Intake
The Nutrients and Their Metabolism
Macronutrients
Carbohydrates are manufactured by plants and are a major source of energy, composing approximately half the total calories in the diet. Carbohydrates are composed of carbon, hydrogen, and oxygen in a ratio of C : O : H2. Important dietary carbohydrates can be categorized as (1) monosaccharides, (2) disaccharides and oligosaccharides, and (3) polysaccharides.
Monosaccharides
Monosaccharides do not normally occur as free molecules in nature but as basic components of disaccharides and polysaccharides. Only a small number of the many monosaccharides found in nature can be absorbed and used by humans. Monosaccharides can have three to seven carbons, but the most important are the six-carbon hexoses: glucose, galactose, and fructose. These hexoses all have the same chemical formula but differ importantly from one another. These differences result from small but significant differences in their chemical structure, some resulting from the presence of chiral carbons with four different atoms or groups attached. These groups can occur in different positions (isomers): glucose and galactose (Figure 3-1).The most important monosaccharide is α-D-glucose. Blood sugar refers to glucose. Because the brain depends on a regular, predictable supply, the body has highly adapted physiologic mechanisms to maintain adequate blood glucose levels.
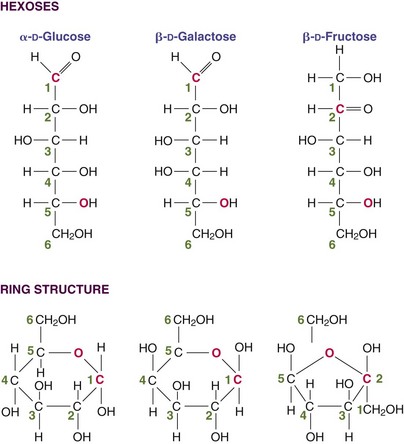
FIGURE 3-1 The three monosaccharides of importance in humans differ from each other in how they are handled metabolically even though they have very similar structures. They are isomers of one another.
Fructose is the sweetest of all monosaccharides (Table 3-1). High-fructose corn syrup is intensely sweet, inexpensive, and manufactured enzymatically by changing the glucose in cornstarch to fructose. Epidemiologic evidence suggests that high-fructose diets (including intake from sweetened beverages) may contribute to obesity and other health conditions, such as the metabolic syndrome. Both galactose and fructose are metabolized in the liver by incorporation into metabolic pathways for glucose, but fructose bypasses a major control enzyme in the glycolytic pathway (Figure 3-2). Galactose is produced from lactose by hydrolysis during the digestive process. Infants born with an inability to metabolize galactose have galactosemia (see Chapter 44).
TABLE 3-1
Sweetness of Sugars and Sugar Substitutes
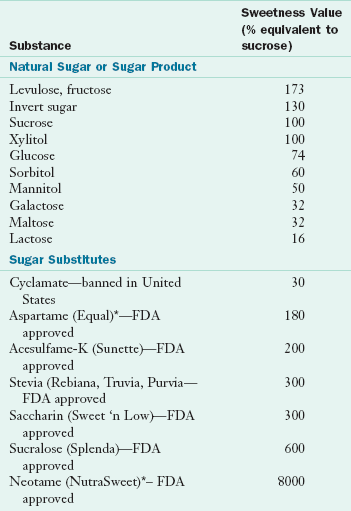
Note: In the United States, six sugar substitutes have been approved for use; stevia, aspartame, sucralose, neotame, acesulfame potassium, and saccharin. Hundreds of new sweeteners are evaluated each year. New sweeteners on the market, such as Swerve and Just Like Sugar, are considered equal in sweetness to sugar.
*Nutritive (has calories).
For more information, see FDA website at: http://www.fda.gov/Food/FoodIngredientsPackaging/ucm094211.htm#qanatural, accessed 1-14-11.
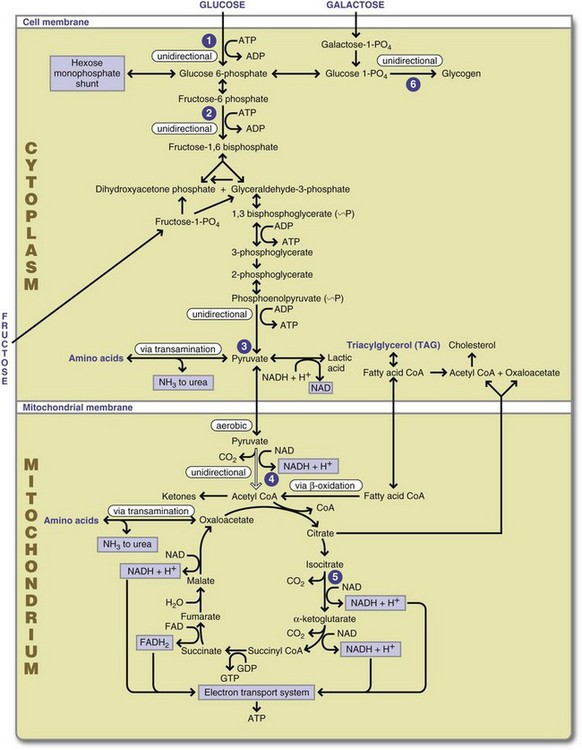
FIGURE 3-2 Overview of macronutrient metabolism. 1, Hexokinase/glucokinase (liver) reaction: uses adenosine triphosphate (ATP), is reversed by glucose 6-phosphotase in gluconeogenesis. 2, Phosphofructokinase reaction: modulated by ATP, positively modified by adenosine monophosphate and adenosine diphosphate (ADP), uses ATP, and is reversed by specific phosphatase in gluconeogenesis. 3, Pyruvate kinase reaction: second example of substrate level phosphorylation of ADP → ATP is not reversible and must be bypassed for gluconeogenesis. 4, Pyruvate dehydrogenase enzyme complex reaction: unidirectional and cannot be reversed. 5, Dehydrogenase reaction: similar to pyruvate dehydrogenase, characterizes the removal of hydrogens in the Krebs cycle. 6, Glycogenesis uses a glycogenic primer reaction and then glycogen synthetase and branching enzymes to synthesize glycogen. The reactions are not reversible. Glycogen is catabolized by a highly controlled phosphorylase. ADP, Adenosine diphosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate. (Courtesy Margie Gallagher, PhD, RD, East Carolina University.)
Disaccharides and Oligosaccharides
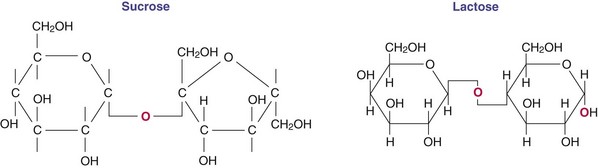
Although a wide variety of disaccharides exist in nature, the three most important disaccharides in human nutrition are sucrose, lactose, and maltose. These sugars are formed from monosaccharides joined by a glycosidic linkage between the active aldehyde or ketone carbon and a specific hydroxyl on another sugar (Figure 3-3). Sucrose occurs naturally in many foods and is also an additive in commercially processed items; it is consumed in large amounts by most Americans. Invert sugar is also a natural form of sugar (unlinked glucose and fructose in a 1 : 1 ratio) that is used commercially because it is sweeter than equal concentrations of sucrose. Invert sugar forms smaller crystals than sucrose and is preferred in the preparation of candies and icings. Honey is an invert sugar. Lactose is made almost exclusively in the mammary glands of lactating animals. Maltose is seldom found naturally in the food supply but is formed by hydrolysis of starch polymers during digestion and is also consumed as an additive in numerous food products. Oligosaccharides are small (3-10 monosaccharide units), readily water soluble, and often sweet (Roberfroid, 2005). Enzymes found in the brush border of the intestine (see Chapter 1) break bonds between molecules in disaccharides and are specific to the particular bond. Larger molecules with linkages that are different are not digestible and are classified as dietary fiber (American Dietetic Association, 2008).
Polysaccharides
Polysaccharides are carbohydrates with more than 10 monosaccharide units. Plants store these carbohydrates as starch granules formed by linking glucose in straight chains and branching into a complex granular structure. Plants make two types of starch, amylose and amylopectin. Amylose is a smaller, linear molecule that is less than 1% branched whereas amylopectin is highly branched. Because of its larger size, amylopectin is more abundant in the food supply, especially in grains and starchy tubers.
Starches from corn, arrowroot, rice, potato, tapioca, and other plants are glucose polymers with the same chemical composition. Their unique character, taste, texture, and absorbability are determined by the relative numbers of glucose units in straight (amylase) and branched configurations (amylopectin) and the degree of accessibility to digestive enzymes.
Raw starch from raw potato or grains is poorly digested. Moist cooking causes the granules to swell, gelatinizes the starch, softens and ruptures the cell wall, and makes the starch more digestible by pancreatic amylase. Starch that remains intact throughout cooking, recrystallizes after cooling, resists enzyme breakdown, and yields limited amounts of glucose for absorption is known as resistant starch. Waxy starch, from corn and rice strains bred for more branched amylopectin chains, forms a smooth paste in water that gels only with a high concentration. Once a gel forms, the product remains thick during freezing and thawing, making it an ideal thickener for commercially frozen fruit pies, sauces, and gravies. Modified food starch is chemically or physically modified to change its viscosity, ability to gel, and other texture properties. Pregelatinized starch, dried on hot drums and made into a porous powder, is rapidly rehydrated with cold liquid. This starch rapidly thickens and is used in instant puddings, salad dressings, pie fillings, gravies, and baby food.
Dextrins result from the digestive process and are large, linear glucose polysaccharides of intermediate lengths cleaved from high amylose starch by α-amylase. Limit dextrins are cleaved from amylopectin containing branch points and are subsequently digested into glucose by the mucosal enzyme isomaltase.
In contrast to plants, animals use carbohydrates primarily to maintain blood glucose concentrations between feedings. To ensure a readily available supply, liver and muscle cells store carbohydrate as glycogen (Figure 3-4). Glycogen is stored hydrated with water; thus the water makes glycogen large, cumbersome, and unsuitable for long-term energy storage. The 70-kg “average” man stores only an 18-hour fuel supply as glycogen, compared with a 2-month supply stored as fat. If all human energy stores were glycogen, humans would need to weigh 60 additional pounds (Alberts et al., 2002). Approximately 150 g of glycogen is stored in muscle, which can be increased fivefold with physical training (see Chapter 24) but is not available to maintain blood glucose directly. It is the glycogen store in the human liver (approximately 90 g) that is involved in the hormonal control of blood sugar.
