Chapter 12 Alimentary System
The alimentary system is the digestive tract from the mouth to the anus with all its associative glands and organs. At the beginning of the fourth week, the primordial gut is closed at its cranial end by the oropharyngeal membrane (Fig. 12-1B) and at its caudal end by the cloacal membrane (Fig. 12-1). The endoderm of the primordial gut gives rise to most of the epithelium and glands of the alimentary system. The epithelium at the cranial and caudal ends of the tract is derived from the ectoderm of the stomodeum and the proctodeum (anal pit), respectively (see Fig. 12-1).
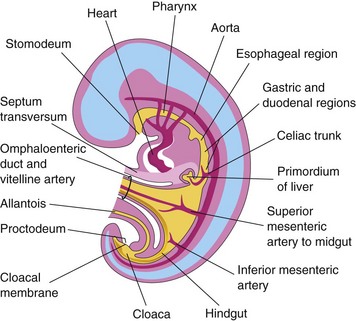
Figure 12–1 Median section of a 4-week embryo, showing the early alimentary system and its blood supply.
The muscular and connective tissue and other layers of the wall of the digestive tract are derived from the splanchnic mesenchyme surrounding the primordial gut. The gut is divided into three parts: foregut, midgut, and hindgut. The regional differentiation of the primordial gut is established by sonic and Indian hedgehog genes (Shh and Ihh) that are expressed in the endoderm and the surrounding mesoderm. The endodermal signaling provides temporal and positional information for the development of the gut.
Foregut
The derivatives of the foregut are as follows:
• The primordial pharynx and its derivatives
• The lower respiratory system
• The duodenum, just distal to the opening of the bile duct
• The liver, biliary apparatus (hepatic ducts, gallbladder, and bile duct), and pancreas
All of the foregut derivatives except the pharynx, the respiratory tract, and most of the esophagus are supplied by the celiac trunk, the artery of the foregut (Figs. 12-1 and 12-2A).
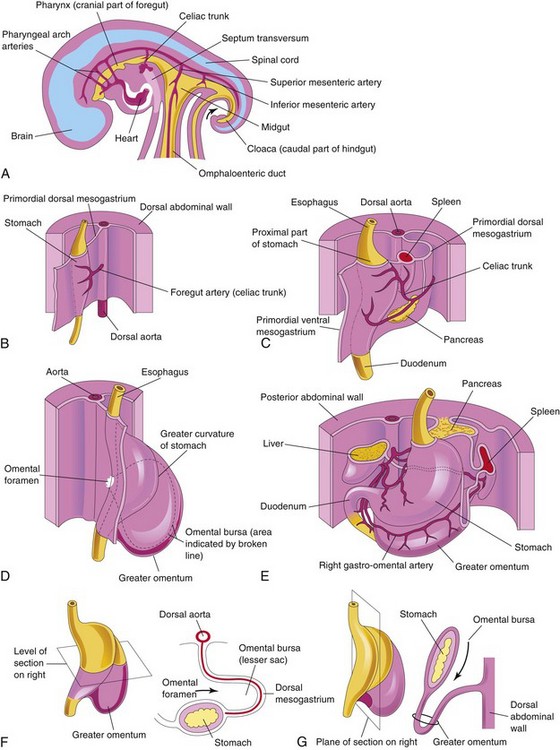
Figure 12–2 Illustrations of the development and rotation of the stomach and the formation of the omental bursa and greater omentum. A, Median section of a 28-day embryo. B, Anterolateral view of a 28-day embryo. C, Embryo at approximately 35 days. D, Embryo at approximately 40 days. E, Embryo at approximately 48 days. F, Lateral view of the stomach and greater omentum of an embryo at approximately 52 days. G, Sagittal section, showing the omental bursa and greater omentum. The arrow in F and G indicates the site of the omental foramen.
Development of Esophagus 
The esophagus elongates rapidly and reaches its final relative length by the seventh week. Its epithelium and glands are derived from the endoderm. The epithelium proliferates and partly or completely obliterates the esophageal lumen; however, recanalization normally occurs by the end of the eighth week. The striated muscle of the esophagus is derived from the mesenchyme in the fourth and sixth pharyngeal arches. The smooth muscle, mainly in the inferior third of the esophagus, develops from the surrounding splanchnic mesenchyme.
Esophageal Atresia
Blockage of the esophagus occurs in approximately 1 in 3000 to 1 in 4500 live births. Approximately one third of affected infants are born prematurely. Esophageal atresia is frequently associated with tracheoesophageal fistula (see Figs. 11-5 and 11-6). Esophageal atresia results from deviation of the tracheoesophageal septum in a posterior direction (see Figs. 11-2 and 11-6); as a result, separation of the esophagus from the laryngotracheal tube is incomplete. In some cases, the atresia results from failure of esophageal recanalization during the eighth week of development. A fetus with esophageal atresia is unable to swallow amniotic fluid, resulting in polyhydramnios.
Esophageal Stenosis
Narrowing of the lumen of the esophagus (stenosis) can occur anywhere along the esophagus, but it usually occurs in the distal one third, either as a web or as a long segment of esophagus with a threadlike lumen. The stenosis usually results from incomplete recanalization of the esophagus during the eighth week.
Development of Stomach 
During the fourth week, a slight dilation of the tubelike foregut indicates the site of the primordial stomach. It first appears as a fusiform enlargement that is oriented in the median plane (Fig. 12-2). The primordial stomach enlarges and broadens ventrodorsally. Its dorsal border grows more quickly than its ventral border. This site of rapid growth demarcates the greater curvature of the stomach (Fig. 12-2D).
Rotation of Stomach
As the stomach enlarges, it rotates 90 degrees in a clockwise direction around its longitudinal axis. The effects of rotation on the stomach are as follows (Figs. 12-2 and 12-3):
• The ventral border (lesser curvature) moves to the right, and the dorsal border (greater curvature) moves to the left (Fig. 12-2C to F).
• Before rotation, the cranial and caudal ends of the stomach are in the median plane (Fig. 12-2B).
• During rotation and growth of the stomach, its cranial region moves to the left and slightly inferiorly, and its caudal region moves to the right and superiorly (Fig. 12-2C to E).
• After rotation, the stomach assumes its final position, with its long axis almost transverse to the long axis of the body (Fig. 12-2E). This rotation and growth explains why the left vagus nerve supplies the anterior wall of the adult stomach and the right vagus nerve innervates its posterior wall.
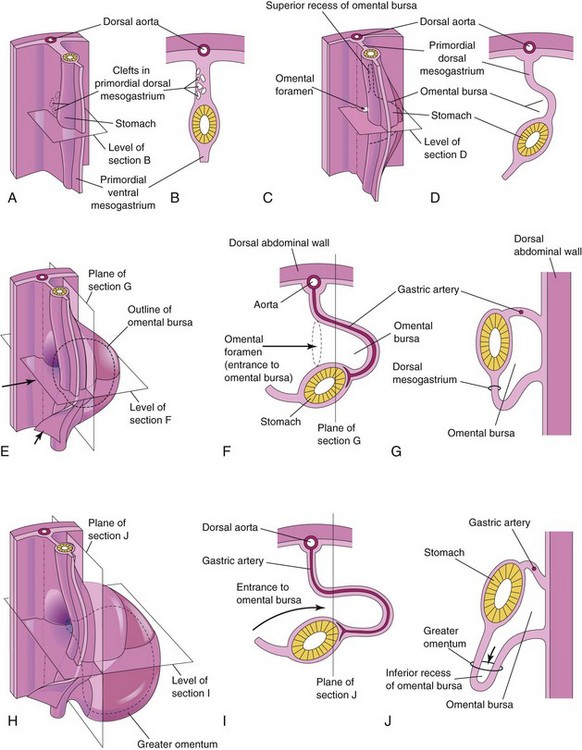
Figure 12–3 Illustrations of the development of the stomach and its mesenteries and the formation of the omental bursa. A, At 5 weeks. B, Transverse section showing clefts in the dorsal mesogastrium. C, Later stage, after coalescence of the clefts to form the omental bursa. D, Transverse section showing the initial appearance of the omental bursa. E, The dorsal mesentery has elongated and the omental bursa has enlarged. F and G, Transverse and sagittal sections, respectively, showing elongation of the dorsal mesogastrium and expansion of the omental bursa. H, At 6 weeks, showing the greater omentum and expansion of the omental bursa. I and J, Transverse and sagittal sections, respectively, showing the inferior recess of the omental bursa and the omental foramen. The arrows in E, F, and I, indicate the site of the omental foramen. In J, the arrow indicates the recess of the omental bursa.
