Chapter 10 Pharyngeal Apparatus
The pharyngeal apparatus (Fig. 10-1) consists of the following: pharyngeal arches, pharyngeal pouches, pharyngeal grooves, and pharyngeal membranes. These embryonic structures contribute to the formation of the face and neck.
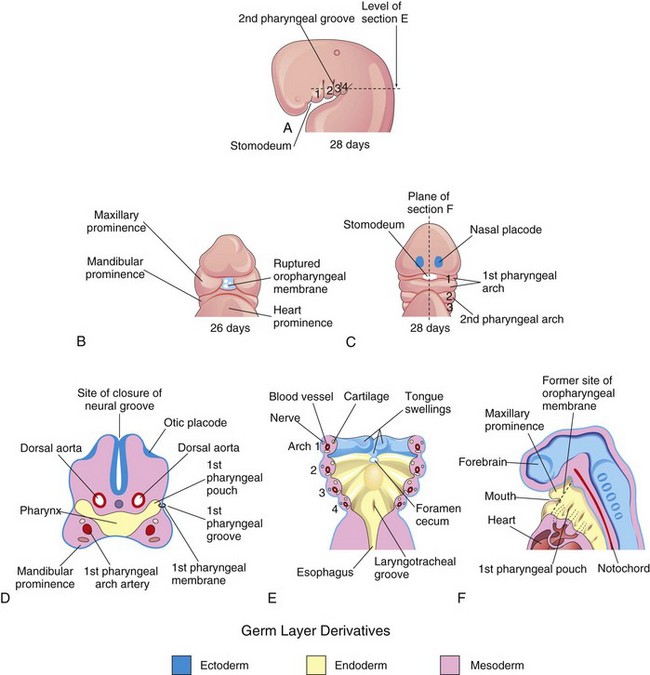
Figure 10–1 Illustrations of the human pharyngeal apparatus. A, Lateral view showing the development of four pharyngeal arches. B and C, Ventral (facial) views showing the relationship of the pharyngeal arches to the stomodeum. D, Frontal section through the cranial region of an embryo. E, Horizontal section showing the arch components and the floor of the primordial pharynx. F, Sagittal section of the cranial region of an embryo, showing the openings of the pharyngeal pouches in the lateral wall of the primordial pharynx.
Pharyngeal Arches 
The pharyngeal arches begin to develop early in the fourth week as neural crest cells migrate from the hindbrain into the mesenchyme of the future head and neck regions. Initially, each pharyngeal arch consists of a core of mesenchyme (embryonic connective tissue) and is covered externally by ectoderm and internally by endoderm (Fig. 10-1D and E). The first pair of arches, the primordia of the jaws, appears as surface elevations lateral to the developing pharynx (Fig. 10-1). Other arches soon appear as obliquely disposed, rounded ridges on each side of the future head and neck regions. By the end of the fourth week, four well-defined pairs of arches are visible (Fig. 10-1A).
The pharyngeal arches support the lateral walls of the primordial pharynx, which is derived from the cranial part of the foregut. The stomodeum (primordial mouth) initially appears as a slight depression of the surface ectoderm (Fig. 10-1A). It is separated from the cavity of the primordial pharynx by a bilaminar membrane—the oropharyngeal membrane—composed of fused ectoderm and endoderm. The oropharyngeal membrane ruptures at approximately 26 days (Fig. 10-1C), bringing the primordial pharynx and foregut into communication with the amniotic cavity. The pharyngeal arches contribute extensively to the formation of the face, nasal cavities, mouth, larynx, pharynx, and neck (Figs. 10-2 to 10-4).
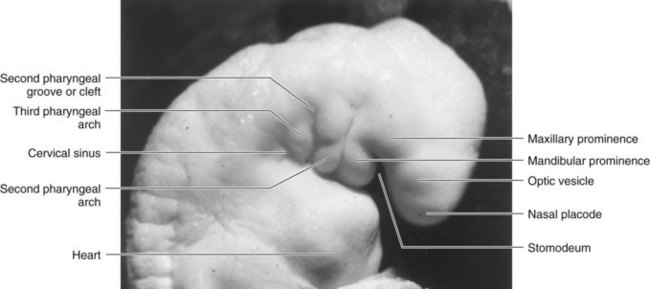
Figure 10–2 A Carnegie stage 13,  -week human embryo.
-week human embryo.
(Courtesy the late Professor Emeritus Dr. K.V. Hinrichsen, Medizinische Fakultät, Institut für Anatomie, Ruhr-Universität Bochum, Bochum, Germany.)
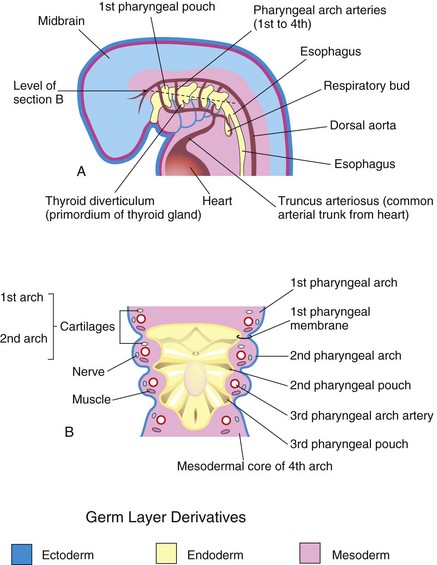
Figure 10–3 A, Illustration of the pharyngeal pouches and pharyngeal arch arteries. B, Horizontal section through the embryo showing the floor of the primordial pharynx and illustrating the germ layer origin of the pharyngeal arch components.
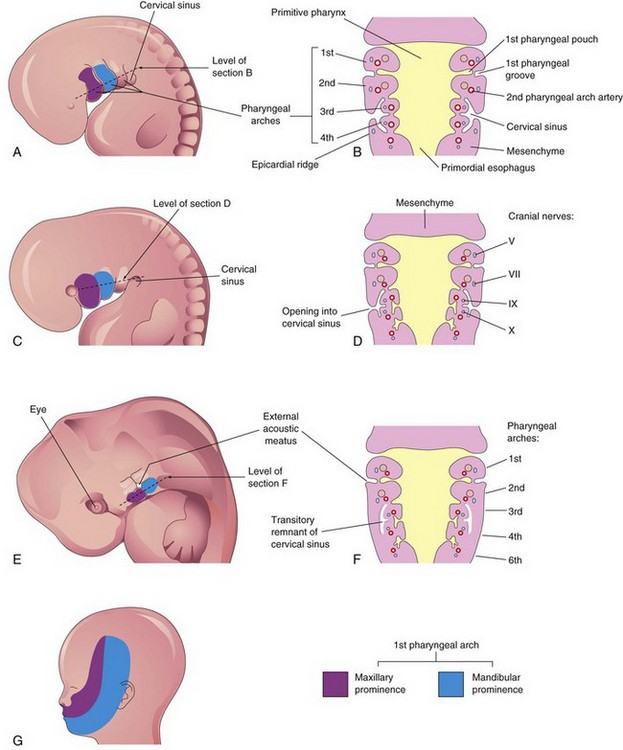
Figure 10–4 A, Lateral view of the head, neck, and thoracic regions of an embryo (approximately 32 days), showing the pharyngeal arches and the cervical sinus. B, Diagrammatic section through the embryo at the level seen in A, showing growth of the second arch over the third and fourth arches. C, An embryo of approximately 33 days. D, Section of the embryo at the level seen in C, showing early closure of the cervical sinus. E, An embryo of approximately 41 days. F, Section of the embryo at the level seen in E, showing the transitory cystic remnant of the cervical sinus. G, Illustration of a 20-week fetus, showing the area of the face derived from the first pair of pharyngeal arches.
The first pharyngeal arch develops two prominences (Figs. 10-1B and 10-2): the smaller maxillary prominence and the larger mandibular prominence. The second pharyngeal arch makes a major contribution to the formation of the hyoid bone (see Fig. 10-5B).
Pharyngeal Arch Components
A typical pharyngeal arch has the following components (Fig. 10-3A and B):
• A pharyngeal arch artery (aortic arch artery) that arises from the truncus arteriosus of the primordial heart and courses around the primordial pharynx to enter the dorsal aorta
• A cartilaginous rod that forms the skeleton of the arch
• A muscular component that is the primordium of the muscles in the head and neck
• A nerve that supplies the mucosa and muscles derived from each arch
Derivatives of Pharyngeal Arch Arteries
The transformation of the pharyngeal arch arteries into the adult arterial pattern of the head and neck is described in the section on the pharyngeal arch artery derivatives in Chapter 14.
Derivatives of Pharyngeal Arch Cartilages
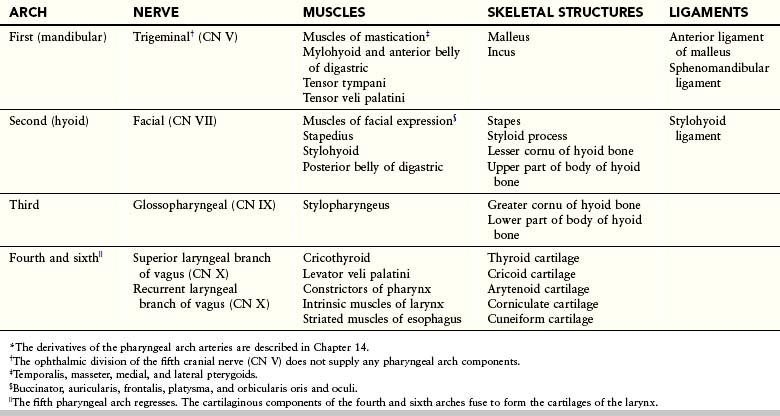
The dorsal end of the first pharyngeal arch cartilage becomes ossified to form two middle ear bones, the malleus and incus (Fig. 10-5 and Table 10-1). The middle section of the cartilage regresses, but its perichondrium forms the anterior ligament of the malleus and the sphenomandibular ligament. Ventral parts of the first arch cartilage form the primordium of the mandible. Each half of the mandible forms lateral to and in close association with its cartilage. The cartilage disappears as the mandible develops around it by intramembranous ossification.
