CHAPTER 2 Anatomy of the Periodontium
The oral mucosa consists of the following three zones:
Clinical Features
In an adult, normal gingiva covers the alveolar bone and tooth root to a level just coronal to the cementoenamel junction. The gingiva is divided anatomically into marginal, attached, and interdental areas. Although each type of gingiva exhibits considerable variation in differentiation, histology, and thickness according to its functional demands, all types are specifically structured to function appropriately against mechanical and microbial damage.4 That is, the specific structure of different gingiva reflects its effectiveness as a barrier to the penetration by microbes and noxious agents into the deeper tissue.
Marginal Gingiva
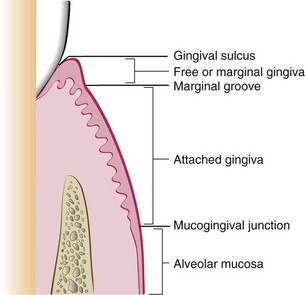
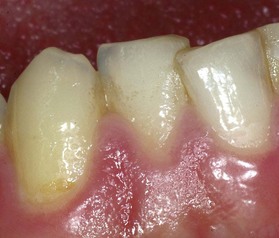
The marginal, or unattached, gingiva is the terminal edge or border of the gingiva surrounding the teeth in collarlike fashion (Figures 2-1 and 2-2). In about 50% of cases, it is demarcated from the adjacent attached gingiva by a shallow linear depression, the free gingival groove.3 Usually about 1 mm wide, the marginal gingiva forms the soft tissue wall of the gingival sulcus. It may be separated from the tooth surface with a periodontal probe.
Figure 2-1 Normal gingiva in a young adult. Note the demarcation (mucogingival line) (arrows) between the attached gingiva and the darker alveolar mucosa.
The most apical point of the marginal gingival scallop is called gingival zenith. Its apicocoronal and mesiodistal dimensions varied between 0.06 and 0.96 mm.81
Gingival Sulcus
The gingival sulcus is the shallow crevice or space around the tooth bounded by the surface of the tooth on one side and the epithelium lining the free margin of the gingiva on the other side. It is V-shaped and barely permits the entrance of a periodontal probe. The clinical determination of the depth of the gingival sulcus is an important diagnostic parameter. Under absolutely normal or ideal conditions, the depth of the gingival sulcus is 0 mm or close to 0 mm.50 These strict conditions of normalcy can be produced experimentally only in germ-free animals or after intense, prolonged plaque control.8,23
In clinically healthy human gingiva, a sulcus of some depth can be found. The depth of this sulcus, as determined in histologic sections, has been reported as 1.8 mm, with variations from 0 to 6 mm90; other studies have reported 1.5 mm136 and 0.69 mm.47 The clinical evaluation used to determine the depth of the sulcus involves the introduction of a metallic instrument, the periodontal probe, and the estimation of the distance it penetrates. The histologic depth of a sulcus does not need to be exactly equal to the depth of penetration of the probe. The so-called probing depth of a clinically normal gingival sulcus in humans is 2 to 3 mm (see Chapter 30).
Attached Gingiva
The attached gingiva is continuous with the marginal gingiva. It is firm, resilient, and tightly bound to the underlying periosteum of alveolar bone. The facial aspect of the attached gingiva extends to the relatively loose and movable alveolar mucosa and is demarcated by the mucogingival junction (see Figure 2-2).
The width of the attached gingiva is another important clinical parameter. It is the distance between the mucogingival junction and the projection on the external surface of the bottom of the gingival sulcus or the periodontal pocket. It should not be confused with the width of the keratinized gingiva because the latter also includes the marginal gingiva (see Figure 2-2).
The width of the attached gingiva on the facial aspect differs in different areas of the mouth.16 It is generally greatest in the incisor region (3.5 to 4.5 mm in maxilla, 3.3 to 3.9 mm in mandible), and narrower in the posterior segments (1.9 mm in maxillary and 1.8 mm in mandibular first premolars)3 (Figure 2-3).
Because the mucogingival junction remains stationary throughout adult life,1 changes in the width of the attached gingiva are caused by modifications in the position of its coronal portion. The width of the attached gingiva increases with age4 and in supraerupted teeth.2 On the lingual aspect of the mandible, the attached gingiva terminates at the junction of the lingual alveolar mucosa, which is continuous with the mucous membrane lining the floor of the mouth. The palatal surface of the attached gingiva in the maxilla blends imperceptibly with the equally firm and resilient palatal mucosa.
Interdental Gingiva
The interdental gingiva occupies the gingival embrasure, which is the interproximal space beneath the area of tooth contact. The interdental gingiva can be pyramidal or can have a “col” shape. In the former the tip of one papilla is located immediately beneath the contact point; the latter presents a valleylike depression that connects a facial and lingual papilla and conforms to the shape of the interproximal contact31 (Figures 2-4 and 2-5).
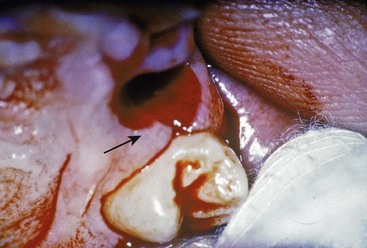
Figure 2-4 Site of extraction showing the facial and palatal interdental papillae and the intervening col (arrow).
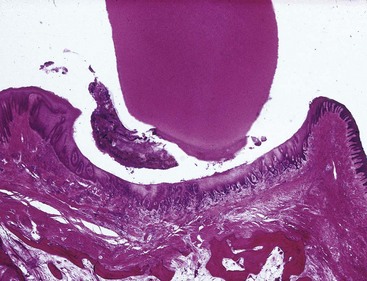
Figure 2-5 Faciolingual section (monkey) showing col between the facial and lingual interdental papillae. The col is covered with nonkeratinized stratified squamous epithelium.
The shape of the gingiva in a given interdental space depends on the contact point between the two adjoining teeth and the presence or absence of some degree of recession. Figure 2-6 depicts the variations in normal interdental gingiva.
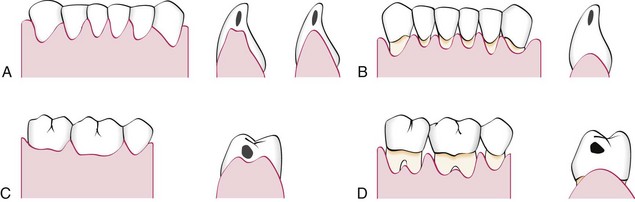
Figure 2-6 Diagram comparing anatomic variations of the interdental col in the normal gingiva (left side) and after gingival recession (right side). A and B, Mandibular anterior segment, facial and buccolingual views, respectively. C and D, Mandibular posterior region, facial and buccolingual views, respectively. Tooth contact points are shown by black marks in lower individual teeth.
The facial and lingual surfaces are tapered toward the interproximal contact area, whereas the mesial and distal surfaces are slightly concave. The lateral borders and tips of the interdental papillae are formed by the marginal gingiva of the adjoining teeth. The intervening portion consists of attached gingiva (Figure 2-7).
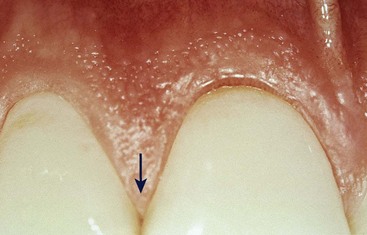
Figure 2-7 Interdental papillae (arrow) with central portion formed by attached gingiva. The shape of the papillae varies according to the dimension of the gingival embrasure.
(Courtesy Dr. Osvaldo Costa.)
If a diastema is present, the gingiva is firmly bound over the interdental bone and forms a smooth, rounded surface without interdental papillae (Figure 2-8).
Microscopic Features
Microscopic examination reveals that gingiva is composed of the overlying stratified squamous epithelium and the underlying central core of connective tissue. Although the epithelium is predominantly cellular in nature, the connective tissue is less cellular and composed primarily of collagen fibers and ground substance. These two tissues are considered separately.*
Gingival Epithelium
General Aspects of Gingival Epithelium Biology
Historically, the epithelial compartment was thought to provide only a physical barrier to infection and the underlying gingival attachment. However, we now believe that epithelial cells play an active role in innate host defense by responding to bacteria in an interactive manner,33 which means the epithelium participates actively in responding to infection, in signaling further host reactions, and in integrating innate and acquired immune responses. For example, epithelial cells may respond to bacteria by increased proliferation, alteration of cell-signaling events, changes in differentiation and cell death, and ultimately, alteration of tissue homeostasis.33 To understand this new perspective of the epithelial innate defense responses and the role of epithelium in gingival health and disease, it is important to understand its basic structure and function (Box 2-1).
BOX 2-1 Modified from Dale BA: Periodontol 2000 30:71, 2002.
Functions and Features of Gingival Epithelium
The gingival epithelium consists of a continuous lining of stratified squamous epithelium, and the three different areas can be defined from the morphologic and functional points of view: the oral or outer epithelium, sulcular epithelium, and junctional epithelium.
The principal cell type of the gingival epithelium, as well as of other stratified squamous epithelia, is the keratinocyte. Other cells found in the epithelium are the clear cells or nonkeratinocytes, which include the Langerhans cells, Merkel cells, and melanocytes.
The main function of the gingival epithelium is to protect the deep structures, while allowing a selective interchange with the oral environment. This is achieved by proliferation and differentiation of the keratinocytes.
Proliferation of keratinocytes takes place by mitosis in the basal layer and less frequently in the suprabasal layers, in which a small proportion of cells remain as a proliferative compartment while a larger number begin to migrate to the surface.
Differentiation involves the process of keratinization, which consists of progressions of biochemical and morphologic events that occur in the cell as they migrate from the basal layer (Figure 2-9). The main morphologic changes are (1) progressive flattening of the cell with an increasing prevalence of tonofilaments, (2) intercellular junctions coupled to the production of keratohyaline granules, and (3) disappearance of the nucleus. (See Schroeder105 for further details.)
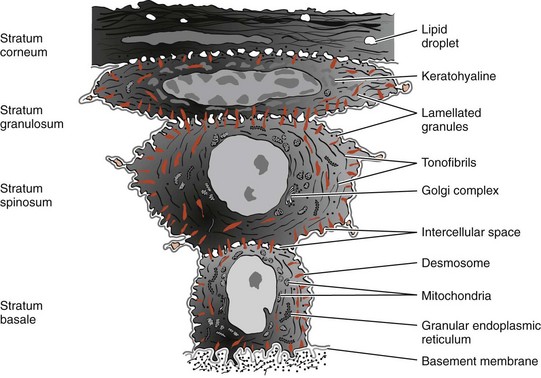
Figure 2-9 Diagram showing representative cells from the various layers of stratified squamous epithelium as seen by electron microscopy.
