CHAPTER 68 Periimplant Anatomy, Biology, and Function
Attempts to replace lost dentition by means of implanted materials can be traced back to the ancient Egyptians, who hammered shaped seashells directly into the jaws for the purpose of replacing teeth.41 Over the last few centuries, a variety of materials have been implanted into jaws in an attempt replace missing teeth. The success of these early implants was extremely poor primarily because they never achieved a stable state of integration with the supporting tissues.85 The typical outcome, regardless of material or design, was healing with a soft tissue layer interposed between the implant and bone (i.e., fibrous encapsulation). Consequently, implants became mobile, infected, and painful, which led to failure.
The history of modern implant dentistry began with the introduction of titanium implants.41 In the 1950s, Per-Ingvar Brånemark, a Swedish professor of anatomy, had a serendipitous finding while studying blood circulation in bone that became a historical breakthrough in medicine. He discovered an intimate bone-to-implant apposition with titanium that offered sufficient strength to cope with load transfer. He called the phenomenon “osseointegration” and developed an implant system with a specific protocol to predictably achieve it. The implants were used to anchor prosthetic replacement teeth in the edentulous jaw,29 and the first patient was successfully treated in 1965.28,70 Subsequent clinical studies proved that commercially pure (CP) titanium implants, placed with a strict protocol, including an unloaded healing period, could predictably achieve osseointegration and retain a full-arch prosthesis in function with long-term success (15 years).9 Since that time, millions of patients worldwide have had missing teeth replaced with implants based on this original concept. Over the years, implant systems with variations in design (geometric and surface characteristics) and modified protocols have been developed and used with equal or better long-term success.
Today, implant designs, surgical placement techniques, healing times, and restorative protocols continue to evolve with the goal of improving outcomes. It is important for clinicians to know periimplant anatomy, to understand the biology, and to appreciate the functional capacity of osseointegrated implants. This chapter reviews implant geometry and surface characteristics, as well as the anatomical and biological relationship of periimplant tissues.
Implant Geometry (Macrodesign)
Numerous implant systems with various geometric (macrodesign) designs have been developed and used before the current implant systems in use today. Previous implant designs included blade vents (narrow, flat shape; tapped into boney trough prepared with rotary burs),67 press-fit cylindrical (bullet shape; pressed or tapped into prepared hole),94 subperiosteal (custom-made framework; adapted to the surface of jawbone),40 and transmandibular (long rods or posts; placed through the anterior mandible).100 Some of these implant systems were initially stable and appeared to be successful over short-term periods (e.g., 5 years) but failed to remain stable, became symptomatic and/or loose, and failed over longer periods.92,117 Lacking predictability, these implant systems are no longer used.
Since the time of the Brånemark studies, millions of patients have been treated worldwide using variations of this techniques using implants with different geometries and surface characteristics. Similar research including that of André Schroeder in Switzerland in the mid1970s contributed to the success of endosseous dental implants. The serendipitous finding of Brånemark was that when a hole is prepared into bone without overheating or otherwise traumatizing the tissues, an inserted biocompatible implantable device would predictably achieve an intimate bone apposition, as long as micromovements at the interface were prevented during the early healing period. The history of the research endeavors in Sweden provides a better understanding of the relevant biologic parameters involved.70
The macroscopic configuration of implants has varied widely; the most common types are listed in Box 68-1. Currently, most endosseous implants have a cylindrical or tapered, screw-shaped/threaded design. The disastrous results with other implant configurations were largely responsible for the evolution toward the current popular designs.14
Endosseous Implants
Blade Implants
Blade implants were designed and developed by Linkow67 and used clinically in 1960s and 1970s. Blade implants were inserted into the jawbone after mucoperiosteal flap elevation and preparation of a channel with a high-speed rotary bur. They were tapped into the narrow trench. One or several posts pierced through the mucoperiosteum after suturing of the flaps. After a few weeks of healing, a fixed prosthesis was fabricated by a classic method and cemented on top of it.
Because the high-speed drilling leads to extensive bone necrosis at the histologic level, fibrous scar tissue formation occurs. This allows downgrowth of the epithelium, which leads to marsupialization of the blade implants (Figure 68-1).57 If a bacterial infection occurs, it can lead to an intractable periimplantitis with ample bone loss. More importantly, removal of such implants after complications implies sacrificing surrounding jawbone. Because of its retentive geometry, the blade implant cannot simply be extracted or removed by a trephine, as with a cylindrical or screw-shaped implant.
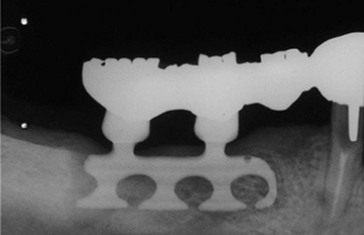
Figure 68-1 Blade implant shows a large radiolucency, indicating that the fibrous encapsulation has led to deep pocketing and subsequent bone loss. Neighboring teeth bear the load of the implant restoration.
The interface between blade vent implant and bone was called fibroosseous integration, which was defined as tissue-to-implant contact: interposition of healthy dense collagen fibers at the interface between the implant and bone.1 At that time, the fibrous tissue was thought to be the replacement of the periodontal ligament112; however, it was fibrous scar tissue following the bone necrosis initiated by high-speed drilling. This allows epithelial downgrowth around the post caused by the bacterial infection, which leads to encapsulation of blade implant with the fibrous scar tissue.
Most of the studies reported less than 50% success rate for 5-year duration with the complication of pocket formation exceeding 6 mm, followed by the significant alveolar bone loss around the implant.36,103 It was also more complicated and invasive surgery to remove the failed implant, which was sacrificing significant surrounding jawbone. As a result of the poor long-term success, as well as the high complication rate, the use of blade vent implants has been significantly reduced. Removal of failed blade implants often requires significant cutting and bone removal despite being mobile.
Pins
Although seldom used at present, in the classic technique, three diverging pins were inserted either transgingivally or after reflection of mucoperiosteal flaps in holes drilled by spiral drills. At the point of convergence, the pins were interconnected with cement to ensure the proper stability because of their divergence. On top of this arrangement, a single tooth could be installed. In edentulous jaws, several of these pin triads could be used to interconnect with a fixed prosthesis.
As with blade implants, the bone necrosis during drilling leads to fibrous encapsulation, marsupialization, and loss of the implants because of infections. A positive aspect, however, is that when such implants must be removed, removing the connection at the place of convergence is sufficient to allow easy extraction of each individual pin. Thus bone loss from removal is minimal.
Disk Implants
Disk implants are rarely used at present. The concept developed by Scortecci is based on the lateral introduction into the jawbone of a pin with a disk on top.96 Once introduced into the bone, the implant has strong retention against vertical extraction forces. Implants have been used with one, two, and even three disks. Unfortunately, as mentioned previously for blade implants, the cutting of the bone by means of high-speed drills leads to a fibrous scar tissue surrounding the implant, as revealed frequently by periimplant radiolucencies. Data on the clinical success of disk implants are mostly anecdotal.
Root Form Implants
Cylindrical Implants
The first implant in this category, designed and developed by Schroeder and colleagues between 1974 and 1985, was called ITI (International Team for Implantology) hollow-cylinder, plasma-splayed, and one-stage implant94 (Figure 68-2). It was thought that the hollow geometry should provide large bone-to-implant contact and holes would favorable for the additional fixation of the implant. However, the survival rates were less favorable compared to the other system.8 Thus this system was withdrawn from clinical use.

Figure 68-2 International Team for Implantology (ITI) hollow cylinder implant. Note the hollow geometry, which should provide large bone-to-implant contact, and holes that theoretically should be favorable for the additional fixation of the implant.
When discussing cylindrical implants, it is important to distinguish between hollow and full cylindrical implants. Straumann and co-workers introduced hollow cylinders in the mid 1970s with the ITI system.104 The idea was that implant stability would benefit from the large bone-to-implant surface provided by means of the hollow geometry. It was also thought that the holes (vents) would favor the ingrowth of bone to offer additional fixation. The same concept was used in the Core-Vent system developed by Niznick.75 Although it was not clarified whether the cause was geometry or the associated surface characteristics (titanium plasma–sprayed surface, titanium alloy), survival statistics were disappointing for hollow cylinder implants.108
Solid cylindrical implants were used by Kirsch and became available under the name IMZ, referring to the internal mobile shock absorber.63 The IMZ system, developed by Kirsch, prevailed in the market (Figure 68-3). The characteristic function of this system was the internal mobile element (IME) shock absorber, which was able to connect with the adjacent natural tooth. The system presented successful results in the short term, but long-term success rates were unacceptable (38% in 10 years), leading to the limited use of this implant design.49 For this reason, this implant is rarely seen in the market.
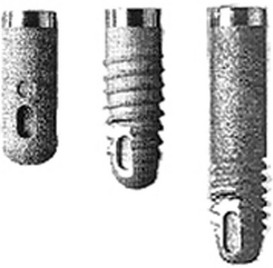
Figure 68-3 The IMZ implants. The roughened surface of the fixture had an advantage of higher integration of bone. Conversely, the surface characteristic added to the high frequency of periimplantitis in long-term use.