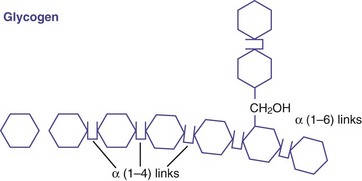
FIGURE 3-4 Glycogen is a branched glucose polymer similar to amylopectin, but the branches in glycogen are shorter and more numerous.
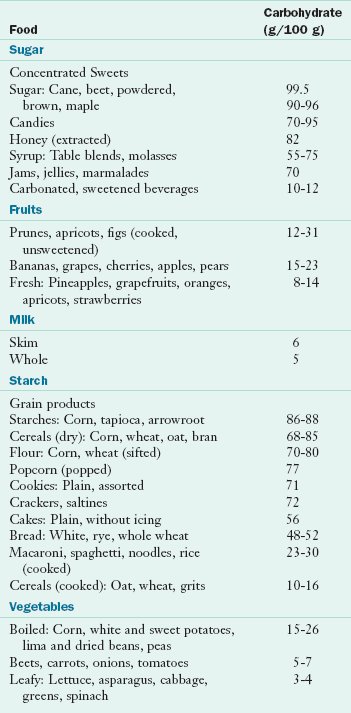
The recommended amount of digestible carbohydrate required in the diet ranges between 45% and 65% of total calories (Institute of Medicine [IOM], Food and Nutrition Board, 2002). The percent carbohydrate of selected foods is given in Table 3-2. The Dietary Guidelines for Americans recommend that consumers select fruits, vegetables, and whole grains for higher fiber intake while decreasing sugar-added food choices (United States Department of Agriculture [USDA], 2005).
Dietary Fiber and Functional Fiber
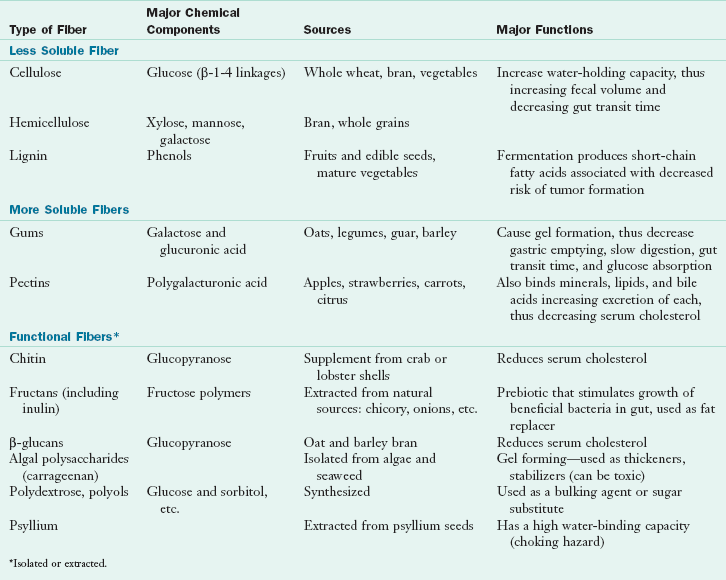
Dietary fiber refers to intact plant components that are not digestible by gastrointestinal (GI) enzymes, whereas functional fiber refers to nondigestible carbohydrates that have been extracted or manufactured from plants. Both of these types of fiber have been shown to have beneficial physiologic functions in the GI tract and in reducing risk of certain disease states. These fibers and their functions are summarized in Table 3-3.
Homopolysaccharides contain repeating units of the same molecule. An example is cellulose, which cannot be hydrolyzed by amylase enzymes. Cellulose is the most abundant organic compound in the world, constituting 50% or more of all the carbon in vegetation. The long cellulose molecule folds back on itself and is held in place by hydrogen bonding, thus giving cellulose fibrils great mechanical strength but limited flexibility. Cellulose is found in carrots and other vegetables. Other homopolymers called beta-glucans (glucopyranose) occur with branching, which makes them more soluble; examples include oats and barley.
Heteropolysaccharides are made by modifying the basic cellulose structure to form compounds with different water solubilities. Hemicellulose is a glucose polymer substituted with different sugar molecules that have different water solubilities. The predominant sugar is used to name the hemicellulose (e.g., xylan, galactan, mannan, arabinose, galactose). Pectins and gums contain sugars and sugar alcohols that make these molecules more water soluble. The galacturonic acid structure of pectin absorbs water and forms a gel that it is widely used for making jams and jellies. The galacturonic acid backbone has rhamnose units inserted at intervals and side chains of arabinose and galactose. Pectin is found in apples, citrus fruit, strawberries, and other fruits. Gums and mucilages (e.g., guar gum) are similar to pectin, except their galactose units are combined with other sugars (e.g., glucose) and polysaccharides. Gums are found in plant secretions and seeds. The specific textural qualities of gums and mucilages are commercially useful when added to processed foods such as ice cream.
Fructans include fructooligosaccharides (FOSs), inulin, inulin-type fructans, and oligofructose and are composed of fructose polymers, often linked with an initial glucose. Inulin is a diverse group of fructose polymers widely distributed in plants as a storage carbohydrate. Oligofructose is a subgroup of inulin with fewer than 10 fructose units. All are poorly digested in the upper GI tract and thus supply only approximately 1 kcal/g (Roberfroid, 2005). Fructans contain fructose; have a sweet, clean flavor; and are half as sweet as sucrose. Major sources of fructans include wheat, onions, garlic, bananas, and chicory; other sources include tomatoes, barley, rye, asparagus, and Jerusalem artichokes. Inulin compounds are widely used to improve the flavor and sweetness of low-calorie foods and to improve the stability and acceptability of fat-reduced foods. Because they are not absorbed in the proximal intestine, fructans have been used as a sugar replacement for diabetic patients.
Prebiotics are nondigestible food substances that selectively stimulate the growth or activity of beneficial bacterial species already resident in the colon (probiotics) and are beneficial to the host. Various prebiotics, including inulin, inulin-type fructans, and FOSs, stimulate the growth of intestinal bacteria, principally bifidobacteria. Fructans (synthesized or extracted) have prebiotic properties and are considered to be functional fiber (Roberfroid, 2007). Functional fiber is commonly added to liquid nutrition supplements and tube feeding formulas.
Algal polysaccharides (e.g., carrageenan) are extracted from seaweed and algae and are used as thickening and stabilizing agents in infant formulas, ice cream, milk pudding, and sour cream products. Algal polysaccharides are used commercially because they form weak gels with proteins and stabilize food mixtures, preventing suspended ingredients from settling. Tobacman (2001) demonstrated that carrageenan damages human cells in culture and destroys human mammary myoepithelial cells at concentrations as low as 0.00014%. With its wide use in commercial foods and uncertainty about the extent of human sensitivity, further investigation of carrageenan is needed.
Polydextrose and other polyols are synthetic polymers of sugar alcohols that are used as sugar substitutes in foods. They are not digestible, contribute to increased fecal bulk, and may be fermented in the small intestine. They are not yet classified as functional fibers (IOM, Food and Nutrition Board, 2002).
Lignin is a woody fiber found in the stems and seeds of fruits and vegetables and the bran layer of cereals. It is not a carbohydrate but is a polymer composed of phenylpropyl alcohols and acids. The phenyl groups contain conjugated double bonds, which make them excellent antioxidants. Flaxseed lignin also has phytoestrogen activity and can mimic estrogen at its receptors on reproductive organs and bone.
Role of Fiber in Digestion and Absorption: The role of fiber in the GI tract varies based on its solubility. Nonabsorbable oligosaccharides and fibers have a significant effect on human physiology. Insoluble fibers such as cellulose increase the water-holding capacity of undigested material, increase fecal volume, increase numbers of stools per day, and decrease GI transit time. On the other hand, soluble fibers form gels, slow GI transit time, bind other nutrients such as cholesterol and minerals, and decrease their absorption. Certain nondigestible oligosaccharides (NDOs), which are fermented by intestinal bacteria, stimulate the intestinal absorption and retention of minerals such as calcium, magnesium, zinc, and iron (Scholz-Ahrens et al., 2001).
Serum lipid concentrations can be modified by both insoluble cellulose and lignin or by soluble pectin and psyllium. They bind fecal bile acids and increase excretion of bile acid–derived cholesterol, thereby reducing lipid absorption. Fermentable oligosaccharides and dietary fiber are converted by intestinal bacteria to short-chain fatty acids (SCFAs), which lower blood lipids. Evidence for the hypocholesterolemic effect of soluble fibers—including FOSs, synthetic polydextrose and polyols, viscous pectin, guar gum, oat bran, psyllium husk, beans, legumes, and fruits and vegetables—is conflicting. Effects vary with the type and amount of fiber (American Dietetic Association, 2008.) Prebiotic modulation by fiber is by fermentation into the SCFAs, acetate, butyrate, and propionate. SCFAs are readily absorbed by the intestinal and colonic mucosa. They enhance sodium and water absorption, colonic blood flow, colonocyte proliferation, GI hormone production, metabolic energy production, and they stimulate the autonomic nervous system via specific receptors in the colon (Tazoe et al., 2008). More than 70% of the fuel for colonocytes is the SCFA butyrate (4C), derived primarily from starch. Propionate (3C) is absorbed and cleared by the liver for hepatic lipid or glucose metabolism. Acetate (2C), produced from undigested carbohydrate, is rapidly metabolized into carbon dioxide by peripheral tissues as a substrate for lipid and cholesterol synthesis (Cummings et al., 2001).
The roles of fiber in the physiology of the GI tract are complex. The adequate intake (AI) of total fiber is set at 38 g/day for men and 25 g/day for women (IOM, Food and Nutrition Board, 2002). Americans’ mean intake of fiber is currently less than half this. In addition to fiber, other nonnutrient components of plants, including tannins, saponins, lectins, and phytates, interact with nutrients, and may reduce their absorption. Phytic acid or phytate, a six-carbon ring with phosphate bound to each carbon, is found in the seed coat of grains and legumes and can bind metal ions, especially calcium, copper, iron, and zinc. Excess phytate can reduce starch hydrolysis if it binds with calcium, which catalyzes the action of amylase.