(Modified from Weinstock A: In Ham AW: Histology, ed 7, Philadelphia, 1974, Lippincott.)
A complete keratinization process leads to the production of an orthokeratinized superficial horny layer similar to that of the skin, with no nuclei in the stratum corneum and a well-defined stratum granulosum (Figure 2-10). Only some areas of the outer gingival epithelium are orthokeratinized; the other gingival areas are covered by parakeratinized or nonkeratinized epithelium,20 considered to be at intermediate stages of keratinization. These areas can progress to maturity or dedifferentiate under different physiologic or pathologic conditions.
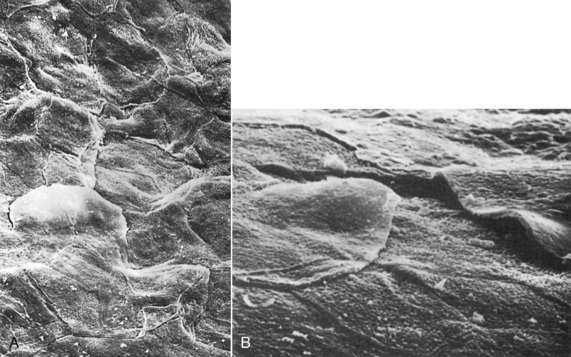
Figure 2-10 A, Scanning electron micrograph of keratinized gingiva showing the flattened keratinocytes and their boundaries on the surface of the gingiva. (×1000.) B, Scanning electron micrograph of gingival margin at edge of gingival sulcus showing several keratinocytes about to be exfoliated. (×3000.)
(From Kaplan GB, Pameijer CH, Ruben MP: J Periodontol 48:446, 1977.)
In parakeratinized epithelia the stratum corneum retains pyknotic nuclei, and the keratohyalin granules are dispersed, not giving rise to a stratum granulosum. The nonkeratinized epithelium (although cytokeratins are the major component, as in all epithelia) has neither granulosum nor corneum strata, whereas superficial cells have viable nuclei.
Immunohistochemistry, gel electrophoresis, and immunoblot techniques have made identification of the characteristic pattern of cytokeratins possible in each epithelial type. The keratin proteins are composed of different polypeptide subunits characterized by their isoelectric points and molecular weights. They are numbered in a sequence contrary to their molecular weight. Generally, basal cells begin synthesizing lower-molecular-weight keratins, such as K19 (40 kD), and express other higher-molecular-weight keratins as they migrate to the surface. K1 keratin polypeptide (68 kD) is the main component of the stratum corneum.29
Other proteins unrelated to keratins are synthesized during the maturation process. The most extensively studied are keratolinin and involucrin, which are precursors of a chemically resistant structure (the envelope) located below the cell membrane, and filaggrin, whose precursors are packed into the keratohyalin granules. In the sudden transition to the horny layer, the keratohyalin granules disappear and give rise to filaggrin, which forms the matrix of the most differentiated epithelial cell, the corneocyte.
Thus, in the fully differentiated state, the corneocytes are mainly formed by bundles of keratin tonofilaments embedded in an amorphous matrix of filaggrin and are surrounded by a resistant envelope under the cell membrane. The immunohistochemical patterns of the different keratin types, envelope proteins, and filaggrin change under normal or pathologic stimuli, modifying the keratinization process.57-59
Electron microscopy reveals that keratinocytes are interconnected by structures on the cell periphery called desmosomes.70 These desmosomes have a typical structure consisting of two dense attachment plaques into which tonofibrils insert and an intermediate, electron-dense line in the extracellular compartment. Tonofilaments, which are the morphologic expression of the cytoskeleton of keratin proteins, radiate in brushlike fashion from the attachment plaques into the cytoplasm of the cells. The space between the cells shows cytoplasmic projections resembling microvilli that extend into the intercellular space and often interdigitate.
Less frequently observed forms of epithelial cell connections are tight junctions (zonae occludens), in which the membranes of the adjoining cells are believed to be fused.127,134 Evidence suggests that these structures allow ions and small molecules to pass from one cell to another.
Cytoplasmic organelle concentration varies among different epithelial strata. Mitochondria are more numerous in deeper strata and decrease toward the surface of the cell.
Accordingly, histochemical demonstration of succinic dehydrogenase, nicotinamide-adenine dinucleotide, cytochrome oxidase, and other mitochondrial enzymes reveals a more active tricarboxylic cycle in basal and parabasal cells, in which the proximity of the blood supply facilitates energy production through aerobic glycolysis.
Conversely, enzymes of the pentose shunt (an alternative pathway of glycolysis), such as glucose-6-phosphatase, increase their activity toward the surface. This pathway produces a larger amount of intermediate products for the production of ribonucleic acid (RNA), which in turn can be used for the synthesis of keratinization proteins. This histochemical pattern is in accordance with the increased volume and the amount of tonofilaments observed in cells reaching the surface; the intensity of the activity is proportional to the degree of differentiation.36,43,56,93
The uppermost cells of the stratum spinosum contain numerous dense granules, keratinosomes or Odland bodies, which are modified lysosomes. They contain a large amount of acid phosphatase, an enzyme involved in the destruction of organelle membranes, which occurs suddenly between the granulosum and corneum strata and during the intercellular cementation of cornified cells. Thus acid phosphatase is another enzyme closely related to the degree of keratinization.18,54,132
Nonkeratinocyte cells are present in gingival epithelium as in other malpighian epithelia. Melanocytes are dendritic cells located in the basal and spinous layers of the gingival epithelium. They synthesize melanin in organelles called premelanosomes or melanosomes30,102,120 (Figure 2-11). These contain tyrosinase, which hydroxylates tyrosine to dihydroxyphenylalanine (dopa), which in turn is progressively converted to melanin. Melanin granules are phagocytosed and contained within other cells of the epithelium and connective tissue called melanophages or melanophores.
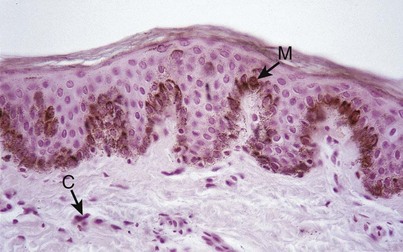
Figure 2-11 Pigmented gingiva of dog showing melanocytes (M) in the basal epithelial layer and melanophores (C) in the connective tissue (Glucksman technique).
Langerhans cells are dendritic cells located among keratinocytes at all suprabasal levels (Figure 2-12). They belong to the mononuclear phagocyte system (reticuloendothelial system) as modified monocytes derived from the bone marrow. They contain elongated granules and are considered macrophages with possible antigenic properties.36 Langerhans cells have an important role in the immune reaction as antigen-presenting cells for lymphocytes. They contain g-specific granules (Birbeck’s granules) and have marked adenosine triphosphatase activity. They are found in oral epithelium of normal gingiva and in smaller amounts in the sulcular epithelium; they are probably absent from the junctional epithelium of normal gingiva.
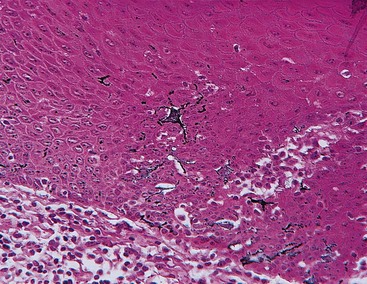
Figure 2-12 Human gingival epithelium, oral aspect. Immunoperoxidase technique showing Langerhans cells.
Merkel cells are located in the deeper layers of the epithelium, harbor nerve endings, and are connected to adjacent cells by desmosomes. They have been identified as tactile perceptors.87
The epithelium is joined to the underlying connective tissue by a basal lamina 300 to 400 Å thick, lying approximately 400 Å beneath the epithelial basal layer.66,110,121 The basal lamina consists of lamina lucida and lamina densa. Hemidesmosomes of the basal epithelial cells abut the lamina lucida, which is mainly composed of the glycoprotein laminin. The lamina densa is composed of type IV collagen. The basal lamina, clearly distinguishable at the ultrastructural level, is connected to a reticular condensation of the underlying connective tissue fibrils (mainly collagen type IV) by the anchoring fibrils.86,95,124 Anchoring fibrils have been measured at 750 nm in length from their epithelial end to their connective tissue end, in which they appear to form loops around collagen fibers. The complex of basal lamina and fibrils is the periodic acid–Schiff (PAS)–positive and argyrophilic line observed at the optical level112,125 (Figure 2-13). The basal lamina is permeable to fluids but acts as a barrier to particulate matter.
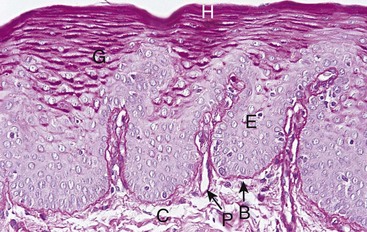
Figure 2-13 Normal human gingiva stained with the periodic acid–Schiff (PAS) histochemical method. The basement membrane (B) is seen between the epithelium (E) and the underlying connective tissue (C). In the epithelium, glycoprotein material occurs in cells and cell membranes of the superficial hornified (H) and underlying granular layers (G). The connective tissue presents a diffuse, amorphous ground substance and collagen fibers. The blood vessel walls stand out clearly in the papillary projections of the connective tissue (P).
Structural and Metabolic Characteristics of Different Areas of Gingival Epithelium
The epithelial component of the gingiva shows regional morphologic variations that reflect tissue adaptation to the tooth and alveolar bone.106 These variations include the oral epithelium, sulcular epithelium, and junctional epithelium. Whereas the oral epithelium and sulcular epithelium are largely protective in function, the junctional epithelium serves many more roles and is of considerable importance in regulating tissue health.11 It is now recognized that epithelial cells are not “passive bystanders” in the gingival tissues; rather, they are metabolically active and capable of reacting to external stimuli by synthesizing a number of cytokines, adhesion molecules, growth factors, and enzymes.11
Oral (Outer) Epithelium
The oral, or outer, epithelium covers the crest and outer surface of the marginal gingiva and the surface of the attached gingiva. On average, the oral epithelium is 0.2 to 0.3 mm in thickness. It is keratinized or parakeratinized or presents various combinations of these conditions (Figure 2-14). The prevalent surface, however, is parakeratinized.14,20,133
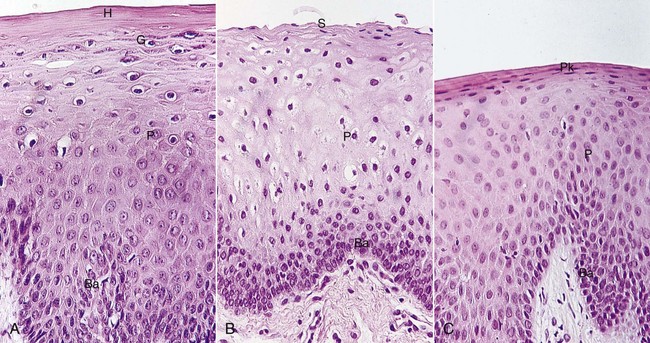
Figure 2-14 Variations in gingival epithelium. A, Keratinized. B, Nonkeratinized. C, Parakeratinized. Horny layer (H), granular layer (G), prickle cell layer (P), basal cell layer (Ba), flattened surface cells (S), parakeratotic layer (Pk).