Even when an intimate bone apposition is achieved, extraction forces on such cylindrical implants lead to strong shear forces at the bone-to-implant interface. Only the microscopic surface irregularities offer some mechanical retention by interdigitation of bone growing onto the implant surface. With a screw-based geometry, forces acting parallel to the long axis of the implants are dispersed in many directions.99
Transmandibular Implants
Transmandibular implants were developed to retain dentures in the edentulous lower jaw. They were indicated for use in the extremely resorbed edentulous mandible with a minimal alveolar ridge height less than 10 mm. The implant was inserted through a submandibular skin incision and required general anesthesia. Two models were available in the 1960s. The first, called the staple-bone implant, was developed by Small. It consisted of a splint adapted to the lower border of the mandible, to which it is fixed by stabilizing pins.101 Two transmandibular screws were driven transgingivally into the mouth. The reported implant survival rate was 93% after 5 years and continued to exceed 90% after 15 years.100,102 The other model, introduced by Bosker, has two metal splints, one below the lower border of the mandible and one intraorally to connect the four posts piercing through the soft tissues.24 The Bosker implant seemed less reliable than the staple-bone implant, achieving only 70% survival after 5 years in the mandibular symphyseal area.108 Both transmandibular implants presented with the gingival hyperplasia or infection with the incidence of 10% to 15% of all cases.100 Despite the good long-term survival data reported (especially for the staple implant), the high incidence of complications and the necessity of using general anesthesia for implant placement, the transmandibular implant design is rarely used today.
Subperiosteal Implants
Subperiosteal implants are customized according to a plaster model derived from an impression of the exposed jawbone, before the surgery planned for implant insertion. The implant was designed with several posts, typically four or more for an edentulous jaw, that passed through the gingival tissues.12
Subperiosteal implants are designed to retain an overdenture, although fixed prostheses have also been cemented onto the posts. As a result of epithelial migration, the framework of subperiosteal implants usually becomes surrounded by fibrous connective tissue (scar), including the space between the implant and the bone surface. The marsupialization, as described earlier, often leads to infectious complications, which often necessitates removal of the implant. Furthermore, while being loaded by jaw function, jawbone resorption occurs rapidly, resulting in a lack of adaptation of the frame to the bone surface. As a result of this type of outcome, subperiosteal implants are now rarely used.
The subperiosteal implant was used in the treatment of atrophic mandibles. The implant is custom designed to fit the mandibular jaw bone using a plaster model obtained from an impression of the exposed mandible. It consisted of a cast metal framework with a subperiosteal component in contact with the bone and a trans-gingival component (posts), which were used to retain the dental prosthesis. The number of transgingival posts on the implants depend on the number of missing teeth, averaging four to six for the totally edentulous jaw. Several clinical studies reported poor survival rates of less than 50% for 5 to 10 years, with the frequent complication of gingival inflammation, involving tissue reaction around the post caused by the lack of soft tissue attachment to the metal post. These complications led to the development of sinus tract resorption of the cortical bone and exposure of framework.76,118 As a result of this higher rate of failure, as well as the significant complications and surgical invasion, subperiosteal implants are rarely used.
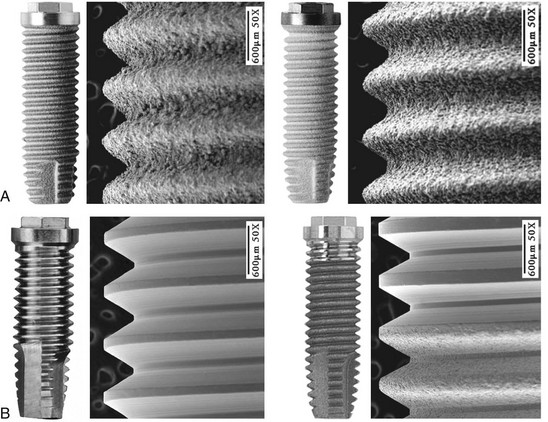
The most common implant design being used today is the screw-shaped or threaded cylindrical implant (Figure 68-4, A). A threaded implant design is preferred because it engages bone well and is able to achieve good primary stabilization. Even systems that started with cylindrical press-fit (nonthreaded) designs progressively evolved to a threaded geometry. The (longitudinal) shape of implants may be parallel or tapered (Figure 68-4, B). Although a vast majority of all implants have been parallel walled, the use of a tapered implant design has recently been advocated because it requires less space in the apical region (i.e., better for placement between roots or in narrow anatomic areas with labial concavities). Tapered implants have also been advocated for use in extraction sockets (Figure 68-5).
Implant Surface Characteristics (Microdesign)
Implant surface characteristics (microtopography) have been shown to positively influence the healing process.30,61,74,81 Accordingly, modification of implant surface characteristics has been a major area of research interest and development over the past 15 to 20 years. Modifications in surface energy, chemical composition, and surface topography are known to influence cellular activity and tissue responses leading to enhanced osteogenesis.25,31,72,111 At the molecular level, modified implant surfaces increase adsorption of serum proteins, mineral ions, and cytokines, which subsequently promote cellular migration and attachment.61,80,95 Implant surface characteristics can also aid in the retention of a fibrin clot, thus providing a migratory pathway for the differentiating osteogenic cells to reach the implant surface.37,38,83 Today, implants are treated with a variety of technologies to modify surface characteristics (microscale or nanoscale) to enhance bone formation.
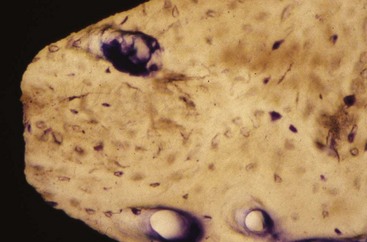
The additive process modifies the microstructure/macrostructure and chemical nature of the implant surface by adding materials or chemicals to the existing surface. There are several methods used to add materials and/or chemicals to the implant surface such as inorganic mineral coatings, plasma spraying, bio-coating with growth factors, fluoride, and particulates or cements containing calcium phosphates, sulfates, or carbonates. The addition of materials, such as hydroxyapatite (Figure 68-6), to the implant surface has been shown to enhance or accelerate the initial bone cells adaptation or proliferation.42,43,53,115 In general, additive surface modifications tend to increase the surface texture greater than subtractive surface modifications, which results in topographically “rougher” implant surfaces (Figure 68-7). Surface roughness can also be increased by oxidizing or adding an oxide layer (Figure 68-8).

Figure 68-6 High magnification (acid fuchsin/toluidine blue; ×100) of hydroxyapatite-coated implant with intimate bone approximation. Parts of the hydroxyapatite coating are observed on the surface.
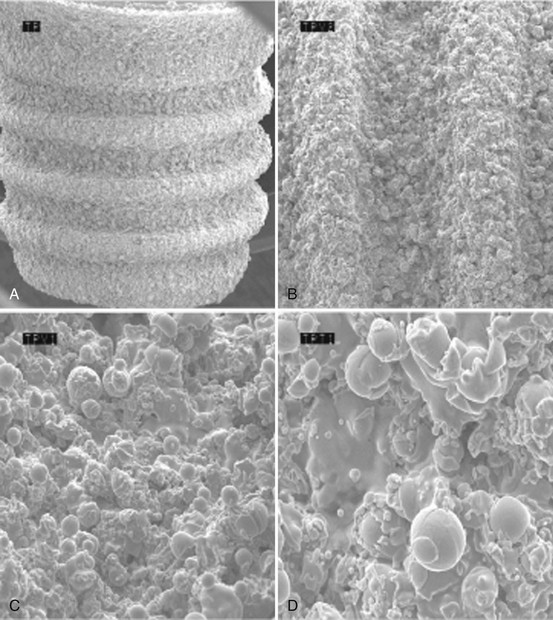
Figure 68-7 Scanning electron microscope (SEM) of titanium plasma-sprayed surface implant with rough surface characteristics. A, Implant with titanium plasma-sprayed surface (×40). B, Notably complex macro-topography on titanium plasma-sprayed surface (×100). C, Titanium plasma-sprayed surface with 1 to 25 µm particles (×500). D, Titanium plasma-sprayed surface with 1–25 µm particles (×1000).
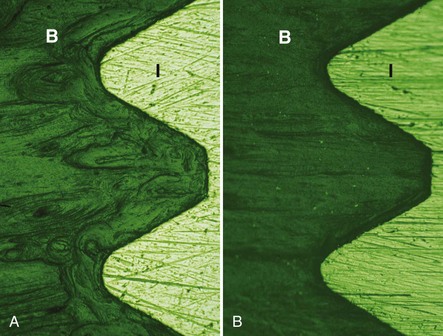
The subtractive process modifies the microstructure and chemical nature of the implant surface by removing or altering the existing surface. The roughness of implant surface can be modified by machining, acid etching, blasting, or a combination of these processes to enhance the amount or speed of osseointegration.35,43,86 The changes are most notable at the microscopic level (Figures 68-9 and Figure 68-10). Implant surfaces that are modified at the microscopic level with techniques, such as acid-etching, are believed to promote favorable cellular responses and increased bone formation in close proximity to the surface16,55 (Figure 68-11).
