CHAPTER 63 Periodontal Plastic and Esthetic Surgery
Terminology
The term mucogingival surgery was initially introduced in the literature by Friedman38 to describe surgical procedures for the correction of relationships between the gingiva and the oral mucous membrane with reference to three specific problem areas: attached gingiva, shallow vestibules, and a frenum interfering with the marginal gingiva. With the advancement of periodontal surgical techniques, the scope of nonpocket surgical procedures has increased, now encompassing a multitude of areas that were not addressed in the past. Recognizing this, the 1996 World Workshop in Clinical Periodontics renamed mucogingival surgery as “periodontal plastic surgery,”3 a term originally proposed by Miller in 1993 and broadened to include the following areas2,3:
Periodontal plastic surgery is defined as the surgical procedures performed to correct or eliminate anatomic, developmental, or traumatic deformities of the gingiva or alveolar mucosa.2,3 Mucogingival therapy is a broader term that includes nonsurgical procedures such as papilla reconstruction by means of orthodontic or restorative therapy. Periodontal plastic surgery includes only the surgical procedures of mucogingival therapy.
This chapter discusses the periodontal plastic surgical techniques included in the traditional definition of mucogingival surgery: (1) widening of attached gingiva (2) deepening of shallow vestibules, and (3) resection of the aberrant frena. In addition, esthetic surgical therapy around the natural dentition and tissue engineering (biologic mediator) are included in this chapter. Other aspects of periodontal plastic surgery, such as periodontal-prosthetic surgery, esthetic surgery around implants, and surgical exposure of teeth for orthodontic therapy, are covered in Chapters 50, 65, and 74.
Objectives
The five objectives of periodontal plastic surgery addressed in this chapter are as follows:
Problems Associated with Attached Gingiva
The ultimate goal of mucogingival surgical procedures is the creation or widening of attached gingiva around teeth and implants.3 The width of the attached gingiva varies in different individuals and on different teeth of the same individual (see Chapter 2). Attached gingiva is not synonymous with “keratinized gingiva” because the latter also includes the free gingival margin.
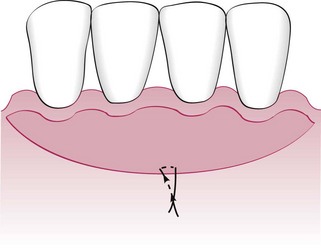
The width of the attached gingiva is determined by subtracting the depth of the sulcus or pocket from the distance between the crest of the gingival margin and the mucogingival junction.
The original rationale for mucogingival surgery was predicated on the assumption that a minimal width of attached gingiva was required to maintain optimal gingival health. However, several studies have challenged the view that a wide, attached gingiva is more protective against the accumulation of plaque than a narrow or a nonexistent zone. No minimum width of attached gingiva has been established as a standard necessary for gingival health. People who practice good, atraumatic oral hygiene may maintain excellent gingival health with almost no attached gingiva.
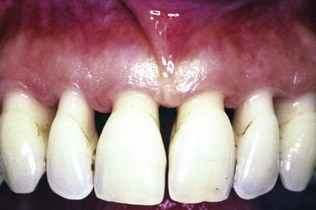
However, those individuals whose oral hygiene practices are less than optimal can be helped by the presence of keratinized gingiva and vestibular depth. Vestibular depth provides space for easier placement of the toothbrush and prevents brushing on mucosal tissue. To improve esthetics, the objective is the coverage of the denuded root surface. The maxillary anterior area, especially the facial aspect of the canine, often presents extensive gingival recession. In such cases, the covering of the denuded root surface not only widens the zone of attached gingiva but also creates a improved esthetic result. This recession and the resultant denuded root surface have a special esthetic concerns for individuals with a high smile line. A wider zone of attached gingiva is also needed around teeth that serve as abutments for fixed or removable partial dentures, as well as in the ridge areas bearing a denture. Teeth with subgingival restorations and narrow zones of keratinized gingiva have higher gingival inflammation scores than teeth with similar restorations and wide zones of attached gingiva.90,91 Therefore, in such cases, techniques for widening the attached gingiva are considered preprosthetic periodontal surgical procedures. Chapter 65 discusses this subject in detail.
Widening the attached gingiva accomplishes the following four objectives:
Problems Associated with Shallow Vestibule
Another objective of periodontal plastic surgery is the creation of vestibular depth when it is lacking. Gingival recession displaces the gingival margin apically, thus reducing vestibular depth, which is measured from the gingival margin to the bottom of the vestibule. As indicated previously, with minimal vestibular depth, proper hygiene procedures are jeopardized. The sulcular brushing technique requires the placement of the toothbrush at the gingival margin, which may not be possible with reduced vestibular depth.
Minimal attached gingiva with adequate vestibular depth may not require surgical correction if proper atraumatic hygiene is practiced with a soft brush. Minimal amounts of keratinized attached gingiva with no vestibular depth benefit from mucogingival correction. Adequate vestibular depth is also necessary for the proper placement of removable prostheses.
Problems Associated with Aberrant Frenum
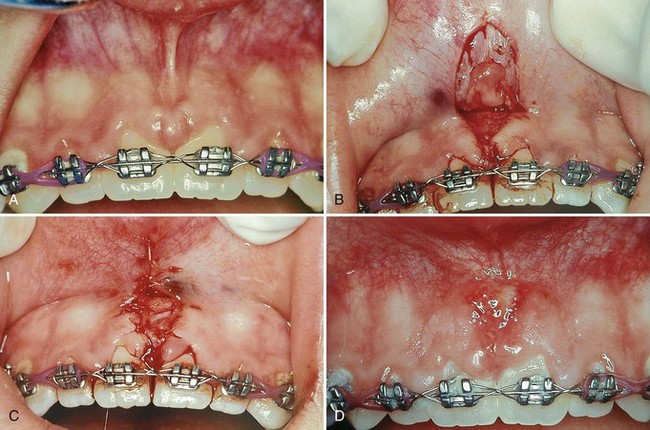
Still another important objective of periodontal plastic surgery is to correct frenal or muscle attachments that may extend coronal to the mucogingival junction. If adequate keratinized, attached gingiva is present coronal to the frenum, it may not be necessary to remove the frenum. A frenum that encroaches on the margin of the gingiva may interfere with plaque removal, and the tension on the frenum may tend to open the sulcus. In such cases, surgical removal of the frenum is indicated.
Esthetic Surgical Therapy
As indicated earlier, the recession of the facial, gingival margin will alter the proper gingival symmetry and result in an esthetic problem. The presence of the interdental papilla is also important to satisfy the esthetic goals of the patient. A missing papilla creates a space which many address as a “black hole.” The regeneration of the lost or reduced papilla is one of the most difficult goals in esthetic periodontal plastic surgery. Another area of concern is the patient who presents an excessive amount of gingiva in the visible area. This condition is often addressed as a “gummy smile” and may be corrected surgically by crown lengthening. The correction of these anatomic defects has become an important part of periodontal plastic surgery.
Tissue Engineering
The future of periodontal plastic surgery will encompass the use of tissue-engineered products at the recipient site to reduce the donor site morbidity. Currently, there are numerous studies, both clinically and in the laboratories, to allow the clinician to utilize this minimally invasive approach to periodontal plastic surgery.