Glucose Absorption and the Glycemic Index: Dietary carbohydrates are digested into glucose, fructose, and galactose through the actions of α-amylase and brush-border digestive enzymes in the upper GI tract. The ability to digest carbohydrates is modified by the relative availability of the starch to enzyme action, the activity of digestive enzymes at the mucosal brush border, and the presence of other dietary factors (such as fat) that slow stomach emptying. Nonabsorbable oligosaccharides and viscous dietary fibers, such as pectins, β-glucans, and gums dilute enzyme concentration. Thus a diet rich in whole foods, such as fruits, vegetables, legumes, nuts, and minimally processed grains, slows the pace of glucose absorption.
Once digested, glucose is actively absorbed across the intestinal cell and transferred to the portal blood for transport to the liver (see Chapter 1). The liver removes approximately 50% of absorbed glucose for oxidation and storage as glycogen. Galactose (absorbed actively) and fructose (absorbed by facilitated diffusion) are also taken up by the liver and incorporated into glucose metabolic pathways. Glucose exits the liver, enters the systemic circulation, and is available for insulin-dependent uptake by peripheral tissues. Major regulators of blood glucose concentration after a meal are the amount and digestibility of ingested carbohydrate, absorption and degree of liver uptake, insulin secretion, and sensitivity of peripheral tissues to insulin action.
The glycemic index is used to rank carbohydrates by their ability to raise blood glucose levels as compared with a reference food. Riccardi et al. (2008) concluded that foods with a low glycemic index have consistently shown beneficial effects on blood glucose control in both the short and long term in diabetic patients. The glycemic index of a diet has a predictable effect on blood glucose levels. However, the Institute of Medicine (IOM) declined to set an upper limit for the glycemic index in the 2002 recommendations because it is difficult to separate the other factors that contribute to blood glucose levels. A metaanalysis by Livesey et al. (2008) concluded that although consumption of diets with reduced glycemic index were followed by positive health markers, fiber (unavailable carbohydrate) was equally important. The glycemic load of a food is the glycemic index of the carbohydrate divided by 100 and multiplied by its amount of available carbohydrate content (i.e. carbohydrates minus fiber) in grams. The glycemic load and dietary fiber also have important implications for individuals manifesting the metabolic syndrome. Published data on the glycemic index of individual foods, using white bread and glucose as reference foods, have been consolidated for the convenience of users. Use of the glycemic index to modify diets and prevent and control chronic disease is still under investigation.
Carbohydrate Regulation of Blood Lipids: Carbohydrate-induced hypertriglyceridemia can result from consuming a high-carbohydrate diet. The body regulates macronutrient levels to provide adequate fuel for body tissues. The brain uses the most of the approximately 200 g of glucose required per day. When blood glucose level falls to less than 40 mg/dL, counterregulatory hormones release macronutrients from stores. When blood glucose level rises to more than 180 mg/dL, glucose is spilled into the urine. High intakes of carbohydrate trigger large releases of insulin for compensatory responses, including insulin-dependent glucose uptake by muscle or fat and active synthesis of glycogen and fat. Blood glucose then drops to a normal range. Approximately 2 hours after a meal, intestinal absorption is complete, but insulin effects persist and blood glucose falls. The body interprets this hypoglycemic state as starvation and secretes counterregulatory hormones to release free fatty acids from fat cells (Ludwig, 2002). Fatty acids are packed into transport lipoproteins (very-low-density lipoproteins [VLDLs]) in the liver, thereby elevating serum triglycerides.
Fats and Lipids
Lipid Structures and Functions
Fats and lipids constitute approximately 34% of the energy in the human diet. Because fat is energy rich and provides 9 kcal/g of energy, humans are able to obtain adequate energy with a reasonable daily consumption of fat-containing foods. Dietary fat is stored in adipose cells. The ability to store and use large amounts of fat enables humans to survive without food for weeks and sometimes months. Some fat deposits are not used effectively during a fast; they are classified as structural fat. Structural fat pads hold the body organs and nerves in position, protecting them against traumatic injury and shock. Fat pads on the palms and buttocks protect the bones from mechanical pressure. A subcutaneous layer of fat insulates the body, preserving body heat and maintaining body temperature.
Dietary fat is essential for the digestion, absorption, and transport of the fat-soluble vitamins and phytochemicals such as carotenoids and lycopenes. Dietary fat depresses gastric secretions, slows gastric emptying, and stimulates biliary and pancreatic flow, thereby facilitating digestion. Fat also conveys important textural properties to foods such as ice creams (smoothness) and baked goods (tenderness—caused by “shortening” strands of gluten). Box 3-1 lists the fat content of some common foods.
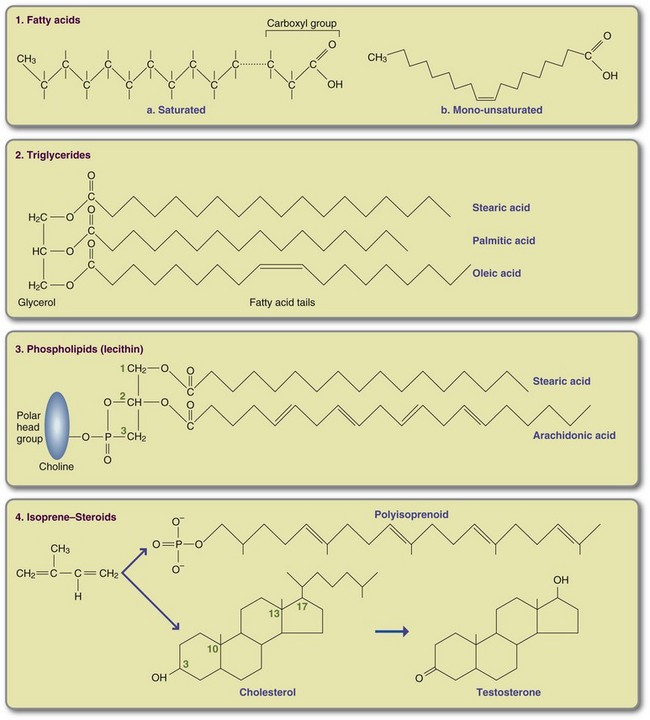
Unlike carbohydrates, lipids are not polymers; they are small molecules extracted from animal and plant tissues. Lipids are composed of a heterogeneous group of compounds characterized by their insolubility in water; they are classified into three major groups (Box 3-2). Figure 3-5 shows some important lipid structures.
Fatty Acids
Fatty acids are rarely free in nature and almost always are linked to other molecules by their hydrophilic carboxylic acid head group. Fatty acids occur primarily as unbranched hydrocarbon chains with an even number of carbons and are classified according to the number of carbons, the number of double bonds, and the position of the double bonds in the chain. Chain length and extent of saturation contribute to the melting temperature of a fat. In general, fats with shorter fatty acid chains or more double bonds are liquid at room temperature. Saturated fats, especially those with long chains, are solid at room temperature. A fat such as coconut oil, which is also highly saturated, is semiliquid at room temperature because the predominant fatty acids are short (8 to 14 carbons). Some manufacturers cool oil and filter out solidified lipid particles before sale; the resulting “winterized” oil remains clear when refrigerated. In general, SCFAs are considered to have 4 to 6 carbons, medium-chain fatty acids to have 8 to14, and long-chain fatty acids (LCFAs), to have 16 to 20 or more.
In a saturated fatty acid (SFA), all carbon binding sites not linked to another carbon are linked to hydrogen and are therefore saturated. There are no double bonds between carbons. Monounsaturated fatty acids (MFAs) contain only one double bond, and polyunsaturated fatty acids (PUFAs) contain two or more double bonds. In MFAs and PUFAs one or more pairs of hydrogen have been removed, and double bonds form between adjacent carbons. Because fatty acids with double bonds are vulnerable to oxidative damage, humans and other warm-blooded organisms store fat predominantly as saturated palmitic fatty acid (C16:0) and stearic fatty acid (C18:0). Cell membranes must be stable and flexible. To achieve this requirement, membrane phospholipids contain one SFA and one highly PUFA, the most abundant of which is arachidonic acid (C20:4). Commonly occurring fatty acids and a typical food source are listed in Table 3-4.
TABLE 3-4
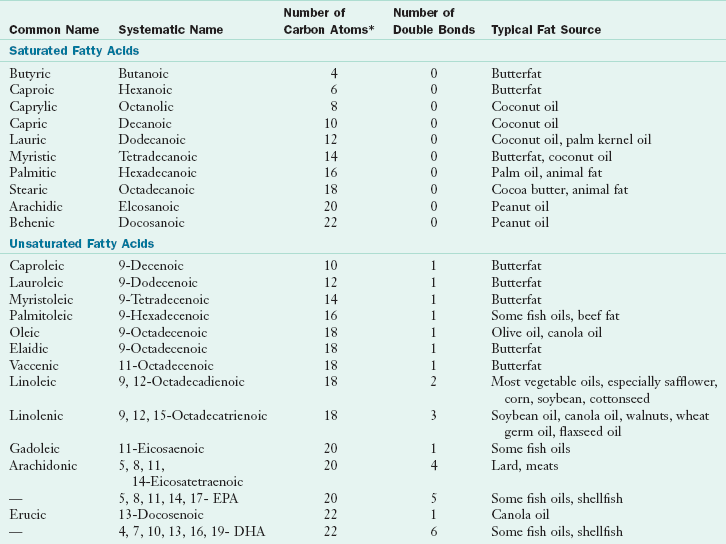
DHA, Docosahexaenoic acid; EPA, eicosapentaenoic acid.
*All double bonds are in the cis configuration except for elaidic acid and vaccenic acid, which are trans.
Modified from Institute of Shortening and Edible Oils: Food fats and oils, ed 6. Washington, DC, 1988, The Institute.
