Chapter 18 Strabismus
Introduction
Definitions
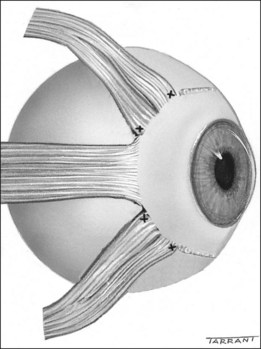
Anatomy of extraocular muscles
Principles
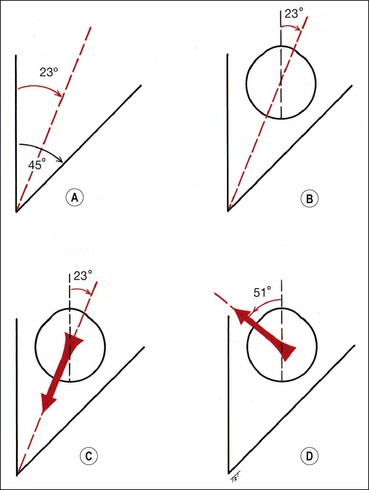
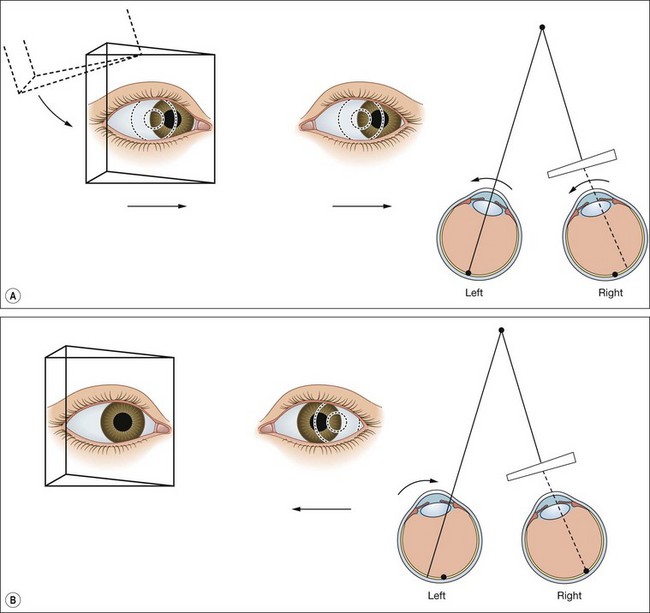
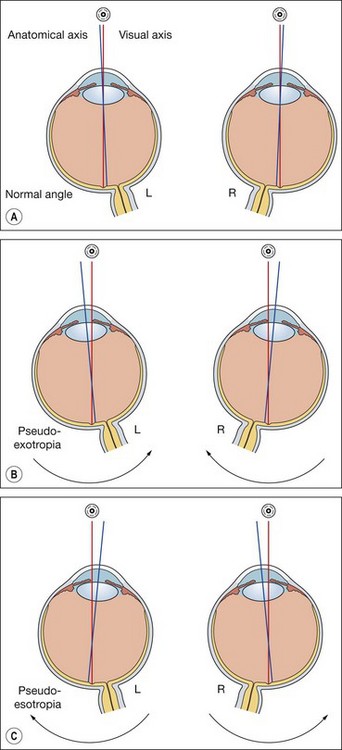
The lateral and medial orbital walls are at an angle of 45° with each other (Fig. 18.2A). The orbital axis therefore forms an angle of 22.5° with both lateral and medial walls. For the sake of simplicity this angle is usually regarded as being 23°.
Horizontal rectus muscles
When the eye is in the primary position, the horizontal recti are purely horizontal movers on the vertical Z axis and have only primary actions.
Vertical rectus muscles
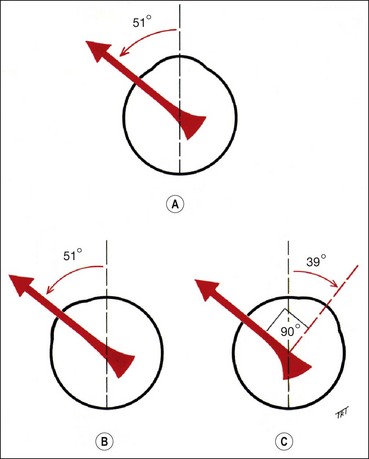
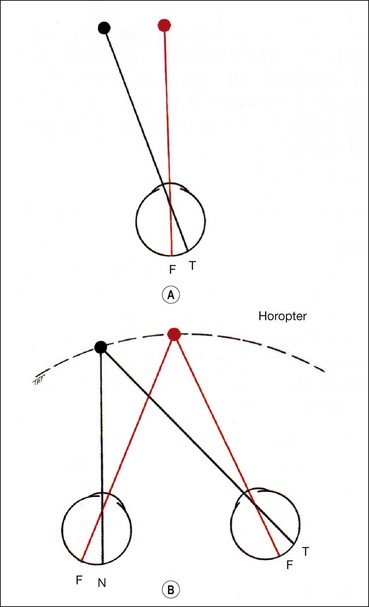
The vertical recti run in line with the orbital axis and are inserted in front of the equator. They therefore form an angle of 23° with the visual axis (see Fig. 18.2C).
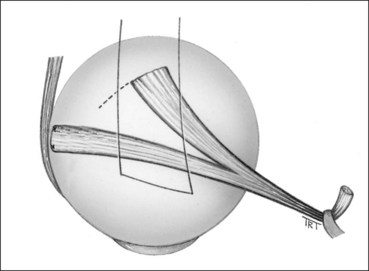
Spiral of Tillaux
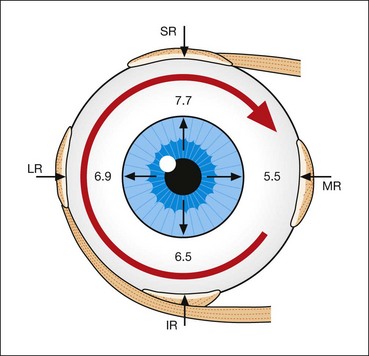
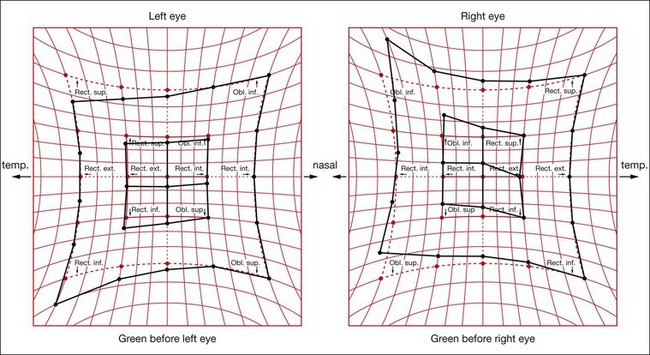
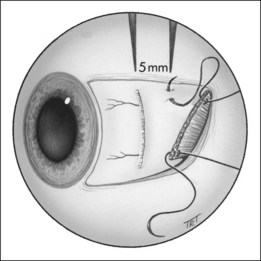
The spiral of Tillaux is an imaginary line joining the insertions of the four recti and is an important anatomical landmark when performing surgery. The insertions are located progressively further away from the limbus in a spiral pattern; the medial rectus insertion is closest (5.5 mm) followed by the inferior rectus (6.5 mm), lateral rectus (6.9 mm) and superior rectus (7.7 mm; Fig. 18.5).
Oblique muscles
The obliques are inserted behind the equator and form an angle of 51° with the visual axis (see Fig. 18.2D).
Muscle pulleys
The four rectus muscles pass through condensations of connective tissue and smooth muscle just posterior to the equator. These condensations act as pulleys and minimize upward and downward movements of the bellies of the medial and lateral rectus muscles during upgaze and downgaze, and horizontal movements of the superior and inferior rectus bellies in left and right gaze.
Ocular movements
Ductions
Ductions are monocular movements around the axes of Fick. They consist of adduction, abduction, elevation, depression, intorsion and extorsion. They are tested by occluding the fellow eye and asking the patient to follow a target in each direction of gaze.