Etiology of Marginal Tissue Recession
The most common cause of gingival recession and the loss of attached gingiva is abrasive and traumatic toothbrushing habits. The bone and soft tissue anatomy of the buccal, radicular surface of the dentition is usually thin, especially around the anterior area. Teeth positioned buccally may have a even thinner bone and gingiva. In many instances, such areas may have a complete absence of bone beneath the thin overlying gingival tissue. Such defect in the bone is called a dehiscence. This anatomic status combined with external trauma from overzealous brushing can lead to the loss of gingival tissue. Recession of the gingival tissue and bone exposes the cemental surface of the root, which results in abrasion and “ditching” of the cemental surface apical to the cementoenamel junction (CEJ). The cementum is softer than enamel and will be destroyed before the enamel surface of the crown.
Another cause for gingival recession is periodontal disease and chronic marginal inflammation. The loss of attachment caused by the inflammation is followed by the loss of bone and gingiva. Advanced periodontal involvement in areas of minimal attached gingiva results in the base of the pocket extending close to, or apical to, the mucogingival junction. Periodontal therapy of these areas also results in gingival recession caused by the loss of gingiva and bone.
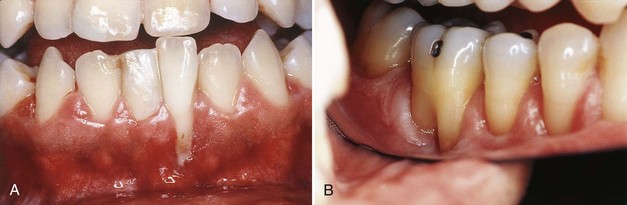
Frenal and muscle attachments that encroach on the marginal gingiva can distend the gingival sulcus, which creates an environment for plaque accumulation. This condition increases the rate periodontal recession and will contribute to the recurrence of the recession even after treatment (Figure 63-1). These problems are more common on facial surfaces, but may also occur on the lingual surface.11
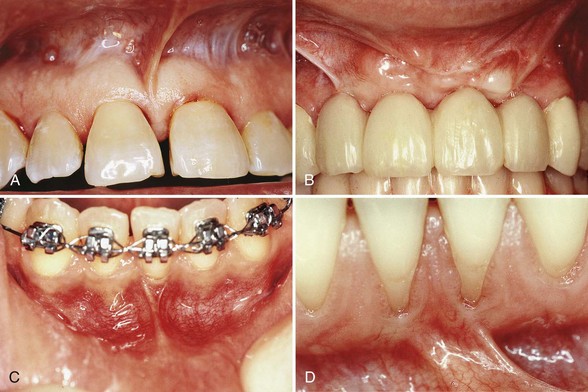
Figure 63-1 High frenum attachments. A, Frenum between maxillary central incisors. B, Frenum attached to facial surface of maxillary lateral incisors. C, Frenum attached to facial surface of mandibular incisor. D, Frenum attached to facial surface of an incisor.
Orthodontic tooth movement through a thin buccal osseous plate may lead to a dehiscence beneath a thin gingiva. This also can lead to the recession of the gingiva45,106 (Figure 63-2).
Factors That Affect Surgical Outcome
Irregularity of Teeth
Abnormal tooth alignment is an important cause of gingival deformities that require corrective surgery and also an important factor in determining the outcome of treatment. The location of the gingival margin, width of the attached gingiva, and alveolar bone height and thickness are all affected by tooth alignment. On teeth that are tilted or rotated labially, the labial bony plate is thinner and located farther apically than on the adjacent teeth; therefore the gingiva is recessed so that the root is exposed.106 On the lingual surface of such teeth, the gingiva is bulbous, and the bone margins are closer to the CEJ. The level of gingival attachment on root surfaces and the width of the attached gingiva after mucogingival surgery are affected as much by tooth alignment as by variations in treatment procedures.
Orthodontic correction is indicated when mucogingival surgery is performed on malposed teeth in an attempt to widen the attached gingiva or to restore the gingiva over denuded roots. If orthodontic treatment is not feasible, the prominent tooth should be reduced to within the borders of the alveolar bone, with special care taken to avoid pulp injury.
Roots covered with thin bony plates present a hazard in mucogingival surgery. Even the most protective type of flap, a partial-thickness flap, creates the risk of bone resorption on the periosteal surface.48 Resorption in amounts that ordinarily are not significant may cause loss of bone height when the bone plate is thin or tapered at the crest.
Mucogingival Line (Junction)
Normally, the mucogingival line in the incisor and canine areas is located approximately 3 mm apical to the crest of the alveolar bone on the radicular surfaces and 5 mm interdentally.92 In periodontal disease and on malposed disease free teeth, the bone margin is located farther apically and may extend beyond the mucogingival line. The distance between the mucogingival line and the CEJ before and after periodontal surgery is not necessarily constant. After inflammation is eliminated, the tissue tends to contract and draw the mucogingival line in the direction of the crown.31
Techniques to Increase Attached Gingiva
To simplify and better understand the techniques and the result of the surgery, the following classifications are presented:
Widening of the keratinized attached gingiva (apical or coronal to the area of recession) can be accomplished by numerous techniques, such as the free gingival autograft, free connective tissue autograft, and lateral pedicle flap, which can be used for either objective.
Gingival Augmentation Apical to Recession
Techniques for gingival augmentation apical to the area of recession include free gingival autograft, free connective tissue autograft,10 and the apically positioned flap.
Free Gingival Autografts
Free gingival grafts are used to create a widened zone of attached gingiva. They were initially described by Bjorn12 in 1963 and have been extensively investigated since that time (Figure 63-3).13
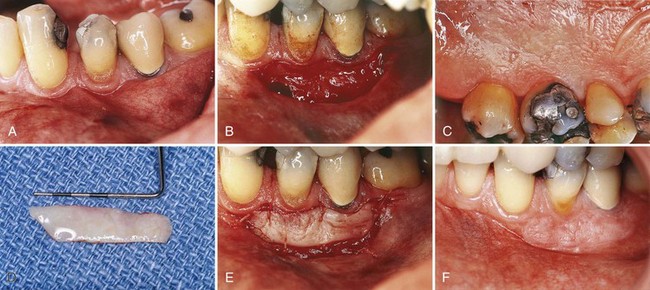
Figure 63-3 Free gingival graft. A, Before treatment; minimal keratinized gingiva. B, Recipient site prepared for free gingival graft. C, Palate will be donor site. D, Free graft. E, Graft transferred to recipient site. F, At 6 months, showing widened zone of attached gingiva.
(Courtesy Dr. Perry Klokkevold, Los Angeles.)
The Classic Technique
Step 1: Prepare the recipient site. The purpose of this step is to prepare a firm connective tissue bed to receive the graft. The recipient site can be prepared by incising at the existing mucogingival junction with a #15 blade to the desired depth, blending the incision on both ends with the existing mucogingival line. Periosteum should be left covering the bone.
Another technique consists of outlining the recipient site with two vertical incisions from the incised gingival margin into the alveolar mucosa. Extend the incisions to approximately twice the desired width of the attached gingiva, allowing for 50% contraction of the graft when healing is complete. The amount of contraction depends on the extent to which the recipient site penetrates the muscle attachments. The deeper the recipient site, the greater is the tendency for the muscles to elevate the graft and reduce the final width of the attached gingiva. The periosteum along the apical border of the graft is sometimes penetrated in an effort to prevent postoperative narrowing of the attached gingiva.
