Chapter 11 Respiratory System
The lower respiratory organs (larynx, trachea, bronchi, and lungs) begin to form during the fourth week. The primordium of the lower respiratory system—the laryngotracheal groove—develops caudal to the fourth pair of pharyngeal pouches (Fig. 11-1A and B). The endodermal lining of the laryngotracheal groove gives rise to the epithelium and glands of the larynx, trachea, bronchi, and pulmonary epithelium. The connective tissue, cartilage, and smooth muscle in these structures develop from the splanchnic mesoderm surrounding the foregut (see Fig. 11-4A). By the end of the fourth week, a pouchlike laryngotracheal diverticulum has formed at the largyngotracheal groove (Figs. 11-1A and 11-2A) and is located ventral to the caudal part of the foregut.
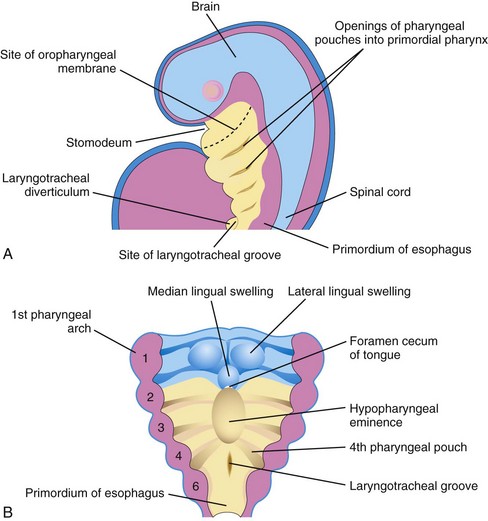
Figure 11–1 A, Sagittal section of the cranial half of the embryo. Lateral view, 4 weeks old. B, Horizontal section of the embryo, showing the floor of the primordial pharynx and the location of the laryngotracheal groove.
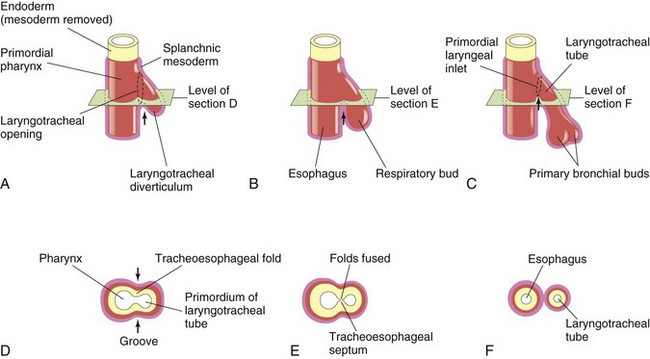
Figure 11–2 Successive stages in the development of the tracheoesophageal septum during the fourth and fifth weeks of development. A to C, Lateral views of the caudal part of the primordial pharynx, showing the laryngotracheal diverticulum and partitioning of the foregut into the esophagus and the laryngotracheal tube. D to F, Transverse sections, showing the formation of the tracheoesophageal septum and how it separates the foregut into the laryngotracheal tube and the esophagus. The arrows represent cellular changes resulting from growth.
As the diverticulum elongates, its distal end enlarges to form a globular respiratory bud (Fig. 11-2B). The laryngotracheal diverticulum soon separates from the primordial pharynx, but it maintains communication with it through the primordial laryngeal inlet (Fig. 11-2A and C). As the diverticulum elongates, it is invested with splanchnic mesoderm (Fig. 11-2B). Longitudinal tracheoesophageal folds develop in the laryngotracheal diverticulum, approach each other, and fuse to form a partition—the tracheoesophageal septum (Fig. 11-2D and E).
This septum divides the cranial part of the foregut into a ventral part, the laryngotracheal tube (primordium of the larynx, trachea, bronchi, and lungs), and a dorsal part (primordium of the oropharynx and esophagus) (Fig. 11-2F). The opening of the laryngotracheal tube into the pharynx becomes the primordial laryngeal inlet (Figs. 11-2F and 11-3C).
