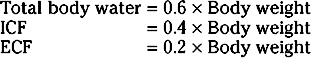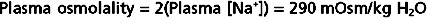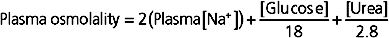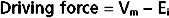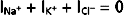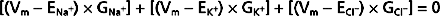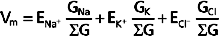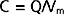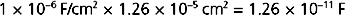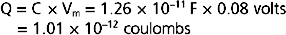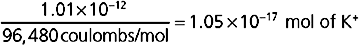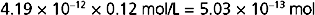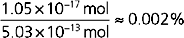CHAPTER 2 Homeostasis of Body Fluids
Normal cellular function requires that the intracellular composition of ions, small molecules, water, pH, and a host of other substances be maintained within a narrow range. This is accomplished by the transport of many substances and water into and out of the cell with the use of membrane transport proteins as described in Chapter 1. In addition, each day food and water are ingested and waste products are excreted from the body. In a healthy individual this occurs without significant changes in either the volume of body fluids or their composition. Such maintenance of steady-state balance, where the volume and composition of body fluids remain constant despite the addition and elimination of water and solutes from the body, to a large degree reflects the function of epithelial cells. These cells, which constitute the interface between the internal environment of the body and the external world, maintain the volume and composition of the fluid bathing all cells (i.e., the extracellular fluid [ECF]) constant. The ECF, in turn, helps cell maintain a constant intracellular environment.
The ability of the body to maintain constant volume and composition of the intracellular fluid (ICF) and ECF is a complex process that involves all organ systems of the body. Transport by the epithelial cells of the gastrointestinal tract, kidneys, and lungs controls both the intake and excretion of numerous substances and water. The cardiovascular system delivers nutrients to and removes waste products from cells and tissues. Finally, the nervous and endocrine systems provide regulation and integration of these important functions.
To provide background for further study of the organ systems, this chapter presents an overview of the concept of steady-state balance, reviews the normal volume and composition of body fluids, and describes how cells maintain their intracellular composition and volume. Included is a presentation on how cells generate and maintain a membrane potential, which is fundamental to understanding the function of excitable cells (e.g., neurons and muscle cells). Finally, because epithelial cells are so central to the process of regulating the volume and composition of body fluids, the principles of solute and water transport by epithelial cells are reviewed.
CONCEPT OF STEADY-STATE BALANCE
The concept of steady-state balance can be illustrated by considering a river on which a dam is built to create an artificial lake. Each day, water enters the lake from the various streams and rivers that feed it. In addition, water is added by rain and snow. At the same time, water is lost through the spillways of the dam and by the process of evaporation. For the level of the lake to remain constant (i.e., steady-state balance), the rate at which water is added, regardless of the source, must be exactly matched by the amount of water lost, again by whichever route. Because the addition of water and loss by evaporation are not easily controlled, the only way to maintain the level of the lake constant is to regulate the amount that is allowed through the spillways. For such a system to work, there must be a “set point,” or a determination of what the optimal level of water in the lake should be. There must also be some way to measure deviations from the set point, such as a measure of the depth of the lake. Finally, there must be a mechanism, or “effector,” that regulates the amount of water that leaves the lake through the spillway. In this example, the dam operator, who controls the spillways, is that effector.
For virtually every substance in the body, the amount or concentration of which must be maintained within a narrow range, there is a set point and mechanism for monitoring deviations from that set point and effector mechanisms to maintain amounts or concentrations of that substance within the body constant, or in steady-state balance.
In keeping with the dam and lake analogy, consider the maintenance of steady-state water balance in humans (see Chapter 34 for details). Each day various volumes of liquid are ingested, and water is produced through cellular metabolism. Importantly, the amount of water added to the body each day is not constant, although it can be regulated to a degree by the thirst mechanism. In addition, water is lost from the body via respiration, sweating, and feces. The amount of water lost by these routes also varies over time, depending on the respiratory rate, physical activity, ambient temperature, and the presence or absence of diarrhea. The only regulated route for excretion of water from the body is the kidneys. The body maintains steady-state water balance by ensuring that the amount of water added to the body each day is exactly balanced by the amount lost or excreted from the body.
The body monitors the amount of water that it contains through changes in the osmolality of ECF. When excess water is added to the body, the osmolality of ECF decreases. Conversely, when excess water is lost from the body, osmolality increases. Cells within the hypothalamus of the brain monitor changes in ECF osmolality around each person’s genetically determined set point. When deviations from the set point occur, neural and hormonal signals are activated (i.e., effectors). For example, when ECF osmolality is increased, neural signals are sent to another region of the hypothalamus to stimulate the sensation of thirst. At the same time, antidiuretic hormone (ADH) is secreted from the posterior pituitary and acts on the kidneys to reduce the excretion of water. Thus, water intake is increased at the same time that its loss from the body is reduced, and the osmolality of ECF returns to its set point. When the osmolality of ECF is decreased, thirst is inhibited, as is the secretion of ADH. As a result, intake of water is reduced, and its excretion by the kidneys is increased. Again, these actions return the osmolality of ECF to the set point.
OVERVIEW OF THE INTRACELLULAR AND EXTRACELLULAR COMPARTMENTS
Definitions and Volumes of Body Fluid Compartments
Water makes up approximately 60% of the body’s weight, with variability among individuals being a function of the amount of adipose tissue. Because the water content of adipose tissue is lower than that of other tissue, increased amounts of adipose tissue reduce the fraction of total body weight attributable to water. The percentage of body weight attributed to water also varies with age. In newborns it is approximately 75%. This decreases to the adult value of 60% by the age of 1 year.
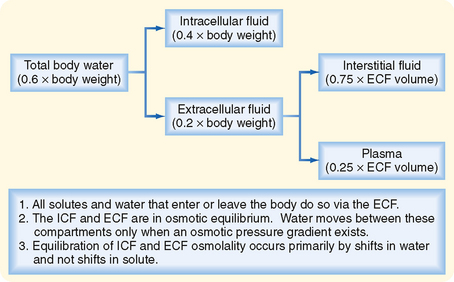
As illustrated in Figure 2-1, total body water is distributed between two major compartments, which are divided by the cell membrane.* The intracellular fluid compartment is the larger compartment and contains approximately two thirds of total body water. The remaining third is contained in the extracellular fluid compartment. Expressed as percentages of body weight, the volumes of total body water, ICF, and ECF are
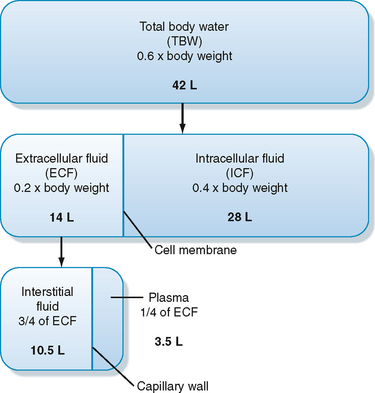
Figure 2-1 Relationship between volumes of the various body fluid compartments. The actual values shown are for an individual weighing 70 kg.
