Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491-2499.
Heidenreich A, Vorreuther R, Neubauer S, et al. The influence of ejaculation on serum levels of prostate specific antigen. J Urol. 1997;157(1):209-211.
Heller JE. Prostatic acid phosphatase: its current clinical status. J Urol. 1987;137(6):1091-1103.
Helo P, Cronin AM, Danila DC, et al. Circulating prostate tumor cells detected by reverse transcription-pcr in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin Chem. 2009;55(4):765-773.
Henttu P, Liao SS, Vihko P. Androgens up-regulate the human prostate-specific antigen messenger ribonucleic acid (mRNA), but down-regulate the prostatic acid phosphatase mRNA in the LNCaP cell line. Endocrinology. 1992;130(2):766-772.
Herschman JD, Smith DS, Catalona WJ. Effect of ejaculation on serum total and free prostate-specific antigen concentrations. Urology. 1997;50(2):239-243.
Hoedemaeker RF, Rietbergen JB, Kranse R, et al. Histopathological prostate cancer characteristics at radical prostatectomy after population based screening. J Urol. 2000;164(2):411-415.
Hugosson J, Aus G, Bergdahl S, et al. Population-based screening for prostate cancer by measuring free and total serum prostate-specific antigen in Sweden. BJU Int. 2003;92(Suppl. 2):39-43.
Hugosson J, Aus G, Lilja H, et al. Prostate specific antigen based biennial screening is sufficient to detect almost all prostate cancers while still curable. J Urol. 2003;169(5):1720-1723.
Humphrey PA, Walther PJ, Currin SM, et al. Histologic grade, DNA ploidy, and intraglandular tumor extent as indicators of tumor progression of clinical stage B prostatic carcinoma. A direct comparison. Am J Surg Pathol. 1991;15(12):1165-1170.
Israeli RS, Miller WHJr, Su SL, et al. Sensitive detection of prostatic hematogenous tumor cell dissemination using prostate specific antigen and prostate specific membrane-derived primers in the polymerase chain reaction. J Urol. 1995;153(3 Pt. 1):573-577.
Iyer RV, Hanlon AL, Pinover WH, et al. Outcome evaluation of the 1997 American Joint Committee on Cancer staging system for prostate carcinoma treated by radiation therapy. Cancer. 1999;85(8):1816-1821.
Jansen FH, Roobol M, Jenster G, et al. Screening for prostate cancer in 2008 II: the importance of molecular subforms of prostate-specific antigen and tissue kallikreins. Eur Urol. 2009;55(3):563-574.
Jewett HJ. Significance of the palpable prostatic nodule. J Am Med Assoc. 1956;160(10):838-839.
Jewett HJ. The present status of radical prostatectomy for stages A and B prostatic cancer. Urol Clin North Am. 1975;2(1):105-124.
Kalish J, Cooner WH, Graham SDJr. Serum PSA adjusted for volume of transition zone (PSAT) is more accurate than PSA adjusted for total gland volume (PSAD) in detecting adenocarcinoma of the prostate. Urology. 1994;43(5):601-606.
Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90(10):766-771.
Kawakami J, Meng MV, Sadetsky N, et al. Changing patterns of pelvic lymphadenectomy for prostate cancer: results from CaPSURE. J Urol. 2006;176(4 Pt. 1):1382-1386.
Keetch DW, Andriole GL, Ratliff TL, et al. Comparison of percent free prostate-specific antigen levels in men with benign prostatic hyperplasia treated with finasteride, terazosin, or watchful waiting. Urology. 1997;50(6):901-905.
Keetch DW, McMurtry JM, Smith DS, et al. Prostate specific antigen density versus prostate specific antigen slope as predictors of prostate cancer in men with initially negative prostatic biopsies. J Urol. 1996;156(2 Pt. 1):428-431.
Khan MA, Partin AW, Rittenhouse HG, et al. Evaluation of proprostate specific antigen for early detection of prostate cancer in men with a total prostate specific antigen range of 4.0 to 10.0 ng/mL. J Urol. 2003;170(3):723-726.
Kindrick AV, Grossfeld GD, Stier DM, et al. Use of imaging tests for staging newly diagnosed prostate cancer: trends from the CaPSURE database. J Urol. 1998;160(6 Pt. 1):2102-2106.
