Medical Nutrition Therapy for Thyroid and Related Disorders
Diabetes mellitus appears to be the most common endocrine-related chronic disease (American Diabetes Association [ADbA], 2007). However, according to the American Association of Clinical Endocrinologists (2005), 27 million Americans have thyroid-related disorders and more than half remain undiagnosed. Furthermore, individuals with diabetes tend to have a higher prevalence of thyroid disorders.
Thyroid-related diseases are often poorly diagnosed, and there is much about their treatment that requires greater clarification and study. For example, radiation exposure of the thyroid at a young age is a risk factor for the development of thyroid cancer, lasting for a lifetime after exposure (Sinnott et al., 2010). Efforts to reduce exposure from medical x-ray examinations can protect the thyroid gland.
Genetic factors promote the endocrine autoimmune diseases. Recent genome-wide association studies (GWAS) have enabled identification of relevant immune response pathways; the same allele that predisposes to a certain autoimmune disease can be protective in another (Wiebolt et al., 2010). Thus endocrine GWAS are needed, especially for Graves’ disease, Hashimoto’s thyroiditis, and Addison’s disease. Each of these disorders has stages beginning with genetic susceptibility, environmental triggers, and active autoimmunity, followed by metabolic derangements with overt symptoms of disease (Michels and Eisenbarth, 2010). Research is needed to clarify how nutrients interact with genetics, especially in the autoimmune thyroid disorders (AITDs).
Thyroid Physiology
The thyroid gland is a small, butterfly-shaped gland found just below the Adam’s apple. Although it weighs less than an ounce, it produces hormones that influence essentially every organ, tissue, and cell in the body, thus having an enormous effect on health. The thyroid gland responds to stimulation by thyroid-stimulating hormone (TSH), a hormone secreted by the pituitary gland. When stimulated, the thyroid gland produces two main hormones: thyroxine (T4), a thyroid hormone named for its four molecules of iodine, and triiodothyronine (T3), a thyroid hormone named for its three molecules of iodine. T3 is the most predominant and active form of thyroid hormone that the body can use. The thyroid gland regulates many processes in the body, including fat and carbohydrate metabolism, body temperature, and heart rate. The thyroid also produces calcitonin, a hormone that helps regulate the amount of blood calcium. Last, reverse T3 (rT3) an isomer of T3, is derived from T4 through the action of deiodinase. The body cannot use rT3.
The synthesis of these hormones requires tyrosine, a key amino acid involved in the production of thyroid hormone, and the trace mineral iodine. Within the cells of the thyroid gland, iodide is oxidized to iodine by hydrogen peroxide, a reaction termed the organification of iodide. Two additional molecules of iodine bind to the tyrosyl ring in a reaction that involves thyroid peroxidase (TPO), an enzyme in the thyroid responsible for thyroid hormone production. Completed thyroid hormones are released into the circulation; however, metabolic effects of thyroid hormones result when the hormones ultimately occupy specific thyroid receptors. It is estimated a cell needs five to seven times more T4 to bind to the nuclear receptors to have a physiologic effect compared with T3.
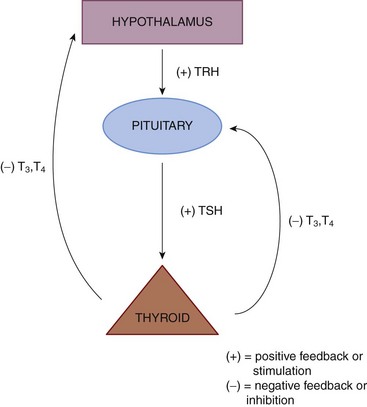
The biosynthetic processes resulting in generation of thyroid hormones within the thyroid gland are controlled by feedback mechanisms within the hypothalamic-pituitary-thyroid axis (HPT axis). The HPT axis is part of the endocrine system responsible for the regulation of metabolism. As its name suggests, it depends on the hypothalamus (a tiny, cone-shaped structure located in the lower center of the brain that communicates between the nervous and endocrine systems), the pituitary gland (the master gland of the endocrine system located at the base of the brain), and the thyroid gland (Figure 32-1).
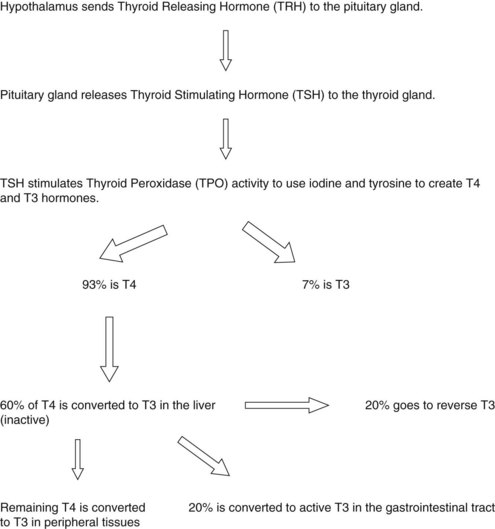
The hypothalamus produces and secretes thyrotropin-releasing hormone (TRH), which travels to the pituitary gland, stimulating it to release TSH, which signals the thyroid gland to upregulate its synthetic machinery. Although T4, T3, and rT3 are generated within the thyroid gland, T4 is quantitatively the major secretory product. All T4 found in circulation is generated in the thyroid unless exogenously administered. Production of T3 and rT3 within the thyroid is relegated to very small quantities and is not considered significant compared with their peripheral production (Figure 32-2).