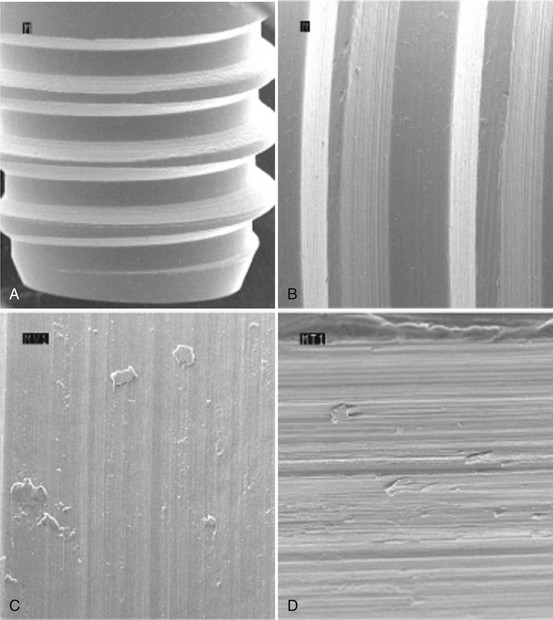
Figure 68-9 Scanning electron microscope (SEM) of machined surface implant with characteristic grooved pattern. A, Implant with machined surface (×40). B, Grooved pattern apparent on machined surface (×100). C, Machined surface with distinct ridges and grooves (×500). D, Machined surface with distinct ridges and grooves (×1000).
(Courtesy Nobel Biocare Services AG, Zurich, Switzerland.)
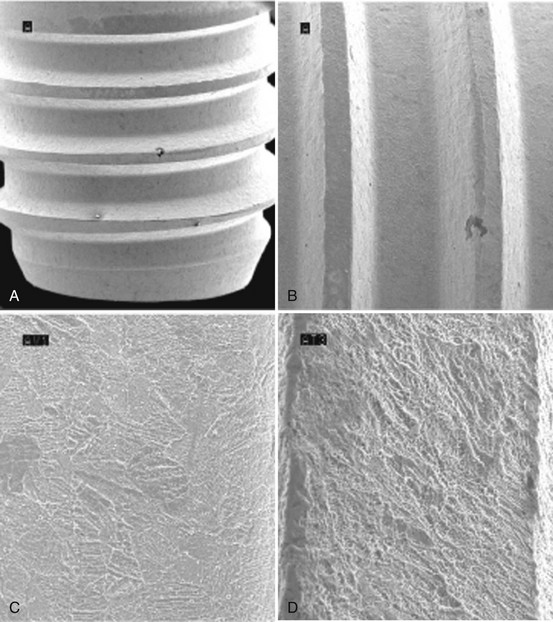
Figure 68-10 Scanning electron microscope (SEM) of acid-etched surface implant with typical microscopic peak-valley appearance. A, Implant with acid etched surface (×40). B, Micro-textured surface alteration on acid-etched surface (×100). C, Acid-etched surface with micropits of 1 to 3 microns (×500). D, Acid-etched surface with micropits of 1 to 3 microns surrounded by larger areas with 6 to 10 µm peak-valley pits (×1000).
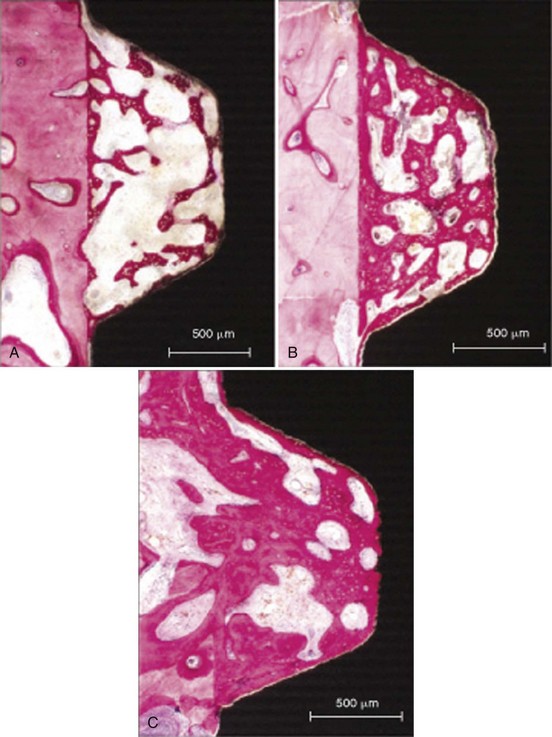
Figure 68-11 Histologic appearance of bone apposition. A, At 2 weeks, bone is deposited on the bony wall of the tissue chamber and on the implant surface. Both layers are connected by a scaffold of tiny trabeculae. Woven bone is characterized by the intense staining of the mineralized matrix and the numerous osteocytes located in large lacunae (undecalcified ground section, surface-stained with toluidine blue and basic fuchsin; bar = 500 µm). B, At 4 weeks, the volume density of this scaffold has increased both by the formation of new trabeculae and by deposition of more mature, parallel-fibered bone onto the primary scaffold. Woven bone is mainly recognized by the numerous large osteocytic lacunae (bright). The gap between bone and implant surface is an artifact (bar = 500 µm). C, At 8 weeks, growth and reinforcement result in a further increase in bone density and an almost perfect coating of the implant surface with bone. Remodeling has started, replacing the primary bone by secondary osteons (arrows; bar = 500 µm).
Implant Surface Chemical Composition
There have been unsuccessful trials with oral implants made of carbon or hydroxyapatite. The lack of resistance, because of material properties, to occlusal forces led to frequent fractures. The so-called noble metals or alloys, on the other hand, do not resist corrosion and have thus been abandoned. Today, the vast majority of oral implants are made of CP titanium or titanium alloys. The following discussion relates to titanium implants.
Titanium is a very reactive metal that oxidizes within nanoseconds when exposed to air. Because of this passive oxide layer, the titanium then becomes very resistant to corrosion in its CP form. Some alloys, such as titanium-aluminum 6%, vanadium 4% (Ti6Al4V), are known to provoke bone resorption as the result of leakage of some toxic components. The oxide layer of CP titanium reaches 10 nm of thickness. It grows over the years when facing a bioliquid. It consists mainly of titanium dioxide (TiO2).
All titanium oxides have dielectric constants, which are higher than most other metal oxides. This factor may explain titanium’s tendency to adsorb biomolecules, as seen during surgery when the blood creeps up the surface during implant insertion. The biomolecules normally appear as folded-up structures to hide their insoluble parts, while putting water-soluble radicals on their surface. Thus they will adhere to the TiO2 surface after displacing the original water molecules sitting on its surface. Although initially attracted by weak van der Waals forces, the high dielectric constant of titanium oxides and the polarizability of the molecules after adsorption will lead to high bond strengths, which are considered irreversible when they surpass 30 kcal/mol.59 In fact, because of its propensity for being covered by an uninterrupted oxide layer, which has ceramic-like properties similar to other metal oxides (e.g., aluminum oxides), titanium makes the coating of implants superfluous. This should be stressed because many authors hope for even better osseointegration potential with calcium phosphate (CaP)–coated surfaces and strongly advocate their use. To date, clinical results with CaP-coated implants have not been encouraging in a long-term perspective.22
Thus the overall view of potential advantages for different implant surface characteristics is complex, and only clinical observations can determine their validity. For good-quality bone, after 15 years of follow-up, clinical success rates of 99% have been reported for implants with a turned surface.65 Enhanced implant surface characteristics are likely to be most beneficial for the more challenging situations, such as poor quality bone and early and immediate loading.
Implant Surface Free Energy and Micro-Roughness
When an implant is brought into contact with bodily tissues and fluids, in this case mostly bone, it faces a “bioliquid,” an aqueous environment. Within milliseconds, water, ions, and small biomolecules are absorbed. One could imagine that this absorbed layer renders all surfaces equal. However, the large molecules and the cells that will subsequently adhere to this surface are influenced by the surface characteristics of this pellicle layer. The composition and structure of the initial layer are largely determined by the underlying surface.89 Thus, the three-dimensional shape of the molecules will be modified during their adherence to this pellicle layer and will unveil different radicals, depending on this metamorphosis.
The surface free energy, often called wettability, is an important parameter for these interactions. It can be assessed through the shape of a standardized drop of liquid put on the clean implant surface. The angle of this drop toward the underlying surface reveals that the cohesive forces between liquid molecules are stronger than the adhesive forces between the liquid and the surface. Thus a ball-shaped drop would reveal a low surface free energy.
Surface topography at the cellular and molecular level means microscopic roughness. A surface roughness can be measured with a profilometer, a stylus that follows the surface and measures the peak-to-valley dimensions (expressed as Ra values) or the spacing between irregularities (expressed as Scx values). Wennerberg and Albrektsson113 provide guidelines for topographic evaluation of implant surfaces.113 No implant surface is smooth, although several reports have incorrectly referred to the “turned” (machined) implant surface as “smooth” (see Figure 68-9). Roughened implant surfaces speed up the bone apposition; as demonstrated in vitro, more prostaglandin E2 (PGE2) and transforming growth factor beta (TGF-β1) are produced on rough than on smooth surfaces.62 Rough surfaces may show some disadvantages such as increased ion leakage and increased adherence of macrophages and subsequent bone resorption.73
It was also reported that in vitro adsorption of fibronectin was higher on smooth than on rough, CP titanium surfaces.44 Fibronectin is a glycoprotein quickly adhering on hard surfaces and known to determine subsequent cell adhesion.78 Microtopography also influences the number and morphology of cell adhesion pseudopods and cell orientation.52 Grooves in an implant surface will guide the cell migration along their direction. Bone growth can enter altered microtopographic features such as pits and porosities with internal dimensions that are only a few microns.
