Nutrition in Pregnancy and Lactation
Optimal preconceptual nutrition supports successful conception when it includes adequate amounts of all of the required vitamins, minerals, and energy-providing macronutrients. Because the developing fetus depends solely on the transfer of substrates from its host, there is simply no other means to acquire nutrition in utero. The cliché that the “fetus is the perfect parasite” implies the fetus takes entirely what it requires at the expense of the host. However, at some point nutritional deficiency can result in premature labor, relieving the host of an ongoing nutritional debt. After birth, quality nutrition during lactation continues the process of providing nutritional building blocks for maximal cerebral development, and growth of all body organs in the neonate.
This time period in the human experience—creating a new human being—sets the stage for the health of future generations. The quality and quantity of in-utero nourishment on the developing zygote, then fetus, then neonate, then adult emerges as one explanation for diseases that manifest in adulthood. This concept is known as fetal origins of disease or developmental origins of health and disease (Niljand, 2008; Solomons, 2009).
Preconception and Fertility
Traditional pregnancy partners were usually “man and wife,” or mother and father. Advances in assisted reproductive technology (ART) mean that “parents” may be egg or sperm donors. ART can involve in vitro fertilization (IVF), cryo embryo transfer, IVF with donor oocytes, intracytoplasmic sperm injection, a gestational carrier, or surrogate mother.
Reproductive Readiness and Fertility
Preconceptual guidance is based on findings that many women enter pregnancy with suboptimal nutrition intake. One study of 249 pregnant women who reported for their first prenatal visit found low dietary intakes of vitamin E, folate, iron, and magnesium in the preconceptual period and during pregnancy (Pinto et al., 2009). Although current public health recommendations promote mostly folate supplementation, there is some evidence that other nutrients also reduce the risk of congenital defects, such as vitamins B12, B6, and niacin, iron, and magnesium (Gaber et al., 2007). Thus a preconceptual multinutrient supplement confers more benefit than single supplements for a pregnant woman, or gravida.
Causes of infertility can be male factor (25% to 40%), ovulation defect (20% to 30%), fallopian tube defect (20% to 30%), unexplained causes (10% to 20%), endometriosis (5% to 10%), and other causes (4%). Infertility may also be due to extremes in body mass index (BMI) in either partner. Women with less than 17% body fat often do not menstruate, and those with less than 22% often do not ovulate. Women at risk include those with excessive exercise regimens, eating disorders, or both.
Dietary changes have been shown to decrease ovulatory disorders and improve fertility. Vitamin D deficiency in both men and women can be associated with infertility (Ozkan et al., 2009). Calcium has been shown to be important in males for spermatogenesis, sperm motility, hyperactivation, and acrosome (area of the sperm that contains digestive enzymes to break down the outer layers of the ovum) reactions. Recommendations include eating a lower glycemic diet (including high-fat dairy products, but reducing trans-fats), obtaining iron from plant sources, consuming a multivitamin daily, and being moderately physically active (Chavarro et al., 2007).
Toxins
Exposure to environmental chemicals such as dioxins, polybrominated biphenyls, phthalate esters, and other industrial products (endocrine disruptors) and heavy metals are known to damage sperm health (Meeker et al., 2008). Healthier sperm counts are associated with avoidance of tobacco and alcohol as well as an optimal diet with zinc, folic acid, and antioxidants (Gaur et al., 2010).
Screening is critical in women for occupational toxin exposure as well as for alcohol, tobacco, and intravenous and recreational drug use (Hannigan et al., 2009). Women with high fish consumption are at risk for entering pregnancy with toxic levels of mercury. Mercury levels decline once fish consumption is reduced. Unfortunately, even when a medical university in Taiwan advised women that fish containing high levels of mercury might be harmful to the brains of their developing fetuses, more than two thirds of the women indicated they would not change their fish intake (Chien et al., 2010).
Maternal caffeine intake and infertility relationships are often debated (Cochrane Update, 2009). A few studies have associated caffeine with increased rates of miscarriage or adverse pregnancy outcome (Jahanfar and Sharifah, 2009). However, caffeinated beverages are not considered to be of high nutritional quality and moderation is encouraged to ensure consumption of fluids with better nutrients, such as soy milk, low-fat dairy, and 100% fruit juices.
Obesity, Endocrine Conditions, and Oxidative Stress
Obesity is often correlated with poor prepregnancy health care, inaccurate self-categorization of weight, unsuccessful weight loss attempts, and insufficient advice regarding the importance of prepregnancy weight loss (Callaway et al., 2009a). In men elevated BMI is associated with lower testosterone levels (Chavarro et al., 2007). Obese women have a higher likelihood of prediabetes, undiagnosed diabetes preconceptually, or prolonged hyperglycemia; they also often have higher rates of fetal congenital anomalies (Selvaraj et al., 2008). Thus reducing obesity preconceptually may lower the risk of birth defects (American Dietetic Association, 2009; Biggio, 2010; Dheen, 2009).
Polycystic ovary syndrome (PCOS) affects 5% to 10% of women of reproductive age. These ovarian cysts alter the testosterone-estrogen balance, which results in insulin resistance and infertility. PCOS is often treated successfully with metformin (Grassi, 2008). See Chapter 32.
Hypothyroidism is also associated with reduced fertility (Hoy-Rosas, 2009). The thyroid hormone requirement increases 20% to 40% during gestation (Yassa et al., 2010). Pregnant women with treated hypothyroidism must increase their T4 levels to prevent transient hypothyroxinemia, associated with preterm birth or low birth weight (LBW) (Yassa et al., 2010). See Chapter 32.
Finally, oxidative stress depletes nutrient stores and contributes to a host of pregnancy complications. A healthy, antioxidant-rich diet and an exercise program help women prepare for an optimal pregnancy outcome. Box 16-1 lists some risk factors for birth defects.
Conception
Conception involves a complex series of endocrine events in which a healthy sperm fertilizes a healthy ovum (egg) within 24 hours of ovulation (see Table 16-1). An optimal environment is needed, including adequate nutrition and the absence of hostile factors. Conception itself does not guarantee successful pregnancy outcome. Low levels of copper and zinc adversely affect the development of the oocyte. Cloning experiments have shown that once the oocyte is fertilized, there is no further genetic material that is incorporated into the genetic sequence of that embryo. Exposures of the embryo or fetus to specific maternal nutrients can turn on or off the imprinting genes that control growth and development.
TABLE 16-1
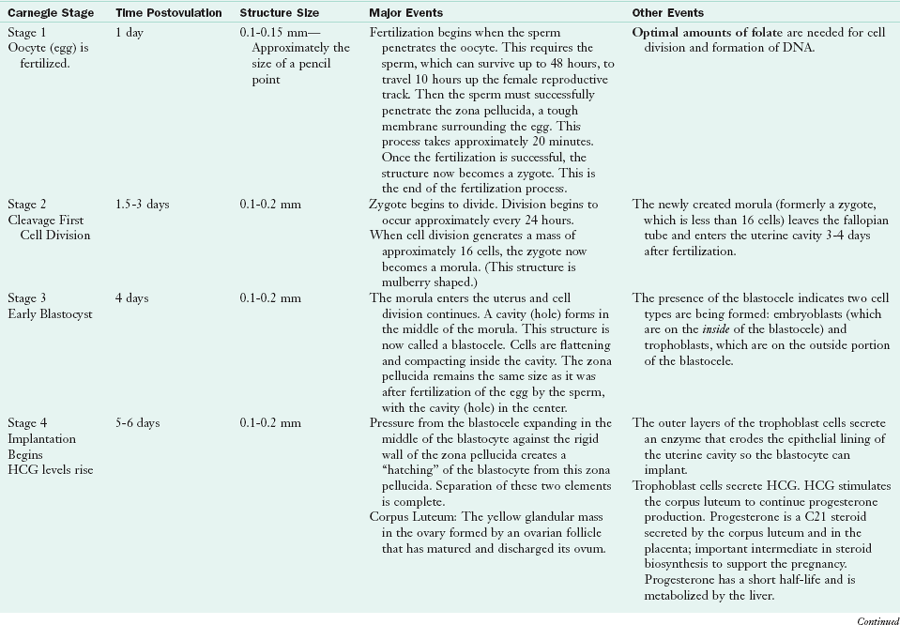





DNA, Deoxyribonucleic acid; HCG, human chorionic gonadotropin; SHH, sonic hedgehog.
Adapted from The Visible Embryo. Accessed 18 June 2010 from www.visembryo.com.
Pregnancy
The eventual infant from a gestational carrier’s womb will not be the same as one that had been carried by the biologic mother, even though the genes themselves are the same (Wilkins-Haug, 2009). This phenomenon reflects the effects of deoxyribonucleic acid (DNA) methylation from the maternal diet, known as epigenetics. Epigenetic effects involve the mechanisms by which DNA transcription is altered in various tissues and at different times without changing the underlying gene sequence. Unfortunately, the biologic changes of early pregnancy are difficult to visualize without sophisticated equipment.
Physiologic Changes of Pregnancy
Blood volume expands approximately 50% by the end of pregnancy. This results in decreased levels of hemoglobin, serum albumin, other serum proteins, and water-soluble vitamins. The decline in serum albumin may be the result of fluid accumulation. The decrease in water-soluble vitamin concentrations makes determination of an inadequate intake or a deficient nutrient state difficult. In contrast, serum concentrations of fat-soluble vitamins and other lipid fractions such as triglycerides, cholesterol, and free fatty acids increase.
Cardiovascular and Pulmonary Function
Increased cardiac output accompanies pregnancy, and cardiac size increases by 12%. Diastolic blood pressure decreases during the first two trimesters because of peripheral vasodilatation, but returns to prepregnancy values in the third trimester. Mild lower extremity edema is a normal condition of pregnancy resulting from the pressure of the expanding uterus on the inferior vena cava. Blood return to the heart decreases, leading to decreased cardiac output, a fall in blood pressure, and lower-extremity edema. Mild physiologic lower extremity edema is associated with slightly larger babies and a lower rate of prematurity.
Maternal oxygen requirements increase and the threshold for carbon dioxide lowers, making the pregnant woman feel dyspneic. Adding to this feeling of dyspnea is the growing uterus pushing the diaphragm upward. Compensation results from more efficient pulmonary gas exchange.
Gastrointestinal Function
During pregnancy the function of the gastrointestinal (GI) tract changes in several ways that affect nutritional status. In the first trimester nausea and vomiting may occur, followed by a return of appetite that may be ravenous (see “Nausea, Vomiting, and Hyperemesis Gravidarium”). Cravings for and aversions to foods are common. Increased progesterone concentrations relax the uterine muscle to allow for fetal growth while also decreasing GI motility with increased reabsorption of water. This often results in constipation. In addition, a relaxed lower esophageal sphincter and pressure on the stomach from the growing uterus can cause regurgitation and gastric reflux (see “Heartburn”).
Gallbladder emptying becomes less efficient because of the effect of progesterone on muscle contractility. Constipation, dehydration, a low-calorie diet, or poor intake are risk factors for gallstone development. During the second and third trimesters, the volume of the gallbladder doubles and its ability to empty efficiently is reduced. Gallbladder disease affects approximately 3.5% of pregnant women.
Celiac disease affects approximately 1 in 333 people, more than previously thought. It adversely affects fertility and absorption of nutrients. Women with celiac disease are at high risk of spontaneous abortion and premature deliveries. Some prenatal supplements may contain gluten or wheat binders and should be avoided. See Chapter 29.
Placenta
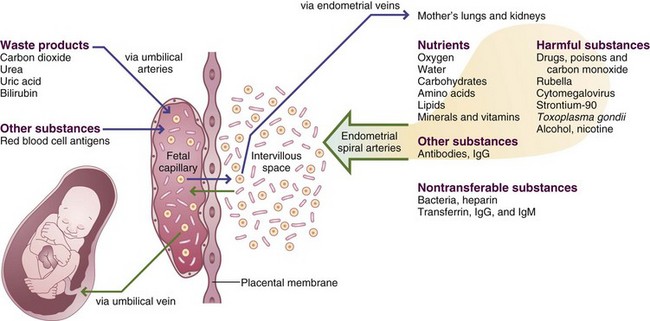
The placenta produces several hormones responsible for regulating fetal growth and development of maternal support tissues. It is the conduit for exchange of nutrients, oxygen, and waste products. Placental insults compromise the ability to nourish the fetus, regardless of how well nourished the mother. Placental insults can be the result of poor placentation from early pregnancy or small infarcts associated with preeclampsia (PET) or hypertension disorders. Placental size can be 15% to 20% lower than normal in fetuses with intrauterine growth restriction (IUGR). A small placenta has a smaller surface area of placental villi, with a reduced functional capacity. Important research about the role of imprinting and epigenetics in placental function is underway (Wilkins-Haug, 2009).
Renal Function
The glomerular filtration rate (GFR) increases by 50% during pregnancy, although the volume of urine excreted each day is not increased. The blood volume increases because of the increased GFR with lower serum creatinine and blood urea nitrogen concentrations. Renal tubular resorption is less efficient than in the nonpregnant state, and glucosuria may occur, along with increased excretion of water-soluble vitamins. Small amounts of glucosuria increase the risk for urinary tract infections. Pregnant women who present with acute pyelonephritis are hospitalized for aggressive antibiotic treatment, as this infection can easily affect the respiratory system.
