CHAPTER 5 Fundamentals in the Methods of Periodontal Disease Epidemiology
The Need for Epidemiology
Beliefs on the cause of a disease impact how diagnosis, treatment, prevention, systemic consequences, and research agendas are considered. In mainstream periodontics, theories on the causes of periodontal disease have typically been specific and strong over the last half century. “Periodontal (gum) diseases, including gingivitis and destructive periodontal disease, are serious infections.” This statement69 from a professional dental organization in 2009 reflects the common and relatively recent belief that periodontal conditions are caused by bacteria.71 Such a tenet on infection led to the following corollaries:
However, additional evidence is required to be reasonably confident in the belief that periodontal disease indeed is an infectious disease. In a modern evidence-based world, case-control studies, cohort studies, and randomized controlled trials are the study designs that can obtain high-level evidence on the causes and management of a disease. These three epidemiologic study designs provide reliable inference on etiology, diagnosis, treatment, and prognosis. This chapter focuses on the role of epidemiology in providing evidence on etiology and on clinical epidemiology and how it relates to diagnosis.
Case-control studies and cohort studies are the two study designs most commonly employed to reliably identify the etiologic factors that cause disease. Smoking, ionizing radiation, hepatitis B, and high blood pressure are examples of causes of common human chronic disease that were identified with these research designs.
Randomized controlled trials offer the most reliable methods to evaluate the diagnosis, management, and prognosis of diseases. Prostate surface antigen (PSA) screening, polio vaccination, and hormone replacement therapy are examples of diagnoses and treatments for chronic diseases that were evaluated by randomized controlled trials.
Suggestions that periodontal diseases are infectious diseases are often justified by studies with a modern evidence-based approach that ranks below these study designs. Some scientific works, such as the experimental gingivitis study or an experimental infection study in nonhuman primates, have been cited frequently to support the “periodontal disease is an infectious disease” assertion. But there are reasons that these studies are ranked as a lower level of evidence than controlled clinical studies. Is the transition from an unnatural inflammation-free condition referred to as “Aarhus superhealthy gingiva”70 to experimental gingivitis44 (which is different from clinical gingivitis) indeed “didactic proof of the essential role of dental plaque bacteria in periodontal diseases”70 or is it a reflection of the unhealthy effects of the typical western diet? Is a “burst” of bone loss22 subsequent to the injection of Bacteroides gingivalis proof that “this microorganism (is) of great importance to the control of destructive periodontal disease”? In a modern evidence-based world, data from these small, uncontrolled case-series studies and experimental animal studies would be considered insufficient evidence to make “periodontal-disease-as-an-infectious-disease” a cornerstone of clinical thinking.
Epidemiologic studies—higher level than case-series or animal studies—have had a powerful impact on the incidence of chronic diseases by reliably identifying the primary causes. Reliable evidence on what causes disease allows laboratory research to focus on interventions on the causal pathways, which can then lead to clinical trials. “Medical science continually passes the baton of discovery from (epidemiologic) observation to laboratory studies to human clinical trials.”40 For example, epidemiologic observations identified hepatitis B as the main cause of liver carcinoma, one of the most common cancers in the world.5 Subsequently, the baton of discovery was passed to basic science in which a recombinant engineered vaccine for hepatitis B was developed. Then, the baton of discovery was passed to clinical epidemiologists who assessed the effectiveness of vaccinations and documented dramatic declines of mortality rates due to liver cancer.39 Similar success stories in the management of chronic diseases in which epidemiology played a critical role include coronary heart disease and blood pressure medication, dental caries and fluoride, and lung cancer and smoking intervention programs.
The emerging high-level epidemiologic evidence on the etiology of periodontal diseases suggests that systemic factors may be the true drivers of the epidemic. Factors, such as cigarette smoking and diabetes, are increasingly recognized as causes of periodontal disease, and their effect on the periodontium appears unrelated to infectious factors.7,61 Organizations, such as the World Health Organization, suggest that periodontal disease prevention be made an integral part of programs that focus on tobacco control, diet, and physical activity.54 The consequences of such a shift from a belief of periodontal disease as an infectious disease to a belief that periodontal disease is a lifestyle disease may impact on several aspects of clinical care. Regardless of your current beliefs on the etiology of periodontal disease, it will become increasingly important to become familiar with epidemiologic methodology to be able to judge this emerging evidence independently and critically.
![]() Science Transfer
Science Transfer
At present, it is difficult to accurately assess epidemiologic data on periodontal disease because of the wide variety of indices and measurements used, as well as the great differences in experience and reliability of the clinical examiners. For example, some epidemiologic surveys evaluated periodontal pocket depth with probes that have a sphere on the tip; in contrast, practicing clinicians measure pocket depths with probes that have a fine, tapered tip. These epidemiologic data may be very different from the detailed day-to-day measurements that are used for diagnosis and treatment planning of individual patients. Thus the reports that periodontal disease prevalence in adults in the United States has decreased over the last decade may or may not reflect an accurate picture.
One common mistake of interpretation of epidemiologic data is to assume a cause and effect relationship when two measurements show a high statistical connection. These statistical connections may or may not be related to cause and effect, and inductive controlled clinical trails are needed to prove if such a relationship exists.
Measuring the Occurrence of Conditions or Diseases
The fundamental tools of epidemiology are simple sums and divisions that reflect how many individuals or sites have or develop a particular condition or disease.
The prevalence is the number of individuals or sites that have a particular condition or disease within a defined population. The prevalence is the sum of the number of individuals or sites divided by the sum of all examined individuals or sites that exhibit the condition or disease of interest. The prevalence of a condition can range from 0% (no one has the condition or disease of interest) to 100% (everyone has the condition or disease of interest).
As an example of prevalence, the Centers for Disease Control (CDC) reported on the prevalence of individuals with at least one periodontal pocket depth of 4 mm or deeper. The CDC reported that in 1988–1994, a little over 1 in 5 Americans had such a condition (a prevalence of a little over 20%), whereas in 1999–2004, only 1 in 10 fell in this category17 (or a prevalence of around 10%). These findings suggests a more than 50% decline in the prevalence of pocket depth greater than or equal to 4 mm for adults 20 to 64 years of age in approximately a decade. These epidemiologic data confirm another report of declining destructive periodontal prevalence in the United States (US).9 Such information on prevalence measures of periodontal conditions may have implications for manpower needs in the US and may provide clues with respect to etiologic factors that drive such changes. The vast majority of countries do not have such prevalence surveillance systems,54 which makes it difficult to determine whether these trends observed in the US are isolated or part of a more general trend.
The risk is the probability than an individual or a site will develop a particular condition or disease during follow-up. The risk for a condition or a disease is (similar to prevalence) a number that ranges between 0% and 100%. The simplest way to estimate risk is to have a fixed number of persons or teeth at risk at some defining moment (time zero [t0]). Individuals or teeth within individuals are followed up over time subsequent to this defining moment. After a follow-up period (from time t0 to time tn), the risk can be calculated as the proportion of persons, or teeth, who develop the clinical outcome of interest during the follow-up.
Since the risk is estimated as a proportion, it is without dimension and ranges between 0 and 1. When a risk is reported, it should be accompanied by a specific time period to which it is applied. A 5% risk for death may be considered small when it refers to a 20-year period but quite high when it refers to a 3-month period.