The oral epithelium is composed of four layers: stratum basale (basal layer), stratum spinosum (prickle cell layer), stratum granulosum (granular layer), and stratum corneum (cornified layer).
The degree of gingival keratinization diminishes with age and the onset of menopause92 but is not necessarily related to the different phases of the menstrual cycle.60 Keratinization of the oral mucosa varies in different areas in the following order: palate (most keratinized), gingiva, ventral aspect of the tongue, and cheek (least keratinized).84
Keratins K1, K2, and K10 to K12, which are specific to epidermal-type differentiation, are immunohistochemically expressed with high intensity in orthokeratinized areas and with less intensity in parakeratinized areas. K6 and K16, characteristic of highly proliferative epithelia, and K5 and K14, stratification-specific cytokeratins, also are present. Parakeratinized areas express K19, which is usually absent from orthokeratinized normal epithelia.15,100
In keeping with the complete or almost-complete maturation, histoenzyme reactions for acid phosphatase and pentose-shunt enzymes are very strong.19,56
Glycogen can accumulate intracellularly when it is not completely degraded by any of the glycolytic pathways. Thus its concentration in normal gingiva is inversely related to the degree of keratinization111,133 and inflammation.35,129,131
Sulcular Epithelium
The sulcular epithelium lines the gingival sulcus (Figure 2-15). It is a thin, nonkeratinized stratified squamous epithelium without rete pegs, and it extends from the coronal limit of the junctional epithelium to the crest of the gingival margin (Figure 2-16). It usually shows many cells with hydropic degeneration.14
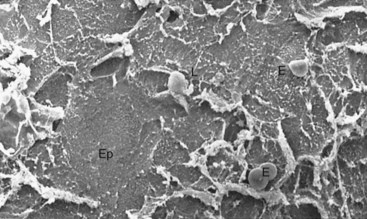
Figure 2-15 Scanning electron microscopic view of epithelial surface facing the tooth in a normal human gingival sulcus. The epithelium (Ep) shows desquamating cells, some scattered erythrocytes (E), and a few emerging leukocytes (L). (×1000.)
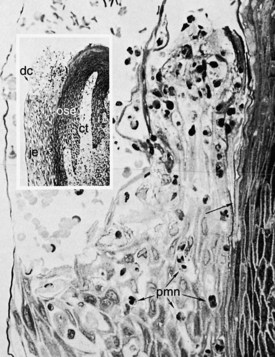
Figure 2-16 Epon-embedded human biopsy specimen showing a relatively normal gingival sulcus. The soft tissue wall of the gingival sulcus is made up of the oral sulcular epithelium (ose) and its underlying connective tissue (ct), whereas the base of the gingival sulcus is formed by the sloughing surface of the junctional epithelium (je). The enamel space is delineated by a dense cuticular structure (dc). A relatively sharp line of demarcation exists between the junctional epithelium and the oral sulcular epithelium (arrow), and several polymorphonuclear leukocytes (pmn) can be seen traversing the junctional epithelium. The sulcus contains red blood cells resulting from the hemorrhage occurring at the time of biopsy. (×391; inset ×55.)
(From Schluger S, Youdelis R, Page RC: Periodontal disease, Philadelphia, 1977, Lea & Febiger.)
As with other nonkeratinized epithelia, the sulcular epithelium lacks granulosum and corneum strata and K1, K2, and K10 to K12 cytokeratins, but it contains K4 and K13, the so-called esophageal-type cytokeratins. It also expresses K19 and normally does not contain Merkel cells.
Histochemical studies of enzymes have consistently revealed a lower degree of activity in the sulcular than in the outer epithelium, particularly in the case of enzymes related to keratinization. Glucose-6-phosphate dehydrogenase expressed a faint and homogeneous reaction in all strata, unlike the increasing gradient toward the surface observed in cornified epithelia.56 Acid phosphatase staining is negative,18 although lysosomes have been described in exfoliated cells.67
Despite these morphologic and chemical characteristics, the sulcular epithelium has the potential to keratinize if (1) it is reflected and exposed to the oral cavity17,21 or (2) the bacterial flora of the sulcus is totally eliminated.22 Conversely, the outer epithelium loses its keratinization when it is placed in contact with the tooth.22 These findings suggest that the local irritation of the sulcus prevents sulcular keratinization.
The sulcular epithelium is extremely important because it may act as a semipermeable membrane through which injurious bacterial products pass into the gingival and tissue fluid from the gingiva seeps into the sulcus.126 Unlike the junctional epithelium, however, the sulcular epithelium is not heavily infiltrated by polymorphonuclear neutrophil leukocytes (PMNs), and it appears to be less permeable.11
Junctional Epithelium
The junctional epithelium consists of a collarlike band of stratified squamous nonkeratinizing epithelium. It is 3 to 4 layers thick in early life, but the number of layers increases with age to 10 or even 20 layers. Also, the junctional epithelium tapers from its coronal end, which may be 10 to 29 cells wide to 1 or 2 cells at its apical termination, located at the cementoenamel junction in healthy tissue. These cells can be grouped in two strata: the basal layer facing the connective tissue and the suprabasal layer extending to the tooth surface. The length of the junctional epithelium ranges from 0.25 to 1.35 mm.
The junctional epithelium is formed by the confluence of the oral epithelium and the reduced enamel epithelium during tooth eruption (Figure 2-17). However, the reduced enamel epithelium is not essential for its formation; in fact, the junctional epithelium is completely restored after pocket instrumentation or surgery, and it forms around an implant.69
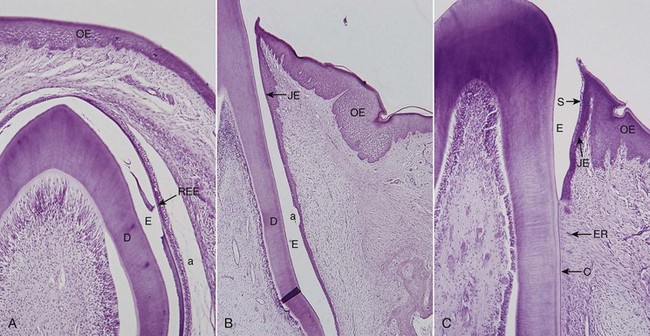
Figure 2-17 Eruption process in cat’s tooth. A, Unerupted tooth. Dentin (D), remnants of enamel matrix (E), reduced enamel epithelium (REE), oral epithelium (OE), artifact (a). B, Erupting tooth forming junctional epithelium (JE). C, Completely erupted tooth. Sulcus with epithelial debris (S), cementum (C), and epithelial rests (ER).
Cell layers not juxtaposed to the tooth exhibit numerous free ribosomes and prominent membrane-bound structures, such as Golgi complexes, and cytoplasmic vacuoles, presumably phagocytic. Lysosome-like bodies also are present, but the absence of keratinosomes (Odland bodies) and histochemically demonstrable acid phosphatase, correlated with the low degree of differentiation, may reflect a low defense power against microbial plaque accumulation in the gingival sulcus. Similar morphologic findings have been described in the gingiva of germ-free rats. PMNs are found routinely in the junctional epithelium of both conventional rats and germ-free rats.137 Research has shown that although numerous migrating PMNs are evident and present around healthy junctional epithelium, a considerable increase in PMN numbers can be expected with the accumulation of dental plaque and gingival inflammation.11
The different keratin polypeptides of junctional epithelium have a particular histochemical pattern. Junctional epithelium expresses K19, which is absent from keratinized epithelia, and the stratification-specific cytokeratins K5 and K14.100 Morgan et al85 reported that reactions to demonstrate K4 or K13 reveal a sudden change between sulcular and junctional epithelia; the junctional area is the only stratified nonkeratinized epithelium in the oral cavity that does not synthesize these specific polypeptides. Another particular behavior of junctional epithelium is the lack of expression of K6 and K16, which is usually linked to highly proliferative epithelia, although the turnover of the cells is very high.
Similar to sulcular epithelium, junctional epithelium exhibits lower glycolytic enzyme activity than outer epithelium, and it lacks acid phosphatase activity.18,56
The junctional epithelium is attached to the tooth surface (epithelial attachment) by means of an internal basal lamina. It is attached to the gingival connective tissue by an external basal lamina that has the same structure as other epithelial–connective tissue attachments elsewhere in the body.71,76 The internal basal lamina consists of a lamina densa (adjacent to the enamel) and a lamina lucida to which hemidesmosomes are attached. Hemidesmosomes have a decisive role in the firm attachment of the cells to the internal basal lamina on the tooth surface. Recent data suggest that the hemidesmosomes may also act as specific sites of signal transduction and thus may participate in regulation of gene expression, cell proliferation, and cell differentiation.61 Organic strands from the enamel appear to extend into the lamina densa.123 The junctional epithelium attaches to afibrillar cementum present on the crown (usually restricted to an area within 1 mm of the cementoenamel junction)108 and root cementum in a similar manner.
Histochemical evidence for the presence of neutral polysaccharides in the zone of the epithelial attachment has been reported.128 Data also have shown that the basal lamina of the junctional epithelium resembles that of endothelial and epithelial cells in its laminin content but differs in its internal basal lamina, which has no type IV collagen.63,99 These findings indicate that the cells of the junctional epithelium are involved in the production of laminin and play a key role in the adhesion mechanism.
The attachment of the junctional epithelium to the tooth is reinforced by the gingival fibers, which brace the marginal gingiva against the tooth surface. For this reason, the junctional epithelium and the gingival fibers are considered a functional unit, referred to as the dentogingival unit.73
In conclusion, it is usually accepted that the junctional epithelium exhibits several unique structural and functional features that contribute to preventing pathogenic bacterial flora from colonizing the subgingival tooth surface.94 First, junctional epithelium is firmly attached to the tooth surface, forming an epithelial barrier against plaque bacteria. Second, it allows access of gingival fluid, inflammatory cells, and components of the immunologic host defense to the gingival margin. Third, junctional epithelial cells exhibit rapid turnover, which contributes to the host-parasite equilibrium and rapid repair of damaged tissue. Also, some investigators indicate that the cells of the junctional epithelium have an endocytic capacity equal to that of macrophages and neutrophils and that this activity might be protective in nature.27
Development of Gingival Sulcus
After enamel formation is complete, the enamel is covered with reduced enamel epithelium (REE), which is attached to the tooth by a basal lamina and hemidesmosomes.72,122 When the tooth penetrates the oral mucosa, the REE unites with the oral epithelium and transforms into the junctional epithelium. As the tooth erupts, this united epithelium condenses along the crown, and the ameloblasts, which form the inner layer of the REE (see Figure 2-17), gradually become squamous epithelial cells. The transformation of the REE into a junctional epithelium proceeds in an apical direction without interrupting the attachment to the tooth. According to Schroeder and Listgarten,108 this process takes between 1 and 2 years.