Congenital Hypertrophic Pyloric Stenosis
Anomalies of the stomach are uncommon, except for hypertrophic pyloric stenosis, which affects 1 in 150 male infants and 1 in 750 female infants. Infants with this anomaly have marked muscular thickening of the pylorus of the stomach, the distal sphincteric region of the stomach. The muscles in the pyloric region are hypertrophied, which results in severe stenosis (narrowing) of the pyloric canal and obstruction to the passage of food. As a result, the stomach becomes markedly distended and its contents are expelled with considerable force (projectile vomiting). Surgical relief of the pyloric obstruction is the usual treatment.
Mesenteries of Stomach
The stomach is suspended from the dorsal wall of the abdominal cavity by the primordial dorsal mesogastrium (Figs. 12-2B and C and 12-3A to D). This mesentery, originally located in the median plane, is carried to the left during rotation of the stomach. The primordial ventral mesogastrium—attaches the stomach and duodenum to the liver and the ventral abdominal wall (Figs. 12-2C and 12-3A and B).
Omental Bursa
Isolated clefts develop in the mesenchyme that forms the dorsal mesogastrium (see Fig. 12-3A and B). The clefts soon coalesce to form a single cavity—the omental bursa (lesser peritoneal sac), a large recess of the peritoneal cavity (Figs. 12-2F and G and 12-3C and D). Rotation of the stomach pulls the dorsal mesogastrium to the left, thereby enlarging the bursa.
The omental bursa lies between the stomach and the posterior abdominal wall. As the stomach enlarges, the omental bursa expands and hangs over the developing intestines. This part of the omental bursa is called the greater omentum (Fig. 12-3G to J and see Fig. 12-13A). The two layers of the greater omentum eventually fuse (see Fig. 12-13F). The omental bursa communicates with the main part of the peritoneal cavity through a small opening—the omental foramen (Figs. 12-2D and F and 12-3C and F).
Development of Duodenum 
Early in the fourth week, the duodenum develops from the caudal part of the foregut and the cranial part of the midgut (Fig. 12-4A). The developing duodenum elongates, forming a C-shaped loop that projects ventrally (Fig. 12-4B to D). As the stomach rotates, the duodenal loop rotates to the right and lies retroperitoneally (external to peritoneum). Because of its derivation from the foregut and midgut, the duodenum is supplied by branches of both the celiac and the superior mesenteric arteries (Fig. 12-1). The lumen of the duodenum is temporarily obliterated because of the proliferation of its epithelial cells, but normally becomes recanalized by the end of the embryonic period.
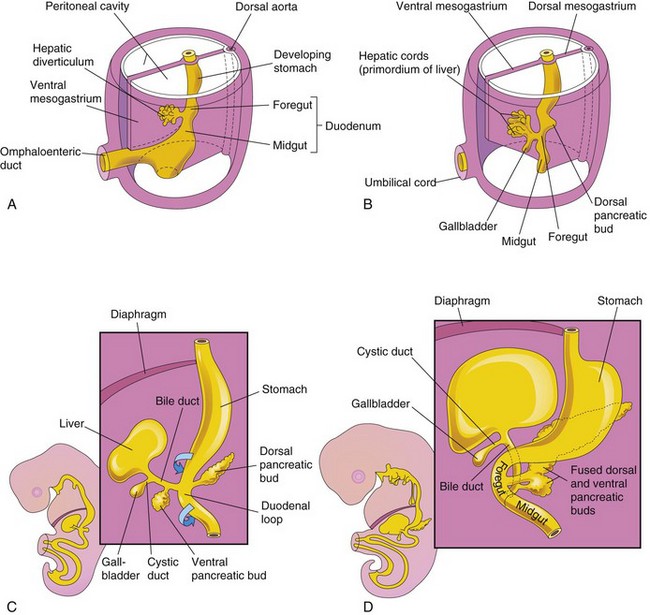
Figure 12–4 Illustrations of progressive stages in the development of the duodenum, liver, pancreas, and extrahepatic biliary apparatus. A, At 4 weeks. B and C, At 5 weeks. D, At 6 weeks.
Duodenal Stenosis
Partial occlusion of the duodenal lumen—duodenal stenosis—is usually caused by incomplete recanalization of the duodenum. Most stenoses involve the horizontal (third) part or the ascending (fourth) part of the duodenum, or both. Because of the occlusion, the stomach contents are often vomited.
Duodenal Atresia
Complete occlusion of the duodenum—duodenal atresia—results from failure of reformation of the lumen (Fig. 12-5B). Most atresias involve the descending and horizontal parts of the duodenum and are located distal to the opening of the bile duct. In infants with duodenal atresia, vomiting begins within a few hours of birth. The vomitus almost always contains bile. Polyhydramnios also occurs because duodenal atresia prevents normal absorption of amniotic fluid by the intestines. A diagnosis of duodenal atresia is suggested by the presence of a “double-bubble sign” on plain radiographs or ultrasound scans. This sign is caused by a distended, gas-filled stomach and the proximal duodenum. Between 20% and 30% of affected infants have Down syndrome, and an additional 20% are premature.
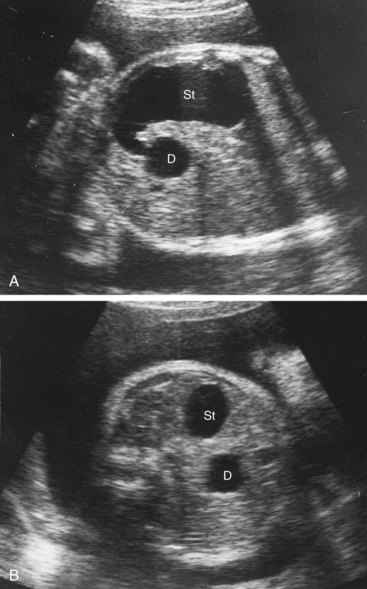
Figure 12–5 Ultrasound scans of a fetus at 33 weeks gestation (31 weeks after fertilization), showing duodenal atresia. A, An oblique scan shows the dilated, fluid-filled stomach (St) entering the proximal duodenum (D), which is also enlarged because of the atresia (blockage) distal to it. B, Transverse ultrasound scan, showing the characteristic “double-bubble” appearance of the stomach and duodenum when there is duodenal atresia.
(Courtesy of Dr. Lyndon M. Hill, Magee-Women’s Hospital, Pittsburgh, PA.)
Development of Liver and Biliary Apparatus 
The liver, gallbladder, and biliary duct system arise as a ventral outgrowth—hepatic diverticulum—from the caudal part of the foregut early in the fourth week (Figs. 12-4A and 12-6A). Wnt/β-catenin signaling is involved in the induction of the hepatic diverticulum. The hepatic diverticulum extends into the septum transversum (Fig. 12-6B), a mass of splanchnic mesoderm between the developing heart and the midgut. The diverticulum enlarges and divides into two parts as it grows between the layers of the ventral mesogastrium (Fig. 12-4A). The larger, cranial part of the diverticulum is the primordium of the liver, and the smaller caudal portion becomes the biliary apparatus. The proliferating endodermal cells give rise to interlacing cords of hepatocytes and to the epithelial lining of the intrahepatic part of the biliary apparatus. The hepatic cords anastomose around endothelium-lined spaces, the primordia of the hepatic sinusoids. The fibrous and hematopoietic tissue and Kupffer cells of the liver are derived from the mesenchyme in the septum transversum.