The #15 blade is used to incise along the gingival margin to separate a flap consisting of epithelium and underlying connective tissue without disturbing the periosteum. Extend the flap to the depth of the vertical incisions. Suture the flap where the apical portion of the free graft will be located. Three to four independent gut sutures are placed. The needle is first passed as a superficial mattress suture perpendicular to the incision and then on the periosteum parallel to the incision (Figure 63-4).
An aluminum foil template of the recipient site can be made to be used as a pattern for the graft.
Grafts can also be placed directly on bone tissue. For this technique, the flap should be separated by blunt dissection with a periosteal elevator. The advantages of this variant are less postoperative mobility of the graft, less swelling, better hemostasis,32 and 1.5 to 2 times less shrinkage.51,52 However, there is a healing lag period that is observed for the first 2 weeks.21,22,36
Step 2: Obtain the graft from the donor site. The classic or conventional free gingival graft technique consists of transferring a piece of keratinized gingiva approximately the size of the recipient site. To avoid the large wound that this procedure sometimes leaves in the donor site, some alternative methods have been proposed. The original technique is described first, followed by several of the most common variants.
For the classic technique, a partial-thickness graft is used. The palate is the usual site from which the donor tissue is removed. The graft should consist of epithelium and a thin layer of underlying connective tissue. Place the template over the donor site, and make a shallow incision around it with a #15 blade. Insert the blade to the desired thickness at one edge of the graft. Elevate the edge and hold it with tissue forceps. Continue to separate the graft with the blade, lifting it gently as separation progresses to provide visibility. Placing sutures at the margins of the graft helps control it during separation and transfer and simplifies placement and suturing to the recipient site.10
Proper thickness is important for survival of the graft. It should be thin enough to permit diffusion of fluid from the recipient site, which is essential in the immediate posttransplant period. A graft that is too thin may necrose and expose the recipient site.73,77 If the graft is too thick, its peripheral layer is jeopardized because of the excessive tissue that separates it from new circulation and nutrients. Thick grafts may also create a deeper wound at the donor site, with the possibility of injuring major palatal arteries.102 The ideal thickness of a graft is between 1.0 and 1.5 mm.73,77 After the graft is separated, remove the loose tissue tags from the undersurface. Thin the edge to avoid bulbous marginal and interdental contours. Special precautions must be taken with grafts from the palate.
The submucosa in the posterior region is thick and fatty and should be trimmed so that it will not interfere with vascularization. Grafts tend to reestablish their original epithelial structure, so mucous glands may occur in grafts obtained from the palate.
A thick graft can be thinned by holding it between two wet wooden tongue depressors and slicing it longitudinally with a sharp #15 blade.
Step 3: Transfer and immobilize the graft. Remove the sponge from the recipient site; reapply it with pressure if necessary until bleeding is stopped. Remove the excess clot. A thick clot interferes with vascularization of the graft.74
Position the graft and adapt it firmly to the recipient site. A space between the graft and the underlying tissue (dead space) impairs vascularization and jeopardizes the graft. Suture the graft at the lateral borders and to the periosteum to secure it in position. The graft must be immobilized. Any movement interferes with healing. Avoid excessive tension, which can distort the graft from the underlying surface. Every precaution should be taken to avoid trauma to the graft. Tissue forceps should be used delicately and a minimum number of sutures used to avoid unnecessary tissue perforation.
Step 4: Protect the donor site. Cover the donor site with a periodontal pack for 1 week, and repeat if necessary. Retention of the pack on the donor site can be a problem. If facial attached gingiva was used, the pack may be retained by locking it through the interproximal spaces onto the lingual surface. If there are no open interdental spaces, the pack can be covered by a plastic stent wired to the teeth. A modified Hawley retainer is useful to cover the pack on the palate and over edentulous ridges.
Variant Techniques
The free gingival graft technique is a predictable procedure, but the donor site (palate) is left with an open wound that must heal by secondary intention. The following variant techniques attempt to minimize the donor site wound by removing the donor tissue in a different configuration and altering the shape to maximize coverage over the recipient site. These techniques are (1) the accordion technique, (2) the strip technique, and (3) the combination epithelial-connective tissue strip technique. All are modifications of the free gingival grafts.
The accordion technique, described by Rateitschak et al,83 attains expansion of the graft by alternate incisions in opposite sides of the graft. This technique increases the donor graft tissue by changing the configuration of the tissue.
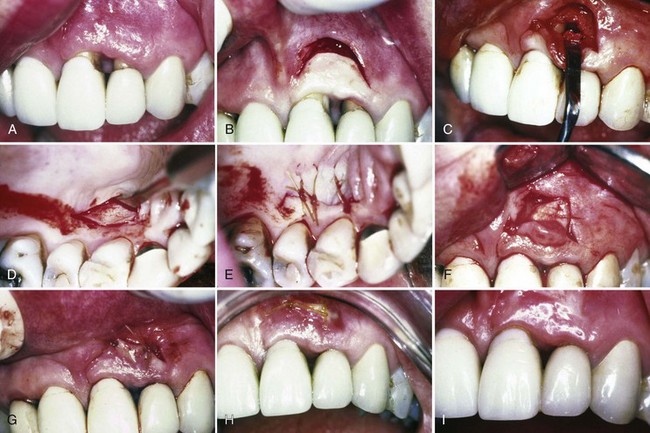
The strip technique, developed by Han et al,46 consists of obtaining two or three strips of gingival donor tissue about 3 to 5 mm wide and long enough to cover the entire length of the recipient site (Figure 63-5). These strips are placed side by side to form one donor tissue and sutured on the recipient site. The area is then covered with aluminum foil and surgical dressing.
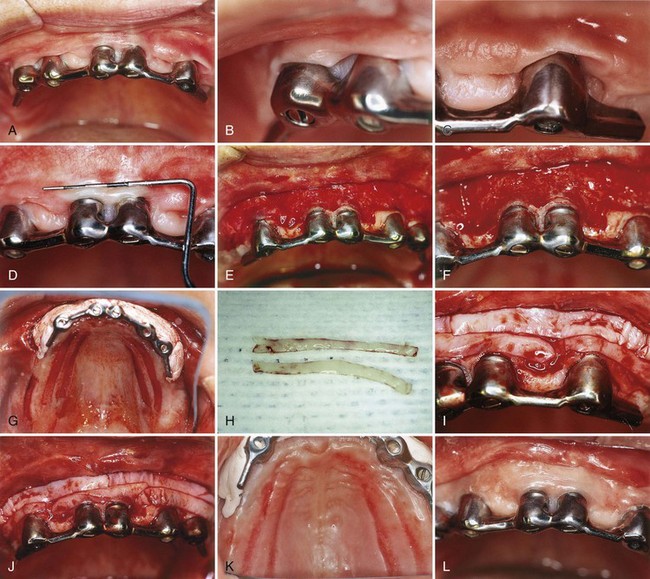
Figure 63-5 Free gingival graft: strip technique. A to D, Mucosal tissue around implants. E and F, Recipient site prepared. G, Donor site with strips of free graft removed. H, Donor strips of free graft. I and J, Strips placed side by side on recipient site. K, Donor area 1 week after graft removal. L, Healing of recipient site after 3 months. Note good keratinized, attached gingiva.
