Chapter 19 Human Birth Defects
Congenital birth defects, developmental anomalies, and malformations are terms currently used to describe disorders that were established during intrauterine life. Birth defects are the leading cause of infant mortality and may be structural, functional, metabolic, behavioral, or hereditary. A birth defect is a structural abnormality of any type; however, not all variations are anomalies. There are four clinically significant types of birth defects: malformation, disruption, deformation, and dysplasia.
Teratology: Study of Abnormal Development
Teratology is the branch of science that studies the causes, mechanisms, and patterns of abnormal development. A fundamental concept in teratology is that certain stages of embryonic development are more vulnerable to disruption than others (see Fig. 19-11).
More than 20% of infant deaths in North America are attributable to birth defects. Major structural anomalies are observed in approximately 3% of newborn infants. Additional defects may only be detected after birth; thus, the incidence of birth defects approaches 6% in 2-year-old infants and 8% in 5-year-old children.
Birth defects may be caused by genetic factors, such as chromosomal abnormalities, as well as environmental factors, such as drugs. However, many common defects are the result of multifactorial inheritance; that is, they are caused by genetic and environmental factors acting together. For 50% to 60% of defects, the causes are unknown (Fig. 19-1). Birth defects may be single or multiple and of major or minor clinical significance.
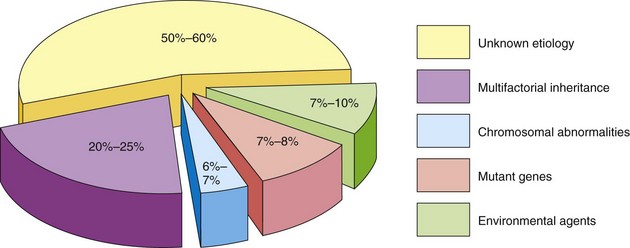
Figure 19–1 Causes of birth defects. Note that the causes of most defects are of unknown etiology and that 20% to 25% of them are caused by a combination of genetic and environmental factors (multifactorial inheritance).
Minor defects are present in approximately 14% of neonates. Anomalies of the external ear, for example, are of no serious medical significance, but they indicate the possible presence of associated major anomalies. Major defects are much more common in early embryos (10%-15%), but most of them abort spontaneously during the first 6 weeks. Chromosomal abnormalities are present in more than 50% to 60% of spontaneously aborted embryos.
Birth Defects Caused by Genetic Factors*
In terms of the sheer number of cases, genetic factors are the most important cause of birth defects. It has been estimated that they cause approximately one third of all defects (see Fig. 19-1). Any mechanism as complex as mitosis or meiosis may occasionally malfunction; thus, chromosomal aberrations are common and are present in 6% to 7% of zygotes. Many of these early embryos never undergo normal cleavage to become blastocysts. The changes may affect the sex chromosomes, the autosomes, or both. Persons with chromosomal abnormalities usually have characteristic phenotypes, such as the physical characteristics of infants with Down syndrome.
Numerical Chromosomal Abnormalities
Numerical aberrations of chromosomes usually result from nondisjunction, an error in cell division in which a chromosome pair or two chromatids of a chromosome do not disjoin during mitosis or meiosis. As a result, the chromosome pair or chromatids pass to one daughter cell, while the other cell receives neither (Fig. 19-2). Nondisjunction may occur during maternal or paternal gametogenesis (see Chapter 2). The chromosomes in somatic (body) cells are normally paired; the homologous chromosomes making up a pair are homologs. Normal human females have 22 pairs of autosomes plus two X chromosomes, whereas normal males have 22 pairs of autosomes plus one X and one Y chromosome.
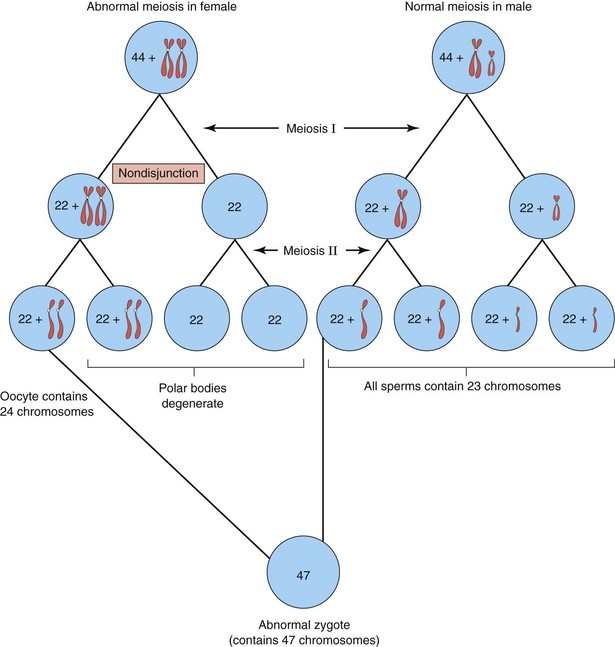
Figure 19–2 Nondisjunction of chromosomes during the first meiotic division of a primary oocyte, resulting in an abnormal oocyte with 24 chromosomes. Subsequent fertilization by a normal sperm produces a zygote with 47 chromosomes—aneuploidy—a deviation from the human diploid number of 46.
Inactivation of Genes
During embryogenesis, one of the two X chromosomes in female somatic cells is randomly inactivated and appears as a mass of sex chromatin. Inactivation of the genes on one X chromosome in the somatic cells of female embryos occurs during implantation.
X-inactivation is important clinically because it means that each cell from a carrier of an X-linked disease has the mutant gene causing the disease, either on the active X chromosome or on the inactivated X chromosome that is represented by sex chromatin. Uneven X-inactivation in monozygotic twins is one reason given for discordance in a variety of birth defects. The genetic basis for discordance is that one twin preferentially expresses the paternal X and the other, the maternal X.
Turner Syndrome
Approximately 1% of female embryos with monosomy X survive (chromosome count 45 and only one X chromosome). The incidence of 45, X—or Turner syndrome—in newborn females is approximately 1 in 8000 live births. Half of the affected individuals have 45, X; the other half have a variety of abnormalities affecting a sex chromosome. The phenotype of Turner syndrome is female (Figure 19-3). Phenotype refers to the morphologic characteristics of an individual, as determined by the genotype and environment in which it is expressed. Secondary sexual characteristics do not develop in 90% of girls with Turner syndrome, necessitating hormone replacement therapy.