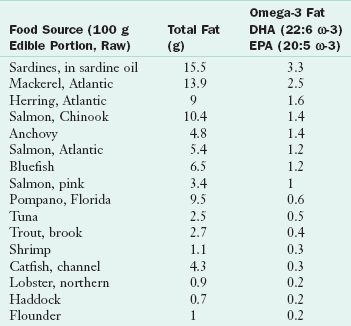
Fatty acids are also characterized by the location of their double bonds. Two notation conventions are used to describe the location of the double bonds (Table 3-5). Omega notation is used in this chapter. In omega notation, a lowercase omega (ω) or n is used to refer to the placement of the first double bond counting from the methyl end (the fatty acid’s omega number). Thus arachidonic acid (20:4 ω-6 or 20:4 n-6), the major highly polyunsaturated fat in membranes of land animals, is an omega-6 (ω-6) fatty acid. It has 20 carbons and four double bonds, the first of which is six carbons from the terminal methyl group. Eicosapentaenoic acid (EPA) (20:5 ω-3 or 20:5 n-3) is found in marine organisms and is an omega-3 (ω-3) fatty acid. It has five double bonds, the first of which is three carbons from the terminal methyl group. Sources of the longer EPA and docosahexaenoic acid (DHA) ω-3 fatty acids are primarily marine, such as cod liver oil, mackerel, salmon, and sardines (Table 3-6).
TABLE 3-5
| α-Linolenic Family (Omega-3) | Linoleic Family (Omega-6) | Oleic Family (Omega-9) |
| 18:3 ω-3 → 18:4 ω-3 | 18:2 ω-6 → 18:3 ω-6 | 18:1 ω-9 → 18:2 ω-9 |
| linolenic | Linoleic | Oleic |
| ↓ | ↓ | ↓ |
| 20:4 ω-3 → 20:5 ω-3 | 20:3 ω-6 → 20:4 ω-6 | 20:2 ω-9 → 20:3 ω-9 |
| Eicosapentaenoic | Arachidonic | Eicosatrienoic* |
| ↓ | ↓ | |
| 22:5 ω-3 → 22:6 ω-3 | 22:4 ω-6 → 22:5 ω-6 | |
| Docosahexanoic | Docosapentaenoic |
Essential Fatty Acids and the Omega-6/Omega-3 Ratio
Only plants (including marine phytoplankton) can synthesize ω-6 and ω-3 fatty acids. Humans and other animals can only place double bonds as low as the ω-9 carbon and cannot produce their own ω-6 and ω-3 fatty acids. But humans can desaturate and elongate linoleic acid (18:2 n-6) to arachidonic acid (20:4 n-6) and alpha-linoleic acid (ALA) (C18:3 ω-3) to EPA (C20:5 ω-3) and DHA (C22:6 ω-3). Therefore both linoleic (18:2 n-6) and ALA (C18:3 ω-3) acids are essential in the diet (IOM, Food and Nutrition Board, 2002).
An essential fatty acid refers to the families of ω-6 and ω-3 fatty acids. Yet it is the longer-chain fatty acids made from them that are components of cell membranes and are precursors of eicosanoids such as prostaglandins, thromboxanes, and leukotrienes. Eicosanoids act as localized (paracrine) hormones and have multiple local functions. They can alter the size and permeability of the blood vessels, alter the activity of platelets and contribute to blood clotting, and modify the processes of inflammation (Figure 3-6). Derivatives of n-3 fatty acids from dietary sources or fish oil can have beneficial effects in a number of disease states (Freemantle et al., 2006; McCowen and Bistrian, 2005), including improved brain function during aging. Roles for ω-3 fatty acids are discussed in chapters related to cardiovascular disease, arthritis and inflammatory conditions, and neurologic disorders.
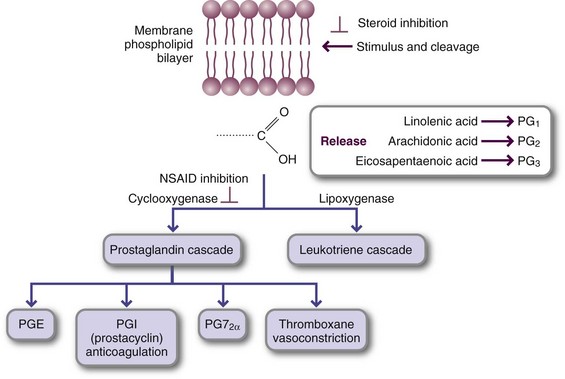
FIGURE 3-6 Eicosanoid synthesis after phospholipid cleavage in the biomembrane. Injury, inflammation, and other stimuli cleave the highly unsaturated fatty acid at the C-2 position of the membrane phospholipid. Arachidonic acid or eicosapentaenoic acid is the major fatty acid released; the pathway entered depends on the degree to which the target tissue expresses the enzyme. The cyclooxygenase pathway leads to prostaglandin, thromboxane, and prostacyclin synthesis. The lipoxygenase pathway, which is common in the lungs and bronchi, leads to leukotriene synthesis and subsequent bronchoconstriction. Note the point at which steroidal and nonsteroidal antiinflammatory drugs act.
An imbalance between dietary ω-3 and ω-6 fatty acids contributes to a wide range of disease states (Wertz, 2009). Excess amounts of ω-6 fatty acids in the diet saturate the enzymes that desaturate and elongate both ω-3 and ω-6 fatty acids; this prevents conversion of ALA into EPA and DHA (Kris-Etherton, 2000). The optimal ω-6/ω-3 ratio has been estimated to be 2 : 1 to 3 : 1, four times lower than the current intake; therefore it is recommended that humans consume more ω-3 fatty acids from vegetable and marine sources. ALA is found in flaxseed (57%), canola oil (8%), soybean oil (7%), and green leaves in a few plants such as purslane.
Trans-Fatty Acids: In natural unsaturated fatty acids, the two carbons participating in a double bond each bind a hydrogen on the same side of the bond (the cis-isomer form), causing the fatty acid to bend (see Figure 3-5). The more double bonds per fatty acid, the more bends in the molecule. Hydrogenation of unsaturated fatty acids is a chemical process that adds hydrogen to oils to form a stable, solid fat such as margarine. Hydrogen can be added both in the natural cis position (with two hydrogens on the same side of the double bond) and in the trans position (with one hydrogen on opposite sides of the double bond). Membrane function depends on the three-dimensional configuration of membrane fatty acids found in phospholipids. The cis double bonds in the membrane bend, allowing the fatty acids to pack loosely, thus making the membrane fluid. Because proteins embedded in a membrane float or sink, depending on the membrane’s fluidity, membrane viscosity is important for membrane protein function.
Trans-fatty acids do not bend; they pack into the membrane as tightly as if they were fully saturated. Trans-fatty acids inhibit the desaturation and elongation of linoleic and ALA, which are critical for fetal brain and organ development. Major sources of trans-fatty acids in the American diet are chemically hydrogenated margarine, shortening, commercial frying fats, high-fat baked goods, and salty snacks containing these fats. Butter and animal fat can also contain trans-fatty acids from bacterial fermentation in the rumen of cows and sheep.
Higher intakes of trans-fatty acids are associated with increased risk for coronary heart disease, cancer, type 2 diabetes, and allergies, possibly because of their potential to influence membrane fluidity (Micha and Mozaffarin, 2009). The U.S. Department of Agriculture Dietary Guidelines for Americans (2005) recommends limiting intake of trans-fatty acids and SFAs to as little as possible.
Conjugated Linoleic Acid: Conjugated linoleic acids (CLAs) are positional and geometric isomers of linoleic acid, not separated by a methylene group as occurs in linoleic acid. These isomers are minor components of the lipids from meat and dairy products. CLA isomers are metabolized in the body through different metabolic pathways with different physiologic outcomes. Eighty percent of CLA is the cis-9, trans-11 isomer. Another notable isomer is trans-10, cis-12, which is more efficiently oxidized and has different biological outcomes. The cis-9, trans-11 isomer appears to be responsible for the anticarcinogenic effect of CLAs; the trans-10, cis-12 isomer reduces body fat and alters blood lipids. Both isomers seem to be responsible for insulin resistance in humans. CLAs are of interest because of these anticarcinogenic, antidiabetogenic, and antiatherogenesis effects. Studies on CLA supplementation demonstrate reduction in body fat percentage and body mass (Baddini et al., 2009; Churrucal et al., 2009).
Triglycerides
The body forms triglycerides (triacylglycerols TAG) by joining three fatty acids to a glycerol side chain (see Figure 3-5, 2), thereby neutralizing reactive fatty acids and making triglycerides water insoluble (hydrophobic). The hydroxyl group on each fatty acid is bound to a hydroxyl group on glycerol, releasing water and forming an ester linkage. Neutral fats can be safely transported in the blood and stored in fat cells (adipocytes) as an energy reserve. Different fatty acids can make up a single triglyceride and depend on the dietary fatty acids and the amount of synthesis taking place. Storage triglycerides from land animals are predominately saturated because SFAs are relatively inert and not susceptible to oxidative damage during storage. Cold-water creatures must maintain their fatty acids in liquid form even at low temperatures; therefore triglycerides in fish oils and marine-derived fats contain longer (C20 and C22), highly unsaturated fatty acids.
Phospholipids
Phospholipids are derivatives of phosphatidic acid, a triglyceride modified to contain a phosphate group at the third position (see Figure 3-5, 3). Phosphatidic acid is esterified into a nitrogen-containing molecule, usually choline, serine, inositol, or ethanolamine, and named for its nitrogenous base (e.g., phosphatidylcholine, phosphatidylserine). Membrane phospholipids usually contain one SFA (C16 to C18) at C-1 and a highly PUFA (C16 to C20) at C-2, usually one of the essential fatty acids. ALA (C18:3 ω-3), arachidonic acid (C20:4 ω-6), and ω-3 substitutes can be cleaved from the lipid bilayer to provide substrate for synthesis of prostaglandins and other mediators of cell activity.
Because it is polar at physiologic pH, the phosphate-containing portion of the molecule forms hydrogen bonds with water, whereas the two fatty acids have hydrophobic interactions with other fatty acids (Figure 3-6). The polar head groups face outward into the aqueous external and cytoplasmic fluids, whereas the centrally placed fatty acid tails participate in hydrophobic interactions at the membrane center. The barrier formed by this lipid bilayer can be crossed only by very small lipid soluble molecules (e.g., oxygen, carbon dioxide, and nitrogen) and to a limited extent by small, uncharged polar molecules such as water and urea.
Lecithin (phosphatidylcholine) is a major phospholipid, and it is the primary component of lipid in the membrane lipid bilayer. Lecithin is also a major component of lipoproteins (i.e., VLDLs, low-density lipoproteins [LDLs], and high-density lipoproteins [HDLs]) used to transport fats and cholesterol. Lecithin is made by the body with arachidonic acid. Because all cells contain lecithin as a lipid bilayer component, animal products, especially liver and egg yolks, are rich sources of lecithin. Plant products such as soybeans, peanuts, legumes, spinach, and wheat germ are also rich sources. Lecithin is widely distributed in the food supply and is added to food products such as margarine, ice cream, snack crackers, and confections as a stabilizer.