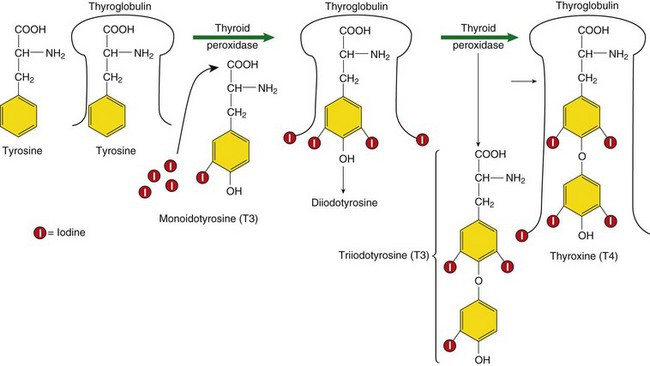
FIGURE 32-2 Constructing thyroid hormones. (1) Accumulation of the raw materials tyrosine and iodide (I-), (2) fabrication or synthesis of the hormone, and (3) secretion of free hormone into the blood.
When T4 is released from the thyroid, it is primarily in a bound form with thyroid-binding globulin (TBG), a protein that transports thyroid hormones through the bloodstream, with lesser amounts bound to T4-binding prealbumin. It is estimated that only 0.03% to 0.05% of T4 within the circulatory system is in a free or unbound form; this unbound T4 is called free T4. In peripheral tissues, approximately 70% of T4 produced is either deiodinated and converted to T3 or rT3, or eliminated. As mentioned, T3 is considered to be the most metabolically active thyroid hormone. Although some T3 is produced in the thyroid, approximately 80% to 85% is generated outside the thyroid, primarily by conversion of T4 in the liver and kidneys. The pituitary and nervous system are capable of converting T4 to T3, so are not reliant on T3 produced in the liver or kidney. Within the liver and kidney, the enzyme responsible for production of T3 is a selenium-dependent enzyme called 5′-deiodinase, an enzyme that removes one molecule of iodine from T4 to form either T3 or rT3 (Figure 32-3).
Assessment in Thyroid Disorders
Assessment begins with an evaluation of thyroid status based on laboratory data such as a full thyroid panel. It may also include a diet history to evaluate micronutrients pertaining to thyroid health along with an evaluation of calorie and carbohydrate intake. Additionally, an assessment of dietary intake of goitrogenic foods may be warranted.
Laboratory Norms: Functional versus Pathological Ranges
A typical (statistical) reference range for TSH in many laboratories is approximately 0.2-5.5 mIU/L. Individuals with TSH values greater than 2 mIU/L have an increased risk of developing overt hypothyroidism during the next 20 years. Subclinical autoimmune thyroid disease is so common in the population that laboratory reference ranges derived from testing apparently healthy subjects could easily be misconstrued for those with disease. Importantly, several studies have detected an increase in TPO antibody positivity with TSH concentrations outside the narrow range of 0.2-1.9 mIU/L (Downs et al., 2008). This fact provides evidence that TSH in the upper reference range is often associated with abnormal pathologic findings (Hak et al., 2000; Saravanan et al., 2002). Additional evidence that thyroid function within the laboratory reference ranges can be associated with adverse outcomes is shown in Table 32-1. Conversely, decreased TSH levels combined with normal to high T4 or T3 levels may be suggestive of hyperthyroidism.
TABLE 32-1
Variation In Thyroid Function within Reference Range and Adverse Outcomes
| TSH > 2 mIU/L* | Increased 20-year risk of hypothyroidism |
| TSH > 2 mIU/L* | Increased frequency of thyroid autoantibodies |
| TSH > 4 mIU/L* | Increased risk of heart disease |
| TSH 2-4 mIU/L* | Cholesterol values respond to thyroxine replacement |
| Free T4 < 10.4 pmol/L† | Impaired psychomotor development of infant if occurs in first trimester of pregnancy |
T3, Triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Changes in 5′-deiodination occur in a number of situations, such as stress, poor nutrition, illness, selenium deficiency, and drug therapy. Toxic metals such as cadmium, mercury and lead have been associated with impaired hepatic 5′-deiodination in animal models. Free radicals are also involved in inhibition of 5′-deiodinase activity. In the course of chronic liver disease such as hepatic cirrhosis, alterations in hepatic deiodination resulting in increased rT3 and a simultaneous decrease in T3 levels have also been observed (Box 32-1).
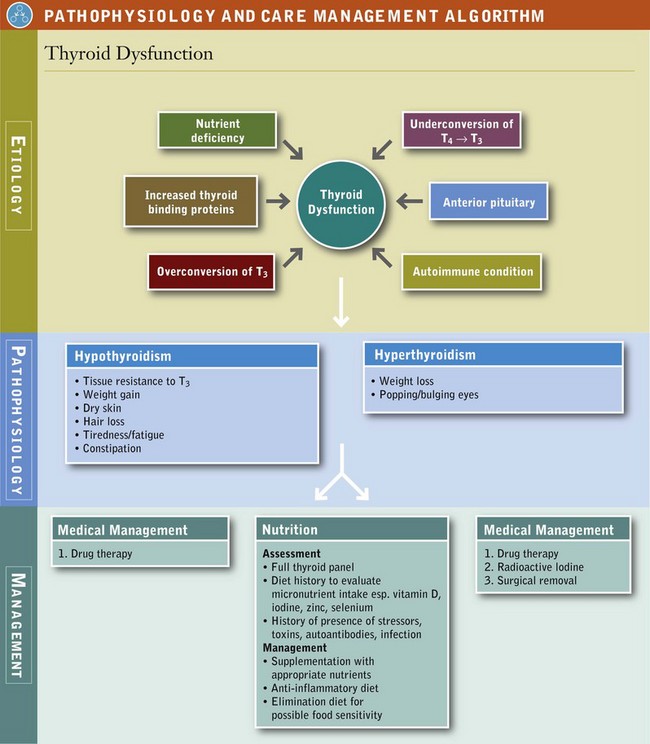
Hypothyroidism
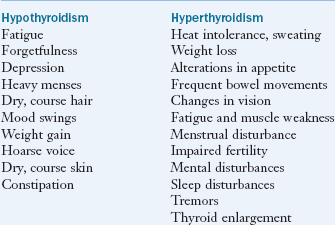
Of the detected cases of underactive thyroid (hypothyroidism), more than half are due to an autoimmune disorder called Hashimoto’s thyroiditis, in which the immune system attacks and destroys thyroid gland tissue. A common clinical presentation of patients with functional changes of the endocrine system is altered thyroid function. Indeed subclinical hypothyroidism represents the first signs of thyroid hormone dysfunction for many individuals. Typical symptoms include low energy, cold hands and feet, fatigue, hypercholesterolemia, muscle pain, depression, and cognitive deficits (Box 32-2). Evaluating thyroid hormone metabolism is needed before thyroid hormone replacement therapy.
