Assessment for Risk Factors in Pregnancy
• Explore the biophysical, psychosocial, sociodemographic, and environmental influences on high risk pregnancy.
• Examine risk factors identified through history, physical examination, and diagnostic techniques.
• Discuss psychologic considerations for the woman and her family experiencing a high risk pregnancy.
• Differentiate among screening and diagnostic techniques, including when they are used in pregnancy and for what purposes.
• Develop a teaching plan to explain screening and diagnostic techniques and implications of findings to women and their families.
Approximately 500,000 of the 4 million births that occur in the United States each year are categorized as high risk because of maternal or fetal complications. A high risk pregnancy is one in which the life or health of the mother or fetus is jeopardized by a disorder coincidental with or unique to pregnancy. Care of these high risk women requires the combined efforts of medical and nursing personnel. Factors associated with a high risk pregnancy are identified in this chapter. Screening and diagnostic techniques often used to monitor the maternal-fetal unit at risk are also described.
Assessment of Risk Factors
Pregnancies can be designated as high risk for any of several undesirable outcomes. Those considered to be at risk for uteroplacental insufficiency (UPI), the gradual decline in delivery of needed substances by the placenta to the fetus, carry a serious threat for fetal growth restriction, intrauterine fetal death, intrapartum fetal distress, and various types of neonatal morbidity.
In the past, risk factors were evaluated only from a medical standpoint. Therefore, only adverse medical, obstetric, or physiologic conditions were considered to place the woman at risk. Today a more comprehensive approach to high risk pregnancy is used, and the factors associated with high risk childbearing are grouped into broad categories based on threats to health and pregnancy outcome. Categories of risk are biophysical, psychosocial, sociodemographic, and environmental (Box 26-1). Risk factors are interrelated and cumulative in their effects.
Biophysical risks include factors that originate within the mother or fetus and affect the development or functioning of either one or both. Examples include genetic disorders, nutritional and general health status, and medical or obstetric-related illnesses. Box 26-2 lists common risk factors for several pregnancy-related problems.
Psychosocial risks consist of maternal behaviors and adverse lifestyles that have a negative effect on the health of the mother or fetus. These risks may include emotional distress and disturbed interpersonal relationships, as well as inadequate social support and unsafe cultural practices.
Sociodemographic risks arise from the mother and her family. These risks may place the mother and fetus at risk. Examples include lack of prenatal care, low income, marital status, and ethnicity (see Box 26-1). Environmental factors include hazards in the workplace and the woman’s general environment and may include environmental chemicals (e.g., lead, mercury), anesthetic gases, and radiation (Chambers & Weiner, 2009; Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010).
Psychologic Considerations Related to High Risk Pregnancy
Once a pregnancy has been identified as high risk, the pregnant woman and her fetus will be monitored carefully throughout the remainder of the pregnancy. All women who undergo antepartal assessments are at risk for real and potential problems and may feel anxious. In most instances the tests are ordered because of suspected fetal compromise, deterioration of a maternal condition, or both. In the third trimester, pregnant women are most concerned about protecting themselves and their fetuses and consider themselves most vulnerable to outside influences. The label of high risk often increases this sense of vulnerability.
When a woman is diagnosed with a high risk pregnancy, she and her family will likely experience stress related to the diagnosis. The woman may exhibit various psychologic responses including anxiety, low self-esteem, guilt, frustration, and inability to function. A high risk pregnancy can also affect parental attachment, accomplishment of the tasks of pregnancy, and family adaptation to the pregnancy. If the woman is fearful for her own well-being, she may continue to feel ambivalence about the pregnancy or may not accept the reality of the pregnancy. She may not be able to complete preparations for the baby or go to childbirth classes if she is placed on restricted activity at home or is hospitalized. The family may become frustrated because they cannot engage in activities that prepare them for parenthood. The nurse can help the woman and her family regain control and balance in their lives by providing support and encouragement, information about the pregnancy problem and its management, and opportunities to make as many choices as possible about the woman’s care.
Antepartum Testing
The major expected outcome of all antepartum testing is the detection of potential fetal compromise. Ideally the technique used identifies fetal compromise before intrauterine asphyxia occurs so that the health care provider can take measures to prevent or minimize adverse perinatal outcomes. Antepartum testing is used primarily in pregnant women at risk for disrupted fetal oxygenation. In most cases, monitoring begins by 32 to 34 weeks of gestation and continues regularly until birth. Assessment tests should be selected based on their effectiveness, and the results must be interpreted in light of the complete clinical picture. Box 26-3 lists common obstetric and medical indications for antepartum testing (Tucker, Miller, & Miller, 2009).
The remainder of this chapter describes maternal and fetal assessment tests that are often used to monitor high risk pregnancies.
Biophysical Assessment
Assessment of fetal activity by the mother is a simple yet valuable method for monitoring the condition of the fetus. The daily fetal movement count (DMFC) (also called kick count) can be assessed at home and is noninvasive, inexpensive, simple to understand, and usually does not interfere with a daily routine. The DFMC is frequently used to monitor the fetus in pregnancies complicated by conditions that may affect fetal oxygenation (see Box 26-2). The presence of movements is generally a reassuring sign of fetal health.
Several different protocols are used for counting. One recommendation is to count once a day for 60 minutes. Another common recommendation is that mothers count fetal activity two or three times daily for 60 minutes each time. Except for establishing a very low number of daily fetal movements or a trend toward decreased motion, the clinical value of the absolute number of fetal movements has not been established, other than in the situation in which fetal movements cease entirely for 12 hours (the so-called fetal alarm signal). A count of fewer than three fetal movements within 1 hour warrants further evaluation by a nonstress test (NST) or a contraction stress test (CST) (oxytocin challenge test [OCT]), biophysical profile, or a combination of these (see later discussion). Women should be taught the significance of the presence or absence of fetal movements (or both), the procedure for counting that is to be used, how to record findings on a daily fetal movement record, and when to notify the health care provider.
Ultrasonography
Sound is a form of wave energy that causes small particles in a medium to oscillate. The frequency of sound, which refers to the number of peaks or waves that move over a given point per unit of time, is expressed in hertz (Hz). Sound with a frequency of 1 cycle, or one peak per second, has a frequency of 1 Hz. When directional beams of sound strike an object, an echo is returned. The time delay between the emission of the sound and the return of the echo and the direction of the echo are noted. From these data the distance and location of an object can be calculated. Ultrasound is sound frequency higher than that detectable by humans (greater than 20,000 Hz). Ultrasound images are a reflection of the strength of the sending beam, the strength of the returning echo, and the density of the medium (e.g., muscle [uterus], bone, tissue [placenta], fluid, or blood) through which the beam is sent and returned.