Lack of load can also be detrimental and can lead to cortical bone resorption. This is well documented in orthopedics and is termed stress shielding.54 This phenomenon has not been properly evaluated for oral implants in which marginal bone resorption is thought to be associated with chronic inflammation of the overlying soft tissues.
The use of finite element analysis (FEA) has become popular but lacks value by itself; invalid assumptions, such as the isotropic nature of the bone, must be used in the modeling. The deviation of FEA data from in vivo data has been well documented.71 FEA data should be considered “descriptive” models that require confirmation by biologic data. However, as with photoelastic studies, FEA analyses do provide some insight on stress concentrations and their relation to implant geometry and the prosthetic superstructures.
Hard Tissue Interface
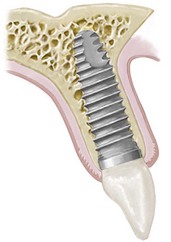
The primary goal of implant installation is to achieve and maintain a stable bone-to-implant connection (i.e., osseointegration).26,28 Histologically, osseointegration is defined as the direct structural and functional connection between ordered, living bone and the surface of a load-bearing implant without intervening soft tissues (Figure 68-12).27,29 Clinically, osseointegration is the asymptomatic rigid fixation of an alloplastic material (implant) in bone with the ability to withstand occlusal forces.13,119 The hard tissue interface is a fundamental requirement for and an essential component of implant success.
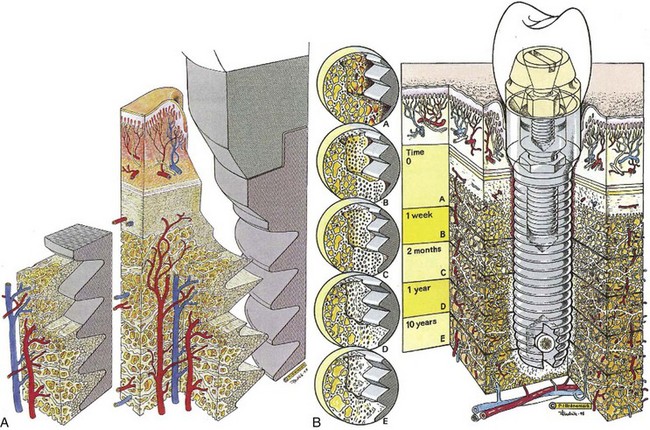
Figure 68-12 A, Three-dimensional diagram of the tissue and titanium interrelationship showing an overall view of the intact interfacial zone around the osseointegrated implant. B, Physiologic evolution of the biology of the interface over time.
The osseointegration process observed after implant insertion can be compared to bone fracture healing. Implant site osteotomy preparation (bone wounding) initiates a sequence of events, including an inflammatory reaction, bone resorption, release of growth factors, and attraction by chemotaxis of osteoprogenitor cells to the site. Differentiation of osteoprogenitor cells into osteoblasts leads to bone formation at the implant surface. Extracellular matrix proteins, such as osteocalcin, modulate apatite crystal growth.116 Specific conditions, optimal for bone formation, must be maintained at the healing site to achieve osseointegration.
Immobility of the implant relative to the bone must be maintained for bone formation at the surface. A mild inflammatory response enhances the bone healing, but moderate inflammation or movement above a certain threshold is detrimental.7 When micromovements at the interface exceed 150 µm, the movement will impair differentiation of osteoblasts and fibrous scar tissue will form between the bone and implant surface.84 Therefore it is important to avoid excessive forces, such as occlusal loading, during the early healing period.
Bone tissue damage and debris created by the osteotomy site preparation must be cleared up by osteoclasts for normal bone healing. These multinuclear cells, originating from the blood, can resorb bone at a pace of 50 to 100 µm per day. There is a coupling between bone apposition and bone resorption (Figure 68-13). Preosteoblasts, derived from primary mesenchymal cells, depend on a favorable oxidation-reduction (redox) potential of the environment. Thus a proper vascular supply and oxygen tension are needed. If oxygen tension is poor, the primary stem cells may differentiate into fibroblasts, form scar tissue, and lead to implant failure (nonintegration).
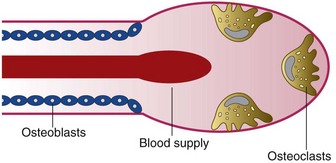
Figure 68-13 The basic multicellular unit is the basic remodeling process for bone renewal. Osteoclasts are imported by the vascular supply, and the resorption lacunae are soon filled by the lining osteoblasts.
If bone is overheated or crushed during preparation, it will become necrotic and may lead to nonmineralized (soft tissue) scar formation or be sequestered. The critical temperature for bone cells that should not be exceeded is 47°C at an exposure time of one minute.7 Thus preparation of implant osteotomy sites require profuse irrigation (cooling) along with gentle, intermittent, moderate-speed drilling using sharp drills. Another complicating factor, well recognized from open wound fractures, is that microbial contamination jeopardizes the normal bone healing. Accordingly, strict aseptic techniques should be maintained.
New bone formation follows a specific sequence of events. Woven bone is quickly formed in the gap between the implant and the bone; it grows fast, up to 100 µm per day, and in all directions. Characterized by a random orientation of its collagen fibrils, high cellularity, and limited degree of mineralization, the biomechanical capacity of woven bone is poor (Figure 68-14, A). Thus any occlusal load should be well controlled or avoided in the early phase of healing. After several months, woven bone is progressively replaced by lamellar bone with organized, parallel layers of collagen fibrils and dense mineralization. Contrary to the fast-growing woven bone, lamellar bone formation occurs at a slow pace (only a few microns per day). Ultimately, after 18 months of healing, a steady state is reached where lamellar bone is continuously resorbed and replaced (Figure 68-14, B).29 At the light microscopic level, an intimate bone-to-implant contact has been extensively reported (Figure 68-15).90 Once the bone-to-implant interface has reached a steady state, it can maintain itself over decades, as ascertained by human histology from implants retrieved because of hardware fractures.11
Clinically, both primary and secondary stability of an implant are critical to success. Primary stability, achieved at the time of surgical placement, depends on the implant geometry (macrodesign), as well as the quality and quantity of bone available for implant anchorage at a specific site. Secondary stability, achieved over time with healing, depends on the implant surface (microdesign), as well as the quality and quantity of adjacent bone, which will determine the percentage of contacts between the implant and bone.18,47,88,106 For example, areas such as the anterior mandible have dense cortical bone and provide rigid primary stabilization and good support throughout the healing process. Conversely, areas such as the posterior maxilla have thin cortical bone and large marrow spaces provide less primary stability. For this reason, the posterior maxilla has been associated with lower success rates compared to other sites with greater bone density and support.17,58
Once osseointegration is achieved, implants can resist and function under the forces of occlusion for many years. Longitudinal biomechanical assessments seem to indicate that during the first weeks after placement of one-stage implants, decreased rigidity is observed.45 This may be indicative of bone resorption during the initial phase of healing. Subsequently, rigidity increases and continues to increase for years.105 Thus, when a prosthesis is installed immediately (in 1 day) or early (in 1 to 2 weeks), care must be taken to control against overload. It is important to recognize that sites with limited primary stability or less bone-to-implant contact (e.g., posterior maxilla) will likely go through a period of even less bone support in the early stages of bone healing due to the initial phase of bone resorption.
Soft Tissue Interface
Not surprisingly, for two decades, research and clinical interest focused on the bone-to-implant interface of osseointegrated implants and the overlying soft tissues were largely ignored. Except for a few descriptive sentences, the classic handbook by Brånemark et al29 presented no data or information about the soft tissue interface. This may be due in part to the fact that most patients were fully edentulous and the Brånemark system implants had turned (machined) surfaces, which are less likely to be associated with soft tissue inflammatory problems.6 Today, there is greater interest in and appreciation for periimplant soft tissues and the soft tissue-to-implant interface as a function of esthetics and maintenance of a seal or barrier against microbial invasion.
Periimplant soft tissues are similar in appearance and structure to periodontal soft tissues (see Chapter 2). Clearly, both implants and teeth emerge through the soft tissues on the alveolar ridge. The soft tissues consist of connective tissue covered by epithelium. There is a gingival/mucosal sulcus, a long junctional epithelial attachment, and a zone of connective tissue above the supporting bone (Figure 68-16). Despite the apparent similarities in soft tissues around teeth and implants, the presence of a periodontal ligament around teeth and not around implants is an important, distinct difference. There are no inserting collagen fibers anywhere along the interface of osseointegrated implants. Bone is in direct contact with the implant surface without intervening soft tissues. Whereas natural teeth have a periodontal ligament with connective tissue fibers suspending them in the alveolar bone, osseointegrated implants do not.