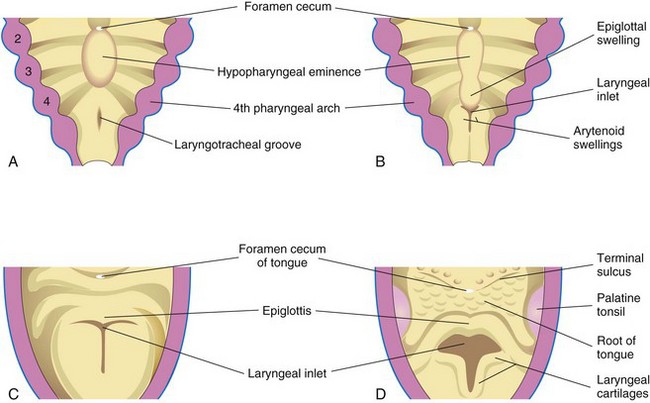
Figure 11–3 Successive stages in the development of the larynx. A, At 4 weeks. B, At 5 weeks. C, At 6 weeks. D, At 10 weeks. The epithelium lining the larynx is of endodermal origin. The cartilages and muscles of the larynx arise from the mesenchyme in the fourth and sixth pairs of pharyngeal arches. Note that the laryngeal inlet changes in shape from a slitlike opening to a T-shaped inlet as the mesenchyme surrounding the developing larynx proliferates.
Development of Larynx 
The epithelial lining of the larynx develops from the endoderm of the cranial end of the laryngotracheal tube. The cartilages of the larynx develop from cell populations in the fourth and sixth pairs of pharyngeal arches (see Chapter 10). The laryngeal cartilages develop from mesenchyme that is derived from neural crest cells. The mesenchyme at the cranial end of the laryngotracheal tube proliferates rapidly, producing paired arytenoid swellings (Fig. 11-3B). These swellings grow toward the tongue, converting the primordial glottis into a T-shaped laryngeal inlet (Fig. 11-3C and D). The laryngeal epithelium proliferates rapidly, resulting in temporary occlusion of the laryngeal lumen. Recanalization of the larynx occurs by the 10th week. The laryngeal ventricles form during this recanalization process. These recesses are bound by folds of mucous membrane that evolve into the vocal folds (cords) and the vestibular folds.
The epiglottis develops from the caudal part of the hypopharyngeal eminence, produced by the proliferation of the mesenchyme in the ventral ends of the third and fourth pharyngeal arches (see Figs. 10-20 and 11-3B to D). The rostral part of this eminence forms the posterior third or pharyngeal part of the tongue (see Fig. 10-20). Because the laryngeal muscles develop from myoblasts in the fourth and sixth pairs of pharyngeal arches, they are innervated by the laryngeal branches of the vagus nerves (CN X) that supply these arches (see Table 10-1). Growth of the larynx and epiglottis is rapid during the first 3 years after birth, following which the epiglottis has reached its adult form. There is gradual descent of both structures during early childhood.
Laryngeal Atresia
Laryngeal atresia (obstruction) is a rare anomaly that results in obstruction of the upper fetal airway; it is also known as congenital high airway obstruction syndrome. Distal to the atresia or stenosis (narrowing), the airways become dilated, the lungs are enlarged and echogenic (capable of producing echoes during ultrasonography because they are filled with liquid), the diaphragm is either flattened or inverted, and fetal ascites, hydrops, or both (accumulation of serous fluid) is present. Prenatal ultrasonography permits diagnosis of these anomalies.
Development of Trachea 
The endodermal lining of the laryngotracheal tube distal to the larynx differentiates into the epithelium and the glands of the trachea and the pulmonary epithelium. The cartilage, connective tissue, and muscles of the trachea are derived from the splanchnic mesoderm surrounding the laryngotracheal tube (Fig. 11-4).