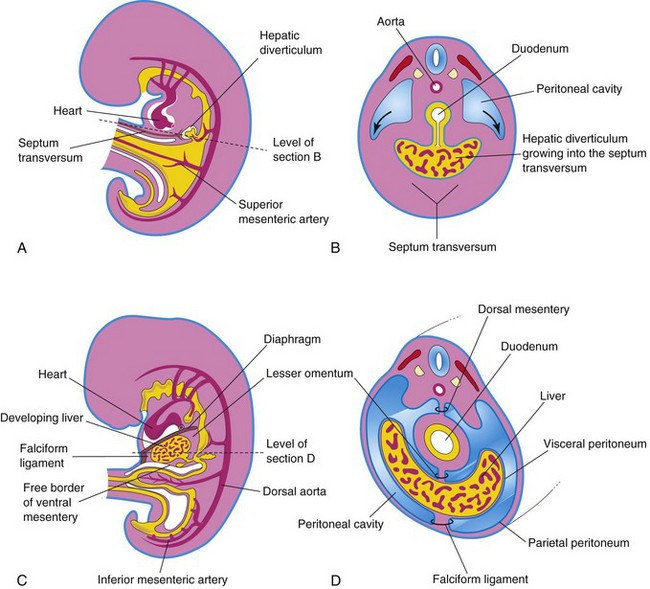
Figure 12–6 Illustrations of how the caudal part of the septum transversum becomes stretched and membranous as it forms the ventral mesentery. A, Median section of a 4-week embryo. B, Transverse section of the embryo, showing expansion of the peritoneal cavity (arrows). C, Sagittal section of a 5-week embryo. D, Transverse section of the embryo after formation of the dorsal and ventral mesenteries.
The liver grows rapidly and fills a large part of the abdominal cavity (Figs. 12-4 and Fig. 12-6C and D). Hematopoiesis (formation of various types of blood cells and other formed elements) begins in the liver during the sixth week. By the ninth week, the liver accounts for approximately 10% of the total weight of the fetus. Bile formation by the hepatic cells begins during the 12th week.
The caudal portion of the hepatic diverticulum becomes the gallbladder; the stalk forms the cystic duct (Fig. 12-4B and C). Initially, the extrahepatic biliary apparatus is occluded with epithelial cells. The stalk connecting the hepatic and cystic ducts to the duodenum becomes the bile duct, which attaches to the ventral aspect of the duodenal loop. As the duodenum grows and rotates, the entrance of the bile duct is carried to the dorsal aspect of the duodenum (Fig. 12-4C and D).
Anomalies of Liver and Biliary Ducts
Minor variations of liver lobulation are common, as are variations of the hepatic ducts, bile duct, and cystic duct. For example, accessory hepatic ducts may be present, and an awareness of their possible presence is important from a surgical perspective. Congenital anomalies of the liver are rare.
Extrahepatic Biliary Atresia
Extrahepatic biliary atresia, the most serious anomaly involving the extrahepatic biliary system, is uncommon. Failure of the bile ducts to canalize often results from persistence of the solid stage of duct development. Jaundice occurs soon after birth.
Ventral Mesentery
The double-layered ventral mesentery (Figs. 12-6C and D and 12-7) gives rise to two structures:
• The lesser omentum, which passes from the liver to the lesser curvature of the stomach (hepatogastric ligament) and from the liver to the duodenum (hepatoduodenal ligament)
• The falciform ligament, which extends from the liver to the ventral abdominal wall
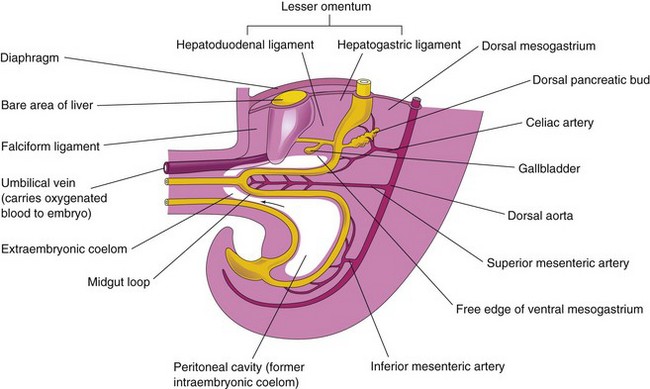
Figure 12–7 Median section of the caudal half of an embryo at the end of the fifth week, showing the liver and its associated ligaments. The arrow indicates the communication of the peritoneal cavity with the extraembryonic coelom. Because of the rapid growth of the liver and the midgut loop, the abdominal cavity temporarily becomes too small to contain the developing intestines; consequently, they enter the extraembryonic coelom in the proximal part of the umbilical cord (see Fig. 12-11B).
The umbilical vein passes in the free border of the falciform ligament on its way from the umbilical cord to the liver. The ventral mesentery, derived from the mesogastrium, also forms the visceral peritoneum of the liver.
Development of Pancreas 
The pancreas develops between the layers of both mesenteries from the dorsal and ventral pancreatic buds, which arise from the caudal part of the foregut (Fig. 12-8A). Most of the pancreas is derived from the dorsal pancreatic bud, which appears first. It grows rapidly between the layers of the dorsal mesentery. Formation of the dorsal pancreatic bud depends on signals from the notochord (activin and fibroblast growth factor 2) that block the expression of sonic hedgehog (Shh) in the endoderm. Expression of pancreatic and duodenal homebox factors (PDX-1 and MafA) is critical for the development of the pancreas.
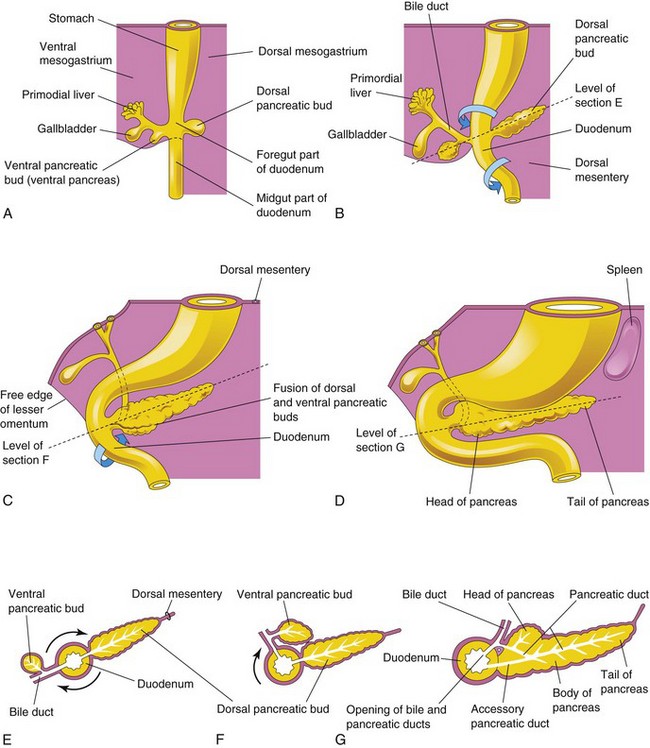
Figure 12–8 A to D, Illustrations of successive stages in the development of the pancreas from the fifth to the eighth weeks. E to G, Transverse sections through the duodenum and the developing pancreas. Growth and rotation (arrows) of the duodenum bring the ventral pancreatic bud toward the dorsal bud; the two buds subsequently fuse. Note that the bile duct initially attaches to the ventral aspect of the duodenum and is carried around to the dorsal aspect as the duodenum rotates. The pancreatic duct is formed by the union of the distal part of the dorsal pancreatic duct and the entire ventral pancreatic duct. The proximal part of the dorsal pancreatic duct usually obliterates, but it may persist as an accessory pancreatic duct.
The ventral pancreatic bud develops near the entry of the bile duct into the duodenum (Fig. 12-8A and B). As the duodenum rotates to the right and becomes C-shaped, the ventral pancreatic bud is carried dorsally with the bile duct (Fig. 12-8C to F). It soon lies posterior to the dorsal pancreatic bud and later fuses with it (Fig. 12-8G). As the pancreatic buds fuse, their ducts anastomose.