Kirkali Z, Kirkali G, Esen A. Effect of ejaculation on prostate-specific antigen levels in normal men. Eur Urol. 1995;27(4):292-294.
Kleer E, Larson-Keller JJ, Zincke H, et al. Ability of preoperative serum prostate-specific antigen value to predict pathologic stage and DNA ploidy. Influence of clinical stage and tumor grade. Urology. 1993;41(3):207-216.
Kleer E, Oesterling JE. PSA and staging of localized prostate cancer. Urol Clin North Am. 1993;20(4):695-704.
Klein EA, Kattan M, Stephenson A, et al. How many lymphadenectomies does it take to cure one patient? Eur Urol. 2008;53(1):13-15. discussion 18–20
Klein LT, Lowe FC. The effects of prostatic manipulation on prostate-specific antigen levels. Urol Clin North Am. 1997;24(2):293-297.
Kundu SD, Roehl KA, Yu X, et al. Prostate specific antigen density correlates with features of prostate cancer aggressiveness. J Urol. 2007;177(2):505-509.
Kurhanewicz J, Vigneron DB, Males RG, et al. The prostate: MR imaging and spectroscopy. Present and future. Radiol Clin North Am. 2000;38(1):115-138. viii–ix
Kwiatkowski MK, Recker F, Piironen T, et al. In prostatism patients the ratio of human glandular kallikrein to free PSA improves the discrimination between prostate cancer and benign hyperplasia within the diagnostic gray zone of total PSA 4 to 10 ng/mL. Urology. 1998;52(3):360-365.
Labrie F, Dupont A, Suburu R, et al. Serum prostate specific antigen as pre-screening test for prostate cancer. J Urol. 1992;147(3 Pt. 2):846-851. discussion 851–2
Lein M, Semjonow A, Graefen M, et al. A multicenter clinical trial on the use of (-5, -7) pro prostate specific antigen. J Urol. 2005;174(6):2150-2153.
Leinonen J, Lovgren T, Vornanen T, et al. Double-label time-resolved immunofluorometric assay of prostate-specific antigen and of its complex with alpha 1-antichymotrypsin. Clin Chem. 1993;39(10):2098-2103.
Lepor H, Wang B, Shapiro E. Relationship between prostatic epithelial volume and serum prostate-specific antigen levels. Urology. 1994;44(2):199-205.
Lilja H. Significance of different molecular forms of serum PSA. The free, noncomplexed form of PSA versus that complexed to alpha 1-antichymotrypsin. Urol Clin North Am. 1993;20(4):681-686.
Lilja H. Prostate-specific antigen: molecular forms and the human kallikrein gene family. Br J Urol. 1997;79(Suppl. 1):44-48.
Lilja H. Biology of prostate-specific antigen. Urology. 2003;62(5 Suppl. 1):27-33.
Lilja H, Ulmert D, Bjork T, et al. Long-term prediction of prostate cancer up to 25 years before diagnosis of prostate cancer using prostate kallikreins measured at age 44 to 50 years. J Clin Oncol. 2007;25(4):431-436.
Lim LS, Sherin K. Screening for prostate cancer in U.S. men ACPM position statement on preventive practice. Am J Prev Med. 2008;34(2):164-170.
Lin K, Lipsitz R, Miller T, et al. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(3):192-199.
Littrup PJ, Kane RA, Williams CR, et al. Determination of prostate volume with transrectal US for cancer screening. Part I. Comparison with prostate-specific antigen assays. Radiology. 1991;178(2):537-542.
Loeb S, Kettermann A, Ferrucci L, et al. PSA doubling time versus PSA velocity to predict high-risk prostate cancer: data from the Baltimore Longitudinal Study of Aging. Eur Urol. 2008;54(5):1073-1080.
Loeb S, Roehl KA, Antenor JA, et al. Baseline prostate-specific antigen compared with median prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology. 2006;67(2):316-320.
Loeb S, Roehl KA, Catalona WJ, et al. Prostate specific antigen velocity threshold for predicting prostate cancer in young men. J Urol. 2007;177(3):899-902.