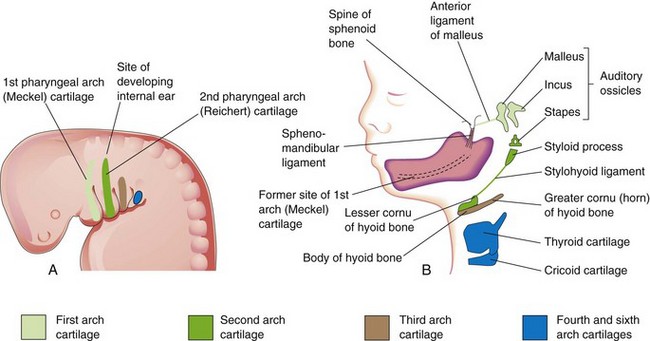
Figure 10–5 A, Schematic lateral view of the head, neck, and thoracic regions of a 4-week embryo, showing the location of the cartilages in the pharyngeal arches. B, Similar view of a 24-week fetus, showing the adult derivatives of the arch cartilages. Note that the mandible is formed by intramembranous ossification of the mesenchymal tissue surrounding the first arch cartilage.
The dorsal end of the second pharyngeal arch cartilage ossifies to form the stapes of the middle ear and the styloid process of the temporal bone. The part of the cartilage between the styloid process and the hyoid bone regresses; its perichondrium forms the stylohyoid ligament. The ventral end of the second arch cartilage ossifies to form the lesser cornu and the superior part of the body of the hyoid bone.
The third pharyngeal arch cartilage ossifies to form the greater cornu and the inferior part of the body of the hyoid bone. The fourth and sixth pharyngeal arch cartilages fuse to form the laryngeal cartilages, except for the epiglottis. The epiglottic and thyroid cartilages appear to develop from neural crest cells (see Fig. 10-22A to C). The cricoid cartilage develops from mesoderm.
Derivatives of Pharyngeal Arch Muscles
The muscular components of the arches form various muscles in the head and neck; for example, the musculature of the first pharyngeal arch forms the muscles of mastication and others (Fig. 10-6A and B and Table 10-1).
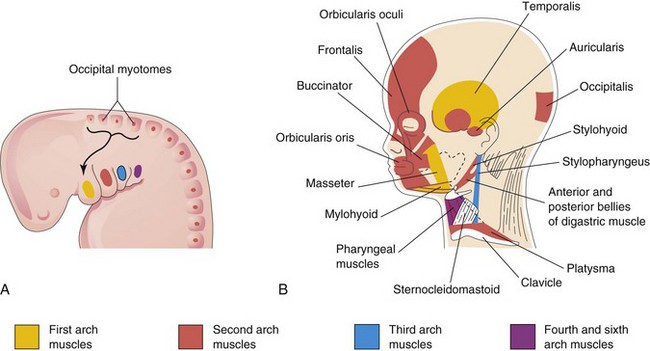
Figure 10–6 A, Lateral view of the head, neck, and thoracic regions of a 4-week embryo showing the muscles derived from the pharyngeal arches. The arrow shows the pathway taken by myoblasts from the occipital myotomes to form the tongue musculature. B, The head and neck regions of a 20-week fetus, showing the muscles derived from the pharyngeal arches. Parts of the platysma and sternocleidomastoid muscles have been removed to show the deeper muscles. Note that myoblasts from the second arch migrate from the neck to the head, where they give rise to the muscles of facial expression. These muscles are supplied by the facial nerve (cranial nerve VII), the nerve of the second pharyngeal arch.
Derivatives of Pharyngeal Arch Nerves
Each arch is supplied by its own cranial nerve (CN). The special visceral efferent (branchial) components of the cranial nerves supply muscles derived from the pharyngeal arches (Fig. 10-7A and Table 10-1). Because the mesenchyme from the pharyngeal arches contributes to the dermis and the mucous membranes of the head and neck, these areas are supplied with the special visceral afferent nerves. The facial skin is supplied by the fifth cranial nerve (CN V, or trigeminal nerve); however, only the caudal two branches (maxillary and mandibular) supply derivatives of the first pharyngeal arch (Fig. 10-7B). CN V is the principal sensory nerve of the head and neck and is the motor nerve for the muscles of mastication. Its sensory branches innervate the face, teeth, and mucous membranes of the nasal cavities, palate, mouth, and tongue (Fig. 10-7C). The seventh cranial nerve (CN VII, or facial nerve), the ninth cranial nerve (CN IX, or glossopharyngeal nerve), and the 10th cranial nerve (CN X, or vagus nerve) supply the second, third, and caudal (fourth to sixth) arches, respectively. The fourth arch is supplied by the superior laryngeal branch of the vagus nerve, whereas the sixth arch is supplied by its recurrent laryngeal branch. The nerves of the second to sixth pharyngeal arches (Fig. 10-7B) innervate the mucous membranes of the tongue, pharynx, and larynx (Fig. 10-7C).
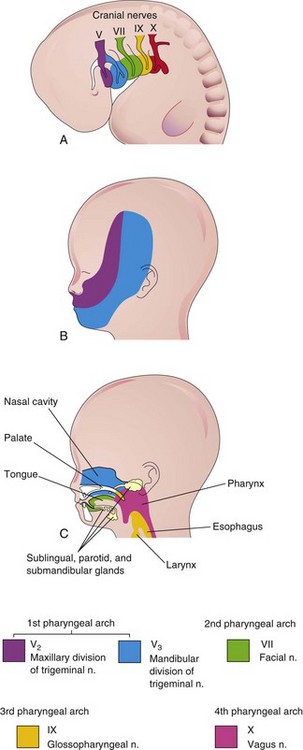
Figure 10–7 A, Lateral view of the head, neck, and thoracic regions of a 4-week embryo, showing the cranial nerves that supply the pharyngeal arches. B, The head and neck regions of a 20-week fetus, showing the superficial distribution of the two caudal branches of the first arch nerve (cranial nerve V). C, Sagittal section of the fetal head and neck, showing the deep distribution of the sensory fibers of the nerves to the teeth and mucosa of the tongue, pharynx, nasal cavity, palate, and larynx.
Pharyngeal Pouches 
The primordial pharynx widens cranially where it joins the stomodeum, and narrows caudally, where it joins the esophagus (Figs. 10-3A and 10-4B). The endoderm of the pharynx lines the internal aspects of the pharyngeal arches and passes into the pharyngeal pouches (Figs. 10-1D and E and 10-8A). These pairs of pouches develop in a craniocaudal sequence between the arches. The first pair of pouches, for example, lies between the first and second pharyngeal arches. Four pairs of pharyngeal pouches are well defined; the fifth pair is absent or rudimentary. The endoderm of the pouches contacts the ectoderm of the pharyngeal grooves, and together they form the double-layered pharyngeal membranes (Fig. 10-3B).
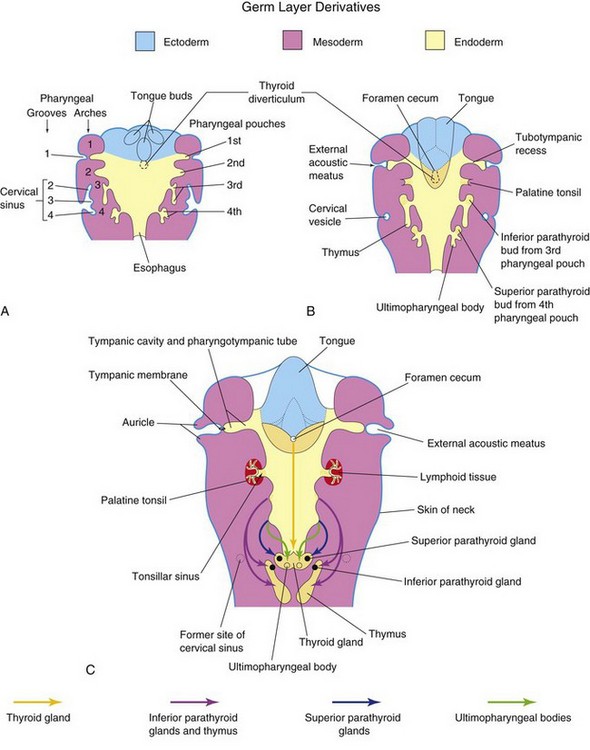
Figure 10–8 Schematic horizontal sections at the level shown in Figure 10-4A, showing the adult derivatives of the pharyngeal pouches. A, At 5 weeks. Note that the second pharyngeal arch grows over the third and fourth arches, burying the second to fourth pharyngeal grooves in the cervical sinus. B, At 6 weeks. C, At 7 weeks. Note the migration of the developing thymus, parathyroid, and thyroid glands into the neck.
Derivatives of Pharyngeal Pouches
The first pharyngeal pouch gives rise to the tubotympanic recess (Fig. 10-8B). The first pharyngeal membrane contributes to the formation of the tympanic membrane (eardrum) (Fig. 10-8C). The cavity of the tubotympanic recess gives rise to the tympanic cavity and the mastoid antrum. The connection of the tubotympanic recess with the pharynx forms the pharyngotympanic tube (auditory tube).
The second pharyngeal pouch is largely obliterated as the palatine tonsil develops (Figs. 10-8C and 10-9). A part of this pouch remains as the tonsillar sinus (fossa). The endoderm of the second pouch proliferates and grows into the underlying mesenchyme. The central parts of these buds break down, forming tonsillar crypts. The pouch endoderm forms the surface epithelium and the lining of the crypts. The mesenchyme around the crypts differentiates into lymphoid tissue, which soon organizes into the lymphatic nodules of the palatine tonsil.
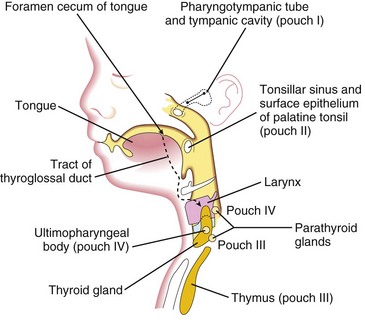
Figure 10–9 A sagittal section of the head, neck, and upper thoracic regions of a 20-week fetus, showing the adult derivatives of the pharyngeal pouches and the descent of the thyroid gland into the neck.