As an example of the clinical utility of the concept of risk, consider concerns on occupational human immunodeficiency virus (HIV) infection for dentists. It has been reported that the risk for developing an HIV infection subsequent to an accidental needlestick with HIV-contaminated blood is 0.3%. Such a statistic has an intuitive appeal and can be related to patients or colleagues. A risk of 0.003 (0.3%) indicates that for every 1000 individuals that have an accidental HIV-contaminated needlestick, 3 are expected to develop an HIV infection.
The odds for an event is the probability that an event happened divided by the probability that an event did not happen. Whereas probability is a value that has to range between 0 and 1, odds values range from 0 to infinity. If the probability for observing an event is small, then the odds and the probability are almost identical. For example, if the probability for a vertical root fracture after an endodontic procedure is 0.001, the odds are 0.001/0.999 or 0.001001.
Odds are commonly reported in studies because they are often easier to estimate with statistical models than probabilities. For example, the odds for developing an HIV infection subsequent to an accidental needlestick with HIV-contaminated blood is 0.003 (0.003/0.997). The odds for developing oral cancer when smoking for 15 years is 0.098 (0.089/0.911).
Incidence rates are an alternative intuitive measure to describe disease occurrence. One of the best examples of an incidence rate is given by the speedometer in a car that displays at any given time the number of miles or kilometers being traveled per hour. In clinical trials or epidemiology, the rate reflects the number of disease occurrences per time unit per person or site. The most commonly used estimator for a disease rate is a ratio in which the numerator is the number of subjects or sites diagnosed with the disease of interest and the denominator is the sum of the time at risk over all subjects or sites in the population.
Incidence rates, as opposed to any of the previously introduced measures of disease occurrence, imply an element of time. The denominator in the incidence rate has time as the dimension. Therefore the dimension of incidence rate is 1/time. This dimension is often referred to as “person-time’ or “site-time” to distinguish the time summation from ordinary clock-time. The magnitude of the incidence rate can vary between 0 and infinity. When there are no new disease onsets during the study period, the incidence rate is 0. When every person observed dies instantaneously at the start of the study, (and thus the sum of the time periods is 0), the incidence rate is infinity.
An example of the application of rates is provided in Figure 5-1, in which the number of teeth lost per 1000 tooth-years is plotted as a function of the maximum probing depth at the start of follow-up. The plot suggests a nonlinear relationship between maximum pocket and tooth loss with a substantial increase in tooth loss rate for teeth that have periodontal pockets of 7 mm or deeper.
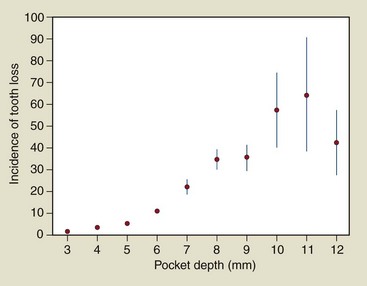
Figure 5-1 Rate of tooth loss (per 1000 tooth/year) as a function of maximum probing depth per tooth in a cohort of 1021 patients (ages 40–65 years) under periodontal specialist care for destructive periodontal disease.
(Data from Hujoel PP, Cunha-Cruz J, Selipsky H, et al: Periodontol 2000 39:22-29, 2005.)
For the study of risk, the population studied is usually limited to those individuals at risk for the outcome of interest. Thus, if the outcome of interest is a disease, the following subjects are excluded from the cohort: persons who already have the disease, persons who have immunity to the disease, or persons who are biologically incapable of developing the disease.
Periodontal Conditions Typically Measured Clinically
A clinical periodontal examination typically can include a variety of measures that reflect on characteristics of the periodontium. Most dental records of periodontal patients contain information on teeth that are present, missing, or impacted, as well as information on the periodontal status of those teeth that are measurable. Typically, a periodontal probe is used and information such as clinical probing depth, bleeding on probing, gingival recession, mobility of teeth, and the presence of furcation involvements are charted. In addition, some clinicians may collect information on the presence of gingivitis by evaluating the color and form of the gingival tissues. These measures may or may not be complemented with radiographic examinations that may provide information on marginal bone levels.
In research settings or in some selected private practices, additional periodontal measures may be collected such as clinical attachment levels, microbiologic measures, gingival crevicular fluid volume, the presence of certain biomarkers in the crevicular fluid, and a variety of indices that measure the amount of gingival inflammation or dental plaque or debris accumulation.
In one survey of clinical studies, it was observed that the gingival index (GI) and bleeding on probing are the two most common methods to assess gingival inflammation.23 The GI was proposed in 1963 as a method for assessing the severity and quantity of gingival inflammation.42,43 With this particular index, only gingival tissues are assessed. Each of the four gingival areas of the tooth (facial, mesial, distal, and lingual) are assessed for inflammation and rated as normal gingiva (a score of 0) to severely inflamed gingiva with a tendency to spontaneously bleed (score of 3). Gingiva that is mildly inflamed but without bleeding on probing is given a GI of 1, whereas moderately inflamed gingiva with bleeding is given a score of 2. The scores can be averaged by patient to provide patient means. Alternative, site-specific analyses can relate both local factors and patient-specific factors to the GI. The cumbersome nature of charting a GI makes it unlikely that it is in common use in clinical practice.
Bleeding on probing is a measure of periodontal inflammation that may be more commonly used by researchers and clinicians. The specific approach to obtain a bleedings measure can vary from one study to the next, as well as from one clinician to another. For example, in the Third National Health and Nutrition Examination Survey (NHANES III),1 bleeding measures were obtained as follows. First, the facial and mesiofacial sites of teeth in two randomly selected quadrants, one maxillary and one mandibular, were selected. A special probe known as the National Institute of Dental Research (NIDR) probe was used in these assessments. This color-coded probe is marked at 2, 4, 6, 8, 10, and 12 mm. To begin the assessment, the examiner dried a quadrant of teeth with air. Then, starting with the most posterior tooth in the quadrant (excluding the third molar), the examiner placed a periodontal probe 2 mm into the gingival sulcus at the facial site and carefully swept the probe from the mesiofacial to the mesial interproximal area. After probing the sites in the quadrant, the examiner assesses the presence or absence of bleeding at each probed site. The same procedure was repeated for the remaining quadrant. As opposed to the NIDR protocol in which gingival bleeding is assessed by sweeping the periodontal probe while inserted only 2 mm into the periodontal sulci or pockets, clinicians may go to the depth of the periodontal sulci or pocket and measure pocket depth in conjunction with bleeding on probing.
Commonly used measures of periodontal tissue destruction in clinical studies—as opposed to periodontal inflammation—are mean probing depth, mean attachment loss, and mean recession level.31 Again, the clinical protocol on how such mean values are collected and calculated can once again vary considerably from one study to the next. One example of description of how such values can be ascertained clinically is described in the National Institute of Dental and Craniofacial Research (NIDCR) “periodontal destruction” examination.1 This examination includes an assessment of periodontal attachment loss58 as the distance (in millimeters) from the cemento-enamel junction to the bottom of the gingival sulcus. This distance was measured at the facial and mesiofacial sites of teeth in a randomly selected maxillary and mandibular quadrant using the indirect measurement method developed by Ramfjord.58
Translating Periodontal Conditions into Traditional Epidemiologic Measures of Disease Occurrence
The application of the traditional epidemiologic methods of risk, prevalence, and rate was challenging because medical epidemiologists typically deal with patients that do or do not have a particular condition of interest. Dental epidemiologists deal with as many of 188 sites per patient that may or may not bleed. In addition, these periodontal sites within patients are correlated, meaning that if a patient has one site that bleeds, it is more likely that this same patient also has other sites that will bleed. It is now well established that gingival bleeding is suppressed in smokers.8 Therefore bleeding on probing in periodontal sites in a smoking patient tends to be more alike than bleeding on probing in periodontal sites among nonsmokers. In others words, sites within patients cannot be assumed to be statistically independent as a result of patient-related factors such as smoking or diet. The statistical methodology to deal with such correlated observations is complex, and for most of the twentieth century, it was challenging to calculate the confidence intervals for site-specific risks or prevalence.33 As a result, clinicians could not obtain reliable information on whether a periodontal site infected with a particular microbiologic species was at an increased risk for periodontal attachment loss. These challenges greatly hampered progress in building causal models of periodontal disease that were based on the higher-level evidence of controlled clinical studies rather than the lower-level evidence of reasoned theory, case-series, and animal studies.