The ventral pancreatic bud forms the uncinate process and part of the head of the pancreas. As the stomach, duodenum, and ventral mesentery rotate, the pancreas comes to lie along the dorsal abdominal wall (Fig. 12-8D and G).
The pancreatic duct forms from the duct of the ventral bud and the distal part of the duct of the dorsal bud (Fig. 12-8G). In approximately 9% of people, the proximal part of the duct of the dorsal bud persists as an accessory pancreatic duct that opens into the minor duodenal papilla. The connective tissue sheath and the interlobular septa of the pancreas develop from the surrounding splanchnic mesenchyme. Insulin secretion begins at approximately 10 weeks. The glucagon- and somatostatin-containing cells develop before differentiation of the insulin-secreting cells occurs. With increasing fetal age, total pancreatic insulin and glucagon content also increases.
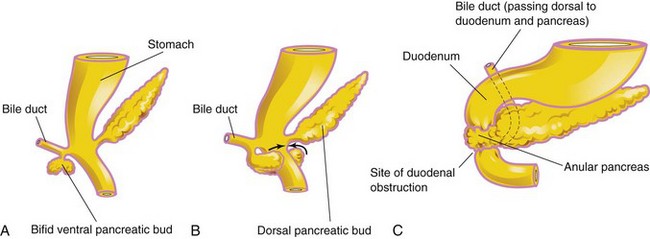
Annular Pancreas
Annular pancreas is an uncommon anomaly and probably results from the growth of a bifid ventral pancreatic bud around the duodenum (Fig. 12-9A to C). The parts of the bifid ventral bud then fuse with the dorsal bud, forming a pancreatic ring. The ringlike, annular part of the pancreas consists of a thin, flat band of pancreatic tissue surrounding the descending, or second, part of the duodenum. An annular pancreas may cause obstruction of the duodenum shortly after birth, but many cases are not diagnosed until adulthood. Males are affected much more frequently than females.
Development of Spleen 
The spleen is derived from a mass of mesenchymal cells located between the layers of the dorsal mesogastrium (Fig. 12-10A and B). The spleen begins to develop during the fifth week, but does not acquire its characteristic shape until early in the fetal period. The spleen is lobulated in the fetus, but the lobules normally disappear before birth. The notches in the superior border of the adult spleen are remnants of the grooves that separated the fetal lobules.
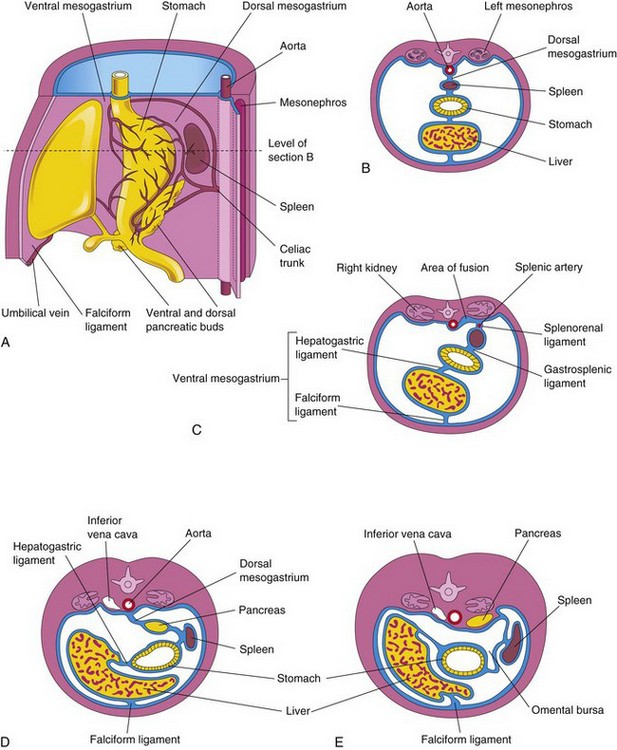
Figure 12–10 A, The left side of the stomach and associated structures at the end of the fifth week. Note that the pancreas, spleen, and celiac trunk are located between the layers of the dorsal mesogastrium. B, Transverse section of the liver, stomach, and spleen at the level shown in A, showing their relationship to the dorsal and ventral mesenteries. C, Transverse section of a fetus, showing fusion of the dorsal mesogastrium with the peritoneum on the posterior abdominal wall. D and E, Similar sections, showing movement of the liver to the right and rotation of the stomach. Observe the fusion of the dorsal mesogastrium to the dorsal abdominal wall, which results in the pancreas becoming retroperitoneal.
Midgut
The derivatives of the midgut are:
• The small intestine, including the duodenum distal to the opening of the bile duct
• The cecum, appendix, ascending colon, and right half to two thirds of the transverse colon
All of these derivatives are supplied by the superior mesenteric artery (Fig. 12-7). The midgut loop is suspended from the dorsal abdominal wall by an elongated mesentery. The midgut elongates and forms a ventral, U-shaped loop that projects into the proximal part of the umbilical cord. This projection of the intestine, occurring at the beginning of the sixth week, is called a physiologic umbilical herniation (Figs. 12-11 and 12-12). Umbilical herniation occurs because there is not enough room in the abdomen for the rapidly growing midgut.
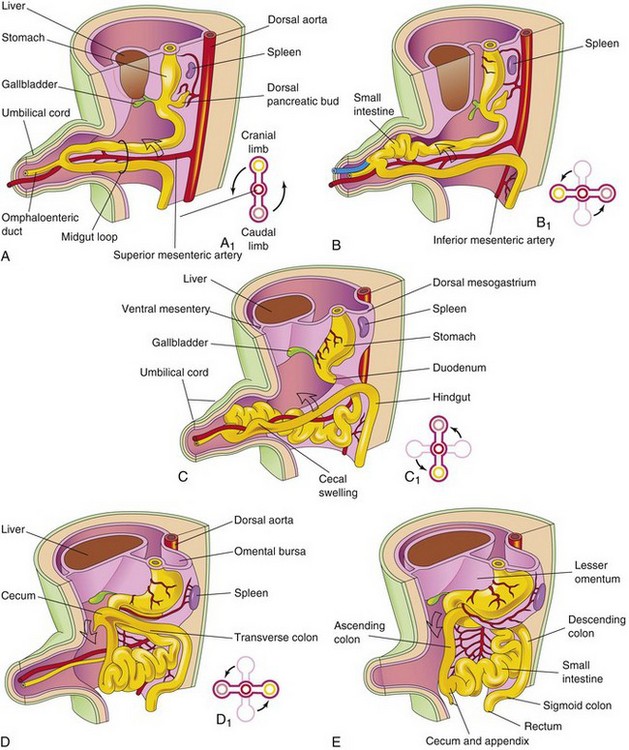
Figure 12–11 Illustrations of the rotation of the midgut, as seen from the left. A, During the sixth week, the midgut loop is situated in the proximal part of the umbilical cord. A1, Transverse section through the midgut loop, showing the initial relationship of the limbs of the midgut loop to the superior mesenteric artery. B, A later stage, showing the beginning of midgut rotation. B1, Illustration of the 90-degree counterclockwise rotation that carries the cranial limb of the midgut to the right. C, At approximately 10 weeks, the intestines return to the abdomen. C1, Illustration of a further rotation of 90 degrees. D, By approximately 11 weeks, all of the intestines return to the abdomen. D1, A further 90-degree rotation of the gut, for a total of 270 degrees. E, The later fetal period, showing the cecum rotating to its normal position in the lower right quadrant of the abdomen.
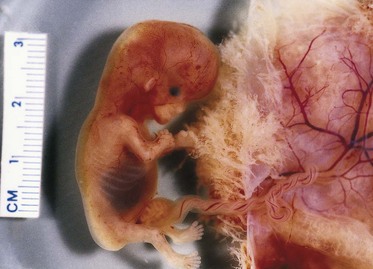
Figure 12–12 Physiologic hernia in a 58-day embryo attached to its chorionic sac. Note the herniated intestine derived from the midgut loop in the proximal part of the umbilical cord.