Uterine Environment
A less than ideal intrauterine environment resulting from maternal infection, stressful events, poor nutrition, or excess saturated fat intake can negatively influence the development of different cell types and organs (Tamashiro and Moran, 2010). Nonetheless, the goal is to support a healthy environment through a proper balance of nutrients and the avoidance of teratogens.
A system depicting embryonic changes was compiled by scientists and embryologists in 1913. This system is known as the “Carnegie criteria,” with 23 stages of developmental milestones. For example, multiple nutrients are involved in the creation of bone (see Box 16-2). Specific nutrients are involved at the different Carnegie stages, see The Visible Embryo from www.visembryo.com
Optimizing outcomes includes adequate prenatal care, minimizing stress, and ensuring a healthy pregnancy diet (Rifas-Shiman et al., 2009). Fortunately, women with poor socioeconomic status can improve their diet quality with nutrition education. Women with preexisting depression are at risk for poor pregnancy outcome and postpartum depression, which not only puts the mother at risk but also the newborn. Inadequate nutrient intake (such as ω-3 fatty acids), poor self-care, or a combination of both, are complex causes but are important to distinguish (Leung et al., 2009).
The effect of poor maternal nutrition follows both infant and mother for decades (Cox and Phelan, 2008). Maternal nutritional status has been evaluated primarily for infant birth weight, risk of neural tube defects (NTDs), and fetal alcohol syndrome (FAS), a major cause of mental retardation and learning disorders. Birth weight is highly correlated with infant mortality and morbidity. Infants born small for gestational age are known to have major organs that are small; they are at increased risk for hypertension, obesity, learning disorders, behavioral problems, glucose intolerance, and cardiovascular disease (see Chapter 43). Intrauterine food restriction or hyperglycemia may reprogram leptin levels and neuropeptide Y, possibly contributing to metabolic conditions later in life (Page et al., 2009). Infants born large for gestational age (LGA) often have hyperglycemia at birth.
Preconceptual vitamin D status is thought to influence 3% of the human genome, including bone health throughout life. Indeed, maternal vitamin D status may program neonatal skeletal development. A study in Finland found that, although the total vitamin D intake met the current recommendations for this nutrient, 71% of women and 15% of newborns were vitamin D–deficient (Viljakainen et al., 2010). A dose of vitamin D that provides for 25-hydroxyvitamin D (25[OH]D) sufficiency in the mother during pregnancy should provide for normal cord blood concentrations of 25(OH)D for the infant.
Effects of Nutritional Status on Pregnancy Outcome
Any adverse maternal condition puts the fetus at risk for being delivered prematurely. Prematurity has significant inherent health risks. One theory for prematurity is the pregnancy is not obtaining adequate nutrients to continue growth and development of the fetus or the placenta. For example, Table 16-2 presents the roles of specific nutrients for neonatal brain development.
TABLE 16-2
Key Nutrients for Fetal and Neonatal Brain Development
| Nutrient | Function in Brain | Effect of Deficiency |
| Energy: protein, carbohydrate, fat | Cell proliferation and differentiation, synaptogenesis, growth factor synthesis | Global effect including cortex, hippocampus, white matter. |
| Iron | Myelin, monoamine synthesis, neuronal and glial energy metabolism. | White matter-striatal-frontal; hippocampus-frontal |
| Zinc | DNA synthesis, neurotransmitter release | Autonomic nervous system, hippocampus, cerebellum |
| Copper | Neurotransmitter synthesis, neuronal and glial energy metabolism, antioxidant activity | Cerebellum |
| Long-chain polyunsaturated fatty acids | Myelin formation, synaptogenesis | Cortex of the brain, the eye |
| Choline | Neurotransmitter synthesis, DNA methylation, myelin synthesis | Hippocampus, white matter |
Adapted from Georgieff MK: Nutrition and the developing brain: nutrient priorities and measurement, Am J Clin Nutr 85:1S, 2007.
Researchers speculate that maternal starvation causes alterations in DNA, regulated by various nutrients very early in pregnancy or at the time of conception. In the early 1900s women with poor nutritional status had adverse pregnancy outcomes with hemorrhage at delivery, prolonged labor, and LBW infants. During World War II, the effects of severe food deprivation on previously well-nourished populations were explored. Higher rates of spontaneous abortion, stillbirths, neonatal deaths, and congenital malformations were noted in offspring born to women who conceived during the famine; surviving infants were smaller. Likewise, results from the Chinese famine of 1959 to 1961 showed similar results in the offspring conceived during this period of maternal malnutrition (Zammit et al., 2007). Smaller organs are found in offspring of mothers who were malnourished during pregnancy (Kyle and Picard, 2006).
Even today, subclinical malnutrition may lead to poor reproductive performance. Women experiencing anorexia nervosa and bulimia nervosa can have amenorrhea, infertility, and reduced rates of pregnancy. Women with a history of eating disorders should therefore be carefully monitored. This includes looking for pregorexia, a form of increased calorie expenditure and caloric restraint during pregnancy (Mathieu, 2009). See Clinical Insight: High-Risk Pregnancies.
The developing fetus may be unable to obtain optimal nutrients from a host who is compromised nutritionally. Compromises in structural or cognitive potential may not be evident when an infant is born, but may manifest later in life when various stages of growth are arrested or altered. Attention deficit disorder in some children may be related to suboptimal gestational iodine or low vitamin D transfer in a depleted mother (Cui et al., 2007).
LBW (<2500 g) and especially very low birth weight (<1500 g) are major factors for perinatal mortality (infant deaths occurring between 28 weeks’ gestation and 4 weeks’ postpartum). These deaths may occur from necrotizing enterocolitis, respiratory distress syndrome, intraventricular hemorrhage, cerebral palsy, or retinopathy of prematurity. Maternal obesity in the African American population has been associated with a 40% higher incidence of stillbirths compared with women with normal BMI status (Salihu et al., 2007).
Maternal Weight Gain
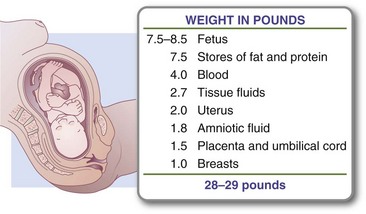
With a singleton gestation, less than half of the total weight gain of a normal-weight pregnant woman resides in the fetus, placenta, and amniotic fluid. The remainder is in maternal reproductive tissues (breast tissues and uterus), interstitial fluid, blood volume, and maternal adipose tissue. Gradually, increased subcutaneous fat in the abdomen, back, and upper thigh serves as an energy reserve for pregnancy and lactation. The normal distribution of weight gain is illustrated in Figure 16-1. Although this chart implies the fetus at term constitutes approximately 27% of the total pregnancy weight gain, this may not be true for all pregnancies.
In normal-weight women living in healthy environments, a gestational weight gain of 25 to 35 lb is associated with a favorable outcome at term. Guidelines issued by the Institute of Medicine (IOM) recommend a weight gain of 25 to 35 lb for women of normal weight (pregravid BMI 18.5 to 24.9), 28 to 40 lb for underweight women (BMI <18.5), and 15 to 25 lb for overweight women (BMI 25 to 29.9) (Rasmussen and Yaktine, 2009; Figure 16-2).
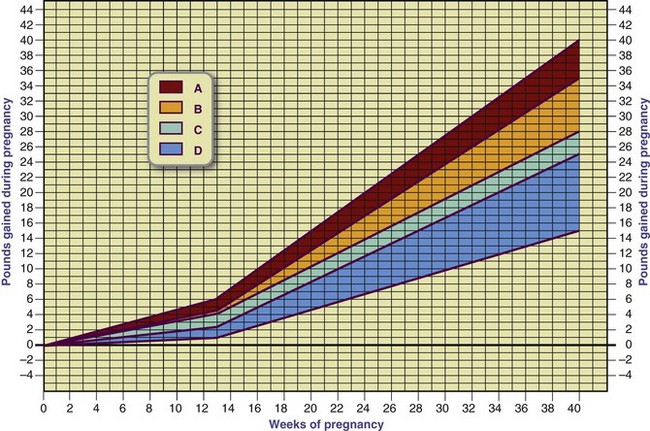
FIGURE 16-2 Desirable weight gain during pregnancy. Females who are of normal weight before their pregnancy should aim for a weight gain in the B to C range (25-35 lb) during pregnancy. Underweight females should gain in the A to B range (28-40 lb). Females who are overweight before pregnancy should gain in the D range (11-20 lb).
Weight loss during pregnancy should be discouraged. As adipose tissue is mobilized, there is concern for the release of semivolatile organic compounds, which can have an effect on the developing fetal brain that is not clear.
Obesity
Pregravid obesity is described as class I (BMI 30-34.9), class II (BMI 35-39.9), and class III (BMI more than 40); optimal gestational gains for these groups are not yet known. Studies indicate that 50% of high-BMI women gain more than the target weight recommendations, thus reflecting the increasing prevalence of obesity among U.S. women (Stotland et al., 2005).
Overweight and obese women are at increased risk for intrauterine fetal demise (IUFD) or miscarriage. The risks for gestational diabetes mellitus (GDM), pregnancy-induced hypertension (PIH), and cesarean section increase in this same group (American Congress of Obstetricians and Gynecologists [ACOG], 2005). When ultrasound studies examined the lean to fat mass in fetuses of women with GDM, their fetuses showed an accelerated rate of growth (de Santis et al., 2010). The risk for delivery of a very preterm (<32 weeks) infant or an infant with a cardiac defect, NTD, or macrosomia (birthweight > 4000 g) increases in women who are obese (Artal et al., 2010). Obesity is associated with a high risk of hypertensive disorders (Callaway et al., 2009b).
An association between maternal obesity and increased incidence of NTDs has not been explained, but obesity itself is an inflammatory state. Low grade systemic inflammation is associated with higher levels of C-reactive protein, interleukin-6, and leptin. A sustained state of hyperglycemia or hyperinsulinism may be related to NTDs as well (Yazdy et al., 2010). Folate intake of 600 mcg/day is less protective against NTDs in obese women than in normal-weight pregnant women; obese women may need more (Scialli and Public Affairs Committee, 2006). There is speculation that the increased body size may require additional supplementation. Since vitamin B12 is a cofactor for methionine synthase, an enzyme that plays a key role in folate metabolism, it may also be required in larger amounts to prevent NTDs. In addition, there is a suggestion that nutrients such as iron, magnesium and niacin may play a role in NDTs (Groenen, 2004). Inadequate choline may be implicated in NDTs since, like folate, it functions as a methyl donor (Zeisel, 2009). A proactive nutritional goal is to choose foods of high antioxidant quality. Indeed, the benefits of prepregnancy maternal weight loss include improved plasma lipids, glucose, and uric acid, which may also reduce pregnancy risk factors.
Postbariatric Surgery: The prevalence of obesity has resulted in more gastric bypass operations for weight reduction, which has tremendous implications for pregnancy. Although prepregnancy weight loss may improve fertility, it has the potential to provide a suboptimal uterine environment for the developing fetus. Pregnancy should be delayed for at least 1 year after this surgery, and adequate nutrient supplementation is essential.
Operative complications are not uncommon, such as intestinal hernias. Deficiencies in iron, vitamins A, D, B12, K, and folate, and calcium can result in maternal complications (severe anemia) or fetal complications, such as congenital abnormalities, smaller kidneys, neonatal rickets, IUGR and failure to thrive (FTT) (Faintuch et al., 2009; Guelinckx et al., 2009). An optimum nutrient prescription and caloric requirement for pregnant women following bariatric surgery has not been determined and thus must be individualized. Low intakes of calcium, vitamin B12, and iron have been noted in this population (Faintuch et al., 2009).
Adolescent Pregnancy
Public health initiatives have helped reduce the incidence of teen pregnancies; however, teenage pregnancy continues as a major public health problem in the United States and is associated with significant medical and nutritional risks. Approximately 1 million U.S. adolescents become pregnant every year, accounting for 25% of U.S. pregnancies. Teens have a higher incidence of delivering a LBW infant. Risk factors for poor outcome in pregnant adolescents are listed in Box 16-3.
Poor outcomes are especially common in obese teens who become pregnant. Excess body fat, particularly of visceral distribution, has been linked with proinflammatory cytokines, chronic low-grade inflammation, low total-body iron metabolism, increased fatigue, and reduced physical activity (Tussing-Humphreys et al., 2009). Many other teens enter pregnancy with suboptimal nutritional status, especially for iron, calcium, and folic acid. Young maternal age is a significant risk factor for developmental defects, such as inadequate neonatal tooth enamel formation (Vello et al., 2009).
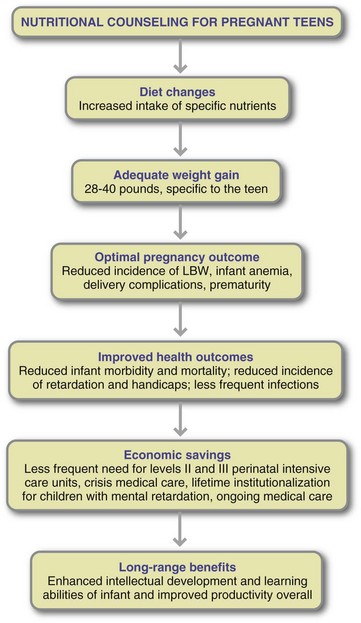
Improved dietary practices can be one of the most important factors for the pregnant teen or young mother. In counseling teen mothers, the nutrition professional must be aware of the teen’s psychosocial, cultural, and literacy level; economic status and dependencies; and any educational frameworks that influence her food choices. Benefits of nutritional counseling for pregnant adolescents are presented in Figure 16-3.