The third pharyngeal pouch expands and develops a solid, bulbar, dorsal part and a hollow, elongate ventral part (Fig. 10-8B). The connection between the pouch and the pharynx is reduced to a narrow duct that soon degenerates. By the sixth week of development, the epithelium of each bulbar dorsal part begins to differentiate into an inferior parathyroid gland. The epithelium of the elongated ventral parts of the third pair of pouches proliferates, obliterating their cavities. These parts come together in the median plane to form the thymus. The primordia of the thymus and parathyroid glands lose their connections with the pharynx. Later, the inferior parathyroid glands separate from the thymus and lie on the dorsal surface of the thyroid gland, whereas the thymus descends into the superior mediastinum (Figs. 10-8C and 10-9). The mesenchyme surrounding the thymic primordium is derived from neural crest cells.
The dorsal part of each fourth pharyngeal pouch develops into a superior parathyroid gland, which lies on the dorsal surface of the thyroid gland (Fig. 10-8B). The parathyroid glands derived from the third pouches descend with the thymus and are carried to a more inferior position than the parathyroid glands that are derived from the fourth pouches (Fig. 10-9). The elongated ventral part of each fourth pouch develops into the ultimopharyngeal body, which fuses with the thyroid gland, giving rise to the parafollicular cells (C cells) of the thyroid gland. These cells produce calcitonin, a hormone involved in the regulation of calcium. C cells differentiate from neural crest cells that migrate from the pharyngeal arches into the fourth pair of pharyngeal pouches.
If the fifth pharyngeal pouch develops, it is rudimentary and becomes part of the fourth pharyngeal pouch.
Pharyngeal Grooves 
The head and neck regions of the embryo exhibit four pharyngeal grooves on each side during the fourth and fifth weeks (Fig. 10-1A). These grooves separate the pharyngeal arches externally. Only one pair of grooves contributes to the adult structures; the first pair persists as the external acoustic meatus (ear canal) (Fig. 10-8C). The other grooves lie in a slit-like depression—the cervical sinus—and are usually obliterated with it as the neck develops (Fig. 10-4B to F).
Congenital Auricular Sinuses and Cysts
Small auricular sinuses and cysts are usually located in a triangular area of skin anterior to the auricle of the external ear (Fig. 10-10D); however, they may occur in other sites around the auricle or in its lobule (earlobe). Although some sinuses and cysts are remnants of the first pharyngeal groove, others represent ectodermal folds sequestered during formation of the auricle from the auricular hillocks (swellings that contribute to the auricle).
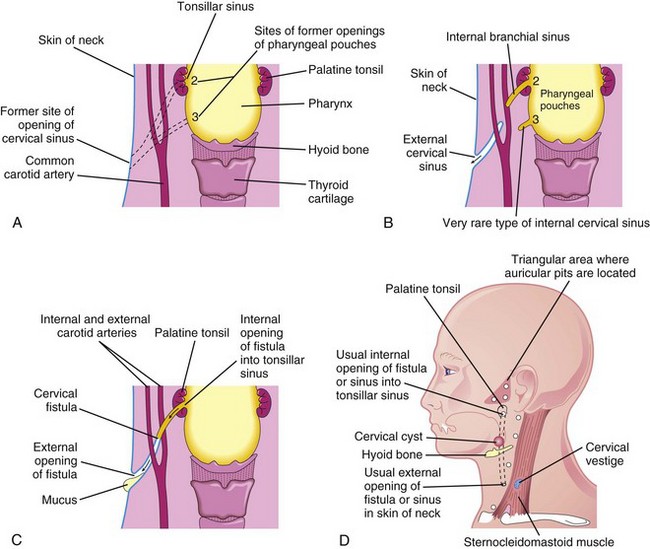
Figure 10–10 A, The adult pharyngeal and neck regions, indicating the former sites of openings of the cervical sinus and the pharyngeal pouches (2 and 3). The broken lines indicate possible courses of cervical fistulas. B, The embryologic basis for various types of cervical sinus. C, Illustration of a cervical fistula resulting from the persistence of parts of the second pharyngeal groove and the second pharyngeal pouch. D, Possible sites of cervical cysts and openings of the cervical sinuses and fistulas. A cervical vestige is also shown.
Cervical (Branchial) Sinuses
Cervical sinuses are uncommon, and almost all that open externally on the side of the neck result from failure of the second pharyngeal groove and the cervical sinus to obliterate (Figs. 10-10B and 10-11A). The sinus typically opens along the anterior border of the sternocleidomastoid muscle in the inferior third of the neck. Anomalies of the other pharyngeal grooves occur in approximately 5% of cases.
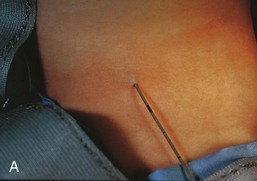
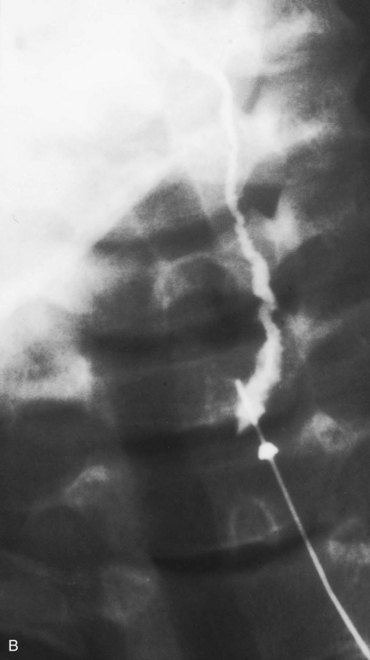
Figure 10–11 A, A child’s neck, showing a catheter inserted into the external opening of a cervical sinus. The catheter allows definition of the length of the tract, which facilitates surgical excision. B, A fistulogram of a complete cervical fistula. The radiograph was taken after injection of a contrast medium to show the course of the fistula through the neck.
(Courtesy Dr. Pierre Soucy, Division of Paediatric Surgery, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada.)
External cervical sinuses are commonly detected during infancy because of the discharge of mucous material from their orifices in the neck. These lateral cervical sinuses are bilateral in approximately 10% of cases and are commonly associated with auricular sinuses.
Internal cervical sinuses open into the pharynx and are very rare. Almost all of these sinuses result from persistence of the proximal part of the second pharyngeal pouch, so they usually open into the tonsillar sinus or near the palatopharyngeal arch (Fig. 10-10B and D). Normally, this pouch disappears as the palatine tonsil develops; its normal remnant is the tonsillar sinus.
Branchial Fistula
An abnormal canal that opens internally into the tonsillar sinus and externally on the side of the neck is a branchial fistula. This rare anomaly results from persistence of parts of the second pharyngeal groove and the second pharyngeal pouch (Figs. 10-10C and D and 10-11B). The fistula ascends from its opening in the neck, through the subcutaneous tissue and the platysma muscle, to reach the tonsillar sinus.
Cervical Cysts
The third and fourth pharyngeal arches are buried in the cervical sinus (Fig. 10-8A). Remnants of parts of the cervical sinus, the second pharyngeal groove, or both may persist and form a spherical or elongated cyst (Fig. 10-10D). Cervical cysts often do not become apparent until late childhood or early adulthood, when they produce a slowly enlarging, painless swelling in the neck (Fig. 10-12). The cysts enlarge because of the accumulation of fluid and cellular debris derived from desquamation of their epithelial linings (Fig. 10-13).
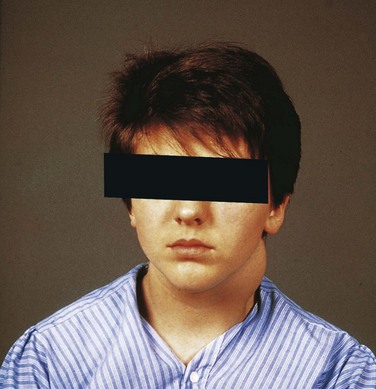
Figure 10–12 A boy with swelling in the neck produced by a cervical cyst. Cervical cysts often lie free in the neck, just inferior to the angle of the mandible, or they may be found anywhere along the anterior border of the sternocleidomastoid muscle.
(Courtesy Dr. Pierre Soucy, Division of Paediatric Surgery, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada.)
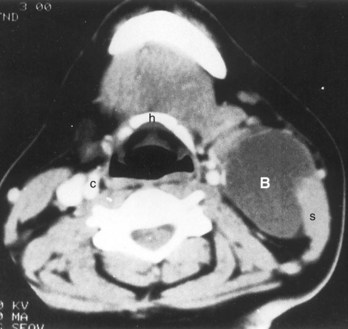
Figure 10–13 A large cervical (cleft) cyst (B) shown by computed tomography of the neck region of a woman who had a “lump” in the neck, similar to that shown in Figure 10-12. The low-density cyst is anterior to the right sternocleidomastoid muscle (s) at the level of the hyoid bone (h). The normal appearance of the left carotid sheath (c) is shown for comparison with the compressed sheath on the right side.
(From McNab T, McLennan MK, Margolis M: Radiology rounds. Can Fam Physician 41:1673, 1995.)
Cervical Vestiges
Normally the pharyngeal cartilages disappear, except for the parts that form ligaments or bones; however, in unusual cases, cartilaginous or bony remnants of the pharyngeal arch cartilages appear under the skin on the side of the neck. These are usually found anterior to the inferior third of the sternocleidomastoid muscle (Fig. 10-10D).
First Arch Syndrome
Abnormal development of the first pharyngeal arch results in various congenital anomalies of the eyes, ears, mandible, and palate that together constitute first pharyngeal arch syndrome (Fig. 10-14). This syndrome is believed to result from insufficient migration of neural crest cells into the first arch during the fourth week. There are two main clinical manifestations of first arch syndrome:
• Treacher Collins syndrome (mandibulofacial dysostosis), is most often caused by an autosomal dominant gene defect (TCOF1), and results in underdevelopment of the zygomatic bones of the face—malar hypoplasia. Characteristic features of the syndrome include down-slanting palpebral fissures, defects of the lower eyelids, deformed external ears, and sometimes abnormalities of the middle and internal ears.