Women are five to eight times more likely than men to suffer from hypothyroidism. In addition, individuals who have celiac disease may be at risk (see Clinical Insight: Was It Gluten That Caused Her Hypothyroidism?)
Pathophysiology
Hashimoto’s thyroiditis is an autoimmune disorder in which the immune system attacks and destroys the thyroid gland. It is the most common form of hypothyroidism. The enlarged, chronically inflamed thyroid gland becomes nonfunctional, with reactive parts of the gland deteriorating after several years. Thyroid autoantibodies indicate the body’s immune system is attacking itself and whether an autoimmune thyroid condition is present, be it hypothyroidism or hyperthyroidism.
Specific antibody tests identify Hashimoto’s thyroiditis. Thyroid peroxidase antibodies (TPO Ab) are immune cells that indicate the immune system is attacking TPO in the thyroid gland. The TPO Ab test is the most important, because TPO is the enzyme responsible for the production of thyroid hormones, and the most frequent target of attack in Hashimoto’s. Thyroglobulin antibodies (TGB Ab) are immune cells that indicate the immune system in attacking thyroglobulin in the thyroid gland. Sometimes this test is necessary as well because it is the second most common target for Hashimoto’s disease.
Schmidt’s syndrome refers to hypothyroidism with other endocrine disorders, including Addison’s disease (adrenal insufficiency), hypoparathyroidism, and diabetes mellitus, all of which may be autoimmune in nature. Euthyroid sick syndrome is hypothyroidism, associated with a severe systemic illness that causes decreased peripheral conversion of T4 to T3, an increased conversion of T3 to the inactive rT3, and decreased binding of thyroid hormones. Conditions commonly associated with this syndrome include protein-calorie malnutrition, surgical trauma, myocardial infarction, chronic renal failure, diabetic ketoacidosis, anorexia nervosa, cirrhosis, thermal injury, and sepsis. Once the underlying cause is treated, the condition is usually resolved (see Pathophysiology and Care Management Algorithm: Thyroid Dysfunction).
Triggers
Adrenal Stress and Oxidative Stress: Low thyroid function is almost always secondary to some other condition, often adrenal fatigue (Abdullatif and Ashraf, 2006). Adrenal fatigue (adrenal stress) denotes a syndrome caused by the decreased ability of the adrenal glands to respond adequately to stress (Wilson, 2008). The adrenal glands are the two glands that sit over the kidneys and are primarily responsible for governing the body’s adaptations to stress of any kind. Chronic adrenal stress causes the following:
• Affects communication between the brain and hormone-secreting glands. The hypothalamus and pituitary gland direct hormone production, including that of the thyroid. When the hypothalamus and pituitary weaken because of chronic adrenal stress, they are not able to communicate well with the thyroid gland.
• Increases thyroid-binding protein activity, so that thyroid hormones cannot get into cells to do their job.
• Hampers the conversion of T4 to active forms of T3 that the body can use.
• Interferes with the detoxification pathways through which unnecessary thyroid hormones exit the body, leading to thyroid hormone resistance.
• Causes cells to lose sensitivity to thyroid hormones.
• Weakens the immune barriers of the digestive tract, lungs, and brain; promotes poor immune regulation.
These factors increase the risk for triggering Hashimoto’s or exacerbating it. These are some of the ways adrenal stress directly affect thyroid function.
Chronic adrenal stress affects other systems of the body, which in turn, decrease thyroid function. For example, the adrenal hormone cortisol plays a big role in thyroid health. Cortisol raises blood sugar when it drops too low. When this happens repeatedly, it exhausts the adrenal and thyroid glands, as well as the hypothalamus and the pituitary gland. Over time, this exhaustion leads to functional hypothyroidism. Additionally, constant cortisol production weakens the gastrointestinal (GI) tract, making it more susceptible to inflammation, dysbiosis, and infection. Thus a vicious cycle weakens the thyroid.
Aging: Maintaining thyroid hormone function throughout the aging process appears to be an important hallmark of healthy aging. The incidence of hypothyroidism (underactive thyroid) increases with age. By age 60, 9% to 17% of men and women have an underactive thyroid. The absence of circulating thyroid autoantibodies in healthy centenarians is noted. Because unhealthy aging is associated with a progressively increasing prevalence of organ-specific and non–organ-specific autoantibodies, the absence of these antibodies may represent a significantly reduced risk for cardiovascular disease and other chronic age-related disorders.
Pregnancy: Thyroid dysfunction has been related to obstetrical complications such as premature delivery, gestational hypertension, preeclampsia, and placental abruption. Nearly 1 out of 50 women in the United States is diagnosed with hypothyroidism during pregnancy. Out of every 100 miscarriages, 6 are associated with thyroid hormone deficiency during pregnancy; up to 18% of women are diagnosed with postpartum thyroiditis; and approximately 25% of women develop permanent hypothyroidism (De Vivo et al., 2010; Yassa et al., 2010).
The World Health Organization (WHO) recently increased the recommended iodine intake during pregnancy from 200 to 250 mcg/d and suggested that a median urinary iodine (UI) concentration of 150-249 mcg/L indicates adequate iodine intake in pregnant women. In areas of severe iodine deficiency, maternal and fetal hypothyroxinemia can cause cretinism and adversely affect cognitive development in children. To prevent fetal damage, iodine should be given before or early in pregnancy. In countries or regions where less than 90% of households are using iodized salt and the median UI concentration in school-age children is less than 100 mcg/L, the WHO recommends iodine supplementation in pregnancy and infancy (Zimmermann, 2009).