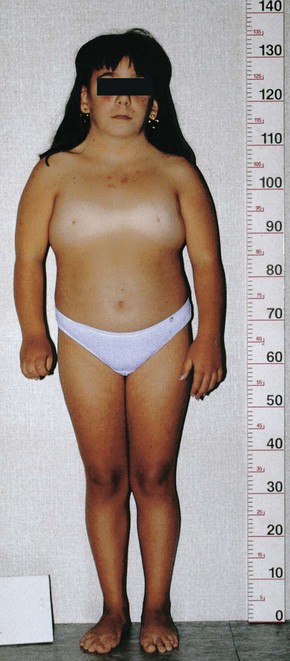
Figure 19–3 Turner syndrome in a 14-year-old girl. Note the classic features of the syndrome: short stature; webbed neck; absence of sexual maturation; broad shield-like chest with widely spaced nipples; and lymphedema of the hands and feet.
(Courtesy of Dr. F. Antoniazzi and Dr. V. Fanos, Department of Pediatrics, University of Verona, Verona, Italy.)
The monosomy X chromosomal abnormality is the most common cytogenetic abnormality observed in live-born infants and fetuses that abort spontaneously; it accounts for approximately 18% of all spontaneous abortions caused by chromosomal abnormalities. In approximately 75% of cases it is the paternal X chromosome that is usually missing.
Aneuploidy and Polyploidy
Changes in chromosome number result in either aneuploidy or polyploidy. Aneuploidy is any deviation from the human diploid number of 46 chromosomes. An aneuploid is an individual or a cell that has a chromosome number that is not an exact multiple of the haploid number of 23 (e.g., 45 or 47). The principal cause of aneuploidy is nondisjunction during cell division (Fig. 19-2), resulting in an unequal distribution of one pair of homologous chromosomes to the daughter cells. One cell has two chromosomes, and the other has neither chromosome of the pair. As a result, the embryo’s cells may be hypodiploid (45, X, or Turner syndrome) (Fig. 19-3) or hyperdiploid (usually 47, as in trisomy 21 or Down syndrome) (Fig. 19-4). Embryos with monosomy—missing a chromosome—usually die. Monosomy of an autosome is extremely uncommon, and approximately 99% of embryos lacking a sex chromosome (45, X) abort spontaneously.
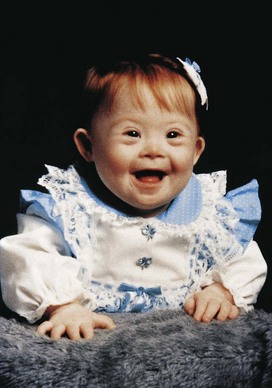
Figure 19–4 A child with Down syndrome (trisomy 21). Note the round face, up-slanted palpebral fissures, and short digits with in-curving of the fifth digit (clinodactyly).
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Trisomy
If three chromosomes of one type are present instead of the usual pair, the abnormality is known as trisomy. Trisomies are the most common abnormalities of chromosome number. The usual cause of this numeric error is meiotic nondisjunction of chromosomes (Fig. 19-2), resulting in a gamete with 24 instead of 23 chromosomes and, subsequently, a zygote with 47 chromosomes.
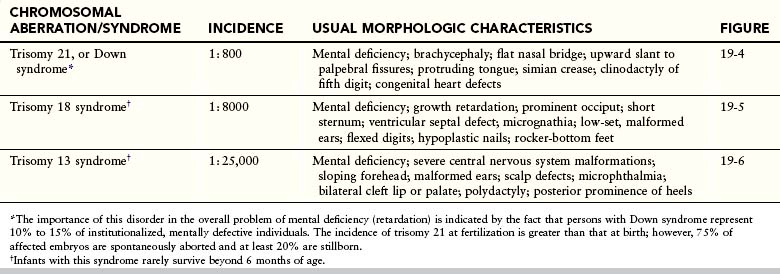
Trisomy of the autosomes is associated mainly with three syndromes (Table 19-1):
• Trisomy 21, or Down syndrome (Fig. 19-4)
• Trisomy 18, or Edwards syndrome (Fig. 19-5)
• Trisomy 13, or Patau syndrome (Fig. 19-6)
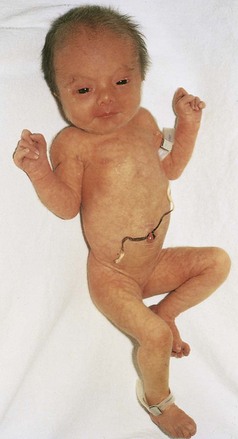
Figure 19–5 Female neonate with trisomy 18. Note the growth retardation, clenched fists with characteristic positioning of the fingers (second and fifth digits overlapping the third and fourth), short sternum, and narrow pelvis.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
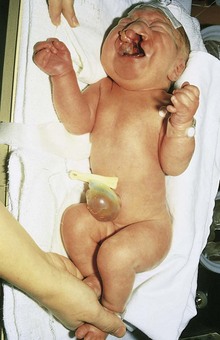
Figure 19–6 Female neonate with trisomy 13. Note the bilateral cleft lip, low-set, malformed ears, and polydactyly (extra digits). A small omphalocele (herniation of viscera into the umbilical cord) is also present.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Infants with trisomy 13 and trisomy 18 are severely malformed and mentally challenged. They usually die early in infancy. More than 50% of trisomic embryos spontaneously abort early. Trisomy of the autosomes occurs with increasing frequency as maternal age increases (Table 19-2).
Table 19–2 Incidence of Down Syndrome in Newborn Infants
| MATERNAL AGE (YEARS) | INCIDENCE |
|---|---|
| 20-24 | 1 : 1400 |
| 25-29 | 1 : 1100 |
| 30-34 | 1 : 700 |
| 35 | 1 : 350 |
| 37 | 1 : 225 |
| 39 | 1 : 140 |
| 41 | 1 : 85 |
| 43 | 1 : 50 |
| 45+ | 1 : 25 |
Mosaicism—two or more cell types containing different numbers of chromosomes (normal and abnormal)—leads to a less severe phenotype and the affected child may have a nearly normal IQ.
Mosaicism
A person who has at least two cell lines with two or more different genotypes (genetic constitutions) is known as a mosaic. Either the autosomes or sex chromosomes may be involved. Usually, the birth defects are less serious than in persons with monosomy or trisomy (e.g., the features of the Turner syndrome are not as evident in 45 X/46, XX mosaic females as in the usual 45, X females). Mosaicism usually results from nondisjunction during early cleavage of the zygote (see Chapter 3). Mosaicism resulting from loss of a chromosome by anaphase lagging also occurs; the chromosomes separate normally, but one of them is delayed in its migration and is eventually lost.