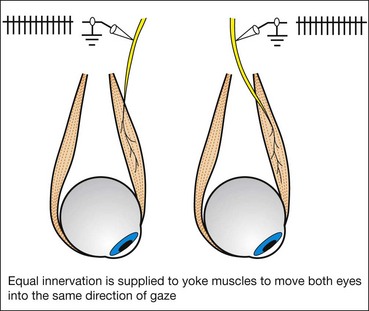
Versions
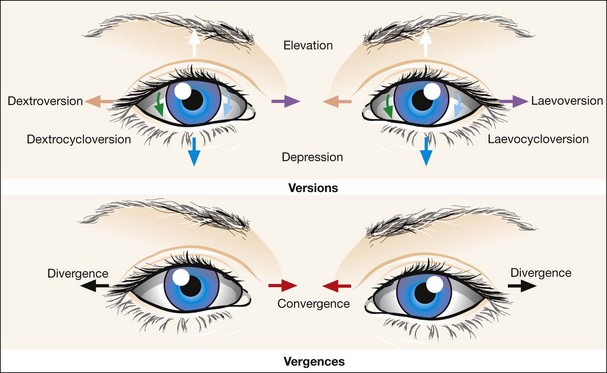
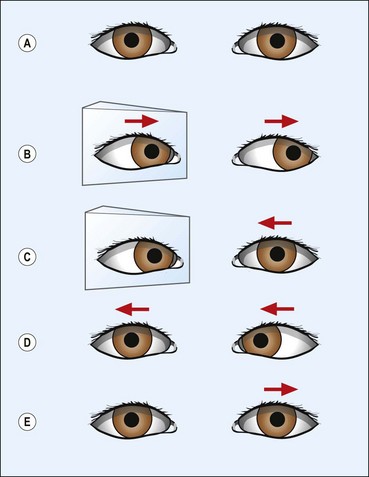
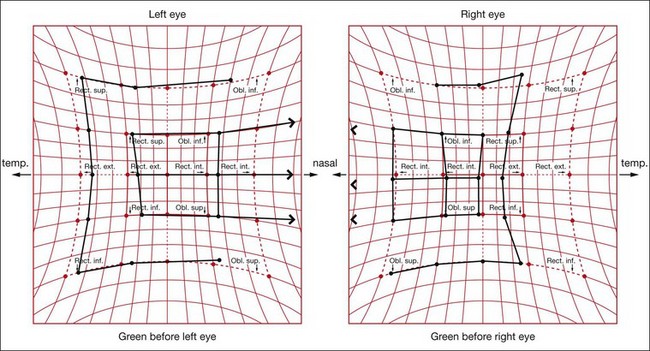
Versions are binocular, simultaneous, conjugate movements in the same direction (Fig. 18.8, top).
Vergences
Vergences are binocular, simultaneous, disjugate or disjunctive movements (in opposite directions) (Fig. 18.8, bottom). Convergence is simultaneous adduction (inward turning); divergence is outwards movement from a convergent position. Convergence may be voluntary or reflex.
Reflex convergence has four components:
Changes in accommodation, convergence and pupil size which occur in concert with a change in the distance of viewing are known as the ‘near triad’.
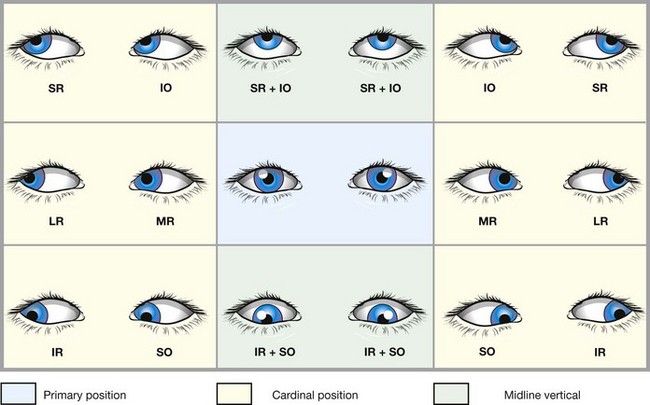
Positions of gaze
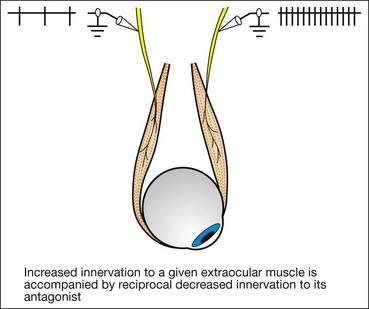
Laws of ocular motility
Sensory considerations
Basic aspects
Fusional convergence helps to control an exophoria whereas fusional divergence helps to control an esophoria. The fusional vergence mechanism may be decreased by fatigue or illness, converting a phoria to a tropia. The amplitude of fusional vergence mechanisms can be improved by orthoptic exercises, particularly in the case of near fusional convergence for the relief of convergence insufficiency.
Sensory adaptations to strabismus
The ocular sensory system in children has the ability to adapt to anomalous states (confusion and diplopia) by two mechanisms: (a) suppression and (b) abnormal retinal correspondence (ARC). These occur because of the plasticity of the developing visual system in children under the age of 6–8 years. Occasional adults who develop sudden-onset strabismus are able to ignore the second image after a time and therefore do not complain of diplopia.
Suppression
Suppression involves active inhibition by the visual cortex of the image from one eye when both eyes are open. Stimuli for suppression include diplopia, confusion and a blurred image from one eye resulting from astigmatism/anisometropia. Clinically, suppression may be:
Abnormal retinal correspondence
Abnormal retinal correspondence (ARC) is a condition in which non-corresponding retinal elements acquire a common subjective visual direction, i.e. fusion occurs in the presence of a small angle manifest squint.
Microtropia
Microtropia is a small angle (<10 Δ) squint in which stereopsis is present but reduced, and there is relative amblyopia of the more ametropic eye. Microtropia has two forms.
Motor adaptation to strabismus
Motor adaptation involves the adoption of an abnormal head posture (AHP) and occurs primarily in children with congenitally abnormal eye movements who use the AHP to maintain BSV. In these children loss of an AHP may indicate loss of binocular function and the need for surgical intervention. These patients may present in adult life with symptoms of decompensation, often unaware of their AHP. Acquired paretic strabismus in adults may be consciously controlled by an AHP provided the deviation is neither too large nor too variable with gaze (incomitance). The AHP eliminates diplopia and helps to centralize the binocular visual field. The patient will turn the head into the direction of the field of action of the weak muscle, so that the eyes are then automatically turned the opposite direction and as far as possible away from its field of action (i.e. the head will turn where the eye cannot). An AHP is analyzed in terms of the following three components:
Amblyopia
Classification
Amblyopia is the unilateral, or rarely bilateral, decrease in best-corrected visual acuity caused by form vision deprivation and/or abnormal binocular interaction, for which there is no identifiable pathology of the eye or visual pathway.
Diagnosis
In the absence of an organic lesion, a difference in best corrected visual acuity of two Snellen lines or more (or >1 log unit) is indicative of amblyopia. Visual acuity in amblyopia is usually better when reading single letters than letters in a row. This ‘crowding’ phenomenon occurs to a certain extent in normal individuals but is more marked in amblyopes and must be taken into account when testing preverbal children.
Treatment
It is essential to examine the fundi to diagnose any visible organic disease prior to commencing treatment for amblyopia. Organic disease and amblyopia may coexist and a trial of patching may still be indicated in the presence of organic disease. If acuity does not respond to treatment, investigations such as electrophysiology or imaging should be reconsidered. The sensitive period during which acuity of an amblyopic eye can be improved is usually up to 7–8 years in strabismic amblyopia and may be longer (into teens) for anisometropic amblyopia where good binocular function is present.
Clinical evaluation
History
Visual acuity
Testing in preverbal children
The evaluation can be separated into the qualitative assessment of visual behaviour and the quantitative assessment of visual acuity using preferential looking tests. Assessment of visual behaviour is achieved as follows:
Testing in verbal children
All of the tests described below are performed at 3 or 4 metres at which it is easier to obtain compliance than at 6 metres, with little or no clinical detriment. It is important to note that amblyopia can only be accurately diagnosed using a crowded test requiring target recognition and that logMAR tests (logarithm of the minimal angle of resolution) provide the best measure against which improvement with amblyopia therapy can be assessed. These are readily available in formats suited to normal children from 2 years onwards.