(Modified from Levy MN, Koeppen BM, Stanton BA: Berne & Levy’s Principles of Physiology, 4th ed. St. Louis, Mosby, 2006.)
The ECF compartment is further subdivided into interstitial fluid and plasma, which are separated by the capillary wall. The interstitial fluid surrounds the cells in the various tissues of the body and accounts for three fourths of the ECF volume. ECF includes water contained within bone and dense connective tissue, as well as cerebrospinal fluid. Plasma represents the remaining fourth of ECF. Under some pathological conditions, additional fluid may accumulate in what is referred to as a “third space.” Third-space collections of fluid are part of the ECF and include, for example, the accumulation of fluid in the peritoneal cavity (ascites) of individuals with liver disease.
Composition of Body Fluid Compartments
Table 2-1 summarizes the composition of the ECF and ICF for a number of important ions and molecules. As discussed in detail later, the composition of ICF is maintained by the action of various specific membrane transport proteins. Principal among these transporters is Na+,K+-ATPase, which converts the energy in ATP into ion and electrical gradients, which in turn can be used to drive the transport of other ions and molecules.
Table 2-1 Ionic Composition of a Typical Cell
| Extracellular Fluid | Intracellular Fluid | |
|---|---|---|
| Na+ (mEq/L) | 135-147 | 10-15 |
| K+ (mEq/L) | 3.5-5.0 | 120-150 |
| Cl− (mEq/L) | 95-105 | 20-30 |
| HCO3− (mEq/L) | 22-28 | 12-16 |
| Ca++ (mmol/L)* | 2.1-2.8 (total) | ≈10−7 (ionized) |
| 1.1-1.4 (ionized) | ||
| Pi (mmol/L)* | 1.0-1.4 (total) | |
| 0.5-0.7 (ionized) | 0.5-0.7 (ionized) |
* Ca++ and Pi (H2PO4−/HPO4−2) are bound to proteins and other organic molecules. In addition, large amounts of Ca++ can be sequestered within cells. Large amounts of Pi are present in cells as part of organic molecules (e.g., ATP).
The composition of the plasma and interstitial fluid compartments of the ECF is similar because they are separated only by the capillary endothelium, a barrier that is freely permeable to ions and small molecules. The major difference between interstitial fluid and plasma is that the latter contains significantly more protein. Although this differential concentration of protein can affect the distribution of cations and anions between these two compartments by the Gibbs-Donnan effect (see later for details), this effect is small, and the ionic composition of interstitial fluid and plasma can be considered to be identical.
Because of its abundance in ECF, Na+ (and its attendant anions, primarily Cl− and HCO3−) is the major determinant of the osmolality of this compartment. Accordingly, a rough estimate of ECF osmolality can be obtained by simply doubling the sodium concentration [Na+]. For example, if a blood sample is obtained from an individual and the [Na+] of plasma is 145 mEq/L, its osmolality can be estimated as
Because water is in osmotic equilibrium across the capillary endothelium and the plasma membrane of cells, measurement of plasma osmolality also provides a measure of the osmolality of the ECF and ICF.
Fluid Exchange between the ICF and ECF
Water moves freely and often rapidly between the various body fluid compartments. Two forces determine this movement: hydrostatic pressure and osmotic pressure. Hydrostatic pressure from pumping of the heart (and the effect of gravity on the column of blood in the vessel) and osmotic pressure exerted by plasma proteins (oncotic pressure) are important determinants of fluid movement across the capillary wall (see Chapter 17). By contrast, because hydrostatic pressure gradients are not present across the cell membrane, only osmotic pressure differences between ICF and ECF cause movement of fluid into and out of cells.
Osmotic pressure differences between ECF and ICF are responsible for movement of fluid between these compartments. Because the plasma membrane of cells contains water channels (aquaporins), water can easily cross the membrane. Hence, a change in the osmolality of either ICF or ECF results in rapid movement (i.e., minutes) of water between these compartments. Thus, except for transient changes, the ICF and ECF compartments are in osmotic equilibrium.
In contrast to water, the movement of ions across cell membranes is more variable from cell to cell and depends on the presence of specific membrane transport proteins (see later). Consequently, as a first approximation, fluid exchange between the ICF and ECF compartments can be analyzed by assuming that appreciable shifts of ions between the compartments do not occur.
A useful approach to understanding the movement of fluids between the ICF and the ECF is outlined in Figure 2-2. To illustrate this approach, consider what happens when solutions containing various amounts of NaCl are added to the ECF.*
In clinical situations a more accurate estimate of plasma osmolality and thus the osmolality of ECF and ICF is obtained by also considering the osmoles contributed by glucose and urea because these are the next most abundant solutes in ECF (the other components of ECF contribute only a few additional milliosmoles). Accordingly, plasma osmolality can be estimated as
The glucose and urea concentrations are expressed in units of mg/dL (dividing by 18 for glucose and 2.8 for urea* allows conversion from the units of mg/dL to mmol/L and thus to mOsm/kg H2O). This estimation of plasma osmolality is especially useful when dealing with patients who have an elevated plasma [glucose] secondary to diabetes mellitus and in patients with chronic renal failure, whose plasma [urea] is elevated.
* The [urea] in plasma is measured as the nitrogen in the urea molecule, or blood urea nitrogen (BUN).
Neurosurgical procedures and cerebrovascular accidents (strokes) often result in the accumulation of interstitial fluid in the brain (i.e., edema) and swelling of neurons. Because the brain is enclosed within the skull, edema can raise intracranial pressure and thereby disrupt neuronal function, eventually leading to coma and death. The blood-brain barrier, which separates the cerebrospinal fluid and brain interstitial fluid from blood, is freely permeable to water but not to most other substances. As a result, excess fluid in brain tissue can be removed by imposing an osmotic gradient across the blood-brain barrier. Mannitol can be used for this purpose. Mannitol is a sugar (molecular weight of 182 g/mol) that does not readily cross the blood-brain barrier and membranes of cells (neurons, as well as other cells in the body). Therefore, mannitol is an effective osmole, and intravenous infusion results in the movement of fluid from brain tissue by osmosis.
Example 1: Addition of Isotonic NaCl to ECF
Addition of an isotonic NaCl solution (e.g., intravenous infusion of 0.9% NaCl, osmolality of ≈290 mOsm/kg H2O)† to ECF increases the volume of this compartment by the volume of fluid administered. Because this fluid has the same osmolality as ECF and therefore also ICF, there will be no driving force for movement of fluid between these compartments, and the volume of ICF will be unchanged. Although Na+ can cross cell membranes, it is effectively restricted to the ECF by the activity of Na+,K+-ATPase, which is present in the plasma membrane of all cells. Therefore, there is no net movement of the infused NaCl into the cells.