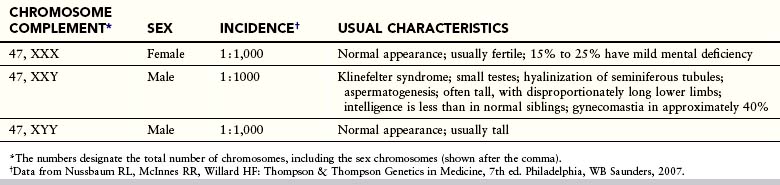
Trisomy of the sex chromosomes is a common condition (Table 19-3); however, because no characteristic physical findings are seen in infants or children, this defect is not usually detected before puberty (Fig. 19-7). The diagnosis is best established by chromosomal and molecular analysis.
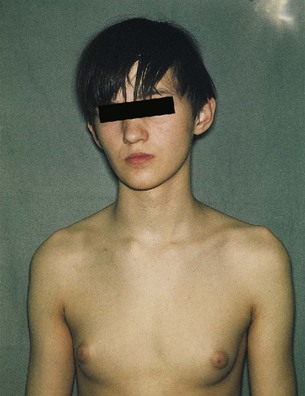
Figure 19–7 A teenage boy with Klinefelter syndrome (XXY trisomy). Note the presence of developed breasts; approximately 40% of males with this syndrome have gynecomastia (excessive development of the male mammary glands) and small testes.
(Courtesy of Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Triploidy
The most common type of polyploidy is triploidy (69 chromosomes). Triploid fetuses have severe intrauterine growth restriction (IUGR), with a disproportionately small trunk as well as other anomalies. Triploidy can result if the second polar body does not separate from the oocyte during the second meiotic division (see Chapter 2); more likely however, triploidy results when an oocyte is fertilized by two sperms (dispermy) almost simultaneously. Triploidy occurs in approximately 2% of embryos but most of them abort spontaneously. Triploid fetuses account for approximately 20% of chromosomally abnormal miscarriages.
Tetraploidy
Doubling the diploid chromosome number to 92 (tetraploidy) probably occurs during the first cleavage division. Division of this abnormal zygote would subsequently result in an embryo with cells containing 92 chromosomes. Tetraploid embryos abort very early; often, all that is recovered is an empty chorionic sac.
Structural Chromosomal Abnormalities
Most abnormalities of chromosome structure result from chromosome breakage followed by reconstitution in an abnormal combination (Fig. 19-8). Chromosome breaks may be induced by various environmental factors, such as irradiation, drugs, chemicals, and viruses. The resulting abnormality in chromosome structure depends on what happens to the broken pieces. The only two aberrations of chromosome structure that are likely to be transmitted from parent to child are structural rearrangements, such as inversion and translocation.
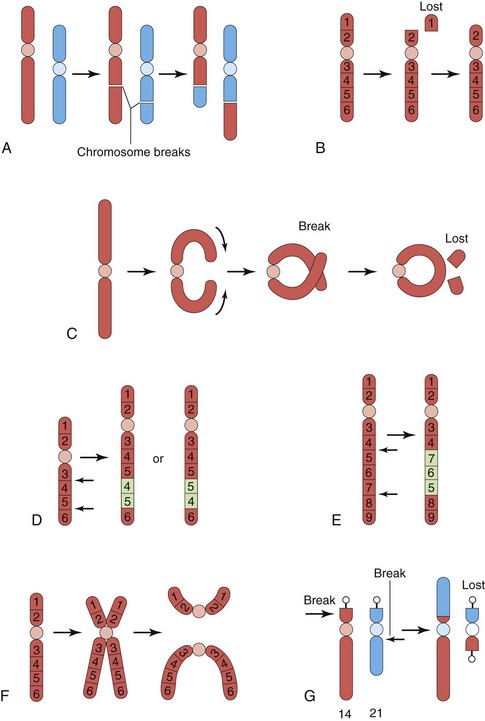
Figure 19–8 Various structural abnormalities of chromosomes. A, Reciprocal translocation. B, Terminal deletion. C, Ring chromosome. D, Duplication. E, Paracentric inversion. F, Isochromosome. G, Robertsonian translocation. Arrows indicate how the structural abnormalities are produced.
Translocation
Translocation is the transfer of a piece of one chromosome to a nonhomologous chromosome. If two nonhomologous chromosomes exchange pieces, it is called a reciprocal translocation (Fig. 19-8A and G). Translocation does not necessarily cause abnormal development. Persons with a translocation between chromosome 21 and chromosome 14, for example (Fig. 19-8G), are phenotypically normal. Such persons are called balanced translocation carriers. They have a tendency, independent of age, to produce germ cells with an abnormal translocation chromosome. Between 3% and 4% of persons with Down syndrome have translocation trisomies; that is, the extra chromosome 21 is attached to another chromosome.
Deletion
When a chromosome breaks, a portion of it may be lost (Fig. 19-8B). A partial terminal deletion from the short arm of chromosome 5 causes cri du chat syndrome. Affected infants have a weak, catlike cry at birth; growth delay with microcephaly (abnormally small head); hypertelorism (wide-set eyes); low-set ears; micrognathia (a small jaw); are severely mentally challenged (retardation); and have congenital heart disease.
A ring chromosome is a type of deletion chromosome from which both ends have been lost and the broken ends have rejoined to form a ring-shaped chromosome (Fig. 19-8C). Ring chromosomes are very rare, but they have been found for all chromosomes. These abnormal chromosomes have been described in persons with Turner syndrome, trisomy 18, and other abnormalities.
Duplications
Duplications may be manifested as a duplicated part of a chromosome located within a chromosome (Fig. 19-8D), attached to a chromosome, or as a separate fragment. Duplications are more common than deletion, and they are less harmful because no loss of genetic material occurs. Duplication may involve part of a gene, a whole gene, or a series of genes.
Inversion
Inversion is a chromosomal aberration in which a segment of a chromosome is reversed. Paracentric inversion is confined to a single arm of the chromosome (Fig. 19-8E), whereas pericentric inversion involves both arms and includes the centromere. Carriers of pericentric inversions are at risk for having offspring with birth defects because of unequal crossing over and malsegregation at meiosis.
Isochromosomes
The abnormality resulting in isochromosomes occurs when the centromere divides transversely instead of longitudinally (Fig. 19-8F). An isochromosome is a chromosome in which one arm is missing and the other is duplicated. It appears to be the most common structural abnormality of the X chromosome. Persons with this chromosomal abnormality are often short in stature and have other stigmata of the Turner syndrome. These characteristics are related to the loss of an arm of an X chromosome.