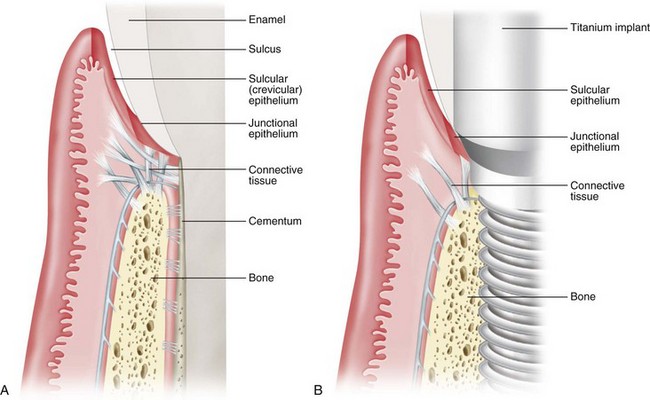
Figure 68-16 Schematic illustration of hard and soft tissue around a tooth and an implant. A, Hard and soft tissue anatomy around a natural tooth demonstrates bone support with a periodontal ligament, a connective tissue zone above the crest of bone with connective tissue fibers (Sharpey’s) inserting into dentin, a long junctional epithelial attachment, a gingival sulcus lined with sulcular epithelium, and oral gingival epithelium (outer surface of gingiva). B, Hard and soft tissue anatomy around an implant demonstrates some similarities and some distinct differences. There is supporting bone in direct approximation to the implant surface without any intervening soft tissues (i.e., no periodontal ligament). A connective tissue zone is present above the level of bone with fibers running parallel to the implant surface and no inserting fibers. There is a long junctional epithelial attachment, a gingival/mucosal sulcus lined with sulcular epithelium and oral gingival/mucosal epithelium (outer surface of soft tissue).
(From Rose LF, Mealey BL: Periodontics: medicine, surgery, and implants, St. Louis, 2004, Mosby.)
As in the natural dentition, the oral epithelium around implants is continuous with a sulcular epithelium that lines inner surface of gingival sulcus; the apical part of the gingival sulcus is lined with long junctional epithelium.69 Ultrastructural examination of the long junctional epithelial attachment adjacent to dental implants has demonstrated that epithelial cells attach with a basal lamina and hemidesmosomes3,5,48,60,98 (Figure 68-17). Histologic studies indicate that these epithelial structures and the surrounding lamina propria cannot be distinguished from those structures around teeth.32 In health, the sulcular epithelium has a thickness of about 0.5 mm.87
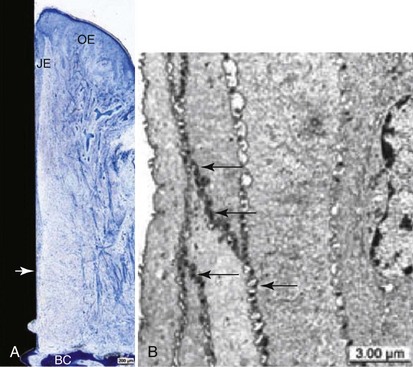
Figure 68-17 A, Overview of ground section showing peri-implant tissues covered with keratinizing oral epithelium (OE). Junctional epithelium (JE) is interposed between connective tissue and alveolar bone crest (BC). Apical end of junctional epithelium (arrow). Toluidine blue stain. (Bar = 200 µm.) B, Transmission electron microscopic view of sulcular epithelium showing tightly sealed intercellular spaces by numerous spot desmosomes (arrows), contributing to low permeability of this portion of periimplant mucosa. Bar = 3 µm.1
The apical edge of the epithelial attachment is about 1.5 to 2.0 mm above the bone margin.82 In healthy periimplant tissues, progressive epithelial downgrowth does not occur, indicating that factors other than inserted collagen fiber bundles (i.e., Sharpey’s fibers in natural dentition) prevent it.
Periimplant connective tissue morphology closely resembles that of the natural dentition except that it lacks a periodontal ligament, cementum, and inserting fibers (Figure 68-18). No significant differences were found at the biochemical level between the periimplant and the periodontal soft tissues.33 Clinically, the thickness of the periimplant soft tissues varies from two to several millimeters (Figure 68-19). An animal study determined the total height of the periimplant “biologic width” to be approximately 3 to 4 mm, where about 2 mm is the epithelial attachment and about 1 to 2 mm is the supracrestal connective tissue zone.19 Consistent with this finding, a human histologic study determined the height of the periimplant “biologic width,” consisting of an epithelial attachment and supracrestal connective tissue, to be about 4 to 4.5 mm46 (Figure 68-20).
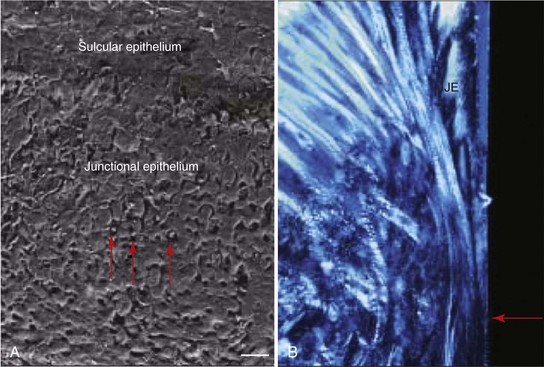
Figure 68-18 A, SEM of the junctional epithelium. Note the neutrophils located between the cells (red arrows). Bar = 40 µm. B, Higher magnification of Figure 68-17 with polarized light showing the apical extent (red arrow) of the junctional epithelium (JE). Note the dense collagen fibers running apicocoronal (i.e., parallel to the implant surface).
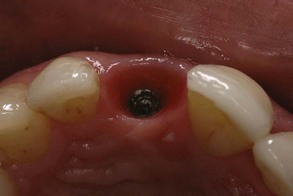
Figure 68-19 Clinical appearance of normal, healthy periimplant tissue with implant restoration removed. Soft tissue thickness varies from site-to-site, depending on quantity and quality of tissue, as well as the anatomy of the surrounding area (e.g., adjacent to natural teeth with healthy periodontal attachment versus adjacent to a space). Note that the intrasulcular tissue appears more erythematous as the result of the thin, nonkeratinized layer of epithelium overlying the connective tissue.
(Courtesy Dr. Stuart Froum, New York, NY.)
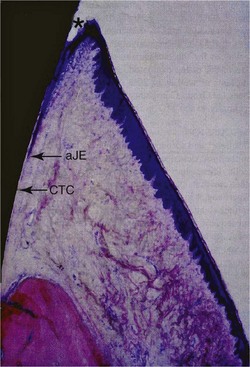
Figure 68-20 Buccolingual section (basic fuchsin stain; original magnification ×12.5; 1-part SLA implant, 3 months unloaded) showing the gingiva and the most coronal part of alveolar bone. Rete peg formation is only apparent in the area of the keratinized oral gingival epithelium. The oral sulcular epithelium exhibits no keratinization. In the area of the most coronal point of the junctional epithelium (cJE), the soft tissues are slightly torn away (artifact) because of non-decalcified histologic processing. The most apical point of the junctional epithelium is indicated (aJE). No rete peg formation is evident adjacent to the basal cell layer of the junctional epithelium (JE), all showing healthy and physiologic soft tissue structures. In addition, the area of connective tissue contact (CTC) adjacent to the machined titanium surface is marked. A slight round cell infiltrate in the connective tissue is indicating a mild inflammation. Note bone remodeling/new bone formation in the crestal bone region indicated by saturated, dark-red stained areas.
Between the epithelial attachment and the marginal bone is a zone of dense connective tissue. This zone of supracrestal connective tissue has an important function in the maintenance of a stable soft tissue–implant interface and as a seal or barrier to the “outside” oral environment. The orientation of connective tissue fibers adjacent to an implant differs from that of periodontal connective tissue fibers. In the absence of cementum and inserting connective tissue fibers (i.e., as in a natural tooth), most periimplant connective tissue fibers run in a direction more or less parallel to the implant surface. Even when the fiber bundles are oriented perpendicularly, which occurs more often in the gingiva than in the mucosa surrounding implants, the bundles are never embedded in the implant surface.
The fiber bundles can also have a “cuff-like” circular orientation.20 The role of these fibers remains unknown, but it appears that their presence helps to create a soft tissue “seal” around the implant. The adaptation of the connective tissue to an implant surface may also be affected by the mobility of the soft tissue around the implant. The connective tissue in direct contact with the implant surface is characterized by an absence of blood vessels and an abundance of fibroblasts interposed between collagen fibers.66 Several animal and human studies have shown that the alignments of connective fibers were circular and horizontal around the implants* (Figure 68-21).
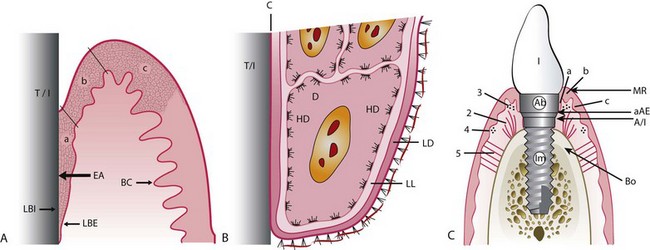
Figure 68-21 A, Histologic scheme of epithelial attachment (EA) (identical for tooth and implant). T/I, Titanium implant; BC, basal complex; LBI, lamina basalis interna; LBE, lamina basalis externa (only location where cell divisions occur); a, long junctional epithelial attachment zone; b, sulcular epithelial zone; c, oral epithelial zone. B, At electron microscopic level, basal complex at epithelial attachment (three most apical cells) and connection with stroma. HD, Hemidesmosomes; D, desmosome; LL, lamina lucida; LD, lamina densa; C, cuticle. C, Implant, abutment (Ab), and crown within alveolar bone and soft tissues. Im, Endosseous part of implant; MR, margin of gingiva/alveolar mucosa; Bo, marginal bone level; 1, implant crown; 2, vertical alveolar-gingival connective tissue fibers; 3, circular gingival connective tissue fibers; 4, circular gingival connective tissue fibers; 5, periosteal-gingival connective tissue fibers; a, junctional epithelium; b, sulcular epithelium; c, oral epithelium; A/I, abutment/implant junction; aAE, apical (point) of attached epithelium.