Multiple Births
The incidence of multiple births in the United States is rising in part because of the increased use of fertility drugs and ART. Infants of multiple-birth pregnancies have a greater risk of premature delivery with accompanying IUGR or LBW than do singletons. Adequate maternal weight gain has been shown to be particularly important in these higher-risk pregnancies.
Multifetal gestations undergo significant maternal physiological adaptations beyond the usual pregnancy changes, including increased plasma volume, metabolic rate, and increased insulin resistance (Goodnight and Newman, 2009). Optimal weight gain and infant gestational ages for this population are presented in Table 16-3.
TABLE 16-3
Weight Gains of Mothers and Incidence of Low Birth Weights in Multiple-Birth Pregnancies

LBW, Low birth weight; VLBW, very low birth weight.
From Luke B: Managing maternal nutrition: prenatal and postpartum, Perinat Nutr Rep 3:2, 1997; Heron et al: Annual summary of vital statistics, 125:4, 2007.
The optimal nutritional requirements for twins and higher-order multiples are not yet known. One nutritional plan is summarized in Table 16-4. Although this plan has not included iodine specifically, it is imperative to meet at least the singleton recommendation of 220 mcg/day, with the upper limit for singleton gestation at 900 mcg for mothers younger than 18 years old and 1100 mcg for those 19 years and older. The dietary reference intakes (DRIs) for choline for the singleton gestation are important as well; include 450 mg/day with a tolerable upper limit of 3 g/day for women younger than 18 and 3.5 grams for women older than 19.
TABLE 16-4
Nutrient Recommendations for Twins
| Nutrient | Twins | Comments |
| Calories | 40-45 kcal/kg normal weight BMI | Weight is not specified—pregravid, ideal or current? |
| Protein | 14.4%-18.1% of total calories | Depending on calorie requirements, could be as high as 175 g/day. |
| Carbohydrate | 350 g/day for normal weight gravid | Encourage low glycemic choices. |
| Fat | 156 g/day for normal weight gravid | Encourage healthy fats. |
| Vitamin D | 1000 IU/day | Assessment of maternal levels should be considered in first and early third trimester to allow alterations in the supplemental dose. |
| Vitamin C | 500-1000 mg/day | This is half of the UL of 1800-2000 mg/day. |
| Vitamin E | 400 mg/day | This is half of the UL of 800-1000 mg/day. |
| Zinc | 15 mg/day (T1); 45 mg/day (T2-3) | Diet alone may not be enough. Supplementation may be required. |
| Iron | 30 mg/day | For nonanemic twins. Rosello-Soberon (2005) reported an estimated 869 mg/day iron requirement for twins compared with 476 mg for a singleton pregnancy. |
| Folic acid | 1000 mcg/day | |
| Calcium | 1500 mg/day (T1); 2500 mg (T2-3) | UL: 2500 mg/day |
| Magnesium | 400 mg/day (T1); 800 mg/day (T2-3) | |
| DHA/EPA | 300-500 mg/day |
BMI, Body mass index; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; T, trimester; UL, tolerable upper limit.
Adapted from Goodnight W, Newman R: Optimal nutrition for improved twin pregnancy outcome, Obstet Gynecol 114:1121, 2009.
Nutritional Supplementation During Pregnancy
Supplementation of a mother’s diet during pregnancy may take the form of additional energy, protein, vitamins, or minerals that exceed her routine daily intake. The more compromised the nutritional status of the woman, the greater is the benefit for pregnancy outcome with improved diet and nutritional supplementation. Judicious use of supplements is needed in high-risk pregnancies and in undernourished women, women with substance abuse, teenage mothers, women with a short interval between pregnancies, women with a history of delivering an LBW infant, and women with a multiple gestation pregnancy.
Under the auspices of the U.S. Department of Agriculture (USDA), pregnant women at nutritional risk are encouraged to enroll in the Special Supplemental Nutrition Program for Women, Infants and Children (WIC). For U.S. citizens, the WIC program serves eligible pregnant women, postpartum women until 6 months’ postpartum, breast-feeding women until 1 year postpartum, and infants and children up to the age of 5 years (see Chapter 10). “Nutritional risk” criteria may include anemia, poor gestational weight gain, inadequate diet, or FTT in the infant or child. Outcome studies show higher mean birth weights and higher mean gestational ages in infants born to WIC participants.
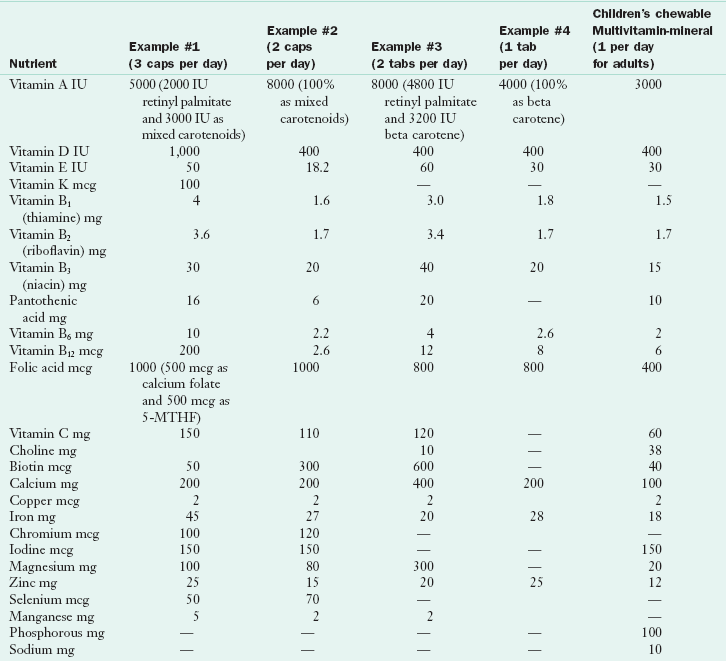
Many pregnant women and some providers have limited knowledge regarding the nutrients in the supplements they have been prescribed or have been advised to purchase over the counter. There is great variability in the composition of supplements as demonstrated in Table 16-5, and formulations change frequently. It is important to read the label of prenatal supplements—some are much more complete than others and some are little more than a children’s chewable multivitamin mineral with extra folic acid and iron. Approximately 60% of prenatal supplements include the recommended dietary allowance (RDA) for iodine, but an analysis found that the actual amount of iodine differed from the amount listed on the label (Leung, 2009). Women often need advice on local, suitable choices for themselves or their children.
Nutrition Requirements
Fetal growth demands additional nutrients and these requirements are defined in the DRIs, which include RDAs and adequate intakes (AIs). These DRIs are found on the inside front cover.
Energy
Additional energy is required during pregnancy to support the metabolic demands of pregnancy and fetal growth. Metabolism increases by 15% in the singleton pregnancy. The 2002 DRIs for energy for the pregnant female are the same as for the nonpregnant female in the first trimester, then increase by 340 to 360 kcal/day during the second trimester and by another 112 kcal/day in the third trimester (Institute of Medicine [IOM], 2002). If maternal weight gain is within the desirable limits, the range of acceptable energy intakes varies widely, given individual differences in energy output and basal metabolic rate.
Exercise: Energy expended in voluntary physical activity is the largest variable in overall energy expenditure. Physical activity increases energy expenditure proportional to body weight. However, most pregnant women compensate for increased weight gain by slowing their work pace; thus total daily energy expenditure may not be substantially greater than before pregnancy.
Excessive exercise, combined with inadequate energy intake, may lead to suboptimal maternal weight gain and poor fetal growth. Therefore a pregnant woman should always discuss exercise with her primary health practitioner. Exercising during pregnancy at high altitudes may compromise fetal oxygen delivery, especially for women who are not acclimatized to higher elevations. Resting uterine blood flow is lower in residents residing at 3100 m than at 1600 m, and blood flow is likely to decrease further during exercise in proportion to the intensity and duration.
Consequences of Energy Restriction: A once-popular concept held that the fetus develops at the expense of the mother during nutritional deprivation. However, evidence from famines clearly contradicts this assumption. It is now accepted that a malnourished mother is proportionately less affected than her fetus. One consequence of severe energy restriction is increased ketone production. Although the fetus has a limited ability to metabolize ketone bodies, the short- and long-term effects of maternal ketonemia are unclear. Both animal and human data indicate that ketone bodies are normally presented to the fetal brain at various times during pregnancy. After an overnight fast, maternal blood ketone body concentrations are greater in pregnant than in nonpregnant women, and even ketonuria can be detected. When ketonuria is present, it indicates a lack of energy-providing macronutrients, which also reduces vitamin and mineral intake. Box 16-4 describes some of the causes of fetal malnutrition.
Protein
There is an additional protein requirement for pregnancy to support the synthesis of maternal and fetal tissues, but the magnitude of this increase is uncertain. Protein requirement increases throughout gestation and is maximized during the third trimester. The current RDA of 0.8 g/kg/day of protein for pregnant women is the same as that for the nonpregnant women in the first half of pregnancy. Needs increase in the second half to 71 g/day, based on 1.1 gm/kg/day of prepregnant weight (IOM, 2002). For each additional fetus, another 25 g/day of protein is recommended; this may be as much as 175 g/day for the normal-weight woman carrying a twin gestation who is consuming 3500 kcal/day (Goodnight and Newman, 2009).
Protein deficiency during pregnancy has adverse consequences. Limited intakes of protein and energy usually occur together, making it difficult to separate the effects of energy deficiency from those of protein deficiency.
Carbohydrates
DRIs for carbohydrates in pregnancy are estimated average requirements (EARs) at 135 g/day; the RDA is 175 g/day (IOM, 2002). This 135 to 175 g/day is recommended to provide enough calories in the diet to prevent ketosis and maintain appropriate blood glucose during pregnancy. With an average 2000 calorie/day regimen, 175 g translates to 700 calories, or 35%. The amount may be greater in women consuming more calories, but careful carbohydrate choices are needed to include all the daily nutrients for pregnancy.
Fiber
Daily consumption of whole-grain breads and cereals, leafy green and yellow vegetables, and fresh and dried fruits should be encouraged to provide additional minerals, vitamins, and fiber. The DRI for fiber during pregnancy is 28 g/day (IOM, 2002) and if met, will help a great deal in managing the constipation that often accompanies pregnancy.
Lipids
There is no DRI for lipids during pregnancy. The amount of fat in the diet should depend on energy requirements for proper weight gain. However, there is a recommendation (an AI of 13 g/day) for the amount of ω-6 polyunsaturated fatty acids (linoleic acid) and an AI of 1.4 g/day for the amount of ω-3 polyunsaturated fatty acids (α-linolenic acid) in the diet (IOM, 2002). The recommendation for docosahexaenoic acid is 300 mg/day. Essential fatty acid requirements can usually be met by one to two portions of fish per week (Simpson et al., 2010b). See Focus On: Omega-3 Fatty Acids in Pregnancy and Lactation.
Vitamins
All vitamins are needed for optimal pregnancy outcome. In some instances the provision of these specific vitamins may be met through diet, and for others a supplement is necessary. Periconceptional multivitamin supplementation has been documented to reduce the risk of heart defects in infants if started very early in pregnancy. Most vitamin and mineral recommendations increase approximately 15% from nonpregnant values. Refer to the DRI tables on the inside cover.
Folic Acid: For nonpregnant adult women, the recommended intake is 400 mcg/day for dietary folate equivalent. Folic acid requirements increase during pregnancy for maternal erythropoiesis, DNA synthesis, and fetal and placental growth. The Centers for Disease Control and Prevention recommends that all women of childbearing age increase their intake of folic acid; obese women even more so. More than half of all U.S. pregnancies are unplanned and the neural tube closes by 28 days of gestation, before most women realize they are pregnant (Goldberg et al., 2006; see Table 16-1). Because the average amount of folic acid received through food fortification (grains) in the United States is only 128 mcg/day, there is a need for the additional 400 mcg/day of synthetic folic acid from supplements or fortified foods (Simpson et al., 2010a).
Folic acid deficiency is marked by a reduced rate of DNA synthesis and mitotic activity in individual cells. White cell morphologic and biochemical changes signaling deficiency precede overt megaloblastic anemia, the latest stage of folate deficiency, which may not present until the third trimester (see Chapter 33). Maternal folate deficiency is associated with an increased incidence of congenital malformations, including cleft lip and palate and NTDs. Indeed, approximately 2500 new cases of NTDs occur in the United States each year; the NTD recurrence rate may be as high as 2% to 10%. Red blood cell folate concentrations exceeding 906 mmol/L (400 ng/mL) are associated with the fewest NTDs.
Women who smoke, consume alcohol moderately or heavily, or use recreational drugs are at risk for marginal folate status, as are those with malabsorption syndromes or genetic differences related to methylation and metabolic use of dietary folate. See Chapters 5 and 8. Malformations can occur in infants of women using the anticonvulsant medications phenytoin, carbamazepine, diphenylhydantoin; oral contraceptives; the diuretic triamterene; and trimethoprim. Women using antiseizure medications must be closely monitored when starting folic acid because folic acid supplementation can reduce seizure threshold.