Loeb S, Roehl KA, Catalona WJ, et al. Is the utility of prostate-specific antigen velocity for prostate cancer detection affected by age? BJU Int. 2008;101(7):817-821.
Loeb S, Roehl KA, Nadler RB, et al. Prostate specific antigen velocity in men with total prostate specific antigen less than 4 ng/mL. J Urol. 2007;178(6):2348-2352. discussion 2352–3
Loeb S, Sutherland DE, D’Amico AV, et al. PSA velocity is associated with Gleason score in radical prostatectomy specimen: marker for prostate cancer aggressiveness. Urology. 2008;72(5):1116-1120. discussion 1120
Lu-Yao G, Stukel TA, Yao SL. Prostate-specific antigen screening in elderly men. J Natl Cancer Inst. 2003;95(23):1792-1797.
Makarov DV, Loeb S, Getzenberg RH, Partin AW. Biomarkers for prostate cancer. Annu Rev Med. 2009;60:139-151.
Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69(6):1095-1101.
Marks LS, Andriole GL, Fitzpatrick JM, et al. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006;176(3):868-874.
Marks LS, Fradet Y, Deras IL, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69(3):532-535.
Mattei A, Fuechsel FG, Bhatta Dhar N, et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. 2008;53(1):118-125.
May M, Knoll N, Siegsmund M, et al. Validity of the CAPRA score to predict biochemical recurrence-free survival after radical prostatectomy. Results from a European multicenter survey of 1,296 patients. J Urol. 2007;178(5):1957-1962. discussion 1962
McCormack RT, Rittenhouse HG, Finlay JA, et al. Molecular forms of prostate-specific antigen and the human kallikrein gene family: a new era. Urology. 1995;45(5):729-744.
Mikolajczyk SD, Catalona WJ, Evans CL, et al. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin Chem. 2004;50(6):1017-1025.
Mikolajczyk SD, Grauer LS, Millar LS, et al. A precursor form of PSA (pPSA) is a component of the free PSA in prostate cancer serum. Urology. 1997;50(5):710-714.
Mikolajczyk SD, Marker KM, Millar LS, et al. A truncated precursor form of prostate-specific antigen is a more specific serum marker of prostate cancer. Cancer Res. 2001;61(18):6958-6963.
Mikolajczyk SD, Millar LS, Wang TJ, et al. A precursor form of prostate-specific antigen is more highly elevated in prostate cancer compared with benign transition zone prostate tissue. Cancer Res. 2000;60(3):756-759.
Mikolajczyk SD, Millar LS, Wang TJ, et al. BPSA, a specific molecular form of free prostate-specific antigen, is found predominantly in the transition zone of patients with nodular benign prostatic hyperplasia. Urology. 2000;55(1):41-45.
Moreno JG, Croce CM, Fischer R, et al. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer Res. 1992;52(21):6110-6112.
Morgan TO, Jacobsen SJ, McCarthy WF, et al. Age-specific reference ranges for prostate-specific antigen in black men. N Engl J Med. 1996;335(5):304-310.
Morgentaler A. Re: the interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2007;177(5):1954.
Morgentaler A, Bruning CO3rd, DeWolf WC. Occult prostate cancer in men with low serum testosterone levels. JAMA. 1996;276(23):1904-1906.
Moul JW, Connelly RR, Perahia B, et al. The contemporary value of pretreatment prostatic acid phosphatase to predict pathological stage and recurrence in radical prostatectomy cases. J Urol. 1998;159(3):935-940.
Moul JW, Sun L, Hotaling JM, et al. Age adjusted prostate specific antigen and prostate specific antigen velocity cut points in prostate cancer screening. J Urol. 2007;177(2):499-503. discussion 503–4
Mueller EJ, Crain TW, Thompson IM, et al. An evaluation of serial digital rectal examinations in screening for prostate cancer. J Urol. 1988;140(6):1445-1447.
Nadler RB, Humphrey PA, Smith DS, et al. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol. 1995;154(2 Pt. 1):407-413.