Birth Defects Caused by Mutant Genes
Between 7% and 8% of birth defects are caused by gene defects (Fig. 19-1). A mutation usually involves a loss or a change in the function of a gene, and is any permanent, heritable change in the sequence of genomic DNA. Because a random change is unlikely to lead to an improvement in development, most mutations are deleterious and some are lethal. The mutation rate can be increased by a number of environmental agents, such as large doses of radiation. Birth defects resulting from gene mutations are inherited according to Mendelian laws; consequently, predictions can be made about the probability of their occurrence in the affected person’s children and other relatives. An example of a dominantly inherited birth defect is achondroplasia—abnormality in conversion of cartilage to bone—(Fig. 19-9), which results from a mutation of the complementary DNA in the fibroblast growth factor receptor 3 (FGFR–3) gene on chromosome 4p. Other birth defects are attributable to autosomal recessive inheritance. Autosomal recessive genes manifest themselves only when homozygous; as a consequence, many carriers of these genes (heterozygous persons) are not identified.
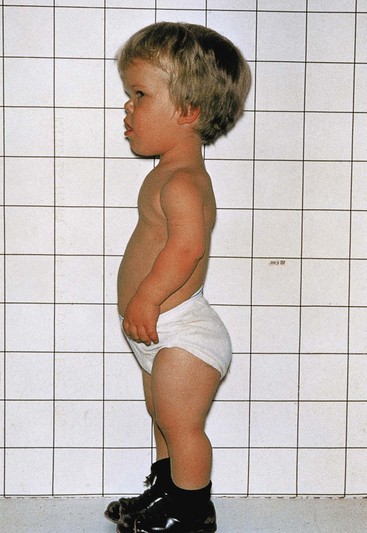
Figure 19–9 A young boy with achondroplasia. Note the short stature, short limbs and fingers, normal length of the trunk, relatively large head, prominent forehead, and depressed nasal bridge.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Fragile X syndrome is the most common inherited cause of moderate mental disorders (Fig. 19-10). Fragile X syndrome has a frequency of 1 in 1500 male births and may account for much of the predominance of males in the mentally challenged population.

Figure 19–10 Fragile X syndrome. A, An 8-year-old, mentally deficient boy with this syndrome exhibiting a relatively normal appearance, with a long face and prominent ears. B, His 6-year-old sister also has this syndrome. She has a mild learning disability and similar features of long face and prominent ears. Note the strabismus (crossed right eye).
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Several genetic disorders have been linked to the expansion of trinucleotides in specific genes. Examples include myotonic dystrophy, Huntington chorea, spinobulbar atrophy (Kennedy disease), and Friedreich ataxia. X-linked recessive genes are usually manifested in affected (homozygous) males and occasionally in carrier (heterozygous) females (e.g., fragile X syndrome).
The human genome comprises an estimated 20,000 to 25,000 genes per haploid set, or 3 billion base pairs. Because of the Human Genome Project and international research collaboration, many disease- and birth defect–causing mutations in genes have been and will continue to be identified. Most genes will be sequenced and their specific function determined. Understanding the cause of birth defects will require an improvement in our understanding of gene expression during early development.
Most genes that are expressed in a cell are expressed in a wide variety of cells. These housekeeping genes are involved in basic cellular metabolic functions, such as nucleic acid and protein synthesis, cytoskeleton and organelle biogenesis, and nutrient transport and mechanisms. The specialty genes are expressed at specific times in specific cells and define the hundreds of different cell types that make up the human organism. An essential aspect of developmental biology is the regulation of gene expression. Regulation is often achieved by transcription factors, which bind to regulatory or promoter elements of specific genes.
Genomic imprinting is an epigenetic process whereby the female and male germ lines confer a sex-specific mark on a chromosome subregion, so that only the paternal or the maternal allele of a gene is active in the offspring. In other words, the sex of the transmitting parent influences the expression or nonexpression of certain genes in the offspring.
Birth Defects Caused by Environmental Factors
Although the embryo is well protected in the uterus, certain environmental agents—teratogens—may cause developmental disruptions after maternal exposure to them (Table 19-4). A teratogen is any agent that can produce a birth defect or increase the incidence of a defect in a population. Environmental factors, such as infections and drugs, may simulate genetic conditions, such as when two or more children of normal parents are affected. The important principle to remember is that not everything that is familial is genetic.
Table 19–4 Some Teratogens Known to Cause Human Birth Defects
| AGENTS | MOST COMMON CONGENITAL ANOMALIES |
|---|---|
| Drugs | |
| Alcohol | Fetal alcohol syndrome (FAS); intrauterine growth restriction (IUGR); mental deficiency; microcephaly; ocular anomalies; joint abnormalities; short palpebral fissures; fetal alcohol spectrum disorders (FASDs); cognitive and neurobehavioral disturbances |
| Androgens and high doses of progestogens | Varying degree of masculinization of female fetuses; ambiguous external genitalia (labial fusion and clitoral hypertrophy) |
| Methotrexate | IUGR; skeletal and renal defects |
| Cocaine | IUGR; prematurity; microcephaly; cerebral infarction; urogenital anomalies; neurobehavioral disturbances |
| Diethylstilbestrol | Abnormalities of uterus and vagina; cervical erosion and ridges |
| Isotretinoin (13-cis-retinoic acid) | Craniofacial abnormalities; neural tube defects such as spina bifida cystica; cardiovascular defects; cleft palate; thymic aplasia |
| Lithium carbonate | Various anomalies, usually involving the heart and great vessels |
| Methotrexate | Multiple anomalies, especially skeletal, involving the face, cranium, limbs, and vertebral column |
| Misoprostol | Abnormal development of the limbs, ocular defects, cranial nerve defects, and autism spectral disorders |
| Phenytoin (Dilantin) | Fetal hydantoin syndrome; IUGR; microcephaly; mental retardation; ridged metopic suture; inner epicanthal folds; eyelid ptosis; broad, depressed nasal bridge; phalangeal hypoplasia |
| Tetracycline | Stained teeth; hypoplasia of enamel |
| Thalidomide | Abnormal development of the limbs; meromelia (partial absence of limb) and amelia (complete absence of limb); facial anomalies; systemic anomalies (e.g., cardiac and kidney defects and ocular anomalies) |
| Trimethadione | Developmental delay; V-shaped eyebrows; low-set ears; cleft lip and/or palate |
| Valproic acid | Craniofacial anomalies; neural tube defects; often hydrocephalus; heart and skeletal defects; poor postnatal cognitive development |
| Warfarin | Nasal hypoplasia; stippled epiphyses; hypoplastic phalanges; eye anomalies; mental deficiency |
| Chemicals | |
| Methylmercury | Cerebral atrophy; spasticity; seizures; mental deficiency |
| Polychlorinated biphenyls | IUGR; skin discoloration |
| Infections | |
| Cytomegalovirus | Microcephaly; chorioretinitis; sensorineural loss; delayed psychomotor and mental development; hepatosplenomegaly; hydrocephaly; cerebral palsy; brain (periventricular) calcification |
| Herpes simplex virus | Skin vesicles and scarring; chorioretinitis; hepatomegaly; thrombocytopenia; petechiae; hemolytic anemia; hydranencephaly |
| Human parvovirus B19 | Fetal anemia; nonimmune hydrops fetalis; fetal death |
| Rubella virus | IUGR; postnatal growth retardation; cardiac and great vessel abnormalities; microcephaly; sensorineural deafness; cataract; microphthalmos; glaucoma; pigmented retinopathy; mental deficiency; neonatal bleeding; hepatosplenomegaly; osteopathy; tooth defects |
| Toxoplasma gondii | Microcephaly; mental deficiency; microphthalmia; hydrocephaly; chorioretinitis; cerebral calcifications; hearing loss; neurologic disturbances |
| Treponema pallidum | Hydrocephalus; congenital deafness; mental deficiency; abnormal teeth and bones |
| Varicella virus | Cutaneous scars (dermatome distribution); neurologic anomalies (e.g., limb paresis, hydrocephaly, seizures); cataracts; microphthalmia; Horner syndrome; optic atrophy; nystagmus; chorioretinitis; microcephaly; mental deficiency; skeletal anomalies (e.g., hypoplasia of limbs, fingers, and toes); urogenital anomalies |
| High levels of ionizing radiation | Microcephaly; mental deficiency; skeletal anomalies; growth retardation; cataracts |
The organs and parts of an embryo are most sensitive to teratogenic agents during periods of rapid differentiation (Fig. 19-11). Because molecular signaling and embryonic induction precede morphologic differentiation, the period during which structures are sensitive to interference by teratogens often precedes the stage of their visible development. Teratogens do not appear to be effective in causing birth defects until cellular differentiation has begun; however, their earlier actions may cause the death of an embryo. The exact mechanisms by which many drugs, chemicals and other environmental factors disrupt embryonic development and induce abnormalities are unclear.