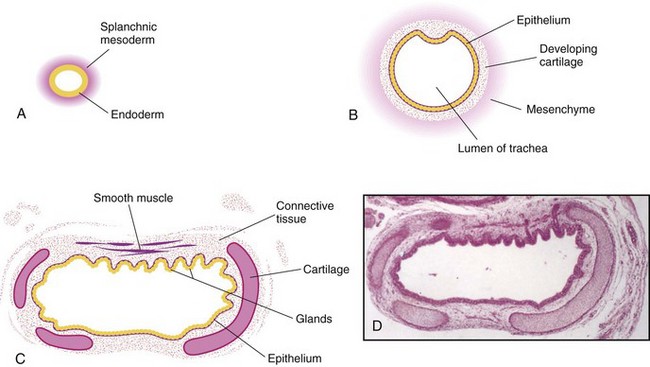
Figure 11–4 Transverse sections through the laryngotracheal tube, showing progressive stages in the development of the trachea. A, At 4 weeks. B, At 10 weeks. C, At 11 weeks. Note that the endoderm of the tube gives rise to the epithelium and the glands of the trachea and that the mesenchyme surrounding the tube forms the connective tissue, muscle, and cartilage (drawing of the micrograph shown in D). D, Photomicrograph of a transverse section of the developing trachea at 12 weeks.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Tracheoesophageal Fistula
A tracheoesophageal fistula (TEF) is an abnormal passage between the trachea and the esophagus (Figs. 11-5 and 11-6A). It occurs at a rate of approximately 1 in 3000 to 1 in 4500 live births and predominantly affects males. In most cases, the fistula is associated with esophageal atresia. TEF results from incomplete division of the cranial part of the foregut into respiratory and esophageal parts during the fourth week. Incomplete fusion of the tracheoesophageal folds results in a defective tracheoesophageal septum and communication between the trachea and esophagus.
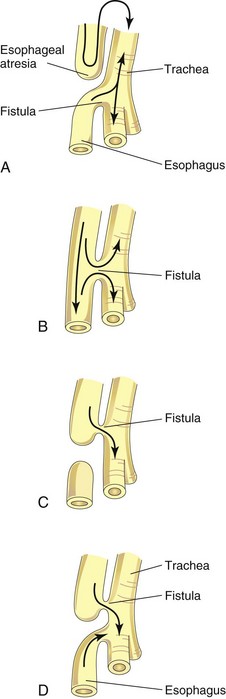
Figure 11–5 The main varieties of tracheoesophageal fistula, shown in order of frequency. Possible directions of the flow of the contents are indicated by arrows. A, Esophageal atresia is associated with tracheoesophageal fistula in more than 85% of cases. B, Fistula between the trachea and the esophagus; this type of anomaly accounts for approximately 4% of cases. C, Atresia of the proximal esophagus ending in a tracheal esophageal fistula with the distal esophagus having a blind pouch. Air cannot enter the distal esophagus and the stomach. D, Atresia of the proximal segment of the esophagus, with fistulas between the trachea and both the proximal and the distal segments of the esophagus. All infants born with tracheoesophageal fistula have esophageal dysmotility, and most have reflux.
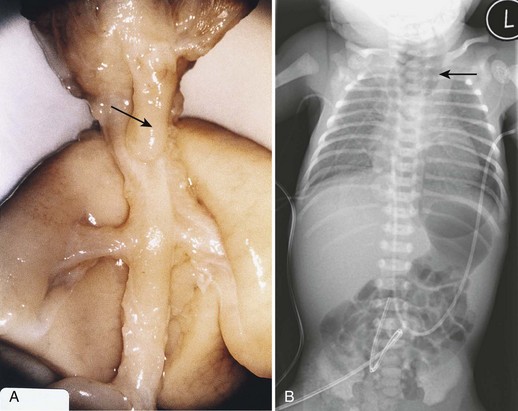
Figure 11–6 A, Tracheoesophageal fistula in a 17-week fetus. The upper esophageal segment ends blindly (pointer). B, Radiograph of an infant with esophageal atresia. Air in the distal gastrointestinal tract indicates the presence of a tracheoesophageal fistula (arrow, blind proximal esophageal sac).
(A, From Kalousek DK, Fitch N, Paradice BA: Pathology of the Human Embryo and Previable Fetus. New York, Springer Verlag, 1990. B, Courtesy of Dr. J. Been, Dr. M. Shuurman, and Dr. S. Robben, Maastricht University Medical Centre, Maastricht, Netherlands.)
TEF is the most common anomaly of the lower respiratory tract. Four main varieties of TEF may develop. The usual anomaly is a blind ending of the superior part of the esophagus (esophageal atresia) and a joining of the inferior part to the trachea near its bifurcation (Figs. 11-5A and 11-6B). Infants with this type of TEF and esophageal atresia cough and choke when swallowing because of the accumulation of excessive amounts of liquid in the mouth and upper respiratory tract. When the infant attempts to swallow milk, it rapidly fills the esophageal pouch and is regurgitated. Gastric contents may also reflux from the stomach through the fistula into the trachea and lungs which may result in pneumonia or pneumonitis (inflammation of the lungs). Other varieties of TEF are shown in Fig. 11-5B to D. Polyhydramnios (see Chapter 8) is often associated with esophageal atresia and TEF. Excess amniotic fluid accumulates because fluid cannot pass to the stomach and intestines for absorption and subsequent transfer through the placenta to the maternal blood for disposal.