The junctional epithelium is a continually self-renewing structure, with mitotic activity occurring in all cell layers.72,122 The regenerating epithelial cells move toward the tooth surface and along it in a coronal direction to the gingival sulcus, where they are shed12 (Figure 2-18). The migrating daughter cells provide a continuous attachment to the tooth surface. The strength of the epithelial attachment to the tooth has not been measured.
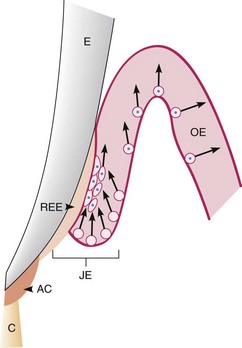
Figure 2-18 Junctional epithelium on an erupting tooth. The junctional epithelium (JE) is formed by the joining of the oral epithelium (OE) and the reduced enamel epithelium (REE). Afibrillar cementum (AC) is sometimes formed on enamel after degeneration of the REE. The arrows indicate the coronal movement of the regenerating epithelial cells, which multiply more rapidly in the JE than in the OE. E, Enamel; C, root cementum. A similar cell turnover pattern exists in the fully erupted tooth.
(Modified from Listgarten MA: J Can Dent Assoc 36:70, 1970.)
The gingival sulcus is formed when the tooth erupts into the oral cavity. At that time, the junctional epithelium and REE form a broad band attached to the tooth surface from near the tip of the crown to the cementoenamel junction.
The gingival sulcus is the shallow V-shaped space or groove between the tooth and gingiva that encircles the newly erupted tip of the crown. In the fully erupted tooth, only the junctional epithelium persists. The sulcus consists of the shallow space that is coronal to the attachment of the junctional epithelium and bounded by the tooth on one side and the sulcular epithelium on the other. The coronal extent of the gingival sulcus is the gingival margin.
Renewal of Gingival Epithelium
The oral epithelium undergoes continuous renewal. Its thickness is maintained by a balance between new cell formation in the basal and spinous layers and the shedding of old cells at the surface. The mitotic activity exhibits a 24-hour periodicity, with the highest and lowest rates occurring in the morning and evening, respectively.123 The mitotic rate is higher in nonkeratinized areas and is increased in gingivitis, without significant gender differences. Opinions differ as to whether the mitotic rate is increased75,76,83 or decreased10 with age.
The mitotic rate in experimental animals varies among different areas of the oral epithelium in descending order: buccal mucosa, hard palate, sulcular epithelium, junctional epithelium, outer surface of the marginal gingiva, and attached gingiva.6,52,75,130 The following have been reported as turnover times for different areas of the oral epithelium in experimental animals: palate, tongue, and cheek, 5 to 6 days; gingiva, 10 to 12 days, with the same or more time required with age; and junctional epithelium, 1 to 6 days.12,119
Regarding junctional epithelium, it was previously thought that only epithelial cells facing the external basal lamina were rapidly dividing. However, evidence indicates that a significant number of the cells, such as the basal cells along the connective tissue, are capable of synthesizing deoxyribonucleic acid (DNA), demonstrating their mitotic activity.97,98 Rapid shedding of cells effectively removes bacteria adhering to the epithelial cells and therefore is an important part of the antimicrobial defense mechanisms at the dentogingival junction.92
Cuticular Structures on the Tooth
The term cuticle describes a thin, acellular structure with a homogeneous matrix, sometimes enclosed within clearly demarcated, linear borders.
Listgarten74 has classified cuticular structures into coatings of developmental origin and acquired coatings. Acquired coatings include those of exogenous origin such as saliva, bacteria, calculus, and surface stains (see Chapters 22 and 23). Coatings of developmental origin are those normally formed as part of tooth development. They include the REE, coronal cementum, and dental cuticle.
After enamel formation is completed, the ameloblastic epithelium is reduced to one or two layers of cells that remain attached to the enamel surface by hemidesmosomes and a basal lamina. This REE consists of postsecretory ameloblasts and cells from the stratum intermedium of the enamel organ.
In some animal species the REE disappears entirely and very rapidly, thereby placing the enamel surface in contact with the connective tissue. Connective tissue cells then deposit a thin layer of cementum known as coronal cementum on the enamel. In humans, thin patches of afibrillar cementum sometimes may be seen in the cervical half of the crown.
Electron microscopy has shown a dental cuticle consisting of a layer of homogeneous organic material of variable thickness (approximately 0.25 µm) overlying the enamel surface. It is nonmineralized and not always present. In some cases near the cementoenamel junction, it is deposited over a layer of afibrillar cementum, which in turn overlies enamel. The cuticle may be present between the junctional epithelium and the tooth. Ultrastructural histochemical studies have shown that the dental cuticle is proteinaceous,64 and it may be an accumulation of tissue fluid components.46,107
Gingival Fluid (Sulcular Fluid)
The value of the gingival fluid is that it can be represented as either a transudate or an exudate. The gingival fluid contains a vast array of biochemical factors, offering potential use as a diagnostic or prognostic biomarker of the biologic state of the periodontium in health and disease.42 The gingival fluid contains components of connective tissue, epithelium, inflammatory cells, serum, and microbial flora inhabiting the gingival margin or the sulcus (pocket).41 In the healthy sulcus the amount of the gingival fluid is very small. During inflammation, however, the gingival fluid flow increases, and its composition start to resemble that of an inflammatory exudate.28
The main route of the gingival fluid diffusion is through the basement membrane, through the relatively wide intracellular spaces of the junctional epithelium, and then into the sulcus.94
The gingival fluid is believed to (1) cleanse material from the sulcus, (2) contain plasma proteins that may improve adhesion of the epithelium to the tooth, (3) possess antimicrobial properties, and (4) exert antibody activity to defend the gingiva (see Chapter 6).
Gingival Connective Tissue
The major components of the gingival connective tissue are collagen fibers (about 60% by volume), fibroblasts (5%), vessels, nerves, and matrix (about 35%).
The connective tissue of the gingiva is known as the lamina propria and consists of two layers: (1) a papillary layer subjacent to the epithelium, which consists of papillary projections between the epithelial rete pegs, and (2) a reticular layer contiguous with the periosteum of the alveolar bone. Connective tissue has a cellular and an extracellular compartment composed of fibers and ground substance. Thus the gingival connective tissue is largely a fibrous connective tissue that has elements originating directly from the oral mucosal connective tissue, as well as some fibers (dentogingival) that originate from the developing dental follicle.11
The ground substance fills the space between fibers and cells, is amorphous, and has a high content of water. It is composed of proteoglycans, mainly hyaluronic acid and chondroitin sulfate, and glycoproteins, mainly fibronectin. Glycoproteins account for the faint PAS-positive reaction of the ground substance.43 Fibronectin binds fibroblasts to the fibers and many other components of the intercellular matrix, helping mediate cell adhesion and migration. Laminin, another glycoprotein found in the basal lamina, serves to attach it to epithelial cells.
The three types of connective tissue fibers are collagen, reticular, and elastic. Collagen type I forms the bulk of the lamina propria and provides the tensile strength to the gingival tissue. Type IV collagen (argyrophilic reticulum fiber) branches between the collagen type I bundles and is continuous with fibers of the basement membrane and blood vessel walls.76
The elastic fiber system is composed of oxytalan, elaunin, and elastin fibers distributed among collagen fibers.26
Therefore densely packed collagen bundles that are anchored into the acellular extrinsic fiber cementum just below the terminal point of the junctional epithelium form the connective tissue attachment. The stability of this attachment is a key factor in limiting the migration of junctional epithelium.27
Gingival Fibers
The connective tissue of the marginal gingiva is densely collagenous, containing a prominent system of collagen fiber bundles called the gingival fibers. They consist of type I collagen.95 The gingival fibers have the following functions:
The gingival fibers are arranged in three groups: gingivodental, circular, and transseptal.65
Gingivodental Group
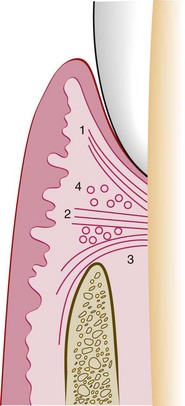
The gingivodental fibers are those on the facial, lingual, and interproximal surfaces. They are embedded in the cementum just beneath the epithelium at the base of the gingival sulcus. On the facial and lingual surfaces, they project from the cementum in fanlike conformation toward the crest and outer surface of the marginal gingiva, terminating short of the epithelium (Figures 2-19 and 2-20). They also extend externally to the periosteum of the facial and lingual alveolar bones, terminating in the attached gingiva or blending with the periosteum of the bone. Interproximally, the gingivodental fibers extend toward the crest of the interdental gingiva.
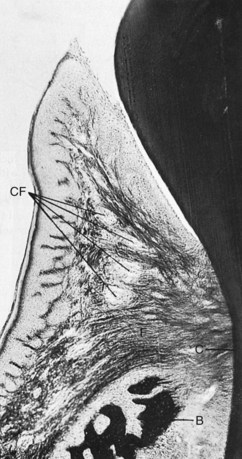
Figure 2-19 Faciolingual section of marginal gingiva showing gingival fibers (F) extending from the cementum (C) to the crest of the gingiva, to the outer gingival surface, and external to the periosteum of the bone (B). Circular fibers (CF) are shown in cross-section between the other groups.
(Courtesy Sol Bernick.)
Circular Group
The circular fibers course through the connective tissue of the marginal and interdental gingivae and encircle the tooth in ringlike fashion.
Transseptal Group
Located interproximally, the transseptal fibers form horizontal bundles that extend between the cementum of approximating teeth into which they are embedded. They lie in the area between the epithelium at the base of the gingival sulcus and the crest of the interdental bone and are sometimes classified with the principal fibers of the periodontal ligament.
Page et al91 also have described (1) a group of semicircular fibers that attach at the proximal surface of a tooth, immediately below the cementoenamel junction, go around the facial or lingual marginal gingiva of the tooth, and attach on the other proximal surface of the same tooth and (2) a group of transgingival fibers that attach in the proximal surface of one tooth, traverse the interdental space diagonally, go around the facial or lingual surface of the adjacent tooth, again traverse diagonally the interdental space, and attach in the proximal surface of the next tooth.