Sphingolipids, Alcohols, Waxes, Isoprenoids, and Steroids: All organisms produce small amounts of complex lipids with specialized, critical functions. Many of these lipids do not contain glycerol and are built from two-carbon acetyl coenzyme A (acetyl CoA) units. Sphingolipids are lipid esters attached to a sphingosine base rather than a glycerol. They are widely distributed in the nervous systems of animals and the membranes of plants and lower eukaryotes such as yeast. Sphingomyelin includes the nitrogenous base choline and makes up more than 25% of the myelin sheath, the lipid-rich structure that protects and insulates cells of the central nervous system. In addition to phosphatidylcholine, sphingomyelin is found in all membranes. Sphingolipidoses comprise a group of genetic lipid-storage diseases in which normal sphingolipid degradation is blocked. Tay-Sachs disease is an example of such a lipid-storage disease.
Long-chain alcohols are metabolic by-products of lipids. The feces contain cetyl alcohol, a by-product of palmitic acid. Beeswax is rich in the alcohol myricyl palmitate. Waxes consist of LCFAs bound to long-chain alcohols. These molecules are almost completely water insoluble and are often used as water repellants, as they are in the feathers of birds and on the leaves of plants.
Isoprenoids, activated derivatives of isoprene, are an extraordinarily large and diverse group of lipids built from one or more five-carbon units. Isoprene contains alternating single and double (conjugated) bonds, an arrangement that can quench free radicals by accepting or donating electrons. Terpene is a generic term for all compounds synthesized from isoprene precursors and includes essential oils of plants (e.g., turpentine from trees and limonene from lemons). Plant pigments that transfer electrons in photosynthesis are also isoprenoids and include lycopene (the red pigment in tomatoes), carotenoids (the yellow and orange pigments in squash and carrots), and the yellow and green chlorophyll group. Fat-soluble vitamins A, D, E, and K and the electron transducer coenzyme Q have isoprenoid structures. Vitamin E, lycopene, and β-carotene are effective antioxidants; nonnutritive phytochemicals with antioxidant function also have an isoprenoid structure.
Steroids constitute a class of lipids derived from a four-membered saturated ring (see Figure 3-5, 4). Cholesterol is the basis for all steroid derivatives made in the body, including glucocorticoids (cortisone) and mineralocorticoids (aldosterone), which are made in the adrenal gland; androgens (testosterone) and estrogens (estradiol), which are made in the testes and ovaries, respectively; and bile acids, which are made in the liver. Vitamin D hormone is made when ultraviolet rays from the sun cleave cholesterol in subcutaneous fat to form cholecalciferol (D3). Synthetic vitamin D is made by irradiating the plant steroid ergosterol to form ergocalciferol (D2).
Cholesterol also plays an important role in membrane function. The rigid, four-ringed cholesterol molecule is bound into the hydrophobic membrane by its hydroxyl group. The stiff, planar rings spread apart and partially immobilize the fatty acid chains near the polar region. At the same time, the nonpolar hydrocarbon tail contributes to greater fluidity in the interior of the membrane. Plasma membranes contain large amounts of cholesterol—up to one molecule for every phospholipid molecule.
Glycolipids include the cerebrosides and gangliosides, which are composed of a sphingosine base and very long-chain (22C) fatty acids. Cerebrosides contain galactose; gangliosides also contain glucose and a complex compound containing an amino sugar. Structurally both compounds are components of nerve tissue and cell membranes, where they play a role in lipid transport.
Synthetic Lipids
Medium-chain triglycerides (MCTs) are SFAs with a chain length of between 6 and 12 carbons. Although MCTs occur naturally in milk fat, coconut oil, and palm kernel oil, they are also produced commercially (MCT oil) as a by-product of margarine production. MCT oils provide 8.25 kcal/g and are of value in a number of clinical situations because they are short enough to be water soluble, require less bile salt for solubilization, are not reesterified in the enterocyte, and are transported as free fatty acids, bound to albumin, through the portal system. Because the portal blood flow rate is approximately 250 times faster than the lymph flow, MCTs are digested quickly and not likely to be affected by intestinal factors that inhibit fat absorption. They are not stored in adipose tissue but are oxidized to acetic acid.
Structured lipids include MCT oil esterified with a desired fatty acid such as linoleic acid or an ω-3 lipid. The combined product is absorbed faster than the long-chain triglyceride alone. Clinically, structured lipids have a role in parenteral and enteral formulas, such as to enhance immune function or athletic performance.
Fat replacers (Table 3-7) are structurally different from fats and do not provide readily absorbable nutrients. Their commercial importance is that they imitate the texture and other sensations of fat, especially in the mouth. Fat replacers differ in their macronutrient base and the extent to which they mimic the characteristics of fat. The caloric value of these substitutes varies between 5 kcal/g (e.g., caprenin) and 0 kcal/g (e.g., olestra, carrageenan). Most fat replacers are derived from plant polysaccharides such as gums, cellulose, dextrins, fiber, maltodextrins, starches, and polydextrose. Olestra is a sucrose polyester in which sucrose is esterified with six to eight fatty acids to form esters. The fatty acid chains range in length from 12 to 24 carbons and are derived from edible oils such as soybean, cottonseed, and corn oils. The product has the physical properties of natural dietary fats. Because they are nonabsorbable, sucrose polyesters do not contribute calories to the diet.
TABLE 3-7
Examples of Fat Replacers and Their Functions and Properties

aCultor Food Science, Inc, Ardsley, N.Y.
bAE Staley manufacturing Co, Decatur, Ill.
cCerestar USA, Inc, Hammond, Ind.
dNational Starch and Chemical Co. Bridgewater, N.J.
eOpta Food Ingredients, Bedford, Mass.
fGrain Processing Corp, Muscatine, Iowa.
gRoquette America, Inc, Keokuk, Iowa.
hAVEBE America Inc, Princeton, N.J.
iZumbro, Inc, Hayfield, Minn.
jRhone-Poulenc, Inc, Cranbury, N.J.
kCanadian Harvest USA, Cambridge, Minn.
lProtein Technologies International, Pryor, Okla.
mMonsanto, Chicago, Ill.
nKelco, Division of Merck, Clark, N.J.
oFMC Corp, Rockland, Me.
pPurity Foods, Okemos, Mich.
qFMC Corp, Philadelphia, Pa.
rAston Chemicals, Aylesbury, Buckinghamshire, England.
sDanisco, New Century, Ky.
tHercules Inc, Wilmington, Del.
uDow Chemical, Midland, Mich.
vFiber Sales and Development Corp, Green Brook, N.J.
wLoders Croklaan, Glen Ellyn, Ill.
xSunsweet Growers, Yuba City, Calif.
yThe Heart Garden Corporation, Los Angeles, Calif.
zNutrasweet, San Diego, Calif.
aaKraft Food Ingredients, Memphis, Ind.
bbCultor Food Science, Ardsley, N.Y.
ccLand O’Lakes Food Division, Arden Hill, Minn.
ddProtein Technologies International, St Louis, Mo.
eeProcter and Gamble, Cincinnati, Ohio.
ffReach Associates, South Orange, N.J.
From American Dietetic Association: Position of the American Dietetic Association: fat replacers, J Am Diet Assoc 105:266, 2005.
Protein-based fat replacers alter the texture of a product in various ways. Microparticulated proteins can act like small ball bearings, providing a fatlike feeling in the mouth. These replacers contribute between 1.3 and 4 kcal/g and augment the protein content of the food. Note that some of these proteins can stimulate an allergic or antigenic response in susceptible individuals (see Chapter 27).
Fat sources can be modified to reduce GI absorption and caloric availability. Monoacylglycerols (monoglycerides) and diacylglycerols (diglycerides) are used as emulsifiers and contribute to the sensory properties of fat but have fewer calories (approximately 5 kcal/g). Salatrim has SFA and LCFA triglyceride molecules and contains 5 kcal/g because of reduced absorbability. Concerns about the long-term effects of fat substitutes are that they may bind essential fatty acids and fat-soluble vitamins and contribute to their malabsorption or have negative effects on fundamental energy intake regulatory mechanisms (McKiernan et al., 2008). However, under most circumstances they appear to be safe, effective, and feasible alternatives for controlling fat and energy in diets (American Dietetic Association, 2005).
Recommendations for Lipid Intake
Recommendations for lipid intake must take into account the documented physiologic and health effects of various lipid components, as well as the worldwide obesity epidemic. For example, SFAs are known to increase LDLs and HDLs, whereas PUFAs decrease the “bad” and “good” lipoproteins. The 2005 Dietary Guidelines for Americans (USDA) recommended the consumption of less than 10% of calories as SFA. SFAs and MFAs, especially those in olive oil, that are similarly thermally stressed do not produce these toxic products. Saturated fat and partially hydrogenated oils have fewer oxygen-binding sites and thereby have increased stability and a longer shelf life; however, their intake is associated with greater risk of cardiovascular disease. On the other hand, too much PUFA can also be dangerous. Double bonds are highly reactive and bind oxygen to form peroxides when exposed to air or heat. When subjected to routine frying or cooking, PUFAs can generate high levels of toxic aldehyde products that promote cardiovascular disease and cancer.
Alcohol (Ethyl Alcohol)
Alcohol has 7 kcal/g and no nutrient value. It is able to permeate all membranes and is absorbed quickly and easily. It is metabolized primarily by the liver enzyme alcohol dehydrogenase (ADH) to acetaldehyde and then to acetyl-CoA, where it can be used to synthesize fat or enter the tricarboxylic acid (TCA) cycle. ADH requires both thiamin and niacin to function. When the amount of alcohol in the cell exceeds the capacity of ADH to metabolize it or when niacin (as NAD+) is depleted, the microsomal ethanol oxidizing system (MEOS) will also metabolize alcohol to acetaldehyde. Chronic alcohol consumption induces both ADH and certain enzymes in the MEOS system. Because the MEOS system is also responsible for the metabolism of many drugs, chronic ingestion of large amounts of alcohol can alter drug responses in unpredictable ways. For example, overall alcoholism leading to induction of the MEOS causes a person to be tolerant not only of alcohol but other drugs as well. But if at any given time the MEOS is saturated with alcohol, drugs are not metabolized at the expected rate and a drug overdose can occur. In addition the production of acetaldehyde in these pathways may be toxic in itself, leading to cirrhosis of the liver.
