Chapter 9 Body Cavities, Mesenteries, and Diaphragm
Early in the fourth week of development, the intraembryonic coelom—the primordium of the body cavities—appears as a horseshoe-shaped cavity (Fig. 9-1A). The curve or bend in this cavity at the cranial end of the embryo represents the future pericardial cavity, and its limbs indicate the future pleural and peritoneal cavities. The distal part of each limb of the intraembryonic coelom is continuous with the extraembryonic coelom at the lateral edges of the embryonic disc (Fig. 9-1B). This communication is important because most of the midgut normally herniates through this communication into the umbilical cord. The intraembryonic coelom provides room for the abdominal organs to develop and move. During embryonic lateral folding, the limbs of the coelom are brought together on the ventral aspect of the embryo (Fig. 9-2A to F).
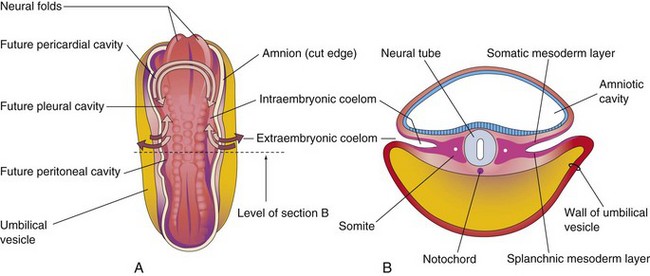
Figure 9–1 A, Dorsal view of a 22-day embryo, showing the outline of the horseshoe-shaped intraembryonic coelom. The amnion has been removed and the coelom is shown as if the embryo were translucent. The continuity of the intraembryonic coelom, as well as the communication of its right and left limbs with the extraembryonic coelom, is indicated by arrows. B, Transverse section through the embryo at the level shown in A.
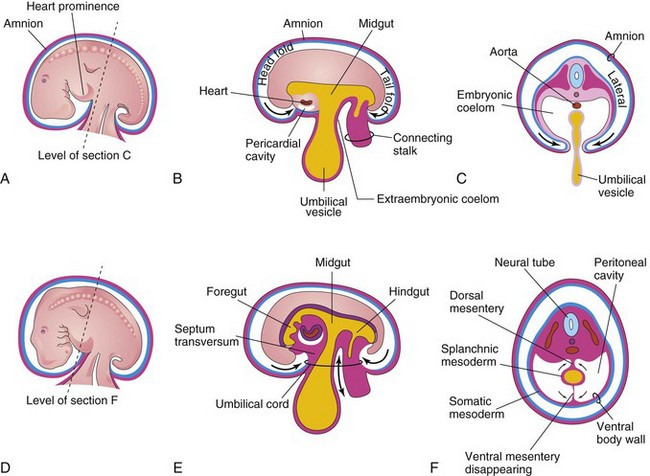
Figure 9–2 Embryonic folding and its effects on the intraembryonic coelom and other structures. A, Lateral view of an embryo (approximately 26 days). B, Schematic sagittal section of the embryo, showing the head and tail folds. C, Transverse section at the level shown in A, indicating how fusion of the lateral folds gives the embryo a cylindric form. D, Lateral view of an embryo (approximately 28 days). E, Schematic sagittal section of the embryo, showing the reduced communication between the intraembryonic and extraembryonic coeloms (double-headed arrow). F, Transverse section, as indicated in D, showing the formation of the ventral body wall and the disappearance of the ventral mesentery. The arrows indicate the junction of the somatic and the splanchnic layers of the mesoderm. The somatic mesoderm will become the parietal peritoneum lining the abdominal wall, and the splanchnic mesoderm will become the visceral peritoneum covering the organs (e.g., stomach).
Embryonic Body Cavity
The intraembryonic coelom gives rise to three well-defined body cavities during the fourth week (Figs. 9-2 and 9-4): a pericardial cavity, two pericardioperitoneal canals connecting the pericardial and the peritoneal cavities, and a large peritoneal cavity.
These body cavities are lined by the mesothelium—a parietal wall derived from the somatic mesoderm and a visceral wall derived from the splanchnic mesoderm (Fig. 9-3E). The mesothelium forms the major portion of the peritoneum. The peritoneal cavity is connected to the extraembryonic coelom at the umbilicus (Fig. 9-4C and D). The peritoneal cavity loses its connection with the extraembryonic coelom during the 10th week as the intestines return to the abdomen from the umbilical cord (see Chapter 12).
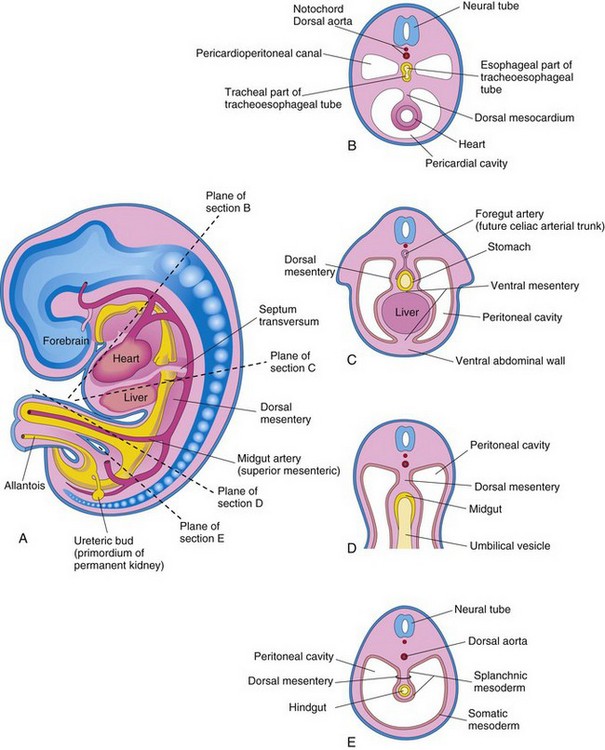
Figure 9–3 Mesenteries and body cavities at the beginning of the fifth week. A, Schematic sagittal section. Note that the dorsal mesentery serves as a pathway for the arteries that supply the developing gut. Nerves and lymphatics also pass between the layers of this mesentery. B to E, Transverse sections through the embryo at the levels shown in A. The ventral mesentery disappears except in the region of the terminal esophagus, stomach, and first part of the duodenum. Note that the right and left parts of the peritoneal cavity, which are separate in C, are continuous in E.
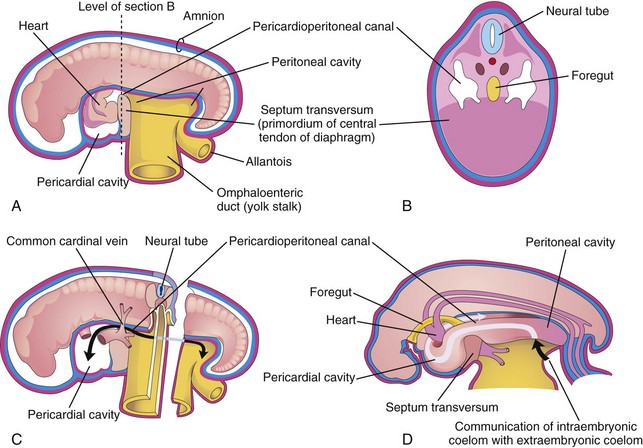
Figure 9–4 Illustration of an embryo (approximately 24 days). A, The lateral wall of the pericardial cavity has been removed to show the primordial heart. B, Transverse section of the embryo, showing the relationship of the pericardioperitoneal canals to the septum transversum and the foregut. C, Lateral view of the embryo, with the heart removed. The embryo has also been sectioned transversely to show the continuity of the intraembryonic and extraembryonic coeloms (black arrow). D, Illustration of the pericardioperitoneal canals that arise from the dorsal wall of the pericardial cavity and pass on each side of the foregut to join the peritoneal cavity. The arrow shows the communication of the extraembryonic coelom with the intraembryonic coelom and the continuity of the intraembryonic coelom at this stage.