Tests for stereopsis
Stereopsis is measured in seconds of arc (1° = 60 minutes of arc; 1 minute = 60 seconds). It is useful to remember that normal spatial visual acuity is 1 minute and normal stereoacuity is 60 seconds (which equals 1 minute). The lower the value the better the acuity. Various tests are employed using different test principles. Random dot tests (e.g. TNO, Frisby) provide the most definitive evidence of high grade BSV. Where this is weak and/or based on ARC, contour-based tests (e.g. Titmus) may give more reliable evidence of stereopsis.
TNO
The TNO random dot test consists of seven plates of randomly distributed paired red and green dots which are viewed with complementary red-green spectacles.
Frisby
The Frisby stereotest consists of three transparent plastic plates of varying thickness.
Lang
The Lang stereotest does not require special spectacles; the targets are seen alternately by each eye through the built-in cylindrical lens elements.
Titmus
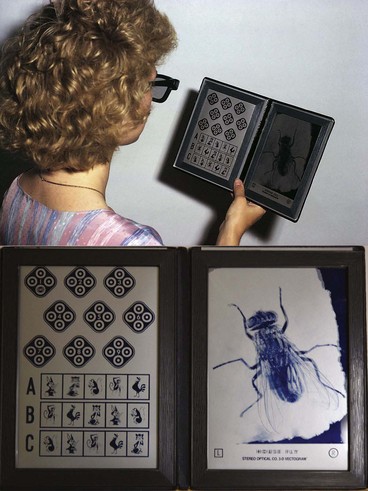
The Titmus test consists of a three-dimensional Polaroid vectograph consisting of two plates in the form of a booklet viewed through Polaroid spectacles. On the right is a large fly, and on the left is a series of circles and animals (Fig. 18.22). The test is performed at a distance of 40 cm.
Tests for binocular fusion in infants without manifest squint
Base-out prism
Base-out prism is a quick and easy method for detecting fusion in children. The test is performed by placing a 20 Δ base-out prism in front of one eye (in this case the right – Fig. 18.23). This displaces the retinal image temporally with resultant diplopia. The examiner observes corrective eye movements as follows:
Most children with good BSV should be able to overcome a 20 Δ prism from the age of 6 months; if not weaker prisms (16 Δ or 12 Δ) may be tried but the response is harder to observe.
Binocular convergence
Simple convergence to an interesting target can be demonstrated from 3 to 4 months. Both eyes should follow the approaching target symmetrically ‘to the nose’. Over-convergence in the infant may indicate an incipient esotropia. Divergence may reflect a tendency to divergence or simply lack of interest in the target.
Tests for sensory anomalies
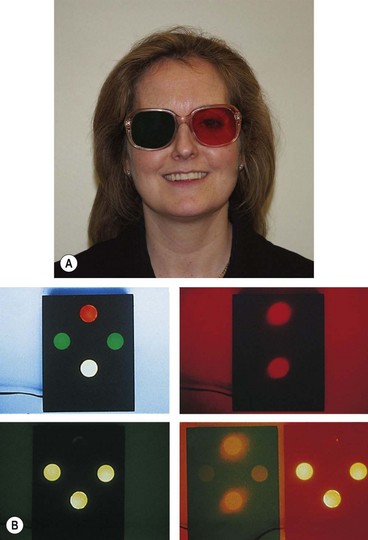
Worth four-dot
This is a dissociation test which can be used with both distance and near fixation and differentiates between BSV, ARC and suppression. Results can only be interpreted if the presence or absence of a manifest squint is known at time of testing.
Bagolini striated glasses
This is a test for detecting BSV, ARC or suppression. Each lens has fine striations which convert a point source of light into a line, as with the Maddox rod (see below).
4 Δ prism test
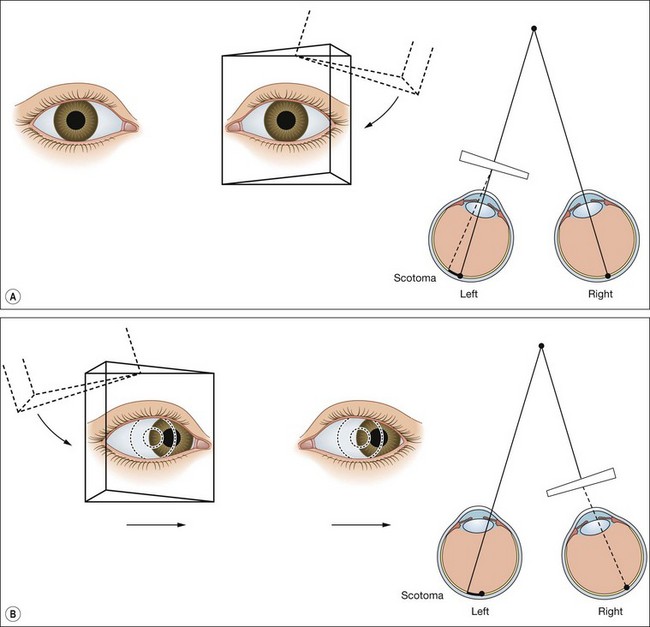
This test differentiates bifoveal fixation (normal BSV) from a central suppression scotoma (CSS) in microtropia and employs the principle described in the 20 Δ test (Hering law and convergence) to overcome diplopia.
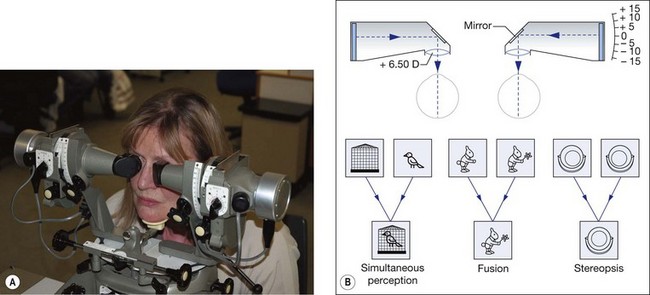
Synoptophore
The synoptophore compensates for the angle of squint and allows stimuli to be presented to both eyes simultaneously (Fig. 18.28A). It can thus be used to investigate the potential for binocular function in the presence of a manifest squint and is of particular value in testing young children (from age 3 years), who generally find it enjoyable. It can also detect suppression and ARC.
Grades of binocular vision
Binocular vision is graded on the synoptophore as follows (Fig. 18.28B, bottom):
Detection of abnormal retinal correspondence
ARC can be detected on the synoptophore as follows:
Measurement of deviation
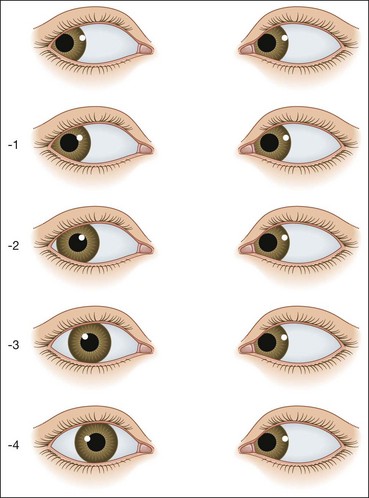
Hirschberg test
The Hirschberg test gives a rough objective estimate of the angle of a manifest strabismus and is especially useful in young or uncooperative patients or when fixation in the deviating eye is poor.
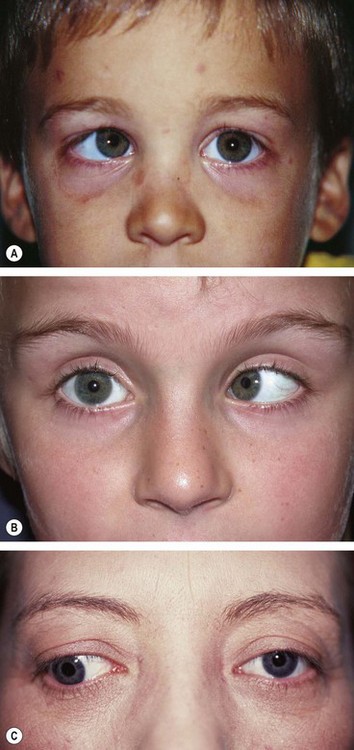
Fig. 18.29 Hirschberg test. (A) The right corneal reflex is near the temporal border of the pupil indicating an angle of about 15°; (B) the left corneal reflex is at the limbus indicating an angle of about 45°; (C) right corneal reflex is at the limbus in a divergent squint
(Courtesy of J Yanguela – fig. A)
Krimsky and prism reflection tests
Corneal reflex assessment can be combined with prisms to give a more accurate approximation of the angle in a manifest deviation.