The most common approach to deal with this challenge of correlated observations was to summarize site-specific periodontal data at patient level. These summaries could be calculated in a variety of ways. Commonly, site-specific information was averaged at a patient level. However, a variety of other approaches has also been utilized. For example, the information on the presence of bleeding in up to 188 periodontal sites in a patient could be summarized as the presence of at least 1 bleeding site, or the presence of at least 5 bleeding sites, regardless of whether patients had 1 tooth or 32 teeth.
Some devised a more sophisticated approach to provide patient-level summary data for periodontal outcome data. For example, the extent and severity index (ESI) of periodontal disease was developed to provide separate estimates of the extent and severity of periodontal disease.11 A threshold of disease must be established to calculate the extent score for an individual. In their initial study of the ESI, Carlos et al11 considered a site to be diseased when attachment loss exceeded 1 mm. For an individual, the extent score is the percentage of sites examined that have attachment loss greater than 1 mm. The severity score for an individual is the average loss of attachment per site among the diseased sites. The ESI is expressed as a bivariate statistic: ESI = (extent, severity). For example, an individual’s ESI of (20, 5.0) would be interpreted as 20% of sites examined had disease and of the diseased sites, the average loss of attachment was 5 mm. The ESI for a population would be the average extent and severity scores for the individuals examined.
The advent of modern statistical techniques to deal with the problem of correlated data made it possible to avoid summarizing site-specific information at a patient level and losing the richness in the clinical data that clinicians need.15,16,27,32,33 These methods allow important clinical questions to be answered, such as determining whether the 3 mm loss of attachment at a site is related to site-specific factors (such as the amount of plaque at that site, the location of the site in the oral cavity, or the microflora present at that site), to host factors (such as glucose control, serum cotinine levels, or intake of fermentable carbohydrates), or to an interaction between a site-specific factor and a host factor.
True and Surrogate Measures of the Periodontal Condition
An important consideration in both epidemiology and clinical practice is the distinction between true and surrogate endpoints of periodontal disease. True endpoints are tangible outcomes that directly measure how a patient feels, functions, or survives.18 A painful periodontal abscess is an example of a tangible event that can be precisely identified or realized by the patient. True endpoints include the oral health–related quality-of-life measurements38,48,65 or self-reported problems such as a positive answer to the question: “When you brush or floss your teeth, do you notice bleeding that is both regular and involves spitting blood-stained saliva?” Synonyms for true endpoints include clinically relevant endpoints, clinically meaningful endpoints, terminal endpoints, or ultimate endpoints.
Surrogate endpoints are intangible to the patient.68 Changes in bleeding on probing or pocket depth are examples of changes that are intangible to the patient. Examples of surrogate endpoints in periodontal research include anatomic measures (e.g., probing depth), measures of inflammation (e.g., gastrointestinal), microbiologic measures, and immunologic measures.15 Surrogate endpoints are often objective because they can be measured by the clinician (rather than relying on self-report by patients) or by laboratory methods. Synonyms for surrogate endpoints include intermediate endpoints, biologic markers, or biomarkers.
Surrogate endpoints can be misleading when the goal is to provide reliable information on clinical decisions related to diagnosis, etiology, treatment, or prognosis. An overview of situations in clinical research in which surrogate endpoints have led to misleading conclusions is provided in Table 82-1 on the Expert Consult website. One periodontal example is systemic antibiotics that may have a beneficial impact on attachment gain20 but a potential increased risk for tooth loss.14
Challenges in Obtaining Epidemiologic Measures of Periodontal Conditions and Diseases
Among the challenges that face the periodontal epidemiologist are the continuous changes in the type of surrogate data collected, the paucity of information on whether surrogate information provides reliable information on outcomes of tangible patient benefit (i.e., outcomes that the patient cares about), the lack of diagnostic codes for the reasons for tooth loss, and the difficulty in estimating the impact of tooth loss on surrogate outcomes.
The diversity of measures to assess periodontal condition or disease is truly staggering. One survey of periodontal clinical trials conducted over a mere 4-year period indicated that a total of 153 distinct surrogate endpoints were defined and that over 80% of the 153 surrogate endpoints were used in fewer than 5 of the 82 trials.23 Another survey similarly identified the diversity in methodologies and definitions as a challenging issue in systematically reviewing evidence.62 This continuous creation of “new and improved” surrogate outcomes in periodontal research is likely an important driver of false-positive conclusions.26
In addition, there is substantial secular variability in the type of periodontal measures that are favored, with new surrogate measures appearing on a regular basis. Russell developed the Periodontal Index,60 which scored the supporting tissues for each tooth in the mouth according to a progressive scale that gives little weight to gingival inflammation and relatively great weight to advanced periodontal disease. Although the Periodontal Index was used in the first NHANES, thereby gaining national prominence in the US, it was not used again in any of the subsequent national surveys.
Similarly, in clinical periodontics, clinical attachment levels were once considered the gold standard for judging a treatment’s efficacy but are now relegated as a mere safety measure in some drug-approval processes.29 As a result of this continuous change in the type of surrogate periodontal measures that are in fashion, it was not possible until recently to provide reliable information on such simple questions as to whether the prevalence of destructive periodontal disease was increasing, decreasing, or remaining constant.
A second challenge in interpreting the clinical relevance is the paucity of information on whether the collected data are informative on any measure that matters to patients such as tooth loss or quality-of-life issues related to oral health. This situation creates challenges in interpreting evidence. It is similar to tracking prostate cancer by measuring inflammation or swelling of the prostate gland by digital palpation, without information on how this information relates to prostate cancer mortality, clinical symptoms of prostate disease, or anything that matters to the patient. This challenge is further compounded by the absence of diagnostic codes for tooth loss, which has largely prevented obtaining reliable information on how many teeth really are lost as the result of periodontal disease opposed to dental caries.
The attempt to track a disease by only collecting a surrogate outcome measure such as probing depth from those teeth that are present leads to a type of bias is typically referred to as survival bias. Most periodontal clinical trials in the twentieth century evaluated the effect of periodontal therapies on those teeth that survived the treatment. As in cancer research, in which studying the effect of chemotherapy on the survivors creates results that cannot be interpreted, studying the effect of periodontal therapies on survivor teeth creates unreliable data. Some methods, such as imputations, can provide an understanding of the extent to which such biases may alter the conclusions of epidemiologic studies.