Tractional forces in the extracellular matrix produced by fibroblasts are believed to be the forces responsible for generating tension in the collagen. This keeps the teeth tightly bound to each other and to the alveolar bone.
Cellular Elements
The preponderant cellular element in the gingival connective tissue is the fibroblast. Numerous fibroblasts are found between the fiber bundles. Fibroblasts are of mesenchymal origin and play a major role in the development, maintenance, and repair of gingival connective tissue. As with connective tissue elsewhere in the body, fibroblasts synthesize collagen and elastic fibers, as well as the glycoproteins and glycosaminoglycans of the amorphous intercellular substance. Fibroblasts also regulate collagen degradation through phagocytosis and secretion of collagenases.
Fibroblast heterogeneity is now a well-established feature of fibroblasts in the periodontium.101 Although the biologic and clinical significance of such heterogeneity is not yet clear, it seems that this is necessary for the normal functioning of tissues in health, disease, and repair.11
Mast cells, which are distributed throughout the body, are numerous in the connective tissue of the oral mucosa and the gingiva.24,116,117,135 Fixed macrophages and histiocytes are present in the gingival connective tissue as components of the mononuclear phagocyte system (reticuloendothelial system) and are derived from blood monocytes. Adipose cells and eosinophils, although scarce, also are present in the lamina propria.
In clinically normal gingiva, small foci of plasma cells and lymphocytes are found in the connective tissue near the base of the sulcus (Figure 2-21). Neutrophils can be seen in relatively high numbers in both the gingival connective tissue and the sulcus. These inflammatory cells usually are present in small amounts in clinically normal gingiva.
Figure 2-21 Section of clinically normal gingiva showing some degree of inflammation, which is almost always present near the base of the sulcus.
Speculations about whether small amounts of leukocytes should be considered a normal component of the gingiva or an incipient inflammatory infiltrate without clinical expression are of theoretic rather than practical importance. Lymphocytes are absent when gingival normalcy is judged by strict clinical criteria or under special experimental conditions,8,89 but they are practically constant in healthy, normal gingiva, even before the complete tooth eruption.68,78,104
Immunohistochemical studies using monoclonal antibodies have identified the different lymphocyte subpopulations. The infiltrate in the area below the junctional epithelium of healthy gingiva in newly erupted teeth in children is mainly composed of T lymphocytes (helper, cytotoxic, suppressor, and natural killer)7,48,115 and thus could be interpreted as a normal lymphoid tissue involved in the early defense recognition system. As time elapses, B lymphocytes and plasma cells appear in greater proportions to elaborate specific antibodies against already-recognized antigens that are always present in the sulcus of clinically normal gingiva.109
Repair of Gingival Connective Tissue
Because of the high turnover rate, the connective tissue of the gingiva has remarkably good healing and regenerative capacity. Indeed, it may be one of the best healing tissues in the body and generally shows little evidence of scarring after surgical procedures. This is likely caused by rapid reconstruction of the fibrous architecture of the tissues.82 However, the reparative capacity of gingival connective tissue is not as great as that of the periodontal ligament or the epithelial tissue.
Blood Supply, Lymphatics, and Nerves
Microcirculatory tracts, blood vessels, and lymphatic vessels play an important role in drainage of tissue fluid and in the spread of inflammation. In gingivitis and periodontitis, the microcirculation and vascular formation change greatly in the vascular network directly under the gingival sulcular epithelium and junctional epithelium.80
![]() Science Transfer
Science Transfer
Understanding normal gingival structure and function provides clinicians with the basis for making clinical decisions that will maintain gingival health. The thick keratinized surface epithelium coupled with the dense type I collagenous gingival connective tissues must be protected from damage during periodontal and restorative therapy. The junctional epithelium and the sulcular epithelium of the gingival sulcus are frequently disrupted by periodontal procedures, such as root planing, and by restorative techniques involving the gingival margins. These epithelial layers have great reparative power; however, their recuperative protective function of providing a barrier and a seal against the tooth depends on an intact dense collagen substructure is provided by the gingival fibers. The clinician has the obligation to minimize trauma to the gingival fibers during therapy so that normal gingival form and function can be maintained.
Gingivitis is initiated by subgingival bacteria causing disruption of the barrier function of sulcular epithelium and subsequent inflammation. Patients need to be able to use oral hygiene techniques that reduce the subgingival bacterial load without causing significant damage to the epithelial structure lining the gingival sulcus. Therapists have to tailor oral hygiene instruction to each patient’s oral gingival anatomy with due regard to the underlying cellular dynamics.
Patients with a thin biotype of gingival architecture are particularly vulnerable to connective tissue loss and epithelial damage, thus they need special atraumatic treatment and oral hygiene techniques. Thick biotypes are more resilient to therapy and may be better able to be treated with more aggressive procedures without risking loss of normal gingival function.
Blood vessels are easily evidenced in tissue sections by means of immunohistochemical reactions against proteins of endothelial cells (factor VIII and adhesion molecules). Before these techniques were developed, vascularization patterns of periodontal tissues had been described using histoenzymatic reactions for alkaline phosphatase and adenosine triphosphatase because of the great activity of these enzymes in endothelial cells.25,138
In experimental animals the perfusion with India ink also was used to study vascular distribution. The injection and subsequent demonstration of peroxidase allow blood vessel identification and permeability studies.114 The PAS reaction also outlines vascular walls by a positive line in their basal membrane.112 Endothelial cells express 5-nucleotidase activity as well.55 Scanning electron microscopy can be used after injection of plastic into the vessels through the carotid artery, followed by corrosion of the soft tissues.44 In addition, laser Doppler flow measurement provides a noninvasive means to observe blood flow modifications related to disease.5
Three sources of blood supply to the gingiva are as follows (Figures 2-22 and 2-23):
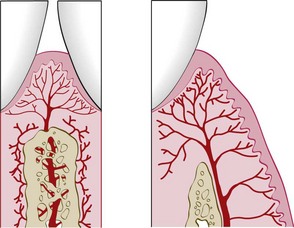
Figure 2-22 Diagram of arteriole penetrating the interdental alveolar bone to supply the interdental tissues (left) and a supraperiosteal arteriole overlying the facial alveolar bone, sending branches to the surrounding tissue (right).
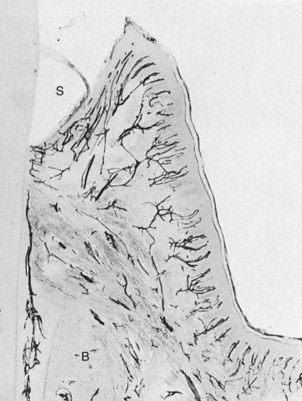
Figure 2-23 Blood supply and peripheral circulation of the gingiva. Tissues perfused with India ink. Note the capillary plexus parallel to the sulcus (S) and the capillary loops in the outer papillary layer. Note also the supraperiosteal vessels external to the bone (B), which supply the gingiva, and a periodontal ligament vessel anastomosing with the sulcus plexus.
(Courtesy Sol Bernick.)
Beneath the epithelium on the outer gingival surface, capillaries extend into the papillary connective tissue between the epithelial rete pegs in the form of terminal hairpin loops with efferent and afferent branches, spirals, and varices25,53 (Figures 2-23 and 2-24). The loops are sometimes linked by cross-communications,44 and flattened capillaries serve as reserve vessels when the circulation is increased in response to irritation.49
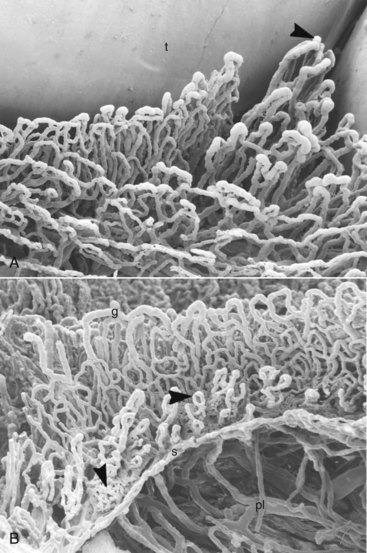
Figure 2-24 Scanning electron microscopic view of gingival tissues of rat molar palatal gingiva after vascular perfusion of plastic and corrosion of soft tissue. A, Oral view of gingival capillaries: t, tooth; interdental papilla (arrowhead). (×180.) B, View from the tooth side. Note the vessels of the plexus next to the sulcular and junctional epithelium. The arrowheads point to vessels in sulcus area with mild inflammatory changes. g, Crest of marginal gingiva; s, bottom of gingival sulcus; pl, periodontal ligament vessels. (×150.)
(Courtesy N.J. Selliseth and K. Selvig, University of Bergen, Norway.)
Along the sulcular epithelium, capillaries are arranged in a flat, anastomosing plexus that extends parallel to the enamel from the base of the sulcus to the gingival margin.25 In the col area, a mixed pattern of anastomosing capillaries and loops occurs.
As mentioned, anatomic and histologic changes have been shown to occur in the gingival microcirculation with gingivitis. Prospective studies of the gingival vasculature in animals have demonstrated that in the absence of inflammation, the vascular network is arranged in a regular, repetitive, and layered pattern.25,96 In contrast, the inflamed gingival vasculature exhibits an irregular vascular plexus pattern, with the microvessels exhibiting a looped, dilated, and convoluted appearance.96
The role of the lymphatic system in removing excess fluids, cellular and protein debris, microorganisms, and other elements is important in controlling diffusion and the resolution of inflammatory processes.79 The lymphatic drainage of the gingiva brings in the lymphatics of the connective tissue papillae.113 It progresses into the collecting network external to the periosteum of the alveolar process, then to the regional lymph nodes, particularly the submaxillary group. In addition, lymphatics just beneath the junctional epithelium extend into the periodontal ligament and accompany the blood vessels.
Neural elements are extensively distributed throughout the gingival tissues. Within the gingival connective tissues, most nerve fibers are myelinated and are closely associated with the blood vessels.77 Gingival innervation is derived from fibers arising from nerves in the periodontal ligament and from the labial, buccal, and palatal nerves.13 The following nerve structures are present in the connective tissue: a meshwork of terminal argyrophilic fibers, some of which extend into the epithelium; Meissner-type tactile corpuscles; Krause-type end bulbs, which are temperature receptors; and encapsulated spindles.9
Correlation of Clinical and Microscopic Features
An understanding of the normal clinical features of the gingiva requires the ability to interpret them in terms of the microscopic structures they represent.
Color
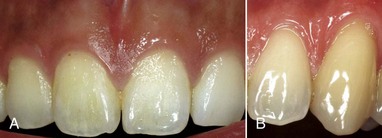
The color of the attached and marginal gingiva is generally described as “coral pink” and is produced by the vascular supply, the thickness and degree of keratinization of the epithelium, and the presence of pigment-containing cells. The color varies among different persons and appears to be correlated with the cutaneous pigmentation. It is lighter in blond individuals with fair complexion than in swarthy, dark-haired individuals (Figure 2-25).