(Courtesy Dr. T. Han and Dr P. Klokkevold, Los Angeles.)
The advantages of this technique are the rapid healing of the donor site. The epithelial migration of the close wound edges (3 to 5 mm) allows rapid epithelialization of the open wound. The donor site usually does not require suturing and heals uneventfully in 1 to 2 weeks.
In some cases, a combination technique can be performed where a deep strip graft is taken from the palate and is split into both an epithelial-connective tissue strip and a pure connective strip. The tissue is obtained as follows: remove a strip of tissue from the palate about 3 to 4 mm thick, place it between two wet tongue depressors, and split it longitudinally with a sharp #15 blade. Both will be used as free grafts. The superficial portion consists of epithelium and connective tissue, and the deeper portion consists only of connective tissue. These donor tissues are placed on the recipient site as in the strip technique. The minimal donor site wound obtained by two donor tissues from one site is the advantage of this technique.
Alternative Donor Tissue
The use of the palate as a donor site for gingival augmentation has numerous disadvantages. Many patients are fearful of palatal surgery from where the donor tissue is procured. There is also a limitation on the amount of tissue that can be removed. Currently, there are numerous clinicians who advocate the use of an acellular dermal matrix (ADM) as a substitute for palatal donor tissue. This product is commercially available under the name AlloDerm and is derived from donated human skin.4 Commercial preparation of this tissue includes a multistep proprietary process that removes both the epidermis and the cells that can lead to tissue rejection and graft failure without damaging the matrix. The remaining ADM consists of a nondenatured three-dimensional arrangement of intact collagen fibers, ground substance, and vascular channels.29 Other techniques to avoid palatal donor site morbidity involve biologic mediators, which is presented later in this chapter.
Healing of the Graft
The success of the graft depends on survival of the connective tissue. Sloughing of the epithelium occurs in most cases, but the extent to which the connective tissue withstands the transfer to the new location determines the fate of the graft. Fibrous organization of the interface between the graft and the recipient bed occurs within 2 to several days.89
The graft is initially maintained by a diffusion of fluid from the host bed, adjacent gingiva, and alveolar mucosa.41 The fluid is a transudate from the host vessels and provides nutrition and hydration essential for the initial survival of the transplanted tissues. During the first day, the connective tissue becomes edematous and disorganized and undergoes degeneration and lysis of some of its elements. As healing progresses, the edema is resolved, and degenerated connective tissue is replaced by new granulation tissue.
Revascularization of the graft starts by the second6 or third43 day. Capillaries from the recipient bed proliferate into the graft to form a network of new capillaries and anastomose with preexisting vessels.53
Many of the graft vessels degenerate and are replaced by new ones, and some of these participate in the new circulation. The central section of the surface is the last to vascularize, but this is complete by the tenth day.
The epithelium undergoes degeneration and sloughing, with complete necrosis occurring in some areas.20,76 It is replaced by new epithelium from the borders of the recipient site. A thin layer of new epithelium is present by the fourth day, with rete pegs developing by the seventh day. Heterotopically placed grafts maintain their structure (keratinized epithelium), even after the grafted epithelium has become necrotic and has been replaced by neighboring areas of nonkeratinized epithelium, which suggests that a genetic predetermination of the specific character of the oral mucosa exists that depends on stimuli originating in the connective tissue.54 This is the basis for the technique that uses grafts composed only of connective tissue obtained from areas in which it is covered by keratinized epithelium.17,30,36
As seen microscopically, healing of a graft of intermediate thickness (0.75 mm) is complete by 10.5 weeks; thicker grafts (1.75 mm) may require 16 weeks or longer.42 The gross appearance of the graft reflects the tissue changes within it. At transplantation, the graft vessels are empty and the graft is pale. The pallor changes to an ischemic grayish white during the first 2 days until vascularization begins and a pink color appears. The plasmatic circulation accumulates and causes softening and swelling of the graft, which are reduced when the edema is removed from the recipient site by the new blood vessels. Loss of epithelium leaves the graft smooth and shiny. New epithelium creates a thin, gray, veil-like surface that develops normal features as the epithelium matures.
Functional integration of the graft occurs by the seventeenth day, but the graft is morphologically distinguishable from the surrounding tissue for months. The graft eventually blends with adjacent tissues, but sometimes, although pink, firm, and healthy, it is somewhat bulbous. This usually presents no problem, but if the graft traps plaque or is esthetically unacceptable, thinning of the graft may be necessary. Thinning the surface of the grafted tissue does reduce the bulbous condition because the surface epithelium tends to proliferate again. The graft should be thinned by making the necessary incisions to elevate it from the periosteum, removing tissue from its undersurface, and suturing it back in place.
Accomplishments
Free gingival grafts effectively widen the attached gingiva. Several biometric studies have analyzed the width of the attached gingiva after the placement of a free gingival graft.19,48,51 After 24 weeks, grafts placed on denuded bone shrink 25%, whereas grafts placed on periosteum shrink 50%.61 The greatest amount of shrinkage occurs within the first 6 weeks.
The placement of a gingival graft does not “improve” the status of the gingiva.34,35,100 Therefore the indication for a free gingival graft should be based on the presence of progressive gingival recession and inflammation. When recession continues to progress after a few months with good plaque control, a graft can be placed to prevent further recession and loss of attached gingiva.
Other materials have been used to replace gingival tissue in gingival extension procedures. Attempts with lyophilized dura mater,88 and sclera have not been satisfactory. The use of irradiated free gingival allograft showed satisfactory results,86 but further research is necessary before it can be considered for clinical use.
Free autogenous gingival grafts have been found to be useful for covering nonpathologic dehiscences and fenestrations. Nonpathologic refers to openings of the bone through the tooth surface not previously exposed to the oral environment and found in the course of flap surgery.33
The use of free gingival autografts to cover denuded roots is described in the section on gingival augmentation coronal to the recession.
Free Connective Tissue Autografts
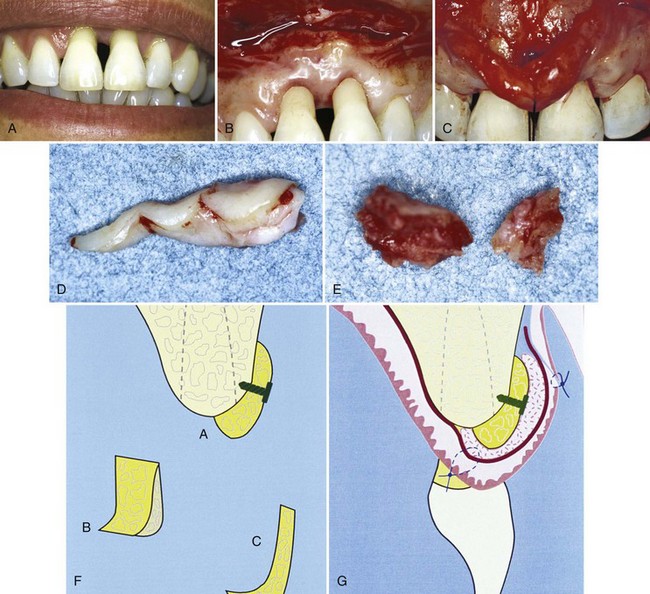
The connective tissue autograft technique was originally described by Edel36 and is based on the fact that the connective tissue carries the genetic message for the overlying epithelium to become keratinized. Therefore only connective tissue from beneath a keratinized zone can be used as a graft (Figure 63-6).