Vitamin B6: Vitamin B6 functions as a cofactor in approximately 50 decarboxylase and transaminase enzymes, especially in amino acid metabolism. Although this vitamin catalyzes a number of reactions involving neurotransmitter production, it is not known whether this function is involved in the relief of nausea or vomiting. Because meat, fish, and poultry are good dietary sources, deficiency is not common and routine prenatal vitamins are sufficient (Simpson et al., 2010a). Megadoses such as 25 mg three times per day has questionable efficacy.
Vitamin B12: Cobalamin is required for enzyme reactions and for generation of methionine and tetrahydrofolate. B12 is found almost exclusively in foods of animal origin (meats, dairy products); therefore vegetarians are at greatest risk for dietary vitamin B12 deficiency and should be supplemented (Simpson et al., 2010a). Deficiencies in both folate and vitamin B12 have been related to depression in adults. There is concern regarding inadequate amounts of these nutrients during fetal brain development affecting infant cognitive and motor development (Black, 2008).
Choline: Choline is an essential nutrient because it cannot be synthesized in sufficient quantities to meet metabolic demands. It is needed for structural integrity of cell membranes, cell signaling, and nerve impulse transmission, and is a major source of methyl groups. Choline has been shown to support adequate neurogenesis in folic-acid deficient mice whose mothers were supplemented (Craciunescu et al., 2010). The IOM recommends choline at 450 mg/day during pregnancy, 25 mg more than for the nonpregnant woman. Choline-rich foods are beef liver, pork, chicken, turkey, fish, egg yolks, soy lecithin, and wheat germ. Pregnant women who are not eating these foods may need supplementation. The prenatal supplement should be evaluated for its choline content; many popular brands do not contain choline.
Vitamin C: Ascorbic acid is involved in collagen synthesis and functions as an antioxidant. Daily consumption of food sources high in this nutrient should be encouraged. At this time there are no recommendations to suggest supplemental vitamin C for the prevention of premature ruptured membranes and pre-eclamptic toxemia (PET).
Vitamin A: Vitamin A deficiency is teratogenic, as noted by xerophthalmia in developing countries. In human cord blood, vitamin A concentrations correlate with birth weight, head circumference, length, and gestation duration. Low maternal vitamin A concentration can result in reduced kidney size in newborns (Goodyer et al., 2007). Infants born prematurely have low vitamin A stores and poor lung function (Darlow and Graham, 2009).
Prenatal vitamin A supplementation is usually not warranted, and in developing countries should not exceed 3000 mcg (10,000 IU)/day (Simpson et al., 2010b). Very high doses of vitamin A (>30,000 IU) may increase the risk for a neural crest defect (Neural Crest and Associated Disorders, 2009). Women who take isotretinoin (Accutane) for acne should stop before they become pregnant. It is a vitamin A analog. As such, the infants of women who become pregnant while on isotretinoin are at extremely high risk for fetal anomalies (NICHD, 2001).
Vitamin D: Vitamin D and its metabolites cross the placenta and appear in fetal blood in the same concentration as in maternal circulation. Vitamin D enhances immune function and brain development (Feron et al., 2005). Vitamin D may have a role in cytokine (Th1 and Th2) regulation, multiple sclerosis, diabetes, and autism. Low vitamin D levels during pregnancy predispose to PET, a hypertensive condition of pregnancy affecting up to 8% of pregnant women (Duley, 2009). Maternal vitamin D deficiency is associated with neonatal hypocalcemia, which can manifest in inadequate fetal bone mineralization, hypoplasia of tooth enamel, or convulsions (Cambadoo et al., 2007).
Vitamin D blood concentrations are often low in infants born to vitamin D–deficient mothers. Vitamin D deficiency is increasingly recognized in dark-skinned and veiled women in the northern latitudes where sun exposure is low (Simpson et al., 2010b). Women who are at risk of entering pregnancy with low vitamin D levels include those with BMI greater than 30 and those with a high use of sunscreen, along with poor dietary intake. Poor muscular performance is associated with vitamin D deficiency. The rate of cesarean section deliveries is found to be inversely related to vitamin D status (Merewood et al., 2009). Vitamin D supplementation may be needed to reach desired serum concentrations of more than 20 ng/ml (50 nmol/L) (Simpson et al., 2010b). Use caution not to overdose; excessive amounts of vitamin D are undesirable.
Vitamin E: Vitamin E requirements are increased during pregnancy. Although deficiency during pregnancy is speculated to cause miscarriage, preterm birth, PET, and IUGR (Gagne et al., 2009), vitamin E deficiency specifically has not yet been reported in human pregnancy. Vitamin E is an important lipophilic antioxidant. Of the many tocopherols and tocotrienols, alpha tocopherol is the most biologically active form and is found in all lipoproteins. See Chapter 3.
Vitamin K: Usual diets do not provide adequate amounts of vitamin K as most food sources are dark leafy green vegetables, and are not consumed in recommended quantities. Vitamin K has an important role in bone health as well as in coagulation homeostasis, so adequate amounts during pregnancy are vital. Vitamin K deficiency has been reported in women who have had hyperemesis gravidarum (HG), Crohn’s disease, and gastric bypass (Brunnetti-Pierri et al., 2007).
Minerals
Calcium: Hormonal factors strongly influence calcium metabolism in pregnancy. Human chorionic somatomammotropin from the placenta increases the rate of maternal bone turnover. Estrogen inhibits bone resorption, provokes a compensatory release of parathyroid hormone, and maintains maternal serum calcium while enhancing maternal absorption of calcium across the gut. The net effect of these changes is the promotion of progressive calcium retention to meet progressively increasing fetal skeletal demands for mineralization. Fetal hypercalcemia and subsequent endocrine adjustments ultimately stimulate the mineralization process.
Approximately 30 g of calcium is accumulated during pregnancy, almost all of it in the fetal skeleton (25 g). The remainder is stored in the maternal skeleton, held in reserve for the calcium demands of lactation. Most fetal accretion occurs during the last trimester of pregnancy, an average of 300 mg/day.
The upper limit for calcium intake during pregnancy is 2500 mg/day. Overconsumption of calcium in food form is not common; however, elevated serum level of calcium can be the result of excess antacid ingestion for heartburn or gastroesophageal reflux disease.
Copper: Diets of pregnant women are often marginal in copper. Copper deficiency alters embryo development and induced-copper deficiency has been shown to be teratogenic. Not only are there genetic mutations, as in Menkes disease, but also secondary deficiencies from excessive zinc or iron intake, certain drugs, and bariatric surgery (Uriu-Adams et al., 2010). These inadequacies cause decreased activity of cuproenzymes, increased oxidative stress, altered iron metabolism, abnormal protein crosslinking, decreased angiogenesis and altered cell signaling (Uriu-Adams et al., 2010). See Chapter 3
Fluoride: The role of fluoride in prenatal development is controversial. Development of primary dentition begins at 10 to 12 weeks’ gestation. From the sixth to the ninth month, the first four permanent molars and eight of the permanent incisors are forming. Thus 32 teeth are developing during gestation. Controversy involves the extent to which fluoride is transported across the placenta and its value in-utero in the development of caries-resistant permanent teeth (see Chapter 26).
Iodine: Iodine is part of the thyroxine molecule, with a critical role in the metabolism of macronutrients. Adequate gestational iodine is associated with a higher intelligence quotient in the child and attention deficit may be associated with milder iodine deficiency (Hoy-Rosas, 2009). In instances in which preconception iodine intake cannot be ensured, supplementation before the end of the second trimester protects the fetal brain from the effects of deficiency (see Chapter 3). To ensure adequate iodine, food is often fortified with iodized salt. Yet many people worldwide are at risk for iodine deficiency caused by low intake of sea products and fish, produce grown in iodine-deficient soils, or food industry use of noniodized salt. Women who emigrate from other countries may have low iodine status because of the low iodine content of their agricultural soil. If urinary iodine levels are low, supplementation is needed (Simpson et al., 2010b). See Table 16-6.
TABLE 16-6
| Urinary iodine Excretion (mcg/L) | Corresponding iodine intake (mcg/day) | Classification and Implication |
| <20 | <30 | Insufficient: severe deficiency |
| 20-49 | 30-47 | Insufficient: moderate deficiency |
| 50-99 | 75-149 | Insufficient: mild deficiency |
| 100-199 | 150-299 | Adequate: optimal nutritional status |
| 200-299 | 300-499 | More than adequate: risk of iodine-induced hyperthyroidism within 5-10 years in susceptible groups |
| >300 | >449 | Risk of adverse health consequences such as iodine-induced hyperthyroidism, autoimmune thyroid disease |
World Health Organization, United Nations Children’s Fund, Assessment of iodine deficiency disorders and monitoring their elimination, pg 33, ISBN 978 92 4 159582 7, 2007.
Iron: A marked increase in the maternal blood supply during pregnancy greatly increases the demand for iron. Normal erythrocyte volume increases by 20% to 30% in pregnancy. A pregnant woman must consume an additional 700 to 800 mg of iron throughout her pregnancy—500 mg for hematopoiesis, and 250 to 300 mg for fetal and placental tissues. Most accretion occurs after the 20th week of gestation when maternal and fetal demands are greatest.
A first trimester ferritin level should be assessed before prescribing iron. Foods containing ascorbic acid enhance absorption. If anemia is not improved with iron therapy, it is advised to check vitamin B6 status. (Hisano et al., 2010). Because many women do not enter pregnancy with sufficient iron stores to cover the physiologic needs of pregnancy, iron supplementation (usually a ferrous salt) is often necessary. Supplementation may be necessary in the third trimester, earlier in pregnancy, or in nonpregnant states if serum ferritin is less than 20 mcg/L, hematocrit is less than 32%, or hemoglobin is less than 10.9 g/dL (Simpson et al., 2010b). Inadequate iron consumption may lead to poor hemoglobin production, followed by compromised delivery of oxygen to the uterus, placenta, and developing fetus. The added workload of the heart from maternal anemia with increased cardiac output can lead to preterm delivery, fetal growth retardation, LBW, or inferior neonatal health.
Although the implications of excessive iron intake for women and their infants are not yet clearly defined, some studies suggest a relationship with GDM (Chen et al., 2009).
Magnesium: The full-term fetus accumulates 1 g of magnesium during gestation. The IOM reports that magnesium supplementation during pregnancy reduces the incidence of PET and IUGR; see “Edema and Leg Cramps.”
Phosphorus: Phosphorus is found in a wide variety of foods and deficiency is rare when one is eating. Low phosphorous levels, indicative of “refeeding syndrome” have been found in women experiencing severe vomiting or other situations resulting in starvation. Hypophosphatemia can be life threatening because phosphorous is important in energy metabolism as a component of adenosine triphosphate (ATP) and must be promptly replenished, as with intravenous phosphorous (Stanza et al., 2008).
Sodium: The hormonal milieu of pregnancy affects sodium metabolism. Increased maternal blood volume leads to increased glomerular filtration of sodium of 5,000 to 10,000 mEq/day. Compensatory mechanisms maintain fluid and electrolyte balance.
Restriction of dietary sodium or the use of diuretics in pregnant women with edema is not recommended. Rigorous sodium restriction stresses the renin-angiotensin-aldosterone system, resulting in water intoxication and renal and adrenal tissue necrosis. Although moderation in the use of salt and other sodium-rich foods is appropriate for everyone, aggressive restriction is usually unwarranted in pregnancy. Consumption of sodium should remain above 2 to 3 g/day. Use of iodized salt should be encouraged.
Zinc: A zinc-deficient diet does not result in the effective mobilization of zinc stored in the maternal skeleton; therefore a compromised zinc status develops rapidly. Zinc deficiency is highly teratogenic and leads to congenital malformations, abnormal brain development in the fetus, and abnormal behavior in the newborn. A low zinc level also adversely affects vitamin A status. Women with low plasma zinc concentrations are at 2.5 times greater risk for delivering an infant weighing less than 2000 g; women younger than 19 years old have an even higher risk (Rwebembera et al., 2005; Scheplyagina, 2005). Evaluating nutritional status using plasma zinc requires caution because homeostatic mechanisms can maintain plasma concentrations for weeks despite inadequate intake (Charney and Malone, 2009). Zinc is available in red meat, seafood, including oysters, and unrefined grains. Extra supplementation is usually not required (Simpson et al., 2010b).
Guide for Eating During Pregnancy
The increased requirements of pregnancy can be met by following the Daily Food Guide (Table 16-7). Box 16-5 provides a summary of nutritional care.
TABLE 16-7
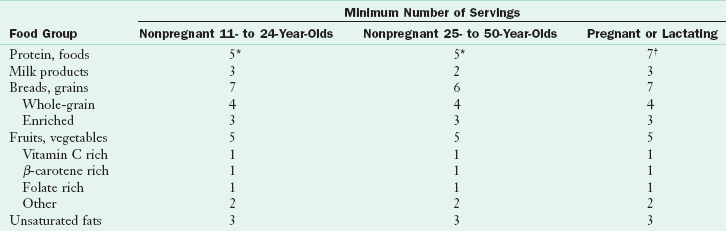
*Equivalent in protein to 5 oz of animal protein; at least three servings per week should be from the vegetable proteins.
†Equivalent in protein to 7 oz of animal protein; at least one of these servings should be a vegetable protein
Modified from Nutrition during pregnancy and the postpartum period: a manual for health care professionals, 1990, California Department of Health Services, Maternal Child Health Branch.