• Pierre Robin sequence consists of hypoplasia of the mandible, cleft palate, and defects of the eye and ear. Many cases of this syndrome are sporadic; however, some appear to have a genetic basis. In Robin morphogenetic complex, the initiating defect is a small mandible (micrognathia), which results in posterior displacement of the tongue and obstruction to full closure of the palatine processes, resulting in bilateral cleft palate.
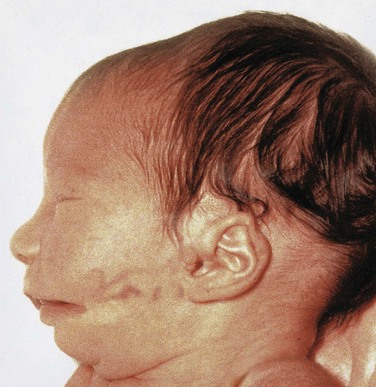
Figure 10–14 An infant with first arch syndrome, a pattern of anomalies resulting from insufficient migration of the neural crest cells into the first pharyngeal arch. Note the following characteristics: deformed auricle of the external ear, preauricular appendage, defect in the cheek between the auricle and the mouth, hypoplasia of the mandible, and macrostomia (large mouth).
Pharyngeal Membranes 
These membranes form where the epithelia of a groove and a pouch approach each other. The pharyngeal membranes appear in the floors of the pharyngeal grooves during the fourth week (Fig. 10-1B and D). Only the first pair contributes to the formation of adult structures, the tympanic membrane (Fig. 10-8C).
Development of Thyroid Gland 
The thyroid gland is the first endocrine gland to develop. It begins to form at approximately 24 days from a median endodermal thickening in the floor of the primordial pharynx (Fig. 10-15A). This thickening soon forms a small outpouching known as the thyroid primordium. As the embryo and tongue grow, the developing thyroid gland descends in the neck, passing ventral to the developing hyoid bone and the laryngeal cartilages. For a short time, it is connected to the tongue by the thyroglossal duct (Fig. 10-15B and C). As a result of rapid cell proliferation, the lumen of the thyroid diverticulum soon obliterates, and then it divides into right and left lobes that are connected by the thyroid isthmus.
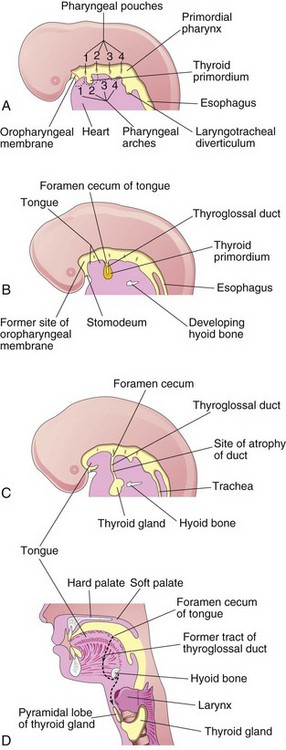
Figure 10–15 Development of the thyroid gland. A to C, Schematic sagittal sections of the head and neck regions of 4-week, 5-week, and 6-week embryos, showing successive stages in the development of the thyroid gland. D, Similar section of an adult head and neck, showing the path taken by the thyroid gland during its embryonic descent (indicated by the former tract of the thyroglossal duct).
DiGeorge Syndrome
Infants with DiGeorge (velocardiofacial) syndrome are born without a thymus and parathyroid glands. The disease is characterized by congenital hypoparathyroidism (hypocalcemia); increased susceptibility to infections (from immune deficiency—specifically, defective T-cell function); palate abnormalities, micrognathia (airway obstruction due to retropositioned tongue); low-set, notched ears; nasal clefts; and cardiac abnormalities (defects of the arch of the aorta and the heart). DiGeorge syndrome occurs when the third and fourth pharyngeal pouches do not differentiate into the thymus and parathyroid glands. The facial abnormalities result primarily from abnormal development of the first arch components during formation of the face and ears. DiGeorge syndrome commonly involves a microdeletion (22q11.2 region), mutation of the HIRA and UFDIL genes, and neural crest cell defects. The incidence of DiGeorge syndrome is one in 2,000 to 4,000 births.
Ectopic Parathyroid Glands
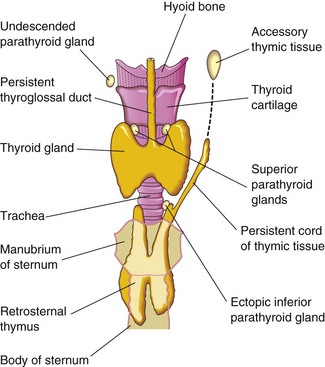
The parathyroids are highly variable in number and location. They may be found anywhere near or within the thyroid gland or the thymus (Fig. 10-16). The superior glands are more constant in position than the inferior ones. Occasionally, an inferior parathyroid gland does not descend and remains near the bifurcation of the common carotid artery. In other cases, it may accompany the thymus into the thorax.
Abnormal Number of Parathyroid Glands
In unusual cases, there may be more than four parathyroid glands. Supernumerary parathyroid glands probably result from division of the primordia of the original glands. Absence of a parathyroid gland results from failure of one of the primordia to differentiate or from atrophy of a gland early in development.
By 7 weeks, the thyroid gland has assumed its definitive shape and has usually reached its final site in the neck (Fig. 10-15D). By this time, the thyroglossal duct has usually degenerated. The proximal opening of the thyroglossal duct persists as a small, blind pit—the foramen cecum in the dorsum of the tongue (Fig. 10-8C). A pyramidal thyroid lobe extends superiorly from the isthmus in approximately 50% of individuals. This lobe may be attached to the hyoid bone by fibrous tissue, smooth muscle, or both.
Thyroglossal Duct Cysts and Sinuses
A remnant of the thyroglossal duct may persist and form a cyst in the tongue or in the anterior part of the neck, usually just inferior to the hyoid bone (Fig. 10-17). The swelling produced by a thyroglossal duct cyst usually develops as a painless, progressively enlarging, movable median mass (Fig. 10-18). The cyst may contain some thyroid tissue. After infection of a cyst, perforation of the skin occurs in some cases, forming a thyroglossal duct sinus that usually opens in the median plane of the neck, anterior to the laryngeal cartilages (Fig. 10-19A).
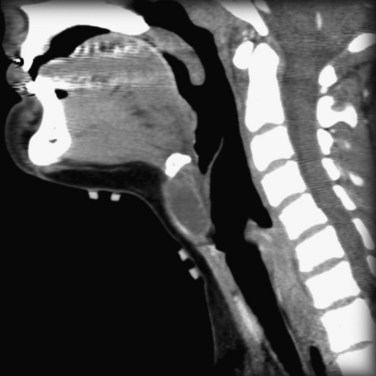
Figure 10–17 CT-Scan of a thyroglossal duct cyst in a child. The cyst is located in the neck anterior to the thyroid cartilage.
(From Dr. Frank Gaillard, Radiopaedia.org, with permission.)
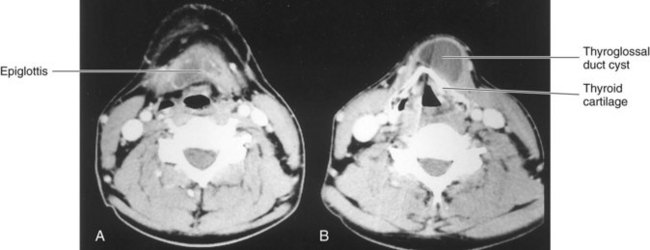
Figure 10–18 Computed tomography scans. A, The level of the thyrohyoid membrane and the base of the epiglottis. B, The level of the thyroid cartilage, which is calcified. The thyroglossal duct cyst extends cranially to the margin of the hyoid bone.
(Courtesy of Dr. Gerald S. Smyser, Altru Health System, Grand Forks, ND.)
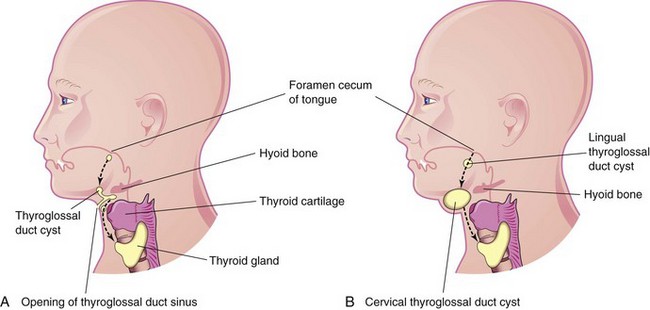
Figure 10–19 A, The head and neck, showing the possible locations of thyroglossal duct cysts. A thyroglossal duct sinus is also shown. The broken line indicates the course taken by the thyroglossal duct during the descent of the developing thyroid gland from the foramen cecum to its final position in the anterior part of the neck. B, Similar sketch showing lingual and cervical thyroglossal duct cysts. Most thyroglossal duct cysts are located just inferior to the hyoid bone.
Ectopic Thyroid Gland
Infrequently, an ectopic thyroid gland is located along the normal route of its descent from the tongue (Fig. 10-15C). Lingual thyroid glandular tissue is the most common type. Incomplete descent of the thyroid gland results in a sublingual thyroid gland that appears high in the neck, at or just inferior to the hyoid bone (Figs. 10-20 and 10-21). As a rule, an ectopic sublingual thyroid gland is the only thyroid tissue present. It is clinically important to differentiate an ectopic thyroid gland from a thyroglossal duct cyst or from accessory thyroid tissue to prevent inadvertent surgical removal of the thyroid gland because this may be the only thyroid tissue present. Failure to recognize the thyroid gland may leave the person permanently dependent on thyroid medication.
