Chapter 14 Cardiovascular System
The cardiovascular system is the first major system to function in the embryo. The primordial heart and vascular system appear in the middle of the third week of development (Fig. 14-1). The heart begins to beat at 22 to 23 days (Fig. 14-2). This precocious development is necessary because the rapidly growing embryo can no longer satisfy its nutritional and oxygen requirements by diffusion alone.
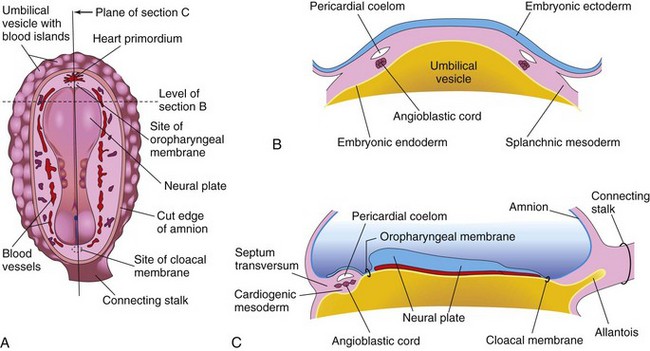
Figure 14–1 Early development of the heart. A, Dorsal view of an embryo (approximately 18 days). B, Transverse section of the embryo, showing angioblastic cords and their relationship to the pericardial coelom. C, Longitudinal section through the embryo, showing the relationship of the angioblastic cords to the oropharyngeal membrane, pericardial coelom, and septum transversum.
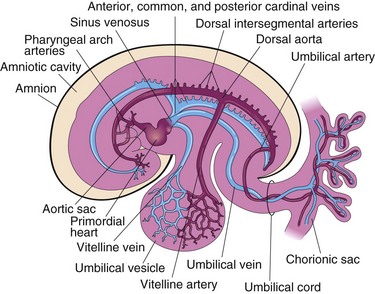
Figure 14–2 The embryonic cardiovascular system (at approximately 26 days), showing vessels on the left side only. The umbilical vein carries well-oxygenated blood and nutrients from the chorion (the embryonic part of the placenta) to the embryo. The umbilical arteries carry poorly oxygenated blood and waste products from the embryo to the chorionic sac.
Early Development of The Heart and Blood Vessels
Paired endothelial strands—angioblastic cords—appear in the cardiogenic mesoderm during the third week of development (Fig. 14-1B and C). These cords canalize to form two heart tubes that soon fuse to form a single heart tube late in the third week (see Fig. 14-5). An inductive influence from the anterior endoderm stimulates early formation of the heart. Cardiac morphogenesis is controlled by a cascade of regulatory genes and transcription factors.
Development of Embryonic Veins Associated with the Heart
Three paired veins drain into the tubular heart of a 4-week embryo (Fig. 14-2):
• Vitelline veins return poorly oxygenated blood from the umbilical vesicle (yolk sac).
• Umbilical veins carry well-oxygenated blood from the chorionic sac (primordial placenta); only the left umbilical vein persists.
• Common cardinal veins return poorly oxygenated blood from the body of the embryo to the heart.
The vitelline veins enter the sinus venosus of the primordial heart (Figs. 14-2 to 14-4A and B). As the liver primordium grows into the septum transversum, the hepatic cords anastomose around preexisting endothelium-lined spaces. These spaces, the primordia of the hepatic sinusoids, later become linked to the vitelline veins. The hepatic veins form from the remains of the right vitelline vein in the region of the developing liver. The portal vein develops from an anastomotic network of vitelline veins around the duodenum (Fig. 14-4B). The fate of the umbilical veins may be summarized as follows (see Fig. 14-4B):
• The right umbilical vein and the cranial part of the left umbilical vein between the liver and the sinus venosus degenerate.
• The persistent caudal part of the left umbilical vein becomes the umbilical vein, which carries well-oxygenated blood from the placenta to the embryo.
• A large venous shunt—the ductus venosus—develops within the liver and connects the umbilical vein with the inferior vena cava (IVC).
The cardinal veins (Figs. 14-2 and 14-3A) constitute the main venous drainage system of the embryo. The anterior and posterior cardinal veins drain the cranial and caudal parts of the embryo, respectively (Fig. 14-3A). These join the common cardinal veins, which enter the sinus venosus (Fig. 14-4A). During the eighth week, the anterior cardinal veins are connected by an oblique anastomosis (Fig. 14-4B) that shunts blood from the left to the right anterior cardinal vein. This shunt becomes the left brachiocephalic vein when the caudal part of the left anterior cardinal vein degenerates (Figs. 14-3D and 14-4C). The superior vena cava (SVC) forms from the right anterior cardinal vein and the right common cardinal vein. The only adult derivatives of the posterior cardinal veins are the root of the azygos vein and the common iliac veins.
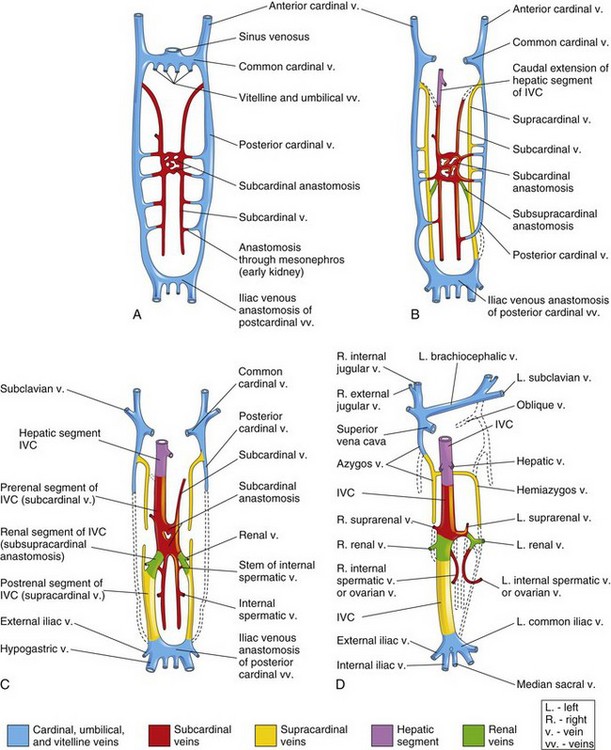
Figure 14–3 Illustrations of the primordial veins of the trunk of an embryo (ventral views). Initially, three systems of veins are present: the umbilical veins from the chorionic sac, the vitelline veins from the umbilical vesicle, and the cardinal veins from the body of the embryo. Next, the subcardinal veins appear, and finally the supracardinal veins develop. A, At 6 weeks. B, At 7 weeks. C, At 8 weeks. D, Adult, showing the transformations that produce the adult venous pattern.
(Modified from Arey LB: Developmental Anatomy, rev. 7th ed. Philadelphia, WB Saunders, 1974).
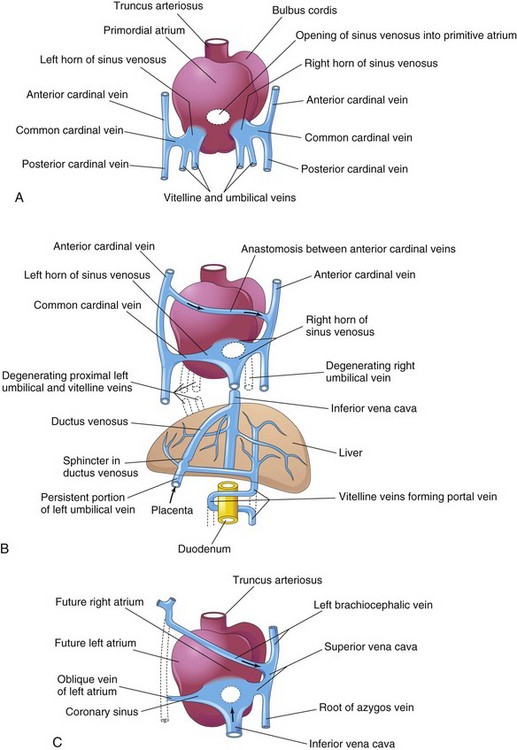
Figure 14–4 Dorsal views of the developing heart. A, During the fourth week (approximately 24 days); the primordial atrium and the sinus venosus, as well as the veins draining into them, are evident. B, At 7 weeks, the right sinual horn is enlarged and the venous circulation through the liver is established. (The organs are not drawn to scale.) C, At 8 weeks, showing the adult derivatives of the cardinal veins. Arrows indicate the flow of blood.
The subcardinal and supracardinal veins gradually replace and supplement the posterior cardinal veins. The subcardinal veins appear first (Fig. 14-3A) and form the stem of the left renal vein, the suprarenal veins, the gonadal veins (testicular and ovarian), and a segment of the inferior vena cava (Fig. 14-3D). The supracardinal veins become disrupted in the region of the kidneys (Fig. 14-3C). Cranial to this, they become united by an anastomosis that forms the azygos and the hemiazygos veins (Figs. 14-3D and 14-4C). Caudal to the kidneys, the left supracardinal vein degenerates but the right supracardinal vein becomes the inferior part of the IVC (Fig. 14-3D). The IVC forms as blood returning from the caudal part of the embryo is shifted from the left to the right side of the body.
Pharyngeal Arch Arteries and Other Branches of the Dorsal Aorta
As the pharyngeal arches form during the fourth and fifth weeks of development, they are supplied by pharyngeal arch arteries that arise from the aortic sac and terminate in the dorsal aortae (Fig. 14-2). Initially, the paired dorsal aortae run through the entire length of the embryo. Later, the caudal portions of the paired dorsal aortae fuse to form a single lower thoracic/abdominal aorta. Of the remaining paired dorsal aortae, the right regresses and the left becomes the primordial aorta.
Intersegmental Arteries
Thirty or so branches of the dorsal aorta, collectively known as the intersegmental arteries, pass between and carry blood to the somites and their derivatives (Fig. 14-2). The intersegmental arteries in the neck join to form the vertebral arteries. Most of the original connections of the intersegmental arteries to the dorsal aorta eventually disappear. In the thorax, the intersegmental arteries persist as intercostal arteries. Most of the intersegmental arteries in the abdomen become lumbar arteries; however, the fifth pair of lumbar intersegmental arteries remains as the common iliac arteries. In the sacral region, the intersegmental arteries form the lateral sacral arteries. The caudal end of the dorsal aorta becomes the median sacral artery.
Fate of Vitelline and Umbilical Arteries
The unpaired ventral branches of the dorsal aorta supply the umbilical vesicle, allantois, and chorion (Fig. 14-2). The vitelline arteries supply the umbilical vesicle (yolk sac) and later, the primordial gut, which forms from the incorporated part of the umbilical vesicle. Only three vitelline arteries remain: the celiac arterial trunk to the foregut; the superior mesenteric artery to the midgut, and the inferior mesenteric artery to the hindgut.
The paired umbilical arteries pass through the connecting stalk (primordial umbilical cord) and join the vessels in the chorion. The umbilical arteries carry poorly oxygenated fetal blood to the placenta (Fig. 14-2). The proximal parts of these arteries become the internal iliac arteries and the superior vesical arteries, whereas the distal parts are obliterated after birth and become the medial umbilical ligaments.
Later Development of The Heart
As the heart tubes fuse, the external layer of the embryonic heart, the primordial myocardium is formed from the splanchnic mesoderm surrounding the pericardial coelom (Figs. 14-5 and 14-6B and C). At this stage, the developing heart is composed of a thin tube, separated from a thick primordial myocardium by gelatinous connective tissue called cardiac jelly (Fig. 14-6C and D). The endothelial tube becomes the internal endothelial lining of the heart, the endocardium, and the primordial myocardium becomes the muscular wall of the heart, the myocardium. The epicardium is derived from the mesothelial cells that arise from the external surface of the sinus venosus (Fig. 14-6F).