Tracheal Stenosis and Atresia
Narrowing (stenosis) and obstruction (atresia) of the trachea are uncommon anomalies that are usually associated with one of the varieties of TEF. Stenoses and atresias probably result from unequal partitioning of the foregut into the esophagus and the trachea. In some cases, a web of tissue obstructs airflow (incomplete tracheal atresia).
Development of Bronchi and Lungs 
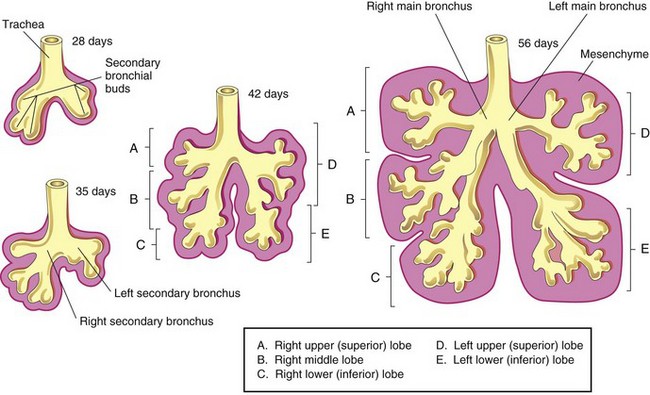
The respiratory bud (lung bud) that developed at the caudal end of the laryngotracheal diverticulum during the fourth week (Fig. 11-2B) soon divides into two outpouchings called primary bronchial buds (Figs. 11-2C and 11-7A). Later, secondary and tertiary bronchial buds form and grow laterally into the pericardioperitoneal canals (Fig. 11-7A). Together with the surrounding splanchnic mesoderm, the bronchial buds differentiate into the bronchi and their ramifications in the lungs (Fig. 11-7B). Early in the fifth week, the connection of each bronchial bud with the trachea enlarges to form the primordia of main bronchi (Fig. 11-8).
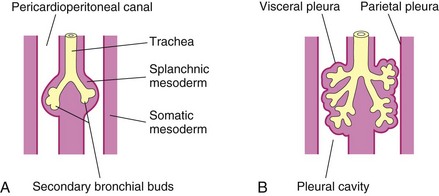
Figure 11–7 Illustrations of the growth of the developing lungs into the splanchnic mesoderm adjacent to the medial walls of the pericardioperitoneal canals (primordial pleural cavities). Development of the layers of the pleura is also shown. A, At 5 weeks. B, At 6 weeks.
The embryonic right main bronchus is slightly larger than the left one and is oriented more vertically. This embryonic relationship persists in adults; consequently, a foreign body is more liable to enter the right main bronchus than the left one. The main bronchi subdivide into secondary bronchi that form lobar, segmental, and intrasegmental branches (Fig. 11-8). On the right, the superior secondary bronchus supplies the upper (superior) lobe of the lung, whereas the inferior secondary bronchus subdivides into two bronchi, one connecting to the middle lobe of the right lung and the other connecting to the lower (inferior) lobe. On the left, the two secondary bronchi supply the upper and lower lobes of the lung. Each secondary bronchus undergoes progressive branching.
The segmental bronchi—10 in the right lung and 8 or 9 in the left lung—begin to form by the seventh week. As this development occurs, the surrounding mesenchyme also divides. Each segmental bronchus, with its surrounding mass of mesenchyme, is the primordium of a bronchopulmonary segment. By 24 weeks, approximately 17 orders of branching have occurred and respiratory bronchioles have developed (Fig. 11-9B). An additional seven orders of airways develop after birth.