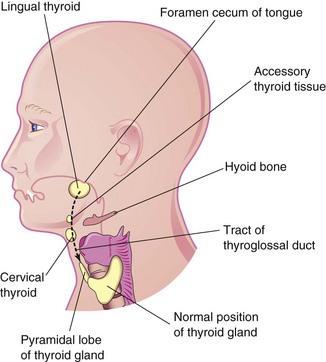
Figure 10–20 The head and neck, showing the usual sites of ectopic thyroid tissue. The broken line indicates the path followed by the thyroid gland during its descent, as well as the former tract of the thyroglossal duct.
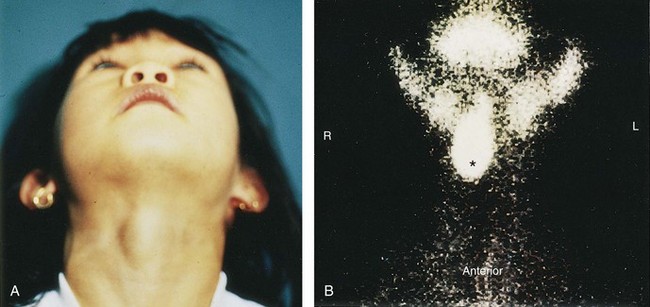
Figure 10–21 A, A sublingual thyroid mass in a 5-year-old girl. B, Technetium 99m pertechnetate scan showing an ectopic sublingual thyroid gland (*) without evidence of functioning thyroid tissue in the lower neck.
(From Leung AKC, Wong AL, Robson WLLM: Ectopic thyroid gland simulating a thyroglossal duct cyst: A case report. Can J Surg 38:87, 1995.)
At 11 weeks, colloid begins to appear in the thyroid follicles; thereafter, iodine concentration and the synthesis of thyroid hormones can be demonstrated.
Development of Tongue 
Near the end of the fourth week, a median triangular elevation appears in the floor of the primordial pharynx, just rostral to the foramen cecum (Fig. 10-22A). This swelling—the median lingual swelling—is the first indication of tongue development. Two oval lateral lingual swellings soon develop on each side of the median swelling. The swellings result from the proliferation of mesenchyme in the ventromedial parts of the first pair of pharyngeal arches. The lateral swellings rapidly increase in size, merge, and overgrow the median tongue swelling.
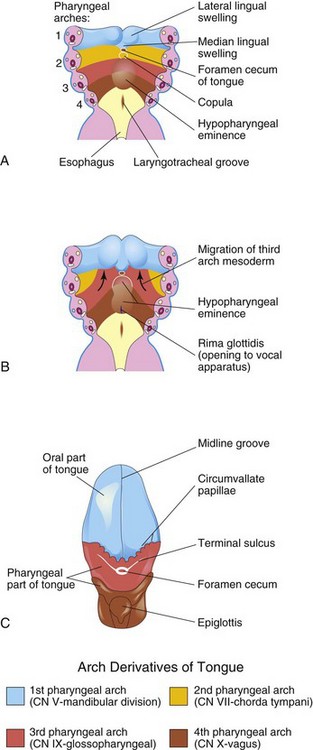
Figure 10–22 A and B, Schematic horizontal sections through the pharynx at the level shown in Figure 10-4A, showing successive stages in the development of the tongue during the fourth and fifth weeks. C, The adult tongue, showing the pharyngeal arch derivation of the nerve supply of its mucosa.
The merged lateral swellings form the anterior two thirds, or the oral part, of the tongue (Fig. 10-22C). The plane of fusion of the lateral swellings is indicated superficially by the midline groove of the tongue and internally by the fibrous lingual septum. The median lingual swelling forms no recognizable part of the adult tongue.
Formation of the posterior third, or the pharyngeal part, of the tongue is indicated by two elevations that develop caudal to the foramen cecum (Fig. 10-22A):
• The copula forms by fusion of the ventromedial parts of the second pair of pharyngeal arches.
• The hypopharyngeal eminence develops caudal to the copula from mesenchyme in the ventromedial parts of the third and fourth pairs of pharyngeal arches.
Congenital Lingual Cysts and Fistulas
Cysts in the tongue may be derived from remnants of the thyroglossal duct (Fig. 10-15B). They may enlarge and produce pharyngeal pain, dysphagia (difficulty in swallowing), or both. Fistulas may also arise as a result of persistence of the lingual parts of the thyroglossal duct; such fistulas open through the foramen cecum into the oral cavity.
The copula is gradually overgrown by the hypopharyngeal eminence and disappears (Fig. 10-22B and C). As a result, the pharyngeal part of the tongue develops from the rostral part of the hypopharyngeal eminence. The line of fusion of the anterior and posterior parts of the tongue is roughly indicated by a V-shaped groove called the terminal sulcus (Fig. 10-22C). The pharyngeal arch mesenchyme forms the connective tissue and vasculature of the tongue. The intrinsic tongue muscles are derived from myoblasts that migrate from the occipital somites (see Fig. 10-6A). The hypoglossal nerve (CN XII) accompanies the myoblasts during their migration and innervates the tongue muscles as they develop.
Ankyloglossia
The lingual frenulum normally connects the inferior surface of the tongue to the floor of the mouth (Fig. 10-23). Ankyloglossia (tongue-tie) occurs in approximately 1 in 300 North American infants, but it is usually of no functional significance. A short frenulum usually stretches with time, making surgical correction of the anomaly unnecessary.
Papillae and Taste Buds of Tongue
The lingual papillae appear by the end of the eighth week. The vallate and foliate papillae appear first, close to the terminal branches of the glossopharyngeal nerve. The fungiform papillae appear later, near the terminations of the chorda tympani branch of the facial nerve. Filiform papillae, the most common papillae, develop during the early fetal period (10–11 weeks). They contain afferent nerve endings that are sensitive to touch.
Taste buds develop during weeks 11 to 13 by inductive interaction between the epithelial cells of the tongue and invading gustatory nerve cells from the chorda tympani, glossopharyngeal, and vagus nerves. Facial responses in the fetus can be induced by bitter-tasting substances at 26 to 28 weeks, indicating that reflex pathways between taste buds and facial muscles are established by this stage.
Nerve Supply of Tongue
The sensory supply to the mucosa of almost the entire anterior tongue (oral part) is from the lingual branch of the mandibular division of the trigeminal nerve (CN V), the nerve of the first pharyngeal arch (Fig. 10-22C). Although the facial nerve is the nerve of the second pharyngeal arch, its chorda tympani branch supplies the taste buds in the anterior tongue, except for the vallate papillae. Because the second arch component, the copula, is overgrown by the third arch component (hypopharyngeal eminence), the facial nerve does not supply any of the tongue mucosa, except for the taste buds. The vallate papillae in the anterior tongue are innervated by the glossopharyngeal nerve of the third pharyngeal arch (Fig. 10-22C). The posterior tongue (pharyngeal part) is innervated mainly by the glossopharyngeal nerve (CN IX). The superior laryngeal branch of the vagus nerve of the fourth arch supplies a small area of the tongue anterior to the epiglottis (Fig. 10-22C). All muscles of the tongue are supplied by the hypoglossal nerve (CN XII), except for the palatoglossus, which is supplied from the pharyngeal plexus by fibers arising from the vagus nerve.
Development of Salivary Glands
During the sixth and seventh weeks, the salivary glands begin as solid epithelial buds from the endoderm of the primordial oral cavity (Fig. 10-7C). The ends of the buds grow into the underlying mesenchyme. The connective tissue in the glands is derived from neural crest cells. All secretory (parenchymal) tissue arises by proliferation of the oral epithelium.
The parotid glands are the first to appear (early in the sixth week). They develop early in the sixth week from the oral ectodermal lining near the angles of the stomodeum. The buds grow toward the ears and branch to form solid cords with rounded ends. Later, the cords canalize and become ducts by approximately 10 weeks. The rounded ends of the cords differentiate into acini, which begin to secrete at 18 weeks. The capsule and the connective tissue develop from the surrounding mesenchyme.
The submandibular glands appear late in the sixth week. They develop from endodermal buds in the floor of the stomodeum. Solid cellular processes grow posteriorly, lateral to the developing tongue. Later they branch and differentiate. Acini begin to form at 12 weeks and secretory activity begins at 16 weeks. Growth of the submandibular glands continues after birth, with the formation of mucous acini. Lateral to the developing tongue, a linear groove forms that soon closes over to form the submandibular duct.
The sublingual glands appear in the eighth week, approximately 2 weeks later than the other salivary glands (Fig. 10-7). They develop from multiple endodermal epithelial buds in the paralingual sulcus. These buds branch and canalize to form 10 to 12 ducts that open independently into the floor of the mouth.
Development of the Face 
The facial primordia begin to appear around the primordial stomodeum early in the fourth week (Fig. 10-24A). Facial development depends on the inductive influence of organizing centers. The prosencephalic organizing center, derived from the prechordal mesoderm that migrates from the primitive streak, is located rostral to the notochord and ventral to the prosencephalon, or forebrain (see Chapter 6). The rhombencephalic organizing center is ventral to the rhombencephalon (hindbrain).
The five facial primordia, which appear around the stomodeum, are:
Both paired prominences are derivatives of the first pair of pharyngeal arches. The prominences are produced by mesenchyme derived from neural crest cells that migrate into the arches during the fourth week of development. These cells are the major source of connective tissue components, including cartilage, bone, and ligaments in the facial and oral regions.
The frontonasal prominence surrounds the ventrolateral part of the forebrain, which gives rise to the optic vesicles that form the eyes (Figs. 10-24A and 10-25). The frontal part of the frontonasal prominence forms the forehead; the nasal part of the frontonasal prominence forms the rostral boundary of the stomodeum and the nose.