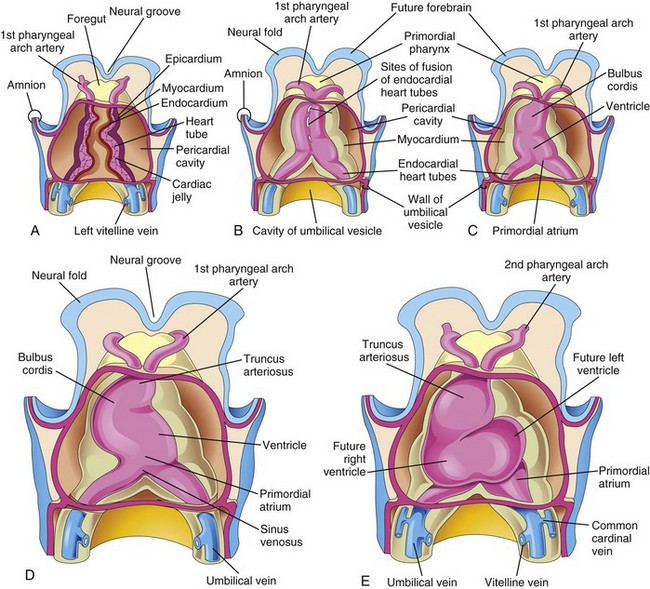
Figure 14–5 A to C, Ventral views of the developing heart and the pericardial region (22–35 days). The ventral pericardial wall has been removed to show the developing myocardium and fusion of the two heart tubes to form a single heart tube. Fusion begins at the cranial ends of the tubes and extends caudally until a single tubular heart is formed. D and E, As the tubular heart elongates, it bends on itself, forming an S-shaped heart.
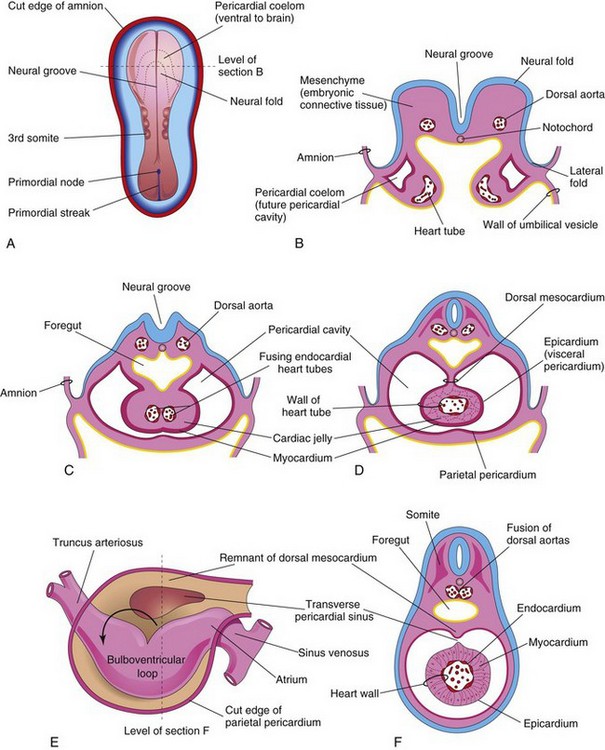
Figure 14–6 A, Dorsal view of an embryo (approximately 20 days). B, Schematic transverse section of the heart region of the embryo illustrated in A, showing the two endocardial heart tubes and the lateral folds of the body. C, Transverse section of a slightly older embryo, showing the formation of the pericardial cavity and the fusing heart tubes. D, Similar section (approximately 22 days), showing the single heart tube suspended by the dorsal mesocardium. E, Schematic drawing of the heart (approximately 28 days), showing degeneration of the central part of the dorsal mesocardium and formation of the transverse sinus of the pericardium. The arrow shows bending of the primordial heart. F, Transverse section of the embryo at the level seen in E, showing the layers of the heart wall.
As folding of the head region occurs, the heart and the pericardial cavity appear ventral to the foregut and caudal to the oropharyngeal membrane (Fig. 14-7A to C). Concurrently, the tubular heart elongates and develops alternate dilations and constrictions (Fig. 14-5C to E): the bulbus cordis (composed of the truncus arteriosus, conus arteriosus, and conus cordis), ventricle, the atrium, and the sinus venosus.
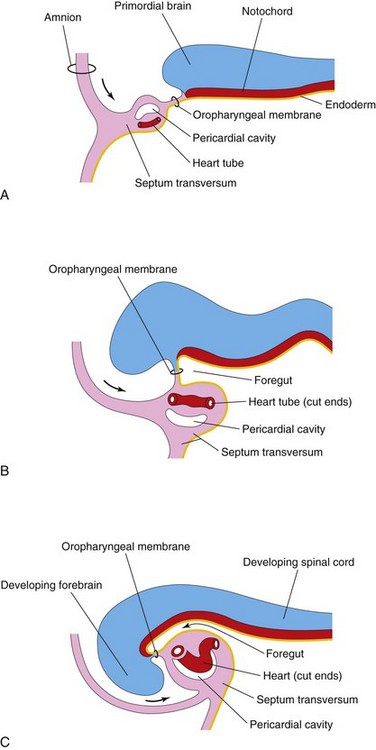
Figure 14–7 Longitudinal sections through the cranial half of human embryos during the fourth week of development. The effect of the head fold (arrows) on the position of the heart and other structures is shown. A and B, As the head fold develops, the heart tube and the pericardial cavity come to lie ventral to the foregut and caudal to the oropharyngeal membrane. C, Note that the positions of the pericardial cavity and the septum transversum have reversed with respect to each other. The septum transversum now lies posterior to the pericardial cavity, where it will form the central tendon of the diaphragm.
The tubular truncus arteriosus (TA) is continuous cranially with the aortic sac (Fig. 14-8A), from which the pharyngeal arch arteries arise. The sinus venosus receives the umbilical, vitelline, and common cardinal veins from the chorion, umbilical vesicle, and embryo, respectively (Fig. 14-4A). The arterial and venous ends of the heart are fixed by the pharyngeal arches and the septum transversum, respectively. Because the bulbus cordis and ventricle grow faster than the other regions, the heart bends on itself, forming a U-shaped bulboventricular loop (Fig. 14-6E). Transforming growth factor β nodal is involved in looping of the heart tube. As the primordial heart bends, the atrium and sinus venosus appear dorsal to the truncus arteriosus, bulbus cordis, and ventricle (Fig. 14-8A and B). By this stage, the sinus venosus has developed lateral expansions, the right and left horns of the sinus venosus.
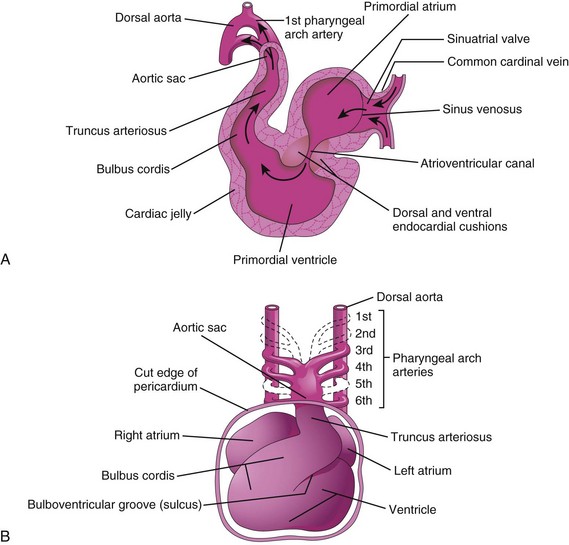
Figure 14–8 A, Sagittal section of the primordial heart (approximately 24 days), showing blood flowing through it (arrows). B, Ventral view of the heart and the pharyngeal arch arteries (approximately 35 days). The ventral wall of the pericardial sac has been removed to show the heart in the pericardial cavity.
As the heart develops, it gradually invaginates the pericardial cavity (Figs. 14-6C and D and 14-7C). The heart is initially suspended from the dorsal wall by a mesentery, the dorsal mesocardium. However, the central part of this mesentery degenerates, forming a communication—the transverse pericardial sinus—between the right and left sides of the pericardial cavity (Fig. 14-6E and F). At this stage, the heart is attached only at its cranial and caudal ends.
Circulation through Primordial Heart
Blood enters the sinus venosus (Fig. 14-8A and 14-4A) from the:
Blood from the sinus venosus enters the primordial atrium; flow from it is controlled by sinuatrial valves (Fig. 14-8A). The blood then passes through the atrioventricular canal into the primordial ventricle. When the ventricle contracts, blood is pumped through the bulbus cordis and truncus arteriosus into the aortic sac, from which it is distributed to the pharyngeal arch arteries (Fig. 14-8B). The blood then passes into the dorsal aortae for distribution to the embryo, umbilical vesicle, and placenta.
Partitioning of Primordial Heart
Partitioning of the atrioventricular (AV) canal, primordial atrium, and ventricle begins at approximately the middle of the fourth week and is essentially completed by the end of the eighth week.
Toward the end of the fourth week, endocardial cushions form on the dorsal and ventral walls of the AV canal (Fig. 14-8A). These cushions approach each other and fuse, dividing the AV canal into right and left AV canals (Fig. 14-9B). These canals partially separate the primordial atrium from the ventricle, and the cushions function as AV valves. The endocardial cushions develop from a specialized extracellular matrix related to the myocardium. Its formation is associated with the expression of transforming growth factor b2 and bone morphogenetic factors 2A and 4.
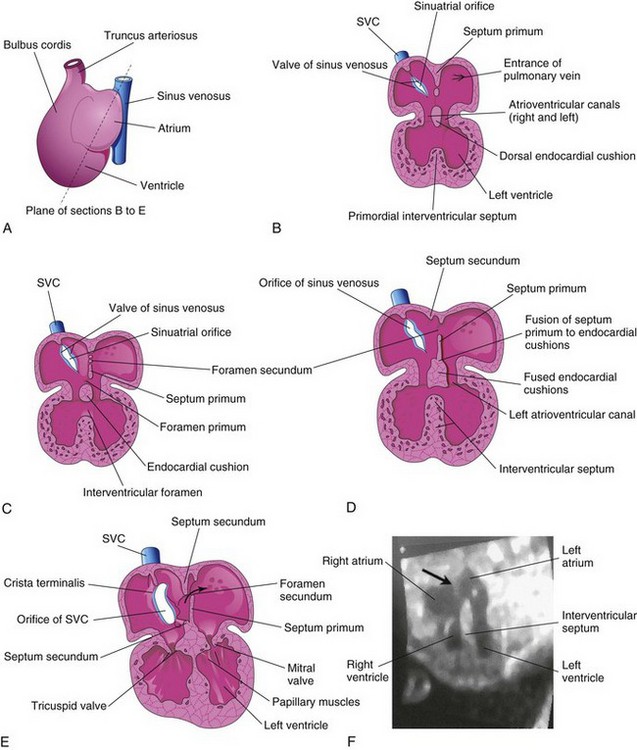
Figure 14–9 The developing heart, showing partitioning of the atrioventricular canal, the primordial atrium, and the ventricle. A, The plane of sections B to E. B, At the fourth week (approximately 28 days), showing the early appearance of the septum primum, the interventricular septum, and the dorsal endocardial cushion. C, Frontal section of the heart (approximately 32 days), showing perforations in the dorsal part of the septum primum. D, Frontal section of the heart (approximately 35 days), showing the foramen secundum. E, At approximately 8 weeks, the heart is partitioned into four chambers. The arrow indicates the flow of well-oxygenated blood from the right to the left atrium. F, Sonogram of a second-trimester fetus, showing the four chambers of the heart. Note the septum secundum (arrow) and the descending aorta.
(F, Courtesy of Dr. G.J. Reid, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Manitoba, Women’s Hospital, Winnipeg, Manitoba, Canada.)