Nadler RB, Loeb S, Roehl KA, et al. Use of 2.6 ng/mL prostate specific antigen prompt for biopsy in men older than 60 years. J Urol. 2005;174(6):2154-2157. discussion 2157
Nakanishi H, Groskopf J, Fritsche HA, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179(5):1804-1809. discussion 1809–10
National Comprehensive Cancer Network. Clinical practice guidelines in oncology. <http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf>, 2009. [accessed 212.01.09]
National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Prostate cancer early detection. <http://www.nccn.org/professionals/physician_gls/PDF/prostate_detection.pdf>, 2007. [accessed 13.04.11]
Naya Y, Stamey TA, Cheli CD, et al. Can volume measurement of the prostate enhance the performance of complexed prostate-specific antigen? Urology. 2002;60(4 Suppl. 1):36-41.
O’Malley KJ, Pound CR, Walsh PC, et al. Influence of biopsy perineural invasion on long-term biochemical disease-free survival after radical prostatectomy. Urology. 2002;59(1):85-90.
Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270(7):860-864.
Okegawa T, Noda H, Nutahara K, et al. Comparison of two investigative assays for the complexed prostate-specific antigen in total prostate-specific antigen between 4.1 and 10.0 ng/mL. Urology. 2000;55(5):700-704.
Okotie OT, Roehl KA, Han M, et al. Characteristics of prostate cancer detected by digital rectal examination only. Urology. 2007;70(6):1117-1120.
Pannek J, Marks LS, Pearson JD, et al. Influence of finasteride on free and total serum prostate specific antigen levels in men with benign prostatic hyperplasia. J Urol. 1998;159(2):449-453.
Parker CC, Husband J, Dearnaley DP. Lymph node staging in clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 1999;2(4):191-199.
Parsons JK, Brawer MK, Cheli CD, et al. Complexed prostate specific antigen (PSA) reduces unnecessary prostate biopsies in the 2.6–4.0 ng/mL range of total PSA. BJU Int. 2004;94(1):47-50.
Partin AW, Carter HB, Chan DW, et al. Prostate specific antigen in the staging of localized prostate cancer: influence of tumor differentiation, tumor volume and benign hyperplasia. J Urol. 1990;143(4):747-752.
Partin AW, Catalona WJ, Finlay JA, et al. Use of human glandular kallikrein 2 for the detection of prostate cancer: preliminary analysis. Urology. 1999;54(5):839-845.
Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277(18):1445-1451.
Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58(6):843-848.
Partin AW, Pound CR, Clemens JQ, et al. Serum PSA after anatomic radical prostatectomy. The Johns Hopkins experience after 10 years. Urol Clin North Am. 1993;20(4):713-725.
Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150(1):110-114.
Patel AA, Chen MH, Renshaw AA, et al. PSA failure following definitive treatment of prostate cancer having biopsy Gleason score 7 with tertiary grade 5. JAMA. 2007;298(13):1533-1538.
Peter J, Unverzagt C, Krogh TN, et al. Identification of precursor forms of free prostate-specific antigen in serum of prostate cancer patients by immunosorption and mass spectrometry. Cancer Res. 2001;61(3):957-962.
Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery—what we have learned and where we are going. J Urol. 1999;162(2):293-306.
Pound CR, Partin AW, Epstein JI, et al. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24(2):395-406.
Prestigiacomo AF, Stamey TA. Physiological variation of serum prostate specific antigen in the 4.0 to 10.0 ng/mL range in male volunteers. J Urol. 1996;155(6):1977-1980.
Purohit RS, Shinohara K, Meng MV, et al. Imaging clinically localized prostate cancer. Urol Clin North Am. 2003;30(2):279-293.
Raaijmakers R, Blijenberg BG, Finlay JA, et al. Prostate cancer detection in the prostate specific antigen range of 2.0 to 3.9 ng/mL: value of percent free prostate specific antigen on tumor detection and tumor aggressiveness. J Urol. 2004;171(6 Pt. 1):2245-2249.
Raaijmakers R, Wildhagen MF, Ito K, et al. Prostate-specific antigen change in the European Randomized Study of Screening for Prostate Cancer, section Rotterdam. Urology. 2004;63(2):316-320.