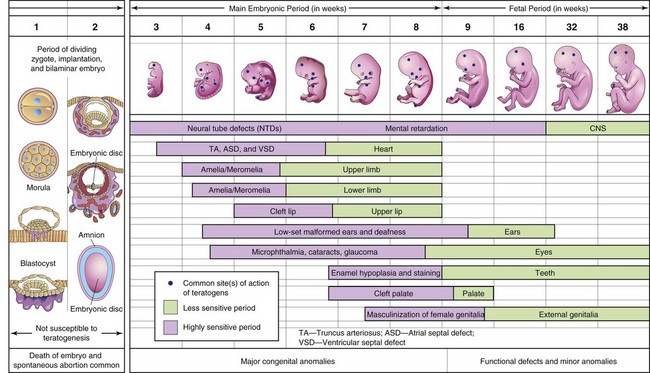
Figure 19–11 Critical periods in human prenatal development. During the first 2 weeks, the embryo is not usually susceptible to teratogens. At this point, a teratogen damages all or most of the cells, resulting in death of the embryo, or damages only a few cells, allowing the conceptus to recover and the embryo to develop without birth defects. The purple areas denote highly sensitive periods, when major defects may be produced (e.g., amelia, absence of limbs). The green sections indicate stages that are less sensitive to teratogens, when minor birth defects may be induced.
Rapid progress in molecular biology is providing additional information on the genetic control of differentiation, as well as the cascade of molecular signals and factors controlling gene expression and pattern formation. Researchers are now directing increasing attention to the molecular mechanisms of abnormal development in an attempt to understand better the pathogenesis of birth defects.
Principles of Teratogenesis
When considering the possible teratogenicity of an agent, such as a drug or a chemical, three factors are important to consider:
Critical Periods of Human Development
An embryo’s susceptibility to a teratogen depends on its stage of development when an agent, such as a drug, is present (Fig. 19-11). The most critical period in development is when cell differentiation and morphogenesis are at their peak. The most critical period for brain development is from 3 to 16 weeks, but its development may be disrupted after this time because the brain is differentiating and growing rapidly at birth.
Teratogens (e.g., drugs) may cause limitation of mental development during the embryonic and fetal periods. Tooth development continues long after birth hence, the development of the permanent teeth may be disrupted by tetracyclines from 18 weeks (prenatal) to 16 years.
The skeletal system has a prolonged critical period of development, extending into childhood; hence, the growth of skeletal tissues provides a good gauge of general growth. Environmental disturbances during the first 2 weeks after fertilization may interfere with cleavage of the zygote and implantation of the blastocyst, which may cause early death and spontaneous abortion of the embryo (Fig. 19-11).
Development of the embryo is most easily disrupted when the tissues and organs are forming (Fig. 19-11). During this organogenetic period, teratogenic agents may induce major birth defects. Physiologic defects—minor morphologic anomalies of the external ear, for example—and functional disturbances, such as limitation of mental development, are likely to result from disruption of development during the fetal period. Each part, tissue, and organ of an embryo has a critical period during which its development may be disrupted (Fig. 19-11). The type of birth defect produced depends on which parts, tissues, and organs are most susceptible at the time the teratogen is active.
Embryologic timetables, such as the one in Fig. 19-11, are helpful when considering the cause of birth defects. However, it is incorrect to assume that defects always result from a single event occurring during the critical period of development, or that it is possible to determine from these tables the day on which a defect was produced. What is known is that the teratogen would have to disrupt development of the tissue, part, or organ before the end of the critical period. The critical period of limb development, for example, is 21 to 36 days after fertilization.
Human Teratogens
Awareness that certain agents can disrupt prenatal development offers the opportunity to prevent some birth defects. For example, if women are made aware of the harmful effects of drugs, environmental chemicals and viruses, most pregnant women will avoid exposure to these teratogenic agents.
Drugs vary considerably in their teratogenicity. Some teratogens, such as thalidomide, cause severe disruption of development if administered during the organogenetic period of certain parts (e.g., the limbs) of the embryo (see Fig. 19-15). Other teratogens cause mental and growth restriction of embryos (Table 19-4). Drug consumption tends to be higher during the critical periods of development among heavy smokers and drinkers. Despite this, fewer than 2% of birth defects are caused by drugs and chemicals. Only a few drugs have been positively implicated as human teratogenic agents but new agents continue to be identified. It is best for women to avoid using all medication during the first trimester unless a strong medical reason exists for its use.
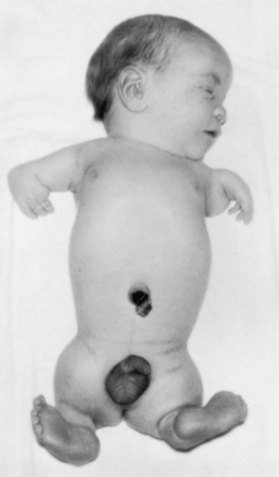
Figure 19–15 Newborn male infant with malformed limbs (meromelia—congenital absence of parts of the limbs) caused by maternal ingestion of thalidomide during the critical period of limb development.