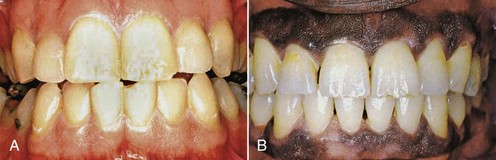
Figure 2-25 A, Clinically normal gingiva in a young adult. B, Heavily pigmented (melanotic) gingiva in a middle-aged adult.
(From Glickman I, Smulow JB: Periodontal disease: clinical, radiographic, and histopathologic features, Philadelphia, 1974, Saunders.)
The attached gingiva is demarcated from the adjacent alveolar mucosa on the buccal aspect by a clearly defined mucogingival line. The alveolar mucosa is red, smooth, and shiny rather than pink and stippled. Comparison of the microscopic structure of the attached gingiva with that of the alveolar mucosa provides an explanation for the difference in appearance. The epithelium of the alveolar mucosa is thinner, is nonkeratinized, and contains no rete pegs (Figure 2-26). The connective tissue of the alveolar mucosa is loosely arranged, and the blood vessels are more numerous.
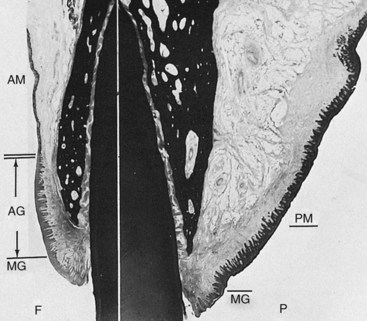
Figure 2-26 Oral mucosa, facial and palatal surfaces. The facial surface (F) shows the marginal gingiva (MG), attached gingiva (AG), and alveolar mucosa (AM). The double line marks the mucogingival junction. Note the differences in the epithelium and connective tissue in the attached gingiva and alveolar mucosa. The palatal surface (P) shows the marginal gingiva (MG) and thick, keratinized palatal mucosa (PM).
Physiologic Pigmentation (Melanin)
Melanin, a non–hemoglobin-derived brown pigment, is responsible for the normal pigmentation of the skin, gingiva, and remainder of the oral mucous membrane. It is present in all normal individuals, often not in sufficient quantities to be detected clinically, but is absent or severely diminished in albinos. Melanin pigmentation in the oral cavity is prominent in black individuals (see Figure 2-25). Ascorbic acid directly downregulates melanin pigmentation in gingival tissues.118
According to Dummett,37 the distribution of oral pigmentation in black individuals is as follows: gingiva, 60%; hard palate, 61%; mucous membrane, 22%; and tongue, 15%. Gingival pigmentation occurs as a diffuse, deep-purplish discoloration or as irregularly shaped, brown and light-brown patches. It may appear in the gingiva as early as 3 hours after birth and often is the only evidence of pigmentation.37
Oral repigmentation refers to the clinical reappearance of melanin pigment after a period of clinical depigmentation of the oral mucosa resulting from chemical, thermal, surgical, pharmacologic, or idiopathic factors.38 Information on the repigmentation of oral tissues after surgical procedures is extremely limited, and no definitive treatment is offered at this time.
Size
The size of the gingiva corresponds with the sum total of the bulk of cellular and intercellular elements and their vascular supply. Alteration in size is a common feature of gingival disease.
Contour
The contour or shape of the gingiva varies considerably and depends on the shape of the teeth and their alignment in the arch, the location and size of the area of proximal contact, and the dimensions of the facial and lingual gingival embrasures. The marginal gingiva envelops the teeth in collarlike fashion and follows a scalloped outline on the facial and lingual surfaces. It forms a straight line along teeth with relatively flat surfaces. On teeth with pronounced mesiodistal convexity (e.g., maxillary canines) or teeth in labial version, the normal arcuate contour is accentuated, and the gingiva is located farther apically. On teeth in lingual version, the gingiva is horizontal and thickened (Figure 2-27). In addition, gingival tissue biotype varies significantly. A thin and clear gingiva is found in one-third of the population, primarily in females with slender teeth with a narrow zone of keratinized tissue, whereas a clear thick gingiva with a broad zone of keratinized tissue is present in two-thirds of the population, primarily in males.34
Shape
The shape of the interdental gingiva is governed by the contour of the proximal tooth surfaces and the location and shape of gingival embrasures. When the proximal surfaces of the crowns are relatively flat faciolingually, the roots are close together, the interdental bone is thin mesiodistally, and the gingival embrasures and interdental gingiva are narrow mesiodistally. Conversely, with proximal surfaces that flare away from the area of contact, the mesiodistal diameter of the interdental gingiva is broad (Figure 2-28). The height of the interdental gingiva varies with the location of the proximal contact. Thus, in the anterior region of the dentition, the interdental papilla is pyramidal in form, whereas the papilla is more flattened in a buccolingual direction in the molar region.
Consistency
The gingiva is firm and resilient and with the exception of the movable free margin, tightly bound to the underlying bone. The collagenous nature of the lamina propria and its contiguity with the mucoperiosteum of the alveolar bone determine the firmness of the attached gingiva. The gingival fibers contribute to the firmness of the gingival margin.
Surface Texture
The gingiva presents a textured surface similar to an orange peel and is referred to as being stippled (see Figure 2-25). Stippling is best viewed by drying the gingiva.
The attached gingiva is stippled; the marginal gingiva is not. The central portion of the interdental papillae is usually stippled, but the marginal borders are smooth. The pattern and extent of stippling vary among individuals and different areas of the same mouth.51,96 Stippling is less prominent on lingual than facial surfaces and may be absent in some persons.
Stippling varies with age. It is absent in infancy, appears in some children at about 5 years of age, increases until adulthood, and frequently begins to disappear in old age.
Microscopically, stippling is produced by alternate rounded protuberances and depressions in the gingival surface. The papillary layer of the connective tissue projects into the elevations, and the elevated and depressed areas are covered by stratified squamous epithelium (Figure 2-29). The degree of keratinization and the prominence of stippling appear to be related.
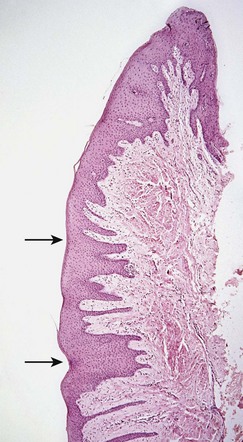
Figure 2-29 Gingival biopsy of patient shown in Figure 2-7, demonstrating alternate elevations and depressions (arrows) in the attached gingiva responsible for stippled appearance.
Scanning electron microscopy has shown considerable variation in shape, but a relatively constant depth of stippling. At low magnification, a rippled surface is seen, interrupted by irregular depressions 50 µm in diameter. At higher magnification, cell micropits are seen.30
Stippling is a form of adaptive specialization or reinforcement for function. It is a feature of healthy gingiva, and reduction or loss of stippling is a common sign of gingival disease. When the gingiva is restored to health after treatment, the stippled appearance returns.
The surface texture of the gingiva is also related to the presence and degree of epithelial keratinization. Keratinization is considered a protective adaptation to function. It increases when the gingiva is stimulated by toothbrushing. However, research on free gingival grafts (see Chapter 63) has shown that when connective tissue is transplanted from a keratinized area to a nonkeratinized area, it becomes covered by a keratinized epithelium.62 This finding suggests a connective tissue–based genetic determination of the type of epithelial surface.
Position
The position of the gingiva refers to the level at which the gingival margin is attached to the tooth. When the tooth erupts into the oral cavity, the margin and sulcus are at the tip of the crown; as eruption progresses, they are seen closer to the root. During this eruption process, as described earlier, the junctional epithelium, oral epithelium, and reduced enamel epithelium undergo extensive alterations and remodeling while maintaining the shallow physiologic depth of the sulcus. Without this remodeling of the epithelia, an abnormal anatomic relationship between the gingiva and the tooth would result.
Continuous Tooth Eruption
According to the concept of continuous eruption,50 eruption does not cease when teeth meet their functional antagonists but continues throughout life. Eruption consists of an active and a passive phase. Active eruption is the movement of the teeth in the direction of the occlusal plane, whereas passive eruption is the exposure of the teeth by apical migration of the gingiva.
This concept distinguishes between the anatomic crown (portion of the tooth covered by enamel) and the anatomic root (portion of the tooth covered by cementum) and between the clinical crown (part of the tooth that has been denuded of its gingiva and projects into the oral cavity) and clinical root (portion of the tooth covered by periodontal tissues). When the teeth reach their functional antagonists, the gingival sulcus and junctional epithelium are still on the enamel, and the clinical crown is approximately two-thirds of the anatomic crown.
Gottlieb and Orban50 believed that active and passive eruption proceed together. Active eruption is coordinated with attrition; the teeth erupt to compensate for tooth substance worn away by attrition. Attrition reduces the clinical crown and prevents it from becoming disproportionately long in relation to the clinical root, thus avoiding excessive leverage on the periodontal tissues. Ideally, the rate of active eruption keeps pace with tooth wear, preserving the vertical dimension of the dentition.
As teeth erupt, cementum is deposited at the apices and furcations of the roots and bone is formed along the fundus of the alveolus and at the crest of the alveolar bone. In this way, part of the tooth substance lost by attrition is replaced by lengthening of the root, and socket depth is maintained to support the root.
Although originally thought to be a normal physiologic process, passive eruption is now considered a pathologic process. Passive eruption is divided into the following four stages (Figure 2-30):
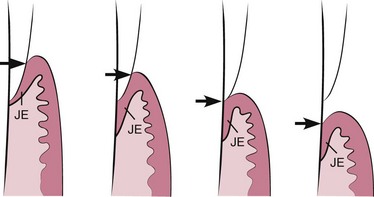
Figure 2-30 Diagrammatic representation of the four steps in passive eruption according to Gottlieb and Orban.50 1, Base of the gingival sulcus (arrow) and the junctional epithelium (JE) are on the enamel. 2, Base of the gingival sulcus (arrow) is on the enamel, and part of the junctional epithelium is on the root. 3, Base of the gingival sulcus (arrow) is at the cementoenamel line, and the entire junctional epithelium is on the root. 4, Base of the gingival sulcus (arrow) and the junctional epithelium are on the root.
As noted, apposition of bone accompanies active eruption. The distance between the apical end of the junctional epithelium and the crest of the alveolus remains constant throughout continuous tooth eruption (1.07 mm).47
Exposure of the tooth by the apical migration of the gingiva is called gingival recession, or atrophy. According to the concept of continuous eruption, the gingival sulcus may be located on the crown, cementoenamel junction, or root, depending on the age of the patient and stage of eruption. Therefore some root exposure with age would be considered normal and referred to as physiologic recession. Again, this concept is not accepted at present. Excessive exposure is termed pathologic recession (see Chapter 22).