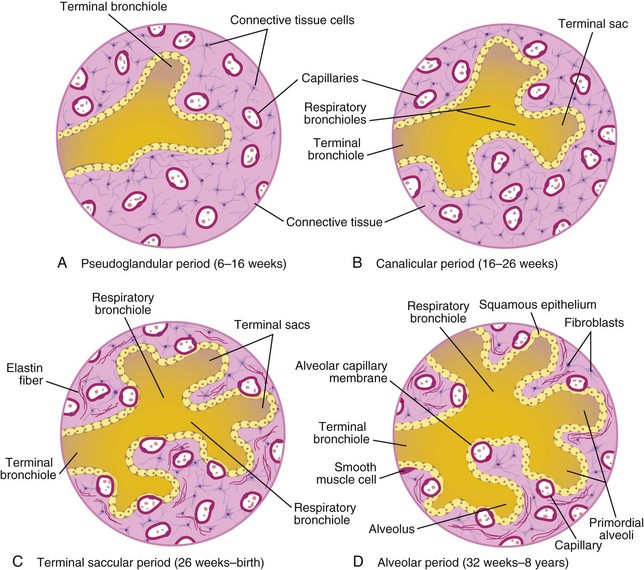
Figure 11–9 Diagram of histologic sections, showing progressive stages of lung development. A and B, Early stages of lung development. In C and D, note that the alveolocapillary membrane is thin and that some capillaries bulge into the terminal saccules.
As the bronchi develop, cartilaginous plates are formed from the surrounding splanchnic mesenchyme. The bronchial smooth muscle and connective tissue and the pulmonary connective tissue and capillaries are also derived from this mesenchyme. As the lungs develop, they acquire a layer of visceral pleura from the splanchnic mesoderm (Fig. 11-7). With expansion, the lungs and pleural cavities grow caudally into the mesenchyme of the body wall and soon lie close to the heart. The thoracic body wall becomes lined by a layer of parietal pleura, derived from the somatic mesoderm (Fig. 11-7B).
Maturation of Lungs
Maturation of the lungs is divided into four stages: pseudoglandular, canalicular, terminal saccular, and alveolar.
Pseudoglandular Period (6–16 Weeks)
The developing lungs resemble, on a histologic basis, an exocrine gland during the early part of this period (Fig. 11-9A). By 16 weeks, all of the major elements of the lung have formed, except those involved with gas exchange. Respiration is not possible; hence, fetuses born during this period are unable to survive.
Canalicular Period (16–26 Weeks)
The canalicular period overlaps the pseudoglandular period because cranial segments of the lungs mature faster than caudal segments. During the canalicular period, the lumina of the bronchi and the terminal bronchioles become larger and the lung tissue becomes highly vascular (Fig. 11-9B). By 24 weeks, each terminal bronchiole has given rise to two or more respiratory bronchioles, each of which then divides into three to six tubular passages called the primordial alveolar ducts. Respiration is possible toward the end of the canalicular stage because some thin-walled terminal sacs (primordial alveoli) have developed at the ends of the respiratory bronchioles and the lung tissue is well vascularized. Although a fetus born at 24 to 26 weeks may survive if given intensive care, it often dies because its respiratory and other systems are relatively immature.
Terminal Sac Stage (26 Weeks to Birth)
During this period, many more terminal sacs develop, their epithelium becomes very thin, and capillaries begin to bulge into these sacs (see Fig. 11-9C). The intimate contact between epithelial and endothelial cells establishes the blood-air barrier, permitting adequate gas exchange for survival. By 26 weeks, the terminal sacs are lined mainly by squamous epithelial cells of endodermal origin—type I pneumocytes—across which gas exchange occurs. The capillary network proliferates rapidly in the mesenchyme around the developing alveoli, and there is concurrent active development of lymphatic capillaries. Scattered among the squamous epithelial cells are rounded secretory epithelial cells—type II pneumocytes—which secrete pulmonary surfactant, a complex mixture of phospholipids and proteins. Surfactant forms as a monomolecular film over the interior walls of the alveolar sacs and counteracts surface tension forces at the air-alveolar interface. This facilitates expansion of the terminal sacs (primordial alveoli).
The maturation of alveolar type II cells and the production of surfactant vary widely in fetuses of different ages. Surfactant production begins by 20 weeks, but surfactant is present in only small amounts in premature infants. It does not reach adequate levels until the late fetal period. Both increased surfactant production induced by antenatal corticosteroids and postnatal surfactant replacement therapy have increased the rates of survival of these infants.