Example 2: Addition of Hypotonic NaCl to ECF
Addition of a hypotonic NaCl solution to ECF (e.g., intravenous infusion of 0.45% NaCl, osmolality of ≈145 mOsm/kg H2O) decreases the osmolality of this fluid compartment and results in movement of water into the ICF. After osmotic equilibration, the osmolality of ICF and ECF is equal but lower than before the infusion, and the volume of each compartment is increased. The increase in ECF volume is greater than the increase in ICF volume.
Example 3: Addition of Hypertonic NaCl to ECF
Addition of a hypertonic NaCl solution to ECF (e.g., intravenous infusion of 3% NaCl, osmolality of ≈1000 mOsm/kg H2O) increases the osmolality of this compartment and results in the movement of water out of cells. After osmotic equilibration, the osmolality of ECF and ICF will be equal but higher than before the infusion. The volume of ECF is increased, whereas that of ICF is decreased.
Fluid and electrolyte disorders are seen commonly in clinical practice (e.g., in patients with vomiting or diarrhea, or both). In most instances these disorders are self-limited, and correction of the disorder occurs without any need for intervention. However, more severe or prolonged disorders may require fluid replacement therapy. Such therapy may be administered orally with special electrolyte solutions, or intravenous fluid may be administered.
Intravenous solutions are available in many formulations. The type of fluid administered to a particular patient is dictated by the patient’s need. For example, if an increase in the patient’s vascular volume is necessary, a solution containing substances that do not readily cross the capillary wall is infused (e.g., 5% protein or dextran solutions). The oncotic pressure generated by the albumin molecules retains fluid in the vascular compartment and thereby expands its volume. Expansion of ECF is accomplished most often by using isotonic saline solutions (e.g., 0.9% NaCl or lactated Ringer’s solution). As already noted, administration of an isotonic NaCl solution does not result in the development of an osmotic pressure gradient across the plasma membrane of cells. Therefore, the entire volume of the infused solution will remain in the ECF. Patients whose body fluids are hyperosmotic need hypotonic solutions. These solutions may be hypotonic NaCl (e.g., 0.45% NaCl or 5% dextrose in water, so-called D5W). Administration of D5W solution is equivalent to the infusion of distilled water because the dextrose is metabolized to CO2 and water. Administration of these fluids increases the volume of both ICF and ECF. Finally, patients whose body fluids are hypotonic need hypertonic solutions. These are typically NaCl-containing solutions (e.g., 3% and 5% NaCl). These solutions expand the volume of ECF but decrease the volume of ICF. Other constituents, such as electrolytes (e.g., K+) or drugs, can be added to intravenous solutions to tailor the therapy to the patient’s fluid, electrolyte, and metabolic needs.
MAINTENANCE OF CELLULAR HOMEOSTASIS
Normal cellular function requires that the composition of ICF be tightly controlled. For example, the activity of some enzymes is dependent on pH. Therefore, intracellular pH must be regulated. The intracellular ionic composition is similarly held within a narrow range. This is necessary for establishment of the membrane potential, a cell property especially important for the normal function of excitable cells (e.g., neurons and muscle cells) and for intracellular signaling (e.g., intracellular [Ca++]–see Chapter 3). Finally, the volume of cells must be maintained because shrinking or swelling of cells can lead to cell damage or death. Regulation of intracellular composition and cell volume is accomplished through the activity of specific transporters in the plasma membrane of cells. This section reviews the mechanisms by which cells maintain their intracellular ionic environment and membrane potential and control their volume.
Ionic Composition of Cells
The intracellular ionic composition of cells varies from tissue to tissue. For example, the intracellular composition of neurons is different from that of muscle cells, which differs from that of blood cells. Nevertheless, there are similar patterns, and these are presented in Table 2-1. When compared with ECF, ICF is characterized by a low [Na+] and a high [K+]. This is the result of the activity of Na+,K+-ATPase, which transports 3 Na+ ions out of the cell and 2 K+ ions into the cell for each molecule of ATP hydrolyzed. As will be discussed, the activity of Na+,K+-ATPase is not only important for establishing the cellular Na+ and K+ gradients but is also involved in indirectly determining the cellular gradients for many other ions and molecules. Because Na+,K+-ATPase transports three cations out of the cell in exchange for two cations, it is electrogenic and thus contributes to the establishment of membrane voltage (cell interior negative). However, Na+,K+-ATPase typically contributes only a few millivolts to the membrane potential. More importantly, it is the leakage of K+ out of the cell through K+-selective channels that is a major determinant of membrane voltage (see later). Thus, Na+,K+-ATPase converts the energy in ATP into ion gradients (i.e., Na+ and K+) and a voltage gradient (i.e., membrane potential) as a result of leakage of K+ out of the cell driven by the K+ concentration gradient across the membrane ([K+]i > [K+]o).
The Na+,K+-ATPase–generated ion and electrical gradients are used to drive the transport of other ions and molecules into or out of the cell (Fig. 2-3). For example, as described in Chapter 1, a number of solute carriers couple the transport of Na+ to that of other ions or molecules. The Na+-glucose and Na+–amino acid symporters use the energy in the Na+ electrochemical gradient, directed to bring Na+ into the cell, to drive the secondary active cellular uptake of glucose and amino acids. Similarly, the inwardly directed Na+ gradient drives the secondary active extrusion of H+ from the cell and thus contributes to the maintenance of intracellular pH. The 3Na+-1Ca++ antiporter, along with plasma membrane Ca++-ATPase, extrudes Ca++ from the cell and thus contributes to maintenance of a low intracellular [Ca++].* Finally, the membrane voltage drives Cl− out of the cell through Cl−-selective channels, thus lowering the intracellular concentration below that of the ECF.
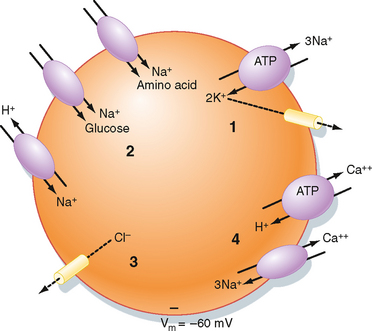
Figure 2-3 Cell model depicting how cellular gradients and the membrane potential (Vm) are established. (1) Na+,K+-ATPase decreases intracellular [Na+] and increases intracellular [K+]. Some K+ exits the cell via K+-selective channels and generates the Vm (cell interior negative). (2) The energy in the Na+ electrochemical gradient drives the transport of other ions and molecules via the use of various solute carriers. (3) The Vm drives Cl− out of the cell through Cl−-selective channels. (4) Ca++-H+-ATPase and the 3Na+-1Ca++ antiporter maintain the low intracellular [Ca++].