During the formation of the head fold, the heart and pericardial cavity move ventrocaudally, anterior to the foregut (Fig. 9-2A, B, D, and E). As a result, the pericardial cavity opens into the pericardioperitoneal canals, which pass dorsal to the foregut (Fig. 9-4B and D). After embryonic folding, the caudal parts of the foregut, midgut, and hindgut are suspended in the peritoneal cavity from the dorsal abdominal wall by the dorsal mesentery (Figs. 9-2F and 9-3B to E).
Mesenteries
A mesentery is a double layer of peritoneum that begins as an extension of the visceral peritoneum that covers an organ. It connects the organ to the body wall and conveys its vessels and nerves. Transiently, the dorsal and ventral mesenteries divide the peritoneal cavity into right and left halves (Fig. 9-3C). The ventral mesentery soon disappears (see Fig. 9-3E), except where it is attached to the caudal part of the foregut (primordium of stomach and proximal part of the duodenum). The peritoneal cavity then becomes a continuous space (Figs. 9-3A and 9-4D). The arteries supplying the primordial gut—celiac arterial trunk (foregut), the superior mesenteric artery (midgut), and the inferior mesenteric artery (hindgut)—pass between the layers of the dorsal mesentery (Fig. 9-3C).
Division of Embryonic Body Cavity
Each pericardioperitoneal canal lies lateral to the proximal part of the foregut (future esophagus) and dorsal to the septum transversum—a thick plate of mesoderm that occupies the space between the thoracic cavity and the omphaloenteric duct (Fig. 9-4A and B). The septum transversum is the primordium of the central tendon of the diaphragm. Partitions form in each pericardioperitoneal canal, separating the pericardial cavity from the pleural cavities; and the pleural cavities from the peritoneal cavity (Fig. 9-3A). Because of the growth of the bronchial buds (primordia of bronchi and lungs) into the pericardioperitoneal canals (Fig. 9-5A), a pair of membranous ridges is produced in the lateral wall of each canal. The cranial ridges—the pleuropericardial folds—are located superior to the developing lungs. The caudal ridges—the pleuroperitoneal folds—are located inferior to the lungs.
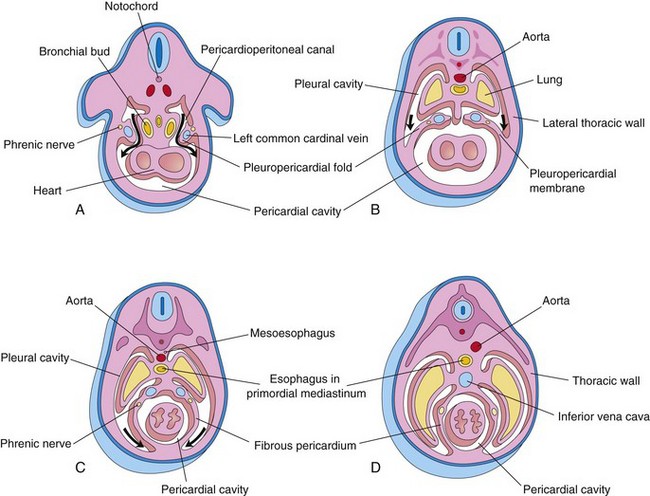
Figure 9–5 Transverse sections through an embryo cranial to the septum transversum, showing successive stages in the separation of the pleural cavities from the pericardial cavity. Growth and development of the lungs, expansion of the pleural cavities, and formation of the fibrous pericardium are also shown. A, At 5 weeks. The arrows indicate the communications between the pericardioperitoneal canals and the pericardial cavity. B, At 6 weeks. The arrows indicate the development of the pleural cavities as they expand into the body wall. C, At 7 weeks. Expansion of the pleural cavities ventrally (arrows) around the heart is evident. The pleuropericardial membranes are now fused in the median plane with each other and with the mesoderm ventral to the esophagus. D, At 8 weeks. Continued expansion of the lungs and the pleural cavities and formation of the fibrous pericardium and the thoracic wall are shown.
As the pleuropericardial folds enlarge, they form partitions that separate the pericardial cavity from the pleural cavities. These partitions—pleuropericardial membranes—contain the common cardinal veins (Fig. 9-5A and B), which drain the venous system into the sinus venosus of the primordial heart (Chapter 14). Subsequently, they grow laterally from the caudal end of the trachea into the pericardioperitoneal canals (future pleural canals). As the primordial pleural cavities expand ventrally around the heart, they extend into the body wall, splitting the mesenchyme into two layers: (1) an outer layer that becomes the thoracic wall and (2) an inner layer (pleuropericardial membrane) that becomes the fibrous pericardium, the outer layer of the pericardial sac that encloses the heart (Fig. 9-5C and D).
The pleuropericardial membranes project into the cranial ends of the pericardioperitoneal canals (Fig. 9-5B). With subsequent growth of the common cardinal veins, positional displacement of the heart, and expansion of the pleural cavities, the pleuropericardial membranes become mesentery-like folds extending from the lateral thoracic wall. By the seventh week, the pleuropericardial membranes fuse with the mesenchyme ventral to the esophagus, separating the pericardial cavity from the pleural cavities (Fig. 9-5C). The primordial mediastinum consists of a mass of mesenchyme that extends from the sternum to the vertebral column, separating the developing lungs (Fig. 9-5D). The right pleuropericardial opening closes slightly earlier than the left one and produces a larger pleuropericardial membrane.
As the pleuroperitoneal folds enlarge, they project into the pericardioperitoneal canals. Gradually, the folds become membranous, forming the pleuroperitoneal membranes (Fig. 9-6B and C). Eventually, these membranes separate the pleural cavities from the peritoneal cavity. The pleuroperitoneal membranes are produced as the developing lungs and pleural cavities expand and invade the body wall. They are attached dorsolaterally to the abdominal wall and their crescentic free edges initially project into the caudal ends of the pericardioperitoneal canals. During the sixth week, the pleuroperitoneal membranes extend ventromedially until their free edges fuse with the dorsal mesentery of the esophagus and the septum transversum (see Fig. 9-6C). This membrane separates the pleural cavities from the peritoneal cavity. Closure of the pleuroperitoneal openings is assisted by the migration of myoblasts (primordial muscle cells) into the pleuroperitoneal membranes. The pleuroperitoneal opening on the right side closes slightly before the left one.
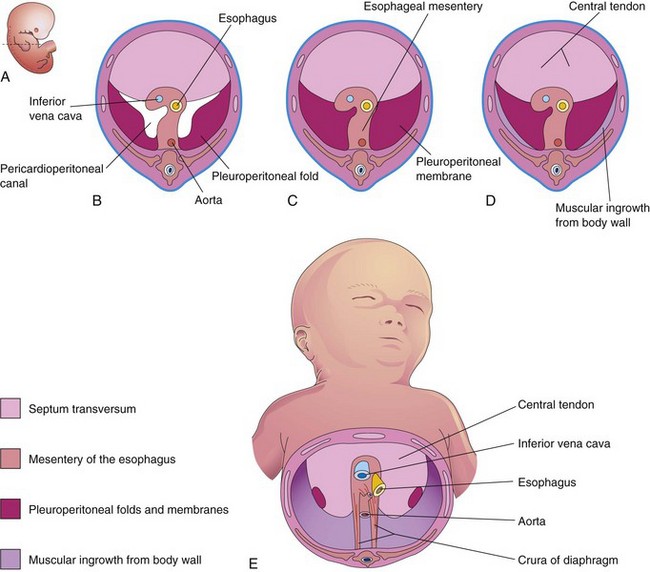
Figure 9–6 Development of the diaphragm. A, Lateral view of an embryo at the end of the fifth week (actual size), indicating the level of B to D sections. B to E show the developing diaphragm as viewed inferiorly. B, Transverse section, showing the unfused pleuroperitoneal membranes. C, Similar section at the end of the sixth week, after fusion of the pleuroperitoneal membranes with the other two diaphragmatic components. D, Transverse section of a 12-week embryo, after ingrowth of the fourth diaphragmatic component from the body wall. E, View of the diaphragm of a newborn infant, indicating the embryologic origin of its components.
Development of Diaphragm
The diaphragm is a dome-shaped, musculotendinous partition that separates the thoracic and abdominal cavities. It is a composite structure that develops from four embryonic components (Fig. 9-6):
Septum Transversum
This transverse septum, which is composed of mesodermal tissue, is the primordium of the central tendon of the diaphragm (Fig. 9-6D and E). The septum transversum grows dorsally from the ventrolateral body wall and forms a semicircular shelf that separates the heart from the liver. After the head folds ventrally during the fourth week, the septum transversum forms a thick incomplete partition between the pericardial and abdominal cavities (Fig. 9-4). The septum transversum expands and fuses with the mesenchyme ventral to the esophagus and the pleuroperitoneal membranes (Fig. 9-6C).
Pleuroperitoneal Membranes
The pleuroperitoneal membranes fuse with the dorsal mesentery of the esophagus and the septum transversum (Fig. 9-6C). This fusion completes the partition between the thoracic and abdominal cavities and forms the primordial diaphragm. The pleuroperitoneal membranes represent relatively small parts of the diaphragm in a neonate (Fig. 9-6E).
Dorsal Mesentery of Esophagus
The septum transversum and the pleuroperitoneal membranes fuse with the dorsal mesentery of the esophagus. This mesentery becomes the median portion of the diaphragm. The crura of the diaphragm—a pair of diverging muscle bundles that cross in the median plane anterior to the aorta (Fig. 9-6E)—develop from myoblasts (primordial muscle cells) that grow into the dorsal mesentery of the esophagus.
Muscular Growth from Lateral Body Walls
During the 9th to 12th weeks, the lungs and pleural cavities enlarge, “burrowing” into the lateral body walls (Fig. 9-5). During this process, the tissue of the body wall is split into two layers:
• An external layer that becomes part of the definitive thoracic and abdominal wall
• An internal layer that contributes muscle to the peripheral portions of the diaphragm, external to the parts derived from the pleuroperitoneal membranes (Fig. 9-6D and E)
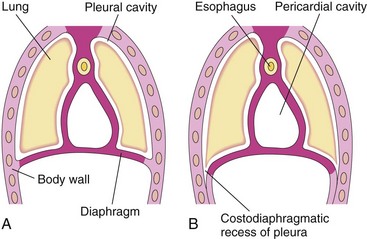
Further extension of the developing pleural cavities into the lateral body walls forms the right and left costodiaphragmatic recesses (Fig. 9-7), establishing the characteristic dome-shaped configuration of the diaphragm.
Positional Changes and Innervation of the Diaphragm
During the fourth week of development, the septum transversum lies opposite the third to fifth cervical somites. During the fifth week, myoblasts from these somites migrate into the developing diaphragm, bringing their nerve fibers with them. Consequently, the phrenic nerves that supply motor innervation to the diaphragm arise from the ventral primary rami of the third, fourth, and fifth cervical spinal nerves, which join together on each side to form a phrenic nerve. The phrenic nerves also supply sensory fibers to the superior and inferior surfaces of the right and left domes of the diaphragm.
Rapid growth of the dorsal part of the embryo’s body results in an apparent descent of the diaphragm. By the sixth week, the developing diaphragm is at the level of the thoracic somites. The phrenic nerves now have a descending course. By the beginning of the eighth week, the dorsal part of the diaphragm lies at the level of the first lumbar vertebra. The phrenic nerves in the embryo enter the diaphragm by passing through the pleuropericardial membranes. For this reason, the phrenic nerves subsequently lie on the fibrous pericardium of the heart, which is derived from the pleuropericardial membranes (Fig. 9-5C and D).
The costal border of the diaphragm receives sensory fibers from the lower intercostal nerves because of the origin of the peripheral part of the diaphragm from the lateral body walls (Fig. 9-6D and E).
Congenital Diaphragmatic Hernia
A posterolateral defect of the diaphragm is the only relatively common congenital anomaly involving the diaphragm (Fig. 9-8A). This diaphragmatic defect occurs in approximately 1 in 2200 newborn infants and is associated with congenital diaphragmatic hernia (CDH) (herniation of abdominal contents into the thoracic cavity).
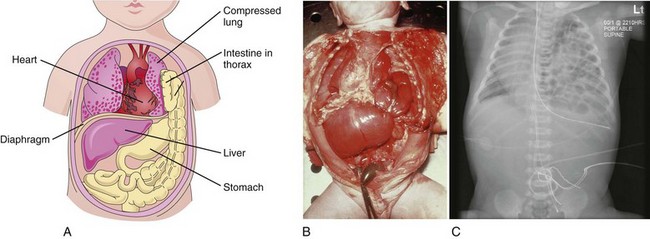
Figure 9–8 A, This “window view” overlooking the thorax and the abdomen shows the herniation of the intestine into the thorax through a posterolateral defect in the left side of the diaphragm. Note that the left lung is compressed and hypoplastic. B, Diaphragmatic hernia. Note the herniation of the stomach and the small intestine into the thorax through a posterolateral defect in the left side of the diaphragm, similar to that shown in Figure 9-8A. Note that the heart is pushed to the right side of the thorax. C, Radiograph showing a diaphragmatic hernia on the left side. Note the loops of small intestine in the thoracic cavity and the displacement of the heart into the right thoracic cavity.
(B, Courtesy of Dr. Nathan E. Wiseman, Professor of Surgery, Children’s Hospital, University of Manitoba, Winnipeg, Manitoba, Canada. C, From Dr. Frank Gaillard, Radiopaedia.org, with permission.)
The most common cause of pulmonary hypoplasia, CDH can lead to life-threatening respiratory difficulties. If severe lung hypoplasia is present, some primordial alveoli may rupture, causing air to enter the pleural cavity (pneumothorax). Usually unilateral, CDH results from defective formation or fusion of the pleuroperitoneal membrane with the other three parts of the diaphragm (Fig. 9-6B). This defect produces a large opening in the posterolateral region of the diaphragm. If a pleuroperitoneal canal is still open when the intestines return to the abdomen from the umbilical cord in the 10th week, some intestine and other viscera may pass into the thorax and compress the lungs. Often the stomach, spleen, and most of the intestines herniate (Figs. 9-8B and 9-8C). The defect usually occurs on the left side, and it is likely related to the earlier closure of the right pleuroperitoneal opening. Ultrasonographic and magnetic resonance imaging can provide a prenatal diagnosis of CDH.
Eventration of Diaphragm
In the uncommon condition of diaphragmatic eventration, part of the diaphragm has defective musculature, causing it to balloon into the thoracic cavity as an aponeurotic (membranous) sheet, forming a large diaphragmatic pouch. Consequently, the abdominal viscera are displaced superiorly into the pocket-like outpouching of the diaphragm. This congenital anomaly results mainly from failure of the muscular tissue from the body wall to extend into the pleuroperitoneal membrane on the affected side.
Retrosternal (Parasternal) Hernia
Herniations may occur through the sternocostal hiatus, the opening for the superior epigastric vessels in the retrosternal area. This hiatus is located between the sternal and the costal parts of the diaphragm. Herniation of the intestine into the pericardial sac may occur or, conversely, part of the heart may descend into the peritoneal cavity in the epigastric region. Large defects are commonly associated with body wall defects in the umbilical region (e.g., omphalocele; see Chapter 12).
Clinically Oriented Questions
1. There have been reports of an infant who was born with its stomach and liver in its chest. Is this possible?
2. Can an infant with most of its abdominal viscera in the chest survive? Some say that diaphragmatic defects can be operated on before birth. Is this true?
3. Do the lungs develop normally in infants who are born with a congenital diaphragmatic hernia?
4. A man underwent routine chest radiography approximately 1 year ago and was told that a small part of his small intestine was in his chest. Is it possible for him to have a congenital diaphragmatic hernia without being aware of it? Would his lung on the affected side be normal?