(From Moore KL: The vulnerable embryo: Causes of malformation in man. Manitoba Med Rev 43:306, 1963.)
Cigarette Smoking
Maternal smoking during pregnancy is a well-established cause of intrauterine growth restriction (IUGR). Despite warnings that cigarette smoking is harmful to the fetus, more than 25% of women continue to smoke during pregnancy. In heavy cigarette smokers (20 per day), premature delivery is twice as frequent as in mothers who do not smoke. In addition, the infants of smokers weigh less than normal.
Nicotine constricts the uterine blood vessels, thereby causing a decrease in uterine blood flow and reducing the supply of oxygen and nutrients available to the embryo or fetus from the maternal blood in the intervillous space of the placenta. High levels of carboxyhemoglobin, resulting from cigarette smoking, appear in the maternal and fetal blood, and may alter the capacity of the blood to transport oxygen. As a result, chronic fetal hypoxia (a decrease in the oxygen level to below normal) may occur, affecting fetal growth and development.
Alcohol
Alcoholism is a drug abuse problem that affects 1% to 2% of women of childbearing age. Both moderate and high levels of alcohol intake during early pregnancy may result in alterations in the growth and morphogenesis of the fetus; the greater the intake, the more severe the signs. Infants born to mothers with chronic alcoholism exhibit a specific pattern of defects, including prenatal and postnatal growth and mental deficiency and other anomalies (Fig. 19-12). This pattern of anomalies, fetal alcohol syndrome, is detected in 1 to 2 infants per 1000 live births. Maternal alcohol abuse is now believed to be the most common cause of mental deficiency.
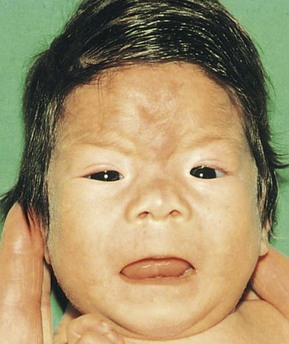
Figure 19–12 Infant with fetal alcohol syndrome. Note the thin upper lip, short palpebral fissures, flat nasal bridge, short nose, and elongated and poorly formed philtrum (vertical groove in the median part of the upper lip). Severe maternal alcohol abuse is believed to be the most common environmental cause of mental deficiency.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Even moderate maternal alcohol consumption (e.g., 1-2 oz daily) may produce fetal alcohol effects—children with behavioral and learning difficulties, for example—especially if the drinking is associated with malnutrition. Binge drinking (heavy consumption of alcohol for 1-3 days during pregnancy) is very likely to produce fetal alcohol effects. The susceptible period of brain development spans the major part of gestation; therefore, the safest advice is total abstinence from alcohol during pregnancy.
Androgens and Progestogens
Androgens and progestogens may affect the female fetus, producing masculinization of the external genitalia (Fig. 19-13). The preparations that should be avoided contain progestins, ethisterone, or norethisterone. From a practical standpoint, the teratogenic risk of these hormones is low. However, progestin exposure during the critical period of development is also associated with an increased prevalence of cardiovascular abnormalities, and exposure of male fetuses during this period may double the incidence of hypospadias in the offspring (see Chapter 13).
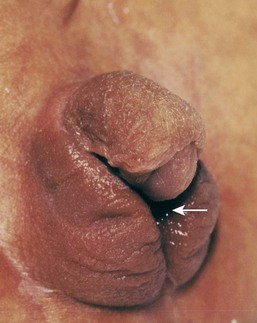
Figure 19–13 Masculinized external genitalia of a female infant with a 46, XX chromosome constitution. Observe the enlarged clitoris and fused labia majora. The arrow indicates the single orifice of a urogenital sinus. The virilization (mature masculine characteristics in a female) was caused by excessive androgens produced by the suprarenal glands during the fetal period (congenital adrenal hyperplasia).
(Courtesy of Dr. Heather Dean, Department of Pediatrics and Child Health and University of Manitoba, Winnipeg, Canada.)
Oral contraceptive (birth control) pills containing progestogens and estrogens, when taken during the early stages of an unrecognized pregnancy, are believed to be teratogenic agents. Many infants of mothers who took progestogen-estrogen birth control pills during the critical period of development have been found to exhibit the VACTERL syndrome—Vertebral, Anal, Cardiac, Tracheal, Esophageal, Renal, and Limb anomalies.
Antibiotics
Tetracyclines cross the placental membrane and are deposited in the embryo’s bones and teeth at sites of active calcification. As little as 1g daily of tetracycline during the third trimester of pregnancy can produce yellow staining of the primary, or deciduous, teeth. Tetracycline therapy during the fourth to ninth months of pregnancy may also cause tooth defects (e.g., enamel hypoplasia), yellow to brown discoloration of the teeth, and diminished growth of the long bones (see Fig. 18-10). Moreover, more than 30 cases of hearing deficit and CN VIII damage have been reported in infants exposed to streptomycin derivatives in utero. By contrast, penicillin has been used extensively during pregnancy and appears to be harmless to the human embryo and fetus.
Anticoagulants
All anticoagulants except heparin cross the placental membrane and may cause hemorrhage in the embryo or fetus. Warfarin, an anticoagulant, is definitely a teratogen. The period of greatest sensitivity is 6 to 12 weeks after fertilization, or 8 to 14 weeks after the last normal menstrual period. Second- and third-trimester exposure may result in mental deficiency, optic atrophy, and microcephaly. Heparin does not cross the placental membrane, and so is the drug of choice for pregnant women requiring anticoagulant therapy.
Anticonvulsants
Epilepsy affects approximately 1 in 200 pregnant women and these women require treatment with an anticonvulsant. Of the anticonvulsant drugs available, phenytoin has been definitively identified as a teratogen. Fetal hydantoin syndrome occurs in 5% to 10% of children born to mothers treated with phenytoins or hydantoin anticonvulsants (Fig. 19-14).
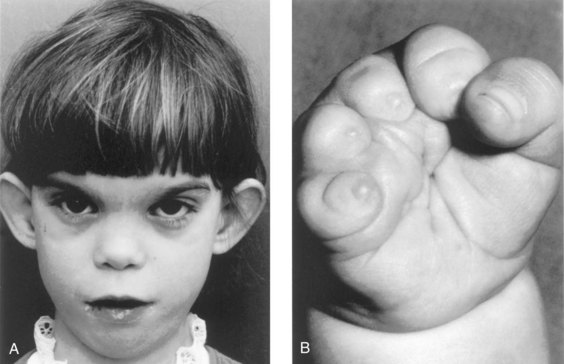
Figure 19–14 Fetal hydantoin syndrome. A, This young girl has a learning disability. Note the unusual ears, the wide spacing of the eyes, the epicanthal folds, the short nose, and the long philtrum. Her mother has epilepsy and took Dilantin throughout her pregnancy. B, Right hand of an infant with severe digital hypoplasia (short fingers), born to a mother who took Dilantin throughout her pregnancy.