This connective tissue interface has been examined by clinical attachment level measurements in patients. Probing attachment levels were consistently found coronal to the alveolar crest in patients with periimplant tissue health, indicating the presence of a zone of direct connective tissue contact to the implant surface. This means that the probing depth measurement performed with a periodontal probe may be about 1.5 mm higher above the bone level in healthy tissues. At inflamed sites, the probe may penetrate to the bone with probing depth measurement reflecting the total soft tissue thickness above bone. In cases with inflammatory periimplant tissue disease, increasing probing depth and reduced attachment levels have been reported.4,39,68,79,110
Questions emerged decades ago, as it did for the natural dentition, about the need for keratinized tissue to surround implants. Prospective and cross-sectional studies, evaluating screw-shaped implants with a machined surface, suggest that the presence or absence of keratinized gingiva is not a prerequisite for long-term stability.93 However, it has been suggested that implants surrounded by mucosa only (i.e., nonkeratinized) are more susceptible to periimplant problems. An animal study observed that ligature-induced periimplantitis occurs more frequently when alveolar mucosa surrounds the implant as compared to when keratinized mucosa surrounds the implant.109
Keratinized mucosa tends to be more firmly anchored by collagen fibers to the underlying periosteum than nonkeratinized mucosa, which has more elastic fibers and tends to be moveable relative to the underlying bone. In clinical studies evaluating intraoral implants, with or without periimplant keratinized mucosa, no clinically significant difference in implant success was reported.64,114 However, when there is a lack of keratinized tissue, patients tend to complain about pain and discomfort while performing oral hygiene procedures or other functions in the area. The symptoms are alleviated by increasing the amount of keratinized (firmly bound) tissue around the implant(s) via soft tissue grafting (see Figures 63-5, A-F and 63-5, G-L). Finally, although it may not be comparable to intraoral implants, mobility of soft tissues surrounding extraoral implants is associated with a higher incidence of implant failure.10
Vascular Supply and Inflammation
The vascular supply of the periimplant gingival or alveolar mucosa may be limited, as compared to periodontal gingiva, due to the lack of a periodontal ligament (Figure 68-22).21 This is especially true in the tissue immediately adjacent to the implant surface. However, capillary loops in the connective tissue under the junctional and sulcular epithelium around implants appear to be anatomically similar to those found in the normal periodontium (Figure 68-23).97
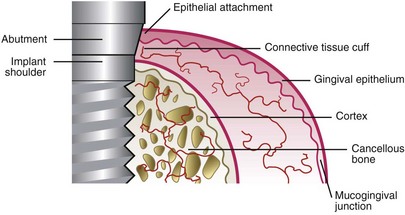
Figure 68-22 Schematic illustration of the blood supply in the connective tissue cuff surrounding the implant/abutment is scarcer than in the gingival complex around teeth because none originates from a periodontal ligament.
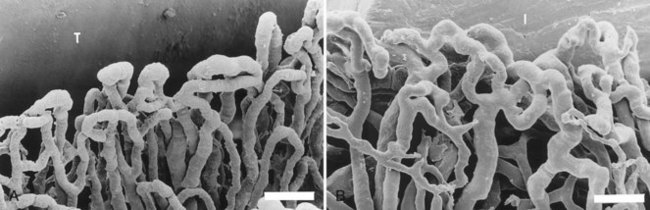
Figure 68-23 A, Microvascular topography surrounding an implant. B, Microvascular topography surrounding a tooth. Bar = 5 µm.
(Courtesy Drs. N. Selliseth and K. Selvig, Bergen, Norway.)
Emerging knowledge indicates that the periimplant gingival or alveolar mucosa has the same morphology as the corresponding tissues around teeth. These soft tissues also react the same way to plaque accumulation. Studies investigating the histology (light microscopic and ultrastructural) of healthy and inflamed tissues surrounding implants in humans have indicated that the inflammatory response to plaque is similar to that observed in periodontal tissues.87 Polymorphonuclear cells and mononuclear cells transmigrate normally through the periimplant sulcular epithelium (Figure 68-24).87
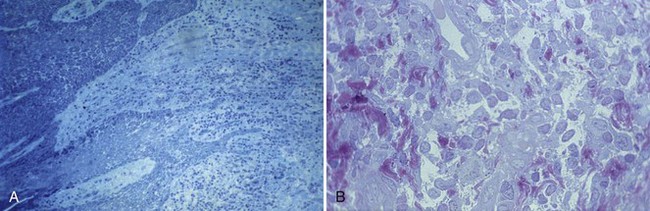
Figure 68-24 A, Histologic slide from healthy gingiva surrounding a well-functioning implant in human patient. No morphologic characteristics differentiate tissue around implant from that around teeth. B, When gingivitis occurs, a profuse migration of inflammatory cells through the pocket epithelium can be observed.
(Courtesy Professor Mariano Sanz, Madrid.)
Clinical Comparison of Teeth and Implants
Although the soft tissue-to-implant (abutment) interface offers striking similarities with tissue surrounding the natural dentition, some differences should be considered. At the bone level, the lack of a periodontal ligament is the most striking difference. The following discussion elaborates on the clinical perspectives of these similarities and differences.
At the bone level the absence of the periodontal ligament surrounding an implant has important clinical consequences. This means that no resilient connection exists between implants and supporting bone. Implants cannot intrude or migrate to compensate for the presence of a premature occlusal contact (as teeth can). Implants and the rigidly attached implant restorations do not move. Thus any occlusal disharmony will have repercussions at either the restoration-to-implant connection, the bone-to-implant interface, or both.
Proprioception in the natural dentition comes from the periodontal ligament. The absence of a periodontal ligament around implants reduces tactile sensitivity56 and reflex function.23 This can become even more challenging when osseointegrated, implant-supported, fixed prostheses are present in both jaws.
The lack of a periodontal ligament and inability of implants to move contraindicates their use in growing individuals. Natural teeth continue to erupt and migrate during growth while implants do not. Implants placed in individuals prior to the completion of growth can lead to occlusal disharmonies with implants.77 Likewise, it may be problematic to place one or more implants in a location adjacent to teeth that are very mobile from the loss of periodontal support because as the teeth move in response to or away from the occlusal forces, the implant(s) will bear the entire load.
Overload, because of improper superstructure design, parafunctional habits, or excessive occlusal load, may cause microstrains and microfractures in the bone, which will lead to bone loss and a fibrous inflammatory tissue at the implant interface.107
Conclusion
Appreciating the osseointegration process is facilitated by a good knowledge and understanding of bone healing. Many factors can interfere with the predictable establishment of a permanent rigid connection between an implant surface and the surrounding bone that is able to sustain occlusal loads. The bone-to-implant interface and its rigidity are a predominant biomechanical aspect of coping with the time and intensity of loading. The soft tissue-to-implant interface also plays an important role in the long-term maintenance of a stable marginal bone levels around implants. Clinicians must familiarize themselves with the underlying molecular and cellular events to evaluate the future evolution of implant design and implant protocols, including surgical placement, restoration, and maintenance.
![]() Science Transfer
Science Transfer
Most current implants are screw-retained cylinders or tapers and are made of titanium, which results in a layer of titanium oxide that has the capacity to enable osseointegration. Roughening of the surfaces resulted in improved blood clot adhesion, osteoprogenitor cell differentiation, and early bone formation, as well as the mechanical advantage of increased surface area. Thus clinicians should utilize any of the variety of new surface modifications available on different implant brands. The use of surface liquids to enhance bone formation, such as fluoride and saline, may accelerate early bone formation.
The soft tissue surrounding implants is not as firmly attached as it is in teeth that have cementum. Probing depths around healthy implants may reach within 1 to 2 mm of the bone margin, but in inflamed tissues the probe will go to the bone level. Clinicians should use light and gentle procedures when measuring pocket depths in the periimplant environment to avoid damage to the fragile soft tissue adhesion to the implant surface. Implants do have healthier soft tissue around them when there is an adequate amount of keratinized gingiva, and in some cases, mucogingival surgery is needed to stabilize gingival health. Plaque control around implants is just as vital to long-term success as it is around teeth, so patients need close supervision of plaque levels during maintenance visits, which should be every 3 or 4 months.
1 American Academy of Implant Dentistry. Glossary of implant terms. J Oral Implantol. 1986;12:284-294.
2 Abrahamsson I, Berglundh T, Moon IS, Lindhe J. Peri-implant tissues at submerged and non-submerged titanium implants. J Clin Periodontol. 1999;26:600-607.