In summary, the fundamental tool of periodontal epidemiology is a measure of the occurrence of periodontal conditions. These measures include epidemiologic statistics, such as prevalence, risk, and rate, and focus on either patient-specific or site-specific markers such as oral health–related quality of life, tooth loss, anatomic measures, or measures of gingival inflammation. This wealth of possibilities in defining periodontal conditions combined with statistical challenges in handling correlated data has made it difficult to answer even such a simple question as to whether a hidden periodontal disease epidemic occurred in the twentieth century.28
Epidemiologic Study Designs
The essence of epidemiology and clinical epidemiology is to relate measures of disease occurrence to suspected causes or interventions. Can the recent dramatic drop in destructive periodontal disease prevalence in the US be attributed to a change in smoking prevalence? Can the presence of particular microbiologic species around a tooth be related to the risk of future tooth loss? Can the rate of tooth loss in a elderly sample be related to the use of an antimicrobial rinse? In an evidence-based approach, these questions can be most reliably answered by three epidemiologic study designs. As mentioned earlier in this chapter and briefly introduced in Chapter 2, these study designs (in order of decreasing reliability) are the randomized controlled trial, the cohort study, and the case-control study. For a comprehensive review of study designs, the reader can consult statistical or epidemiologic textbooks.
Randomized Controlled Trials
Randomized controlled trials in periodontics typically assign patients or some teeth within a patient randomly to a treatment and subsequent outcomes are assessed. Table 5-1 provides two examples of randomized controlled trials.
TABLE 5-1 Examples of Periodontal Randomized Controlled Trials
| Periodontal Treatment | Outcome | Sample Size |
|---|---|---|
| Scaling and root planing in pregnant women50 | Infant low birth weight | 823 |
| Biphasic calcium phosphate ceramic51 | Clinical attachment level | 137 |
The randomized controlled trial is the only study design that can provide a probabilistic basis for making causal inference between an intervention and an outcome. Reliable inference on the causality of associations is only possible if there is a specific pretrial hypothesis that specifies the endpoint, the treatments to be compared, the patient population, and the degree of required precision and if the trial conduct adheres strictly to design and analysis principles of definitive trials. Factors important in obtaining reliable answers include a secure randomization process, masking of patients and clinicians, the presence of an independent data and safety monitoring board, and a strict adherence to the pretrial hypothesis, including an intent-to-treat analysis. Such trials are rare in any field including periodontal research. As a result, most of the trials published in the literature are in the category of exploratory trials typically reporting no pretrial hypothesis and concluding that the intervention was successful when compared to the control.23 Such conclusions are almost always false-positive conclusions.26 Only definitive trials can provide reliable answers on treatment efficacy. Unfortunately, these are the trials that are least likely to be published.
Cohort Studies
Cohort studies are sometimes referred to as exposure-based study designs. Subjects are classified with respect to an exposure such as cigarette smoking or diabetes and followed longitudinally for the assessment of periodontal outcomes. Table 5-2 provides two examples of cohort studies.
TABLE 5-2 Examples of Periodontal Cohort Studies
| Periodontal Exposure | Outcome | Sample Size |
|---|---|---|
| Periodontal disease and tooth loss34 | Coronary heart disease | 51,529 |
| Gingivitis10 | Tooth loss | >500 |
Cohorts can be defined by a geographic area, records, exposure status, or a combination of different criteria. In one study on causal factors of edentulism, the population of interest was defined as the inhabitants of the town of Tecumseh, Michigan. Persons within this community were examined in 1959 as part of a community-wide health study. Twenty-eight years later a subset of these patients was reexamined to study the risk factors for edentulism.1 Several natural disease history studies of destructive periodontal disease have been conducted based on geographic location. Examples include the Norwegian Longitudinal Study,3 the Veterans Administration Longitudinal Study,37 and the Sri Lanka study.4 A cohort can be defined by records (schools, health insurance plans, unions, industries, or professional organizations). Many cohort studies on periodontal disease outcomes are performed in patients belonging to a particular dental insurance company14 or to a professional group.34 Finally, cohorts can be defined based on a specific exposure. For example, different levels of fluoride concentration in the water supply has been used for the definition of cohorts.
Case-Control Studies
Case-control studies are typically referred to as outcome-based study designs. Persons with a condition or outcome of interest (cases) are compared to persons without a condition of interest (controls) with respect to the past history of the suspected causal factors. Many people intuitively think along the lines of a case-control study when evaluating disease etiology. For example, if one suffers from food poisoning after a party, he is likely to compare his past food intake history with the past food intake history of those individuals at the party that did not have food poisoning. Similarly, if one is diagnosed with a serious illness, a common reaction is: “Why me?” followed by a comparison of past history of exposures with other individuals that did not develop the serious illness. The primary goal in a case-control study is to find out what past exposures or factors are different between diseased patients (cases) and nondiseased patients? Table 5-3 provides two examples of case-control studies.
TABLE 5-3 Examples of Periodontal Case-Control Studies
| Case-Control Criteria | Investigated Risk Factors | Sample Size |
|---|---|---|
| Destructive periodontal disease3 | Smoking | |
| Acute myocardial infarction4 | Dental health |
The case-control study is the most challenging type of study to conduct. Trying to minimize the role of bias in case-control studies requires careful planning, conduct, and analysis, and even when everything is done perfectly, one can come up with the wrong conclusions in case-control studies. A recent review of the quality of periodontal case-control studies suggested that they are frequently inadequately conducted and reported.46
Two important elements of the case-control study design are the definitions of case and control. A case is a person in the population or study group identified as having a particular disease, health disorder, or condition.12 The case definition should be rigorous to minimize bias and misclassification and can be based on symptoms, signs, or results of diagnostic tests. For example, the case definition for a myocardial infarction in a case-control study on the relationship between dental health and acute myocardial infarction was as follows4:
Three different types of cases can be distinguished based on the relationship between the date of disease onset and the data of study initiation. Incident cases are those whose date of diagnosis is after the date of study initiation. Using this approach, cases are interviewed at the closest possible time to the etiologic exposure. Factors affecting survival of cases may not have yet started to act. As a result, case-control studies with incident cases are least likely to confuse causes of disease with consequences of a disease. Prevalent cases are those cases whose date of diagnosis precedes the date of study initiation. The selection of prevalent cases has only one advantage: cases are readily available. Prevalent cases may represent the survivors of all cases. Under such circumstances, factors that affect survival may appear as etiologic factors. The latter is of particular concern in dental case-control studies in which diagnosis of case status is based on surviving teeth. Decedent cases are those cases who died before the date of study initiation. Decedent cases possess all the disadvantages of prevalent cases. In addition, it may be that proxies need to be interviewed to investigate past exposure histories.
In a case-control study, the controls should be at risk for developing the investigated disease and come from the same population that generated the cases. For example, if the investigated disease is root caries, the controls should be at risk for developing root caries (i.e., have exposed root surfaces) and originate from the same population that generated the cases that have root caries.
Etiology
Human chronic diseases, such as cancer, diabetes, and destructive periodontal disease, have complex etiologies. The terms, necessary cause, component cause, and sufficient cause, help define the challenges in determining the etiology of a disease and in verbalizing the complexity of chronic disease etiology.59
The set of causes that initiate a chronic disease is referred to as a sufficient cause. Each sufficient cause consists of multiple component causes. Consider the hypothetical example where four sufficient causes exist for noniatrogenic destructive periodontal disease (Figure 5-2). The first sufficient cause in this example includes the following component causes: smoking, delayed neutrophil apoptosis, an interleukin-1 (IL-1) gene defect, dental plaque, a tooth, and an unspecified gene defect. These different elements of a sufficient cause are referred to as component causes. All component causes of a sufficient cause need to be present in order for the disease process to be initiated. Multiple sufficient causes may be responsible for a given disease. For example, two sufficient causes exist for destructive periodontal diseases that do not include smoking.