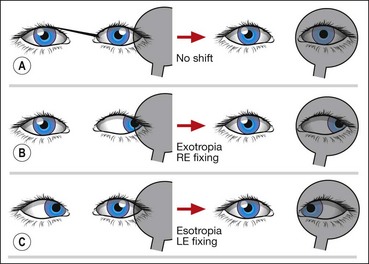
Cover–uncover test
The cover–uncover test consists of two parts:
Most examiners perform the cover test and the uncover test sequentially, hence the term cover–uncover test.
Alternate cover test
The alternate cover test is a dissociation test which reveals the total deviation when fusion is suspended. It should be performed after the cover–uncover test.
Prism cover test
The prism cover test measures the angle of deviation on near or distance fixation and in any gaze position. It combines the alternate cover test with prisms and is performed as follows:
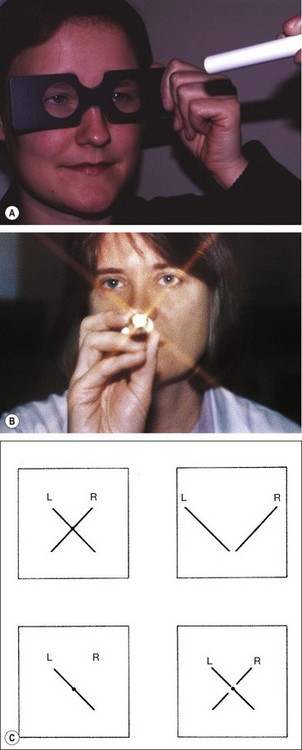
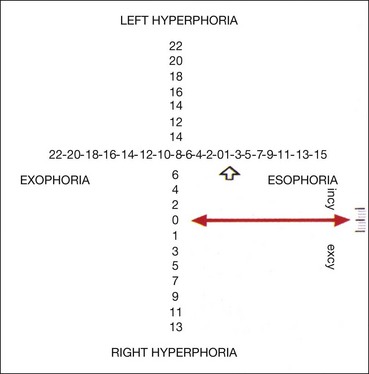
Maddox wing
The Maddox wing dissociates the eyes for near fixation (1/3 m) and measures heterophoria. The instrument is constructed in such a way that the right eye sees only a white vertical arrow and a red horizontal arrow, whereas the left eye sees only horizontal and vertical rows of numbers (Fig. 18.36). Measurements are made as follows:
Maddox rod
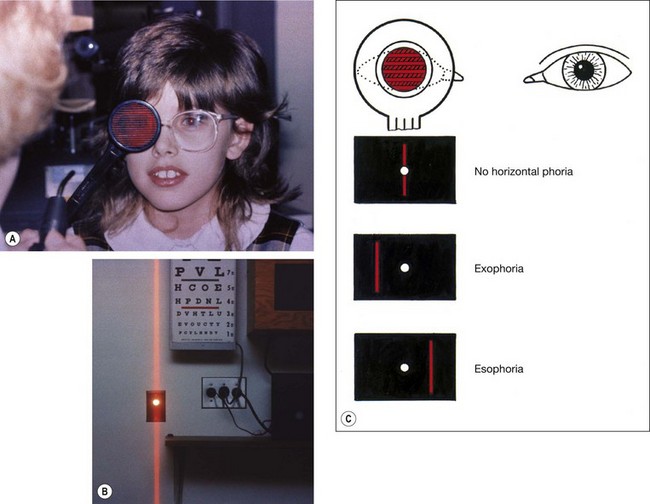
The Maddox rod consists of a series of fused cylindrical red glass rods which convert the appearance of a white spot of light into a red streak. The optical properties of the rods cause the streak of light to be at an angle of 90° with the long axis of the rods; when the glass rods are held horizontally, the streak will be vertical and vice versa. The test is performed as follows:
Motility tests
Ocular movements
Examination of ocular movements involves assessment of smooth pursuit movements followed by that of saccadic movements.
Near point of convergence
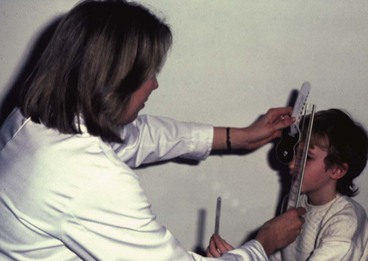
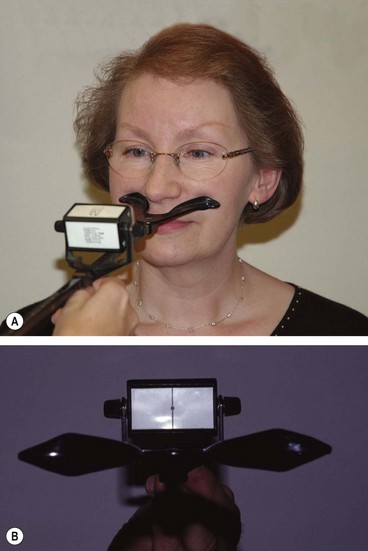
The near point of convergence (NPC) is the nearest point on which the eyes can maintain binocular fixation. It can be measured with the RAF rule which rests on the patient’s cheeks (Fig. 18.39A). A target (Fig. 18.39B) is slowly moved along the rule towards the patient’s eyes until one eye loses fixation and drifts laterally (objective NPC). The subjective NPC is the point at which the patient reports diplopia. Normal NPC should be nearer than 10 cm without undue effort.
Near point of accommodation
The near point of accommodation (NPA) is the nearest point on which the eyes can maintain clear focus. It can also be measured with the RAF rule. The patient fixates a line of print, which is then slowly moved towards the patient until it becomes blurred. The distance at which this is first reported is read off the rule and denotes the NPA. The NPA recedes with age; when sufficiently far away to render reading difficult without optical correction, presbyopia is present. At the age of 20 years the NPA is 8 cm and by the age of 50 years it has receded to approximately 46 cm. The amplitude of accommodation can also be assessed using concave lenses in 0.5 DS steps whilst fixating the 6/6 Snellen line and reporting when the vision blurs.
Fusional amplitudes
Fusional amplitudes measure the efficacy of vergence movements. They may be tested with prisms bars or the synoptophore. An increasingly strong prism is placed in front of one eye, which will then abduct or adduct (depending on whether the prism is base-in or base-out), in order to maintain bifoveal fixation. When a prism greater than the fusional amplitude is reached, diplopia is reported or one eye drifts the other way, indicating the limit of vergence ability.
Postoperative diplopia test
This simple test is mandatory prior to strabismus surgery in all non-binocular patients over 7–8 years of age to assess the risk of diplopia after surgery.