Alveolar Period (32 Weeks to 8 Years)
Exactly when the terminal saccular period ends and the alveolar period begins depends on the definition of the term alveolus (Fig. 11-9D). At the beginning of the alveolar period, each respiratory bronchiole terminates in a cluster of thin-walled terminal sacs that are separated from one another by loose connective tissue. These terminal sacs represent future alveolar ducts. The alveolocapillary membrane (pulmonary diffusion barrier, or respiratory membrane) is sufficiently thin to allow gas exchange. The transition from dependence on the placenta for gas exchange to autonomous gas exchange after birth requires the following adaptive changes in the lungs:
• Production of surfactant in the alveolar sacs
• Transformation of the lungs into gas-exchanging organs
• Establishment of parallel pulmonary and systemic circulations
Approximately 95% of characteristic mature alveoli develop postnatally. Before birth, the primordial alveoli appear as small bulges on the walls of the respiratory bronchioles and terminal sacs (future alveolar ducts). After birth, the primordial alveoli enlarge as the lungs expand; however, most of the increase in the size of the lungs results from a continued increase in the number of respiratory bronchioles and primordial alveoli rather than from an increase in the size of the alveoli. Alveolar development is largely complete by 3 years of age, but new alveoli may be added until approximately 8 years of age. Unlike mature alveoli, immature alveoli have the potential for forming additional primordial alveoli.
Approximately 150 million primordial alveoli, one half the number in adults, are present in the lungs of a full-term newborn infant. On chest radiographs, therefore, the lungs of newborn infants appear denser than adult lungs. Between the third and the eighth year, the adult complement of 300 million alveoli is achieved.
Three factors that are essential for normal lung development are:
Fetal breathing movements occur before birth, exerting sufficient force to cause aspiration of some amniotic fluid into the lungs. These fetal breathing movements occur approximately 50% of the time and only during rapid eye movement sleep. These movements stimulate lung development, possibly by creating a pressure gradient between the lungs and the amniotic fluid. By birth, the fetus has had the advantage of several months of breathing exercise. Fetal breathing movements increase as the time of delivery approaches.
At birth, the lungs are approximately half-filled with fluid derived from the amniotic cavity, the lungs, and the tracheal glands. Aeration of the lungs at birth occurs not so much by the inflation of empty collapsed organs as by the rapid replacement of intra-alveolar fluid by air. The fluid in the lungs is cleared at birth by three routes:
• Through the mouth and nose by pressure on the thorax during vaginal delivery
• Into the pulmonary capillaries and pulmonary arteries and veins
Oligohydramnios and Lung Development
When oligohydramnios (an insufficient amount of amniotic fluid) is severe and chronic (e.g., because of amniotic fluid leakage), lung development is retarded because restriction of the fetal thorax by the uterine walls impedes lung growth. Pulmonary hypoplasia occurs as a result and may be severe.
Respiratory Distress Syndrome
Respiratory distress syndrome (RDS) affects approximately 2% of live newborn infants, and those born prematurely are the most susceptible. RDS is also known as hyaline membrane disease. Affected infants have rapid, labored breathing shortly after birth. An estimated 30% of all neonatal disease results from RDS or its complications. Surfactant deficiency is a major cause of RDS. The lungs are underinflated and the alveoli contain fluid with a high protein content—hyaline membrane. This membrane is believed to be derived from a combination of substances in the circulation and from the injured pulmonary epithelium. Prolonged intrauterine asphyxia may produce irreversible changes in type II alveolar cells, making them incapable of producing surfactant. Not all of the growth factors and hormones that control surfactant production have been identified, but corticosteroids and thyroxine are potent stimulators of surfactant production.
Lungs of Newborn Infants
Fresh, healthy neonatal lungs always contain some air; consequently, pulmonary tissue samples float in water. By contrast, a diseased lung that is partially filled with fluid may not float. Of medicolegal significance is the fact that the lungs of a stillborn infant are firm and sink when placed in water because they contain fluid, not air.
Lung Hypoplasia
In infants with a congenital diaphragmatic hernia (see Chapter 9), the lung is unable to develop normally because it is compressed by the abnormally positioned abdominal viscera. Lung hypoplasia is characterized by markedly reduced lung volume. Many infants with a congenital diaphragmatic hernia die of pulmonary insufficiency, despite optimal postnatal care, because their lungs are too hypoplastic to support extrauterine life.
Clinically Oriented Questions
1. What stimulates the infant to start breathing at birth? Is “slapping the buttocks” necessary?
2. An infant reportedly died approximately 72 hours after birth from the effects of respiratory distress syndrome. What is respiratory distress syndrome? By what other name is this condition known? Is its cause genetic or environmental?