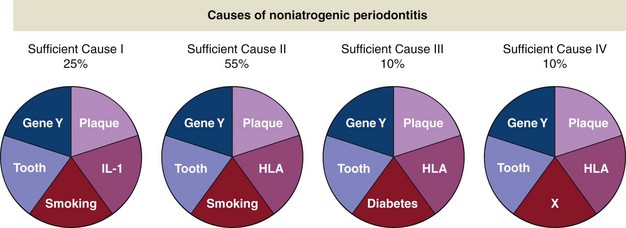
Figure 5-2 Causes of noniatrogenic periodontitis.
(Data from Rothman KJ: Am J Epidemiol 141(2):90-95, 1995.)
A component cause, which is an element of all the sufficient causes for a given disease, is referred to as a necessary cause. For example, fermentable carbohydrates are a necessary cause for dental caries. However, there are few examples of necessary causes—smoking is not a necessary cause of lung cancer or destructive periodontal disease, hepatitis B infection is not a necessary cause of liver cancer, or Streptococcus viridans is not a necessary cause of bacterial endocarditis. The search for necessary causes of disease is important, since its elimination would eradicate a disease.
The proportion of disease caused by different component causes does not add up to 100%. The component cause “smoking” is responsible for 80% of the destructive periodontal disease cases, plaque for 100%, and diabetes for 10%.
The complex causal web leading to the initiation and progression of chronic disease makes the reliable identification of causal components difficult. Over the last 50 years, epidemiology has succeeded in reliably identifying the highly prevalent causes for human chronic diseases. Now that those component causes responsible for a large proportion of cases for particular diseases (e.g., smoking for lung cancer) have been identified, the search for new causes is becoming significantly more challenging. For example, the hope existed that the genome project would lead to quick advances, but these hopes have not yet been fulfilled. Chronic diseases are typically caused not by one gene but by a set of many different genes, each one responsible for only a small proportion of the cases and acting in a variety of synergistic mechanisms on disease initiation.
Suspected Modifiable Etiologic Factors for Periodontal Disease
Tobacco Smoking
Tobacco smoking is recognized by several organizations as one of the primary drivers in periodontal disease epidemiology.7 Many criteria for causality have been satisfied,19 and smoking cessation has shown to slow the progression of periodontal disease.36,37,55 The strong impact of tobacco smoking on periodontal disease has the potential to induce spurious causal associations in other suspected risk factors for periodontal disease. For example, smoking is a risk factor for both type II diabetes72 and periodontal disease, making associations between type II diabetes and periodontal disease susceptible to biases. To obtain reliable inference on causal factors other than smoking, studies on periodontal disease epidemiology may need to be restricted to those who have never smoked.
Carbohydrate Metabolism
Several studies have shown relationships between periodontal disease and a variety of conditions that center on carbohydrate metabolism, including intake of dietary carbohydrates, exercise, obesity, prediabetes and diabetes. A systematic review of randomized controlled trials on carbohydrates suggested that an increased intake of fermentable carbohydrates will cause an increase in gingivitis.24 Two systematic reviews suggested diabetes as a risk factor for destructive periodontal disease.61,64
Dental Plaque
Several systematic reviews have provided evidence that chemotherapeutic and mechanical plaque control will reduce gingival inflammation. Essential oils6 and cetylpyridinium chloride-containing mouth rinses21 may reduce gingival inflammation. Interdental brushes may reduce dental plaque, bleeding, and probing pocket depth.66 Power-driven toothbrushes may be more effective than manual toothbrushes in removing plaque and reducing inflammation.63 Self-performed dental flossing may not be effective in reducing plaque and gingival inflammation.6 Although these systematic reviews provide evidence on the role of dental plaque in gingival inflammation, such studies do not necessarily suggest that dental plaque is the primary cause of gingival inflammation. Similar to the fluoride needed to prevent decay caused by fermentable carbohydrates, a toothbrush may be necessary to prevent gingivitis as the result of an unhealthy diet.
There is no reliable randomized controlled trial evidence that oral hygiene has a beneficial impact on the prevention of periodontal destruction.25
The Cause of Periodontal Disease for the Patient Sitting in Your Chair
In clinical epidemiology, in a court of a law, and in modern-day clinical practice, uncertainty regarding the “cause” is a key consideration when discussing causality. The term attributable risk percentage is used to express the probability that a disease is caused by a suspected etiologic agent. In a smoker with lung cancer, there may be a 20% probability that the lung cancer was caused by a factor other than smoking (e.g., radon). In an obese person with diabetes, there may be a 10% chance that smoking played no role in the onset of diabetes. For a worker with leukemia in the nuclear industry, there may be an 80% chance that the leukemia was not caused by the low-level protracted radiation exposures. One can almost never determine with certainty what caused a particular condition or disease to appear in a patient. All one can do is to assign probabilities to the likelihood that a particular causal factor was responsible for the disease diagnosed in a patient. Destructive periodontal disease and periodontal inflammation are no exception to this general rule of uncertainty in determining disease etiology. As a result, diagnostic names, such as plaque-induced gingival disease or non-plaque–induced inflammatory gingival lesions,41 can be considered misnomers, since such names imply diagnostic certainty that lead to circular reasoning.4 In the absence of high-level evidence on the causes of these diseases, a declaration of certainty on causality reflects the strong belief in the low-level evidence that suggests that the cause of periodontal disease is infection. The principle of diagnostic uncertainty is important not only when considering disease etiology but also when it comes to diagnosing periodontal conditions.
Diagnosis
Periodontal Conditions Versus Periodontal Diseases
Disease is defined as an attribute or a characteristic of a person, and diagnosis is the clinician’s belief that the person has the attribute.67 The World Health Organization (WHO) defined disease as those adverse health consequences that include physical or psychological impairment, activity restrictions, and/or role limitations.67 Certain periodontal conditions have been associated with such adverse consequences and thus certain periodontal conditions qualify as diseases under the WHO definition. In one study, about 1 in 5 patients presenting to a periodontal specialist reported that teeth, gums, or dentures had an impact fairly often or very often on either eating; relaxing; avoiding going out; or feeling self-conscious, pain, or discomfort. In this same study, 4 out of 10 patients rated their oral health as fair or poor.13 Other studies have shown that gingival conditions, such as necrotizing ulcerative gingivitis or attachment loss in high school students, are similarly related to oral health–related quality of life.45
An important question in periodontal diagnosis is determining which periodontal conditions can be diagnosed as “diseased.” Can a patient with a couple of sites with 1 or 2 mm of attachment loss be classified as diseased? How about a patient with such subtle gingival inflammation that the majority of clinicians would not notice the inflammation and that even highly trained clinical examiners agree poorly on the presence of gingivitis? Disagreements on such questions is one of the reasons why the prevalence of gingivitis and destructive periodontal disease can range widely, depending on which reference levels are considered to be the cut-off for normal versus diseased.