Investigation of diplopia
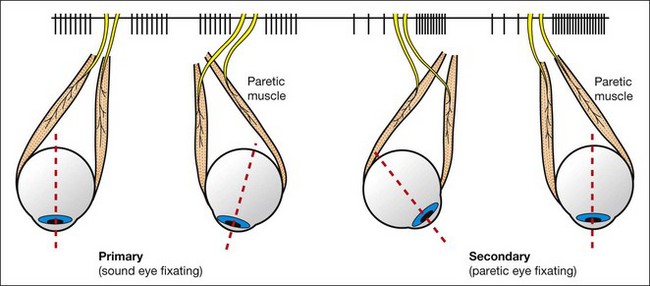
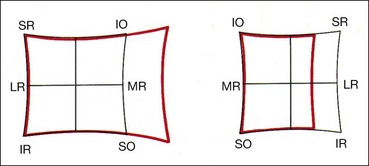
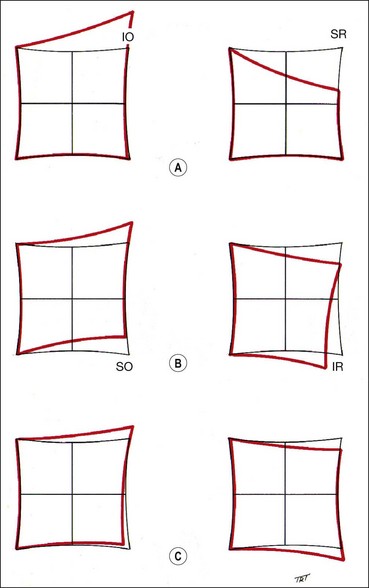
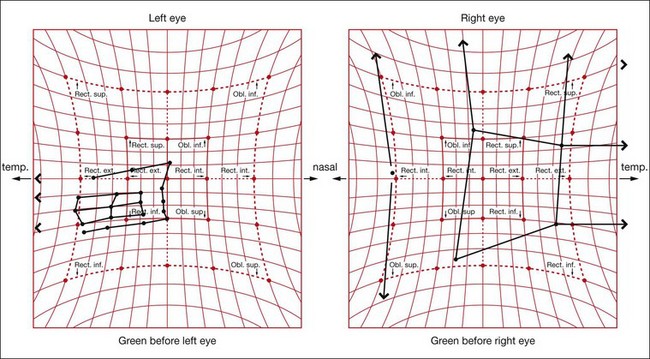
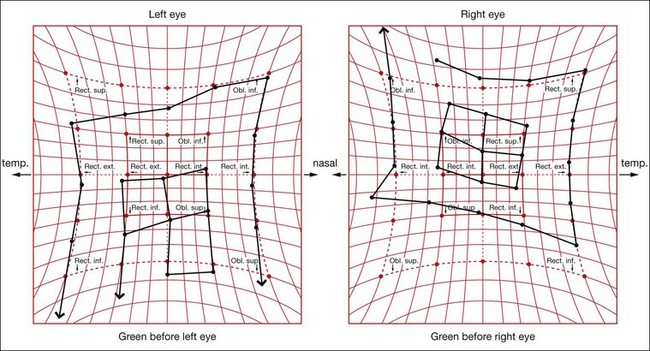
The Hess screen and the Lees screen are two similar tests that plot the dissociated ocular position as a function of extraocular muscle action and enable differentiation of paretic strabismus caused by neurological pathology from restrictive myopathy such as in thyroid eye disease or a blow-out fracture of the orbit, and recent onset paresis from long-standing. They also allow quantitative monitoring of progress in a range of conditions.
Electronic Hess test
The screen contains a tangent pattern (2D projection of a spherical surface) printed onto a dark grey background. Red lights that can be individually illuminated by a control panel indicate the cardinal positions of gaze within a central field (15° from primary position) and a peripheral field (30°); each square represents 5° of ocular rotation.
Lees screen
The apparatus consists of two opalescent glass screens at right-angles to each other, bisected by a two-sided plane mirror which dissociates the two eyes (Fig. 18.40). Each screen has a tangent pattern marked onto the back surface which is revealed only when the screen is illuminated.
Changes with time
Changes with time are very useful as a prognostic guide.
Clinical examples
By analyzing the following examples familiarization is gained with ocular motor nerve palsies as discussed in Chapter 19.
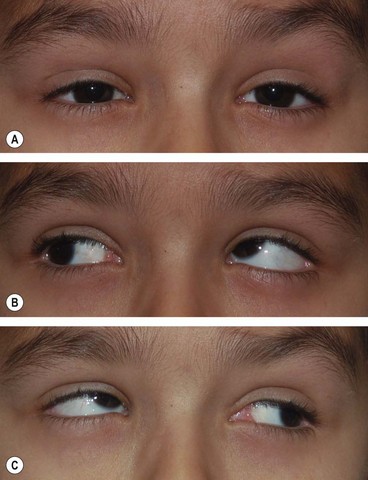
In inferior rectus palsy, the function of the superior oblique muscle can only be assessed by observing intorsion on attempted depression. This is best performed by observing a conjunctival landmark on the slit-lamp.
Refraction and fundoscopy
It should be emphasized that dilated fundoscopy is mandatory in the context of strabismus, to exclude any underlying ocular pathology such as macular scarring, optic disc hypoplasia or retinoblastoma. Strabismus is often secondary to refractive error and hypermetropia (hyperopia), astigmatism, anisometropia and myopia may all be associated.
Cycloplegia
The commonest refractive error causing strabismus is hypermetropia. Accurate measurements of hypermetropia necessitate effective paralysis of the ciliary muscle (cycloplegia), in order to neutralize the effect of accommodation, which masks the true degree of this refractive error.
Change of refraction
Because refraction changes with age, it is important to check this in patients with strabismus at least every year and more frequently in younger children and if acuity is reduced. At birth most babies are hypermetropic. After the age of 2 years there may be an increase in hypermetropia and a decrease in astigmatism. Hypermetropia may continue to increase until the age of about 6 years, levelling off between the ages of 6 and 8 and subsequently.
When to prescribe
Most children are mildly hypermetropic (1 to 3 D). There is some evidence that fully correcting hypermetropia in a normal child may reduce physiological emmetropization.
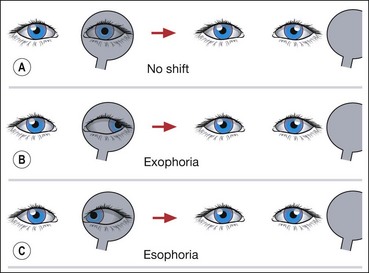
Heterophoria
Heterophoria may present clinically with associated visual symptoms, particularly at times of stress or poor health, when the fusional amplitudes are insufficient to maintain alignment.
Vergence abnormalities
Convergence insufficiency
Convergence insufficiency (CI) typically affects individuals, such as students, with excessive near visual demand.
Divergence insufficiency
Divergence paresis or paralysis is a rare condition typically associated with underlying neurological disease, such as intracranial space-occupying lesions, cerebrovascular accidents and head trauma. Presentation may be at any age and may be difficult to differentiate from 6th nerve palsy, but is primarily a concomitant esodeviation with reduced or absent divergence fusional amplitudes. It is difficult to treat; prisms are the best option.
Near reflex insufficiency
Spasm of the near reflex
Spasm of the near reflex is a functional condition affecting patients of all ages (mainly females).
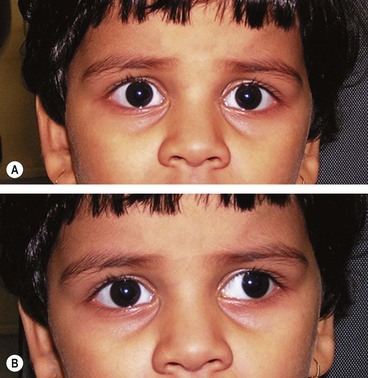
Esotropia
Esotropia (manifest convergent squint) may be concomitant or incomitant. In a concomitant esotropia the variability of the angle of deviation is within 5 Δ in different horizontal gaze positions. In an incomitant deviation the angle differs in various positions of gaze as a result of abnormal innervation or restriction. This section deals only with concomitant esotropia. A classification is shown in Table 18.1. However, all squints are different and not all fit neatly into a classification. For example, a microtropia may occur with a number of the other categories. It is more important to understand the part played by binocular function, refractive error and accommodation in the pathophysiology of each individual squint and to tailor treatment accordingly.
Table 18.1 Classification of esotropia
| 1. Accommodative |
| 2. Non-accommodative |
Early-onset esotropia
Up to the age of 4 months, infrequent episodes of convergence are normal but thereafter ocular misalignment is abnormal. Early-onset (congenital, essential, infantile) esotropia is an idiopathic esotropia developing within the first 6 months of life in an otherwise normal infant with no significant refractive error and no limitation of ocular movements.
Signs
Initial treatment
Early ocular alignment gives the best change of the child developing some degree of binocular function. Ideally, the eyes should be surgically aligned by the age of 12 months, and at the very latest by the age of 2 years, but only after amblyopia or significant refractive errors have been corrected.