(Courtesy of Dr. D.K. Kalousek, Department of Pathology, University of British Columbia, Children’s Hospital, Vancouver, British Columbia, Canada.)
The midgut loop communicates with the umbilical vesicle (yolk sac) through the narrow omphaloenteric duct (yolk stalk) until the 10th week (Fig. 12-11A and C). The cranial limb of the loop grows rapidly and forms most of the small intestine. The caudal limb undergoes very little change, except for the development of the cecal diverticulum, which is the primordium of the cecum and appendix (Fig. 12-11C to E).
Rotation of Midgut Loop 
While in the umbilical cord, the midgut loop rotates 90 degrees counterclockwise around the axis of the superior mesenteric artery (see Fig. 12-11B). This rotation brings the cranial limb (small intestine) of the midgut loop to the right and the caudal limb (large intestine) to the left.
Return of Midgut to Abdomen
During the 10th week, with enlargement of the abdominal cavity, the intestines return to the abdomen (reduction of the physiologic midgut hernia) (Fig. 12-11C and D). The small intestine returns first, passing posterior to the superior mesenteric artery, and occupies the central part of the abdomen. As the large intestine returns, it undergoes a further 180-degree counterclockwise rotation (Fig. 12-11C1 and D1). Later, it comes to occupy the right side of the abdomen. The ascending colon becomes recognizable as the posterior abdominal wall progressively elongates (Figs. 12-11E and 12-13A).
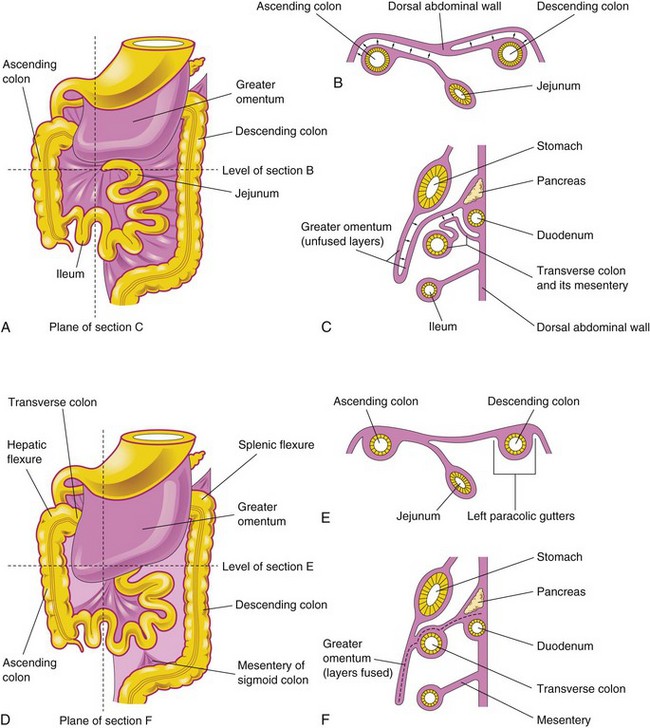
Figure 12–13 Fixation of the intestines. A, Ventral view of the intestines before their fixation. B, Transverse section at the level shown in A. The arrows indicate areas of subsequent fusion. C, Sagittal section at the plane shown in A, illustrating the greater omentum overhanging the transverse colon. The arrows indicate areas of subsequent fusion. D, Ventral view of the intestines after their fixation. E, Transverse section at the level shown in D after disappearance of the mesentery of the ascending and descending colon. F, Sagittal section at the plane shown in D, illustrating fusion of the greater omentum with the mesentery of the transverse colon and fusion of the layers of the greater omentum.
Fixation of Intestines
Rotation of the stomach and duodenum causes the duodenum and pancreas to fall to the right, where they are pressed against the posterior abdominal wall by the colon. The adjacent layers of peritoneum fuse and subsequently disappear (see Fig. 12-13C and F); consequently, most of the duodenum and the head of the pancreas become retroperitoneal (posterior to peritoneum). The mesentery of the ascending colon fuses with the parietal peritoneum on the posterior abdominal wall. The mesentery of the ascending colon becomes retroperitoneal (see Fig. 12-13B and E). The other derivatives of the midgut loop retain their mesenteries.
Cecum and Appendix 
The primordium of the cecum and the appendix appears in the sixth week as a swelling on the antimesenteric border of the caudal limb of the midgut loop (Figs. 12-11C to E and 12-14A). Initially, the appendix is a small diverticulum of the cecum. It subsequently increases rapidly in length so that at birth it is a relatively long tube arising from the distal end of the cecum (Fig. 12-14D). After birth, the unequal growth of the walls of the cecum results in the appendix entering its medial side (see Fig. 12-14E). The appendix is subject to considerable variation in position. As the ascending colon elongates, the appendix may pass posterior to the cecum (retrocecal appendix) or the colon (retrocolic appendix).
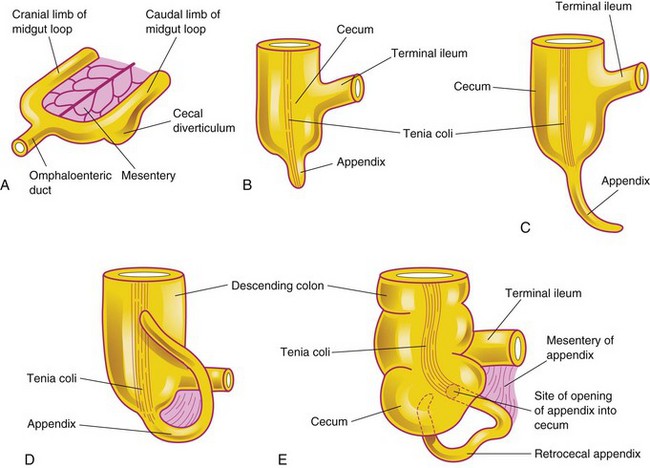
Figure 12–14 Successive stages in the development of the cecum and appendix. A, At 6 weeks. B, At 8 weeks. C, At 12 weeks. D, At birth. Note that the appendix is relatively long and is continuous with the apex of the cecum. E, Adult. Note that the appendix is now relatively short and is located posterior to the cecum.
Congenital Omphalocele
Congenital omphalocele results in persistence of the herniation of the abdominal contents into the proximal part of the umbilical cord (Figs. 12-15 and 12-16). This is caused by failure of the body walls to fuse at the umbilical ring because of defective growth of mesenchyme. Herniation of the intestines occurs in approximately 1 in 5000 births; herniation of the liver and intestines occurs less frequently (1 in 10,000 births). The size of the hernia depends on its contents. The abdominal cavity is proportionately small when an omphalocele is present because the impetus for it to grow is absent.
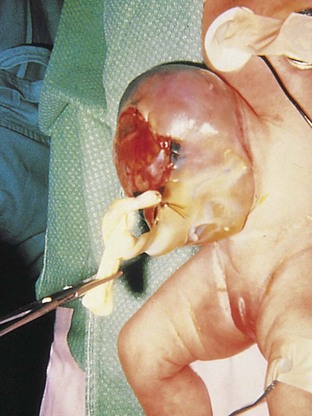
Figure 12–15 An infant with an omphalocele. The defect resulted in herniation of intra-abdominal structures (liver and intestine) into the proximal end of the umbilical cord. The omphalocele is covered by a membrane composed of peritoneum and amnion.
(Courtesy of Dr. N.E. Wiseman, Department of Surgery, Children’s Hospital, Winnipeg, Manitoba, Canada.)
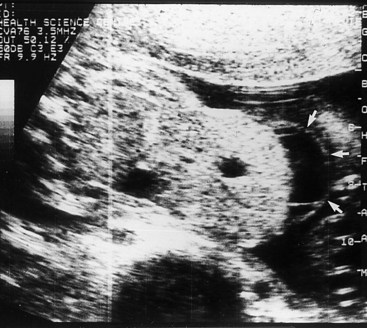
Figure 12–16 Ultrasonogram of the abdomen of a fetus (28 weeks’ gestation), showing a large omphalocele, with much of the liver protruding from the abdominal wall. The mass also contains a small, membrane-covered sac (arrows). The umbilical cord was integrally involved in the anomaly.