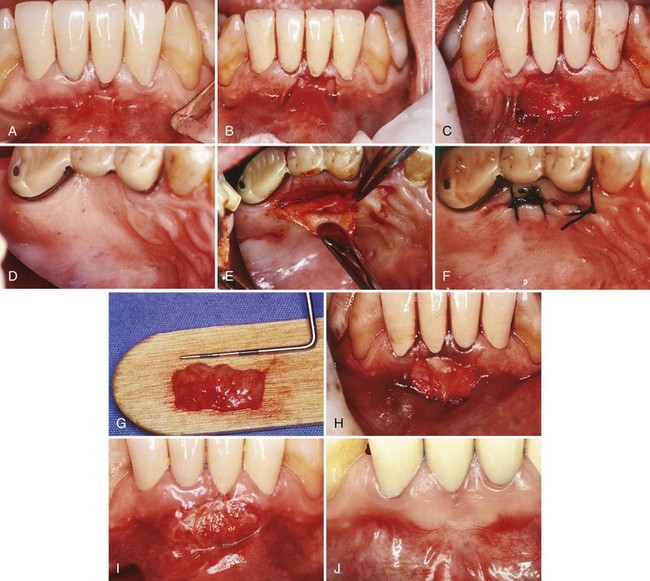
Figure 63-6 Free connective tissue graft. A, Lack of keratinized, attached gingiva buccal to central incisor. B, Vertical incisions to prepare recipient site. C, Recipient site prepared. D, Palate from which connective tissue will be removed for donor tissue. E, Removal of connective tissue. F, Donor site sutured. G, Connective tissue for graft. H, Free connective tissue placed at donor site. I, Postoperative healing at 10 days. J, Final healing at 3 months. Note wide, keratinized, attached gingiva.
(Courtesy Dr. M. Orisini, Italy.)
The advantage of this technique is that the donor tissue is obtained from the undersurface of the palatal flap, which is sutured back in primary closure; therefore healing is by first intention. The patient has less discomfort postoperatively at the donor site.
Another advantage of the free connective tissue autograft is that improved esthetics can be achieved because of a better color match of the grafted tissue to the adjacent areas.
Apically Displaced Flap
This technique utilizes the apically positioned flap, either partial thickness or full thickness, to increase the zone of keratinized gingiva. Chapter 59 provides a step-by-step description of the surgical technique for apically displaced flaps, and Figure 63-7 illustrates the procedure.
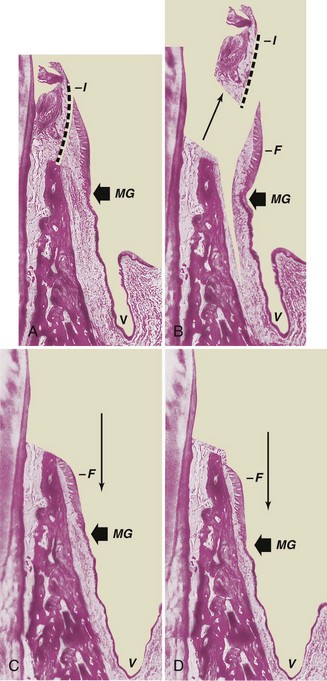
Figure 63-7 Apically displaced partial-thickness flap. A, Internal bevel incision (I) separates inner wall of periodontal pocket. MG, Mucogingival junction; V, vestibular fornix. B, Partial-thickness flap (F) separated, leaving periosteum and a layer of connective tissue on the bone. The inner wall of the periodontal pocket (I) is removed, and the tooth is scaled and planed. C, Partial-thickness flap (F) displaced apically, with edge of the flap at crest of the bone. Note that the vestibular fornix is also moved apically. D, Partial-thickness flap (F) displaced apically, with edge of the flap several millimeters below crest of the bone.
Accomplishments
The apically displaced flap technique increases the width of the keratinized gingiva but cannot predictability deepen the vestibule with attached gingiva. Adequate vestibular depth must be present before the surgery to allow apical positioning of the flap. The edge of the flap may be located in three positions in relation to the bone, as follows:
Placing the flap short of the crest increases the risk of a slight reduction in bone height,28 but the advantage of a well-formed gingival margin compensates for this.
Other Techniques
The “vestibular extension technique,” originally described by Edlan and Mejchar,37 produced statistically significant widening of attached nonkeratinized tissue. This increase in width in the mandibular area reportedly persisted in patients observed for up to 5 years.37,87,101 Currently, this technique is of historic interest only.
The fenestration operation was designed to widen the zone of attached gingiva with a minimum loss of bone height.84,85 It has also been called the periosteal separation technique.26 It uses a partial-thickness flap, except in a rectangular area at the base of the operative field, where the periosteum is removed, exposing the bone. This is the area of fenestration. Its purpose is to create a scar that is firmly bound to the bone.23 It prevents soft tissue separation from the bone and postsurgical narrowing of the attached zone. Results obtained with this technique are not as predictable as with the free gingival graft; therefore it is not widely performed except for small isolated areas.
Gingival Augmentation Coronal to Recession (Root Coverage)
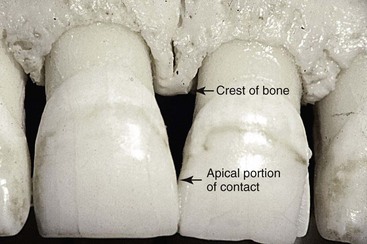
Understanding the different stages and conditions of gingival recession is necessary for predictable root coverage. Several classifications of denuded roots have been proposed. In the 1960s, Sullivan and Atkins94 classified gingival recession into four anatomic categories: (1) shallow-narrow, (2) shallow-wide, (3) deep-narrow, and (4) deep-wide.
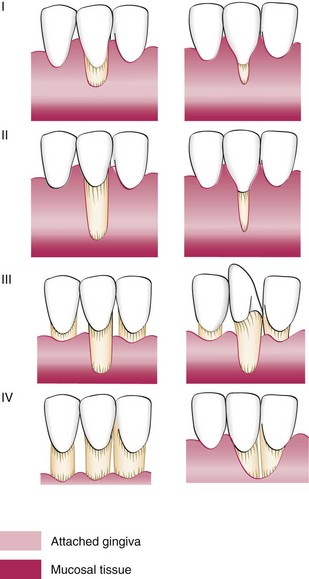
This early classification helped categorize the lesion but did not enable the clinician to predict the outcome of therapy. The predictability of root coverage can be enhanced by the presurgical examination and the correlation of the recession by using the classification proposed by Miller,71 as follows (Figure 63-8):
In general, the prognoses for Classes I and II are good to excellent; whereas for Class III, only partial coverage can be expected. Class IV has a very poor prognosis with current techniques.
The following is a list of techniques used for gingival augmentation coronal to the recession (root coverage):
Some of the techniques used for widening the attached gingiva apical to the area of recession can also be used for root coverage. Both the free gingival and the connective tissue autograft used for apical widening can be used for coronal augmentation by incorporating some modifications. In using the free grafts for root coverage, the recipient bed surrounding the denuded root surface must be extended wider to allow for better blood supply to the donor free graft. This is necessary because a portion of the donor tissue overlies the root surface, which does not have blood supply.