Diagnostic ultrasonography is an important, safe technique in antepartum fetal surveillance. It provides critical information to health care providers regarding fetal activity and gestational age, normal versus abnormal fetal growth curves, visual assistance with which invasive tests may be performed more safely, fetal and placental anatomy, and fetal well-being (Richards, 2007). Ultrasound examination can be performed abdominally or transvaginally during pregnancy. Both methods produce a three-dimensional view from which a pictorial image is obtained. Newer machines produce a four-dimensional view (Fig. 26-1, A and B). Abdominal ultrasonography is more useful after the first trimester, when the pregnant uterus becomes an abdominal organ. During the procedure the woman should have a full bladder to displace the uterus upward to provide a better image of the fetus. Transmission gel or paste is applied to the woman’s abdomen before a transducer is moved over the skin to enhance transmission and reception of the sound waves. She is positioned with small pillows under her head and knees. The display panel is positioned so that she or her partner (or both) can observe the images on the screen if they desire.
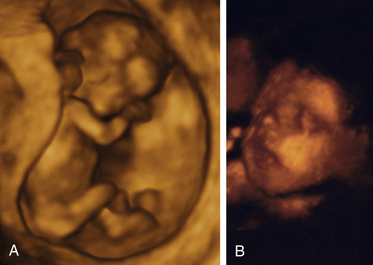
FIG. 26-1 Fetus seen on three-dimensional ultrasound. A, Full body view of fetus at 11 weeks and 6 days of gestation. B, Close-up view of fetal face later in pregnancy. (A, Courtesy Shannon Perry, Phoenix, AZ; B, courtesy Margaret Spann, New Johnsonville, TN.)
Transvaginal ultrasonography, in which the probe is inserted into the vagina, allows pelvic anatomic features to be evaluated in greater detail and intrauterine pregnancy to be diagnosed earlier. A transvaginal ultrasound examination is well tolerated by most pregnant women because it removes the need for a full bladder. It is especially useful in obese women whose thick abdominal layers cannot be penetrated adequately with an abdominal approach. A transvaginal ultrasound may be performed with the woman in a lithotomy position or with her pelvis elevated by towels, cushions, or a folded pillow. This pelvic tilt is optimal to image the pelvic structures. A protective cover such as a condom, the finger of a clean surgical glove, or a special probe cover provided by the manufacturer is used to cover the transducer probe. The probe is lubricated with a water-soluble gel and placed in the vagina either by the examiner or by the woman herself. During the examination the position of the probe or the tilt of the examining table may be changed so that the complete pelvis is in view. The procedure is not physically painful, although the woman will feel pressure as the probe is moved. Transvaginal ultrasonography is optimally used in the first trimester to detect ectopic pregnancies, monitor the developing embryo, help identify abnormalities, and help establish gestational age. In some instances it may be used as an adjunct to abdominal scanning to evaluate preterm labor in second- and third-trimester pregnancies.
Levels of Ultrasonography
The American College of Obstetricians and Gynecologists (ACOG) (2004) described three levels of ultrasonography. The standard examination is used most frequently and can be performed by ultrasonographers or other heath care professionals, including nurses, who have had special training. Indications for standard ultrasonography are described in detail in the next section. Its primary use is to detect fetal viability, determine the presentation of the fetus, assess gestational age, locate the placenta, examine the fetal anatomic structures for malformations, and determine amniotic fluid volume (AFV). Limited examinations are performed for specific indications such as identifying fetal presentation during labor or evaluating fetal heart rate (FHR) activity when it is not detected by other methods (ACOG). Specialized or targeted examinations are performed if a woman is suspected of carrying an anatomically or a physiologically abnormal fetus. Indications for this comprehensive examination include abnormal findings on clinical examination, especially with polyhydramnios or oligohydramnios, elevated alpha-fetoprotein (AFP) levels, and a history of offspring with anomalies that can be detected by ultrasound examination. Specialized ultrasonography is performed by highly trained and experienced personnel (ACOG).
Indications For Use
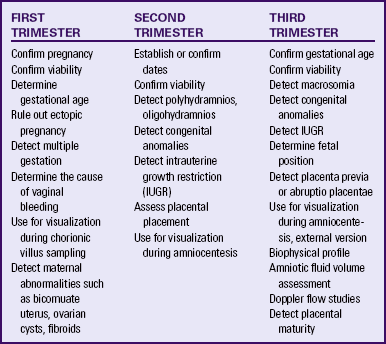
Major indications for obstetric sonography are listed by trimester in Table 26-1. During the first trimester ultrasound examination is performed to obtain information regarding the number, size, and location of gestational sacs; the presence or absence of fetal cardiac and body movements; the presence or absence of uterine abnormalities (e.g., bicornuate uterus or fibroids) or adnexal masses (e.g., ovarian cysts or an ectopic pregnancy); and pregnancy dating (by measuring the crown-rump length).
During the second and third trimesters information regarding the following conditions is sought: fetal viability, number, position, gestational age, growth pattern, and anomalies; AFV; placental location and maturity; presence of uterine fibroids or anomalies; presence of adnexal masses; and cervical length.
Ultrasonography provides earlier diagnoses, allowing therapy to be instituted sooner in the pregnancy, thereby decreasing the severity and duration of morbidity, both physical and emotional, for the family. For instance, early diagnosis of a fetal anomaly gives the family choices such as intrauterine surgery or other therapy for the fetus, termination of the pregnancy, or preparation for the care of an infant with a disorder.
Fetal Heart Activity: Fetal heart activity can be demonstrated as early as 6 to 7 weeks of gestation by real-time echo scanners and at 10 to 12 weeks by Doppler mode. By 9 to 10 weeks, gestational trophoblastic disease can be diagnosed. Fetal death can be confirmed by lack of heart motion, the presence of fetal scalp edema, and maceration and overlap of the cranial bones.
Gestational Age: Gestational dating by ultrasonography is indicated for conditions such as uncertain dates for the last normal menstrual period, recent discontinuation of oral contraceptives, a bleeding episode during the first trimester, uterine size that does not agree with dates, and other high risk conditions. In fact, growing evidence suggests that pregnancies should be dated by an ultrasound performed before 22 weeks of gestation rather than by menstrual dates because the ultrasound dating is more accurate than even “sure” menstrual dates (Richards, 2007). The methods of fetal age estimation used include determination of gestational sac dimensions (at approximately 8 weeks), measurement of crown-rump length (between 7 and 12 weeks), measurement of the biparietal diameter (BPD) (after 12 weeks), and measurement of femur length (after 12 weeks) (Fig. 26-2). An ultrasound examination performed for pregnancy dating between 14 and 22 weeks of gestation is comparable to one performed during the first trimester in terms of accuracy. After that time, however, ultrasound dating is less reliable because of variability in fetal size (Richards).
Fetal Growth: Fetal growth is determined by both intrinsic growth potential and environmental factors. Conditions that require ultrasound assessment of fetal growth include poor maternal weight gain or pattern of weight gain, previous pregnancy with intrauterine growth restriction (IUGR), chronic infections, ingestion of drugs (tobacco, alcohol, and over-the-counter and street drugs), maternal diabetes mellitus, hypertension, multifetal pregnancy, and other medical or surgical complications.