Membrane Potential
As described earlier, the Na+,K+-ATPase and K+-selective channels in the plasma membrane are important determinants of the membrane potential (Vm) of the cell. For all cells within the body, the resting membrane potential is oriented with the interior of the cell electrically negative with respect to ECF. However, the magnitude of Vm can vary widely.
To understand what determines the magnitude of Vm it is important to recognize that any transporter that transfers charge across the membrane has the potential to influence Vm. Such transporters are said to be electrogenic. As might be expected, the contribution of various electrogenic transporters to Vm is highly variable from cell to cell. For example, Na+,K+-ATPase transfers one net positive charge across the membrane. However, the direct contribution of Na+,K+-ATPase to the Vm of most cells is only a few millivolts at the most. Similarly, the contribution of other electrogenic transporters, such as the 3Na+-1Ca++ antiporter and the Na+-glucose symporter, is minimal. The major determinants of Vm are ion channels. The type (i.e., selectivity), number, and activity (i.e., gating) of these channels determine the magnitude of Vm. As described in Chapter 5, rapid changes in ion channel activity underlie the action potential in neurons and other excitable cells such as skeletal and cardiac muscle (see Chapters 12 and 13).
As ions move across the membrane through a channel, they generate a current. As described in Chapter 1, this current can be measured, even at the level of a single channel. By convention, the current generated by the movement of cations into the cell or the movement of anions out of the cell is defined as negative current. Conversely, the movement of cations out of the cell or the movement of anions into the cell is defined as positive current. Also by convention, the magnitude of Vm is expressed with respect to the outside of the cell. Thus, for a cell with a Vm of −80 mV, the interior of the cell is electrically negative with respect to the outside of the cell.
The current carried by ions moving through a channel depends on the driving force for that ion and the conductance of the channel. As described in Chapter 1, the driving force is determined by the energy in the concentration gradient for the ion across the membrane, as calculated by the Nernst equation (Ei), and by Vm.
Thus, as defined by Ohm’s law, the ion current through the channel (Ii) is determined as follows:
where gi is the conductance of the channel. For a cell, the conductance of the membrane to a particular ion (gi) is determined by the number of ion channels in the membrane and the amount of time that each channel is in the open state.
As illustrated in Figure 2-4, Vm is the voltage at which there is no net ion flow into or out of the cell. Thus, for a cell having ion channels selective for Na+, K+, and Cl−,
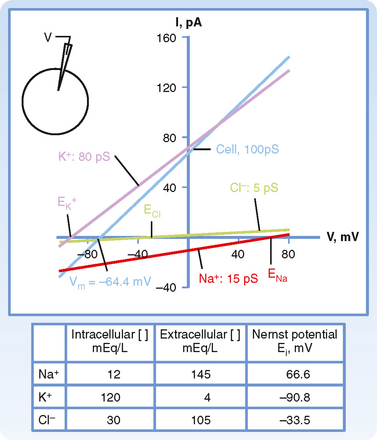
Figure 2-4 Current-voltage relationship of a hypothetical cell containing Na+-, K+-, and Cl−-selective channels. The current-voltage relationship for each ion is shown, as is the relationship for the whole cell. Because 80% of cell conductance is due to K+, the resting membrane voltage (Vm) of −64.4 mV is close to that of the Nernst equilibrium potential for K+.
or
where ΣG = GNa+ = GK+ = GCl−
Inspection of Equation 2-6, which is often called the chord conductance equation, reveals that Vm will be close to the Nernst potential of the ion to which the membrane has the highest conductance. In Figure 2-4, 80% of the membrane conductance is attributable to K+; as a result, Vm is close to the Nernst potential for K+ (EK+). For most cells at rest, the membrane has high conductance for K+, and thus Vm approximates EK+. Moreover, Vm will be greatly influenced by the magnitude of EK+, which in turn will be greatly influenced by changes in [K+] of the ECF. For example, if intracellular [K+] is 120 mEq/L and extracellular [K+] is 4 mEq/L, EK+ has a value of −90.8 mV. If extracellular [K+] is increased to 7 mEq/L, EK+ would be −79.9 mV. This change in EK+ will depolarize Vm (i.e., Vm is less negative). Conversely, if extracellular [K+] is decreased to 2 mEq/L, EK+ becomes −109.4 mV, and the Vm hyperpolarizes (i.e., Vm is more negative).
Changes in extracellular [K+] can have important effects on excitable cells, especially the heart. A decrease in extracellular [K+] (hypokalemia) hyperpolarizes the Vm of cardiac myocytes and in so doing makes it more difficult to initiate an action potential because a larger depolarizing current is needed to reach threshold (see Chapter 16). If severe, hypokalemia can lead to cardiac arrhythmias and eventually the heart can stop contracting (asystole). An increase in extracellular [K+] (hyperkalemia) can be equally deleterious to cardiac function. With hyperkalemia, Vm is depolarized, thus making it easier to initiate an action potential. However, as depolarization of Vm progresses, Na+ channels, the opening of which initiates the action potential, become inactivated. When this occurs, cardiac arrhythmias develop, and as with hypokalemia, the heart can stop contracting.
Equation 2-6 also defines the limits for the membrane potential. Again looking at the example depicted in Figure 2-4, it is apparent that Vm cannot be more negative than EK+ (−90.8 mV), as would be the case if the membrane were conductive only to K+. Conversely, Vm could not be more positive than ENa+ (66.6 mV), a condition met if the membrane were conductive only to Na+. The dependence of Vm on conductance of the membrane to specific ions is the basis by which action potentials in excitable cells are generated (Fig. 2-5). In all excitable cells the membrane at rest is predominantly conductive to K+, and thus Vm is near EK+. When an action potential is initiated, Na+ channels open and the membrane is now predominantly conductive to Na+. As a result, Vm now approaches ENa+. The generation of action potentials is discussed in more detail in Chapter 5.
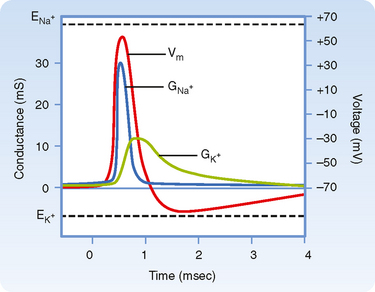
Figure 2-5 Nerve action potential showing the changes in Na+ (GNa+) and K+ (GK+) conductance and membrane potential (Vm). At rest, the membrane has high K+ conductance and Vm is near the Nernst equilibrium potential for K+ (EK+). With initiation of the action potential there is a large increase in Na+ conductance of the membrane, and Vm approaches the Nernst potential for Na+ (ENa+). The increase in Na+ conductance is transient, and K+ conductance then increases above its value before the action potential. This hyperpolarizes the cell as Vm approaches EK+. As K+ conductance returns to its baseline value, Vm returns to its resting value of −70 mV.