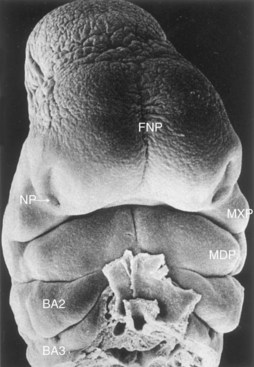
Figure 10–25 Scanning electron micrograph showing a ventral view of a human embryo at approximately 33 days (stage 15; crown–rump length [CRL], 8 mm). Observe the prominent frontonasal prominence (FNP) surrounding the telencephalon (forebrain). Also observe the nasal pits (NP) located in the ventrolateral regions of the frontonasal prominence. Medial and lateral nasal prominences surround these pits. The wedge-shaped maxillary prominences (MXP) form the lateral boundaries of the stomodeum. The fusing mandibular prominences (MDP) are located just caudal to the stomodeum. The second pharyngeal arch (BA2) is clearly visible and shows overhanging margins (opercula). The third pharyngeal (branchial) arch (BA3) is also clearly visible.
(From Hinrichsen K: The early development of morphology and patterns of the face in the human embryo. Adv Anat Embryol Cell Biol 98:1, 1985.)
The maxillary prominences form the lateral boundaries of the stomodeum, whereas the mandibular prominences constitute the caudal boundary of the primordial mouth (Figs. 10-24A and 10-25). The lower jaw and the lower lip are the first parts of the face to form. They result from merging of the medial ends of the mandibular prominences.
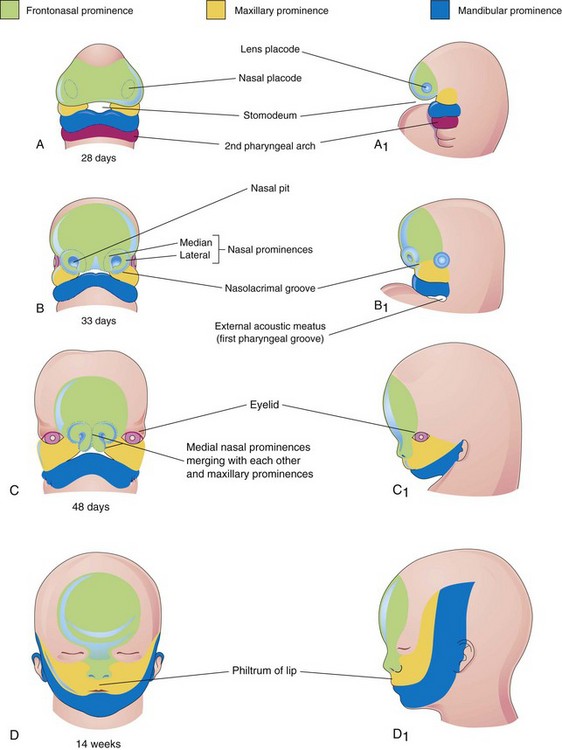
By the end of the fourth week, bilateral oval thickenings of the surface ectoderm—nasal placodes—have developed on the inferolateral parts of the frontonasal prominence (Figs. 10-25 and 10-26A and B). Initially, these placodes are convex, but later, they are stretched to produce a flat depression in each placode. The mesenchyme in the margins of the placodes proliferates, producing horseshoe-shaped elevations—the medial and lateral nasal prominences (Figs. 10-24B and 10-26D and E). As a result, the nasal placodes lie in depressions, called nasal pits (Figs. 10-24B and 10-26C and D). These pits are the primordia of the anterior nares (nostrils) and nasal cavities (Fig. 10-26E). Proliferation of mesenchyme in the maxillary prominences causes them to enlarge and grow medially toward each other and the nasal prominences (Figs. 10-24B and C and 10-25). The medial migration of the maxillary prominences moves the medial nasal prominences toward the median plane and each other. Each lateral nasal prominence is separated from the maxillary prominence by a cleft called the nasolacrimal groove (Fig. 10-24B).
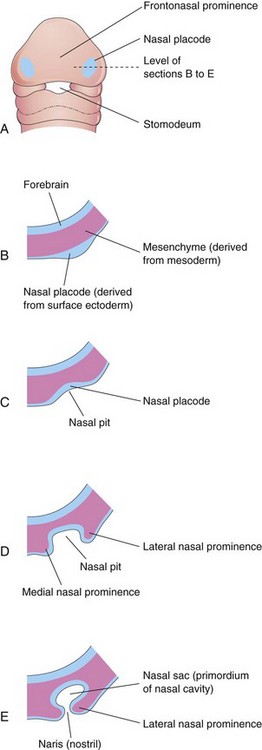
Figure 10–26 Progressive stages in the development of a human nasal sac (primordial nasal cavity). A, Ventral view of an embryo at approximately 28 days. B to E, Transverse sections through the left side of the developing nasal sac.
By the end of the fifth week, six auricular hillocks—primordia of the auricle—(mesenchymal swellings) form around the first pharyngeal groove (three on each side), the primordium of the external acoustic meatus (canal). Initially, the external ears are positioned in the neck region; however, as the mandible develops, they ascend to the side of the head at the level of the eyes (Fig. 10-24B and C). By the end of the sixth week, each maxillary prominence has begun to merge with the lateral nasal prominence along the line of the nasolacrimal groove (Fig. 10-27A and B). This establishes continuity between the side of the nose, formed by the lateral nasal prominence, and the cheek region, formed by the maxillary prominence.
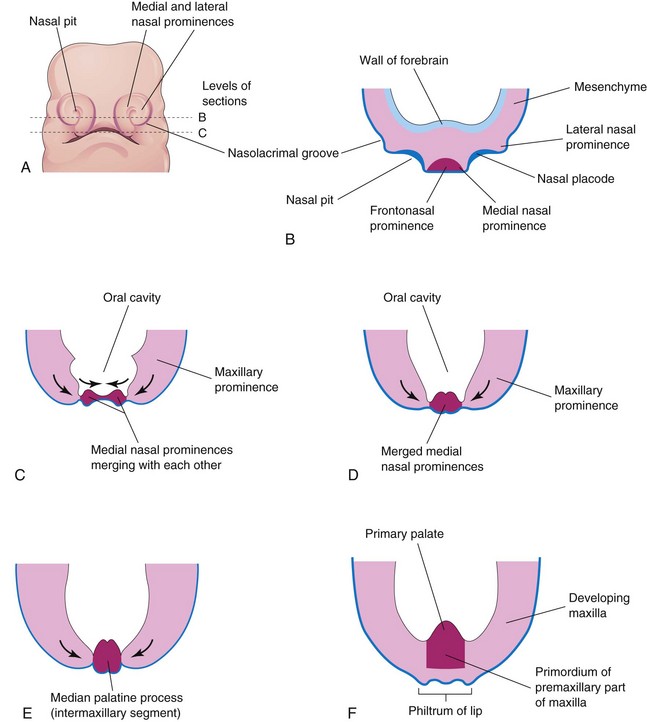
Figure 10–27 Illustrations of the early development of the maxilla, palate, and upper lip. A, Facial view of a 5-week embryo. B and C, Sketches of horizontal sections at the levels shown in A. The arrows indicate subsequent growth of the maxillary and medial nasal prominences toward the median plane and merging of the prominences with each other. D to F, Similar sections of older embryos showing merging of the medial nasal prominences with each other and with the maxillary prominences to form the upper lip.
The nasolacrimal duct develops from a rod-like thickening of ectoderm in the floor of the nasolacrimal groove. This thickening gives rise to a solid epithelial cord that separates from the ectoderm and sinks into the mesenchyme. Later, as a result of apoptosis (programmed cell death), the cord canalizes to form the nasolacrimal duct. The cranial end of this duct expands to form the lacrimal sac. In the late fetal period, the nasolacrimal duct drains into the inferior meatus in the lateral wall of the nasal cavity. The duct usually becomes completely patent only after birth.
Between weeks 7 and 10, the medial nasal prominences merge with each other and with the maxillary and lateral nasal prominences (Fig. 10-24C), resulting in disintegration of their contacting surface epithelia. This causes intermingling of the underlying mesenchyme. Merging of the medial nasal and maxillary prominences results in continuity of the upper jaw and lip and separation of the nasal pits from the stomodeum. As the medial nasal prominences merge, they form an intermaxillary segment (Figs. 10-27C to F). The intermaxillary segment gives rise to the following:
• The deep median part of the upper lip
• The premaxillary part of the maxilla and its associated gingiva (gum)
The lateral parts of the upper lip, most of the maxilla, and the secondary palate form from the maxillary prominences (Fig. 10-24D). These prominences merge laterally with the mandibular prominences. Recent studies show that the lower part of the medial nasal prominences becomes deeply positioned and are covered by medial extensions of the maxillary prominences to form the philtrum. The primordial lips and cheeks are invaded by myoblasts from the second pair of pharyngeal arches, which differentiate into the facial muscles (see Fig. 10-6 and Table 10-1). The myoblasts from the first pair of arches differentiate into the muscles of mastication.
Development of Nasal Cavities 
As the face develops, the nasal placodes become depressed, forming nasal pits (Figs. 10-25 and 10-26). Proliferation of the surrounding mesenchyme forms the medial and lateral nasal prominences and results in deepening of the nasal pits and formation of primordial nasal sacs. Each nasal sac grows dorsally, ventral to the developing forebrain (Fig. 10-28A). At first, the nasal sacs are separated from the oral cavity by the oronasal membrane. This membrane ruptures by the end of the sixth week of development, bringing the nasal and oral cavities into communication (Fig. 10-28B and C). The regions of continuity between the nasal and oral cavities are the primordial choanae, which lie posterior to the primary palate. After the secondary palate develops, the choanae are located at the junction of the nasal cavity and pharynx (Fig. 10-28D). While these changes are occurring, the superior, middle, and inferior conchae develop as elevations of the lateral walls of the nasal cavities (see Fig. 10-30D). Concurrently, the ectodermal epithelium in the roof of each nasal cavity becomes specialized to form the olfactory epithelium. Some epithelial cells differentiate into olfactory receptor cells. The axons of these cells constitute the olfactory nerves, which grow into the olfactory bulbs of the brain (Fig. 10-28C and D).