1 Ainamo A. Influence of age on the location of the maxillary mucogingival junction. J Periodontal Res. 1978;13:189.
2 Ainamo A, Ainamo J. The width of attached gingiva on supraerupted teeth. J Periodontal Res. 1978;13:194.
3 Ainamo J, Löe H. Anatomical characteristics of gingiva: a clinical and microscopic study of the free and attached gingiva. J Periodontol. 1966;37:5.
4 Ainamo J, Talari A. The increase with age of the width of attached gingiva. J Periodontal Res. 1976;11:182.
5 Ambrosini P, Cherene S, Miller N, et al. A laser Doppler study of gingiva blood flow variations following periosteal stimulation. J Clin Periodontol. 2002;29:103.
6 Anderson GS, Stern IB. The proliferation and migration of the attachment epithelium on the cemental surface of the rat incisor. Periodontics. 1966;4:15.
7 Armitt KL. Identification of T cell subsets in gingivitis in children. Periodontology. 1986;7:3.
8 Attstrom R, Graf-de Beer M, Schroeder HE. Clinical and histologic characteristics of normal gingiva in dogs. J Periodontal Res. 1975;10:115.
9 Avery JK, Rapp R. Pain conduction in human dental tissues. Dent Clin North Am. July 1959:489.
10 Barakat NJ, Toto PD, Choukas NC. Aging and cell renewal of oral epithelium. J Periodontol. 1969;40:599.
11 Bartold PM, Walsh LJ, Narayanan AS. Molecular and cell biology of the gingiva. Periodontol 2000. 2000;24:28.
12 Beagrie GS, Skougaard MR. Observations on the life cycle of the gingival epithelial cells of mice as revealed by autoradiography. Acta Odontol Scand. 1962;20:15.
13 Bernick S. Innervation of the teeth and periodontium. Dent Clin North Am. July 1959:503.
14 Biolcati EL, Carranza FAJr, Cabrini RL. Variaciones y alteraciones de la queratinizacion en encias humanas clinicamente sanas. Rev Asoc Odontol Argent. 1953;41:446.
15 Bosch FX, Ouhayoun JP, Bader BL, et al. Extensive changes in cytokeratin expression patterns in pathologically affected human gingiva. Virchows Arch B Cell Pathol. 1989;58:59.
16 Bowers GM. A study of the width of the attached gingiva. J Periodontol. 1963;34:210.
17 Bral MM, Stahl SS. Keratinizing potential of human crevicular epithelium. J Periodontol. 1977;48:381.
18 Cabrini RL, Carranza FAJr. Histochemical study on alkaline phosphatase in gingivae, varying the pH and the substrate. J Dent Res. 1951;30:28.
19 Cabrini RL, Carranza FAJr. Histochemistry of periodontal tissues: a review of the literature. Int Dent J. 1966;16:466.
20 Cabrini R, Cabrini RL, Carranza FAJr. Estudio histologico de la queratinizacion del epitelio gingival y de la adherencia epitelial. Rev Asoc Odontol Argent. 1953;41:212.
21 Caffesse RG, Karring T, Nasjleti CE. Keratinizing potential of sulcular epithelium. J Periodontol. 1977;48:140.
22 Caffesse RG, Nasjleti CE, Castelli WA. The role of sulcular environment in controlling epithelial keratinization. J Periodontol. 1979;50:1.
23 Caffesse RG, Kornman KS, Nasjleti CE. The effect of intensive antibacterial therapy on the sulcular environment in monkeys. Part II. Inflammation, mitotic activity and keratinization of the sulcular epithelium. J Periodontol. 1980;5:155.
24 Carranza FAJr, Cabrini RL. Mast cells in human gingiva. J Oral Surg. 1955;8:1093.
25 Carranza FAJr, Itoiz ME, Cabrini RL, et al. A study of periodontal vascularization in different laboratory animals. J Periodontal Res. 1966;1:120.
26 Chavier C. Elastic fibers of healthy human gingiva. J Periodontol. 1990;9:29.
27 Cho MI, Garant PR. Development and general structure of the periodontium. Periodontol 2000. 2000;24:9.
28 Cimasoni G. Crevicular fluid updated. Monogr Oral Sci. 1983;12:1.
29 Clausen H, Moe D, Buschard K, et al. Keratin proteins in human oral mucosa. J Oral Pathol. 1986;15:36.
30 Cleaton-Jones P, Buskin SA, Volchansky A. Surface ultrastructure of human gingiva. J Periodontal Res. 1978;13:367.
31 Cohen B. Morphological factors in the pathogenesis of periodontal disease. Br Dent J. 1959;107:31.
32 Cohen L. ATPase and DOPA oxidase activity in human gingival epithelium. Arch Oral Biol. 1967;12:1241.
33 Dale BA. Periodontal epithelium: a newly recognized role in health and disease. Periodontol 2000. 2002;30:70.
34 De Rouck T, Eghbali R, Collys K, et al. The gingival biotype revisited: transparency of the periodontal probe through the g ingival margin as a method to discriminate thin from thick gingiva. J Clin Periodontol.. 2009 May;36(5):428-433.
35 Dewar MR. Observations on the composition and metabolism of normal and inflamed gingivae. J Periodontol. 1955;26:29.
36 DiFranco CF, Toto PD, Rowden G, et al. Identification of Langerhans cells in human gingival epithelium. J Periodontol. 1985;56:48.
37 Dummett CO. Physiologic pigmentation of the oral and cutaneous tissues in the Negro. J Dent Res. 1946;25:421.
38 Dummett CO, et al. Oral pigmentation: physiologic and pathologic, NY State. Dent J. 1959;25:407.
39 Egelberg J. The topography and permeability of blood vessels at the dento-gingival junction in dogs. J Periodontal Res. 1967;1:1.
40 Eichel B, Shahrik HA, Lisanti VF. Cytochemical demonstration and metabolic significance of reduced diphospho-pyridinenucleotide and triphospho-pyridinenucleotide reductases in human gingiva. J Dent Res. 1964;43:92.
41 Embery G, Waddington R. Gingival crevicular fluid: biomarkers of periodontal tissue activity. Adv Dent Res. 1994;8:329.
42 Embery G, Waddington RJ, Hall RC, et al. Connective tissue elements as diagnostic aids in periodontology. Periodontol 2000. 2000;24:193.
43 Engel MB. Water-soluble mucoproteins of the gingiva. J Dent Res. 1953;32:779.
44 Folke LE, Stallard RE. Periodontal microcirculation as revealed by plastic microspheres. J Periodontal Res. 1967;2:53.
45 Forsslund G. Structure and function of capillary system in the gingiva in man: development of stereophotogrammetric method and its application for study of the subepithelial blood vessels in vivo. Acta Odontol Scand. 1959;17:9.
46 Frank RM, Cimasoni G. On the ultrastructure of the normal human gingivo-dental junction. Z Zellforsch Mikrosk Anat. 1970;109:356.
47 Gargiulo AW, Wentz FM, Orban B. Dimensions and relations of the dentogingival junction in humans. J Periodontol. 1961;32:261.
48 Gillett R, Cruchley A, Johnson NW. The nature of the inflammatory infiltrates in childhood gingivitis, juvenile periodontitis and adult periodontitis: immunohistochemical studies using monoclonal antibody to HLADr. J Clin Periodontol. 1986;13:281.
49 Glickman I, Johannessen LB. Biomicroscopic (slit-lamp) evaluation of the normal gingiva of the albino rat. J Am Dent Assoc. 1950;41:521.
50 Gottlieb B, Orban B. Active and passive continuous eruption of teeth. J Dent Res. 1933;13:214.
51 Greene AH. A study of the characteristics of stippling and its relation to gingival health. J Periodontol. 1962;33:176.
52 Hansen ER. Mitotic activity of the gingival epithelium in colchicinized rats. Odontol Tidskr. 1966;74:229.
53 Hansson BO, Lindhe J, Branemark PI. Microvascular topography and function in clinically healthy and chronically inflamed dento-gingival tissues: a vital microscopic study in dogs. Periodontics. 1968;6:264.
54 Itoiz ME, Carranza FAJr, Cabrini RL. Histotopographic distribution of alkaline and acid phosphatase in periodontal tissues of laboratory animals. J Periodontol. 1964;35:470.
55 Itoiz ME, Carranza FAJr, Cabrini RL. Histotopographic study of esterase and 5-nucleotidase in periodontal tissues of laboratory animals. J Periodontol. 1967;38:130.
56 Itoiz ME, Carranza FAJr, Gimenez IB, et al. Microspectrophotometric analysis of succinic dehydrogenase and glucose-6-phosphate dehydrogenase in human oral epithelium. J Periodontal Res. 1972;7:14.
57 Itoiz ME, Lanfranchi HE, Gimenez-Conti IB, et al. Immunohistochemical demonstration of keratins in oral mucosa lesions. Acta Odontol Latinoam. 1984;1:47.
58 Itoiz ME, Conti CJ, Lanfranchi HE, et al. Immunohistochemical detection of filaggrin in preneoplastic and neoplastic lesions of the human oral mucosa. Am J Pathol. 1985;119:456.
59 Itoiz ME, Conti CJ, Gimenez IB, et al. Immunodetection of involucrin in lesions of the oral mucosa. J Oral Pathol. 1986;15:205.
60 Iusem R. A cytology study of the cornification of the oral mucosa in women: a preliminary report. J Oral Surg. 1950;3:1516.
61 Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488.
62 Karring T, Lang NP, Löe H. The role of gingival connective tissue in determining epithelial differentiation. J Periodontal Res. 1975;10:1.
63 Kobayashi K, Rose GG. Ultrastructural histochemistry of the dento-epithelial junction. II. Colloidal thorium and ruthenium red. J Periodontal Res. 1978;13:164.
64 Kobayashi K, Rose GG. Ultrastructural histochemistry of the dento-epithelial junction. III. Chloramine T–silver methenamine. J Periodontal Res. 1979;14:123.
65 Kronfeld R. Histopathology of the teeth and their surrounding structures. Philadelphia: Lea & Febiger; 1939.
66 Kurahashi Y, Takuma S. Electron microscopy of human gingival epithelium. Bull Tokyo Dent Coll. 1962;3:29.
67 Lange D, Camelleri GE. Cytochemical demonstration of lysosomes in exfoliated epithelial cells of the gingival cuff. J Dent Res. 1967;46:625.
68 Laurell L, Rylander H, Sundin Y. Histologic characteristics of clinically healthy gingiva in adolescents. Scand J Dent Res. 1987;95:456.