Partitioning of Primordial Atrium
The primordial atrium is divided into right and left atria by the formation and subsequent modification and fusion of two septa, the septum primum and the septum secundum (Figs. 14-9A to E and 14-10).
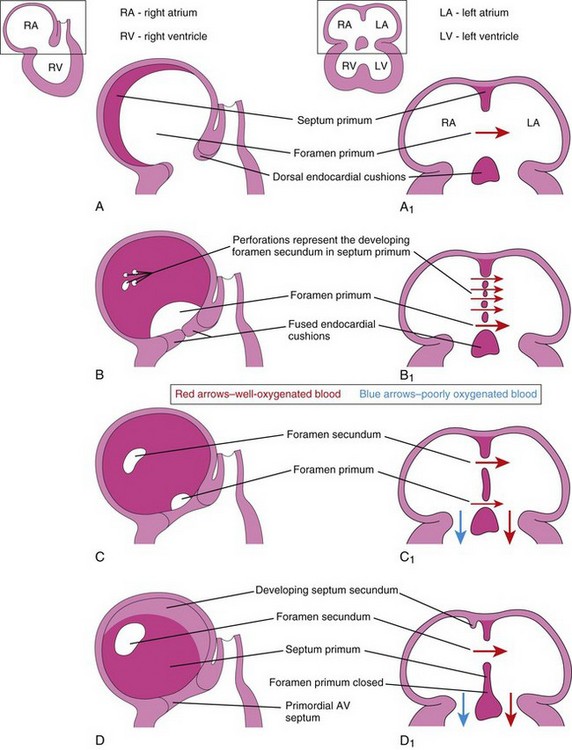
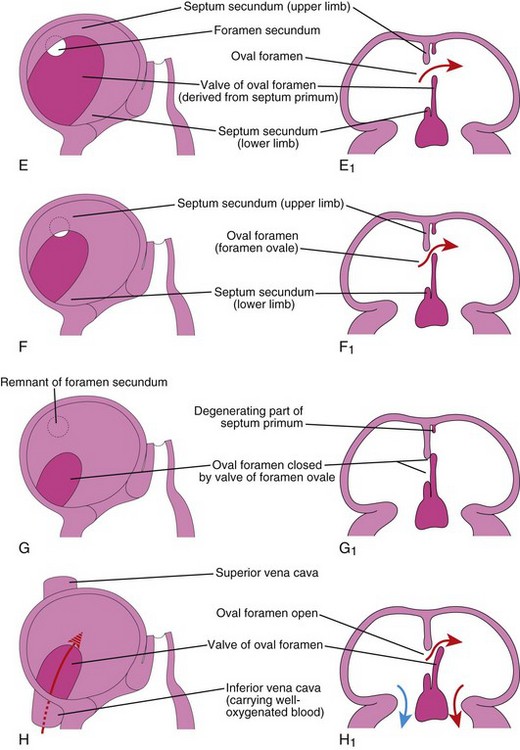
Figure 14–10 Progressive stages in the partitioning of the primordial atrium. A to H, Views of the developing interatrial septum, as viewed from the right side. A1 to H1, Frontal sections of the developing interatrial septum. As the septum secundum grows, note that it overlaps the opening (foramen secundum) in the septum primum Observe the valve of the oval foramen in G1 and H1.
The septum primum grows toward the fusing endocardial cushions from the roof of the primordial atrium, partially dividing the atrium into right and left halves. As this curtain-like septum develops, a large opening—the foramen primum—forms between its free edge and the endocardial cushions (Figs. 14-9C and 14-10A to C). The foramen allows shunting of oxygenated blood from the right to the left atrium. The foramen becomes progressively smaller and disappears as the septum primum fuses with the fused endocardial cushions to form the primordial AV septum (see Fig. 14-10D and D1).
Before the foramen primum disappears, perforations, produced by apoptosis (programmed cell death), appear in the central part of the septum primum. As the free edge of the septum primum fuses with the left side of the fused endocardial cushions, obliterating the foramen primum (Figs. 14-9D and 14-10D), the perforations coalesce to form another opening—the foramen secundum (Fig. 14-10C). The foramen secundum ensures continued shunting of oxygenated blood from the right to the left atrium.
The septum secundum grows from the ventrocranial wall of the atrium, immediately to the right of the septum primum (Fig. 14-10D1). As this crescentic, muscular septum grows during the fifth and sixth weeks, it gradually overlaps the foramen secundum in the septum primum (Fig. 14-10E and F). The septum secundum forms an incomplete partition between the atria; the opening in the foramen secundum is called the oval foramen (foramen ovale). The cranial part of the septum primum gradually disappears (Fig. 14-10G1). The remaining part of the septum primum, attached to the endocardial cushions, forms the valve of the oval foramen.
Before birth, the oval foramen allows most of the oxygenated blood entering the right atrium from the IVC to pass into the left atrium (Fig. 14-10H1). It also prevents the passage of blood in the opposite direction—the septum primum would close against the relatively rigid septum secundum (Fig. 14-10G1). After birth, the oval foramen functionally closes due to higher pressure in the left atrium, and the valve of the oval foramen fuses with the septum secundum, forming the oval fossa (fossa ovalis). As a result, the interatrial septum becomes a complete partition between the atria.
Changes in Sinus Venosus
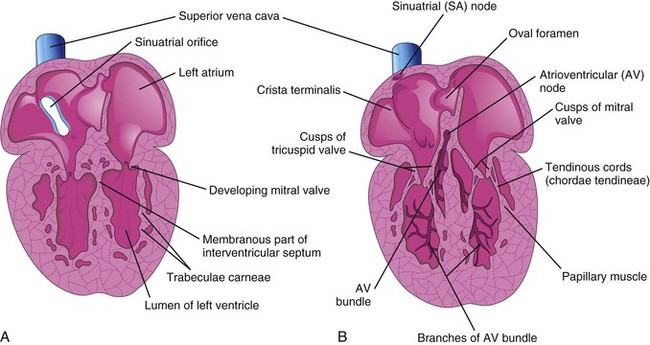
Initially, the sinus venosus opens into the posterior wall of the primordial atrium (sinuatrial orifice). By the end of the fourth week of development, the right sinual horn becomes larger than the left sinual horn (Fig. 14-11A and B). As this occurs, the sinuatrial orifice moves to the right and opens in the part of the primordial atrium that will become the adult right atrium (Fig. 14-12C). As the right sinual horn enlarges, it receives all of the blood from the head and neck through the SVC, and from the placenta and the caudal regions of the body through the IVC.
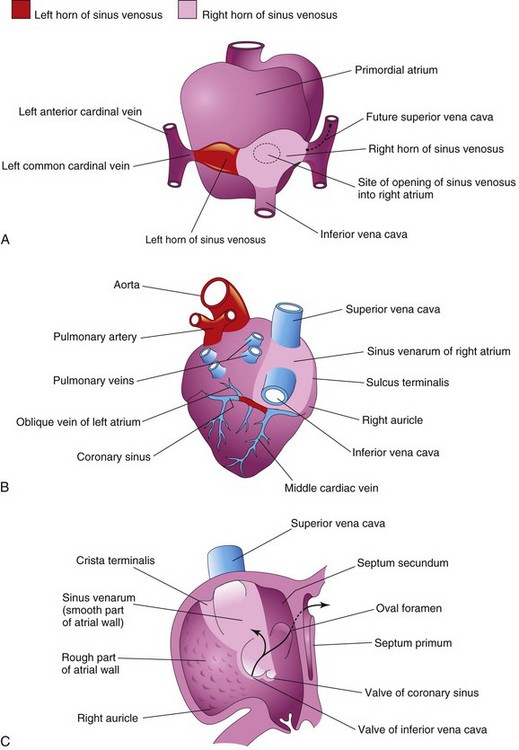
Figure 14–11 Illustrations of the fate of the sinus venosus. A, Dorsal view of the heart (approximately 26 days), showing the primordial atrium and sinus venosus. B, Dorsal view at 8 weeks, after incorporation of the right sinual horn into the right atrium. The left sinual horn has become the coronary sinus. C, Internal view of the fetal right atrium, showing the smooth part of the wall of the right atrium (sinus venarum), derived from the right sinual horn. The crista terminalis and the valves of the inferior vena cava and the coronary sinus, derived from the right sinuatrial valve are also shown. The primordial right atrium becomes the right auricle, a conical muscular pouch. Arrows indicate the flow of blood.
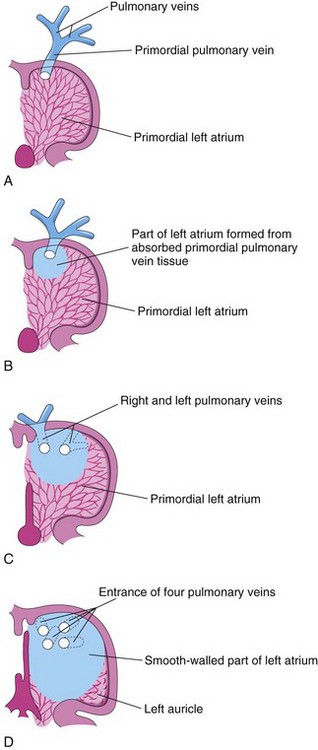
Figure 14–12 Illustrations of absorption of the pulmonary vein into the left atrium. A, At 5 weeks, showing the primordial pulmonary vein opening into the primordial left atrium. B, Later stage, showing partial absorption of the primordial pulmonary vein. C, At 6 weeks, showing the openings of two pulmonary veins into the left atrium as a result of absorption of the primordial pulmonary vein. D, At 8 weeks, showing four pulmonary veins with separate atrial orifices. The primordial left atrium becomes the left auricle, a tubular pouch of the atrium. Most of the left atrium is formed by absorption of the primordial pulmonary vein and its branches.
The left sinual horn becomes the coronary sinus, and the right sinual horn is incorporated into the wall of the right atrium (Fig. 14-11B and C) and becomes the smooth part of the internal wall of the right atrium is called the sinus venarum (Fig. 14-11B and C). The remainder of the anterior internal surface of the wall of the right atrium, as well as that of the auricle, has a rough, trabeculated appearance. These latter two parts are derived from the primordial atrium. The smooth part and the rough part are demarcated internally in the right atrium by a vertical ridge, termed the crista terminalis, or terminal crest (Fig. 14-11C), and externally by a shallow groove, the sulcus terminalis, or terminal groove (Fig. 14-11B). The crista terminalis represents the cranial part of the right sinuatrial valve (Fig. 14-11C); the caudal part of this valve forms the valves of the IVC and the coronary sinus. The left sinuatrial valve fuses with the septum secundum and is incorporated with it into the interatrial septum.
Primordial Pulmonary Vein and Formation of Left Atrium
Most of the wall of the left atrium is smooth because it is formed by the incorporation of the primordial pulmonary vein (Fig. 14-12A). This vein develops as an outgrowth of the dorsal atrial wall, just to the left of the septum primum. As the atrium expands, the primordial pulmonary vein and its main branches are gradually incorporated into the wall of the left atrium (Fig. 14-12B). As a result, four pulmonary veins are formed (Fig. 14-12C and D). The small left auricle is derived from the primordial atrium; its internal surface has a rough, trabeculated appearance.
Partitioning of Primordial Ventricle
Division of the primordial ventricle into two ventricles is first indicated by a median ridge—the muscular interventricular (IV) septum—in the floor of the ventricle, near its apex (Fig. 14-9B). This fold has a concave superior free edge (Fig. 14-13A). Initially, most of its increase in height results from dilation of the ventricles on each side of the muscular IV septum (Fig. 14-13B). Myocytes from both the right and left primordial ventricles contribute to the formation of the muscular part of the IV septum. Until the seventh week, there is a crescent-shaped opening (IV foramen) between the free edge of the IV septum and the fused endocardial cushions. The IV foramen permits communication between the right and left ventricles (Figs. 14-13B and 14-14B). The IV foramen usually closes by the end of the seventh week as the bulbar ridges fuse with the endocardial cushion (Fig. 14-14C to E). Closure of the IV foramen and formation of the membranous part of the IV septum result from the fusion of tissues from three sources: the right bulbar ridge, the left bulbar ridge, and the endocardial cushion.