Diagnostic Tests Available to Assess Periodontal Conditions
Diagnostic tests for periodontal disease can include anatomic measures of tissue destruction such as probing pocket depth and clinical attachment loss; measures of gingival inflammation such as redness, suppuration, bleeding, bleeding on probing, elevated gingival temperature, and a panoply of gingival crevicular fluid markers; radiographic measures of bone destruction and tooth mobility; and microbiologic measures. These test results combined with factors, such as age, past dental history, and systemic conditions, can be translated into a distinct set of periodontal diagnoses.
Translating Periodontal Diagnostic Test Results into Periodontal Disease Diagnosis
Three different methods can be distinguished to translate clinical conditions into diseases: (1) normative or arbitrary values, (2) risk-based reference values, or (3) treatment-based reference values.30
Normative or Arbitrary Values to Diagnose Periodontal Disease
Diseases can be defined based on normative or arbitrary reference values. If the normal periodontium is assumed to have no pocket depths deeper than 3 mm, then one could define destructive periodontal disease as a patient with any pocket deeper than or equal to 4 mm, or a patient with 3 pockets ≥5 mm could be classified as having destructive periodontal disease.
Alternatively, normative values could be based on parametric or nonparametric percentage cut-off values. For instance, the 97.5th percentile of the age-specific number of pockets deeper than 5 mm could be used to define destructive periodontal disease. Based on the NHANES III data, a 28-year-old individual with 2 pockets deeper than 5 mm could be diagnosed as having destructive periodontal disease, whereas 5 periodontal pockets deeper than 5 mm would be required in a 58-year-old individual.30
Diagnoses based on normative or arbitrary cut-offs result in normative or arbitrary disease prevalence levels, regardless of the distribution of underlying risk factors. Regardless of whether 5% or 95% of the population smoked 2 packs-a-day for 40 years, the prevalence of destructive periodontal disease would remain equal to the selected cut-off value. If all human chronic diseases were defined based on an arbitrary tenth percentile cut-off value, the prevalence of all chronic disease would be equal to 5% (e.g., 5% of the population would have blood pressure that is too high or 5% would have a blood glucose level that is too high).
Risk-Based Reference Values to Diagnose Periodontal Disease
The diagnosis of disease can be set at that point on the diagnostic marker in which a steep increased risk for adverse health outcomes is present. The cut-off is still somewhat arbitrary but is connected to clinical realities in terms of the risk of adverse health outcomes. There is a trade-off between the dangers of missed diagnoses when the cut-off is made too high (more specific) and the dangers of false-positive diagnoses when the cut-off is too low (more sensitive).
A risk-based diagnosis of destructive periodontal disease requires the conduct of longitudinal studies in which pocket depth at baseline is related to the risk of subsequent adverse outcomes such as tooth loss. Figure 5-1 represents such a plot and suggests that a pocket depth of 6 mm could be a diagnostic marker for destructive periodontal disease because a distinctive increased risk for tooth loss is associated with pocket depth values 6 mm or deeper.
Risk-based diagnosis of chronic diseases, similar to the use of normative or arbitrary values, can do more harm than good. A diagnosis of obesity based on a body mass index (BMI) of 28 may do more harm than good if weight loss treatments increase mortality risk.35 A diagnosis of high blood pressure57 or diabetes49 may cause more harm than good if the prescribed treatment further increases the mortality risk. Similarly, a diagnosis of destructive periodontal disease based on the presence of periodontal pockets 6 mm or deeper may cause more harm than good if the suggested periodontal treatments increase periodontal morbidity.
Therapeutic Reference Values to Diagnose Periodontal Disease
A more attractive definition of disease is the therapeutic, or treatment-based, diagnosis. Under this definition, a person is defined as diseased only if the diagnosis of disease leads to tangible benefits. Most commonly, it is best to avoid diagnosing disease unless it can be shown that the diagnosis and the subsequent treatment actually provide tangible patient outcomes. Based on such an approach to diagnoses, periodontal disease should only be diagnosed if it leads to less morbidity.
Periodontal Disease Diagnoses
The Medical Subject Heading (MESH) term headings for periodontal disease, the classification systems for periodontal diseases developed by professional organizations, and a sampling of English-language periodontal textbooks indicates that periodontal disease diagnoses come and go at a fast rate. On PubMed, seven different entry terms are currently listed under the MESH heading of Periodontitis, reflecting some of the distinct periodontal diagnoses that have been used in the literature since 1965. A recent conference consensus2 concluded that five of these seven terms listed in MESH are obsolete. The American Academy of Periodontology (AAP) reported 10 different classification systems in 20 years.4 Periodontal textbooks have similarly reported different sets of periodontal diagnoses every decade.
Periodontal dystrophies provide one example on the apparent arbitrariness by which periodontal diagnoses come and go. Periodontal dystrophies were commonly reported from the eighteenth century until the 1960s. This diagnosis was rendered obsolete after two World Conferences in the 1970s concluded that some “as yet unclassified microorganism was found deep in the periodontal pocket.”56 Consequently, subsequent periodontal textbooks no longer referred to the diagnosis of periodontosis. Recently, an argument was made that this diagnosis should be resurrected.53
This example illustrates how profoundly the belief that “periodontal disease is an infectious disease” has influenced all aspects of clinical periodontics, including the system for classifying periodontal conditions. Periodontal diagnoses are currently based on the premise that periodontal diseases “follow an infection/host paradigm in which is it held that noxious materials from dental plaque bacteria induce an inflammatory response in the adjacent periodontal tissue … Central to this paradigm is the notion that the destruction of periodontal tissues is accompanied by an inflammatory response.”3 This means that even if the inflammatory response is not seen clinically, as in periodontosis, and if the microorganism cannot be identified, as is the case for periodontosis, it can still be theorized that the infection paradigm must be true and that inflammation that cannot be clinically observed and unidentifiable microbes must be at play.
Such beliefs on “periodontal-disease-as-an-infection” for creating a diagnostic classification of periodontal diseases are questionable on two points. First, high-level evidence from epidemiologic studies is required to determine that the belief on periodontal disease as an infection is valid. The emerging evidence suggests that smoking and diabetes may cause destructive periodontal disease independent of microbial colonizations, bringing into question this premise. Second, for chronic diseases with multiple causes, it is not possible to determine the cause of the disease and therefore it is of little clinical value to name the disease after a suspected cause. For example, periodontal disease in a diabetic patient cannot be referred to diabetic periodontitis. The clinician can only say that there is certain probability that the periodontal disease in a diabetic patient can be attributed to the diabetic condition.
From a clinical perspective, the ever-changing diagnostic classification systems that result from consensus conferences are largely irrelevant, since no reliable evidence exists that clinical use of such diagnostic systems improve patient outcomes. From a clinical perspective, the classification system developed by the American Dental Association (ADA) and the AAP has more clinical relevance because it provides information to the patient with respect to severity and prognosis and is free of assumptions when it comes to potential etiologic factors. The following four periodontal diagnoses are commonly recognized in the permanent dentition:
Such simple diagnostic systems of periodontal disease have many advantages in patient care because they provide useful information on both the severity of the disease and the prognosis.
United States Prevalence Data of Periodontal Disease
The US is one of the few countries where detailed information on changes in periodontal disease prevalence has been documented with national surveys (Dye, 2005) (see Tables 5-4 and 5-5). As a result, it may be worthwhile to take a closer look at the secular disease trends in this country as it may provide useful insights what may be happening worldwide.