(Courtesy of Dr. C.R. Harman, Department of Obstetrics, Gynecology and Reproductive Sciences, Women’s Hospital and University of Maryland, Baltimore, MD.)
Umbilical Hernia
When the intestines herniate through an imperfectly closed umbilicus, an umbilical hernia forms. This common type of hernia differs from an omphalocele. In umbilical hernias, the protruding mass (usually consisting of part of the greater omentum and small intestine) is covered by subcutaneous tissue and skin. The hernia protrudes during crying, straining, or coughing.
Hindgut 
The derivatives of the hindgut are:
• The left third to half of the transverse colon, the descending colon and sigmoid colon, the rectum, and the superior part of the anal canal
• The epithelium of the urinary bladder and most of the urethra
All of these derivatives are supplied by the inferior mesenteric artery (Fig. 12-7). The descending colon becomes retroperitoneal as its mesentery fuses with the peritoneum on the left posterior abdominal wall (Fig. 12-13B and E). The mesentery of the sigmoid colon is retained.
Gastroschisis and Congenital Epigastric Hernia
Gastroschisis results from a defect near the median plane of the abdominal wall (Fig. 12-17). The viscera protrude into the amniotic cavity and are bathed by amniotic fluid. The term gastroschisis, which literally means “split stomach,” is a misnomer because it is the anterior abdominal wall, not the stomach, that is split. The defect usually occurs on the right side, lateral to the median plane, and is more common in boys than in girls. The anomaly results from incomplete closure of the lateral folds during the fourth week of development (see Chapter 6).
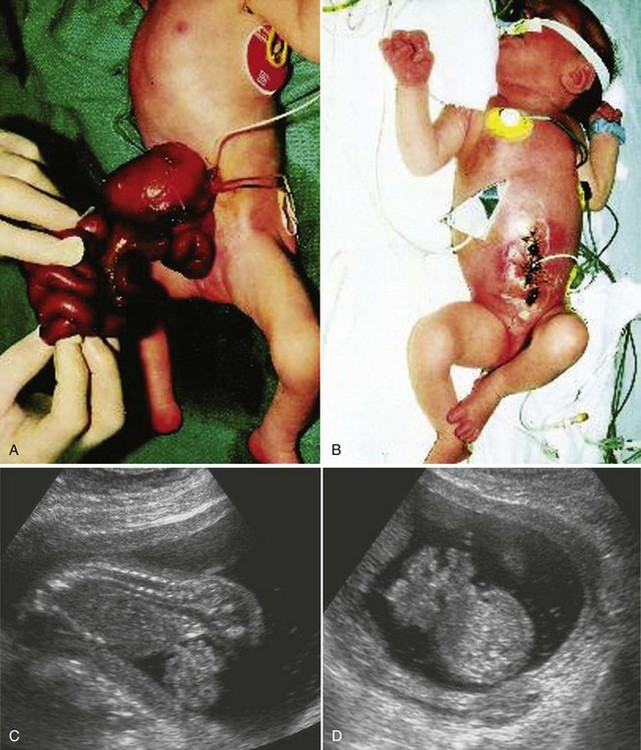
Figure 12–17 A, A newborn infant with an anterior abdominal wall defect—gastroschisis. The defect was relatively small (2-4 cm long) and involved all layers of the abdominal wall. It was located to the right of the umbilicus. B, The same infant after the viscera were returned to the abdomen and the defect was surgically closed. C, D, Sonogram of an 18-week fetus with gastroschisis. Loops of bowel can be seen in the amniotic fluid ventral to the fetus on the sagittal scan (C) and the axial scan (D) of the fetal abdomen.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital, Winnipeg, Manitoba, Canada. C and D, Courtesy of Dr. E. A. Lyons, Professor of Radiology, Obstetrics and Gynecology, and Anatomy, Health Sciences Centre, University of Manitoba, Winnipeg, Manitoba, Canada.)
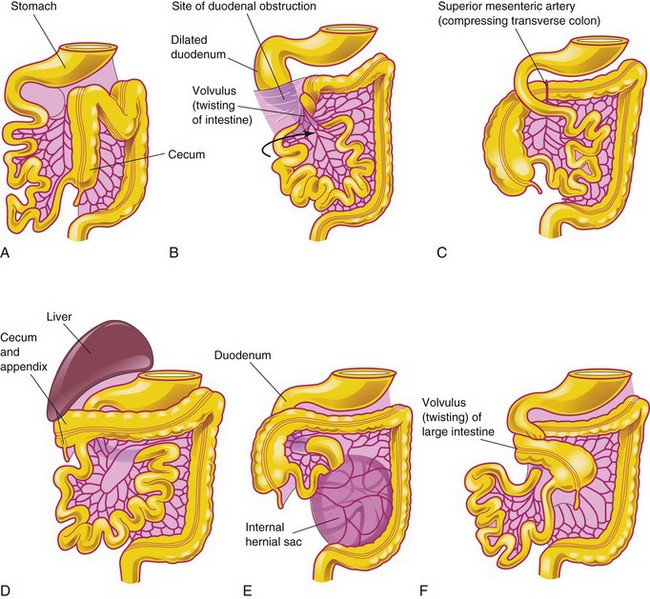
Nonrotation of Midgut
Nonrotation of the midgut (left-sided colon) is a relatively common condition (Fig. 12-18A and B) resulting in the caudal limb of the midgut loop returning to the abdomen first. The small intestine then lies on the right side of the abdomen, and the entire large intestine lies on the left. Although patients are generally asymptomatic, if volvulus (twisting) occurs, the superior mesenteric artery may be obstructed, resulting in infarction and gangrene of the associated intestine.
Mixed Rotation and Volvulus
With mixed rotation and volvulus, the cecum lies just inferior to the pylorus of the stomach and is fixed to the posterior abdominal wall by peritoneal bands that pass over the duodenum (Fig. 12-18B). These bands and the volvulus usually cause duodenal obstruction. This type of malrotation results from failure of the midgut loop to complete the final 90 degrees of rotation (Fig. 12-11D); consequently, the terminal part of the ileum returns to the abdomen first.
Reversed Rotation
In very unusual cases, the midgut loop rotates in a clockwise rather than a counterclockwise direction (Fig. 12-18C). As a result, the duodenum lies anterior to the superior mesenteric artery rather than posterior to it, and the transverse colon lies posterior to the superior mesenteric artery instead of anterior to it. In these infants, the transverse colon may be obstructed by pressure from the superior mesenteric artery.
Subhepatic Cecum and Appendix
If the cecum adheres to the inferior surface of the liver when it returns to the abdomen (Fig. 12-11D), it is drawn superiorly with the liver. As a result, the cecum remains in its fetal position (Fig. 12-18D). Subhepatic cecum and appendix are more common in males than in females. Subhepatic cecum is not common in adults; when it occurs, it may create problems in the diagnosis and surgical removal of the appendix.
Internal Hernia
In the case of an internal hernia, the small intestine passes into the mesentery of the midgut loop during the return of the intestines to the abdomen (Fig. 12-18E). As a result, a hernia-like sac forms. This very uncommon condition usually does not produce symptoms and is often detected at autopsy or during an anatomical dissection.
Midgut Volvulus
Midgut volvulus is an anomaly in which the small intestine does not enter the abdominal cavity normally and the mesenteries do not undergo normal fixation. As a result, twisting (volvulus) of the intestines occurs (Fig. 12-18F). Only two parts of the intestine—the duodenum and the proximal colon—are attached to the posterior abdominal wall. The small intestine hangs by a narrow stalk that contains the superior mesenteric artery and vein. These vessels are usually twisted in this stalk and become obstructed at or near the duodenojejunal junction. The circulation to the twisted intestine is often restricted; if the vessels are completely obstructed, necrosis develops.