Subsequent treatment
Accommodative esotropia
Near vision involves both accommodation and convergence. Accommodation is the process by which the eye focuses on a near target, by altering the curvature of the crystalline lens. Simultaneously, the eyes converge, in order to fixate bi-foveally on the target. Both accommodation and convergence are quantitatively related to the proximity of the target, and have a fairly constant relationship to each other (AC/A ratio) as described previously. Abnormalities of the AC/A ratio are an important cause of certain types of esotropia.
Refractive accommodative esotropia
In this type of accommodative esotropia, the AC/A ratio is normal and esotropia is a physiological response to excessive hypermetropia, usually between +2.00 and +7.00 D. The considerable degree of accommodation required to focus clearly on even a distant target is accompanied by a proportionate amount of convergence, which is beyond the patient’s fusional divergence amplitude. It cannot therefore be controlled, and a manifest convergent squint results. The magnitude of the deviation varies little (usually <10 Δ) between distance and near. The deviation typically presents at the age of 18 months–3 years (range 6 months–7 years).
Non-refractive accommodative esotropia
In this type of accommodative esotropia the AC/A ratio is high so that a unit increase of accommodation is accompanied by a disproportionately large increase in convergence. This occurs independently of refractive error, although hypermetropia frequently coexists. It can be subdivided into:
Treatment
Microtropia
Microtropia (monofixation syndrome), may be primary or follow surgery for a large deviation. It may occur in apparent isolation, but it is often associated with other conditions such as anisometropic amblyopia. Microtropia is more a description of binocular status than a specific diagnosis, for example a patient with fully accommodative esotropia may control to a microtropia rather than true bifoveal BSV with glasses. It is characterized by the following:
Others esotropias
Secondary (sensory) esotropia
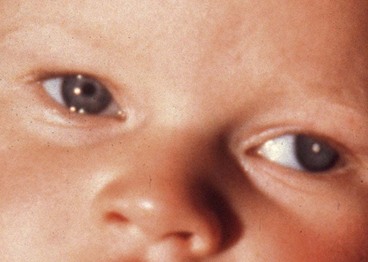
Secondary esotropia is caused by a unilateral reduction in visual acuity which interferes with or abolishes fusion; causes include cataract, optic atrophy or hypoplasia, macular scarring or retinoblastoma. Fundus examination under mydriasis is therefore essential in all children with strabismus.
Consecutive esotropia
Consecutive esotropia follows surgical overcorrection of an exodeviation. If it occurs following surgery for an intermittent exotropia in a child it should not be allowed to persist for more than 6 weeks without further intervention.
Cyclic esotropia
Cyclic esotropia is a very rare condition characterized by alternating manifest esotropia with suppression and BSV, each typically lasting 24 hours. The condition may persist for months or years and the patient may eventually develop a constant esotropia requiring surgery. Earlier correction of the full manifest angle can be successfully performed during the intermittent phase.
High myopia esotropia
Patients with high myopia may have instability of the muscle pulleys that stabilize the superior rectus and lateral rectus muscles. This results in nasal displacement of the superior rectus and inferior displacement of the lateral rectus. The possibility of this condition should be considered in high myopes with acquired esotropia; MR scan is mandatory in making the diagnosis. Treatment involves plication of the superior and lateral recti with a non-absorbable suture.
Exotropia
Constant (early-onset) exotropia
Intermittent exotropia
Diagnosis
Classification
Treatment
Sensory exotropia