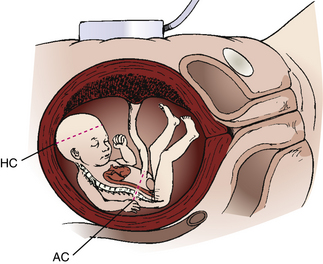
Serial evaluations of BPD, limb length, and abdominal circumference (AC) can allow differentiation among size discrepancy resulting from inaccurate dates, true IUGR, and macrosomia. IUGR may be symmetric (the fetus is small in all parameters) or asymmetric (head and body growth vary). Symmetric IUGR reflects a chronic or long-standing insult and may be caused by low genetic growth potential, intrauterine infection, undernutrition, heavy smoking, or chromosomal aberration. Asymmetric growth suggests an acute or late-occurring deprivation, such as placental insufficiency resulting from hypertension, renal disease, or cardiovascular disease. Reduced fetal growth is still one of the most frequent conditions associated with stillbirth. Macrosomic infants (those weighing 4000 g or more) are at increased risk for traumatic injury and asphyxia during birth. Macrosomia may also be characterized as symmetric or asymmetric.
Fetal Anatomy: Anatomic structures that can be identified by ultrasonography (depending on the gestational age) include the following: head (including ventricles and blood vessels), neck, spine, heart, stomach, small bowel, liver, kidneys, bladder, and limbs. Ultrasonography permits the confirmation of normal anatomy, as well as the detection of major fetal malformations. The presence of an anomaly may influence the location of birth (e.g., a subspecialty center versus a basic care center) and the method of birth (vaginal versus cesarean) to optimize neonatal outcomes. For example, plans are often made for a fetus with a condition that will require immediate surgery to be born in or near a hospital able to provide that care, rather than in a small community hospital that is totally unequipped to meet the newborn’s needs.
The number of fetuses and their presentations also may be assessed by ultrasonography, allowing plans for therapy and mode of birth to be made in advance.
Fetal Genetic Disorders and Physical Anomalies: A prenatal screening technique called nuchal translucency (NT) screening uses ultrasound measurement of fluid in the nape of the fetal neck between 10 and 14 weeks of gestation to identify possible fetal abnormalities (Fig. 26-3). A fluid collection that is greater than 3 mm is considered abnormal. When combined with low maternal serum marker levels, elevated NT indicates a possible increased risk of certain chromosomal abnormalities in the fetus, including trisomies 13, 18, and 21. An elevated NT alone indicates an increased risk of fetal cardiac disease. If the NT is abnormal, diagnostic genetic testing is recommended (ACOG, 2007; Gilbert, 2011).
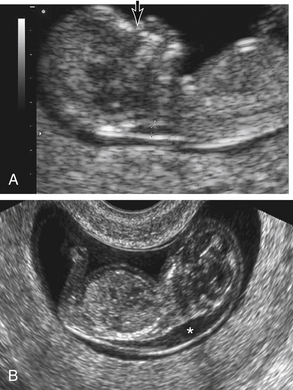
FIG. 26-3 Fetal nuchal translucency. A, Nuchal lucency (calipers) and nasal bone (arrow) in 12-week fetus. B, Increased nuchal translucency. Transvaginal ultrasound performed at 12 weeks demonstrates a sonolucent area (asterisk) over the posterior neck and upper thorax.(From Martin, R., Fanaroff, A., & Walsh, M. [2006]. Fanaroff and Martin’s neonatalperinatal medicine: Diseases of the fetus and infant [8th ed.]. Philadelphia: Mosby.)
Placental Position and Function: The pattern of uterine and placental growth and the fullness of the maternal bladder influence the apparent location of the placenta by ultrasonography. During the first trimester, differentiation between the endometrium and small placenta is difficult. By 14 to 16 weeks the placenta is clearly defined; but if it is seen to be low lying, its relationship to the internal cervical os can sometimes be dramatically altered by varying the fullness of the maternal bladder. In approximately 4% to 6% of all pregnancies in which ultrasound scanning is performed during the second trimester, the placenta seems to be overlying the os. However, more than 90% of cases of placenta previa diagnosed during the second trimester will have resolved by term, primarily because of the elongation of the lower uterine segment as pregnancy advances. Therefore, if placenta previa is diagnosed before 24 weeks of gestation, an ultrasound examination should be repeated between 28 and 32 weeks of gestation to confirm the diagnosis (Francois & Foley, 2007).
Another use for ultrasonography is grading of placental aging. Calcium and fibrin deposits in an aging placenta result in intervillous hemorrhagic infarcts. Also as blood vessels in the placenta age and thicken, oxygen transport is affected. Whether these placental changes adversely affect fetal outcomes in postterm pregnancies is unknown, however, given that most fetuses continue to grow (Gilbert, 2011).
Adjunct to Other Invasive Tests: The safety of amniocentesis is increased when the positions of the fetus, placenta, and pockets of amniotic fluid can be identified accurately. Ultrasound scanning has reduced risks previously associated with amniocentesis, such as fetomaternal hemorrhage from a pierced placenta. Percutaneous umbilical blood sampling and chorionic villus sampling also are guided by ultrasonography to identify the cord and chorion frondosum accurately.
Fetal Well-being: Physiologic parameters of the fetus that can be assessed with ultrasound scanning include AFV, vascular waveforms from the fetal circulation, heart motion, fetal breathing movements (FBMs), fetal urine production, and fetal limb and head movements. Assessment of these parameters, alone or in combination, yields a fairly reliable picture of fetal well-being. The significance of these findings is discussed in the following sections.
Doppler Blood Flow Analysis: One of the major advances in perinatal medicine is the ability to study blood flow noninvasively in the fetus and placenta with ultrasound. Doppler blood flow analysis is a helpful adjunct in the management of pregnancies at risk because of hypertension, IUGR, diabetes mellitus, multiple fetuses, and preterm labor.
When a sound wave is reflected from a moving target, a change occurs in the frequency of the reflected wave relative to the transmitted wave, called the Doppler effect. An ultrasound beam scattered by a group of red blood cells (RBCs) is an example of this effect. The velocity of the RBCs can be determined by measuring the change in the frequency of the sound wave reflected off the cells (Fig. 26-4).
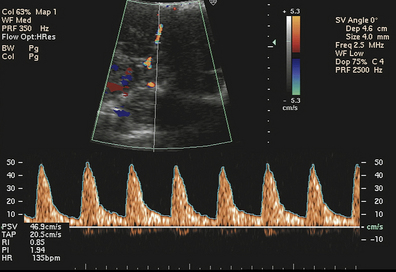
FIG. 26-4 Umbilical artery velocity waveform. (From Callen, P. [2000]: Ultrasonography in obstetrics and gynecology [4th ed.]. Philadelphia: Saunders.)
The shifted frequencies can be displayed as a plot of velocity versus time, and the shape of these waveforms can be analyzed to give information about blood flow and resistance in a given circulation. Velocity waveforms from umbilical and uterine arteries, reported as systolic/diastolic (S/D) ratios, can be first detected at 15 weeks of pregnancy. Because of the progressive decline in resistance in both the umbilical and uterine arteries, this ratio normally decreases as pregnancy advances. IUGR is seen more often in fetuses whose ratios remain elevated for their gestational age (Druzin, Smith, Gabbe, & Reed, 2007). Severely restricted uterine artery blood flow is indicated by absent or reversed flow during diastole (Tucker et al., 2009). In postterm pregnancies evaluated by Doppler umbilical flow studies an elevated S/D ratio indicates a poorly perfused placenta. Abnormal results also are seen with certain chromosome abnormalities (trisomy 13 and 18) in the fetus and with lupus erythematosus in the mother. Exposure to nicotine from maternal smoking also has been reported to increase the S/D ratio (see Fig. 26-4).
Amniotic Fluid Volume: Abnormalities in AFV are frequently associated with fetal disorders. Subjective determinants of oligohydramnios (decreased fluid) include the absence of fluid pockets in the uterine cavity and the impression of crowding of small fetal parts. An objective criterion of decreased AFV is met if the largest pocket of fluid measured in two perpendicular planes is less than 2 cm (Harman, 2009). Increased amniotic fluid is called polyhydramnios or sometimes just hydramnios. Subjective criteria for polyhydramnios include multiple large pockets of fluid, the impression of a floating fetus, and free movement of fetal limbs. Hydramnios is usually defined as pockets of amniotic fluid measuring more than 8 cm (Gilbert, 2007).
The total AFV can be evaluated by a method in which the vertical depths (in centimeters) of the largest pocket of amniotic fluid in all four quadrants surrounding the maternal umbilicus are totaled, providing an amniotic fluid index (AFI). A normal AFI is 10 cm or greater, with the upper range of normal around 25 cm. AFI values between 5 and 10 cm are considered to be low normal, whereas an AFI of less than 5 cm indicates oligohydramnios. With polyhydramnios the AFI would be above 25 cm (Tucker et al., 2009). Oligohydramnios is associated with congenital anomalies (e.g., renal agenesis), growth restriction, and fetal distress during labor. Polyhydramnios is associated with neural tube defects (NTDs), obstruction of the fetal gastrointestinal tract, multiple fetuses, and fetal hydrops.
Biophysical Profile: Real-time ultrasound permits detailed assessment of the physical and physiologic characteristics of the developing fetus and cataloging of normal and abnormal biophysical responses to stimuli. The biophysical profile (BPP) is a noninvasive dynamic assessment of a fetus that is based on acute and chronic markers of fetal disease. The BPP includes AFV, FBMs, fetal movements, and fetal tone determined by ultrasound and FHR reactivity determined by means of NST. The BPP may therefore be considered a physical examination of the fetus, including determination of vital signs. FHR reactivity, FBMs, fetal movement, and fetal tone reflect current central nervous system (CNS) status, whereas the AFV demonstrates
the adequacy of placental function over a longer period (Tucker et al., 2009). BPP scoring and management are detailed in Tables 26-2 and 26-3.
TABLE 26-2
| BIOPHYSICAL VARIABLE | NORMAL (SCORE = 2) | ABNORMAL (SCORE = 0) |
| Fetal breathing movements | At least one episode of >30 seconds’ duration in 30 minutes’ observation | Absent or no episode of ≥30 seconds’ duration in 30 minutes |
| Gross body movement | At least three discrete body/limb movements in 30 minutes (episodes of active continuous movement considered a single movement) | Up to two episodes of body/limb movements in 30 minutes |
| Fetal tone | At least one episode of active extension with return to flexion of fetal limb(s) or trunk, opening and closing of hand considered normal tone | Either slow extension with return to partial flexion or movement of limb in full extension or absent fetal movement |
| Reactive fetal heart rate | At least two episodes of acceleration of ≥15 beats/min and 15 seconds’ duration associated with fetal movement in 30 minutes | Fewer than two accelerations or acceleration <15 beats/min in 30 minutes |
| Qualitative amniotic fluid volume | At least one pocket of amniotic fluid measuring 2 cm in two perpendicular planes | Either no amniotic fluid pockets or a pocket <2 cm in two perpendicular planes |
Source: Druzin, M., Smith, J., Gabbe, S., & Reed, K. (2007). Antepartum fetal evaluation. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
TABLE 26-3
BIOPHYSICAL PROFILE MANAGEMENT
| SCORE | INTERPRETATION | MANAGEMENT |
| 10 | Normal infant; low risk of chronic asphyxia | Repeat testing at weekly intervals; repeat twice weekly in women with diabetes and women at 41 weeks of gestation |
| 8 | Normal infant; low risk of chronic asphyxia | Repeat testing at weekly intervals; repeat testing twice weekly in women with diabetes and women at 41 weeks of gestation; oligohydramnios is an indication for delivery |
| 6 | Suspect chronic asphyxia | If 36 weeks of gestation and conditions are favorable, deliver; if at >36 weeks and L/S <2, repeat test in 4-6 hours; deliver if oligohydramnios is present |
| 4 | Suspect chronic asphyxia | If 36 weeks of gestation, deliver; if <32 weeks of gestation, repeat test |
| 0-2 | Strongly suspect chronic asphyxia | Extend testing time to 120 minutes; if persistent score ≤4, deliver, regardless of gestational age |
Source: Druzin, M., Smith, J., Gabbe, S., & Reed, K. (2007). Antepartum fetal evaluation. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
The BPP is used very frequently for antepartum fetal testing because it is a reliable predictor of fetal well-being. A BPP of 8 to 10 with a normal AFV is considered normal. Advantages of the test include excellent sensitivity and a low false-negative rate (Tucker et al., 2009). One limitation of the test is that if the fetus is in a quiet sleep state, the BPP can require a long period of observation. Also unless the ultrasound examination is videotaped, it cannot be reviewed (Druzin et al., 2007).
Nursing Role
Although a growing number of nurses perform ultrasound scans and BPPs in certain centers, the main role of nurses is in counseling and educating women about the procedure. Ultrasound is
widely used and in fact is considered a standard part of current prenatal care. Unlike many diagnostic tests, most women look forward to and enjoy their prenatal ultrasound. In the 30 years that diagnostic ultrasonography has been used, no evidence of any harmful effects on humans has emerged (Richards, 2007).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a noninvasive radiologic technique used for obstetric and gynecologic diagnosis. Similar to computed tomography (CT), MRI provides excellent pictures of soft tissue. Unlike CT, ionizing radiation is not used. Therefore, vascular structures within the body can be visualized and evaluated without injecting an iodinated contrast medium, thus eliminating any known biologic risk. Similar to sonography, MRI is noninvasive and can provide images in multiple planes, but no interference occurs from skeletal, fatty, or gas-filled structures, and imaging of deep pelvic structures does not require a full bladder.
With MRI the examiner can evaluate fetal structure (CNS, thorax, abdomen, genitourinary tract, musculoskeletal system) and overall growth, the placenta (position, density, and presence of gestational trophoblastic disease), and the quantity of amniotic fluid. Maternal structures (uterus, cervix, adnexa, and pelvis), the biochemical status (pH, adenosine triphosphate content) of tissues and organs, and soft-tissue, metabolic, or functional anomalies can also be evaluated.
The woman is placed on a table in the supine position and moved into the bore of the main magnet, which is similar in appearance to a CT scanner. Depending on the reason for the study the procedure may take from 20 to 60 minutes, during which time the woman must be perfectly still except for short respites. Because of the long time needed to produce MRIs, the fetus will probably move, which will obscure anatomic details. The only way to ensure that this problem does not occur is to administer a sedative to the mother, but this approach should be reserved for selected cases in which visualization of fetal detail is critical.
MRI has little effect on the fetus. Concerns that the FHR or fetal movement would decrease have not been supported. ![]()
Biochemical Assessment
Biochemical assessment involves biologic examination (e.g., as chromosomes in exfoliated cells) and chemical determinations (e.g., lecithin/sphingomyelin [L/S] ratio surfactant/albumin [S/A] ratio, [TDX FLM assay], and bilirubin level) (Table 26-4). Procedures used to obtain the needed specimens include amniocentesis, percutaneous umbilical blood sampling, chorionic villus sampling, and maternal sampling (Box 26-4).
TABLE 26-4
SUMMARY OF BIOCHEMICAL MONITORING TECHNIQUES
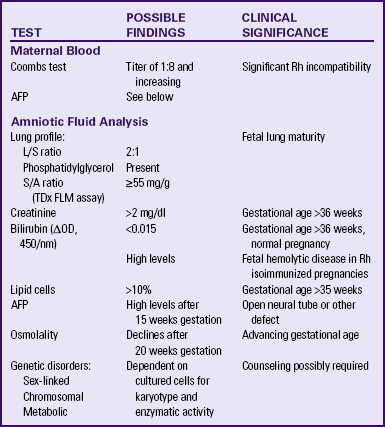
AFP, Alpha-fetoprotein; L/S, lecithin/sphingomyelin; S/A, surfactant/albumin. TDx FLM assay, name of specific test used to determine S/A ratio.
Amniocentesis
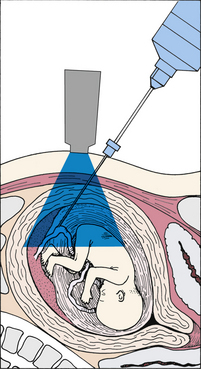
Amniocentesis is performed to obtain amniotic fluid, which contains fetal cells. Under direct ultrasonographic visualization, a needle is inserted transabdominally into the uterus, amniotic fluid is withdrawn into a syringe, and the various assessments are performed (Fig. 26-5). Amniocentesis is possible after week 14 of pregnancy, when the uterus becomes an abdominal organ, and sufficient amniotic fluid is available for testing. Indications for the procedure include prenatal diagnosis of genetic disorders or congenital anomalies (NTDs in particular), assessment of pulmonary maturity, and diagnosis of fetal hemolytic disease.
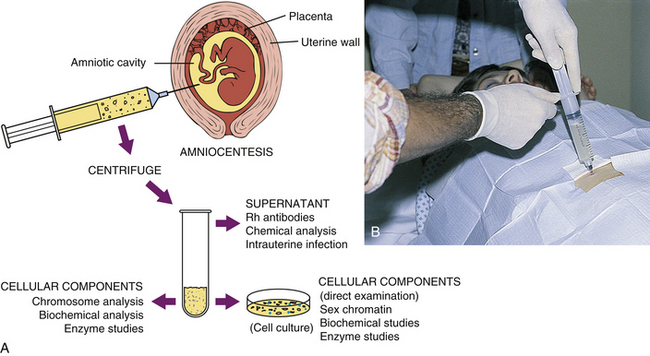
FIG. 26-5 A, Amniocentesis and laboratory use of amniotic fluid aspirant. B, Transabdominal amniocentesis. (B, Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
Complications in the mother and fetus occur in less than 1% of the cases and include the following:
• Maternal: hemorrhage, fetomaternal hemorrhage with possible maternal Rh isoimmunization, infection, labor, placental abruptio, inadvertent damage to the intestines or bladder, and anaphylactoid syndrome of pregnancy (amniotic fluid embolism)
• Fetal: death, hemorrhage, infection (amnionitis), direct injury from the needle, miscarriage or preterm labor, and leakage of amniotic fluid
Many of the complications have been minimized or eliminated by using ultrasonography to direct the procedure.
Indications for Use
Genetic Concerns: Historically, prenatal assessment of genetic disorders focused on women older than 35 years (Box 26-5), with a previous child with a chromosomal abnormality, or with a family history of chromosomal anomalies. Inherited errors of metabolism (such as Tay-Sachs disease), hemophilia, and thalassemia and other disorders for which marker genes are known also can be detected by prenatal screening. Fetal cells can be cultured for karyotyping of chromosomes (see Chapter 3). Karyotyping also permits determination of fetal sex, which is important if an X-linked disorder (occurring almost always in a male fetus) is suspected.
Biochemical analysis of enzymes in amniotic fluid can detect inborn errors of metabolism. For example, AFP levels in amniotic fluid are assessed as a follow-up for elevated levels in maternal serum. High AFP levels in amniotic fluid help confirm the diagnosis of an NTD such as spina bifida or anencephaly or an abdominal wall defect such as omphalocele. The elevation results from the increased leakage of cerebrospinal fluid into the amniotic fluid through the closure defect. AFP levels may also be elevated in a normal multifetal pregnancy and with intestinal atresia, presumably caused by lack of fetal swallowing.
A concurrent test that finds the presence of acetylcholinesterase almost always indicates a fetal defect (Wapner, Jenkins, & Khalek, 2009). In such instances, follow-up ultrasound examination is recommended.
Fetal Maturity: Late in pregnancy, accurate assessment of fetal lung maturity is possible by examining amniotic fluid for the presence of phosphatidylglycerol (PG). Determination of the lecithin/sphingomyelin (L/S) ratio and the surfactant/albumin (S/A) ratio [TDx FLM assay] are other methods to determine fetal lung maturity. The FLM assay is often used as the primary test for determining fetal lung maturity in clinical practice because it is simple to perform and accurate. FLM test results are similar to those of the PG test and the L/S ratio in terms of predicting pulmonary maturity (Mercer, 2009) (see Table 26-4).
Fetal Hemolytic Disease: Another indication for amniocentesis is the identification and follow-up of fetal hemolytic disease in cases of isoimmunization. The procedure is usually not performed until the mother’s antibody titer reaches 1:8 and is increasing. Although percutaneous umbilical blood sampling is still the procedure of choice to treat fetal hemolytic disease, it is used less frequently for evaluating this condition. Instead, doppler velocimetry of the fetal middle cerebral artery is used to predict anemia associated with fetal hemolytic disease accurately and noninvasively (Tucker et al., 2009).
Chorionic Villus Sampling
The combined advantages of earlier diagnosis and rapid results have made chorionic villus sampling (CVS) a popular technique for genetic studies in the first trimester, although some risks to the fetus exist. Indications for CVS are similar to those for amniocentesis, although CVS cannot be used for maternal serum marker screening because no fluid is obtained. CVS performed in the second trimester carries no greater risk of pregnancy loss than amniocentesis and is considered equal to amniocentesis in diagnostic accuracy (Simpson & Otano, 2007). ![]()
The procedure can be performed in the first or second trimester, ideally between 10 and 13 weeks of gestation and involves the removal of a small tissue specimen from the fetal portion of the placenta (figs. 26-6 and 26-7). Because chorionic villi originate in the zygote, this tissue reflects the genetic makeup of the fetus (Gilbert, 2011).