Amino Acids and Protein
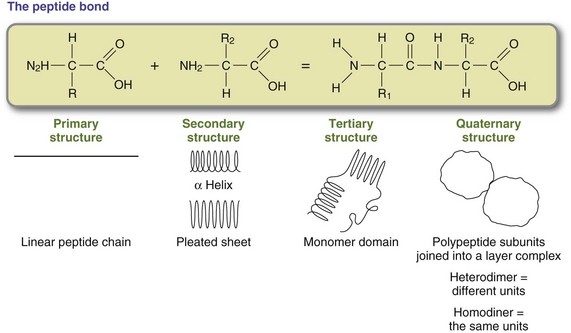
Whereas plant structures are composed primarily of carbohydrates, the body structure of humans and animals is built on protein. Proteins differ molecularly from carbohydrates and lipids in that they contain nitrogen. Primary roles for proteins in the body include structural protein, enzymes, hormones, transport, and immunoproteins. Proteins are composed of amino acids (Figure 3-7) linked by peptide bonds (Figure 3-8).
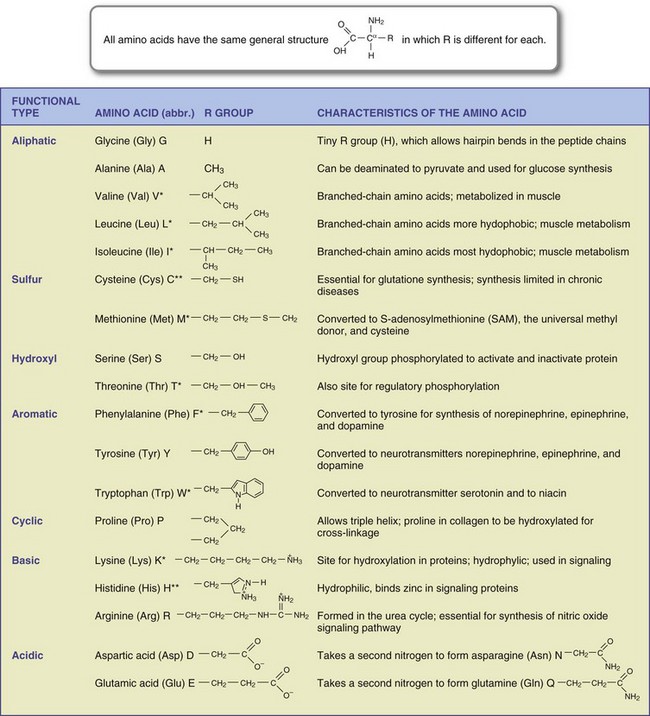
FIGURE 3-7 Structures and functions of the 20 amino acids required by humans. All amino acids have the same general structure, but the R group is different for each. Amino acids are abbreviated using a three-letter and single-letter code. Amino acids marked with an asterisk (*) are essential; those marked with double asterisks (**) are essential for infants and those with certain chronic diseases.
The sequence of the amino acids determines the ultimate structure and function of the protein and is determined by the genetic code stored in the cell nucleus as deoxyribonucleic acid (DNA). As illustrated in Figure 3-9 and in Chapter 5, protein synthesis is a complex process through which the protein pattern is copied from DNA to ribonucleic acid (RNA). The pattern for protein synthesis is carried to the rough endoplasmic reticulum via messenger RNA (mRNA). New proteins are built by attaching amino acids as dictated by the mRNA in a precise linear sequence. When the protein has been built, it detaches from the message and is ready to be used or further processed for use (see Chapter 5).
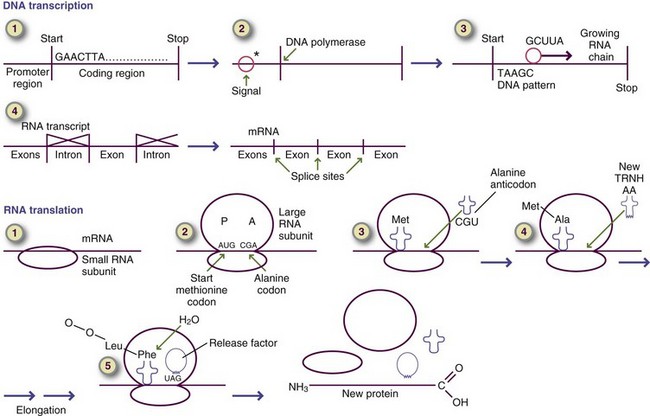
FIGURE 3-9 Summary of deoxyribonucleic acid transcription and ribonucleic acid translation in the eukaryotic cell.
Proper folding of the completed linear amino acid chain is essential for a protein to perform its unique functions. The linear sequence of individual amino acids dictates the configuration of the mature protein. R groups protrude from the newly synthesized peptide chain and are in position to react with each other. Folding is accomplished through hydrogen bonding, ionic bonding, and hydrophobic and other interactions between individual R groups on each amino acid. For example, a negative charge on one amino acid R group forms an attraction with a positive charge on another, forming a precise, three-dimensional structure. Proteins have the following four levels of structure:
1. Primary structure: Peptide bonds are formed between sequential amino acids according to directions on mRNA. The completed protein is a linear chain of amino acids.
2. Secondary structure: Attractions between R groups of amino acids create helices and pleated sheet structures.
3. Tertiary structure: Helices and pleated sheets are folded into compact domains. Small proteins have one domain, and large proteins have multiple domains.
4. Quaternary structure: Individual polypeptides can serve as subunits in the formation of larger assemblies, or complexes. Subunits are bound together by numerous weak, noncovalent interactions; sometimes they are stabilized by disulfide bonds. For example, four hemoglobin monomers are joined to form the tetramer hemoglobin molecule.
Protein structure is a critical component of protein function. The active and catalytic sites at which protein action occurs are formed by juxtaposing functional groups from nearby but occasionally distant R groups. If the linear protein sequence is altered, as in genetic diseases, the protein is unable to form active sites, and its activity may be reduced or eliminated entirely.
Essential Amino Acids
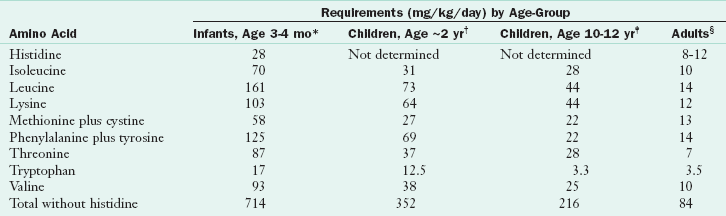
Synthesis of proteins requires the presence of all necessary amino acids during the process. Chemically, amino acids are carboxylic acids with an amino group attached to the α-carbon. All amino acids have this same general structure; the side chain is also attached to the α-carbon (the R group), which dictates the identity and function of each amino acid. Note that the α-carbon is a chiral carbon, and isomers can be formed. The L-isomer is functional in the human body. Many amino acids can be synthesized from carbon skeletons produced as intermediates in the major metabolic pathways by a process called transamination, which adds an amino group from another amino acid without actually producing a free amino group. Transamination is an important process because it allows for the production of nonessential amino acids from metabolic intermediates while using free amino groups, so that they are not left to produce toxic ammonia. For example, pyruvate formed during glycolysis is easily converted to the amino acid alanine by adding an amino group via the enzyme alanine aminotransaminase. On the other hand, essential amino acids have carbon skeletons that humans cannot synthesize in adequate amounts and therefore must obtain from the diet (Table 3-8). Protein can also be an energy source. Proteins contain 5 kcal/g; removal of the amino group and the formation and excretion of urea (deamination) has a metabolic cost of 1 kcal/g. Therefore the resulting carbon skeleton product can be used for energy at the rate of 4 kcal/g. These carbon skeletons can also be used to produce glucose. When the diet is low in carbohydrate or an individual is starving, protein is the only good source of de novo synthesis of glucose available; this process is called gluconeogenesis. Oxaloacetate is moved out of the mitochondria and converted to phosphoenolpyruvate (PEP) (see Figure 3-2). From PEP, the glycolytic pathway can be reversed because all the enzymes with the exception of phosphofructokinase and glucokinase are reversible. Both of these enzymes can be reversed by specific phosphatase enzyme when there is a need for blood glucose. Because glucokinase is found primarily in the liver, it is only reversed there, making the liver the primary site for gluconeogenesis. Amino acids that produce carbon skeletons that can be converted to glucose are called glucogenic amino acids. Only two of the 20 amino acids cannot be used to produce at least some glucose. These amino acids are lysine and threonine. They produce products that are converted to ketones and used for energy; thus they are known as ketogenic amino acids.
TABLE 3-8
Estimates of Amino Acid Requirements
*Based on amounts of amino acids in human milk or cow’s milk formulas fed at levels that supported good growth.
†Based on achievement of nitrogen balance sufficient to support adequate lean tissue gain (16 mg nitrogen/kg/day).
‡Based on upper range of requirement for positive nitrogen balance.
§Based on highest estimate of requirement to achieve nitrogen balance.
Modified from World Health Organization: Energy and protein requirements report of a joint FAO/WHO/UNU expert consultation, Technical Report Series 724, p 65, Geneva, 1985, WHO.
According to current recommendations, a healthy adult human requires 0.8 g of protein per kilogram of healthy body weight (IOM, Food and Nutrition Board, 2002). To obtain this quantity of protein, humans benefit when dietary protein makes up approximately 10% to 15% of total energy intake. Protein requirements increase during times of stress and disease. Protein-rich foods are obtained primarily from animal flesh or animal products such as eggs and milk. Most plant foods are relatively poor sources of protein, with the exception of legumes and beans.
Dietary Protein Quality
Because the synthesis of proteins for the body depends on the availability of all necessary amino acids, the quality of a dietary protein depends on its amino acid makeup and their bioavailability. A number of methods have been used to measure the quality of proteins based on these properties. More than 50 years ago, Block and Mitchel (1946) determined that a protein’s biologic value could be determined by the essential amino acid profile compared with human requirements. The essential amino acid that occurred in the least concentration compared with human requirement was the limiting amino acid, from which a “chemical score” of protein quality could be calculated.