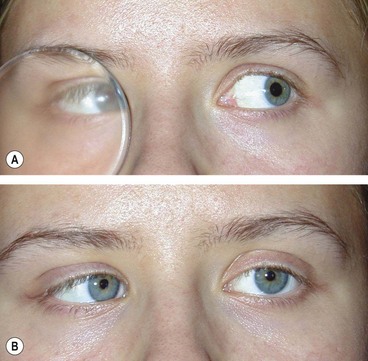
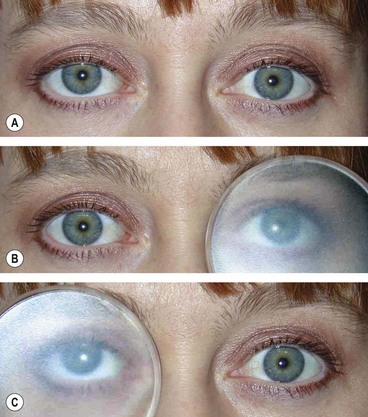
Secondary (sensory) exotropia is the result of monocular or binocular visual impairment by acquired lesions, such as cataract (Fig. 18.57) or other media opacities. Treatment consists of correction of the visual deficit, if possible, followed by surgery if appropriate. A minority of patients develop intractable diplopia due to loss of fusion, even when good visual acuity is restored to both eyes and the eyes are realigned.
Consecutive exotropia
Consecutive exotropia develops spontaneously in an amblyopic eye, or more frequently following surgical correction of an esodeviation. In early postoperative divergence muscle slippage must be considered. Most cases present in adult life with concerns about cosmesis and social function, and can be greatly helped by surgery. Careful evaluation of the risk of postoperative diplopia is required, although serious problems are uncommon. About 75% of patients are still well-aligned 10 years after surgery, although re-divergence may occur.
Special syndromes
Recent genetic and neuropathological studies have shown that a group of congenital neuromuscular disorders are the result of developmental errors in the innervation of ocular and facial muscles. These conditions are now referred to as congenital cranial dysinnervation disorders (CCDD) and include Duane syndrome, Möbius syndrome, congenital fibrosis of the extraocular muscles, Marcus Gunn jaw-winking syndrome (see Ch. 1), congenital ptosis and congenital facial palsy.
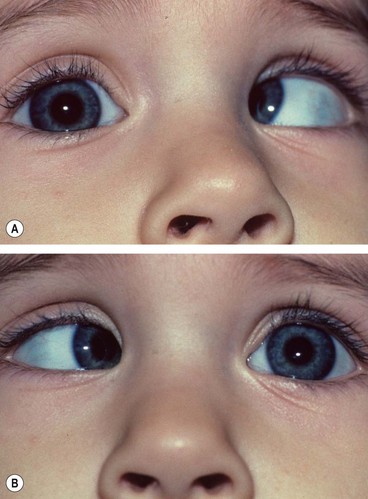
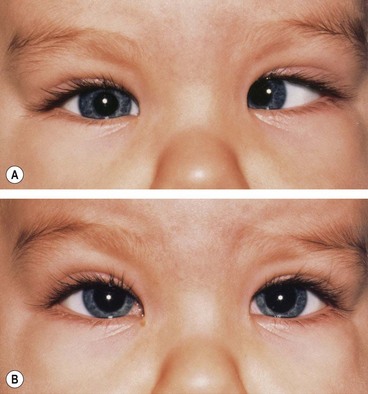
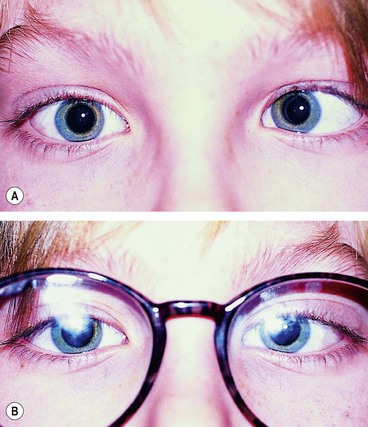
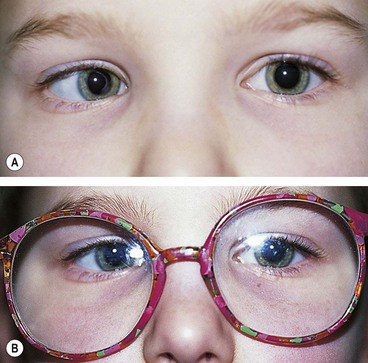
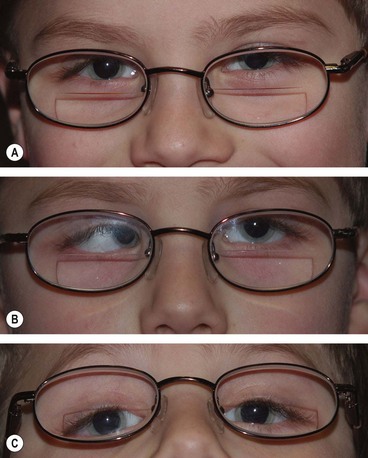
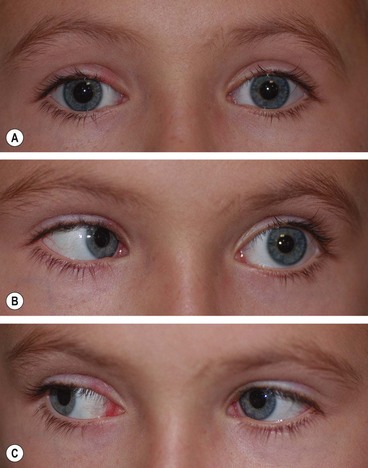
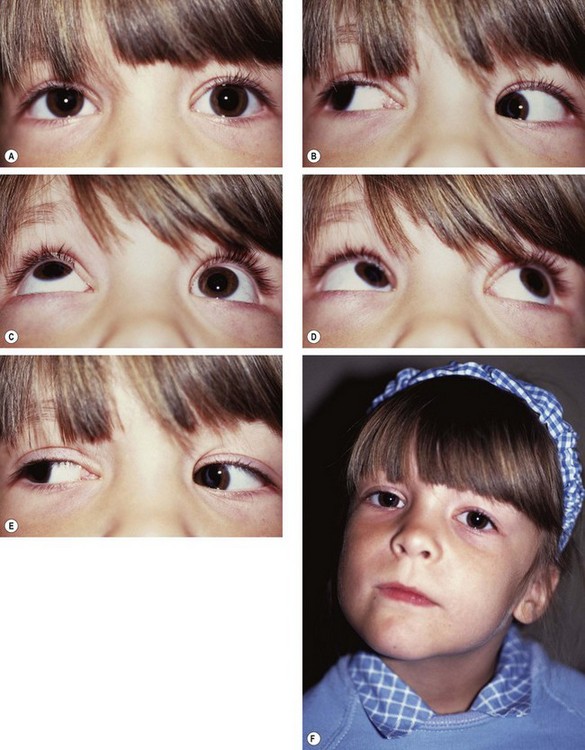
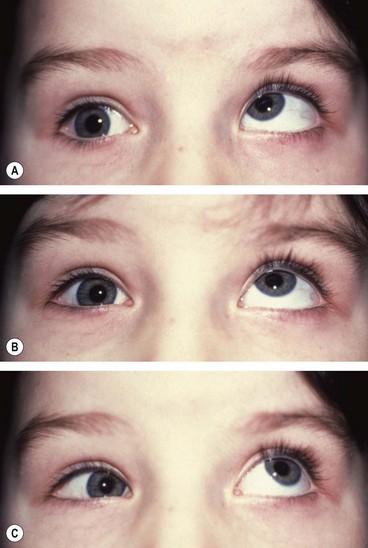
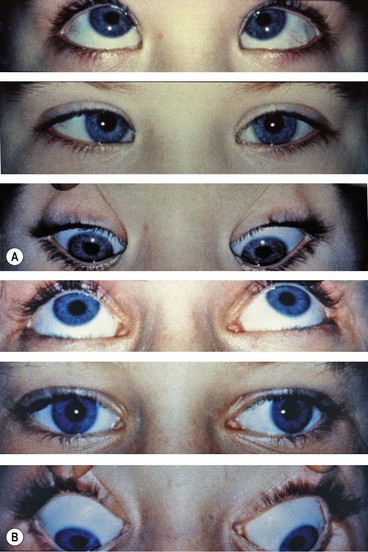
Duane retraction syndrome
In Duane retraction syndrome there is failure of innervation of the lateral rectus by the 6th nerve, with anomalous innervation of the lateral rectus by fibres from the 3rd nerve. The condition is often bilateral, although frequently involvement in one eye may be very subtle. Some children have associated congenital defects such as perceptive deafness and speech disorder.
Signs
There is usually BSV in the primary position, often with a face turn. The affected eye shows the following motility defects (Figs 18.58-18.60).
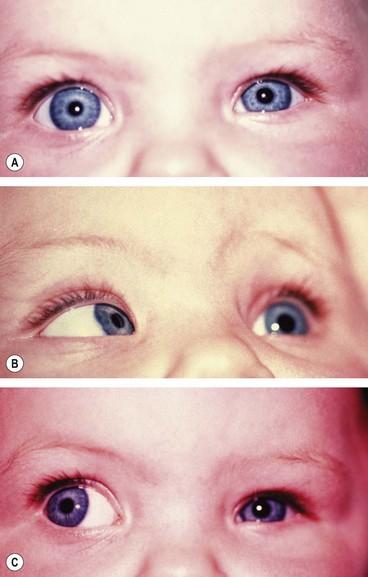
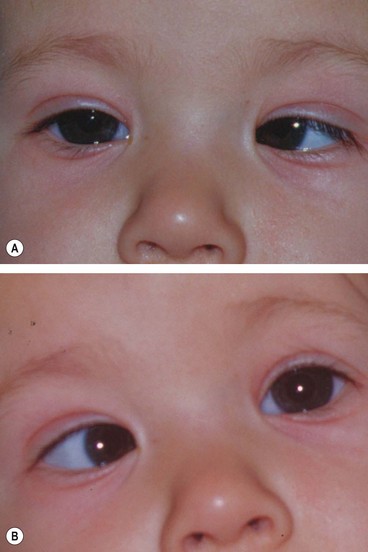
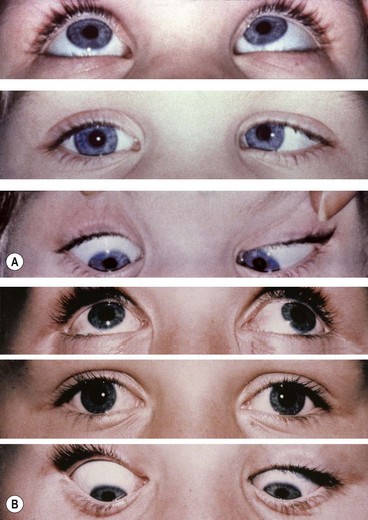
Fig. 18.58 Duane syndrome Huber type III in an infant. (A) Straight eyes in the primary position; (B) limited left abduction with widening of the left palpebral fissure; (C) gross limitation of left adduction with narrowing of the left palpebral fissure
(Courtesy of K Nischal)
Classification (Huber)
The underlying pathophysiology is similar in all three types, the differences being due to variation in the degree of anomaly in the innervation to the lateral and medial recti.
Treatment
The majority of patients with Duane syndrome do not need surgical intervention.
Brown syndrome
Brown syndrome is a condition involving mechanical restriction. It is usually congenital but occasionally acquired:
Treatment
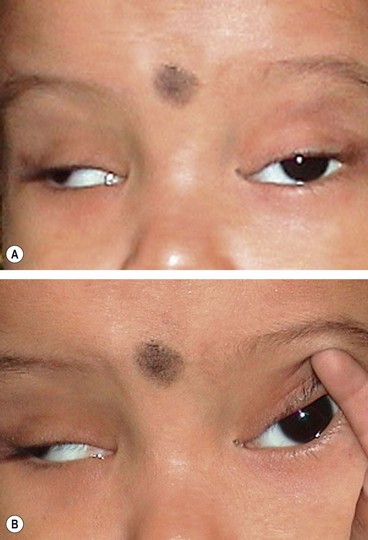
Monocular elevator deficit
Monocular elevator palsy, sometimes also referred to as double elevator palsy, is a rare sporadic condition. It is thought to be caused either by a tight or contracted inferior rectus muscle or a hypoplastic or ineffective superior rectus muscle.
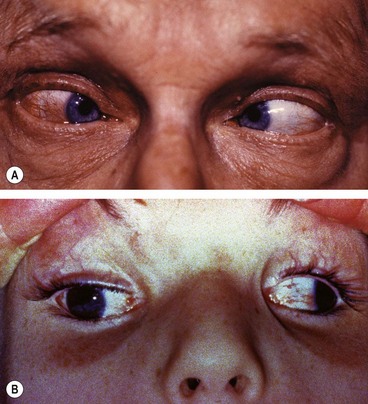
Möbius syndrome
Möbius syndrome is a very rare congenital, sporadic condition.