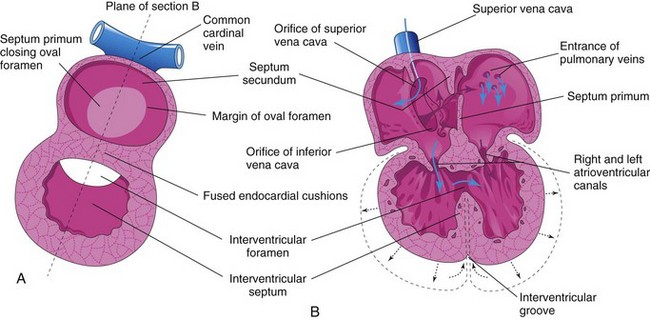
Figure 14–13 Illustrations of partitioning of the primordial heart. A, Sagittal section late in the fifth week, showing the cardiac septa and foramina. B, Frontal section at a slightly later stage, showing the directions of blood flow through the heart (blue arrows) and the expansion of the ventricles (dotted arrows).
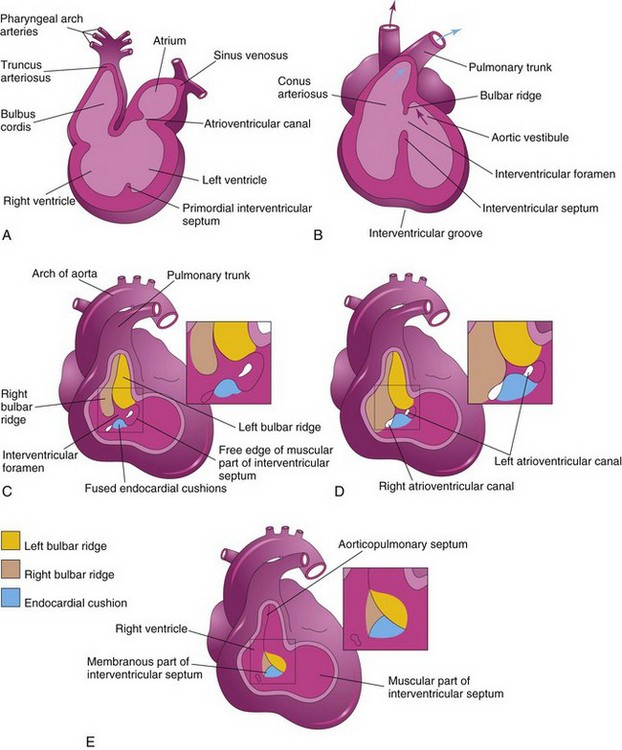
Figure 14–14 Illustrations of the incorporation of the bulbus cordis into the ventricles and partitioning of the bulbus cordis and truncus arteriosus into the aorta and the pulmonary trunk. A, Sagittal section at 5 weeks, showing the bulbus cordis as one of the chambers of the primordial heart. B, Schematic coronal section at 6 weeks, after the bulbus cordis has been incorporated into the ventricles to become the conus arteriosus (infundibulum) of the right ventricle and the aortic vestibule of the left ventricle. The arrows indicate blood flow. C to E, Closure of the interventricular (IV) foramen and formation of the membranous part of the IV septum. The walls of the truncus arteriosus, the bulbus cordis, and the right ventricle have been removed. C, At 5 weeks, showing the bulbar ridges and fused endocardial cushions. D, At 6 weeks, showing how the proliferation of subendocardial tissue diminishes the IV foramen. E, At 7 weeks, showing the fused bulbar ridges, the membranous part of the IV septum formed by extensions of tissue from the right side of the endocardial cushions, and closure of the IV foramen.
The membranous part of the IV septum is derived from an extension of tissue from the right side of the endocardial cushion to the muscular part of the IV septum. This tissue merges with the aorticopulmonary septum and the thick, muscular part of the IV septum (Fig. 14-15B). Closure of the IV foramen and formation of the membranous part of the IV septum results in communication of the pulmonary trunk with the right ventricle and the aorta communicates with the left ventricle (Fig. 14-14E). A process of cavitation in the ventricular walls forms a sponge-like mass of muscular bundles. Some bundles remain as the trabeculae carneae. Other bundles become the papillary muscles and tendinous cords (Latin chordae tendineae). The tendinous cords run from the papillary muscles to the AV valves (Fig. 14-15B).
Partitioning of Bulbus Cordis and Truncus Arteriosus
During the fifth week, active proliferation of the mesenchymal cells in the walls of the bulbus cordis results in the formation of bulbar ridges (Figs. 14-14C and D and 14-16B and C). Similar ridges form in the truncus arteriosus; these are continuous with the bulbar ridges. The bulbar and truncal ridges are derived mainly from the neural crest mesenchyme. Bone morphogenetic protein and other signaling systems, such as Wnt and fibroblast growth factor, have been implicated in the induction and migration of neural crest cells through the primordial pharynx and the pharyngeal arches. Concurrently, the bulbar and truncal ridges undergo 180-degree spiraling. The spiral orientation of the bulbar and truncal ridges, possibly caused in part by the streaming of blood from the ventricles, results in the formation of a spiral aorticopulmonary septum when the ridges fuse (Fig. 14-16D to G). This septum divides the bulbus cordis and the truncus arteriosus into two arterial channels, the aorta, and the pulmonary trunk. Because of the spiraling of the aorticopulmonary septum, the pulmonary trunk twists around the ascending aorta (see Fig. 14-16H).
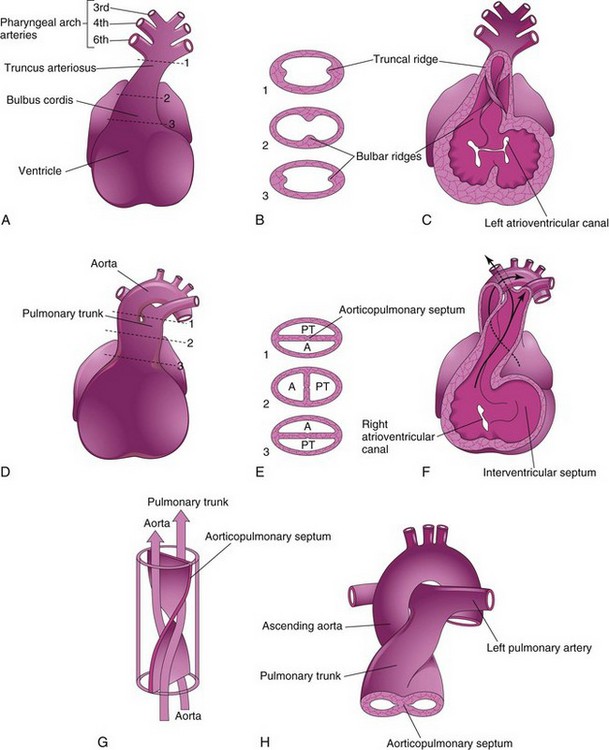
Figure 14–16 Partitioning of the bulbus cordis and truncus arteriosus. A, Ventral aspect of the heart at 5 weeks. The broken lines and arrows indicate the levels of the sections shown in B. B, Transverse sections of the truncus arteriosus and bulbus cordis, showing the truncal and bulbar ridges. C, The ventral wall of the heart and the truncus arteriosus has been removed to show these ridges. D, Ventral aspect of the heart after partitioning of the truncus arteriosus. The broken lines and arrows indicate the levels of the sections shown in E. E, Sections through the newly formed aorta (A) and the pulmonary trunk (PT) show the aorticopulmonary septum. F, At 6 weeks. The ventral wall of the heart and the pulmonary trunk has been removed to show the aorticopulmonary septum. G, The spiral form of the aorticopulmonary septum is shown. H, The ascending aorta and pulmonary trunk are shown as they twist around each other and they leave the heart.
The bulbus cordis is incorporated into the walls of the definitive ventricles in several ways (see Fig. 14-14A and B):
• In the right ventricle, the bulbus cordis is represented by the conus arteriosus (infundibulum), which gives origin to the pulmonary trunk.
• In the left ventricle, the bulbus cordis forms the walls of the aortic vestibule, the part of the ventricular cavity just inferior to the aortic valve.
Fetal Cardiac Ultrasonography
Echocardiography and Doppler ultrasonography have made it possible for sonographers to recognize normal and abnormal fetal cardiac anatomy. Most studies are performed at 18 to 22 weeks gestation, when the heart is large enough to be examined easily; however, real-time ultrasound images of the fetal heart can be obtained at 16 weeks.
Development of Cardiac Valves
The semilunar valves develop from three swellings of subendocardial tissue around the orifices of the aorta and pulmonary trunk (Fig. 14-17B to F). These swellings are hollowed out and reshaped to form three thin-walled cusps. The AV valves (tricuspid and mitral valves) develop similarly from localized proliferations of tissue around the AV canals.
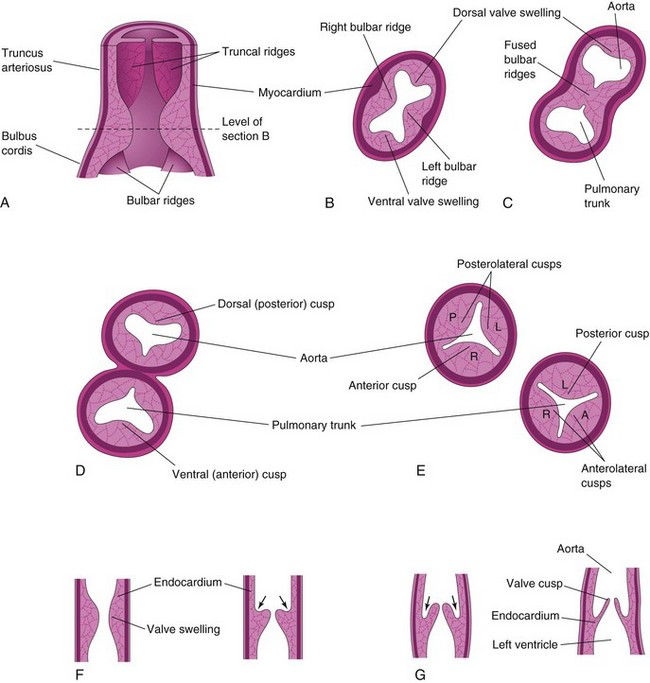
Figure 14–17 Development of the semilunar valves of the aorta and pulmonary trunk. A, Section of the truncus arteriosus and the bulbus cordis, showing the valve swellings. B, Transverse section of the bulbus cordis. C, Similar section after fusion of the bulbar ridges. D, Formation of the walls and valves of the aorta and the pulmonary trunk. E, Rotation of the vessels has established the adult positions of the valves in relation to each other. F and G, Longitudinal sections of the aorticoventricular junction, showing successive stages in the hollowing (arrows) and thinning of the valve swellings to form the valve cusps. L, left; P, posterior; R, right.
Conducting System of Heart
Initially, the muscle layers of the atrium and the ventricle are continuous. The primordial atrium acts as the interim pacemaker of the heart, but the sinus venosus soon takes over this function. The sinuatrial node develops during the fifth week. This node is located in the right atrium, near the entrance of the superior vena cava (SVC) (Fig. 14-15B). After incorporation of the sinus venosus into the heart, cells from its left wall are found in the base of the interatrial septum, near the opening of the coronary sinus. Together with cells from the AV region, they form the AV node and the AV bundle, located just superior to the endocardial cushions. The fibers arising from the AV bundle pass from the atrium into the ventricle and split into right and left bundle branches, which are distributed throughout the ventricular myocardium (Fig. 14-15B). The sinuatrial node, AV node, and AV bundle are richly supplied by nerves; however, the primordial conducting system is developed before these nerves enter the heart.