Medical Management
When the thyroid is underactive (hypothyroidism) because of autoimmune disease (Hashimoto’s disease), radioactive iodine treatment, congenital defects, or surgical removal (thyroidectomy), the conventional pharmacologic approach for treatment is prescription thyroid hormone replacement medication. Table 32-2 provides an overview of key forms of thyroid hormone replacement. With the further elucidation of the effects of genetics, new agents are likely to become available as adjunct therapy (Anderson, 2008).
TABLE 32-2
Pharmacologic Treatments for Hypothyroidism
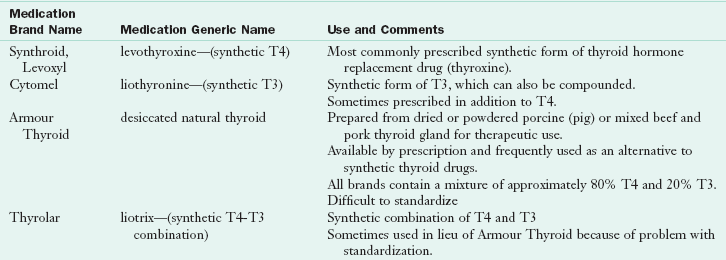
T3, Triiodothyronine; T4, thyroxine.
From Shomon M: All about thyroid drugs, 2007. Accessed May 16, 2011 from http://thyroid.about.com/cs/thyroiddrugs/a/overview.htm.
Medical Nutrition Therapy
It is well established that several nutrients are involved in thyroid health, particularly iodine and selenium. Because of the critical role of iodine in the synthesis of thyroid hormone, this trace mineral has received the most attention historically with respect to thyroid disorders. Other deficiencies of micronutrients such as iron, selenium, vitamin A, and possibly zinc may interact with iodine nutriture and thyroid function (Hess, 2010).
Fasting or Restrictive Diets: Calorie and carbohydrate restriction may substantially reduce thyroid hormone activity. There is a wide range of variation between individuals; genetics, obesity, gender, and the macronutrient content of the hypocaloric diet influence the response. Nutritional status and energy expenditure both influence thyroid function centrally at the level of TSH secretion, deiodination, and possibly elsewhere. Because an increase of rT3 is found at the expense of T3 during caloric restriction, it is possible that the hepatic pathways play a substantial role in metabolic control during energy balance. However, when caloric restriction is longer than three weeks, T4 and rT3 levels return to normal values.
Fasting also exerts a powerful influence on the metabolism of thyroid hormones. Mild elevations in endogenous cortisol levels might be partly responsible. Ketones generated from calorie deprivation do not appear to suppress T3 generation and hepatic 5′-deiodinase activity. However, it is not clear whether ketones have a similar effect in a calorie-sufficient diet. On a low-calorie diet, elimination of rT3 by 5′-deiodination is decreased. Calories and energy balance might also influence thyroid hormone metabolism during increased caloric consumption, during which the clearance of rT3 by 5′-deiodination is actually increased. On a low-calorie diet, elimination of rT3 by 5′-deiodination is decreased; however, the clearance of rT3 by 5′-deiodination is actually increased with a high calorie diet.
Goitrogens: Cyanogenic plant foods (cauliflower, broccoli, cabbage, Brussel sprouts, mustard seed, turnip, radish, bamboo shoot, and cassava) exert antithyroid activity through inhibition of TPO. The hydrolysis of some glucosinolates found in cruciferous vegetables (e.g., progoitrin) may yield goitrin, a compound known to interfere with thyroid hormone synthesis. The hydrolysis of indole glucosinolates results in the release of thiocyanate ions, which can compete with iodine for uptake by the thyroid gland. Increased exposure to thiocyanate ions from cruciferous vegetable consumption, however, does not increase the risk of hypothyroidism unless accompanied by iodine deficiency.
Soybean, an important source of protein in many developing countries, also has goitrogenic properties when iodine intake is limited. The isoflavones, genistein and daidzein, inhibit the activity of TPO and can lower thyroid hormone synthesis. Furthermore, soybean interrupts the enterohepatic cycle of thyroid hormone metabolism. However, high intakes of soy isoflavones do not appear to increase the risk of hypothyroidism when iodine consumption is adequate.
Since the addition of iodine to soy-based formulas in the 1960s, there have been no further reports of hypothyroidism developing in soy formula-fed infants. Soybeans are by far the most concentrated source of isoflavones in the human diet. Small amounts are found in a number of legumes, grains, and vegetables. Average dietary isoflavone intakes in Asian countries, in particular in Japan and China, range from 11-47 mg/day because of intake of the traditional foods made from soybeans, including tofu, tempeh, miso, and matte, whereas intakes are considerably lower in Western countries (2 mg/day). Soy products (meat substitutes, soy milk, soy cheese, and soy yogurt), however, are gaining popularity in Western countries. Although research has not determined the exact effect of soy on the metabolic fate of thyroid hormones, excessive soy consumption is best approached cautiously in those with suspected impairment of thyroid metabolic pathways.
Iodine: As a trace element, iodine is present in the human body in amounts of 10-15 mg and 70% to 80% of it is located in the thyroid gland (Melse-Boonstra and Jaiswal, 2010) (see Chapter 3). Ninety percent of it is organically bound to thyroglobulin (Tg). Iodide is actively absorbed in the thyroid gland to help produce the biochemically active thyroid hormones, T4 and T3 (see Figure 32-2).It is estimated the thyroid gland must capture a minimum of 60 mcg of iodide (the ionic form of iodine) daily to ensure an adequate supply for the production of thyroid hormone (Gropper et al., 2009). Inadequate intake of iodine impairs thyroid function and results in a spectrum of disorders. Randomized controlled intervention trials in iodine deficient populations have shown that providing iron along with iodine results in greater improvements in thyroid function and volume than providing iodine alone (Hess, 2010). It is also vital to thyroid function, as it is a major cofactor and stimulator for the enzyme TPO.
In autoimmune Hashimoto’s, supplementing with iodine may exacerbate the condition. Because iodine stimulates production of TPO, this in turn increases the levels of TPO antibodies (TPO Abs) dramatically, indicating an autoimmune flare-up. Some people develop symptoms of an overactive thyroid, whereas others have no symptoms despite tests showing an elevated level of TPO Abs. Therefore one must be cautious regarding the use of iodine. Furthermore, although iodine deficiency is the most common cause of hypothyroidism for most of the world’s population (Melse-Boonstra and Jaiswal, 2010), in the United States and other westernized countries, Hashimoto’s accounts for the majority of cases (Ebert, 2010; Sloka et al., 2005).
Although the risk of iodine deficiency for populations living in iodine-deficient areas without adequate iodine fortification programs is well recognized, concerns have been raised that certain subpopulations may not consume adequate iodine in countries considered iodine-sufficient. Vegetarian and nonvegetarian diets that exclude iodized salt, fish, and seaweed have been found to contain very little iodine. Furthermore, UI excretion studies suggest that iodine intakes are declining in Switzerland, New Zealand, and the United States, possibly because of increased adherence to dietary recommendations to restrict salt intake for reducing the incidence of hypertension.
Severe iodine deficiency during pregnancy has been shown to increase the risk of stillbirths, spontaneous abortions, and congenital abnormalities. The most severe is cretinism, which is a state of mental retardation mostly in combination with dwarfism, deaf-mutism, and spasticity (Chen and Hetzel, 2010). These conditions are largely irreversible. The consequences of severe iodine deficiency during pregnancy on pregnancy outcome and early infant development have been described extensively (Zimmermann, 2009); other details can be found in Chapter 16.
Iron: Historically, it has been thought that low thyroid function may cause anemia. Recent studies suggest that low thyroid function may be secondary to low iron status or anemia. The reason for this is because TPO is a glycosylated heme enzyme that is iron-dependent. The insertion of heme iron into TPO is necessary for the enzyme to translocate to the apical cell surface of thyrocytes (or thyroid epithelial cells), thus assisting TPO to catalyze the two initial steps of thyroid hormone synthesis (Zimmermann, 2006). A full assessment of iron status could likely help to identify the cause of many cases of thyroid malfunction (Titchenal et al., 2009).
Selenium: Selenium, as selenocysteine, is a cofactor for 5′-deiodinase. If selenium is deficient, the deiodinase activity is impaired, resulting in a decreased ability to deiodinate T4 to T3. In animals, deficiencies of selenium are associated with impaired 5′-deiodinase activity in the liver and kidney, as well as reduced T3 levels. Evidence suggests a strong linear association between lower T3/T4 ratios and reduced selenium status, even among individuals considered to be euthyroid based on standard laboratory parameters. This association is particularly strong in older adults, possibly as the result of impaired peripheral conversion. An inverse relationship between T3 and breast cancer is associated with decreased selenium status, even when plasma T4 and TSH concentrations may be similar. This combination of factors strongly suggests that low T3 may be due to faulty conversion of T4 to T3 expected in selenium deficiency.
Selenium participates in the antioxidant network. It assists in detoxification as part of glutathione peroxidase, an enzyme whose main biologic role is to protect the organism from oxidative damage. Several studies reported on the benefit of selenium treatment both in Hashimoto’s thyroiditis and Graves’ disease.
Evidence also suggests that high intakes of selenium might exert a detrimental influence on thyroid hormone metabolism. Although individuals exposed to high dietary levels of selenium typically have normal levels of T4, T3, and TSH, a significant inverse correlation has been found between T3 and selenium. Some researchers have hypothesized the activity of 5′-deiodinase might become depressed after a high dietary intake of selenium, suggesting a safe level of dietary selenium at or below 500 mcg daily (Kohrle and Gartner, 2009).
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a common endocrine disorder of unknown cause that affects an estimated 3% to 12% of women of reproductive age in Western societies (Moran et al., 2010). The condition is characterized by reproductive issues such as amenorrhea or other menstrual irregularities, anovulation, enlarged ovaries with multiple cysts, and infertility. More generalized symptoms include acne, hirsutism (excessive or abnormal distribution of hair growth), male-pattern baldness, obesity, and sleep apnea (Table 32-3).
Pathophysiology
Biochemical and endocrine abnormalities in women with PCOS include elevated levels of androgens (dehydroepiandrosterone, testosterone, and androstenedione), hyperinsulinemia (which results from insulin resistance), impaired glucose tolerance, and hyperlipidemia. Hyperandrogenism is responsible for many of the symptoms of PCOS, such as reproductive and menstrual abnormalities, hirsutism, and acne. Elevated androgen levels, in turn, appear to be due in part to hyperinsulinemia, which triggers the increase in androgen production. Thus interventions that improve insulin resistance and hyperinsulinemia may reverse some of the manifestations of PCOS.
The insulin resistance seen in women with PCOS is unique in that it occurs independent of body weight to some extent and is not always corrected by weight loss. It appears to result from a postreceptor defect in an insulin-mediated signaling pathway (Diamanti-Kandarkis and Papavassiliou, 2006). Conventional treatment of PCOS includes diet and exercise to promote weight loss. In women who are obese, weight loss may improve insulin resistance, decrease androgen levels and hirsutism, and restore ovulation in some cases. Low–glycemic index diets have historically been recommended without evidence of their clinical effectiveness. However, the capacity of dietary carbohydrates to increase postprandial blood sugar response may be an important consideration for optimizing metabolic and clinical outcomes in PCOS. Furthermore, independent of weight loss, a low–glycemic index diet appears to result in greater improvements in health, including improved insulin sensitivity, improved menstrual regularity, better emotion scores (on a questionnaire designed to detect changes in quality of life), and decreased markers of inflammation as compared with a conventional low-fat diet when matched closely for macronutrient and fiber content (Marsh et al., 2010).
Medical Management
Hypothyroidism occurs in some cases of PCOS. Laboratory tests for thyroid function are frequently normal in patients with clinical evidence of hypothyroidism, and treatment with thyroid hormone results in clinical improvement in many patients. Therefore an empirical trial of thyroid hormone should be considered for patients with PCOS who have clinical evidence of hypothyroidism.
Thyroid antibody status should be taken into account when considering empirical treatment with thyroid hormone in women with PCOS. Metformin is frequently prescribed to improve insulin resistance, and treatment with this drug may lead to resumption of ovulation. Other therapies include the drugs clomiphene citrate (to induce ovulation) and spironolactone (an antiandrogen), as well as oral contraceptives (to treat menstrual irregularities and hirsutism).
Medical Nutrition Therapy
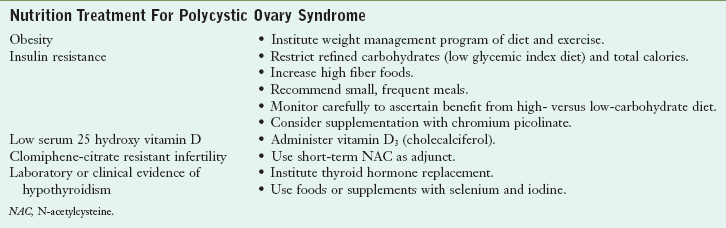
Nutritional interventions that may be beneficial for women with PCOS include dietary modifications designed to enhance insulin sensitivity. This includes restricting refined carbohydrates and total calories; consuming high-fiber foods; and eating small, frequent meals. Some patients with insulin resistance fare better on a diet high in complex carbohydrates (approximately 60% of total calories), whereas others respond better to a low-carbohydrate diet (≤40% of total calories). Additionally, supplementation with vitamin D3 (800-1200 IU/day), and chromium picolinate (200-1000 mcg/day) has been reported to improve glucose tolerance, insulin secretion, and insulin sensitivity (Lydic et al., 2006). Short-term treatment with N-acetylcysteine (600 mg twice a day) may be useful as an adjunct to clomiphene citrate in women with clomiphene citrate-resistant infertility (Rizk et al., 2005). In addition, treatment with thyroid hormone may be beneficial for women who have laboratory or clinical evidence of hypothyroidism (Table 32-3).
Hyperthyroidism
Graves’ disease is an autoimmune disease in which the thyroid is diffusely enlarged (goiter) and overactive, producing an excessive amount of thyroid hormones. It is the most common cause of hyperthyroidism (overactive thyroid) in the United States. Physical symptoms frequently include red, dry, swollen, puffy, and bulging eyes (exophthalmos), heat intolerance, difficulty sleeping, and anxiety (see Box 32-2). However the most common sign of Graves’ disease is goiter or thyroid enlargement (Figure 32-4).The excessive thyroid hormones may cause a serious metabolic imbalance, thyrotoxicosis. The prevalence of maternal thyrotoxicosis is approximately 1 case per 500 persons, with maternal Graves’ disease being the most common cause (American Thyroid Association, 2008).
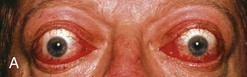
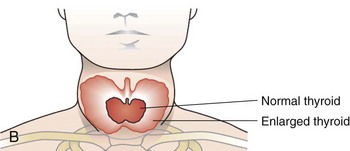
FIGURE 32-4 A, Exophthalmos. (From SPL/Photo Researchers, Inc.) B, Thyroid enlargement. (From Buck C: 2011 ICD-9-CM, for Hospitals, Volumes 1, 2 and 3, Professional Edition, St. Louis, 2011, Saunders.)
Pathophysiology
Commonly, patients have a family history involving a wide spectrum of autoimmune thyroid diseases, such as Graves’ disease, Hashimoto’s thyroiditis, or postpartum thyroiditis. In Graves’s disease, the TRH receptor itself is the primary autoantigen and is responsible for the manifestation of hyperthyroidism. The thyroid gland is under continuous stimulation by circulating autoantibodies against the TRH receptor, and pituitary TSH secretion is suppressed because of the increased production of thyroid hormones. These thyroid-stimulating antibodies cause release of thyroid hormone and Tg and they also stimulate iodine uptake, protein synthesis, and thyroid gland growth.
The Tg and TPO Abs appear to have little role in Graves’ disease. However, as mentioned earlier, they are markers of Hashimoto’s autoimmune disease against the thyroid. A TSH antibody—typically referred to as thyroid-stimulating immunoglobulin—test is used to identify hyperthyroidism, or Graves’ disease.
Triggers
Graves’ disease is an autoimmune disorder, influenced by a combination of environmental and genetic factors. Genetic factors contribute to approximately 20% to 30% of overall susceptibility. Other factors include infection, excessive iodide intake, stress, female gender, steroids, and toxins. Smoking has been implicated in the worsening of Graves’ ophthalmopathy. Graves’ disease has also been associated with infectious agents such as Yersinia enterocolitica and Borrelia burgdorferi.
Genetics: Several autoimmune thyroid disease susceptibility genes have been identified and appear to be specific to either Graves’ disease or Hashimoto’s thyroiditis, whereas others confer susceptibility to both conditions. The genetic predisposition to thyroid autoimmunity may interact with environmental factors or events to precipitate the onset of Graves’ disease. HLA-DRB1 and HLA-DQB1 appear to be associated with Graves’ disease susceptibility.
Stress: Stress can be a factor for thyroid autoimmunity. Acute stress-induced immunosuppression may be followed by immune system hyperactivity, which could precipitate autoimmune thyroid disease. This may occur during the postpartum period, in which Graves’ disease may occur 3-9 months after delivery. Estrogen may influence the immune system, particularly the β-cells. Trauma to the thyroid has also been reported to be associated with Graves’ disease. This may include surgery of the thyroid gland, percutaneous injection of ethanol, and infarction of a thyroid adenoma.
Medical Management
For patients with sustained forms of hyperthyroidism, such as Graves’s disease or toxic nodular goiter, antithyroid medications can be used. The goal with this form of drug therapy is to prevent the thyroid from producing hormones (Table 32-4).
The effects of immunotherapy are also being evaluated (Michels and Eisenbarth, 2010). See Pathophysiology and Care Management Algorithm: Thyroid Dysfunction.
Managing Imbalances Of The Hypothalamus-Pituitary-Thyroid Axis
The thyroid has a relationship to hypothalamic, pituitary, immune, adrenal, and cardiovascular functions that affect clinical, cellular, and molecular outcomes. A checklist of considerations is found in Box 32-3, and is discussed here.
1. Provide adequate precursors for the formation of T4. Iodide is a limiting nutrient in many individuals for the production of T4. Adequate levels of organic iodide, which can come from sea vegetables, iodized salt, and seafood, are important in T4 production. Adequate dietary protein intake is important in establishing proper protein calorie nutrition. Supplementation with tyrosine does not appear to have a beneficial effect on elevating thyroid hormones.
2. Reduce antithyroidal antibodies. A variety of food antigens could induce antibodies that crossreact with the thyroid gland. A food elimination diet using gluten-free grains and possible elimination of casein, the predominant milk protein, might be considered for hypothyroidism of unexplained origin. It has also been suggested that environmental toxins may play a role in inducing autoimmune thyroiditis and thyroid dysfunction. Implementing nutritional support and providing adequate levels of vitamin D to support the immune system may be beneficial.
3. Improve the conversion of T4 to T3. Nutritional agents that help support proper deiodination by the type 1 5′-deiodinase enzyme include selenomethionine (as L-selenomethionine) and zinc (as zinc glycinate or zinc citrate). Human studies have repeatedly demonstrated consequent reduced concentrations of thyroid hormones when a zinc deficiency is present (Blazewicz et al., 2010). In children with Down syndrome, zinc sulfate may reduce thyroidal antibodies, improve thyroid function, and reduce the incidence of subclinical hypothyroidism.
4. Enhance T3 influence on mitochondrial bioenergetics. A number of important nutritional relationships improve thyroid hormones’ effect on the mitochondria. Selenium supplementation in animals can improve the production of T3 and lower autoantibodies to thyroid hormones, while improving energy production. Supplementation with selenomethionine results in improved deiodination of T4, which may improve adenosine triphosphate formation by supporting improved mitochondrial activity. Food sources of selenium include the Brazil nut, snapper, cod, halibut, yellow fin tuna, salmon, sardines, shrimp, mushrooms, and barley.
5. Monitor use of botanical products. Based on animal studies, it appears that certain botanical preparations influence thyroid activity. The most significant products include Commiphora mukul (guggulsterones, from guggul extract) and Withania somnifera (ashwagandha). Commiphora mukul demonstrates strong thyroid stimulatory action. Its administration (1 mg/100 g body weight) increases iodine uptake by the thyroid, increases TPO activity, and decreases lipid peroxidation, suggesting that increased peripheral generation of T3 might be mediated by this plant’s antioxidant effects. Withania somnifera (ashwagandha) root extract (1.4 g/kg) may increase T3 and T4 concentrations without changing 5′-deiodinase activity.
6. Avoid disruption of thyroid hormone metabolism from flavonoids. Flavonoids, both natural and synthetic, have the potential to disrupt thyroid hormone metabolism. Synthetic flavonoid derivatives can decrease serum T4 concentrations and inhibit both the conversion of T4 toT3 and the metabolic clearance of rT3 by the selenium-dependent 5′-deiodinase. Naturally occurring flavonoids appear to have a similar inhibitory effect. Of the naturally occurring flavonoids, luteolin (most often found in leaves, but also seen in celery, thyme, dandelion, green pepper, thyme, perilla, camomile tea, carrots, olive oil, peppermint, rosemary, and oregano) is the most active inhibitor of 5′-deiodinase activity. Because isolated or concentrated flavonoids are increasingly used as therapeutic interventions, more research on the potential influence of these substances on thyroid hormone metabolism is desirable.
7. Use caution with supplements. Lipoic acid reduces the conversion of T4 to T3. Because it is usually not a therapeutic advantage to decrease peripheral activation of T3 subsequent to T4 therapy, use of lipoic acid supplements in hypothyroid patients receiving exogenous hormone therapy should be approached with caution.
8. Maintain vitamin sufficiency. One nutrient that is critically important for establishing immune balance and preventing the production of autoantibodies is vitamin D. Vitamin D is considered a prohormone with antiproliferative, differentiating, and immunosuppressive activities. Vitamin D is an effective immune modulator (Baeke et al., 2010) and may suppress the development of autoimmune diseases, such as arthritis and multiple sclerosis. Conversely, a vitamin D deficiency is associated with numerous autoimmune conditions, including Hashimoto’s. More than 90% of people with autoimmune thyroid disease have a genetic defect affecting their ability to metabolize vitamin D (Lin et al.,2006; Stefanic et al., 2008). Vitamin D also appears to work with other nutritional factors to help regulate immune sensitivity and may protect against development of autoantibodies. After exposure to heavy metals, decreases in a variety of hepatic antioxidant lipid peroxidation (the oxidative degradation of lipids) systems have been observed. Ascorbic acid has been shown to be effective in preventing cadmium-induced decreases in T3 and hepatic 5′-deiodination.