Congenital fibrosis of the extraocular muscles
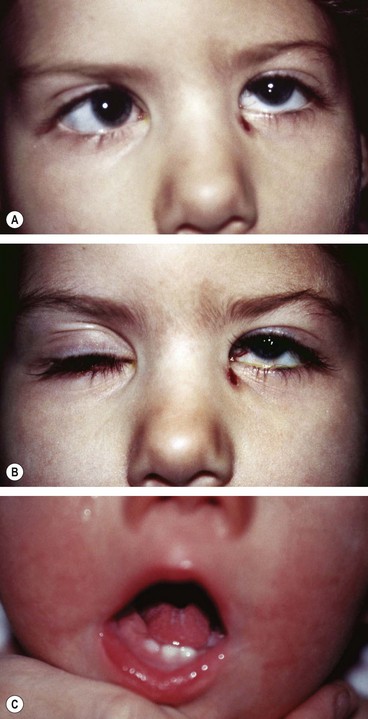
Congenital fibrosis of the extraocular muscles (CFOEM) is a rare non-progressive, usually AD, disorder characterized by bilateral ptosis and restrictive external ophthalmoplegia (Fig. 18.64).
Strabismus fixus
Strabismus fixus is a very rare condition in which both eyes are fixed by fibrous tightening of the medial recti (convergent strabismus fixus – Fig. 18.65A), or the lateral recti (divergent strabismus fixus – Fig. 18.65B).
Alphabet patterns
‘V ‘or ‘A’ patterns may occur when the relative contributions of the superior rectus and inferior oblique to elevation, or of the inferior rectus and superior oblique to depression are abnormal, resulting in abnormal balance of their horizontal vectors in up- and downgaze. They can also be caused by anomalies in the position of the rectus muscle pulleys leading to abnormal lines of action of the muscles. They are assessed by measuring horizontal deviations in the primary position, upgaze and downgaze and may occur regardless of whether a deviation is concomitant or incomitant.
‘V’ pattern
A ‘V’ pattern is said to be significant when the difference between upgaze and downgaze is ≥15 Δ (allowing for a small physiological variation).
Causes
Treatment
By inferior oblique weakening or superior oblique strengthening when oblique dysfunction is present. Without oblique muscle dysfunction treatment is as follows:
‘A’ pattern
An ‘A’ pattern is considered significant if the difference between upgaze and downgaze is ≥10 Δ. In a binocular patient it may cause problems with reading.
Causes
Treatment
Patients with oblique dysfunction are treated by superior oblique posterior tenotomy. Treatment of cases without oblique muscle dysfunction is as follows:
Surgery
The most common aims of surgery on the extraocular muscles are to correct misalignment to improve appearance, and if possible to restore BSV. Surgery can also be used to reduce an abnormal head posture and to expand or centralize a field of BSV. However, the first step in the management of childhood strabismus involves correction of any significant refractive error and/or treatment of amblyopia. Once maximal visual potential is reached in both eyes, any residual deviation can be treated surgically. The three main types of procedure are: (a) weakening, which decreases the pull of a muscle, (b) strengthening, which enhances the pull of a muscle and (c) procedures that change the direction of muscle action.
Weakening procedures
The procedures for weakening the action of a muscle are: (a) recession, (b) disinsertion (or myectomy) and (c) posterior fixation suture.
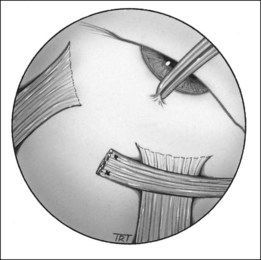
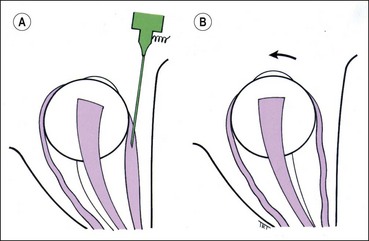
Recession
Recession slackens a muscle by moving it away from its insertion. It can be performed on any muscle except the superior oblique.
Disinsertion
Disinsertion involves detaching the muscle from its insertion without reattachment. It is most commonly used to weaken an overacting inferior oblique muscle when the technique is the same as for a recession except that the muscle is not sutured. Very occasionally, the procedure is performed on a severely contracted rectus muscle.
Posterior fixation suture
The principle of this (Faden) procedure is to suture the muscle belly to the sclera posteriorly so as to decrease the pull of the muscle in its field of action without affecting the eye in the primary position. The Faden procedure may be used on the medial rectus to reduce convergence in a convergence excess esotropia and on the superior rectus to treat DVD. When treating DVD, the superior rectus muscle may also be recessed. The belly of the muscle is then anchored to the sclera with a non-absorbable suture about 12 mm behind its insertion.
Strengthening procedures
Treatment of paretic strabismus
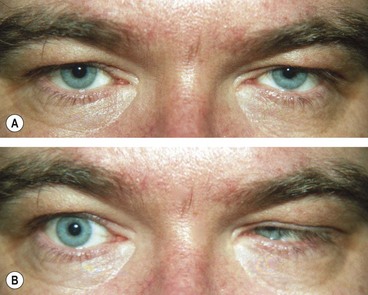
Lateral rectus palsy
Surgical intervention for 6th nerve palsy should be considered only when it is clear that spontaneous improvement will not occur. This is usually after at least 3 months have elapsed without improvement, typically at least 6 months from onset of the palsy. Treatment of partial and complete lateral rectus palsies is different.
Three rectus muscles should not be detached from the globe at the same procedure because of the risk of anterior segment ischaemia.
Superior oblique palsy
Surgical intervention should be considered to improve troublesome diplopia or an abnormal head posture. The treatment of unilateral and bilateral palsies is different. General principles are as follows:
Adjustable sutures
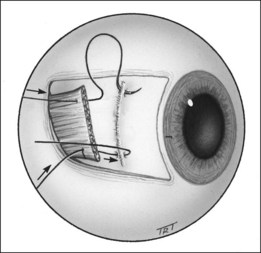
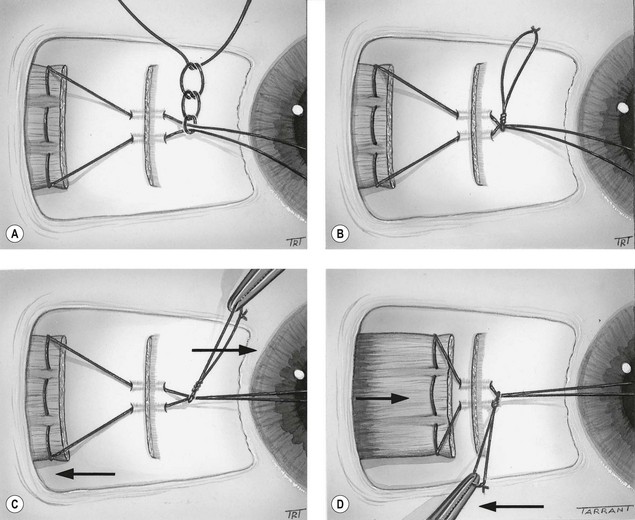
Indications
The results of strabismus surgery can be improved by the use of adjustable suture techniques on the rectus muscles. These are particularly indicated when a precise outcome is essential and when the results with more conventional procedures are likely to be unpredictable; for example, acquired vertical deviations associated with thyroid myopathy or following a blow-out fracture of the floor of the orbit. Other indications include 6th nerve palsy, adult exotropia and re-operations in which scarring of surrounding tissues may make the final outcome unpredictable. The main contraindication is patients who are too young or unwilling to cooperate during postoperative suture adjustment.
Initial steps
Postoperative adjustment
This is performed under topical anaesthesia, usually a few hours after surgery when the patient is fully awake.
A similar technique can be used for rectus muscle resection.
Botulinum toxin chemodenervation
Temporary paralysis of an extraocular muscle can be created by an injection of botulinum toxin under topical anaesthesia and EMG control. The effect takes several days to develop, is usually maximal at 1–2 weeks following injection and has generally worn off by 3 months. Side-effects are uncommon, although about 5% of patients may develop some degree of temporary ptosis. The following are the main indications for chemodenervation: