Chapter 6 Development During Weeks Four to Eight
All major external and internal structures are established during the fourth to eighth weeks. By the end of this organogenetic period, all of the main organ systems have begun to develop. Exposure of embryos to teratogens (e.g., drugs) during this period may cause major congenital anomalies (see Chapter 19). As the tissues and organs form, the shape of the embryo changes so that, by the eighth week, the embryo has a distinctly human appearance.
Folding of Embryo
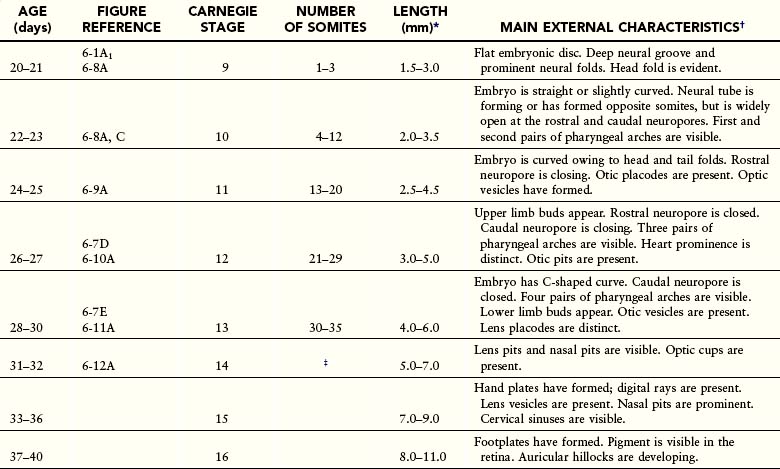
A significant event in the establishment of body form is folding of the flat trilaminar embryonic disc into a somewhat cylindric embryo (Fig. 6-1). Folding results from rapid growth of the embryo, particularly the brain and the spinal cord. Folding at the cranial and caudal ends and at the sides of the embryo occurs simultaneously. Concurrently, a relative constriction occurs at the junction of the embryo and the umbilical vesicle. Head and tail folds cause the cranial and caudal regions to move ventrally as the embryo elongates (see Fig. 6-1A2 to D2).
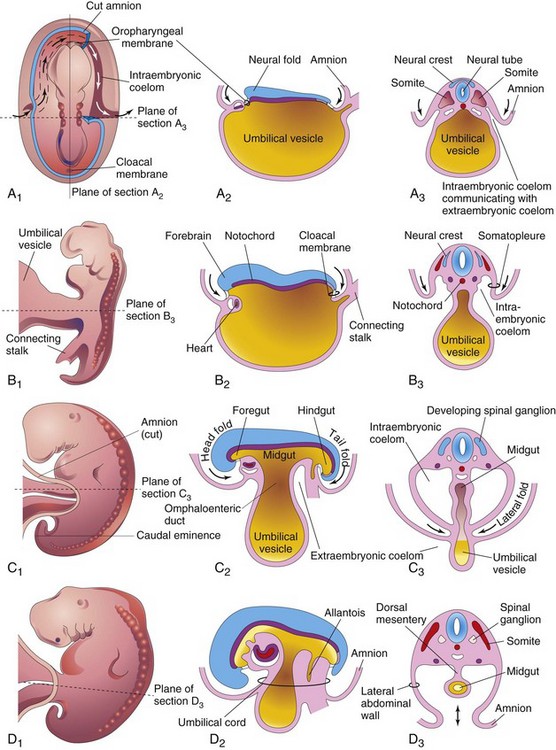
Figure 6–1 Folding of embryos during the fourth week. A1, Dorsal view of an embryo early in the fourth week. Three pairs of somites are visible. The continuity of the intraembryonic coelom and the extraembryonic coelom is shown on the right side by removal of a part of the embryonic ectoderm and mesoderm. B1, C1, and D1, Lateral views of embryos at 22, 26, and 28 days, respectively. A2, B2, C2, and D2, Sagittal sections at the plane shown in A1. A3, B3, C3, and D3, Transverse sections at the levels indicated in A1 to D1.
Head and Tail Folds
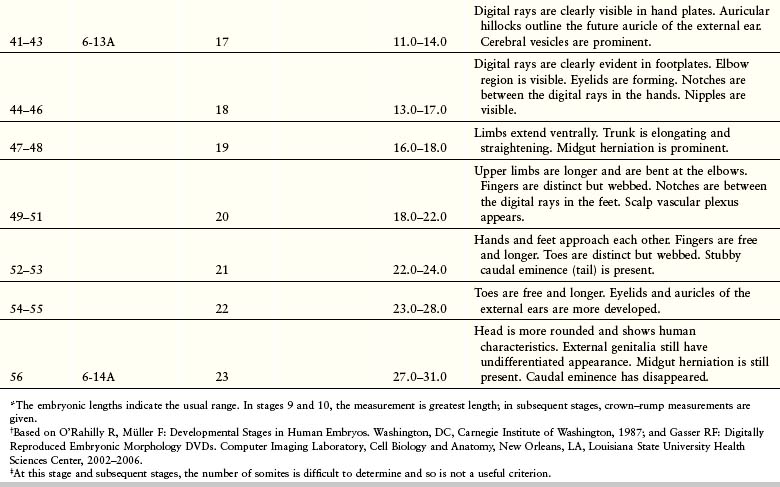
By the beginning of the fourth week, the neural folds in the cranial region form the primordium of the brain. Later, the developing forebrain grows cranially beyond the oropharyngeal membrane and overhangs the developing heart. Concomitantly, the primordial heart and the oropharyngeal membrane move onto the ventral surface of the embryo (Fig. 6-2). During lateral (longitudinal) folding, part of the endoderm of the umbilical vesicle is incorporated into the embryo as the foregut (Fig. 6-2C). The foregut lies between the brain and the heart, and the oropharyngeal membrane separates the foregut from the stomodeum (primordial mouth).
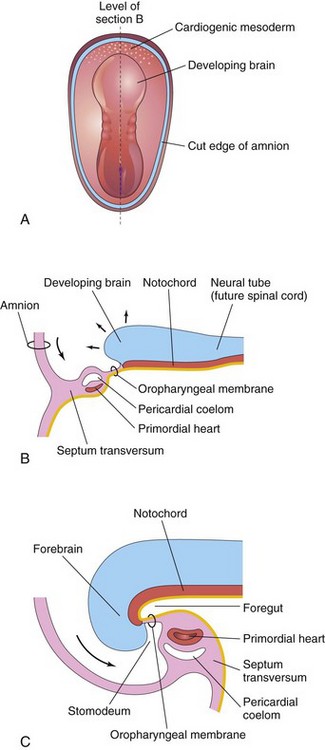
Figure 6–2 Folding of cranial end of the embryo. A, Dorsal view of an embryo at 21 days. B, Sagittal section of the cranial part of the embryo at the plane in A, showing the ventral movement of the heart. C, Sagittal section of an embryo at 26 days. Note that the septum transversum, heart, pericardial coelom, and oropharyngeal membrane have moved to the ventral surface of the embryo. Observe also that part of the umbilical vesicle is incorporated into the embryo as the foregut.
Folding of the caudal end of the embryo results primarily from growth of the distal part of the neural tube, the primordium of the spinal cord. As the embryo grows, the tail region projects over the cloacal membrane (future site of the anus) (Fig. 6-3B). During folding, part of the endodermal germ layer is incorporated into the embryo as the hindgut (see Fig. 6-3C). The terminal part of the hindgut soon dilates to form the cloaca (see Fig. 6-3B and C). The connecting stalk (primordium of the umbilical cord) is now attached to the ventral surface of the embryo, and the allantois—an endodermal diverticulum of the umbilical vesicle—is partially incorporated into the embryo (Fig. 6-1D2 and 6-3C).
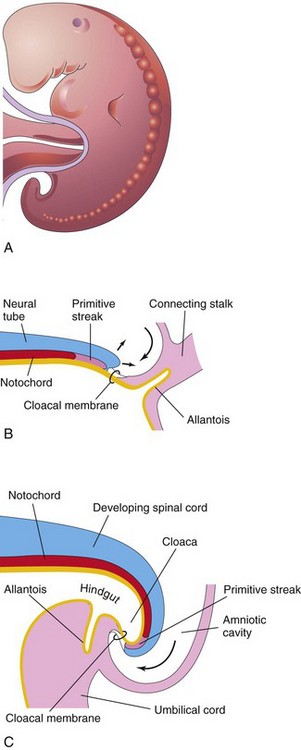
Figure 6–3 Folding of caudal end of the embryo. A, Lateral view of a 4-week embryo. B, Sagittal section of the caudal part of the embryo at the beginning of the fourth week. C, Similar section at the end of the fourth week. Note that part of the umbilical vesicle is incorporated into the embryo as the hindgut and that the terminal part of the hindgut has dilated to form the cloaca. Observe also the change in position of the primitive streak, allantois, cloacal membrane, and connecting stalk.
Lateral Folds
Folding of the sides of the embryo is thought to result from growth of the somites, which produces right and left lateral folds (Fig. 6-1A3 to D3). The lateral body wall folds toward the median plane, rolling the edges of the embryonic disc ventrally and forming a roughly cylindric embryo. As the abdominal walls form by fusion of the lateral folds, part of the endoderm germ layer is incorporated into the embryo as the midgut. Initially, there is a wide connection between the midgut and the umbilical vesicle (Fig. 6-1A2). After lateral folding, the connection is reduced to an omphaloenteric duct, formerly called the yolk stalk (Fig. 6-1C2). As the umbilical cord forms from the connecting stalk, ventral fusion of the lateral folds reduces the region of communication between the intraembryonic and extraembryonic coelomic cavities (Fig. 6-1C2). As the amniotic cavity expands and obliterates most of the extraembryonic coelom, the amnion forms the epithelial covering of the umbilical cord (Fig. 6-1D2).
Germ Layer Derivatives
The three germ layers (ectoderm, mesoderm, and endoderm) formed during gastrulation give rise to the primordia of all tissues and organs (Fig. 6-4). The cells of each germ layer divide, migrate, aggregate, and differentiate in rather precise patterns as they form the various organ systems (organogenesis).
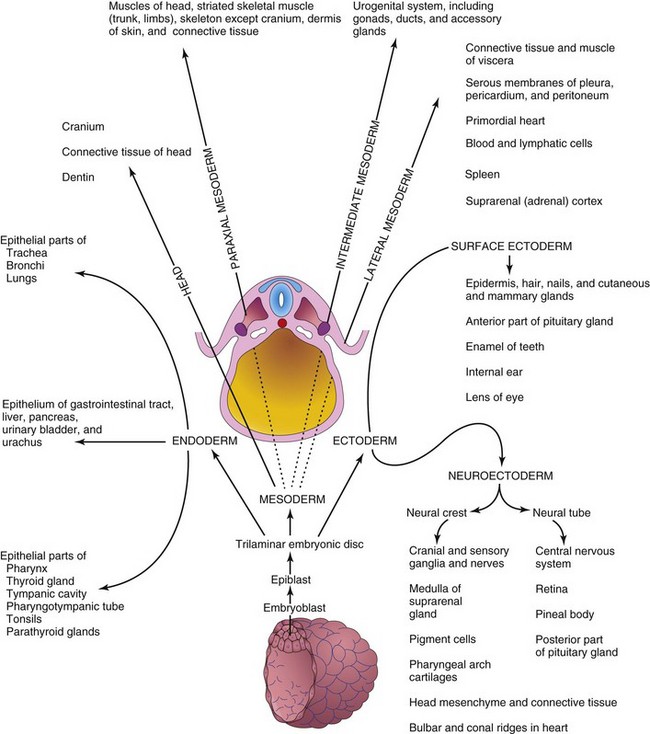
Figure 6–4 Illustration of derivatives of the three germ layers: ectoderm, endoderm, and mesoderm. Cells from these layers contribute to the formation of different tissues and organs; for example, the endoderm forms the epithelial lining of the gastrointestinal tract and the mesoderm gives rise to connective tissues and muscles.
Control of Embryonic Development
Embryonic development results from genetic plans in the chromosomes. Knowledge of the genes that control human development is increasing exponentially. Most developmental processes depend on a precisely coordinated interaction of genetic and environmental factors. Several control mechanisms guide differentiation and ensure synchronized development, such as tissue interactions, regulated migration of cells and cell colonies, controlled proliferation, and apoptosis (programmed cell death). Each system of the body has its own developmental pattern, and most processes of morphogenesis are regulated by complex molecular mechanisms.
Embryonic development is essentially a process of growth and increasing complexity of structure and function. Growth is achieved by mitosis, together with the production of extracellular matrices, whereas complexity is achieved through morphogenesis and differentiation. The cells that make up the tissues of very early embryos are pluripotential; that is, depending on the circumstances, they are able to follow more than one pathway of development. This broad developmental potential becomes progressively restricted as tissues acquire the specialized features necessary for increased sophistication of structure and function. Such restriction presumes that choices must be made to achieve tissue diversification. Most evidence indicates that these choices are determined not as a consequence of cell lineage, but rather in response to cues from the immediate surroundings, including the adjacent tissues. As a result, the architectural precision and coordination that are often required for the normal function of an organ appear to be achieved by the interaction of its constituent parts during development.
The interaction of tissues during development is a recurring theme in embryology. The interactions that lead to a change in the course of development of at least one of the interactants are called inductions. Numerous examples of such inductive interactions can be found in the literature; for example, during the development of the eye, the optic vesicle induces the development of the lens from the surface ectoderm of the head. When the optic vesicle is absent, the eye does not develop. Moreover, if the optic vesicle is removed and placed in association with surface ectoderm that is not usually involved in eye development, lens formation can be induced. Clearly then, the development of a lens depends on the ectoderm acquiring an association with a second tissue. In the presence of the neuroectoderm of the optic vesicle, the surface ectoderm of the head follows a pathway of development that it would not otherwise have taken. Similarly, many of the morphogenetic tissue movements that play such important roles in shaping the embryo also provide for the changing tissue associations that are fundamental to inductive tissue interactions.
The fact that one tissue can influence the developmental pathway adopted by another tissue presumes that a signal passes between the two interactants. Analysis of the molecular defects in mutant strains that show abnormal tissue interactions during embryonic development and studies of the development of embryos with targeted gene mutations have begun to reveal the molecular mechanisms of induction. The mechanism of signal transfer appears to vary with the specific tissues involved. In some cases, the signal appears to take the form of a diffusible molecule that passes from the inductor to the reacting tissue. In other instances, the message appears to be mediated through a nondiffusible extracellular matrix that is secreted by the inductor and that comes into contact with the reacting tissue. In still other cases, the signal appears to require physical contact between the inducing tissue and the responding tissue. Regardless of the mechanism of intercellular transfer involved, the signal is translated into an intracellular message that influences the genetic activity of the responding cells.
To be competent to respond to an inducing stimulus, the cells of the reacting system must express the appropriate receptor for the specific inducing signal molecule, the components of the particular intracellular signal transduction pathway, and the transcription factors that mediate the particular response. Experimental evidence suggests that the acquisition of competence by the responding tissue is often dependent on its previous interactions with other tissues. For example, the lens-forming response of the head ectoderm to the stimulus provided by the optic vesicle appears to be dependent on a previous association of the head ectoderm with the anterior neural plate (see Chapter 20).
Estimation of Embryonic Age
Estimates of the age of recovered embryos (e.g., after spontaneous abortion) are determined from their external characteristics and measurements of their length (see Table 6-1). Size alone may be an unreliable criterion because some embryos undergo a progressively slower rate of growth before death. The appearance of the developing limbs is a very helpful criterion for estimating embryonic age. Because embryos are straight in the third and early fourth weeks (Fig. 6-5A), their measurements indicate the greatest length. The sitting height, or crown–rump length, is used to estimate the age of older embryos (see Fig. 6-5B and C). Standing height, or crown–heel length, is sometimes measured in 8-week embryos (see Fig. 6-5D). The Carnegie Embryonic Staging System is used internationally (see Table 6-1) for comparison.
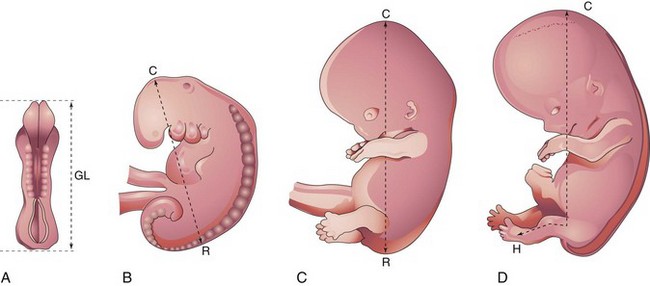
Figure 6–5 Methods used to measure the length of embryos. A, Greatest length (GL). B and C, Crown–rump length (CRL). D, Crown–heel length (CHL).
Ultrasonographic Examination of Embryos
Most women seeking obstetrical care have at least one ultrasonographic examination during their pregnancy for one or more of the following reasons:
• Estimation of gestational age for confirmation of clinical dating
• Evaluation of embryonic growth when intrauterine growth restriction (IUGR) is suspected
• Guidance during chorionic villus or amniotic fluid sampling
The size of an embryo in a pregnant woman can be estimated using ultrasonographic measurements. Transvaginal or endovaginal ultrasonography permits accurate measurement of crown–rump length in early pregnancy (Fig. 6-6).
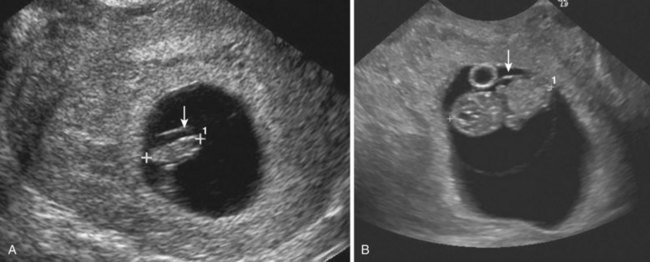
Figure 6–6 Endovaginal scan of embryos. A, Endovaginal scan of a 5-week embryo (CRL 10 mm, calipers) surrounded by the amniotic membrane (arrow). B, Coronal scan of a 7-week embryo (CRL 22 mm, calipers). Amnion seen anterior (arrow). Umbilical vesicle (yolk sac) anterior.
(Courtesy E. A. Lyons, M.D., Professor of Radiology and Obstetrics and Gynecology, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
Highlights of Development During Weeks Four to Eight
The criteria for estimating developmental stages in human embryos are listed in Table 6-1.
Fourth Week
In the fourth week the somites produce conspicuous surface elevations and the neural tube is open at the rostral and caudal neuropores (Figs. 6-7A and 6-8A). By 24 days, the pharyngeal arches have appeared (Fig. 6-7A to C). The embryo now develops a curved shape because of the head and tail folds. The early heart produces a large ventral prominence and pumps blood (Figs. 6-9 and 6-10). The rostral neuropore is closed at 26 days. The forebrain produces a prominent elevation of the head and the long, curved caudal eminence (tail-like structure) is present. Upper limb buds are recognizable by day 26 or 27 as small swellings on the ventrolateral body walls (Fig. 6-11A and B). The otic pits, the primordia of the internal ears, are also visible. Ectodermal thickenings, called lens placodes, indicating the future lenses of the eyes, are visible on the sides of the head. The fourth pair of pharyngeal arches and the lower limb buds are visible by the end of the fourth week (see Fig. 6-7C). Rudiments of many organ systems, especially the cardiovascular system, are established.
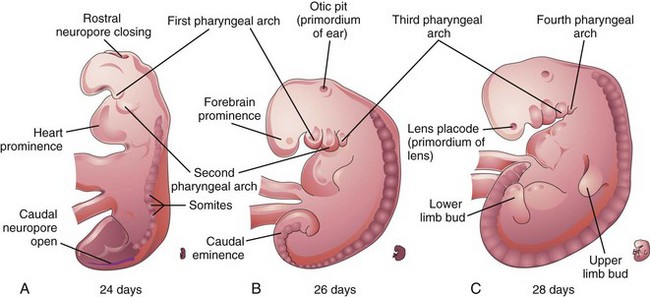
Figure 6–7 A, B, and C, Lateral views of older embryos, showing 16, 27, and 33 somites, respectively. The rostral neuropore is normally closed by 25 to 26 days, and the caudal neuropore is usually closed by the end of the fourth week.
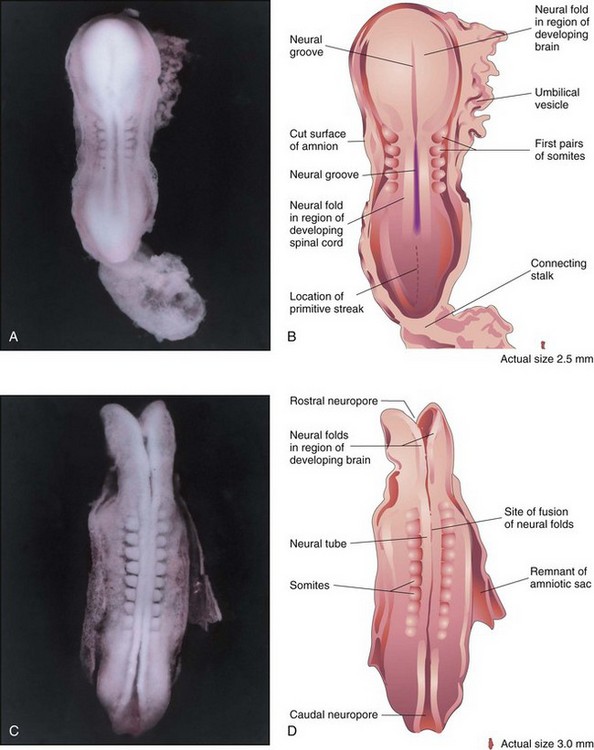
Figure 6–8 A, Dorsal view of a five-somite embryo at Carnegie stage 10, approximately 22 days, showing the neural folds and the neural groove. The neural folds in the cranial region have thickened to form the primordium of the brain. B, Illustration of the structures shown in A. Most of the amniotic and chorionic sacs have been cut away to expose the embryo. C, Dorsal view of a 10-somite embryo at Carnegie stage 10, approximately 23 days. The neural folds have fused opposite the somites to form the neural tube (primordium of the spinal cord in this region). The neural tube is in open communication with the amniotic cavity at the cranial and caudal ends through the rostral and caudal neuropores, respectively. D, Diagram of the structures shown in C.
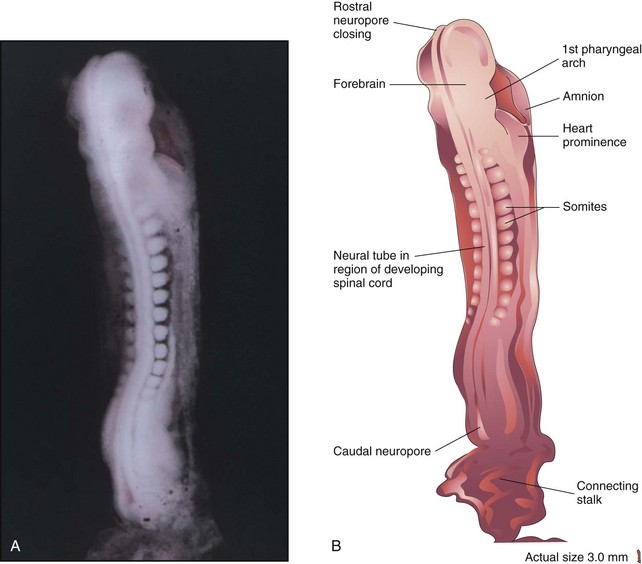
Figure 6–9 A, Dorsal view of a 13-somite embryo at Carnegie stage 11, approximately 24 days. The rostral neuropore is closing, but the caudal neuropore is wide open. B, Illustration of the structures shown in A. The embryo is curved because of folding of the cranial and caudal ends.
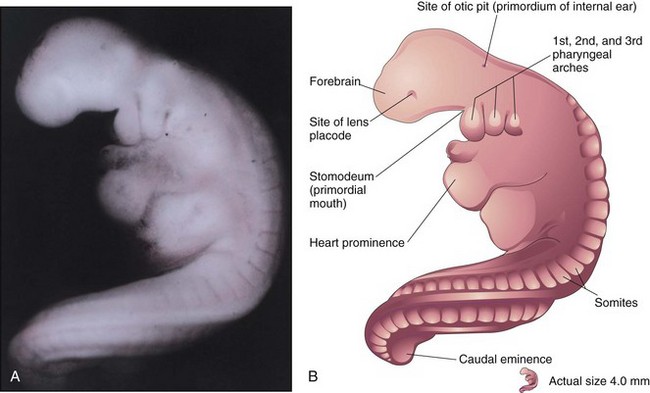
Figure 6–10 A, Lateral view of a 27-somite embryo at Carnegie stage 12, approximately 26 days. The embryo is very curved, especially its long, tail-like caudal eminence. The lens placode is the primordium of the lens of the eye. The otic pit indicates early development of the internal ear. B, Illustration of the structures shown in A. The rostral neuropore is closed, and three pairs of pharyngeal arches are present.
(A, From Nishimura H, Semba H, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
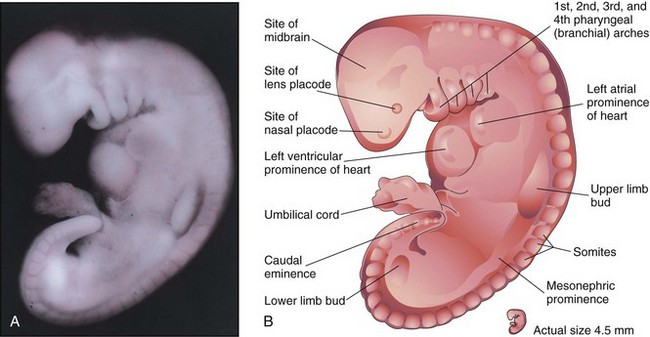
Figure 6–11 A, Lateral view of an embryo at Carnegie stage 13, approximately 28 days. The primordial heart is large and is divided into a primordial atrium and a ventricle. The rostral and caudal neuropores are closed. B, Illustration of the structures shown in A. The embryo has a characteristic C-shaped curvature, four pharyngeal arches, and upper and lower limb buds.
(A, From Nishimura H, Semba H, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
Fifth Week
Changes in body form are minor during the fifth week compared with those that occurred during the fourth week. Growth of the head exceeds that of other regions (Fig. 6-12A and B), which is caused mainly by the rapid development of the brain and facial prominences. The face soon contacts the heart prominence. The mesonephric ridges indicate the site of the mesonephric kidneys. The mesonephric kidneys are the primordia of the permanent kidneys (see Fig. 6-12A and B).
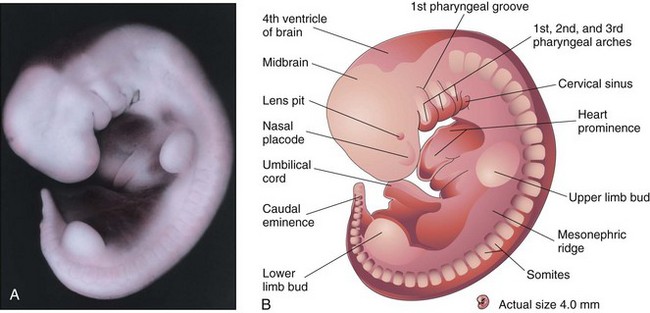
Figure 6–12 A, Lateral view of an embryo at Carnegie stage 14, approximately 32 days. The second pharyngeal arch has overgrown the third arch, forming a depression known as the cervical sinus. The mesonephric ridge indicates the site of the mesonephric kidney, an interim functional kidney. B, Illustration of the structures shown in A. The upper limb buds are paddle-shaped, whereas the lower limb buds are flipper-like.
(A, From Nishimura H, Semba H, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
Sixth Week
It has been reported that embryos in the sixth week show spontaneous movements, such as twitching of the trunk and limbs. Embryos at this stage show reflex responses to touch. The primordia of the digits—the digital rays—begin to develop (Fig. 6-13A and B). Development of the lower limbs occurs 4 to 5 days later than that of the upper limbs. Several small swellings—auricular hillocks—develop and contribute to the formation of the auricle, the shell-shaped part of the external ear. The eyes are now obvious largely because retinal pigment has formed. The head is much larger relative to the trunk and is bent over the large heart prominence. This head position results from bending in the cervical (neck) region. The trunk then begins to straighten. During the sixth week, the intestines enter the extraembryonic coelom in the proximal part of the umbilical cord. This umbilical herniation is a normal event in the embryo, occurring because the abdominal cavity is too small at this stage to accommodate the rapidly growing intestines.
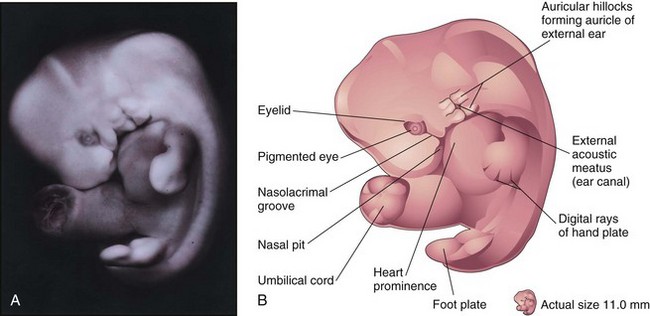
Figure 6–13 A, Lateral view of an embryo at Carnegie stage 17, approximately 42 days. Digital rays are visible in the hand plate, indicating the future site of the digits (fingers). B, Illustration of the structures shown in A. The eye, auricular hillocks, and external acoustic meatus are now clearly discernible.
Seventh Week
The limbs undergo considerable change during the seventh week. Notches appear between the digital rays in the hand plates, partially separating the future digits. Communication between the primordial gut and the umbilical vesicle is now reduced to a relatively slender duct, the omphaloenteric duct.
Eighth Week
At the beginning of this final week of the embryonic period, the digits of the hand are separated, but noticeably webbed. Notches are clearly visible between the digital rays of the feet. The scalp vascular plexus has appeared and forms a characteristic band around the head. At the end of the fetal period, the digits have lengthened and are separated (Fig. 6-14A and B). Coordinated limb movements first occur during this week. Primary ossification begins in the femur. All evidence of the tail-like caudal eminence has disappeared by the end of the eighth week. The hands and feet each approach each other ventrally. At the end of the eighth week, the embryo has visually distinct human characteristics; however, the head is still disproportionately large, constituting almost half of the embryo. The neck region is established. The eyelids are closing, and by the end of the eighth week, they begin to unite by epithelial fusion. The intestines are still in the proximal portion of the umbilical cord. The auricles of the external ears begin to assume their final shape, but are still low-set on the head. Although sex differences exist in the appearance of the external genitalia, they are not distinctive enough to permit accurate sex identification.
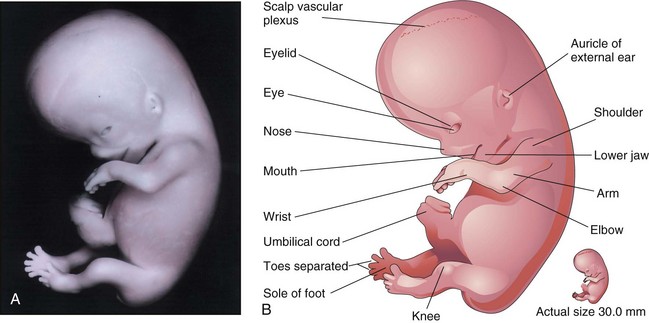
Figure 6–14 A, Lateral view of an embryo at Carnegie stage 23, approximately 56 days. B, Illustration of the structures shown in A.
(A, From Nishimura H, Semba H, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)