Protein quality is also determined by measuring the amount of protein actually used by an organism; net protein utilization (NPU) is the one method of doing so. Dietary protein is equated with its metabolic products by measuring nitrogen in the diet and biologic samples and converting it to the amount of protein on the basis of the formula (nitrogen [grams] × 6.25 = protein [grams]). The gain in nitrogen is compared with the nitrogen intake, and the proportion of nitrogen retained in the body is computed to obtain the NPU. The NPU ranges from approximately 40 to 94, with protein from animal products scoring higher and protein from vegetables scoring lower. However, care must be taken when using animals in trials for determining the quality of a protein for humans.
The World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA) have adopted a protein digestibility corrected amino acid score (PDCAAS) as the official assay for evaluating protein quality in humans. The PDCAAS is based on amino acid requirements of children ages 2 to 5 years and represents the amino acid score after correcting for digestibility. After being corrected for digestibility, proteins that provide amino acids equal to or in excess of requirements receive a PDCAAS of 1. Gilani et al. (2008) have identified the need for more accurate, standardized methods that include bioactive peptides.
Digestibility is a major factor affecting protein quality and is affected by multiple factors. Meat preparation procedures often involve wine or vinegar marinades and moist heat to tenderize tough cuts of meat through denaturation. Proteins are kept in their functional three-dimensional structure by hydrogen and ionic interactions; these bonds loosen in the presence of acid, salt, and heat. Because they denature proteins, these methods also soften gristle, or connective tissue proteins, and release muscle proteins from their attachments, thereby making all proteins more available to digestive enzymes.
Vegetable protein is less efficient than animal protein; it is encased in carbohydrate and is less available to digestive enzymes. Some plants contain enzymes that interfere with protein digestion and require heat inactivation before consumption. For example, soybeans contain a trypsinase that inactivates trypsin, the major protein-digesting enzyme in the intestine. Although research suggests that plant protein may benefit blood pressure control, little has been published regarding protein sources in diets of U.S. adults and factors influencing these choices (Lin et al., 2010). Protein source frequencies in the lifestyle-changing PREMIER trial were poultry, dairy, refined grains and beef; animal protein was two thirds of intake, and one third was from plant protein, varying by sex, race, age, and body weight status (Lin et al., 2010).
Food processing can damage amino acids and reduce their digestive availability in several ways. Mild heat treatment in the presence of reducing milk sugars (glucose and galactose) during processing causes a loss of available lysine. Lactose reacts with lysine side chains and renders them unavailable. This browning (the Maillard reaction) causes significant lysine loss at high temperatures. Under severe heating conditions in the presence (or even absence) of sugars or oxidized lipids, all amino acids in food proteins become resistant to digestion. When protein is exposed to severe treatment with alkali, the amino acids lysine and cysteine can react together and form a potentially toxic lysinoalanine. Exposure to sulfur dioxide and other oxidative conditions can result in loss of methionine. Thermal processing and low-moisture storage of proteins can also result in reductive binding of vitamin B6 to lysine residues, thereby inactivating the vitamin. Therefore proper handling of protein foods is necessary to maintain their integrity and usefulness.
As noted, if the amino acid profile of a food does not match human needs, those in short supply are “limiting.” The quality of dietary protein can be improved by combining protein sources with different limiting amino acids. Diets based on a single plant food staple do not foster optimal growth because the diet does not have enough of the limiting amino acid to provide substrates for protein synthesis. If another plant protein that contains an excess of the limiting amino acid is added to the diet, the protein combination is complemented; essential amino acids are adequate to support human protein synthesis. The concept of complementary proteins is important for populations without animal protein intake or at risk from insufficiently diverse foods. Complementary foods that, when eaten together, provide all of the essential amino acids are shown in Table 3-9. It is not necessary to eat complementary amino acids during a single meal, but they should be eaten within the same day (American Dietetic Association, 2009). Children, pregnant women, and nursing mothers who have vegan diets need to plan their diets carefully to include a mixture of amino acid–containing foods.
TABLE 3-9
Food Combinations Providing All Essential Amino Acids
| Excellent Combinations* | Examples |
| Grains and legumes | Rice and beans, pea soup and toast, lentil curry and rice |
| Grains and dairy | Pasta and cheese, rice pudding, cheese sandwich |
| Legumes and seeds | Garbanzo beans and sesame seeds; hummus as dip, falafel, or soup |
*Other combinations, such as dairy and seeds, dairy and legumes, grains and seeds, are less effective because the chemical scores are similar and not effectively complementary.
For food lists containing high protein, see http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w203.pdf
Nitrogen Balance
Homeostatic regulations control the concentrations of specific amino acids in the amino acid pool and the rate at which muscle and plasma proteins are synthesized and broken down. Body protein synthesis and turnover are regulated. In healthy individuals the amount of protein taken in is balanced by protein used for body maintenance and excreted in feces, in urine, and from skin, resulting in a zero protein balance (Figure 3-10). This balance reflects homeostatic regulations within tissues.
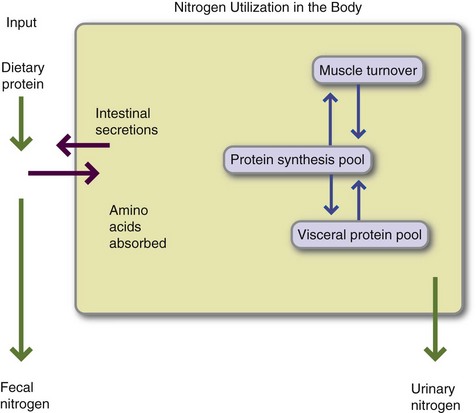
FIGURE 3-10 Nitrogen use in the body. Protein supplies nitrogen in the form of amino acids, according to the formula (nitrogen [grams] = protein [grams] ÷ 6.25). Dietary protein and protein from endogenous secretions are available for absorption across the gastrointestinal tract. More than 95% of protein is normally absorbed and enters the synthetic pool. Muscle proteins and visceral (i.e., plasma) proteins are broken down and built up daily. Nitrogen is converted to urea and excreted in the urine. Minor amounts of nitrogen are lost in the menstrual flow and the normal secretions and turnover of skin and its appendages. In a healthy individual, nitrogen intake equals nitrogen losses; the person is in zero protein balance. (Modified from Crim MC, Munro HN: Proteins and amino acids. In Shils ME et al., editors: Modern nutrition in health and disease, Philadelphia, 1994, Lea & Febiger.)
Muscle mass (somatic protein) is maintained with the circulating amino acid pool such that similar quantities of muscle protein are destroyed and rebuilt daily. Muscle mass can be estimated using the creatinine/height index and the midarm muscle circumference. Amino acids are also required for synthesis of visceral proteins by the liver and other tissues. A person with an infection or a traumatic injury excretes more nitrogen than is ingested; inflammatory cytokines cause nitrogen loss and negative nitrogen balance under these conditions. The pregnant woman and her growing child use ingested protein for growth and to retain more protein than is lost daily (positive nitrogen balance).
Nitrogen in the form of ammonia (NH3) is highly toxic, easily crosses membranes, and cannot be allowed to travel unbound throughout the body. In the fed state, pyruvate and other carbon skeletons take up nitrogen (via transamination) and transport it to the liver as nonessential amino acids, usually alanine and glutamic acid (from α-ketoglutarate). When these amino acids reach the liver, they are deaminated or transaminated back into the carbon skeleton. A deaminated ammonia ion is combined with carbon dioxide in the presence of high-energy phosphate and magnesium by the enzyme carbamoyl phosphate synthase to form carbamoyl phosphate, the first intermediate of the urea cycle. A second amino group enters the urea cycle via aspartic acid. Thus with each urea molecule formed, two excess amino groups can be excreted. Urea makes up 90% of urinary nitrogen in the fed state. Arginine, one of the basic amino acids is also a product of the urea cycle. Arginine is required for formation of nitric oxide and other mediators of the inflammatory response (Gropper et al., 2005). Although classified as a nonessential amino acid, arginine may be essential for critically ill individuals.
Macronutrient Use and Storage in the Fed State
Absorbed carbohydrates are transported as plasma glucose in the portal vein. An increase in the glucose level in the portal vein stimulates pre-formed insulin secretion from the pancreas. One of the most dramatic effects of insulin is its effects on the glucose transporters (GLUT 4) in insulin-dependent adipose and muscle. However, the liver is the first organ to receive portal blood glucose. The liver takes up approximately 50% of absorbed glucose via noninsulin-dependent transporters (GLUT 2) and immediately phosphorylates glucose into glucose-6-phosphate using the high-capacity enzyme glucokinase, thereby retaining glucose in the liver cells (see Figure 3-2).
Insulin enhances the oxidation of glucose in the glycolytic pathway by increasing the activity of glucokinase. Pyruvate dehydrogenase is also stimulated, increasing glycolysis and acetyl CoA production in both the liver and the muscle to generate adenosine triphosphate (ATP). In addition, insulin increases glycogen synthase activity in the liver and muscle, maximizing glucose storage as glycogen storage under fed conditions. Muscle glycogen is used within the muscle cell to provide ATP for muscle contraction. Its concentration in the muscle depends on the physical activity of the individual and can be greatly increased by physical training.
Liver glycogen serves as a reservoir, providing a readily available supply of glucose to maintain blood glucose levels during the fasting state. If carbohydrate intake exceeds the body’s oxidative and storage capacities, the cells convert carbohydrate into fat. Elevated insulin levels increase the activity of fatty acid and triglyceride synthesis enzymes such as acetyl CoA carboxylase in the liver, lipoprotein lipase (LPL) in the adipose tissue, and fatty acid synthetase.
Because they are fat soluble, lipids cannot be transported unbound through the aqueous media of the body. Absorbed fatty acids and monoglycerides are reesterified into triglycerides within mucosal cells, and the fat-soluble center is surrounded with a thin layer of protein and phospholipid for transport. The protein component includes apoproteins (Apo) B, A, C, and E with specific functions. The resulting chylomicrons contain only 2% protein; the rest are triglycerides (84%), cholesterol, and phospholipids. The lipid-rich particles leave the mucosal cells and travel through lymphatic channels to the thoracic duct that empties into the right side of the heart. Rapid blood flow in the heart prevents the large, lipid-rich chylomicrons from forming clumps and fat emboli. Chylomicrons transport dietary fat and are found in blood only after meals, making the plasma appear milky after a high-fat meal.