(Modified from Levy MN, Koeppen BM, Stanton BA: Berne & Levy’s Principles of Physiology, 4th ed. St. Louis, Mosby, 2006.)
Regulation of Cell Volume
As already noted, changes in cell volume can lead to cell damage and death. Consequently, cells have developed mechanisms to regulate their volume. Most cells are highly permeable to water because of the presence of aquaporins in their plasma membranes. As discussed in Chapter 1, the osmotic pressure gradients across the cell membrane that are generated by effective osmoles will cause water to move either into or out of the cell and result in changes in cell volume. Thus, cells swell when in hypotonic solutions and shrink when placed in hypertonic solutions (see later). However, even when a cell is placed in an isotonic solution, maintenance of cell volume is an active process requiring the expenditure of ATP and specifically the activity of Na+,K+-ATPase.
Establishment of Vm requires separation of charge across the plasma membrane. However, the number of ions that must move across the membrane is a tiny fraction of the total number of ions in the cell. For example, consider a spherical cell with a diameter of 20 μm and a Vm of −80 mV. Furthermore, assume that the −80 mV is the result of K+ diffusing out of the cell and that intracellular [K+] is 120 mEq/L. The amount of K+ that would have to diffuse out of the cell to establish the −80-mV Vm is then calculated as follows.
First, the charge separation across the membrane needs to be calculated. This is done by knowing that the plasma membrane behaves electrically like a capacitor, the capacitance (C) of which is approximately 1 microfarad/cm2 (1 μF/cm2), and
where Q is charge and has the units of coulombs. Given that the surface area of the cell is 4πr2, or 1.26 × 10−5 cm2, the capacitance of the cell is calculated as
Thus, the charge separation across the membrane is calculated as
Because 1 mol of K+ contains 96,480 coulombs, the amount of K+ that had to diffuse across the membrane to establish the Vm of −80 mV is
With a cell volume of 4.19 × 10−12 L (volume = 4 πr3/3) and an intracellular [K+] of 120 mEq/L, the total intracellular [K+] is
Thus, the diffusion of 1.05 × 10−17 mol of K+ out of the cell represents only a 0.002% change in intracellular [K+]:
Thus, the intracellular composition of the cell is not appreciably altered by diffusion of K+ out of the cell.
Isotonic Cell Volume Regulation
The importance of Na+,K+-ATPase in isotonic regulation of cell volume can be appreciated by the observation that red blood cells swell when chilled (i.e., reduced ATP synthesis) or when Na+,K+-ATPase is inhibited by cardiac glycosides (e.g., ouabain). The necessity for energy expenditure to maintain cell volume in an isotonic solution is a result of the effect of intracellular proteins on the distribution of ions across the plasma membrane, the so-called Gibbs-Donnan effect (Fig. 2-6).
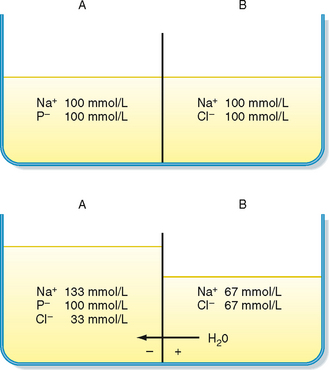
Figure 2-6 The Gibbs-Donnan effect. Top panel, Two solutions are separated by a membrane that is permeable to Na+, Cl−, and H2O, but not permeable to protein (P−). The osmolality of solution A is identical to that of solution B. Bottom panel, Cl− diffuses from solution B to A down its concentration gradient. This causes solution A to become electrically negative with respect to solution B. This membrane voltage then drives the diffusion of Na+ from solution B to A. The accumulation of additional Na+ and Cl− in solution A increases its osmolality and causes water to flow from B to A.
The Gibbs-Donnan effect occurs when a membrane separating two solutions is permeable to some but not all the molecules in solution. As noted previously, this effect accounts for the small differences in the ionic composition of plasma versus interstitial fluid. In this case, the capillary endothelium represents the membrane, and the plasma proteins are the molecules whose permeability across the capillary is restricted. For cells, the membrane is the plasma membrane, and the impermeant molecules are the intracellular proteins and organic molecules.
As depicted in Figure 2-6, the presence of impermeant molecules (e.g., protein) in one compartment results over time in the accumulation of permeable molecules/ions in the same compartment. This increases the number of osmotically active particles in the compartment containing the impermeant anions, which in turn increases osmotic pressure, and water enters the compartment. For cells, the Gibbs-Donnan effect would increase the number of osmotically active particles in the cell and result in cell swelling. However, the activity of Na+,K+-ATPase counteracts the Gibbs-Donnan effect by actively extruding cations (3 Na+ ions are extruded while 2 K+ ions are brought into the cell). In addition, the K+ gradient established by Na+,K+-ATPase allows for development of the Vm (cell interior negative), which in turn drives Cl− out of the cell. Thus, through the activity of Na+,K+-ATPase the number of intracellular osmotically active particles is reduced from what would occur as a result of the Gibbs-Donnan effect, and cell volume is maintained in isotonic solutions.
Nonisotonic Cell Volume Regulation
Most cells throughout the body are bathed with isotonic ECF, the composition of which is tightly regulated. However, certain regions within the body are not isotonic (e.g., the medulla of the kidney), and with disorders in water balance, the ECF can become either hypotonic or hypertonic. When this occurs, cells will either swell or shrink. Because cell swelling or shrinkage can result in cell damage or death, many cells have mechanisms that limit the degree to which cell volume changes. These mechanisms are particularly important for neurons, where swelling within the confined space of the skull can lead to serious neurological damage.
In general, when a cell is exposed to nonisotonic ECF volume, regulatory responses are activated within seconds to minutes to restore cell volume (Fig. 2-7). In the case of cell swelling, a regulatory volume decrease (RVD) response transports osmotically active particles (osmolytes) out of the cell and reduces intracellular osmotic pressure, thereby restoring cell volume to normal. Conversely with cell shrinking, a regulatory volume increase (RVI) response transports osmolytes into the cell and raises intracellular osmotic pressure, thereby restoring cell volume to normal. These osmolytes include ions and organic molecules such as polyols (sorbitol and myoinositol), methylamines (glycerophosphorylcholine and betaine), and some amino acids (taurine, glutamate, and β-alanine). If exposed to the nonisotonic ECF for an extended period, the cell alters intracellular levels of the organic osmolytes through metabolic processes.