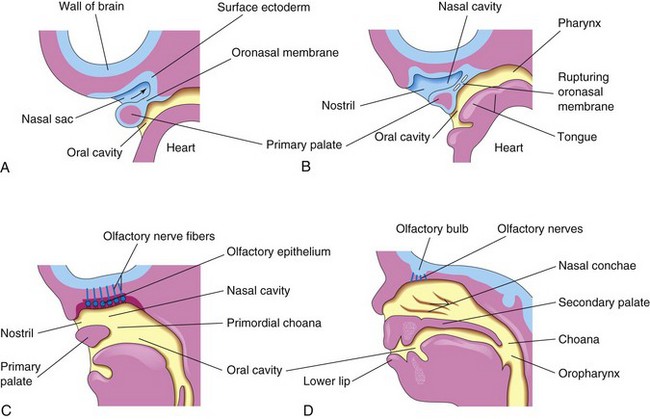
Figure 10–28 Sagittal sections of the head, showing development of the nasal cavities. The nasal septum has been removed. A, At 5 weeks. B, At 6 weeks, showing breakdown of the oronasal membrane. C, At 7 weeks, showing the nasal cavity communicating with the oral cavity and the development of the olfactory epithelium. D, At 12 weeks. The palate and the lateral wall of the nasal cavity are evident.
Paranasal Sinuses
Some paranasal sinuses, in particular, the maxillary sinuses, begin to develop during late fetal life; the remainder of them develop after birth. They form from outgrowths (diverticula) of the walls of the nasal cavities, becoming pneumatic (air-filled) extensions of the nasal cavities in the adjacent bones. The original openings of the diverticula persist as the orifices of the adult sinuses.
Paranasal Sinuses in Neonatal and Postnatal Development
Most of the paranasal sinuses are rudimentary or absent in newborns. The maxillary sinuses are small at birth. They grow slowly until puberty and are not fully developed until all of the permanent teeth have erupted in early adulthood. No frontal or sphenoid sinuses are present at birth. The ethmoid cells (sinuses) are small before 2 years, and they do not begin to grow rapidly until 6 to 8 years. At approximately 2 years, the two most anterior ethmoid cells grow into the frontal bone, forming a frontal sinus on each side. Usually, the frontal sinuses are visible on radiographs by 7 years. The two most posterior ethmoid cells grow into the sphenoid bone at approximately 2 years, forming two sphenoid sinuses. Growth of the paranasal sinuses is important in altering the size and shape of the face during infancy and childhood and in adding resonance to the voice during adolescence.
Development of Palate 
The palate develops from two primordia: the primary palate and the secondary palate.
Palatogenesis begins in the sixth week but is not completed until the 12th week. The critical period of development of the palate is from the end of the sixth week until the beginning of the ninth week.
Primary Palate
Early in the sixth week, the primary palate (median palatine process) begins to develop from the deep part of the intermaxillary segment of the maxilla (Figs. 10-27F and 10-28). Initially, this segment is a wedge-shaped mass of mesenchyme between the internal surfaces of the maxillary prominences of the developing maxillae. The primary palate forms the premaxillary part of the maxilla (Fig. 10-29B). It represents only a small part of the adult hard palate (the part anterior to the incisive fossa).
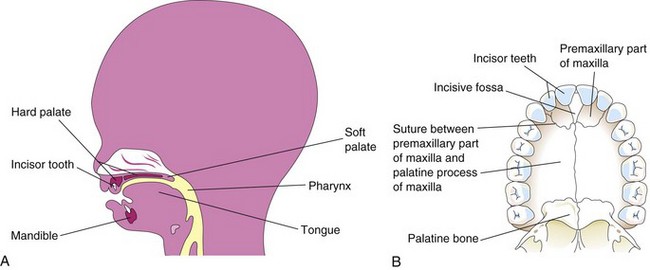
Figure 10–29 A, Sagittal section of the head of a 20-week fetus, showing the location of the palate. B, The bony palate and the alveolar arch of a young adult. The suture between the premaxillary part of the maxilla and the fused palatal processes of the maxillae is usually visible in the crania of young persons.
Secondary Palate
The secondary palate is the primordium of the hard and soft parts of the palate (Figs. 10-28D and 10-29A and B). The secondary palate begins to develop early in the sixth week from two mesenchymal projections that extend from the internal aspects of the maxillary prominences. Initially, these structures—lateral palatine processes (palatal shelves)—project inferomedially on each side of the tongue (Figs. 10-30A to C). As the jaws develop, the tongue becomes relatively smaller and moves inferiorly. During the seventh and eighth weeks, the lateral palatine processes elongate and ascend to a horizontal position superior to the tongue. Gradually, the processes approach each other and fuse in the median plane (Fig. 10-30D to H). They also fuse with the nasal septum and the posterior part of the primary palate. Elevation of the palatine processes to the horizontal position is believed to be caused by an intrinsic force that is generated by the hydration of hyaluronic acid in the mesenchymal cells within the palatine processes. The medial epithelial seam at the edges of the palatine shelves breaks down, allowing for the fusion of the palatine shelves.
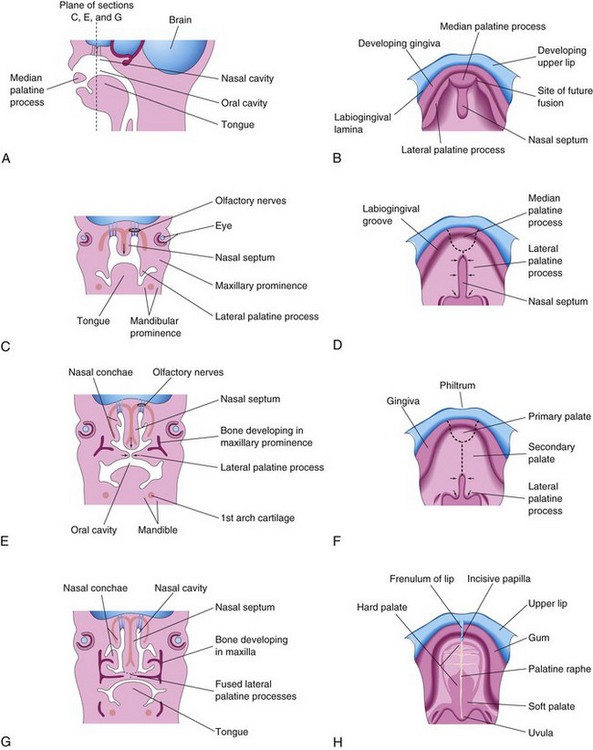
Figure 10–30 A, Sagittal section of the embryonic head at the end of the sixth week, showing the median palatine process, or primary palate. B, D, F, and H, The roof of the mouth from the sixth to 12th weeks, showing the development of the palate. The broken lines indicate the sites of fusion of the palatine processes. The arrows indicate medial and posterior growth of the lateral palatine processes. C, E, and G, Frontal sections of the head, showing fusion of the lateral palatine processes with each other and with the nasal septum, and separation of the nasal and oral cavities.
The nasal septum develops in a downward growth pattern from internal parts of the merged medial nasal prominences (Fig. 10-30C, E, and G). The fusion between the nasal septum and the palatine processes begins anteriorly during the ninth week and is completed posteriorly by the 12th week, superior to the primordium of the hard palate (Fig. 10-30D and F). Bone gradually develops by intramembranous ossification (see Chapter 15) in the primary palate, forming the premaxillary part of the maxilla, which lodges the incisor teeth (Fig. 10-29B). Concurrently, bone extends from the maxillae and palatine bones into the lateral palatine processes to form the hard palate (Fig. 10-30E and G). The posterior parts of these processes do not become ossified; they extend posteriorly beyond the nasal septum and fuse to form the soft palate, including its conical projection, the uvula (Fig. 10-30D, F, and H). The median palatine raphe indicates the line of fusion of the lateral palatine processes. A small nasopalatine canal persists in the median plane of the palate, between the premaxillary part of the maxilla and the palatine processes of the maxillae. This canal is represented in the adult hard palate by the incisive fossa (Fig. 10-29B). An irregular suture runs from the incisive fossa to the alveolar process of the maxilla, between the lateral incisor and the canine teeth on each side, indicating where the embryonic primary and secondary palates fused.
Cleft Lip and Cleft Palate
Clefts of the upper lip and palate are common. The defects are classified according to developmental criteria, with the incisive fossa and papilla used as reference landmarks (Fig. 10-29B and see Fig. 10-34A). Cleft and cleft palate are especially conspicuous because they result in an abnormal facial appearance and defective speech (Fig. 10-31). Two major groups of cleft lip and cleft palate are recognized (Figs. 10-32 to 10-34):
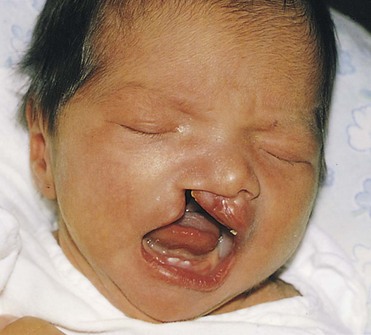
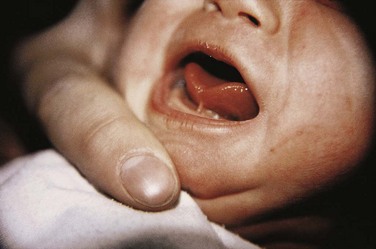
Figure 10–31 Infant with unilateral cleft lip and cleft palate. Clefts of the lip, with or without a cleft palate, occur in approximately 1 in 1000 births; most affected individuals are boys.
(Courtesy of A.E. Chudley, M.D., professor of pediatrics and child health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
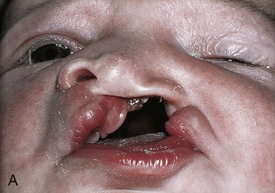
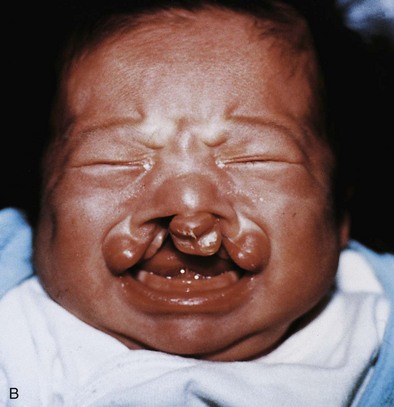
Figure 10–32 Congenital anomalies of the lip and palate. A, Infant with a left unilateral cleft lip and a cleft palate. B, Infant with a bilateral cleft lip and a cleft palate.