Chylomicrons leave the heart through the aorta and are dispersed into the general circulation and transported to the adipocytes. The enzyme LPL is expressed on the membrane of endothelial cells lining capillaries in the region of the adipocytes. LPL is activated by lipoprotein-bound Apo C to bind chylomicrons and cleave triglycerides, releasing fatty acids and monoglycerides that cross the fatty lipid membrane, enter the adipocytes, and become reesterified into triglyceride for safe and hydrophobic storage. Note that insulin, the predominant hormone in the fed state, activates LPL and facilitates fat storage. The chylomicron remnant, relieved of some of its triglyceride content, is bound to liver receptors and recycled.
The liver receives fat from numerous sources: chylomicron remnants, circulating fatty acids, uptake of intermediate lipoproteins and other lipoproteins, and endogenous synthesis. The liver reesterifies fat from all sources and forms VLDLs, which are richer in cholesterol compared with chylomicrons but still contain a large proportion of triglycerides. VLDLs also contain Apos B, E, and C and adsorb Apo A as they circulate. In the fed state numerous VLDLs are formed and transported to the adipocytes, where triglycerides are again hydrolyzed, reesterified, and stored. Even in fasting, VLDLs are formed to carry endogenous lipids.
Dietary cholesterol is transported via chylomicrons and VLDLs but is not removed by LPL. After LPL has cleaved the maximum triglyceride from VLDLs, the remnant remaining is called an intermediate-density lipoprotein. Once the maximum triglyceride is removed, the lipoprotein is known as an LDL and primarily carries cholesterol. Although LDLs can be taken up by the liver on receptors for Apo B and Apo E, they are first taken up by specific LDL receptors that bind these cholesterol-rich particles. After uptake, endocytic vesicles containing LDL fuse with a lysosome. The digestive enzymes in the lysosome break down the protein and phospholipids, leaving free cholesterol. Free cholesterol regulates cholesterol synthesis and LDL uptake within the cell by inhibiting 3-hydroxy-3-methylglutaryl (HMG) CoA reductase, the rate-limiting enzyme for cholesterol synthesis from acetyl CoA. It downregulates cellular synthesis of the LDL receptor and reduces receptor expression on the membrane. Free cholesterol also increases the esterification of cholesterol for storage.
Cholesterol is removed from the cell membrane and other lipoproteins by HDLs. HDL particles are formed in the liver and other tissues as disk-shaped lipoproteins. They circulate in the bloodstream and accumulate free cholesterol, which they esterify with fatty acid from their phosphatidylcholine (lecithin) structure. The ability of HDLs to function as a cholesterol transporter depends on the activity of their copper-dependent enzyme lecithin-cholesterol acyltransferase, which esterifies cholesterol and stores it in its hydrophobic center. When it has accumulated sufficient lipid to become spherical, HDL is taken up by the liver and recycled. Recycled cholesterol is used for bile acid synthesis, stored in subcutaneous tissue, formed into vitamin D, or secreted as VLDL.
The LDLs that remain in circulation too long are susceptible to oxidative damage and macrophage scavenging. Macrophages are large cells that engulf other particles. They are distributed throughout the body, play a major role in immune defense, and inhabit the arteries, where they serve as a surveillance mechanism against foreign and microbial agents in the blood. Although macrophages do not recognize and ingest normal lipoproteins, they do recognize as foreign those lipoproteins that have undergone oxidation. Macrophages ingest oxidized LDLs and accumulate ingested fat within their cytoplasm, giving them a foamy appearance (thus the name foam cells). LDL ingestion activates macrophages and stimulates them to secrete mediators that trigger multiple inflammatory and proliferative cascades, some of which lead to atherosclerosis.
Macronutrient Catabolism in the Fasted State
The body has a remarkable ability to withstand food deprivation, allowing humans to survive cycles of feast and famine. Adaptive changes allow the body to access stored macronutrients to provide for routine activities.
Individuals with protein-energy malnutrition (PEM) or protein-calorie malnutrition (PCM) can have varying symptoms, determined by the cause of the malnutrition. Starvation of both protein and calories leads to marasmus at one end of the PEM continuum. At the opposite end is protein deprivation that occurs in individuals who are consuming carbohydrates almost entirely. Kwashiorkor is the Ghanaian word for the disease that develops when a mother’s first child is weaned from protein-rich breast milk to a protein-poor carbohydrate food source. The condition is caused by severe protein deficiency and hypoalbuminemia. In adults, the correct term is protein-energy malnutrition, not the pediatric term, kwashiorkor. The starving adult has simply “malnutrition” described more fully in Chapter 14.
Glucose is an obligate nutrient for the brain and nervous system, red and white blood cells, and other glucose-requiring tissues. To maintain function, the blood glucose level must be maintained within a normal range at all times. During early fasting, glucose is obtained from glycogen by the action of the hormones glucagon and epinephrine; these stores are depleted in 18 to 24 hours. At this point, new glucose must be synthesized using protein as a substrate. The catabolic hormones epinephrine, thyroxine, and glucagon stimulate the release of muscle protein and other available substrates for gluconeogenesis. The most common amino acid substrate for gluconeogenesis is alanine; when its nitrogen is removed, alanine becomes pyruvate. Note that glycogen is never totally depleted, even during long-term starvation. A small amount of pre-formed glycogen is carefully guarded as a primer for glycogen resynthesis.
As fasting is prolonged and the body adapts to starvation conditions, liver gluconeogenesis decreases from producing 90% of the glucose to less than 50%, with the remainder being supplied by the kidney. Although the muscle and brain are unable to release free glucose, the muscle can release pyruvate and lactate for gluconeogenesis in the Cori cycle. Muscles also release glutamine and alanine. These amino acids can be deaminated or transaminated into α-ketoglutarate or pyruvate, respectively, and converted into oxaloacetate, then to glucose. During prolonged fasting the kidney requires ammonia to excrete acidic metabolic products. Muscle-derived glutamine is used for this purpose; deaminated glutamine (α-ketoglutarate) can then be used to produce glucose. Thus, during starvation, glucose production by the kidney increases while production by the liver decreases.
In addition to glucose, a reliable energy source is required during fasting. The best source is fat that is stored in adipocytes and used primarily by muscles, including the heart muscle, to make ATP. Fatty acid release and use require low insulin levels and an increase of the antiinsulin hormones glucagon, cortisone, epinephrine, and growth hormone. Antiinsulin hormones activate the hormone-sensitive lipase enzyme on the adipocyte membrane. This enzyme cleaves stored triglycerides, releasing fatty acids and glycerol from fat cells. Fatty acids travel to the liver bound to serum albumin and easily enter the liver cells. Once inside the cell, fatty acids enter the liver mitochondria via the carnitine acyltransferase transport system, which carries fatty acid carnitine esters across the mitochondrial membrane. Once inside the mitochondria, acetyl CoA is formed from fatty acid CoA via the process of β-oxidation. During starvation, excess acetyl CoA molecules accumulate in the liver because the liver is able to obtain all necessary energy from the process of β-oxidation and form ketones, which then enter the bloodstream and act as a source of energy for the muscles, thus sparing protein.
Adaptation to starvation depends on ketone production. As the blood ketone level rises during fasting, the brain and nervous system, although obligate glucose consumers, begin to use ketones as an energy source. Because the brain is using a fuel other than glucose, the demand on muscle protein for gluconeogenesis declines, thereby reducing the rate of muscle catabolism. Reduced muscle catabolism reduces the amount of ammonia received by the liver. Liver synthesis of urea decreases precipitously, reflecting the slower rate of muscle protein deamination. If the fast extends for weeks, the rate of urea synthesis and excretion is minimized. In an individual who has adapted to starvation, urea is excreted from the kidney at approximately the same rate as uric acid.
Thus, in an individual who is adapting to starvation, protein losses are minimized and lean body mass spared. Although fat cannot be converted into glucose, it does provide fuel for the muscle and brain as ketones. As long as water is available, a normal-weight individual can fast for a month. Relatively normal nutritional indices, immune function, and other system function are maintained. However, when fat stores are exhausted, protein is used, and death is the ultimate consequence.
In certain cases of trauma and sepsis, the individual is not able to adapt to fasting or starvation. If an individual who is fasting develops an infection, inflammatory mediators such as interleukin-1 and tumor necrosis factor stimulate insulin secretion and prevent the development of mild ketosis. Without ketones the brain and other tissues continue to depend on glucose, thereby limiting the person’s ability to adapt to starvation. Muscle mass erodes to provide glucose substrates. A fasting person with an infection rapidly develops a negative nitrogen balance. When 50% of the protein stores is exhausted, recovery from infection is poor.
Adaptation to starvation is also not possible for those with protein malnutrition because the carbohydrate intake stimulates insulin production. Insulin is a storage hormone that prevents fat stores from being accessed for fuel. It also inhibits fat from being formed into ketones, thereby limiting adaptation to starvation. Insulin secretion inhibits muscle breakdown. Protein cannot be used to make albumin and other visceral proteins. Edema results because albumin exerts osmotic pressure in the vessels. If the albumin concentration is low, fluid remains in the extracellular spaces and causes edema. Compromised neural function or GI absorption, decreased cardiac output, immune function, fatigue, and other symptoms of malnutrition result from inadequate protein synthesis, inadequate ATP production, and fluid accumulation in the tissues.
Nonadapted malnutrition is dangerous. Not only can unremitting protein loss become life threatening by compromising the muscles of the heart and respiratory system, but it also compromises the immune system. The individual becomes susceptible to a vicious cycle of infections, diarrhea, additional nutrient loss, an even weaker immune system, and finally opportunistic infections and death. Iatrogenic, or “physician-induced,” malnutrition was recognized long ago as a danger for hospitalized patients and remains so to this day (Kruizenga et al., 2005). Often lung failure occurs from weakened respiratory muscles. Pneumonia yields the mortal blow, yet malnutrition is the actual underlying cause.
 cup
cup cup
cup cup
cup cup
cup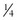 of 12 inch
of 12 inch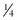 cup
cup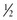 medium
medium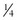 cup
cup of 9 inch
of 9 inch pie
pie