Calcium Intake
Milk is one choice for a calcium source for the pregnant woman to meet her increased calcium requirements. A number of milk choices are available: whole milk, low fat milk, skim milk, nonfat powdered milk, buttermilk, acidophilus milk, Lactaid-treated milk, evaporated milk, enriched soy milk, enriched rice and other grain milks, enriched nut milks, and yogurt. Goat milk is also available, but usually has low folate content. Approximately  cup of dried skim milk is equivalent to 1 cup of fluid milk. Milk can be made richer in calcium, protein, and calories by adding 2 tablespoons of dried nonfat milk to a glass of fluid milk.
cup of dried skim milk is equivalent to 1 cup of fluid milk. Milk can be made richer in calcium, protein, and calories by adding 2 tablespoons of dried nonfat milk to a glass of fluid milk.
Not all milk products are fortified with vitamin D3, a derivative from an animal source. Some soy milks are fortified with vitamin D2; because this is a nonanimal source, it may be preferred by vegans. Vitamin D2 potency is less than one third that of vitamin D3.
There are many other calcium-containing foods such as spinach, kale, and other dark green leafy vegetables, tofu, canned salmon, almonds, and calcium-fortified drinks and juices. See Table 3-25 in Chapter 3. Many women, primarily non–white women, are less able to digest the disaccharide lactose present in milk, unless it is taken in small amounts or milk is in a cooked product. If necessary, calcium supplements such as calcium lactate or calcium carbonate may be prescribed (see Chapter 25).
Fluids
Drinking 8 to 10 glasses of quality fluid daily, mainly water, is encouraged. Although the 2004 report by the National Academies set the AI at 1.5 L/day, with an upper limit of 2.3 L/day, evaluation of a woman’s body size as well as climatic conditions are important considerations. Adequate hydration improves the overall sense of well being. Frequent urination is often a complaint from pregnant women; however, optimal hydration reduces risks for urinary tract infections, kidney stones, and constipation.
Alcohol
Abundant evidence from both animal studies and human experience associates maternal alcohol consumption with teratogenicity and a specific pattern of abnormalities in the neonate. Features of fetal alcohol syndrome (FAS) include prenatal and postnatal growth failure, developmental delay, microcephaly, eye changes (including involvement of the epicanthal fold), facial abnormalities, and skeletal joint abnormalities (Figure 16-4). However, many children adversely affected by maternal drinking during pregnancy cannot be identified early in life using current diagnostic criteria for fetal alcohol spectrum disorder. Alcohol has been shown to alter gene expression. Changes involve proteins associated with central nervous system development; organ morphogenesis; immunologic responses; endocrine function; ion homeostasis; and skeletal, cardiovascular, and cartilage development.
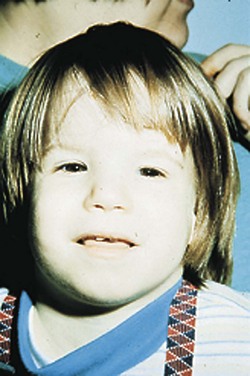
FIGURE 16-4 One-year-old child with fetal alcohol syndrome. (From Streissguth AP et al: Teratogenic effects of alcohol in humans and laboratory animals, Science 209:353, 1980.)
Use of alcohol during pregnancy has been associated with an increased rate of spontaneous abortion, placenta abruptio, LBW deliveries, mental retardation, and cognitive compromise. The American Congress of Obstetricians and Gynecologists (ACOG), as well as the March of Dimes and other professional organizations, recommend that alcohol not be used during pregnancy. Reduced-alcohol wines and beers do contain small amounts of alcohol and are also contraindicated. Despite the multiple warnings of fetal injury caused by alcohol, it has been shown that some women continue to consume alcohol in pregnancy (Crozier et al., 2009; Hannigan et al., 2009).
Nonnutritive Substances in Foods
Artificial Sweeteners: The artificial sweeteners sold in the United States have chemical names of saccharin, acesulfame-K, sucralose, and aspartame; brand names are Sweet ‘n’ Low, Sunette (or Sweet One), Splenda, Equal, or NutraSweet, respectively.
Saccharin is a weak carcinogenic in rats in very high doses. However, its consumption in pregnancy has not been restricted. Acesulfame-K consumption by pregnant women is classified as safe, even without long-term studies during human pregnancy. Both saccharin and acesulfame-K cross the placenta and appear in breast milk, but have no known adverse effect on the fetus or infant. Sucralose, a carbohydrate derived from sucrose, was approved for general use in all foods by the Food and Drug Administration (FDA) in 1998. It has not been found to be mutagenic or teratogenic in high doses in animals.
Aspartame is unsafe for women with phenylketonuria (PKU), regardless of whether they are pregnant. Aspartame is metabolized to phenylalanine and aspartic acid. Women with PKU are advised to follow a lifelong low-protein diet and should always be followed by qualified nutrition specialists, especially if they become pregnant. High circulating concentrations of phenylalanine are known to damage the fetal brain (see Chapter 44).
The plant derived sweetener stevia, has not been found to affect fetal development.
Bisphenol-A: Bisphenol-A, or BPA, an endocrine disruptor, may affect thyroid function in humans, especially in the fetus. It may also decrease the serum T4 half-life by activating hepatic enzymes (Pearce and Braverman, 2009). It should be eliminated if possible from the diet of the pregnant woman, and has been eliminated from plastic bottles and dishes used in feeding the newborn in the US and many other countries (Kubwabo et al., 2009).
Lead and Other Contaminants: Contaminants in food are the exception rather than the rule in the United States, but they do occur. In high concentrations, they can pass across the placenta to the fetus (Figure 16-5). Poorly glazed dishware and leaded crystal decanters often contain high amounts of lead. Old cookware coated with polytetrafluoroethylene (Teflon) are sources of contamination and need to be avoided. Pregnant women should be advised against using dolomite as a calcium supplement because it comes from seashells or sea coral, which have been shown to contain heavy metals, such as lead, the result of dumping industrial wastes in the oceans.
Listeria Monocytogenes: Listeria monocytogenes infects 2500 Americans each year; 500 of those infected die. Pregnant women are 20 times more likely than other healthy adults to become infected with Listeria. It is a known cause of spontaneous abortion and meningitis of the fetus and newborn. Listeria is a soilborne organism; infection results from eating contaminated foods of animal origin and raw vegetables. Raw milk, smoked seafood, frankfurters, pâté, soft cheeses, cold cuts from the deli counter, and uncooked meats are likely sources. Produce irrigated with wastewater needs to be carefully washed with potable water before ingestion.
Melamine: Melamine is a chemical additive that was criminally added to infant formula from China to increase the nitrogen content; this was discovered when analyzed by modern technology. It is a toxic substance and leads to renal damage or death in infants who ingest it. (Wen et al., 2010).
Mercury: In January 2001 the USDA and the FDA issued a warning for pregnant and lactating women and women of childbearing age to limit consumption of shark, mackerel, tilefish, tuna, and swordfish to no more than two times a week in 4-oz portions. Traces of methyl mercury are found in most fish, but concentrations may be higher in fish from waters close to areas of industrial mercury pollution. The usual concentration of methyl mercury in most fish ranges from less than 0.01 ppm to 0.5 ppm. Few species of fish reach the FDA limit for human consumption of 1 ppm except shark, swordfish, large tuna (the type used to make sushi or fresh steaks), tilefish, and king mackerel. Seafoods (canned tuna, shrimp, pollock, salmon, cod, catfish, clams, flatfish, crabs, and scallops) are continually at risk of mercury contamination and women are advised to regularly check the latest advisories. Farm-raised fish are subject to mercury contamination via acid rain.
Polychlorinated Biphenyls: More than 1.2 billion pounds of polychlorinated biphenyls (PCBs) were produced in the United States before 1976, and half still remain in the water systems. Although PCBs can be absorbed through the skin and lungs, they primarily enter the body from ingestion of contaminated fatty fish such as salmon, lake trout, and carp. They readily pass through the placenta and breast milk; thus pregnant and nursing women and women of childbearing age should avoid eating fish from water known to be contaminated with PCBs. Questions regarding mercury, PCBs, and other contaminants can be directed to state land and natural resource departments.
Cravings and Harmful Beliefs, Avoidances, and Aversions
Most women change their diets during their pregnancies. Change may be due to medical advice, cultural beliefs, or a change in food preference and appetite. Food avoidances may not reflect a mother’s conscious choice to eliminate certain foods during her pregnancy. Some reasons for food avoidance may include smell adversity caused by enhanced perception of aromas, a heightened gag response, getting ill while eating or smelling a particular food, or altered gastric comfort.
Cravings and Aversions: Cravings and aversions are powerful urges toward or away from foods, including foods about which women experience no unusual attitudes when not pregnant. The most commonly craved foods are sweets and dairy products or foods that are quick to eat. The most common aversions reported are to alcohol, coffee, other caffeinated drinks, and meats. However, cravings and aversions are not limited to any particular food or food groups.
Pica: Consumption of nonfood substances (pica) during pregnancy most often involves geophagia (consumption of dirt or clay) or amylophagia (consumption of starch such as laundry starch). Other substances include ice, paper, burnt matches, stones or gravel, charcoal, soot, cigarette ashes, antacid tablets, milk of magnesia, baking soda, and coffee grounds.
The incidence of pica is not limited to any one geographic area, race, sex, culture, or social status; nor is it limited to pregnancy. Its cause in pregnancy is poorly understood. One theory suggests that pica relieves nausea and vomiting. It has also been hypothesized that a deficiency of an essential nutrient such as calcium or iron results in the eating of nonfood substances that contain these nutrients. Malnutrition can be a consequence when nonfood substances displace essential nutrients in the diet. Starch in excessive amounts can contribute to obesity and can be deleterious in managing diabetes mellitus. Some substances contain toxic compounds or heavy metals; others interfere with the absorption of iron or other minerals. Excessive intake of starch and clay can lead to intestinal obstruction.
Complications and Nutritional Implications
Pregnant women become constipated if they fail to consume adequate water and fiber. Women who are treated with ondansetron (Zofran) for nausea and vomiting often experience severe constipation. Straining during stooling (val salva) increases the risk for hemorrhoids. Increased consumption of fluids, fiber-rich foods (see Appendix 41), and dried fruits (especially prunes and apricots), and nuts usually controls these problems. Some women may also require a bulking type of stool softener.
Diabetes Mellitus
The Hyperglycemia Adverse Pregnancy Outcome (HAPO) trial recently defined GDM as having one positive glucose reading after a 75-g glucose challenge (Table 16-8). Glucose intolerance may be associated with obesity. Women with recurrent preterm births are often treated with 17 α-hydroxyprogesterone caproate, which increases insulin resistance and the rate of GDM (Waters et al., 2009). Women diagnosed with GDM are at risk for future type 2 diabetes mellitus and cardiovascular disease. Although low serum 25(OH)D concentrations correlate with impaired glucose intolerance (von Hurst et al., 2009), no recommendations for nutrient supplementation have been made.
TABLE 16-8
Recommendations for the Diagnosis of GDM
| Time | HAPO* Recommendations | Current Standards |
| Fasting | <92 mg/dL | <95 mg/dL |
| 1 hour | <180 mg/dL | <180 mg/dL |
| 2 hour | <153 mg/dL | <155 mg/dL |
| 3 hour | Not performed | <140 mg/dL |
GDM, Gestational diabetes mellitus; HAPO, Hyperglycemia Adverse Pregnancy Outcome trial.
(*The challenge is proposed to by 75 grams of glucola)
References:
Hadar E et al: Towards new diagnostic criteria for diagnosing GDM: the HAPO study, J Perinat Med 37:447, 2009.
Metzger B et al: Hyperglycemia and adverse pregnancy outcomes, N Engl J Med 358:1991, 2008.
Fetuses of mothers with type 1 or type 2 diabetes are at risk for cardiac defects, such as transposition of the great vessels, double outlet of the right ventricle, tetralogy of Fallot, and mitral and pulmonary atresia (Corrigan et al., 2009). Fetuses of women with GDM are at risk for hypoglycemia at birth, a neonatal intensive care stay, macrosomia, or shoulder dystocia (HAPO Study Cooperative Research Group, 2010). These infants may have lower levels of potassium, zinc, manganese, and chromium (Afridi et al., 2009).
Approaches to reduce the incidence of GDM include providing women with supplemental probiotics before and during pregnancy. Probiotics appear to alter maternal microbiota, change the immune response (Luoto, 2010), and support better glucose tolerance and lower body weight (Laitinen et al., 2009). Women with GDM or elevated first-trimester uric acid concentrations may benefit from a low glycemic prenatal diet (Laughton et al., 2009). See Chapter 31.
Edema and Leg Cramps
Mild, physiologic edema is usually present in the extremities in the third trimester and should not be confused with the pathologic, generalized edema associated with PIH. Normal edema in the lower extremities in pregnancy is caused by the pressure of the enlarging uterus on the vena cava, obstructing the return of blood flow to the heart. When a woman reclines on her side, the mechanical effect is removed, and extravascular fluid is mobilized and eventually eliminated by increased urine output. No dietary intervention is required. Magnesium supplementation has been recommended to reduce leg cramps in pregnancy; however, it may not be effective for every pregnant woman (Nygaard et al., 2008; Sohrabvand et al., 2006).
Heartburn
Gastric esophageal reflux is common during the latter part of pregnancy, and often occurs at night. In most cases this is an effect of pressure of the enlarged uterus on the intestines and stomach, which, in combination with the relaxation of the esophageal sphincter, may result in regurgitation of stomach contents into the esophagus. Relief may occur by suggesting that the pregnant woman eat frequent small meals and stay upright for at least 3 hours after a meal and before lying down. Smaller plates can be used to remind a woman about reduced gastric volume. See Chapter 28.