TABLE 5-4 Periodontal Status of the United States Population among Adults
| Adults (20–64 years) | 1988–1994 | 1999–2004 |
|---|---|---|
| Number of teeth present | 24 | 25 |
| Edentulism | 6% | 4% |
| Periodontal disease ( one site with ≥3-mm attachment loss and ≥4-mm pocket depth | 15% | 9% |
| Periodontal disease among poor | 28% | 14% |
| Dental visits | 66% | 6% |
| Mean pocket depth | 1.47 mm | 1.02 mm |
| Mean loss of attachment | 1.07 mm | 0.72 mm |
| ≥2 mm recession in at least one site | 32% | 21% |
| ≥4 mm pocket depth in at least one site | 23% | 10% |
| ≥4 mm attachment loss in at least one site | 25% | 17% |
TABLE 5-5 Periodontal Status of the United States Population among Seniors
| Seniors (65 years and older) | 1988–1994 | 1999–2004 |
|---|---|---|
| Number of teeth present | 18 | 19 |
| Edentulism | 34% | 27% |
| Periodontal disease ( one site with ≥3-mm attachment loss and ≥4-mm pocket depth | 19.5% | 10.5% |
| Periodontal disease among poor | 26.3 | 16.6 |
| Dental visits | 54% | 55% |
| Mean pocket depth | 1.47 mm | 1.07 mm |
| Mean loss of attachment | 2.04 mm | 1.55 mm |
| ≥2-mm recession in at least one site | 73% | 48% |
| ≥4-mm pocket depth in at least one site | 22% | 12% |
| ≥4-mm attachment loss in at least one site | 59% | 50% |
But, before delving into the results of this report, the issue of defining periodontal disease needs to briefly revisited here (Section X). The use of expert-definitions of periodontal disease, as opposed to evidence-based definitions, causes the same issues in challenges in interpreting national survey data, as it does in interpreting research on treatment or etiology. Consider the following example: in the 1994–2000 survey, it was reported that approximately 1 in 10 seniors 75 years and older have periodontal disease (11.3%). If 1 in 10 seniors have periodontal disease, it appears plainly impossible that 2 in 10 seniors could have moderate-to-severe periodontitis (20.75%). Yet these are the statistics that are reported. Although several explanations are possible for such apparently contradictory findings, the arbitrary definitions of periodontal disease offer one plausible explanation (see Section X). The myriad of non-evidence based decisions for defining periodontal disease allows for a myriad of prevalence estimates that are not always logically consistent with each other. These expert decisions include which periodontal outcome measures to select, which cut-off values to choose for each of the selected outcome measures, at which periodontal locations these particular conditions need to be present, and whether these conditions need to co-occur at the same site. This multiplicity of choices is problematic for much of the periodontal research, including national surveys and can make prevalence estimates swing widely.
Regardless of these challenges in interpreting national survey data, the reported changes in periodontal disease parameters between the NHANES 1998–2004 survey and the 1994–2004 survey—and other sources of data—consistently suggest substantial improvements in periodontal disease prevalence (at least in terms of pocket depth and attachment levels) (Dye, 2005). Among adults, edentulism dropped by 2%, average number of teeth present in the oral cavity increased by one tooth per mouth, prevalence of moderate-to-severe periodontitis dropped by 4.3%, and prevalence of dental visits dropped by 6%. Among poor adults, prevalence of periodontitis dropped by 14%. Similar large changes occurred among the seniors. Edentulism dropped by 7%, average number of teeth present in the oral cavity increased by one tooth, prevalence of moderate-to-severe periodontitis dropped by 9%, and prevalence of dental visits remained unchanged. Among the poor seniors, prevalence of periodontitis dropped by almost 10%. If it would turn out that periodontal pockets are a valid surrogate for periodontal disease, these data imply that a fraction of the observed reduction in edentulism and tooth loss in the US population may be due to a reduction in periodontal disease severity.
Although relating population-level statistics to suspected causal factors can be misleading, hypotheses regarding the causes of these rapid changes can be analyzed. Large declines in periodontal disease prevalence appear difficult to explain by improved periodontal care or more effective periodontal procedures. The dramatic declines in periodontal disease prevalence are most pronounced among those with the least access to dental care: the poor. Moreover, these large declines occurred when studies reported prior large decreases in periodontal treatment utilization. A more plausible explanation for the decrease in periodontal disease prevalence is cigarette smoking. The large declines may at least in part be attributed to the continued decline in smoking. While these surveys were conducted, the prevalence of current smokers in the United States continued to decline (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm). Worldwide changes in smoking habits and periodontitis prevalence will provide further opportunities to either refute or confirm cigarette smoking as a primary driver of the worldwide periodontitis epidemic. Other suspected etiological factors of periodontal disease appear opposite of expectations. Two suspected causal factors of periodontal disease, diabetes and obesity, increased during this decade suggesting that the prevalence of periodontal pockets and periodontal attachment loss would similarly increase. Yet the opposite trend was documented.
Causal hypotheses based on aggregate data have led to misleading conclusions on topics such as sugar consumption and dental caries, therefore one should be cautious in generating hypotheses regarding the primary drivers of the periodontal disease epidemic based on successive national survey data. More detailed patient-level epidemiological analyses will be required to explore answers to these questions as to why the periodontal disease prevalence as measured in terms of periodontal pocket depths and attachment levels is decreasing so rapidly. However, what can be concluded is that prevalence of periodontal pockets and periodontal treatment needs were opposite of predicted: both the need and the actual utilization for periodontal services appears to be decreasing as rapidly as the disease itself is decreasing.
A final note of caution should be considered when interpreting these national prevalence data. It would be wrong to imply that the pandemic of dental diseases has been conquered. Substantial improvements have occurred in unequivocal parameters of dental suffering such as edentulism. However, dental diseases remain more prevalent than most systemic chronic diseases and remain one of the most costly chronic conditions to treat. The cost-effective primary prevention of dental diseases appears to remain an elusive goal for most civilized societies.
1 National Health and Nutrition Examination Survey, III 1988-94 (NHANES III). U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, pp. 1 CD-ROM. 1996.
2 International Workshop for a Classification of Periodontal Diseases and Conditions. Ann Periodontol. 1999;4:1-112.
3 Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9-21.
4 Baelum V, Lopez R. Defining and classifying periodontitis: need for a paradigm shift? Eur J Oral Sci. 2003;111:2-6.
5 Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133.
6 Berchier CE, Slot DE, Haps S, Van der Weijden GA. The efficacy of dental floss in addition to a toothbrush on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6:265-279.
7 Bergstrom J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92:1-8.
8 Bergstrom J, Bostrom L. Tobacco smoking and periodontal hemorrhagic responsiveness. J Clin Periodontol. 2001;28:680-685.
9 Borrell LN, Burt BA, Taylor GW. Prevalence and trends in periodontitis in the USA: the [corrected] NHANES, 1988 to 2000. J Dent Res. 2005;84:924-930.
10 Burt BA, Ismail AI, Morrison EC, Beltran ED. Risk factors for tooth loss over a 28-year period. Journal of Dental Research. 1990;69:1126-1130.
11 Carlos JP, Wolfe MD, Kingman A. The extent and severity index: a simple method for use in epidemiologic studies of periodontal disease. J Clin Periodontol. 1986;13:500-505.
12 Chen C, Slots J. The current status and future prospects of altering the pathogenic microflora of periodontal disease. Curr Opin Periodontol. 1993:71-77.
13 Cunha-Cruz J, Hujoel PP, Kressin NR. Oral health-related quality of life of periodontal patients. J Periodontal Res. 2007;42:169-176.