3 Abrahamsson I, Berglundh T, Wennstrom J, Lindhe J. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implants Res. 1996;7:212-219.
4 Abrahamsson I, Soldini C. Probe penetration in periodontal and peri-implant tissues. An experimental study in the beagle dog. Clin Oral Implants Res. 2006;17:601-605.
5 Abrahamsson I, Zitzmann NU, Berglundh T, Linder E, Wennerberg A, Lindhe J. The mucosal attachment to titanium implants with different surface characteristics: an experimental study in dogs. J Clin Periodontol. 2002;29:448-455.
6 Adell R, Lekholm U, Rockler B, et al. Marginal tissue reactions at osseointegrated titanium fixtures (I). A 3-year longitudinal prospective study. Int J Oral Maxillofac Surg. 1986;15:39-52.
7 Albrektsson T. Bone Tissue Response. In: Branemark PI, Zarb G, Albrektsson T, editors. Tissue-Integrated Prothesis: Osseointegration in Clinical Dentistry. Chicago: Quintessence, 1987.
8 Albrektsson T. The host-implant interface: biology. Int J Prosthodont. 2003;16(Suppl):29-30. discussion 47-51
9 Albrektsson T, Branemark PI, Hansson HA, Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155-170.
10 Albrektsson T, Branemark PI, Jacobsson M, Tjellstrom A. Present clinical applications of osseointegrated percutaneous implants. Plast Reconstr Surg. 1987;79:721-731.
11 Albrektsson T, Eriksson AR, Friberg B, et al. Histologic investigations on 33 retrieved Nobelpharma implants. Clin Mater. 1993;12:1-9.
12 Albrektsson T, Sennerby L. State of the art in oral implants. J Clin Periodontol. 1991;18:474-481.
13 Albrektsson T, Wennerberg A. The impact of oral implants—past and future, 1966-2042. J Can Dent Assoc. 2005;71:327.
14 Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11-25.
15 Arvidson K, Fartash B, Hilliges M, Kondell PA. Histological characteristics of peri-implant mucosa around Branemark and single-crystal sapphire implants. Clin Oral Implants Res. 1996;7:1-10.
16 Arvidsson A, Sater BA, Wennerberg A. The role of functional parameters for topographical characterization of bone-anchored implants. Clin Implant Dent Relat Res. 2006;8:70-76.
17 Balshi TJ, Wolfinger GJ. Management of the posterior maxilla in the compromised patient: historical, current, and future perspectives. Periodontol 2000. 2003;33:67-81.
18 Barewal RM, Oates TW, Meredith N, Cochran DL. Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants. 2003;18:641-651.
19 Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996;23:971-973.
20 Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2:81-90.
21 Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994;21:189-193.
22 Block MS, Kent JN. Long-term follow-up on hydroxylapatite-coated cylindrical dental implants: a comparison between developmental and recent periods. J Oral Maxillofac Surg. 1994;52:937-943. discussion 944
23 Bonte B, van Steenberghe D. Masseteric post-stimulus EMG complex following mechanical stimulation of osseointegrated oral implants. J Oral Rehabil. 1991;18:221-229.
24 Bosker H, van Dijk L. The transmandibular implant: a 12-year follow-up study. J Oral Maxillofac Surg. 1989;47:442-450.
25 Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137-146.
26 Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399-410.
27 Branemark PI. The Osseointegration Book: from Calvarium to Calcaneus. Berlin; Chicago: Quitessenz Verlags; 2005.
28 Branemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3:81-100.
29 Branemark PI, Zarb G, Albrektsson T. Tissue-Integrated Prothesis: Osseointegration in Clinical Dentistry. Chicago: Quintessence; 1987.
30 Buser D, Broggini N, Wieland M, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529-533.
31 Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889-902.
32 Buser D, Weber HP, Donath K, Fiorellini JP, Paquette DW, Williams RC. Soft tissue reactions to non-submerged unloaded titanium implants in beagle dogs. J Periodontol. 1992;63:225-235.
33 Chavrier C, Couble ML, Hartmann DJ. Qualitative study of collagenous and noncollagenous glycoproteins of the human healthy keratinized mucosa surrounding implants. Clin Oral Implants Res. 1994;5:117-124.
34 Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 1997;68:186-198.
35 Cooper LF, Zhou Y, Takebe J, et al. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006;27:926-936.
36 Cranin AN, Rabkin MF, Garfinkel L. A statistical evaluation of 952 endosteal implants in humans. J Am Dent Assoc. 1977;94:315-320.
37 Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998;11:391-401.
38 Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67:932-949.
39 DeAngelo SJ, Kumar PS, Beck FM, Tatakis DN, Leblebicioglu B. Early soft tissue healing around one-stage dental implants: clinical and microbiologic parameters. J Periodontol. 2007;78:1878-1886.
40 Dhal. Om Mojligheten for implantationi kaken ac metallskelette som bas dller retention for fasta eller avtagbara proteser. Odont Tidskr. 1943.
41 Driskell TD. History of implants. Cda J. 1987;15:16-25.
42 Ellingsen JE, Johansson CB, Wennerberg A, Holmen A. Improved retention and bone-tolmplant contact with fluoride-modified titanium implants. Int J Oral Maxillofac Implants. 2004;19:659-666.
43 Ellingsen JE, Thomsen P, Lyngstadaas SP. Advances in dental implant materials and tissue regeneration. Periodontol 2000. 2006;41:136-156.
44 Francois P, Vaudaux P, Taborelli M, Tonetti M, Lew DP, Descouts P. Influence of surface treatments developed for oral implants on the physical and biological properties of titanium. (II) Adsorption isotherms and biological activity of immobilized fibronectin. Clin Oral Implants Res. 1997;8:217-225.
45 Friberg B, Sennerby L, Linden B, Grondahl K, Lekholm U. Stability measurements of one-stage Branemark implants during healing in mandibles. A clinical resonance frequency analysis study. Int J Oral Maxillofac Surg. 1999;28:266-272.
46 Glauser R, Schupbach P, Gottlow J, Hammerle CH. Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: A light-microscopic overview and histometric analysis. Clin Implant Dent Relat Res. 2005;7(Suppl 1):S44-S51.
47 Glauser R, Sennerby L, Meredith N, et al. Resonance frequency analysis of implants subjected to immediate or early functional occlusal loading. Successful vs. failing implants. Clin Oral Implants Res. 2004;15:428-434.
48 Gould TR, Westbury L, Brunette DM. Ultrastructural study of the attachment of human gingiva to titanium in vivo. J Prosthet Dent. 1984;52:418-420.
49 Haas R, Mensdorff-Pouilly N, Mailath G, Watzek G. Survival of 1,920 IMZ implants followed for up to 100 months. Int J Oral Maxillofac Implants. 1996;11:581-588.
50 Hermann JS, Buser D, Schenk RK, Higginbottom FL, Cochran DL. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000;11:1-11.
51 Hermann JS, Buser D, Schenk RK, Schoolfield JD, Cochran DL. Biologic Width around one- and two-piece titanium implants. Clin Oral Implants Res. 2001;12:559-571.
52 Hong HL, Brunette DM. Effect of cell shape on proteinase secretion by epithelial cells. J Cell Sci. 1987;87(Pt 2):259-267.
53 Huang YH, Xiropaidis AV, Sorensen RG, Albandar JM, Hall J, Wikesjo UM. Bone formation at titanium porous oxide (TiUnite) oral implants in type IV bone. Clin Oral Implants Res. 2005;16:105-111.
54 Huiskes R, Weinans H, Dalstra M. Adaptive bone remodeling and biomechanical design considerations for noncemented total hip arthroplasty. Orthopedics. 1989;12:1255-1267.
55 Ivanoff CJ, Widmark G, Johansson C, Wennerberg A. Histologic evaluation of bone response to oxidized and turned titanium micro-implants in human jawbone. Int J Oral Maxillofac Implants. 2003;18:341-348.
56 Jacobs R, van Steenberghe D. Role of periodontal ligament receptors in the tactile function of teeth: a review. J Periodontal Res. 1994;29:153-167.
57 James RA. Peri-implant considerations. Dent Clin North Am. 1980;24:415-420.
58 Jemt T. Failures and complications in 391 consecutively inserted fixed prostheses supported by Branemark implants in edentulous jaws: a study of treatment from the time of prosthesis placement to the first annual checkup. Int J Oral Maxillofac Implants. 1991;6:270-276.
59 Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent. 1983;49:832-837.
60 Kawahara H, Kawahara D, Mimura Y, Takashima Y, Ong JL. Morphologic studies on the biologic seal of titanium dental implants. Report II. In vivo study on the defending mechanism of epithelial adhesions/attachment against invasive factors. Int J Oral Maxillofac Implants. 1998;13:465-473.
61 Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7:329-345.
62 Kieswetter K, Schwartz Z, Hummert TW, et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32:55-63.
63 Kirsch A. The two-phase implantation method using IMZ intramobile cylinder implants. J Oral Implantol. 1983;11:197-210.
64 Lekholm U, Ericsson I, Adell R, Slots J. The condition of the soft tissues at tooth and fixture abutments supporting fixed bridges. A microbiological and histological study. J Clin Periodontol. 1986;13:558-562.
65 Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res. 1996;7:329-336.