(A, Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada. B, From Chodirker BN, Chudley AE, Persaud TVN: Possible prenatal hydantoin effect in child born to a nonepileptic mother. Am J Med Genet 27:373, copyright © 1987. Reprinted by permission of Wiley-Liss, a division of John Wiley and Sons, Inc.)
Valproic acid has been the drug of choice for the management of different types of epilepsy; however, its use by pregnant women has led to a pattern of anomalies consisting of poorer postnatal cognitive development and craniofacial, heart, and limb defects. There is also an increased risk of neural tube defects. Phenobarbital is considered a safe antiepileptic drug for use during pregnancy.
Antineoplastic Agents
Tumor-inhibiting chemicals are highly teratogenic. This is not surprising because these agents inhibit mitosis in rapidly dividing cells. It is recommended that they be avoided, especially during the first trimester of pregnancy. Methotrexate, a folic acid antagonist and a derivative of aminopterin, is a known potent teratogen that produces major congenital anomalies.
Angiotensin-Converting Enzyme Inhibitors
Exposure of the fetus to angiotensin-converting enzyme inhibitors, used as antihypertensive agents, causes oligohydramnios, fetal death, long-lasting hypoplasia of the bones of the calvaria, IUGR, and renal dysfunction.
Retinoic Acid (Vitamin A)
Isotretinoin (13-cis-retinoic acid), used for the oral treatment of severe cystic acne, is teratogenic in humans, even at very low doses. The critical period for exposure appears to be from the third week to the fifth week (5-7 weeks after the last normal menstrual period). The risk of spontaneous abortion and birth defects after exposure to retinoic acid is high. Postnatal follow-up studies of children exposed to isotretinoin in utero showed significant neuropsychological impairment. Vitamin A is a valuable and necessary nutrient during pregnancy, but long-term exposure to large doses of vitamin A is unwise because of insufficient evidence to rule out a teratogenic risk.
Salicylates
Acetylsalicylic acid, or aspirin, is the most commonly ingested drug during pregnancy. Large doses are potentially harmful to the embryo or fetus. Studies indicate that low doses appear not to be teratogenic.
Thyroid Drugs
Iodides readily cross the placental membrane and interfere with thyroxin production. They may also cause thyroid enlargement and cretinism (arrested physical and mental development and dystrophy of bones and soft tissue). Maternal iodine deficiency may cause congenital cretinism. The administration of antithyroid drugs for the treatment of maternal thyroid disorders may cause congenital goiter if the dose administered exceeds that required to control the disease.
Tranquilizers
Thalidomide is a potent teratogen. Nearly 12,000 infants have been born with defects caused by this drug. The characteristic feature of thalidomide syndrome is meromelia—phocomelia, or “seal limbs” (Fig. 19-15). It has been well established clinically that the period when thalidomide causes congenital anomalies is from 20 to 36 days after fertilization (34-50 days after the last normal menstrual period). Thalidomide is absolutely contraindicated in women of childbearing age.
Psychotropic Drugs
Lithium is the drug of choice for long-term maintenance therapy in people with the mental illness known as bipolar disorder; however, it has been known to cause birth defects, mainly of the heart and great vessels, in infants born to mothers given the drug early in pregnancy. Although lithium carbonate is a human teratogen, the Food and Drug Administration has stated that the agent may be used during pregnancy if “in the opinion of the physician the potential benefits outweigh the possible hazards.” Benzodiazepine derivatives are psychoactive drugs that are frequently used by pregnant women. These include diazepam and oxazepam, which readily cross the placental membrane. The use of these drugs during the first trimester of pregnancy is associated with transient withdrawal symptoms and craniofacial anomalies in the neonate. Selective serotonin reuptake inhibitors (SSRIs) are used for the treatment of depression. Use of these drugs by the mother may lead to transient neurobehavioral disturbances and persistent pulmonary hypertension in the neonate.
Illicit Drugs
Cocaine is one of the most commonly abused illicit drugs in North America, and its increasing use by women of childbearing age is of major concern. Many reports deal with the prenatal effects of cocaine; these include spontaneous abortion, prematurity, and diverse anomalies in the offspring.
Methadone, used for the treatment of heroin addiction, is considered a “behavioral teratogen,” as is heroin. Infants born to narcotic-dependent women, lower birth weights, and receiving maintenance methadone therapy have been found to have central nervous system dysfunction and smaller head circumferences than nonexposed infants. There is also concern about the long-term postnatal developmental effects of methadone.
Environmental Chemicals as Teratogens
In recent years, there has been increasing concern about the possible teratogenicity of environmental, industrial, and agricultural chemicals, pollutants, and food additives.
Organic Mercury
Infants of mothers whose main diet during pregnancy consists of fish containing abnormally high levels of organic mercury acquire fetal Minamata disease and exhibit neurologic and behavioral disturbances resembling those associated with cerebral palsy. Methylmercury is a teratogen that causes cerebral atrophy, spasticity, seizures, and mental retardation.
Infectious Agents as Teratogens
Rubella (German or Three-Day Measles)
The rubella virus crosses the placental membrane and infects the embryo or fetus. In cases of primary maternal infection during the first trimester of pregnancy, the overall risk of embryonic or fetal infection is approximately 20%. The clinical features of congenital rubella syndrome are cataracts, congenital glaucoma, cardiac defects, and deafness (Fig. 19-16). The earlier in pregnancy that maternal rubella infection occurs, the greater the danger that the embryo will be malformed.
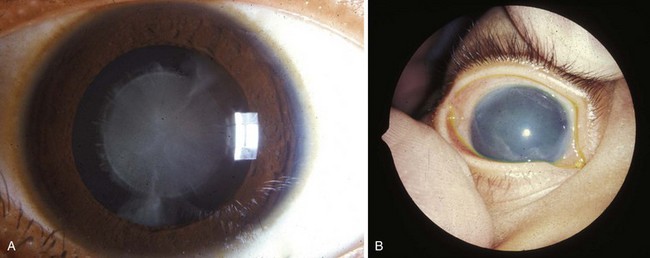
Figure 19–16 A, Typical appearance of a congenital cataract that may be caused by the rubella virus. Cardiac defects and deafness are other congenital defects common to this infection. B, Clouding of the cornea caused by congenital glaucoma. Corneal clouding may also result from infection, trauma, or metabolic disorders.