Nausea, Vomiting, and Hyperemesis Gravidarum
Morning sickness, nausea and vomiting in pregnancy (NVP), affects 50% to 90% of all pregnant women during the first trimester and usually resolves at approximately 17 weeks gestation. Motion, loud noises, bright lights, and adverse climate conditions may trigger the nausea (Erick, 2004). Fortunately, most women with NVP are functional, able to work, do not lose weight, and are helped by simple dietary measures. Small, frequent snacks of carbohydrate foods reduce nausea for some, whereas protein foods may help others. Diets high in ginger and protein can reduce symptoms of nausea (Levine et al., 2008). Ginger reduces symptoms of NVP better than vitamin B6 (Chittumma et al., 2007; Ensiyeh and Sakineh, 2009). Other therapies suggested include crackers or potato chips, elastic wrist bands, electronic wrist bands (“Relief Bands”), special lollipops (“Preggie Pops”), red raspberry leaf tea, noise reduction, acupuncture, and hypnosis. Some even try “Morning Sickness Magic,” a tonic containing peach, ginger, raspberry leaf, vitamin B6, and folate.
Some women do not tolerate the odors from hot foods, and room-temperature foods may be preferred. Smelling lemons may help block noxious odors (Erick, 2004). Unfortunately, there is no cure-all. Women suffering with nausea should eat whatever reduces the sensation of nausea and avoid odors that trigger nausea.
When early pregnancy is characterized by excessive vomiting and weight loss, fluid and electrolyte imbalances can occur. Here, “morning sickness” becomes hyperemesis gravidarum (HG); this occurs in approximately 1% to 2% of pregnancies. Hospitalization for nutrition support and hydration is usually indicated. Appropriate weight gain for pregnancy; correction of fluid and electrolyte deficits; avoidance of ketosis; control of HG symptoms; and achievement of nitrogen, vitamin, and mineral balance are the goals in management (Austin, 2010).
Complications of HG vary, but may include global subluxation (Zeller, 2007), chondrodysplasia punctata, splenic avulsion (Nguyen et al., 1995), ruptured esophagus, and gestational malnutrition (Fejzo et al., 2009). A nuisance factor is ptyalism gravidarum, or excess saliva. Salivary output can be substantial and can be a source of lost electrolytes. Output of 500-1000 mL/day is not uncommon. Another difficult to identify but serious complication may be Wernicke encephalopathy with at least 49 cases being reported worldwide by 2006 (Chiossi et al., 2006) (see Chapter 3). Here intravenous administration of 100-mg thiamin for several days may be required (Austin, 2010).
In HG, enteral nutrition has variable effectiveness. Part of the challenge is that many obstetricians lack knowledge in tube placement, and women with severe emesis and retching have often dislodged tubes and are sometimes reluctant for replacement. If tubes are placed during a hospitalization, frequent nursing checks during the night disrupt sleep and add to sleep deprivation issues, which have not been widely recognized. Parenteral nutrition is used if the GI tract is not accessible, or if enteral nutrition is not tolerated (Austin, 2010). Because pregnancy is a condition of accelerated starvation, refeeding syndrome is often seen (Majumdar and Dada, 2010). Electrolytes such as phosphorous, magnesium, and potassium must be evaluated daily because low levels may result in cardiac irregularities and respiratory failure (Stanza et al., 2008). See Chapter 14.
Pregnancy-Induced Hypertension
Pregnancy-induced hypertension (PIH) includes gestational hypertension, PET, and eclampsia. Gestational hypertension is a maternal blood pressure equal to or greater than 140/90 with no proteinuria that develops after mid-pregnancy. These women may develop pre-eclamptic toxemia (PET), defined as a systolic blood pressure of 140 or more, or a diastolic blood pressure of more than 90, plus urinary protein of more than 300 mg from a 24-hour urine sample. Severe PET is defined as a systolic blood pressure of more than 160 or a diastolic blood pressure of more than 110, plus more than 5 g of protein in a 24-hour urine sample. PET is associated with decreased uterine blood flow from vasospasm, leading to reduced placental size, compromised fetal nourishment, and an IUGR fetus.
The cause of PET is unknown; however, the disease complicates 5% to 8% of pregnancies (Getahun et al., 2007). Theories regarding causes are varied and include vascular injury to the placental blood vessels, high BMI, nulliparity, advanced maternal age, multifetal gestation, non-white race, renal disease, high pregnancy weight gain, and low levels of vitamin D (Getahun et al., 2007). Very young women with first pregnancies are also known to have higher incidences of PET. The incidence of PET is also increased in women with autoimmune diseases such as type 1 diabetes and rheumatoid arthritis.
PET is more common in dark-skinned women living in northern latitudes and others with a high prevalence of hypovitaminosis D. Low levels of vitamin D produce elevated cytokines. There is a reduction in the levels of circulating 1,25(OH)2D3 in preeclamptic patients (Spinnato, 2007) ; this may be caused by a disturbance in 1β-hydroxylation within the placenta.
Eclampsia is PIH resulting in grand mal seizures. Symptoms that precede seizure are dizziness, headache, visual disturbances, facial edema, right-sided epigastric pain, anorexia, nausea, and vomiting. Fetal death often results in women who develop eclampsia. A small percent of cases of eclampsia present in the postpartum period. Eclampsia can be fatal to the mother if not treated promptly. Intravenous magnesium supplementation may be used.
Lactation
Exclusive breast-feeding is unequivocally the preferred method of infant feeding for the first 4 to 6 months of life. Both the American Dietetic Association and the American Academy of Pediatrics (AAP) have issued position statements in support of breast-feeding (James and Lessen, 2009). Other supporters include Healthy People 2010, The WIC program, and the U.S. Breastfeeding Committee.
Benefits
Breast-feeding can reduce the incidence of both types 1 and 2 diabetes (Gunderson et al., 2007; Malcova et al., 2006). An excellent way to promote breast-feeding is to discuss its benefits, as shown in Box 16-6.
In 1991 the World Health Organization and the United Nations Children’s Fund adopted the Baby-Friendly Hospital Initiative, a global effort to increase the incidence and duration of breast-feeding. To become “baby friendly,” a hospital must show to an outside review board that it implements the “Ten Steps to Successful Breast-feeding,” a guideline for mother-baby management in the hospital; see Clinical Insight: Baby-Friendly Hospital Initiative: Ten Steps to Successful Breast-Feeding.
Contraindications
Breast-feeding is contraindicated for infants with galactosemia and for mothers who have active untreated tuberculosis or are positive for human T-cell lymphotropic virus type 1 or 2, who use drugs of abuse, have human immunodeficiency virus (in the United States), or who take certain medications (i.e., antimetabolites and chemotherapeutic agents). The use of radioactive isotopes requires temporary cessation of breast-feeding (Lawrence and Lawrence, 2005).
Physiology of Lactation
Mammary gland growth during menarche and pregnancy prepares the woman for lactation. Hormonal changes markedly increase breast, areola, and nipple size. In pregnancy, hormones significantly increase ducts and alveoli and influence mammary growth. Late in pregnancy the lobules of the alveolar system are maximally developed, and small amounts of colostrum may be released for several weeks before term and for a few days after delivery. After birth there is a rapid drop in circulating levels of estrogen and progesterone accompanied by a rapid increase in prolactin secretion, setting the stage for a copious milk supply.
The usual stimulus for milk production and secretion is suckling. Subcutaneous nerves of the areola send a message via the spinal cord to the hypothalamus, which in turn transmits a message to the pituitary gland, where both the anterior and posterior areas are stimulated. Prolactin from the anterior pituitary stimulates alveolar cell milk production, as shown in Figure 16-6.
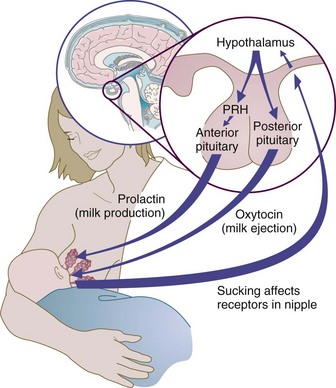
FIGURE 16-6 Physiology of milk production and the let-down reflex. PRH, Pituitary-releasing hormone.
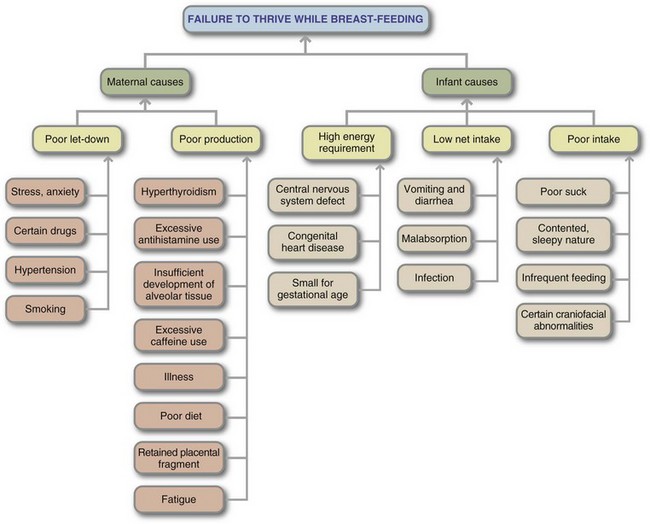
Oxytocin from the posterior pituitary stimulates the myoepithelial cells of the mammary gland to contract, causing movement of milk through the ducts and lactiferous sinuses, a process referred to as let-down. “Let-down” is highly sensitive. Oxytocin may be released by visual, tactile, olfactory, and auditory stimuli; and even by thinking about the infant. Oxytocin secretion can also be inhibited by pain, emotional and physical stress, fatigue, and anxiety. Women who have diabetes, who are obese, who are stressed during delivery, or who have retained placental fragments in the uterus are at risk for delayed milk production, when signs of lactogenesis are absent 72 hours after birth (Lawrence and Lawrence, 2005).
Nutritional Requirements of Lactation
Lactation is nutritionally demanding, especially for the woman who nurses her infant exclusively for a number of months. Increased intake of most nutrients is advised. The nutritional status of lactating women who have previously undergone gastric bypass surgery needs close attention because suboptimal levels of iron, vitamin A, vitamin D, vitamin K, folate, and calcium have been reported (Guelinckx et al., 2009).
Milk production is most affected by the frequency of suckling and maternal hydration. However, milk composition varies according to the mother’s diet. For example, the fatty acid composition of a mother’s milk reflects her dietary intake. In addition, milk concentrations of selenium, iodine, and some of the water-soluble B vitamins reflect the maternal diet. Breast milk of malnourished mothers has been shown to have lower levels of various nutrients, reflecting the foods she has available to eat.
Energy
Milk production is 80% efficient: production of 100 mL of milk (approximately 75 kcal) requires an 85-kcal expenditure (Lawrence and Lawrence, 2005). During the first 6 months of lactation, average milk production is 750 mL/day, with a range of 550 to more than 1200 mL/day. Because production is a function of the frequency, duration, and intensity of infant suckling, infants who feed well are likely to stimulate the production of larger volumes of milk.
The DRI for energy during lactation is 330 kcal greater during the first 6 months of lactation and 400 kcal greater during the second 6 months of lactation over that for a nonpregnant woman. It is the same as the DRI during the second trimester of pregnancy (IOM, 2002). The obese and overweight woman may not require the full 330 to 400 extra kcal/day. Maternal fat stores accumulated during pregnancy provide approximately 100 to 150 kcal per day to support the early months of lactation. When the reserve fat stores have been depleted, dietary energy support for lactation must be increased if the mother intends to provide all or most of her infant’s nutrition through breast milk alone. During the second 6 months of lactation, production generally drops to an average of 600 mL/day or approximately 20 oz/day.
Healthy breast-feeding women can lose as much as 1 pound per week and still supply adequate milk to maintain their infants’ growth. Breast-feeding women should be reminded of the energy expenditure required to produce milk: approximately 0.67 kcal per 1 cc of breast milk output. Milk production decreases in mothers whose intakes are suboptimal; less than 1800 calories/day, and breast milk will reflect the nutrient profile of the maternal diet. Appropriate fluid intake (such as drinking for thirst) and adequate rest are also needed.
Protein
The DRI suggests an additional 25 g of protein a day for lactation, or 71 g of protein a day. This is based on an RDA of 1.1 gm/kg/day of prepregnancy weight. Clinical judgment is necessary with protein recommendations because 71 g/day may be too low in an overweight woman and too high for the woman with a lower BMI. Women with surgical delivery and women who enter pregnancy in poor nutritional shape may need additional protein. The average protein requirement for lactation is estimated from milk composition data and the mean daily volume of 750 mL, assuming 70% efficiency in the conversion of dietary protein to milk protein.
Breast milk has a whey/casein ratio of 90 : 10 early in lactation, which changes to 80 : 20 as an average, and to 60 : 40 as the baby gets older. It is speculated that this ratio makes breast milk more digestible. In contrast, the whey/casein ratio of cow’s milk protein is 18 : 82.
Carbohydrates
The RDA for carbohydrate is 210 g/day (IOM, 2002). This 210 g/day is the recommended amount to provide enough calories in the diet for adequate volumes of milk and to maintain an adequate energy level during lactation. This may need to be adjusted depending on activity of the mother and the amount of breast-feeding. The woman with a poor gestation weight gain may require more carbohydrates.