Abnormalities of Conducting System
Abnormalities of the conducting tissue may cause unexpected death during infancy, as in “crib death,” or sudden infant death syndrome. Most likely, no single mechanism is responsible for the sudden, unexpected deaths of apparently healthy infants. A brainstem developmental abnormality or maturational delay related to neuroregulation of cardiorespiratory control has been suggested.
Birth Defects of the Heart and Great Vessels
Congenital heart defects (CHDs) occur with a frequency of 6 to 8 in 1000 births. Some cases of CHD are caused by single-gene or chromosomal mechanisms; others result from exposure to teratogens such as the rubella virus. Most CHDs are believed to be caused by multiple factors, both genetic and environmental (i.e., multifactorial inheritance). Recent technology, such as real-time, 3D echocardiography, has permitted the detection of fetal CHDs as early as the 17th or 18th week of gestation.
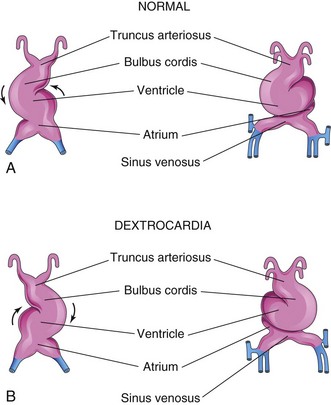
Dextrocardia
If the heart tube bends to the left instead of to the right, the heart is displaced to the right (Fig. 14-18), and there is transposition whereby the heart and its vessels are reversed, left to right, as in a mirror image. Dextrocardia is the most frequent positional abnormality of the heart. In dextrocardia with situs inversus (transposition of abdominal viscera), the incidence of accompanying cardiac defects is low. In isolated dextrocardia, the abnormal position of the heart is not accompanied by displacement of other viscera.
Ectopia Cordis
In ectopia cordis, an extremely rare condition, the heart is located outside the thoracic cavity. The most common thoracic form of ectopia cordis results from faulty development of the sternum and pericardium secondary to incomplete fusion of the lateral folds in the formation of the thoracic wall during the fourth week. Death occurs in most cases during the first few days after birth, usually as a result of infection, cardiac failure, or hypoxemia. If no severe cardiac defects are present, surgical therapy usually consists of covering the heart with skin.
Atrial Septal Defects
Atrial septal defects (ASDs) occur more frequently in girls than in boys. The most common form of ASD is a patent oval foramen (Figs. 14-19A and 14-20A to D). A small, isolated patent oval foramen is of no hemodynamic significance. However, if other defects are present (e.g., pulmonary atresia), blood is shunted through the oval foramen into the left atrium, producing cyanosis.
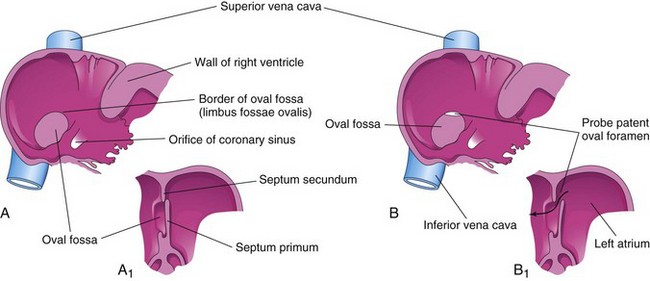
Figure 14–19 A, Normal postnatal appearance of the right side of the interatrial septum after adhesion of the septum primum to the septum secundum. A1, Section of the interatrial septum, showing the formation of the oval fossa in the interatrial septum. Note that the floor of this fossa is formed by the septum primum. B and B1, Similar views of a probe patent oval foramen resulting from incomplete adhesion of the septum primum to the septum secundum.
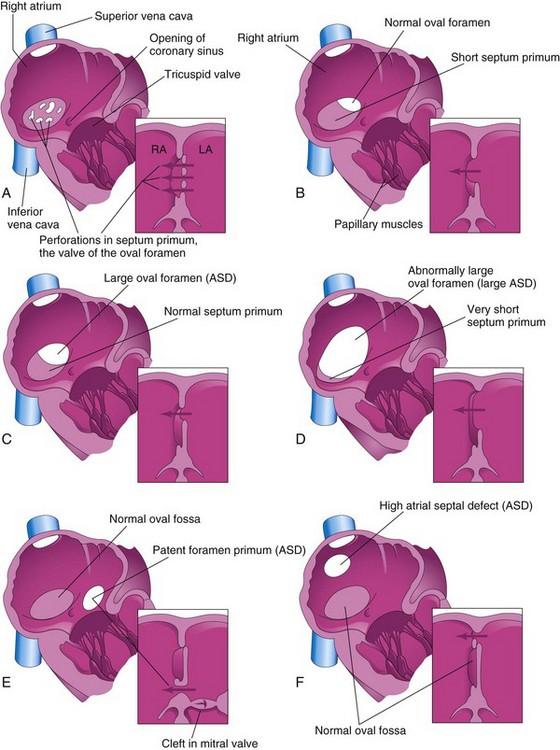
Figure 14–20 Illustrations of the right aspect of the interatrial septum. The adjacent sketches of sections of the septa show various types of atrial septal defects (ASD). A, Patent oval foramen resulting from resorption of the septum primum in abnormal locations. B, Patent oval foramen caused by excessive resorption of the septum primum (“short flap defect”). C, Patent oval foramen resulting from an abnormally large oval foramen. D, Patent oval foramen resulting from an abnormally large oval foramen and excessive resorption of the septum primum. E, Endocardial cushion defect with a primum-type ASD. The adjacent section shows the cleft in the anterior cusp of the mitral valve. F, Sinus venosus ASD. The high septal defect resulted from abnormal absorption of the sinus venosus into the right atrium. In E and F, note that the oval fossa has formed normally. Arrows indicate the direction of flow of blood.
A probe patent oval foramen is present in up to 25% of people. In this abnormality, a probe can be passed from one atrium to the other through the superior part of the floor of the oval fossa; the defect is not clinically significant. A probe patent oval foramen results from incomplete adhesion between the flap of the valve of the oval foramen and the septum secundum after birth.
There are four clinically significant types of ASD (Fig. 14-20), of which the first two are relatively common:
Ostium secundum ASDs (Fig. 14-20A to D) occur in the area of the oval fossa and include defects of both the septum primum and the septum secundum. Females with ASDs outnumber males 3 to 1. Ostium secundum ASDs are one of the most common, yet least severe types of congenital heart disease (CHD). The patent oval foramen usually results from abnormal resorption of the septum primum during the formation of the foramen secundum. If resorption occurs in abnormal locations, the septum primum is fenestrated, or net-like (Fig. 14-20A). If excessive resorption of the septum primum occurs, the resulting short septum primum does not close the oval foramen (Fig. 14-20B). If an abnormally large oval foramen develops as a result of defective development of the septum secundum, a normal septum primum does not close the abnormal oval foramen at birth. Large ostium secundum ASDs may also occur because of a combination of excessive resorption of the septum primum and a large oval foramen.
Endocardial cushion defects with a patent foramen primum (Fig. 14-20E) are less common forms of ASD. The septum primum does not fuse with the endocardial cushions, resulting in a patent foramen primum. Usually, there is also a cleft in the anterior cusp of the mitral valve.
Sinus venosus ASDs are located in the superior part of the interatrial septum, close to the entry of the SVC (Fig. 14-20F). These defects result from incomplete absorption of the sinus venosus into the right atrium, abnormal development of the septum secundum, or both. Common atrium occurs in patients with all three types of defects: ostium secundum, ostium primum, and sinus venosus.
Ventricular Septal Defects
Ventricular septal defects (VSDs) are the most common type of CHD, accounting for approximately 25% of cases. VSDs occur more frequently in males than in females. Most VSDs involve the membranous part of the IV septum (Fig. 14-21B). Many small VSDs close spontaneously, usually during the first year of life. Most people with a large VSD have massive left to right shunting of blood. Muscular VSD is a less common type of defect that may appear anywhere in the muscular part of the IV septum. Transposition of the great arteries (Fig. 14-22) and a rudimentary outlet chamber are present in most infants with this severe type of CHD.
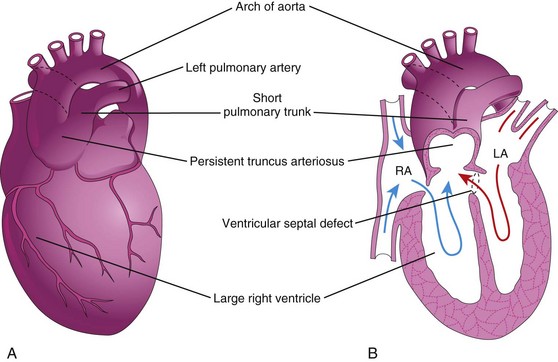
Figure 14–21 The main types of persistent truncus arteriosus (PTA). A, The common trunk divides into the aorta and a short pulmonary trunk. B, Frontal section of the heart shown in A. Observe the circulation in this heart (arrows) and the ventricular septal defect (VSD). LA, left atrium; RA, right atrium.
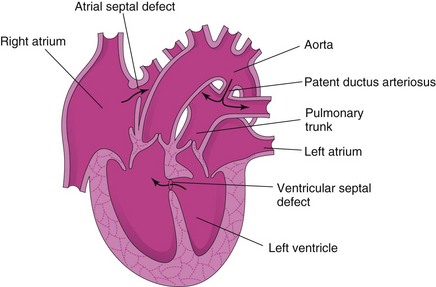
Figure 14–22 Frontal section of a malformed heart, showing transposition of the great arteries. The ventricular septal defect (VSD) and the atrial septal defect (ASD) allow mixing of the arterial and venous blood. Transposition of the great arteries is the most common single cause of cyanotic heart disease in newborn infants. As is shown here, it is often associated with other cardiac anomalies (VSD and ASD). The arrows indicate the flow of blood. In TGA, when there is an ASD, blood flows from the right atrium to the left atrium.
Persistent Truncus Arteriosus
Persistent truncus arteriosus results from failure of the truncal ridges and the aorticopulmonary septum to develop normally and to divide the truncus arteriosus into the aorta and the pulmonary trunk (Fig. 14-21). The most common type of persistent truncus arteriosus is a single arterial trunk (truncus arteriosus) that branches to form the pulmonary trunk and the ascending aorta (Fig. 14-21A and B) supplying the systemic, pulmonary, and coronary circulations. A VSD is always present with truncus arteriosus, and the truncus arteriosus straddles the VSD (Fig. 14-21B).
Transposition of Great Arteries
Transposition of the great arteries is the most common cause of cyanotic heart disease in newborn infants (Fig. 14-22). In typical cases, the aorta lies anterior and to the right of the pulmonary trunk and arises anteriorly from the morphologic right ventricle, whereas the pulmonary trunk arises from the morphologic left ventricle. There is also an ASD, with or without an associated patent ductus arteriosus (PDA) and VSD. This defect is believed to result from failure of the conus arteriosus to develop normally during incorporation of the bulbus cordis into the ventricles. Defective neural crest cell migration may also be involved.
Unequal Division of Truncus Arteriosus
Unequal division of the truncus arteriosus (Figs. 14-21 and 14-23A and B) results when partitioning of the truncus arteriosus superior to the valves is disparate, producing one large great artery and one small one. As a result, the aorticopulmonary septum is not aligned with the IV septum, and a VSD results. The larger vessel (aorta or pulmonary trunk) usually straddles the VSD (Fig. 14-23A and B). In pulmonary valve stenosis, the cusps of the pulmonary valve are fused together to form a dome with a narrow central opening. In infundibular stenosis, the conus arteriosus of the right ventricle is underdeveloped. The two types of pulmonary stenosis may be concurrent. Depending on the degree of obstruction to blood flow, there is a variable degree of hypertrophy of the right ventricle (see Fig. 14-23B).
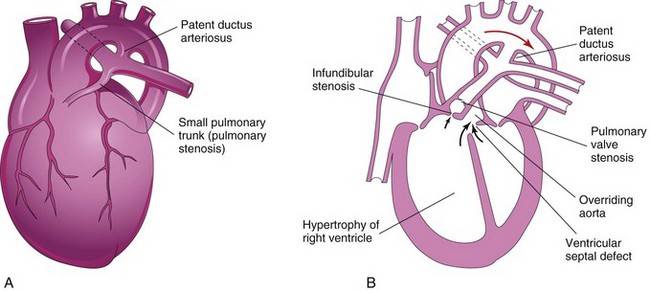
Figure 14–23 A, An infant’s heart showing a small pulmonary trunk (pulmonary stenosis) and a large aorta resulting from unequal partitioning of the truncus arteriosus. There is also hypertrophy of the right ventricle and a patent ductus arteriosus. B, Frontal section of a heart, showing a tetralogy of Fallot. Observe the four cardiac defects of this tetralogy: pulmonary valve stenosis, ventricular septal defect, overriding aorta, and hypertrophy of the right ventricle. In this case, infundibular stenosis is also shown. The arrows indicate the flow of blood into the great vessels (aorta and pulmonary trunk).
Tetralogy of Fallot
The classic group of four cardiac defects known as tetralogy of Fallot (see Fig. 14-23A and B) consists of the following:
• Pulmonary stenosis (obstructed right ventricular outflow)
• Dextroposition of the aorta (straddling or overriding both ventricles)
The pulmonary trunk is usually small, and there may be varying degrees of pulmonary artery stenosis as well.
Aortic Stenosis and Aortic Atresia
In aortic valve stenosis, the edges of the valve are usually fused to form a dome with a narrow opening. This anomaly may be present at birth (congenital), or it may develop after birth (acquired). The valvular stenosis causes extra work for the heart and results in hypertrophy of the left ventricle and abnormal heart sounds (heart murmurs). In subaortic stenosis, there is often a band of fibrous tissue just inferior to the aortic valve. The narrowing of the aorta results from persistence of tissue that normally degenerates as the valve forms. Aortic atresia is present when obstruction of the aorta or its valve is complete.
Derivatives of Pharyngeal Arch Arteries
As the pharyngeal arches develop during the fourth week, they are supplied by pharyngeal arch arteries that arise from the aortic sac (Fig. 14-24B). The pharyngeal arch arteries terminate in the dorsal aorta on the ipsilateral side. Although six pairs of pharyngeal arch arteries usually develop, they are not all present at the same time (Fig. 14-6).
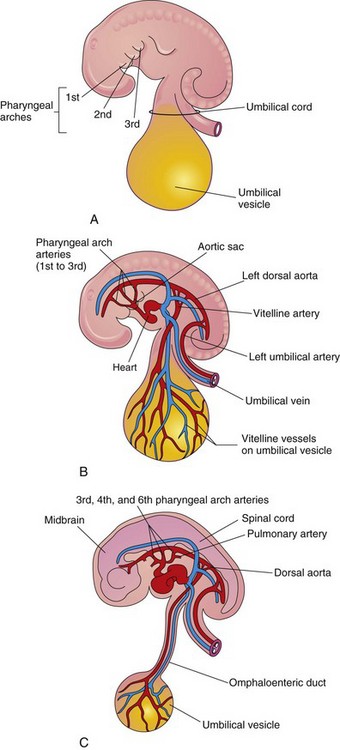
Figure 14–24 The pharyngeal arches and pharyngeal arch arteries. A, Left side of an embryo (approximately 26 days). B, Illustration of this embryo, showing the left pharyngeal arch arteries arising from the aortic sac, running through the pharyngeal arches, and terminating in the left dorsal aorta. C, An embryo (approximately 37 days). Note the single dorsal aorta and the mostly degenerated first two pairs of pharyngeal arch arteries.
Derivatives of First Pair of Pharyngeal Arch Arteries
The first pair of arteries largely disappears but remnants of them form parts of the maxillary arteries, which supply the ears, teeth, muscles of the eyes and face.
Derivatives of Second Pair of Pharyngeal Arch Arteries
Dorsal parts of the second pair of pharyngeal arch arteries persist and form the stems of the small stapedial arteries, which run through the ring of the stapes (but undergo atrophy before birth).
Derivatives of Third Pair of Pharyngeal Arch Arteries
Proximal parts of the third pair of pharyngeal arch arteries form the common carotid arteries, which supply structures in the head (Fig. 14-25D). Distal parts of the third pair of pharyngeal arch arteries join with the dorsal aortas to form the internal carotid arteries, and they supply the ears, the orbits, and the brain and its meninges.
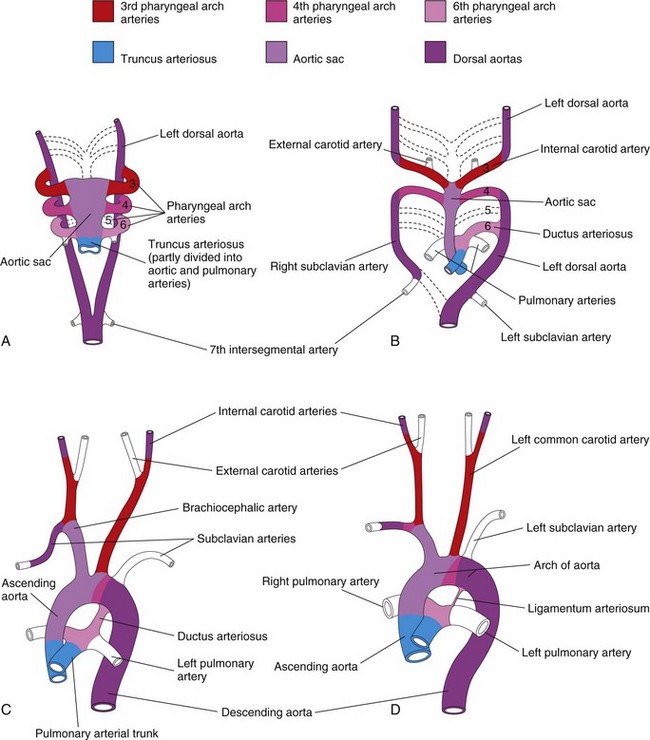
Figure 14–25 Illustration of the arterial changes that occur during transformation of the truncus arteriosus, aortic sac, pharyngeal arch arteries, and dorsal aortas into the adult arterial pattern. The vessels that are not colored are not derived from these structures. A, Pharyngeal arch arteries at 6 weeks. By this stage, the first two pairs of pharyngeal arch arteries have largely disappeared. B, Pharyngeal arch arteries at 7 weeks. The parts of the dorsal aortas and pharyngeal arch arteries that normally disappear are indicated with broken lines. C, Arterial arrangement at 8 weeks. D, Arterial vessels of a 6-month-old infant. Note that the ascending aorta and the pulmonary arteries are considerably smaller in C than in D. This represents the relative flow through these vessels at the different stages of development. Observe the large size of the ductus arteriosus in C; it is essentially a direct continuation of the pulmonary trunk. Eventually, the ductus arteriosus becomes the ligamentum arteriosum, as shown in D.
Derivatives of Fourth Pair of Pharyngeal Arch Arteries
The left fourth pharyngeal arch artery forms part of the arch of the aorta (Fig. 14-25C and D). The proximal part of the arch artery develops from the aortic sac, whereas the distal part is derived from the left dorsal aorta. The right fourth pharyngeal arch artery becomes the proximal part of the right subclavian artery. The distal part of the subclavian artery forms from the right dorsal aorta and the right seventh intersegmental artery. The left subclavian artery is not derived from a pharyngeal arch artery; it forms from the left seventh intersegmental artery (Fig. 14-25A). As development proceeds, differential growth shifts the origin of the left subclavian artery cranially. Consequently, it comes to lie close to the origin of the left common carotid artery (Fig. 14-25D).
Fate of Fifth Pair of Pharyngeal Arch Arteries
In approximately 50% of embryos, the fifth pair of pharyngeal arch arteries comprises rudimentary vessels that soon degenerate, leaving no vascular derivatives. In other embryos, these arches do not develop.
Derivatives of Sixth Pair of Pharyngeal Arch Arteries
The left sixth pharyngeal arch artery develops as follows (Fig. 14-25B and C):
• The proximal part of the artery persists as the proximal part of the left pulmonary artery.
• The distal part of the artery passes from the left pulmonary artery to the dorsal aorta and forms a prenatal shunt, the ductus arteriosus.
The right sixth pharyngeal arch artery develops as follows:
The transformation of the sixth pair of pharyngeal arch arteries explains why the course of the recurrent laryngeal nerves differs on the two sides. These nerves supply the sixth pair of pharyngeal arches and hook around the sixth pair of pharyngeal arch arteries on their way to the developing larynx (Fig. 14-26A). On the right, because the distal part of the right sixth pharyngeal arch artery degenerates, the right recurrent laryngeal nerve moves superiorly and hooks around the proximal part of the right subclavian artery, the derivative of the fourth pharyngeal arch artery (Fig. 14-26B). On the left, the left recurrent laryngeal nerve hooks around the ductus arteriosus formed by the distal part of the sixth pharyngeal arch artery. When this arterial shunt involutes after birth, the nerve remains around the ligamentum arteriosum (remnant of the ductus arteriosus) and the arch of the aorta (Fig. 14-26C).
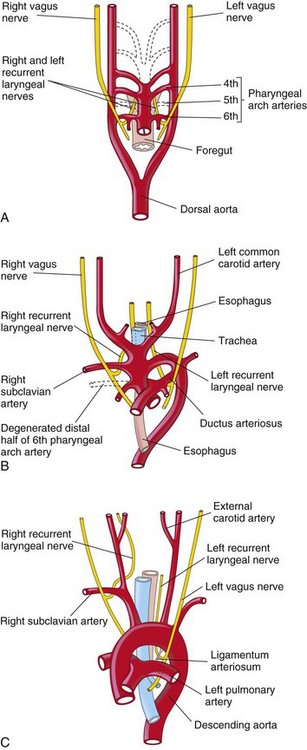
Figure 14–26 The relation of the recurrent laryngeal nerves to the pharyngeal arch arteries. A, At 6 weeks, showing that the recurrent laryngeal nerves are hooked around the sixth pair of pharyngeal arch arteries. B, At 8 weeks, showing that the right recurrent laryngeal nerve is hooked around the right subclavian artery and the left recurrent laryngeal nerve is hooked around the ductus arteriosus and the arch of the aorta. C, In a child, the left recurrent laryngeal nerve is hooked around the ligamentum arteriosum and the arch of the aorta.
Coarctation of Aorta
Aortic coarctation (constriction) occurs in approximately 10% of children and adults with CHDs. Coarctation is characterized by a constriction of the aorta of varying length (Fig. 14-27). Most constrictions occur distal to the origin of the left subclavian artery, at the entrance of the ductus arteriosus (juxtaductal coarctation). A classification system of preductal and postductal coarctations is commonly used; however, in most cases, the coarctation is directly opposite the ductus arteriosus. Coarctation of the aorta occurs twice as often in males as in females. Coarctation of the aorta is caused by genetic factors, environmental factors, or both.
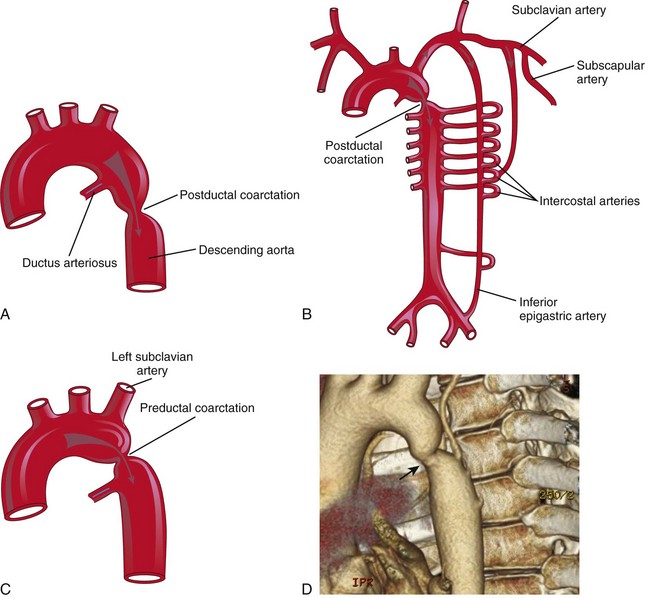
Figure 14–27 A, Postductal coarctation of the aorta. B, Common routes of the collateral circulation that develop in association with postductal coarctation of the aorta. C, Preductal coarctation. Arrows indicate flow of blood. D, Preductal coarctation (arrow) in aorta in an adult.
(D, Courtesy of Dr. James Koenig, Department of Radiology, Health Sciences Centre, Winnipeg, Manitoba, Canada.)
Double Pharyngeal Arch Artery
Double pharyngeal arch artery is a rare anomaly that is characterized by a vascular ring around the trachea and the esophagus (Fig. 14-28). The vascular ring results from failure of the distal part of the right dorsal aorta to disappear (see Fig. 14-28A); as a result, right and left arches form. Usually, the right arch of the aorta is the larger one and it passes posterior to the trachea and the esophagus (see Fig. 14-28B).
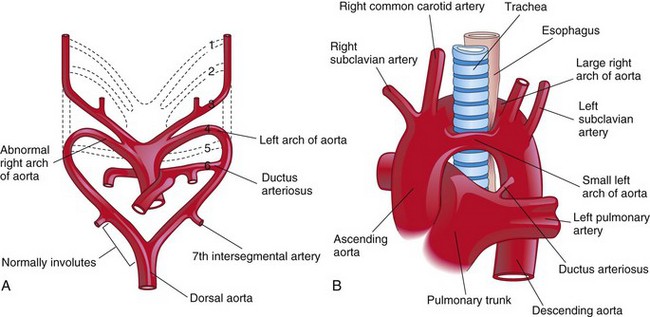
Figure 14–28 A, Embryonic pharyngeal arch arteries, showing the embryologic basis of a double arch of the aorta. The distal portion of the right dorsal aorta persists and forms a right pharyngeal arch artery. B, A large right arch of the aorta and a small left arch of the aorta arise from the ascending aorta and form a vascular ring around the trachea and the esophagus. Note the compression of the esophagus and the trachea. The right common carotid and subclavian arteries arise separately from the large right arch of the aorta.
Right Arch of Aorta
When the entire right dorsal aorta persists (Fig. 14-29A) and the distal part of the left dorsal aorta involutes, a right pharyngeal arch artery results. There are two main types:
• Right arch of the aorta without a retroesophageal component (see Fig. 14-29B). The ductus arteriosus (or ligamentum arteriosum) passes from the right pulmonary artery to the right arch of the aorta.
• Right arch of the aorta with a retroesophageal component (see Fig. 14-29C). Originally, a small left arch of the aorta probably involuted, leaving the right arch of the aorta posterior to the esophagus. The ductus arteriosus (or ligamentum arteriosum) attaches to the distal part of the arch of the aorta and forms a ring that may constrict the esophagus and the trachea.
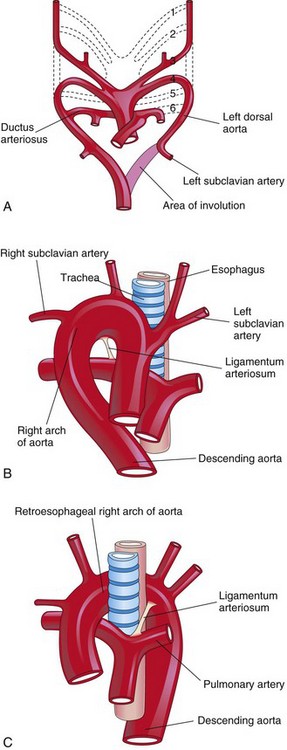
Figure 14–29 A, Pharyngeal arch arteries showing abnormal involution of the distal portion of the left dorsal aorta. There is also persistence of the entire right dorsal aorta and the distal part of the right sixth pharyngeal arch artery. B, Right pharyngeal arch artery without a retroesophageal component. C, Right arch of the aorta with a retroesophageal component. The abnormal right arch of the aorta and the ligamentum arteriosum (postnatal remnant of the ductus arteriosus) form a vascular ring that compresses the esophagus and the trachea.
Anomalous Right Subclavian Artery
The right subclavian artery normally arises from the distal part of the arch of the aorta and passes posterior to the trachea and the esophagus to supply the right upper limb (Fig. 14-30). A retroesophageal right subclavian artery occurs when the right fourth pharyngeal arch artery and the right dorsal aorta disappear cranial to the seventh intersegmental artery. As a result, the right subclavian artery forms from the right seventh intersegmental artery and the distal part of the right dorsal aorta. As development proceeds, differential growth shifts the origin of the right subclavian artery cranially, until it comes to lie close to the origin of the left subclavian artery.
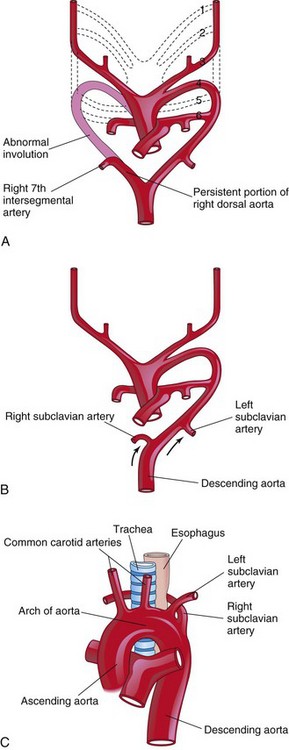
Figure 14–30 The possible embryologic basis for an abnormal origin of the right subclavian artery. A, The right fourth pharyngeal arch artery and the cranial part of the right dorsal aorta have involuted. As a result, the right subclavian artery forms from the right seventh intersegmental artery and the distal segment of the right dorsal aorta. B, As the arch of the aorta forms, the right subclavian artery is carried cranially (arrows) with the left subclavian artery. C, The abnormal right subclavian artery arises from the aorta and passes posterior to the trachea and the esophagus.
Pharyngeal Arch Artery Anomalies
Because of the many changes involved in the transformation of the embryonic pharyngeal arch system of arteries into the adult arterial pattern, it is understandable why anomalies may occur. Most irregularities result from the persistence of parts of the pharyngeal arch arteries that usually disappear or from the disappearance of parts that normally persist.
Fetal and Neonatal Circulation
The fetal circulation (Fig. 14-31) is designed to serve prenatal needs. Modifications at birth establish the neonatal pattern (Fig. 14-32). Before birth, the lungs do not provide gas exchange and the pulmonary vessels are vasoconstricted. Three shunts are essential in the transitional circulation: the ductus venosus, oval foramen, and ductus arteriosus.
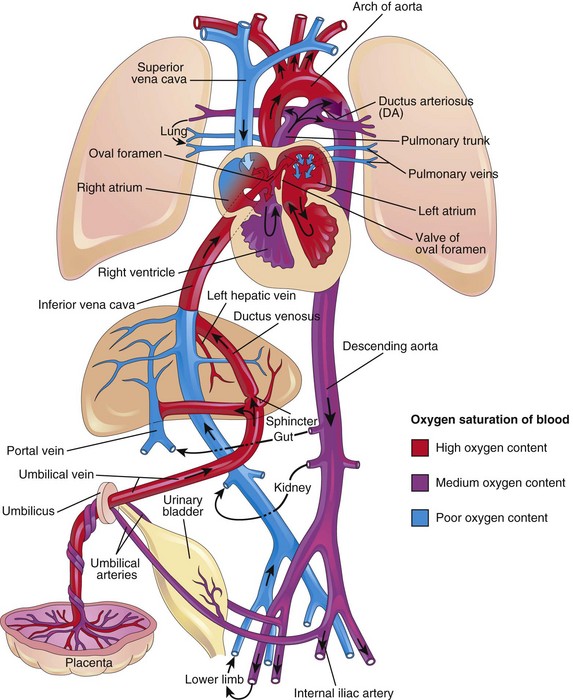
Figure 14–31 Fetal circulation. The colors indicate the oxygen saturation of the blood, and the arrows show the course of the blood from the placenta to the heart. The organs are not drawn to scale. A small amount of highly oxygenated blood from the inferior vena cava remains in the right atrium and mixes with poorly oxygenated blood from the superior vena cava. Observe that three shunts permit most of the blood to bypass the liver and lungs: (1) the ductus venosus, (2) the oval foramen, and (3) the ductus arteriosus. The poorly oxygenated blood returns to the placenta for oxygen and nutrients through the umbilical arteries.
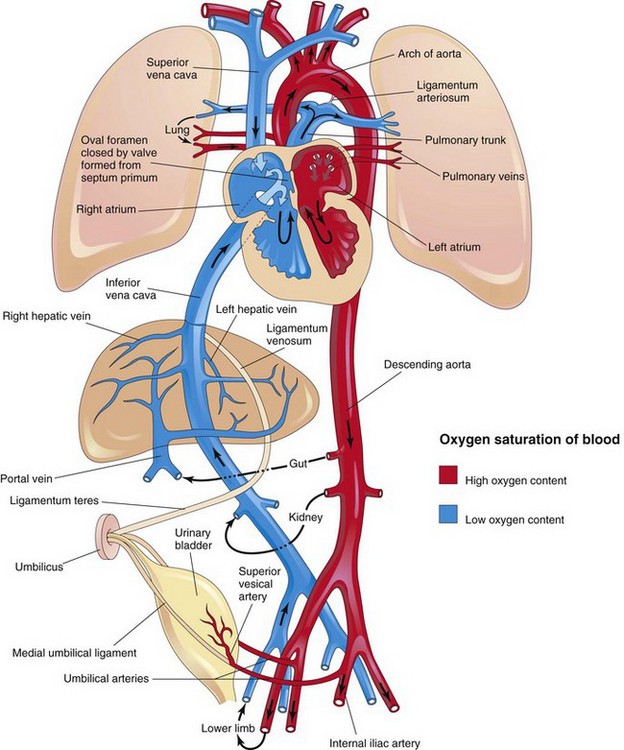
Figure 14–32 Neonatal circulation. The adult derivatives of the fetal vessels and structures that become nonfunctional at birth are shown. The arrows indicate the course of the blood in the infant. The organs are not drawn to scale. After birth, the three fetal shunts cease to function, and the pulmonary and systemic circulations become separated.
Fetal Circulation
Highly oxygenated, nutrient-rich blood returns under high pressure from the placenta in the umbilical vein (see Fig. 14-31). On approaching the liver, approximately one half of the blood passes directly into the ductus venosus, a fetal vessel connecting the umbilical vein to the IVC; consequently this blood bypasses the liver. The other half of the blood in the umbilical vein flows into the sinusoids of the liver and enters the IVC through the hepatic veins. Blood flow through the ductus venosus is regulated by a sphincter mechanism close to the umbilical vein. After a short course in the IVC, all of the blood enters the right atrium of the heart. Most blood from the IVC is directed by the inferior border of the septum secundum (crista dividens), through the oval foramen, and into the left atrium. There, it mixes with the relatively small amount of poorly oxygenated blood returning from the lungs through the pulmonary veins. Fetal lungs use the oxygen from the blood instead of replenishing it. From the left atrium, the blood then passes to the left ventricle and leaves through the ascending aorta. The arteries to the heart, head, neck, and upper limbs receive well-oxygenated blood. The liver also receives well-oxygenated blood from the umbilical vein.