69 Lavelle CLB. Mucosal seal around endosseous dental implants. J Oral Implantol. 1981;9:357.
70 Listgarten MA. The ultrastructure of human gingival epithelium. Am J Anat. 1964;114:49.
71 Listgarten MA. Electron microscopic study of the gingivo-dental junction of man. Am J Anat. 1966;119:147.
72 Listgarten MA. Phase-contrast and electron microscopic study of the junction between reduced enamel epithelium and enamel in unerupted human teeth. Arch Oral Biol. 1966;11:999.
73 Listgarten MA. Changing concepts about the dento-gingival junction. J Can Dent Assoc. 1970;36:70.
74 Listgarten MA. Structure of surface coatings on teeth: a review. J Periodontol. 1976;47:139.
75 Löe H, Karring T. Mitotic activity and renewal time of the gingival epithelium of young and old rats. J Periodontal Res. 1969;4:18.
76 Löe H, Karring T. A quantitative analysis of the epithelium–connective tissue interface in relation to assessments of the mitotic index. J Dent Res. 1969;48:634.
77 Luthman J, Johansson O, Ahlström U, et al. Immunohistochemical studies of the neurochemical markers CGRP, enkephalin, galanin, gamma-MSH, NPY, PHI, proctolin, PTH, somatostatin, SP, VIP, tyrosine hydroxylase and neurofilament in nerves and cells of the human attached gingiva. Arch Oral Biol. 1988;33:149.
78 Magnusson B. Mucosal changes in erupting molars in germ-free rats. J Periodontal Res. 1969;4:181.
79 Marchetti C, Poggi P. Lymphatic vessels in the oral cavity: different structures for the same function. Microsc Res Tech. 2002;56:42.
80 Matsuo M, Takahashi K. Scanning electron microscopic observation of microvasculature in periodontium. Microsc Res Tech. 2002;56:3.
81 Mattos CM, Santana RB. A quantitative evaluation of the spatial maxillary anterior dentition. J Periodontol. 2008 Oct;79(10):1880-1885.
82 Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256.
83 Meyer J, Marwah AS, Weinmann JP. Mitotic rate of gingival epithelium in two age groups. J Invest Dermatol. 1956;27:237.
84 Miller SC, Soberman A, Stahl SS. A study of the cornification of the oral mucosa of young male adults. J Dent Res. 1951;30:4.
85 Morgan PR, Leigh IM, Purkis PE, et al. Site variation in keratin expression in human oral epithelia: an immunocytochemical study of individual keratins. Epithelia. 1987;1:31.
86 Moss ML. Phylogeny and comparative anatomy of oral ectodermal-ectomesenchymal inductive interactions. J Dent Res. 1969;48:732.
87 Ness KH, Morton TH, Dale BA. Identification of Merkel cells in oral epithelium using antikeratin and antineuroendocrine monoclonal antibodies. J Dent Res. 1987;66:1154.
88 Nuki K, Hock J. The organisation of the gingival vasculature. J Periodontal Res. 1974;9:305.
89 Oliver RC, Holm-Pederen P, Löe H. The correlation between clinical scoring, exudate measurements and microscopic evaluation of inflammation in the gingiva. J Periodontol. 1969;40:201.
90 Orban B, Kohler J. Die physiologische Zahn-fleischtasche, Epithelansatz und Epitheltiefenwucherung. Z Stomatol. 1924;22:353.
91 Page RC, Ammons WF, Schectman LR, et al. Collagen fibre bundles of the normal marginal gingiva in the marmoset. Arch Oral Biol. 1972;19:1039.
92 Papic M, Glickman I. Keratinization of the human gingiva in the menstrual cycle and menopause. Oral Surg Oral Med Oral Pathol. 1950;3:504.
93 Person P, Felton J, Fine A. Biochemical and histochemical studies of aerobic oxidative metabolism of oral tissues. 3. Specific metabolic activities of enzymatically separated gingival epithelium and connective tissue components. J Dent Res. 1965;44:91.
94 Pöllänen MT, Salonen JI, Uitto VJ. Structure and function of the tooth-epithelial interface in health and disease. Periodontol 2000. 2003;31:12.
95 Romanos GE, Bernimoulin JP. Collagen as a basic element of the periodontium: immunohistochemical aspects in the human and animal. 1. Gingiva and alveolar bone. Parodontologie. 1990;4:363.
96 Rosenberg HM, Massler M. Gingival stippling in young adult males. J Periodontol. 1967;38:473.
97 Salonen JI. Proliferative potential of the attached cells of human junctional epithelium. J Periodontal Res. 1994;29:41.
98 Salonen JI, Pöllänen MT. Junctional epithelial cells directly attached to the tooth. Acta Med Dent Helv. 1997;2:137.
99 Sawada T, Yamamoto T, Yanagisawa T, et al. Electron-immunocytochemistry of laminin and type-IV collagen in the junctional epithelium of rat molar gingiva. J Periodontal Res. 1990;25:372.
100 Sawaf MH, Ouhayoun JP, Forest N. Cytokeratin profiles in oral epithelial: a review and a new classification. J Biol Buccale. 1991;19:187.
101 Schor SL, Ellis I, Irwin CR, et al. Subpopulations of fetal-like gingival fibroblasts: characterisation and potential significance for wound healing and the progression of periodontal disease. Oral Dis. 1996;2:155.
102 Schroeder HE. Melanin containing organelles in cells of the human gingiva. J Periodontal Res. 1969;4:1.
103 Schroeder HE. Ultrastructure of the junctional epithelium of the human gingiva. Helv Odontol Acta. 1969;13:65.
104 Schroeder HE. Transmigration and infiltration of leucocytes in human junctional epithelium. Helv Odontol Acta. 1973;17:6.
105 Schroeder HE. Differentiation of human oral stratified epithelia. New York: Karger; 1981.
106 Schroeder HE. Gingiva. In: Oksche A, Vollrath L, editors. The periodontium handbook of microscopic anatomy, vol. 5. Berlin: Springer-Verlag; 1986.
107 Schroeder HE. The periodontium. Berlin: Springer-Verlag; 1986.
108 Schroeder HE, Listgarten MA. Fine structure of the developing epithelial attachment of human teeth. In: Monographs in developmental biology. Basel: Karger; 1971.
109 Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol 2000. 1997;13:91.
110 Schroeder HE, Theilade J. Electron microscopy of normal human gingival epithelium. J Periodontal Res. 1966;1:95.
111 Schultz-Haudt SD, From S. Dynamics of periodontal tissues. I. The epithelium. Odont T. 1961;69:431.
112 Schultz-Haudt SD, Paus S, Assev S. Periodic acid–Schiff reactive components of human gingiva. J Dent Res. 1961;40:141.
113 Schweitzer G. Lymph vessels of the gingiva and teeth. Arch Mik Anat Ent. 1907;69:807.
114 Schwint AE, Itoiz ME, Cabrini RL. A quantitative histochemical technique for the study of vascularization in tissue sections using horseradish peroxidase. Histochem J. 1984;16:907.
115 Seymour GJ, Crouch MS, Powell RN, et al. The identification of lymphoid cell subpopulations in sections of human lymphoid tissue and gingivitis in children using monoclonal antibodies. J Periodontal Res. 1982;17:247.
116 Shapiro S, Ulmansky M, Scheuer M. Mast cell population in gingiva affected by chronic destructive periodontal disease. J Periodontol. 1969;40:276.
117 Shelton LE, Hall WB. Human gingival mast cells. J Periodontal Res. 1968;3:214.
118 Shimada Y, Tai H, Tanaka A, et al. Effects of ascorbic acid on gingival melanin pigmentation in vitro and in vivo. J Periodontol.. 2009 Feb;80(2):317-323.
119 Skougaard MR, Beagrie GS. The renewal of gingival epithelium in marmosets (Callithrix jacchus) as determined through autoradiography with thymidine-H3. Acta Ondontol Scand. 1962;20:467.
120 Squier CA, Waterhouse JP. The ultrastructure of the melanocyte in human gingival epithelium. J Dent Res. 1967;46:113.
121 Stern IB. Electron microscopic observations of oral epithelium. I. Basal cells and the basement membrane. Periodontics. 1965;3:224.
122 Stern IB: The fine structure of the ameloblast-enamel junction in rat incisors, epithelial attachment and cuticular membrane. Presented at 5th International Congress for Electron Microscopy, 1966, p 6.
123 Stern IB: Further electron microscopic observations of the epithelial attachment, International Association for Dental Research Abstracts, 45th general meeting, 1967, p 118.
124 Susi F: Histochemical, autoradiographic and electron microscopic studies of keratinization in oral mucosa, Tufts University, 1967 (PhD thesis).
125 Swift JA, Saxton CA. The ultrastructural location of the periodate-Schiff reactive basement membrane of the dermoepidermal junctions of human scalp and monkey gingiva. J Ultrastruct Res. 1967;17:23.
126 Thilander H. Permeability of the gingival pocket epithelium. Int Dent J. 1964;14:416.
127 Thilander H, Bloom GD. Cell contacts in oral epithelia. J Periodontal Res. 1968;3:96.
128 Thonard JC, Scherp HW. Histochemical demonstration of acid mucopolysaccharides in human gingival epithelial intercellular spaces. Arch Oral Biol. 1962;7:125.
129 Trott JR. An investigation into the glycogen content of the gingivae. Dent Pract. 1957;7:234.
130 Trott JR, Gorenstein SL. Mitotic rates in the oral and gingival epithelium of the rat. Arch Oral Biol. 1963;8:425.
131 Turesky S, Glickman I, Litwin T. A histochemical evaluation of normal and inflamed human gingivae. J Dent Res. 1951;30:792.
132 Waterhouse JP. The gingival part of the human periodontium, its ultrastructure and the distribution in it of acid phosphatase in relation to cell attachment and the lysosome concept. Dent Pract Dent Rec. 1965;15:409.
133 Weinmann JP, Meyer J. Types of keratinization in the human gingiva. J Invest Dermatol. 1959;32:87.
134 Weinstock A, Albright JT. Electron microscopic observations on specialized structures in the epithelium of the normal human palate. J Dent Res. 1966;45:79.
135 Weinstock A, Albright JT. The fine structure of mast cells in normal human gingiva. J Ultrastruct Res. 1967;17:245.
136 Weski O. Die chronische marginales Enzundungen des Alveolar-fortsatzes mit besonderer Berucksichtigung der Alveolarpyorrhoe. Vierteljahrschr Zahnheilk. 1922;38:1.
137 Yamasaki A, Nikai H, Niitani K, et al. Ultrastructure of the junctional epithelium of germ-free rat gingiva. J Periodontol. 1979;50:641.
138 Zander HA. The distribution of phosphatase in gingival tissue. J Dent Res. 1941;20:347.