Lipid
The amount and type of fat in breast milk directly reflects the maternal diet. The “fore” milk or first milk in a feeding is lower in fat than the “hind” milk at the end of a feeding. Dietary fat choices by the mother can increase or decrease specific fatty acids in her milk. Severe restriction of energy intake results in mobilization of body fat, and the milk produced has a fatty acid composition resembling that of the mother’s body fat.
There is no DRI for total lipids during lactation because it depends on the amount of energy required by the mother to maintain milk production. DRIs state a recommended amount of specific long-chain polyunsaturated fatty acids in human milk because their presence in the maternal diet is crucial for fetal and infant brain development. The AI for ω-6 polyunsaturated fatty acids is 13 g/day, and the AI for ω-3 polyunsaturated fatty acids is 1.3 g/day (IOM, 2002). Trans fats should be avoided completely by the nursing mother so that the potential for their appearance in her breast milk is reduced.
Human milk contains 10 to 20 mg/dL of cholesterol, resulting in an approximate consumption of 100 mg/day by the infant. The amount of cholesterol in milk does not reflect the mother’s diet; however, the cholesterol content of the milk decreases over time as lactation progresses.
Vitamins and Minerals
The vitamin D content of milk is related to maternal vitamin D intake and the degree of sun exposure. Numerous case reports document marginal or significant vitamin D deficiency in pregnant women and in infants of lactating women who are veiled, dark skinned, with BMI of more than 30, who use sunscreens heavily, or who live in northern latitudes with decreased sun exposure. Women with lactose intolerance who do not drink vitamin D–fortified milk or take a vitamin supplement may be at higher risk for vitamin D deficiency. Because of reports of clinical rickets, the AAP recommends that all breast-fed infants receive an additional 200 IU (5 mcg) of vitamin D daily beginning at 2 months of age (Lawrence and Lawrence, 2005). During lactation, supplements administered directly to the infant allow the infant to easily achieve vitamin D sufficiency; the mother may need much higher doses (100 mcg or 4000 IU per day) to achieve adult-normal 25(OH)D concentrations and vitamin D adequacy in her exclusively breast-fed infant.
The calcium content of breast milk is not related to maternal intake, and there is no convincing evidence that maternal change in bone mineral density is influenced by calcium intake across a broad range of intakes up to 1600 mg/day. In a community study of 210 Sri Lankan women, prolonged lactation was not shown to have detrimental effects on bone density mass (Lenora et al., 2009).
The amount of iodine in breast milk may not always reflect maternal intake. See “Iodine” earlier in this chapter. In the US the presence of the industrial pollutant, perchlorate, has been shown to inhibit iodine uptake. Perchlorate has been found in mothers’ milk as well as in the water supply (Dasgupta et al., 2008). This may explain why iodine levels in some individuals are low, despite what appears to be an adequate amount of iodine in the diet.
The requirements for zinc during lactation are greater than those during pregnancy. In the process of normal lactation the zinc content of breast milk drops dramatically during the first few months from 2 to 3 mg/day to 1 mg/day by the third month after birth.
Breast-Feeding an Infant
The advantages of breast-feeding should be presented throughout the childbearing years (Figure 16-7). During the last months of pregnancy, counseling on the process of lactation should be made available to women who have decided to breast-feed. Fathers or partners should be encouraged to participate in counseling sessions because the emotional support they provide contributes to the success of lactation.
Colostrum
Colostrum is the thin, yellow, milky fluid that is the first milk available after birth. It is higher in protein and lower in fat and carbohydrate than mature milk. Colostrum provides approximately 20 kcal/oz and is a rich source of antibodies (Lawrence and Lawrence, 2005). The unique properties of colostrum include that it is lower in lactose than mature milk, facilitates the passage of meconium (first stool of neonate), is high in antioxidants, and is lower in water-soluble vitamins than mature milk. Colostrum is also higher in fat-soluble vitamins, protein, sodium, potassium, chloride, zinc, and immunoglobulins than mature milk.
The Technique
Breast-feeding is a learned skill for both mother and her infant. The baby should be put to breast soon after birth and remain in direct skin-to-skin contact until the first feed is accomplished (American Academy of Pediatrics, 2005). Lactating women may experience a tingling sensation in the breast signaling the let-down reflex. Practice, patience, and perseverance are necessary. Within 48 to 96 hours after birth the breasts become fuller and firmer as the milk volumes increase. Women should be encouraged to pump and store breast milk in the likelihood of needing to be away from the infant for a feeding or two. Use of a breast pump facilitates breast milk expression; pump rentals may be covered by insurance.
Women who have insulin-dependent diabetes may experience “lactation hypoglycemia” as they increase their breast-feeding sessions. Those who have had cesarean deliveries or those who are taking pain medication may have difficulty with discerning let-down from low glucose levels. Frequent glucose monitoring needs to be emphasized to ensure safety for both mother and baby.
There is no need to offer breast-fed babies additional water because 87% of breast milk is water. However, cases of hypernatremic dehydration caused by suboptimal breast-feeding do occur. Most cases involve young and first-time mothers who may feel intimidated and overwhelmed at delivery, lack breast-feeding education, and are unaware of the consequences of dehydration. Extreme heat or hot weather may also contribute to this problem. The consequence of hypernatremic dehydration can be permanent brain damage or death. Therefore it is vital to evaluate the breast-feeding in the clinic at followup. Problems identified can then be addressed, and a plan of care can be implemented.
All breast-fed newborns should be seen within 3 to 5 days of birth by an experienced health care professional (AAP, 2005).
Exclusive breast-feeding is recommended for the first 6 months, but may continue for the first year or as long as it is mutually desired by mother and child (AAP, 2005).
Exercise and Breast-Feeding
The breast-feeding mother should be encouraged to get back to exercise a few weeks after delivery, after lactation is well established. Aerobic exercise at 60% to 70% of maximum heart rate has no adverse effect on lactation; infants gain weight at the same rate, and the mother’s cardiovascular fitness improves. Exercise also improves plasma lipids and insulin response in lactating women.
Transfer of Drugs into Human Milk
Almost all drugs taken by the mother appear in her milk to some degree. The amount that usually transfers is quite small. Many factors influence how medications transfer into human milk: milk/plasma ratio, molecular weight of the drug, and the protein binding and lipid solubility of the drug.
It is estimated that more than 500,000 pregnancies in the United States each year involve women who have psychiatric illnesses that either antedate the pregnancy or emerge during pregnancy and an estimated one third of them require a psychotropic medication (ACOG 2009; Voyer and Moretti, 2009). The need for medication must be balanced against the risk of an untreated illness. Websites that can provide more information include the AAP, www.aap.org, and the National Institutes of Health, http://www.nimh.nih.gov/health/publications/mental-health-medications/complete-index.shtml.
Failure to Thrive in the Breast-Fed Infant
Insufficient milk supply is rarely a problem for the well-fed, well-rested, and unstressed mother. Sucking stimulates the flow of milk; thus feeding on demand should supply ample amounts of milk to the infant. If the baby continues to gain weight and length steadily, has at least six to eight wet diapers daily, and has frequent stools, the milk supply is probably adequate.
Occasionally, however, an infant fails to thrive while seeming to nurse properly. Figure 16-8 illustrates potential problems in the mother or the infant that should be investigated during the course of evaluation. If the cause of the problem cannot be identified or the defined problem cannot be corrected, it may be necessary to encourage the mother to use commercial infant formula for at least partial nutritional support of the infant. A thorough assessment of the maternal diet and health habits is always necessary. Women who consume diets low in vitamins D or B12 or iodine produce milk with low levels; the result is FTT in the breast-fed infant.
Three cases of dilated cardiomyopathy in male neonates caused by low vitamin D intakes in the lactating mothers have been reported. Two cases from the United Arab Emirates found congestive cardiac failure in two 9-month-old babies who were also noted to have rickets (Amirlak et al., 2008). Both mothers were also diagnosed with vitamin D deficiencies, being heavily clothed. Dilated cardiomyopathy associated with hypocalcemic rickets has been reported in the United States in breast-fed black infants (Brown et al., 2009).
Sometimes the infant may become intolerant or allergic to something the mother has ingested. Cow’s milk protein, notably casein, has been implicated, as have peanuts. At this time, the amount or the frequency has not been conclusively determined (Sicherer SH et al., 2010; DesRoches A et al., 2010; Lopez-Exposito I et al., 2009). When suspicious foods are removed from the mother’s diet, it is important to assess the nutritional quality of her diet and supplement her appropriately (see Chapter 27).
Other Problems of Breast-Feeding
Overweight lactating women can restrict their energy intake by 500 kcal per day by decreasing consumption of foods high in fat and simple sugars, but they must increase their intake of foods high in calcium, vitamin D, vitamin A, vitamin C, and ω-3 fats to provide key nutrients for their milk supply.
Breast augmentation, a procedure in which an implant is inserted into the breast to enlarge it, is a common elective breast procedure. Periareolar and transareolar incisions can cause lactation insufficiency. These mothers should be encouraged to breast-feed, and their infants monitored for appropriate weight gain.
Reduction mammoplasty is often recommended for women with extremely large breasts who suffer from back, shoulder, or neck pain or poor body image. There are wide variations in milk production, from a little to full production, depending on the amount of tissue removed and the type of surgical incision. These mothers should also be encouraged to breast-feed and be given anticipatory guidance and support; their infants should be monitored closely for appropriate weight gain.
There can be a number of other hurdles to overcome to breast-feed successfully. These problems and their solutions are discussed in Table 16-9.
TABLE 16-9
Management of Breast-Feeding Difficulties
| Problem | Approaches to Management |
| Retracted nipples | Before feeding the infant, roll the nipple gently between the fingers until erect. |
| Baby’s mouth not open wide enough | Before feeding, depress the infant’s lower jaw with one finger as the nipple is guided into the mouth. |
| Baby sucks poorly | Stimulate sucking motions by pressing upward under the baby’s chin. Expression of colostrums often occurs, and the taste may stimulate sucking. |
| Baby demonstrates rooting but does not grasp the nipple; eventually cries in frustration | Interrupt the feeding, comfort the infant; the mother should take time to relax before trying again. |
| Baby falls asleep while nursing | If the infant falls asleep early in the feeding, the mother should awaken the infant by holding him or her upright, rubbing his or her back, talking to him or her, or providing similar quiet stimuli; another effort at feeding can then be made. If the baby falls asleep again, the feeding should be postponed. |
Agency for Toxic Substances and Disease Registry, Polychlorinated Biphenyls
http://www.atsdr.cdc.gov/tfacts17.html
Breastfeeding After Breast Reduction
Centers for Disease Control, Listeriosis
http://www.cdc.gov/nczved/divisions/dfbmd/diseases/listeriosis/
References
Afridi, HI, et al. Status of essential trace metals in biological samples of diabetic mothers and their neonates. Arch Gynecol Obstet. 2009;280:415.
Amirlak, I, et al. Dilated cardiomyopathy secondary to nutritional rickets. Ann Trop Paediatr. 2008;93:227.
American Dietetic Association. Position of the American Dietetic Association and American Society for Nutrition: obesity, reproduction and pregnancy outcome. J Am Diet Assoc. 2009;109:918.
American Academy of Pediatrics. breastfeeding and the use of human milk. Pediatrics. 2005;115:496.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion number 315, Sept 2005. Obesity in pregnancy. Obstet Gynecol. 2005;106(3):671.
American Congress of Obstetricians and Gynecologists (ACOG). ACOG Practice Bulletin. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2009;111:1001.
Artal, R, et al. Weight gain recommendations in pregnancy and the obesity epidemic. Obstet Gynecol. 2010;115:152.
Arterburn, LM, et al. Algal-oil capsules and cooked salmon: nutritionally equivalent sources of docosahexaenoic acid. J Am Diet Assoc. 2008;108:1204.
Austin, T. Nutrition management of the hyperemesis gravidarum patient. Nutrition Support Line. 2010;32(2):16.
Biggio, JR, et al. Fetal anomalies in obese women: the contribution of diabetes. Ob Gyn. 2010;115:290.
Black, MM. Effect of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull. 2008;29:S126.
Brown, J, et al. Hypocalcemic rickets and dilated cardiomyopathy: case reports and review of the literature. Pediatric Cardiol. 2009;30:6.
Brunetti-Pierri, N, et al. Gray matter heterotopias and brachytelephalangic chondrodysplasia punctata: a complication of hyperemesis gravidarum induced vitamin K deficiency? Am J Gen Med. 2007;143:200. [Part A].
Callaway, LK, et al. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191:425.
Callaway, LK, et al. Obesity and the hypertensive disorders of pregnancy. Hypertens Preg. 2009;28:473.
Cambadoo, L, et al. Maternal vitamin D deficiency associated with neonatal hypocalcaemic convulsions. Nutr J. 2007;6:23.
Charney, P, Malone, AM. ADA pocket guide to nutrition assessment, ed 2. Chicago: American Dietetic Association; 2009.
Chen, KK, et al. Iron supplementation in pregnancy and development of gestational diabetes—a randomized placebo-controlled trial. BJOG. 2009;116:389.
Chavarro, JE, et al. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050.
Chien, LC, et al. Hair mercury concentration and fish consumption: risk and perception of risk among women of childbearing age. Environ Res. 2010;110:123.