Stenosis and Atresia of Intestine
Partial occlusion (stenosis) and complete occlusion (atresia) of the intestinal lumen (Fig. 12-5) account for approximately one third of cases of intestinal obstruction. The obstructive lesion occurs most often in the ileum (50%) and duodenum (25%). These anomalies result from failure of recanalization of the intestine. Most atresias of the ileum are probably caused by infarction of the fetal bowel as a result of impairment of its blood supply secondary to volvulus. This impairment most likely occurs during the 10th week as the intestines return to the abdomen.
Ileal Diverticulum and Other Omphaloenteric Duct Remnants
A congenital ileal diverticulum (Meckel diverticulum) (Fig. 12-19) occurs in 2% to 4% of infants and is three to five times more prevalent in males than in females. It represents a remnant of the proximal portion of the omphaloenteric duct. It typically appears as a finger-like pouch approximately 3 to 6 cm long that arises from the antimesenteric border of the ileum, 40 to 50 cm from the ileocecal junction. An ileal diverticulum is of clinical significance because it sometimes becomes inflamed and causes symptoms that mimic appendicitis. The wall of the diverticulum contains all layers of the ileum and may also contain small patches of gastric and pancreatic tissues. The gastric mucosa often secretes acid, producing ulceration and bleeding (Fig. 12-20A to C). An ileal diverticulum may be connected to the umbilicus by a fibrous cord or an omphaloenteric fistula (Fig. 12-20B and C); other possible remnants of the omphaloenteric duct are shown in Fig. 12-20D to F.
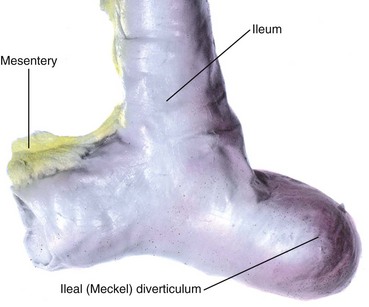
Figure 12–19 A typical ileal diverticulum (cadaveric specimen), commonly referred to clinically as Meckel diverticulum.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
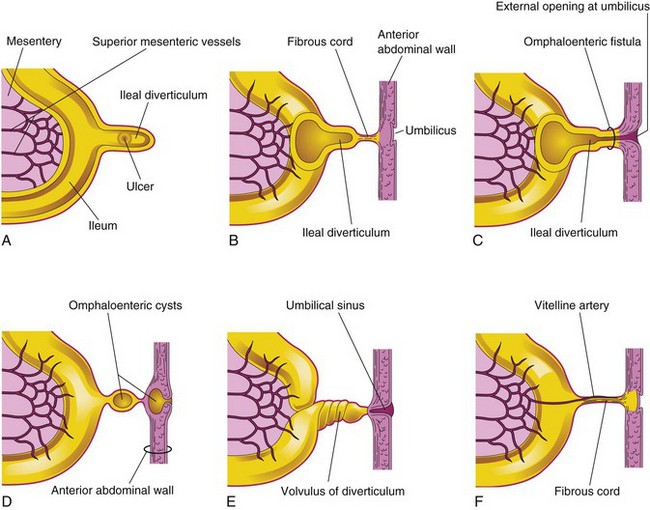
Figure 12–20 Ileal diverticula and other remnants of the omphaloenteric duct. A, Section of the ileum and a diverticulum with an ulcer. B, A diverticulum connected to the umbilicus by a fibrous cord. C, Omphaloenteric fistula resulting from persistence of the entire intra-abdominal portion of the omphaloenteric duct. D, Omphaloenteric cysts at the umbilicus and in a fibrous remnant of the omphaloenteric duct. E, Umbilical sinus resulting from the persistence of the omphaloenteric duct near the umbilicus. F, The omphaloenteric duct has persisted as a fibrous cord connecting the ileum with the umbilicus. A persistent vitelline artery extends along the fibrous cord to the umbilicus.
Cloaca 
The expanded terminal part of the hindgut, the cloaca, is an endoderm-lined chamber that is in contact with the surface ectoderm at the cloacal membrane (Fig. 12-21A and B). This membrane is composed of the endoderm of the cloaca and the ectoderm of the proctodeum (Fig. 12-21C and D). The cloaca receives the allantois ventrally (Fig. 12-21A).
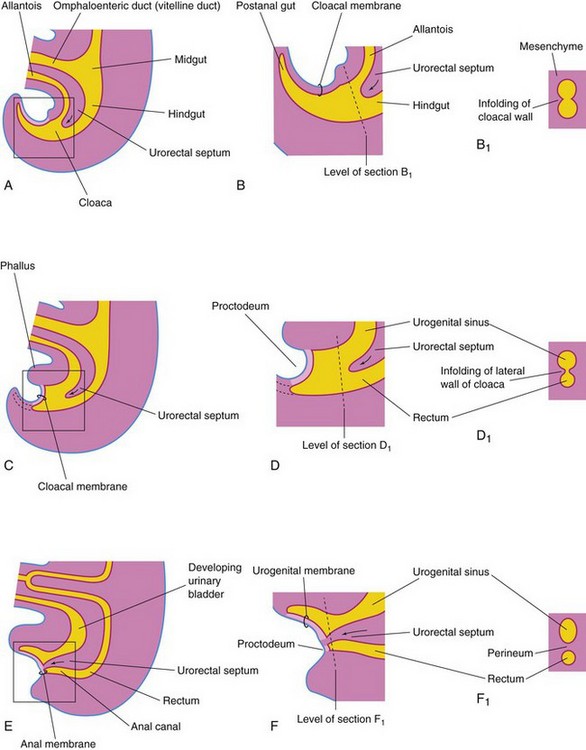
Figure 12–21 Illustrations of successive stages in the partitioning of the cloaca into the rectum and the urogenital sinus by the urorectal septum. A, C, and E, Views from the left side at 4, 6, and 7 weeks, respectively. B, D, and F, Enlargements of the cloacal region. B1, D1, and F1, Transverse sections of the cloaca at the levels shown in B, D, and F, respectively. Note that the postanal gut, or tailgut (shown in B), degenerates and disappears as the rectum forms from the dorsal part of the cloaca (shown in C). The arrows indicate the growth of the urorectal septum.
Partitioning of Cloaca
The cloaca is divided into dorsal and ventral parts by mesenchyme—the urorectal septum—that develops in the angle between the allantois and the hindgut (Fig. 12-21C and D). As the septum grows toward the cloacal membrane, it develops forklike extensions that produce infoldings of the lateral walls of the cloaca (Fig. 12-21B1). These folds grow toward each other and fuse, forming a partition that divides the cloaca into two parts (Fig. 12-21D to F1)—the rectum and the cranial part of the anal canal dorsally, and the urogenital sinus ventrally.
By the seventh week, the urorectal septum has fused with the cloacal membrane, dividing it into a smaller, dorsal anal membrane and a larger, ventral urogenital membrane (Fig. 12-21E and F). The area of fusion of the urorectal septum with the cloacal membrane is represented in the adult by the perineal body, the tendinous center of the perineum.
Mesenchymal proliferations produce elevations of the surface ectoderm around the anal membrane. As a result, this membrane is soon located at the bottom of an ectodermal depression—the proctodeum (Fig. 12-21F). The anal membrane usually ruptures at the end of the eighth week of development.
Anal Canal 
The superior two thirds of the adult anal canal are derived from the hindgut; the inferior one third develops from the proctodeum (Fig. 12-22). The junction of the epithelium derived from the ectoderm of the proctodeum and that derived from the endoderm of the hindgut is roughly indicated by an irregular pectinate line located at the inferior limit of the anal valves (Fig. 12-22). This line also indicates the approximate former site of the anal membrane. At the anus, the epithelium is keratinized and continuous with the skin around it. The other layers of the wall of the anal canal are derived from splanchnic mesenchyme.
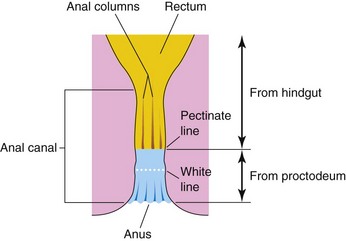
Figure 12–22 The rectum and anal canal, showing their developmental origins. Note that the superior two thirds of the anal canal are derived from the hindgut, whereas the inferior one third of the anal canal is derived from the proctodeum. Because of their different embryologic origins, the superior and inferior parts of the anal canal are supplied by different arteries and nerves and have different venous and lymphatic drainages.