The small amount of well-oxygenated blood from the IVC that remains in the right atrium mixes with poorly oxygenated blood from the SVC and the coronary sinus and passes into the right ventricle. This blood, with medium oxygen content, leaves the heart through the pulmonary trunk. Because of the high pulmonary vascular resistance in fetal life, pulmonary blood flow is low. Approximately 10% of the blood goes to the lungs, but most of it passes through the ductus arteriosus into the aorta to the fetal body. It then returns to the placenta through the umbilical arteries (Fig. 14-31). Approximately 10% of blood from the ascending aorta enters the descending aorta to supply the viscera and the inferior part of the body. Most of the blood in the descending aorta passes into the umbilical arteries and is returned to the placenta for reoxygenation.
Transitional Neonatal Circulation
Important circulatory adjustments occur at birth, when the circulation of fetal blood through the placenta ceases and the infant’s lungs expand and begin to function (Fig. 14-32). As soon as the infant is born, the oval foramen, ductus arteriosus, ductus venosus, and umbilical vessels are no longer needed. The sphincter in the ductus arteriosus constricts and all blood entering the liver passes through the hepatic sinusoids. This, combined with occlusion of the placental circulation, causes an immediate decrease in blood pressure in the IVC and the right atrium.
Because of increased pulmonary blood flow, the pressure in the left atrium is higher than in the right atrium. The increased left atrial pressure closes the oval foramen by pressing the valve of the foramen against the septum secundum (Fig. 14-32). The output from the right ventricle then flows entirely into the pulmonary circulation. Because pulmonary vascular resistance is lower than systemic vascular resistance, blood flow in the ductus arteriosus reverses, passing from the aorta to the pulmonary trunk.
The ductus arteriosus begins to constrict at birth, but for a few days there is often a small shunt of blood from the aorta to the pulmonary trunk in healthy, full-term infants. In premature infants and those with persistent hypoxia, the ductus arteriosus may remain open much longer. In full-term infants, oxygen is the most important factor in controlling closure of the ductus arteriosus, and it appears to be mediated by bradykinin and prostaglandins.
The umbilical arteries constrict at birth, preventing loss of the infant’s blood. The umbilical cord is not tied for a minute or so; consequently, blood flow through the umbilical vein continues, transferring fetal blood from the placenta to the infant.
The change from the fetal to the adult pattern of blood circulation is not a sudden occurrence. Some changes occur with the first breath; others take place over hours and days. The closure of fetal shunts and the oval foramen is initially a functional change. Later, anatomical closure results from the proliferation of endothelial and fibrous tissues.
Derivatives of Fetal Vascular Structures
Because of the changes in the cardiovascular system at birth, certain vessels and structures are no longer required after birth.
Umbilical Vein and Round Ligament of the Liver
The intra-abdominal part of the umbilical vein eventually becomes the round ligament of the liver (Latin ligamentum teres) (Fig. 14-32). The umbilical vein remains patent for a considerable period and may be used for blood transfusions during early infancy. These transfusions are often performed to prevent brain damage and death in infants with anemia as a result of erythroblastosis fetalis.
Ductus Venosus and Ligamentum Venosum
The ductus venosus becomes the ligamentum venosum; however, its closure is more prolonged than that of the ductus arteriosus. The ligamentum venosum passes through the liver from the left branch of the portal vein to the IVC, to which it is attached (Fig. 14-33).
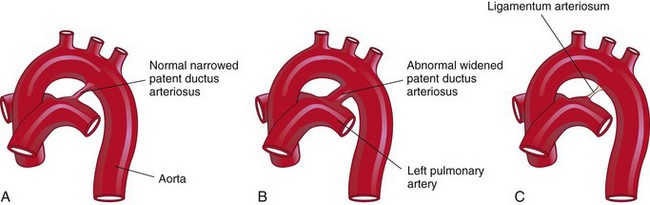
Figure 14–33 Closure of the ductus arteriosus. A, The ductus arteriosus of a newborn infant. B, Abnormal patent ductus arteriosus in a 6-month-old infant. C, The ligamentum arteriosum in a 6-month-old infant.
Patent Ductus Arteriosus
A common anomaly, PDA occurs two to three times more frequently in females than in males (see Fig. 14-33B). Functional closure of the ductus arteriosus usually occurs soon after birth; however, if it remains patent, aortic blood is shunted into the pulmonary artery. PDA is the most common congenital anomaly associated with maternal rubella infection during early pregnancy. Premature infants, infants born at high altitude, and those with certain chromosomal anomalies may also have a PDA. The embryologic basis of PDA is failure of the ductus arteriosus to involute after birth and form the ligamentum arteriosum.
Umbilical Arteries and Abdominal Ligaments
Most of the intra-abdominal parts of the umbilical arteries become the medial umbilical ligaments (see Fig. 14-32); the proximal parts of these vessels persist as the superior vesical arteries, which supply the urinary bladder.
Oval Foramen and Oval Fossa
The oval foramen normally closes functionally at birth. Anatomical closure occurs by the third month and results from tissue proliferation and adhesion of the septum primum to the left margin of the septum secundum. The septum primum forms the floor of the oval fossa. The inferior edge of the septum secundum forms a rounded fold, the border of the oval fossa (Latin limbus fossae ovalis), which marks the former boundary of the oval foramen (Fig. 14-19).
Development of THE Lymphatic System
The lymphatic system begins to develop at the end of the sixth week. Lymphatic vessels develop in a manner similar to that described for blood vessels, and they make connections with the venous system. The early lymphatic capillaries join each other to form a network of lymphatics. Six primary lymph sacs exist at the end of the embryonic period (Fig. 14-34A):
• Two jugular lymph sacs near the junction of the subclavian veins with the anterior cardinal veins (future internal jugular veins)
• Two iliac lymph sacs near the junction of the iliac veins with the posterior cardinal veins
• One retroperitoneal lymph sac in the root of the mesentery on the posterior abdominal wall
• One cisterna chyli (chyle cistern ) located dorsal to the retroperitoneal lymph sac
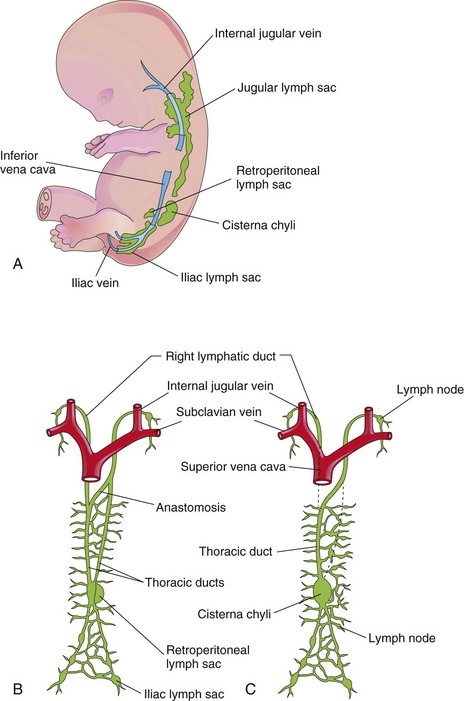
Figure 14–34 Development of the lymphatic system. A, Left side of a  week embryo, showing the primary lymph sacs. B, Ventral view of the lymphatic system at 9 weeks, showing the paired thoracic ducts. C, Later in the fetal period, showing formation of the thoracic duct and the right lymphatic duct.
week embryo, showing the primary lymph sacs. B, Ventral view of the lymphatic system at 9 weeks, showing the paired thoracic ducts. C, Later in the fetal period, showing formation of the thoracic duct and the right lymphatic duct.
Lymphatic vessels soon join the lymph sacs and accompany main veins; to the head, neck, and upper limbs from the jugular lymph sacs; to the lower trunk and lower limbs from the iliac lymph sacs; and to the primordial gut from the retroperitoneal lymph sac and the cisterna chyli. Two large channels (right and left thoracic ducts) connect the jugular lymph sacs with this cistern. Soon, a large anastomosis forms between these channels (Fig. 14-34B).
The thoracic duct develops from:
• The caudal part of the right thoracic duct
• The anastomosis between the thoracic ducts and the cranial part of the left thoracic duct
The right lymphatic duct is derived from the cranial part of the right thoracic duct (Fig. 14-34C). The thoracic duct and the right lymphatic duct connect with the venous system at the venous angle between the internal jugular vein and the subclavian vein.
Development of Lymph Nodes
Except for the superior part of the cisterna chyli, the lymph sacs are transformed into groups of lymph nodes during the early fetal period. Mesenchymal cells invade each lymph sac and form a network of lymphatic channels, the primordia of the lymph sinuses. Other mesenchymal cells give rise to the capsules and connective tissue framework of the lymph nodes.
The lymphocytes are derived originally from primordial stem cells in the umbilical vesicle mesenchyme and later from the liver and spleen. The lymphocytes eventually enter the bone marrow, where they divide to form lymphoblasts. The lymphocytes that appear in the lymph nodes before birth are derived from the thymus, a derivative of the third pair of pharyngeal pouches (Chapter 10). Small lymphocytes leave the thymus and circulate to other lymphoid organs. Later, some mesenchymal cells in the lymph nodes also differentiate into lymphocytes.
Anomalies of Lymphatic System
Congenital anomalies of the lymphatic system are uncommon. There may be diffuse swelling of a part of the body, termed congenital lymphedema. This condition may result from dilation of the primordial lymphatic channels or from congenital hypoplasia of the lymphatic vessels. Cystic hygromas are large swellings that usually appear in the inferolateral part of the neck and consist of large, single or multilocular, fluid-filled cavities. Hygromas may be present at birth, but they often enlarge and become evident during later infancy. Hygromas are believed to arise from parts of a jugular lymph sac that are pinched off, or from lymphatic spaces that do not establish connections with the main lymphatic channels.
Development of Spleen and Tonsils
The spleen develops from an aggregation of mesenchymal cells in the dorsal mesentery of the stomach (see Chapter 12). The palatine tonsils develop primarily from the second pair of pharyngeal pouches and the nearby mesenchyme. The tubal tonsils develop from aggregations of lymph nodules around the pharyngeal openings of the pharyngotympanic tubes. The pharyngeal tonsils (adenoids) develop from an aggregation of lymph nodules in the wall of the nasopharynx. The lingual tonsil develops from an aggregation of lymph nodules in the root of the tongue. Lymph nodules also develop in the mucosa of the respiratory and alimentary systems.
Clinically Oriented Questions
1. A pediatrician diagnosed a heart murmur in a newborn infant. What does this mean? What causes this condition, and what does it indicate?
2. Are congenital anomalies of the heart common? What is the most common congenital heart defect in neonates?
3. What are the causes of congenital anomalies of the cardiovascular system? Can drugs taken by the mother during pregnancy cause congenital cardiac defects? One mother who drank heavily during her pregnancy had a child with a heart defect. Could her drinking have caused her infant’s heart defect?
4. Can viral infections cause congenital heart disease? Is it true that if a mother has measles during pregnancy, her infant will have an abnormality of the cardiovascular system? Is it true that pregnant women can be given a vaccine that will protect their unborn child against certain viruses?
5. In an infant, the aorta arose from the right ventricle and the pulmonary artery arose from the left ventricle. The infant died during the first week. What is this anomaly called, and how common is this disorder? Can the condition be corrected surgically? If so, how is this done?
6. During a routine examination of identical twin sisters in their forties, it was found that one of them had a reversed heart. Is this a serious heart anomaly? How common is this among identical twins, and what causes this condition to develop?