(Courtesy of Dr. Barry H. Grayson and Dr. Bruno L. Vendittelli, New York University Medical Center, Institute of Reconstructive Plastic Surgery, New York, NY.)
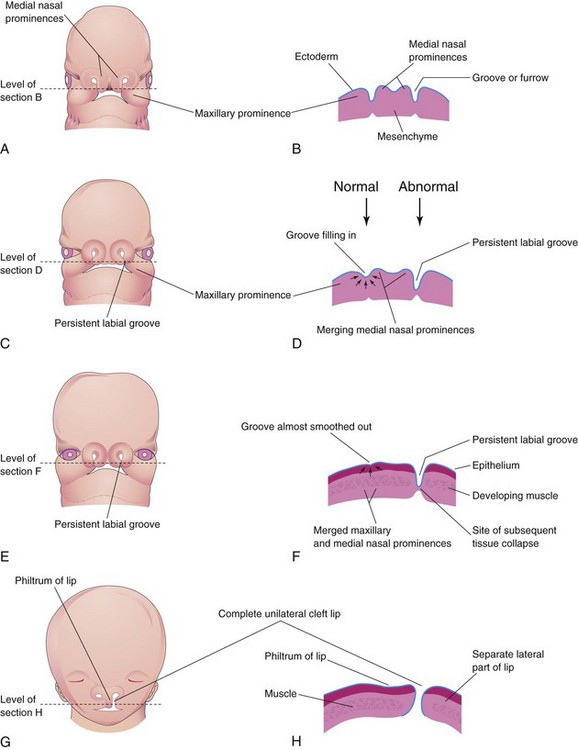
Figure 10–33 Illustrations of the embryologic basis for a complete unilateral cleft lip. A, A 5-week embryo. B, Horizontal section through the head, showing the grooves between the maxillary prominences and the merging medial nasal prominences. C, A 6-week embryo, showing a persistent labial groove on the left side. D, Horizontal section through the head, showing the groove gradually filling in on the right side after proliferation of the mesenchyme (arrows). E, A 7-week embryo. F, Horizontal section through the head, showing that the epithelium on the right has almost been pushed out of the groove between the maxillary and medial nasal prominences. G, A 10-week fetus with a complete unilateral cleft lip. H, Horizontal section through the head after stretching of the epithelium and breakdown of the tissues in the floor of the persistent labial groove on the left side, resulting in the formation of a complete unilateral cleft lip.
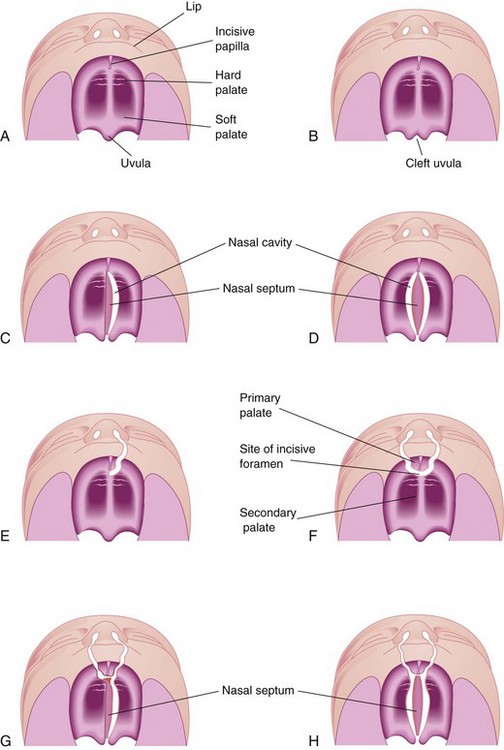
Figure 10–34 Various types of cleft lip and cleft palate. A, Normal lip and palate. B, Cleft uvula. C, Unilateral cleft of the posterior, or secondary, palate. D, Bilateral cleft of the posterior palate. E, Complete unilateral cleft of the lip and the alveolar process of the maxilla, with a unilateral cleft of the anterior, or primary, palate. F, Complete bilateral cleft of the lip and the alveolar processes of the maxillae, with bilateral cleft of the anterior palate. G, Complete bilateral cleft of the lip and the alveolar processes of the maxillae, with bilateral cleft of the anterior palate and unilateral cleft of the posterior palate. H, Complete bilateral cleft of the lip and the alveolar processes of the maxillae, with complete bilateral cleft of the anterior and posterior palate.
Anterior cleft anomalies include cleft lip, with or without cleft of the alveolar part of the maxilla. A complete cleft anomaly is one in which the cleft extends through the lip and the alveolar part of the maxilla to the incisive fossa, separating the anterior and posterior parts of the palate (Fig. 10-34E and F). Anterior cleft anomalies result from a deficiency of mesenchyme in the maxillary prominences and the median palatine process (Fig. 10-27D and E).
Posterior cleft anomalies include clefts of the secondary, or posterior, palate that extend through the soft and hard regions of the palate to the incisive fossa, separating the anterior and posterior parts of the palate (Fig. 10-34G and H). Posterior cleft anomalies are caused by defective development of the secondary palate and result from growth distortions in the lateral palatine processes that, in turn, prevent the medial migration and fusion of these processes.
Clefts involving the upper lip, with or without cleft palate, occur in approximately 1 in 1000 births; however, their frequency varies widely, and 60% to 80% of those affected are boys. The clefts vary in severity from small notches in the vermilion border of the lip (Fig. 10-33G) to larger clefts that extend into the floor of the nostril and through the alveolar part of the maxilla (Figs. 10-32A and 10-34E). Cleft lip can be unilateral or bilateral.
Unilateral cleft lip (Fig. 10-32A) results from failure of the maxillary prominence on the affected side to unite with the merged medial nasal prominences (Fig. 10-33A to H), in turn, causing a persistent labial groove. The tissues in the floor of the persistent groove break down. As a result, the lip is divided into medial and lateral parts. Sometimes, a bridge of tissue, called a Simonart band, joins the parts of the incomplete cleft lip.
Bilateral cleft lip (Figs. 10-32B and 10-34F) results from failure of the mesenchymal masses in the maxillary prominences to meet and unite with the merged medial nasal prominences. When there is a complete bilateral cleft of the lip and the alveolar part of the maxilla, the intermaxillary segment hangs free and projects anteriorly. These defects are especially deforming because of the loss of continuity of the orbicularis oris muscle, which closes the mouth and purses the lips.
Median cleft lip is an extremely rare defect. It results from partial or complete failure of the medial nasal prominences to merge and form the intermaxillary segment. Median cleft of the lower lip is also very rare and is caused by failure of the mandibular prominences to merge completely.
The landmark for distinguishing anterior from posterior cleft anomalies is the incisive fossa. Anterior and posterior cleft anomalies are embryologically distinct.
Cleft palate, with or without cleft lip, occurs in approximately 1 in 2500 births and is more common in girls than in boys. The cleft may involve only the uvula, giving it a fish-tail appearance (Fig. 10-34B), or it may extend through the soft and hard regions of the palate (Fig. 10-34C and D). In severe cases associated with cleft lip, the cleft in the palate extends through the alveolar part of the maxilla and the lips on both sides (Fig. 10-34G and H).
Unilateral and bilateral clefts in the palate are classified into three groups:
• Clefts of the anterior palate result from failure of the lateral palatine processes to meet and fuse with the primary palate (Fig. 10-34F).
• Clefts of the posterior palate result from failure of the lateral palatine processes to meet and fuse with each other and with the nasal septum (Fig. 10-30E).
• Clefts of the anterior and posterior parts of the palate result from failure of the lateral palatine processes to meet and fuse with the primary palate, with each other, and with the nasal septum.
Most clefts of the lip and palate result from multiple factors (multifactorial inheritance; see Chapter 19). Some clefts of the lip, palate, or both appear as part of syndromes determined by single mutant genes. Other clefts are features of chromosomal syndromes, especially trisomy 13. A few cases of cleft lip or cleft palate appear to be caused by teratogenic agents (e.g., anticonvulsant drugs). A sibling of a child with a cleft palate has an elevated risk of having a cleft palate, but no increased risk of cleft lip. A cleft of the lip and the alveolar process of the maxilla that continues through the palate is usually transmitted through a male sex-linked gene.
Facial Clefts
Various types of facial cleft occur but they are extremely rare. Severe clefts are usually associated with gross anomalies of the head. Oblique facial clefts (orbitofacial fissures) are often bilateral and extend from the upper lip to the medial margin of the orbit. When this occurs, the nasolacrimal ducts are open grooves (persistent nasolacrimal grooves). Oblique facial clefts associated with cleft lip result from failure of the maxillary prominences to merge with the lateral and medial nasal prominences. Lateral, or transverse, facial clefts run from the mouth toward the ear. Bilateral clefts result in a very large mouth, a condition called macrostomia. In severe cases, the clefts in the cheeks extend almost to the ears.
Clinically Oriented Questions
1. What kind of lip defect is a “harelip”? What is the clinical name for this birth defect?
2. Some say that embryos have cleft lips and that this common facial anomaly represents a persistence of this embryonic condition. Are these statements accurate?
3. Neither Clare nor her husband has a cleft lip or a cleft palate, and no one in either one of their families is known to have or to have had these anomalies. What are their chances of having a child with a cleft lip, with or without a cleft palate?
4. Mary’s son has a cleft lip and a cleft palate. Her brother has a similar defect involving his lip and palate. Although Mary does not plan to have any more children, her husband says that Mary is entirely to blame for their son’s birth defects. Was the defect likely inherited only from Mary’s side of the family?
5. A patient’s son has minor anomalies involving his external ears, but he does not have hearing problems or a facial malformation. Would his ear abnormalities be considered pharyngeal (branchial) defects?