Successful and predictable root coverage has been reported using free gingival autografts.70,72
The Classic Technique
Miller72 applied the classic free gingival autograft described previously with a few modifications.
This technique results in predictable coverage of the denuded root surface but may present esthetic color discrepancies with the adjacent gingiva because of a lighter color.
Free Connective Tissue Autograft
The free connective tissue technique was described by Levine in 1991.57 The difference between this technique and the free gingival autograft is that the donor tissue is connective tissue (see Figure 63-6).
The following is a step-by-step surgical description of the free connective tissue autograft technique:
Laterally (Horizontally) Displaced Pedicle Flap
The displaced pedicle flap technique, originally described by Grupe and Warren in 1956,44 was the standard technique for many years and is still indicated in some cases. The laterally positioned flap can be used to cover isolated, denuded root surfaces that have adequate donor tissue laterally. The vestibular depth must also be present (Figures 63-9 and 63-10).
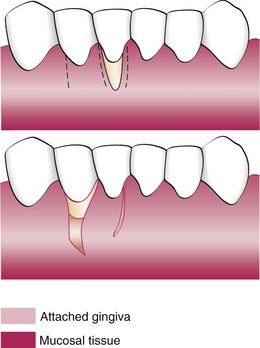
Figure 63-9 Laterally displaced flap for coverage of denuded root. Top, Incisions removing the gingival margin around the exposed root and outlining the flap. Bottom, After the gingiva around the exposed root is removed, the flap is separated, transferred, and sutured.
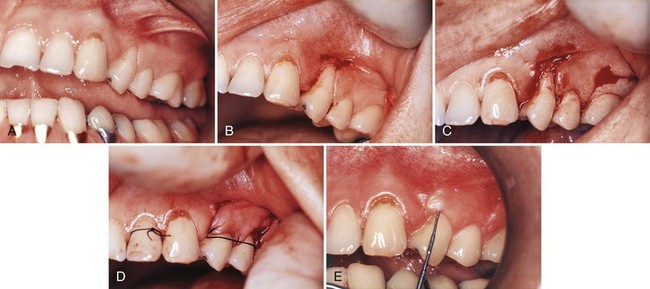
Figure 63-10 Laterally displaced flap. A, Preoperative view, maxillary bicuspid. B, Recipient site is prepared by exposing the connective tissue around the recession. C, Incisions are made at the donor site in preparation of moving the tissue laterally. D, Pedicle flap is sutured in position. E, Postoperative result at 1 year.
(Courtesy Dr. E.B. Kenney, Los Angeles.)
The following is a step-by-step surgical description:
With a #15 blade, make a vertical incision from the gingival margin to outline a flap adjacent to the recipient site. Incise to the periosteum, and extend the incision into the oral mucosa to the level of the base of the recipient site. The flap should be sufficiently wider than the recipient site to cover the root and provide a broad margin for attachment to the connective tissue border around the root. The interdental papilla at the distal end of the flap, or a major portion of it, should be included to secure the flap in the interproximal space between the donor and the recipient teeth.
Make a vertical incision along the gingival margin and interdental papilla, and separate a flap consisting of epithelium and a thin layer of connective tissue, leaving the periosteum on the bone.
A releasing incision is sometimes needed to avoid tension on the base of the flap, which can impair the blood supply when the flap is moved. To do this, make a short oblique incision into the alveolar mucosa at the distal corner of the flap, in the direction of the recipient site.
Variant Techniques
There are many variations in the incisions for the laterally displaced flap. A common alternative is the use of converging oblique incisions over the recipient site and a vertical or oblique incision at the distal end of the donor site so that the transposed flap is slightly wider at its base. In another modification, the marginal attachment at the donor site is preserved to reduce the likelihood of recession and marginal bone resorption, but this requires a donor site with a wider zone of attached gingiva.
Sliding partial-thickness grafts from neighboring edentulous areas (pedicle grafts)43 can be used to restore attached gingiva on teeth adjacent to edentulous spaces with denuded roots and a small, vestibular fornix, often complicated by tension from a frenum. The “double-papilla flap” attempts to cover roots denuded by isolated gingival defects with a flap formed by joining the contiguous halves of the adjacent interdental papillae.25,49 Results with this technique are unpredictable because blood supply is impaired by suturing the two flaps over the root surface.
Accomplishments of Pedicle Autograft
Coverage of the exposed root surface with the sliding-flap technique has been reported to be 60%,41 61%, and 72%.83 Histologic studies in animals have reported 50% coverage.24,104
The extent to which the flap establishes a new attachment to the root with the formation of new cementum and the embedding of new connective tissue fibers has not been established. New attachment on artificially denuded roots in experimental animals104 and in some clinical studies of humans has been reported,93,95 but it does not occur consistently enough to be predictable.
In the donor site, there is uneventful repair and restoration of gingival health and contours, with some loss of radicular bone (0.5 mm) and recession (1.5 mm) reported with full-thickness flaps.
Coronally Displaced Flap
The purpose of the coronally displaced flap procedure is to create a split-thickness flap in the area apical to the denuded root and position it coronally to cover the root. Two techniques are available for this purpose.
First Technique
Variations to First Technique
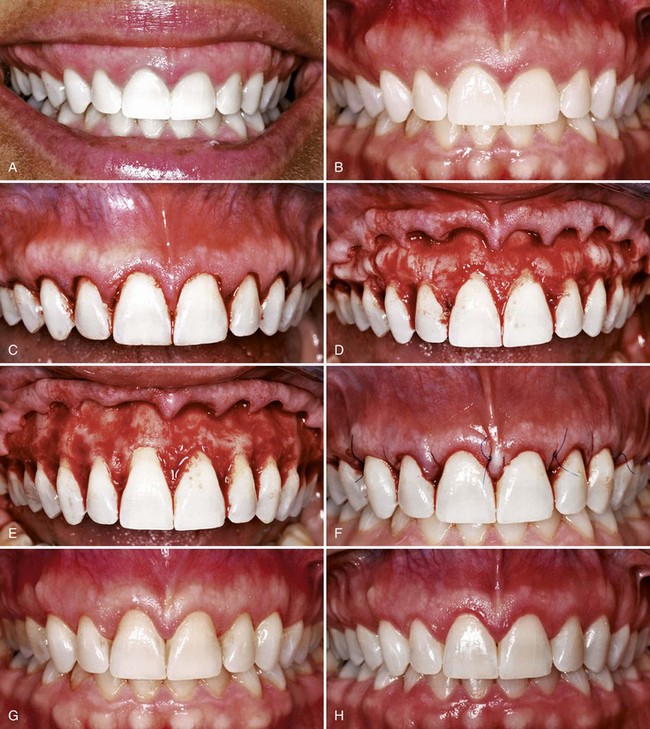
Results with the coronally displaced flap technique are often unfavorable45 because of insufficient keratinized gingiva apical to the recession. To overcome this problem and to increase the chances of success, a gingival augmentation procedure with a free autogenous graft can be performed before the coronally positioned graft, as described earlier in this chapter. This creates several millimeters of attached keratinized gingiva apical to the denuded root (Figure 63-11).