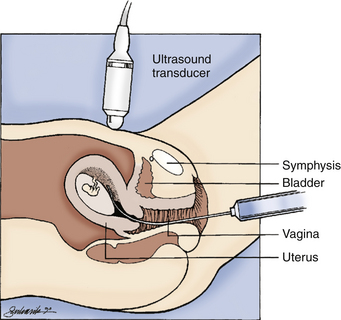
FIG. 26-6 Transcervical chorionic villus sampling. (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone.)
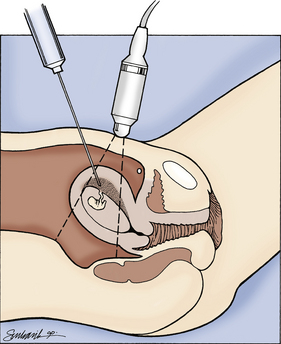
FIG. 26-7 Transabdominal chorionic villus sampling. (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone.)
CVS procedures can be accomplished transcervically or transabdominally. In transcervical sampling a sterile catheter is introduced into the cervix under continuous ultrasonographic guidance, and a small portion of the chorionic villi is aspirated with a syringe. The aspiration cannula and obturator must be placed at a suitable site, and rupture of the amniotic sac must be avoided (see Fig 26-6). The transcervical procedure is contraindicated if a cervical infection, such as chlamydia or herpes, is present (Gilbert, 2011).
If the abdominal approach is used, an 18-gauge spinal needle with stylet is inserted under sterile conditions through the abdominal wall into the chorion frondosum under ultrasound guidance. The stylet is then withdrawn, and the chorionic tissue is aspirated into a syringe (see Fig. 26-7).
Complications of the procedure include vaginal spotting or bleeding immediately afterward, miscarriage (0.3% of cases), rupture of membranes (0.1% of cases), and chorioamnionitis (0.5% of cases). Controversy exists concerning fetal limb reduction defects associated with CVS. Any increased risk appears to
exist before 10 weeks of gestation. For this reason CVS is usually not performed until after 9 menstrual weeks of gestation (Simpson & Otano, 2007).
Use of amniocentesis and CVS is declining because of advances in noninvasive screening techniques. These techniques include measurement of NT, maternal serum screening tests in the first and second trimesters, and ultrasonography in the second trimester (Wapner et al., 2009).
Percutaneous Umbilical Blood Sampling
Direct access to the fetal circulation during the second and third trimesters is possible through percutaneous umbilical blood sampling (PUBS) (also called cordocentesis), which is the most widely used method for fetal blood sampling and transfusion. PUBS involves the insertion of a needle directly into a fetal umbilical vessel, preferably the vein, under ultrasound guidance. Ideally, the umbilical cord is punctured near its insertion into the placenta (Figs. 26-8 and 26-9). At this point the cord is well anchored and will not move, and the risk of maternal blood contamination (from the placenta) is slight. Generally, a small amount of blood is removed and tested immediately by the Kleihauer-Betke procedure (Apt test) to ensure that it is fetal in origin (Simpson & Otano, 2007). Indications for use of PUBS include prenatal diagnosis of inherited blood disorders, karyotyping of malformed fetuses, detection of fetal infection, and assessment and treatment of isoimmunization and thrombocytopenia in the fetus (Wapner et al., 2009). Complications that can occur include loss of the pregnancy, hematomas, bleeding from the puncture site in the umbilical cord, transient fetal bradycardia, and fetomaternal hemorrhage. Maternal complications are rare, but include hemorrhage and transplacental hemorrhage (Simpson & Otano).
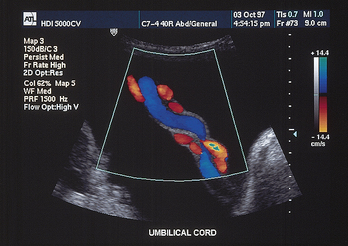
FIG. 26-9 Umbilical cord as seen on ultrasound at 26 weeks of gestation. (Courtesy Advanced Technology Laboratories, Bothell, WA.)
In fetuses at risk for isoimmune hemolytic anemia, PUBS permits precise identification of fetal blood type and RBC count and may prevent the need for further intervention. If the fetus is positive for the presence of maternal antibodies, a direct blood test can confirm the degree of anemia resulting from hemolysis. Intrauterine transfusion of severely anemic fetuses can be performed 4 to 5 weeks earlier than through the intraperitoneal route.
Follow-up includes continuous FHR monitoring for 1 to 2 hours after the procedure. Women should also be taught to do fetal movement counting at home (Gilbert, 2011).
Maternal Assays
Maternal serum alpha-fetoprotein (MSAFP) levels are used as a screening tool for NTDs in pregnancy. Through this technique, approximately 80% to 85% of all open NTDs and open abdominal wall defects can be detected early in pregnancy. Screening is recommended for all pregnant women.
The cause of NTDs is not well understood, but 95% of all affected infants are born to women with no family history of similar anomalies (Wapner et al., 2009). The defect occurs in approximately 2 of 1000 births in the United States. The rate of NTDs is decreasing as a result of the use of folate preconceptionally and during early pregnancy for prevention of this condition (Manning, 2009). ![]()
Alpha-Fetoprotein (AFP) is produced by the fetal liver, and increasing levels are detectable in the serum of pregnant women from 14 to 34 weeks of gestation. Although amniotic fluid AFP measurement is diagnostic for NTD, MSAFP is a screening tool only and identifies candidates for the more definitive procedures of amniocentesis and ultrasound examination. MSAFP screening can be performed with reasonable reliability any time between 15 and 20 weeks of gestation (16 to 18 weeks being ideal) (Wapner et al., 2009).
Once the maternal level of AFP is determined, it is compared with normal values for each week of gestation. Values also should be correlated with maternal age, weight, race, presence of a multifetal pregnancy, and whether the woman has insulin-dependent diabetes. If findings are abnormal, follow-up procedures include genetic counseling for families with a history of NTD, repeated AFP, specialized ultrasound examination, and possibly, amniocentesis (Cunningham et al., 2010).
Multiple Marker Screens
Screening to detect fetal chromosomal abnormalities, particularly Trisomy 21 (Down syndrome) is now available, beginning in the first trimester of pregnancy. This first trimester screen is done at 11 to 14 weeks of gestation. It includes measurement of two maternal biochemical markers, pregnancy-associated placental protein (PAPP-A) and human chorionic gonadotropin (hCG) or the free beta-human chorionic gonadotropin (β-hCG) subunit, and evaluation of fetal NT, or a combination of both. In the first trimester, hCG levels are higher than normal while PAPP-A levels are lower than normal in the presence of a fetus with Trisomy 21. First trimester screening using PAPP-A and hCG or β-hCG levels has been shown to be as accurate for detecting fetuses with Trisomy 21 as triple screening in the second trimester (Cunningham et al., 2010; Wapner, et al., 2009).