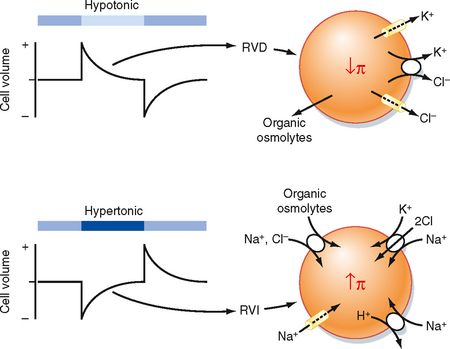
Figure 2-7 Volume regulation of cells in hypotonic and hypertonic media. Top panel, When cells are exposed to a hypotonic medium, they swell and then undergo a regulatory volume decrease (RVD). The RVD involves loss of KCl and organic osmolytes from the cell. The reduction in cellular KCl and organic osmolytes decreases intracellular osmotic pressure, water leaves the cell, and the cell returns to near its original volume. Lower panel, When cells are exposed to a hypertonic medium, they shrink and then undergo a regulatory volume increase (RVI). During the RVI NaCl and organic osmolytes enter the cell. Na+,K+-ATPAse (not depicted) exchanges Na+ for K+, so the KCl content of the cell is increased. The increase in cellular KCl and organic osmolytes increases intracellular osmotic pressure and brings water back into the cell, and the cell returns to near its original volume.
The ECF of individuals with disorders in water balance may be either hypotonic (positive water balance) or hypertonic (negative water balance). With longstanding positive water balance, for example, as often occurs in individuals with inappropriate secretion of ADH (see Chapter 34), the neurons and glial cells in the brain reduce intracellular osmolytes to minimize cell swelling. If the disturbed water balance is corrected too quickly, the reduced osmolytes within the neurons and glial cells lead to shrinking and damage of the cell. Damage to the glial cells that synthesize myelin within the brain can result in demyelinization. This demyelinization response, termed osmotic demyelinization syndrome, can affect any of the white matter of the brain, but especially regions of the pons. These effects are often irreversible. Thus, correction of disorders in water balance is usually accomplished slowly to avoid neurological complications.
The RVI response results in rapid uptake of NaCl and a number of organic osmolytes. With cell shrinking there is activation of the Na+-H+ antiporter (NHE-1), the 1Na+,1K+,2Cl− symporter (NKCC1), and a number of cation-selective channels that together bring NaCl into the cell. Na+,K+-ATPase then extrudes the Na+ in exchange for K+, so ultimately the KCl content of the cell is increased. Several organic osmolyte transporters are also activated by cell shrinkage. These include a 3Na+,1Cl−-taurine symporter, a 3Na+,2Cl−-betaine symporter, a 2Na+-myoinositol symporter, and an Na+–amino acid symporter. These transporters use the energy in the Na+ and Cl− gradients to drive the secondary active uptake of these organic osmolytes.
The RVD response results in the loss of KCl and organic osmolytes from the cell. Loss of KCl occurs through the activation of a wide range of K+-selective, Cl−-selective, and anion channels (the specific channels involved vary depending on the cell), as well as by activation of K+-Cl− symporters. Some of the organic osmolytes appear to leave the cell via anion channels (e.g., volume-sensitive organic osmolyte anion channels–VSOAC).
There are several mechanisms involved in activation of these various transporters during the volume regulatory responses. Changes in cell size appear to be monitored by the cytoskeleton, by changes in macromolecular crowding and ionic strength of the cytoplasm, and by channels whose gating is influenced, either directly or indirectly, by stretching of the plasma membrane (e.g., stretch-activated cation channels). A number of second messenger systems may also be involved in these responses (e.g., calmodulin, protein kinase A, and protein kinase C), but the precise mechanisms have not been completely defined.
PRINCIPLES OF EPITHELIAL TRANSPORT
Epithelial cells are arranged in sheets and provide the interface between the external world and the internal environment (i.e., ECF) of the body. Depending on their location, epithelial cells serve several important functions, such as establishing a barrier to microorganisms (lungs, gastrointestinal tract, and skin), prevention of loss of water from the body (skin), and maintenance of a constant internal environment (lungs, gastrointestinal tract, and kidneys). This latter function is a result of the ability of epithelial cells to carry out regulated vectorial transport (i.e., transport from one side of the epithelial cell to the opposite side). In this section the principles of epithelial transport are reviewed. The transport functions of specific epithelial cells are discussed in the appropriate chapters throughout the book.
Epithelial Structure
Figure 2-8 shows a schematic representation of an epithelial cell. The free surface of the epithelial layer is referred to as the apical membrane. It is in contact with the external environment (e.g., air within the alveoli and larger airways of the lung and the contents of the gastrointestinal tract) or with the ECF (e.g., glomerular filtrate in the nephrons of the kidneys and secretions of the ducts of the pancreas or sweat glands). The basal side of the epithelium rests on a basal lamina, which is secreted by the epithelial cells, and this in turn is attached to the underlying connective tissue.
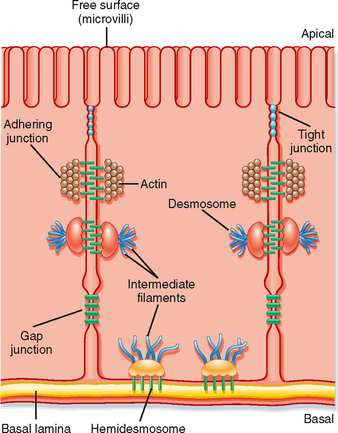
Figure 2-8 Schematic of an epithelial cell illustrating the various adhering junctions. The tight junction separates the apical membrane from the basolateral membrane (see text for details).
Epithelial cells are connected to one another and to the underlying connective tissue by a number of specialized junctions (Fig. 2-8). The adhering junction, desmosomes, and hemidesmosomes provide mechanical adhesion by linking the cytoskeleton of adjacent cells together. The gap junction and tight junction play important physiological roles. Gap junctions provide low-resistance connections between cells.* The functional unit of the gap junction is the connexon. A connexon is composed of six integral membrane protein subunits called connexins. A connexon in one cell is aligned with the connexon in the adjacent cell to form a channel. The channel may be gated and, when open, allows the movement of ions and small molecules between the cells. Because of their low electrical resistance, they effectively couple one cell to the adjacent cell electrically. The tight junction constitutes a pathway for the movement of molecules from one side of the epithelium to the other. This paracellular pathway, as it is called, is described in detail later.