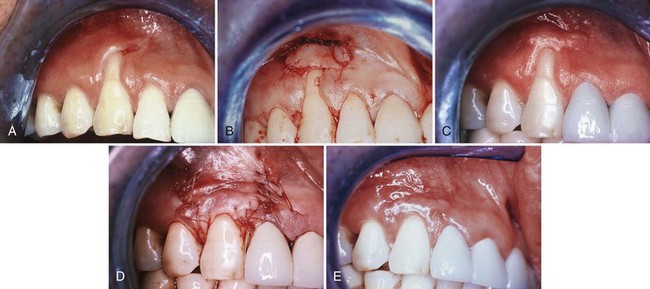
Figure 63-11 Coronally displaced flap. A, Preoperative view. Note the recession and the lack of attached gingiva. B, After placement of a free gingival graft. C, Three months after placement of the graft. D, Flap, including the graft, positioned coronally and sutured. E, Six months later. Note the root coverage and the presence of attached gingiva. Compare with A.
(Courtesy Dr. T.J. Han, Los Angeles.)
Two months after this surgery, a second-stage procedure is performed, coronally positioning the flap that includes the free autogenous graft. The use of citric acid with a pH 1.0 for conditioning the root surface has been suggested.50,58
A significant degree of reduction in recession treated by this double-step procedure was reported after 2 years by Bernimoulin et al11 and confirmed by others.18,60,61
Second Technique
Tarnow has described the semilunar coronally repositioned flap to cover isolated denuded root surfaces97 (Figure 63-12).
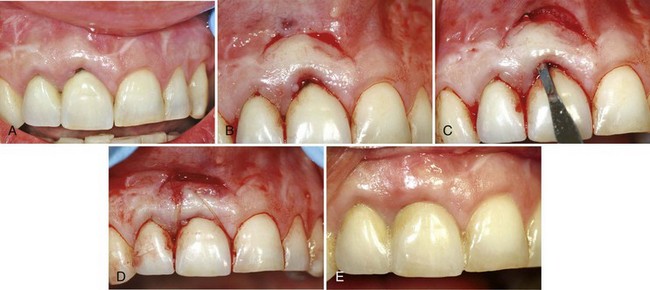
Figure 63-12 Semilunar coronally positioned flap. A, Slight recession in facial of the upper left canine. B, After thorough scaling and root planing of the area, a semilunar incision is made and the tissue separated from the underlying bone. The flap collapses, covering the recession. C, Appearance after 7 weeks. Note coverage of the previous root denudation.
(Courtesy Dr. J.J. Elbaz, Beverly Hills, California.)
Subepithelial Connective Tissue Graft (Langer)
The subepithelial connective tissue procedure is indicated for larger and multiple defects with good vestibular depth and gingival thickness to allow a split-thickness flap to be elevated. Adjacent to the denuded root surface, the donor connective tissue is sandwiched between the split flap (Figures 63-13 and 63-14). This technique was described by Langer and Langer in 1985.56 Similar approaches had been previously reported by Perez-Fernandez79 and Raetzke.82
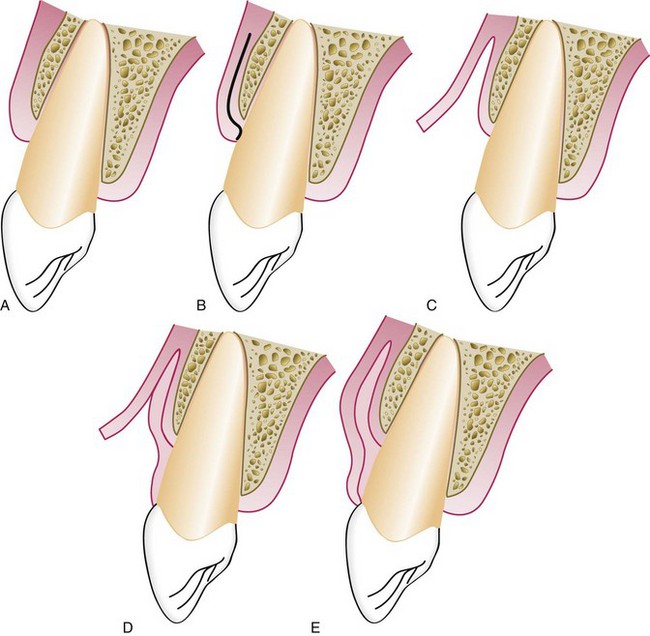
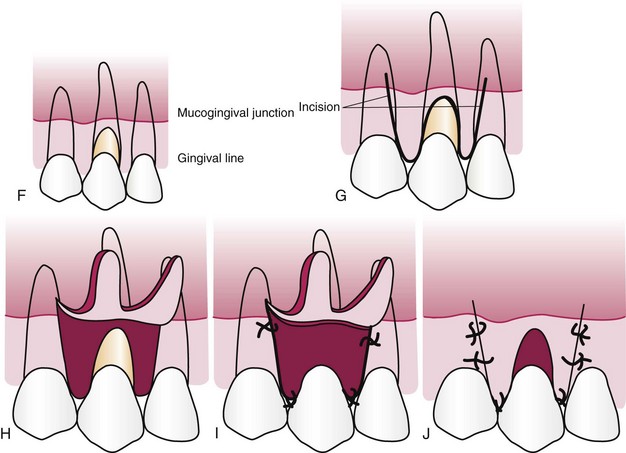
Figure 63-13 Subepithelial connective tissue graft for root coverage. A to E, Sagittal views. A, Preoperative view of facial recession on maxillary central incisor. B, Split-thickness incision for recipient site. C, Split-thickness flap reflected. D, Connective tissue placed over denuded root surface. Note that apical portion of the donor tissue is placed between the split flap. E, Recipient flap is closed. Subepithelial connective tissue graft for root coverage. F, Gingival recession. G, Vertical incisions to prepare recipient site. H, Split-thickness flap reflected. I, Connective tissue sutured over denuded root surface. J, Split-thickness flap sutured over donor connective tissue.
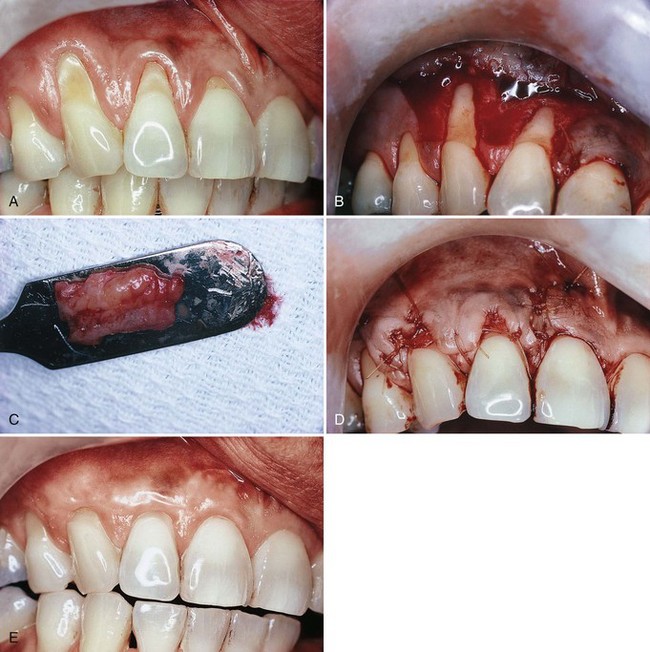
Figure 63-14 Langer technique for root coverage. A, Preoperative view. Note the recession on teeth #6 to #8. B, Split-thickness flap elevated on teeth #6 and #7. Note that the interdental papillae are not included in the flap, nor is the gingival margin area of tooth #8, which was treated by means of a coronally displaced flap. C, Connective tissue from palate. D, graft placed under the flap and covering receded areas approximately to the cementoenamel junction. Sutures in place. E, Roots covered after complete healing. Note the thickness of the tissue in the area covered and excellent color.