Ravery V, Boccon-Gibod LA, Dauge-Geffroy MC, et al. Systematic biopsies accurately predict extracapsular extension of prostate cancer and persistent/recurrent detectable PSA after radical prostatectomy. Urology. 1994;44(3):371-376.
Riehmann M, Rhodes PR, Cook TD, et al. Analysis of variation in prostate-specific antigen values. Urology. 1993;42(4):390-397.
Rietbergen JB, Hoedemaeker RF, Kruger AE, et al. The changing pattern of prostate cancer at the time of diagnosis: characteristics of screen detected prostate cancer in a population based screening study. J Urol. 1999;161(4):1192-1198.
Rifkin MD. MRI of the prostate. Crit Rev Diagn Imaging. 1990;31(2):223-262.
Roehl KA, Antenor JA, Catalona WJ. Robustness of free prostate specific antigen measurements to reduce unnecessary biopsies in the 2.6 to 4.0 ng/mL range. J Urol. 2002;168(3):922-925.
Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172(3):910-914.
Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60(3):434-441.
Rommel FM, Agusta VE, Breslin JA, et al. The use of prostate specific antigen and prostate specific antigen density in the diagnosis of prostate cancer in a community based urology practice. J Urol. 1994;151(1):88-93.
Roobol MJ, Kranse R, de Koning HJ, et al. Prostate-specific antigen velocity at low prostate-specific antigen levels as screening tool for prostate cancer: results of second screening round of ERSPC (Rotterdam). Urology. 2004;63(2):309-313. discussion 313–15
Ross KS, Carter HB, Pearson JD, et al. Comparative efficiency of prostate-specific antigen screening strategies for prostate cancer detection. JAMA. 2000;284(11):1399-1405.
Ross KS, Guess HA, Carter HB. Estimation of treatment benefits when PSA screening for prostate cancer is discontinued at different ages. Urology. 2005;66(5):1038-1042.
Ross LE, Coates RJ, Breen N, et al. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004;38(6):732-744.
Schaeffer EM, Carter HB, Kettermann A, et al. Prostate specific antigen testing among the elderly—when to stop? J Urol. 2009;181(4):1606-1614. discussion 1613–14
Schroder FH. Screening for prostate cancer. Urol Clin North Am. 2003;30(2):239-251. viii
Schroder FH, Carter HB, Wolters T, et al. Early detection of prostate cancer in 2007. Part 1: PSA and PSA kinetics. Eur Urol. 2008;53(3):468-477.
Schroder FH, Hermanek P, Denis L, et al. The TNM classification of prostate cancer. Prostate Suppl. 1992;4:129-138.
Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320-1328.
Schroder FH, Roobol MJ, van der Kwast TH, et al. Does PSA velocity predict prostate cancer in pre-screened populations? Eur Urol. 2006;49(3):460-465. discussion 465
Schroder FH, van der Maas P, Beemsterboer P, et al. Evaluation of the digital rectal examination as a screening test for prostate cancer. Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 1998;90(23):1817-1823.
Schwartz LM, Woloshin S, Fowler FJJr, et al. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71-78.
Seaman E, Whang M, Olsson CA, et al. PSA density (PSAD). Role in patient evaluation and management. Urol Clin North Am. 1993;20(4):653-663.
Sengupta S, Myers RP, Slezak JM, et al. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol. 2005;174(6):2191-2196.
Shingleton WB, Terrell F, Kolski J, et al. Prostate specific antigen measurements after minimally invasive surgery of the prostate in men with benign prostatic hypertrophy. Prostate Cancer Prostatic Dis. 2000;3(3):200-202.
Simak R, Madersbacher S, Zhang ZF, et al. The impact of ejaculation on serum prostate specific antigen. J Urol. 1993;150(3):895-897.
Singh H, Canto EI, Shariat SF, et al. Predictors of prostate cancer after initial negative systematic 12 core biopsy. J Urol. 2004;171(5):1850-1854.
Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289(11):1414-1420.
Smith DS, Catalona WJ. Rate of change in serum prostate specific antigen levels as a method for prostate cancer detection. J Urol. 1994;152(4):1163-1167.
Smith DS, Catalona WJ. Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology. 1995;45(1):70-74.