Because of its hindgut origin, the superior two thirds of the anal canal are supplied mainly by the superior rectal artery, the continuation of the inferior mesenteric artery. Its nerves are from the autonomic nervous system. The inferior one third of the anal canal, because of its origin from the proctodeum, is supplied mainly by the inferior rectal arteries, branches of the internal pudendal artery. The inferior part of the anal canal is supplied by the inferior rectal nerve and is sensitive to pain, temperature, touch, and pressure.
The differences in blood supply, nerve supply, and venous and lymphatic drainage of the two parts of the anal canal are important clinically because the characteristics of carcinomas involving the two parts differ. Tumors in the superior part are painless and arise from the columnar epithelium, whereas those in the inferior part are painful and arise from the squamous epithelium.
Congenital Megacolon
In infants with congenital megacolon, or Hirschsprung disease (Fig. 12-23), a part of the colon is dilated because of the absence of autonomic ganglion cells in the myenteric plexus distal to the dilated segment of colon. The enlarged colon—megacolon—has the normal number of ganglion cells. The dilation results from failure of peristalsis in the aganglionic segment, which prevents movement of the intestinal contents. Males are affected more than females (4 to 1). Congenital megacolon results from failure of neural crest cells to migrate into the wall of the colon during the fifth to seventh weeks of development. Of the genes involved in the pathogenesis of Hirschsprung disease, the RET proto-oncogene accounts for most cases.
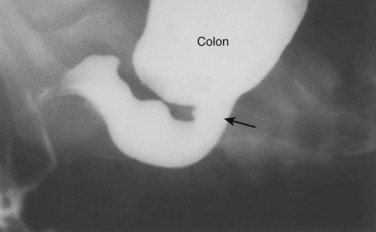
Figure 12–23 Radiograph of the colon, after a barium enema, in a 1-month-old infant with megacolon (Hirschsprung disease). The distal aganglionic segment is narrow, with a dilated proximal colon full of fecal material. Note the transition zone (arrow).
(Courtesy of Dr. Martin H. Reed, Department of Radiology, University of Manitoba and Children’s Hospital, Winnipeg, Manitoba, Canada.)
Imperforate Anus and Anorectal Anomalies
Imperforate anus occurs in approximately 1 in 5000 newborn infants, and it is more common in boys (Figs. 12-24 and 12-25C). Most anorectal anomalies result from abnormal development of the urorectal septum, resulting in incomplete separation of the cloaca into urogenital and anorectal parts (Fig. 12-25A). Lesions are classified as low or high, depending on whether the rectum ends superior or inferior to the puborectalis muscle.
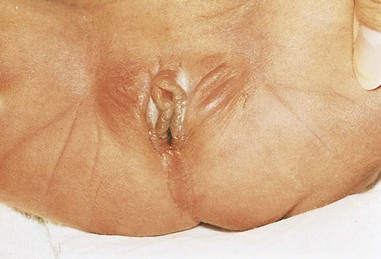
Figure 12–24 Female neonate with membranous anal atresia (imperforate anus). In most cases of anal atresia, a thin layer of tissue separates the anal canal from the exterior.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital, Winnipeg, Manitoba, Canada.)
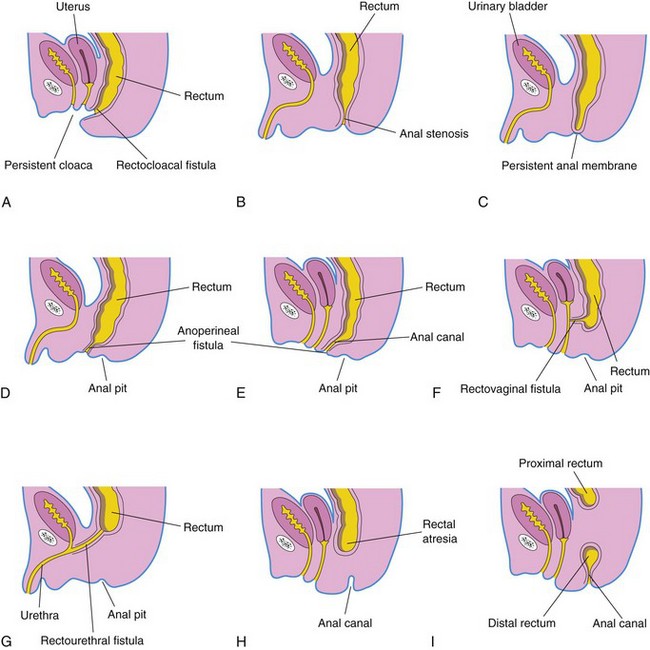
Figure 12–25 Illustrations of various types of anorectal anomalies. A, Persistent cloaca. Note the common outlet of the intestinal, urinary, and reproductive tracts. B, Anal stenosis. C, Membranous anal atresia. D and E, Anal agenesis with a perineal fistula. F, Anorectal agenesis with a rectovaginal fistula. G, Anorectal agenesis with a rectourethral fistula. H and I, Rectal atresia.
Low Rectal Anomalies
Anal Agenesis, With or Without a Fistula
The anal canal may end blindly or there may be an ectopic anus or an anoperineal fistula that opens into the perineum (Fig. 12-25D and E). The abnormal canal may, however, open into the vagina or the urethra in males (Fig. 12-25F and G). Most low anorectal anomalies are associated with an external fistula. Anal agenesis with a fistula results from incomplete separation of the cloaca by the urorectal septum.
Anal Stenosis
In anal stenosis, the anus is in a normal position but the anus and the anal canal are narrow (Fig. 12-25B). This anomaly is probably caused by a slight dorsal deviation of the urorectal septum as it grows caudally to fuse with the cloacal membrane.
Membranous Atresia of Anus
In membranous anal atresia, the anus is in the normal position but a thin layer of tissue separates the anal canal from the exterior (Figs. 12-24 and 12-25C). The anal membrane is thin enough to bulge on straining. This anomaly results from failure of the anal membrane to perforate at the end of the eighth week.
High Anorectal Anomalies
Anorectal Agenesis, With or Without a Fistula
In anorectal agenesis, the rectum ends superior to the puborectalis muscle. This is the most common type of anorectal anomaly, and it accounts for approximately two thirds of anorectal defects. Although the rectum ends blindly, there is usually a fistula to the bladder (rectovesical fistula) or the urethra (rectourethral fistula) in males, or to the vagina (rectovaginal fistula) or the vestibule of the vagina (rectovestibular fistula) (Fig. 12-25F and G). Anorectal agenesis with a fistula is the result of incomplete separation of the cloaca by the urorectal septum.
Rectal Atresia
In rectal atresia, the anal canal and the rectum are present but are separated (Fig. 12-25H and I). Sometimes the two segments of bowel are connected by a fibrous cord, the remnant of the atretic portion of the rectum. The cause of rectal atresia may be abnormal recanalization of the colon or, more likely, a defective blood supply.
Clinically Oriented Questions
1. About 2 weeks after birth, an infant began to vomit shortly after feeding. Each time, the vomitus was propelled approximately 2 feet. The physician told the mother that the infant had an obstructing benign growth that causes a narrow outlet from the stomach. Is there an embryologic basis for this anomaly?
2. Do infants with Down syndrome have an increased incidence of duodenal atresia? Can the condition be corrected?
3. A man claimed that his appendix was on his left side. Is this possible and, if so, how could this happen?
4. A patient reported that she had two appendices and separate operations to remove them. Do people ever have two appendices?
5. What is Hirschsprung disease? Some sources state that it is a congenital condition resulting from large bowel obstruction. Is this correct? If so, what is its embryologic basis?
6. A nurse observed what appeared to be feces being expelled from a baby’s umbilicus. How could this happen? What conditions would likely be present?