About one third of all fetuses with an increased NT will have a chromosome abnormality; half of these are Trisomy 21. Combining the serum marker and NT values results in the detection of Down syndrome in 79% to 87% of cases. These results are comparable to those obtained with quad screening in the second trimester (Cunningham et al., 2010).
In the second trimester, triple and quad screening is available to screen for fetuses with Trisomy 21 and Trisomy 18. The triple-marker screen, performed at 16 to 18 weeks of gestation, measures the levels of three maternal serum markers: MSAFP, unconjugated estriol, and hCG. In the presence of a fetus with Trisomy 21 the MSAFP and unconjugated estriol levels are low, whereas the hCG level is elevated. Low values in all three markers are associated with Trisomy 18 (Cunningham et al., 2010; Gilbert, 2011).
The quad-screen adds an additional marker, a placental hormone called inhibin A, to increase the accuracy of screening for Down syndrome in women less than 35 years of age. Low inhibin A levels indicate the possibility of Down syndrome (Gilbert, 2011). The addition of inhibin A to the other three markers increases the detection rate for Down syndrome in the entire population from 70% to 80% (Simpson & Otano, 2007). Similar to triple marker screening, the optimal time to perform the quad-screen is between 16 and 18 weeks of gestation (Gilbert).
The ability of multiple marker tests to detect chromosomal abnormalities depends on the accuracy of gestational age assessment. These tests are screening procedures only and are not diagnostic. A positive screening test result indicates an increased risk but is not diagnostic of Trisomy 21 or another chromosomal abnormality. Women with positive results should be offered diagnostic testing by amniocentesis or fetal blood sampling for fetal karyotyping (Cunningham et al., 2010).
Coombs Test
The indirect Coombs test is a screening tool for Rh incompatibility. If the maternal titer for Rh antibodies is greater than 1:8, amniocentesis for determination of bilirubin in amniotic fluid is indicated to establish the severity of fetal hemolytic anemia. The Coombs test can also detect other antibodies that may place the fetus at risk for incompatibility with maternal antigens.
Antepartal Assessment Using Electronic Fetal Monitoring
First- and second-trimester antepartal assessment is directed primarily at the diagnosis of fetal anomalies. The goal of third-trimester testing is to determine whether the intrauterine environment continues to be supportive to the fetus. The testing is often used to determine the timing of childbirth for women at risk for UPI. Gradual loss of placental function results first in inadequate nutrient delivery to the fetus, leading to IUGR. Subsequently, respiratory function also is compromised, resulting in fetal hypoxia. Common indications for both the nonstresss test (NST) and the contraction stress test CST, sometimes called the oxytocin challenge test (OCT), are listed in Box 26-6.
No clinical contraindications exist for the NST, but results may not be conclusive if gestation is 26 weeks or less. In general the CST cannot be performed on women who should not give birth vaginally at the time the test is performed. Absolute contraindications for the CST are the following: preterm labor, placenta previa, vasa previa, cervical incompetence, multiple gestation, and previous classic incision for cesarean birth (Tucker et al., 2009).
Nonstress Test
The NST is the most widely applied technique for antepartum evaluation of the fetus. It is an ideal screening test and is the primary method of antepartum fetal assessment at most sites. The basis for the NST is that the normal fetus will produce characteristic heart rate patterns in response to fetal movement. In the term fetus, accelerations are associated with movement more than 85% of the time (Druzin et al., 2007). The most common reason for the absence of FHR accelerations is the quiet fetal sleep state. However, medications such as narcotics, barbiturates, and beta-blockers, maternal smoking, and the presence of fetal malformations can also adversely affect the test (Druzin et al.; Gilbert, 2011). The NST can be performed easily and quickly in an outpatient setting because it is noninvasive, is relatively inexpensive, and has no known contraindications. Disadvantages include the requirement for twice-weekly testing and a high false-positive rate. The test also is slightly less sensitive in detecting fetal compromise than the CST or BPP (Tucker et al., 2009).
Procedure
The woman is seated in a reclining chair (or in semi-Fowler position) with a slight lateral tilt to optimize uterine perfusion and prevent supine hypotension. The FHR is recorded with a Doppler transducer, and a tocodynamometer is applied to detect uterine contractions or fetal movements. The tracing is observed for signs of fetal activity and a concurrent acceleration of FHR. If evidence of fetal movement is not apparent on the tracing, the woman may be asked to depress a button on a handheld event marker connected to the monitor when she feels fetal movement. The movement is then noted on the tracing. Because almost all accelerations are accompanied by fetal movement, the movements need not be recorded for the test to be considered reactive. The test is usually completed within 20 to 30 minutes, but more time may be required if the fetus must be awakened from a sleep state.
Caregivers sometimes suggest that the woman drink orange juice or be given glucose to increase her blood sugar level and thereby stimulate fetal movements. ![]() Although this practice is common, research has not proven it to be effective (Druzin et al., 2007).
Although this practice is common, research has not proven it to be effective (Druzin et al., 2007).
Vibroacoustic stimulation is often used to stimulate fetal activity if the initial NST result is nonreactive and thus hopefully shortens the time required to complete the test (Druzin et al., 2007).
Interpretation
NST results are either reactive (Fig. 26-10) or nonreactive (Fig. 26-11). Box 26-7 lists criteria for both results.
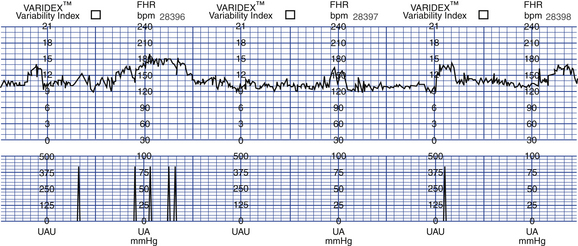
FIG. 26-10 Reactive nonstress test. (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone.)
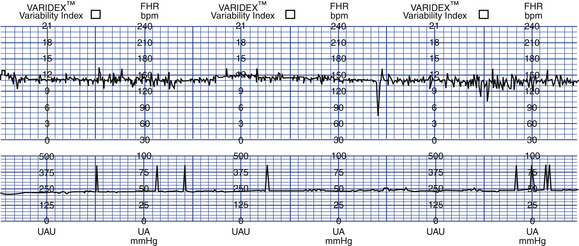
FIG. 26-11 Nonnreactive nonstress test. (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone.)
A nonreactive test requires further evaluation. The testing period is often extended, usually for an additional 20 minutes, with the expectation that the fetal sleep state will change and the test will become reactive. During this time, vibroacoustic stimulation (see later discussion) may be used to stimulate fetal activity. If the test does not meet the criteria after 40 minutes, a CST or BPP will usually be performed. Once NST testing is initiated, it is usually repeated once or twice weekly for the remainder of the pregnancy (Druzin et al., 2007; Tucker et al., 2009).