Smith DS, Catalona WJ, Herschman JD. Longitudinal screening for prostate cancer with prostate-specific antigen. JAMA. 1996;276(16):1309-1315.
Sokoll LJ, Ellis W, Lange P, et al. A multicenter evaluation of the PCA3 molecular urine test: pre-analytical effects, analytical performance, and diagnostic accuracy. Clin Chim Acta. 2008;389(1–2):1-6.
Southwick PC, Catalona WJ, Partin AW, et al. Prediction of post-radical prostatectomy pathological outcome for stage T1c prostate cancer with percent free prostate specific antigen: a prospective multicenter clinical trial. J Urol. 1999;162(4):1346-1351.
Stamey TA, Caldwell M, McNeal JE, et al. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172(4 Pt. 1):1297-1301.
Stamey TA, Kabalin JN, Ferrari M. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. III. Radiation treated patients. J Urol. 1989;141(5):1084-1087.
Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909-916.
Stein A, deKernion JB, Dorey F. Prostatic specific antigen related to clinical status 1 to 14 years after radical retropubic prostatectomy. Br J Urol. 1991;67(6):626-631.
Stenman UH, Hakama M, Knekt P, et al. Serum concentrations of prostate specific antigen and its complex with alpha 1-antichymotrypsin before diagnosis of prostate cancer. Lancet. 1994;344(8937):1594-1598.
Stenner J, Holthaus K, Mackenzie SH, et al. The effect of ejaculation on prostate-specific antigen in a prostate cancer-screening population. Urology. 1998;51(3):455-459.
Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98(10):715-717.
Steuber T, Niemela P, Haese A, et al. Association of free-prostate specific antigen subfractions and human glandular kallikrein 2 with volume of benign and malignant prostatic tissue. Prostate. 2005;63(1):13-18.
Stone NN, Stock RG, Parikh D, et al. Perineural invasion and seminal vesicle involvement predict pelvic lymph node metastasis in men with localized carcinoma of the prostate. J Urol. 1998;160(5):1722-1726.
Stone NN, Stock RG, Unger P. Indications for seminal vesicle biopsy and laparoscopic pelvic lymph node dissection in men with localized carcinoma of the prostate. J Urol. 1995;154(4):1392-1396.
Taneja SS, Tran K, Lepor H. Volume-specific cutoffs are necessary for reproducible application of prostate-specific antigen density of the transition zone in prostate cancer detection. Urology. 2001;58(2):222-227.
Tchetgen MB, Song JT, Strawderman M, et al. Ejaculation increases the serum prostate-specific antigen concentration. Urology. 1996;47(4):511-516.
Tempany CM, Zhou X, Zerhouni EA, et al. Staging of prostate cancer: results of Radiology Diagnostic Oncology Group project comparison of three MR imaging techniques. Radiology. 1994;192(1):47-54.
Terris MK, Klonecke AS, McDougall IR, et al. Utilization of bone scans in conjunction with prostate-specific antigen levels in the surveillance for recurrence of adenocarcinoma after radical prostatectomy. J Nucl Med. 1991;32(9):1713-1717.
Terris MK, McNeal JE, Freiha FS, et al. Efficacy of transrectal ultrasound-guided seminal vesicle biopsies in the detection of seminal vesicle invasion by prostate cancer. J Urol. 1993;149(5):1035-1039.
Terris MK, Pham TQ, Issa MM, et al. Routine transition zone and seminal vesicle biopsies in all patients undergoing transrectal ultrasound guided prostate biopsies are not indicated. J Urol. 1997;157(1):204-206.
Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/mL or lower. JAMA. 2005;294(1):66-70.
Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529-534.
Thompson IM, Pauler Ankerst D, Chi C, et al. Prediction of prostate cancer for patients receiving finasteride: results from the Prostate Cancer Prevention Trial. J Clin Oncol. 2007;25(21):3076-3081.
Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246.
Thompson IM, Rounder JB, Teague JL, et al. Impact of routine screening for adenocarcinoma of the prostate on stage distribution. J Urol. 1987;137(3):424-426.
Tremblay RR, Deperthes D, Tetu B, et al. Immunohistochemical study suggesting a complementary role of kallikreins hK2 and hK3 (prostate-specific antigen) in the functional analysis of human prostate tumors. Am J Pathol. 1997;150(2):455-459.
Troyer JK, Beckett ML, Wright GLJr. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30(4):232-242.
Ts’o PO, Pannek J, Wang ZP, et al. Detection of intact prostate cancer cells in the blood of men with prostate cancer. Urology. 1997;49(6):881-885.
Turner RD, Belt E. The results of 1,694 consecutive simple perineal prostatectomies. J Urol. 1957;77(6):853-863.
U.S. Preventive Services Task Force. Screening for prostate cancer. U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185-191.
Vallancien G, Bochereau G, Wetzel O, et al. Influence of preoperative positive seminal vesicle biopsy on the staging of prostatic cancer. J Urol. 1994;152(4):1152-1156.
van der Cruijsen-Koeter IW, Roobol MJ, Wildhagen MF, et al. Tumor characteristics and prognostic factors in two subsequent screening rounds with four-year interval within prostate cancer screening trial, ERSPC Rotterdam. Urology. 2006;68(3):615-620.
van Gils MP, Hessels D, Hulsbergen-van de Kaa CA, et al. Detailed analysis of histopathological parameters in radical prostatectomy specimens and PCA3 urine test results. Prostate. 2008;68(11):1215-1222.
Veneziano S, Pavlica P, Querze R, et al. Correlation between prostate-specific antigen and prostate volume, evaluated by transrectal ultrasonography: usefulness in diagnosis of prostate cancer. Eur Urol. 1990;18(2):112-116.
Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008;6:19.
Vickers AJ, Savage C, O’Brien MF, et al. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27(3):398-403.
Vieira JG, Nishida SK, Pereira AB, et al. Serum levels of prostate-specific antigen in normal boys throughout puberty. J Clin Endocrinol Metab. 1994;78(5):1185-1187.
Vis AN, Hoedemaeker RF, Roobol M, et al. Tumor characteristics in screening for prostate cancer with and without rectal examination as an initial screening test at low PSA (0.0–3.9 ng/mL). Prostate. 2001;47(4):252-261.
Walsh PC. Urological survey. J Urol. 2008;179:543.
Walsh PC, Jewett HJ. Radical surgery for prostatic cancer. Cancer. 1980;45(Suppl. 7):1906-1911.
Walter LC, Bertenthal D, Lindquist K, et al. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336-2342.
Wang MC, Papsidero LD, Kuriyama M, et al. Prostate antigen: a new potential marker for prostatic cancer. Prostate. 1981;2(1):89-96.
Welch HG. Should I be tested for cancer? Maybe not and here’s why. Berkeley: University of California Press; 2004.
Whitman EJ, Groskopf J, Ali A, et al. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J Urol. 2008;180(5):1975-1978. discussion 1978–9
Whittemore AS, Cirillo PM, Feldman D, et al. Prostate specific antigen levels in young adulthood predict prostate cancer risk: results from a cohort of Black and White Americans. J Urol. 2005;174(3):872-876. discussion 876
Whitmore WFJr. Hormone therapy in prostatic cancer. Am J Med. 1956;21(5):697-713.
Wolf JSJr, Cher M, Dall’era M, et al. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol. 1995;153(3 Pt. 2):993-999.
Woodrum DL, Brawer MK, Partin AW, et al. Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol. 1998;159(1):5-12.
Yavascaoglu I, Savci V, Oktay B, et al. The effects of ejaculation on serum prostate-specific antigen (PSA). Int Urol Nephrol. 1998;30(1):53-58.
Young CY, Montgomery BT, Andrews PE, et al. Hormonal regulation of prostate-specific antigen messenger RNA in human prostatic adenocarcinoma cell line LNCaP. Cancer Res. 1991;51(14):3748-3752.
Yu KK, Scheidler J, Hricak H, et al. Prostate cancer: prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999;213(2):481-488.
Yu X, Han M, Loeb S, et al. Comparison of methods for calculating prostate specific antigen velocity. J Urol. 2006;176(6 Pt. 1):2427-2431. discussion 2431
Zincke H, Oesterling JE, Blute ML, et al. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994;152(5 Pt. 2):1850-1857.