The apical surface of epithelial cells may have specific structural features. One such feature is microvilli (Fig. 2-8). Microvilli are small (typically 1 to 2 μm in length), nonmotile projections of the apical plasma membrane that serve to increase surface area. They are commonly seen on cells that must transport large quantities of ions, water, and molecules (e.g., epithelial cells lining the small intestine and cells of the renal proximal tubule). The core of microvilli is composed of actin filaments and a number of accessory proteins (e.g., villin, fimbrin, fascin, and myosin1). This actin core is connected to the cytoskeleton of the cell via the terminal web (a network of actin fibers at the base of the microvilli) and provides structural support for the microvilli. Another surface feature is stereocilia. Stereocilia are long (3 to 5 μm), nonmotile membrane projections that, like microvilli, increase the surface area of the apical membrane. They are found in the epididymis of the testis and the hair cells of the inner ear. Their core contains actin filaments and the accessory proteins erzin and fimbrin. A third apical membrane feature is cilia. Cilia may be either motile or nonmotile. Motile cilia contain a microtubule core arranged in a characteristic 9=2 pattern (nine pairs of microtubules around the circumference of the cilium and one pair of microtubules in the center). Dynein is the molecular motor that drives the movement of cilia. Cilia are characteristic features of the epithelial cells that line the respiratory tract. They “beat” in a synchronized manner and serve to transport mucus and inhaled particulates out of the lung, a process termed mucociliary transport (see Chapter 25). Nonmotile cilia, also called primary cilia, serve as mechanoreceptors and are involved in determining the left-right asymmetry of organs during embryological development, as well as sensing the flow rate of tubular fluid in the nephron of the kidneys (see Chapter 33). Only a single primary cilium is found in the apical membrane of the cell. It has a microtubule core (9=0 arrangement) and lacks a molecular motor protein.
Tight junctions (also called zonula occludens) are composed of linear arrays of several integral membrane proteins, including occludins, claudins, and several members of the immunoglobulin superfamily. The tight junction complex allows selective diffusion of ions or water, or both, between cells. Junctional proteins (e.g., occludins and claudins) are transmembrane proteins that span the membrane of one cell and link to the extracellular portion of the same molecule in the adjacent cell. Cytoplasmic linker proteins (e.g., ZO-1, ZO-2, and ZO-3) then link the membrane-spanning proteins to the cytoskeleton of the cell. Of these junctional proteins, claudins appear to be important in determining the permeability characteristics of the tight junction. For example, claudin-16 is critical for determining the permeability of the tight junctions to divalent cations in the thick ascending limb of Henle’s loop in the kidney. Claudin-4 has been shown in cultured kidney cells to control the permeability of the tight junction to Na+, whereas claudin-15 determines whether a tight junction is permeable to cations or anions. Thus, the permeability characteristics of the tight junction are determined, at least in part, by the specific claudins expressed by the cells.
The tight junction effectively divides the plasma membrane of epithelial cells into two domains: an apical surface and a basal surface. Because the tight junction is near the apical pole of the cell, the lateral surface of the cell is continuous with the basal surface. Consequently, the term basolateral membrane is often used when referring to this surface domain of the epithelial cell. The basolateral membrane of many epithelial cells is folded or invaginated. This is especially so for epithelial cells that have high transport rates. These invaginations serve to increase the membrane’s surface area to accommodate the large number of membrane transporters (e.g., Na+,K+-ATPase) needed in the membrane.
Vectorial Transport
Because the tight junction divides the plasma membrane into two domains (i.e., apical and basolateral), epithelial cells are capable of vectorial transport, whereby an ion or molecule can be transported from one side of the epithelial sheet to the opposite side (Fig. 2-9). The act of vectorial transport requires that specific membrane transport proteins be targeted to and remain in one or the other of the membrane domains. In the example shown in Figure 2-9, the Na+ channel is present only in the apical membrane, whereas Na+,K+-ATPase and the K+ channel are confined to the basolateral membrane. The operation of Na+,K+-ATPase and leakage of K+ out of the cell across the basolateral membrane set up a large electrochemical gradient for Na+ to enter the cell across the apical membrane through the Na+ channel (intracellular [Na+] < extracellular [Na+] and Vm is oriented cell interior negative). The Na+ is then pumped out of the cell by Na+,K+-ATPase, and vectorial transport from the apical side of the epithelium to the basolateral side of the epithelium occurs. Transport from the apical side to the basolateral side of an epithelium is termed absorption or reabsorption. For example, uptake of nutrients from the lumen of the gastrointestinal tract is termed absorption, and transport of NaCl and water from the lumen of renal nephrons is termed reabsorption. Transport from the basolateral side of the epithelium to the apical side is termed secretion.
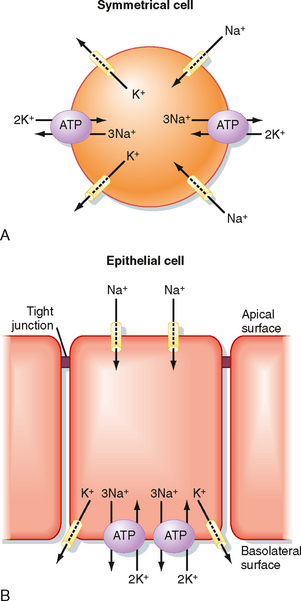
Figure 2-9 A, Symmetrical cells (e.g., red blood cell) have membrane transport proteins distributed over the entire surface of the cell. B, Epithelial cells target various membrane transport proteins to either the apical or the basolateral membrane. By confining the transporters to a membrane domain, vectorial transport can occur. In the cell depicted, Na+ is transported from the apical surface to the basolateral surface.
As noted previously, Na+,K+-ATPase and K+-selective channels play an important role in establishing cellular ion gradients for Na+ and K+ and generating Vm. In all epithelial cells, except the choroid plexus,* Na+,K+-ATPase is located in the basolateral membrane of the cell. Numerous K+-selective channels are found in epithelial cells and may be located in either membrane domain. By the establishment of these chemical and voltage gradients, transport of other ions and solutes can be driven (e.g., Na+-glucose symporter, Na+-H+ antiporter, 1Na+,1K+,2Cl− symporter, 1Na+-3HCO3− symporter). The direction of transepithelial transport (reabsorption or secretion) simply depends on the membrane domain in which the transporter is located. Because of the dependence on Na+,K+-ATPase, epithelial transport requires the expenditure of energy. Other ATP-dependent transporters are also involved in epithelial transport, including H+-ATPase, H+,K+-ATPase, and a host of ATP-binding cassette (ABC) transporters such as pGP and MRP2, which transport xenobiotics (drugs), and the cystic fibrosis transmembrane regulator (CFTR).