(Courtesy Dr. T.J. Han, Los Angeles.)
The following is the step-by-step surgical description:
A variant of the subepithelial connective tissue graft, called a subpedicle (bilaminar) connective tissue graft, was described by Nelson in 1987.75 This technique uses a pedicle over the connective tissue that covers the denuded root surface. Therefore the blood supply is increased over the donor tissue and the gingival margin is thickened for better marginal stability.
Guided Tissue Regeneration Technique for Root Coverage
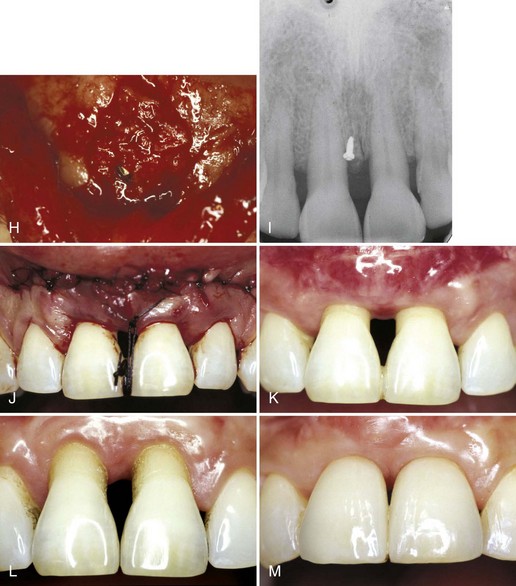
Pini-Prato et al80 described a technique based on the principle of guided tissue regeneration (GTR) (see Chapter 61). Theoretically, GTR should result in reconstruction of the attachment apparatus, along with coverage of the denuded root surface (Figure 63-15).
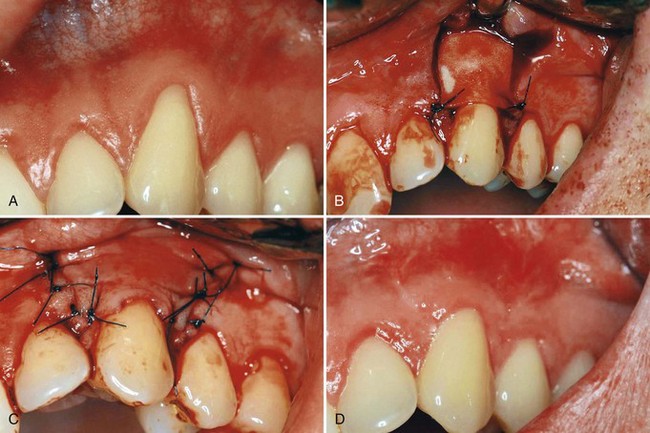
Figure 63-15 Guided tissue regeneration technique for root coverage. A, Marked recession of maxillary left cuspid. B, Vertical incisions made and membrane placed over recession. C, Flap sutured over the membrane. D, Postoperative result. Note complete coverage of recession.
(Courtesy Dr. Zoran Aleksic, Belgrade, Serbia.)
The following is a step by step description of the surgery:
Tinti and Vincenzi99 used titanium-reinforced membranes to create space beneath the membrane. Resorbable membranes have also been used to achieve root coverage. The inability to create space between the resorbable membrane and the denuded root, because of its softness, may present a problem, even though not needing a second surgery is an advantage.
Clinical studies comparing this technique with the coronally displaced flap have shown that the GTR technique is better when the recession is greater than 4.98 mm apicocoronally.80 Histologically, one case reported 3.66 mm of new connective tissue attachment associated with 2.48 mm of new cementum and 1.84 mm of bone growth.27
Pouch and Tunnel Technique (Coronally Advanced Tunnel Technique)
To minimize incisions and the reflection of flaps and to provide abundant blood supply to the donor tissue, the placement of the subepithelial donor connective tissue into pouches beneath papillary tunnels allows for intimate contact of donor tissue to the recipient site.107 The positioning of the graft in the pouch and through the tunnel and the coronal placement of the recessed gingival margins completely covers the donor tissue. Therefore the esthetic result is excellent. The technique is especially effective for the anterior maxillary area in which vestibular depth is adequate and there is good gingival thickness (Figure 63-16).
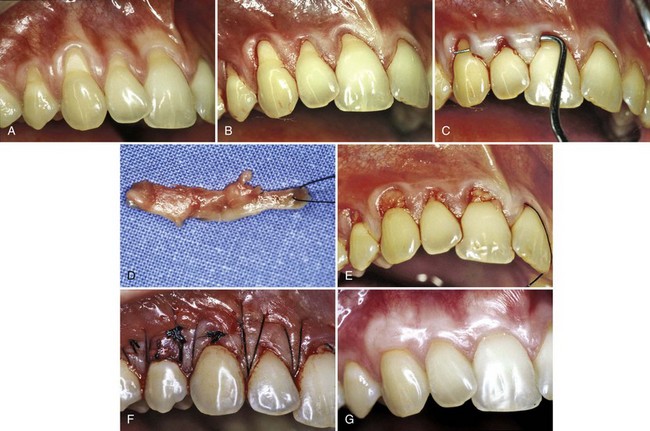
Figure 63-16 Pouch and tunnel technique for root coverage. A, Preoperative view. Note gingival recession. B, Sulcular incision is made from the mesial to the facial line angles. C, A tunnel is made through the papilla using a blunt incision. D, A connective tissue graft is taken from the palate. E, The connective tissue is placed through the papillary tunnel and apically beneath the pouch. F, The facial gingival margin covers the connective tissue using horizontal mattress sutures interdentally. G, Postoperative view. Note complete root coverage and thickened gingival margin at 3 months.
(Courtesy Dr. Robert R. Azzi, Paris.)
One of the advantages to this technique is the thickening of the gingival margin after healing. The thicker gingival margin is stable to allow for the possibility of “creeping reattachment” of the margin. The use of small, contoured blades enables the surgeon to incise and split the gingival tissues to create the recipient pouches and tunnels (Figure 63-17). The work by Azzi et al8 in this area of surgery has contributed to a better understanding of the technique and outcome of this procedure. This surgery is also referred to as the coronally advanced tunnel technique.
Following is a step-by-step description of the surgical procedure as outlined by Azzi (see Figure 63-16):
The area is not covered with periodontal dressing.
The patient is instructed to rinse daily with chlorhexidine gluconate and to avoid touching the sutures during oral hygiene procedures.
Antibiotics can be administered (amoxicillin 500 mg three times a day) if deemed necessary.