66 Linkevicius T, Apse P. Biologic width around implants. An evidence-based review. Stomatologija. 2008;10:27-35.
67 Linkow LI. The endosseous blade vent. Newsl Am Acad Implant Dent. 1969;18:15-24.
68 Listgarten MA. Soft and hard tissue response to endosseous dental implants. Anat Rec. 1996;245:410-425.
69 Listgarten MA, Lang NP, Schroeder HE, Schroeder A. Periodontal tissues and their counterparts around endosseous implants [corrected and republished with original paging, article orginally printed in Clin Oral Implants Res 1991 Jan-Mar;2(1):1-19]. Clin Oral Implants Res. 1991;2:1-19.
70 McClarence E. Close to the Edge: Branemark and the Development of Osseointegration. London: Quintessence; 2003.
71 Mellal A, Wiskott HW, Botsis J, Scherrer SS, Belser UC. Stimulating effect of implant loading on surrounding bone. Comparison of three numerical models and validation by in vivo data. Clin Oral Implants Res. 2004;15:239-248.
72 Mendonca G, Mendonca DB, Aragao FJ, Cooper LF. Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials. 2008;29:3822-3835.
73 Murray DW, Rae T, Rushton N. The influence of the surface energy and roughness of implants on bone resorption. J Bone Joint Surg Br. 1989;71:632-637.
74 Nanci A, Wuest JD, Peru L, et al. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J Biomed Mater Res. 1998;40:324-335.
75 Niznick GA. The Core-Vent implant system. J Oral Implantol. 1982;10:379-418.
76 Obwegeser HL. Experiences with subperiosteal implants. Oral Surg Oral Med Oral Pathol. 1959;12:777-786.
77 Op Heij DG, Opdebeeck H, van Steenberghe D, Quirynen M. Age as compromising factor for implant insertion. Periodontol 2000. 2003;33:172-184.
78 Pearson BS, Klebe RJ, Boyan BD, Moskowicz D. Comments on the clinical application of fibronectin in dentistry. J Dent Res. 1988;67:515-517.
79 Pontes AE, Ribeiro FS, Iezzi G, Piattelli A, Cirelli JA, Marcantonio EJr. Biologic width changes around loaded implants inserted in different levels in relation to crestal bone: histometric evaluation in canine mandible. Clin Oral Implants Res. 2008;19:483-490.
80 Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311-2321.
81 Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res. 2002;13:1-19.
82 Quirynen M, van Steenberghe D, Jacobs R, Schotte A, Darius P. The reliability of pocket probing around screw-type implants. Clin Oral Implants Res. 1991;2:186-192.
83 Raghavendra S, Wood MC, Taylor TD. Early wound healing around endosseous implants: a review of the literature. Int J Oral Maxillofac Implants. 2005;20:425-431.
84 Recum AF, Shannon CE, Cannon CE, Long KJ, Kooten TG, Meyle J. Surface roughness, porosity, and texture as modifiers of cellular adhesion. Tissue Eng. 1996;2:241-253.
85 Ring ME. A thousand years of dental implants: a definitive history–part 1. Compend Contin Educ Dent. 1995;16:1060. 1062, 1064 passim
86 Ronold HJ, Lyngstadaas SP, Ellingsen JE. A study on the effect of dual blasting with TiO2 on titanium implant surfaces on functional attachment in bone. J Biomed Mater Res A. 2003;67:524-530.
87 Sanz M, Alandez J, Lazaro P, Calvo JL, Quirynen M, van Steenberghe D. Histo-pathologic characteristics of peri-implant soft tissues in Branemark implants with 2 distinct clinical and radiological patterns. Clin Oral Implants Res. 1991;2:128-134.
88 Scarano A, Degidi M, Iezzi G, Petrone G, Piattelli A. Correlation between implant stability quotient and bone-implant contact: a retrospective histological and histomorphometrical study of seven titanium implants retrieved from humans. Clin Implant Dent Relat Res. 2006;8:218-222.
89 Schakenraad JM, Busscher HJ, Wildevuur CR, Arends J. The influence of substratum surface free energy on growth and spreading of human fibroblasts in the presence and absence of serum proteins. J Biomed Mater Res. 1986;20:773-784.
90 Schenk RK, Buser D. Osseointegration: a reality. Periodontol 2000. 1998;17:22-35.
91 Schierano G, Ramieri G, Cortese M, Aimetti M, Preti G. Organization of the connective tissue barrier around long-term loaded implant abutments in man. Clin Oral Implants Res. 2002;13:460-464.
92 Schnitman PA, Shulman LB. Recommendations of the consensus development conference on dental implants. J Am Dent Assoc. 1979;98:373-377.
93 Schou S, Holmstrup P, Hjorting-Hansen E, Lang NP. Plaque-induced marginal tissue reactions of osseointegrated oral implants: a review of the literature. Clin Oral Implants Res. 1992;3:149-161.
94 Schroeder A, Pohler O, Sutter F. [Tissue reaction to an implant of a titanium hollow cylinder with a titanium surface spray layer]. SSO Schweiz Monatsschr Zahnheilkd. 1976;86:713-727.
95 Schwartz Z, Boyan BD. Underlying mechanisms at the bone-biomaterial interface. J Cell Biochem. 1994;56:340-347.
96 Scortecci G. Immediate function of cortically anchored disk-design implants without bone augmentation in moderately to severely resorbed completely edentulous maxillae. J Oral Implantol. 1999;25:70-79.
97 Selliseth NJ, Selvig KA. Microvascular adaptation to transmucosal implants. A scanning electron microscopic study in the rat. Clin Oral Implants Res. 1995;6:205-212.
98 Shioya K, Sawada T, Miake Y, Inoue S, Yanagisawa T. Ultrastructural study of tissues surrounding replanted teeth and dental implants. Clin Oral Implants Res. 2009;20:299-305.
99 Siegele D, Soltesz U. Numerical investigations of the influence of implant shape on stress distribution in the jaw bone. Int J Oral Maxillofac Implants. 1989;4:333-340.
100 Small IA. Chalmers J. Lyons memorial lecture: metal implants and the mandibular staple bone plate. J Oral Surg. 1975;33:571-585.
101 Small IA, Metz H, Kobernick S. The mandibular staple implant for the atrophic mandible. J Biomed Mater Res. 1974;8:365-371.
102 Small IA, Misiek D. A sixteen-year evaluation of the mandibular staple bone plate. J Oral Maxillofac Surg. 1986;44:60-66.
103 Smithloff M, Fritz ME. The use of blade implants in a selected population of partially edentulous adults. A five-year report. J Periodontol. 1976;47:19-24.
104 Sutter F, Schroeder A, Straumann F. Engineering and design aspects of the I.T.I. hollow-basket implants. J Oral Implantol. 1983;10:535-551.
105 Tricio J, Laohapand P, van Steenberghe D, Quirynen M, Naert I. Mechanical state assessment of the implant-bone continuum: a better understanding of the Periotest method. Int J Oral Maxillofac Implants. 1995;10:43-49.
106 Trisi P, Lazzara R, Rao W, Rebaudi A. Bone-implant contact and bone quality: evaluation of expected and actual bone contact on machined and osseotite implant surfaces. Int J Periodontics Restorative Dent. 2002;22:535-545.
107 Ueda M, Matsuki M, Jacobsson M, Tjellstrom A. Relationship between insertion torque and removal torque analyzed in fresh temporal bone. Int J Oral Maxillofac Implants. 1991;6:442-447.
108 Versteegh PA, van Beek GJ, Slagter AP, Ottervanger JP. Clinical evaluation of mandibular overdentures supported by multiple-bar fabrication: a follow-up study of two implant systems. Int J Oral Maxillofac Implants. 1995;10:595-603.
109 Warrer K, Buser D, Lang NP, Karring T. Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa. An experimental study in monkeys. Clin Oral Implants Res. 1995;6:131-138.
110 Weber HP, Kim DM, Ng MW, Hwang JW, Fiorellini JP. Peri-implant soft-tissue health surrounding cement- and screw-retained implant restorations: a multi-center, 3-year prospective study. Clin Oral Implants Res. 2006;17:375-379.
111 Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25:4731-4739.
112 Weiss CM. Tissue integration of dental endosseous implants: description and comparative analysis of the fibro-osseous integration and osseous integration systems. J Oral Implantol. 1986;12:169-214.
113 Wennerberg A, Albrektsson T. Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants. 2000;15:331-344.
114 Wennstrom JL, Bengazi F, Lekholm U. The influence of the masticatory mucosa on the peri-implant soft tissue condition. Clin Oral Implants Res. 1994;5:1-8.
115 Wikesjo UM, Huang YH, Xiropaidis AV, et al. Bone formation at recombinant human bone morphogenetic protein-2-coated titanium implants in the posterior maxilla (Type IV bone) in non-human primates. J Clin Periodontol. 2008;35:992-1000.
116 Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11:1103-1113.
117 Yanase R. Management of subperiosteal implant denture problems. In: International Symposium on Preprosthetic Surgery. California: Palm Springs; 1985.
118 Yurkatas. The current state of implants in prosthodontics. Newsletter AM Acad Implant Dent. 1967.
119 Zarb G, Albrektsson T. Osseointegration– a requiem for the periodontal ligament? The International Journal of Periodontics and Restorative Dentistry. 1991;14:251-262.