(Reprinted from Otolaryngologic Clinics of North America 40(1), Guercio J, Martyn L, Congenital malformations of the eye and orbit, 113–140, Copyright 2007, with permission from Elsevier.)
Cytomegalovirus
Cytomegalovirus is the most common viral infection of the human fetus. Because the disease seems to be fatal when it affects the embryo, most pregnancies likely end in spontaneous abortion when the infection occurs during the first trimester. Later in pregnancy, cytomegalovirus infection may result in IUGR and severe fetal anomalies. Of particular concern are cases of asymptomatic cytomegalovirus infection, which are often associated with audiologic, neurologic, and neurobehavioral disturbances in infancy.
Herpes Simplex Virus
Maternal infection with herpes simplex virus in early pregnancy increases the abortion rate threefold, and infection after the 20th week is associated with an increased rate of prematurity as well as birth defects (e.g., microcephaly and mental deficiency). Infection of the fetus with herpes simplex virus usually occurs very late in pregnancy, probably most often during delivery.
Varicella (Chickenpox)
Varicella and herpes zoster (shingles) are caused by the same virus, varicella-zoster virus. There is convincing evidence that maternal varicella infection during the first 4 months of pregnancy causes several birth defects (such as muscle atrophy and mental deficiency). There is a 20% incidence of these or other defects when the infection occurs during the critical period of development (Fig. 19-11).
Human Immunodeficiency Virus
Human immunodeficiency virus (HIV) is the retrovirus that causes acquired immune deficiency syndrome (AIDS). Infection of pregnant women with HIV is associated with serious health problems in the fetus. These include infection of the fetus, preterm delivery, low birth weight, IUGR, microcephaly, and craniofacial abnormalities. Transmission of the HIV virus to the fetus can occur during pregnancy, labor, or delivery.
Toxoplasmosis
Maternal infection with the intracellular parasite Toxoplasma gondii is usually through one of the following routes:
• Eating raw or poorly cooked meat (usually pork or lamb containing Toxoplasma cysts)
• Close contact with infected domestic animals (usually cats) or infected soil
The T. gondii organism crosses the placental membrane and infects the fetus, causing destructive changes in the brain that result in mental deficiency and other birth defects. Mothers of infants with congenital defects are often unaware of having had toxoplasmosis. Because animals (cats, dogs, rabbits, and other domestic and wild animals) may be infected with this parasite, pregnant women should avoid contact with them. In addition, unpasteurized milk should be avoided.
Congenital Syphilis
Syphilis infection affects approximately 3 in 10,000 live-born infants in the United States. Treponema pallidum, the small, spiral microorganism that causes syphilis, rapidly crosses the placental membrane as early as 9 to 10 weeks of gestation. The fetus can become infected at any stage of the disease or at any stage of pregnancy. Primary maternal infections (acquired during pregnancy and left untreated) nearly always cause serious fetal infection and birth defects. However, adequate treatment of the mother kills the organism. Secondary maternal infections (acquired before pregnancy) seldom result in fetal disease and anomalies. If the mother remains untreated, stillbirths occur in approximately 25% of cases.
Radiation as a Teratogen
Exposure to high levels of ionizing radiation may injure embryonic cells, resulting in cell death, chromosomal injury, and retardation of mental development and physical growth. The severity of the embryonic damage is related to the absorbed dose, the dose rate, and the stage of embryonic or fetal development when the exposure occurs. Accidental exposure of pregnant women to radiation is a common cause for anxiety.
No conclusive proof exists that human congenital anomalies have been caused by diagnostic levels of radiation. Scattered radiation from a radiographic examination of a part of the body that is not near the uterus (e.g., thorax, sinuses, teeth) produces a dose of only a few millirads, which is not teratogenic to the embryo. The recommended limit of maternal exposure of the whole body to radiation from all sources is 500 mrad (0.005 Gy) for the entire gestational period.
Maternal Factors as Teratogens
Poorly controlled diabetes mellitus in the mother with persisting hyperglycemia and ketosis, particularly during embryogenesis, is associated with a two- to three-fold higher incidence of birth defects. The infant of a diabetic mother is usually large (macrosomia). The common anomalies include holoprosencephaly (failure of the forebrain to divide into hemispheres), meroencephaly (partial absence of the brain), sacral agenesis, vertebral anomalies, congenital heart defects, and limb anomalies. If left untreated, women who are homozygous for phenylalanine hydroxylase deficiency—phenylketonuria—and those with hyperphenylalaninemia are at increased risk for having offspring with microcephaly, cardiac defects, mental retardation, and IUGR. The congenital anomalies can be prevented if the mother with phenylketonuria follows a phenylalanine-restricted diet before and during pregnancy.
Mechanical Factors as Teratogens
Clubfoot and congenital dislocation of the hip may be caused by mechanical forces, particularly in a malformed uterus. Such deformations may be caused by any factor that restricts the mobility of the fetus, thereby causing prolonged compression in an abnormal posture. A significantly reduced quantity of amniotic fluid (oligohydramnios) may result in mechanically induced deformation of the limbs such as hyperextension of the knee. Intrauterine amputations or other anomalies caused by local constriction during fetal growth may result from amniotic bands (see Fig. 8-14), rings formed as a result of rupture of the amnion during early pregnancy.
Birth Defects Caused by Multifactorial Inheritance
Many common birth defects (e.g., cleft lip, with or without cleft palate) have familial distributions consistent with multifactorial inheritance (Fig. 19-1). Multifactorial inheritance may be represented by a model in which one’s “liability” for a disorder is a continuous variable determined by a combination of genetic and environmental factors, with a developmental threshold dividing individuals with the anomaly from those without it. Multifactorial traits are often single major defects, such as cleft lip, isolated cleft palate, and neural tube defects. Some of these anomalies may also occur as part of the phenotype in syndromes determined by single-gene inheritance, chromosomal abnormality, or an environmental teratogen. The recurrence risks used for genetic counseling of families having birth defects that have been determined by multifactorial inheritance are empirical risks based on the frequency of the anomaly in the general population and in different categories of relatives. In individual families, such estimates may be inaccurate because they are usually averages for the population rather than precise probabilities for the individual family.
Clinically Oriented Questions
1. If a pregnant woman takes aspirin in normal doses, will it cause congenital anomalies?
2. If a woman is a drug addict, will her child show signs of drug addiction?
3. Are all drugs tested for teratogenicity before they are marketed? If the answer is “yes,” why are these teratogens still sold?
4. Is cigarette smoking during pregnancy harmful to the embryo or fetus? If the answer is “yes,” would refraining from inhaling cigarette smoke be safer?
5. Are any drugs safe to take during pregnancy? If so, what are they?