Chiossi, G, et al. Hyperemesis gravidarum complicated by Wernicke’s encephalopathy: background, case report and review of the literature. Obstet Gynecol Surv. 2006;61(4):255.
Chittumma, P, et al. Comparison of the effectiveness of ginger and vitamin B6 for the treatment of nausea and vomiting in early pregnancy: a randomized double-blind controlled trial. J Med Assoc Thai. 2007;90:15.
Cochrane Update. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Obstet Gynecol. 2009;14:161.
Corrigan, N, et al. Fetal cardiac effects of maternal hyperglycemia during pregnancy. Birth Defects Res A Clin Mol Teratol. 2009;85:523.
Craciunescu, CN, et al. Dietary choline reverses some, but not all, effects of folate deficiency on neurogenesis and apoptosis in fetal mouse brain. J Nutr. 2010;140:1162.
Crozier, SR, et al. Do women change their health behaviours in pregnancy? Findings from the Southampon Women’s Survey. Paediatric Perinatal Epidemiol. 2009;23:446.
Cui, X, et al. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int J Dev Neurosci. 2007;25:227.
Cox, JT, Phelan, ST. Nutrition during pregnancy. Obstet Gynecol Clin North Am. 2008;35:369.
Darlow, BA, Graham, P, Vitamin A supplementation to prevent mortality and short term and long term morbidity in very low birthweight infants (review). The Cochrane Collaborative, The Cochrane Library 2008;4. Accessed from. http://www.thecochranelibrary.com
Dasgupta, PK, et al. Intake of iodine and perchlorate and excretion in human milk. Environ Sci Technol. 2008;42:81.
Dheen, ST, et al. Recent studies on neural tube defects in embryos of diabetic pregnancy: an overview. Curr Med Chem. 2009;16:2345.
De Santis, MS, et al. Growth of fetal lean and fat mass in gestational diabetes. Ultrasound Obst Gynecol. 2010;36:328.
DesRoches, A, Infante-Rivard, C, et al. Peanut allergy: is maternal transmission of antigen during pregnancy and breastfeeding a risk factor? J Investig Allergol Clin Immunol. 2010;20(4):289.
Duley, L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130.
Ensiyeh, J, Sakineh, MA. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: a randomized controlled trial. Midwifery. 2009;25:649.
Erick, M. Managing morning sickness: a survival guide for pregnant women. Boulder, Colo: Bull Publishing; 2004.
Faintuch, J, et al. Pregnancy nutritional indices and birth weight after Roux-en-Y gastric bypass. Obes Surg. 2009;19:583.
Fejzo, MS, et al. Symptoms and pregnancy outcomes associated with extreme weight loss among women with hyperemesis gravidarum. J Women’s Health. 2009;18:1981.
Feron, F, et al. Developmental vitamin D3 deficiency alters the adult brain. Brain Res Bull. 2005;65:14.
Gaber, KR, et al. Maternal vitamin B12 and risk of neural tube defects in Egyptian patients. Clin Lab. 2007;53:69.
Gagne, A, et al. Absorption, transport, and bioavailability of vitamin E and its role in pregnant women. J Obstet Gynaecol Can. 210, 2009.
Gaur, DS, et al. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male infertility. Indian J Pathol Microbiol. 2010;53:35.
Georgieff, MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:1S.
Getahun, D, et al. Primary preeclampsia in the second pregnancy: effect of changes in prepregnancy body mass index between pregnancies. Ob Gyn. 2007;110:1319.
Goldberg, BB, et al. Prevalence of periconceptual folic acid use and perceived barriers to postgestational continuance of supplemental folic acid: survey from a Teratogen Information Service. Birth Defects Res A Clin Mol Teratol. 2006;76:193.
Goodnight, W, Newman, R. Optimal nutrition for improved twin pregnancy outcome. Obstet Gynecol. 2009;114:1121.
Goodyer, P, et al. Effects of maternal vitamin A status on kidney development: a pilot study. Pediatr Nephrol. 2007;22:209.
Grassi, A. Recognition and treatment approaches for polycystic ovary syndrome. In: Women’s health report. Chicago, Ill: American Dietetic Association, Women’s Health Dietetic Practice Group; Summer 2008.
Groenen, PM, et al. Low maternal dietary intakes of iron, magnesium and niacin are associated with spina bifida in the offspring. J Nutr. 2004;134:1516.
Guelinckx, I, et al. Reproductive outcome after bariatric surgery: a critical review. Hum Reprod Update. 2009;15:189.
Gunderson, EP, et al. Lactation and changes in maternal metabolic risk factors. Obstet Gynecol. 2007;109:729.
Hadar, E, et al. Towards new diagnostic criteria for diagnosing GDM: the HAPO study. J Perinat Med. 2009;37:447.
HAPO Study Cooperative Research Group, Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. associations with maternal body mass index. Am J Ostet Gynecol. 2010;202:255.
Hannigan, JH, et al. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2009;44:583.
Hisano, M, et al. Vitamin B6 deficiency and anemia in pregnancy. Eur J Clin Nutr. 2010;64:221.
Hoy-Rosas, J. Iodine and reproductive nutrition. In: Women’s health report. Chicago, Ill: American Dietetic Association, Women’s Health Dietetic Practice Group; Spring 2009.
Institute of Medicine (IOM), Food and Nutrition Board. Dietary reference intakes for energy, macronutrients, carbohydrates, fiber, fat and fatty acids. Washington, DC: National Academies Press; 2002.
Jahanfar S, Sharifah H: Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome, Cochrane Database of Systemic Reviews. 2:CD006965, 2009.
James, J, Lessen, R. Position of the American Dietetic Association: promoting and supporting breastfeeding. J Am Diet Assoc. 2009;109:1926.
Kubwabo, C, et al. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009;26:928.
Kyle, UG, Picard, C. The Dutch Famine of 1944-45: a pathological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006;9:388.
Laitinen, K, et al. Probiotics and dietary counseling contribute to glucose regulation during and after pregnancy: a randomized controlled trial. Br J Nutr. 2009;101:1679.
Laughton, SK. Elevated first trimester uric acid concentrations are associated with the development of gestational diabetes. Am J Ob Gyn. 2009;201:402.
Lawrence, RA, Lawrence, RM. Breastfeeding: a guide for the medical profession. Philadelphia: Mosby; 2005.
Lenora, J, et al. Effects of multiparity and prolonged breast-feeding on maternal bone density: a community-based cross-sectional study. BMC Women’s Health. 2009;9:19.
Levine, ME, et al. Protein and ginger for the treatment of chemo-induced delayed nausea. J Alter Complement Med. 2008;14:545.
Leung, AM, et al. Iodine content of prenatal multivitamins in the United States. N Engl J Med. 2009;360:939.
Leung, BMT, Kaplan, BJ. Perinatal depression: prevalence, risks and the nutrition link—A review of the literature. J Am Diet Assoc. 2009;109:1566.
Lopez-Exposito, I, Song, Y, et al. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J Allergy Clin Immunol. 2009 Nov;124:1039.
Luoto, R, et al. Impact of maternal probiotic-supplemented dietary couselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103(12):1792.
Majumdar, S, Dada, B. Refeeding syndrome: a serious and potentially life-threatening complication of severe hyperemesis gravidarum. J Obstet Gynaecol. 2010;30:416.
Malcova, H, et al. Absence of breast-feeding is associated with the risk of type I diabetes: a case-control study in a population with rapidly increasing incidence. Eur J Pediatr. 2006;165:114.
Mathieu, J. What is pregorexia? J Am Dent Assoc. 2009;109:976.
Meeker, JD, et al. Cadmium, lead and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008;116:1473.
Merewood, A, et al. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrin Metab. 2009;94:940.
Metzger, B, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991.
National Institute for Child Health and Human Development (NICHD), National Institutes of Health. Moderate doses of vitamin A do not pose risk of birth defects. http://www.nichd.nih.gov//new/releases/vitama.cfm, 2001. [Accessed 1 December 2006 from].
Neural Crest and Associated Disorders. Vertebrate embryology. Accessed 11 November 2009 from http://www.brown.edu/Courses/BI0032/neurcrst/migrate.htm.
Niljand, MJ. Prenatal origins of adult disease. Curr Opin Ob Gyn. 2008;20:132.
Nguyen, N, et al. Splenic avulsion in a pregnant patient with vomiting. Can J Surg. 1995;38:464.
Nygaard, IH, et al. Does oral magnesium substitution relieve pregnancy-induced leg cramps? Eur J Obstet Gynecol Reprod Biol. 2008;141:23.
Ozkan, S, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 7 July 2009. [[Epub ahead of print.]].
Palacios, C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006;46(8):621.
Page, KC, et al. Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am J Physiol Regul Integr Comp Physiol. 2009;297:1049.
Pearce, EN, Braverman, LE. Environmental pollutants and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:801.
Pinto, E, et al. Dietary intake and nutritional adequacy prior to conception and during pregnancy: a follow-up study in the north of Portgal. Publ Health Nutr. 2009;12:922.
Rasmussen, KM, Yaktine, AL. editors: Weight gain during pregnancy: re-examining the recommendations. Washington, DC: The National Academies Press; 2009.
Rifas-Shiman, SL, et al. Dietary quality during pregnancy varies by maternal characteristics in project VIVA: a US cohort. J Am Diet Assoc. 2009;109:1004.
Rosello-Soberon, ME, et al. Twin pregnancies: eating for three? Maternal nutrition update. Nutr Rev. 2005;63(9):95–302.
Rwebembera, AA, et al. Relationship between infant birth weight <2000 g and maternal zinc levels at Muhimbili National Hospital, Dar Es Salamm, Tanzania. J Trop Pediatr. 2005;52:118.
Salihu, HM, et al. Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol. 2007;110:552.
Scheplyagina, LA. Impact of the mother’s zinc deficiency on the woman’s and newborn’s health status. J Trace Elem Med Biol. 2005;19:29.
Scialli, AR, Public Affairs Committee of the Teratology Society. Teratology public affairs committee position paper: maternal obesity and pregnancy. Birth Defects Res A Clin Mol Teratol. 2006;76:73.
Selvaraj, N, et al. Oxidative stress: does it play a role in the genesis of early glycated proteins? Med Hypotheses. 2008;70:265.
Sicherer, SH, Wood, RA, et al. Maternal consumption of peanuts during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010;126:1191.
Simpson, JL, et al. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part I—folate, vitamin B12 and vitamin B6. J Matern Fetal Neonatal Med. 2010;23:1323.
Simpson, JL, et al. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part II—vitamin D, vitamin A, iron, zinc, iodine, essential fatty acids. J Matern Fetal Neonatal Med. 2011;24:1.
Sohrabvand, F, et al. Vitamin B supplementation for leg cramps during pregnancy. Intl J Gynaecol Obstet. 2006;95:48.
Solomons, NW. Developmental origins of health and disease: concepts, caveats and consequences for public health nutrition. Nutr Rev. 2009;67:S12.
Spinnato, JA, II., et al. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstet Gynecol. 2007;110:1311.
Stanza, Z, et al. Nutrition in clinical practice—the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr. 2008;62:687.
Stotland, N, et al. Body mass index, provider advice and target gestational weight gain. Obstet Gynecol. 2005;105:633.
Tamashiro, KL, Moran, TH. Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav. 2010;100:560.
Tussing-Humphreys, LM, et al. Excess adiposity, inflammation and iron-deficiency in female adolescents. J Am Diet Assoc. 2009;109:297.
Uriu-Adams, JY, et al. Influence of copper on early development: prenatal and postnatal considerations. Biofactors. 2010;36:136.
Vello, M, et al. Prenatal and neonatal risk factors for the development of enamel defects in low birth weight children. Oral Dis. 2009.
Viljakainen, HT, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749.
Von Hurst, PR, et al. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficit: a randomized, placebo controlled trial. Br J Nutr. 2009;28:1.
Voyer Lavigne, S, Moretti, M. Medication in pregnancy and lactation. Obstet Gynecol. 2009;114:166.
Wai-Man See, A, et al. A nutritional model of late embryonic vitamin A deficiency produces defects in organogenesis at a high penetrance and reveals new roles for the vitamin in skeletal development. Dev Biol. 2008;316:171.
Waters, TP, et al. Effect of 17 a-hydroxyprogesterone caproate on glucose intolerance in pregnancy. Obstet Gynecol. 2009;14:45.
Wen, JG, et al. Melamine related bilateral renal calculi in 50 children: single center experience in diagnosis and treatment. J Urol. 2010;183:1533.
Wilkins-Haug, L. Epigenetics and assisted reproduction. Curr Opin Obstet Gynecol. 2009;21:201.
Yassa, L, et al. Thyroid Hormone Early Adjustment in Pregnancy (The THERAPY) Trial. J Clin Endocrinol Metab. 12 May 2010. [[Epub ahead of print.]].
Yazdy, MM, et al. Maternal dietary glycemic intake and the risk of neural tube defects. Am J Epidemiol. 2010;171:407.
Zammit, S, et al. Schizophrenia and neural tube defects: comparisons from an epidemiological perspective. Schizophr Bull. 2007;33(4):853–858.
Zeisel, SH, daCosta, K. Choline: an essential nutrient for public health. Nutrition Reviews. 2009;67:615.
Zeller, J, et al. Spontaneous globe subluxation in a patient with hyperemesis gravidarum: a case report and review of the literature. J Emerg Med. 2007;32:285.