Other Endocrine System Disorders
In Cushing’s syndrome, too much cortisol remains in the bloodstream over a long period. The exogenous form occurs when individuals take steroids or other similar medications and ceases when the medication is stopped. Endogenous Cushing’s syndrome is rare and occurs as the result of a tumor on the adrenal or pituitary gland. Weight gain, easy bruising, depression, muscle loss, and weakness are common symptoms. A weight management protocol may be needed.
Addison’s Disease
Primary adrenal insufficiency, also known as Addison’s disease, is rare. In this condition, insufficient steroid hormones are produced in spite of adequate levels of the hormone ACTH. Regulation of blood glucose levels and stress management are affected. Loss of appetite, fatigue, low blood pressure, nausea and vomiting, and darkening of skin on the face and neck may occur. Patients with Addison’s disease should not restrict their salt intake unless they have concurrent hypertension. Those patients who live in warm climates and therefore have increased losses through perspiration, may need to increase salt intake.
References
Abdullatif, H, Ashraf, A. Reversible subclinical hypothyroidism in the presence of adrenal insufficiency. Endocr Pract. 2006;12:572.
American Association of Clinical Endocrinologists. Facts about thyroid disease. Accessed 16 August 2010 from http://www.aace.com/public/awareness/tam/2005/pdfs/thyroid_disease_fact_sheet.pdf, 2005.
American Diabetes Association (ADbA). Diabetes Statistics. Accessed July 15, 2010 from http://www.diabetes.org/diabetes-basics/diabetes-statistics/, 2007.
American Thyroid Association. Iodine deficiency. Accessed July 15, 2010 from http://www.thyroid.org/patients/patient_brochures/iodine?deficiency.html, 2008.
Anderson, MS. Update in endocrine autoimmunity. J Clin Endocrinol Metab. 2008;93:3663.
Baeke, F, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482.
Barker, J, Liu, E. Celiac disease: pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr. 2008;55:349.
Blazewicz, A, et al. Determination of cadmium, cobalt, copper, iron, manganese, and zinc in thyroid glands of patients with diagnosed nodular goitre using ion chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:34.
Cassio, A, et al. Long-term clinical significance of thyroid autoimmunity in children with celiac disease. J Pediatr. 2010;156:292.
Chen, Z, Hetzel, B. Cretinism revisited. Best Pract Res Clin Endocrinol Metab. 2010;24:39.
De Vivo, A, et al. Thyroid function in women found to have early pregnancy loss. Thyroid. 2010;20:633.
Diamanti-Kandarakis, E, Papavassiliou, A. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12:324.
Downs, H, et al. Clinical inquiries: How useful are autoantibodies in diagnosing thyroid disorders? J Fam Pract. 2008;57:615.
Duntas, L. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190.
Ebert, E. The thyroid and the gut. J Clin Gastroenterol. 2010;44:402.
Gropper, S, et al. Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Cengage Learning; 2009.
Hak, AE, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med. 2000;132:270.
Hess, S. The impact of common micronutrient deficiencies on iodine and thyroid metabolism: the evidence from human studies. Best Pract Res Clin Endocrinol Metab. 2010;24:117.
Kohrle, J, Gartner, R. Selenium and Thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:815.
Lin, W, et al. Vitamin D receptor gene polymorphisms are associated with risk of Hashimoto’s thyroiditis in Chinese patients in Taiwan. J Clin Lab Anal. 2006;20:109.
Lydic, M, et al. Chromium picolinate improves insulin sensitivity in obese subjects with polycystic ovary syndrome. Fertil Steril. 2006;86:243.
Marsh, K, et al. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92:83.
Meloni, A, et al. Prevalence of autoimmune thyroiditis in children with celiac disease and effect of gluten withdrawal. J Pediatr. 2009;155:51.
Melse-Boonstra, A, Jaiswal, N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab. 2010;24:29.
Michels, AW, Eisenbarth, GS. Immunologic endocrine disorders. J Allergy Clin Immunol. 2010;125:S226.
Moran, L, et al. Polycystic ovary syndrome and weight management. Womens Health. 2010;6:271.
Rizk, A, et al. N-acetyl-cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate-resistant patients with polycystic ovary syndrome. Fertil Steril. 2005;83:367.
Saravanan, P, et al. Psychological well-being in patients on “adequate” doses of L-thyroxine: results of a large controlled community based questionnaire study. Clin Endocrinol. 2002;57:577.
Shomon, M. All about thyroid drugs. Accessed July 15, 2010 from http://thyroid.about.com/cs/thyroiddrugs/a/overview.htm, 2007.
Shomon, M. Thyroid disease symptoms—hypothyroidism and hyperthyroidism. Accessed from http://thyroid.about.com/cs/basics_starthere/a/symptoms.htm, 2008.
Sinnott, B, et al. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev. 2010;31:756.
Sloka, J, et al. Co-occurrence of autoimmune thyroid disease in a multiple sclerosis cohort. J Autoimmune Dis. 2005;2:9.
Stefanic, M, et al. Association of vitamin D receptor gene 3′-variants with Hashimoto’s thyroiditis in the Croatian population. Int J Immunogenet. 2008;35:125.
Titchenal, A, et al. Iron plays an important role for the thyroid. Accessed July 10, 2010 from http://www.nutritionatc.hawaii.edu/HO/2009/415.htm, 2009.
Wiebolt, J, et al. Endocrine autoimmune disease: genetics become complex. Eur J Clin Invest. 2010;40:1144.
Wilson, J. Adrenal fatigue: the 21st century stress syndrome. Petaluma, CA: Smart Publications; 2008.
Yassa, L, et al. Thyroid Hormone Early Adjustment in Pregnancy (the THERAPY) Trial. J Clin Endocrinol Metab. 2010;95:3234.
Zimmermann, M. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr. 2009;89:668S.
Zimmermann, M. The influence of iron status on iodine utilization and thyroid function. Ann Rev Nutr. 2006;26:367.