Solutes and water can be transported across an epithelium by traversing both the apical and basolateral membranes (transcellular transport) or by moving between cells across the tight junction (paracellular transport). Solute transport via the transcellular route is a two-step process, with the solute molecule being transported across both the apical and basolateral membranes. Uptake into the cell or transport out of the cell may be either a passive or an active process. Typically, one of the steps is passive and the other is active. For the example shown in Figure 2-9, B, uptake of Na+ into the cell across the apical membrane through the Na+-selective channel is passive and driven by the electrochemical gradient for Na+. Exit of Na+ from the cell across the basolateral membrane is achieved by primary active transport via Na+,K+-ATPase. Because a transepithelial gradient for Na+ can be generated by this process (i.e., the [Na+] in the apical compartment can be reduced below that of the basolateral compartment), the overall process of transepithelial Na+ transport is said to be active. Any solute that is actively transported across an epithelium must be transported via the transcellular pathway.
Depending on the epithelium, the paracellular pathway is an important route for the transepithelial transport of solute and water. As noted, the permeability characteristics are determined, at least in part, by the specific claudins expressed by the cell. Thus, tight junctions can have low permeability to solutes or water, or to both. Alternatively, tight junctions can have very high permeability. For epithelia in which rates of transepithelial transport are high, the tight junctions typically have high permeability (i.e., are leaky). Examples of such epithelia include the proximal tubule of the renal nephron and the early segments of the small intestine (e.g., jejunum). If the epithelium must establish large transepithelial gradients for solutes or water (or for both), the tight junctions typically have low permeability (i.e., are tight). Examples of this type of epithelium include the collecting duct of the renal nephron and the terminal portion of the colon. In addition, the tight junction may be selective for certain solutes (e.g., selective for cations versus anions).
All solute transport that occurs through the paracellular pathway is passive in nature. The two driving forces for this transport are the transepithelial concentration gradient for the solute and, if the solute is charged, the transepithelial voltage (Fig. 2-10). The transepithelial voltage may be oriented with the apical surface electrically negative with respect to the basolateral surface, as shown in Figure 2-10, or it may be oriented with the apical surface electrically positive with respect to the basolateral surface. The polarity and magnitude of the transepithelial voltage are determined by the specific membrane transporters in the apical and basolateral membranes, as well as by the permeability characteristics of the tight junction.
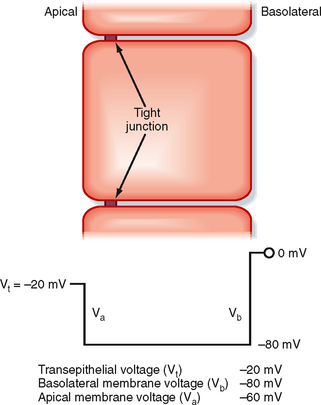
Figure 2-10 Electrical profile across an epithelial cell. The magnitude of the membrane voltages and the transepithelial voltage are determined by the various membrane transport proteins in the apical and basolateral membranes (see text for details).
It is important to recognize that transcellular transport processes set up the transepithelial chemical and voltage gradients, which in turn can drive paracellular transport. This is illustrated in Figure 2-11 for an epithelium that reabsorbs NaCl and for an epithelium that secretes NaCl. In both epithelia, the transepithelial voltage is oriented with the apical surface electrically negative with respect to the basolateral surface. For the NaCl-reabsorbing epithelium, the transepithelial voltage is generated by the active transcellular reabsorption of Na+. This voltage in turn drives Cl− reabsorption through the paracellular pathway. In contrast, for the NaCl-secreting epithelium, the transepithelial voltage is generated by the active transcellular secretion of Cl−. Na+ is then secreted passively via the paracellular pathway.
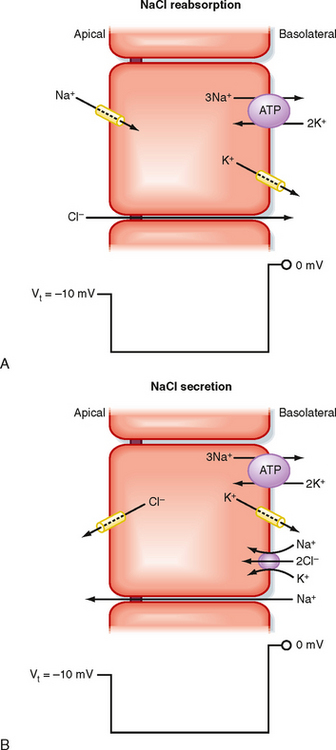
Figure 2-11 Role of the paracellular pathway in epithelial transport. A, Na+ transport through the cell generates a transepithelial voltage that then drives the passive movement of Cl− through the tight junction. NaCl reabsorption results. B, Cl− transport through the cell generates a transepithelial voltage that then drives the passive transport of Na+ through the tight junction. NaCl secretion results.
Transepithelial Water Movement
Water movement across epithelia is passive and driven by transepithelial osmotic pressure gradients. Water movement can occur by a transcellular route involving aquaporins in both the apical and basolateral membranes.* In addition, water may also move through the paracellular pathway. In the NaCl-reabsorbing epithelium depicted in Figure 2-11, A, reabsorption of NaCl from the apical compartment lowers the osmotic pressure in that compartment, whereas the addition of NaCl to the basolateral compartment raises the osmotic pressure in that compartment. As a result, a transepithelial osmotic pressure gradient is established that drives the movement of water from the apical to the basolateral compartment (i.e., reabsorption). The opposite occurs with NaCl-secreting epithelium (see Fig. 2-11, B), where the transepithelial secretion of NaCl establishes a transepithelial osmotic pressure gradient that drives water secretion.
In some epithelia (e.g., proximal tubule of the renal nephron), movement of water across the epithelium via the paracellular pathway can drive the movement of additional solute. This process is termed solvent drag and reflects the fact that solutes dissolved in the water will traverse the tight junction with the water.
As is the case with the establishment of transepithelial concentration and voltage gradients, the establishment of transepithelial osmotic pressure gradients requires transcellular transport of solutes by the epithelial cells.
Regulation of Epithelial Transport
Epithelial transport must be regulated to meet the homeostatic needs of the individual. Depending on the epithelium, this regulation involves neural or hormonal mechanisms, or both. For example, the enteric nervous system of the gastrointestinal tract regulates solute and water transport by the epithelial cells that line the intestine and colon. Similarly, the sympathetic nervous system regulates transport by the epithelial cells of the renal nephron. Aldosterone, a steroid hormone produced by the adrenal cortex (see Chapter 42), is an example of a hormone that regulates NaCl transport by the epithelial cells of the colon, renal nephron, and sweat ducts. Epithelial cell transport can also be regulated by locally produced and locally acting substances, a process termed paracrine regulation. Regulation of HCl secretion in the stomach by histamine is an example of this process. Cells that are located near the epithelial cells of the stomach release histamine, which then stimulates HCl-secreting cells of the stomach (parietal cells) to secrete HCl.
When acted on by a regulatory signal, the epithelial cell may respond in several different ways, including
The first two mechanisms can occur quite rapidly (seconds to minutes), whereas the synthesis of transporters takes additional time (minutes to days).