14 Cunha-Cruz J, Hujoel PP, Maupome G, Saver B. Systemic antibiotics and tooth loss in periodontal disease. J Dent Res. 2008;87:871-876.
15 DeRouen TA, Hujoel PP, Mancl LA. Statistical issues in periodontal research. J Dent Res. 1995;74:1731-1737.
16 DeRouen TA, Mancl L, Hujoel P. Measurement of associations in periodontal diseases using statistical methods for dependent data. J Periodontal Res. 1991;26:218-229.
17 Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat. 2007;11(248):1-92.
18 Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605-613.
19 Gelskey SC. Cigarette smoking and periodontitis: methodology to assess the strength of evidence in support of a causal association. Community Dent Oral Epidemiol. 1999;27:16-24.
20 Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8:115-181.
21 Haps S, Slot DE, Berchier CE, Van der Weijden GA. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6:290-303.
22 Holt SC, Ebersole J, Felton J, et al. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55-57.
23 Hujoel PP. Definitive versus Exploratory Periodontal Trials: A Survey of Published Studies. J Dent Res. 1995;74:1453-1459.
24 Hujoel PP. Dietary carbohydrates and dental-systemic disease. J Dent Res. 2009;88:490-502.
25 Hujoel PP. Endpoints in periodontal trials: the need for an evidence-based research approach. Periodontol 2000. 2004;36:196-204.
26 Hujoel PP, Sample size, Hypotheses and Clinical Significance; Limiting False Conclusions, Giannobile WVG, Burt R, editors, Clinical Research in Oral Health, 2009
27 Hujoel PP, Baab DA, DeRouen TA. Measures of treatment efficacy. J Clin Periodontol. 1993;20:601-605.
28 Hujoel PP, Bergström J, del Aguila MA, DeRouen TA. A hidden periodontitis epidemic during the 20th century? Community Dent Oral Epidemiol. 2002;30:1-6.
29 Hujoel PP, Cunha-Cruz J, Loesche WJ, Robertson PB. Personal oral hygiene and chronic periodontitis: a systematic review. Periodontol 2000. 2005;37:29-34.
30 Hujoel PP, Cunha-Cruz J, Selipsky H, Saver BG. Abnormal pocket depth and gingival recession as distinct phenotypes. Periodontol 2000. 2005;39:22-29.
31 Hujoel PP, DeRouen TA. A survey of endpoint characteristics in periodontal clinical trials published 1988-1992, and implications for future studies. J Clin Periodontol. 1995;22:397-407.
32 Hujoel PP, Loesche WJ, DeRouen TA. Assessment of relationships between site-specific variables. J Periodontol. 1990;61:368-372.
33 Hujoel PP, Weyant RJ, DeRouen TA. Measures of dental disease occurrence. Community Dent Oral Epidemiol. 1991;19:252-256.
34 Joshipura KJ, Rimm EB, Douglass CW, et al. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631-1636.
35 Kassirer JP, Angell M. Losing weight–an ill-fated New Year’s resolution. N Engl J Med. 1998;338:52-54.
36 Krall EA, Dawson-Hughes B, Garvey AJ, Garcia RI. Smoking, smoking cessation, and tooth loss. J Dent Res. 1997;76:1653-1659.
37 Krall EA, Dietrich T, Nunn ME, Garcia RI. Risk of tooth loss after cigarette smoking cessation. Prev Chronic Dis. 2006;3:A115.
38 Leao A, Sheiham A. The development of a socio-dental measure of dental impacts on daily living. Community Dent Health. 1996;13:22-26.
39 Lee CL, Hsieh KS, Ko YC. Trends in the incidence of hepatocellular carcinoma in boys and girls in Taiwan after large-scale hepatitis B vaccination. Cancer Epidemiol Biomarkers Prev. 2003;12:57-59.
40 Levy D, Brink S. A change of heart: how the Framingham heart study helped unravel the mysteries of cardiovascular disease. New York: Knopf: Distributed by Random House; 2005.
41 Lindhe J, Karring T, Lang NP. Clinical periodontology and implant dentistry. Oxford, UK; Malden, MA: Blackwell; 2003.
42 Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 38(Suppl), 1967.
43 Loe H, Silness J. Periodontal disease in pregnancy. Acat Odontologica Scandinavica. 1963;21:533-551.
44 Löe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36:177-187.
45 Lopez R, Baelum V. Oral health impact of periodontal diseases in adolescents. J Dent Res. 2007;86:1105-1109.
46 Lopez R, Scheutz F, Errboe M, Baelum V. Selection bias in case-control studies on periodontitis: a systematic review. Eur J Oral Sci. 2007;115:339-343.
47 http://www.mayoclinic.com/health/periodontitis/DS00369, Access date: June 19, 2009
48 McGrath C, Bedi R. An evaluation of a new measure of oral health related quality of life–OHQoL-UK(W). Community Dent Health. 2001;18:138-143.
49 Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789-830.
50 Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885-1894.
51 Nery EB, Lee KK, Czajkowski S, et al. A Veterans Administration Cooperative Study of biphasic calcium phosphate ceramic in periodontal osseous defects. J Periodontol. 1990;61:737-744.
52 O’Connor CM, Dunne MW, Pfeffer MA, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. Jama. 2003;290:1459-1466.
53 Page RC, Sturdivant EC. Noninflammatory destructive periodontal disease (NDPD). Periodontol 2000. 2002;30:24-39.
54 Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol. 2005;76:2187-2193.
55 Preshaw PM, Heasman L, Stacey F, et al. The effect of quitting smoking on chronic periodontitis. J Clin Periodontol. 2005;32:869-879.
56 Prichard JF. The diagnosis and treatment of periodontal disease. Philadelphia: Saunders; 1979.
57 Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. Jama. 1995;274:620-625.
58 Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30:51-59.
59 Rothman KJ: Modern Epidemiology, Little, Brown and Company
60 Russell AL. A system of classification and scoring for prevalence surveys of periodontal disease. J Dent Res. 1956;35:350-359.
61 Ryan ME, Carnu O, Kamer A. The influence of diabetes on the periodontal tissues. J Am Dent Assoc. 2003;134:34S-40S. Spec No
62 Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36:458-467.
63 Sicilia A, Arregui I, Gallego M, et al. A systematic review of powered vs manual toothbrushes in periodontal cause-related therapy. J Clin Periodontol. 2002;29(Suppl 3):39-54. discussion 90-91
64 Skamagas M, Breen TL, LeRoith D. Update on diabetes mellitus: prevention, treatment, and association with oral diseases. Oral Dis. 2008;14:105-114.
65 Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11:3-11.
66 Slot DE, Dorfer CE, Van der Weijden GA. The efficacy of interdental brushes on plaque and parameters of periodontal inflammation: a systematic review. Int J Dent Hyg. 2008;6:253-264.
67 Temple LK, McLeod RS, Gallinger S, Wright JG. Essays on science and society. Defining disease in the genomics era. Science. 2001;293:807-808.
68 Temple RJ. A regulatory authority’s opinion about surrogate endpoints. In: Nimmo WS, Tucker GT, editors. Clinical Measurement in Drug Evaluation. New York: J Wiley, 1995.
69 The American Academy of Periodontology: Causes of gum Disease, Chicago. 2009.
70 Theilade E. This week’s citation classic. Current Contents. 16, 1987.
71 Genco RJ, Goldman HM, Cohen DW. Contemporary periodontics. St. Louis: Mosby; 1990.
72 Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654-2664.