Vibroacoustic Stimulation
Vibroacoustic stimulation (also called the fetal acoustic stimulation test) is another method of testing antepartum FHR response. This test is generally performed in conjunction with the NST and uses a combination of sound and vibration to stimulate the fetus. Whether the acoustic or the vibratory component alters the fetal state is unclear. The test takes approximately 15 minutes to complete, with the fetus monitored for 5 to 10 minutes before stimulation to obtain a baseline FHR. If the fetal baseline pattern is nonreactive, the sound source (usually a laryngeal stimulator) is then activated for 3 seconds on the maternal abdomen over the fetal head. Monitoring continues for another 5 minutes, after which the monitor tracing is assessed. The desired result is a reactive NST. The accelerations produced may have a significant increase in duration (Fig 26-12). The test may be repeated at 1-minute intervals up to three times when no response is noted. Further evaluation is needed with BPP or CST if the pattern is still nonreactive (Druzin et al., 2007).
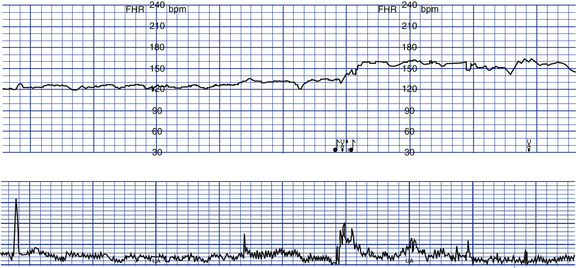
FIG. 26-12 Reactive nonstress test after vibroacoustic stimulation. The stimulus was applied at the point marked by the musical notes. A sustained fetal heart rate acceleration was produced. (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone.)
Contraction Stress Test
The CST (or OCT) was the first widely used electronic fetal assessment test. It was devised as a graded stress test of the fetus, and its purpose was to identify the jeopardized fetus that was stable at rest but showed evidence of compromise after stress. Uterine contractions decrease uterine blood flow and placental perfusion. If this decrease is sufficient to produce hypoxia in the fetus, a deceleration in FHR will result.
The CST provides an earlier warning of fetal compromise than the NST and with fewer false-positive results. However, in addition to the contraindications described earlier the CST is more time consuming and expensive than the NST. It is also an invasive procedure if oxytocin stimulation is required. Because of these disadvantages, the CST is used infrequently.
Procedure
The woman is placed in semi-Fowler position or sits in a reclining chair with a slight lateral tilt to optimize uterine perfusion and avoid supine hypotension. She is monitored electronically with the fetal ultrasound transducer and uterine tocodynamometer. The tracing is observed for 10 to 20 minutes for baseline rate and variability and the possible occurrence of spontaneous contractions. The two methods of CST are the nipple-stimulated contraction test and the more commonly used oxytocin-stimulated contraction test.
Nipple-Stimulated Contraction Test: Several methods of nipple stimulation have been described. In one approach the woman applies warm, moist washcloths to both breasts for several minutes. The woman is then asked to massage one nipple for 10 minutes. Massaging the nipple causes a release of oxytocin from the posterior pituitary. An alternative approach is for her to massage one nipple through her clothes for 2 minutes, rest for 5 minutes, and repeat the cycles of massage and rest as necessary to achieve adequate uterine activity. When adequate contractions or hyperstimulation (defined as uterine contractions lasting more than 90 seconds or five or more contractions in 10 minutes) occurs, stimulation should be stopped (Druzin et al., 2007).
Oxytocin-Stimulated Contraction Test: Exogenous oxytocin also can be used to stimulate uterine contractions. An intravenous (IV) infusion is begun, and a dilute solution of oxytocin (e.g., 30 units in 500 ml of fluid) is infused into the tubing of the main IV device through a piggyback port and delivered by an infusion pump to ensure an accurate dose. One method of oxytocin infusion is to begin at 0.5 milliunits/min and double the dose every 20 minutes until three uterine contractions of good quality, each lasting 40 to 60 seconds, are observed within a 10-minute period. A rate of 10 milliunits/min is usually adequate to elicit uterine contractions (Druzin et al., 2007).
Interpretation
CST results are either negative, positive, equivocal, suspicious, or unsatisfactory. If no late decelerations are observed with the contractions, the findings are considered negative (Fig. 26-13, A). Repetitive late decelerations render the test results positive (see Fig. 26-13, B). Box 26-8 lists criteria for each possible test result.
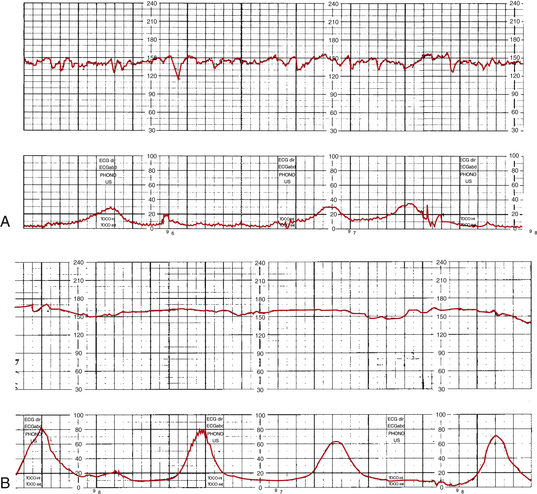
FIG. 26-13 Contraction stress test (CST). A, Negative CST. B, Positive CST. (From Tucker, S. [2004]. Pocket guide to fetal monitoring and assessment [5th ed.]. St. Louis: Mosby.)
The desired CST result is negative because it has consistently been associated with good fetal outcomes. With a negative result the test is repeated in 1 week. Positive CST results have been associated with intrauterine fetal death, late FHR decelerations in labor, IUGR, and meconium-stained amniotic fluid. A positive CST result usually leads to hospitalization for further close observation or birth. Unsatisfactory, suspicious, and equivocal tests must be repeated within 24 hours (Druzin et al., 2007; Tucker et al., 2009).
Nurses’ Role in Assessment of the High Risk Pregnancy
The nurse’s role is primarily that of educator and support person when the woman is undergoing such examinations as ultrasonography, MRI, CVS, PUBS, and amniocentesis. In some instances the nurse may assist the physician with the procedure. In many settings, nurses perform NSTs, CSTs, and BPPs; conduct an initial assessment; and begin necessary interventions for nonreassuring results. These nursing procedures are accomplished after additional education and training, under guidance of established protocols, and in collaboration with obstetric providers. Client teaching, which is an integral component of this role, involves preparing the woman for the procedure, interpreting the findings, and providing psychosocial support when needed.
KEY POINTS
• A high risk pregnancy is one in which the life or well-being of the mother or infant is jeopardized by a biophysical or psychosocial disorder coincidental with or unique to pregnancy.
• Biophysical, sociodemographic, psychosocial, and environmental factors place the pregnancy and fetus or neonate at risk.
• Biophysical assessment techniques include DFMCs, ultrasonography, and MRI.
• Biochemical monitoring techniques include amniocentesis, PUBS, CVS, MSAFP, and multiple marker screens.
• Reactive NSTs and negative CSTs suggest fetal well-being.
• Most assessment tests have some degree of risk for the mother and fetus and usually cause some anxiety for the woman and her family.
• The nurse’s role in assessment of the high risk pregnancy is primarily that of educator and support person.
![]() Audio Chapter Summaries Access an audio summary of the Key Points on
Audio Chapter Summaries Access an audio summary of the Key Points on ![]()